Abstract
Screening mammography reduces breast cancer mortality; however, when used to examine women with dense breasts, its performance and resulting benefits are reduced. Increased breast density is an independent risk factor for breast cancer. Digital breast tomosynthesis (DBT), ultrasound (US), molecular breast imaging (MBI), MRI, and contrast-enhanced mammography (CEM) each have shown improved cancer detection in dense breasts when compared with 2D digital mammography (DM). DBT is the preferred mammographic technique for producing a simultaneous reduction in recalls (i.e., additional imaging). US further increases cancer detection after DM or DBT and reduces interval cancers (cancers detected in the interval between recommended screening examinations), but it also produces substantial additional false-positive findings. MBI improves cancer detection with an effective radiation dose that is approximately fourfold that of DM or DBT but is still within accepted limits. MRI provides the greatest increase in cancer detection and reduces interval cancers and late-stage disease; abbreviated techniques will reduce cost and improve availability. CEM appears to offer performance similar to that of MRI, but further validation is needed. Dense breast notification will soon be a national standard; therefore, understanding the performance of mammography and supplemental modalities is necessary to optimize screening for women with dense breasts.
Keywords: breast density, contrast-enhanced mammography, molecular breast imaging, MRI, tomosynthesis, ultrasound
Mammography and Breast Density
Mammography is the only imaging modality proven to reduce breast cancer mortality, as shown in both randomized controlled trials and observational studies [1, 2]. In the United States, women with an average risk for breast cancer are advised to commence mammographic screening between 40 and 50 years old and continue screening annually or biennially through age 74 years or for as long as they are in good health [3–5]. The greatest benefit is observed when annual mammography begins at age 40 years [6]. With the peak breast cancer incidence occurring in Hispanic and Black women at a younger age (in their forties [7]), it is particularly important to begin annual screening of these populations by age 40 years.
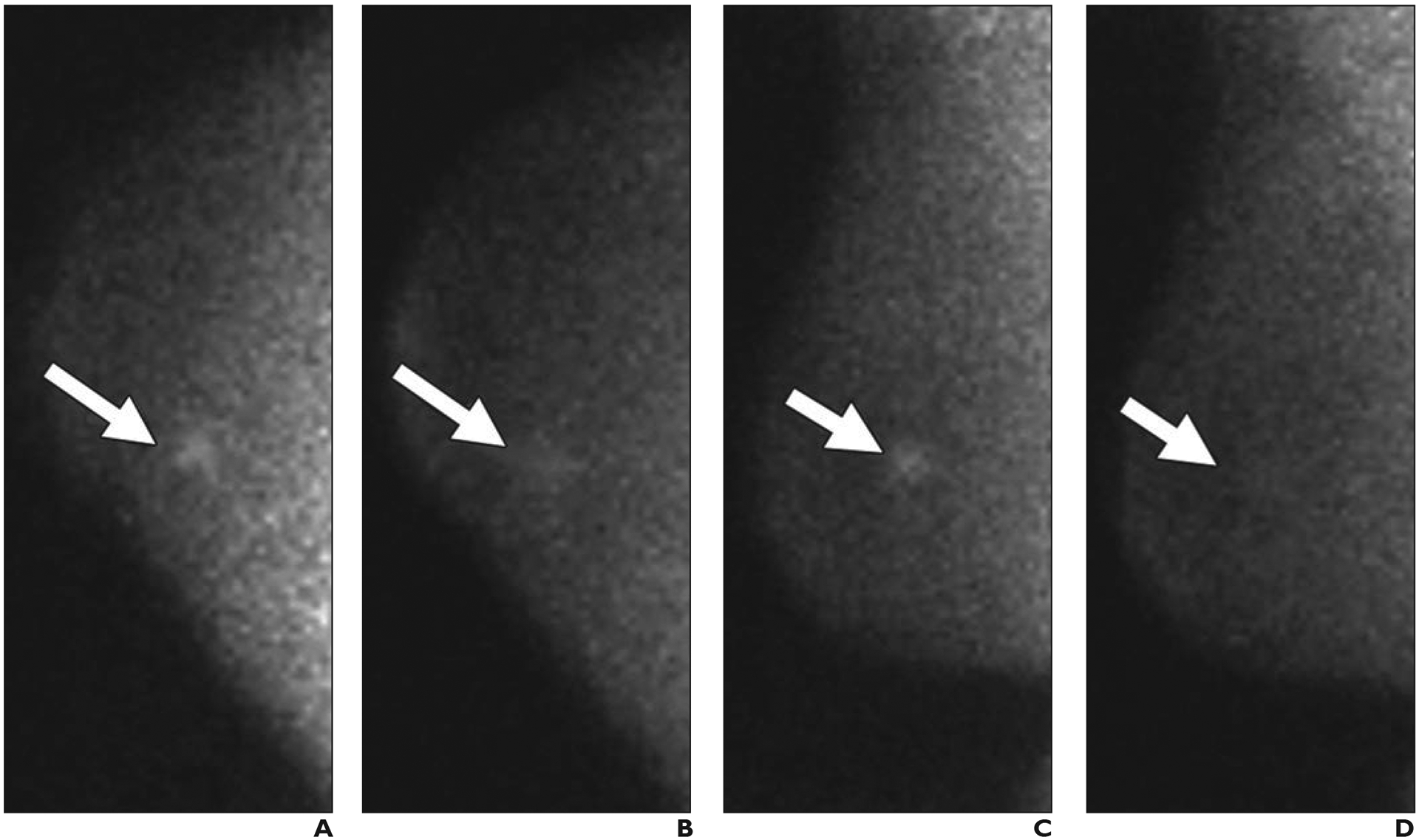
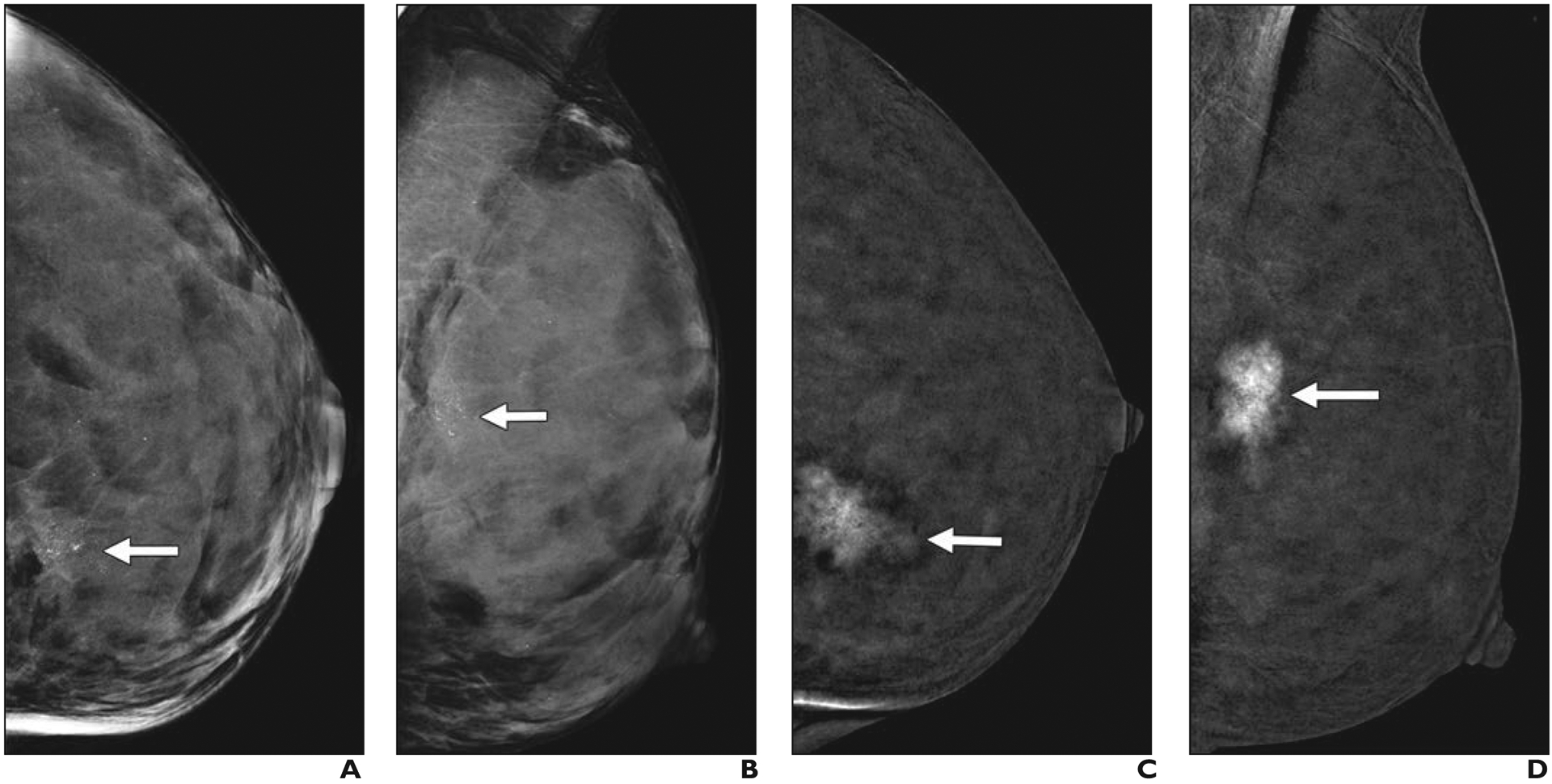
However, not all women benefit equally from mammography. Mammographic performance is dependent on breast density. Breast density refers to the amount of fibroglandular tissue relative to fat and is determined either visually or quantitatively on mammography. The BI-RADS 5th edition delineates four categories of breast density (from least to most fibroglandular tissue): category A (fatty), B (scattered), C (heterogeneously dense), and D (extremely dense) [8] (Fig. 1). Categories C and D denote dense tissue. Although malignant calcifications remain well visualized, noncalcified cancers (approximately 45% of invasive cancers [9]) can be masked by dense tissue. The sensitivity range of mammography is 81–93% for fatty breasts, 84–90% for breasts with scattered fibroglandular density, 69–81% for heterogeneously dense breasts, and 57–71% for extremely dense breasts [10].
Fig. 1—
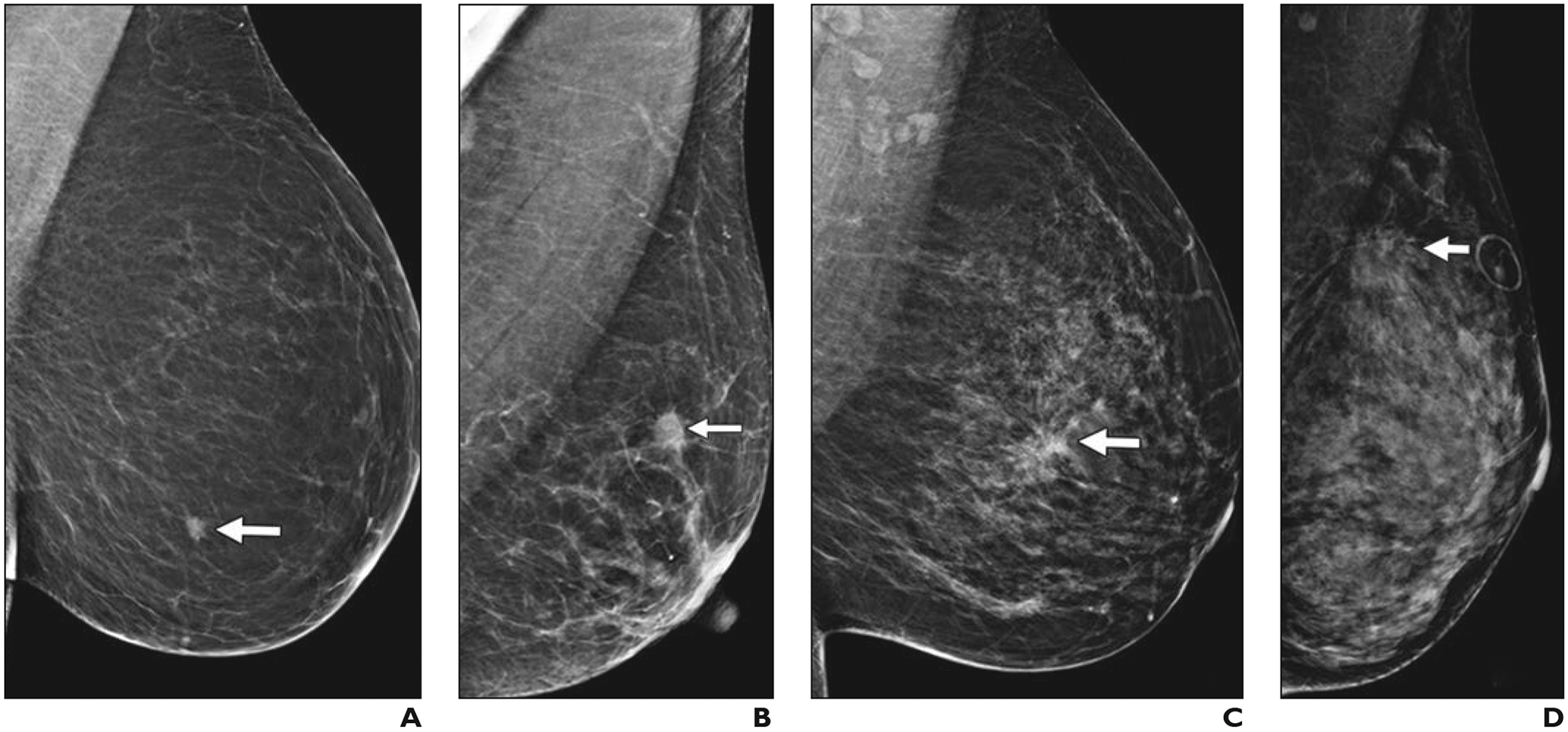
Left mediolateral oblique digital mammography (DM) images of cancer in breasts representing each of four BI-RADS breast density categories.
A, 61-year-old woman with pathogenic BRCA1 mutation. Screening DM image shows fatty breast and spiculated mass (arrow) that proved to be a 0.9-cm grade 3 invasive ductal carcinoma (IDC) that was estrogen receptor (ER) negative, progesterone receptor (PR) negative, and human epidermal growth factor receptor 2 (HER2 [also known as ERBB2]) negative with associated ductal carcinoma in situ (DCIS). Ki-67 proliferation index was high (90%). Three sentinel nodes were negative for metastasis.
B, 65-year-old woman with scattered fibroglandular breast tissue density. Screening DM image shows spiculated mass (arrow) that proved to be 1.1-cm grade 2 IDC with extensive DCIS that was ER positive, PR positive, and HER2 negative. Ki-67 proliferation index was high (30%), and metastatic sentinel node showed focal extracapsular extension.
C, 40-year-old woman with heterogeneously dense parenchyma, which may obscure small masses. Baseline screening DM image shows irregular mass (arrow) with distortion in central left breast. Core biopsy specimen showed 1.2-cm IDC that was ER positive, PR positive, and HER2 negative. Ki-67 proliferation index was moderate (15%). Patient went elsewhere for treatment.
D, 43-year-old woman with extremely dense parenchyma, which lowers sensitivity of mammography. Baseline screening DM image shows spiculated mass (arrow) with associated distortion in upper posterior left breast. This proved to be T2 (> 2 cm) grade 2 IDC with associated DCIS that was ER positive, PR positive, and HER2 negative. Ki-67 proliferation index was low (10%), and sentinel node biopsy was negative for metastasis.
Dense breasts are common. Approximately 36% of women more than 40 years old have heterogeneously dense breasts, and 7% have extremely dense breasts [11]. To date, 38 states and the District of Columbia have enacted dense breast notification laws to alert patients to the possibility of false-negative mammography results caused by dense tissue masking cancer detection; specific information and recommendations (if any) provided to patients vary from state to state. National standardized reporting criteria are being developed by the U.S. Food and Drug Administration [12].
Knowledge of breast density is critical because mammographic performance and resultant screening outcomes are significantly impacted. Both increased false-positives findings [10, 13] and reduced cancer detection are seen for women with dense breasts [14]. Lower reductions in breast cancer mortality have been shown for women with dense breasts [15, 16], who have more interval cancers than do women with nondense breasts [17].
Interval cancer rates vary from 0.8 cancers per 1000 screenings in annual screening programs to 2.1 cancers per 1000 screenings in biennial screening programs [18, 19]. Some interval cancers reflect more aggressive biology (triple-negative or human epidermal growth factor receptor 2 [HER2; also known as ERBB2]–positive cancers) with rapid growth. Other interval cancers, however, are histologically similar to cancers detected on screening mammography and are likely masked in dense breast tissue [20, 21]. Interval cancers are 13–18 times more common among women with extremely dense versus fatty breasts [22, 23]. In tracking long-term outcomes from screening, Webb et al. [24] reported 609 deaths from breast cancer in a cohort screened for more than 10 years, with 60 of 609 deaths (9.9%) attributable to interval cancers. More effective screening should reduce the frequency of interval cancers.
In addition to masking detection of some cancers, dense parenchyma is one of the strongest and most prevalent risk factors for breast cancer [25]. Compared to women with fatty breasts, women with extremely dense breasts have a four- to fivefold greater risk of breast cancer [22, 26]. Depending on age and hormonal status, women with heterogeneously and extremely dense tissue are 1.4–1.6 and 1.5–2.1 times more likely to have breast cancer develop, respectively, compared with women with scattered fibroglandular density [27].
Data on the histologic type, aggressiveness, size, and nodal status of breast cancers in dense breasts are sparse. Pooled analysis found that younger women (those < 55 years old) with dense breasts are more likely to have estrogen receptor–negative tumors than older women [28]; increasing tumor size and positive lymph node status correlated with increasing breast density.
Lymph node status remains the most important prognostic factor for breast cancer outcomes. Indeed, across randomized trials of mammography, only those that increased detection of node-negative invasive cancers reduced mortality [29]. Similarly, reducing late-stage disease significantly contributes to decreased mortality from breast cancer [30].
Although mammography has proven value in reducing breast cancer mortality, the negative impact of breast density on mammographic performance highlights the need for more effective screening strategies. Digital breast tomosynthesis (DBT), ultrasound (US), molecular breast imaging (MBI), MRI, and contrast-enhanced mammography (CEM) each have shown value in improving breast cancer detection in women with dense breasts. The metrics associated with improved outcomes (increased incremental cancer detection rate [ICDR], especially of node-negative invasive cancers; reduced interval cancers; and reduced late-stage disease) as well as recall rates will be examined for each modality. This comparative analysis of modality performance, in conjunction with information regarding availability and cost, can guide screening strategies for women with dense breasts.
Digital Breast Tomosynthesis
DBT is a digital mammographic technique in which multiple, angled, low-dose projection images are acquired and reconstructed into thin (typically 1 mm) slices [31]. By minimizing the impact of superimposed structures, tomosynthesis enhances lesion visibility and reduces unnecessary recalls due to summation artifacts [32]. Introduction of synthetic reconstructed 2D mammography has allowed the radiation dose to remain comparable to that of conventional 2D DM [33] and has permitted DBT with synthetic reconstructions to replace DM.
DBT has been proven effective in screening. A multiinstitutional retrospective analysis revealed an ICDR of 1.2 cancers per 1000 screening examinations (95% CI, 0.8–1.6; p < 0.001) when DBT is added to DM [34]. Two prospective European screening trials [35, 36] showed increases of 2.3 and 2.7 cancers per 1000 screening examinations with DBT. Of note, an improved cancer detection rate (CDR) is attributable to additional invasive tumors rather than ductal carcinoma in situ, lessening the potential for overdiagnosis. Limited data exist regarding biologic subtypes of invasive cancers detected with DBT only, suggesting a trend toward detection of smaller (< 2 cm) tumors with luminal A–like characteristics [37, 38]. Using TMIST (Tomosynthesis Mammographic Imaging Screening Trial) criteria (metastasis [including nodal metastasis], size ≥ 1 cm and either HER2-positive status or estrogen receptor– and progesterone receptor–negative status or both, or size ≥ 2 cm), Conant et al. [39] reported greater detection of cancers with poor prognosis using DBT versus DM, although differences were not significant. A doubling of the rate of detection of invasive lobular carcinoma has been observed [34], likely because of increased conspicuity of tumor spiculation and architectural distortion.
An ongoing criticism of screening mammography is its generation of false-positive recall findings, which prompt unnecessary additional imaging and intervention [4]. DBT reduces false-positive recalls due to summation artifact [32] and also because of its better depiction of multiplicity and bilaterality of circumscribed masses (allowing benign assessment [40]). In conjunction with gains in cancer detection, DBT achieves a simultaneous 2% absolute recall rate reduction (relative reduction, 15–17%) [34–36]. Conant et al. [39] showed that these gains were sustained over multiple rounds of DBT screening. However, interval cancer rates were not significantly impacted by DBT [39], as was also reported by Bahl et al. [41].
Given the inverse relationship between mammographic sensitivity and increasing density, stratification of DBT performance by breast density is of particular interest. Table 1 summarizes four studies [13, 35, 42, 43] that evaluated screening outcomes for women who underwent DBT plus DM versus DM alone, as stratified by breast density. DBT consistently increased the CDR and decreased recalls for both dense and nondense tissue. Rafferty et al. [43] observed the greatest gain in cancer detection and recall reduction for women with heterogeneously dense breasts (Fig. 2). No improvement in cancer detection was noted for women with extremely dense breasts (Fig. 3), although false-positive recalls were reduced [43]. Østerås et al. [42] observed improved CDR but no reduction in recalls for women with extremely dense breasts (Table 1). Lowry et al. [44] observed reduced recalls and improved CDR on incidence screening DBT (versus DM) for women with heterogeneously dense breasts but not for women with extremely dense breasts.
TABLE 1:
Summary of Studies Showing Incremental Increase in Cancer Detection and Decrease in Recall Rates After Addition of DBT to DM for Evaluation of Dense and Nondense Breasts
| Finding, Breast Density, and Modality | Ciatto et al., 2013 [35]a | Rafferty et al., 2016 [43]b | Østerås et al., 2019 [42]a,c | Conant et al., 2019 [13]b | ||||
|---|---|---|---|---|---|---|---|---|
| Value | P | Value | P | Value | P | Value | P | |
| CDR per 1000 screening examinations | ||||||||
| Nondense | ||||||||
| DM and DBT | 8.4 (51/6079) | 5.1 (455/89,171) | 7.7 (122/15,785) | 5.6 (87/15,612) | ||||
| DM | 5.6 (34/6079) | 4.2 (610/146,910) | 6.0 (94/15,785) | 4.5 (301/66,664) | ||||
| Differenced | 2.8 | < 0.0001 | 0.95 | < 0.001 | 1.8 | < 0.001 | 1.1 | 0.091 |
| Dense | ||||||||
| DM and DBT | 6.6 (8/1215) | 5.8 (495/84,243) | 12.5 (106/8466) | 7.8 (73/9321) | ||||
| DM | 4.1 (5/1215) | 4.5 (597/131,996) | 9.7 (82/8466) | 5.4 (192/35,309) | ||||
| Differenced | 2.5 | 0.25 | 1.4 | < 0.001 | 2.8 | < 0.001 | 2.4 | < 0.0001 |
| Heterogeneously dense | ||||||||
| DM and DBT | NR | 6.1 (450/72,481) | 12.5 (83/6645) | NR | ||||
| DM | NR | 4.5 (528/113,290) | 9.6 (64/6645) | NR | ||||
| Differenced | 1.6 | < 0.001 | 2.9 | < 0.001 | ||||
| Extremely dense | ||||||||
| DM and DBT | NR | 3.9 (45/11,762) | 12.7 (23/1821) | NR | ||||
| DM | NR | 3.8 (69/18,706) | 9.9 (18/1821) | NR | ||||
| Differenced | 0.1 | 0.88 | 2.8 | 0.06 | ||||
| Recall rate per 1000 screening examinationse | ||||||||
| Nondense | ||||||||
| DM and DBT | 39 (233/6028) | 79 (6955/89,171) | 79 (1246/15,785) | 76 (2334/30,839) | ||||
| DM | 45 (273/6028) | 90 (12,845/146,910) | 96 (1515/15,785) | 100 (8549/85,275) | ||||
| Differenced | −6 | 0.0035 | −12 | < 0.001 | −17 | < 0.001 | −24 | < 0.001 |
| Dense | ||||||||
| DM and DBT | 66 (80/1207) | 109 (9030/84,243) | 125 (1061/8466) | 105 (2109/20,070) | ||||
| DM | 73 (88/1207) | 127 (16,582/131,996) | 133 (1126/8466) | 135 (5931/44,094) | ||||
| Differenced | −7 | 0.35 | −18 | < 0.001 | −8 | < 0.001 | −30 | < 0.0001 |
| Heterogeneously dense | ||||||||
| DM and DBT | NR | 110 (7852/72,481) | 120 (804/6645) | NR | ||||
| DM | NR | 128 (14,484/113,290) | 132 (879/6645) | NR | ||||
| Differenced | −18 | < 0.001 | −12 | < 0.001 | ||||
| Extremely dense | ||||||||
| DM and DBT | NR | 98 (1178/11,762) | 141 (257/1821) | NR | ||||
| DM | NR | 114 (2098/18,706) | 136 (247/1821) | NR | ||||
| Differenced | −16 | < 0.001 | 5 | 0.82 | ||||
Note—Nondense tissue was defined as almost entirely fatty tissue or scattered fibroglandular density, and dense tissue was defined as heterogeneously dense or extremely dense tissue. CDR = cancer detection rate, DBT = digital breast tomosynthesis, DM = 2D full-field digital mammography, NR = not reported.
Each patient had both standard DM and DBT examinations and served as her own control.
Historical controls for DM results.
Results are reported at the breast level and inferred at the participant level; one woman had bilateral breast cancer.
Difference between DM plus DBT versus DM alone.
Fig. 2—
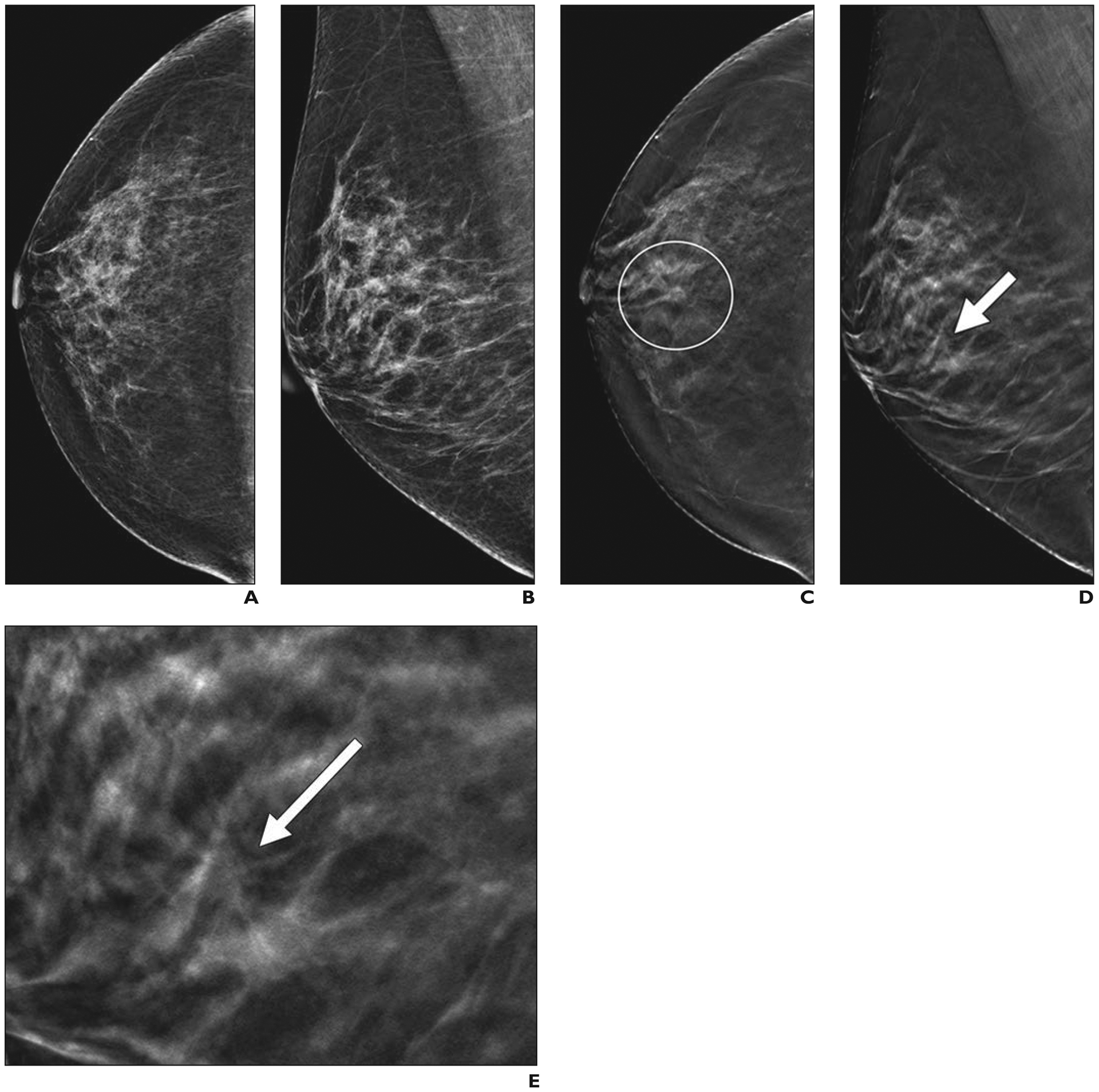
46-year-old woman with cancer seen on annual screening tomosynthesis examination only.
A and B, Two-dimensional craniocaudal (A) and mediolateral oblique (B) digital mammograms show heterogeneously dense breast tissue.
C and D, Craniocaudal digital breast tomosynthesis image (C) shows spiculated mass (within circle), which has much more subtle appearance on mediolateral oblique image (arrow, D).
E, Close-up of mediolateral oblique digital breast tomosynthesis image in D shows mass (arrow) in detail. Core biopsy and excision revealed 0.7-cm grade 2 invasive ductal carcinoma that was estrogen receptor positive, progesterone receptor positive, and human epidermal growth factor receptor 2 (HER2 [also known as ERBB2]) negative. Sentinel node was negative for metastasis.
Fig. 3—
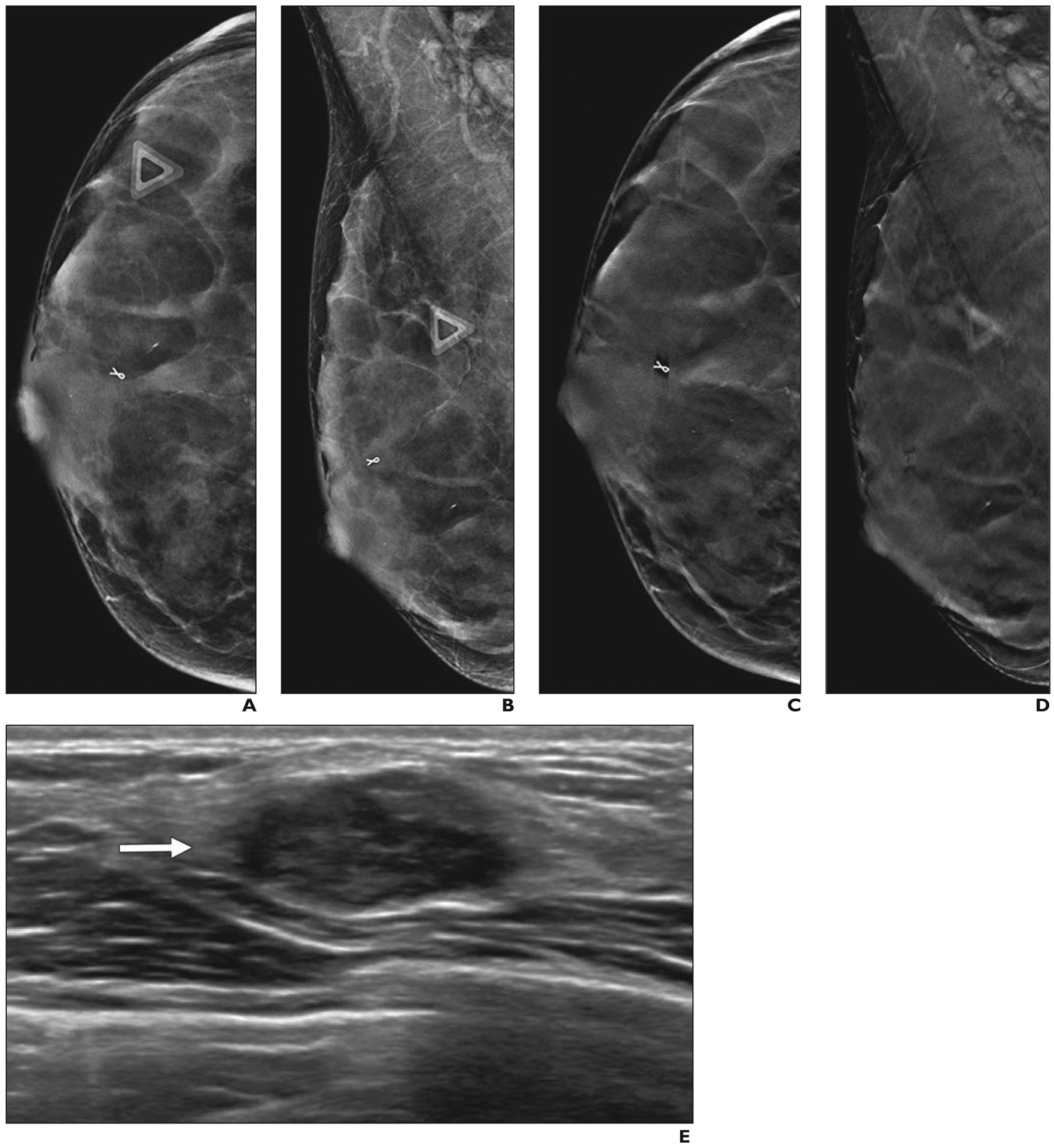
51-year-old woman with right breast pain who had cancer that was masked by extremely dense breast tissue on tomosynthesis.
A and B, Two-dimensional craniocaudal (A) and mediolateral oblique (B) digital mammograms show extremely dense parenchyma with ribbon clip from prior benign biopsy and no abnormality at site of pain (triangles).
C and D, Craniocaudal (C) and mediolateral oblique (D) digital breast tomosynthesis images show no abnormality.
E, Directed ultrasound image of area of focal pain shows 1.6-cm irregular, hypoechoic mass (arrow). Ultrasound-guided core needle biopsy revealed grade 3 invasive ductal carcinoma that was triple receptor negative. Excision after neoadjuvant chemotherapy showed no residual tumor. Sentinel node was negative for metastasis.
The improved CDR and reduced recalls of DBT versus DM are expected to produce improved health care outcomes at an equivalent or reduced cost [45–47]. Most major insurance carriers now cover DBT, and at least 17 states and the District of Columbia mandate insurance coverage for screening DBT [48]. Despite the use of DBT, limitations in detecting cancer persist, particularly in women with extremely dense breasts. Such women receive the greatest benefit from supplemental screening strategies.
Screening Ultrasound: Technique
Unlike other methods of supplemental screening, screening US requires neither ionizing radiation nor IV contrast agents. There are several methods of performing whole-breast US, including handheld US (HHUS) performed by a physician (usually a radiologist), HHUS performed by a technologist, and automated US (AUS). Training is necessary; for HHUS, the minimum experience required of American College of Radiologic Imaging Network (ACRIN) 6666 investigators was 500 breast US examinations [49]. US billing is currently coded as either limited breast US (Current Procedural Terminology [CPT] code 76642) or complete breast US (CPT code 76641), which does not specify screening or diagnostic use, with a charge designated for each breast (using a right or left modifier). ICD-10 (International Classification of Diseases, 10th revision) code R92.2 (incomplete examination due to dense breasts) is used with ICD-10 diagnosis code Z12.39 (other screening). Insurance typically will cover screening US when it is ordered by a health care provider, although deductibles and copayments apply in most states.
HHUS methods should use a high-frequency linear-array transducer of at least 12 MHz and, ideally, 17–18 MHz. Surveying is most efficient when performed in the transverse and sagittal planes; the axilla can be electively included. For negative examinations, documentation should include at least one image from each quadrant and the retroareolar region, as validated in the ACRIN 6666 protocol [49]. For simple cysts, a single image is sufficient, and documentation is encouraged to reduce recalls from mammography and DBT (the ACRIN 6666 protocol required that only the largest simple cyst in each quadrant be documented). For lesions other than simple cysts, orthogonal images should be obtained without and with calipers in a manner that captures their longest diameter (often radial [like a clock hand] along the duct system), with horizontal and anteroposterior diameters documented on that image and the horizontal diameter documented on the orthogonal view. Power Doppler imaging can be used to document slow flow. Harmonics can help clear artifactual internal echoes in small cysts or clustered microcysts. Elastography can be applied to assess lesion stiffness. A cine loop can be obtained for later review when doing batch reading and can be helpful when technologists performing HHUS are uncertain about vague findings.
Technical recall occasionally is needed to distinguish artifactual shadowing at the interface of fat lobules, or intraductal debris, from a true mass. An isolated, circumscribed, oval hypoechoic or isoechoic mass that is seen on US only can be assessed as BI-RADS category 3 (probably benign), and follow-up at the annual screening US examination is reasonable [50]. Failure to document a mass that is undergoing monitoring can also prompt a technical recall (at no additional charge).
The most common AUS method uses a wide (15 cm), curved transducer to acquire transverse images with coronal and sagittal reconstructions. A minimum of three but sometimes five to six acquisitions are needed for breast coverage. A semiautomated approach uses an automated arm and standard transducer [51]. A final assessment typically can be rendered with HHUS, including BI-RADS category 4 or 5 lesions for which biopsy is recommended (with a process that should include contacting the patient and physician), whereas additional targeted US is needed for most AUS recalls; Doppler and elastography are not available during AUS.
Outcomes of Screening Ultrasound Examinations
For women with dense breasts, the average ICDR was 2.0–2.7 cancers per 1000 screening examinations, using either an AUS or HHUS technique, and 88% of cancers detected only on screening US were invasive [52] (Fig. 4). Where details were available, when HHUS was performed by a physician, 497 of 554 invasive cancers (89.7%) detected were node negative, and when performed by a technologist, 102 of 123 invasive cancers (82.9%) were node negative; similarly, 63 of 69 invasive cancers (92%) detected on AUS were node negative [52]. In nearly every series, the mean size range of invasive cancers was 7–14 mm, and invasive lobular cancers represented 15–20% of those detected by screening US only.
Fig. 4—
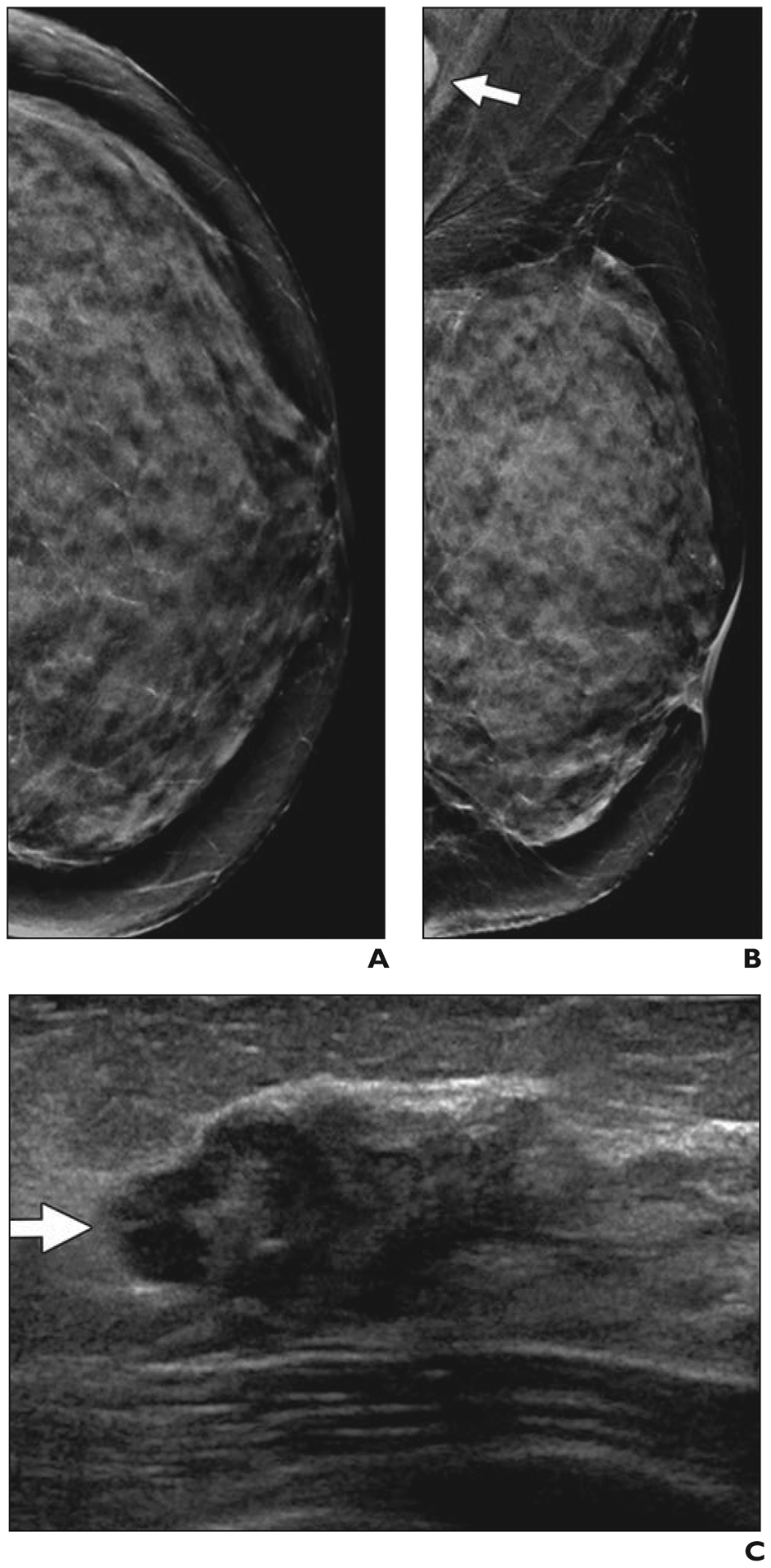
58-year-old woman with extremely dense breasts who had cancer seen on screening ultrasound (US) only.
A and B, Craniocaudal (A) and mediolateral oblique (B) synthetic 2D mammograms show normal extremely dense parenchyma, which was also interpreted as negative on digital breast tomosynthesis. In retrospect, portion of dense node is seen in left axilla (arrow, B). Handheld screening US performed by technologist was conducted bilaterally with standard documentation.
C, Radial US image of left breast shows irregular, hypoechoic 1.9-cm mass (arrow) located in 12-o’clock position 6 cm from nipple. US-guided core biopsy revealed grade 2 invasive ductal carcinoma that was estrogen receptor positive, progesterone receptor negative, and human epidermal growth factor receptor 2 (HER2 [also known as ERBB2]) amplified by fluorescence in situ hybridization. Ki-67 proliferation index was high (40%). US-guided core biopsy of left axillary node confirmed metastatic disease. Patient received primary chemotherapy and had no residual invasive carcinoma and few foci of ductal carcinoma in situ at lumpectomy. Targeted axillary dissection (with seed-localized excision of known metastatic node) showed one metastatic node with treatment effect and three normal nodes.
On average, during the prevalence US screening round, biopsy will be recommended for approximately 3% of women [52], with a wide range of malignancy rates (2–30%) observed for suspicious findings seen on US only. The average absolute increase in recalls from screening US is 7.5% among those who undergo HHUS and 10.6% among those who undergo AUS [52]; as with all modalities, recall rates are lower with incidence screening than with prevalence screening.
Adding screening US to mammography reduces rates of interval cancer rates for women with dense breasts. The Japanese Strategic Anti-Cancer Randomized Trial randomized women 40–49 years old who had breast densities of all types to undergo either mammography alone or mammography plus HHUS and showed increased detection of node-negative invasive cancer in women undergoing screening US as well as half the interval cancer rate (at 0.5 cancers per 1000 screens) [53]. In the ACRIN 6666 protocol, the interval cancer rate was low (nine cases per 111 cancers overall), accounting for 8% of all cancers identified across 7473 screening examinations (i.e., 1.2 cancers per 1000 screening examinations) [54]. Similarly, Corsetti et al. [55] found an interval cancer rate of 1.1 cancers per 1000 women with dense breasts when screening US was added, which was comparable to the rate of 1.0 cancer per 1000 women with fatty breasts screened with mammography alone. Screening US has not been proven to reduce late-stage disease.
A few studies have evaluated the use of US after DBT for women with dense breasts, with a resulting ICDR of 0.9–2.6 invasive cancers detected per 1000 screening examinations with HHUS [56–58]. One series reported similar results with the use of AUS after DBT [59].
Barriers to implementing screening US include a high prevalence of benign cystic lesions [60], a shortage of trained personnel (for HHUS), and large numbers of images (for AUS). Artificial intelligence may facilitate interpretation of both HHUS and AUS (as reviewed in [52]). Among women who undergo screening MRI, screening US offers no added benefit [61].
Molecular Breast Imaging
MBI is a nuclear medicine technique (subject to state licensing requirements) that uses a dedicated gamma camera to depict preferential uptake of 99mTc-sestamibi in mitotically active breast tissue. Commercial MBI technology includes single-head dedicated sodium iodide cameras (formerly known as breast-specific gamma imaging) or cadmium zinc telluride radiation detectors in a dual-head configuration.
The MBI examination consists of IV administration of 99mTc-sestamibi, typically 8 mCi (296 MBq), with imaging commencing immediately after injection. Patient preparation may include fasting for 3 hours before injection and use of peripheral blanket warming to increase breast uptake [62]. Bilateral craniocaudal and mediolateral oblique views are acquired for 7–10 minutes each (total time required for a routine four-view examination, 28–40 minutes), with the patient seated and with light compression applied to limit motion [62, 63].
Because MBI exploits functional behavior rather than x-ray attenuation, it can detect cancers masked by dense breast tissue [64]. In a single-center prospective trial of 1585 women with dense breasts and 21 cancers [65], addition of MBI increased sensitivity to 90% (19/21) relative to 24% (5/21) with mammography alone (p < 0.001), but it decreased specificity from 89% for mammography to 83% for mammography plus MBI (p < 0.001) and led to additional biopsies being performed for 47 of 1585 women (3.0%). The ICDR of MBI was 6.9 invasive cancers per 1000 screening examinations or 8.8 cancers per 1000 screening examinations when DCIS was included. The rate of interval cancer detection was 1.3 cancers per 1000 screens. Shermis et al. [66] reported similar results from a retrospective review of 1696 women who were undergoing supplemental screening with MBI in clinical practice: after negative results on DM, MBI yielded an invasive ICDR of 6.5 cancers per 1000 screening examinations and an overall ICDR of 7.7 cancers per 1000 screening examinations. Across five studies assessing the performance of screening MBI in detecting 63 breast cancers, 52 (83%) were detected by supplemental MBI only, with 37 (71%) of these 52 cancers found to be invasive; 26 of 30 invasive cancers for which node status was reported (87%) were node negative [64] (Fig. 5). Cancers not detected by MBI (n = 6) included three DCIS, two grade 1 invasive lobular cancers (size, 0.6 and 0.7 cm), and one grade 2 invasive ductal cancer (size, 0.3 cm) [64].
Fig. 5—
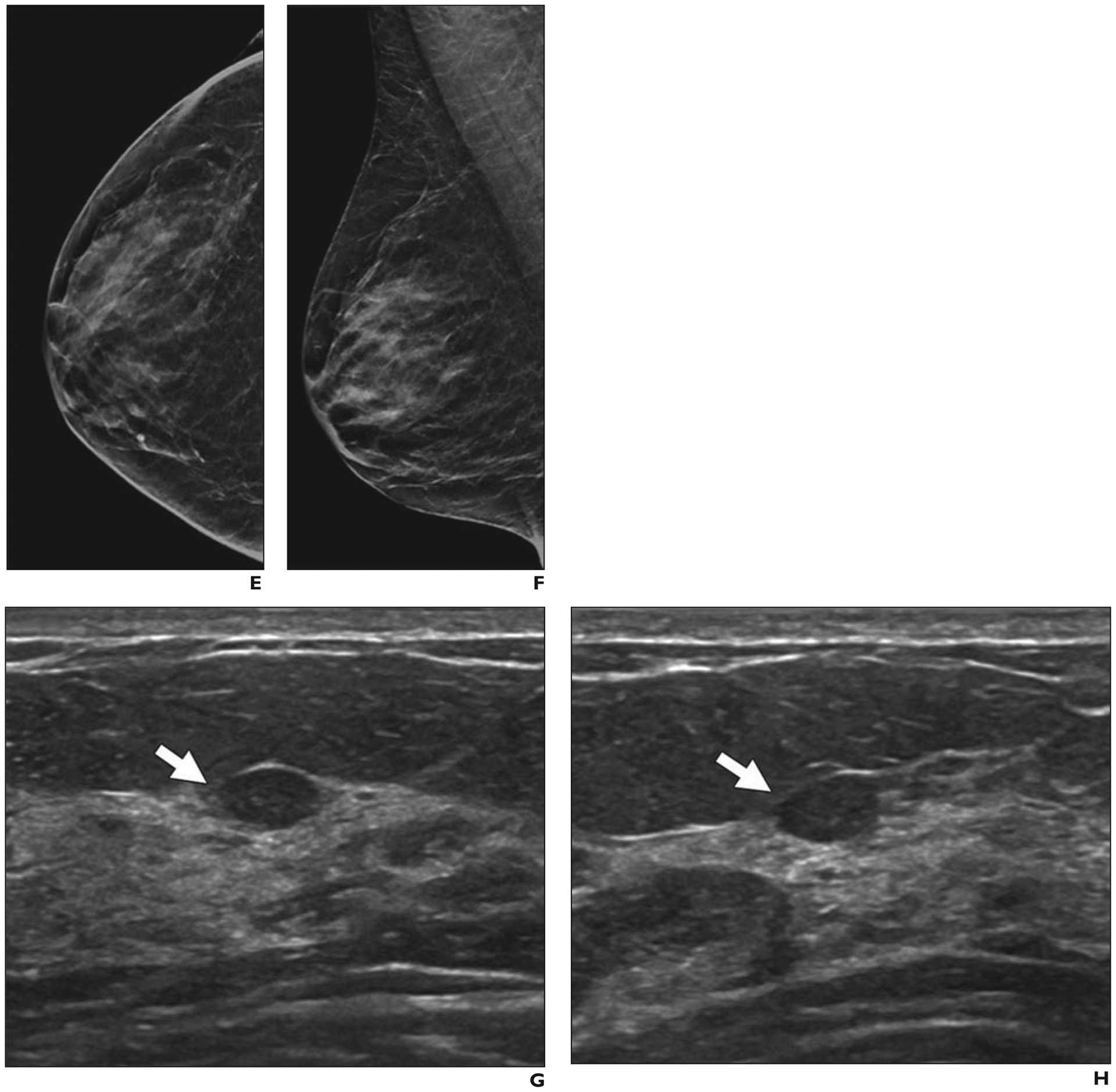
47-year-old woman with heterogeneously dense breasts who had cancer detected on screening molecular breast imaging (MBI) only. (Courtesy of Hunt KN, Mayo Clinic, Rochester, MN)
A–D, Craniocaudal views from upper (A) and lower (B) detector heads and mediolateral oblique views from medial (C) and lateral (D) detector heads obtained during MBI performed after IV injection of 7.5-mCi (277.5 MBq) 99mTc-sestamibi show 9-mm focal area of moderate-intensity uptake (arrows) in upper inner right breast at middle depth.
E and F, Synthetic 2D craniocaudal (E) and mediolateral oblique (F) mammograms from digital breast tomosynthesis acquisition show heterogeneously dense parenchyma and no abnormality. Spot compression mediolateral oblique view (not shown) was also normal.
G and H, Targeted transverse (G) and longitudinal (H) ultrasound images show oval, circumscribed 0.7-cm isoechoic mass (arrows) thought to correspond to MBI finding. Ultrasound-guided core biopsy and surgical excision revealed 0.4-cm grade 1 invasive ductal carcinoma that was estrogen receptor positive, progesterone receptor positive, and human epidermal growth factor receptor 2 (HER2 [also known as ERBB2]) negative. Ki-67 proliferation index was low at 8% and low-grade ductal carcinoma in situ was present. Sentinel node biopsy was negative for metastasis.
The ongoing prospective multicenter Density MATTERS (Molecular Breast Imaging and Tomosynthesis to Eliminate the Reservoir of Cancers) trial compares MBI with DBT for screening women with dense breasts. Inclusion of two annual screening rounds will provide data that are currently lacking regarding the impact of MBI on rates of interval cancer and advanced breast cancer (defined in this trial as cancer that is ≥ 2 cm or node positive).
MBI is an alternative for women who cannot undergo MRI [63]. The mean billing cost is $450, and reimbursement is approximately $300; coverage can require preauthorization. The benefit-to-risk ratios calculated for MBI screening of dense breasts overlap those for mammography [67]; however, concerns persist regarding the radiation risk from MBI.
Barriers to the use of MBI include the minimum examination time of 40 minutes and systemic whole-body radiation exposure. Dosimetry models show an effective dose of approximately 2 mSv for MBI versus 0.5 for DM or DBT with synthetic reconstructions; 2 mSv is less than the annual background radiation limit (mean, 3.1 mSv) and therefore is considered low risk for harmful effects. Work is under way to validate processing algorithms to allow further reductions in administered activity or acquisition time for MBI [68] that may reduce barriers to adoption. Direct biopsy capability is available from one vendor (GE Healthcare) and is in development by another (CMR Naviscan) [64].
MRI
Contrast-enhanced MRI is highly sensitive for breast cancer detection, is not limited by breast density, and is without ionizing radiation. The reported sensitivity of breast MRI alone is generally greater than 80%. Its specificity ranges from 83% to more than 98% and typically exceeds 90% on incidence screening rounds [69]. Cancer detection depends on differential vascularity and enhancement and therefore requires the use of gadolinium-based contrast agent. Low rates of adverse events (0.17%; 95% CI, 0.15–0.19%) [70] are observed, including nephrogenic systemic fibrosis [71]. Intracranial gadolinium deposition has been observed, but to date, gadolinium deposition has no clear adverse effects and is reduced with use of macrocyclic chelates [71].
Most literature on screening MRI is limited to women with a high risk for breast cancer, for whom breast MRI markedly reduces interval cancers and late-stage disease. Among 1592 screening examinations of 501 women, Sardanelli et al. [72] reported 16 additional cancers detected by MRI after mammography plus US (ICDR of MRI, 10 cancers per 1000 screening examinations), three of which were interval cancers (interval cancer rate 1.9 cancers per 1000 screening examinations). Vreemann et al. [73] reported findings for 2773 women undergoing 9571 mammography and MRI examinations. Of 129 screen-detected cancers, 118 were seen on MRI (sensitivity, 91.5%). Forty-one interval cancers were reported: 16 produced symptoms (interval cancer rate 1.7 cancers per 1000 screening examinations, with most occurring in women with pathogenic BRCA1 mutations) and 25 were diagnosed at prophylactic mastectomy. Warner et al. [74] observed a 70% reduction in stage II–IV breast cancers among 445 women with pathogenic BRCA mutations who were undergoing screening MRI, with one interval cancer in the MRI group and 38 in the matched comparison group. The average size of the invasive cancers was 0.9 cm in the MRI cohort versus 1.8 cm in the comparison group (p < 0.001). On the basis of findings from 1275 high-risk screening MRI examinations that diagnosed 114 cancers (including 10 interval cancers [8.8%]), Heijnsdijk et al. [75] predicted a reduction in mortality of 48–61% with the use of MRI alone. Although MRI primarily has been studied for supplemental screening, increasing evidence indicates that mammography provides little additional benefit for some high-risk populations, particularly women younger than 40 years old who have pathogenic BRCA1 mutations [75–77].
Current guidelines from the American Cancer Society [78] and the National Comprehensive Cancer Network [79] recommend screening MRI for women at high risk for breast cancer, with screening beginning at age 25 years and with mammography included by age 30 years for those with known or suspected pathogenic BRCA1 or BRCA2 mutations; they also recommend that MRI be considered for women with lobular carcinoma in situ or atypical hyperplasia. Screening MRI should be stopped by age 75 years [79]. American College of Radiology guidelines also endorse screening MRI for women with a personal history of breast cancer and dense breasts or diagnosis by age 50 years [80].
Increasing support exists for MRI screening of women with dense breasts, even when additional risk factors are not present. The MRI substudy from the ACRIN 6666 trial showed an ICDR of 14.7 cancers per 1000 women with the use of MRI after mammography plus US [54] (Table 2). Kuhl et al. [81] evaluated supplemental MRI in women with a lifetime risk of breast cancer of less than 15%; for the 60% (1282/2120) of women with dense breasts, the ICDR was at least 26 cancers per 1282 women screened (20.3 cancers per 1000 screening examinations) and 11 cancers per 1282 women screened (8.6 cancers per 1000 screening examinations) for incidence screening.
TABLE 2:
Summary of Results of Studies of Supplemental Breast MRI for Screening Women With Dense Breasts
| Study Finding or Feature | ACRIN 6666 Trial [54] | Kuhl et al. 2017 [81] | DENSE Trial [82] | ECOG-ACRIN 1141 Trial [87] | |
|---|---|---|---|---|---|
| Breast density | Density C or Da | All densities; 1282 (60.5%) of women had density C or D | Density D | Density C or D | |
| Cancer risk | ≥ 2.5% 5-y risk by Claus or Gail model OR ≥ 1.7% 5-y risk if patient had extremely dense breasts, or PHBC | Average risk (< 15% lifetime risk by Gail model) | Risk level not specified | Median 5-year BCSC risk 1.6% (range, 0.3–7.8%) | |
| Type of conventional imaging performed | Film mammography or 2D DM plus US | 2D DM; 1335 (63%) underwent screening US | 2D DM | Tomosynthesis | |
| Total no. of women and MRI examinations | 612 | 3861 MRI examinations in 1021 women | 4783 | 1444 | |
| No. of women with PHBC | 275 | 0 | NR | 8 | |
| Breast imaging modality | Full-protocol MRI | Full-protocol MRI | Full-protocol MRI | DBT | |
| Incremental CDR per 1000 women screened (no. of women with cancer/total no. of women screened) | 14.7 (9/612) | Overall, 15.5 (60/3861) | 16.5 (79/4783) | 0.7 (1/1444)b | |
| Prevalence screening, 22.6 (48/2120); incidence screening, 6.9 (12/1741) | |||||
| Prevalence screening of dense breasts only, 20.3 (26/1282) | |||||
| Incremental invasive CDR per 1000 women screened (no. of women with cancer/total no. of women) | 13.1 (8/612) | Prevalence screening, 14.2 (30/2120); incidence screening, 5.7 (10/1741) | 13.4 (64/4783) | 0 | |
| Median size of invasive cancer (mm) | 8.5 | 8 | 9.5 | NR | |
| Staged, node-negative invasive cancers, % (of those staged, no. of node-negative invasive cancer/total no. of women with invasive cancer) | 100 (7/7) | 90 (37/41) | 86 (55/64) | NR | |
| Recall rate, % (no. of recalls/total no. of examinations)c | 25.9 (159/612) | 16.3 (346/2120) | 9.5 (454/4783) | 10.1 (146/1444) | |
| PPV3, malignancies as % of of biopsies performed | 23.1 (12/52) | 35.7 (61/174) | 26.3 (79/300) | 31.0 (9/29) | |
| Sensitivity, %d (n/N) | 87.5 (14/16) | 100 (61/61) | 95.2 (79/83) | 39.1 (9/23) | |
| Specificity, %e | 75.7 (451/596) | Prevalence screening, 95.8 (1986/2072); incidence screening, 98.4 (1701/1728) | 92.1 (4329/4700)f | 97.4 (1371/1407) | |
| Interval cancer rate per 1000 screening examinations | NR | 0 | 0.8 (4/4783) | NA | |
Note—ACRIN = American College of Radiologic Imaging Network, ECOG = Eastern Cooperative Oncology Group, PHBC = personal history of breast cancer, BCSC = Breast Cancer Surveillance Consortium, DM = digital mammography, NR = not reported, DBT = digital breast tomosynthesis, CDR = cancer detection rate, PPV3 = positive predictive value 3, NA = not applicable.
BI-RADS breast density category C denotes heterogeneously dense breasts and category D denotes extremely dense breasts.
Of 17 invasive cancers, 10 were seen on abbreviated MRI only. Of six ductal carcinomas in situ, four were seen on abbreviated MRI only, one was seen on DBT only, and one was seen on both abbreviated MRI and DBT.
Abnormal interpretation rate.
Values in parentheses denote number of cancers detected by supplemental MRI/cancers diagnosed within screening interval.
Values in parentheses denote number of true-negative MRI examinations/women without a cancer diagnosis within the screening interval.
The published study reported 92.0%; however, it appears that four interval cancers were erroneously included in the denominator when the percentage was calculated.
The Dutch DENSE trial (Dense Tissue and Early Breast Neoplasm Screening) trial invited women 50–75 years old who had normal findings on screening mammography and extremely dense breasts to undergo biennial screening with MRI and mammography versus mammography alone [82], with an acceptance rate of 59% (similar to the acceptance rate of 58% in the ACRIN 6666 trial [83]). The first screening round yielded an MRI ICDR of 79 cases per 4783 women screened (16.5 cancers per 1000 screening examinations), including 64 invasive cancers and 15 DCIS (for an invasive ICDR of 13.4 cancers per 1000 screening examinations); 55 of 64 (86%) invasive cancers were node negative [82]. Use of MRI reduced the rate of interval cancers from 4.9 cancers per 1000 screening examinations to 0.8 cancers per 1000 screening examinations. Preliminary results from the second MRI screening round showed substantial reduction in both the ICDR (5.9 cases per 1000 women with cancer of all types; 4.1 cancers per 1000 women with invasive cancers) and false-positive recalls (21 recalls per 1000 screening examinations in the second round vs 80 recalls per 1000 screening examinations in the first round) [84]. Barriers to screening MRI include claustrophobia, fear or intolerance of contrast injection, inconvenience, and fear of false-positive findings [83, 85].
Accredited breast MRI protocols currently require an unenhanced series, at least two contrast-enhanced series, and a T2-weighted series, resulting in typical scanning times of 15–30 minutes. To reduce cost and increase availability, Kuhl et al. [86] introduced abbreviated MRI that uses an unenhanced and a single contrast-enhanced T1-weighted acquisition with subtracted and maximum intensity projections (Fig. 6).
Fig. 6—
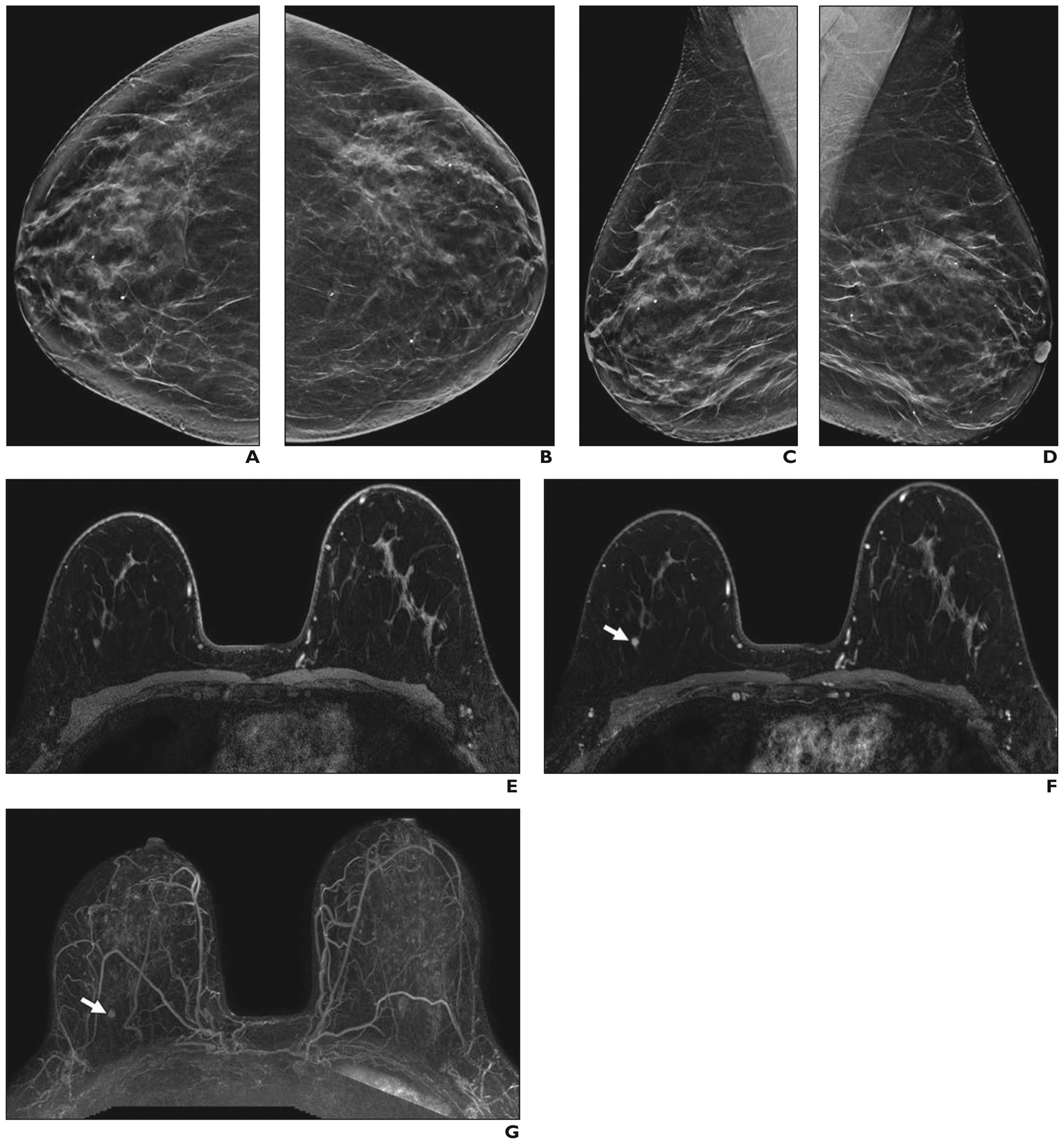
69-year-old woman with dense breasts and history of lobular carcinoma in situ that had been excised 2 years previously who had cancer seen on screening abbreviated MRI only.
A–D, Craniocaudal (A and B) and mediolateral oblique (C and D) synthetic 2D views from screening digital breast tomosynthesis show heterogeneously dense breasts with no abnormalities.
E–G, Images from baseline abbreviated MRI screening examination, which consisted of unenhanced 3D fat-suppressed T1-weighted acquisition (E) and single contrast-enhanced 3D T1-weighted acquisition (F) from which maximum-intensity-projection subtracted image (G) was created, show enhancing, round 0.6-cm mass (arrows, F and G) with indistinct margins and heterogeneous internal enhancement in upper outer right breast. MRI-guided biopsy and excision confirmed 0.6-cm grade 1 invasive ductal carcinoma that was estrogen receptor positive, progesterone receptor positive, and human epidermal growth factor receptor 2 (HER2 [also known as ERBB2]) negative. Sentinel node biopsy was negative for metastasis.
In the ECOG-ACRIN Cancer Research Group 1141 multicenter trial, Comstock et al. [87] compared prevalence screening with abbreviated MRI (including T2-weighted images) with incidence DBT screening. Abbreviated MRI had superior sensitivity (96% vs 39%) but reduced specificity (87% vs 97%) among 1444 women with 26 cancers. Abbreviated MRI alone detected all 17 invasive cancers (with 16 of 17 [94%] node negative) and five of six DCIS (83%), missing one 7-cm high-grade DCIS seen on DBT; DBT detected seven of 17 invasive cancers (41%) and two of six DCIS (33%), yielding an ICDR for abbreviated MRI of 14 cancers among 1444 women screened (9.7 cancers per 1000 screening examinations) including 10 invasive cancers among 1444 women screened (for an invasive ICDR of 6.9 cancers per 1000 screening examinations; p = 0.002) (Table 2). The additional imaging rate (for recall or short-term follow-up) was 7.5% (108 additional examinations for 1444 women screened) for abbreviated MRI and 10.1% (146 additional examinations for 1444 screening examinations) for DBT (p = 0.02). The biopsy rate associated with abbreviated MRI was nearly fourfold that associated with DBT (107 vs 29 biopsies, representing 7.4% and 2.0% of women, respectively) with a lower positive predictive value 3 (PPV3) for biopsy (19% for MRI vs 35.5% for DBT; p = 0.08).
Although MRI clearly depicts additional cancers, concern regarding overdiagnosis remains. Of importance, MRI depicts relatively more invasive cancers than DCIS [88]. Although MRI is more sensitive than mammography (89–92% vs 55–46%) for showing DCIS [89, 90], it appears relatively insensitive to low- and intermediate-grade DCIS [90, 91], potentially mitigating its contribution to overdiagnosis.
A few states and the District of Columbia require insurance coverage for screening MRI for women with dense breasts, or women at high risk for breast cancer, or both. In New Jersey and Pennsylvania, insurance is required to cover screening MRI for extremely dense breasts without other risk factors [48]. Nearly all health insurance will cover screening MRI for women at high risk, although many require preauthorization, and deductibles and copayments apply. Insofar as there is no CPT code for abbreviated MRI insurance billing, facilities typically bill patients directly, charging $250–500 [92]. Unenhanced MRI using DWI holds promise, but it is in the early stages of clinical validation at this time [93].
Contrast-Enhanced Mammography
CEM, also known as contrast-enhanced digital mammography or contrast-enhanced spectral mammography, capitalizes on vascular enhancement from injected iodinated contrast medium to depict cancers on mammography. Craniocaudal and mediolateral oblique views of each breast are obtained with low energy (24–30 kVp) and high energy (typically 40–45 kVp) technique; the high-energy images exploit an abrupt increase in x-ray absorption at the K-edge of iodine (approximately 33 keV). Two images per view result: the low-energy image mimics standard 2D mammography, and the subtracted image shows areas of enhancement only (Figs. 7 and 8). Overall radiation exposure is approximately twice that observed with standard mammography but is well within accepted limits. Most data on CEM performance are from patients with newly diagnosed cancer. In a meta-analysis of 13 studies comparing CEM and MRI, Xiang et al. [94] found that the overall sensitivity of CEM mirrored that of MRI (97% each), but specificity was higher with CEM (0.66; 95% CI, 0.59–0.71) than with MRI (0.52; 95% CI, 0.46–0.58).
Fig. 7—
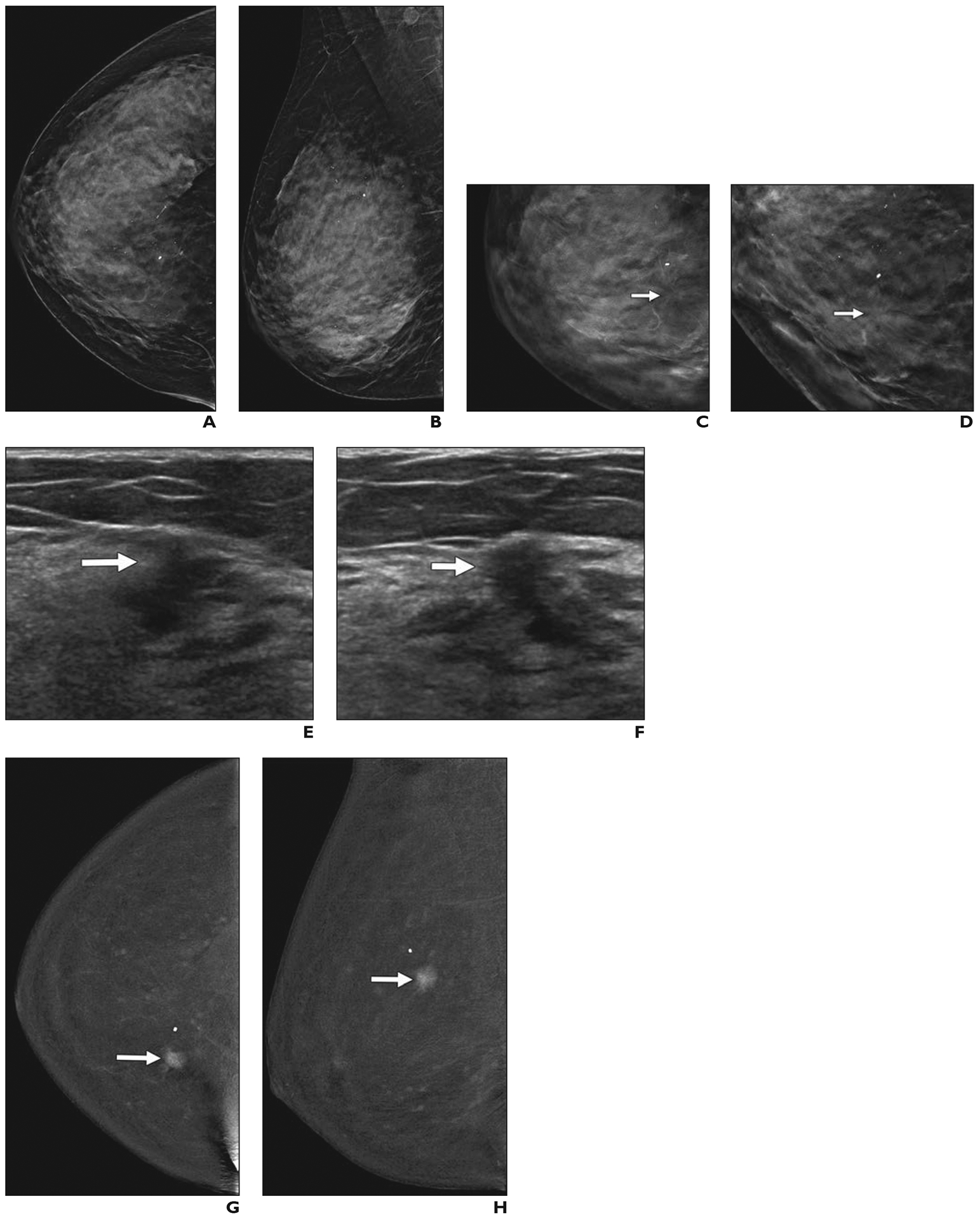
57-year-old woman with dense breasts who had invasive ductal cancer shown on contrast-enhanced mammography.
A and B, Screening craniocaudal (A) and mediolateral oblique (B) 2D synthetic mammography images show heterogeneously dense parenchyma and few calcifications.
C and D, Enlarged views of craniocaudal tomosynthesis image of inner right breast (C) and angled spot compression craniocaudal tomosynthesis image (D) show subtle distortion (arrows).
E and F, Targeted radial (E) and antiradial (F) ultrasound images of right breast show irregular, hypoechoic 0.9-cm mass (arrows) in 1-o’clock position located 6 cm from nipple, although findings of screening handheld ultrasound examination were negative. Before biopsy, patient underwent research contrast-enhanced mammography performed 2.5 minutes after IV injection of 125 mL of iopamidol (370 mg I/mL; Isovue 370, Bracco).
G and H, Craniocaudal (G) and mediolateral oblique (H) subtracted contrast-enhanced mammography images show strongly enhancing 1.2-cm irregular mass (arrows) and mild background parenchymal enhancement. Ultrasound-guided core biopsy revealed grade 2 invasive ductal carcinoma with ductal carcinoma in situ that was estrogen receptor positive, progesterone receptor positive, and human epidermal growth factor receptor 2 (HER2 [also known as ERBB2]) negative. Ki-67 proliferation index was low (10%). Invasive carcinoma measured 1.3 cm at lumpectomy, and four sentinel nodes were negative for metastasis.
Fig. 8—
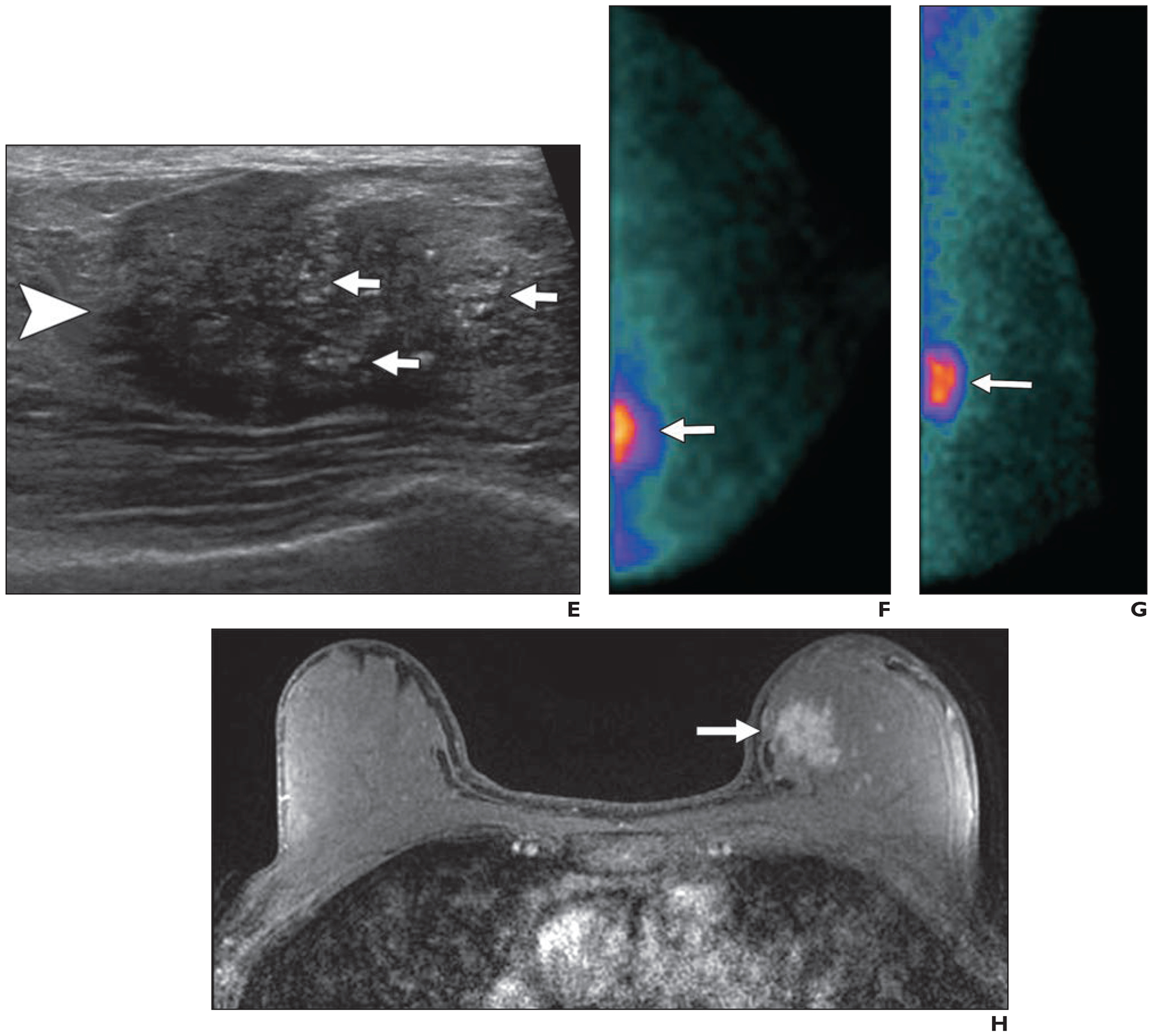
51-year-old woman with extremely dense breasts who had invasive ductal carcinoma visualized using multiple modalities.
A and B, Screening craniocaudal (A) and mediolateral oblique (B) mammography images of left breast show regional amorphous and pleomorphic calcifications (arrows) that are well seen despite extremely dense parenchyma.
C and D, Subtracted craniocaudal (C) and mediolateral oblique (D) images from research contrast-enhanced mammography performed after IV injection of 125 mL of 300 mg I/mL iohexol (Omnipaque 300, GE Healthcare) show strong enhancement of irregular 2.7-cm mass (arrows) at site of calcifications.
E, Targeted ultrasound image shows partially circumscribed, partially indistinctly marginated, slightly hypoechoic 3.1-cm mass (arrowhead) with echogenic calcifications (arrows). Stereotactic biopsy was performed to ensure optimal sampling of calcifications and showed grade 2 invasive ductal carcinoma with ductal carcinoma in situ that was estrogen receptor negative, progesterone receptor negative, and human epidermal growth factor receptor 2 (HER2 [also known as ERBB2]) negative. Ki-67 proliferation index was low (10%).
F and G, Craniocaudal (F) and mediolateral oblique (G) views from research molecular breast imaging (10-minute acquisitions obtained after IV injection of 7.3 mCi [270.1 MBq] of 99mTc-sestamibi) performed after diagnosis shows intense uptake in irregular 3.4-cm mass (arrows). Note slight reduction in inclusion of extreme posterior tissues relative to mammography.
H, Axial fat-suppressed T1-weighted MR image shows intense enhancement of irregular 3.0-cm mass (arrow) at site of known malignancy. Patient had partial response to primary chemotherapy with two sentinel nodes negative for metastases (or therapy-related changes).
Sorin et al. [95] reported results for 611 women who underwent screening CEM, 568 (93%) of whom had dense breasts and 295 (48.3%) of whom had a family or personal history of breast cancer. Of 21 malignancies, 11 were seen on mammography and 19 were seen on CEM (ICDR of CEM, eight cancers per 611 women screened; 13.1 cancers per 1000 screening examinations; 95% CI, 6.2–21.1). Of eight malignancies seen on CEM only, seven were invasive, and two of four that underwent node staging had metastases. Sung et al. [96] reported 904 women who were undergoing CEM, 700 of whom had dense breasts (including the 307 women reported in [97]), with 15 women (1.7%) having reactions to contrast medium, one of which was moderate (dyspnea, which required diphenhydramine) and the remainder of which were mild (e.g., nausea or hives). Sixteen cancers were found; 14 (88%) were found on CEM and two interval cancers (interval cancer rate 2.2 cancers per 1000 screening examinations); were found, one of which was seen on MRI and one of which was seen on screening US 10 months later. Cancer was detected in 12 women with dense breasts; six of the cancers (50%) were detected on low-energy images, and 10 (83%) were detected on CEM. Six of the 12 cancers in dense breasts were seen on CEM only (an ICDR of 6.0 cancers per 700 women screened or 8.6 cancers per 1000 screening examinations); four invasive cancers were found (median size, 0.8 cm), all of which were node negative. For CEM, overall specificity was 93.7% with a PPV3 of 29.4% (15 biopsies were malignant of 51 biopsies performed because of suspicious findings on CEM). These promising results have prompted initiation of a multicenter trial [98] that will compare performance of CEM (to DBT plus US) in women with dense breasts who have an average to intermediate risk of breast cancer.
Several barriers to CEM adoption remain. Across 84 publications, encompassing results for 14,012 women who underwent CEM, Zanardo et al. [99] reported that the pooled rate of adverse reactions was 0.82% (95% CI, 0.64–1.05%), and staff should be trained to recognize reactions to contrast medium. CEM currently lacks widespread direct biopsy capability for findings not visible on mammography or US, uncommonly resulting in the need for MRI-guided biopsy. Most centers use the CPT code for 2D mammography for billing CEM; billing and reimbursement for the contrast component is variable.
Fig. 9—
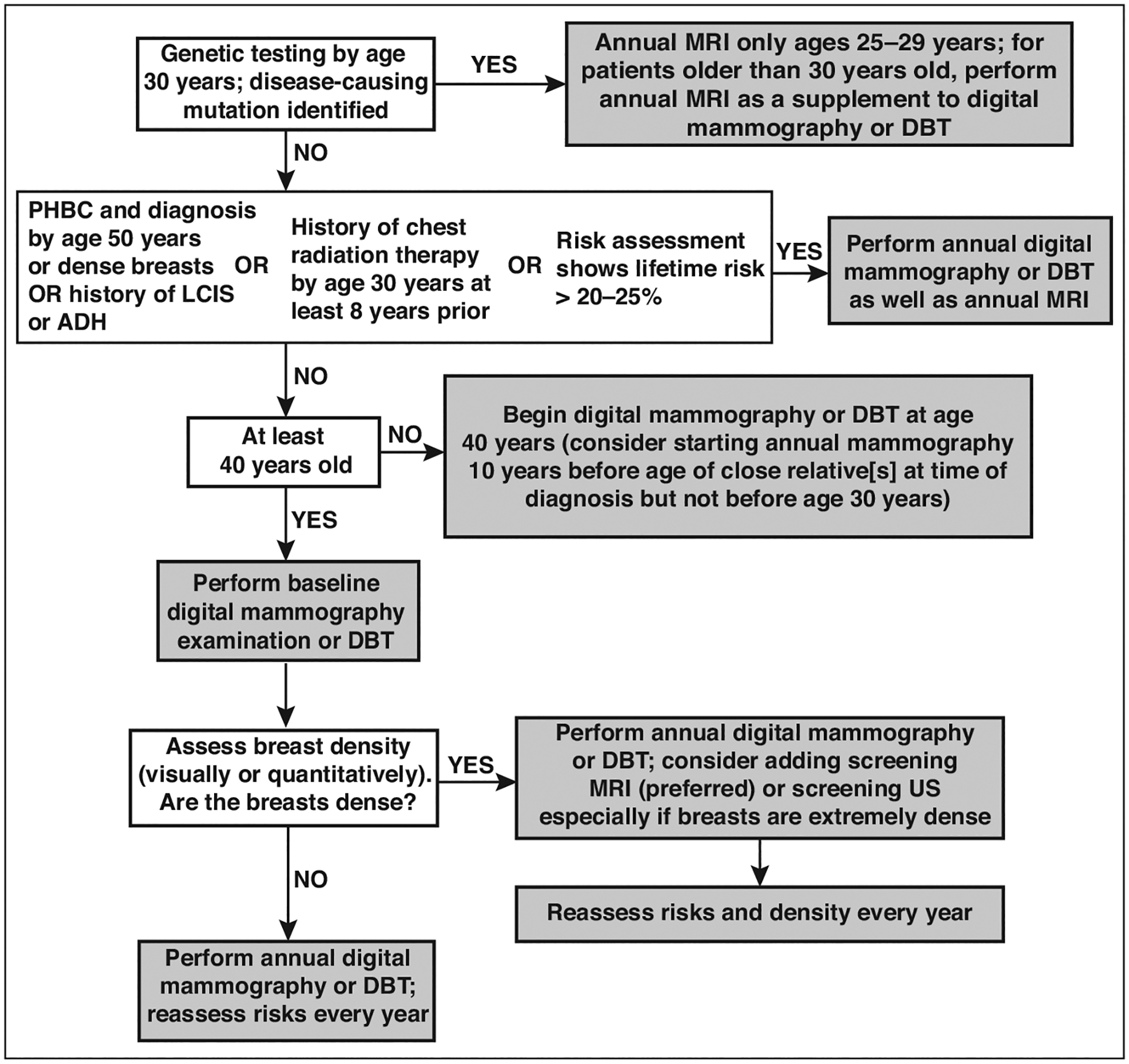
Flowchart illustrates current approaches to supplemental screening in context of risk factors, including breast density. If not performed by age 30 years, genetic testing can be performed at time of diagnosis of breast or ovarian cancer, when appropriate family history is identified, or when family member is found to have pathogenic mutation. Women with high risk for breast cancer who are pregnant or lactating may consider screening ultrasound (US) during that time. Similar performance has been observed for abbreviated MRI and full-protocol diagnostic MRI. For women who cannot tolerate MRI, US is most widely available alternative but produces less gain in cancer detection than MRI. Molecular breast imaging (MBI) or contrast-enhanced mammography (CEM) appear to produce cancer detection similar to that of MRI but are not yet widely available alternatives when MRI is not possible; further validation is needed. If screening MRI is performed, additional supplemental screening with US, MBI, or CEM is not needed. Supplemental screening MRI should stop by age 75 even among high-risk women. PHBC = personal history of breast cancer, LCIS = lobular carcinoma in situ, ADH = atypical ductal hyperplasia, DBT = digital breast tomosynthesis.
TABLE 3:
Summary of Comparative Impact of Supplemental Screening Beyond 2D Mammography for Women With Dense Breasts and Surrogate Endpoint Validation
| Screening Modality | No. of Patients | Incremental CDRa | Incremental Invasive CDRa | Node-Negative Invasive Cancers, %b | Incremental Recall Ratea | Reduced Interval Cancers | Reduced Late-Stage Disease |
|---|---|---|---|---|---|---|---|
| DBT | 103,245c | 1.7c | 1.4d | Not evaluated | −20c | No | Not evaluated |
| US | 452,743e | 2.0–2.7e | 1.8–2.3e | 88.6 (635/717) | 76–106 | Yes | Not evaluated |
| MBIf | 4277 | 8.1 | 6.2 | 85 (23/27) | 67 | Not yet evaluated | Not yet evaluated |
| MRI | 9256g | 16.0 | 12.1 | 88 (99/112) | 104 | Yes | Yesh |
| MRI after DBT | 1444i | 9.7 | 6.9 | 94 (16/17) | 215 | Not yet evaluated | |
| CEM | 1311j | 10.7 | 8.4 | 75 (6/8) | 150j | Not yet evaluated | Not yet evaluated |
Note—CDR = cancer detection rate, DBT = digital breast tomosynthesis, US = ultrasound, MBI = molecular breast imaging, CEM = contrast-enhanced mammography.
Per 1000 women screened.
Data in parentheses are number of invasive breast cancers staged that were node negative/total number of invasive breast cancers seen only on that modality.
The CDR of DBT was 682 cancers detected among 103,245 women (6.61 cancers detected per 1000 women screened) across the four studies presented in Table 1 [13, 35, 42, 43] versus 876 cancers detected among 176,986 women (4.95 cancers detected per 1000 women screened) for 2D mammography, for a difference of 1.7 cancers detected per 1000 women screened; there were 12,280 recalls per 113,986 DBT examinations (10.8%) versus 23,727 recalls per 185,763 2D examinations (12.8%), for a difference of 2.0% or 20 recalls per 1000 examinations.
Data are from [43] only; incremental invasive CDR data were not reported in the other studies.
As summarized in Berg et al. [52], results are from 361,562 US examinations performed by physicians, 64,018 US examinations performed by technologists, and 27,163 automated screening US examinations, with an average incremental CDR of 2.0 cancers (88% of which were invasive cancers) per 1000 women screened for handheld US performed by a physician and 2.7 cancers (86% of which were invasive cancers) per 1000 women screened for US performed by a technologist, and 2.5 cancers (91% of which were invasive cancers) per 1000 women screened for automated US.
Data from three series [65, 66, 100] limited to women with dense breasts on prior or current mammogram.
Data from three of the series [54, 81, 82] summarized in Table 2. In Kuhl et al. [81], the incremental CDR for prevalence screening versus incidence screening was 22.6 cancers per 1000 women screened versus 6.9 cancers per 1000 women screened, respectively. Other findings are for prevalence screening.
Reduced late-stage disease has been shown only for women with known pathogenic BRCA1 or BRCA2 mutations screened using MRI [74].
Prevalence screening with abbreviated MRI compared with tomosynthesis [87], as described in Table 2.
Data are from two series [95, 96]. Cancer findings are shown for the 700 women with dense breasts in the series evaluated by Sung et al. [96], whereas recall rates include false-positive and true-positive recalls for all 904 women because results were not distinguished for the subset of women with dense breasts.
Consensus Opinions.
An increased risk for cancer and masking of noncalcified cancers reduce the potential benefit of mammographic screening in women with dense breasts: supplemental screening should be discussed, with patient tolerance and preferences considered. Table 3 summarizes performance characteristics and the extent of validation across the modalities discussed. Figure 9 presents the current approaches to supplemental screening in the context of other risk factors in addition to breast density.
The use of screening DBT with synthetic reconstructions improves cancer detection, reduces recalls, and is achieved with a radiation dose comparable to that of DM; it therefore is the preferred mammographic technique for women with heterogeneously dense breasts. As measured by clinically detected interval cancers after a false-negative screening examination, the sensitivity of mammography in women with extremely dense breasts, however, is as low as 57% and is not appreciably improved by DBT: supplemental screening should be performed.
Although there is a relatively small additional cancer yield from US after mammography or DBT, interval cancer rates are reduced by screening US.
MBI improves cancer detection in women with dense breasts, and assessment of the impact on interval cancer rates after DBT is in progress; however, this approach currently requires a 40-minute examination time and incurs whole-body radiation exposure.
The most validated approach producing the greatest improvement in cancer detection is contrast-enhanced MRI, even when it is performed after DBT. Interval cancer rates are decreased by MRI, as is late-stage disease (with the latter proven in high-risk women only). Abbreviated MRI will reduce cost and improve availability, but claustrophobia and other patient tolerance issues must be considered. MRI should be stopped by age 75 years, even for high-risk women.
CEM appears to have performance characteristics similar to those of MRI, but further validation is necessary, as is improved availability of direct biopsy capability.
Dense breast notification will soon be the national standard; therefore, understanding the performance of mammography and supplemental screening options is incumbent on all physicians to optimize the screening of women with dense breasts. Out-of-pocket costs and the availability of technology influence implementation. In national databases, data directly attributing recalls and cancers detected to each modality used in screening would facilitate both audits and outcomes analyses.
Acknowledgments
W. A. Berg has a research grant from Koios Medical, Inc.; E. A. Rafferty is a consultant to Hologic, Inc.; S. M. Friedewald has a research grant from Hologic and a travel grant from Siemens Healthcare; C. B. Hruska receives royalties for technology licensed to CMR Naviscan; and H. Rahbar is a coinvestigator in research supported by GE Healthcare and has provided voluntary consulting to Philips Healthcare.
Supported by National Cancer Institute, National Institutes of Health grants R01CA187593 (to W. A. Berg) and R01CA203883 and R01CA207290 (to H. Rahbar) and Breast Cancer Research Foundation grant 19-015 (to W. A. Berg).
References
- 1.Coldman A, Phillips N, Wilson C, et al. Pan-Canadian study of mammography screening and mortality from breast cancer. J Natl Cancer Inst 2014; 106:dju261. [DOI] [PubMed] [Google Scholar]
- 2.Tabár L, Vitak B, Chen TH, et al. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology 2011; 260:658–663 [DOI] [PubMed] [Google Scholar]
- 3.Monticciolo DL, Newell MS, Hendrick RE, et al. Breast cancer screening for average-risk women: recommendations from the ACR Commission on Breast Imaging. J Am Coll Radiol 2017; 14:1137–1143 [DOI] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Fontham ET, Etzioni R, et al. ; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 2015; 314:1599–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siu AL US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2016; 164:279–296 [DOI] [PubMed] [Google Scholar]
- 6.Helvie MA, Bevers TB. Screening mammography for average-risk women: the controversy and NCCN’s position. J Natl Compr Canc Netw 2018; 16:1398–1404 [DOI] [PubMed] [Google Scholar]
- 7.Stapleton SM, Oseni TO, Bababekov YJ, Hung YC, Chang DC. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg 2018; 153:594–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS Mammography, 5th ed. In: D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA, et al. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology, 2013 [Google Scholar]
- 9.Gajdos C, Tartter PI, Bleiweiss IJ, et al. Mammographic appearance of non-palpable breast cancer reflects pathologic characteristics. Ann Surg 2002; 235:246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerlikowske K, Zhu W, Tosteson AN, et al. ; Breast Cancer Surveillance Consortium. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med 2015; 162:673–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst 2014; 106:dju255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DenseBreast-Info website. Legislation and regulation. densebreast-info.org/legislation.aspx. Published March 28, 2019. Updated October 22, 2020. Accessed November 25, 2020
- 13.Conant EF, Barlow WE, Herschorn SD, et al. ; Population-Based Research Optimizing Screening Through Personalized Regimen (PROSPR) Consortium. Association of digital breast tomosynthesis vs digital mammography with cancer detection and recall rates by age and breast density. JAMA Oncol 2019; 5:635–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Destounis S, Johnston L, Highnam R, Arieno A, Morgan R, Chan A. Using volumetric breast density to quantify the potential masking risk of mammographic density. AJR 2017; 208:222–227 [DOI] [PubMed] [Google Scholar]
- 15.van der Waal D, Ripping TM, Verbeek AL, Broeders MJ. Breast cancer screening effect across breast density strata: a case-control study. Int J Cancer 2017; 140:41–49 [DOI] [PubMed] [Google Scholar]
- 16.Chiu SY, Duffy S, Yen AM, Tabár L, Smith RA, Chen HH. Effect of baseline breast density on breast cancer incidence, stage, mortality, and screening parameters: 25-year follow-up of a Swedish mammographic screening. Cancer Epidemiol Biomarkers Prev 2010; 19:1219–1228 [DOI] [PubMed] [Google Scholar]
- 17.Wanders JOP, Holland K, Karssemeijer N, et al. The effect of volumetric breast density on the risk of screen-detected and interval breast cancers: a cohort study. Breast Cancer Res 2017; 19:67–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houssami N, Hunter K. The epidemiology, radiology and biological characteristics of interval breast cancers in population mammography screening. NPJ Breast Cancer 2017; 3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology 2017; 283:49–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holm J, Humphreys K, Li J, et al. Risk factors and tumor characteristics of interval cancers by mammographic density. J Clin Oncol 2015; 33:1030–1037 [DOI] [PubMed] [Google Scholar]
- 21.Domingo L, Salas D, Zubizarreta R, et al. ; INCA Study Group. Tumor pheno-type and breast density in distinct categories of interval cancer: results of population-based mammography screening in Spain. Breast Cancer Res 2014; 16:R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med 2007; 356:227–236 [DOI] [PubMed] [Google Scholar]
- 23.Ciatto S, Visioli C, Paci E, Zappa M. Breast density as a determinant of interval cancer at mammographic screening. Br J Cancer 2004; 90:393–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webb ML, Cady B, Michaelson JS, et al. A failure analysis of invasive breast cancer: most deaths from disease occur in women not regularly screened. Cancer 2014; 120:2839–2846 [DOI] [PubMed] [Google Scholar]
- 25.Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K; Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer. JAMA Oncol 2017; 3:1228–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2006; 15:1159–1169 [DOI] [PubMed] [Google Scholar]
- 27.Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol 2010; 28:3830–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertrand KA, Scott CG, Tamimi RM, et al. Dense and nondense mammographic area and risk of breast cancer by age and tumor characteristics. Cancer Epidemiol Biomarkers Prev 2015; 24:798–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned? Radiol Clin North Am 2004; 42:793–806, v [DOI] [PubMed] [Google Scholar]
- 30.Duffy SW, Tabár L, Yen AM, et al. Mammography screening reduces rates of advanced and fatal breast cancers: results in 549,091 women. Cancer 2020; 126:2971–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niklason LT, Christian BT, Niklason LE, et al. Digital tomosynthesis in breast imaging. Radiology 1997; 205:399–406 [DOI] [PubMed] [Google Scholar]
- 32.Rafferty EA, Park JM, Philpotts LE, et al. Assessing radiologist performance using combined digital mammography and breast tomosynthesis compared with digital mammography alone: results of a multicenter, multi-reader trial. Radiology 2013; 266:104–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skaane P, Bandos AI, Eben EB, et al. Two-view digital breast tomosynthesis screening with synthetically reconstructed projection images: comparison with digital breast tomosynthesis with full-field digital mammographic images. Radiology 2014; 271:655–663 [DOI] [PubMed] [Google Scholar]
- 34.Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311:2499–2507 [DOI] [PubMed] [Google Scholar]
- 35.Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013; 14:583–589 [DOI] [PubMed] [Google Scholar]
- 36.Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267:47–56 [DOI] [PubMed] [Google Scholar]
- 37.Wang WS, Hardesty L, Borgstede J, Takahashi J, Sams S. Breast cancers found with digital breast tomosynthesis: a comparison of pathology and histologic grade. Breast J 2016; 22:651–656 [DOI] [PubMed] [Google Scholar]
- 38.Kim JY, Kang HJ, Shin JK, et al. Biologic profiles of invasive breast cancers detected only with digital breast tomosynthesis. AJR 2017; 209:1411–1418 [DOI] [PubMed] [Google Scholar]
- 39.Conant EF, Zuckerman SP, McDonald ES, et al. Five consecutive years of screening with digital breast tomosynthesis: outcomes by screening year and round. Radiology 2020; 295:285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leung JW, Sickles EA. Multiple bilateral masses detected on screening mammography: assessment of need for recall imaging. AJR 2000; 175:23–29 [DOI] [PubMed] [Google Scholar]
- 41.Bahl M, Gaffney S, McCarthy AM, Lowry KP, Dang PA, Lehman CD. Breast cancer characteristics associated with 2D digital mammography versus digital breast tomosynthesis for screening-detected and interval cancers. Radiology 2018; 287:49–57 [DOI] [PubMed] [Google Scholar]
- 42.Østerås BH, Martinsen ACT, Gullien R, Skaane P. Digital mammography versus breast tomosynthesis: impact of breast density on diagnostic performance in population-based screening. Radiology 2019; 293:60–68 [DOI] [PubMed] [Google Scholar]
- 43.Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315:1784–1786 [DOI] [PubMed] [Google Scholar]
- 44.Lowry KP, Coley RY, Miglioretti DL, et al. Screening performance of digital breast tomosynthesis vs digital mammography in community practice by patient age, screening round, and breast density. JAMA Netw Open 2020; 3:e2011792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lowry KP, Trentham-Dietz A, Schechter CB, et al. Long-term outcomes and cost-effectiveness of breast cancer screening with digital breast tomosynthesis in the United States. J Natl Cancer Inst 2020; 112:582–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller JD, Bonafede MM, Herschorn SD, Pohlman SK, Troeger KA, Fajardo LL. Value analysis of digital breast tomosynthesis for breast cancer screening in a US Medicaid population. J Am Coll Radiol 2017; 14:467–474.e5 [DOI] [PubMed] [Google Scholar]
- 47.Hunter SA, Morris C, Nelson K, Snyder BJ, Poulton TB. Digital breast tomosynthesis: cost-effectiveness of using private and Medicare insurance in community-based health care facilities. AJR 2017; 208:1171–1175 [DOI] [PubMed] [Google Scholar]
- 48.Densebreast-info.org website. Comparative analysis of state density inform efforts and insurance coverage. densebreast-info.org/img/comparative-analysis-state-density-inform-efforts-insurance-coverage.pdf. Revised October 10, 2020. Accessed November 25, 2020
- 49.Berg WA, Blume JD, Cormack JB, et al. ; ACRIN 6666 Investigators. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 2008; 299:2151–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barr RG, Zhang Z, Cormack JB, Mendelson EB, Berg WA. Probably benign lesions at screening breast US in a population with elevated risk: prevalence and rate of malignancy in the ACRIN 6666 trial. Radiology 2013; 269:701–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol 2010; 20:734–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berg WA, Vourtsis A. Screening breast ultrasound using hand-held or automated technique in women with dense breasts. J Breast Imaging 2019; 1:283–296 [DOI] [PubMed] [Google Scholar]
- 53.Ohuchi N, Suzuki A, Sobue T, et al. ; J-START Investigator Groups. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-Cancer Randomized Trial (J-START): a randomised controlled trial. Lancet 2016; 387:341–348 [DOI] [PubMed] [Google Scholar]
- 54.Berg WA, Zhang Z, Lehrer D, et al. ; ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012; 307:1394–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer 2011; 47:1021–1026 [DOI] [PubMed] [Google Scholar]
- 56.Berg WA, Bandos AI, Gur D, et al. Second reading DBT versus supplemental screening US in dense breasts: interim analysis from the DBTUST. (abstract) Radiological Society of North America 2019 annual meeting website. rsna2019.rsna.org/program/. Accessed December 18, 2020 [Google Scholar]
- 57.Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial. J Clin Oncol 2016; 34:1882–1888 [DOI] [PubMed] [Google Scholar]
- 58.Tagliafico AS, Mariscotti G, Valdora F, et al. A prospective comparative trial of adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts (ASTOUND-2). Eur J Cancer 2018; 104:39–46 [DOI] [PubMed] [Google Scholar]
- 59.Chough DM, Berg WA, Bandos AI, et al. A prospective study of automated breast ultrasound (ABUS) screening of women with dense breasts in a digital breast tomosynthesis-based practice. J Breast Imaging 2020; 2:125–133 [DOI] [PubMed] [Google Scholar]
- 60.Berg WA, Sechtin AG, Marques H, Zhang Z. Cystic breast masses and the ACRIN 6666 experience. Radiol Clin North Am 2010; 48:931–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR 2009; 192:390–399 [DOI] [PubMed] [Google Scholar]
- 62.Swanson T, Tran TD, Ellingson L, et al. Best practices in molecular breast imaging: a guide for technologists. J Nucl Med Technol 2018; 46:3–11 [DOI] [PubMed] [Google Scholar]
- 63.American College of Radiology website. ACR practice parameter for the performance of molecular breast imaging (MBI) using a dedicated gamma camera. www.acr.org/-/media/ACR/Files/Practice-Parameters/MBI.pdf?la=en. Adopted 2017. Accessed October 13, 2020
- 64.Hruska CB. Molecular breast imaging for screening in dense breasts: state of the art and future directions. AJR 2017; 208:275–283 [DOI] [PubMed] [Google Scholar]
- 65.Rhodes DJ, Hruska CB, Conners AL, et al. Journal club: molecular breast imaging at reduced radiation dose for supplemental screening in mammographically dense breasts. AJR 2015; 204:241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shermis RB, Wilson KD, Doyle MT, et al. Supplemental breast cancer screening with molecular breast imaging for women with dense breast tissue. AJR 2016; 207:450–457 [DOI] [PubMed] [Google Scholar]
- 67.Brown M, Covington MF. Comparative benefit to risk of molecular breast imaging, 2D full-field digital mammography with and without tomosynthesis, and synthetic mammography with tomosynthesis. Radiol Imaging Cancer 2019; 1:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tao AT, Hruska CB, Conners AL, et al. Dose reduction in molecular breast imaging with a new image-processing algorithm. AJR 2020; 214:185–193 [DOI] [PubMed] [Google Scholar]
- 69.Mann RM, Kuhl CK, Moy L. Contrast-enhanced MRI for breast cancer screening. J Magn Reson Imaging 2019; 50:377–390s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sodagari F, Mozaffary A, Wood CG 3rd, Schmitz B, Miller FH, Yaghmai V. Reactions to both nonionic iodinated and gadolinium-based contrast media: incidence and clinical characteristics. AJR 2018; 210:715–719 [DOI] [PubMed] [Google Scholar]
- 71.Costelloe CM, Amini B, Madewell JE. Risks and benefits of gadolinium-based contrast enhanced MRI. Semin Ultrasound CT MR 2020; 41:260–274 [DOI] [PubMed] [Google Scholar]
- 72.Sardanelli F, Podo F, Santoro F, et al. High Breast Cancer Risk Italian 1 (HIB-CRIT-1) Study. Multicenter surveillance of women at high genetic breast cancer risk using mammography, ultrasonography, and contrast-enhanced magnetic resonance imaging (the High Breast Cancer Risk Italian 1 Study): final results. Invest Radiol 2011; 46:94–105 [DOI] [PubMed] [Google Scholar]
- 73.Vreemann S, Gubern-Mérida A, Schlooz-Vries MS, et al. Influence of risk category and screening round on the performance of an MR imaging and mammography screening program in carriers of the BRCA mutation and other women at increased risk. Radiology 2018; 286:443–451 [DOI] [PubMed] [Google Scholar]
- 74.Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol 2011; 29:1664–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heijnsdijk EA, Warner E, Gilbert FJ, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening: MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev 2012; 21:1458–1468 [DOI] [PubMed] [Google Scholar]
- 76.Phi XA, Saadatmand S, De Bock GH, et al. Contribution of mammography to MRI screening in BRCA mutation carriers by BRCA status and age: individual patient data meta-analysis. Br J Cancer 2016; 114:631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vreemann S, van Zelst JCM, Schlooz-Vries M, et al. The added value of mammography in different age-groups of women with and without BRCA mutation screened with breast MRI. Breast Cancer Res 2018; 20:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saslow D, Boetes C, Burke W, et al. ; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007; 57:75–89 [DOI] [PubMed] [Google Scholar]
- 79.National Comprehensive Cancer Network (NCCN). Breast cancer screening and diagnosis, version 1. 2020. www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf. Published September 17, 2020. Accessed September 29, 2020
- 80.Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol 2018; 15(3 Pt A):408–414 [DOI] [PubMed] [Google Scholar]
- 81.Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology 2017; 283:361–370 [DOI] [PubMed] [Google Scholar]
- 82.Bakker MF, de Lange SV, Pijnappel RM, et al. ; DENSE Trial Study Group. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med 2019; 381:2091–2102 [DOI] [PubMed] [Google Scholar]
- 83.Berg WA, Blume JD, Adams AM, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology 2010; 254:79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bakker MF, de Lange SV, Pijnappel RM, et al. MRI in addition to mammography screening in women with extremely dense breasts: primary outcome of the randomized DENSE trial. (abstract) Radiological Society of North America; 2019. annual meeting website.rsna2019.rsna.org/program/. Accessed December 18, 2020 [Google Scholar]
- 85.de Lange SV, Bakker MF, Monninkhof EM, et al. Reasons for (non)participation in supplemental population-based MRI breast screening for women with extremely dense breasts. Clin Radiol 2018; 73:759.e1–759.e9 [DOI] [PubMed] [Google Scholar]
- 86.Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. J Clin Oncol 2014; 32:2304–2310 [DOI] [PubMed] [Google Scholar]
- 87.Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA 2020; 323:746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sung JS, Stamler S, Brooks J, et al. Breast cancers detected at screening MR imaging and mammography in patients at high risk: method of detection reflects tumor histopathologic results. Radiology 2016; 280:716–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Berg WA, Gutierrez L, NessAiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology 2004; 233:830–849 [DOI] [PubMed] [Google Scholar]
- 90.Kuhl CK, Schrading S, Bieling HB, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet 2007; 370:485–492 [DOI] [PubMed] [Google Scholar]
- 91.Kuhl CK, Strobel K, Bieling H, et al. Impact of preoperative breast MR imaging and MR-guided surgery on diagnosis and surgical outcome of women with invasive breast cancer with and without DCIS component. Radiology 2017; 284:645–655 [DOI] [PubMed] [Google Scholar]
- 92.Heacock L, Reig B, Lewin AA, Toth HK, Moy L, Lee CS. Abbreviated breast MRI: road to clinical implementation. J Breast Imaging 2020; 2:201–214 [DOI] [PubMed] [Google Scholar]
- 93.Amornsiripanitch N, Bickelhaupt S, Shin HJ, et al. Diffusion-weighted MRI for unenhanced breast cancer screening. Radiology 2019; 293:504–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xiang W, Rao H, Zhou L. A meta-analysis of contrast-enhanced spectral mammography versus MRI in the diagnosis of breast cancer. Thorac Cancer 2020; 11:1423–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sorin V, Yagil Y, Yosepovich A, et al. Contrast-enhanced spectral mammography in women with intermediate breast cancer risk and dense breasts. AJR 2018; 211:[web]W267–W274 [DOI] [PubMed] [Google Scholar]
- 96.Sung JS, Lebron L, Keating D, et al. Performance of dual-energy contrast-enhanced digital mammography for screening women at increased risk of breast cancer. Radiology 2019; 293:81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jochelson MS, Pinker K, Dershaw DD, et al. Comparison of screening CEDM and MRI for women at increased risk for breast cancer: a pilot study. Eur J Radiol 2017; 97:37–43 [DOI] [PubMed] [Google Scholar]
- 98.American College of Radiology website. Contrast enhanced mammography imaging screening trial. www.acr.org/Research/Clinical-Research/CMIST. Accessed October 13, 2020
- 99.Zanardo M, Cozzi A, Trimboli RM, et al. Technique, protocols and adverse reactions for contrast-enhanced spectral mammography (CESM): a systematic review. Insights Imaging 2019; 10:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rhodes DJ, Hruska CB, Phillips SW, Whaley DH, O’Connor MK. Dedicated dual-head gamma imaging for breast cancer screening in women with mammographically dense breasts. Radiology 2011; 258:106–118 [DOI] [PubMed] [Google Scholar]


