Highlights
-
•
Persons with binge eating disorder show increased impulsivity.
-
•
We investigated cognitive control to food cues using fNIRS.
-
•
Compared to healthy controls, binge eaters show weaker activation of the prefrontal cortex.
-
•
After behavioral therapy, binge eaters increase prefrontal cortex activation.
Keywords: Binge eating disorder, Cognitive control, fNIRS, Impulsivity, Prefrontal cortex, Response inhibition
Abstract
Background
Behavioral and cognitive control are vital for healthy eating behavior. Patients with binge eating disorder (BED) suffer under recurrent binge eating episodes accompanied by subjective loss of control that results, among other factors, from increased impulsivity.
Methods
In the current study, we investigated the frontal network using functional near-infrared spectroscopy (fNIRS) during a food specific go/nogo task to assess response inhibition in 24 patients with BED (BMI range 22.6–59.7 kg/m2) compared to 12 healthy controls (HC) (BMI range 20.9–27 kg/m2). Patients with BED were invited to undergo fNIRS measurements before an impulsivity-focused cognitive behavioral group treatment, directly after this treatment and 3 months afterwards. As this was a planned subgroup analysis of the randomized controlled IMPULS trial, patients with BED were randomized either to the treatment group (n = 14) or to a control group (n = 10). The treatment group received 8 weekly sessions of the IMPULS treatment.
Results
We found a significant response inhibition effect (nogo minus go), in terms of an increased oxygenated hemoglobin response in the bilateral prefrontal cortex in both groups. The greatest response was observed when participants were instructed to go for healthy and withhold their response to unhealthy high caloric food cues. The healthy nogo condition failed to show a significant prefrontal inhibitory response, which was probably related to the task design, as the condition was considered more demanding. BED patients, especially those with higher trait impulsivity, showed a weaker activation of the prefrontal cortex during response inhibition, predominantly in the right hemisphere. Interestingly, three months after the treatment, patients of the treatment group increased their right prefrontal cortex activity during response inhibition. Likewise, increased prefrontal cortex activation correlated with decreased trait impulsivity after treatment.
Conclusions
Our results suggest that patients with BED have limited resources to activate the prefrontal cortex when asked to inhibit a reaction onto food-specific stimuli. However, this effect could be partly driven by differences in BMI between the HC and BED group. Cognitive-behavioral therapy targeting impulsive eating behavior may improve prefrontal cortex recruitment during response inhibition.
1. Introduction
The fight against obesity is one of the most challenging tasks of modern times. Healthcare costs associated with the treatment of obesity and its comorbidities constitute an estimated annual burden of about $ 29 billion Euro in Germany (Effertz et al., 2016). At the same time, there is an enormous increase in eating disorders in all age groups worldwide (Hudson et al., 2007). Binge eating disorder (BED) strongly associates with severe obesity, a finding consistently reported in both clinical and community based samples (Hudson et al., 2007). According to the Fifth Edition (DSM-5) of the Diagnostic and Statistical Manual, BED is defined as constantly recurring eating of very large food portions in the absence of hunger in a discrete period of time. During these episodes, patients with BED exhibit a subjective loss of control. This behavioral pattern is often associated with a lack of impulse control. Beside increased reward sensitivity, decreased inhibition is a major factor in the concept of impulsivity, and the interaction of both factors constitutes the syndrome of the eating disorder (Dawe and Loxton, 2004). Specifically, patients with BED show, according to several systematic reviews, enhanced reward sensitivity to high calorie food items, increased attention to food stimuli and reduced behavioral response inhibition (Chami et al., 2019, Giel et al., 2017, Schag et al., 2013a, Stojek et al., 2018). There is still an ongoing debate whether impulsivity deficits in patients with BED are general or food-specific. For example, BED is associated with increased trait impulsivity (Gerlach et al., 2015) and particularly, Manasse and colleagues (2016) reported a general response inhibition deficit. However, according to the systematic reviews cited above (Chami et al., 2019, Giel et al., 2017, Schag et al., 2013a, Stojek et al., 2018), most experimental studies including behavioral as well as brain imaging data point to diminished inhibitory control or a tendency for more rash behaviors specifically towards food in persons with BED. Most studies investigating behavioral inhibition in obesity and BED use a stop-signal task (SST) or a go/nogo paradigm, frequently with neutral and food items. In both paradigms, participants have to withhold a prepotent response when confronted with rare stimuli, while reacting to more frequent stimuli. For successful inhibition, trait impulsivity seems to be an important factor as performance of patients with BED in inhibitory control tasks correlate with trait impulsivity (Hege et al., 2015, Muhlberg et al., 2016, Schag et al., 2013b). However, there are only a limited number of studies that examined food-specific impulsive behavior in obese persons with and without BED (Batterink et al., 2010, Jasinska et al., 2012, Loeber et al., 2012). Thus, to further specify the food-specific inhibitory control deficits in patients with BED, we compare inhibitory control using healthy vs. unhealthy foods in a go/nogo task in the current study.
In addition to behavioral parameters, the neurophysiological signature of inhibitory control to food stimuli is of particular interest in BED (Kessler et al., 2016). Successful inhibition activates the prefrontal control network resulting in an increase in neuroelectrical activity (Folstein and Van Petten, 2008, Hege et al., 2014) and an increase in prefrontal hemodynamic responses (Carbine et al., 2018, Oliva et al., 2019). For instance, first electroencephalography (EEG) studies in patients with BED indicate that diminished conflict processing as well as increased frontal beta activity are positively associated with inhibitory control (Leehr et al., 2018, Tammela et al., 2010). Furthermore, patients with BED show a hypoactivity in the prefrontal control network during response inhibition, particularly individuals with increased behavioral impulsiveness (Hege et al., 2015). Functional magnetic resonance imaging (fMRI) measurements, investigating regional hemodynamic changes, report elevated food-cue reactivity in reward-related brain regions, including the orbitofrontal cortex, in persons with BED (Romei et al., 2020, Schienle et al., 2009). This supports the notion of heightened sensitivity to rewarding stimuli. In particular, one pilot fMRI study detected that reward system activity discriminates between patients who recover from BED after treatment and patients who do not (Balodis et al., 2014). However, no fMRI study thus far investigated neural correlates of food-specific inhibitory behavior in patients with BED before and after treatment. This is in part due to the methodological limitations of neuroimaging techniques. Individuals diagnosed with BED often suffer from severe or morbid obesity, which makes it difficult to assess brain activation for example in a supine position using fMRI.
Functional near-infrared spectroscopy (fNIRS) is an cost-effective and highly adaptable neuroimaging tool that allows measurements in a natural environmental setting (for example while sitting, standing etc.) in healthy participants as well as various clinical samples (Ehlis et al., 2014). Unlike fMRI, subjects are not confined to a supine position permitting the implementation of more complex tasks. Both fMRI and fNIRS are non-invasive techniques measuring the hemodynamic response as a proxy of neural activity. fNIRS uses near-infrared light to measure oxygenated and deoxygenated hemoglobin in superficial layers of brain tissue, which correlates with the fMRI-based BOLD signal (Steinbrink et al., 2006). In patients with eating disorders, two studies report diminished prefrontal activity during a cognitive task. Suda et al. (2010) reported less activity in frontal regions during a verbal fluency task in persons with an eating disorder particularly with an increasing number of binge eating scores. In response to food cues, one study identified diminished prefrontal cortex (PFC) reactivity in children with Anorexia Nervosa (Nagamitsu et al., 2011). Moreover, Rosch et al. (2020) showed PFC hyporesponsivity in obese patients with and without BED in an fNIRS study with a food-specific go/nogo task. However, no study so far investigated the neurophysiological signature of inhibitory control towards food stimuli in patients with BED undergoing cognitive-behavioral therapy.
Therefore, in the present study we investigated the frontal network using fNIRS during a food-specific go/nogo task to assess inhibitory control in patients with BED compared to healthy controls and compared before vs. after treatment. In general, cognitive behavioral treatment (CBT) for BED is considered the evidence-based treatment of choice (Agras et al., 2017, Hay, 2013, Hilbert et al., 2019, Vocks et al., 2010). Schag et al. (2019) developed a specific food-related and impulsivity-focused cognitive behavioral treatment to enhance control over eating and decrease the number of binge eating episodes. Besides traditional CBT, this outpatient group therapy included two main interventions, a) reducing the risk for binge eating episodes by implementation of individual stimulus and response control strategies, and b) food-related cue exposure with response prevention. It is suggested that these two interventions reduce food-related impulsivity, i.e. reduce hyper-responsivity of the reward system towards food stimuli and increase inhibitory control, i.e. enhance frontal cortex activity while inhibiting reactions towards food. Thus, we expect that patients with higher deficits in impulsivity, i.e. reduced activity in prefrontal cortex, should benefit more from the treatment. The IMPULS treatment has already been investigated concerning its efficacy in the randomized controlled IMPULS trial (Schag et al., 2019). Patients with BED were randomized to either the treatment group or a control group. The treatment group received eight weekly sessions of the impulsivity-focused group intervention; the BED control group received no treatment. A subgroup of these patients from the IMPULS trial were invited to undergo fNIRS measurements before, directly after treatment and 3 months after completing the therapy.
We hypothesize that before treatment, BED patients compared to the healthy control group exhibit reduced prefrontal activation during food-specific response inhibition (Rosch et al., 2020). Furthermore, we conjecture that the prefrontal activation pattern at baseline is a significant predictor for treatment success (i.e. eating disorder pathology at the end of treatment) and correlates with trait impulsivity. Moreover, we expect improved impulse control with enhanced prefrontal activity in the treatment group after treatment, but not in the BED control group.
2. Methods
2.1. Participants
Patients with BED were recruited through the Department of Psychosomatic Medicine and Psychotherapy at the University Hospital of Tübingen from the IMPULS trial (Schag et al., 2019, Schag et al., 2021). BED was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria. All BED patients were invited to undergo NIRS measurements before treatment (T0), at the end of treatment (T1) and 3 months after completing treatment (T2). Exclusion criteria for both groups including patients with BED comprised somatic diseases influencing weight or eating behavior and instable medication, pregnancy or lactation, psychoactive medication except antidepressants, a history of head injury, neurological diseases, psychosis, bipolar I disorder or substance addiction.
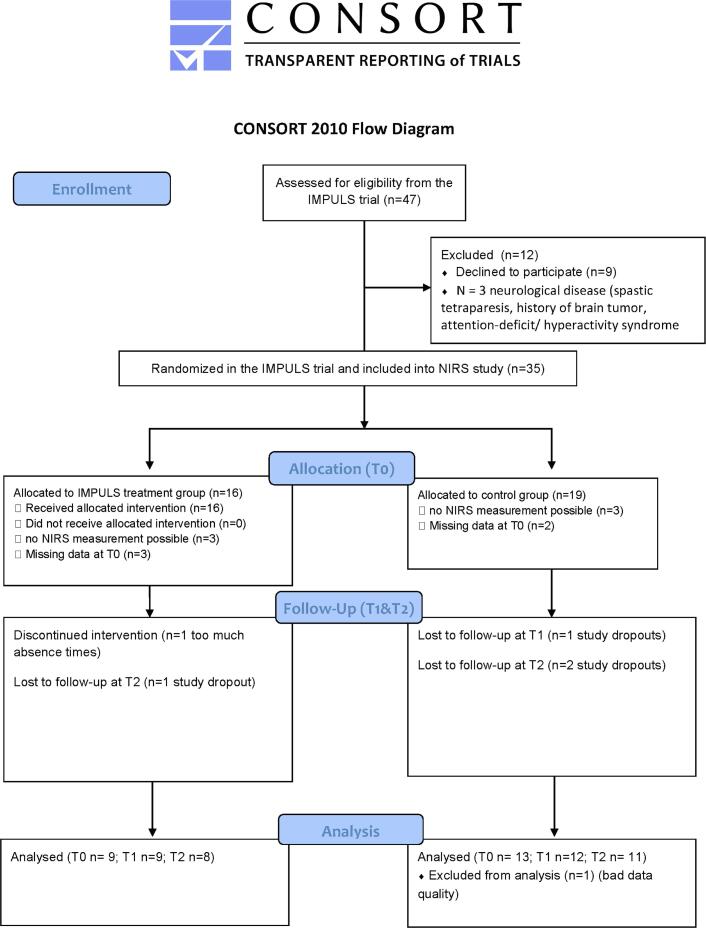
As the current NIRS study started one year into the IMPULS trial, 47 out of the 80 patients from the IMPULS trial (Schag et al., 2019) were asked to participate in the NIRS study (see CONSORT flow chart Fig. 1). 35 volunteers of this subsample (74,5%) participated in the NIRS study, 16 in the IMPULS treatment group and 19 in the control group. In 6 patients, NIRS measurements were not possible due to poor optical contact based on obstruction by hair (e.g. thick hair) so that finally 29 patients with BED were assessed. Based on missing data at baseline (due to technical difficulties (failure to save log files) and participants failing to show for baseline NIRS appointment T0), 5 further participants were excluded from the analysis leaving 24 patients with BED at T0 (20 women and 4 men; age range 19–63 years, BMI range 22.6–59.7 kg/m2). Of those, 3 (30%) patients in the treatment group (TG) and 4 (29%) patients in the control group (CG) presented at least one comorbid mental disorder at baseline. In total, 12 (75%) anxiety disorders were currently diagnosed as well as 3 affective disorders (19%) and one somatoform disorder (6%).
Fig. 1.
Patient flow chart of the NIRS analysis in the IMPULS trial.
Additionally, 15 healthy normal weight or overweight controls (HC) (7 women and 5 men; age range 24–60 years, BMI range 20.9–27 kg/m2) participated in the study at T0 of which three were not assessable by NIRS due to poor optical contact based on obstruction by hair. HC were recruited through local advertisement and reported no history of serious or chronic illness including neurological and mental disease, in particular no eating disorder diagnosis. The sample characteristics of the HC and BED patients at baseline are presented in Table 1. The sample characteristics of the TG and CG are presented in Table 2.
Table 1.
Participants’ characteristics at baseline (T0).
| Healthy controls (HC) | Binge eating disorderpatients (BED) | P-value | |
|---|---|---|---|
| Gender (f/m) | 7/5 | 20/4 | 0.126§ |
| Age (years) | 39.2 ± 12.0 | 42.5 ± 12.7 | 0.463 |
| BMI (kg/m2) | 23.7 ± 1.6 | 39.3 ± 9.7 | <0.001 |
| Hunger prior to fNIRS (VAS) | 2.2 ± 2.3 | 3.6 ± 2.8 | 0.150 |
| Education (school graduation) N (%)Low/High | 0/12 | 2/22 | 0.543§ |
Displayed are mean ± SD; P-values based on one-way ANOVA; §based on Chi-square test.
Abbreviations: VAS, Visual Analogue Scale.
Table 2.
Characteristics of BED patients at all three measurement time points.
| Time point |
T0 |
T1 |
T2 |
|||
|---|---|---|---|---|---|---|
| Group | TG | CG | TG | CG | TG | CG |
| N | 10 | 14 | 9 | 12 | 8 | 11 |
| Age (years) | 38.8 ± 12.7 | 45.2 ± 12.6 | ||||
| BMI (kg/m2) | 37.4 ± 10.4 | 40.5 ± 9.4 | 36.0 ± 10.6 | 40.1 ± 10.3 | 36.5 ± 12.3 | 40.7 ± 10.2 |
| BIS-15 | ||||||
| Total | 35.8 ± 6.1 | 36.7 ± 8.3 | 34.1 ± 5.6 | 35.8 ± 8.4 | 33.3 ± 5.3 | 34.9 ± 7.1 |
| Motor | 11.8 ± 2.6 | 12.5 ± 2.7 | 11.5 ± 3 | 11.9 ± 3.3 | 10.5 ± 3.3 | 12 ± 2.3 |
| Attention | 10.9 ± 2.8 | 11.2 ± 3.1 | 10.5 ± 2.6 | 11 ± 3.1 | 10.3 ± 3.0 | 10.7 ± 3.0 |
| Nonplanning | 13.1 ± 3.2 | 13 ± 3.7 | 12 ± 1.4 | 12.8 ± 3.9 | 12.5 ± 1.6 | 12.5 ± 3.8 |
| EDE-Q total* | 2.8 ± 0.5 | 2.5 ± 0.7 | 2 ± 0.9 | 2.2 ± 0.7 | 1.9 ± 1.12 | 2.2 ± 1.0 |
| BDI II sum score | 16.1 ± 10.8 | 14.0 ± 11.3 | 10.8 ± 8.9 | 9.25 ± 6.8 | 11.1 ± 13.4 | 10.9 ± 10.7 |
| Hunger prior to fNIRS (VAS) | 3.7 ± 2.6 | 2.8 ± 2.1 | 1.5 ± 1.9 | 2.1 ± 2.2 | 1.2 ± 1.9 | 2.4 ± 2.3 |
Displayed are mean ± SD; There is no significant difference between the CG and TG group for all listed variables at time point T0, T1 and T2 (one-way ANOVA; p < 0.05); *Within-group analysis shows a significant decrease in EDE-Q total score for the TG group (p < 0.05); Abbreviations: BIS-15, Barratt Impulsivity Scale short version; BDI II, Becks Depression Inventory; EDE-Q, Eating Disorder Examination Questionnaire; TG, Treatment group; CG, Control group.
This study was approved by the local ethics committee at the University of Tübingen. All participants gave written informed consent before the experiment (Project number: 245/2015BO2).
2.2. Procedure
Prior to the fNIRS experiment, BED patients had an additional study appointment to assess height, weight, socioeconomic variables and BED diagnosis as well as comorbid disorders using the Structured Clinical Interview for Axis I Disorders (SCID-I (Wittchen et al., 1997)). In addition, participants reported eating disorder pathology using the Eating Disorder Examination Questionnaire (EDE-Q (Hilbert and Tuschen-Caffier, 2006)), depressive symptoms in the Becks Depression inventory (Hautzinger et al., 2006), and the BIS-15 (Meule et al., 2011) was used to assess different aspects of impulsivity as a personality trait. Besides the total sum score, we assessed the three second-order factors of the questionnaire including attentional, motor and non-planning impulsiveness.
Patients with BED received a standardized typical German breakfast at the Department of Psychosomatic Medicine and Psychotherapy, and healthy controls were instructed to eat their usual breakfast before coming to the NIRS experiment. To assess subjective feeling of hunger, participants rated their current hunger level before and after the experiment on a visual analogue scale (0: not hungry at all, 10: very hungry). Recording sessions started between 9:00 and 13:00 h, on average 2 h after breakfast, and lasted for 1 h. All participants had normal or corrected-to-normal vision.
2.2.1. Stimuli and go/nogo task
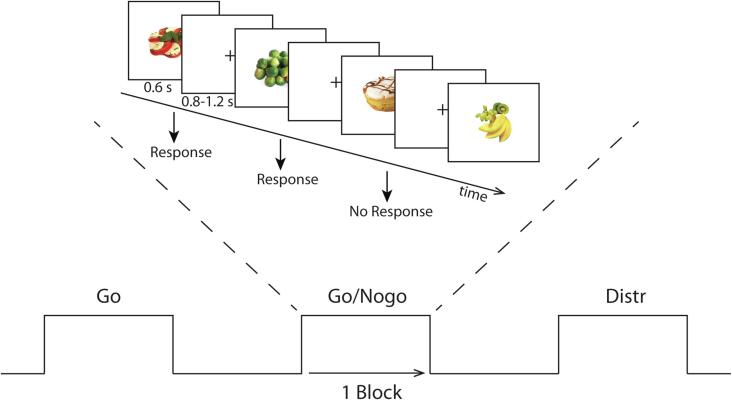
During the fNIRS recording, participants performed a visual go/nogo task in a block design with two different conditions lasting 14 min each. In the unhealthy nogo condition, participants were instructed to withhold their respond to unhealthy food pictures and respond to healthy food pictures (i.e. unhealthy food as nogo cues, healthy food as go cues). In the healthy nogo condition, participants were instructed to withhold their response to healthy food pictures and respond to unhealthy food pictures (i.e. healthy food as nogo cues, unhealthy food as go cues). Participants were either asked to respond to healthy food or unhealthy food pictures by pressing a button with their right index finger using a computer mouse. Each condition consisted of 14 blocks of data collection. Each block contained 16 food stimuli presented for 600 ms with an interstimulus interval of 800–1200 ms (see Fig. 2). Of the 14 blocks, six go blocks contained only go cues; six go/nogo blocks contained 4 or 6 nogo cues and two distraction blocks contained 8 nogo or only 2 nogo cues. As a result, a total of 184 go trials and 40 nogo trials were presented in each session. The blocks were presented in a pseudorandomized order and counterbalanced across groups. The distraction blocks were used to ensure that the participants could not predict whether the current block was a go or mixed go/nogo block.
Fig. 2.
Go/nogo paradigm during fNIRS recording. Each session consisted of 6 go blocks, 6 go/nogo blocks and 2 distraction blocks. The experiment consisted of two conditions: a healthy and an unhealthy condition. The figure displays the unhealthy nogo condition; participants were instructed to withhold their response to unhealthy food stimuli (unhealthy nogo, healthy go). For the healthy condition, participants were instructed to withhold their response to healthy food stimuli.
The stimulus material was selected from the food-pics database (http://food-pics.sbg.ac.at, (Blechert et al., 2014)). The unhealthy food images had significantly more kilocalories per 100 g (t(78) = 9.86, p < 0.001) and more kilocalories in total (unhealthy: 611.61 (SD 796.02) kcal, healthy: 151.59 (139.94) kcal; t(78) = 3.60, p < 0.001) in comparison with healthy food items. The pictures did not differ in terms of complexity, intensity and spatial frequencies (see Supplementary Table 1 for a detailed description of the picture set). Prior to the task, participants rated each food stimulus for tastiness and healthiness on a scale of 1 to 5 (1: very tasty up to 5: not at all tasty; 1: very healthy up to 5: very unhealthy). Both HC and patients with BED correctly identified stimuli as healthy and unhealthy by 98%.
The reaction times for correct responses during go trials and unsuccessful inhibition during nogo trials were recorded. Furthermore, commission errors (i.e., “go” responses for nogo trials (false alarms)) were calculated based on the individual ratings. The percentage of commission errors was calculated as the total number of failures of inhibition divided by the total number of nogo trials multiplied by 100.
2.2.2. fNIRS recording and preprocessing
The multi-channel NIRS device OXYMON MK III (ARTINIS MEDICAL SYSTEMS, Elst, Netherlands) was used to record oxygenated (O2Hb, wavelength: 847 nm) and deoxygenated (HHb, wavelength: 761.5 nm) hemoglobin concentrations at a sampling rate of 50 Hz. Two 4x4 optode probesets consisting of 2 × 10 channels were used (see Supplementary Fig. 1). The distance between each transmitter and receiver was 3.5 cm. The center of the optical probes was placed either at F3 (left hemisphere) or F4 (right hemisphere) according to the international 10–20 EEG system (Jasper, 1958). The positioning of the channels covered large parts of the prefrontal cortex.
The software oxysoft from Artinis (Artinis Medical Systems, Elst, The Netherlands) was used to convert the raw data into relative changes in oxygenated (oxy-Hb) and deoxygenated hemoglobin (deoxy-Hb). Different path length factors were calculated in relation to the age of the subjects. Events were defined based on the markers in the trigger channel. For preprocessing and first level analyses, established matlab scripts were used (Haeussinger et al., 2014, Hudak et al., 2017, Metzger et al., 2017). For physiological noise removal (heartbeat and respiration), a band pass filter was applied to the data (frequency band: 0.01 to 0.1 Hz). Each file was individually checked for motion artifacts. Events with signal amplitudes above 1.5 mmol*mm in both oxy-Hb and HHb were marked and excluded from further analyses. As a result, 3.07% (range between subjects: 0–16.6%) of all events were discarded. Noisy channels were interpolated using three surrounding channels. This concerned particularly channel 7 and 17, which were positioned at the border of the probeset.
For further analyses, oxy-Hb was investigated. While both oxy-Hb and deoxy-Hb give vital information about cortical activation, oxy-Hb is considered a more reliable parameter (Plichta et al., 2006). Furthermore, previous NIRS protocols using go/nogo identified stable oxy-Hb increases during go/nogo (Herrmann et al., 2005).
2.3. Statistical analyses
2.3.1. fNIRS data
2.3.1.1. Data processing
Event-related averages for oxy-Hb and deoxy-Hb were computed using an interval of 50 s (10 s baseline, 30 s task, 10 s task offset) for each block (nogo, go, distraction), separately for the healthy and unhealthy condition (see the time courses for each channel during nogo-blocks, Supplementary Fig. 2). The signal change between the entire block of each event (30 s) and the preceding baseline was calculated using Ordinary Least Squares (OLS) linear regression. The resulting parameter estimates (betas) for each subject, channel, condition and block were extracted and further analyzed. The distraction blocks were discarded from further analysis.
2.3.1.2. First visit (T0): Healthy controls and BED patients
For all statistical analyses, IBM SPSS Statistics 27 was used. The parameter estimates (betas) were analyzed, for each channel, by creating contrasts of nogo minus go for the healthy and unhealthy condition separately. We first evaluated response inhibition (nogo minus go) for the BED and HC group separately using a one-sample t-test. Furthermore, we used General Estimating Equations (GEE) to investigate group specific effects; subject identification was used as subject variable and condition (healthy /unhealthy) as within subjects variable. At T0, the factors group (HC vs. BED) and condition (healthy vs. unhealthy) were used. Furthermore, age was used as a covariate as it strongly influences hemodynamic brain activations during cognitive control processes (Vasta et al., 2017). In the GEE model, we investigated the main effects condition, group and age, as well as the group × condition interaction. For testing the effects in the model, a Wald Chi Square test was used (p < 0.05). For all contrasts, a p-value of 0.05 (Dubey/Armitage-Parmar corrected for multiple testing) was considered significant. The Dubey/Armitage-Parmar correction procedure was used (Sankoh et al., 1997) to correct for multiple comparisons over all channels. This approach takes into account the correlation between neighboring channels and has been applied in several NIRS studies (Artemenko et al., 2019, Ehlis et al., 2009, Heinzel et al., 2013). For significant interactions pairwise comparison were performed using Bonferroni-Holm adjustment for multiple comparisons. Topographical plots were generated with established matlab scripts to display t-values of nogo minus go response.
2.3.1.3. Effect of IMPULS treatment in BED patients
Due to the limited number of participants, a composite score of the left (Channels 2,3,5,7,8,9) and right (Channels 12,13,15,17,18,19) channels were created to analyze the effect of treatment on the left and right hemisphere prefrontal inhibition response, instead of each channel separately. We used the nogo minus go contrasts of the left and right prefrontal cortex to calculate per protocol analyses using GEE. The unhealthy and healthy conditions were separately analyzed. The subject identification was defined as subject variable and time (time points T0, T1 and T2) were considered as within-subject variables. For the analysis, we defined time and treatment (BED treatment group versus BED control group) as factors. Age, BMI and BIS-15 total score at T0 were used as covariates. For significant effects pairwise comparison were performed using sequential Bonferroni Holm adjustment for multiple comparisons (p < 0.05).
2.3.2. Behavioral data (Go/Nogo task)
2.3.2.1. First visit (T0): Healthy controls and BED patients
Concerning sample characteristics, one-way ANOVAs were used to report group differences in BMI, age, gender and self-reported hunger.
For the go/nogo task, the commission errors (false alarm rate) were analyzed using a GEE with binomial link function; this is an extension of the generalized linear model for dependent measurements. The reaction times during go blocks were analyzed using a GEE with a linear variable without transformation. At T0, the factors group (HC vs. BED) and condition (healthy vs. unhealthy) were used. Furthermore, age was used as a covariate. For testing the effects in the model, a Wald Chi Square test was used (p < 0.05).
2.3.2.2. Effect of treatment in BED patients
Concerning sample characteristics, repeated measurement ANOVAs with the within-subjects factor time point T0, T1 and T2 and between-subject factor BED treatment group versus BED control group were computed.
Concerning the go/no-no task, the treatment effect was examined on commission errors by including all three time points into the GEE model. Thus, we analyzed the GEE with the factors treatment (BED TG vs. BED CG), condition (healthy vs. unhealthy) and time (T0, T1 and T2). The continuous variables age and BIS-15 total as well as BMI at T0 were used as covariates.
2.3.3. Associations between brain activity and behavioral data
Pearson correlations were calculated between the eating disorder pathology (EDEQ total score), trait impulsivity (BIS-15 total score) and prefrontal response during the task. For this purpose, we used the composite score of the left (Channels 2,3,5,7,8,9) and right (Channels 12,13,15,17,18,19) prefrontal cortex inhibitory response (nogo minus go). Further, we correlated performance in the go/nogo task with impulsivity (BIS-15 total score) and BMI. For all analyses, a statistical threshold of p < 0.05 was considered as significant.
3. Results
3.1. Response inhibition task (go/ nogo)
3.1.1. First visit (T0): Healthy controls and BED patients
For the go/nogo paradigm, a significant effect of condition and age was observed for the commission errors and reaction time at T0 [commission errors: condition (χ2(1) = 35.10, p < 0.001, age χ2(1) = 12.65, p < 0.001); reaction time: condition (χ2(1) = 16.32, p < 0.001, age χ2(1) = 23.22, p < 0.001)]. Independent of the group, in the healthy condition, the reaction times were slower (464.36 (SD 64.68) ms versus 431.60 (SD 61.30) ms) and the error rates were higher (22.91% versus 12.85%) compared to the unhealthy condition. We found no group differences (commission errors: χ2(1) = 0.598, p = 0.439; reaction times: (χ2(1) = 0.136, p = 0.712) or group × condition interactions (commission errors: (χ2(1) = 0.362, p = 0.547); reaction times: (χ2(1) = 1.242,p = 0.265). Median and SD of reaction time and commission errors are reported in Table 3.
Table 3.
Go/nogo behavioral data at baseline (T0).
| Condition | HC | BED | P-value |
|---|---|---|---|
| Reaction time (ms) for correct go trials | |||
| Unhealthy | 416.82 ± 41.5 | 438.9 ± 68.7 | 0.313 |
| Healthy | 448.80 ± 62.7 | 457.1 ± 66.7 | 0.721 |
| Commission error % | |||
| Unhealthy | 12.89 ± 5.59 | 12.84 ± 8.8 | 0.984 |
| Healthy | 21.56 ± 12.37 | 23.59 ± 8.8 | 0.618 |
3.1.2. Effect of treatment in BED patients
The IMPULS treatment had no significant effect on commission errors and reaction time during the go/nogo task, i.e. there was no significant time × condition or time × treatment interaction (GEE model; all p > 0.05). However, there was a distinct condition effect with more commission errors and slower reaction times in the healthy compared to the unhealthy condition (commission errors: χ2(1) = 45.60, p < 0.001; reaction times; χ2(1) = 65.24, p < 0.001;). BMI and BIS-15 total showed no effect, but age showed a significant effect (commission errors: χ2(1) = 4.45, p = 0.035; reaction times: χ2(1) = 9.65, p = 0.002). Regarding the reaction times there was a significant treatment × condition interaction (χ2(1) = 5.07, p = 0.024). Post hoc pairwise comparisons revealed faster reaction times in the unhealthy condition compared to the healthy condition in both BED groups (mean difference in reaction time of −29.65 ± 4.48 for the BED treatment and −16.83 ± 3.55 for the BED control group) (both p < 0.001, Bonferroni corrected).
3.1.3. Correlation with go/nogo behavior in BED patients at baseline (T0)
There was a significant association between BMI at T0 and the number of commission errors in the healthy condition adjusted for age (radj = 0.50, padj = 0.014), such that patients with a higher BMI made more errors during nogo. No significant correlation was observed between BIS-15 and commission errors (p > 0.05).
3.2. fNIRS data
3.2.1. Prefrontal cortex activation during food-specific response inhibition in HC and BED patients at baseline (T0)
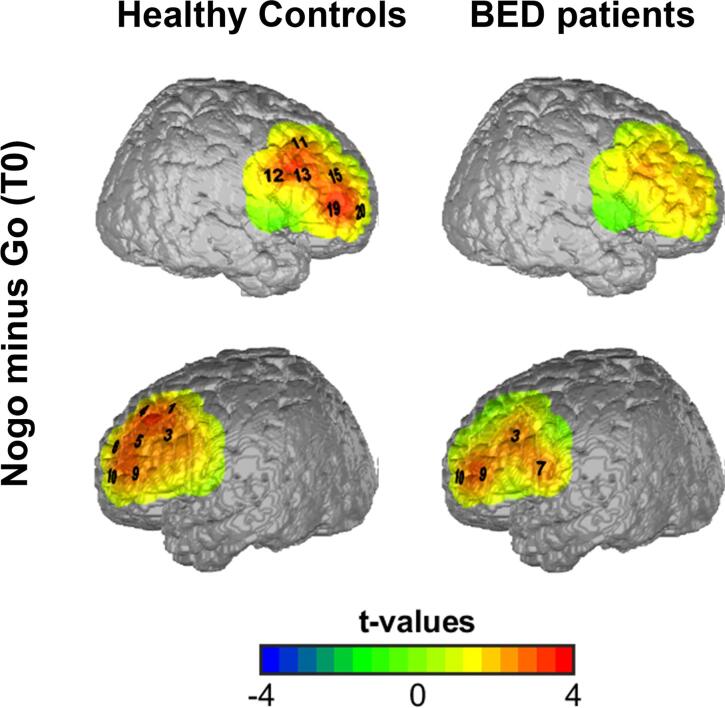
In the unhealthy condition, we observed in the HC group an increase in oxy-Hb for nogo minus go in most frontal channels (channel 1,3,4,5,6,9,10,11,12,13,15,19, and 20, p < 0.05, one-sample t-tests D/AP corrected) (supplementary Table 2), which includes the bilateral prefrontal regions; in the BED patients, we found an increase in oxy-Hb only in four channels of the left prefrontal cortex (channel 3,7,9 and 10; p < 0.05, one-sample t-tests D/AP corrected) (supplementary Table 2; Fig. 3). In the healthy condition, we observed no significant differences for nogo minus go in both, the HC and BED group.
Fig. 3.
Topograhic plot of prefrontal activation during response inhibition (nogo minus go) during the unhealthy condition. Significant oxy-Hb increase in right (upper panel) and left (lower panel) frontal channels for nogo versus go projected on a brain map in healthy volunteers and BED patients. Channels marked in black numbers survived D/AP correction for multiple comparisons. The colors indicate t-values.
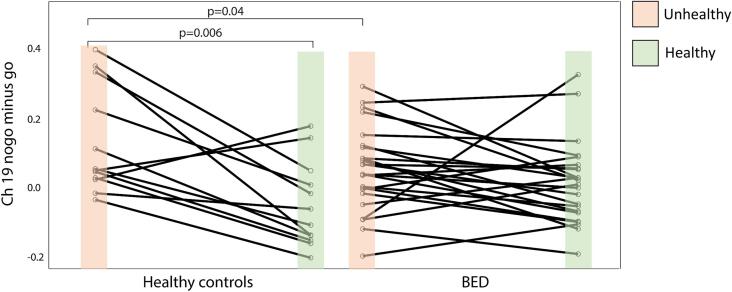
To investigate group differences and interactions, we used a GEE with group and condition as categorical factors and age as covariate. We found a significant main effect of condition (unhealthy vs. healthy) for nogo minus go in channel 1,2,3,5,6,7,8,9,10,12,13,15,16, 18, 19 and 20 (all p-values < 0.05, D/AP corrected). No main effect of group (HC vs. BED at T0) or age was identified. However, a significant group × condition interaction was found in the right prefrontal cortex, namely channel 10, 13, 15 and 19, although only channel 19 survived D/AP correction (χ2(1) = 6.09, p = 0.02) (Fig. 4). Pairwise comparisons of Channel 19 revealed that the HC group showed a significant difference between the unhealthy compared to the healthy condition for nogo minus go (p = 0.002 Bonferroni-Holm corrected), while BED patients showed no difference between conditions (p = 0.29).
Fig. 4.
Significant group × condition interaction in response inhibition of the right prefrontal cortex at T0. Spaghetti plot shows differential oxy-Hb beta weight for nogo minus go in channel 19 for the unhealthy and healthy condition. HC show an increased brain activity in response inhibition during the unhealthy compared to the healthy condition (p = 0.002, Bonferroni corrected). BED patients show a weaker response inhibition in the right prefrontal cortex (Channel 19) than HC during the unhealthy condition (p = 0.04, uncorrected for multiple comparisons).
3.2.2. Correlations between prefrontal inhibitory response and impulsivity at T0
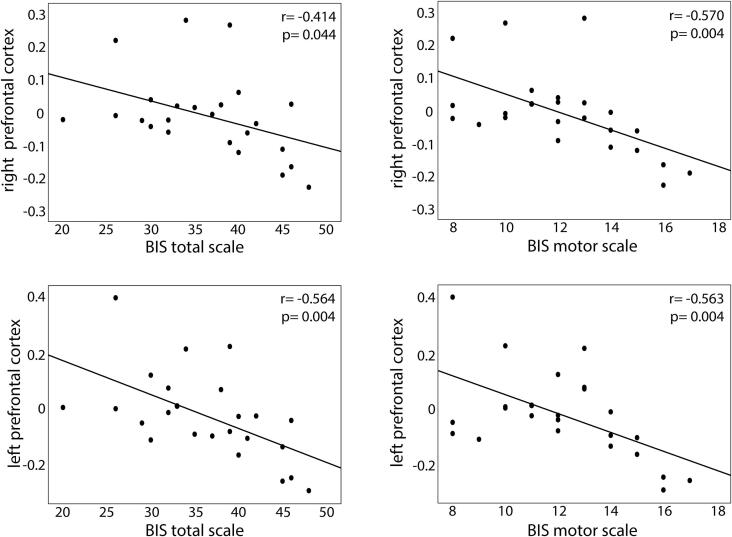
Furthermore, BED patients showed a significant relationship between trait impulsivity and prefrontal inhibitory response during nogo minus go. Higher impulsivity scores, particularly the total scale and the motor subscale, correlated with decreased left and right prefrontal cortex inhibitory response at baseline (T0). This was only found for the healthy condition (Fig. 5). Not all results remained significant after adjusting for age and BMI (p < 0.05) (see supplementary Table 3).
Fig. 5.
Relationship between prefrontal inhibitory response (nogo minus go) in the healthy condition and trait impulsivity in BED patients at baseline (T0). Plots show significant negative correlations between response inhibition in the right and left prefrontal cortex with the total BIS scale and the BIS motor scale (based on BIS-15 questionnaire).
3.2.3. Effect of IMPULS treatment on prefrontal cortex activation during response inhibition
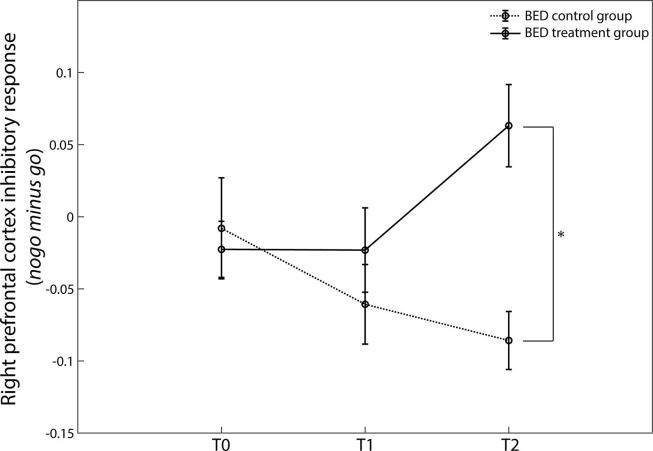
In a further step, we investigated the effect of the IMPULS treatment on the inhibitory response (nogo minus go) of the left and right prefrontal cortex in BED patients. In the right PFC, we identified a significant main effect of treatment (χ2(1) = 8.434, p = 0.004) with stronger response inhibition in the BED treatment opposed to the BED control group in the healthy condition. Moreover, a time × treatment interaction was found for the nogo minus go response in the right prefrontal for the healthy food condition (χ2(1) = 10.498, p = 0.005). Pairwise comparisons revealed a significant difference for the T2 right prefrontal inhibitory response between the treatment and the control group (p < 0.001 corrected) (Fig. 6). No significant difference was found between the treatment and control group for the T0 and T1 right prefrontal inhibitory response in the healthy food condition (p > 0.05). BED patients undergoing treatment increased their right prefrontal activity 3 months (T2) after completing the therapy (p < 0.001 corrected). BED patients of the control group showed no significant change in response inhibition of the right prefrontal cortex. For the unhealthy condition, no significant treatment effect or interactions were found in the right DLPFC.
Fig. 6.
Effect of IMPULS treatment on the response of the right prefrontal cortex for nogo minus go during the healthy condition. Diagram shows response inhibition at three time points (T0: before treatment, T1: directly after treatment, and T2: three months follow-up). Only BED patients undergoing treatment increased their prefrontal activity from T0 to T2 (*p < 0.05 for interaction group × time points adjusted for BMI, age and BIS-15 at baseline).
In the left DLPFC, we found for the healthy condition a significant main effect of treatment (χ2(1) = 5.357, p = 0.021) and a main effect of BIS-15 total at T0 (χ2(1) = 15.153, p < 0.001), while for the unhealthy condition a significant effect of age was observed (χ2(1) = 5.879, p = 0.015). No significant time × treatment interaction was found for the left DLPFC nogo minus go response (p > 0.05).
3.2.4. Associations between changes of prefrontal inhibitory response, impulsivity and eating disorder pathology
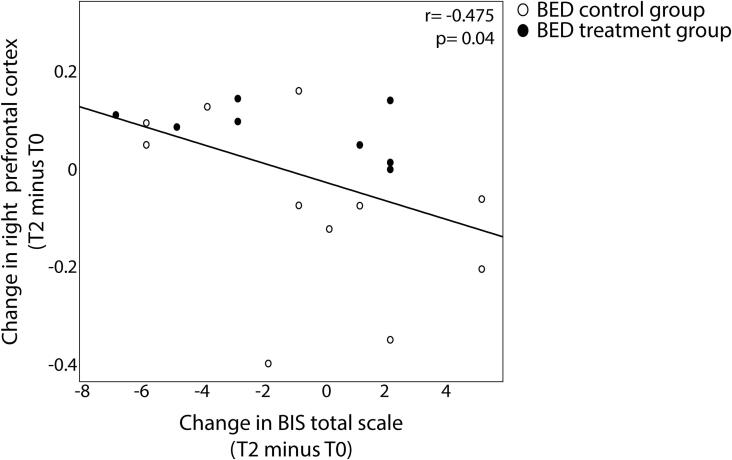
Moreover, we found a negative relationship between the increase in right prefrontal activity from visit T0 to T2 in the healthy condition and the decrease in impulsivity 3 months after treatment (ΔBIS-15 total score T2 minus T0) (r = -0.475, p = 0.04) (Fig. 7). Hence, participants showing a more pronounced increase in right prefrontal activity 3 months after completing the treatment have a more prominent decrease in trait impulsivity at follow-up.
Fig. 7.
Relationship between change in right prefrontal inhibitory response (nogo minus go) in the healthy condition and change in trait impulsivity in BED patients from before treatment (T0) to after three months follow-up (T2). Plots show significant negative correlations between change in response inhibition in the right prefrontal cortex with the change in total BIS-15 scale. Hence, persons reducing trait impulsivity after three months follow up show an increase in right prefrontal cortex activity.
Left and right prefrontal response inhibition (oxy-Hb) at T0 did not correlate with changes in eating disorder pathology (p > 0.05; based on EDEQ and BIS-15 questionnaire). Right prefrontal response inhibition at T1 in the unhealthy condition (directly after 8-wks therapy), however, correlated with the eating disorder pathology 3 months after treatment (ΔEDEQ sum score- T2 minus T0) (r = -0.588, p = 0.006).
4. Discussion
Prefrontal cortex activation during inhibition is vital for the control of eating behavior. This is the first study to investigate inhibitory control to food cues in healthy controls and patients with BED before and after a specific cognitive-behavioral therapy. Moreover, this is the first study implementing fNIRS in individuals with severe obesity (BMI > 40 kg/m2) to evaluate treatment success on the neurophysiological signature of inhibitory control. We identified a specific response inhibition signature in terms of an increased concentration of oxygenated hemoglobin in the bilateral prefrontal cortex using healthy and unhealthy food images in both groups. Patients with BED, exemplifying higher trait impulsivity, showed attenuated prefrontal cortex activation, particularly in the right prefrontal cortex. At a behavioral level, we found no group differences; however, with increasing BMI, BED patients made more errors during response inhibition. Three months after an impulsivity-focused cognitive treatment, BED patients increased their right prefrontal activity during response inhibition, which significantly correlated with a reduction in trait impulsivity. Contrary to our hypothesis, prefrontal cortex activity during response inhibition prior to the intervention did not predict treatment outcome. However, prefrontal cortex activity directly after treatment predicted eating behavior pathology 3 months later.
4.1. Effect of healthy versus unhealthy condition on response inhibition
In the current study, a modified experimental block design was developed to implement a classical food go/nogo task for fNIRS requirements. Based on the clinical picture of BED, we used both unhealthy high caloric food and healthy low calorie food pictures as nogo cues in a counterbalanced order. In both groups, we see the greatest prefrontal inhibitory response in the unhealthy condition, when participants were instructed to go for healthy and withhold their response to unhealthy high caloric food cues. To our surprise, no significant prefrontal response inhibition effect (nogo minus go) was observed in the healthy nogo condition in both healthy controls and BED patients. This is reflected by the behavioral findings showing significantly slower reaction times and more commission errors (false alarm rates) in the healthy condition in both groups. Nonetheless, all participants showed a similar pattern suggesting that the go/nogo task in the healthy condition was considered more difficult resulting in more errors, slower reaction times and a corresponding failure to show a prominent prefrontal inhibitory response. Previous behavioral studies comparing food-specific versus general inhibitory control revealed a higher commission error rate to food cues, particularly high caloric food, compared to neutral stimuli, as for example toys (Teslovich et al., 2014). So far, there are very few studies, though, that implemented different types of food stimuli within one inhibition paradigm. He and colleagues (He et al., 2019, He et al., 2014) report higher commission error rates to high calorie nogo stimuli as opposed to low calorie nogo stimuli in mostly normal-weight young participants, which is probably due to the higher salience of the high calorie food stimuli.
Based on the current study, we postulate that the behavioral differences observed using healthy vs. unhealthy nogo stimuli could be based on the fact that it is contrary to good judgement that low-calorie healthy food should be avoided and high-calorie unhealthy food preferred. Hence, the healthy nogo condition was probably considered more demanding, which lead to the observed increased errors and slower reactions and the failure to show a significant response inhibition in the PFC.
4.2. Reduced prefrontal cortex activation in BED patients
Our study is in line with recent imaging studies in obesity showing reduced prefrontal cortex activation and increased errors during inhibitory control (Batterink et al., 2010, He et al., 2019, He et al., 2014, Hege et al., 2015, Tuulari et al., 2015). This exacerbated PFC decrease seems to be even more pronounced in patients with BED compared to obese individuals without BED (Balodis et al., 2013, Hege et al., 2015). Moreover, even persons of normal weight with binge-eating episodes show lower prefrontal activity during response inhibition (Oliva et al., 2019). Using fNIRS, two studies confirm the notion of prefrontal hypoactivity by reporting diminished prefrontal activity across different tasks in persons with binge eating disorder (Rosch et al., 2020, Suda et al., 2010). Likewise, in our study, BED patients fail to increase right prefrontal activity when withholding the response to unhealthy food stimuli. Healthy participants, on the other hand, showed a distinct increase in activation in the left and right prefrontal cortex during response inhibition to unhealthy food. While it remains unclear whether the left versus right PFC has a specific contribution to pathological eating behavior, our study is consistent with the notion that dietary self-control is dependent on the capacity to regulate or modulate PFC activity (Kohl et al., 2019, Lowe et al., 2019, Neseliler et al., 2019). The increase in PFC activity has repeatedly been seen when individuals were asked to suppress the desire to eat (Batterink et al., 2010, Hollmann et al., 2012, Kohl et al., 2019) and predicts reduced food intake (Lopez et al., 2017). There is also some evidence supporting the left–right dichotomy (Lowe et al., 2019), allocating a special role of the right PFC to inhibitory control and reward-based learning (Alonso-Alonso and Pascual-Leone, 2007) and the left PFC to decision making processing including self-regulatory abilities (Lowe et al., 2019). This is in accordance with our finding that BED patients with impulse deficits, as measured with the BIS impulsivity scale, manifest diminished inhibition particularly in the right PFC compared to patients with less inhibitory deficits in the more demanding healthy condition.
4.3. Enhanced right prefrontal activation during food-specific response inhibition after IMPULS treatment in BED patients
There are several studies showing that cognitive-behavioral therapy is an effective treatment for eating disorders (Hay, 2013, Hilbert et al., 2019, Ricca et al., 2000). However, the remission rate is still about 50% in BED and weight-loss is rarely achieved (Hay, 2013). In a recent study, Schag et al. (2019) showed that the impulsivity aspect is a decisive factor for successful treatment. BED patients of the current study took part in this recently published impulsivity-focused treatment (Schag et al., 2019). The study reports a significant improvement in eating disorder pathology, showing a reduction in the number of binge eating episodes compared with the BED control group three months after treatment (Schag et al., 2019) and improved inhibitory control (Schag et al., 2021). Concomitantly, we describe a significant increase in right PFC activity during response inhibition to food cues three months after the treatment. Therefore, we speculate that the impulsivity-focused treatment modified implicit evaluation processes to food stimuli, which might be a prerequisite for effective regulation of maladaptive eating behavior. Interestingly, participants of the BED treatment groups enhanced prefrontal inhibitory activity during the more cognitive demanding healthy condition from before to after treatment. This may indicate that cognitive therapy in BED can improve prefrontal recruitment in cognitively demanding tasks.
In the current project, no significant change in brain activity or behavior was observed immediately following the 8-wks intervention. Moreover, BED patients randomized to the control group improved neither their eating disorder pathology nor prefrontal cortex inhibitory-related activity. In contrast, both the patients from the treatment group and from the control group reduced binge eating frequency in the IMPULS trial from Schag et al. (2019) directly after treatment. A potential explanation of these differences between the studies is two-fold: First, it might be that the short-term effect after treatment in the CG, reported in the IMPULS trial (Schag et al., 2019) was due to an increased motivation or self-observation to regulate eating behavior. This could not be sustained in the long term, as it did not consolidate in neurobiological processes. Second, patients from the treatment group might have been able to transfer the learned mechanisms of the IMPULS treatment into everyday life. Thus, they show an improvement in behavioral outcomes, i.e. binge eating frequency. However, to transfer the learned mechanisms to related behaviors e.g. while performing the Go/nogo task may take more time. As patients from the treatment group further reduced binge eating frequency after treatment (Schag et al., 2019), this could explain the delayed changes of brain activity in the Go/nogo task from the current study only at follow-up. Another possible explanation of the results is that other treatments might have influenced brain activity of the patients. However, only one patient from the treatment group started a psychotherapy during the follow-up and only one other patient started a guided weight reduction group program. We think it is unlikely that this small proportion of additional treatment affected our results. Last, it is worth noting that only a subsample (N = 24) of the IMPULS trial (N = 80) was included in the presented NIRS study. It could be that this subsample did not benefit as much from the IMPULS treatment as the other patients from the IMPULS trial by chance or - due to the smaller sample size, the NIRS subsample might lack statistical power to detect smaller effects directly after treatment.
There are only few studies investigating the effect of an impulsivity-focused training on the brain with inconclusive findings. Two studies employed EEG to capture the neurophysiological signature of inhibitory control. Blackburne et al. (2016) used a mobile-phone based intervention to train inhibitory control in overweight and obese persons. They show an enhanced P3 amplitude during response inhibition and modified food consumption, suggesting improved cognitive control (Blackburne et al., 2016). In a more recent EEG study, this finding could not be confirmed in BED patients. Although the intervention yielded promising behavioral results, no changes in N2 or P3 amplitudes of the EEG were identified (Chami et al., 2020b). Using fMRI, on the other hand, Balodis et al. (2014) found that BED patients who lowered their binge eating episodes after treatment revealed higher prefrontal cortex activity during reward processing. This leads to the assumption that the reported findings are presumably task and stimuli specific, which is further substantiated by the fact that food-specific interventions show greater success in reducing binge eating episodes (Chami et al., 2020a).
In the current study, there is a significant brain-behavior relationship in response to treatment. Patients who enhanced their right PFC response also showed the greatest improvement in controlling impulsive behavior and reduced eating behavior pathology 3 months after the intervention. However, contrary to our hypothesis, PFC response prior to the intervention did not predict treatment success. Nonetheless, right PFC activity directly after the 8-wk intervention predicted eating behavior pathology 3 months later. Likewise, a recent longitudinal fMRI study on dietary restriction showed that the prefrontal food-cue responsivity 1 month into the intervention predicted weight loss 3 months later (Neseliler et al., 2019). Particularly the interaction between brain circuits predicted the success in weight loss. Successful dieters showed enhanced functional connectivity between the dorsolateral PFC (cognitive control) and the ventromedial PFC (reward value) (Neseliler et al., 2019, Weygandt et al., 2013). Future studies in BED need to include interactions between brain circuitries, by investigating functional connectivity between prefrontal regions (cf. (Kroczek et al., 2017)), which might play a crucial role in facilitating self-control over eating behavior.
4.4. Limitations
The small sample size limits our statistical power and the generalizability of our findings. A further issue is the dropout rate in particular in the follow-up measurement 3 months after the therapy, which might have biased our results. Due to our study design, we cannot conclude whether differences in BMI or other comorbidities as depression could drive the differences in prefrontal response inhibition between healthy controls and BED patients. Furthermore, we cannot conclude whether BED patients showed general impulsive behavior changes. Hence, further studies using nonfood pictures or objects as control stimuli need to evaluate food-specific versus general inhibitory control in persons of overweight and obesity and BED. Furthermore, the failure to show a significant prefrontal response inhibition effect in the healthy nogo condition may also lie within the design of the study. Based on the fNIRS task requirements, we used a go/nogo block design, which has already been implemented successfully several years ago (Herrmann et al., 2005). However, the nogo blocks engage, besides inhibitory control, other cognitive functions such as attentional processes and working memory. For future studies, an event-related design could shed more light on the issue focusing on the inhibitory response and factoring out other cognitive processes. In general, studies using fNIRS are limited regarding both spatial and depth resolution making it difficult to generalize our findings. fMRI – with its increased depth resolution – shows that subcortical (striatal) regions also play an important role in go/nogo behavior (besides the prefrontal cortex). However, subcortical activation cannot be detected with most fNIRS devices, so that insight is only provided regarding cortical aspects of the broad network involved in the cognitive control of food-cue processing and eating behavior.
5. Conclusions
As obesity is worldwide on the rise, it is vital to implement neuroimaging tools that provide the possibility to investigate the underlying neurophysiological mechanisms of cognitive control in eating behavior in the entire spectrum of obesity. Using fNIRS, we could show a concentration increase in oxygenated hemoglobin in the prefrontal cortex during response inhibition using food stimuli in all weight groups. BED patients with high trait impulsivity revealed the most prominent decrements in right prefrontal inhibitory control in response to high caloric food cues. This suggests that patients with BED have limited resources to activate the prefrontal cortex when asked to inhibit a certain behavior. Cognitive-behavioral therapy targeting impulsive eating behavior, however, has the potential to improve prefrontal cortex recruitment, resulting in a more favorable treatment outcome.
Funding
The study was supported in part by a grant from the German Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD), and by the European Union Seventh Framework Programme (FP7/2007–2013) under Grant Agreement 607310 (Nudge-it). Furthermore, this work was supported by the Medical Faculty Tübingen within the applied clinical science funding, grant number 323–0-1 and personal funding of Kathrin Schag by the “Tübinger Frauenförderung”, grant numbert 2624–0-0.
CRediT authorship contribution statement
Ralf Veit: Methodology, Validation, Formal analysis, Writing - original draft. Kathrin Schag: Conceptualization, Methodology, Project administration, Writing - review & editing. Eric Schopf: Methodology, Investigation, Writing - review & editing. Maike Borutta: Investigation, Project administration. Jann Kreutzer: Methodology, Investigation, Writing - review & editing. Ann-Christine Ehlis: Software, Resources, Writing - review & editing. Stephan Zipfel: Conceptualization, Resources, Writing - review & editing, Supervision, Funding acquisition. Katrin E. Giel: Conceptualization, Resources, Writing - review & editing, Supervision, Funding acquisition. Hubert Preissl: Conceptualization, Methodology, Resources, Writing - review & editing, Supervision, Funding acquisition. Stephanie Kullmann: Conceptualization, Methodology, Validation, Formal analysis, Writing - original draft, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102679.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Agras W.S., Fitzsimmons-Craft E.E., Wilfley D.E. Evolution of cognitive-behavioral therapy for eating disorders. Behav. Res. Ther. 2017;88:26–36. doi: 10.1016/j.brat.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Alonso M., Pascual-Leone A. The right brain hypothesis for obesity. JAMA. 2007;297:1819–1822. doi: 10.1001/jama.297.16.1819. [DOI] [PubMed] [Google Scholar]
- Artemenko C., Soltanlou M., Bieck S.M., Ehlis A.C., Dresler T., Nuerk H.C. Individual Differences in Math Ability Determine Neurocognitive Processing of Arithmetic Complexity: A Combined fNIRS-EEG Study. Front. Hum. Neurosci. 2019;13:227. doi: 10.3389/fnhum.2019.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis I., Grilo C., Kober H., Worhunsky P., White M., Stevens M., Pearlson G., Potenza M. A Pilot Study Linking Reduced Fronto-Striatal Recruitment during Reward Processing to Persistent Bingeing Following Treatment for Binge-Eating Disorder. Int. J. Eat. Disord. 2014;47:376–384. doi: 10.1002/eat.22204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis I.M., Molina N.D., Kober H., Worhunsky P.D., White M.A., Rajita S., Grilo C.M., Potenza M.N. Divergent neural substrates of inhibitory control in binge eating disorder relative to other manifestations of obesity. Obesity (Silver Spring) 2013;21:367–377. doi: 10.1002/oby.20068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterink L., Yokum S., Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage. 2010;52:1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburne T., Rodriguez A., Johnstone S.J. A Serious Game to Increase Healthy Food Consumption in Overweight or Obese Adults: Randomized Controlled Trial. JMIR Serious Games. 2016;4 doi: 10.2196/games.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert J., Meule A., Busch N.A., Ohla K. Food-pics: an image database for experimental research on eating and appetite. Front. Psychol. 2014;5:617. doi: 10.3389/fpsyg.2014.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbine K.A., Duraccio K.M., Kirwan C.B., Muncy N.M., LeCheminant J.D., Larson M.J. A direct comparison between ERP and fMRI measurements of food-related inhibitory control: Implications for BMI status and dietary intake. Neuroimage. 2018;166:335–348. doi: 10.1016/j.neuroimage.2017.11.008. [DOI] [PubMed] [Google Scholar]
- Chami R., Cardi V., Lautarescu A., Mallorquí Bagué N., McLoughlin G. Neural responses to food stimuli among individuals with eating and weight disorders: a systematic review of event-related potentials. Int. Rev. Psych. 2019;31:1–14. doi: 10.1080/09540261.2019.1622515. [DOI] [PubMed] [Google Scholar]
- Chami R., Cardi V., Lawrence N., MacDonald P., Rowlands K., Hodsoll J., Treasure J. Targeting binge eating in bulimia nervosa and binge eating disorder using inhibitory control training and implementation intentions: a feasibility trial. Psychol. Med. 2020:1–10. doi: 10.1017/S0033291720002494. [DOI] [PubMed] [Google Scholar]
- Chami R., Treasure J., Cardi V., Lozano-Madrid M., Eichin K.N., McLoughlin G., Blechert J. Exploring changes in event-related potentials after a feasibility trial of inhibitory training for bulimia nervosa and binge eating disorder. Front. Psychol. 2020;11:1056. doi: 10.3389/fpsyg.2020.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe S., Loxton N.J. The role of impulsivity in the development of substance use and eating disorders. Neurosci. Biobehav. Rev. 2004;28:343–351. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Effertz T., Engel S., Verheyen F., Linder R. The costs and consequences of obesity in Germany: a new approach from a prevalence and life-cycle perspective. Eur. J. Health Econ. 2016;17:1141–1158. doi: 10.1007/s10198-015-0751-4. [DOI] [PubMed] [Google Scholar]
- Ehlis A.C., Ringel T.M., Plichta M.M., Richter M.M., Herrmann M.J., Fallgatter A.J. Cortical correlates of auditory sensory gating: a simultaneous near-infrared spectroscopy event-related potential study. Neuroscience. 2009;159:1032–1043. doi: 10.1016/j.neuroscience.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Ehlis A.C., Schneider S., Dresler T., Fallgatter A.J. Application of functional near-infrared spectroscopy in psychiatry. Neuroimage. 2014;85(Pt 1):478–488. doi: 10.1016/j.neuroimage.2013.03.067. [DOI] [PubMed] [Google Scholar]
- Folstein J.R., Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach G., Herpertz S., Loeber S. Personality traits and obesity: a systematic review. Obes. Rev. 2015;16:32–63. doi: 10.1111/obr.12235. [DOI] [PubMed] [Google Scholar]
- Giel K.E., Teufel M., Junne F., Zipfel S., Schag K. Food-Related Impulsivity in Obesity and Binge Eating Disorder-A Systematic Update of the Evidence. Nutrients. 2017;9:1170. doi: 10.3390/nu9111170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeussinger F.B., Dresler T., Heinzel S., Schecklmann M., Fallgatter A.J., Ehlis A.C. Reconstructing functional near-infrared spectroscopy (fNIRS) signals impaired by extra-cranial confounds: an easy-to-use filter method. Neuroimage. 2014;95:69–79. doi: 10.1016/j.neuroimage.2014.02.035. [DOI] [PubMed] [Google Scholar]
- Hautzinger M., Keller F., Kühner C., editors. BDI-II. Beck Depressions Inventar Revision - Manual. Harcourt Test Services; Frankfurt: 2006. [Google Scholar]
- Hay P. A systematic review of evidence for psychological treatments in eating disorders: 2005–2012. Int. J. Eat. Disord. 2013;46:462–469. doi: 10.1002/eat.22103. [DOI] [PubMed] [Google Scholar]
- He Q., Huang X., Zhang S., Turel O., Ma L., Bechara A. Dynamic Causal Modeling of Insular, Striatal, and Prefrontal Cortex Activities During a Food-Specific Go/NoGo Task. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:1080–1089. doi: 10.1016/j.bpsc.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q., Xiao L., Xue G., Wong S., Ames S.L., Schembre S.M., Bechara A. Poor ability to resist tempting calorie rich food is linked to altered balance between neural systems involved in urge and self-control. Nutr. J. 2014;13:92. doi: 10.1186/1475-2891-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hege M.A., Preissl H., Stingl K.T. Magnetoencephalographic signatures of right prefrontal cortex involvement in response inhibition. Hum. Brain Mapp. 2014;35:5236–5248. doi: 10.1002/hbm.22546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hege M.A., Stingl K.T., Kullmann S., Schag K., Giel K.E., Zipfel S., Preissl H. Attentional impulsivity in binge eating disorder modulates response inhibition performance and frontal brain networks. Int J Obes (Lond) 2015;39:353–360. doi: 10.1038/ijo.2014.99. [DOI] [PubMed] [Google Scholar]
- Heinzel S., Metzger F.G., Ehlis A.C., Korell R., Alboji A., Haeussinger F.B., Hagen K., Maetzler W., Eschweiler G.W., Berg D., Fallgatter A.J., Consortium T.S. Aging-related cortical reorganization of verbal fluency processing: a functional near-infrared spectroscopy study. Neurobiol. Aging. 2013;34:439–450. doi: 10.1016/j.neurobiolaging.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Herrmann M.J., Plichta M.M., Ehlis A.C., Fallgatter A.J. Optical topography during a Go-NoGo task assessed with multi-channel near-infrared spectroscopy. Behav. Brain Res. 2005;160:135–140. doi: 10.1016/j.bbr.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Hilbert A., Petroff D., Herpertz S., Pietrowsky R., Tuschen-Caffier B., Vocks S., Schmidt R. Meta-analysis of the efficacy of psychological and medical treatments for binge-eating disorder. J. Consult. Clin. Psychol. 2019;87:91–105. doi: 10.1037/ccp0000358. [DOI] [PubMed] [Google Scholar]
- Hilbert A., Tuschen-Caffier B. Verlag für Psychotherapie; Münster: 2006. Eating Disorder Examination: Deutschsprachige Übersetzung. [Google Scholar]
- Hollmann M., Hellrung L., Pleger B., Schlogl H., Kabisch S., Stumvoll M., Villringer A., Horstmann A. Neural correlates of the volitional regulation of the desire for food. Int J Obes (Lond) 2012;36:648–655. doi: 10.1038/ijo.2011.125. [DOI] [PubMed] [Google Scholar]
- Hudak J., Blume F., Dresler T., Haeussinger F.B., Renner T.J., Fallgatter A.J., Gawrilow C., Ehlis A.C. Near-Infrared Spectroscopy-Based Frontal Lobe Neurofeedback Integrated in Virtual Reality Modulates Brain and Behavior in Highly Impulsive Adults. Front. Hum. Neurosci. 2017;11:425. doi: 10.3389/fnhum.2017.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J.I., Hiripi E., Pope H.G., Jr., Kessler R.C. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol. Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska A.J., Yasuda M., Burant C.F., Gregor N., Khatri S., Sweet M., Falk E.B. Impulsivity and inhibitory control deficits are associated with unhealthy eating in young adults. Appetite. 2012;59:738–747. doi: 10.1016/j.appet.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper H. Report of committee on methods of clinical exam in EEG. Electroencephalogr. Clin. Neurophysiol. Suppl. 1958:370–375. [Google Scholar]
- Kessler R.M., Hutson P.H., Herman B.K., Potenza M.N. The Neurobiological Basis of Binge-Eating Disorder. Neurosci. Biobehav. Rev. 2016 doi: 10.1016/j.neubiorev.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Kohl S.H., Veit R., Spetter M.S., Gunther A., Rina A., Luhrs M., Birbaumer N., Preissl H., Hallschmid M. Real-time fMRI neurofeedback training to improve eating behavior by self-regulation of the dorsolateral prefrontal cortex: A randomized controlled trial in overweight and obese subjects. Neuroimage. 2019;191:596–609. doi: 10.1016/j.neuroimage.2019.02.033. [DOI] [PubMed] [Google Scholar]
- Kroczek A.M., Haeussinger F.B., Fallgatter A.J., Batra A., Ehlis A.C. Prefrontal functional connectivity measured with near-infrared spectroscopy during smoking cue exposure. Addict. Biol. 2017;22:513–522. doi: 10.1111/adb.12344. [DOI] [PubMed] [Google Scholar]
- Leehr E.J., Schag K., Dresler T., Grosse-Wentrup M., Hautzinger M., Fallgatter A.J., Zipfel S., Giel K.E., Ehlis A.C. Food specific inhibitory control under negative mood in binge-eating disorder: Evidence from a multimethod approach. Int. J. Eat. Disord. 2018;51:112–123. doi: 10.1002/eat.22818. [DOI] [PubMed] [Google Scholar]
- Loeber S., Grosshans M., Korucuoglu O., Vollmert C., Vollstadt-Klein S., Schneider S., Wiers R.W., Mann K., Kiefer F. Impairment of inhibitory control in response to food-associated cues and attentional bias of obese participants and normal-weight controls. Int J Obes (Lond) 2012;36:1334–1339. doi: 10.1038/ijo.2011.184. [DOI] [PubMed] [Google Scholar]
- Lopez R.B., Chen P.A., Huckins J.F., Hofmann W., Kelley W.M., Heatherton T.F. A balance of activity in brain control and reward systems predicts self-regulatory outcomes. Soc. Cogn. Affect Neurosci. 2017;12:832–838. doi: 10.1093/scan/nsx004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe C.J., Reichelt A.C., Hall P.A. The prefrontal cortex and obesity: a health neuroscience perspective. Trends Cogn. Sci. 2019;23:349–361. doi: 10.1016/j.tics.2019.01.005. [DOI] [PubMed] [Google Scholar]
- Manasse S.M., Goldstein S.P., Wyckoff E., Forman E.M., Juarascio A.S., Butryn M.L., Ruocco A.C., Nederkoorn C. Slowing down and taking a second look: Inhibitory deficits associated with binge eating are not food-specific. Appetite. 2016;96:555–559. doi: 10.1016/j.appet.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger F.G., Ehlis A.C., Haeussinger F.B., Schneeweiss P., Hudak J., Fallgatter A.J., Schneider S. Functional brain imaging of walking while talking - An fNIRS study. Neuroscience. 2017;343:85–93. doi: 10.1016/j.neuroscience.2016.11.032. [DOI] [PubMed] [Google Scholar]
- Meule A., Vögele C., Kübler A. Psychometrische Evaluation der deutschen Barratt Impulsiveness Scale-Kurzversion (BIS-15) Diagnostica. 2011:126–133. [Google Scholar]
- Muhlberg C., Mathar D., Villringer A., Horstmann A., Neumann J. Stopping at the sight of food - How gender and obesity impact on response inhibition. Appetite. 2016;107:663–676. doi: 10.1016/j.appet.2016.08.121. [DOI] [PubMed] [Google Scholar]
- Nagamitsu S., Araki Y., Ioji T., Yamashita F., Ozono S., Kouno M., Iizuka C., Hara M., Shibuya I., Ohya T., Yamashita Y., Tsuda A., Kakuma T., Matsuishi T. Prefrontal brain function in children with anorexia nervosa: a near-infrared spectroscopy study. Brain Dev. 2011;33:35–44. doi: 10.1016/j.braindev.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Neseliler S., Hu W., Larcher K., Zacchia M., Dadar M., Scala S.G., Lamarche M., Zeighami Y., Stotland S.C., Larocque M., Marliss E.B., Dagher A. Neurocognitive and Hormonal Correlates of Voluntary Weight Loss in Humans. Cell Metab. 2019;29(39–49) doi: 10.1016/j.cmet.2018.09.024. [DOI] [PubMed] [Google Scholar]
- Oliva R., Morys F., Horstmann A., Castiello U., Begliomini C. The impulsive brain: Neural underpinnings of binge eating behavior in normal-weight adults. Appetite. 2019;136:33–49. doi: 10.1016/j.appet.2018.12.043. [DOI] [PubMed] [Google Scholar]
- Plichta M.M., Herrmann M.J., Baehne C.G., Ehlis A.C., Richter M.M., Pauli P., Fallgatter A.J. Event-related functional near-infrared spectroscopy (fNIRS): are the measurements reliable? Neuroimage. 2006;31:116–124. doi: 10.1016/j.neuroimage.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Ricca V., Mannucci E., Zucchi T., Rotella C.M., Faravelli C. Cognitive-behavioural therapy for bulimia nervosa and binge eating disorder. A review. Psychother Psychosom. 2000;69:287–295. doi: 10.1159/000012410. [DOI] [PubMed] [Google Scholar]
- Romei A., Voigt K., Verdejo-Garcia A. A Perspective on Candidate Neural Underpinnings of Binge Eating Disorder: Reward and Homeostatic Systems. Curr. Pharm. Des. 2020;26:2327–2333. doi: 10.2174/1381612826666200309152321. [DOI] [PubMed] [Google Scholar]
- Rosch S.A., Schmidt R., Luhrs M., Ehlis A.C., Hesse S., Hilbert A. Evidence of fNIRS-Based Prefrontal Cortex Hypoactivity in Obesity and Binge-Eating Disorder. Brain Sci. 2020;11 doi: 10.3390/brainsci11010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankoh A.J., Huque M.F., Dubey S.D. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat. Med. 1997;16:2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Schag K., Leehr E., Meneguzzo P., Martus P., Zipfel S., Giel K. Food-related impulsivity assessed by longitudinal laboratory tasks is reduced in patients with binge eating disorder in a randomized controlled trial. Sci. Rep. 2021 doi: 10.1038/s41598-021-87231-w. PMID: 33859214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schag K., Rennhak S.K., Leehr E.J., Skoda E.M., Becker S., Bethge W., Martus P., Zipfel S., Giel K.E. IMPULS: Impulsivity-Focused Group Intervention to Reduce Binge Eating Episodes in Patients with Binge Eating Disorder - A Randomised Controlled Trial. Psychother. Psychosom. 2019;88:141–153. doi: 10.1159/000499696. [DOI] [PubMed] [Google Scholar]
- Schag K., Schönleber J., Teufel M., Zipfel S., Giel K.E. Food-related impulsivity in obesity and Binge Eating Disorder - a systematic review. Obes. Rev. 2013;14:477–495. doi: 10.1111/obr.12017. [DOI] [PubMed] [Google Scholar]
- Schag K., Teufel M., Junne F., Preissl H., Hautzinger M., Zipfel S., Giel K.E. Impulsivity in binge eating disorder: food cues elicit increased reward responses and disinhibition. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0076542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle A., Schafer A., Hermann A., Vaitl D. Binge-eating disorder: reward sensitivity and brain activation to images of food. Biol. Psychiatry. 2009;65:654–661. doi: 10.1016/j.biopsych.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Steinbrink J., Villringer A., Kempf F., Haux D., Boden S., Obrig H. Illuminating the BOLD signal: combined fMRI-fNIRS studies. Magn. Reson. Imaging. 2006;24:495–505. doi: 10.1016/j.mri.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Stojek M., Shank L.M., Vannucci A., Bongiorno D.M., Nelson E.E., Waters A.J., Engel S.G., Boutelle K.N., Pine D.S., Yanovski J.A., Tanofsky-Kraff M. A systematic review of attentional biases in disorders involving binge eating. Appetite. 2018;123:367–389. doi: 10.1016/j.appet.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda M., Uehara T., Fukuda M., Sato T., Kameyama M., Mikuni M. Dieting tendency and eating behavior problems in eating disorder correlate with right frontotemporal and left orbitofrontal cortex: a near-infrared spectroscopy study. J. Psychiatr. Res. 2010;44:547–555. doi: 10.1016/j.jpsychires.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Tammela L.I., Pääkkönen A., Karhunen L.J., Karhu J., Uusitupa M.I.J., Kuikka J.T. Brain electrical activity during food presentation in obese binge-eating women. Clin. Physiol. Funct. Imaging. 2010;30:135–140. doi: 10.1111/j.1475-097X.2009.00916.x. [DOI] [PubMed] [Google Scholar]
- Teslovich T., Freidl E.K., Kostro K., Weigel J., Davidow J.Y., Riddle M.C., Helion C., Dreyfuss M., Rosenbaum M., Walsh B.T., Casey B.J., Mayer L. Probing behavioral responses to food: development of a food-specific go/no-go task. Psychiatry Res. 2014;219:166–170. doi: 10.1016/j.psychres.2014.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuulari J.J., Karlsson H.K., Hirvonen J., Salminen P., Nuutila P., Nummenmaa L. Neural circuits for cognitive appetite control in healthy and obese individuals: an fMRI study. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0116640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasta R., Cutini S., Cerasa A., Gramigna V., Olivadese G., Arabia G., Quattrone A. Physiological Aging Influence on Brain Hemodynamic Activity during Task-Switching: A fNIRS Study. Front. Aging Neurosci. 2017;9:433. doi: 10.3389/fnagi.2017.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocks S., Tuschen-Caffier B., Pietrowsky R., Rustenbach S.J., Kersting A., Herpertz S. Meta-analysis of the effectiveness of psychological and pharmacological treatments for binge eating disorder. Int. J. Eat. Disord. 2010;43:205–217. doi: 10.1002/eat.20696. [DOI] [PubMed] [Google Scholar]
- Weygandt M., Mai K., Dommes E., Leupelt V., Hackmack K., Kahnt T., Rothemund Y., Spranger J., Haynes J.D. The role of neural impulse control mechanisms for dietary success in obesity. Neuroimage. 2013;83:669–678. doi: 10.1016/j.neuroimage.2013.07.028. [DOI] [PubMed] [Google Scholar]
- Wittchen, H.U., Wunderlich, U., Gruschwitz, S., Zaudig, M., 1997. SKID-I. Strukturiertes Klinisches Interview für DSM-IV. Achse I: Psychische Störungen. Göttingen, Germany:Hogrefe.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.