Summary
Adipose-derived mesenchymal stromal cells (ADSCs) play important roles in the alleviation of inflammation and autoimmune diseases. Interleukin-33 (IL-33), a member of the IL-1 family, has been shown to regulate innate and adaptive immunity. However, it is still unknown whether ADSCs regulate immune responses via IL-33. We show here that ADSCs produced IL-33 in response to IL-1β stimulation, which depended on TAK1, ERK, and p38 pathways. ADSCs-derived IL-33 drove the proliferation of CD4+Foxp3+ST2+ regulatory T cells (Tregs) and alleviated experimental autoimmune Sjögren syndrome in mice. Importantly, human ADSCs also produced IL-33 in response to IL-1β. Thus, we have revealed a previously unrecognized immunoregulatory function of ADSCs by IL-33 production in experimental autoimmunity, which may have clinical applications for human immunopathology.
Subject areas: Immunology, Cell Biology
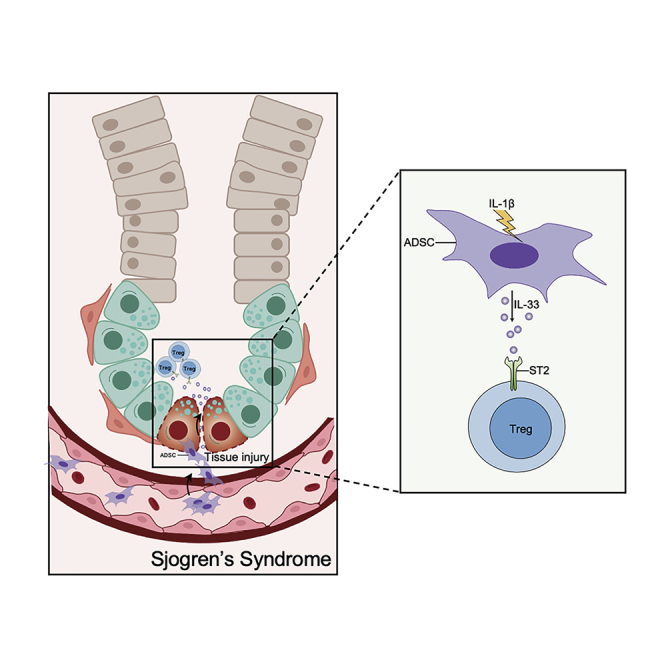
Graphical abstract
Highlights
-
•
Human and mouse ADSCs express IL-33 in response to IL-β stimulation
-
•
mADSC-derived IL-33 inhibits inflammation in salivary glands in SS model
-
•
mADSC-derived IL-33 expand ST2+ Tregs in vitro and in SS model
Immunology ; Cell Biology
Introduction
Adipose-derived mesenchymal stromal cells (ADSCs), a subtype of mesenchymal stromal cells (MSCs), played important roles in regenerative medicine to differentiating in bone and adipocytes and in modulating immune responses and alleviating inflammation or autoimmune diseases including arthritis, colitis, and autoimmune diabetes (Bassi et al., 2012; Gonzalez et al., 2009a, 2009b; Mizuno et al., 2012; Razmkhah et al., 2015; Shang et al., 2015; Zuk et al., 2001). The MSCs-tracking analysis showed that exogenously delivered MSCs could be detected in inflamed zones for the first week after injection, suggesting their interaction with inflammatory cells and cytokines. Indeed, studies reported that inflammatory cytokines such as interferon (IFN)-γ, interleukin (IL)-1β, and tumor necrosis factor (TNF)-α could be critical for MSC-mediated immunoregulation (Castelo-Branco et al., 2012; Chen et al., 2013; Tanaka et al., 2008; Xu et al., 2012). The inflammatory cytokines drive the immunosuppression function of MSCs through expression and release of different factors and cytokines in the inflammatory “niches” (Krampera et al., 2006; Meisel et al., 2004; Mougiakakos et al., 2011; Ren et al., 2008, 2009; Sheng et al., 2008).
IL-33 was first reported as a novel member of the IL-1 family in 2005 (Schmitz et al., 2005) and is now recognized as a crucial factor in influencing innate and adaptive immunity (Ali et al., 2011). Among its pleiotropic functions (Baumann et al., 2015; Gao et al., 2015; Hepworth et al., 2012; Price et al., 2010; Reichenbach et al., 2015; Yang et al., 2011), IL-33 drives proliferation of CD4+Foxp3+ regulatory T cells (Tregs) and group 2 innate lymphoid cells (ILC2s) and regulates immune responses in lymphoid organs, gut, lungs, and adipose tissues (Arpaia et al., 2015; Matta et al., 2016; Molofsky et al., 2015b; Mougiakakos et al., 2011; Schiering et al., 2014). IL-33 also functions for the maintenance of tissue homeostasis and repair of tissue damage (Liew et al., 2016; Molofsky et al., 2015a). Typically IL-33 is expressed at high levels in the nuclei of various cell types in human and mouse tissues in the steady state, including endothelial cells (Chen et al., 2015), epithelial cells (Nakanishi et al., 2013), macrophages, and fibroblast-like cells (Moussion et al., 2008; Pichery et al., 2012). Besides, it was recently reported that some subsets of MSCs expressing IL-33 were involved in the homeostasis and metabolism of adipocytes (Mahlakoiv et al., 2019). However, it remains largely unknown what drives MSCs to produce IL-33 and whether the IL-33 has immunoregulatory effects on distant organs and tissues beyond adipose tissues.
Results
Murine ADSCs produce IL-33 in response to IL-1β
ADSCs from C57BL/6 mice and IL-33-deficient mice (C57BL/6J-Il33tm1b(EUCOMM)Cln, Il33−/−) were isolated from subcutaneous fat tissues. ADSCs were determined based on their positivity for MSC markers SCA-1, CD44, CD90, and CD105 and negativity for epithelial cell marker CD31 and hematopoietic cell marker CD45 (Bourin et al., 2013) (Figure S1A). Also, ADSCs were functionally capable of differentiating into adipocytes, osteoblasts, and chondroblasts under standard differentiation culture conditions (Figure S1B). ADSCs exhibited no difference in their surface markers and multiple differentiation potential from wild-type (WT) and Il33−/− mice.
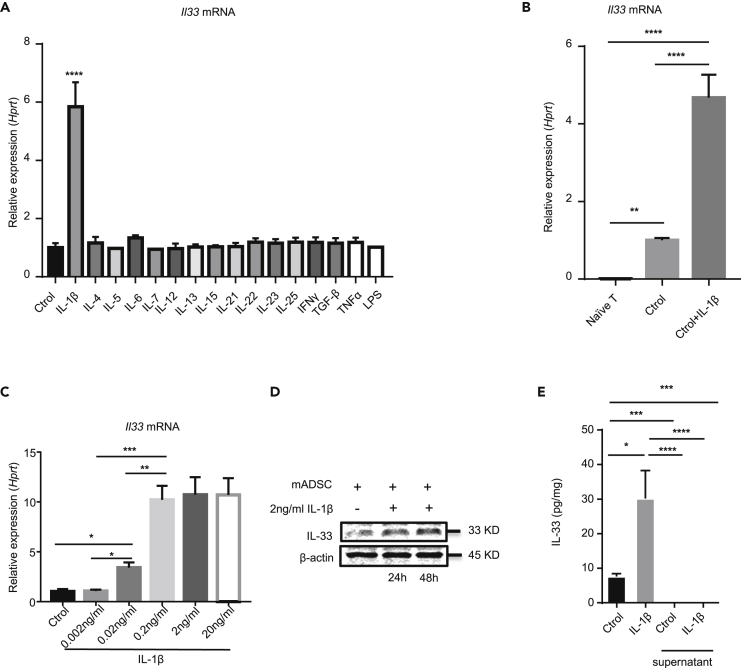
To mimic IL-33 production from mADSCs in the inflammatory “niches,” we stimulated ADSCs that were isolated from C57BL/6 mice with a panel of inflammatory cytokines in cultures for 6 h Il33 mRNA was determined by real-time PCR. Among the comprehensive panel of the cytokines we examined, only IL-1β was able to significantly upregulate Il33 mRNA expression in ADSCs (Figure 1A). ADSCs expressed spontaneous base levels of Il33 mRNA higher than did T cells (Figure 1B). IL-1β driving Il33 mRNA expression in ADSCs was dose-dependent and reached a plateau at 0.2 ng/mL of IL-1β (Figure 1C). Consistent with Il33 mRNA, western blot analysis showed a substantially higher level of IL-33 protein in IL-1β-treated ADSCs compared with untreated cells (Figure 1D). ELISA analysis also showed higher levels of IL-33 protein in the cell lysates from IL-1β-treated ADSCs when compared with the untreated cells, although IL-33 was hardly detected in the cell culture supernatants from all the cultures (Figure 1E). Of note, although IFN-γ alone was unable to induce Il33 mRNA, it enhanced IL-β-induced IL-33 in mouse ADSCs (Figure S2). Thus, mADSCs produce IL-33 upon IL-1β stimulation.
Figure 1.
mADSC can produce IL-33 with IL-1β stimulation
(A) Il33 mRNA expression in mADSC stimulated for 6 h with different cytokines as indicated. (2 ng/mL IL-1β, 10 ng/mL IL-4, 10 ng/mL IL-5, 50 ng/mL IL-6, 10 ng/mL IL-7, 10 ng/mL IL-12, 10 ng/mL IL-13, 10 ng/mL IL-15, 10 ng/mL IL-21, 10 ng/mL IL-22, 10 ng/mL IL-23, 10 ng/mL IL-25,10 ng/mL IFNγ, 2 ng/mL TGF-β, 10 ng/mL TNFα, 10 ng/mL LPS).
(B) Il33 mRNA can be produced in mADSC stimulated for 6 h with 10 ng/mL IL-1β.
(C) Il33 mRNA in mADSC stimulated for 6 h with different dose of concentration of IL-1β (0, 0.002, 0.02, 0.2, 2 and 20 ng/mL).
(D) Western blot of IL-33 and β-actin in mADSC stimulated with or without 2 ng/mL IL-1β for 24 or 48 h
(E) ELISA of IL-33 in mADSC lysate and supernatant stimulated with or without 10 ng/mL IL-1β for 72 h. Data are pooled from three (A and C) independent experiments or are representative of three (B, D, and E) independent experiments. In (A), (B), (C), and (E) one-way ANOVA was used. Bars, mean; error bars, SEM; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
IL-1β upregulates IL-33 through TAK1-ERK/p38 pathways in mADSCs
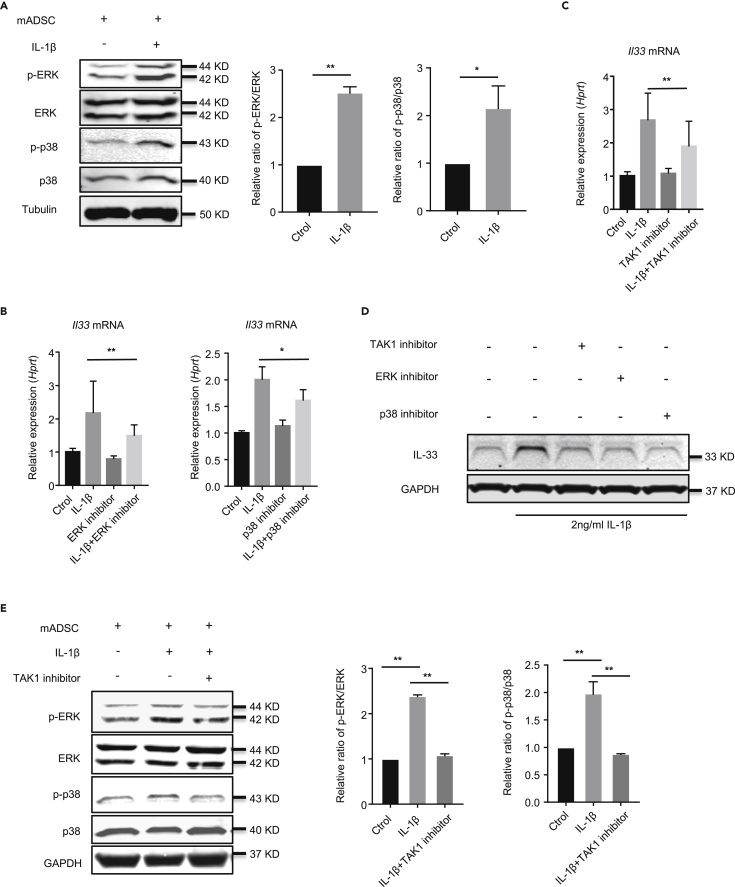
We next investigated the signaling pathways by which IL-33 was induced by IL-1β stimulation in ADSCs. We first analyzed the expression and activation of ERK, p38, JNK, and nuclear factor (NF)-κB proteins in ADSCs stimulated with IL-1β. We found that the relative ratio of phosphorylated ERK (p-ERK)/ERK and phosphorylated p38 (p-p38)/p38 were increased in ADSCs treated with IL-1β compared with the untreated cells (Figure 2A). However, there were no significant differences between the phosphorylated JNK and NF-κB (p65) proteins between IL-1β-treated and untreated ADSCs (Figures S3A and S3B).
Figure 2.
IL-1β upregulates IL-33 expression by promoting TAK1, ERK, and p38 pathway in mADSC
(A) Western blot of p-ERK, ERK, p-p38, p38, and Tubulin in mADSC stimulated with or without IL-1β for 2 h
(B) Il33 mRNA expression in mADSC stimulated with or without IL-1β, ERK inhibitor, or p38 inhibitor for 6 h
(C) Il33 mRNA expression in mADSC stimulated with or without IL-1β, TAK1 inhibitor for 6 h.
(D) Western blot of IL-33 and GAPDH in mADSC stimulated with or without IL-1β, TAK1 inhibitor, ERK inhibitor, or p38 inhibitor for 24 h.
(E) Western blot of p-ERK, ERK, p-p38, p38, and GAPDH in mADSC stimulated with or without IL-1β, TAK1 inhibitor for 1 h. Data are pooled from three (B and C) independent experiments or are representative of three (A, D, and E) independent experiments. In (A), Student's t test was used. In (B), (C), and (E) one-way ANOVA was used. Bars, mean; error bars, SEM; ∗p < 0.05, ∗∗p < 0.01.
Next, we investigated which of the aforementioned pathways was involved in IL-1β-driven IL-33 induction in ADSCs by utilizing the selective protein kinase inhibitors 0.2 μM U0126 (for ERK), 20 μM SB203580 (for p38 MAPK), 2 μM SP600125 (for JNK), and 2 μM JSH23 (for NF-κB) to block the activity of the indicated proteins. We found that inhibition of ERK and p38 MAPK activity significantly reduced IL-1β-induced Il33 mRNA and IL-33 protein (Figures 2B and 2D), whereas inhibition of JNK and NF-κB failed to do so (Figures S3C and S3D). In addition, suppression of TAK1 activity also decreased Il33 mRNA and IL-33 protein in ADSCs (Figures 2C and 2D). To explore the mechanism of the TAK1 signal pathway during IL-1β-driven IL-33, we examined whether TAK1 inhibition affects the phosphorylation of p38 and ERK. Our result showed that the phosphorylation of p38 and ERK was significantly reduced in TAK1 inhibitor (100 nM) group after IL-1β treatment (Figure 2E). These data suggest that IL-1β activates TAK1, up-regulating phosphorylation of p38 and ERK, and subsequently mediates IL-33 expression.
mADSCs-derived IL-33 regulates ST2 expression in Tregs in vitro
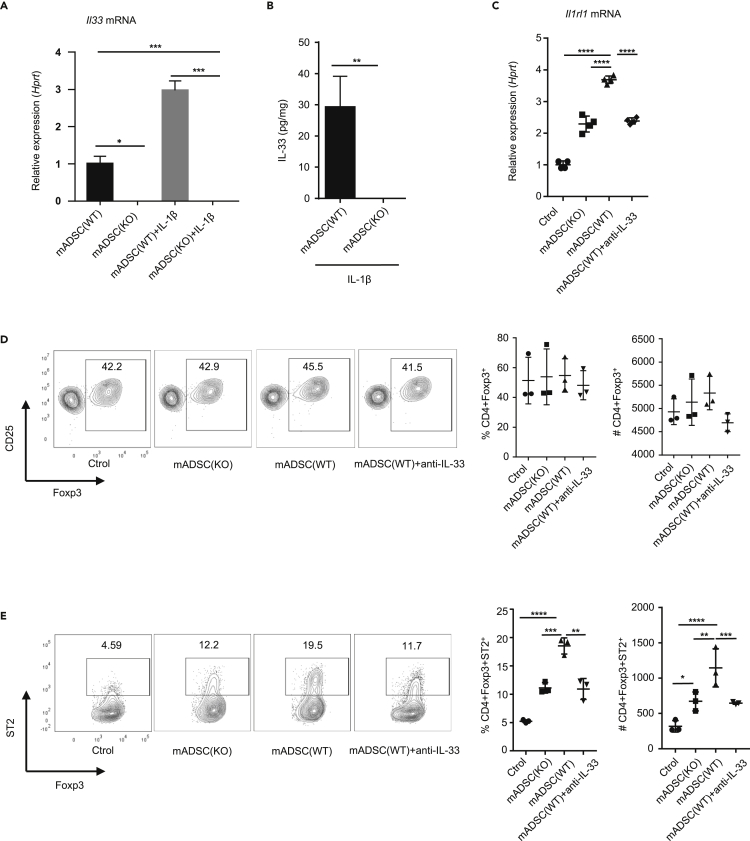
To determine the immunoregulatory function of IL-33 produced by ADSCs, we next studied the effects of IL-1β-treated mADSCs lysates on CD4+CD25+Foxp3+ Tregs (Chen et al., 2003), because IL-33 has been shown to promote the proliferation of Tregs (Matta et al., 2016; Vasanthakumar et al., 2015). ADSCs from WT or Il33−/− mice were cultured with or without IL-1β to confirm that Il33 mRNA and IL-33 protein were detected only in WT but not Il33−/− cells (Figures 3A and 3B). CD4+CD25+ Tregs isolated from spleen and lymph nodes in C57BL/6 mice were cultured with IL-1β-stimulated WT or Il33−/− ADSCs lysates. We found that IL-1β-stimulated WT ADSCs lysates significantly upregulated Il1rl1 mRNA (receptor for IL-33, ST2) expression in Tregs, which was abrogated by the inclusion of anti-IL-33 antibody in the cultures (Figure 3C). Consistently, IL-1β-stimulated Il33−/− ADSCs lysates exhibited significantly lower activity to upregulate Il1rl1 mRNA in Tregs than did WT ADSCs lysates (Figure 3C). Consequently, the frequency and the absolute number of ST2+ Treg subsets within the CD4+Foxp3+ Tregs cultured with IL-1β-treated WT ADSCs were much higher than those of ST2+ Treg subsets in Tregs treated with Il33−/− ADSCs (Figures 3D and 3E). Consistently, the increase in ST2+ Tregs in Tregs stimulated with WT ADSCs was entirely abolished by neutralization of IL-33 with anti-IL33 antibody (Figure 3E). The data collectively indicate that IL-33 from mADSCs upregulates ST2+ Tregs in cultures.
Figure 3.
IL-33 from mADSC affects ST2 expression on Treg in vitro
(A) Il33 mRNA expression in ADSC from WT and Il33−/− mice stimulated for 6 h with or without 10 ng/mL IL-1β.
(B) ELISA of IL-33 protein in mADSC stimulated with 10 ng/mL IL-1β for 72 h
(C) Il1rl1 mRNA expression in CD4+CD25+ T cell from spleen with anti-CD3/CD28 plus stimulated with or without ADSC lysate from WT or Il33−/− mice or ADSC soap from WT mice with anti-IL-33 for 24 h.
(D) Frequency and absolute number of Foxp3+ T cells in CD4+CD25+ T cell from spleen with anti-CD3/CD28 plus stimulated with or without ADSC lysate from WT or Il33−/− mice or ADSC soap from WT mice with anti-IL-33 for 72 h.
(E) Frequency and absolute number of ST2+ T cells in CD4+Foxp3+ T cell from spleen with anti-CD3/CD28 plus stimulated with or without ADSC lysate from WT or Il33−/− mice or ADSC soap from WT mice with anti-IL33 for 72 h. Data are pooled from three (A, C, D, and E) independent experiments or are representative of three (B) independent experiments. In (B), Student's t test was used. In (A), (C), (D), and (E), one-way ANOVA was used. Bars, mean; error bars, SEM; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
WT but not Il33−/− mADSCs improve Sjögren syndrome by suppressing inflammation in salivary glands in NOD mice
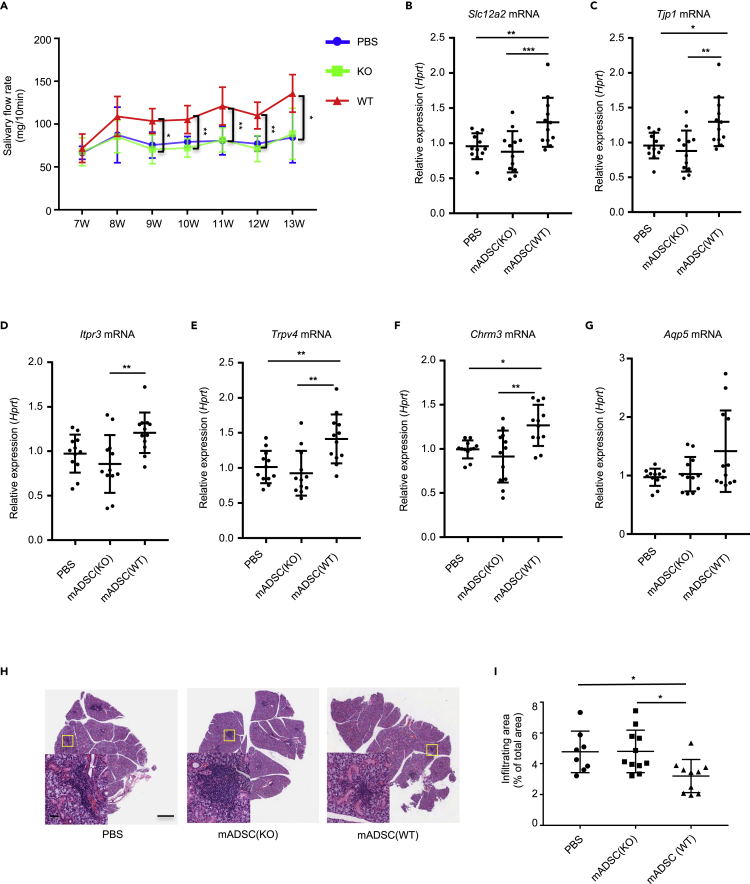
The expansion of ST2+ Tregs by ADSCs-derived IL-33 encouraged us to investigate the immunoregulatory and therapeutic function of ADSCs in autoimmunity and inflammation in vivo. For this, we intravenously injected WT and Il33−/− mADSCs (both on C57BL/6 background) into allogeneic NOD/ShiLtj mice (NOD, on Cataract Shionogi background) that have been well known to develop an autoimmune Sjögren syndrome (SS) spontaneously. The rationale underlying the utilization of allogeneic ADSC transplantation was based on the published animal and clinical studies that show negligible allorejection and side effects of allogeneic MSCs transplantation due to the non/low MHC II expression on MSCs, yet exhibition of beneficial and immunoregulatory function (Alexeev et al., 2014; Xu et al., 2012). We injected allogeneic ADSCs from WT or Il33−/− mice or PBS into NOD/ShiLtj mice at 7 weeks of age when the inflammation starts to occur in the submandibular glands. Yet, the secretory function of the submandibular glands has not been compromised (Delaleu et al., 2011). The saliva flow rates were measured weekly from 7 through 13 weeks (Figure S4A). We found that WT ADSCs transplantation significantly improved the saliva flow rates 2 weeks after injection in NOD/ShiLtj mice compared with PBS- or Il33−/− mADSCs-treated mice (Figure 4A). We then examined the expression of genes, including Slc12a2, Tjp1, Aqp5, Itpr3, Trpv4, and Chrm3, which have been known to be relevant to submandibular gland function at the 13th week (Markadieu and Delpire, 2014; Wang et al., 2017). The expressions of all those genes except Aqp5 mRNA in submandibular glands were significantly increased in WT ADSCs-treated mice compared with the mice treated with Il33−/− mADSCs or PBS (Figures 4B–4G). Consistent with the improvement of saliva flow and the upregulation of the genes associated with submandibular gland function, histological analysis of the submandibular gland tissues revealed that transplantation of WT ADSCs resulted in a substantial reduction of inflammatory areas in the submandibular glands compared with Il33−/− ADSCs- or PBS-treated groups (Figures 4H and 4I). These findings altogether indicate that allogeneic ADSCs suppress inflammation and improve the secretory function of salivary glands in an IL-33-dependent manner.
Figure 4.
WT but not Il33−/− mADSCs suppress inflamed tissue damage and improve submandibular gland function in NOD/ShiLtj mice
(A) Salivary flow rate of NOD/ShiLtj treated with PBS, ADSC from WT or Il33−/− mice. PBS group (n = 12), KO group (n = 12), WT group (n = 12). (B) Slc12a2 mRNA expression in the submandibular glands of NOD/ShiLtj mice injected with PBS, ADSC from WT or Il33−/− mice.
(C) Tjp1 mRNA expression in the submandibular glands of NOD/ShiLtj mice injected with PBS, ADSC from WT or Il33−/− mice.
(D) Itpr3 mRNA expression in the submandibular glands of NOD/ShiLtj mice injected with PBS, ADSC from WT or Il33−/− mice.
(E) Trpv4 mRNA expression in the submandibular glands of NOD/ShiLtj mice injected with PBS, ADSC from WT or Il33−/− mice.
(F) Chrm3 mRNA expression in the submandibular glands of NOD/ShiLtj mice injected with PBS, ADSC from WT or Il33−/− mice.
(G) Aqp5 mRNA expression in the submandibular glands of NOD/ShiLtj mice injected with PBS, ADSC from WT or Il33−/− mice.
(H) Histology of the submandibular glands of NOD/ShiLtj mice injected with PBS, ADSC from WT or Il33−/− mice.
(I) The infiltrating area in the submandibular glands of NOD/ShiLtj mice injected with PBS, ADSC from WT or Il33−/− mice. Data are pooled from three independent experiments. One-way ANOVA was used. Scale bars, 100 μm and 50 μm; bars, mean; error bars, SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
ADSC-derived IL-33 upregulates ST2+ Tregs in NOD mice
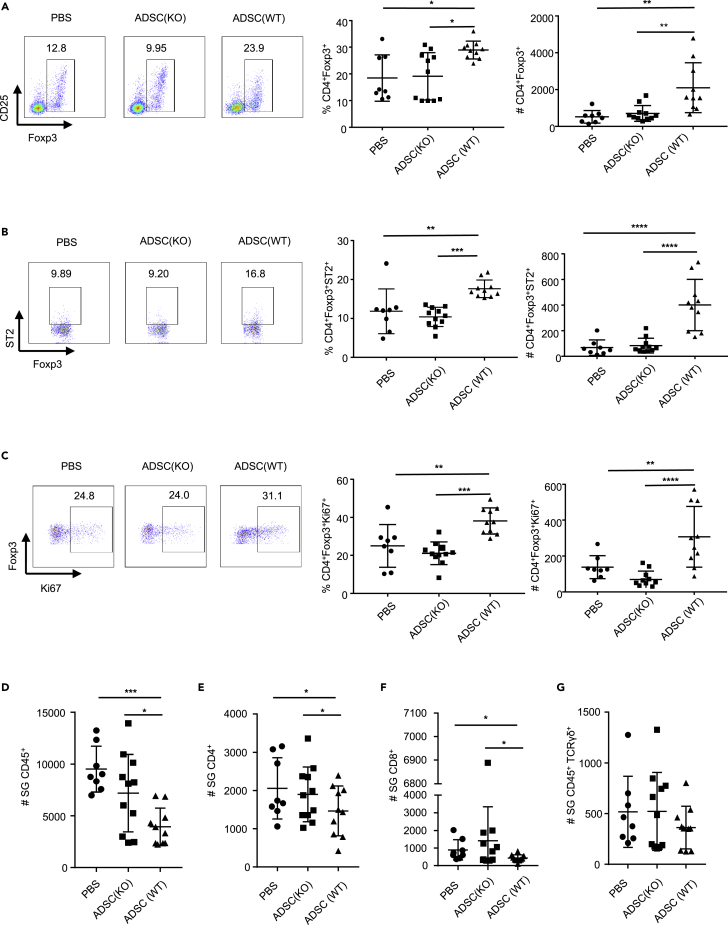
To understand the underlying mechanisms by which allogeneic ADSCs IL-33 suppressed inflammation and improved the function of submandibular glands, we examined the changes of Tregs and proinflammatory effector cells in the submandibular glands of NOD mice. Strikingly, WT ADSCs treatment resulted in a significant increase in both the frequency and absolute number of CD4+Foxp3+ Tregs in the submandibular glands in SS-like NOD mice compared with other groups (Figure 5A). More importantly, WT ADSCs treatment, but not Il33−/− ADSCs treatment, led to a significant increase in the frequency and total number of ST2+ Tregs (Figure 5B). Further analysis of in vivo Treg expansion with Ki67 staining revealed that WT ADSCs treatment caused significantly more Ki67+ Tregs in the submandibular glands than did Il33−/− ADSCs or PBS treatment, suggesting IL-33-driven Treg proliferation upon WT ADSCs transplantation (Figure 5C).
Figure 5.
WT ADSCs increase the frequency and an absolute number of CD4+Foxp3+ST2+ Tregs in the submandibular glands of NOD/ShiLtj mice
(A) Frequency and absolute number of Foxp3+ T cells in the submandibular glands of NOD/ShiLtj mice injected with PBS, ADSC from WT or Il33−/− mice.
(B) Frequency and absolute number of ST2+ T cells in CD4+Foxp3+ T cell in the submandibular glands of NOD/ShiLtj mice injected with PBS, ADSC from WT or Il33−/− mice.
(C) Frequency and absolute number of Ki67+ in CD4+Foxp3+ T cells in the submandibular glands of NOD/ShiLtj mice injected with PBS, ADSC from WT or Il33−/− mice.
(D) Absolute number of CD45+ cells in the submandibular glands of NOD/ShiLtj mice injected with PBS, ADSC from WT or Il33−/− mice.
(E) Absolute number of CD45+TCRβ+CD4+ cells per mg of submandibular gland of NOD/ShiLtj mice injected with PBS, ADSC from WT or Il33−/− mice.
(F) Absolute number of CD45+TCRβ+CD8+ cells in the submandibular glands of NOD/ShiLtj mice injected with PBS, ADSC from WT or Il33−/− mice.
(G) Absolute number of CD45+TCRγδ+ cells in the submandibular glands of NOD/ShiLtj mice injected with PBS, ADSC from WT or Il33−/− mice. Data are pooled from three independent experiments. One-way ANOVA was used. Bars, mean; error bars, SEM; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
As IL-33 is also associated with the differentiation and function of various lymphocytes including type 2 helper T (Th2) cells and ILC2s (Licona-Limon et al., 2013; Molofsky et al., 2013; Moro et al., 2010; Schmitz et al., 2005) in addition to Tregs, we studied Th2 (IL-4+, IL-13+) CD4+ T cells and ILC2s in the submandibular glands among all the groups of NOD mice and no significant differences could be observed (Figures S4–S7). Furthermore, the frequency of IL-13-expressing (Lin−CD45+GATA3+ST2+IL-13+) and IL-4-producing (Lin−CD45+GATA3+ST2+IL-4+) ILC2 subsets also did not exhibit changes after ADSCs treatment (Figures S8A–S8C). Interestingly, the frequency of CD8+TCRαβ+IL-4+ T cells increased in the submandibular glands of WT ADSCs-treated group compared with Il33−/− ADSCs- or PBS-treated groups (Figure S6D), but the significance of the increase remains unknown.
We next examined the CD45+ inflammatory cells in the submandibular glands. WT ADSCs treatment caused significantly fewer CD45+ cells in the submandibular glands than Il33−/− ADSCs or PBS-treated mice (Figure 5D). The absolute number of CD4+ (Figure 5E) and CD8+ (Figure 5F) T cells, but not TCRγδ+ T cells (Figure 5G), significantly decreased in WT ADSC-treated mice compared with IIl33−/− ADSCs- or PBS- treated groups. Consequently, the total number of IFN-γ+CD4+ T cells (Th1) and IFN-γ+CD8+ T cells were decreased in WT ADSC-treated NOD mice, although the frequency did not change significantly (Figures S4B–S4E, S5A, S5E, S6A, S6E, S7A, and S7E). Interestingly, both WT and Il33−/− mADSCs treatment showed a similar reduction of IL-17+ T cells compared with the PBS group, suggesting that the decrease of IL-17 was independent of IL-33 (Figures S5B and S7B). In contrast to the submandibular glands, there was no significant difference in the frequency and number of CD4+Foxp3+ Tregs, ST2+Tregs and Ki67+ Tregs in the spleen, and armpit, inguinal and submandibular lymph nodes of all groups of NOD/ShiLtj mice (Figure S9). Taken together, these data indicate that mADSCs-IL-33-mediated suppression of inflammation and improvement of submandibular gland function was mainly ascribed to the enhanced number of ST2+ Tregs and reduced the number of CD45+ proinflammatory cells especially IFN-γ+ T cells in the glands.
IL-1β upregulates IL-33 in human ADSCs
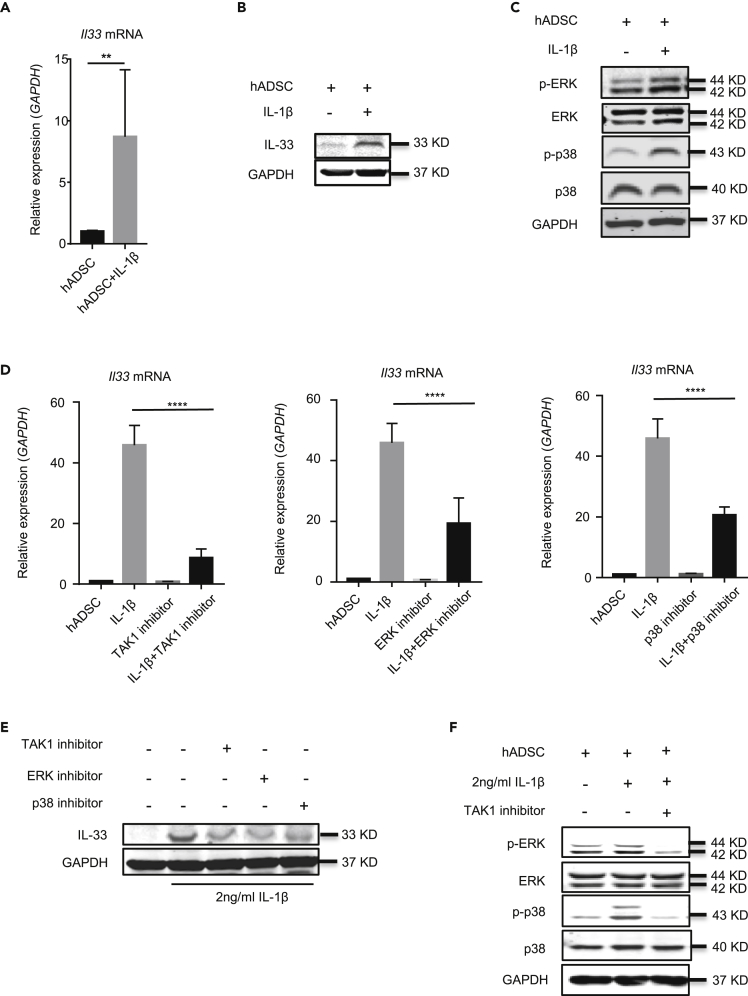
We next investigated whether human ADSCs also expressed IL-33 and IL-1β upregulated its expression. For this, we cultured human ADSCs with or without 2 ng/mL IL-1β for 6 and 24 h and then checked IL33 mRNA and IL-33 protein, respectively. Untreated human ADSCs hardly exhibited IL-33; however, IL-1β treatment significantly upregulated the amount of IL33 mRNA in human ADSCs at 6 h (Figure 6A). IL-33 protein was also increased significantly by IL-1β treatment in human ADSCs at 24 h (Figure 6B). We also studied the signal pathways for IL-33 production by human ADSCs in response to IL-1β. Consistent with murine ADSCs, the levels of phosphorylated ERK and p38 proteins were significantly increased in human ADSCs after IL-1β stimulation compared with the unstimulated cells (Figure 6C). Moreover, the selective protein kinase inhibitors (5Z)-7-oxozeaenol, U0126, and SB203580, which inhibit TAK1, ERK, and p38 MAPK, respectively, significantly decreased the inductive effect of IL-1β on IL33 mRNA and IL-33 protein in human ADSCs (Figures 6D and 6E). TAK1 inhibitor also decreased the relative ratio of phosphorylated ERK/ERK and phosphorylated p38/p38 (Figure 6F). These data indicated that human ADSCs also produce IL-33 by IL-1β stimulation through TAK1-ERK/p38 signal pathways, suggesting a translational relevance and significance of the murine ADSCs treatment in animal SS models.
Figure 6.
IL-1β upregulates IL-33 expression in human ADSCs
(A) IL33 mRNA expression in human ADSC stimulated for 6 h with or without 2 ng/mL IL-1β.
(B) Western blot of IL-33 and GAPDH in human ADSC stimulated with or without 2 ng/mL IL-1β for 24 h.
(C) Western blot of p-ERK, ERK, p-p38, p38, and GAPDH in human ADSC stimulated with or without IL-1β for 2 h
(D) IL33 mRNA expression in human ADSC stimulated for 6 h with or without IL-1β, TAK1 inhibitor, ERK inhibitor, or p38 inhibitor for 6 h.
(E) Western blot of IL-33 and GAPDH in human ADSC stimulated with or without IL-1β, TAK1 inhibitor, ERK inhibitor, or p38 inhibitor for 24 h.
(F) Western blot of p-ERK, ERK, p-p38, p38, and GAPDH in human ADSC stimulated with or without IL-1β, TAK1 inhibitor for 1 h. Data are pooled from three (A and D) independent experiments or are representative of three ( B, C, E and F ) independent experiments. In (A), Student's t test was used. In (D), one-way ANOVA was used. Bars, mean; error bars, SEM; ∗∗p < 0.01, ∗∗∗∗p < 0.0001.
Discussion
In this article, we demonstrated that ADSCs represent an important cellular source of IL-33 that could be induced specifically by IL-1β among the dozens of immune cytokines tested. We further elucidated that IL-1β-induced IL-33 production by ADSCs was mediated by TAK1-ERK/p38 signaling pathways. By using Il33−/− mice, we have uncovered that allogeneic ADSCs transplantation was able to suppress the inflammation and improve the saliva secretory function of salivary glands in NOD mice via an IL-33-dependent manner. Importantly, we have elucidated that the immunoregulatory function in SS by ADSCs-derived IL-33 is mediated mainly through upregulation of ST2+ Tregs in the submandibular glands. Strikingly, we have shown that human ADSCs also produce IL-33 in response to IL-1β stimulation in vitro, suggesting a clinical relevance and significance in human diseases such as SS.
Several conclusions can be drawn from our findings. First, ADSCs are an important cellular source of IL-33 and IL-1β is the most important cytokine to induce IL-33 in ADSCs. IL-33 has been recognized as a unique but crucial regulatory cytokine in immunoregulation (Baumann et al., 2015; Gao et al., 2015; Hepworth et al., 2012; Price et al., 2010; Reichenbach et al., 2015; Yang et al., 2011) in several physiological and pathological settings such as autoimmunity, obesity, and allergy/asthma (Nakanishi et al., 2013; Nechama et al., 2018; Scott et al., 2018; Zhu et al., 2017). Several types of cells have been reported to produce IL-33 including endothelial cells (Chen et al., 2015), epithelial cells (Nakanishi et al., 2013), macrophages, dendritic cells, mast cells, and fibroblast-like cells (Moussion et al., 2008; Pichery et al., 2012) (Su et al., 2013; Xu et al., 2008) in response to certain stimuli such as LPS, IFN-γ, TNF-α, IL-3, IL-4, IL-17, and IL-1β (Meephansan et al., 2012, 2013; Su et al., 2013; Xu et al., 2008; Zhao and Hu, 2012). However, it was unknown whether MSCs could produce IL-33 until recently. Few studies have started to show that MSCs can produce IL-33 (Mahlakoiv et al., 2019), but what is the factor(s)/cytokines to stimulate MSCs to produce IL-33 remained unknown. We have here extended the studies by unambiguously proving that ADSCs indeed produce IL-33. Importantly, we have discovered that IL-1β is the critical factor in driving IL-33 expression in ADSCs. This finding is significant as it provides a previously unrecognized mechanism underlying MSCs-mediated immunoregulatory activities in the inflammatory tissues and organs and the paradoxical requirement of proinflammatory cytokines such as IL-1β (Bassi et al., 2012; Ding et al., 2010; Liu et al., 2013; Meisel et al., 2004). The specific stimulatory function for IL-1β in MSC-IL-33 production is unexpected but exciting. Although it is presently unknown why IL-1β, but not other proinflammatory cytokines such as TNF-α and IL-6, is the key to induce IL-33 secretion by ADSCs, it does suggest a possible functional link between IL-1β-producing cells and ADSCs. As IL-1β can be a cell membrane-bound form, this finding also suggests an interaction and functional regulation via a cell-cell contact manner, a fascinating question to be explored. The specific role of IL-1β in inducing IL-33 by ADSCs is further supported by the downstream molecular pathways that are involved in the effect, which is mainly mediated by ERK and p38 and TAK1, rather than NF-κB and JNK. As shown by qPCR analysis in Figures 1 and 5, we observed that human ADSCs expressed higher levels of IL-33 than did mouse ADSCs in response to their respective IL-1β stimulation. Although mechanistically still elusive, the species difference was reported before. For example, a study reported that human IL-33 was converted into more stable mature forms; however, mouse IL-33 was rapidly degraded (Cayrol et al., 2018). Thus further work is needed to understand the specific variation in the future.
Second, ADSCs can regulate inflammation and suppress autoimmunity in distant tissues and organs by producing IL-33. Supporting this conclusion includes that administration by intravenous injection of ADSCs from WT mice, but not from Il33−/− mice, effectively suppressed the ongoing inflammation in the submandibular glands and protected the secretary function of saliva in NOD mice. Our findings here provided a mechanism for the immunoregulatory effect of allogenic ADSCs through an IL-33-dependent mechanism.
Moreover, we found that the immunoregulatory function of ADSC-derived IL-33 is mainly through the expansion of ST2+ Tregs in the submandibular glands. Several pieces of evidence support this conclusion: in vitro, WT, but not Il33−/−, ADSCs-mediated expansion of Tregs are primarily restricted to the ST2+ Tregs. In contrast, ADSCs fail to convert naive CD4+ T cells to Foxp3+ Tregs, consistent with the notion that TGF-β (Chen et al., 2003) rather than IL-33 is the primary driving force to induce Foxp3 from naive CD4+ T cells. IL-33 is the cytokine to expand ST2+ Tregs. This IL-33-driven ST2+ Treg expansion was confirmed in the submandibular glands in SS NOD mice. Administration of WT, but not Il33−/−, ADSCs significantly expand ST2+ Tregs in the submandibular glands of SS mice, which consequently protects the function of salivary glands. Notably, the increase in ST2+ Tregs in the submandibular glands is not only restricted to the frequency but also the absolute number of the ST2+ Tregs, further confirming the real growth of the Tregs by IL-33. The significant expansion of ST2+ Tregs in the submandibular glands plays an important role in suppressing the inflammation of the tissue. This is supported by the significant decrease in the absolute number of infiltrated CD45+ proinflammatory cells in the submandibular glands of SS-like NOD mice treated with WT ADSCs. IL-13-producing T cells have been implicated in the pathogenesis of an SS animal model that is induced by the deficiency of transcriptional factor Id3 (Li et al., 2004). However, we did not find that IL-13+ T cells were changed by ADSCs treatment, suggesting the ADSCs-IL-33 functioning NOD model was not primarily by affecting IL-13+ Th2 cells. Of note, WT ADSCs treatment surprisingly increased the frequency and number of CD8+IL-4+ T cells in the submandibular glands; although the overall frequency is low, it remains to be known whether these IL-4+ T cells play any function in the immunoregulatory effects on SS. The suppressive function of Tregs and ST2+ Tregs in immune cells and responses has been validated in many in vitro and in vivo settings in the literature, including in NOD mice (Alunno et al., 2015; Burzyn et al., 2013; Katsifis et al., 2007; Sarigul et al., 2010).
Third, IL-33 can be produced by ADSC with IL-1β stimulation, although IL-33 but cannot be secreted. Supporting this conclusion includes that IL-33 was hardly detected in the cell culture supernatants, although ELISA analysis also showed higher levels of IL-33 protein in the cell lysates from IL-1β-treated ADSCs compared with the untreated cells. Moreover, the cell lysates from IL-1β-stimulated WT ADSCs significantly upregulated Il1rl1 mRNA (receptor for IL-33, ST2) expression in Tregs. There must be a way for MSCs in vivo to be equipped functionally and recruited accurately to damaged tissue. Several researches have proved that following intravenous infusion of MSC populations expanded in vitro, almost 80% MSCs are trapped in the lungs, and these cells in lung disappeared with a half-life of about 24 h; some MSCs subsequently become home to damaged tissue (Barbash et al., 2003; Lee et al., 2009). We have also previously reported that allogenic MSCs could exhibit protective effects on SS development in mice by showing that MSCs could migrate into the submandibular glands to function (Xu et al., 2012). Therefore, the therapeutic effects of MSCs may depend mainly on the release of IL-33 of dead MSCs to regulate inflammation and tissue homeostasis.
Finally, the ADSCs-IL-33 may also play an important role in MSC-mediated immunoregulation in relevant human autoimmune diseases like SS. Supporting this conclusion include our findings that human ADSCs also produce IL-33, which is also triggered by IL-1β stimulation in vitro. Although it still needs to be confirmed in the patients, our experimental evidence indeed shows the clinical relevance of our animal studies.
In sum, we have discovered that adipose tissues contain MSCs that produce IL-33 in response to proinflammatory cytokine IL-1β. This MSC-derived IL-33 can suppress inflammation in organ-specific autoimmunity such as SS through specifically expanding ST2+ Tregs. Our findings may be clinically and translationally relevant, as human ADSCs also produce IL-33 in response to IL-1β. These findings collectively should have implications for better manipulation of allogeneic MSCs in relevant human autoimmune diseases such as SS.
Limitations of the study
There are still some limitations in our study. For example, it remains to be elucidated how important IL-1β-mediated increase in IL-33 from ADSC is in the SS model. The technical challenge and difficulty prevented us to use ST2 knockout Tregs to provide further evidence of IL-33/ST2 pathway in Treg-mediated suppression of SS models, which we will address in the future. Moreover, it would be interesting to investigate whether ADSC-derived IL-33 has any effects on Treg function through their ST2 receptors in patients with SS.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Wanjun Chen(wchen@dir.nidcr.nih.gov).
Materials availability
This study did not generate new unique reagents.
Data and code accessibility
This study did not generate/analyze datasets and code.
Methods
All methods can be found in the accompanying Transparent methods supplemental file.
Acknowledgments
We thank Dr. M. Colonna, Dept of Pathology and Immunology, Washington University, MO, USA, for C57BL/6J-Il33tm1b(EUCOMM)Cln. We also thank the NIDCR CTRC and VRC for their technical support. This work was supported by the Intramural Research Program of the National Institute of Dental and Craniofacial Research (US National Institutes of Health). This work was also supported by the grant from the National Natural Science Foundation of China (81970905 to O.S.L.).
Author contributions
O.L. and J.X. designed and performed experiments, analyzed and interpreted the data, and drafted the manuscript; F.W., W.J., N.G., P.Z., N.G., Y.H., and A.B. performed experiments; G.M., S.W., and Z. T. provided critical input and/or support. W.C. conceived and supervised the whole study, designed the experiments, and wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: May 21, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102446.
Supplemental information
References
- Alexeev V., Arita M., Donahue A., Bonaldo P., Chu M.L., Igoucheva O. Human adipose-derived stem cell transplantation as a potential therapy for collagen VI-related congenital muscular dystrophy. Stem Cell Res. Ther. 2014;5:21. doi: 10.1186/scrt411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S., Mohs A., Thomas M., Klare J., Ross R., Schmitz M.L., Martin M.U. The dual function cytokine IL-33 interacts with the transcription factor NF-kappaB to dampen NF-kappaB-stimulated gene transcription. J. Immunol. 2011;187:1609–1616. doi: 10.4049/jimmunol.1003080. [DOI] [PubMed] [Google Scholar]
- Alunno A., Carubbi F., Bistoni O., Caterbi S., Bartoloni E., Mirabelli G., Cannarile F., Cipriani P., Giacomelli R., Gerli R. T regulatory and T helper 17 cells in primary Sjogren's syndrome: facts and perspectives. Mediators Inflamm. 2015;2015:243723. doi: 10.1155/2015/243723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia N., Green J.A., Moltedo B., Arvey A., Hemmers S., Yuan S., Treuting P.M., Rudensky A.Y. A distinct function of regulatory T cells in tissue protection. Cell. 2015;162:1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbash I.M., Chouraqui P., Baron J., Feinberg M.S., Etzion S., Tessone A., Miller L., Guetta E., Zipori D., Kedes L.H. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- Bassi E.J., Moraes-Vieira P.M., Moreira-Sa C.S., Almeida D.C., Vieira L.M., Cunha C.S., Hiyane M.I., Basso A.S., Pacheco-Silva A., Camara N.O. Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes. 2012;61:2534–2545. doi: 10.2337/db11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann C., Bonilla W.V., Frohlich A., Helmstetter C., Peine M., Hegazy A.N., Pinschewer D.D., Lohning M. T-bet- and STAT4-dependent IL-33 receptor expression directly promotes antiviral Th1 cell responses. Proc. Natl. Acad. Sci. U S A. 2015;112:4056–4061. doi: 10.1073/pnas.1418549112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin P., Bunnell B.A., Casteilla L., Dominici M., Katz A.J., March K.L., Redl H., Rubin J.P., Yoshimura K., Gimble J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzyn D., Kuswanto W., Kolodin D., Shadrach J.L., Cerletti M., Jang Y., Sefik E., Tan T.G., Wagers A.J., Benoist C. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo-Branco M.T., Soares I.D., Lopes D.V., Buongusto F., Martinusso C.A., do Rosario A., Jr., Souza S.A., Gutfilen B., Fonseca L.M., Elia C. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS One. 2012;7:e33360. doi: 10.1371/journal.pone.0033360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayrol C., Duval A., Schmitt P., Roga S., Camus M., Stella A., Burlet-Schiltz O., Gonzalez-de-Peredo A., Girard J. Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33. Nat. Immunol. 2018;19:375–385. doi: 10.1038/s41590-018-0067-5. [DOI] [PubMed] [Google Scholar]
- Chen Q.Q., Yan L., Wang C.Z., Wang W.H., Shi H., Su B.B., Zeng Q.H., Du H.T., Wan J. Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses. World J. Gastroenterol. 2013;19:4702–4717. doi: 10.3748/wjg.v19.i29.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Jin W., Hardegen N., Lei K.J., Li L., Marinos N., McGrady G., Wahl S.M. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.Y., Hong J., Gannon J., Kakkar R., Lee R.T. Myocardial pressure overload induces systemic inflammation through endothelial cell IL-33. Proc. Natl. Acad. Sci. U S A. 2015;112:7249–7254. doi: 10.1073/pnas.1424236112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaleu N., Nguyen C.Q., Peck A.B., Jonsson R. Sjogren's syndrome: studying the disease in mice. Arthritis Res. Ther. 2011;13:217. doi: 10.1186/ar3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G., Liu Y., Wang W., Wei F., Liu D., Fan Z., An Y., Zhang C., Wang S. Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cells. 2010;28:1829–1838. doi: 10.1002/stem.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Wang X., Yang Q., Zhao X., Wen W., Li G., Lu J., Qin W., Qi Y., Xie F. Tumoral expression of IL-33 inhibits tumor growth and modifies the tumor microenvironment through CD8+ T and NK cells. J. Immunol. 2015;194:438–445. doi: 10.4049/jimmunol.1401344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M.A., Gonzalez-Rey E., Rico L., Buscher D., Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- Gonzalez M.A., Gonzalez-Rey E., Rico L., Buscher D., Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–1019. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- Hepworth M.R., Maurer M., Hartmann S. Regulation of type 2 immunity to helminths by mast cells. Gut Microbes. 2012;3:476–481. doi: 10.4161/gmic.21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsifis G.E., Moutsopoulos N.M., Wahl S.M. T lymphocytes in Sjogren's syndrome: contributors to and regulators of pathophysiology. Clin. Rev. Allergy Immunol. 2007;32:252–264. doi: 10.1007/s12016-007-8011-8. [DOI] [PubMed] [Google Scholar]
- Krampera M., Cosmi L., Angeli R., Pasini A., Liotta F., Andreini A., Santarlasci V., Mazzinghi B., Pizzolo G., Vinante F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- Lee R.H., Pulin A.A., Seo M.J., Kota D.J., Ylostalo J., Larson B.L., Semprun-Prieto L., Delafontaine P., Prockop D.J. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Dai M., Zhuang Y. A T cell intrinsic role of Id3 in a mouse model for primary Sjogren's syndrome. Immunity. 2004;21:551–560. doi: 10.1016/j.immuni.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Licona-Limon P., Kim L.K., Palm N.W., Flavell R.A. TH2, allergy and group 2 innate lymphoid cells. Nat. Immunol. 2013;14:536–542. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- Liew F.Y., Girard J.P., Turnquist H.R. Interleukin-33 in health and disease. Nat. Rev. Immunol. 2016;16:676–689. doi: 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- Liu O., Xu J., Ding G., Liu D., Fan Z., Zhang C., Chen W., Ding Y., Tang Z., Wang S. Periodontal ligament stem cells regulate B lymphocyte function via programmed cell death protein 1. Stem Cells. 2013;31:1371–1382. doi: 10.1002/stem.1387. [DOI] [PubMed] [Google Scholar]
- Mahlakoiv T., Flamar A.L., Johnston L.K., Moriyama S., Putzel G.G., Bryce P.J., Artis D. Stromal cells maintain immune cell homeostasis in adipose tissue via production of interleukin-33. Sci. Immunol. 2019;4:eaax0416. doi: 10.1126/sciimmunol.aax0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markadieu N., Delpire E. Physiology and pathophysiology of SLC12A1/2 transporters. Pflugers Arch. 2014;466:91–105. doi: 10.1007/s00424-013-1370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta B.M., Reichenbach D.K., Zhang X., Mathews L., Koehn B.H., Dwyer G.K., Lott J.M., Uhl F.M., Pfeifer D., Feser C.J. Peri-alloHCT IL-33 administration expands recipient T-regulatory cells that protect mice against acute GVHD. Blood. 2016;128:427–439. doi: 10.1182/blood-2015-12-684142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meephansan J., Komine M., Tsuda H., Karakawa M., Tominaga S., Ohtsuki M. Expression of IL-33 in the epidermis: the mechanism of induction by IL-17. J. Dermatol. Sci. 2013;71:107–114. doi: 10.1016/j.jdermsci.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Meephansan J., Tsuda H., Komine M., Tominaga S., Ohtsuki M. Regulation of IL-33 expression by IFN-gamma and tumor necrosis factor-alpha in normal human epidermal keratinocytes. J. Invest. Dermatol. 2012;132:2593–2600. doi: 10.1038/jid.2012.185. [DOI] [PubMed] [Google Scholar]
- Meisel R., Zibert A., Laryea M., Gobel U., Daubener W., Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- Mizuno H., Tobita M., Uysal A.C. Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30:804–810. doi: 10.1002/stem.1076. [DOI] [PubMed] [Google Scholar]
- Molofsky A.B., Nussbaum J.C., Liang H.E., Van Dyken S.J., Cheng L.E., Mohapatra A., Chawla A., Locksley R.M. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky A.B., Savage A.K., Locksley R.M. Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity. 2015;42:1005–1019. doi: 10.1016/j.immuni.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky A.B., Van Gool F., Liang H.E., Van Dyken S.J., Nussbaum J.C., Lee J., Bluestone J.A., Locksley R.M. Interleukin-33 and interferon-gamma counter-regulate group 2 innate lymphoid cell activation during immune perturbation. Immunity. 2015;43:161–174. doi: 10.1016/j.immuni.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro K., Yamada T., Tanabe M., Takeuchi T., Ikawa T., Kawamoto H., Furusawa J., Ohtani M., Fujii H., Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Mougiakakos D., Jitschin R., Johansson C.C., Okita R., Kiessling R., Le Blanc K. The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells. Blood. 2011;117:4826–4835. doi: 10.1182/blood-2010-12-324038. [DOI] [PubMed] [Google Scholar]
- Moussion C., Ortega N., Girard J.P. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel 'alarmin'? PLoS One. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi W., Yamaguchi S., Matsuda A., Suzukawa M., Shibui A., Nambu A., Kondo K., Suto H., Saito H., Matsumoto K. IL-33, but not IL-25, is crucial for the development of house dust mite antigen-induced allergic rhinitis. PLoS One. 2013;8:e78099. doi: 10.1371/journal.pone.0078099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechama M., Kwon J., Wei S., Kyi A.T., Welner R.S., Ben-Dov I.Z., Arredouani M.S., Asara J.M., Chen C.H., Tsai C.Y. The IL-33-PIN1-IRAK-M axis is critical for type 2 immunity in IL-33-induced allergic airway inflammation. Nat. Commun. 2018;9:1603. doi: 10.1038/s41467-018-03886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichery M., Mirey E., Mercier P., Lefrancais E., Dujardin A., Ortega N., Girard J.P. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J. Immunol. 2012;188:3488–3495. doi: 10.4049/jimmunol.1101977. [DOI] [PubMed] [Google Scholar]
- Price A.E., Liang H.E., Sullivan B.M., Reinhardt R.L., Eisley C.J., Erle D.J., Locksley R.M. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. U S A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razmkhah M., Abedi N., Hosseini A., Imani M.T., Talei A.R., Ghaderi A. Induction of T regulatory subsets from naive CD4+ T cells after exposure to breast cancer adipose derived stem cells. Iran. J. Immunol. 2015;12:1–15. [PubMed] [Google Scholar]
- Reichenbach D.K., Schwarze V., Matta B.M., Tkachev V., Lieberknecht E., Liu Q., Koehn B.H., Pfeifer D., Taylor P.A., Prinz G. The IL-33/ST2 axis augments effector T-cell responses during acute GVHD. Blood. 2015;125:3183–3192. doi: 10.1182/blood-2014-10-606830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G., Su J., Zhang L., Zhao X., Ling W., L'Huillie A., Zhang J., Lu Y., Roberts A.I., Ji W. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- Ren G., Zhang L., Zhao X., Xu G., Zhang Y., Roberts A.I., Zhao R.C., Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Sarigul M., Yazisiz V., Bassorgun C.I., Ulker M., Avci A.B., Erbasan F., Gelen T., Gorczynski R.M., Terzioglu E. The numbers of Foxp3 + Treg cells are positively correlated with higher grade of infiltration at the salivary glands in primary Sjogren's syndrome. Lupus. 2010;19:138–145. doi: 10.1177/0961203309348234. [DOI] [PubMed] [Google Scholar]
- Schiering C., Krausgruber T., Chomka A., Frohlich A., Adelmann K., Wohlfert E.A., Pott J., Griseri T., Bollrath J., Hegazy A.N. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J., Owyang A., Oldham E., Song Y., Murphy E., McClanahan T.K., Zurawski G., Moshrefi M., Qin J., Li X. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Scott I.C., Majithiya J.B., Sanden C., Thornton P., Sanders P.N., Moore T., Guscott M., Corkill D.J., Erjefalt J.S., Cohen E.S. Interleukin-33 is activated by allergen- and necrosis-associated proteolytic activities to regulate its alarmin activity during epithelial damage. Sci. Rep. 2018;8:3363. doi: 10.1038/s41598-018-21589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Q., Bai Y., Wang G., Song Q., Guo C., Zhang L., Wang Q. Delivery of adipose-derived stem cells attenuates adipose tissue inflammation and insulin resistance in obese mice through remodeling macrophage phenotypes. Stem Cells Dev. 2015;24:2052–2064. doi: 10.1089/scd.2014.0557. [DOI] [PubMed] [Google Scholar]
- Sheng H., Wang Y., Jin Y., Zhang Q., Zhang Y., Wang L., Shen B., Yin S., Liu W., Cui L. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008;18:846–857. doi: 10.1038/cr.2008.80. [DOI] [PubMed] [Google Scholar]
- Su Z., Lin J., Lu F., Zhang X., Zhang L., Gandhi N.B., de Paiva C.S., Pflugfelder S.C., Li D.Q. Potential autocrine regulation of interleukin-33/ST2 signaling of dendritic cells in allergic inflammation. Mucosal Immunol. 2013;6:921–930. doi: 10.1038/mi.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka F., Tominaga K., Ochi M., Tanigawa T., Watanabe T., Fujiwara Y., Ohta K., Oshitani N., Higuchi K., Arakawa T. Exogenous administration of mesenchymal stem cells ameliorates dextran sulfate sodium-induced colitis via anti-inflammatory action in damaged tissue in rats. Life Sci. 2008;83:771–779. doi: 10.1016/j.lfs.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Vasanthakumar A., Moro K., Xin A., Liao Y., Gloury R., Kawamoto S., Fagarasan S., Mielke L.A., Afshar-Sterle S., Masters S.L. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat. Immunol. 2015;16:276–285. doi: 10.1038/ni.3085. [DOI] [PubMed] [Google Scholar]
- Wang S.Q., Wang Y.X., Hua H. Characteristics of labial gland mesenchymal stem cells of healthy individuals and patients with Sjogren's syndrome: a preliminary study. Stem Cells Dev. 2017;26:1171–1185. doi: 10.1089/scd.2017.0045. [DOI] [PubMed] [Google Scholar]
- Xu D., Jiang H.R., Kewin P., Li Y., Mu R., Fraser A.R., Pitman N., Kurowska-Stolarska M., McKenzie A.N., McInnes I.B. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc. Natl. Acad. Sci. U S A. 2008;105:10913–10918. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Wang D., Liu D., Fan Z., Zhang H., Liu O., Ding G., Gao R., Zhang C., Ding Y. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjogren syndrome. Blood. 2012;120:3142–3151. doi: 10.1182/blood-2011-11-391144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Li G., Zhu Y., Liu L., Chen E., Turnquist H., Zhang X., Finn O.J., Chen X., Lu B. IL-33 synergizes with TCR and IL-12 signaling to promote the effector function of CD8+ T cells. Eur. J. Immunol. 2011;41:3351–3360. doi: 10.1002/eji.201141629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W.H., Hu Z.Q. Up-regulation of IL-33 expression in various types of murine cells by IL-3 and IL-4. Cytokine. 2012;58:267–273. doi: 10.1016/j.cyto.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Zhu J., Xu Y., Zhu C., Zhao J., Meng X., Chen S., Wang T., Li X., Zhang L., Lu C. IL-33 induces both regulatory B cells and regulatory T cells in dextran sulfate sodium-induced colitis. Int. Immunopharmacol. 2017;46:38–47. doi: 10.1016/j.intimp.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Zuk P., Zhu M., Mizuno H., Huang J., Futrell J., Katz A., Benhaim P., Lorenz H., Hedrick M. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.