Abstract
Postmenopausal atrophic vaginitis, along with vasomotor symptoms and sleep disorders, is one of the most troublesome symptoms of menopause. However, many women do not manage this symptom properly due to insufficient knowledge of the symptoms or sexual embarrassment. With appropriate treatment, many postmenopausal women can experience relief from discomforts, including burning sensation or dryness of the vagina and dyspareunia. Topical lubricants and moisturizers, systemic and local estrogens, testosterones, intravaginal dehydroepiandrosterones (DHEAs), selective estrogen receptor modulators, and energy-based therapies are possible treatment modalities. Systemic and local estrogen therapies effectively treat genitourinary syndrome of menopause (GSM), but they are contraindicated in patients with breast cancer, for whom lubricants and moisturizers must be considered as the primary treatment. Intravaginal DHEA and ospemifene can be recommended for moderate to severe GSM; however, there is insufficient data on the use of intravaginal DHEA or ospemifene in patients with breast cancer, and further studies are needed. Energy-based devices such as vaginal laser therapy reportedly alleviate GSM symptoms; however, the U.S. Food and Drug Administration warning has recently been issued because of complications such as chronic pain and burning sensations of the vagina. To summarize, clinicians should provide appropriate individualized treatment options depending on women's past history, symptom severity, and chief complaints.
Keywords: Atrophic vaginitis, Dyspareunia, Genitourinary syndrome of menopause, Menopause
INTRODUCTION
Genitourinary syndrome of menopause (GSM) is a newly coined term that refers to a series of concerning symptoms related to estrogen deficiency, and involves changes in not only the genital area but also the urinary tract [1]. This term was first proposed in 2014 by the North American Menopause Society (NAMS) and the International Society for the Study of Women's Sexual Health to provide a more accurate representation of vulvovaginal atrophy (VVA) in menopause.
Both the female genital and lower urinary tract arise from the urogenital sinus; estrogen receptors are widely distributed in these organs, such as the vagina, vestibule, and bladder trigone [1,2]. A decrease in circulating estrogen after menopause or the abrupt deficiency of estrogen in certain conditions, such as surgical menopause, radiation or chemotherapy-induced ovarian insufficiency, results in structural changes in the urogenital epithelium and connective tissue. This causes a series of changes, such as vaginal blood flow reduction, loss of lactobacilli, altered microbiome, decreased lubrication, and increased vaginal pH [3]. These changes ultimately lead to unpleasant genitourinary symptoms, such as vaginal irritation, impaired sexual function, and recurrent urinary infection [1]. In addition to the above clinical symptoms, vaginal pH or vaginal maturation index (VMI) may be helpful in diagnosing VVA. Normal vaginal pH is 3.8 to 4.5 or below, and it increases to pH of 4.6 or above in VVA because of decrease in lactobacilli. VMI assesses the relative proportion of parabasal, intermediate, and superficial epithelial cells in the vaginal tract; in VVA, the relative proportion of superficial cells is less than 5% [3]. When diagnosing VVA, it must be differentiated from other conditions that can similarly cause bothersome vaginal symptoms—for example: lichen sclerosis, lichen planus, hyperkeratosis, contact dermatitis, vulvar cancer, vulvar intraepithelial neoplasm, extramammary Paget disease, and vaginal infections [4]. An assessment of history of vaginal hygiene and cosmetic use, vaginal discharge, and skin condition should be carried out, and proper biopsy must be performed in cases of uncertain diagnosis.
A large survey conducted in Britain in 1993 reported that about 50% of postmenopausal women had urogenital symptoms that negatively affected their quality of life [5,6]. Despite the high prevalence of GSM, many women are not adequately treated. The major reason for a small number of women receiving treatment is that most women either hesitate to disclose their discomfort owing to sexual embarrassment or have beliefs that these symptoms represent a natural aging process [7]. In some cases, women refuse GSM treatment for fear of hormonal treatment, which is widely used as part of GSM treatment. In addition, healthcare providers are not adequately responsive to this issue. According to the REVIVE-EU (REal Women's VIews of Treatment Options for Menopausal Vaginal ChangEs-Europe) survey conducted in 2014, only 10.3% of healthcare providers had initiated inquiry with patients about GSM [7]. These results suggest that postmenopausal women do not have enough information about GSM and its available treatment options.
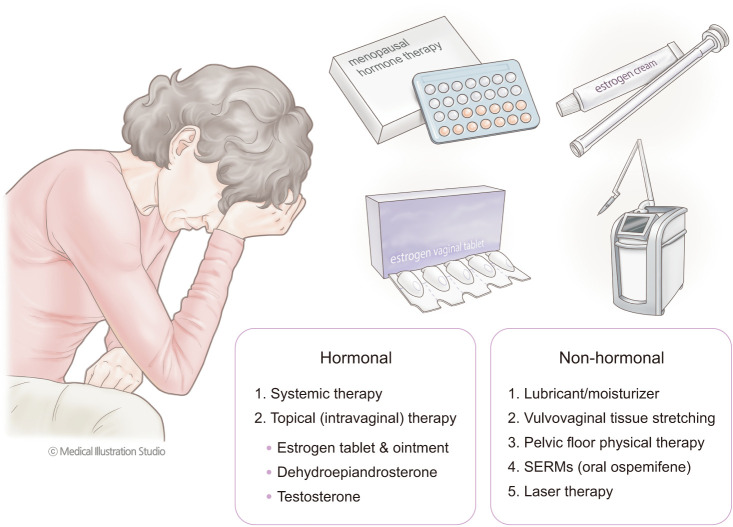
The examples of treatment options that are approved in Korea are presented in Table 1. In this paper, we reviewed the various management options of GSM, which includes individually tailored treatments and their potential risks and benefits (Fig. 1).
Table 1. Commonly used therapeutic options available for genitourinary syndrome of menopause in Korea.
| Product name | Ingredients | |
|---|---|---|
| Lubricants | Lubri-Feel, personal lubricant cleanser | Purified water, glycerin, polyquaternium, propylene glycol, chlorhexidine gluconate, allantoin, disodium ethylenediaminetetraacetic acid (EDTA), methylparaben |
| Astroglide Gel Lubricant | Purified water, glycerin, hydroxyethylcellulose, chlorhexidine gluconate, methylparaben, glucono delta lactone, sodium hydroxide | |
| Moisturizer | Hyal G | Purified water, sodium hyaluronate, lactic acid, propylene glycol, hydroxyethyl cellulose, 1,2-hexanediol, allantoin, disodium EDTA |
| Vaginal estrogen | ||
| Vaginal cream | Premarin vaginal cream | Conjugated estrogens 0.625 mg/g |
| Vaginal tablet | Gynoflor vaginal tablet | Estriol 0.03 mg + Lactobacillus acidophilus 50 mg |
| Ovestin vaginal suppositories | Estriol 0.5 mg | |
| Estriol Ovulum Jenapharm vaginal suppositories | Estriol 0.5 mg |
Fig. 1. Various treatment options for genitourinary syndrome of menopause. SERMs: selective estrogen receptor modulators.
MANAGEMENT OF GSM
Topical moisturizers and lubricants
Vaginal moisturizers and non-hormonal lubricants are effective primary treatments for GSM. They reduce vaginal friction, and temporarily improve vaginal dryness and pain during sexual activity.
Vaginal moisturizers are intended to provide relatively long-term vaginal symptom relief. When applied once every 1 to 3 days, it adheres to the vaginal mucosa, changes the fluid content of the endothelium, and lowers vaginal pH, retaining its functionality for a relatively longer time than that of lubricants. The efficacy of vaginal moisturizers was compared with that of placebo and low-dose vaginal estrogen treatment (ET) [8]. The group using vaginal moisturizer every 3 days showed comparable results to the group using topical ET in terms of improvement in vaginal dryness, and there was no statistically significant difference [9].
Vaginal lubricants provide temporary relief of vaginal dryness and are particularly beneficial for women whose chief complaint is vaginal dryness during sexual intercourse. Depending on the ingredient, lubricants can be divided into water-, silicone-, and oil-based lubricants. Water-based lubricants are most widely accessible, and cause fewer adverse genital symptoms than that observed with lubricants containing other type ingredients [10]. These water-based lubricants contain a bio-adhesive hydrophilic polymer to maintain the moisturizing effect, preservatives to prevent bacterial contamination, and other excipients to maintain pH and osmolality. Although lubricants are generally thought to be low-risk products, the World Health Organization (WHO) demonstrated that the use of lubricants with a pH and osmolality that are physiologically too different from the normal vaginal environment can make women susceptible to bacterial vaginosis. Ideally, personal lubricants with osmolality less than 380 mOsm/kg and a pH of approximately 4.5 is recommended to prevent vaginal mucosal damage, contact dermatitis, and sexually transmitted infections [11]. Patients can use natural oils such as olive, coconut, and mineral oil for vaginal moisturization, but clinical trials are lacking [12,13]. The choice of drug type and its duration of use differ depending on a woman's condition and indications [14].
Another non-hormonal alternative, topical lidocaine, can be used for dyspareunia. It can reduce sexual discomfort by numbing the nerves that are stimulated during intercourse. In a double-blind, randomized study of breast cancer survivors who lack systemic circulating estrogen, dyspareunia symptoms were improved by applying topical 4% aqueous lidocaine in the vulvar vestibule before intercourse [15].
Low-dose vaginal ET
Low-dose topical vaginal ET is an extremely effective treatment option when GSM persists after non-hormonal treatments. Low-dose vaginal ET involves administering estrogen doses to postmenopausal women to maintain estrogen in the normal range. The NAMS recommends low-dose vaginal ET in moderate-to-severe GSM or when other treatment results are not satisfactory [16]. When this topical hormone is absorbed into the vaginal mucosa, it improves vaginal blood flow and thickens the vaginal epithelium. According to the CLarifying vaginal atrophy's impact On SEx and Relationships (CLOSER) survey, women had decreased coital pain (56%) and increased sexual satisfaction (41%) with the aid of low-dose vaginal ET [17].
Various preparations are available as vaginal ET, including vaginal estradiol tablets, ointments, and rings. Tablets and ointments have been approved in Korea. Serum estrogen levels are generally maintained at the menopausal level with low-dose vaginal ET. According to Shifren [18], a low-dose vaginal estrogen ring (7.5 µg/day) or tablet (10 µg) did not change serum estradiol levels from baseline, and remained in a range of 3 to 11 pg/mL. In case of vaginal estrogen creams (estradiol or conjugated estrogens [CEs]), the serum estradiol concentration varied depending on the frequency of administration and dose administered [19,20]. However, the use of low-dose vaginal estrogen must be carefully decided in women with breast cancer, especially those using an aromatase inhibitor, because of a potential risk of increasing estrogen levels in the blood even by the slightest degree.
Estetrol (E4), a fetal selective estrogen receptor modulator, is a natural estrogen produced exclusively by the human fetal liver during pregnancy. It relieves GSM symptoms by activating nuclear estrogen receptor α without affecting coagulation factors, lipid profile, and breast cells. A randomized study showed that treatment with estetrol in a dose range of 2 to 40 mg per day significantly improved VVA and vaginal cytology in postmenopausal women [21,22].
After the initiation of vaginal ET, improvements in the vaginal environment appeared within a few weeks, and the optimal effect appeared after 2 to 3 months. Currently, all globally approved forms of vaginal estrogens are effective to some extent for the treatment of GSM.
Systemic estrogen therapy
Both systemic and vaginal estrogen therapies are effective in improving GSM symptoms. They promote vaginal blood flow, improve vaginal secretions, and reduce vaginal pH to promote the proliferation of normal vaginal flora and improve vaginal microenvironment.
Notably, systemic estrogen therapy is suitable for not only relieving GSM symptoms but also improving additional conditions such as vasomotor symptoms and osteoporosis. However, systemic estrogen therapy is considered only after a thorough risk-benefit analysis. Although the lowest effective dose of systemic estrogen therapy is generally recommended, a patient's situation should be accurately determined after treatment as the therapy may affect the endometrium or breast depending on the patient's condition.
Intravaginal dehydroepiandrosterone (DHEA)
The U.S. Food and Drug Administration (FDA) allowed the use of intravaginal DHEA for the treatment of moderate-to-severe dyspareunia caused by GSM. DHEA is transformed to estrogens in vaginal mucosal cells and improves symptoms of vaginal irritation. In a randomized, double-blind, placebo-controlled study, 0.5% (6.5 mg) vaginal DHEA was used daily for 12 weeks, effectively improving both objective vaginal health index scores and subjective discomfort compared to the placebo group [23]. In this trial, serum hormone levels, such as that of estradiol and testosterone, were maintained within the menopausal baseline range [23,24]. A recent review article concluded that intravaginal DHEA was superior to placebo and as efficacious as vaginal estrogen in relieving dyspareunia and GSM symptoms. The most common side effect reported was vaginal discharge due to dissolution of the formulation, and abnormalities in the cervical smear [25]. Although the use of intravaginal DHEA is not contraindicated in women with breast cancer, further studies are required on this subject, because estrogen is a metabolite of DHEA; moreover, there are no largescale studies on the long-term safety of intravaginal DHEA in patients with hormone-dependent cancer [16].
Systemic or local testosterone
Studies on the effects of androgens on the genitourinary system have mainly been conducted in animals. Likewise, several studies have demonstrated that androgens play an important role in the human urogenital system [26], but data so far are scarce. In a study by Salinger [27], menopausal women aged 54 to 85 years were administered intramuscular testosterone propionate daily or every other day to a total of 125 mg over 1 week. Subsequently, vaginal tissue biopsy was performed to evaluate the hormonal effect—vaginal superficial cells, epithelial intermediates, and glycogen deposition were increased [27]. However, current studies of systemic transdermal testosterone therapy, alone or in combination with ET, have not proven its effects on vaginal health [28,29].
A study of postmenopausal women using intravaginal testosterone showed decreased vaginal pH and increased vaginal lactobacilli compared to the group using lubricating gel [30]. Another study compared the group using intravaginal testosterone (5 mg/dose) in combination with conjugated equine estrogen (CEE) with the group using CEE alone. However, as CEE was administered to both groups, there was a limitation in elucidating the effect of vaginal testosterone alone [31].
According to the 2020 position statement of the NAMS, to date, there is not enough data to confirm the safety and efficacy of systemic local testosterone therapy for the treatment of GSM [16]. To date, hypoactive sexual desire disorder/dysfunction is the only evidence-based indication for testosterone use in postmenopausal women [32].
Selective estrogen receptor modulators (SERMs)
SERMs are synthetic nonsteroidal agents that exhibit a mixture of estrogenic or anti-estrogenic effects, depending on the target tissues. Ospemifene is the only SERM indicated for the treatment of GSM-related dyspareunia. A previous study has shown that taking ospemifene 60 mg orally daily significantly improved patient discomfort and objective quality assessment conditions such as vaginal pH and VMI [33]. However, ospemifene has not yet been approved for use in breast cancer patients, although drug-based endometrial safety has been confirmed in a one-year study. Moreover, the use of ospemifene may increase the risk of vasomotor symptoms or venous thromboembolism; hence, this should be considered before prescribing ospemifene [18].
Further, studies have evaluated the role of bazedoxifene (BZA), a SERM, for the treatment of GSM. In a multicenter, randomized, double-blind clinical trial, BZA/CE, BZA alone, and placebo were administered to postmenopausal women, and their effect on VVA was evaluated. The percentage of superficial cells of the vagina and subjective symptoms were significantly improved in the BZA/CE group but not in the BZA alone or placebo group [34].
Vaginal laser therapy
Minimally ablative fractional laser therapy may stimulate cell repair and tissue growth. It also mediates cell proliferation via an immunologic mechanism stimulating the release of anti-inflammatory cytokines that control cell-mediated immunity [35]. Previous small-scale studies demonstrated that fractional carbon dioxide laser therapy effectively treats GSM symptoms [36,37]. In a study involving 21 perimenopausal women with GSM, 3 vaginal laser treatments were performed at 4-week intervals, and the subjects' vaginal symptoms and sexual function scores after 24 weeks were significantly higher than those at baseline [37]. Non-ablative laser therapies, such as erbium:YAG laser therapy, are also being trialed to relieve GSM symptoms. The wavelength of laser pulses induces the rupture of water molecules and generates reactive oxygen species, causing the contraction of collagen fibers, fibroblastic stimulation, and neo-collagenesis [38]. According to a pilot study, vaginal erbium laser treatment effectively improves dryness and dyspareunia for up to 24 weeks after treatment [39]. The large, multicenter Vaginal Erbium Laser Academy Study (VELAS) is evaluating the safety and efficacy of vaginal erbium lasers for the treatment of GSM [40].
However, the effectiveness and safety of vaginal laser treatment has not been fully established, and these energy-based therapies have not yet been approved by the FDA for this indication [16]. In conclusion, the use of laser therapy for GSM treatment is still controversial, and further investigation is needed on the objective effects of vaginal laser treatment and its outcomes compared to hormone treatment.
Radiofrequency therapy
With high-frequency alternating current, radiofrequency can temporarily increase the intracellular temperature, leading to cellular membrane rupture. This leads to neo-collagenesis of the vaginal mucosal wall; this is a mechanism similar to that of laser treatment. A pilot study showed that 14 women with GSM, treated with microablative fractional radiofrequency 3 times with a 1-month interval, experienced improvement in symptom severity and sexual satisfaction [41]. However, this energy-based device is also not FDA approved for the treatment of VVA, and more studies are needed.
Non-pharmacotherapeutic options
Women should know that VVA, which occurs with estrogen loss, is one of the typical menopausal symptoms. They should be educated that GSM symptoms do not improve naturally, and that symptoms can be improved using appropriate treatment options.
Vulvovaginal tissue stretching promotes vaginal elasticity. In addition, masturbation and self-stimulation are available options for patients. A vaginal dilator can help stretch the vaginal tissues. Women can alleviate pain during sexual activity by using a vaginal dilator that helps in vaginal relaxation [42].
Women who suffer from GSM-related dyspareunia may have nonrelaxing pelvic floor dysfunction, and in this case, pelvic floor physical therapy can be tried to relieve the symptoms [43].
CONCLUSION
GSM is a newly coined term that not only explains vaginal symptoms, but also makes it easy to understand the overall spectrum of genitourinary symptoms caused by decreased estrogen levels during menopause. Various symptoms of GSM are known to have serious negative effects on women's quality of life after menopause, but they still remain largely under-diagnosed and under-treated. However, these symptoms can be overcome by understanding the various treatment options and finding options optimized for each patient.
Vaginal lubricants or moisturizers can be used as a first-line treatment; however, in case of lack of response to non-hormonal treatments, low-dose vaginal ET is the standard treatment. However, several other effective treatment strategies are available, including the use of ospemifene and intravaginal DHEA, as described above. Modern treatments such as vaginal laser therapy require additional research to demonstrate long-term efficacy.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Portman DJ, Gass ML Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. Maturitas. 2014;79:349–354. doi: 10.1016/j.maturitas.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Robinson D, Cardozo LD. The role of estrogens in female lower urinary tract dysfunction. Urology. 2003;62(4 Suppl 1):45–51. doi: 10.1016/s0090-4295(03)00676-9. [DOI] [PubMed] [Google Scholar]
- 3.Mac Bride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc. 2010;85:87–94. doi: 10.4065/mcp.2009.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Connell TX, Nathan LS, Satmary WA, Goldstein AT. Non-neoplastic epithelial disorders of the vulva. Am Fam Physician. 2008;77:321–326. [PubMed] [Google Scholar]
- 5.Calleja-Agius J, Brincat MP. Urogenital atrophy. Climacteric. 2009;12:279–285. doi: 10.1080/13697130902814751. [DOI] [PubMed] [Google Scholar]
- 6.Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women's VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10:1790–1799. doi: 10.1111/jsm.12190. [DOI] [PubMed] [Google Scholar]
- 7.Nappi RE, Palacios S, Panay N, Particco M, Krychman ML. Vulvar and vaginal atrophy in four European countries: evidence from the European REVIVE Survey. Climacteric. 2016;19:188–197. doi: 10.3109/13697137.2015.1107039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Baghdadi O, Ewies AA. Topical estrogen therapy in the management of postmenopausal vaginal atrophy: an up-to-date overview. Climacteric. 2009;12:91–105. doi: 10.1080/13697130802585576. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Geng L, Song X, Li H, Giordan N, Liao Q. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575–1584. doi: 10.1111/jsm.12125. [DOI] [PubMed] [Google Scholar]
- 10.Herbenick D, Reece M, Hensel D, Sanders S, Jozkowski K, Fortenberry JD. Association of lubricant use with women's sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202–212. doi: 10.1111/j.1743-6109.2010.02067.x. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO/UNFPA/FHI360: advisory note. Geneva: World Health Organization; 2012. Report No.: WHO/RHR/12.33. [Google Scholar]
- 12.Sandhu RS, Wong TH, Kling CA, Chohan KR. In vitro effects of coital lubricants and synthetic and natural oils on sperm motility. Fertil Steril. 2014;101:941–944. doi: 10.1016/j.fertnstert.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 13.Voeller B, Coulson AH, Bernstein GS, Nakamura RM. Mineral oil lubricants cause rapid deterioration of latex condoms. Contraception. 1989;39:95–102. doi: 10.1016/0010-7824(89)90018-8. [DOI] [PubMed] [Google Scholar]
- 14.Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition. Climacteric. 2016;19:151–161. doi: 10.3109/13697137.2015.1124259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33:3394–3400. doi: 10.1200/JCO.2014.60.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The North American Menopause Society (NAMS) The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020;27:976–992. doi: 10.1097/GME.0000000000001609. [DOI] [PubMed] [Google Scholar]
- 17.Simon JA, Nappi RE, Kingsberg SA, Maamari R, Brown V. Clarifying Vaginal Atrophy's Impact on Sex and Relationships (CLOSER) survey: emotional and physical impact of vaginal discomfort on North American postmenopausal women and their partners. Menopause. 2014;21:137–142. doi: 10.1097/GME.0b013e318295236f. [DOI] [PubMed] [Google Scholar]
- 18.Shifren JL. Genitourinary syndrome of menopause. Clin Obstet Gynecol. 2018;61:508–516. doi: 10.1097/GRF.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 19.Eugster-Hausmann M, Waitzinger J, Lehnick D. Minimized estradiol absorption with ultra-low-dose 10 microg 17beta-estradiol vaginal tablets. Climacteric. 2010;13:219–227. doi: 10.3109/13697137.2010.483297. [DOI] [PubMed] [Google Scholar]
- 20.Santen RJ, Pinkerton JV, Conaway M, Ropka M, Wisniewski L, Demers L, et al. Treatment of urogenital atrophy with low-dose estradiol: preliminary results. Menopause. 2002;9:179–187. doi: 10.1097/00042192-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Coelingh Bennink HJ, Verhoeven C, Zimmerman Y, Visser M, Foidart JM, Gemzell-Danielsson K. Clinical effects of the fetal estrogen estetrol in a multiple-rising-dose study in postmenopausal women. Maturitas. 2016;91:93–100. doi: 10.1016/j.maturitas.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 22.North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause. 2007;14(3 Pt 1):355–369. doi: 10.1097/gme.0b013e31805170eb. quiz 370-1. [DOI] [PubMed] [Google Scholar]
- 23.Labrie F, Archer DF, Koltun W, Vachon A, Young D, Frenette L, et al. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2018;25:1339–1353. doi: 10.1097/GME.0000000000001238. [DOI] [PubMed] [Google Scholar]
- 24.Ke Y, Gonthier R, Simard JN, Archer D, Lavoie L, Martel C, et al. Serum steroids remain within the same normal postmenopausal values during 12-month intravaginal 0.50% DHEA. Horm Mol Biol Clin Investig. 2015;24:117–129. doi: 10.1515/hmbci-2015-0035. [DOI] [PubMed] [Google Scholar]
- 25.Sauer U, Talaulikar V, Davies MC. Efficacy of intravaginal dehydroepiandrosterone (DHEA) for symptomatic women in the perior postmenopausal phase. Maturitas. 2018;116:79–82. doi: 10.1016/j.maturitas.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Traish AM, Vignozzi L, Simon JA, Goldstein I, Kim NN. Role of androgens in female genitourinary tissue structure and function: implications in the genitourinary syndrome of menopause. Sex Med Rev. 2018;6:558–571. doi: 10.1016/j.sxmr.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Salinger SL. Proliferative effect of testosterone propionate on human vaginal epithelium. Acta Endocrinol (Copenh) 1950;4:265–284. doi: 10.1530/acta.0.0040265. [DOI] [PubMed] [Google Scholar]
- 28.Panay N, Al-Azzawi F, Bouchard C, Davis SR, Eden J, Lodhi I, et al. Testosterone treatment of HSDD in naturally menopausal women: the ADORE study. Climacteric. 2010;13:121–131. doi: 10.3109/13697131003675922. [DOI] [PubMed] [Google Scholar]
- 29.Simon J, Braunstein G, Nachtigall L, Utian W, Katz M, Miller S, et al. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J Clin Endocrinol Metab. 2005;90:5226–5233. doi: 10.1210/jc.2004-1747. [DOI] [PubMed] [Google Scholar]
- 30.Bell RJ, Rizvi F, Islam RM, Davis SR. A systematic review of intravaginal testosterone for the treatment of vulvovaginal atrophy. Menopause. 2018;25:704–709. doi: 10.1097/GME.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 31.Raghunandan C, Agrawal S, Dubey P, Choudhury M, Jain A. A comparative study of the effects of local estrogen with or without local testosterone on vulvovaginal and sexual dysfunction in postmenopausal women. J Sex Med. 2010;7:1284–1290. doi: 10.1111/j.1743-6109.2009.01667.x. [DOI] [PubMed] [Google Scholar]
- 32.Davis SR, Baber R, Panay N, Bitzer J, Cerdas Perez S, Islam RM, et al. Global consensus position statement on the use of testosterone therapy for women. Climacteric. 2019;22:429–434. doi: 10.1080/13697137.2019.1637079. [DOI] [PubMed] [Google Scholar]
- 33.Portman DJ, Bachmann GA, Simon JA. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20:623–630. doi: 10.1097/gme.0b013e318279ba64. [DOI] [PubMed] [Google Scholar]
- 34.Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause. 2010;17:281–289. doi: 10.1097/GME.0b013e3181b7c65f. [DOI] [PubMed] [Google Scholar]
- 35.Becorpi A, Campisciano G, Zanotta N, Tredici Z, Guaschino S, Petraglia F, et al. Fractional CO2 laser for genitourinary syndrome of menopause in breast cancer survivors: clinical, immunological, and microbiological aspects. Lasers Med Sci. 2018;33:1047–1054. doi: 10.1007/s10103-018-2471-3. [DOI] [PubMed] [Google Scholar]
- 36.Alexiades MR. Fractional CO2 laser treatment of the vulva and vagina and the effect of postmenopausal duration on efficacy. Lasers Surg Med. 2020 doi: 10.1002/lsm.23247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arroyo C. Fractional CO2 laser treatment for vulvovaginal atrophy symptoms and vaginal rejuvenation in perimenopausal women. Int J Womens Health. 2017;9:591–595. doi: 10.2147/IJWH.S136857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elia D, Gambacciani M, Berreni N, Bohbot JM, Druckmann R, Geoffrion H, et al. Genitourinary syndrome of menopause (GSM) and laser VEL: a review. Horm Mol Biol Clin Investig. 2019;41 doi: 10.1515/hmbci-2019-0024. [DOI] [PubMed] [Google Scholar]
- 39.Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: the second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric. 2015;18:757–763. doi: 10.3109/13697137.2015.1045485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gambacciani M, Torelli MG, Martella L, Bracco GL, Casagrande AG, Albertin E, et al. Rationale and design for the Vaginal Erbium Laser Academy Study (VELAS): an international multicenter observational study on genitourinary syndrome of menopause and stress urinary incontinence. Climacteric. 2015;18 Suppl 1:43–48. doi: 10.3109/13697137.2015.1071608. [DOI] [PubMed] [Google Scholar]
- 41.Kamilos MF, Borrelli CL. New therapeutic option in genitourinary syndrome of menopause: pilot study using microablative fractional radiofrequency. Einstein (Sao Paulo) 2017;15:445–451. doi: 10.1590/S1679-45082017AO4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faubion SS, Sood R, Kapoor E. Genitourinary syndrome of menopause: management strategies for the clinician. Mayo Clin Proc. 2017;92:1842–1849. doi: 10.1016/j.mayocp.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 43.Faubion SS, Shuster LT, Bharucha AE. Recognition and management of nonrelaxing pelvic floor dysfunction. Mayo Clin Proc. 2012;87:187–193. doi: 10.1016/j.mayocp.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]