Abstract
Background:
There is limited research investigating maternal dietary practices and health care provider recommendations when providing breast milk (BM) to children with IgE-mediated food allergy.
Objective:
This study explored health care provider recommendations and maternal practices when providing BM to children with IgE-mediated food allergy. We also assessed for possible IgE-mediated reactions to BM while the mother consumed the food to which her child was allergic.
Methods:
A web-based survey was distributed to breastfeeding (BF) mothers of children with IgE-mediated food allergies. Reported reactions to BM were scored by an allergist, provided only with details of the possible reaction and not the suspect allergen or route of exposure, as to the likelihood that the reaction was IgE-mediated.
Results:
133 mothers completed the survey. After food allergy diagnosis, 43.4% ( n = 63) of mothers reported they were advised by their health care provider to continue BF without dietary restriction, 17.3% ( n = 23) were advised to avoid eating the food(s) their child was allergic to while BF, and in 28.6% ( n = 38) this concern was not addressed. A minority of mothers (12%, n = 16/133) reported their child experienced an allergic reaction to BM. An allergist evaluated most of these reactions (75%, n = 12/16) as not likely IgE-mediated.
Conclusion:
This study exposed inconsistent recommendations for mothers providing BM to children with IgE-mediated food allergies. Most mothers were able to consume the food their child was allergic to without adverse sequelae. Standardized, evidence-based recommendations would enhance the well-being of these mother/infant dyads.
Keywords: Breast milk, Breastfeeding, IgE-mediated, Food allergy, Allergy, Atopy, Anaphylaxis, Provider Recommendations, Avoidance, Maternal Diet
Introduction
Human breast milk (BM) is the gold standard nutrition for infants until 6 months of age, with continued BM thereafter until at least 1 year of age while introducing complementary foods.1,2 BM contains a plethora of biologically active substances such as immune cells, cytokines, hormones, human milk oligosaccharides (HMOs), microorganisms, and non-human proteins.3–10 There is conflicting evidence regarding the influence of BM, if any, on the development of food allergy and other atopic diseases in children.11,12 There is a temporal association between potential BM exposure and the development of food allergies, as 4 – 10% of children in Western countries have a diagnosed IgE-mediated food allergy by 12 months of age.12–17 However, maternal allergen avoidance during lactation is not clearly protective against food allergy development even in high risk children for atopic disease, and therefore, is not advised as a preventative measure.18–20 There are no current consensus guidelines addressing maternal dietary practices when providing BM to a child who has already been diagnosed with an IgE-mediated food allergy.
It is clear allergenic proteins from a variety of common food allergens cross into maternal breast milk.8,21–25 However, how often such proteins lead to allergic reactions in BF infants is not clear, nor is it clear if these allergens lead to sensitization or tolerance induction in the infant. A few prospective studies have conducted BM challenges in food allergic children while the mother consumed the food the child was allergic to, but these studies are limited by their broad definitions of food allergy and/or a lack of double-blind placebo-controlled food challenges (DBPCFC) to document clinical reactions.26–28 Exceedingly rare case reports have documented IgE-mediated reactions to allergenic proteins found within human BM.29–34 Several of the reported reactions appear inconsistent with standard definitions of IgE-mediated food allergy. The full spectrum of risks and benefits of allergen exposure or avoidance through BM for children with IgE-mediated food allergy are largely unknown.
The purpose of this study was to determine what advice, if any, mothers who were providing BM to their children with IgE-mediated food allergy had been given about this practice. This study also aimed to explore for any possible IgE-mediated reactions to BM among food allergic infants while mothers consumed the food(s) their child was allergic to. In doing so, we aimed to help identify any potential risks and benefits of this practice in order to better optimize the care provided to these mother/infant dyads.
Methods
Participants
This study underwent IRB review at Scripps Health and was approved as an exempted study. Participants were recruited through online social media groups intended for breastfeeding mothers or mothers of food allergic children. Several of these social media groups were specifically for physician mothers. The total number of mothers meeting inclusion criteria is unavailable given the mode of distribution, and therefore, a response rate could not be calculated. A total of N = 133 mothers participated in the survey.
Materials
Survey responses were collated on REDCap electronic data capture tools and consisted of a maximum of 41 questions.35,36 The survey was voluntary, anonymous, and uncompensated. The response format was variable with a combination of multiple choice, check box, yes/no, true/false, and open-response questions. Branching logic was utilized in a manner such that some respondents may not have received a particular question based upon their responses to previous questions.
Participants were initially asked 5 mandatory questions that served as the study inclusion criteria (Table 1). Any participant that did not meet inclusion criteria or did not fully answer these questions could not proceed with the remaining survey questions. Only subjects meeting all of the inclusion criteria (N = 133) were included in the remaining analysis. Of the remaining survey questions, 28 explored details regarding the mother’s dietary practices and whether they were consuming the food(s) to which their child was allergic while providing BM to their child. Mothers were also asked what advice health care providers had given regarding the maternal diet. It was not specified whether the child’s allergist or primary care provider gave this advice. Additional questions explored if a mother believed her child had experienced an allergic reaction to BM while consuming the food(s) to which her child was allergic. If any such reaction was reported, the nature of reaction(s) was assessed in detail including the symptoms, onset, duration, treatment, recurrence, cross-contamination, etc. The remaining survey questions requested non-identifying demographic information.
Table 1.
Survey inclusion criteria.
| Survey Inclusion Criteria |
|---|
| Respondent’s child was diagnosed with an IgE-mediated food allergy by a licensed healthcare provider before 2 years of age |
| Child’s IgE-mediated food allergy was confirmed by positive blood or skin testing |
| Respondent listed the specific food(s) the child had an IgE-mediated allergy to |
| Child had been seen by an allergist who confirmed the presence of an IgE-mediated food allergy |
| Respondent provided breast milk to child before child was diagnosed with an IgE-mediated food allergy |
Procedure
The survey was open for participation from April 3rd, 2020 through April 10th, 2020. There were a high number of daily posts on the social media groups where the survey was shared, and older posts resulted in lower priority viewing. Therefore, the majority of responses were collected in the first few days. After approximately 24 hours with no additional responses, the survey was closed. Responses were exported from REDCap for statistical analyses. Of respondents who reported a possible allergic reaction in their infant to BM, a board-certified allergist rated the description of the child’s reaction on a scale of 1–5 based on how consistent the reaction was with an IgE-mediated allergic reaction: 1 = highly inconsistent, 2 = likely inconsistent, 3 = unclear if consistent, 4 = likely consistent, 5 = highly consistent. The allergist was blinded to the suspect allergen, route of exposure (BM), and the overall study aims. For reactions that scored 4 or 5, a grade level was assigned to the reaction using World Allergy Association (WAO) modified 2019 grading system for allergic reactions.37
Statistical Analyses
Survey response data were descriptively summarized as categorical data with both percentages of all responses and frequency of responses reported. Unless the denominator is specified due to item non-response or subgroup analysis, the denominator of each percentage is the full sample N = 133. Further, descriptive subgroup analyses summarized percentages and frequencies of categorical response data among those reporting versus not reporting allergic reactions to breastmilk, and among those reporting reactions broken down by the IgE likelihood scoring. All analyses were conducted using R v. 3.5.3 and GraphPad Prism v. 8.
Results
Respondent Demographics
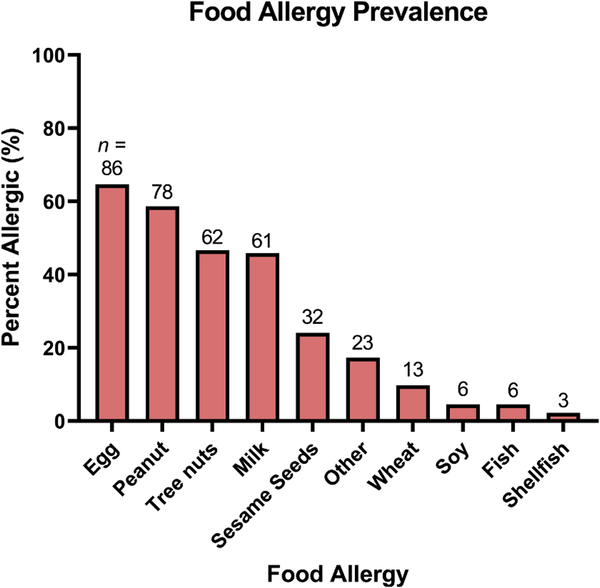
A majority of respondents were greater than 36 years of age (62.4%, n = 83) at the time of survey completion (Table 2). Most respondents’ children were diagnosed with an IgE-mediated food allergy before 12 months of age (85.7%, n = 114) and of these, 30.8% were diagnosed before 6 months of age (n = 41) (Table 2). At the time of survey completion, a majority of children were ≥ 3 years old (56.4%, n = 75) and 29.3% were ≥ 5 years old (n = 39). Most respondents (93.2%, n = 124) were located in a relatively even geographic distribution across the U.S. Racial and ethnic distribution of respondents are listed in Table 2. The three most common IgE-mediated food allergies observed in this study were egg (64.7%, n = 86), peanut (58.7%, n = 78), and tree nuts (46.6%, n = 62) (Figure 1) and the majority of food allergic children had multiple IgE-mediated food allergies (72.2%, n = 96). Among children first diagnosed with a food allergy before 6 months of age (30.8%, 41/133), the three most common IgE-mediated food allergies observed were egg (65.9%, 27/41), peanut (58.5%, 24/41), and milk (56.1%, 23/41). The prevalence of sesame allergy was 24.1%, (32/133) and was more common than wheat, soy, fish, and shellfish allergies (Figure 1). For those who listed “other” food allergies, responses are reported in eTable 1. The remaining food allergy distribution is shown in Figure 1.
Table 2.
Respondent demographics.
| Respondent Demographics | N (%) |
|---|---|
| Age of respondent at time of survey completion | |
| < 30 years | 9 (6.8%) |
| 31–35 years | 41 (30.8%) |
| 36–40 years | 53 (39.8%) |
| >40 years | 30 (22.6%) |
| Age of child when first diagnosed with food allergy | |
| 0–6 months | 41 (30.8%) |
| 7–12 months | 73 (54.9%) |
| 13–18 months | 16 (12.0%) |
| 19–24 months | 2 (1.5%) |
| > 24 months* | 1 (0.8%) |
| Age of child at the time of survey completion | |
| < 1 year | 7 (5.3%) |
| 1–2 years old | 51 (38.3%) |
| 3–4 years old | 36 (27.1%) |
| ≥ 5 years old | 39 (29.3%) |
| Mother provided breast milk to child for a duration of | |
| < 6 months | 0 (0.0%) |
| 7–12 months | 36 (27.0%) |
| 13–18 months | 63 (47.4%) |
| 19–24 months | 17 (12.8%) |
| >24 months | 17 (12.8%) |
| Identified Ethnicity | |
| Hispanic or Latino | 5 (3.8%) |
| Not Hispanic or Latino | 115 (86.5%) |
| Item non-response | 13 (9.8%) |
| Identified Race(s)** | |
| American Indian or Alaskan Native | 0 (0.0%) |
| Asian | 34 (25.6%) |
| Black or African American | 4 (3.0%) |
| Native Hawaiian or other Pacific Islander | 0 (0.0%) |
| White (Caucasian) | 93 (69.9%) |
| Other | 6 (4.5%) |
| Location | |
| Northeast United States | 33 (24.8%) |
| Southeast United States | 29 (21.8%) |
| Midwest United States | 30 (22.6%) |
| Western United States | 32 (24.1%) |
| Canada | 7 (5.3%) |
| Oceania (New Zealand and Australia) | 2 (1.5%) |
Food allergy diagnosis < 2 years was an inclusion criteria question, though one respondent gave a differing response in a second survey question.
Could check multiple boxes for respondents who identified as multiracial
Figure 1:
Prevalence of IgE-mediated food allergies among children of survey respondents, listed in descending order of frequency. Bar labels reflect the total number of children with each respective food allergy, percent allergic (%) reflects n / 133.
eTable 1.
My child was diagnosed with an allergy to “other” food(s).
| Other Food Allergies: | N (%) |
|---|---|
| Chickpeas | 4 (3.0%) |
| Avocado | 3 (2.3%) |
| Pea (unspecified) | 3 (2.3%) |
| Lentils | 3 (2.3%) |
| Banana | 2 (1.5%) |
| Kiwi | 2 (1.5%) |
| Oats | 2 (1.5%) |
| Green peas | 2 (1.5%) |
| Sunflower | 2 (1.5%) |
| Legumes | 2 (1.5%) |
| Casein | 1 (0.08%) |
| Mustard | 1 (0.08%) |
| Pepperd | 1 (0.08%) |
| Turkey | 1 (0.08%) |
| Coconut | 1 (0.08%) |
| Tomatoes | 1 (0.08%) |
| Couple type of trees | 1 (0.08%) |
| Sesame* | 1 (0.08%) |
One individual wrote-in sesame as a food allergy, and this individual’s response was added to the group of sesame allergic children
Health Care Provider Recommendations
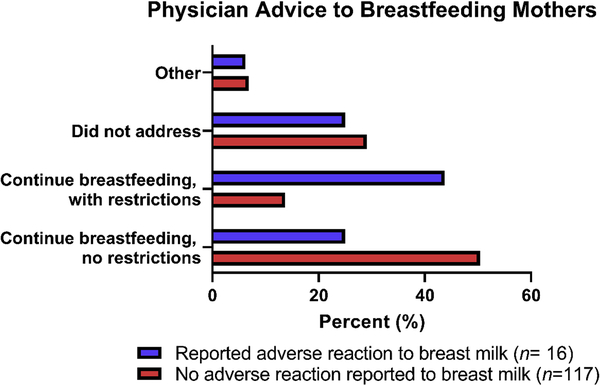
Less than half of women surveyed reported their health care provider encouraged them to continue BF without dietary restrictions (43.4%, n = 63). Some were advised to continue BF, but to avoid eating the food(s) their child was allergic to (17.3%, n = 23) while in others this issue was not addressed at all (28.6%, n = 38). A small number responded “other” to this question (6.8%, n = 9). No mothers were encouraged by their health care provider to stop BF entirely. Health care providers recommended food avoidance to BF mothers at a significantly increased frequency to mothers who believed their child had experienced an allergic reaction specifically to BM (43.8%, 7/16) versus those who did not (13.7%, 16/117) (χ2= 6.9, p = 0.009) (Figure 2). When asked if a mother had received conflicting advice from healthcare providers on what she should (or should not) eat while providing breast milk to her food allergic child, 30.3% (n = 40) of mothers responded that they had received conflicting advice.
Figure 2.
Physician advice to nursing mothers with regard to maternal diet, plotted in relation to the mother’s report of IgE-mediated food allergic child experiencing an allergic reaction to maternal breast milk.
Maternal Breastfeeding Practices
All respondents (n = 133) provided BM to their child for a minimum duration of 7 months (Table 2). Additionally, all respondents provided BM to their child before their child’s IgE-mediated food allergy diagnosis (100%, n = 133). Most continued to BF after their child was diagnosed with a food allergy (89.4%, 118/132; 1 mother did not respond). For those that discontinued BF after their child’s food allergy diagnosis (10.5%, 14/132), none reported that BF cessation was related to their child’s food allergy diagnosis. Among mothers who did not provide BM to their child after food allergy diagnosis (n = 14), most (71.4%, 10/14) reported their health care provider did not provide counseling regarding maternal dietary practices at the time of their child’s food allergy diagnosis.
Following their child’s food allergy diagnosis, 9.2% (12/131; 2 mothers did not respond) of mothers reported throwing away expressed BM that had been stored in the freezer. Of the mothers who threw away BM, only 16.7% (2/12) believed their child had an allergic reaction to BM; most (66.7%, 8/12) completely avoided eating the food(s) their child was allergic to after their child’s food allergy diagnosis and thus discarded BM that was obtained while they were still consuming the food in question. An elimination diet had been advised by health care providers for most (66.7%, 8/12) of these mothers.
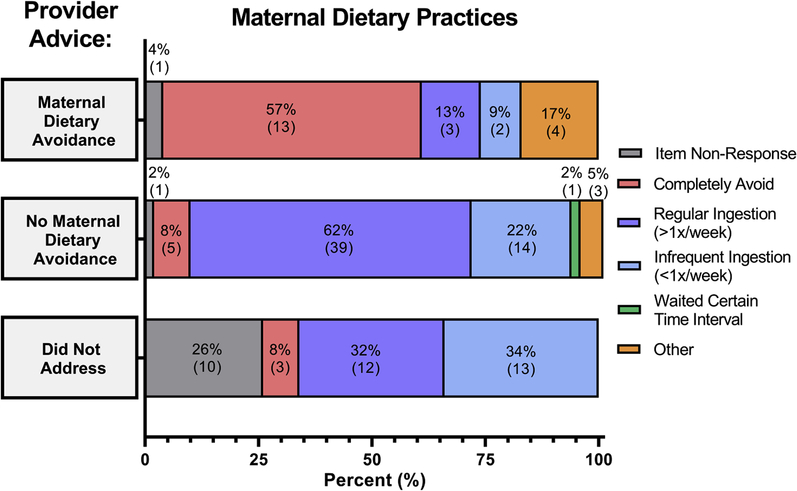
For the 89.4% (n = 118) of mothers that continued to BF after their child’s food allergy diagnosis, 46.6% (55/118) continued to eat the food their child was allergic to on a regular basis (greater than once per week) and did not alter how they provided BM to their child. An additional 25.4% (30/118) continued to eat the food their child was allergic to on an infrequent basis (less than once per week) without altering how they provided BM to their child. One mother avoided providing BM to her child for a certain time interval after consuming the foods to which her child was allergic (0.8%, 1/118). The remaining respondents completely avoided eating the food(s) their child was allergic to (20.3%, 24/118) or responded “other” to this question (6.8%, 8/118). The relationship between health care provider advice and the dietary practices of mothers is summarized in Figure 3.
Figure 3.
Relationship between provider advice and maternal dietary practices with regard to maternal ingestion of the food(s) to which her child was allergic while providing breast milk to her child. Not depicted in the figure are mothers who responded their provider’s advice was “other” and no mothers responded that their provider recommended total cessation of BF.
Breast Milk Reactions
Most mothers noticed no allergic reaction in their child while consuming the food their child was allergic to (88.0%, 117/133). A minority of mothers (12.0%, 16/133) believed their child had a possible allergic reaction to BM. Among the 16 reported allergic reactions to BM, most (56.3%, 9/16) were scored as “highly” or “likely inconsistent” with an IgE-mediated allergic reaction, 3/16 were “unknown” if consistent, and a minority (25.0%, 4/16) were “likely consistent” with an IgE-mediated allergic reaction. No reactions were scored as “highly consistent” with an IgE-mediated allergic reaction.
Of the 4 reactions to BM in children that were scored as “likely” IgE-mediated, all occurred during BF as opposed to with expressed BM. All 4 children had multiple IgE-mediated food allergies, and it was not asked which specific food allergen the mother had consumed prior to the described reaction in the child. None reported any cross-contamination of food into the BM. Three of the 4 possible reactions to BM had symptoms isolated to the skin (erythematous rash around mouth, face, and/or body with/without swelling of eyes, lips, and/or tongue) while one had further symptoms including abdominal pain and vomiting. None received injectable epinephrine. One was treated with antihistamines. Three of the 4 “likely” IgE-mediated reactions were scored as mild-grade allergic reactions, and one was scored as moderate-grade 3/5. Following this reaction, all 4 mothers reported they completely eliminated the food(s) their child was allergic to from their own diet while continuing to provide BM to their child (Table 3).
Table 3.
Characteristics of reported BM reactions that were scored as likely IgE-Mediated based solely on reaction details.
| Characteristics | Dyad #1 | Dyad #2 | Dyad #3 | Dyad #4 |
|---|---|---|---|---|
| Food allergies in child: | egg, milk | egg, milk, peanut, tree nut(s), shellfish | wheat, tree nut(s), sesame seeds, other | milk, tree nut(s) |
| Mother last consumed food:† | 4–8 hours prior | 16–24 hours prior | 72 hours – 1 week prior | 1–4 hours prior |
| BF or expressed BM: | BF | BF | BF | BF |
| Cross-contamination: | None | None | None | None |
| Summary of symptoms: | Rash (face, body) | Rash (face), swelling, abdominal pain/vomiting, mild respiratory symptoms | Rash (body) | Rash (face, body) |
| Medical treatment: | None | None | None | Antihistamines |
| Mothers diet following: | Strictly avoid‡ | Strictly avoid‡ | Strictly avoid‡ | Strictly avoid‡ |
| Total duration of BF: | 16–18 months | 13–15 months | 13–15 months | 22–24 months |
| Child’s current§ age: | 1 year | 3 years | > 5 years | > 5 years |
| Child’s current§ allergy status: | Still allergic | Still allergic | Other: “outgrown all but sesame” | Still allergic |
| Reaction score: | 4/5 (likely IgE-mediated) | 4/5 (likely IgE-mediated) | 4/5 (likely IgE-mediated) | 4/5 (likely IgE-mediated) |
| Grade of reaction‖: | 1/5 | 3/5 | 1/5 | 1/5 |
It was not asked which specific food allergen the mother had consumed prior to the described reaction in the child
Mother chose to strictly avoid eating the food(s) her child was allergic to following the reported adverse reaction in child to BM.
At time of survey completion.
Assigned unblinded scores using the modified WAO grading system37
In addition to IgE-mediated reactions, 14 mothers described how their child’s atopic dermatitis (AD) worsened with BM exposure while the mother consumed the food(s) to which her child was allergic. Among these mothers, a minority (28.6%, 4/14) also reported allergic reactions to BM in the child. The remaining 69.2% (9/14) of mothers who noted worsening of the child’s AD had not observed an allergic reaction to BM in the child. Of mothers reporting worsening AD, 42.9% (6/14) completely avoided eating the food their child was allergic to, 28.6% (4/14) continued to eat the food(s) their child was allergic to, and one mother waited a certain time interval to BF after eating the food(s) their child was allergic to. 21.4% (3/14) responded “other” to this question.
Discussion
A majority of children in this study were diagnosed with an IgE-mediated food allergy while still BF and most continued to BF after their food allergy diagnosis. This study captured differing advice regarding maternal dietary practices provided by health care providers to BF mothers of children with food allergies. Some mothers were told to continue to BF without dietary restriction, others were told to continue to BF but eliminate the food the child was allergic to from their diet, and with others the issue was not addressed at all. Such discrepancies have likely emerged at least in part due to the paucity of studies exploring the impact of food antigen exposure through BM, and by extension the scarcity of consensus opinion on the matter.18,19 There is a need for standardization of recommendations across the U.S., as inconsistent medical advice has been correlated with threatened therapeutic relationships between patients and providers.38
Health care provider recommendations appeared to be strongly influenced by maternal perceptions of risk, as dietary avoidance was advised at significantly increased frequency for mothers who believed their child had experienced an allergic reaction to food antigens within BM. While understandable, this advice may be unfounded. In our study, mothers who provided BM to children with IgE-mediated food allergies rarely had reactions to BM that were consistent with an IgE-mediated process, and none were “highly likely” to be IgE-mediated based on our scoring system. For the select few that were scored as “likely” IgE-mediated reactions, the majority were graded as mild (grade 1) in severity. None of the BM reactions required treatment with injectable epinephrine and only one was treated with antihistamines. These findings suggests that although small quantities of food proteins from food allergens may pass into maternal BM,7,8,21–23 the likelihood of this causing a serious adverse reaction for a child with an IgE-mediated food allergy is low. The decreased risk of severe reactions is also supported by fact that infants are at lower risk for life-threatening episodes of anaphylaxis in comparison to older children.39,40 These potential associations could be better addressed with prospective studies.
Maternal Practices
Maternal dietary practices when BF a child with IgE-mediated food allergies was variable, perhaps in part due to inconsistent recommendations on the practice from health care providers. The majority of mothers continued to eat the food(s) their child was allergic to while providing BM to their food allergic child. Most mothers avoided the food in their diet if recommended to do so by their health care provider. Among mothers in whom health care providers advised no maternal dietary changes, we noted increased maternal tendency to continue providing BM without dietary restrictions. This tendency was also observed in mothers in whom health care providers did not address this practice. There was a higher rate of item non-response in this subgroup, as more of these mothers did not provide BM to their child after food allergy diagnosis. The observed relationship between a lack of provider counseling and a reduced likelihood of providing BM after food allergy diagnosis may have been corollary, as perhaps providers did not address the mother’s diet because she had ceased or was weaning BF. It is also possible that the lack of counseling increased the likelihood of BF cessation after food allergy diagnosis. No mothers reported that BF cessation was related to the child’s food allergy diagnosis, indicating this was more likely the former. Our results suggest that health care provider recommendations influence the BF practices of mothers and we propose that providers should address this concern with all mothers providing BM to a child with an IgE-mediated food allergy.
It is worth noting that even when counseled by their health care provider regarding maternal dietary practices, the advice offered was not always followed. Most mothers did not perceive an adverse reaction in their food allergic child while BF and consuming the food(s) to which their child was allergic. Therefore, in such contexts, mothers may have ignored the advice of health care providers who recommended dietary avoidance. In circumstances in which providers recommended no maternal elimination diet and yet mothers chose to avoid eating the food(s) to which the child was allergic, this may have occurred due to a variety of outside influences on maternal perception of risk including online sources of misinformation. Additionally, BF mothers may feel pressured to pursue elimination diets by family and peers.41 These potential barriers to counseling require consideration in patient-provider interactions.
Almost 1/10 mothers discarded frozen BM following food allergy diagnosis, suggesting concern about the presence of potentially allergenic proteins secreted within BM, to the point that it significantly impacts how they provide BM to their child. Our study suggests this practice is not likely necessary, as most mothers may safely continue to provide BM to their IgE-mediated food allergic child that was expressed while the mother consumed the food(s) to which the child was allergic. In the rare event that an evidence-based indication for a maternal elimination diet emerges, the provider should consider recommending saving the frozen BM to potentially be used at a later date.
Breast Milk Adverse Reactions
We found a higher prevalence of milk, tree nut, wheat, and shellfish allergy in the 4 children with “likely” IgE-mediated reactions to BM relative to children with reactions that were scored as “unlikely” or “unknown” if IgE mediated, though this subgroup was very small and underpowered for statistical analysis. It has been hypothesized that certain food protein qualities may make them more resistant to digestion and able to pass intact across the intestinal border, which has been demonstrated with casein from cow’s milk.42 It is theoretically possible that certain allergenic proteins within BM may have qualities that make them more or less likely to trigger the possible, but rare IgE-mediated responses to maternal BM observed in this study.
Beyond the reported IgE-mediated allergic reactions to BM, this study captured a spectrum of additional concerns regarding adverse reactions to BM while mothers consumed the food(s) to which her child was allergic. Several mothers reported their child’s AD worsened in these circumstances. One described a reaction that appeared consistent with food protein-induced allergic proctocolitis (FPIAP) while others reported allergies in their child to common food protein-induced enterocolitis syndrome (FPIES) foods such as oat, avocado, and banana. Some described symptoms in the infant such as gas or infantile crying/colic. In AD, an elimination diet may potentially improve a child’s symptoms, but it has not been shown to significantly change the disease trajectory and there are nutritional risks associated with dietary elimination in both the child and the mother.43,44 Additionally, though it is unknown if food allergens in BM induce tolerance, any potential for this would be lost with a maternal elimination diet in high risk children with AD. It is therefore imperative to carefully weigh the risks and benefits of maternal elimination diets in AD. Evidence does not strongly support maternal elimination diets for conditions such as infantile colic or gas.41,45,46 The results of this study highlight the importance of probing into the specifics of adverse BM reactions and the provision of education if the reported reactions do not warrant a maternal elimination diet.
Limitations
This study had several limitations. Given the ability to share information widely on social media, this study is limited in that it is unknown how many individuals were invited for survey participation. As such, a response rate could not be calculated. Sampling bias may have influenced which mothers responded to this survey and selection bias may have influenced which social media groups were targeted for participation in the survey. Recall bias may have influenced recollection of past events. Another limitation was that several of the social media groups where this survey was shared were for physician mothers, so the percentage of respondents that were physicians was likely higher than the general population. As the profession of respondents was not addressed, the extent to which this influenced data cannot be fully elucidated. There may be less generalizability of this study if a high number of respondents were physician mothers. We observed a low enrollment of Hispanic and Black respondents which may also impact this study’s generalizability. Dishonest answers and/or confusion regarding survey questions could also bias study results. Also, the gold standard for diagnosis of IgE-mediated food allergy (oral food challenge) could not be employed and it was not stratified which type of health care provider (such as an allergist or primary care provider) was providing the dietary advice to mothers.47
There were also limitations in data analysis. Respondents did not answer every question, which limits subgroup analysis. Additionally, many of the subgroup analyses presented resulted in small sample sizes, and hence, only descriptive statistics were possible. A larger future study is warranted to confirm these findings, and statistical inference would be needed to determine whether many descriptive findings are statistically significant when adequately powered.
Conclusion
In conclusion, this study exposed inconsistent recommendations from health care providers with regard to the mother’s own diet when providing BM to children with IgE-mediated food allergies. Such inconsistencies have likely emerged due to a scarcity of supporting literature specifically addressing this topic. Given the importance of BM for infant health, in conjunction with the challenges associated with food elimination diets and the high number of women in the U.S. who stop BF prior to the recommended minimum duration of one year,48 health care providers should be encouraged to address this topic with all lactating mothers who provide BM to IgE-mediated food allergic children, if only to highlight the scarcity of data in this area. This study suggests there is a low level of risk for an IgE-mediated allergic reaction in the infant when the mother continues to eat the food(s) to which the child is allergic.
Given the limited evidence of serious risk to date, we propose some recommendations for practice. We encourage BF without dietary restrictions for most mothers of children with IgE-mediated food allergies. Should a mother express concern regarding an allergic reaction to BM in her food allergic child, a careful history should be conducted. We observed minimal evidence of IgE-mediated allergic reactions in the majority of reported adverse reactions to BM in our study. In such instances, reassurance may be considered without changing the mother’s diet, if clinically appropriate. We acknowledge there are non-IgE mediated adverse reactions to BM such as FPIAP that may necessitate a maternal elimination diet, and clinical discretion is required.
In the specific event that a reaction to BM is suspected to represent an IgE-mediated allergic reaction, shared decision making between mother and provider should help guide next steps in management. We theorize there may be benefits to continuing to BF without maternal dietary restrictions even in cases of mild grade, likely IgE-mediated reactions to BM, including but not limited to possible tolerance induction in the food-allergic child, reducing stress and nutritional risks associated with food avoidance diets, and prolonging BF in the mother.44 Further research with prospective studies would be beneficial to further explore best practices for these mother/infant dyads.
Acknowledgments
Funding Source: UL1TR002550 (PI: Topol)
Abbreviations/Acronyms:
- BM
Breast milk
- BF
Breastfeeding
Footnotes
Conflict of Interest
HW – No conflicts of interest
SSB –No conflicts of interest
JK– No conflicts of interest
KL– No conflicts of interest
CC – No conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Breastfeeding and the Use of Human Milk. Pediatrics. 2012;129(3): e827–e841. [DOI] [PubMed] [Google Scholar]
- 2.Breastfeeding. Breastfeeding Web site. https://www.who.int/health-topics/breastfeeding#tab=tab_2. Published 2018. Accessed 2020. [Google Scholar]
- 3.Kim SY, Yi DY. Components of human breast milk: from macronutrient to microbiome and microRNA. Clin Exp Pediatr. 2020;63(8):301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munblit D, Peroni DG, Boix-Amoros A, et al. Human Milk and Allergic Diseases: An Unsolved Puzzle. Nutrients. 2017;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez JM. The origin of human milk bacteria: is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv Nutr. 2014;5(6):779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajani PS, Seppo AE, Jarvinen KM. Immunologically Active Components in Human Milk and Development of Atopic Disease, With Emphasis on Food Allergy, in the Pediatric Population. Front Pediatr. 2018;6:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stuart CA, Twiselton R, Nicholas MK, Hide DW. Passage of cows’ milk protein in breast milk. Clin Allergy. 1984;14(6):533–535. [DOI] [PubMed] [Google Scholar]
- 8.Kilshaw PJ, Cant AJ. The passage of maternal dietary proteins into human breast milk. Int Arch Allergy Appl Immunol. 1984;75(1):8–15. [DOI] [PubMed] [Google Scholar]
- 9.Jarvinen KM. Variations in Human Milk Composition: Impact on Immune Development and Allergic Disease Susceptibility. Breastfeed Med. 2018;13(S1):S11–S13. [DOI] [PubMed] [Google Scholar]
- 10.Hunt KM, Foster JA, Forney LJ, et al. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS One. 2011;6(6):e21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panel NI-SE, Boyce JA, Assa’ad A, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 Suppl):S1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venter C, Brown KR, Maslin K, Palmer DJ. Maternal dietary intake in pregnancy and lactation and allergic disease outcomes in offspring. Pediatr Allergy Immunol. 2017;28(2):135–143. [DOI] [PubMed] [Google Scholar]
- 13.Osborne NJ, Koplin JJ, Martin PE, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127(3):668–676 e661–662. [DOI] [PubMed] [Google Scholar]
- 14.Gupta RS, Warren CM, Smith BM, et al. The Public Health Impact of Parent-Reported Childhood Food Allergies in the United States. Pediatrics. 2018;142(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kvenshagen B, Halvorsen R, Jacobsen M. Is there an increased frequency of food allergy in children delivered by caesarean section compared to those delivered vaginally? Acta Paediatr. 2009;98(2):324–327. [DOI] [PubMed] [Google Scholar]
- 16.Eller E, Kjaer HF, Host A, Andersen KE, Bindslev-Jensen C. Food allergy and food sensitization in early childhood: results from the DARC cohort. Allergy. 2009;64(7):1023–1029. [DOI] [PubMed] [Google Scholar]
- 17.Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1):e9–17. [DOI] [PubMed] [Google Scholar]
- 18.Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol. 2014;134(5):1016–1025 e1043. [DOI] [PubMed] [Google Scholar]
- 19.Greer FR, Sicherer SH, Burks AW, Committee On N, Section On A, Immunology. The Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Hydrolyzed Formulas, and Timing of Introduction of Allergenic Complementary Foods. Pediatrics. 2019;143(4). [DOI] [PubMed] [Google Scholar]
- 20.Fleischer DM, Chan ES, Venter C, et al. A Consensus Approach to the Primary Prevention of Food Allergy Through Nutrition: Guidance from the American Academy of Allergy, Asthma, and Immunology; American College of Allergy, Asthma, and Immunology; and the Canadian Society for Allergy and Clinical Immunology. J Allergy Clin Immunol Pract. 2020. [DOI] [PubMed] [Google Scholar]
- 21.Fukushima Y, Kawata Y, Onda T, Kitagawa M. Consumption of cow milk and egg by lactating women and the presence of beta-lactoglobulin and ovalbumin in breast milk. Am J Clin Nutr. 1997;65(1):30–35. [DOI] [PubMed] [Google Scholar]
- 22.Vadas P, Wai Y, Burks W, Perelman B. Detection of peanut allergens in breast milk of lactating women. JAMA. 2001;285(13):1746–1748. [DOI] [PubMed] [Google Scholar]
- 23.Picariello G, De Cicco M, Nocerino R, et al. Excretion of Dietary Cow’s Milk Derived Peptides Into Breast Milk. Front Nutr. 2019;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu J, Garrigues L, Van den Toorn H, Stahl B, Heck AJR. Discovery and Quantification of Nonhuman Proteins in Human Milk. J Proteome Res. 2019;18(1):225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luskin K; Leibel S; Ghassemian M; Chambers C; Kitsen J; Mortazavi KBBG D. Analysis of Non-Human Proteins/Peptides in Human Breast Milk by Mass Spectrometry. J Allergy Clin Immunol. 2020;145:AB163. [Google Scholar]
- 26.Arne Host SH, and Osterballe Ole. A Prospective Study of Cow’s Milk Allergy in Exclusively Breast-Fed Infants. Acta Paediatr Scand. 1988;77:663–670. [DOI] [PubMed] [Google Scholar]
- 27.Kirsi-Marjut Jarvinen SM-K, Hanna Suomalainen. Cow’s milk challenge through human milk evokes immune responses in infants with cow’s milk allergy. The Journal of Pediatrics. 1999;135(4):506–512. [DOI] [PubMed] [Google Scholar]
- 28.Martin-Munoz MF, Pineda F, Garcia Parrado G, et al. Food allergy in breastfeeding babies. Hidden allergens in human milk. Eur Ann Allergy Clin Immunol. 2016;48(4):123–128. [PubMed] [Google Scholar]
- 29.Lifschitz CHH, H.K.; Guerra C; Byrd N Anaphylactic Shock Due to Cow’s Milk Protein Hypersensitivity in a Breast-Fed Infant. Journal of Pediatric Gastroenterology and Nutrition. 1988;7:141–144. [DOI] [PubMed] [Google Scholar]
- 30.Warner JO. Food allergy in fully breast-fed infants. Clinical Allergy. 1980;10:133–136. [DOI] [PubMed] [Google Scholar]
- 31.Durgakeri PJ, B. A rare case of lactation anaphylaxis. AMJ. 2015;8(3):103–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Des Roches AP, L.; Singer S; Seidman E An allergic reaction to peanut in an exclusively breastfed infant. Allergy Net. 2015;60:266–267. [DOI] [PubMed] [Google Scholar]
- 33.Monti GM, L.; Libanore V; Peltran A; Muratore M; Silvestro L Anaphylaxis due to fish hypersensitivity in an exclusively breastfed infant. Acta Paediatr. 2006;11:1514–1515. [DOI] [PubMed] [Google Scholar]
- 34.Arima TC-A, E ; Funakoshi H; Inoue Y; Tomiita M; Kohno Y; Shimojo N Immediate systemic allergic reaction in an infant to fish allergen ingested through breast milk. Asia Pacific Allergy. 2016;6:257–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sánchez-Borges M; Ansotegui LC I. World Allergy Organization Grading System for Systemic Allergic Reactions: it Is Time to Speak the Same Language When it Comes to Allergic Reactions. Curr Treat Options Allergy. 2019;6:388–395. [Google Scholar]
- 38.Corr L, Rowe H, Fisher J. Mothers’ perceptions of primary health-care providers: thematic analysis of responses to open-ended survey questions. Aust J Prim Health. 2015;21(1):58–65. [DOI] [PubMed] [Google Scholar]
- 39.Ramsey NB, Guffey D, Anagnostou K, Coleman NE, Davis CM. Epidemiology of Anaphylaxis in Critically Ill Children in the United States and Canada. J Allergy Clin Immunol Pract. 2019;7(7):2241–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samady W, Trainor J, Smith B, Gupta R. Food-induced anaphylaxis in infants and children. Ann Allergy Asthma Immunol. 2018;121(3):360–365. [DOI] [PubMed] [Google Scholar]
- 41.Kidd M, Hnatiuk M, Barber J, Woolgar MJ, Mackay MP. “Something is wrong with your milk”: Qualitative study of maternal dietary restriction and beliefs about infant colic. Can Fam Physician. 2019;65(3):204–211. [PMC free article] [PubMed] [Google Scholar]
- 42.Sakurai N, Nishio S, Akiyama Y, et al. Apical-to-basolateral transepithelial transport of cow’s milk caseins by intestinal Caco-2 cell monolayers: MS-based quantitation of cellularly degraded alpha- and beta-casein fragments. J Biochem. 2018;164(2):113–125. [DOI] [PubMed] [Google Scholar]
- 43.Eigenmann PA, Beyer K, Lack G, et al. Are avoidance diets still warranted in children with atopic dermatitis? Pediatr Allergy Immunol. 2020;31(1):19–26. [DOI] [PubMed] [Google Scholar]
- 44.Rajani PS, Martin H, Groetch M, Jarvinen KM. Presentation and Management of Food Allergy in Breastfed Infants and Risks of Maternal Elimination Diets. J Allergy Clin Immunol Pract. 2020;8(1):52–67. [DOI] [PubMed] [Google Scholar]
- 45.Sarasu JM, Narang M, Shah D. Infantile Colic: An Update. Indian Pediatr. 2018;55(11):979–987. [PubMed] [Google Scholar]
- 46.Zeevenhooven J, Browne PD, L’Hoir MP, de Weerth C, Benninga MA. Infant colic: mechanisms and management. Nat Rev Gastroenterol Hepatol. 2018;15(8):479–496. [DOI] [PubMed] [Google Scholar]
- 47.Hammond C, Lieberman JA. Unproven Diagnostic Tests for Food Allergy. Immunol Allergy Clin North Am. 2018;38(1):153–163. [DOI] [PubMed] [Google Scholar]
- 48.Center for Disease Control and Prevention. Breastfeeding Report Card. Published 2018. Accessed2020.