Abstract
Study Objectives
Aerobic fitness (AF) and sleep are major determinants of health in adolescents and impact neurocognitive and psychological development. However, little is known about the interactions between AF and sleep during the developmental transition experienced across adolescence. This study aimed to consider the relationships between AF and habitual sleep patterns and sleep neurophysiology in healthy adolescents.
Methods
Subjects (mean age = 14.6 ± 2.3 years old, range 11–17, 11 females) were evaluated for AF (peak VO2 assessed by ramp-type progressive cycle ergometry in the laboratory), habitual sleep duration and efficiency (7–14 days actigraphy), and topographic patterns of spectral power in slow wave, theta, and sleep spindle frequencies in non-rapid eye movement (NREM) sleep using overnight polysomnography (PSG) with high-density electroencephalography (hdEEG, 128 channels).
Results
Significant relationships were observed between peak VO2 and habitual bedtime (r = −0.650, p = .009) and wake-up time (r = −0.603, p = .017), with greater fitness associated with going to bed and waking up earlier. Peak VO2 significantly predicted slow oscillations (0.5–1 Hz, p = .018) and theta activity (4.5–7.5 Hz, p = .002) over anterior frontal and central derivations (p < .001 and p = .001, respectively) after adjusting for sex and pubertal development stage. Similar associations were detected for fast sleep spindle activity (13–16 Hz, p = .006), which was greater over temporo-parietal derivations.
Conclusions
Greater AF was associated with a more mature pattern of topographically-specific features of sleep EEG known to support neuroplasticity and cognitive processes and which are dependent on prefrontal cortex and hippocampal function in adolescents and adults. AF was also correlated with a smaller behavioral sleep phase delay commonly seen during adolescence.
Keywords: aerobic fitness, sleep, EEG, PSG, adolescents, development
Statement of Significance.
This study found that greater aerobic fitness (AF) was associated with earlier habitual sleep times and with a more mature pattern of fronto-central slow wave and parietal fast frequency sleep spindle oscillations during non-rapid eye movement (NREM) sleep. AF may be involved in minimizing the phase delay commonly observed during adolescence and may enhance topographically specific features of sleep physiology known to mechanistically support neuroplasticity and cognitive processes.
Introduction
Aerobic (or cardiopulmonary) fitness (AF) reflects the integrated ability to deliver oxygen to the mitochondria and support muscle activity during exercise. It is considered to reflect total body health and can be measured directly in the laboratory, expressed as maximal oxygen consumption (peak VO2) [1]. AF is used as an indicator of overall health, and in adolescents it is a strong predictor of future physical health outcomes (e.g. cardiovascular disease) [2]. There is also a strong relationship between AF and cognitive performance, with stronger effect sizes when peak VO2 measures are used as opposed to indirect methods of AF evaluation [3]. A recent study in 167 Spanish teenagers (ages 14–15) reported that peak VO2 was the best predictor of neurocognitive battery test scores (e.g. attention, concentration, cognitive flexibility, and processing speed) and psychosocial variables (e.g. self-efficacy and general health) relative to other fitness indicators such as percentage of body fat mass, explosive force, speed, and weekly amount of physical exercise [4]. AF has also been associated with improved executive control and hippocampal-dependent memory in 9- to 10-year-old children [5], as well as enhanced neuronal pruning evidenced by a reduction in prefrontal grey matter volume in adolescents [6–8]. Hypothesized mechanisms supporting relationships between AF and cognitive benefits include increased production of neurotrophins [9, 10], cerebral structural changes [11], and increased cerebral blood flow [12].
Sleep is a major health determinant across the lifespan and is vital for cognitive health of the developing child [13, 14]. Similar to AF, which shows a strong maturational effect with nearly linear increases in AF across maturity status and age [15, 16], sleep also undergoes distinct maturational changes during adolescence marked by a decrease in the duration of total sleep, largely due to a decline in amount and intensity of non-rapid eye movement (NREM) slow-wave sleep (SWS) [17]. Overnight polysomnography (PSG) with high-density electroencephalography (hdEEG; 128 channels) reveals topographical patterns of sleep oscillations that have been specifically linked to neuroplasticity, neurodevelopment, and cognitive function [6]. Slow-wave activity (SWA) tracks the synaptic pruning supporting cortical brain maturation during adolescent brain development [7, 8, 18], and several studies in adolescents have confirmed a relationship between cognitive improvement and plastic changes in neuronal function evident during sleep [6, 18–22]. Indeed, the topographic distribution (i.e. cortical location on the scalp) of SWA peaks over occipital regions in early childhood and migrates to anterior frontal cortex in later pubertal development stages, similar to, and correlated with, observed trajectories in grey matter brain development [6–8]. Furthermore, the intensity of SWA tracks closely with changes in synaptic density over the course of development [18].
Other NREM frequencies (e.g. theta activity and sleep spindles) also show maturational changes in topographic expression and are linked to neuroplasticity [6, 19–22]. Both SWA and theta activity show the largest reductions in absolute spectral power across development, with NREM theta activity showing declines of ~60% between ages 11–16.5 years, preceding developmental changes in delta activity [6, 8]. The mirroring of this effect with the differential synaptic pruning of neurons in cortical layers 3 and 5 suggests that both delta and theta may track distinct aspects of cortical maturation during adolescent development [8]. Similar to SWA, theta activity becomes more frontal with increasing age, tracking the trajectory of cortical maturation [6]. Sleep spindles also change during adolescence, with mean sleep spindle frequencies becoming faster across development [6]. The topographic changes in spindle activity are complex, with slower frequency spindles becoming more frontal-dominant, while faster frequency spindles become more parietal-dominant in peak expression [6, 22], ultimately mimicking the canonical topographic expression of these sleep spindle subtypes observed in adults [23]. This developmental increase in mean sleep spindle frequency has been shown to be directly related to improvements in sleep-dependent memory across adolescence, whereas increases in slow spindles over anterior frontal electrodes are associated with improvements in cognitive ability more broadly [22].
As both sleep and AF are fundamental for health, impact cognitive function, and undergo simultaneous maturation across adolescence, it is possible that AF is associated with sleep patterns and sleep-related neurodevelopmental processes. While some studies have suggested the possibility that higher AF (using peak VO2 indirect measures) is associated with better subjective sleep quality [24, 25], we are not aware of any studies evaluating the relationships between the direct measure of AF (i.e. peak VO2) and measures of subjective and objective sleep, including local expression of sleep EEG oscillations across frequencies. This study aimed to assess the relationships between AF (i.e. peak VO2) and comprehensive evaluation of sleep using gold-standard assessment methodology, including subjective questionnaires, habitual sleep measures (i.e. actigraphy and sleep diary), and in-laboratory sleep neurophysiology (i.e. PSG with hdEEG), in adolescents. We hypothesized that greater AF would be associated with healthier sleep patterns, including timing and amounts of sleep, when controlling for sex, maturation, and physical activity. We further hypothesized that greater AF would be associated with a more mature pattern of local sleep expression within frequency ranges associated with both neuroplasticity and brain development; i.e. that AF would be positively associated with NREM SWA and theta activity over anterior prefrontal EEG derivations and positively associated with fast sigma activity over posterior parietal EEG derivations.
Methods
Study participants
Twenty healthy adolescents (average age = 14.6 years, ranged 11–17, 11 females), with no clinical psychological symptoms, participated in the study (full demographics are provided in Table 1). Participants were recruited directly from the Pediatric Exercise and Genomics Research Center (PERC) Participant Registry of motivated adolescents interested in research participation. Additionally, a flyer was distributed in various schools and community areas. The study aimed to sample middle- or high-school age (grades 6–12 and 11- to 17-year-old) participants across pubertal development stages (equivalent to Tanner stages I-V [26]), who were in good health (determined by history), reported no evidence of disease or disability that would impair their participation in AF evaluation and/or overnight sleep study, and fluent in the English language. Exclusion criteria included reports of diagnosed chronic illness, pregnancy or breastfeeding, use of illegal drugs or alcohol in the last month (by history), and regular use of anti-inflammatory medications parenterally, orally, or as inhaled agents. Participants were recruited from the Orange County, CA area. They were not allowed to travel outside the time zone within 2-weeks of study participation. This study was approved by the Institutional Review Board at the University of California, Irvine (UCI), and informed consent/assent were obtained for all participants.
Table 1.
Descriptive characteristics of the participants (N = 20) and correlation coefficients between sleep and aerobic fitness
| Correlation with peak VO2a | |||
|---|---|---|---|
| Demographics | Mean (SD) | r | p |
| Age (years) | 14.61 (2.25) | ||
| Sex | |||
| Female (n; %) | 11 (55.0) | ||
| Male (n; %) | 9 (45.0) | ||
| Pubertal Development Stage | |||
| I (n; %) | 2 (10.0) | ||
| II (n; %) | 3 (15.0) | ||
| III (n; %) | 2 (10.0) | ||
| IV (n; %) | 10 (50.0) | ||
| V (n; %) | 3 (15.0) | ||
| Anthropometrics | |||
| Height (cm) | 162.96 (13.70) | ||
| Weight (kg) | 53.61 (12.91) | ||
| BMI percentile | 47.44 (23.23) | ||
| Aerobic fitness | |||
| Peak VO2 (mL/kg/minute) | 44.75 (8.48) | ||
| Habitual sleep (averaged across 7–14 days and nights) | |||
| Subjective (sleep diary) | |||
| Bedtime | 22:55 (1:06) | −0.583* | .014 |
| Lights off time | 23:28 (1:12) | −0.671** | .003 |
| Wakeup time | 8:07 (1:16) | −0.587* | .013 |
| Get up time | 8:23 (1:19) | −0.607* | .010 |
| Total time in bed (minutes) | 568.2 (68.5) | −0.188 | .471 |
| Total sleep time (minutes) | 538.8 (61.2) | −0.152 | .561 |
| Sleep onset latency (minutes) | 11.5 (5.7) | 0.019 | .943 |
| Wake after sleep onset (minutes) | 2.5 (3.0) | −0.286 | .266 |
| Sleep efficiency (%) | 94.8 (2.5) | 0.227 | .381 |
| Objective (actigraphy) | |||
| Bedtime | 22:47 (0:58) | −0.650** | .009 |
| Sleep onset time | 23:37 (1:10) | −0.656** | .008 |
| Wakeup time | 7:52 (1:18) | −0.603* | .017 |
| Get up time | 8:24 (1:20) | −0.618* | .014 |
| Total time in bed (minutes) | 577.0 (58.7) | −0.257 | .355 |
| Total sleep time (minutes) | 424.7 (56.7) | 0.062 | 0.825 |
| Sleep onset latency (minutes) | 49.9 (25.8) | −0.214 | .444 |
| Wake after sleep onset (minutes) | 70.1 (46.7) | −0.244 | .380 |
| Sleep efficiency (%) | 74.0 (8.5) | 0.268 | .334 |
| Objective sleep (in-laboratory polysomnography) | |||
| Total time in bed (minutes) | 570.3 (66.3) | −0.133 | .610 |
| Total sleep time (minutes) | 527.2 (78.0) | −0.179 | .492 |
| Sleep onset latency (minutes) | 3.7 (2.6) | 0.029 | .911 |
| Wake after sleep onset (minutes) | 37.7 (23.7) | 0.201 | .440 |
| Sleep efficiency (%) | 92.2 (5.1) | −0.184 | .479 |
| Stage 1 sleep (%) | 4.2 (1.7) | 0.043 | .870 |
| Stage 2 sleep (%) | 44.4 (7.5) | 0.069 | .791 |
| Stage 3 sleep (%) | 29.3 (6.4) | 0.044 | .868 |
| NREM sleep (%) | 77.9 (4.1) | 0.200 | .442 |
| REM sleep (%) | 22.1 (4.1) | −0.198 | .446 |
aPartial correlation adjusted for sex, pubertal development stage, and physical activity. BMI, body mass index; NREM, non-rapid eye movement; REM, rapid eye movement.
*p < .05, **p < .01.
Study design
All evaluations took place during the summer (July–September) of 2019, when participants were not restricted by school schedules.
Visit 1–UCI pediatric exercise and genomic research center human performance laboratory
Physical examination.
Anthropometric measurements (height and weight) and subjective self-assessment of pubertal development stage (corresponding to Tanner stages I-V [26]) were completed.
Physical activity.
Participants completed the modifiable exercise questionnaire (MAQ) [27], which was revised to ask about activities during the last week and yielded the average time of engagement in moderate or vigorous physical activity. Participants were also asked if they were involved in organized competitive sports, and if training required early morning practice.
AF (Cardiopulmonary Exercise Test).
Participants performed a ramp-type progressive cycle ergometry exercise test using the SensorMedics metabolic system (Vmax 229, Yorba Linda, CA). Gas exchange was measured breath-by-breath and peak VO2 was calculated as the highest 20-second rolling average in the last 2 minutes of exercise. All participants achieved respiratory exchange ratio (RER) ≥ 1.1 at the end of the test.
At-home ambulatory assessment
Habitual sleep and activity patterns.
After completion of Visit 1, participants were provided with daily sleep diaries [28] with instructions for completion and were given a lightweight accelerometer/actigraph (Actiwatch Spectrum Plus; Philips Respironics, Bend, OR) to wear on their non-dominant wrist for 7–14 days, up until the time they came to the Sleep Center for their overnight assessment. For scheduling flexibility, overnight assessment was scheduled between 7 and 14 days post-AF assessment. The actigraphy recording was continuous and started on the day of the AF evaluation and lasted up to 2 full weeks. The actigraph assesses movements and has been validated and frequently utilized as a proxy measure of objective sleep [29]. Scoring followed expert consensus guidelines [29] and used the validated proprietary Actiware software algorithm [30, 31]. The Actiwatch has off-wrist detection methodology and off-wrist times are classified as missing data rather than no activity during these times. We set an a priori definition for compliance: participants with <5 consecutive days of recordings and/or having >2 full nights missing were excluded. Those with >3 consecutive hours missing data on more than 2 days were reviewed by the authors (ABN and IYC) to ensure that these missing data did not impact habitual sleep data outcomes. We refer here to actigraphy-derived sleep outcomes as habitual sleep which here reflects the individual behavioral and familial pattern and habits rather than free-living which tend to reflect uninhibited behavior and ad libitum sleep. While not in formal school, they still were restricted to familial routines and other responsibilities (e.g. organized sports and other scheduled activities) and thus would not be considered free-living. Variables extracted from both sleep diary and actigraphy included total sleep time (TST), sleep onset latency (SOL), wake after sleep onset (WASO), sleep efficiency (SE), and sleep onset/offset times. Sleep diary included a daily report of caffeine use.
Visit 2–UCI sleep center
Clinical assessment.
Semi-structured psychiatric evaluation [32] with sleep history was completed by mental health providers specializing in sleep (either ABN or IYC).
Overnight PSG with hdEEG.
Participants were instructed to avoid caffeinated beverages after noontime on their sleep study day. They were allowed to sleep up to 11 hours in the sleep laboratory. In-lab bedtimes were set approximately one hour later than actigraphy-defined habitual bedtimes as they completed other experimental protocols (i.e. assessment of dim light melatonin onset, DLMO) during the evening (data not reported in this manuscript). Standard clinical PSG with hdEEG (128 channels), including electrooculogram (EOG), electromyogram (EMG), electrocardiogram (ECG), and respiratory monitoring, was recorded simultaneously via an integrated system (Natus), following standard procedures [33, 34]. EEG, EOG, ECG, and EMG signals were digitized at 1024 Hz. Sleep variables, including sleep stages and clinical events, were visually scored in 30-second epochs according to standard criteria [34] by Registered Polysomnographic Technologists, and was reviewed by a board-certified sleep medicine specialist (R.M.B.). PSG variables of interest included TST, SOL, WASO, SE, percent of the sleep period spent in sleep stages N1, N2, N3, and R.
hdEEG processing.
Data preprocessing, including semi-automated artifact rejection [33, 35, 36], and analyses of sleep data focusing on NREM stages (N2 and N3) were performed using custom MATLAB scripts as previously described [37]. EEG data were imported into EEGLAB (http://sccn.ucsd.edu/eeglab/), downsampled to 256 Hz, and filtered between 0.3 and 40 Hz. EEG channels with significant artifact were removed and interpolated using surrounding channels employing the spherical interpolation method [38, 39]. Fast Fourier transform (FFT) was applied to the artifact-free filtered EEG signal in 30-second intervals employing a multi-taper approach using 11 discrete prolate spheroidal Slepian sequence (DPSS) tapers (0.25 Hz bandwidth, 16.667% moving window overlap, 0 order padding) to estimate power spectral density (PSD) [40–42]. Spectral power estimates were derived from the median across epochs for each bin by sleep stage at each EEG derivation, to minimize the influence of epochs containing outliers. Spectral estimates were averaged across frequency bands to construct absolute spectral power estimates for each a priori defined frequency bands of interest due to their known associations with neuroplasticity and neurodevelopment (i.e. slow oscillation: 0.5–1 Hz, delta: 1–4.5 Hz, theta: 4.5–7.5 Hz, alpha: 7.5–11 Hz, slow sigma: 11–13 Hz, fast sigma: 13–16 Hz, beta: 16–28 Hz, and gamma: 28–40 Hz) [6, 18–22]. While absolute power is commonly reported, comparisons across adolescence are confounded by a brain state and frequency-independent decrease in total power across pubertal development stages [43, 44]. To address this concern, relative power in each frequency bin was derived for each epoch at each derivation by dividing absolute power estimates by estimates of total power. Relative power for each frequency band of interest was derived using the same procedure used for absolute power and was normalized by z-transforming relative power estimates across derivations. One participant was excluded from analysis due to the presence of significant EEG artifact.
Analyses
Distribution statistics were calculated for each demographic, sleep and fitness measure for the overall sample as well as stratified by sex. Bivariate analyses (correlations, one-way regressions, chi-square tests) were performed to examine the relationship between sex and pubertal development stage (subjectively reported Tanner stage) on each sleep outcome. Due to the lack of one-to-one mapping between objectively and subjectively assessed pubertal development stages [45], effect sizes characterizing sex and pubertal development stage were calculated from the bivariate results, whereby participants with subjective pubertal development stage I–III (early pubertal development) were compared to participants in stages IV–V (late pubertal development). Peak VO2 was evaluated in relationship to habitual sleep patterns and overnight PSG with hdEEG. For hdEEG topographical analyses, nonparametric threshold-free cluster enhancement (TFCE) was implemented as previously described [46] to correct for multiple comparisons using 5000 permutations [47] and the corrected significance threshold was set to p < .05. Multiple regression analyses were performed to examine the relationship between peak VO2 and sleep parameters, controlling for sex, pubertal development stage, and physical activity. Due to the exploratory nature of the evaluations as well as the use of hdEEG with 128 channels, significance was considered at p < .05 after controlling for sex and pubertal stage.
Results
All participants completed the fitness assessment, and valid overnight PSG was obtained by 19 (95%) participants (data from one participant was not eligible due to equipment failure). Actigraphy-derived habitual sleep patterns were obtained for 18 (90%) participants (two participants did not meet actigraphy adherence definition) over an average of 8.6 ± 2.1 days and nights (range of 7–14 days and nights). Average missing data was 2.3% of the entire recording time (ranged 0%–9.4%). There were minimal data missing from sleep diaries; only 3.9% of 181 days of sleep recording had missing entries and no participant missed an entire day of entries (most commonly participants did not report SOL). Only 1 participant reported daily use of caffeine (averaging 2 cups of caffeinated beverage per day), and an additional 6 reported occasional use of caffeine in a rate of <1 serving per day. In this study cohort, 80% reported engagement in competitive sports (e.g. water polo, swimming, soccer, taekwondo, and tennis). Three participants (15%) reported that their sports required an early morning awakening. The sample engaged in moderate or vigorous physical activity for a median of 41.4 minutes (range: 5 minutes to 5 hours) per day.
Fitness characterization
Aerobic fitness.
Peak VO2 was normally distributed, yet the majority of the sample had average-to-high levels of AF as indicated by pediatric VO2 norms [48], with a mean peak VO2 = 44.8 ± 8.5 (mL/kg/minute). The relatively high fitness level was evident in both sexes (peak VO2–Males = 50.7 ± 4.9 and peak VO2–Females = 39.9 ± 7.8). Nineteen participants were within normal body mass index (BMI) percentile ranges [49] and one was overweight (overall range: 8th‒90th). In this sample, the relationship between peak VO2 and reported engagement in moderate or vigorous physical activity was not statistically significant when controlling for sex and pubertal development stage (p = .1), indicating that it is unlikely that engagement in physical activity fully explains any reported effects of AF on sleep expression.
Sleep characterization
No significant sleep disorders were reported or observed. Habitual sleep evaluation suggested that the majority of this sample was likely sleep-deprived (average habitual actigraphic TST = 7.1 ± 0.9 hours; range 5.6–9.1 hours), with only three adolescents (16.8%) sleeping over 8 hours on average during the ambulatory assessment. During overnight PSG, participants fell asleep relatively quickly (SOL = 3.7 ± 2.6 minutes; range 0.1–11) with high SE (92.2 ± 5.1%; range 81.0%–98.1%), which are typically signs of chronic sleep restriction and accumulated sleep debt [50, 51]. This may also be partly influenced by delaying sleep onset by 1 hour past their habitual bedtime due to the protocol design (i.e. completion of DLMO protocol). As participants were allowed ad libitum sleep (but no more than 11 hours) and with possible increased sleep drive resulting from the study procedures, TST on PSG (8.8 ± 1.3 hours range 6.3–10.8) was greater than that seen during actigraphy assessment (t = −5.39, p < .001). No significant differences were noted in habitual sleep duration between weekdays and weekends on actigraphy (weekday TST = 6.98 ± 1.08 hours [range 5.2–9.1], weekend TST = 7.3 ± 0.98 hours [range 5.2–8.9], t = −1.38, p = .185).
Pubertal development stage and NREM sleep oscillations.
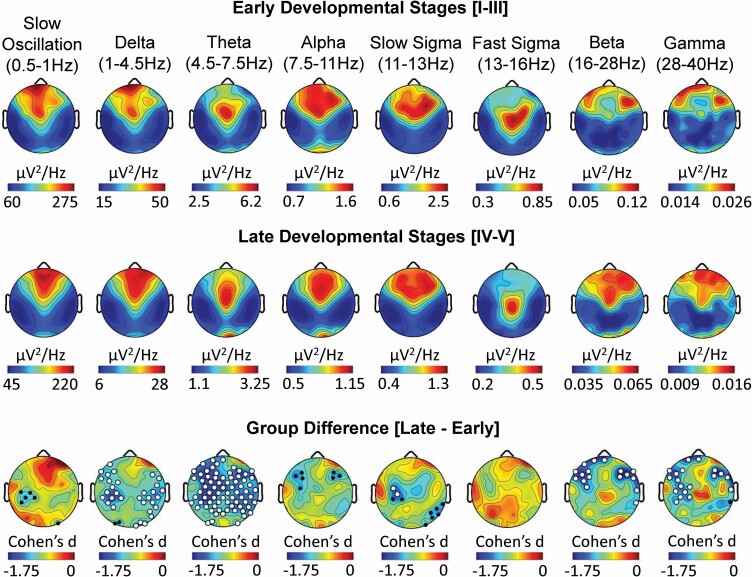
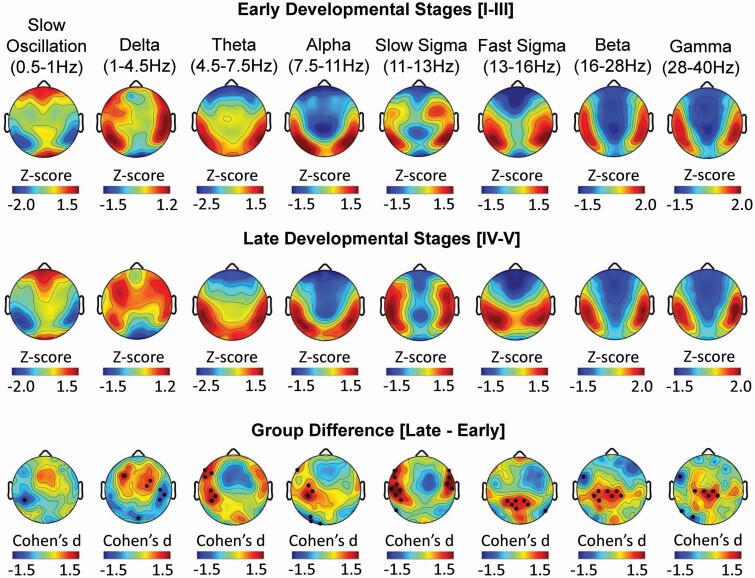
Global differences in absolute spectral power were observed between early/late pubertal development groups across the scalp in nearly all frequency bands of interest. Reductions in delta, theta, slow sigma, beta, and gamma power at multiple derivations remained significant following TFCE correction for multiple comparisons (Figure 1). Consistent with the effects of pubertal status, negative associations between age and absolute spectral power during NREM sleep were identified globally across frequencies (Supplementary Figure S1). Relative SWA (i.e. slow oscillation and delta frequency bands), a measure controlling for the natural total power decrease during brain maturation, was higher at fronto-central EEG derivations and lower at temporal-parietal derivations in late compared to early pubertal development groups (Figure 2). Furthermore, slow sigma power was greater at lateral fronto-central derivations and fast sigma power was greater at central-parietal derivations in the late relative to the early pubertal development group. In each of these cases, large effect sizes were detected (all Cohen’s d >1.2). Greater lateral fronto-central theta and alpha power were detected in late relative to early pubertal development groups, while beta and gamma power were greater over central-parietal derivations and lower over frontal derivations (Figure 2). Similar findings were detected when relating relative spectral power to age rather than pubertal development stage (Supplementary Figure S1). Importantly, the distribution of sex did not differ significantly between pubertal development groups (p > .05), minimizing the possibility that results were biased by sex differences. These findings indicate that after accounting for the absolute differences in total power across frequencies, relative increases in power were observed in adolescents at later stages of pubertal development, with SWA being more frontal, theta and slow spindle activity being more lateral frontal, and fast spindle activity being more parietal relative to adolescents in early stages of pubertal development.
Figure 1.
Pubertal development stage impacts the topographic expression of absolute spectral power during NREM sleep. Topography of absolute power during NREM sleep at each EEG derivation (128 channels) are presented for eight frequency bands for 19 adolescents in early (top row) and late (middle row) pubertal development stages. Topographic plots exhibiting Cohen’s d effect sizes of the difference between early and late pubertal development stages at each EEG derivation are presented for each frequency band (bottom row). In each topographic plot, color scales reflect absolute spectral power values (µV2/Hz; top and middle row) or Cohen’s d effect sizes (bottom row), with warmer colors reflecting higher positive values and cooler colors reflecting more negative values. White dots (TFCE corrected; p < .05) and black dots (uncorrected; p < .05) indicate electrode derivations exhibiting significant pubertal development group differences (early vs. late).
Figure 2.
Pubertal development stage impacts the topographic expression of relative spectral power during NREM sleep. Topography of relative power during NREM sleep at each EEG derivation are presented for eight frequency bands for 19 adolescents in early (top row) and late (middle row) pubertal development stages. Topographic plots exhibiting Cohen’s d effect sizes of the difference between early and late pubertal development stages at each EEG derivation are presented for each frequency band (bottom row). In each topographic plot, color scales reflect Z-scores (top and middle row) or Cohen’s d effect sizes (bottom row), with warmer colors reflecting higher positive values and cooler colors reflecting more negative values. White dots (TFCE corrected; p < .05) and black dots (uncorrected; p < .05) indicate electrode derivations exhibiting significant pubertal development group differences (early vs. late)
Relationships between habitual sleep and AF
When adjusting for sex, pubertal development stage, and physical activity, negative relationships were observed between peak VO2 with subjective sleep diary and objective habitual bedtime and wake-up time (Table 1), indicating those who were more fit had an earlier sleep schedule, both by going to bed and waking up earlier. We observed no significant relationships between peak VO2 with habitual sleep summaries, including TST and SE. Three children reported engagement in organized competitive sports which required to have early morning awakenings for practice during the assessment week. Removing these individuals from the analyses did not change the interpretation of the results.
Relationships between AF and NREM sleep oscillations.
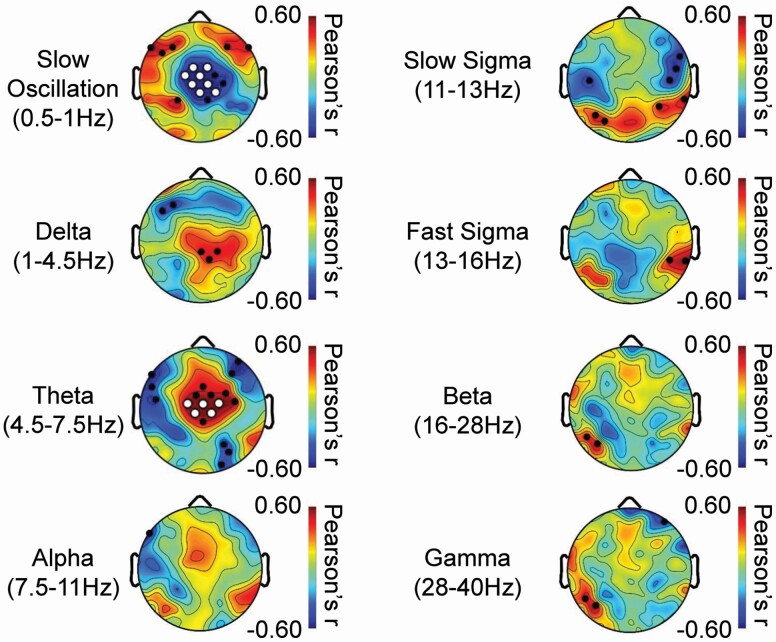
SWA in the slow oscillation frequency range was greater over anterior frontal EEG derivations and lower over central EEG derivations in individuals with higher peak VO2, the latter of which remained significant following TFCE correction (Figure 3). In contrast, SWA in the delta frequency range and NREM theta activity was higher over central EEG derivations in individuals with higher peak VO2, the latter remained significant following TFCE correction. Peak VO2 was also negatively associated with temporo-frontal slow sigma activity and positively associated with temporo-parietal sigma activity (Figure 3).
Figure 3.
Aerobic fitness impacts the topographic expression of relative spectral power during NREM sleep. Topography of Pearson’s correlation coefficients relating peak VO2 with relative power during NREM sleep at each EEG derivation in 19 adolescents are presented for eight frequency bands. In each topographic plot, color scales reflect Pearson’s correlation coefficients, with warmer colors reflecting more positive r values and cooler colors reflecting more negative r values. In each topographic plot, white dots (TFCE corrected; p < .05) black dots (uncorrected; p < .05) indicate electrode derivations exhibiting significant associations.
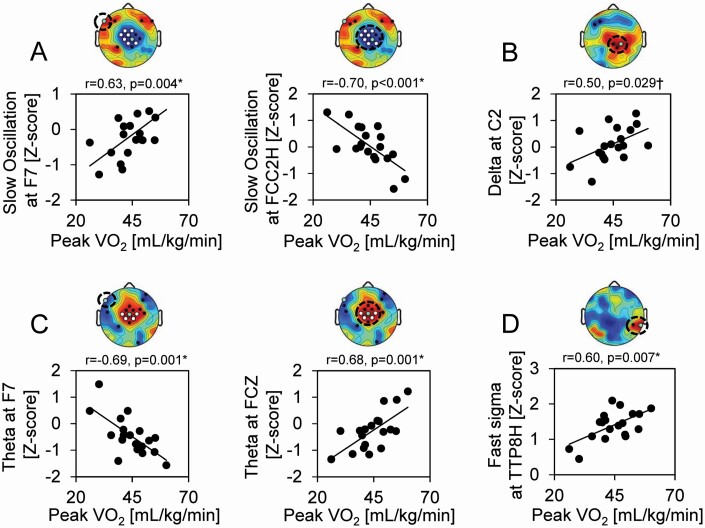
After controlling for sex and pubertal development stage, peak VO2 remained a significant predictor of SWA in the slow oscillation frequency range over anterior frontal (partial r = 0.567, SE = 0.019, p = .018, FDR corrected p = .024) and central (partial r = −0.765, SE = 0.018, p < .001, FDR corrected p < .002) EEG derivations (Figure 4a), and trended to predict SWA in the delta frequency range over central EEG derivations (partial r = 0.434, SE = 0.019, p = .082 FDR-corrected p = .082; Figure 4b). Peak VO2 also remained a significant predictor of left anterior frontal theta (partial r = −0.715, SE = 0.016, p = .001, FDR corrected p = .003) and central theta activity (partial r = 0.725, SE = 0.018, p = .001, FDR corrected p = .003) after controlling for covariates (Figure 4c). A significant association was also detected between peak VO2 and fast sigma activity over temporo-parietal EEG derivations (partial r = 0.646, SE = 0.012, p = .005, FDR corrected p = .009) after controlling for sex and pubertal development stage (Figure 4d). Similar findings were also reported when adjusting for sex and age (Figure 4, legend).
Figure 4.
Scatterplots of peak associations between aerobic fitness and relative spectral power during NREM sleep. (a) Scatter plots depicting peak positive (left) and negative (right) associations between peak VO2 and relative power during NREM sleep in the slow oscillation (0.5–1 Hz) frequency range. (b) Scatter plot depicting the peak positive association between peak VO2 and relative power during NREM sleep in the delta (1–4.5 Hz) frequency range. (c) Scatter plots depicting peak positive (left) and negative (right) associations between peak VO2 and relative power during NREM sleep in the theta (5.5–7.5 Hz) frequency range. (d) Scatter plot depicting the peak positive association between peak VO2 and relative power during NREM sleep in the fast sigma (13–16 Hz) frequency range. Topographic distribution of Pearson’s r values for correlations between relative power in (a) slow oscillation, (b) delta, (c) theta, and (d) fast sigma frequency bands and peak VO2 are presented above each respective scatter plot, with white dots (TFCE corrected; p < .05) black dots (uncorrected; p < .05) indicating electrode derivations exhibiting significant associations and green dots depicting the EEG derivation plotted in the scatterplot below. *denotes associations that remained significant (p < .05) and †denotes associations that trended towards significance (p < .10) in multiple regression models adjusting for sex and pubertal developmental stage. All these associations remained significant in multiple regression models adjusting for sex and age, including positive (a; partial r = 0.555, p = .021, FDR corrected p = .024) and negative (a; partial r = −0.775, p < .001, FDR corrected p < .002) associations between relative slow oscillation power and peak VO2, positive associations between relative delta power (b; partial r = 0.552, p = .022, FDR corrected p = .024) and peak VO2, positive (c; partial r = 0.706, p = .002, FDR corrected p = .005) and negative (c; partial r = −0.679, p = .003, FDR corrected p = .006) associations between relative theta power and peak VO2, and positive associations between fast sigma power (d; partial r = 0.625, p = .007, FDR corrected p = .011) and peak VO2.
Discussion
This study found significant relationships between AF and comprehensive characteristics of sleep across adolescence. In this study, we analyzed AF (i.e. peak VO2, a gold standard test for AF assessment), which has been shown to be especially important for overall health and neurocognitive performance [52–54]. AF was associated with the timing of habitual sleep patterns and with the expression of NREM sleep oscillations. These preliminary data suggest that higher AF may be associated with a smaller behavioral sleep delay during adolescence, and that AF is associated with frequency-specific and topographically-specific expression of NREM sleep oscillations, including those known to be influenced by development and are linked to neural plasticity in adolescence [6, 18–22].
Habitual sleep and AF
Aerobically fit adolescents, regardless of sex, pubertal development stage, and physical activity levels had earlier bedtimes and waketimes. This relationship between AF and habitual sleep-wake patterns (i.e. sleep times) was distinct from the effects of pubertal development on sleep timing, which prior studies found resulted in later bedtimes [55, 56]. Importantly, 80% of our sample reported participation in organized competitive sports with 15% requiring a regular and earlier wakeup time. Removing the three study participants from the analyses did not significantly change the results and thus early awakenings for sports practice were unlikely to have acted to reduce the magnitude of the typically observed natural circadian phase delay in sleep-wake patterns in developing adolescents. Of note, despite our original hypothesis, no relationships were observed between AF with habitual TST and SE in any method used for assessment, including sleep history, sleep diary, actigraphy, and PSG. In addition, we did not observe significant weekday/weekend sleep differences, possibly as this study was conducted during summer when adolescents were not restricted to school schedule, allowing for an evaluation of sleep patterns that may be more aligned to participants’ endogenous preference and reduces the possible confounds of social jetlag caused by school schedules.
Sleep neurophysiology and AF
We found significant correlations between AF and sleep EEG topography, after adjusting for sex and pubertal development stage. Greater AF (higher peak VO2) was associated with greater anterior prefrontal SWA in the slow oscillation frequency range (0.5–1 Hz), and lower slow oscillation power at central EEG derivations. The negative associations of slow oscillations with AF over central EEG derivations were particularly robust, remaining significant following correction for multiple comparisons across the 128 channel EEG array. The opposite pattern was observed for SWA in the delta (1–4.5 Hz) frequency range and theta activity (4.5–7.5 Hz), with positive associations with theta remaining significant following whole head correction for multiple comparisons. The differential effect of AF on slow-wave expression across frequencies is consistent with prior literature indicating that slow oscillations (i.e. <1 Hz slow waves) and delta waves (1–4.5 Hz slow waves) have distinct neurobiological origins [57] and topographic expression profiles [58, 59], are affected differentially by the buildup and dissipation of homeostatic sleep pressure [59, 60], and have distinct effects on cognition [61]. In terms of topography, in both adolescents and adults [58, 59], slow oscillations show a more anterior frontal expression peak and delta waves have a peak concentrated over fronto-central EEG derivations. In the current study, AF appeared to accentuate these differential topographic patterns of slow oscillation and delta activity, which may have biological and cognitive implications that should be examined in future studies. Posterior tempo-parietal fast sigma activity was also greater in adolescents who exhibited greater AF. Notably, slow oscillations and parietal fast sleep spindles have been associated with sleep-dependent memory processing and cognitive ability (e.g. executive functioning, attention) in adolescents and adults [21, 22, 62–67]. Directly manipulating the expression of these oscillations through stimulation enhances cognitive function such as memory retention [68–70]. The observed positive association between peak VO2 and prefrontal SWA in the slow oscillation frequency range and parietal fast sigma activity support the hypothesis that AF is associated with topographically-specific elements of sleep physiology known to mechanistically support neuroplasticity and cognitive processes (e.g. sleep-dependent memory, executive functioning, and attention) which are dependent on prefrontal cortex and hippocampal function in adolescents and adults.
Sleep neurophysiology and pubertal development status
Prior studies indicate that the topographical expression of sleep oscillations during NREM sleep transforms across advancing age in adolescents [6, 18–21]. For example, the peak expression of SWA migrates from derivations at occipital cortex to those at prefrontal cortex from early childhood to late adolescence [6, 8, 18]. Similar age-related changes are seen in intra-hemisphere coherence during sleep spindles and traveling distance of slow waves [21, 71]. Development across childhood and adolescence is accompanied by overall reductions in absolute power across frequencies, including in SWA and theta activity [6, 18], both of which may track distinct aspects of cortical maturation across adolescent development [8]. Supporting this possibility, these developmental changes in SWA are associated with grey matter volumetric changes and white matter myelination across development [71, 72]. With respect to the effects of pubertal development stage, our findings are consistent with the literature examining the effects of age, demonstrating developmental-related changes in the topographic expression of sleep oscillations across distinct frequency bands. For example, SWA exhibited a more prefrontal-dominant pattern in later relative to earlier pubertal development stages, while theta and slow sigma activity became more lateral prefrontal. In contrast, faster frequency wave forms, including fast sigma, beta, and gamma power peaked over central and parietal EEG derivations in late relative to early pubertal development stages. These effects were consistent with our analyses evaluating age, and reflect findings reported in the literature [6, 22]. If these developmental changes in SWA and theta activity are in fact directly tied to cortical brain maturation, the association of AF with the expression of these sleep oscillations could have important implications for the role of AF in optimal brain development during adolescence.
Strength and limitations
Results of the present study should be interpreted in light of several methodological limitations. Due to the small sample size, error protections were not employed and thus findings require replication. Nevertheless, for many of the reported findings, effect sizes are large and robust against adjustment for confounding factors. This study was not powered to examine possible interaction effect (e.g. sex and pubertal development stage). We used a self-reported questionnaire to assess habitual physical activity (over the last week). In this sample, we did not observe a significant relationship between self-reported amount of engagement in moderate to vigorous physical activity and peak VO2 when controlling for sex and developmental maturation, and self-reported habitual physical activity levels were not associated with sleep timing in this sample. Future studies utilizing validated objective and gold standard approaches for habitual physical activity are needed to better clarify the possible impact of habitual physical activity on the relationship between sleep and AF. Additionally, ambulatory assessment data suggested that the sample was likely sleep-deprived. Indeed, short SOL (ranged from 0.1 to 11 minutes) was observed during in-laboratory PSG in all participants. Sleep restriction is common in adolescents, and as seen in our findings, less than 16.8% exhibited more than 8 hours of sleep. While sleep patterns in the sleep laboratory may not be representative of sleep at home (and may reflect recovery sleep as they were allowed to sleep ad lib and bedtimes were 1 hour delayed due to experimental design), the use of hdEEG allows focusing on sleep oscillations and their topography which are stable within subjects and largely independent of TST [73, 74]. Of note, TST and SOL were not associated with AF in either sleep diary, actigraphy, or PSG assessment. Therefore, these discrepancies are unlikely to impact the relationships that these independent measures—actigraphy and sleep EEG topography—have with AF. Additionally, in our data, none of the significant effects between local sleep and developmental stage or local sleep and AF were significantly associated with TST or SOL from the overnight study, suggesting that neither the habitual sleep restriction nor the protocol (that shifted bedtimes by 1 hour) likely explain the observed effects in this sample. Variability between weekday and weekend sleep are common in this age group [55, 56], and as this study was completed during summer vacation aiming to maximize free-living conditions, this could potentially impact the generalizability of these data. Large cross-sectional studies are necessary to evaluate the relationship between AF and habitual sleep while considering the possible impact of seasons, school schedule, habitual physical activity, exercise, mental health, and circadian rhythms. Despite these limitations, the present study provides the first characterization of phenotypic sleep using habitual sleep and sleep neurophysiology measures along with gold-standard in-laboratory assessment of AF in healthy adolescents.
Conclusion
In this study of a group of 11–17 year-olds, AF was associated with an earlier habitual sleep pattern (i.e. going to sleep early and waking up early) and with topographically specific expression of sleep EEG in adolescents. AF was further associated with a more mature pattern of expression of NREM sleep oscillations that are involved in cognitive function in both children and adults. These findings, while preliminary, highlight the relevance of AF as a factor that may support biopsychosocial health and cognitive function through impacting sleep processes.
Supplementary Material
Acknowledgments
The authors would like to thank all the participants evaluated in this study. We would also like to thank all Registered Polysomnographic Technologists at the UCI Sleep Center and the PERC staff for their invaluable assistance with data collection and scoring of sleep stages.
Funding
The work presented in this article was supported by National Center for Advancing Translational Sciences (NCATS) grant #UL1TR001414 and Pediatric Exercise and Genomics Research Center (PERC) Systems Biology Fund.
Disclosure Statement
Dr. Benca has served as a consultant to Eisai, Genomind, Idorsia, Jazz, Merck, Sage, and Sunovion. There are no non-financial conflicts of interest to disclose for any of the authors.
References
- 1. Ross R, et al. ; American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; Council on Functional Genomics and Translational Biology; Stroke Council. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134(24):e653–e699. [DOI] [PubMed] [Google Scholar]
- 2. Mintjens S, et al. Cardiorespiratory fitness in childhood and adolescence affects future cardiovascular risk factors: a systematic review of longitudinal studies. Sports Med. 2018;48(11):2577–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Etnier JL, et al. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev. 2006;52(1):119–130. [DOI] [PubMed] [Google Scholar]
- 4. Reigal RE, et al. Physical exercise, fitness, cognitive functioning, and psychosocial variables in an adolescent sample. Int J Environ Res Public Health.. 2020;17(3):1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khan NA, et al. The relation of childhood physical activity and aerobic fitness to brain function and cognition: a review. Pediatr Exerc Sci. 2014;26(2):138–146. [DOI] [PubMed] [Google Scholar]
- 6. Kurth S, et al. Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalogram study. J Neurosci. 2010;30(40):13211–13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchmann A, et al. EEG sleep slow-wave activity as a mirror of cortical maturation. Cereb Cortex. 2011;21(3):607–615. [DOI] [PubMed] [Google Scholar]
- 8. Campbell IG, et al. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci U S A. 2009;106(13):5177–5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang T, et al. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: a review. Scand J Med Sci Sports. 2014;24(1):1–10. [DOI] [PubMed] [Google Scholar]
- 10. Vaynman S, et al. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19(4):283–295. [DOI] [PubMed] [Google Scholar]
- 11. Ruotsalainen I, et al. Aerobic fitness, but not physical activity, is associated with grey matter volume in adolescents. Behav Brain Res. 2019;362:122–130. [DOI] [PubMed] [Google Scholar]
- 12. Swain RA, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117(4):1037–1046. [DOI] [PubMed] [Google Scholar]
- 13. Matricciani L, et al. Children’s sleep and health: a meta-review. Sleep Med Rev. 2019;46:136–150. [DOI] [PubMed] [Google Scholar]
- 14. Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Armstrong N, et al. Aerobic fitness and trainability in healthy youth: gaps in our knowledge. Pediatr Exerc Sci. 2016;28(2):171–177. [DOI] [PubMed] [Google Scholar]
- 16. Armstrong N, et al. Peak oxygen uptake in relation to growth and maturation in 11- to 17-year-old humans. Eur J Appl Physiol. 2001;85(6):546–551. [DOI] [PubMed] [Google Scholar]
- 17. Carskadon MA. Sleep in adolescents: the perfect storm. Pediatr Clin North Am. 2011;58(3):637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feinberg I, et al. Gamma distribution model describes maturational curves for delta wave amplitude, cortical metabolic rate and synaptic density. J Theor Biol. 1990;142(2):149–161. [DOI] [PubMed] [Google Scholar]
- 19. Gorgoni M, et al. Sleep electroencephalography and brain maturation: developmental trajectories and the relation with cognitive functioning. Sleep Med. 2020;66:33–50. [DOI] [PubMed] [Google Scholar]
- 20. Goldstone A, et al. Sleep spindle characteristics in adolescents. Clin Neurophysiol. 2019;130(6):893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tarokh L, et al. Early adolescent cognitive gains are marked by increased sleep EEG coherence. PLoS One. 2014;9(9):e106847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hahn M, et al. Developmental changes of sleep spindles and their impact on sleep-dependent memory consolidation and general cognitive abilities: a longitudinal approach. Dev Sci. 2019;22(1):e12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Gennaro L, et al. Sleep spindles: an overview. Sleep Med Rev. 2003;7(5):423–440. [DOI] [PubMed] [Google Scholar]
- 24. Mota J, et al. Associations between sleep quality with cardiorespiratory fitness and BMI among adolescent girls. Am J Hum Biol. 2010;22(4):473–475. [DOI] [PubMed] [Google Scholar]
- 25. Lee AJ, et al. Association between sleep quality and physical fitness in female young adults. J Sports Med Phys Fitness. 2007;47(4):462–467. [PubMed] [Google Scholar]
- 26. Petersen AC, et al. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 1988;17(2):117–133. [DOI] [PubMed] [Google Scholar]
- 27. Vuillemin A, et al. Self-administered questionnaire compared with interview to assess past-year physical activity. Med Sci Sports Exerc. 2000;32(6):1119–1124. [DOI] [PubMed] [Google Scholar]
- 28. Carney CE, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ancoli-Israel S, et al. The SBSM guide to actigraphy monitoring: clinical and research applications. Behav Sleep Med. 2015;13 Suppl 1:S4–S38. [DOI] [PubMed] [Google Scholar]
- 30. Oakley NR. Validation with polysomnography of the sleep-watch sleep/wake scoring algorithm used by the Actiwatch activity monitoring system. Technical report to Mini Mitter Co., Inc; 1997. [Google Scholar]
- 31. Hyde M, et al. Validation of actigraphy for determining sleep and wake in children with sleep disordered breathing. J Sleep Res. 2007;16(2):213–216. [DOI] [PubMed] [Google Scholar]
- 32. Kaufman J, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. [DOI] [PubMed] [Google Scholar]
- 33. Jones SG, et al. Regional reductions in sleep electroencephalography power in obstructive sleep apnea: a high-density EEG study. Sleep. 2014;37(2):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berry RB, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.5 ed. Darien, IL: American Academy of Sleep Medicine; 2018. [Google Scholar]
- 35. Plante DT, et al. Altered slow wave activity in major depressive disorder with hypersomnia: a high density EEG pilot study. Psychiatry Res. 2012;201(3):240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Riedner BA, et al. Regional patterns of elevated alpha and high-frequency electroencephalographic activity during nonrapid eye movement sleep in chronic insomnia: a pilot study. Sleep. 2016;39(4):801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Riedner BA, et al. Sleep homeostasis and cortical synchronization: III. A high-density EEG study of sleep slow waves in humans. Sleep. 2007;30(12):1643–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Perrin F, et al. Spherical splines for scalp potential and current density mapping. Electroencephalogr Clin Neurophysiol. 1989;72(2):184–187. [DOI] [PubMed] [Google Scholar]
- 39. Ferree TC. Spline interpolation of the scalp EEG. Technical note, Electrical Geodesics. 2000. www.egi.com/Technotes/SplineInterpolation.pdf. Accessed March 26, 2006.
- 40. Babadi B, et al. A review of multitaper spectral analysis. IEEE Trans Biomed Eng. 2014;61(5):1555–1564. [DOI] [PubMed] [Google Scholar]
- 41. Helfrich RF, et al. Old brains come uncoupled in sleep: slow wave-spindle synchrony, brain atrophy, and forgetting. Neuron. 2018;97(1):221–230.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Prerau MJ, et al. Sleep neurophysiological dynamics through the lens of multitaper spectral analysis. Physiology (Bethesda). 2017;32(1):60–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Whitford TJ, et al. Brain maturation in adolescence: concurrent changes in neuroanatomy and neurophysiology. Hum Brain Mapp. 2007;28(3):228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tarokh L, et al. Developmental changes in the human sleep EEG during early adolescence. Sleep. 2010;33(6): 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Koopman-Verhoeff ME, et al. Classifying pubertal development using child and parent report: comparing the pubertal development scales to tanner staging. J Adolesc Health. 2020;66(5):597–602. [DOI] [PubMed] [Google Scholar]
- 46. Plante DT, et al. Establishing the objective sleep phenotype in hypersomnolence disorder with and without comorbid major depression. Sleep. 2019;42(6). doi: 10.1093/sleep/zsz060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fattinger S, et al. Theta waves in children’s waking electroencephalogram resemble local aspects of sleep during wakefulness. Sci Rep. 2017;7(1):11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bongers BC, et al. Pediatric Norms for Cardiopulmonary Exercise Testing: In Relation to Sex and Age. ‘s-Hertogenbosch, the Netherlands: Uitgeverij BOXPress BV; 2014. [Google Scholar]
- 49. Centers for Disease Control and Prevention. BMI Percentile Calculator for Child and Teen. https://www.cdc.gov/healthyweight/bmi/calculator.html. Accessed May 17, 2020.
- 50. Banks S, et al. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3(5):519–528. [PMC free article] [PubMed] [Google Scholar]
- 51. Ong JL, et al. EEG changes accompanying successive cycles of sleep restriction with and without naps in adolescents. Sleep. 2017;40(4). doi: 10.1093/sleep/zsx030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Armstrong N, et al. Assessment and interpretation of aerobic fitness in children and adolescents. Exerc Sport Sci Rev. 1994;22:435–476. [PubMed] [Google Scholar]
- 53. Herting MM, et al. Exercise, cognition, and the adolescent brain. Birth Defects Res. 2017;109(20):1672–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Herting MM, et al. Aerobic fitness relates to learning on a virtual Morris Water Task and hippocampal volume in adolescents. Behav Brain Res. 2012;233(2):517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Crowley SJ, et al. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8(6):602–612. [DOI] [PubMed] [Google Scholar]
- 56. Crowley SJ, et al. An update on adolescent sleep: new evidence informing the perfect storm model. J Adolesc. 2018;67:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Steriade M, et al. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13(8):3252–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Markovic A, et al. Heritability of sleep EEG topography in adolescence: results from a longitudinal twin study. Sci Rep. 2018;8(1):7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bersagliere A, et al. Mapping slow waves by EEG topography and source localization: effects of sleep deprivation. Brain Topogr. 2018;31(2):257–269. [DOI] [PubMed] [Google Scholar]
- 60. Werth E, et al. Fronto-occipital EEG power gradients in human sleep. J Sleep Res. 1997;6(2):102–112. [DOI] [PubMed] [Google Scholar]
- 61. Kim J, et al. Competing roles of slow oscillations and delta waves in memory consolidation versus forgetting. Cell. 2019;179(2):514–526.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chatburn A, et al. Sleep spindle activity and cognitive performance in healthy children. Sleep. 2013;36(2):237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Munz MT, et al. Slow oscillating transcranial direct current stimulation during non-rapid eye movement sleep improves behavioral inhibition in attention-deficit/hyperactivity disorder. Front Cell Neurosci. 2015;9:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mander BA, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16(3):357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mander BA, et al. White matter structure in older adults moderates the benefit of sleep spindles on motor memory consolidation. J Neurosci. 2017;37(48):11675–11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mander BA, et al. Wake deterioration and sleep restoration of human learning. Curr Biol. 2011;21(5):R183–R184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Saletin JM, et al. The role of sleep in directed forgetting and remembering of human memories. Cereb Cortex. 2011;21(11):2534–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Marshall L, et al. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–613. [DOI] [PubMed] [Google Scholar]
- 69. Lustenberger C, et al. Feedback-controlled transcranial alternating current stimulation reveals a functional role of sleep spindles in motor memory consolidation. Curr Biol. 2016;26(16):2127–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Prehn-Kristensen A, et al. Transcranial oscillatory direct current stimulation during sleep improves declarative memory consolidation in children with attention-deficit/hyperactivity disorder to a level comparable to healthy controls. Brain Stimul. 2014;7(6):793–799. [DOI] [PubMed] [Google Scholar]
- 71. Kurth S, et al. Traveling slow oscillations during sleep: a marker of brain connectivity in childhood. Sleep. 2017;40(9). doi: 10.1093/sleep/zsx121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Goldstone A, et al. The mediating role of cortical thickness and gray matter volume on sleep slow-wave activity during adolescence. Brain Struct Funct. 2018;223(2):669–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ong JL, et al. Trait-like characteristics of sleep EEG power spectra in adolescents across sleep opportunity manipulations. J Sleep Res. 2019;28(5):e12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. De Gennaro L, et al. An electroencephalographic fingerprint of human sleep. Neuroimage. 2005;26(1):114–122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.