ABSTRACT
Candida albicans is a major fungal pathogen of humans. It exists as a commensal in the oral cavity, gut or genital tract of most individuals, constrained by the local microbiota, epithelial barriers and immune defences. Their perturbation can lead to fungal outgrowth and the development of mucosal infections such as oropharyngeal or vulvovaginal candidiasis, and patients with compromised immunity are susceptible to life-threatening systemic infections. The importance of the interplay between fungus, host and microbiota in driving the transition from C. albicans commensalism to pathogenicity is widely appreciated. However, the complexity of these interactions, and the significant impact of fungal, host and microbiota variability upon disease severity and outcome, are less well understood. Therefore, we summarise the features of the fungus that promote infection, and how genetic variation between clinical isolates influences pathogenicity. We discuss antifungal immunity, how this differs between mucosae, and how individual variation influences a person's susceptibility to infection. Also, we describe factors that influence the composition of gut, oral and vaginal microbiotas, and how these affect fungal colonisation and antifungal immunity. We argue that a detailed understanding of these variables, which underlie fungal-host-microbiota interactions, will present opportunities for directed antifungal therapies that benefit vulnerable patients.
Keywords: Candida, Candida infections, antifungal immunity, microbiota, mycobiota, fungus-host-microbiota interactions, patient variability, fungal variability, microbiota variability
The complexity and variability of FunHoMic interactions between the fungal pathogen, its human host and the Microbiota strongly influence the development and outcomes of the superficial and systemic Candida albicans infections that plague human health worldwide.
INTRODUCTION
Fungal pathogens have a major global impact upon human health. Estimates suggest that, at any given time, over a quarter of the world's population have a fungal infection of the skin, that 75% of women suffer at least one episode of vulvovaginal candidiasis during their lifetime, and that over a million people die each year from an invasive fungal infection (Brown et al. 2012). Mortality rates for those suffering systemic fungal infections are unacceptably high, reaching 50% in many cases. This is because fungal infections are often difficult to diagnose, and are particularly challenging to treat (Perlroth, Choi and Spellberg 2007; Brown et al. 2012; Köhler, Casadevall and Perfect 2014). There is a clear and urgent medical need for more accurate diagnostics, for safer and more effective antifungal drugs, and for host-directed therapies. The search for antifungal drug targets is somewhat constrained by the fact that, as eukaryotes, fungi share fundamental mechanisms of cell growth and division with humans. The search for diagnostic markers that can distinguish infection from fungal commensalism is especially challenging. Therefore, the development of potent new clinical tools is dependent upon a comprehensive understanding of fungal pathogenicity and antifungal immunity.
Candida species are amongst the top fungal killers (Brown et al. 2012). Of these, Candida albicans remains the most common cause of life-threatening systemic candidiasis, although the frequent prophylactic use of azole antifungal drugs has led to the emergence of other Candida species with intrinsic resistance to these drugs (Nguyen et al. 1996; Silva et al. 2012; Chowdhary, Sharma and Meis 2017). Nevertheless, in this review we focus on C. albicans, because a combination of three main factors arguably makes this species unique amongst fungal pathogens: (a) its lifestyle as both a commensal and potent pathogen; (b) the range and frequency of infections that it causes; and (c) its pathobiology has been studied in greater depth than most other fungal pathogens.
Candida albicans is an opportunistic pathogen that exists as a commensal in most individuals, and is a frequent cause of mucosal and systemic infections (See The Fungus). Unlike most fungal pathogens, C. albicans is generally considered to be obligately associated with warm-blooded animals (Odds 1988). Environmental isolates of C. albicans continue to be reported (Bensasson et al. 2019; Maciel et al. 2019; Opulente et al. 2019). However, although the existence of an environmental reservoir cannot be excluded, it is apparently not necessary for human colonisation.
Candida albicans is transmitted vertically from mother to child, and infections arise predominantly from the endogenous microbiota rather than other sources (d Enfert 2009; Miranda et al. 2009; Zhai et al. 2020) (see The Microbiota). This contrasts with other major pathogens such as Aspergillus, Cryptococcus and Histoplasma species, which are fundamentally environmental fungi that have evolved traits that promote pathogenicity in humans, possibly through their transient passage in niches that have similarities with those encountered in the human host, for example, their association with rodents or contact and evasion of amoebic predation in the environment (Steenbergen, Shuman and Casadevall 2001; Malliaris, Steenbergen and Casadevall 2004; Van Waeyenberghe et al. 2013; Hillmann et al. 2015). Pneumocystis jirovecii is obligately associated with humans, but this major pathogen differs from C. albicans in that it is unable to thrive outside its host (Liu, Fahle and Kovacs 2018). Consequently, key aspects of Pneumocystis jirovecii biology remain unexplored. The lifestyle of C. albicans even differs considerably from its distant cousin, C. (Brunke and Hube 2013; Kasper, Seider and Hube 2015). Genetic evidence suggests that, although it is often presumed to be a human commensal such as C. albicans, C. glabrata seems to be only secondarily associated with humans and is likely to have environmental reservoirs (Gabaldón and Fairhead 2019).
The biology, epidemiology, pathogenicity and immunology of C. albicans have been studied in greater depth than for any other fungal pathogen. This depth of knowledge provides a strong platform for studies of the relationships between the fungal pathogen, host immunity and local microbiota that lie at the heart of fungal infection (Casadevall and Pirofski 1999, 2003, 2015; Jabra-Rizk et al. 2016) (Fig. 1). Other major fungal pathogens infect humans by different routes to C. albicans, but many principles that are emerging for C. albicans may be applicable to these pathogens. Therefore, we present underlying principles of C. albicans colonisation and infection, antifungal immune defences, and the protective properties of the local microbiota in the gastrointestinal (GI) tract, oral cavity and vagina. We also address the variability that influences the Fungus-Host-Microbiota interplay and how this impacts infection. A detailed understanding of this tripartite interplay is essential to optimise therapeutic strategies for individual patients (d Enfert 2009; Pirofski and Casadevall 2020).
Figure 1.
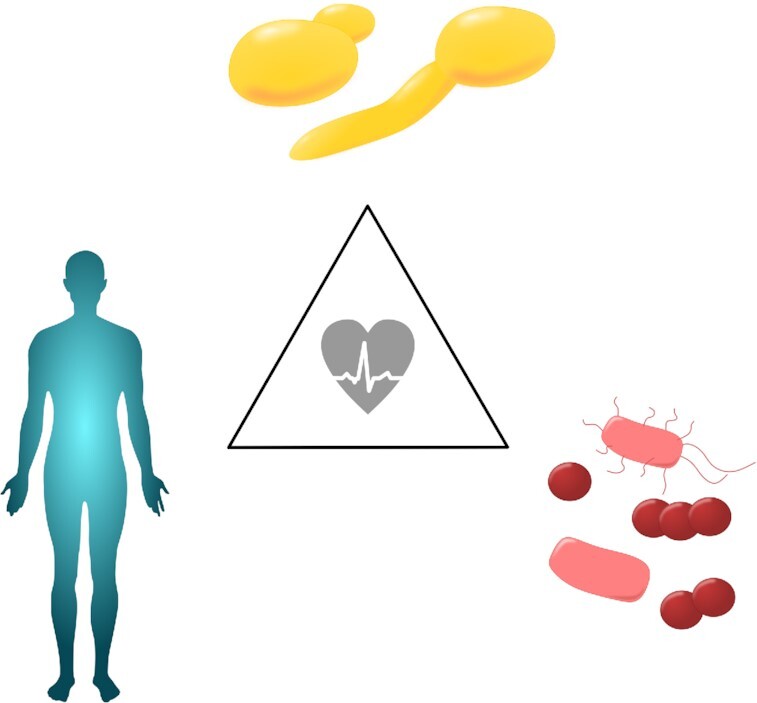
Three-way interactions between the fungus, the host and the local microbiota strongly influence the likelihood and severity of C. albicans infections. See text.
THE FUNGUS
C. albicans commensalism and pathogenicity
C. albicans frequently inhabits the oral, vaginal and GI mucosa of healthy individuals as a harmless commensal (Ghannoum et al. 2010; Drell et al. 2013; Nash et al. 2017) (Fig. 2). Indeed, C. albicans is present on the mucosa of most people in most human populations (Neville, d Enfert and Bougnoux 2015; Prieto et al. 2016; Mishra and Koh 2018). However, this fungus can cause infections if the local microbiota becomes perturbed, normal tissue barriers are weakened or immune defences become compromised.
Figure 2.
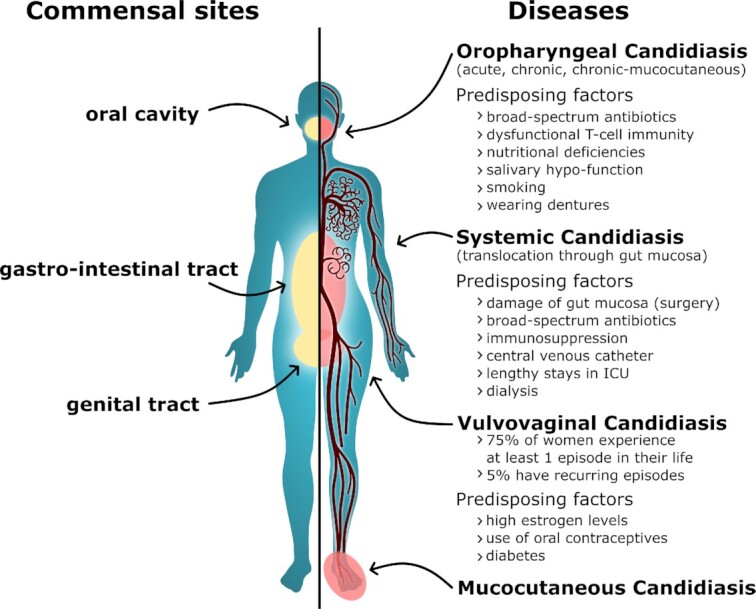
Sites of C. albicans commensalism and disease on the human body. Sites of C. albicans commensalism (left side) include the oral cavity, gastrointestinal tract (gut) and the genital tract. C. albicans can infect these sites (right side) to cause oropharyngeal or vulvovaginal candidiasis. C. albicans can also cause systemic infections of the blood and internal organs, which often arise via translocation of C. albicans from the gut into the bloodstream. Candida albicans also causes mucocutaneous infections of the skin and nails. Factors that predispose individuals to such infections are listed. See text.
Mucosal infections, characterised by fungal colonisation (i.e. overgrowth) associated with an inflammatory host response, are extremely common and can have a major impact upon the quality of life for many individuals (Fig. 2). For instance, most women of reproductive age (75%) will experience at least one episode of VVC (‘thrush’) in their lifetime, and up to 9% suffer from recurrent VVC, as defined by multiple episodes of vaginitis per annum (Foxman et al. 2013; Yano et al. 2019; Rosati, Bruno, Jaeger, Ten Oever et al. 2020). Risk factors for VVC include high estrogen levels, the use of oral contraceptives and uncontrolled diabetes. However, episodes can be idiopathic (i.e. of unknown cause) and VVC, unlike oral candidiasis, can occur in apparently healthy individuals (see Innate antifungal responses).
Oropharyngeal candidiasis (OPC) can broadly be classified into three main conditions, namely acute, chronic and chronic mucocutaneous candidiasis syndromes (Vila et al. 2020) (Fig. 2). Predisposing factors include nutritional deficiencies, local dysbiosis, salivary hypo-function, smoking, wearing dentures and dysfunctional T-cell immunity due to genetic alterations or other infections. Indeed, OPC is the most frequently diagnosed oral opportunistic infection in HIV-positive individuals and many acute cases are caused by broad-spectrum antibiotic treatments (Samaranayake 1992; Vila et al. 2020).
Life-threatening systemic C. albicans infections can arise when the fungus enters the bloodstream (Fig. 2). Candidaemia is the fourth most common nosocomial bloodstream infection in North America (Pfaller and Diekema 2010), but the incidence of invasive candidiasis in European countries is generally lower (Meyer et al. 2013; Yapar 2014). The presence of a central venous catheter, dialysis, antibiotic treatment, lengthy stays in intensive care units (ICUs), recent major surgery, and receiving total parenteral nutrition are among the predisposing factors for systemic candidiasis (Pappas et al. 2018). Most disseminated infections arise from Candida escaping the patient's own GI tract (Miranda et al. 2009; Gouba and Drancourt 2015; Zhai et al. 2020). Systemic infections arise when host defences are compromised by, for example, damage to the intestinal barrier (e.g. surgery or trauma), medically induced immunosuppression (corticosteroids or chemotherapy-induced neutropenia), or the use of broad-spectrum antibiotics (Pappas et al. 2018). A combination of these factors is typically needed to allow C. albicans to translocate from the gut (Koh et al. 2008; Papon, Bougnoux and d Enfert 2020). Once in the blood, C. albicans can disseminate to almost all organs including kidney, liver, and spleen (Pappas et al. 2018). The mortality rate for these infections, which varies across geographical regions, is reported to lie between 10% and 47% despite the availability of antifungal therapies (Brown et al. 2012). This is unacceptably high.
Clearly, knowledge about the factors and conditions that promote C. albicans commensalism or opportunism is important for an understanding of the mechanisms that underlie the transition from commensalism to pathogenicity. Much work has focussed on the virulence factors and fitness attributes that promote C. albicans infection (see Virulence Factors and Fitness attributes). However, the pathogenesis of C. albicans also depends on the host site of colonisation (Fidel et al. 2020). Candida albicans asymptomatically inhabits the oral mucosa and only causes infection when host defences are weakened. In contrast, C. albicans is an immunoreactive coloniser during vulvovaginal infection, eliciting host damage via a hyperactive immune response. Meanwhile, systemic infections are mostly nosocomial and are generally associated with predisposing conditions. The fungus is able to cause these different types of infection by tuning the expression of its arsenal of virulence factors and fitness attributes to the local niche.
Virulence factors
Cellular polymorphism
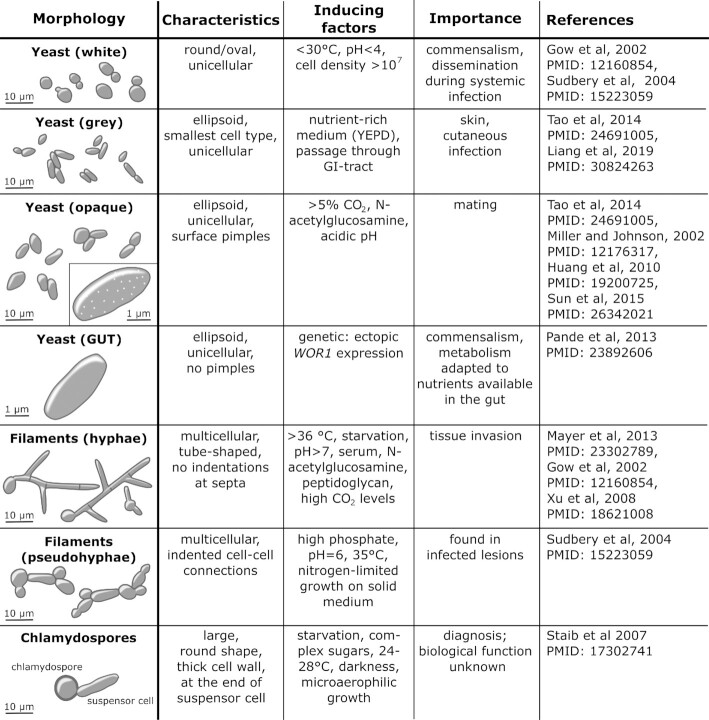
The polymorphic nature of C. albicans is integral to both commensalism and pathogenesis. This fungus is able to switch reversibly between different growth forms and morphologies (Noble, Gianetti and Witchley 2017) (Fig. 3). Depending upon the environmental conditions, C. albicans can grow as unicellular yeast cells, pseudohyphae, or true hyphae that lack invaginations at septal junctions (Sudbery, Gow and Berman 2004). Also, depending on the presence of certain environmental cues, C. albicans can undergo phenotypic switching to interchange reversibly between white, grey and opaque phenotypes, each of which displays distinct yeast cell and colony morphologies, and gene expression profiles. Furthermore, a gastrointestinally induced transition (GUT) phenotype has been described for C. albicans cells that ectopically overexpress the Wor1 regulator which, together with Efg1, controls white-grey-opaque switching (Pande, Chen and Noble 2013). Phenotypic switching is a strictly regulated process that seems to be associated with commensalism, host niche adaptation, mating, immune evasion and virulence (Miller and Johnson 2002; Morschhäuser 2010; Pande, Chen and Noble 2013; Xie et al. 2013; Tao et al. 2014). Finally, C. albicans can differentiate to form chlamydospores, enlarged thick-walled cells, under nutrient limitation, low temperature and microaerophilia (Staib and Morschhäuser 2007; Böttcher et al. 2016) (Fig. 3).
Figure 3.
Candida albicans is polymorphic, displaying a range of cellular growth forms. C. albicans yeast cells can undergo phenotypic switching between white, grey and opaque growth forms that present with different shapes and cell surface characteristics (Gow, Brown and Odds 2002; Sudbery, Gow and Berman 2004; Xu et al. 2008; Huang et al. 2009; Mayer, Wilson and Hube 2013; Tao et al. 2014; Sun et al. 2015). These forms are induced in response to different environmental inputs, and hence are associated with different types of infection (Gow, Brown and Odds 2002). Significantly, the opaque form is associated with efficient mating in C. albicans (Miller and Johnson 2002), with grey cells displaying an intermediate mating competence between opaque and white cells (Tao et al. 2014). The gastrointestinally induced transition (GUT) phenotype is observed in C. albicans cells that ectopically express WOR1 (Pande, Chen and Noble 2013), a key regulator of commensalism. The transition from (white) yeast cells to pseudohyphae or hyphae is stimulated by a wide variety of environmental inputs, which include elevated temperatures, pH and peptidoglycan. Pseudohyphae can be distinguished from hyphae on the basis of the position of the septal junction between a mother yeast cell and its filamentous daughter, and by the presence of invaginations at these septal junctions in pseudohyphae, but not hyphae (Merson-Davies and Odds 1989; Sudbery 2001; Sudbery, Gow and Berman 2004). Candida albicans can be induced to form chlamydospores under specific environmental conditions (Jansons and Nickerson 1970), but the biological significance of this growth form remains obscure (Staib and Morschhäuser 2007). See text.
Both yeast and hyphal morphologies are necessary for the full virulence of C. albicans (Lo et al. 1997; Murad et al.2001; Saville et al. 2003; Jacobsen et al. 2012) (Fig. 4). However, it is generally thought that yeast cells are well suited to dissemination, and hyphal cells to tissue invasion (Gow, Brown and Odds 2002). The yeast-to-hypha transition is accompanied by an extensive change in gene expression profile, in cell wall structure, and by the expression of many virulence factors (Jacobsen et al. 2012; Mayer, Wilson and Hube 2013; Chen et al. 2020). The change in morphology can be triggered by many environmental factors present in host niches, such as physiological temperatures (>36°C), starvation, an ambient pH of >7, the presence of serum, N-acetylglucosamine, or elevated CO2 levels (Mayer, Wilson and Hube 2013). Furthermore, hyphal development is triggered by the bacterial cell wall component, peptidoglycan (Xu et al. 2008), which is of particular relevance to fungus-host-microbiota interactions. Not surprisingly given the complexity of environmental inputs and cellular outputs, yeast-hypha morphogenesis is regulated by a complex signalling network that includes the cAMP-protein kinase A, Efg1, Cph1, Czf1, Hog1 and Nrg1 pathways (Basso et al. 2019; Kadosh 2019; Kornitzer 2019).
Figure 4.
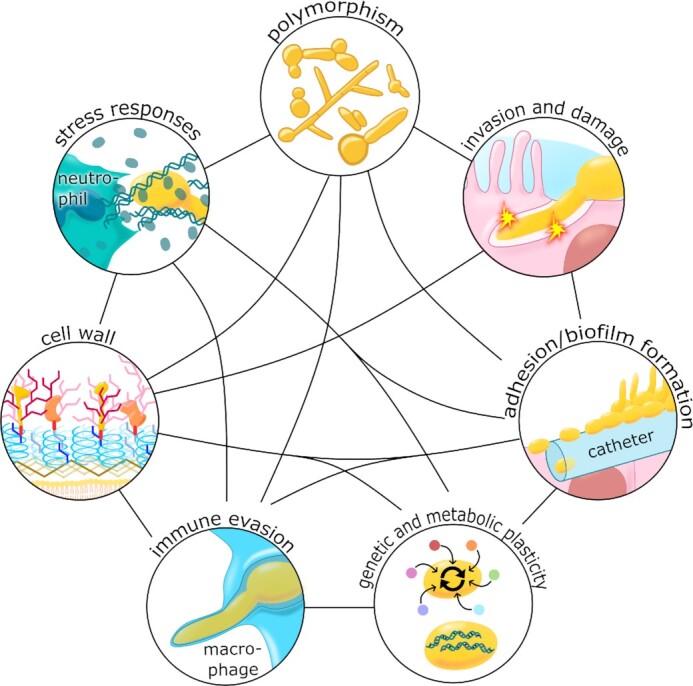
A combination of virulence factors and fitness attributes promote C. albicans virulence. Polymorphism: The ability of C. albicans to undergo morphological transitions allows it to adapt to different growth conditions, adhere to biotic and abiotic surfaces, invade cells and tissue, and escape from immune cells. Invasion and damage: A combination of induced endocytosis and active penetration promote fungal invasion of host tissues, and the accumulation of the toxin, candidalysin, in the invasion pocket leads to pore formation and host cell damage. Adhesion/biofilm formation: The battery of adhesins promotes fungal adhesions to biological and abiotic surfaces, which can lead to the development of biofilms, for example on medical devices such as catheters. Genetic and metabolic plasticity: Candida albicans displays a high degree of metabolic flexibility, which allows it to adapt rapidly to diverse host niches. This fungus also displays great genetic plasticity, which permits rapid evolutionary adaptation to selective pressures and stresses such as exposure to antifungal drugs. Stress responses: Candida albicans activates robust stress responses following exposure to host imposed stresses, including ROS and RNS, which enhances fungal survival following immune attack, for example. Cell wall: As well as maintaining cell morphology, the robust cell wall provides protection against host-imposed stresses including changes in osmolarity. Immune evasion: Candida albicans has evolved a variety of immune evasion strategies that include the modulation of PAMP exposure at the cell surface to evade immune recognition, and phagocytic escape mechanisms to evade killing by innate immune cells. See text.
During experimental colonisation of the murine GI tract, C. albicans was found to thrive in the yeast form (Vautier et al. 2015). The basis for the predominance of the yeast morphology during gut colonisation remains unclear, but unknown selective pressures favour growth in the yeast form during experimental GI colonisation in mice during GI dysbiosis (Tso et al. 2018). Furthermore, mucus covering the epithelium, tight junctions between epithelial cells, and the lamina propria serve as physical barriers that limit C. albicans translocation and dissemination from the gut (Yan, Yang and Tang 2013; Arevalo and Nobile 2020). Mucin, the main component of mucus, prevents hyphal formation (Kavanaugh et al. 2014) and reduces the adherence of C. albicans to epithelial cells (de Repentigny et al. 2000). Similarly, saliva can exert anti-Candida effects in the oral cavity (Hibino et al. 2009) (see Oral cavity). More recent work suggests that filamentous forms can exist in certain parts of the GI tract where the microenvironment favours hyphal development (Witchley et al. 2019). Only under certain circumstances, for example when a perturbed microbiota and a compromised immune system lose control over C. albicans growth (see The Host and The Microbiota), the fungus can switch from commensalism to pathogenicity (Gow et al. 2011).
Significantly, the host can exploit the yeast-to-hypha transition to discriminate between colonisation and infection. This involves a biphasic innate immune response at the epithelial barrier (Moyes et al. 2010; Roselletti et al. 2019). The first signalling event is triggered by fungal cell wall components, notably β-glucans and mannans, irrespective of cell morphology (Moyes et al. 2010). The second, danger response, is only induced once a high fungal burden is achieved, hypha formation occurs, and the hypha-associated toxin candidalysin is expressed (see Host damage) (Moyes et al. 2010, 2016). This leads to the secretion of pro-inflammatory cytokines and phagocyte infiltration, which promote fungal clearance. In addition, phagocytes can distinguish hyphae from yeast cells based on the shorter cell wall mannan fibrils of hyphal cells (Cheng et al. 2011). Macrophages also respond to hyphal load, in part through the degree of metabolic competition between host and pathogen, displaying reduced activation of the NLRP3-inflammasome pathway at low hyphal burdens (Tucey et al. 2020; Westman et al. 2020). Thus, while hypha formation is critical for invasion (see Invasion mechanisms), the host has developed mechanisms to recognise the invasive form of C. albicans. Therefore, hypha formation seems to be detrimental for C. albicans commensalism.
Adhesion to abiotic and biotic surfaces
Candida albicans cells can adhere to each other as well as to host cells and abiotic surfaces, such as catheters or dental implants, which promotes colonisation and the formation of biofilms (de Groot et al. 2013; Lohse et al. 2018) (Fig. 4). Candida albicans forms hyphae upon sensing contact to a surface (Kumamoto 2008) and hyphae express specific adhesins that promote adhesion to such surfaces (de Groot et al. 2013).
The Agglutinin-Like Sequence (ALS) genes represent one family of adhesins in C. albicans, some of which are morphogenetically regulated (Hoyer and Cota 2016). Analogous adhesin families are present in other pathogenic and non-pathogenic fungi (Butler et al. 2009). Als adhesins have a three-domain structure: the N-terminal ligand-binding domain (Lin et al. 2014); internal tandem repeats; and the C-terminal domain, which binds the cell wall via a modified glycosylphosphatidylinisotol (GPI)-anchor. In C. albicans, the ALS gene family has nine members, each of which displays a high degree of variability between alleles and strains, particularly in the length of the central repetitive domain (Hoyer and Cota 2016). Als3, the best-studied Als family member, has multiple functions. It binds heterogenous ligands including cadherins, ferritin and a Streptococcus gordonii surface protein (Phan et al. 2007; Almeida et al. 2008; Bamford et al. 2015). Als3 also acts as an invasin that promotes fungal invasion of host cells (Phan et al. 2007) and iron assimilation (Almeida et al. 2008). This makes Als3 an asset for the fungus during infection, but also a potential target for anti-Candida therapies (Edwards et al. 2018; Marc et al. 2018; Kioshima et al. 2019).
The hyphal wall protein 1 (Hwp1), is specifically expressed during hyphal growth (Staab, Ferrer and Sundstrom 1996), and is the founding member of a second family of five adhesins in C. albicans (de Groot et al. 2013). Members of the Hwp family are required for both virulence and mating. The N-terminus of Hwp1 is enriched in glutamine residues that become cross-linked to the host extracellular matrix by host transglutaminases (Staab et al. 1999). In contrast, Yeast wall protein 1 (Ywp1) appears to counteract adhesion leading to the release of yeast cells from surfaces, which might promote fungal dissemination during systemic candidiasis (Granger 2012).
A third family of putative adhesins is encoded by the twelve-member HYR gene family (de Groot et al. 2013). The founding member of this family, HYR1, like ALS3 and HWP1, is expressed during hyphal development (Bailey et al. 1996). This HYR family has been less well characterised than the ALS and HWP families. Nevertheless, it adds to the adhesins that C. albicans expresses to promote robust adhesion to each other, abiotic surfaces or the host.
The cell wall
Both cellular polymorphism and adhesion are intimately associated with the C. albicans cell wall, the organelle that maintains the morphology of the C. albicans cell and that supplies the scaffold for most adhesin proteins (Klis, de Groot and Hellingwerf 2001; de Groot et al. 2004; Gow, Latge and Munro 2017) (Fig. 4). The cell wall also provides osmotic stability and protects against environmental stresses. It is robust in exerting control of cell shape, and yet elastic during responses to acute osmotic stress (Ene et al. 2015). Furthermore, the cell wall is a highly flexible organelle, in that it displays a high capacity to adapt and remodel itself in response to environmental challenges or antifungal drugs (Sosinska et al. 2008; Ene et al. 2012; Childers et al. 2019).
The C. albicans cell wall is a two-layered structure. The inner layer consists of chitin, β-1,3- and β-1,6-glucans and mannoproteins. The outer layer is enriched in mannan fibrils that are anchored to mannoproteins cross-linked to the inner layer of the wall (Kapteyn et al. 2000; Gow et al. 2011; Gow, Latge and Munro 2017). Chitin comprises about 2%–3% of the mass of the yeast cell wall, but represents an important structural component that is essential for the integrity of the cell wall. The main structural polysaccharide of the C. albicans cell wall is β-glucan, which accounts for 50%–60% of the mass of the yeast cell wall (Shepherd 1987; Klis, de Groot and Hellingwerf 2001). The β-1,3-glucan network provides the platform for covalent attachment of chitin, β-1,6-glucan and mannoproteins.
Two main classes of cell wall mannoproteins have been defined in C. albicans. GPI-anchored proteins are the more abundant class. As their name suggests, these are linked via modified GPI anchors to β-1,6-glucan which, in turn, are covalently attached to β-1,3-glucan (Kapteyn et al. 2000). Pir proteins (proteins with internal repeats) are covalently attached to β-1,3-glucan directly (Kapteyn et al. 2000). C. albicans cell wall mannoproteins contribute 30–40% of the mass of the yeast cell wall (Kapteyn et al. 2000) and are adorned with N- and/or O-linked oligosaccharides. The O-linked oligosaccharides are often linked to serine-threonine-rich repeats (e.g. in ALS adhesins: see Adhesion to abiotic and biotic surfaces) and are thought to confer rod-like structures to these domains (Gatti et al. 1994). N-linked mannans are highly branched structures that form the fibrils in the outer layer of the wall (Gow, Latge and Munro 2017; Childers et al. 2019). The functions of about 70% of cell wall mannoproteins remain obscure, but some are known or suspected to be involved in the infection process (De Groot, Ram and Klis 2005; Richard and Plaine 2007).
The cell wall is an attractive target for antifungal therapy because it is essential for fungal viability and not present on human cells. Consequently, β-1,3-glucan synthesis is the target for a major class of antifungal drugs in clinical use—the echinocandins (Odds, Brown and Gow 2003). Significantly, in the context of this review, the cell wall is also the first point of direct contact with the host, and therefore a prime target for immune recognition (see Fungal recognition) (Netea et al. 2008; Erwig and Gow 2016).
Biofilm formation
Candida albicans can form florid biofilms on biological surfaces and also abiotic surfaces such as catheters, dentures and prosthetic joints (Fig. 4). Biofilms are a common source of nosocomial infection (Ramage et al. 2005; Nobile and Johnson 2015), and they increase therapeutic challenges by enhancing the resistance to antifungal drugs (Taff et al. 2013).
Biofilm formation is initiated by adhesion of C. albicans cells to the surface (see Adhesion to abiotic and biotic surfaces). Surface contact stimulates hyphal growth (see Cellular polymorphism), the development of the biofilm and the production of extracellular matrix, and the biofilm matures into an organised and robust structure (Nobile and Johnson 2015). Biofilm formation is a complex process that is controlled by a network of transcription factors and that integrates the expression of adhesins, cellular morphogenesis and the production of extracellular matrix. Accordingly, biofilm formation is controlled by a complex transcriptional network of over 1000 genes (Finkel and Mitchell 2011; Nobile et al. 2012; Lohse et al. 2018). These target genes include members of the ALS family, which are essential for biofilm formation and enhance aggregation between fungal cells via amyloid formation (Dehullu et al. 2019; Vida Ho et al. 2019).
Biofilm maturation is followed by the dispersal of yeast cells from the biofilm, which promotes fungal dissemination. Candida albicans cells dispersed from biofilms are distinct from planktonically grown yeast. These dispersed cells are transcriptionally reprogrammed to utilise alternative carbon sources and they acquire nutrients, such as zinc and amino acids, with higher efficiency (Uppuluri et al. 2018).
Candida albicans clinical isolates display a high degree of heterogeneity with respect to their capacity to form biofilms and the underlying regulatory network (Sherry et al. 2017; Huang et al. 2019), and biofilm-forming ability has been associated with high mortality rates in patients (Rajendran et al. 2016). In the clinical setting, the situation is further complicated by the formation of multispecies biofilms. For example, C. albicans is commonly associated with Streptococcus and Actinomyces species in dental samples, with Lactobacillus species in vaginal specimens, and with Pseudomonas in the lungs of cystic fibrosis patients (Hogan, Vik and Kolter 2004; Falagas, Betsi and Athanasiou 2006; Bamford et al. 2009; Bandara et al. 2009; Cruz et al. 2013; Bamford et al. 2015) (see Synergistic and antagonistic interactions between kingdoms). These inter-kingdom associations affect C. albicans growth, morphogenesis and drug resistance (Hogan, Vik and Kolter 2004).
Invasion mechanisms
The invasion of host cells and tissues provides an effective strategy to access more nutrients, avoid competition with other members of the microbiota, and potentially escape antimicrobial treatment (Fig. 4). Two distinct routes for the invasion of epithelia and endothelia are known for C. albicans: induced endocytosis and active penetration (Dalle et al. 2010; Wächtler et al. 2012). Induced endocytosis is mediated by the fungal proteins Ssa1 and Als3 (the adhesin-invasin, mentioned above), both of which are present on the cell wall. These proteins bind to E- and N- cadherins on epithelial and endothelial cells, as well as to the epithelial growth factor receptor of oral epithelial cells, to induce the uptake of fungal cells through remodelling of the host cytoskeleton (Phan et al. 2007; Moreno-Ruiz et al. 2009; Sun et al. 2010; Solis et al. 2017). Active penetration is achieved through the growth of hyphae into host tissue. This is the dominant route of fungal invasion into oral epithelial cells and the only observed route in enterocytes (Dalle et al. 2010; Wächtler et al. 2012).
As stated, the GI tract is a major reservoir for resident C. albicans (Nucci and Anaissie 2001; Gouba and Drancourt 2015), and hence fungal translocation across intestinal barriers is a common source of systemic candidiasis. This translocation can be promoted by injury, GI pathologies or medical interventions. Nevertheless, the translocation of C. albicans cells through enterocytes in a transcellular manner, and subsequent necrotic host cell death, is a major mechanism by which the fungus crosses the epithelial barrier (Allert et al. 2018). C. albicans directs physical force against cell membranes to stretch and rupture host cell membranes via a combination of hyphal growth and secreted virulence factors (Wächtler et al. 2012). Meanwhile, host cells employ several mechanisms to expand and repair membranes to limit this damage (Westman, Hube and Fairn 2019). This leads to the formation of the so-called ‘invasion pocket’ where the invading hypha is surrounded by host membrane (Moyes et al. 2016). The confined space around the hypha, within the invasion pocket, permits the accumulation of C. albicans secreted virulence factors to high local concentrations that cause further damage and stress to the host (Dalle et al. 2010; Moyes et al. 2016; Allert et al. 2018).
Host damage
The ability to damage host cells provides C. albicans with access to cytoplasmic nutrients, and the fungus possesses an extensive weaponry to impose damage (Fig. 4). Damaging factors that accumulate in the invasion pocket include secreted hydrolases such as phospholipase B1, lipases and secreted aspartic proteases (Saps) that degrade host membranes, proteins and extracellular matrix releasing nutrients (Mukherjee et al. 2001; Naglik, Challacombe and Hube 2003; Schofield et al. 2005). Candida albicans also expresses candidalysin—a pore forming α-helical peptide toxin that is encoded by the ECE1 gene (Moyes et al. 2016). Pores formed in the host cell membrane by candidalysin probably leak cytoplasmic contents into the invasion pocket, thereby providing additional nutrients for the fungus. This may include access to essential micronutrients such as iron and zinc. Specific proteins bind these micronutrients, which are then endocytosed or transported across the fungal cell membrane via specific transporters. For example, members of the Rbt5-family transport heme across the cell wall (Kuznets et al. 2014; Nasser et al. 2016). Also, zinc is acquired via the zincophore Pra1 (pH-regulated antigen 1), which is released into the extracellular space and then, when loaded with zinc, is transported back into the fungus by the zinc transporter Ztr1 (Citiulo et al. 2012).
Fitness attributes
Fitness attributes are factors that promote fungal virulence by enhancing the physiological robustness of the fungus in host niches, rather than by interacting directly with the host. In C. albicans, fitness attributes include metabolic flexibility combined with potent nutrient acquisition systems, and robust stress response mechanisms (Mayer, Wilson and Hube 2013; Brown, Budge et al. 2014; Brown, Brown, et al. 2014). These promote the success of C. albicans both as a commensal and as a pathogen of humans.
Flexible metabolic adaptation
Metabolic adaptability is critical during C. albicans transitions between commensalism and pathogenicity (Fig. 4). This was highlighted by an elegant screen for regulatory circuitry that drives the commensal and pathogenic states in C. albicans (Pérez, Kumamoto and Johnson 2013). Much of this circuitry is involved in the regulation of metabolism. Metabolic regulation in C. albicans is integrated with the control of virulence factors and stress resistance through major regulatory hubs such as Efg1, Tup1, Nrg1, Hog1 and Gcn4 (Murad et al. 2001; Tripathi et al. 2002; Doedt et al. 2004; Alonso-Monge et al. 2009). Therefore, metabolic adaptation is essential for commensalism and virulence, and is intimately linked with other pathogenicity traits (Mayer, Wilson and Hube 2013; Brown, Brown, et al. 2014).
Glucose is a preferred carbon source for C. albicans, but under glucose-limiting conditions, such as in the colon or after entrapment in the phagosome, C. albicans tunes its metabolism to feed on alternative carbon sources (Lorenz, Bender and Fink 2004; Barelle et al. 2006). Even when glucose becomes available, C. albicans can simultaneously utilise alternative carbon sources through multiple pathways (Sandai et al. 2012; Childers et al. 2016). This metabolic flexibility allows the fungus to adapt to contrasting host niches. Significantly, it also influences the tolerance of C. albicans to antifungal drugs and environmental stresses (Ene et al. 2012). For example, growth on lactate protects against osmotic and cell wall stresses while utilisation of amino acids and N-acetylglucosamine (GlcNAc) increases fungal resistance to reactive oxygen and nitrogen species (ROS and RNS, respectively) (Williams and Lorenz 2020). These alternative carbon sources appear to serve as niche-specific signals that prime the fungus for impending challenges, pointing to the dexterity of C. albicans not only to adapt, but also to anticipate, local stress conditions (Brown, Budge et al. 2014; Alistair J P Brown et al. 2019; Williams and Lorenz 2020). The metabolic flexibility of C. albicans extends well beyond carbon metabolism to include nitrogen, phosphate and micronutrient assimilation (Lorenz, Bender and Fink 2004; Yin et al. 2004; Vylkova et al. 2011; Ene et al. 2014; Ikeh et al. 2016).
Micronutrients, such as iron and zinc, are essential for structural integrity and physiological processes in C. albicans. However, in response to infection, through a process called nutritional immunity, the host limits the availability of these micronutrients and exposes the fungus to toxic levels of other species such as copper ions (Noble 2013; Potrykus et al. 2013; Mackie et al. 2016; Sprenger et al. 2018). In response, the fungus activates efficient micronutrient acquisition strategies. High affinity iron uptake involving a cyclic iron reduction pathway (iron reductase, multicopper ferroxidase and iron permease) is activated to take over from low affinity ferritin-iron uptake via the protein Als3, which is operational in hyphae during iron-replete conditions (Wilson, Naglik and Hube 2016; Bairwa, Hee Jung and Kronstad 2017). Candidaalbicans can also assimilate iron from heme and hemoglobin using Common in Fungal Extracellular Membrane (CFEM) proteins, and can scavenge siderophores synthesised by other microorganisms using the Arn1/Sit1 ferrichrome transporter (Bairwa, Hee Jung and Kronstad 2017). Transcriptional circuitry involving Sef1, Sfu1 and Hap43 control iron homeostasis by activating iron assimilation mechanisms when iron is limiting, and by repressing iron uptake when it is in excess (Chen et al. 2011; Noble 2013). Candida albicans utilises two uptake mechanisms to scavenge zinc. The first, which operates mainly at acidic pHs, involves uptake via the Zrt2 transporter into the cytoplasm (Crawford et al. 2018). The second, which is functional at neutral pHs, entails zincophore-mediated zinc scavenging through a secreted protein, Pra1 and uptake via the transporter Zrt1 (Citiulo et al. 2012; Wilson 2015; Crawford et al. 2018). C. albicans responds to zinc limitation by forming goliath cells (enlarged and spherical yeasts that exhibit enhanced adhesion) and avoids zinc toxicity by rapidly compartmentalizing zinc in storage vacuoles called zincosomes (Malavia et al. 2017; Crawford et al. 2018).
Robust stress responses
Fungal pathogens generally display robust responses to certain stresses, particularly oxidative stress (Brown et al. 2017) (Fig. 4). Candida albicans is resistant to significantly higher levels of ROS than its distant cousin, Saccharomyces cerevisiae (Jamieson, Stephen and Terrière 1996; Nikolaou et al. 2009) and this helps the fungus to counter toxic ROS produced by innate immune cells, before and during phagocytic attack (Miramón et al. 2012). C. albicans and other fungal pathogens counteract acute exogenous oxidative stresses by inducing genes involved in ROS detoxification (e.g. catalase and superoxide dismutases), the synthesis of antioxidants (e.g. glutathione and thioredoxin), and the repair of ROS-mediated damage to DNA, proteins and lipids (Enjalbert, Nantel and Whiteway 2003, Enjalbert et al. 2006; Znaidi et al. 2009). The inactivation of key regulators of the response in C. albicans (Cap1, Skn7 and Hog1) compromises oxidative stress resistance (Alarco and Raymond 1999; Singh et al. 2004; Smith et al. 2004). Virulence is attenuated by the inactivation of the Hog1 stress activated protein kinase (Alonso-Monge et al. 1999; Cheetham et al. 2011), but only to a minor extent by the loss of Cap1 or Skn7 (Singh et al. 2004; Jain et al. 2013). The overexpression of catalase, which detoxifies hydrogen peroxide, enhances oxidative stress resistance in vitro, and yet, counterintuitively, reduces the virulence of C. albicans (Román et al. 2016; Pradhan et al. 2017). This is because overexpression of this abundant ferroprotein places an undue demand for the essential micronutrient, iron, under iron limiting conditions in vivo (Pradhan et al. 2017). Clearly, numerous and potentially opposing, selective pressures must be balanced to optimise fungal fitness in a particular host niche.
While much attention has focussed on oxidative stress, C. albicans faces other forms of environmental stress in the host, including nitrosative, osmotic and thermal stresses. Innate immune cells expose C. albicans to RNS) in an attempt to kill and clear the fungus. C. albicans responds by activating genes involved in RNS detoxification (such as the flavohemoglobin, Yhb1), glutathione synthesis and recycling, and the repair of RNS-mediated damage (Hromatka, Noble and Johnson 2005; Tillmann et al. 2015). The response to nitrosative stress is driven by the transcription factor Cta4 and Hog1 (Chiranand et al. 2008; Herrero-de-Dios et al. 2018). The inactivation of YHB1, CTA4 or HOG1 attenuates nitrosative stress resistance and virulence (Alonso-Monge et al. 1999; Hromatka, Noble and Johnson 2005; Chiranand et al. 2008; Cheetham et al. 2011; Miramón et al. 2012).
Candida albicans cells thrive in niches with different osmolarities (e.g. on skin, in the oral cavity or GI tract), and yet must maintain osmo-homeostasis to grow. Hypo- and hyper-osmotic challenges are countered by modulating the levels of intracellular osmolytes. For example, C. albicans upregulates the synthesis and accumulation of glycerol and arabitol in response to hyperosmotic challenges (San José et al. 1996; Kayingo and Wong 2005). This response is regulated at both transcriptional and post-transcriptional levels by the evolutionarily conserved Hog1 MAP kinase signalling pathway (Smith et al. 2004; Enjalbert et al. 2006).
Candida albicans must also restore and maintain proteostasis in the face of thermal challenges, even within the mammalian host (Nicholls et al. 2011). Even mild increases in temperature lead to activation of the so-called heat shock response (Leach, Tyc et al. 2012), which is regulated by an autoregulatory circuit involving the heat shock transcription factor (Hsf1) and heat shock protein 90 (Hsp90) (Leach, Budge et al. 2012). The response involves the induction of functions involved in protein refolding and protein degradation to repair or recycle damaged proteins (Nicholls et al. 2009; Leach et al. 2016). The heat shock response is integrated with key virulence attributes in C. albicans such as yeast-hypha morphogenesis, adhesion and the ability to damage epithelial cells (Shapiro et al. 2009; Leach et al. 2016). Consequently, the inactivation of the response attenuates virulence (Nicholls et al. 2011).
Candida albicans can thrive over an extremely wide range of ambient pHs, from pH 2 to 10 (Vylkova et al. 2011). pH responses are particularly relevant given the ability of C. albicans to colonise host niches with contrasting pHs such as the vagina (acidic), GI tract (acidic to mildly alkaline) and blood (neutral). These pH responses, which are regulated in part by the evolutionarily conserved Rim101 pathway (Davis, Wilson and Mitchell 2000), are tightly integrated with metabolic adaptation, nutrient acquisition and morphogenesis (Davis et al. 2000). Yeast-hypha morphogenesis in C. albicans is regulated in response to ambient pH (Buffo, Herman and Soll 1984; Porta et al. 1999; Chen et al. 2020; Villa et al. 2020). Ambient pH also affects trace metal solubility, and consequently, micronutrient assimilation strategies in C. albicans are regulated in response to pH (Noble 2013; Wilson 2015; Crawford et al. 2018). Significantly, C. albicans is not simply reactive to pH: it can proactively alkalinise its microenvironment through the catabolism of polyamines and amino acids, leading to the release of ammonia and/or CO2 (Mayer et al. 2012; Vylkova and Lorenz 2014; Danhof et al. 2016; Vylkova 2017). Interestingly, lactate production by a co-commensal in the oral cavity, Streptococcus mutans, provides carboxylic acid substrates that are sufficient to promote C. albicans-mediated alkalinisation of the microenvironment (Danhof et al. 2016; Willems et al. 2016).
Immune evasion
Immune evasion can be viewed as an additional type of fitness attribute because it promotes the physiological robustness of the fungus in the host (Fig. 4). Candida albicans has evolved a variety of mechanisms through which it can reduce recognition by immune cells, decrease the efficacy of antimicrobial killing mechanisms, escape immune cells following engulfment, and manipulate the immune system (see Innate antifungal responses and Fungal countermeasures for more detail). During co-evolution with its host, C. albicans has even developed mechanisms by which it can anticipate, and protect itself against, imminent immune attack.
Clearly, C. albicans possesses an array of powerful fitness attributes through which this fungus tunes its physiology to counter environmental challenges presented by the host. Significantly, the fungus not only adapts to host-defined conditions, but can also anticipate impending challenges, and actively modulate its microenvironment.
Candida albicans epidemiology and variability
The flexibility of C. albicans, which underlies its success as a commensal and a pathogen, is also reflected at the genetic level (Fig. 4). Clinical isolates of C. albicans are generally diploid, with a haploid genome size of 16 Mb, organised into eight chromosomes. However, isolates display high levels of sequence heterozygosity between homologous chromosomes (Selmecki, Forche and Berman 2006; Ford et al. 2014; Hirakawa et al. 2015) and a high degree of genome plasticity driven by ploidy changes, karyotypic variations due to partial and whole chromosome aneuploidies, point mutations, short and long-range loss of heterozygosity (LOH) events and copy number variations (Chibana, Beckerman and Magee 2000; Selmecki, Forche and Berman 2006; Ford et al. 2014; Hirakawa et al. 2015; Ropars et al. 2018; Sitterlé et al. 2019). Furthermore, haploid and tetraploid strains have been observed both in vitro and in vivo (Hull, Raisner and Johnson 2000; Magee and Magee 2000; Hickman et al. 2013).
Multilocus sequence typing (MLST) and genome sequencing studies have revealed that C. albicans isolates are distributed amongst at least 23 genetic clusters (1–18, A-E) (Bougnoux et al. 2006; Odds et al. 2007; Odds 2010; Ropars et al. 2018). In general, there are no clear phenotypic associations with these clusters (Bougnoux et al. 2006; MacCallum et al. 2009). However, some clusters do exhibit geographical enrichment (Odds et al. 2007; MacCallum et al. 2009; Shin et al. 2011), suggesting independent recent evolutionary histories for these clusters. Cluster 13 is somewhat exceptional in that it represents a highly clonal lineage of isolates that exhibit low heterozygosity (Ropars et al. 2018). Isolates in cluster 13 are distributed worldwide (Fakhim et al. 2020), despite being called Candida africana strains (Tietz et al. 2001). They are isolated predominantly from the genital niche and display unusual morphological and phenotypic features that include slow growth, an inability to produce chlamydospores and assimilate aminosugars, and decreased virulence (Tietz et al. 2001; Romeo, De Leo and Criseo 2011; Borman et al. 2013). In contrast to other C. albicans clusters, cluster 13 isolates harbour a unique pattern of single nucleotide polymorphisms (SNPs) and a significantly lower level of heterozygosity (Ropars et al. 2018). In addition, in cluster 13 isolates, genes important for morphogenesis and virulence have undergone pseudogenisation, which probably explains the decreased virulence and apparent genital niche restriction of these isolates (Ropars et al. 2018).
Once thought to be an asexual obligate diploid organism, C. albicans has been shown to undergo a parasexual cycle (Magee and Magee 2000; Bennett and Johnson 2003; Ene and Bennett 2014). The majority of C. albicans diploid strains are incapable of mating, being heterozygous at the mating type-like (MTL) locus. However, mating can occur mainly between strains that have become homozygous at the MTL locus on chromosome 5, and have complementary MTL genotypes (i.e. are MTLa/a and MTLα/α). Additionally, mating in C. albicans is also dependent on a phenotypic switch from the mainly sterile ‘white’ phenotype to the mating competent ‘opaque’ phenotype (Miller and Johnson 2002). Mating between competent isolates of opposite mating-type results in tetraploid cells. These can subsequently undergo concerted chromosome loss, which can restore the diploid state in a meiosis-independent manner (Bennett and Johnson 2003; Hickman et al. 2015). However, this process yields diverse intermediate aneuploid states (Hickman et al. 2015). Hence, this mode of parasexual reproduction provides a means of generating genetic and phenotypic diversity in C. albicans (Forche et al. 2008; Hickman et al. 2015). Indeed, recombination has been shown to occur three orders of magnitude more frequently during concerted chromosome loss than during mitosis (Anderson et al. 2019). Interestingly, recombination during concerted chromosome loss is highly dependent on two meiosis-specific genes, SPO11 and REC8 (Forche et al. 2008; Anderson et al. 2019). The involvement of meiosis-specific genes in concerted chromosome loss has led to the suggestion that this process ‘blurs the boundaries’ between meiosis and mitosis, and that this ‘parameiosis’ might provide insight into the evolution of meiosis (Anderson et al. 2019).
The view that the parasexual cycle rarely occurs in the host is supported by population genetics, which shows that C. albicans populations are predominantly clonal (Pujol et al. 1993; McManus and Coleman 2014). Nevertheless, the conservation of mating genes suggests that this process is associated with an evolutionary advantage. Furthermore, because the parasexual cycle is stimulated by environmental stress, it may be a diversity-enhancing process that enhances adaptation and survival under hostile conditions (Selmecki, Forche and Berman 2010; Zhang et al. 2015; Hirakawa et al. 2017; Popp et al. 2019). This idea is corroborated by evidence of recombination and gene flow in natural isolates, despite the largely clonal structures of C. albicans populations (Odds et al. 2007; Bougnoux et al. 2008; Zhang et al. 2015; Ropars et al. 2018). This could explain why C. albicans isolates maintain a high degree of genetic diversity despite their predominantly clonal reproduction.
The diversity of C. albicans populations has arisen partly through changes in ploidy and aneuploidy. These mechanisms have provided C. albicans with a means of evolving rapidly in response to environmental challenges (Selmecki, Forche and Berman 2006; Diogo et al. 2009; Bennett, Forche and Berman 2014). The association of genome rearrangements with antifungal resistance acquisition has been well documented, with genomes of antifungal-resistant strains often exhibiting copy number variations and chromosome aneuploidies (Selmecki, Forche and Berman 2010). Indeed, a striking example of segmental aneuploidy was reported in fluconazole resistant strains, consisting of an isochromosome composed of the two left arms of chromosome 5 (Selmecki, Forche and Berman 2006, Selmecki et al. 2008). Trisomy of chromosome 2 or R has also been reported to enhance antifungal drug resistance in C. albicans (Xingxing Li et al. 2015; Yang et al. 2019). Large-scale chromosome rearrangements occur in C. albicans as an adaptation mechanism in both oral and GI niches (Ene et al. 2018; Forche et al. 2018). Similar observations have been made in isolates collected from a single human individual (Sitterlé et al. 2019). Genome sequencing of clinical isolates from patients that received antifungal therapy revealed that eight of the 21 isolates underwent karyotypic changes, with the majority being trisomic for chromosome 4 or 7 (Hirakawa et al. 2015). However, a more recent study of 182 clinical isolates might suggest that both segmental and whole chromosome aneuploidies are relatively infrequent events (Ropars et al. 2018). Changes in ploidy are known to provide a selective advantage under stress conditions, but can confer long-term fitness defects when grown under nonselective conditions, as illustrated by decreased growth and virulence (Hickman et al. 2015, 2013; Hirakawa et al. 2015). Therefore, the extent to which these events are observed in the genomes of C. albicans isolates must reflect the frequency of these types of genetic event and the nature of the selective pressures that these isolates recently faced.
Diversity has also arisen through high rates of mutation at the nucleotide level (SNPs, insertions and deletions). Candida albicans isolates display high levels of natural heterozygosity, with one heterozygous SNP occurring per 200–300 bp on average (Jones et al. 2004; Butler et al. 2009; Hirakawa et al. 2015; Ropars et al. 2018). The levels of heterozygosity are influenced by large LOH events, which can affect all chromosomes and are common in C. albicans isolates. LOH events are significantly elevated under stress conditions, such as exposure to antifungal agents, heat or oxidative stress (Forche et al. 2011; Ropars et al. 2018). Rapid phenotypic and genetic changes have been observed in various infection and colonisation models as well as in clinical isolates (Forche, May and Magee 2005; Bougnoux et al. 2006, 2009; Cheng et al. 2007; Bougnoux et al. 2008; Diogo et al. 2009; Lüttich et al. 2013; Ene et al. 2018; Forche et al. 2018; Sitterlé et al. 2020). This microevolution is driven primarily by de novo base substitution and short-range LOH events (Ene et al. 2018), and can clearly impact the relationship between fungus and host (Wartenberg et al. 2014; Tso et al. 2018; Liang and Bennett 2019) as well as resistance to antifungal therapy (Coste et al. 2006; Ford et al. 2014).
THE HOST
Mammals are constantly exposed to microbes on the skin and mucosal surfaces of the GI, respiratory and reproductive tracts. Therefore, epithelial surfaces in the mucosal tissues represent primary sites of interaction between C. albicans and the host (Lim et al. 2012). To prevent microbial overgrowth on the epithelial barriers and microbial invasion of tissues, the host actively surveys and protects its barrier surfaces via two distinct, complementary and cooperating branches of the immune system: innate and adaptive immunity (Fig. 5). As well as forming a physical barrier, epithelial cells contribute to the host response through active recognition of microbes and evaluation of their pathogenic potential. This is complemented by myeloid cells of the innate immune system, which exploit evolutionarily conserved pattern recognition receptors (PRRs) to recognise microbial pathogen-associated molecular patterns (PAMPs). Recognition of PAMPs by PRRs triggers phagocytosis of the microbial target and/or antimicrobial effector responses with the purpose to eradicate the pathogen. In addition, the innate immune system, and dendritic cells (DCs) in particular, activate the adaptive immune system. T helper (Th) cells are activated in an antigen-specific manner to coordinate epithelial defenses, improve innate immune function, activate antibody responses, and ultimately control the fungal load and resolve inflammation. Through the development of immunological memory, adaptive immunity provides long-lasting protection against microbes. We address the cellular and molecular mechanisms of innate and adaptive immunity that provide critical protection against C. albicans infection at epithelial barriers where interactions between the fungus, host and microbiota play out. These interactions are dependent on tissue type and are influenced by variations between individuals that affect susceptibility to fungal infection.
Figure 5.
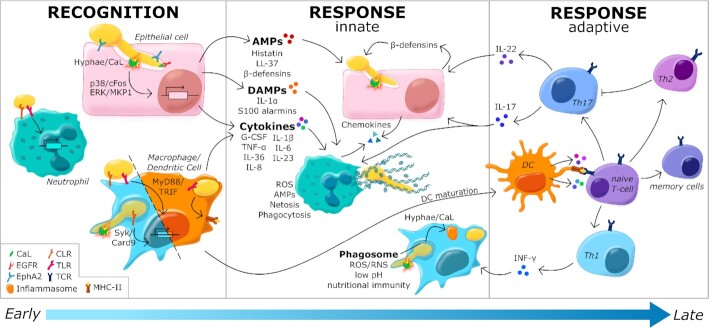
Immune recognition of, and immune responses against, C. albicans. Candida albicans yeast and hyphal cells are recognised by neutrophils, macrophages and dendritic cells via pattern recognition receptors (see key). This recognition activates the expression and release of proinflammatory cytokines and chemokines that promote the recruitment of macrophages and neutrophils to the site of infection. Epithelial cells respond to hypha formation and the subsequent secretion of candidalysin by the fungus, by activating the expression and release of AMPs, DAMPs, chemokines and cytokines via p38/cFos and ERK/MKP1 signalling. The AMPs attenuate fungal growth and invasion, while DAMPs and cytokines promote inflammation. Myeloid cells promote fungal killing and clearance through a combination of phagocytosis and NETosis in the case of neutrophils. Fungal recognition leads to the maturation of dendritic cells, and their surface presentation of fungal antigens to naïve T-cells, which stimulates adaptive immunity. The interactions between antigen-presenting dendritic cells and naïve T-cells induces T-cell activation and differentiation into various effector T cell subsets that regulate mucosal immunity largely via IL-17 and IL-22 secretion, and stimulate macrophages via IFN-γ. See text.
Innate immunity
Fungal recognition
The innate immune system is the first line of defense against C. albicans infection (Fig. 5). Epithelial cells (Richardson, Ho and Naglik 2018; Nikou et al. 2019; Swidergall 2019) combine with innate immune cells (Naglik et al. 2017; Verma, Gaffen and Swidergall 2017; Richardson et al. 2019) to provide this defense system, initiating anti-Candida immunity in response to fungal recognition.
Tissue-resident phagocytes, such as macrophages and DCs, are crucial in maintaining mucosal homeostasis (Ramirez-Ortiz and Means 2012; Xu and Shinohara 2017; Watanabe et al. 2019). However, innate immune cell populations differ between tissues, resulting in tissue-specific variation in the induction of innate and adaptive immune responses (see Variability in the immune response). Following hypha formation and C. albicans invasion, neutrophils and monocytes are rapidly recruited to the site of infection to mediate pathogen clearance through various antifungal responses (see Antifungal response) (Richardson et al. 2019).
Myeloid cells recognise specific microbial PAMPs using specific PRRs that fall into four main families: Toll-like receptors (TLRs), C-type lectin receptors (CLRs), nucleotide oligomerisation domain (NOD)-like receptors (NLRs) and RigI-helicase receptors (RLRs). CLRs are critical for fungal recognition (Hardison and Brown 2012). Several types of CLR recognise C. albicans, including Dectin-1, Dectin-2, Mincle, DC-Sign, and the mannose receptor (MR) (Hardison and Brown 2012; Dambuza et al. 2017; Goyal et al. 2018; Swidergall 2019). Dectin-1 recognises fungal β-glucans, which triggers the Card9-Syk pathway, leading to Nuclear Factor-kappa B (NFκB) activation and consequent cytokine and chemokine release (Drummond et al. 2011). In addition, dectin-1 induces phagocytosis and inflammasome activation (Kankkunen et al. 2010; Goodridge, Underhill and Touret 2012; Swidergall 2019). Dectin-2 recognises α-mannans (McGreal et al. 2006; Saijo et al. 2010) and induces the formation of Neutrophil Extracellular Traps (NETs) after recognising unopsonised C. albicans cells (Wu et al. 2019). In addition, Dectin-2 forms heterodimers with Dectin-3 and binds α-mannans on the surfaces of C. albicans hyphae (Zhu et al. 2013). Mannans are also recognised by Mincle, DC-Sign and the MR (Hardison and Brown 2012; Erwig and Gow 2016; Dambuza et al. 2017).
TLR-mediated PAMP recognition activates MyD88-dependent and TRIF signalling pathways in innate immune cells to regulate the inflammatory response (Kawasaki and Kawai 2014; Swidergall 2019). TLR2 and TLR4 recognise mannoproteins, while TLR9 recognises fungal DNA (Naglik et al. 2017). In addition, together with TLR9, the cytosolic NLR receptor NOD2 senses chitin particles (Wagener et al. 2014). NOD2-mediated recognition of chitin was found to down-regulate inflammatory responses (Wagener et al. 2014), which explains why NOD2 was initially described as being redundant for the induction of inflammatory responses against C. albicans (van der Graaf et al. 2006; van de Veerdonk et al. 2009). Recently, the epithelial Ephrin type-A receptor 2 (EphA2) was described as a non-classical PRR that recognises β-glucan (Swidergall et al. 2018). This receptor is expressed on neutrophils and stimulates antifungal activity during oropharyngeal candidiasis (OPC) (Swidergall, Solis et al. 2019). Meanwhile, the melanoma differentiation-associated factor 5 (MDA5), a member of the RIG-I-like receptor (RLR) family that senses viral RNA, has been reported to also trigger an antifungal immune response, although its ligand remains obscure (Jaeger, van der Lee et al. 2015) (Table 1).
Table 1.
Pattern recognition receptors in epithelial and innate immune cells that recognise C. albicans pathogen-associated molecular patterns.
| PRR family | PRR | Fungal PAMP | Expressed in | Reference |
|---|---|---|---|---|
| TLRs | TLR2 | Phospholipomannans | Neutrophils, macrophages, DCs, Epithelial cells (oral, vaginal, intestinal) | (Kurt-Jones et al. 2002; Jouault et al. 2003; Fazeli, Bruce and Anumba 2005; Décanis, Savignac and Rouabhia 2009; McClure and Massari 2014) |
| TLR4 | O-linked mannans | Neutrophils, monocyte, macrophages, DCs, epithelial cells (oral, vaginal, intestinal) | (Netea et al. 2006; Hyung Sook Kim et al. 2016; Fazeli, Bruce and Anumba 2005; Weindl et al. 2007; McClure and Massari 2014) | |
| TLR9 | Fungal DNA Chitin | DCs, Neutrophils, macrophages, epithelial cells (oral, vaginal, intestinal) | (Miyazato et al. 2009; Kasperkovitz et al. 2011; McClure and Massari 2014; Wagener et al. 2014) | |
| CLRs | Dectin-1 | β-glucans | Macrophages, monocytes, neutrophils, DCs, epithelial cells (oral, intestinal) | (Brown and Gordon 2001; Brown et al. 2002; Taylor et al. 2002; Ariizumi, Shen, Shikano, Xu et al. 2000; Cohen-Kedar et al. 2014) |
| Dectin-2 | Mannoproteins (a-mannans) | Macrophages, DCs | (Taylor et al. 2005; Ariizumi, Shen, Shikano, Ritter, et al. 2000) | |
| Dectin-3 | Mannoproteins (a-mannans) | Macrophages, | (Zhu et al. 2013) | |
| DC SIGN | Mannoproteins | Macrophages, DCs | (Cambi et al. 2003; Rappocciolo et al. 2006) | |
| Mincle | Mannoproteins | Neutrophils, macrophages, DCs | (Wells et al. 2008; Vijayan et al. 2012; Martínez-López et al. 2019) | |
| MR | Mannoproteins Chitin | DCs, macrophages | (van de Veerdonk et al. 2009; Martinez-Pomares 2012; Wagener et al. 2014) | |
| NA | EphA2 | β-glucans | Oral epithelial cells, neutrophils | (Swidergall, Solis, et al. 2019) |
| Galectin-3 | β-mannosides | Monocytes, macrophages, DCs, neutrophils, epithelial cells | (Jouault et al. 2006) | |
| RLRs | MDA5 | Unknown | Monocytes, DCs, macrophages, epithelial cells | (Plato, Hardison and Brown 2015) |
| NLRs | NOD2 | Chitin | Monocytes, DCs, macrophages | (Wagener et al. 2014) |
PRRs involved in the recognition of C. albicans by myeloid cells have been well characterised (above), but less is known about epithelial cell PRRs that recognise C. albicans. Epithelial cells use several types of PRR to sense C. albicans, including TLR2, TLR4, dectin-1 and EphA2 (Weindl et al. 2007; Décanis, Savignac and Rouabhia 2009; Cohen-Kedar et al. 2014; Swidergall et al. 2018). Despite its primordial role in the recognition of C. albicans by myeloid cells, dectin-1 is thought to play a limited role in epithelial cells (Moyes et al. 2010; Verma et al. 2017; Richardson, Ho and Naglik 2018). Rather, sensing of fungal β-glucans by epithelial cells is achieved mainly by EphA2, which activates MAPK and STAT3 signalling to induce the secretion of inflammatory cytokines and antimicrobial peptides by oral epithelial cells (Swidergall et al. 2018). PRR expression patterns vary amongst epithelial cell types and this, together with differential myeloid cell types, contributes to niche-specific variations in mucosal responses against C. albicans (Nikou et al. 2019; Swidergall 2019) (see Tissue-specific immune responses).
Epithelial cells can be activated by the C. albicans peptide toxin, candidalysin, as well as through PRR-PAMP interactions. This cytolytic peptide damages epithelial cells and activates the epithelial growth factor receptor (EGFR) (Jemima Ho et al. 2019). This, in turn, activates p38/cFos and ERK/MKP1 signalling, leading to the initiation of various effector responses (see Innate antifungal responses). The epithelial response to candidalysin is particularly relevant to the transition of C. albicans from commensalism to pathogenicity, because candidalysin is synthesised during hyphal growth and accumulates in the invasion pocket as the fungus invades the epithelial surface (Moyes et al. 2016) (see Invasion mechanisms). This response to candidalysin endows epithelial cells with the ability to respond to the invasive hyphal form of C. albicans, rather than its relatively benign commensal state (Moyes et al. 2010; Naglik et al. 2017).
Innate antifungal responses
Following recognition of C. albicans by phagocytic receptors, phagocytes such as neutrophils and macrophages can engulf the target C. albicans cell by phagocytosis, the purpose being to entrap and kill the pathogen (Brown 2011) (Fig. 5). Phagocytosis involves rapid reorganisation of the plasma membrane and cytoskeleton, and the imposition of mechanical force to engulf the fungal cell and entrap it within a phagosome (Ostrowski, Grinstein and Freeman 2016; Huse 2017). The phagosome then undergoes a series of plasma-membrane phosphoinositide- and Rab-dependent membrane fusion and fission events with endolysosomal compartments that promote the assimilation of microbicidal and lytic enzymes, and the progressive acidification of the organelle, to form the mature phagolysosome (Brown 2011; Fairn and Grinstein 2012; Miramón, Kasper and Hube 2013; Erwig and Gow 2016; Walpole, Grinstein and Westman 2018). In an attempt to kill the fungus, the phagocyte exposes its fungal cargo to a low pH, a nutrient limiting microenvironment and a potent mix of proteases, reactive chemical species ROS and RNS, cation fluxes and AMPs (Lorenz, Bender and Fink 2004; Brown 2011; Miramón, Kasper and Hube 2013; Erwig and Gow 2016). However, these skirmishes between phagocyte and fungus do not always achieve fungal clearance. This is because C. albicans has evolved molecular mechanisms that help it to evade phagocytic recognition, escape the phagocyte following engulfment, and resist phagocytic killing mechanisms (Austermeier et al. 2020) (see Fitness attributes and Immune evasion).
PAMP-PRR interactions activate host cell signalling, which in turn, induces a myriad of effector responses that are specific to the cell and tissue type (Brown et al. 2002; Roeder et al. 2004). Epithelial cells secrete antimicrobial peptides (AMPs) such as LL-37, histatins and β-defensins. These AMPs exert their antifungal effects by a variety of mechanisms that include binding to the fungal cell wall or permeabilizing the fungal plasma membrane (Krishnakumari, Rangaraj and Nagaraj 2009; Chang et al. 2012; Swidergall and Ernst 2014). In the oral epithelium, nitric oxide and human-β-defensin (hBD)-2 production contribute to the early defensive response following direct contact with C. albicans and intra-epithelial invasion (Casaroto et al. 2019). In the GI tract, mucins produced by goblet cells suppress the yeast-hypha transition, surface adhesion and biofilm formation of C. albicans, thereby minimizing the capacity of the fungus to attach to, invade, and damage the epithelium (Kavanaugh et al. 2014) (see Virulence factors).
When C. albicans does manage to colonise the epithelium, the fungal toxin, candidalysin, plays a central role in triggering downstream responses (Kasper et al. 2018; Jemima Ho et al. 2019; Swidergall, Khalaji et al. 2019). The damage caused by candidalysin causes epithelial cells to passively alert professional immune cells through their release of danger-associated molecular patterns (DAMPs) or alarmins (Yang and Oppenheim 2009). For example, S100 alarmins produced by the vaginal epithelium are a potent driver of neutrophil influx during vaginitis in a murine model of infection (Yano et al. 2010, 2014). Similarly, damage to oral epithelial cells results in their release of the alarmin IL-1ɑ, which triggers the neutrophil response to C. albicans in the oral mucosa via IL-1 signalling (Dongari-Bagtzoglou, Kashleva and Villar 2004; Altmeier et al. 2016). Epithelial cells also produce pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, G-CSF, TNF, and IL-36 (Villar et al. 2005; Verma et al. 2018). IL-8 acts as a chemoattractant that mobilises neutrophils from the circulation to the infection site. These neutrophils engage the fungus directly. They also engage in cross talk with local epithelial cells via TNF, thereby promoting TLR4-mediated signalling in the epithelium to enhance protection against fungal invasion and cell damage during oral candidiasis (Weindl et al. 2007).
Neutrophils are central players in antifungal defences due to their rapid activation of the fungicidal oxidative burst, (Peltroche-Llacsahuanga et al. 2000), their formation of NETs (Kenno et al. 2016), and their release of AMPs via degranulation (Urban et al. 2009) (Fig 5). Mice with C. albicans colonisation in their GI tract display enhanced neutrophil responsiveness and fungus-specific CD4 + T-cell responses during systemic candidiasis (Shao et al. 2019). This contrasts with observations during VVC in humans and mice, where fungal susceptibility is associated with uncontrolled inflammation and neutrophil influx (Black et al. 1998; Fidel et al. 2004). These observations reinforce the context-dependent nature of local immune responses.
Macrophages contribute to fungal clearance through their uptake of fungal cells, displaying a greater phagocytic capacity, but lower uptake rate, than polymorphonuclear leukocytes, (PMNs) (Rudkin et al. 2013) (Fig. 5). The hyphal form of C. albicans is relatively resistant to phagocytosis (Lewis et al. 2012). Nevertheless, macrophages still engulf portions of the hyphae, which can become trapped in ‘frustrated phagosomes’ (Maxson et al. 2018). After phagocytosis by macrophages, C. albicans yeast cells can undergo morphogenesis to generate hyphae. The yeast-hypha transition activates the NOD-and pyrin domain-containing protein 3 (NLRP3) inflammasome. This is essential for the release by the macrophage of pro-inflammatory IL-18 and IL-1β, which further promote Th1/Th17 activity during infection (Joly et al. 2009; van de Veerdonk, Joosten et al. 2011; Kasper et al. 2018). However, hyphal development within the phagolysosome can help C. albicans evade macrophage killing by inducing pyroptosis, rupture and death of the macrophage in vitro (Vázquez-Torres and Balish 1997; Uwamahoro et al. 2014; Wellington et al. 2014; Kasper et al. 2018; O'Meara et al. 2018; Westman et al. 2018; Austermeier et al. 2020). Nevertheless, macrophages provide an important contribution to antifungal defences during systemic infection. For example, the functionality of resident renal macrophages, which is dependent on expression of the chemokine receptor CX3CR1, is important for controlling C. albicans in the early stages of a systemic infection, and for survival of the host (Lionakis et al. 2013). Similarly, microglia play an important role in antifungal immunity in the central nervous system, promoting neutrophil recruitment via candidalysin induced IL-1β and CXCL1 signalling (Drummond et al. 2019).
Mast cells modulate the antifungal potency of macrophages. Activated mast cells enhance macrophage functionality by improving their crawling ability and chemotaxis in response to C. albicans stimulation (De Zuani et al. 2018). Meanwhile, resting mast cells inhibit the phagocytosis of C. albicans by macrophages, which suggests a role for mast cells in the maintenance of commensalism (De Zuani et al. 2018). Inflammatory monocytes expressing CCR2 and Ly6C also contribute to fungal clearance during the early stages of systemic infection. Clearance is enhanced in the kidney and brain, but less so in the liver and spleen, indicating an organ-specific role for these monocytes during disseminated infection (Ngo et al. 2014).
Fungal countermeasures
During co-evolution of fungus and host, the antifungal responses of the immune system have imposed strong selective pressures upon C. albicans to evade these responses. Consequently, the fitness of the fungus in vivo has been enhanced by the development of a variety of fungal countermeasures that promote immune evasion and manipulation (Underhill 2007; Marcos et al. 2016).
A number of the fitness attributes and virulence factors, described above, promote immune evasion (see Virulence factors and Fitness attributes). For example, the formation of biofilms shields C. albicans cells from immunological attack (Kernien et al. 2017). The ability of C. albicans to resist pH extremes and to actively resist phagolysosomal acidification reduces the antifungal potency of phagocytes (Vylkova et al. 2011; Bain, Gow and Erwig 2015; Vylkova and Lorenz 2017; Westman et al. 2018). Also, the activation of robust oxidative and nitrosative stress responses provides a degree of protection against the toxic ROS and RNS generated by innate immune cells (Miramón et al. 2012). These responses include secreted and cell wall bound ROS detoxifying enzymes that help to counter immune attack (Crowe et al. 2003; Fradin et al. 2005; Dantas et al. 2015). However, C. albicans is sensitive to certain combinations of stress encountered within the phagosome (Kaloriti et al. 2014; Kos et al. 2016).
Hypha formation reduces the exposure of C. albicans to phagocytic killing because lengthy hyphal cells are harder to engulf, and hyphae have been reported to display lower levels of the inflammatory MAMP, β-1,3-glucan, at their cell surface (Gantner, Simmons and Underhill 2005; Bain et al. 2014; Mukaremera et al. 2017). Furthermore, C. albicans can undergo yeast-hypha morphogenesis following phagocytosis by macrophages, rupturing the phagosome and eventually leading to host cell death and fungal escape (Lewis et al. 2012; Ermert et al. 2013; Vylkova and Lorenz 2017). Indeed, the fungus is capable of triggering pyroptosis, inflammasome activation and cell death in a macrophage that has engulfed it (Uwamahoro et al. 2014; Wellington et al. 2014; O'Meara et al. 2015; Kasper et al. 2018), and can also induce host cell death through metabolic competition for glucose (Tucey et al. 2018; Tucey et al. 2020). Like other fungal pathogens, C. albicans may also escape the macrophage without lysing the host cell (Bain et al. 2012), although this mode of escape is thought to be rare.
Members of the secreted aspartic protease family (Sap1-3) promote immune evasion by degrading complement proteins (C3b, C4b, C5) thereby reducing the inhibitory potential of the complement system (Gropp et al. 2009). Candida albicans also expresses complement binding proteins at its cell surface that reduce the efficacy of the complement system (Poltermann et al. 2007; Zipfel and Skerka 2009). Pra1, which promotes zinc assimilation in C. albicans (see Fitness attributes), also interacts with complement regulators and plasminogen. In addition, Pra1 was the first protein described to bind to C4BP, which regulates the classical and lectin complement pathways and avoids C3b and C4b deposition on the fungal surface when captured by C. albicans, impeding complement cascade progression (Luo et al. 2009, 2011; Zipfel, Hallström and Riesbeck 2013). Furthermore, C. albicans secretes prostaglandins that modulate host immunity by downregulating chemokine and TNF production (Noverr et al. 2001). On the other hand, host immune mediators such as IFNγ, IL-17, TNF and PGE2 influence C. albicans growth, filamentation and biofilm formation (Kalo-Klein and Witkin 1990; Noverr and Huffnagle 2004; Zelante et al. 2012; Rocha et al. 2017).
More recently, it was found that C. albicans yeast cells can evade phagocytic recognition by actively masking β-1,3-glucan at their cell surface. Interestingly the fungus exploits host signals, such as lactate, hypoxia, iron limitation and ambient pH, to modulate its β-1,3-glucan exposure (Ballou et al. 2016; Sherrington et al. 2017; Lopes et al. 2018; Pradhan et al. 2018; Cottier et al. 2019; Pradhan et al. 2019). Reducing the levels of β-1,3-glucan exposure leads to the attenuation of anti-Candida immune responses (Ballou et al. 2016; Sherrington et al. 2017; Lopes et al. 2018; Pradhan et al. 2018, 2019) and promotes disease progression (Lopes et al. 2018). Indeed, the fungus appears to use these host signals to anticipate impending immune attack and to protect itself by activating immune evasion mechanisms (Alistair J P Brown et al. 2019). These, and other anticipatory responses (Rodaki et al. 2009; Brunke and Hube 2014), provide strong evidence for the co-evolution of C. albicans with its host (Brown, Larcombe and Pradhan 2020).
Adaptive immunity
The adaptive immune system evolved to establish long-term protection through its ability to generate immunological memory (Fig. 5). The key role played by this arm of the immune system in providing surveillance of commensal organisms is reflected in the fungal dysbiosis that occurs in the absence of adaptive immunity (Lanternier, Cypowyj et al. 2013). The adaptive immune system involves B and T cells. B cells are essential for the production of antibodies, whereas T helper (Th) cells provide essential support for mucosal host defense and the innate immune response.
Candida albicans-specific antibodies are detectable in individuals that have been exposed to the fungus (Swoboda et al. 1993; López-Ribot et al. 2004; Pitarch et al. 2006). Their role in the control of fungal colonisation remains unclear, although the presence of anti-C. albicans antibodies might provide protection to mice against a potentially lethal systemic challenge (Matthews et al. 1991), as does gut colonisation through the development of pronounced anti-C. albicans IgG levels (Huertas et al. 2017). For some time, it has been thought that antibodies may have diagnostic as well as therapeutic value (Matthews et al. 1988). Recent studies have reinforced their diagnostic potential (Wang et al. 2020), and recombinant anti-C. albicans antibodies have been shown to display therapeutic potential in preclinical models of infection by improving phagocytosis (Rudkin et al. 2018).
T cells exist as various subtypes that contribute differentially to antifungal immunity (Borghi et al. 2014; Verma et al. 2014; Lionakis and Levitz 2018) (Fig. 5). Among CD4+ T cells, Th1 and Th17 cells promote the phagocytic clearance of fungal cells through the release of inflammatory cytokines such as IFN-γ and IL-17A/F, respectively, and these T cell subsets are critical for protective antifungal immunity. On the other hand, Th2 cells counter-regulate Th1 and Th17 responses, which can favour fungal persistence and promote allergic manifestations. Regulatory T cells (Tregs) maintain the homeostatic balance between these responses and limit inflammation as the infection is cleared. Th17 cells represent a major subset, and Th1 and Th2 cells minor subsets, of the human C. albicans-specific T helper cell population (Becattini et al. 2015; Bacher et al. 2019). However, additional T helper cell subsets have been described more recently (Eyerich et al. 2011; Becker et al. 2016). Moreover, T helper cells express plasticity. For example, C. albicans-specific Th17 cells can adopt the ability to produce additional cytokines, such as the Th1 prototypic cytokine IFN-γ (Zielinski et al. 2012). Cytotoxic (CD8+) T-cells may also play a role in anti-Candida immunity (Beno, Stöver and Mathews 1995; Marquis et al. 2006).
The major protective role of Th17 cells in antifungal immunity is illustrated by the strong association of human defects in this T cell compartment and IL-17 signalling with uncontrolled fungal growth on mucosal surfaces and the skin (Puel et al. 2011; Ling et al. 2015; Li et al. 2017; Puel 2020). Consistently, mice with defects in the IL-17 signalling pathway display a reduced ability to cope with C. albicans administered via oropharyngeal or epicutaneous routes (Conti et al. 2009; Gladiator et al. 2013; Kashem, Igyarto et al. 2015), while IFN-γ-producing Th1 cells may have a disease-promoting effect (Igyártó et al. 2011). Also, the expansion of fungus-specific Th1 and Th17 cells in response to mucosal colonisation enhances protection against subsequent systemic C. albicans infections in mice (Romani et al. 1994; Shao et al. 2019). However, T cell- and IL-17-defects do not alter susceptibility to systemic infection in humans (Lionakis 2014).
CD4 + T cells are characterised by their ability to respond in an antigen-specific manner. Antigen-specific activation of (naïve) T cells depends on their interactions with DCs that present antigen on MHC-II molecules, and provide co-stimulatory and polarising cytokine signals. DCs are divisible into several subsets, most of which reside in peripheral tissues in close proximity to the microbiota where they interact with C. albicans. In response to PRR-mediated activation, DCs undergo a maturation program and migrate to the draining lymph nodes, where they encounter, activate, and prime antigen-specific T cells. This process relies on a tightly coordinated interplay between the innate and adaptive immune system (Fig. 5). The priming of T cells comprises of three steps. First, the recognition of peptide-MHC-II complexes by T cells via their T cell receptor (TCR) defines the antigen-specificity of the response. Second, this interaction is supported by adhesion and co-stimulatory molecules, which are induced at the cell surface of DCs in response to microbial stimulation, and these form an immune synapse that stimulates T cell proliferation. Third, the cytokine microenvironment directs the T cell differentiation towards distinct Th lineages via STAT (signal transducer and activator of transcription) signalling and the induction of fate-determining transcription factors (Wüthrich, Deepe and Klein 2012).
While antigens and the polarisation-inducing microbial signals are functionally distinct, the physical connection between antigen and PAMP enhances the efficiency of the T cell activation and differentiation process. Some of the few naturally processed and presented C. albicans antigens identified so far are glycosylated cell wall proteins, such as Mp65 (Pietrella et al. 2008) and Als3 (Bär et al. 2012). These mannoproteins can therefore serve concomitantly as a source of MHC-II antigen cargo as well as PAMPs. Such antigens support the coordinated process of antigen presentation and T cell polarisation.
The process of DC maturation is shaped by the specific PRR pathways that become activated in DCs following a microbial encounter (Fig. 5). This then determines the profile of cytokines that are produced, and hence directs the fate of the Th cell polarisation. Fungal cell wall components such as mannans and β-1,3-glucans trigger Syk- and CARD9-dependent cytokine signatures characterised by IL-23, IL-6, and IL-1β, which collectively instruct Th17 differentiation (LeibundGut-Landmann et al. 2007; Robinson et al. 2009; Saijo et al. 2010). IL-6 and IL-1β, together with TGF-β in mice, drive the commitment of Th17 cells, while IL-23 promotes lineage maintenance in a STAT3- and RORγt-dependent manner (Korn et al. 2009). Th17 cell differentiation is further modulated by the antigen dose and by tissue-specific cues.
Th17 cells produce the IL-17 family of effector cytokines: IL-17A and IL-17F as well as IL-22. These cytokines act primarily on epithelial cells and control the expression of genes linked to antimicrobial defense and tissue repair (Conti et al. 2009, 2016). IL-17 can also play an important role in promoting neutrophil recruitment (Liang et al. 2007), although, in the oral mucosa, the neutrophil response against C. albicans is largely independent of IL-17 (K Trautwein-Weidner et al. 2015). Instead, it depends on IL-1 and chemokines produced by epithelial cells in response to virulent C. albicans strains (Altmeier et al. 2016). While the functions of IL-17A and IL-17F are related, they do play non-redundant roles in host defense (Gladiator et al. 2013; Whibley et al. 2016). Similar to IL-17A and IL-17F, IL-22 also induces AMPs and contributes to fungal control (Liang et al. 2006). However, in contrast to IL-17A and IL-17F, defects in the IL-22 pathway have a minor impact on fungal control in experimentally infected mice (Conti et al. 2009; De Luca et al. 2013). Lately, IL-22 and IL-17A/F have been found to function nonredundantly during OPC, and IL-22 was shown to regulate the responsiveness of the epithelium to IL-17 (Aggor et al. 2020).
CD4 + T cells are the major source of IL-17 during responses to C. albicans at barrier tissues, but other sources may also contribute. CD8+ αβ T cells can produce IL-17 in response to C. albicans (and other fungi), and these cells may play a compensatory role in the absence of CD4 + T cells (Nanjappa et al. 2012; Hernández-Santos et al. 2013). Moreover, innate lymphocytes and innate lymphoid cells (ILCs) can generate IL-17 (Cua and Tato 2010; Gladiator et al. 2013). In experimental models of oral infection, where naïve mice were exposed to a virulent C. albicans strain, the antifungal response was characterised by induction of IL-17 in ILCs, γδ T cells and a tissue-resident population of αβ T cells that respond in a TCR-independent manner (Sparber et al. 2018; Conti, Peterson et al. 2014; Kashem, Riedl et al. 2015; Verma et al. 2017). These three cellular subsets act in a partially redundant manner (Conti, Peterson et al. 2014; Gladiator et al. 2013). Therefore, although small in size, the IL-17-producing ILC population can compensate for the absence of αβ and γδ T cells during acute OPC (Gladiator et al. 2013). The extent to which innate sources of IL-17 contribute to antifungal defense in humans to maintain host-fungus homeostasis is not yet clear.
As a result of their exposure to C. albicans in the microbiota, most humans produce C. albicans-specific memory Th17 cells. In the circulation, these cells display the phenotype of effector memory T cells, which can respond rapidly to fungal exposure (Acosta-Rodriguez et al. 2007). In the skin, their expression of CD69 and CD103 characterises these C. albicans-specific memory Th17 cells as tissue-resident memory cells (Park et al. 2018). The maintenance of C. albicans-specific T cells is dependent on the persistence of the fungus in the host (Park et al. 2018; Shao et al. 2019; Kirchner and LeibundGut-Landmann 2020). The relevance of tissue-resident memory T cells for local immunosurveillance of C. albicans in barrier tissues was confirmed recently in a model of stable C. albicans commensalism, where tissue-resident memory T cells were sufficient to prevent fungal overgrowth (Kirchner and LeibundGut-Landmann 2020). The relationship between circulating and tissue-resident memory T cells directed against C. albicans remains to be determined, although their shared T cell receptor sequences suggest a common origin for both populations of memory Th17 cells (Park et al. 2018). Clearly, IL-17 immunity plays an important protective role in antifungal immunity. However, IL-17 signalling also has pathogenic potential, such as in the context of some autoimmune disorders (Eyerich, Dimartino and Cavani 2017) (see Immunopathology in candidiasis).
FoxP3 + IL-2Rα(CD25+) regulatory T cells (Tregs) are key mediators of immune regulation that provide endogenous regulatory mechanisms that can prevent potentially harmful immune responses. These Tregs confer immune tolerance through the expression of IL-10 and TGF-β, the consumption of IL-2, and the expression of inhibitory receptors that target T cells directly or indirectly via modulation of DC functionality (Romano et al. 2019). Furthermore, Tregs are developmentally linked to Th17 as they can promote Th17 differentiation by consumption of IL-2 (a cytokine that constrains Th17 differentiation) and, in mice, by providing TGF-β (which promotes Th17 polarisation) (Pandiyan et al. 2011). While Tregs directed against C. albicans are largely expanded in the physiological T cell repertoire in humans (Bacher et al. 2014), their contribution to the maintenance of stable C. albicans homeostasis in barrier tissues remains unclear. In a murine model of C. albicans commensalism, Tregs were dispensable for stable fungal colonisation and an absence of Tregs did not result in dysregulation of the antifungal Th17 response (Kirchner et al. 2019). Instead, the kynurenine pathway, which regulates tryptophan catabolism, might contribute to antifungal tolerance and limit inflammation in mucosal tissues (De Luca et al. 2013).
To summarise, a combination of epidemiological data, association studies in human primary immunodeficiency (PID) syndromes, in vitro challenges with primary human cells, and experiments in various mouse models of superficial candidiasis, have highlighted the importance of Th17 immunity during long-term colonisation of barrier tissues by C. albicans, and the fine lines between fungal commensalism and pathogenicity, and health and disease.
Tissue-specific variability of the mucosal immune response
Candida albicans colonises and causes infections in a range of different tissues, each of which characterised by a different architecture, nutrient supply, metabolic environment, and immune cell composition. Consequently, distinct host defense mechanisms against C. albicans exist in each tissue. Adaptive T cell immunity predominates in fungal control at the skin and most mucosal barriers (except for the vaginal mucosa), whereas innate myeloid cell-mediated mechanisms dominate the immune response to systemic infection (Lionakis 2014). Neutrophils and inflammatory monocytes have also been linked to antifungal immunity in barrier tissues. This notion has arisen primarily from experiments involving acute infections of previously C. albicans-naïve mice with highly virulent C. albicans strains, which trigger a strong inflammatory response and tissue damage. Under such conditions, inflammatory leukocytes (primarily neutrophils) are rapidly recruited to the infected tissues (Conti et al. 2009; K Trautwein-Weidner et al. 2015; Bai et al. 2020) where they prevent deep tissue invasion and mediate the rapid elimination of C. albicans (K Trautwein-Weidner et al. 2015). In contrast, C. albicans colonisation of barrier tissues is not generally accompanied by tissue inflammation (Schönherr et al. 2017), just as fungal commensalism in healthy individuals is not associated with inflammation.
In the vaginal mucosa, pathogenesis is thought to arise largely as a consequence of neutrophil-mediated immunopathology rather than a defect in T cell immunity (Fidel et al. 2004; Giraldo et al. 2012; Rosati, Bruno, Jaeger, Kullberg et al. 2020). Symptomatic infection correlates with elevated infiltration of neutrophils that are not able to limit the fungal burden (Yano, Noverr and Fidel 2017; Ardizzoni et al. 2020).
In contrast to the vaginal mucosa, where Th17 cells do not provide a major protective contribution, Th17 immunity is crucial for controlling the commensal colonisation of C. albicans on the skin and the mucosa of the oral cavity and GI tract (Sparber and LeibundGut-Landmann 2019). Experiments in mice have shown that the mechanisms of Th17 induction vary depending on the tissue. This is probably due to differences in the composition of antigen-presenting cells between the different tissues. Langerhans cells (LCs) are the predominant DC subset in the skin epidermis, but this cell type only represents a fraction of the DC population in the oral and vaginal epithelium (Hovav 2018). In the skin, LCs prime C. albicans-specific Th17 cells (Kashem, Igyarto et al. 2015), but they appear dispensable in the oral mucosa where conventional migratory DCs and monocyte-derived inflammatory DCs execute this task (Kerstin Trautwein-Weidner et al. 2015). In the gut, CX3CR1 + mononuclear phagocytes are essential for the initiation of adaptive immunity against C. albicans (Leonardi et al. 2018). Meanwhile, in the vaginal mucosa, plasmacytoid DCs may dominate and instruct a primarily tolerogenic response (LeBlanc, Barousse and Fidel 2006). Therefore, DCs are central coordinators of antifungal immunity. This relates not only to T cell activation in barrier tissues, but also to systemic candidiasis where DCs are indispensable for organising neutrophil-mediated innate immunity (Whitney et al. 2014).
Explanatory Box 1: Immunopathology
Neutrophils are amongst the first immune cells to be recruited from the bloodstream to the site of infection or tissue injury. Their recruitment is a multi-step process initiated by changes in the endothelium, and is induced by inflammatory mediators secreted by epithelial and tissue-resident immune cells (Kolaczkowska and Kubes 2013). At the site of infection, neutrophils clear pathogens through a combination of mechanisms including phagocytosis, degranulation, and NET formation (Selders et al. 2017; Rosales 2018). However, the secretion of ROS, proteolytic enzymes and AMPs by neutrophils can also lead to tissue injury and collateral damage (Wang 2018). Neutrophils die during the process of NETosis and release their nuclear and cytoplasmic contents. This can result in the presentation of auto-antigens and the production of pro-inflammatory cytokines, DAMPs and alarmins (Wang 2018; Wilgus 2018). DAMPs induce further neutrophil recruitment (Pittman and Kubes 2013), promoting a hyperinflammatory loop that, if not dampened by anti-inflammatory mechanisms, can exaggerate inflammation and tissue damage (Tisoncik et al. 2012). The adaptive immune system also mediates immunopathology via T cells, and Th17 cells in particular. C. albicans-specific Th17 cells promote inflammation and mediate immunopathological effects (Bacher et al. 2019; Shao et al. 2019; Hurabielle et al. 2020). Key anti-inflammatory mechanisms are mediated by Treg cells, myeloid suppressor cells, and anti-inflammatory molecules such as IL-1-family cytokines (IL-1Ra, IL-37, IL-38, IL-36Ra), IL-10, α1-antitrypsin, soluble cytokine receptors, and cytokine binding proteins (Netea et al. 2017; Dinarello 2018). The resolution of inflammation is an active process comprising of numerous signalling pathways that inhibit the inflammatory loop and limit tissue injury, as well as promoting pathogen clearance (Netea et al. 2017).
Immunopathology in candidiasis
The innate and adaptive immune responses provide essential protection against mucosal and life-threatening systemic infections, but uncontrolled inflammation can contribute to disease by causing immunopathology (Explanatory Box 1). There is a balance between immune protection and immunopathology. Using mouse models of systemic candidiasis, some investigators found that type I interferons promote fatal immunopathology through the recruitment and activation of inflammatory monocytes and neutrophils (Majer et al. 2012), whereas others observed reduced survival and concluded that type I interferons are crucial for immunity against C. albicans (del Fresno et al. 2013). Neutrophil accumulation in the kidneys and lung has been shown to cause immunopathology and organ failure in murine models (Lionakis et al. 2011; Desai and Lionakis 2018; Lee et al. 2018). During VVC in mice and humans, candidalysin-induced mucosal damage allows DAMPs and proinflammatory cytokine secretion, which promotes neutrophil recruitment and the exacerbation of inflammation (Richardson et al. 2018). Moreover, activation of the NLRP3 inflammasome and unrestrained IL-1β production can induce a hyperinflammatory state at the vaginal mucosa and acute symptoms of VVC (Rosati, Bruno, Jaeger, Ten Oever et al. 2020). This is influenced by endogenous anti-inflammatory mediators and environmental conditions (Rosati, Bruno, Jaeger, Ten Oever et al. 2020) such as short-chain fatty acids (SCFAs) derived from resident bacteria, which also play a crucial role in the immunopathology of oral candidiasis in mice (Bhaskaran et al. 2018). Th17 polarisation associated with intestinal C. albicans colonisation can be deemed as protective as it can cross-protect against systemic disease (Shao et al. 2019). However, these specific Th17 cells also contribute to allergic airway inflammation (Bacher et al. 2019; Shao et al. 2019) through cross-reactivity to the lung pathogen Aspergillus fumigatus (Bacher et al. 2019). C. albicans-specific Th17 cells can also promote inflammation in the skin and thereby contribute to psoriaform pathology (Hurabielle et al. 2020).
Several endogenous mechanisms regulate inflammation to maintain the balance between immune protection and immunopathology (Netea et al. 2017). The neutrophil response protects against C. albicans by inducing neutrophil chemokines (Mengesha and Conti 2017; Sparber and LeibundGut-Landmann 2019), but these also promote inflammation. The IL-1 family of cytokines, which drive neutrophil responses (Altmeier et al. 2016; Verma et al. 2018), are regulated by endogenous anti-inflammatory cytokines. For example, IL-37 compromises protection against systemic infection by reducing neutrophil influx (van de Veerdonk et al. 2014), but the capacity to reduce this influx potentially makes IL-37 a key player for preventing immunopathology. Other endogenous regulators include IL-1Ra, which neutralises IL-1 signalling and dampens NLRP3 Inflammasome activity, thereby contributing to reduced immunopathology (Borghi et al. 2015). The anti-inflammatory cytokines IL-36Ra and IL-38 can also attenuate the C. albicans-induced Th17 response (van de Veerdonk et al. 2012).
Clearly, molecules that target the IL-17 and IL-1 signalling pathways may have potential therapeutic value as treatments for immunopathology associated with candidiasis. Targeting the NLRP3 inflammasome has also been suggested as a potential strategy to ameliorate inflammation during VVC (Bruno et al. 2015; Richardson et al. 2018). However, the fine balance between protection and pathology must be deciphered before the accurate therapeutic modulation of these pathways can be achieved. Furthermore, the role and therapeutic applications of immunomodulators such as Indoleamine-pyrrole 2,3-dioxygenase 1 (IDO1), an enzyme producing tolerogenic kynurenines (De Luca et al. 2013), should be further evaluated.
Trained Immunity
The classical paradigm of host immune defense is based on the ability of the innate immune system to provide short term protection, combined with the capacity of adaptive immunity to mount immunological memory and provide long-lasting protection against the same pathogen. A growing body of evidence now shows that the innate immune system is able to generate immunological memory, independently of adaptive immunity. This phenomenon, which is termed ‘trained immunity’, has been described in invertebrates, plants, and mammals (Kurtz and Franz 2003; Durrant and Dong 2004; Netea, Quintin and van der Meer 2011), and is based on functional reprogramming of innate immune cells.
Candida albicans, and individual components of its cell wall, are potent immune modulators (see Cell wall). Even in mice that are deficient in T and B lymphocytes (i.e. lack adaptive immunity), an initial non-lethal exposure to C. albicans provides protection against a subsequent C. albicans infection (Bistoni et al. 1986). This resistance to re-infection was described as a macrophage-dependent mechanism associated with enhanced production of the proinflammatory cytokines TNF, IFN-γ, and IL-1β (Vecchiarelli et al. 1989). Moreover, protection was not restricted to disseminated candidiasis: cross-protection to unrelated pathogens such as Staphylococcus aureus was also induced (Bistoni et al. 1986; Netea, Quintin and van der Meer 2011). Further studies demonstrated that stimulation with C. albicans or β-glucan, leading to activation of the dectin-1/PI3K-Akt-mTOR axis (Quintin et al. 2012; Cheng et al. 2014), elicits epigenetic remodeling of the transcriptional repertoire (Saeed et al. 2014). This leads to a shift in immune cell metabolism from oxidative phosphorylation to aerobic glycolysis (the Warburg effect) (Cheng et al. 2014), and enhanced pro-inflammatory cytokine production (Quintin 2019; Netea et al. 2020). Further studies revealed that β-glucan-primed monocytes differentiate into macrophages that display highly active metabolic activity and increased glucose consumption (Leonhardt et al. 2018). Interestingly, C. albicans-induced trained immunity is defective in chronic mucocutaneous candidiasis (CMC) patients, indicating that STAT-1 signalling is involved in the induction of trained immunity (Ifrim et al. 2015).
The induction of trained immunity depends strongly on the nature of the ligand and the PRR that is activated. For example, while TLR4 activation by lipopolysaccharide (LPS) can lead to a state of immunotolerance or immunoparalysis that compromises antifungal host defense (Grondman et al. 2019), the TLR4 agonist, monophosphoryl lipid A (MPLA), has been recently reported as an inducer of trained immunity (Fensterheim et al. 2018). Immunotolerance in sepsis patients increases the risk of secondary infections, including candidiasis (Otto et al. 2011). Conversely, trained immunity induced by C. albicans can enhance protection against sepsis in mice (Cheng et al. 2014). In addition, C. albicans colonisation of the GI tract provides protection against a variety of systemic pathogens (Tso et al. 2018). Therefore, the temporary transcriptional and metabolic rewiring via β-glucan-administration might provide a strategy to revert the LPS-induced tolerance of innate immune cells. Indeed, pharmacological targeting in myeloid cells, for example, by inhibition of the phosphatase SHIP-1 (Saz-Leal et al. 2018) or the IRG1-itaconate-SDH axis (Domínguez-Andrés et al. 2019), could play a pivotal role in harnessing beneficial effects of trained immunity (Mulder et al. 2019).
Variability amongst individuals
Variation between individuals influences the host-fungus interaction and susceptibility to fungal infection. The identification of candidate genetic traits is, therefore, pivotal for the selection of patients that would benefit from host-directed therapy or antifungal prophylaxis.
The effectiveness of a person's anti-fungal immune response is severely impaired if they acquire an immunocompromised status, for example through HIV-induced AIDS, neutropenia induced by cytostatic therapy, or immunosuppressive therapy during organ transplantation. Furthermore, certain genetic variations compromise the efficacy of immune pathways and exert strong detrimental effects upon antifungal immunity. Genetic susceptibility to fungal infection has been comprehensively studied and reviewed (Lionakis 2012). Mutations in STAT1, for instance, predispose individuals to CMC (Puel et al. 2011; van de Veerdonk, Plantinga et al. 2011). Also, inborn errors in Th17 or CARD9 immunity are associated with recurrent mucosal and invasive candidiasis, respectively (Puel 2020). Interestingly, genetic immunodeficiencies often lead to different susceptibilities to fungal infections of the mucosal surfaces, skin, and nails. Similarly, HIV patients develop oropharyngeal candidiasis (OPC) more often than vaginal infections (VVC) (Fidel 2002). This is consistent with the existence of distinct anti-Candida immune mechanisms in different mucosal niches (see Tissue-specific variability of the immune response).
Many genetic polymorphisms in PRRs have been associated with impaired antifungal host defense (Jaeger, Stappers et al. 2015). For instance, SNPs in the TLR1 and TLR4 genes increase the risk of candidaemia (Plantinga, Johnson et al. 2012; Van der Graaf et al. 2006), and a variable number tandem repeat (VNTR) polymorphism in the NLRP3 gene is associated with increased susceptibility to VVC (Jaeger et al. 2016). Susceptibility to mucosal or systemic candidiasis varies depending on the nature of the receptor or effector molecule that is mutated. For example, a homozygous mutation in the dectin-1 gene is more likely to predispose the individual to CMC (Ferwerda et al. 2009), whereas defects in CARD9 result in systemic, mucosal, and subcutaneous candidiasis (Drewniak et al. 2013; Lionakis and Holland 2013; Lanternier, Pathan et al. 2013). Interestingly, CARD9-deficient individuals are prone to fungal proliferation in the central nervous system (CNS), but not in the kidney, spleen, or liver (Drummond et al. 2015), which highlights an organ-specific CARD9-dependent immune mechanism, such as IL-1β/CXCL1-mediated neutrophil recruitment by microglial cells (Drummond et al. 2019).
Genome-wide association studies (GWAS) have been performed to identify genetic polymorphisms associated with susceptibility to infectious diseases (Newport and Finan 2011), and overviews of comprehensive multi-omic systems approaches towards an understanding of host-fungal interactions have been published (Horn et al. 2012; Culibrk, Croft and Tebbutt 2016). The first GWAS analysis for fungal infections identified three novel risk loci associated with increased susceptibility and severity of candidaemia: CD58, LCE4A-C1orf68, and TAGAP (Kumar et al. 2014). Although GWAS is an ideal approach for the identification of novel genetic associations with susceptibility to fungal infections, it is difficult to achieve a high level of statistical significance (<5 × 10–8) with the generally small cohorts of candidaemia patients available (Manolio 2010; Chapman and Hill 2012). Hence, the power of GWAS can be enhanced by combining the outputs with systems biology, transcriptomics and available knowledge of immunology and microbiology, to pinpoint disease-associated genetic determinants. The functional validation of putative hits in an independent cohort can underline the relevance of newly identified genetic associations. For instance, the integration of gene expression data and functional genomics revealed the importance of type I IFNs in the host response against C. albicans (Smeekens et al. 2013; Jaeger, van der Lee et al. 2015). Also, using a computational approach based on publicly available transcript profiling data sets, MALT1, SERPINE1, ICAM1, IL8, and IL1A were discovered as common immune response-inducing genes during fungal infection (Kidane, Lawrence and Murali 2013). Furthermore, combining genetic data from candidaemia cohorts with immune-profiling of C. albicans-stimulated cells, the MAP3K8 and SERPINA1 genes were shown to contribute to candidaemia susceptibility (Matzaraki et al. 2017). Mapping genetic determinants to variability in transcription or cytokine levels can lead to the identification of expression quantitative trait loci (eQTL) or cytokine-quantitative trait loci (cQTL), respectively (i.e. the genetic variation associated with different levels of transcriptional and cytokine responses). The analysis of eQTLs is leading to an understanding of how human genetic variation affects the anti-Candida host response and of the populations of cells involved in the clearance of the pathogen (de Vries et al. 2020). The investigation of cQTL datasets revealed SIGLEC15 as a susceptibility factor for RVVC (M Jaeger et al. 2019) as well as susceptibility pathways, such as lipid homeostasis and inflammation, that affect the response of monocytes to fungal bloodstream infections (Martin Jaeger et al. 2019).
In addition to genetic variability, external factors such as broad-spectrum antibiotics or immunosuppression regimens negatively influence the microbial community. This, in turn, affects metabolic homeostasis (Zarrinpar et al. 2018) and host resistance to both antibiotic-resistant microbes and fungal pathogens (Ubeda and Pamer 2012). For instance, preexposure to antibiotics not only increases Candida colonisation levels in the GI tract, but also facilitates disruption of the mucosal barrier and leads to C. albicans bloodstream infections (Das et al. 2011; Gutierrez et al. 2020). This could be due to the loss of protection from microbiota-derived metabolites, such as short-chain fatty acids (Guinan et al. 2019; Gutierrez et al. 2020). Moreover, antibiotic-induced dysbiosis can reduce pro-inflammatory cytokine production towards LPS stimulation (Lankelma et al. 2016). Thus, supplementation with probiotics may represent a useful strategy to counterbalance the negative effects of antibiotic-induced therapy and improve the host immune response against fungal infections (Ubeda and Pamer 2012).
THE MICROBIOTA
Gastrointestinal (GI) tract
The collection of microbes that colonises the GI tract is termed the ‘gut microbiota’, and is composed of bacteria, archaea, eukaryotic microbes and viruses. Thousands of microbial strains have been detected in the human gut, and these microbes can be important contributors to human health and disease (Fig. 6). For example, the gut microbiota plays key roles in nutrition (by degrading dietary components that would otherwise pass through the GI tract undigested), in host immune development and maintenance, and in protecting the host against pathogenic microbes, including C. albicans. This latter process, termed ‘colonisation resistance’, is multifactorial. It involves both microbe-microbe interactions (such as competition for nutrients, niches and binding sites, and the release of antimicrobial substances), and host-microbe interactions (whereby the microbiota can stimulate the host's immune system or strengthen the gut epithelial barrier against invading pathogens) (Lawley and Walker 2013). Significantly, an individual's degree of colonisation resistance is thought to be strongly influenced by the composition of their gut microbiota, with some individuals being more intrinsically resistant to infection than others (Ubeda et al. 2010).
Figure 6.
Oral, vaginal and GI microbiota, and factors that influence C. albicans colonisation of these body sites. The major microbial groups (family level for bacteria and genus level for fungi) of the healthy oral cavity (only for bacteria) (Bik et al. 2010; Dewhirst et al. 2010), GI tract (Booijink et al. 2010; Arumugam et al. 2011; Zhou et al. 2013; Villmones et al. 2018) and vagina (Human Microbiome Project Consortium 2012) are listed in decreasing order of abundance. Pie charts indicate the relative abundance of the phyla in a representative healthy oral cavity and colon (see key for colour code). The fungal component of the oral microbiota is extremely variable, and many fungi present in this compartment are likely to be transient (see text). Therefore, for the oral cavity, the fungal genera are not presented in descending order of abundance, and no pie chart is provided. The lower panel summarises factors that influence the degree of C. albicans colonisation (yellow) and likelihood of infection (brown arrows) of these mucosal surfaces: arrows up, increased likelihood of colonisation/infection; arrows down, decreased likelihood of colonisation/infection. See text.
Most gut microbiota studies have focussed on the bacterial component, which accounts for the greatest proportion of biomass present by far (Qin et al. 2010; Arumugam et al. 2011). These gut bacteria display both antagonistic and mutualistic relationships with C. albicans and other members of the fungal community (mycobiota) that help to maintain homeostasis in the human GI tract. Fungi represent just ∼0.1% of the GI tract biosphere, which makes the fungal mycobiota more challenging to study than the bacterial microbiota (Underhill and Iliev 2014). Also, mechanical lysis steps are best employed during DNA extraction to recover reasonable quantities of fungal DNA (Angebault et al. 2018), and because different extraction and sequencing methods have been used, gut mycobiota compositional analyses are difficult to compare between studies (Bellemain et al. 2010; Tedersoo et al. 2015; Tedersoo and Lindahl 2016; Huseyin et al. 2017; Angebault et al. 2018). Many mycobiota studies provide information about genera (e.g. Candida) rather than species (e.g. C. albicans). This section discusses causes of variability in the bacterial microbiota of the human GI tract and, where possible, the impact upon the GI mycobiota (fungal microbiota), and C. albicans in particular.
Variability along the GI tract
The compartments of the human GI tract, including the small intestine, caecum and large intestine (colon), have variable physiology and, as a result, each harbours distinct microbial communities (Fig. 6). Compared to the colon, the small intestine contains comparatively high levels of stomach acids, oxygen and antimicrobials, and is characterised by a short transit time. The small intestine also contains higher concentrations of bile acids, which are bactericidal to certain microbial species (Donaldson, Lee and Mazmanian 2016). Accordingly, the microbial community of the small intestine is less diverse than the colonic microbiota, and tends to be dominated by fast-growing facultative anaerobes such as streptococci and Proteobacteria that have the ability to adhere to epithelia or mucus (Zoetendal et al. 2012).
Moving from the small intestine, the caecum is the gateway to the colon. In the caecum, relatively long transit times and the prevailing environmental conditions favour the growth of fermentative anaerobes that can degrade complex polysaccharides, notably members of the Firmicutes and Bacteroidetes phyla (Donaldson, Lee and Mazmanian 2016). In contrast to the small intestine, the structure and physiology of the colon allows the survival of a more dense and diverse bacterial community, which can reach densities of up to 1011 cells per gram of colonic contents, one of the highest microbial concentrations in nature. The colon contains two layers of mucus, secreted by goblet cells, which separate colonic epithelial cells from the bacterial mass. The inner, firmly attached mucus layer is nearly sterile, whereas the outer layer is in direct contact with the luminal contents and can be a rich niche for microbial colonisation (Johansson et al. 2008, 2010). The luminal and mucosal compartments of the colon are often colonised by different profiles of bacterial populations. In a mouse model, C. albicans cells are visible in the lumen as well as the mucus layer (Witchley et al. 2019).
The fungal component of the GI tract is less diverse than its bacterial counterpart (Chehoud et al. 2015; Nash et al. 2017). Levels of fungal colonisation are lower in the small intestine, compared to the oral cavity and colon (Schulze and Sonnenborn 2009). Nevertheless, C. albicans can colonise the stomach, small intestine, caecum, and colon of mice (Witchley et al. 2019). The human intestinal mycobiota is characterised by a high inter- and intra-individual variability, which makes it difficult to define a ‘normal’ or healthy GI mycobiota composition (Nash et al. 2017; Raimondi et al. 2019). However, Ascomycota and Basidiomycota represent the two dominant phyla in the GI tract (Chehoud et al. 2015; Nash et al. 2017). The most frequently identified genera are Candida (e.g. C. albicans), Saccharomyces (e.g. S. cerevisiae), Galactomyces, Penicillium, Aspergillus, Malasezzia and Debaryomyces. Some of these fungi may not be true colonisers of the GI tract, but transient species that are brought by food or the environment. Consequently, an individual's lifestyle has a strong influence on their GI mycobiota and its variability (Auchtung et al. 2018; Raimondi et al. 2019).
Variability between individuals
Each individual has a distinct intestinal microbiota composition and structure. Many factors such as birth delivery mode, environmental exposure to colonising microbes, host genetics, host diet and lifestyle contribute to the unique nature of a given individual's intestinal bacterial and fungal microbiota (Qin et al. 2010; Salonen et al. 2014; Mehta et al. 2018), including their carriage of C. albicans (Neville, d Enfert and Bougnoux 2015) (Fig. 6).
Variability across lifespan
The mode of childbirth strongly affects the initial structure of the gut microbial community in neonates (Reyman et al. 2019). Bacteria such as Enterobacter, Haemophilus, Staphylococcus and Veillonella species are found in relatively high abundances in the faecal microbiota of babies born via caesarean (C)-section (Bäckhed et al. 2015). In contrast, the faecal microbiota of vaginally-delivered babies is enriched in Bifidobacterium, Lactobacillus, Prevotella and Atopobium spp., which are typically derived from the vagina of mothers (Dominguez-Bello et al. 2010). Babies delivered vaginally appear to be twice as likely to become colonised by C. albicans compared to those born by C-section (Parm et al. 2011).
Following birth, the main driver of gut microbiota composition is infant diet (breastfeeding versus formula milk). The colon of infants that are exclusively breast-fed is characterised by high numbers of milk oligosaccharide-utilising Bifidobacterium species (Yatsunenko et al. 2012; Odamaki et al. 2016; Hill et al. 2017), whereas formula-fed infants tend to possess more diverse microbiota that are less dominated by bifidobacteria (Rubaltelli et al. 1998; Klaassens et al. 2009; Lee et al. 2015). Following the introduction of solid food, alpha-diversity (i.e. the variation of microbes in a single sample) increases and the microbiota transitions towards an adult-like composition (Yatsunenko et al. 2012; Schei et al. 2017). Little is known about C. albicans GI primocolonisation, but this species has been detected in newborns and infants (Bliss et al. 2008; Schei et al. 2017) and in maternal milk, suggesting that breastfeeding could be a source of colonisation (Boix-Amorós et al. 2017).
Once established, the adult bacterial microbiota is generally considered to be quite stable over many decades (Faith et al. 2013), albeit allowing for temporary disruptions by factors such as antibiotic treatment or inflammation. Every adult has a distinct microbiota at the species/strain level. Nevertheless, in the mature adult gut, obligate anaerobes generally predominate, the microbiota typically containing high levels of Firmicutes and Bacteroidetes spp. (Qin et al. 2010). There is significant variation between studies (see Variability due to diet and geography), but Candida spp. are thought to be present in the GI tracts of over half of adults (Odds 2010; Hoffmann et al. 2013). However, the fungal mycobiota of the adult GI tract appears to be less stable than the bacterial microbiota (Dollive et al. 2013).
Further changes in the microbiota occur in the elderly (generally over 65), possibly associated with altered dietary habits and living environments, reduced metabolism and immune function, and increased antibiotic usage (Lovat 1996; Simon, Hollander and McMichael 2015). Accumulating evidence suggests that old age can be associated with a decrease in ‘beneficial’ bacteria and an increase in ‘harmful’ species (Xu, Zhu and Qiu 2019), potentially making the elderly population more susceptible to C. albicans colonisation (Kauffman 2001; Miranda et al. 2009).
Variability arising from host genetics, diet and geography
The composition of the gut microbiota might also be shaped to some degree by the genetics of the individual, although recent work has shown that this only has a minor impact (between 1.9% and 8.1% of gut microbiota variability) compared to other factors such as environmental exposure and host diet (Rothschild et al. 2018) Studies of twins have indicated that certain types of bacteria might be more influenced by host genetics than others. For example, the Christensenellaceae family is more likely to be influenced by host genetics, while Bacteroidetes carriage is most likely shaped by environmental factors (Simões et al. 2013; Goodrich et al. 2014).
An individual's dietary choices have major impacts on the composition of their bacterial community, and diet is therefore an important driver of inter-individual variation (Walker et al. 2011; David, Maurice et al. 2014). The main energy sources for colonic microbiota are complex plant fibres. These can be recalcitrant to degradation by host enzymes in the small intestine and therefore pass into the colon relatively intact. Additional energy sources include residual peptides and host secretions such as mucus (Derrien et al. 2004, 2008; Hai Li et al. 2015; Van den Abbeele et al. 2010; Lukovac et al. 2014; Van Herreweghen et al. 2017; Van den Abbeele, Gérard et al. 2011; Tramontano et al. 2018). The type and availability of these various nutrients exert selective effects on numerous groups of bacteria. The starkest difference is between animal-based (high fat and protein content) and plant-based diets (rich in plant polysaccharides). Plant-based diets lead to increases in Prevotella species and Firmicutes, whereas animal-based/lower fibre diets stimulate an increase in Bacteroides and bile-tolerant species such as Bilophilia and Alistipes (Wu et al. 2011; David, Maurice et al. 2014; De Filippis et al. 2016; Pareek et al. 2019).
Differences in dietary habits between people inhabiting different regions of the world (He et al. 2018), and between those living in urbanised versus rural settings, are considered to be a main driver of geographical variation in microbiota composition. Indeed, the migration of people from rural to westernised settings greatly impacts the composition of their resident intestinal microbiota (Vangay et al. 2018). Those living in less urbanised societies typically consume greater amounts of dietary fibre and less meat and processed foods. Consequently, they tend to have a greater predominance and prevalence of more specialist fibre-degrading bacteria in their gut. In contrast, people living in cities or more urbanised countries, are characterised by their consumption of more refined, high protein and high fat diets, and they harbour microbial communities with reduced diversity (Schnorr et al. 2014).
Geographic location also affects the prevailing mycobiota, especially as many of the fungi detected in the GI tract are not true colonisers, but only transient species brought in as a result of different diets/environmental exposures (Auchtung et al. 2018; Raimondi et al. 2019). For example, Aspergillus oryzae, a species used to ferment soybeans to make soy sauce, is often present in the guts of healthy Japanese (Motooka et al. 2017). Also, the relatively high abundance of Penicillium and Debaryomyces spp. in Sardinian volunteers has been linked with high levels of cheese consumption in this region (Wu et al. 2020). Interestingly, the GI tracts of Wayampi Amerindians harbour a relatively high abundance of Candida krusei and S. cerevisiae, and less C. albicans, compared to individuals with more industrialised lifestyles (Angebault et al. 2013). However, the carriage of Candida spp. in the gut has been negatively associated with amino acid-, protein-, and fatty acid-rich diets (Hoffmann et al. 2013), which are characteristic of urbanised societies, suggesting that geographically-related factors other than diet may also affect the likelihood of Candida carriage in the gut. These may include exposure to environmental stressors and pollutants, including antibiotics (Jin et al. 2017; Karl et al. 2018) (see below).
Variability due to lifestyle and xenobiotics
A range of non-dietary lifestyle factors can also impact the gut microbiota and its resilience against invading pathogens. For example, exposure to stress is thought to lower the numbers of potentially beneficial gut bacteria such as Lactobacillus spp., and this has been postulated to have multiple effects on colonic motor activity via the gut-brain axis (Grenham et al. 2011; Galley et al. 2014; Murakami et al. 2017). Therefore, Lactobacillus spp. have been proposed as candidates for probiotic intervention (Bravo et al. 2011). Interestingly, preliminary studies have shown that Lactobacillus might reduce C. albicans overgrowth (Drutz 1992; Ceresa et al. 2015; Morais et al. 2017), and reductions in the prevalence of Lactobacillus spp. in the gut may be associated with stress-induced candidiasis (Meyer, Goettlicher and Mendling 2006; Akimoto-Gunther et al. 2016). Indeed, L. rhamnosus has been shown to reduce the capity of C. albicans to damage epithelial barriers and translocate into the ‘bloodstream’ in an intestine-on-chip model (Graf et al. 2019; Maurer et al. 2019).
Many xenobiotics interact with, and influence, the gut microbiota. In turn, these may increase the risk of developing opportunistic infections by disrupting colonisation resistance. Antibiotics have been the most studied xenobiotics. In addition to treating the aetiological agent of a disease, long-term broad-spectrum antibiotics can exert collateral damage upon beneficial indigenous gut bacteria (Dethlefsen et al. 2008; Fouhy et al. 2012; Burdet et al. 2019). This can have the unintended effect of suppressing colonisation resistance, leading to the outgrowth of opportunistic pathogens. This includes C. albicans, as antibiotic treatments permit persistent C. albicans colonisation of the GI tract in mice that are normally resistant to colonisation (Fan et al. 2015). Several studies have attempted to define the mechanisms underlying this outgrowth (Guinan et al. 2019; Gutierrez et al. 2020; Zhai et al. 2020). Cefoperazone-treated mice display reduced levels of the short-chain fatty acids generated by the gut microbiota, which enhances C. albicans growth, morphogenesis and biofilm formation (Guinan et al. 2019). On the other hand, the outgrowth of C. albicans in antibiotic-treated mice has been linked to increased levels of carbohydrates, sugar alcohols and primary bile acids as well as decreases in carboxylic acids and secondary bile acids (Gutierrez et al. 2020). Although the effect of antibiotics on the gut mycobiota of healthy humans remains largely unknown, the administration of antibiotics to immunocompromised patients has been associated with decreases in the diversity of the gut microbiota and marked expansions in the burdens of pathogenic Candida species (Zhai et al. 2020). The extent of overall microbiota recovery after cessation of antibiotic treatment depends on the spectrum of activity of the antibiotic, the length of time it was administered, and the underlying composition of the baseline gut microbiota. In general, the microbiota appears to be reasonably resilient to short courses of certain antibiotics, displaying an ability to recover after treatment with, for example, ciprofloxacin (Pop et al. 2016) or azithromycin (Wei et al. 2018). However, recovery is not always complete (Dethlefsen et al. 2008; Fouhy et al. 2012).
Attention has also turned towards the susceptibility of the microbiota to non-antibiotic xenobiotics, many of which are commonly used drugs (Jackson et al. 2018; Maier et al. 2018; Vich Vila et al. 2020). Proton pump inhibitors (PPIs) have been the most studied non-antibiotic xenobiotics (Jackson et al. 2018; Vich Vila et al. 2020). Some evidence suggests that the use of PPIs increases the risk of Candida colonisation in intensive care patients (Mojazi Amiri et al. 2012; Jacobs et al. 2015). Histamine-2 receptor blockers also disturb colonisation resistance against opportunistic infections, primarily C. albicans (Saiman et al. 2001).
Variability associated with illness
Perturbations in the GI tract microbiota are associated with a multitude of disorders such as Inflammatory Bowel Disease (IBD), diabetes, obesity, colorectal cancer and cirrhosis. IBD includes conditions such as Crohn's disease (CD) and ulcerative colitis (UC). These diseases can further drive variability within the gut microbiota. Increasing evidence suggests that people suffering from some of these conditions display even more inter-individual variability than healthy controls (Zaneveld, McMinds and Vega Thurber 2017). IBD patients tend to have reduced overall microbiota diversity with decreased prevalence of potentially beneficial Firmicutes lineages such as Faecalibacterium prausnitzii. They also have increased levels of opportunistic pathogens such as Enterobacteriaceae, which are better able to thrive in an inflammatory environment than many other obligately anaerobic gut commensals (Manichanh et al. 2006; Sokol et al. 2009; Pascal et al. 2017; Franzosa et al. 2019; Lloyd-Price et al. 2019). IBD patients often show a disequilibrium in the diversity of bacteria and fungi in their GI tracts (Wheeler et al. 2016), which suggested that Candida spp. might also play a role in IBD pathogenesis. C. albicans, and the Candida genus in general, are more abundant in IBD patients (Ott et al. 2008; Kumamoto 2011; Chehoud et al. 2015; Sokol et al. 2017). Recent data indicate that Malassezia, rather than Candida, is associated which Crohn's disease (Limon et al. 2019). Nevertheless, a positive clinical response to faecal microbiota transplantation in ulcerative colitis patients has been associated with high levels of Candida spp. colonisation before treatment and decreased Candida abundance in the gut following treatment (Leonardi et al. 2020).
Patients with primary sclerosing cholangitis (PSC) also harbour decreased bacterial diversity, while the fungal diversity in their GI tract is increased (Lemoinne et al. 2020). Patients with Clostridioides difficile infections that have received a faecal microbiota transplant, often show reduced fungal diversity and C. albicans outgrowth in their gut. Indeed, a high abundance of C. albicans in the donor's gut might compromise the success of the faecal transplantation (Zuo et al. 2018). Alcoholic hepatitis has been associated with an increase in the abundance of Candida spp. in the gut mycobiota and a decrease of fungal diversity (Lang et al. 2020), while an outgrowth of Candida spp. has also been observed in children suffering from autistic spectrum disorders (Strati et al. 2017). Therefore, changes in the gut mycobiota are associated with, and potentially contribute to, a wide range of pathologies.
Oral cavity
Defining the core oral microbiota for a healthy individual is complicated by the fact that the oral cavity is a primary entry point for microbes in food and from the environment. Thus, microbes identified in the oral cavity may be transient, and washed out through saliva before having any impact upon health, rather than being active colonisers of this niche. Nevertheless, the oral cavity does harbour the second largest microbiota, in terms of diversity, compared to other body sites (Zhou et al. 2013) (Fig. 6).
Variability between individuals
Many of the factors that contribute to the variability of GI tract microbiota have a similar impact upon the microbiota present at other body sites, such as the oral environment (Fig. 6).
Variability across lifespan
The development of the oral microbiota in infants is influenced by their mode of delivery (Lif Holgerson et al. 2011; Dzidic et al. 2018). Infants born by C-section initially have more oral colonisers, such as Staphylococcus, Corynebacterium and Propionibacterium spp., which are derived from human skin (Dominguez-Bello et al. 2010). In contrast, babies born vaginally have bacterial communities reflecting their mothers’ vaginal bacterial communities, dominated by Lactobacillus, Prevotella and Sneathia spp. Candida spp. are identified more frequently in the oral microbiota of newborns that were vaginally born, especially by mothers whose vagina was colonised by Candida (Al-Rusan, Darwazeh and Lataifeh 2017).
After 6 months of age, the impact of delivery mode is gradually eliminated as microbial patterns converge to that observed for older individuals. The oral microbial communities then evolve together over time with the host. This applies to both the oral bacterial and fungal microbiota. However, no consistent pattern has emerged for fungal colonisation, with conflicting results observed between studies (Baley et al. 1986; Caramalac et al. 2007; Farmaki et al. 2007; Bliss et al. 2008; Siavoshi et al. 2013; Filippidi et al. 2014; Stecksén-Blicks et al. 2015; Ward et al. 2018). Some studies have suggested vertical transmission from mother to child (Caramalac et al. 2007; Filippidi et al. 2014). Other studies consider breastmilk to be a source of fungal colonisation, with Malassezia (44%), Candida (19%) and Saccharomyces (12%) being the main taxa detected within one month of birth (Boix-Amorós et al. 2017). However, once again, no consistent pattern has emerged (Darwazeh and al-Bashir 1995; Matee et al. 1996; Mattos-Graner et al. 2001; Kadir, Uygun and Akyüz 2005; Neves et al. 2015; Stecksén-Blicks et al. 2015). The development of the oral mycobiota can also be influenced by nail biting and finger sucking, which might enhance the colonisation by microbes usually found on the skin (e.g. Malassezia spp.) (Dupuy et al. 2014), and the use of pacifiers has also been correlated with increased fungal colonisation (Darwazeh and al-Bashir 1995; Mattos-Graner et al. 2001; Zöllner and Jorge 2003).
By the age of three, children have developed a complex oral microbial community, although they carry higher levels than older children of Pseudomonadaceae, Moraxellaceae and Enterobacteriaceae, which are not usually associated with healthy commensal oral microbiota (Crielaard et al. 2011). The oral bacterial microbiota of healthy adults is marked by increased proportions of Bacteroidetes (Prevotella spp.), Spirochaetes, Actinobacteria and Firmicutes (Keijser et al. 2008; Crielaard et al. 2011). The fungal taxa most frequently isolated from the oral cavity are Candida spp. and S. cerevisiae (foodborne) (Baley et al. 1986; Darwazeh and al-Bashir 1995; Matee et al. 1996; Mattos-Graner et al. 2001; Zöllner and Jorge 2003; Kadir, Uygun and Akyüz 2005; Farmaki et al. 2007; Filippidi et al. 2014; Neves et al. 2015; Ward et al. 2018). C. albicans is the Candida species most frequently isolated from the oral cavity, although other species such as C. tropicalis, C. krusei, C. kefyr and C. glabrata have also been detected.
After maturation of the microbiota, the oral cavity is thought to have the most stable microbial profile among all body sites (Zhou et al. 2013). Several studies have analysed temporal variation in the salivary microbiota (Caporaso et al. 2011; David, Materna et al. 2014; Flores et al. 2014; Belstrøm et al. 2016). This revealed high variability in the relative abundances of taxa, with, for instance, greater stability in individuals harbouring a more diverse tongue community (Flores et al. 2014). As with the GI tract microbiota, there is evidence that the oral microbiota can be influenced by birthplace and current geographic residence (Xu and Mitchell 2003; Wang et al. 2013).
Supragingival, tongue and salivary communities display strong inter-individual and inter-site differences (Hall et al. 2017). Among all the anatomical sites of the oral cavity, the supragingival plaque community is distinct from that of the tongue plaque and the saliva, with high similarity between the tongue and saliva. The supragingival plaque harbours a bacterial community with much lower diversity compared with that of the tongue and the salivary communities. Saliva has the highest number of bacterial taxa while supragingival plaque has the lowest. Hall and co-workers (Hall et al. 2017) identified 26 core taxa, belonging to five phyla (Actinobacteria, Bacteroidetes, Firmicutes, Fusobacterium, and Proteobacteria), across all sites. Few taxa were shared among all sites.
Variability arising from diet
Dietary factors contribute to the variation of oral microbial communities. In general, foods are swallowed quickly after a short period of mastication. Nevertheless, the introduction or sudden lack of certain nutrients can cause shifts in the oral microbiota (Adler et al. 2013; Zheng et al. 2015). For example, microbes that contribute to folate biosynthesis, such as Streptococcus, increase after long-term deprivation of fresh fruit and vegetables, which are rich in folic acid (Zheng et al. 2015). Vegetarians and non-vegetarians display similar rates of C. albicans carriage in their oral microflora (Patil et al. 2017). Fungi are introduced to the oral cavity via food and drink. Therefore, fungi commonly derived from fermented beverages such as beer are often isolated from the oral cavity (Fan et al. 2018).
Variability associated with illness
The most common oral conditions include tooth decay, periodontal disease and oral cancer. While many intestinal diseases have been associated with gut dysbiosis, there is still debate as to whether oral diseases are correlated with oral microbial diversity. For example, periodontal disease patients display more diverse and complex oral microbial communities than peri-implantitis patients (Kumar et al. 2012; Liu et al. 2012). Nevertheless, pathogenic Streptococcus spp. promote caries by lowering oral pH, which results in the demineralisation of enamel (Ajdić et al. 2002; Mei et al. 2013; Ito et al. 2019). Interactions between bacteria and fungi are likely to be of relevance to oral health. Interestingly, a study assessing Candida load and the bacterial composition of saliva in a Dutch cohort revealed that a low diversity of salivary microbiota characterised by dominant acidogenic bacilli (streptococci and lactobacilli) is positively correlated with elevated Candida burdens and possible overgrowth (Kraneveld et al. 2012). However, only certain diseases correlate with fungal colonisation of the oral cavity. These include, but are not limited to, HIV/AIDS (Cassone and Cauda 2012), cancer treatments (Silk 2014), dental caries (Falsetta et al. 2014) and oral lesions (ulcerations, nodules or granulomas) (Muzyka and Epifanio 2013). All of these conditions are linked either to the creation of novel niches that are not naturally present, or to perturbation of immune function. They are often correlated with Candida overgrowth (C. albicans in 70–80% cases), leading to oropharangeal candidiasis, particularly in immunocompromised individuals (Millsop and Fazel 2016).
Vaginal mucosa
Variability between individuals
Interactions between the resident microbiota and C. albicans in the vaginal tract are important for pathogenesis (Fig. 6). The vaginal bacterial microbiota of healthy reproductive-age women is generally dominated by Lactobacillus spp. (Ravel et al. 2011). Lactic acid production by these bacteria contributes to a healthy vaginal pH that is commonly lower than 4.5 (Ravel et al. 2011). The vaginal bacterial microbiota can be further sub-classified into five main community state types (CSTs): CST-I (Lactobacillus crispatus-dominated); CST-II (L. gasseri-dominated); CST-III (L. iners-dominated); and CST-V (L. jensenii-dominated) (Ravel et al. 2011). The CST-IV state is extremely diverse compared to the other types, comprising anaerobes and species linked to bacterial vaginosis (BV). CST-IV has been further divided into subgroups: CST-IVA (containing some lactobacilli); CST-IVB (high prevalence of Atopobium spp.); CST-IVC (Gardnerella subgroup A-dominated); and CST-IVD (Gardnerella subgroup C-associated) (Gajer et al. 2012; Albert et al. 2015). Women can transition between these CST states, for example, during menses (Gajer et al. 2012). CST-I has been associated with C. albicans colonisation (Sarah E Brown et al. 2019), but more studies are required to fully understand the complexity of the vaginal microbiota and its potential association with disease.
Less is known about the mycobiota of the human vagina. Culture-dependent studies indicate that C. albicans is the most abundant fungal species, although its abundance has been shown to vary according to lifestyle, age, ethnicity, hygiene habits and contraceptive methods (Fischer 2010; Wei, Feng and Luo 2010; Fischer and Bradford 2011; Shaaban et al. 2015; Donders et al. 2017, 2018). Indeed, intrauterine contraceptive systems have been reported to be associated with a rise in C. albicans colonisation, while progesterone-only pills result in lower rates of colonisation (Donders et al. 2017, 2018). However, due to the relatively low sensitivity of conventional culture approaches, these studies may underestimate the true fungal diversity of the vagina (Guo et al. 2012; Drell et al. 2013). Using 18S rRNA gene sequencing, Guo and co-workers demonstrated that the healthy vaginal mycobiota was mainly composed of Ascomycota (∼70% relative abundance), with the Candida genus dominating, and C. albicans as the main species. Basidiomycota were also detected, but with a lower proportional abundance (Guo et al. 2012). These results were confirmed using ITS1 pyrosequencing (Drell et al. 2013). Taking these findings together, the most abundant fungi in the healthy vaginal tract appear to be C. albicans, S. cerevisiae and C. tropicalis (Guo et al. 2012). Recent data indicate an association between the type of Lactobacillus species present and the likelihood of Candida colonisation (Tortelli et al. 2020).
Variability associated with age and pregnancy
The vaginal bacterial microbiota is influenced by oestrogen levels and is most stable when these are high (Gajer et al. 2012) (Fig. 6). Prepuberty is characterised by a bacterial microbiota comprised of anaerobes, diphtheroids, lactobacilli, streptococci, Staphylococcus epidermidis, and Escherichia coli (Hammerschlag et al. 1978). During puberty, increased oestrogen stimulates thickening of the glycogen-rich vaginal epithelium and establishes a vaginal microbiota dominated by lactobacilli (Miller et al. 2016). High oestrogen levels in reproductive women create unique features for the vaginal mucosa (Kalia, Singh and Kaur 2020), inducing a tolerogenic immune repertoire through immunomodulation of the neutrophil response (Willems et al. 2020). When glycogen is degraded by host α-amylases, products such as maltose and maltotriose foster the growth of Lactobacillus, leading to a reduced vaginal pH (Spear et al. 2014).
Lactobacillus spp. typically dominate the vaginal microbiota during pregnancy, and increased levels of these bacteria were reported in Lactobacillus-dominated CSTs compared to non-pregnant women (Aagaard et al. 2012; Romero et al. 2014; MacIntyre et al. 2015; Freitas et al. 2017). During pregnancy, the microbiota is also characterised by a lower occurrence of Mollicutes, and by members of the orders Clostridiales, Bacteroidales, and Actinomycetales (Aagaard et al. 2012; Freitas et al. 2017). Sampling six weeks postpartum revealed that bacterial diversity increases following birth and the vaginal microbiota readily assumes CST-IV (MacIntyre et al. 2015).
Fluctuations in oestrogen levels probably underlie variations in the abundance of C. albicans. Indeed, oestrogen injection is required to promote C. albicans colonisation of the vagina in rats and mice (Cheng, Yeater and Hoyer 2006). This is probably linked to the stimulatory effects of oestrogen upon C. albicans morphogenesis (White and Larsen 1997; Tarry et al. 2005). In humans, rising oestrogen levels during pregnancy has also been associated with an increase in C. albicans colonisation (Goplerud, Ohm and Galask 1976), which can potentially lead to premature birth (Roberts et al. 2011).
Postmenopausal women are also more likely to display a CST-IV microbiota (Brotman, Shardell et al. 2014). Menopausal women frequently experience a loss of lactobacilli and an increased vaginal pH (Brotman, Shardell et al. 2014; Gliniewicz et al. 2019). This elevation in vaginal pH, combined with an increase in vaginal glycogen levels, may contribute to the reduced incidence of VVC observed after the menopause (Hillier and Lau 1997; Spinillo et al. 1997). The reduced levels of oestrogen may also explain the decreased rates of VVC in postmenopausal women (Nwokolo and Boag 2000). Consequently, hormone replacement therapy (HRT) is a risk factor for VVC in these women (Fischer and Bradford 2011). HRT can restore a Lactobacillus dominated microbiota, similar to that of premenopausal women (Gliniewicz et al. 2019), and this treatment increases the likelihood of postmenopausal women succumbing to VVC (Fischer and Bradford 2011). However, more comprehensive studies are needed for a better understanding of the relationship between the overall vaginal mycobiota and health and disease.
Variability relating to geography and ethnicity
Independent of geography, the vaginal microbiota of women is dominated by Lactobacillus (Anukam et al. 2006; Shi et al. 2009; Zhou et al. 2010; Ravel et al. 2011; Pendharkar et al. 2013; Albert et al. 2015; Madhivanan et al. 2015). Nevertheless, the dominating species of the Lactobacillus genus may differ between geographical regions. Similar rates of vaginal colonisation by Candida spp. (11–17%) have been reported for asymptomatic women from European, South American and Middle Eastern countries (Gonçalves et al. 2016). However, VVC rates differ significantly for symptomatic women around the world, ranging from 12% to 57%, and most cases are caused by C. albicans (Gonçalves et al. 2016).
Regarding ethnic differences, Asian and Caucasian women from North America are mainly colonised by Lactobacillus spp. (CST-I, II, III and V), whereas black and Hispanic women are more likely to be colonised by CST-IV communities (Ravel et al. 2011). Women of African, American and European ancestry are more likely to be colonised by L. iners and L. crispatus, respectively (Fettweis et al. 2014). However, the basis for these differences is not clear (Gupta, Kakkar and Bhushan 2019). The evidence for different VVC rates between ethnic groups is limited (Wei, Feng and Luo 2010). Further studies would be required to define whether significant differences exist, and to parse apart the basis for any apparent differences.
Variability arising from lifestyle and xenobiotics
A number of factors influence the vaginal microbiota and, consequently, may predispose women to infection or aid in preventing infection (Fig. 6). The effects of antibiotics on the microbiota of women with vaginal infections are well studied. Metronidazole treatment of women with BV has been shown to increase prevalence of Lactobacillus spp. (Mayer et al. 2015). Similarly, the vaginal microbiota becomes dominated by L. iners when azithromycin is administered to treat Chlamydia trachomatis (Tamarelle et al. 2020). The composition of the lactobacilli community can shift in response to antibiotics, since vaginal Lactobacillus spp. have varying antibiotic sensitivity profiles (Melkumyan et al. 2015). Pregnant women frequently become colonised with Staphylococcus when receiving antibiotic treatment (Stokholm et al. 2014). It is well known that antibiotic treatments predispose individuals to VVC if they are already colonised with Candida spp. (Sobel 2007).
Not much is known regarding the impact of diet on the vaginal bacterial microbiota. Individuals consuming fibre-rich diets are less likely to have BV-associated microbiota (Shivakoti et al. 2020). Ingestion of micronutrients such as the zwitterion betaine (an osmolyte and methyl donor) may result in a microbiota that is predominantly lactobacilli (Tuddenham et al. 2019). In addition, smoking reduces vaginal lactobacilli (potentially via amines, anti-oestrogenic effects and bacteriophage induction) and increases the probability of acquiring a CST-IV microbiota (Brotman, He et al. 2014). As the vaginal microbiota influences the vaginal mycobiota (Sobel 2007), these effects are likely to influence C. albicans colonisation.
Translational opportunities
Given the impact that the microbiota appears to have on susceptibility to Candida infections, there are clear potential therapeutic benefits to bolstering our microbial communities at various body sites. Probiotics provide a means of altering the microbiota. Probiotics are defined as ‘live microorganisms that, when administered in adequate amounts, confer a health benefit on the host’ (Hill et al. 2014). Currently, robust evidence for clinical efficacy is limited to a relatively narrow set of conditions. However, there is clear potential to widen this applicability to IBD, for example (Rondanelli et al. 2017). Interactions between the microbiota and host are thought to play key roles in Candida colonisation and pathogenesis, and therefore, live biotherapeutic products (LBPs) that exert anti-Candida effects are worthy of further study. (LBPs are products containing live microbes that are used to prevent or treat a medical condition.) Some are under development (Poupet et al. 2019). These have the potential to dramatically ameliorate the economic and health burden imposed by this fungus and reduce the risk of vulnerable individuals to Candida infections. Several types of patient cohort may benefit from such an approach.
Premature neonates are among those most at risk of developing systemic Candida infections. Compared to full-term healthy babies, premature newborns have an altered microbiota and can be colonised by opportunistic pathogens (Hill et al. 2017; Korpela et al. 2018). In addition to weakened colonisation resistance, their immature immune system places them at risk of late-onset sepsis caused by C. albicans, which has colonised their GI tract through vertical transmission from their mothers, or from the hospital environment (Bliss et al. 2008). Supplementation with a Lactobacillus probiotic (L. rhamnosus and L. reuteri) results in lower GI tract colonisation of Candida spp. compared to controls (Manzoni et al. 2006; Romeo et al. 2011). L. reuteri was found to be as effective as the antifungal nystatin in preventing candidaemia (Oncel et al. 2015). Replacing prophylactic antifungal treatments with LBP-based therapy would have the advantage of reducing the selection for antifungal drug resistance. Additionally, LBPs may benefit premature neonates by preventing GI symptoms such as regurgitation, vomiting and abdominal distension (Indrio et al. 2008; Rougé et al. 2009; Romeo et al. 2011), while reducing hospitalisation time (Romeo et al. 2011). Consequently, the use of LBPs for premature neonates may help decrease their risk of developing nosocomial infections.
HIV positive individuals are another group at high risk of developing C. albicans infections, especially oropharyngeal candidiasis (Patil et al. 2018). Administration of probiotic strains with anti-Candida activity could prevent the development of such infections by reducing levels of fungal colonisation (Hu et al. 2013).
Studies have shown that LBPs are more successful than placebos at preventing recurrence of vulvovaginal candidiasis (Vladareanu et al. 2018). Furthermore, therapies that combine LBPs with azoles have been shown to improve drug efficacy by restoring the local bacterial community (Kovachev and Vatcheva-Dobrevska 2015; Russo et al. 2019). Increasing the efficacy of the antifungal drug in this way may allow drug doses to be reduced.
Taken together, current evidence is encouraging and suggests that probiotics can be used to prevent C. albicans infections in vulnerable cohorts. However, further studies are required to identify optimal LBP candidates, and to understand the underlying mechanisms of action that result in clinical efficacy.
THE FUNGUS-HOST-MICROBIOTA INTERPLAY
The previous sections describe the multifaceted nature of C. albicans interactions with the host, how antifungal immunity influences these interactions, and how the microbiota is closely related to host physiology and impacts C. albicans colonisation. These tripartite interactions between host, fungus and microbiota are incredibly complex and strongly influence the likelihood and outcomes of Candida infections. Here we discuss the nature of interdependencies within the fungus-host-microbiota interplay and their impact on health and Candida infections, in particular.
Synergistic and antagonistic interactions between kingdoms
Many researchers have focused on antagonistic interactions between C. albicans and bacteria species because these could potentially be exploited in therapeutic approaches (see Impact of a changing microbiota on the Fungus-Host-Microbiota interplay and Translational opportunities). However, it is estimated that approximately 30% of all Candida bloodstream infections are polymicrobial and involve both fungi and bacteria (Klotz et al. 2007). This suggests that synergism can occur between C. albicans and certain bacterial species (Fig. 7). [In this context, ‘synergism’ describes polymicrobial interactions during which one microorganism promotes colonisation or infection by another (Brogden, Guthmiller and Taylor 2005)].
Figure 7.
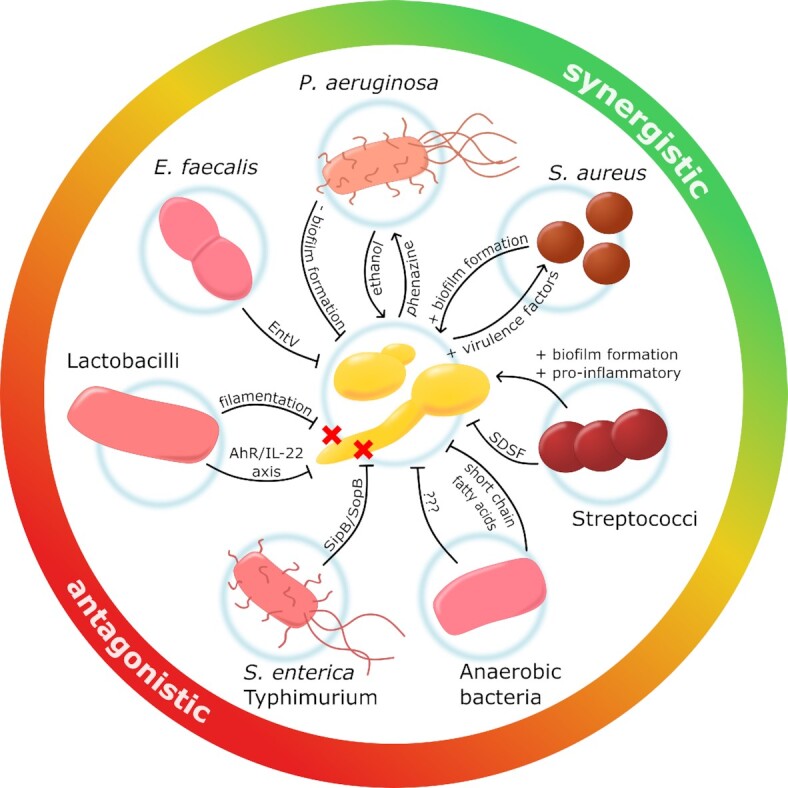
The interplay between C. albicans and certain bacterial present in human microbiotas. The growth of C. albicans in mucosal niches is generally constrained by the local bacterial microbiota via colonisation resistance. However, specific interactions with certain bacteria present in the vagina, oral cavity and/or GI tract influence the growth and/or virulence of C. albicans more directly. These interactions can be antagonistic (i.e. reduce the growth and virulence of the fungus) or synergistic (i.e. enhance the growth or virulence of the fungus). Anaerobic bacteria antagonise C. albicans colonisation by mechanisms that include the production of short chain fatty acids. S. enterica Typhimurium kills C. albicans hyphae by injecting effectors into the fungus via the SopB translocase. Lactobacilli antagonise C. albicans colonisation acidifying the local environment which reduces filamentation, and by generating metabolites that enhance IL-22-mediated immunity. E. faecalis blocks yeast-hypha morphogenesis and biofilm formation using EntV. Interactions between C. albicans and P. aeruginosa mutually enhance their virulence via cross-talk involving ethanol production by the fungus, which promotes toxic phenazine production by the bacterium, and this in turn promotes alcohol production by C. albicans. S. aureus binds C. albicans hyphae and promotes biofilm formation and antimicrobial resistance. Furthermore, co-infection with S. aureus significantly enhances the lethality of C. albicans. Streptococci block the formation of C. albicans hyphae via the diffusible factor, SDSF, but potentially co-exist as commensals with C. albicans. See text.
Candida albicans interacts with different types of resident microorganisms depending on the body site (see The Microbiota). Candida albicans synergises with various streptococcal species that are abundant in the oral cavity, through physical interactions that enhance bacterial growth and adhesion, lead to more pronounced biofilm formation and, in some cases, increase fungal invasion (Silverman et al. 2010; Diaz et al. 2012; Metwalli et al. 2013; Xu et al. 2016; Koo, Andes and Krysan 2018; Vila et al. 2020). Molecules involved in these physical interactions include bacterial adhesins (Holmes, McNab and Jenkinson 1996; Silverman et al. 2010), specific fungal surface proteins (Holmes, McNab and Jenkinson 1996; Dutton et al. 2014; Xu et al. 2017), and components of the extracellular polysaccharide matrix produced in biofilms (Falsetta et al. 2014). Communication within mixed biofilms involves bacterial and fungal quorum sensing systems that influence the expression of virulence factors and bacterial metabolism (Sztajer et al. 2014; He et al. 2017; Kim et al. 2017). In addition to this direct synergism, streptococcal co-infection stimulates complex immune reactions that promote the expression of proinflammatory cytokines and enhanced tissue inflammation in a murine model of C. albicans thrush (Xu et al. 2014). The clinical importance of these synergistic interactions is suggested by co-colonisation of bacteria with fungi in oral diseases such as childhood caries, periodontitis, and denture stomatitis (O'Donnell et al. 2015). Furthermore, a causal relationship between bacterial-fungal co-infection and disease severity has been demonstrated for caries in a rat model (Falsetta et al. 2014). Fungal colonisation also affects oral microbiota composition, which encourages invasive infection (Bertolini et al. 2019).
The synergistic cross-kingdom interactions between C. albicans and Staphylococcus aureus have been comparatively well studied (Carolus, Van Dyck and Van Dijck 2019) (Fig. 7). Staphylococcus aureus can bind to C. albicans hyphae, which indirectly enhances the attachment of the bacterium to abiotic surfaces and promotes the formation of mixed biofilms with increased resistance to antimicrobial compounds (Shirtliff, Peters and Jabra-Rizk 2009; Harriott and Noverr 2010; Peters et al. 2010; Harriott and Noverr 2011; Kong et al. 2016; Kean et al. 2017). Even more striking, though, is the enhanced lethality observed following co-infection in a mouse model (Peters and Noverr 2013). A synergistic enhancement of virulence occurs, independent of the ability of C. albicans to form filaments (Nash et al. 2016). This synergy is driven by an augmented host immune response (Nash et al. 2016, 2014; Peters and Noverr 2013). In addition, the presence of C. albicans increases the expression of staphylococcal virulence factors by modifying the environment (Todd et al. 2019; Todd, Noverr and Peters 2019). Significantly, this synergistic virulence depends on the Candida species involved, and was not observed for the closely related, but less virulent species, Candida dubliniensis.
Some relationships are difficult to place within a dichotomous scheme of synergism or antagonism. In some cases, diffusible molecules underlie inter-kingdom interactions (Deveau et al. 2018) as well as microbiota-induced immunomodulation of the host (Blacher et al. 2017). Most bacterial molecules target C. albicans virulence factors. Salmonella enterica serovar Typhimurium uses SipB translocase to inject SopB effectors and induce killing of the fungal hypha (Kim and Mylonakis 2011). Enterococcus faecalis restricts biofilm development by preventing yeast-to-hypha transition via the bacteriocin inhibitor EntV (Graham et al. 2017). Streptococcus mutans prevents hypha formation by targeting HWP1 (a hyphal-specific gene) using Streptococcus Diffusible Signal Factor (SDSF), a fatty acid. (Vílchez et al. 2010). These molecules do not act on the yeast form, indicating a potential propensity for commensal co-existence.
The host responds to, and influences, some fungal-bacterial interactions. This has been observed for interactions between C. albicans and Pseudomonas aeruginosa, which engage in an interactive molecular dialogue that leads to mutual enhancement of their virulence (Chen et al. 2014) (Fig. 7). C. albicans produces ethanol, which favours the production of more toxic classes of phenazines by P. aeruginosa, such as pyocyanin, phenazine methosulfate and phenazine-1-carboxylate. As well as inhibiting filamentation and biofilm formation, these phenazines induce C. albicans to produce more ethanol by compromising mitochondrial functionality (Morales et al. 2013; Lindsay et al. 2014). Ethanol reduces the ability of macrophages to clear P. aeruginosa (Greenberg et al. 1999), while phenazines cause damage to respiratory epithelial tissues (Rada and Leto 2013). The mammalian host contributes actively to this interplay by responding to phenazines via the Aryl hydrocarbon Receptor (AhR) to enhance antimicrobial defences (Moura-Alves et al. 2014). Significantly, there is a strong association between ethanol production by Candida and the development of oral cancer (Alnuaimi et al. 2016).
The mammalian AhR is a multi-class receptor that modulates disease resistance (by activating IL-17A/IL-22 production) and disease tolerance (via TGF β activated Treg cell differentiation) (Cheng et al. 2010; Zelante et al. 2013; Bessede et al. 2014) (see Adaptive immunity). AhR functions within the indoleamine 2,3-dioxygenase 1 (IDO1)-catalysed pathway that converts tryptophan to L-kynurenine (Bessede et al. 2014). This pathway plays a dual role as microbial commensals use it to enhance host resistance, while pathogenic populations exploit it to dampen immune responses (Cheng et al. 2010; Zelante et al. 2013) (Fig. 7). Lactobacilli can switch their usage of carbon sources from sugar to tryptophan and utilise this pathway to initiate strain- and location-specific host effects that protect against C. albicans infection (Zelante et al. 2013). L. reuteri produces indole-3-aldehyde (3-IAld), which binds AhR and triggers the production of IL-22 in the gut. Meanwhile, L. acidophilus utilises the AhR/IL-22 axis against C. albicans in the vagina. However, C. albicans is able to switch tryptophan degradation mechanisms from L-kynurenine to 5-hydroxyltryptophan, which inhibits IL-17 production and impairs the host response against infections (Cheng et al. 2010). These examples illustrate the complexity of interactions between the fungus, the host and the microbiota.
Impact of a changing microbiota on the Fungus-Host-Microbiota interplay
The host microbiota contributes to anti-Candida defences through colonisation resistance. Consequently, perturbations of the healthy oral, gut and vaginal microbiota can predispose the host to C. albicans infection (see The Microbiota) (Fig. 8). However, C. albicans is not a passive player in these interactions (see The Fungus). For example, the fungus actively promotes oral microbiota perturbations under conditions of immunosuppression by increasing the prevalence of enterococci, which negatively impacts the integrity of the epithelial barrier and enhances C. albicans invasion (Bertolini et al. 2019). The complexity of interactions between the fungus, host and microbiota are also evident in the vagina, where high oestrogen levels promote Lactobacillus spp. colonisation, and affect C. albicans morphogenesis, thereby influencing the risk of C. albicans colonisation (see Vaginal mucosa). Colonisation resistance against C. albicans arises though a number of synergistic mechanisms, many of which target fungal virulence traits or modulate the host's response.
Figure 8.
The complexity of fungus-host-microbiota interactions is dramatically increased by variability between C. albicans clinical isolates, between individuals, and in their microbiotas. Fungal variability arises through significant genetic and phenotypic variation between clinical isolates of C. albicans. The immune-competence of individuals varies significantly depending on their genetics, age and lifestyle. Furthermore, the microbiotas of the GI tract, oral cavity and vagina can each vary dramatically, depending on the age and health of the individual, and their diet, possible medications and life circumstances. Therefore, variation in each of the three elements of the fungus-host-microbiota interplay strongly influences the susceptibility of an individual to C. albicans infection as well as the outcome of that infection. See text.
Several members of the gut microbiota that contribute to colonisation resistance against C. albicans produce short chain fatty acids (SCFAs). Antibiotic treatment leads to a reduction in colonic SCFAs and, consequently, an increase in the susceptibility of mice to C. albicans infection (Noverr and Huffnagle 2004; Guinan et al. 2019). Butyrate, in particular, has a profound impact on C. albicans growth, biofilm and hypha formation (Nguyen et al. 2011; Guinan et al. 2019). In the colon, such effects are unlikely to be mediated by weak acid stress (Ramsdale et al. 2008) given that the ambient pH of this niche is above the pKa for this SCFA. Rather, butyrate perturbs iron homeostasis (Cottier et al. 2015) and inhibits the metabolic activity of the fungus (Nguyen et al. 2011; Guinan et al. 2019) via Mig1 regulation of HGT16, which encodes a putative glucose transporter (Cottier et al. 2017). In vitro studies have demonstrated that SCFAs impair C. albicans morphogenesis and biofilm formation, in part by reducing the ambient pH (Noverr and Huffnagle 2004; Nguyen et al. 2011; Guinan et al. 2019). Similarly, lactic acid, generated by lactobacilli, maintains an acidic vaginal pH that inhibits C. albicans morphogenesis (Köhler, Assefa and Reid 2012). Although Lactobacillus spp. also produce hydrogen peroxide, it is believed that lactic acid is the main basis for anti-Candida activity in the vagina (Strus et al. 2005; Köhler, Assefa and Reid 2012).
Both hypha and biofilm formation promote the pathogenicity of C. albicans. The formation of C. albicans biofilms depends upon the yeast-to-hypha transition, and is a significant clinical challenge (see Virulence factors). Some members of the microbiota have been shown to hinder C. albicans morphogenesis and biofilm formation via secreted enzymes (Allonsius et al. 2019) or other products (Jarosz et al. 2009; Vílchez et al. 2010; James et al. 2016; Oliveira et al. 2016; Hager et al. 2019; Jang et al. 2019). Nevertheless, C. albicans can form polymicrobial biofilms with some members of the oral and gut microbiota, which display elevated drug and host resistance and can strongly influence clinical outcomes (Harriott and Noverr 2011; Fox et al. 2014; Cavalcanti et al. 2015).
Competition for adhesion sites and nutrients, especially glucose, by members of the microbiota also contributes to colonisation resistance against C. albicans in the gut, vagina and oral cavity (Boris et al. 1998; Basson 2000; Graf et al. 2019). L. rhamnosus GG is a common gut and oral isolate (Ahrné et al. 1998) that has been shown to prevent C. albicans-induced damage and invasion through both nutrient depletion and blocking of adhesion sites (Mailänder-Sánchez et al. 2017). L. rhamnosus can also reduce C. albicans proliferation and induce shedding from the epithelial barrier, thereby preventing invasion of the fungus into the tissue (Graf et al. 2019). Interactions between the microbiota, including microbial-associated factors, and the host immune system help to regulate C. albicans and prevent dissemination.
The microbiota also influences the colonisation of C. albicans indirectly by influencing host functionality. Macrophages exposed to microbiota-produced butyrate are more efficient at phagocytosing C. albicans cells, and they produce increased levels of nitric oxide, which enhances eradication of the pathogen (Nguyen et al. 2011). In response to butyrate generated by the microbiota, colon epithelial cells express the AMP, LL-37 (Schauber et al. 2003), which exerts candidacidal effects (see Innate antifungal responses). These host cells also activate LL-37 production in response to microbiota-induced hypoxia via HIF-1α (Hypoxia Induced Factor 1α) (Fan et al. 2015). Blautia producta and Bacteroides thetaiotaomicron, both common members of the human gut microbiota, promote colonisation resistance and eliminate C. albicans by stimulating LL-37 production in mice (Fan et al. 2015). Colonisation resistance against C. albicans is also provided by IL-22, which is produced by the host and induced by lactobacilli (Zelante et al. 2013). In addition, L. rhamnosus GG modulates the inflammatory response of epithelial cells by reducing IL-1α and GM-CSF production (Mailänder-Sánchez et al. 2017). By limiting the C. albicans-induced proinflammatory response of vaginal cells (with the exception of IL-1α and IL-1β), lactobacilli can alleviate symptomatic vulvovaginal candidiasis while sustaining an antifungal immune response (Wagner and Johnson 2012). Similarly, L. crispatus reduces epithelial TLR2/4 expression and IL-8 levels in the presence of C. albicans, but maintains antifungal activity by increasing β-defensin production (Rizzo, Losacco and Carratelli 2013). Clearly, the changes in the microbiota strongly influence the iterative interactions between fungus, host and microbiota. Specific probiotic bacteria, including Bifidobacterium breve, L. rhamnosus, and Lactobacillus casei can also modulate specific PRR ligand- and C. albicans-induced cytokine responses (Plantinga, van Bergenhenegouwen et al. 2012).
Impact of patient variability upon the Fungus-Host-Microbiota interplay
The nature of an individual affects the types and outcomes of fungal-microbiota interactions directly and indirectly by: (i) genetic determinants that influence immune responses; (ii) personal environment and lifestyle, which affect the microbiota and (iii) iatrogenic interventions that target the microbiota or host response (Fig. 8). As outlined above, the microbiota is critical for colonisation resistance, leading to host protection, but on the other hand, certain combinations of opportunistic pathogens synergise to promote infection (see Synergistic and antagonistic interactions between kingdoms). It is well known that diet strongly influences the human gut microbiota (David, Maurice et al. 2014; Jeziorek, Frej-Mądrzak and Choroszy-Król 2019). Fundamental differences in diet, and possibly also exposure to microbes, are the most likely reason for the observed differences in C. albicans colonisation rates between industrialised and rural countries, which can differ by over 10-fold (Angebault et al. 2013) (see Gastrointestinal (GI) tract). Antibiotic treatments are probably the most common iatrogenic intervention that directly affects the microbiota, and one of the main predisposing factors to candidiasis in general. More specific iatrogenic factors include oral contraceptives and dental prostheses, which alter the local mucosal environment and thereby promote vaginal and oral candidiasis, respectively (Mothibe and Patel 2017; Jacob et al. 2018).
A healthy immune system is crucial for protection against fungal infections (see The Host). Individuals vary in their susceptibility to C. albicans infection because of genetic differences that affect susceptibility, and the existence of coexisting morbidities in some individuals. Genetic variations in key receptors or molecular effectors have been shown to increase the risk of Candida infections (see Variability amongst individuals). For instance, monogenic primary immunodeficiency syndromes highly predispose an individual to haematogenously disseminated candidiasis and mucosal candidiasis (e.g. OPC, skin, nails). However, the genetic mutations defined to date do not explain the observed variation in susceptibility to candidiasis within not-at-risk subjects. Phenotypic variation occurs also in healthy individuals. For instance, if primary immune cells from healthy immunocompetent individuals are challenged with C. albicans in vitro, different outcomes are observed due to variation in their immune cell populations (Misme-Aucouturier et al. 2017). This can arise through genetic variation at the CR1 locus, which encodes a master regulator of C. albicans-specific immune responses (Piasecka et al. 2018). Thus, inter-individual variability in innate and adaptive responses against Candida spp. are likely to influence the degree of host-mediated damage during infection (Alvarez-Rueda et al. 2020). Consequently, understanding the basis of subject-to-subject diversity, and how this affects Candida pathogenicity, is likely to prove important for prevention and therapeutic strategies.
Comorbidities and treatment of other diseases can also affect a patient's susceptibility to C. albicans infection (see C. albicans commensalism and pathogenicity). Uncontrolled diabetes, for example, favours both bacterial and yeast infections due to metabolic alterations and impaired antimicrobial activity (Rodrigues, Rodrigues and Henriques 2019). Admission to an ICU, medical surgery, hematopoietic stem cell transplantation, and the use of external devices are independent risk factors for candidaemia and, together with the duration of hospitalisation, affect the mortality rates for candidiasis infections (Ortega et al. 2005; Das et al. 2011; Falcone et al. 2017; Poissy et al. 2020). These patients are commonly immunocompromised, either as a result of their primary disease, or through treatment. For example, OPC is a hallmark of HIV positive individuals and cancer patients (Samaranayake 1992; Redding et al. 1999). Moreover, as mentioned, dysregulated innate immunity is associated with failure to clear Candida spp., for example in neutropenic patients or neutrophil-related disorders (Nucci et al. 1997; Desai and Lionakis 2018). Glucocorticoids (Fan et al. 2012) and chemotherapy (Teoh and Pavelka 2016) weaken the host defence and increase the risk for invasive candidiasis.
Impact of fungal variability on the Fungus-Host-Microbiota interplay
Variability in C. albicans-host relationships is driven by the fungus as well as the host and its microbiota (Fig. 8). Clinical isolates of C. albicans display a high degree of genetic and phenotypic diversity (see Candida albicans epidemiology and variability). This fungal diversity can be observed at the genetic level (Tavanti et al. 2006; Cavalieri et al. 2017; Schönherr et al. 2017) as well as the transcriptional level (Thewes et al. 2008). The variation impacts multifarious aspects of C. albicans biology, such as stress and nutrient responses, and virulence properties such as morphogenesis, adhesion and invasion, that consequently, influence initial host-pathogen interactions, as well as colonisation and infection (see The Fungus). Therefore, it is not surprising that fungal variation affects the fitness of a given C. albicans strain in the host, and also disease outcome (Thewes et al. 2007, 2008; Cavalieri et al. 2017; Schönherr et al. 2017; Kirchner et al. 2019).
In principle, C. albicans strains can be classified on the basis of their virulence, rather than their epidemiological relationship. Comparative studies of various C. albicans isolates have identified genes whose expression or lack of expression strongly influences the virulence potential of these strains. Examples include EFG1, encoding a key transcription factor involved in morphogenesis (Hirakawa et al. 2015), and DFG16, encoding a pH sensor (Thewes et al. 2007). Strains displaying reduced expression of EFG1 or DFG16 display reduced virulence in mouse models of systemic infection (Thewes et al. 2007; Hirakawa et al. 2015). Even the development of hemizygosity at the EFG1 locus is sufficient to promote commensalism, rather than pathogenicity, in C. albicans (Liang et al. 2019).
A number of studies have highlighted the significance of variabilities between C. albicans isolates. The three C. albicans isolates, SC5314, 101 and ATCC10231 are all able to form hyphae. Nevertheless, SC5314 displays enhanced tissue invasion compared to ATCC10231 and 101 (Thewes et al. 2007; Schönherr et al. 2017), resulting in higher virulence. The strain SC5314 triggers rapid neutrophil recruitment and the production of proinflammatory cytokines leading to fungal clearance of the oral mucosa. In contrast, strains with lower virulence induce slower and weaker immune responses, which lead to fungal persistence (Schönherr et al. 2017). Similar results have been observed in a murine model of vaginal colonisation, where a less immunostimulatory C. albicans strain is able to persist over five weeks, in contrast to SC5314, which is cleared by week three (Rahman et al. 2007). The genetic background of C. albicans also influences survival in the phagosome (Tavanti et al. 2006; Cavalieri et al. 2017), the relative importance of specific PRRs for fungal clearance in vivo (Marakalala et al. 2013) and even the polarisation of the immune response (Cavalieri et al. 2017). Clearly, the intraspecies diversity of C. albicans has major consequences for the outcome of host-pathogen interactions.
NEW CHALLENGES
Elaborating the complexity of the microbiota
Meta-omics
The ability to define the complexity of relevant microbiotas rapidly and accurately represents a major challenge. It is vital that we address this challenge to establish phenotypic associations with specific members of the microbial community. Meta-omics, which refers to culture-independent functional and sequence-based analysis of the collective microbial genomes, transcriptomes, proteomes or metabolomes in a given sample (Handelsman et al. 1998; Riesenfeld, Schloss and Handelsman 2004), includes a powerful set of approaches to achieve this.
To date, DNA sequence-based studies have often been based on the analysis of amplicons generated from the microbial community with specific primers that are typically targeted towards bacterial 16S ribosomal RNA genes, and fungal 18S rRNA genes or internal transcribed spacer (ITS) regions. These approaches can provide comprehensive overviews of microbiota compositions, without directly assessing functional capabilities. However, the continuing development of DNA sequencing and genome analysis bioinformatics tools is permitting more widespread use of full shotgun metagenomics instead. This approach does not rely on amplification of marker genes since the extracted DNA is sequenced directly. The approach is more expensive and computationally intensive than marker gene sequencing because it requires sequencing to be carried out at a much higher depth. However, it has the advantages of avoiding biases associated with the amplification step, and generating information on both the function and composition of microbiotas, thereby providing information at much greater resolution (Walker et al. 2014; Almeida et al. 2019). For example, a recent metagenomic analysis of the human gut microbiota from over eleven thousand individuals identified 1952 candidate bacterial species that have not yet been cultured and increased the known phylogenetic diversity of the gut microbiota almost three-fold (Almeida et al. 2019). Furthermore, these new genomes were estimated to encode hundreds of new biosynthetic gene clusters, revealing valuable clues about the potential functionalities of these novel candidate species.
In principle, full shotgun metagenomics can also be applied to mycobiota studies, but further advances are essential before fungal metagenomes can be analyzed more accurately. In particular, the lack of non-redundant and comprehensive fungal databases presents one of the most significant limitations. The accuracy of sequence classification depends fundamentally on the quality of the reference database, and, due to the large number of microbial species that have not yet been identified or genome sequenced, the existing databases are incomplete. This problem is gradually being lessened however, by the continual addition of genomes from newly isolated species (see Culturomics) and metagenome-assembled genomes (MAGs), which can provide reasonably accurate draft genomes for uncultured organisms.
Metagenomics can also be complemented by other -omic approaches to increase the power of meta-analyses. For example, metagenomics is being combined with meta-transcriptomic [i.e. the combined transcriptomes of the microbial community as a whole (Martinez et al. 2016; Franzosa et al. 2018)], proteomic (Van Belkum et al. 2018; Zhou et al. 2019), and metabolomic data sets (Smirnov et al. 2016; Yachida et al. 2019). Major software challenges must be addressed to improve the efficiency with which these different data sets can be integrated. For example, linking a metabolic gene to its transcript is relatively straightforward, but linking these to the corresponding enzyme and metabolic reaction is less so. Nevertheless, studies such as these are enhancing the associations between the composition of the gut microbiota and disease state for numerous conditions, including cancer, diabetes and inflammatory bowel diseases (Erickson et al. 2012; Smirnov et al. 2016; Zhang et al. 2018; Yachida et al. 2019; Zhou et al. 2019). These technologies have the potential to revolutionise our understanding of fungus-host-microbiota interactions and, as a result, our ability to develop personalised therapeutic strategies for individuals at risk of life-threatening fungal infections.
Culturomics
In the early 2010s, the use of high throughput culturing coupled to MALDI-ToF mass spectrometry (MS) revolutionised clinical microbiology (Seng et al. 2009; Bizzini et al. 2010; van Veen, Claas and Kuijper 2010). This has since been termed ‘culturomics’ (Lagier et al. 2012). In brief, culturomics can identify atypical bacteria by combining multiple culture conditions (Beijerinck 1901; Weinstein 1996) with MALDI-ToF MS and 16S rRNA gene sequencing. The pioneering study used 212 culture conditions (Lagier et al. 2012), which was subsequently reduced to 18 conditions (Lagier et al. 2015) and recently the overall workload has been further reduced (Chang et al. 2019). Culturomics permits the identification of microbial minorities present at concentrations lower than 1e + 05 CFU/mL, which can encompass up to 65% of bacterial species in a given sample (Lagier et al. 2012). This not only enables a better description of the bacterial diversity (Dubourg et al. 2014), but also provides viable microbes for downstream analysis. Downstream characterisations of the new species can include pathogenic potential, metabolic functionality and interactions with other residents of the microbiota studied.
Newly identified species whose genomes have been sequenced can be used to identify previously found, yet unidentified, operational taxonomic units (OTUs), thus filling gaps in sequence-based analyses (Rinke et al. 2013; Lagier et al. 2016). Between 2015, when 2172 different species cultured from different human body sites were reported (Hugon et al. 2015), and 2018, 288 new species were isolated by culturomics (Bilen et al. 2018). Therefore, culturomics and metagenomics are complementary techniques, with an overlap as small as 15% of detected species in a given sample (Lagier et al. 2012, 2015; Pfleiderer et al. 2013; Dubourg et al. 2014; Mailhe et al. 2018).
Models for the experimental dissection of Fungus-Host-Microbiota interactions
Model experimental systems are essential for the detailed mechanistic dissection of disease establishment and progression in humans. Models of fungal infection can simulate the process with some degree of accuracy, but they never recapitulate human infections perfectly. Therefore, selecting an appropriate model is a crucial step that requires consideration of many parameters, such as similarity to the human situation, cost, workload, throughput and ethical concerns (Maccallum 2012; Brunke et al. 2015; Poupet et al. 2020). It is important to reconsider the relevance of a model to the human condition, and to clearly define the limitations of the model as well as the aspects of the human infection that are recapitulated by the model.
Mucosa simulating models
Rodents, particularly mouse models, have been used extensively to study vaginal and oral candidiasis (Rahman et al. 2007; Solis and Filler 2012; Cassone and Sobel 2016), as well as systemic candidiasis (MacCallum and Odds 2005; Szabo and MacCallum 2011; Brunke et al. 2015), allowing investigators to better understand the pathogenicity of C. albicans. However, there are significant differences between the immune systems of mice and humans (Mestas and Hughes 2004). Also, most laboratory mice are not naturally colonised by C. albicans and therefore do not develop a primed immunity to this opportunistic pathogen (Cassone and Sobel 2016). Moreover, the GI microbiota established in laboratory rodents generally mediates colonisation resistance against C. albicans. Thus, antibiotic treatment is required for prolonged high-level colonisation of the murine gut by C. albicans (Conti, Huppler et al. 2014). Oral models generally focus on infection, rather than colonisation, and immunosuppressive treatment is usually required to induce OPC (Solis and Filler 2012). Also, to study VVC in mice, oestrogen treatment is necessary to facilitate vaginal colonisation by C. albicans (Cassone and Sobel 2016).
Alternative in vivo mammalian models have been used to study Fungus-Host-Microbiota interactions, such as piglets and non-human primates, which are naturally colonised by C. albicans. However, these are cost- and labour-intensive and present ethical challenges (Steele, Ratterree and Fidel 1999; Cassone and Sobel 2016; M Jaeger et al. 2019). Under these circumstances, model hosts of lower phylogenetic or ontogenetic stage can provide alternative platforms to study C. albicans infections. Non-mammalian models that have been exploited to study C. albicans pathogenesis include a chorio-allantoic membrane chicken embryo model, zebrafish, nematodes and insects (Brennan et al. 2002; Gow et al. 2003; Jacobsen et al. 2011; Tobin, May and Wheeler 2012; Brunke et al. 2015).
In vitro cell culture systems also provide useful models of C. albicans infection. These are less expensive, provide higher throughput and present fewer ethical concerns compared to in vivo models. For example, static cell culture models that mimic C. albicans interactions with intestinal epithelial cells have been used to dissect processes involved in translocation through intestinal barriers (Allert et al. 2018). Also, in vitro circulatory C. albicans-endothelium interaction models have been used to study endothelial adhesion events under conditions of physiological blood pressure (Wilson and Hube 2010). Reconstituted Human Epithelium (RHE) uses Transwell® technology to form polarised epithelia and allows easy access to the apical and basolateral compartments for infection studies (Schaller et al. 2006). Such models closely recapitulate the histology of normal vaginal and oral mucosae and relevant aspects of innate immune responses (Schaller et al. 2005; Yadev et al. 2011) and can mimic epithelial interactions with phagocytes (Weindl et al. 2007). However, RHE models do have limitations, such as the lack of supporting cell types, the absence of mucins, non-constant desquamation, and the overgrowth of microbes due to static conditions (Tabatabaei, Moharamzadeh and Tayebi 2020). These limitations need to be addressed to gain accurate views of fungal infection.
Recently, human cell lines were incorporated into an oral-mucosa-on-a-chip model to study host-microbiota interactions (Rahimi et al. 2018). Also, oral mucosa organoids, which recapitulate the original tissue genetically, histologically and functionally, have been established (Driehuis et al. 2019). In principle, these organoids could be developed to integrate, for example, supporting cells and saliva, to further enhance their relevance to the natural oral mucosa. Organ-on-a-chip models of vaginal infection are under development (https://gtr.ukri.org/projects?ref=studentship-1818626; https://ncats.nih.gov/tissuechip/chip/female). Ideally, these models would include iron restriction and hypoxia as these conditions are known to influence the behaviour of C. albicans (Moosa et al. 2004; Sosinska et al. 2008; Rastogi et al. 2016; Pradhan et al. 2018, 2019). In the future, organ-on-a-chip models of oral and vaginal infection will exploit microfluidic platforms to combine patient-derived primary cells and microbes to represent donor variability and permit the development of predictive and potentially personalized infection models.
Gut simulating models
Models are also critical for the experimental dissection of host-microbiota interactions in the GI tract. These include organoid models as well as specialised fermentation systems (Fehlbaum et al. 2015; Park et al. 2017; Bein et al. 2018; Pearce et al. 2018; Pham et al. 2019), but the co-culturing of human and microbial cells remains a technical challenge. L. rhamnosus has been shown recently to modulate C. albicans pathogenicity in a commensal-like co-culture model (Graf et al. 2019). A similar model, involving co-culture of intestinal epithelial cells and M-cells, revealed that C. albicans translocate preferentially through the M-cell (Albac et al. 2016). These models are high-throughput, cost-efficient and able to recapitulate epithelial cell diversity by co-culturing different epithelial cell types. Nevertheless, such models do not provide the complex tissue architecture of the intestinal epithelium in vivo and, due to the static conditions, they only offer a short assay window before rapid microbial overgrowth occurs (Albac et al. 2016; Park et al. 2017; Pearce et al. 2018; Graf et al. 2019).
On-chip technologies permit the culture of human cells under perfusion, enabling their differentiation into a polarised columnar epithelium (Hyun Jung Kim et al. 2016; Trietsch et al. 2017). This has been extended to develop an immunocompetent intestine-on-a-chip model using caco-2 epithelial cells, endothelial cells and peripheral blood mononuclear cells (PBMCs) to study the interaction between C. albicans and probiotic L. rhamnosus (Maurer et al. 2019). Although this model already provides three-dimensional structures that resemble organotypic microanatomical structures and mimic microphysiological niches of the human intestine, further improvements to increase mucosa cellular diversity and mucus production are possible (Pan et al. 2015; Pearce et al. 2018). In the future, long-term cultures of patient-derived intestinal organoids may be feasible, which opens new avenues for the development of gut models that are even more physiologically relevant (Sato et al. 2009; Mottawea et al. 2019). Patient-derived ileal organoids and faecal samples have been used to culture a complex microbiota in an anaerobic gut-on-a-chip model for up to 5 days (Jalili-Firoozinezhad et al. 2019). This type of model is important because it permits the analysis of donor variability and potentially allows the development of personalised therapies.
Fermentation-based models provide powerful in vitro tools that permit the dissection of microbial processes in the human GI tract. Static batch fermentations with faecal inocula are the simplest and most frequently used models (Walker et al. 2005). These have provided a powerful first approach to study bacterial-fungal interactions and to screen novel therapeutics, but they do not recapitulate the richness of GI compartments (Hillman et al. 2017). Therefore, multicompartmental models have been developed (Guerra et al. 2012; Venema and van den Abbeele 2013). These often contain three-stage culture reactors (Gibson, Cummings and Macfarlane 1988) that can reproduce differences between proximal (acidic, carbohydrate-rich) and distal colonic regions (neutral, carbohydrate-depleted). The multicompartmental M-SHIME system is a powerful tool that permits the analysis of complex, rich and relatively stable microbial communities within GI compartments from the stomach to descending colon (Van de Wiele et al. 2015; Molly, Vande Woestyne and Verstraete 1993). This model has been used to study bacterial-bacterial interactions and the impact of diet and drugs on these interactions (Sivieri et al. 2014; Van den Abbeele et al. 2016; Marzorati et al. 2017; Rivière et al. 2018; Lambrecht et al. 2019).
Despite their power, these fermentation systems have rarely been used to examine fungal- bacterial interactions. In two studies, C. albicans colonisation and outgrowth was shown to be strongly correlated with antibiotic treatment, but mitigated by L. plantarum (Payne et al. 2003; Wynne et al. 2004). However, more recent work has shown that it is important to include the mucus layer to properly simulate the human gut environment in vitro (Van den Abbeele, Van de Wiele et al. 2011; Van den Abbeele, Gérard et al. 2011; Van den Abbeele et al. 2013). Indeed, the presence of mucus influenced interactions between the yeast Saccharomyces boulardii and L. rhamnosus GG and their ability to limit the outgrowth of toxigenic E. coli (Moens et al. 2019). Therefore, a M-SHIME-based system that includes a mucus-rich environment (Van den Abbeele et al. 2012) would seem most appropriate for the dissection of C. albicans-microbiota interactions in the GI tract.
SUMMARY AND OUTLOOK
To summarise, it is clear that the interactions between C. albicans, the human host, and the local microbiota have a major impact upon the likelihood of mucosal and systemic infections and the severity of these infections. It is also apparent that these fungus-host-microbiota interactions are dynamic, iterative and enormously complex (Fig. 8). This immense complexity is increased further by the genetic and phenotypic variation within the species of C. albicans, and by numerous factors that contribute to the variability of individuals and their microbiotas. Yet this complexity must be addressed and defined if the research community is to develop: (i) sensitive and accurate diagnostics capable of distinguishing C. albicans infection from commensalism, and at an early stage when the infection is more amenable to therapy; (ii) novel and efficacious anti-fungal therapies that complement the limited antifungal drugs that are currently available, and that address the problematic emergence of drug resistance and drug resistant species; (iii) tests that quickly establish whether a particular patient is at risk of developing severe candidaemia or recurrent candidiasis and (iv) personalised therapeutic strategies that address the specific make-up and needs of the individual patient.
Despite these challenges, our increased understanding of antifungal immunity and responses is offering potential immunotherapeutic opportunities (De Luca et al. 2013; Davidson, Netea and Kullberg 2018). Effective anti-Candida vaccines, which have proven elusive for so long, are now in sight (Cassone 2015; De Bernardis et al. 2018; Edwards et al. 2018). Our deeper comprehension of fungal immune evasion strategies affords the potential to block these phenotypes and thereby enhance the efficacy of natural antifungal immunity mechanisms (Childers et al. 2020). Significantly, the dramatic expansion in genomic and phenotypic datasets for clinical isolates of C. albicans is providing a much clearer picture of the nature of fungal variability and in-patient evolution (Selmecki, Forche and Berman 2006; Ford et al. 2014; Hirakawa et al. 2015; Ropars et al. 2018; Sitterlé et al. 2019). This information is vital because it will reveal ways in which this microevolution might be inhibited or exploited therapeutically. This information will also highlight essential fungal processes that are less prone to variability and hence present better therapeutic targets. Rapid advances in metagenomics and culturomics are highlighting fungal-bacterial associations between the gut microbiota that are likely to yield useful prognostic tools for patients at risk of systemic candidiasis (Yachida et al. 2019). Our increased knowledge of local fungus-microbiota interactions is facilitating the development of probiotic therapies to address VVC, OPC and C. albicans colonisation of the GI tract (Romeo et al. 2011; Hu et al. 2013; Morais et al. 2017; Vladareanu et al. 2018). In the future, the availability of effective probiotics should help to reduce our dependence on antifungal drugs while, at the same time, enhancing antifungal immunity (Ubeda and Pamer 2012).
How relevant are these points to other fungal pathogens of humans, such as Aspergillus, Cryptococcus, Pneumocystis and other pathogenic Candida species? Pathogenic Aspergillus and Cryptococcus species are environmental fungi that infect humans via the lung. Therefore, while fundamental principles relating to local antifungal immunity, immunotherapy and microbiota-mediated colonisation resistance are clearly of relevance (Armstrong-James et al. 2017; Dumas et al. 2018; Hernández-Santos et al. 2018; Drummond and Lionakis 2019; Maschirow, Suttorp and Opitz 2019; Warris, Bercusson and Armstrong-James 2019), the specific details will differ significantly. Pneumocystis jirovecii also infects the lung, but this fungus is an intracellular parasite that is obligately associated with its human host. In this case, the lung microbiota has not been particularly informative in distinguishing infected from uninfected patients (Kehrmann et al. 2017). Although other Candida pathogens may infect from environmental reservoirs, these species cause similar types of infection to C. albicans, and therefore the points raised in this review will be of general relevance to these species. However, some differences in tissue tropism and patient type exist between species (Sullivan et al. 1995; Silva et al. 2012; Pammi et al. 2013), and some species differ in their immune avoidance strategies (Brunke and Hube 2013; Kasper, Seider and Hube 2015). Nevertheless, the importance of the general principles discussed in this C. albicans-oriented review cannot be understated, most notably the major impact of variability in the fungus, the individual host, and the local microbiota upon disease severity and outcome (Carvalho et al. 2010; Farrer et al. 2015; Hube 2015; Ballard et al. 2018; Stone et al. 2019; Vandeplassche et al. 2019).
ACKNOWLEDGEMENTS
We thank our friends and colleagues in the medical mycology, fungal immunology and microbiota fields for many thought-provoking discussions.
Contributor Information
Christophe d'Enfert, Unité Biologie et Pathogénicité Fongiques, Département de Mycologie, Institut Pasteur, USC 2019 INRA, 25, rue du Docteur Roux, 75015 Paris, France.
Ann-Kristin Kaune, Aberdeen Fungal Group, Institute of Medical Sciences, University of Aberdeen, Ashgrove Road West, Foresterhill, Aberdeen AB25 2ZD, UK.
Leovigildo-Rey Alaban, BIOASTER Microbiology Technology Institute, 40 avenue Tony Garnier, 69007 Lyon, France; Université de Paris, Sorbonne Paris Cité, 25, rue du Docteur Roux, 75015 Paris, France.
Sayoni Chakraborty, Microbial Immunology Research Group, Emmy Noether Junior Research Group Adaptive Pathogenicity Strategies, and the Department of Microbial Pathogenicity Mechanisms, Leibniz Institute for Natural Product Research and Infection Biology – Hans Knöll Institute, Beutenbergstraße 11a, 07745 Jena, Germany; Institute of Microbiology, Friedrich Schiller University, Neugasse 25, 07743 Jena, Germany.
Nathaniel Cole, Gut Microbiology Group, Rowett Institute, University of Aberdeen, Ashgrove Road West, Foresterhill, Aberdeen AB25 2ZD, UK.
Margot Delavy, Unité Biologie et Pathogénicité Fongiques, Département de Mycologie, Institut Pasteur, USC 2019 INRA, 25, rue du Docteur Roux, 75015 Paris, France; Université de Paris, Sorbonne Paris Cité, 25, rue du Docteur Roux, 75015 Paris, France.
Daria Kosmala, Unité Biologie et Pathogénicité Fongiques, Département de Mycologie, Institut Pasteur, USC 2019 INRA, 25, rue du Docteur Roux, 75015 Paris, France; Université de Paris, Sorbonne Paris Cité, 25, rue du Docteur Roux, 75015 Paris, France.
Benoît Marsaux, ProDigest BV, Technologiepark 94, B-9052 Gent, Belgium; Center for Microbial Ecology and Technology (CMET), Department of Biotechnology, Faculty of Bioscience Engineering, Ghent University, Coupure Links, 9000 Ghent, Belgium.
Ricardo Fróis-Martins, Immunology Section, Vetsuisse Faculty, University of Zurich, Winterthurerstrasse 266a, Zurich 8057, Switzerland; Institute of Experimental Immunology, University of Zurich, Winterthurerstrasse 190, Zürich 8057, Switzerland.
Moran Morelli, Mimetas, Biopartner Building 2, J.H. Oortweg 19, 2333 CH Leiden, The Netherlands.
Diletta Rosati, Department of Internal Medicine and Radboud Center for Infectious Diseases, Radboud University Medical Center, Geert Grooteplein 28, 6525 GA Nijmegen, The Netherlands.
Marisa Valentine, Microbial Immunology Research Group, Emmy Noether Junior Research Group Adaptive Pathogenicity Strategies, and the Department of Microbial Pathogenicity Mechanisms, Leibniz Institute for Natural Product Research and Infection Biology – Hans Knöll Institute, Beutenbergstraße 11a, 07745 Jena, Germany.
Zixuan Xie, Gut Microbiome Group, Vall d'Hebron Institut de Recerca (VHIR), Vall d'Hebron Hospital Universitari, Vall d'Hebron Barcelona Hospital Campus, Passeig Vall d'Hebron 119–129, 08035 Barcelona, Spain.
Yoan Emritloll, Unité Biologie et Pathogénicité Fongiques, Département de Mycologie, Institut Pasteur, USC 2019 INRA, 25, rue du Docteur Roux, 75015 Paris, France.
Peter A Warn, Magic Bullet Consulting, Biddlecombe House, Ugbrook, Chudleigh Devon, TQ130AD, UK.
Frédéric Bequet, BIOASTER Microbiology Technology Institute, 40 avenue Tony Garnier, 69007 Lyon, France.
Marie-Elisabeth Bougnoux, Unité Biologie et Pathogénicité Fongiques, Département de Mycologie, Institut Pasteur, USC 2019 INRA, 25, rue du Docteur Roux, 75015 Paris, France.
Stephanie Bornes, Université Clermont Auvergne, INRAE, VetAgro Sup, UMRF0545, 20 Côte de Reyne, 15000 Aurillac, France.
Mark S Gresnigt, Microbial Immunology Research Group, Emmy Noether Junior Research Group Adaptive Pathogenicity Strategies, and the Department of Microbial Pathogenicity Mechanisms, Leibniz Institute for Natural Product Research and Infection Biology – Hans Knöll Institute, Beutenbergstraße 11a, 07745 Jena, Germany.
Bernhard Hube, Microbial Immunology Research Group, Emmy Noether Junior Research Group Adaptive Pathogenicity Strategies, and the Department of Microbial Pathogenicity Mechanisms, Leibniz Institute for Natural Product Research and Infection Biology – Hans Knöll Institute, Beutenbergstraße 11a, 07745 Jena, Germany.
Ilse D Jacobsen, Microbial Immunology Research Group, Emmy Noether Junior Research Group Adaptive Pathogenicity Strategies, and the Department of Microbial Pathogenicity Mechanisms, Leibniz Institute for Natural Product Research and Infection Biology – Hans Knöll Institute, Beutenbergstraße 11a, 07745 Jena, Germany.
Mélanie Legrand, Unité Biologie et Pathogénicité Fongiques, Département de Mycologie, Institut Pasteur, USC 2019 INRA, 25, rue du Docteur Roux, 75015 Paris, France.
Salomé Leibundgut-Landmann, Immunology Section, Vetsuisse Faculty, University of Zurich, Winterthurerstrasse 266a, Zurich 8057, Switzerland; Institute of Experimental Immunology, University of Zurich, Winterthurerstrasse 190, Zürich 8057, Switzerland.
Chaysavanh Manichanh, Gut Microbiome Group, Vall d'Hebron Institut de Recerca (VHIR), Vall d'Hebron Hospital Universitari, Vall d'Hebron Barcelona Hospital Campus, Passeig Vall d'Hebron 119–129, 08035 Barcelona, Spain.
Carol A Munro, Aberdeen Fungal Group, Institute of Medical Sciences, University of Aberdeen, Ashgrove Road West, Foresterhill, Aberdeen AB25 2ZD, UK.
Mihai G Netea, Department of Internal Medicine and Radboud Center for Infectious Diseases, Radboud University Medical Center, Geert Grooteplein 28, 6525 GA Nijmegen, The Netherlands.
Karla Queiroz, Mimetas, Biopartner Building 2, J.H. Oortweg 19, 2333 CH Leiden, The Netherlands.
Karine Roget, NEXBIOME Therapeutics, 22 allée Alan Turing, 63000 Clermont-Ferrand, France.
Vincent Thomas, BIOASTER Microbiology Technology Institute, 40 avenue Tony Garnier, 69007 Lyon, France.
Claudia Thoral, NEXBIOME Therapeutics, 22 allée Alan Turing, 63000 Clermont-Ferrand, France.
Pieter Van den Abbeele, ProDigest BV, Technologiepark 94, B-9052 Gent, Belgium.
Alan W Walker, Gut Microbiology Group, Rowett Institute, University of Aberdeen, Ashgrove Road West, Foresterhill, Aberdeen AB25 2ZD, UK.
Alistair J P Brown, MRC Centre for Medical Mycology, Department of Biosciences, University of Exeter, Geoffrey Pope Building, Stocker Road, Exeter EX4 4QD, UK.
FUNDING
We received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie action, Innovative Training Network: FunHoMic; grant N° 812969. CdE received funding from the French Government ‘Investissement d'Avenir’ program (Laboratoire d'Excellence Integrative Biology of Emerging Infectious Diseases, ANR-10-LABX-62-IBEID), the Agence Nationale de la Recherche (ERA-Net Infect-ERA, FUNCOMPATH, ANR-14-IFEC-0004), the EU Horizon2020 consortium ‘Host-Directed Medicine in invasive FUNgal infections’—HDM-FUN (Grant Agreement 847507). SLL and CdE received funding from the Swiss National Science Foundation (Sinergia program, #CRSII5_173863). BIOASTER received funding from the French Government ‘Investissement d'Avenir’ program (Grant No. ANR-10-AIRT-03). MSG was supported by a Humboldt Research Fellowship for Postdoctoral Researchers by the Alexander von Humboldt-Foundation and the Deutsche Forschungsgemeinschaft (DFG) Emmy Noether Program (project no. 434385622/GR 5617/1-1). BH was supported by the Deutsche Forschungsgemeinschaft (DFG) project Hu 532/20-1, project C1 within the Collaborative Research Centre (CRC)/Transregio 124 FungiNet and the Balance of the Microverse Cluster under Germany´s Excellence Strategy—EXC 2051–Project-ID 390713860, the EU Horizon2020 consortium ‘Host-Directed Medicine in invasive FUNgal infections’—HDM-FUN (Grant Agreement 847507), the Leibniz Association Campus InfectoOptics SAS-2015-HKI-LWC and the Wellcome Trust (215599/Z/19/Z). IDJ was supported by the Deutsche Forschungsgemeinschaft (DFG) project C5 within the Collaborative Research Centre (CRC)/Transregio 124 FungiNet and the Balance of the Microverse Cluster under Germany´s Excellence Strategy—EXC 2051–Project-ID 390713860, the Leibniz Association Campus InfectoOptics SAS-2015-HKI-LWC and the Wellcome Trust (Grant 215599/Z/19/Z). CM received funding from the the Instituto de Salud Carlos III/FEDER. MGN was supported by an ERC Advanced Grant (#833247) and a Spinoza grant of the Netherlands Organization for Scientific Research. CAM was supported by EU Horizon2020 consortium ‘Host-Directed Medicine in invasive FUNgal infections’—HDM-FUN (Grant Agreement 847507) and the Wellcome Trust Strategic Award for Medical Mycology and Fungal Immunology (097377/Z/11/Z). AWW receives core funding support from the Scottish Government's Rural and Environment Science and Analytical Services (RESAS). AJPB was supported by a programme grant from the UK Medical Research Council (MR/M026663/1) and by the Medical Research Council Centre for Medical Mycology at the University of Exeter (MR/N006364/1).
Conflicts of Interest
None declared.
REFERENCES
- Aagaard K, Riehle K, Ma Ket al. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One. 2012;7:e36466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Rodriguez EV, Rivino L, Geginat Jet al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–46. [DOI] [PubMed] [Google Scholar]
- Adler CJ, Dobney K, Weyrich LSet al. Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions. Nat Genet. 2013;45:450–5., 455e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggor FEY, Break TJ, Trevejo-Nuñez Get al. Oral epithelial IL-22/STAT3 signaling licenses IL-17-mediated immunity to oral mucosal candidiasis. Sci Immunol. 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrné S, Nobaek S, Jeppsson Bet al. The normal Lactobacillus flora of healthy human rectal and oral mucosa. J Appl Microbiol. 1998;85:88–94. [DOI] [PubMed] [Google Scholar]
- Ajdić D, McShan WM, McLaughlin REet al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci USA. 2002;99:14434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimoto-Gunther L, Bonfim-Mendonça P, de Set al. Highlights regarding host predisposing factors to recurrent vulvovaginal candidiasis: chronic stress and reduced antioxidant capacity. PLoS One. 2016;11:e0158870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rusan RM, Darwazeh AMG, Lataifeh IM. The relationship of Candida colonization of the oral and vaginal mucosae of mothers and oral mucosae of their newborns at birth. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:459–63. [DOI] [PubMed] [Google Scholar]
- Alarco AM, Raymond M. The bZip transcription factor Cap1p is involved in multidrug resistance and oxidative stress response in Candida albicans. J Bacteriol. 1999;181:700–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albac S, et al. Candida albicans is able to use M cells as a portal of entry across the intestinal barrier in vitro. Cell Microbiol. 2016;18:195–210. [DOI] [PubMed] [Google Scholar]
- Albert AYK, et al. A Study of the Vaginal Microbiome in Healthy Canadian Women Utilizing cpn60-Based Molecular Profiling Reveals Distinct Gardnerella Subgroup Community State Types. PLoS One. 2015;10:e0135620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allert S, et al. Candida albicans-Induced Epithelial Damage Mediates Translocation through Intestinal Barriers. MBio. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allonsius CN, Vandenheuvel D, Oerlemans EFMet al. Inhibition of Candida albicans morphogenesis by chitinase from Lactobacillus rhamnosus GG. Sci Rep. 2019;9:2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida A, Mitchell AL, Boland Met al. A new genomic blueprint of the human gut microbiota. Nature. 2019;568:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida RS, Brunke S, Albrecht Aet al. the hyphal-associated adhesin and invasin Als3 of Candida albicans mediates iron acquisition from host ferritin. PLoS Pathog. 2008;4:e1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnuaimi AD, Ramdzan AN, Wiesenfeld Det al. Candida virulence and ethanol-derived acetaldehyde production in oral cancer and non-cancer subjects. Oral Dis. 2016;22:805–14. [DOI] [PubMed] [Google Scholar]
- Alonso-Monge R, Carvaihlo S, Nombela Cet al. The Hog1 MAP kinase controls respiratory metabolism in the fungal pathogen Candida albicans. Microbiology (Reading, Engl). 2009;155:413–23. [DOI] [PubMed] [Google Scholar]
- Alonso-Monge R, Navarro-García F, Molero Get al. Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J Bacteriol. 1999;181:3058–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmeier S, Toska A, Sparber Fet al. IL-1 Coordinates the Neutrophil Response to C. albicans in the Oral Mucosa. PLoS Pathog. 2016;12:e1005882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Rueda N, Rouges C, Touahri Aet al. In vitro immune responses of human PBMCs against Candida albicans reveals fungal and leucocyte phenotypes associated with fungal persistence. Sci Rep. 2020;10:6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MZ, Thomson GJ, Hirakawa MPet al. A “parameiosis” drives depolyploidization and homologous recombination in Candida albicans. Nat Commun. 2019;10:4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angebault C, Ghozlane A, Volant Set al. Combined bacterial and fungal intestinal microbiota analyses: Impact of storage conditions and DNA extraction protocols. PLoS One. 2018;13:e0201174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angebault C, et al. Candida albicans is not always the preferential yeast colonizing humans: a study in Wayampi Amerindians. J Infect Dis. 2013;208:1705–16. [DOI] [PubMed] [Google Scholar]
- Anukam KC, Osazuwa EO, Ahonkhai Iet al. Lactobacillus vaginal microbiota of women attending a reproductive health care service in Benin city, Nigeria. Sex Transm Dis. 2006;33:59–62. [DOI] [PubMed] [Google Scholar]
- Ardizzoni A, et al. Perinuclear Anti-Neutrophil Cytoplasmic Antibodies (pANCA) Impair Neutrophil Candidacidal Activity and Are Increased in the Cellular Fraction of Vaginal Samples from Women with Vulvovaginal Candidiasis. J Fungi (Basel). 2020;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo AV, Nobile CJ. Interactions of microorganisms with host mucins: a focus on Candida albicans. FEMS Microbiol Rev. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariizumi K, Shen GL, Shikano Set al. Cloning of a second dendritic cell-associated C-type lectin (dectin-2) and its alternatively spliced isoforms. J Biol Chem. 2000;275:11957–63. [DOI] [PubMed] [Google Scholar]
- Ariizumi K, Shen GL, Shikano Set al. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J Biol Chem. 2000;275:20157–67. [DOI] [PubMed] [Google Scholar]
- Armstrong-James D, Brown GD, Netea MGet al. Immunotherapeutic approaches to treatment of fungal diseases. Lancet Infect Dis. 2017;17:e393–402. [DOI] [PubMed] [Google Scholar]
- Arumugam M, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchtung TA, Fofanova TY, Stewart CJet al. Investigating colonization of the healthy adult gastrointestinal tract by fungi. mSphere. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austermeier S, Kasper L, Westman Jet al. I want to break free - macrophage strategies to recognize and kill Candida albicans, and fungal counter-strategies to escape. Curr Opin Microbiol. 2020;58:15–23. [DOI] [PubMed] [Google Scholar]
- Bacher P, et al. Antigen-specific expansion of human regulatory T cells as a major tolerance mechanism against mucosal fungi. Mucosal Immunol. 2014;7:916–28. [DOI] [PubMed] [Google Scholar]
- Bacher P, et al. Human Anti-fungal Th17 Immunity and Pathology Rely on Cross-Reactivity against Candida albicans. Cell. 2019;176:1340–55..e15. [DOI] [PubMed] [Google Scholar]
- Bailey DA, Feldmann PJ, Bovey Met al. The Candida albicans HYR1 gene, which is activated in response to hyphal development, belongs to a gene family encoding yeast cell wall proteins. J Bacteriol. 1996;178:5353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain J, Gow NAR, Erwig L-P. Novel insights into host-fungal pathogen interactions derived from live-cell imaging. Semin Immunopathol. 2015;37:131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain JM, Lewis LE, Okai Bet al. Non-lytic expulsion/exocytosis of Candida albicans from macrophages. Fungal Genet Biol. 2012;49:677–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain JM, et al. Candida albicans hypha formation and mannan masking of β-glucan inhibit macrophage phagosome maturation. MBio. 2014;5:e01874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairwa G, Hee Jung W, Kronstad JW. Iron acquisition in fungal pathogens of humans. Metallomics. 2017;9:215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai W, Wang Q, Deng Zet al. TRAF1 suppresses antifungal immunity through CXCL1-mediated neutrophil recruitment during Candida albicans intradermal infection. Cell Commun Signal. 2020;18:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baley JE, Kliegman RM, Boxerbaum Bet al. Fungal colonization in the very low birth weight infant. Pediatrics. 1986;78:225–32. [PubMed] [Google Scholar]
- Ballard E, Melchers WJG, Zoll Jet al. In-host microevolution of Aspergillus fumigatus: A phenotypic and genotypic analysis. Fungal Genet Biol. 2018;113:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou ER, et al. Lactate signalling regulates fungal β-glucan masking and immune evasion. Nat Microbiol. 2016;2:16238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford CV, d Mello A, Nobbs AHet al. Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication. Infect Immun. 2009;77:3696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford CV, Nobbs AH, Barbour MEet al. Functional regions of Candida albicans hyphal cell wall protein Als3 that determine interaction with the oral bacterium Streptococcus gordonii. Microbiology (Reading, Engl). 2015;161:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandara HMHN, Yau JYY, Watt RMet al. Escherichia coli and its lipopolysaccharide modulate in vitro Candida biofilm formation. J Med Microbiol. 2009;58:1623–31. [DOI] [PubMed] [Google Scholar]
- Barelle CJ, Priest CL, Maccallum DMet al. Niche-specific regulation of central metabolic pathways in a fungal pathogen. Cell Microbiol. 2006;8:961–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson NJ. Competition for glucose between Candida albicans and oral bacteria grown in mixed culture in a chemostat. J Med Microbiol. 2000;49:969–75. [DOI] [PubMed] [Google Scholar]
- Basso V, d Enfert C, Znaidi Set al. From Genes to Networks: The Regulatory Circuitry Controlling Candida albicans Morphogenesis. Curr Top Microbiol Immunol. 2019;422:61–99. [DOI] [PubMed] [Google Scholar]
- Becattini S, et al. T cell immunity. Functional heterogeneity of human memory CD4+ T cell clones primed by pathogens or vaccines. Science. 2015;347:400–6. [DOI] [PubMed] [Google Scholar]
- Becker KL, Rösler B, Wang Xet al. Th2 and Th9 responses in patients with chronic mucocutaneous candidiasis and hyper-IgE syndrome. Clin Exp Allergy. 2016;46:1564–74. [DOI] [PubMed] [Google Scholar]
- Beijerinck MW. Über oligonitrophile Mikroben. Centralblatt für Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene, Abteilung II. 1901;7:561–82. [Google Scholar]
- Bein A, Shin W, Jalili-Firoozinezhad Set al. Microfluidic Organ-on-a-Chip Models of Human Intestine. Cell Mol Gastroenterol Hepatol. 2018;5:659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellemain E, Carlsen T, Brochmann Cet al. ITS as an environmental DNA barcode for fungi: an in silico approach reveals potential PCR biases. BMC Microbiol. 2010;10:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belstrøm D, Holmstrup P, Bardow Aet al. Temporal stability of the salivary microbiota in oral health. PLoS One. 2016;11:e0147472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett RJ, Forche A, Berman J. Rapid mechanisms for generating genome diversity: whole ploidy shifts, aneuploidy, and loss of heterozygosity. Cold Spring Harb Perspect Med. 2014;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett RJ, Johnson AD. Completion of a parasexual cycle in Candida albicans by induced chromosome loss in tetraploid strains. EMBO J. 2003;22:2505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beno DW, Stöver AG, Mathews HL. Growth inhibition of Candida albicans hyphae by CD8+ lymphocytes. J Immunol. 1995;154:5273–81. [PubMed] [Google Scholar]
- Bensasson D, Dicks J, Ludwig JMet al. Diverse Lineages of Candida albicans Live on Old Oaks. Genetics. 2019;211:277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini M, Ranjan A, Thompson Aet al. Candida albicans induces mucosal bacterial dysbiosis that promotes invasive infection. PLoS Pathog. 2019;15:e1007717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessede A, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511:184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran N, Quigley C, Paw Cet al. Role of short chain fatty acids in controlling tregs and immunopathology during mucosal infection. Front Microbiol. 2018;9:1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bik EM, et al. Bacterial diversity in the oral cavity of 10 healthy individuals. ISME J. 2010;4:962–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilen M, Dufour J-C, Lagier J-Cet al. The contribution of culturomics to the repertoire of isolated human bacterial and archaeal species. Microbiome. 2018;6:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistoni F, Vecchiarelli A, Cenci Eet al. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect Immun. 1986;51:668–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzini A, Durussel C, Bille Jet al. Performance of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of bacterial strains routinely isolated in a clinical microbiology laboratory. J Clin Microbiol. 2010;48:1549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacher E, Levy M, Tatirovsky Eet al. Microbiome-Modulated Metabolites at the Interface of Host Immunity. J Immunol. 2017;198:572–80. [DOI] [PubMed] [Google Scholar]
- Black CA, Eyers FM, Russell Aet al. Acute neutropenia decreases inflammation associated with murine vaginal candidiasis but has no effect on the course of infection. Infect Immun. 1998;66:1273–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss JM, Basavegowda KP, Watson WJet al. Vertical and horizontal transmission of Candida albicans in very low birth weight infants using DNA fingerprinting techniques. Pediatr Infect Dis J. 2008;27:231–5. [DOI] [PubMed] [Google Scholar]
- Boix-Amorós A, Martinez-Costa C, Querol Aet al. Multiple Approaches Detect the Presence of Fungi in Human Breastmilk Samples from Healthy Mothers. Sci Rep. 2017;7:13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booijink CCGM, El-Aidy S, Rajilić-Stojanović Met al. High temporal and inter-individual variation detected in the human ileal microbiota. Environ Microbiol. 2010;12:3213–27. [DOI] [PubMed] [Google Scholar]
- Borghi M, Renga G, Puccetti Met al. Antifungal th immunity: growing up in family. Front Immunol. 2014;5:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghi M, et al. Pathogenic NLRP3 Inflammasome Activity during Candida Infection Is Negatively Regulated by IL-22 via Activation of NLRC4 and IL-1Ra. Cell Host Microbe. 2015;18:198–209. [DOI] [PubMed] [Google Scholar]
- Boris S, Suárez JE, Vázquez Fet al. Adherence of human vaginal lactobacilli to vaginal epithelial cells and interaction with uropathogens. Infect Immun. 1998;66:1985–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman AM, Szekely A, Linton CJet al. Epidemiology, antifungal susceptibility, and pathogenicity of Candida africana isolates from the United Kingdom. J Clin Microbiol. 2013;51:967–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougnoux M-E, Pujol C, Diogo Det al. Mating is rare within as well as between clades of the human pathogen Candida albicans. Fungal Genet Biol. 2008;45:221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougnoux ME, et al. Multilocus sequence typing reveals intrafamilial transmission and microevolutions of Candida albicans isolates from the human digestive tract. J Clin Microbiol. 2006;44:1810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MVet al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M, Thomas DY, Whiteway Met al. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol Med Microbiol. 2002;34:153–7. [DOI] [PubMed] [Google Scholar]
- Brogden KA, Guthmiller JM, Taylor CE. Human polymicrobial infections. Lancet. 2005;365:253–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman RM, He X, Gajer Pet al. Association between cigarette smoking and the vaginal microbiota: a pilot study. BMC Infect Dis. 2014;14:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman RM, Shardell MDet al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. 2014;21:450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJP, Brown GD, Netea MGet al. Metabolism impacts upon Candida immunogenicity and pathogenicity at multiple levels. Trends Microbiol. 2014;22:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJP, Budge Set al. Stress adaptation in a pathogenic fungus. J Exp Biol. 2014;217:144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJP, Cowen LE, di Pietro Aet al. Stress Adaptation. Microbiol Spectr. 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJP, Gow NAR, Warris Aet al. Memory in fungal pathogens promotes immune evasion, colonisation, and infection. Trends Microbiol. 2019;27:219–30. [DOI] [PubMed] [Google Scholar]
- Brown AJP, Larcombe DE, Pradhan A. Thoughts on the evolution of Core Environmental Responses in yeasts. Fungal Biol. 2020;124:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Denning DW, Gow NARet al. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv13. [DOI] [PubMed] [Google Scholar]
- Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. [DOI] [PubMed] [Google Scholar]
- Brown GD, Taylor PR, Reid DMet al. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med. 2002;196:407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol. 2011;29:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SE, et al. The Vaginal Microbiota and Behavioral Factors Associated With Genital Candida albicans Detection in Reproductive-Age Women. Sex Transm Dis. 2019;46:753–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunke S, Hube B. Adaptive prediction as a strategy in microbial infections. PLoS Pathog. 2014;10:e1004356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunke S, Hube B. Two unlike cousins: Candida albicans and C. glabrata infection strategies. Cell Microbiol. 2013;15:701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunke S, et al. Of mice, flies–and men? Comparing fungal infection models for large-scale screening efforts. Dis Model Mech. 2015;8:473–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno VM, Shetty AC, Yano Jet al. Transcriptomic analysis of vulvovaginal candidiasis identifies a role for the NLRP3 inflammasome. MBio. 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffo J, Herman MA, Soll DR. A characterization of pH-regulated dimorphism in Candida albicans. Mycopathologia. 1984;85:21–30. [DOI] [PubMed] [Google Scholar]
- Burdet C, et al. Ceftriaxone and Cefotaxime Have Similar Effects on the Intestinal Microbiota in Human Volunteers Treated by Standard-Dose Regimens. Antimicrob Agents Chemother. 2019;63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler G, et al. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature. 2009;459:657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F, et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe. 2015;17:690–703. [DOI] [PubMed] [Google Scholar]
- Bär E, Gladiator A, Bastidas Set al. A novel Th cell epitope of Candida albicans mediates protection from fungal infection. J Immunol. 2012;188:5636–43. [DOI] [PubMed] [Google Scholar]
- Böttcher B, Pöllath C, Staib Pet al. Candida species Rewired Hyphae Developmental Programs for Chlamydospore Formation. Front Microbiol. 2016;7:1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambi A, et al. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur J Immunol. 2003;33:532–8. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, et al. Moving pictures of the human microbiome. Genome Biol. 2011;12:R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramalac DA, da Silva Ruiz L, de Batista GCMet al. Candida isolated from vaginal mucosa of mothers and oral mucosa of neonates: occurrence and biotypes concordance. Pediatr Infect Dis J. 2007;26:553–7. [DOI] [PubMed] [Google Scholar]
- Carolus H, Van Dyck K, Van Dijck P. Candida albicans and Staphylococcus Species: A Threatening Twosome. Front Microbiol. 2019;10:2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A, Cunha C, Pasqualotto ACet al. Genetic variability of innate immunity impacts human susceptibility to fungal diseases. Int J Infect Dis. 2010;14:e460–8. [DOI] [PubMed] [Google Scholar]
- Casadevall A, Pirofski L-A. Host-pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect Immun. 1999;67:3703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, Pirofski L-A. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol. 2003;1:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, Pirofski L-A. What is a host? Incorporating the microbiota into the damage-response framework. Infect Immun. 2015;83:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaroto AR, et al. Candida albicans-Cell Interactions Activate Innate Immune Defense in Human Palate Epithelial Primary Cells via Nitric Oxide (NO) and β-Defensin 2 (hBD-2). Cells. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone A, Cauda R. Candida and candidiasis in HIV-infected patients: where commensalism, opportunistic behavior and frank pathogenicity lose their borders. AIDS. 2012;26:1457–72. [DOI] [PubMed] [Google Scholar]
- Cassone A, Sobel JD. Experimental models of vaginal candidiasis and their relevance to human candidiasis. Infect Immun. 2016;84:1255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone A. Vulvovaginal Candida albicans infections: pathogenesis, immunity and vaccine prospects. BJOG. 2015;122:785–94. [DOI] [PubMed] [Google Scholar]
- Cavalcanti YW, et al. Virulence and pathogenicity of Candida albicans is enhanced in biofilms containing oral bacteria. Biofouling. 2015;31:27–38. [DOI] [PubMed] [Google Scholar]
- Cavalieri D, et al. Genomic and Phenotypic Variation in Morphogenetic Networks of TwoCandida albicansIsolates Subtends Their Different Pathogenic Potential. Front Immunol. 2017;8:1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceresa C, Tessarolo F, Caola Iet al. Inhibition of Candida albicans adhesion on medical-grade silicone by a Lactobacillus-derived biosurfactant. J Appl Microbiol. 2015;118:1116–25. [DOI] [PubMed] [Google Scholar]
- Chang H-T, Tsai P-W, Huang H-Het al. LL37 and hBD-3 elevate the β-1,3-exoglucanase activity of Candida albicans Xog1p, resulting in reduced fungal adhesion to plastic. Biochem J. 2012;441:963–70. [DOI] [PubMed] [Google Scholar]
- Chang Y, et al. Optimization of culturomics strategy in human fecal samples. Front Microbiol. 2019;10:2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SJ, Hill AVS. Human genetic susceptibility to infectious disease. Nat Rev Genet. 2012;13:175–88. [DOI] [PubMed] [Google Scholar]
- Cheetham J, MacCallum DM, Doris KSet al. MAPKKK-independent regulation of the Hog1 stress-activated protein kinase in Candida albicans. J Biol Chem. 2011;286:42002–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehoud C, et al. Fungal signature in the gut microbiota of pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1948–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AI, et al. Candida albicans ethanol stimulates Pseudomonas aeruginosa WspR-controlled biofilm formation as part of a cyclic relationship involving phenazines. PLoS Pathog. 2014;10:e1004480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Pande K, French SDet al. An iron homeostasis regulatory circuit with reciprocal roles in Candida albicans commensalism and pathogenesis. Cell Host Microbe. 2011;10:118–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Yeater KM, Hoyer LL. Cellular and molecular biology of Candida albicans estrogen response. Eukaryotic Cell. 2006;5:180–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Clancy CJ, Zhang Zet al. Uncoupling of oxidative phosphorylation enables Candida albicans to resist killing by phagocytes and persist in tissue. Cell Microbiol. 2007;9:492–501. [DOI] [PubMed] [Google Scholar]
- Cheng S-C, van de Veerdonk F, Smeekens Set al. Candida albicans dampens host defense by downregulating IL-17 production. J Immunol. 2010;185:2450–57. [DOI] [PubMed] [Google Scholar]
- Cheng S-C, et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S-C, et al. The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. J Leukoc Biol. 2011;90:357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhou X, Ren Bet al. The regulation of hyphae growth in Candida albicans. Virulence. 2020;11:337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibana H, Beckerman JL, Magee PT. Fine-resolution physical mapping of genomic diversity in Candida albicans. Genome Res. 2000;10:1865–77. [DOI] [PubMed] [Google Scholar]
- Childers DS, Avelar GM, Bain JMet al. Epitope shaving promotes fungal immune evasion. MBio. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers DS, Avelar GM, Bain JMet al. Impact of the Environment upon the Candida albicans Cell Wall and Resultant Effects upon Immune Surveillance. Curr Top Microbiol Immunol. 2019. [DOI] [PubMed] [Google Scholar]
- Childers DS, et al. The rewiring of ubiquitination targets in a pathogenic yeast promotes metabolic flexibility, host colonization and virulence. PLoS Pathog. 2016;12:e1005566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiranand W, McLeod I, Zhou Het al. CTA4 transcription factor mediates induction of nitrosative stress response in Candida albicans. Eukaryotic Cell. 2008;7:268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhary A, Sharma C, Meis JF. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017;13:e1006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citiulo F, Jacobsen ID, Miramón Pet al. Candida albicans scavenges host zinc via Pra1 during endothelial invasion. PLoS Pathog. 2012;8:e1002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Kedar S, Baram L, Elad Het al. Human intestinal epithelial cells respond to β-glucans via Dectin-1 and Syk. Eur J Immunol. 2014;44:3729–40. [DOI] [PubMed] [Google Scholar]
- Conti HR, Huppler AR, Whibley Net al. Animal models for candidiasis. Curr Protoc Immunol. 2014;105:19.6.1–19.6.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, Peterson ACet al. Oral-resident natural Th17 cells and γδ T cells control opportunistic Candida albicans infections. J Exp Med. 2014;211:2075–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, et al. IL-17 Receptor Signaling in Oral Epithelial Cells Is Critical for Protection against Oropharyngeal Candidiasis. Cell Host Microbe. 2016;20:606–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste A, Turner V, Ischer Fet al. A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics. 2006;172:2139–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottier F, Tan ASM, Chen Jet al. The transcriptional stress response of Candida albicans to weak organic acids. G3 (Bethesda). 2015;5:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottier F, Tan ASM, Yurieva Met al. The Transcriptional Response of Candida albicans to Weak Organic Acids, Carbon Source, and MIG1 Inactivation Unveils a Role for HGT16 in Mediating the Fungistatic Effect of Acetic Acid. G3 (Bethesda). 2017;7:3597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottier F, et al. Remasking of Candida albicans β-Glucan in Response to Environmental pH Is Regulated by Quorum Sensing. MBio. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, et al. Biphasic zinc compartmentalisation in a human fungal pathogen. PLoS Pathog. 2018;14:e1007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crielaard W, Zaura E, Schuller AAet al. Exploring the oral microbiota of children at various developmental stages of their dentition in the relation to their oral health. BMC Med Genomics. 2011;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe JD, Sievwright IK, Auld GCet al. Candida albicans binds human plasminogen: identification of eight plasminogen-binding proteins. Mol Microbiol. 2003;47:1637–51. [DOI] [PubMed] [Google Scholar]
- Cruz MR, Graham CE, Gagliano BCet al. Enterococcus faecalis inhibits hyphal morphogenesis and virulence of Candida albicans. Infect Immun. 2013;81:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–89. [DOI] [PubMed] [Google Scholar]
- Culibrk L, Croft CA, Tebbutt SJ. Systems Biology Approaches for Host-Fungal Interactions: An Expanding Multi-Omics Frontier. OMICS. 2016;20:127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Enfert C. Hidden killers: persistence of opportunistic fungal pathogens in the human host. Curr Opin Microbiol. 2009;12:358–64. [DOI] [PubMed] [Google Scholar]
- Dalle F, Wächtler B, L'Ollivier Cet al. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol. 2010;12:248–71. [DOI] [PubMed] [Google Scholar]
- Dambuza IM, Levitz SM, Netea MGet al. Fungal recognition and host defense mechanisms. Microbiol Spectr. 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danhof HA, Vylkova S, Vesely EMet al. Robust Extracellular pH Modulation by Candida albicans during Growth in Carboxylic Acids. MBio. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas A da S, Day A, Ikeh Met al. Oxidative stress responses in the human fungal pathogen, Candida albicans. Biomolecules. 2015;5:142–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwazeh AM, al-Bashir A. Oral candidal flora in healthy infants. J Oral Pathol Med. 1995;24:361–4. [DOI] [PubMed] [Google Scholar]
- Das I, Nightingale P, Patel Met al. Epidemiology, clinical characteristics, and outcome of candidemia: experience in a tertiary referral center in the UK. Int J Infect Dis. 2011;15:e759–63. [DOI] [PubMed] [Google Scholar]
- David LA, Materna AC, Friedman Jet al. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014;15:R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LA, Maurice CFet al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson L, Netea MG, Kullberg BJ. Patient Susceptibility to Candidiasis-A Potential for Adjunctive Immunotherapy. J Fungi (Basel). 2018;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D, Edwards JE, Mitchell APet al. Candida albicans RIM101 pH response pathway is required for host-pathogen interactions. Infect Immun. 2000;68:5953–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D, Wilson RB, Mitchell AP. RIM101-dependent and-independent pathways govern pH responses in Candida albicans. Mol Cell Biol. 2000;20:971–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bernardis F, Graziani S, Tirelli Fet al. Candida vaginitis: virulence, host response and vaccine prospects. Med Mycol. 2018;56:26–31. [DOI] [PubMed] [Google Scholar]
- De Filippis F, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–21. [DOI] [PubMed] [Google Scholar]
- de Groot PWJ, Bader O, de Boer ADet al. Adhesins in human fungal pathogens: glue with plenty of stick. Eukaryotic Cell. 2013;12:470–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot PWJ, de Boer AD, Cunningham Jet al. Proteomic analysis of Candida albicans cell walls reveals covalently bound carbohydrate-active enzymes and adhesins. Eukaryotic Cell. 2004;3:955–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot PWJ, Ram AF, Klis FM. Features and functions of covalently linked proteins in fungal cell walls. Fungal Genet Biol. 2005;42:657–75. [DOI] [PubMed] [Google Scholar]
- Dehullu J, Valotteau C, Herman-Bausier Pet al. Fluidic Force Microscopy Demonstrates That Homophilic Adhesion by Candida albicans Als Proteins Is Mediated by Amyloid Bonds between Cells. Nano Lett. 2019;19:3846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Fresno C, Soulat D, Roth Set al. Interferon-β production via Dectin-1-Syk-IRF5 signaling in dendritic cells is crucial for immunity to C. albicans. Immunity. 2013;38:1176–86. [DOI] [PubMed] [Google Scholar]
- De Luca A, et al. IL-22 and IDO1 affect immunity and tolerance to murine and human vaginal candidiasis. PLoS Pathog. 2013;9:e1003486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Repentigny L, Aumont F, Bernard Ket al. Characterization of binding of Candida albicans to small intestinal mucin and its role in adherence to mucosal epithelial cells. Infect Immun. 2000;68:3172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien M, Collado MC, Ben-Amor Ket al. The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl Environ Microbiol. 2008;74:1646–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien M, Vaughan EE, Plugge CMet al. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54:1469–76. [DOI] [PubMed] [Google Scholar]
- Desai JV, Lionakis MS. The role of neutrophils in host defense against invasive fungal infections. Curr Clin Microbiol Rep. 2018;5:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen L, Huse S, Sogin MLet al. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveau A, et al. Bacterial-fungal interactions: ecology, mechanisms and challenges. FEMS Microbiol Rev. 2018;42:335–52. [DOI] [PubMed] [Google Scholar]
- de Vries DH, Matzaraki V, Bakker OBet al. Integrating GWAS with bulk and single-cell RNA-sequencing reveals a role for LY86 in the anti-Candida host response. PLoS Pathog. 2020;16:e1008408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard Jet al. The human oral microbiome. J Bacteriol. 2010;192:5002–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zuani M, Paolicelli G, Zelante Tet al. Mast Cells Respond to Candida albicans Infections and Modulate Macrophages Phagocytosis of the Fungus. Front Immunol. 2018;9:2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz PI, Xie Z, Sobue Tet al. Synergistic interaction between Candida albicans and commensal oral streptococci in a novel in vitro mucosal model. Infect Immun. 2012;80:620–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281:8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogo D, Bouchier C, d Enfert Cet al. Loss of heterozygosity in commensal isolates of the asexual diploid yeast Candida albicans. Fungal Genet Biol. 2009;46:159–68. [DOI] [PubMed] [Google Scholar]
- Doedt T, Krishnamurthy S, Bockmühl DPet al. APSES proteins regulate morphogenesis and metabolism in Candida albicans. Mol Biol Cell. 2004;15:3167–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollive S, et al. Fungi of the murine gut: episodic variation and proliferation during antibiotic treatment. PLoS One. 2013;8:e71806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras Met al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107:11971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Andrés J, et al. The itaconate pathway is a central regulatory node linking innate immune tolerance and trained immunity. Cell Metab. 2019;29:211–20..e5. [DOI] [PubMed] [Google Scholar]
- Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 2016;14:20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donders G, Bellen G, Janssens Det al. Influence of contraceptive choice on vaginal bacterial and fungal microflora. Eur J Clin Microbiol Infect Dis. 2017;36:43–48. [DOI] [PubMed] [Google Scholar]
- Donders GGG, Bellen G, Ruban Ket al. Short- and long-term influence of the levonorgestrel-releasing intrauterine system (Mirena®) on vaginal microbiota and Candida. J Med Microbiol. 2018;67:308–13. [DOI] [PubMed] [Google Scholar]
- Dongari-Bagtzoglou A, Kashleva H, Villar CC. Bioactive interleukin-1alpha is cytolytically released from Candida albicans-infected oral epithelial cells. Med Mycol. 2004;42:531–41. [DOI] [PubMed] [Google Scholar]
- Drell T, et al. Characterization of the vaginal micro- and mycobiome in asymptomatic reproductive-age Estonian women. PLoS One. 2013;8:e54379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewniak A, et al. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood. 2013;121:2385–92. [DOI] [PubMed] [Google Scholar]
- Driehuis E, et al. Oral mucosal organoids as a potential platform for personalized cancer therapy. Cancer Discov. 2019;9:852–71. [DOI] [PubMed] [Google Scholar]
- Drummond RA, Lionakis MS. Organ-specific mechanisms linking innate and adaptive antifungal immunity. Semin Cell Dev Biol. 2019;89:78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond RA, Saijo S, Iwakura Yet al. The role of Syk/CARD9 coupled C-type lectins in antifungal immunity. Eur J Immunol. 2011;41:276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond RA, et al. CARD9+ microglia promote antifungal immunity via IL-1β- and CXCL1-mediated neutrophil recruitment. Nat Immunol. 2019;20:559–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond RA, et al. CARD9-Dependent Neutrophil Recruitment Protects against Fungal Invasion of the Central Nervous System. PLoS Pathog. 2015;11:e1005293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drutz DJ. Lactobacillus prophylaxis for Candida vaginitis. Ann Intern Med. 1992;116:419–20. [DOI] [PubMed] [Google Scholar]
- Dubourg G, et al. Culturomics and pyrosequencing evidence of the reduction in gut microbiota diversity in patients with broad-spectrum antibiotics. Int J Antimicrob Agents. 2014;44:117–24. [DOI] [PubMed] [Google Scholar]
- Dumas A, Bernard L, Poquet Yet al. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol. 2018;20:e12966. [DOI] [PubMed] [Google Scholar]
- Dupuy AK, David MS, Li Let al. Redefining the human oral mycobiome with improved practices in amplicon-based taxonomy: discovery of Malassezia as a prominent commensal. PLoS One. 2014;9:e90899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant WE, Dong X. Systemic acquired resistance. Annu Rev Phytopathol. 2004;42:185–209. [DOI] [PubMed] [Google Scholar]
- Dutton LC, Nobbs AH, Jepson Ket al. O-mannosylation in Candida albicans enables development of interkingdom biofilm communities. MBio. 2014;5:e00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzidic M, Collado MC, Abrahamsson Tet al. Oral microbiome development during childhood: an ecological succession influenced by postnatal factors and associated with tooth decay. ISME J. 2018;12:2292–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Décanis N, Savignac K, Rouabhia M. Farnesol promotes epithelial cell defense against Candida albicans through Toll-like receptor 2 expression, interleukin-6 and human beta-defensin 2 production. Cytokine. 2009;45:132–40. [DOI] [PubMed] [Google Scholar]
- Edwards JE, et al. A Fungal Immunotherapeutic Vaccine (NDV-3A) for Treatment of Recurrent Vulvovaginal Candidiasis-A Phase 2 Randomized, Double-Blind, Placebo-Controlled Trial. Clin Infect Dis. 2018;66:1928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ene IV, Adya AK, Wehmeier Set al. Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cell Microbiol. 2012;14:1319–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ene IV, Bennett RJ. The cryptic sexual strategies of human fungal pathogens. Nat Rev Microbiol. 2014;12:239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ene IV, Brunke S, Brown AJPet al. Metabolism in fungal pathogenesis. Cold Spring Harb Perspect Med. 2014;4:a019695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ene IV, Farrer RA, Hirakawa MPet al. Global analysis of mutations driving microevolution of a heterozygous diploid fungal pathogen. Proc Natl Acad Sci USA. 2018;115:E8688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ene IV, Walker LA, Schiavone Met al. Cell wall remodeling enzymes modulate fungal cell wall elasticity and osmotic stress resistance. MBio. 2015;6:e00986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjalbert B, Nantel A, Whiteway M. Stress-induced gene expression in Candida albicans: absence of a general stress response. Mol Biol Cell. 2003;14:1460–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjalbert B, Smith DA, Cornell MJet al. Role of the Hog1 stress-activated protein kinase in the global transcriptional response to stress in the fungal pathogen Candida albicans. Mol Biol Cell. 2006;17:1018–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson AR, et al. Integrated metagenomics/metaproteomics reveals human host-microbiota signatures of Crohn's disease. PLoS One. 2012;7:e49138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermert D, Niemiec MJ, Röhm Met al. Candida albicans escapes from mouse neutrophils. J Leukoc Biol. 2013;94:223–36. [DOI] [PubMed] [Google Scholar]
- Erwig LP, Gow NAR. Interactions of fungal pathogens with phagocytes. Nat Rev Microbiol. 2016;14:163–76. [DOI] [PubMed] [Google Scholar]
- Eyerich K, Dimartino V, Cavani A. IL-17 and IL-22 in immunity: Driving protection and pathology. Eur J Immunol. 2017;47:607–14. [DOI] [PubMed] [Google Scholar]
- Eyerich S, et al. IL-22 and TNF-α represent a key cytokine combination for epidermal integrity during infection with Candida albicans. Eur J Immunol. 2011;41:1894–901. [DOI] [PubMed] [Google Scholar]
- Fairn GD, Grinstein S. How nascent phagosomes mature to become phagolysosomes. Trends Immunol. 2012;33:397–405. [DOI] [PubMed] [Google Scholar]
- Faith JJ, et al. The long-term stability of the human gut microbiota. Science. 2013;341:1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhim H, Vaezi A, Javidnia Jet al. Candida africana vulvovaginitis: Prevalence and geographical distribution. J Mycol Med. 2020;100966. [DOI] [PubMed] [Google Scholar]
- Falagas ME, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother. 2006;58:266–72. [DOI] [PubMed] [Google Scholar]
- Falcone M, et al. Assessment of risk factors for candidemia in non-neutropenic patients hospitalized in Internal Medicine wards: A multicenter study. Eur J Intern Med. 2017;41:33–38. [DOI] [PubMed] [Google Scholar]
- Falsetta ML, et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun. 2014;82:1968–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D, et al. Activation of HIF-1α and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat Med. 2015;21:808–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, et al. Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Microbiome. 2018;6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y-C, Li W-G, Zheng M-Het al. Invasive fungal infection in patients with systemic lupus erythematosus: experience from a single institute of Northern China. Gene. 2012;506:184–7. [DOI] [PubMed] [Google Scholar]
- Farmaki E, et al. Fungal colonization in the neonatal intensive care unit: risk factors, drug susceptibility, and association with invasive fungal infections. Am J Perinatol. 2007;24:127–35. [DOI] [PubMed] [Google Scholar]
- Farrer RA, et al. Genome Evolution and Innovation across the Four Major Lineages of Cryptococcus gattii. MBio. 2015;6:e00868–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli A, Bruce C, Anumba DO. Characterization of Toll-like receptors in the female reproductive tract in humans. Hum Reprod. 2005;20:1372–8. [DOI] [PubMed] [Google Scholar]
- Fehlbaum S, Chassard C, Haug MCet al. Design and Investigation of PolyFermS In Vitro Continuous Fermentation Models Inoculated with Immobilized Fecal Microbiota Mimicking the Elderly Colon. PLoS One. 2015;10:e0142793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fensterheim BA, et al. The TLR4 agonist monophosphoryl lipid A drives broad resistance to infection via dynamic reprogramming of macrophage metabolism. J Immunol. 2018;200:3777–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferwerda B, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361:1760–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettweis JM, et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology (Reading, Engl). 2014;160:2272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidel PL, Barousse M, Espinosa Tet al. An intravaginal live Candida challenge in humans leads to new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect Immun. 2004;72:2939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidel PL, Yano J, Esher SKet al. Applying the Host-Microbe Damage Response Framework to Candida Pathogenesis: Current and Prospective Strategies to Reduce Damage. J Fungi (Basel). 2020;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidel PL. Distinct protective host defenses against oral and vaginal candidiasis. Med Mycol. 2002;40:359–75. [PubMed] [Google Scholar]
- Filippidi A, Galanakis E, Maraki Set al. The effect of maternal flora on Candida colonisation in the neonate. Mycoses. 2014;57:43–48. [DOI] [PubMed] [Google Scholar]
- Finkel JS, Mitchell AP. Genetic control of Candida albicans biofilm development. Nat Rev Microbiol. 2011;9:109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer G, Bradford J. Vulvovaginal candidiasis in postmenopausal women: the role of hormone replacement therapy. J Low Genit Tract Dis. 2011;15:263–7. [DOI] [PubMed] [Google Scholar]
- Fischer G. Chronic vulvitis in pre-pubertal girls. Australas J Dermatol. 2010;51:118–23. [DOI] [PubMed] [Google Scholar]
- Flores GE, et al. Temporal variability is a personalized feature of the human microbiome. Genome Biol. 2014;15:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forche A, Abbey D, Pisithkul Tet al. Stress alters rates and types of loss of heterozygosity in Candida albicans. MBio. 2011;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forche A, Alby K, Schaefer Det al. The parasexual cycle in Candida albicans provides an alternative pathway to meiosis for the formation of recombinant strains. PLoS Biol. 2008;6:e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forche A, Magee PT, Selmecki Aet al. Evolution in Candida albicans populations during a single passage through a mouse host. Genetics. 2009;182:799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forche A, May G, Magee PT. Demonstration of loss of heterozygosity by single-nucleotide polymorphism microarray analysis and alterations in strain morphology in Candida albicans strains during infection. Eukaryotic Cell. 2005;4:156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forche A, et al. Rapid Phenotypic and Genotypic Diversification After Exposure to the Oral Host Niche in Candida albicans. Genetics. 2018;209:725–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CB, et al. The evolution of drug resistance in clinical isolates of Candida albicans. Elife. 2014;4:e00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouhy F, et al. High-throughput sequencing reveals the incomplete, short-term recovery of infant gut microbiota following parenteral antibiotic treatment with ampicillin and gentamicin. Antimicrob Agents Chemother. 2012;56:5811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox EP, Cowley ES, Nobile CJet al. Anaerobic bacteria grow within Candida albicans biofilms and induce biofilm formation in suspension cultures. Curr Biol. 2014;24:2411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman B, Muraglia R, Dietz J-Pet al. Prevalence of recurrent vulvovaginal candidiasis in 5 European countries and the United States: results from an internet panel survey. J Low Genit Tract Dis. 2013;17:340–45. [DOI] [PubMed] [Google Scholar]
- Fradin C, De Groot P, MacCallum Det al. Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol Microbiol. 2005;56:397–415. [DOI] [PubMed] [Google Scholar]
- Franzosa EA, et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol. 2019;4:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzosa EA, et al. Species-level functional profiling of metagenomes and metatranscriptomes. Nat Methods. 2018;15:962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas AC, Chaban B, Bocking Aet al. The vaginal microbiome of pregnant women is less rich and diverse, with lower prevalence of Mollicutes, compared to non-pregnant women. Sci Rep. 2017;7:9212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabaldón T, Fairhead C. Genomes shed light on the secret life of Candida glabrata: not so asexual, not so commensal. Curr Genet. 2019;65:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajer P, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012;4:132ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley JD, Nelson MC, Yu Zet al. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014;14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 2005;24:1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti E, Popolo L, Vai Met al. O-linked oligosaccharides in yeast glycosyl phosphatidylinositol-anchored protein gp115 are clustered in a serine-rich region not essential for its function. J Biol Chem. 1994;269:19695–700. [PubMed] [Google Scholar]
- Ghannoum MA, Jurevic RJ, Mukherjee PKet al. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010;6:e1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GR, Cummings JH, Macfarlane GT. Use of a three-stage continuous culture system to study the effect of mucin on dissimilatory sulfate reduction and methanogenesis by mixed populations of human gut bacteria. Appl Environ Microbiol. 1988;54:2750–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo PC, de Carvalho JBJ, do Amaral RLGet al. Identification of immune cells by flow cytometry in vaginal lavages from women with vulvovaginitis and normal microflora. Am J Reprod Immunol. 2012;67:198–205. [DOI] [PubMed] [Google Scholar]
- Gladiator A, Wangler N, Trautwein-Weidner Ket al. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol. 2013;190:521–5. [DOI] [PubMed] [Google Scholar]
- Gliniewicz K, Schneider GM, Ridenhour BJet al. Comparison of the vaginal microbiomes of premenopausal and postmenopausal women. Front Microbiol. 2019;10:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves B, Ferreira C, Alves CTet al. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905–27. [DOI] [PubMed] [Google Scholar]
- Goodrich JK, et al. Human genetics shape the gut microbiome. Cell. 2014;159:789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodridge HS, Underhill DM, Touret N. Mechanisms of Fc receptor and dectin-1 activation for phagocytosis. Traffic. 2012;13:1062–71. [DOI] [PubMed] [Google Scholar]
- Goplerud CP, Ohm MJ, Galask RP. Aerobic and anaerobic flora of the cervix during pregnancy and the puerperium. Am J Obstet Gynecol. 1976;126:858–68. [DOI] [PubMed] [Google Scholar]
- Gouba N, Drancourt M. Digestive tract mycobiota: a source of infection. Med Mal Infect. 2015;45:9–16. [DOI] [PubMed] [Google Scholar]
- Gow NAR, Brown AJP, Odds FC. Fungal morphogenesis and host invasion. Curr Opin Microbiol. 2002;5:366–71. [DOI] [PubMed] [Google Scholar]
- Gow NAR, Knox Y, Munro CAet al. Infection of chick chorioallantoic membrane (CAM) as a model for invasive hyphal growth and pathogenesis of Candida albicans. Med Mycol. 2003;41:331–8. [DOI] [PubMed] [Google Scholar]
- Gow NAR, Latge J-P, Munro CA. The fungal cell wall: structure, biosynthesis, and function. Microbiol Spectr. 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow NAR, van de Veerdonk FL, Brown AJPet al. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol. 2011;10:112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S, Castrillón-Betancur JC, Klaile Eet al. The Interaction of Human Pathogenic Fungi With C-Type Lectin Receptors. Front Immunol. 2018;9:1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf K, et al. Keeping Candida commensal: how lactobacilli antagonize pathogenicity of Candida albicans in an in vitro gut model. Dis Model Mech. 2019;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CE, Cruz MR, Garsin DAet al. Enterococcus faecalis bacteriocin EntV inhibits hyphal morphogenesis, biofilm formation, and virulence of Candida albicans. Proc Natl Acad Sci USA. 2017;114:4507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger BL. Insight into the antiadhesive effect of yeast wall protein 1 of Candida albicans. Eukaryotic Cell. 2012;11:795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg SS, Zhao X, Hua Let al. Ethanol inhibits lung clearance of Pseudomonas aeruginosa by a neutrophil and nitric oxide-dependent mechanism, in vivo. Alcohol Clin Exp Res. 1999;23:735–44. [PubMed] [Google Scholar]
- Grenham S, Clarke G, Cryan JFet al. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondman I, et al. Frontline Science: Endotoxin-induced immunotolerance is associated with loss of monocyte metabolic plasticity and reduction of oxidative burst. J Leukoc Biol. 2019;106:11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropp K, Schild L, Schindler Set al. The yeast Candida albicans evades human complement attack by secretion of aspartic proteases. Mol Immunol. 2009;47:465–75. [DOI] [PubMed] [Google Scholar]
- Guerra A, Etienne-Mesmin L, Livrelli Vet al. Relevance and challenges in modeling human gastric and small intestinal digestion. Trends Biotechnol. 2012;30:591–600. [DOI] [PubMed] [Google Scholar]
- Guinan J, Wang S, Hazbun TRet al. Antibiotic-induced decreases in the levels of microbial-derived short-chain fatty acids correlate with increased gastrointestinal colonization of Candida albicans. Sci Rep. 2019;9:8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R, Zheng N, Lu Het al. Increased diversity of fungal flora in the vagina of patients with recurrent vaginal candidiasis and allergic rhinitis. Microb Ecol. 2012;64:918–27. [DOI] [PubMed] [Google Scholar]
- Gupta S, Kakkar V, Bhushan I. Crosstalk between Vaginal Microbiome and Female Health: A review. Microb Pathog. 2019;136:103696. [DOI] [PubMed] [Google Scholar]
- Gutierrez D, et al. Antibiotic-induced gut metabolome and microbiome alterations increase the susceptibility to Candida albicans colonization in the gastrointestinal tract. FEMS Microbiol Ecol. 2020;96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager CL, Isham N, Schrom KPet al. Effects of a Novel Probiotic Combination on Pathogenic Bacterial-Fungal Polymicrobial Biofilms. MBio. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MW, Singh N, Ng KFet al. Inter-personal diversity and temporal dynamics of dental, tongue, and salivary microbiota in the healthy oral cavity. npj Biofilms and Microbiomes. 2017;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschlag MR, Alpert S, Onderdonk ABet al. Anaerobic microflora of the vagina in children. Am J Obstet Gynecol. 1978;131:853–6. [DOI] [PubMed] [Google Scholar]
- Handelsman J, Rondon MR, Brady SFet al. Molecular biological access to the chemistry of unknown soil microbes: a new frontier for natural products. Chem Biol. 1998;5:R245–9. [DOI] [PubMed] [Google Scholar]
- Hardison SE, Brown GD. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol. 2012;13:817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harriott MM, Noverr MC. Ability of Candida albicans mutants to induce Staphylococcus aureus vancomycin resistance during polymicrobial biofilm formation. Antimicrob Agents Chemother. 2010;54:3746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harriott MM, Noverr MC. Importance of Candida-bacterial polymicrobial biofilms in disease. Trends Microbiol. 2011;19:557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Kim D, Zhou Xet al. RNA-Seq Reveals Enhanced Sugar Metabolism in Streptococcus mutans Co-cultured with Candida albicans within Mixed-Species Biofilms. Front Microbiol. 2017;8:1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Santos N, Huppler AR, Peterson ACet al. Th17 cells confer long-term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunol. 2013;6:900–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Santos N, Wiesner DL, Fites JSet al. Lung Epithelial Cells Coordinate Innate Lymphocytes and Immunity against Pulmonary Fungal Infection. Cell Host Microbe. 2018;23:511–22..e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero-de-Dios C, Day AM, Tillmann ATet al. Redox Regulation, Rather than Stress-Induced Phosphorylation, of a Hog1 Mitogen-Activated Protein Kinase Modulates Its Nitrosative-Stress-Specific Outputs. MBio. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, et al. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat Med. 2018;24:1532–5. [DOI] [PubMed] [Google Scholar]
- Hibino K, Samaranayake LP, Hägg Uet al. The role of salivary factors in persistent oral carriage of Candida in humans. Arch Oral Biol. 2009;54:678–83. [DOI] [PubMed] [Google Scholar]
- Hickman MA, Paulson C, Dudley Aet al. Parasexual Ploidy Reduction Drives Population Heterogeneity Through Random and Transient Aneuploidy in Candida albicans. Genetics. 2015;200:781–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman MA, et al. The “obligate diploid” Candida albicans forms mating-competent haploids. Nature. 2013;494:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14. [DOI] [PubMed] [Google Scholar]
- Hill CJ, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier SL, Lau RJ. Vaginal microflora in postmenopausal women who have not received estrogen replacement therapy. Clin Infect Dis. 1997;25 Suppl 2:S123–6. [DOI] [PubMed] [Google Scholar]
- Hillman ET, Lu H, Yao Tet al. Microbial Ecology along the Gastrointestinal Tract. Microbes Environ. 2017;32:300–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmann F, Novohradská S, Mattern DJet al. Virulence determinants of the human pathogenic fungus Aspergillus fumigatus protect against soil amoeba predation. Environ Microbiol. 2015;17:2858–69. [DOI] [PubMed] [Google Scholar]
- Hirakawa MP, Chyou DE, Huang Det al. Parasex Generates Phenotypic Diversity de Novo and Impacts Drug Resistance and Virulence in Candida albicans. Genetics. 2017;207:1195–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa MP, et al. Genetic and phenotypic intra-species variation in Candida albicans. Genome Res. 2015;25:413–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C, Dollive S, Grunberg Set al. Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PLoS One. 2013;8:e66019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan DA, Vik A, Kolter R. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol Microbiol. 2004;54:1212–23. [DOI] [PubMed] [Google Scholar]
- Ho Jemima, et al. Candidalysin activates innate epithelial immune responses via epidermal growth factor receptor. Nat Commun. 2019;10:2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AR, McNab R, Jenkinson HF. Candida albicans binding to the oral bacterium Streptococcus gordonii involves multiple adhesin-receptor interactions. Infect Immun. 1996;64:4680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn F, Heinekamp T, Kniemeyer Oet al. Systems biology of fungal infection. Front Microbiol. 2012;3:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovav A-H. Mucosal and Skin Langerhans Cells - Nurture Calls. Trends Immunol. 2018;39:788–800. [DOI] [PubMed] [Google Scholar]
- Ho Vida, Herman-Bausier P, Shaw Cet al. An Amyloid Core Sequence in the Major Candida albicans Adhesin Als1p Mediates Cell-Cell Adhesion. MBio. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer LL, Cota E. Candida albicans Agglutinin-Like Sequence (Als) Family Vignettes: A Review of Als Protein Structure and Function. Front Microbiol. 2016;7:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hromatka BS, Noble SM, Johnson AD. Transcriptional response of Candida albicans to nitric oxide and the role of the YHB1 gene in nitrosative stress and virulence. Mol Biol Cell. 2005;16:4814–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Srikantha T, Sahni Net al. CO(2) regulates white-to-opaque switching in Candida albicans. Curr Biol. 2009;19:330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MY, Woolford CA, May Get al. Circuit diversification in a biofilm regulatory network. PLoS Pathog. 2019;15:e1007787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hube B. Virulence profile: Bernhard Hube. Virulence. 2015;6:523–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huertas B, Prieto D, Pitarch Aet al. Serum Antibody Profile during Colonization of the Mouse Gut by Candida albicans: Relevance for Protection during Systemic Infection. J Proteome Res. 2017;16:335–45. [DOI] [PubMed] [Google Scholar]
- Hugon P, Dufour J-C, Colson Pet al. A comprehensive repertoire of prokaryotic species identified in human beings. Lancet Infect Dis. 2015;15:1211–19. [DOI] [PubMed] [Google Scholar]
- Hu H, Merenstein DJ, Wang Cet al. Impact of eating probiotic yogurt on colonization by Candida species of the oral and vaginal mucosa in HIV-infected and HIV-uninfected women. Mycopathologia. 2013;176:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull CM, Raisner RM, Johnson AD. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science. 2000;289:307–10. [DOI] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium . Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurabielle C, et al. Immunity to commensal skin fungi promotes psoriasiform skin inflammation. Proc Natl Acad Sci USA. 2020;117:16465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse M. Mechanical forces in the immune system. Nat Rev Immunol. 2017;17:679–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huseyin CE, Rubio RC, O'Sullivan Oet al. The fungal frontier: A comparative analysis of methods used in the study of the human gut mycobiome. Front Microbiol. 2017;8:1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifrim DC, Quintin J, Meerstein-Kessel Let al. Defective trained immunity in patients with STAT-1-dependent chronic mucocutaneaous candidiasis. Clin Exp Immunol. 2015;181:434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igyártó BZ, et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35:260–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeh MAC, et al. Pho4 mediates phosphate acquisition in Candida albicans and is vital for stress resistance and metal homeostasis. Mol Biol Cell. 2016;27:2784–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indrio F, Riezzo G, Raimondi Fet al. The effects of probiotics on feeding tolerance, bowel habits, and gastrointestinal motility in preterm newborns. J Pediatr. 2008;152:801–6. [DOI] [PubMed] [Google Scholar]
- Ito S, et al. Specific strains of Streptococcus mutans, a pathogen of dental caries, in the tonsils, are associated with IgA nephropathy. Sci Rep. 2019;9:20130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabra-Rizk MA, Kong EF, Tsui Cet al. Candida albicans Pathogenesis: Fitting within the Host-Microbe Damage Response Framework. Infect Immun. 2016;84:2724–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MA, et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat Commun. 2018;9:2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob L, John M, Kalder Met al. Prevalence of vulvovaginal candidiasis in gynecological practices in Germany: A retrospective study of 954,186 patients. Curr Med Mycol. 2018;4:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs DM, Beyda ND, Asuphon Oet al. Host factors and clinical outcomes of Candida colonization in critically ill patients. Mycopathologia. 2015;179:87–93. [DOI] [PubMed] [Google Scholar]
- Jacobsen ID, Grosse K, Berndt Aet al. Pathogenesis of Candida albicans infections in the alternative chorio-allantoic membrane chicken embryo model resembles systemic murine infections. PLoS One. 2011;6:e19741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen ID, Wilson D, Wächtler Bet al. Candida albicans dimorphism as a therapeutic target. Expert Rev Anti Infect Ther. 2012;10:85–93. [DOI] [PubMed] [Google Scholar]
- Jaeger M, Stappers MHT, Joosten LABet al. Genetic variation in pattern recognition receptors: functional consequences and susceptibility to infectious disease. Future Microbiol. 2015;10:989–1008. [DOI] [PubMed] [Google Scholar]
- Jaeger M, van der Lee Ret al. The RIG-I-like helicase receptor MDA5 (IFIH1) is involved in the host defense against Candida infections. Eur J Clin Microbiol Infect Dis. 2015;34:963–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger Martin, et al. A Genome-Wide Functional Genomics Approach Identifies Susceptibility Pathways to Fungal Bloodstream Infection in Humans. J Infect Dis. 2019;220:862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger M, et al. Association of a variable number tandem repeat in the NLRP3 gene in women with susceptibility to RVVC. Eur J Clin Microbiol Infect Dis. 2016;35:797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger M, et al. A systems genomics approach identifies SIGLEC15 as a susceptibility factor in recurrent vulvovaginal candidiasis. Sci Transl Med. 2019;11. [DOI] [PubMed] [Google Scholar]
- Jain C, Pastor K, Gonzalez AYet al. The role of Candida albicans AP-1 protein against host derived ROS in in vivo models of infection. Virulence. 2013;4:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalili-Firoozinezhad S, et al. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat Biomed Eng. 2019;3:520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James KM, MacDonald KW, Chanyi RMet al. Inhibition of Candida albicans biofilm formation and modulation of gene expression by probiotic cells and supernatant. J Med Microbiol. 2016;65:328–36. [DOI] [PubMed] [Google Scholar]
- Jamieson DJ, Stephen DW, Terrière EC. Analysis of the adaptive oxidative stress response of Candida albicans. FEMS Microbiol Lett. 1996;138:83–88. [DOI] [PubMed] [Google Scholar]
- Jang SJ, Lee K, Kwon Bet al. Vaginal lactobacilli inhibit growth and hyphae formation of Candida albicans. Sci Rep. 2019;9:8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansons VK, Nickerson WJ. Induction, morphogenesis, and germination of the chlamydospore of Candida albicans. J Bacteriol. 1970;104:910–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosz LM, Deng DM, van der Mei HCet al. Streptococcus mutans competence-stimulating peptide inhibits Candida albicans hypha formation. Eukaryotic Cell. 2009;8:1658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeziorek M, Frej-Mądrzak M, Choroszy-Król I. The influence of diet on gastrointestinal Candida spp. colonization and the susceptibility of Candida spp. to antifungal drugs. Rocz Panstw Zakl Hig. 2019;70:195–200. [DOI] [PubMed] [Google Scholar]
- Jin Y, Wu S, Zeng Zet al. Effects of environmental pollutants on gut microbiota. Environ Pollut. 2017;222:1–9. [DOI] [PubMed] [Google Scholar]
- Johansson MEV, Gustafsson JK, Sjöberg KEet al. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS One. 2010;5:e12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MEV, Phillipson M, Petersson Jet al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly S, Ma N, Sadler JJet al. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol. 2009;183:3578–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T, et al. The diploid genome sequence of Candida albicans. Proc Natl Acad Sci USA. 2004;101:7329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouault T, El Abed-El Behi M, Martínez-Esparza Met al. Specific recognition of Candida albicans by macrophages requires galectin-3 to discriminate Saccharomyces cerevisiae and needs association with TLR2 for signaling. J Immunol. 2006;177:4679–87. [DOI] [PubMed] [Google Scholar]
- Jouault T, Ibata-Ombetta S, Takeuchi Oet al. Candida albicans phospholipomannan is sensed through toll-like receptors. J Infect Dis. 2003;188:165–72. [DOI] [PubMed] [Google Scholar]
- Kadir T, Uygun B, Akyüz S. Prevalence of Candida species in Turkish children: relationship between dietary intake and carriage. Arch Oral Biol. 2005;50:33–37. [DOI] [PubMed] [Google Scholar]
- Kadosh D. Regulatory mechanisms controlling morphology and pathogenesis in Candida albicans. Curr Opin Microbiol. 2019;52:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia N, Singh J, Kaur M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: a critical review. Ann Clin Microbiol Antimicrob. 2020;19:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalo-Klein A, Witkin SS. Prostaglandin E2 enhances and gamma interferon inhibits germ tube formation in Candida albicans. Infect Immun. 1990;58:260–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaloriti D, et al. Mechanisms underlying the exquisite sensitivity of Candida albicans to combinatorial cationic and oxidative stress that enhances the potent fungicidal activity of phagocytes. MBio. 2014;5:e01334–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankkunen P, Teirilä L, Rintahaka Jet al. (1,3)-beta-glucans activate both dectin-1 and NLRP3 inflammasome in human macrophages. J Immunol. 2010;184:6335–42. [DOI] [PubMed] [Google Scholar]
- Kapteyn JC, Hoyer LL, Hecht JEet al. The cell wall architecture of Candida albicans wild-type cells and cell wall-defective mutants. Mol Microbiol. 2000;35:601–11. [DOI] [PubMed] [Google Scholar]
- Karl JP, Hatch AM, Arcidiacono SMet al. Effects of psychological, environmental and physical stressors on the gut microbiota. Front Microbiol. 2018;9:2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashem SW, Igyarto BZet al. Candida albicans morphology and dendritic cell subsets determine T helper cell differentiation. Immunity. 2015;42:356–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashem SW, Riedl MS, Yao Cet al. Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity. Immunity. 2015;43:515–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasperkovitz PV, Khan NS, Tam JMet al. Toll-like receptor 9 modulates macrophage antifungal effector function during innate recognition of Candida albicans and Saccharomyces cerevisiae. Infect Immun. 2011;79:4858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper L, Seider K, Hube B. Intracellular survival of Candida glabrata in macrophages: immune evasion and persistence. FEMS Yeast Res. 2015;15:fov042. [DOI] [PubMed] [Google Scholar]
- Kasper L, et al. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat Commun. 2018;9:4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman CA. Fungal infections in older adults. Clin Infect Dis. 2001;33:550–5. [DOI] [PubMed] [Google Scholar]
- Kavanaugh NL, Zhang AQ, Nobile CJet al. Mucins suppress virulence traits of Candida albicans. MBio. 2014;5:e01911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayingo G, Wong B. The MAP kinase Hog1p differentially regulates stress-induced production and accumulation of glycerol and D-arabitol in Candida albicans. Microbiology (Reading, Engl). 2005;151:2987–99. [DOI] [PubMed] [Google Scholar]
- Kean R, et al. Candida albicans Mycofilms Support Staphylococcus aureus Colonization and Enhances Miconazole Resistance in Dual-Species Interactions. Front Microbiol. 2017;8:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrmann J, et al. The lung microbiome in patients with pneumocystosis. BMC Pulm Med. 2017;17:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijser BJF, Zaura E, Huse SMet al. Pyrosequencing analysis of the oral microflora of healthy adults. J Dent Res. 2008;87:1016–20. [DOI] [PubMed] [Google Scholar]
- Kenno S, Perito S, Mosci Pet al. Autophagy and Reactive Oxygen Species Are Involved in Neutrophil Extracellular Traps Release Induced by C. albicans Morphotypes. Front Microbiol. 2016;7:879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernien JF, Snarr BD, Sheppard DCet al. The Interface between Fungal Biofilms and Innate Immunity. Front Immunol. 2017;8:1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidane YH, Lawrence C, Murali TM. Computational approaches for discovery of common immunomodulators in fungal infections: towards broad-spectrum immunotherapeutic interventions. BMC Microbiol. 2013;13:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, et al. Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Sci Rep. 2017;7:41332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Hyung Sook, et al. Curdlan activates dendritic cells through dectin-1 and toll-like receptor 4 signaling. Int Immunopharmacol. 2016;39:71–78. [DOI] [PubMed] [Google Scholar]
- Kim Hyun Jung, Li H, Collins JJet al. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci USA. 2016;113:E7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Mylonakis E. Killing of Candida albicans filaments by Salmonella enterica serovar Typhimurium is mediated by sopB effectors, parts of a type III secretion system. Eukaryotic Cell. 2011;10:782–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioshima ES, Shinobu-Mesquita CS, Abadio AKRet al. Selection of potential anti-adhesion drugs by in silico approaches targeted to ALS3 from Candida albicans. Biotechnol Lett. 2019;41:1391–401. [DOI] [PubMed] [Google Scholar]
- Kirchner FR, LeibundGut-Landmann S. Tissue-resident memory Th17 cells maintain stable fungal commensalism in the oral mucosa. Mucosal Immunol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner FR, et al. Persistence of Candida albicans in the Oral Mucosa Induces a Curbed Inflammatory Host Response That Is Independent of Immunosuppression. Front Immunol. 2019;10:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassens ES, Boesten RJ, Haarman Met al. Mixed-species genomic microarray analysis of fecal samples reveals differential transcriptional responses of bifidobacteria in breast- and formula-fed infants. Appl Environ Microbiol. 2009;75:2668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klis FM, de Groot P, Hellingwerf K. Molecular organization of the cell wall of Candida albicans. Med Mycol. 2001;39 Suppl 1:1–8. [PubMed] [Google Scholar]
- Klotz SA, Chasin BS, Powell Bet al. Polymicrobial bloodstream infections involving Candida species: analysis of patients and review of the literature. Diagn Microbiol Infect Dis. 2007;59:401–6. [DOI] [PubMed] [Google Scholar]
- Koh AY, Köhler JR, Coggshall KTet al. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog. 2008;4:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–75. [DOI] [PubMed] [Google Scholar]
- Kong EF, Tsui C, Kucharíková Set al. Commensal Protection of Staphylococcus aureus against Antimicrobials by Candida albicans Biofilm Matrix. MBio. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H, Andes DR, Krysan DJ. Candida-streptococcal interactions in biofilm-associated oral diseases. PLoS Pathog. 2018;14:e1007342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornitzer D. Regulation of Candida albicans Hyphal Morphogenesis by Endogenous Signals. J Fungi (Basel). 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka Met al. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. [DOI] [PubMed] [Google Scholar]
- Korpela K, et al. Intestinal microbiota development and gestational age in preterm neonates. Sci Rep. 2018;8:2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos I, Patterson MJ, Znaidi Set al. Mechanisms Underlying the Delayed Activation of the Cap1 Transcription Factor in Candida albicans following Combinatorial Oxidative and Cationic Stress Important for Phagocytic Potency. MBio. 2016;7:e00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovachev SM, Vatcheva-Dobrevska RS. Local Probiotic Therapy for Vaginal Candida albicans Infections. Probiotics Antimicrob Proteins. 2015;7:38–44. [DOI] [PubMed] [Google Scholar]
- Kraneveld EA, Buijs MJ, Bonder MJet al. The relation between oral Candida load and bacterial microbiome profiles in Dutch older adults. PLoS One. 2012;7:e42770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnakumari V, Rangaraj N, Nagaraj R. Antifungal activities of human beta-defensins HBD-1 to HBD-3 and their C-terminal analogs Phd1 to Phd3. Antimicrob Agents Chemother. 2009;53:256–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto CA. Inflammation and gastrointestinal Candida colonization. Curr Opin Microbiol. 2011;14:386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto CA. Molecular mechanisms of mechanosensing and their roles in fungal contact sensing. Nat Rev Microbiol. 2008;6:667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PS, Mason MR, Brooker MRet al. Pyrosequencing reveals unique microbial signatures associated with healthy and failing dental implants. J Clin Periodontol. 2012;39:425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, et al. Immunochip SNP array identifies novel genetic variants conferring susceptibility to candidaemia. Nat Commun. 2014;5:4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones EA, Mandell L, Whitney Cet al. Role of toll-like receptor 2 (TLR2) in neutrophil activation: GM-CSF enhances TLR2 expression and TLR2-mediated interleukin 8 responses in neutrophils. Blood. 2002;100:1860–68. [PubMed] [Google Scholar]
- Kurtz J, Franz K. Innate defence: evidence for memory in invertebrate immunity. Nature. 2003;425:37–38. [DOI] [PubMed] [Google Scholar]
- Kuznets G, et al. A relay network of extracellular heme-binding proteins drives C. albicans iron acquisition from hemoglobin. PLoS Pathog. 2014;10:e1004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler GA, Assefa S, Reid G. Probiotic interference of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 with the opportunistic fungal pathogen Candida albicans. Infect Dis Obstet Gynecol. 2012;2012:636474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler JR, Casadevall A, Perfect J. The spectrum of fungi that infects humans. Cold Spring Harb Perspect Med. 2014;5:a019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier J-C, Hugon P, Khelaifia Set al. The rebirth of culture in microbiology through the example of culturomics to study human gut microbiota. Clin Microbiol Rev. 2015;28:237–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier J-C, et al. Culture of previously uncultured members of the human gut microbiota by culturomics. Nat Microbiol. 2016;1:16203. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lagier JC, et al. Microbial culturomics: paradigm shift in the human gut microbiome study. Clin Microbiol Infect. 2012;18:1185–93. [DOI] [PubMed] [Google Scholar]
- Lambrecht E, Van Coillie E, Van Meervenne Eet al. Commensal E. coli rapidly transfer antibiotic resistance genes to human intestinal microbiota in the Mucosal Simulator of the Human Intestinal Microbial Ecosystem (M-SHIME). Int J Food Microbiol. 2019;311:108357. [DOI] [PubMed] [Google Scholar]
- Lang S, et al. Intestinal fungal dysbiosis and systemic immune response to fungi in patients with alcoholic hepatitis. Hepatology. 2020;71:522–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankelma JM, Belzer C, Hoogendijk AJet al. Antibiotic-Induced Gut Microbiota Disruption Decreases TNF-α Release by Mononuclear Cells in Healthy Adults. Clin Transl Gastroenterol. 2016;7:e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanternier F, Cypowyj S, Picard Cet al. Primary immunodeficiencies underlying fungal infections. Curr Opin Pediatr. 2013;25:736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanternier F, Pathan Set al. Deep dermatophytosis and inherited CARD9 deficiency. N Engl J Med. 2013;369:1704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley TD, Walker AW. Intestinal colonization resistance. Immunology. 2013;138:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach MD, Budge S, Walker Let al. Hsp90 orchestrates transcriptional regulation by Hsf1 and cell wall remodelling by MAPK signalling during thermal adaptation in a pathogenic yeast. PLoS Pathog. 2012;8:e1003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach MD, Tyc KM, Brown AJPet al. Modelling the regulation of thermal adaptation in Candida albicans, a major fungal pathogen of humans. PLoS One. 2012;7:e32467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach MD, et al. Hsf1 and Hsp90 orchestrate temperature-dependent global transcriptional remodelling and chromatin architecture in Candida albicans. Nat Commun. 2016;7:11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc DM, Barousse MM, Fidel PL. Role for dendritic cells in immunoregulation during experimental vaginal candidiasis. Infect Immun. 2006;74:3213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EKS, et al. Leukotriene B4-Mediated Neutrophil Recruitment Causes Pulmonary Capillaritis during Lethal Fungal Sepsis. Cell Host Microbe. 2018;23:121–33..e4. [DOI] [PubMed] [Google Scholar]
- Lee SA, Lim JY, Kim B-Set al. Comparison of the gut microbiota profile in breast-fed and formula-fed Korean infants using pyrosequencing. Nutr Res Pract. 2015;9:242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeibundGut-Landmann S, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–8. [DOI] [PubMed] [Google Scholar]
- Lemoinne S, et al. Fungi participate in the dysbiosis of gut microbiota in patients with primary sclerosing cholangitis. Gut. 2020;69:92–102. [DOI] [PubMed] [Google Scholar]
- Leonardi I, et al. CX3CR1+ mononuclear phagocytes control immunity to intestinal fungi. Science. 2018;359:232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi I, et al. Fungal Trans-kingdom Dynamics Linked to Responsiveness to Fecal Microbiota Transplantation (FMT) Therapy in Ulcerative Colitis. Cell Host Microbe. 2020;27:823–829.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt J, et al. Candida albicans β-Glucan Differentiates Human Monocytes Into a Specific Subset of Macrophages. Front Immunol. 2018;9:2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis LE, Bain JM, Lowes Cet al. Stage specific assessment of Candida albicans phagocytosis by macrophages identifies cell wall composition and morphogenesis as key determinants. PLoS Pathog. 2012;8:e1002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S-H, Anderson MZ, Hirakawa MPet al. Hemizygosity enables a mutational transition governing fungal virulence and commensalism. Cell Host Microbe. 2019;25:418–431.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S-H, Bennett RJ. The Impact of Gene Dosage and Heterozygosity on The Diploid Pathobiont Candida albicans. J Fungi (Basel). 2019;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SC, Tan X-Y, Luxenberg DPet al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SC, et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179:7791–9. [DOI] [PubMed] [Google Scholar]
- Lif Holgerson P, Harnevik L, Hernell Oet al. Mode of birth delivery affects oral microbiota in infants. J Dent Res. 2011;90:1183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Hai, et al. The outer mucus layer hosts a distinct intestinal microbial niche. Nat Commun. 2015;6:8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Vinh DC, Casanova J-Let al. Inborn errors of immunity underlying fungal diseases in otherwise healthy individuals. Curr Opin Microbiol. 2017;40:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CSY, Rosli R, Seow HFet al. Candida and invasive candidiasis: back to basics. Eur J Clin Microbiol Infect Dis. 2012;31:21–31. [DOI] [PubMed] [Google Scholar]
- Limon JJ, et al. Malassezia Is Associated with Crohn's Disease and Exacerbates Colitis in Mouse Models. Cell Host Microbe. 2019;25:377–388.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay AK, Morales DK, Liu Zet al. Analysis of Candida albicans mutants defective in the Cdk8 module of mediator reveal links between metabolism and biofilm formation. PLoS Genet. 2014;10:e1004567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y, et al. Inherited IL-17RC deficiency in patients with chronic mucocutaneous candidiasis. J Exp Med. 2015;212:619–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Oh S-H, Jones Ret al. The peptide-binding cavity is essential for Als3-mediated adhesion of Candida albicans to human cells. J Biol Chem. 2014;289:18401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionakis MS, Holland SM. CARD9: at the intersection of mucosal and systemic antifungal immunity. Blood. 2013;121:2377–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionakis MS, Levitz SM. Host Control of Fungal Infections: Lessons from Basic Studies and Human Cohorts. Annu Rev Immunol. 2018;36:157–91. [DOI] [PubMed] [Google Scholar]
- Lionakis MS, Lim JK, Lee C-CRet al. Organ-specific innate immune responses in a mouse model of invasive candidiasis. J Innate Immun. 2011;3:180–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionakis MS. Genetic susceptibility to fungal infections in humans. Curr Fungal Infect Rep. 2012;6:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionakis MS. New insights into innate immune control of systemic candidiasis. Med Mycol. 2014;52:555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionakis MS, et al. CX3CR1-dependent renal macrophage survival promotes Candida control and host survival. J Clin Invest. 2013;123:5035–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, et al. Deep sequencing of the oral microbiome reveals signatures of periodontal disease. PLoS One. 2012;7:e37919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Fahle GA, Kovacs JA. Inability to Culture Pneumocystis jirovecii. MBio. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yang F, Li Det al. Trisomy of chromosome R confers resistance to triazoles in Candida albicans. Med Mycol. 2015;53:302–9. [DOI] [PubMed] [Google Scholar]
- Lloyd-Price J, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo HJ, Köhler JR, DiDomenico Bet al. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–49. [DOI] [PubMed] [Google Scholar]
- Lohse MB, Gulati M, Johnson ADet al. Development and regulation of single- and multi-species Candida albicans biofilms. Nat Rev Microbiol. 2018;16:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes JP, Stylianou M, Backman Eet al. Evasion of Immune Surveillance in Low Oxygen Environments Enhances Candida albicans Virulence. MBio. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz MC, Bender JA, Fink GR. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryotic Cell. 2004;3:1076–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovat LB. Age related changes in gut physiology and nutritional status. Gut. 1996;38:306–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukovac S, Belzer C, Pellis Let al. Differential modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. MBio. 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Blom AM, Rupp Set al. The pH-regulated antigen 1 of Candida albicans binds the human complement inhibitor C4b-binding protein and mediates fungal complement evasion. J Biol Chem. 2011;286:8021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Poltermann S, Kunert Aet al. Immune evasion of the human pathogenic yeast Candida albicans: Pra1 is a Factor H, FHL-1 and plasminogen binding surface protein. Mol Immunol. 2009;47:541–50. [DOI] [PubMed] [Google Scholar]
- López-Ribot JL, Casanova M, Murgui Aet al. Antibody response to Candida albicans cell wall antigens. FEMS Immunol Med Microbiol. 2004;41:187–96. [DOI] [PubMed] [Google Scholar]
- Lüttich A, Brunke S, Hube Bet al. Serial passaging of Candida albicans in systemic murine infection suggests that the wild type strain SC5314 is well adapted to the murine kidney. PLoS One. 2013;8:e64482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum DM, Castillo L, Nather Ket al. Property differences among the four major Candida albicans strain clades. Eukaryotic Cell. 2009;8:373–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum DM, Odds FC. Temporal events in the intravenous challenge model for experimental Candida albicans infections in female mice. Mycoses. 2005;48:151–61. [DOI] [PubMed] [Google Scholar]
- Maccallum DM. Hosting infection: experimental models to assay Candida virulence. Int J Microbiol. 2012;2012:363764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciel NO, et al. Occurrence, antifungal susceptibility, and virulence factors of opportunistic yeasts isolated from Brazilian beaches. Mem Inst Oswaldo Cruz. 2019;114:e180566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre DA, et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci Rep. 2015;5:8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie J, Szabo EK, Urgast DSet al. Host-Imposed Copper Poisoning Impacts Fungal Micronutrient Acquisition during Systemic Candida albicans Infections. PLoS One. 2016;11:e0158683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhivanan P, et al. Identification of culturable vaginal Lactobacillus species among reproductive age women in Mysore, India. J Med Microbiol. 2015;64:636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee BB, Magee PT. Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science. 2000;289:310–13. [DOI] [PubMed] [Google Scholar]
- Maier L, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555:623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailhe M, et al. Repertoire of the gut microbiota from stomach to colon using culturomics and next-generation sequencing. BMC Microbiol. 2018;18:157. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mailänder-Sánchez D, et al. Antifungal defense of probiotic Lactobacillus rhamnosus GG is mediated by blocking adhesion and nutrient depletion. PLoS One. 2017;12:e0184438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majer O, Bourgeois C, Zwolanek Fet al. Type I interferons promote fatal immunopathology by regulating inflammatory monocytes and neutrophils during Candida infections. PLoS Pathog. 2012;8:e1002811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavia D, Lehtovirta-Morley LE, Alamir Oet al. Zinc Limitation Induces a Hyper-Adherent Goliath Phenotype in Candida albicans. Front Microbiol. 2017;8:2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malliaris SD, Steenbergen JN, Casadevall A. Cryptococcus neoformans var. gattii can exploit Acanthamoeba castellanii for growth. Med Mycol. 2004;42:149–58. [DOI] [PubMed] [Google Scholar]
- Manichanh C, et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolio TA. Genomewide association studies and assessment of the risk of disease. N Engl J Med. 2010;363:166–76. [DOI] [PubMed] [Google Scholar]
- Manzoni P, Mostert M, Leonessa MLet al. Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by Candida species in preterm neonates: a randomized study. Clin Infect Dis. 2006;42:1735–42. [DOI] [PubMed] [Google Scholar]
- Marakalala MJ, et al. Differential adaptation of Candida albicans in vivo modulates immune recognition by dectin-1. PLoS Pathog. 2013;9:e1003315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc G, Araniciu C, Oniga SDet al. New N-(oxazolylmethyl)-thiazolidinedione Active against Candida albicans Biofilm: Potential Als Proteins Inhibitors. Molecules. 2018;23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos CM, et al. Anti-Immune Strategies of Pathogenic Fungi. Front Cell Infect Microbiol. 2016;6:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis M, Lewandowski D, Dugas Vet al. CD8+ T cells but not polymorphonuclear leukocytes are required to limit chronic oral carriage of Candida albicans in transgenic mice expressing human immunodeficiency virus type 1. Infect Immun. 2006;74:2382–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pomares L. The mannose receptor. J Leukoc Biol. 2012;92:1177–86. [DOI] [PubMed] [Google Scholar]
- Martinez X, Pozuelo M, Pascal Vet al. MetaTrans: an open-source pipeline for metatranscriptomics. Sci Rep. 2016;6:26447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-López M, et al. Microbiota Sensing by Mincle-Syk Axis in Dendritic Cells Regulates Interleukin-17 and -22 Production and Promotes Intestinal Barrier Integrity. Immunity. 2019;50:446–461.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzorati M, Vilchez-Vargas R, Bussche JVet al. High-fiber and high-protein diets shape different gut microbial communities, which ecologically behave similarly under stress conditions, as shown in a gastrointestinal simulator. Mol Nutr Food Res. 2017;61. [DOI] [PubMed] [Google Scholar]
- Maschirow L, Suttorp N, Opitz B. Microbiota-Dependent Regulation of Antimicrobial Immunity in the Lung. Am J Respir Cell Mol Biol. 2019;61:284–9. [DOI] [PubMed] [Google Scholar]
- Matee MI, Samaranayake LP, Scheutz Fet al. Biotypes of oral Candida albicans isolates in a Tanzanian child population. APMIS. 1996;104:623–8. [PubMed] [Google Scholar]
- Matthews R, Burnie J, Smith Det al. Candida and AIDS: evidence for protective antibody. Lancet. 1988;2:263–6. [DOI] [PubMed] [Google Scholar]
- Matthews RC, Burnie JP, Howat Det al. Autoantibody to heat-shock protein 90 can mediate protection against systemic candidosis. Immunology. 1991;74:20–24. [PMC free article] [PubMed] [Google Scholar]
- Mattos-Graner RO, de Moraes AB, Rontani RMet al. Relation of oral yeast infection in Brazilian infants and use of a pacifier. ASDC J Dent Child. 2001;68:10. [PubMed] [Google Scholar]
- Matzaraki V, et al. An integrative genomics approach identifies novel pathways that influence candidaemia susceptibility. PLoS One. 2017;12:e0180824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M, et al. A three-dimensional immunocompetent intestine-on-chip model as in vitro platform for functional and microbial interaction studies. Biomaterials. 2019;220:119396. [DOI] [PubMed] [Google Scholar]
- Maxson ME, Naj X, O'Meara TRet al. Integrin-based diffusion barrier separates membrane domains enabling the formation of microbiostatic frustrated phagosomes. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer BT, Srinivasan S, Fiedler TLet al. Rapid and profound shifts in the vaginal microbiota following antibiotic treatment for bacterial vaginosis. J Infect Dis. 2015;212:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013;4:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer FL, Wilson D, Jacobsen IDet al. The novel Candida albicans transporter Dur31 Is a multi-stage pathogenicity factor. PLoS Pathog. 2012;8:e1002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure R, Massari P. TLR-Dependent Human Mucosal Epithelial Cell Responses to Microbial Pathogens. Front Immunol. 2014;5:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGreal EP, Rosas M, Brown GDet al. The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology. 2006;16:422–30. [DOI] [PubMed] [Google Scholar]
- McManus BA, Coleman DC. Molecular epidemiology, phylogeny and evolution of Candida albicans. Infect Genet Evol. 2014;21:166–78. [DOI] [PubMed] [Google Scholar]
- Mehta RS, et al. Stability of the human faecal microbiome in a cohort of adult men. Nat Microbiol. 2018;3:347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei M-L, Chu C-H, Low K-Het al. Caries arresting effect of silver diamine fluoride on dentine carious lesion with S. mutans and L. acidophilus dual-species cariogenic biofilm. Med Oral Patol Oral Cir Bucal. 2013;18:e824–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkumyan AR, Priputnevich TV, Ankirskaya ASet al. Effects of antibiotic treatment on the lactobacillus composition of vaginal microbiota. Bull Exp Biol Med. 2015;158:766–8. [DOI] [PubMed] [Google Scholar]
- Mengesha BG, Conti HR. The Role of IL-17 in Protection against Mucosal Candida Infections. J Fungi (Basel). 2017;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merson-Davies LA, Odds FC. A morphology index for characterization of cell shape in Candida albicans. J Gen Microbiol. 1989;135:3143–52. [DOI] [PubMed] [Google Scholar]
- Mestas J, Hughes CCW. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–8. [DOI] [PubMed] [Google Scholar]
- Metwalli KH, Khan SA, Krom BPet al. Streptococcus mutans, Candida albicans, and the human mouth: a sticky situation. PLoS Pathog. 2013;9:e1003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E, Geffers C, Gastmeier Pet al. No increase in primary nosocomial candidemia in 682 German intensive care units during 2006 to 2011. Euro Surveill. 2013;18. [PubMed] [Google Scholar]
- Meyer H, Goettlicher S, Mendling W. Stress as a cause of chronic recurrent vulvovaginal candidosis and the effectiveness of the conventional antimycotic therapy. Mycoses. 2006;49:202–9. [DOI] [PubMed] [Google Scholar]
- Miller EA, Beasley DE, Dunn RRet al. Lactobacilli dominance and vaginal ph: why is the human vaginal microbiome unique? Front Microbiol. 2016;7:1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MG, Johnson AD.. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell. 2002;110:293–302. [DOI] [PubMed] [Google Scholar]
- Millsop JW, Fazel N. Oral candidiasis. Clin Dermatol. 2016;34:487–94. [DOI] [PubMed] [Google Scholar]
- Miramón P, Dunker C, Windecker Het al. Cellular responses of Candida albicans to phagocytosis and the extracellular activities of neutrophils are critical to counteract carbohydrate starvation, oxidative and nitrosative stress. PLoS One. 2012;7:e52850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miramón P, Kasper L, Hube B. Thriving within the host: Candida spp. interactions with phagocytic cells. Med Microbiol Immunol. 2013;202:183–95. [DOI] [PubMed] [Google Scholar]
- Miranda LN, et al. Candida colonisation as a source for candidaemia. J Hosp Infect. 2009;72:9–16. [DOI] [PubMed] [Google Scholar]
- Mishra AA, Koh AY. Adaptation of Candida albicans during gastrointestinal tract colonization. Curr Clin Microbiol Rep. 2018;5:165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misme-Aucouturier B, Albassier M, Alvarez-Rueda Net al. Specific Human and Candida Cellular Interactions Lead to Controlled or Persistent Infection Outcomes during Granuloma-Like Formation. Infect Immun. 2017;85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazato A, et al. Toll-like receptor 9-dependent activation of myeloid dendritic cells by Deoxynucleic acids from Candida albicans. Infect Immun. 2009;77:3056–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens F, Duysburgh C, van den Abbeele Pet al. Lactobacillus rhamnosus GG and Saccharomyces cerevisiae boulardii exert synergistic antipathogenic activity in vitro against enterotoxigenic Escherichia coli. Benef Microbes. 2019;10:923–35. [DOI] [PubMed] [Google Scholar]
- Mojazi Amiri H, Frandah W, Colmer-Hamood Jet al. Risk factors of Candida colonization in the oropharynx of patients admitted to an intensive care unit. J Mycol Med. 2012;22:301–7. [DOI] [PubMed] [Google Scholar]
- Molly K, Vande Woestyne M, Verstraete W. Development of a 5-step multi-chamber reactor as a simulation of the human intestinal microbial ecosystem. Appl Microbiol Biotechnol. 1993;39:254–8. [DOI] [PubMed] [Google Scholar]
- Moosa M-YS, Sobel JD, Elhalis Het al. Fungicidal activity of fluconazole against Candida albicans in a synthetic vagina-simulative medium. Antimicrob Agents Chemother. 2004;48:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais IMC, Cordeiro AL, Teixeira GSet al. Biological and physicochemical properties of biosurfactants produced by Lactobacillus jensenii P6A and Lactobacillus gasseri P65. Microb Cell Fact. 2017;16:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales DK, Grahl N, Okegbe Cet al. Control of Candida albicans metabolism and biofilm formation by Pseudomonas aeruginosa phenazines. MBio. 2013;4:e00526–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Ruiz E, Galán-Díez M, Zhu Wet al. Candida albicans internalization by host cells is mediated by a clathrin-dependent mechanism. Cell Microbiol. 2009;11:1179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morschhäuser J. Regulation of white-opaque switching in Candida albicans. Med Microbiol Immunol. 2010;199:165–72. [DOI] [PubMed] [Google Scholar]
- Mothibe JV, Patel M. Pathogenic characteristics of Candida albicans isolated from oral cavities of denture wearers and cancer patients wearing oral prostheses. Microb Pathog. 2017;110:128–34. [DOI] [PubMed] [Google Scholar]
- Motooka D, et al. Fungal ITS1 Deep-Sequencing Strategies to Reconstruct the Composition of a 26-Species Community and Evaluation of the Gut Mycobiota of Healthy Japanese Individuals. Front Microbiol. 2017;8:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottawea W, Butcher J, Li Jet al. The mucosal-luminal interface: an ideal sample to study the mucosa-associated microbiota and the intestinal microbial biogeography. Pediatr Res. 2019;85:895–903. [DOI] [PubMed] [Google Scholar]
- Moura-Alves P, et al. AhR sensing of bacterial pigments regulates antibacterial defence. Nature. 2014;512:387–92. [DOI] [PubMed] [Google Scholar]
- Moyes DL, et al. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe. 2010;8:225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyes DL, et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532:64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaremera L, Lee KK, Mora-Montes HMet al. Candida albicans Yeast, Pseudohyphal, and Hyphal Morphogenesis Differentially Affects Immune Recognition. Front Immunol. 2017;8:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee PK, Seshan KR, Leidich SDet al. Reintroduction of the PLB1 gene into Candida albicans restores virulence in vivo. Microbiology (Reading, Engl). 2001;147:2585–97. [DOI] [PubMed] [Google Scholar]
- Mulder WJM, Ochando J, Joosten LABet al. Therapeutic targeting of trained immunity. Nat Rev Drug Discov. 2019;18:553–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad AM, d'Enfert C, Gaillardin Cet al. Transcript profiling in Candida albicans reveals new cellular functions for the transcriptional repressors CaTup1, CaMig1 and CaNrg1. Mol Microbiol. 2001;42:981–93. [DOI] [PubMed] [Google Scholar]
- Murad AM, Leng P, Straffon Met al. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. EMBO J. 2001;20:4742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, et al. Changes in intestinal motility and gut microbiota composition in a rat stress model. Digestion. 2017;95:55–60. [DOI] [PubMed] [Google Scholar]
- Muzyka BC, Epifanio RN. Update on oral fungal infections. Dent Clin North Am. 2013;57:561–81. [DOI] [PubMed] [Google Scholar]
- Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev. 2003;67:400–28., table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naglik JR, König A, Hube Bet al. Candida albicans-epithelial interactions and induction of mucosal innate immunity. Curr Opin Microbiol. 2017;40:104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjappa SG, Heninger E, Wüthrich Met al. Tc17 cells mediate vaccine immunity against lethal fungal pneumonia in immune deficient hosts lacking CD4+ T cells. PLoS Pathog. 2012;8:e1002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash AK, et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome. 2017;5:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash EE, Peters BM, Fidel PLet al. Morphology-Independent Virulence of Candida Species during Polymicrobial Intra-abdominal Infections with Staphylococcus aureus. Infect Immun. 2016;84:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash EE, Peters BM, Palmer GEet al. Morphogenesis is not required for Candida albicans-Staphylococcus aureus intra-abdominal infection-mediated dissemination and lethal sepsis. Infect Immun. 2014;82:3426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser L, Weissman Z, Pinsky Met al. Structural basis of haem-iron acquisition by fungal pathogens. Nat Microbiol. 2016;1:16156. [DOI] [PubMed] [Google Scholar]
- Netea MG, Brown GD, Kullberg BJet al. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6:67–78. [DOI] [PubMed] [Google Scholar]
- Netea MG, Quintin J, van der Meer JWM. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9:355–61. [DOI] [PubMed] [Google Scholar]
- Netea MG, et al. A guiding map for inflammation. Nat Immunol. 2017;18:826–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest. 2006;116:1642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves AB, Lobo LA, Pinto KCet al. Comparison between Clinical Aspects and Salivary Microbial Profile of Children with and without Early Childhood Caries: A Preliminary Study. J Clin Pediatr Dent. 2015;39:209–14. [DOI] [PubMed] [Google Scholar]
- Neville BA, d'Enfert C, Bougnoux M-E. Candida albicans commensalism in the gastrointestinal tract. FEMS Yeast Res. 2015;15. [DOI] [PubMed] [Google Scholar]
- Newport MJ, Finan C. Genome-wide association studies and susceptibility to infectious diseases. Brief Funct Genomics. 2011;10:98–107. [DOI] [PubMed] [Google Scholar]
- Ngo LY, Kasahara S, Kumasaka DKet al. Inflammatory monocytes mediate early and organ-specific innate defense during systemic candidiasis. J Infect Dis. 2014;209:109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LN, Lopes LCL, Cordero RJBet al. Sodium butyrate inhibits pathogenic yeast growth and enhances the functions of macrophages. J Antimicrob Chemother. 2011;66:2573–80. [DOI] [PubMed] [Google Scholar]
- Nguyen MH, Peacock JE, Morris AJet al. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am J Med. 1996;100:617–23. [DOI] [PubMed] [Google Scholar]
- Nicholls S, Leach MD, Priest CLet al. Role of the heat shock transcription factor, Hsf1, in a major fungal pathogen that is obligately associated with warm-blooded animals. Mol Microbiol. 2009;74:844–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls S, MacCallum DM, Kaffarnik FARet al. Activation of the heat shock transcription factor Hsf1 is essential for the full virulence of the fungal pathogen Candida albicans. Fungal Genet Biol. 2011;48:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaou E, Agrafioti I, Stumpf Met al. Phylogenetic diversity of stress signalling pathways in fungi. BMC Evol Biol. 2009;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikou S-A, Kichik N, Brown Ret al. Candida albicans Interactions with Mucosal Surfaces during Health and Disease. Pathogens. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile CJ, Fox EP, Nett JEet al. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell. 2012;148:126–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile CJ, Johnson AD. Candida albicans Biofilms and Human Disease. Annu Rev Microbiol. 2015;69:71–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble SM, Gianetti BA, Witchley JN. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat Rev Microbiol. 2017;15:96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble SM. Candida albicans specializations for iron homeostasis: from commensalism to virulence. Curr Opin Microbiol. 2013;16:708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noverr MC, Huffnagle GB. Regulation of Candida albicans morphogenesis by fatty acid metabolites. Infect Immun. 2004;72:6206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noverr MC, Phare SM, Toews GBet al. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect Immun. 2001;69:2957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucci M, Anaissie E. Revisiting the source of candidemia: skin or gut? Clin Infect Dis. 2001;33:1959–67. [DOI] [PubMed] [Google Scholar]
- Nucci M, Colombo AL, Spector Net al. Breakthrough candidemia in neutropenic patients. Clin Infect Dis. 1997;24:275–6. [DOI] [PubMed] [Google Scholar]
- Nwokolo NC, Boag FC. Chronic vaginal candidiasis. Management in the postmenopausal patient. Drugs Aging. 2000;16:335–9. [DOI] [PubMed] [Google Scholar]
- O'Donnell LE, Millhouse E, Sherry Let al. Polymicrobial Candida biofilms: friends and foe in the oral cavity. FEMS Yeast Res. 2015;15. [DOI] [PubMed] [Google Scholar]
- O'Meara TR, Veri AO, Ketela Tet al. Global analysis of fungal morphology exposes mechanisms of host cell escape. Nat Commun. 2015;6:6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Meara TR, et al. High-Throughput Screening Identifies Genes Required for Candida albicans Induction of Macrophage Pyroptosis. MBio. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odamaki T, Kato K, Sugahara Het al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds FC, Brown AJP, Gow NAR. Antifungal agents: mechanisms of action. Trends Microbiol. 2003;11:272–9. [DOI] [PubMed] [Google Scholar]
- Odds FC. Candida and candidosis: a review and bibliography. 2nd ed. London: Baillière Tindall, 1988. [Google Scholar]
- Odds FC. Molecular phylogenetics and epidemiology of Candida albicans. Future Microbiol. 2010;5:67–79. [DOI] [PubMed] [Google Scholar]
- Odds FC, et al. Molecular phylogenetics of Candida albicans. Eukaryotic Cell. 2007;6:1041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira VMC, Santos SSF, Silva CRGet al. Lactobacillus is able to alter the virulence and the sensitivity profile of Candida albicans. J Appl Microbiol. 2016;121:1737–44. [DOI] [PubMed] [Google Scholar]
- Oncel MY, et al. Comparison of Lactobacillus reuteri and nystatin prophylaxis on Candida colonization and infection in very low birth weight infants. J Matern Fetal Neonatal Med. 2015;28:1790–4. [DOI] [PubMed] [Google Scholar]
- Opulente DA, et al. Pathogenic budding yeasts isolated outside of clinical settings. FEMS Yeast Res. 2019;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega M, Rovira M, Almela Met al. Bacterial and fungal bloodstream isolates from 796 hematopoietic stem cell transplant recipients between 1991 and 2000. Ann Hematol. 2005;84:40–6. [DOI] [PubMed] [Google Scholar]
- Ostrowski PP, Grinstein S, Freeman SA. Diffusion barriers, mechanical forces, and the biophysics of phagocytosis. Dev Cell. 2016;38:135–46. [DOI] [PubMed] [Google Scholar]
- Otto GP, Sossdorf M, Claus RAet al. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit Care. 2011;15:R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott SJ, et al. Fungi and inflammatory bowel diseases: Alterations of composition and diversity. Scand J Gastroenterol. 2008;43:831–41. [DOI] [PubMed] [Google Scholar]
- Pammi M, Holland L, Butler Get al. Candida parapsilosis is a significant neonatal pathogen: a systematic review and meta-analysis. Pediatr Infect Dis J. 2013;32:e206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande K, Chen C, Noble SM. Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nat Genet. 2013;45:1088–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiyan P, Conti HR, Zheng Let al. CD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity. 2011;34:422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Han L, Zhang Yet al. Optimization of Caco-2 and HT29 co-culture in vitro cell models for permeability studies. Int J Food Sci Nutr. 2015;66:680–5. [DOI] [PubMed] [Google Scholar]
- Papon N, Bougnoux M-E, d'Enfert C. Tracing the origin of invasive fungal infections. Trends Microbiol. 2020;28:240–2. [DOI] [PubMed] [Google Scholar]
- Pappas PG, Lionakis MS, Arendrup MCet al. Invasive candidiasis. Nat Rev Dis Primers. 2018;4:18026. [DOI] [PubMed] [Google Scholar]
- Pareek S, et al. Comparison of Japanese and Indian intestinal microbiota shows diet-dependent interaction between bacteria and fungi. npj Biofilms and Microbiomes. 2019;5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CO, et al. Staged development of long-lived T-cell receptor αβ TH17 resident memory T-cell population to Candida albicans after skin infection. J Allergy Clin Immunol. 2018;142:647–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park G-S, Park MH, Shin Wet al. Emulating Host-Microbiome Ecosystem of Human Gastrointestinal Tract in Vitro. Stem Cell Rev and Rep. 2017;13:321–34. [DOI] [PubMed] [Google Scholar]
- Parm U, Metsvaht T, Sepp Eet al. Risk factors associated with gut and nasopharyngeal colonization by common Gram-negative species and yeasts in neonatal intensive care units patients. Early Hum Dev. 2011;87:391–9. [DOI] [PubMed] [Google Scholar]
- Pascal V, et al. A microbial signature for Crohn's disease. Gut. 2017;66:813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S, Majumdar B, Sarode SCet al. Oropharyngeal Candidosis in HIV-Infected Patients-An Update. Front Microbiol. 2018;9:980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S, Rao RS, Raj ATet al. Oral Candidal Carriage in Subjects with Pure Vegetarian and Mixed Dietary Habits. J Clin Diagn Res. 2017;11:ZC22–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S, Gibson G, Wynne Aet al. In vitro studies on colonization resistance of the human gut microbiota to Candida albicans and the effects of tetracycline and Lactobacillus plantarum LPK. Curr Issues Intest Microbiol. 2003;4:1–8. [PubMed] [Google Scholar]
- Pearce SC, Coia HG, Karl JPet al. Intestinal in vitro and ex vivo Models to Study Host-Microbiome Interactions and Acute Stressors. Front Physiol. 2018;9:1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltroche-Llacsahuanga H, Schnitzler N, Schmidt Set al. Phagocytosis, oxidative burst, and killing of Candida dubliniensis and Candida albicans by human neutrophils. FEMS Microbiol Lett. 2000;191:151–5. [DOI] [PubMed] [Google Scholar]
- Pendharkar S, Magopane T, Larsson P-Get al. Identification and characterisation of vaginal lactobacilli from South African women. BMC Infect Dis. 2013;13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlroth J, Choi B, Spellberg B. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med Mycol. 2007;45:321–46. [DOI] [PubMed] [Google Scholar]
- Peters BM, Jabra-Rizk MA, Scheper MAet al. Microbial interactions and differential protein expression in Staphylococcus aureus -Candida albicans dual-species biofilms. FEMS Immunol Med Microbiol. 2010;59:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BM, Noverr MC. Candida albicans-Staphylococcus aureus polymicrobial peritonitis modulates host innate immunity. Infect Immun. 2013;81:2178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36:1–53. [DOI] [PubMed] [Google Scholar]
- Pfleiderer A, Lagier JC, Armougom Fet al. Culturomics identified 11 new bacterial species from a single anorexia nervosa stool sample. Eur J Clin Microbiol Infect Dis. 2013;32:1471–81. [DOI] [PubMed] [Google Scholar]
- Pham VT, Chassard C, Rifa Eet al. Lactate metabolism is strongly modulated by fecal inoculum, ph, and retention time in polyferms continuous colonic fermentation models mimicking young infant proximal colon. mSystems. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan QT, Myers CL, Fu Yet al. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007;5:e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecka B, et al. Distinctive roles of age, sex, and genetics in shaping transcriptional variation of human immune responses to microbial challenges. Proc Natl Acad Sci USA. 2018;115:E488–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrella D, Lupo P, Rachini Aet al. A Candida albicans mannoprotein deprived of its mannan moiety is efficiently taken up and processed by human dendritic cells and induces T-cell activation without stimulating proinflammatory cytokine production. Infect Immun. 2008;76:4359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirofski L-A, Casadevall A. Antimicrobial Therapy in the Context of the Damage-Response Framework: the Prospect of Optimizing Therapy by Reducing Host Damage. Antimicrob Agents Chemother. 2020;64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitarch A, Jiménez A, Nombela Cet al. Decoding serological response to Candida cell wall immunome into novel diagnostic, prognostic, and therapeutic candidates for systemic candidiasis by proteomic and bioinformatic analyses. Mol Cell Proteomics. 2006;5:79–96. [DOI] [PubMed] [Google Scholar]
- Pittman K, Kubes P. Damage-associated molecular patterns control neutrophil recruitment. J Innate Immun. 2013;5:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantinga TS, Johnson MDet al. Toll-like receptor 1 polymorphisms increase susceptibility to candidemia. J Infect Dis. 2012;205:934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantinga TS, van Bergenhenegouwen J, Jacobs Cet al. Modulation of Toll-like receptor ligands and Candida albicans-induced cytokine responses by specific probiotics. Cytokine. 2012;59:159–65. [DOI] [PubMed] [Google Scholar]
- Plato A, Hardison SE, Brown GD. Pattern recognition receptors in antifungal immunity. Semin Immunopathol. 2015;37:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poissy J, et al. Risk factors for candidemia: a prospective matched case-control study. Crit Care. 2020;24:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltermann S, Kunert A, von der Heide Met al. Gpm1p is a factor H-, FHL-1-, and plasminogen-binding surface protein of Candida albicans. J Biol Chem. 2007;282:37537–44. [DOI] [PubMed] [Google Scholar]
- Pop M, et al. Individual-specific changes in the human gut microbiota after challenge with enterotoxigenic Escherichia coli and subsequent ciprofloxacin treatment. BMC Genomics. 2016;17:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp C, Ramírez-Zavala B, Schwanfelder Set al. Evolution of Fluconazole-Resistant Candida albicans Strains by Drug-Induced Mating Competence and Parasexual Recombination. MBio. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta A, Ramon AM, Fonzi WA. PRR1, a homolog of Aspergillus nidulans palF, controls pH-dependent gene expression and filamentation in Candida albicans. J Bacteriol. 1999;181:7516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrykus J, Stead D, Maccallum DMet al. Fungal iron availability during deep seated candidiasis is defined by a complex interplay involving systemic and local events. PLoS Pathog. 2013;9:e1003676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poupet C, Chassard C, Nivoliez Aet al. Caenorhabditis elegans, a Host to Investigate the Probiotic Properties of Beneficial Microorganisms. Frontiers in nutrition. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poupet C, Veisseire P, Bonnet Met al. Curative Treatment of Candidiasis by the Live Biotherapeutic Microorganism Lactobacillus rhamnosus Lcr35® in the Invertebrate Model Caenorhabditis elegans: First Mechanistic Insights. Microorganisms. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan A, et al. Elevated catalase expression in a fungal pathogen is a double-edged sword of iron. PLoS Pathog. 2017;13:e1006405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan A, et al. Hypoxia Promotes Immune Evasion by Triggering β-Glucan Masking on the Candida albicans Cell Surface via Mitochondrial and cAMP-Protein Kinase A Signaling. MBio. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan A, et al. Non-canonical signalling mediates changes in fungal cell wall PAMPs that drive immune evasion. Nat Commun. 2019;10:5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto D, Correia I, Pla Jet al. Adaptation of Candida albicans to commensalism in the gut. Future Microbiol. 2016;11:567–83. [DOI] [PubMed] [Google Scholar]
- Puel A. Human inborn errors of immunity underlying superficial or invasive candidiasis. Hum Genet. 2020;139:1011–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol C, Reynes J, Renaud Fet al. The yeast Candida albicans has a clonal mode of reproduction in a population of infected human immunodeficiency virus-positive patients. Proc Natl Acad Sci USA. 1993;90:9456–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez JC, Kumamoto CA, Johnson AD. Candida albicans commensalism and pathogenicity are intertwined traits directed by a tightly knit transcriptional regulatory circuit. PLoS Biol. 2013;11:e1001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintin J. Fungal mediated innate immune memory, what have we learned?. Semin Cell Dev Biol. 2019;89:71–77. [DOI] [PubMed] [Google Scholar]
- Quintin J, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. 2012;12:223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada B, Leto TL. Pyocyanin effects on respiratory epithelium: relevance in Pseudomonas aeruginosa airway infections. Trends Microbiol. 2013;21:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi C, Rahimi B, Padova Det al. Oral mucosa-on-a-chip to assess layer-specific responses to bacteria and dental materials. Biomicrofluidics. 2018;12:054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman D, Mistry M, Thavaraj Set al. Murine model of concurrent oral and vaginal Candida albicans colonization to study epithelial host-pathogen interactions. Microbes Infect. 2007;9:615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi S, et al. Longitudinal survey of fungi in the human gut: ITS profiling, phenotyping, and colonization. Front Microbiol. 2019;10:1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran R, et al. Biofilm formation is a risk factor for mortality in patients with Candida albicans bloodstream infection-Scotland, 2012–2013. Clin Microbiol Infect. 2016;22:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage G, Saville SP, Thomas DPet al. Candida biofilms: an update. Eukaryotic Cell. 2005;4:633–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ortiz ZG, Means TK. The role of dendritic cells in the innate recognition of pathogenic fungi (A. fumigatus, C. neoformans and C. albicans). Virulence. 2012;3:635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsdale M, Selway L, Stead Det al. MNL1 regulates weak acid-induced stress responses of the fungal pathogen Candida albicans. Mol Biol Cell. 2008;19:4393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappocciolo G, Jenkins FJ, Hensler HRet al. DC-SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages. J Immunol. 2006;176:1741–9. [DOI] [PubMed] [Google Scholar]
- Rastogi R, Su J, Mahalingam Aet al. Engineering and characterization of simplified vaginal and seminal fluid simulants. Contraception. 2016;93:337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel J, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. 2011;108 Suppl 1:4680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding SW, et al. Epidemiology of oropharyngeal Candida colonization and infection in patients receiving radiation for head and neck cancer. J Clin Microbiol. 1999;37:3896–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyman M, et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat Commun. 2019;10:4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard ML, Plaine A. Comprehensive analysis of glycosylphosphatidylinositol-anchored proteins in Candida albicans. Eukaryotic Cell. 2007;6:119–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JP, Ho J, Naglik JR. Candida-Epithelial Interactions. J Fungi (Basel). 2018;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JP, Moyes DL, Ho Jet al. Candida innate immunity at the mucosa. Semin Cell Dev Biol. 2019;89:58–70. [DOI] [PubMed] [Google Scholar]
- Richardson JP, Willems HMEet al. Candidalysin drives epithelial signaling, neutrophil recruitment, and immunopathology at the vaginal mucosa. Infect Immun. 2018;86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesenfeld CS, Schloss PD, Handelsman J. Metagenomics: genomic analysis of microbial communities. Annu Rev Genet. 2004;38:525–52. [DOI] [PubMed] [Google Scholar]
- Rinke C, et al. Insights into the phylogeny and coding potential of microbial dark matter. Nature. 2013;499:431–7. [DOI] [PubMed] [Google Scholar]
- Rivière A, Selak M, Geirnaert Aet al. Complementary Mechanisms for Degradation of Inulin-Type Fructans and Arabinoxylan Oligosaccharides among Bifidobacterial Strains Suggest Bacterial Cooperation. Appl Environ Microbiol. 2018;84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo A, Losacco A, Carratelli CR. Lactobacillus crispatus modulates epithelial cell defense against Candida albicans through Toll-like receptors 2 and 4, interleukin 8 and human β-defensins 2 and 3. Immunol Lett. 2013;156:102–9. [DOI] [PubMed] [Google Scholar]
- Roberts CL, Rickard K, Kotsiou Get al. Treatment of asymptomatic vaginal candidiasis in pregnancy to prevent preterm birth: an open-label pilot randomized controlled trial. BMC Pregnancy Childbirth. 2011;11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJ, et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med. 2009;206:2037–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha FAC, Alves AMCV, Rocha MFGet al. Tumor necrosis factor prevents Candida albicans biofilm formation. Sci Rep. 2017;7:1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodaki A, Bohovych IM, Enjalbert Bet al. Glucose promotes stress resistance in the fungal pathogen Candida albicans. Mol Biol Cell. 2009;20:4845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues CF, Rodrigues ME, Henriques M. Candida sp. Infections in Patients with Diabetes Mellitus. J Clin Med. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder A, Kirschning CJ, Rupec RAet al. Toll-like receptors and innate antifungal responses. Trends Microbiol. 2004;12:44–49. [DOI] [PubMed] [Google Scholar]
- Romani L, Mencacci A, Tonnetti Let al. IL-12 is both required and prognostic in vivo for T helper type 1 differentiation in murine candidiasis. J Immunol. 1994;153:5167–75. [PubMed] [Google Scholar]
- Romano M, Fanelli G, Albany CJet al. Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front Immunol. 2019;10:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo MG, Romeo DM, Trovato Let al. Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: incidence of late-onset sepsis and neurological outcome. J Perinatol. 2011;31:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo O, De Leo F, Criseo G. Adherence ability of Candida africana: a comparative study with Candida albicans and Candida dubliniensis. Mycoses. 2011;54:e57–61. [DOI] [PubMed] [Google Scholar]
- Romero R, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román E, Prieto D, Martin Ret al. Role of catalase overproduction in drug resistance and virulence in Candida albicans. Future Microbiol. 2016;11:1279–97. [DOI] [PubMed] [Google Scholar]
- Rondanelli M, Faliva MA, Perna Set al. Using probiotics in clinical practice: Where are we now? A review of existing meta-analyses. Gut Microbes. 2017;8:521–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropars J, et al. Gene flow contributes to diversification of the major fungal pathogen Candida albicans. Nat Commun. 2018;9:2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front Physiol. 2018;9:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati D, Bruno M, Jaeger Met al. An exaggerated monocyte-derived cytokine response to Candida hyphae in patients with recurrent vulvovaginal candidiasis. J Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati D, Bruno M, Jaeger Met al. Recurrent vulvovaginal candidiasis: an immunological perspective. Microorganisms. 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselletti E, Perito S, Sabbatini Set al. Vaginal Epithelial Cells Discriminate Between Yeast and Hyphae of Candida albicans in Women Who Are Colonized or Have Vaginal Candidiasis. J Infect Dis. 2019;220:1645–54. [DOI] [PubMed] [Google Scholar]
- Rothschild D, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–5. [DOI] [PubMed] [Google Scholar]
- Rougé C, et al. Oral supplementation with probiotics in very-low-birth-weight preterm infants: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2009;89:1828–35. [DOI] [PubMed] [Google Scholar]
- Rubaltelli FF, Biadaioli R, Pecile Pet al. Intestinal flora in breast- and bottle-fed infants. J Perinat Med. 1998;26:186–91. [DOI] [PubMed] [Google Scholar]
- Rudkin FM, Bain JM, Walls Cet al. Altered dynamics of Candida albicans phagocytosis by macrophages and PMNs when both phagocyte subsets are present. MBio. 2013;4:e00810–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudkin FM, et al. Single human B cell-derived monoclonal anti-Candida antibodies enhance phagocytosis and protect against disseminated candidiasis. Nat Commun. 2018;9:5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R, Superti F, Karadja Eet al. Randomised clinical trial in women with Recurrent Vulvovaginal Candidiasis: Efficacy of probiotics and lactoferrin as maintenance treatment. Mycoses. 2019;62:328–35. [DOI] [PubMed] [Google Scholar]
- Saeed S, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;345:1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo S, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–91. [DOI] [PubMed] [Google Scholar]
- Saiman L, et al. Risk factors for Candida species colonization of neonatal intensive care unit patients. Pediatr Infect Dis J. 2001;20:1119–24. [DOI] [PubMed] [Google Scholar]
- Salonen A, et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014;8:2218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranayake LP. Oral mycoses in HIV infection. Oral Surg Oral Med Oral Pathol. 1992;73:171–80. [DOI] [PubMed] [Google Scholar]
- Sandai D, et al. The evolutionary rewiring of ubiquitination targets has reprogrammed the regulation of carbon assimilation in the pathogenic yeast Candida albicans. MBio. 2012;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San José C, Monge RA, Pérez-Díaz Ret al. The mitogen-activated protein kinase homolog HOG1 gene controls glycerol accumulation in the pathogenic fungus Candida albicans. J Bacteriol. 1996;178:5850–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–5. [DOI] [PubMed] [Google Scholar]
- Saville SP, Lazzell AL, Monteagudo Cet al. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryotic Cell. 2003;2:1053–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saz-Leal P, Del Fresno C, Brandi Pet al. Targeting SHIP-1 in Myeloid Cells Enhances Trained Immunity and Boosts Response to Infection. Cell Rep. 2018;25:1118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M, Korting HC, Borelli Cet al. Candida albicans-secreted aspartic proteinases modify the epithelial cytokine response in an in vitro model of vaginal candidiasis. Infect Immun. 2005;73:2758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M, Zakikhany K, Naglik JRet al. Models of oral and vaginal candidiasis based on in vitro reconstituted human epithelia. Nat Protoc. 2006;1:2767–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber J, et al. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut. 2003;52:735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schei K, Avershina E, Øien Tet al. Early gut mycobiota and mother-offspring transfer. Microbiome. 2017;5:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnorr SL, et al. Gut microbiome of the Hadza hunter-gatherers. Nat Commun. 2014;5:3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield DA, Westwater C, Warner Tet al. Differential Candida albicans lipase gene expression during alimentary tract colonization and infection. FEMS Microbiol Lett. 2005;244:359–65. [DOI] [PubMed] [Google Scholar]
- Schulze J, Sonnenborn U. Yeasts in the gut: from commensals to infectious agents. Dtsch Arztebl Int. 2009;106:837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönherr FA, et al. The intraspecies diversity of C. albicans triggers qualitatively and temporally distinct host responses that determine the balance between commensalism and pathogenicity. Mucosal Immunol. 2017;10:1335–50. [DOI] [PubMed] [Google Scholar]
- Selders GS, Fetz AE, Radic MZet al. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen Biomater. 2017;4:55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmecki A, Forche A, Berman J. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science. 2006;313:367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmecki A, Forche A, Berman J. Genomic plasticity of the human fungal pathogen Candida albicans. Eukaryotic Cell. 2010;9:991–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmecki A, Gerami-Nejad M, Paulson Cet al. An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol Microbiol. 2008;68:624–41. [DOI] [PubMed] [Google Scholar]
- Seng P, Drancourt M, Gouriet Fet al. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis. 2009;49:543–51. [DOI] [PubMed] [Google Scholar]
- Shaaban OM, Abbas AM, Moharram AMet al. Does vaginal douching affect the type of candidal vulvovaginal infection? Med Mycol. 2015;53:817–27. [DOI] [PubMed] [Google Scholar]
- Shao T-Y, et al. Commensal Candida albicans Positively Calibrates Systemic Th17 Immunological Responses. Cell Host Microbe. 2019;25:404–17..e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro RS, Uppuluri P, Zaas AKet al. Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr Biol. 2009;19:621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd MG. Cell envelope of Candida albicans. Crit Rev Microbiol. 1987;15:7–25. [DOI] [PubMed] [Google Scholar]
- Sherrington SL, Sorsby E, Mahtey Net al. Adaptation of Candida albicans to environmental pH induces cell wall remodelling and enhances innate immune recognition. PLoS Pathog. 2017;13:e1006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry L, Kean R, McKloud Eet al. Biofilms Formed by Isolates from Recurrent Vulvovaginal Candidiasis Patients Are Heterogeneous and Insensitive to Fluconazole. Antimicrob Agents Chemother. 2017;61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, et al. Genetic diversity among Korean Candida albicans bloodstream isolates: assessment by multilocus sequence typing and restriction endonuclease analysis of genomic DNA by use of BssHII. J Clin Microbiol. 2011;49:2572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtliff ME, Peters BM, Jabra-Rizk MA. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol Lett. 2009;299:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakoti R, Tuddenham S, Caulfield LEet al. Dietary macronutrient intake and molecular-bacterial vaginosis: Role of fiber. Clin Nutr. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Chen L, Tong Jet al. Preliminary characterization of vaginal microbiota in healthy Chinese women using cultivation-independent methods. J Obstet Gynaecol Res. 2009;35:525–32. [DOI] [PubMed] [Google Scholar]
- Siavoshi F, Taghikhani A, Malekzadeh Ret al. The role of mother's oral and vaginal yeasts in transmission of Helicobacter pylori to neonates. Arch Iran Med. 2013;16:288–94. [PubMed] [Google Scholar]
- Silk H. Diseases of the mouth. Prim Care. 2014;41:75–90. [DOI] [PubMed] [Google Scholar]
- Silva S, Negri M, Henriques Met al. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev. 2012;36:288–305. [DOI] [PubMed] [Google Scholar]
- Silverman RJ, Nobbs AH, Vickerman MMet al. Interaction of Candida albicans cell wall Als3 protein with Streptococcus gordonii SspB adhesin promotes development of mixed-species communities. Infect Immun. 2010;78:4644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282:20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões CD, Maukonen J, Kaprio Jet al. Habitual dietary intake is associated with stool microbiota composition in monozygotic twins. J Nutr. 2013;143:417–23. [DOI] [PubMed] [Google Scholar]
- Singh P, Chauhan N, Ghosh Aet al. SKN7 of Candida albicans: mutant construction and phenotype analysis. Infect Immun. 2004;72:2390–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitterlé E, Maufrais C, Sertour Net al. Within-Host Genomic Diversity of Candida albicans in Healthy Carriers. Sci Rep. 2019;9:2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitterlé E, et al. Large-scale genome mining allows identification of neutral polymorphisms and novel resistance mutations in genes involved in Candida albicans resistance to azoles and echinocandins. J Antimicrob Chemother. 2020;75:835–48. [DOI] [PubMed] [Google Scholar]
- Sivieri K, Morales MLV, Saad SMIet al. Prebiotic effect of fructooligosaccharide in the simulator of the human intestinal microbial ecosystem (SHIME® model). J Med Food. 2014;17:894–901. [DOI] [PubMed] [Google Scholar]
- Smeekens SP, et al. Functional genomics identifies type I interferon pathway as central for host defense against Candida albicans. Nat Commun. 2013;4:1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov KS, Maier TV, Walker Aet al. Challenges of metabolomics in human gut microbiota research. Int J Med Microbiol. 2016;306:266–79. [DOI] [PubMed] [Google Scholar]
- Smith DA, Nicholls S, Morgan BAet al. A conserved stress-activated protein kinase regulates a core stress response in the human pathogen Candida albicans. Mol Biol Cell. 2004;15:4179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961–71. [DOI] [PubMed] [Google Scholar]
- Sokol H, et al. Fungal microbiota dysbiosis in IBD. Gut. 2017;66:1039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H, et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15:1183–9. [DOI] [PubMed] [Google Scholar]
- Solis NV, Filler SG. Mouse model of oropharyngeal candidiasis. Nat Protoc. 2012;7:637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis NV, Swidergall M, Bruno VMet al. The Aryl Hydrocarbon Receptor Governs Epithelial Cell Invasion during Oropharyngeal Candidiasis. MBio. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosinska GJ, de Groot PWJ, Teixeira de Mattos MJet al. Hypoxic conditions and iron restriction affect the cell-wall proteome of Candida albicans grown under vagina-simulative conditions. Microbiology (Reading, Engl). 2008;154:510–20. [DOI] [PubMed] [Google Scholar]
- Sparber F, Dolowschiak T, Mertens Set al. Langerin+ DCs regulate innate IL-17 production in the oral mucosa during Candida albicans-mediated infection. PLoS Pathog. 2018;14:e1007069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparber F, LeibundGut-Landmann S. Interleukin-17 in Antifungal Immunity. Pathogens. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear GT, et al. Human α-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. J Infect Dis. 2014;210:1019–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinillo A, Bernuzzi AM, Cevini Cet al. The relationship of bacterial vaginosis, Candida and Trichomonas infection to symptomatic vaginitis in postmenopausal women attending a vaginitis clinic. Maturitas. 1997;27:253–60. [DOI] [PubMed] [Google Scholar]
- Sprenger M, Kasper L, Hensel Met al. Metabolic adaptation of intracellular bacteria and fungi to macrophages. Int J Med Microbiol. 2018;308:215–27. [DOI] [PubMed] [Google Scholar]
- Staab JF, Bradway SD, Fidel PLet al. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science. 1999;283:1535–8. [DOI] [PubMed] [Google Scholar]
- Staab JF, Ferrer CA, Sundstrom P. Developmental expression of a tandemly repeated, proline-and glutamine-rich amino acid motif on hyphal surfaces on Candida albicans. J Biol Chem. 1996;271:6298–305. [DOI] [PubMed] [Google Scholar]
- Staib P, Morschhäuser J. Chlamydospore formation in Candida albicans and Candida dubliniensis–an enigmatic developmental programme. Mycoses. 2007;50:1–12. [DOI] [PubMed] [Google Scholar]
- Stecksén-Blicks C, Granström E, Silfverdal SAet al. Prevalence of oral Candida in the first year of life. Mycoses. 2015;58:550–6. [DOI] [PubMed] [Google Scholar]
- Steele C, Ratterree M, Fidel PL. Differential susceptibility of two species of macaques to experimental vaginal candidiasis. J Infect Dis. 1999;180:802–10. [DOI] [PubMed] [Google Scholar]
- Steenbergen JN, Shuman HA, Casadevall A. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc Natl Acad Sci USA. 2001;98:15245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokholm J, et al. Antibiotic use during pregnancy alters the commensal vaginal microbiota. Clin Microbiol Infect. 2014;20:629–35. [DOI] [PubMed] [Google Scholar]
- Stone NR, et al. Dynamic ploidy changes drive fluconazole resistance in human cryptococcal meningitis. J Clin Invest. 2019;129:999–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strati F, et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome. 2017;5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strus M, Kucharska A, Kukla Get al. The in vitro activity of vaginal Lactobacillus with probiotic properties against Candida. Infect Dis Obstet Gynecol. 2005;13:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbery P, Gow N, Berman J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004;12:317–24. [DOI] [PubMed] [Google Scholar]
- Sudbery PE. The germ tubes of Candida albicans hyphae and pseudohyphae show different patterns of septin ring localization. Mol Microbiol. 2001;41:19–31. [DOI] [PubMed] [Google Scholar]
- Sullivan DJ, Westerneng TJ, Haynes KAet al. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology (Reading, Engl). 1995;141:1507–21. [DOI] [PubMed] [Google Scholar]
- Sun JN, et al. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog. 2010;6:e1001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Cao C, Jia Wet al. pH Regulates White-Opaque Switching and Sexual Mating in Candida albicans. Eukaryotic Cell. 2015;14:1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidergall M, Ernst JF. Interplay between Candida albicans and the antimicrobial peptide armory. Eukaryotic Cell. 2014;13:950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidergall M, Khalaji Met al. Candidalysin Is Required for Neutrophil Recruitment and Virulence During Systemic Candida albicans Infection. J Infect Dis. 2019;220:1477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidergall M, Solis NV, Lionakis MSet al. EphA2 is an epithelial cell pattern recognition receptor for fungal β-glucans. Nat Microbiol. 2018;3:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidergall M, Solis NV, Wang Zet al. EphA2 Is a Neutrophil Receptor for Candida albicans that Stimulates Antifungal Activity during Oropharyngeal Infection. Cell Rep. 2019;28:423–433.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidergall M. Candida albicans at Host Barrier Sites: Pattern Recognition Receptors and Beyond. Pathogens. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoboda RK, Bertram G, Hollander Het al. Glycolytic enzymes of Candida albicans are nonubiquitous immunogens during candidiasis. Infect Immun. 1993;61:4263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo EK, MacCallum DM. The contribution of mouse models to our understanding of systemic candidiasis. FEMS Microbiol Lett. 2011;320:1–8. [DOI] [PubMed] [Google Scholar]
- Sztajer H, Szafranski SP, Tomasch Jet al. Cross-feeding and interkingdom communication in dual-species biofilms of Streptococcus mutans and Candida albicans. ISME J. 2014;8:2256–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabaei F, Moharamzadeh K, Tayebi L. Three-Dimensional In Vitro Oral Mucosa Models of Fungal and Bacterial Infections. Tissue Eng Part B Rev. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taff HT, Mitchell KF, Edward JAet al. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013;8:1325–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamarelle J, et al. Nonoptimal Vaginal Microbiota After Azithromycin Treatment for Chlamydia trachomatis Infection. J Infect Dis. 2020;221:627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L, et al. Discovery of a “white-gray-opaque” tristable phenotypic switching system in candida albicans: roles of non-genetic diversity in host adaptation. PLoS Biol. 2014;12:e1001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarry W, Fisher M, Shen Set al. Candida albicans: the estrogen target for vaginal colonization. J Surg Res. 2005;129:278–82. [DOI] [PubMed] [Google Scholar]
- Tavanti A, Campa D, Bertozzi Aet al. Candida albicans isolates with different genomic backgrounds display a differential response to macrophage infection. Microbes Infect. 2006;8:791–800. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Brown GD, Reid DMet al. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol. 2002;169:3876–82. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Reid DM, Heinsbroek SEMet al. Dectin-2 is predominantly myeloid restricted and exhibits unique activation-dependent expression on maturing inflammatory monocytes elicited in vivo. Eur J Immunol. 2005;35:2163–74. [DOI] [PubMed] [Google Scholar]
- Tedersoo L, Lindahl B. Fungal identification biases in microbiome projects. Environ Microbiol Rep. 2016;8:774–9. [DOI] [PubMed] [Google Scholar]
- Tedersoo L, et al. Shotgun metagenomes and multiple primer pair-barcode combinations of amplicons reveal biases in metabarcoding analyses of fungi. MC. 2015;10:1–43. [Google Scholar]
- Teoh F, Pavelka N. How chemotherapy increases the risk of systemic candidiasis in cancer patients: current paradigm and future directions. Pathogens. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thewes S, Kretschmar M, Park Het al. In vivo and ex vivo comparative transcriptional profiling of invasive and non-invasive Candida albicans isolates identifies genes associated with tissue invasion. Mol Microbiol. 2007;63:1606–28. [DOI] [PubMed] [Google Scholar]
- Thewes S, Moran GP, Magee BBet al. Phenotypic screening, transcriptional profiling, and comparative genomic analysis of an invasive and non-invasive strain of Candida albicans. BMC Microbiol. 2008;8:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietz HJ, Hopp M, Schmalreck Aet al. Candida africana sp. nov., a new human pathogen or a variant of Candida albicans? Mycoses. 2001;44:437–45. [DOI] [PubMed] [Google Scholar]
- Tillmann AT, et al. Contribution of Fdh3 and Glr1 to Glutathione Redox State, Stress Adaptation and Virulence in Candida albicans. PLoS One. 2015;10:e0126940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisoncik JR, Korth MJ, Simmons CPet al. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin DM, May RC, Wheeler RT. Zebrafish: a see-through host and a fluorescent toolbox to probe host-pathogen interaction. PLoS Pathog. 2012;8:e1002349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd OA, Fidel PL, Harro JMet al. Candida albicans Augments Staphylococcus aureus Virulence by Engaging the Staphylococcal agr Quorum Sensing System. MBio. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd OA, Noverr MC, Peters BM. Candida albicans Impacts Staphylococcus aureus Alpha-Toxin Production via Extracellular Alkalinization. mSphere. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortelli BA, Lewis WG, Allsworth JEet al. Associations between the vaginal microbiome and Candida colonization in women of reproductive age. Am J Obstet Gynecol. 2020;222:471.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontano M, et al. Nutritional preferences of human gut bacteria reveal their metabolic idiosyncrasies. Nat Microbiol. 2018;3:514–22. [DOI] [PubMed] [Google Scholar]
- Trautwein-Weidner K, Gladiator A, Kirchner FRet al. Antigen-Specific Th17 Cells Are Primed by Distinct and Complementary Dendritic Cell Subsets in Oropharyngeal Candidiasis. PLoS Pathog. 2015;11:e1005164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautwein-Weidner K, Gladiator A, Nur Set al. IL-17-mediated antifungal defense in the oral mucosa is independent of neutrophils. Mucosal Immunol. 2015;8:221–31. [DOI] [PubMed] [Google Scholar]
- Trietsch SJ, et al. Membrane-free culture and real-time barrier integrity assessment of perfused intestinal epithelium tubes. Nat Commun. 2017;8:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi G, Wiltshire C, Macaskill Set al. Gcn4 co-ordinates morphogenetic and metabolic responses to amino acid starvation in Candida albicans. EMBO J. 2002;21:5448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso GHW, et al. Experimental evolution of a fungal pathogen into a gut symbiont. Science. 2018;362:589–95. [DOI] [PubMed] [Google Scholar]
- Tucey TM, Verma J, Olivier FABet al. Metabolic competition between host and pathogen dictates inflammasome responses to fungal infection. PLoS Pathog. 2020;16:e1008695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucey TM, et al. Glucose Homeostasis Is Important for Immune Cell Viability during Candida Challenge and Host Survival of Systemic Fungal Infection. Cell Metab. 2018;27:988–1006.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuddenham S, et al. Associations between dietary micronutrient intake and molecular-Bacterial Vaginosis. Reprod Health. 2019;16:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda C, Pamer EG. Antibiotics, microbiota, and immune defense. Trends Immunol. 2012;33:459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda C, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120:4332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill DM, Iliev ID. The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol. 2014;14:405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill DM. Escape Mechanisms from the Immune Response. In: Brown GD, Netea MG (eds.). Immunology of fungal infections. Dordrecht: Springer Netherlands, 2007, 429–42. [Google Scholar]
- Uppuluri P, Acosta Zaldívar M, Anderson MZet al. Candida albicans Dispersed Cells Are Developmentally Distinct from Biofilm and Planktonic Cells. MBio. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban CF, Ermert D, Schmid Met al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwamahoro N, et al. The pathogen Candida albicans hijacks pyroptosis for escape from macrophages. MBio. 2014;5:e00003–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Belkum A, Broadwell D, Lovern Det al. Proteomics and metabolomics for analysis of the dynamics of microbiota. Expert Rev Proteomics. 2018;15:101–4. [DOI] [PubMed] [Google Scholar]
- Van den Abbeele P, Belzer C, Goossens Met al. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013;7:949–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Abbeele P, Gérard Pet al. Arabinoxylans and inulin differentially modulate the mucosal and luminal gut microbiota and mucin-degradation in humanized rats. Environ Microbiol. 2011;13:2667–80. [DOI] [PubMed] [Google Scholar]
- Van den Abbeele P, Marzorati M, Derde Met al. Arabinoxylans, inulin and Lactobacillus reuteri 1063 repress the adherent-invasive Escherichia coli from mucus in a mucosa-comprising gut model. npj Biofilms and Microbiomes. 2016;2:16016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Abbeele P, Van de Wiele T, Verstraete Wet al. The host selects mucosal and luminal associations of coevolved gut microorganisms: a novel concept. FEMS Microbiol Rev. 2011;35:681–704. [DOI] [PubMed] [Google Scholar]
- Van den Abbeele P, et al. Incorporating a mucosal environment in a dynamic gut model results in a more representative colonization by lactobacilli. Microb Biotechnol. 2012;5:106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Abbeele P, et al. Microbial community development in a dynamic gut model is reproducible, colon region specific, and selective for Bacteroidetes and Clostridium cluster IX. Appl Environ Microbiol. 2010;76:5237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeplassche E, Tavernier S, Coenye Tet al. Influence of the lung microbiome on antibiotic susceptibility of cystic fibrosis pathogens. Eur Respir Rev. 2019;28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Graaf CAA, Netea MG, Franke Bet al. Nucleotide oligomerization domain 2 (Nod2) is not involved in the pattern recognition of Candida albicans. Clin Vaccine Immunol. 2006;13:423–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Graaf CAA, Netea MG, Morré SAet al. Toll-like receptor 4 Asp299Gly/Thr399Ile polymorphisms are a risk factor for Candida bloodstream infection. Eur Cytokine Netw. 2006;17:29–34. [PubMed] [Google Scholar]
- van de Veerdonk FL, Gresnigt MS, Oosting Met al. Protective host defense against disseminated candidiasis is impaired in mice expressing human interleukin-37. Front Microbiol. 2014;5:762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk FL, Joosten LAB, Shaw PJet al. The inflammasome drives protective Th1 and Th17 cellular responses in disseminated candidiasis. Eur J Immunol. 2011;41:2260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk FL, Plantinga TSet al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. 2011;365:54–61. [DOI] [PubMed] [Google Scholar]
- van de Veerdonk FL, et al. IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist. Proc Natl Acad Sci USA. 2012;109:3001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk FL, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5:329–40. [DOI] [PubMed] [Google Scholar]
- Van de Wiele T, Van den Abbeele P, Ossieur Wet al. The Simulator of the Human Intestinal Microbial Ecosystem (SHIME®). In: Verhoeckx K, Cotter P, López-Expósito Iet al. (eds.). The Impact of Food Bioactives on Health: in vitro and ex vivo models [Internet]. Cham: Springer International Publishing, 2015, 305–17. [Google Scholar]
- Vangay P, et al. US immigration westernizes the human gut microbiome. Cell. 2018;175: 962–72..e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Herreweghen F, et al. In vitro colonisation of the distal colon by Akkermansia muciniphila is largely mucin and pH dependent. Benef Microbes. 2017;8:81–96. [DOI] [PubMed] [Google Scholar]
- van Veen SQ, Claas ECJ, Kuijper EJ. High-throughput identification of bacteria and yeast by matrix-assisted laser desorption ionization-time of flight mass spectrometry in conventional medical microbiology laboratories. J Clin Microbiol. 2010;48:900–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Waeyenberghe L, Baré J, Pasmans Fet al. Interaction of Aspergillus fumigatus conidia with Acanthamoeba castellanii parallels macrophage-fungus interactions. Environ Microbiol Rep. 2013;5:819–24. [DOI] [PubMed] [Google Scholar]
- Vautier S, et al. Candida albicans colonization and dissemination from the murine gastrointestinal tract: the influence of morphology and Th17 immunity. Cell Microbiol. 2015;17:445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchiarelli A, Cenci E, Puliti Met al. Protective immunity induced by low-virulence Candida albicans: cytokine production in the development of the anti-infectious state. Cell Immunol. 1989;124:334–44. [DOI] [PubMed] [Google Scholar]
- Venema K, van den Abbeele P. Experimental models of the gut microbiome. Best Pract Res Clin Gastroenterol. 2013;27:115–26. [DOI] [PubMed] [Google Scholar]
- Verma A, Wüthrich M, Deepe Get al. Adaptive immunity to fungi. Cold Spring Harb Perspect Med. 2014;5:a019612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma AH, et al. IL-36 and IL-1/IL-17 Drive Immunity to Oral Candidiasis via Parallel Mechanisms. J Immunol. 2018;201:627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma Akash, Gaffen SL, Swidergall M. Innate immunity to mucosal candida infections. J Fungi (Basel). 2017;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma Akash H, et al. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor candidalysin. Sci Immunol. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vich Vila A, et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat Commun. 2020;11:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan D, Radford KJ, Beckhouse AGet al. Mincle polarizes human monocyte and neutrophil responses to Candida albicans. Immunol Cell Biol. 2012;90:889–95. [DOI] [PubMed] [Google Scholar]
- Vila T, Sultan AS, Montelongo-Jauregui Det al. Oral candidiasis: A disease of opportunity. J Fungi (Basel). 2020;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar CC, Kashleva H, Mitchell APet al. Invasive phenotype of Candida albicans affects the host proinflammatory response to infection. Infect Immun. 2005;73:4588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa S, Hamideh M, Weinstock Aet al. Transcriptional control of hyphal morphogenesis in Candida albicans. FEMS Yeast Res. 2020;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villmones HC, Haug ES, Ulvestad Eet al. Species level description of the human ileal bacterial microbiota. Sci Rep. 2018;8:4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladareanu R, Mihu D, Mitran Met al. New evidence on oral L. plantarum P17630 product in women with history of recurrent vulvovaginal candidiasis (RVVC): a randomized double-blind placebo-controlled study. Eur Rev Med Pharmacol Sci. 2018;22:262–7. [DOI] [PubMed] [Google Scholar]
- Vylkova S, Carman AJ, Danhof HAet al. The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. MBio. 2011;2:e00055–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vylkova S, Lorenz MC. Modulation of phagosomal pH by Candida albicans promotes hyphal morphogenesis and requires Stp2p, a regulator of amino acid transport. PLoS Pathog. 2014;10:e1003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vylkova S, Lorenz MC. Phagosomal Neutralization by the Fungal Pathogen Candida albicans Induces Macrophage Pyroptosis. Infect Immun. 2017;85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vylkova S. Environmental pH modulation by pathogenic fungi as a strategy to conquer the host. PLoS Pathog. 2017;13:e1006149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Torres A, Balish E. Macrophages in resistance to candidiasis. Microbiol Mol Biol Rev. 1997;61:170–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vílchez R, Lemme A, Ballhausen Bet al. Streptococcus mutans inhibits Candida albicans hyphal formation by the fatty acid signaling molecule trans-2-decenoic acid (SDSF). Chembiochem. 2010;11:1552–62. [DOI] [PubMed] [Google Scholar]
- Wagener J, et al. Fungal chitin dampens inflammation through IL-10 induction mediated by NOD2 and TLR9 activation. PLoS Pathog. 2014;10:e1004050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner RD, Johnson SJ. Probiotic lactobacillus and estrogen effects on vaginal epithelial gene expression responses to Candida albicans. J Biomed Sci. 2012;19:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AW, Duncan SH, Louis Pet al. Phylogeny, culturing, and metagenomics of the human gut microbiota. Trends Microbiol. 2014;22:267–74. [DOI] [PubMed] [Google Scholar]
- Walker AW, Duncan SH, McWilliam Leitch ECet al. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl Environ Microbiol. 2005;71:3692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AW, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walpole GFW, Grinstein S, Westman J. The role of lipids in host-pathogen interactions. IUBMB Life. 2018;70:384–92. [DOI] [PubMed] [Google Scholar]
- Wang H, Xu J, Guo Het al. Patterns of human oral yeast species distribution on Hainan Island in China. Mycopathologia. 2013;176:359–68. [DOI] [PubMed] [Google Scholar]
- Wang J. Neutrophils in tissue injury and repair. Cell Tissue Res. 2018;371:531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, et al. Diagnostic value of Candida mannan antigen and anti-mannan IgG and IgM antibodies for Candida infection. Mycoses. 2020;63:181–8. [DOI] [PubMed] [Google Scholar]
- Ward TL, Dominguez-Bello MG, Heisel Tet al. Development of the Human Mycobiome over the First Month of Life and across Body Sites. mSystems. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warris A, Bercusson A, Armstrong-James D. Aspergillus colonization and antifungal immunity in cystic fibrosis patients. Med Mycol. 2019;57:S118–26. [DOI] [PubMed] [Google Scholar]
- Wartenberg A, et al. Microevolution of Candida albicans in macrophages restores filamentation in a nonfilamentous mutant. PLoS Genet. 2014;10:e1004824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Alexander M, Misharin AVet al. The role of macrophages in the resolution of inflammation. J Clin Invest. 2019;129:2619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindl G, Naglik JR, Kaesler Set al. Human epithelial cells establish direct antifungal defense through TLR4-mediated signaling. J Clin Invest. 2007;117:3664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein MP. Current blood culture methods and systems: clinical concepts, technology, and interpretation of results. Clin Infect Dis. 1996;23:40–46. [DOI] [PubMed] [Google Scholar]
- Wei S, Mortensen MS, Stokholm Jet al. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: A double-blind, randomized, placebo-controlled trial. EBioMedicine. 2018;38:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y-P, Feng J, Luo Z-C. Isolation and genotyping of vaginal non-albicans Candida spp. in women from two different ethnic groups in Lanzhou, China. Int J Gynaecol Obstet. 2010;110:227–30. [DOI] [PubMed] [Google Scholar]
- Wellington M, Koselny K, Sutterwala FSet al. Candida albicans triggers NLRP3-mediated pyroptosis in macrophages. Eukaryotic Cell. 2014;13:329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells CA, et al. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol. 2008;180:7404–13. [DOI] [PubMed] [Google Scholar]
- Westman J, Hube B, Fairn GD. Integrity under stress: Host membrane remodelling and damage by fungal pathogens. Cell Microbiol. 2019;21:e13016. [DOI] [PubMed] [Google Scholar]
- Westman J, Moran GP, Mogavero Set al. Candida albicans Hyphal Expansion Causes Phagosomal Membrane Damage and Luminal Alkalinization. MBio. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman J, Walpole GFW, Kasper Let al. Lysosome Fusion Maintains Phagosome Integrity during Fungal Infection. Cell Host Microbe. 2020. [DOI] [PubMed] [Google Scholar]
- Wheeler ML, et al. Immunological consequences of intestinal fungal dysbiosis. Cell Host Microbe. 2016;19:865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whibley N, et al. Antibody blockade of IL-17 family cytokines in immunity to acute murine oral mucosal candidiasis. J Leukoc Biol. 2016;99:1153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S, Larsen B. Candida albicans morphogenesis is influenced by estrogen. Cell Mol Life Sci. 1997;53:744–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney PG, Bär E, Osorio Fet al. Syk signaling in dendritic cells orchestrates innate resistance to systemic fungal infection. PLoS Pathog. 2014;10:e1004276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilgus TA. Alerting the body to tissue injury: The role of alarmins and DAMPs in cutaneous wound healing. Curr Pathobiol Rep. 2018;6:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems HM, Kos K, Jabra-Rizk MAet al. Candida albicans in oral biofilms could prevent caries. Pathog Dis. 2016;74. [DOI] [PubMed] [Google Scholar]
- Willems HME, Ahmed SS, Liu Jet al. Vulvovaginal candidiasis: A current understanding and burning questions. J Fungi (Basel). 2020;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RB, Lorenz MC. Multiple Alternative Carbon Pathways Combine To Promote Candida albicans Stress Resistance, Immune Interactions, and Virulence. MBio. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D, Hube B. Hgc1 mediates dynamic Candida albicans-endothelium adhesion events during circulation. Eukaryotic Cell. 2010;9:278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D, Naglik JR, Hube B. The Missing Link between Candida albicans Hyphal Morphogenesis and Host Cell Damage. PLoS Pathog. 2016;12:e1005867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. An evolutionary perspective on zinc uptake by human fungal pathogens. Metallomics. 2015;7:979–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witchley JN, Penumetcha P, Abon NVet al. Candida albicans Morphogenesis Programs Control the Balance between Gut Commensalism and Invasive Infection. Cell Host Microbe. 2019;25:432–443.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GD, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Zeng T, Deligios Met al. Age-Related Variation of Bacterial and Fungal Communities in Different Body Habitats across the Young, Elderly, and Centenarians in Sardinia. mSphere. 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S-Y, Weng C-L, Jheng M-Jet al. Candida albicans triggers NADPH oxidase-independent neutrophil extracellular traps through dectin-2. PLoS Pathog. 2019;15:e1008096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne AG, McCartney AL, Brostoff Jet al. An in vitro assessment of the effects of broad-spectrum antibiotics on the human gut microflora and concomitant isolation of a Lactobacillus plantarum with anti-Candida activities. Anaerobe. 2004;10:165–9. [DOI] [PubMed] [Google Scholar]
- Wächtler B, Citiulo F, Jablonowski Net al. Candida albicans-epithelial interactions: dissecting the roles of active penetration, induced endocytosis and host factors on the infection process. PLoS One. 2012;7:e36952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüthrich M, Deepe GS, Klein B. Adaptive immunity to fungi. Annu Rev Immunol. 2012;30:115–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, et al. White-opaque switching in natural MTLa/α isolates of Candida albicans: evolutionary implications for roles in host adaptation, pathogenesis, and sex. PLoS Biol. 2013;11:e1001525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Zhu H, Qiu P. Aging progression of human gut microbiota. BMC Microbiol. 2019;19: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Sobue T, Bertolini Met al. S. oralis activates the Efg1 filamentation pathway in C. albicans to promote cross-kingdom interactions and mucosal biofilms. Virulence. 2017;8:1602–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Sobue T, Bertolini Met al. Streptococcus oralis and Candida albicans Synergistically Activate μ-Calpain to Degrade E-cadherin From Oral Epithelial Junctions. J Infect Dis. 2016;214:925–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Sobue T, Thompson Aet al. Streptococcal co-infection augments Candida pathogenicity by amplifying the mucosal inflammatory response. Cell Microbiol. 2014;16:214–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mitchell TG. Geographical differences in human oral yeast flora. Clin Infect Dis. 2003;36:221–4. [DOI] [PubMed] [Google Scholar]
- Xu S, Shinohara ML. Tissue-Resident Macrophages in Fungal Infections. Front Immunol. 2017;8:1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X-L, Lee RTH, Fang H-Met al. Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p. Cell Host Microbe. 2008;4:28–39. [DOI] [PubMed] [Google Scholar]
- Yachida S, et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat Med. 2019;25:968–76. [DOI] [PubMed] [Google Scholar]
- Yadev NP, Murdoch C, Saville SPet al. Evaluation of tissue engineered models of the oral mucosa to investigate oral candidiasis. Microb Pathog. 2011;50:278–85. [DOI] [PubMed] [Google Scholar]
- Yang D, Oppenheim JJ. Alarmins and antimicrobial immunity. Med Mycol. 2009;47 Suppl 1:S146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Teoh F, Tan ASMet al. Aneuploidy Enables Cross-Adaptation to Unrelated Drugs. Mol Biol Evol. 2019;36:1768–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Yang C, Tang J. Disruption of the intestinal mucosal barrier in Candida albicans infections. Microbiol Res. 2013;168:389–95. [DOI] [PubMed] [Google Scholar]
- Yano J, Lilly E, Barousse Met al. Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candida vaginitis. Infect Immun. 2010;78:5126–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano J, Noverr MC, Fidel PL. Vaginal Heparan Sulfate Linked to Neutrophil Dysfunction in the Acute Inflammatory Response Associated with Experimental Vulvovaginal Candidiasis. MBio. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano J, Palmer GE, Eberle KEet al. Vaginal epithelial cell-derived S100 alarmins induced by Candida albicans via pattern recognition receptor interactions are sufficient but not necessary for the acute neutrophil response during experimental vaginal candidiasis. Infect Immun. 2014;82:783–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano J, Sobel JD, Nyirjesy Pet al. Current patient perspectives of vulvovaginal candidiasis: incidence, symptoms, management and post-treatment outcomes. BMC Womens Health. 2019;19:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapar N. Epidemiology and risk factors for invasive candidiasis. Ther Clin Risk Manag. 2014;10:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, et al. Proteomic response to amino acid starvation in Candida albicans and Saccharomyces cerevisiae. Proteomics. 2004;4:2425–36. [DOI] [PubMed] [Google Scholar]
- Zaneveld JR, McMinds R, Vega Thurber R. Stress and stability: applying the Anna Karenina principle to animal microbiomes. Nat Microbiol. 2017;2:17121. [DOI] [PubMed] [Google Scholar]
- Zarrinpar A, Chaix A, Xu ZZet al. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat Commun. 2018;9:2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante T, et al. Sensing of mammalian IL-17A regulates fungal adaptation and virulence. Nat Commun. 2012;3:683. [DOI] [PubMed] [Google Scholar]
- Zelante T et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–85. [DOI] [PubMed] [Google Scholar]
- Zhai B, et al. High-resolution mycobiota analysis reveals dynamic intestinal translocation preceding invasive candidiasis. Nat Med. 2020;26:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Magee BB, Magee PTet al. Selective Advantages of a Parasexual Cycle for the Yeast Candida albicans. Genetics. 2015;200:1117–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, et al. Metaproteomics reveals associations between microbiome and intestinal extracellular vesicle proteins in pediatric inflammatory bowel disease. Nat Commun. 2018;9:2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, et al. Metagenomic sequencing reveals altered metabolic pathways in the oral microbiota of sailors during a long sea voyage. Sci Rep. 2015;5:9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, et al. Longitudinal multi-omics of host-microbe dynamics in prediabetes. Nature. 2019;569:663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Hansmann MA, Davis CCet al. The vaginal bacterial communities of Japanese women resemble those of women in other racial groups. FEMS Immunol Med Microbiol. 2010;58:169–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, et al. Biogeography of the ecosystems of the healthy human body. Genome Biol. 2013;14:R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L-L, Zhao X-Q, Jiang Cet al. C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity. 2013;39:324–34. [DOI] [PubMed] [Google Scholar]
- Zielinski CE, Mele F, Aschenbrenner Det al. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature. 2012;484:514–8. [DOI] [PubMed] [Google Scholar]
- Zipfel PF, Hallström T, Riesbeck K. Human complement control and complement evasion by pathogenic microbes–tipping the balance. Mol Immunol. 2013;56:152–60. [DOI] [PubMed] [Google Scholar]
- Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–40. [DOI] [PubMed] [Google Scholar]
- Znaidi S, Barker KS, Weber Set al. Identification of the Candida albicans Cap1p regulon. Eukaryotic Cell. 2009;8:806–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoetendal EG, et al. The human small intestinal microbiota is driven by rapid uptake and conversion of simple carbohydrates. ISME J. 2012;6:1415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo T, et al. Gut fungal dysbiosis correlates with reduced efficacy of fecal microbiota transplantation in Clostridium difficile infection. Nat Commun. 2018;9:3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöllner MSA da C, Jorge AOC. Candida spp. occurrence in oral cavities of breastfeeding infants and in their mothers’ mouths and breasts. Pesqui Odontol Bras. 2003;17:151–5. [DOI] [PubMed] [Google Scholar]