Abstract
In clinical trials evaluating HIV-1 prevention products, ex vivo exposure of mucosal tissue to HIV-1 is performed to inform drug levels needed to suppress viral infection. Understanding assay and participant variables that influence HIV-1 replication will help with assay implementation. Demographic and behavioral data were obtained from 61 healthy women aged 21–45. Paired cervical tissue (CT) and vaginal tissue (VT) biopsies were collected and treated with HIV-1BaL or HIV-1JR-CSF, washed, and cultured. On days 3, 7, and/or 11, culture supernatant was collected, and viral replication was monitored by p24 ELISA. Tissue was extracted at study end, and HIV-1 relative RNA copies were determined by polymerase chain reaction. Cumulative p24 and RNA were log-transformed and analyzed using a linear mixed model, t-test, and an intraclass correlation coefficient (ICC). HIV replication was similar between CT and VT for each virus, but HIV-1BaL had 1.5 log10 and 0.9 log10 higher levels of p24 than HIV-1JR-CSF in CT and VT, respectively (p < .001), which correlated with HIV-1 relative RNA copies. Cumulative p24 and RNA copies in both tissues demonstrated low intraperson correlation for both viruses (ICC ≤0.513 HIV-1BaL; ICC ≤0.419 HIV-1JR-CSF). Enrollment into previous clinical studies in which genital biopsies were collected modestly decreased the HIV-1BaL cumulative p24 for CT, but not for VT. To improve the ex vivo challenge assay, viruses should be evaluated for replication in mucosal tissue before study implementation, baseline mucosal tissue is not needed if a placebo/no treatment group is included within the clinical trial, and previous biopsy sites should be avoided.
Keywords: : ex vivo challenge assay, pharmacodynamics, HIV prevention, efficacy biomarker
Introduction
In recent HIV prevention phase 1/2 clinical trials, emphasis has been placed on developing pharmacokinetic (PK)/pharmacodynamic (PD) relationships to define effective concentrations of drug needed to prevent HIV infection. The PD data are generated using mucosal tissue biopsies obtained from trial participants and exposed to HIV in the laboratory, termed the “ex vivo challenge assay.” Viral replication is monitored after a short culture period (11–14 days) through the detection of a viral protein, p24, in the culture supernatant. Drug effectiveness is assessed by comparing p24 concentrations from baseline tissue (before product use) or placebo product user's tissue to p24 concentrations from active product user's tissue. However, the capacity of mucosal tissue collected from baseline visits or placebo users to robustly replicate HIV-1 has shown a great deal of heterogeneity as is exemplified by cumulative p24 values which range over 4 log10.1–9 The p24 heterogeneity in colorectal tissue is tempered somewhat due to the greater numbers of tissue biopsies that are collected for PD assessments (up to 4) and are averaged.1,2,8,9 For cervical tissue (CT) and vaginal tissue (VT), a single biopsy is used for PD assessments, and the breadth of p24 values is greater than colorectal tissue.3–5,7 This is due to the limited numbers of cervical/vaginal biopsies that can be collected at any one visit for each participant during the clinical trial.
Understanding the inherent variability of HIV-1 replication in mucosal tissue is important for defining effective concentrations of antiretroviral drugs. If tissues from baseline or placebo/untreated group do not demonstrate sufficient viral replication, the effective concentration of an active product may be more difficult to determine. Several variables could impact the interpretation of the ex vivo challenge assay results. These include those associated with the assay directly, such as the viral isolate used to infect mucosal tissue and the number of days the supernatant is collected, and/or those associated with the study participants, such as the number of biopsies collected per participant and whether mucosal biopsies have been taken in previous studies. Our goal was to systematically evaluate these variables to inform the use of the ex vivo challenge assay in future clinical studies intended to define a biomarker of drug efficacy for HIV-1 prevention.
Materials and Methods
Reagents
Tissue culture base medium was purchased from Mediatech, Inc. (Manassas, VA); supplements were purchased from Gemini Bio-Products (West Sacramento, CA) or Lonza (Walkersville, MD); and interleukin-2 was purchased from Roche (Indianapolis, IN). HIV-1BaL was expanded in activated human peripheral blood mononuclear cells (PBMCs; Central Blood Bank, Pittsburgh, PA), and HIV-1JR-CSF was an infectious molecular clone transfected into 293T cells (gift from C. Ochsenbauer, University of Alabama, Birmingham); both viruses were obtained from the NIH AIDS Reagent Program. Tissue culture infectious doses at 50% (TCID50) were determined in PBMCs.10
Participant recruitment and tissue collection
Healthy women aged 18–45 years old provided written informed consent (PRO13120447; approved by the University of Pittsburgh Institutional Review Board). The participants were free from abnormal vaginal discharge and sexually transmitted diseases, including Trichomonas vaginalis, Neisseria gonorrhoeae, and Chlamydia trachomatis. HIV rapid tests confirmed their negative serostatus. Pregnant and menopausal women were excluded. Demographic (race/ethnicity) and behavioral data were collected. The behavioral data encompassed the date of their most recent vaginal intercourse, participation in previous clinical trials in which genital biopsies were collected, and smoking status.
Using Tischler forceps, full-thickness tissue biopsies measuring 5 × 3 mm were obtained from the ectocervix (CT) with a weight of 15.5 ± 28.6 mg (mean ± standard deviation) and from the proximal vaginal wall (VT) with a weight of 9.9 ± 5.6 mg. The CT and VT were brought to the laboratory within 30 min of collection.
HIV replication in mucosal tissue
For the first 31 women, 4 CT and 4 VT were collected; 2 of each CT and VT were exposed separately to 5 × 104 TCID50 of HIV-1BaL or HIV-1JR-CSF for 2 h, washed, weighed, and then placed in individual wells of a 48-well plate with complete medium. On days 4, 7, and 11, culture supernatant was collected and stored; fresh medium was replenished on days 4 and 7.
For the next 30 women, 2 CT and 2 VT biopsies were obtained and exposed to 5 × 104 TCID50 of HIV-1BaL for 2 h, washed, weighed, and then placed in individual wells of a 48-well plate with complete medium. Viral p24 from one CT and VT was quantified through day 7 of culture, and the second CT and VT were quantified through day 11 of culture to determine optimal day for completion of the assay. Culture supernatant was collected on days 4 and 7 for all biopsies and on day 11 for those remaining in culture until day 11.
Culture supernatant was evaluated for p24gag production by the high sensitivity AlphaLISA (PerkinElmer, Waltham, MA). Cumulative p24 was obtained by adding the p24 values from days 4, 7, and/or 11 and correcting for the initial biopsy weight. HIV replication was defined as cumulative p24 of >7.6 pg/mg for CT and >11.9 pg/mg, which was twofold above the mean weight corrected lower limit of detection (117.6 pg/ml) of the assay.
Tissue at the end of study was placed in RNA later (Invitrogen, Carlsbad, CA) and stored at −80°C until the RNA was extracted using the RNeasy Mini Kit (Qiagen, Germantown, MD). RNA was converted to cDNA using the SuperScript VILO cDNA Synthesis Kit (Invitrogen) and amplified for HIV-1 with primers designed to cross the donor/acceptor site. An endogenous control of CD3ɛ (Thermo Fisher) was also amplified to provide an estimate of T cells in the tissue. The copies of HIV-1 and CD3ɛ RNA were related to a titration of cDNA from the ACH-2 cell line, which is a T cell clone with one proviral HIV-1 copy per cell,11 and reported as relative copies.
Statistical analyses
Viral p24 values were corrected for their weight by dividing p24 by weight and log-transformed. Intraclass correlation coefficients (ICCs) were calculated to examine the intraperson variation. To compare cumulative p24 between tissues infected by HIV-1BaL and HIV-1JR-CSF or between CT and VT biopsies, we used a linear mixed model (LMM) with log10 (weight-corrected cumulative p24) as response variable, HIV type (or tissue type) as fixed effect, and subjects as random effects, to adjust for intraperson correlation. A t-test, which assumes that all samples are independent (no intraperson correlation), was also run.
To compare day 7 versus day 11 p24, a LMM was fit with log10 (p24_day 11) − log10 (p24_day 7) as response variable and subjects as random effects and followed by paired t-test, which assumes independence of the tissues.
To inform the design of the future clinical trial, we calculated sample size needed to detect a 1 log10 reduction in cumulative p24 in the active treatment group relative to the placebo treatment group with 80% power.
All analyses were performed using R 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org); the sample size calculation was conducted using PASS 14 [PASS 14 Power Analysis and Sample Size Software (2015); NCSS, LLC, Kaysville, UT].
Results
Study population
Women recruited to participate in this study were 29 years old (mean; range 20–45) (Table 1). Approximately 40% of the women had enrolled in one or more previous clinical studies that obtained cervical and/or vaginal biopsies. Our study population resembled those typically recruited for HIV prevention trials, which were composed of healthy reproductive-aged women.3,4,12,13
Table 1.
Participant Demographic and Behavior Measurements
| Demographic | n (%) |
|---|---|
| Race | |
| Asian | 3 (4.9) |
| Black | 17 (27.9) |
| White | 34 (55.7) |
| Other | 7 (11.5) |
| Ethnicity | |
| Hispanic | 1 (1.6) |
| Not Hispanic | 60 (98.4) |
| Behavior | |
| Current smoker | 14 (23.0) |
| Contraceptiona | |
| Oral pills | 12 (19.7) |
| Depot medroxyprogesterone acetate injection | 2 (3.8) |
| Intrauterine device | 13 (21.3) |
| Condom | 19 (31.1) |
| Bi-tubal ligation | 5 (8.2) |
| Not sexually active with a man | 9 (14.8) |
| Participated in previous clinical study collecting cervical/vaginal biopsies? | |
| Yes | 24 (39.3) |
| No | 37 (60.7) |
Contraception grouping is not mutually exclusive.
Reproducibility of HIV-1 replication in CT and VT biopsies
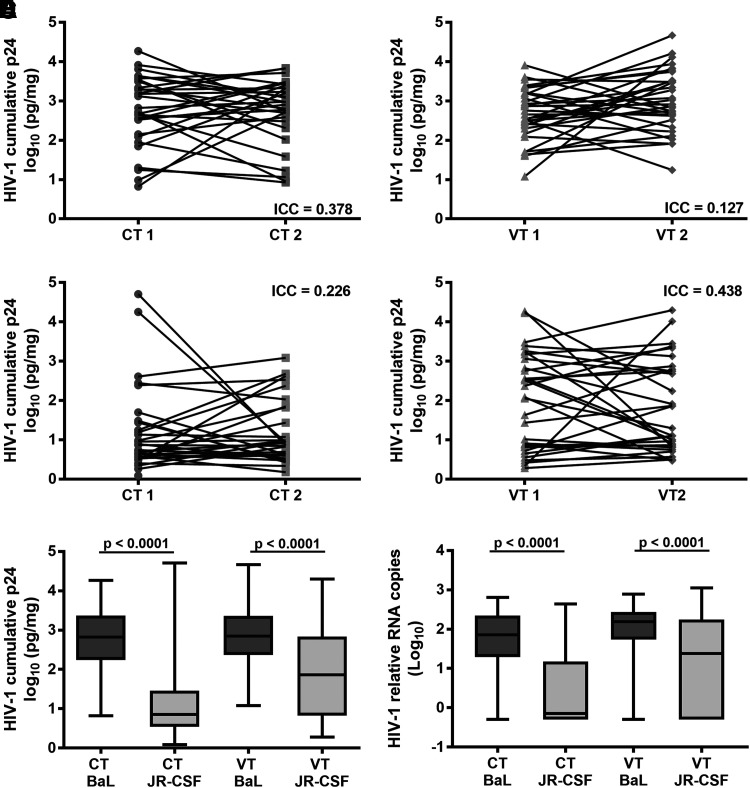
Four CT and four VT biopsies were obtained, and paired CT and VT biopsies were exposed to HIV-1BaL or HIV-1JR-CSF in the same manner as would be used in the ex vivo challenge assay. Cumulative p24 through day 11 of culture showed significant intraperson variation as characterized by the low (≤0.438) ICC for HIV-1BaL (Fig. 1A, B) and HIV-1JR-CSF (Fig. 1C, D) replicating in either CT (Fig. 1A, C) or VT (Fig. 1B, D).
FIG. 1.
Reproducibility of HIV-1 replication in mucosal tissue. Two CT and VT biopsies were collected from 31 participants and exposed to either HIV-1BaL or HIV-1JR-CSF and followed in culture for 11 days. Cumulative p24 was corrected for biopsy weight and log-transformed. Intraperson variability was tested using ICC. HIV-1BaL (A, B) and HIV-1JR-CSF (C, D) ICC was poor (<0.4) to fair (0.4–0.59). Overall, HIV-1BaL replicated to higher levels than HIV-1JR-CSF. HIV-1BaL demonstrated better cumulative p24 (E) and HIV-1 relative RNA copies (F) than HIV-1JR-CSF in CT and VT. p values were the same for the LMM and t-test (p < .0001). CT, cervical tissue; ICC, intraclass correlation coefficient; LMM, linear mixed model; VT, vaginal tissue.
HIV-1BaL cumulative p24 was greater in CT and VT than HIV-1JR-CSF. Within the CT, 11 paired biopsies did not replicate HIV-1JR-CSF (Fig. 1C) compared to zero paired biopsies not replicating HIV-1BaL (Fig. 1A). Within the VT, six paired biopsies did not replicate HIV-1JR-CSF (Fig. 1D) compared to zero paired biopsies not replicating HIV-1BaL (Fig. 1B). HIV-1BaL produced 1.5 log10 in CT and 0.9 log10 in VT higher quantities of cumulative p24 pg/mg than HIV-1JR-CSF (LMM and t-test p < .0001) (Fig. 1E). HIV-1BaL replicated equally well in CT and VT (LMM p = .9439, t-test p = .9543), while HIV-1JR-CSF replicated better in VT compared to CT (LMM p = .0016, t-test p = .0036).
Comparison of cumulative p24 to viral RNA
The use of cumulative p24 has become the standard in reporting results for the ex vivo challenge assay.1–9,14 However, viral RNA or DNA could also be used for quantification. We investigated tissue-associated viral RNA as a second measure of viral replication in the tissue. Similar to the cumulative p24, the intraperson variation as measured by HIV-1 relative RNA copies was characterized by low ICCs in CT and VT for HIV-1BaL (ICC = 0.513 and 0.408, respectively) and for HIV-1JR-CSF (ICC = 0.336 and 0.252, respectively). The HIV-1 relative RNA copies demonstrated positive correlations (p ≤ .0078) to the cumulative p24 for HIV-1BaL and HIV-1JR-CSF in CT (Supplementary Fig. S1A, C; Supplementary Data are available online at www.liebertpub.com/aid) and VT (Supplementary Fig. S1B, D).
Viral RNA copies generally were not correlated with CD3ɛ or 18s RNA expression (data not shown). VT expressed higher relative copies of CD3ɛ compared to CT (ANOVA, Dunn's multiple comparisons p < .003) (Supplementary Fig. S1E), indicating either greater numbers of or more active transcription in the CD3ɛ expressing cell population. No difference in the relative copies of 18s was noted between CT and VT (ANOVA, Kruskal–Wallis test) (Supplementary Fig. S1F), suggesting consistency between the tissues for general RNA levels. The relative HIV-1 RNA copies were consistent with the cumulative p24 data, which showed that HIV-1BaL replicated to significantly higher quantities than HIV-1JR-CSF (LMM and t-test p < .0001) (Fig. 1F).
Impact of previous biopsy collection on HIV-1 replication
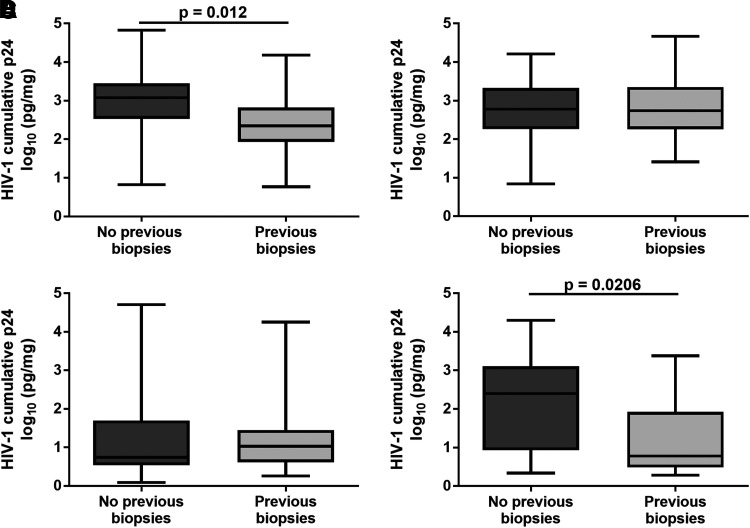
Recruitment of participants in phase 1 clinical trials often is done through use of lists of volunteers who make themselves available for studies that interest them. Thus some participants may have joined in previous clinical studies in which mucosal biopsies were collected. Approximately 40% previously participated in a clinical study that collected cervical and/or vaginal biopsies. Comparing the cumulative p24 of HIV-1BaL in those women who had not participated in clinical studies collecting biopsies with those women who did, a five-fold decrease in cumulative p24 was found in CT (LMM p = .012; t-test p = .006) (Fig. 2A), but not in VT (Fig. 2B). When comparing the cumulative p24 of HIV-1JR-CSF, the opposite results were found; no difference in cumulative p24 was found in CT (Fig. 2C), but a 41-fold decrease was found in VT (LMM p = .0206; t-test p = .0027) (Fig. 2D) in those women participating in previous clinical studies collecting biopsies.
FIG. 2.
HIV-1 replication in mucosal tissue collected from women with a history of previous biopsy collection. Of the overall 61 participants, 91 CT (A, C) and VT (B, D) biopsies were exposed with HIV-1BaL (A, B), and 61 CT and VT were exposed to HIV-1JR-CSF (C, D). Cumulative p24 was compared between those women who had no previous CT or VT biopsies and those women who previously participated in a clinical trial with CT and/or VT biopsy collection. Significant differences were defined using a LMM.
Effect of culture period on HIV-1 replication
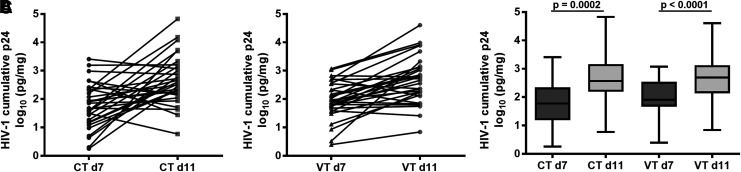
Because HIV-1BaL had more robust replication, it was used to evaluate the length of the culture period. Paired CT and VT biopsies were collected, and one of each was cultured through day 7 and the other through day 11. Cumulative p24 was 1.18 log10 pg/mg for CT (Fig. 3A) and 1.09 log10 pg/mg for VT (Fig. 3B) higher for day 11 than day 7 of culture. While this was a significant increase in cumulative p24 overall for day 11 (LMM and t-test p < .0001) (Fig. 3C), some tissues had lower cumulative p24 on day 11 compared to day 7 (Fig. 3A, B).
FIG. 3.
Influence of the ex vivo challenge day of culture on cumulative p24. Paired CT (A) and VT (B) biopsies were collected from 30 participants and exposed to HIV-1BaL and followed in culture for 7 or 11 days. Cumulative p24 was corrected for biopsy weight and log-transformed. Significantly greater cumulative p24 was observed on day 11 compared to day 7 (C) (LMM and t-test).
Sample size calculation
One of the goals of defining tissue PD is to show a reduced cumulative p24 of the active treatment group relative to the baseline specimens or placebo treatment group. A sample size calculation was done to determine how many tissue biopsies would be needed to detect a 1 log10 reduction in cumulative p24 (Table 2). Comparable numbers of CT and VT biopsies (26 and 22, respectively) were needed for HIV-1BaL, which reflects the similar cumulative p24 in both tissue types. However, more VT biopsies would be needed than CT biopsies (48 and 32, respectively) for HIV-1JR-CSF, due to the greater variability in HIV replication in VT compared to CT.
Table 2.
Summary and Power Analysis of Cumulative p24
| Tissue, HIV-1 | Cumulative log10 p24, pg/mg, mean ± SD | N per groupa |
|---|---|---|
| Cervix, BaL | 2.712627 ± 0.8662082 | 26 |
| Cervix, JR-CSF | 1.172574 ± 0.9503544 | 32 |
| Vagina, BaL | 2.782427 ± 0.772591 | 22 |
| Vagina, JR-CSF | 1.903719 ± 1.191362 | 48 |
The number of tissue biopsies needed to detect a 1 log10 difference in cumulative p24 with 80% statistical power at alpha = 0.05.
SD, standard deviation.
Discussion
The ex vivo challenge assay was created to define drug concentrations needed to prevent HIV-1 infection of mucosal tissue. The assay can provide reassurance to product developers that the drug was effective in the tissue most likely to first encounter HIV-1. Reproducible, robust HIV-1 replication in mucosal tissue used in the ex vivo challenge assay is needed to establish the background viral replication to define the PD effects of antiretroviral drugs. However, variable viral replication in the baseline and/or placebo treated tissues could underestimate the drug's effective concentration determined by the model, so assay and participant variables were evaluated. Assay variables showed that a robust replicating virus was needed to generate high p24 concentrations or viral RNA copies, which were also dependent on the day of culture. Participant variables showed inherent intraperson variability in HIV-1 replication, and previous biopsy collection had a modest effect on the capacity of tissue to replicate HIV. Based on these data, a relatively high sample size of ∼24 biopsies would be needed to detect a 1 log10 reduction in cumulative p24.
Characterization of HIV-1 from early mucosal transmission events has shown these viruses to be CCR5 dependent.15–17 Consequently, CCR5-dependent HIV-1 was used for the ex vivo challenge assay. HIV-1BaL has been used most often as it replicates well in mucosal tissue and retains the use of CCR5.2,18 However, HIV-1BaL was isolated in the early 1980s from lung tissue of a child with AIDS19 and it has been propagated in the laboratory since that time. While HIV-1JR-CSF was isolated from the cerebrospinal fluid of an HIV-1 infected person with dementia in the 1980s as well,20 an infectious molecular clone was created so viral evolution by laboratory propagation would be minimized.
Despite both viruses utilizing CCR5, HIV-1BaL infects macrophage and T cells equally well, while HIV-1JR-CSF infects macrophage poorly and T cells efficiently.21 Infection predominately in CCR5+/CD4(low)+ T cells is a characteristic of viruses isolated soon after transmission,16,22 which is why HIV-1JR-CSF was included in this study as it has these early transmission characteristics. HIV-1JR-CSF replicated less well than HIV-1BaL as evidenced by lower cumulative p24 and relative RNA copies. This was unexpected as others have shown that HIV-1JR-CSF will replicate in VT.23 However, 2–3 log10 more HIV-1JR-CSF was used to infect the VT in the referenced study compared to the amount of virus used in this study. We have shown that HIV-1JR-CSF does replicate well in activated ectocervical and colonic explant tissue,24,25 but the tissue used in the ex vivo challenge assay was not activated. HIV-1JR-CSF may require higher levels of dNTP pools or lower activity of the sterile alpha motif and HD-domain containing protein 1 (SAMHD1), which are found in activated cells,26,27 compared to HIV-1BaL.
While only HIV-1BaL and HIV-1JR-CSF were the focus in this study, other viruses representative of the geographic regions where the HIV-1 prevention product will be used or even infectious drug resistant viruses should be considered to fully evaluate the potential efficacy of an HIV-1 prevention product. Our data suggest that before use for a clinical study, the viruses should be evaluated for their capacity to replicate in mucosal tissue to ensure their fitness.
While there are abundant HIV-1 immune targets such as Langerhans cells, dendritic cells, lymphocytes, and macrophages in the cervix and vagina,28–30 there is heterogeneity of immune cell populations distributed across the organ with identified lymphoid aggregates.29 CT and VT supported HIV-1 infection and replication as noted in this study, but the tissues from the same person demonstrated considerable variability in cumulative p24 and viral RNA transcripts, similar to tissues between different women reflecting the heterogeneity of immune cell populations within the CT and VT.
When we evaluated if participation in previous clinical studies collecting cervical and/or vaginal biopsies influenced the capacity to replicate HIV-1, HIV-1BaL replication was modestly affected with a five-fold reduction in cumulative p24 in CT, but no significant difference in VT was found. The opposite result was noted for HIV-1JR-CSF; VT demonstrated a significant reduction in cumulative p24 with no effect in viral replication from CT. The impact of repeat biopsy collection on HIV-1JR-CSF replication could be influenced by the overall poor replication of this virus in mucosal tissue. Due to the greater surface area, the likelihood of repeatedly taking biopsies from the same sites in the vagina is less compared to the smaller surface area of the cervix. Repeated tissue biopsy collection may result in a reduction or alteration in immune cell composition resulting in the modest decrease in the capacity of HIV-1 to infect the biopsied tissue. We were limited on the amount of tissue collected and could not confirm these findings by characterizing the immune cell populations in the CT or VT. While we did quantify the relative RNA copies of CD3ɛ expressed, representative of T cells, in the tissue, which were greater in the VT than the CT, precisely quantifying cell numbers will require additional studies. However, some of the repeat biopsy effects could be minimized if chart notes are made of the biopsy locations for each participant, and those locations could be avoided in subsequent studies.
Semen can influence the immune cell composition of the female genital tract. By 12 h, an increase in immune cell recruitment in the cervix has been documented after coitus without a condom, but not after coitus with a condom,31 and was associated with increased production of inflammatory cytokines and chemokines, which influenced the leukocyte migration.31–33 The increase in potential target cells along with a possible pro-inflammatory milieu could increase HIV-1 susceptibility. All of the participants in this study, if they were sexually active, had their last sexual act greater than 48 h from the time the CT and VT were collected, thus making a temporal linkage between recent sexual activity and HIV-1 replication difficult. How long semen can influence genital immune cell populations and a pro-inflammatory milieu has not been defined. Moreover, it is not known if multiple exposures to semen have an additive effect on immune cell recruitment or if a steady state of immune cells is maintained. While some clinical trials are now incorporating coitus in to their design to understand the impact of intercourse/semen on drug PK,34,35 women recruited into phase 1/2 clinical trials typically are expected to refrain from coitus for at least 48 h before their visit so the impact of semen should be minimized.
The variability of viral replication in CT and VT resulted in ∼24 persons that would need to be enrolled or biopsies to be collected in a clinical trial to determine a 1 log10 decrease in p24. Furthermore, the 1 log10 decrease in p24 was dependent on the HIV-1 isolate used for the ex vivo challenge assay as more persons would need to be enrolled if using a poorly replicating virus, such as HIV-1JR-CSF. In another analysis evaluating ex vivo challenge data from several laboratories, high CT and VT p24 variability, similar to our findings, was reported.14 A power analysis noted that the number of tissues needed for a 1 log10 decrease in HIV-1BaL p24 was 21 for CT (range 13–28) and 10 for VT (range 4–24) for participants/biopsies. To reduce the number of participants needed for the clinical trial, the number of tissue biopsies collected per participant could be increased. Unlike colorectal tissue where up to 20–30 tissue biopsies have been collected per participant with 4 of them used for the ex vivo challenge assay,8,9 the number of tissue biopsies from CT and VT is limited and typically has been up to 4 and 5 in total, respectively.7 Despite these limited numbers, PK/PD associations using the ex vivo challenge assay with CT and VT have been characterized in several clinical trials.3–7 With the refinements noted in this study, more precise PK/PD associations may be possible.
Assay and participant variables have been identified that can be incorporated into the design of clinical trials using the ex vivo challenge to define PK/PD correlations. While the goal is to characterize effective drug concentrations for HIV-1 suppression, there are limitations to the assay. It should be noted that the amount of drug needed to suppress HIV-1 in the ex vivo challenge assay due to high viral titers used in the assay may exceed the amount of drug needed to prevent HIV-1 acquisition in persons participating in a randomized control trial as the concentrations of HIV-1 in semen, even during primary infection, are likely lower.36,37 Moreover, PK assessments capture drug levels at specified times after dosing as tissues are immediately frozen when collected, while PD assessments are cultured for a short period of time during which drug can be washed away.5 This may result in an overestimation of the amount of drug needed for viral suppression. However, pharmacometric modeling work is being done to help interpret these assay nuances.38,39 The work presented in this study should refine the ex vivo challenge assay to optimize its incorporation into the clinical study design.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Bill & Melinda Gates Foundation (OPP1084465). The authors thank the participants for their altruism by volunteering for medical research. This work could not have been done without the help from the Reproductive Infectious Disease Research Clinical Team, in particular Tracy Campbell, Carol Priest, and Tationna Smalley, and additional laboratory support provided by Cory Shetler, Krishna Majmundar, and Ratiya Pamela Kunjara Na Ayudhya.
Preliminary data of this work were presented (Abstract No. P06.04) at the HIV Research for Prevention meeting in Chicago, IL on October 19, 2016.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Anton PA, Cranston RD, Kashuba A, et al. : RMP-02/MTN-006: A phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses 2012;28:1412–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anton PA, Saunders T, Elliott J, et al. : First phase 1 double-blind, placebo-controlled, randomized rectal microbicide trial using UC781 gel with a novel index of ex vivo efficacy. PLoS One 2011;6:e23243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunge KE, Dezzutti CS, Rohan LC, et al. : A phase 1 trial to assess the safety, acceptability, pharmacokinetics, and pharmacodynamics of a novel dapivirine vaginal film. J Acquir Immune Defic Syndr 2016;71:498–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen BA, Panther L, Marzinke MA, et al. : Phase 1 safety, pharmacokinetics, and pharmacodynamics of dapivirine and maraviroc vaginal rings: A double-blind randomized trial. J Acquir Immune Defic Syndr 2015;70:242–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dezzutti CS, Richardson-Harman N, Rohan LC, et al. : Pharmacodynamic correlations using fresh and cryopreserved tissue following use of vaginal rings containing dapivirine and/or maraviroc in a randomized, placebo controlled trial. Medicine (Baltimore) 2016;95:e4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox J, Tiraboschi JM, Herrera C, et al. : Brief report: Pharmacokinetic/pharmacodynamic investigation of single-dose oral maraviroc in the context of HIV-1 pre-exposure prophylaxis. J Acquir Immune Defic Syndr 2016;73:252–257 [DOI] [PubMed] [Google Scholar]
- 7.Robinson JA, Marzinke MA, Bakshi RP, et al. : Comparison of dapivirine vaginal gel and film formulation pharmacokinetics and pharmacodynamics (FAME 02B). AIDS Res Hum Retroviruses 2017;33:339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGowan I, Cranston RD, Duffill K, et al. : A phase 1 randomized, open label, rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of three formulations of tenofovir 1% gel (the CHARM-01 Study). PLoS One 2015;10:e0125363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGowan I, Dezzutti CS, Siegel A, et al. : Long-acting rilpivirine as potential pre-exposure prophylaxis for HIV-1 prevention (the MWRI-01 study): An open-label, phase 1, compartmental, pharmacokinetic and pharmacodynamic assessment. Lancet HIV 2016;3:e569–e578 [DOI] [PubMed] [Google Scholar]
- 10.Reed LJ, Muench H: A simple method of estimating fifty per cent endpoints. Am J Hygiene 1938;27:493–497 [Google Scholar]
- 11.Clouse KA, Powell D, Washington I, et al. : Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol 1989;142:431–438 [PubMed] [Google Scholar]
- 12.Hendrix CW, Chen BA, Guddera V, et al. : MTN-001: Randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS One 2013;8:e55013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz JL, Rountree W, Kashuba AD, et al. : A multi-compartment, single and multiple dose pharmacokinetic study of the vaginal candidate microbicide 1% tenofovir gel. PLoS One 2011;6:e25974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson-Harman N, Parody R, Anton PA, et al. : Analytical advances in the ex vivo challenge efficacy assay. AIDS Res Hum Retroviruses 2017;33:395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baalwa J, Wang S, Parrish NF, et al. : Molecular identification, cloning and characterization of transmitted/founder HIV-1 subtype A, D and A/D infectious molecular clones. Virology 2013;436:33–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. : Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 2008;105:7552–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parrish NF, Wilen CB, Banks LB, et al. : Transmitted/founder and chronic subtype C HIV-1 use CD4 and CCR5 receptors with equal efficiency and are not inhibited by blocking the integrin alpha4beta7. PLoS Pathog 2012;8:e1002686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dezzutti CS, Uranker K, Bunge KE, Richardson-Harman N, Macio I, Hillier SL: HIV-1 infection of female genital tract tissue for use in prevention studies. J Acquir Immune Defic Syndr 2013;5:548–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gartner S, Markovits P, Markovitz DM, Kaplan MH, Gallo RC, Popovic M: The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 1986;233:215–219 [DOI] [PubMed] [Google Scholar]
- 20.Koyanagi Y, Miles S, Mitsuyasu RT, Merrill JE, Vinters HV, Chen IS: Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science 1987;236:819–822 [DOI] [PubMed] [Google Scholar]
- 21.Chikere K, Webb NE, Chou T, et al. : Distinct HIV-1 entry phenotypes are associated with transmission, subtype specificity, and resistance to broadly neutralizing antibodies. Retrovirology 2014;11:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ping LH, Joseph SB, Anderson JA, et al. : Comparison of viral Env proteins from acute and chronic infections with subtype C human immunodeficiency virus type 1 identifies differences in glycosylation and CCR5 utilization and suggests a new strategy for immunogen design. J Virol 2013;87:7218–7233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicol MR, Emerson CW, Prince HM, et al. : Models for predicting effective HIV chemoprevention in women. J Acquir Immune Defic Syndr 2015;68:369–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dezzutti CS, Russo J, Wang L, et al. : Development of HIV-1 rectal-specific microbicides and colonic tissue evaluation. PLoS One 2014;9:e102585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott YM, Park SY, Dezzutti CS: Broadly neutralizing anti-HIV antibodies prevent HIV infection of mucosal tissue ex vivo. Antimicrob Agents Chemother 2016;60:904–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amie SM, Noble E, Kim B: Intracellular nucleotide levels and the control of retroviral infections. Virology 2013;436:247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franzolin E, Pontarin G, Rampazzo C, et al. : The deoxynucleotide triphosphohydrolase SAMHD1 is a major regulator of DNA precursor pools in mammalian cells. Proc Natl Acad Sci U S A 2013;110:14272–14277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen R, Richter HE, Smith PD: Interactions between HIV-1 and mucosal cells in the female reproductive tract. Am J Reprod Immunol 2014;71:608–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pudney J, Quayle AJ, Anderson DJ: Immunological microenvironments in the human vagina and cervix: Mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod 2005;73:1253–1263 [DOI] [PubMed] [Google Scholar]
- 30.Trifonova RT, Lieberman J, van Baarle D: Distribution of immune cells in the human cervix and implications for HIV transmission. Am J Reprod Immunol 2014;71:252–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA: Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol 2012;188:2445–2454 [DOI] [PubMed] [Google Scholar]
- 32.Chen JC, Johnson BA, Erikson DW, et al. : Seminal plasma induces global transcriptomic changes associated with cell migration, proliferation and viability in endometrial epithelial cells and stromal fibroblasts. Hum Reprod 2014;29:1255–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharkey DJ, Macpherson AM, Tremellen KP, Mottershead DG, Gilchrist RB, Robertson SA: TGF-beta mediates proinflammatory seminal fluid signaling in human cervical epithelial cells. J Immunol 2012;189:1024–1035 [DOI] [PubMed] [Google Scholar]
- 34.Herold BC, Chen BA, Salata RA, et al. : Impact of sex on the pharmacokinetics and pharmacodynamics of 1% tenofovir gel. Clin Infect Dis 2016;62:375–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keller MJ, Mesquita PM, Torres NM, et al. : Postcoital bioavailability and antiviral activity of 0.5% PRO 2000 gel: Implications for future microbicide clinical trials. PLoS One 2010;5:e8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baeten JM, Kahle E, Lingappa JR, et al. : Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med 2011;3:77ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stekler J, Sycks BJ, Holte S, et al. : HIV dynamics in seminal plasma during primary HIV infection. AIDS Res Hum Retroviruses 2008;24:1269–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richardson-Harman N, Hendrix CW, Bumpus NN, et al. : Correlation between compartmental tenofovir concentrations and an ex vivo rectal biopsy model of tissue infectibility in the RMP-02/MTN-006 phase 1 study. PLoS One 2014;9:e111507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang KH, Hendrix C, Bumpus N, et al. : A multi-compartment single and multiple dose pharmacokinetic comparison of rectally applied tenofovir 1% gel and oral tenofovir disoproxil fumarate. PLoS One 2014;9:e106196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.