Abstract
Aim:
To present the pre-specified analyses of >5-years follow-up of the Phase III ALTTO trial.
Patients and Methods:
8381 patients with stage I-III HER2 positive breast cancer randomized to chemotherapy plus 1-year of: trastuzumab (T), oral lapatinib (L; no longer evaluated), trastuzumab followed by lapatinib (T→L), and lapatinib + trastuzumab (L+T). Primary endpoint was disease-free survival (DFS). Secondary analysis examined DFS treatment effects by hormone receptor status, nodal status and chemotherapy timing; time to recurrence; overall survival (OS) and safety (overall and cardiac).
Results:
At a median follow-up of 6.9 years, 705 DFS events for L+T vs. T were observed. Hazard Ratio (HR) for DFS was 0.86 (95% CI, 0.74–1.00) for L+T vs. T and 0.93 (95% CI, 0.81–1.08) for T→L vs. T. The 6-year DFS were 85%, 84%, and 82% for L+T, T→L, and T, respectively. HR for OS was 0.86 (95% CI, 0.70–1.06) for L+T vs. T and 0.88 (95% CI, 0.71–1.08) for T→L vs. T. The 6-year OS were 93%, 92%, and 91% for L+T, T→L, and T, respectively. Subset analyses showed a numerically better HR for DFS in favour of L+T vs. T for the hormone-receptor-negative [HR 0.80 (95% CI, 0.64–1.00; 6-yr DFS%=84% vs. 80%)] and the sequential chemotherapy [HR 0.83 (95% CI, 0.69–1.00; 6-yr DFS%=83% vs.79%)] subgroups.
Conclusion:
T+L did not significantly improve DFS and OS over T alone, both with chemotherapy, and, therefore, cannot be recommended for adjuvant treatment of early stage HER2-positive breast cancer.
Trial Registration:
clinicaltrials.gov Identifier NCT00490139
Keywords: Early breast cancer, HER2, lapatinib, trastuzumab, adjuvant chemotherapy
Introduction
Since 2005, adjuvant chemotherapy plus 1 year of the monoclonal antibody trastuzumab (T), has become standard of care therapy for patients with early stage HER2 positive breast cancer.1–6 Lapatinib (L), an oral anti-HER1–2 tyrosine kinase inhibitor (TKI), demonstrated significant clinical activity against HER2 positive breast cancer in the metastatic setting7–11 and the combination of lapatinib and trastuzumab significantly improved rates of pathological complete response (pCR) compared with either drug alone in the Neo-ALTTO trial.12 The Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization (ALTTO) trial is the first large prospective randomized trial to report three anti-HER2 strategies that incorporated L and T and compared these with T alone as adjuvant therapy for patients with early stage HER2-positive breast cancer. The primary analysis of this trial was presented in 2014 and demonstrated that adjuvant treatment that includes L did not significantly improve DFS compared with T alone but added toxicity.13,14 The current manuscript reports the pre-specified analyses foreseen when all patients had completed ≥5 years of follow-up.
Material and methods
Study Design and Settings
The ALTTO trial (NCT00490139) is a prospective randomized, phase 3, open-label, multicenter study conducted in 946 centers in 44 countries from 4 different continents. The patients were enrolled between June 2007 and July 2011. Participating medical centers’ institutional review boards (or Ethics Committees) approved the study, and all patients provided written informed consent prior to study entry. The trial was coordinated by the Breast International Group (BIG) for Europe and rest of the world and by the North Central Cancer Treatment Group (NCCTG: now Alliance) for the United States of America (US) and Canada. Support was provided by the US National Cancer Institute (NCI), National Cancer Institute of Canada Clinical Trials Group (now Canadian Clinical Trials Group), Glaxo-Smith-Kline from its development until November 30, 2015 and by Novartis since then. Clinical database was located at the BrEAST Data Center (Institut Jules Bordet, Brussels) and statistical analyses were performed by Frontier Science Scotland (FSS, Scotland).
Participants
Eligible patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status 0–1, be at least 18 years of age, with clinical or pathologically confirmed stage I (pT=1–2 cm) to stage III, centrally confirmed HER2 positive (3+ by IHC and/or FISH positive) invasive breast cancer. The hematologic, renal and hepatic functions were to be adequate and a baseline left ventricular ejection fraction (LVEF) ≥50%, measured by echocardiography or MUGA scan, was required.
Procedures
Patients were randomly assigned centrally in a 1:1:1:1 ratio to receive the anti-HER2 targeted therapy in one of 4 arms: in Arm 1 patients received intravenous T (at a loading dose of 4 mg/kg once and then 2 mg/kg weekly during chemotherapy or at a loading dose of 8 mg/kg once and then 6 mg/kg every 3 weeks when given alone); in Arm 2 they received oral L (750 mg/day during chemotherapy and 1,500 mg/day when given alone); in Arm 3 a sequence of the two agents (T→L) that started with 12 weekly doses of T, followed after a 6-week washout, by 34 weeks of L at 1,500 mg/day; and in Arm 4 the combination of the two anti-HER2 agents (L+T) with T at the aforementioned dosages and L at 750 mg/day during chemotherapy (reduced from an initial dose of 1,000 mg/day based on safety data), with an escalation to 1,000 mg/day after the completion of chemotherapy.
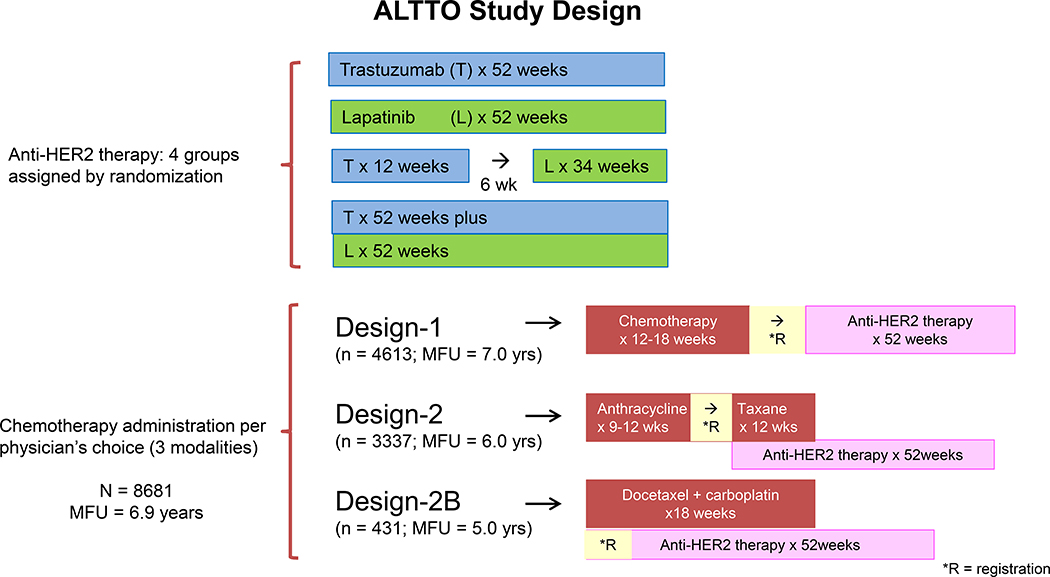
The adjuvant chemotherapy regimens and timing of anti-HER2 were based on patients/physicians choice. The anti-HER 2 therapy was administered either sequentially at the completion of all chemotherapy (Design 1), concurrently with the taxane component of the chemotherapy (Design 2), or concurrently with a non-anthracycline regimen (Design 2B) (Figure 1). The chemotherapy regimens for Designs 1 and 2 consisted of at least 4 cycles of an anthracycline based regimen (several regimens available to choose from), followed by a taxane (weekly paclitaxel or 3-weekly docetaxel), and the regimen of Design 2B consisted of 6 cycles of every 3-week docetaxel and carboplatin.
Figure 1.
ALTTO Study Design. ALTTO indicates Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization; MFU (median follow up); *R, registration.
Adjuvant endocrine therapy was given to patients with hormone receptor–positive disease. Radiotherapy was mandatory in cases of breast-conserving surgery and in accordance with institutional guidelines in cases of mastectomy. Both treatments were given after completion of all chemotherapy and concomitantly with anti-HER2 treatment.
Chemotherapy adverse effects were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.15
Statistical Analyses
Randomization used permuted blocks stratified by timing of chemotherapy (Design 1 vs. Design 2/2B), central hormone receptor status (positive [≥ 1%] vs. negative), and lymph node status (not applicable [neoadjuvant chemotherapy], node negative, 1–3, ≥4 positive nodes). The primary endpoint was disease-free survival (DFS) defined as time from randomization to recurrence of invasive breast cancer at local, regional, or distant sites; contralateral invasive breast cancer; second non-breast malignancy; or death as a result of any cause, whichever occurred first. Secondary endpoints were overall survival (OS), safety in general and cardiac safety, time to recurrence, time to distant recurrence, and time to first brain metastasis.
Sample size calculations were based on the two-sided superiority comparison between the L+T arm and the T arm with 850 DFS events calculated to provide an 80% power to detect a hazard ratio (HR) of 0.80 at α = .0167, using a log-rank test stratified by hormone receptor status (2 groups), nodal status (4 groups) and chemotherapy timing (2 groups). Estimation of hazard ratios and 95% confidence intervals were by Cox’s proportional hazards model stratified by three stratification factors (16 strata)16; and estimation of survival functions were by the Kaplan-Meier method with standard errors calculated using Greenwood’s formula.17,18 For efficacy analyses the intent to treat (ITT) population was utilized and for the safety analysis only those patients who received at least one dose of the anti-HER2 therapy were considered.
The L monotherapy arm is not evaluated in this manuscript because it was closed in 2011 due to statistical inferiority to T monotherapy at the first interim efficacy analysis, and patients free of disease were offered adjuvant T. For the 2014 Primary Analysis, the type I error of 5% was split evenly between the two comparisons of the remaining lapatinib combination arms versus T, and therefore all subsequent analyses are descriptive, not inferential.
Results
Participants
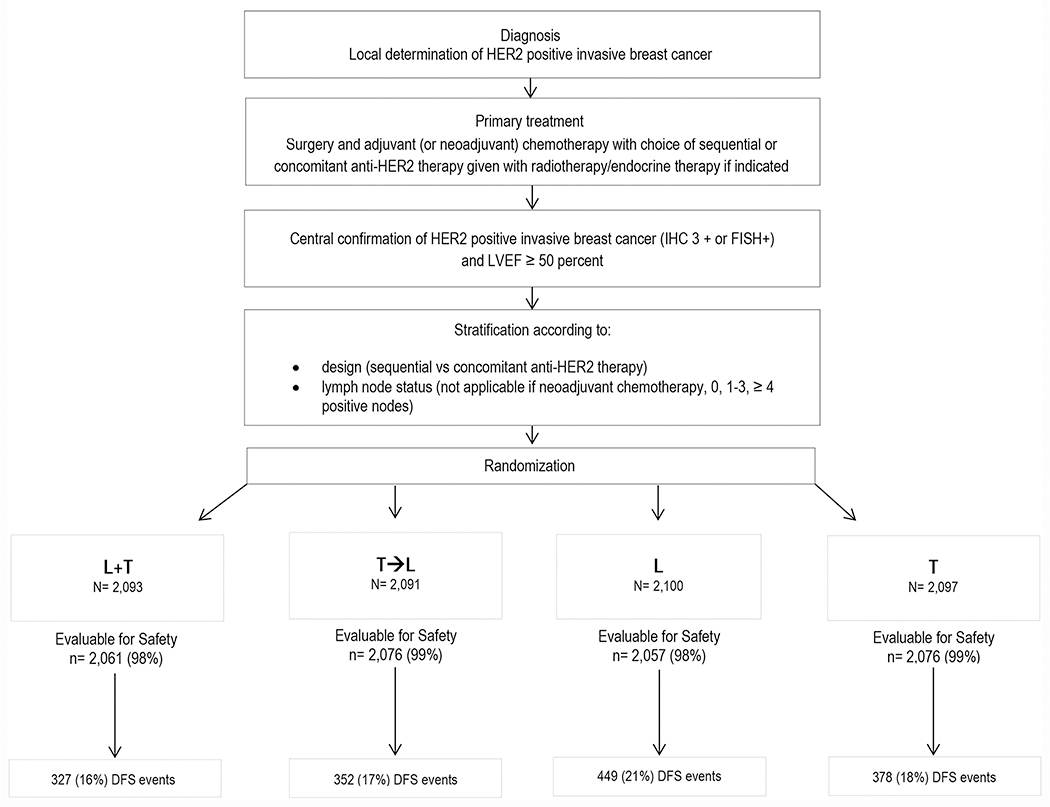
The trial accrued 8381 patients (Figure 2, CONSORT flow diagram); 2097 patients received T for 52 weeks, 2100 patients received L for 52 weeks, 2091 patients received T for 12 weeks followed by a wash out period of 6 weeks and then L for 34 weeks, and 2093 patients received L concurrently with T for 52 weeks. Design 1 recruited rapidly and closed enrollment in March 2009 to permit sufficient enrollment to Design 2, and Design 2B was only incorporated in 2009. Thus, the number of patients treated by a certain chemotherapy timing and the duration of follow-up differ among the different designs. Approximately 55% of patients (n = 4613) were treated under the sequential Design 1 and have a median follow-up of 7 years; 40% of patients (n = 3337) were treated under Design 2 and have a median follow-up of 6 years; and only 5% of patients were treated under Design 2B (n = 431) and the median follow-up of this group is 5 years. The median duration of follow-up for all patients is 6.9 years.
Figure 2.
CONSORT Chart. DFS indicates disease-free survival; FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IHC, immunohistochemistry; L, lapatinib; LVEF, left ventricular ejection fraction; T, trastuzumab.
Detailed baseline characteristics of the intent-to-treat population (ITT), the tumors and the primary treatments were previously described13 and pertinent baseline characteristics based on primary treatment by design and by treatment arm can be found in Supplement 1. Briefly, in the ITT population the median age was 51 years (range 18–82), 57% of patients were post-menopausal, 51% had node-positive disease, 50% had a tumor size >2 cm, and 57% had hormone receptor–positive (HR+) disease. Approximately 8% of patients (12% on Design-1 and 6% on Design-2) received the chemotherapy in the neoadjuvant setting and were eligible as long as all chemotherapy was completed prior to randomization (Design-1) or the anthracycline-based chemotherapy was completed prior to receiving the targeted therapy concomitantly with the taxane (Design-2).
Safety
The incidence of adverse events (AEs) was higher in L-containing arms than in the T arm. AEs related to study treatment were 93% in the L+T arm vs. 64% in the T arm. There were also more discontinuations of study treatment (23% vs 8%), dose reductions (20% vs 4%) and dose interruptions/delays (46% vs. 20%) due to toxicity in the L+T arm vs. the T arm; and this was more pronounced when targeted therapy was provided concurrently with the chemotherapy (design 2/2B). Generally, the most common AEs related to L and leading to dose changes were diarrhea, neutropenia, and rash. The incidence of primary or secondary cardiac endpoints was low in all treatment arms: 1% for L+T, 0.5% for T→L and 0.9% for T for primary endpoints. Additional safety results are available in Supplement 1.
Efficacy
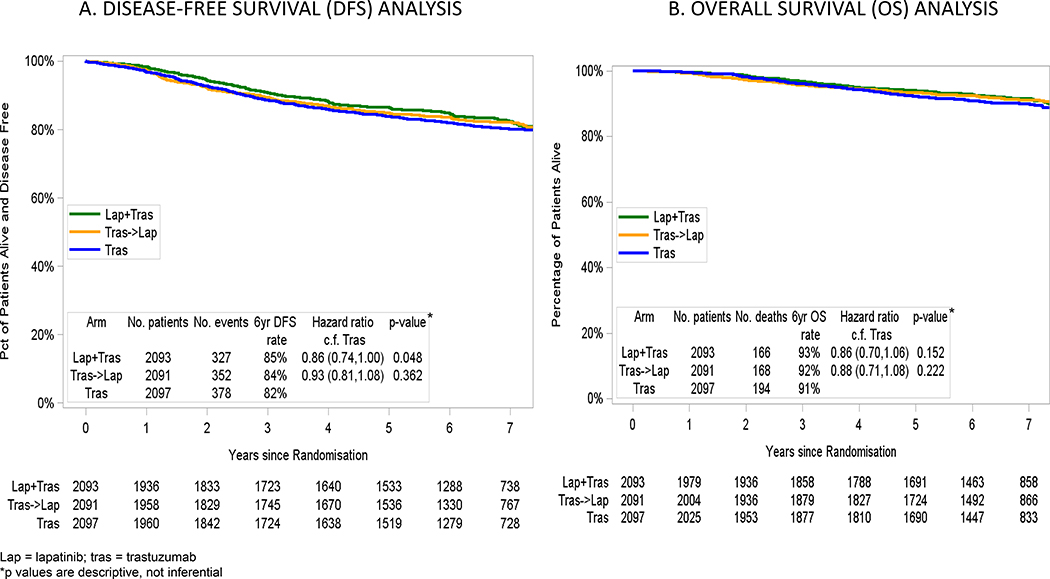
The overall median follow-up of this study is 6.9 years (range, 1 day to 9.0 years). At this time there are still less than the anticipated number of events with only 705 total DFS events between the L+T and T arms (327 DFS events in the L+T arm vs. 378 events in the T arm). This is lower than the expected 850 events that were anticipated to occur. As one would expect with the passing of time, the number of events per 1000 patient-years has declined since the primary analysis (31.9 vs. 37.6 events per 1000 patient-years for L+T vs. T at the 2014 Primary analysis and 20.3 vs. 21.4 events per 1000 patient-years in L+T vs T since then). The 6-year DFS rates were 82% for T, 84% for T→L and 85% for L+T with HR of 0.93 (95% CI, 0.81–1.08) and 0.86 (95% CI, 0.74–1.00) for T→L vs. T and L+T vs. T, respectively (Figure 3A). The 6-year OS rates were 91% for T, 92% for T→L and 93% for L+T with HR of 0.88 (95% CI, 0.71–1.08) and 0.86 (95% CI, 0.70–1.06) for T→L vs. T and L+T vs. T, respectively (Figure 3B). There were no differences in sites of first DFS events according to treatment arm for central nervous system (2% for the 3 arms), loco-regional (≤2% for the 3 arms), or distant recurrences (≤5% for visceral recurrence and 2% for bone recurrence in the 3 arms). The rate of contralateral invasive breast cancer was 1% in each of the 3 arms and second (non-breast) primary malignancy occurred in ≤3% of patients in all arms. Time to recurrence (TTR) seemed to be shorter in the T vs. L+T arm (HR 0.86; 95% CI, 0.74–1.00).
Figure 3.
3A. DISEASE-FREE SURVIVAL (DFS) ANALYSIS; 3B. OVERALL SURVIVAL (OS) ANALYSIS Kaplan-Meier curves. *p-values are descriptive, not inferential. DFS indicates disease-free survival; Lap, lapatinib; OS, overall survival; and Tras, trastuzumab.
Efficacy in Stratification Subgroups
Outcome analysis was stratified by timing of chemotherapy (design 1 vs. design 2/2B), central hormone receptor (ER/PgR) status (positive [≥ 1%] vs. negative), and lymph node (LN) status (not applicable [neoadjuvant chemotherapy], node negative, 1–3, ≥4 positive nodes) and results are outlined in Table 1. Subset analyses showed a better HR for DFS in favour of L+T vs. T for the hormone-receptor-negative [HR 0.80 (95% CI, 0.64–1.00; 6-yr DFS%=84% vs. 80%)] and the sequential chemotherapy [HR 0.83 (95% CI, 0.69–1.00; 6-yr DFS%=83% vs.79%)] subgroups
Table 1.
DFS Overall and in Stratification Subgroups
| 6-yr DFS | Hazard Ratio | ||
|---|---|---|---|
| L+T vs T | |||
| T→L vs T | |||
| Overall | L+T | 85% | 0.86 (95% CI, 0.74–1.00) |
| T→L | 84% | 0.93 (95% CI, 0.81–1.08) | |
| T | 82% | ||
| Design-1 | L+T | 83% | 0.83 (95% CI, 0.69–1.00) |
| T→L | 82% | 0.87 (95% CI, 0.73–1.05) | |
| T | 79% | ||
| Design-2/2B | L+T | 87% | 0.92 (95% CI, 0.72–1.18) |
| T→L | 85% | 1.05 (95% CI, 0.82–1.33) | |
| T | 86% | ||
| Hormone receptor positive | L+T | 85% | 0.91 (95% CI, 0.75–1.11) |
| T→L | 85% | 0.90 (95% CI, 0.74–1.10) | |
| T | 83% | ||
| Hormone receptor negative | L+T | 84% | 0.80 (95% CI, 0.64–1.00) |
| T→L | 82% | 0.97 (95% CI, 0.79–1.20) | |
| T | 80% | ||
| pN0 | L+T | 91% | 0.91 (95% CI, 0.67–1.22) |
| T→L | 90% | 1.02 (95% CI, 0.76–1.36) | |
| T | 89% | ||
| pN1 | L+T | 87% | 0.78 (95% CI, 0.58–1.05) |
| T→L | 87% | 0.76 (95% CI, 0.57–1.02) | |
| T | 84% | ||
| pN2–3 | L+T | 75% | 0.92 (95% CI, 0.71–1.18) |
| T→L | 71% | 1.09 (95% CI, 0.86–1.39) | |
| T | 73% |
DFS: disease-free survival
L+T: trastuzumab concurrently with lapatinib
T→L: trastuzumab followed by lapatinib
T: trastuzumab alone
Discussion
A very meaningful improved clinical outcome (progression-free survival and OS) with dual HER2 blockade (T+ the monoclonal antibody pertuzumab [P]) was demonstrated in the metastatic setting in the phase III CLEOPATRA trial, which led to a new standard of care.25,26Additionally, a few phase II and phase III neoadjuvant clinical trials have demonstrated improved clinical outcome (pCR) with dual HER2 blockade (T plus either L or P), especially in the hormone receptor-negative population and when targeted therapy is given concurrently with chemotherapy.12,19–24 The ALTTO trial was the first large phase III adjuvant trial to test the hypothesis that dual HER2 blockade could further improve survival outcomes of patients with early stage HER2-positive breast cancer compared with adjuvant T. In this study, patients with hormone receptor-negative tumors, pN1disease and those receiving the targeted agents sequentially following the chemotherapy seemed to derive a non-statistically significant benefit from dual HER2 blockade. The overall conclusion of the trial is that ALTTO failed to demonstrate a statistically significant improvement in clinical outcomes (DFS/OS). However, in 2011 when the L monotherapy arm was discontinued, the Hochberg step-wise alpha-spending procedure was replaced by the Bonferroni procedure, which required a 2-sided p-value of ≤ 0.025 for statistical significance. Thus, the observed value of p = 0.048 was declared as not statistically significant. If, the original Hochberg procedure had been retained, both pairwise comparisons would have been declared statistically significant because both p-values were ≤ 0.0527.
Other phase III trials tested the efficacy of dual HER2 blockade in the adjuvant setting. The APHINITY trial28 tested T+ pertuzumab (P) vs. T + placebo (Pla) along with chemotherapy as adjuvant therapy for stage I-III HER2 positive breast cancer patients. The primary analysis results at a median follow-up of 45.4 months were published in 2017 and demonstrated a statistically significant p=0.045 result for the primary invasive-DFS (IDFS) analysis. Updated APHINITY results29 at a median follow up of 74.1 months show 6-year IDFS of 90.6% in the T+P group and 87.8% in the T group (HR 0.76; 95% CI, 0.64–0.91). By contrast, the results of ALTTO reported here at a median follow-up of 83 months had 6-year IDFS of 85% in the T+L group and 82% in the T group (HR 0.86; 95% CI, 0.74–1.00). Thus, despite the differences with respect to statistical significance observed at the primary analysis (one p=048 [not significant] and the other p=0.045 [significant]), the efficacy results with respect to IDFS seen for T+L vs T in ALTTO and T+P vs T in APHINITY are remarkably similar.
Another phase III trial evaluating sequential T plus the oral irreversible pan-HER2 tyrosine kinase inhibitor neratinib (N) demonstrated improved outcome in the adjuvant setting. The ExteNET trial30–31 compared N vs placebo (Pla), both given for 12 months after completion of 1 year of T. Neratinib led to a 2.5% absolute improvement in 5-year IDFS (90.2% for N vs 87.7% for Pla; HR 0·73, 95% CI 0·57–0·92, p=0·0083). Patients with HR+ BC were allowed to take adjuvant endocrine therapy (ET) concurrently with N and they derived the largest benefit (an absolute 5.1% benefit in 5-year IDFS for patients with HR+/HER2+ BC who started N within 1 year of completing adjuvant T; HR 0.58; 95% CI, 0.41–0.82). This was contrary of what was observed in ALTTO and also contrary of what was observed using N in the neoadjuvant I-SPY trial32 and in the metastatic NALA33 trial. Although in the I-SPY and NALA trials ET was not allowed while patients received N plus chemotherapy, 89% of HR+ patients in the ALTTO trial received adjuvant ET.
Conclusion
T+L did not significantly improve DFS and OS over T alone and added significant non-cardiac toxicity. Therefore, it cannot be recommended for adjuvant treatment of early stage HER2-positive breast cancer. Over 90% of patients who participated in ALTTO are alive at this longer follow-up evaluation, a testimony of the continued improvement in the outcome of all patients with early stage HER2 positive breast cancer.
Supplementary Material
Supplement 1. Baseline Patient Characteristics and Detailed Safety Analyses
Supplement 2. ALTTO protocol
Highlights.
First phase III study of adjuvant dual HER2 blockade for HER2+ breast cancer
8381 patients with stage I-III HER2+ breast cancer, followed for at least 5 years
Compared chemotherapy + trastuzumab vs chemotherapy + 3 lapatinib-containing arms
Lapatinib associated with increased non-cardiac toxicity
Chemotherapy + lapatinib/trastuzumab not superior to chemotherapy + trastuzumab
Acknowledgment
We thank each and every one of the patients who participated in the Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization (ALTTO) study; the Breast European Adjuvant Study Team (BrEAST) Data Center; the Frontier Science team; the Breast International Group (BIG) headquarters; the US National Cancer Institute (NCI); the North Central Cancer Treatment Group (NCCTG; Alliance); the ALTTO Executive and Steering Committee members; the Independent Data Monitoring Committee (IDMC) members; the Cardiac Advisory Board members; the three central pathology laboratories; Novartis; Florentine Hilbers, Sarra El-Abed, Vasiliki Balta, Celine Schurmans, Daniela D. Rosa, Kamal Saini, Otto Metzger Filho, Robin McConnell, Vicki Paterson, Christine Campbell, Eleanor McFadden, Emma Paterson and Garrick Kassab for their scientific and/or project management support; and the physicians, nurses, trial coordinators, and pathologists.
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), UG1CA233180, UG1CA233196, UG1CA233329, UG1CA232760; U10CA180863 to the Canadian Clinical Trials Group and grant 704970 from the Canadian Cancer Society; GlaxoSmithKline from its initial development until November 30, 2015, and Novartis since December 2015. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. https://acknowledgments.alliancefound.org
Conflict of interest:
1. Dr. Moreno-Aspitia – Institutional research funding from GlaxoSmithKline, Genentech, Daiichi Sankyo, Novartis, Merck, Pfizer, AbbVie and Eli Lilly;
2. Dr. Holmes – none;
3. Dr. Jackish – honoraria from GlaxoSmithKline and honoraria and travel/accommodations/expenses from Roche;
4. Dr de Azambuja – honoraria and/or advisory board from Roche/Genentech, Novartis, Seattle Genetics Zodiac, Libbs and Pierre Fabre;; travel grants from Roche/Genentech and GSK/Novartis; research grant to his institution from Roche/Genentech, Astra-Zeneca, GSK, Novartis and Servier;
5. Dr. Boyle – consulting or advisory role and travel/accommodations/expenses from Novartis, consulting or advisory role with Eli Lilly, Pfizer, and Roche, and relationship with Paxman;
6. Mr. Hillman – none;
7. Dr. Korde – none;
8. Dr. Fumagalli – grants to her institution from GSK and Novartis during conduct of study. DF reports institutional grants from AstraZeneca, Roche/Genentech, Servier, Tesaro, and Pfizer outside submitted work;
9. Mr. Izquierdo – employment and stock/other ownership interests with Novartis;
10. Dr. McCullough – none;
11. Dr. Wolff – consulting or advisory role and research funding (Inst) from Pfizer, research funding (Inst) from Myriad Genetics, and named as inventor on one or more issued patents or pending patent applications relating to methylation in breast cancer, and has assigned his rights to JHU, participating in a royalty sharing agreement with JHU;
12. Dr. Pritchard – honoraria, consulting or advisory role, and travel/accommodations/expenses from AstraZeneca, Eisai, GlaxoSmithKline, Novartis, Pfizer, and Roche;
13. Dr. Untch –research funding (Inst) or honoraria (Inst) for consulting or advisory role from Abbvie, Astra Zeneca, Amgen, MSD, Celgene, Mundipharma, Pfizer, Roche, Novartis, Lilly, Pierre Fabre;
14. Mr. Guillaume – none;
15. Dr. Ewer – consulting agreements with Astra-Zeneca, Bayer and Boehringer Ingelheim;
16. Dr. Shao – none;
17. Dr. Sim – none;
18. Dr. Aziz – none;
19. Dr. Demetriou – honoraria, consulting or advisory role with Pfizer, Mylan, Eli Lilly, Novartis, MSD, Astra Zeneca, Sanofi, Merck Serono and Roche;
20. Dr. Andersson – none;
21. Dr. Mehta – none;
22. Dr. Toi – research grantor honoraria for lecture or consulting or advisory role from JBCRG association, Chugai Pharma, Takeda, Pfizer, Kyowa-Hakko-Kirin, Taiho, Eisai, Novartis, Daiichi-Sankyo, Astra-Zeneca, Eli Lilly, Konica Minolta, Yakult, Shimadzu, Kyowa Kirin, Bertis, BMS and Athenex Oncology;
23. Dr. Lang – none;
24. Dr. Xu – Consulting or advisory role from Novartis, Roche, Astra-Zeneca;
25. Dr. Smith – none;
26. Dr. Barrios – Research grants to the institution from Pfizer, Novartis, Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Roche/Genentech, Lilly, Sanofi, Taiho Pharmaceutical, Mylan, Merrimack, Merck, AbbVie, Astellas Pharma, BioMarin, Bristol-Myers Squibb, Daiichi Sankyo, Abraxis Biosciences, AB Science, Asana Biosciences, Medivation, Exelixis, ImClone Systems, LEO Pharma, Millennium, Merck KGaA, Shanghai Henlius Biotech, Polyphor, PharmaMar. Consulting and Ad Boards from Boehringer-Ingelheim, GSK, Novartis, Pfizer, Roche/Genentech, Eisai, Bayer, MSD, Astra Zeneca, Zodiac;
27. Dr. Baselga – leadership and stock/other ownership interests with AstraZeneca, Infinity Pharmaceuticals and Varian Medical Systems, stock/other ownership interests with GRAIL, Juno Therapeutics, and PMV Pharma, and consulting or advisory role at Eli Lilly, Novartis, and GRAIL;
28. Dr. Gelber – research funding (Inst) from AstraZeneca, Celgene, GlaxoSmithKline, Merck, Novartis, Pfizer, Ipsen, and Roche;
29. Dr. Piccart-Gebhart – consulting or advisory role with HUYA Bioscience International, Oncolytics, consulting or advisory role and research funding (inst) from AstraZeneca, Debiopharm Group, Eli Lilly, MSD, Novartis, Pfizer, Radius Health, Roche/Genentech and Servier.
Footnotes
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alvaro Moreno-Aspitia, Jacoby Center for Breast Health, Mayo Clinic, Jacksonville, Florida, USA.
Eileen M. Holmes, Dundee Epidemiology and Statistics Unit, University of Dundee, Dundee, UK..
Christian Jackisch, Department of Gynecology and Obstetrics, Sana Klinikum Offenbach GmbH, Germany.
Evandro de Azambuja, Institute Jules Bordet and l’ Université Libre de Bruxelles (U.L.B), Brussels, Belgium.
Frances Boyle, Patricia Ritchie Centre for Cancer Care and Research, University of Sydney, Australia.
David W. Hillman, Alliance Statistics and Data Center, Mayo Clinic, Rochester, Minnesota, USA.
Larissa Korde, Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Bethesda, Maryland, USA.
Debora Fumagalli, Breast International Group, Brussels, Belgium.
Miguel A. Izquierdo, Novartis Pharma AG, Basel, Switzerland.
Ann E. McCullough, Division of Anatomic Pathology, Mayo Clinic, Scottsdale, Arizona, USA.
Antonio C. Wolff, Johns Hopkins Kimmel Cancer Center, Baltimore, Maryland, USA.
Kathleen I. Pritchard, Institute Jules Bordet and l’ Université Libre de Bruxelles (U.L.B), Brussels, Belgium.
Michael Untch, Helios Klinikum Berlin-Buch, Berlin, Germany.
Sébastien Guillaume, Institute Jules Bordet and l’ Université Libre de Bruxelles (U.L.B), Brussels, Belgium.
Michael S. Ewer, MD Anderson Cancer Center, University of Texas, Houston, Texas, USA.
Zhimin Shao, Fudan University Shanghai Cancer Center, Shanghai, People’s Republic of China.
Sung Hoon Sim, Center for Breast Cancer, National Cancer Centre, Gyeonggi-do, Korea.
Zeba Aziz, Allama Iqbal Medical College, Lahore, Pakistan.
Georgia Demetriou, University of the Witwatersrand, Johannesburg, South Africa.
Ajay O. Mehta, Central India Cancer Research Institute, Nagpur, Maharashtra, India.
Michael Andersson, Department of Oncology, Rigshospitalet, Copenhagen, Denmark.
Masakazu Toi, Graduate School of Medicine, Kyoto University, Kyoto, Japan.
Istvan Lang, National Institute of Oncology, Budapest, Hungary.
Binghe Xu, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Ian E. Smith, The Royal Marsden Hospital NHS Foundation Trust, London, England.
Carlos H. Barrios, Latin American Cooperative Oncology Group (LACOG), Oncoclínicas, Porto Alegre, Brazil.
Jose Baselga, Oncology Research & Development, Astra-Zeneca, Cambridge, UK.
Richard D. Gelber, Dana-Farber/Partners CancerCare, Harvard Medical School, Harvard TH Chan School of Public Health and Frontier Science Technology Research Foundation, Boston, Massachusetts, USA.
Martine Piccart-Gebhart, Canadian Cancer Trials Group (CCTG), Kingston,Ontario, Canada.
References
- 1.Romond EH, Perez EA, Bryant J, et al. : Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353:1673–84, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Perez EA, Romond EH, Suman VJ, et al. : Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 32:3744–52, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. : Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353:1659–72, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Gianni L, Dafni U, Gelber RD, et al. : Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol 12:236–44, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. : 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet 382:1021–8, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Slamon D, Eiermann W, Robert N, et al. : Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365:1273–83, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burris HA 3rd, Hurwitz HI, Dees EC, et al. : Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol 23:5305–13, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Gomez HL, Doval DC, Chavez MA, et al. : Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol 26:2999–3005, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Geyer CE, Forster J, Lindquist D, et al. : Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355:2733–43, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Gelmon KA, Boyle FM, Kaufman B, et al. : Lapatinib or Trastuzumab Plus Taxane Therapy for Human Epidermal Growth Factor Receptor 2-Positive Advanced Breast Cancer: Final Results of NCIC CTG MA.31. J Clin Oncol 33:1574–83, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Blackwell KL, Burstein HJ, Storniolo AM, et al. : Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol 30:2585–92, 2012 [DOI] [PubMed] [Google Scholar]
- 12.de Azambuja E, Holmes AP, Piccart-Gebhart M, et al. : Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol 15:1137–46, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Piccart-Gebhart M, Holmes AP, Baselga J, et al. : First results from the phase III ALTTO trial (BIG 2–06; NCCTG [Alliance] N063D) comparing one year of anti-HER2 therapy with lapatinib alone (L), trastuzumab alone (T), their sequence (T→L), or their combination (T+L) in the adjuvant treatment of HER2-positive early breast cancer (EBC). J Clin Oncol 32:LBA4, 2014 [Google Scholar]
- 14.Piccart-Gebhart M, Holmes E, Baselga J, et al. : Adjuvant Lapatinib and Trastuzumab for Early Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Results From the Randomized Phase III Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization Trial. J Clin Oncol 34:1034–42, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Cancer Institute: NCI Common Terminology Criteria for Adverse Events (CTCAE) Files, 2018 [Google Scholar]
- 16.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481, 1958 [Google Scholar]
- 17.Greenwood M: The Natural Duration of Cancer. Rep Public Health Med Subj (Lond) 33:1–26, 1926 [Google Scholar]
- 18.Cox DR: Regression models and life-tables. J R Stat Soc Series B Stat Methodol 34:187–220, 1972 [Google Scholar]
- 19.Baselga J, Bradbury I, Eidtmann H, et al. : Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 379:633–40, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gianni L, Pienkowski T, Im YH, et al. : Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13:25–32, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Guarneri V, Frassoldati A, Bottini A, et al. : Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. J Clin Oncol 30:1989–95, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Rimawi MF, Mayer IA, Forero A, et al. : Multicenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer: TBCRC 006. J Clin Oncol 31:1726–31, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robidoux A, Tang G, Rastogi P, et al. : Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. Lancet Oncol 14:1183–92, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Schneeweiss A, Chia S, Hickish T, et al. : Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 24:2278–84, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Baselga J, Cortes J, Kim SB, et al. : Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366:109–19, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swain SM, Baselga J, Kim SB, et al. : Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 372:724–34, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmes EM, Bradbury I, Williams LS, Korde L, et al. : Are we assuming too much with our statistical assumptions? Lessons learned from the ALTTO trial. Annals of Oncology 30: 1507–1513, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Minckwitz G, Procter M, de Azambuja E, et al. : Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med 377:122–131, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piccart M, Procter M, Fumagalli D, et al. : GS1–04. Adjuvant Pertuzumab and Trastuzumab in early HER2-positive Breast Cancer in the APHINITY trial: 6 years’ follow-up. J Clin Onc [DOI] [PubMed] [Google Scholar]
- 30.Martin M, Holmes FA, Ejlertsen B, et al. : Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 18:1688–1700, 2017 [DOI] [PubMed] [Google Scholar]
- 31.Chan A, Moy B, Mansi J, et al. : Final efficacy results of neratinib in HER2-positive hormone receptor-positive early-stage breast cancer for the phase III ExteNET trial. Clinical Breast Cancer October 6, 2020; 10.1016/j.clbc.2020.09.014 [DOI] [PubMed] [Google Scholar]
- 32.Park JW, Liu MC, Yee D, et al. : Adaptive randomization of neratinib in early breast cancer. N Engl J Med 375:11–22, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saura C, Oliveira M, Feng Y-H, et al. : Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With ‡ 2 HER2-Directed Regimens: Phase III NALA Trial. J Clin Oncol 38:3138–3149 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1. Baseline Patient Characteristics and Detailed Safety Analyses
Supplement 2. ALTTO protocol