Abstract
The oral and maxillofacial regions have complex anatomical structures and different tissue types, which have vital health and aesthetic functions. Biodegradable metals (BMs) is a promising bioactive materials to treat oral and maxillofacial diseases. This review summarizes the research status and future research directions of BMs for oral and maxillofacial applications. Mg-based BMs and Zn-based BMs for bone fracture fixation systems, and guided bone regeneration (GBR) membranes, are discussed in detail. Zn-based BMs with a moderate degradation rate and superior mechanical properties for GBR membranes show great potential for clinical translation. Fe-based BMs have a relatively low degradation rate and insoluble degradation products, which greatly limit their application and clinical translation. Furthermore, we proposed potential future research directions for BMs in the oral and maxillofacial regions, including 3D printed BM bone scaffolds, surface modification for BMs GBR membranes, and BMs containing hydrogels for cartilage regeneration, soft tissue regeneration, and nerve regeneration. Taken together, the progress made in the development of BMs in oral and maxillofacial regions has laid a foundation for further clinical translation.
Keywords: Biodegradable metals, Oral and maxillofacial region, Bone fracture, Bone fixation, Bone defect, Guided bone regeneration
Graphical abstract
Highlights
-
•
The research status of biodegradable metals for oral and maxillofacial applications is systematically reviewed.
-
•
The future research directions of biodegradable metals or oral and maxillofacial applications are prospected. .
-
•
Zn-based GBR membranes show great potential for clinical translation.
1. Introduction
The oral and maxillofacial region is one of the important parts of the human body because of their many functions and aesthetic values, which have a complex anatomical structure and several tissue types. Once damaged, it is difficult to repair. Bone substitutions including autograft, allograft, xenograft, and artificial materials are used to reconstruct oral and maxillofacial regions. Autograft has great osteogenic effect and is considered the gold standard for bone augmentation. However, autograft has some shortcomings such as extra trauma in donor site and limited bone available [1]. Xenograft with a wide range of sources is commonly used bone augmentation in clinic. However, there are some problems in xenograft, such as immune rejection, ethical controversy, and unsatisfactory osseointegration [2,3]. Dentine grafts and allografts may be effective methods for bone augmentation, but they have not been widely studied and applied in clinical practice [[4], [5], [6]]. Bioceramics is difficult to balance good degradability and mechanical strength, which limits their clinical application [7]. Non-biodegradable metallic materials, such as titanium (Ti) and stainless steel, are widely applied in maxillofacial surgery because of their excellent mechanical strength and biocompatibility. However, they require secondary surgery to remove the implants, causing surgical pain and economic burden. In addition, the difference in the elastic modulus of these metals and bone causes stress shielding, leading to resorption of the surrounding bone [8]. While biodegradable polymer has the disadvantages of low mechanical strength and acidic degradation products [9,10].
Biodegradable metals (BMs) have biodegradability properties, excellent mechanical strength, ductility, formability, osteogenic capacity, and antibacterial properties. In 2014, Zheng et al., provided the first formal definition of the term “Biodegradable metals (BMs)" [11]. In 2019, to make it identical to the term “Absorbable metallic biomaterials” proposed by the ASTM, Zheng et al. modified the definition as follows: “metals expected to corrode gradually in vivo, with an appropriate host response elicited by released corrosion products, which can pass through or be metabolized or assimilated by cells and/or tissue, and then dissolve completely upon fulfilling the mission to assist with tissue healing with no implant residues” [12]. The most representative BMs are Mg-based BMs, Zn-based BMs, and Fe-based BMs. Mg-based and Fe-based BMs have a long history of biomedical use. Fe-based BMs have been used to manufacture biodegradable medical sutures since the 17th century [13]. The earliest recorded application of Mg was as a ligature for bleeding vessels in 1878. By the early 20th century, Mg implants were being used for orthopedic fixation [14]. Mg-based BMs are the most thoroughly studied and widely used BMs because its elastic modulus is similar to human bones, and its biosafety, biodegradability, and visualization on radiographs. Over time, Mg-based BMs have been applied in manufacturing fixation plates, screws, pins, wires, and stents [15]. Fe-based BMs have a relatively low degradation rate and insoluble degradation products, which greatly limit their application [16]. Zn-based BMs with a moderate degradation rate and superior mechanical properties show great potential for clinical translation. In recent years, Zn-based BMs have attracted more and more attention for orthopedic and cardiovascular applications.
To date, there has no review on BMs for oral and maxillofacial applications. This review focuses on the latest applications of three representative BMs (Mg-based BMs, Fe-based BMs, and Zn-based BMs) in the oral and maxillofacial regions, including bone, cartilage, teeth, and soft tissue. In addition, we summarize the application of composite materials containing BMs or BM compounds in oral and maxillofacial region. We also discuss the obstacles for the application of BMs in the oral and maxillofacial regions, and its prospects and possible development directions in the future.
2. Biodegradation of biodegradable metals
Unlike in vitro degradation, the degradation of BMs in complex in vivo environments is often difficult to predict. The degradation of BMs in vivo depends to a great extent on the conditions at the site of implantation [17,18]. Some researchers believe that the implants used for bone fixation must maintain their mechanical stability for at least 12 weeks; however, for children, a shorter stable time is acceptable [19,20].
2.1. Biodegradation of Mg-based BMs
In the nearly neutral physiological environment, Mg-based BMs react with water to form Mg hydroxide and release hydrogen gas that has very low solubility in the blood, which is a natural electrochemical reaction.
| Mg → Mg2+ +2e− (anodic reaction) |
| 2H2O + 2e− → H2 + 2OH− (cathodic reaction) |
| Mg+2H2O → Mg(OH)2+ H2 (overall reaction) |
| Mg(OH)2+ 2Cl− → MgCl2+2OH− |
| 5Ca2++3PO43−+OH− ⇌ Ca5(PO4)3OH |
Mg hydroxide formed on the surface of Mg-based BMs, a non-toxic and harmless precipitate, has loose, porous and non-adhesive properties. Therefore, the degradation product Mg hydroxide can only provide partial protection for Mg-based BMs to prevent further corrosion [21]. In a physiological environment, abundant aggressive chloride ions react with Mg hydroxide to form Mg chloride. Chloride ions at concentrations above 30 mmol/L will destroy the passivation film of surface corrosion products and accelerate the corrosion of Mg alloys by pitting corrosion [19]. The increase of local pH and the saturation of calcium ions and phosphate in body fluids promote the formation of calcium phosphate compounds that can inhibit the degradation of Mg implants [22,23]. The composition of calcium phosphate deposits is similar to the calcium salts found in natural bone and contributes to the formation of new surrounding bone [23,24].
The degradation of Mg implants in vivo leads to the production of excess Mg ions. Mg, an essential micronutrient, is consumed at about 350–400 mg/day [25]. Cells can tolerate concentrations of Mg ions up to 16 mM, which is about 16 times the physiological range [26]. The excessive Mg ions produced by the degradation of regular volume Mg implants in vivo do not change the concentration of Mg ions in serum or affect biosecurity, because they are excreted out of the body through the urinary system [27,28]. An Mg alloy implanted into the cranial bone produced no Mg deposition in lymph nodes after degradation [29]. Based on in vivo experiments, the excess Mg ions produced by Mg implants resulted in no adverse health risks to rats with chronic renal failure [30]. Furthermore, Mg ions can promote the formation of callus and accelerate local bone mineralization and remodeling. Mg ions promote fracture healing by upregulating the expression of the calcitonin gene-related peptide, thereby reducing delayed fracture healing and nonunion of fractures [31]. The newly formed bone at the fracture site fixed with Mg and Ti has a similar histological structure and mechanical properties [32,33]. What's more, Mg alloys are relatively safe for children's bone development [[34], [35], [36]].
However, the rapid degradation of Mg results in an increase in the local pH level, although blood and tissue fluid flowing through the body can buffer pH changes. The high alkaline extracellular environment caused by the rapid corrosion of Mg is not conducive to the survival and osteogenic differentiation of human bone marrow-derived stem cells (hBMSCs) [37], and even leads to alkalosis [38]. Relatively large amounts of hydrogen are produced in the early stages after Mg implantation, possibly leading to the formation of localized gas cavities, especially if the soft tissue seals well. To some extent, the hydrogen gas cavity compresses the surrounding tissues, which is harmful to cell adhesion, proliferation, and differentiation. Hydrogen gas accumulation interferes with implant osseointegration and bone formation around the Mg implant [17,39]. Therefore, it is important to reduce the degradation rate of Mg-based BMs.
2.2. Biodegradation of Zn-based BMs
Zn is the second-most abundant trace element in the human body. Dietary allowance in the United States recommends 8–11 mg/d of Zn. According to the standards of the Food and Drug Administration, adult Zn tolerance is 40 mg/d [40]. Zn-based BMs degrade naturally in the physiological environment without producing gas and harmful products. Many preclinical experiments showed that the Zn-based BMs implanted in the rabbit abdominal and rat femur condlye have good biocompatibility [41]. Degradation products of Zn-based BMs mainly include Zn oxide, Zn hydroxide, and Zn phosphate, among which Zn phosphate might be a key degradation product to improve the biocompatibility of Zn-based implants [41,42]. Similarly, chlorine ions in the physiological environment can also react with Zn(OH)2 to form more soluble chlorine salts, which will disrupt the equilibrium of dissolution and formation of Zn(OH)2 and promote further dissolution of the Zn-based BMs [21].
| Zn → Zn2+ + 2e− (anodic reaction) |
| 2H2O + O2 + 4e− → 4OH− (cathodic reaction) |
| 2Zn2+ + 2H2O + O2 → 2Zn (OH)2 (overall reaction) |
| Zn2+ + 2OH− → ZnO + H2O |
| 3Zn2+ + 2HPO42− + 2OH− +2H2O → Zn3(PO4)2 |
The degradation rate of Zn-based BMs was moderate, being lower than that of Mg-based BMs and higher than that of Fe-based BMs. Previous experiments showed that the degradation rate of Zn-based BMs in vivo is about 0.07–0.22 mm/year [43,44].
2.3. Biodegradation of Fe-based BMs
Iron plays a crucial role in the biochemical activities of the body and is an essential nutrient with limited bioavailability. The human body contains about 3–5 g iron, which is stored mainly in hemoglobin, liver and muscle [45]. To compensate for iron depletion due to skin and mucous membrane shedding, menstruation, etc., the recommended daily intake of iron is 1–2 mg/day [46]. Although iron is a physiological element, its redox property determines its harm to cells and tissues. In the process of iron degradation, in addition to producing Fe2+ and Fe3+, it can also produce reactive oxygen and free radicals, resulting in cell and tissue damage [45]. Fe can catalyze the formation of reactive oxygen species and free radicals, resulting in cell and tissue damage. The human body has no effective physiological mechanism for excreting Fe, therefore, Fe intake must be strictly controlled to prevent excess [47]. Thus, some researchers believe that the current Fe-based BMs cannot be considered as safe or biocompatible materials [45].
| Fe → Fe2+ +2e− (anodic reaction) |
| 2H2O + 2e− → H2 + 2OH− (cathodic reaction) |
| Fe + 2H2O → Fe(OH)2+ H2 (overall reaction) |
| 2Fe(OH)2 + H2O + 1/2O2 → 2Fe(OH)3 |
| Fe2+ + 2Cl- → FeCl2 |
| Fe2+ + CO32− → FeCO3 |
Several animal experiments have shown that the degradation rate of Fe-based BMs implants in vivo is extremely slow [48]. Pins of pure Fe and two Fe-based BMs (Fe–10Mn–1Pd and Fe–21Mn–0.7C–1Pd, in wt.%) remained largely intact for a year after placement in the femur of rats [49]. The slow degradation rate of Fe-based alloys might not only hinder the formation of new tissue, but also causes side effects similar to those of permanent implants [50]. The passivation layer formed by the degradation of Fe-based BMs in the physiological environment can inhibit the further degradation of the alloys [51]. Solid Fe degradation products are stable and insoluble in the physiological environment, which might cause metabolic complications [52].
3. Mg-based BMs for oral and maxillofacial application
3.1. Application of Mg-based BMs in hard tissue
As a cofactor for enzymes involved in carbohydrate and lipid metabolism, Mg plays an essential role in many metabolic processes [53]. In adults, the total amount of Mg stored in the body is about 25 g, of which 66% is stored in the bones [54]. As the fourth most abundant cation in the body, Mg2+ plays a significant role in the development and mineralization of hard tissue. Mg, a stimulant of bone conduction and bone growth, is closely related to bone metabolism [55]. Mg deficiency affects the immune response and leads to a decrease in the number of osteoblasts and an increase in osteoclasts, leading to bone loss [56]. Mg binds to ion channels on the cell membrane and can affect molecular mobility and permeability. The Mg ion channel, transient receptor potential cation channel subfamily M member 7 (TRPM7), is highly expressed in ameloblasts, odontoblasts, and osteoblasts [57]. Low concentrations of Mg ions can enhance the osteogenic activity of calvarial osteoblasts by activating the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/protein kinase B (Akt) pathway [58]. In vitro and in vivo experiments have confirmed that Mg promotes the mineralization of hard tissues, including enamel, dentin, and craniofacial bone. In the oral and maxillofacial region, the application of Mg in hard tissue mainly includes the following: Screws and plates for maxillofacial bone fixation, guided bone regeneration (GBR) membranes and bone substitutes for bone defects, surface modification of dental implants, and cartilage and tooth regeneration.
3.1.1. Mg-based fracture fixation systems
Maxillofacial fractures are often caused by interpersonal violence and traffic accidents, which affect the appearance of the patient and their normal physiological functions such as chewing and speech [59,60]. Open reduction and internal fixation are the most common methods for fracture fixation treatment [61,62]. This method is also used in orthognathic surgery and bone graft reconstruction after tumor resection in oral and maxillofacial surgery. Bone fixation systems can bear part or all the functional load of the fracture site, thus preventing rotation and displacement of the broken end of the fracture [63]. Although Ti internal fixation systems were regarded as the gold standard, they have many disadvantages, including infection, exposure, pain, cold intolerance, and palpability [64]. The inert metal fixation plates and screws usually have to be removed after the healing of bone tissue. The plate removal rate after surgery reported in the literature varies greatly, from 5 to 55% [65,66], of which 64.4% were removed because of complications [64].
Biodegradable fracture fixation systems solve the disadvantages brought about by the inert metal fixture, which have been used in oral and maxillofacial region surgery since the early 1970s [67]. Current biodegradable medical implants are usually based on polymers, such as poly-l-lactic acid (PLLA) and polylactic acid-glycolic acid (PLGA) [68]. The fixation screws of degradable polymers have poor mechanical properties, leading to early fracture, and usually need tedious additional procedures to make up for these disadvantageous mechanical properties [65,69]. Moreover, the inflammation and foreign body reactions caused by the acidic degradation products of synthetic polymers also limit their practical applications [9,10]. Clinical studies and meta-analyses have shown that the degradable polymer fixation systems used in maxillofacial surgery do not have adequate safety profiles [70,71].
Mg-based BMs with excellent mechanical strength and good biocompatibility are considered promising candidates for biodegradable bone fixation screws and plates. Mg-based alloys screws are the only BM bone fixation device that have achieved clinical translation [23,72]. The elastic modulus of Mg-based alloys is very close to those of natural bone, especially natural cortical bone, which greatly weakens the “stress shielding” effect, thus, decreasing bone resorption [19,73]. The fracture strength and compressive yield strength of Mg-based alloys are lower than those of Ti alloys and cobalt-chromium alloys [19], and are 3–16 times stronger than those of polymers [74]. Except for the mandible, the flat bones of the maxillofacial region usually bear a lower load than long bones [18]. Researchers have found that Mg-based alloy fixation provides sufficient mechanical strength for maxillofacial bone healing [75]. In vitro pull-out experiments showed that pure Mg and AZ31 Mg alloy screws have similar fixation strengths to stainless steel screws [18]. Mg screws can closely bond with the surrounding bone, similar to stainless steel screws [76,77]. A study has shown that bone-implant interface strength of Mg alloy was stronger than those of Ti alloy. After 24 weeks, an Mg rod implanted into the rat femur maintained the main part and could provide mechanical strength [20]. In summary, current experiments show that Mg implants can undertake the functional load of the maxillofacial bone.
To date, many studies have used Mg-based fixation plates and screws to fix maxillofacial fractures (Table 1). In the oral and maxillofacial region, most publications about Mg-based alloy fixation systems are preclinical studies, including finite element analysis. Clinical trials have demonstrated that Mg-based screws provide adequate fixation and are effective in the treatment of hallux valgus surgery and medial malleolar fracture surgery [23,[78], [79], [80]]. Most of the published literature indicates that Mg-based alloy plates and screws are good candidates for bone fixation.
Table 1.
Mg-based fracture fixation systems for the oral and maxillofacial regions.
| Implant site | Materials | Fixation types | Experimental method | Evaluation method | Results | First author, year, ref | |
|---|---|---|---|---|---|---|---|
| Mandible fracture | Posterior mandibular body | Mg | Screw | Finite element modeling (FEM) | Stress Distribution | Mg screws can maintain the mechanical stability of the mandible in advancement and setback sagittal split ramus osteotomy. | Lee J.Y., 2014 [91] |
| Posterior mandibular body | Mg–Ca–Zn alloy | Screw | FEM | Stress Distribution; Deformation caused by masticatory loading | Mg–Ca–Zn alloy screws can maintain the stability of the mandible position after sagittal split ramus osteotomy successfully. | Lee J.H., 2017 [92] | |
| The lower edge of the mandible just posterior to the molars | Mg; AZ31 | Screw | FEM; Animal experiment (rabbits) | In-vitro pull-out test; Micro-CT; Histological analysis |
Mg and AZ31 screws promote craniofacial bone remodeling and exhibit a similar holding strength to stainless steel screws. | Henderson S.E., 2014 [18] | |
| The lower edge of mandibular angle | WE43 | Screw; Plate |
Animal experiment (miniature pigs) | Micro-CT; Histological analysis | Three kinds (fluoridated, hydrogenated, and non-modified) of WE43 plates and screws can fix mandibular angle osteotomy. | Naujokat H., 2020 [84] | |
| Mandibular Angle | WE43 | Rivet | Animal experiment (miniature pigs) | Micro-CT; Histological analysis | The design of rivet-screw for the manufacture of Mg fixation is feasible. | Schaller B., 2016 [85] | |
| Condylar head | MgYREZr alloy | Cannulated headless screw | Clinical experiment | Cone-beam CT | MAGNEZIX® CS Screws can fix condylar head fracture successfully. | Leonhardt H.,2017 [88] | |
| Midface fracture | Supraorbital rim and Zygomatic arch | WE43 | Nonlocking screw; Plate |
Animal experiment (miniature pigs) | Standardized CT; Micro-CT; Histological analysis; Histomorphometry |
WE43 fixation systems show excellent fixation effect in mid-face fractures. | Schaller B., 2018 [96] |
| Zygomatic arch | WE43 | Screw; Plate |
Animal experiment (beagles) | Radiographs; Micro-CT; Mechanical Testing; Histological analysis; |
WE43 fixation systems show good biocompatibility, initial stability, and Osteogenic ability in fixation of zygomatic arch fractures. | Kim B.J., 2018 [97] | |
| Maxilla (LeFort I osteotomy) | WE43 | Screw; Plate |
Animal experiment (beagles) | Micro-CT; Histological analysis; |
WE43 fixation is clinically feasible for LeFort I osteotomy, but further treatment is needed to reduce the degradation rate. | Byun S.H., 2020 [98] | |
| Maxilla (LeFort I osteotomy) | ZK60 | Screw; Plate |
Animal experiment (beagles) | Micro-CT | LLA-coated ZK60 fixation has sufficient mechanical strength but an unsuitable rapid biodegradation rate. | Byun S.H., 2020 [99] | |
| Frontal fracture | Frontal bone | WE43 | Screw; Plate |
Animal experiment (miniature pigs) | Standardized CT; Micro-CT; Histological analysis; |
WE43 plates and screws do not interfere with bone healing and are suitable for the fixation of minipig frontal bone. | Naujokat H., 2017 [102] |
| Frontal bone | WE43 | Screw; Plate |
Animal experiment (miniature pigs) | Standardized CT; Micro-CT; Histological analysis; |
Mg plates and screws show good biocompatibility and stability in the frontal bone of minipigs. | Schaller B., 2016 [103] |
3.1.1.1. Mandibular fracture
Mandibular fractures are the most common facial fractures requiring surgical intervention, accounting for 41.6–75.2% [81], which affect the patient's occlusion, speech, and facial symmetry [82]. Mandibular fractures usually require open reduction fixation with rigid plates and screws [83]. In recent years, biodegradable Mg plates and screws used for mandibular fracture fixation and orthognathologic fixation have attracted the attention of researchers. Henderson et al. inserted Mg-based screws (pure Mg and AZ31 Mg alloy) into a rabbit's mandible and found that the Mg screws promoted new bone remodeling. Compared with stainless steel screws, the bone above the head of both Mg-based screws was overgrown, suggesting that Mg-based alloys might contribute to periosteum osteogenesis [18]. Naujokat et al. implanted three kinds of WE43 Mg alloy plates and screws in an osteotomy at the mandibular angle in miniature pigs, namely, the unmodified group, the hydrogenated group, and the fluorinated group [84]. Within eight weeks of implantation, histological analysis showed that all three types of WE43 fixations promoted bone regeneration and remodeling (Fig. 1). Surface modification by hydrogenation and fluoridation didn't significantly affect the degradation rate, biocompatibility, and fracture healing of Mg fixation systems. However, the torsional strength of Mg alloys was limited. Therefore, inserting poor self-tapping Mg screws into the bone with great torque and compression force might cause Mg screw fracture. To eliminate these disadvantages, Schaller's group developed a new type of expandable Mg cylindrical hollow rivet-screw, which were successfully implanted into the mandible of pigs. By pulling the mandrel out of the hollow rivet-screw, the Mg rivet-screw expands and is pressed tightly against the bone. In vivo experiments showed that the Mg rivet-screws maintained sufficient stability after 12–24 weeks. Although Mg rivet-screws exhibited inferior osseointegration and osteogenic capability to Ti rivet-screws, surface coating improved the performance of Mg rivet-screws [85]. Overall, the design of Mg rivet-screw for fixation is feasible.
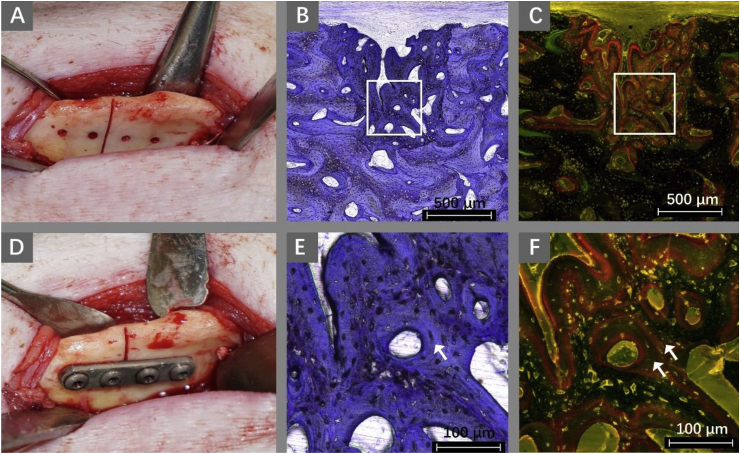
Fig. 1.
Surgical procedure and histological specimens of mandibular fractures in miniature pigs fixed with WE43 Mg alloy plates and screws. (A) Screw holes were drilled and then the osteotomy was performed. (D) The mandibular is fixed using an Mg alloy plate and four screws. After 8 weeks of fixation, the mandibular osteotomy healed completely. Toluidine blue staining image shows the newly healed bone(B) and healed bone presents lamellar structures (arrows in E). In the fluorescence microscopic image, two concentric bands of red fluorescence indicate new bone formation (C, F) [84].
Condylar fractures account for 25–35% of mandibular fractures, representing one of the most common maxillofacial fractures [86]. Open reduction and rigid internal fixation of a mandibular condylar fracture can achieve better results because it can better restore the pre-traumatic anatomical relationship and promotes the rapid healing of fractures [87]. The biodegradable Mg-based headless MAGNEZIX® CS Screws (MAGNEZIX® CS, Syntellix AG, Hanover, Germany) have been used in patients with a condylar head fracture for clinical study. A short-term observation after open reduction and internal fixation showed that Mg showed excellent biomechanical stability and no other complications [88].
Orthognathic surgery is an effective method to correct mandibular deformity. The sagittal split ramus osteotomy (SSRO) of the mandible can correct the retrognathism or prognathism while maintaining a condylar position [89]. In the finite element analysis model of SSRO, the stress distribution on the mandible of Mg and its alloy screws was similar to that of Ti screws, but quite different from that of polymer screws [90]. Lee's group compared the biomechanical stability of Mg, Ti, and polymer as orthopedic screw material for SSRO, based on three-dimensional finite element analysis [91]. In advancement SSRO, five Mg screws (three upper, two lower) could maintain the mechanical stability of the mandibular propulsive force, equivalent to the stability provided by three to four Ti screws. However, five polymer screws provided insufficient stability. Three Mg screws (two upper, one lower) are recommended for the setback SSRO operation. Later, these researchers also used finite element analysis to study the feasibility of biodegradable Mg–Ca–Zn alloy screws as fixation screws in setback SSRO of the mandible [92]. After the osteotomy of the SSRO of the mandible, after which the chewing load increased from 132 N to 1000 N, the deformation of the Mg screw was almost the same as that of the Ti screw. The deformation of the Mg screw was less than 0.21 mm under the initial functional load (132 N). Results showed that the biodegradable Mg–Ca–Zn alloy screws could bear more stress, reduce the stress distributed in the mandible, and successfully maintain the stability of the mandible position after SSRO. In conclusion, for mandibular orthognathic surgery, biodegradable Mg screws provide sufficient mechanical strength and stability as a potential bone fixation. However, this strategy needs to be confirmed in future animal experiments and clinical trials.
3.1.1.2. Midface fracture
The midface bone is not only the attachment point for many muscles, but also affects the occlusal relationship and protects key structures, such as the eye. Maxillary and orbital fractures account for 39.8% of facial fractures, respectively, representing common facial fractures [93]. The zygoma connects with many bones in the craniofacial region, which can absorb and buffer the force from the cranial base [94]. Surgical intervention is usually required for midface fracture [95]. Researchers have demonstrated the feasibility of WE43 Mg alloy plates and screws for several different midface fractures.
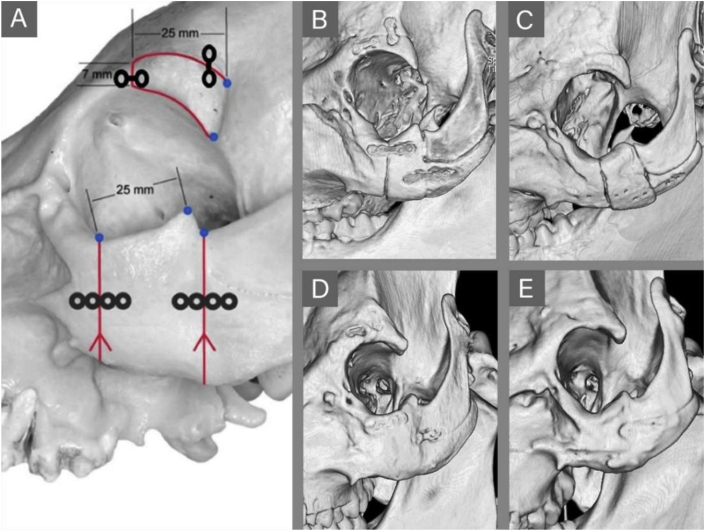
Schaller et al. simulated two different types of midface fractures via osteotomies of the supraorbital rim and zygomatic arch of minipigs, and used WE43 Mg plates and screws to fix them (Fig. 2) [96]. After 9 months, the WE43 fixation system had a good fixation effect on the midface fracture and showed excellent performance in promoting fracture healing. Compared with that of the PLGA control group, the Mg alloy fixation plates and screws demonstrated adequate mechanical properties and a reduction in size. Kim's group came to a similar conclusion, that Mg alloy screws and plates provide excellent initial stability and osteogenic ability for the fixation of zygomatic arch fractures in beagles and could bear the load in the early stage of fracture healing [97].
Fig. 2.
(A) Schematic presentation of osteotomies of the supraorbital rim and zygomatic arch of minipigs fixed by plates and screws. Computed tomography (CT) images of midface osteotomies fixed in Mg alloy (middle side) and polymer (right side) groups at 1 month (B, C) and 9 months (D, E) after surgery. At 1 month, the fracture line becomes blurred in the Mg alloy group (B). At 9 months, the fracture line and bone surface are completely healed (D, E) [96].
In 2020, Byun et al. used WE43 Mg alloy pretreated with an extrusion process to manufacture screws and L-shaped plates [98]. The mechanical strength of the Mg screws and plates was enhanced by extrusion. A LeFort I osteotomy model was built in beagles to evaluate the fixation effect of the extruded WE43 plates and screws on midface fixation. The histological results showed new bone formed around the Mg plates and screws. At 24 weeks, the disappearance of the osteotomy lines indicated complete bone healing, and the Mg fixation system was completely degraded. However, self-eliminating gas buildup, swelling, and inflammation have been observed in some experimental animals. Therefore, WE43 Mg alloy fixation is clinical feasible for midface fracture fixation; however, further surface treatment or alloying is needed to reduce the degradation rate. In the same year, the properties of PLLA-coated ZK60 plates and screws for midfacial bone fixation were verified in a beagle model. ZK60 fixation systems showed better mechanical properties than WE43 fixation systems; however, the biodegradation rate was too fast, leading to an inflammatory response. PLLA coating failed to prevent the rapid absorption of ZK60 because of microcracks [99].
3.1.1.3. Frontal fracture
Frontal fractures on the upper third of the face are relatively rare facial fractures, accounting for only 5–15% of all maxillofacial fractures [100]. However, because the frontal bone is close to important structures and organs, such as the eyes and brain, frontal bone fracture is often associated with intracranial injury and ophthalmic diseases, making it a troublesome maxillofacial fracture [101].
In 2017, Naujokat's team applied WE43 Mg alloy fixation to a cranial fracture model for the first time. They implanted standard-sized plates and screws made of WE43 Mg alloy and Ti in nine minipigs to fix cranio-osteoplasty, following similar surgical procedures to those used on humans. Although the adjacent bone in the Mg alloy group showed in a lower bone-implant contact ratio and a less homogeneous structure than that of the Ti group, the results indicated no significant differences between Mg-based and Ti-based osteosynthesis during bone healing of the osteotomies. WE43 Mg alloy plates and screws displayed good biocompatibility and did not interfere with minipig frontal bone healing [102]. Schaller et al. implanted WE43 Mg alloy fixations, with and without plasma electrolytic surface coating, into the frontal bone of adult miniature pigs [103]. The Mg plates and screws showed good stability in the frontal bone of pigs. No signs of Mg implant displacement and deformation, nor complications of inflammation and allergies, were observed. In a few cases, a slight subcutaneous emphysema occurred around the uncoated Mg implants in the early post-implantation period, which decreased gradually without affecting wound healing. Although the bone around WE43 Mg alloy fixations has lower bone density and bone-implant contact area than Ti screws, plasma electrolytic surface coating can significantly reduce the degradation rate of the Mg fixation and increase the bone-implant contact area. These studies indicated that Mg alloy plates and screws are promising candidates for the fixation of frontal bone fractures.
3.1.2. Mg for bone augmentation
3.1.2.1. Mg-based alloys for bone regeneration
GBR is one of the most common and effective methods to treat maxillofacial defects. The GBR membrane acts as a barrier between hard and soft tissues, providing enough space for bone regeneration [104]. In general, GBR membranes are divided into absorbable membranes (usually made of collagen or PLA) and non-absorbable membranes (usually Ti or polytetrafluoroethylene) [105]. Absorbable GBR membranes are widely used in the clinic because of their good biocompatibility and the advantage of not requiring secondary surgery. However, the deformation caused by their insufficient strength and rapid degradation rate lead to the collapse of the osteogenic space, which will have a negative impact on osteogenesis [106]. Currently, the main challenges for absorbable membranes are rapid degradation and low mechanical strength, especially in areas with large bone defects [107].
In recent years, some novel GBR membranes have been fabricated using Mg-based alloys. Meshes made from Mg and its alloys have sufficient mechanical properties to maintain the space of the osteogenic site and can be used in large bone defects. For example, an Mg–Al–Zn alloy mesh (diameter 8 mm, thickness 250 μm, square hole 1.5 × 1.5 mm) could greatly improve the flexural stress and modulus of polymer/demineralized bone matrix hybrid scaffold, which facilitated the reconstruction of rat calvarial critical-sized bone defect [108]. Zhao et al. Developed Mg–Zn–RE(Y, Gd, La and Ce) alloy membranes (diameter 5 mm, thickness 110 μm) with excellent biocompatibility and peomoted bone formation in critical-sized rat calvarial fefects [109]. Gao et al. found that WE43 and Mg3Gd Mg alloys membranes (diameter 6 mm, thickness 100 μm) had good biocompatibility but pure corrosion resistance [110]. A variety of surface modification methods have been used to reduce the degradation rate of Mg menbranes. For instance, Wu et al. formed a dense protective layer on the pure Mg mesh (diameter 10 mm, thickness 0.1 mm, aperture 0.4 mm) using plasma electrolytic oxidation (PEO) and hydrothermal treatment (HT), which delayed the biodegradation of Mg mesh and increase the bone volume and bone density of calvarial defects in rats [111]. Peng et al. prepared a calcium phosphate coated Mg membrane and confirmed that calcium phosphate coating reduced the degradation rate of pure Mg membrane and promoted early healing of rabbit calvarial defect better than that of Ti membrane [112]. Steigmann et al. fabricated physical vapor deposition coated pure Mg membranes and found it alleviated numbers of anti-inflammatory macrophages compared with pure Mg membranes [113]. Lin et al. integrated solution heat-treatment and fluoride coating technique to treat Mg–5Zn-0.5Zr alloys for GBR membranes (diameter 7 mm, thickness 0.4 mm, no pores), which could improve the corrosion resistance and minimize the effects of stress corrosion and crevice corrosion [114]. Guo et al. prepared composite Mg chitosan (CS–Mg) membranes (diameter 6 mm, thickness 1 mm, no pores) by dipping the Mg3Gd alloy into a chitosan solution [115]. Mg promoted cell adhesion and osteogenic activity of the CS-Mg membrane. Similar to previous studies [116,117], the chitosan coating reduced the degradation rate of Mg3Gd alloy. In addition, chitosan neutralizes the cytotoxicity of high concentration Mg alloy extracts. CS-Mg membrane showed good ability to repair the rabbit calvarial defect, which was not inferior to that induced by a commercial collagen GBR membrane (Heal-All®). The CS-Mg membrane with high porosity, adequate pore diameter, and pore interconnectivity might be more osteogenic, which has excellent potential in GBR. However, Byun et al. manufactured a highly pure Mg mesh (diameter 12 mm; thickness 0.05 mm; aperture 0.3 mm) with a HA coating, which did not show bone regeneration in a rat calvarial defect model [118]. This undesirable result may be caused by the large pore spacing, low porosity, and pore interconnectivity.
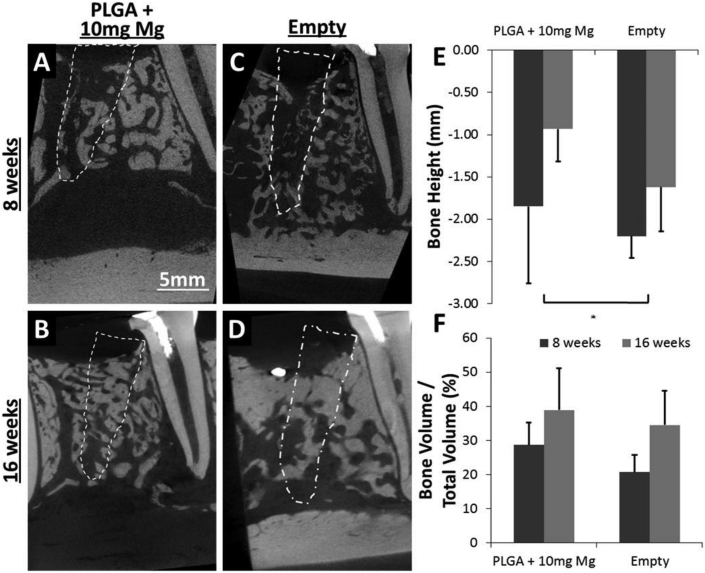
Mg-based alloys can also be used as bone filling materials. Liu Y et al. synthesized a Na micro-alloyed MgSnZnNa alloy with enhanced hardness and corrosion resistance, Mg6Sn5Zn0.3Na with 5 mm in diameter and 0.3 mm in thickness with 600 μm holes promoted the repair of rat calvarial defects through co-release of Mg and Na for osteogenesis and angiogenesis [119]. Yu et al. manufactured a mineralized collagen/Mg–Ca alloy combined scaffold by physical combination of three Mg–Ca alloy rods and porous mineralized collagen. The Mg–Ca alloy rods improved the mechanical properties of mineralized collagen, which could withstand the physiological force in the mouth and maintain the osteogenic space without collapse. Hematological examination showed that the Mg2+ released by the alloy rod degradation was safe, however, the combined scaffold did not appear to achieve the desired effect in repairing alveolar buccal bone defects in dogs [120]. A canine socket preservation experiment showed that the mineralized collagen/Mg–Ca group was superior to the mineralized collagen group according to several osteogenic indexes [121]. Brown et al. added pure Mg particles (0, 10, 20, 40 mg) to PLGA (40 mg) to overcome the shortcomings of the low mechanical strength of PLGA and the possible inflammation caused by acidic by-products [122]. The Mg/PLGA scaffolds not only facilitated the proliferation of BMSCs, but also increased the bone height in a canine premolar tooth socket preservation model (Fig. 3). Those indicate that Mg-alloys are promising candidates for bone regeneration.
Fig. 3.
Micro-CT images displaying the implantation of polylactic acid-glycolic acid (PLGA)+ 10 mg Mg scaffolds (A, C), which increased the bone height compared with empty defects (B, D). (E) Bone height was found to be better preserved by the Mg/PLGA scaffold compared with the empty defect (p < 0.05). (F) Bone volume as a percentage of total defect volume was higher for Mg/PLGA than for the empty defects; however, this increase was not statistically different [122].
3.1.2.2. Mg for surface modification of dental implants
The elementary theory of dental implants is the osseointegration between the implant and the alveolar bone [123]. In recent years, surface modification to increase osseointegration has become a research hotspot. Researchers have developed various methods to modify the roughness, microscopic morphology, wetness, chemical properties, and bioactivity of the implant surfaces [124,125]. Surface modification of implants with BMs is considered a promising method for the development of Ti implants with early osteogenic ability [126,127].
Many studies have shown that the incorporation of Mg could enhance osseointegration and the stability of Ti implants [128,129]. Compared with Ca modification, surface Mg modification was more effective in promoting osteogenic differentiation of BMSCs, possibly by enhancing cell adhesion and inhibiting the phosphorylation of β-catenin [127]. Song et al. prepared a novel Mg-SLA-Ti implant using a vacuum arc source ion implantation method by adding Mg to the sand-blasted and acid-etched (SLA) Ti implant [130]. The Mg-SLA-Ti implants were implanted into the mandible of dogs to study the bone healing response. The results of histomorphological analysis and resonance frequency analysis showed that Mg increased the bone-implant contact and implant stability. In addition, Galli et al. constructed Ti implants with a mesoporous surface loaded with Mg and concluded that Mg released from the implant surfaces promoted osseointegration at the early stage of healing (0–3 weeks), which is highly desirable for implant early loading [131].
According to the results of Sul's group and Cho's group, the most appropriate Mg ion concentration to promote osseointegration for Mg-doped Ti implants produced by plasma ion implantation and microarc oxidation was 9% [126,132,133]. In addition to the surface composition, the microscopic morphology, roughness, hydrophilicity, pore configurations, oxide thickness, crystal structure, and other surface characteristics of the implant could also affect cell behavior and the osseointegration of the implants [134]. However, the specific effects and roles of many of the above-mentioned factors remain unclear; therefore, the extensive application of Mg-rich Ti implants requires further study.
What's more, infection cause soft tissue inflammation and surrounding bone loss, which is an important cause of implant failure. When bacteria form biofilms, it is difficult for external antibiotics to clear the bacteria on the surface of the implants because they are not sensitive to antimicrobials [135]. The fight against bacterial infection and biofilm formation, and the regulation of inflammation, is a new research direction for Ti implants [136]. Mg is used to modify the surface of Ti implants, playing simultaneous osteogenic and anti-infection roles [137,138]. For example, Shen et al. constructed a metal-organic framework coating that was rich in Mg and Zn ions on an alkali-heat treated Ti surface, which could release large amounts of Mg in the early postoperative period to kill pathogenic bacteria. This coating not only demonstrated high antibacterial and anti-inflammatory properties, but also greatly improved the formation of the new bone around the implant [139]. Furthermore, Yu et al. developed Zn/Mg ion co-implantation Ti dental implants (Zn/Mg-PIII) using plasma immersion ion implantation (PIII) [140]. Zn and Mg ions released by Zn/Mg-PIII not only enhanced osteogenesis and osseointegration at the bone-implant interface, but also inhibited the adhesion and growth of typical oral bacteria, including Porphyromonas gingivalis, Fusobacterium nucleatum, and Streptococcus mutans. The broad spectrum antimicrobial activity of Mg helps dental implants to fight bacterial infections in a complex, germ-infested oral environment.
3.1.2.3. Mg-containing bone substitutes
Hydroxyapatite, bovine bone matrix, and bioglasses are the most common bone substitutes to prevent the collapse of hard and soft tissues and to maintain blood supply [141]. In recent years, bone substitutes containing Mg have achieved encouraging results [142].
Mg can be incorporated into HA to enhance the biological and physicochemical properties of HA. Mg effectively inhibited the harmful crystallization of HA by adsorbing onto the HA surface and blocking the crystallization site [143,144]. Mg-doped hydroxyapatite (MgHA) is relatively well studied Mg-based bone substitute material, whose commercial product is SintLife®. Compared with pure HA, the solubility of MgHA is improved under the physiological PH value (pH = 7.4), which increases the local concentration of Mg ions and phosphate, and promotes the formation of nucleation sites and the growth of apatite [145,146]. In vitro and in vivo experiments demonstrated that the addition of Mg enhanced the bone induction of porous HA in a dose-dependent manner [147]. After being implanted in human alveolar sockets for 4 months, histological examinations showed extensive newly formed bone, consisting of lamellar and braided tissues, and no inflammatory infiltration in all treated sites. Some MgHA particles were integrated by bone, and the residual biomaterial graft particles account for 32.2–38% [141,148]. Recently, MgHA has been applied to produce bone substitutes with excellent biocompatibility and osteogenic activity [[149], [150], [151], [152]]. Caneva's team evaluated the performance of small particles of MgHA (Ca10-xMgx[PO4]6[OH]2; SintLife®, Finceramica, Faenza, Italy) as a bone substitute in the immediate implantation of dog posterior teeth. Histomorphometric analysis showed that, although not statistically significant, MgHA implantation preserved the lateral buccal bone wall better on average [153]. Crespi et al. compared the effects on reducing alveolar bone resorption and increasing alveolar bone formation of MgHA and Calcium sulfate (CS) in clinical trials. They found that the reduction of alveolar bone in the MgHA group was smaller than that in the CS group, and MgHA had a better effect of maintaining bone contours [154]. They also found that the ability of MgHA to reduce alveolar bone resorption and promote bone formation in alveolar fossae was similar to that of allograft porcine bone graft in similar clinical trials [148]. These conclusions are consistent with the systematic review by Barallat, who conclude that MgHA is one of the most effective bone substitutes for alveolar preservation, showing similar osteogenic effect to CS and porcine-derived bone grafts [155].
MgHA can also be applied as in human maxillary sinus lifting surgery. Histomorphological results showed that the MgHA granules have good osseointegration properties, although weaker than autogenous bone. Considering that autologous bone is regarded as the gold standard for bone regeneration materials, the osteogenic effect of MgHA in maxillary sinus augmentation is acceptable [156]. A prospective two-year clinical study demonstrated the feasibility of nanostructured MgHA as a reinforced filler for vertical ridge augmentation [157]. The nanostructured MgHA was successful in increasing the alveolar ridge height, even in cases with early implant loading. In addition, compared with pure HA scaffolds, MgHA scaffolds had stronger osteogenesis and angiogenesis activities in vitro and could significantly improve the restoration effect of goat calvarial defects [158].
MgHA was also applied in combination with collagen scaffolds for alveolar socket preservation. Recently, a double-blinded prospective clinical trial revealed that the effect of the combination of MgHA and collagen scaffolds on alveolar socket preservation was consistent with that of the deproteinized bovine bone matrix [159]. The MgHA composite scaffold was almost completely replaced by newly formed bone tissue after 6 months of implantation. The MgHA composite scaffold with high porosity has good operability after being wetted with liquid and can be considered as a potential bone substitute with better effect.
The Mg-doped wollastonite (CaSiO3; CSi) ceramic improved the mechanical strength or osteogenesis of the original scaffolds, thus having great potential to repair thin-walled bone defects [160]. An increase in the Mg content promoted the expression of osteogenic genes and the production of more skeletons and osteoids. The higher the Mg doping ratio, the higher the densification and the lower the degradation rate of wollastonite bioceramic [161]. SrO and MgO doped TCP scaffolds enhanced the mechanical and in vivo biological performance of TCP scaffolds [162]. In addition, adding Mg into deproteinized porcine cancellous bone substitute could enhance its osteoconductivity and repair rabbit calvarial defects more effectively [163].
Mg-based bone cement named OsteoCrete® (Bone Solutions Inc., Colleyville, TX, USA) with adhesive qualities to bone and bone regeneration ability have been used as a bone gap filler for human long bones and the pelvis, which is receiving increased research attention [[164], [165], [166]]. Sehlke et al. used OsteoCrete® for bone grafting of immediate implant in mongrel dog posterior teeth. Results proved that the Mg-based bone cement successfully filled the bone defect and restored the complex outline of bone defects. However, due to lack of pores, OsteoCrete® is difficult to be completely replace by new bone when applied to large bone defects; however, it can be applied to small bone defects around the implant [167].
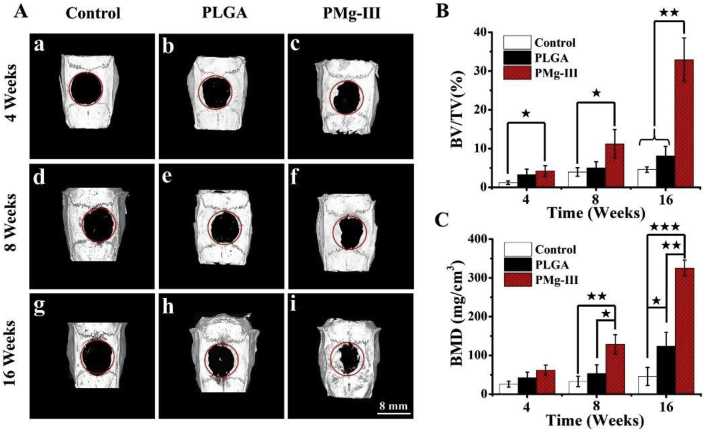
Zhang's group manufactured a nanocomposite hydrogel based on hyaluronic acid and self-assembled bisphosphonate-Mg (BP–Mg) nanoparticles [168]. This novel hydrogel, which controls the release Mg ions for a long time, effectively improved the progress of calvarial defect healing in rats. In addition, Yuan et al. synthesized novel injectable Mg-rich PLGA microspheres by compounding MgO and MgCO3 into PLGA scaffolds, which could effectively promote the restoration of rat critical-sized calvarial defects, as well as the formation of vessel-like structures through the load and release behavior of Mg ions (Fig. 4) [169]. Liu et al. prepared poly (l-lactic acid)/gelatin degradable membranes containing MgO nanoparticles (nMgO) using electrospinning [170]. The nMgO improved the mechanical properties of the membrane significantly, adjusted the degradation rate of the membrane to suit the rate of periodontal tissue regeneration, and effectively repaired the periodontal bone defects in rats in a dose-dependent manner. On the whole, the incorporation of Mg and its compounds into polymers is effective to achieve better performance.
Fig. 4.
(A) Micro-CT images of the reconstruction of a calvarial defect in rats at 4, 8, and 16 weeks after operation. (B, C) The osteogenesis effect of the Mg-rich PLGA microsphere microsphere group were significantly higher in comparison with the control group and PLGA group. ★(p < 0.05), ★★(p < 0.01), ★★★(p < 0.001) [169].
3.1.3. Mg for teeth
Dental caries, trauma, acidosis, abrasion, and other common oral diseases can lead to tooth tissue damage. However, dental hard tissue is non-renewable or hard to regenerate; therefore, the development of tooth tissue engineering is important [171]. Regenerative endodontics can construct a functional dentin-pulp complex and promote the regeneration and repair of new dental tissue [172,173]. The concentration of Mg in cells is relatively high, which has a catalytic effect and is an important cofactor in many metabolic processes in vivo [174]. Mg is involved in the mineralization of teeth and directly affects the formation of apatite crystals, relating to the quality of hard tissue and the anatomical structure of teeth [175,176]. Lack of Mg ions will interfere with the normal mineralization of the dental tissues, resulting in insufficient mineralization of dentin and enamel, and even a reduction in tooth length [177,178].
Mg can be used as a growth factor in dental tissue engineering to stimulate various cells’ odontogenic differentiation and the formation of mineralized tissue. Mg ions enter human dental pulp cells (hDPSCs) via ion channel TRPM7, and are involved in cell proliferation, migration, and osteogenic differentiation, and participate in the process of pulp repair [179]. The addition of 2.5% Mg carbonate apatite to a glass ionomer cement promoted the proliferation and mineralization of hDPSCs, which showed a potential for dentin regeneration and repair [180]. Kong et al. added different concentrations of MgCl2 to the culture medium and found that the transcriptome of hDPSCs suggested significant odontogenic differentiation through RNA sequencing. An extracellular microenvironment with high Mg concentration activates extracellular signal-regulated kinase (ERK)/BMP2/Smads signal transduction by increasing the intracellular Mg concentration, thus promoting odontogenic differentiation in hDPSCs [181]. Using a similar method, Zheng et al. found that Mg ions entered the cells through Magnesium transporter 1 (MagT1) and affected the odontogenic differentiation of BMSCs through the ERK/mitogen-activated protein kinase (MAPK) signaling pathway [182].
Theocharidou et al. produced Mg-doped and Zn-doped bioceramic scaffolds with high porosity and porous interconnectivity. These scaffolds provide a microenvironment rich in Mg and Zn ions, which is conducive to the odontogenic differentiation of hDPSCs and the formation of mineralized tissue with a Ca/P ratio close to that of biological apatite [183]. Qu et al. combined organic nanofibrous gelatin with inorganic Mg phosphate to create a bionic composite scaffold for dentin regeneration. Organic nanofibrous gelatin with high porosity is close to the matrix composition and structure of natural dentin. Mg phosphate increased the mechanical strength of the nanofiber gelatin scaffolds significantly. The controlled release of Mg ions from the scaffolds significantly enhanced the proliferation and odontogenic differentiation of hDPSCs in vitro and generated more dentin like mineralized structures in vivo [184].
3.2. Mg for the oral mucosa
About 27% of Mg in adults is stored in soft tissues [185]. There is a close relationship between Mg and soft tissue. The adhesion and growth of fibroblasts are the first steps of soft tissue regeneration. Studies have shown that the concentration of Mg ions affects the migration and adhesion of human fibroblasts [186,187]. Amberg et al. found that Mg ions promoted the growth of oral soft tissue and establish a soft tissue barrier in a palatal defects model in beagles [188].
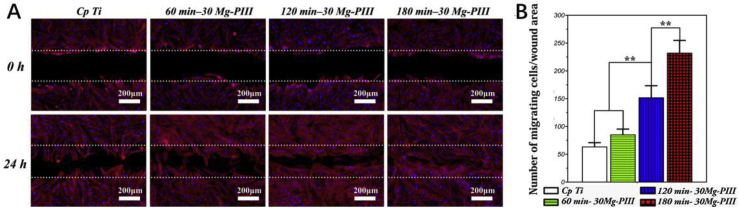
Histologically, the soft tissue barrier around the implant is weaker than the natural gingiva, which is also the reason why bacteria are more likely to erode the tissue around the implants [189,190]. Therefore, it is important to establish a good sealing soft tissue barrier in the neck of dental implant and strengthen the combination between soft tissue and implant. Mg ions can be applied to modify Ti surface to improve their combination with soft tissue, because of their excellent biocompatibility and antibacterial effect. In 2012, Okawachi et al. employed hydrothermal treatment of Ti with an Mg solution, which improved the integration of gingival epithelial cells and fibroblasts with Ti. This study provided a new strategy to increase the quality of the soft tissue seal around the implant [191]. Zhu's group immobilized Mg ions onto the Ti substrate using an Mg plasma immersion ion implantation technique [192]. The Mg-modified Ti surface could regulate the expression of integrins, and greatly enhanced cell adhesion, proliferation, migration, and extracellular matrix components synthesis by activating the PI3K/Akt signaling pathway (Fig. 5). Furthermore, Mg increased the expression of wound healing gene TGFB1 (encoding transforming growth factor beta 1 (TGFβ1)) and remodeling related gene expression, which helped to establish functional soft-tissue barriers around dental implants. These studies indicated that the modification of Ti surface by Mg is beneficial to the integration of soft tissues around the neck of Ti implants, which can be an ideal strategy to promote the development of a soft tissue barrier around the dental implant.
Fig. 5.
Wound healing assay: (A) 4′,6-diamidino-2-phenylindole (DAPI) and rhodamine-phalloidin staining images exhibited more apparent migration tendency at 0 and 24 h after creating the cell-free gap. (B) The quantification of migrating fibroblasts. ∗ Represents p < 0.05 and ∗∗ represents p < 0.01 [192].
4. Zn-based BMs for oral and maxillofacial application
In recent years, Zn and its alloys have gradually become research hotspot of biodegradable biomaterials. Zn is a cofactor in many enzymes and regulatory proteins, and is found in about 2–3 g in the adult body, with 60% in muscle tissue, 30% in bone and 5% in skin [193,194]. Zn is the most abundant trace element in cells, and plays a regulatory role in gene transcription, cell signal transduction, hormone release, and cell apoptosis [40]. Zn also functions in bone growth and development, wound healing, and tissue maintenance. Zn intake deficiency will hinder the growth and development of maxillofacial bones and teeth [195]. Zn can also effectively inhibit the formation of gingivitis, plaque, and calculus, which are closely related to periodontal health [196,197]. In the oral and maxillofacial region, Zn-based alloy fracture fixation systems can be applied for maxillofacial surgery. Zn can also promote hard tissue regeneration and wound healing.
4.1. Zn-based fracture fixation systems
Zn and its alloys have good biological safety and osteogenic properties. The degradation rate of Zn is moderate, between those of Fe and Mg, which is a major competitive advantage of Zn [198]. Zn-based alloys overcome the disadvantages of Mg-based alloy degradation resulting in gas and Fe-based alloy degradation resulting in insoluble substances. Zn-based alloys have low melting points and reactivity, and good machinability, allowing them to be processed at low temperature without shielding gas [199]. Oral intake of Zn can increase serum Zn and alkaline phosphatase (ALP) activity and promote fracture healing in patients [200]. Local injection of ZnCl2 promoted fracture healing of bones in rats, and increased the mechanical strength and stability after fracture healing [201,202].
Some scholars think that the minimum mechanical requirements for biodegradable orthopedic implants are a yield strength greater than 230 MPa, a tensile strength greater than 300 MPa, and elongation greater than 15–18% might be a reference for the mechanical properties required for craniofacial applications. Although the mechanical properties of pure Zn are poor, the mechanical properties of Zn-based alloy are similar to those of Mg-based alloy, which can satisfy the requirements for its clinical application in maxillofacial surgery [44]. Researchers have developed Zn-based alloys for maxillofacial surgery and verified their performance in vitro and in vivo. Ping Li et al. suggested that the rolled Zn–4Cu alloy was a promising biodegradable implant material for maxillofacial bone. The rolled Zn–4Cu alloy showed excellent mechanical properties, a uniform corrosion pattern, and good biocompatibility. In addition, it improved the proliferative activity of cells and inhibited mixed bacterial adhesion and biofilm formation in the oral cavity [198]. Kubásek et al. implanted Zn–2Mg alloy hemispherical implants into the calvarial defects of rats without any adverse effects, which showed that the Zn–2Mg alloy had good biocompatibility [199]. The compressive and tensile strength of the Zn–2Mg alloy were similar to those of Mg–4Y-3RE alloy, which can meet the mechanical requirements for maxillofacial applications. The corrosion rate of the Zn–2Mg alloy in vivo was 0.1 mm/year, one-ninth of that of the Mg–4Y-3RE alloy. However, low ductility (≈5%) and high elastic modulus (≈90 GPa) were disadvantages of Zn–2Mg alloys.
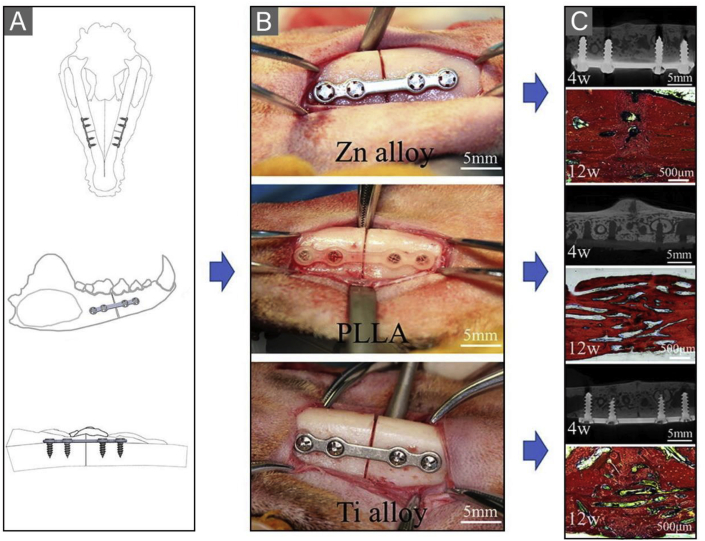
Currently, there is only one preclinical study on a Zn-based alloy fracture fixation system for maxillofacial fractures. Wang et al. evaluated the efficacy of a Zn–Mg–Fe alloy internal fixation system for maxillofacial fracture fixation using a beagle bilateral mandible fracture model [203]. Results showed that the Zn–Mg–Fe alloy could provide stable fixation for canine mandible fractures and ensured good mechanical properties of the healed mandible (Fig. 6). The effect of the Zn–Mg–Fe alloy fracture fixation system on the mandible was comparable to that of Ti alloy and superior to that of PLLA. Compared with the PLLA group and Ti group, Zn–Mg–Fe alloy fixation had an advantage in promoting new bone formation, which was caused by the osteogenesis effect of Zn ions produced during degradation.
Fig. 6.
Bilateral mandibular fractures of a beagle were fixed with plates and screws of three materials. (A) Operation simulation diagram. (B) Fixation of mandible fractures with three fracture fixation systems. From top: Zn alloy group, PLLA group, Ti alloy group. (C) Micro-CT cross-sectional images of the mandible at week 4 and picrofuchsin staining images of the mandible, from top: Zn alloy group, PLLA group, and Ti alloy group [203].
4.2. Zn for bone augmentation
Zn is an important trace element in growth and development, 85% of which is stored in bone and muscle, and plays an indispensable role in bone metabolism [204]. Most Zn binds to metalloprotein complexes, such as ALP [205]. Zn ions can inhibit osteoclast-mediated bone resorption, stimulate the proliferation of osteoblasts, and enhance osteogenesis by increasing ALP activity and collagen synthesis [206]. Zn transporters regulate the expression of osteoblast differentiation proteins such as ALP, osteopontin, runt-related transcription factor 2 (Runx2), and bone sialoprotein, which induces an osteoblast phenotype [207]. Zn deficiency can cause bone retardation by inhibiting Runx2 regulation and extracellular matrix mineralization [207]. Zn supplementation promotes bone formation in patients with thalassemia and those receiving hemodialysis [205]. Tokudome et al. found that local injection of zinc octanate and zinc stearate around alveolar bone could improve alveolar bone formation in ovariectomized Zn-deficient osteoporosis rats [208]. Zn combined with puerarin prevented mandible loss in ovariectomized rats by inhibiting osteoclast activity [209].
4.2.1. Zn for GBR membranes
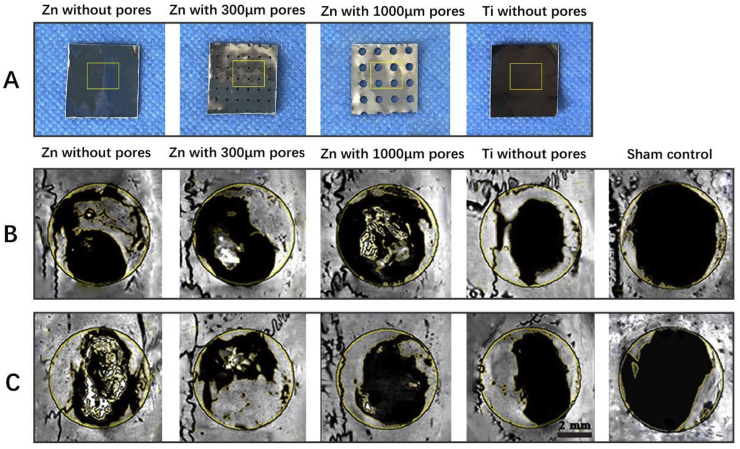
Zn can be used as GBR membranes for bone regeneration. Guo et al. prepared three kinds of novel biodegradable pure Zn membranes for GBR: non-porous pure Zn membranes, pure Zn membranes with 300 μm holes, and pure Zn membranes with 1000 μm holes (Fig. 7) [210]. Except for the pure Zn membranes with 1000 μm pores, the pure Zn membranes showed sufficient mechanical properties and a suitable degradation rate. In a rat calvarial defect model, the pure Zn membranes with 300 μm holes showed the most significant osteogenic capacity, which were comparable to non-porous Ti membranes. This study was the first systematic in vitro and in vivo investigation of Zn-Based GBR membranes. Zhang et al. produced Zn-0.8Li-0.2 Mg and Zn-0.8Li-0.2Ag alloys for GBR membranes by adding alloying elements to enhance the mechanical properties and corrosion resistance of a Zn–Li alloy [211]. The addition of Mg increased the yield strength and ultimate tensile strength of the Zn–Li alloy, but decreased its elongation and corrosion resistance. The Zn-0.8Li-0.2Ag alloy had the strongest tensile properties and corrosion resistance. The hot-extruded Zn-0.5Cu-0.2Fe alloys manufactured by Zhang's team with excellent biosafety and antibacterial properties were candidate materials for biodegradable GBR membrane materials [212]. Hot-extrusion and Cu improved the mechanical properties of the Zn–Cu–Fe alloys, and Fe alloying improved the degradation rate of the Zn–Cu–Fe alloys. The osteogenic differentiation ability of these Zn-based alloys for GBR membrane requires further in vitro and in vivo experiments.
Fig. 7.
(A) Macroscopic images of three types of pure Zn membranes and pure Ti membranes. (B, C) Representative micro-CT results of rat calvarias covered with three kinds of pure Zn membranes at week 6 and week 10, with a Ti membrane as a control, and a bone defect without a membrane as sham control group [210].
In addition to the manufacture of Zn-based GBR membranes, Zn have been combined with degradable polymers to prepare composite GBR membranes with osteogenic effects. Researchers combined Zn-containing bioactive glass with PLA to produce a degradable GBR membrane for periodontal bone regeneration with flexibility and operability [213]. Compared with the incorporation of Zn-free bioactive glass, the incorporation of Zn-containing bioactive glass into GBR membranes significantly improved the osteogenic differentiation and mineralization ability of rat BMSCs in vitro. Chou's group prepared a Zn-HAp GBR membrane by heat treatment and compared its osteogenic effects with a commercially available collagen membrane and unfilled defect groups using a rat calvarial defect model. Micro-CT and histological analysis showed that the bone regeneration in the Zn-HAp group was markedly higher than that in the collagen membrane group and the control unfilled defect group [214]. Toledano et al. produced Zn-containing methylmethacrylate membranes for GBR [215]. Compared with the incorporation of Ca, TiO2, and BMP, the incorporation of Zn into the methylmethacrylate membrane more effectively promoted the repair of rabbit calvarial defects and induced more new bone formation. The same research group also prepared Zn-containing composite polymer GBR membranes that promoted angiogenesis and regulated the macrophage polarization toward a healing phenotype [216]. In vivo results proved that Zn-containing composite polymer GBR membranes had a stronger osteogenic ability than that of the blank control group and the doxycycline-containing composite polymer GBR membranes. Thus Zn can be used as an effective osteogenic biological activity regulator in manufacturing GBR membranes.
4.2.2. Zn for surface modification of dental implants
Zn can be used for surface modification of Ti dental implants. Zn could help improve osseointegration and antibacterial properties of Ti dental implants to reduce the risk of osseointegration delay and infection [217,218]. For instance, Jin et al. incorporated Zn into etched Ti using plasma immersion ion implantation [219]. The addition of Zn promoted the adhesion, osteogenic differentiation, and extracellular matrix mineralization of rat mesenchymal stem cells, and enhance the broad-spectrum antibacterial activity. He et al. prepared Ti implants with porous Zn-containing coatings by PEO [220]. Compared with sandblasted Ti implants and Ti implants with Ca/P coatings, the Ti implants with Zn coatings promoted bone integration and bone reconstruction, and improved the bone-implant bonding strength in rabbit mandibles. Other researchers developed a TiO2/ZnO coating for Ti implants. The TiO2/ZnO coating not only promoted osteogenic differentiation of BMSCs and osteoblasts by releasing appropriate concentrations of Zn ions [[221], [222], [223]], but also induced balanced activation of macrophages, regulated bone immunity, and accelerated bone healing [224]. In vitro experiments showed that the TiO2/ZnO coating inhibited bacteria in a concentration-dependent manner [223]. Studies have shown that an ZnO coating for Ti implants could inhibit facultative anaerobes and Streptococcus sp. in the oral cavity by releasing Zn ions or reactive oxygen species [225,226].
Zn can also be combined with other bioactive materials to modify the surface of Ti dental implants. Feng et al. prepared an active Zn/Ca/P coating on Ti implants using PEO. They proved that this Zn/Ca/P coating had a stronger bone healing effect and bone bonding strength than Ti implants with a Ca/P coating only and sandblasted Ti implants after implantation into the mandible of rabbits [227]. In addition, in vitro and in vivo experiments in rats proved that Zn/Ag co-implanted Ti had excellent osteogenic and antibacterial abilities, which were related to the synergistic effect of Zn and Ag [228]. The synergistic effect is induced by the release of Zn ions into the microenvironment, which a long-range role, while Ag on the sample surface has a short-range role. In addition, the micro-galvanic couples of Zn and Ag were also attributed to the synergistic function of the Ti surface [229]. Roguska et al. also confirmed the anti-infection role of Ag/Zn coatings for dental implants [230].
4.2.3. Zn-containing bone substitutes
Zn-doped bone substitutes also play an important role in the oral and maxillofacial regions. Sil-Oss® (Azurebio, Madrid, MA, Spain) is the only commercially available Zn-doped bone substitute at present, which is a synthetic and inorganic bone graft material, mainly composed of Zn-substituted monetite [231]. It can release Zn ions and other inorganic ions to promote the differentiation of osteoblasts and inhibit osteoclasts, which can be used as a bone substitute for oral and maxillofacial regions. Sil-Oss® promotes bone regeneration, showing the advantages of faster absorption and replacement of vascularized new bone [232]. Mario et al. found that Sil-Oss® shows similar bone regeneration ability to a Bio-Oss® xenograft and a higher ability of new mineralized tissue formation and absorption rate using a beagle model of bone defects around implants. The results showed that Sil-Oss® was suitable for GBR bone-grafting and periodontal bone repair in fenestrated areas, which proved its potential for periodontal bone regeneration [233]. A randomized, controlled clinical trial conducted by Fraile et al. demonstrated the safety and effectiveness of Sil-Oss® in alveolar bone preservation after tooth extraction. The ability of Sil-Oss® to maintain alveolar bone contours is similar to that of Bio-Oss®, with the advantage of being absorbed more quickly and inducing new bone formation [232]. Moreover, through a 9-month randomized controlled clinical trial, Chandra et al. found that Sil-Oss® is effective to treat periodontal three wall interproximal defects, showing a better bone filling effect, bone density, and mineralization rate than HA [234]. Furthermore, Wang et al. synthesized Zn-passivated carbon dots (Zn-CDs). In vitro results showed that the Zn-CDs promoted the proliferation, osteogenic differentiation, and mineralization of rat BMSCs at a low concentration. The rat calvarial defect model results showed that Zn-CD-loaded gelatin/HA nanofibrous scaffolds promoted significantly more (2-fold) bone regeneration than that of pure HA nanofiber scaffolds [235].
Furthermore, Zn with antibacterial biological activity and can be used to prepare functional bone substitutes [236]. Zn incorporated HA (ZnHA) not only increased the growth and osteogenic differentiation indicators of human amniotic mesenchymal cells, but also showed broad spectrum antibiotic activity [[237], [238], [239]]. The addition of Zn improved the osteogenic activity and reduced the inflammatory response of HA by affecting cytokines production [240]. Bhardwaj et al. conducted a 1-year clinical trial on 45 sites of intraoral bone defects to compare the osteogenic properties of ZnHA and HA in repairing periodontal bone defects. According to clinical examination and radiographic examination, ZnHA had a significant effect on the treatment of periodontal intrabony defects compared with HA alone [236]. Suruagy et al. implanted nanostructured porous HA scaffold containing 2 wt% Zn (nZnHA) and nanostructured porous HA (nHA) scaffolds into the calvarial defects of rabbits. The results showed that nZnHA promoted the formation of new bone and improved bone repair to a greater extent than nHA, but with no significant difference. The results of this experiment were not as clear cut as previous experiments, possibly because the addition of Zn changed the microstructure of the scaffolds [241].
4.3. Zn for teeth
Zn exists naturally in saliva, teeth, and the oral mucosa [193]. Zn has good oral compatibility, can stay in the mouth for several to 10 h, and can exert an antibacterial role, after using oral health products containing Zn [242]. Zn can inhibit the growth of dental plaque and dental calculus by changing the growth of calcium phosphate crystals [243]. Zn plays an important role in tooth development and enamel maturation and mineralization, and affects the dynamic demineralization and remineralization balance of teeth. Toothpaste containing low doses of Zn can reduce demineralization of tooth enamel [244]. Zn deficiency decreased Zn levels in the enamel and dentin of progeny rats and increased the incidence of dental caries [245,246]. Some researchers believe that the distribution and content of Zn in enamel might affect the occurrence of caries [247].
Although high concentrations of Zn (10 and 20 ppm) are toxic to hDPSCs, low dose Zn (0–5 ppm) has good biosafety and can induce the proliferation and differentiation of hDPSCs. As a growth factor, Zn ions promote the osteogenic differentiation of hDPSCs [248,249]. Lin et al. found that Zn chloride promotes odontogenic differentiation of hDPSCs by up regulating metallothionein gene expression [250]. Huang et al. manufactured Zn-containing bioactive glasses, which promoted the odontogenic differentiation and mineralization of hDPSCs via in vitro experiments. The possible mechanism is that Zn-containing bioactive glasses upregulate the expression of vascular endothelial growth factor (VEGF) in the odontogenic and osteogenic differentiation pathways [251].
5. Fe-based BMs for oral and maxillofacial application
Fe and Fe-based alloys were one of the earliest BMs to be used in medical devices. Fe is a necessary trace element for the human body. Compared with Mg-based and Zn-based alloys, Fe-based alloys have excellent mechanical strength similar to stainless steel, which is the greatest advantage of Fe-based alloys [11]. However, Fe-based alloys also have obvious disadvantages, such as a slow degradation rate and insoluble degradation products, which greatly limit their application and clinical transformation. Also, ferromagnetism interferes with the performance of some radiological examinations, such as magnetic resonance imaging. Another controversial issue is the biological safety of Fe in osteogenesis [47].
Fe overload can lead to systemic bone loss, which causes a range of bone diseases [[252], [253], [254]]. Fe deficiency restricted mineralization and damaged bone morphology, strength, and density [255,256]. Both Fe overload and Fe deficiency are detrimental to bone homeostasis [257]. Some researchers believe that Fe influences osteogenesis in a dose-dependent manner. High concentrations of Fe decrease differentiation and osteogenic activity of osteoblasts and increase osteoclast activity [258]. Low doses of Fe do not affect intracellular Fe content and osteoblast function [259]. Zhao et al. considered that the intracellular Fe slightly lower than the normal concentration promoted the osteoblast activity, while the intracellular Fe seriously lower than the normal concentration inhibited the osteoblast activity [260].
Compared with Mg-based alloys and Zn-based alloys, Fe-based alloys are used less frequently in soft and hard oral and maxillofacial tissues because of their degradation properties and biological activities. In recent years, researchers have improved the degradation rate of Fe-based materials by alloying [261,262], surface coating [52,263], changing the material structure [264], or forming composite materials [52,265,266]. The addition of manganese (Mn) is an alloying strategy to speed up the corrosion of Fe-based alloys. For example, Hong et al. developed Fe–Mn and Fe–Mn–1Ca alloys for 3D printing in maxillofacial bone regeneration, which showed good biological activity, cellular compatibility, and corrosion rates [267].
However, there have been no preclinical and clinical trials on the application of Fe-based alloy fracture fixation in maxillofacial surgery. There are several studies on the application of Fe-containing scaffolds in maxillofacial bone regeneration. For example, Manchón et al. manufactured Fe-containing β-TCP (tricalcium phosphate) ceramic bone tissue engineering scaffolds with cytocompatibility and bone conduction properties [268]. The presence of Fe accelerated the degradation rate of β-TCP, which releases more Ca ions to the surrounding, promotes bone formation, and provides growth space for new bone tissue. Compared with the pure β-TCP scaffold, this Fe-containing β-TCP scaffold showed stronger bone regeneration and better restoration of rabbit calvarial defects.
Iron oxide nanoparticles (IONPs), mainly comprising magnetite (Fe3O4) and maghemite (γ-Fe2O3), are useful for oral and maxillofacial bone regeneration. They can be used to manufacture magnetic response scaffolds to promote bone repair and regeneration [269]. IONPs promote osteogenic differentiation of hBMSCs through the MAPK pathway [270]. In addition, magnetic IONPs promoted osteogenic differentiation of MSCs by upregulating the expression of long noncoding RNA INZEB2 [271]. Under a magnetic field, IONPs promote osteoblast differentiation via activation of integrin alpha-3, inhibit osteoclast activity, and exert an anti-inflammatory effect. Xia et al. used superparamagnetic IONPs to prepare magnetic calcium phosphate cement scaffolds. IONPs not only improved the mechanical strength and biocompatibility of calcium phosphate cement scaffolds, but also induced osteogenic differentiation and mineralization of hDPSC in vitro via the WNT pathway [272]. Magnetic IONP-doped scaffolds with an external static magnetic field have better bone induction ability. Scaffolds containing IONPs promoted four times more bone regeneration in mandibular defects in rats than calcium phosphate scaffolds [273]. In addition, the authors manufactured injectable calcium phosphate scaffolds containing IONPs and demonstrated their osteogenic effect in vitro [274]. Furthermore, Brett et al. combined IONPs with an HA-PLGA scaffold to manufacture a magnetic response scaffold [275]. Under the effect of an external magnetic field, the prefabricated magnetic nanoparticle scaffolds promoted magnetic transfection and increased the expression of the target gene, BCL2 (encoding B-cell lymphoma 2), which stimulated the survival of cells. The combination of magnetic IONPs-doped scaffolds and hASCs promoted bone regeneration of mouse critical calvarial defect.
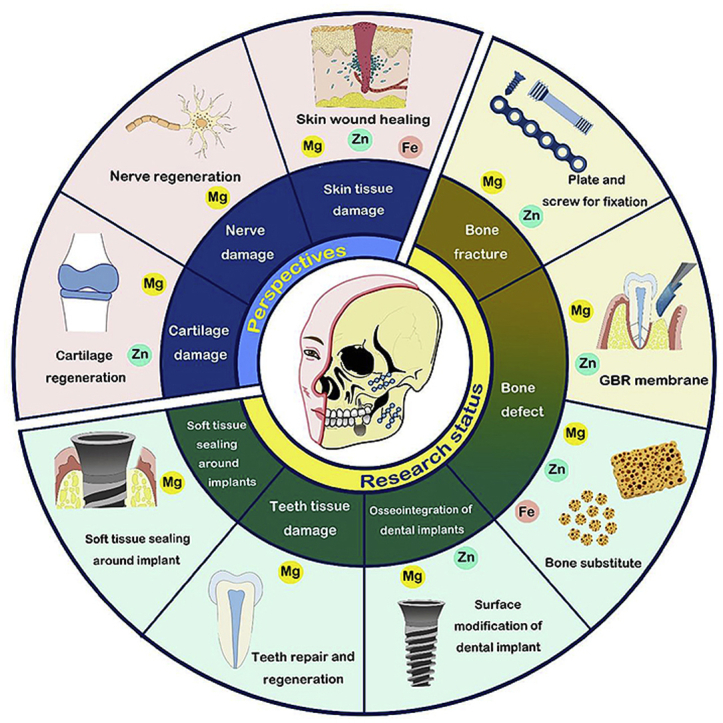
6. Perspectives
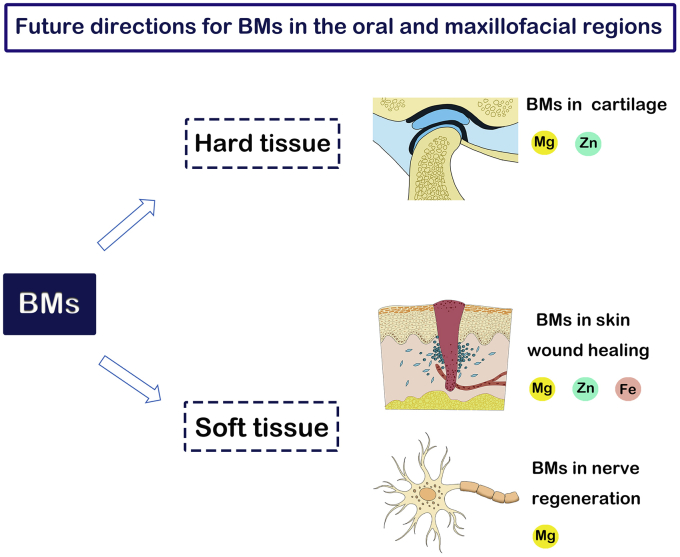
Currently, the application of BMs in the oral and maxillofacial regions is still in its infancy. Most preclinical studies of BM fixation systems for maxillofacial surgery have yielded encouraging results. Considering the advantages of BMs mentioned above, they have great potential in future application. Although BMs and BM ions have not been applied to cartilage regeneration, nerve regeneration, and skin wound healing in oral and maxillofacial regions, we believe that these research fields have great research value and potential. Future application areas for BMs in the oral and maxillofacial regions are summarized in Fig. 8. In addition, structural optimization design and new fabricating technology are needed to improve BMs instruments for oral and maxillofacial application.
Fig. 8.
Future application areas for BMs in the oral and maxillofacial regions.
6.1. Cartilage regeneration
More than 25% of people have symptoms of temporomandibular joint (TMJ) disorders (TMDs) [276]. The cartilage of the joint consists of the TMJ disc and TMJ condylar cartilage, both of which are fibrocartilage with a high content of type I collagen [277]. The lack of blood supply and the difficulty in self-repair mean that when a TMD progresses to the later stage, invasive treatments, such as arthrodesis and joint replacement, are needed [276].
Previous studies have demonstrated that Mg deficiency can lead to the production of inflammatory factors [278], cartilage damage [279], osteoarthritis [280,281], chondrocalcification [282], and other diseases. Mg reduced the production of inflammatory mediators, inhibited abnormal mineralization of cartilage, exerted an analgesic effect on joint diseases, and had a protective effect on cartilage [185,283,284]. Mg enhanced the adhesion of synovial mesenchymal stem cells by affecting integrin α3 and β1, and promotes the synthesis of the cartilage matrix in the early stage [285]. A non-physiological high extracellular Mg concentration directly affects the metabolism of chondrocytes and promotes their proliferation and redifferentiation [286]. In addition, Mg ions enhanced chondrogenic differentiation of hBMSCs by inhibiting the adverse effects of activated macrophage induced inflammation [278]. A highly pure Mg screw stimulated fibrocartilaginous entheses regeneration by upregulating the expression levels of BMP-2 and VEGF [[287], [288], [289], [290]]. Furthermore, Mg and Mg(OH)2 nanoparticles can be combined with polymers to make a biomimetic scaffold for cartilage regeneration and treatment of osteochondral lesions [291,292].
Zn has a well-known insulin-mimetic property, which is conducive to cartilage regeneration [293]. Local application of ZnCl2 enhanced cartilage formation and improved endochondral ossification [201,202]. In vitro experiments showed that a low concentration of Zn ions could increase the proliferation rate of growth plate chondrocytes by 40–50% and increased the synthesis of highly charged proteoglycan molecules, thus reducing mineralization [294]. Zn deficiency significantly inhibited chondrocyte proliferation, promoted cell differentiation, and induced apoptosis of growth plate cells, resulting in serious injury in the growth plate of young chickens [295]. The combination of Zn and other drugs reduced cartilage damage and prevented the development of arthritis [296,297]. Zn ions inhibited the progression of arthritis through in vitro cell culture and an in vivo rat osteoarthritis model by activating the p-Akt/nuclear factor erythroid 2-related factor (Nrf2) pathway to resist oxidative stress and reduce pro-inflammatory cytokines [298]. Moreover, ZnO can be used to synthesize ZnO composite fiber scaffolds to promote cartilage regeneration [299].
Although BMs have not been reported to be directly used in maxillofacial regions, published preclinical and clinical trials of Mg in cartilage applied to other parts of the body have achieved positive results [300,301]. We believe that the application of Mg-based BMs and Zn-based BMs has potential for the repair of temporomandibular joint cartilage.
6.2. Wound healing
A new research direction is the application of BMs in wound healing. Compared with the oral mucosa, skin wound healing takes longer and produces more scar tissue [302]. The treatment of wounds has always been a thorny issue because of the enormous impact on the patient's appearance and psychological health, especially of the wound is in the maxillofacial region. There is an increasing evidence that BM elements are essential for wound healing, particularly Zn ions [303,304].
Zn is an important coenzyme in tissue repair and is a component of many proteins, playing an important role in a series of wound healing processes [305], such as coagulation [306], cellular immune regulation [307], epithelial regeneration, angiogenesis, and extracellular matrix deposition [307,308]. Disorders of Zn metabolism and Zn deficiency may cause a variety of skin diseases, such as poor wound healing, skin rash, acral and pluriorificial dermatitis, and alopecia [309]. Since the 1980s, Zn-doped products for wound healing, such as Zn paste bandages, Zn-impregnated bandages, topical Zn ointment, have appeared to promote the wound healing and fight infection [310,311]. Supplementation with a Zn preparation is not only beneficial to the healing of pressure injuries in the elderly and foot ulcers in patients with diabetes [[312], [313], [314]], but also promotes wound healing of patients with burns [315,316]. Khan et al. found that local injection of ZnO-modified gold nanoparticles significantly promoted wound healing in mice in a full-thickness skin trauma model [317]. ZnO was an important application form of Zn that promotes wound healing, and exhibits broad-spectrum antibacterial properties [318]. ZnO nanoparticles (nZnO) have a high surface area and excellent antibacterial activity [319]. ZnO and nZnO can be used to prepare a variety of functional dressings.
Mg ions are indispensable cofactors involved in protein and collagen synthesis during wound healing, and the control and regulation of the functional properties of the integrin family, thereby affecting cell differentiation, wound healing, and hemostasis [320]. Li W et al. inserted Mg–2Zn pins into the leg muscles of rats and found that the pins had good soft tissue biocompatibility and maintained mechanical strength. Although the degradation rate remains to be improved, the authors suggested that Mg–2Zn alloy pins may be an effective candidate for oral soft tissue oral stapler [321]. Mg–Zn–Zr (ZK60) alloys can release appropriate concentration of Mg ions (13.2 mM), which significantly promoted the viability and migration of fibroblast and wound healing [322]. Coger et al. proposed that wound healing might be enhanced by time-specific supplementation of Mg from the late inflammation until the mid-proliferation phase [323]. Sasaki's group manufactured new Mg-smectite that promoted collagen formation and angiogenesis, and accelerates the wound healing process, by releasing bioactive ions such as Mg ions and Si ions [324]. Furthermore, Mg has alkaline degradation products and an inhibitory effect on gram-negative and gram-positive bacteria, which can prevent wound infection [[325], [326], [327], [328]]. A randomized study has proven that Mg ions accelerate wound shrinking, promote wound healing, and control infection of abdominal and perineal incisional wounds [329]. Hu et al. designed light-responsive multifunctional nanoparticles loaded with Mg ions, which could significantly accelerate wound healing in a rat wound infection model under laser irradiation [330].
Fe is an essential element in hemostasis, inflammation, re-epithelialization, extracellular proteins deposition, and extracellular matrix remodeling [[331], [332], [333]]. Fe also affects wound healing and tissue repair by influencing macrophage function [334,335]. Coger et al. suggested that supplementing Fe during the late stage of inflammation to the middle stage of proliferation might promote wound healing [336]. Fe with antibacterial properties and can be used for functional wound dressing [337]. Moniri et al. developed an anti-bacterial nanocellulose dressing containing Fe3O4 nanoparticles that could continuously release Fe3+ and upregulate the critical genes for wound healing [338]. Tian's group produced a hydrogel containing Fe3+ complexes with antibacterial activity and self-healing ability, which improved wound healing in the back of mice. Although Fe ions have been used to promote wound healing, studies suggested that Fe ions might be involved in the process of tissue damage, which was harmful to the healing of ulcers and wounds [339,340]. Considering that skin wound healing is a complex biological process involving multiple stages, the application of Fe to promote soft tissue healing requires further research.
6.3. Nerve regeneration
The application of BMs, represented by Mg, in repairing nerve injury is also a promising direction. Mg ion is involved in neurotransmission in vivo and is an effective neuroprotective agent [341], e.g., by preventing hypoxic synaptic damage [342]. The details of the mechanism are that Mg blocks the calcium channel of the n-methyl-d-aspartic acid receptor and inhibits the production of glutamatergic excitation signals [343,344]. Mg deficiency is associated with many nervous system diseases, such as cerebral vasospasm, Alzheimer's disease, stroke, and migraine [345]. In vivo experiments in horses have shown that oral or intravenous Mg might reduce the signs of trigeminal neuralgia [346,347]. In addition, reduced serum Mg levels are associated with peripheral neuropathy in type 2 diabetes [348]. Fei et al. found that ZN (Mg–2Zn–Nd) and Mg–10Li alloys have no obvious neurotoxicity and have the potential for nerve repair applications [349]. Crush injury models of the sciatic nerve in mice and rats demonstrated that Mg supplementation inhibited Schwann cell apoptosis and promoted nerve regeneration and nerve structure recovery by downregulating inflammatory responses, exerting protective effects on blood vessels and nerves [350,351]. Similarly, sciatic nerve axotomy in neonatal rats demonstrated that subcutaneous injection of an Mg solution reduced motor neuron death and increased motor unit survival [352]. Furthermore, an appropriate concentration of Mg ions could promote the proliferation of cultured neural stem cells by regulating mitochondrial function [353,354]. The placement of an Mg wire in the hollow nerve growth conduits is beneficial for nerve repair, especially to the injured sciatic nerves with a short gap in adult rats. Mg wires improved axonal parameters and had anti-inflammatory effects compared with those of a single hollow nerve conduit or a Ti wire conduit [355]. In the oral and maxillofacial area, nerve injury caused by implant operation is a common complication during mandibular third molar extraction [356]. The recovery of nerve injury has always been a difficult clinical problem. It is believed that Mg can play a significant role in oral and maxillofacial nerve repair.
6.4. New fabricating technology for BMs
The shape of the maxillofacial flat bone is irregular, which has higher symmetry and aesthetic requirements. Additive manufacturing (AM) technology makes it possible to produce porous BM implants with custom geometric shapes. AM technology, especially the laser powder bed fusion (L-PBF) technology, is expected to significantly and accurately control the microstructure and degradation performance of BM scaffolds, thus improving their mechanical properties and osteogenesis, which has a strong advantage in manufacturing tissue engineering scaffolds [357]. In 2019, Wen et al. successfully manufactured a porous pure Zn scaffold through customized gas circulation system and process optimization [358]. Studies by Chou et al. [359] and Yang et al. [360] have shown that after optimization of structure and porosity, the compressive yield strength and Young's modulus of pure Fe and Fe–30Mn alloy scaffolds are similar to that of natural bone, which makes it possible for Fe-based alloy to be applied inbone tissue engineering. In 2017, Jauer et al. manufactured a WE43 Mg alloy porous scaffold that can be customized geometrically based on the CT data of the mandible [361]. In the future, the application of BM instruments fabricated by AM technology needs extensive and in-depth study.
7. Conclusions
In summary, BMs have a broad application prospect in the oral and maxillofacial regions. BMs can not only be applied for bone fracture fixation, but also promote the regeneration of hard tissue, including bone, cartilage, and teeth. Mg-based BMs degrades fast with gas formation, Fe-based BMs have a relatively low degradation rate and insoluble degradation products, which greatly limits their application and clinical transformation. Zn-based alloys have a uniform and moderate degradation rate, and do not produce gas during degradation, which are more promising than Mg-based and Fe-based alloys for fracture fixation systems and GBR membranes. However, further research is still needed to develop new structure and multi-functional surface coatings and modification techniques to improve the degradation properties and biological effects of fracture fixation systems and GBR membranes. To pave the way for oral and maxillofacial clinical translation of BMs, long-term studies in large animal trials and clinical trials are needed.
Declaration of competing interest
The authors declared that they have no conflicts of interest to this paper. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant numbers 51901003, 51931001, 51871004, and 81771039]; the National Key R&D Program of China [grant number 2018YFE0104200]; the Key Research and Development Program of Ningxia Hui Autonomous Region [grant number 2018BEG02012], and the Open Project of NMPA Key laboratory for Dental Materials [grant number PKUSS20200401].
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Yufeng Zheng, Email: yfzheng@pku.edu.cn.
Yunsong Liu, Email: liuyunsong@hsc.pku.edu.cn.
References
- 1.Shibuya N., Jupiter D.C. Bone graft substitute: allograft and xenograft. Clin. Podiatr. Med. Surg. 2015;32(1):21–34. doi: 10.1016/j.cpm.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin P., Li D.J., Auston D.A., Mir H.S., Yoon R.S., Koval K.J. Autograft, allograft, and bone graft substitutes: clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J. Orthop. Trauma. 2019;33(4):203–213. doi: 10.1097/bot.0000000000001420. [DOI] [PubMed] [Google Scholar]
- 3.Sheikh Z., Hamdan N., Ikeda Y., Grynpas M., Ganss B., Glogauer M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: a review. Biomater. Res. 2017;21:9. doi: 10.1186/s40824-017-0095-5. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakhshalian N., Nowzari H., Ahn K.M., Arjmandi B.H. Demineralized dentin matrix and bone graft: a review of literature. J. West Soc. Periodontol. Periodontal. Abstr. 2014;62(2):35–38. [PubMed] [Google Scholar]
- 5.Minetti E., Palermo A., Savadori P., Barlattani A., Jr., Franco R., Michele M., Gianfreda F., Bollero P. Autologous tooth graft: a histological comparison between dentin mixed with xenograft and dentin alone grafts in socket preservation. J. Biol. Regul. Homeost. Agents. 2019;33(6 Suppl. 2):189–197. [PubMed] [Google Scholar]
- 6.Serrano Méndez C.A., Lang N.P., Caneva M., Ramírez Lemus G., Mora Solano G., Botticelli D. Comparison of allografts and xenografts used for alveolar ridge preservation. A clinical and histomorphometric RCT in humans. Clin. Implant Dent. Relat. Res. 2017;19(4):608–615. doi: 10.1111/cid.12490. [DOI] [PubMed] [Google Scholar]
- 7.Thavornyutikarn B., Chantarapanich N., Sitthiseripratip K., Thouas G.A., Chen Q. Bone tissue engineering scaffolding: computer-aided scaffolding techniques. Prog. Biomater. 2014;3:61–102. doi: 10.1007/s40204-014-0026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagels J., Stokdijk M., Rozing P.M. Stress shielding and bone resorption in shoulder arthroplasty. J. Shoulder Elbow Surg. 2003;12(1):35–39. doi: 10.1067/mse.2003.22. [DOI] [PubMed] [Google Scholar]
- 9.Amini A.R., Wallace J.S., Nukavarapu S.P. Short-term and long-term effects of orthopedic biodegradable implants. J. Long Term Eff. Med. Implants. 2011;21(2):93–122. doi: 10.1615/jlongtermeffmedimplants.v21.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamata M., Sakamoto Y., Kishi K. Foreign-body reaction to bioabsorbable plate and screw in craniofacial surgery. J. Craniofac. Surg. 2019;30(1):e34–e36. doi: 10.1097/scs.0000000000004945. [DOI] [PubMed] [Google Scholar]
- 11.Zheng Y.F., Gu X.N., Witte Biodegradable metals. Mater. Sci. Eng. 2014;77:1–34. [Google Scholar]
- 12.Liu Y., Zheng Y., Chen X.-H., Yang J.-A., Pan H., Chen D., Wang L., Zhang J., Zhu D., Wu S.J.A.F.M. Fundamental theory of biodegradable metals-definition, criteria, and design. Adv. Funct. Mater. 2019;29(18) 1805402.1-181805402.21. [Google Scholar]
- 13.Seitz J.M., Durisin M., Goldman J., Drelich J.W. Recent advances in biodegradable metals for medical sutures: a critical review. Adv. Healthc. Mater. 2015;4(13):1915–1936. doi: 10.1002/adhm.201500189. [DOI] [PubMed] [Google Scholar]
- 14.Witte F. The history of biodegradable magnesium implants: a review. Acta Biomater. 2010;6(5):1680–1692. doi: 10.1016/j.actbio.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 15.Hang R., Wang C., Yu Z., Li Z., Xiao Y. Biodegradable metallic wires in dental and orthopedic applications: a review. Metals. 2018;8:212. doi: 10.3390/met8040212. [DOI] [Google Scholar]
- 16.Pierson D., Edick J., Tauscher A., Pokorney E., Bowen P., Gelbaugh J., Stinson J., Getty H., Lee C.H., Drelich J., Goldman J. A simplified in vivo approach for evaluating the bioabsorbable behavior of candidate stent materials. J. Biomed. Mater. Res. B Appl. Biomater. 2012;100(1):58–67. doi: 10.1002/jbm.b.31922. [DOI] [PubMed] [Google Scholar]
- 17.Kraus T., Fischerauer S.F., Hanzi A.C., Uggowitzer P.J., Loffler J.F., Weinberg A.M. Magnesium alloys for temporary implants in osteosynthesis: in vivo studies of their degradation and interaction with bone. Acta Biomater. 2012;8(3):1230–1238. doi: 10.1016/j.actbio.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Henderson S.E., Verdelis K., Maiti S., Pal S., Chung W.L., Chou D.T., Kumta P.N., Almarza A.J. Magnesium alloys as a biomaterial for degradable craniofacial screws. Acta Biomater. 2014;10(5):2323–2332. doi: 10.1016/j.actbio.2013.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staiger M.P., Pietak A.M., Huadmai J., Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006;27(9):1728–1734. doi: 10.1016/j.biomaterials.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Castellani C., Lindtner R.A., Hausbrandt P., Tschegg E., Stanzl-Tschegg S.E., Zanoni G., Beck S., Weinberg A.M. Bone-implant interface strength and osseointegration: biodegradable magnesium alloy versus standard titanium control. Acta Biomater. 2011;7(1):432–440. doi: 10.1016/j.actbio.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Liu X., Sun J., Qiu K., Yang Y., Pu Z., li l., Zheng Y. Effects of alloying elements (Ca and Sr) on microstructure, mechanical property and in vitro corrosion behavior of biodegradable Zn-1.5Mg alloy. J. Alloys Compd. 2015;664 doi: 10.1016/j.jallcom.2015.10.116. [DOI] [Google Scholar]
- 22.Jang Y., Collins B., Sankar J., Yun Y. Effect of biologically relevant ions on the corrosion products formed on alloy AZ31B: an improved understanding of magnesium corrosion. Acta Biomater. 2013;9(10):8761–8770. doi: 10.1016/j.actbio.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 23.Lee J.W., Han H.S., Han K.J., Park J., Jeon H., Ok M.R., Seok H.K., Ahn J.P., Lee K.E., Lee D.H., Yang S.J., Cho S.Y., Cha P.R., Kwon H., Nam T.H., Han J.H., Rho H.J., Lee K.S., Kim Y.C., Mantovani D. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. Proc. Natl. Acad. Sci. USA. 2016;113(3):716–721. doi: 10.1073/pnas.1518238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witte F., Kaese V., Haferkamp H., Switzer E., Meyer-Lindenberg A., Wirth C.J., Windhagen H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials. 2005;26(17):3557–3563. doi: 10.1016/j.biomaterials.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 25.Karunakaran R., Ortgies S., Tamayol A., Bobaru F., Sealy M.P. Additive manufacturing of magnesium alloys. Bioact. Mater. 2020;5(1):44–54. doi: 10.1016/j.bioactmat.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maradze D., Musson D., Zheng Y., Cornish J., Lewis M., Liu Y. High magnesium corrosion rate has an effect on osteoclast and mesenchymal stem cell role during bone remodelling. Sci. Rep. 2018;8(1):10003. doi: 10.1038/s41598-018-28476-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang E., Xu L., Yu G., Pan F., Yang K. In vivo evaluation of biodegradable magnesium alloy bone implant in the first 6 months implantation. J. Biomed. Mater. Res. 2009;90(3):882–893. doi: 10.1002/jbm.a.32132. [DOI] [PubMed] [Google Scholar]
- 28.Draxler J., Martinelli E., Weinberg A.M., Zitek A., Irrgeher J., Meischel M., Stanzl-Tschegg S.E., Mingler B., Prohaska T. The potential of isotopically enriched magnesium to study bone implant degradation in vivo. Acta Biomater. 2017;51:526–536. doi: 10.1016/j.actbio.2017.01.054. [DOI] [PubMed] [Google Scholar]
- 29.Torroni A., Xiang C., Witek L., Rodriguez E.D., Coelho P.G., Gupta N. Biocompatibility and degradation properties of WE43 Mg alloys with and without heat treatment: in vivo evaluation and comparison in a cranial bone sheep model. J. Cranio-Maxillo-Fac. Surg. 2017;45(12):2075–2083. doi: 10.1016/j.jcms.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Wang J., Xu J., Liu W., Li Y., Qin L. Biodegradable magnesium (Mg) implantation does not impose related metabolic disorders in rats with chronic renal failure. Sci. Rep. 2016;6:26341. doi: 10.1038/srep26341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian L., Sheng Y., Huang L., Chow D.H., Chau W.H., Tang N., Ngai T., Wu C., Lu J., Qin L. An innovative Mg/Ti hybrid fixation system developed for fracture fixation and healing enhancement at load-bearing skeletal site. Biomaterials. 2018;180:173–183. doi: 10.1016/j.biomaterials.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 32.Chaya A., Yoshizawa S., Verdelis K., Noorani S., Costello B.J., Sfeir C. Fracture healing using degradable magnesium fixation plates and screws. J. Oral Maxillofac. Surg. 2015;73(2):295–305. doi: 10.1016/j.joms.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Chaya A., Yoshizawa S., Verdelis K., Myers N., Costello B.J., Chou D.T., Pal S., Maiti S., Kumta P.N., Sfeir C. In vivo study of magnesium plate and screw degradation and bone fracture healing. Acta Biomater. 2015;18:262–269. doi: 10.1016/j.actbio.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Pichler K., Kraus T., Martinelli E., Sadoghi P., Musumeci G., Uggowitzer P.J., Weinberg A.M. Cellular reactions to biodegradable magnesium alloys on human growth plate chondrocytes and osteoblasts. Int. Orthop. 2014;38(4):881–889. doi: 10.1007/s00264-013-2163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kraus T., Fischerauer S., Treichler S., Martinelli E., Eichler J., Myrissa A., Zotsch S., Uggowitzer P.J., Loffler J.F., Weinberg A.M. The influence of biodegradable magnesium implants on the growth plate. Acta Biomater. 2018;66:109–117. doi: 10.1016/j.actbio.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 36.Song M.H., Yoo W.J., Cho T.J., Park Y.K., Lee W.J., Choi I.H. Vivo response of growth plate to biodegradable Mg-Ca-Zn alloys depending on the surface modification. Int. J. Mol. Sci. 2019;20(15) doi: 10.3390/ijms20153761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang C., Yuan G., Zhang J., Tang Z., Zhang X., Dai K. Effects of magnesium alloys extracts on adult human bone marrow-derived stromal cell viability and osteogenic differentiation. Biomed. Mater. 2010;5(4) doi: 10.1088/1748-6041/5/4/045005. [DOI] [PubMed] [Google Scholar]
- 38.Kamrani S., Fleck C. Biodegradable magnesium alloys as temporary orthopaedic implants: a review. Biometals. 2019;32(2):185–193. doi: 10.1007/s10534-019-00170-y. [DOI] [PubMed] [Google Scholar]
- 39.Persaud-Sharma D., McGoron A. Biodegradable magnesium alloys: a review of material development and applications. J. Biomim. Biomater. Tissue Eng. 2012;12:25–39. doi: 10.4028/www.scientific.net/JBBTE.12.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livingstone C. Zinc: physiology, deficiency, and parenteral nutrition. Nutr. Clin. Pract. 2015;30(3):371–382. doi: 10.1177/0884533615570376. [DOI] [PubMed] [Google Scholar]
- 41.Su Y., Yang H., Gao J., Qin Y.X., Zheng Y., Zhu D. Interfacial zinc phosphate is the key to controlling biocompatibility of metallic zinc implants. Adv. Sci. 2019;6(14):1900112. doi: 10.1002/advs.201900112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su Y., Wang K., Gao J., Yang Y., Qin Y.X., Zheng Y., Zhu D. Enhanced cytocompatibility and antibacterial property of zinc phosphate coating on biodegradable zinc materials. Acta Biomater. 2019;98:174–185. doi: 10.1016/j.actbio.2019.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao C., Wang L., Ren Y., Sun S., Zhang E., Yan C., Liu Q., Sun X., Shou F., Duan J., Wang H., Qin G. Indirectly extruded biodegradable Zn-0.05wt%Mg alloy with improved strength and ductility: in vitro and in vivo studies. J. Mater. Sci. Technol. 2018;34(9):1618–1627. doi: 10.1016/j.jmst.2018.01.006. [DOI] [Google Scholar]
- 44.Li H.F., Xie X.H., Zheng Y.F., Cong Y., Zhou F.Y., Qiu K.J., Wang X., Chen S.H., Huang L., Tian L., Qin L. Development of biodegradable Zn-1X binary alloys with nutrient alloying elements Mg, Ca and Sr. Sci. Rep. 2015;5 doi: 10.1038/srep10719. 10719-10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scarcello E., Lison D. Are Fe-based stenting materials biocompatible? A critical review of in vitro and in vivo studies. J. Funct. Biomater. 2019;11(1):2. doi: 10.3390/jfb11010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papanikolaou G., Pantopoulos K. Iron metabolism and toxicity. Toxicol. Appl. Pharmacol. 2005;202(2):199–211. doi: 10.1016/j.taap.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 47.Punzo F., Tortora C., Argenziano M., Casale M., Perrotta S., Rossi F. Iron chelating properties of Eltrombopag: investigating its role in thalassemia-induced osteoporosis. PLoS One. 2018;13(12) doi: 10.1371/journal.pone.0208102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peuster M., Hesse C., Schloo T., Fink C., Beerbaum P., von Schnakenburg C. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials. 2006;27(28):4955–4962. doi: 10.1016/j.biomaterials.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 49.Kraus T., Moszner F., Fischerauer S., Fiedler M., Martinelli E., Eichler J., Witte F., Willbold E., Schinhammer M., Meischel M., Uggowitzer P.J., Löffler J.F., Weinberg A. Biodegradable Fe-based alloys for use in osteosynthesis: outcome of an in vivo study after 52weeks. Acta Biomater. 2014;10(7):3346–3353. doi: 10.1016/j.actbio.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 50.Peuster M., Hesse C., Schloo T., Fink C., Beerbaum P., von Schnakenburg C. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials. 2006;27(28):4955–4962. doi: 10.1016/j.biomaterials.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 51.Drynda A., Hassel T., Bach F.W., Peuster M. In vitro and in vivo corrosion properties of new iron-manganese alloys designed for cardiovascular applications. J. Biomed. Mater. Res. B Appl. Biomater. 2015;103(3):649–660. doi: 10.1002/jbm.b.33234. [DOI] [PubMed] [Google Scholar]
- 52.Lin W.-J., Zhang D.-Y., Zhang G., Sun H.-T., Qi H.-P., Chen L.-P., Liu Z.-Q., Gao R.-L., Zheng W. Design and characterization of a novel biocorrodible iron-based drug-eluting coronary scaffold. Mater. Des. 2016;91:72–79. doi: 10.1016/j.matdes.2015.11.045. [DOI] [Google Scholar]
- 53.Grober U., Schmidt J., Kisters K. Magnesium in prevention and therapy. Nutrients. 2015;7(9):8199–8226. doi: 10.3390/nu7095388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanbay M., Goldsmith D., Uyar M.E., Turgut F., Covic A. Magnesium in chronic kidney disease: challenges and opportunities. Blood Purif. 2010;29(3):280–292. doi: 10.1159/000276665. [DOI] [PubMed] [Google Scholar]
- 55.Liu C., Ren Z., Xu Y., Pang S., Zhao X., Zhao Y. Biodegradable magnesium alloys developed as bone repair materials: a review. Scanning. 2018:2018. doi: 10.1155/2018/9216314. 9216314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rude R.K., Singer F.R., Gruber H.E. Skeletal and hormonal effects of magnesium deficiency. J. Am. Coll. Nutr. 2009;28(2):131–141. doi: 10.1080/07315724.2009.10719764. [DOI] [PubMed] [Google Scholar]
- 57.Nakano Y., Le M.H., Abduweli D., Ho S.P., Ryazanova L.V., Hu Z., Ryazanov A.G., Den Besten P.K., Zhang Y. A critical role of TRPM7 as an ion channel protein in mediating the mineralization of the craniofacial hard tissues. Front. Physiol. 2016;7:258. doi: 10.3389/fphys.2016.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J., Ma X.Y., Feng Y.F., Ma Z.S., Ma T.C., Zhang Y., Li X., Wang L., Lei W. Magnesium ions promote the biological behaviour of rat calvarial osteoblasts by activating the PI3K/akt signalling pathway. Biol. Trace Elem. Res. 2017;179(2):284–293. doi: 10.1007/s12011-017-0948-8. [DOI] [PubMed] [Google Scholar]
- 59.Viozzi C.F. Maxillofacial and mandibular fractures in sports. Clin. Sports Med. 2017;36(2):355–368. doi: 10.1016/j.csm.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Lee K.H. Interpersonal violence and facial fractures. J. Oral Maxillofac. Surg. 2009;67(9):1878–1883. doi: 10.1016/j.joms.2009.04.117. [DOI] [PubMed] [Google Scholar]
- 61.Koso R.E., Terhoeve C., Steen R.G., Zura R. Healing, nonunion, and re-operation after internal fixation of diaphyseal and distal femoral fractures: a systematic review and meta-analysis. Int. Orthop. 2018;42(11):2675–2683. doi: 10.1007/s00264-018-3864-4. [DOI] [PubMed] [Google Scholar]
- 62.Duplantier N.L., Mitchell R.J., Zambrano S., Stone A.C., Delgado D.A., Lambert B.S., Moreno M.R., Harris J.D., McCulloch P.C., Lintner D.M., Varner K.E. A biomechanical comparison of fifth metatarsal jones fracture fixation methods. Am. J. Sports Med. 2018;46(5):1220–1227. doi: 10.1177/0363546517753376. [DOI] [PubMed] [Google Scholar]
- 63.Yeoh M., Cunningham L.L., Jr. Concepts of rigid fixation in facial fractures. Atlas Oral Maxillofac. Surg. Clin. North Am. 2019;27(2):107–112. doi: 10.1016/j.cxom.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 64.Nagase D.Y., Courtemanche D.J., Peters D.A. Plate removal in traumatic facial fractures: 13-year practice review. Ann. Plast. Surg. 2005;55(6):608–611. doi: 10.1097/01.sap.0000189666.13860.c0. [DOI] [PubMed] [Google Scholar]
- 65.Al-Moraissi E.A., Ellis E., 3rd Biodegradable and titanium osteosynthesis provide similar stability for orthognathic surgery. J. Oral Maxillofac. Surg. 2015;73(9):1795–1808. doi: 10.1016/j.joms.2015.01.035. [DOI] [PubMed] [Google Scholar]
- 66.Borstlap W.A., Stoelinga P.J., Hoppenreijs T.J., van't Hof M.A. Stabilisation of sagittal split advancement osteotomies with miniplates: a prospective, multicentre study with two-year follow-up. Part I. Clinical parameters. Int. J. Oral Maxillofac. Surg. 2004;33(5):433–441. doi: 10.1016/j.ijom.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 67.Suuronen R., Kontio R., Ashammakhi N., Lindqvist C., Laine P. Bioabsorbable self-reinforced plates and screws in craniomaxillofacial surgery. Bio Med. Mater. Eng. 2004;14(4):517–524. [PubMed] [Google Scholar]
- 68.Hayden Gephart M.G., Woodard J.I., Arrigo R.T., Lorenz H.P., Schendel S.A., Edwards M.S., Guzman R. Using bioabsorbable fixation systems in the treatment of pediatric skull deformities leads to good outcomes and low morbidity. Childs Nerv. Syst. 2013;29(2):297–301. doi: 10.1007/s00381-012-1938-y. [DOI] [PubMed] [Google Scholar]
- 69.Tuovinen V., Suuronen R., Teittinen M., Nurmenniemi P. Comparison of the stability of bioabsorbable and titanium osteosynthesis materials for rigid internal fixation in orthognathic surgery. A prospective randomized controlled study in 101 patients with 192 osteotomies. Int. J. Oral Maxillofac. Surg. 2010;39(11):1059–1065. doi: 10.1016/j.ijom.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 70.Gareb B., van Bakelen N.B., Buijs G.J., Jansma J., de Visscher J., Hoppenreijs T.J.M., Bergsma J.E., van Minnen B., Stegenga B., Bos R.R.M. Comparison of the long-term clinical performance of a biodegradable and a titanium fixation system in maxillofacial surgery: a multicenter randomized controlled trial. PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0177152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang L., Xu M., Jin X., Xu J., Lu J., Zhang C., Tian T., Teng L. Complications of absorbable fixation in maxillofacial surgery: a meta-analysis. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0067449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Konneker S., Krockenberger K., Pieh C., von Falck C., Brandewiede B., Vogt P.M., Kirschner M.H., Ziegler A. Comparison of SCAphoid fracture osteosynthesis by MAGnesium-based headless Herbert screws with titanium Herbert screws: protocol for the randomized controlled SCAMAG clinical trial. BMC Muscoskel. Disord. 2019;20(1):357. doi: 10.1186/s12891-019-2723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian P., Liu X. Surface modification of biodegradable magnesium and its alloys for biomedical applications. Regen. Biomater. 2015;2(2):135–151. doi: 10.1093/rb/rbu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yazdimamaghani M., Razavi M., Vashaee D., Moharamzadeh K., Boccaccini A.R., Tayebi L. Porous magnesium-based scaffolds for tissue engineering. Mater. Sci. Eng. C, Mater. Biol. Appl. 2017;71:1253–1266. doi: 10.1016/j.msec.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 75.On S.-W., Cho S.-W., Byun S.-H., Yang B.-E. Bioabsorbable osteofixation materials for maxillofacial bone surgery: a review on polymers and magnesium-based materials. Biomedicines. 2020;8(9) doi: 10.3390/biomedicines8090300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Erdmann N., Angrisani N., Reifenrath J., Lucas A., Thorey F., Bormann D., Meyer-Lindenberg A. Biomechanical testing and degradation analysis of MgCa0.8 alloy screws: a comparative in vivo study in rabbits. Acta Biomater. 2011;7(3):1421–1428. doi: 10.1016/j.actbio.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 77.Waizy H., Weizbauer A., Maibaum M., Witte F., Windhagen H., Lucas A., Denkena B., Meyer-Lindenberg A., Thorey F. Biomechanical characterisation of a degradable magnesium-based (MgCa0.8) screw. J. Mater. Sci. Mater. Med. 2012;23(3):649–655. doi: 10.1007/s10856-011-4544-8. [DOI] [PubMed] [Google Scholar]
- 78.Kose O., Turan A., Unal M., Acar B., Guler F. Fixation of medial malleolar fractures with magnesium bioabsorbable headless compression screws: short-term clinical and radiological outcomes in eleven patients. Arch. Orthop. Trauma Surg. 2018;138(8):1069–1075. doi: 10.1007/s00402-018-2941-x. [DOI] [PubMed] [Google Scholar]
- 79.Atkinson H.D., Khan S., Lashgari Y., Ziegler A. Hallux valgus correction utilising a modified short scarf osteotomy with a magnesium biodegradable or titanium compression screws - a comparative study of clinical outcomes. BMC Muscoskel. Disord. 2019;20(1):334. doi: 10.1186/s12891-019-2717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Acar B., Kose O., Turan A., Unal M., Kati Y.A., Guler F. Comparison of bioabsorbable magnesium versus titanium screw fixation for modified distal chevron osteotomy in hallux valgus. BioMed Res. Int. 2018;2018:5242806. doi: 10.1155/2018/5242806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gomez Rosello E., Quiles Granado A.M., Artajona Garcia M., Juanpere Marti S., Laguillo Sala G., Beltran Marmol B., Pedraza Gutierrez S. Facial fractures: classification and highlights for a useful report. Insights Imag. 2020;11(1):49. doi: 10.1186/s13244-020-00847-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reddy L., Lee D., Vincent A., Shokri T., Sokoya M., Ducic Y. Secondary management of mandible fractures. Facial Plast. Surg. 2019;35(6):627–632. doi: 10.1055/s-0039-1700887. [DOI] [PubMed] [Google Scholar]
- 83.Burns B., Fields J.M., Farinas A., Pollins A., Perdikis G., Thayer W. Comparing maximal forces in resorbable poly-L-lactic acid and titanium plates for mandibular fracture fixation. Heliyon. 2020;6(4) doi: 10.1016/j.heliyon.2020.e03705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Naujokat H., Ruff C.B., Kluter T., Seitz J.M., Acil Y., Wiltfang J. Influence of surface modifications on the degradation of standard-sized magnesium plates and healing of mandibular osteotomies in miniature pigs. Int. J. Oral Maxillofac. Surg. 2020;49(2):272–283. doi: 10.1016/j.ijom.2019.03.966. [DOI] [PubMed] [Google Scholar]
- 85.Schaller B., Saulacic N., Beck S., Imwinkelried T., Goh B.T., Nakahara K., Hofstetter W., Iizuka T. In vivo degradation of a new concept of magnesium-based rivet-screws in the minipig mandibular bone. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;69:247–254. doi: 10.1016/j.msec.2016.06.085. [DOI] [PubMed] [Google Scholar]
- 86.Emam H.A., Jatana C.A., Ness G.M. Matching surgical approach to condylar fracture type. Atlas Oral Maxillofac. Surg. Clin. North Am. 2017;25(1):55–61. doi: 10.1016/j.cxom.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 87.Yao S., Zhou J., Li Z. Contrast analysis of open reduction and internal fixation and non-surgical treatment of condylar fracture: a meta-analysis. J. Craniofac. Surg. 2014;25(6):2077–2080. doi: 10.1097/scs.0000000000001010. [DOI] [PubMed] [Google Scholar]
- 88.Leonhardt H., Franke A., McLeod N.M.H., Lauer G., Nowak A. Fixation of fractures of the condylar head of the mandible with a new magnesium-alloy biodegradable cannulated headless bone screw. Br. J. Oral Maxillofac. Surg. 2017;55(6):623–625. doi: 10.1016/j.bjoms.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 89.Reyneke J.P. Basic guidelines for the surgical correction of mandibular anteroposterior deficiency and excess. Clin. Plast. Surg. 2007;34(3):501–517. doi: 10.1016/j.cps.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 90.Paeng J.Y., Hong J., Kim C.S., Kim M.J. Comparative study of skeletal stability between bicortical resorbable and titanium screw fixation after sagittal split ramus osteotomy for mandibular prognathism. J. Cranio-Maxillo-Fac. Surg. 2012;40(8):660–664. doi: 10.1016/j.jcms.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 91.Lee J.Y., Lee J.W., Pang K.M., Kim H.E., Kim S.M., Lee J.H. Biomechanical evaluation of magnesium-based resorbable metallic screw system in a bilateral sagittal split ramus osteotomy model using three-dimensional finite element analysis. J. Oral Maxillofac. Surg. 2014;72(2):402. doi: 10.1016/j.joms.2013.10.003. e1-13. [DOI] [PubMed] [Google Scholar]
- 92.Lee J.H., Han H.S., Kim Y.C., Lee J.Y., Lee B.K. Stability of biodegradable metal (Mg-Ca-Zn alloy) screws compared with absorbable polymer and titanium screws for sagittal split ramus osteotomy of the mandible using the finite element analysis model. J. Cranio-Maxillo-Fac. Surg. 2017;45(10):1639–1646. doi: 10.1016/j.jcms.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 93.Ludi E.K., Rohatgi S., Zygmont M.E., Khosa F., Hanna T.N. Do radiologists and surgeons speak the same language? A retrospective review of facial trauma. AJR Am. J. Roentgenol. 2016;207(5):1070–1076. doi: 10.2214/ajr.15.15901. [DOI] [PubMed] [Google Scholar]
- 94.Bergeron J.M., Raggio B.S. StatPearls Publishing StatPearls Publishing LLC.; Treasure Island (FL): 2020. Zygomatic Arch Fracture, StatPearls. [PubMed] [Google Scholar]
- 95.Reiter M.J., Schwope R.B., Theler J.M. Postoperative CT of the midfacial skeleton after trauma: review of normal appearances and common complications. AJR Am. J. Roentgenol. 2017;209(4):W238–w248. doi: 10.2214/ajr.17.17875. [DOI] [PubMed] [Google Scholar]
- 96.Schaller B., Matthias Burkhard J.P., Chagnon M., Beck S., Imwinkelried T., Assad M. Fracture healing and bone remodeling with human standard-sized magnesium versus polylactide-Co-glycolide plate and screw systems using a mini-swine craniomaxillofacial osteotomy fixation model. J. Oral Maxillofac. Surg. 2018;76(10):2138–2150. doi: 10.1016/j.joms.2018.03.039. [DOI] [PubMed] [Google Scholar]
- 97.Kim B.J., Piao Y., Wufuer M., Son W.C., Choi T.H. Biocompatibility and efficiency of biodegradable magnesium-based plates and screws in the facial fracture model of beagles. J. Oral Maxillofac. Surg. 2018;76(5):1055. doi: 10.1016/j.joms.2018.01.015. e1-1055.e9. [DOI] [PubMed] [Google Scholar]
- 98.Byun S.H., Lim H.K., Cheon K.H., Lee S.M., Kim H.E., Lee J.H. Biodegradable magnesium alloy (WE43) in bone-fixation plate and screw. J. Biomed. Mater. Res. B Appl. Biomater. 2020 doi: 10.1002/jbm.b.34582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Byun S.-H., Lim H.-K., Lee S.-M., Kim H.J., Kim S.-M., Lee J. Biodegradable magnesium alloy (ZK60) with a poly(l-lactic)-acid polymer coating for maxillofacial surgery. Metals. 2020;10:724. doi: 10.3390/met10060724. [DOI] [Google Scholar]
- 100.Arnold M.A., Tatum S.A., 3rd Frontal sinus fractures: evolving clinical considerations and surgical approaches. Craniomaxillofacial Trauma Reconstr. 2019;12(2):85–94. doi: 10.1055/s-0039-1678660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jeyaraj P. Frontal bone fractures and frontal sinus injuries: treatment paradigms. Ann. Maxillofac. Surg. 2019;9(2):261–282. doi: 10.4103/ams.ams_151_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Naujokat H., Seitz J.M., Acil Y., Damm T., Moller I., Gulses A., Wiltfang J. Osteosynthesis of a cranio-osteoplasty with a biodegradable magnesium plate system in miniature pigs. Acta Biomater. 2017;62:434–445. doi: 10.1016/j.actbio.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 103.Schaller B., Saulacic N., Imwinkelried T., Beck S., Liu E.W., Gralla J., Nakahara K., Hofstetter W., Iizuka T. In vivo degradation of magnesium plate/screw osteosynthesis implant systems: soft and hard tissue response in a calvarial model in miniature pigs. J. Cranio-Maxillo-Fac. Surg. 2016;44(3):309–317. doi: 10.1016/j.jcms.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 104.Turri A., Elgali I., Vazirisani F., Johansson A., Emanuelsson L., Dahlin C., Thomsen P., Omar O. Guided bone regeneration is promoted by the molecular events in the membrane compartment. Biomaterials. 2016;84:167–183. doi: 10.1016/j.biomaterials.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 105.Villar C.C., Cochran D.L. Regeneration of periodontal tissues: guided tissue regeneration. Dent Clin North Am. 2010;54(1):73–92. doi: 10.1016/j.cden.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 106.Liu J., Kerns D.G. Mechanisms of guided bone regeneration: a review. Open Dent. J. 2014;8:56–65. doi: 10.2174/1874210601408010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pihlstrom B.L., Michalowicz B.S., Johnson N.W. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. doi: 10.1016/s0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 108.Chen Y., Ye S.H., Sato H., Zhu Y., Shanov V., Tiasha T., D'Amore A., Luketich S., Wan G., Wagner W.R. Hybrid scaffolds of Mg alloy mesh reinforced polymer/extracellular matrix composite for critical-sized calvarial defect reconstruction. J. Tissue Eng. Regen. Med. 2018;12(6):1374–1388. doi: 10.1002/term.2668. [DOI] [PubMed] [Google Scholar]
- 109.Zhao M., Liu G., Li Y., Yu X., Yuan S., Nie Z., Wang J., Han J., Tan C., Guo C. Degradation behavior, transport mechanism and osteogenic activity of Mg–Zn–RE alloy membranes in critical-sized rat calvarial defects. Coatings. 2020;10:496. doi: 10.3390/coatings10050496. [DOI] [Google Scholar]
- 110.Guo Y., Liu W., Ma S., Wang J., Zou J., Liu Z., Zhao J., Zhou Y. A preliminary study for novel use of two Mg alloys (WE43 and Mg3Gd) J. Mater. Sci. Mater. Med. 2016;27(5):82. doi: 10.1007/s10856-016-5691-8. [DOI] [PubMed] [Google Scholar]
- 111.Wu S., Jang Y.S., Kim Y.K., Kim S.Y., Ko S.O., Lee M.H. Surface modification of pure magnesium mesh for guided bone regeneration: in vivo evaluation of rat calvarial defect. Materials. 2019;12(17) doi: 10.3390/ma12172684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peng W., Chen J.X., Shan X.F., Wang Y.C., He F., Wang X.J., Tan L.L., Yang K.J.R.M. 2019. Mg-based Absorbable Membrane for Guided Bone Regeneration (GBR): a Pilot Study. [Google Scholar]
- 113.Steigmann L., Jung O., Kieferle W., Stojanovic S., Proehl A., Görke O., Emmert S., Najman S., Barbeck M., Rothamel D. Biocompatibility and immune response of a newly developed volume-stable magnesium-based barrier membrane in combination with a PVD coating for guided bone regeneration (GBR) Biomedicines. 2020;8(12) doi: 10.3390/biomedicines8120636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin D.J., Fei-Yi H., Hung-Pang L., Metals Y.M.-L.J. Development of a novel degradation-controlled magnesium-based regeneration membrane for future guided bone regeneration (GBR) therapy. Metals. 2017;7(11):481–498. [Google Scholar]
- 115.Guo Y., Yu Y., Han L., Ma S., Zhao J., Chen H., Yang Z., Zhang F., Xia Y., Zhou Y. Biocompatibility and osteogenic activity of guided bone regeneration membrane based on chitosan-coated magnesium alloy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;100:226–235. doi: 10.1016/j.msec.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 116.Heise S., Virtanen S., Boccaccini A.R. Tackling Mg alloy corrosion by natural polymer coatings-A review. J. Biomed. Mater. Res. 2016;104(10):2628–2641. doi: 10.1002/jbm.a.35776. [DOI] [PubMed] [Google Scholar]
- 117.Zhao J., Chen L.-j., Yu K., Chen C., Dai Y.-l., Qiao X.-y., Yan Y., Yu Z.-m. Effects of chitosan coating on biocompatibility of Mg–6%Zn–10%Ca3(PO4)2 implant. Trans. Nonferrous Metals Soc. China. 2015;25(3):824–831. doi: 10.1016/S1003-6326(15)63669-X. [DOI] [Google Scholar]
- 118.Byun S.H., Lim H.K., Kim S.M., Lee S.M., Kim H.E., Lee J.H. The bioresorption and guided bone regeneration of absorbable hydroxyapatite-coated magnesium mesh. J. Craniofac. Surg. 2017;28(2):518–523. doi: 10.1097/scs.0000000000003383. [DOI] [PubMed] [Google Scholar]
- 119.Liu Y., Li H., Xu J., TerBush J., Li W., Setty M., Guan S., Nguyen T.D., Qin L., Zheng Y. Biodegradable metal-derived magnesium and sodium enhances bone regeneration by angiogenesis aided osteogenesis and regulated biological apatite formation. Chem. Eng. J. 2021 doi: 10.1016/j.cej.2020.127616. 410 127616. [DOI] [Google Scholar]
- 120.Guo C.-W., Yu Q., Sun B.-Z., Wang C.-Y., Yang J.-X. Evaluation of alveolar bone repair with mineralized collagen block reinforced with Mg–Ca alloy rods. J. Biomater. Tissue Eng. 2018;8:1–10. doi: 10.1166/jbt.2018.1709. [DOI] [Google Scholar]
- 121.Yu Q., Wang C., Yang J., Guo C., Zhang S. Mineralized collagen/Mg-Ca alloy combined scaffolds with improved biocompatibility for enhanced bone response following tooth extraction. Biomed. Mater. 2018;13(6) doi: 10.1088/1748-605X/aadb47. [DOI] [PubMed] [Google Scholar]
- 122.Brown A., Zaky S., Ray H., Jr., Sfeir C. Porous magnesium/PLGA composite scaffolds for enhanced bone regeneration following tooth extraction. Acta Biomater. 2015;11:543–553. doi: 10.1016/j.actbio.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 123.Ogle O.E. Implant surface material, design, and osseointegration. Dent Clin North Am. 2015;59(2):505–520. doi: 10.1016/j.cden.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 124.Jemat A., Ghazali M.J., Razali M., Otsuka Y. Surface modifications and their effects on titanium dental implants. BioMed Res. Int. 2015;2015:791725. doi: 10.1155/2015/791725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Smeets R., Stadlinger B., Schwarz F., Beck-Broichsitter B., Jung O., Precht C., Kloss F., Grobe A., Heiland M., Ebker T. Impact of dental implant surface modifications on osseointegration. BioMed Res. Int. 2016;2016:6285620. doi: 10.1155/2016/6285620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sul Y.T., Kang B.S., Johansson C., Um H.S., Park C.J., Albrektsson T. The roles of surface chemistry and topography in the strength and rate of osseointegration of titanium implants in bone. J. Biomed. Mater. Res. 2009;89(4):942–950. doi: 10.1002/jbm.a.32041. [DOI] [PubMed] [Google Scholar]
- 127.Park J.W., Hanawa T., Chung J.H. The relative effects of Ca and Mg ions on MSC osteogenesis in the surface modification of microrough Ti implants. Int. J. Nanomed. 2019;14:5697–5711. doi: 10.2147/ijn.S214363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Park J.W., An C.H., Jeong S.H., Suh J.Y. Osseointegration of commercial microstructured titanium implants incorporating magnesium: a histomorphometric study in rabbit cancellous bone. Clin. Oral Implants Res. 2012;23(3):294–300. doi: 10.1111/j.1600-0501.2010.02144.x. [DOI] [PubMed] [Google Scholar]
- 129.Sul Y.T., Jonsson J., Yoon G.S., Johansson C. Resonance frequency measurements in vivo and related surface properties of magnesium-incorporated, micropatterned and magnesium-incorporated TiUnite, Osseotite, SLA and TiOblast implants. Clin. Oral Implants Res. 2009;20(10):1146–1155. doi: 10.1111/j.1600-0501.2009.01767.x. [DOI] [PubMed] [Google Scholar]
- 130.Song W.W., Heo J.H., Lee J.H., Park Y.M., Kim Y.D. Osseointegration of magnesium-incorporated sand-blasted acid-etched implant in the dog mandible: resonance frequency measurements and histomorphometric analysis. Tissue Eng. Regen. Med. 2016;13(2):191–199. doi: 10.1007/s13770-016-9126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Galli S., Naito Y., Karlsson J., He W., Andersson M., Wennerberg A., Jimbo R. Osteoconductive potential of mesoporous titania implant surfaces loaded with magnesium: an experimental study in the rabbit. Clin. Implant Dent. Relat. Res. 2015;17(6):1048–1059. doi: 10.1111/cid.12211. [DOI] [PubMed] [Google Scholar]
- 132.Sul Y.T., Johansson C., Wennerberg A., Cho L.R., Chang B.S., Albrektsson T. Optimum surface properties of oxidized implants for reinforcement of osseointegration: surface chemistry, oxide thickness, porosity, roughness, and crystal structure. Int. J. Oral Maxillofac. Implants. 2005;20(3):349–359. [PubMed] [Google Scholar]
- 133.Cho L.R., Kim D.G., Kim J.H., Byon E.S., Jeong Y.S., Park C.J. Bone response of Mg ion-implanted clinical implants with the plasma source ion implantation method. Clin. Oral Implants Res. 2010;21(8):848–856. doi: 10.1111/j.1600-0501.2009.01862.x. [DOI] [PubMed] [Google Scholar]
- 134.Sul Y.T., Byon E., Wennerberg A. Surface characteristics of electrochemically oxidized implants and acid-etched implants: surface chemistry, morphology, pore configurations, oxide thickness, crystal structure, and roughness. Int. J. Oral Maxillofac. Implants. 2008;23(4):631–640. [PubMed] [Google Scholar]
- 135.Arciola C.R., Campoccia D., Ehrlich G.D., Montanaro L. Biofilm-based implant infections in orthopaedics. Adv. Exp. Med. Biol. 2015;830:29–46. doi: 10.1007/978-3-319-11038-7_2. [DOI] [PubMed] [Google Scholar]
- 136.Spriano S., Yamaguchi S., Baino F., Ferraris S. A critical review of multifunctional titanium surfaces: new frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018;79:1–22. doi: 10.1016/j.actbio.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 137.Jin G., Qin H., Cao H., Qian S., Zhao Y., Peng X., Zhang X., Liu X., Chu P.K. Synergistic effects of dual Zn/Ag ion implantation in osteogenic activity and antibacterial ability of titanium. Biomaterials. 2014;35(27):7699–7713. doi: 10.1016/j.biomaterials.2014.05.074. [DOI] [PubMed] [Google Scholar]
- 138.Jin G., Qin H., Cao H., Qiao Y., Zhao Y., Peng X., Zhang X., Liu X., Chu P.K. Zn/Ag micro-galvanic couples formed on titanium and osseointegration effects in the presence of S. aureus. Biomaterials. 2015;65:22–31. doi: 10.1016/j.biomaterials.2015.06.040. [DOI] [PubMed] [Google Scholar]
- 139.Shen X., Zhang Y., Ma P., Sutrisno L., Luo Z., Hu Y., Yu Y., Tao B., Li C., Cai K. Fabrication of magnesium/zinc-metal organic framework on titanium implants to inhibit bacterial infection and promote bone regeneration. Biomaterials. 2019:212. doi: 10.1016/j.biomaterials.2019.05.008. 1-16. [DOI] [PubMed] [Google Scholar]
- 140.Yu Y., Jin G., Xue Y., Wang D., Liu X., Sun J. Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 2017;49:590–603. doi: 10.1016/j.actbio.2016.11.067. [DOI] [PubMed] [Google Scholar]
- 141.Canullo L., Pellegrini G., Canciani E., Heinemann F., Galliera E., Dellavia C. Alveolar socket preservation technique: effect of biomaterial on bone regenerative pattern. Ann. Anat. 2016 doi: 10.1016/j.aanat.2015.05.007. 206 73-9. [DOI] [PubMed] [Google Scholar]
- 142.Wei J., Jia J., Wu F., Wei S., Zhou H., Zhang H., Shin J.W., Liu C. Hierarchically microporous/macroporous scaffold of magnesium-calcium phosphate for bone tissue regeneration. Biomaterials. 2010;31(6):1260–1269. doi: 10.1016/j.biomaterials.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 143.Nabiyouni M., Bruckner T., Zhou H., Gbureck U., Bhaduri S.B. Magnesium-based bioceramics in orthopedic applications. Acta Biomater. 2018;66:23–43. doi: 10.1016/j.actbio.2017.11.033. [DOI] [PubMed] [Google Scholar]
- 144.Meininger S., Mandal S., Kumar A., Groll J., Basu B., Gbureck U. Strength reliability and in vitro degradation of three-dimensional powder printed strontium-substituted magnesium phosphate scaffolds. Acta Biomater. 2016;31:401–411. doi: 10.1016/j.actbio.2015.11.050. [DOI] [PubMed] [Google Scholar]
- 145.Landi Elena, Tampieri Anna, Mattioli-Belmonte Monica, Celotti Giancarlo, Sandri Monica, Gigante Antonio, Fava Paola, Biagini Graziella. Biomimetic Mg- and Mg,CO3-substituted hydroxyapatites: synthesis characterization and in vitro behaviour. J. Eur. Ceram. Soc. 2006;26(13):2593–2601. [Google Scholar]
- 146.Cacciotti Ilaria, Bianco Alessandra, Lombardi Mariangela, Montanaro Laura. Mg-substituted hydroxyapatite nanopowders: synthesis, thermal stability and sintering behaviour. J. Eur. Ceram. Soc. 2009;29(14):2969–2978. [Google Scholar]
- 147.Xu T., He X., Chen Z., He L., Lu M., Ge J., Weng J., Mu Y., Duan K. Effect of magnesium particle fraction on osteoinduction of hydroxyapatite sphere-based scaffolds. J. Mater. Chem. B. 2019;7(37):5648–5660. doi: 10.1039/c9tb01162e. [DOI] [PubMed] [Google Scholar]
- 148.Crespi R., Cappare P., Gherlone E. Comparison of magnesium-enriched hydroxyapatite and porcine bone in human extraction socket healing: a histologic and histomorphometric evaluation. Int. J. Oral Maxillofac. Implants. 2011;26(5):1057–1062. [PubMed] [Google Scholar]
- 149.Felice P., Lizio G., Marchetti C., Checchi L., Scarano A. Magnesium-substituted hydroxyapatite grafting using the vertical inlay technique. Int. J. Periodontics Restor. Dent. 2013;33(3):355–363. doi: 10.11607/prd.0893. [DOI] [PubMed] [Google Scholar]
- 150.R. Sedghi, A. Shaabani, N. Sayyari, Electrospun triazole-based chitosan nanofibers as a novel scaffolds for bone tissue repair and regeneration, Carbohydr. Polym. 2020, 230 115707. doi: 10.1016/j.carbpol.2019.115707. [DOI] [PubMed]
- 151.Scalera F., Palazzo B., Barca A., Gervaso F. Sintering of magnesium-strontium doped hydroxyapatite nanocrystals: towards the production of 3D biomimetic bone scaffolds. J. Biomed. Mater. Res. 2020;108(3):633–644. doi: 10.1002/jbm.a.36843. [DOI] [PubMed] [Google Scholar]
- 152.Chen S., Shi Y., Zhang X., Ma J. Biomimetic synthesis of Mg-substituted hydroxyapatite nanocomposites and three-dimensional printing of composite scaffolds for bone regeneration. J. Biomed. Mater. Res. 2019;107(11):2512–2521. doi: 10.1002/jbm.a.36757. [DOI] [PubMed] [Google Scholar]
- 153.Caneva M., Botticelli D., Stellini E., Souza S.L., Salata L.A., Lang N.P. Magnesium-enriched hydroxyapatite at immediate implants: a histomorphometric study in dogs. Clin. Oral Implants Res. 2011;22(5):512–517. doi: 10.1111/j.1600-0501.2010.02040.x. [DOI] [PubMed] [Google Scholar]
- 154.Crespi R., Cappare P., Gherlone E. Magnesium-enriched hydroxyapatite compared to calcium sulfate in the healing of human extraction sockets: radiographic and histomorphometric evaluation at 3 months. J. Periodontol. 2009;80(2):210–218. doi: 10.1902/jop.2009.080400. [DOI] [PubMed] [Google Scholar]
- 155.Barallat L., Ruiz-Magaz V., Levi P.A., Jr., Mareque-Bueno S., Galindo-Moreno P., Nart J. Histomorphometric results in ridge preservation procedures comparing various graft materials in extraction sockets with nongrafted sockets in humans: a systematic review. Implant Dent. 2014;23(5):539–554. doi: 10.1097/id.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 156.Crespi R., Mariani E., Benasciutti E., Cappare P., Cenci S., Gherlone E. Magnesium-enriched hydroxyapatite versus autologous bone in maxillary sinus grafting: combining histomorphometry with osteoblast gene expression profiles ex vivo. J. Periodontol. 2009;80(4):586–593. doi: 10.1902/jop.2009.080466. [DOI] [PubMed] [Google Scholar]
- 157.Canullo L., Sisti A. Early implant loading after vertical ridge augmentation (VRA) using e-PTFE titanium-reinforced membrane and nano-structured hydroxyapatite: 2-year prospective study. Eur. J. Oral Implant. 2010;3(1):59–69. [PubMed] [Google Scholar]
- 158.Deng L., Li D., Yang Z., Xie X., Kang P. Repair of the calvarial defect in goat model using magnesium-doped porous hydroxyapatite combined with recombinant human bone morphogenetic protein-2. Bio Med. Mater. Eng. 2017;28(4):361–377. doi: 10.3233/bme-171678. [DOI] [PubMed] [Google Scholar]
- 159.Taschieri S., Del Fabbro M., Panda S., Goker F., Babina K.S., Tampieri A., Mortellaro C. Prospective clinical and histologic evaluation of alveolar socket healing following ridge preservation using a combination of hydroxyapatite and collagen biomimetic xenograft versus demineralized bovine bone. J. Craniofac. Surg. 2019;30(4):1089–1094. doi: 10.1097/scs.0000000000005416. [DOI] [PubMed] [Google Scholar]
- 160.Sun M., Liu A., Shao H., Yang X., Ma C., Yan S., Liu Y., He Y., Gou Z. Systematical evaluation of mechanically strong 3D printed diluted magnesium doping wollastonite scaffolds on osteogenic capacity in rabbit calvarial defects. Sci. Rep. 2016;6:34029. doi: 10.1038/srep34029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xie J., Yang X., Shao H., Ye J., He Y., Fu J., Gao C., Gou Z. Simultaneous mechanical property and biodegradation improvement of wollastonite bioceramic through magnesium dilute doping. J. Mech. Behav. Biomed. Mater. 2016;54:60–71. doi: 10.1016/j.jmbbm.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 162.Tarafder S., Dernell W.S., Bandyopadhyay A., Bose S. SrO- and MgO-doped microwave sintered 3D printed tricalcium phosphate scaffolds: mechanical properties and in vivo osteogenesis in a rabbit model. J. Biomed. Mater. Res. B Appl. Biomater. 2015;103(3):679–690. doi: 10.1002/jbm.b.33239. [DOI] [PubMed] [Google Scholar]
- 163.Park J.W., Ko H.J., Jang J.H., Kang H., Suh J.Y. Increased new bone formation with a surface magnesium-incorporated deproteinized porcine bone substitute in rabbit calvarial defects. J. Biomed. Mater. Res. 2012;100(4):834–840. doi: 10.1002/jbm.a.34017. [DOI] [PubMed] [Google Scholar]
- 164.Kim M.S., Kovacevic D., Milks R.A., Jun B.J., Rodriguez E., DeLozier K.R., Derwin K.A., Iannotti J.P. Bone graft substitute provides metaphyseal fixation for a stemless humeral implant. Orthopedics. 2015;38(7):e597–603. doi: 10.3928/01477447-20150701-58. [DOI] [PubMed] [Google Scholar]
- 165.Roller B.L., Kuroki K., Bozynski C.C., Pfeiffer F.M., Cook J.L. Use of a novel magnesium-based resorbable bone cement for augmenting anchor and tendon fixation. Am. J. Orthoped. 2018;47(2) doi: 10.12788/ajo.2018.0010. [DOI] [PubMed] [Google Scholar]
- 166.Schendel S.A., Peauroi J. Magnesium-based bone cement and bone void filler: preliminary experimental studies. J. Craniofac. Surg. 2009;20(2):461–464. doi: 10.1097/SCS.0b013e31819b9819. [DOI] [PubMed] [Google Scholar]
- 167.Sehlke B.M., Wilson T.G., Jones A.A., Yamashita M., Cochran D.L. The use of a magnesium-based bone cement to secure immediate dental implants. Int. J. Oral Maxillofac. Implants. 2013;28(6):e357–e367. doi: 10.11607/jomi.te16. [DOI] [PubMed] [Google Scholar]
- 168.Zhang K., Lin S., Feng Q., Dong C., Yang Y., Li G., Bian L. Nanocomposite hydrogels stabilized by self-assembled multivalent bisphosphonate-magnesium nanoparticles mediate sustained release of magnesium ion and promote in-situ bone regeneration. Acta Biomater. 2017;64:389–400. doi: 10.1016/j.actbio.2017.09.039. [DOI] [PubMed] [Google Scholar]
- 169.Yuan Z., Wei P., Huang Y., Zhang W., Chen F., Zhang X., Mao J., Chen D., Cai Q., Yang X. Injectable PLGA microspheres with tunable magnesium ion release for promoting bone regeneration. Acta Biomater. 2019;85:294–309. doi: 10.1016/j.actbio.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 170.Liu X., He X., Jin D., Wu S., Wang H., Yin M., Aldalbahi A., El-Newehy M., Mo X., Wu J. A biodegradable multifunctional nanofibrous membrane for periodontal tissue regeneration. Acta Biomater. 2020;108:207–222. doi: 10.1016/j.actbio.2020.03.044. [DOI] [PubMed] [Google Scholar]
- 171.Abou Neel E.A., Chrzanowski W., Salih V.M., Kim H.W., Knowles J.C. Tissue engineering in dentistry. J. Dent. 2014;42(8):915–928. doi: 10.1016/j.jdent.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 172.Saoud T.M.A., Ricucci D., Lin L.M., Gaengler P. Regeneration and repair in endodontics-A special issue of the regenerative endodontics-A new era in clinical endodontics. Dent. J. 2016;4(1) doi: 10.3390/dj4010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kim S.G., Malek M., Sigurdsson A., Lin L.M., Kahler B. Regenerative endodontics: a comprehensive review. Int. Endod. J. 2018;51(12):1367–1388. doi: 10.1111/iej.12954. [DOI] [PubMed] [Google Scholar]
- 174.Wolf F.I., Cittadini A. Chemistry and biochemistry of magnesium. Mol. Aspect. Med. 2003;24(1–3):3–9. doi: 10.1016/s0098-2997(02)00087-0. [DOI] [PubMed] [Google Scholar]
- 175.Wiesmann H.P., Tkotz T., Joos U., Zierold K., Stratmann U., Szuwart T., Plate U., Hohling H.J. Magnesium in newly formed dentin mineral of rat incisor. J. Bone Miner. Res. 1997;12(3):380–383. doi: 10.1359/jbmr.1997.12.3.380. [DOI] [PubMed] [Google Scholar]
- 176.Scibior A., Adamczyk A., Mroczka R., Niedzwiecka I., Golebiowska D., Fornal E. Effects of vanadium (V) and magnesium (Mg) on rat bone tissue: mineral status and micromorphology. Consequences of V-Mg interactions. Metallomics. 2014;6(12):2260–2278. doi: 10.1039/c4mt00234b. [DOI] [PubMed] [Google Scholar]
- 177.Luder H.U., Gerth-Kahlert C., Ostertag-Benzinger S., Schorderet D.F. Dental phenotype in Jalili syndrome due to a c.1312 dupC homozygous mutation in the CNNM4 gene. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0078529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sano T., Morohashi T., Amano H., Yamada S., Nakamura M. Distributions of magnesium and sulphur in defect layers of incisor dentine in magnesium-deficient rats. Arch. Oral Biol. 2005;50(8):715–725. doi: 10.1016/j.archoralbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 179.Cui L., Xu S.M., Ma D.D., Wu B.L. The effect of TRPM7 suppression on the proliferation, migration and osteogenic differentiation of human dental pulp stem cells. Int. Endod. J. 2014;47(6):583–593. doi: 10.1111/iej.12193. [DOI] [PubMed] [Google Scholar]
- 180.Laiteerapong A., Lochaiwatana Y., Hirata I., Okazaki M., Mori K., Murakami S., Poolthong S. A novel glass ionomer cement containing MgCO(3 )apatite induced the increased proliferation and differentiation of human pulp cells in vitro. Dent. Mater. J. 2012;31(5):772–778. doi: 10.4012/dmj.2012-096. [DOI] [PubMed] [Google Scholar]
- 181.Kong Y., Hu X., Zhong Y., Xu K., Wu B., Zheng J. Magnesium-enriched microenvironment promotes odontogenic differentiation in human dental pulp stem cells by activating ERK/BMP2/Smads signaling. Stem Cell Res. Ther. 2019;10(1):378. doi: 10.1186/s13287-019-1493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zheng J.M., Kong Y.Y., Li Y.Y., Zhang W. MagT1 regulated the odontogenic differentiation of BMMSCs induced byTGC-CM via ERK signaling pathway. Stem Cell Res. Ther. 2019;10(1):48. doi: 10.1186/s13287-019-1148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Theocharidou A., Bakopoulou A., Kontonasaki E., Papachristou E., Hadjichristou C., Bousnaki M., Theodorou G., Papadopoulou L., Kantiranis N., Paraskevopoulos K., Koidis P. Odontogenic differentiation and biomineralization potential of dental pulp stem cells inside Mg-based bioceramic scaffolds under low-level laser treatment. Laser Med. Sci. 2017;32(1):201–210. doi: 10.1007/s10103-016-2102-9. [DOI] [PubMed] [Google Scholar]
- 184.Qu T., Jing J., Jiang Y., Taylor R.J., Feng J.Q., Geiger B., Liu X. Magnesium-containing nanostructured hybrid scaffolds for enhanced dentin regeneration. Tissue Eng. Part A. 2014;20(17–18):2422–2433. doi: 10.1089/ten.TEA.2013.0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li Y., Yue J., Yang C. Unraveling the role of Mg(++) in osteoarthritis. Life Sci. 2016;147:24–29. doi: 10.1016/j.lfs.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 186.Lange T.S., Kirchberg J., Bielinsky A.K., Leuker A., Bank I., Ruzicka T., Scharffetter-Kochanek K. Divalent cations (Mg2+, Ca2+) differentially influence the beta 1 integrin-mediated migration of human fibroblasts and keratinocytes to different extracellular matrix proteins. Exp. Dermatol. 1995;4(3):130–137. doi: 10.1111/j.1600-0625.1995.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 187.Lange T.S., Bielinsky A.K., Kirchberg K., Bank I., Herrmann K., Krieg T., Scharffetter-Kochanek K. Mg2+ and Ca2+ differentially regulate beta 1 integrin-mediated adhesion of dermal fibroblasts and keratinocytes to various extracellular matrix proteins. Exp. Cell Res. 1994;214(1):381–388. doi: 10.1006/excr.1994.1271. [DOI] [PubMed] [Google Scholar]
- 188.Amberg R., Elad A., Rothamel D., Fienitz T., Szakacs G., Heilmann S., Witte F. Design of a migration assay for human gingival fibroblasts on biodegradable magnesium surfaces. Acta Biomater. 2018;79:158–167. doi: 10.1016/j.actbio.2018.08.034. [DOI] [PubMed] [Google Scholar]
- 189.Heitz-Mayfield L.J., Lang N.P. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontology. 2000;53:167–181. doi: 10.1111/j.1600-0757.2010.00348.x. 2010. [DOI] [PubMed] [Google Scholar]
- 190.Takamori Y., Atsuta I., Nakamura H., Sawase T., Koyano K., Hara Y. Histopathological comparison of the onset of peri-implantitis and periodontitis in rats. Clin. Oral Implants Res. 2017;28(2):163–170. doi: 10.1111/clr.12777. [DOI] [PubMed] [Google Scholar]
- 191.Okawachi H., Ayukawa Y., Atsuta I., Furuhashi A., Sakaguchi M., Yamane K., Koyano K. Effect of titanium surface calcium and magnesium on adhesive activity of epithelial-like cells and fibroblasts. Biointerphases. 2012;7(1–4):27. doi: 10.1007/s13758-012-0027-9. [DOI] [PubMed] [Google Scholar]
- 192.Zhu Y., Zhang C.N., Gu Y.X., Shi J.Y., Mo J.J., Qian S.J., Qiao S.C., Lai H.C. The responses of human gingival fibroblasts to magnesium-doped titanium. J. Biomed. Mater. Res. 2020;108(2):267–278. doi: 10.1002/jbm.a.36813. [DOI] [PubMed] [Google Scholar]
- 193.Lynch R.J. Zinc in the mouth, its interactions with dental enamel and possible effects on caries; a review of the literature. Int. Dent. J. 2011;61(Suppl 3):46–54. doi: 10.1111/j.1875-595X.2011.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kaur K., Gupta R., Saraf S.A., Saraf S.K. Zinc: the metal of life. Compr. Rev. Food Sci. Food Saf. 2014;13(4):358–376. doi: 10.1111/1541-4337.12067. [DOI] [PubMed] [Google Scholar]
- 195.Kara C., Orbak R., Dagsuyu I.M., Orbak Z., Bilici N., Gumustekin K. In vivo assessment of zinc deficiency on craniofacial growth in a rat model. Eur. J. Dermatol. 2009;3(1):10–15. [PMC free article] [PubMed] [Google Scholar]
- 196.Zhong Y., Li X., Hu D.Y., Mateo L.R., Morrison B.M., Jr., Delgado E., Zhang Y.P. Control of established gingivitis and dental plaque using a 1450 ppm fluoride/zinc-based dentifrice: a randomized clinical study. J. Clin. Dent. 2015;26(4):104–108. [PubMed] [Google Scholar]
- 197.Seyedmajidi S.A., Seyedmajidi M., Moghadamnia A., Khani Z., Zahedpasha S., Jenabian N., Jorsaraei G., Halalkhor S., Motallebnejad M. Effect of zinc-deficient diet on oral tissues and periodontal indices in rats. Int. J. Mol. Cell Med. 2014;3(2):81–87. [PMC free article] [PubMed] [Google Scholar]
- 198.Li P., Zhang W., Dai J., Xepapadeas A.B., Schweizer E., Alexander D., Scheideler L., Zhou C., Zhang H., Wan G., Geis-Gerstorfer J. Investigation of zinc-copper alloys as potential materials for craniomaxillofacial osteosynthesis implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;103:109826. doi: 10.1016/j.msec.2019.109826. [DOI] [PubMed] [Google Scholar]
- 199.Kubásek J., Dvorský D., Šedý J., Msallamová Š., Levorová J., Foltán R., Vojtěch D. The fundamental comparison of Zn-2Mg and Mg-4Y-3RE alloys as a perspective biodegradable materials. Materials. 2019;12(22) doi: 10.3390/ma12223745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sadighi A., Roshan M.M., Moradi A., Ostadrahimi A. The effects of zinc supplementation on serum zinc, alkaline phosphatase activity and fracture healing of bones. Saudi Med. J. 2008;29(9):1276–1279. [PubMed] [Google Scholar]
- 201.Wey A., Cunningham C., Hreha J., Breitbart E., Cottrell J., Ippolito J., Clark D., Lin H.N., Benevenia J., O'Connor J.P., Lin S.S., Paglia D.N. Local ZnCl2 accelerates fracture healing. J. Orthop. Res. 2014;32(6):834–841. doi: 10.1002/jor.22593. [DOI] [PubMed] [Google Scholar]
- 202.Krell E.S., Ippolito J.A., Montemurro N.J., Lim P.H., Vincent R.A., Hreha J., Cottrell J., Sudah S.Y., Muñoz M.F., Pacific K.P., Benevenia J., OʼConnor J.P., Lin S.S. Local zinc chloride release from a calcium sulfate carrier enhances fracture healing. J. Orthop. Trauma. 2017;31(3):168–174. doi: 10.1097/bot.0000000000000748. [DOI] [PubMed] [Google Scholar]
- 203.Wang X., Shao X., Dai T., Xu F., Zhou J.G., Qu G., Tian L., Liu B., Liu Y. In vivo study of the efficacy, biosafety, and degradation of a zinc alloy osteosynthesis system. Acta Biomater. 2019;92:351–361. doi: 10.1016/j.actbio.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 204.Tapiero H., Tew K.D. Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed. Pharmacother. 2003;57(9):399–411. doi: 10.1016/s0753-3322(03)00081-7. [DOI] [PubMed] [Google Scholar]
- 205.Shiota J., Tagawa H., Izumi N., Higashikawa S., Kasahara H. Effect of zinc supplementation on bone formation in hemodialysis patients with normal or low turnover bone. Ren. Fail. 2015;37(1):57–60. doi: 10.3109/0886022x.2014.959412. [DOI] [PubMed] [Google Scholar]
- 206.Seo H.J., Cho Y.E., Kim T., Shin H.I., Kwun I.S. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr. Res. Pract. 2010;4(5):356–361. doi: 10.4162/nrp.2010.4.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kwun I.S., Cho Y.E., Lomeda R.A., Shin H.I., Choi J.Y., Kang Y.H., Beattie J.H. Zinc deficiency suppresses matrix mineralization and retards osteogenesis transiently with catch-up possibly through Runx 2 modulation. Bone. 2010;46(3):732–741. doi: 10.1016/j.bone.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 208.Tokudome Y., Otsuka M. Possibility of alveolar bone promoting enhancement by using lipophilic and/or hydrophilic zinc related compounds in zinc-deficient osteoporosis rats. Biol. Pharm. Bull. 2012;35(9):1496–1501. doi: 10.1248/bpb.b12-00218. [DOI] [PubMed] [Google Scholar]
- 209.Liu H., Li W., Jia S., Li B. Puerarin and zinc additively prevent mandibular bone loss through inhibiting osteoclastogenesis in ovariectomized rats. Histol. Histopathol. 2017;32(8):851–860. doi: 10.14670/hh-11-855. [DOI] [PubMed] [Google Scholar]
- 210.Guo H., Xia D., Zheng Y., Zhu Y., Liu Y., Zhou Y. A pure zinc membrane with degradability and osteogenesis promotion for guided bone regeneration: in vitro and in vivo studies. Acta Biomater. 2020;106:396–409. doi: 10.1016/j.actbio.2020.02.024. [DOI] [PubMed] [Google Scholar]
- 211.Zhang Y., Yan Y., Xu X., Lu Y., Chen L., Li D., Dai Y., Kang Y., Yu K. Investigation on the microstructure, mechanical properties, in vitro degradation behavior and biocompatibility of newly developed Zn-0.8%Li-(Mg, Ag) alloys for guided bone regeneration. Mater. Sci. Eng. C, Mater. Biol. Appl. 2019;99:1021–1034. doi: 10.1016/j.msec.2019.01.120. [DOI] [PubMed] [Google Scholar]
- 212.Zhang W., Li P., Shen G., Mo X., Zhou C., Alexander D., Rupp F., Geis-Gerstorfer J., Zhang H., Wan G. Appropriately adapted properties of hot-extruded Zn–0.5Cu–xFe alloys aimed for biodegradable guided bone regeneration membrane application. Bioactive Mater. 2020;6(4):975–989. doi: 10.1016/j.bioactmat.2020.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Oh S.A., Won J.E., Kim H.W. Composite membranes of poly(lactic acid) with zinc-added bioactive glass as a guiding matrix for osteogenic differentiation of bone marrow mesenchymal stem cells. J. Biomater. Appl. 2012;27(4):413–422. doi: 10.1177/0885328211408944. [DOI] [PubMed] [Google Scholar]
- 214.Chou J., Komuro M., Hao J., Kuroda S., Hattori Y., Ben-Nissan B., Milthorpe B., Otsuka M. Bioresorbable zinc hydroxyapatite guided bone regeneration membrane for bone regeneration. Clin. Oral Implants Res. 2016;27(3):354–360. doi: 10.1111/clr.12520. [DOI] [PubMed] [Google Scholar]
- 215.Toledano M., Gutierrez-Pérez J.L., Gutierrez-Corrales A., Serrera-Figallo M.A., Toledano-Osorio M., Rosales-Leal J.I., Aguilar M., Osorio R., Torres-Lagares D. Novel non-resorbable polymeric-nanostructured scaffolds for guided bone regeneration. Clin. Oral Invest. 2020;24(6):2037–2049. doi: 10.1007/s00784-019-03068-8. [DOI] [PubMed] [Google Scholar]
- 216.Toledano M., Toledano-Osorio M., Osorio R., Carrasco-Carmona Á., Gutiérrez-Pérez J.L., Gutiérrez-Corrales A., Serrera-Figallo M.A., Lynch C.D., Torres-Lagares D. Doxycycline and zinc loaded silica-nanofibrous polymers as biomaterials for bone regeneration. Polymers. 2020;12(5) doi: 10.3390/polym12051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.El-Wassefy N.A., Reicha F.M., Aref N.S. Electro-chemical deposition of nano hydroxyapatite-zinc coating on titanium metal substrate. Int. J. Implant Dent. 2017;3(1) doi: 10.1186/s40729-017-0095-1. 39-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Qiao Y., Zhang W., Tian P., Meng F., Zhu H., Jiang X., Liu X., Chu P.K. Stimulation of bone growth following zinc incorporation into biomaterials. Biomaterials. 2014;35(25):6882–6897. doi: 10.1016/j.biomaterials.2014.04.101. [DOI] [PubMed] [Google Scholar]
- 219.Jin G., Cao H., Qiao Y., Meng F., Zhu H., Liu X. Osteogenic activity and antibacterial effect of zinc ion implanted titanium. Colloids Surf. B Biointerfaces. 2014;117:158–165. doi: 10.1016/j.colsurfb.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 220.He J., Feng W., Zhao B.H., Zhang W., Lin Z. Vivo effect of titanium implants with porous zinc-containing coatings prepared by plasma electrolytic oxidation method on osseointegration in rabbits. Int. J. Oral Maxillofac. Implants. 2018;33(2):298–310. doi: 10.11607/jomi.5764. [DOI] [PubMed] [Google Scholar]
- 221.Zhang R., Xu N., Liu X., Yang X., Yan H., Ma J., Feng Q., Shen Z. Micro/nanostructured TiO(2)/ZnO coating enhances osteogenic activity of SaOS-2 cells. Artif. Cells Nanomed. Biotechnol. 2019;47(1):2838–2845. doi: 10.1080/21691401.2018.1546187. [DOI] [PubMed] [Google Scholar]
- 222.Zhang R., Liu X., Xiong Z., Huang Q., Yang X., Yan H., Ma J., Feng Q., Shen Z. Novel micro/nanostructured TiO2/ZnO coating with antibacterial capacity and cytocompatibility. Ceram. Int. 2018;44(8):9711–9719. doi: 10.1016/j.ceramint.2018.02.202. [DOI] [Google Scholar]
- 223.Hu H., Zhang W., Qiao Y., Jiang X., Liu X., Ding C. Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium. Acta Biomater. 2012;8(2):904–915. doi: 10.1016/j.actbio.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 224.Zhang R., Liu X., Xiong Z., Huang Q., Yang X., Yan H., Ma J., Feng Q., Shen Z. The immunomodulatory effects of Zn-incorporated micro/nanostructured coating in inducing osteogenesis. Artif. Cells Nanomed. Biotechnol. 2018;46(sup1):1123–1130. doi: 10.1080/21691401.2018.1446442. [DOI] [PubMed] [Google Scholar]
- 225.Abdulkareem E.H., Memarzadeh K., Allaker R.P., Huang J., Pratten J., Spratt D. Anti-biofilm activity of zinc oxide and hydroxyapatite nanoparticles as dental implant coating materials. J. Dent. 2015;43(12):1462–1469. doi: 10.1016/j.jdent.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 226.Memarzadeh K., Sharili A.S., Huang J., Rawlinson S.C., Allaker R.P. Nanoparticulate zinc oxide as a coating material for orthopedic and dental implants. J. Biomed. Mater. Res. 2015;103(3):981–989. doi: 10.1002/jbm.a.35241. [DOI] [PubMed] [Google Scholar]
- 227.Feng W., Zhao B.H., Zhang W., Lin Z. [An experimental study of Zn/Ca/P-containing coatings on titanium implant surface modified by plasma electrolytic oxidation] Zhonghua kou qiang yi xue za zhi = Zhonghua kouqiang yixue zazhi = Chinese journal of stomatology. 2019;54(1):46–51. doi: 10.3760/cma.j.issn.1002-0098.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 228.Jin G., Qin H., Cao H., Qian S., Zhao Y., Peng X., Zhang X., Liu X., Chu P.K. Synergistic effects of dual Zn/Ag ion implantation in osteogenic activity and antibacterial ability of titanium. Biomaterials. 2014;35(27):7699–7713. doi: 10.1016/j.biomaterials.2014.05.074. [DOI] [PubMed] [Google Scholar]
- 229.Jin G., Qin H., Cao H., Qiao Y., Zhao Y., Peng X., Zhang X., Liu X., Chu P.K. Zn/Ag micro-galvanic couples formed on titanium and osseointegration effects in the presence of S. aureus. Biomaterials. 2015;65:22–31. doi: 10.1016/j.biomaterials.2015.06.040. [DOI] [PubMed] [Google Scholar]
- 230.Roguska A., Belcarz A., Zalewska J., Hołdyński M., Andrzejczuk M., Pisarek M., Ginalska G. Metal TiO(2) nanotube layers for the treatment of dental implant infections. ACS Appl. Mater. Interfaces. 2018;10(20):17089–17099. doi: 10.1021/acsami.8b04045. [DOI] [PubMed] [Google Scholar]
- 231.Padilla S., Castro A., Garzón-Gutiérrez A., Benito L., Sanz S., Canillas M., Carrodeguas R. Novel nanostructured Zn-substituted monetite based biomaterial for bone regeneration. J. Nanomed. Nanotechnol. 2015;6 doi: 10.4172/2157-7439.1000325. [DOI] [Google Scholar]
- 232.Flores Fraile J., López-Valverde N., García de Castro Andews A., Santos Marino J.A., Ramírez J.M., Gómez de Diego R., Montero J., López-Valverde A., Blanco Antona L.A. Safety and efficacy of a new synthetic material based on monetite, silica gel, PS-wallastonite, and a hydroxyapatite calcium deficient: a randomized comparative clinic trial. Medicina. 2020;56(2):46. doi: 10.3390/medicina56020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Pérez-Sayáns M., Lorenzo-Pouso A.I., Galindo-Moreno P., Muñoz-Guzón F., González-Cantalapiedra A., López-Peña M., Somoza-Martín M., Gallas-Torreira M., García-García A. Evaluation of a new tricalcium phosphate for guided bone regeneration: an experimental study in the beagle dog. Odontology. 2019;107(2):209–218. doi: 10.1007/s10266-018-0384-z. [DOI] [PubMed] [Google Scholar]
- 234.Chandra R., Deshoju A., Reddy A., Reddy B., Nagarajan S., Naveen A. Efficacy of a novel Zn-substituted monetite-based scaffold in the treatment of periodontal osseous defects. J. Int. Acad. Periodontol. 2017;19:2–9. [PubMed] [Google Scholar]
- 235.Wang B., Yang M., Liu L., Yan G., Yan H., Feng J., Li Z., Li D., Sun H., Yang B. Osteogenic potential of Zn(2+)-passivated carbon dots for bone regeneration in vivo. Biomater. Sci. 2019;7(12):5414–5423. doi: 10.1039/c9bm01181a. [DOI] [PubMed] [Google Scholar]
- 236.Bhardwaj V.A., Deepika P.C., Basavarajaiah S. Zinc incorporated nano hydroxyapatite: a novel bone graft used for regeneration of intrabony defects. Contemp. Clin. Dent. 2018;9(3):427–433. doi: 10.4103/ccd.ccd_192_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Chung R.J., Hsieh M.F., Huang C.W., Perng L.H., Wen H.W., Chin T.S. Antimicrobial effects and human gingival biocompatibility of hydroxyapatite sol-gel coatings. J. Biomed. Mater. Res. B Appl. Biomater. 2006;76(1):169–178. doi: 10.1002/jbm.b.30365. [DOI] [PubMed] [Google Scholar]
- 238.Stanic V., Dimitrijevic-Brankovic S., Stankovic J., Mitrić M., Jokic B., Plećaš I., Raičević S. Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders. Appl. Surf. Sci. 2010;256:6083–6089. doi: 10.1016/j.apsusc.2010.03.124. [DOI] [Google Scholar]
- 239.Thian E.S., Konishi T., Kawanobe Y., Lim P.N., Choong C., Ho B., Aizawa M. Zinc-substituted hydroxyapatite: a biomaterial with enhanced bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 2013;24(2):437–445. doi: 10.1007/s10856-012-4817-x. [DOI] [PubMed] [Google Scholar]
- 240.Grandjean-Laquerriere A., Laquerriere P., Jallot E., Nedelec J.M., Guenounou M., Laurent-Maquin D., Phillips T.M. Influence of the zinc concentration of sol-gel derived zinc substituted hydroxyapatite on cytokine production by human monocytes in vitro. Biomaterials. 2006;27(17):3195–3200. doi: 10.1016/j.biomaterials.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 241.Suruagy A.A., Alves A.T., Sartoretto S.C., Calasans-Maia J.A., Granjeiro J.M., Calasans-Maia M.D. Physico-chemical and histomorphometric evaluation of zinc-containing hydroxyapatite in rabbits calvaria. Braz. Dent. J. 2016;27(6):717–726. doi: 10.1590/0103-6440201601028. [DOI] [PubMed] [Google Scholar]
- 242.Prasad K.V., Therathil S.G., Agnihotri A., Sreenivasan P.K., Mateo L.R., Cummins D. The effects of two new dual zinc plus arginine dentifrices in reducing oral bacteria in multiple locations in the mouth: 12-hour whole mouth antibacterial protection for whole mouth health. J. Clin. Dent. 2018;29(Spec No A):A25–32. [PubMed] [Google Scholar]
- 243.Sowinski J., Petrone D.M., Battista G., Simone A.J., Crawford R., Patel S., Petrone M.E., DeVizio W., Volpe A.R., Proskin H.M. Clinical efficacy of a dentifrice containing zinc citrate: a 12-week calculus clinical study in adults. Comp. Cont. Educ. Dent. 1998;19(2 Suppl):16–19. [PubMed] [Google Scholar]
- 244.Churchley D., Newby C.S., Willson R., Haider A., Schemehorn B., Lynch R.J. Protection against enamel demineralisation using toothpastes containing o-cymen-5-ol, zinc chloride and sodium fluoride. Int. Dent. J. 2011;61(3):55–59. doi: 10.1111/j.1875-595X.2011.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Cerklewski F.L. Effect of suboptimal zinc nutrition during gestation and lactation on rat molar tooth composition and dental caries. J. Nutr. 1981;111(10):1780–1783. doi: 10.1093/jn/111.10.1780. [DOI] [PubMed] [Google Scholar]
- 246.Rapisarda E., Longo A. [Effects of zinc and vitamin B 6 in experimental caries in rats] Minerva Stomatol. 1981;30(4):317–320. [PubMed] [Google Scholar]
- 247.Rahman M.T., Hossain A., Pin C.H., Yahya N.A. Zinc and metallothionein in the development and progression of dental caries. Biol. Trace Elem. Res. 2019;187(1):51–58. doi: 10.1007/s12011-018-1369-z. [DOI] [PubMed] [Google Scholar]
- 248.An S., Gong Q., Huang Y. Promotive effect of zinc ions on the vitality, migration, and osteogenic differentiation of human dental pulp cells. Biol. Trace Elem. Res. 2017;175(1):112–121. doi: 10.1007/s12011-016-0763-7. [DOI] [PubMed] [Google Scholar]
- 249.Yusa K., Yamamoto O., Takano H., Fukuda M., Iino M. Zinc-modified titanium surface enhances osteoblast differentiation of dental pulp stem cells in vitro. Sci. Rep. 2016 doi: 10.1038/srep29462. 6 29462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lin C.Y., Lin H.H., Tsai M.H., Lin S.P., Chen M.H. Zinc chloride for odontogenesis of dental pulp stem cells via metallothionein up-regulation. J. Endod. 2011;37(2):211–216. doi: 10.1016/j.joen.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 251.Huang M., Hill R.G., Rawlinson S.C.F. Zinc bioglasses regulate mineralization in human dental pulp stem cells. Dent. Mater. 2017;33(5):543–552. doi: 10.1016/j.dental.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 252.Zhang J., Zheng L., Wang Z., Pei H., Hu W., Nie J., Shang P., Li B., Hei T.K., Zhou G. Lowering iron level protects against bone loss in focally irradiated and contralateral femurs through distinct mechanisms. Bone. 2019;120:50–60. doi: 10.1016/j.bone.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 253.Rossi F., Perrotta S., Bellini G., Luongo L., Tortora C., Siniscalco D., Francese M., Torella M., Nobili B., Di Marzo V., Maione S. Iron overload causes osteoporosis in thalassemia major patients through interaction with transient receptor potential vanilloid type 1 (TRPV1) channels. Haematologica. 2014;99(12):1876–1884. doi: 10.3324/haematol.2014.104463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yuan Y., Xu F., Cao Y., Xu L., Yu C., Yang F., Zhang P., Wang L., Shen G., Wang J., Xu Y. Iron accumulation leads to bone loss by inducing mesenchymal stem cell apoptosis through the activation of Caspase3. Biol. Trace Elem. Res. 2019;187(2):434–441. doi: 10.1007/s12011-018-1388-9. [DOI] [PubMed] [Google Scholar]
- 255.Parelman M., Stoecker B., Baker A., Medeiros D. Iron restriction negatively affects bone in female rats and mineralization of hFOB osteoblast cells. Exp. Biol. Med. (Maywood, N.J.) 2006;231(4):378–386. doi: 10.1177/153537020623100403. [DOI] [PubMed] [Google Scholar]
- 256.Medeiros D.M., Plattner A., Jennings D., Stoecker B. Bone morphology, strength and density are compromised in iron-deficient rats and exacerbated by calcium restriction. J. Nutr. 2002;132(10):3135–3141. doi: 10.1093/jn/131.10.3135. [DOI] [PubMed] [Google Scholar]
- 257.Balogh E., Paragh G., Jeney V. Influence of iron on bone homeostasis. Pharmaceuticals. 2018;11(4):107. doi: 10.3390/ph11040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.He Y.F., Ma Y., Gao C., Zhao G.Y., Zhang L.L., Li G.F., Pan Y.Z., Li K., Xu Y.J. Iron overload inhibits osteoblast biological activity through oxidative stress. Biol. Trace Elem. Res. 2013;152(2):292–296. doi: 10.1007/s12011-013-9605-z. [DOI] [PubMed] [Google Scholar]
- 259.Messer J.G., Kilbarger A.K., Erikson K.M., Kipp D.E. Iron overload alters iron-regulatory genes and proteins, down-regulates osteoblastic phenotype, and is associated with apoptosis in fetal rat calvaria cultures. Bone. 2009;45(5):972–979. doi: 10.1016/j.bone.2009.07.073. [DOI] [PubMed] [Google Scholar]
- 260.Zhao G.Y., Zhao L.P., He Y.F., Li G.F., Gao C., Li K., Xu Y.J. A comparison of the biological activities of human osteoblast hFOB1.19 between iron excess and iron deficiency. Biol. Trace Elem. Res. 2012;150(1–3):487–495. doi: 10.1007/s12011-012-9511-9. [DOI] [PubMed] [Google Scholar]
- 261.Traverson M., Heiden M., Stanciu L.A., Nauman E.A., Jones-Hall Y., Breur G.J. Vivo evaluation of biodegradability and biocompatibility of Fe30Mn alloy. Vet. Comp. Orthop. Traumatol. 2018;31(1):10–16. doi: 10.3415/vcot-17-06-0080. [DOI] [PubMed] [Google Scholar]
- 262.Kraus T., Moszner F., Fischerauer S., Fiedler M., Martinelli E., Eichler J., Witte F., Willbold E., Schinhammer M., Meischel M., Uggowitzer P.J., Löffler J.F., Weinberg A. Biodegradable Fe-based alloys for use in osteosynthesis: outcome of an in vivo study after 52 weeks. Acta Biomater. 2014;10(7):3346–3353. doi: 10.1016/j.actbio.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 263.Oriňaková R., Oriňak A., Giretová M., Medvecký L., Kupková M., Hrubovčáková M., Maskal'ová I., Macko J., Kal'avský F. A study of cytocompatibility and degradation of iron-based biodegradable materials. J. Biomater. Appl. 2016;30(7):1060–1070. doi: 10.1177/0885328215615459. [DOI] [PubMed] [Google Scholar]
- 264.Li Y., Jahr H., Lietaert K., Pavanram P., Yilmaz A., Fockaert L.I., Leeflang M.A., Pouran B., Gonzalez-Garcia Y., Weinans H., Mol J.M.C., Zhou J., Zadpoor A.A. Additively manufactured biodegradable porous iron. Acta Biomater. 2018;77:380–393. doi: 10.1016/j.actbio.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 265.Montufar E.B., Casas-Luna M., Horynová M., Tkachenko S., Fohlerová Z., Diaz-de-la-Torre S., Dvořák K., Čelko L., Kaiser J. High strength, biodegradable and cytocompatible alpha tricalcium phosphate-iron composites for temporal reduction of bone fractures. Acta Biomater. 2018;70:293–303. doi: 10.1016/j.actbio.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 266.Tkachenko S., Horynová M., Casas-Luna M., Diaz-de-la-Torre S., Dvořák K., Celko L., Kaiser J., Montufar E.B. Strength and fracture mechanism of iron reinforced tricalcium phosphate cermet fabricated by spark plasma sintering. J. Mech. Behav. Biomed. Mater. 2018;81:16–25. doi: 10.1016/j.jmbbm.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 267.Hong D., Chou D.-T., Velikokhatnyi O.I., Roy A., Lee B., Swink I., Issaev I., Kuhn H.A., Kumta P.N. Binder-jetting 3D printing and alloy development of new biodegradable Fe-Mn-Ca/Mg alloys. Acta Biomater. 2016;45:375–386. doi: 10.1016/j.actbio.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 268.Manchón A., Hamdan Alkhraisat M., Rueda-Rodriguez C., Prados-Frutos J.C., Torres J., Lucas-Aparicio J., Ewald A., Gbureck U., López-Cabarcos E. A new iron calcium phosphate material to improve the osteoconductive properties of a biodegradable ceramic: a study in rabbit calvaria. Biomed. Mater. 2015;10(5) doi: 10.1088/1748-6041/10/5/055012. [DOI] [PubMed] [Google Scholar]
- 269.Li Y., Ye D., Li M., Ma M., Gu N. Adaptive materials based on iron oxide nanoparticles for bone regeneration. ChemPhysChem : Eur. J. Chem. Phys. Phys. Chem. 2018;19(16):1965–1979. doi: 10.1002/cphc.201701294. [DOI] [PubMed] [Google Scholar]
- 270.Wang Q., Chen B., Cao M., Sun J., Wu H., Zhao P., Xing J., Yang Y., Zhang X., Ji M., Gu N. Response of MAPK pathway to iron oxide nanoparticles in vitro treatment promotes osteogenic differentiation of hBMSCs. Biomaterials. 2016;86:11–20. doi: 10.1016/j.biomaterials.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 271.Qiwei W., Bo C., Fang M., Shikang L., Meng C., Yan L., Ning G. Magnetic iron oxide nanoparticles accelerate osteogenic differentiation of mesenchymal stem cells via modulation of long noncoding RNA INZEB2. Nano Res. 2017;10(2):626–642. [Google Scholar]
- 272.Xia Y., Guo Y., Yang Z., Chen H., Ren K., Weir M.D., Chow L.C., Reynolds M.A., Zhang F., Gu N., Xu H.H.K. Iron oxide nanoparticle-calcium phosphate cement enhanced the osteogenic activities of stem cells through WNT/β-catenin signaling. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;104:109955. doi: 10.1016/j.msec.2019.109955. [DOI] [PubMed] [Google Scholar]
- 273.Xia Y., Chen H., Zhao Y., Zhang F., Li X., Wang L., Weir M.D., Ma J., Reynolds M.A., Gu N., Xu H.H.K. Novel magnetic calcium phosphate-stem cell construct with magnetic field enhances osteogenic differentiation and bone tissue engineering. Mater. Sci. Eng. C. 2019;98:30–41. doi: 10.1016/j.msec.2018.12.120. [DOI] [PubMed] [Google Scholar]
- 274.Xia Y., Chen H., Zhang F., Wang L., Chen B., Reynolds M.A., Ma J., Schneider A., Gu N., Xu H.H.K. Injectable calcium phosphate scaffold with iron oxide nanoparticles to enhance osteogenesis via dental pulp stem cells. Artif. Cells Nanomed. Biotechnol. 2018;46(sup1):423–433. doi: 10.1080/21691401.2018.1428813. [DOI] [PubMed] [Google Scholar]
- 275.Brett E., Zielins E.R., Luan A., Ooi C.C., Shailendra S., Atashroo D., Menon S., Blackshear C., Flacco J., Quarto N., Wang S.X., Longaker M.T., Wan D.C. Magnetic nanoparticle-based upregulation of B-cell lymphoma 2 enhances bone regeneration. Stem Cells Transl. Med. 2017;6(1):151–160. doi: 10.5966/sctm.2016-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Murphy M.K., MacBarb R.F., Wong M.E., Athanasiou K.A. Temporomandibular disorders: a review of etiology, clinical management, and tissue engineering strategies. Int. J. Oral Maxillofac. Implants. 2013;28(6):e393–414. doi: 10.11607/jomi.te20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Lowe J., Almarza A.J. A review of in-vitro fibrocartilage tissue engineered therapies with a focus on the temporomandibular joint. Arch. Oral Biol. 2017;83:193–201. doi: 10.1016/j.archoralbio.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Hu T., Xu H., Wang C., Qin H., An Z. Magnesium enhances the chondrogenic differentiation of mesenchymal stem cells by inhibiting activated macrophage-induced inflammation. Sci. Rep. 2018;8(1):3406. doi: 10.1038/s41598-018-21783-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Gruber H.E., Ingram J., Norton H.J., Wei L.Y., Frausto A., Mills B.G., Rude R.K. Alterations in growth plate and articular cartilage morphology are associated with reduced SOX9 localization in the magnesium-deficient rat. Biotech. Histochem. 2004;79(1):45–52. doi: 10.1080/10520290410001697913. [DOI] [PubMed] [Google Scholar]
- 280.Li H., Zeng C., Wei J., Yang T., Gao S.G., Li Y.S., Luo W., Xiao W.F., Xiong Y.L., Lei G.H. Serum calcium concentration is inversely associated with radiographic knee osteoarthritis: a cross-sectional study. Medicine (Baltim.) 2016;95(6) doi: 10.1097/md.0000000000002838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Zeng C., Wei J., Li H., Yang T., Zhang F.J., Pan D., Xiao Y.B., Yang T.B., Lei G.H. Relationship between serum magnesium concentration and radiographic knee osteoarthritis. J. Rheumatol. 2015;42(7):1231–1236. doi: 10.3899/jrheum.141414. [DOI] [PubMed] [Google Scholar]
- 282.Zeng C., Wei J., Terkeltaub R., Yang T., Choi H.K., Wang Y.L., Xie D.X., Hunter D.J., Zhang Y., Li H., Cui Y., Li L.J., Lei G.H. Dose-response relationship between lower serum magnesium level and higher prevalence of knee chondrocalcinosis. Arthritis Res. Ther. 2017;19(1):236. doi: 10.1186/s13075-017-1450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Pfister K., Mazur D., Vormann J., Stahlmann R. Diminished ciprofloxacin-induced chondrotoxicity by supplementation with magnesium and vitamin E in immature rats. Antimicrob. Agents Chemother. 2007;51(3):1022–1027. doi: 10.1128/aac.01175-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Lee C.H., Wen Z.H., Chang Y.C., Huang S.Y., Tang C.C., Chen W.F., Hsieh S.P., Hsieh C.S., Jean Y.H. Intra-articular magnesium sulfate (MgSO4) reduces experimental osteoarthritis and nociception: association with attenuation of N-methyl-D-aspartate (NMDA) receptor subunit 1 phosphorylation and apoptosis in rat chondrocytes. Osteoarthritis Cartilage. 2009;17(11):1485–1493. doi: 10.1016/j.joca.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 285.Shimaya M., Muneta T., Ichinose S., Tsuji K., Sekiya I. Magnesium enhances adherence and cartilage formation of synovial mesenchymal stem cells through integrins. Osteoarthritis Cartilage. 2010;18(10):1300–1309. doi: 10.1016/j.joca.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 286.Feyerabend F., Witte F., Kammal M., Willumeit R. Unphysiologically high magnesium concentrations support chondrocyte proliferation and redifferentiation. Tissue Eng. 2006;12(12):3545–3556. doi: 10.1089/ten.2006.12.3545. [DOI] [PubMed] [Google Scholar]
- 287.Cheng P., Han P., Zhao C., Zhang S., Wu H., Ni J., Hou P., Zhang Y., Liu J., Xu H., Liu S., Zhang X., Zheng Y., Chai Y. High-purity magnesium interference screws promote fibrocartilaginous entheses regeneration in the anterior cruciate ligament reconstruction rabbit model via accumulation of BMP-2 and VEGF. Biomaterials. 2016;81:14–26. doi: 10.1016/j.biomaterials.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 288.Diekmann J., Bauer S., Weizbauer A., Willbold E., Windhagen H., Helmecke P., Lucas A., Reifenrath J., Nolte I., Ezechieli M. Examination of a biodegradable magnesium screw for the reconstruction of the anterior cruciate ligament: a pilot in vivo study in rabbits. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;59:1100–1109. doi: 10.1016/j.msec.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 289.Fu Y.M., Yin Y., Guan J.W., Ji Q.B., Wang Y., Zhang Q. The evaluation of a degradable Magnesium alloy Bio-Transfix nail system compounded with bone morphogenetic protein-2 in a beagle anterior cruciate ligament reconstruction model. J. Biomater. Appl. 2019;34(5):687–698. doi: 10.1177/0885328219865069. [DOI] [PubMed] [Google Scholar]
- 290.Wang J., Xu J., Fu W., Cheng W., Chan K., Yung P.S., Qin L. Biodegradable magnesium screws accelerate fibrous tissue mineralization at the tendon-bone insertion in anterior cruciate ligament reconstruction model of rabbit. Sci. Rep. 2017;7:40369. doi: 10.1038/srep40369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Park K.S., Kim B.J., Lih E., Park W., Lee S.H., Joung Y.K., Han D.K. Versatile effects of magnesium hydroxide nanoparticles in PLGA scaffold-mediated chondrogenesis. Acta Biomater. 2018;73:204–216. doi: 10.1016/j.actbio.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 292.Condello V., Filardo G., Madonna V., Andriolo L., Screpis D., Bonomo M., Zappia M., Dei Giudici L., Zorzi C. Use of a biomimetic scaffold for the treatment of osteochondral lesions in early osteoarthritis. BioMed Res. Int. 2018;2018:7937089. doi: 10.1155/2018/7937089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Koerner J.D., Vives M.J., O'Connor J.P., Chirichella P., Breitbart E.A., Chaudhary S.B., Uko L., Subramanian S., Fritton J.C., Benevenia J., Lin S.S. Zinc has insulin-mimetic properties which enhance spinal fusion in a rat model. Spine J. 2016;16(6):777–783. doi: 10.1016/j.spinee.2016.01.190. [DOI] [PubMed] [Google Scholar]
- 294.Rodríguez J.P., Rosselot G. Effects of zinc on cell proliferation and proteoglycan characteristics of epiphyseal chondrocytes. J. Cell. Biochem. 2001;82(3):501–511. doi: 10.1002/jcb.1178. [DOI] [PubMed] [Google Scholar]
- 295.Wang X., Fosmire G.J., Gay C.V., Leach R.M., Jr. Short-term zinc deficiency inhibits chondrocyte proliferation and induces cell apoptosis in the epiphyseal growth plate of young chickens. J. Nutr. 2002;132(4):665–673. doi: 10.1093/jn/132.4.665. [DOI] [PubMed] [Google Scholar]
- 296.Lee S.Y., Lee S.H., Jhun J., Seo H.B., Jung K.A., Yang C.W., Park S.H., Cho M.L. A combination with probiotic complex, zinc, and coenzyme Q10 attenuates autoimmune arthritis by regulation of Th17/treg balance. J. Med. Food. 2018;21(1):39–46. doi: 10.1089/jmf.2017.3952. [DOI] [PubMed] [Google Scholar]
- 297.Kwon J.Y., Lee S.H., Jhun J., Choi J., Jung K., Cho K.H., Kim S.J., Yang C.W., Park S.H., Cho M.L. The combination of probiotic complex, rosavin, and zinc improves pain and cartilage destruction in an osteoarthritis rat model. J. Med. Food. 2018;21(4):364–371. doi: 10.1089/jmf.2017.4034. [DOI] [PubMed] [Google Scholar]
- 298.Huang T.C., Chang W.T., Hu Y.C., Hsieh B.S., Cheng H.L., Yen J.H., Chiu P.R., Chang K.L. Zinc protects articular chondrocytes through changes in Nrf2-mediated antioxidants, cytokines and matrix metalloproteinases. Nutrients. 2018;10(4) doi: 10.3390/nu10040471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Khader A., Arinzeh T.L. Biodegradable zinc oxide composite scaffolds promote osteochondral differentiation of mesenchymal stem cells. Biotechnol. Bioeng. 2020;117(1):194–209. doi: 10.1002/bit.27173. [DOI] [PubMed] [Google Scholar]
- 300.Biber R., Pauser J., Gesslein M., Bail H.J. Magnesium-based absorbable metal screws for intra-articular fracture fixation. Case Rep. Orthop. 2016;2016:9673174. doi: 10.1155/2016/9673174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Aktan C., Ertan M.B., Turan A., Kose O. Fixation of small osteochondral fragments in a comminuted distal humerus fracture with magnesium bioabsorbable screws: a case report. Cureus. 2018;10(12) doi: 10.7759/cureus.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Sculean A., Gruber R., Bosshardt D.D. Soft tissue wound healing around teeth and dental implants. J. Clin. Periodontol. 2014;41(Suppl 15) doi: 10.1111/jcpe.12206. S6-22. [DOI] [PubMed] [Google Scholar]
- 303.Stechmiller J.K. Understanding the role of nutrition and wound healing. Nutr. Clin. Pract. 2010;25(1):61–68. doi: 10.1177/0884533609358997. [DOI] [PubMed] [Google Scholar]
- 304.Denda M., Katagiri C., Hirao T., Maruyama N., Takahashi M. Some magnesium salts and a mixture of magnesium and calcium salts accelerate skin barrier recovery. Arch. Dermatol. Res. 1999;291(10):560–563. doi: 10.1007/s004030050454. [DOI] [PubMed] [Google Scholar]
- 305.Ogawa Y., Kawamura T., Shimada S. Zinc and skin biology. Arch. Biochem. Biophys. 2016;611:113–119. doi: 10.1016/j.abb.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 306.Taylor K.A., Pugh N. The contribution of zinc to platelet behaviour during haemostasis and thrombosis. Metallomics. 2016;8(2):144–155. doi: 10.1039/c5mt00251f. [DOI] [PubMed] [Google Scholar]
- 307.Lin P.-H., Sermersheim M., Li H., Lee P.H.U., Steinberg S.M., Ma J. Zinc in wound healing modulation. Nutrients. 2017;10(1):16. doi: 10.3390/nu10010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Gawronska-Kozak B. Scarless skin wound healing in FOXN1 deficient (nude) mice is associated with distinctive matrix metalloproteinase expression. Matrix Biol. 2011;30(4):290–300. doi: 10.1016/j.matbio.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Glutsch V., Hamm H., Goebeler M. Zinc and skin: an update. J. Dtsch Dermatol. Ges. 2019;17(6):589–596. doi: 10.1111/ddg.13811. [DOI] [PubMed] [Google Scholar]
- 310.O'Connor S., Murphy S. Chronic venous leg ulcers: is topical zinc the answer? A review of the literature. Adv. Skin Wound Care. 2014;27(1):35–44. doi: 10.1097/01.Asw.0000439173.79541.96. quiz 45-6. [DOI] [PubMed] [Google Scholar]
- 311.Lansdown A.B., Mirastschijski U., Stubbs N., Scanlon E., Agren M.S. Zinc in wound healing: theoretical, experimental, and clinical aspects. Wound Repair Regen. 2007;15(1):2–16. doi: 10.1111/j.1524-475X.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 312.Heintschel M., Heuberger R. The potential role of zinc supplementation on pressure injury healing in older adults: a review of the literature. Wounds. 2017;29(2):56–61. [PubMed] [Google Scholar]
- 313.Cereda E., Gini A., Pedrolli C., Vanotti A. Disease-specific, versus standard, nutritional support for the treatment of pressure ulcers in institutionalized older adults: a randomized controlled trial. J. Am. Geriatr. Soc. 2009;57(8):1395–1402. doi: 10.1111/j.1532-5415.2009.02351.x. [DOI] [PubMed] [Google Scholar]
- 314.Momen-Heravi M., Barahimi E., Razzaghi R., Bahmani F., Gilasi H.R., Asemi Z. The effects of zinc supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Wound Repair Regen. 2017;25(3):512–520. doi: 10.1111/wrr.12537. [DOI] [PubMed] [Google Scholar]
- 315.Kurmis R., Greenwood J., Aromataris E. Trace element supplementation following severe burn injury: a systematic review and meta-analysis. J. Burn Care Res. 2016;37(3):143–159. doi: 10.1097/bcr.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 316.Wang X.X., Zhang M.J., Li X.B. [Advances in the research of zinc deficiency and zinc supplementation treatment in patients with severe burns] Zhonghua Shaoshang Zazhi. 2018;34(1):57–59. doi: 10.3760/cma.j.issn.1009-2587.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 317.Khan M.I., Behera S.K., Paul P., Das B., Suar M., Jayabalan R., Fawcett D., Poinern G.E.J., Tripathy S.K., Mishra A. Biogenic Au@ZnO core-shell nanocomposites kill Staphylococcus aureus without provoking nuclear damage and cytotoxicity in mouse fibroblasts cells under hyperglycemic condition with enhanced wound healing proficiency. Med. Microbiol. Immunol. 2019;208(5):609–629. doi: 10.1007/s00430-018-0564-z. [DOI] [PubMed] [Google Scholar]
- 318.Król A., Pomastowski P., Rafińska K., Railean-Plugaru V., Buszewski B. Zinc oxide nanoparticles: synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017;249:37–52. doi: 10.1016/j.cis.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 319.Xie Y., He Y., Irwin P.L., Jin T., Shi X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011;77(7):2325–2331. doi: 10.1128/aem.02149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Li X., Gao P., Wan P., Pei Y., Shi L., Fan B., Shen C., Xiao X., Yang K., Guo Z. Novel bio-functional magnesium coating on porous Ti6Al4V orthopaedic implants: in vitro and in vivo study. Sci. Rep. 2017;7:40755. doi: 10.1038/srep40755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Li W., Yuan F., Bai J., Cheng J., Li H., Zheng J., Bai W., Lyu P. In vivo evaluation of bending strengths and degradation rates of different magnesium pin designs for oral stapler. J. Appl. Biomater. Funct. Mater. 2020:18. doi: 10.1177/2280800019836400. [DOI] [PubMed] [Google Scholar]
- 322.Lin D.J., Hung F.Y., Yeh M.L., Lui T.S. Microstructure-modified biodegradable magnesium alloy for promoting cytocompatibility and wound healing in vitro. J. Mater. Sci. Mater. Med. 2015;26(10):248. doi: 10.1007/s10856-015-5572-6. [DOI] [PubMed] [Google Scholar]
- 323.Coger V., Million N., Rehbock C., Sures B., Nachev M., Barcikowski S., Wistuba N., Strauss S., Vogt P.M. Tissue concentrations of zinc, iron, copper, and magnesium during the phases of full thickness wound healing in a rodent model. Biol. Trace Elem. Res. 2019;191(1):167–176. doi: 10.1007/s12011-018-1600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Sasaki Y., Sathi G.A., Yamamoto O. Wound healing effect of bioactive ion released from Mg-smectite. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;77:52–57. doi: 10.1016/j.msec.2017.03.236. [DOI] [PubMed] [Google Scholar]
- 325.Robinson D.A., Griffith R.W., Shechtman D., Evans R.B., Conzemius M.G. In vitro antibacterial properties of magnesium metal against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Acta Biomater. 2010;6(5):1869–1877. doi: 10.1016/j.actbio.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 326.Rahim M.I., Eifler R., Rais B., Mueller P.P. Alkalization is responsible for antibacterial effects of corroding magnesium. J. Biomed. Mater. Res. 2015;103(11):3526–3532. doi: 10.1002/jbm.a.35503. [DOI] [PubMed] [Google Scholar]
- 327.Lellouche J., Kahana E., Elias S., Gedanken A., Banin E. Antibiofilm activity of nanosized magnesium fluoride. Biomaterials. 2009;30(30):5969–5978. doi: 10.1016/j.biomaterials.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 328.Chakravarty S., Melton C.N., Bailin A., Yahr T.L., Anderson G.G. Pseudomonas aeruginosa magnesium transporter MgtE inhibits type III secretion system gene expression by stimulating rsmYZ transcription. J. Bacteriol. 2017;199(23) doi: 10.1128/jb.00268-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Pastorfide G.B., Gorgonio N.M., Ganzon A.R., Alberto R.M. Zinc chloride spray--magnesium hydroxide ointment dual topical regimen in the treatment of obstetric and gynecologic incisional wounds. Clin. Therapeut. 1989;11(2):258–263. [PubMed] [Google Scholar]
- 330.Hu C., Zhang F., Kong Q., Lu Y., Zhang B., Wu C., Luo R., Wang Y. Synergistic chemical and photodynamic antimicrobial therapy for enhanced wound healing mediated by multifunctional light-responsive nanoparticles. Biomacromolecules. 2019;20(12):4581–4592. doi: 10.1021/acs.biomac.9b01401. [DOI] [PubMed] [Google Scholar]
- 331.Wilkinson H.N., Upson S.E., Banyard K.L., Knight R., Mace K.A., Hardman M.J. Reduced iron in diabetic wounds: an oxidative stress-dependent role for STEAP3 in extracellular matrix deposition and remodeling. J. Invest. Dermatol. 2019;139(11):2368–2377. doi: 10.1016/j.jid.2019.05.014. e7. [DOI] [PubMed] [Google Scholar]
- 332.Recalcati S., Gammella E., Buratti P., Doni A., Anselmo A., Locati M., Cairo G. Macrophage ferroportin is essential for stromal cell proliferation in wound healing. Haematologica. 2019;104(1):47–58. doi: 10.3324/haematol.2018.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Wilkinson H.N., Roberts E.R., Stafford A.R., Banyard K.L., Matteucci P., Mace K.A., Hardman M.J. Tissue iron promotes wound repair via M2 macrophage polarization and the chemokine (C-C motif) ligands 17 and 22. Am. J. Pathol. 2019;189(11):2196–2208. doi: 10.1016/j.ajpath.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 334.Sindrilaru A., Peters T., Wieschalka S., Baican C., Baican A., Peter H., Hainzl A., Schatz S., Qi Y., Schlecht A., Weiss J.M., Wlaschek M., Sunderkötter C., Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Invest. 2011;121(3):985–997. doi: 10.1172/jci44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Ganz T. Macrophages and iron metabolism. Microbiol. Spectr. 2016;4(5) doi: 10.1128/microbiolspec.MCHD-0037-2016. [DOI] [PubMed] [Google Scholar]
- 336.Coger V., Million N., Rehbock C., Sures B., Nachev M., Barcikowski S., Wistuba N., Strauß S., Vogt P.M. Tissue concentrations of zinc, iron, copper, and magnesium during the phases of full thickness wound healing in a rodent model. Biol. Trace Elem. Res. 2019;191(1):167–176. doi: 10.1007/s12011-018-1600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ezraty B., Barras F. The 'liaisons dangereuses' between iron and antibiotics. FEMS Microbiol. Rev. 2016;40(3):418–435. doi: 10.1093/femsre/fuw004. [DOI] [PubMed] [Google Scholar]
- 338.Moniri M., Boroumand Moghaddam A., Azizi S., Abdul Rahim R., Zuhainis Saad W., Navaderi M., Arulselvan P., Mohamad R. Molecular study of wound healing after using biosynthesized BNC/Fe(3)O(4) nanocomposites assisted with a bioinformatics approach. Int. J. Nanomed. 2018;13:2955–2971. doi: 10.2147/ijn.S159637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Wlaschek M., Singh K., Sindrilaru A., Crisan D., Scharffetter-Kochanek K. Iron and iron-dependent reactive oxygen species in the regulation of macrophages and fibroblasts in non-healing chronic wounds. Free Radic. Biol. Med. 2019;133:262–275. doi: 10.1016/j.freeradbiomed.2018.09.036. [DOI] [PubMed] [Google Scholar]
- 340.Ferris A.E., Harding K.G. An overview of the relationship between anaemia, iron, and venous leg ulcers. Int. Wound J. 2019;16(6):1323–1329. doi: 10.1111/iwj.13192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Kaptanoglu E., Beskonakli E., Solaroglu I., Kilinc A., Taskin Y. Magnesium sulfate treatment in experimental spinal cord injury: emphasis on vascular changes and early clinical results. Neurosurg. Rev. 2003;26(4):283–287. doi: 10.1007/s10143-003-0272-y. [DOI] [PubMed] [Google Scholar]
- 342.Libien J., Sacktor T.C., Kass I.S. Magnesium blocks the loss of protein kinase C, leads to a transient translocation of PKC(alpha) and PKC(epsilon), and improves recovery after anoxia in rat hippocampal slices. Brain Res. Mol. Brain Res. 2005;136(1–2):104–111. doi: 10.1016/j.molbrainres.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 343.Kirkland A.E., Sarlo G.L., Holton K.F. The role of magnesium in neurological disorders. Nutrients. 2018;10(6):730. doi: 10.3390/nu10060730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Clerc P., Young C.A., Bordt E.A., Grigore A.M., Fiskum G., Polster B.M. Magnesium sulfate protects against the bioenergetic consequences of chronic glutamate receptor stimulation. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0079982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Xue W., You J., Su Y., Wang Q. The effect of magnesium deficiency on neurological disorders: a narrative review article. Iran. J. Public Health. 2019;48(3):379–387. [PMC free article] [PubMed] [Google Scholar]
- 346.Sheldon S.A., Aleman M., Costa L.R.R., Santoyo A.C., Howey Q., Madigan J.E. Intravenous infusion of magnesium sulfate and its effect on horses with trigeminal-mediated headshaking. J. Vet. Intern. Med. 2019;33(2):923–932. doi: 10.1111/jvim.15410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Sheldon S.A., Aleman M., Costa L.R.R., Weich K., Howey Q., Madigan J.E. Effects of magnesium with or without boron on headshaking behavior in horses with trigeminal-mediated headshaking. J. Vet. Intern. Med. 2019;33(3):1464–1472. doi: 10.1111/jvim.15499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Zhang Q., Ji L., Zheng H., Li Q., Xiong Q., Sun W., Zhu X., Li Y., Lu B., Liu X., Zhang S. Low serum phosphate and magnesium levels are associated with peripheral neuropathy in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2018;146:1–7. doi: 10.1016/j.diabres.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 349.Fei J., Wen X., Lin X., Saijilafu, Wang W., Ren O., Chen X., Tan L., Yang K., Yang H., Yang L. Biocompatibility and neurotoxicity of magnesium alloys potentially used for neural repairs. Mater. Sci. Eng. C. 2017;78:1155–1163. doi: 10.1016/j.msec.2017.04.106. [DOI] [PubMed] [Google Scholar]
- 350.Chen Y.J., Cheng F.C., Chen C.J., Su H.L., Sheu M.L., Sheehan J., Pan H.C. Down-regulated expression of magnesium transporter genes following a high magnesium diet attenuates sciatic nerve crush injury. Neurosurgery. 2019;84(4):965–976. doi: 10.1093/neuros/nyy120. [DOI] [PubMed] [Google Scholar]
- 351.Pan H.C., Sheu M.L., Su H.L., Chen Y.J., Chen C.J., Yang D.Y., Chiu W.T., Cheng F.C. Magnesium supplement promotes sciatic nerve regeneration and down-regulates inflammatory response. Magnes. Res. 2011;24(2):54–70. doi: 10.1684/mrh.2011.0280. [DOI] [PubMed] [Google Scholar]
- 352.Gougoulias N., Hatzisotiriou A., Kapoukranidou D., Albani M. Magnesium administration provokes motor unit survival, after sciatic nerve injury in neonatal rats. BMC Muscoskel. Disord. 2004;5(1):33. doi: 10.1186/1471-2474-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Jia S., Mou C., Ma Y., Han R., Li X. Magnesium regulates neural stem cell proliferation in the mouse hippocampus by altering mitochondrial function. Cell Biol. Int. 2016;40(4):465–471. doi: 10.1002/cbin.10569. [DOI] [PubMed] [Google Scholar]
- 354.Vennemeyer J.J., Hopkins T., Kuhlmann J., Heineman W.R., Pixley S.K. Effects of elevated magnesium and substrate on neuronal numbers and neurite outgrowth of neural stem/progenitor cells in vitro. Neurosci. Res. 2014;84:72–78. doi: 10.1016/j.neures.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 355.Hopkins T.M., Little K.J., Vennemeyer J.J., Triozzi J.L., Turgeon M.K., Heilman A.M., Minteer D., Marra K., Hom D.B., Pixley S.K. Short and long gap peripheral nerve repair with magnesium metal filaments. J. Biomed. Mater. Res. 2017;105(11):3148–3158. doi: 10.1002/jbm.a.36176. [DOI] [PubMed] [Google Scholar]
- 356.Bataineh A.B. Sensory nerve impairment following mandibular third molar surgery. J. Oral Maxillofac. Surg. 2001;59(9):1012–1017. doi: 10.1053/joms.2001.25827. discussion 1017. [DOI] [PubMed] [Google Scholar]
- 357.Putra N.E., Mirzaali M.J., Apachitei I., Zhou J., Zadpoor A.A. Multi-material additive manufacturing technologies for Ti-, Mg-, and Fe-based biomaterials for bone substitution. Acta Biomater. 2020;109:1–20. doi: 10.1016/j.actbio.2020.03.037. [DOI] [PubMed] [Google Scholar]
- 358.Wen P., Qin Y., Chen Y., Voshage M., Jauer L., Poprawe R., Schleifenbaum J.H. Laser additive manufacturing of Zn porous scaffolds: shielding gas flow, surface quality and densification. J. Mater. Sci. Technol. 2019;35(2):368–376. doi: 10.1016/j.jmst.2018.09.065. [DOI] [Google Scholar]
- 359.Chou D.-T., Wells D., Hong D., Lee B., Kuhn H., Kumta P.N. Novel processing of iron–manganese alloy-based biomaterials by inkjet 3-D printing. Acta Biomater. 2013;9(10):8593–8603. doi: 10.1016/j.actbio.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 360.Yang C., Huan Z., Wang X., Wu C., Chang J. 3D printed Fe scaffolds with HA nanocoating for bone regeneration. ACS Biomater. Sci. Eng. 2018;4 doi: 10.1021/acsbiomaterials.7b00885. [DOI] [PubMed] [Google Scholar]
- 361.Qin Y., Wen P., Guo H., Xia D., Zheng Y., Jauer L., Poprawe R., Voshage M., Schleifenbaum J.H. Additive manufacturing of biodegradable metals: current research status and future perspectives. Acta Biomater. 2019;98:3–22. doi: 10.1016/j.actbio.2019.04.046. [DOI] [PubMed] [Google Scholar]