Key Points
Question
How are brain morphometric features associated with later psychosis conversion in individuals at clinical high risk (CHR) for developing psychosis?
Findings
In this case-control study including 3169 participants, lower cortical thickness, but not cortical surface area or subcortical volume, was more pronounced in individuals at CHR in a manner highly consistent with thinner cortex in individuals with established psychosis. Regions that displayed lower cortical thickness in individuals at CHR who later developed a psychotic disorder additionally displayed abnormal associations with age.
Meaning
In this study, CHR status and later transition to psychosis was robustly associated with lower cortical thickness; abnormal age associations and specificity to cortical thickness may point to aberrant postnatal brain development in individuals at CHR, including pruning and myelination.
This case-control study investigates baseline structural magnetic resonance imaging (MRI) differences between individuals at clinical high risk and healthy controls as well as between participants at clinical high risk who later developed a psychotic disorder and those who did not.
Abstract
Importance
The ENIGMA clinical high risk (CHR) for psychosis initiative, the largest pooled neuroimaging sample of individuals at CHR to date, aims to discover robust neurobiological markers of psychosis risk.
Objective
To investigate baseline structural neuroimaging differences between individuals at CHR and healthy controls as well as between participants at CHR who later developed a psychotic disorder (CHR-PS+) and those who did not (CHR-PS−).
Design, Setting, and Participants
In this case-control study, baseline T1-weighted magnetic resonance imaging (MRI) data were pooled from 31 international sites participating in the ENIGMA Clinical High Risk for Psychosis Working Group. CHR status was assessed using the Comprehensive Assessment of At-Risk Mental States or Structured Interview for Prodromal Syndromes. MRI scans were processed using harmonized protocols and analyzed within a mega-analysis and meta-analysis framework from January to October 2020.
Main Outcomes and Measures
Measures of regional cortical thickness (CT), surface area, and subcortical volumes were extracted from T1-weighted MRI scans. Independent variables were group (CHR group vs control group) and conversion status (CHR-PS+ group vs CHR-PS− group vs control group).
Results
Of the 3169 included participants, 1428 (45.1%) were female, and the mean (SD; range) age was 21.1 (4.9; 9.5-39.9) years. This study included 1792 individuals at CHR and 1377 healthy controls. Using longitudinal clinical information, 253 in the CHR-PS+ group, 1234 in the CHR-PS− group, and 305 at CHR without follow-up data were identified. Compared with healthy controls, individuals at CHR exhibited widespread lower CT measures (mean [range] Cohen d = −0.13 [−0.17 to −0.09]), but not surface area or subcortical volume. Lower CT measures in the fusiform, superior temporal, and paracentral regions were associated with psychosis conversion (mean Cohen d = −0.22; 95% CI, −0.35 to 0.10). Among healthy controls, compared with those in the CHR-PS+ group, age showed a stronger negative association with left fusiform CT measures (F = 9.8; P < .001; q < .001) and left paracentral CT measures (F = 5.9; P = .005; q = .02). Effect sizes representing lower CT associated with psychosis conversion resembled patterns of CT differences observed in ENIGMA studies of schizophrenia (ρ = 0.35; 95% CI, 0.12 to 0.55; P = .004) and individuals with 22q11.2 microdeletion syndrome and a psychotic disorder diagnosis (ρ = 0.43; 95% CI, 0.20 to 0.61; P = .001).
Conclusions and Relevance
This study provides evidence for widespread subtle, lower CT measures in individuals at CHR. The pattern of CT measure differences in those in the CHR-PS+ group was similar to those reported in other large-scale investigations of psychosis. Additionally, a subset of these regions displayed abnormal age associations. Widespread disruptions in CT coupled with abnormal age associations in those at CHR may point to disruptions in postnatal brain developmental processes.
Introduction
The clinical high-risk (CHR) paradigm is a widely used framework to investigate mechanisms underlying psychosis vulnerability. Help-seeking individuals who do not meet diagnostic criteria for a psychotic disorder but typically present with subthreshold psychotic symptoms and accumulating risk factors are considered at CHR for developing psychosis.1 An estimated 18% to 20% of individuals at CHR develop a psychotic disorder within 2 years of identification,2 although conversion rates vary, likely due to heterogeneous recruitment and sampling strategies as well as interventions applied.3 However, despite decades of research, the nature of morphometrical differences associated with psychosis conversion remains largely unknown. Here, we aim to address this question by combining all available structural neuroimaging data in CHR to date in an attempt to better understand group differences associated with psychosis risk and conversion in this population.
A large body of work has used structural magnetic resonance imaging (sMRI) to investigate morphometric brain differences in individuals at CHR.4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20 However, the extent to which characteristic baseline (ie, when participants are initially ascertained and assessed at a first study visit) structural neuroimaging differences exist between those at CHR who later develop a psychotic disorder (CHR-PS+) compared with those who do not (CHR-PS−) is debated. Many studies failed to find baseline differences between these 2 groups,4,14,21,22 although a meta-analysis16 and multicenter study23 found lower prefrontal and temporal volumes or cortical thickness measured by MRI (which we will refer to as CT) in individuals at CHR who developed a psychotic disorder. High attrition rates in samples of individuals at CHR24 coupled with low psychosis conversion rates2,25 often yielded insufficient power to detect between-group structural brain differences. Moreover, small sample sizes can be associated with inflated effect sizes,26 so effect sizes of prior studies that found structural brain differences in individuals at CHR may be overestimated. Although multisite consortia aim to address these challenges, to our knowledge, the largest published sMRI studies to date included fewer than 50 individuals at CHR who later developed a psychotic disorder.21,23 Furthermore, it is currently unknown whether group differences are robust enough to predict outcomes.
Importantly, many participants at CHR are adolescents or young adults, a time frame associated with psychosis onset.27,28 Prefrontal-temporal brain regions, which are typically implicated in psychosis, show protracted developmental courses continuing through adolescence,29,30 suggesting that morphometric differences associated with psychosis risk vary with age. Indeed, there are developmental influences on psychotic symptom presentation,31 perhaps driven by differences in regional brain changes. It is not fully understood how age-related patterns in brain morphometry in individuals at CHR differ from normal development. Thus, using a developmental framework to examine whether morphometric differences in individuals at CHR are influenced by age may provide important insights into mechanisms associated with psychosis risk and the stability of neuroimaging measures associated with psychosis risk across development.
Finally, to our knowledge, it is unknown whether baseline brain differences associated with future conversion to psychosis resemble those observed in other large-scale psychosis studies. Understanding whether morphometric differences in individuals at CHR overlap with those observed in individuals who have schizophrenia32,33 and individuals with a genetic subtype of psychosis34,35 will provide insights into convergent or distinct differences across the psychosis spectrum.
To address these questions, we founded the Enhancing Neuro Imaging Genetics Through Meta-Analysis (ENIGMA) Clinical High Risk for Psychosis Working Group in 2018. Using baseline sMRI data and longitudinal clinical information from 31 sites, this study addressed the following questions:
Do participants at CHR and healthy controls differ in CT, surface area (SA), and/or subcortical volumes?
Is there a neuroanatomic signature associated with future transition to a psychotic disorder (CHR-PS+ group vs CHR-PS− group vs control group)?
Do structural neuroimaging measures identified in aims 1 and 2 display group differences in age associations suggestive of abnormal developmental trajectories?
Is the pattern of morphometric alteration associated with psychosis conversion similar to that observed in other ENIGMA studies of psychosis?
Methods
Participants
We included 1792 individuals at CHR, including 253 in the CHR-PS+ group, 1234 in the CHR-PS− group, and 305 without follow-up data, and 1377 healthy controls from 31 sites participating in the ENIGMA Clinical High Risk for Psychosis Working Group (Table). Participants met the Comprehensive Assessment of At-Risk Mental States (CAARMS; n = 821) or Structured Interview for Prodromal Syndromes (SIPS; n = 971) CHR criteria (eMethods in the Supplement). Site-specific inclusion and exclusion criteria are detailed in eTable 1 in the Supplement. All sites obtained local institutional review board approval prior to data collection. Informed written consent was obtained from every participant or the participant’s guardian for participants younger than 18 years. All studies were conducted in accordance with the Declaration of Helsinki.36
Table. Age and Sex Information for Healthy Controls and Participants at Clinical High Risk (CHR) for Psychosis at Each Site.
| Sitea | Control group | Individuals at CHR | CHR-PS+ groupb | CHR-PS− groupb | Transition rate, % | Follow-up length, mean (SD), mo | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Female, No. (%) | Age, mean (SD; range), y | No. | Female, No. (%) | Age, mean (SD; range), y | No. | Female, No. (%) | Age, mean (SD; range), y | No. | Female, No. (%) | Age, mean (SD; range), y | |||
| Amsterdam | 23 | 13 (57) | 23.4 (2.8; 19-32) | 16 | 10 (62) | 23.6 (2.5; 20-30) | 0 | NAc | NAc | 16 | 10 (62) | 23.6 (2.5; 20-30) | NAc | 24.0 (0) |
| Barcelona-HSJD | 44 | 18 (41) | 15.6 (1.6; 13-18) | 55 | 32 (58) | 15.2 (1.6; 11-18) | 7 | 3 (43) | 15.7 (2.4; 12-18) | 35 | 23 (66) | 15.2 (1.5; 11-18) | 16.7 | 17.4 (4.8) |
| Bern | 17 | 6 (35) | 20.2 (6.1; 13-34) | 43 | 22 (51) | 18.5 (5.1; 10-35) | 0 | NAc | NAc | 19 | 11 (58) | 16.8 (3.8; 10-26) | NAc | 15.8 (5.7) |
| Columbia1 | 9 | 2 (22) | 24.4 (4.1; 20-33) | 17 | 9 (53) | 22.9 (5.0; 15-31) | 3 | 2 (67) | 19.4 (7.3; 15-28) | 14 | 7 (50) | 23.6 (4.3; 16-31) | 17.6 | 20.6 (10.3) |
| Columbia2 | 15 | 9 (60) | 26.2 (2.2; 22-30) | 19 | 7 (37) | 23.3 (3.6; 18-31) | NAc | NAc | NAc | NAc | NAc | NAc | NAc | NAc |
| Columbia3 | 37 | 12 (32) | 22.9 (3.6; 15-30) | 58 | 15 (26) | 21.0 (4.0; 14-29) | 14 | 4 (29) | 21.1 (4.8; 15-29) | 44 | 11 (25) | 20.9 (3.8; 14-27) | 24.1 | 24.0 (0) |
| Copenhagen | 59 | 29 (49) | 24.8 (3.3; 20-35) | 163 | 86 (52.8) | 24.2 (4.2; 18-38) | 13 | 5 (38) | 23.1 (3.4; 18-29) | 95 | 54 (57) | 24.5 (4.6; 18-38) | 12.0 | 11.8 (1.2) |
| CSU | 59 | 25 (42) | 21.5 (3.1; 15-30) | 52 | 24 (46) | 19.5 (5.0; 13-35) | 21 | 12 (57) | 19.4 (5.1; 13-35) | 31 | 12 (39) | 19.5 (5.0; 13-30) | 40.4 | 14.7 (7.6) |
| Glasgow | 46 | 30 (65) | 22.8 (3.6; 18-32) | 80 | 63 (79) | 22.2 (4.8; 17-34) | 6 | 5 (83) | 18.5 (1.9; 17-22) | 74 | 58 (78) | 22.5 (4.8; 17-34) | 7.5 | 20.2 (9.9) |
| Heidelberg | 33 | 16 (48) | 15.7 (0.9; 14-17) | 22 | 13 (59) | 15.1 (1.1; 14-17) | 0 | NAc | NAc | 18 | 12 (67) | 15.1 (1.0; 14-17) | NAc | 9.7 (3.3) |
| IDIBAPS | 54 | 35 (65) | 15.9 (1.6; 11-18) | 74 | 49 (66) | 15.3 (1.7; 10-18) | 17 | 11 (65) | 15.1 (1.4; 13-17) | 42 | 28 (67) | 15.5 (1.9; 10-18) | 28.8 | 14.3 (5.5) |
| ISMMS | 12 | 5 (42) | 27.9 (3.7; 23-35) | 25 | 13 (52) | 23.4 (5.4; 17-35) | 1 | 0 | 26.3 (0; 26-26) | 12 | 7 (58) | 23.6 (5.5; 17-35) | 7.7 | 6.0 (0) |
| London | 29 | 10 (34) | 24.5 (4.7; 20-36) | 81 | 25 (31) | 22.6 (4.4; 18-38) | 6 | 0 | 24.0 (4.4; 18-29) | 62 | 20 (32) | 22.3 (4.6; 18-38) | 8.8 | 18.8 (8.7) |
| Maastricht | 38 | 12 (32) | 25.6 (5.7; 18-39) | 48 | 14 (29) | 20.2 (4.1; 12-29) | 6 | 2 (33) | 20.5 (5.2; 15-26) | 25 | 8 (32) | 18.6 (3.4; 12-27) | 19.4 | NAc |
| Melbourne | 92 | 42 (46) | 21.9 (3.6; 15-30) | 249 | 124 (49.8) | 19.7 (3.4; 14-29) | 71 | 31 (44) | 19.5 (3.5; 14-29) | 165 | 85 (51.5) | 19.8 (3.4; 14-28) | 30.1 | 71.4 (53.7) |
| Mexico City | 38 | 10 (26) | 20.9 (3.4; 15-28) | 33 | 7 (21) | 19.5 (4.1; 13-31) | 7 | 2 (29) | 20.4 (6.0; 15-31) | 26 | 5 (19) | 19.3 (3.6; 13-27) | 21.2 | 24.0 (0) |
| MHRC | 51 | 0 | 22.2 (2.8; 16-27) | 38 | 0 | 20.1 (2.5; 16-28) | 3 | 0 | 19.0 (2.4; 16-21) | 35 | 0 | 20.2 (2.5; 17-28) | 7.9 | 30.2 (6.6) |
| MPRC | 20 | 8 (40) | 17.8 (4.3; 12-24) | 31 | 16 (52) | 17.0 (3.2; 12-22) | 3 | 1 (33) | 16.0 (4.4; 13-21) | 10 | 5 (50) | 15.8 (2.7; 12-20) | 23.1 | 7.1 (1.1) |
| Newcastle | 17 | 13 (76) | 20.3 (1.9; 17-24) | 45 | 24 (53) | 19.6 (2.2; 15-24) | 1 | 0 | 17.4 (0; 17-17) | 41 | 22 (54) | 19.6 (2.2; 15-24) | 2.4 | 21.5 (12) |
| Oslo region | 63 | 24 (38) | 19.8 (3.6; 15-29) | 21 | 8 (38) | 19.9 (3.6; 16-29) | 2 | 1 (50) | 20.0 (5.4; 16-24) | 18 | 7 (39) | 19.9 (3.7; 16-29) | 10.0 | 12.5 (1.4) |
| Pitt | 65 | 26 (40) | 23.0 (5.9; 14-40) | 26 | 14 (54) | 20.8 (5.3; 12-36) | 2 | 1 (50) | 17.1 (0.8; 16-18) | 11 | 7 (64) | 19.5 (6.4; 12-36) | 15.4 | 13.1 (3.6) |
| RUMC | 29 | 19 (66) | 23.9 (3.2; 18-30) | 67 | 31 (46) | 22.7 (3.9; 16-30) | 4 | 2 (50) | 24.5 (2.9; 21-27) | 60 | 28 (47) | 22.6 (3.9; 16-30) | 6.2 | 8.3 (4.5) |
| Singapore | 53 | 25 (47) | 22.0 (4.2; 14-30) | 100 | 32 (32.0) | 21.9 (3.6; 14-30) | 11 | 3 (27) | 20.1 (3.1; 15-26) | 88 | 28 (32) | 22.1 (3.6; 14-30) | 11.1 | 18.9 (5.9) |
| SNUH | 74 | 24 (32) | 21.2 (2.5; 17-27) | 74 | 19 (26) | 20.7 (3.8; 15-34) | 9 | 3 (33) | 22.0 (5.0; 16-33) | 46 | 11 (24) | 20.4 (3.7; 15-34) | 16.4 | 30.9 (17.2) |
| Stavanger | 33 | 16 (48) | 17.0 (3.1; 13-27) | 37 | 22 (59) | 16.6 (2.4; 13-25) | 5 | 4 (80) | 16.2 (2.7; 13-19) | 31 | 18 (58) | 16.7 (2.3; 14-25) | 13.9 | 24.0 (0) |
| Toho | 16 | 8 (50) | 23.2 (2.9; 18-28) | 40 | 28 (70) | 23.7 (6.9; 13-39) | 4 | 3 (75) | 19.0 (4.4; 14-24) | 36 | 25 (69) | 24.2 (7.0; 13-39) | 10.0 | 12.0 (0) |
| Tokyo | 25 | 12 (48) | 22.1 (2.8; 16-25) | 39 | 18 (46) | 20.9 (3.5; 14-29) | 3 | 0 | 22.7 (4.7; 19-28) | 25 | 15 (60) | 20.5 (3.1; 14-27) | 10.7 | 26.5 (12.1) |
| Toronto | 39 | 16 (41) | 25.5 (5.2; 18-38) | 27 | 12 (44) | 20.8 (1.8; 18-27) | 0 | NAc | NAc | 4 | 2 (50) | 19.7 (1.1; 18-21) | NAc | 24.0 (0) |
| Toyama | 141 | 67 (47.5) | 25.1 (4.2; 18-38) | 79 | 35 (44) | 18.5 (4.0; 13-31) | 10 | 3 (30) | 20.8 (5.7; 15-31) | 69 | 32 (46) | 18.1 (3.7; 13-30) | 12.7 | 37.4 (34.2) |
| UCSF | 103 | 43 (41.7) | 23.7 (7.5; 13-40) | 71 | 34 (48) | 19.3 (4.4; 12-32) | 13 | 5 (38) | 21.4 (4.4; 16-29) | 47 | 23 (49) | 19.0 (4.1; 12-29) | 21.7 | 21.0 (5.4) |
| Zurich | 43 | 22 (51) | 22.2 (5.6; 13-36) | 62 | 25 (40) | 19.1 (4.9; 13-35) | 11 | 2 (18) | 18.5 (3.4; 14-24) | 35 | 14 (40) | 19.1 (5.3; 13-35) | 23.9 | 20.3 (12.5) |
Abbreviations: NA, not available; CHR-PS+, individuals at CHR who later developed a psychotic disorder; CHR-PS−, individuals at CHR who did not later develop a psychotic disorder.
Site name abbreviations as follows: Amsterdam, Vrije Universiteit Amsterdam, Amsterdam, the Netherlands; Barcelona-HSJD, Hospital Sant Joan de Déu Barcelona, Barcelona, Spain; Bern, University Hospital of Child and Adolescent Psychiatry and Psychotherapy, University of Bern, Bern, Switzerland; Columbia1, New York State Psychiatric Institute, Columbia University, New York; Columbia2, New York State Psychiatric Institute, Columbia University; Columbia3, New York State Psychiatric Institute, Columbia University; Copenhagen, Mental Health Center Copenhagen and CINS, Mental Health Center Glostrup, University of Copenhagen, Copenhagen, Denmark; CSU, Central South University, Changsha, China; Glasgow, Institute of Neuroscience and Psychology, University of Glasgow, Glasgow, Scotland; Heidelberg, Heidelberg University Hospital, Heidelberg, Germany; IDIBAPS, August Pi I Sunyer Biomedical Research Institute, Barcelona, Spain; ISMMS, Icahn School of Medicine at Mount Sinai, New York, New York; London, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, United Kingdom; Maastricht, Maastricht University, Maastricht, the Netherlands; Melbourne, University of Melbourne, Melbourne, Australia; Mexico City, Instituto Nacional de Neurología y Neurocirugía, Mexico City, Mexico; MHRC, Mental Health Research Center Moscow, Moscow, Russia; MPRC, Maryland Psychiatric Research Center, University of Maryland School of Medicine, Baltimore; Newcastle, University of Newcastle, Newcastle, Australia; Oslo region, NORMENT, University of Oslo and Oslo University Hospital, Oslo, Norway; Pitt, University of Pittsburgh, Pittsburgh, Pennsylvania; RUMC, Rush University Medical Center, Chicago, Illinois; Singapore, Institute of Mental Health and National University of Singapore, Singapore; SNUH, Seoul National University, Seoul, Republic of Korea; Stavanger, Stavanger University Hospital, Stavanger, Norway; Toho, Department of Neuropsychiatry, Toho University School of Medicine, Tokyo, Japan; Tokyo, Department of Neuropsychiatry, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan; Toronto, Centre for Addiction and Mental Health, University of Toronto, Toronto, Ontario, Canada; Toyama, University of Toyama Graduate School of Medicine and Pharmaceutical Sciences, Toyama, Japan; UCSF, University of California, San Francisco; Zurich, Psychiatric Hospital, University of Zurich, Zurich, Switzerland. Additional site details can be found in eTable 1 in the Supplement.
Values for the CHR-PS+ and CHR-PS− groups do not always sum to the number of individuals at CHR at each site because there were individuals at CHR without follow-up data (n = 305).
Data were not available either because no individuals at CHR transitioned to a psychotic disorder or because follow-up data were not available.
Image Acquisition and Processing
A total of 31 sites contributed T1-weighted MRI brain scans from 50 MRI scanners, including 42 3-T scanners and 8 1.5-T scanners (eTable 2 in the Supplement). Scanners were manufactured by Siemens (n = 23), Philips (n = 8), GE (n = 18), and Toshiba (n = 1). A breakdown of the number of scans obtained for those in the CHR-PS+, CHR-PS−, and control groups for each scanner is reported in eTable 3 in the Supplement. After processing the data using Freesurfer analysis software (eTable 2 in the Supplement),37,38,39 we extracted 68 CT, 68 SA, and 16 subcortical volume measures. We also examined 3 global neuroimaging measures—total intracranial volume (ICV), mean CT, and total SA—resulting in 155 neuroimaging measures. We implemented the ENIGMA consortium quality assessment pipeline.32,33,34,35,40,41 A priori power calculations are included in eMethods in the Supplement.
Statistical Analyses
Group-Related and Conversion-Related Differences in sMRI Metrics
We assessed group differences using general linear models (GLMs) within a mega-analysis framework, with each sMRI measure (ie, CT, SA, or subcortical volume) as the dependent variable and group (CHR or healthy control) or conversion status (CHR-PS+, CHR-PS−, or control) as the independent variable. We included age, age2, sex, and estimated total ICV as covariates in all models and corrected for multiple comparisons (n = 155) using the false discovery rate method.42 q Values less than .05 were considered statistically significant. Significance for P values for minimal-effects testing was set at P < .05 and were 1-tailed; all other P and q values were 2-tailed. All analyses were conducted using R version 3.6.3 (The R Foundation).
For all structural neuroimaging measures, we calculated Cohen d effect sizes from the GLMs between 2 (individuals at CHR vs healthy controls) or 3 groups of interest (CHR-PS+ group vs control group; CHR-PS+ group vs CHR-PS− group; CHR-PS− group vs control group). Based on recent work demonstrating that neuroComBat harmonization increases statistical power within a mega-analytic framework,43 primary analyses were conducted within a mega-analysis framework using data that were corrected for site and scanner associations using neuroComBat harmonization. Additional analyses were conducted to assess the robustness of results obtained using this approach (eMethods in the Supplement). For all neuroimaging measures, we investigated sMRI differences associated with the specific psychosis-risk syndromes (eg, attenuated positive symptom syndrome) (eMethods in the Supplement).
To evaluate the stability of group and conversion status differences, we performed analyses statistically controlling for baseline psychotropic medication exposure. To assess site effects, we conducted jackknife resampling analyses, ie, iteratively removing one site’s data and rerunning respective analyses.44 sMRI measures that failed to show a group or conversion status association at a q value less than .05 in more than 10% jackknife iterations (ie, 4 of 31 sites) were considered unstable.
To assess the meaningfulness of obtained effect sizes, we used 2 analytic approaches: equivalence testing (to assess whether observed differences fell within the upper and lower bounds of a predefined smallest effect size of interest, providing support for the absence of a meaningful effect) and minimal-effects testing (to assess whether observed effects were greater than the same predefined effect size).45 Upper and lower bounds (representing the positive and negative predefined smallest effect size of interest) were set to a Cohen d of 0.15 and −0.15, respectively (eMethods in the Supplement).
Group and Conversion-Related Differences in sMRI Age Associations
We used general additive models (GAMs)46,47 to model group and conversion status differences in the association between age and sMRI measures (eMethods in the Supplement). First, we examined the interaction between group (individuals at CHR vs healthy controls) and age in the 56 neuroimaging measures that differed at a q value less than .05 between healthy controls and individuals at CHR. Next, we conducted GAM analyses on the 4 sMRI measures on which the CHR-PS+, CHR-PS−, and control groups differed from each other (ie, left paracentral CT, right paracentral CT, left fusiform CT, right superior temporal CT) in analyses of psychosis conversion. We examined the associations of baseline age and group/conversion status as well as the interaction between the 2 variables. Sex and estimated ICV were included as covariates. Similar to previous work examining age associations during adolescent development,48,49 we restricted our sample’s age range to 12 to 25 years (eTable 4 in the Supplement). Details on post hoc analyses for significant interaction associations are provided in the eMethods in the Supplement.
Comparison of Psychosis Conversion Effect Sizes With Findings of Other ENIGMA Studies
We computed Spearman rank correlations to assess the extent to which the pattern of observed effect sizes (Cohen d for CHR-PS+ and CHR-PS− groups vs control group) correlated with the pattern found in prior psychosis studies, specifically the ENIGMA Schizophrenia Working Group (individuals with schizophrenia vs healthy controls)32,33 and ENIGMA 22q11.2 Deletion Syndrome Working Group (individuals with 22q11.2 deletion syndrome with psychosis vs those with 22q11.2 deletion syndrome without psychosis).34,35 As a control, we compared the effect sizes of the CHR-PS+ group and CHR-PS− group vs control group with the effect sizes of the major depressive disorder (MDD) group vs control group published by the ENIGMA Major Depressive Disorder Working Group (eMethods in the Supplement).40,41
Results
Sample Characteristics
Of the 3169 included participants, 1428 (45.1%) were female, and the mean (SD; range) age was 21.1 (4.9; 9.5-39.9) years (Table). Intelligence quotient (IQ) comparisons between healthy controls and individuals at CHR are reported in eTable 5 in the Supplement. Within each site, baseline IQ measures were largely similar in all participants at CHR, including those in the CHR-PS+ group, CHR-PS− group, and participants at CHR without follow-up information (eTable 6 in the Supplement). For symptom measures, participants at CHR without follow-up data had less severe baseline positive, negative, and disorganized symptoms on the SIPS compared with participants in the CHR-PS+ and CHR-PS− groups (eTable 7 in the Supplement). Compared with the CHR-PS+ and CHR-PS− groups, those without follow-up data had less severe cognitive changes on the CAARMS (eTable 7 in the Supplement). Few participants at CHR reported typical (less than 1%) and/or atypical (12.4%) antipsychotic medication use (eTable 8 in the Supplement).
CT in Participants at CHR vs Healthy Controls
In neuroComBat-harmonized GLM mega-analyses, participants at CHR had smaller global neuroimaging measures compared with healthy controls (estimated ICV: Cohen d = −0.13; 95% CI, −0.20 to −0.06; mean CT: Cohen d = −0.18; 95% CI, −0.25 to −0.11; total SA: Cohen d = −0.15; 95% CI, −0.22 to −0.08). We also observed significant group associations in 53 additional GLMs (eTable 9 in the Supplement). The largest group associations were observed for widespread lower CT in individuals at CHR vs healthy controls (42 of 68 comparisons; Cohen d range, −0.17 to −0.09) (Figure 1A; eTable 9 and eFigure 1 in the Supplement). Few subcortical (3 of 16) and SA (8 of 68) group differences were observed. No group × sex interactions were detected.
Figure 1. Effect Sizes for Mega-analysis of Group and Conversion Status.

A, The top row reflects the results of the overall general linear model. A deeper purple indicates a greater group association (healthy controls vs individuals at clinical high risk [CHR]) in this region. We observed the greatest group associations in cortical thickness measures. The second row indicates the pairwise effect sizes for healthy controls vs individuals at CHR in regions that were statistically significant (q < .05) in the overall comparison (top row). Regions that were not statistically significant in the overall comparison are gray. Compared with healthy controls, individuals at CHR exhibited lower cortical thickness across the cortex. Red indicates that healthy controls had a larger value compared with individuals at CHR for this region. B, The top row reflects the results of the overall general linear model. A deeper purple indicates a greater conversion status association (control group vs individuals at CHR who later developed a psychotic disorder [CHR-PS+] vs individuals at CHR who did not later develop a psychotic disorder [CHR-PS−]) in this region. The second and third rows indicate the pairwise effect sizes for the control group vs CHR-PS+ group and CHR-PS− group vs CHR-PS+ group, respectively. Pairwise comparisons are presented in regions that were statistically significant (q < .05) in the overall comparison (top row). Regions that were not statistically significant in the overall comparison are gray. Regions in which the CHR-PS+ group had lower cortical thickness compared with the control group and the CHR-PS− group are highlighted in yellow.
We present results of possible confound analyses, including ICV, medication and site associations, equivalence testing, and results of neuroComBat harmonization in eTables 10 to 13 and eFigures 1 and 2 in the Supplement. No sMRI measures were uniquely sensitive to psychosis-risk syndrome (eResults and eTables 14 to 17 in the Supplement).
Association of Paracentral, Fusiform, and Superior Temporal CT With Psychosis Conversion
A total of 48 structural neuroimaging measures exhibited a significant overall association with psychosis conversion status in GLM mega-analyses using neuroComBat harmonized data (Figure 1B; eTable 18 and eFigure 3 in the Supplement). Most significant differences were observed for CT measures (n = 37). Within these 48 regions, we conducted pairwise GLMs between the CHR-PS+ and control groups, CHR-PS− and control groups, and CHR-PS+ and CHR-PS− groups. Of these 48 regions, the CHR-PS+ group differed from the CHR-PS− and control groups on 4 neuroimaging measures.
Compared with the control and CHR-PS− groups, the CHR-PS+ group exhibited lower CT in bilateral paracentral, right superior temporal, and left fusiform regions (mean Cohen d of 4 sMRI measures = −0.22; 95% CI, −0.35 to 0.10). Similar findings were observed for the left superior temporal and right fusiform regions. Moreover, the CHR-PS+ and CHR-PS− groups exhibited thinner cortex in bilateral paracentral, superior temporal, and fusiform regions compared with the control group (Figure 2). Using minimal-effects testing, we observed that effect sizes for bilateral paracentral (left hemisphere: z = −2.43; P = .02; right hemisphere: z = −1.86; P = .06), right superior temporal (z = −2.29; P = .02), and left fusiform (z = −2.00; P = .05) in the CHR-PS+ group vs the control group were all greater than 0.15, except for the right paracentral region, underscoring the presence of notable group differences.
Figure 2. Bar Graphs for Regions in Which Individuals at Clinical High Risk Who Later Developed a Psychotic Disorder (CHR-PS+) Had Lower Cortical Thickness Compared With Those Who Did Not Later Develop a Psychotic Disorder (CHR-PS−) and Healthy Controls.
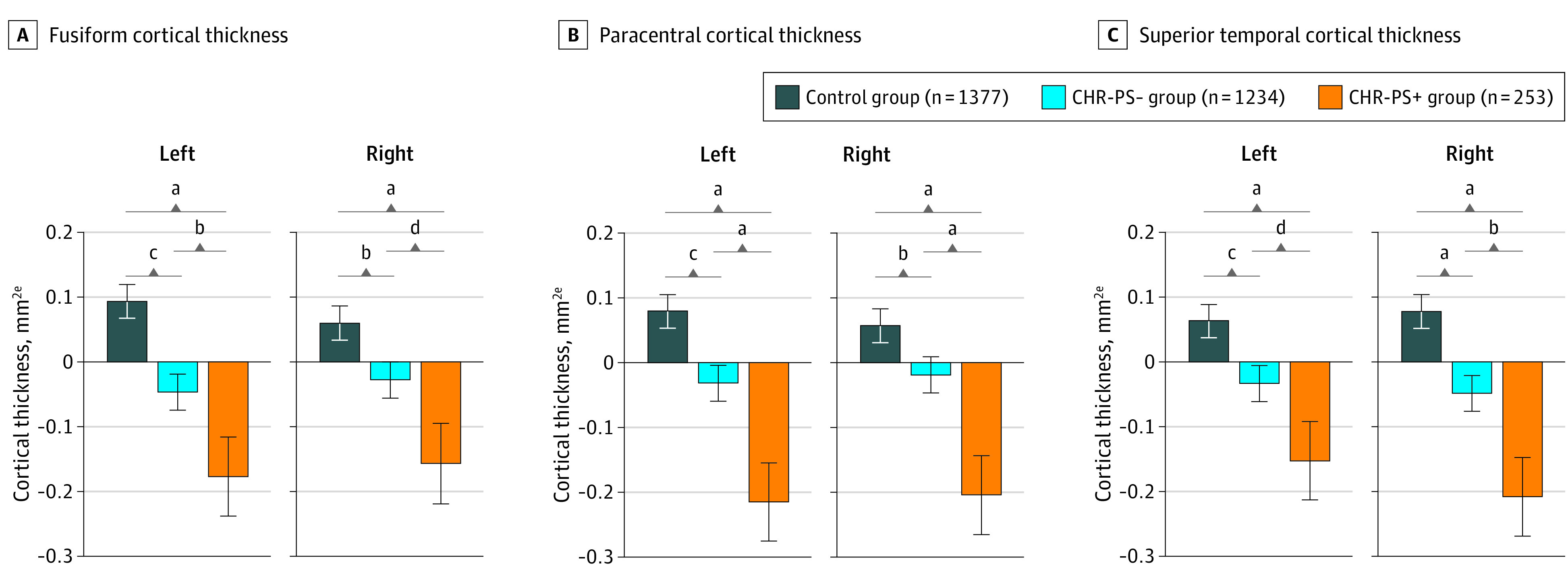
aP ≤ .001.
bP < .05.
cP ≤ .01.
dP < .10.
eCortical thicknesses normalized to a mean of 0.
In all remaining comparisons of regions that exhibited a statistically significant association with psychosis conversion status, the CHR-PS+ and CHR-PS− groups significantly differed from the control group at P < .05. However, the CHR-PS+ group did not differ from the CHR-PS− group in any remaining comparisons (eTable 18 in the Supplement). We observed no conversion status × sex interactions, and results remained stable when length of follow-up period was included as a covariate.
We present results of confound analyses (medication, site associations, equivalence testing) in the eResults, eTables 19 to 21, and eFigure 2 in the Supplement. There were no statistically significant psychosis risk syndrome × conversion status interactions (eResults and eTable 22 in the Supplement).
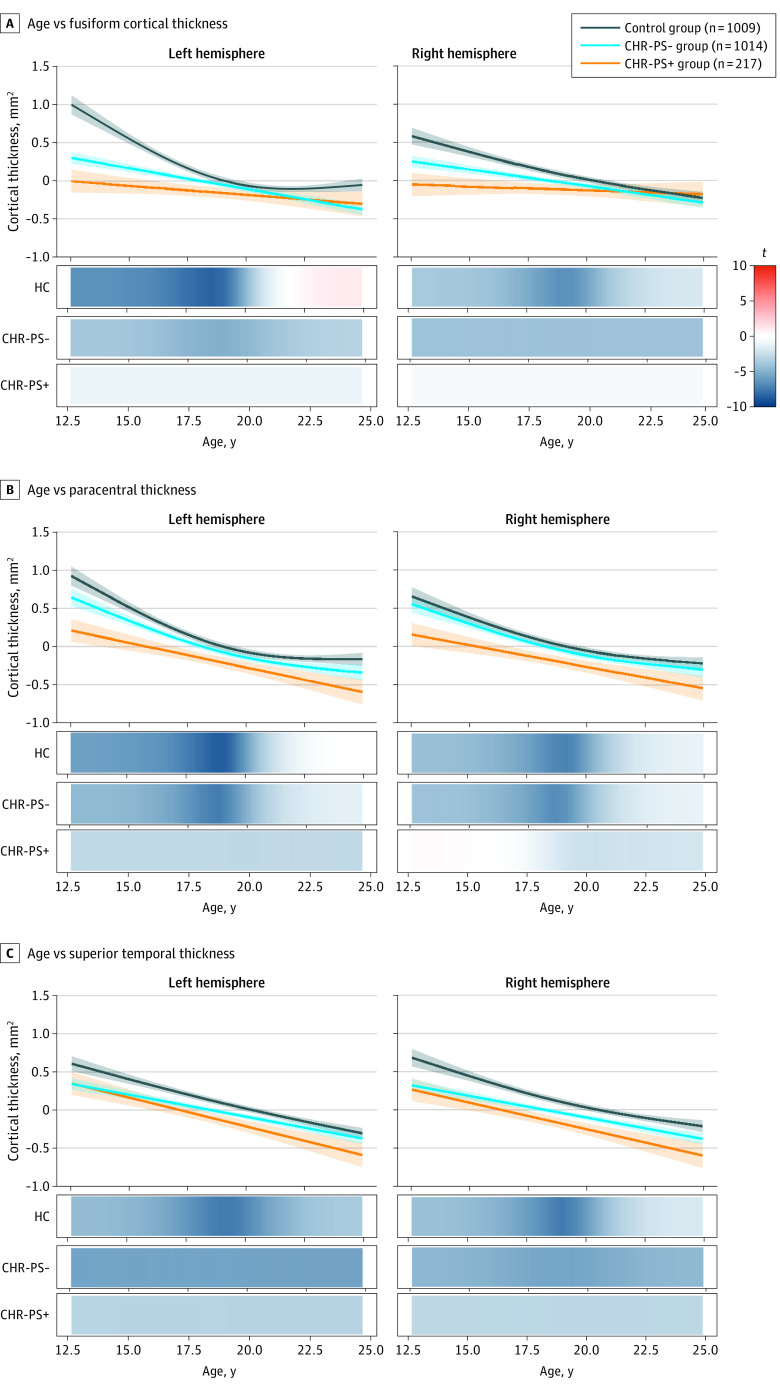
Age Associations in CHR-PS+ and CHR-PS− Groups Compared With Control Group
In GAM analyses, we observed no statistically significant group × age interactions for the 56 neuroimaging measures that differed between individuals at CHR and healthy controls (eTable 23 in the Supplement). We then conducted GAM analyses on the 4 sMRI measures on which the CHR-PS+ group displayed lower CT compared with the CHR-PS− and control groups in psychosis conversion group analyses. These sMRI measures were left paracentral CT, right paracentral CT, left fusiform CT, and right superior temporal CT. Two measures displayed a significant psychosis conversion status × age interaction. For each group × age interaction analysis, we assessed group differences in age associations (ie, CHR-PS+ group vs control group, CHR-PS− group vs control group, and CHR-PS+ group vs CHR-PS− group).
In left fusiform, the association of age with CT differed between the CHR-PS+ group and control group (F = 9.8; P < .001; q < .001) and the CHR-PS− group and control group (F = 8.7; P < .001; q < .001) (Figure 2A) but not between the CHR-PS+ group and CHR-PS− group (F = 1.3; P = .31; q = .45). Between ages 12 and 16 years, those in the control group showed a stronger negative association of age with CT compared with those in the CHR-PS+ and CHR-PS− groups. Although the interaction was not statistically significant, a similar pattern emerged for the right fusiform CT (Figure 3A; eTable 24 in the Supplement).
Figure 3. Age Associations of Regions That Exhibited an Association of Conversion Status.
Each graph has a line of best fit for the association of age with the respective neuroimaging measures. Shading around the line indicates the SE. The bars underneath the age plots reflect the derivative of the slope, ie, the rate of change taking place at a particular age, scaled as a pseudo t statistic, based on the posterior simulation. CHR-PS+ indicates individuals at clinical high risk who later developed a psychotic disorder; CHR-PS−, individuals at clinical high risk who did not later develop a psychotic disorder.
Age associations in the left paracentral CT differed between the CHR-PS+ group and the control group (F = 5.9; P = .005; q = .02) (Figure 2B) but not between the CHR-PS− group and control group (F = 0.2; P = .68; q = .74) or the CHR-PS+ group and CHR-PS− group (F = 1.9; P = .18; q = .45). Among individuals aged 12 to 15.8 years, those in the control group showed a stronger negative association of age with CT compared with those in the CHR-PS+ group. The association of age with CT did not differ between the CHR-PS− group and control group (F = 0.2; P = .69; q = .74). This pattern of results was not observed for the right paracentral CT (Figure 3B; eTable 24 in the Supplement). We found no significant age × conversion status interactions for the superior temporal CT (Figure 3C; eTable 24 in the Supplement); all groups showed negative associations of age with CT.
CT in the ENIGMA CHR Group Compared With Other ENIGMA Working Groups
eFigure 4 in the Supplement provides a visual overview of CT differences in the CHR-PS+ group, individuals with schizophrenia (using published data from the ENIGMA Schizophrenia Working Group32,33), and individuals with 22q11.2 deletion syndrome and a psychotic disorder (using published data from the ENIGMA 22q11.2 Deletion Syndrome Working Group34,35).
The overall pattern of baseline CT differences in the CHR-PS+ group relative to the control group was significantly correlated with that observed in individuals with schizophrenia (ρ = 0.35; 95% CI, 0.12 to 0.55; P = .004) and in individuals with 22q11.2 deletion syndrome and psychosis (ρ = 0.43; 95% CI, 0.20 to 0.61; P = .001) (eFigure 4 in the Supplement). CT differences in the CHR-PS+ group relative to the control group were not correlated with CT differences observed in individuals with MDD (ρ = −0.03), and the slopes for the correlation between (1) CT differences in the CHR-PS+ group and individuals with schizophrenia and (2) CT differences in the CHR-PS+ group and individuals with MDD were significantly different (Steiger z = 2.06; P = .008).
No significant correlations were observed for SA (schizophrenia: ρ = −0.03; 22q11.2 deletion syndrome with psychosis: ρ = −0.06) (eFigures 5 and 6 in the Supplement). Subcortical volume differences in the CHR-PS+ group relative to the control group were correlated with those observed in individuals with schizophrenia (ρ = 0.54; P = .03). A similar nonsignificant correlation was observed for the correlation analysis involving individuals with 22q11.2 deletion syndrome and psychosis (ρ = 0.46; P = .07) (eFigures 5 and 6 in the Supplement). Associations involving CHR-PS− vs control CT, SA, and subcortical volume differences were similar to those reported here (eResults in the Supplement).
Discussion
We conducted, to our knowledge, the largest multisite neuroimaging investigation to date in participants at CHR, examining baseline structural neuroimaging measures associated with later transition to psychosis. We found widespread lower CT in individuals at CHR, consistent with previously reported CT differences in individuals with an established psychotic disorder. Compared with those in the CHR-PS− and control groups, at baseline, those in the CHR-PS+ group exhibited thinner cortex in bilateral paracentral, right fusiform, and left superior temporal regions, with effect sizes significantly greater than what we considered to be meaningful a priori. Our results were robust to associations of medication exposure, sex, site, and length of follow-up period. Findings from this international effort suggest that conversion to psychosis among those at CHR is associated with lower CT at baseline.
We identified widespread regional lower CT in individuals at CHR compared with healthy controls. Lower CT has been observed in individuals with schizophrenia as well as other psychiatric disorders.32,40,50 Importantly, the overall pattern of lower CT in those in the CHR-PS+ and CHR-PS− groups resembled that observed in individuals with schizophrenia and individuals with 22q11.2 deletion syndrome and a psychotic disorder but not in individuals with MDD. For the CHR-PS+ group, correlations with CT differences in individuals with schizophrenia were significantly greater than the association observed with CT differences in individuals with MDD. Taken together, our results suggest that the overall constellation of reported CT differences in individuals at CHR resembles the general pattern of CT differences observed in individuals with schizophrenia and genetic disorders associated with psychosis and thus suggest that widespread thinner cortex in individuals at CHR may be associated with their increased risk of psychosis.
We also found that lower CT in paracentral, superior temporal, and fusiform regions was associated with psychosis conversion; individuals in the CHR-PS+ group exhibited significantly lower CT than those in the CHR-PS− group and control group in these regions. Lower baseline CT and/or volume in these regions has previously been reported in individuals at CHR who later developed a psychotic disorder17,18 (data not used here). Furthermore, longitudinal CT decreases in these regions have been associated with transition to psychosis in those at CHR.6,19,20 The magnitude of altered CT in individuals in the CHR-PS+ group in the paracentral, superior temporal, and fusiform regions was highly consistent with findings in individuals with schizophrenia,33,51,52 and lower fusiform and paracentral CT has been observed in individuals who hear voices but do not have a diagnosis of schizophrenia.53 Given that both help-seeking and non–help-seeking individuals on the psychosis spectrum exhibit alterations in these regions, CT in the paracentral, superior temporal, and fusiform areas may display a dose-response association with psychosis risk. While this interpretation also aligns with our observation that CT in these regions differed between the CHR-PS+, CHR-PS−, and control groups (with the lowest CT for those in the CHR-PS+ group), this explanation remains speculative in light of the cross-sectional nature of the data.
Consistent with previous CHR studies examining baseline neuroimaging associations with later conversion to psychosis,17 we did not observe widespread subcortical volume or SA differences associated with later psychosis transition. Taken together, these results suggest that CT reductions may be among the most widespread, robust, and specific morphometric changes associated with psychosis risk and conversion compared with SA or subcortical volume.
An intriguing pattern of findings emerged from the psychosis conversion × age analyses. Compared with the control group, the CHR-PS+ and CHR-PS− groups exhibited significantly lower paracentral and fusiform region CT among those aged 12 to 16 years. Our analyses investigating age-associated rates of change (estimated using cross-sectional data) seemed to indicate a steeper decline in slope for those in the control group during this time frame, which reached a plateau in adulthood. However, those in the CHR-PS− group displayed a slower decline, and results in the CHR-PS+ group were indicative of a reduced or delayed rate of change. Relative to the normative timetable in healthy controls, these findings may suggest an accelerated developmental decrease in paracentral and/or fusiform CT in the CHR-PS+ and CHR-PS− groups, with the greatest declines occurring in the CHR-PS+ group. If indeed normative CT decreases during adolescence represent a period of specialization (where higher-level systems that contribute to adult outcomes are formed54,55), lower CT, most apparent in those in the CHR-PS+ group, could reflect impairments in optimal specialization. However, these observations are speculative, and the veracity of these patterns will be most accurately captured with longitudinal analyses that encompass a wide age range (eg, early childhood through adulthood).
The neuroanatomic pattern of group differences and age-associated disruptions observed in individuals at CHR may provide important insights into mechanisms underlying increased risk of psychosis. Preclinical models56,57 and recent genome-wide association studies58 suggest that genetic variants associated with SA are linked to the regulation of neural progenitor cells during fetal development, while genetic markers associated with CT are associated with regulatory processes in adulthood. Thus, CT differences may be the end result of maladaptive maturation-related mechanisms that occur during postfetal development, including proliferation, synaptic pruning, and/or myelination.59,60,61,62 Thinner CT, particularly in early adolescence (Figure 3), could reflect abnormal synaptic plasticity or pruning, which have both been implicated in in vitro schizophrenia models.63 Although excessive synaptic pruning is one plausible explanation for thinner cortex associated with psychosis transition, recent evidence suggests that intracortical myelination and/or expression of myelin-related genes may be mechanisms of cortical thinning.64,65 To better understand neurobiological mechanisms underlying psychosis transition in individuals at CHR, investigations of concomitant measures of CT, macroscale white matter tracts, and intracortical myelination are necessary. Finally, it is also possible that lower CT is not a mechanism of psychosis and can instead be attributed to environmental factors or social determinants associated with psychosis,66,67 or that lower CT occurs in response to other possible biological mechanisms underlying psychosis (eg, hypothalamic-pituitary-adrenal stress response68).
Even if CT reductions in individuals at CHR were robust, effect sizes for between-group differences were nevertheless small to moderate and accounted for approximately 1% of the variance in comparisons between the CHR-PS+ and CHR-PS− groups. The subtle nature of these morphometric differences underscores the importance of adequate statistical power, achievable only through large-scale multisite collaborations. Consistent with recent work showing that schizophrenia polygenic risk scores only improved differentiation of individuals in the CHR-PS+ group from controls (and not those in the CHR-PS+ group from those in the CHR-PS− group),69 we anticipate that baseline, univariate sMRI metrics will have a similar impact on psychosis risk prediction algorithms. Given the logistic and financial challenges that MRI brings, the use of MRI metrics in isolation may not be feasible or useful for psychosis risk prediction. A viable solution may be to adopt sequential assessment frameworks, as recently implemented.70 Alternatively, sMRI differences may be a better predictor of general psychopathology and would be better suited for transdiagnostic risk prediction models.71
Limitations
Our study had limitations. One limitation common to multisite studies is that data were collected from multiple scanners, although leave-1-out analyses suggest that site associations were not prominent. Second, this initial study focused on baseline cross-sectional data and did not investigate progressive sMRI changes associated with psychosis conversion, as identified in prior work.6,18,19,20,21,72 Additionally, CHR status is associated with heterogeneous outcomes,73,74,75 and neuroimaging phenotypes may differentiate among variability in psychosocial functioning and/or among other psychiatric diagnoses (eg, mood and anxiety disorders). These are two future goals of the ENIGMA Clinical High Risk for Psychosis Working Group, now that feasibility of this collaboration has been established.
Conclusions
In the largest study of brain abnormalities in individuals at CHR to date, we found robust evidence of a subtle, widespread pattern of CT differences, consistent with observations in psychosis. The specificity of these differences to CT—as well as age-associated deviations in regions sensitive to psychosis conversion—may point to abnormal development processes. These findings also point to age ranges (ie, early adolescence) when morphometric abnormalities in individuals at CHR might be greatest.
eMethods.
eResults.
eTable 1. Inclusion and exclusion participant criteria per site.
eTable 2. Scanner and acquisition parameters by site.
eTable 3. N HC vs. CHR vs. CHR-PS+ vs. CHR-PS- vs. CHR-UNK by site and scanner.
eTable 4. Age and sex of HC vs. CHR vs. CHR-PS+ vs. CHR-PS- in 12-25 year old age range (Age-associated analyses).
eTable 5. Intelligence quotient comparisons of HC vs. CHR by site.
eTable 6. Intelligence quotient comparisons of CHR-PS+ vs. CHR-PS- vs. CHR-UNK by site.
eTable 7. Symptom differences between CHR groups (CHR-PS+, CHR-PS-, CHR-UNK) as measured by the SIPS or CAARMS.
eTable 8. CHR current medication information reported by site.
eTable 9. CHR vs. HC effect size overview (post-ComBat mega analysis).
eTable 10. CHR vs. HC effect size overview, ICV excluded (post-ComBat mega analysis).
eTable 11. CHR vs. HC antipsychotic medication effects.
eTable 12. CHR vs. HC equivalence testing results.
eTable 13. CHR vs. HC effect size overview (pre-ComBat mega and meta analysis).
eTable 14. CHR subgroup (APS vs. BIPS vs. GRD vs. HC) effect size overview.
eTable 15. APS subgroup (APS vs. no APS assignment vs. HC) effect size overview .
eTable 16. BIPS subgroup (BIPS vs. no BIPS assignment vs. HC) effect size overview.
eTable 17. GRD subgroup (GRD vs. no GRD assignment vs. HC) effect size overview.
eTable 18. CHR-PS+ vs. CHR-PS- vs. HC effect size overview (post-ComBat mega analysis).
eTable 19. CHR-PS+ vs. CHR-PS- vs. HC effect size overview, ICV excluded (post-ComBat mega analysis).
eTable 20. CHR-PS+ vs. CHR-PS- vs. HC antipsychotic medication effects.
eTable 21. CHR-PS+ vs. CHR-PS- vs. HC equivalence testing results.
eTable 22. CHR subgroup-by-psychosis conversion interaction analyses effect size overview.
eTable 23. Age-by-group effects of CHR vs. HC.
eTable 24. Age-by-conversion effects on CHR-PS+ vs. CHR-PS- vs. HC.
eFigure 1. CHR vs. HC effect size overview (post-ComBat mega analysis).
eFigure 2. Jackknife resampling analysis for neuroimaging regions meeting statistical significance (q<.05) in Aim 1.
eFigure 3. CHR-PS+ vs. CHR-PS- vs. HC effect size overview (post-ComBat mega analysis).
eFigure 4. Cortical thickness alterations in CHR-PS+, schizophrenia, and 22q.11DS.
eFigure 5. CHR-PS+ vs. SZ vs. 22q11DS effect size overview.
eFigure 6. Surface area, subcortical volume and global measure correlations between CHR-PS+, SZ, and 22q11DS.
eReferences.
References
- 1.Yung AR, McGorry PD, McFarlane CA, Jackson HJ, Patton GC, Rakkar A. Monitoring and care of young people at incipient risk of psychosis. Schizophr Bull. 1996;22(2):283-303. doi: 10.1093/schbul/22.2.283 [DOI] [PubMed] [Google Scholar]
- 2.Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220-229. doi: 10.1001/archgenpsychiatry.2011.1472 [DOI] [PubMed] [Google Scholar]
- 3.Hartmann JA, Yuen HP, McGorry PD, et al. Declining transition rates to psychotic disorder in “ultra-high risk” clients: investigation of a dilution effect. Schizophr Res. 2016;170(1):130-136. doi: 10.1016/j.schres.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 4.Zikidi K, Gajwani R, Gross J, et al. Grey-matter abnormalities in clinical high-risk participants for psychosis. Schizophr Res. 2020;226:120-128. doi: 10.1016/j.schres.2019.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velakoulis D, Wood SJ, Wong MTH, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63(2):139-149. doi: 10.1001/archpsyc.63.2.139 [DOI] [PubMed] [Google Scholar]
- 6.Sun D, Phillips L, Velakoulis D, et al. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophr Res. 2009;108(1-3):85-92. doi: 10.1016/j.schres.2008.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fornito A, Yung AR, Wood SJ, et al. Anatomic abnormalities of the anterior cingulate cortex before psychosis onset: an MRI study of ultra-high-risk individuals. Biol Psychiatry. 2008;64(9):758-765. doi: 10.1016/j.biopsych.2008.05.032 [DOI] [PubMed] [Google Scholar]
- 8.Tomyshev AS, Lebedeva IS, Akhadov TA, Omelchenko MA, Rumyantsev AO, Kaleda VG. Alterations in white matter microstructure and cortical thickness in individuals at ultra-high risk of psychosis: a multimodal tractography and surface-based morphometry study. Psychiatry Res Neuroimaging. 2019;289:26-36. doi: 10.1016/j.pscychresns.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 9.Kwak YB, Kim M, Cho KIK, Lee J, Lee TY, Kwon JS. Reduced cortical thickness in subjects at clinical high risk for psychosis and clinical attributes. Aust N Z J Psychiatry. 2019;53(3):219-227. doi: 10.1177/0004867418807299 [DOI] [PubMed] [Google Scholar]
- 10.Iwashiro N, Suga M, Takano Y, et al. Localized gray matter volume reductions in the pars triangularis of the inferior frontal gyrus in individuals at clinical high-risk for psychosis and first episode for schizophrenia. Schizophr Res. 2012;137(1-3):124-131. doi: 10.1016/j.schres.2012.02.024 [DOI] [PubMed] [Google Scholar]
- 11.Takayanagi Y, Kulason S, Sasabayashi D, et al. Reduced thickness of the anterior cingulate cortex in individuals with an at-risk mental state who later develop psychosis. Schizophr Bull. 2017;43(4):907-913. doi: 10.1093/schbul/sbw167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung Y, Allswede D, Addington J, et al. ; North American Prodrome Longitudinal Study (NAPLS) Consortium . Cortical abnormalities in youth at clinical high-risk for psychosis: findings from the NAPLS2 cohort. Neuroimage Clin. 2019;23:101862. doi: 10.1016/j.nicl.2019.101862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koutsouleris N, Meisenzahl EM, Davatzikos C, et al. Use of neuroanatomical pattern classification to identify subjects in at-risk mental states of psychosis and predict disease transition. Arch Gen Psychiatry. 2009;66(7):700-712. doi: 10.1001/archgenpsychiatry.2009.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klauser P, Zhou J, Lim JKW, et al. Lack of evidence for regional brain volume or cortical thickness abnormalities in youths at clinical high risk for psychosis: findings from the Longitudinal Youth at Risk study. Schizophr Bull. 2015;41(6):1285-1293. doi: 10.1093/schbul/sbv012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziermans TB, Durston S, Sprong M, et al. No evidence for structural brain changes in young adolescents at ultra high risk for psychosis. Schizophr Res. 2009;112(1-3):1-6. doi: 10.1016/j.schres.2009.04.013 [DOI] [PubMed] [Google Scholar]
- 16.Fusar-Poli P, Borgwardt S, Crescini A, et al. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2011;35(5):1175-1185. doi: 10.1016/j.neubiorev.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 17.Del Re EC, Stone WS, Bouix S, et al. Baseline cortical thickness reductions in clinical high risk for psychosis: brain regions associated with conversion to psychosis versus non-conversion as assessed at one-year follow-up in the Shanghai-At-Risk-for-Psychosis (SHARP) study. Schizophr Bull. 2021;47(2):562-574. doi: 10.1093/schbul/sbaa127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borgwardt SJ, McGuire PK, Aston J, et al. Structural brain abnormalities in individuals with an at-risk mental state who later develop psychosis. Br J Psychiatry Suppl. 2007;51:s69-s75. doi: 10.1192/bjp.191.51.s69 [DOI] [PubMed] [Google Scholar]
- 19.Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361(9354):281-288. doi: 10.1016/S0140-6736(03)12323-9 [DOI] [PubMed] [Google Scholar]
- 20.Takahashi T, Wood SJ, Yung AR, et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66(4):366-376. doi: 10.1001/archgenpsychiatry.2009.12 [DOI] [PubMed] [Google Scholar]
- 21.Cannon TD, Chung Y, He G, et al. ; North American Prodrome Longitudinal Study Consortium . Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77(2):147-157. doi: 10.1016/j.biopsych.2014.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakuma A, Obara C, Katsura M, et al. No regional gray matter volume reduction observed in young Japanese people at ultra-high risk for psychosis: a voxel-based morphometry study. Asian J Psychiatr. 2018;37:167-171. doi: 10.1016/j.ajp.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 23.Mechelli A, Riecher-Rössler A, Meisenzahl EM, et al. Neuroanatomical abnormalities that predate the onset of psychosis: a multicenter study. Arch Gen Psychiatry. 2011;68(5):489-495. doi: 10.1001/archgenpsychiatry.2011.42 [DOI] [PubMed] [Google Scholar]
- 24.Farris MS, Devoe DJ, Addington J. Attrition rates in trials for adolescents and young adults at clinical high-risk for psychosis: a systematic review and meta-analysis. Early Interv Psychiatry. 2020;14(5):515-527. doi: 10.1111/eip.12864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon AE, Borgwardt S, Riecher-Rössler A, Velthorst E, de Haan L, Fusar-Poli P. Moving beyond transition outcomes: meta-analysis of remission rates in individuals at high clinical risk for psychosis. Psychiatry Res. 2013;209(3):266-272. doi: 10.1016/j.psychres.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 26.Button KS, Ioannidis JPA, Mokrysz C, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14(5):365-376. doi: 10.1038/nrn3475 [DOI] [PubMed] [Google Scholar]
- 27.Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344(8934):1398-1402. doi: 10.1016/S0140-6736(94)90569-X [DOI] [PubMed] [Google Scholar]
- 28.Lauronen E, Miettunen J, Veijola J, Karhu M, Jones PB, Isohanni M. Outcome and its predictors in schizophrenia within the Northern Finland 1966 Birth Cohort. Eur Psychiatry. 2007;22(2):129-136. doi: 10.1016/j.eurpsy.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 29.Vijayakumar N, Allen NB, Youssef G, et al. Brain development during adolescence: a mixed-longitudinal investigation of cortical thickness, surface area, and volume. Hum Brain Mapp. 2016;37(6):2027-2038. doi: 10.1002/hbm.23154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamnes CK, Herting MM, Goddings A-L, et al. Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J Neurosci. 2017;37(12):3402-3412. doi: 10.1523/JNEUROSCI.3302-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schimmelmann BG, Michel C, Martz-Irngartinger A, Linder C, Schultze-Lutter F. Age matters in the prevalence and clinical significance of ultra-high-risk for psychosis symptoms and criteria in the general population: findings from the BEAR and BEARS-Kid studies. World Psychiatry. 2015;14(2):189-197. doi: 10.1002/wps.20216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Erp TGM, Hibar DP, Rasmussen JM, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA Consortium. Mol Psychiatry. 2016;21(4):547-553. doi: 10.1038/mp.2015.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Erp TGM, Walton E, Hibar DP, et al. ; Karolinska Schizophrenia Project . Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium. Biol Psychiatry. 2018;84(9):644-654. doi: 10.1016/j.biopsych.2018.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ching CRK, Gutman BA, Sun D, et al. Mapping subcortical brain alterations in 22q11.2 deletion syndrome: effects of deletion size and convergence with idiopathic neuropsychiatric illness. Am J Psychiatry. 2020;177(7):589-600. doi: 10.1176/appi.ajp.2019.19060583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun D, Ching CRK, Lin A, et al. Large-scale mapping of cortical alterations in 22q11.2 deletion syndrome: convergence with idiopathic psychosis and effects of deletion size. Mol Psychiatry. 2020;25(8):1822-1834. doi: 10.1038/s41380-018-0078-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 37.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195-207. doi: 10.1006/nimg.1998.0396 [DOI] [PubMed] [Google Scholar]
- 38.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050-11055. doi: 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341-355. doi: 10.1016/S0896-6273(02)00569-X [DOI] [PubMed] [Google Scholar]
- 40.Schmaal L, Veltman DJ, van Erp TGM, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2016;21(6):806-812. doi: 10.1038/mp.2015.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmaal L, Hibar DP, Sämann PG, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2017;22(6):900-909. doi: 10.1038/mp.2016.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benjamini Y, Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc B (Methodological). 1995;57(1):289-300. [Google Scholar]
- 43.Radua J, Vieta E, Shinohara R, et al. ; ENIGMA Consortium Collaborators . Increased power by harmonizing structural MRI site differences with the ComBat batch adjustment method in ENIGMA. Neuroimage. 2020;218:116956. doi: 10.1016/j.neuroimage.2020.116956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Efron B, Stein C.. The jackknife estimate of variance. Ann Statist. 1981;9(3):586-596. doi: 10.1214/aos/1176345462 [DOI] [Google Scholar]
- 45.Lakens D. Equivalence tests: a practical primer for t tests, correlations, and meta-analyses. Soc Psychol Personal Sci. 2017;8(4):355-362. doi: 10.1177/1948550617697177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wood SN. Generalized Additive Models: An Introduction With R. 2nd ed. CRC Press; 2017. doi: 10.1201/9781315370279 [DOI] [Google Scholar]
- 47.Hastie T, Tibshirani R.. Generalized additive models. Statist Sci. 1986;1(3):297-310. doi: 10.1214/ss/1177013604 [DOI] [PubMed] [Google Scholar]
- 48.Jalbrzikowski M, Larsen B, Hallquist MN, Foran W, Calabro F, Luna B. Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: associations with anxiety and depression. Biol Psychiatry. 2017;82(7):511-521. doi: 10.1016/j.biopsych.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marek S, Hwang K, Foran W, Hallquist MN, Luna B. The contribution of network organization and integration to the development of cognitive control. PLoS Biol. 2015;13(12):e1002328. doi: 10.1371/journal.pbio.1002328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Writing Committee for the Attention-Deficit/Hyperactivity Disorder, Autism Spectrum Disorder, Bipolar Disorder, Major Depressive Disorder, Obsessive-Compulsive Disorder, and Schizophrenia ENIGMA Working Groups . Virtual histology of cortical thickness and shared neurobiology in 6 psychiatric disorders. JAMA Psychiatry. 2021;78(1):47-63. doi: 10.1001/jamapsychiatry.2020.2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Onitsuka T, Shenton ME, Kasai K, et al. Fusiform gyrus volume reduction and facial recognition in chronic schizophrenia. Arch Gen Psychiatry. 2003;60(4):349-355. doi: 10.1001/archpsyc.60.4.349 [DOI] [PubMed] [Google Scholar]
- 52.Takahashi T, Suzuki M, Zhou S-Y, et al. Temporal lobe gray matter in schizophrenia spectrum: a volumetric MRI study of the fusiform gyrus, parahippocampal gyrus, and middle and inferior temporal gyri. Schizophr Res. 2006;87(1-3):116-126. doi: 10.1016/j.schres.2006.04.023 [DOI] [PubMed] [Google Scholar]
- 53.van Lutterveld R, van den Heuvel MP, Diederen KMJ, et al. Cortical thickness in individuals with non-clinical and clinical psychotic symptoms. Brain. 2014;137(pt 10):2664-2669. doi: 10.1093/brain/awu167 [DOI] [PubMed] [Google Scholar]
- 54.Murty VP, Calabro F, Luna B. The role of experience in adolescent cognitive development: integration of executive, memory, and mesolimbic systems. Neurosci Biobehav Rev. 2016;70:46-58. doi: 10.1016/j.neubiorev.2016.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calabro FJ, Murty VP, Jalbrzikowski M, Tervo-Clemmens B, Luna B. Development of hippocampal-prefrontal cortex interactions through adolescence. Cereb Cortex. 2020;30(3):1548-1558. doi: 10.1093/cercor/bhz186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pontious A, Kowalczyk T, Englund C, Hevner RF. Role of intermediate progenitor cells in cerebral cortex development. Dev Neurosci. 2008;30(1-3):24-32. doi: 10.1159/000109848 [DOI] [PubMed] [Google Scholar]
- 57.Rakic P. Specification of cerebral cortical areas. Science. 1988;241(4862):170-176. doi: 10.1126/science.3291116 [DOI] [PubMed] [Google Scholar]
- 58.Grasby KL, Jahanshad N, Painter JN, et al. ; Alzheimer’s Disease Neuroimaging Initiative; CHARGE Consortium; EPIGEN Consortium; IMAGEN Consortium; SYS Consortium; Parkinson’s Progression Markers Initiative; Enhancing NeuroImaging Genetics through Meta-Analysis Consortium (ENIGMA)—Genetics working group . The genetic architecture of the human cerebral cortex. Science. 2020;367(6484):eaay6690. doi: 10.1126/science.aay6690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huttenlocher PR. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163(2):195-205. doi: 10.1016/0006-8993(79)90349-4 [DOI] [PubMed] [Google Scholar]
- 60.Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232(4747):232-235. doi: 10.1126/science.3952506 [DOI] [PubMed] [Google Scholar]
- 61.Petanjek Z, Judas M, Kostović I, Uylings HBM. Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: a layer-specific pattern. Cereb Cortex. 2008;18(4):915-929. doi: 10.1093/cercor/bhm124 [DOI] [PubMed] [Google Scholar]
- 62.Natu VS, Gomez J, Barnett M, et al. Apparent thinning of human visual cortex during childhood is associated with myelination. Proc Natl Acad Sci U S A. 2019;116(41):20750-20759. doi: 10.1073/pnas.1904931116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sellgren CM, Gracias J, Watmuff B, et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci. 2019;22(3):374-385. doi: 10.1038/s41593-018-0334-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whitaker KJ, Vértes PE, Romero-Garcia R, et al. ; NSPN Consortium . Adolescence is associated with genomically patterned consolidation of the hubs of the human brain connectome. Proc Natl Acad Sci U S A. 2016;113(32):9105-9110. doi: 10.1073/pnas.1601745113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parker N, Patel Y, Jackowski AP, et al. ; Saguenay Youth Study and the IMAGEN Consortium . Assessment of neurobiological mechanisms of cortical thinning during childhood and adolescence and their implications for psychiatric disorders. JAMA Psychiatry. 2020;77(11):1127-1136. doi: 10.1001/jamapsychiatry.2020.1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murray RM, Mondelli V, Stilo SA, et al. The influence of risk factors on the onset and outcome of psychosis: what we learned from the GAP study. Schizophr Res. 2020;225:63-68. doi: 10.1016/j.schres.2020.01.011 [DOI] [PubMed] [Google Scholar]
- 67.Anglin DM, Galea S, Bachman P. Going upstream to advance psychosis prevention and improve public health. JAMA Psychiatry. 2020;77(7):665-666. doi: 10.1001/jamapsychiatry.2020.0142 [DOI] [PubMed] [Google Scholar]
- 68.Pruessner M, Cullen AE, Aas M, Walker EF. The neural diathesis-stress model of schizophrenia revisited: an update on recent findings considering illness stage and neurobiological and methodological complexities. Neurosci Biobehav Rev. 2017;73:191-218. doi: 10.1016/j.neubiorev.2016.12.013 [DOI] [PubMed] [Google Scholar]
- 69.Perkins DO, Olde Loohuis L, Barbee J, et al. Polygenic risk score contribution to psychosis prediction in a target population of persons at clinical high risk. Am J Psychiatry. 2020;177(2):155-163. doi: 10.1176/appi.ajp.2019.18060721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koutsouleris N, Kambeitz-Ilankovic L, Ruhrmann S, et al. ; PRONIA Consortium . Prediction models of functional outcomes for individuals in the clinical high-risk state for psychosis or with recent-onset depression: a multimodal, multisite machine learning analysis. JAMA Psychiatry. 2018;75(11):1156-1172. doi: 10.1001/jamapsychiatry.2018.2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McGorry PD, Hartmann JA, Spooner R, Nelson B. Beyond the “at risk mental state” concept: transitioning to transdiagnostic psychiatry. World Psychiatry. 2018;17(2):133-142. doi: 10.1002/wps.20514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dazzan P, Soulsby B, Mechelli A, et al. Volumetric abnormalities predating the onset of schizophrenia and affective psychoses: an MRI study in subjects at ultrahigh risk of psychosis. Schizophr Bull. 2012;38(5):1083-1091. doi: 10.1093/schbul/sbr035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rutigliano G, Valmaggia L, Landi P, et al. Persistence or recurrence of non-psychotic comorbid mental disorders associated with 6-year poor functional outcomes in patients at ultra high risk for psychosis. J Affect Disord. 2016;203:101-110. doi: 10.1016/j.jad.2016.05.053 [DOI] [PubMed] [Google Scholar]
- 74.Michel C, Ruhrmann S, Schimmelmann BG, Klosterkötter J, Schultze-Lutter F. Course of clinical high-risk states for psychosis beyond conversion. Eur Arch Psychiatry Clin Neurosci. 2018;268(1):39-48. doi: 10.1007/s00406-016-0764-8 [DOI] [PubMed] [Google Scholar]
- 75.Woods SW, Walsh BC, Addington J, et al. Current status specifiers for patients at clinical high risk for psychosis. Schizophr Res. 2014;158(1-3):69-75. doi: 10.1016/j.schres.2014.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eResults.
eTable 1. Inclusion and exclusion participant criteria per site.
eTable 2. Scanner and acquisition parameters by site.
eTable 3. N HC vs. CHR vs. CHR-PS+ vs. CHR-PS- vs. CHR-UNK by site and scanner.
eTable 4. Age and sex of HC vs. CHR vs. CHR-PS+ vs. CHR-PS- in 12-25 year old age range (Age-associated analyses).
eTable 5. Intelligence quotient comparisons of HC vs. CHR by site.
eTable 6. Intelligence quotient comparisons of CHR-PS+ vs. CHR-PS- vs. CHR-UNK by site.
eTable 7. Symptom differences between CHR groups (CHR-PS+, CHR-PS-, CHR-UNK) as measured by the SIPS or CAARMS.
eTable 8. CHR current medication information reported by site.
eTable 9. CHR vs. HC effect size overview (post-ComBat mega analysis).
eTable 10. CHR vs. HC effect size overview, ICV excluded (post-ComBat mega analysis).
eTable 11. CHR vs. HC antipsychotic medication effects.
eTable 12. CHR vs. HC equivalence testing results.
eTable 13. CHR vs. HC effect size overview (pre-ComBat mega and meta analysis).
eTable 14. CHR subgroup (APS vs. BIPS vs. GRD vs. HC) effect size overview.
eTable 15. APS subgroup (APS vs. no APS assignment vs. HC) effect size overview .
eTable 16. BIPS subgroup (BIPS vs. no BIPS assignment vs. HC) effect size overview.
eTable 17. GRD subgroup (GRD vs. no GRD assignment vs. HC) effect size overview.
eTable 18. CHR-PS+ vs. CHR-PS- vs. HC effect size overview (post-ComBat mega analysis).
eTable 19. CHR-PS+ vs. CHR-PS- vs. HC effect size overview, ICV excluded (post-ComBat mega analysis).
eTable 20. CHR-PS+ vs. CHR-PS- vs. HC antipsychotic medication effects.
eTable 21. CHR-PS+ vs. CHR-PS- vs. HC equivalence testing results.
eTable 22. CHR subgroup-by-psychosis conversion interaction analyses effect size overview.
eTable 23. Age-by-group effects of CHR vs. HC.
eTable 24. Age-by-conversion effects on CHR-PS+ vs. CHR-PS- vs. HC.
eFigure 1. CHR vs. HC effect size overview (post-ComBat mega analysis).
eFigure 2. Jackknife resampling analysis for neuroimaging regions meeting statistical significance (q<.05) in Aim 1.
eFigure 3. CHR-PS+ vs. CHR-PS- vs. HC effect size overview (post-ComBat mega analysis).
eFigure 4. Cortical thickness alterations in CHR-PS+, schizophrenia, and 22q.11DS.
eFigure 5. CHR-PS+ vs. SZ vs. 22q11DS effect size overview.
eFigure 6. Surface area, subcortical volume and global measure correlations between CHR-PS+, SZ, and 22q11DS.
eReferences.