Abstract
Objective
To examine the association between hyperkalemia and long-term cardiovascular and renal outcomes in patients with chronic kidney disease.
Patients and Methods
An observational retrospective cohort study was performed using a Japanese hospital claims registry, Medical Data Vision (April 1, 2008, to September 30, 2018). Of 1,208,894 patients with at least 1 potassium measurement, 167,465 patients with chronic kidney disease were selected based on International Classification of Diseases, Tenth Revision codes or estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73 m2. Hyperkalemia was defined as at least 2 potassium measurements of 5.1 mmol/L or greater within 12 months. Normokalemic controls were patients without a record of potassium levels of 5.1 mmol/L or greater and 3.5 mmol/L or less. Changes in eGFRs and hazard ratios of death, hospitalization for cardiac events, heart failure, and renal replacement therapy introduction were assessed between propensity score–matched hyperkalemic patients and normokalemic controls.
Results
Of 16,133 hyperkalemic patients and 11,898 normokalemic controls eligible for analyses, 5859 (36.3%) patients and 5859 (49.2%) controls were selected after propensity score matching. The mean follow-up period was 3.5 years. The 3-year eGFR change in patients and controls was −5.75 and −1.79 mL/min/1.73 m2, respectively. Overall, hyperkalemic patients had higher risks for death, hospitalization for cardiac events, heart failure, and renal replacement therapy introduction than controls, with hazard ratios of 4.40 (95% CI, 3.74 to 5.18), 1.95 (95% CI, 1.59 to 2.39), 5.09 (95% CI, 4.17 to 6.21), and 7.54 (95% CI, 5.73 to 9.91), respectively.
Conclusion
Hyperkalemia was associated with significant risks for mortality and adverse clinical outcomes, with more rapid decline of renal function. These findings underscore the significance of hyperkalemia as a predisposition to future adverse events in patients with chronic kidney disease.
Abbreviations and Acronyms: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HF, heart failure; ICD-10, International Classification of Diseases, Tenth Revision; LDL, low-density lipoprotein; MDV, Medical Data Vision; MRA, mineralocorticoid receptor antagonist; PS, propensity score; RAASi, renin-angiotensin-aldosterone system inhibitor; RRT, renal replacement therapy; S-K, serum potassium
Hyperkalemia, characterized by abnormally elevated serum potassium (S-K) levels, develops when there is excessive production or inadequate elimination of potassium, and it is often found in patients with chronic kidney disease (CKD) during routine outpatient and inpatient care.1, 2, 3, 4, 5, 6 Recent reports have indicated that hyperkalemia potentially is not merely an electrolyte disturbance but also serves as a marker reflecting patients’ general conditions.7 Several studies have documented the increased risks for adverse clinical outcomes in hyperkalemic patients, wherein direct associations between elevated S-K levels and adverse clinical outcomes have been demonstrated in various geographic regions, documenting a U-shaped association between mortality risk and S-K level.8, 9, 10, 11 A hospital-based cohort study of 923 Korean patients found 31% in-hospital mortality for patients with S-K levels of 6.5 mmol/L or greater.12 These findings were consistent regardless of underlying comorbid conditions such as CKD and heart failure (HF).13,14
However, few studies have examined the association between abnormal S-K levels and long-term cardiovascular and renal outcomes over several years.15 Moreover, previous studies primarily examined cardiovascular outcomes and death; therefore, information regarding renal outcomes is limited. Statistically, previous pharmacoepidemiologic studies have estimated event risks based on time-updated or a single S-K measurement and then applied the models such as generalized additive models,8 regression models,9, 10, 11 or generalized estimating equations.13,14 These models are presumed to be suitable for assessing direct and short-term associations; however, in assessing long-term associations, S-K values vary throughout the clinical course.16 Additionally, stratification based on a single S-K measurement may result in the inclusion of patients with temporary S-K level fluctuation or pseudo-hyperkalemia. Therefore, a rigorous definition of patients with hyperkalemia and normokalemic controls is required to assess long-term associations between hyperkalemia and adverse clinical outcomes.
In this study, we investigated the association between hyperkalemia and long-term adverse clinical outcomes in patients with CKD and continuous medical care in a large-scale nationwide administrative data set. To rigorously assess the prognostic effect of hyperkalemia, we required multiple S-K measurements within a 12-month period. The normokalemic control group was based on careful examination of patient characteristics in the pre-index 12 months. Furthermore, we assessed renal outcomes, including the introduction of renal replacement therapy (RRT) and estimated glomerular filtration rate (eGFR) decline in addition to cardiovascular outcomes and death, to evaluate the effect of hyperkalemia on prognosis from a broad clinical perspective.
Patients and Methods
Data Source
Data were obtained from Medical Data Vision (MDV), one of the largest hospital claims registries in Japan. With a diagnostic and procedural coding system, MDV is built from hospital claims data including individual prescriptions, procedures, examinations, and laboratory data. Medical Data Vision uses International Classification of Diseases, Tenth Revision (ICD-10) coding. Data collection began in April 2008. As of September 2018, MDV had collected 25,570,000 individual patient records from 374 hospitals across Japan.
Study Design and Patient Selection
There were 1,208,894 adult patients (aged ≥18 years) who had at least 1 S-K measurement during the study period (April 1, 2008, to September 30, 2018). A total of 167,465 patients (13.9%) had either CKD based on ICD-10 codes or an average eGFR less than 60 mL/min/1.73 m2.
Hyperkalemia was defined as patients with at least 2 S-K readings of 5.1 mmol/L or greater within a 12-month interval.17 A cohort of normokalemic controls included patients without any record of S-K levels of 3.5 mmol/L or less and 5.1 mmol/L or greater. The index date was the first hyperkalemic episode (S-K ≥5.1 mmol/L) for hyperkalemic patients. Normokalemic controls were followed up from the first visit after 12 months from their initial hospital record to obtain the 12-month pre-index period. Patients were followed up until the date of emigration from the database, date of death, or end of the study period, whichever came first.
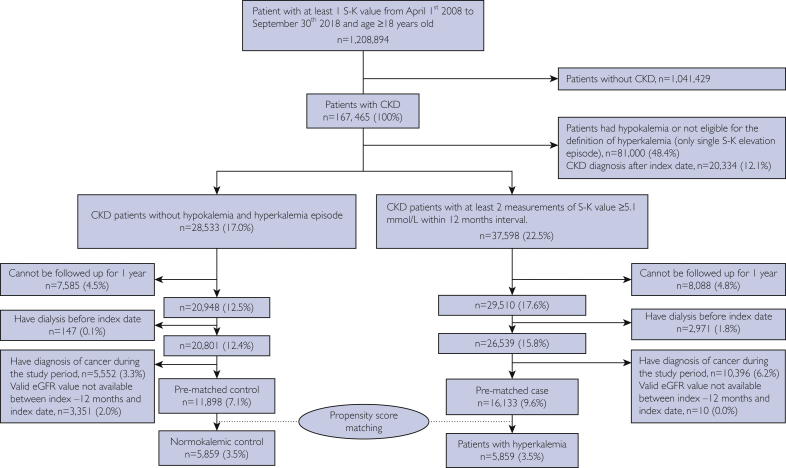
For this analysis, patients with hypokalemia or only 1 record of S-K level of 5.1 mmol/L or greater (n=81,000 of 167,465; 48.4%) and patients who were first had CKD diagnosed after the index date (n=20,334 of 167,465; 12.1%) were excluded. We also excluded patients who could not be followed up for at least 12 months (n=15,673 of 167,465; 9.4%), those with a cancer diagnosis (n=15,948 of 167,465; 9.5%), those undergoing dialysis before the index date (n=3118 of 167,465; 1.9%), and patients without valid eGFR values (n=3361 of 167,465; 2.0%). A total of 16,133 hyperkalemic patients and 11,898 normokalemic controls were examined (Figure 1).
Figure 1.
Flow diagram of patient inclusion into the study. CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate; S-K = serum potassium.
Covariates and Clinical Outcomes
Known high-risk comorbid conditions of hyperkalemia,4,5,18 including CKD, HF, diabetes mellitus (DM), and hypertension, were defined based on ICD-10 codes and eGFR values (Supplemental Table 1, available online at https://mcpiqojournal.org). Other comorbid conditions are listed in Supplemental Table 2 (available online at https://mcpiqojournal.org) and were used to calculate the Charlson Comorbidity Index score. Drug treatment records were collected for 120 days before the index date based on commonly used intervals of drug prescription in Japanese clinical practice. Information on hyperkalemia treatment including diuretics (thiazide and loop diuretics), glucose injection, calcium gluconate, sodium bicarbonate, and potassium binders was collected. Information on drugs associated with hyperkalemia, including renin-angiotensin-aldosterone system inhibitors (RAASis; ie, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, mineralocorticoid receptor antagonists (MRAs), and other drugs associated with hyperkalemia, including azole antifungals, β-blockers, calcium channel blockers, cyclosporin, digoxin, heparin, nonsteroidal anti-inflammatory drugs, potassium supplements, tacrolimus, trimethoprim, and systemic corticosteroids, was also assessed. Moreover, treatment for comorbid conditions including HF, DM, and dyslipidemia were collected.
The outcomes of interest included all-cause death; hospitalization for cardiac events as a composite of myocardial infarction, arrhythmia, and cardiac arrest; hospitalization for HF; and introduction of RRT. Definitions of clinical outcomes are listed in Supplemental Table 3 (available online at https://mcpiqojournal.org). Changes in eGFR values during the 3-year period were also assessed for the eGFR decline.
Statistical Analyses
Continuous variables were reported as mean ± SD and median. Frequency and percentage were used to document categorical measures of interest. Cumulative incidences of the first occurrence of clinical outcomes were estimated using the cumulative incidence function, with death as a competing risk. The Kaplan-Meier method was used to estimate the cumulative incidence of death.
A propensity score (PS) for hyperkalemia was developed using covariates including age, sex, index year, length of patient follow-up, prescription of RAASi and other drugs associated with hyperkalemia, diuretics, drug treatment for HF, and antidiabetic medications. Other covariates included CKD stage; the presence of comorbid conditions including HF, DM, hypertension , dyslipidemia, atrial fibrillation or flutter, valvular heart disease, acute kidney injury, sepsis, and peripheral edema; CCI score; and history of hospitalization for 3 or more consecutive days before the index date. Based on the PS, hyperkalemic patients were matched 1:1 with normokalemic controls, with a caliper width of 0.1. The validity of PS matching was assessed by evaluating standardized differences of patient characteristics. A significant imbalance was considered to be present if a greater than 10% standardized difference was present between the 2 groups after matching.
After PS matching, 5859 hyperkalemic patients and 5859 normokalemic controls were selected for the study (Figure 1). We compared the incidence of clinical outcomes between PS-matched patients and controls. The Cox proportional hazard model was used to estimate the hazard ratios with a 95% CI for clinical outcomes. The subgroup of interest included patients with or without HF and analysis by CKD stages. To assess the long-term prognostic effect of hyperkalemia on adverse clinical outcomes, we also compared the incidence of clinical outcomes after 12 months between hyperkalemic patients and normokalemic controls by re-indexing the patients’ follow-up at 12 months after the first hyperkalemic episode.
To evaluate changes in eGFRs, we built a mixed-effects model for repeated measurements to generate a least square mean change from baseline eGFR. The index date for hyperkalemic patients was the date of the first hyperkalemic episode; however, some patients did not have a 12-month pre-index record. Although this inclusion allowed the assessment of broad patient types with an increased sample size, the difference in pre-index period may cause potential immortal time biases19 and underestimation of risk factors, treatments, and medical history. Therefore, we performed a sensitivity analysis including only patients whose pre-index medical records for 12 months were available to confirm the stability of the results. Statistical analyses were performed using SAS, version 9.4 (SAS Institute).
Because patient records were anonymized and deidentified, informed consent was not required. The use of deidentified data was in accordance with local regulations. This study was reviewed and approved by an independent ethics committee (Clinical Research Promotion Network Japan; 2440023).
Results
Baseline Characteristics
Table 1 shows characteristics of hyperkalemic patients and normokalemic controls before and after PS matching. After PS matching, baseline characteristics were well-balanced between the 2 groups, with standardized differences of less than 10% in all variables used for PS. The mean age of both groups was 71 years, with a mean of 3.6 years of follow-up. Mean S-K levels were 5.3±0.3 and 4.3±0.3 mmol/L in patients and controls, respectively. Approximately 26% (1548 of 5859) and 49% (2887 of 5859) of patients had HF and DM, respectively. A total of 2718 (46.4%) patients and 2754 (47.0%) controls were on RAASi therapy. Inotropes and MRAs for HF, sodium bicarbonate, and potassium binders were more frequently prescribed in hyperkalemic patients.
Table 1.
Characteristics of Hyperkalemic Patients and Normokalemic Controls Before and After Propensity Score Matchinga,b
| Before Matching |
After Matching |
|||||
|---|---|---|---|---|---|---|
| Hyperkalemia (n = 16,133) | Normokalemia (n = 11,898) | Standardized Difference (%)c | Hyperkalemia (n = 5859) | Normokalemia (n = 5859) | Standardized Difference (%)c | |
| Age (y), mean ± SD | 73.4±12.6 | 66.6±14.3 | 50.2 | 71.2±12.5 | 71.4±11.9 | 1.9 |
| Age group (y), no. (%) | ||||||
| 18-64 | 3576 (22.2) | 4565 (38.4) | 45.7 | 1567 (26.8) | 1504 (25.7) | 2.9 |
| 65-79 | 6850 (42.5) | 5179 (43.5) | 2710 (46.3) | 2786 (47.6) | ||
| ≥80 | 5707 (35.4) | 2154 (18.1) | 1582 (27.0) | 1569 (26.8) | ||
| Male sex, no. (%) | 9109 (56.5) | 6791 (57.1) | 1.2 | 3423 (58.4) | 3398 (58.0) | 0.9 |
| Length of follow-up (mo), mean ± SD | 37.5±24.3 | 45.7±22.6 | 35.2 | 43.0±24.4 | 43.6±22.0 | 2.3 |
| S-K level at index date (mmol/L), mean ± SD | 5.4±0.5 | 4.3±0.3 | 300.0 | 5.3±0.3 | 4.3±0.3 | 346.2 |
| S-K level group (mmol/L), no. (%) | ||||||
| ≥5.1-<5.5 | 11,221 (69.6) | 4619 (78.8) | ||||
| ≥5.5-<6.0 | 3460 (21.4) | 1021 (17.4) | ||||
| ≥6.0-<6.5 | 898 (5.6) | 170 (2.9) | ||||
| ≥6.5-<7.0 | 314 (2.0) | 30 (0.5) | ||||
| ≥7.0 mmol/L | 240 (1.5) | 19 (0.3) | ||||
| CKD stage, no. (%) | 16,133 (100) | 11,898 (100) | 0 | 5859 (100) | 5859 (100) | 0 |
| 1 | 200 (1.2) | 1086 (9.1) | 149.7 | 195 (3.3) | 194 (3.3) | 3.2 |
| 2 | 1140 (7.1) | 3821 (32.1) | 1091 (18.6) | 1070 (18.3) | ||
| 3 | 2655 (16.5) | 4652 (39.1) | 2264 (38.6) | 2301 (39.3) | ||
| 3b | 4128 (25.6) | 1815 (15.3) | 1745 (29.8) | 1770 (30.2) | ||
| 4 | 4745 (29.4) | 379 (3.2) | 394 (6.7) | 379 (6.5) | ||
| 5 | 3265 (20.2) | 145 (1.2) | 170 (2.9) | 145 (2.5) | ||
| eGFR (mL/min/1.73 m2), mean ± SD | 32.3±20.4 | 59.8±21.3 | 131.7 | 49.1±20.5 | 50.4±18.4 | 6.5 |
| HF, no. (%) | 6394 (39.6) | 2090 (17.6) | 50.4 | 1548 (26.4) | 1575 (26.9) | 1.0 |
| Prescribed medical treatment for HF, no. (%) | 4856 (76.0) | 1485 (71.1) | 11.1 | 1144 (73.9) | 1178 (74.8) | 2.0 |
| ACEi or ARB | 3627 (74.7) | 1105 (74.4) | 0.6 | 846 (74.0) | 878 (74.5) | 1.3 |
| β-Blocker | 2658 (54.7) | 835 (56.2) | 3.0 | 654 (57.2) | 674 (57.2) | 0.1 |
| Digoxin | 539 (11.1) | 151 (10.2) | 3.0 | 127 (11.1) | 134 (11.4) | 0.9 |
| Inotrope | 1115 (23.0) | 194 (13.1) | 26.0 | 227 (19.8) | 154 (13.1) | 18.3 |
| MRA | 2048 (42.2) | 335 (22.6) | 42.9 | 447 (39.1) | 279 (23.7) | 33.6 |
| Diabetes, no. (%) | 7717 (47.8) | 5521 (46.4) | 2.9 | 2887 (49.3) | 2876 (49.1) | 0.4 |
| Prescription of antidiabetics, no. (%) | 5821 (36.1) | 3552 (29.9) | 13.3 | 2018 (34.4) | 2043 (34.9) | 0.9 |
| Hypertension, no. (%) | 12,196 (75.6) | 7675 (64.5) | 24.4 | 4158 (71.0) | 4206 (71.8) | 1.8 |
| Dyslipidemia, no. (%) | 5057 (31.3) | 3979 (33.4) | 4.5 | 1970 (33.6) | 2011 (34.3) | 1.5 |
| Total cholesterol (mg/dL), mean ± SD | 176.4±46.8 | 188.7±37.1 | 29.0 | 181.3±45.2 | 184.9±37.8 | 8.6 |
| HDL cholesterol (mg/dL), mean ± SD | 51.1±16.4 | 55.0±15.4 | 24.5 | 53.7±16.7 | 53.8±15.1 | 0.7 |
| LDL cholesterol (mg/dL) Mean ± SD | 102.1±36.0 | 108.8±29.6 | 20.2 | 105.4±35.3 | 106.4±29.3 | 3.4 |
| Other comorbid conditions, no. (%) | ||||||
| Myocardial infarction | 668 (4.1) | 285 (2.4) | 9.8 | 210 (3.6) | 202 (3.4) | 0.7 |
| Peripheral vascular disease | 2705 (16.8) | 1463 (12.3) | 12.7 | 848 (14.5) | 966 (16.5) | 5.6 |
| Cerebrovascular disease | 3904 (24.2) | 2147 (18.0) | 15.1 | 1301 (22.2) | 1354 (23.1) | 2.2 |
| Chronic pulmonary disease | 2569 (15.9) | 1434 (12.1) | 11.2 | 875 (14.9) | 845 (14.4) | 1.5 |
| Atrial fibrillation or flutter | 2399 (14.9) | 996 (8.4) | 20.4 | 651 (11.1) | 687 (11.7) | 1.9 |
| Valvular heart disease | 1776 (11.0) | 697 (5.9) | 18.6 | 471 (8.0) | 480 (8.2) | 0.6 |
| Acute kidney injury | 676 (4.2) | 53 (0.4) | 25.1 | 55 (0.9) | 50 (0.9) | 0.9 |
| Sepsis | 1608 (10.0) | 869 (7.3) | 9.5 | 509 (8.7) | 529 (9.0) | 1.2 |
| Peripheral edema | 478 (3.0) | 126 (1.1) | 13.6 | 86 (1.5) | 88 (1.5) | 0.3 |
| Charlson Comorbidity Index score, mean ± SD | 1.1±1.2 | 0.9±1.0 | 20.8 | 1.0±1.1 | 1.1±1.1 | 1.9 |
| RAASi treatment, no. (%) | 8314 (51.5) | 4635 (39.0) | 25.5 | 2718 (46.4) | 2754 (47.0) | 1.2 |
| ACEi | 1649 (10.2) | 644 (5.4) | 18.0 | 501 (8.6) | 404 (6.9) | 6.2 |
| ARB | 6391 (39.6) | 4013 (33.7) | 12.2 | 2145 (36.6) | 2341 (40.0) | 6.8 |
| MRA | 2401 (14.9) | 376 (3.2) | 41.8 | 586 (10.0) | 297 (5.1) | 18.8 |
| Other drugs associated with hyperkalemia,d no. (%) | 10,325 (64.0) | 5087 (42.8) | 43.6 | 3196 (54.5) | 3248 (55.4) | 1.8 |
| Hyperkalemia treatment at index date, no. (%) | ||||||
| Thiazide diuretics | 481 (3.0) | 227 (1.9) | 7.0 | 98 (1.7) | 188 (3.2) | 10.0 |
| Loop diuretics | 3693 (22.9) | 522 (4.4) | 56.0 | 563 (9.6) | 475 (8.1) | 5.3 |
| Glucose injection + insulin | 342 (2.1) | 4 (0.0) | 20.3 | 23 (0.4) | 4 (0.1) | 6.8 |
| Calcium gluconate | 306 (1.9) | 40 (0.3) | 14.9 | 44 (0.8) | 21 (0.4) | 5.3 |
| Sodium bicarbonate | 1339 (8.3) | 310 (2.6) | 25.3 | 341 (5.8) | 179 (3.1) | 13.5 |
| Potassium binder | 1444 (9.0) | 20 (0.2) | 43.1 | 302 (5.2) | 18 (0.3) | 30.1 |
ACEi = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein; HF = heart failure; LDL = low-density lipoprotein; MRA = mineralocorticoid receptor antagonist; RAASi = renin-angiotensin-aldosterone system inhibitor; S-K = serum potassium.
SI conversion factors: To convert total, HDL, and LDL cholesterol values to mmol/L, multiply by 0.0259.
Other drugs associated with hyperkalemia include nonsteroidal anti-inflammatory drugs, azole antifungals, β-blockers, calcium channel blockers, cyclosporin, digoxin, heparin, potassium supplements, tacrolimus, trimethoprim, and systemic corticosteroids.
Standardized difference greater than 0.1 was considered a non-negligible difference.
Relative Risk for Clinical Outcomes in Hyperkalemic Patients Compared With Normokalemic Controls
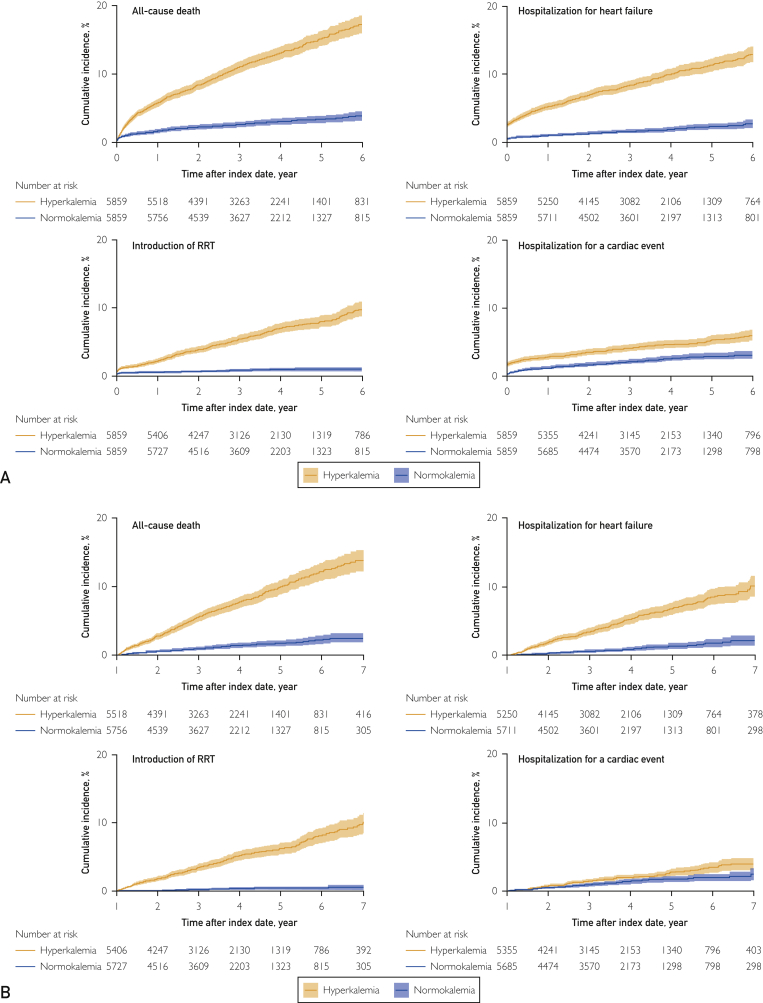
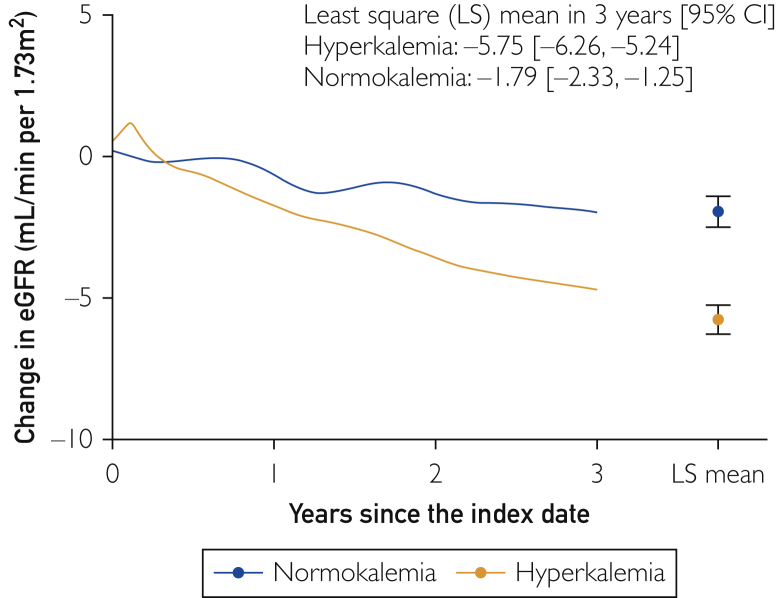
Figure 2 shows the cumulative incidences of clinical outcomes in hyperkalemic patients and normokalemic controls after PS matching. Compared with controls, hyperkalemic patients had higher risks for death, hospitalization for cardiac events, HF, and RRT introduction than controls, with hazard ratios of 4.40 (95% CI, 3.74 to 5.18), 1.95 (95% CI, 1.59 to 2.39), 5.09 (95% CI, 4.17 to 6.21), and 7.54 (95% CI, 5.73 to 9.91), respectively. The higher incidence of adverse clinical outcomes was still present when clinical outcomes were assessed after 12 months by re-indexing the patients’ follow-up at 12 months after the original index date, with incidence rate ratios (incidence rate per 100 patient-years in hyperkalemia vs normokalemia) of death, hospitalization for cardiac events, hospitalization for HF, and RRT introduction of 5.48 (2.63; 95% CI, 2.39 to 2.90 vs 0.48; 95% CI, 0.39 to 0.61), 1.66 (0.73; 95% CI, 0.60 to 0.88 vs 0.44; 95% CI, 0.35 to 0.56), 5.19 (1.87; 95% CI, 1.66 to 2.11 vs 0.36; 95% CI, 0.28 to 0.47), and 16.73 (1.84; 95% CI, 1.63 to 2.07 vs 0.11; 95% CI, 0.07 to 0.18), respectively. The mean 3-year change in eGFR in patients was −5.75 mL/min/1.73 m2 vs −1.79 mL/min/1.73 m2 in controls (Figure 3).
Figure 2.
Cumulative incidence of clinical outcomes in hyperkalemic patients and normokalemic controls after matching (A) overall follow-up period and (B) adverse clinical outcomes after 12 months. RRT = renal replacement therapy.
Figure 3.
Estimated glomerular filtration rate (eGFR) changes in hyperkalemic patients and normokalemic controls after matching. LS = least square.
Sensitivity and Subgroup Analyses
The sensitivity analysis, which was restricted to the 2711 matched pairs whose medical records were available 12 months before the index date, showed consistent trends for all clinical outcomes including death, with a hazard ratio of 5.23 (95% CI, 4.02 to 6.80), hospitalization for cardiac events hazard ratio of 1.52 (95% CI, 1.11 to 2.09), hospitalization for HF hazard ratio of 5.11 (95% CI, 3.73 to 7.01), and introduction of RRT hazard ratio of 7.53 (95% CI, 4.63 to 12.23). In this patient cohort, the mean 3-year change in eGFR was −4.33 mL/min/1.73 m2 in patients and −1.89 mL/min/1.73 m2 in controls.
The subgroup of hyperkalemic patients with HF had a higher cumulative incidence of death (with HF: 33.5%; 95% CI, 28.2% to 39.5% vs without HF: 16.5%; 95% CI, 14.4% to 19.0%), hospitalization for cardiac events (14.4%; 95% CI, 9.4% to 20.3% vs 6.0%; 95% CI, 4.3% to 8.2%), and hospitalization for HF (35.2%; 95% CI, 29.3% to 41.3% vs 10.4%; 95% CI, 8.3% to 12.7) and an equivalent or slightly lower cumulative incidence of RRT introduction (12.4%; 95% CI, 6.5% to 20.1% vs 15.0%; 95% CI, 12.6% to 17.5%) compared with patients without HF.
In both subgroups of patients with or without HF, higher risks for adverse clinical outcomes in hyperkalemic patients compared with normokalemic controls were consistent with the overall population (Table 2). The association of hyperkalemia with death event was the highest in patients with CKD stage 3a and getting lower toward more advanced stages of CKD with hazard ratios of 5.35 (95% CI, 3.93 to 7.27) for stage 3a, 3.75 (95% CI, 2.82 to 5.00) for stage 3b, 1.79 (95% CI, 1.18 to 2.70) for stage 4, and 1.04 (95% CI, 0.62 to 1.76) for stage 5.
Table 2.
Subgroup Analysis in Patients With or Without Heart Failure After Matchinga
| Hyperkalemia |
Normokalemia |
Relative Risks |
||||
|---|---|---|---|---|---|---|
| No. of Events | % | No. of Events | % | Hazard Ratio | 95% CI | |
| Overall | n=5859 | n=5859 | ||||
| Death | 745 | 12.7 | 172 | 2.9 | 4.40 | 3.74-5.18 |
| Hospitalization for cardiac eventsb | 278 | 4.7 | 140 | 2.4 | 1.95 | 1.59-2.39 |
| Hospitalization for HF | 581 | 9.9 | 114 | 1.9 | 5.09 | 4.17-6.21 |
| Renal replacement therapy introduction | 402 | 6.9 | 52 | 0.9 | 7.54 | 5.73-9.91 |
| With HF | n=1548 | n=1575 | ||||
| Death | 308 | 19.9 | 81 | 5.1 | 4.05 | 3.18-5.16 |
| Hospitalization for cardiac eventsb | 141 | 9.1 | 68 | 4.3 | 2.11 | 1.58-2.81 |
| Hospitalization for HF | 371 | 24.0 | 86 | 5.5 | 4.67 | 3.71-5.89 |
| Renal replacement therapy introduction | 80 | 5.2 | 17 | 1.1 | 4.60 | 2.78-7.62 |
| Without HF | n=4311 | n=4284 | ||||
| Death | 437 | 10.1 | 91 | 2.1 | 4.81 | 3.83-6.03 |
| Hospitalization for cardiac eventsb | 137 | 3.2 | 72 | 1.7 | 1.84 | 1.38-2.45 |
| Hospitalization for HF | 210 | 4.9 | 28 | 0.7 | 7.17 | 4.84-10.62 |
| Renal replacement therapy introduction | 322 | 7.5 | 35 | 0.8 | 8.93 | 6.38-12.51 |
HF = heart failure.
Cardiac event includes myocardial infarction, arrhythmia, and cardiac arrest.
Discussion
This study assessed associations between hyperkalemia and long-term adverse clinical outcomes in patients with CKD by rigorously defining hyperkalemic patients and PS-matched normokalemic CKD controls. We found significantly increased risks for mortality and adverse clinical outcomes, accompanied by a more rapid decline in renal function in hyperkalemic patients. Higher risks for adverse clinical outcomes associated with hyperkalemia were consistent both in patients with or without HF at baseline. These findings highlight the potential role of hyperkalemia as an independent predictor of adverse cardiovascular and renal outcomes in a contemporary population of patients with CKD recorded from 2008 to 2018.
In our study, the relative risk for mortality was 4.40 and the risk continued to increase over time as determined by the linear tendency of increased mortality risks after 12 months, which is consistent with previously published studies. A Danish registry study with more than 30,000 patients with HF reported a 3.39-fold higher risk for death within 6 months after hyperkalemic episodes when compared with those without hyperkalemia.20 Likewise, studies from the United Kingdom reported a 2- to 3-fold increase in mortality with an S-K level of 6.0 mmol/L or greater as compared with the reference S-K level of 4.5 to less than 5.0 mmol/L in both patients with CKD and patients with HF.13,14
In a US cohort study of patients with HF with a median follow-up of 2.79 years, the time-dependent exposure to abnormal S-K levels was assessed.21 As with the previous reports,8,13,14 a nonlinear U-shaped association with mortality risk was observed. In this study, associations between state of S-K level control, that is, normo-, hypo-, or hyperkalemia, and mortality risk were modeled using the multilevel survival analysis and notably, potassium level normalization was independently associated with lower mortality risk. In the subgroup analysis by CKD stages, the association of hyperkalemia with death event was lower in advanced CKD stages.
Similar findings were reported in a previous study based on a Swedish registry, showing lower relative risks for 90-day mortality associated with hyperkalemia in CKD stage 4 to 5 when compared with CKD stage 1 to 2.22 A physiologic adaptation to chronic hyperkalemia in these population may partially explain these results but cannot be ascertained from our observational analyses.23,24
Importantly, none of the studies noted investigated renal outcomes. In our study, there was a slight increase in eGFR immediately after the index hyperkalemic episode, which may be partially explained by renal recovery in hospitalized patients with acute conditions. After the initial increase in eGFR, a steeper eGFR decline was observed over 3 years in hyperkalemic patients when compared with normokalemic controls, suggesting the detrimental effect of hyperkalemia on the progression of renal dysfunction. A previous study that attempted to develop a reference value for eGFR decline rate in Japanese patients reported that eGFR decline in Japanese patients with stage 3a CKD was approximately −0.5 to −0.6 mL/min/1.73 m2 per year, which was comparable with the eGFR decline found in normokalemic controls in our study.25
Although a cause-effect relationship between hyperkalemia and an accelerated eGFR decline remains unclear, the abnormal physiologic effect from a high potassium load may directly and indirectly lead to progression of renal dysfunction. Hyperkalemia is reported to induce renal and cardiotoxicity in animals,26, 27, 28, 29 whereas findings in animals are not necessarily identical to those in humans and warrant further investigations.30 Hyperkalemia may also constitute constraints of the treatment for CKD. Discontinuations of the treatment for renal disease such as RAASi therapy due to hyperkalemia may be associated with the hyperfiltration in a short-term and more rapid renal function decline for a long-term period.31 It should also be noted that the higher proportion of sodium bicarbonate use in hyperkalemic patients indicates that they were prone to have metabolic acidosis. It is known that hyperkalemia induces metabolic acidosis by impaired renal ammonia excretion due to reduced ammonium production by proximal tubules and ammonium transport in collecting ducts.29,32 Therefore, appropriate treatment for hyperkalemia may potentially be a key factor to improve the renal outcome in patients with CKD.
Our study has several strengths, including the large sample size, drawn from a nationwide claims registry representing real-world practice, and a rigorous definition of hyperkalemia, which allowed us to examine its association with long-term adverse clinical outcomes.
Despite these advantages, this study also has several limitations. First, this study used hospital claims data. Hence, the data were not collected for specific research purposes. Sociodemographic factors such as nutritional status, quality of life, socioeconomic status, living conditions, and physical activities could not be retrieved from the database.33,34 Hyperkalemia may impede consuming a healthy diet, which potentially may cause worse nutritional status and physical condition and lower quality of life and eventually increase the risk for poor clinical outcomes. The lower cardiovascular and mortality risks associated with higher fruit and vegetable intake have been reported in various populations, including those undergoing maintenance hemodialysis.35,36 Likewise, studies have reported the association between fruit and vegetable consumption and health-related quality of life.37,38 A prospective cohort study design may be suitable to rigorously collect nutritional status and patient-reported outcomes; and this study may address the importance of more strict S-K level control, not only for better clinical outcomes but also for improving quality of life and for long-term clinical management of hyperkalemic patients.
One advantage of hospital claims data is that patient records were collected systematically and electronically as part of routine clinical practice, which helps avoid recall bias in collecting clinical information. Nearly 100% of all prescription information was captured in the data set. Furthermore, data were obtained from 374 hospitals across Japan, which improved the generalizability of the results. Although we tried to adjust for patient background and conditions as much as possible, some covariates had residual imbalances. For instance, the use of inotropes and MRAs was more prevalent in hyperkalemic patients. The selection of variables included in the PS modeling can affect both the validity and efficiency of the effect estimates.39,40 Therefore, we cannot be completely positive that the choice of some variables did not affect the outcome. Finally, because this is an observational study, the results need to be interpreted carefully and the associations found cannot be considered indicative of a causal relationship.
Conclusion
We reported the association between hyperkalemia and long-term adverse clinical outcomes in patients with CKD under continuous care. Hyperkalemia was associated with a significant risk for mortality and adverse clinical outcomes. The more rapid decline in renal function that we found may be related to the risk for adverse clinical outcomes. Our findings underscore the significance of hyperkalemic condition as a precursor of future adverse events. Continuous S-K level management in high-risk patients with CKD with hyperkalemia would be important for better clinical outcomes.
Acknowledgments
The authors thank Masafumi Okada, ZhenZhen Fang, Yiyun Lin, and all project members of IQVIA Solutions, KK, for providing technical and editorial support, including data analysis under the guidance of the authors.
Footnotes
Grant Support: This study and the corresponding analyses were supported and funded by AstraZeneca KK. AstraZeneca also manufactures a drug (sodium zirconium cyclosilicate) that is used to treat hyperkalemia. Authors from AstraZeneca participated in the organization of the study design and interpretation of the results and contributed to the manuscript drafts and revisions and the decision to approve publication of the final manuscript.
Potential Competing Interests: Dr Kohsaka reports investigator-initiated grant funding from Bayer and Daiichi Sankyo and personal fees from AstraZeneca, Bayer, Bristol-Myers Squibb, and Pfizer. Drs Okami and Yajima are employed by AstraZeneca KK. Drs Kanda and Kashihara report consultancy and speakers bureau from AstraZeneca KK.
Supplemental material can be found online at https://mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Gumz M.L., Rabinowitz L., Wingo C.S. An integrated view of potassium homeostasis. N Engl J Med. 2015;373(1):60–72. doi: 10.1056/NEJMra1313341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer B.F. Regulation of potassium homeostasis. Clin J Am Soc Nephrol. 2015;10(6):1050–1060. doi: 10.2215/CJN.08580813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaitman M., Dixit D., Bridgeman M.B. Potassium-binding agents for the clinical management of hyperkalemia. P.T. 2016;41(1):43–50. [PMC free article] [PubMed] [Google Scholar]
- 4.Khanagavi J., Gupta T., Aronow W.S., et al. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10(2):251–257. doi: 10.5114/aoms.2014.42577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer B.F. Managing hyperkalemia caused by inhibitors of the renin–angiotensin–aldosterone system. N Engl J Med. 2004;351(6):585–592. doi: 10.1056/NEJMra035279. [DOI] [PubMed] [Google Scholar]
- 6.Kovesdy C.P. Updates in hyperkalemia: outcomes and therapeutic strategies. Rev Endocr Metab Disord. 2017;18(1):41–47. doi: 10.1007/s11154-016-9384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tromp J., van der Meer P. Hyperkalaemia: aetiology, epidemiology, and clinical significance. Eur Heart J Suppl. 2019;21(suppl A):A6–A11. doi: 10.1093/eurheartj/suy028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo J., Brunelli S.M., Jensen D.E., Yang A. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol. 2016;11(1):90–100. doi: 10.2215/CJN.01730215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins A.J., Pitt B., Reaven N., et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46(3):213–221. doi: 10.1159/000479802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krogager M.L., Torp-Pedersen C., Mortensen R.N., et al. Short-term mortality risk of serum potassium levels in hypertension: a retrospective analysis of nationwide registry data. Eur Heart J. 2017;38(2):104–112. doi: 10.1093/eurheartj/ehw129. [DOI] [PubMed] [Google Scholar]
- 11.Kashihara N., Kohsaka S., Kanda E., Okami S., Yajima T. Hyperkalemia in real-world patients under continuous medical care in Japan. Kidney Int Rep. 2019;4(9):1248–1260. doi: 10.1016/j.ekir.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.An J.N., Lee J.P., Jeon H.J., et al. Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care. 2012;16(6):R225. doi: 10.1186/cc11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linde C., Qin L., Bakhai A., et al. Serum potassium and clinical outcomes in heart failure patients: results of risk calculations in 21334 patients in the UK. ESC Heart Fail. 2019;6(2):280–290. doi: 10.1002/ehf2.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuland H., McEwan P., Evans M., et al. Serum potassium as a predictor of adverse clinical outcomes in patients with chronic kidney disease: new risk equations using the UK clinical practice research datalink. BMC Nephrol. 2018;19(1):211. doi: 10.1186/s12882-018-1007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovesdy C.P., Matsushita K., Sang Y., et al. CKD Prognosis Consortium Serum potassium and adverse outcomes across the range of kidney function: a CKD prognosis consortium meta-analysis. Eur Heart J. 2018;39(17):1535–1542. doi: 10.1093/eurheartj/ehy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie D., Yang W., Jepson C., et al. Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. Statistical methods for modeling time-updated exposures in cohort studies of chronic kidney disease. Clin J Am Soc Nephrol. 2017;12(11):1892–1899. doi: 10.2215/CJN.00650117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Betts K.A., Woolley J.M., Mu F., McDonald E., Tang W., Wu E.Q. The prevalence of hyperkalemia in the United States. Curr Med Res Opin. 2018;34(6):971–978. doi: 10.1080/03007995.2018.1433141. [DOI] [PubMed] [Google Scholar]
- 18.Kovesdy C.P. Management of hyperkalaemia in chronic kidney disease. Nat Rev Nephrol. 2014;10(11):653–662. doi: 10.1038/nrneph.2014.168. [DOI] [PubMed] [Google Scholar]
- 19.Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf. 2007;16(3):241–249. doi: 10.1002/pds.1357. [DOI] [PubMed] [Google Scholar]
- 20.Thomsen R.W., Nicolaisen S.K., Hasvold P., et al. Elevated potassium levels in patients with congestive heart failure: occurrence, risk factors, and clinical outcomes: a Danish population-based cohort study. J Am Heart Assoc. 2018;7(11):e008912. doi: 10.1161/JAHA.118.008912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Núñez J., Bayés-Genís A., Zannad F., et al. Long-term potassium monitoring and dynamics in heart failure and risk of mortality. Circulation. 2018;137(13):1320–1330. doi: 10.1161/CIRCULATIONAHA.117.030576. [DOI] [PubMed] [Google Scholar]
- 22.Gasparini A., Evans M., Barany P., et al. Plasma potassium ranges associated with mortality across stages of chronic kidney disease: the Stockholm CREAtinine Measuments (SCREAM) project. Nephrol Dial Transplant. 2019;34(9):1534–1541. doi: 10.1093/ndt/gfy249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allon M. Hyperkalemia in end-stage renal disease: mechanisms and management. J Am Soc Nephrol. 1995;6(4):1134–1142. doi: 10.1681/ASN.V641134. [DOI] [PubMed] [Google Scholar]
- 24.Fennari F.J., Segal A.S. Hyperkalemia: an adaptive response in chronic renal insufficiency. Kidney Int. 2002;62(1):1–9. doi: 10.1046/j.1523-1755.2002.00350.x. [DOI] [PubMed] [Google Scholar]
- 25.Baba M., Shimbo T., Horio M., et al. Longitudinal study of the decline in renal function in healthy subjects. PLoS One. 2015;10(6):e0129036. doi: 10.1371/journal.pone.0129036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiatt N., Katayanagi T., Miller A. Cardiac sensitivity to hyperkalemia in adrenalectomized dogs. Proc Soc Exp Biol Med. 1975;149(1):168–171. doi: 10.3181/00379727-149-38765. [DOI] [PubMed] [Google Scholar]
- 27.Coulter D.B., Swenson M.J. Effects of potassium intoxication on porcine electrocardiograms. Am J Vet Res. 1970;31(11):2001–2011. [PubMed] [Google Scholar]
- 28.Fisch C. Relation of electrolyte disturbances to cardiac arrhythmias. Circulation. 1973;47(2):408–419. doi: 10.1161/01.cir.47.2.408. [DOI] [PubMed] [Google Scholar]
- 29.Harris A.N., Grimm P.R., Lee H.W., et al. Mechanism of hyperkalemia-induced metabolic acidosis. J Am Soc Nephrol. 2018;29(5):1411–1425. doi: 10.1681/ASN.2017111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montford J.R., Linas S. How dangerous is hyperkalemia? J Am Soc Nephrol. 2017;28(11):3155–3165. doi: 10.1681/ASN.2016121344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wetmore JB, Yan H, Horne L, Peng Y, Gilbertson DT. Risk of hyperkalemia from renin-angiotensin-aldosterone system inhibitors and factors associated with treatment discontinuities in a real-world population [published online ahead of print December 17, 2019. Nephrol Dial Transplant.https://doi.org/10.1093/ndt/gfz263. [DOI] [PubMed]
- 32.DuBose T.D., Jr. Hyperkalemic metabolic acidosis. Am J Kidney Dis. 1999;33(5):xlv–xlviii. doi: 10.1016/s0272-6386(99)70412-9. [DOI] [PubMed] [Google Scholar]
- 33.Saydah S.H., Imperatore G., Beckles G.L. Socioeconomic status and mortality: contribution of health care access and psychological distress among U.S. adults with diagnosed diabetes. Diabetes Care. 2013;36(1):49–55. doi: 10.2337/dc11-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lear S.A., Hu W., Rangarajan S., et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet. 2017;390(10113):2643–2654. doi: 10.1016/S0140-6736(17)31634-3. [DOI] [PubMed] [Google Scholar]
- 35.Saglimbene V.M., Wong G., Ruospo M., et al. Fruit and vegetable intake and mortality in adults undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2019;14(2):250–260. doi: 10.2215/CJN.08580718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly J.T., Palmer S.C., Wai S.N., et al. Healthy dietary patterns and risk of mortality and ESRD in CKD: a meta-analysis of cohort studies. Clin J Am Soc Nephrol. 2017;12(2):272–279. doi: 10.2215/CJN.06190616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nanri H., Yamada Y., Itoi A., et al. Frequency of fruit and vegetable consumption and the oral health-related quality of life among Japanese elderly: a cross-sectional study from the Kyoto-Kameoka study. Nutrients. 2017;9(12):1362. doi: 10.3390/nu9121362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steptoe A., Perkins-Porras L., Hilton S., Rink E., Cappuccio F.P. Quality of life and self-rated health in relation to changes in fruit and vegetable intake and in plasma vitamins C and E in a randomised trial of behavioural and nutritional education counselling. Br J Nutr. 2004;92(1):177–184. doi: 10.1079/BJN20041177. [DOI] [PubMed] [Google Scholar]
- 39.Brookhart M.A., Schneeweiss S., Rothman K.J., Glynn R.J., Avorn J., Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brookhart M.A., Wyss R., Layton J.B., Stürmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6(5):604–611. doi: 10.1161/CIRCOUTCOMES.113.000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.