Abstract
Ha and colleagues describe a previously unappreciated diversity of microbes in the mesenteric adipose tissue (MAT) surrounding the GI tract. Viable bacteria that are mislocalized from the gut microbiota and metabolically adapted to the MAT contribute to the “creeping fat” of Crohn’s disease.
Insects play countless important roles in our lives, pollinating fruit blossoms, degrading dead trees, and serving as predators and prey in the greater ecosystem. However, the frenzy induced by a bee flying through the living room or a termite in the baseboards highlights that invasion of these beneficial creatures into our homes can instantly change them into pests. In this report, (Ha et al., 2020) describe a previously unappreciated microbial diversity present in mesenteric adipose tissue (MAT) surrounding the GI tract, identifying viable bacteria in both healthy and inflamed tissues. The mesentery is a critical, yet understudied, structure attached to the intestine with a stromal, immune and adipose network that serves as a conduit for the intestine’s blood supply, lymphatic drainage, and nervous system input (Coffey and O’Leary, 2016). In inflammatory bowel disease (IBD), microbe-host interactions are altered, with altered intestinal microbial communities and barrier defects leading to translocation of microbes outside of the intestinal (Kruis et al., 2014; Laffineur et al., 1992; Peyrin-Biroulet et al., 2012; Sartor and Wu, 2017). Both forms of IBD, ulcerative colitis (UC) and Crohn’s disease (CD) differ in their inflammatory patterns, while UC is focused in the mucosa of the large intestine and continuous, CD involves the full thickness of the intestine and often the mesentery and is spatially intermittent. Long appreciated, but of unclear significance, fibrotic mesenteric fat hypertrophy around the circumference of the intestine (“Creeping Fat”), is a well-known feature of Crohn’s Disease, particularly in stricturing disease of the ileum, that was described in 1932 by Dr. Burrill Crohn during his initial landmark report describing Crohn’s Disease(Crohn et al., 1984).
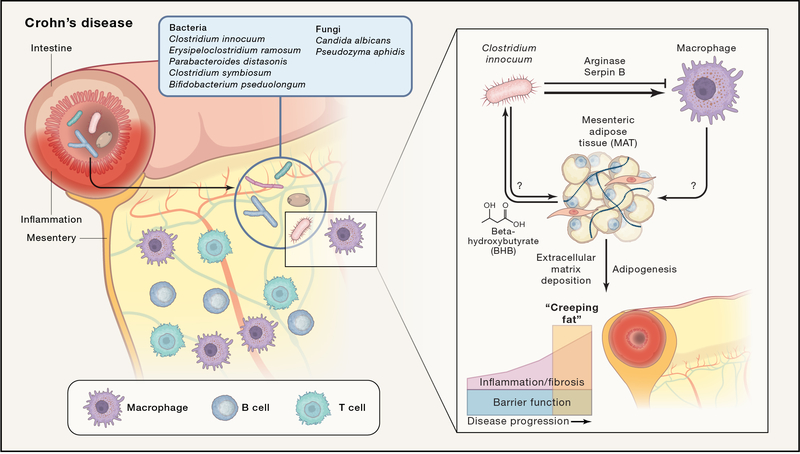
(Ha et al., 2020) test the hypothesis that Creeping Fat (CrF) formation is a response to local microbial translocation into the mesentery. They use CrF and MAT samples from patients with Crohn’s disease compared to MAT from Ulcerative Colitis or healthy controls to examine host gene expression as well as the presence of microbes via genetic and culture-based techniques. Sequencing-based analysis identified diverse bacteria and fungi in nearly all samples; the microbial signatures of diversity and composition generally matched gut luminal and mucosal microbial communities, suggesting MAT enmeshes microbes that migrate across intestine in health and disease. Culture based methods recovered 229 isolates from CD and UC MAT, falling into 84 different species including the unique presence of five species in Crohn’s MAT(Fig. 1).
Figure 1.
Microbial translocation into mesenteric adipose tissue contributes to Crohn’s disease “Creeping Fat”.
(Left side) This study finds that the mesenteric adipose tissue (MAT) in Crohn’s disease (CD) is enriched for an immune infiltrate that includes: T cells, B cells and macrophages with a mixed tissue remodeling type 2 and an inflammatory type 1 program. They find the unique presence of five bacterial and two fungal species in CD MAT listed above.
(Right side) Isolates of C. innocuum from CD MAT as compared to CD mucosa are enriched for metabolism of beta-hydroxybutyrate, a substrate from lipolysis abundant in fat, as well as expression of serpin B and arginase, enzymes that protect against neutrophil elastase and inhibit macrophage nitric oxide production, respectively. In vitro experiments revealed no significant response from primary MAT stromal and stem cells when Crohn’s MAT-specific bacteria are added, but do demonstrate expression of collagen genes when they are exposed to macrophage conditioned media, suggesting that translocated bacteria lead to tissue remodeling and fibrosis seen in Creeping Fat via macrophage activation. The direct response of adipocytes to bacterial stimuli remains untested and will be of interest going forward. Given the large T and B cell component present in CrF and inflamed MAT, it will be important to elucidate the role of adaptive immunity in this complex microbial-host dialogue.
(Ha et al., 2020) narrowed their focus onto Clostridium innocuum, given its abundance in and exclusivity to CD MAT. Genome sequencing of 30 isolates of C. innocuum collected from mucosa or MAT and two reference strains revealed genes mediating protection from oxidative damage, immune evasion, lipid utilization, and type IV pili, which led the authors to test and confirm a previously unappreciated ability for twitching motility. Isolates of C. innocuum from CD MAT as compared to CD mucosa are enriched for metabolic adaptations and immune evasion functions to facilitate survival in the adipose tissue microenvironment (Fig. 1). These data highlight microbial adaptation at the isolate level to colonize CD MAT and CrF by C. innocuum.
When (Ha et al., 2020) turned their attention to analysis of CrF as compared to other forms of MAT, they found single cell transcriptional signatures of inflammation and fibrosis. Both T cells and B cells are expanded as well as macrophages highly expressing TLR2 and NLRP3. Pathways for extracellular matrix production are enriched in progenitor cells of the CrF. Via in vitro experimental follow-up with patient derived primary cells, the authors propose a model in which translocated bacteria lead to tissue remodeling and fibrosis via macrophage activation (Fig. 1).
(Ha et al., 2020) employed gnotobiotic mice colonized with 8 anaerobic bacteria with or without C. innocuum to experimentally test causation. C. innocuum was recovered from MAT in healthy mice and those with intestinal inflammation incited by dextran sodium sulfate. Adipogenesis and extracellular matrix genes were upregulated within the MAT in both healthy and inflamed settings when C. innocuum was present compared to the 8 bacteria alone, consistent with this strain promoting adipogenesis and remodeling within the MAT. Intriguingly, additional species are found to translocate with C. innocuum, suggesting C. innocuum is facilitating the spread of other species as well. Whether C. innocuum can be leveraged as a diagnostic tool to predict outcomes in CD or if it can be targeted as a therapeutic warrants further study.
Increased study of adipose tissue, partly driven by the global scourge of obesity, is revealing tremendous functional diversity, particularly as a key driver of inflammation(Chait and den Hartigh, 2020; Hotamisligil, 2017). (Ha et al., 2020) add to this emerging recognition that the adipose tissue is not a passive depot for storing energy, but rather integrates microbial and nutritional signals with both inflammatory and metabolic outputs. MAT and CrF may serve to contain gut bacteria that have mislocalized--markers of systemic bacterial exposure in serum in CD are similar to healthy controls, but raised in UC, indicating MAT and CrF may play a protective role in CD. Indeed, the fat body in Drosophila serves critical roles in both immune defense and metabolism and is proposed to be the anatomic and functional equivalent of the vertebrate mesentery and liver(Hotamisligil, 2017). Thus, MAT may function as a potent nutritional and immunologic rheostat and further study is likely to further elucidate our microbiota’s contribution to inflammatory and metabolic disease.
A broad body of literature supports impaired barrier function as a result of the microbiota’s response to the industrialized diet—the equivalent of tears in our screen doors and cracks in our foundations that allow insects to infiltrate our homes. The loss of dietary fiber, high fat content, and chemical additives, such as emulsifiers, in mouse models lead to loss of gut barrier integrity, increased mucosal and systemic inflammation, and metabolic dysfunction (Dabke et al., 2019). A provocative extension of this work suggests intestinal barrier dysfunction specific to industrialized nations contributes to the rise in inflammatory and metabolic disease via increased disseminated microbial signals that drive adipose tissue inflammation. For instance, independent of obesity, increased mesenteric adiposity is a risk factor for insulin resistance, cardiovascular disease, and metabolic dysfunction associated fatty liver (MAFLD)(Chait and den Hartigh, 2020). In metabolic dysfunction, is the microbial composition of MAT altered leading to inflammation-induced increases in visceral adiposity that augment systemic inflammation? The lifestyle, microbial, and host factors that drive this spectrum of diseases are complex, but in shedding light on this long-standing mystery of CrF in Crohn’s disease the authors take a significant step in advancing our knowledge about how adipose tissue responds to and possibly protects us during commensal invasion.
Acknowledgments:
S.P.S. is supported by NIH Training Grant T32DK007056. J.L.S. is a Chan Zuckerberg Biohub Investigator and acknowledges support from the National Institutes of Health R01-DK085025 and DP1-AT00989203. The authors declare no competing interests.
References
- Chait A, and den Hartigh LJ (2020). Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front Cardiovasc Med 7, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey JC, and O’Leary DP (2016). The mesentery: structure, function, and role in disease. Lancet Gastroenterol Hepatol 1, 238–247. [DOI] [PubMed] [Google Scholar]
- Crohn BB, Ginzburg L, and Oppenheimer GD (1984). Landmark article Oct 15, 1932. Regional ileitis. A pathological and clinical entity. By Crohn Burril B., Ginzburg Leon, and Oppenheimer Gordon D.. JAMA 251, 73–79. [DOI] [PubMed] [Google Scholar]
- Dabke K, Hendrick G, and Devkota S (2019). The gut microbiome and metabolic syndrome. J Clin Invest 129, 4050–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha CWY, Martin A, Sepich-Poore GD, Shi B, Wang Y, Gouin K, Humphrey G, Sanders K, Ratnayake Y, Chan KSL, et al. (2020). Translocation of Viable Gut Microbiota to Mesenteric Adipose Drives Formation of Creeping Fat in Humans. Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS (2017). Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185. [DOI] [PubMed] [Google Scholar]
- Kruis T, Batra A, and Siegmund B (2014). Bacterial translocation - impact on the adipocyte compartment. Front Immunol 4, 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffineur G, Lescut D, Vincent P, Quandalle P, Wurtz A, and Colombel JF (1992). [Bacterial translocation in Crohn disease]. Gastroenterol Clin Biol 16, 777–781. [PubMed] [Google Scholar]
- Peyrin-Biroulet L, Gonzalez F, Dubuquoy L, Rousseaux C, Dubuquoy C, Decourcelle C, Saudemont A, Tachon M, Béclin E, Odou MF, et al. (2012). Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn’s disease. Gut 61, 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor RB, and Wu GD (2017). Roles for Intestinal Bacteria, Viruses, and Fungi in Pathogenesis of Inflammatory Bowel Diseases and Therapeutic Approaches. Gastroenterology 152, 327–339.e324. [DOI] [PMC free article] [PubMed] [Google Scholar]