Abstract
Background
Earlier, we reported that the microRNA (miR)-155 expression in dendritic cells (DCs) from Behcet’s disease (BD) patients was decreased and affected cytokine production of DCs. In this study, we investigated the mechanisms whereby miR-155 regulates cytokine production by DCs.
Methods
The formation of autophagosomes in DCs was detected by transmission electron microscopy. Western blotting was used to detect the protein levels of LC3, Beclin-1, P62, p-mTOR, and p-Akt in DCs. TNF-α, IL-6, and IL-1β expression were investigated by ELISA. MiR-155 mimics were transfected to DCs to evaluate its effects on autophagy and cytokine production. RNA interference was used to downregulate the expression of TAB2.
Results
The formation of autophagosomes was found in DCs of active BD patients. The expressions of LC3-II, Beclin-1, and P62 were significantly increased in DCs of active BD patients compared to that of inactive BD patients and healthy controls. The expressions of IL-6, IL-1β, and TNF-α were significantly increased in DCs of active BD patients compared to that of healthy controls. The autophagy promoter (3-MA) and inhibitor (rapamycin) significantly decreased or increased the expression of TNF-α, IL-6, and IL-1β by DCs. The expression of LC3-II and Beclin-1 was significantly increased, but the expression of P62 proteins was decreased in DCs transfected with miR-155 mimics or after TAB2 was downregulated. The expression of TNF-α, IL-6, and IL-1β was decreased in DCs after miR-155 was upregulated or TAB2 was downregulated. The ratios of p-Akt/Akt and p-mTOR/mTOR were decreased in DCs after miR-155 was upregulated.
Conclusions
These results suggest that miR-155 affects the production of TNF-α, IL-6, and IL-1β by DCs through activation of the Akt/mTOR signaling pathway and by affecting the process of autophagy.
Keywords: Behcet’s disease, Autophagy, MicroRNA-155
Introduction
Behcet’s disease (BD) is a major uveitis entity in China, which symptoms include skin lesions, genital ulcers, oral aphthae, and recurrent uveitis [1–3]. BD is now considered as an autoinflammatory disease. Many studies have demonstrated that dendritic cells (DCs) play a key role in modulating the aberrant immune reaction during the development of BD [4–7]. Earlier research from our group showed that the expression of miR-155 in DCs of BD patients was decreased [8]. However, the exact mechanism of how miR-155 regulates the function of DCs in patients with BD is still not completely elucidated and was therefore the subject of the study reported here, whereby we focused on the possible role of autophagy.
Autophagy is an evolutionarily catabolic pathway that degrades abnormal proteins, damaged organelles, and recycles cellular components [9]. This process is initiated by a double-membraned autophagosome, in which the autophagosomes fuse with the lysosomes, resulting in their degradation by acidic hydrolases. Lysosomes subsequently release the end-products of autophagic digestion into the cytoplasm, which then participate in cellular metabolism [10]. Autophagy has been shown to play a crucial role in maintaining normal intracellular homeostasis of eukaryotic cells and the dysfunction of autophagy may be involved in the pathogenesis of various diseases, including cancer, inflammation, neurodegenerative disease, and autoimmune disease [11–15]. Our earlier studies showed that autophagy-related gene (ATGs) polymorphisms were associated with the development of BD [16]. MiR-155 can modulate the process of autophagy and participates in the development of various diseases [17–19]. In this study, we show that autophagy was activated in DCs from active BD patients, but that autophagic degradation was decreased. We furthermore show that autophagy was involved in the miR-155-dependent regulation of cytokine release from DCs.
Materials and methods
Subjects
Twenty-three active BD patients (13 males and 10 females, with an average age of 36.5 years), 8 inactive BD patients (5 males and 3 females, with an average age of 37.1 years) and 28 healthy individuals (16 males and 12 females, with an average age of 34.4 years) were included in this study (some patients were included in two or three experiments) (Table 1). All subjects were enrolled between July 2015 and November 2020. The BD patients were diagnosed according to the criteria of the International Study Group for Behcet’s Disease [20]. None of the active BD patients enrolled in this study were taking immunosuppressive agents when first visiting our hospital. The inactive BD patients were enrolled after complete control of the intraocular inflammation and termination of all medications for at least 6 months. The healthy controls had no clinical history of systemic diseases or uveitis. Venous blood samples were taken from patients and controls. Written consent was collected from each healthy control and BD patient. This study obeyed the tenets of the Declaration of Helsinki and was approved by our Clinical Ethical Research Committee.
Table 1.
Clinical parameters
| Active BD patients (23) | Inactive BD patients (8) | Normal controls (28) | P value | |
|---|---|---|---|---|
| Age | 36±2 | 37±5 | 34±3 | P>0.05 |
| Gender (male/female) | 13/10 | 5/3 | 16/12 | P>0.05 |
Values are expressed as mean±SD
Cell culture
Peripheral blood mononuclear cells (PBMCs) and CD14+ monocytes were separated from the peripheral blood and purified according to the methods described earlier [21]. Immature monocyte-derived DCs were obtained as described earlier [22]. DCs were treated with autophagy activator (rapamycin (100nM, Sigma-Aldric, St Louis, MO, USA) or inhibitor (3-MA(10mM, Sigma-Aldrich, St Louis, MO, USA) together with LPS (Sigma-Aldrich, St Louis, MO, USA) for 24 h.
Transmission electron microscopy
The collected DCs were prepared according to the methods described earlier [23]. An H-7500 transmission electron microscope (Hitachi, Japan) was used to identify autophagosome-like vesicles at 80 kV.
Western blotting
Western blotting was performed following methods described earlier [8]. All the band detection is within the linear range.
MiRNAs and siRNAs transfection
The miR-155 mimics from GenePharma (Shanghai, China) were transfected into DCs according to methods described previously [8]. The siTAB2-RNAi-LV and pGC-FU-RNAi-NC-LV as controls were from GeneChem (Shanghai, China) and transfected into DCs according to the user’s manual.
ELISA
The concentrations of TNF-α, IL-6, and IL-1β in cell supernatants were measured by ELISA (Human DuoSet ELISA Development Kit; R&D Systems, Minneapolis, MN).
Statistical analysis
One-way ANOVA and Student’s t test were carried out by using SPSS17.0 software (SPSS Inc., Chicago, IL, USA). P values < 0.05 were considered significant.
Results
Autophagy is activated, but the capability of autophagic degradation is decreased in DCs from active BD patients
The most important manifestation of autophagy is the formation of autophagosomes and this was investigated by TEM. Our results showed that autophagosomes were only found in DCs from active BD patients, but not in DCs from inactive BD patients and healthy controls (Fig. 1a). To further identify whether autophagy was activated in patients with active BD, the expression of Beclin-1 and LC3, which are two important markers of autophagy, were investigated by Western blot. The results showed that compared with inactive BD patients and healthy controls, and the protein levels of Beclin-1 and LC3-II were significantly increased in DCs from active BD patients after stimulation with LPS (Fig. 1b–d). There was no difference concerning the expression of LC3 and Beclin-1 between the inactive BD patients and healthy controls. Taken together, these results suggested that autophagy was activated in DCs from active BD patients.
Fig. 1.
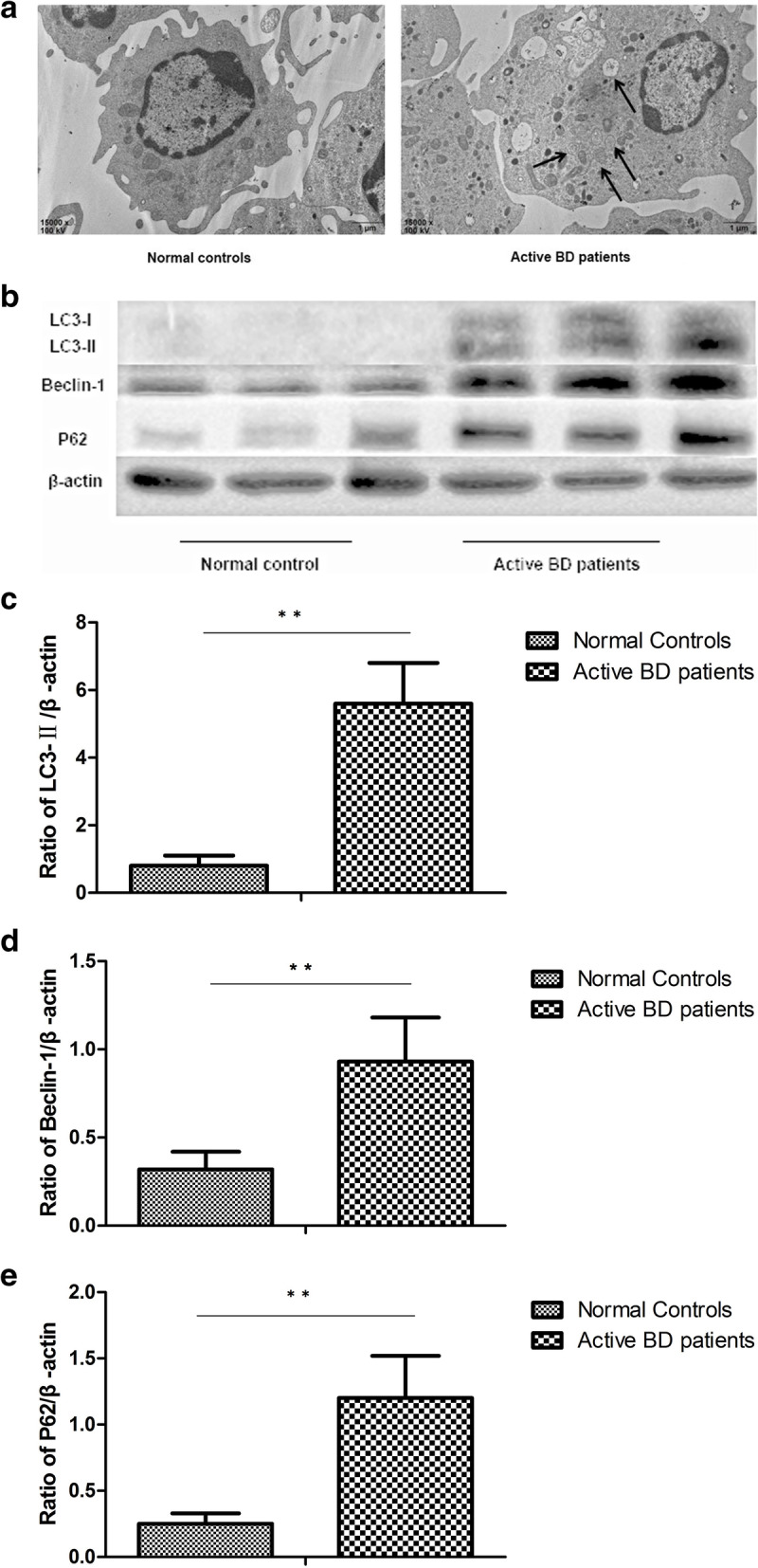
Autophagy is involved in the cytokine production of DCs. a Representative TEM photomicrographs of DCs from normal controls (n=3), inactive BD patients (n=3), and active BD patients (n=3). b Expression levels of LC3-I, LC3-II, Beclin-1, and P62 protein in DCs from normal controls (n=8), inactive BD patients (n=8), and active BD patients (n=8) were quantified by Western blot. c–e Quantification of expression levels of LC3-II, Beclin-1, and P62 proteins. *p<0.05, **p<0.01
P62/SQSTM1 (hereafter referred to as P62), serving as anautophagic substrate, is degraded during autophagy [24]. P62 accumulates in cells when degradation capacity via autophagy is impaired. We next investigated the expression of P62 in DCs from active BD patients. The results showed that the protein expression level of P62 was significantly higher in DCs of active BD patients than that of inactive BD patients and healthy controls, which indicates that autophagic degradation was decreased in active BD patients, despite the presence of a large number of autophagosomes (Fig. 1b, e).
Autophagy is involved in cytokine production of DCs
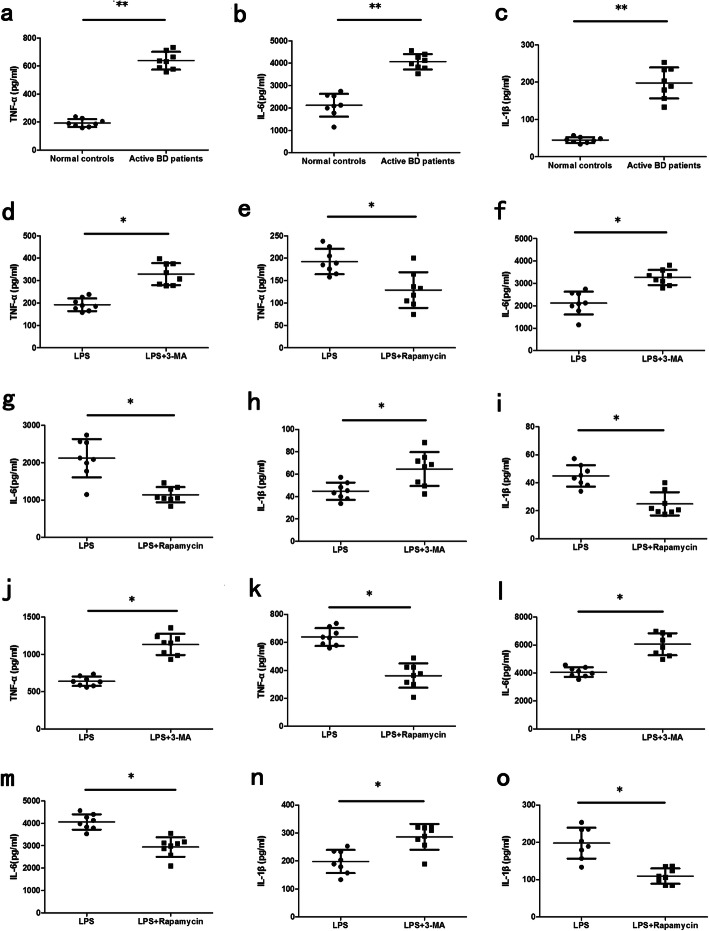
In view of the dysfunctional autophagy in DCs of active BD patients, further experiments were performed to examine whether autophagy had an effect on cytokine production by DCs. The results demonstrated that the expression of TNF-α, IL-6, and IL-1β of LPS-treated DCs from active BD patients was significantly higher than that of healthy controls (Fig. 2a–c). It has been proven that 3-MA could inhibit autophagy through PI3K pathway, and rapamycin could activate autophagy by mTOR pathway [25, 26]. The results showed that 3-MA and rapamycin, which are the inhibitor or promoter of autophagy, significantly increased or decreased the expression levels of TNF-α, IL-6, and IL-1β by DCs treated with LPS (Fig. 2d–o). These data suggest that autophagy plays a role as a negative regulator of inflammation by regulating the expression of inflammatory cytokines.
Fig. 2.
Autophagy is involved in the cytokine production of DCs. DCs from normal controls (n=8) and active BD patients (n=8) were assessed. a–c DCs were stimulated with LPS (100ng/ml) for 24 h. Cytokines including TNF-α, IL-1β, and IL-6 were measured in the culture supernatants by ELISA. d–i DCs from the normal controls were treated with 3-MA (10mM) or rapamycin (100nM) together with LPS (100ng/ml) for 24 h. TNF-α, IL-1β, and IL-6 were measured in the culture supernatants by ELISA. j–o DCs from active BD patients were treated with 3-MA (10mM) or rapamycin (100nM) together with LPS (100ng/ml) for 24 h. TNF-α, IL-1β, and IL-6 were measured in the culture supernatants by ELISA. *p<0.05, **p<0.01
MiR-155 is involved in the cytokine production of DCs induced by dysfunctional autophagy
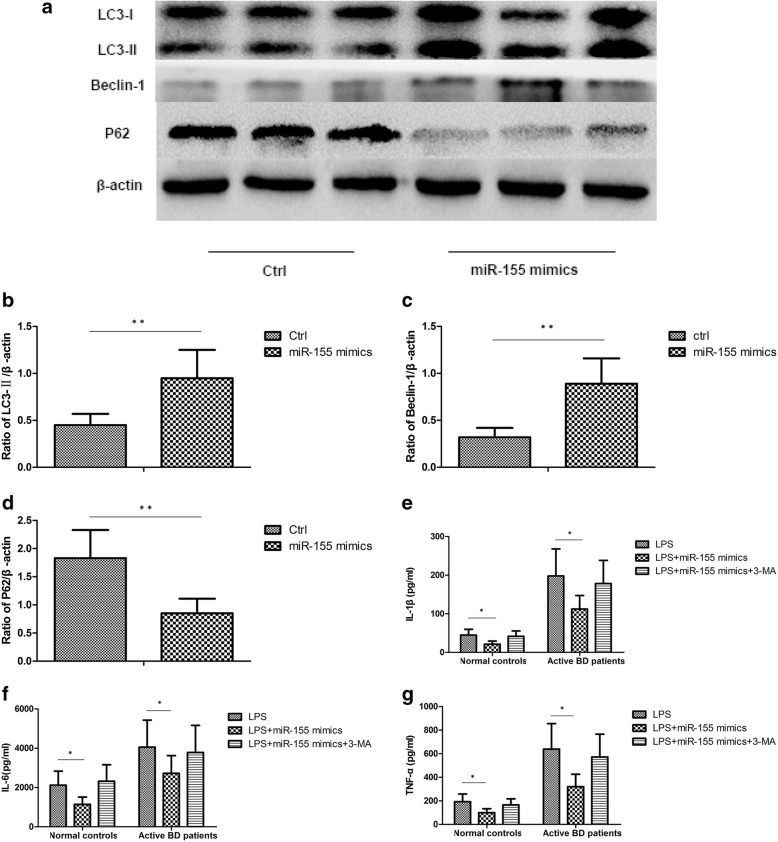
Earlier, we showed that the expression of miR-155 was decreased in DCs from active BD patients [8]. Moreover, miR-155 has been reported to be involved in autophagy regulation [17, 27–29]. A further experiment was carried out to investigate if miR-155 was associated with cytokine production by DCs induced by dysfunctional autophagy. The results showed that in DCs overexpressing miR-155, the expression of LC3-II and Beclin-1 was significantly increased after stimulation with LPS. The expression of P62 was significantly decreased in DCs overexpressing miR-155 after stimulation with LPS (Fig. 3a–d). As compared with DCs transfected with the control sequence, TNF-α, IL-6, and IL-1β production were significantly decreased in DCs overexpressing miR-155. However, there was no difference concerning the expression of TNF-α, IL-6, and IL-1β in LPS-treated DCs overexpressing miR-155 after treatment with 3-MA compared to DCs transfected with the control sequence (Fig. 3e–g).
Fig. 3.
MiR-155 is involved in the cytokine production of DCs induced by dysfunctional autophagy. DCs from normal controls (n=8) and active BD patients (n=8) were assessed. DCs were transfected with control mimics and miR-155 mimics at a final concentration of 100 nM. After 48 h, DCs were stimulated with 100ng/mL LPS for 24 h. a Expression levels of LC3-I, LC3-II, Beclin-1, and P62 protein in DCs from normal controls and active BD patients were quantified by Western blot. b–d Quantification of expression levels of LC3-II, Beclin-1, and P62 proteins. e–g TNF-α, IL-1β, and IL-6 were measured in the culture supernatants by ELISA. *p<0.05, **p<0.01
TAB2 is involved in the effects of MiR-155 on autophagy
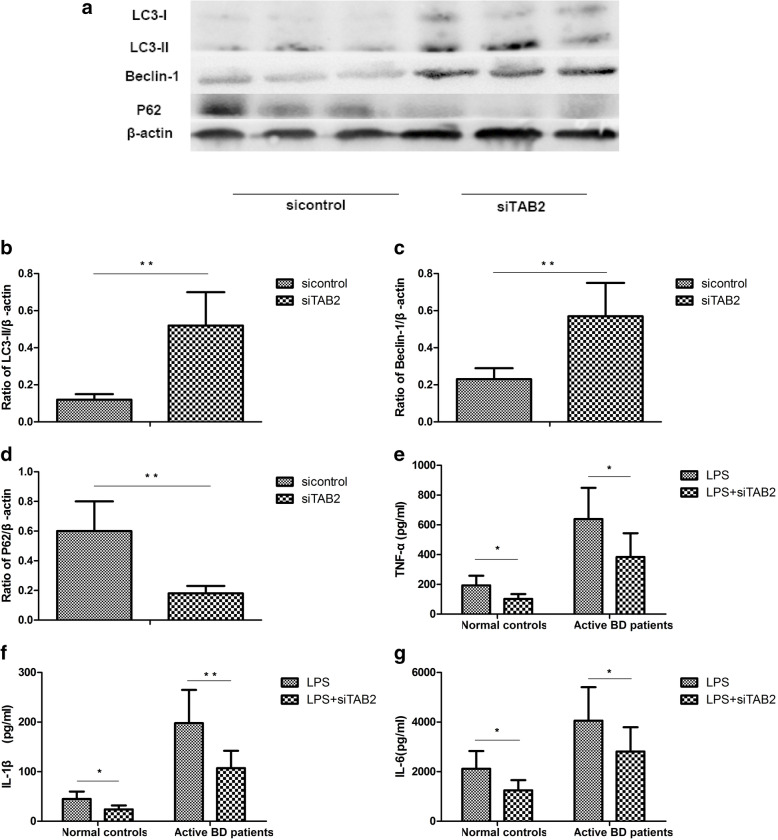
Transforming growth factor β-activated kinase 1-binding protein 2 (TAB2) is the direct target of miR-155. The expression of TAB2 in DCs from active BD patients is increased, and its expression can be suppressed by miR-155 [8]. Since the above findings showed that miR-155 was involved in the cytokine production of DCs induced by autophagy, a further experiment was performed to investigate whether TAB2 was involved in the effects of miR-155 on autophagy. The results showed that the expression of LC3-II and Beclin-1 was significantly increased, but that the expression of P62 was decreased in DCs after TAB2 was downregulated (Fig. 4a–d). The expression of TNF-α, IL-6, and IL-1β by DCs treated with LPS was significantly decreased after TAB2 was downregulated (Fig. 4e–g).
Fig. 4.
TAB2 is involved in the effects of miR-155 on autophagy. DCs from normal controls (n=8) and active BD patients (n=8) were assessed. DCs were transfected with siRNA or control siRNA and then stimulated with 100ng/mL LPS for 24 h. a Expression levels of LC3-I, LC3-II, Beclin-1, and P62 protein in DCs from the normal controls and active BD patients were quantified by Western blot. b–d Quantification of expression levels of LC3-II, Beclin-1, and P62 proteins. e–g TNF-α, IL-1β, and IL-6 were measured in the culture supernatants by ELISA. *p<0.05, **p<0.01
MiR-155 regulates autophagy through Akt/mTOR pathway
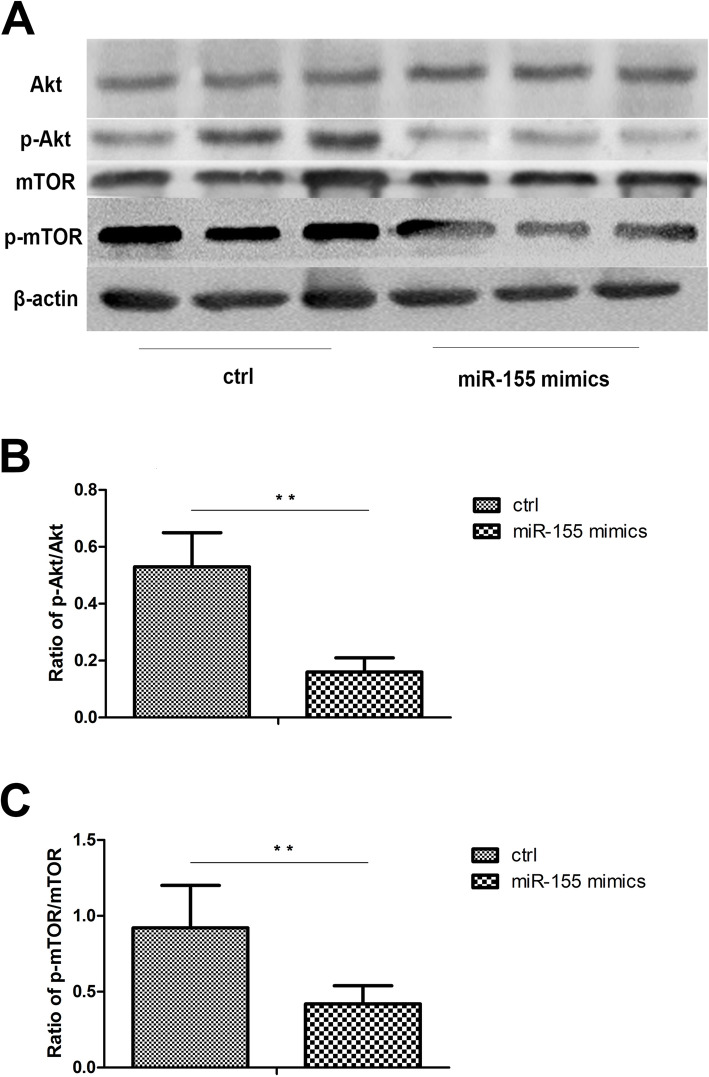
The aforementioned results showed that miR-155 could regulate cytokine production of DCs by controlling autophagy. The Akt/mTOR pathway is the most common signaling pathway involved in autophagy. We further examined whether miR-155 exerted its effects on autophagy by activating the Akt/mTOR pathway. The results showed that the ratios of p-Akt/Akt and p-mTOR/mTOR were significantly increased in DCs treated with LPS after transfection with miR-155 mimics (Fig. 5a–c). These results suggested that miR-155 may activate autophagy via the Akt/mTOR signaling pathway.
Fig. 5.
MiR-155 regulates autophagy through Akt/mTOR pathway. DCs from normal controls (n=8) and active BD patients (n=8) were assessed. After 48 h, DCs were stimulated with 100ng/mL LPS for 24 h. a Total levels of Akt and mTOR, together with phosphorylation levels of Akt and mTOR in DCs were determined by Western blotting. b–c Quantification of expression levels of p-Akt/Akt and p-mTOR/mTOR proteins. *p<0.05, **p<0.01
Discussion
In this study, we found that the autophagy process was activated in DCs from active BD patients. Autophagic degradation was however decreased, which, in turn, results in proinflammatory cytokine release by DCs. Moreover, we found that miR-155 and its target protein TAB2 were involved in the proinflammatory cytokine production of DCs by regulating autophagy through the Akt/mTOR signaling pathway. These results indicate that miR-155 might take part in the development of BD by controlling autophagy. In other words, our findings indicate that certain triggers in a genetically predisposed individual stimulates autophagosome formation, whereby a defective control of the subsequent degradation of autophagic products leads to a local stimulation of proinflammatory cytokine release.
It has been reported that autophagy was associated with the development of animal uveitis models [30, 31]. However, the role of autophagy in the pathogenesis of human uveitis is still unknown. In this study, we first detected the activity level of autophagy in DCs from active and inactive BD patients. The results showed that the autophagosomes were found in DCs from active BD patients. Furthermore, we found that as compared with inactive BD patients and healthy controls, the protein levels of two important markers of autophagy activity, Beclin-1 and LC3-II, were significantly increased in DCs derived from active BD patients. Additionally, the protein expression level of P62 was significantly increased in DCs of active BD patients as compared with inactive BD patients and healthy controls, which indicated that there is an increased number of autophagosomes but a concurrent buildup of autophagic degradation products.
In view of the impaired autophagic flux in DCs of active BD patients, we further examined if the activity of autophagy is associated with the cytokine production by DCs. Our results showed that compared with healthy controls, the production of TNF-α, IL-6, and IL-1β by DCs from active BD patients stimulated with LPS were significantly increased. We also showed that the autophagy inhibitor (3-MA) or promoter (rapamycin) could upregulate or downregulate the production of TNF-α, IL-6, and IL-1β. These results proved that the autophagy activity level correlated with the production of cytokines by DCs. These results are consistent with previous studies showing that IL-1β, IL-17, and IL-18 levels were downregulated in murine DCs after autophagy was inhibited [32]. Others showed that the production of IL-6, IFN-β, and TNF-α by DCs were decreased after Beclin-1 was knocked out [33]. These results indicate that a defective control of autophagy may trigger cytokine production by DCs.
Earlier, we reported that the expression of miR-155 was downregulated in DCs obtained from active BD patients and was involved in cytokine production by DCs [8]. It has also been reported that the expression of miR-155 was increased or decreased in various diseases and was involved in the development of these diseases by regulating autophagy [17, 19, 34, 35]. In view of the aforementioned reports, we next investigated whether miR-155 exerted its effects on cytokine production by DCs via the process of autophagy. To answer this question, an experiment with miR-155 mimic transfection was performed. The results showed that an miR-155 mimic could promote the expression of LC3-II and Beclin-1, which meant that autophagy activity was increased. More importantly, the protein level of P62 was downregulated in DCs after miR-155 mimic was transfected, showing that autophagic degradation was increased. Additionally, miR-155 mimic transfection reduced the production of TNF-α, IL-1β, and IL-6. The autophagy inhibitor, 3-MA, was able to counteract the effects of miR-155 on cytokine production. All these results suggested that autophagy was involved in the effects of miR-155 on cytokine production by DCs.
TAB2 has been reported to be the target of miR-155, and its protein expression level was elevated in DCs derived from active BD patients [8]. It also been reported that TAB2 could bind to Beclin1 or ATG13 to regulate autophagy [36–38]. A further experiment was performed to investigate whether miR-155 regulates the process of autophagy through TAB2. The results showed that downregulation of TAB2 could promote the autophagic flux. The expression of TNF-α, IL-6, and IL-1β was decreased after TAB2 was downregulated. Taken together, these data indicate that miR-155 regulates autophagy by controlling the expression of TAB2. Our results are in agreement with previous studies that showed that miR-155 promoted autophagy via a decrease in TAB2 expression, thereby stimulating osteoclast formation [17].
The Akt/mTOR pathway is one of the most common signaling pathways involved in the control of autophagy [39, 40]. Rapamycin, as the inhibitor of mTOR, has been shown to alleviate inflammation of the retina [41]. It has also been reported that the use of this mTOR inhibitor was safe and effective in treating non-infectious uveitis [42–44]. In this study, we found that miR-155 could downregulate the phosphorylation level of mTOR and Akt in DCs.
The limitation of our research is that we did not investigate the function of autophagy in other types of uveitis and the detailed mechanisms on how a decreased autophagic degradation results in increased cytokine production. Further experiments are needed to explore the effects of treatment on the function of autophagy in DCs and if the dysfunction of autophagy is a common phenomenon of uveitis.
Conclusions
Collectively, these results show that miR-155 can control the production of cytokines by DCs via the process of autophagy. Our study provides a greater depth of understanding about the mechanism on how miR-155 is involved in the development of BD and provides evidence for the use of autophagy as a potential therapeutic target of uveitis.
Acknowledgements
We thank all the patients and medical staff who generously contributed to this study.
Abbreviations
- miR
MicroRNA
- BD
Behcet’s disease
- DCs
Dendritic cells
- ATGs
Autophagy-related gene
- PBMCs
Peripheral blood mononuclear cells
- TAB2
Transforming growth factor β-activated kinase 1-binding protein 2
Authors’ contributions
LL and Q-YZ conceived and designed the study, performed the experiments and statistical analysis, and drafted the manuscript. L-FQ participated in performing the experiments and revised the manuscript. All authors critically revised the manuscript for important intellectual content and approved its final version. The authors contributed to the final manuscript.
Funding
This work was supported by the Basic Research program of Chongqing (cstc2020jcyj-msxmX0898), Basic Research program of Chongqing (cstc2015jcyjA10112), Natural Science Foundation Major International (Regional) Joint Research Project (grant no. 81720108009), Natural Science Foundation Project of Chongqing (cstc2017shmsA130073), Chongqing Key Laboratory of Ophthalmology (grant no. CSTC, 2008CA5003), Chongqing Science & Technology Platform and Base Construction Program (grant no. cstc2014pt-sy10002), Science and Technology Project Foundation of Chongqing (cstc2016jcyjA0597), and National Natural Science Foundation Project (grant no 81870673).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study obeyed the tenets of the Declaration of Helsinki and was approved by our Clinical Ethical Research Committee. Written consent was collected from each healthy control and BD patient.
Consent for publication
All participants have approved to publish the data in this manuscript.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Suzuki Kurokawa M, Suzuki N. Behcet’s disease. Clin Exp Med. 2004;4(1):10–20. doi: 10.1007/s10238-004-0033-4. [DOI] [PubMed] [Google Scholar]
- 2.Bonfioli AA, Orefice F. Behcet’s disease. Semin Ophthalmol. 2005;20(3):199–206. doi: 10.1080/08820530500231953. [DOI] [PubMed] [Google Scholar]
- 3.Deuter CM, Kotter I, Wallace GR, Murray PI, Stubiger N, Zierhut M. Behcet’s disease: ocular effects and treatment. Progress Retin Eye Res. 2008;27(1):111–136. doi: 10.1016/j.preteyeres.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Wang C, Ye Z, Kijlstra A, Zhou Y, Yang P. Activation of the aryl hydrocarbon receptor affects activation and function of human monocyte-derived dendritic cells. Clin Exp Immunol. 2014;177(2):521–530. doi: 10.1111/cei.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He Y, Wang C, Su G, Deng B, Ye Z, Huang Y, Yuan G, Aize K, Li H, Yang P. Decreased expression of A20 is associated with ocular Behcet’s disease (BD) but not with Vogt-Koyanagi-Harada (VKH) disease. Br J Ophthalmol. 2018;102(8):1167–1172. doi: 10.1136/bjophthalmol-2017-311707. [DOI] [PubMed] [Google Scholar]
- 6.Wan CK, He C. Cutting edge: IL-1 receptor signaling is critical for the development of autoimmune uveitis. J Immunol. 2016;196(2):543–6. [DOI] [PMC free article] [PubMed]
- 7.Ture-Ozdemir F, Tulunay A, Elbasi MO, Tatli I, Maurer AM, Mumcu G, Direskeneli H, Eksioglu-Demiralp E. Pro-inflammatory cytokine and caspase-1 responses to pattern recognition receptor activation of neutrophils and dendritic cells in Behcet’s disease. Rheumatology (Oxford) 2013;52(5):800–805. doi: 10.1093/rheumatology/kes399. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Q, Xiao X, Wang C, Zhang X, Li F, Zhou Y, Kijlstra A, Yang P. Decreased microRNA-155 expression in ocular Behcet’s disease but not in Vogt Koyanagi Harada syndrome. Invest Ophthalmol Vis Sci. 2012;53(9):5665–5674. doi: 10.1167/iovs.12-9832. [DOI] [PubMed] [Google Scholar]
- 9.Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176(1-2):11–42. doi: 10.1016/j.cell.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12(9):814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boutouja F, Stiehm CM, Platta HW. mTOR: a cellular regulator interface in health and disease. Cells.2019;8(1):18. 10.3390/cells8010018. [DOI] [PMC free article] [PubMed]
- 12.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368(7):651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 13.Lin DS, Huang YW, Ho CS, Hung PL, Hsu MH, Wang TJ, Wu TY, Lee TH, Huang ZD, Chang PC, et al. Oxidative insults and mitochondrial DNA mutation promote enhanced autophagy and mitophagy compromising cell viability in pluripotent cell model of mitochondrial disease. Cells. 2019;8(1):65. 10.3390/cells8010065. [DOI] [PMC free article] [PubMed]
- 14.Ma S, Attarwala I, Xie XS. SQSTM1/p62: a potential target for neurodegenerative disease. ACS Chem Neurosci. 2019;10(5):2094–2114. doi: 10.1021/acschemneuro.8b00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin TA, Wu VC. Autophagy in chronic kidney diseases. Cells. 2019;8(1):61. 10.3390/cells8010061. [DOI] [PMC free article] [PubMed]
- 16.Zheng M, Yu H, Zhang L, Li H, Liu Y, Kijlstra A, Yang P. Association of ATG5 gene polymorphisms with Behcet’s disease and ATG10 gene polymorphisms with VKH syndrome in a Chinese Han population. Invest Ophthalmol Vis Sci. 2015;56(13):8280–8287. doi: 10.1167/iovs.15-18035. [DOI] [PubMed] [Google Scholar]
- 17.Sul OJ, Sung YB, Rajasekaran M, Ke K, Yu R, Back SH, Choi HS. MicroRNA-155 induces autophagy in osteoclasts by targeting transforming growth factor beta-activated kinase 1-binding protein 2 upon lipopolysaccharide stimulation. Bone. 2018;116:279–289. doi: 10.1016/j.bone.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Zheng ZJ, Jia YJ, Yang YL, Xue YM. Role of p53/miR-155-5p/sirt1 loop in renal tubular injury of diabetic kidney disease. J Transl Med. 2018;16(1):146. [DOI] [PMC free article] [PubMed]
- 19.Yang Y, Zhang N, Wang S, Wen Y. MicroRNA-155 regulates inflammatory response in ischemic cerebral tissues through autophagy. Curr Neurovasc Res. 2018;15(2):103–110. doi: 10.2174/1567202615666180601081409. [DOI] [PubMed] [Google Scholar]
- 20.Criteria for diagnosis of Behcet’s disease. International Study Group for Behcet’s Disease. Lancet. 1990, 335(8697):1078-1080. [PubMed]
- 21.Liang L, Tan X, Zhou Q, Zhu Y, Tian Y, Yu H, Kijlstra A, Yang P. IL-1beta triggered by peptidoglycan and lipopolysaccharide through TLR2/4 and ROS-NLRP3 inflammasome-dependent pathways is involved in ocular Behcet’s disease. Invest Ophthalmol Vis Sci. 2013;54(1):402–414. doi: 10.1167/iovs.12-11047. [DOI] [PubMed] [Google Scholar]
- 22.Wang C, Tian Y, Lei B, Xiao X, Ye Z, Li F, Kijlstra A, Yang P. Decreased IL-27 expression in association with an increased Th17 response in Vogt-Koyanagi-Harada disease. Invest Ophthalmol Vis Sci. 2012;53(8):4668–4675. doi: 10.1167/iovs.12-9863. [DOI] [PubMed] [Google Scholar]
- 23.Lopes de Faria JM, Duarte DA, Montemurro C, Papadimitriou A, Consonni SR, Lopes de Faria JB. Defective autophagy in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2016;57(10):4356–4366. doi: 10.1167/iovs.16-19197. [DOI] [PubMed] [Google Scholar]
- 24.Katsuragi Y, Ichimura Y, Komatsu M. p62/SQSTM1 functions as a signaling hub and an autophagy adaptor. FEBS J. 2015;282(24):4672–4678. doi: 10.1111/febs.13540. [DOI] [PubMed] [Google Scholar]
- 25.Noda T. Regulation of autophagy through TORC1 and mTORC1. Biomolecules. 2017;7(3):52. 10.3390/biom7030052. [DOI] [PMC free article] [PubMed]
- 26.Wu Y, Wang X, Guo H, Zhang B, Zhang XB, Shi ZJ, Yu L. Synthesis and screening of 3-MA derivatives for autophagy inhibitors. Autophagy. 2013;9(4):595–603. doi: 10.4161/auto.23641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Etna MP, Sinigaglia A, Grassi A. Mycobacterium tuberculosis-induced miR-155 subverts autophagy by targeting ATG3 in human dendritic cells. PLoS Pathog. 2018;14(1):e1006790. [DOI] [PMC free article] [PubMed]
- 28.Liu F, Nie C, Zhao N, Wang Y, Liu Y, Li Y, Zeng Z, Ding C, Shao Q, Qing C, et al. MiR-155 alleviates septic lung injury by inducing autophagy via inhibition of transforming growth factor-beta-activated binding protein 2. Shock. 2017;48(1):61–68. doi: 10.1097/SHK.0000000000000839. [DOI] [PubMed] [Google Scholar]
- 29.Zarogoulidis P, Petanidis S, Domvri K, Kioseoglou E, Anestakis D, Freitag L, Zarogoulidis K, Hohenforst-Schmidt W, Eberhardt W. Autophagy inhibition upregulates CD4(+) tumor infiltrating lymphocyte expression via miR-155 regulation and TRAIL activation. Mol Oncol. 2016;10(10):1516–1531. doi: 10.1016/j.molonc.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santeford A, Wiley LA, Park S, Bamba S, Nakamura R, Gdoura A, Ferguson TA, Rao PK, Guan JL, Saitoh T, Akira S, Xavier R, Virgin HW, Apte RS. Impaired autophagy in macrophages promotes inflammatory eye disease. Autophagy. 2016;12(10):1876–1885. doi: 10.1080/15548627.2016.1207857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia X, Li J, Shi D, Zhao Y, Dong Y, Ju H, Yang J, Sun J, Li X, Ren H. Grouping annotations on the subcellular layered interactome demonstrates enhanced autophagy activity in a recurrent experimental autoimmune uveitis T cell line. Plos one. 2014;9(8):e104404. doi: 10.1371/journal.pone.0104404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mantegazza AR, Wynosky-Dolfi MA. Increased autophagic sequestration in adaptor protein-3 deficient dendritic cells limits inflammasome activity and impairs antibacterial immunity. PLoS Pathog. 2017;13(12):e1006785. [DOI] [PMC free article] [PubMed]
- 33.Zang F, Chen Y, Lin Z, Cai Z, Yu L, Xu F, Wang J, Zhu W, Lu H. Autophagy is involved in regulating the immune response of dendritic cells to influenza A (H1N1) pdm09 infection. Immunology. 2016;148(1):56–69. doi: 10.1111/imm.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu K, Zhu C, Yao Y, Wang X, Song J, Zhai J. MicroRNA-155-enhanced autophagy in human gastric epithelial cell in response to Helicobacter pylori. Saudi J Gastroenterol. 2016;22(1):30–36. doi: 10.4103/1319-3767.173756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du F, Yu F, Wang Y, Hui Y, Carnevale K, Fu M, Lu H, Fan D. MicroRNA-155 deficiency results in decreased macrophage inflammation and attenuated atherogenesis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2014;34(4):759–767. doi: 10.1161/ATVBAHA.113.302701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takaesu G, Kobayashi T, Yoshimura A. TGFβ-activated kinase 1 (TAK1)-binding proteins (TAB) 2 and 3 negatively regulate autophagy. J Biochem. 2012;151(2):157–166. doi: 10.1093/jb/mvr123. [DOI] [PubMed] [Google Scholar]
- 37.Criollo A, Niso-Santano M, Malik SA, Michaud M, Morselli E, Mariño G, Lachkar S, Arkhipenko AV, Harper F, Pierron G, Rain JC, Ninomiya-Tsuji J, Fuentes JM, Lavandero S, Galluzzi L, Maiuri MC, Kroemer G. Inhibition of autophagy by TAB2 and TAB3. EMBO J. 2011;30(24):4908–4920. doi: 10.1038/emboj.2011.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niso-Santano M, Criollo A, Malik SA, Michaud M, Morselli E, Mariño G, Lachkar S, Galluzzi L, Maiuri MC, Kroemer G. Direct molecular interactions between Beclin 1 and the canonical NFκB activation pathway. Autophagy. 2012;8(2):268–270. doi: 10.4161/auto.8.2.18845. [DOI] [PubMed] [Google Scholar]
- 39.Paglin S, Lee NY, Nakar C, Fitzgerald M, Plotkin J, Deuel B, Hackett N, McMahill M, Sphicas E, Lampen N, Yahalom J. Rapamycin-sensitive pathway regulates mitochondrial membrane potential, autophagy, and survival in irradiated MCF-7 cells. Cancer Res. 2005;65(23):11061–11070. doi: 10.1158/0008-5472.CAN-05-1083. [DOI] [PubMed] [Google Scholar]
- 40.Takeuchi H, Kondo Y, Fujiwara K, Kanzawa T, Aoki H, Mills GB, Kondo S. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005;65(8):3336–3346. doi: 10.1158/0008-5472.CAN-04-3640. [DOI] [PubMed] [Google Scholar]
- 41.Okamoto T, Ozawa Y, Kamoshita M, Osada H, Toda E, Kurihara T, Nagai N, Umezawa K, Tsubota K. The neuroprotective effect of rapamycin as a modulator of the mTOR-NF-kappaB axis during retinal inflammation. Plos one. 2016;11(1):e0146517. doi: 10.1371/journal.pone.0146517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blair J, Barry R, Moore DJ, Denniston AK. A comprehensive review of mTOR-inhibiting pharmacotherapy for the treatment of non-infectious uveitis. Curr Pharm Des. 2017;23(20):3005–3014. doi: 10.2174/1381612823666170111125550. [DOI] [PubMed] [Google Scholar]
- 43.Blair J, Barry R, Murray PI, Moore DJ, Denniston AK: mTOR-inhibiting pharmacotherapy for the treatment of non-infectious uveitis: a systematic review protocol. Syst Rev. 2018;7(1):83. [DOI] [PMC free article] [PubMed]
- 44.Ibrahim MA, Sepah YJ, Watters A, Bittencourt M, Vigil EM, Do DV, Nguyen QD. One-year outcomes of the SAVE study: sirolimus as a therapeutic approach for UVEitis. Transl Vis Sci Technol. 2015;4(2):4. doi: 10.1167/tvst.4.2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.