Abstract
In December 2019, all nations learnt about the emergence of a pandemic of coronavirus disease (COVID-19), induced by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is a member of the β-coronavirus group. As SARS-CoV-2 has the potentiality of leading to life-threatening respiratory failure, its transmission routes need to be characterized. Yet, the possibility of airborne transmission is still debated. This study was performed to evaluate potential hospital indoor air viral quality in order to detect SARS-COV-2. For this purpose, an impinger method was used to monitor the SARS-COV-2 virus in the air. Thus, 33 samples were collected from 8 different hospital locations. The sampling time was between 50 and 60 min with a sampling flow rate of 28 L/min. Air samples were taken from 2 to 5 m away from the patients’ beds. Temperature, relative humidity, and CO2 concentration were 28, 37, and 438 ppm, respectively. The results indicated that air samples which were 2 to 5 m away from the patients’ beds were negative for the presence of the virus. According to the obtained results, it is suggested that airborne transmission may not have much effect on this pandemic. However, as the patients with SARS-CoV-2 were hospitalized in rooms with negative air pressure, the results might have been negatively affected.
Graphical abstract
Keywords: SARS-coronavirus-2, Airborne transmission, Hospital indoor air, Impinger method, COVID-19 patient wards, Temperature, Relative humidity
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel coronavirus disease, was initially identified in Wuhan City, China, in December 2019 (Lu et al. 2020; Xu et al. 2020; Zandian et al. 2021) and has subsequently become a global health problem due to its highly contagious nature (Sarailoo et al. 2021; Yongjian et al. 2020). This disease has revealed sever effects on different parts of human life such as health, economy, and environment (Dargahi et al. 2021b; Shakil et al. 2020). Considering SARS-COV-2 as an international concern, the World Health Organization (WHO) has announced the disease as a public health emergency and declared the outbreak of SARS-COV-2 on January 30, 2020 (Dargahi et al. 2021a; Kandel et al. 2020; Noorimotlagh et al. 2020). Based on the WHO report, the number of confirmed cases and deaths until April 08, 2021, was 133,695,421 and 2,901,124 over the world, respectively. These statistics led to an announcement by this organization on the same day that evaluated the condition as a global pandemic (Karami et al. 2021; Noorimotlagh et al. 2020). Due to severe acute respiratory syndrome of SARS-CoV-2, the SARS-COV-2 epidemic has spread to more than 221 countries and regions (Hu et al. 2021). Considering the Ministry of Health and Medical Education, the first official report of death caused by SARS-CoV-2 in Iran was recorded on February 19, 2020 (Takian et al. 2020). On February 11, 2021, the number of COVID-19 cases and deaths caused by SARS-COV-2 was 1,984,348 and 63,699, respectively, which led to ranking Iran as the 15th country in terms of involvement with this virus among 221 countries and territories with SARS-COV-2 outbreak. It has been reported that most of SARS-CoV-2-infected patients have generally mild symptoms, e.g., fever, dry cough, and sore throat. However, severe and even fatal complications such as acute respiratory distress syndrome (ARDS) are observed in some patients (Chan et al. 2020; Chia et al. 2020; Noorimotlagh et al. 2020; Sohrabi et al. 2020). The human-to-human transmissibility of SARS-COV-2 has been previously confirmed which has increased the attentions and concerns over the world. Since SARS-COV-2 is rapidly spreading, numerous researches are being conducted to better recognize different routes of the virus transmission and to understand the clinical features and severity of the disease. Although the person-to-person transmission of SARS-COV-2 has been mainly discussed in previously conducted investigations, the airborne transmission of this virus is still controversial (Faridi et al. 2020b). The incubation time for person-to-person transmission routes of SARS-CoV-2 has been previously reported to be between 2 and 10 days (Chan et al. 2020; Klompas et al. 2020) and its transmission happens through direct personal contact, droplets, hands, or contaminated surfaces (Chan et al. 2020; Masoumbeigi et al. 2020). Based on laboratory experiments, this virus is capable of remaining infectious in aerosols for hours and on surfaces for days (Van Doremalen et al. 2020). Evidently, the existence of high viral loads in the respiratory tract of admitted infected persons released in the environment through droplets or spread via coughing or sneezing can increase the virus dispersion and finally expedite its transfer in hospital settings (Rothan and Byrareddy 2020). Since the droplets are able to remain suspended in closed and stationary environments, e.g., hospital wards, for more than 10 min and can cover long distances, controlling the disease has been considered far complicated (Bourouiba 2020; Ong et al. 2020; Razzini et al. 2020). According to the existing reports, airborne transmission has a critical role in the epidemiology of the two zoonotic coronaviruses, i.e., SARS-CoV and MERS-CoV, which have emerged since two decades ago (Van Doremalen et al. 2020). So far, researchers have not been able to provide a comprehensive conclusion on SARS-CoV-2 airborne transmission (Ghinai et al. 2020; Kenarkoohi et al. 2020; Van Doremalen et al. 2020). Morawska and Cao (2020), considering the results of previous studies which confirmed the air as the foremost transmission route of SARS-CoV-2 in particular indoor environments, suggested the probability of same transmission for the novel coronavirus (Liu et al. 2020; Morawska and Cao 2020). Despite this evidence, no country or authority has yet taken very effective regulations and procedures for avoiding the spread of SARS-COV-2 through the air for preventing its transmission through indoor air. Therefore, it is necessary for the authorities to be certain whether the virus can spread through air. Necessary preventive measures should be taken to impede or decline the spread of the SARS-COV-2 virus, especially by eliminating the virus droplets with proper ventilation (Morawska and Cao 2020). In this regard, some important measures encompass increasing the frequency and amount of ventilation, employing natural ventilation, preventing air circulation, preventing other people from being in direct air flow, and minimizing the number of people who are in a common environment (Qian and Zheng 2018). Overall, concerning the prevalence extent of SARS-COV-2 around the world and the high mortality rate and incidence of this disease, various researchers around the world are seeking to investigate its transmissibility among people through various routes, including air and wastewater. Hence, it is very important to study the possible transmission paths of this disease to prevent its further spread. Therefore, this study was performed to estimate the viral quality of indoor air in a hospital to spot the presence of SARS-CoV-2 virus.
Materials and methods
Approval statement
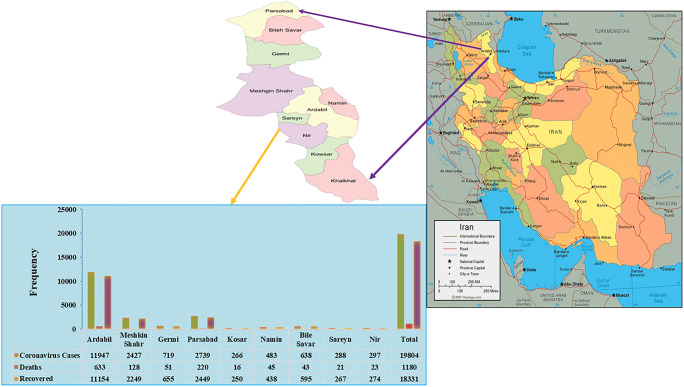
This project was carried out according to the health protocols in Ardabil, Iran (ethic code IR.ARUMS.REC.1399.312). Ardebil is the capital of Ardabil province, located in the northwest of Iran, with a population of 625,000. Ardabil is among the mostly affected cities by SARS-CoV-2 in Iran (Fig. 1).
Fig. 1.
Percentage of infection, mortality, and recovery in cities of Ardabil province located in northwestern Iran during the SARS-CoV-2 pandemic
Specimen collection, storage, and transfer
Material procurement
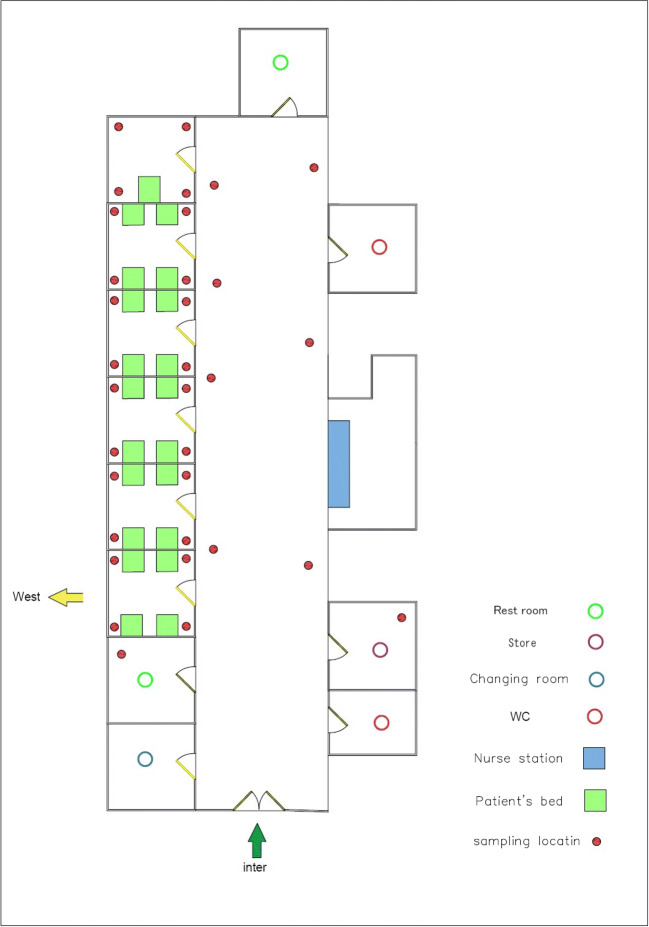
Decontamination and cleaning of all work sites, centrifuges, lab dishes, and other instruments were done prior to the phase of specimen collection. Disinfectants including 70% ethanol, 10% bleach, and DNAZap™ or RNase AWAY® were consumed to decrease the potential contamination of nucleic acid. Regarding the exposure time that was between 1, 2, and 3 h, an impinger connected to a pump with a flow rate of 28 L/min (SKC, England) was used to collect the SARS-COV-2 virus in the air. VTM culture medium, including 15 mL of DMEM (Dulbecco’s Modified Eagle’s Medium) 10,000 units/mL of penicillin, 10,000 μg/mL of streptomycin, and 25 μg/mL of fungizone (amphotericin B), used for the prevention and elimination of bacterial contaminants, was used to collect samples. Samples were collected from different hospital wards (Fig. 2) including respiratory section-1 (COVID-19), laboratory section, CT section, respiratory section-2 (COVID-19), respiratory section-1 (COVID-19) checkup room, respiratory section-2 (COVID-19) station section, emergency section, and intensive care unit (ICU). We set up our research operations in corona wards at the height of about 1.0, 1.2, 1.5, 1.8, and 2.0 m and the approximate distance from each patient was 2.0 to 5.0 m. There were several symptoms for patients such as sever coughing during the sampling. From each ward, 10 samples were taken by suction of the air into the sterile impinge (Sarkhosh et al. 2021). Indoor relative humidity and temperature were recorded by HD and110 KIMO (Sauermann Group). Also, we recorded CO2 concentration in the air of the wards using AQ110. The collected samples were kept at the temperature of − 20 °C until the corona lab test results arrived. After each sampling, sampler device was disinfected with alcohol 70%.
Fig. 2.
Air sample location of Imam Khomeini Hospital in Ardabil province
Viral genome extraction
A nucleic acid extraction kit (Gene Favor) was used to extract the virus genome and the extracted genomes were preserved in the freezer for the next stage at − 70 °C.
Running real-time PCR and data analysis
To identify the SARS-CoV-2 virus, the isolated genome was put in the experiment micro-tube along with other reagents. Then, depending on the package protocol, the real-time PCR findings were evaluated and then, the results were defined as positive and negative. There were several steps for the identification of virus: Reasonable reagent quantities, the cycle of temperature, and appropriate replication were performed for the preliminary screening stage in the first phase. The probe real-time reverse transcriptase-polymerase chain reaction (RT-PCR) and specific primer targeting ORF1ab and N genes (nucleoprotein gene) were carried out to determine the viral genomes of the SARS-CoV-2 in the samples collected from the hospital indoor air. Applied Biosystems™ Real-Time PCR System 7500 software v2.0.5 was operated to set up RT-PCR. Permissible amount of reagents for real-time PCR Master Mix required for synthesis is presented in Table 1. All experiments were done according to the safety protocols (Corman et al. 2020; Waggoner et al. 2020) (Table 1).
Table 1.
Primers and probes used in this study
| Organisms | Target gene | Sequence (5′–3′) | Cycling parameters | Reference |
|---|---|---|---|---|
| SARS-CoV-2 |
Probe and primer ORF1a/b |
FACAGGTGGAACCTCATCAGGAGATGC-BBQ F-GTGARATGGTCATGTGTGGCGG R-CARATGTTAAASACACTATTAGCATA |
55°C 10′ 94°C 3′ 94°C 15″ 58°C 30″ 45x. | Corman et al |
|
Primer and probe N gene |
F-AAATTTTGGGGACCAGGAAC R-TGGCAGCTGTGTAGGTCAA PFAM-ATGTCGCGCATTGGCATGGA-BHQ |
55°C 10′ 94°C 3′ 94°C 15″ 58°C 30″ 45x. | Waggoner et al |
Proper amount of the synthesis reagents for Real time PCR Master Mix: per reaction H2O (RNAse free) 0.6 μL 2× reaction mix* 12.5 μL MgSO4 (50mM) 0.4 μL BSA (1 mg/mL)** 1 μL primer ORF1a/b _SARSr-F (10 μM stock solution), 1.5 μL primer ORF1a/b SARSr-R (10 μM stock solution), 2 μL probe RdRP_SARSr-P1 (10 μM stock solution), 0.5 μL probe RdRP_SARSr-P2 (10 μM stock solution), 0.5 μL of SARS-CoV-2 primer and probe sets s Cov RdRP and N gens. SSIII/Taq EnzymeMix* 1 μL template RNA, add 5 μL, total reaction mix 20 μL. Thermal cycler: 55°C 10′ 94°C 3′ 94°C 15″ 58°C 30″ 45x
Positive and negative control
In this study, 200 copies/mL was the detection limit of the kit.
Control materials SARS-CoV-2 PCR-positive control
A positive template control was carried out for each detection run to assess whether the rRT-PCR method worked appropriately.
Control materials SARS-CoV-2 PCR-negative control
A “no template” (negative) control was carried out for each detection run to assess whether there was any contamination for the rRT-PCR course. The Wuhan CoV N-gene kit (TIB Molbiol, Berlin, Germany) was used for internal control in the extraction stage to validate the extraction and PCR amplification process.
Results and discussion
Table 1 shows the specifications of all samples of SARS-CoV-2 collected in the air of Imam Khomeini Hospital in Ardabil and Table 2 presents additional information about the environmental conditions of patients’ rooms with SARS-CoV-2 in this hospital. As shown in Table 1, 55 patients with SARS-CoV-2 were hospitalized, of whom 28 patients received oxygen masks and 24 patients underwent intubation. There were 35 staff members in a routine hospital shift. In this study, 32 samples were taken from 8 different hospital locations. According to Table 2, air samples which were 2 to 5 m away from the patients’ beds were negative. Therefore, it is suggested that airborne transmission may not have much effect on this pandemic.
Table 2.
SARS-CoV-2 in the air samples of hospital wards (n = 32)
| Hospital wards | Number of air samples | Status of COVID-19 patients | Number of staffs | SARS-CoV-2 in air sample |
|---|---|---|---|---|
| Respiratory section-1 (COVID-19) | S1 | 25 (oxygen mask: 15, intubated: 10) | 8 | Neg |
| S2 | Neg | |||
| S10 | Neg | |||
| S11 | Neg | |||
| S13 | Neg | |||
| S19 | Neg | |||
| S20 | Neg | |||
| S31 | Neg | |||
| S32 | Neg | |||
| Laboratory section | S3 | Presence of people suspected to have the disease | 5 | Neg |
| S16 | Neg | |||
| S26 | Neg | |||
| S27 | Neg | |||
| CT section | S4 | Presence of people suspected to have the disease | 3 | Neg |
| S23 | Neg | |||
| S34 | Neg | |||
| Respiratory section-2 (COVID-19) | S5 | 22 (oxygen mask: 13, intubated: 9) | 8 | Neg |
| S14 | Neg | |||
| S22 | Neg | |||
| S28 | Neg | |||
| S30 | Neg | |||
| Respiratory section-1 (COVID-19) checkup room | S6 | Presence of people suspected to have the disease | 2 | Neg |
| Respiratory section-2 (COVID-19) station section | S7 | Presence of people suspected to have the disease | 2 | Neg |
| Emergency section | S8 | 3 (oxygen mask: 0, intubated: 0) and presence of people suspected to have the disease | 5 | Neg |
| S12 | Neg | |||
| S17 | Neg | |||
| S18 | Neg | |||
| S25 | Neg | |||
| S29 | Neg | |||
| S33 | Neg | |||
| ICU | S9 | 2 | Neg | |
| S15 | 5 (oxygen mask: 0, intubated: 5) | Neg |
S sample number, Neg negative result
According to Table 3, all windows were closed during the sampling phase and the ventilation system was mechanical/natural. Wards where patients were transient or hospitalized were enclosed with space ranging between 20 and 200 m2. The lowest and highest temperatures in the respiratory section-1 (COVID-19) and CT section were 19.5 °C and 28 °C, respectively. On the other hand, the highest humidity rate was 41% in the ICU and the lowest humidity was 33% in the respiratory section-2 (COVID-19). The highest CO2 in air observed in the respiratory section-1 (COVID-19) was 438 ppm and the lowest CO2 in air ppm observed in the respiratory section-1 (COVID-19) checkup room was 315. As patients with COVID-19 were hospitalized in rooms with negative air pressure in this study, we assume that this condition may affect the results.
Table 3.
Additional information on environmental status of patients’ rooms with confirmed COVID-19
| Hospital wards | Status of windows | Area of patient rooms (m2) | Ventilation system | Temperature (°C) | Relative humidity (%) | CO2 (ppm) |
|---|---|---|---|---|---|---|
| Respiratory section-1 (COVID-19) | 5 (close) | 125 | Mechanical/natural | 28 | 37 | 438 |
| Laboratory section | 4 (close) | 60 | Mechanical/natural | 24.5 | 37 | 351 |
| CT section | 2 (close) | 50 | Mechanical/natural | 19.5 | 39 | 325 |
| Respiratory section-2 (COVID-19) | 4 (close) | 100 | Mechanical/natural | 26.5 | 33 | 417 |
| Respiratory section-1 (COVID-19) checkup room | 1 (close) | 25 | Mechanical/natural | 24.5 | 37 | 315 |
| Respiratory section-2 (COVID-19) station section | 1 (close) | 20 | Mechanical/natural | 26.5 | 33 | 342 |
| Emergency section | 8 (close) | 200 | mechanical/natural | 23.5 | 40 | 425 |
| ICU | 2 (close) | 70 | Mechanical/natural | 24.1 | 41 | 412 |
Worldwide case studies have established that the behavior change of SARS-CoV-2 virus has been unprecedentedly unique with more persistence and possibility viable rates in the air. Atzrodt et al. 2020) tested a combination of environmental elements on HCoV-229E persistence, specially two temperatures typical of the two extreme indoor atmospheric conditions in temperate countries (6 and 20 °C) and three relative humidity demonstrating low (30%), medium (50%), and high (80%) conditions in both indoor and outdoor environments (Ijaz et al. 1985). The nosocomial transmission during the pandemic by airborne SARS-CoV-2 virus-laden aerosols in healthcare facilities can be expected. Hence, clearly defined rules, medical and scientific administration, and clinical and physical measures are of principal importance to end the SARS-CoV-2 pandemic over the world. Mask wearing and observing social distancing have been considered a general training worldwide in combating the contraction of SARS-CoV-2. Undeniably, although such measures may determine to control the SARS-CoV-2 pandemic to a great extent, the broad control span of virus-laden aerosols and droplet transmission is poorly understood (Jayaweera et al. 2020). Faridi et al. (2020a) have shown that all air samples collected in the selected large hospital indoor air were negative; however, we recommend more in vivo investigations be directed using actual patient breathing, coughing, and sneezing aerosols in order to show the option of generation of the airborne size transporter aerosols and the viability portion of the surrounded virus in those carrier aerosols. Moreover, according to the study of Van Doremalen et al. (2020), SARS-CoV-2 remained viable and infectious in aerosols for hours and on special surfaces for up to days, which indicates a possibility of droplet, aerosol, and fomite transmission. Similarly, Fears et al. (2020) report that SARS-CoV-2 generally remains infectious as it passes through an air space over short distances and linger as respiratory droplets when generated by respiratory particles produced by an infected person (Fears et al. 2020).
Furthermore, Masoumbeigi et al. found all the samples collected from the air were negative for SARS-CoV-2 existence. These results revealed that SARS-CoV-2 could not be transmitted through airborne path in hospital indoor air. Jin et al. found that all samples taken from surface and air environments from the staff’s personal protective equipment dressing room were negative, but the air sample from the isolation room was highly positive (SARS-CoV-2) (Jin et al. 2021). The reported median half-life estimate of SARS-CoV-2 in aerosols ranges from approximately 1.1 to 1.2 h (95% confidence intervals of 0.64–2.64) (Van Doremalen et al. 2020). Lindsey C. Marr et al. have examined the presence and survival of coronavirus in air with generated aerosols of asymptomatic infection. It is generally believed that aerosol physics play a critical role in the prevention and control of SARS-CoV-2 (Allen and Marr 2020). Table 4 inspects the conflicting studies on the possibility of aerosol transmission route of SARS-CoV-2.
Table 4.
Compression of conflicting studies about the possibility of airborne transmission SARS-CoV-2
| Raw | study | Results | References |
|---|---|---|---|
| 1 | Airborne transmission of SARS-CoV-2: the world should face the reality | Their study showed that larger droplets with viral content deposit close to the emission point. | Morawska et al. |
| 2 | A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran | All air samples were negative. | Faridi et al. |
| 3 | Detection of air and surface contamination by severe acute respiratory syndrome coronavirus (SARS-CoV-2) in hospital rooms of infected patients | SARS-COV-2 viral RNA in air has been detected in hospitals, including at distances greater than 2 m from patients. | Chia et al. |
| 4 | Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals | They found that the majority of these viruses was associated with microscopic droplets of diameter 2.5 microns and smaller. | Liu et al. |
| 5 | Hospital indoor air quality monitoring for the detection of SARS-CoV-2 (COVID-19) virus | They found two positive air samples (in the ICU) out of 14 ones taken from different wards with confirmed COVID-19 patients. | Kenarkoohi et al. |
| 6 | SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19 ward of a hospital in Milan, Italy | All the air samples collected from the contaminated area were positive while viral RNA was not detected in either semi contaminated or clean areas. | Razzini et al. |
| 7 | Investigation of hospital indoor air quality for the presence of SARS-Cov-2 | All of the samples were negative for SARS-Cov-2 occurrence. | Masoumbeigi et al. |
| 8 | Airborne transmission of SARSCoV-2: theoretical considerations and available evidence. | They reported no perfect experimental data are proving or disproving droplet vs. aerosol-based transmission of SARS-CoV-2. | Klompas et al. |
| 9 | Inspecting hospital indoor air quality for possible presence of SARS-CoV-2 (Covid-19) | All the air samples collected from the different wards of hospital were negative. | This study |
We think that positive air samples were quite scarce throughout the relevant literature. This can possibly be attributed to ventilation in hospitals where pollutants originating in the building are diluted so the virus is also diluted with clean air by an airflow rate which change the air at a noticeable rate. Thus, it seems SARS-COV-2 airborne transmission may not have much effect on this pandemic. The generalizability of this finding is limited as we lacked samplers such as gelatin filers, PTFE filters, and cyclones which show efficient performance for catching SARS-CoV-2 and MERS-CoV viruses followed by PCR investigation. Despite efforts to clarify the virus transmission paths, specifically in indoor settings, certain features of SARS-CoV-2 dispersion are still uncovered.
Conclusion
A comprehensive understanding of the transmission pathways of SARS-CoV-2 can allow a better preventive plan. Such pathways include the airborne transmission path which triggers the discussion that the virus goes far outside 1 m reported by the WHO. This should be observed as a protection distance especially in healthcare settings. Our study has several limitations. In fact, we only focused on the association between air parameters and SARS-CoV-2-confirmed cases and not the causal effect of air on SARS-CoV-2 infection.
Authorship contribution
Mehdi Vosoughi: methodology, validation, formal analysis, investigation, resources, writing - original draft; Chiman Karami: conceptualization, methodology, validation, formal analysis, investigation, project administration; Abdollah Dargahi: methodology, validation, resources, writing - original draft; Farhad Jeddi: validation, formal analysis; Kamyar Mazloum Jalali: methodology, validation; Aidin Hadisi: methodology, validation, resources and supervision; Somayeh Biparva Haghighi: analyses, writing and text revision; Hadi Peeri Dogahe: supervision project; Zahra Noorimotlagh: validation, formal analysis, resources, administration; Seyyed Abbas Mirzaee: conceptualization, methodology, validation, resources.
Funding
The current study was financially supported by Ardabil University of Medical Sciences, Ardabil, Iran (ARUMS) under grant number 3852 and by INSF under grant number 99004900.
Data availability
The datasets analyzed during the current study are available from the corresponding authors on realistic demand.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Highlights
• Temperature and relative humidity have critical roles in potential airborne transmission of SARS-COV-2.
• Microclimate condition seems to have much effect on this airborne transmission.
• Understanding the routes of transmission can best determine appropriate preventive measures.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chiman Karami, Email: chkarami.chiman@gmail.com.
Abdollah Dargahi, Email: a.dargahi29@yahoo.com.
References
- Allen JG, Marr LC. Recognizing and controlling airborne transmission of SARS-CoV-2 in indoor environments. Indoor Air. 2020;30:557–558. doi: 10.1111/ina.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzrodt CL, Maknojia I, McCarthy RD, Oldfield TM, Po J, Ta KT, Stepp HE, Clements TP. A Guide to COVID-19: a global pandemic caused by the novel coronavirus SARS-CoV-2. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourouiba LJJ (2020) Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. 323, 1837-1838. [DOI] [PubMed]
- Chan JF-W, Yuan S, Kok K.-H, To KK-W, Chu H, Yang J, Xing F, Liu J, Yip CC-Y, Poon RW-SJTL (2020) A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. 395, 514-523. [DOI] [PMC free article] [PubMed]
- Chia PY, Coleman KK, Tan YK, Ong SWX, Gum M, Lau SK, Sutjipto S, Lee PH, Young BE, Milton DK (2020) Detection of air and surface contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in hospital rooms of infected patients. medRxiv.
- Corman V, Bleicker T, Brünink S, Drosten C, Zambon M (2020) Diagnostic detection of 2019-nCoV by real-time RT-PCR. World Health Organization (Jan 17)
- Dargahi A, Jeddi F, Ghobadi H, Vosoughi M, Karami C, Sarailoo M, Hadisi A, Mokhtari SA, Haghighi SB, Sadeghi H. Evaluation of masks’ internal and external surfaces used by health care workers and patients in coronavirus-2 (SARS-CoV-2) wards. Environ Res. 2021;196:110948. doi: 10.1016/j.envres.2021.110948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargahi A, Jeddi F, Vosoughi M, Karami C, Hadisi A, Mokhtarie SA, Alighadri M, Haghighi SB, Sadeghi H. Investigation of SARS CoV-2 virus in environmental surface. Environ Res. 2021;195:110765. doi: 10.1016/j.envres.2021.110765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faridi S, Niazi S, Sadeghi K, Naddafi K, Yavarian J, Shamsipour M, Jandaghi NZS, Sadeghniiat K, Nabizadeh R, Yunesian M (2020a) A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci Total Environ 138401 [DOI] [PMC free article] [PubMed]
- Faridi, S., Niazi, S., Sadeghi, K., Naddafi, K., Yavarian, J., Shamsipour, M., Jandaghi, N.Z.S., Sadeghniiat, K., Nabizadeh, R., Yunesian, M.J.SoTTE (2020b) A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. 138401. [DOI] [PMC free article] [PubMed]
- Fears AC, Klimstra WB, Duprex P, Hartman A, Weaver SC, Plante K, Mirchandani D, Plante J, Aguilar PV, Fernandez DJM (2020) Comparative dynamic aerosol efficiencies of three emergent coronaviruses and the unusual persistence of SARS-CoV-2 in aerosol suspensions.
- Ghinai I, McPherson TD, Hunter JC, Kirking HL, Christiansen D, Joshi K, Rubin R, Morales-Estrada S, Black SR, Pacilli MJTL. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Ni W, Wang Z, Ma G, Pan B, Dong L, Gao R, Jiang F. The distribution of SARS-CoV-2 contamination on the environmental surfaces during incubation period of COVID-19 patients. Ecotoxicol Environ Saf. 2021;208:111438. doi: 10.1016/j.ecoenv.2020.111438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz M, Brunner A, Sattar S, Nair RC, Johnson-Lussenburg C. Survival characteristics of airborne human coronavirus 229E. J Gen Virol. 1985;66:2743–2748. doi: 10.1099/0022-1317-66-12-2743. [DOI] [PubMed] [Google Scholar]
- Jayaweera, M., Perera, H., Gunawardana, B., Manatunge, J., 2020. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environmental Research, 109819. [DOI] [PMC free article] [PubMed]
- Jin T, Li J, Yang J, Li J, Hong F, Long H, Deng Q, Qin Y, Jiang J, Zhou X, Song Q (2021) SARS-CoV-2 presented in the air of an intensive care unit (ICU). Sustain Cities Soc 65:102446 [DOI] [PMC free article] [PubMed]
- Kandel N, Chungong S, Omaar A, Xing JJTL (2020) Health security capacities in the context of COVID-19 outbreak: an analysis of International Health Regulations annual report data from 182 countries [DOI] [PMC free article] [PubMed]
- Karami C, Normohammadi A, Dargahi A, Vosoughi M, Zandian H, Jeddi F, Mokhtari SA, Moradi-Asl E. Investigation of SARS-CoV-2 virus on nozzle surfaces of fuel supply stations in north west of Iran. Sci Total Environ. 2021;780:146641. doi: 10.1016/j.scitotenv.2021.146641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenarkoohi A, Noorimotlagh Z, Falahi S, Amarloei A, Mirzaee SA, Pakzad I, Bastani E. Hospital indoor air quality monitoring for the detection of SARS-CoV-2 (COVID-19) virus. Sci Total Environ. 2020;748:141324. doi: 10.1016/j.scitotenv.2020.141324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompas M, Baker MA, Rhee C (2020) Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. Jama [DOI] [PubMed]
- Liu Y, Ning Z, Chen Y, Guo M, Liu Y, Gali NK, Sun L, Duan Y, Cai J, Westerdahl D. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Lu H, Stratton CW, Tang YWJJ. Outbreak of pneumonia of unknown etiology in Wuhan. China: the mystery and the miracle. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoumbeigi H, Ghanizadeh G, Arfaei RY, Heydari S, Goodarzi H, Sari RD, Tat M, Engineering (2020) Investigation of hospital indoor air quality for the presence of SARS-Cov-2. J Environ Health Sci:1–5 [DOI] [PMC free article] [PubMed]
- Morawska L, Cao JJEI (2020) Airborne transmission of SARS-CoV-2: the world should face the reality. 105730. [DOI] [PMC free article] [PubMed]
- Noorimotlagh Z, Karami C, Mirzaee SA, Kaffashian M, Mami S, Azizi MJIi (2020) Immune and bioinformatics identification of T cell and B cell epitopes in the protein structure of SARS-CoV-2: a systematic review. 106738. [DOI] [PMC free article] [PubMed]
- Ong SWX, Tan YK, Chia PY, Lee TH, Ng OT, Wong MSY, Marimuthu KJJ (2020) Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. 323:1610-1612. [DOI] [PMC free article] [PubMed]
- Qian H, Zheng XJJotd (2018) Ventilation control for airborne transmission of human exhaled bio-aerosols in buildings. 10:S2295. [DOI] [PMC free article] [PubMed]
- Razzini K, Castrica M, Menchetti L, Maggi L, Negroni L, Orfeo NV, Pizzoccheri A, Stocco M, Muttini S, Balzaretti CMJSoTTE (2020) SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19 ward of a hospital in Milan, Italy. 140540. [DOI] [PMC free article] [PubMed]
- Rothan HA, Byrareddy SNJJoa (2020) The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. 102433. [DOI] [PMC free article] [PubMed]
- Sarailoo M, Matin S, Vosoughi M, Dargahi A, Gholizadeh H, Rajabi Damavandi M, Abbasi-Ghahramanloo A, Kamran A. Investigating the relationship between occupation and SARS-CoV2. Work. 2021;68:27–32. doi: 10.3233/WOR-205066. [DOI] [PubMed] [Google Scholar]
- Sarkhosh M, Najafpoor AA, Alidadi H, Shamsara J, Amiri H, Andrea T, Kariminejad F. Indoor Air Quality associations with sick building syndrome: an application of decision tree technology. Build Environ. 2021;188:107446. doi: 10.1016/j.buildenv.2020.107446. [DOI] [Google Scholar]
- Shakil MH, Munim ZH, Tasnia M, Sarowar SJSotTE (2020) COVID-19 and the environment: a critical review and research agenda. 141022. [DOI] [PMC free article] [PubMed]
- Sohrabi C, Alsafi Z, O’Neill N, Khan M, Kerwan A, Al-Jabir A, Iosifidis C, Agha RJIJoS (2020) World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19). [DOI] [PMC free article] [PubMed]
- Takian A, Raoofi A, Kazempour-Ardebili SJL (2020) COVID-19 battle during the toughest sanctions against Iran. 395, 1035. [DOI] [PMC free article] [PubMed]
- Van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN et al (2020) Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 382(16):1564–1567 [DOI] [PMC free article] [PubMed]
- Waggoner JJ, Stittleburg V, Pond R, Saklawi Y, Sahoo MK, Babiker A, Hussaini L, Kraft CS, Pinsky BA, Anderson E. Triplex real-time RT-PCR for severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26:1633–1635. doi: 10.3201/eid2607.201285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu LJTLrm (2020) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. 8, 420-422. [DOI] [PMC free article] [PubMed]
- Yongjian Z, Jingu X, Fengming H, Liqing CJSotte (2020) Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. 138704. [DOI] [PMC free article] [PubMed]
- Zandian H, Sarailoo M, Dargahi S, Gholizadeh H, Vosoughi M, Dargahi A. Evaluation of knowledge and health behavior of university of medical sciences students about the prevention of COVID-19. Work. 2021;68:543–549. doi: 10.3233/WOR-203395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding authors on realistic demand.