Abstract
The adenosine A2A receptor is a major target of caffeine, the most widely used psychoactive substance worldwide. Large epidemiological studies have long shown caffeine consumption is a strong inverse predictor of Parkinson’s disease (PD). In this review, we first examine the epidemiology of caffeine use vis-à-vis PD and follow this by looking at the evidence for adenosine A2A receptor antagonists as potential neuroprotective agents. There is a wealth of accumulating biological, epidemiological and clinical evidence to support the further investigation of selective adenosine A2A antagonists, as well as caffeine, as promising candidate therapeutics to fill the unmet need for disease modification of PD.
Keywords: Adenosine, A2A receptors, Caffeine, Neuroprotection, Parkinson’s disease
1. Introduction
The adenosine A2A receptor is a major target of caffeine, the most widely used psychoactive substance worldwide. Large epidemiological investigations have identified caffeine consumption as a strong inverse predictor of Parkinson’s disease (PD) (and independent from the similarly inverse association of cigarette smoking) [1,2]. The risk of PD among men routinely consuming a daily intake of 2–3 cups of coffee is nearly half that of men who do not consume caffeine, whereas decaffeinated coffee affords no such reduced risk [3–6]. In this review, we first examine the epidemiology of caffeine in PD and follow this by examining evidence for caffeine and other, more selective antagonists of the adenosine A2A receptor as potential neuroprotective agents. Finally, we suggest how the clinical relevance of this evidence might be tested in future clinical trials pursuing disease-modification in PD.
1.1. Epidemiology of caffeine in PD
1.1.1. Caffeine, a major ‘reduced risk’ factor for PD
Other than cigarette smoking, dietary caffeine is the environmental exposure most robustly and reproducibly linked to a lower risk of developing PD [7]. In addition to numerous case-control studies demonstrating lower caffeine intake in association with PD [1,2], well-designed, rigorously conducted epidemiological studies of prospectively followed cohorts have convincingly shown that the risk of PD is significantly and dose-dependently lower among those who consume coffee, tea and other caffeinated beverages [3,6,8–10]. The lack of effect for decaffeinated drinks [3,4] implicates caffeine as the molecular component of coffee driving its inverse correlation with PD; however, other components remain possibilities given that caffeine is not the only molecule removed during the decaffeination process [11]. Indeed, although caffeine itself is CNS-active and is reproducibly protective in multiple PD models (vide infra) – and possibly in PD if it actually intervenes against its causes – other constituents of coffee or tea have shown neuroprotective properties in animal models of the disease [12–14]. Of note, the design of longitudinal epidemiological studies finding a reduced risk of PD among those consuming less caffeine is based on prospective assessment of caffeine intake years before PD diagnosis, and indeed these predictive associations persisted in lag analyses that exclude PD cases that occurred within 2 [4] or even 6 years [15] from dietary survey. This temporal gap between exposure ascertainment and future PD diagnoses greatly reduces – but does not eliminate – the possibility of reverse causation; i.e., the possibility that early PD features (preceding diagnosis) caused a reduction in caffeine consumption behavior.
While reduced PD risk is strongly associated with caffeine consumption among men, the relationship is more complex in women, among whom caffeine’s overall link to PD is generally smaller or absent [3]. Several studies [4,9,15,16] (but not all [6]) have found that the weaker association between PD and women may be explained by estrogen status, with an inverse relationship in women who are not receiving post-menopausal hormone replacement, whereas those taking replacement therapy have shown no consistent PD link. Preclinical experiments support the biological plausibility for an inverse association due to a protective effect of caffeine that can be modulated by estrogen replacement. Using a standard (high-dose MPTP) toxin model of PD, we found a protective effect of caffeine in male, but not female mice unless they were ovariectomized, and while ovarectomy restored the protective effect of caffeine in older female mice, that restoration was prevented by chronic estrogen replacement treatment [17]. Estrogen administered to male mice eliminated caffeine’s neuroprotective actions. Taken together, these convergent epidemiological and laboratory data, with the caveats of epidemiological data consistency and mouse model relevance notwithstanding, raise the possibility of a competitive interaction between estrogen and caffeine’s protective effects in PD.
Epidemiological studies of whether the caffeine-PD link is modified by caffeine metabolism – inferred from genetic (CYP1A2 polymorphism) determinants of slow versus fast caffeine metabolizers – have provided inconsistent evidence. While two cohort studies suggest a stronger protective association in ‘slow metabolizers’ [18,19], several others have found no difference [20–22]. Although, in humans, caffeine has a serum clearance half-life typically less than 6 h, it is demethylated by CYP1A2 to paraxanthine (the primary metabolite of caffeine) and other dimethyl-xanthines (e.g., theophylline), which, like caffeine, are also non-selective adenosine A2A antagonists with neuroprotective properties in an MPTP model of PD [23]. Thus, the lack of a clear interaction between CYP1A2 polymorphism and reduced PD risk with caffeine may be consistent with the protective potential of caffeine and its metabolites.
1.1.2. Caffeine, a less certain predictor of PD progression
While greater caffeine use in healthy populations generally predicts a reduced risk of developing PD, the relationship between caffeine consumption by those already diagnosed with PD and their subsequent disease progression is less straightforward. Evaluation of PD patients who were surveyed for daily caffeine intake and then followed prospectively in multiple observational and interventional study cohorts, showed total caffeine ingestion from coffee, tea and other dietary sources was inconsistently correlated with clinical decline. In an Italian study that enrolled 79 early, untreated, ‘de novo’ PD patients [24] higher levels of caffeine intake were associated with significantly slower rates of decline in motor and non-motor symptoms, although the latter was surveyed at the end of the 4-year follow-up period (limiting conclusions on the predictive value of caffeine usage). In contrast, a larger (n = 413) but shorter (1-year) study of de novo participants in NET-PD cohorts (FS1 and FS-TOO) [25] reported no consistent association between caffeine intake and motor progression. The latter was assessed, as in the Italian study, by the time to disability warranting initiation of dopaminergic medication, and by change in Unified Parkinson Disease Rating Scale [UPDRS] score. Similarly, in the even larger LS-1 trial cohort (n = 1549), which was followed for longer (for up to 5 years) [26], caffeine intake was not associated with slower disease progression. In fact, higher caffeine intake among participants randomized to the active study arm (oral creatine treatment) was actually associated with a faster rate of progression by UPDRS score change. However, the LS-1 trial participants were enrolled at a later stage of disease (after initiation of dopaminergic medications) and were not surveyed for their caffeine usage until 1–2 years after baseline – adding to the complexity of data interpretation.
Another intriguing findings is that caffeine use is consistently associated with reduced rates of developing levodopa-induced dyskinesias (LID), a sometimes disabling motor complication of antiparkinsonian dopaminergic drug treatment. Using data from the CALM-PD trial cohort, we reported that early PD participants who reported higher daily intake of caffeine were less likely to develop dyskinesias after nearly six years of treatment with levodopa or a dopaminergic agonist [27]. Similarly in a case-control study of 485 PD patients in Italy, ever-having-been a coffee drinker and greater number of cups ingested per day were associated with a significantly lower likelihood of manifesting dyskinesias [28]. Combined with preclinical evidence that adenosine A2A receptors play a critical role in the development of LID in murine and primate models [29,30], these findings support a hypothesis that coadministration of caffeine or a more selective A2A antagonist may mitigate against the development of LID [27,31,32].
1.1.3. Caffeine, as an emerging marker of resistance to genetic PD
The increasingly routine identification of discrete genetic subtypes of PD (most commonly attributed to mutations in the GBA and LRRK2 genes) provides an opportunity to characterize gene-environment and pharmacogenetic interactions that may influence the development and progression of PD. The relationship between PD associations with LRRK2 and caffeine may be a prototype for such gene-environment interaction based on emerging evidence that links external (dietary [33–35] and internal (plasma [36]) caffeine exposure with a reduced likelihood of developing PD among carriers of a pathogenic LRRK2 mutation. This seems to occur to an even greater extent than among PD patients who do not carry LRRK2 mutation [33,36]. Given caffeine’s association with LRRK2 PD resistance, its neuroprotective properties in PD models (vide infra), and its general safety at doses of at least 400 mg/day [37] (less than the exposure from drinking two Dunkin’ Donut Coffees or two Starbucks Café Americanos per day [38]), caffeine is a reasonable candidate for interventional investigations in PD prevention trials targeting those at high risk for the disease based on the presence of a LRRK2 mutation.
1.2. Evidence for A2A antagonists as potential neuroprotective agents
1.2.1. Neuroprotection by A2A antagonists in PD models
The first experimental evidence for a neuroprotective effect of A2A receptor antagonists including caffeine arose 20 years ago with the demonstration that genetic inactivation and pharmacological blockade of the A2A receptor attenuate dopaminergic neurotoxicity and neurodegeneration in rodent models of parkinsonism. These models were created using mitochondrial toxins (systemically-administered MPTP and brain infusion of 6-OHDA), which produce relatively selective neuronal cell death [39–41]. A2A receptor antagonist-mediated neuroprotection against dopaminergic neurodegeneration has been confirmed by follow-up studies and has been extended to other neurotoxin models of PD, such as the rotenone rat PD model [42,43]. Protection against dopaminergic neurotoxicity was achieved by selective A2A antagonists (including SCH58261, KW-6002, DMPX, CSC, MSX-3 and IDPU), but not the A1 antagonist DPCPX [39,44–46]. Systemic administration of the A2A antagonist SCH58261 for five days also improved synaptic currents, spine morphology, and dendritic excitability of the striatopallidal neurons after dopamine depletion in mice [47]. Furthermore, acute or chronic treatment with the non-selective adenosine antagonist caffeine attenuates MPTP-induced loss of dopamine content and dopaminergic terminals in the striatum [39,41] as well as loss of dopaminergic neurons in the substantia nigra [40,41,48]. Caffeine conferred protection against the degeneration of dopamine cell bodies in the substantia nigra even when it was administered after the onset of the neurodegenerative process in a chronic MPTP-exposure paradigm [49]. While reproducible in traditional toxin models of PD neurodegeneration, caffeine’s neuroprotective properties are not universal. Indeed caffeine has been shown to exacerbate methamphetamine-induced dopaminergic cell death and neuroinflammation in mouse substantia nigra [50,51]. Overall, these studies demonstrate a potential neurobiological basis for the relationship between increased caffeine consumption and reduced risk of developing PD, and support the clinical potential for A2A antagonism as a disease-modifying drug target against PD.
Another line of experimental evidence for A2A receptor mediated neuroprotection against dopaminergic neurodegeneration came from the demonstration of A2A receptor modulation of α-synuclein-mediated neurotoxicity in transgenic PD models. Alpha-synuclein aggregation and misfolding plays a central role in PD pathogenesis and the appearance of α-synuclein inclusions within in neurons is highly correlated with the onset of neuronal loss [52–54]. A2A receptor blockade decreases α-synuclein aggregation in SynT-Synphilin-1 neuroglioma cells [55], and A2A receptor knock out prevents loss of dopaminergic neurons caused by the transgenic overexpression of the human α-synuclein gene containing both A53T and A30P mutations (hm(2)-αSYN) [56]. Furthermore, intrastriatal injection of preformed A53T α-synuclein fibrils has been recently developed as a model of PD that recapitulates the robust formation of α-synuclein inclusions characteristic of PD pathology, with the abnormal seeding and spreading of α-synuclein inclusions throughout the brain alongside dopaminergic neuron loss [57,58]. In this α-synu-clein fibril model of PD, genetic deletion of A2A receptors [59] or chronic caffeine treatment [60] attenuates neurodegeneration induced by intra-striatal injection of preformed A53T α-synuclein fibril. Collectively, these findings define aberrant A2A receptor signaling as a critical pathogenic mechanism of α-synuclein-triggered neurodegeneration in PD models.
1.2.2. Up-regulation of the adenosine A2AR signaling in PD brains
Adenosine A2A receptor signaling is important not only in physiological homeostatic regulation, but also in many pathophysiological processes including inflammatory diseases [61,62], ischemia and reperfusion [63] and neurodegenerative disorders [64,65]. The brain response to a variety of insults is characterized by upregulated A2A receptor signaling, which may contribute to development of neurological disorders, including PD. For example, recent studies show that dopamine depleting 6-OHDA/MPTP treatment increased ATP release and upregulated ecto-5'-nucleotidase (CD73, a marker for mesenchymal stem cells) and A2A receptors in striatal synaptosomes [66] and in the striatum of MPTP-treated mice [67]. Furthermore, A2A receptor upregulation in neurons and glial cells of hippocampus is induced by intra-hippocampal injection of preformed A53T α-Syn fibrils [68].
Importantly, A2A receptor upregulation has also been clearly documented in putamen of early stage of PD (Braak stage 1–2) [69]. PET studies have also confirmed up-regulation of striatal A2A receptors in PD with dyskinesia, compared to PD patients without dyskinesia [70,71]. Moreover, a recent study suggests a self-regulating feed-forward adenosine formation (via CD73) can activate A2A receptors and promote neuroinflammation as CD73 inactivation suppressed A2A receptor induction and A2A-mediated pro-inflammatory responses [67]. Thus, increased A2A receptor signaling represents a useful biomarker as well as a therapeutic target of PD. Consequently, the potential to protect against dopaminergic neurodegeneration represents the most exciting and yet-to-be realized aspect of A2A antagonists as a novel therapy for PD.
1.3. Mechanisms of neuroprotection via A2A antagonism in PD models
How genetic deletion or pharmacological blockade of the A2A receptor affords protection against dopaminergic neuron degeneration in PD remains unclear. Several critical pathological processes at cellular and molecular levels have been identified in PD pathogenesis, including mitochondrial dysfunction, neuroinflammation, and dysregulated proteasome and autophagy pathways [72,73]. In the next part of this review, we examine how A2A receptor activity may influence these pathogenic processes to confer neuroprotection against dopaminergic neurotoxicity.
1.3.1. A2A receptor modulation of mitochondrial dysfunction
PD pathogenesis is characterized by mitochondrial dysfunction. Studies in MPTP-mice models show reduced (13)C labeling of GluC 4, GABAC2 and GlnC4 from [1,6-(13)C2]glucose in the cerebral cortex, striatum, thalamus and cerebellum, suggesting impaired glutamatergic and GABAergic neuronal activity and neurotransmission [74]. However, pre-treatment with caffeine has been shown to partially preserve the (13)C labeling of amino acids of MPTP-treated mice compared to control values and A2A antagonists reduced the mitochondrial toxin rotenone-induced loss of corticostriatal field potential amplitude as well as membrane depolarization in striatal slices [75]. In MitoPark mice—a genetic model of mitochondrial dysfunction with dopamine neurodegeneration, pre-treatment with the A2A antagonist MSX-3 prevented the reduction of spontaneous locomotor activity [76], lending further support for A2A receptor modulation of mitochondrial dysfunction.
1.3.2. A2A receptor interaction with NMDA receptor
Abnormal activation of A2A receptors might lead to overactivation of NMDA receptors, which is a prominent synaptic event resulting in excitotoxicity [77]. A2A receptors are known to facilitate NMDA receptors function in the hippocampus (possibly by facilitation of presynaptic release of glutamate [78]) and also attenuate traumatic brain injury by control of glutamate-mediated neurotoxicity [79]. In SH-SY5Y cells, A2A receptor antagonists also rescue α-synuclein toxicity via an NMDA receptor interaction [80]. However, it is also possible that A2A antagonists confer neuroprotective actions at non-neuronal cells (such as glial cells) since A2A antagonists and caffeine remain neuroprotective in forebrain neuron-specific A2A knockout mice [81,82,90].
1.3.3. A2A receptor modulation of microglial activity and neuroinflammation
Extracellular aggregated α-synuclein can trigger massive microglial activation and neuroinflammation by binding to Toll-like receptor 2 (TLR2), CD11b receptors and integrin β1 subunit on microglia, resulting in neuronal death [83–87]. It is thought that A2A receptors modulate microglial reactivity and control neuroinflammatory processes to modify α-synuclein-induced neurodegeneration [88,89]. Consistent with this view, α-synuclein-triggered reactive microglial activation, NF-κB p65 activation neuroinflammatory responses were largely reverted in A2A receptor knockout mice [59]. Furthermore, genetic inactivation of CD73 reduces LPS-induced microglial release of pro-inflammatory cytokines, but increases microglia process extension, movement and morphological transformation in acute MPTP model of PD [67]. CD73 modulation of microglial activity is associated with microglia-mediated neuroinflammation and protects against dopaminergic neurodegeneration and increases motor behaviors in MPTP model of PD. A2A receptor activation can inhibit dopamine-mediated anti-inflammation in glial cells to exacerbate inflammation in the brain. On the other hand, astrocytic A2A receptors may not be involved in neuroprotection as caffeine’s protective properties were undiminished in astrocytic A2A receptor conditional knockout mice [82]. Thus, the homeostatic balance between adenosine and dopamine signaling is critical for the control of microglia immunoresponses.
1.3.4. Direct interactions of A2A receptors with α-synuclein
Studies in a yeast proteotoxicity model with aggregation of recombinant α-synuclein show that caffeine can reduce the toxicity of oligomers and aggregates, and increase cell survival with concomitant reduction in intracellular oxidative stress and decreased oxidative proteome damage [91]. This was achieved presumably by caffeine directly binding to α-synuclein to alter the native conformation of mature α-synuclein aggregates displaying amorphous as well as fibrillar morphology [91]. This concept is supported by a recent study showing that A2A antagonists decrease the percentage of cells displaying α-synuclein inclusions in cultured cells [80]. It is thought that A2A receptor deletion may protect against α-synuclein aggregation and neurodegeneration by decreasing the phosphorylation of α-synuclein on Ser 129, a safeguard procedure for control of α-synuclein toxicity. The notion that A2A receptors may modulate phosphorylation modifications of pathological proteins corroborates the recent finding that A2A antagonist MSX-3 significantly improved memory and reduced Tau hyperphosphorylation in THY-Tau 22 mice [92].
1.3.5. A2A receptor modulation of proteosome and autophagy pathway
Increasing evidence demonstrates that aberrant regulation of autophagy contributes to α-synuclein aggregation and α-synuclein-induced neurodegeneration in PD [93–95]. It is postulated that A2A receptor activity may confer protection by control of ubiquitin-proteasome system (UPS) and autolysosome activity associated with degradation of the accumulated α-synuclein [96,97]. However, this assumption of A2A receptor modulation of UPS is apparently not supported by recent demonstrations that A2A activation enhances proteasome activity and reduces mutant huntingtin aggregations through a PKA-dependent pathway [98,99]. On the other hand, both caffeine and A2A receptor signaling can regulate autophagy activity under different conditions in several cell types [100,101]. Indeed, in the α-synuclein fibril model of PD, chronic caffeine treatment did not affect autophagy processes in the normal striatum, but selectively reversed α-synuclein-induced defects in macroautophagy (by enhancing microtubule-associated protein 1 light chain 3, and reducing the receptor protein sequestosome 1, SQSTM1/p62) and chaperone-mediated autophagy (CMA, by enhancing LAMP2A) [125]. The ability of caffeine to modify autophagy is correlated with that chronic caffeine-mediated attenuation of the pathological cascade leading to α-synucleinopathy, including pSer129α-Syn-rich aggregates, apoptotic neuronal cell death, and microglia and astroglia reactivation [125]. Thus, caffeine may represent a novel pharmacological therapy for PD by targeting the autophagy pathway.
1.4. The challenges in developing A2A receptor-based disease-modification therapy for PD
Despite considerable strengths of epidemiological evidence and consistent demonstration that A2A antagonists afford neuroprotection against MPTP- or 6-OHDA- and alpha-synuclein induced dopaminergic neurotoxicity, the therapeutic potential of A2A antagonists (including caffeine) remains unclear. Significant knowledge gaps remain to be closed before the therapeutic potential of A2A antagonists in disease-modifying effect in PD can be realized. First, it would be important to validate and cement the neuroprotective effect of A2A antagonists in a non-human primate model of PD which has much evolutional similarity to humans. Second, the mechanism by which A2A inactivation protects against the loss of dopaminergic neurons remains unknown with the particular challenge lying in explaining the apparent dichotomy between restricted expression of the A2A receptor in striatopallidal neurons and neuroprotection against degeneration of dopaminergic neurons in the substantia nigra where only scattered (if any) expression of A2A receptors is detected. Third, we need to develop better biomarkers to closely monitor any neuroprotective effect of A2A antagonism given the relatively short duration of clinical trials with respect to the slow pace of neurodegeneration in PD pathogenesis. Sensitive and reliable biomarkers may directly detect effects of A2A antagonists on dopaminergic neuron degeneration (rather than indirectly assess them based on changes in worsening motor symptoms). Tackling these challenges would greatly facilitate development of novel and promising strategies in PD by targeting adenosine production and A2A receptor signaling.
1.4.1. Disease modification trials of A2A receptor antagonism in PD
Although caffeine [37,102] and more selective adenosine A2A receptor antagonists (such as istradefylline [KW-6002] [103], preladenant [SCH420814/MK 3814] [104], tozadenant [SYN115] [105], and vipadenant [BIIB014] [106,107]) have been extensively tested in Phase 2 and 3 randomized, placebo-controlled trials (RCTs) as candidate therapeutics for symptomatic indications in PD, few trials have encompassed designs capable of assessing long-term disease modification with these agents and early trial terminations have precluded these assessments.
Preladenant was tested as monotherapy in 1007 early stage PD participants in a large, 52-week long phase 3 double-blind RCT initially under a delayed-start, two-period trial design [108], which was structured to support regulatory approval for a ‘disease modification’ indication [109,110]. In keeping with such a goal, the trial included an active comparator treatment arm with rasagiline, a drug that had been employed in a delayed-start, two-period trial [111] also in pursuit of a disease-modification indication. However, nine months after starting enrollment, the study design was modified to eliminate the delayed start treatment arm (see [108] and ClinicalTrial.gov registration NCT01155479 [112] entries under “History of Changes” from June 30, 2010 until April 6, 2011), in which placebo was used during the first 26-week period before replacement by preladenant during the second 26-week period.
Similarly, caffeine was investigated for disease modification in PD as a secondary outcome of a smaller (n = 121), but longer (planned for 5 years), Phase 3, two-arm, double-blind RCT, with a primary outcome focused on assessing improvement in motor symptoms. Its ambitious two-period, delayed-start study design entailed comparison of PD patients who were enrolled on stable symptomatic therapy and then randomized to receive either caffeine at 200 mg twice daily for 5 years, or placebo twice daily for 4.5 years before being switched to caffeine treatment for the final 6 months [113]. Unfortunately, with the primary outcome analysis demonstrating no significant symptomatic benefit after 6 months [37], the study was terminated early, precluding the opportunity to test for disease modification by caffeine treatment.
1.4.2. Future designs with caffeine and more selective A2A receptor antagonists
Despite these missed clinical trial opportunities to test the hypothesis of neuroprotection by adenosine A2A receptor antagonism, new prospects are appearing on the horizon. With advances in identifying large numbers of individuals who are at risk (genetically and/or prodromally) of developing PD, but otherwise healthy, it is now plausible to begin planning trials for prevention of the disease. Carriers of pathogenic LRRK2 mutations with a lifetime penetrance of PD generally exceeding 30%, who have family members suffering from the disease as well as children or grandchildren who are also at-risk carriers, may be motivated to enroll in prevention trials testing compelling and low-risk candidate protectants like caffeine. The preclinical and biomarker data mentioned above, which suggest a greater potential benefit in LRRK2 mutation-driven as compared to idiopathic PD, further encourage consideration of such trials. At present the greatest limitation to embarking on prevention trials may be the inadequacy of currently envisioned outcome measures. While phenoconversion to manifest disease is intuitive and a seemingly definitive endpoint, the wait for it might be prohibitively long and would require a large sample size. Enriching a target population for older participants (e.g., >60 years among genetically at-risk subjects) and/or for additional or prodromal risk factors (e.g., presence of hyposmia or REM sleep behavior disorder) may lessen this challenge. Alternatively, sensitive tracking of prodromal motor features (e.g., subtle motor slowing assessed by wearable or smartphone sensors) or neuroimaging (e.g., dopamine transporter brain scan changes) markers of pre-diagnostic progression may become sufficiently validated to employ in a proof-of-concept prevention trial designs.
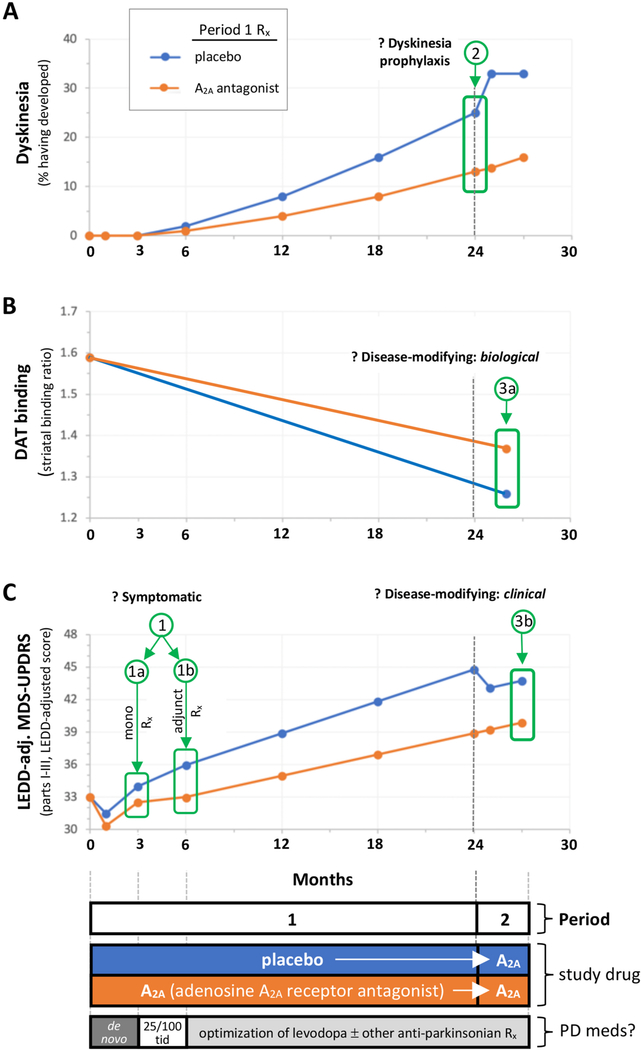
Finally, there may be an opportunity to build on the established safety and efficacy of istradefylline, now that it has secured regulatory approval in Japan and the United States, for use as adjunctive treatment of PD symptoms among those experiencing wearing off of levodopa benefit. As reviewed above, there is considerable evidence for the disease-modifying potential of istradefylline and other adenosine A2A receptor antagonists in PD. Collectively, the data suggest that this strategy might slow not only the neurodegeneration underlying the inexorably progressive loss of motor function, but also help prevent the maladaptive neuroplasticity that leads to dyskinetic motor complications in PD. Among many trial strategies, one powerful Phase 2 design that could explore and support several indications by employing staggered co-primary outcomes would entail enrolling early, untreated PD patients – who are not heavy caffeine consumers and who are expected to presently warrant treatment with dopaminergic therapy. In this scenario (Fig. 1), participants would be randomized to receive an A2A receptor antagonist (or one of two doses) or placebo in equal proportions. After an initial treatment period (e.g., of 12 weeks) to assess for effects on motor (and other) features, then all participants would initiate standard carbidopa/levodopa 25/100 dosing three times daily while continuing on and blinded to treatment with an A2A receptor antagonist or placebo for another two to four years as levodopa and other antiparkinsonian medications are adjusted to optimize management, before a final 12–24 week period when the placebo is replaced with the A2A receptor antagonist. This delay-start design would sequentially elucidate the potential for valuable (extended) indications for symptomatic benefit as monotherapy or as early adjuntive therapy (Fig. 1C; panel C/ outcomes 1a and 1b), and then for preventing dyskinesia (Fig. 1A; panel A/outcome 2), and finally for reducing long-term parkinsonian deficits and/or of dopamine transporter (DAT) binding site loss, a marker for dopaminergic neuron degeneration (Fig. 1B; panels B/outcome 3a and panel C/outcome 3b).
Fig. 1. Envisioned design for a phase 2, randomized, double-blind clinical trial of an adenosine A2A receptor antagonist to investigate its multiple potential indications spanning short-term symptomatic and long-term, disease course benefits in PD.
The study proposed here would evaluate three co-primary outcomes based on three measures: development of dyskinesia, a motor complication induced by repeated levodopa treatment (A), striatal dopamine transporter (DAT) binding, a marker nigrostriatal neuron integrity, whose progressive loss can reflect dopaminergic neuron degeneration (B), and levodopa equivalent daily dose (LEDD)-adjusted Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), which can show progressive clinical worsening (increasing score) over years in early PD [116] as well as short-term placebo or medication effects (C).
By enrolling only participants who have been recently diagnosed, who have a DAT deficit on neuroimaging [117] and who are not expected to require anti-parkinsonian medication until at least a few months after enrollment, the trial would likely be more sensitive to interventions targeting A2A-dependent pathophysiology in the early stages of PD, which has been implicated by epidemiological and preclinical data (see text). And by enrolling only participants with modest caffeine intake (< 100 mg/day) the study would enrich for those whose targeted A2A receptors are available (i.e., given that normal caffeine use in humans may substantially block striatal A2A receptors) [118]. Alternatively, simply monitoring caffeine consumption would allow stratification or adjustment of results by caffeine levels, and may improve interpretation of the findings [31,119]. Of note, negative results of prior trials of selective A2A antagonists as symptomatic therapy, particularly as monotherapy targeting de novo PD subjects [e.g., [114]], may be attributed at least in part to enrollment of a small but significant proportion of non-PD patients who do not have a striatal dopaminergic deficit, and of patients whose caffeine use greatly reduced the availability of striatal A2A receptors. The proposed trial’s exclusion of people who have DAT scans without evidence of dopaminergic deficit (SWEDDs), and of people regularly consuming ≥100mg or more mg of caffeine daily, increases the likelihood of identifying benefits of selective A2A antagonist therapy in early PD.
Sequential outcomes would be analyzed to test 3 hypotheses, that beginning treatment with an A2A antagonist prior to levodopa initiation in this enriched early PD population 1) improves parkinsonian symptoms and deficits (assessed on the MDS-UPDRS) in the short-term, both as monotherapy (1a) and perhaps more substantially as an adjunct (1b) to initial levodopa treatment with 25/100 carbidopa/levodopa t.i.d. for 3 months (panel C); 2) delays or prevents the development dyskinesia (LID), which can be experienced in a quarter of PD within 18 months of starting standard levodopa treatment [120] (panel A); and 3) slows long-term progression of dopaminergic parkinsonian deficits measured radiographically by serial DAT imaging (3a, Panel B; with DAT scans conducted at screening/baseline and thereafter including 1–2 months into Period 2 in order to avoid any acute confounding effect A2A antagonist treatment may have on DAT signal) or clinically by adjusted MDS-UPDRS (panel C). The latter scale can be used to gauge differences in progression of clinical disability after years of A2A antaongist versus placebo treatment, based not only on the residual group difference after delayed-start of the drug in the 2nd period (3b) but also on a slope difference in Period 1, and on lack of convergence of slopes in Period 2 – as expected in a classic 2-period design [110], which is particularly well suited to candidate neuroprotectants that possess symptomatic antiparkinsonian properties like A2A antagonists. Note also that A2A antagonist are well known to modestly exacerbate or unmask LID after chronic treated with levodopa [e.g., [115], their potential for LID prophylaxis notwithstanding, as depicted for in panel A upon addition of A2A antagonist at the start of Period 2 at 24 months in the (early) placebo arm.
Although the MDS-UPDRS score is a well characterized composite patient- and clinician-reported outcome designed for in-clinic assessment, it may be modified for remote evaluation via televisits [121]. This variant and other remote assessments of parkinsonism currently being developed or validated (e.g., [122]) may offer attractive alternative measures of clinical progression given the convergence of impediments to long-term serial in-person clinic visits (e.g., the 2020 pandemic [123]) with advances in digital and wearable technologies [124].
Overall, a remarkable convergence of biological, epidemiological and clinical data support the further investment in selective adenosine A2A antagonists (as well as the possibility of caffeine fulfilling this role) as promising candidate therapeutics to fill the unmet need for disease-modifying treatment of people with PD.
Acknowledgements
Supported by the Farmer Family Foundation Parkinson’s Research Initiative and NIH grant NS110879. Trial design insights of Eric Macklin, PhD were incorporated and appreciated.
Footnotes
Disclosure statement
This article is published as part of a supplement supported by Kyowa Kirin, Inc.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Costa J, Lunet N, Santos C, Santos J, Vaz-Carneiro A, Caffeine exposure and the risk of Parkinson’s disease: a systematic review and meta-analysis of observational studies, J Alzheimers Dis 20 (Suppl 1) (2010) S221–S238. [DOI] [PubMed] [Google Scholar]
- [2].Bakshi R, Macklin EA, Hung AY, Hayes MT, Hyman BT, Wills AM, Gomperts SN, Growdon JH, Ascherio A, Scherzer CR, Schwarzschild MA, Associations of lower caffeine intake and plasma urate levels with idiopathic Parkinson’s disease in the harvard biomarkers study, J. Parkinsons Dis 10 (2) (2020) 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ascherio A, Zhang SM, Hernan MA, Kawachi I, Colditz GA, Speizer FE, Willett WC, Prospective study of caffeine consumption and risk of Parkinson’s disease in men and women, Ann. Neurol. 50 (1) (2001) 56–63. [DOI] [PubMed] [Google Scholar]
- [4].Palacios N, Gao X, McCullough ML, Schwarzschild MA, Shah R, Gapstur S, Ascherio A, Caffeine and risk of Parkinson’s disease in a large cohort of men and women, Mov. Disord. 27 (10) (2012) 1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hong CT, Chan L, Bai C-H, The effect of caffeine on the risk and progression of Parkinson’s disease: a meta-analysis, Nutrients 12 (6) (2020) 1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liu R, Guo X, Park Y, Huang X, Sinha R, Freedman ND, Hollenbeck AR, Blair A, Chen H, Caffeine intake, smoking, and risk of Parkinson disease in men and women, Am. J. Epidemiol. 175 (11) (2012) 1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ascherio A, Schwarzschild MA, The epidemiology of Parkinson’s disease: risk factors and prevention, Lancet Neurol. 15 (12) (2016) 1257–1272. [DOI] [PubMed] [Google Scholar]
- [8].Ross GW, Abbott RD, Petrovitch H, Morens DM, Grandinetti A, Tung KH, Tanner CM, Masaki KH, Blanchette PL, Curb JD, Popper JS, White LR, Association of coffee J Am. Med. Assoc 283 (20) (2000) 2674–2679. [DOI] [PubMed] [Google Scholar]
- [9].Ascherio A, Chen H, Schwarzschild MA, Zhang SM, Colditz GA, Speizer FE, Caffeine, postmenopausal estrogen, and risk of Parkinson’s disease, Neurology 60 (5) (2003) 790–795. [DOI] [PubMed] [Google Scholar]
- [10].Sääksjärvi K, Knekt P, Rissanen H, Laaksonen MA, Reunanen A, Männistö S, Prospective study of coffee consumption and risk of Parkinson’s disease, Eur. J. Clin. Nutr 62 (7) (2008) 908–915. [DOI] [PubMed] [Google Scholar]
- [11].Sales AL, dePaula J, Mellinger Silva C, Cruz A, Lemos Miguel MA, Farah A, Effects of regular and decaffeinated roasted coffee (Coffea arabica and Coffea canephora) extracts and bioactive compounds on in vitro probiotic bacterial growth, Food Func 11 (2) (2020) 1410–1424. [DOI] [PubMed] [Google Scholar]
- [12].Trinh K, Andrews L, Krause J, Hanak T, Lee D, Gelb M, Pallanck L, Decaffeinated coffee and nicotine-free tobacco provide neuroprotection in Drosophila models of Parkinson’s disease through an NRF2-dependent mechanism, J. Neurosci. 30 (16) (2010) 5525–5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhou ZD, Xie SP, Saw WT, Ho PGH, Wang H, Lei Z, Yi Z, Tan EK, The therapeutic implications of tea polyphenols against dopamine (DA) neuron degeneration in Parkinson’s disease (PD), Cells 8 (8) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yan R, Zhang J, Park HJ, Park ES, Oh S, Zheng H, Junn E, Voronkov M, Stock JB, Mouradian MM, Synergistic neuroprotection by coffee components eicosanoyl-5-hydroxytryptamide and caffeine in models of Parkinson’s disease and DLB, Proc. Natl. Acad. Sci. U. S. A. 115 (51) (2018) E12053–e12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim IY, O’Reilly É J, Hughes KC, Gao X, Schwarzschild MA, Ascherio A, Differences in Parkinson’s disease risk with caffeine intake and postmenopausal hormone use, J. Parkinsons Dis. 7 (4) (2017) 677–684. [DOI] [PubMed] [Google Scholar]
- [16].Palacios N, Weisskopf M, Simon K, Gao X, Schwarzschild M, Ascherio A, Polymorphisms of caffeine metabolism and estrogen receptor genes and risk of Parkinson’s disease in men and women, Park. Relat. Disord 16 (6) (2010) 370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xu K, Xu Y, Brown-Jermyn D, Chen JF, Ascherio A, Dluzen DE, Schwarzschild MA, Estrogen prevents neuroprotection by caffeine in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease, J. Neurosci. 26 (2) (2006) 535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Popat RA, Van Den Eeden SK, Tanner CM, Kamel F, Umbach DM, Marder K, Mayeux R, Ritz B, Ross GW, Petrovitch H, Topol B, McGuire V, Costello S, Manthripragada AD, Southwick A, Myers RM, Nelson LM, Coffee, ADORA2A, and CYP1A2: the caffeine connection in Parkinson’s disease, Eur. J. Neurol. 18 (5) (2011) 756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chuang YH, Lill CM, Lee PC, Hansen J, Lassen CF, Bertram L, Greene N, Sinsheimer JS, Ritz B, Gene-environment interaction in Parkinson’s disease: coffee, ADORA2A, and CYP1A2, Neuroepidemiology 47 (3–4) (2016) 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tan EK, Chua E, Fook-Chong SM, Teo YY, Yuen Y, Tan L, Zhao Y, Association between caffeine intake and risk of Parkinson’s disease among fast and slow metabolizers, Pharmacogenetics Genom. 17 (11) (2007) 1001–1005. [DOI] [PubMed] [Google Scholar]
- [21].Hill-Burns EM, Hamza TH, Zabetian CP, Factor SA, Payami H, An attempt to replicate interaction between coffee and CYP1A2 gene in connection to Parkinson’s disease, Eur. J. Neurol. 18 (9) (2011) e107–e108, author reply e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kim IY, O’Reilly E J,´ Hughes KC, Gao X, Schwarzschild MA, McCullough ML, Hannan MT, Betensky RA, Ascherio A, Interaction between caffeine and polymorphisms of glutamate ionotropic receptor NMDA type subunit 2A (GRIN2A) and cytochrome P450 1A2 (CYP1A2) on Parkinson’s disease risk, Mov. Disord. 33 (3) (2018) 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Xu K, Xu YH, Chen JF, Schwarzschild MA, Neuroprotection by caffeine: time course and role of its metabolites in the MPTP model of Parkinson’s disease, Neuroscience 167 (2) (2010) 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Moccia M, Erro R, Picillo M, Vitale C, Longo K, Amboni M, Pellecchia MT, Barone P, Caffeine consumption and the 4-year progression of de novo Parkinson’s disease, Park. Relat. Disord. 32 (2016) 116–119. [DOI] [PubMed] [Google Scholar]
- [25].Simon DK, Swearingen CJ, Hauser RA, Trugman JM, Aminoff MJ, Singer C, Truong D, Tilley BC, Caffeine and progression of Parkinson disease, Clin. Neuropharmacol. 31 (4) (2008) 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Simon DK, Wu C, Tilley BC, Wills AM, Aminoff MJ, Bainbridge J, Hauser RA, Schneider JS, Sharma S, Singer C, Tanner CM, Truong D, Wong PS, Caffeine and progression of Parkinson disease: a deleterious interaction with creatine, Clin. Neuropharmacol 38 (5) (2015) 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wills AM, Eberly S, Tennis M, Lang AE, Messing S, Togasaki D, Tanner CM, Kamp C, Chen JF, Oakes D, McDermott MP, Schwarzschild MA, Caffeine consumption and risk of dyskinesia in CALM-PD, Mov. Disord. 28 (3) (2013) 380–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nicoletti A, Zappia M, Coffee consumption and risk of levodopa-induced dyskinesia in Parkinson’s disease: the FRAGAMP study, Mov. Disord. 30 (13) (2015) 1854–1856. [DOI] [PubMed] [Google Scholar]
- [29].Bibbiani F, Oh JD, Petzer JP, Castagnoli N Jr., Chen JF, Schwarzschild MA, Chase TN, A2A antagonist prevents dopamine agonist-induced motor complications in animal models of Parkinson’s disease, Exp. Neurol. 184 (1) (2003) 285–294. [DOI] [PubMed] [Google Scholar]
- [30].Xiao D, Bastia E, Xu YH, Benn CL, Cha JH, Peterson TS, Chen JF, Schwarzschild MA, Forebrain adenosine A2A receptors contribute to L-3,4-dihydroxyphenylalanine-induced dyskinesia in hemiparkinsonian mice, J. Neurosci. 26 (52) (2006) 13548–13555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chen JF, Fredduzzi S, Bastia E, Yu L, Moratalla R, Ongini E, Schwarzschild MA, Adenosine A2A receptors in neuroadaptation to repeated dopaminergic stimulation: implications for the treatment of dyskinesias in Parkinson’s disease, Neurology 61 (11 Suppl 6) (2003) S74–S81. [DOI] [PubMed] [Google Scholar]
- [32].Jenner P, A cup of coffee a day keeps dyskinesia away? Mov. Disord. 28 (3) (2013) 265–267. [DOI] [PubMed] [Google Scholar]
- [33].Kumar PM, Paing SS, Li H, Pavanni R, Yuen Y, Zhao Y, Tan EK, Differential effect of caffeine intake in subjects with genetic susceptibility to Parkinson’s Disease, Sci. Rep. 5 (2015) 15492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tanner CMC, Meng C, Marder K, Bressman S, Saunders-Pullman R, Alcalay R, Tolosa E, Brice A, Goldman S, Schuele B, Lang A, Goldwurm S, Riboldazzi G, Ferreira J, Klein C, Berg D, Brockmann K, Tazir M, Aasly J, Marti-Masso J, Marti-Masso J, Munhoz R, Rieder C, San Luciano M, Mellick G, Sue C, Hasegawa K, Tan E, Langston J, M. LRRK2 Cohort-Consortium, Caffeinated drinks, LRRK2 genotype and PD [abstract], Mov. Disord. 32 (suppl 2) (2017). [Google Scholar]
- [35].Yahalom G, Rigbi A, Israeli-Korn S, Krohn L, Rudakou U, Ruskey JA, Benshimol L, Tsafnat T, Gan-Or Z, Hassin-Baer S, Greenbaum L, Age at onset of Parkinson’s disease among Ashkenazi Jewish patients: contribution of environmental factors, LRRK2 p.G2019S and GBA p.N370S mutations, J. Parkinsons Dis 10 (3) (2020) 1123–1132. [DOI] [PubMed] [Google Scholar]
- [36].Crotty GF, Maciuca R, Macklin EA, Wang J, Montalban M, Davis SS, Alkabsh JI, Bakshi R, Chen X, Ascherio A, Astarita G, Huntwork-Rodriguez S, Schwarzschild MA, Association of caffeine and related analytes with resistance to Parkinson’s disease among LRRK2 mutation carriers: a metabolomic study. Neurology, 2020. MS ID# 103689 [in press; online 2020–09-30]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Postuma RB, Anang J, Pelletier A, Joseph L, Moscovich M, Grimes D, Furtado S, Munhoz RP, Appel-Cresswell S, Moro A, Borys A, Hobson D, Lang AE, Caffeine as symptomatic treatment for Parkinson disease (Café-PD): a randomized trial, Neurology 89 (17) (2017) 1795–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Caffeine Chart. https://cspinet.org/eating-healthy/ingredients-of-concern/caffeine-chart. (Accessed 18 September 2020).
- [39].Chen JF, Xu K, Petzer JP, Staal R, Xu YH, Beilstein M, Sonsalla PK, Castagnoli K, Castagnoli N Jr., Schwarzschild MA, Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson’s disease, J. Neurosci. 21 (10) (2001) RC143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ikeda K, Kurokawa M, Aoyama S, Kuwana Y, Neuroprotection by adenosine A2A receptor blockade in experimental models of Parkinson’s disease, J. Neurochem. 80 (2) (2002) 262–270. [DOI] [PubMed] [Google Scholar]
- [41].Xu K, Xu YH, Chen JF, Schwarzschild MA, Caffeine’s neuroprotection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity shows no tolerance to chronic caffeine administration in mice, Neurosci. Lett. 322 (1) (2002) 13–16. [DOI] [PubMed] [Google Scholar]
- [42].Soliman AM, Fathalla AM, Moustafa AA, Dose-dependent neuroprotective effect of caffeine on a rotenone-induced rat model of parkinsonism: a histological study, Neurosci. Lett. 623 (2016) 63–70. [DOI] [PubMed] [Google Scholar]
- [43].Fathalla AM, Soliman AM, Moustafa AA, Selective A(2A) receptors blockade reduces degeneration of substantia nigra dopamine neurons in a rotenone-induced rat model of Parkinson’s disease: a histological study, Neurosci. Lett. 643 (2017) 89–96. [DOI] [PubMed] [Google Scholar]
- [44].Alfinito PD, Wang SP, Manzino L, Rijhsinghani S, Zeevalk GD, Sonsalla PK, Adenosinergic protection of dopaminergic and GABAergic neurons against mitochondrial inhibition through receptors located in the substantia nigra and striatum, respectively, J. Neurosci. 23 (34) (2003) 10982–10987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pierri M, Vaudano E, Sager T, Englund U, KW-6002 protects from MPTP induced dopaminergic toxicity in the mouse, Neuropharmacology 48 (4) (2005) 517–524. [DOI] [PubMed] [Google Scholar]
- [46].Kumari N, Agrawal S, Kumari R, Sharma D, Luthra PM, Neuroprotective effect of IDPU (1-(7-imino-3-propyl-2,3-dihydrothiazolo [4,5-d]pyrimidin-6(7H)-yl) urea) in 6-OHDA induced rodent model of hemiparkinson’s disease, Neurosci. Lett. 675 (2018) 74–82. [DOI] [PubMed] [Google Scholar]
- [47].Peterson JD, Goldberg JA, Surmeier DJ, Adenosine A2a receptor antagonists attenuate striatal adaptations following dopamine depletion, Neurobiol. Dis. 45 (1) (2012) 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Oztas E, Xu K, Kalda A, Irrizary M, Schwarzschild MA, J-F C, Caffeine attenutes MPTP-induced loss of dopaminergic neurons in substantial nigra in mice, Annual meeting of Society for Neuroscience, Orlando, FL, 2002, 2002. [Google Scholar]
- [49].Sonsalla PK, Wong LY, Harris SL, Richardson JR, Khobahy I, Li W, Gadad BS, German DC, Delayed caffeine treatment prevents nigral dopamine neuron loss in a progressive rat model of Parkinson’s disease, Exp. Neurol. 234 (2) (2012) 482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sinchai T, Plasen S, Sanvarinda Y, Jaisin Y, Govitrapong P, Morales NP, Ratanachamnong P, Plasen D, Caffeine potentiates methamphetamine-induced toxicity both in vitro and in vivo, Neurosci. Lett. 502 (1) (2011) 65–69. [DOI] [PubMed] [Google Scholar]
- [51].Frau L, Costa G, Porceddu PF, Khairnar A, Castelli MP, Ennas MG, Madeddu C, Wardas J, Morelli M, Influence of caffeine on 3,4-methylenediox-ymethamphetamine-induced dopaminergic neuron degeneration and neuroinflammation is age-dependent, J. Neurochem. 136 (1) (2016) 148–162. [DOI] [PubMed] [Google Scholar]
- [52].Goedert M, Alpha-synuclein and neurodegenerative diseases, Nat. Rev. Neurosci. 2 (7) (2001) 492–501. [DOI] [PubMed] [Google Scholar]
- [53].Wong YC, Krainc D, alpha-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies, Nat. Med. 23 (2) (2017) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lashuel HA, Overk CR, Oueslati A, Masliah E, The many faces of alpha-synuclein: from structure and toxicity to therapeutic target, Nat. Rev. Neurosci 14 (1) (2013) 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ferreira DG, Batalha VL, Vicente Miranda H, Coelho JE, Gomes R, Goncalves FQ, Real JI, Rino J, Albino-Teixeira A, Cunha RA, Outeiro TF, Lopes LV, Adenosine A2A receptors modulate alpha-synuclein aggregation and toxicity, Cerebr. Cortex 27 (1) (2017) 718–730. [DOI] [PubMed] [Google Scholar]
- [56].Kachroo A, Schwarzschild MA, Adenosine A2A receptor gene disruption protects in an alpha-synuclein model of Parkinson’s disease, Ann. Neurol. 71 (2) (2012) 278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM, Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death, Neuron 72 (1) (2011) 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM, Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice, Science 338 (6109) (2012) 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hu QD, Ren XP, Liu Y, Li ZH, Zhang LP, Chen XJ, He CX, Chen JF, Aberrant adenosine A2A receptor signaling contributes to neurodegeneration and cognitive impairments in a mouse model of synucleinopathy, Exp. Neurol. 283 (2016) 213–223. [DOI] [PubMed] [Google Scholar]
- [60].Luan Y, Ren X, Zheng W, Zeng Z, Guo Y, Hou Z, Guo W, Chen X, Li F, Chen JF, Chronic caffeine treatment protects against α-synucleinopathy by reestablishing autophagy activity in the mouse striatum, Front. Neurosci. 12 (2018) 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hasko G, Linden J, Cronstein B, Pacher P, Adenosine receptors: therapeutic aspects for inflammatory and immune diseases, Nat. Rev. Drug Discov 7 (9) (2008) 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Eltzschig HK, Carmeliet P, Hypoxia and inflammation, N. Engl. J. Med 364 (7) (2011) 656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Olanow CW, Kieburtz K, Schapira AH, Why have we failed to achieve neuroprotection in Parkinson’s disease? Ann. Neurol. 64 (Suppl 2) (2008) S101–S110. [DOI] [PubMed] [Google Scholar]
- [64].Burnstock G, Fredholm BB, Verkhratsky A, Adenosine and ATP receptors in the brain, Curr. Top. Med. Chem 11 (8) (2011) 973–1011. [DOI] [PubMed] [Google Scholar]
- [65].Fredholm BB, Adenosine, an endogenous distress signal, modulates tissue damage and repair, Cell Death Differ. 14 (7) (2007) 1315–1323. [DOI] [PubMed] [Google Scholar]
- [66].Carmo M, Gonçalves FQ, Canas PM, Oses JP, Fernandes FD, Duarte FV, Enhanced ATP release and CD73-mediated adenosine formation sustain adenosine A(2A) receptor over-activation in a rat model of Parkinson’s disease 176 (18) (2019) 3666–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Meng F, Guo Z, Hu Y, Mai W, Zhang Z, Zhang B, Ge Q, Lou H, Guo F, Chen J, Duan S, Gao Z, CD73-derived adenosine controls inflammation and neurodegeneration by modulating dopamine signalling, Brain 142 (3) (2019) 700–718. [DOI] [PubMed] [Google Scholar]
- [68].Hu Q, Ren X, Liu Y, Li Z, Zhang L, Chen X, He C, Chen JF, Aberrant adenosine A2A receptor signaling contributes to neurodegeneration and cognitive impairments in a mouse model of synucleinopathy, Exp. Neurol. 283 (Pt A) (2016) 213–223. [DOI] [PubMed] [Google Scholar]
- [69].Villar-Menendez I, Porta S, Buira SP, Pereira-Veiga T, Diaz-Sanchez S, Albasanz JL, Ferrer I, Martin M, Barrachina M, Increased striatal adenosine A2A receptor levels is an early event in Parkinson’s disease-related pathology and it is potentially regulated by miR-34b, Neurobiol. Dis. 69 (2014) 206–214. [DOI] [PubMed] [Google Scholar]
- [70].Ramlackhansingh AF, Bose SK, Ahmed I, Turkheimer FE, Pavese N, Brooks DJ, Adenosine 2A receptor availability in dyskinetic and nondyskinetic patients with Parkinson disease, Neurology 76 (21) (2011) 1811–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mishina M, Ishiwata K, Naganawa M, Kimura Y, Kitamura S, Suzuki M, Hashimoto M, Ishibashi K, Oda K, Sakata M, Hamamoto M, Kobayashi S, Katayama Y, Ishii K, Adenosine A(2A) receptors measured with [C]TMSX PET in the striata of Parkinson’s disease patients, PloS One 6 (2) (2011), e17338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Rocha EM, De Miranda B, Sanders LH, Alpha-synuclein: pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease, Neurobiol. Dis. 109 (Pt B) (2018) 249–257. [DOI] [PubMed] [Google Scholar]
- [73].Kalia LV, Lang AE, Parkinson’s disease, Lancet 386 (9996) (2015) 896–912. [DOI] [PubMed] [Google Scholar]
- [74].Bagga P, Chugani AN, Patel AB, Neuroprotective effects of caffeine in MPTP model of Parkinson’s disease: a (13)C NMR study, Neurochem. Int. 92 (2016) 25–34. [DOI] [PubMed] [Google Scholar]
- [75].Belcastro V, Tozzi A, Tantucci M, Costa C, Di Filippo M, Autuori A, Picconi B, Siliquini S, Luchetti E, Borsini F, Calabresi P, A2A adenosine receptor antagonists protect the striatum against rotenone-induced neurotoxicity, Exp. Neurol. 217 (1) (2009) 231–234. [DOI] [PubMed] [Google Scholar]
- [76].Marcellino D, Lindqvist E, Schneider M, Muller CE, Fuxe K, Olson L, Galter D, Chronic A2A antagonist treatment alleviates parkinsonian locomotor deficiency in MitoPark mice, Neurobiol. Dis. 40 (2) (2010) 460–466. [DOI] [PubMed] [Google Scholar]
- [77].Besancon E, Guo S, Lok J, Tymianski M, Lo EH, Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke, Trends Pharmacol. Sci. 29 (5) (2008) 268–275. [DOI] [PubMed] [Google Scholar]
- [78].Rebola N, Lujan R, Cunha RA, Mulle C, Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses, Neuron 57 (1) (2008) 121–134. [DOI] [PubMed] [Google Scholar]
- [79].Dai SS, Zhou YG, Li W, An JH, Li P, Yang N, Chen XY, Xiong RP, Liu P, Zhao Y, Shen HY, Zhu PF, Chen JF, Local glutamate level dictates adenosine A2A receptor regulation of neuroinflammation and traumatic brain injury, J. Neurosci. 30 (16) (2010) 5802–5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ferreira DG, Batalha VL, Vicente Miranda H, Coelho JE, Gomes R, Goncalves FQ, Real JI, Rino J, Albino-Teixeira A, Cunha RA, Outeiro TF, Lopes LV, Adenosine A2A receptors modulate α-synuclein aggregation and toxicity, Cereb Cortex 27 (1) (2017) 718–730, 10.1093/cercor/bhv268. [DOI] [PubMed] [Google Scholar]
- [81].Shen HY, Coelho JE, Ohtsuka N, Canas PM, Day YJ, Huang QY, Rebola N, Yu L, Boison D, Cunha RA, Linden J, Tsien JZ, Chen JF, A critical role of the adenosine A2A receptor in extrastriatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum and forebrain A2A receptor knock-outs, J. Neurosci. 28 (12) (2008) 2970–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Xu K, Di Luca DG, Orrú M, Xu Y, Chen JF, Schwarzschild MA, Neuroprotection by caffeine in the MPTP model of Parkinson’s disease and its dependence on adenosine A2A receptors, Neuroscience 322 (2016) 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Sacino AN, Brooks M, McKinney AB, Thomas MA, Shaw G, Golde TE, Giasson BI, Brain injection of alpha-synuclein induces multiple proteinopathies, gliosis, and a neuronal injury marker, J. Neurosci. 34 (37) (2014) 12368–12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Yasuda T, Nakata Y, Mochizuki H, alpha-Synuclein and neuronal cell death, Mol. Neurobiol. 47 (2) (2013) 466–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, Wenning GK, Stefanova N, Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia, Glia 61 (3) (2013) 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Tansey MG, Goldberg MS, Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention, Neurobiol. Dis. 37 (3) (2010) 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Lee HJ, Kim C, Lee SJ, Alpha-synuclein stimulation of astrocytes: potential role for neuroinflammation and neuroprotection, Oxid Med Cell Longev 3 (4) (2010) 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Brambilla R, Cottini L, Fumagalli M, Ceruti S, Abbracchio MP, Blockade of A2A adenosine receptors prevents basic fibroblast growth factor-induced reactive astrogliosis in rat striatal primary astrocytes, Glia 43 (2) (2003) 190–194. [DOI] [PubMed] [Google Scholar]
- [89].Boison D, Chen JF, Fredholm BB, Adenosine signaling and function in glial cells, Cell Death Differ. 17 (7) (2010) 1071–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hui CW, Zhang Y, Herrup K, Non-neuronal cells are required to mediate the effects of neuroinflammation: results from a neuron-enriched culture system, PloS One 11 (1) (2016), e0147134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kardani J, Roy I, Understanding caffeine’s role in attenuating the toxicity of alpha-synuclein aggregates: implications for risk of Parkinson’s disease, ACS Chem. Neurosci 6 (9) (2015) 1613–1625. [DOI] [PubMed] [Google Scholar]
- [92].Laurent C, Burnouf S, Ferry B, Batalha VL, Coelho JE, Baqi Y, Malik E, Mariciniak E, Parrot S, Van der Jeugd A, Faivre E, Flaten V, Ledent C, D’Hooge R, Sergeant N, Hamdane M, Humez S, Muller CE, Lopes LV, Buee L, Blum D, A2A adenosine receptor deletion is protective in a mouse model of Tauopathy, Mol. Psychiatr 21 (1) (2016) 97–107. [DOI] [PubMed] [Google Scholar]
- [93].Poehler AM, Xiang W, Spitzer P, May VE, Meixner H, Rockenstein E, Chutna O, Outeiro TF, Winkler J, Masliah E, Klucken J, Autophagy modulates SNCA/alpha-synuclein release, thereby generating a hostile microenvironment, Autophagy 10 (12) (2014) 2171–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ebrahimi-Fakhari D, Wahlster L, McLean PJ, Protein degradation pathways in Parkinson’s disease: curse or blessing, Acta Neuropathol. 124 (2) (2012) 153–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Xilouri M, Brekk OR, Stefanis L, Autophagy and alpha-synuclein: relevance to Parkinson’s disease and related synucleopathies, Mov. Disord. 31 (2) (2016) 178–192. [DOI] [PubMed] [Google Scholar]
- [96].Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC, Alpha-Synuclein is degraded by both autophagy and the proteasome, J. Biol. Chem. 278 (27) (2003) 25009–25013. [DOI] [PubMed] [Google Scholar]
- [97].Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D, Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy, Science 305 (5688) (2004) 1292–1295. [DOI] [PubMed] [Google Scholar]
- [98].Huang CL, Yang JM, Wang KC, Lee YC, Lin YL, Yang YC, Huang NK, Gastrodia elata prevents huntingtin aggregations through activation of the adenosine A(2)A receptor and ubiquitin proteasome system, J. Ethnopharmacol. 138 (1) (2011) 162–168. [DOI] [PubMed] [Google Scholar]
- [99].Chiang MC, Chen HM, Lai HL, Chen HW, Chou SY, Chen CM, Tsai FJ, Chern Y, The A2A adenosine receptor rescues the urea cycle deficiency of Huntington’s disease by enhancing the activity of the ubiquitin-proteasome system, Hum. Mol. Genet. 18 (16) (2009) 2929–2942. [DOI] [PubMed] [Google Scholar]
- [100].Sinha RA, Farah BL, Singh BK, Siddique MM, Li Y, Wu Y, Ilkayeva OR, Gooding J, Ching J, Zhou J, Martinez L, Xie S, Bay BH, Summers SA, Newgard CB, Yen PM, Caffeine stimulates hepatic lipid metabolism by the autophagy-lysosomal pathway in mice, Hepatology 59 (4) (2014) 1366–1380. [DOI] [PubMed] [Google Scholar]
- [101].Liu YW, Yang T, Zhao L, Ni Z, Yang N, He F, Dai SS, Activation of Adenosine 2A receptor inhibits neutrophil apoptosis in an autophagy-dependent manner in mice with systemic inflammatory response syndrome, Sci. Rep 6 (2016) 33614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Postuma RB, Lang AE, Munhoz RP, Charland K, Pelletier A, Moscovich M, Filla L, Zanatta D, Rios Romenets S, Altman R, Chuang R, Shah B, Caffeine for treatment of Parkinson disease: a randomized controlled trial, Neurology 79 (7) (2012) 651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Sako W, Murakami N, Motohama K, Izumi Y, Kaji R, The effect of istradefylline for Parkinson’s disease: a meta-analysis, Sci. Rep 7 (1) (2017) 18018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Hauser RA, Cantillon M, Pourcher E, Micheli F, Mok V, Onofrj M, Huyck S, Wolski K, Preladenant in patients with Parkinson’s disease and motor fluctuations: a phase 2, double-blind, randomised trial, Lancet Neurol. 10 (3) (2011) 221–229. [DOI] [PubMed] [Google Scholar]
- [105].Hauser RA, Olanow CW, Kieburtz KD, Pourcher E, Docu-Axelerad A, Lew M, Kozyolkin O, Neale A, Resburg C, Meya U, Kenney C, Bandak S, Tozadenant (SYN115) in patients with Parkinson’s disease who have motor fluctuations on levodopa: a phase 2b, double-blind, randomised trial, Lancet Neurol. 13 (8) (2014) 767–776. [DOI] [PubMed] [Google Scholar]
- [106].Brooks DJ, Papapetropoulos S, Vandenhende F, Tomic D, He P, Coppell A, O’Neill G, An open-label, positron emission tomography study to assess adenosine A2A brain receptor occupancy of vipadenant (BIIB014) at steady-state levels in healthy male volunteers, Clin. Neuropharmacol. 33 (2) (2010) 55–60. [DOI] [PubMed] [Google Scholar]
- [107].Dose-finding safety study of BIIB014 in combination with levodopa in moderate to late stage Parkinson’s disease. https://clinicaltrials.gov/ct2/show/NCT00438607.
- [108].Stocchi F, Rascol O, Hauser RA, Huyck S, Tzontcheva A, Capece R, Ho TW, Sklar P, Lines C, Michelson D, Hewitt DJ, Randomized trial of preladenant, given as monotherapy, in patients with early Parkinson disease, Neurology 88 (23) (2017) 2198–2206. [DOI] [PubMed] [Google Scholar]
- [109].McDermott MP, Hall WJ, Oakes D, Eberly S, Design and analysis of two-period studies of potentially disease-modifying treatments, Contr. Clin. Trials 23 (6) (2002) 635–649. [DOI] [PubMed] [Google Scholar]
- [110].D’Agostino RB Sr., The delayed-start study design, N. Engl. J. Med. 361 (13) (2009) 1304–1306. [DOI] [PubMed] [Google Scholar]
- [111].Olanow CW, Rascol O, Hauser R, Feigin PD, Jankovic J, Lang A, Langston W, Melamed E, Poewe W, Stocchi F, Tolosa E, A double-blind, delayed-start trial of rasagiline in Parkinson’s disease, N. Engl. J. Med. 361 (13) (2009) 1268–1278. [DOI] [PubMed] [Google Scholar]
- [112].A placebo- and active-controlled study of preladenant in early Parkinson’s disease (PD) (P05664) (PARADYSE). https://clinicaltrials.gov/ct2/show/NCT01155479.
- [113].Caffeine as a therapy for Parkinson’s disease. https://clinicaltrials.gov/ct2/show/NCT01738178.
- [114].Fernandez HH, Greeley DR, Zweig RM, Wojcieszek J, Mori A, Sussman NM, Istradefylline as monotherapy for Parkinson disease: results of the 6002-US-051 trial, Park. Relat. Disord 16 (1) (2010) 16–20. [DOI] [PubMed] [Google Scholar]
- [115].Lewitt PA, Guttman M, Tetrud JW, Tuite PJ, Mori A, Chaikin PC, Sussman NM, Adenosine A(2A) receptor antagonist istradefylline (KW-6002) reduces "off" time in Parkinson’s disease: a double-blind, randomized, multicenter clinical trial (6002-US-005), Ann. Neurol. (2008). [DOI] [PubMed] [Google Scholar]
- [116].Parkinson Study Group STEADY-PD III Investigators, Isradipine versus placebo in early Parkinson disease: a randomized trial, Ann. Intern. Med. 172 (9) (2020) 591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Stephenson D, Hill D, Cedarbaum JM, et al. , The qualification of an enrichment biomarker for clinical trials targeting early stages of Parkinson’s disease, J. Parkinsons Dis 9 (3) (2019) 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Tavares AA, Batis JC, Papin C, et al. , Kinetic modeling, test-retest, and dosimetry of 123I-MNI-420 in humans, J. Nucl. Med. 54 (10) (2013) 1760–1767. [DOI] [PubMed] [Google Scholar]
- [119].Xu K, Bastia E, Schwarzschild M, Therapeutic potential of adenosine A(2A) receptor antagonists in Parkinson’s disease, Pharmacol. Ther. 105 (3) (2005) 267–310. [DOI] [PubMed] [Google Scholar]
- [120].Parkinson Study Group, Pramipexole vs levodopa as initial treatment for Parkinson disease: a randomized controlled trial. Parkinson Study Group, J. Am. Med. Assoc. 284 (15) (2000) 1931–1938. [DOI] [PubMed] [Google Scholar]
- [121].Abdolahi A, Scoglio N, Killoran A, Dorsey ER, Biglan KM, Potential reliability and validity of a modified version of the Unified Parkinson’s Disease Rating Scale that could be administered remotely, Park. Relat. Disord 19 (2) (2013) 218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Assessing Tele-health outcomes in multiyear extensions of PD trials (AT-HOME PD), https://clinicaltrials.gov/ct2/show/NCT03538262.
- [123].Schneider RB, Myers TL, Tarolli CG, et al. , Remote Administration of the MDS-UPDRS in the Time of COVID-19 and beyond [published online ahead of print, 2020 Jul 15], J. Parkinsons Dis (2020), 10.3233/JPD-202121. [DOI] [PubMed] [Google Scholar]
- [124].Espay AJ, Hausdorff JM, et al. , Sáanchez-Ferro Á, A roadmap for implementation of patient-centered digital outcome measures in Parkinson’s disease obtained using mobile health technologies, Mov. Disord. 34 (5) (2019) 657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Luan Yanan, Ren Xiangpeng, Zheng Wu, Zeng Zhenhai, Guo Yingzi, Hou Zhidong, Guo Wei, Chen Xingjun, Li Fei, Chen Jiang-Fan, Chronic Caffeine Treatment Protects Against α-Synucleinopathy by Reestablishing Autophagy Activity in the Mouse Striatum, Front. Neurosci 12 (2018) 301, 10.3389/fnins.2018.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]