Abstract
Background: Shufeng Jiedu capsule has been widely used in China for acute upper respiratory tract infections (AURTIs). The aim of this study was to evaluate its effectiveness and safety for AURTIs.
Methods: Randomized controlled trials comparing SFJD with conventional drug for patients with AURTIs were included. Eight databases were searched from their inceptions to February 2021. Data was synthesized using risk ration (RR) or mean difference (MD) with their 95% confidence interval (CI). The primary outcome was resolution time of typical symptoms.
Results: Twenty-five RCTs involving 3410 patients were included. SFJD in combination with conventional drug was associated with; in common cold shortening the duration of fever (MD −1.54 days, 95% CI [−2.15,−0.92], I2 = 80%, n = 385, 3 trials) and cough (MD −1.22 days, 95% CI [−1.52, −0.93]); in herpangina, shortening the duration of fever (MD -0.68 days, 95% CI [−1.15, −0.21], I2 = 68%, n = 140, 2 trials) and blistering (MD −0.99 days, 95% CI [−1.23, −0.76], n = 386, 3 trials); in acute tonsillitis and acute pharyngitis shortening the duration of fever (MD −1.13 days, 95% CI [−1.36, −0.90], I2 = 33%, n = 688, 7 trials) and sore throat (MD −1.13 days, 95% CI [−1.40, −0.86], I2 = 84.1%, n = 1194, 10 trials). SFJD also improving their cure rate with a range (1–5 days). No serious adverse events were reported.
Conclusion: Low certainty evidence suggests that SFJD appears to shorten the duration of symptoms in AURTIs, improve cure rate and seems safe for application. However, high quality placebo controlled trials are warranted to confirm its benefit.
Keywords: Chinese herbal medicine, Shufeng Jiedu, Respiratory tract infections, Randomized controlled trials, Systematic review
1. Introduction
Upper respiratory tract infections (URTIs) are one of the most commonly occurring diseases. In 2017, the global incidence of URTIs was 17.1 billion worldwide 1, which posed substantial socioeconomic burden to public health. The global 6090,503 disability-adjusted life years due to URTIs were lost in 20162. Acute upper respiratory tract infections (AURTIs) are the most common type of URTIs. According to the International Statistical Classification of Diseases (ICD-10), AURTIs include common cold, acute sinusitis, acute pharyngitis, acute tonsillitis, acute laryngitis and tracheitis, acute epiglottitis, and acute upper respiratory infections of multiple and unspecified sites 3. AURTIs are predominantly viral, and most AURTIs are mild and self-limited within 7 days 4. Antibiotics are recommended only for specific URTIs and validated clinical indications (such as acute pharyngitis and acute tonsillitis caused by streptococcus) in existing guidelines, for other AURTIs, symptomatic treatment were recommended 5,6. It was reported that the majority of antibiotic prescriptions for AURTIs were unnecessary and ineffective7, 8, 9. Additionally, inappropriate use of antibiotics may lead to bacterial resistance, and as many as 35,000 people die each year as a result in US 10.
Shufeng Jiedu (SFJD) capsule as an oral Chinese patent herbal medicine has been widely used in China for the treatment of AURTIs for 30 years 11. The ingredients of SFJD were presented in (Supplementary material). SFJD has been recommended in several Chinese national guidelines for the treatment of AURTIs 12, 13, 14. There are many clinical trials comparing the effect of SFJD with conventional drug for the treatment of AURTIs. Additionally, an in vitro study of antibacterial testing suggested that SFJD capsule had a broad-spectrum antibacterial effect 15, moreover, SFJD combined with oseltamivir had synergistic antiviral effects on respiratory infection 16. However, there is no systematic review to evaluate the effectiveness of SFJD on AURTIs. Therefore, this systematic review aims to collect all relevant randomized controlled trials (RCTs) on SFJD capsule for patients with AURTIs and to evaluate its therapeutic effect and safety.
2. Methods
This systematic review was reported following PRISMA 17 statement, and the protocol has been registered on INPLASY (INPLASY202050083)18.
2.1. Inclusion and exclusion criteria
2.1.1. Inclusion criteria
Parallel group RCTs regardless of blinding were included. The study population (P) was patients diagnosed with AURTIs or one of the classification of AURTIs regardless of gender, age or ethnicity from primary care or outpatient department of hospitals. AURTIs might include common cold, acute rhinitis, herpangina, acute pharyngitis or acute tonsillitis. The interventions (I) were SFJD capsule used alone or in combination with conventional drug. Conventional drug referred to symptomatic treatment (e.g., antipyretic and decongestant, etc.), symptomatic treatment plus antivirals or antibiotics. The controls (C) were placebo or conventional drug. The primary outcome (O) was the duration of typical symptoms in AURTIs (time to fever, cough, blisters or sore throat resolution). The secondary outcomes were cure rate and adverse events. Follow up of outcome measurements were all limited to within 5 days of treatment.
2.1.2. Exclusion criteria
Studies with a course of treatment over 7 days, and studies with other Chinese patent medicine controlled or studies failed to report the minimal required outcomes were excluded.
2.2. Search strategy
PubMed, the Cochrane Library, EMBASE, Web of Science, China National Knowledge Infrastructure (CNKI), Chinese Scientific Journal Database (VIP), SinoMed and Wanfang databases were searched from their inceptions to February 2021. A systematic search was also conducted in Clinical Trials.gov (www.clinicaltrials.gov) and Chinese Clinical Trial Registry (http://www.chictr.org.cn/index.aspx). Search strategy for PubMed: Shufengjiedu[Title/Abstract] OR Shu Feng Jie Du [Title/Abstract] OR Shu-Feng-Jie-Du [Title/Abstract] OR Shufeng Jiedu [Title/Abstract] OR Shufeng-Jiedu [Title/Abstract]. There was no limitation of language.
2.3. Study selection and data extraction
After removing duplicates, two authors (YY Zhang and RY Xia) independently screened studies by titles and abstracts. In the full text screening process, uncertainty and insufficient information was determined for eligibility through obtaining full texts, and discrepancies were resolved by discussion or determined by a third-party adjudication. Reasons for excluding studies were recorded at this stage. A predefined data extraction form was used by paired reviewers (YY Zhang and SB Liang; YL Li and LY Zhao) to extract data independently, including study ID, population, interventions, comparisons, outcomes, and design characteristics (sample size, setting and funding).
2.4. Quality assessments
The quality assessment of individual trial was performed using Cochrane Risk of Bias tool 2.019. This revised tool includes five items as following: randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome and selection of the reported result. The included trials were assessed as low risk of bias, some concerns or high risk of bias in each domain19,20. The method of GRADE 21 (Grading of Recommendations Assessment, Development and Evaluation) was employed to rate the certainty of evidence in five domains (risk of bias, directness, precision, consistency, and the possibility of publication bias).
2.5. Data synthesis
For dichotomous data, we calculated risk ratio (RR) with 95% confidence interval (CI); for continuous data, we calculated mean difference (MD) with 95% CI. Cochrane Review Manager 5.4 software was employed for data analyses. In meta-analysis, Cochrane Q test and I² statistic were employed to assess statistical heterogeneity 20. A fixed-effects model was considered when I2 < 30%, otherwise, a random-effects model was used. According to the classification of AURTIs in ICD-103, there is no substantial pathophysiologic difference between common cold and acute rhinitis, and therefore we combined them together. Additionally, conventional drug refers to symptomatic treatment used alone or in combination with antibiotics or antivirals, they were considered as a whole. We lumped them together to demonstrate the overall differences of therapeutic effect between SFJD and conventional drug, as well as to make the results more clearly. Funnel plots were performed through Review Manager 5.4 software to detect the possibility of publication bias, if ten or more studies were included in a meta-analysis. To explain heterogeneity, we predefined subgroup analysis in terms of the severity of AURTIs and different ages of patients (adults, children aged between 2 and 14 years old). When the primary outcomes showed clinically meaningful differences between groups, sensitivity analysis was employed to test the robustness of the results in following methodological domains: comparison between clear and unclear randomization concealment; comparison between placebo used and not used; comparison between reported loss- to-follow-up and not reported.
3. Results
3.1. Screening
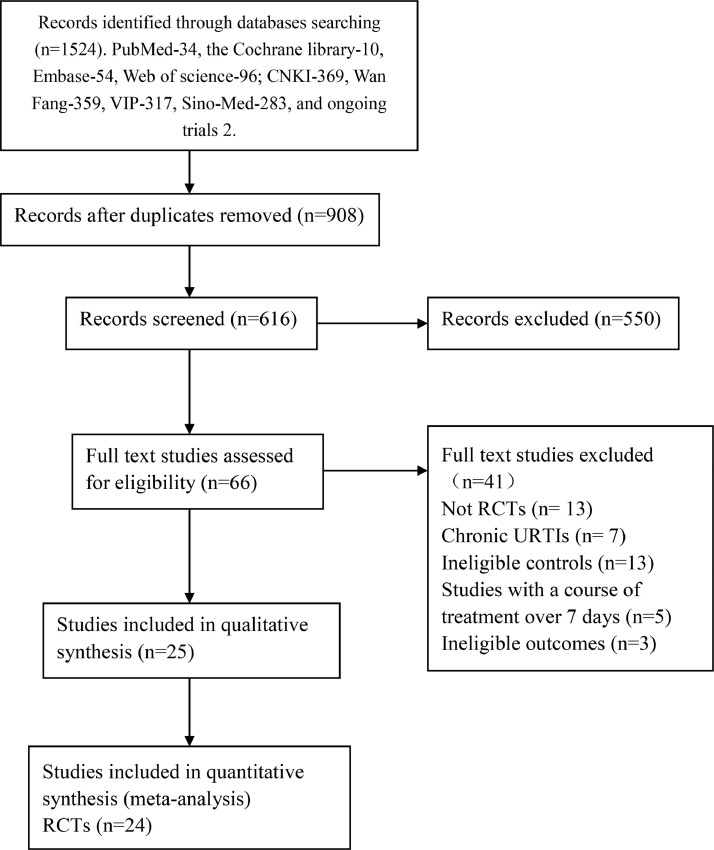
Initially 1524 records were retrieved. Among which 908 duplicates were removed. 550 records were excluded after title and abstract screening, remaining 66 records. Full-text screening identified 25 RCTs involving 3410 participants, of which 1069 were children. One placebo controlled trial on common cold 29 was narratively described and hence could not be included in the quantitative synthesis (Fig. 1).
Fig. 1.
Flow chart of study selection. RCT: randomized controlled trials; URTIs: upper respiratory tract infections.
3.2. Study characteristics
There were ten trials 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 focused on common cold, three 33, 34, 35 on herpangina, four 36, 37, 38, 39on acute pharyngitis, seven 40, 41, 42, 43, 44, 45, 46 on acute tonsillitis and one 32 on acute rhinitis. All trials were conducted in China and there was only one 29 multi-center trial, the rest were single center trials. None of the trials reported the funding or conflicts of interest. Sample size varied from 60 to 246 and the mean age varied from 2.4 to 70.9 years. Baseline major symptoms reported were fever, cough and stuffy nose in common cold; fever and blisters in herpangina; fever and sore throat in acute pharyngitis and acute tonsillitis. The duration of treatment varied from 3 to 7 days, and the outcomes were all measured within 5 days from starting treatment. The baseline body temperature in trials were presented in (Table 1). The criteria details of cure rate in individual studies were presented in (Supplementary material). The dose reported in these trials was three times daily administration of SFJD, for adults and children over 10 years old was 4 capsules per time, 3 capsules for 6 to 10 years old, 2 capsules for 3 to 6 years old, and 1 capsule for 2 to 3 years old. Conventional drug referred to symptomatic treatment used alone or in combination with antibiotics or antivirals. Symptomatic treatment included antipyretic 22,24,33,45, decongestant 26,36, antitussive 22, 23, 24, expectorants 25, 28 and saline nasal spray 32; antiviral drugs included ribavirin 22-23,34-35 and interferon 33; antibiotics included amoxicillin 41,45, cefaclor 40,46 and cefuroxime 42,44. Only one included trial was placebo controlled 29. The characteristics of included trials were shown in Table 1.
Table 1.
Characteristics of included randomized trials on SFJD capsules for AURTIs.
| Study ID | Population | Sample size | Average age (year) | Male (%) | Time from symptom onset when included (Hours) | TCM syndrome differentiation | Baseline major symptoms | Intervention | Comparison | Treatment duration (days) | Measurement time of outcomes (days) | Outcomes reporteda |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Common cold | Fever, cough | |||||||||||
| Chen H 2016 [22] | Children | 78/78 | 6.3/6.5 | 44.9/52.6 | NR | NR | >37.3℃ | SFJD+ST +ribavirin | ST +ribavirin | 7d | 5d | 2,3,6 |
| Zhou XF 2016[23] | Children | 70/70 | 5.1/4.8 | 48.6/44.348 | 48hr | Wind-heat syndrome | 37.3–41.6℃ | SFJD +ST+ ribavirin | ST+ ribavirin | 5d | 5d | 2,3,6 |
| Wang Q 2018[24] | Children | 45/44 | 7.15/7.2 | 53.3/52.27 | NR | NR | >37.3℃ | SFJD +ST+ antivirals | ST+ antivirals | 5d | 5d | 2,3,6 |
| Zhang YP 2014[25] | Adults | 110/110 | 40.2/39.81 | 54.5/59.0 | NR | NR | >37.3℃ | SFJD+ST | ST | 7d | 1d | 1 |
| Ye XQ 2013[26] | Adults | 100/100 | 38.55/37.85 | 52/49 | 48hr | Wind-heat syndrome | 37.3–39.0℃ | SFJD+ST | ST | 3d | 3d | 1,2,6 |
| Zhao JL 2018[27] | Adults | 61/62 | 45.69/45.21 | 49.1/53.2 | NR | NR | >37.3℃ | SFJD+ST | ST | 3d | 3d | 1,6 |
| Zhang B 2020[28] | Adults | 119/118 | 31.94/35.94 | 56.8/54.6 | NR | NR | 37.3–39.0℃ | SFJD+ST | ST | 3d | 3d | 1,6 |
| Xu YL 2015[29] | Adults | 120/120 | 49.24/47.77 | 60/57.9 | 36hr | Wind-heat syndrome | 37.3–39.0℃ | SFJD | placebo | 7d | 3d | 1,2,6 |
| Wu XJ 2015[30] | Adults | 112/119 | 70.9/70.8 | 59.8/59.8 | NR | NR | >37.3℃ | SFJD+ST+ antibiotics | ST+ antibiotics | 7d | 3d | 1 |
| Zhao LB 2020 [31] | Adults | 78/78 | 37.3/36.8 | 52.3/51.3 | 36h | Wind-heat syndrome | 37.1–39.1℃ | SFJD +ST+ antivirals | ST+ antivirals | 5d | 5d | 6 |
| Acute rhinitis | Fever, stuffy nose | |||||||||||
| Li Y 2015[32] | Adults | 30/30 | 49.2/50.3 | 43.3/46.7 | 72hr | Wind-heat syndrome | >37.3℃ | SFJD+ST | ST | 7d | 5d | 6 |
| Herpangina | Fever, blisters | |||||||||||
| Yang Y 2019[33] | Children | 35/35 | 4.55/4.2 | 51.4/42.9 | 48hr | NR | >37.3℃ | SFJD +ST+ interferon | ST+ interferon | 5d | 5d | 2,5,6 |
| Yang ML 2016[34] | Children | 123/123 | 4.2/4.1 | 49.6/47.9 | 48hr | NR | >37.3℃ | SFJD+ST+ ribavirin | ST+ ribavirin | 5d | 5d | 2,5,6 |
| Liu CX 2015[35] | Children | 37/33 | 2.4/2.5 | NR | 48hr | NR | 37.3–41.0℃ | SFJD +ST+ ribavirin | ST+ ribavirin | 5d | 5d | 2,5,6 |
| Acute pharyngitis | Fever, sore throat | |||||||||||
| Zhu SM 2019[36] | Adults | 38/38 | 41.34/41.25 | 57.9/52.6 | 48hr | Wind-heat syndrome | 37.3–38.5℃ | SFJD+ ST | ST | 5d | 5d | 4,6 |
| Jiang JQ 2018[37] | Adults | 48/48 | 37/39.81 | 47.9/43.8 | 48hr | NR | >37.3℃ | SFJD+ST | ST | 7d | 5d | 4,6 |
| Dong W 2020[38] | Adults | 45/45 | 30.1/32.1 | 48.89/53.33 | 36h | NR | >37.3℃ | SFJD+ST | ST | 6s | 5d | 2,4 |
| Zhou QQ 2020[39] | Adults | 106/106 | 32.75/30.63 | 69.8/74.53 | 48h | NR | NR | SFJD+ST | ST | 7d | 5d | 4 |
| Acute tonsillitis | Fever, sore throat | |||||||||||
| Zhang JY 2015[40] | Adults | 45/45 | 22.94/24.17 | 68.9/60 | 48hr | NR | 37.3–39.1℃ | SFJD +ST+ cefaclor | ST+ cefaclor | 7d | 5d | 2,4,6 |
| Wang ZB 2018[41] | Adults | 55/55 | 16.39/20.73 | 56.4/49.1 | 72hr | NR | >37.3℃ | SFJD +ST+ amoxicillin | ST+ amoxicillin | 7d | 5d | 2,4,6 |
| Li G 2017[42] | Adults | 50/50 | 27.1/27.3 | 56/52 | NR | NR | ≥38.0 ℃ | SFJD +ST+ cefuroxime | ST+ cefuroxime | 5d | 5d | 2,4,6 |
| Li BR 2020[43] | Adults | 50/50 | 22/21 | 52/42 | NR | NR | >37.3℃ | SFJD+ST+ penicillin | ST+ penicillin | 7d | 5d | 2,4,6 |
| Zhao ZY 2015[44] | Children | 58/50 | 5.9/6.1 | 53.4/54 | 72hr | NR | ≥38.0 ℃ | SFJD +ST+ cefuroxime | ST+ cefuroxime | 5d | 5d | 2,4,6 |
| Yang X2017 [45] | Children | 35/35 | 3.52/3.53 | 48.6/51.4 | 48hr | NR | 38.4–39.8 ℃ | SFJD +ST+ amoxicillin | ST+ amoxicillin | 5d | 5d | 2,4,6 |
| Huang PL 2016[46] | Children | 60/60 | 7.12/6.74 | 51.7/53.3 | 72h | Wind-heat syndrome | >37.3℃ | SFJD +ST+ cefaclor | ST+ cefaclor | 6d | 5d | 2,4,6 |
Notes: AURTIs, acute upper respiratory tract infections; d, days; hr, hours; NR, not reported; SFJD, Shufeng Jiedu; ST, symptomatic treatment.
Outcomes: 1, Resolution rate of fever at day 3 of treatment; 2, Time to fever resolution; 3, Time to cough resolution; 4, Time to sore throat resolution; 5, Time to blister resolution; 6, Cure rate.
1–5 were primary outcomes, 6 was secondary outcome.
3.3. Risk of bias assessment
In terms of randomization process, the baseline data were comparable in all the included trials.
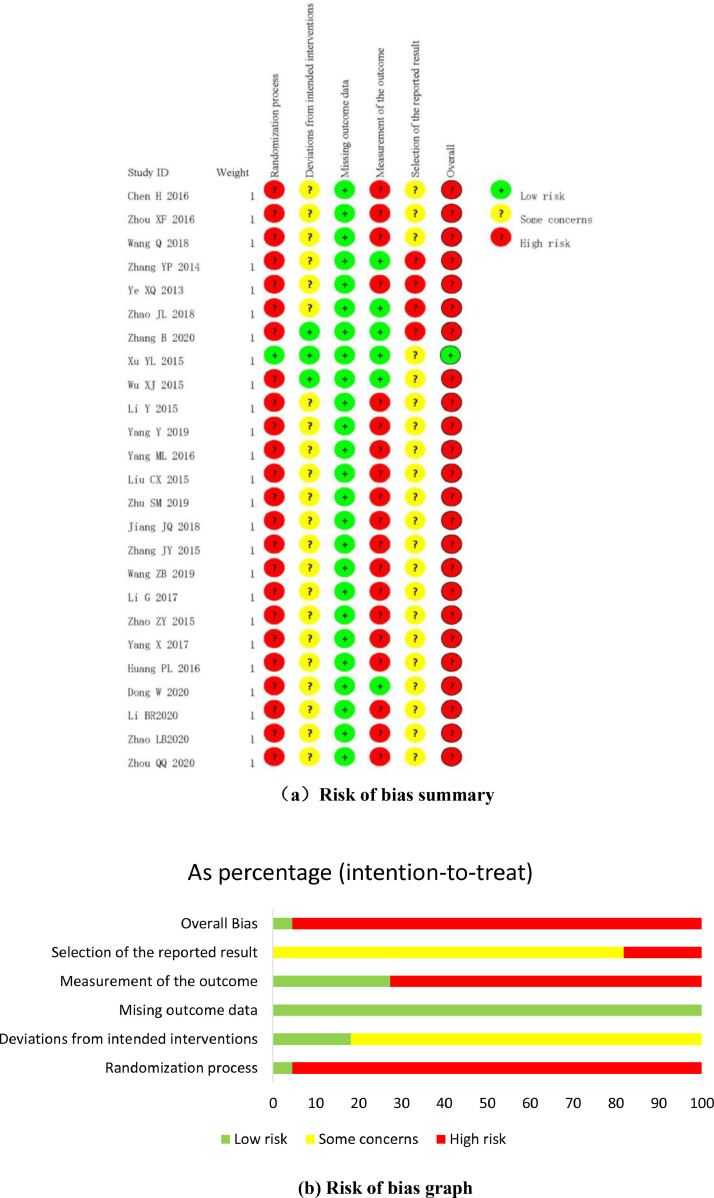
Clear random allocation sequence and allocation concealment were reported in only one trial29, and which was assessed as low risk of bias. Besides, the remaining 24 trials 22, 23, 24, 25, 26, 27, 28,30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 only mentioned random without describing detailed randomization methods. In terms of deviations from intended interventions, participants and personnel were blinded in only two trials28-29. Apart from that, deviations from interventions were not mentioned in other 23 trials 22, 23, 24, 25, 26, 27,30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, and therefore the majority of the included trials were assessed as some concerns in this domain. All trials were considered as low risk of bias in missing outcome data domain due to complete outcome data or there was evidence that the result was not biased by the missing data. In terms of measurement of outcomes, nineteen trials 22, 23, 24, 26,32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 were assessed as high risk of bias due to the outcomes were patients self-reported and likely influenced, other 6 trials 25,27, 28, 29, 30, 31 were of low risk of bias since the outcomes were objective or outcome assessors were not aware of the interventions received by participants. In terms of selection of the reported result, twenty-one trials 22, 23, 24,29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 were assessed as some concerns due to lack of pre-specified analysis plan, four trials 25, 26, 27, 28 were assessed as high risk of bias due to lack of pre-specified analysis plan and multiple time points outcome measurements. The overall bias was assessed as low for only one trial 29 and high for the remaining 24 trials 22, 23, 24, 25, 26, 27, 28,30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46. The methodological quality of the included trials were shown in Fig. 2.
Fig. 2.
(a) Risk of bias summary.
Fig. 2(b) Risk of bias graph.
3.4. Effect estimates
3.4.1. Common cold/acute rhinitis
There were 10 trials 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 focused on common cold, of these, three trials22, 23, 24 reported the time to fever and cough resolution, as well as the cure rate, the other 7 trials25, 26, 27, 28, 29, 30, 31 reported the cure rate. Only one trial32 on acute rhinitis and reported the cure rate.
3.4.1.1. Time to fever and cough resolution
Three trials 22, 23, 24 compared SFJD plus conventional drug (symptomatic treatment and antivirals) with conventional drug for common cold in children, and reported the time to fever and cough resolution. The pooled results showed that SFJD was superior to control group in shortening the time to fever resolution (MD −1.54 days, 95% CI [−2.15, −0.92], I2 = 80%, n = 385, 3 trials, very low certainty) and cough resolution (MD −1.22 days, 95% CI [−1.52, −0.93], n = 385, 3 trials, low certainty) (Table 2, Supplementary material).
Table 2.
Evidence summary of common cold/ acute rhinitis: SFJD +conventional drug versus conventional drug.
| Certainty assessment |
No of patients |
Effect |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | SFJD+ conventional drug | conventional drug | Relative(95% CI) | Absolute(95% CI) | ||
| Time to fever resolution | ||||||||||||
| 3 | randomized trials | seriousa | serious b | not serious | serious c | undetected | 193 | 192 | - | MD 1.54 lower(2.15 lower to 0.92 lower) | ⊕◯◯◯VERY LOW | CRITICAL |
| Time to cough resolution | ||||||||||||
| 3 | randomized trials | serious a | not serious | not serious | serious c | undetected | 193 | 192 | - | MD 1.22 lower(1.52 lower to 0.93 lower) | ⊕⊕◯◯LOW | CRITICAL |
| Cure rate | ||||||||||||
| 10 | randomized trials | serious a | not serious | serious d | not serious | undetected | 554/792 (69.9%) | 446/801 (57.7%) | RR 1.26(1.17 to 1.35) | 145 more per 1000(from 95 more to 195 more) | ⊕⊕◯◯LOW | IMPORTANT |
| Cure rate in adults | ||||||||||||
| 7 | randomized trials | serious a | not serious | not serious | not serious | undetected | 463/599 (77.3%) | 372/609 (61.1%) | RR 1.27(1.18 to 1.36) | 165 more per 1000(from 110 more to 2more) | ⊕⊕⊕◯MODERATE | IMPORTANT |
| Cure rate in children | ||||||||||||
| 3 | randomized trials | serious a | not serious | not serious | seriouse | undetected | 91/193 (47.2%) | 74/192 (38.5%) | RR 1.22(0.97 to 1.54) | 85 more per 1000(from 12 fewer to 208 more) | ⊕⊕◯◯LOW | IMPORTANT |
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
GRADE Working Group grades of evidence.
Moderate certainty (⊕⊕⊕◯): We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty (⊕⊕◯◯): Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect.
Very low certainty (⊕◯◯◯): We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
Notes: CI, confidence interval; RR, risk ratio; SFJD, ShuFeng JieDu.
The blinding method was not used.
I square value was large.
Number of patients included was less than 400.
Indirectness of age (adults and children).
A small number of events of less than 300.
3.4.1.2. Clinical cure
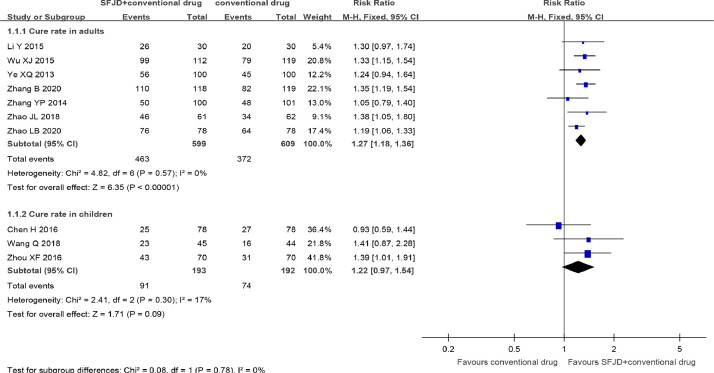
SFJD combined with conventional drug (symptomatic treatment alone 25, 26, 27, 28,32, symptomatic treatment plus antivirals 22, 23, 24,31 or symptomatic treatment plus antibiotics30) were also found more effective than conventional drug in improving the cure rate in common cold with a range (1–5 days) of treatment (RR 1.26, 95% CI [1.17, 1.35], n = 1593, 10 trials 22, 23, 24, 25, 26, 27, 28,30, 31, 32, low certainty) (Fig. 3, Table 2). Subgroup analysis by age suggested that SFJD in combination with conventional drug was still superior to conventional drug in improving the cure rate in adults (RR 1.27, 95%CI [1.18, 1.36], n = 1208, 7 trials 25, 26, 27, 28, 30, 31, 32, moderate certainty). But this effect was not significant in children (RR 1.22, 95%CI [0.97, 1.54], n = 385, 3 trials 22, 23, 24 low certainty) (Fig. 3, Table 2). There was only one double blinded, placebo-controlled trial29 which comparing the effect of SFJD with placebo on common cold, the result was narratively described. The cure rate was 44.2% in SFJD group and 9.3% in placebo group (RR 4.74, 95%CI [2.61, 8.61], n = 238, 1 trial, moderate certainty) (Supplementary material).
Fig. 3.
Cure rate with a range (1–5days) of treatment in common cold. SFJD, Shufeng Jiedu.
3.4.2. Herpangina
3.4.2.1. Time to fever and blisters resolution
Three trials33, 34, 35 compared SFJD in combination with conventional drug (symptomatic treatment plus antivirals) with conventional drug on herpangina. Which showed that SFJD plus conventional drug was superior to conventional drug in shortening the duration of fever (MD −0.68 days, 95% CI [−1.15, −0.21], I2 = 68%, n = 140, 2 trials, very low certainty) and blisters (MD −0.99 days, 95% CI [−1.23, −0.76], n = 386, 3 trials, low certainty) (Supplementary material).
3.4.2.2. Clinical cure
The cure rate was defined as resolution of fever and blisters with a range (1–5 days) of treatment. SFJD plus conventional drug was superior to conventional drug for cure rate (RR 1.30, 95% CI [1.12, 1.51], n = 386, 3 trials, low certainty) (Supplementary material).
3.4.3. Acute tonsillitis and acute pharyngitis
3.4.3.1. Time to fever and sore throat resolution
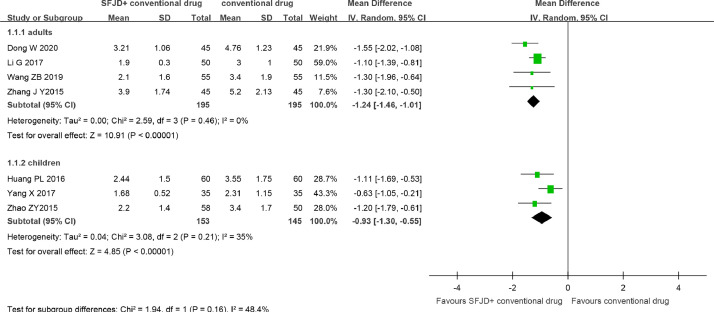
SFJD plus conventional drug (symptomatic treatment and antibiotics) compared with conventional drug for acute tonsillitis and acute pharyngitis. Seven trials 40, 41, 42, 43, 44, 45, 46 indicated that the duration of fever was shorter in SFJD group than conventional drug group (MD −1.13 days, 95% CI [−1.36, −0.90], I2 = 33%, n = 688, 7 trials, moderate certainty) (Fig. 4, Table 3). This effect was still observed regardless of adults (MD −1.24 days, 95% CI [−1.46, −1.01], n = 390, 4 trials, low certainty) or children (MD −0.93 days, 95% CI [−1.30, −0.55], n = 298, 3 trials, low certainty).
Fig. 4.
Time to fever resolution (d) in acute tonsillitis and acute pharyngitis.SFJD, Shufeng Jiedu.
Table 3.
Evidence summary of acute tonsillitis and acute pharyngitis: SFJD + conventional drug versus conventional drug.
| Certainty assessment |
№ of patients |
Effect |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | SFJD +conventional drug | conventional drug | Relative(95% CI) | Absolute(95% CI) | ||
| Time to fever resolution | ||||||||||||
| 7 | RCTs | serious a | not serious | serious b | not serious | undetected | 348 | 340 | - | MD 1.13 d lower(1.36 lower to 0.90 lower) | ⊕⊕⊕◯MODERATE | CRITICAL |
| Time to fever resolution- adults | ||||||||||||
| 4 | RCTs | serious a | not serious | not serious | serious c | undetected | 195 | 195 | - | MD 1.24 d lower(1.46 lower to 1.01 lower) | ⊕⊕◯◯LOW | CRITICAL |
| Time to fever resolution - children | ||||||||||||
| 3 | RCTs | serious a | not serious | not serious | serious c | undetected | 153 | 145 | - | MD 0.93 d lower(1.30 lower to 0.55 lower) | ⊕⊕◯◯LOW | CRITICAL |
| Time to sore throat resolution | ||||||||||||
| 10 | RCTs | serious a | not serious | serious b | not serious | undetected | 601 | 593 | - | MD 1.13 d lower(1.40 lower to 0.86 lower) | ⊕⊕◯◯LOW | CRITICAL |
| Time to sore throat resolution - adults | ||||||||||||
| 7 | RCTs | serious a | serious d | not serious | not serious | undetected | 448 | 448 | - | MD 1.29 d lower(1.60 lower to 0.97 lower) | ⊕⊕◯◯LOW | CRITICAL |
| Time to sore throat resolution – children | ||||||||||||
| 3 | RCTs | serious a | not serious | not serious | serious c | undetected | 153 | 145 | - | MD 0.73 d lower(1.03 lower to 0.43 lower) | ⊕⊕◯◯LOW | CRITICAL |
| Cure rate | ||||||||||||
| 10 | RCTs | serious a | not serious | serious b | serious | undetected | 289/545 (53.0%) | 228/537(42.5%) | RR 1.26(1.13 to 1.41) | 110more per 1000(from 55 more to 174 more) | ⊕⊕◯◯LOW | IMPORTANT |
| cure rate - adults | ||||||||||||
| 7 | RCTs | serious a | not serious | not serious | serious | undetected | 239/392(61.0%) | 197/392 (50.3%) | RR 1.21(1.09 to 1.35) | 106 more per 1000(from 45 fewer to 176 more) | ⊕⊕⊕◯MODERATE | IMPORTANT |
| cure rate - children | ||||||||||||
| 3 | RCTs | serious a | not serious | not serious | serious e | undetected | 50/153 (32.7%) | 31/145 (21.4%) | RR 1.54(1.05 to 2.26) | 115 more per 1000(from 11 more to 269 more) | ⊕⊕◯◯LOW | IMPORTANT |
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
GRADE Working Group grades of evidence.
Low certainty (⊕⊕◯◯): Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect;
Very low certainty (⊕◯◯◯): We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect;
Notes: CI, Confidence interval; MD, Mean difference; RCTs, Randomized controlled trial; RR, Risk ratio; SFJD, ShuFeng JieDu.
The blinding method was not used.
Indirectness of age (adults and children).
Number of patients included was less than 400.
I square value was large.
A small number of events of less than 300.
SFJD was also more effective than conventional drug (symptomatic treatment alone or symptomatic treatment plus antibiotics) in shortening the time to sore throat resolution in acute tonsillitis and acute pharyngitis (MD −1.13 days, 95% CI [−1.40, −0.86], I2 = 84.1%, n = 1194, 10 trials, low certainty). This effect was still observed regardless of adults (MD −1.29 days, 95% CI [−1.60, −0.97], I2 = 70%, n = 896, 7 trials, low certainty) or children (MD −0.73 days, 95% CI [−1.03, −0.43], n = 298, 3 trials, low certainty) (Table 3, Supplementary material).
3.4.3.2. Clinical cure
The criteria for cure rate was resolution of sore throat and fever with a range (1–5 days). SFJD was superior to conventional drug (symptomatic treatment alone or symptomatic treatment plus antibiotics) for cure rate (RR 1.26, 95% CI [1.13, 1.41], I2 = 29.4%, n = 1082, 10 trials, low certainty) (Table 3, Supplementary material). This effect was still observed regardless of the age of patients.
3.4.4. Adverse events
There were 7 trials 25-26,31,36,38,40,46 reported transient and minor gastrointestinal adverse events both in SFJD group (nausea in 6 cases, diarrhea in 4 cases) and in conventional drug group (nausea in 9 cases, diarrhea in 3 cases). SFJD was not judged as directly related to nausea and diarrhea in these trials, and no serious adverse events were reported in the included trials.
3.4.5. Publication bias
Funnel plots of cure rate in common cold seems to be symmetrical (Supplementary materials: Fig. 8), and funnel plots of time to sore throat resolution in acute tonsillitis and acute pharyngitis seems to be asymmetrical (Supplementary materials: Fig. 9). Suggesting that we cannot rule out the possibility of publication bias.
3.4.6. Additional analysis
We were unable to conduct sensitivity analysis for placebo or allocation concealment in any meaningful primary outcomes, since the allocation concealment was clearly described in only one placebo-controlled trial 29 and the trial was narratively described. Two trials 25,29 reported drop outs but under different outcomes. Predefined subgroup analysis via the age of patients (adults and children) was conducted for 3 outcomes under common cold, acute tonsillitis and acute pharyngitis, we were unable to conduct other meaningful subgroup analysis due to limited trials.
3.4.7. Certainty of evidence
GRADE approaches were employed to rate the certainty of evidence from all available outcomes. The quality of the evidence was downgraded to low or very low certainty due to lack of the blinding, imprecision (small number of events of less than 300 or number of patients included was less than 400), inconsistency (I square value was large) or indirectness of age (adults and children). The detailed evidence summary of outcomes were presented in (Table. 2,3, Supplementary material).
4. Discussion
4.1. Main findings
Low certainty evidence suggested that SFJD capsule as an adjunctive treatment to conventional drug was more beneficial than conventional drug alone to shorten the duration of symptoms in AURTIs, as well as improve cure rate with a range (1–5 days) of treatment. In common cold we found that SFJD was superior to conventional drug alone in shortening the duration of cough (−1.22 days) and fever (−1.54 days), as well as improving cure rate by 14.1%. In herpangina, SFJD plus conventional drug was more effective in shortening the duration of fever (−0.68 days), blisters (−0.99 days) and improving the cure rate by 15.8%. In acute tonsillitis and acute pharyngitis, SFJD combined with conventional drug was superior to conventional drug in shortening the duration of fever (−1.13 days), sore throat (−1.13 days) and increasing cure rate by 11%. These effects were still observed regardless of age. However, due to high risk of bias and small sample size, we downgraded these outcomes from high to low or very low certainty. In addition, there was no serious adverse events reported in the included trials.
4.2. Relation to previous research
Currently, there was one systematic review47 on SFJD for chronic obstructive pulmonary disease (COPD) suggested that SFJD may be beneficial to shorten the length of hospitalization and improve symptoms related to COPD. Another prior systematic review11 narratively reviewed the clinical and experimental findings of Chinese herbal medicine for the treatment of AURTIs. Suggesting that SFJD capsule was effective in improving symptoms of AURTIs, such as fever and cough. Which was consistent with our study. However, only one RCT on SFJD for AURTIs was identified and meta-analysis was not performed in this previous review. We conducted a more comprehensive search and meta-analysis was performed for more available outcomes. In addition, the protocol of this systematic review was registered prospectively and GRADE approaches were employed to assess the certainty of evidence.
4.3. Strengths and limitations
This is the first systematic review and meta-analysis based on RCTs to assess therapeutic effect and safety of Chinese patent herbal medicine SFJD capsule for AURTIs. We comprehensively searched the Chinese and English databases and identified all available RCTs. The outcomes were all assessed within 5 days of treatment, since many types of AURTIs were self-limited in 7 days4. The protocol of this systematic review was registered on INPLASY, and subgroup analysis via the age of patients was conducted. The methodological quality of RCTs were evaluated comprehensively by the latest Cochrane Risk of Bias tool 2.0, and GRADE criteria was also employed to determine the certainty of evidence.
However, there are several limitations. Firstly, antibiotics are not recommended for most AURTIs in guidelines, but they were used in the included trials. Secondly, predefined outcomes of symptom improvement rate and the severity of AURTIs were not reported in included trials. Additionally, only one double-blinded, placebo-controlled trial was assessed as having low risk of bias, the remaining included trials were of high risk of bias.
4.4. Implications for clinical practice
The diagnosis and treatment of AURTIs should strictly following guidelines. Antibiotics are recommended only for specific AURTIs (such as acute pharyngitis and acute tonsillitis caused by streptococcus) in existing guidelines, for other AURTIs, symptomatic treatment were recommended5,6. Standard care following guidelines are recommended, considering the potential effect of SFJD capsule, it might be a safe symptomatic treatment for AURTIs.
4.5. Implications for research
SFJD may have a role to play for the relief of symptoms from common AURTIs, but there is uncertainty due to the high risk of bias in the current studies. High quality placebo controlled RCTs with SFJD used alone or in combination with standard care are needed. Outcome reporting needs to be improved, the time from symptoms onset when included and the time point for every outcome measured should be clearly reported, since most AURTIs resolve from 3 to 7 days 4. In addition, cure rate was widely used in these trials as composite outcomes48 (usually included a set of symptoms and signs), but the detailed measurement of individual symptoms or signs could not be determined.
4.6. Conclusion
Currently, low certainty evidence suggests that SFJD may be effective in shortening the duration of typical symptoms in AURTIs, and improve cure rate with a range (1–5 days) of treatment. No serious adverse event was reported. However, high quality placebo-controlled trials are needed to confirm its benefits.
4.7. Author contributions
Conceptualization: YY Zhang, JP Liu and RY Xia. Methodology: RY Xia and S B Liang. Software: YY Zhang and S B Liang; YL Li and LY Zhao. Formal Analysis: YY Zhang and RY Xia. Data Curation: YY Zhang and RY Xia. Writing – Original Draft: YY Zhang. Writing – Review & Editing: RY Xia, SB Liang, XY Hu, MY Dai, YL Li, LY Zhao, M Moore, YT Fei and JP Liu. Funding Acquisition: JP Liu. YY Zhang and RY Xia are co-first authors. All authors approved the final manuscript.
Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
Funding
This work is supported by the National Key Research and Development Project: Adding Chinese herbal medicine to antibiotic treatment for acute exacerbation of chronic obstructive pulmonary disease (Grant no. 2018YFE0102300).
Ethical statement
This work did not require an ethical approval as it does not involve any human or animal experiment.
Data availability
The data will be made available upon request.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.imr.2021.100726.
Appendix. Supplementary materials
References
- 1.James S.L., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet North Am Ed. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geneva . WHO; 2020. Disease Burden and Mortality Estimates.http://www.who.int/gho/mortality_burden_disease/en/index.html website. Published 2018. Accessed June 20. [Google Scholar]
- 3.Vol. 23. WHO; 2021. http://www.bmj.com/content/346/bmj.f2914 (International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10)). website. Published 2019. Accessed February. [Google Scholar]
- 4.King D., Mitchell B., Williams C.P., Spurling G. Saline nasal irrigation for acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015;(4) doi: 10.1002/14651858.CD006821.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris A.M., Hicks L.A., Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the american college of physicians and the centers for disease control and prevention. Ann Intern Med. 2016;164(6):425–434. doi: 10.7326/M15-1840. [DOI] [PubMed] [Google Scholar]
- 6.Hersh A.L., Jackson M.A., Hicks L.A., DISEASES TCOI Principles of judicious antibiotic prescribing for upper respiratory tract infections in pediatrics. Pediatr Off Publ Am Acad Pediatr. 2013;132(6):1146–1154. doi: 10.1542/peds.2013-3260. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro D.J., Hicks L.A., Pavia A.T., Hersh A.L. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemoth. 2014;(1):234–240. doi: 10.1093/jac/dkt301. [DOI] [PubMed] [Google Scholar]
- 8.Wang J., Wang P., Wang X., Zheng Y., Xiao Y. Use and prescription of antibiotics in primary health care settings in China. JAMA Intern Med. 2014;174(12):1914. doi: 10.1001/jamainternmed.2014.5214. [DOI] [PubMed] [Google Scholar]
- 9.Alves Galvão M.G., Rocha Crispino Santos M.A., Alves Da Cunha A. Antibiotics for preventing suppurative complications from undifferentiated acute respiratory infections in children under five years of age. Cochrane Database Syst Rev. 2016;(2) doi: 10.1002/14651858.CD007880.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CDC. 2019 AR Threats Report, CDC Newsroom. https://www.cdc.gov/media/releases/2019/t1113-ar-threats.html. Published 2019. Accessed June 25, 2020.
- 11.Huang Z.S., Pan X.Y., Zhou J., Leung W.T., Li C.T., Wang L. Chinese herbal medicine for acute upper respiratory tract infections and reproductive safety: a systematic review. Biosci Trends. 2019;13(2):117–129. doi: 10.5582/bst.2018.01298. [DOI] [PubMed] [Google Scholar]
- 12.Li J., Yu X. Guidelines for the diagnosis and treatment of common cold in traditional Chinese medicine. J Tradit Chin Med. 2016;57(08):716–720. [Google Scholar]
- 13.China Association of Traditional Chinese Medicine . Beijing: China Press of Traditional Chinese Medicine; 2012. Guidelines for Diagnosis and Treatment of Common Diseases of Otolaryngology in Traditional Chinese. [Google Scholar]
- 14.National Administration of Traditional Chinese Medicine (NATCM) NATCM; 2021. The Office of the NATCM Issued a Circular on TCM Clinical Pathways and TCM Diagnosis and Treatment Plans (2017 edition) for 92 Diseases Including Stroke (cerebral infarction)http://www.satcm.gov.cn/yizhengsi/gongzuodongtai/2018-03-24/2651.html website. Published in 2018. Accessed February 10. [Google Scholar]
- 15.Bao Y.Y., Gao Y.J., Cui X.L. Effect of Shufeng Jiedu capsules as a broad-spectrum antibacterial. Biosci Trends. 2016;10(1):74–78. doi: 10.5582/bst.2015.01172. [DOI] [PubMed] [Google Scholar]
- 16.Ji S., Bai Q., Wu X., Zhang D.W., Wang S., Shen J.L. Unique synergistic antiviral effects of Shufeng Jiedu Capsule and oseltamivir in influenza A viral-induced acute exacerbation of chronic obstructive pulmonary disease. Biomed Pharmacother. 2020;121 doi: 10.1016/j.biopha.2019.109652. [DOI] [PubMed] [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaffa J., Altmane D.G. Preferred Reporting items for systematic reviews and meta-analyses: the PRISMA statement. Revista Espanola de Nutricion Humana y Dietetica. 2014;18(3):172–181. [Google Scholar]
- 18.Zhang Y.Y., Xia R.Y., Liang S.B., Dai M.Y., Hu X.Y., Willcox M., et al. Chinese Patent Medicine ShufengJiedu Capsule for Acute Upper Respiratory Tract Infections: A Protocol of a Systematic Review of Randomized Clinical Trials. Inplasy protocol 202050083. doi: 10.37766/inplasy2020.5.0083. [DOI]
- 19.Higgins J.P.T., Savović J., Page M.J., Elbers R.G., Sterne J.A.C. Chapter 8: assessing risk of bias in a randomized trial. In: Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. Cochrane Handbook For Systematic Reviews of Interventions Version 6.0 (updated July 2019) Cochrane; 2019. Available from www.training.cochrane.org/handbook. Accessed September 22, 2020. [Google Scholar]
- 20.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J. Cochrane8, 2020; 2019. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019)www.training.cochrane.org/handbook Available from. Accessed September 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H., Su Y.M., Luan J.Q. Clinical observation of Shufeng Jiedu capsule in the treatment of acute upper respiratory tract infection in children. Word J Integr Tradit West Med. 2016;11(05):716–718. [Google Scholar]
- 23.Zhou X.F., Wang X.J. Clinical observation of Shufeng Jiedu capsule on wind-heat syndrome of children with acute viral upper respiratory tract infection. Beijing J Tradit Chin Med. 2016;25(01):84–86. [Google Scholar]
- 24.Wang Q., Liu K., Chen F., Zhao L. Therapeutic effect of Shufeng Jiedu capsule on acute upper respiratory tract infection in children. J Clin Pulm Med. 2018;23(10):1842–1845. [Google Scholar]
- 25.Zhang Y.P., Lin Y., Min M., Xi P.L., Tong C.Y. Effects of Shufengjiedu capsules on serum amyloid A in the treatment of patients with an acute viral upper respiratory tract infection. J Pathogen Biol. 2014;9(08):734–736. [Google Scholar]
- 26.Ye X.Q., Zeng D.Z., Luo S.F. Clinical observation of Shufeng Jiedu capsule in the treatment of wind-heat syndrome of cold. Anhui Med Pharm J. 2013;17(04):664–666. [Google Scholar]
- 27.Zhao J.L., Xu D.F., Li C.Y. Effect of Shufeng Jiedu capsule on acute upper respiratory tract infection. Chin Arch Tradit Chin Med. 2018;36(05):1222–1225. [Google Scholar]
- 28.Zhang B., Zhang W. Clinical effect of Shufeng Jiedu Capsules for acute upper respiratory tract infection and its effect on inflammatory factors. J New ChinMed. 2020;52(04):50–53. [Google Scholar]
- 29.Wu X.J., Xue M.M., Huang J.L., Zhang Y.P., Wang C.L., Song Z.J. Effect of Shufeng Jiedu capsule combined with antibiotics on aged patients with bacterial acute upper respiratory tract infection. Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease. 2015;23(03):97–99. [Google Scholar]
- 30.Xu Y.L., Xue Y.L., Zhang H.H., Lv F.Y., Tian Z.J., Xing Z.H. Clinical observation on treatment of acute upper respiratory infection wind-heat syndrome with Shufeng Jiedu capsules randomize-controlled double-blind test. J Tradit Chin Med. 2015;56(08):676–679. [Google Scholar]
- 31.Zhao L.B., Wang Z.B. Clinical observation of Shufeng Jiedu capsule in treating acute upper respiratory tract infection (wind-heat syndrome) J Emerg Tradit Chin Med. 2020;29(07):1278–1279. [Google Scholar]
- 32.Li Y., Wang X.J. Clinical observation on the treatment of acute rhinitis (Wind Heat Syndrome) with Shufeng Jiedu capsule. Guid J Tradit Chin Med Pharm. 2015;21(21):49–51. [Google Scholar]
- 33.Yang Y., Li D.Y. Effect of Shufeng Jiedu capsule combined with recombinant alpha 1B interferon on children with herpetic pharyngitis. J Emerg Tradit Chin Med. 2019;28(05):899–900. [Google Scholar]
- 34.Yang M.L. Effect of Shufeng Jiedu capsule on 123 children with herpetic pharyngitis. J Emerg Tradit Chin Med. 2016;25(12):2364–2365. [Google Scholar]
- 35.Liu C.X. Treatment of 37 cases of infantile herpetic angina with Shufeng Jiedu Capsule. Henan Tradit Chin Med. 2015;35(07):1695–1697. [Google Scholar]
- 36.Zhu S.M., Mao X.P., Liu Q. Clinical observation on the treatment of acute pharyngitis (Wind Heat Syndrome) with Shufeng Jiedu capsule combined with budesonide atomization. Chin Med Innov. 2019;16(08):67–71. [Google Scholar]
- 37.Jiang J.Q., Cheng L.L., Li Q. Clinical observation on the treatment of acute pharyngitis with TCM combined with Western medicine. J Emerg Tradit Chin Med. 2018;27(08):1448–1450. [Google Scholar]
- 38.Dong W., Zheng R.H. Effect of Shufeng Jiedu capsule combined with dexamethasone ultrasonic atomization inhalation on TNF-α and IL-6 in patients with acute pharyngitis. Mod J Integr Tradit Chin West Med. 2020;29(35):3936–3939. [Google Scholar]
- 39.Zhou Q.Q., Jl Zhu, Pu L.C. Clinical study on Shufeng Jiedu capsules for acute laryngopharyngitis. J New Chin Med. 2020;52(10):96–98. [Google Scholar]
- 40.Zhang J.Y. Clinical observation on the treatment of acute attacks of chronic tonsillitis with Shufeng Jiedu capsule. J Emerg Tradit Chin Med. 2015;24(12):2230–2232. [Google Scholar]
- 41.Wang Z.B., Zhao L.B. Clinical observation on the treatment of Acute tonsillitis with Shufeng Jiedu capsule. J Emerg Tradit Chin Med. 2019;28(10):1827–1829. [Google Scholar]
- 42.Li G., Ding Y., Wang S.S., Xie H., Xu D.M. Clinical observation on the treatment of acute suppurative tonsillitis with Shufeng Jiedu capsule combined with cefuroxime sodium. Anti-Infect Pharm. 2017;14(02):475–476. [Google Scholar]
- 43.Li B.R., Yang L.L., Si J.Y. Clinical study of Shufeng Jiedu capsule for acute tonsillitis. J Emerg Tradit Chin Med. 2020;29(10):1849–1851. [Google Scholar]
- 44.Zhao Z.Y. Treatment of 58 cases of acute tonsillitis in children with Shufeng Jiedu Capsule. Henan Tradit Chin Med. 2015;35(07):1690–1692. [Google Scholar]
- 45.Yang X., Yang X.W. Observation on efficacy of Shufengjiedu capsules in treatment of acute Suppurative Tonsillitis. Eval Anal Drug-Use Hosp China. 2017;17(01) [Google Scholar]
- 46.Huang P.L., Pan Y.D. Clinical observation on the treatment of Tonsillitis in children (Wind Heat Syndrome) with Shufeng Jiedu capsule combined with Cefaclor for oral suspension. J Emerg Tradit Chin Med. 2016;25(10):1984–1986. [Google Scholar]
- 47.Xia R.Y., Hu X.Y., Fei Y.T., Willcox M., Wen L.Z., Yu M.K. Shufeng Jiedu capsules for treating acute exacerbations of chronic obstructive pulmonary disease: a systematic review and meta-analysis. BMC Complement Med Ther. 2020;20(1):151. doi: 10.1186/s12906-020-02924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christopher M.C. Understanding the use of composite endpoints in clinical trials. West J Emerg Med. 2018;19(4):631–634. doi: 10.5811/westjem.2018.4.38383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be made available upon request.