Abstract
Background.
Reward processing deficits have been implicated in the etiology of depression. A blunted reward positivity (RewP), an event-related potential elicited by feedback to monetary gain relative to loss, predicts new onsets and increases in depression symptoms. Etiological models of depression also highlight stressful life events. However, no studies have examined whether stressful life events moderate the effect of the RewP on subsequent depression symptoms. We examined this question during the key developmental transition from childhood to adolescence.
Methods.
A community sample of 369 children (mean age of 9) completed a self-report measure of depression symptoms. The RewP to winning vs. losing was elicited using a monetary reward task. Three years later, we assessed stressful life events occurring in the year prior to the follow-up. Youth depressive symptoms were rated by the children and their parents at baseline and follow-up.
Results.
Stressful life events moderated the effect of the RewP on depression symptoms at follow-up such that a blunted RewP predicted higher depression symptoms in individuals with higher levels of stressful life events. This effect was also evident when events that were independent of the youth’s behavior were examined separately.
Conclusions.
These results suggest that the RewP reflects a vulnerability for depression that is activated by stress.
Introduction
Anhedonia — or a deficit in the ability to experience pleasure — is a core symptom of depression. This has generated a large literature examining the role of behavioral, physiological, and neural measures of various aspects of reward in depressed individuals (Forbes and Dahl, 2005, 2012; Goldstein and Klein, 2014; Pizzagalli, 2014; Proudfit et al. 2015; Kujawa and Burkhouse, 2017; Keren et al. 2018). For instance, depressed individuals exhibit aberrant reward-related behavior and neural activity when anticipating or receiving rewards (Pizzagalli et al. 2005; Forbes et al. 2006; McFarland and Klein, 2009; Pizzagalli et al. 2009; Eshel and Roiser, 2010; Robinson et al. 2012; Stringaris et al. 2015).
Aberrant reward-related behavior and neural activity may also indicate risk for depression (Forbes et al. 2006; Luking et al. 2016b; Kujawa and Burkhouse, 2017; Keren et al. 2018). Offspring of depressed mothers exhibit a different pattern of neural activity during anticipation or receipt of reward compared to offspring of never depressed mothers (Gotlib et al. 2010; McCabe et al. 2012; Luking et al. 2016a). Moreover, longitudinal studies using electrophysiology and functional magnetic resonance imaging (fMRI) have found that blunted responsivity to rewards predicts increases in symptoms or onsets of depressive disorders (Hanson et al. 2015; Stringaris et al. 2015; Nelson et al. 2016).
Event related potentials (ERPs), particularly the reward positivity (RewP; Proudfit, 2015), provides an index of reward responsiveness. RewP is a positive deflection in the ERP signal following positive information (e.g., monetary gain) and is either reduced or absent when receiving negative information (e.g., monetary loss). Although previously referred to as the medial frontal negativity and feedback negativity, we refer to this ERP as the RewP as evidence suggests that this component is best characterized by a positive deflection in the ERP signal during reward trials that is diminished or absent in response to no reward. Supporting its construct validity, the RewP has been linked to behavioral and self-report measures of reward responsiveness and neural activation in reward-related regions like the ventral striatum (Carlson et al., 2011; Bress and Hajcak, 2013).
Children, adolescents, and adults with depression exhibit a smaller RewP to monetary reward compared to individuals without depression (Foti and Hajcak, 2009; Bress et al. 2012; Liu et al. 2014; Belden et al. 2016). A blunted RewP has also been found in children and adolescents of depressed parents compared to offspring of non-depressed parents (Foti et al. 2011; Kujawa et al. 2014). Longitudinal studies indicate that a blunted RewP predicts increases in depressive symptoms and first onsets of depressive disorders in adolescents (Bress et al. 2013; Nelson et al. 2016; Kujawa et al. 2018).
This literature suggests that reduced reward responsiveness, as measured by RewP, could reflect a predisposition to depression (Kujawa and Burkhouse, 2017). If so, then whether or not individuals with a blunted RewP manifest depressive symptoms may depend on their exposure to life stress (Meehl, 1975; Auerbach et al. 2014). Most theoretical and empirical work on stress and reward has focused on the deleterious effects of stress exposure on reward-related behavior and neural function (Willner et al. 1987; Bogdan and Pizzagalli, 2006; Auerbach et al. 2014). In contrast, there is a paucity of research regarding stress as moderating the effect of reward processing on depression. One recent study reported that the interaction between ventral striatal activity in a gambling task and stressful life events was associated with concurrent depression symptoms in youth (Luking et al. 2018). Another study found that interactions of ventral striatal activity during a monetary incentive task with early and recent life stress were associated with concurrent anhedonic depressive symptoms in young adults (Corral-Frías et al. 2015). To our knowledge, only one longitudinal study has examined interactions between reward responsiveness and stress on depression. Retrospective reports of early childhood maltreatment and slower reaction time on a monetary incentive delay task interacted to predict subsequent depression symptoms in older adolescents (Dennison et al. 2016). The present study extends this literature by examining whether stressful life events moderate the effect of the RewP in late childhood on predicting depression symptoms in early adolescence.
When exploring stress as a moderator of the RewP in predicting depression, it is important to distinguish between independent and dependent life events (Brown and Harris, 1978; Shrout et al. 1989). Independent, or fateful, events are stressors that occur irrespective of an individual’s own behavior (e.g., illness of a family member; moving to a new city and school because of a parent’s job). With dependent events, an individual’s own behavior may play a role in generating the event (e.g., romantic relationship break-up; failing a class or losing a job). Both independent and dependent events predict depression (Kendler et al. 1999; Hankin et al. 2007; Kendler et al. 2002; Stroud et al. 2011; Vrshek-Schallhorn et al. 2015). However, the causal role of independent events is clearer, as dependent events may result from prior predispositions or symptoms that account for their relationship with depression (Kendler et al. 1999; Hammen, 2006; Kercher et al. 2009; Kendler and Gardner, 2010). There are also developmental considerations, as independent life events may play a greater role earlier in development, as children and younger adolescents have less control over their environments, and therefore fewer opportunities to “generate” dependent events (Rudolph and Hammen, 1999; Rice et al. 2003; Hammen, 2006). In addition, children and younger adolescents may be more susceptible to stressors occurring to others on whom they depend (e.g., parental divorce or unemployment), which are generally independent of the youth’s behavior.
In summary, few studies have examined the moderating role of stressful life events on reward processing in predicting depression, and to our knowledge none have used a neural measure of reward responsivity in a longitudinal design. The goal of this paper is to examine whether life stressors moderate the association between the RewP and depression symptoms from late childhood to early adolescence, which marks the beginning of the risk period for onsets of depression (Salk et al. 2017). We assessed the RewP and depression symptoms in a sample of 9-year-old children. Three years later current depression and stressful life events over the past year were assessed. We hypothesized that stressful life events would moderate the effect of the RewP on future depression symptoms, such that adolescents with both a decreased RewP and greater stress would exhibit the largest increases in depressive symptoms from age 9 to 12. We also explored independent and dependent life events separately given the stronger causal inferences afforded by independent events and their relevance in childhood and early adolescence.
Methods
Participants were drawn from the Stony Brook Temperament study, a longitudinal examination of temperament and psychopathology (Klein and Finsaas, 2017). Three-year-old children and their families (N = 559) were included if at least one English-speaking biological parent could participate and if the child did not have significant medical or developmental disabilities. Three years later, an additional group of six-year-olds from racial/ethnic minority groups (N =50) were added to increase the sample’s diversity. Parents provided consent and children provided assent to participate. Procedures were approved by the Stony Brook University Institutional Review Board.1
This study uses data from the age 9 and 12 assessments. Of the 470 children who participated at the age 9 assessment, we excluded 45 participants for poor quality RewP data, 2 with missing depressive symptom data from that wave, and 4 who had a lifetime DSM-IV diagnosis of MDD or dysthymia assessed via the Kiddie Schedule for Affective Disorders and Schizophrenia (Kaufman et al. 1997), resulting in N =419. An additional 34 participants did not attend the age 12 follow-up, 2 participants were missing depressive symptom data from that wave, and 13 were missing the UCLA Life Stress Interview (Hammen et al. 1987), resulting in sample of 370. One additional participant with outlier data was removed, leaving a final sample of 369.
Of these 369 participants, 54.7% (n =202) were male and 81.3% (n = 300) were non-Hispanic Caucasian. Participants with complete data had a mean age of 9.16 years (SD = 0.37) at baseline and 12.65 years (SD = 0.44) at follow-up. The 369 included participants did not significantly differ from the 101 excluded participants on sex, χ²(1, N =470) = 0.003, p > .05 or racial/ethnic minority status, χ²(1, N =470) = 3.15, p > .05. Excluded participants were slightly older at baseline, t(468)=2.12, p=.03, hence age was used as a covariate in regression analyses.
Measures
Depression Symptoms.
At the age 9 and 12 assessments, children and both parents completed the Children’s Depression Inventory (CDI; Kovacs, 1992), a measure of depressive symptoms occurring during the past two weeks that is designed for ages 7–17. The 6-week test-retest stability of the CDI has been reported as .67 (Finch Jr et al. 1987). In our sample, internal consistency of the CDI was good (median alpha = .76, range .74 – .79 youth, mother, and father report at age 9; median alpha = .80, range .79 – .83 for age 12). The mean CDI scores at age 9 were 4.89 (SD = 4.14), 7.13 (SD = 4.80), and 7.13 (SD = 4.14) for youth, mother, and father reports, respectively. At age 12, the mean CDI scores were 4.53 (SD = 5.00), 6.88 (SD = 4.89), and 7.28 (SD = 4.88) for youth, mother, and father reports, respectively. Correlations between mother and father reports were r = .41 at age 9, and r = .50, at age 12. Correlations among child and parent reports at ages 9 and 12, respectively, were: mother-child r=.22 and .32; and father-child r=.14 and .34. The youth’s, mother’s and father’s CDI reports were z-scored and averaged.2 Participants were included if at least two informants completed the CDI. Of the 369 participants, 325 and 298 had data from all three informants at the age 9 and 12 assessments, respectively.
Life Stress.
At the age 12 assessment, children and a parent were each administered the UCLA Life Stress Interview (LSI; Hammen et al. 1987). The LSI assesses episodic and chronic stressors involving the youth during the past 12 months by content domains including social life, friendships, family relationships, and work/school. Events reported in this study occurred in the year prior to the age 12 follow-up and at least two years after the initial age 9 RewP assessment. Following Brown and Harris (1978), the interviewer presented a description of all events reported by the youth and/or parent and the circumstances surrounding the event without describing the participant’s affective reactions to a team of raters for consensus ratings of objective threat using a 5-point scale ranging from 1 (“minimal or no effect”) to 5 (“great effect”). Raters also indicated the degree of behavioral dependence for each event using a 3-point scale ranging from 1(“completely dependent”) to 3 (“completely independent”). We summed the total number of events an individual experienced during the assessment interval. Events were counted as independent if behavioral dependence was rated as a 3 and dependent when given a score of 1. Events that were coded as 2 (ambiguous) were not included in either the dependent or independent categories. We then created separate continuous total scores of independent and dependent events by summing the total number events for each type. Previous reports have found inter-rater reliability regarding the impact of events and behavioral dependence to be excellent (r = .85 and .97, respectively; Rudolph and Hammen, 1999).
Reward task.
The RewP was elicited using a computerized monetary reward task, which was described to participants as a guessing task where they could earn up to $5 (Foti and Hajcak, 2009; Beldin et al. 2016). Participants were presented with two doors and instructed to select one by clicking the right or left mouse button, revealing whether the door yielded monetary gain or loss. Reward feedback was random and not dependent on the participant’s choice. After selecting a door, a fixation mark appeared on screen for 1000 milliseconds, which was followed by gain feedback indicated by a green arrow pointing up or loss feedback represented by a red arrow pointing down. Feedback displayed for 2000 milliseconds. Participants completed 60 trials comprised of an equal number of gain and loss trials presented in a random order. Participants were told that gain trials yielded $0.50 to add to their total and loss trials would subtract $0.25. In our study, the split-half reliability of activity elicited during gain and loss trials was .79 and .63. In previous studies, the 2-year test-retest reliability of gain and loss trials was .64 and .67, respectively (Bress et al. 2015).
EEG data acquisition and processing.
EEG was recorded using Biosemi with 34 channels based on the 10/20 system. Participants were fitted with a 32 channel Lycra cap with additional electrodes for Iz and FCz. Data were referenced to electrodes placed on the right and left mastoids during offline processing. Additional facial electrodes were placed above and below the left eye, to the left of the left eye, and to the right of the right eye in order to correct for eye blinks. The data was sampled at a rate of 1024 Hz. The data was further processed offline using Brain Vision Analyzer (Brain Products). Data was filtered using 0.01 and 30 Hz cutoffs. The data was segmented so that a trial began 500 ms before feedback onset and ended at 1000 ms after feedback onset. Artifacts were flagged if a voltage difference of 300 μV occurred within a given trial, the voltage changed more than 50 μV between data points, or there was a difference in voltage of less than .50 μV within 100 ms intervals. The data were then visually inspected to remove additional artifacts. Participants had on average 28.73 loss trials and 28.96 gain trails retained after artifact rejection. The 500 ms interval before feedback onset was used to baseline correct the data. Following recent papers (e,g., Kessel et al. 2015), the RewP was formed by taking the average mean amplitude across gain trials and subtracting the average signal to loss trials occurring at 275–375 ms following task feedback. The RewP at FCz and Cz were pooled to reduce noise from a single electrode source, and because this is where the difference between reward and loss was maximal in the overall sample.
Data-analytic approach
We conducted descriptive statistics and bivariate correlation analyses among major study variables using SPSS version 22 (IBM). Descriptive statistics were used to illustrate the overall levels of depressive symptoms, life events, and values of the RewP for the sample. Bivariate correlations were examined amongst major study variables to show the relationships between variables and the stability of depressive symptoms from age 9 to 12. We then conducted hierarchical multiple regression analyses using Mplus version 8 (Muthén & Muthén, 2017). All predictor variables including covariates (age, sex, and baseline age 9 depressive symptoms) were centered before being entered in the model. In the first regression, the interaction term was formed by taking the product of the centered life events and RewP scores. In the second regression, we created two interaction terms, the first comprised of the product of the centered dependent life events and RewP, and a second comprised of independent life events and the RewP. Simple slopes were plotted using the regression equation for the full sample and points to plot were selected based on 16th, 50th, and 84th percentile ranks.
Results
Descriptive statistics and correlations are presented in Table 1; Figure 1 depicts the RewP waveform and scalp distribution. Depression symptoms were moderately stable over time. The total number of stressful life events, as well as independent and dependent stressful life events, in the year prior to follow-up were associated with depression symptoms at age 12. At age 9, males experienced greater depression symptoms than females, but by age 12 this gender difference was non-significant. Consistent with prior analyses, the RewP was significantly larger in males (Kujawa et al., 2015). Females were significantly more likely to experience independent life events.3
Table 1.
Correlations among major study variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. Gender (female =0 & male =1) | .01 | .21*** | .18*** | −.06 | .01 | −.11* | .09 | |
| 2. Age at baseline | - | .07 | −.01 | −.06 | −.02 | −.07 | .07 | |
| 3. Age 9 CDI | - | −.01 | .10 | .06 | .09 | .55*** | ||
| 4. Age 9 RewP | - | .02 | .01 | .02 | −.09 | |||
| 5. Total Stress | - | .76*** | .77*** | .17*** | ||||
| 6. Dependent events | - | .18** | .12* | |||||
| 7. Independent events | - | .14** | ||||||
| 8. Age 12 CDI | - | |||||||
|
M (SD) |
54.7% | 9.16 (0.37) |
0.01 (0.73) |
5.28 (7.71) |
4.54 (2.77) |
1.97 (1.78) |
2.54 (1.82) |
0.02 (0.78) |
CDI = Children’s Depression Inventory; RewP = Reward Positivity.
= p< .05;
= p< .01;
= p< .001
Figure 1.

ERP waveform and RewP scalp distribution
FCz and Cz electrode channels were pooled to generate this waveform. The scale distribution of the RewP is depicted as the mean amplitude of gain trials subtracted from loss trials occurring at 275–375 ms following monetary feedback.
RewP and total stressful life events
Multiple regression analysis was used to examine whether stressful life events in the year prior to the age 12 assessment moderated the effects of age 9 RewP in predicting depressive symptoms at age 12, adjusting for sex, age, and baseline depression. The RewP X total life stress interaction term significantly predicted depressive symptoms at follow up (Table 2). Simple slopes were calculated at low (16th percentile), intermediate (50th percentile), and high (84th percentile) values of total stressful life events, as shown in Figure 2A. Simple slopes were significant at high levels of stress (b= −0.023, SE = 0.006, 95% CI [−0.034, −0.011], p < .001), but were not significant at intermediate (b= −0.007, SE = 0.004, 95% CI [−0.016, 0.001], p = .09) or low levels of total stressful life events (b= 0.008, SE = 0.006, 95% CI [−0.005, 0.021],p = .21).4
Table 2.
Age 12 depression symptoms predicted by an interaction between REWP and total stressful life events
| Β | 95% CI Lower - Upper |
t | p | |
|---|---|---|---|---|
| Gender (male) | 0.01 | [−0.08, 0.09] | 0.13 | .89 |
| Age at baseline | 0.05 | [−0.03, 0.14] | 1.25 | .21 |
| Age 9 Depression Symptoms | 0.54 | [0.47, 0.61] | 14.30 | <.001 |
| Age 9 RewP | −0.08 | [−0.16, 0.01] | −1.85 | .06 |
| Total stressful life events | 0.12 | [0.03, 0.20] | 2.75 | <.01 |
| Total stressful life events X Age 9 RewP | −0.15 | [−0.23, −0.07] | −3.50 | <.001 |
| R2 = 0.35 |
B is a standardized regression coefficient; RewP = Reward Positivity.
Figure 2.
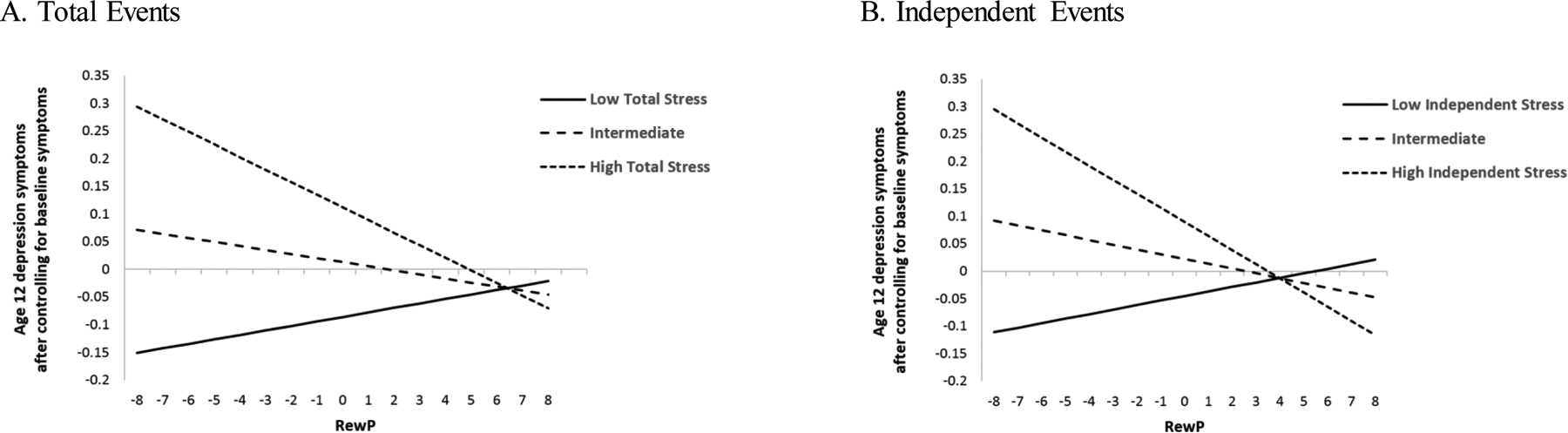
Depression symptoms at age 12 follow-up predicted by interactions between stress and RewP
CDI = Children’s Depression Inventory; RewP = Reward Positivity. Simple slopes for total stressful life events and independent life events were plotted at the 16th, 50th, and 84th percentiles. CDI scores are calculated by a composite of z-scores based on youth and parent’s report of depression symptoms and are adjusted for age 9 symptoms and covariates.
RewP, dependent, and independent stressful life events
Next, dependent and independent life events were entered simultaneously in a multiple regression model (Table 3). Neither the main effect for dependent events nor the RewP X dependent events interaction were significant. In contrast, independent life events significantly moderated the RewP-depression relationship. Simple slopes were calculated at low, intermediate, and high values (16th, 50th, and 84th percentiles) of independent life events, as shown in Figure 2B. Simple slopes were significant at high (b= −0.026, SE = 0.007, 95% CI [−0.039, −0.012], p < .001) and intermediate (b= −0.009, SE = 0.004, 95% CI [−0.017, 0.00], p <.05) levels of independent stress, but were not significant at low levels of independent stress (b= 0.009, SE = 0.007, 95% CI [−0.003, 0.021], p = .19).5
Table 3.
Age 12 depression symptoms predicted by an interaction of RewP and independent and dependent life events
| Β | 95% CI Lower - Upper |
t | p | |
|---|---|---|---|---|
| Gender (male) | 0.002 | [−0.09, 0.09] | 0.04 | .97 |
| Age at baseline | 0.05 | [−0.03, 0.14] | 1.25 | .21 |
| Age 9 Depression Symptoms | 0.54 | [0.47, 0.61] | 14.44 | <.001 |
| Age 9 RewP | −0.08 | [−0.16, 0.01] | −1.78 | .08 |
| Dependent events | 0.07 | [−0.02, 0.15] | 1.55 | .12 |
| Independent events | 0.08 | [−0.01, 0.16] | 1.81 | .07 |
| Dependent events X age 9 RewP | −0.05 | [−0.13, 0.04] | −1.06 | .29 |
| Independent events X age 9 RewP | −0.14 | [−0.23, −0.06] | −3.29 | <.001 |
| R2 = 0.35 |
B is a standardized regression coefficient; RewP = Reward Positivity.
Discussion
This is the first prospective study examining life events as moderating a neural measure of reward in predicting subsequent depressive symptoms. We found that episodic life events moderated the effects of the RewP on depression symptoms at follow-up, even after adjusting for baseline symptoms. Children with more blunted RewP and higher stress exhibited the greatest depression symptoms at follow-up in early adolescence. Additionally, independent stress specifically moderated the effects of the RewP on depression. These findings indicate that exposure to life stress influences whether blunted reward sensitivity will lead to greater depression in early adolescence, a period that marks the beginning of a rapid increase in rates of depression (Salk et al. 2017). Moreover, these data suggest that a reward-based vulnerability to depression is evident as early as middle childhood, well before the post-pubertal surge in depression symptoms and diagnoses. Thus, there may be a window of at least several years for preventive interventions targeting blunted reward sensitivity.
Our results are consistent with the limited previous research examining the interaction between reward processing and stress on depression. Prior studies were mostly cross-sectional designs or relied on retrospective reports of early childhood stress (Corral-Frías et al. 2015; Dennison et al. 2016; Luking et al. 2018). Most previous studies also used self-report questionnaires to assess life events, which have much lower validity than interview assessments (Harkness and Monroe, 2016). Our study is novel in that we used a prospective, longitudinal design and a state-of-the-art semi-structured interview for life events. Additionally, we examined independent and dependent life events separately. This is important because independent events afford clearer causal interpretations, whereas associations between dependent events and depression may due to third variables such as genes, personality, cognitive style, or even prior abnormalities in reward processing (Kendler et al. 2010; Auerbach et al. 2014). Interestingly, we found effects for independent but not dependent events despite consistent evidence for the depressogenic effects of dependent events in many studies of older adolescents and adults (Kendler et al. 1999, 2010). This may be because children and younger adolescents have fewer dependent events due to the social and familial constraints on their autonomy, and because they are more affected by stressors occurring to others on whom they depend (Rudolph and Hammen, 1999; Rice et al. 2003).
This study extends the broader literature and theoretical perspectives regarding stress and reward. The majority of previous research has focused on the effects of stress in disrupting reward systems (Auerbach et al. 2014; Pizzagalli, 2014), with studies observing reward-related changes in behavior and neural function following exposure to stress in rodents (e.g., Willner et al. 1987) and humans (e.g., Berenbaum and Connelly, 1993). We instead took the perspective of a diathesis-stress model, where stress serves to activate pre-existing vulnerabilities, in this case blunted reward processing, which then leads to depression. However, finding that life events moderate the effect of the RewP on depression does not contradict previous studies. Rather, simultaneously incorporating the effects of prior stress on reward function along with the moderating effect of later stress on the relationship between reward processing and subsequent depression may yield a more dynamic and comprehensive account of the development of depressive disorders.
Previous work has demonstrated that the RewP is associated with concurrent depression (Foti and Hajcak, 2009; Bress et al. 2012; Liu et al. 2014; Belden et al. 2016) and familial risk for depression (Foti et al. 2011; Kujawa et al. 2014). However, there has been little attention to potential moderators, such as stressful life events, of the association between the RewP and depression. Consideration of moderators may clarify for whom and under what circumstances a blunted RewP leads to depression, indicating who may benefit most from preventive measures. Unlike some moderators (demographic characteristics, family history), one’s capacity to cope with stressors is potentially modifiable and therefore a good target for prevention.
While this study extends the literature on the associations between reward processing, stress, and depression, it has several limitations. First, the ERP task used indexes reward as the difference between gain and loss trials, making it difficult to disentangle whether positive or negative feedback drives results. However, supplementary analyses (footnote 4) suggested that response to gains may be driving the interaction with independent events. Second, the task uses monetary rewards; it is possible that other stimuli, like social reward, could yield different, perhaps stronger, findings. Third, we had to examine symptoms rather than episode onsets of depression due to low rate of depression diagnoses at age 12. However, it is increasingly recognized that depression exists on a continuum and that episodes represent relatively arbitrary demarcations (Watson, 2005). Nevertheless, it will be important to follow this sample further into adolescence and adulthood to determine if the pattern of results holds for predicting depression diagnoses. Fourth, we only assessed life events that occurred 12 months prior to the follow-up, meaning that some life events occurring earlier in the three-year follow-up interval may not have been captured. Lastly, in adjusting for baseline symptoms, we may not have captured symptom increases occurring after the age 9 wave but before the events occurred. However, the fact that we observed effects for independent events indicates that even if symptoms had begun to increase prior to events, they did not play a causal role in the events’ occurrence.
In summary, we found that episodic stressful life events moderated the effects of reduced reward responsiveness at age 9 on depression symptoms 3 years later, adjusting for baseline symptoms. Moreover, these findings were evident even when analyses were limited to independent events that could not have been influenced by the youth’s behavior. Additional studies using reward paradigms that employ non-monetary stimuli, examine other developmental periods, and incorporate the effects of prior stress on reward functioning will help elucidate the complex relationships between reward processing, stress, and depression.
Footnotes
We have previously reported that the RewP at age 9 moderated the effect of a maternal history of depression on depression symptoms at age 12 (Kujawa et al. 2018). The current paper differs by focusing on recent life stress. Nevertheless, we included maternal history of depression and the interaction between maternal depression and the RewP as covariates in our regression analyses and found that it did not influence our findings.
We also examined children’s reports and the aggregation of both parents’ reports separately for each of the main analyses. The results reported for RewP X life events interactions using the composite measure were the same when using either children’s or parents’ reports alone.
Although the RewP was not significantly correlated with depressive symptoms at age 12, when we examined ERPs to loss and gain separately, the gain RewP was inversely correlated with age 12 depression symptoms, with more blunted gain amplitude associated with higher symptoms. The loss RewP was not significantly associated with symptoms.
We also conducted additional regression analyses examining interactions with either sex or pubertal status and found that neither variable significantly moderated the RewP by stressful life event interaction term. Pubertal status was assessed at ages 9 (reported by children and both parents) and at age 12 (reported by children and one parent) using the Puberty Development Scale (Peterson et al., 1988). A parent child aggregate was formed by z-scoring their responses.
We conducted additional regressions examining interactions between separate gain and loss wave forms with total, independent, and dependent life events. The pattern of results for response to gain interacting with both total and independent events was consistent with the pattern of results observed using the RewP difference score. Response to loss did not interact with life events in any analyses, suggesting that the results for the RewP difference score were driven by responsivity to gain. We also conducted additional analyses examining gain and loss as residual scores instead of using the RewP difference score. The same pattern emerged again in which the gain residual score significantly interacted with life events.
Reference List
- Auerbach RP, Admon R, Pizzagalli DA (2014). Adolescent depression: Stress and reward dysfunction. Harvard Review of Psychiatry 22, 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden AC, Irvin K, Hajcak G, Kappenman ES, Kelly D, Karlow S, Luby JL, Barch DM (2016). Neural correlates of reward processing in depressed and healthy preschool-age children. Journal of the American Academy of Child & Adolescent Psychiatry 55, 1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum H, Connelly J (1993). The effect of stress on hedonic capacity. Journal of Abnormal Psychology 102, 474–481. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli DA (2006). Acute stress reduces reward responsiveness: Implications for depression. Biological Psychiatry, 60, 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress JN, Foti D, Kotov R, Klein DN, Hajcak G (2013). Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology 50, 74–81. [DOI] [PubMed] [Google Scholar]
- Bress JN, Hajcak G (2013). Self-report and behavioral measures of reward sensitivity predict the feedback negativity. Psychophysiology 50, 610–616. [DOI] [PubMed] [Google Scholar]
- Bress JN, Meyer A, Proudfit GH (2015). The stability of the feedback negativity and its relationship with depression during childhood and adolescence. Development and Psychopathology 27, 1285–1294. [DOI] [PubMed] [Google Scholar]
- Bress JN, Smith E, Foti D, Klein DN, Hajcak G (2012). Neural response to reward and depressive symptoms in late childhood and early adolescence. Biological Psychology 89, 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GW, Harris T (1978). Social origins of depression: A study of psychiatric disorder in women. Travistock Publications Limited: London. [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G (2011). Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortial activity: A combined ERP and fMRI study. NeuroImage 57, 1608–1616. [DOI] [PubMed] [Google Scholar]
- Corral-Frías NS, Nikolova YS, Michalski LJ, Baranger DAA, Hariri AR, Bogdan R (2015). Stress-related anhedonia is associated with ventral stirautm reactivity to reward and trasndiagnostic psychiatric symptomatology. Psychological Medicine 45, 2605–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison M, Sheridan MA, Busso DS, Jenness JL, Peverill M, Rosen ML, McLaughlin KA (2016). Neurobehavioral markers of resilience to depression amongst adolescents exposed to child abuse. Journal of Abnormal Psychology 125, 1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel N, Roiser JP (2010). Reward and punishment processing in depression. Biological Psychiatry 68, 118–124. [DOI] [PubMed] [Google Scholar]
- Finch AJ Jr, Saylor CF, Edwards GL, McIntosh JA (1987). Children’s Depression Inventory: Reliability over repeated administrations. Journal of Clinical Child Psychology 16, 339–341. [Google Scholar]
- Forbes EE, Christopher May J, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, Birmaher B, Axelson DA, Dahl RE (2006). Reward-related decision-making in pediatric major depressive disorder: An fMRI study. Journal of Child Psychology and Psychiatry and Allied Disciplines 47, 1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE (2005). Neural systems of positive affect: Relevance to understanding child and adolescent depression? Development and Psychopathology 17, 827–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE (2012). Research Review: Altered reward function in adolescent depression: What, when and how? Journal of Child Psychology and Psychiatry and Allied Disciplines 35 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Hajcak G (2009). Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biological Psychology 81, 1–8. [DOI] [PubMed] [Google Scholar]
- Foti D, Kotov R, Klein DN, Hajcak G (2011). Abnormal neural sensitivity to monetary gains versus losses among adolescents at risk for depression. Journal of Abnormal Child Psychology 39, 913–924. [DOI] [PubMed] [Google Scholar]
- Goldstein BL, Klein DN (2014). A review of selected candidate endophenotypes for depression. Clinical Psychology Review 35, 417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J (2010). Neural processing of reward and loss in girls at risk for major depression. Archives of General Psychiatry 67, 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C (2006). Stress generation in depression: Reflections on origins, research, and future directions. Journal of clinical psychology 62, 1065–1082. [DOI] [PubMed] [Google Scholar]
- Hammen CL, Gordon D, Burge D, Adrian C, Jaenicke C, Hiroto D (1987). Maternal affective disorders, illness, and stress: Risk for children’s psychopathology. American Journal of Psychiatry 144, 736–741. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Mermelstein R, Roesch L (2007). Sex differences in adolescent depression: Stress exposure and reactivity models. Child Development 78, 279–295. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Hariri AR, Williamson DE (2015). Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biological Psychiatry 78, 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness KL, Monroe SM (2016). The assessment and measurement of adult life stress: Basic premises, operational principles, and design requirements. Journal of Abnormal Psychology 125, 727–745. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao UM, Flynn C, Moreci P, Williamson D, Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry 36, 980–988. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO (2010). Dependent stressful life events and prior depressive episodes in the prediction of major depression: the problem of causal inference in psychiatric epidemiology. Archives of general psychiatry 67, 1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA (2002). Toward a comprehensive developmental model for major depression in women. American Journal of Psychiatry 159, 1133–1145. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA (1999). Causal relationship between stressful life events and the onset of major depression. American Journal of Psychiatry 156, 837–841. [DOI] [PubMed] [Google Scholar]
- Kercher AJ, Rapee RM, Schniering CA (2009). Neuroticism, life events and negative thoughts in the development of depression in adolescent girls. Journal of Abnormal Child Psychology 37, 903–915. [DOI] [PubMed] [Google Scholar]
- Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, Pan PM, Meffert L, Kaiser A, Wolke S, Pine DS, Stringaris A (2018). Reward processing in depression: A conceptual and meta-analytic review across fMRI and EEG studies. American Journal of Psychiatry 175, 1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel EM, Kujawa A, Hajcak Proudfit G, Klein DN (2015). Neural reactivity to monetary rewards and losses differentiates social from generalized anxiety in children. Journal of child psychology and psychiatry 56, 792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DN, Finsaas MC (2017). The Stony Brook Temperament Study: Early antecedents and pathways to emotional disorders. Child Development Perspectives 11, 257–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M (1992). Children’s depression inventory: manual. Multi-Health Systems. [Google Scholar]
- Kujawa A, Burkhouse KL (2017). Vulnerability to depression in youth: Advances from affective neuroscience. : Biological Psychiatry Cognitive Neuroscience and Neuroimaging 2, 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, Klein DN (2018). Reduced reward responsiveness moderates the effect of maternal depression on depressive symptoms in offspring: evidence across levels of analysis. Journal of Child Psychology and Psychiatry 60, 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Proudfit GH, Klein DN (2014). Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. Journal of Abnormal Psychology 123, 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Proudfit GH, Laptook R, Klein DN (2015). Early parenting moderates the association between parental depression and neural reactivity to rewards and losses in offspring. Clinical Psychological Science 3 503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WH, Wang LZ, Shang HR, Shen Y, Li Z, Cheung EF, Chan RC (2014). The influence of anhedonia on feedback negativity in major depressive disorder. Neuropscyhologia 53, 213–220. [DOI] [PubMed] [Google Scholar]
- Luking KR, Nelson BD, Infantolino ZP, Sauder CL, Hajcak G (2018). Ventral striatal function interacts with positive and negative life events to predict concurrent youth depressive symptoms. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 1, 937–946. [DOI] [PubMed] [Google Scholar]
- Luking KR, Pagliaccio D, Luby JL, Barch DM (2016a). Depression risk predicts blunted neural responses to gains and enhanced response to loses in healthy children. Journal of the American Academy of Child and Adolescent Psychiatry 55, 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking KR, Pagliaccio D, Luby JL, Barch DM (2016b). Reward processing and risk for depression across development. Trends in Cognitive Sciences 20, 456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Woffindale C, Harmer CJ, Cowen PJ (2012). Neural processing of reward and punishment in young people at increased familial risk of depression. Biological Psychiatry 72, 588–594. [DOI] [PubMed] [Google Scholar]
- McFarland BR, Klein DN (2009). Emotional reactivity in depression: Diminished responsiveness to anticipated reward but not to anticipated punishment or to nonreward or avoidance. Depression and Anxiety 26, 117–122. [DOI] [PubMed] [Google Scholar]
- Meehl PE (1975). Hedonic capacity: Some conjectures. Bulletin of the Menninger Clinic 39, 295–307. [PubMed] [Google Scholar]
- Muthén L, & Muthén BO (2017). Mplus user’s guide (version 8.0). Mplus user’s guide (seventh edition) (Seventh Ed). Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Nelson BD, Perlman G, Klein DN, Kotov R, Hajcak G (2016). Blunted neural response to rewards as a prospective predictor of the development of depression in adolescent girls. American Journal of Psychiatry 173, 1223–1230. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence 17, 117–133. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA (2014). Depression, stress, and anhedonia: Toward a synthesis and integrated model. Annual Review of Clinical Psychology 10, 393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M (2009). Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. Journal of Psychiatric Research 43, 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP (2005). Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biological Psychiatry 57, 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit GH (2015). The reward positivity: From basic research on reward to a biomarker for depression. Psychophysiology 52, 449–459. [DOI] [PubMed] [Google Scholar]
- Proudfit GH, Bress JN, Foti D, Kujawa A, Klein DN (2015). Depression and event related potentials: Emotional disengagement and reward insensitivity. Current Opinion in Psychology 4, 110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice F, Harold GT, Thapar A (2003). Negative life events as an account of age‐related differences in the genetic aetiology of depression in childhood and adolescence. Journal of Child Psychology and Psychiatry 44, 977–987. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Cools R, Carlisi CO, Sahakian BJ, Drevets WC (2012). Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. American Journal of Psychiatry 169, 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Hammen CL (1999). Age and gender as determinant of stress exposure, generation and reactions in youngsters: A transactional perspective. Child and Development 70, 660–677. [DOI] [PubMed] [Google Scholar]
- Salk RH, Hyde JS, Abramson LY (2017). Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychological Bulletin 143, 783–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Link BG, Dohrenwend BP, Skodol AE, Stueve A, Mirotznik J (1989). Characterizing life events as risk factors for depression: The role of fateful loss events. Journal of Abnormal Psychology 98, 460–467. [DOI] [PubMed] [Google Scholar]
- Stringaris A, Vidal-Ribas Belil P, Artiges E, Lemaitre H, Gollier-Briant F, Wolke S, Vulser H, Miranda R, Penttilä J, Struve M, Fadai T, Kappel V, Grimmer Y, Goodman R, Poustka L, Conrod P, Cattrell A, Banashewski T, Bokde ALW, Bromberg U, Büchel C, Flor H, Frouin V, Gallinat J, Garavan H, Gowland P, Heinz A, Ittermann B, Nees F, Papadopoulos D, Paus T, Smolka MN, Walter H, Whelan R, Martinot JL, Schumann G, Paillère-Martinot ML, IMAGEN Consortium (2015). The brain’s response to reward anticipation and depression in adolescence: Dimensionality, specificity, and longitudinal predictions in a community-based sample. American Journal of Psychiatry 172, 1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud CB, Davila J, Hammen C, Vrshek-Schallhorn S (2011). Severe and nonsevere events in first onsets versus recurrences of depression: Evidence for stress sensitization. Journal of Abnormal Psychology 120, 142–154. [DOI] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S, Stroud CB, Mineka S, Hammen C, Zinbarg RE, Wolitzky-Taylor K, Craske MG (2015). Chronic and episodic interpersonal stress as statistically unique predictors of depression in two samples of emerging adults. Journal of Abnormal Psychology 124, 918–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D (2005). Rethinking the mood and anxiety disorders: A quantitative hierarchical model for DSM-V. Journal of Abnormal Psychology 114, 522–536. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R (1987). Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 93, 358–364. [DOI] [PubMed] [Google Scholar]


