Abstract
Excess body fat and sedentary behavior are associated with increased breast cancer risk and mortality, including in normal weight women. To investigate underlying mechanisms, we examined whether adiposity and exercise impact the breast microenvironment (e.g., inflammation and aromatase expression) and circulating metabo-inflammatory factors. In a cross-sectional cohort study, breast white adipose tissue (WAT) and blood were collected from 100 women undergoing mastectomy for breast cancer risk reduction or treatment. Self-reported exercise behavior, body composition measured by dual-energy x-ray absorptiometry (DXA), and waist:hip ratio were obtained prior to surgery. Breast WAT inflammation (B-WATi) was assessed by immunohistochemistry and aromatase expression was assessed by quantitative PCR. Metabolic and inflammatory blood biomarkers that are predictive of breast cancer risk and progression were measured. B-WATi was present in 56/100 patients and was associated with older age, elevated BMI, postmenopausal status, decreased exercise, hypertension and dyslipidemia (Ps<0.001). Total body fat and trunk fat correlated with B-WATi and breast aromatase levels (Ps<0.001). Circulating C-reactive protein, interleukin-6, insulin and leptin positively correlated with body fat and breast aromatase levels, while negative correlations were observed for adiponectin and sex hormone binding globulin (P<0.001). Inverse relationships were observed with exercise (Ps<0.05). In a subgroup of 39 women with normal BMI, body fat levels positively correlated with B-WATi and aromatase expression (Ps<0.05). In conclusion, elevated body fat levels and decreased exercise are associated with pro-tumorigenic micro- and host environments in normal, overweight, and obese individuals. These findings support the development of BMI-agnostic lifestyle interventions that target adiposity.
Keywords: obesity, body fat, insulin, inflammation, metabolic syndrome, breast cancer, exercise
Introduction
Obesity, classically defined by elevated body mass index (BMI), increases the risk of hormone receptor (HR)-positive breast cancer in postmenopausal women (1, 2). By contrast, in premenopausal women, obesity has been observed to confer a mildly protective effect against breast cancer incidence (3-7). After breast cancer diagnosis, obesity is associated with increased risk of recurrence in pre- and postmenopausal women across all tumor subtypes (8-11). However, some women with a normal BMI may also have unfavorable breast cancer risk or outcomes. For example, postmenopausal women with a normal BMI but excess body fat are at increased risk for invasive breast cancer (12). High levels of total body fat are also associated with increased mortality after breast cancer diagnosis, independent of BMI (13). Adipose tissue dysfunction, including inflammation and effects on adipokines, hormones and proinflammatory mediators have been implicated in the pathogenesis and progression of breast cancer in obese and normal weight individuals (12, 14-18). In this study, we investigated whether the tumor-promoting effects of adipose tissue dysfunction correlate with adiposity independent of BMI, which could explain the higher risk of breast cancer incidence and mortality in women with excess body fat.
Chronic inflammation is linked to the development and progression of multiple malignancies (19-23). There are numerous causes of chronic inflammation including tobacco use, infection and obesity (24). The link between obesity and white adipose tissue inflammation was first reported in 2003 (25). A pathological hallmark of white adipose tissue inflammation is the crown-like structure (CLS) (26). CLS are easily recognized in adipose tissue and reflect a dead or dying adipocyte surrounded by macrophages. The adipocyte appears to be stressed or die secondary to hypoxia and possibly endoplasmic reticulum stress (27). The surrounding macrophages phagocytize the dead adipocytes and become foam cells (28).
Breast white adipose tissue inflammation (B-WATi), as defined by the presence of CLS, is found in the majority of overweight and obese women but also in some women with a normal BMI (14, 18). B-WATi has been associated with an increased risk of breast cancer in women who have a history of benign breast disease (29). In addition to its potentially procarcinogenic impact, B-WATi appears to support the progression of established breast tumors. Specifically, we carried out a retrospective study of women with recurrent breast cancer despite curative-intent treatment for primary disease and showed that the presence of B-WATi at the time of mastectomy was associated with shorter distant recurrence-free survival (15). Another group reported that B-WATi at the time of mastectomy was associated with reduced overall survival in breast cancer patients (16). Our previous work suggests that the connection between inflamed adipose tissue and cancer can be explained, in part, by local effects in the breast as well as related systemic effects (23). For example, B-WATi is associated with elevated levels of aromatase, the rate-limiting enzyme for estrogen biosynthesis (30). B-WATi is also associated with the metabolic syndrome and altered levels of systemic metabo-inflammatory factors (15). Thus, B-WATi is associated with systemic changes that have been linked to both tumor development and recurrence.
We previously reported that women with B-WATi have larger breast adipocytes, suggestive of higher overall body fat levels (14, 31). Accordingly, we investigated whether body fat level measured by dual-energy x-ray absorptiometry (DXA) is an independent determinant of B-WATi and its associated tumor-promoting effects including elevated aromatase expression in the breast and altered circulating metabo-inflammatory factors. We also examined whether exercise was associated with B-WATi in a cohort of women undergoing breast surgery.
Materials and Methods
Study Population and Clinical Data Collection
Written informed consent was provided by women who were scheduled to undergo mastectomy for breast cancer risk reduction or treatment at Memorial Sloan Kettering Cancer Center (MSKCC). Institutional Review Board approval was obtained from MSKCC and Weill Cornell Medicine (New York, NY) to conduct this study. Prior to surgery, body fat was measured by DXA using a Lunar Prodigy multiple detector fan-beam densitometer (GE Healthcare). After calibration, single-beam, whole-body scanning was conducted in the supine position. Height and weight were prospectively recorded prior to surgery and used to calculate BMI. Standard definitions were used to categorize BMI as under- or normal weight (BMI< 25), overweight (BMI 25.0 – 29.9), or obese (BMI ≥ 30). Waist circumference was measured using a paper measuring tape placed horizontally halfway between the lower border of the ribs, and the iliac crest. Hip circumference was measured at the widest point over the buttocks. For each parameter, the average of 2 measurements was taken and the waist:hip ratio was calculated. Clinicopathological data (age, race, and menopause status) were systematically extracted from the electronic medical record by research staff and physicians, and independent data review was carried out for quality assurance. Menopause status was categorized as either premenopausal or postmenopausal based on National Comprehensive Cancer Network (NCCN) criteria (32). Specifically, women were classified as postmenopausal if they had bilateral oophorectomy or reported permanent cessation of menses for 12 or more months in the absence of chemotherapy or endocrine therapy.
Exercise Behavior
Self-reported exercise was quantified using the Godin Lesion Time Exercise Questionnaire (GLTEQ) (33). The GLTEQ assesses the number of times per week an individual performs mild, moderate, and vigorous exercise for more than 20 minutes during free time. Total exercise behavior was calculated as follows: the frequency of exercise sessions per week within each intensity category was multiplied by the average reported duration, weighted by an estimate of the metabolic equivalent (MET), summed across all intensities, and reported as average MET-hours per week. Standard MET weightings including examples for each level of exercise intensity are as follows: mild (3 METs, e.g., easy walking, yoga), moderate (5 METs, e.g., brisk walking, tennis), and strenuous (9 METs, e.g., running, vigorous swimming).
Biospecimen Acquisition
Breast white adipose tissue (WAT) specimens were obtained prospectively under a standard tissue acquisition protocol. For each subject, paraffin blocks were prepared from breast WAT not involved by tumor on the day of surgery. Frozen samples were stored in the presence or absence of RNAlater (Ambion). A 30 mL fasting blood sample was obtained preoperatively on the day of surgery. Blood was separated into serum and plasma by centrifugation within 3 hours of collection and stored at −80° C.
Detection and Measurement of B-WATi
The presence or absence of B-WATi was determined by histologic assessment as previously described (14). Briefly, B-WATi was detected by the presence of CLS of the breast (CLS-B), which are comprised of a dead or dying adipocyte surrounded by CD68-positive macrophages (19). From each mastectomy sample, five formalin-fixed paraffin-embedded (FFPE) blocks were prepared and one section per FFPE block (5 µm thick and approximately 2 cm in diameter) was generated such that five sections were stained for CD68, a macrophage marker (mouse monoclonal KP1 antibody; Dako; dilution 1:4,000). Immunostained tissue sections were examined by the study pathologist (DG) using light microscopy to detect the presence or absence of CLS-B and record the number of CLS-B per slide. Digital photographs of each slide were generated and WAT area was measured with Image J Software (NIH, Bethesda, MD). The severity of B-WATi was quantified as number of CLS-B per square centimeter of WAT (CLS-B/cm2).
Adipocyte Measurement
Two H&E stained sections were generated from FFPE breast tissue in order to measure adipocyte diameters as previously described (14). The H&E sections were photographed at 20X using an Olympus BX43 or BX50 microscopes equipped with an Olympus DP27 or MicroFire (Optronics) digital camera, respectively. Mean diameters were calculated using measurements from 30 individual adipocytes for each patient using the linear dimensional tool in the Canvas 11 Software (ACD Systems International, Inc.), which was calibrated using a microscope stage micrometer.
Quantitative PCR
Total RNA was isolated from human breast tissue using the RNeasy Mini Kit (Qiagen). RNA (2 µg) was reverse transcribed using the qScript cDNA Synthesis Kit (QuantaBio), and the resulting cDNA used for real-time PCR amplification with Fast SYBR green PCR master mix on a 7500 HT real-time PCR system (Applied Biosystems). GAPDH was used as an endogenous normalization control. Commercial primers for SIRT1 (QT00051261), and GAPDH (QT00079247) were purchased from Qiagen. In-house primers for aromatase (forward: 5’-CACATCCTCAATACCAGGTCC-3’ and reverse: 5’-CAGAGATCCAGACTCGCATG-3’) were synthesized by Sigma-Aldrich. Relative fold-induction was determined using the ΔΔCTanalysis protocol.
Blood Assays
Plasma levels of leptin, adiponectin, high sensitivity C-reactive protein (hsCRP), sex hormone binding globulin (SHBG), interleukin-6 (IL-6; R&D Systems, Minneapolis, MN), and insulin (Mercodia, Uppsala, Sweden), were measured in a single batch by enzyme-linked immunosorbent assay.
Biostatistical Analyses
For continuous variables, differences between patients with and without B-WATi were examined using the non-parametric Wilcoxon rank-sum test. Categorical variables were examined using Fisher’s exact test. Levels of a circulating factor and levels of relative expression of a gene were log transformed and CLS-B/cm2 was square root transformed for improving clarity in data visualization. Correlation between two continuous variables was examined using Spearman’s method. For inflammation markers, absolute quantification was made for duplicates of samples per patient. Average values from duplicate samples per patient were used in subsequent analysis. For gene expression measured using qPCR, reference samples from the same sources were included in all the batches and Δ Δ cycle threshold (Δ Δ Ct) values were calculated by normalizing against the Δ Ct values for the reference samples. These analyses were carried out in the overall study population and in the subpopulation with BMI < 25. For all analyses, statistical significance was set at two-tailed P<.05. All statistical analyses were conducted using R software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study population
From April 2016 through August 2018, 100 women were enrolled and underwent DXA scans prior to mastectomy. Breast WAT and blood samples were obtained from all 100 women at the time of surgery. In the overall study population, median age was 49 years (range, 29-82). Median BMI was 26.1 (range, 17.5-42), 52% were premenopausal and 45% were postmenopausal. B-WATi, as defined by CLS-B positivity, was observed in 56% of participants (Fig. 1A). Additional baseline characteristics stratified by the presence or absence of CLS-B are summarized in Table 1.
Figure 1. Breast aromatase levels correlate with adiposity and B-WATi.
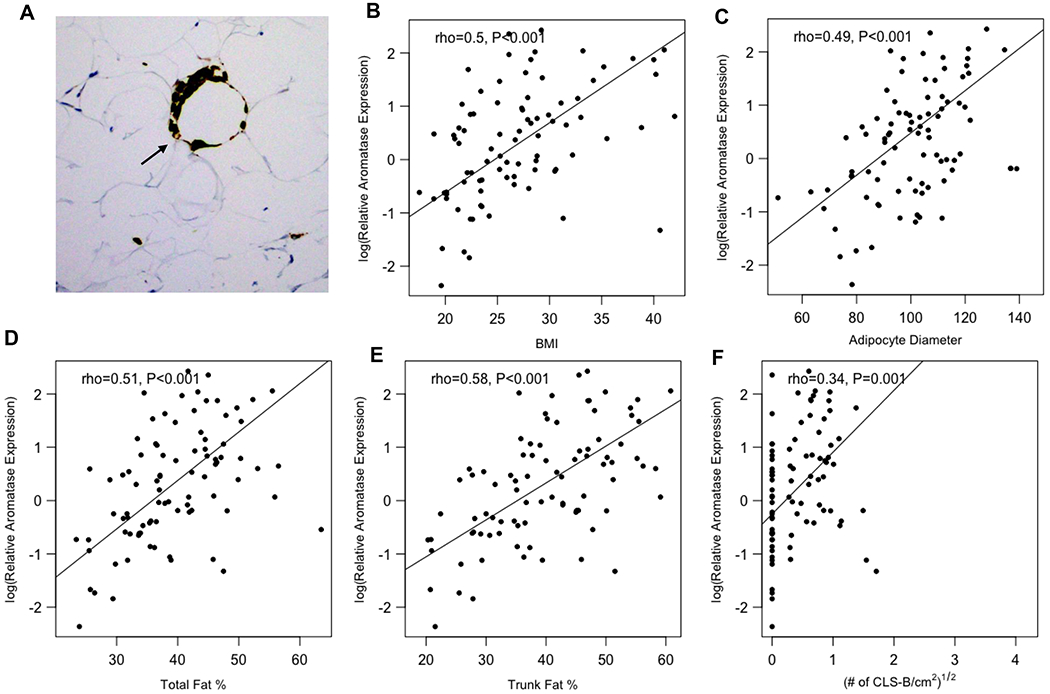
(A) Anti-CD68 immunostaining showing CLS-B (200x). Aromatase expression in the breast positively correlated with (B) BMI, (C) adipocyte diameter, (D) total fat percentage, (E) trunk fat percentage, and (F) the severity of B-WATi.
Table 1.
Baseline characteristics of study population.
| Variables | All (n=100) | B-WATi Absent (n=44) | B-WATi Present (n=56) | P |
|---|---|---|---|---|
| Age (years), median (range) | 49 (29 to 82) | 45 (29 to 79) | 52 (31 to 82) | 0.001 |
| BMI, median (range) | 26.1 (17.5 to 42.0) | 23.7 (17.5 to 32.2) | 28.3 (20.1 to 42.0) | <0.001 |
| BMI (%) | ||||
| Normal / Underweight | 39 (39%) | 25 (58%) | 14 (25%) | |
| Overweight | 37 (37%) | 15 (35%) | 22 (39%) | |
| Obese | 23 (23%) | 3 (7%) | 20 (36%) | <0.001 |
| Menopausal Status, n (%) | ||||
| Pre | 52 (54%) | 32 (74%) | 20 (37%) | |
| Post | 45 (46%) | 11 (26%) | 34 (63%) | |
| Not reported | 3 (3%) | 1 (2%) | 2 (4%) | <0.001 |
| Race, n (%) | ||||
| Asian | 14 (14%) | 4 (9%) | 10 (18%) | |
| Black | 8 (8%) | 4 (9%) | 4 (7%) | |
| White | 68 (68%) | 32 (73%) | 36 (64%) | |
| Not reported | 10 (10%) | 4 (9%) | 6 (11%) | 0.625 |
| Adipocyte Diameter (¼) | ||||
| Median (range) | 102.5 (51.1 to 145.1) | 95.9 (51.1 to 118.2) | 105.8 (72.1 to 145.1) | <0.001 |
| Exercise (MET-hours/week)a | ||||
| Median (range) | 12.5 (0 to 137.5) | 22.5 (0 to 137.5) | 8 (0 to 78) | 0.005 |
| Hypertension, n (%) | 18 (18%) | 4 (9%) | 14 (26%)b | 0.038 |
| Diabetes, n (%) | 3 (3%) | 0 (0%) | 3 (5%)c | 0.252 |
| Dyslipidemia, n (%) | 13 (13%) | 1 (2%) | 12 (22%)b | 0.005 |
| Tumor Subtype, n (%) | ||||
| HR+, HER2-negative | 56 (88%) | 28 (88%) | 28 (88%) | |
| HER2+ | 7 (11%) | 4 (12%) | 3 (10%) | |
| Triple negative | 1 (1%) | 0 (0%) | 1 (2%) | 1.00 |
| Non-invasive or benign | 36 (36%) | 12 (27%) | 24 (43%) | 0.142 |
No data:
, n=6 (B-WATi absent) and n=11 (B-WATi present);
, n=2;
, n=1
Abbreviations: B-WATi, breast white adipose tissue inflammation; BMI, body mass index; MET, metabolic equivalent task; HR, hormone receptor
B-WATi correlates with metabolic disorders, decreased exercise, and excess body fat
On average, patients with B-WATi were older, had higher BMI and larger adipocytes, and were more likely to be postmenopausal compared to women without inflammation (Table 1). The incidence of hypertension (P=0.038) and dyslipidemia (P=0.005) was higher in women with B-WATi. Self-reported exercise participation, standardized as MET-hours per week, was lower in women with B-WATi compared to those without inflammation. Several measures of adiposity including total body fat and trunk fat were higher in women with B-WATi (Table 2). When adjusted for menopausal status, the associations among BMI and body fat parameters with B-WATi remained similar (Ps<0.05). Notably, waist:hip ratio did not correlate with B-WATi.
Table 2.
Body Composition Measurements and Breast White Adipose Tissue Inflammation
| Variables | All (n=100) | B-WATi Absent (n=44) | B-WATi Present (n=56) | P |
|---|---|---|---|---|
| Total body fat (%) | ||||
| Median (range) | 38.8 (21.7 to 63.5) | 34.7 (21.7 to 63.5) | 42.5 (31.7 to 56.5) | <0.001 |
| Total fat mass (kg) | ||||
| Median (range) | 25.46 (9.82 to 55.04) | 20.23 (9.82 to 42.65) | 29.34 (15.89 to 55.04) | <0.001 |
| Total lean mass (kg) | ||||
| Median (range) | 39.14 (19.57 to 57.66) | 37.55 (19.57 to 46.73) | 40.08 (29.97 to 57.66) | 0.043 |
| Fat:Lean Ratio | ||||
| Median (range) | 0.64 (0.28 to 1.3) | 0.56 (0.28 to 1.27) | 0.74 (0.46 to 1.3) | <0.001 |
| Trunk fat (%)a | ||||
| Median (range) | 40 (18.2 to 60.8) | 32.85 (18.2 to 59.1) | 45.4 (30 to 60.8) | <0.001 |
| Trunk fat mass (kg)a | ||||
| Median (range) | 12.77 (3.73 to 29.11) | 9.11 (3.73 to 21.9) | 15.44 (7.07 to 29.11) | <0.001 |
| Waist:Hip Ratiob | ||||
| Median (range) | 0.84 (0.59 to 1.33) | 0.82 (0.59 to 1.32) | 0.84 (0.62 to 1.33) | 0.268 |
No data:
, n=1 (B-WATi absent);
, n=8 (B-WATi absent) and n=14 (B-WATi present)
Abbreviations: B-WATi, breast white adipose tissue inflammation
Breast aromatase levels correlate with amounts of body fat and B-WATi
Aromatase mRNA levels positively correlated with measurements of body fat and B-WATi. Levels of aromatase mRNA positively correlated with BMI, breast adipocyte diameter, total fat, and trunk fat (Ps<0.001) (Fig. 1). Additionally, levels of aromatase were increased in women with B-WATi (P<0.001) and correlated with the severity of B-WATi (P=0.001). When adjusted for menopausal status, the correlations among BMI and body fat parameters with relative aromatase expression remained similar (Ps<0.05).
Systemic factors correlate with adiposity, B-WATi and aromatase expression
Blood biomarkers that have been linked to the pathogenesis of breast cancer were measured. Levels of hsCRP, IL-6, leptin and insulin all increased in a stepwise manner from underweight/normal to overweight to obese participants (Ps<0.001; Table S1). In contrast, levels of SHBG decreased in association with elevated BMI (P<0.001; Table S1). Next levels of each of these blood biomarkers were correlated with measurements of adiposity, total MET hours (TMetHr) of exercise, and intra-breast histological and molecular endpoints (adipocyte diameter, severity of B-WATi, SIRT1 and aromatase mRNA levels) (Table 3). Blood levels of hsCRP, IL-6, leptin, and insulin positively correlated with BMI, total fat mass and trunk fat mass (Ps<0.001). Blood levels of adiponectin (P<0.05) and SHBG (P<0.001) were inversely related to BMI. SHBG levels were also inversely correlated with total fat mass and trunk fat mass (Ps<0.001). Levels of hsCRP (P=0.034), IL-6 (P=.018), and leptin (P=0.001) were inversely related to levels of exercise (TMetHr). By contrast, blood levels of adiponectin (P=0.040) and SHBG (P=0.003) positively correlated with levels of exercise.
Table 3.
Correlations among Blood Biomarkers, Adiposity, Exercise and Breast Endpoints
| Blood Marker | BMI | Total Fat Mass | Trunk Fat Mass | TMetHr | Breast |
|||
|---|---|---|---|---|---|---|---|---|
| Adipocyte Diameter | CLS-B/cm2 | SIRT1 | Aromatase | |||||
| hsCRP | ||||||||
| ρ (Spearman’s) | 0.68 | 0.67 | 0.66 | −0.25 | 0.47 | 0.42 | −0.3 | 0.47 |
| P | <0.001 | <0.001 | <0.001 | 0.034 | <0.001 | <0.001 | 0.010 | <0.001 |
| IL-6 | ||||||||
| ρ (Spearman’s) | 0.48 | 0.48 | 0.50 | −0.28 | 0.47 | 0.28 | −0.46 | 0.43 |
| P | <0.001 | <0.001 | <0.001 | 0.018 | <0.001 | 0.009 | <0.001 | <0.001 |
| Leptin | ||||||||
| ρ (Spearman’s) | 0.81 | 0.85 | 0.82 | −0.37 | 0.56 | 0.29 | −0.46 | 0.43 |
| P | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | 0.007 | <0.001 | <0.001 |
| Adiponectin | ||||||||
| ρ (Spearman’s) | −0.21 | −0.14 | −0.21 | 0.24 | −0.33 | −0.28 | 0.27 | −0.19 |
| P | 0.045 | 0.201 | 0.053 | 0.040 | 0.001 | 0.008 | 0.020 | 0.107 |
| Insulin | ||||||||
| ρ (Spearman’s) | 0.57 | 0.51 | 0.50 | −0.19 | 0.40 | 0.15 | −0.31 | 0.37 |
| P | <0.001 | <0.001 | <0.001 | 0.108 | <0.001 | 0.157 | 0.007 | <0.001 |
| SHBG | ||||||||
| ρ (Spearman’s) | −0.40 | −0.37 | −0.43 | 0.35 | −0.41 | −0.28 | 0.21 | −0.29 |
| P | <0.001 | <0.001 | <0.001 | 0.003 | <0.001 | 0.008 | 0.076 | 0.012 |
Abbreviations: BMI, body mass index; TMetHr, total metabolic equivalent task hours; CLS-B, crown-like structures of the breast; hsCRP, high sensitivity C-reactive protein; IL-6, interleukin-6; SHBG, sex hormone binding globulin
Although blood biomarkers are routinely used in studies that evaluate the link between obesity and breast cancer, to our knowledge, blood biomarkers have not been correlated with relevant intra-breast measurements. Notably, levels of hsCRP, IL-6, leptin and insulin were positively correlated with breast adipocyte diameter (Ps<0.001). Each of these blood biomarkers except insulin also correlated with the severity of B-WATi (CLS-B/cm2). Breast SIRT1 mRNA levels inversely correlated with body fat (Fig. S1) and blood levels of hsCRP, IL-6, leptin and insulin (Table 3). Conversely, levels of adiponectin (P=0.020) and SHBG (P=0.076) were positively correlated with SIRT1 mRNA levels. Breast aromatase mRNA levels were positively correlated with blood levels of hsCRP, IL-6, leptin and insulin (Ps<0.001). In contrast, an inverse relationship was observed between blood levels of SHBG (P=0.012) and aromatase mRNA levels.
To investigate a potential impact of medications for hypertension or diabetes, sensitivity analyses were carried out by excluding patients taking these medications (18 patients were taking anti-hypertensive medications and 3 patients were taking medications for diabetes). The results remained consistent with analyses in the overall population (Table S2).
Adiposity correlates with B-WATi and breast aromatase levels in normal weight women
A subgroup of 39 out of 100 women in our study had BMI < 25. Of these, 14 (36%) had B-WATi and 25 (64%) did not. Normal weight women with B-WATi had enlarged adipocytes and a higher incidence of dyslipidemia compared to women without inflammation (Table S3). Participants with higher body fat levels were more likely to have B-WATi despite having a normal BMI (Table 4). Next we evaluated the relationship between body fat, B-WATi and aromatase mRNA levels in these normal weight women. Although a clear relationship between BMI and aromatase mRNA levels was not detected, adipocyte diameter (P=0.06) and DXA-derived measures of total body fat and trunk fat positively correlated with breast aromatase levels (Ps=0.02; Fig. 2). In women with a BMI<25, levels of aromatase mRNA were increased in those with B-WATi (P=0.045) and correlated with the severity of B-WATi (P=0.03) (Fig. 2).
Table 4.
Body Composition Measurements and Breast White Adipose Tissue Inflammation in Patients with BMI <25
| Variables | All (n=39) |
B-WATi Absent (n=25) |
B-WATi Present (n=14) |
P |
|---|---|---|---|---|
| Total fat (%) | ||||
| Median (range) | 33.5 (21.7 to 43.8) | 31 (21.7 to 38.7) | 35.9 (31.7 to 43.8) | 0.002 |
| Total fat mass (kg) | ||||
| Median (range) | 17.74 (9.82 to 25.74) | 16.51 (9.82 to 25.74) | 20.46 (15.89 to 24.45) | 0.004 |
| Total lean mass (kg) | ||||
| Median (range) | 35.73 (19.57 to 45.59) | 36.31 (19.57 to 45.59) | 34.76 (29.97 to 43.01) | 0.117 |
| Fat:Lean Ratio | ||||
| Median (range) | 0.51 (0.28 to 0.84) | 0.46 (0.28 to 0.84) | 0.56 (0.46 to 0.78) | 0.007 |
| Trunk fat (%)a | ||||
| Median (range) | 30.3 (18.2 to 48.1) | 27.8 (18.2 to 37.7) | 36.2 (30 to 48.1) | <0.001 |
| Trunk fat mass (kg)a | ||||
| Median (range) | 7.56 (3.73 to 13.99) | 6.98 (3.73 to 11.39) | 9.69 (7.07 to 13.99) | <0.001 |
| Waist:Hip Ratiob | ||||
| Median (range) | 0.8 (0.59 to 1.33) | 0.79 (0.59 to 1.32) | 0.81 (0.7 to 1.33) | 0.662 |
No data:
, n=1 (B-WATi present);
, n=4 (B-WATi absent) and n=3 (B-WATi present)
Abbreviations: B-WATi, breast white adipose tissue inflammation
Figure 2. Breast aromatase levels correlate with adiposity and B-WATi in patients with BMI < 25.
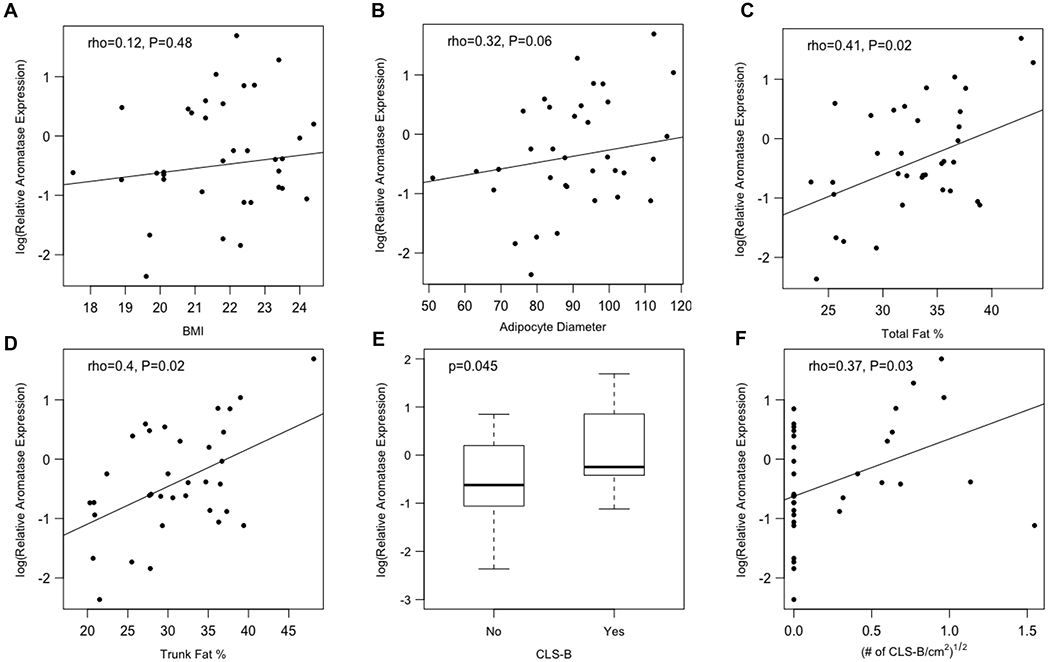
Among the 39 normal weight patients in this study, correlations among aromatase expression in the breast and (A) BMI, (B) adipocyte diameter, (C) total fat percentage, (D) trunk fat percentage, (E) the presence of B-WATi, and (F) the severity of B-WATi.
Discussion
Here we report that elevated body fat levels are associated with breast inflammation, higher aromatase expression in the breast, and dysregulation of circulating metabo-inflammatory factors. Decreased exercise was associated with both breast inflammation and dysregulated circulating metabo-inflammatory factors. Additionally, we report that commonly measured blood biomarkers are predictive of body fat levels and histological and molecular changes in the breast that have been linked to the development and progression of breast cancer.
Findings from this study confirm prior evidence that B-WATi correlates with elevated BMI, the postmenopausal state and features of the metabolic syndrome (14, 15). Collectively, these findings suggest that inflammation contributes to the increased risk of breast cancer associated with excess body fat, the postmenopausal state and metabolic syndrome. Because of the inherent limitations of using BMI as an objective measurement of body fat, it was important to utilize DXA scans to determine if excess body fat correlated with both B-WATi and aromatase levels. Adiposity assessment by DXA provides highly accurate and reproducible measurements, whereas anthropometric measures such as waist circumference have variable sensitivity for diagnosing excess body fat (34, 35). Our findings indicate that measurement of body fat using DXA scan provides a more sensitive diagnosis of B-WATi than BMI or waist-hip ratio. Interestingly, increased body fat including in women with a normal BMI correlated with both B-WATi and elevated breast aromatase levels. The fact that increased body fat correlated with elevated breast aromatase levels in normal BMI women provides important mechanistic insights into why increased body fat is associated with a doubling in the risk of HR-positive breast cancer in normal BMI postmenopausal women (12). Although the precise mechanism by which aromatase levels increase in the context of excess body fat is incompletely understood, one likely contributor is leptin, a proinflammatory adipokine (36). Additionally, B-WATi may promote the growth of hormone independent tumors via metabolic dysregulation (e.g., elevated levels of insulin).
Numerous observational and preclinical studies have suggested that exercise protects against the development and progression of breast cancer (37–40). Exercise has a variety of effects that can potentially explain this protective effect ranging from modulating immune function to altering hormone levels (41-46). Here we show for the first time that reduced exercise is associated with B-WATi which would be predicted, in turn, to increase the risk of breast cancer or potentially worsen prognosis. Certainly, this finding strengthens the rationale for incorporating exercise into lifestyle interventions aimed at improving body composition and reducing B-WATi. This finding also underscores the need to include women with normal BMI and elevated body fat levels in exercise intervention trials.
Blood biomarkers are commonly measured in studies that focus on the link between obesity and cancer (47-51). Our observation that blood levels of hsCRP, IL-6, leptin and insulin increased whereas SHBG decreased in association with elevated BMI, total and trunk fat mass is consistent with previously published reports. A negative correlation was observed between the amount of exercise and blood levels of hsCRP, IL-6, and leptin whereas a positive correlation was found with blood levels of adiponectin and SHBG; these correlations with exercise are interesting but may simply reflect the inverse relationship between exercise and body fat. Importantly, very little is known about the relationship between these commonly measured blood biomarkers and the state of the breast microenvironment. Here we found that blood levels of hsCRP, IL-6, leptin and insulin positively correlate with breast adipocyte size, the severity of B-WATi or both. Excess body fat manifests as adipocyte hypertrophy at the microscopic level, and these observations are consistent with our prior studies in which increased breast adipocyte size and B-WATi were associated with elevated levels of inflammatory biomarkers (hsCRP, IL-6), an adipokine (leptin) and insulin resistance. These findings suggest that B-WATi may also promote tumor growth via estrogen-independent mechanisms. A negative correlation was observed for blood adiponectin and SHBG levels and both breast adipocyte size and the severity of B-WATi. This result is consistent with excess body fat being associated with both suppression of adiponectin gene expression (52) and insulin resistance; elevated insulin levels, a known consequence of insulin resistance, down regulate levels of SHBG (53). In addition to inflammation and expansion of the breast fat depot, we found that the measured blood biomarkers are predictive of aromatase levels in the breast. Aromatase is the rate limiting enzyme in estrogen biosynthesis and is the target of widely used medications (i.e., aromatase inhibitors) for breast cancer prevention and treatment. In this study, blood levels of hsCRP, IL-6, leptin and insulin all positively correlated with breast aromatase levels. Since each of these blood biomarkers increases with excess body fat as does breast aromatase, it is not surprising that the blood biomarkers correlate with aromatase levels. Blood levels of SHBG inversely correlated with breast aromatase levels. The reduced levels of SHBG should result in increased free estradiol levels. At the same time, a reduction in blood SHBG predicts for elevated levels of aromatase and presumably increased breast estrogens. Reduced levels of SIRT1 occur in the breast tissue of obese women and contribute to elevated aromatase expression (54, 55). In line with other findings, levels of proinflammatory biomarkers including hsCRP, IL-6 and leptin were all inversely related to breast SIRT1 levels whereas SIRT1 levels were positively correlated with blood levels of adiponectin. Although previous studies have demonstrated a relationship between these blood biomarkers and either the development or progression of breast cancer, we demonstrate that these blood biomarkers provide a window to important histological and molecular changes in the breast that are linked to cancer. Additional studies will be needed to determine if measurements of body fat levels in conjunction with blood biomarkers are more useful than blood biomarkers alone for determining cancer risk.
Finally, we note that this is a cross-sectional study and that the observed correlations do not indicate causation. Furthermore, women included in this study underwent mastectomy for breast cancer treatment or as prophylaxis due to high risk. Therefore, future prospective trials are needed to rule out reverse causation and test whether lowering levels of body fat impact breast cancer risk and/or outcomes. Such trials should be conducted in both the primary and secondary risk settings for better applicability and generalizability. Our finding that aromatase levels are increased in the breasts of women with excess body fat suggests that either an aromatase inhibitor or selective estrogen receptor modulator (SERM) might reduce the risk of HR-positive breast cancer in this high risk group. In fact, it is known that aromatase inhibitors are beneficial at reducing the risk of HR-positive breast cancer even in normal BMI women (56, 57). However, due to safety concerns and side effects, most women are reluctant to use anti-estrogens long term to reduce the risk of breast cancer (58). Alternate strategies warrant consideration. In the future, it is possible that a role for weight loss medications will be identified. Lifestyle interventions that aim to improve body composition ranging from time-restricted eating to whole food plant-based diets used alone or in combination with exercise also warrant testing. It is possible, for example, that normal BMI postmenopausal women with excess body fat will benefit from such an intervention. The current results suggest that future intervention trials should include blood biomarkers that correlate with both body composition and breast biology.
Supplementary Material
Prevention Relevance Statement:
We report that individuals with high body fat and low exercise levels have breast inflammation, higher breast aromatase expression, and levels of circulating metabo-inflammatory factors that have been associated with increased breast cancer risk. These findings support interventions to lower adiposity, even among normal weight individuals, to prevent tumor growth.
Acknowledgements
This work was supported by grants, NIH/NCI U54 CA210184-01 (to A.J. Dannenberg), Conquer Cancer Foundation of the American Society of Clinical Oncology (to N.M. Iyengar), the Breast Cancer Research Foundation (to N.M Iyengar and A.J. Dannenberg), the Botwinick-Wolfensohn Foundation (in memory of Mr. and Mrs. Benjamin Botwinick; to A.J. Dannenberg), Myrna and Bernard Posner (to N.M. Iyengar), and the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Competing Interests:
Dr. Iyengar receives consulting fees from Novartis and Seattle Genetics. Dr. Morrow receives consulting fees from Genomic Health.
Footnotes
Presentations:
This work was presented in part at the 2019 Annual Meeting of the American Society of Clinical Oncology.
References
- 1.Trentham-Dietz A, Newcomb PA, Storer BE, Longnecker MP, Baron J, Greenberg ER, et al. Body size and risk of breast cancer. American journal of epidemiology. 1997;145:1011–9. [DOI] [PubMed] [Google Scholar]
- 2.Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and cancer: local and systemic mechanisms. Annual review of medicine. 2015;66:297–309. [DOI] [PubMed] [Google Scholar]
- 3.Premenopausal Breast Cancer Collaborative G, Schoemaker MJ, Nichols HB, Wright LB, Brook MN, Jones ME, et al. Association of Body Mass Index and Age With Subsequent Breast Cancer Risk in Premenopausal Women. JAMA Oncol 2018;4:e181771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warner ET, Hu R, Collins LC, Beck AH, Schnitt S, Rosner B, et al. Height and Body Size in Childhood, Adolescence, and Young Adulthood and Breast Cancer Risk According to Molecular Subtype in the Nurses’ Health Studies. Cancer Prev Res (Phila) 2016;9:732–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baer HJ, Tworoger SS, Hankinson SE, Willett WC. Body fatness at young ages and risk of breast cancer throughout life. American journal of epidemiology. 2010;171:1183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh H, Boeke CE, Tamimi RM, Smith-Warner SA, Wang M, Willett WC, et al. The interaction between early-life body size and physical activity on risk of breast cancer. International journal of cancer Journal international du cancer. 2015;137:571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michels KB, Terry KL, Willett WC. Longitudinal study on the role of body size in premenopausal breast cancer. Archives of internal medicine. 2006;166:2395–402. [DOI] [PubMed] [Google Scholar]
- 8.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. The New England journal of medicine. 2003;348:1625–38. [DOI] [PubMed] [Google Scholar]
- 9.Ewertz M, Jensen MB, Gunnarsdottir KA, Hojris I, Jakobsen EH, Nielsen D, et al. Effect of obesity on prognosis after early-stage breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:25–31. [DOI] [PubMed] [Google Scholar]
- 10.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast cancer research and treatment. 2010;2010:23. [DOI] [PubMed] [Google Scholar]
- 11.Sparano JA, Zhao F, Martino S, Ligibel JA, Perez EA, Saphner T, et al. Long-Term Follow-Up of the E1199 Phase III Trial Evaluating the Role of Taxane and Schedule in Operable Breast Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iyengar NM, Arthur R, Manson JE, Chlebowski RT, Kroenke CH, Peterson L, et al. Association of Body Fat and Risk of Breast Cancer in Postmenopausal Women With Normal Body Mass Index: A Secondary Analysis of a Randomized Clinical Trial and Observational Study. JAMA Oncol. 2019;5:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, et al. Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol. 2018;4:798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iyengar NM, Morris PG, Zhou XK, Gucalp A, Giri D, Harbus MD, et al. Menopause is a determinant of breast adipose inflammation. Cancer Prev Res (Phila). 2015;8:349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iyengar NM, Zhou XK, Gucalp A, Morris PG, Howe LR, Giri D, et al. Systemic Correlates of White Adipose Tissue Inflammation in Early-Stage Breast Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koru-Sengul T, Santander AM, Miao F, Sanchez LG, Jorda M, Gluck S, et al. Breast cancers from black women exhibit higher numbers of immunosuppressive macrophages with proliferative activity and of crown-like structures associated with lower survival compared to non-black Latinas and Caucasians. Breast cancer research and treatment. 2016;158:113–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun X, Casbas-Hernandez P, Bigelow C, Makowski L, Joseph Jerry D, Smith Schneider S, et al. Normal breast tissue of obese women is enriched for macrophage markers and macrophage-associated gene expression. Breast cancer research and treatment. 2012;131:1003–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iyengar NM, Brown KA, Zhou XK, Gucalp A, Subbaramaiah K, Giri DD, et al. Metabolic Obesity, Adipose Inflammation and Elevated Breast Aromatase in Women with Normal Body Mass Index. Cancer Prev Res (Phila). 2017;10:235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annual review of physiology. 2010;72:219–46. [DOI] [PubMed] [Google Scholar]
- 20.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators of inflammation. 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunter MJ, Wang T, Cushman M, Xue X, Wassertheil-Smoller S, Strickler HD, et al. Circulating Adipokines and Inflammatory Markers and Postmenopausal Breast Cancer Risk. Journal of the National Cancer Institute. 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunter MJ, Xie X, Xue X, Kabat GC, Rohan TE, Wassertheil-Smoller S, et al. Breast cancer risk in metabolically healthy but overweight postmenopausal women. Cancer research. 2015;75:270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34:4270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of clinical investigation. 2003;112:1821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. Journal of lipid research. 2005;46:2347–55. [DOI] [PubMed] [Google Scholar]
- 27.Gregor MF, Hotamisligil GS. Thematic review series: Adipocyte Biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. Journal of lipid research. 2007;48:1905–14. [DOI] [PubMed] [Google Scholar]
- 28.Haka AS, Barbosa-Lorenzi VC, Lee HJ, Falcone DJ, Hudis CA, Dannenberg AJ, et al. Exocytosis of macrophage lysosomes leads to digestion of apoptotic adipocytes and foam cell formation. Journal of lipid research. 2016;57:980–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carter JM, Hoskin TL, Pena MA, Brahmbhatt R, Winham SJ, Frost MH, et al. Macrophagic “Crown-like Structures” Are Associated with an Increased Risk of Breast Cancer in Benign Breast Disease. Cancer Prev Res (Phila). 2018;11:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila). 2011;4:1021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iyengar NM, Chen IC, Zhou XK, Giri DD, Falcone DJ, Winston LA, et al. Adiposity, Inflammation, and Breast Cancer Pathogenesis in Asian Women. Cancer Prev Res (Phila). 2018;11:227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology Version 3.2014: Breast Cancer. 2014; Available from: http://www.nccn.org
- 33.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–6. [PubMed] [Google Scholar]
- 34.Molarius A, Seidell JC, Sans S, Tuomilehto J, Kuulasmaa K. Varying sensitivity of waist action levels to identify subjects with overweight or obesity in 19 populations of the WHO MONICA Project. J Clin Epidemiol. 1999;52:1213–24. [DOI] [PubMed] [Google Scholar]
- 35.Molarius A, Seidell JC, Visscher TL, Hofman A. Misclassification of high-risk older subjects using waist action levels established for young and middle-aged adults--results from the Rotterdam Study. J Am Geriatr Soc. 2000;48:1638–45. [DOI] [PubMed] [Google Scholar]
- 36.Brown KA, McInnes KJ, Hunger NI, Oakhill JS, Steinberg GR, Simpson ER. Subcellular localization of cyclic AMP-responsive element binding protein-regulated transcription coactivator 2 provides a link between obesity and breast cancer in postmenopausal women. Cancer research. 2009;69:5392–9. [DOI] [PubMed] [Google Scholar]
- 37.Bernstein L, Henderson BE, Hanisch R, Sullivan-Halley J, Ross RK. Physical exercise and reduced risk of breast cancer in young women. Journal of the National Cancer Institute. 1994;86:1403–8. [DOI] [PubMed] [Google Scholar]
- 38.Gammon MD, John EM, Britton JA. Recreational and occupational physical activities and risk of breast cancer. Journal of the National Cancer Institute. 1998;90:100–17. [DOI] [PubMed] [Google Scholar]
- 39.Friedenreich CM, Neilson HK, Farris MS, Courneya KS. Physical Activity and Cancer Outcomes: A Precision Medicine Approach. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22:4766–75. [DOI] [PubMed] [Google Scholar]
- 40.Jones LW, Kwan ML, Weltzien E, Chandarlapaty S, Sternfeld B, Sweeney C, et al. Exercise and Prognosis on the Basis of Clinicopathologic and Molecular Features in Early-Stage Breast Cancer: The LACE and Pathways Studies. Cancer research. 2016;76:5415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iyengar NM, Jones LW. Development of Exercise as Interception Therapy for Cancer: A Review. JAMA Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedenreich CM, Woolcott CG, McTiernan A, Ballard-Barbash R, Brant RF, Stanczyk FZ, et al. Alberta physical activity and breast cancer prevention trial: sex hormone changes in a year-long exercise intervention among postmenopausal women. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:1458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McTiernan A, Tworoger SS, Ulrich CM, Yasui Y, Irwin ML, Rajan KB, et al. Effect of exercise on serum estrogens in postmenopausal women: a 12-month randomized clinical trial. Cancer research. 2004;64:2923–8. [DOI] [PubMed] [Google Scholar]
- 44.Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Randomized controlled trial of exercise and blood immune function in postmenopausal breast cancer survivors. J Appl Physiol (1985). 2005;98:1534–40. [DOI] [PubMed] [Google Scholar]
- 45.Koelwyn GJ, Quail DF, Zhang X, White RM, Jones LW. Exercise-dependent regulation of the tumour microenvironment. Nature reviews Cancer. 2017;17:620–32. [DOI] [PubMed] [Google Scholar]
- 46.Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell metabolism. 2016;23:554–62. [DOI] [PubMed] [Google Scholar]
- 47.McTiernan A, Irwin M, Vongruenigen V. Weight, physical activity, diet, and prognosis in breast and gynecologic cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:4074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Irwin ML, McTiernan A, Bernstein L, Gilliland FD, Baumgartner R, Baumgartner K, et al. Relationship of obesity and physical activity with C-peptide, leptin, and insulin-like growth factors in breast cancer survivors. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005;14:2881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Irwin ML, Varma K, Alvarez-Reeves M, Cadmus L, Wiley A, Chung GG, et al. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ligibel JA, Campbell N, Partridge A, Chen WY, Salinardi T, Chen H, et al. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:907–12. [DOI] [PubMed] [Google Scholar]
- 51.Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:42–51. [DOI] [PubMed] [Google Scholar]
- 52.Pietilainen KH, Kannisto K, Korsheninnikova E, Rissanen A, Kaprio J, Ehrenborg E, et al. Acquired obesity increases CD68 and tumor necrosis factor-alpha and decreases adiponectin gene expression in adipose tissue: a study in monozygotic twins. The Journal of clinical endocrinology and metabolism. 2006;91:2776–81. [DOI] [PubMed] [Google Scholar]
- 53.Gallagher EJ, LeRoith D. The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol Metab. 2010;21:610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holloway KR, Barbieri A, Malyarchuk S, Saxena M, Nedeljkovic-Kurepa A, Cameron Mehl M, et al. SIRT1 positively regulates breast cancer associated human aromatase (CYP19A1) expression. Molecular endocrinology. 2013;27:480–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Subbaramaiah K, Iyengar NM, Morrow M, Elemento O, Zhou XK, Dannenberg AJ. Prostaglandin E2 down-regulates sirtuin 1 (SIRT1), leading to elevated levels of aromatase, providing insights into the obesity-breast cancer connection. The Journal of biological chemistry. 2019;294:361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cuzick J, Sestak I, Forbes JF, Dowsett M, Cawthorn S, Mansel RE, et al. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395:117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. The New England journal of medicine. 2011;364:2381–91. [DOI] [PubMed] [Google Scholar]
- 58.Aktas B, Sorkin M, Pusztai L, Hofstatter EW. Uptake of exemestane chemoprevention in postmenopausal women at increased risk for breast cancer. Eur J Cancer Prev. 2016;25:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


