Virus entry and cell-cell fusion mediated by HSV require four essential glycoproteins, gD, gH/gL, gB, and a gD receptor. Virus-neutralizing antibodies directed against any of these proteins bind to residues within key functional sites and interfere with essential steps in the fusion pathway.
KEYWORDS: cell-cell fusion, herpes simplex virus, monoclonal antibodies, neutralization, protein-protein interactions
ABSTRACT
Herpes simplex virus (HSV) entry and cell-cell fusion require glycoproteins gD, gH/gL, and gB. HSV entry begins with gD binding its receptor (nectin-1), which then activates gH/gL to enable the conversion of prefusion gB to its active form to promote membrane fusion. Virus-neutralizing monoclonal antibodies (Mabs) interfere with one or more of these steps, and the localization of their epitopes identifies functional sites on each protein. Utilizing this approach, we have identified the gH/gL binding face on gD and the corresponding gD binding site on gH/gL. Here, we used combinations of these Mabs to define the orientations of gD and gH/gL relative to each other. We reasoned that if two Mabs, one directed at gD and the other at gH/gL, block fusion more effectively than when either Mab was used alone (additive), then their epitopes would be spatially distanced, and the binding of one would not directly interfere with the binding of the other during fusion. However, if the two Mabs blocked fusion with an efficacy equal to or lesser than that when either Mab was used alone (indifferent), we propose that their epitopes would be in close proximity in the complex. Using a live-cell fusion assay, we found that some Mab pairings blocked the fusion with different mechanisms, while others had similar mechanisms of action. Grouping the different combinations of antibodies into indifferent and additive groups, we present a model for the orientation of gD vis-à-vis gH/gL in the complex.
IMPORTANCE Virus entry and cell-cell fusion mediated by HSV require four essential glycoproteins, gD, gH/gL, gB, and a cellular gD receptor. Virus-neutralizing antibodies directed against any of these proteins bind to residues within key functional sites and interfere with essential steps in the fusion pathway. Thus, the epitopes of these Mabs overlap and point to critical, functional sites on their target proteins. Here, we combined gD and gH/gL antibodies to determine whether they work in an additive or a nonadditive (indifferent) fashion to block specific events in glycoprotein-driven cell-cell fusion. Identifying combinations of antibodies that have additive effects will help in the rational design of an effective therapeutic “polyclonal antibody” to treat HSV disease. In addition, identification of the exact contact regions between gD and gH/gL can inform the design of small molecules that would interfere with gD-gH/gL complex formation, thus preventing the virus from entering the host cell.
INTRODUCTION
The entry of herpes simplex virus (HSV) into cells, either by fusion at the plasma membrane or by endocytosis, involves four essential glycoproteins (gB, gD, and gH/gL) and a cellular receptor (either nectin-1 or herpes virus entry mediator [HVEM]) (reviewed in references 1–4). This process can be disrupted by antibodies that target any of the specific proteins that are important at different stages of the virus entry process. It is generally accepted that neutralizing and virus-to-cell spread-blocking antibodies bind epitopes in their target protein at or near a functional site, thereby interfering with essential steps in the virus life cycle as well as in the cell-cell fusion pathway. Understanding the mechanism by which a neutralizing antibody inhibits a specific step of virus entry, especially at the initial stages, is of high interest in vaccine development, immunoglobulin therapies, and antiviral drug design.
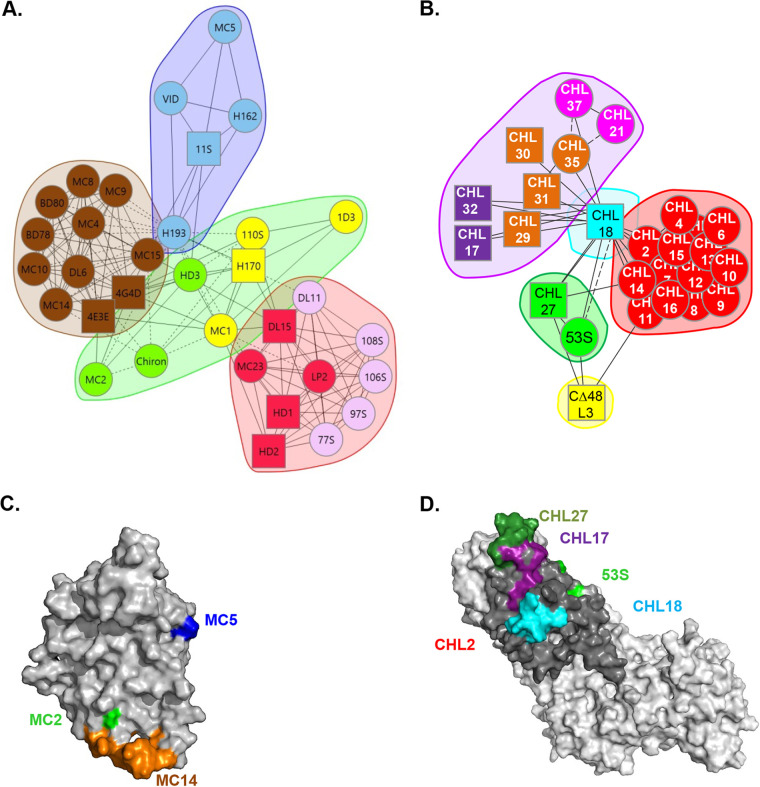
The overall HSV fusion model shows that receptor binding by gD opens an interaction domain on gD that can now bind to the regulator complex gH/gL, which in turn interacts with and activates the fusion protein gB. Cairns et al. provided a deeper insight into the HSV fusion apparatus by localizing the gH/gL binding site on gD (5) and, more recently, the gD binding site on gH/gL (6). Using competition analysis, we have organized our extensive collection of monoclonal antibodies (Mabs) against gD and gH/gL into “trees” (7, 8) and, more recently, into community maps (6, 9) (Fig. 1A and B). The gD neutralizing Mabs were initially thought to inhibit virus entry and cell fusion through two distinct mechanisms: (i) blocking the binding of virus to one of its receptors (Mabs in the red, pink, and yellow communities in Fig. 1A) or (ii) a post-receptor-binding step, which we presumed to be the interaction of gD with the fusion regulator gH/gL (green, blue, and brown) (9). With the recent identification of a direct interaction between gD and gH/gL (5), we have further separated the second group of Mabs into those that induce post-receptor-binding conformational changes of gD (green) and those that sterically block the gD-gH/gL interaction (blue, brown, and red). Furthermore, we used the known epitopic location of the MC5 (blue), MC14 (brown), and MC23 (red) Mabs that block the gD-gH/gL interaction to define a putative gH/gL binding site on gD (Fig. 1C) (5).
FIG 1.
Antibody community maps and epitope location. (A and B) gD (A) and gH/gL (B) monoclonal antibodies were organized into communities based on competition, phenotype, and relationship with other antibodies. For each protein, the Mabs were divided into communities and colored accordingly. Antibody names in a circle indicate that competition was measured as both a ligand and an analyte; antibody names in a square indicate that competition was measured in one direction only, as either a ligand or an analyte. Solid connecting lines specify that competition between the two Mabs was seen as both a ligand and an analyte for each. Dashed connecting lines identify that the competition between Mabs was seen in one direction only. (C and D) Epitopes/virus escape residues of relevant Mabs used in this study shown on three-dimensional (3D) structures of gD (C) and gH/gL (D).
We have applied a similar strategy with the gH/gL antibody collection and organized them into competition communities and associated representatives with their ability to bock fusion. When screened for their ability to block the gD-gH/gL interaction, these gH/gL Mabs formed two functional groups: Mabs that blocked this interaction (cyan, green, and dark blue in Fig. 1B) and Mabs that did not but that surprisingly stabilized the interaction (red and purple) and in the process inhibited fusion. Using the known location of epitopes of the gH/gL Mabs that blocked the interaction, we have localized four antigenic sites on the gH/gL structure (6), three of which (represented by CHL17, CHL27, and CHL18) are assigned to flexible regions in the N terminus of gH and the C terminus of gL (Fig. 1D); the fourth epitope (recognized by 53S Mab) was recently localized by mutagenesis (6). Despite the identification of three gH/gL binding sites on gD and four gD binding sites on gH/gL, it is still unknown whether in a complex site 1 on gD contacts site 1, 2, or 3 on gH/gL.
The goal of this study was to determine the relative orientations of gD and gH/gL vis-à-vis each other in the complex by using pairs of gD and gH/gL Mabs that are known to block the interaction and determining their consequent effect on fusion using a modified kinetic split luciferase assay (SLA). The expectation was that in a preformed glycoprotein-antibody complex, Mabs that bound epitopic sites that were physically close on the two interacting proteins would inhibit fusion through a similar mechanism, while those that were spatially separated would have different mechanisms in blocking fusion or affect different parts of an overall mechanism. Because the gD and gH/gL Mabs used did no compete for binding to their respective proteins, the contribution of the two Mabs to the inhibition of fusion was evaluated using the Bliss independence model (10–13). This model assumes that two inhibitors of function (here, antibodies) have independent binding sites and independent mechanisms of action and act in such a manner that neither of them interferes with each other but both contribute to the overall inhibitory event (10).
Using homologous and heterologous gD and gH/gL Mab pairs, we found two situations. In the first case, combinations (gD-gD, gH/gL-gH/gL, or gD-gH/gL Mabs) were more effective in blocking fusion (additive effect), suggesting that the two Mabs blocked fusion through independent mechanisms. In the second case, inhibition was similar whether or not the antibodies were used alone or in combinations (indifferent effect), suggesting that the two Mabs had similar mechanisms in the fusion-blocking process.
We show that Mabs that target different regions of the complex have an additive effect, while indifferent combinations target sites that are closer to each other. Using the additive/indifferent relationship between different sets of gD and gH/gL Mab heterologous combinations, we present a model for the orientation of the gD molecule in relation to gH/gL.
RESULTS
Considerations in application of the Bliss independence model.
According to the Bliss independence model, the effect of “inhibitor” combinations is expected to fit into one of four possible outcomes: additive, synergistic, indifferent, and antagonistic. For our purposes, we simplified this classification into two categories: (i) “indifferent” (Fig. 2C) for combinations that are only as effective as when one of the Mabs is used alone (the location and overall mechanisms of action are the same) and (ii) “additive” for combinations where each antibody binds to a distinct functional site and acts independently of the other and both contribute to the final effect. In this case, the effect of the combination closely resembles the calculated theoretical curve (Fig. 2D).
FIG 2.
Expected outcomes when two antibodies targeting two proteins are used to simultaneously inhibit cell-cell fusion. (A and B) gD (cyan) and gH/gL (pink) Mabs known to block the physical interaction between gD and gH/gL were used to inhibit fusion. These Mabs could recognize epitopes that are spatially close (A) or separated (B) in the complex. (B and C) A Bliss independence model was used to evaluate the effect of combinations of Mabs. A theoretical additive curve was calculated based on the ability of each Mab to inhibit fusion. (C) If the measured combined effect was worse than the theoretical curve, the combination was deemed indifferent, and the two Mabs were considered to have similar mechanisms of inhibition. (D) If the measured combined effect of the two Mabs was similar to the calculated additive curve, the combination was considered additive, and the two Mabs were considered to have different mechanisms of action.
Our organization of antibodies into community groups was based in part on their ability to compete for binding to identical or overlapping epitopes on the same protein (either gD or gH/gL). Accordingly, if two antibodies recognized separate epitopes, both Mabs should bind independently of each other provided that the binding of one Mab does not change the presentation of the epitope for the second Mab. From a functional standpoint, two noncompeting inhibitory Mabs could simultaneously bind their target epitopes, resulting in an additive effect on fusion inhibition. However, if the two Mabs recognized the same epitope on a protein, then their competition for binding should result in an indifferent effect, and the combination of the two would inhibit fusion at levels similar to that of the more active Mab. A combination of two Mabs, one against gD and the other against gH/gL (heterologous combination), would have an additive effect on the inhibition of fusion (Fig. 2D) if the two Mabs inhibited through different mechanisms or bound different parts of the binding site (Fig. 2B). However, if the combination had an indifferent effect (Fig. 2C), then we would interpret that as an indication that the two Mabs bound sites that were close to each other (Fig. 2A).
Effect of competing and noncompeting gD Mabs on the kinetics of fusion.
We previously demonstrated that gD and gH/gL Mabs that blocked gD-gH/gL complex formation as assessed by surface plasmon resonance (SPR) also blocked cell-cell fusion (5, 6). We hypothesized that the epitopes of these Mabs are near or at functional sites and define the gD-gH/gL interface. To begin to characterize the location of this interaction, we used combinations of two Mabs to inhibit cell-cell fusion. To determine the effect of two Mabs, we modified our split luciferase assay (see Materials and Methods), in which target and effector cells express complementary, but inactive, halves of luciferase and fusion is measured by the restoration of luciferase activity. For gH/gL Mabs, effector cells (expressing gB and gH/gL) were preincubated with a gH/gL Mab for 1 h at 37°C. For gD Mabs, soluble gD2306 protein was preincubated with the indicated Mab for 1 h at 4°C. Fusion was triggered by the simultaneous addition of nectin-1 target cells and the gD-Mab complex to the effector cell/gH/gL Mab mix. Fusion was monitored for 2 h using a plate reader to assess luciferase activity. We chose soluble gD instead of full-length gD for two reasons: first, to allow for the formation of gD-Mab and gH/gL-Mab complexes before fusion is triggered, for a better understanding of the mechanism of action of the Mabs and the availability of epitopes during fusion, and, second, to partly match the SPR experiments (where both gD and gH/gL were in soluble forms) that provided the competition data for all the Mabs examined.
Before we determined the effect of combinations of Mabs, we first established the concentration at which each Mab would block ∼50% of fusion (50% inhibitory concentration [IC50]). The IC50 varied between 0.3 and 2.5 μg/ml (Fig. 3). Each Mab was then used to block fusion at the IC50, either alone (colored curves in Fig. 4 to 7) or in combinations (black curves). Second, based on the inhibitory activity of each Mab at the IC50, we calculated the theoretical curve (gray curves in Fig. 4 to 7) using the Bliss model. This calculation assumes that both Mabs block in an additive way. Third, we calculated the excess over Bliss (eob) coefficient, based on the difference between the measured and the calculated inhibition activities. A close overlap between the measured and the theoretical curves (Fig. 2D) resulting in a null or positive eob would indicate that the two Mabs in the combination had an “additive effect” due to independent mechanisms of action in fusion. However, a negative eob value and a divergence of the two curves (with the measured black curve resembling the inhibitory effect of the more active Mab) would indicate that the combination was indifferent (Fig. 2C) and that the two Mabs worked through the same mechanism, i.e., inhibited the same step in fusion.
FIG 3.
Mab titration. To determine the IC50, serial 2-fold dilutions of each antibody were used to block fusion in the split luciferase assay. The effect of each concentration was evaluated over a 2-h time course. The luciferase readings of the “no-antibody” sample at 2 h were set at 100%.
FIG 4.
Effect of combinations of anti-gD antibodies. (A) Schematic representation of the gD crystal structure (in gray) and the location of epitopes of Mabs used (colored regions). (B to E) Competing (B) and noncompeting (C to E) gD antibodies were evaluated for their inhibitory activity in a fusion assay. Curves from a representative experiment (three independent experiments, each done in duplicate) are shown. The combined effect of two antibodies (black curves) was evaluated over a 2-h time course. A theoretical, additive curve (gray) was calculated based on the ability of each Mab (colored curves) to block ∼50% of fusion. (B and C) Combinations of competing Mabs like MC5 and H162 have an indifferent (less-than-additive) inhibitory effect (B), while noncompeting Mabs MC2 and MC14 have an additive effect (C). (D and E) An indifferent effect was also seen for noncompeting Mabs MC2 and MC5 (D) or Mabs that block the same step in the fusion process, like MC5 and MC14 (E). (F) Excess over Bliss (eob) values were calculated (on a scale of 1) as the difference between the measured and the calculated inhibitory activities of each pair of Mabs. Combinations of antibodies with a positive eob are considered to have an additive effect on fusion. A negative eob indicates pairs that are indifferent. Averages from three independent experiments, each done in duplicate, are shown. (G) Diagram of antibody relationships.
FIG 5.
Effect of gH/gL combination antibodies. (A) Schematic representation of the gH/gL 3D structure (in gray). The location of binding sites for the antibodies used is shown in color. (B to H) Competing (B) and noncompeting (C to H) gH/gL antibodies were evaluated for their blocking activity. Curves from a representative experiment (three independent experiments, each done in duplicate) are shown. The combined effect of two antibodies (black curves) was evaluated over a 2-h time course. A theoretical, additive curve (gray) was calculated based on the ability of each Mab (colored curves) to inhibit fusion. (B) Combinations of competing Mabs like CHL2 and CHL4 have an indifferent effect. (C) Conformational changes allow competing Mabs CHL27 and 53S to bind and inhibit fusion in an additive fashion. (D to H) Other noncompeting Mab pairs, CHL17-53S (D), CHL18-53S (E), CHL17-CHL27 (F), CHL18-CHL27 (G), and CHL17-CHL18 (H), also have an additive effect. (I) Excess over Bliss (eob) was calculated (on a scale of 1) as the difference between the measured and the calculated inhibitory activities of each pair of Mabs. Averages from three independent experiments, each done in duplicate, are shown. (J) Diagram of antibody relationships.
FIG 6.
Effect of heterologous gD and gH/gL Mab combinations. (A) A gD Mab that does not block the gD-gH/gL interaction (MC2) has an additive effect when combined with any of the gH/gL Mabs that block complex formation. (B) A similar relationship was found between a gH/gL Mab that does not block the interaction (CHL2) and the two gD blocking Mabs MC5 and MC14. Curves from a representative experiment (three independent experiments, each done in duplicate) are shown. (C) Excess over Bliss was calculated (on a scale of 1) as the difference between the measured and the calculated inhibitory activities of each pair of Mabs. Averages from three independent experiments, each done in duplicate, are shown. (D) Diagram of antibody relationships.
FIG 7.
Effect of heterologous blocking gD and gH/gL Mab combinations. (A) The effect of two antibodies (black curves) was evaluated over a 2-h time course. A theoretical, additive curve (gray) was calculated based on the ability of MC5 and CHL17 or CHL18 to block ∼50% of fusion. MC5-CHL17 and MC5-CHL18 are additive. MC5-CHL27 and MC5-53S are less than additive. (B) Effect of MC14 combined with CHL17, CHL18, CHL27, or 53S. Unlike MC5, MC14 combined with CHL18 is less than additive, while MC14-CHL27 and MC14-53S are additive. (C) Excess over Bliss was calculated (on a scale of 1) as the difference between the measured and the calculated inhibitory activities of each pair of Mabs. (D) Diagram of antibody relationships.
As a proof of principle for this approach, we first chose two pairs of gD Mabs: in one pair, the two Mabs (MC5 and H162) compete for binding to an epitope (14), and in the second pair, the Mabs (MC2 and MC14) do not compete and synergize in a virus neutralization assay (8).
(i) Effect of combination of competing gD Mabs.
MC5 and H162 are part of the same competition community (9) (Fig. 1A). Although their epitopes share essential residues (Table 1) (14), they have distinct neutralization profiles: H162 neutralizes HSV-1 only, while MC5 neutralizes both HSV-1 and HSV-2. As expected from the Bliss model, which predicts that two Mabs that compete for binding will exhibit an indifferent fusion-blocking ability (Fig. 2D), combining the two competing Mabs MC5 and H162 (black curve in Fig. 4B) resulted in an indifferent effect; the inhibition was as efficient as that of the more active Mab (H162 [in light blue]).
TABLE 1.
Properties of monoclonal antibodies used in this studyg
| Mab | Epitope/virus escape mutation position(s) | Type specificity (binding)h | Virus neutralization | Virus spread |
|---|---|---|---|---|
| gD | ||||
| MC2 | 67a | T2 | Yes | NDg |
| MC5 | 54a | TC | Yes | ND |
| MC14 | 262–272b | TC | No | +f |
| gH/gL | ||||
| CHL2 | gH 116c | T2 | No | +c |
| CHL17 | gH 19–38c | T2 | Yes | +c |
| CHL27 | gH 37–47d | TC | Yes | ND |
| CHL18 | gL 209–218c | T2 | No | +c |
| 53S | gL 48,e 77d | TC | Yes | ND |
(ii) Effect of combination of noncompeting gD Mabs.
MC2 and MC14 have distinct epitopes (Fig. 1A) and biological functions: MC2 is HSV-2 specific, and MC14 is a type-common Mab that does not neutralize but inhibits virus spread and cell-cell fusion (8, 14, 15). This pair of Mabs has a synergistic effect on virus neutralization (8). As expected, we found that the combination of MC2-MC14 (black curve in Fig. 4C) inhibited fusion significantly more efficiently than either of the single Mabs (green and brown curves). These results show that inhibitory, noncompeting Mabs that work through independent mechanisms satisfy the additive prediction of the Bliss model.
Next, we focused on the effect of gD Mab combinations from different communities. First, we tested the combination of MC5 and MC14, which have nonoverlapping epitopes (8, 14). However, because each Mab blocks the physical interaction between gD and gH/gL (5), we would anticipate that the pair would have an indifferent effect on fusion inhibition. Indeed, the MC5-MC14 (black) combination was only as efficient as either MC5 (blue) or MC14 (orange) alone (Fig. 4D). We conclude that MC5 and MC14 block fusion through the same mechanism.
Next, we assessed the combination of MC2 and MC5, both of which block a post-receptor-binding function (8) and have distinct, nonoverlapping epitopes (14). While MC5 blocks gD-gH/gL binding, MC2 does not (5). Contrary to expectation, we found that the pair was indifferent when inhibiting fusion (Fig. 4E), suggesting an overall similarity of the fusion inhibition mechanisms.
We conclude that selected combinations of gD Mabs affect the kinetics of fusion in different ways and that this is dependent on the known properties of each Mab. Fitting with our predictions, combinations of noncompeting Mabs (MC2-MC14) have an additive effect, while competing antibodies MC5 and H162 are indifferent. Interestingly, noncompeting Mabs MC2 and MC5 have an indifferent effect, suggesting that their individual inhibitory effects utilize the same mechanism. A summary diagram of all gD Mab combinations is shown in Fig. 4G.
Since the experimental data fit the theoretical model, we utilized it to study the effect of gH/gL Mabs and the combination of gD and gH/gL Mabs on the inhibition of fusion.
Effect of homologous gH/gL Mab pairs that block gD binding.
We next applied a similar approach to determine the effect of combinations of gH/gL Mabs on fusion inhibition, particularly those that physically block binding to gD (6). A summary diagram of all gH/gL combinations is shown in Fig. 5J.
As a control pair, we used CHL2 and CHL4. These Mabs compete for binding, share residue gH 116 as essential for binding (Table 1) (7), and fail to block the physical binding of gD to gH/gL (6, 7). As expected for a pair of competing Mabs, the effect of the combination of the two on fusion inhibition was indifferent (Fig. 5B).
We hypothesized that the gH/gL Mabs that blocked gD-gH/gL complex formation (CHL17, CHL18, CHL27, and 53S) would have a similar mechanism of action. However, the distinct location of each epitope on gH/gL (Fig. 1D and Fig. 5A) suggested that in some pairs, the Mabs might block independently of each other. Strikingly, unlike CHL2-CHL4, all of the other pairs of gH/gL Mabs had an additive effect on fusion inhibition (Fig. 5C to H), as evidenced by positive eob values (Fig. 5I). Below is a description of all the combinations. A summary diagram is presented in Fig. 5J.
(i) CHL17 and CHL27.
Although both CHL17 and CHL27 Mabs bind to the N terminus, they have distinct, consecutive, nonoverlapping linear epitopes (gH 19–38 and gH 37–47, respectively) (6, 7) located in a highly disordered region in gH/gL (16). Despite their close epitopic location, the CHL17-CHL27 combination had an additive effect on fusion inhibition (Fig. 5C). This suggested that the flexible gH N terminus is physically split into two separate functional regions, each making a distinct contribution to the interaction with gD.
(ii) CHL17 and CHL18.
The additive effect of CHL17-CHL18 (Fig. 5D) and CHL18-CHL27 (Fig. 5E) shows that there was no competition for binding or function between CHL18 and either CHL17 or CHL27. This confirmed our previous hypothesis that both the gH N terminus (where CHL17 and CHL27 map) and the gL C terminus (CHL18 epitope) are distinct, flexible functional regions that do not interfere with each other during fusion/interaction with gD (6, 17).
(iii) CHL17 and 53S.
The additive relationship between 53S and either CHL17 (Fig. 5F) or CHL18 (Fig. 5G) identifies a fourth, independent functional site, represented by the epitope of 53S (which includes residues gL 48 and gL 77 [6]), which is distinct from the gH N-terminal (CHL17) or gL C-terminal (CHL18) epitopes.
(iv) CHL27 and 53S.
The effect of the CHL27-53S pair (Fig. 5H) was of particular interest as it allowed us to clarify the special relationship between these two Mabs. Previous biosensor studies showed that CHL27 and 53S, whether as IgG or Fab, compete for binding to the surface of gH/gL and were placed in the same antigenic community (Fig. 1B) since both Mabs blocked the physical interaction between gD and gH/gL (6). However, mapping data showed that these Mabs actually had distinct binding properties. CHL27 recognizes a linear epitope (gH 37–47) within the flexible N terminus of gH (Table 1) (6). In contrast, 53S targets a conformational epitope that requires both gH and gL (18–22) and includes gL residues P48 (21) and P77 (6). Figure 5H shows that the combination of CHL27 and 53S was very efficient and that the effect was additive, consistent with the mapping data.
We conclude that despite their shared ability to block the gD-gH/gL interaction, CHL17, CHL18, CHL27, and 53S Mabs target different regions of the complex and act in an additive manner. The epitopes recognized by these Mabs form four distinct functional regions, each with distinct contributions to gD-gH/gL complex formation.
Effect of heterologous Mab combinations that prevent gD-gH/gL complex formation.
Here, we used heterologous combinations of gD and gH/gL Mabs to determine if they would affect fusion in an additive or indifferent manner. We predicted that the pairs of antibodies that block either side of a direct gD-gH/gL interface (Fig. 2A) would inhibit fusion by the same mechanism and thus would not be expected to give additive inhibition under the Bliss model. In contrast, pairs of Mabs that block at different sites (Fig. 2B) will have an additive effect.
First, we analyzed the effects of two control Mabs, MC2 (anti-gD) and CHL2 (anti-gH/gL): MC2 was combined with gH/gL blocking Mabs, and CHL2 was combined with gD blocking Mabs.
(i) Combination of MC2 (nonblocking gD Mab) with gH/gL Mabs that block gD-gH/gL complex formation.
MC2 inhibits cell-cell fusion but does not block the gD-gH/gL interaction (5). As expected, the combination of MC2 with any of the gH/gL Mabs that block the physical interaction (CHl17, CHL18, CHL27, or 53S) had an additive effect and showed positive eob values (Fig. 6A), inhibiting fusion more efficiently than either of the Mabs alone (compare black and colored curves).
(ii) Combination of CHL2 (nonblocking gH/gL Mab) with gD Mabs that block gD-gH/gL complex formation.
CHL2 fails to block the physical binding of gD to gH/gL (6), and similar to MC2, CHL2 had an additive effect when combined with either MC5 or MC14 (Fig. 6B). We interpret these data to indicate that combinations of noncompeting gD and gH/gL Mabs that have different mechanisms of action have an additive effect on fusion (Fig. 6C).
(iii) Combination of MC5 (anti-gD Mab blocking gH/gL binding) with anti-gH/gL Mabs that block the interaction with gD.
Figure 7A shows the effect of MC5 when combined with the gH/gL Mabs CHL17, CHL18, CHL27, and 53S. We found that the MC5-CHL17 combination was additive, indicating that both Mabs bound to their respective protein and inhibited fusion independently of each other. Interestingly, MC5 combined with CHL27, whose binding site lies on the structure just after the CHL17 epitope (6, 7) (Fig. 1D), had an indifferent effect. Similar indifferent inhibition was observed when MC5 was combined with 53S, suggesting that the mechanisms of inhibition by MC5 with either CHL27 or 53S are somehow similar. In contrast, the MC5-CHL18 pair inhibited fusion in an additive fashion, supporting a different mechanism of action for this antibody pair.
(iv) Combination of MC14 (anti-gD Mab blocking binding to gH/gL) with anti-gH/gL Mabs blocking gD binding.
Similar to MC5, MC14 had an additive effect on fusion when combined with CHL17 (Fig. 7B). However, the MC14-CHL18 combination was indifferent, while MC14 with either CHL27 or 53S was additive. This suggested that MC14 and CHL18 had similar blocking mechanisms and were likely quite close spatially. However, MC14 and either CHL27 or 53S worked independently, placing the epitopes further apart.
We conclude that although the Mabs used in this study can block the gD-gH/gL interaction, some of them do so independently of each other, while others block the same way. Using the split luciferase assay as a readout, we used the mutual ability of the two Mabs to either dampen or increase their individual effects on fusion to postulate their relative position to each other on a model (Fig. 8). Due to their indifferent effect, the binding sites of CHL27 (Fig. 8, dark green) and 53S (light green) on gH/gL were positioned close to the MC5 epitope (blue) on gD at one end of the complex. At the other end, CHL18 (cyan) was placed near MC14 (orange) to account for their indifferent effects on fusion inhibition.
FIG 8.
Model for the gD-gH/gL interface. (A and B) Two possible models for the positioning of gD and gH/gL relative to each other. Diagrams show the epitopes of Mabs that block the gD-gH/gL interaction. The epitopes of CHL17, CHL27, 53S, and CHL18 (colored triangles) define the gD binding site on gH/gL (left). The reciprocal gH/gL binding site on gD is defined by MC5, MC14, and MC23 (middle). In a complex, the CHL27/53S region in gH/gL can contact gD at the epitope for MC5 (A) or MC14 (B). (C) 3D model of the gD-gH/gL complex with putative transmembrane and cytoplasmic tails. The positioning of the two proteins is based on the ability of pairs of antibodies to inhibit fusion in an additive (CHL27-MC14, 53S-MC14, and CHL18-MC5) fashion. The regions that contain the epitopes for CHL17 (purple) and CHL27 (green) in the N terminus and CHL18 (cyan) in the gL C terminus were artificially modeled using the structure under PDB accession number 3MC1 as a starting point. For gD, the C terminus of gD (PDB accession number 2C36) was modeled onto gD285 (PDB accession number 1L2G) to mimic the post-receptor-binding conformation. gH/gL is shown in an open state to allow the access of gD to the 53S epitope (light green) on gH/gL. The extended conformation of gD accounts for the ability of gH/gL Mabs from the magenta group to stabilize the complex and inhibit fusion by tethering gD onto gH/gL.
DISCUSSION
In this study, we have analyzed the mechanism of interaction between gD and gH/gL using a modified split luciferase assay to reveal how fusion is impacted by homologous or heterologous combinations of Mabs with known epitopes and blocking function. While several research studies focused on the impact of combinations of broadly neutralizing antibodies, such as antibodies against HIV (23–25), chikungunya virus (26), rabies virus (27), hepatitis C virus (HCV) (28), or herpesviruses (8, 29–31), with the aim of using combinations in passive immunization for treatment of viral infections, the choice of antibodies was based on their overall neutralizing capability and not on an understanding of their mechanisms of action. Here, we have used anti-gD and anti-gH/gL Mabs to identify pairs of antibodies that have an additive effect, thus enabling the identification of antibody pairs that are more likely to work together effectively in future combination therapies.
Each of the antibodies used in our studies was capable of physically interfering with the formation of the gD-gH/gL complex by SPR and also impacted either virus entry, virus spread, or cell-cell fusion inhibition (7, 8, 14, 15, 17). At the center of our approach was the concept of “competition/no competition.” If two noncompeting antibodies could simultaneously bind their targets in a functional assay, each Mab should contribute to the final inhibitory activity in an additive way. Conversely, a combination of Mabs that compete for binding to the same epitope would not be more effective than either of the Mabs alone.
The innovative aspect of this study was the use of heterologous pairs of Mabs that target gD and gH/gL. Of the 11 homologous combinations of gD and gH/gL Mabs tested, 7 were found to have an additive effect on cell fusion: MC2-MC14 for gD Mabs and CHL17-CHL27, CHL17-CHL18, CHL18-CHL27, CHL18-53S, CHL17-53S, and CHL27-53S for gH/gL Mabs. Many of these combinations confirmed our original hypothesis that competing antibodies (MC5-H162 for gD [Fig. 4B] or CHL2-CHL4 for gH/gL [Fig. 5B]) or antibodies that have similar mechanisms of action (MC5-MC14 [Fig. 4D]) will have an indifferent effect. The indifferent effect of MC2-MC5 was somehow unexpected as these Mabs do not compete for binding. When originally characterized, they were found to be the only gD Mabs that neutralized virus by blocking a post-receptor-binding step (8) and were hypothesized to block the gD-gH/gL interaction. However, only MC5 was found to physically block this interaction (6). This suggests that the binding of one Mab induces conformational changes that, although not preventing the binding of the second Mab, block or change its activity, making their relationship and their mechanism of action closer than anticipated.
The additive effect of all homologous gH/gL Mabs was surprising considering that all these Mabs were shown to block the gD-gH/gL interaction (6). This suggests that any of these pairs would be good candidates for passive immunizations, especially considering the lack of humoral responses to gH/gL in naturally infected people (32–34), despite the essential role that gH/gL plays in the virus life cycle and the antigenicity of the protein from either serotype (7, 35, 36). From a mechanistic point, we propose that although all these Mabs can block the gD-gH/gL interaction, they probably target different regions of the complex and act through different mechanisms or as different parts of an overall mechanism.
We found particularly interesting the indifferent relationships between specific pairs, which we believe may inform on the orientation of gD vis-à-vis gH/gL. In the formation of a complex, the gH/gL region defined by the CHL27 and 53S epitopes could contact gD at either the MC5 (Fig. 8A) or the MC14 (Fig. 8B) epitope. The epitopes of CHL27 and 53S are spatially close enough to form a unit at the N terminus of gH/gL, while CHL18 forms a separate unit at the C terminus of gL (Fig. 1D) (6). Both CHL27 and 53S have an additive effect on cell fusion when combined with MC14. CHL18 is additive with MC5. If we interpret that the additive effect is a consequence of the ability of both Mabs to bind different regions of the complex/block through different mechanisms and that both contribute to fusion inhibition, then the region defined by CHL27/53S epitopes on gH/gL should be spatially separated from the MC14 epitope on gD, and the CHL18 epitope on gH/gL should be separated from the region on gD defined by the MC5 epitope (Fig. 8A). When the two proteins are positioned like this, the CHL27/53S region on gH/gL is very close to the MC5 epitope on gD, and CHL18 on gH/gL is closer to MC14 (Fig. 8A), which explains their indifferent contribution to fusion inhibition. We propose that because these regions are physically close to each other, the binding of one of the Mabs is enough to block the interaction, and the effect is not enhanced by the addition of the second Mab (Fig. 7).
We propose that there are three contact regions between gD and gH/gL (Fig. 8C). The first contact region is defined by the epitopes of MC5 on gD (gD54 and gD 74–77) and of CHL27 (gH 37–47) and 53S (gL 48 and gL 77) on gH/gL. The second region of contact is defined by MC14 (gD 262–272) and CHL18 (gL 209–218). Because Mab CHL17 (gH 19–38) showed an additive inhibitory effect with both MC5 and MC14, the CHL17 epitope belongs to a third contact site on gH/gL. Interestingly, CHL17 and CHL27 have distinct mechanisms of action despite the adjacent location of their epitopes.
Remarkably, the three sites in gH/gL defined by CHL17, CHL27, and CHL18 epitopes are in flexible regions (17, 22, 37–41) at the gH N terminus and the gL C terminus and might have a role in the tethering of gD in the complex. Whether these flexible regions move in response to the receptor-activated gD or as a consequence of gD binding to gH/gL is yet unknown. It has been proposed that the displacement of the N terminus of gH followed by the C terminus of gL exposes a region in gH/gL that is important for the interaction with gD (17), suggesting that the epitopes of 53S on gH/gL and of MC5 on gD act as possible functional binding/docking sites (Fig. 8B). The movement of the gH N terminus is supported by the phenotype of two Mabs, CHL27 and 53S. Although these Mabs have distinct epitopes (6), they compete for binding to soluble gH/gL, suggesting that the protein is in a closed conformation that allows the binding of only one Mab at a time. In the context of cell surface-expressed full-length gH/gL, there is sufficient mobility to fully expose both sites (Fig. 5).
The proposed extended conformation of gD, with the C terminus lying over gH/gL (Fig. 8C), is based in part on the location of 52S Mab. 52S Mab is an HSV-1-specific gH/gL Mab with escape mutations at positions gH 536 and gH 537 (42) (magenta in Fig. 8) that was proposed to block fusion by affecting the interaction between gD and gH/gL (16). The proposed model is further supported by the crystal structure of Epstein-Barr virus (EBV) gp42, a functional homologue of HSV gD, in complex with EBV gH/gL (43). Electron microscopy and crystallography studies showed that the N-terminal domain of gp42 adopts an extended conformation that wraps around the external surface of three gH domains and establishes five distinct binding sites, tethering the C-terminal domain to the complex (43, 44). While the high-affinity interaction between gp42 and gH/gL and the multiple tethering sites are required for membrane fusion (43, 44), the interaction between HSV gD and gH/gL is less stable and has been detected only by biosensor studies using truncated proteins (5, 6). It is likely that the stability of the complex is affected by the number of tethering sites between gD and gH/gL, especially between the gD C terminus and gH/gL. In biosensor studies, the complex can be stabilized by specific gH/gL Mabs (6), and some of them (CHL37 and CHL21) are part of the same community as 52S, which was proposed to block gD binding to gH/gL (16).
Studies are under way to determine the effect of combinations of gD, gH/gL, and gB Mabs on viral infection in vitro, and we anticipate that Mabs that compete for the same epitope will have the same effect as that observed in the cell fusion assay. It would be interesting to determine the contribution of Mabs known to block virus spread only, as there is a possibility that some of them might improve the neutralizing activity of other Mabs, like MC14 does for MC2 (8). Cumulatively, assessing the effect of combinations of Mabs will provide essential insights into not only the mechanism driving viral fusion but also the development of therapeutic antibody cocktails for use in the treatment of herpes disease. With the development of various platforms for antibody production, there has been an increase in passive or active antibody transfer studies for the treatment or prevention of various viral infections (45, 46). Studies in mouse models have shown that passive immunization with HSV-neutralizing antibodies provided significant protection against disease and partial protection against death (15, 47–51). Thus, our findings will help in identifying which Mabs will be more likely to protect when provided as a therapeutic cocktail for passive or active immunization.
MATERIALS AND METHODS
Cells and soluble protein.
Mouse melanoma cells (B78H1) were grown in 5% fetal bovine serum (FBS)–Dulbecco’s modified Eagle’s medium (DMEM). For B78H1 cells expressing nectin-1 (designated C10), the medium was supplemented with 500 μg/ml G418 (52). Soluble gD protein from HSV-2 was purified from baculovirus-infected insect cells (Sf9) as previously described (53).
Plasmids.
Split luciferase Rluc8(1–7) and Rluc8(8–11) and the wild-type (wt) glycoprotein constructs pTC580 (gB2), pTC510 (gH2), and pTC579 (gL2) have all been described previously (37, 41, 54, 55).
Antibodies.
gD Mabs in the MC series as well as gH/gL Mab 53S and the CHL series were previously described (6–8).
Split luciferase assay.
The split luciferase assay (SLA) was described elsewhere (41, 54, 56). Briefly, 5 × 104 B78 cells (effector cells) were seeded on white, cell culture-treated 96-well plates. A total of 4 × 105 B78-C10 cells (target cells) were seeded on 6-well plates. Transfection was performed the following day. A master mix containing 125 ng each of the gB, gH, gL, and Rluc8(1–7) plasmids was split over three wells of effector cells. Target cells were transfected with 1 μg of Rluc8(8–11) plasmid per well. At 24 h posttransfection, effector cells were preincubated for 1 h at 37°C with EnduRen substrate (Promega) diluted 1:1,000 in fusion medium (DMEM without phenol red supplemented with 50 mM HEPES and 5% FBS). Fusion was triggered by the addition of 30 μg/ml soluble gD2306 protein and target cells to effector cells. Luciferase production was monitored over 2 h with measurements every 5 min using a BioTek plate reader.
Inhibition of fusion with Mabs.
The assay for inhibition of fusion with Mabs was performed essentially as described previously (14, 56). To identify the concentration at which a Mab inhibited approximately 50% of fusion (IC50), each Mab was titrated by 2-fold dilution, beginning with 10 μg/ml. A negative control (transfected effector cells overlaid with target cells but with no gD added) was also included.
For gH/gL Mabs, effector cells were preincubated with both EnduRen substrate and gH/gL Mabs for 1 h at 37°C. For gD Mabs, 30 μg/ml gD2306 protein was preincubated with the indicated Mabs for 1 h at 4°C.
Data analysis.
The Bliss independence model (10) was applied to determine the effect of combinations of antibodies. The activity of each individual Mab was used to calculate a theoretical additive curve (shown in gray in all graphs) using the following formula for probabilistic independence: EA + EB(1 − EA) = EA + EB − EAEB.
The excess over Bliss (eob) score was calculated (on a scale of 1) as the difference between the observed response (EAB) and the Bliss-predicted response (ZAB) at the same combination dose (57–59). A pair of Mabs with an eob greater than ∼0 was considered additive or better; a pair with an eob less than −0.05 was considered “indifferent” or worse.
ACKNOWLEDGMENTS
We thank Leslie King (School of Veterinary Medicine, University of Pennsylvania) and Claude Krummenacher (Rowan University) for critical readings of the manuscript.
This research was supported by grants AI-18289, AI-142940, and AI-139618 and by a grant from BioNTech, Inc.
We dedicate this article to the memory of Roselyn J. Eisenberg.
REFERENCES
- 1.Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. 2012. Herpes virus fusion and entry: a story with many characters. Viruses 4:800–832. doi: 10.3390/v4050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connolly SA, Jackson JO, Jardetzky TS, Longnecker R. 2011. Fusing structure and function: a structural view of the herpesvirus entry machinery. Nat Rev Microbiol 9:369–381. doi: 10.1038/nrmicro2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vallbracht M, Backovic M, Klupp BG, Rey FA, Mettenleiter TC. 2019. Common characteristics and unique features: a comparison of the fusion machinery of the alphaherpesviruses Pseudorabies virus and Herpes simplex virus. Adv Virus Res 104:225–281. doi: 10.1016/bs.aivir.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Sathiyamoorthy K, Chen J, Longnecker R, Jardetzky TS. 2017. The COMPLEXity in herpesvirus entry. Curr Opin Virol 24:97–104. doi: 10.1016/j.coviro.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cairns TM, Ditto NT, Atanasiu D, Lou H, Brooks BD, Saw WT, Eisenberg RJ, Cohen GH. 2019. Surface plasmon resonance reveals direct binding of herpes simplex virus glycoproteins gH/gL to gD and locates a gH/gL binding site on gD. J Virol 93:e00289-19. doi: 10.1128/JVI.00289-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cairns TM, Atanasiu D, Saw WT, Lou H, Whitbeck JC, Ditto NT, Bruun B, Browne H, Bennett L, Wu C, Krummenacher C, Brooks BD, Eisenberg RJ, Cohen GH. 2020. Localization of the interaction site of herpes simplex virus glycoprotein D (gD) on the membrane fusion regulator, gH/gL. J Virol 94:e00983-20. doi: 10.1128/JVI.00983-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cairns TM, Shaner MS, Zuo Y, Ponce-de-Leon M, Baribaud I, Eisenberg RJ, Cohen GH, Whitbeck JC. 2006. Epitope mapping of herpes simplex virus type 2 gH/gL defines distinct antigenic sites, including some associated with biological function. J Virol 80:2596–2608. doi: 10.1128/JVI.80.6.2596-2608.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazear E, Whitbeck JC, Ponce-de-Leon M, Cairns TM, Willis SH, Zuo Y, Krummenacher C, Cohen GH, Eisenberg RJ. 2012. Antibody-induced conformational changes in herpes simplex virus glycoprotein gD reveal new targets for virus neutralization. J Virol 86:1563–1576. doi: 10.1128/JVI.06480-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cairns TM, Ditto NT, Lou H, Brooks BD, Atanasiu D, Eisenberg RJ, Cohen GH. 2017. Global sensing of the antigenic structure of herpes simplex virus gD using high-throughput array-based SPR imaging. PLoS Pathog 13:e1006430. doi: 10.1371/journal.ppat.1006430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bliss CI. 1939. The toxicity of poisons applied jointly. Ann Appl Biol 26:585–615. doi: 10.1111/j.1744-7348.1939.tb06990.x. [DOI] [Google Scholar]
- 11.El Hassouni B, Mantini G, Li Petri G, Capula M, Boyd L, Weinstein HNW, Valles-Marti A, Kouwenhoven MCM, Giovannetti E, Westerman BA, Peters GJ, EORTC PAMM Group. 2019. To combine or not combine: drug interactions and tools for their analysis. Reflections from the EORTC-PAMM course on preclinical and early-phase clinical pharmacology. Anticancer Res 39:3303–3309. doi: 10.21873/anticanres.13472. [DOI] [PubMed] [Google Scholar]
- 12.Goldoni M, Johansson C. 2007. A mathematical approach to study combined effects of toxicants in vitro: evaluation of the Bliss independence criterion and the Loewe additivity model. Toxicol In Vitro 21:759–769. doi: 10.1016/j.tiv.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Roell KR, Reif DM, Motsinger-Reif AA. 2017. An introduction to terminology and methodology of chemical synergy—perspectives from across disciplines. Front Pharmacol 8:158. doi: 10.3389/fphar.2017.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atanasiu D, Saw WT, Lazear E, Whitbeck JC, Cairns TM, Lou H, Eisenberg RJ, Cohen GH. 2018. Using antibodies and mutants to localize the presumptive gH/gL binding site on herpes simplex virus gD. J Virol 92:e01694-18. doi: 10.1128/JVI.01694-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hook LM, Cairns TM, Awasthi S, Brooks BD, Ditto NT, Eisenberg RJ, Cohen GH, Friedman HM. 2018. Vaccine-induced antibodies to herpes simplex virus glycoprotein D epitopes involved in virus entry and cell-to-cell spread correlate with protection against genital disease in guinea pigs. PLoS Pathog 14:e1007095. doi: 10.1371/journal.ppat.1007095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowdary TK, Cairns TM, Atanasiu D, Cohen GH, Eisenberg RJ, Heldwein EE. 2010. Crystal structure of the conserved herpesvirus fusion regulator complex gH-gL. Nat Struct Mol Biol 17:882–888. doi: 10.1038/nsmb.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atanasiu D, Cairns TM, Whitbeck JC, Saw WT, Rao S, Eisenberg RJ, Cohen GH. 2013. Regulation of herpes simplex virus gB-induced cell-cell fusion by mutant forms of gH/gL in the absence of gD and cellular receptors. mBio 4:e00046-13. doi: 10.1128/mBio.00046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cairns TM, Milne RS, Ponce-de-Leon M, Tobin DK, Cohen GH, Eisenberg RJ. 2003. Structure-function analysis of herpes simplex virus type 1 gD and gH-gL: clues from gDgH chimeras. J Virol 77:6731–6742. doi: 10.1128/jvi.77.12.6731-6742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan Q, Longnecker R, Connolly SA. 2014. Substitution of herpes simplex virus 1 entry glycoproteins with those of saimiriine herpesvirus 1 reveals a gD-gH/gL functional interaction and a region within the gD profusion domain that is critical for fusion. J Virol 88:6470–6482. doi: 10.1128/JVI.00465-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan Q, Longnecker R, Connolly SA. 2015. A functional interaction between herpes simplex virus 1 glycoprotein gH/gL domains I and II and gD is defined by using alphaherpesvirus gH and gL chimeras. J Virol 89:7159–7169. doi: 10.1128/JVI.00740-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan Q, Lin E, Spear PG. 2009. Insertional mutations in herpes simplex virus type 1 gL identify functional domains for association with gH and for membrane fusion. J Virol 83:11607–11615. doi: 10.1128/JVI.01369-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou W, Chen F, Klyachkin Y, Sham YY, Geraghty RJ. 2014. Mutations in the amino terminus of herpes simplex virus type 1 gL can reduce cell-cell fusion without affecting gH/gL trafficking. J Virol 88:739–744. doi: 10.1128/JVI.02383-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doria-Rose NA, Georgiev I, O’Dell S, Chuang GY, Staupe RP, McLellan JS, Gorman J, Pancera M, Bonsignori M, Haynes BF, Burton DR, Koff WC, Kwong PD, Mascola JR. 2012. A short segment of the HIV-1 gp120 V1/V2 region is a major determinant of resistance to V1/V2 neutralizing antibodies. J Virol 86:8319–8323. doi: 10.1128/JVI.00696-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendoza P, Gruell H, Nogueira L, Pai JA, Butler AL, Millard K, Lehmann C, Suarez I, Oliveira TY, Lorenzi JCC, Cohen YZ, Wyen C, Kummerle T, Karagounis T, Lu CL, Handl L, Unson-O’Brien C, Patel R, Ruping C, Schlotz M, Witmer-Pack M, Shimeliovich I, Kremer G, Thomas E, Seaton KE, Horowitz J, West AP, Jr, Bjorkman PJ, Tomaras GD, Gulick RM, Pfeifer N, Fatkenheuer G, Seaman MS, Klein F, Caskey M, Nussenzweig MC. 2018. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 561:479–484. doi: 10.1038/s41586-018-0531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagh K, Bhattacharya T, Williamson C, Robles A, Bayne M, Garrity J, Rist M, Rademeyer C, Yoon H, Lapedes A, Gao H, Greene K, Louder MK, Kong R, Karim SA, Burton DR, Barouch DH, Nussenzweig MC, Mascola JR, Morris L, Montefiori DC, Korber B, Seaman MS. 2016. Optimal combinations of broadly neutralizing antibodies for prevention and treatment of HIV-1 clade C infection. PLoS Pathog 12:e1005520. doi: 10.1371/journal.ppat.1005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pal P, Dowd KA, Brien JD, Edeling MA, Gorlatov S, Johnson S, Lee I, Akahata W, Nabel GJ, Richter MK, Smit JM, Fremont DH, Pierson TC, Heise MT, Diamond MS. 2013. Development of a highly protective combination monoclonal antibody therapy against chikungunya virus. PLoS Pathog 9:e1003312. doi: 10.1371/journal.ppat.1003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bakker AB, Marissen WE, Kramer RA, Rice AB, Weldon WC, Niezgoda M, Hanlon CA, Thijsse S, Backus HH, de Kruif J, Dietzschold B, Rupprecht CE, Goudsmit J. 2005. Novel human monoclonal antibody combination effectively neutralizing natural rabies virus variants and individual in vitro escape mutants. J Virol 79:9062–9068. doi: 10.1128/JVI.79.14.9062-9068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mankowski MC, Kinchen VJ, Wasilewski LN, Flyak AI, Ray SC, Crowe JE, Jr, Bailey JR. 2018. Synergistic anti-HCV broadly neutralizing human monoclonal antibodies with independent mechanisms. Proc Natl Acad Sci U S A 115:E82–E91. doi: 10.1073/pnas.1718441115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Criscuolo E, Clementi N, Mancini N, Burioni R, Miduri M, Castelli M, Clementi M. 2018. Synergy evaluation of anti-herpes simplex virus type 1 and 2 compounds acting on different steps of virus life cycle. Antiviral Res 151:71–77. doi: 10.1016/j.antiviral.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Sanna PP, Ramiro-Ibanez F, De Logu A. 2000. Synergistic interactions of antibodies in rate of virus neutralization. Virology 270:386–396. doi: 10.1006/viro.2000.0276. [DOI] [PubMed] [Google Scholar]
- 31.Vanarsdall AL, Chin AL, Liu J, Jardetzky TS, Mudd JO, Orloff SL, Streblow D, Mussi-Pinhata MM, Yamamoto AY, Duarte G, Britt WJ, Johnson DC. 2019. HCMV trimer- and pentamer-specific antibodies synergize for virus neutralization but do not correlate with congenital transmission. Proc Natl Acad Sci U S A 116:3728–3733. doi: 10.1073/pnas.1814835116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cairns TM, Huang ZY, Gallagher JR, Lin Y, Lou H, Whitbeck JC, Wald A, Cohen GH, Eisenberg RJ. 2015. Patient-specific neutralizing antibody responses to herpes simplex virus are attributed to epitopes on gD, gB, or both and can be type specific. J Virol 89:9213–9231. doi: 10.1128/JVI.01213-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cairns TM, Huang Z-Y, Whitbeck JC, Ponce de Leon M, Lou H, Wald A, Krummenacher C, Eisenberg RJ, Cohen GH. 2014. Dissection of the antibody response against herpes simplex virus glycoproteins in naturally infected humans. J Virol 88:12612–12622. doi: 10.1128/JVI.01930-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitbeck JC, Huang Z-Y, Cairns TM, Gallagher JR, Lou H, Ponce-de-Leon M, Belshe RB, Eisenberg RJ, Cohen GH. 2014. Repertoire of epitopes recognized by serum IgG from humans vaccinated with herpes simplex virus 2 glycoprotein D. J Virol 88:7786–7795. doi: 10.1128/JVI.00544-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gompels UA, Minson AC. 1989. Antigenic properties and cellular localization of herpes simplex virus glycoprotein H synthesized in a mammalian cell expression system. J Virol 63:4744–4755. doi: 10.1128/JVI.63.11.4744-4755.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buckmaster EA, Gompels U, Minson A. 1984. Characterisation and physical mapping of an HSV-1 glycoprotein of approximately 115 × 10(3) molecular weight. Virology 139:408–413. doi: 10.1016/0042-6822(84)90387-8. [DOI] [PubMed] [Google Scholar]
- 37.Cairns TM, Friedman LS, Lou H, Whitbeck JC, Shaner MS, Cohen GH, Eisenberg RJ. 2007. N-terminal mutants of herpes simplex virus type 2 gH are transported without gL but require gL for function. J Virol 81:5102–5111. doi: 10.1128/JVI.00097-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng T, Ponce de Leon M, Novotny MJ, Jiang H, Lambris JD, Dubin G, Spear PG, Cohen GH, Eisenberg RJ. 1998. Structural and antigenic analysis of a truncated form of the herpes simplex virus glycoprotein gH-gL complex. J Virol 72:6092–6103. doi: 10.1128/JVI.72.7.6092-6103.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng T, Ponce-de-Leon M, Jiang H, Dubin G, Lubinski JM, Eisenberg RJ, Cohen GH. 1998. The gH-gL complex of herpes simplex virus (HSV) stimulates neutralizing antibody and protects mice against HSV type 1 challenge. J Virol 72:65–72. doi: 10.1128/JVI.72.1.65-72.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klyachkin YM, Stoops KD, Geraghty RJ. 2006. Herpes simplex virus type 1 glycoprotein L mutants that fail to promote trafficking of glycoprotein H and fail to function in fusion can induce binding of glycoprotein L-dependent anti-glycoprotein H antibodies. J Gen Virol 87:759–767. doi: 10.1099/vir.0.81563-0. [DOI] [PubMed] [Google Scholar]
- 41.Atanasiu D, Saw WT, Eisenberg RJ, Cohen GH. 2016. Regulation of herpes simplex virus glycoprotein-induced cascade of events governing cell-cell fusion. J Virol 90:10535–10544. doi: 10.1128/JVI.01501-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Showalter SD, Zweig M, Hampar B. 1981. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun 34:684–692. doi: 10.1128/IAI.34.3.684-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sathiyamoorthy K, Hu YX, Mohl BS, Chen J, Longnecker R, Jardetzky TS. 2016. Structural basis for Epstein-Barr virus host cell tropism mediated by gp42 and gHgL entry glycoproteins. Nat Commun 7:13557. doi: 10.1038/ncomms13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sathiyamoorthy K, Jiang J, Hu YX, Rowe CL, Mohl BS, Chen J, Jiang W, Mellins ED, Longnecker R, Zhou ZH, Jardetzky TS. 2014. Assembly and architecture of the EBV B cell entry triggering complex. PLoS Pathog 10:e1004309. doi: 10.1371/journal.ppat.1004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sparrow E, Friede M, Sheikh M, Torvaldsen S, Newall AT. 2016. Passive immunization for influenza through antibody therapies, a review of the pipeline, challenges and potential applications. Vaccine 34:5442–5448. doi: 10.1016/j.vaccine.2016.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salazar G, Zhang N, Fu TM, An Z. 2017. Antibody therapies for the prevention and treatment of viral infections. NPJ Vaccines 2:19. doi: 10.1038/s41541-017-0019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang Y, Leib D. 2017. Preventing neonatal herpes infections through maternal immunization. Future Virol 12:709–711. doi: 10.2217/fvl-2017-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang Y, Patel CD, Manivanh R, North B, Backes IM, Posner DA, Gilli F, Pachner AR, Nguyen LN, Leib DA. 2017. Maternal antiviral immunoglobulin accumulates in neural tissue of neonates to prevent HSV neurological disease. mBio 8:e00678-17. doi: 10.1128/mBio.00678-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chu CF, Meador MG, Young CG, Strasser JE, Bourne N, Milligan GN. 2008. Antibody-mediated protection against genital herpes simplex virus type 2 disease in mice by Fc gamma receptor-dependent and -independent mechanisms. J Reprod Immunol 78:58–67. doi: 10.1016/j.jri.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bourne N, Pyles RB, Bernstein DI, Stanberry LR. 2002. Modification of primary and recurrent genital herpes in guinea pigs by passive immunization. J Gen Virol 83:2797–2801. doi: 10.1099/0022-1317-83-11-2797. [DOI] [PubMed] [Google Scholar]
- 51.Bystricka M, Petrikova M, Zatovicova M, Solarikova L, Kostolansky F, Mucha V, Russ G. 1997. Monoclonal antibodies to the distinct antigenic sites on glycoproteins C and B and their protective abilities in herpes simplex virus infection. Acta Virol 41:5–12. [PubMed] [Google Scholar]
- 52.Miller CG, Krummenacher C, Eisenberg RJ, Cohen GH, Fraser NW. 2001. Development of a syngenic murine B16 cell line-derived melanoma susceptible to destruction by neuroattenuated HSV-1. Mol Ther 3:160–168. doi: 10.1006/mthe.2000.0240. [DOI] [PubMed] [Google Scholar]
- 53.Willis SH, Rux AH, Peng C, Whitbeck JC, Nicola AV, Lou H, Hou W, Salvador L, Eisenberg RJ, Cohen GH. 1998. Examination of the kinetics of herpes simplex virus glycoprotein D binding to the herpesvirus entry mediator, using surface plasmon resonance. J Virol 72:5937–5947. doi: 10.1128/JVI.72.7.5937-5947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Atanasiu D, Saw WT, Gallagher JR, Hannah BP, Matsuda Z, Whitbeck JC, Cohen GH, Eisenberg RJ. 2013. Dual split protein-based fusion assay reveals that mutations to herpes simplex virus (HSV) glycoprotein gB alter the kinetics of cell-cell fusion induced by HSV entry glycoproteins. J Virol 87:11332–11345. doi: 10.1128/JVI.01700-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kondo N, Miyauchi K, Matsuda Z. 2011. Monitoring viral-mediated membrane fusion using fluorescent reporter methods. Curr Protoc Cell Biol Chapter 26:Unit 26.9. doi: 10.1002/0471143030.cb2609s50. [DOI] [PubMed] [Google Scholar]
- 56.Saw WT, Matsuda Z, Eisenberg RJ, Cohen GH, Atanasiu D. 2015. Using a split luciferase assay (SLA) to measure the kinetics of cell-cell fusion mediated by herpes simplex virus glycoproteins. Methods 90:68–75. doi: 10.1016/j.ymeth.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foucquier J, Guedj M. 2015. Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect 3:e00149. doi: 10.1002/prp2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goswami CP, Cheng L, Alexander PS, Singal A, Li L. 2015. A new drug combinatory effect prediction algorithm on the cancer cell based on gene expression and dose-response curve. CPT Pharmacometrics Syst Pharmacol 4:e9. doi: 10.1002/psp4.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Q, Yin X, Languino LR, Altieri DC. 2018. Evaluation of drug combination effect using a Bliss independence dose-response surface model. Stat Biopharm Res 10:112–122. doi: 10.1080/19466315.2018.1437071. [DOI] [PMC free article] [PubMed] [Google Scholar]