Viruses in the Reoviridae family include important pathogens of humans and other animals and have segmented RNA genomes. Recombination in RNA virus populations can facilitate novel host exploration and increased disease severity.
KEYWORDS: Reoviridae, defective viral genome, double-stranded RNA virus, recombination, reovirus, rotavirus, segmented
ABSTRACT
For viruses with segmented genomes, genetic diversity is generated by genetic drift, reassortment, and recombination. Recombination produces RNA populations distinct from full-length genome segments and can influence viral population dynamics, persistence, and host immune responses. Viruses in the Reoviridae family, including rotavirus and mammalian orthoreovirus (reovirus), have been reported to package segments containing rearrangements or internal deletions. Rotaviruses with RNA segments containing rearrangements have been isolated from immunocompromised and immunocompetent children and in vitro following serial passage at relatively high multiplicity. Reoviruses that package small, defective RNA segments have established chronic infections in cells and in mice. However, the mechanism and extent of Reoviridae RNA recombination are undefined. To fill this gap in knowledge, we determined the titers and RNA segment profiles for reovirus and rotavirus following serial passage in cultured cells. The viruses exhibited occasional titer reductions characteristic of interference. Reovirus strains frequently accumulated segments that retained 5′- and 3′-terminal sequences and featured large internal deletions, while similarly fragmented segments were rarely detected in rotavirus populations. Using next-generation RNA sequencing to analyze RNA molecules packaged in purified reovirus particles, we identified distinct recombination sites within individual viral genome segments. Recombination junctions were frequently but not always characterized by short direct sequence repeats upstream and downstream that spanned junction sites. Taken together, these findings suggest that reovirus accumulates defective genome segments featuring internal deletions during passage and undergoes sequence-directed recombination at distinct sites.
IMPORTANCE Viruses in the Reoviridae family include important pathogens of humans and other animals and have segmented RNA genomes. Recombination in RNA virus populations can facilitate novel host exploration and increased disease severity. The extent, patterns, and mechanisms of Reoviridae recombination and the functions and effects of recombined RNA products are poorly understood. Here, we provide evidence that mammalian orthoreovirus regularly synthesizes RNA recombination products that retain terminal sequences but contain internal deletions, while rotavirus rarely synthesizes such products. Recombination occurs more frequently at specific sites in the mammalian orthoreovirus genome, and short regions of identical sequence are often detected at junction sites. These findings suggest that mammalian orthoreovirus recombination events are directed in part by RNA sequences. An improved understanding of recombined viral RNA synthesis may enhance our capacity to engineer improved vaccines and virotherapies in the future.
INTRODUCTION
Genetic drift and reassortment are typically considered the primary mechanisms by which segmented RNA viruses acquire genetic diversity. However, recombination also occurs regularly during the replication of these viruses and yields noncanonical RNA molecules that differ from full-length genome segments (1, 2). These noncanonical RNAs may be packaged and can influence viral population dynamics, persistence, and host immune responses. A subset of noncanonical RNAs resulting from viral recombination events are known as defective viral genomes (DVGs) because they are unable to replicate in the absence of functional trans-complementation by the full-length parental RNA and its translation product(s). The most frequently reported type of RNA DVG arises from recombination events resulting in large deletions that may remove much of the coding region of a viral RNA while retaining elements required for polymerase binding, replication, and packaging (2–4). Functions of DVGs are poorly defined for RNA viruses, although some configurations have demonstrated roles in viral replication interference, innate immune antagonism, and virus evolution (2). For segmented Reoviridae viruses, the need to successfully package a multipartite genome likely imposes additional restrictions on the variety of noncanonical RNAs that are tolerated. Overall for the Reoviridae, there have been limited studies of recombination events and the recombined noncanonical RNAs they generate.
For the Reoviridae family of segmented, double-stranded RNA (dsRNA) viruses, RNA segment termini are predicted to direct packaging and other viral replication processes. Rotavirus is the leading cause of diarrheal mortality among unvaccinated children under 5 years of age (5). Mammalian orthoreovirus (reovirus) has been linked to loss of oral tolerance associated with celiac disease and is in advanced clinical trials as an oncolytic virus (6). Reoviridae particles are nonenveloped, multilayered, and encapsidate 9 to 12 dsRNA genome segments (7). Following entry into target cells and outer capsid removal, subvirion particles function as nanoscale factories that transcribe capped, positive-sense viral RNA (+RNA) species that are ∼0.7 to 4 kb in length (7–11). The 10 reovirus segments are classified as large (L1 to L3), medium (M1 to M3), or small (S1 to S4), while the 11 rotavirus RNA segments are continuously numbered, with g1 representing the largest and g11 the smallest segment. Most segments contain a single open reading frame (ORF) flanked by 5′ and 3′ untranslated regions (UTRs) (12–14). Extensive base pairing between 5′- and 3′-terminal regions, interrupted by secondary structures, has been predicted for many segments (15–21). Reoviridae +RNA sequences required for packaging, assortment, transcription, or translation encompass the UTR and extend into the ORF (18, 22–25). Since Reoviridae viruses can package noncanonical segments, at least some level of divergence from the consensus sequence is tolerated (10, 26–34). However, the extent of recombination and degree to which noncanonical segments are packaged are unknown.
For the Reoviridae, noncanonical genome segments have been reported. Rotaviruses with RNA segments containing rearrangements have been isolated from immunocompromised and immunocompetent children and in vitro following serial passage at relatively high multiplicity (31, 33, 35, 36). Most reported rearrangements involve partial head-to-tail duplications ligated after the termination codon of the functional ORF (35). In some cases, rotavirus rearrangements occur at preferred sites, with direct repeat sequences or RNA secondary structures proposed as recombination hot spots (27, 30, 33). Rearranged segments have also been detected in orbiviruses (29, 32). Finally, reoviruses establishing chronic infections in vitro and in vivo may package small, defective RNA segments (25, 26, 34, 37, 38). Together, these observations suggest that recombination is not a rare occurrence during Reoviridae replication. However, previous work often has not approached studies of Reoviridae recombination and noncanonical segments in a systematic manner. The mechanism of Reoviridae recombination, the frequency with which recombined RNAs are synthesized or packaged, and the functions of recombined RNAs remain poorly understood.
In the current study, we sought to elucidate the type and frequency of noncanonical RNA synthesis by Reoviridae viruses. We determined the titers and RNA segment profiles of reovirus (recombinant strain [rs] T1L and rsT3DI) and rotavirus (rsSA11) laboratory strains that had been rescued by reverse genetics then serially passaged 10 times, each in triplicate lineages, in cultured cells. Viruses exhibited occasional reductions in titer that are characteristic of interference by defective interfering viral genes. The two reoviruses accumulated noncanonical RNAs that retain 5′ and 3′ termini and feature one or more large internal deletions, while the rotavirus rarely accumulated such DVGs. Analyses of next-generation RNA sequencing data sets from purified rsT1L reovirus RNA revealed many junctions, with hot spots for recombination in specific viral genome segments. Further, direct sequence repeats of 3 to 9 bp were favored at the recombination junction sites, suggesting that DVG formation is primarily directed by sequence complementarity. These findings suggest that reovirus frequently synthesizes and packages DVGs that contain internal deletions and undergoes recombination at distinct sites across the genome that encode key sequence features. This work provides rationale for future studies that will reveal detailed mechanisms of DVG synthesis and DVG effects on viral population dynamics and host responses.
RESULTS
Virus titer patterns differ among serially passaged Reoviridae viruses.
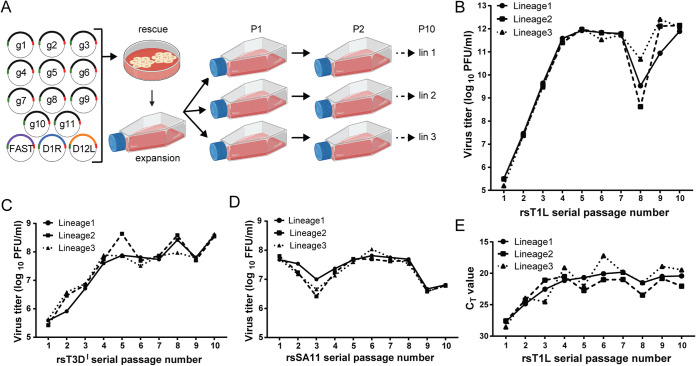
To compare virus population replication in cultured cells over multiple infections between reovirus and rotavirus, we utilized a serial passage approach with two strains of reovirus, T1L and T3DI, and one strain of rotavirus, SA11. T1L and T3DI represent distinct human reovirus serotypes (12). T3D induces necrosis and substantially more apoptosis than T1L in murine L929 fibroblasts (L cells) (39, 40). SA11 is a simian rotavirus that induces necrosis in African green monkey kidney epithelial (MA104) cells (41). For the initial passage, viruses were recovered using plasmid-based reverse genetics and amplified once in cultured cells (Fig. 1A). rsT1L, rsT3DI, and rsSA11 were serially passaged 10 times, each in three lineages, and infectious virus titers were determined. In passage 1 (P1), cells were infected at a multiplicity of infection (MOI) of 1 PFU/cell (rsT1L and rsT3DI) or 0.25 PFU/cell (rsSA11). In P2 to P10, cells were infected blindly with a fixed volume of lysate from the previous passage, and MOIs were back calculated (Table 1). While the three lineages of each virus strain exhibited relatively similar virus titer patterns, rsT1L, rsT3DI, and rsSA11 exhibited notable differences in virus titer pattern across the passage series (Fig. 1B to D). Virus titers for rsT1L reovirus climbed steadily to ∼1012 PFU/ml at P5, remained high through P7, decreased by ∼10- to 1,000-fold at P8, and then rebounded through P10 (Fig. 1B). The average MOI for P6 to P8 was >100,000 PFU/cell (Table 1). For serially passaged rsT3DI reovirus, virus titers climbed to ∼6 × 107 PFU/ml at P5 and then fluctuated between ∼3 × 107 and 4 × 108 PFU/ml through P10 (Fig. 1C). The average MOI varied from ∼10 to 50 PFU/cell from P5 to P10 (Table 1). By P1, rsSA11 rotavirus had already reached titers of ∼5 × 107 PFU/ml (Fig. 1D). The titers then decreased by ∼5- to 25-fold at P3 and P9 and rebounded during intervening passages. MOIs were ∼1 to 15 focus-forming units (FFU)/cell for most rsSA11 passages, suggesting lower virus production (Table 1). Titer patterns in serially passaged rsSA11 rotavirus lineages resembled those of rsT3DI reovirus in that peak titers were lower and stayed within a narrower range, but they resembled those of rsT1L reovirus in that distinct titer dips and rebounds were observed concurrently for all three lineages (Fig. 1B to D).
FIG 1.
Serial passage workflow and virus titers. (A) Workflow for rotavirus serial passaging. Baby hamster kidney cells expressing T7 RNA polymerase were transfected with plasmids encoding the 11 rotavirus positive-sense viral RNAs (+RNAs) (g1 to g11) and helper plasmids encoding capping enzymes (D1R and D12L) and a cell-cell fusion protein (FAST) and then cocultured with MA104 cells to promote recombinant virus rescue. rsSA11 was amplified by a single passage in MA104 cells, and the virus stock titer was determined. To generate P1 stocks, MA104 monolayers in three flasks were adsorbed at an MOI of 0.25 PFU/cell, washed, and incubated with fresh medium for 48 h prior to lysis by multiple rounds of freezing and thawing. Subsequent passages (P2 to P10) were generated by adsorption of MA104 monolayers with 3 ml of cleared lysate from the previous passage, with three lineages each passaged in an independent series. A similar workflow was used for rescue and passaging of reovirus, employing standard reverse genetics approaches (23, 71) and with passages conducted in suspension rather than monolayer culture. Created with BioRender.com. (B and C) Graphs showing titers for three lineages of rsT1L (B) or rsT3DI (C) reoviruses across 10 serial passages, quantified by plaque assay. (D) Graphs showing titers for three lineages of rsSA11 rotavirus across all 10 serial passages, quantified by fluorescent focus assay. (E) Graph showing CT values from S4 RT-qPCR analysis of RNA extracted from three lineages of rsT1L across 10 passages.
TABLE 1.
Average infection multiplicity for three lineages by passage
| Passage | rsT1L MOI (PFU/cell) |
rsT3DI MOI (PFU/cell) |
rsSA11 MOI (FFU/cell) |
|---|---|---|---|
| 1 | 1.00 | 1.00 | 0.25a |
| 2 | 0.05 | 0.07 | 10.67 |
| 3 | 5.13 | 0.50 | 4.45 |
| 4 | 726.67 | 1.32 | 1.14 |
| 5 | 62,666.67 | 11.57 | 3.63 |
| 6 | 184,666.67 | 38.83 | 9.04 |
| 7 | 113,333.33 | 9.77 | 14.84 |
| 8 | 116,666.67 | 13.33 | 10.31 |
| 9 | 3,454.67 | 49.57 | 8.51 |
| 10 | 265,800.00 | 11.70 | 0.82 |
Passage 1 MOI for rsSA11 was measured in PFU/cell.
To determine whether viral RNA levels correlated with decreases in titer during serial passage, we used quantitative reverse transcription-PCR (RT-qPCR). Using primers that bound near the 3′ terminus of segment S4, we found that the cycle threshold (CT) value for detection of RNA extracted from lysates of rsT1L decreased through P7 and increased at P8 before decreasing again for the final passages (Fig. 1E). While not all DVGs may have been detected using this approach, this observation suggests that overall reovirus RNA levels decreased in conjunction with virus titer at P8. No consistent correlation between CT value and decrease in titer was detected for rsSA11 (data not shown).
Reovirus and rotavirus produce small noncanonical segments during serial passage.
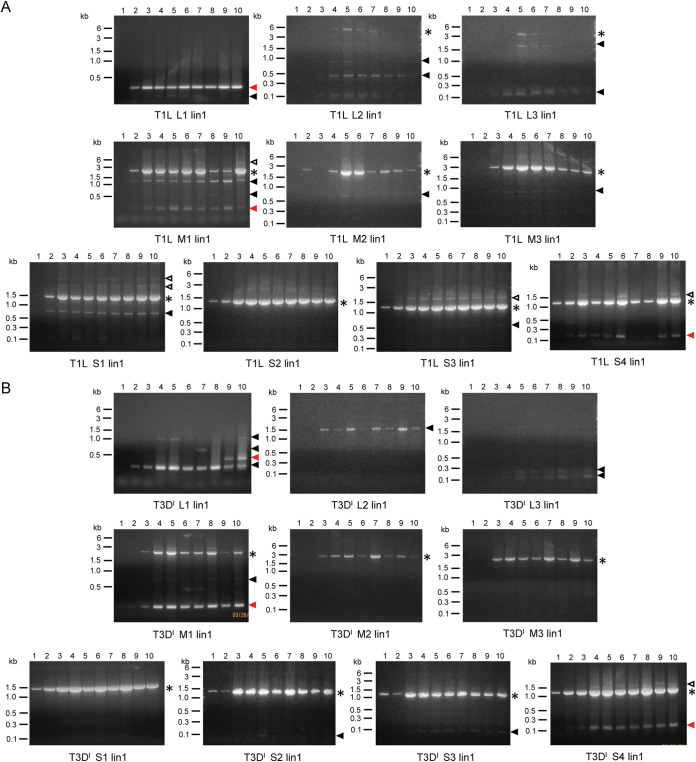
Alterations to virus titer patterns during serial passage can be due to the presence of DVGs, which may interfere with viral replication (4, 42). To detect noncanonical segments in lysates from reovirus and rotavirus passages, we used reverse transcription-PCR (RT-PCR). RNA molecules containing both the 5′ and 3′ termini of a specific viral genome segment were amplified and visualized. Using this approach, we consistently detected full-length viral genome segments of ≤3.3 kb in length, which included M and S reovirus segments and all rotavirus segments (g1 to g11) (Fig. 2 and 3). Longer full-length segments, such as reovirus L segments, were amplified infrequently, due to limitations of the enzymes used for amplification. Thus, our assay conditions permitted detection of small RNA molecules that contained native viral 5′ and 3′ segment termini. For rsT1L reovirus lineage 1 (lin1), RT-PCR products smaller than the full-length segment were amplified from multiple passages for each segment, except S2 (Fig. 2A). Some noncanonical segments (L1, M1, and S1) were detected almost continuously across passages, while others (L2, L3, M2, and M3) were detected only transiently. For segments M1, S1, and S4, RNA products slightly longer than the full-length genome segment were detected. While no noncanonical segment clearly correlated with the change in titer at P8, a noncanonical L3 segment was detected from P5 to P7, and a faint, transient, noncanonical M1 segment was detected only in P8 and P9 (Fig. 1B and 2A). Additionally, segments L2, L3, and M2 decreased in intensity prior to P8. For rsT3DI reovirus lin1, RT-PCR products smaller than the full-length segment were amplified from multiple passages for 7 of 10 segments (Fig. 2B). Like the patterns observed for rsT1L, these products often were detected almost continuously across rsT3DI passages. However, some products were detected transiently, with products derived from the rsT3DI L1 segment providing the most striking example of this property. In contrast to the frequent detection of noncanonical segments smaller than the parental segment for serially passaged reoviruses, such a product was detected for only 1 of 11 rotavirus segments, g5 (Fig. 3). For several segments, indistinct bands high on the gels suggested the presence of RT-PCR products that were longer than the full-length products. Taken together, lin1 RNA profiles suggest that reovirus and rotavirus differ in noncanonical RNA species accumulation during serial passage in cultured cells, with reovirus frequently accumulating RNAs smaller than parental segments but with identical termini and rotavirus accumulating such an RNA species only for g5.
FIG 2.
Serial passage lineage 1 reovirus segment profiles. RNA extracted from serial passage lysates was used as the template in RT-PCRs, along with primers that bind to the 3′- and 5′-terminal sequences of each reovirus genome segment. Products from reactions in which template RNA was extracted from rsT1L lin1 P1 to P10 (A) or rsT3DI lin1 P1 to P 10 (B) were resolved on 1.2% ethidium bromide-stained agarose gels. The identity of the reovirus segment (L1 to L3, M1 to M3, and S1 to S4) recognized by the terminal primers is indicated. Black asterisks indicate the position of the full-length segment. Filled triangles indicate noncanonical RNA products smaller than the full-length segment. Red triangles indicate products that were excised and sequenced. Open triangles indicate products larger than the full-length segment.
FIG 3.
Serial passage lineage 1 rotavirus segment profiles. RNA extracted from serial passage lysates was used as the template in RT-PCRs, along with primers that bind to the 3′- and 5′-terminal sequences of each rotavirus genome segment. Products from reactions in which template RNA was extracted from rsSA11 lin1 P1 to P10 were resolved on 1.2% ethidium bromide-stained agarose gels. The identity of the rotavirus segment (g1 to g11) recognized by the terminal primers is indicated. Black asterisks indicate the position of the full-length segment. Filled triangles indicate products smaller than the full-length segment.
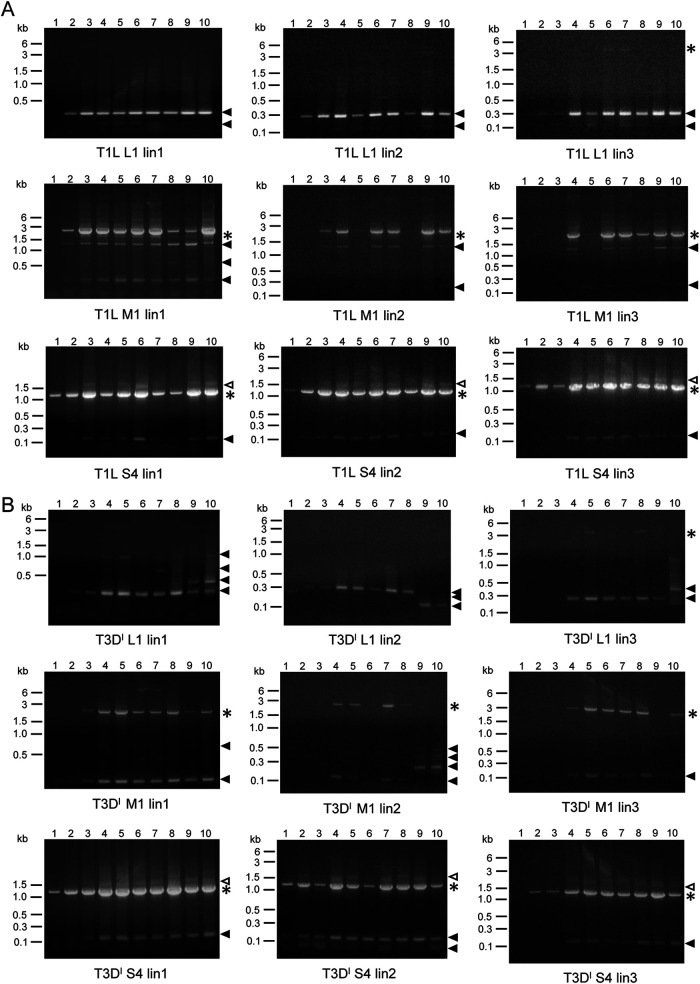
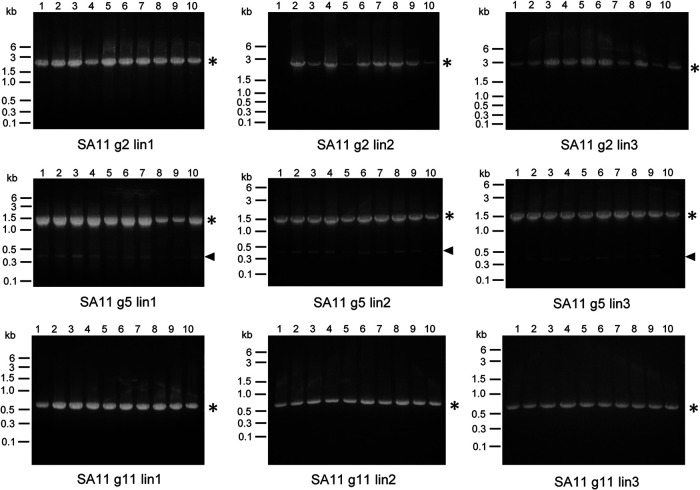
After examining RNA profiles for all 10 or 11 segments of lin1, we compared RNA profiles among all three lineages for each virus passage series following amplification by RT-PCR and resolution in 1.2% agarose, as in Fig. 2 and 3. For rsT1L and rsT3DI reoviruses, we used primers that anneal to the termini of L1, M1, and S4, and for rsSA11 rotavirus, we used primers that anneal to g2, g5, and g11. These segments were chosen to compare RNA profiles between representative small, medium, and large viral genome segments. For rsT1L reovirus, we found that profiles of putative DVGs were mostly conserved across the three independently passaged lineages (Fig. 4A). RNA profiles of rsT3DI reovirus were less well conserved across the three lineages (Fig. 4B). For example, for rsT3DI L1, all three lineages contained a small noncanonical RNA segment, ∼0.3 kb in size, present in many passages. However, lin1 contained transient, larger noncanonical RNA segments. Ln2 contained smaller, transient rsT3DI L1 noncanonical RNA segments instead of the ∼0.3-kb segment in P1, P9, and P10, and lin3 contained a larger noncanonical rsT3DI L1 segment in later passages. RNA profiles for rsSA11 rotavirus were very similar across the three lineages, featuring a single noncanonical RNA segment of ∼0.4 kb only for g5 (Fig. 5). As noted for lin1, for some segments in lin2 and lin3, indistinct bands high on the gels suggested the presence of RT-PCR products longer than the full-length products. The observation that similarly sized products that differ from full-length segments were amplified by primers specific for the 5′ and 3′ segment termini arose in multiple independent passages of rsT1L reovirus and rsSA11 rotavirus suggests that these viruses preferentially accumulate certain noncanonical RNAs.
FIG 4.
Serial passage lineage 1 to 3 reovirus segment profiles. RNA extracted from serial passage lysates was used as the template in RT-PCRs, along with primers that bind to the 3′- and 5′-terminal sequences of reovirus genome segments L1, M1, and S4. Products from reactions in which template RNA was extracted from rsT1L reovirus lin2 or lin3 P1 to P10 (A) or rsT3DI reovirus lin2 or lin3 P1 to 10 (B) were resolved on 1.2% ethidium bromide-stained agarose gels. Gels from lin1 are identical to those shown in Fig. 2 but are included for comparison. Black asterisks indicate the position of the full-length segment. Filled triangles indicate products smaller than the full-length segment. Open triangles indicate products larger than the full-length segment.
FIG 5.
Serial passage lineage 1 to 3 rotavirus segment profiles. RNA extracted from serial passage lysates was used as the template in RT-PCRs, along with primers that bind to the 3′- and 5′-terminal sequences of rotavirus genome segments g2, g5, and g11. Products from reactions in which template RNA was extracted from rsSA11 rotavirus lin2 or lin3 P1 to 10 were resolved on 1.2% ethidium bromide-stained agarose gels. Gels from lin1 are identical to those shown in Fig. 3 but are included for comparison. Black asterisks indicate the position of the full-length segment. Filled triangles indicate products smaller than the full-length segment.
Noncanonical reovirus segments feature large deletions.
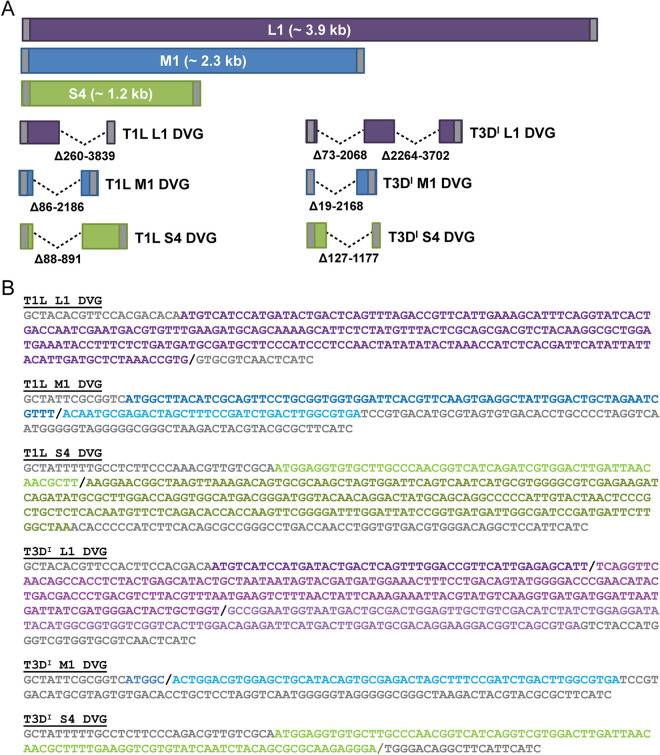
To gain insight into the identities of noncanonical RNA products detected in lysates of serially passaged reovirus, we excised bands from agarose gels on which RT-PCR products had been resolved (Fig. 2) and used the Sanger method to determine their sequences. We selected bright, low-molecular-weight bands amplified using primers specific for the termini of rsT1L and rsT3D reovirus L1, M1, and S4. The sequenced segments ranged from ∼7% to 33% of the length of the parental segments. Comparison with reference sequences revealed that each product was a DVG that contained relatively short intact termini and one or two large internal deletions (Fig. 6A). The largest single deletion, in the rsT1L L1 DVG, was of 3,581 nucleotides and removed the majority of the ORF and about half of the 3′ UTR. In every case, the 5′ UTR was left intact, and for five of the six sequenced DVGs, more than 50 nucleotides of the 5′ end of the ORF also was left intact (Fig. 6B). The shortest terminal regions on either side of a novel DVG junction were only 15 to 20 nucleotides in length and were observed at the 5′ terminus of the rsT3DI M1 DVG and the 3′ termini of the rsT1L L1 DVG and the rsT3DI S4 DVG. For the rsT1L L1 DVG and the rsT3DI S4 DVG, part of the 3′ UTR was deleted during its emergence. For the rsT1L M1 DVG, less than 50 nucleotides of the ORF preceding the 3′ UTR was retained, but at 80 nucleotides, this UTR is relatively long. Together, these observations suggest that serially passaged reoviruses regularly undergo recombination events resulting in generation of DVGs that retain 5′ and 3′ termini and feature one or multiple large internal deletions.
FIG 6.
Schematics of reovirus DVGs. RT-PCR products amplified using primers that bind the 5′ and 3′ termini of the L1, M1, and S4 reovirus segments, smaller than the full-length segments, and indicated in Fig. 2 were excised from agarose gels and sequenced. (A) Schematics, drawn to scale, of reovirus L1, M1, and S4 segments and sequenced rsT1L and rsT3DI reovirus DVGs are shown. The ORF is colored purple (L1), blue (M1), or green (S4), and the 5′ and 3′ UTRs are colored gray. Deletions are indicated by dashed lines, and deleted nucleotides are described. (B) Sequences of sequenced rsT1L and rsT3DI L1, M1, and S4 DVGs. 5′ and 3′ UTRs are colored gray, recombination sites are indicated by a black forward slash, and ORF sequences upstream or downstream of recombination sites are colored in shades of purple (L1), blue (M1), or green (S4).
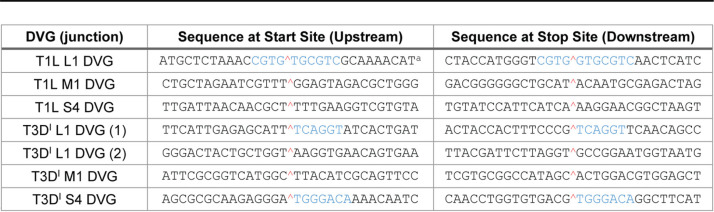
To better understand reovirus recombination, we examined sequences immediately surrounding the new junctions created in DVGs (Table 2). In several cases, recombination appeared nonhomologous (35). However, for the rsT1L L1 DVG, the first rsT3DI L1 DVG junction, and the rsT3DI S4 DVG, short regions of sequence similarity (6 or 7 nucleotides) were detected downstream from the recombination site. For the rsT1L L1 DVG, the upstream four nucleotides also were identical. Thus, these junctions were identical before and after recombination, or in the case of the rsT1L L1 DVG contained a single inserted nucleotide.
TABLE 2.
Nucleotides surrounding sequenced reovirus DVG recombination junctions
Sequences colored blue indicate regions of identical sequence between start and stop sites. The red caret indicates the recombination site.
Reovirus recombination occurs at distinct sites.
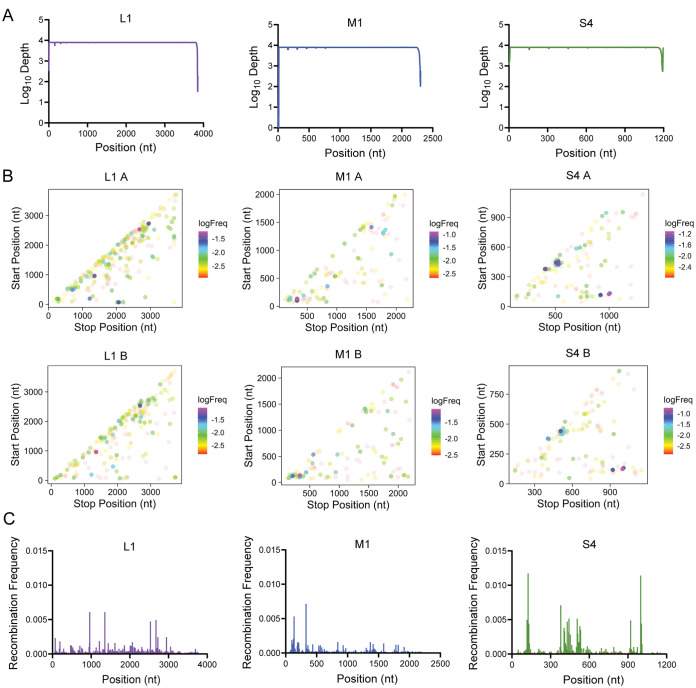
To detect reovirus recombination biases, we extracted and sequenced RNA from gradient-purified, benzonase-treated (to remove extravirion nucleic acids) rsT1L particles. These viruses had been amplified only a few times following recovery using plasmid-based reverse genetics and were not anticipated to have accumulated numerous DVGs. We aligned high-quality reads to reference rsT1L sequences for each genome segment using ViReMa (Virus Recombination Mapper), a platform that detects recombination events in viral genomes from input next-generation sequencing data, and analyzed recombination events with a custom bioinformatic pipeline (43, 44). Our sequencing averaged ∼104 reads per site across most genome segments, with reduced coverage at the extreme 5′ and 3′ termini (Fig. 7A). Approximately 99% of reads mapped to viral genome segments, demonstrating that the virion particles had been sufficiently purified (Table 3). We detected a genome-wide recombination frequency of approximately 0.01% and identified many noncanonical junctions within individual viral genome segments (Fig. 7B and Table 3). In accordance with previous reports, a recombination junction was defined as a deletion greater than 5 bp flanked both upstream and downstream by a 25-bp high-quality alignment (43, 44). Forward, 5′-to-3′ junctions were filtered for each segment. Small, internal deletions of less than 150 bp with an average size of ∼66 bp were especially prevalent in the L segments, as indicated by the concentration of points along the diagonal axes of junction plots, though similar deletions were detected in all segments (Fig. 7B and data not shown) (45). The S segments more frequently exhibited fusion of one segment terminus to the other than did the larger segments, as indicated by the concentration of points in the lower right corners of junction plots (Fig. 7B and data not shown) (45). Several segments exhibited hot spots for recombination (Fig. 7C). A hot spot was defined as a position or clustering of positions where the recombination frequency exceeded that of the overall genome. Further, positions of interest were identified if the recombination frequency was higher than that of the rest of the segment. In segment S4, hot spots were detected around nucleotides 117 to 131, 404 to 452, 506 to 532, 921, and 999 to 1005. However, for a few segments (L2, S2, and S3), recombination frequency was low across the entire genome segment length (data not shown) (45). These data demonstrate that reovirus recombination occurs at key, distinct sites across genome segments where the recombination frequency was up to 100-fold higher than the mean frequency across all segments.
FIG 7.
rsT1L reovirus junction frequency. (A) Graphs showing sequence depth at each nucleotide position across the indicated rsT1L genome segment. The mean coverage depth for two samples of sequenced virion RNA is shown. (B) Graphs showing recombination junction site location and frequency in sequenced rsT1L virion RNA for the indicated reovirus genome segment. Junction sites are indicated by dots whose position corresponds to upstream and downstream sequences that are merged to form a novel junction. Junction frequency is indicated by dot color, according to the legend to the right of each image. Junction maps for independently purified and sequenced virion RNA preparations are shown in separate graphs labeled A or B. (C) Graphs showing recombination frequency at each nucleotide position across the indicated rsT1L genome segment. Mean positional recombination frequency for two samples of sequenced virion RNA is shown.
TABLE 3.
Alignment statistics
| Alignment statistic | Segment | S199 (A) | S200 (B) |
|---|---|---|---|
| % reads mapped to virus | 98.96 | 99.60 | |
| Total no. of reads | 76,327,059 | 73,209,162 | |
| % recombined basesa | 0.013 | 0.008 | |
| No. of reads mapped to viral genome segment |
L1 | 19,484,338 | 16,469,190 |
| L2 | 8,971,901 | 7,223,829 | |
| L3 | 8,540,478 | 7,958,444 | |
| M1 | 4,951,438 | 4,425,385 | |
| M2 | 7,114,085 | 8,009,169 | |
| M3 | 7,501,592 | 9,128,523 | |
| S1 | 5,272,443 | 4,961,923 | |
| S2 | 4,393,207 | 4,603,800 | |
| S3 | 4,557,003 | 4,867,022 | |
| S4 | 4,743,900 | 5,271,611 |
Percent recombined bases is the number of nucleotides in all ViReMa-detected junctions divided by the total number of nucleotides mapping to the viral genome segments.
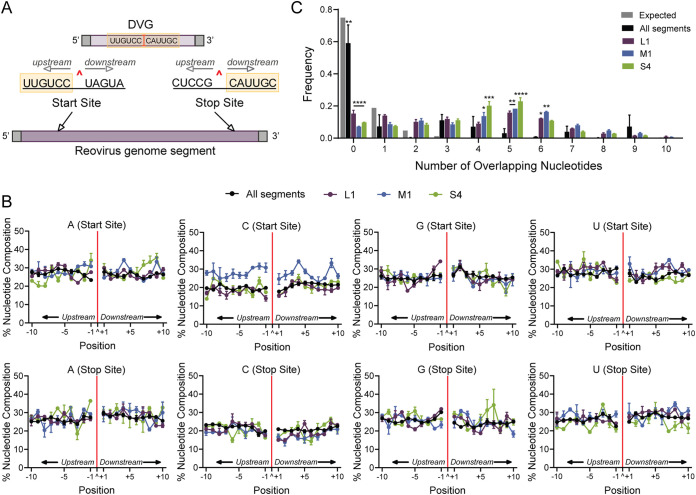
To test whether reovirus DVGs encode specific sequence preference, we extracted and quantified the upstream and downstream sequences flanking both the junction start and stop sites (Fig. 8A). The percent adenosine (A), cytosine (C), guanosine (G), and uracil (U) was calculated and plotted for each position in a 10-nucleotide (nt) window flanking the junction site represented by a red line and caret (^) (Fig. 8B). To exclude potential biases for overrepresented species, junctions were not weighted by depth. The global composition including all segments was compared to the L1, M1, and S4 genome segments individually (Fig. 8B). Neither the overall reovirus genome nor the L1, M1, and S4 segments displayed strong nucleotide preferences at recombination sites.
FIG 8.
rsT1L reovirus junction site composition. Nucleotide composition and sequence homology were quantified from junction start and stop sites. (A) Schematic of DVG recombination junctions in the context of the parental genome segment. The junction is labeled with a red caret (^), and example sequences are shown. DVG junction sites are characterized by a 5′ start site and a 3′ stop site. Sequences upstream of the start site and downstream of the stop site (orange highlight) form the junction-spanning sequence of the DVG. (B) Nucleotide composition was calculated as the percent adenosine (A), cytosine (C), guanine (G), and uracil (U) at each position in a 10-bp region surrounding the DVG start and stop sites. The junction is labeled as a caret (^) and denoted with a solid red line. Positions upstream (−10 to −1) and downstream (+1 to +10) of the junction position are indicated. Each point represents a mean (n = 2), and error bars represent standard error. (C) Sequence microhomology distributions of all rsT1L genome segments (black) and the L1 (purple), M1 (blue), and S4 (green) genome segments were compared to an expected probability distribution (gray). The frequency of each overlap is displayed as a mean (n = 2), and error bars represent standard error.
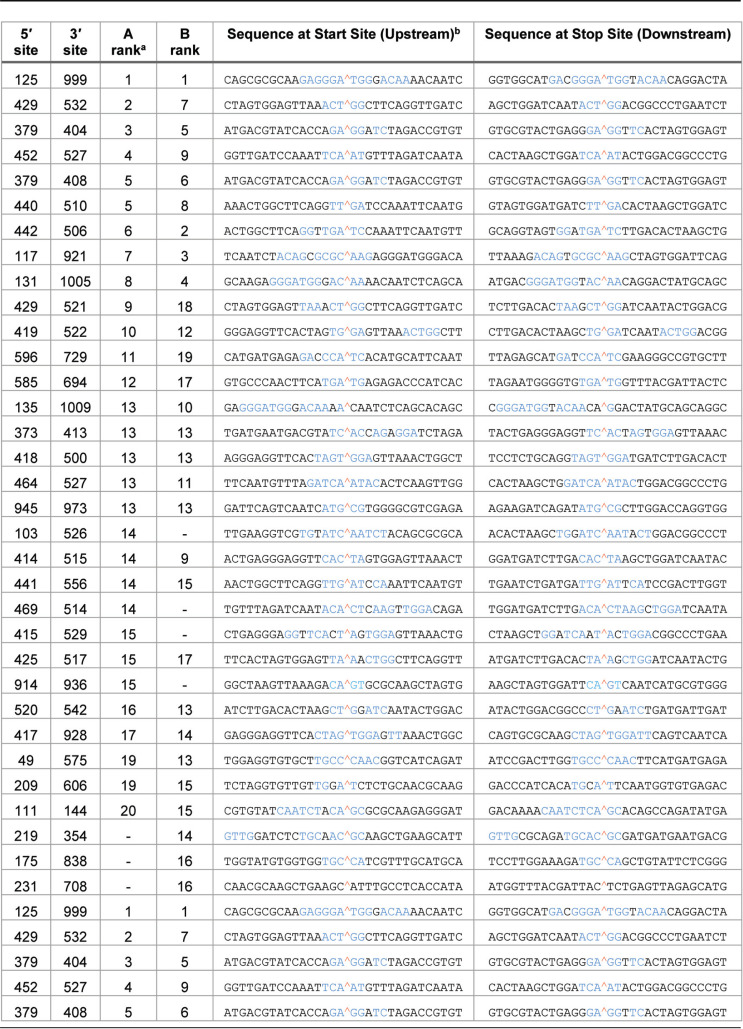
We further tested whether reovirus recombination occurs in regions of sequence microhomology, similar to other RNA viruses, such as dengue virus and Flock House virus (FHV) (46). Microhomology was defined as a region of identical sequence between 2 and 20 bp in length. When microhomology at ViReMa-detected junctions was compared to an expected probability distribution, there was significant enrichment for 4 to 6 bp of overlap at junction sites for segments L1, M1, and S4 (Fig. 8C). To ascertain any preference for sequence homology in the most frequently detected recombination junctions, we examined sequences upstream and downstream from the start and stop sites of the 25 most frequently identified S4 recombination junctions (Table 4). At least four nucleotides of identical sequence were detected for all but one of the top-ranking recombination junctions (Table 4). Typically, regions of sequence similarity spanned the recombination junction, such that sequences immediately surrounding the newly recombined junction site were identical to those at both the start and stop junction sites. Together, these observations suggest that RNA sequence homology plays a role in reovirus recombination site selection.
TABLE 4.
Nucleotides surrounding rsT1L S4 recombination junctions
Shown are sequences flanking the 25 most frequently detected recombination junctions from two rsT1L preparations, A and B, and rank based on read count.
Sequences colored blue indicate regions of identical sequence between start and stop sites. A red caret indicates the recombination site.
DISCUSSION
While the synthesis and packaging of noncanonical segments, including DVGs, have been reported for multisegmented, dsRNA viruses of the Reoviridae family (25, 27, 29–35, 37, 38), the frequency and mechanism of recombination yielding these RNA products are unknown. Here, we serially passaged two strains of reovirus and one strain of rotavirus in cultured cells at moderate to very high MOI and compared the virus titers and RNA segment profiles. We demonstrated that small, noncanonical RNA products that retain the termini and feature large internal deletions are synthesized frequently by both strains of reovirus (Fig. 2, 4, and 6). Some small reovirus DVGs were maintained throughout the passage series, while other DVGs were transient (Fig. 2 and 4). Transcriptome sequencing (RNA-Seq) analysis of RNA extracted from two independent preparations of purified, low-passage, benzonase-treated rsT1L reovirus revealed specific junction locations and recombination hot spots in individual genome segments (Fig. 7). Sequences surrounding the recombination junction sites in packaged rsT1L RNA revealed significant nucleotide overlap and regions of sequence microhomology at junction sites (Fig. 8C and Table 4). Together, these findings suggest that reovirus frequently synthesizes and packages defective genome segments featuring internal deletions and that recombination can be directed by sequence and occurs at distinct sites.
Multiple factors may influence virus titer during serial passage. T3D reovirus induces necrosis and substantially more apoptosis than T1L in L cells, and SA11 induces necrosis in MA104 cells (39–41). Once most cells in the culture have become infected, cell death may limit the peak titer that can be achieved for rsT3DI and rsSA11, which may in part explain their narrower titer ranges compared with rsT1L. Another potential influence on virus titer is defective interfering particles. A repeating pattern of dips and rebounds in titer has been reported for serially and continuously cultured viruses and associated with generation of defective interfering particles (4, 26, 36, 42, 47). DVGs may interfere with viral replication through competition with full-length genomes for the viral polymerase and structural proteins (48, 49). Small DVGs may be transcribed or replicated with higher efficiency than the full-length genome, permitting eventual monopolization of RNA-binding or enzymatic sites in viral proteins. Particles encapsidating defective genomes may also activate innate immune responses, interfering with viral replication (4). The frequency of the titer dips and rebounds of rsT3DI is more consistent with that previously reported for serially passaged bovine rotavirus than the frequency exhibited by rsT1L or rsSA11 (36). However, whether DVGs detected in the current study interfere with replication remains to be determined. Interestingly, viruses with the most consistent titer patterns among lineages, rsT1L and rsSA11, also had the most consistent RT-PCR profiles, while the virus with the most variability in titer among lineages, rsT3DI, also exhibited the most diverse RNA segment profiles, suggesting a potential link between these phenotypes (Fig. 1B to D and Fig. 4 and 5). We anticipate that the true diversity and abundance of DVGs present in reovirus and rotavirus populations is much greater than revealed by RT-PCR and electrophoretic analysis, which likely was limited to the most abundant populations of small DVGs with termini that match those of a specific genome segment.
Differences in DVG profiles among Reoviridae genera and reovirus strains detected in this study suggest underlying differences in recombination properties. It is unclear why serially passaged rsSA11 rotavirus lysates contained so few DVGs with matched segment termini and internal deletions, while such DVGs were detected for most rsT1L and rsT3DI reovirus segments in most passages (Fig. 2 and 5). For both reovirus and rotavirus, we detected putative noncanonical segments of greater length than the parental segment. In the literature, head-to-tail duplications (concatemers) resulting in longer segments have been most often reported for rotavirus, whereas internal deletions have been reported for reoviruses (25, 35, 37). Importantly, packaging of DVGs that lack part of the ORF, assuming they replace the parental segment, will yield defective virus particles that are unable to replicate independently. In contrast, rotavirus noncanonical segments containing partial duplications that maintain the ORF have been shown to be replication competent and genetically stable (35). Thus, these RNA species are anticipated to mediate very different downstream effects during infection. One potential explanation for recombination differences between reovirus and rotavirus is that cell type influences recombination outcomes. However, as small DVGs have been previously detected for reovirus and long noncanonical segments have been previously detected for rotavirus, a more likely explanation is that Reoviridae virion structure influences noncanonical RNA length. Reoviruses have turrets at the icosahedral vertices, through which newly synthesized viral +RNAs exit the particle, whereas rotaviruses are nonturreted Reoviridae family members. For both reovirus and rotavirus, the RNA-dependent RNA polymerase (RdRp) has a similar structure and occupies a space just inside and slightly off-center from the 5-fold icosahedral vertex (50–54). However, while RNA templates bind the reovirus RdRp in the correct register for initiation, viral RNA templates overshoot the rotavirus RdRp initiation register (53, 54). Additionally, while the reovirus turret performs most of the capping functions as viral transcripts exit the particle, the rotavirus capping enzyme resides in the interior of the core shell, adjacent to the RdRp (52, 55). These differences between turreted and nonturreted Reoviridae may influence RNA synthesis or recombination outcomes. Accordingly, like rotavirus, nonturreted orbiviruses package concatemers that are significantly longer than full-length segments (29). While specific mechanisms underlying differences in noncanonical viral segment synthesis remain unknown, infrequent detection of small rotavirus DVGs and frequent detection of small reovirus DVGs in this study (Fig. 2 and 5) are consistent with published findings.
It is unclear why rsT1L reovirus exhibited similar DVG profiles across lineages, while rsT3DI exhibited much greater variability (Fig. 4). Consistency in RT-PCR profiles among rsT1L lineages and the finding that junction sites in packaged rsT1L RNA from two different low-passage-number virus preparations were extremely well conserved support a model in which rsT1L recombination occurs at distinct sites and yields specific DVGs (Fig. 4 and 7B). Alternatively, rsT1L replication may tolerate DVG diversity poorly and limit the range of detected variants through purifying selection. The variability in RNA segment profiles among rsT3DI lineages indicates that the products are not random artifacts of detection and suggests reduced recombination specificity or increased recombination frequency compared to rsT1L (Fig. 4). For influenza virus, a segmented negative-strand RNA virus, the abundance of DVGs can be significantly affected by specific amino acid substitutions in the viral RdRp, suggesting an important role for this enzyme in DVG synthesis (56, 57). Further, recombination in other RNA viruses has been linked to the activity of the RdRps in resisting mutations (58–62). Thus, viral RdRps including the reovirus RdRp may encode several important, potentially interconnected functions, including RNA synthesis, high-fidelity replication, and recombination. These functions, including recombination, may function together to direct viral evolution. For reovirus, the viral RdRp also likely plays a prominent role in recombination potential, with other viral and host factors potentially influencing recombination properties. For example, viral RdRp cofactor μ2 has been shown to differentially influence T1L and T3D transcriptional activity and replication efficiency in specific cell types (63, 64). Thus, identifying key determinants of recombination and noncanonical segment synthesis will be important areas of future study.
In comparison to other recent reports on RNA virus recombination, reovirus demonstrated ∼37-fold-lower recombination frequency than coronavirus and ∼20-fold-lower recombination frequency than FHV (44, 65). While microhomology can occur as an artifact of chimeric reads generated in vitro during Illumina RNA-seq library preparation, artifactual recombination typically joins fragments in opposite orientations, involves short deletions, and lacks site specificity across the genome, whereas biological recombination by the virus often will join fragments in the same orientation and may occur at hot spots in the genome (43, 46, 65). Potential artifacts were removed by filtering for forward recombination junctions and removing short deletions from data sets presented here. Thus, despite the low reovirus recombination frequency detected in this study, the bioinformatic quality controls, orientation of joined fragments, and presence of hot spots at specific sites in the genome support the biological relevance for recombination detected by RNA-seq (Fig. 7). Detection and sequencing of DVGs containing large internal deletions following reovirus passage in cultured cells further support the validity of these findings (Fig. 2, 4, and 6). Recombination frequency for FHV increases during passage and may also do so for passaged reoviruses (65).
The molecular mechanism of reovirus recombination is unknown. For influenza virus, DVGs containing internal deletions are common, and the 5′ and 3′ sequences of a DVG are always derived from the same segment and polarity (66). While our methodology cannot exclude the presence of other types of DVGs, our deep-sequencing data and previously reported findings suggest that reovirus synthesizes DVGs that feature internal deletions and 5′ and 3′ termini from a single segment of the same polarity (Fig. 6) (25). Consistent with the detection of internal deletions of the same polarity, evidence suggests that each of the RdRp-containing replication complexes inside the core shell of rotavirus and cytoplasmic polyhedrosis virus, another Reoviridae virus, services only a single, dedicated RNA segment (67, 68). Influenza virus DVGs containing internal deletions are proposed to arise from pausing of the RdRp during nascent strand synthesis while continuing to process along the template molecule and then resuming synthesis at a downstream point on the template (1). Downstream RdRp reinitiation may be guided by complementarity between the nascent strand and the site of reinitiation on the template (69, 70). Given the similarity in DVG composition and conservation of sequences at junction sites detected for multiple segments (Fig. 6 and 8C and Table 4), reovirus may synthesize DVGs using a mechanism similar to that proposed for influenza virus. RT-PCR products differing in size from full-length rsT1L S2, which exhibited low recombination frequency across the segment, were not detected (Fig. 2A). However, such RT-PCR products were detected for L2 and S3, which also exhibited low overall recombination frequencies. Aside from the presence of small stretches of homologous direct repeats, it is unclear why recombination occurs more frequently at some sites than others throughout the rsT1L reovirus genome (Fig. 7C). It is possible that, in addition to sequence, +RNA secondary structure also contributes to recombination site selection. This hypothesis is supported by the observation that not all recombination sites contain direct repeat sequences (Tables 2 and 4).
Effects of noncanonical RNA species, including DVGs, during Reoviridae infection are poorly understood. In addition to their effects on viral populations and the host, studies of noncanonical segments will reveal principles governing assortment and packaging, which may enable more sophisticated engineering of Reoviridae-based vaccines and therapeutics using reverse genetics platforms. While passaging at relatively high MOI and detection by RT-PCR suggest the existence of small DVGs in reovirus populations, these approaches are relatively insensitive (Fig. 1 and 6). The frequent detection of novel junctions resulting from recombination events via next-generation sequencing in relatively low-passage-number reovirus stocks suggests that these events are common even in the absence of high-passage-number conditions (Fig. 7 and 8). Continued application of sensitive approaches will enable a deeper understanding of recombination frequency, and the identities of noncanonical RNA species, mechanisms by which they are generated, and their impacts on virus populations and the host.
MATERIALS AND METHODS
Cells.
Spinner-adapted L929 cells were grown in suspension culture in Joklik’s minimum essential medium (JMEM; U.S. Biological) supplemented to contain 5% fetal bovine serum (FBS) (Gibco) and 2 mM l-glutamine (Corning). All media were additionally supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin (Corning), and 25 ng/ml amphotericin B (Corning). All cells were maintained at 37°C with 5% CO2. Baby hamster kidney cells expressing T7 RNA polymerase under the control of a cytomegalovirus promoter (BHK-T7) (71) were maintained in Dulbecco’s minimum essential medium (DMEM; Corning) supplemented to contain 5% FBS (Gibco), with 1 mg/ml Geneticin (Gibco) added during alternate passages. MA104 cells were grown in minimum essential medium with Earle’s salts (EMEM; Corning) supplemented to contain 5% FBS.
Viruses.
Laboratory stocks of reovirus strains rsT1L and rsT3DI were engineered using plasmid-based reverse genetics (23, 71). rsT3DI differs from rsT3D in that it contains an engineered T249I mutation that prevents proteolytic cleavage of attachment protein σ1 (23). The T249I mutation was introduced in the pBacT7-S1T3D plasmid by ‘round the horn PCR (72) with mutagenic primers. Monolayers of BHK-T7 cells at ∼50% confluence in six-well plates were cotransfected using TransIT-LT1 transfection reagent (Mirus Bio LLC) with 0.8 μg of each of 10 plasmid constructs representing the T1L or T3DI reovirus genome. After 5 days of incubation, cells were lysed by two rounds of freezing and thawing, and rsT1L or rsT3DI in lysates was amplified once in L cells prior to use in serial passage experiments. Viral titers were determined by plaque assay using L cells as previously described (73).
A laboratory stock of rsSA11 was engineered using reverse genetics (74). Confluent monolayers of BHK-T7 cells in 12-well plates were cotransfected using TransIT-LT1 transfection reagent (Mirus Bio LLC) with 0.27 μg of each of 11 plasmid constructs representing the SA11 rotavirus genome and of each of two plasmids encoding vaccinia virus mRNA capping enzymes and 0.005 μg of a plasmid encoding the Nelson Bay virus fusion-associated small transmembrane protein. After 1 day of incubation, supernatants were removed and replaced with serum-free DMEM. After one additional day of incubation, supernatants were removed, and 3.3 × 104 MA104 cells in serum-free DMEM containing 0.5 μg/ml of trypsin (Worthington, bovine pancreas, chromatographically purified) were added to each well, followed by incubation at 37°C for 3 more days. Cells were then lysed by three rounds of freezing and thawing, and rsSA11 in lysates was amplified once in MA104 cells prior to use in serial passage experiments. Virus titer was determined by plaque assay using MA104 cells as previously described (75).
Reovirus serial passage in L cells.
For P1, 5 × 107 L cells, which support vigorous reovirus replication, were pelleted and adsorbed with rsT1L or rsT3DI in 10 ml JMEM at an MOI of 1 PFU/cell for 1 h at 37°C. Cell-virus mixtures were transferred to 1-liter bottles containing a magnetic stirring rod, and JMEM was added to a total volume of 100 ml. Spinner bottles were incubated at 37°C with stirring for 48 h prior to two rounds of freezing and thawing and removal of large cellular debris by centrifugation at 1,000 × g for 5 min. For subsequent passages (P2 to P10), an identical protocol was followed, except that 5 × 107 L cells were pelleted and resuspended in an adsorption inoculum of 10 ml of cleared lysate from the previous passage. For each virus, passages were conducted in triplicate lineages, which were maintained throughout the series. Virus titer in each passage for each lineage was determined by plaque assay (73). Average MOI from lin1 to lin3 for P2 to P10 was calculated based on the volume and titer of the inoculum (lysate from previous passage) divided by the number of cells adsorbed with inoculum.
Rotavirus serial passage in MA104 cells.
For P1, rsSA11 was activated by incubation with 10 μg/ml of trypsin for 1 h at 37°C and then diluted to a final concentration of <2 μg/ml of trypsin. A confluent monolayer of MA104 cells (∼1.5 × 107), which support vigorous rotavirus replication, in a T150 flask was washed with serum-free EMEM adsorbed with rsSA11 in 3 ml EMEM at an MOI of 0.25 PFU/cell for 1 h at 37°C. The inoculum was removed, and the monolayer was washed prior to the addition of 20 ml serum-free EMEM containing 0.5 μg/ml of trypsin. Cells were incubated at 37°C for 48 h prior to two rounds of freezing and thawing and removal of large cellular debris by centrifugation at 1,000 × g for 5 min. For subsequent passages (P2 to P10), an identical protocol was followed, except that 3 ml of cleared rsSA11 lysate from the previous serial passage was activated by incubation with 1 μg/ml of trypsin for 1 h at 37°C and then used as the adsorption inoculum for the subsequent passage. Passages were conducted in triplicate lineages, which were maintained throughout the series. Virus titer in each passage for each lineage was determined by fluorescent focus assay (FFA). The average MOI from lin1 to lin3 for P2 to P10 was calculated based on the volume and titer of the inoculum (lysate from previous passage) divided by the number of cells adsorbed with inoculum.
Quantitative reverse transcription-PCR.
RNA was isolated from cleared serial passage lysates using TRIzol LS reagent (Invitrogen), according to the manufacturer’s protocol. Equal volumes of RNA were quantified using the Superscript III First Strand cDNA Synthesis System (Invitrogen) and PowerUp SYBR green Master Mix (Applied Biosystems), according to the manufacturer’s protocols with minor modification. qPCRs were performed in duplicate. To quantify S4 RNA, we used random hexamers in the reverse transcription reaction to produce cDNA and used S4-specific primers in the qPCR (T1S4_995F [GGGATGGTACAACAGGACTATG] and T1S4_1111R [CGCCAATCATCACCGGATAA]). Primers were annealed at 65°C for 5 min and then cooled to 4°C. Reverse transcription was conducted with incubation at 50°C for 50 min. The reaction was terminated by incubation at 85°C for 5 min and then treated with RNase H. Subsequently, 40 cycles of qPCR were conducted at 95°C for 15 s, followed by incubation at 60°C for 1 min using a StepOnePlus real-time PCR system (Applied Biosciences). Product specificity was checked by dissociation curve analysis.
Rotavirus fluorescent focus assay.
MA104 cells were seeded into 96-well, black-walled plates to achieve a density of ∼2.25 × 104. Rotavirus stocks were activated by incubation with 10 μg/ml trypsin for 1 h at 37°C and then diluted serially in serum-free EMEM. Cells were washed twice with serum-free EMEM and then adsorbed with serial dilutions of rotavirus at 37°C for 1 h. Inocula were removed, and cells were washed and incubated in fresh medium at 37°C for 12 to 18 h. Cells were then fixed with cold methanol, and rotavirus proteins were detected by incubation with polyclonal rotavirus antiserum (Invitrogen) at a 1:1,000 dilution in phosphate-buffered saline (PBS) containing 0.5% Triton X-100 at 37°C, followed by incubation with Alexa Fluor 488-labeled secondary IgG (Invitrogen) and 4′,6′-diamidino-2-phenylindole (DAPI). Images were captured for four fields of view per well using an ImageXpress Micro XL automated microscope imager (Molecular Devices). Total and infected cells were quantified using MetaXpress high-content image acquisition and analysis software (Molecular Devices). The number of fluorescent focus units per milliliter of virus stock was calculated based on the surface area of the well quantified and virus inoculum volume and dilution.
RT-PCR and electrophoretic analysis of DVG profiles.
RNA was isolated from cleared serial passage lysates using TRIzol LS reagent (Invitrogen), according to the manufacturer’s protocol. Genome segments and DVGs were amplified using a OneStep RT-PCR kit (Qiagen), using the isolated serial passage RNA as a template and gene-specific primers that bind at or adjacent to the 5′ and 3′ RNA termini. Primer sequences are available upon request (45). Reaction and thermal cycler conditions were as described by the manufacturer. An extension time of 1 min was used for genome segments of <2 kb, and 3 min was used for segments of >2 kb. RT-PCR products were resolved by electrophoresis in 1.2% agarose in the presence of ethidium bromide and imaged with a VWR photo imager and variable intensity UV transilluminator. RT-PCR product sizes were analyzed in comparison to migration of markers in 100-bp and 1-kb ladders (New England Biolabs).
DVG sequencing.
Selected DNA fragments were excised from agarose gels, purified using a QIAquick gel extraction kit (Qiagen), and sequenced using the Sanger method (Genewiz) and the same primers used for RT-PCR amplification. Sequences were aligned to the reference viral genome using CLC Sequence Viewer 8 (Qiagen).
rsT1L purification, library preparation, and next-generation sequencing.
In two independent preparations, L cells (2 × 108) were pelleted and adsorbed with laboratory stocks of rsT1L reovirus in 20 ml JMEM at an MOI of 10 PFU/cell for 1 h at 37°C. Cell-virus mixtures were transferred to 1-liter bottles containing a magnetic stirring rod, and JMEM was added to a total volume of 400 ml. Spinner bottles were incubated at 37°C with stirring for 48 h, then cells were pelleted by centrifugation at 1,800 × g for 10 min at 4°C. Cell pellets were resuspended in homogenization buffer (25 mM NaCl, 10 mM Tris-HCl [pH 7.4], 10 mM β-mercaptoethanol) and frozen at −80°C. Cell pellets were thawed and incubated with 0.14% (vol/vol) deoxycholate for 30 min on ice prior to the addition of Vertrel XF and sonication. Virus particles were concentrated by centrifugation at 25,000 × g for ∼16 h in a 1.2- to 1.4-g/cm3 cesium chloride density gradient. The reovirus-containing fraction was collected and dialyzed at 4°C in virion storage buffer (150 mM NaCl, 15 mM MgCl, 10 mM Tris-HCl [pH 7.4]). Purified rsT1L particles were treated with a final concentration of 1 U/μl benzonase (Millipore) for 1 h at 37°C to degrade extraparticle nucleic acids. RNA was isolated from benzonase-treated particles using TRIzol LS reagent (Invitrogen) according to the manufacturer’s protocol.
RNA libraries were prepared from RNA isolated from cesium chloride gradient-purified, benzonase-treated rsT1L virions. In accord with the manufacturer’s instructions for NEBNext Ultra II RNA library preparation, contaminating DNA was degraded by treatment with RNase-free DNase I (New England Biolabs) for 10 min at 37°C. RNA was isolated by using TRIzol LS reagent (Invitrogen), according to the manufacturer’s protocol, and the concentration and quality of RNA were quantified using a Bioanalyzer (Agilent). Library preparation for Illumina sequencing was performed using the NEBNext Ultra II RNA Library Prep kit for Illumina (New England Biolabs) and a minimum of 5 ng of RNA according to the manufacturer’s instructions. Briefly, RNA was fragmented prior to first-strand and second-strand synthesis and RNAClean XP (Beckman Coulter) purification. PCR enrichment of adapter-ligated DNA was performed using NEBNext Multiplex Oligos for Illumina (New England Biolabs) to produce Illumina-ready libraries. Libraries were sequenced by 150-bp paired-end sequencing on a NovaSeq 6000 sequencing system (Illumina). Assistance with quality control and next-generation sequencing was provided by the Vanderbilt Technologies for Advanced Genomics (VANTAGE) research core.
Illumina RNA-seq processing and alignment.
Raw reads were processed by first removing the Illumina TruSeq adapter using Trimmomatic (76) default settings (command line parameters java -jar trimmomatic.jar PE sample_R1.fastq.gz sample_R2.fastq.gz output_paired_R1.fastq output_unpaired_R1.fastq output_paired_R2.fastq output_unpaired_R2_unpaired.fastq ILLUMINACLIP:TruSeq3-PE.fa:2:30:10 LEADING:3 TRAILING:3 SLIDINGIWINDOW:4:15 MINLEN:36). Reads shorter than 36 bp were removed and low-quality bases (Q score < 30) were trimmed from read ends. The raw FASTQ files were aligned to the reovirus genome segments (NCBI or GenBank accession numbers M24734.1, AF378003.1, AF129820.1, AF461682.1, AF490617.1, AF174382.1, M14779.1, L19774.1, M14325.1, and M13139.1) using the Python2 script ViReMa (Viral Recombination Mapper, version 0.15) (43) using the command line parameters python2 ViReMa.py reference_index input.fastq output.sam --OuputDir sample_virema/--OutputTag sample_virema -BED --MicroIndelLength 5. The sequence alignment map (SAM) file was processed using the samtools (77) suite to calculate nucleotide depth at each position in a sorted binary alignment map (BAM) file (using command line parameters samtools depth -a -m 0 sample_virema.sorted.bam > sample_virema.coverage).
Recombination junction analysis.
Recombination junctions were first filtered in the forward (5′ → 3′) direction using the dpylr package (RStudio). The frequency of each junction was calculated by comparing the depth of the unique junction to the total number of nucleotides in all detected junctions in a library. Junctions were plotted according to the genomic position and colored according to log10 of the frequency using ggplot2 in RStudio. Recombination frequency was calculated at each genomic position by dividing the number of nucleotides in any junction mapping to the position divided by the total number of nucleotides sequenced at the position.
Nucleotide composition analysis and sequence microhomology quantification.
Nucleotide composition at each position surrounding DVG junctions was determined. To avoid bias of highly replicated DVGs and to more closely reflect the stochastic nature of RNA recombination, each unique detected junction was counted equally rather than weighting by read count (65). Sequences were extracted from a sorted BED file listing the junctions using Rec_Site_Extraction.py with a 30-bp window. Start site and stop site sequences were separated in Microsoft Excel, and the nucleotide frequency at each position was calculated using the Biostrings (78) package in RStudio (78). The length of microhomology at junction sites was extracted from ViReMa SAM files using the Compiler_Module.py of ViReMa and -FuzzEntry --Defuzz 0 flags. The frequency of overlaps ranging from 0 to 10 bp was calculated and compared to an expected probability distribution using uHomology.py.
For junction analyses shown in Tables 2 and 4, regions of sequence similarity were defined as stretches of at least four sequential, identical nucleotides located in the same position or removed by a single nucleotide, relative to the junction site. A single mismatch, shown in black, was tolerated in identical sequences longer than four nucleotides, if at least two additional identical nucleotides were adjacent to it.
Data availability.
Data generated from Illumina RNA-seq can be accessed at the NCBI Sequence Read Archive (SRA) under BioProject accession number PRJNA669717. Code utilized in this report can be accessed at https://github.com/DenisonLabVU/rna-seq-pipeline.
ACKNOWLEDGMENTS
We thank the staff at VANTAGE for assistance with next-generation sequencing and James Chappell for critical reading of the manuscript.
This work was supported by U.S. Public Health Service Awards T32 AI112541 (to S.C.S.), T32 GM065086 (to J.G.), and F31 AI147560 (to J.G.) and by Clinical and Translational Science Award UL1 TR002243 from the National Center for Advancing Translational Sciences (to K.M.O.). Contents are solely the responsibility of the authors and do not necessarily represent official views of the National Institutes of Health or the National Center for Advancing Translational Sciences.
REFERENCES
- 1.Alnaji FG, Brooke CB. 2020. Influenza virus DI particles: defective interfering or delightfully interesting? PLoS Pathog 16:e1008436. doi: 10.1371/journal.ppat.1008436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vignuzzi M, Lopez CB. 2019. Defective viral genomes are key drivers of the virus-host interaction. Nat Microbiol 4:1075–1087. doi: 10.1038/s41564-019-0465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poirier EZ, Vignuzzi M. 2017. Virus population dynamics during infection. Curr Opin Virol 23:82–87. doi: 10.1016/j.coviro.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Rezelj VV, Levi LI, Vignuzzi M. 2018. The defective component of viral populations. Curr Opin Virol 33:74–80. doi: 10.1016/j.coviro.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 5.GBD 2015 Child Mortality Collaborators. 2016. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1725–1774. doi: 10.1016/S0140-6736(16)31575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouziat R, Hinterleitner R, Brown JJ, Stencel-Baerenwald JE, Ikizler M, Mayassi T, Meisel M, Kim SM, Discepolo V, Pruijssers AJ, Ernest JD, Iskarpatyoti JA, Costes LM, Lawrence I, Palanski BA, Varma M, Zurenski MA, Khomandiak S, McAllister N, Aravamudhan P, Boehme KW, Hu F, Samsom JN, Reinecker HC, Kupfer SS, Guandalini S, Semrad CE, Abadie V, Khosla C, Barreiro LB, Xavier RJ, Ng A, Dermody TS, Jabri B. 2017. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science 356:44–50. doi: 10.1126/science.aah5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attoui H, Becnel J, Belaganahalli S, Bergoin M, Brussaard CP, Chappell JD, Ciarlet M, del Vas M, Dermody TS, Dormitzer PR, Duncan R, Fang Q, Graham R, Guglielmi KM, Harding RM, Hillman B, Makkay A, Marzachì C, Matthijnssens J, Mertens PPC, Milne RG, Mohd Jaafar F, Mori H, Noordeloos AA, Omura T, Patton JT, Rao S, Maan M, Stoltz D, Suzuki N, Upadhyaya NM, Wei C, Zhou H. 2012. Part II: The viruses – the double stranded RNA viruses - family Reoviridae, p 541–637. In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (ed), Virus taxonomy: classification and nomenclature: ninth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego, CA. [Google Scholar]
- 8.Guglielmi KM, McDonald SM, Patton JT. 2010. Mechanism of intraparticle synthesis of the rotavirus double-stranded RNA genome. J Biol Chem 285:18123–18128. doi: 10.1074/jbc.R110.117671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald SM, Patton JT. 2011. Assortment and packaging of the segmented rotavirus genome. Trends Microbiol 19:136–144. doi: 10.1016/j.tim.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy P. 2017. Bluetongue virus structure and assembly. Curr Opin Virol 24:115–123. doi: 10.1016/j.coviro.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Trask SD, McDonald SM, Patton JT. 2012. Structural insights into the coupling of virion assembly and rotavirus replication. Nat Rev Microbiol 10:165–177. doi: 10.1038/nrmicro2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dermody TS, Parker JS, Sherry B. 2013. Orthoreoviruses, p 1304–1346. In Knipe DM, Howley PM (ed), Fields virology, 6th ed, vol 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 13.Estes MK, Greenberg HB. 2013. Rotaviruses, p 1347–1401. In Knipe DM, Howley PM (ed), Fields virology, 6th ed, vol 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 14.Roy P. 2013. Orbiviruses, p 1402–1423. In Knipe DM, Howley PM (ed), Fields virology, 6th ed, vol 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 15.Biswas S, Li W, Manktelow E, Lever J, Easton LE, Lukavsky PJ, Desselberger U, Lever AM. 2014. Physicochemical analysis of rotavirus segment 11 supports a ‘modified panhandle’ structure and not the predicted alternative tRNA-like structure (TRLS). Arch Virol 159:235–248. doi: 10.1007/s00705-013-1802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chapell JD, Goral MI, Rodgers SE, dePamphilis CW, Dermody TS. 1994. Sequence diversity within the reovirus S2 gene: reovirus genes reassort in nature, and their termini are predicted to form a panhandle motif. J Virol 68:750–756. doi: 10.1128/JVI.68.2.750-756.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, Manktelow E, von Kirchbach JC, Gog JR, Desselberger U, Lever AM. 2010. Genomic analysis of codon, sequence and structural conservation with selective biochemical-structure mapping reveals highly conserved and dynamic structures in rotavirus RNAs with potential cis-acting functions. Nucleic Acids Res 38:7718–7735. doi: 10.1093/nar/gkq663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuo E, Roy P. 2009. Bluetongue virus VP6 acts early in the replication cycle and can form the basis of chimeric virus formation. J Virol 83:8842–8848. doi: 10.1128/JVI.00465-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borodavka A, Dykeman EC, Schrimpf W, Lamb DC. 2017. Protein-mediated RNA folding governs sequence-specific interactions between rotavirus genome segments. Elife 6:e27453. doi: 10.7554/eLife.27453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bravo JPK, Bartnik K, Venditti L, Gail EH, Davidovich C, Lamb DC, Tuma R, Calabrese AN, Borodavka A. 2020. Structural basis of rotavirus RNA chaperone displacement and RNA annealing. bioRxiv 10.1101/2020.10.26.354233. [DOI] [PMC free article] [PubMed]
- 21.Bravo JPK, Borodavka A, Barth A, Calabrese AN, Mojzes P, Cockburn JJB, Lamb DC, Tuma R. 2018. Stability of local secondary structure determines selectivity of viral RNA chaperones. Nucleic Acids Res 46:7924–7937. doi: 10.1093/nar/gky394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demidenko AA, Blattman JN, Blattman NN, Greenberg PD, Nibert ML. 2013. Engineering recombinant reoviruses with tandem repeats and a tetravirus 2A-like element for exogenous polypeptide expression. Proc Natl Acad Sci U S A 110:E1867–E1876. doi: 10.1073/pnas.1220107110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi T, Antar AA, Boehme KW, Danthi P, Eby EA, Guglielmi KM, Holm GH, Johnson EM, Maginnis MS, Naik S, Skelton WB, Wetzel JD, Wilson GJ, Chappell JD, Dermody TS. 2007. A plasmid-based reverse genetics system for animal double-stranded RNA viruses. Cell Host Microbe 1:147–157. doi: 10.1016/j.chom.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roner MR, Joklik WK. 2001. Reovirus reverse genetics: incorporation of the CAT gene into the reovirus genome. Proc Natl Acad Sci U S A 98:8036–8041. doi: 10.1073/pnas.131203198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou S, Brown EG. 1992. Identification of sequence elements containing signals for replication and encapsidation of the reovirus M1 genome segment. Virology 186:377–388. doi: 10.1016/0042-6822(92)90003-8. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed R, Graham AF. 1977. Persistent infections in L cells with temperature-sensitive mutants of reovirus. J Virol 23:250–262. doi: 10.1128/JVI.23.2.250-262.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alam MM, Kobayashi N, Ishino M, Nagashima S, Paul SK, Chawla-Sarkar M, Krishnan T, Naik TN. 2008. Identical rearrangement of NSP3 genes found in three independently isolated virus clones derived from mixed infection and multiple passages of rotaviruses. Arch Virol 153:555–559. doi: 10.1007/s00705-007-0004-7. [DOI] [PubMed] [Google Scholar]
- 28.Boyce M, McCrae MA, Boyce P, Kim JT. 2016. Inter-segment complementarity in orbiviruses: a driver for co-ordinated genome packaging in the Reoviridae? J Gen Virol 97:1145–1157. doi: 10.1099/jgv.0.000400. [DOI] [PubMed] [Google Scholar]
- 29.Eaton BT, Gould AR. 1987. Isolation and characterization of orbivirus genotypic variants. Virus Res 6:363–382. doi: 10.1016/0168-1702(87)90067-0. [DOI] [PubMed] [Google Scholar]
- 30.Gault E, Schnepf N, Poncet D, Servant A, Teran S, Garbarg-Chenon A. 2001. A human rotavirus with rearranged genes 7 and 11 encodes a modified NSP3 protein and suggests an additional mechanism for gene rearrangement. J Virol 75:7305–7314. doi: 10.1128/JVI.75.16.7305-7314.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kojima K, Taniguchi K, Kawagishi-Kobayashi M, Matsuno S, Urasawa S. 2000. Rearrangement generated in double genes, NSP1 and NSP3, of viable progenies from a human rotavirus strain. Virus Res 67:163–171. doi: 10.1016/s0168-1702(00)00139-8. [DOI] [PubMed] [Google Scholar]
- 32.Ramig RF, Samal SK, McConnell S. 1985. Genome RNAS of virulent and attenuated strains of bluetongue virus serotypes 10, 11, 13 and 17. Prog Clin Biol Res 178:389–396. [PubMed] [Google Scholar]
- 33.Schnepf N, Deback C, Dehee A, Gault E, Parez N, Garbarg-Chenon A. 2008. Rearrangements of rotavirus genomic segment 11 are generated during acute infection of immunocompetent children and do not occur at random. J Virol 82:3689–3696. doi: 10.1128/JVI.01770-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spandidos DA, Graham AF. 1976. Generation of defective virus after infection of newborn rats with reovirus. J Virol 20:234–247. doi: 10.1128/JVI.20.1.234-247.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desselberger U. 1996. Genome rearrangements of rotaviruses. Adv Virus Res 46:69–95. doi: 10.1016/s0065-3527(08)60070-6. [DOI] [PubMed] [Google Scholar]
- 36.Hundley F, Biryahwaho B, Gow M, Desselberger U. 1985. Genome rearrangements of bovine rotavirus after serial passage at high multiplicity of infection. Virology 143:88–103. doi: 10.1016/0042-6822(85)90099-6. [DOI] [PubMed] [Google Scholar]
- 37.Ni Y, Kemp MC. 1994. Subgenomic S1 segments are packaged by avian reovirus defective interfering particles having an S1 segment deletion. Virus Res 32:329–342. doi: 10.1016/0168-1702(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 38.Nonoyama M, Watanabe Y, Graham AF. 1970. Defective virions of reovirus. J Virol 6:226–236. doi: 10.1128/JVI.6.2.226-236.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berger AK, Danthi P. 2013. Reovirus activates a caspase-independent cell death pathway. mBio 4:e00178-13. doi: 10.1128/mBio.00178-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tyler KL, Squier MK, Rodgers SE, Schneider BE, Oberhaus SM, Grdina TA, Cohen JJ, Dermody TS. 1995. Differences in the capacity of reovirus strains to induce apoptosis are determined by the viral attachment protein sigma 1. J Virol 69:6972–6979. doi: 10.1128/JVI.69.11.6972-6979.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castilho JG, Botelho MV, Lauretti F, Taniwaki N, Linhares RE, Nozawa C. 2004. The in vitro cytopathology of a porcine and the simian (SA-11) strains of rotavirus. Mem Inst Oswaldo Cruz 99:313–317. doi: 10.1590/s0074-02762004000300013. [DOI] [PubMed] [Google Scholar]
- 42.Bangham CR, Kirkwood TB. 1993. Defective interfering particles and virus evolution. Trends Microbiol 1:260–264. doi: 10.1016/0966-842X(93)90048-V. [DOI] [PubMed] [Google Scholar]
- 43.Routh A, Johnson JE. 2014. Discovery of functional genomic motifs in viruses with ViReMa–a Virus Recombination Mapper–for analysis of next-generation sequencing data. Nucleic Acids Res 42:e11. doi: 10.1093/nar/gkt916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gribble J, Stevens LJ, Agostini ML, Anderson-Daniels J, Chappell JD, Lu X, Pruijssers AJ, Routh AL, Denison MR. 2021. The coronavirus proofreading exoribonuclease mediates extensive viral recombination. PLoS Pathog 17:e1009226. doi: 10.1371/journal.ppat.1009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith S, Gribble J, Diller JR, Wiebe MR, Thoner TW, Denison MR, Ogden KM. 2020. Reovirus RNA recombination is sequence directed and generates internally deleted defective genome segments during passage. bioRxiv 10.1101/2020.10.19.346031. [DOI] [PMC free article] [PubMed]
- 46.Peccoud J, Lequime S, Moltini-Conclois I, Giraud I, Lambrechts L, Gilbert C. 2018. A survey of virus recombination uncovers canonical features of artificial chimeras generated during deep sequencing library preparation. G3 (Bethesda) 8:1129–1138. doi: 10.1534/g3.117.300468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tapia F, Laske T, Wasik MA, Rammhold M, Genzel Y, Reichl U. 2019. Production of defective interfering particles of influenza A virus in parallel continuous cultures at two residence times–insights from qPCR measurements and viral dynamics modeling. Front Bioeng Biotechnol 7:275. doi: 10.3389/fbioe.2019.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Portner A, Kingsbury DW. 1971. Homologous interference by incomplete Sendai virus particles: changes in virus-specific ribonucleic acid synthesis. J Virol 8:388–394. doi: 10.1128/JVI.8.4.388-394.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang AS, Baltimore D. 1970. Defective viral particles and viral disease processes. Nature 226:325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- 50.Ding K, Celma CC, Zhang X, Chang T, Shen W, Atanasov I, Roy P, Zhou ZH. 2019. In situ structures of rotavirus polymerase in action and mechanism of mRNA transcription and release. Nat Commun 10:2216. doi: 10.1038/s41467-019-10236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jenni S, Salgado EN, Herrmann T, Li Z, Grant T, Grigorieff N, Trapani S, Estrozi LF, Harrison SC. 2019. In situ structure of rotavirus VP1 RNA-dependent RNA polymerase. J Mol Biol 431:3124–3138. doi: 10.1016/j.jmb.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X, Walker SB, Chipman PR, Nibert ML, Baker TS. 2003. Reovirus polymerase lambda 3 localized by cryo-electron microscopy of virions at a resolution of 7.6 A. Nat Struct Biol 10:1011–1018. doi: 10.1038/nsb1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu X, McDonald SM, Tortorici MA, Tao YJ, Vasquez-Del Carpio R, Nibert ML, Patton JT, Harrison SC. 2008. Mechanism for coordinated RNA packaging and genome replication by rotavirus polymerase VP1. Structure 16:1678–1688. doi: 10.1016/j.str.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tao Y, Farsetta DL, Nibert ML, Harrison SC. 2002. RNA synthesis in a cage–structural studies of reovirus polymerase lambda3. Cell 111:733–745. doi: 10.1016/S0092-8674(02)01110-8. [DOI] [PubMed] [Google Scholar]
- 55.Kumar D, Yu X, Crawford SE, Moreno R, Jakana J, Sankaran B, Anish R, Kaundal S, Hu L, Estes MK, Wang Z, Prasad BVV. 2020. 2.7 Å cryo-EM structure of rotavirus core protein VP3, a unique capping machine with a helicase activity. Sci Adv 6:eaay6410. doi: 10.1126/sciadv.aay6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fodor E, Mingay LJ, Crow M, Deng T, Brownlee GG. 2003. A single amino acid mutation in the PA subunit of the influenza virus RNA polymerase promotes the generation of defective interfering RNAs. J Virol 77:5017–5020. doi: 10.1128/jvi.77.8.5017-5020.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vasilijevic J, Zamarreno N, Oliveros JC, Rodriguez-Frandsen A, Gomez G, Rodriguez G, Perez-Ruiz M, Rey S, Barba I, Pozo F, Casas I, Nieto A, Falcon A. 2017. Reduced accumulation of defective viral genomes contributes to severe outcome in influenza virus infected patients. PLoS Pathog 13:e1006650. doi: 10.1371/journal.ppat.1006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Domingo E, Holland JJ. 1997. RNA virus mutations and fitness for survival. Annu Rev Microbiol 51:151–178. doi: 10.1146/annurev.micro.51.1.151. [DOI] [PubMed] [Google Scholar]
- 59.Kempf BJ, Peersen OB, Barton DJ. 2016. Poliovirus polymerase Leu420 facilitates RNA recombination and ribavirin resistance. J Virol 90:8410–8421. doi: 10.1128/JVI.00078-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kempf BJ, Watkins CL, Peersen OB, Barton DJ. 2019. Picornavirus RNA recombination counteracts error catastrophe. J Virol 93:e00652-19. doi: 10.1128/JVI.00652-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li C, Wang H, Shi J, Yang D, Zhou G, Chang J, Cameron CE, Woodman A, Yu L. 2019. Senecavirus-specific recombination assays reveal the intimate link between polymerase fidelity and RNA recombination. J Virol 93:e00576-19. doi: 10.1128/JVI.00576-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poirier EZ, Mounce BC, Rozen-Gagnon K, Hooikaas PJ, Stapleford KA, Moratorio G, Vignuzzi M. 2015. Low-fidelity polymerases of alphaviruses recombine at higher rates to overproduce defective interfering particles. J Virol 90:2446–2454. doi: 10.1128/JVI.02921-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ooms LS, Kobayashi T, Dermody TS, Chappell JD. 2010. A post-entry step in the mammalian orthoreovirus replication cycle is a determinant of cell tropism. J Biol Chem 285:41604–41613. doi: 10.1074/jbc.M110.176255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yin P, Cheang M, Coombs KM. 1996. The M1 gene is associated with differences in the temperature optimum of the transcriptase activity in reovirus core particles. J Virol 70:1223–1227. doi: 10.1128/JVI.70.2.1223-1227.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jaworski E, Routh A. 2017. Parallel ClickSeq and Nanopore sequencing elucidates the rapid evolution of defective-interfering RNAs in Flock House virus. PLoS Pathog 13:e1006365. doi: 10.1371/journal.ppat.1006365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lazzarini RA, Keene JD, Schubert M. 1981. The origins of defective interfering particles of the negative-strand RNA viruses. Cell 26:145–154. doi: 10.1016/0092-8674(81)90298-1. [DOI] [PubMed] [Google Scholar]
- 67.Cui Y, Zhang Y, Zhou K, Sun J, Zhou ZH. 2019. Conservative transcription in three steps visualized in a double-stranded RNA virus. Nat Struct Mol Biol 26:1023–1034. doi: 10.1038/s41594-019-0320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Periz J, Celma C, Jing B, Pinkney JN, Roy P, Kapanidis AN. 2013. Rotavirus mRNAS are released by transcript-specific channels in the double-layered viral capsid. Proc Natl Acad Sci U S A 110:12042–12047. doi: 10.1073/pnas.1220345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lui WY, Yuen CK, Li C, Wong WM, Lui PY, Lin CH, Chan KH, Zhao H, Chen H, To KKW, Zhang AJX, Yuen KY, Kok KH. 2019. SMRT sequencing revealed the diversity and characteristics of defective interfering RNAs in influenza A (H7N9) virus infection. Emerg Microbes Infect 8:662–674. doi: 10.1080/22221751.2019.1611346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saira K, Lin X, DePasse JV, Halpin R, Twaddle A, Stockwell T, Angus B, Cozzi-Lepri A, Delfino M, Dugan V, Dwyer DE, Freiberg M, Horban A, Losso M, Lynfield R, Wentworth DN, Holmes EC, Davey R, Wentworth DE, Ghedin E, INSIGHT FLU003 Study Group. 2013. Sequence analysis of in vivo defective interfering-like RNA of influenza A H1N1 pandemic virus. J Virol 87:8064–8074. doi: 10.1128/JVI.00240-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kobayashi T, Ooms LS, Ikizler M, Chappell JD, Dermody TS. 2010. An improved reverse genetics system for mammalian orthoreoviruses. Virology 398:194–200. doi: 10.1016/j.virol.2009.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hemsley A, Arnheim N, Toney MD, Cortopassi G, Galas DJ. 1989. A simple method for site-directed mutagenesis using the polymerase chain reaction. Nucleic Acids Res 17:6545–6551. doi: 10.1093/nar/17.16.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berard A, Coombs KM. 2009. Mammalian reoviruses: propagation, quantification, and storage. Curr Protoc Microbiol Chapter 15:Unit15C.1. doi: 10.1002/9780471729259.mc15c01s14. [DOI] [PubMed] [Google Scholar]
- 74.Kanai Y, Komoto S, Kawagishi T, Nouda R, Nagasawa N, Onishi M, Matsuura Y, Taniguchi K, Kobayashi T. 2017. Entirely plasmid-based reverse genetics system for rotaviruses. Proc Natl Acad Sci U S A 114:2349–2354. doi: 10.1073/pnas.1618424114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arnold M, Patton JT, McDonald SM. 2009. Culturing, storage, and quantification of rotaviruses. Curr Protoc Microbiol Chapter 15:Unit 15C.13. doi: 10.1002/9780471729259.mc15c03s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pagès H, Aboyoun P, Gentleman R, DebRoy S. 2020. Biostrings: efficient manipulation of biological strings. R package version 2560.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated from Illumina RNA-seq can be accessed at the NCBI Sequence Read Archive (SRA) under BioProject accession number PRJNA669717. Code utilized in this report can be accessed at https://github.com/DenisonLabVU/rna-seq-pipeline.