Abstract
Reconsolidation may be a viable therapeutic target to inhibit pathological fear memories. In the clinic, incidental or imaginal reminders are used to safely retrieve traumatic memories of experiences that occurred elsewhere. However, it is unknown whether indirectly retrieved traumatic memories are sensitive to disruption. Here we used a backward conditioning procedure to indirectly retrieve and manipulate a hippocampus-dependent contextual fear engram in male rats. We show that conditioned freezing to a backward conditioned stimulus is mediated by fear to the conditioning context, activates hippocampal ensembles that can be covertly captured and chemogenetically activated to drive fear, and is impaired by post-retrieval protein synthesis inhibition. These results reveal that indirectly retrieved contextual fear memories reactivate hippocampal ensembles and undergo protein synthesis-dependent reconsolidation. Clinical interventions that rely on indirect retrieval of traumatic memories, such as imaginal exposure, may open a window for editing or erasing neural representations that drive pathological fear.
Reporting Summary:
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Cognitive behavioral therapies, such as prolonged exposure therapy, are widely used treatments for a number of debilitating fear-related and anxiety disorders1,2. Similar to extinction learning in rodents, prolonged exposure therapy attempts to extinguish maladaptive fear responses by exposing patients to trauma-related stimuli (often using imaginal exposure) in a safe environment. Despite efficacy in most patients, clinical interventions are nonetheless susceptible to relapse3. Accordingly, there is significant interest in developing therapeutic strategies that selectively target and eliminate traumatic fear memories.
Studies in rodents have shown that consolidated fear memories become labile upon retrieval and undergo a protein synthesis-dependent phase of reconsolidation4,5. Memory attenuated in this way may be less susceptible to relapse6 suggesting an effective therapeutic strategy to provide long-term relief7. Although reconsolidation-based therapies have high therapeutic potential8, translating findings from experimentally controlled situations to real-world clinical scenarios is a challenge. In animal models, for example, contextual fear memories are reactivated by direct exposure to shock-associated contexts9,10. In patients, however, these memories must be retrieved indirectly using trauma-related cues or imaginal exposure. Although the development of virtual reality exposure therapy holds promise for enhancing exposure-based treatment outcomes in humans11, a critical question is whether reactivation using indirect reminders yields episodic retrieval of traumatic fear memories that are sensitive to reconsolidation manipulations12,13.
To accomplish “covert” memory retrieval in rats, we utilized a backward (BW) fear conditioning procedure14,15. Critically, this procedure does not require returning the animal to the conditioning context in order to retrieve an aversive memory of that place. In this procedure, rats are placed into a novel chamber and presented with several trials in which an aversive footshock unconditioned stimulus (US) is immediately followed by the presentation of an auditory conditioned stimulus (CS). In this procedure, the CS does not become directly associated with the US, but nevertheless evokes conditioned fear (indexed by freezing behavior). It does so by reactivating a memory of the conditioning context and indirectly retrieving a memory of the aversive US16. Given the critical role for the hippocampus in contextual fear memory17, we hypothesized that a backward CS reactivates a contextual fear engram in the hippocampus in the absence of re-exposure to the conditioning context. This would allow for the capture and manipulation of an indirectly retrieved contextual fear memory, similar to the way in which a clinician might use an incidental reminder to facilitate the episodic recollection of a traumatic experience in the clinic.
Results
Effects of context extinction on fear to a forward or backward CS
To demonstrate that conditioned freezing to a backward CS is mediated by fear to the conditioning context, animals underwent forward or backward conditioning followed by extinction of the conditioning context (Fig. 1a). We hypothesized that context extinction would undermine freezing to the backward but not forward CS. During conditioning (Fig. 1b), all rats exhibited low freezing prior to the first trial, but showed increased freezing across the conditioning trials [repeated measures: main effect of trial; [F(4, 112) = 99.7, p < 0.0001]. On the following two days, half of the rats in each group were placed into the conditioning context (A; ‘Ext’), while the other half were simply exposed to a novel context (C; ‘No Ext’) for an equivalent amount of time. As expected, freezing behavior in rats exposed to the conditioning context was elevated initially and decreased across days; rats exposed to the neutral context showed low levels of freezing behavior in both sessions. A repeated measures ANOVA revealed a main effect of time [F(1, 28) = 14.4, p = 0.0007], a main effect of extinction procedure [F(1, 28) = 10.2, p = 0.003], and a significant time × extinction interaction [F(1, 28) = 14.6, p = 0.0007]. Importantly, there were no statistical differences between groups in average freezing during the second day of extinction (p’s > 0.11; Fig. 1b).
Figure 1: Conditioned freezing to a backward CS is mediated by a contextual fear memory and engages the dorsal hippocampus.
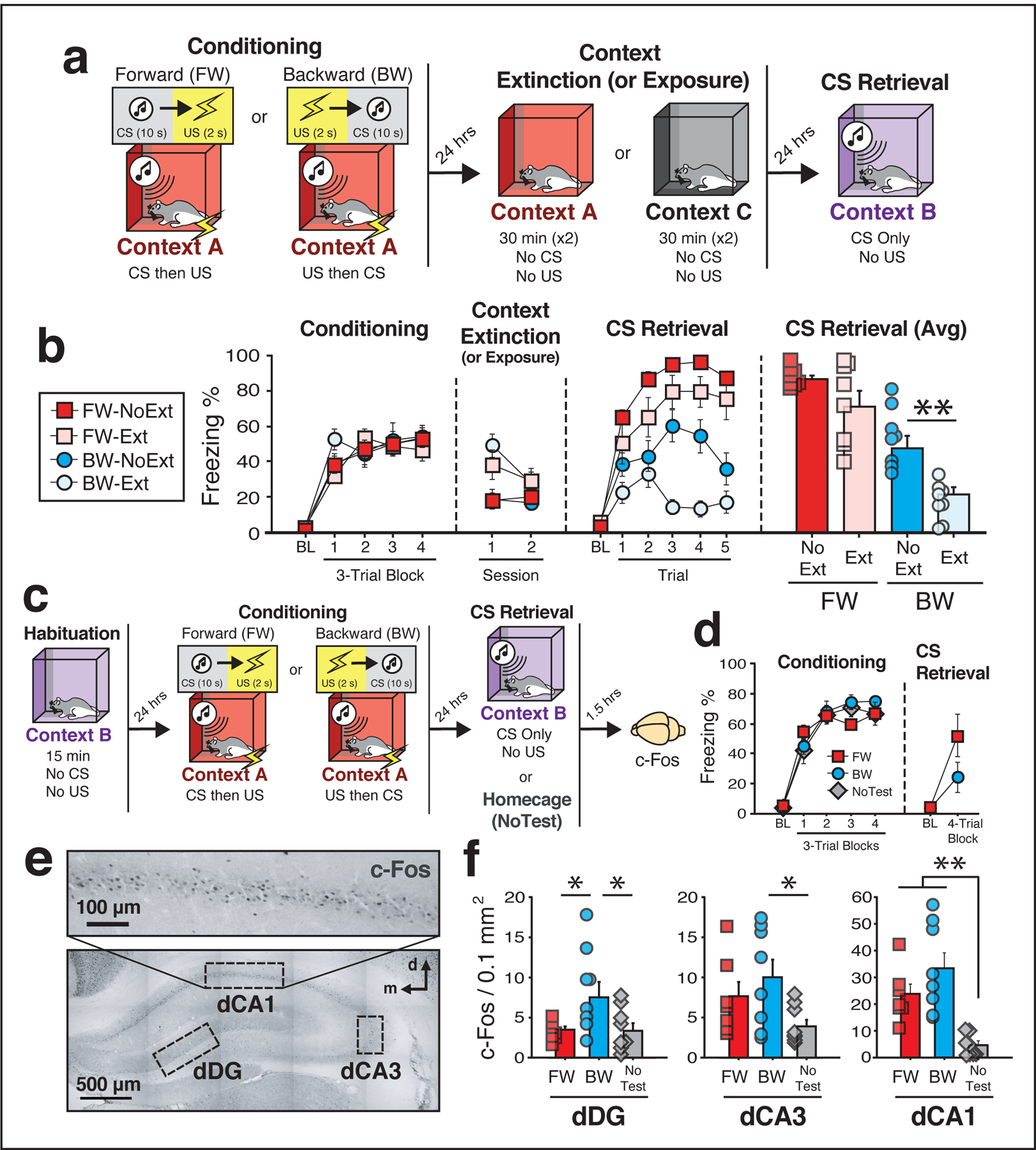
(a) Behavioral schematic. (b) Freezing behavior during conditioning, extinction, and retrieval testing. For conditioning, the left panel depicts mean percentage freezing for each group during the 5-min baseline period (BL) and across each conditioning block. For extinction, data are shown as the mean percentage freezing across the entire session for each day. For CS retrieval, data represent the mean percentage freezing during the 5-min BL and across each test trial (each trial was composed of a 10-s CS and a 60-s ISI). The right panel depicts average freezing across all test trials. Although extinction of the conditioning context did not significantly affect freezing to the FW CS, it significantly reduced freezing elicited by the BW CS (BW-NoExt vs. BW-Ext, p = 0.005), two-way analysis of variance (ANOVA) (repeated measures) followed by Bonferroni’s multiple comparisons post hoc test. Groups: [FW-NoExt (n = 8); FW-Ext (n = 8), BW-NoExt (n = 8), BW-Ext (n = 8)]. (c) Behavioral schematic. (d) Freezing behavior during conditioning and retrieval. For conditioning, the left panel depicts freezing during the 5-min baseline (BL) period and across each conditioning block. For retrieval, the right panel depicts average freezing across four retrieval trials (each trial composed of a 10-s CS and a 60-s ISI). Animals were sacrificed for c-Fos immunohistochemistry 90 min after the first retrieval trial. (e) Representative photomicrograph depicting c-Fos labeling and regions counted within the dHPC. (f) Mean c-Fos positive cells for each of the quantified regions (four to six images per subject; standardized to 0.1 mm2). In the CA1 region, presentation of either the BW or FW CS resulted in elevated levels of c-Fos expression relative to controls (BW vs. NoTest, p < 0.0001; FW vs. NoTest, p = 0.004), whereas in DG the BW CS resulted in increased levels of c-Fos relative to all other groups (BW vs. NoTest, p = 0.027; BW vs. FW, p = 0.037), one-way analysis of variance (ANOVA) followed by Fisher’s PLSD post hoc test. Groups: [FW (n = 7); BW (n = 8); NoTest (n = 8)]. All data are represented as means ± s.e.m.
Twenty-four hours after the final extinction session all rats were tested for conditioned freezing to the forward or backward CS (Fig. 1b). Analysis of freezing across the five test trials (excluding the baseline) revealed a main effect of trial [repeated measures: F(4, 112) = 8.04, p < 0.0001], a main effect of conditioning procedure [F(1, 28) = 54.3, p < 0.0001], and a main effect of extinction procedure [F(1, 28) = 12.3, p = 0.002]. Importantly, the analysis also yielded a significant trial × conditioning procedure × extinction procedure interaction [F(4, 112) = 2.82, p = 0.028], suggesting that the effects of context extinction differentially affect freezing to the backward and forward CSs. Indeed, post hoc comparisons revealed that extinction dramatically impaired freezing to the backward CS (p = 0.005; Fig. 1b), without affecting freezing to the forward CS. Together these data support the hypothesis that the expression of fear to a backward CS is mediated by the retrieval of a contextual fear memory.
Effects of CS exposure on c-Fos activity in the dHPC
Given that freezing to the backward CS is mediated by retrieval of a contextual fear memory, we asked whether the backward CS engages the dorsal hippocampus (dHPC), a brain region known to be important for both contextual fear and higher-order conditioning17. Three experimental groups were compared: rats conditioned and tested to a forward CS (‘FW’), rats conditioned and tested to a backward CS (‘BW’), and rats conditioned to either a forward or backward CS (evenly split) but remaining in their homecage during the retrieval session (‘NoTest’). Prior to conditioning, rats underwent a habituation session in what would later be the test context (see Fig. 1c for behavioral schematic). This session was conducted in an effort to bias c-Fos expression towards cells activated by CS retrieval rather than the test context. Twenty-four hours after habituation, rats underwent forward or backward conditioning in a distinct context. Freezing was low during the baseline period and increased significantly across the duration of the session [main effect of trial: F(4, 76) = 143.3, p < 0.0001; Fig. 1d]. Although the analysis revealed a significant trial × conditioning procedure interaction [F(4, 76) = 2.54, p = 0.047), post hoc comparisons indicated that there were no statistical differences between any of the groups across the conditioning session (p’s > 0.47). Twenty-four hours after conditioning rats received a retrieval test in a familiar, safe context; control rats (NoTest) remained in their homecage and were perfused alongside retrieval animals (Fig. 1d). During the retrieval test, freezing was low prior to the first trial and was significantly increased by CS presentation in both forward and backward conditioned rats [main effect of trial; repeated measures: F(1, 21) = 18.6, p = 0.0003; no other main effects or interactions (F < 2.98, p’s > 0.09)].
Ninety minutes after the retrieval test, rats were sacrificed and their brains processed for c-Fos immunohistochemistry; c-Fos-positive (c-Fos+) nuclei were counted in three dHPC subregions (Fig. 1e). As shown in Fig. 1f, presentation of either the forward or backward CS increased the number of c-Fos+ cells in the dHPC relative to NoTest controls. One-way ANOVAs comparing c-Fos counts within each region revealed significant main effects of group in dCA1 [F(2, 20) = 12.90, p = 0.0003], dDG [F(2, 20) = 3.61, p = 0.04], and a trend in dCA3 [F(2, 20) = 3.47, p = 0.051]. Within the dCA1, both the forward and the backward CS produced similar increases in the number of c-Fos+ cells relative to NoTest controls (BW vs NoTest, p < 0.0001; FW vs NoTest, p = 0.004), whereas within the dentate gyrus (DG) the backward CS produced greater increases in the number of c-Fos+ cells relative to all of the other groups (BW vs NoTest, p = 0.027; BW vs FW, p = 0.037; Fig. 1f). These findings reveal that the dHPC is engaged during expression of conditioned freezing, and that the DG may be preferentially engaged by the contextual memory retrieved by a backward CS.
Impact of CS exposure on c-Fos activity in a dHPC fear engram
An important question is whether presentation of the backward CS during a retrieval test reactivates DG cells active during backward conditioning. To examine this possibility, we infused the dHPC with a viral cocktail (AAV-Fos-tTA and AAV-TRE-hM3Dq-mCherry) to achieve activity-dependent expression of “designer receptors exclusively activated by designer drugs” (DREADDs; Fig. 2a–b). To restrict tTA-dependent expression of hM3Dq-mCherry to the conditioning session, rats were maintained on a doxycycline (DOX) diet until conditioning.
Figure 2: A backward CS results in the reactivation of a contextual fear engram.
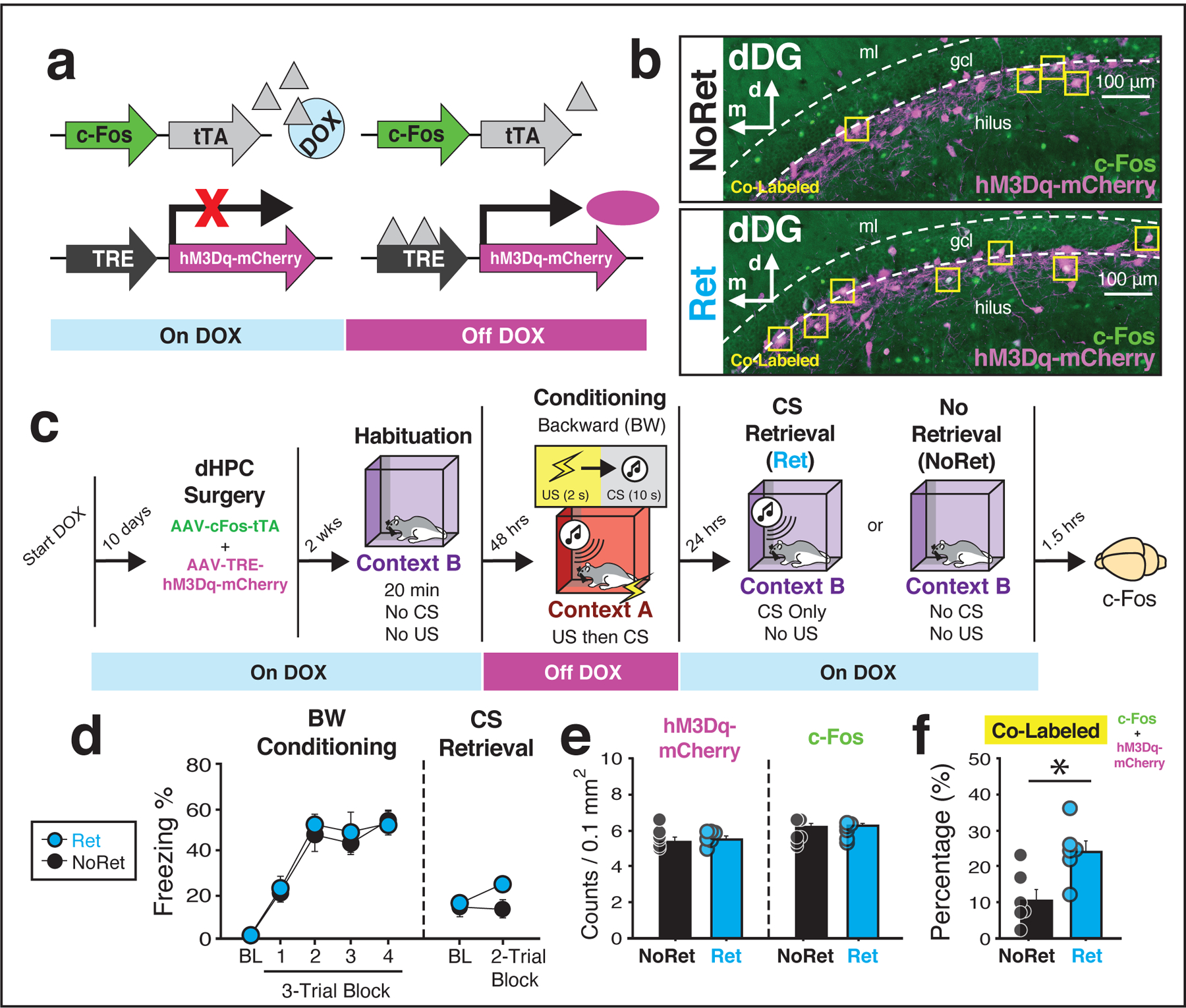
(a) Schematic of the viral strategy. (b) Representative images (20×) from the dentate gyrus (DG). Yellow squares indicate cells that are doubled-labeled for hM3Dq-mCherry (purple) and c-Fos (green). (c) Behavioral schematic. (d) For conditioning, the left panels depict the mean percentage of freezing behavior for each group during the 5-min baseline (BL) period and across each conditioning block. For retrieval, the right panel depicts average freezing during the 3-min baseline period and across the first two retrieval trials (each trial composed of a 10-s CS and a 60-s interstimulus interval) Note that while rats in the NoRet group did not receive any CS presentations, the 2-trial block is defined as an equivalent amount of time (i.e., 140 sec after the baseline or the equivalent of two CS trials). (e) Quantification of cells tagged during conditioning (mCherry+) and activated by the CS retrieval procedure (endogenous c-Fos; four to six images per subject; standardized to 0.1 mm2). (f) Although there were no differences between groups in the number of cells labeled during conditioning (mCherry+) or retrieval (c-Fos+), presentation of the BW CS resulted in significant increases in the proportion of double-labeled cells (NoRet vs. Ret, p = 0.012), one-way analysis of variance (ANOVA). Groups: [NoRet (n = 6); Ret (n = 6)].
Prior to conditioning, rats were given a brief exposure session in which they were habituated to the retrieval context and were immediately taken off DOX to open a cell labeling window for the conditioning session (see Fig. 2c for behavioral schematic). Two days later, all rats underwent BW conditioning and were immediately placed back on DOX. Conditioning was similar to previous experiments [main effect of trial: F(4, 40) = 71.5, p < 0.0001]; there were no other main effects or interactions (F’s < 0.29, p’s > 0.74; Fig. 2d). The next day, half of the rats were given a backward CS (‘Ret’) retrieval session to examine the extent to which cells activated within the DG during conditioning (mCherry) were reactivated by the presentation of the BW CS (overlapping endogenous c-Fos protein); the other half of rats served as controls and were simply exposed to the retrieval context for an equivalent amount of time. Note that animals did not receive drug injections for this test; hM3Dq-mCherry labeling was simply used as a proxy for dHPC activity at conditioning.
Analysis of freezing across the five-trial retrieval test (Fig. 2d) revealed no differences between groups [no main effect of group or trial × group interaction; (F’s < 1.89, p’s > 0.11)]. However, close inspection of the data revealed that average freezing across the first two trials was significantly elevated in rats that were presented with the CS [main effect of group; repeated measures: F(1, 10) = 4.97, p = 0.049]. Importantly, although we found no differences between groups in the overall number of cells activated by conditioning (hM3Dq-mCherry+) or CS retrieval (c-Fos+), rats that received backward CS presentations during the retrieval test displayed a significant increase in the percentage of cells that were double-labeled [Fig. 2b, e–f; factorial ANOVA: F(1, 10) = 9.53, p = 0.01]. This suggests that presentation of the backward CS resulted in the reactivation of neural ensembles within the DG that encode contextual representations during backward conditioning.
Chemogenetic activation of a covertly captured HPC ensemble
Collectively, these experiments suggest that the backward CS functions as an indirect retrieval cue to “covertly” reactivate a hippocampal-dependent contextual fear memory. If so, chemogenetic activation of a covertly captured HPC ensemble should be sufficient to drive conditional fear in a safe context, as has been demonstrated for direct reactivation of HPC ensembles18. Accordingly, rats were injected with the same viral cocktail described above to achieve DOX-regulated and c-Fos-dependent expression of the chemogenetic actuator hM3Dq-mCherry in the dHPC. Prior to conditioning, and while on the DOX diet, all rats were habituated to the retrieval context in an effort to minimize the animal’s tendency to generalize fear across contexts (Fig. 3a). The next day, all rats underwent backward conditioning. All groups exhibited reliable conditioning [main effect of trial; repeated measures: F(4, 144) = 145.3, p < 0.0001]. There were no other significant main effects or interactions (F’s < 1.8, p’s > 0.15). After conditioning, rats were immediately returned to their home cages and the DOX diet was replaced with normal chow.
Figure 3: Chemogenetic activation of a covertly captured hippocampal neural ensemble drives freezing behavior.
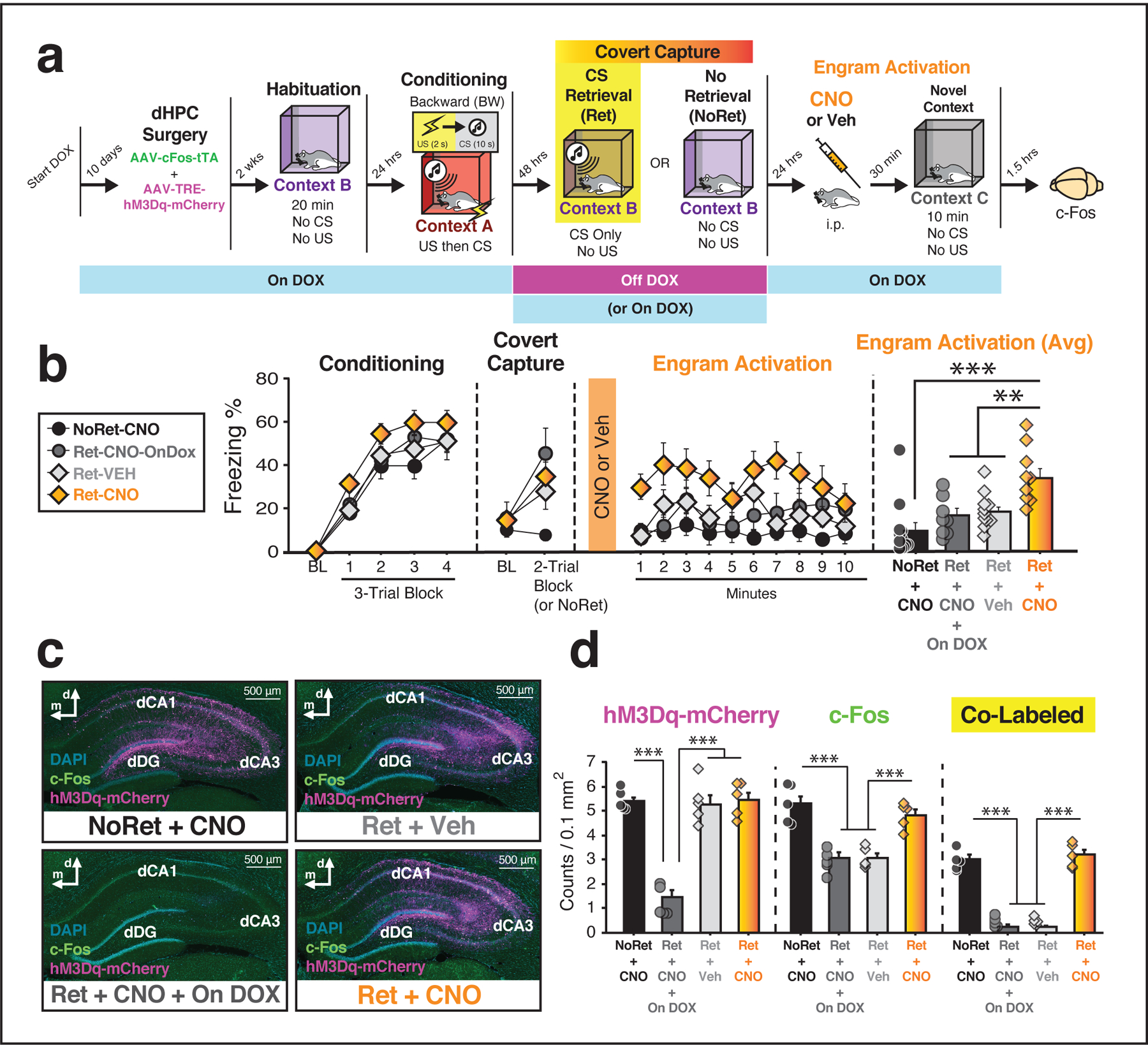
(a) Behavioral schematic. (b) Freezing behavior for conditioning, retrieval, and engram activation sessions. For conditioning, the panel depicts freezing during the 5-min baseline (BL) and across conditioning blocks. For retrieval (“covert capture”), the panel depicts average freezing during the 3-min baseline (BL) and average freezing across the first two retrieval trials (each trial consists of a 10-s CS and a 60-s ISI). During the covert capture session, animals were removed from the DOX diet to capture dHPC ensembles activated by presentation of the backward CS. During the test session (“engram activation”), systemic CNO administration increased freezing in Ret-CNO relative to all other groups. The right panel shows average freezing across the engram activation session for each group (Ret-CNO vs. Ret-VEH, p = 0.004; Ret-CNO vs. NoRet-CNO, p < 0.0001; Ret-CNO vs. Ret-CNO-OnDOX, p = 0.004), one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparisons post hoc test. Groups: [Ret-CNO (n = 9); Ret-VEH (n = 11); Ret-CNO-OnDOX (n = 8); NoRet-CNO (n = 12)]. (c) Representative images from each group showing expression of hM3Dq-mCherry; c-Fos expression was quantified in a random subset of animals. (d) Removal of DOX prior to CS retrieval resulted in robust expression of hM3Dq-mCherry relative to OnDOX controls [factorial ANOVA with Bonferroni multiple comparisons test: F(3, 16) = 41.57, p < 0.0001]. For animals that were taken off DOX, CNO administration prior to testing resulted in significant increases in c-Fos expression [factorial ANOVA with Bonferroni multiple comparisons test: F(3, 16) = 22.65, p < 0.0001]. Groups: [NoRet-CNO (n = 5); Ret-CNO-OnDOX (n = 5); Ret-VEH (n = 5); Ret-CNO (n = 5)]. All data are represented as means ± s.e.m. **, *** denote p < 0.005, p < 0.0001, respectively.
Two days later, rats were given a retrieval session in which they were presented with the backward CS to capture and tag active HPC ensembles; after the retrieval session they were immediately placed back on DOX. Analysis of freezing behavior across the five-trial retrieval session revealed a significant main effect of trial [repeated measures: F(5, 180) = 13.4, p < 0.0001], a significant main effect of group [F(3, 36) = 4.00, p = 0.015], and a significant trial × group interaction [F(15, 180) = 3.41, p < 0.0001]. Similar to our previous experiment, we found that freezing was maximal during the first two retrieval trials and was significantly elevated in rats that were presented with a CS [Fig. 3b; main effect of Ret vs NoRet; repeated measures: F(1, 38) = 11.7, p = 0.002]. Importantly, presentation of the backward CS increased hM3Dq-mCherry expression in animals removed from the DOX diet relative to control rats that remained on DOX throughout the duration of the experiment [main effect of group; factorial ANOVA: F(3, 16) = 41.55, p < 0.0001]. Post hoc analyses confirmed that rats that remained on DOX were statistically different than all other groups (p’s < 0.0001; Fig. 3c–d).
Twenty-four hours after retrieval session, rats received systemic injections of either VEH or the DREADD ligand, clozapine-N-oxide (CNO, 3 mg/kg), to activate the captured HPC ensemble; freezing responses were assessed during a 10-minute test session in a novel context. As shown in Fig. 3b, CNO increased freezing behavior in rats that received the backward CS off DOX (Ret-CNO) relative to all of the other control groups. A repeated measures ANOVA revealed a main effect of group [F(3, 36) = 7.94, p = 0.0003]; there were no other significant main effects or interactions (F’s < 1.6, p’s > .14). Post hoc comparisons confirmed that freezing behavior in the Ret-CNO group was significantly elevated relative to controls (p’s < 0.005). This indicates that chemogenetic reactivation of the HPC neuronal ensemble representing the contextual memory covertly retrieved by a BW CS is sufficient to drive conditional freezing.
As shown in Fig. 3d, mCherry labeling was increased in all animals undergoing a retrieval test off the DOX diet, independent of whether the BW CS was presented (Ret-CNO; Ret-VEH) or not (NoRet-CNO). This indicates that context-exposure alone was sufficient to drive activity-dependent expression of hM3Dq in the dHPC, and implies that this may have accounted for mCherry expression in the animals also presented with the BW CS. Moreover, CNO delivery increased c-Fos expression in both the NoRet-CNO and the Ret-CNO groups, as well as the total number of cells positive for both c-Fos and hM3Dq-mCherry (co-labeled) within the DG. Critically, however, only rats in the Ret-CNO group exhibited increased levels of freezing behavior after CNO administration. This suggests that cells tagged after presentation of the backward CS (Ret-CNO), but not mere placement in the retrieval context (NoRet-CNO), represented a contextual fear memory.
Inhibition of protein synthesis in the dHPC following retrieval of a forward or backward CS
These experiments support the hypothesis that a backward CS evokes freezing behavior by retrieving a hippocampus-dependent contextual fear engram. This suggests the backward CS serves as an indirect retrieval cue to covertly access a contextual fear memory in the HPC. Although directly reactivated contextual fear memories undergo a period of reconsolidation in which they are sensitive to protein synthesis inhibition, it is not known whether this is true for clinically relevant indirect retrieval procedures. To explore this question, rats were implanted with bilateral cannula targeting the dorsal DG and, after recovery, were subject to either forward or backward fear conditioning (Fig. 4a–c). During conditioning (Fig. 4d), freezing was low prior to the first trial and increased across the conditioning trials [main effect of trial: F(4, 196) = 213.68, p < 0.0001]; there were no other significant main effects or interactions (F’s < 2.09, p’s > 0.17). Next, rats underwent a retrieval session in which they were presented with the forward or backward CS to reactivate the fear memory and immediately thereafter received an intra-HPC infusion of the protein synthesis inhibitor rapamycin (1.5 μg/side) or VEH and were returned to their homecages. During the reactivation session (‘reactivation’; Fig. 4d), FW and BW groups differed in their levels of conditioned freezing. A repeated measures ANOVA revealed a main effect of trial [F(1, 49) = 115.5, p < 0.0001], a main effect of conditioning procedure [F(1, 49) = 8.36, p = 0.006] and a significant trial × conditioning procedure interaction [F(1, 49) = 23.2, p < 0.0001]. Post hoc analyses revealed that although there were no differences within the FW and BW groups (p’s > 0.31), rats that were conditioned to a forward CS showed increased average levels of freezing during the retrieval trials relative to groups conditioned to a BW CS (p = 0.0003).
Figure 4: The covert retrieval of a contextual fear memory results in labile memory trace that is vulnerable to disruption by protein synthesis inhibition.
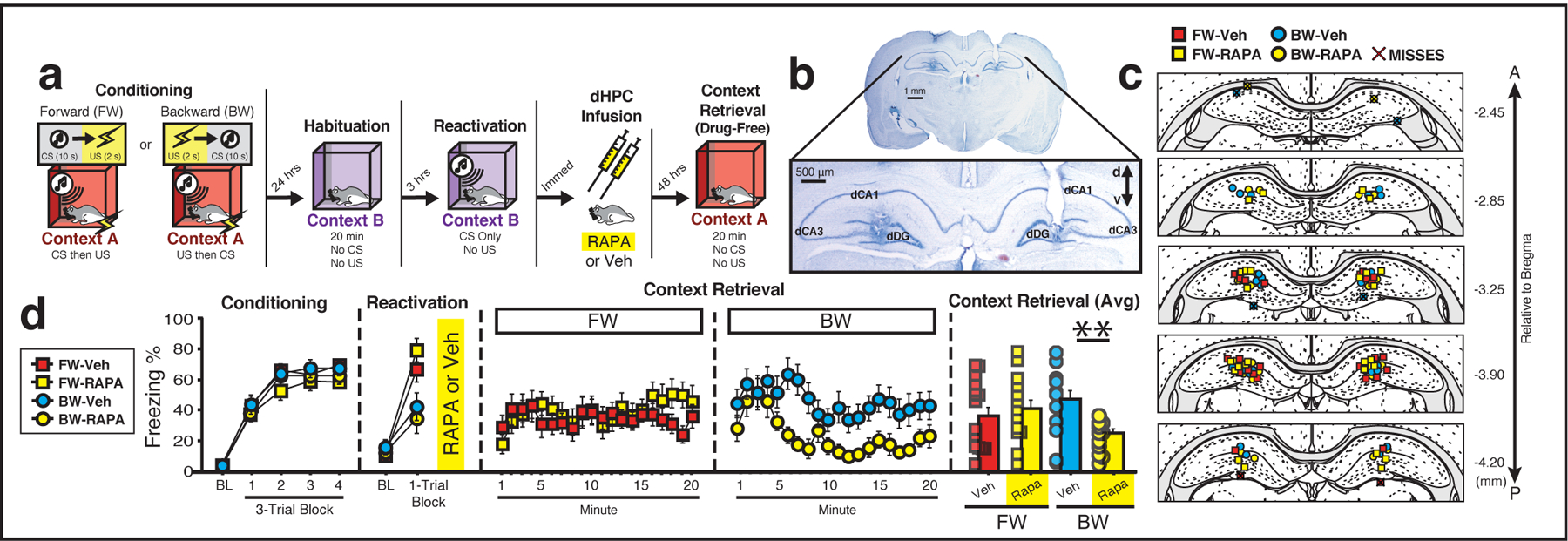
(a) Behavioral schematic. (b) Representative photomicrograph depicting bilateral dHPC cannula placements. (c) Documentation of cannula placements in the dHPC. Symbols denote the location of the injector tips of the cannula tracts for each animal for each group35. (d) Freezing behavior during conditioning, reactivation, and the context test. For conditioning, the left panel depicts the mean percentage freezing for each group during the 5-min baseline period (BL) and across each conditioning block. For reactivation, the panel depicts freezing during 3-min baseline (BL) period and across one retrieval trial (the trial consisted of one 10-s CS and the 60-s post-tone interval). Administration of rapamycin into the dHPC immediately after presentation of a BW, but not FW, CS impaired freezing behavior during the subsequent drug-free context test. The right panel depicts average freezing across the entire 20-min context test for each group (BW-VEH vs. BW-Rapa, p = 0.006), two-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparisons post hoc test. Groups: [FW-VEH (n = 15); FW-Rapa (n = 13); BW-VEH (n = 14); BW-Rapa (n = 11)]. All data are represented as means ± s.e.m.
Forty-eight hours later, freezing to the conditioning context was assessed in a 20-min test session. As shown in Fig. 4d, post-retrieval rapamycin infusions into the dHPC impaired contextual freezing in backward-, but not forward-conditioned, rats relative to VEH-treated controls. A repeated measures ANOVA revealed a significant main effect of time [F(19, 931) = 2.72, p = 0.0001], a significant time × conditioning procedure interaction [F(19, 931) = 2.42, p = 0.0006] and, importantly, a significant conditioning procedure × drug group interaction [F(1, 49) = 6.44, p = 0.01]. Post hoc analyses indicated that rapamycin reduced freezing in rats when administered after presentation of the BW (p = 0.006), but not forward CS (p = 0.52), relative to VEH-treated rats. Thus, presentation of the backward CS covertly retrieved a contextual fear memory that was sensitive to hippocampal protein synthesis inhibition. Importantly, this experiment demonstrates that contextual fear memory could be indirectly reactivated and attenuated without exposing the animals to the conditioning context. This suggests that therapeutic strategies that rely on indirect retrieval in a clinical setting may be viable therapeutic options for inhibiting pathological fear.
Discussion
Here we combined an innovative implementation of a classic behavioral procedure (backward conditioning) to investigate whether indirectly retrieved contextual fear memories within the HPC could be targeted and manipulated. We show that fear to a backward CS is mediated through the conditioning context and recruits hippocampal neurons to a greater degree than a forward CS. We also found that exposure to the indirect CS reinstated conditioning-related activity in a HPC ensemble. Moreover, HPC ensembles retrieved by the BW CS could be captured using activity-dependent expression of DREADDs and pharmacologically reactivated to drive freezing in a context never paired with shock. Lastly, we observed that intra-hippocampal protein synthesis inhibition disrupted the reconsolidation of a contextual fear memory retrieved covertly by the backward CS. In total, our work describes for the first time HPC representations for covertly retrieved memories and provides novel evidence that HPC engrams reactivated by covert retrieval cues are sensitive to protein synthesis inhibition.
Previous studies employing activity-dependent labeling strategies have shown that the reactivation of contextual fear engrams within the HPC is both necessary and sufficient for the expression of contextual fear18–21. However, in contrast to the current work, these studies have captured HPC ensembles during conditioning. Although this has been fundamental to our understanding of processes underlying memory encoding and retrieval22–25, it does not inform clinical interventions for pathological fear memories in individuals that have prior histories of trauma. Accordingly, a critical question is whether retrieval methods used to facilitate episodic recollection of trauma in a clinical setting result in the reactivation of neuronal populations that encoded the initial trauma. This is particularly relevant to studies of reconsolidation, in which neural manipulations target the physical memory trace. Here we show that covert retrieval of a contextual fear memory results in the reactivation of a contextual fear engram and that the chemogenetic activation of this ensemble supports conditioning-related behavior in a neutral context. Moreover, reconsolidation of this indirectly retrieved memory could be disrupted by hippocampal protein synthesis inhibition. Thus, a critical finding from the current study is that indirect retrieval of a contextual fear memory permits the reactivation and attenuation of a hippocampal engram representing that memory.
Although our results suggest that clinical interventions that rely on indirect retrieval methods (such as imaginal exposure) may be effective for opening a window to modify, edit or erase neural representations of unwanted traumatic fear memories, an important question is whether indirectly reactivated memories are sensitive to amnesic agents during reconsolidation26. Given that memories integrate into complex associative structures (including outside the hippocampus), it is unclear if the reactivation of one element of the associative network results in the reactivation of other parts of the associative network in a way that renders them sensitive to reconsolidation manipulations. Indeed, a previous study using second-order conditioning procedures with discrete CSs found that directly—but not indirectly—reactivated fear memories undergo reconsolidation within the amygdala27. However, here we report that reconsolidation of an indirectly retrieved contextual fear memory is disrupted by hippocampal protein synthesis inhibition. This is consistent with previous work showing that presentation of a trace-conditioned CS also renders an associated contextual fear memory sensitive to hippocampal protein synthesis inhibition13. Although we did not explore whether amygdala protein synthesis is necessary for reconsolidation of fear to a backward CS, these results suggest that the HPC may have a privileged role in this process, which is consistent with its proposed role in episodic memory.
Lastly, although the ultimate goal of reconsolidation-based therapies is to erase traumatic memories, several studies have demonstrated that retrograde amnesia produced by protein synthesis inhibitors is either transient or recoverable28–32. For instance, a recent study found that systemic administration of a protein synthesis inhibitor after a contextual fear conditioning resulted in robust impairments in the expression of that memory that could be recovered by artificial (e.g., optogenetic) activation of the contextual fear engram within the HPC29,30. Based on these results the authors suggest that although the time-limited protein synthesis following learning is dispensable for memory storage, it may be required for effective memory retrieval processes. Although we found that intra-hippocampal rapamycin impaired reconsolidation of a covertly retrieved context memory, it is possible that this reflects a retrieval deficit, as opposed to memory erasure. Indeed, recent reports have challenged the idea that contextual and auditory fear memories in rats undergo protein synthesis-dependent reconsolidation33,34. Indeed, we observed spared freezing in rapamycin-treated rats during the early portions of the context test in the current study (Fig. 4d). However, it is possible this reflects an incomplete attenuation of protein synthesis within the dHPC or sparing of engram ensembles outside of the dHPC (including extra-hippocampal regions). Whether this is true for older memories that are less dependent on the hippocampus10,24 is an important avenue for future work.
In conclusion, our results reveal that indirect retrieval of a contextual fear memory results in a labile memory trace in the hippocampus that is vulnerable to disruption. This process may contribute to the efficacy of clinical interventions, such as imaginal exposure, that rely on indirect retrieval and manipulation of traumatic memories. Developing retrieval-based behavioral or neural interventions that target hippocampal ensembles may prove particularly effective in attenuating traumatic fear memories humans.
Materials and Methods
Subjects
Adult experimentally naïve male Long-Evans rats (200 – 240 g upon arrival; 10 – 12 weeks old) were obtained from a commercial supplier (Envigo) and used for all experiments. Rats were individually housed in clear plastic cages on rotating racks in a climate-controlled vivarium with a fixed 14/10 hour light/dark cycle (lights on at 7:00 AM) and were given access to standard rodent chow (with exception of the reactivation experiments, see below) and water ad libitum. All experiments were conducted during the light phase. Upon arrival, all rats were handled by the experimenter (~30 sec/rat/day) for a minimum of 5 days prior to the start of any surgical or behavioral procedures. All experimental procedures were conducted in accordance with the US National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the Texas A&M University Institutional Animals Care and Use Committee (IACUC).
Viruses and Drugs
Plasmids were a generous gift from the laboratory of Dr. Susumu Tonegawa and were packaged at the University of Pennsylvania Vector Core. From these plasmids, and only for the activity-dependent cell labeling experiments, rats received a 50:50 viral cocktail containing AAV9-TRE-hM3Dq-mCherry-rBG (titer: ≥ 5 × 1013 GC/mL) and AAV9-cFos-tTA-bGH (titer: ≥ 5 × 1013 GC/mL) as described below. Clozapine-N-oxide (CNO) was provided by the Chemical Synthesis and Drug Supply Program of the National Institute of Mental Health (NIMH). Rapamycin was obtained from LC Laboratories. Doxycycline-containing rodent chow (DOX; 40 mg/kg) was obtained from Envigo. For the tagging experiments (described below), subjects were fed the DOX diet for at least 10 days prior to any surgical procedure.
Surgeries
For all surgeries, rats were anesthetized with isoflurane (5% for induction, 1–2% for maintenance) and placed into a stereotaxic frame (Kopf Instruments). The hair on the scalp was shaved, povidine-iodine was applied to the skin, and a small incision was made in the scalp to expose the top of the skull. The skull was leveled by placing bregma and lambda in the same horizontal plane.
For experiments involving activity-dependent cell labeling, rats received bilateral infusions of the viral cocktail (described above) into the dHPC (same coordinates as above; 700 nL total infusion volume/hemisphere) using a microinfusion pump (KD Scientific). Specifically, 10-μl syringes (Hamilton) were mounted on the microinfusion pump; polyethylene tubing (PE-20; Brain Tree Scientific) connected the syringe to stainless steel injection needles (26 gauge) that were backfilled with the viral cocktail immediately prior to injection. Virus was infused at a rate of 100 nL/min and injector tips were left in the brain for five additional minutes to allow for diffusion. After the infusion procedure, the incision was closed with sutures and post-operative procedures were conducted as described above. Rats were given a two-week recovery period after surgery and prior to behavioral testing to allow for viral infection.
For experiments involving intracranial microinfusions of rapamycin, small holes were drilled into the skull for placement of two to three anchoring screws. Bilateral stainless-steel guide cannulas (5 mm; 26 gauge; Plastics One) were inserted into the dorsal hippocampus (dHPC) at the following coordinates (relative to bregma): anteroposterior (A/P), −3.5 mm; mediolateral (M/L), ±2.45 mm; dorsoventral (D/V), −3.0 mm (relative to dura). Dental cement was used to secure the guide cannulas to the skull. Stainless-steel dummy guides (5 mm; 31 gauge; Plastics One) were inserted into the guide cannulas. Topical antibiotic (Triple Antibiotic Plus; G&W Laboratories) was applied to the surgical site and one chewable carprofen tablet (2 mg; Bio-Serv) was provided for post-operative pain management. Rats were given a minimum of one week to recover prior to the beginning of behavioral testing.
Drug Injections
For post-reactivation dHPC microinfusions, rats were transported from the behavioral testing room to an adjacent infusion room and the dummy guides were removed from the guide cannula. Stainless steel injectors (5 mm, 33 gauge) were connected to polyethylene tubing (PE-20; Brain Tree Scientific); the other end of the tubing was connected to a 10 μl syringe (Hamilton) which was mounted on an infusion pump (KD Scientific). Rapamycin (LC Laboratories) was dissolved in 100% DMSO to a concentration of 5μg/μl36 and rats received bilateral infusions (0.3 μl/hemisphere) of rapamycin or vehicle (100% DMSO) at a rate of 0.275 μl/min. Injectors remained in the guide cannulas for 1 min after the infusion to allow for diffusion of drug and rats were immediately transported back the their home cages following the infusion process.
Behavioral Apparatus
All behavioral experiments were conducted within two distinct rooms within the laboratory. Each room housed 8 identical rodent conditioning chambers (30 × 24 × 21 cm; Med Associates). Each chamber was housed in a larger external sound-attenuating cabinet and consisted of two aluminum sidewalls and a rear wall, ceiling, and a hinged front door made from Plexiglas. The grid floor consisted of 19 stainless steel rods that were wired to a shock source and solid-state grid scrambler for delivery of the footshock US (Med Associates). Each chamber contained a 15-W house light and ventilation fan to provide ambient background noise (~60 dB). Digital cameras were mounted above each chamber for visual recording and observation of behavior. Cues were manipulated to generate three distinct contexts. For context A, the house light was turned off and the overhead white lights and ventilation fans were turned on. Cabinet doors remained open for the duration of each session. Chambers were wiped with 1.0 % ammonium hydroxide prior to each behavioral session. Rats were transported to context A in black plastic boxes. For context B, house lights were turned on, fans were turned off, and the room was dimly lit by overhead fluorescent red lights. Cabinet doors remained closed for the duration of each behavioral session. Black Plexiglas floors were placed over the grid and each chamber was wiped down with a 3.0 % acetic acid solution prior to each behavioral session. Rats were transported to context B in white plastic boxes with a clean layer of bedding. For context C, both the house light and overhead white lights were turned on, fans were turned on, and cabinet doors remained open. Chambers were wiped with 70% ethanol prior to each behavioral session and rats were transported to context C in white plastic boxes with a clean layer of bedding.
For unbiased measurements of freezing behavior, each behavioral chamber rested on a load cell platform used to detect chamber displacement in response to each rat’s motor activity37. During behavioral testing, load-cell values (ranging from −10 to +10 V) were recorded and digitized at 5 Hz using Threshold Activity software (Med Associates). These values were then transformed to generate absolute values ranging from 0 to 100 with lower values indicating less cage displacement. Freezing was quantified by computing the number of observations for each rat that had a value less than the freezing threshold (load-cell values of 10 or less) for a minimum of 5 consecutive observations (1 s or more).
Histological Procedures
Upon completion of the experiment, rats were overdosed with sodium pentobarbital (Fatal Plus; 100 mg/mL, 0.5 mL, i.p.) and perfused transcardially with physiological saline followed by 10% formalin. Brains were extracted and stored overnight (at 4° C) in 10 % formalin after which they were transferred to a 30 % sucrose solution for a minimum of 3 days. After fixation and cryoprotection, brains were flash frozen on dry ice and sections were collected using a cryostat (Leica Microsystems) at −20° C. To verify the activity-dependent expression of hM3Dq-mCherry, coronal sections underwent fluorescent immunostaining (below) to visualize the localization and extent of mCherry expression in the dHPC.
For behavioral experiments involving c-Fos quantification (but in the absence of surgical procedures), coronal section (40 μm) containing the dHPC were collected into well plates containing phosphate buffered saline (1× PBS, 7.4 pH) with 0.01% sodium azide and stored at 4° C until immunohistochemistry was performed. Identical procedures were used for experiments involving viral manipulations, however 30 μm coronal sections of the dHPC were collected.
For cannula experiments, coronal sections (40 μm) were dry mounted on subbed microscope slides and stained with thionin (0.25 %) for cannula tract visualization. Specifically, tissue slides were submerged for 5 min each in 95% EtOH and 100% EtOH, followed by 10 min of submersion in CitriSolv (Fisher Scientific). Mounted tissue was then submerged in 100% EtOH (3 min), 95% EtOH (2 min), 70% EtOH (2 min), dH2O (2 min), followed by 0.25% thionin for ~15 sec. The tissue was then rinsed in dH2O, followed by submersion in 70% EtOH and 0.01% acetic acid (1 min), 70% EtOH (1 min), 95% EtOH (2 min; twice), 100% EtOH (2 min, twice), before submersion in CitriSolv for 10 min prior to cover slipping. Glass coverslips were mounted on slides using Permount mounting medium (Fisher Scientific) and coronal sections were imaged at 10× using a Leica Microscope (MZFLIII) with Leica Firecam software.
Immunohistochemistry
For immunohistochemistry to detect c-Fos, slices were first rinsed three times in Tris-buffered saline (TBS; 1×, 7.4 pH). All rinses were ~30 sec each; each step was done at room temperature and on a plate shaker. Tissue was transferred across wells using mesh well inserts. The tissue was then placed in 0.3 % H2O2 (in TBS) for 15 min followed by three rinses in TBS. Sections were then incubated overnight in primary antibody [rabbit anti-c-Fos, 1:10,000; Millipore, ABE457 (Antibodyregistry.org: AB_2631318)] in TBS containing Tween-20 (TBST). The next day, sections were rinsed three times in TBS and then transferred to secondary antibody for 1 hr [biotinylated goat anti-rabbit, 1:1000 in TBST; Jackson Laboratories, Code No 111-065-003, (Antibodyregistry.org: AB_2337959)]. After three more rinses in TBS, the tissue was incubated in avidin biotin complex (ABC, 1:1000 in TBST; Vector Labs) for 45 min. After three washes in TBS, sections were then transferred to wells containing 3, 3’ diaminobenzidine [(DAB) 5% stock, 1:200], nickel ammonium sulfate (5% stock, 1:10), and 30% H2O2 (1:2,000) in TBS for 10 minutes to generate chromophore products. Finally, tissue was rinsed three more times in TBS, mounted on subbed slices and coverslipped with Permount mounting medium (Fisher Scientific).
For fluorescent immunostaining, slices were first rinsed three times (10 min/wash) in 1× PBST (PBS with 0.1% Triton-X; pH 7.4) and then placed in 10 % normal donkey serum (NDS in PBST) for one hour. All steps occurred at room temperature and on a plate shaker, unless stated otherwise. Tissue was transferred using mesh well inserts. Slices were then incubated with one or more primary antibodies (1:500 dilution in PBS) at room temperature for 24 hrs [guinea pig anti-c-Fos, Synaptic Systems, Cat No 226 005 (Antibodyregistry.org: AB_2800522); rabbit anti-RFP, Rockland, Cat No 600-401-379 (Antibodyregistry.org: AB_2209751)]. The next day, slices were again rinsed in PBS-T three times and then incubated with one or more secondary antibodies (1:500 dilution in PBS) for two hours at room temperature in 1 % NDS in PBST [Alexa Fluor 488 donkey anti-guinea pig, Jackson ImmunoResearch, Cat No 706-545-148 (Antibodyregistry.org: AB_ 2340472); Cy3 donkey anti-rabbit, Jackson ImmunoResearch, Cat No 711-165-152 (Antibodyregistry.org: AB_2307443)]. After a final rinse in PBS, stained brain sections were then wet-mounted on gel-subbed slides and coverslipped with DAPI-containing fluoromount mounting medium (Invitrogen).
Image Analysis
All imaging and cell counting was conducted by experimenters that were blind to group assignments. For c-Fos DAB quantifications, four to six brightfield images (20×) of the bilateral dHPC were taken at different A/P levels (ranging from approximately −2.85 mm to −4.6 mm relative to bregma) using a Zeiss microscope and Axio Imager software (Zen Pro 2012). Counts were confined to the following areas of interest: (1) dorsal DG ‘dDG’ (area of 619 μm × 247 μm, positioned at the middle of the upper blade of the dDG), (2) dorsal CA3 ‘dCA3’ (an area of 247 μm × 371 μm, positioned with its midpoint at the center of dCA3), and (3) dorsal CA1 ‘dCA1’ (an area of 774 μm × 247 μm, positioned in the middle of dCA1). The number of c-Fos+ cells within each area for each image were counted, averaged and divided by the surface area (standardized to 0.1 mm2). ImageJ software was used for c-Fos counting38.
For fluorescent viral expression and c-Fos quantification, four to six fluorescent images were taken at different A/P levels (ranging approximately from −2.85 to −4.60 mm relative to bregma) at 20× magnification [Fig. 2b, dDG: 676 μm × 307 μm; Fig. 3c, dDG: 845 μm × 404 μm] using a Zeiss microscope and Axio Imager software (Zen Pro 2012). ImageJ software was used to count cells38. The number of c-Fos+, mCherry+, and co-labeled cells for each image were averaged and divided by the surface area (standardized to 0.1 mm2), unless stated otherwise.
Statistics
All data were analyzed using conventional parametric statistics (Statview; SAS Institute). Two-way ANOVA and repeated-measures ANOVA were used to assess main effects and interactions (α = 0.05). For post hoc group comparisons involving three means, Fisher’s protected least significant differences (PLSD) was used; for group comparisons involving four or more means, Bonferroni’s post hoc test was used. The distribution of the data was assumed to be normal, but this was not formally tested. No statistical methods were used to pre-determine group sizes; group sizes were determined based on prior work and what is common in the field14,30,39,40. All data are represented as means ± s.e.m.
Behavioral Procedures
Overviews of each behavioral experiments are provided in the figures. In all experiments, the conditioned stimulus (CS) was an auditory tone (80 dB, 2 kHz, 10 sec) and the unconditioned stimulus (US) was a scrambled footshock (2 sec, 1 mA) delivered through the grid floor. During behavioral testing, the experimenters were not blind to group assignments, however all freezing data were collected using an unbiased data acquisition system (Threshold Activity, described above).
Effects of context extinction on freezing to a forward or backward CS.
In a 2 × 2 design, rats (n = 32, no exclusions) were randomly assigned to receive forward (FW) or backward (BW) conditioning procedures (day 1). After conditioning, rats were either returned to the conditioning context (‘Ext’) or were simply exposed to a novel context alone (‘NoExt’) for an equivalent amount of time (days 2 and 3) prior to a CS retrieval test (day 4). This design resulted in the following group numbers [BW-NoExt (n = 8); BW-Ext (n = 8); FW-NoExt (n = 8); FW-Ext (n = 8)]. For conditioning, FW- and BW-conditioned rats were run in alternating squads; extinction assignments were counterbalanced for chamber position in all sessions. For FW conditioning, rats were placed into the conditioning context (A) and, following a 5-min baseline period, were presented with twelve CS-then-US trials (CS offset immediately preceded US) each separated by a 58-sec inter-stimulus interval (ISI). Rats remained in the chamber for one minute after the last trial at which time they were returned to their homecages. Backward conditioning was conducted in an identical fashion with the exception that the arrangement of the CS and US were switched such that CS presentation immediately followed the delivery of the US (i.e., US-then-CS).
For context extinction or novel context exposure, rats in both the BW and FW groups were exposed to either the conditioning context (A; ‘Ext’) or a novel context (C; ‘NoExt’) for thirty minutes/day for two consecutive days. No stimuli were presented during these sessions and rats were immediately transported back to their home cages following each session.
Twenty-four hours after the last extinction session all rats underwent a CS retrieval test. Rats were transported from the vivarium to context B and received five presentations of the CS (in the absence of the US) after a 5-min baseline; each CS presentation was separated by a 60-s ISI. Rats remained in the chamber for 1 min after the last CS presentation, at which point they were removed and returned to their home cages.
Effects of CS exposure on c-Fos activity in the dHPC.
Rats (n = 24, before exclusions) were randomly assigned to receive a forward (FW)-or backward (BW)-conditioned CS at testing, or no CS retrieval at test (NoTest). The NoTest group was divided such that half of the rats in that group received FW conditioning, while the other half received BW conditioning. One rat was excluded from the analysis due to poor tissue quality. This resulted in the following group numbers: FW (n = 7); BW (n = 8); NoTest (FW-conditioned: n = 4; BW-conditioned: n = 4).
One day prior to conditioning all rats were given a 15-min exposure session to what would be the retrieval context (context B). For conditioning, all rats (in squads of eight, groups intermixed) were transported to context A and received either FW or BW conditioning as described above. Twenty-four hours after conditioning, rats in the FW and BW groups were transported from the vivarium to a neutral context B and after a 3-min baseline period were presented with four CS-alone trials. Each CS presentation was separated by a 60-s ISI and rats remained in chamber for one minute after last CS presentation before being transported back the vivarium. Rats were perfused ninety minutes after the first CS of the test. Rats in the NoTest group (with FW- and BW-conditioned animals intermixed) were not given a CS retrieval session but were perfused alongside groups of rats in the FW and BW groups.
Effect of backward CS exposure on c-Fos activity in a HPC fear engram.
All rats (n = 14, before exclusions) were given a 20-min exposure session to what would be the retrieval context (B). After the exposure session, all rats were taken off DOX and 48 hours later received BW conditioning in context A as described above. Immediately after conditioning, animals were placed back on the DOX diet to prevent further labeling. Twenty-four hours later, half of the rats were randomly assigned to received five CS-only presentations, while the other half of rats were simply exposed to the same context for an identical amount of time. Note that groups were run in different (alternating) squads. Ninety minutes after the first CS presentation of the retrieval session, rats were sacrificed for c-Fos/mCherry immunohistochemistry. Although NoRet rats did not receive CS presentations, they were perfused at an equivalent time point as rats in the Ret group. Lastly, two rats were excluded due to poor viral infection and expression resulting in the following group numbers: Ret (n = 6); NoRet (n = 6).
Chemogenetic activation of a covertly captured HPC neuronal ensemble.
After an exposure session (day 1), all rats (n = 64, prior to exclusions) were received BW conditioning (day 2) and 48 hrs later were given a retrieval session in which they were presented with the BW CS to label and capture putative engram cells in the dHPC (day 5). The next day we examined the impact of chemogenetic engram cell activation on freezing responses in a novel context during a 10-min test session (day 6).
For the exposure session, rats were transported from the vivarium and placed into context B for 20 min; no additional stimuli were presented during this session. This session was conducted in an effort to bias cell labeling during the subsequent capture session to the backward CS presentation, rather than context B itself. The next day, rats received BW conditioning as described above. Immediately after conditioning, rats were taken off DOX (replaced with standard chow) for 48 hours to open a labeling window for cell tagging. In addition, we included a control group that remained on DOX throughout the duration of the experiment (‘OnDOX’); note that all rats were randomly assigned to the experimental and control groups prior to the start of behavioral testing. For the activity-dependent capture session, groups of rats were placed into context B and after a 3-min baseline period received five CS presentations each separated by a 60-s ISI (‘Ret’). Rats remained in the chamber for 1 min after the last CS presentation at which time they were returned to their homecages. A control group was included that was exposed to context B for an equivalent amount of time, but did not receive any CS presentations (‘NoRet’). After being returned to their homecages, all rats were immediately placed back on the DOX diet to prevent further cell labeling. Twenty-four hours after cell labeling, rats were injected with CNO (3 mg/kg, i.p.) or VEH and were placed into a novel context C to assess whether reactivation of the tagged BW CS cell ensemble was sufficient to drive conditioned freezing. Lastly, 90 min after testing, a random subset of rats from each group was sacrificed for quantification of c-Fos and mCherry expression [Ret-CNO (n = 5); Ret-CNO-OnDOX (n = 5); Ret-VEH (n = 5); NoRet-CNO (n = 5)]. In addition, histological verification of activity-dependent hM3Dq-mCherry expression in all rats was performed as described above.
During the experiment, one rat became ill and was immediately euthanized [Ret-CNO (n = 1)], and any animal (aside from OnDOX animals) that did not exhibit bilateral expression of mCherry in the dHPC was excluded from the analysis [Ret-CNO (n = 4); Ret-VEH (n = 4); NoRet-CNO (n = 2)]. Lastly, several rats in the NoRet group (n = 4) exhibited high levels of freezing behavior during the capture session (> 25%), suggesting that contextual fear had generalized to the retrieval context, at least in these animals. These animals were excluded from the analyses to ensure that we did not inadvertently capture a generalized context fear memory in the NoRet animals. This resulted in the following final group numbers for the behavioral experiment: NoRet-CNO (n = 12); Ret-VEH (n = 11); Ret-CNO (n = 9); Ret-CNO-OnDOX (n = 8). This behavioral experiment was performed in two replications with similar outcomes in each and were therefore combined for statistical analysis.
Inhibition of protein synthesis in the dHPC after retrieval of a forward or backward CS.
In a 2 × 2 design rats (n = 64, prior to exclusions) were randomly assigned to receive either FW or BW fear conditioning (day1); infusion of the protein synthesis inhibitor rapamycin (‘RAPA’) or it’s vehicle (‘VEH’) were given immediately following a single CS retrieval session (day 2) and contextual fear responses were subsequently examined in a drug-free test session (day 4). During the experiment, two rats had their headcaps come loose; they were sacrificed and excluded (n = 1, FW-RAPA; n = 1, BW-RAPA). Three additional rats did not complete the study due to illness (n = 1, FW-RAPA; n = 1, BW-RAPA; n = 1, BW-VEH). Lastly, technical errors during the infusion procedure (n = 1, FW–RAPA; n = 1, BW-RAPA) and off-target cannula placements outside of the dHPC (n = 1, FW–VEH; n = 1, BW–RAPA; n = 1, BW–VEH) resulted in the following group numbers: FW-VEH (n = 15); FW-RAPA (n = 13); BW-VEH (n = 14); BW-RAPA (n = 11). Note that one additional rat in the BW-RAPA group was marked as an outlier (± 2 standard deviations from the group mean) during the context test and was removed from analysis (the above group sizes reflect this).
For conditioning, rats were transported from the vivarium to context A and received either FW or BW conditioning in alternating squads; chambers were counterbalanced for drug assignments in all sessions. Twenty-four hours after conditioning (day 2), rats were given a 20-min exposure session to the retrieval context (B) in the absence of the CS or the US. This exposure session was conducted to reduce any fear that may have generalized across contexts and to ensure that drug manipulations following the subsequent retrieval session were molecular events associated with the reconsolidation of the CS-evoked memory. After exposure (later that same day), FW and BW rats (intermixed in each squad) were returned to the retrieval context (B) and presented with a single CS after a 3-min baseline period. The rats remained in the chamber for 1 min (4 min and 10 sec for entire session) after which they were immediately transported to an adjacent room and received intra-DG infusions of either RAPA or VEH. Rats were returned to their homecages immediately after the infusion process.
Forty-eight hours after drug infusion, rats were returned to the conditioning context (A) for a 20-min context test. No additional stimuli were presented during this session and rats were transported to the vivarium following the conclusion of the test. Note that this behavioral experiment was performed in two replications with similar outcomes in each and were therefore combined for statistical analysis.
Supplementary Material
Acknowledgments:
We thank S. Tonegawa for kindly providing pAAV.TRE.hM3Dq.mCherry and pAAV.cFos.tTA plasmids. We also thank J. Liu and A. Martinez for technical assistance, and S. Ramirez and A. Milton for their helpful reviews of the manuscript. This work was supported by NIH grants F31MH107113 (TDG), R01MH065961 and R01MH117852 (SM), and a Brain & Behavioral Research Foundation Distinguished Investigator grant (SM).
Footnotes
Conflict of interest: The authors declare no competing interests.
Data availability statement: The data that support the findings of this study are available from the corresponding author upon request.
References
- 1.Craske MG, Treanor M, Conway CC, Zbozinek T & Vervliet B Maximizing exposure therapy: an inhibitory learning approach. Behav. Res. Ther 58, 10–23 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNally RJ Mechanisms of exposure therapy: how neuroscience can improve psychological treatments for anxiety disorders. Clin. Psychol. Rev 27, 750–759 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Vervliet B, Craske MG & Hermans D Fear extinction and relapse: state of the art. Annu. Rev. Clin. Psychol 9, 215–248 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Nader K, Schafe GE & Le Doux JE Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406, 722–726 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Przybyslawski J & Sara SJ Reconsolidation of memory after its reactivation. Behav. Brain Res 84, 241–246 (1997). [DOI] [PubMed] [Google Scholar]
- 6.Duvarci S & Nader K Characterization of fear memory reconsolidation. J. Neurosci 24, 9269–9275 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kindt M, Soeter M & Vervliet B Beyond extinction: erasing human fear responses and preventing the return of fear. Nat. Neurosci 12, 256–258 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Phelps EA & Hofmann SG Memory editing from science fiction to clinical practice. Nature 572, 43–50 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Blundell J, Kouser M & Powell CM Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiol. Learn. Mem 90, 28–35 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debiec J, LeDoux JE & Nader K Cellular and systems reconsolidation in the hippocampus. Neuron 36, 527–538 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Eshuis LV et al. Efficacy of immersive PTSD treatments: A systematic review of virtual and augmented reality exposure therapy and a meta-analysis of virtual reality exposure therapy. J. Psychiatr. Res (2020). doi: 10.1016/j.jpsychires.2020.11.030 [DOI] [PubMed] [Google Scholar]
- 12.Soeter M & Kindt M Retrieval cues that trigger reconsolidation of associative fear memory are not necessarily an exact replica of the original learning experience. Front. Behav. Neurosci 9, 122 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Runyan JD & Dash PK Inhibition of hippocampal protein synthesis following recall disrupts expression of episodic-like memory in trace conditioning. Hippocampus 15, 333–339 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Goode TD, Ressler RL, Acca GM, Miles OW & Maren S Bed nucleus of the stria terminalis regulates fear to unpredictable threat signals. Elife 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ressler RL, Goode TD, Evemy C & Maren S NMDA receptors in the CeA and BNST differentially regulate fear conditioning to predictable and unpredictable threats. Neurobiol. Learn. Mem 174, 107281 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang RC, Blaisdell AP & Miller RR Backward conditioning: mediation by the context. J Exp Psychol Anim Behav Process 29, 171–183 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Maren S, Phan KL & Liberzon I The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci 14, 417–428 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka KZ et al. Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron 84, 347–354 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Denny CA et al. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 83, 189–201 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen BK et al. Artificially Enhancing and Suppressing Hippocampus-Mediated Memories. Curr. Biol 29, 1885–1894.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Josselyn SA & Tonegawa S Memory engrams: Recalling the past and imagining the future. Science (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goode TD, Tanaka KZ, Sahay A & McHugh TJ An integrated index: engrams, place cells, and hippocampal memory. Neuron 107, 805–820 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tonegawa S, Morrissey MD & Kitamura T The role of engram cells in the systems consolidation of memory. Nat. Rev. Neurosci 19, 485–498 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Davis P & Reijmers LG The dynamic nature of fear engrams in the basolateral amygdala. Brain Res. Bull 141, 44–49 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alfei JM et al. Generalization and recovery of post-retrieval amnesia. J. Exp. Psychol. Gen 149, 2063–2083 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Debiec J, Doyère V, Nader K & Ledoux JE Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proc. Natl. Acad. Sci. USA 103, 3428–3433 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lattal KM & Abel T Behavioral impairments caused by injections of the protein synthesis inhibitor anisomycin after contextual retrieval reverse with time. Proc. Natl. Acad. Sci. USA 101, 4667–4672 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan TJ, Roy DS, Pignatelli M, Arons A & Tonegawa S Memory. Engram cells retain memory under retrograde amnesia. Science 348, 1007–1013 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy DS, Muralidhar S, Smith LM & Tonegawa S Silent memory engrams as the basis for retrograde amnesia. Proc. Natl. Acad. Sci. USA 114, E9972–E9979 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trent S, Barnes P, Hall J & Thomas KL Rescue of long-term memory after reconsolidation blockade. Nat. Commun 6, 7897 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gisquet-Verrier P et al. Integration of New Information with Active Memory Accounts for Retrograde Amnesia: A Challenge to the Consolidation/Reconsolidation Hypothesis? J. Neurosci 35, 11623–11633 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schroyens N, Sigwald EL, Van Den Noortgate W, Beckers T & Luyten L Reactivation-dependent amnesia for contextual fear memories: Evidence for publication bias. Eneuro (2020). doi: 10.1523/ENEURO.0108-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luyten L, Schnell AE, Schroyens N & Beckers T Lack of drug-induced post-retrieval amnesia for auditory fear memories in rats. BMC Biol. 19, 17 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swanson LW Brain maps 4.0-Structure of the rat brain: An open access atlas with global nervous system nomenclature ontology and flatmaps. J. Comp. Neurol 526, 935–943 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gafford GM, Parsons RG & Helmstetter FJ Consolidation and reconsolidation of contextual fear memory requires mammalian target of rapamycin-dependent translation in the dorsal hippocampus. Neuroscience 182, 98–104 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maren S Overtraining does not mitigate contextual fear conditioning deficits produced by neurotoxic lesions of the basolateral amygdala. J. Neurosci 18, 3088–3097 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider CA, Rasband WS & Eliceiri KW NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marek R et al. Hippocampus-driven feed-forward inhibition of the prefrontal cortex mediates relapse of extinguished fear. Nat. Neurosci 21, 384–392 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shrestha P et al. Cell-type-specific drug-inducible protein synthesis inhibition demonstrates that memory consolidation requires rapid neuronal translation. Nat. Neurosci 23, 281–292 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


