Abstract
Introduction
Edinburgh and Lothians’ Viral Intervention Study Kids is a parallel, open-label, randomised controlled trial of hypertonic saline (HS) nose drops (~2.6% sodium chloride) vs standard care in children <7 years of age with symptoms of an upper respiratory tract infection (URTI).
Methods and analysis
Children are recruited prior to URTI or within 48 hours of developing URTI symptoms by advertising in areas such as local schools/nurseries, health centres/hospitals, recreational facilities, public events, workplaces, local/social media. Willing parents/guardians, of children <7 years of age will be asked to contact the research team at their local site. Children will be randomised to either a control arm (standard symptomatic care), or intervention arm (three drops/nostril of HS, at least four times a day, until 24 hours after asymptomatic or a maximum of 28 days). All participants are requested to provide a nasal swab at the start of the study (intervention arm: before HS drops) and then daily for four more days. Parent/guardian complete a validated daily diary, an end of illness diary, a satisfaction questionnaire and a wheeze questionnaire (day 28). The parent/guardian of a child in the intervention arm is taught to prepare HS nose drops. Parent/guardian of children asymptomatic at recruitment are requested to inform the research team within 48 hours of their child developing an URTI and follow the instructions already provided. The day 28 questionnaire determines if the child experienced a wheeze following illness. Participation in the study ends on day 28.
Ethics and dissemination
The study has been approved by the West of Scotland Research Ethics Service (18/WS/0080). It is cosponsored by Academic and Clinical Central Office for Research and Development—a partnership between the University of Edinburgh and National Health Service Lothian Health Board. The findings will be disseminated through peer-reviewed publications, conference presentations and via the study website.
Trial registration number
Keywords: paediatric infectious disease & immunisation, virology, primary care, community child health, neonatology
Strengths and limitations of this study.
Open-label community-based randomised controlled study investigating the effectiveness of hypertonic saline (HS) versus standard treatment in children under 7 years of age with a common cold.
Parents in the intervention arm are taught to safely make HS at home.
Nose swabs are collected to identify the virus(es) and to measure change in viral shedding.
A validated symptom score diary (Canadian Acute Respiratory Illness and Flu Scale) is used as part of the daily diary.
There is no placebo arm.
Introduction
Viral upper respiratory tract infections (URTIs) are very common in childhood resulting in a significant burden on the population and health services.1 The annual incidence rate of URTI in childhood can vary between 6 and 12 episodes.2 3 Children have a longer duration of illness (mean 1.8 weeks; SD: 1.3 weeks) compared with adults.3 4 In Scotland, URTI is the leading cause of general practitioner (GP) visits in children <5 years (n=84 710, 574/1000 population) and the fourth most common cause of consultation in children aged 5–14 (n=33 790, 116.5/1000 population).5 In secondary care, in 2015–16, 13.3% (n=16 644) and 19.7% (14 600) of all admissions/emergency admissions respectively in children were due to URTI/lower respiratory tract infections.6 A total of 12%–14% of children who develop an URTI will go on to develop lower respiratory tract disease (viral induced wheeze: 80/1000,7 bronchiolitis: 46/10008 and pneumonia: 0.27 episodes/child year.9 Hence interventions that reduce URTI severity may considerably benefit patients/carers and reduce pressure on the National Health Service (NHS). Since >200 viruses10 can cause an URTI, individually targeted antiviral therapy is impractical and measures that work against all viruses are required.
New data suggest that hypertonic saline (HS) may be able to suppress viral replication, regardless of viral type and hence be a potential respiratory antiviral agent with clinical application. Saline irrigation is commonly used in clinical practice, mostly to deliver a mucolytic effect. However, we have recently identified that human epithelial (cervical-HeLa, respiratory-A549) cells use sodium chloride (NaCl) to mount a broad-spectrum antiviral effect against representative DNA, RNA enveloped and non-enveloped viruses.11 The antiviral effect is dependent on the entry of chloride ions into the cell and the production of intracellular hypochlorous acid (HOCl).11 HOCl is the active ingredient of bleach, which can inactivate most viruses.12–15 A polymorphism causing reduction in HOCl production has been reported in individuals with cervical cancer.16 Since cervical cancer follows infection with high-risk types of human papilloma viruses, it suggests a key role for local antiviral mechanisms. HOCl production is an important antibacterial mechanism in human neutrophils.17 Increased HOCl production is reported within gut epithelial cell of fruit flies after bacterial lysate ingestion.18 These data suggest an anti-infective role of HOCl and its precursor NaCl in epithelial cells.
In addition, it has recently been shown that accumulation of Na+ ions in human skin helps fight bacterial/parasitic infections.19 20 A high salt diet increased Na+ in skin leading to a hypertonic environment, increased nitric oxide (NO) production in macrophages and thereby pathogen removal.19 20 While our laboratory data point to the importance of Cl− in combating viral infections, Jantsch et al have shown the importance of Na+ in fighting bacterial infections. Taken together, these data suggest that innate immunity may be dependent on NaCl in epithelial cells helping to clear bacterial and viral infection.
Nebulised HS has been used to treat bronchiolitis; an acute viral infection of young children caused by a variety of respiratory viruses. Meta-analyses of current trials suggest a possible reduction in length of hospital stay,21 22 but the association is weak and there have been concerns about the replicability of this finding23–27 potentially as HS has been given at the peak of disease when viral load is maximum and lower respiratory tract disease (with potential dysregulated immune response) is established. More positive signals have been demonstrated when HS has been administered in accident and emergency department contexts to reduce hospital admission rates: data remain conflicting and a systematic review has been started to analyse data as they emerge.26–28
The role of saline (isotonic or hypertonic) in children with viral URTI has been explored in two published studies. In children aged 6–10 years administered isotonic saline as a spray (six times/day), there was a significant reduction in reported sore throat, nasal secretions and use of nasal decongestants/mucolytic (vs standard care, no placebo).29 In children <2 years treated with saline/sea water drops (three times a day for 5 days), there was a significant reduction in URTI symptoms reported when compared with untreated children.30 However, a Cochrane review concluded that no definitive conclusions could be drawn as the available studies were small and had major methodological limitations: baseline symptom score was calculated over 7 days (not at the point of entry) and groups had different characteristics at baseline.31
We recently completed the Edinburgh and Lothians’ Viral Intervention Study (ELVIS), an open label pilot randomised controlled trial (RCT) of HS nasal irrigation and gargling (HSNIG) in 66 adults with an URTI (www.elvisstudy.com).4 Most participants were infected with rhinoviruses/coronaviruses. The intervention arm had a 22% reduction in duration of illness (mean (SD) of intervention arm: 6.8 days (2.2) and control arm 8.7 days (3.3), difference of 1.9 days; p=0.01). Ninety-three per cent believed HSNIG helped improve symptoms of the cold. There was 36% reduction in over-the-counter medication use (p=0.003), transmission within household was reduced by 35% (p=0.006) and viral shedding reduced at a faster rate of ≥0.5 log10/day in those receiving HSNIG (p=0.04).4 The reduction in viral shedding and transmission within household were supportive of our laboratory data and consistent with a direct cellular antiviral action by NaCl.
Given the laboratory evidence, supported by our demonstration of clinical benefits in adults, an RCT in children with URTI to study the effects of HS on duration of illness and viral shedding is now needed. No suitable placebo is available: Sodium bicarbonate and plain water cause discomfort when administered to nasal mucosa and normal saline, a commonly used, safe, placebo contains NaCl and so may not act as a placebo. For these reasons, the study will not be placebo controlled.
Methods and analysis
Study objectives
Primary objective
To investigate whether the use of parent/guardian-initiated HS nose drops administered to children with symptoms consistent with acute viral URTI reduces the duration of symptoms when compared with children managed using standard care.
Secondary objectives
To determine the effect of HS nose drops on:
Severity of all symptoms.
Duration and severity of individual symptoms.
Contact with NHS 24, out of hours primary care (OOH), and primary care (GP).
Hospital attendance (ie, A&E attendance and/or hospital admission) and diagnosis.
Reduction in wheeze.
Over the counter medication use.
Duration, reduction or rate of reduction in viral shedding.
Transmission within the household.
Side effects associated with the use of saline nose drops.
Adverse events (AEs) associated with the use of saline nose drops.
Time off from school/nursery for child and workdays lost for parent/guardian.
Cost associated with illness (over the counter medication costs and NHS costs).
Study design and sample size
ELVIS Kids is a parallel, open label, RCT of HS nose drops (~2.6% NaCl) versus standard care in children <7 years of age with symptoms of an URTI. The aim is to recruit a total of 480 children (240/arm).
The study will run over ~42 months at participating sites in Scotland (sites are as listed on ClinicalTrials.gov). Children are recruited prior to, or within 48 hours of developing URTI symptoms by advertising in areas such as local schools, nurseries, health centres, hospitals, recreational facilities, workplaces public events and the community as well as local and social media. For the purposes of this study an URTI is defined as: at least two respiratory symptoms (nasal congestion, runny nose, cough, sore throat) or one respiratory symptoms and at least one systemic symptom (low energy/tired, muscle aches/pains, headache, fever ≥38°C). Willing parents/guardians, will be directed by the study advertising to contact the research team at their local site) if they are interested in participating.
Children will be randomised to either a control arm of standard symptomatic care, or an intervention arm of three drops each nostril of HS at least four times a day and up to a maximum of 12 times a day until asymptomatic or maximum of 28 days. All parents/guardians will be requested to obtain a mid-turbinate nasal swab from the participant first thing in the morning (before nose drops in the intervention arm) for five consecutive days (unless the child is well before then), a daily diary (a global severity question, Canadian Acute Respiratory Illness and Flu Scale (CARIFS),32 a validated illness measure in the UK, side effects and compliance with trial procedures) until they report the child as ‘not unwell’, an end of illness diary (infection in household contacts, ease of use and acceptability of intervention, medication and healthcare use, acceptability, time taken off usual activities, wheezing and whistling in the chest), a satisfaction questionnaire and AEs. Parents/guardians of the children allocated to the intervention arm will be taught how to prepare the HS (including sterilisation instructions for children under a year). Parents/guardians of children who are asymptomatic at recruitment are requested to inform their local research team when the child develops an URTI (within 48 hours) and follow the instructions already provided to them. On day 28, parents/guardians will be contacted to determine if their child suffered from wheezing or whistling in the chest either during the illness or at any point until day 28. Participation in the study will end on day 28.
Eligibility and consent
Prescreening for eligibility to participate will be completed by a member of the research team at the clinical trials unit when parents/guardians phone to express interest in the study. If parents/guardians attend an appointment and take part in the study, the study number will be recorded on the screening log and details of eligibility will be recorded in the study database.
Inclusion criteria
Children between corrected gestational age of ≥40 weeks and <7 years of age.
Children without URTI OR ≤48 hours of URTI* starting.
*An URTI being defined as at least two respiratory symptoms (nasal congestion (ie, stuffy nose), runny nose, cough, sore throat) or one respiratory symptom+at least one systemic symptom (low energy/tired, muscle aches/pains, headache, fever 38°C).
Exclusion criteria
Children needing immediate medical attention.
Children using saline drops/sprays at the time of randomisation.
Children on immunosuppressive medication, regular oral/inhaled steroids, regular antibiotics (use of antibiotics is allowed as long as the child does not need regular antibiotics).
Children with a known chronic illness (eg, cystic fibrosis, cardiac, renal, liver, lung, neurological conditions) apart from wheeze or asthma which are not exclusions if the child is otherwise well and not on regular steroids).
Children being followed up for developmental delay.
Children receiving the nasal influenza vaccine ≤14 days ago.
Children taking part in another interventional trial.
If parents/guardians indicating that they are unable to comply with the study protocol prior to randomisation.
If parents/guardians are unable to understand written or spoken English.
Children randomised to ELVIS KIDS on a previous episode of URTI.
Children with a concurrently participating sibling.
All ineligible and non-recruited participants will be recorded on the ELVIS Kids screening log with a reason given.
Obtaining consent
Only trained and delegated members of the trial team will take consent—this will usually be the research nurse. The participant information sheet (PIS), which will explain the aims of the study and the potential risks and benefits of the study treatment, are provided to parents/guardians when they meet the research team (also available online). A children’s PIS will be available to discuss with older children attending the appointment with the option of giving their assent (online supplemental file 1).
bmjopen-2021-049964supp001.pdf (1MB, pdf)
If the parent/guardian wishes to participate in the study, then they will be asked to sign the informed consent form (ICF) (online supplemental file 1). Both the parent/guardian and the person delegated to take consent will sign and personally date the ICF. The original signed ICF must be kept by the Investigator in the investigator site file, one copy is provided to the parent/guardian, one copy is placed in TRAK. The same would apply in the case of assent being given.
Randomisation and treatment allocation
A member of the research team from the clinical research facility will perform the randomisation using a web-based randomisation service managed by the Edinburgh Clinical Trials Unit (ECTU). Children will be allocated to receive either HS nose drops or standard care in a 1:1 ratio using minimisation based on age (0–2, >2 years) and sex and allocated to receive the treatment which minimises the imbalance with a probability 0.8. The study is not blinded apart from those carrying out lab assessments of nasal swabs.
Sea salt will be provided by Cornish Sea Salt company in 225 g pots. They will be supplied to local pharmacies where they will be labelled and stored. A working stock will be issued to the research team. If a child is allocated to an intervention arm, the parent/guardian will be given instructions on the preparation and use of HS nose drops using instructional video, verbal and written information. Parents/guardians will be asked to add one level measure of sea salt to a fixed volume of freshly boiled water using the measuring spoon and clean glass jar provided. This provides a NaCl concentration of ~2.6% and the drops can be used once cooled. Two glass jars are provided so that the parent/guardian could use one and have a clean spare to prepare solution the next day. Two dropper bottles are provided with which nose drops can be applied (one in use, and a clean spare).
Withdrawal of study participants
Parents/guardians are free to withdraw their child from the study at any point. If withdrawal occurs, the primary reason for withdrawal will be documented in the participant’s case report form (CRF), if given. All data and swabs collected before withdrawal will be retained for analysis in cases where participants withdraw.
Study assessments
The protocol is designed in accordance with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT). The trial overview of the study assessments is available as a SPIRIT figure (table 1). At the appointment, a member of the research team will train the parent/guardian how to identify an URTI, how to measure temperature, how to complete the diaries and how to collect and return the mid-turbinate swabs. In addition, those in the intervention arm will be given instructions on how to prepare and apply nose drops. Baseline information on the child, contact details of parent/guardian and number of household members at the time of recruitment will be collected in the electronic CRF (eCRF). If recruited when symptomatic, parents will be instructed to start the study assessments the same day. If recruited when asymptomatic, and there are no changes to the child’s medical information, parent/guardians will be asked to start the study assessments and to inform the study team the same day if possible or at least within 2 days of onset of illness. If recruited when asymptomatic, and there are changes to the child’s medical information, parent/guardians will be asked to contact the study team to ensure the child still meets the eligibility criteria before starting the study procedures. If it is not suitable for the child to take part during this URTI (eg, received the influenza vaccine in the past 2 weeks) they will remain on study and be asked to contact the team at the onset of the next URTI.
Table 1.
Assessments and timepoints
| Study timepoints | Prescreen ing | Baseline | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Days 5–8 | Days 5–28 (as applicable) | Day 28 |
| Prescreening | X | |||||||||
| Informed consent | X | |||||||||
| Eligibility criteria | X | |||||||||
| Eligibility review if change in health information | X | |||||||||
| Randomisation/treatment allocation | X | |||||||||
| Baseline case report form | X | |||||||||
| Nose swab collection (if child remains unwell) | X | X | X | X | X | |||||
| Nose swab return | X | |||||||||
| Intervention arm—HS drops | X | X | X | X | X | X | ||||
| Daily diary | X | X | X | X | X | X | ||||
| Adverse events | X | X | X | X | X | X | ||||
| End of illness diary |

|
|||||||||
| Satisfaction questionnaire |

|
|||||||||
| Return of diaries | X | |||||||||
| Day 28 wheeze question | X | |||||||||
HS, hypertonic saline.
Parents/guardians are requested to collect a nasal (mid-turbinate) swab as soon as possible on day 1 and first thing in the morning (and prior to HS nose drops being applied) on days 2–5 if children remain unwell. These samples are to be packed in the transport box provided, stored in the fridge and returned in the prepaid envelope or as soon as possible after completing collection. If samples are not received by day 10, a reminder will be sent to the parent/guardian.
Parents/guardians will complete a daily diary (online supplemental file 1) which records any symptoms the child is experiencing, compliance to nasal swabs and HS drops, any side effects and use of healthcare services. Parents will be taught how to measure temperature with TempaDot. Parents are advised to measure the temperature only if they think the child has a fever. If the child has an axillary temperature of ≥38°C, it should be recorded as a fever in the daily diary. The diaries will be provided as an online form (unless parents cannot access this in which case a paper copy can be provided). If the online Daily Diary is not completed a reminder will be sent.
An end of Illness diary (online supplemental file 1) and satisfaction questionnaire (online supplemental file 1) will be completed by the parent/guardian once the child is asymptomatic for >24 hours or after a maximum of 28 days. On day 28, the parent/guardian will be contacted by email and sent a text message to ask if their child has experienced any wheeze since the end of illness diary was completed (online supplemental file 1).
Participants will be sent a £30 voucher by email as compensation for any inconvenience once they have returned the study data.
Analysis and storage of samples
Up to five nasal swabs will be collected and posted to the Department of Laboratory Medicine, Royal Infirmary of Edinburgh, 51 Little France Crescent, Edinburgh, EH16 4SA, where they will be stored and processed. Day 1 samples will be analysed by the respiratory panel and the cycle threshold (CT) of positive samples recorded. If the day one sample is missing, the first available sample will be tested to identify the virus. If an agent is identified, all samples (days 1–5) will be tested in parallel to estimate change in viral shedding and the CT recorded. If a sample is positive on day 1 and negative on subsequent days, they may be tested for human DNA to confirm a sample was collected. Log conversion of each positive result will be done using the following formula: (40-CT of specimen)/3.3 to estimate change in shedding. All nucleic acid extracts and remaining original samples will be stored in the Lothian NHS research Scotland BioResource biobank (REC reference 15/ES/0094) and can be used in future ethically approved studies.
Outcomes/endpoints
Primary endpoint
Duration of illness (measured as the number of days until the parent reports the child to be well).
Secondary endpoints
Severity of all symptoms as measured by CARIFS.
The length of time for individual symptoms to resolve.
Severity of individual symptoms.
Contacting healthcare (NHS 24, OOH, GP) (number of participants and frequency of contacts).
Participants needing GP appointments (number of participants and frequency of contacts).
Participants attending hospital and diagnosis (number of participants and frequency of contacts).
Length of stay in hospital if admitted.
Number of participants reporting wheeze during illness and between end of illness to 28 days.
Number of participants reporting over the counter medication use.
Duration of viral shedding.
Reduction in viral shedding.
Rate of reduction in viral shedding.
Reduction in transmission to household contacts.
Number of participants reporting side effects associated with nasal drops.
Number of participants reporting AEs associated with nasal drops.
Types and severity of side effects/AEs reported.
Number of days lost from school/nursery for child.
Number of days lost from work for parent/guardian.
Cost of over the counter medication used.
NHS costs associated with illness.
Participant timeline
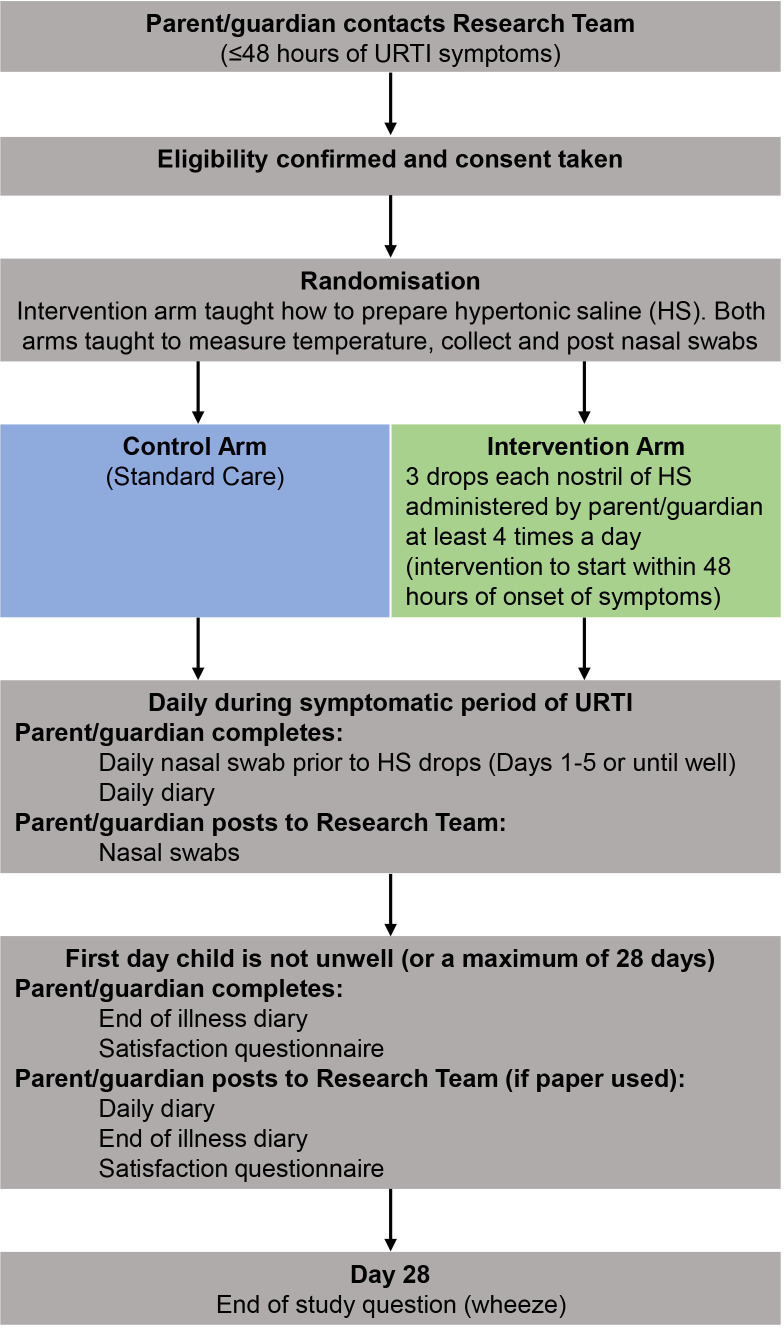
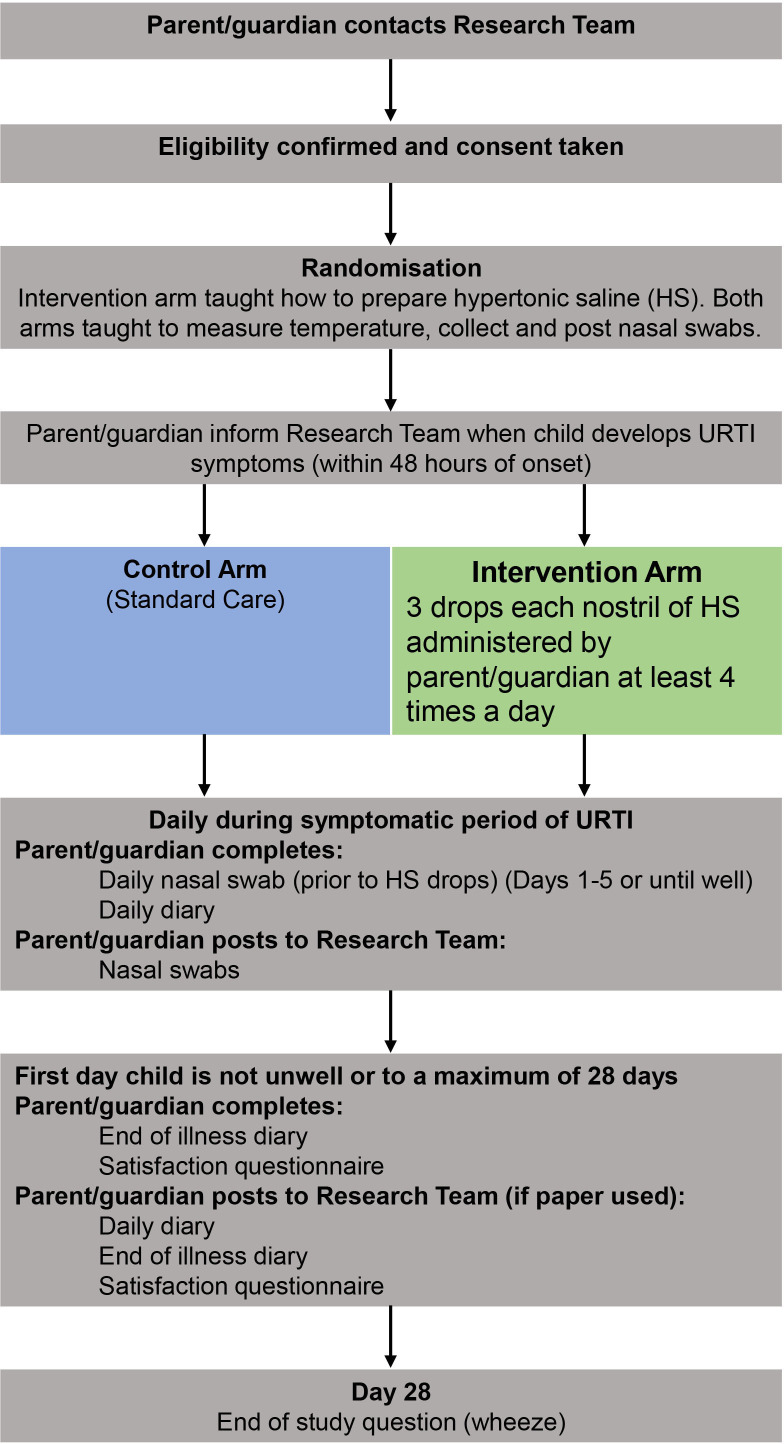
The participant pathways can be seen in figures 1 and 2. Participants will be active in the study for 28 days. There are no long-term follow-up assessments after day 28 of URTI developing.
Figure 1.
ELVIS Kids Patient Pathway when the child has an URTI at recruitment. ELVIS, Edinburgh and Lothians’ Viral Intervention Study; URTI, upper respiratory tract infection.
Figure 2.
ELVIS Kids Patient Pathway when the child does not have an URTI at recruitment. ELVIS, Edinburgh and Lothians’ Viral Intervention Study; URTI, upper respiratory tract infection.
Data collection
Baseline data will be collected on the baseline eCRF by a member of the research team. Parents/guardians will record study data onto either an online form which will be saved into the eCRF. A paper CRF (pCRF) option is available if a parent/guardian prefers it. pCRF will be returned to the local research team and transcribed by a member of the research team into the database and cross-checked by another.
Virological results are downloaded (identifiable by study number) on a weekly basis on to a specific drive by the laboratory information management and technology team. These will be emailed to the ECTU on a monthly basis and uploaded into the study database.
The trial database will be created and maintained by ECTU. Trained and delegated members of the research team will be given password-protected logins to the database to complete data entry. Data completed online by parents/guardians will be transmitted into the study database. The data will be stored in a secure server in the University of Edinburgh for at least the archiving period.
Adverse events
Symptoms and side effects from the daily diary will be recorded in the CRF but will not be recorded as an AE or adverse reaction. Hospitalisation is a study outcome and is exempt from reporting to the sponsor as a serious AE.
Any other AEs identified between day 1 and 28 of the study will be recorded. Any events reaching seriousness criteria will be reported to the sponsor within 24 hours.
Sample size calculation and statistical analysis
Sample size calculation is based on mean (SD) duration of illness values in a control population from Gruber et al of 13 (9) days. To detect a 20% difference in mean duration, that is, 3 days, using a two sided, two-sample test with 5% level of significance, 90% power and common SD of 9 days we will need a sample of 191 per treatment arm, without drop-outs. Hence, we will recruit 240 participants per arm to allow for up to 20% drop-outs.
Statistical analysis will be conducted according to the details specified in the prespecified statistical analysis plan. Differences in illness duration between treatment arms will be compared using a two-sample t-test or non-parametric equivalent, as appropriate. This method will also be employed to examine differences between treatment arms for other continuous outcome measures such as average symptom score, viral shedding between treatment arms. For binary categorical data, for example, the proportion of participants per arm attending their GP, attending hospital, etc, we will compare the treatment arms using a binomial test for the comparison of proportions. Where we have categorical data with more than two categories a χ2 test will be used to examine the relationships between treatment arms. If the number of cases of individual viruses are sufficient, the above analysis will be repeated by virus type.
Oversight arrangements
The study is cosponsored by Academic and Clinical Central Office for Research and Development, a partnership between the University of Edinburgh and NHS Lothian Health Board based at QMRI, 47 Little France Crescent, Edinburgh Email: enquiries@accord.scot. The trial will be coordinated by a Project Management Group, consisting of the chief investigator, coinvestigators, trial manager, statistician and coordinating nurse. The trial manager will oversee the study and will be accountable to the chief investigator. ECTU is responsible for trial management and oversight of data collection. The Edinburgh Clinical Research Facility are responsible for the statistical analysis. A Delegation Log will be prepared, detailing the responsibilities of each member of staff working on the trial. A trial steering committee (TSC) has been established to oversee the conduct progress. As there will be no data monitoring committee for this project, the TSC will review safety information as part of their remit.
Ethics and dissemination
The study will be conducted in accordance with the principles of the International Conference on Harmonisation Tripartite Guideline for Good Clinical Practice (ICH). The study has been approved by the West of Scotland Research Ethics Service (reference: 18/WS/0080). Any changes in research activity, except those necessary to remove an apparent, immediate hazard to the participant in the case of an urgent safety measure, will be reviewed and approved by the chief investigator. Amendments will be submitted to the sponsor for review and authorisation before being submitted in writing to the appropriate REC, and local research and development office for approval prior to participants being enrolled into an amended protocol. The findings will be disseminated through peer-reviewed publications, conference presentations and on the study website.
Confidentiality
All laboratory specimens, evaluation forms, reports, and other records must be identified in a manner designed to maintain participant confidentiality. All records must be kept in a secure storage area with limited access. Clinical information will not be released without the written permission of the participant’s parent/guardian. The Investigator and study site staff involved with this study may not disclose or use for any purpose other than performance of the study, any data, record or other unpublished, confidential information disclosed to those individuals for the purpose of the study. Prior written agreement from the sponsor or its design must be obtained for the disclosure of any said confidential information to other parties.
All Investigators and study site staff involved with this study must comply with the requirements of general data protection regulations with regard to the collection, storage, processing and disclosure of personal information and will uphold the Act’s core principles. Access to collated participant data will be restricted to individuals from the research team treating the participants, representatives of the sponsor(s) and representatives of regulatory authorities. Computers used to collate the data will have limited access measures via user names and passwords. Published results will not contain any personal data that could allow identification of individual participants.
Patient and public involvement
Feedback was obtained from patient and public involvement (PPI) representatives on the study protocol, information sheets, diaries and consent forms and necessary modifications made prior to starting the study. A PPI representative is also invited to attend the trial steering committee meetings.
Access to data
Ownership of the data arising from this study resides with the study team. On completion of the study, the study data will be analysed and tabulated, and a clinical study report will be prepared in accordance with ICH guidelines.
Trial status
This paper describes study protocol version V4 (06/01/2020). The trial opened on 2 November 2018. The first participant was recruited on 6 November 2018. The planned study end date is 30 November 2021. At the time of submission, study recruitment was suspended due to the COVID-19 pandemic.
Discussion
The study is based on the recently discovered evidence that epithelial cells have an innate antiviral effect.11 This effect can be augmented by supplying the cells with chloride ion through NaCl. Saline, commonly used as a placebo cannot be used in that role here as it contains NaCl—the substrate being tested. We are hence measuring viral shedding as an independent measure of any antiviral effect. The results from this trial will help determine if a simple and low-cost intervention could help to reduce the duration of symptoms of URTI in children. Changes to the duration of individual symptoms, wheeze, transmission within the household, over-the-counter medication use, need for further treatment, days lost and cost of illness are all secondary outcomes.
Conclusion
Since numerous viruses can cause URTI and in the absence of an antiviral agent/vaccine against the vast majority of viruses, if successful, this low cost and easily accessible intervention can easily be rolled out globally.
Supplementary Material
Footnotes
Collaborators: The ELVIS Kids Trial Investigators are: Brittney Abernathy, Julie Baggot, Gillian Black, Louise Frampton, Naomi Matos, Elinore McGivern, Rachel McKernan, Debbie Miller, Margaret Millar, Sheila Mortimer, Finny Paterson, Maxine Ramsay, Joan Thomson, Emma Ward, Jacqueline Waters. Department of Laboratory Medicine, Royal Infirmary of Edinburgh: Alistair Scott, Jenny Dove, Marianne Cunningham, Lisa Marie Wilson. Edinburgh Clinical Trials Unit (ECTU): Ruth Armstrong, Christine Campbell, Gina Cranswick, Ronnie Harkess, Lynsey Milne, Pamela Sinclair, Michelle Steven. Pharmacy NHS Lothian. Ruaridh Buchan, Jacqueline Waters. Edinburgh Clinical Research Facility: Emily Evans. NHS Tayside: Principal Investigator - Dr Clare Webster; Research Nurses - Anne Macleod, Susan Macfarlane, Debbie Rice. NHS Lanarkshire: Principal Investigator - Dr Carol Dryden; Research Nurses - Karen Leitch, Berni Welsh, Angela Brown, Jackie Quigley NHS Ayrshire: Principal Investigator - Dr Tim Adams; Research Nurse - Claire Bell.
Contributors: SR: Planned the study and wrote the protocol and the manuscript. CG: Planned the study, revised protocol and approved the final manuscript. KO, PR: Revised protocol, obtained approvals and approved the final manuscript. ASt: Revised protocol and approved the final manuscript. ASh and SC: Planned the study and revised protocol and approved the final manuscript.
Funding: This work was supported by the Chief Scientist Office, grant number TCS/17/12. Sea Salt was provided by Cornish Sea Salt Company. Swabs and transport medium were supplied by Copan, Italy. Both Cornish Sea Salt Company and Copan, Italy had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results. ASh is supported by HDR UK and BREATHE—The Health Data Research Hub for Respiratory Health (MC_PC_19004). BREATHE is funded through the UK Research and Innovation Industrial Strategy Challenge Fund.
Disclaimer: The funder had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
ELVIS Kids Trial Investigators:
Brittney Abernathy, Julie Baggot, Ruaridh Buchan, Gillian Black, Louise Frampton, Naomi Matos, Elinore McGivern, Rachel McKernan, Debbie Miller, Margaret Millar, Sheila Mortimer, Finny Paterson, Maxine Ramsay, Joan Thomson, Emma Ward, and Jacqueline Waters
References
- 1. Toivonen L, Schuez-Havupalo L, Karppinen S, et al. Rhinovirus infections in the first 2 years of life. Pediatrics 2016;138:e20161309. 10.1542/peds.2016-1309 [DOI] [PubMed] [Google Scholar]
- 2. Tarafder MR, Carabin H, Gyorkos TW, et al. Diarrhea and colds in child day care centers: impact of various numerator and denominator definitions of illness episodes. Epidemiology 2009;20:796–9. 10.1097/EDE.0b013e3181ba468e [DOI] [PubMed] [Google Scholar]
- 3. Grüber C, Keil T, Kulig M, et al. History of respiratory infections in the first 12 yr among children from a birth cohort. Pediatr Allergy Immunol 2008;19:505–12. 10.1111/j.1399-3038.2007.00688.x [DOI] [PubMed] [Google Scholar]
- 4. Ramalingam S, Graham C, Dove J, et al. A pilot, open labelled, randomised controlled trial of hypertonic saline nasal irrigation and gargling for the common cold. Sci Rep 2019;9:1015. 10.1038/s41598-018-37703-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scotland I. How often do people in Scotland consult their GP practice and why? Reasons for consulting the GP practice: top 10 most common conditions seen, 2016. http://www.isdscotland.org/Health-Topics/General-Practice/GP-Consultations/ [Accessed 08 Feb 2016].
- 6. Scotland I. Annual acute hospital activity and NHS beds information in Scotland - year ending 31 March 2016. Available: https://www.isdscotland.org/Health-Topics/Hospital-Care/Publications/data-tables.asp?id=8582016
- 7. Schuez-Havupalo L, Karppinen S, Toivonen L, et al. Association between infant swimming and rhinovirus-induced wheezing. Acta Paediatr 2014;103:1153–8. 10.1111/apa.12736 [DOI] [PubMed] [Google Scholar]
- 8. Green CA, Yeates D, Goldacre A, et al. Admission to hospital for bronchiolitis in England: trends over five decades, geographical variation and association with perinatal characteristics and subsequent asthma. Arch Dis Child 2016;101:140–6. 10.1136/archdischild-2015-308723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. le Roux DM, Myer L, Nicol MP, et al. Incidence and severity of childhood pneumonia in the first year of life in a South African birth cohort: the Drakenstein child health study. Lancet Glob Health 2015;3:e95–103. 10.1016/S2214-109X(14)70360-2 [DOI] [PubMed] [Google Scholar]
- 10. Centre for Disease Control . Common Cold and Runny Nose, 2016. Available: https://www.cdc.gov/getsmart/community/for-patients/common-illnesses/colds.html [Accessed 25 Dec 2016].
- 11. Ramalingam S, Cai B, Wong J, et al. Antiviral innate immune response in non-myeloid cells is augmented by chloride ions via an increase in intracellular hypochlorous acid levels. Sci Rep 2018;8:13630. 10.1038/s41598-018-31936-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim HJ, Lee J-G, Kang JW, et al. Effects of a low concentration hypochlorous acid nasal irrigation solution on bacteria, fungi, and virus. Laryngoscope 2008;118:1862–7. 10.1097/MLG.0b013e31817f4d34 [DOI] [PubMed] [Google Scholar]
- 13. Yu MS, Park HW, Kwon HJ, et al. The effect of a low concentration of hypochlorous acid on rhinovirus infection of nasal epithelial cells. Am J Rhinol Allergy 2011;25:40–4. 10.2500/ajra.2011.25.3545 [DOI] [PubMed] [Google Scholar]
- 14. Rutala WA, Weber DJ. Uses of inorganic hypochlorite (bleach) in health-care facilities. Clin Microbiol Rev 1997;10:597–610. 10.1128/CMR.10.4.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jordan WE, Jones DV, Klein M. Antiviral effectiveness of chlorine bleach in household laundry use. Am J Dis Child 1969;117:313–6. 10.1001/archpedi.1969.02100030315010 [DOI] [PubMed] [Google Scholar]
- 16. Castelão C, da Silva AP, Matos A, et al. Association of myeloperoxidase polymorphism (G463A) with cervix cancer. Mol Cell Biochem 2015;404:1–4. 10.1007/s11010-015-2359-5 [DOI] [PubMed] [Google Scholar]
- 17. Winterbourn CC, Kettle AJ. Redox reactions and microbial killing in the neutrophil phagosome. Antioxid Redox Signal 2013;18:642–60. 10.1089/ars.2012.4827 [DOI] [PubMed] [Google Scholar]
- 18. Chen X, Lee K-A, Ha E-M, et al. A specific and sensitive method for detection of hypochlorous acid for the imaging of microbe-induced HOCl production. Chem Commun 2011;47:4373–5. 10.1039/c1cc10589b [DOI] [PubMed] [Google Scholar]
- 19. Jantsch J, Schatz V, Friedrich D, et al. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab 2015;21:493–501. 10.1016/j.cmet.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Minton K. Antibacterial immunity: a pinch of salt. Nat Rev Immunol 2015;15:202. 10.1038/nri3835 [DOI] [PubMed] [Google Scholar]
- 21. Zhang L, Mendoza-Sassi RA, Wainwright C. Nebulised hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database Syst Rev 2013;7:CD006458. [DOI] [PubMed] [Google Scholar]
- 22. Zhang L, Mendoza-Sassi RA, Klassen TP, et al. Nebulized hypertonic saline for acute bronchiolitis: a systematic review. Pediatrics 2015;136:687–701. 10.1542/peds.2015-1914 [DOI] [PubMed] [Google Scholar]
- 23. Silver AH, Esteban-Cruciani N, Azzarone G, et al. 3% Hypertonic Saline Versus Normal Saline in Inpatient Bronchiolitis: A Randomized Controlled Trial. Pediatrics 2015;136:1036–43. 10.1542/peds.2015-1037 [DOI] [PubMed] [Google Scholar]
- 24. Everard ML, Hind D, Ugonna K, et al. SABRE: a multicentre randomised control trial of nebulised hypertonic saline in infants hospitalised with acute bronchiolitis. Thorax 2014;69:1105–12. 10.1136/thoraxjnl-2014-205953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cunningham S, Unger SA. Nebulised hypertonic saline in bronchiolitis: take it with a pinch of salt. Thorax 2014;69:1065–6. 10.1136/thoraxjnl-2014-206210 [DOI] [PubMed] [Google Scholar]
- 26. Grewal S, Klassen TP. The tale of 2 trials: disentangling contradictory evidence on hypertonic saline for acute bronchiolitis. JAMA Pediatr 2014;168:607–9. 10.1001/jamapediatrics.2014.423 [DOI] [PubMed] [Google Scholar]
- 27. Baron J, El-Chaar G. Hypertonic saline for the treatment of bronchiolitis in infants and young children: a critical review of the literature. J Pediatr Pharmacol Ther 2016;21:7–26. 10.5863/1551-6776-21.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Badgett RG, Vindhyal M, Stirnaman JT, et al. A living systematic review of nebulized hypertonic saline for acute bronchiolitis in infants. JAMA Pediatr 2015;169:788–9. 10.1001/jamapediatrics.2015.0681 [DOI] [PubMed] [Google Scholar]
- 29. Slapak I, Skoupá J, Strnad P, et al. Efficacy of isotonic nasal wash (seawater) in the treatment and prevention of rhinitis in children. Arch Otolaryngol Head Neck Surg 2008;134:67–74. 10.1001/archoto.2007.19 [DOI] [PubMed] [Google Scholar]
- 30. Köksal T, Çizmeci MN, Bozkaya D, et al. Comparison between the use of saline and seawater for nasal obstruction in children under 2 years of age with acute upper respiratory infection. Turk J Med Sci 2016;46:1004–13. 10.3906/sag-1507-18 [DOI] [PubMed] [Google Scholar]
- 31. Kassel JC, King D, Spurling GK. Saline nasal irrigation for acute upper respiratory tract infections. Cochrane Database Syst Rev 2010;3:CD006821. 10.1002/14651858.CD006821.pub2 [DOI] [PubMed] [Google Scholar]
- 32. Jacobs B, Young NL, Dick PT, et al. Canadian acute respiratory illness and flu scale (CARIFS): development of a valid measure for childhood respiratory infections. J Clin Epidemiol 2000;53:793–9. 10.1016/s0895-4356(99)00238-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-049964supp001.pdf (1MB, pdf)