This systematic review and meta-analysis uses data from clinical trials to compare treatments for cocaine use disorder and examines whether any treatment approaches are associated with reductions in cocaine use among adults who are actively using cocaine.
Key Points
Question
What treatments for cocaine use disorder are associated with objective reductions in cocaine use among adults?
Findings
In this meta-analysis of 157 clinical trials comprising 402 treatment groups and 15 842 participants, only contingency management programs were significantly associated with an increased likelihood of having a negative test result for the presence of cocaine, and this association remained significant in all sensitivity analyses.
Meaning
The findings suggest that contingency management programs may be beneficial for the treatment of cocaine use disorder among adults who actively use cocaine.
Abstract
Importance
In the US and the United Kingdom, cocaine use is the second leading cause of illicit drug overdose death. Psychosocial treatments for cocaine use disorder are limited, and no pharmacotherapy is approved for use in the US or Europe.
Objective
To compare treatments for active cocaine use among adults.
Data Sources
PubMed and the Cochrane Database of Systematic Reviews were searched for clinical trials published between December 31, 1995, and December 31, 2017.
Study Selection
This meta-analysis was registered on Covidence.org (study 8731) on December 31, 2015. Clinical trials were included if they (1) had the term cocaine in the article title; (2) were published between December 31, 1995, and December 31, 2017; (3) were written in English; (4) enrolled outpatients 18 years or older with active cocaine use at baseline; and (5) reported treatment group size, treatment duration, retention rates, and urinalysis results for the presence of cocaine metabolites. A study was excluded if (1) more than 25% of participants were not active cocaine users or more than 80% of participants had negative test results for the presence of cocaine metabolites at baseline and (2) it reported only pooled urinalysis results indicating the presence of multiple substances and did not report the specific proportion of positive test results for cocaine metabolites. Multiple reviewers reached criteria consensus. Of 831 records screened, 157 studies (18.9%) met selection criteria and were included in the analysis.
Data Extraction and Synthesis
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline. Search results were imported from PubMed XML into Covidence.org then Microsoft Excel. Data extraction was completed in 2 iterations to ensure fidelity. Analyses included a multilevel random-effects model, a multilevel mixed-effects meta-regression model, and sensitivity analyses. Treatments were clustered into 11 categories (psychotherapy, contingency management programs, placebo, opioids, psychostimulants, anticonvulsants, dopamine agonists, antidepressants, antipsychotics, miscellaneous medications, and other therapies). Missing data were imputed using multiple imputation by chained equations. The significance threshold for all analyses was P = .05. Data were analyzed using the metafor and mice packages in R software, version 3.3.2 (R Foundation for Statistical Computing). Data were analyzed from January 1, 2018, to February 28, 2021.
Main Outcomes and Measures
The primary outcome was the intention-to-treat logarithm of the odds ratio (OR) of having a negative urinalysis result for the presence of cocaine metabolites at the end of each treatment period compared with baseline. The hypothesis, which was formulated after data collection, was that no treatment category would have a significant association with objective reductions in cocaine use.
Results
A total of 157 studies comprising 402 treatment groups and 15 842 participants were included. Excluding other therapies, the largest treatment groups across all studies were psychotherapy (mean [SD] number of participants, 40.04 [36.88]) and contingency management programs (mean [SD] number of participants, 37.51 [25.51]). Only contingency management programs were significantly associated with an increased likelihood of having a negative test result for the presence of cocaine (OR, 2.13; 95% CI, 1.62-2.80), and this association remained significant in all sensitivity analyses.
Conclusions and Relevance
In this meta-analysis, contingency management programs were associated with reductions in cocaine use among adults. Research efforts and policies that align with this treatment modality may benefit those who actively use cocaine and attenuate societal burdens.
Introduction
After years of decreasing rates, the prevalence of cocaine use has been increasing since 2012; cocaine is currently the second leading cause of overdose death (with opioids the first) associated with illicit drug use in the US1,2 and the United Kingdom.3 Cocaine use has taken a particular toll on certain vulnerable populations; for example, it is the leading cause of overdose death among Black persons.4 However, treatments for cocaine use disorders are limited and, despite the performance of many clinical trials over several decades, no pharmacotherapy has been approved by government agencies in the US or Europe. The lack of approved treatments for cocaine use disorder is in contrast to the approval of naltrexone, methadone, and buprenorphine medications for the treatment of opioid use disorder5,6,7,8; naltrexone, acamprosate, and disulfiram medications for the treatment of alcohol use disorder9,10; and varenicline, bupropion, and nicotine replacement medications for the treatment of tobacco use disorder.11,12
The absence of a standard treatment for cocaine use disorders has hampered clinical treatment. With no guiding prototype available, the development of new treatments has proven challenging. Furthermore, current understanding of the pathophysiologic characteristics of cocaine use disorders remains insufficient for the development of beneficial pharmacological treatments. Numerous meta-analyses have attempted to search for a signal of treatment benefit by pooling results from multiple clinical trials. However, meta-analytic investigations have reported no improvement in outcomes among those receiving anticonvulsant,13,14,15 antidepressant,16 antipsychotic,17,18,19 acupuncture,20 disulfiram,21 dopamine agonist,22 opioid,23 and psychostimulant24,25,26 therapies. Meta-analyses of psychosocial interventions have reported variable effect sizes given the heterogeneity of approaches.23,27,28 Meta-analyses of contingency management programs, which comprise positive reinforcement of drug abstinence, have indicated beneficial outcomes; however, these analyses have been limited to studies with distinct populations, studies with specific comparison groups,23,29,30,31 or studies that exclusively examined contingency management interventions.32 Furthermore, leaders in the field of substance use disorders continue to classify contingency management as a treatment with limited benefits,33 making its comparative role in the treatment of cocaine use disorder unclear. We performed a comprehensive meta-analysis of all treatments for cocaine use disorders published over 22 years, inclusive of all clinical trials and cocaine-using populations, to examine which treatment approaches, if any, were associated with a reduction in cocaine use. The hypothesis, which was formulated after data collection and based on the results reported in most previously published meta-analyses, was that no treatment category would have a significant association with objective reductions in cocaine use.
Methods
Search Strategy and Selection Criteria
In this systematic review and meta-analysis, all methods were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.34 This meta-analysis was prospectively registered on Covidence.org (study 8731) on December 31, 2015. Covidence.org was used to store search results, identify duplicates, and track screening decisions. The PubMed database was searched for clinical trials with the term cocaine in the article title that were published between December 31, 1995, and December 31, 2015. This search was temporally expanded and repeated on December 31, 2016, and December 31, 2017, to update the analysis with relevant studies. Only English language articles were included; this criterion excluded 6 of 831 abstracts, with only 2 of those 6 abstracts describing small studies relevant to this meta-analysis. The exact search string was as follows: cocaine[title] AND (Clinical Trial[ptyp] AND “loattrfull text”[sb] AND (“1995/12/31”[PDAT]: “2017/12/31”[PDAT]) AND English[lang]). In addition, all references within the Cochrane Database of Systematic Reviews that were identified as meta-analyses of treatments for cocaine use disorders, all references of clinical trials that were identified during full-text screening, and all references that were cited within identified references were included in the abstract screening.
All clinical trial designs were included if their goal was to assess the efficacy of a treatment for reducing cocaine use. Participants had to be 18 years or older with active cocaine use at baseline that was identified by self-report or urinalysis testing. Studies were excluded if more than 25% of participants were not active cocaine users based on self-report or if more than 80% of participants had negative test results for the presence of cocaine metabolites at baseline. Only studies that reported treatment group size, treatment duration, retention rates, and treatment outcomes using urinalysis testing for cocaine metabolites were included. Studies that reported treatment outcomes only as pooled urinalysis results across multiple drugs (ie, urinalysis results not reporting the specific proportion of negative and positive test results for the presence of cocaine metabolites) were excluded.
Study authors were not contacted, and unpublished data were not sought. Data included in the meta-analysis were extracted at the summary estimate level. One reviewer (B.S.B.) performed the search, full-text screening, data extraction, and data analysis. Two reviewers (B.S.B. and S.N.) screened all abstracts and references, and 2 reviewers (B.S.B and S.S.H.) performed the data analysis. Disagreements regarding inclusion were resolved via reconsideration by 1 reviewer (B.S.B.); however, no disagreements occurred. A detailed outline of the study protocol is available in eMethods 1 in the Supplement.
Outcome Measures
The start and end of treatment were defined as the first and last points at which participants were exposed to the treatment; posttreatment data were not included. The primary outcome was defined as the intention-to-treat (ITT) logarithm of the odds ratio (log OR) of having a negative urinalysis result for the presence of cocaine metabolites at the end of the treatment period compared with baseline. Baseline urinalysis data were either reported directly, inferred based on the requirement of having a positive test result for cocaine at study entry, or estimated based on urinalysis results during the first week of treatment. The type of baseline data reported was coded as a dummy variable and included in the statistical analysis. For studies reporting multiple baseline types, direct baseline testing was preferred to positive test results at screening, which was in turn preferred to estimation based on the first week of treatment. Outcome urinalysis data were reported either directly as ITT outcomes or calculated based on retention rates and non-ITT outcomes. Urinalysis data from the end of treatment were either reported at the last treatment point or as the mean urinalysis result for the entire treatment period. The type of outcome data reported was also coded as a dummy variable and included in the statistical analysis. For studies reporting multiple outcome types, data from the last treatment point were preferred to mean urinalysis results across the treatment period, and direct ITT reporting was preferred to calculation of ITT outcomes based on retention and non-ITT outcomes.
Data Extraction
All search results were imported from PubMed XML output into Covidence.org, with duplicates automatically removed during importation. Two reviewers (B.S.B. and S.N.) independently assessed references and abstracts. If both reviewers agreed that the clinical trial did or did not meet eligibility criteria, it was included or excluded, respectively. The full text of all remaining articles was obtained, and the same eligibility criteria were used to determine which, if any, articles should be excluded at this stage. Any disagreements were resolved via discussion and were ultimately decided based on the discretion of 2 reviewers (B.S.B. and C.H.H). One reviewer (B.S.B.) read each full-text article, determined whether the study met inclusion criteria, and extracted the data to a Microsoft Excel database, which was backed up continuously to offsite storage. Data extraction was completed in 2 iterations, with the second iteration ensuring the fidelity of the first.
In addition to urinalysis results and retention data, the information extracted included study characteristics (lead author, publication year, double-blindedness, randomization, and multisitedness), participant characteristics (age, sex, years of cocaine use, self-reported days of cocaine use per week, reported scores on the Addiction Severity Index drug composite subscale (score range, 0-1, with higher scores indicating more severe problems associated with drug use), and intervention details (treatment category, specific treatment, treatment dose, and duration of treatment).
Eleven treatment categories were defined based on categories used in previous systematic reviews of treatments for cocaine use disorders13,14,15,16,17,18,19,22,23,24,25,27,28,29,31,32; these categories comprised psychotherapy, contingency management programs, placebo, opioids, psychostimulants, anticonvulsants, dopamine agonists, antidepressants, antipsychotics, miscellaneous medications (medications that did not fit in other medication categories), and other therapies (nonmedications that did not fit in any treatment category). All treatments to which participants were simultaneously exposed in a treatment arm within a study were coded by category. For example, a single treatment group may have been treated with fluoxetine and methadone medications and cognitive behavioral therapy concomitantly, and this treatment group would have been coded in the antidepressant, psychotherapy, and opioid treatment categories.
Statistical Analysis
All statistical analyses were performed using the metafor35 (meta-regression) and mice36 (multiple imputation by chained equations) packages in R software, version 3.3.2 (R Foundation for Statistical Computing).37 All R scripts with associated text output can be found in eMethods 2 and eMethods 3 in the Supplement. The escalc function within the metafor package was used to calculate the log OR (yi = ln [(p/1 − p)/(q/1 − q)]) and variability (vi) for each treatment group based on the group size and proportion of urinalysis results that were negative for the presence of cocaine metabolites at the start (q) and end (p) of treatment. This method took into account the number of participants in each treatment group with decreasing variability with increasing group size.
The mice package in R software was used to impute missing data. The mice package is a robust method of data imputation that creates multiple estimates for each missing value and pools the results to account for uncertainty in the imputations and to estimate accurate SEs.38 Predictive mean matching was used to impute missing baseline data on urinalysis results, duration of treatment, participant age, proportion of male participants, years of cocaine use, and score on the Addiction Severity Index39 drug composite subscale. In brief, predictive mean matching uses linear regression with all observed data from all variables to build a predictor matrix. The algorithm then finds cases with observed data in which the predicted value of the observed data point is proximal to the predicted value of the missing data point. The algorithm then selects 5 donors from the closest matches, randomly samples an observed value from one of the donors, and uses this value to impute the missing data point. Given that imputed data are always observed values within the data set, they always meet boundary criteria and have the same distribution. The type of baseline urinalysis data (inferred by positive urinalysis results for the presence of cocaine as a requirement at study entry, measured at baseline, or measured during the first week) was imputed using a bayesian polytomous regression model built from all observed data. Data imputation was performed 5 times to build 5 separate imputed data sets.
In the primary analyses, we constructed a multilevel random-effects model, with treatment groups nested within studies and studies nested within the first author (ie, multiple studies conducted by the first author would be nested together). The intraclass correlation coefficient was used to assess the effect of nesting, and the Higgins I2 statistic was used to estimate heterogeneity. Collinearity was defined as a variance inflation factor greater than 5. Statistical significance was set at P = .05. A multilevel mixed-effects meta-regression analysis was conducted for the data set composed of studies with complete data for baseline urinalysis results, treatment duration, proportion of male participants, and mean participant age. Data from the Addiction Severity Index were not included in the nonimputed analyses, as most studies did not report them. This analysis was then repeated for the 5 imputed data sets, and the results of the 5 analyses were pooled. These 2 analyses (multilevel without imputed data and pooled results of 5 multilevel models with imputed data) served as the primary analyses.
Sensitivity analyses were conducted by examining the results of several other random-effects models, comprising models with treatment factors only, models with retention rate as a covariate, single-level models, and models assessing the results of each of the 5 imputed data sets on an individual basis. Bias was assessed by regressing effect size against variance using the method for multilevel models described by Sterne et al40 and Egger et al.41 Data were analyzed from January 1, 2018, to February 28, 2021.
Results
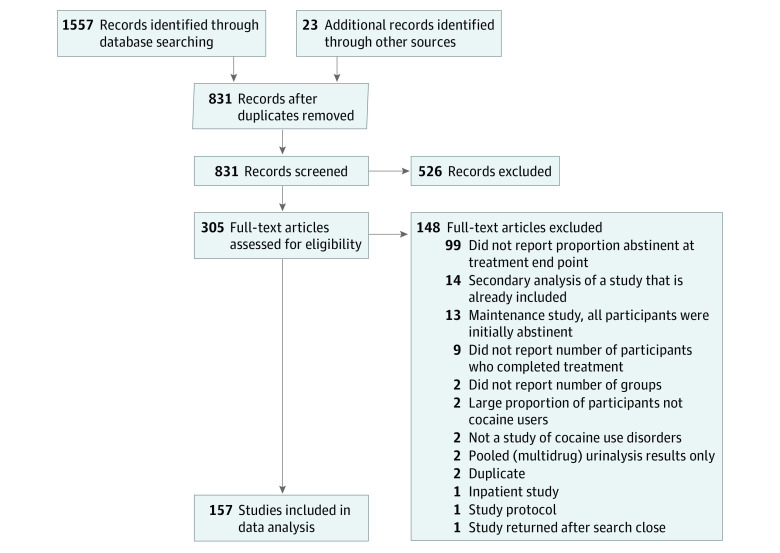
In total, 1580 records were identified, and 831 records were screened; of those, 305 full-text articles were assessed for eligibility. After exclusions, 157 studies (18.9%) with 402 treatment groups and 15 842 participants were included in the meta-analysis.42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198 The PRISMA34 diagram shows detailed information on the number of ineligible studies and the reasons for exclusion (Figure).
Figure. Study Selection.
Table 1 includes summary statistics for each treatment category and covariate. Among 15 842 total participants, the mean (SD) age was 38.27 (4.30) years, and 10 541 of 15 262 participants (69.1%) were male. Excluding other therapies, the largest treatment groups across all studies were psychotherapy (mean [SD] number of participants, 40.04 [36.88]) and contingency management programs (mean [SD] number of participants, 37.51 [25.51]). The mean (SD) cocaine use per week was 3.25 (1.00) days, and the mean (SD) number of years of cocaine use was 11.82 (3.56). A total of 4258 of 15 842 participants (26.9%) completed treatment and were cocaine-free at the end of treatment.
Table 1. Summary Statistics for Treatment Categories and Covariates.
| Characteristic | Treatment category, mean (SD) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anticonvulsants | Antidepressants | Antipsychotics | Contingency management | Dopamine agonists | Miscellaneous medications | Opioids | Other therapies | Placebo | Psychostimulants | Psychotherapy | |
| Treatment groups, No. | 20 | 18 | 12 | 88 | 20 | 54 | 159 | 26 | 96 | 24 | 330 |
| Total participants, No. | 683 | 574 | 281 | 3301 | 710 | 1751 | 5139 | 1805 | 3381 | 702 | 13213 |
| Participants per treatment group | 34.15 (20.64) | 31.89 (15.84) | 23.42 (10.99) | 37.51 (25.51) | 35.50 (19.19) | 32.43 (28.86) | 32.32 (18.61) | 69.42 (83.73) | 35.22 (25.41) | 29.25 (17.00) | 40.04 (36.88) |
| Age, y | 36.52 (4.92) | 36.03 (2.74) | 39.46 (4.99) | 38.12 (4.06) | 38.65 (4.69) | 39.44 (4.46) | 37.64 (3.36) | 38.39 (4.18) | 38.26 (4.63) | 38.01 (4.41) | 37.82 (4.27) |
| No. of treatment groups with unreported values | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 3 | 1 | 0 | 4 |
| Proportion of male participants | 0.78 (0.15) | 0.68 (0.29) | 0.77 (0.19) | 0.61 (0.18) | 0.79 (0.12) | 0.76 (0.14) | 0.60 (0.19) | 0.71 (0.15) | 0.74 (0.18) | 0.73 (0.12) | 0.70 (0.20) |
| No. of treatment groups with unreported values | 0 | 1 | 0 | 2 | 0 | 2 | 4 | 0 | 1 | 1 | 7 |
| Cocaine use, d/wk | 3.93 (1.10) | 3.26 (0.86) | 2.56 (0.76) | 3.40 (1.13) | 3.41 (0.62) | 3.29 (0.95) | 3.47 (0.78) | 3.49 (0.59) | 3.34 (0.83) | 3.01 (0.68) | 3.25 (0.96) |
| No. of treatment groups with unreported values | 3 | 1 | 7 | 33 | 6 | 7 | 43 | 6 | 18 | 9 | 110 |
| Cocaine use, y | 11.51 (2.95) | 9.75 (1.61) | 15.10 (4.94) | 10.81 (3.27) | 10.54 (3.19) | 12.67 (3.47) | 10.51 (3.40) | 11.50 (4.22) | 11.89 (3.79) | 12.02 (2.15) | 11.36 (3.60) |
| No. of treatment groups with unreported values | 11 | 14 | 9 | 37 | 3 | 15 | 74 | 10 | 39 | 11 | 127 |
| Score on ASI drug composite subscalea | 0.31 (0.08) | 0.35 (0.07) | 0.24 (0.01) | 0.25 (0.09) | 0.23 (0.02) | 0.27 (0.09) | 0.35 (0.10) | 0.29 (0.05) | 0.27 (0.07) | 0.22 (0.02) | 0.25 (0.09) |
| No. of treatment groups with unreported values | 12 | 15 | 9 | 56 | 12 | 42 | 121 | 17 | 69 | 20 | 215 |
| Proportion cocaine-free at baseline | 0.26 (0.19) | 0.37 (0.02) | 0.33 (0.19) | 0.24 (0.19) | 0.30 (0.13) | 0.25 (0.15) | 0.19 (0.14) | 0.17 (0.17) | 0.28 (0.16) | 0.23 (0.15) | 0.28 (0.18) |
| No. of treatment groups with unreported values | 8 | 16 | 4 | 43 | 9 | 37 | 81 | 12 | 57 | 11 | 183 |
| Treatment duration, wk | 0.61 (0.23) | 0.51 (0.21) | 0.44 (0.26) | 0.64 (0.24) | 0.43 (0.15) | 0.64 (0.22) | 0.72 (0.17) | 0.71 (0.21) | 0.58 (0.24) | 0.56 (0.19) | 0.60 (0.24) |
| No. of treatment groups with unreported values | 0 | 0 | 0 | 5 | 0 | 0 | 2 | 0 | 0 | 0 | 7 |
| Proportion completing treatment | 0.48 (0.25) | 0.54 (0.23) | 0.35 (0.22) | 0.60 (0.24) | 0.48 (0.25) | 0.44 (0.26) | 0.41 (0.16) | 0.48 (0.29) | 0.40 (0.21) | 0.45 (0.24) | 0.45 (0.22) |
| No. of treatment groups with unreported values | 6 | 5 | 2 | 51 | 7 | 23 | 61 | 8 | 31 | 13 | 135 |
| Proportion completing treatment and cocaine-free at end of treatment | 0.29 (0.15) | 0.26 (0.12) | 0.20 (0.18) | 0.38 (0.19) | 0.20 (0.15) | 0.25 (0.18) | 0.29 (0.13) | 0.31 (0.18) | 0.22 (0.15) | 0.23 (0.11) | 0.28 (0.18) |
| No. of treatment groups with unreported values | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviation: ASI, Addiction Severity Index.
Score range, 0 to 1, with higher scores indicating more severe problems associated with drug use.
Table 2 includes main outcome measures for the primary analyses, which comprised multilevel models with covariates with and without imputed data. For the primary multilevel analysis with covariates, several treatments were associated with a significant increase in the ITT likelihood of producing a negative urinalysis result in the nonimputed data set; however, only contingency management programs were significant for both imputed (OR, 2.13; 95% CI, 1.62-2.80) and nonimputed (OR, 2.09; 95% CI, 1.59-2.75) data sets (Table 2). This statistical significance persisted across all sensitivity testing (Table 3). After contingency management programs, the treatment category that was significantly associated with the ITT likelihood of producing a negative urinalysis result across most analyses was opioids. Opioid agonist therapies were significantly associated with a reduction in cocaine use in 6 of 9 analyses that did not include completion rate as a covariate and in 1 of 8 analyses that included completion rate as a covariate. A total of 330 of 402 treatment groups incorporated some form of psychotherapy; however, none of the primary or sensitivity analyses indicated a significant association between psychotherapy and change in the ITT likelihood of producing a negative urinalysis. Notably, placebo was not associated with a significant change in the ITT likelihood of producing a negative urinalysis result for the imputed (OR, 1.03; 95% CI, 0.59-1.80) or nonimputed (OR, 1.48; 95% CI, 0.86-2.53) data set in the primary analyses, and this nonsignificance was also observed in all sensitivity analyses. When completion rate was added as a covariate to the primary nonimputed multilevel model, only contingency management programs remained significantly associated with outcomes (OR, 2.06; 95% CI, 1.53-2.77).
Table 2. Outcomes for Primary Analyses.
| Treatment category | Without imputed data | With imputed data | ||||||
|---|---|---|---|---|---|---|---|---|
| No. | OR (95% CI) | No. | OR (95% CI) | |||||
| Studies | Treatment groups | Participants | Studies | Treatment groups | Participants | |||
| Anticonvulsants | 14 | 17 | 570 | 2.32 (1.15-4.67)a | 16 | 20 | 683 | 1.39 (0.59-3.29) |
| Antidepressants | 8 | 15 | 482 | 1.96 (0.91-4.25) | 11 | 18 | 574 | 1.36 (0.63-2.91) |
| Antipsychotics | 8 | 11 | 241 | 1.59 (0.68-3.71) | 9 | 12 | 281 | 1.02 (0.41-2.53) |
| Contingency management | 38 | 69 | 2744 | 2.09 (1.59-2.75)b | 50 | 88 | 3301 | 2.13 (1.62-2.80)b |
| Dopamine agonists | 10 | 18 | 645 | 1.55 (0.80-3.00) | 12 | 20 | 710 | 1.04 (0.51-2.14) |
| Miscellaneous medications | 26 | 38 | 1455 | 1.64 (0.88-3.06) | 37 | 54 | 1751 | 1.14 (0.60-2.17) |
| Opioids | 49 | 142 | 4686 | 2.32 (1.54-3.49)b | 55 | 159 | 5139 | 1.70 (0.98-2.94) |
| Other therapies | 12 | 21 | 1577 | 2.19 (1.07-4.49)c | 15 | 26 | 1805 | 1.35 (0.72-2.53) |
| Placebo | 65 | 78 | 2889 | 1.48 (0.86-2.53) | 80 | 96 | 3381 | 1.03 (0.59-1.80) |
| Psychostimulants | 13 | 19 | 645 | 2.48 (1.27-4.85)d | 17 | 24 | 702 | 1.74 (0.81-3.74) |
| Psychotherapy | 98 | 258 | 10 773 | 1.08 (0.74-1.58) | 131 | 330 | 13 213 | 1.18 (0.81-1.73) |
Abbreviation: OR, odds ratio.
P = .02.
P < .001.
P = .03.
P = .008.
Table 3. Sensitivity Analysesa.
| Variable | Single-level analysis | Multilevel analysis | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments only | Treatments with covariates | Treatments with covariates plus completion rate | Treatments with covariatesb | Treatments with covariates plus completion rate | Treatments with covariates imputed: model 1 | Treatments with covariates imputed: model 2 | Treatments with covariates imputed: model 3 | Treatments with covariates imputed: model 4 | Treatments with covariates imputed: model 5 | Treatments with covariates imputed: pooled b | Treatments with covariates imputed plus completion rate: model 1 | Treatments with covariates imputed plus completion rate: model 2 | Treatments with covariates imputed plus completion rate: model 3 | Treatments with covariates imputed plus completion rate: model 4 | Treatments with covariates imputed plus completion rate: model 5 | Treatments with covariates imputed plus completion rate: pooled | |
| Treatment category | |||||||||||||||||
| Anticonvulsants | N | Y (P = .02) | N | Y (P = .02) | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Antidepressants | Y (P = .001) | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Antipsychotics | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Contingency management | Y (P = .003) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) |
| Dopamine agonists | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Miscellaneous medications | Y (P = .02) | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Opioids | Y (P < .001) | Y (P < .001) | Y (P = .02) | Y (P < .001) | N | Y (P = .04) | N | Y (P = .005) | N | Y (P = .004) | N | N | N | N | N | N | N |
| Other | Y (P = .02) | Y (P = .008) | Y (P = .006) | Y (P = .03) | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Placebo | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Psychostimulants | N | N | Y (P = .02) | Y (P = .008) | N | Y (P = .01) | N | N | N | N | N | N | N | N | N | N | N |
| Psychotherapy | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Study type | |||||||||||||||||
| Double-blind | NA | Y (P = .01) | N | Y (P = .02) | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Multisite | NA | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Randomized | NA | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Baseline typec | |||||||||||||||||
| 2d | NA | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P = .002) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) |
| 3e | NA | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | N | Y (P < .001) | Y (P < .001) | Y (P < .001) | N | N | N | Y (P < .001) | Y (P < .001) | Y (P < .001) | N (P < .001) | N |
| Outcome typef | |||||||||||||||||
| 2g | NA | Y (P = .001) | N | N | N | N | Y (P = .03) | Y (P = .03) | N | N | N | N | N | N | N | N | N |
| 3h | NA | Y (P < .001) | Y (P = .003) | Y (P = .04) | N | N | Y (P < .001) | Y (P < .001) | N | Y (P = .005) | N | N | Y (P = .04) | N | N | Y (P = .009) | N |
| 4i | NA | Y (P < .001) | N | N | N | N | Y (P < .001) | Y (P < .001) | Y (P = .02) | Y (P = .02) | N | Y (P = .04) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P = .002) | N |
| Other variables | |||||||||||||||||
| Treatment duration | NA | N | Y (P = .01) | N | Y (P = .04) | Y (P = .02) | N | N | Y (P = .02) | N | N | Y (P = .005) | N | N | Y (P = .005) | Y (P = .03) | N |
| Male sex, % | NA | Y (P = .001) | Y (P = .02) | N | N | Y (P = .02) | N | Y (P = .009) | N | N | N | Y (P = .02) | N | Y (P = .01) | N | N | N |
| Age, y | NA | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Baseline cocaine use, d/wk | NA | NA | NA | NA | NA | N | N | Y (P = .03) | N | N | N | N | N | Y (P = .01) | Y (P = .008) | N | N |
| Year of study publication | NA | Y (P = .006) | Y (P = .03) | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Score on ASI drug composite subscale | NA | NA | NA | NA | NA | N | N | N | N | N | N | N | N | N | N | N | N |
| Completion rate | NA | NA | Y (P < .001) | NA | Y (P < .001) | NA | NA | NA | NA | NA | NA | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) | Y (P < .001) |
Abbreviations: ASI, Addiction Severity Index; NA, not applicable.
Statistically significant result, Y or N.
Primary analysis.
All baseline types were compared with baseline type 1, which comprised all participants with positive urinalysis results for cocaine at screening (study inclusion criterion).
Proportion of participants with positive urinalysis results for cocaine reported at baseline.
Proportion of participants with positive urinalysis results for cocaine reported during first week of treatment;
All outcome types were compared with outcome type 1, which comprised the proportion of participants with positive urinalysis results for cocaine reported as intention-to-treat during last week of treatment.
Intention-to-treat proportion of participants with positive urinalysis results for cocaine calculated from retention rate and outcome during last week of treatment.
Mean proportion of participants with positive urinalysis results for cocaine reported as intention-to-treat during treatment.
Intention-to-treat mean proportion of participants with positive urinalysis results for cocaine calculated from retention rate and outcome during treatment.
Forest plots with unadjusted outcomes for all studies with baseline data are shown by treatment category in eFigures 1 to 11 in the Supplement. Baseline data type was the only covariate significantly associated with outcome for the primary multilevel analysis with covariates, with baseline urinalysis results that were reported before receipt of treatment being associated with lower ITT likelihood of producing a negative urinalysis result for both imputed (OR, 0.26; 95% CI, 0.13-0.55) and nonimputed (OR, 0.13; 95% CI, 0.08-0.20) data sets compared with the reference variable, which was the OR of having a positive urinalysis result for the presence of cocaine as a requirement for study entry. This finding was in contrast to baseline data type being reported as the urinalysis results for week 1 of treatment, which was significant for the nonimputed (OR, 0.11; 95% CI, 0.06-0.20) but not the imputed (OR, 0.42; 95% CI, 0.03-5.45) data set.
Heterogeneity (I2) was 80.96% in the base random-effects model without any treatment category factors or covariates, 41.79% in the model with all treatment categories and covariates (with the exception of completion rate), 22.57% in the model with completion rate also included as a covariate, 58.54% in the multilevel model with all treatment categories and covariates (with the exception of completion rate), and 49.40% in the model with completion rate also included as a covariate. The I2 ranged from 72.69% to 76.72% across the 5 imputed data sets that did not include completion rate as a covariate and from 68.35% to 72.78% for the 5 studies that included completion rate as a covariate. When data were divided into subsets for each treatment category, the I2 was greater than 50% for placebo, psychotherapy, contingency management programs, opioids, and miscellaneous medications.
The intraclass correlation coefficient was 0.498 for the multilevel model that did not include completion rate as a covariate (treatments with covariates) and 0.392 for the model that included completion rate as a covariate (treatments with covariates plus completion rate;Table 3). The intraclass correlation coefficient ranged from 0.222 to 0.608 for the 5 imputed models that did not include completion rate as a covariate (treatments with covariates imputed: models 1-5) and from 0.107 to 0.548 for the 5 imputed models that included completion rate as a covariate (treatments with covariates imputed plus completion rate: models 1-5).
Examination of funnel plots across models and across treatment categories did not indicate any major sources of bias (eFigure 12 and eFigure 13 in the Supplement), and Egger tests were nonsignificant for the full multilevel model without imputed data. However, Egger tests did indicate bias in 4 of 5 imputed data sets and in several treatment categories, including placebo (with the exception of antipsychotics, psychotherapy, psychostimulants, miscellaneous medications, and other therapies). Collinearity was not detected for any treatment category or covariate by variance inflation factor.
Discussion
This meta-analysis was constructed to maximize inclusivity to detect a broad treatment category that was both generalizable and beneficial in reducing cocaine use. Although several treatment categories were associated with benefits in the nonimputed data set, when data were imputed to include the complete data set, only contingency management programs were consistently associated with a significant reduction in urinalysis-confirmed cocaine use. Other treatment categories were not significantly associated with this outcome. This finding is in contrast to placebo, which was consistently not associated with a significant change in objective cocaine use in any of the primary or sensitivity analyses.
The a priori hypothesis was that no treatment category would have a significant association with objective cocaine use. However, the positive association between contingency management treatment approaches and a significant reduction in objective cocaine use was not entirely unexpected. Previous meta-analyses of contingency management programs for reducing cocaine use have suggested benefits among particular clinical populations,23,29,30 and a 2018 high-quality meta-analysis that used comparison groups to assess psychosocial treatments for cocaine use disorders found contingency management to be the most beneficial treatment.31 Moreover, large-scale implementation of contingency management programs for the treatment of substance use disorders by the US Department of Veterans Affairs has indicated both clinical benefits similar to those reported in clinical trials and low costs.199 Given the results of our study and the fact that the Department of Veterans Affairs is the largest integrated provider of addiction services in the US,200 consideration of the implementation of contingency management programs on a national level or within other major health care systems in the US is warranted.
After contingency management programs, the treatment category associated with the most benefit across analyses was opioids. Opioid agonist therapies were significantly associated with a reduction in cocaine use in 6 of 9 analyses that did not include completion rate as a covariate and 1 of 8 analyses that included completion rate as a covariate, suggesting that the benefit of opioids for reducing ITT cocaine use was enhanced through an increase in retention rate. Notably, all studies that included opioid therapies (buprenorphine and methadone) as treatments were conducted among populations with concomitant opioid use disorders. These medications have been significantly associated with increases in retention rates7 and, consistent with our findings, the only other meta-analysis to examine the treatment of cocaine use disorder using opioid therapies did not find a significant association outside of this increased retention rate.23
Our analysis did not reveal a significant association between psychotherapy and reductions in cocaine use. Meta-analyses of psychosocial interventions have reported variable effect sizes that have been associated with the heterogeneity of approaches.23,27,28 Our analysis also did not take into account the type or dose (ie, session length and frequency) of psychotherapy provided. A total of 330 of 402 treatment groups incorporated some form of psychotherapy, and open-label as well as noncontrolled study designs were included. Hence, if a general association between psychotherapy and cocaine use was present, it would have likely been detected. However, our approach cannot rule out benefits associated with specific approaches or doses.
Strengths and Limitations
This study has several strengths. Broad inclusivity is its major strength, as this inclusivity provided an increased likelihood of detecting an association that was consistently present in a treatment category (vs a response that was present only for a specific treatment within a category). In addition, our analyses have at least 2 other distinct aspects, which are the inclusion of all eligible studies regardless of quality and the calculation of effect sizes from baseline to end of treatment rather than the measurement of treatment benefits in comparison with a control group, even when a control group was present. Both of these approaches were used to maximize the sensitivity for detecting a beneficial treatment.
This study also has limitations. Given the study’s broad inclusivity, we were unable to resolve the effect size of specific treatments or identify the potential benefits of a specific treatment within a broad treatment category. The approaches used to maximize the sensitivity of detecting a beneficial treatment also increased the probability of a type I error, which is a major limitation; however, we decided to trade this limitation for the benefit of inclusiveness, as previous meta-analyses of treatments for cocaine use disorders have reported mostly negative results. We compensate for this limitation by reporting the results of all sensitivity analyses and limiting our conclusion to the significant association between contingency management programs and reductions in cocaine use, as contingency management was the only treatment category with positive results in both the primary analyses and all sensitivity analyses. Furthermore, study quality was not found to be associated with outcomes, and we did not find data indicating that the inclusion of low-quality studies biased the results.
Conclusions
In this systematic review and meta-analysis, we specifically designed our approach to search for a signal of treatment benefit present among the broad treatment categories defined by previous systematic reviews. Given the largely negative results of published meta-analyses of treatments for cocaine use disorders, we expanded our search beyond the typical restrictions that lead to data exclusion. This approach allowed us to look broadly across the extant literature; however, this broad reach came at the expense of granularity and strength of conclusion. Our comprehensive analyses suggested that contingency management approaches were associated with reductions in cocaine use. Thus, there may not be a case for therapeutic pessimism regarding cocaine use disorder. Prioritizing implementation research that informs health care systems regarding beneficial and viable adoption approaches (eg, examining current limits on patient incentive programs)201 may produce greater public health benefits than additional efforts to assess whether contingency management programs are generally beneficial for the treatment of cocaine use disorders.
eMethods 1. Study Protocol
eMethods 2. R Scripts With Associated Output
eMethods 3. Model Outputs in Data Sets (Subsetted by Treatment Category)
eFigure 1. Forest Plot Without Imputed Data: Anticonvulsants
eFigure 2. Forest Plot Without Imputed Data: Antidepressants
eFigure 3. Forest Plot Without Imputed Data: Antipsychotics
eFigure 4. Forest Plot Without Imputed Data: Contingency Management
eFigure 5. Forest Plot Without Imputed Data: Dopamine Agonists
eFigure 6. Forest Plot Without Imputed Data: Miscellaneous Medications
eFigure 7. Forest Plot Without Imputed Data: Opioids
eFigure 8. Forest Plot Without Imputed Data: Other Interventions
eFigure 9. Forest Plot Without Imputed Data: Placebo
eFigure 10. Forest Plot Without Imputed Data: Psychostimulants
eFigure 11. Forest Plot Without Imputed Data: Psychotherapy
eFigure 12. Funnel Plots (Full Models)
eFigure 13. Funnel Plots (By Treatment Category)
eReferences
References
- 1.Jalal H, Buchanich JM, Roberts MS, Balmert LC, Zhang K, Burke DS. Changing dynamics of the drug overdose epidemic in the United States from 1979 through 2016. Science. 2018;361(6408):eaau1184. doi: 10.1126/science.aau1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hedegaard H, Minino AM, Warner M. Drug overdose deaths in the United States, 1999-2018. NCHS Data Brief. 2020;(356):1-8. [PubMed] [Google Scholar]
- 3.European Monitoring Centre for Drugs and Drug Addiction . United Kingdom country drug report 2019. European Monitoring Centre for Drugs and Drug Addiction; 2019. Accessed April 4, 2020. https://www.emcdda.europa.eu/system/files/publications/11355/united-kingdom-cdr-2019.pdf
- 4.Shiels MS, Freedman ND, Thomas D, Berrington de Gonzalez A. Trends in U.S. drug overdose deaths in non-Hispanic Black, Hispanic, and non-Hispanic White persons, 2000-2015. Ann Intern Med. 2018;168(6):453-455. doi: 10.7326/M17-1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen S, Larance B, Lintzeris N. Opioid agonist treatment for patients with dependence on prescription opioids. JAMA. 2017;317(9):967-968. doi: 10.1001/jama.2017.0001 [DOI] [PubMed] [Google Scholar]
- 6.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2003;(2):CD002209. doi: 10.1002/14651858.CD002209 [DOI] [PubMed] [Google Scholar]
- 7.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2004;(3):CD002207. doi: 10.1002/14651858.CD002207.pub2 [DOI] [PubMed] [Google Scholar]
- 8.Tanum L, Solli KK, Latif ZEH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205. doi: 10.1001/jamapsychiatry.2017.3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anton RF, O’Malley SS, Ciraulo DA, et al. ; COMBINE Study Research Group . Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017. doi: 10.1001/jama.295.17.2003 [DOI] [PubMed] [Google Scholar]
- 10.Skinner MD, Lahmek P, Pham H, Aubin HJ. Disulfiram efficacy in the treatment of alcohol dependence: a meta-analysis. PLoS One. 2014;9(2):e87366. doi: 10.1371/journal.pone.0087366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cahill K, Lindson-Hawley N, Thomas KH, Fanshawe TR, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2016;2016(5):CD006103. doi: 10.1002/14651858.CD006103.pub7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329. doi: 10.1002/14651858.CD009329.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minozzi S, Cinquini M, Amato L, et al. Anticonvulsants for cocaine dependence. Cochrane Database Syst Rev. 2015;(4):CD006754. doi: 10.1002/14651858.CD006754.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez Y, Farre M, Fonseca F, Torrens M. Anticonvulsant drugs in cocaine dependence: a systematic review and meta-analysis. J Subst Abuse Treat. 2010;38(1):66-73. doi: 10.1016/j.jsat.2009.07.001 [DOI] [PubMed] [Google Scholar]
- 15.Singh M, Keer D, Klimas J, Wood E, Werb D. Topiramate for cocaine dependence: a systematic review and meta-analysis of randomized controlled trials. Addiction. 2016;111(8):1337-1346. doi: 10.1111/add.13328 [DOI] [PubMed] [Google Scholar]
- 16.Pani PP, Trogu E, Vecchi S, Amato L. Antidepressants for cocaine dependence and problematic cocaine use. Cochrane Database Syst Rev. 2011;(12):CD002950. doi: 10.1002/14651858.CD002950.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alvarez Y, Perez-Mana C, Torrens M, Farre M. Antipsychotic drugs in cocaine dependence: a systematic review and meta-analysis. J Subst Abuse Treat. 2013;45(1):1-10. doi: 10.1016/j.jsat.2012.12.013 [DOI] [PubMed] [Google Scholar]
- 18.Indave BI, Minozzi S, Pani PP, Amato L. Antipsychotic medications for cocaine dependence. Cochrane Database Syst Rev. 2016;3:CD006306. doi: 10.1002/14651858.CD006306.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kishi T, Matsuda Y, Iwata N, Correll CU. Antipsychotics for cocaine or psychostimulant dependence: systematic review and meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2013;74(12):e1169-e1180. doi: 10.4088/JCP.13r08525 [DOI] [PubMed] [Google Scholar]
- 20.Gates S, Smith LA, Foxcroft DR. Auricular acupuncture for cocaine dependence. Cochrane Database Syst Rev. 2006;(1):CD005192. doi: 10.1002/14651858.CD005192.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pani PP, Trogu E, Vacca R, Amato L, Vecchi S, Davoli M. Disulfiram for the treatment of cocaine dependence. Cochrane Database Syst Rev. 2010;(1):CD007024. doi: 10.1002/14651858.CD007024.pub2 [DOI] [PubMed] [Google Scholar]
- 22.Minozzi S, Amato L, Pani PP, et al. Dopamine agonists for the treatment of cocaine dependence. Cochrane Database Syst Rev. 2015;2015(5):CD003352. doi: 10.1002/14651858.CD003352.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castells X, Kosten TR, Capella D, Vidal X, Colom J, Casas M. Efficacy of opiate maintenance therapy and adjunctive interventions for opioid dependence with comorbid cocaine use disorders: a systematic review and meta-analysis of controlled clinical trials. Am J Drug Alcohol Abuse. 2009;35(5):339-349. doi: 10.1080/00952990903108215 [DOI] [PubMed] [Google Scholar]
- 24.Castells X, Casas M, Pérez-Mañá C, Roncero C, Vidal X, Capellà D. Efficacy of psychostimulant drugs for cocaine dependence. Cochrane Database Syst Rev. 2010;(2):CD007380. doi: 10.1002/14651858.CD007380.pub3 [DOI] [PubMed] [Google Scholar]
- 25.Castells X, Casas M, Vidal X, et al. Efficacy of central nervous system stimulant treatment for cocaine dependence: a systematic review and meta-analysis of randomized controlled clinical trials. Addiction. 2007;102(12):1871-1887. doi: 10.1111/j.1360-0443.2007.01943.x [DOI] [PubMed] [Google Scholar]
- 26.Sangroula D, Motiwala F, Wagle B, Shah VC, Hagi K, Lippmann S. Modafinil treatment of cocaine dependence: a systematic review and meta-analysis. Subst Use Misuse. 2017;52(10):1292-1306. doi: 10.1080/10826084.2016.1276597 [DOI] [PubMed] [Google Scholar]
- 27.Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179-187. doi: 10.1176/appi.ajp.2007.06111851 [DOI] [PubMed] [Google Scholar]
- 28.Knapp WP, Soares BGO, Farrel M, Lima MS. Psychosocial interventions for cocaine and psychostimulant amphetamines related disorders. Cochrane Database Syst Rev. 2007;(3):CD003023. doi: 10.1002/14651858.CD003023.pub2 [DOI] [PubMed] [Google Scholar]
- 29.Schumacher JE, Milby JB, Wallace D, et al. Meta-analysis of day treatment and contingency-management dismantling research: Birmingham Homeless Cocaine Studies (1990-2006). J Consult Clin Psychol. 2007;75(5):823-828. doi: 10.1037/0022-006X.75.5.823 [DOI] [PubMed] [Google Scholar]
- 30.Rash CJ, Alessi SM, Petry NM. Substance abuse treatment patients in housing programs respond to contingency management interventions. J Subst Abuse Treat. 2017;72:97-102. doi: 10.1016/j.jsat.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Crescenzo F, Ciabattini M, D’Alo GL, et al. Comparative efficacy and acceptability of psychosocial interventions for individuals with cocaine and amphetamine addiction: a systematic review and network meta-analysis. PLoS Med. 2018;15(12):e1002715. doi: 10.1371/journal.pmed.1002715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis DR, Kurti AN, Skelly JM, Redner R, White TJ, Higgins ST. A review of the literature on contingency management in the treatment of substance use disorders, 2009-2014. Prev Med. 2016;92:36-46. doi: 10.1016/j.ypmed.2016.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volkow ND, Boyle M. Neuroscience of addiction: relevance to prevention and treatment. Am J Psychiatry. 2018;175(8):729-740. doi: 10.1176/appi.ajp.2018.17101174 [DOI] [PubMed] [Google Scholar]
- 34.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1-48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 36.van Buuren S, Groothuis-Oudshoorn K.. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1-67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 37.The R project for statistical computing. R Foundation; 2016. Accessed June 5, 2018. https://www.R-project.org/
- 38.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40-49. doi: 10.1002/mpr.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients: the Addiction Severity Index. J Nerv Ment Dis. 1980;168(1):26-33. doi: 10.1097/00005053-198001000-00006 [DOI] [PubMed] [Google Scholar]
- 40.Sterne JAC, Juni P, Schulz KF, Altman DG, Bartlett C, Egger M. Statistical methods for assessing the influence of study characteristics on treatment effects in ‘meta-epidemiological’ research. Stat Med. 2002;21(11):1513-1524. doi: 10.1002/sim.1184 [DOI] [PubMed] [Google Scholar]
- 41.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rowan-Szal GA, Bartholomew NG, Chatham LR, Simpson DD. A combined cognitive and behavioral intervention for cocaine-using methadone clients. J Psychoactive Drugs. 2005;37(1):75-84. doi: 10.1080/02791072.2005.10399750 [DOI] [PubMed] [Google Scholar]
- 43.Rawson RA, Huber A, McCann M, et al. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Arch Gen Psychiatry. 2002;59(9):817-824. doi: 10.1001/archpsyc.59.9.817 [DOI] [PubMed] [Google Scholar]
- 44.Avants SK, Margolin A, DePhilippis D, Kosten TR. A comprehensive pharmacologic-psychosocial treatment program for HIV-seropositive cocaine- and opioid-dependent patients: preliminary findings. J Subst Abuse Treat. 1998;15(3):261-265. doi: 10.1016/S0740-5472(97)00226-2 [DOI] [PubMed] [Google Scholar]
- 45.Malcolm R, LaRowe S, Cochran K, et al. A controlled trial of amlodipine for cocaine dependence: a negative report. J Subst Abuse Treat. 2005;28(2):197-204. doi: 10.1016/j.jsat.2004.12.006 [DOI] [PubMed] [Google Scholar]
- 46.Kennedy AP, Gross RE, Whitfield N, Drexler KPG, Kilts CD. A controlled trial of the adjunct use of D-cycloserine to facilitate cognitive behavioral therapy outcomes in a cocaine-dependent population. Addict Behav. 2012;37(8):900-907. doi: 10.1016/j.addbeh.2012.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Covi L, Hess JM, Schroeder JR, Preston KL. A dose response study of cognitive behavioral therapy in cocaine abusers. J Subst Abuse Treat. 2002;23(3):191-197. doi: 10.1016/S0740-5472(02)00247-7 [DOI] [PubMed] [Google Scholar]
- 48.Kampman KM, Dackis C, Pettinati HM, Lynch KG, Sparkman T, O’Brien CP. A double-blind, placebo-controlled pilot trial of acamprosate for the treatment of cocaine dependence. Addict Behav. 2011;36(3):217-221. doi: 10.1016/j.addbeh.2010.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kampman KM, Dackis C, Lynch KG, et al. A double-blind, placebo-controlled trial of amantadine, propranolol, and their combination for the treatment of cocaine dependence in patients with severe cocaine withdrawal symptoms. Drug Alcohol Depend. 2006;85(2):129-137. doi: 10.1016/j.drugalcdep.2006.04.002 [DOI] [PubMed] [Google Scholar]
- 50.Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O’Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30(1):205-211. doi: 10.1038/sj.npp.1300600 [DOI] [PubMed] [Google Scholar]
- 51.Dackis CA, Kampman KM, Lynch KG, et al. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. J Subst Abuse Treat. 2012;43(3):303-312. doi: 10.1016/j.jsat.2011.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winhusen T, Somoza E, Ciraulo DA, et al. A double-blind, placebo-controlled trial of tiagabine for the treatment of cocaine dependence. Drug Alcohol Depend. 2007;91(2-3):141-148. doi: 10.1016/j.drugalcdep.2007.05.028 [DOI] [PubMed] [Google Scholar]
- 53.Somoza EC, Winship D, Gorodetzky CW, et al. A multisite, double-blind, placebo-controlled clinical trial to evaluate the safety and efficacy of vigabatrin for treating cocaine dependence. JAMA Psychiatry. 2013;70(6):630-637. doi: 10.1001/jamapsychiatry.2013.872 [DOI] [PubMed] [Google Scholar]
- 54.Kampman KM, Pettinati H, Lynch KG, Sparkman T, O’Brien CP. A pilot trial of olanzapine for the treatment of cocaine dependence. Drug Alcohol Depend. 2003;70(3):265-273. doi: 10.1016/S0376-8716(03)00009-7 [DOI] [PubMed] [Google Scholar]
- 55.Kampman KM, Pettinati H, Lynch KG, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 2004;75(3):233-240. doi: 10.1016/j.drugalcdep.2004.03.008 [DOI] [PubMed] [Google Scholar]
- 56.Reid MS, Angrist B, Baker S, et al. A placebo-controlled screening trial of celecoxib for the treatment of cocaine dependence. Addiction. 2005;100(suppl 1):32-42. doi: 10.1111/j.1360-0443.2005.00989.x [DOI] [PubMed] [Google Scholar]
- 57.Reid MS, Casadonte P, Baker S, et al. A placebo-controlled screening trial of olanzapine, valproate, and coenzyme Q10/L-carnitine for the treatment of cocaine dependence. Addiction. 2005;100(suppl 1):43-57. doi: 10.1111/j.1360-0443.2005.00990.x [DOI] [PubMed] [Google Scholar]
- 58.Johnson BA, Roache JD, Ait-Daoud N, et al. A preliminary randomized, double-blind, placebo-controlled study of the safety and efficacy of ondansetron in the treatment of cocaine dependence. Drug Alcohol Depend. 2006;84(3):256-263. doi: 10.1016/j.drugalcdep.2006.02.011 [DOI] [PubMed] [Google Scholar]
- 59.Margolin A, Kantak K, Copenhaver M, Avants SK. A preliminary, controlled investigation of magnesium L-aspartate hydrochloride for illicit cocaine and opiate use in methadone-maintained patients. J Addict Dis. 2003;22(2):49-61. doi: 10.1300/J069v22n02_04 [DOI] [PubMed] [Google Scholar]
- 60.Alterman AI, Snider EC, Cacciola JS, et al. A quasi-experimental comparison of the effectiveness of 6- versus 12-hour per week outpatient treatments for cocaine dependence. J Nerv Ment Dis. 1996;184(1):54-56. doi: 10.1097/00005053-199601000-00010 [DOI] [PubMed] [Google Scholar]
- 61.Avants SK, Margolin A, Holford TR, Kosten TR. A randomized controlled trial of auricular acupuncture for cocaine dependence. Arch Intern Med. 2000;160(15):2305-2312. doi: 10.1001/archinte.160.15.2305 [DOI] [PubMed] [Google Scholar]
- 62.Halikas JA, Crosby RD, Pearson VL, Graves NM. A randomized double-blind study of carbamazepine in the treatment of cocaine abuse. Clin Pharmacol Ther. 1997;62(1):89-105. doi: 10.1016/S0009-9236(97)90155-7 [DOI] [PubMed] [Google Scholar]
- 63.Carroll KM, Nich C, Petry NM, Eagan DA, Shi JM, Ball SA. A randomized factorial trial of disulfiram and contingency management to enhance cognitive behavioral therapy for cocaine dependence. Drug Alcohol Depend. 2016;160:135-142. doi: 10.1016/j.drugalcdep.2015.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petry NM, Alessi SM, Rash CJ. A randomized study of contingency management in cocaine-dependent patients with severe and persistent mental health disorders. Drug Alcohol Depend. 2013;130(1-3):234-237. doi: 10.1016/j.drugalcdep.2012.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petry NM, Barry D, Alessi SM, Rounsaville BJ, Carroll KM. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. J Consult Clin Psychol. 2012;80(2):276-285. doi: 10.1037/a0026883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Silverman K, Wong CJ, Needham M, et al. A randomized trial of employment-based reinforcement of cocaine abstinence in injection drug users. J Appl Behav Anal. 2007;40(3):387-410. doi: 10.1901/jaba.2007.40-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Silverman K, Robles E, Mudric T, Bigelow GE, Stitzer ML. A randomized trial of long-term reinforcement of cocaine abstinence in methadone-maintained patients who inject drugs. J Consult Clin Psychol. 2004;72(5):839-854. doi: 10.1037/0022-006X.72.5.839 [DOI] [PubMed] [Google Scholar]
- 68.Petitjean SA, Dürsteler-MacFarland KM, Krokar MC, et al. A randomized, controlled trial of combined cognitive-behavioral therapy plus prize-based contingency management for cocaine dependence. Drug Alcohol Depend. 2014;145:94-100. doi: 10.1016/j.drugalcdep.2014.09.785 [DOI] [PubMed] [Google Scholar]
- 69.Dürsteler-MacFarland KM, Farronato NS, Strasser J, et al. A randomized, controlled, pilot trial of methylphenidate and cognitive-behavioral group therapy for cocaine dependence in heroin prescription. J Clin Psychopharmacol. 2013;33(1):104-108. doi: 10.1097/JCP.0b013e31827bfff4 [DOI] [PubMed] [Google Scholar]
- 70.Cornish JW, Maany I, Fudala PJ, Ehrman RN, Robbins SJ, O’Brien CP. A randomized, double-blind, placebo-controlled study of ritanserin pharmacotherapy for cocaine dependence. Drug Alcohol Depend. 2001;61(2):183-189. doi: 10.1016/S0376-8716(00)00140-X [DOI] [PubMed] [Google Scholar]
- 71.Brown ES, Todd JP, Hu LT, et al. A randomized, double-blind, placebo-controlled trial of citicoline for cocaine dependence in bipolar I disorder. Am J Psychiatry. 2015;172(10):1014-1021. doi: 10.1176/appi.ajp.2015.14070857 [DOI] [PubMed] [Google Scholar]
- 72.Raby WN, Rubin EA, Garawi F, et al. A randomized, double-blind, placebo-controlled trial of venlafaxine for the treatment of depressed cocaine-dependent patients. Am J Addict. 2014;23(1):68-75. doi: 10.1111/j.1521-0391.2013.12065.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silverman K, Svikis D, Robles E, Stitzer ML, Bigelow GE. A reinforcement-based therapeutic workplace for the treatment of drug abuse: six-month abstinence outcomes. Exp Clin Psychopharmacol. 2001;9(1):14-23. doi: 10.1037/1064-1297.9.1.14 [DOI] [PubMed] [Google Scholar]
- 74.Shoptaw S, Kintaudi PC, Charuvastra C, Ling W. A screening trial of amantadine as a medication for cocaine dependence. Drug Alcohol Depend. 2002;66(3):217-224. doi: 10.1016/S0376-8716(01)00205-8 [DOI] [PubMed] [Google Scholar]
- 75.Margolin A, Kleber HD, Avants SK, et al. Acupuncture for the treatment of cocaine addiction: a randomized controlled trial. JAMA. 2002;287(1):55-63. doi: 10.1001/jama.287.1.55 [DOI] [PubMed] [Google Scholar]
- 76.García-Fernández G, Secades-Villa R, García-Rodríguez O, Sánchez-Hervás E, Fernández-Hermida JR, Higgins ST. Adding voucher-based incentives to community reinforcement approach improves outcomes during treatment for cocaine dependence. Am J Addict. 2011;20(5):456-461. doi: 10.1111/j.1521-0391.2011.00154.x [DOI] [PubMed] [Google Scholar]
- 77.Malcolm R, Herron J, Sutherland SE, Brady KT. Adverse outcomes in a controlled trial of pergolide for cocaine dependence. J Addict Dis. 2001;20(1):81-92. doi: 10.1300/J069v20n01_08 [DOI] [PubMed] [Google Scholar]
- 78.Grabowski J, Rhoades H, Stotts A, et al. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology. 2004;29(5):969-981. doi: 10.1038/sj.npp.1300392 [DOI] [PubMed] [Google Scholar]
- 79.Kampman K, Volpicelli JR, Alterman A, et al. Amantadine in the early treatment of cocaine dependence: a double-blind, placebo-controlled trial. Drug Alcohol Depend. 1996;41(1):25-33. doi: 10.1016/0376-8716(96)01225-2 [DOI] [PubMed] [Google Scholar]
- 80.McKay JR, Van Horn DHA, Lynch KG, et al. An adaptive approach for identifying cocaine dependent patients who benefit from extended continuing care. J Consult Clin Psychol. 2013;81(6):1063-1073. doi: 10.1037/a0034265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mardikian PN, LaRowe SD, Hedden S, Kalivas PW, Malcolm RJ. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):389-394. doi: 10.1016/j.pnpbp.2006.10.001 [DOI] [PubMed] [Google Scholar]
- 82.Wardle MC, Vincent JN, Suchting R, Green CE, Lane SD, Schmitz JM. Anhedonia is associated with poorer outcomes in contingency management for cocaine use disorder. J Subst Abuse Treat. 2017;72:32-39. doi: 10.1016/j.jsat.2016.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beresford TP, Clapp L, Martin B, Wiberg JL, Alfers J, Beresford HF. Aripiprazole in schizophrenia with cocaine dependence: a pilot study. J Clin Psychopharmacol. 2005;25(4):363-366. doi: 10.1097/01.jcp.0000169419.38899.5b [DOI] [PubMed] [Google Scholar]
- 84.Walsh SL, Middleton LS, Wong CJ, et al. Atomoxetine does not alter cocaine use in cocaine dependent individuals: double blind randomized trial. Drug Alcohol Depend. 2013;130(1-3):150-157. doi: 10.1016/j.drugalcdep.2012.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Donlin WD, Knealing TW, Needham M, Wong CJ, Silverman K. Attendance rates in a workplace predict subsequent outcome of employment-based reinforcement of cocaine abstinence in methadone patients. J Appl Behav Anal. 2008;41(4):499-516. doi: 10.1901/jaba.2008.41-499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Monti PM, Rohsenow DJ, Michalec E, Martin RA, Abrams DB. Brief coping skills treatment for cocaine abuse: substance use outcomes at three months. Addiction. 1997;92(12):1717-1728. doi: 10.1111/j.1360-0443.1997.tb02892.x [DOI] [PubMed] [Google Scholar]
- 87.Bernstein J, Bernstein E, Tassiopoulos K, Heeren T, Levenson S, Hingson R. Brief motivational intervention at a clinic visit reduces cocaine and heroin use. Drug Alcohol Depend. 2005;77(1):49-59. doi: 10.1016/j.drugalcdep.2004.07.006 [DOI] [PubMed] [Google Scholar]
- 88.Silverman K, Wong CJ, Umbricht-Schneiter A, Montoya ID, Schuster CR, Preston KL. Broad beneficial effects of cocaine abstinence reinforcement among methadone patients. J Consult Clin Psychol. 1998;66(5):811-824. doi: 10.1037/0022-006X.66.5.811 [DOI] [PubMed] [Google Scholar]
- 89.Handelsman L, Rosenblum A, Palij M, et al. Bromocriptine for cocaine dependence: a controlled clinical trial. Am J Addict. 1997;6(1):54-64. [PubMed] [Google Scholar]
- 90.Gorelick DA, Wilkins JN. Bromocriptine treatment for cocaine addiction: association with plasma prolactin levels. Drug Alcohol Depend. 2006;81(2):189-195. doi: 10.1016/j.drugalcdep.2005.06.010 [DOI] [PubMed] [Google Scholar]
- 91.Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR. Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Arch Gen Psychiatry. 1997;54(8):713-720. doi: 10.1001/archpsyc.1997.01830200041006 [DOI] [PubMed] [Google Scholar]
- 92.Ling W, Hillhouse MP, Saxon AJ, et al. Buprenorphine + naloxone plus naltrexone for the treatment of cocaine dependence: the Cocaine Use Reduction with Buprenorphine (CURB) study. Addiction. 2016;111(8):1416-1427. doi: 10.1111/add.13375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Bupropion hydrochloride versus placebo, in combination with cognitive behavioral therapy, for the treatment of cocaine abuse/dependence. J Addict Dis. 2008;27(1):13-23. doi: 10.1300/J069v27n01_02 [DOI] [PubMed] [Google Scholar]
- 94.Levin FR, Evans SM, McDowell DM, Brooks DJ, Nunes E. Bupropion treatment for cocaine abuse and adult attention-deficit/hyperactivity disorder. J Addict Dis. 2002;21(2):1-16. doi: 10.1300/J069v21n02_01 [DOI] [PubMed] [Google Scholar]
- 95.Sofuoglu M, Poling J, Babuscio T, et al. Carvedilol does not reduce cocaine use in methadone-maintained cocaine users. J Subst Abuse Treat. 2017;73:63-69. doi: 10.1016/j.jsat.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.González G, Desai R, Sofuoglu M, et al. Clinical efficacy of gabapentin versus tiagabine for reducing cocaine use among cocaine dependent methadone-treated patients. Drug Alcohol Depend. 2007;87(1):1-9. doi: 10.1016/j.drugalcdep.2006.07.003 [DOI] [PubMed] [Google Scholar]
- 97.Mancino MJ, McGaugh J, Chopra MP, et al. Clinical efficacy of sertraline alone and augmented with gabapentin in recently abstinent cocaine-dependent patients with depressive symptoms. J Clin Psychopharmacol. 2014;34(2):234-239. doi: 10.1097/JCP.0000000000000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sigmon SC, Correia CJ, Stitzer ML. Cocaine abstinence during methadone maintenance: effects of repeated brief exposure to voucher-based reinforcement. Exp Clin Psychopharmacol. 2004;12(4):269-275. doi: 10.1037/1064-1297.12.4.269 [DOI] [PubMed] [Google Scholar]
- 99.Sayers SL, Campbell EC, Kondrich J, et al. Cocaine abuse in schizophrenic patients treated with olanzapine versus haloperidol. J Nerv Ment Dis. 2005;193(6):379-386. doi: 10.1097/01.nmd.0000165089.14736.bf [DOI] [PubMed] [Google Scholar]
- 100.Martell BA, Orson FM, Poling J, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66(10):1116-1123. doi: 10.1001/archgenpsychiatry.2009.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Haney M, Gunderson EW, Jiang H, Collins ED, Foltin RW. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol Psychiatry. 2010;67(1):59-65. doi: 10.1016/j.biopsych.2009.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Epstein DH, Hawkins WE, Covi L, Umbricht A, Preston KL. Cognitive-behavioral therapy plus contingency management for cocaine use: findings during treatment and across 12-month follow-up. Psychol Addict Behav. 2003;17(1):73-82. doi: 10.1037/0893-164X.17.1.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Secades-Villa R, Sánchez-Hervás E, Zacarés-Romaguera F, García-Rodríguez O, Santonja-Gómez FJ, García-Fernández G. Community Reinforcement Approach (CRA) for cocaine dependence in the Spanish public health system: 1 year outcome. Drug Alcohol Rev. 2011;30(6):606-612. doi: 10.1111/j.1465-3362.2010.00250.x [DOI] [PubMed] [Google Scholar]
- 104.Secades-Villa R, García-Rodríguez O, Higgins ST, Fernández-Hermida JR, Carballo JL. Community reinforcement approach plus vouchers for cocaine dependence in a community setting in Spain: six-month outcomes. J Subst Abuse Treat. 2008;34(2):202-207. doi: 10.1016/j.jsat.2007.03.006 [DOI] [PubMed] [Google Scholar]
- 105.Higgins ST, Sigmon SC, Wong CJ, et al. Community reinforcement therapy for cocaine-dependent outpatients. Arch Gen Psychiatry. 2003;60(10):1043-1052. doi: 10.1001/archpsyc.60.9.1043 [DOI] [PubMed] [Google Scholar]
- 106.Carroll KM, Kiluk BD, Nich C, et al. Computer-assisted delivery of cognitive-behavioral therapy: efficacy and durability of CBT4CBT among cocaine-dependent individuals maintained on methadone. Am J Psychiatry. 2014;171(4):436-444. doi: 10.1176/appi.ajp.2013.13070987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.García-Fernández G, Secades-Villa R, García-Rodríguez O, Peña-Suárez E, Sánchez-Hervás E. Contingency management improves outcomes in cocaine-dependent outpatients with depressive symptoms. Exp Clin Psychopharmacol. 2013;21(6):482-489. doi: 10.1037/a0033995 [DOI] [PubMed] [Google Scholar]
- 108.Miguel AQC, Madruga CS, Cogo-Moreira H, et al. Contingency management is effective in promoting abstinence and retention in treatment among crack cocaine users in Brazil: a randomized controlled trial. Psychol Addict Behav. 2016;30(5):536-543. doi: 10.1037/adb0000192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Petry NM, Alessi SM, Carroll KM, et al. Contingency management treatments: reinforcing abstinence versus adherence with goal-related activities. J Consult Clin Psychol. 2006;74(3):592-601. doi: 10.1037/0022-006X.74.3.592 [DOI] [PubMed] [Google Scholar]
- 110.Baldaçara L, Diniz TA, Parreira BL, Milhomem JJ, Almeida LJ, Fernandes CC. Could disulfiram be a new treatment for crack cocaine dependence? a pilot study. Braz J Psychiatry. 2013;35(1):97-98. doi: 10.1016/j.rbp.2012.10.006 [DOI] [PubMed] [Google Scholar]
- 111.Reis AD, Castro LA, Faria R, Laranjeira R. Craving decrease with topiramate in outpatient treatment for cocaine dependence: an open label trial. Braz J Psychiatry. 2008;30(2):132-135. doi: 10.1590/S1516-44462008005000012 [DOI] [PubMed] [Google Scholar]
- 112.Gonçalves-Ferreira A, do Couto FS, Rainha Campos A, Lucas Neto LP, Gonçalves-Ferreira D, Teixeira J. Deep brain stimulation for refractory cocaine dependence. Biol Psychiatry. 2016;79(11):e87-e89. doi: 10.1016/j.biopsych.2015.06.023 [DOI] [PubMed] [Google Scholar]
- 113.Kosten T, Oliveto A, Feingold A, et al. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug Alcohol Depend. 2003;70(3):315-325. doi: 10.1016/S0376-8716(03)00032-2 [DOI] [PubMed] [Google Scholar]
- 114.Oliveto AH, Feingold A, Schottenfeld R, Jatlow P, Kosten TR. Desipramine in opioid-dependent cocaine abusers maintained on buprenorphine vs methadone. Arch Gen Psychiatry. 1999;56(9):812-820. doi: 10.1001/archpsyc.56.9.812 [DOI] [PubMed] [Google Scholar]
- 115.Grabowski J, Rhoades H, Schmitz J, et al. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21(5):522-526. doi: 10.1097/00004714-200110000-00010 [DOI] [PubMed] [Google Scholar]
- 116.George TP, Chawarski MC, Pakes J, Carroll KM, Kosten TR, Schottenfeld RS. Disulfiram versus placebo for cocaine dependence in buprenorphine-maintained subjects: a preliminary trial. Biol Psychiatry. 2000;47(12):1080-1086. doi: 10.1016/S0006-3223(99)00310-8 [DOI] [PubMed] [Google Scholar]
- 117.Salloum IM, Douaihy A, Cornelius JR, Kirisci L, Kelly TM, Hayes J. Divalproex utility in bipolar disorder with co-occurring cocaine dependence: a pilot study. Addict Behav. 2007;32(2):410-415. doi: 10.1016/j.addbeh.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 118.Schubiner H, Saules KK, Arfken CL, et al. Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol. 2002;10(3):286-294. doi: 10.1037/1064-1297.10.3.286 [DOI] [PubMed] [Google Scholar]
- 119.Elkashef A, Fudala PJ, Gorgon L, et al. Double-blind, placebo-controlled trial of selegiline transdermal system (STS) for the treatment of cocaine dependence. Drug Alcohol Depend. 2006;85(3):191-197. doi: 10.1016/j.drugalcdep.2006.04.010 [DOI] [PubMed] [Google Scholar]
- 120.Licata SC, Penetar DM, Ravichandran C, et al. Effects of daily treatment with citicoline: a double-blind, placebo-controlled study in cocaine-dependent volunteers. J Addict Med. 2011;5(1):57-64. doi: 10.1097/ADM.0b013e3181d80c93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J. Effects of oral methamphetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2009;101(1-2):34-41. doi: 10.1016/j.drugalcdep.2008.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kablinger AS, Lindner MA, Casso S, et al. Effects of the combination of metyrapone and oxazepam on cocaine craving and cocaine taking: a double-blind, randomized, placebo-controlled pilot study. J Psychopharmacol. 2012;26(7):973-981. doi: 10.1177/0269881111430745 [DOI] [PubMed] [Google Scholar]
- 123.Garcia-Rodriguez O, Secades-Villa R, Higgins ST, et al. Effects of voucher-based intervention on abstinence and retention in an outpatient treatment for cocaine addiction: a randomized controlled trial. Exp Clin Psychopharmacol. 2009;17(3):131-138. doi: 10.1037/a0015963 [DOI] [PubMed] [Google Scholar]
- 124.Carroll KM, Nich C, Shi JM, Eagan D, Ball SA. Efficacy of disulfiram and Twelve Step Facilitation in cocaine-dependent individuals maintained on methadone: a randomized placebo-controlled trial. Drug Alcohol Depend. 2012;126(1-2):224-231. doi: 10.1016/j.drugalcdep.2012.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oliveto A, Poling J, Sevarino KA, et al. Efficacy of dose and contingency management procedures in LAAM-maintained cocaine-dependent patients. Drug Alcohol Depend. 2005;79(2):157-165. doi: 10.1016/j.drugalcdep.2005.01.007 [DOI] [PubMed] [Google Scholar]
- 126.Baldaçara L, Cogo-Moreira H, Parreira BL, et al. Efficacy of topiramate in the treatment of crack cocaine dependence: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2016;77(3):398-406. doi: 10.4088/JCP.14m09377 [DOI] [PubMed] [Google Scholar]
- 127.Dunn KE, Fingerhood M, Wong CJ, Svikis DS, Nuzzo P, Silverman K. Employment-based abstinence reinforcement following inpatient detoxification in HIV-positive opioid and/or cocaine-dependent patients. Exp Clin Psychopharmacol. 2014;22(1):75-85. doi: 10.1037/a0034863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rosenblum A, Magura S, Palij M, Foote J, Handelsman L, Stimmel B. Enhanced treatment outcomes for cocaine-using methadone patients. Drug Alcohol Depend. 1999;54(3):207-218. doi: 10.1016/S0376-8716(98)00166-5 [DOI] [PubMed] [Google Scholar]
- 129.McKee SA, Carroll KM, Sinha R, et al. Enhancing brief cognitive-behavioral therapy with motivational enhancement techniques in cocaine users. Drug Alcohol Depend. 2007;91(1):97-101. doi: 10.1016/j.drugalcdep.2007.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Egelko S, Galanter M, Dermatis H, DeMaio C. Evaluation of a multisystems model for treating perinatal cocaine addiction. J Subst Abuse Treat. 1998;15(3):251-259. doi: 10.1016/S0740-5472(97)00225-0 [DOI] [PubMed] [Google Scholar]
- 131.Levin FR, Mariani JJ, Specker S, et al. Extended-release mixed amphetamine salts vs placebo for comorbid adult attention-deficit/hyperactivity disorder and cocaine use disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(6):593-602. doi: 10.1001/jamapsychiatry.2015.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schmitz JM, Averill P, Stotts AL, Moeller FG, Rhoades HM, Grabowski J. Fluoxetine treatment of cocaine-dependent patients with major depressive disorder. Drug Alcohol Depend. 2001;63(3):207-214. doi: 10.1016/S0376-8716(00)00208-8 [DOI] [PubMed] [Google Scholar]
- 133.Myrick H, Henderson S, Brady KT, Malcolm R. Gabapentin in the treatment of cocaine dependence: a case series. J Clin Psychiatry. 2001;62(1):19-23. doi: 10.4088/JCP.v62n0105 [DOI] [PubMed] [Google Scholar]
- 134.Margolin A, Avants SK, Malison RT, Kosten TR. High- and low-dose mazindol for cocaine dependence in methadone-maintained patients: a preliminary evaluation. Subst Abuse. 1997;18(3):125-131. doi: 10.1080/08897079709511358 [DOI] [Google Scholar]
- 135.Milby JB, Schumacher JE, McNamara C, et al. Initiating abstinence in cocaine abusing dually diagnosed homeless persons. Drug Alcohol Depend. 2000;60(1):55-67. doi: 10.1016/S0376-8716(00)80008-3 [DOI] [PubMed] [Google Scholar]
- 136.Kosten TR, Oliveto A, Sevarino KA, Gonsai K, Feingold A. Ketoconazole increases cocaine and opioid use in methadone maintained patients. Drug Alcohol Depend. 2002;66(2):173-180. doi: 10.1016/S0376-8716(01)00198-3 [DOI] [PubMed] [Google Scholar]
- 137.Schmitz JM, Mooney ME, Moeller FG, Stotts AL, Green C, Grabowski J. Levodopa pharmacotherapy for cocaine dependence: choosing the optimal behavioral therapy platform. Drug Alcohol Depend. 2008;94(1-3):142-150. doi: 10.1016/j.drugalcdep.2007.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Petry NM, Martin B. Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. J Consult Clin Psychol. 2002;70(2):398-405. doi: 10.1037/0022-006X.70.2.398 [DOI] [PubMed] [Google Scholar]
- 139.Schottenfeld RS, Chawarski MC, Pakes JR, Pantalon MV, Carroll KM, Kosten TR. Methadone versus buprenorphine with contingency management or performance feedback for cocaine and opioid dependence. Am J Psychiatry. 2005;162(2):340-349. doi: 10.1176/appi.ajp.162.2.340 [DOI] [PubMed] [Google Scholar]
- 140.Nuijten M, Blanken P, van den Brink W, Hendriks V. Modafinil in the treatment of crack-cocaine dependence in the Netherlands: results of an open-label randomised controlled feasibility trial. J Psychopharmacol. 2015;29(6):678-687. doi: 10.1177/0269881115582151 [DOI] [PubMed] [Google Scholar]
- 141.Hersh D, Van Kirk JR, Kranzler HR. Naltrexone treatment of comorbid alcohol and cocaine use disorders. Psychopharmacology (Berl). 1998;139(1-2):44-52. doi: 10.1007/s002130050688 [DOI] [PubMed] [Google Scholar]
- 142.Galanter M, Dermatis H, Keller D, Trujillo M. Network therapy for cocaine abuse: use of family and peer supports. Am J Addict. 2002;11(2):161-166. doi: 10.1080/10550490290087938 [DOI] [PubMed] [Google Scholar]
- 143.Magura S, Rosenblum A, Lovejoy M, Handelsman L, Foote J, Stimmel B. Neurobehavioral treatment for cocaine-using methadone patients: a preliminary report. J Addict Dis. 1994;13(4):143-160. doi: 10.1300/J069v13n04_03 [DOI] [PubMed] [Google Scholar]
- 144.Kampman KM, Rukstalis M, Ehrman R, et al. Open trials as a method of prioritizing medications for inclusion in controlled trials for cocaine dependence. Addict Behav. 1999;24(2):287-291. doi: 10.1016/S0306-4603(98)00040-9 [DOI] [PubMed] [Google Scholar]
- 145.Shoptaw S, Majewska MD, Wilkins J, Twitchell G, Yang X, Ling W. Participants receiving dehydroepiandrosterone during treatment for cocaine dependence show high rates of cocaine use in a placebo-controlled pilot study. Exp Clin Psychopharmacol. 2004;12(2):126-135. doi: 10.1037/1064-1297.12.2.126 [DOI] [PubMed] [Google Scholar]
- 146.Margolin A, Avants SK, Kosten TR. Pemoline for the treatment of cocaine dependence in methadone-maintained patients. J Psychoactive Drugs. 1996;28(3):301-304. doi: 10.1080/02791072.1996.10472491 [DOI] [PubMed] [Google Scholar]
- 147.Kosten TR, Wu G, Huang W, et al. Pharmacogenetic randomized trial for cocaine abuse: disulfiram and dopamine β-hydroxylase. Biol Psychiatry. 2013;73(3):219-224. doi: 10.1016/j.biopsych.2012.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Crosby RD, Pearson VL, Eller C, Winegarden T, Graves NL. Phenytoin in the treatment of cocaine abuse: a double-blind study. Clin Pharmacol Ther. 1996;59(4):458-468. doi: 10.1016/S0009-9236(96)90116-2 [DOI] [PubMed] [Google Scholar]
- 149.Shearer J, Wodak A, van Beek I, Mattick RP, Lewis J. Pilot randomized double blind placebo-controlled study of dexamphetamine for cocaine dependence. Addiction. 2003;98(8):1137-1141. doi: 10.1046/j.1360-0443.2003.00447.x [DOI] [PubMed] [Google Scholar]
- 150.Mooney ME, Herin DV, Specker S, Babb D, Levin FR, Grabowski J. Pilot study of the effects of lisdexamfetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2015;153:94-103. doi: 10.1016/j.drugalcdep.2015.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gilgun-Sherki Y, Eliaz RE, McCann DJ, et al. Placebo-controlled evaluation of a bioengineered, cocaine-metabolizing fusion protein, TV-1380 (AlbuBChE), in the treatment of cocaine dependence. Drug Alcohol Depend. 2016;166:13-20. doi: 10.1016/j.drugalcdep.2016.05.019 [DOI] [PubMed] [Google Scholar]
- 152.Petry NM, Martin B, Simcic F Jr. Prize reinforcement contingency management for cocaine dependence: integration with group therapy in a methadone clinic. J Consult Clin Psychol. 2005;73(2):354-359. doi: 10.1037/0022-006X.73.2.354 [DOI] [PubMed] [Google Scholar]
- 153.Petry NM, Tedford J, Austin M, Nich C, Carroll KM, Rounsaville BJ. Prize reinforcement contingency management for treating cocaine users: how low can we go, and with whom? Addiction. 2004;99(3):349-360. doi: 10.1111/j.1360-0443.2003.00642.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sofuoglu M, Poling J, Gonzalez G, Gonsai K, Oliveto A, Kosten TR. Progesterone effects on cocaine use in male cocaine users maintained on methadone: a randomized, double-blind, pilot study. Exp Clin Psychopharmacol. 2007;15(5):453-460. doi: 10.1037/1064-1297.15.5.453 [DOI] [PubMed] [Google Scholar]
- 155.Epstein DH, Schmittner J, Umbricht A, Schroeder JR, Moolchan ET, Preston KL. Promoting abstinence from cocaine and heroin with a methadone dose increase and a novel contingency. Drug Alcohol Depend. 2009;101(1-2):92-100. doi: 10.1016/j.drugalcdep.2008.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tapp A, Wood AE, Kennedy A, Sylvers P, Kilzieh N, Saxon AJ. Quetiapine for the treatment of cocaine use disorder. Drug Alcohol Depend. 2015;149:18-24. doi: 10.1016/j.drugalcdep.2014.12.037 [DOI] [PubMed] [Google Scholar]
- 157.Brown ES, Nejtek VA, Perantie DC, Bobadilla L. Quetiapine in bipolar disorder and cocaine dependence. Bipolar Disord. 2002;4(6):406-411. doi: 10.1034/j.1399-5618.2002.02229.x [DOI] [PubMed] [Google Scholar]
- 158.Kirby KC, Carpenedo CM, Dugosh KL, et al. Randomized clinical trial examining duration of voucher-based reinforcement therapy for cocaine abstinence. Drug Alcohol Depend. 2013;132(3):639-645. doi: 10.1016/j.drugalcdep.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Weinstein SP, Gottheil E, Sterling RC. Randomized comparison of intensive outpatient vs. individual therapy for cocaine abusers. J Addict Dis. 1997;16(2):41-56. doi: 10.1300/J069v16n02_04 [DOI] [PubMed] [Google Scholar]
- 160.Shoptaw S, Watson DW, Reiber C, et al. Randomized controlled pilot trial of cabergoline, hydergine and levodopa/carbidopa: Los Angeles Cocaine Rapid Efficacy Screening Trial (CREST). Addiction. 2005;100(suppl 1):78-90. doi: 10.1111/j.1360-0443.2005.00991.x [DOI] [PubMed] [Google Scholar]
- 161.Shoptaw S, Yang X, Rotheram-Fuller EJ, et al. Randomized placebo-controlled trial of baclofen for cocaine dependence: preliminary effects for individuals with chronic patterns of cocaine use. J Clin Psychiatry. 2003;64(12):1440-1448. doi: 10.4088/JCP.v64n1207 [DOI] [PubMed] [Google Scholar]
- 162.Preston KL, Ghitza UE, Schmittner JP, Schroeder JR, Epstein DH. Randomized trial comparing two treatment strategies using prize-based reinforcement of abstinence in cocaine and opiate users. J Appl Behav Anal. 2008;41(4):551-563. doi: 10.1901/jaba.2008.41-551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Montoya ID, Gorelick DA, Preston KL, et al. Randomized trial of buprenorphine for treatment of concurrent opiate and cocaine dependence. Clin Pharmacol Ther. 2004;75(1):34-48. doi: 10.1016/j.clpt.2003.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Petry NM, Alessi SM, Hanson T, Sierra S. Randomized trial of contingent prizes versus vouchers in cocaine-using methadone patients. J Consult Clin Psychol. 2007;75(6):983-991. doi: 10.1037/0022-006X.75.6.983 [DOI] [PubMed] [Google Scholar]
- 165.McKay JR, Lynch KG, Coviello D, et al. Randomized trial of continuing care enhancements for cocaine-dependent patients following initial engagement. J Consult Clin Psychol. 2010;78(1):111-120. doi: 10.1037/a0018139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ghitza UE, Epstein DH, Schmittner J, Vahabzadeh M, Lin JL, Preston KL. Randomized trial of prize-based reinforcement density for simultaneous abstinence from cocaine and heroin. J Consult Clin Psychol. 2007;75(5):765-774. doi: 10.1037/0022-006X.75.5.765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Oliveto A, Poling J, Mancino MJ, et al. Randomized, double blind, placebo-controlled trial of disulfiram for the treatment of cocaine dependence in methadone-stabilized patients. Drug Alcohol Depend. 2011;113(2-3):184-191. doi: 10.1016/j.drugalcdep.2010.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Brodie JD, Case BG, Figueroa E, et al. Randomized, double-blind, placebo-controlled trial of vigabatrin for the treatment of cocaine dependence in Mexican parolees. Am J Psychiatry. 2009;166(11):1269-1277. doi: 10.1176/appi.ajp.2009.08121811 [DOI] [PubMed] [Google Scholar]
- 169.Grabowski J, Roache JD, Schmitz JM, Rhoades H, Creson D, Korszun A. Replacement medication for cocaine dependence: methylphenidate. J Clin Psychopharmacol. 1997;17(6):485-488. doi: 10.1097/00004714-199712000-00008 [DOI] [PubMed] [Google Scholar]
- 170.Plebani JG, Lynch KG, Yu Q, Pettinati HM, O’Brien CP, Kampman KM. Results of an initial clinical trial of varenicline for the treatment of cocaine dependence. Drug Alcohol Depend. 2012;121(1-2):163-166. doi: 10.1016/j.drugalcdep.2011.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Grabowski J, Rhoades H, Silverman P, et al. Risperidone for the treatment of cocaine dependence: randomized, double-blind trial. J Clin Psychopharmacol. 2000;20(3):305-310. doi: 10.1097/00004714-200006000-00003 [DOI] [PubMed] [Google Scholar]
- 172.Jones HE, Johnson RE, Bigelow GE, Silverman K, Mudric T, Strain EC. Safety and efficacy of L-tryptophan and behavioral incentives for treatment of cocaine dependence: a randomized clinical trial. Am J Addict. 2004;13(5):421-437. doi: 10.1080/10550490490512753 [DOI] [PubMed] [Google Scholar]
- 173.Mooney ME, Schmitz JM, Moeller FG, Grabowski J. Safety, tolerability and efficacy of levodopa-carbidopa treatment for cocaine dependence: two double-blind, randomized, clinical trials. Drug Alcohol Depend. 2007;88(2-3):214-223. doi: 10.1016/j.drugalcdep.2006.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kirby KC, Marlowe DB, Festinger DS, Lamb RJ, Platt JJ. Schedule of voucher delivery influences initiation of cocaine abstinence. J Consult Clin Psychol. 1998;66(5):761-767. doi: 10.1037/0022-006X.66.5.761 [DOI] [PubMed] [Google Scholar]
- 175.Preston KL, Umbricht A, Wong CJ, Epstein DH. Shaping cocaine abstinence by successive approximation. J Consult Clin Psychol. 2001;69(4):643-654. doi: 10.1037/0022-006X.69.4.643 [DOI] [PubMed] [Google Scholar]
- 176.Poling J, Oliveto A, Petry N, et al. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch Gen Psychiatry. 2006;63(2):219-228. doi: 10.1001/archpsyc.63.2.219 [DOI] [PubMed] [Google Scholar]
- 177.Maude-Griffin PM, Hohenstein JM, Humfleet GL, Reilly PM, Tusel DJ, Hall SM. Superior efficacy of cognitive-behavioral therapy for urban crack cocaine abusers: main and matching effects. J Consult Clin Psychol. 1998;66(5):832-837. doi: 10.1037/0022-006X.66.5.832 [DOI] [PubMed] [Google Scholar]
- 178.Silverman K, Higgins ST, Brooner RK, et al. Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Arch Gen Psychiatry. 1996;53(5):409-415. doi: 10.1001/archpsyc.1996.01830050045007 [DOI] [PubMed] [Google Scholar]
- 179.Shorter D, Lindsay JA, Kosten TR. The alpha-1 adrenergic antagonist doxazosin for treatment of cocaine dependence: a pilot study. Drug Alcohol Depend. 2013;131(1-2):66-70. doi: 10.1016/j.drugalcdep.2012.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Katz EC, Robles-Sotelo E, Correia CJ, Silverman K, Stitzer ML, Bigelow G. The brief abstinence test: effects of continued incentive availability on cocaine abstinence. Exp Clin Psychopharmacol. 2002;10(1):10-17. doi: 10.1037/1064-1297.10.1.10 [DOI] [PubMed] [Google Scholar]
- 181.Kampman KM, Rukstalis M, Pettinati H, et al. The combination of phentermine and fenfluramine reduced cocaine withdrawal symptoms in an open trial. J Subst Abuse Treat. 2000;19(1):77-79. doi: 10.1016/S0740-5472(99)00076-8 [DOI] [PubMed] [Google Scholar]
- 182.Coviello DM, Alterman AI, Rutherford MJ, Cacciola JS, McKay JR, Zanis DA. The effectiveness of two intensities of psychosocial treatment for cocaine dependence. Drug Alcohol Depend. 2001;61(2):145-154. doi: 10.1016/S0376-8716(00)00136-8 [DOI] [PubMed] [Google Scholar]
- 183.Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat. 2001;21(4):193-198. doi: 10.1016/S0740-5472(01)00202-1 [DOI] [PubMed] [Google Scholar]
- 184.Liu S, Green CE, Lane SD, et al. The influence of dopamine β-hydroxylase gene polymorphism rs1611115 on levodopa/carbidopa treatment for cocaine dependence: a preliminary study. Pharmacogenet Genomics. 2014;24(7):370-373. doi: 10.1097/FPC.0000000000000055 [DOI] [PubMed] [Google Scholar]
- 185.Poling J, Rounsaville B, Gonsai K, Severino K, Sofuoglu M. The safety and efficacy of varenicline in cocaine using smokers maintained on methadone: a pilot study. Am J Addict. 2010;19(5):401-408. doi: 10.1111/j.1521-0391.2010.00066.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Holtyn AF, Koffarnus MN, DeFulio A, et al. The therapeutic workplace to promote treatment engagement and drug abstinence in out-of-treatment injection drug users: a randomized controlled trial. Prev Med. 2014;68:62-70. doi: 10.1016/j.ypmed.2014.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gonzalez G, Sevarino K, Sofuoglu M, et al. Tiagabine increases cocaine-free urines in cocaine-dependent methadone-treated patients: results of a randomized pilot study. Addiction. 2003;98(11):1625-1632. doi: 10.1046/j.1360-0443.2003.00544.x [DOI] [PubMed] [Google Scholar]
- 188.Umbricht A, DeFulio A, Winstanley EL, et al. Topiramate for cocaine dependence during methadone maintenance treatment: a randomized controlled trial. Drug Alcohol Depend. 2014;140:92-100. doi: 10.1016/j.drugalcdep.2014.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Milby JB, Schumacher JE, Vuchinich RE, Freedman MJ, Kertesz S, Wallace D. Toward cost-effective initial care for substance-abusing homeless. J Subst Abuse Treat. 2008;34(2):180-191. doi: 10.1016/j.jsat.2007.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Brodie JD, Figueroa E, Dewey SL. Treating cocaine addiction: from preclinical to clinical trial experience with gamma-vinyl GABA. Synapse. 2003;50(3):261-265. doi: 10.1002/syn.10278 [DOI] [PubMed] [Google Scholar]
- 191.Levin FR, Evans SM, Brooks DJ, Garawi F. Treatment of cocaine dependent treatment seekers with adult ADHD: double-blind comparison of methylphenidate and placebo. Drug Alcohol Depend. 2007;87(1):20-29. doi: 10.1016/j.drugalcdep.2006.07.004 [DOI] [PubMed] [Google Scholar]
- 192.Focchi GRA, Leite MC, Andrade AG, Scivoletto S. Use of dopamine agonist pergolide in outpatient treatment of cocaine dependence. Subst Use Misuse. 2005;40(8):1169-1177. doi: 10.1081/JA-200030552 [DOI] [PubMed] [Google Scholar]
- 193.Feingold A, Oliveto A, Schottenfeld R, Kosten TR. Utility of crossover designs in clinical trials: efficacy of desipramine vs. placebo in opioid-dependent cocaine abusers. Am J Addict. 2002;11(2):111-123. doi: 10.1080/10550490290087884 [DOI] [PubMed] [Google Scholar]
- 194.Kosten TR, Domingo CB, Shorter D, et al. Vaccine for cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend. 2014;140:42-47. doi: 10.1016/j.drugalcdep.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Martell BA, Mitchell E, Poling J, Gonsai K, Kosten TR. Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol Psychiatry. 2005;58(2):158-164. doi: 10.1016/j.biopsych.2005.04.032 [DOI] [PubMed] [Google Scholar]
- 196.Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: effects of reinforcement magnitude. Psychopharmacology (Berl). 1999;146(2):128-138. doi: 10.1007/s002130051098 [DOI] [PubMed] [Google Scholar]
- 197.Dallery J, Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of opiate plus cocaine abstinence in treatment-resistant methadone patients: effects of reinforcer magnitude. Exp Clin Psychopharmacol. 2001;9(3):317-325. doi: 10.1037/1064-1297.9.3.317 [DOI] [PubMed] [Google Scholar]
- 198.Petry NM, Alessi SM, Marx J, Austin M, Tardif M. Vouchers versus prizes: contingency management treatment of substance abusers in community settings. J Consult Clin Psychol. 2005;73(6):1005-1014. doi: 10.1037/0022-006X.73.6.1005 [DOI] [PubMed] [Google Scholar]
- 199.DePhilippis D, Petry NM, Bonn-Miller MO, Rosenbach SB, McKay JR. The national implementation of contingency management (CM) in the Department of Veterans Affairs: attendance at CM sessions and substance use outcomes. Drug Alcohol Depend. 2018;185:367-373. doi: 10.1016/j.drugalcdep.2017.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.US Department of Veterans Affairs. Restoring trust in veterans health care: fiscal year 2016 annual report. Veterans Health Administration; 2016. Accessed March 26, 2020. https://www.va.gov/HEALTH/docs/VHA_AR16.pdf
- 201.Glass JE, Nunes EV, Bradley KA. Contingency management: a highly effective treatment for substance use disorders and the legal barriers that stand in its way. Health Affairs. March 11, 2020. Accessed February 28, 2021. https://www.healthaffairs.org/do/10.1377/hblog20200305.965186/full/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Study Protocol
eMethods 2. R Scripts With Associated Output
eMethods 3. Model Outputs in Data Sets (Subsetted by Treatment Category)
eFigure 1. Forest Plot Without Imputed Data: Anticonvulsants
eFigure 2. Forest Plot Without Imputed Data: Antidepressants
eFigure 3. Forest Plot Without Imputed Data: Antipsychotics
eFigure 4. Forest Plot Without Imputed Data: Contingency Management
eFigure 5. Forest Plot Without Imputed Data: Dopamine Agonists
eFigure 6. Forest Plot Without Imputed Data: Miscellaneous Medications
eFigure 7. Forest Plot Without Imputed Data: Opioids
eFigure 8. Forest Plot Without Imputed Data: Other Interventions
eFigure 9. Forest Plot Without Imputed Data: Placebo
eFigure 10. Forest Plot Without Imputed Data: Psychostimulants
eFigure 11. Forest Plot Without Imputed Data: Psychotherapy
eFigure 12. Funnel Plots (Full Models)
eFigure 13. Funnel Plots (By Treatment Category)
eReferences