Abstract
Background & Aims
Type 2 diabetes is a major driver of fatty liver disease and its long-term complications. The aim of this study was to investigate the individual contribution of inborn and acquired risk factors for severe liver disease in individuals with type 2 diabetes from the UK Biobank study.
Methods
A total of 22,812 UK Biobank participants of European descent without clinical history of liver disease and liver cancer were prospectively followed for the development of severe liver disease, defined as a composite diagnosis of cirrhosis, decompensated liver disease, hepatocellular carcinoma, and/or liver transplantation from the National Health Service records. The contribution of inborn and acquired risk factors to the risk of incident severe liver disease was assessed by Cox proportional hazards models.
Results
During a median follow-up of 8.9 years (IQR 8.1–9.6), there were 279 individuals with severe liver disease, including 255 with cirrhosis and/or decompensated liver disease, 47 with hepatocellular carcinoma, and 5 with liver transplantation; death from severe liver disease occurred in 83 individuals. Risk factors independently associated with increased risk of incident severe liver disease included abnormal aspartate aminotransferase (adjusted hazard ratio [aHR] 4.85, 95% CI 2.76–8.54), decrease in serum albumin (aHR 2.39, 95% CI 1.76–3.24) and platelet count (aHR 1.12, 95% CI 1.09–1.16), cardiovascular disease (aHR 1.86, 95% CI 1.23–2.79), microalbuminuria (aHR 1.55, 95% CI 1.04–2.30), PNPLA3 rs738409 (aHR 1.67, 95% CI 1.27–2.18) and TM6SF2 rs58542926 (aHR 1.63, 95% CI 1.12–2.39), while the net effect of male sex was protective (aHR 0.49, 95% CI 0.26–0.94).
Conclusions
These findings may help in clinical care to identify individuals with type 2 diabetes at risk of severe liver disease, in turn leading to personalised risk prediction and prevention strategies.
Lay summary
Type 2 diabetes is a key driver of severe liver disease, namely cirrhosis, hepatocellular carcinoma, and liver-related mortality. In Europeans with type 2 diabetes from the prospective UK Biobank study, abnormal liver function, cardiovascular disease, microalbuminuria, and genetic variants in PNPLA3 and TM6SF2 genes are the major independent risk factors for severe liver disease. These findings may contribute in clinical care to identify and closely monitor individuals with type 2 diabetes at risk of developing severe liver disease, requiring more intensive follow-up strategies.
Keywords: Cirrhosis, Hepatocellular carcinoma, Fatty liver, Type 2 diabetes
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; FLD, fatty liver disease; HbA1c, glycated haemoglobin; HR, hazard ratio; ICD-10, International Classification of Diseases 10th edition; SLD, severe liver disease; UACR, urinary albumin-to-creatinine ratio
Graphical abstract
Highlights
-
•
Prospective cohort study of more than 20,000 European participants with type 2 diabetes from the UK Biobank.
-
•
Severe liver disease endpoint defined as a composite diagnosis of cirrhosis, liver cancer, and liver transplantation.
-
•
Inborn risk factors for severe liver disease: genetic loci in PNPLA3 and TM6SF2.
-
•
Acquired risk factors for severe liver disease: abnormal liver function, cardiovascular and kidney complications.
-
•
Clinical risk estimation for predicting severe liver disease in individuals with type 2 diabetes.
Introduction
The growing epidemic of diabetes is a serious global public health issue with nearly half a billion individuals living with diabetes and approximately 90% of them have type 2 diabetes.1 An overwhelming body of evidence supports that type 2 diabetes is a key risk factor for fatty liver disease (FLD), which is the most common chronic liver disease worldwide.2,3 The global prevalence of FLD in individuals with type 2 diabetes is more than 2-fold higher than in the general population (55% vs. 25%, respectively), with the highest rate reported in Europe (68%).2,4
Strikingly, among FLD comorbidities (e.g. obesity, hypertension, and dyslipidaemia), type 2 diabetes seems to be the strongest risk factor for the progression of liver disease to its long-term complications, namely cirrhosis and hepatocellular carcinoma, and for mortality.2 The risk of life-threatening liver-related complications increases with the increase in the number of features of metabolic syndrome.5 Harmful alcohol consumption is the other major cause of non-viral cirrhosis and hepatocellular carcinoma in Europe and worldwide.[6], [7], [8] In addition to the well-established metabolic and environmental risk factors, in recent years common genetic variants in several genes were found to robustly contribute to FLD and the entire spectrum of its complications.9,10
Within this context, to identify and closely monitor those who are at risk of progressive liver disease, it will be key to identify drivers and predictors of liver damage and fibrosis in individuals with type 2 diabetes. Björkström et al.11 have recently examined the contribution of clinical risk factors for developing severe liver disease (SLD) in a very large cohort of individuals with type 2 diabetes from the Swedish National Diabetes Register. They found older age, male sex, higher BMI, hypertension, lower kidney function, microalbuminuria, and smoking as independent risk factors, whereas statins were protective against SLD. However, the predictive value of biochemical proxies of liver damage and function was not examined. Moreover, the contribution of human genetics and alcohol use in this context remains to be investigated.
Therefore, the aim of this study was to examine the major inborn and acquired independent risk factors contributing to SLD among participants with type 2 diabetes from the prospective UK Biobank study.
Materials and methods
Study population and data collection
The study design and methods of the UK Biobank have been described in detail previously.12 Briefly, the UK Biobank is a large prospective cohort study with approximately 500,000 participants aged 40–69 years, recruited between 2006 and 2010 from 22 assessment centres across the UK. The UK Biobank study has been approved by the North West Multicenter Research Ethics Committee (reference number 11/NW/0382). All participants provided informed consent to the study.
Potential participants were identified from the National Health Service patient registers and invited to attend the local assessment centre. At the baseline assessment visit, they completed a touch-screen self-administered questionnaire and a computer-assisted interview regarding medical history, current pharmacological therapy, sociodemographic characteristics, smoking status, alcohol consumption, dietary habits, physical activity, and family history of major diseases. Baseline anthropometric measures (e.g. height, weight, and waist circumference) were assessed by trained staff using standardised procedures. Blood samples were collected for genome-wide genotyping and biochemical analyses, including glycated haemoglobin (HbA1c) (VARIANT II TURBO Hemoglobin Testing System, Bio-Rad), serum glucose, alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin and creatinine (AU5800, Beckman Coulter), and urine albumin (AU5400, Randox Bioscience) and creatinine (AU5400, Beckman Coulter). The protocol for samples collecting, processing, and storage was developed using a highly automated and validated approach.13 Further information about the study protocol and methods is available in the UK Biobank website (https://www.ukbiobank.ac.uk/).
Definition of baseline exclusion criteria
Baseline exclusion criteria were as follows: (1) self-reported history of liver disease; (2) hospital diagnosis of chronic viral hepatitis, SLD, and/or other causes of liver disease occurred before the baseline assessment visit and defined according to the International Classification of Diseases 10th edition (ICD-10 B18, B19, C22.0, E83.0, E83.1, I85.0, I85.9, K70.3, K70.4, K70.9, K71, K72.1, K72.9, K74.1, K74.2, K74.3, K74.4, K74.5, K74.6, K75.2, K75.3, K75.4, K75.8, K75.9, K76.6, K76.7, K76.8, K76.9, R18, Z94.4); (3) self-reported history of liver cancer; (4) diagnosis of liver cancer based on cancer register occurred before the baseline assessment visit (ICD-10 C22); (5) self-reported non-European ancestry (i.e. all ethnic groups other than White British, White Irish, and any other White background); (6) participants with withdrawn consent. A total of 466,783 participants were included for the final analyses. Details of baseline exclusion criteria are provided in Tables S1–S3.
Definition of baseline type 2 diabetes
Baseline previously diagnosed type 2 diabetes was defined by at least 1 of the following criteria: (1) self-reported history of type 2 or unspecified diabetes; (2) hospital diagnosis of type 2 or unspecified diabetes that occurred before the baseline assessment visit (ICD-10 E11, E14); (3) current insulin treatment and/or use of oral hypoglycaemic drugs. Among individuals without a prior diagnosis of diabetes, undiagnosed type 2 diabetes was defined by at least 1 of the following criteria: (1) serum glucose level ≥11.1 mmol/L (200 mg/dl); (2) HbA1c ≥48 mmol/mol (6.5%). The threshold of 11.1 mmol/L (200 mg/dl) for serum glucose was chosen to avoid false positives, as blood samples were collected not necessarily fasting. The final baseline population included 22,812 participants with type 2 diabetes.
Definition of covariates and comorbidities
Baseline anthropometric measures were assessed by trained staff using standardised procedures. Height and weight were measured using the Seca 202 height measure (Seca, Hamburg, Germany) and the Tanita BC-418 MA body composition analyser (Tanita Europe, Amsterdam, Netherlands), respectively. BMI was calculated by dividing the weight (kg) by the square of the height (m2). Waist circumference was measured at the umbilicus level using the Wessex non-stretchable sprung tape measure (Wessex, UK).
Socioeconomic status was defined using the Townsend deprivation index.14 Data on family history of diabetes, smoking, alcohol consumption, and physical activity were collected through a baseline touch-screen questionnaire. A positive family history of diabetes was defined as participants who had one or more first-degree relatives (i.e. parents and/or siblings) diagnosed as having diabetes. Smoking status was categorised into 2 groups: current smoking and never/former smoking. Frequency of daily alcohol consumption (g/day) was quantified based on the average weekly alcohol intake or on the average monthly alcohol intake (when the average weekly data was missing). Alcohol grams for each type of drink (i.e. red wine, white wine or champagne, beer or cider, spirits, fortified wine, and other alcoholic drinks) were derived from the corresponding reference alcohol content reported in the National Health Service UK guidelines (1 unit of alcohol = 8 g of alcohol, https://www.nhs.uk/live-well/alcohol-support/calculating-alcohol-units/ – accessed on January 2020). Excessive alcohol consumption was defined when daily alcohol intake was ≥30 g and ≥20 g for men and women, respectively.15 A detailed explanation of the alcohol consumption extraction pipeline is provided in the supplementary material. Regarding physical activity, participants were categorised into 2 groups if they underwent physical exercise or not according to the UK physical activity recommendations (i.e. ≥150 min/week and ≥75 min/week for moderate and vigorous physical activity, respectively; UK Biobank data-field 22035).
Estimated glomerular filtration rate (eGFR) was calculated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.16 Albuminuria categories were defined based on a single sample spot urinary albumin-to-creatinine ratio (UACR) (i.e. 3–29 mg/mmol and ≥30 mg/mmol for micro- and macro-albuminuria, respectively).17
Baseline dyslipidaemia was defined as self-reported history of high cholesterol or use of lipid-lowering drugs. Similarly, baseline hypertension was defined as self-reported history of hypertension or use of antihypertensive drugs. Baseline cardiovascular disease was defined as self-reported history or hospital diagnosis of angina, myocardial infarction, stroke, or transient ischaemic attack (ICD-10 I20–I25, I60–I64, I69, G45).
Genotyping
Detailed information about genotyping and arrays used in the UK Biobank study has been provided elsewhere.18 Genotype data were available for approximately 490,000 participants. PNPLA3 rs738409 C>G (p.I148M), TM6SF2 rs58542926 C>T (p.E167K), MBOAT7 rs641738 C>T, GCKR rs1260326 C>T (p.P446L), and HSD17B13 rs72613567:TA were assayed using 2 similar genotyping arrays (i.e. Affymetrix UK BiLEVE and UK Biobank Axiom arrays) and coded as 0, 1, or 2 for non-carriers, heterozygous carriers, and homozygous carriers of the minor allele, respectively. For PNPLA3 rs738409, TM6SF2 rs58542926, MBOAT7 rs641738, and GCKR rs1260326 the minor allele (i.e. G allele, T allele, T allele, and T allele, respectively) was the risk-increasing allele, whereas for HSD17B13 rs72613567 the minor TA allele had a protective effect.[19], [20], [21], [22], [23]
Follow-up outcome
Follow-up data on health-related events and mortality were obtained through linkage of the National Health Service records, including in-hospital admissions, death register, and cancer register (UK Biobank data-fields 41270, 40001, 40002, and 40006). Detailed information regarding the linkage procedure is available in the UK Biobank website (https://www.ukbiobank.ac.uk/wp-content/uploads/2011/11/UK-Biobank-Protocol.pdf – accessed on January 2020). The study outcome was incident SLD, defined as a composite diagnosis of cirrhosis, decompensated liver disease (i.e. esophageal varices with or without bleeding, portal hypertension, hepatorenal syndrome, liver failure), hepatocellular carcinoma, and/or liver transplantation (ICD-10 C22.0, I85.0, I85.9, K70.3, K70.4, K72.1, K72.9, K74.1, K74.2, K74.6, K76.6, K76.7, Z94.4) in any of the aforementioned records. A list of all the diagnoses used to define SLD is presented in Table S3. Among those with SLD, individuals were excluded if they received a hospital diagnosis of chronic viral hepatitis or other causes of liver disease (ICD-10 B18, B19, E83.0, E83.1, K71, K74.3, K74.4, K74.5, K75.2, K75.3, K75.4, K75.8, K75.9) before the diagnosis of the outcome of interest. The length of follow-up for each participant was calculated from the date of baseline assessment visit up to the first date of SLD diagnosis, the date of death, or the date of end of follow-up for the assessment centre attended (31 January 2018), whichever occurred first. The study flowchart is provided in Fig. S1.
Statistical analysis
Continuous variables were shown as mean ± SD if normally distributed or median (IQR) if skewed. Categorical variables were shown as number (percentage).
In UK Biobank participants with type 2 diabetes, the following risk factors for SLD were tested: age (continuous), sex, family history of diabetes, duration of diabetes (continuous), hypertension, dyslipidaemia, BMI ≥30 kg/m2, waist circumference ≥94 cm and ≥80 cm (for men and women, respectively24), alcohol consumption (complete abstinence, low-moderate intake, and excessive intake [i.e. ≥30 g/day and ≥20 g/day for men and women, respectively15]), current smoking status, physical activity ≥150 min/week and ≥75 min/week (for moderate and vigorous physical activity, respectively), HbA1c (continuous), ALT >30 U/L and >19 U/L (for men and women, respectively25), AST >30 U/L and >19 U/L (for men and women, respectively), serum albumin (continuous), platelet count (continuous), eGFR <60 ml/min/1.73 m2, micro- and macro-albuminuria (i.e. UACR 3–29 mg/mmol and ≥30 mg/mmol, respectively17), PNPLA3 rs738409, TM6SF2 rs58542926, MBOAT7 rs641738, GCKR rs1260326, HSD17B13 rs72613567, use of oral hypoglycaemic drugs, insulin treatment, and use of statins. The association of the abovementioned risk factors with incident SLD was assessed by Cox proportional hazards models, including age, sex, BMI, duration of diabetes, alcohol intake, and all predictor variables with a value of p <0.05 in the univariate model. The contribution of genetic factors was estimated by assuming an additive or recessive model, separately. Missing data for any of the covariates were removed from the analyses.
Two sensitivity analyses were performed: (1) excluding participants with excessive alcohol consumption at baseline; (2) stratifying by sex.
Cumulative incidence curves were computed using the Aalen-Johansen estimator, with mortality and liver diagnoses other than FLD entered as the competing events for SLD and analysed according to the different genetic variants. Comparisons were carried out using the log-rank test.
All analyses were performed using R statistical software, version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics, genotyping, and incidence of SLD
A total of 22,812 participants of European descent from the UK Biobank with type 2 diabetes were included in the analyses (see Fig. S1 for selection criteria). We defined SLD as diagnosis of cirrhosis and its complications, namely hepatic decompensation, hepatocellular carcinoma, and liver transplantation. The baseline characteristics of the study participants stratified by incident SLD status are shown in Table 1 (see Table S4 for baseline characteristics of the entire UK Biobank population stratified by type 2 diabetes status). In the overall cohort, 2 out of 3 participants were men and approximately half of them had a positive family history of diabetes. The mean ± SD age was 60.1 ± 7 years and BMI was 31.6 ± 5.9 kg/m2, indicating that a large number of individuals with type 2 diabetes are obese and overweight. The median (IQR) duration of diabetes was 4.5 (2.5–9.4) years whereas HbA1c was 50.3 (43.2–59.7) mmol/mol.
Table 1.
Baseline characteristics of UK Biobank participants of European descent with type 2 diabetes stratified by incident sever liver disease status.
| Total (n = 22,812) | No severe liver disease (n = 22,533) | Severe liver disease (n = 279) | p value | |
|---|---|---|---|---|
| Age, years | 60.1 ± 7 | 60.1 ± 7 | 61.5 ± 6.2 | 0.002 |
| Men, n (%) | 14,273 (63%) | 14,066 (62%) | 207 (74%) | <0.001 |
| Townsend deprivation index | -1.5 (-3.3–1.7) | -1.5 (-3.3–1.7) | -0.6 (-2.8–2.4) | <0.001 |
| Family history of diabetes, n (%) | 9,692 (43%) | 9,591 (43%) | 101 (37%) | 0.11 |
| Duration of diabetes, years | 4.5 (2.5–9.4) | 4.5 (2.5–9.4) | 6.3 (2.5–9.5) | 0.49 |
| BMI, kg/m2 | 31.6 ± 5.9 | 31.6 ± 5.9 | 33.7 ± 6 | <0.001 |
| Waist circumference, cm | 103.4 ± 14.6 | 103.4 ± 14.6 | 110.3 ± 13.7 | <0.001 |
| Lifestyle | ||||
| Current smoking, n (%) | 2,505 (11%) | 2,472 (11%) | 33 (12%) | 0.61 |
| Alcohol intake, g/day | 5.8 (0–19.4) | 5.7 (0–19.4) | 9.7 (0–30.9) | <0.001 |
| Alcohol intake ≥30/20 g/day, n (%) | 3,566 (16%) | 3,491 (15%) | 75 (27%) | <0.001 |
| Physical activity ≥150/75 min/week, n (%) | 7,980 (45%) | 7,883 (45%) | 97 (45%) | 0.99 |
| Clinical chemistry | ||||
| HbA1c, mmol/mol | 50.3 (43.2–59.7) | 50.3 (43.2–59.7) | 50 (42.5–60.1) | 0.59 |
| ALT, U/L | 25.3 (18.8–34.7) | 25.2 (18.7–34.5) | 36.4 (26–53.5) | <0.001 |
| AST, U/L | 25.2 (21–31.1) | 25.1 (21–30.9) | 41.8 (29.5–57.7) | <0.001 |
| Albumin, g/dl | 4.5 ± 0.3 | 4.5 ± 0.3 | 4.3 ± 0.3 | <0.001 |
| Platelet count, ∗109/L | 247.4 ± 65 | 248 ± 64.7 | 198.2 ± 71 | <0.001 |
| eGFR, ml/min/1.73 m2 | 88.3 ± 16.5 | 88.3 ± 16.5 | 87.5 ± 19 | 0.48 |
| Microalbuminuria, n (%) | 3,110 (27%) | 3,052 (26%) | 58 (37%) | 0.004 |
| Macroalbuminuria, n (%) | 488 (4%) | 480 (4%) | 8 (5%) | 0.64 |
| Comorbidities | ||||
| Hypertension, n (%) | 16,958 (74%) | 16,734 (74%) | 224 (80%) | 0.18 |
| Dyslipidaemia, n (%) | 18,213 (80%) | 17,996 (80%) | 217 (78%) | 0.082 |
| Cardiovascular disease, n (%) | 5,068 (22%) | 4,972 (22%) | 96 (34%) | <0.001 |
| Drugs | ||||
| Metformin, n (%) | 12,278 (54%) | 12,105 (54%) | 173 (62%) | 0.005 |
| Thiazolidinediones, n (%) | 1,726 (8%) | 1,695 (8%) | 31 (11%) | 0.042 |
| Sulfonylureas, n (%) | 4,584 (20%) | 4,512 (20%) | 72 (26%) | 0.043 |
| Insulin, n (%) | 4,530 (20%) | 4,483 (20%) | 47 (17%) | 0.46 |
| Statins, n (%) | 16,500 (72%) | 16,304 (72%) | 196 (70%) | 0.13 |
Continuous variables are shown as mean ± SD or median and (IQR) if normally distributed or skewed, respectively. Categorical variables are shown as number and (proportion). Values of p are from generalised linear models adjusted for age, sex, and assessment centre. Values of p <0.05 were considered statistically significant.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin.
Individuals with development of SLD during follow-up were older, had higher BMI and waist circumference, higher alcohol intake, higher transaminases, lower serum albumin, lower platelet count, and higher prevalence of cardiovascular disease compared with those without. Moreover, they were more likely to be treated with metformin, thiazolidinediones, and sulfonylureas. There were no differences in glycaemic control, duration of diabetes, family history of diabetes, and use of insulin therapy between the 2 groups.
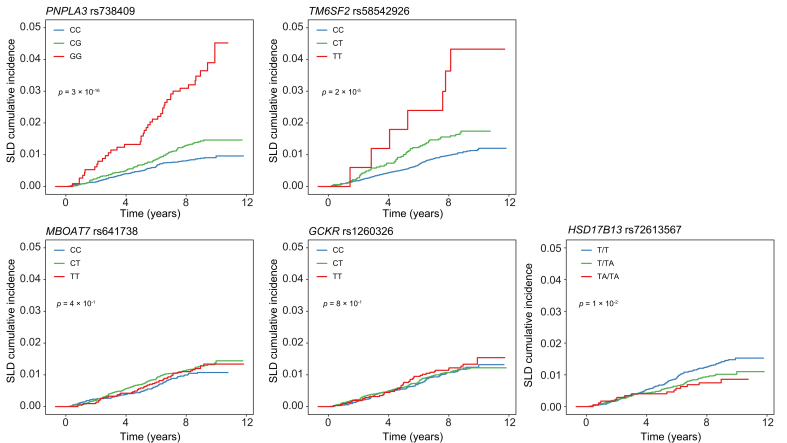
Minor allele frequencies of known genetic variants associated with SLD in the general population were consistent with previous reports in Europeans19,21,23,26,27 and genotype frequency distribution of these variants was in Hardy-Weinberg equilibrium. Genotype frequency stratified by incident SLD status is shown in Table 2. As expected, there was an enrichment of the minor allele for the PNPLA3 rs738409 and the TM6SF2 rs58542926 variants in individuals who developed SLD compared with those without, whereas the HSD17B13 rs72613567 variant was less common in this group.
Table 2.
Genotype frequency of PNPLA3 rs738409, TM6SF2 rs58542926, MBOAT7 rs641738, GCKR rs1260326, and HSD17B13 rs72613567 in UK Biobank participants of European descent with type 2 diabetes stratified by incident severe liver disease status.
| Total (n = 22,812) | No severe liver disease (n = 22,533) | Severe liver disease (n = 279) | p value | |
|---|---|---|---|---|
| PNPLA3 rs738409 | ||||
| CC, n (%) | 13,436 (61%)∗ | 13,318 (61%) | 118 (45%) | |
| CG, n (%) | 7,540 (34%) | 7,435 (34%) | 105 (40%) | <0.001 |
| GG, n (%) | 1,133 (5%) | 1,092 (5%) | 41 (15%) | |
| TM6SF2 rs58542926 | ||||
| CC, n (%) | 18,626 (84%)∗ | 18,425 (84%) | 201 (76%) | |
| CT, n (%) | 3,274 (15%) | 3,219 (15%) | 55 (21%) | <0.001 |
| TT, n (%) | 167 (1%) | 160 (1%) | 7 (3%) | |
| MBOAT7 rs641738 | ||||
| CC, n (%) | 6,869 (31%)∗ | 6,797 (31%) | 72 (28%) | |
| CT, n (%) | 10,765 (49%) | 10,629 (49%) | 136 (52%) | 0.30 |
| TT, n (%) | 4,279 (20%) | 4,226 (20%) | 53 (20%) | |
| GCKR rs1260326 | ||||
| CC, n (%) | 8,556 (39%)∗ | 8,455 (39%) | 101 (38%) | |
| CT, n (%) | 10,320 (47%) | 10,200 (47%) | 120 (46%) | 0.61 |
| TT, n (%) | 3,150 (14%) | 3,109 (14%) | 41 (16%) | |
| HSD17B13 rs72613567 | ||||
| T/T, n (%) | 11,525 (52%)∗ | 11,365 (52%) | 160 (61%) | |
| T/TA, n (%) | 8,770 (40%) | 8,682 (40%) | 88 (34%) | 0.004 |
| TA/TA, n (%) | 1,738 (8%) | 1,724 (8%) | 14 (5%) | |
Values of p are from generalised linear models adjusted for age, sex, and assessment centre. Values of p <0.05 were considered statistically significant.
Genotype distribution is in Hardy-Weinberg equilibrium.
During a median (IQR) follow-up of 8.9 (8.1–9.6) years, there were 279 individuals with SLD, including 255 with cirrhosis and/or decompensated liver disease, 47 with hepatocellular carcinoma, and 5 that underwent liver transplantation; death from SLD occurred in 83 individuals.
Cumulative incidence of SLD for the different genetic variants across genotypes in the entire cohort is shown in Fig. 1.
Fig. 1.
Cumulative incidence of severe liver disease for PNPLA3 rs738409, TM6SF2 rs58542926, MBOAT7 rs641738, GCKR rs1260326, and HSD17B13 rs7261356 across genotypes in the entire cohort with type 2 diabetes.
Blue, green, and red lines represent non-carriers, heterozygous carriers, and homozygous carriers of the minor allele, respectively. Values of p are from the log-rank test for trend. SLD, severe liver disease.
Risk factors for SLD
Risk factors independently associated with increased risk of incident SLD in European participants with type 2 diabetes are shown in Table 3 and included: AST >30/19 U/L (adjusted hazard ratio [aHR] 4.85, 95% CI 2.76–8.54), decrease in serum albumin (aHR 2.39, 95% CI 1.76–3.24) and platelet count (aHR 1.12, 95% CI 1.09–1.16), cardiovascular disease (aHR 1.86, 95% CI 1.23–2.79), microalbuminuria (aHR 1.55, 95% CI 1.04–2.30), PNPLA3 rs738409 (aHR 1.67, 95% CI 1.27–2.18 and aHR 2.32, 95% CI 1.36–3.96 for the additive and recessive models, respectively), and TM6SF2 rs58542926 (aHR 1.63, 95% CI 1.12–2.39 and aHR 4.33, 95% CI 1.74–10.80 for the additive and recessive models, respectively), whereas the net effect of male sex was protective (aHR 0.49, 95% CI 0.26–0.94). In sensitivity analyses, after excluding participants with excessive alcohol consumption and stratifying by sex, results were substantially similar to the main model except that in women higher BMI was positively correlated with SLD (aHR 1.41, 95% CI 1.03–1.93) (Tables S5 and S6).
Table 3.
Risk factors for severe liver disease in UK Biobank participants of European descent with type 2 diabetes (n = 22,812).
| Variable | HR (95% CI) | p value | aHR (95% CI) | p value |
|---|---|---|---|---|
| Age, years | 1.03 (1.02–1.05) | <0.001 | 1.01 (0.98–1.05) | 0.39 |
| Male sex | 1.76 (1.35–2.31) | <0.001 | 0.49 (0.26–0.94) | 0.031 |
| Family history of diabetes | 0.76 (0.60–0.98) | 0.031 | 0.74 (0.50–1.10) | 0.13 |
| Duration of diabetes, years | 1.01 (0.99–1.03) | 0.18 | 1.00 (0.97–1.03) | 0.97 |
| Comorbidities | ||||
| Hypertension | 1.44 (1.07–1.93) | 0.016 | 0.70 (0.42–1.18) | 0.18 |
| Dyslipidaemia | 0.89 (0.67–1.18) | 0.42 | ||
| BMI ≥30 kg/m2 | 1.84 (1.42–2.38) | <0.001 | 1.02 (0.64–1.64) | 0.93 |
| Waist circumference ≥94/80 cm | 2.80 (1.69–4.64) | <0.001 | 1.19 (0.49–2.92) | 0.70 |
| Cardiovascular disease | 1.94 (1.52–2.49) | <0.001 | 1.86 (1.23–2.79) | 0.003 |
| Lifestyle | ||||
| Low–moderate alcohol intake∗ | 0.71 (0.53–0.94) | 0.018 | 0.80 (0.49–1.31) | 0.38 |
| Excessive alcohol intake∗ | 1.63 (1.19–2.23) | 0.003 | 1.59 (0.93–2.71) | 0.091 |
| Current smoking status | 1.13 (0.79–1.63) | 0.51 | ||
| Physical activity ≥150/75 min/week | 1.00 (0.83–1.21) | 0.99 | ||
| Clinical chemistry | ||||
| HbA1c, mmol/mol | 1.00 (0.99–1.01) | 0.74 | ||
| ALT >30/19 U/L | 2.26 (1.76–2.90) | <0.001 | 1.37 (0.83–2.24) | 0.22 |
| AST >30/19 U/L | 5.18 (4.09–6.55) | <0.001 | 4.85 (2.76–8.54) | <0.001 |
| Albumin, per 0.5 g/dl decrease | 2.45 (2.01–2.99) | <0.001 | 2.39 (1.76–3.24) | <0.001 |
| Platelet count, per 10∗109/L decrease | 1.16 (1.13–1.18) | <0.001 | 1.12 (1.09–1.16) | <0.001 |
| eGFR <60 ml/min/1.73 m2 | 1.68 (1.12–2.51) | 0.012 | 1.22 (0.66–2.25) | 0.52 |
| Microalbuminuria | 1.70 (1.23–2.35) | 0.001 | 1.55 (1.04–2.30) | 0.03 |
| Macroalbuminuria | 1.37 (0.67–2.79) | 0.38 | ||
| Genetic risk factors | ||||
| PNPLA3 rs738409 genotype | ||||
| Additive model | 1.92 (1.61–2.30) | <0.001 | 1.67 (1.27–2.18) | <0.001 |
| Recessive model | 3.47 (2.49–4.84) | <0.001 | 2.32 (1.36–3.96)† | 0.002 |
| TM6SF2 rs58542926 genotype | ||||
| Additive model | 1.69 (1.32–2.17) | <0.001 | 1.63 (1.12–2.39) | 0.011 |
| Recessive model | 3.59 (1.70–7.62) | <0.001 | 4.33 (1.74–10.80)† | 0.002 |
| MBOAT7 rs641738 genotype | ||||
| Additive model | 1.09 (0.92–1.30) | 0.30 | ||
| Recessive model | 1.05 (0.78–1.42) | 0.75 | ||
| GCKR rs1260326 genotype | ||||
| Additive model | 1.04 (0.87–1.24) | 0.67 | ||
| Recessive model | 1.12 (0.80–1.56) | 0.52 | ||
| HSD17B13 rs72613567 genotype | ||||
| Additive model | 0.74 (0.60–0.91) | 0.004 | 0.74 (0.54–1.03) | 0.076 |
| Recessive model | 0.66 (0.38–1.13) | 0.13 | ||
| Drugs | ||||
| Metformin | 1.40 (1.10–1.78) | 0.006 | 1.46 (0.96–2.23) | 0.076 |
| Thiazolidinediones | 1.51 (1.04–2.20) | 0.03 | 1.48 (0.82–2.67) | 0.19 |
| Sulfonylureas | 1.40 (1.07–1.83) | 0.014 | 1.32 (0.87–1.99) | 0.19 |
| Insulin | 0.83 (0.61–1.14) | 0.26 | ||
| Statins | 0.91 (0.70–1.17) | 0.45 | ||
HRs with 95% CIs were calculated by Cox proportional hazards models. Age, sex, BMI, alcohol intake, duration of diabetes, and all predictor variables with a value of p <0.05 in the univariate model were included in the multivariate model.
aHR, adjusted HR; ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HR, hazard ratio.
Low–moderate (<20/30 g/day) and excessive (≥20/30 g/day) alcohol intake tested against abstainers.
aHR calculated assuming recessive model instead of additive model.
Discussion
In this work we investigate for the first time in the UK Biobank the acquired and inborn independent risk factors for SLD among Europeans with type 2 diabetes. Among the acquired, we demonstrate that abnormal AST levels, decrease in serum albumin and platelet count, cardiovascular disease, and microalbuminuria are independent markers of SLD. Among the inborn, genetic variants in PNPLA3 and TM6SF2 genes increase the risk of SLD in this population.
In our analyses, we started by: (1) selecting individuals with type 2 diabetes as those with diagnosis of type 2 or unspecified diabetes and/or self-reported history of these conditions, and (2) excluding at baseline those with diagnosis of all major causes of liver disease and liver cancer and/or self-reported history of these conditions. Then, we prospectively examined the incidence of SLD, defined as a composite diagnosis of severe chronic liver disease including non-viral cirrhosis, decompensated liver disease, hepatocellular carcinoma, and liver transplantation.
Our results provide several clues regarding the risk prediction of SLD in individuals with type 2 diabetes. Indeed, biochemical proxies of hepatocellular damage, that is transaminases, are the strongest predictor of SLD with an elevation above the upper normal limit associated with an approximately 5-fold increased risk. Interestingly, abnormal AST seems to predict adverse liver outcomes more accurately than abnormal ALT. High AST levels may indicate mitochondrial damage as a result of alcohol abuse and they correlate with liver fibrosis better than ALT.28 As a result, transaminases remain, also in individuals with type 2 diabetes, a main screening test to define the risk level of developing life-threatening liver-related complications. However, it should be borne in mind that chronic liver damage and advanced fibrosis may develop even with normal liver enzymes.29
Biochemical proxies of reduced liver function (low albumin) and portal hypertension (low platelet count) are strong markers of SLD in individuals with type 2 diabetes. This is likely because of the fact that they mirror the presence of an underlying and unknown advanced liver disease. Consistent with the present study, where we have excluded at baseline only those with self-reported or diagnosed SLD, FLD may progress to advanced fibrosis without having being diagnosed.30,31 This may suggest that, during follow-up of individuals with type 2 diabetes, special attention is required towards lowering of platelet count and albumin levels. Indeed, in individuals with diabetes low albumin levels may be more frequently attributed to proteinuria attributable to advanced chronic kidney disease.
Excess alcohol intake is well-known to cause liver damage and to exacerbate liver injury induced by other causes. Moreover, it is also associated with development of type 2 diabetes and with worse glycaemic control.32 Here we find an almost doubling of the risk of SLD in individuals with type 2 diabetes and excessive alcohol consumption even if not statistically significant, which is consistent with the risk observed in the general population.33 Notably, low-moderate alcohol intake appears not to increase the risk of SLD. Future studies are warranted to prove if a complete abstinence is not required to prevent liver disease progression among individuals with type 2 diabetes.
Cardiovascular disease represents the first cause of death in individuals suffering from FLD.5 Consistently, cardiovascular disease resulted as a strong risk factor for developing SLD in our cohort, as well as the presence of microalbuminuria. These data support the notion that individuals with type 2 diabetes and cardiovascular/kidney complications should be screened for liver disease. Taking all this together, liver disease may be considered among diabetes-related complications.
Unfavourable genetics is a robust independent risk factor for SLD. Of note, this is the first prospective study specifically evaluating the impact of genetic risk variants on the risk of developing advanced liver disease in individuals of European descent with type 2 diabetes. In particular, our data demonstrate that PNPLA3 rs738409 and TM6SF2 rs58542926, the two strongest genetic variants increasing the risk of SLD in the general population,19,20 confer also a strong susceptibility to SLD in those with type 2 diabetes. Notably, unlike traditional risk factors that may vary over time, the risk conferred by genetic variants is very stable and constitutes a lifetime burden. As a consequence, genetic testing for PNPLA3 rs738409 and TM6SF2 rs58542926 might be useful to identify individuals with type 2 diabetes at high-risk for progressive liver disease, thus requiring more intensive follow-up strategies or specific lifestyle changes (e.g. reduction in alcohol and fructose intake).
Surprisingly, increased BMI was not associated with increase in the risk of SLD in the overall cohort. This may be because the mean BMI of the cohort was in the range of class I obesity and the net contribution of obesity to SLD is likely diluted by the absence of normal-weight individuals. Notably, obesity had a greater impact on SLD risk in women than in men, supporting the presence of sexual dysmorphism underlying human disease. Indeed, this could be a result of the complex interaction between genetic factors, sex, and its related biological components. In agreement, data in the literature report a stronger association between increased BMI and diabetes risk in women than in men.34 However, tailored studies with larger sample size, specifically focusing on sex differences in liver disease, are needed to ascertain this issue.
To date, there is a lack of prospective cohort studies evaluating the contribution of multiple risk factors for advanced liver disease in type 2 diabetes. In a large prospective cohort study of over 400,000 Swedish participants with type 2 diabetes, Björkström et al.11 have recently shown that risk factors independently associated with SLD were older age, male sex, higher BMI, hypertension, lower eGFR, microalbuminuria, and smoking, whereas use of statins conferred decreased risk. However, in this study the contribution of biochemical proxies of liver function and damage, alcohol consumption, and genetic variations was not investigated.
We confirm the role of microalbuminuria as independent risk factor for SLD. Additionally, we show that microalbuminuria is correlated with increased risk of SLD especially in men, in line with the well-documented higher frequency of albuminuric renal impairment in men with type 2 diabetes compared with women.35 Notwithstanding the significantly higher incidence of SLD in men, we find that male sex is protective against SLD. This is likely because we included the main mediators of the association between male sex and SLD in the multivariate Cox regression analysis. Further studies specifically aimed at evaluating the effect of sex on the risk of SLD are required to verify this finding. We do not find age, high BMI, hypertension, low eGFR, and smoking associated with SLD. These results might be explained by the fact that we included in the multivariate model additional stronger risk factors for SLD, such as biochemical proxies of liver damage, harmful alcohol consumption, and genetic variants. Alternatively, these data might be attributable to the different category of variables included in the multivariate model (binary vs. continuous) or to the relatively lower sample size of our study compared with that by Björkström et al.11
Another major difference between the study by Björkström et al.11 and our study is that we included alcoholic-related diagnoses in the definition of SLD. This is because metabolic and alcoholic liver disease share similar molecular pathways10,[19], [20], [21],27,36 and also because a clear distinction is often difficult to assess in the real life. Moreover, the sensitivity analysis excluding participants with excessive alcohol consumption showed similar results to the main model.
Additionally, in both studies HbA1c was found to be not related to increased risk of SLD. Because of the well-established association of HbA1c with higher risk of chronic micro- and macro-vascular complications of type 2 diabetes,37 this finding might be explained by the fact that HbA1c does not accurately reflect glycaemic control in individuals with diabetes and cirrhosis.[38], [39], [40]
The major strengths of our study are the following: (1) the large sample size, including more than 20,000 individuals with type 2 diabetes from the general population; (2) the prospective study design; (3) the use of standardised procedures and centrally validated protocol for blood samples collecting, processing, and storage of the UK Biobank. Furthermore, this is the first prospective study collectively examining the impact of indices of liver function and damage, alcohol consumption, and genetic variants on the risk of developing SLD in European individuals with type 2 diabetes.
This study also has limitations. First, some cases of asymptomatic liver disease (e.g. compensated cirrhosis or early stages of hepatocellular carcinoma) may have been underdiagnosed. Similarly, some cases of chronic viral hepatitis may be unknown or sometimes the viral aetiology of cirrhosis may be not specified in hospital records. However, we tried to reduce this bias by linking liver-related diagnoses from multiple registers (i.e. hospital records, death register, and cancer register). Second, we included participants with previous history and/or hospital diagnosis of unspecified diabetes, those treated with insulin, and those with undiagnosed diabetes based on circulating glucose and/or HbA1c tests. As a result, although type 2 diabetes accounts for approximately 90% of people with diabetes,1 few participants may be affected by type 1 diabetes or latent autoimmune diabetes of adulthood. Third, since we had only 1 baseline urine sample to establish albuminuria categories, the diagnosis of moderately and severely increased albuminuria could be overestimated, although an Italian multicentre prospective cohort study of over 15,000 patients with type 2 diabetes reported similar rates.41 Finally, the results were obtained in Europeans and further studies are needed to validate them in other ethnic groups.
In conclusion, we demonstrate that in Europeans with type 2 diabetes: (1) abnormal AST levels, decrease in serum albumin and platelet count, cardiovascular disease, and microalbuminuria are independent markers of SLD; (2) genetic variants in PNPLA3 and TM6SF2 genes increase the risk of SLD. Our findings may help to identify individuals with type 2 diabetes at-risk for SLD by identifying acquired and inborn risk factors. This may contribute to estimate a personalised risk prediction and to implement strategies to prevent SLD.
Financial support
This work was supported by the Swedish Research Council (Vetenskapsrådet [VR], 2016-01527), the Swedish state under the agreement between the Swedish government and the county councils (the ALF agreement) (SU 2018-04276), the Swedish Diabetes Foundation (DIA 2017-205), the Swedish Heart-Lung Foundation (20120533), the Wallenberg Academy Fellows from the Knut and Alice Wallenberg Foundation (KAW 2017.0203), the Astra Zeneca Agreement for Research, the Swedish Foundation for Strategic Research (SSF ITM17-0384), and Novo Nordisk Project Grants in Endocrinology & Metabolism - Nordic Region 2020.
Authors’ contributions
Contributed to study concept and design: UVG, SR. Contributed to drafting the manuscript: FT, ADV, UVG, SR. Contributed to perform the statistical analysis: ADV. Contributed to analysis and interpretation of data, critically revised the manuscript for important intellectual content, and approved the final version for submission: all authors. Full access to all the data in the study and responsibility for the integrity of the data and the accuracy of the data analysis: all authors
Data availability statement
UK Biobank data are available through a procedure described at http://www.ukbiobank.ac.uk/using-the-resource/.
Conflict of interest
The authors declare no financial or other relationships with drug manufacturers that could lead to a conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We thank the staff and the participants of the UK Biobank study. This research has been conducted using the UK Biobank resource (application 37142).
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2021.100262.
Supplementary data
The following are the supplementary data to this article:
References
- 1.International Diabetes Federation . 9th edn. International Diabetes Federation; Brussels: 2019. IDF Diabetes Atlas. [Google Scholar]
- 2.Younossi Z.M., Golabi P., de Avila L., Paik J.M., Srishord M., Fukui N. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Tavaglione F., Targher G., Valenti L., Romeo S. Human and molecular genetics shed lights on fatty liver disease and diabetes conundrum. Endocrinol Diabetes Metab. 2020;3 doi: 10.1002/edm2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 5.Younossi Z.M. Non-alcoholic fatty liver disease – a global public health perspective. J Hepatol. 2019;70:531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 6.Blachier M., Leleu H., Peck-Radosavljevic M., Valla D.C., Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593–608. doi: 10.1016/j.jhep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Tsochatzis E.A., Bosch J., Burroughs A.K. Liver cirrhosis. Lancet. 2014;383:1749–1761. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 8.Forner A., Llovet J.M., Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 9.Eslam M., Valenti L., Romeo S. Genetics and epigenetics of NAFLD and NASH: clinical impact. J Hepatol. 2018;68:268–279. doi: 10.1016/j.jhep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Romeo S., Sanyal A., Valenti L. Leveraging human genetics to identify potential new treatments for fatty liver disease. Cell Metab. 2020;31:35–45. doi: 10.1016/j.cmet.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Björkström K., Franzén S., Eliasson B., Miftaraj M., Gudbjörnsdottir S., Trolle-Lagerros Y. Risk factors for severe liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2019;17:2769–2775. doi: 10.1016/j.cgh.2019.04.038. e2764. [DOI] [PubMed] [Google Scholar]
- 12.Sudlow C., Gallacher J., Allen N., Beral V., Burton P., Danesh J. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott P., Peakman T.C., Biobank U. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. 2008;37:234–244. doi: 10.1093/ije/dym276. [DOI] [PubMed] [Google Scholar]
- 14.Townsend P., Phillimore P., Beattie A. Croom Helm; London: 1988. Health and Deprivation: Inequality and the North. [Google Scholar]
- 15.European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., Feldman H.I. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association Standards of medical care in diabetes. Diabetes Care. 2020;43:S135–S151. doi: 10.2337/dc20-S011. [DOI] [PubMed] [Google Scholar]
- 18.Bycroft C., Freeman C., Petkova D., Band G., Elliott L.T., Sharp K. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Cox D., Pennacchio L.A. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozlitina J., Smagris E., Stender S., Nordestgaard B.G., Zhou H.H., Tybjærg-Hansen A. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancina R.M., Dongiovanni P., Petta S., Pingitore P., Meroni M., Rametta R. The MBOAT7-TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology. 2016;150:1219–1230. doi: 10.1053/j.gastro.2016.01.032. e1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valenti L., Alisi A., Nobili V. Unraveling the genetics of fatty liver in obese children: additive effect of P446L GCKR and I148M PNPLA3 polymorphisms. Hepatology. 2012;55:661–663. doi: 10.1002/hep.25617. [DOI] [PubMed] [Google Scholar]
- 23.Abul-Husn N.S., Cheng X., Li A.H., Xin Y., Schurmann C., Stevis P. A protein-truncating HSD17B13 variant and protection from chronic liver disease. N Engl J Med. 2018;378:1096–1106. doi: 10.1056/NEJMoa1712191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alberti K.G., Zimmet P., Shaw J. Metabolic syndrome – a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 25.Prati D., Taioli E., Zanella A., Della Torre E., Butelli S., Del Vecchio E. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 26.Dongiovanni P., Petta S., Maglio C., Fracanzani A.L., Pipitone R., Mozzi E. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology. 2015;61:506–514. doi: 10.1002/hep.27490. [DOI] [PubMed] [Google Scholar]
- 27.Speliotes E.K., Yerges-Armstrong L.M., Wu J., Hernaez R., Kim L.J., Palmer C.D. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Botros M., Sikaris K.A. The de ritis ratio: the test of time. Clin Biochem Rev. 2013;34:117–130. [PMC free article] [PubMed] [Google Scholar]
- 29.Verma S., Jensen D., Hart J., Mohanty S.R. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD) Liver Int. 2013;33:1398–1405. doi: 10.1111/liv.12226. [DOI] [PubMed] [Google Scholar]
- 30.Doycheva I., Cui J., Nguyen P., Costa E.A., Hooker J., Hofflich H. Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther. 2016;43:83–95. doi: 10.1111/apt.13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwok R., Choi K.C., Wong G.L., Zhang Y., Chan H.L., Luk A.O. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65:1359–1368. doi: 10.1136/gutjnl-2015-309265. [DOI] [PubMed] [Google Scholar]
- 32.Lomonaco R., Bril F., Portillo-Sanchez P., Ortiz-Lopez C., Orsak B., Biernacki D. Metabolic impact of nonalcoholic steatohepatitis in obese patients with type 2 diabetes. Diabetes Care. 2016;39:632–638. doi: 10.2337/dc15-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roerecke M., Vafaei A., Hasan O.S.M., Chrystoja B.R., Cruz M., Lee R. Alcohol consumption and risk of liver cirrhosis: a systematic review and meta-analysis. Am J Gastroenterol. 2019;114:1574–1586. doi: 10.14309/ajg.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kautzky-Willer A., Harreiter J., Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 2016;37:278–316. doi: 10.1210/er.2015-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penno G., Solini A., Bonora E., Fondelli C., Orsi E., Zerbini G. Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J Hypertens. 2011;29:1802–1809. doi: 10.1097/HJH.0b013e3283495cd6. [DOI] [PubMed] [Google Scholar]
- 36.Buch S., Stickel F., Trépo E., Way M., Herrmann A., Nischalke H.D. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet. 2015;47:1443–1448. doi: 10.1038/ng.3417. [DOI] [PubMed] [Google Scholar]
- 37.Stratton I.M., Adler A.I., Neil H.A., Matthews D.R., Manley S.E., Cull C.A. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elkrief L., Rautou P.E., Sarin S., Valla D., Paradis V., Moreau R. Diabetes mellitus in patients with cirrhosis: clinical implications and management. Liver Int. 2016;36:936–948. doi: 10.1111/liv.13115. [DOI] [PubMed] [Google Scholar]
- 39.Orsi E., Grancini V., Menini S., Aghemo A., Pugliese G. Hepatogenous diabetes: is it time to separate it from type 2 diabetes? Liver Int. 2017;37:950–962. doi: 10.1111/liv.13337. [DOI] [PubMed] [Google Scholar]
- 40.Bhattacharjee D., Vracar S., Round R.A., Nightingale P.G., Williams J.A., Gkoutos G.V. Utility of HbA1c assessment in people with diabetes awaiting liver transplantation. Diabet Med. 2019;36:1444–1452. doi: 10.1111/dme.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pugliese G., Solini A., Bonora E., Fondelli C., Orsi E., Nicolucci A. Chronic kidney disease in type 2 diabetes: lessons from the Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicentre study. Nutr Metab Cardiovasc Dis. 2014;24:815–822. doi: 10.1016/j.numecd.2014.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
UK Biobank data are available through a procedure described at http://www.ukbiobank.ac.uk/using-the-resource/.