Key Points
Question
Does breast conservation offer a survival benefit compared with mastectomy when results are adjusted for main confounders such as comorbidity and socioeconomic status?
Findings
In this large cohort study based on prospectively collected national data from 48 986 patients with breast cancer, overall and breast cancer–specific survival were significantly better after breast-conserving surgery followed by radiotherapy than after mastectomy with or without radiotherapy despite stepwise adjustment for tumor characteristics, treatment, demographics, comorbidity, and socioeconomic background.
Meaning
Breast conservation seems to offer a survival benefit independent of measured confounders and should be given priority if both breast conservation and mastectomy are valid options.
Abstract
Importance
Cohort studies show better survival after breast-conserving surgery (BCS) with postoperative radiotherapy (RT) than after mastectomy (Mx) without RT. It remains unclear whether this is an independent effect or a consequence of selection bias.
Objective
To determine whether the reported survival benefit of breast conservation is eliminated by adjustment for 2 pivotal confounders, comorbidity and socioeconomic status.
Design, Setting, and Participants
Cohort study using prospectively collected national data. Swedish public health care; nationwide clinical data from the National Breast Cancer Quality Register, comorbidity data from Patient Registers at the National Board of Health and Welfare, and individual-level education and income data from Statistics Sweden. The cohort included all women diagnosed as having primary invasive T1-2 N0-2 breast cancer and undergoing breast surgery in Sweden from 2008 to 2017. Data were analyzed between August 19, 2020, and November 12, 2020.
Exposures
Locoregional treatment comparing 3 groups: breast-conserving surgery with radiotherapy (BCS+RT), mastectomy without radiotherapy (Mx-RT), and mastectomy with radiotherapy (Mx+RT).
Main Outcomes and Measures
Overall survival (OS) and breast cancer–specific survival (BCSS). Main outcomes were determined before initiation of data retrieval.
Results
Among 48 986 women, 29 367 (59.9%) had BCS+RT, 12413 (25.3%) had Mx-RT, and 7206 (14.7%) had Mx+RT. Median follow-up was 6.28 years (range, 0.01-11.70). All-cause death occurred in 6573 cases, with death caused by breast cancer in 2313 cases; 5-year OS was 91.1% (95% CI, 90.8-91.3) and BCSS was 96.3% (95% CI, 96.1-96.4). Apart from expected differences in clinical parameters, women receiving Mx-RT were older, had a lower level of education, and lower income. Both Mx groups had a higher comorbidity burden irrespective of RT. After stepwise adjustment for all covariates, OS and BCSS were significantly worse after Mx-RT (hazard ratio [HR], 1.79; 95% CI, 1.66-1.92 and HR, 1.66; 95% CI, 1.45-1.90, respectively) and Mx+RT (HR, 1.24; 95% CI, 1.13-1.37 and HR, 1.26; 95% CI, 1.08-1.46, respectively) than after BCS+RT.
Conclusions and Relevance
Despite adjustment for previously unmeasured confounders, BCS+RT yielded better survival than Mx irrespective of RT. If both interventions are valid options, mastectomy should not be regarded as equal to breast conservation.
This study determines whether the reported survival benefit of breast conservation is eliminated by adjustment for 2 pivotal confounders, comorbidity and socioeconomic status.
Introduction
Since the publication of key trials1,2 confirming the oncological equivalence of breast-conserving surgery (BCS) followed by adjuvant radiotherapy (RT) and mastectomy (Mx), BCS is recommended for patients with early breast cancer. Additionally, in case of advanced lymph node involvement, Mx does not confer any survival benefit.3 The same is true for the younger breast cancer population and in specific subtypes such as triple-negative breast cancer (TNBC).4,5,6 Recently, population-based studies have reported improved overall survival after BCS with RT over Mx without RT.7,8,9,10 Mastectomy has subsequently been questioned as an equally valid surgical alternative. However, there are important confounders that may have biased these results.
When deciding on 1 of the basic 2 surgical options (BCS vs Mx), many interacting contributory factors are taken into consideration: anticipated resection volume and its association with breast volume, the tumor location in the breast and the feasibility of postoperative RT, as well as patient comorbidities, age, preferences, and beliefs.
The consequences of BCS vs Mx differ as measured by complication rates, length of hospital stay, rehabilitation time, patient-reported symptoms,11 body image, and quality of life.12 Commonly, Mx is proposed as a means to avoid postoperative RT and may therefore be more prevalent in rural areas where patients need to travel further to receive RT. However, it is important to consider that RT is not only indicated after BCS: in Sweden, postmastectomy RT is recommended for T3 tumors and extensive tumor multifocality as well as in node-positive disease, with few exceptions.
While the previously mentioned studies deliver evidence encouraging the use of BCS, it remains unclear why such survival differences would exist.7,8,9,10 Theories of a negative effect of larger surgery on recurrence rates and survival through the systemic release of growth factors and inflammatory effects have not been sufficiently corroborated, so Mx in itself may not be an independent factor for worse survival.13 Selection mechanisms and unmeasured confounders must be suspected. For example, BCS is less common in women with a lower socioeconomic status,14 which in turn is associated with multimorbidity,15 a more advanced stage at presentation,16 lower rates of adjuvant chemotherapy,17 and worse survival.18,19,20 Furthermore, comorbidity is associated with choice of systemic and locoregional treatment21,22,23 and survival.21,24
To further dissect the association of locoregional treatment with survival, this large population-based cohort study investigates the association of socioeconomic factors and comorbidity with overall and breast cancer–specific survival after BCS with RT, Mx with, and Mx without postoperative RT.
Methods
This cohort study used prospectively collected data from the Swedish National Breast Cancer Register (NKBC), with national coverage since 1992 and harmonized online reporting since 2008. The NKBC includes date of diagnosis, age, sex, invasiveness, primary tumor and lymph node characteristics, metastases, date and type of surgery, oncological treatment, and follow-up. The register is 98% to 99% complete, and a 2019 validation showed a greater than 90% overlap between NKBC and validation data.25
From the NKBC, we included all patients diagnosed as having primary invasive breast cancer from January 1, 2008, until December 31, 2017, who underwent breast surgery with known surgery date, known tumor size of up to 50 mm (T1-2), no more than 10 positive lymph nodes (N0-2), and available data on planned or given adjuvant RT. These inclusion criteria were chosen to select patients in whom choice of locoregional treatment may have an independent survival effect and who likely would have had a choice between BCS and Mx. For women with bilateral breast cancer, we selected the side with the larger tumor and/or more nodal metastases. The cohort was individually linked to the Swedish National Patient Registers including inpatient and outpatient care, the Cause of Death Register at the National Board of Health and Welfare, and population registers at Statistics Sweden holding demographic and socioeconomic information, using the personal identification number assigned to all Swedish residents. One woman with a registered death date prior to diagnosis was excluded, as were 46 women with reused personal identification numbers.
Locoregional Treatment
Locoregional treatment was categorized as BCS with RT (BCS+RT), Mx with RT (Mx+RT), or Mx without RT (Mx-RT). The use of reconstructive or oncoplastic procedures was not considered. Because the omission of whole-breast irradiation after BCS was not in accordance with Swedish guidelines, 2390 women treated with BCS but not receiving adjuvant RT were excluded, leaving 48 986 women for the final analysis (eFigure 1 in the Supplement).
Radiotherapy target and dose were not sufficiently available, and adjuvant RT was therefore treated as a binary variable (yes/no). According to Swedish guidelines for the relevant years, RT to regional lymph nodes was recommended in case of regional macrometastatic disease independent of the type of axillary surgery performed. In case of neoadjuvant chemotherapy, clinical node positivity or a macrometastasis on pretreatment sentinel node biopsy was an indication for regional RT, regardless of the type and results of postchemotherapy axillary staging. After neoadjuvant chemotherapy, any size of axillary metastasis was an indication for regional RT. Micrometastatic axillary disease was no indication for adjuvant regional RT, and in case of 1 single macrometastasis in a low-grade tumor and BCS, regional RT could be omitted.
Locoregional treatment not following national guidelines (ie, no RT after mastectomy despite nodal involvement) occurred in 2542 women (5.2%). Potential overtreatment with RT after mastectomy in T1N0 and T2N0 (1701 women, 3.5%) was possibly owing to extensive multifocality because only the largest tumor focus was registered.
Tumor Characteristics and Treatment
In the NKBC, clinical pretreatment tumor size and nodal status are registered in accordance with the TNM classification (cN; cT), while exact invasive tumor size (in millimeters), number, and size of nodal metastases are registered postoperatively. Status variables unaffected by treatment were selected, ie, pretreatment clinical variables in case of neoadjuvant treatment (cT; cN) and histopathological variables in case of primary surgery (pT; pN). Likewise, data on tumor biology (estrogen receptor [ER] and progesterone receptor [PR] status, Erb-B2 receptor tyrosine kinase 2 [ERBB2] amplification, and proliferation) were based on the pretreatment core biopsy and on the tumor specimen, respectively. Tumor size (T) was categorized into T1 and T2, and lymph node status into N0, N1, and N2 in accordance with the eighth edition of the AJCC Cancer Staging Manual.26 Both variables were then combined into prognostic groups (T1N0, T1N1, T1N2, T2N0, T2N1, and T2N2). Estrogen receptor and PR were considered negative if less than 10%. The ERBB2 amplification was confirmed by an immunohistochemistry (IHC) score of 3+ or by in situ hybridization, performed in case of score of 2+. Hormone receptor–positive (HR+) tumors were ER+ and/or PR+, and hormone receptor negative (HR−) tumors were ER− and PR−. Subtypes were classified as HR+ERBB2−, HR+ERBB2+, HR−ERBB2+, and HR−ERBB2−. Oncological treatment included RT, chemotherapy (CT) (yes/no), endocrine treatment (yes/no), and targeted therapy (yes/no).
Comorbidities
From the National Patient Registers, both main and contributing diagnoses of any comorbidity between 2008 and 2017 and within 12 months before treatment, listed in the Royal College of Surgeons Charlson Comorbidity Index (CCI; eTable 1 in the Supplement), were extracted.27 For patients with breast cancer diagnosed in 2008, comorbidities registered in 2008 were used.
Education, Income, and Country of Birth
From the Longitudinal Integrated Database for Health Insurance and Labour Market Studies (LISA) database at Statistics Sweden, information on education and income for 2008 to 2017 was individually linked. The highest attained education by the year preceding the diagnosis of breast cancer was categorized as 9 years or less (primary), 10 to 13 years (secondary), and more than 13 years (tertiary). Family income in the calendar year prior to cancer surgery was used to reflect socioeconomic status and categorized into quartiles (low [Q1: 0 to 25%], middle [Q2-Q3: 25% to 75%], and high [Q4: 75% to 100%]). Income was adjusted for inflation over the study period. Country of birth was categorized as Sweden, Europe except Sweden, and any other countries. For women diagnosed in 2008, education level and income were based on data from 2008.
Follow-up for Death
Date and cause of death was obtained from the Cause of Death Register and complemented with information from the Total Population Register at Statistics Sweden if dates were incomplete. Death owing to breast cancer was defined as death with International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) code C50 as the registered cause of death.
Ethical Considerations
The study was approved by the regional Ethical Review Authority in Stockholm (2017/2493-31), under the explicit condition that no specific informed consent was obtained other than the general consent for the use of personal data when accepting registration in NKBC. Registration in the remaining national registers is mandatory by law without consent.
Statistical Methods
Start of follow-up was from date of surgery until end of follow-up at death or end of study in September 2019. We assessed death owing to any cause (overall survival [OS]) and death owing to breast cancer (breast cancer–specific survival [BCSS]), for which deaths owing to other causes than breast cancer were censored. Unadjusted survival proportions were estimated using the Kaplan-Meier method and compared using the log-rank test. Overall and breast cancer–specific mortality rates were modeled and adjusted using Cox regression, with time since surgery as the underlying timescale. Associations between locoregional treatment (BCS+RT, Mx-RT, and Mx+RT) and mortality rates are reported as hazard ratios (HRs) with 95% CIs. First, models were stepwise adjusted for confounders (age, year, region, prognostic group, Nottingham grade, subtype, socioeconomic factors, and CCI). The adjustment for grade and subtype was by stratification with separate baseline hazards, thereby accounting for nonproportional hazards in these variables. Second, we estimated HRs for locoregional treatment by prognostic group. In a final step, HRs for locoregional treatment were estimated for short (0-5 years) and long (>5 years) follow-up separately. In the final model, all variables except CCI fulfilled the proportional hazard assumption. Hence, we assessed a model with a time-varying effect of CCI as a sensitivity analysis, the results of the exposure variable of interest (locoregional treatment); however, this remained unchanged to the second decimal. Thus, the final model used in the main analysis did not include time-varying effects in CCI. Women with missing information on any covariates in the models were excluded. The significance level was .05 and all tests were 2-sided. All statistical analyses were performed using R, version 4.0.1 (R Foundation).
Results
Among 48 986 women, 29 367 (59.9%) had received BCS+RT, 12 413 (25.3%) received Mx-RT, and 7206 (14.7%) received Mx+RT; Table 1. Median follow-up was 6.28 years (range, 0.01-11.70 years). Women in the BCS+RT group were more often within the age span of Swedish mammography screening (40-74 years) and had smaller tumors with less nodal involvement than women in the Mx+RT group. Women in the Mx-RT group had the highest mean age and similar rates of nodal involvement as the BCS+RT group. Neoadjuvant treatment, adjuvant chemotherapy, and targeted treatment were most common in the Mx+RT group where the proportion of larger tumors (T2) and of nodal involvement (both N1 and N2) was largest, which was mirrored in the distribution over the prognostic groups. There were fewer HR+/ERBB2− and more high-grade tumors in the Mx+RT group compared with BCS+RT. Women with Mx-RT had lower education levels and a lower family income, while women in both Mx groups had more comorbidities than those with BCS+RT. The distribution of locoregional treatments varied across Swedish regions but not over calendar periods.
Table 1. Patient, Tumor, and Treatment Characteristics by Locoregional Treatment.
| Characteristic | No. (%) | P value | |||
|---|---|---|---|---|---|
| BCS+RT (n = 29 367) | Mx-RT (n = 12 413) | Mx+RT (n = 7206) | Total (n = 48 986) | ||
| Follow-up time from surgery, median (range), y | 6.35 (0.02-11.70) | 6.20 (0.01-11.69) | 6.02 (0.12-11.66) | 6.28 (0.01-11.70) | <.001 |
| No. of deaths | |||||
| OS | 2272 (7.7) | 2912 (23.5) | 1389 (19.3) | 6573 (13.4) | <.001 |
| BCSS | 727 (2.5) | 771 (6.2) | 815 (11.3) | 2313 (4.7) | <.001 |
| Age at diagnosis, y | |||||
| <40 | 736 (2.5) | 451 (3.6) | 643 (8.9) | 1830 (3.7) | <.001 |
| 40-49 | 4170 (14.2) | 1457 (11.7) | 1503 (20.9) | 7130 (14.6) | |
| 50-64 | 12 424 (42.3) | 3212 (25.9) | 2366 (32.8) | 18 002 (36.7) | |
| 65-74 | 10 134 (34.5) | 3383 (27.3) | 1629 (22.6) | 15 146 (30.9) | |
| ≥75 | 1903 (6.5) | 3910 (31.5) | 1065 (14.8) | 6878 (14.0) | |
| Mean (SD) | 60.8 (10.5) | 66.3 (14.2) | 58.7 (13.9) | 61.9 (12.4) | <.001 |
| Median (range) | 62 (22-94) | 68 (21-97) | 59 (19-95) | 63 (19-97) | <.001 |
| Year of surgery | |||||
| 2008-2009 | 5044 (17.2) | 2665 (21.5) | 1404 (19.5) | 9113 (18.6) | <.001 |
| 2010-2011 | 6076 (20.7) | 2934 (23.6) | 1636 (22.7) | 10 646 (21.7) | |
| 2012-2013 | 6759 (23.0) | 2782 (22.4) | 1571 (21.8) | 11 112 (22.7) | |
| 2014-2015 | 7437 (25.3) | 2683 (21.6) | 1691 (23.5) | 11 811 (24.1) | |
| 2016-2017 | 4051 (13.8) | 1349 (10.9) | 904 (12.5) | 6304 (12.9) | |
| T stagea | |||||
| T1 | 23 266 (79.2) | 7114 (57.3) | 2643 (36.7) | 33 023 (67.4) | <.001 |
| T2 | 6101 (20.8) | 5299 (42.7) | 4563 (63.3) | 15 963 (32.6) | |
| T stageb | |||||
| T1mi | 177 (0.6) | 116 (0.9) | 43 (0.6) | 336 (0.7) | <.001 |
| T1a | 1146 (3.9) | 435 (3.5) | 97 (1.3) | 1678 (3.4) | |
| T1b | 7082 (24.1) | 1479 (11.9) | 379 (5.3) | 8940 (18.3) | |
| T1c | 14 737 (50.2) | 5035 (40.6) | 1935 (26.9) | 21 707 (44.3) | |
| T1 | 124 (0.4) | 49 (0.4) | 189 (2.6) | 362 (0.7) | |
| T2 | 6101 (20.8) | 5299 (42.7) | 4563 (63.3) | 15 963 (32.6) | |
| Lymph node status | |||||
| N0 | 22 933 (78.1) | 9871 (79.5) | 1701 (23.6) | 34 505 (70.4) | <.001 |
| N1 | 5666 (19.3) | 2268 (18.3) | 3989 (55.4) | 11 923 (24.3) | |
| N2 | 768 (2.6) | 274 (2.2) | 1516 (21.0) | 2558 (5.2) | |
| Prognostic groups | |||||
| T1N0 | 19 147 (65.2) | 6021 (48.5) | 805 (11.2) | 25 973 (53.0) | <.001 |
| T1N1 | 3732 (12.7) | 1036 (8.3) | 1420 (19.7) | 6188 (12.6) | |
| T1N2 | 387 (1.3) | 57 (0.5) | 418 (5.8) | 862 (1.8) | |
| T2N0 | 3786 (12.9) | 3850 (31.0) | 896 (12.4) | 8532 (17.4) | |
| T2N1 | 1934 (6.6) | 1232 (9.9) | 2569 (35.7) | 5735 (11.7) | |
| T2N2 | 381 (1.3) | 217 (1.7) | 1098 (15.2) | 1696 (3.5) | |
| Primary treatment | |||||
| Surgery | 28 859 (98.3) | 12 149 (97.9) | 6038 (83.8) | 47 046 (96.0) | <.001 |
| Neoadjuvant systemic treatment | 508 (1.7) | 264 (2.1) | 1168 (16.2) | 1940 (4.0) | |
| Bilateral breast cancer | |||||
| No | 28 761 (97.9) | 11 965 (96.4) | 7034 (97.6) | 47 760 (97.5) | <.001 |
| Yes | 606 (2.1) | 448 (3.6) | 172 (2.4) | 1226 (2.5) | |
| Histological invasive subtype | |||||
| Ductal | 23 607 (80.4) | 9367 (75.5) | 4920 (68.3) | 37 894 (77.4) | <.001 |
| Lobular | 3052 (10.4) | 1906 (15.4) | 935 (13.0) | 5893 (12.0) | |
| Other | 2114 (7.2) | 848 (6.8) | 208 (2.9) | 3170 (6.5) | |
| Missing | 594 (2.0) | 292 (2.4) | 1143 (15.9) | 2029 (4.1) | |
| Nottingham grade | |||||
| 1 | 7388 (25.2) | 2086 (16.8) | 514 (7.1) | 9988 (20.4) | <.001 |
| 2 | 14 346 (48.9) | 6296 (50.7) | 2878 (39.9) | 23 520 (48.0) | |
| 3 | 6873 (23.4) | 3602 (29.0) | 2596 (36.0) | 13071 (26.7) | |
| Missing | 760 (2.6) | 429 (3.5) | 1218 (16.9) | 2407 (4.9) | |
| ERBB2 amplification | |||||
| Yes | 2932 (10.0) | 1619 (13.0) | 1425 (19.8) | 5976 (12.2) | <.001 |
| No | 25 304 (86.2) | 9979 (80.4) | 5454 (75.7) | 40 737 (83.2) | |
| Missing | 1131 (3.9) | 815 (6.6) | 327 (4.5) | 2273 (4.6) | |
| ER status | |||||
| Positive | 23 512 (80.1) | 9091 (73.2) | 5066 (70.3) | 37 669 (76.9) | <.001 |
| Negative | 2197 (7.5) | 1091 (8.8) | 933 (12.9) | 4221 (8.6) | |
| Missing | 3658 (12.5) | 2231 (18.0) | 1207 (16.7) | 7096 (14.5) | |
| PR status | |||||
| Positive | 19 541 (66.5) | 7185 (57.9) | 3982 (55.3) | 30 708 (62.7) | <.001 |
| Negative | 5529 (18.8) | 2622 (21.1) | 1849 (25.7) | 10 000 (20.4) | |
| Missing | 4297 (14.6) | 2606 (21.0) | 1375 (19.1) | 8278 (16.9) | |
| Subtype | |||||
| HR+ERBB2- | 21 177 (72.1) | 7852 (63.3) | 4193 (58.2) | 33 222 (67.8) | <.001 |
| HR+ERBB2+ | 1960 (6.7) | 919 (7.4) | 786 (10.9) | 3665 (7.5) | |
| HR-ERBB2+ | 519 (1.8) | 346 (2.8) | 356 (4.9) | 1221 (2.5) | |
| HR-ERBB2- | 1536 (5.2) | 678 (5.5) | 519 (7.2) | 2733 (5.6) | |
| Missing | 4175 (14.2) | 2618 (21.1) | 1352 (18.8) | 8145 (16.6) | |
| Chemotherapy | |||||
| Yes | 8168 (27.8) | 2727 (22.0) | 4377 (60.7) | 15 272 (31.2) | <.001 |
| No | 21 199 (72.2) | 9686 (78.0) | 2829 (39.3) | 33 714 (68.8) | |
| Endocrine treatment | |||||
| Yes | 18 932 (64.5) | 8004 (64.5) | 4794 (66.5) | 31 730 (64.8) | .003 |
| No | 10 435 (35.5) | 4409 (35.5) | 2412 (33.5) | 17 256 (35.2) | |
| Targeted treatment | |||||
| Yes | 2174 (7.4) | 960 (7.7) | 1164 (16.2) | 4298 (8.8) | <.001 |
| No | 27 193 (92.6) | 11 453 (92.3) | 6042 (83.8) | 44 688 (91.2) | |
| Country of birth | |||||
| Sweden | 25 208 (85.8) | 10 797 (87.0) | 6051 (84.0) | 42 056 (85.9) | <.001 |
| Europe, not Sweden | 2791 (9.5) | 1206 (9.7) | 717 (10.0) | 4714 (9.6) | |
| Asia, Africa, North/South America, Australia, and Oceania | 1354 (4.6) | 401 (3.2) | 431 (6.0) | 2186 (4.5) | |
| Missing | 14 | 9 (0.1) | 7 (0.1) | 30 (0.1) | |
| Region of residence | |||||
| Stockholm/Gotland | 7377 (25.1) | 1997 (16.1) | 1770 (24.6) | 11 144 (22.7) | <.001 |
| Uppsala/Örebro | 6222 (21.2) | 2313 (18.6) | 1743 (24.2) | 10 278 (21.0) | |
| Southeast | 2631 (9.0) | 1437 (11.6) | 1006 (14.0) | 5074 (10.4) | |
| South | 5323 (18.1) | 2889 (23.3) | 1226 (17.0) | 9438 (19.3) | |
| West | 4978 (17.0) | 2769 (22.3) | 939 (13.0) | 8686 (17.7) | |
| North | 2836 (9.7) | 1008 (8.1) | 522 (7.2) | 4366 (8.9) | |
| Family income | |||||
| Low | 5527 (18.8) | 4282 (34.5) | 1609 (22.3) | 11 418 (23.3) | <.001 |
| Middle | 15 580 (53.1) | 5720 (46.1) | 3524 (48.9) | 24 824 (50.7) | |
| High | 8211 (28.0) | 2399 (19.3) | 2063 (28.6) | 12 673 (25.9) | |
| Missing | 49 (0.2) | 12 (0.1) | 10 (0.1) | 71 (0.1) | |
| Highest attained education | |||||
| ≤9 y (Primary) | 5596 (19.1) | 3748 (30.2) | 1447 (20.1) | 10 791 (22.0) | <.001 |
| 10-13 y (Secondary) | 12 867 (43.8) | 4819 (38.8) | 2962 (41.1) | 20 648 (42.2) | |
| >13 y (Tertiary) | 10 664 (36.3) | 3680 (29.6) | 2707 (37.6) | 17 051 (34.8) | |
| Missing | 240 (0.8) | 166 (1.3) | 90 (1.2) | 496 (1.0) | |
| Charlson comorbidity index within 1 y before treatment | |||||
| Mean (SD) | 0.268 (1.12) | 0.525 (1.53) | 1.05 (2.27) | 0.449 (1.47) | <.001 |
| Median (range) | 0 (0-12.0) | 0 (0-14.0) | 0 (0-10.0) | 0 (0-14.0) | <.001 |
| Charlson Comorbidity Index within 1 y before treatment | |||||
| 0 | 26 810 (91.3) | 10 209 (82.2) | 5664 (78.6) | 42 683 (87.1) | <.001 |
| ≥1 | 2557 (8.7) | 2204 (17.8) | 1542 (21.4) | 6303 (12.9) | |
Abbreviations: BCS, breast-conserving surgery; BCSS, breast cancer–specific survival; ERBB2, Erb-B2 receptor tyrosine kinase 2; ER, estrogen receptor; HR, hormone receptor status; Mx, mastectomy; OS, overall survival; PR, progesterone receptor; RT, radiotherapy.
Histopathologic tumor size for primarily operated patients but clinical T stage for patients receiving neoadjuvant treatment.
Histopathologic tumor size available only for primarily operated-on patients.
In total, 6573 deaths occurred during follow-up, of which 2313 (35.2%) were owing to breast cancer. Five-year survival was 91.1% (OS) and 96.3% (BCSS), while 10-year survival was 79.5% (OS) and 93.1% (BCSS). In the unadjusted analysis, Mx-RT was associated with the lowest OS (Figure 1A) and Mx+RT with the lowest BCSS (Figure 1B). The corresponding 5-year and 10-year survival proportions by locoregional treatment groups are presented in Table 2. When stratified by prognostic group, Mx-RT was particularly associated with lower BCSS among prognostic groups with a clear indication for adjuvant RT, ie, T1N2 and T2N2 (Figure 2). Mastectomy with RT was associated with a lower BCSS than BCS+RT across all prognostic groups (Figure 2), and with the lowest BCSS in all age groups (eFigure 2 in the Supplement). For OS, Mx-RT was associated with the lowest survival in all prognostic groups (eFigure 3 in the Supplement), and Mx+RT with the lowest survival across all age groups (eFigure 4 in the Supplement).
Figure 1. Survival Proportions by Locoregional Treatment Group for Overall Survival (A) and Breast Cancer–Specific Survival (B).
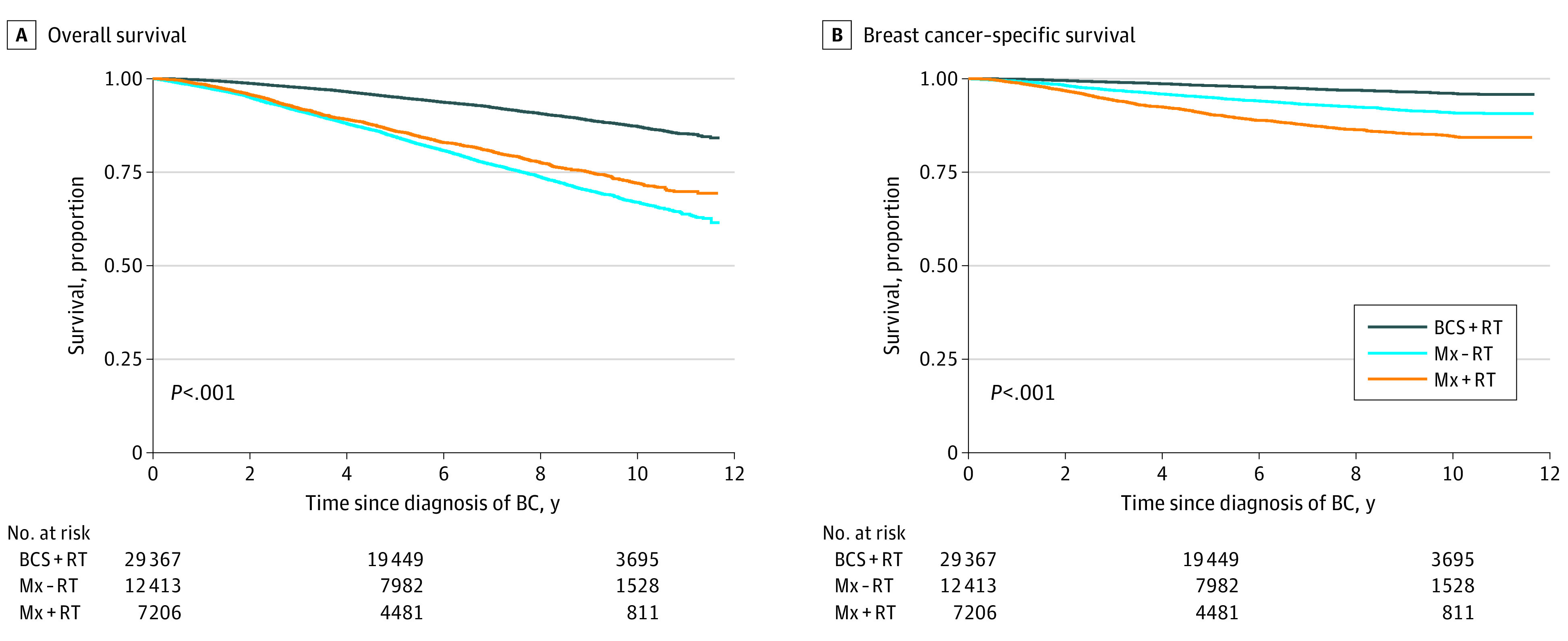
BC indicates breast cancer; BCS, breast-conserving surgery; Mx, mastectomy; RT, radiotherapy.
Table 2. Hazard Ratios of OS and BCSS for Locoregional Treatment Adjusted Stepwise for Tumor Characteristics, Treatment, Socioeconomic Status, and Charlson Comorbidity Index.
| Variable | Survival % (95% CI) | HR (95% CI) | ||||
|---|---|---|---|---|---|---|
| 5-y | 10-y | Model 1a | Model 2b | Model 3c | Model 4d | |
| OS | ||||||
| BCS+RT | 95.1 (94.9-95.4) | 87.3 (86.7-87.9) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Mx-RT | 84.5 (83.9-85.2) | 67.0 (65.9-68.2) | 1.94 (1.82-2.06) | 1.91 (1.78-2.06) | 1.83 (1.70-1.97) | 1.79 (1.66-1.92) |
| Mx+RT | 86.0 (85.2-86.9) | 72.1 (70.7-73.7) | 2.36 (2.21-2.53) | 1.24 (1.13-1.37) | 1.25 (1.13-1.38) | 1.24 (1.13-1.37) |
| BCSS | ||||||
| BCS+RT | 98.2 (98.0-98.3) | 96.1 (95.8-96.5) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Mx-RT | 95.0 (94.6-95.4) | 91.0 (90.3-91.7) | 1.89 (1.69-2.10) | 1.71 (1.50-1.96) | 1.67 (1.46-1.91) | 1.66 (1.45-1.90) |
| Mx+RT | 90.5 (89.7-91.2) | 84.6 (83.5-85.8) | 4.30 (3.88-4.76) | 1.25 (1.08-1.45) | 1.25 (1.08-1.46) | 1.26 (1.08-1.46) |
Abbreviations: BCS, breast-conserving surgery; BCSS, breast cancer–specific survival; Mx, mastectomy; OS, overall survival; RT, radiotherapy.
Adjusted for age, calendar year, and region of residence at diagnosis.
Adjusted for same variables as model 1 plus Nottingham grade, prognostic group, and subtype.
Adjusted for same variables as model 2 plus education, family income, and country of birth.
Adjusted for same variables as model 3 plus Charlson Comorbidity Index one year prior to operation.
Figure 2. Survival Proportions for Breast Cancer-Specific Survival by Locoregional Treatment Group and Prognostic Group.
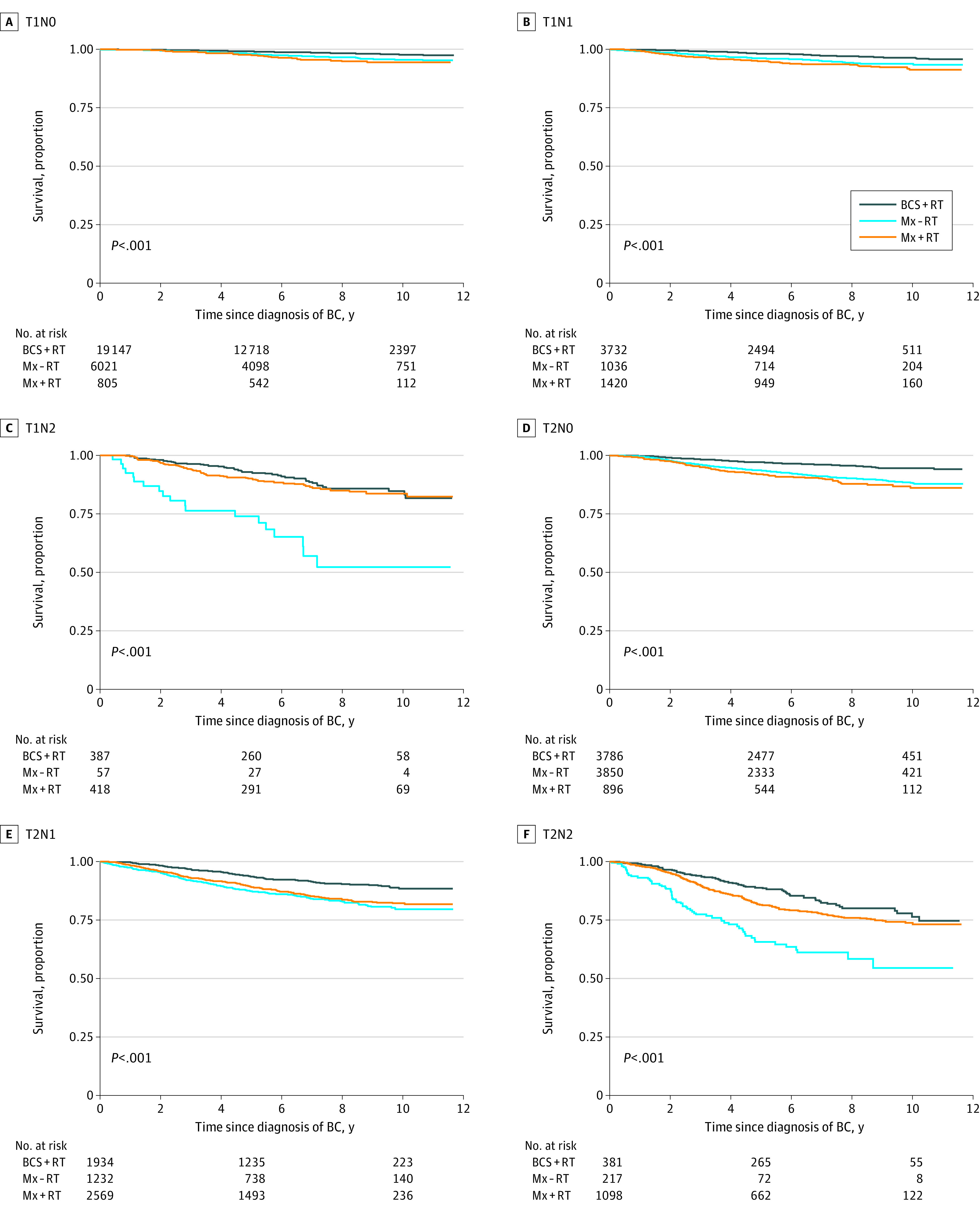
BC indicates breast cancer; BCS, breast-conserving surgery; Mx, mastectomy; RT, radiotherapy.
In age-, year-, and region-adjusted Cox regression models, Mx-RT was associated with an increased overall mortality rate (HR, 1.94; 95% CI, 1.82-2.06) and breast-cancer-specific mortality rate (HR, 1.89; 95% CI, 1.69-2.10) (Table 2, Model 1). The associations were even stronger for Mx+RT (OS: HR, 2.36; 95% CI, 2.21-2.53; BCSS: HR, 4.30; 95% CI, 3.88-4.76, model 1). After adjustment for tumor stage, subtype, and grade, the associations were reduced but remained nevertheless significant (model 2). Further adjustments for education level, family income, and country of birth (model 3) and the addition of CCI (model 4) did not alter the estimates substantially.
When stratifying by prognostic group, the associations varied substantially (Table 3). Mastectomy without RT was associated with increased overall and breast cancer–specific mortality rates compared with BCS+RT regardless of prognostic group, with the exception of T1N1 where no association was found (BCSS: HR, 0.99; 95% CI, 0.60-1.64). Mastectomy with RT was associated with an increased overall mortality rate in T1N0, T1N1, and T2N0, but not T1N2. Among T2N1 and T2N2, Mx+RT was not associated with increased overall mortality rates, although point estimates were moderately increased.
Table 3. Hazard Ratios of OS and BCSS for Locoregional Treatment by Prognostic Groups.
| Variable | OS | BCSS | ||||
|---|---|---|---|---|---|---|
| No. (deaths) | HR (95% CI)a | P value | No. (deaths) | HR (95% CI)a | P value | |
| Overall | ||||||
| BCS+RT | 29 367 (2272) | 1 [Reference] | NA | 29 367 (727) | 1 [Reference] | NA |
| Mx-RT | 12 413 (2912) | 1.79 (1.66-1.92) | <.001 | 12 413 (771) | 1.66 (1.45-1.91) | <.001 |
| Mx+RT | 7206 (1389) | 1.24 (1.13-1.37) | <.001 | 7206 (815) | 1.28 (1.10-1.49) | .001 |
| T1N0 | ||||||
| BCS+RT | 19 147 (1233) | 1 [Reference] | NA | 19 147 (243) | 1 [Reference] | NA |
| Mx-RT | 6021 (947) | 1.56 (1.40-1.74) | <.001 | 6021 (166) | 1.68 (1.31-2.15) | <.001 |
| Mx+RT | 805 (76) | 1.37 (1.03-1.84) | .03 | 805 (30) | 1.79 (1.08-2.99) | .03 |
| T1N1 | ||||||
| BCS+RT | 3732 (269) | 1 [Reference] | NA | 3732 (89) | 1 [Reference] | NA |
| Mx-RT | 1036 (261) | 2.02 (1.61-2.53) | <.001 | 1036 (49) | 0.99 (0.60-1.64) | .97 |
| Mx+RT | 1420 (190) | 1.43 (1.13-1.81) | .003 | 1420 (86) | 1.51 (1.02-2.25) | .04 |
| T1N2 | ||||||
| BCS+RT | 387 (66) | 1 [Reference] | NA | 387 (43) | 1 [Reference] | NA |
| Mx-RT | 57 (36) | 3.79 (2.10-6.83) | <.001 | 57 (19) | 5.05 (2.32-10.95) | <.001 |
| Mx+RT | 418 (96) | 1.31 (0.88-1.96) | .18 | 418 (56) | 1.29 (0.77-2.15) | .34 |
| T2N0 | ||||||
| BCS+RT | 3786 (372) | 1 [Reference] | NA | 3786 (140) | 1 [Reference] | NA |
| Mx-RT | 3850 (1021) | 1.70 (1.46-1.98) | <.001 | 3850 (299) | 1.66 (1.29-2.13) | <.001 |
| Mx+RT | 896 (140) | 1.53 (1.17-2.00) | .002 | 896 (85) | 1.64 (1.07-2.51) | .02 |
| T2N1 | ||||||
| BCS+RT | 1934 (244) | 1 [Reference] | NA | 1934 (149) | 1 [Reference] | NA |
| Mx-RT | 1232 (494) | 2.08 (1.70-2.55) | <.001 | 1232 (175) | 1.53 (1.13-2.08) | .006 |
| Mx+RT | 2569 (519) | 1.32 (1.08-1.60) | .007 | 2569 (328) | 1.21 (0.93-1.59) | .16 |
| T2N2 | ||||||
| BCS+RT | 381 (88) | 1 [Reference] | NA | 381 (63) | 1 [Reference] | NA |
| Mx-RT | 217 (153) | 2.77 (1.92-3.98) | <.001 | 217 (63) | 2.37 (1.47-3.82) | <.001 |
| Mx+RT | 1098 (368) | 1.29 (0.95-1.74) | .10 | 1098 (230) | 1.26 (0.87-1.82) | .23 |
Abbreviations: BCS, breast-conserving surgery; BCSS, breast cancer–specific survival; HR, hazard ratio; Mx, mastectomy; OS, overall survival; RT, radiotherapy.
Adjusted for age and calendar year at diagnosis, region, country of birth, family income, highest education, Nottingham grade, hormone receptor status (estrogen receptor/progesterone receptor), ERBB2, and Charlson Comorbidity Index 1 year prior to surgery.
When stratifying by follow-up time, the adjusted associations for all prognostic groups combined did not vary by short (0-5 years) or long (>5 years) follow-up for overall mortality rates, whereas the associations for breast cancer–specific mortality rates were stronger shortly (0-5 years) after surgery (eTable 2 in the Supplement). These association patterns were similar when stratified by prognostic groups, but the pattern was less consistent for breast cancer–specific mortality, where some prognostic groups had stronger associations with surgical treatment in the longer follow-up (Mx+RT: T1N0, T2N0).
Of special clinical interest are women who would probably have the choice of 2 guideline-adherent locoregional treatment alternatives: women with T1-2N0 tumors are commonly suitable for BCS+RT or Mx-RT, and women with T1-2N1-2 tumors are often suitable for BCS+RT or Mx+RT. In T1-2N0, adjusted HRs for both OS and BCSS showed a significant benefit of BCS+RT over Mx-RT. Among women with T1-2N1-2, those with T1N1 had lower mortality rates (OS and BCSS) with BCS+RT than with Mx+RT, and those with T2N1 had a lower mortality rate for OS only. For the remaining groups, no significant associations were found (Table 3).
Discussion
The findings of this report confirm the superiority of BCS with RT over Mx with an overall and breast cancer–specific relative survival gain of 56% to 70% in node-negative patients. This association resisted adjustment for tumor biology and status, socioeconomic background, and comorbidities. The same association was observed in lower-burden, node-positive disease, but not in women with higher nodal stage. Because there was no inferior survival for BCS in node-positive patients, this report gives no support to advocate Mx in women without specific risk factors, such as a strong family history or gene mutations.
There are complex interactions between breast cancer survival, socioeconomic status, and comorbidity. Individuals with a lower socioeconomic status present with more advanced disease, have a lower adherence to mammography screening, are less likely to receive chemotherapy, and have inferior survival rates.16,17,18 In addition, lifestyle factors increasing cancer risk and impacting survival, such as obesity and smoking, are more common in socioeconomically deprived groups, in addition to comorbidities that negatively affect completion rates of systemic therapy.28 Comorbidity is a mediator of death and will thus be associated with OS but may also be associated with BCSS by modulating adjuvant treatments. While a significant association of these factors with survival differences between BCS and Mx was anticipated, the adjustment for socioeconomic background and comorbidity was not associated with HRs for OS or BCSS. It is unlikely to assume that BCS would have some intrinsic positive association with survival, although it provides better health-related quality of life and was associated with fewer postoperative complications than Mx.29 Although tangential whole-breast irradiation after BCS reduces the risk of axillary recurrence in patients with node-negative disease,30 no independent association of RT could be observed in this study. Thus, further unmeasured confounding must be suspected: first, no Swedish register provides information on smoking or body mass index, and second, the CCI lacks important potential contributory diagnoses, such as alcohol and drug abuse as well as psychiatric disorders. Furthermore, there may be complex and synergistic interactions between multiple confounders that are difficult to control for in an observational setting.
The decision for BCS vs Mx is multifaceted. Importantly, it is influenced by the degree of patient-perceived information and involvement, fear of cancer recurrence, the perception that health outweighs breast retention, and the risk of reoperation in case of positive margins.14,31 These obstacles can be overcome by dedicated patient information and education, and a collaborative weighing of pros and cons by the treating clinician and the patient. It is striking that extensive breast surgery is more prevalent in node-positive disease despite suitability for breast conservation, indicating a misconception of safety, probably both from a patient and a physician perspective. In short, more extensive breast surgery does not appear to save any lives.
Limitations
The strength of this work is the population-based setting, providing a representative sample with complete follow-up and detailed clinical data. To our knowledge, this is the first report that integrates socioeconomic status and comorbidity in survival analyses juxtaposing locoregional treatments. Limitations include the lack of potential confounders, such as smoking and body mass index, and the potential underestimation of unlisted comorbid conditions. However, in terms of capturing comorbidities, both hospital and outpatient data and both main and contributing diagnoses were included, assuring a sound validity of CCI. Follow-up is still short considering late recurrences, especially in luminal-type breast cancer. Therefore, it will be important to replicate this survival analysis in additional studies.
Conclusions
In conclusion, this report adds evidence to support the recommended use of BCS with RT in both node-negative and node-positive breast cancer. Neither socioeconomic background and comorbidity nor the addition of postoperative RT after mastectomy diminished survival differences. This report casts additional doubt on the practice to offer mastectomy to patients who are suitable candidates for breast conservation.
eTable 1. ICD-10 codes based on the Royal College of Surgeons’ adaptation of the Charlson Comorbidity Index
eTable 2. Hazard ratios of OS and BCSS for locoregional treatment and prognostic group by short (0-5 years) and long (>5 years) follow-up
eFigure 1. CONSORT diagram for all primary breast cancer cases diagnosed in Sweden 2008-2017
eFigure 2. Survival proportions for breast cancer-specific survival by locoregional treatment group and age
eFigure 3. Survival proportions for overall survival by locoregional treatment group and prognostic group
eFigure 4. Survival proportions for overall survival by locoregional treatment group and age
References
- 1.Fisher B, Redmond C, Poisson R, et al. Eight-year results of a randomized clinical trial comparing total mastectomy and lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1989;320(13):822-828. doi: 10.1056/NEJM198903303201302 [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227-1232. doi: 10.1056/NEJMoa020989 [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Deng JP, Sun JY, et al. Noninferior outcome after breast-conserving treatment compared to mastectomy in breast cancer patients with four or more positive lymph nodes. Front Oncol. 2019;9:143. doi: 10.3389/fonc.2019.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vila J, Gandini S, Gentilini O. Overall survival according to type of surgery in young (≤40 years) early breast cancer patients: a systematic meta-analysis comparing breast-conserving surgery versus mastectomy. Breast. 2015;24(3):175-181. doi: 10.1016/j.breast.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 5.Abdulkarim BS, Cuartero J, Hanson J, Deschênes J, Lesniak D, Sabri S. Increased risk of locoregional recurrence for women with T1-2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol. 2011;29(21):2852-2858. doi: 10.1200/JCO.2010.33.4714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye JC, Yan W, Christos PJ, Nori D, Ravi A. Equivalent survival with mastectomy or breast-conserving surgery plus radiation in young women aged < 40 years with early-stage breast cancer: a national registry-based stage-by-stage comparison. Clin Breast Cancer. 2015;15(5):390-397. doi: 10.1016/j.clbc.2015.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Maaren MC, de Munck L, de Bock GH, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol. 2016;17(8):1158-1170. doi: 10.1016/S1470-2045(16)30067-5 [DOI] [PubMed] [Google Scholar]
- 8.de Boniface J, Frisell J, Bergkvist L, Andersson Y. Breast-conserving surgery followed by whole-breast irradiation offers survival benefits over mastectomy without irradiation. Br J Surg. 2018;105(12):1607-1614. doi: 10.1002/bjs.10889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofvind S, Holen Å, Aas T, Roman M, Sebuødegård S, Akslen LA. Women treated with breast conserving surgery do better than those with mastectomy independent of detection mode, prognostic and predictive tumor characteristics. Eur J Surg Oncol. 2015;41(10):1417-1422. doi: 10.1016/j.ejso.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 10.Agarwal S, Pappas L, Neumayer L, Kokeny K, Agarwal J. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg. 2014;149(3):267-274. doi: 10.1001/jamasurg.2013.3049 [DOI] [PubMed] [Google Scholar]
- 11.Davis LE, Fulton C, Bubis LD, et al. Patient-reported symptoms following mastectomy alone or lumpectomy plus radiation for early stage breast cancer: a cohort study. Breast Cancer Res Treat. 2019;175(3):721-731. doi: 10.1007/s10549-019-05196-x [DOI] [PubMed] [Google Scholar]
- 12.Al-Ghazal SK, Fallowfield L, Blamey RW. Comparison of psychological aspects and patient satisfaction following breast conserving surgery, simple mastectomy and breast reconstruction. Eur J Cancer. 2000;36(15):1938-1943. doi: 10.1016/S0959-8049(00)00197-0 [DOI] [PubMed] [Google Scholar]
- 13.Adam H, Docherty Skogh AC, Edsander Nord Å, et al. Risk of recurrence and death in patients with breast cancer after delayed deep inferior epigastric perforator flap reconstruction. Br J Surg. 2018;105(11):1435-1445. doi: 10.1002/bjs.10866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frisell A, Lagergren J, Halle M, de Boniface J. Socioeconomic status differs between breast cancer patients treated with mastectomy and breast conservation, and affects patient-reported preoperative information. Breast Cancer Res Treat. 2020;179(3):721-729. doi: 10.1007/s10549-019-05496-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pathirana TI, Jackson CA. Socioeconomic status and multimorbidity: a systematic review and meta-analysis. Aust N Z J Public Health. 2018;42(2):186-194. doi: 10.1111/1753-6405.12762 [DOI] [PubMed] [Google Scholar]
- 16.Lundqvist A, Andersson E, Ahlberg I, Nilbert M, Gerdtham U. Socioeconomic inequalities in breast cancer incidence and mortality in Europe-a systematic review and meta-analysis. Eur J Public Health. 2016;26(5):804-813. doi: 10.1093/eurpub/ckw070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dreyer MS, Nattinger AB, McGinley EL, Pezzin LE. Socioeconomic status and breast cancer treatment. Breast Cancer Res Treat. 2018;167(1):1-8. doi: 10.1007/s10549-017-4490-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal S, Ying J, Boucher KM, Agarwal JP. The association between socioeconomic factors and breast cancer-specific survival varies by race. PLoS One. 2017;12(12):e0187018. doi: 10.1371/journal.pone.0187018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vona-Davis L, Rose DP. The influence of socioeconomic disparities on breast cancer tumor biology and prognosis: a review. J Womens Health (Larchmt). 2009;18(6):883-893. doi: 10.1089/jwh.2008.1127 [DOI] [PubMed] [Google Scholar]
- 20.Halmin M, Bellocco R, Lagerlund M, Karlsson P, Tejler G, Lambe M. Long-term inequalities in breast cancer survival: a ten year follow-up study of patients managed within a National Health Care System (Sweden). Acta Oncol. 2008;47(2):216-224. doi: 10.1080/02841860701769768 [DOI] [PubMed] [Google Scholar]
- 21.Lee L, Cheung WY, Atkinson E, Krzyzanowska MK. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: a systematic review. J Clin Oncol. 2011;29(1):106-117. doi: 10.1200/JCO.2010.31.3049 [DOI] [PubMed] [Google Scholar]
- 22.Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin. 2016;66(4):337-350. doi: 10.3322/caac.21342 [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues G, Sanatani M. Age and comorbidity considerations related to radiotherapy and chemotherapy administration. Semin Radiat Oncol. 2012;22(4):277-283. doi: 10.1016/j.semradonc.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 24.Land LH, Dalton SO, Jørgensen TL, Ewertz M. Comorbidity and survival after early breast cancer: a review. Crit Rev Oncol Hematol. 2012;81(2):196-205. doi: 10.1016/j.critrevonc.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 25.Löfgren L, Eloranta S, Krawiec K, Asterkvist A, Lönnqvist C, Sandelin K; steering group of the National Register for Breast Cancer . Validation of data quality in the Swedish National Register for Breast Cancer. BMC Public Health. 2019;19(1):495. doi: 10.1186/s12889-019-6846-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cancer Staging Manual AJCC. 8 ed: Springer International Publishing; 2017. [Google Scholar]
- 27.Armitage JN, van der Meulen JH; Royal College of Surgeons Co-morbidity Consensus Group . Identifying co-morbidity in surgical patients using administrative data with the Royal College of Surgeons Charlson Score. Br J Surg. 2010;97(5):772-781. doi: 10.1002/bjs.6930 [DOI] [PubMed] [Google Scholar]
- 28.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37-43. doi: 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 29.Ng ET, Ang RZ, Tran BX, et al. Comparing quality of life in breast cancer patients who underwent mastectomy versus breast-conserving surgery: a meta-analysis. Int J Environ Res Public Health. 2019;16(24):E4970. doi: 10.3390/ijerph16244970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gentilini O, Botteri E, Leonardi MC, et al. Ipsilateral axillary recurrence after breast conservative surgery: The protective effect of whole breast radiotherapy. Radiother Oncol. 2017;122(1):37-44. doi: 10.1016/j.radonc.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 31.Lee WQ, Tan VKM, Choo HMC, et al. Factors influencing patient decision-making between simple mastectomy and surgical alternatives. BJS Open. 2018;3(1):31-37. doi: 10.1002/bjs5.50105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. ICD-10 codes based on the Royal College of Surgeons’ adaptation of the Charlson Comorbidity Index
eTable 2. Hazard ratios of OS and BCSS for locoregional treatment and prognostic group by short (0-5 years) and long (>5 years) follow-up
eFigure 1. CONSORT diagram for all primary breast cancer cases diagnosed in Sweden 2008-2017
eFigure 2. Survival proportions for breast cancer-specific survival by locoregional treatment group and age
eFigure 3. Survival proportions for overall survival by locoregional treatment group and prognostic group
eFigure 4. Survival proportions for overall survival by locoregional treatment group and age


