Abstract
Objective
To assess the functional effects of a variant, c.89 G > A (p.Arg30Gln), in the transient receptor potential melastatin 8 (TRPM8) cold-sensing, nonselective cation channel, which we have previously identified in a patient with familial trigeminal neuralgia.
Methods
We carried out Ca2+ imaging and whole-cell patch-clamp recording.
Results
The TRPM8 mutation enhances channel activation, increases basal current amplitude and intracellular [Ca2+] in cells carrying the mutant channel, and enhances the response to menthol.
Conclusions
We propose that Arg30Gln confers gain-of-function attributes on TRPM8, which contribute to pathogenesis of trigeminal neuralgia in patients carrying this mutation.
Trigeminal neuralgia (TN) is a unique pain disorder in which affected individuals suffer from paroxysms of severe pain in the distribution of the trigeminal nerve, i.e., over the face, usually with onset in older adults.1 The disorder is often unilateral, and in many cases, although not all, it is associated with neurovascular compression of the trigeminal root.2 Although most cases of trigeminal neuralgia are sporadic, familial occurrence has been reported.3–5 Di Stefano et al.6 recently reported a positive family history for 12 patients with trigeminal neuralgia within a series of 88 patients enrolled consecutively for assessment at a specialized neuropathic pain center. The occurrence of familial TN suggests a genetic contribution to this disorder. Whole-exome sequencing of genes within the electrogenisome known to be expressed within trigeminal ganglion revealed multiple variants of ion channels including sodium channels, potassium channels, chloride channels, calcium channels, and transient receptor potential (TRP) channels in the genomes of these familial patients with TN,6 but the possible contribution of these variants to TN must be empirically assessed. Here, we use Ca2+ imaging and whole-cell patch clamp to assess a variant in the transient receptor potential melastatin 8 (TRPM8) cold-sensing, nonselctive cation channel, c.89 G > A (p.Arg30Gln), in a patient with familial trigeminal neuralgia. We demonstrate that this TRPM8 mutation displays multiple gain-of-function characteristics including enhanced channel activation, increased basal current amplitude and intracellular [Ca2+] in cells carrying the mutant channel, and enhanced response to menthol. We suggest a model in which these gain-of-function attributes contribute to the pathogenesis of trigeminal neuralgia.
Methods
Patient
The patient was a 61-year-old woman with severe attacks of unilateral facial pain, beginning at the age of 60, triggered by chewing, talking, and light touch of the nasal wing (case 1 in DiStefano et al., 20206). Two brothers also had a history of trigeminal neuralgia. MRI revealed neurovascular compression with atrophy and dislocation of the trigeminal root. Oxcarbazepine provided pain relief. The study was approved by the Ethic Committee of Sapienza University (reference 649/17). Written informed consent was obtained from the participant.
DNA analysis revealed that this patient and her older brother, also carrying a diagnosis of trigeminal neuralgia, share a 9-bp duplication (c.642_650dup; 27/190,394 on gnomAD v2) in potassium channel gene KCNC3 and a synonymous splice site variant (c.1467 G > A; 3/250,442 on gnomAD v2) in KCND2 predicted to reduce splicing efficiency using Alternative Splice Site Predictor (wangcomputing.com/assp/) and Human Splicing Finder (umd.be/HSF/). The patient carried a novel missense variant c.1489 G > A (p.Gly497Ser) in KCNH2 and a rare missense variant c.6032 G > A (p.Arg2011Gln; 4/282,448 on gnomAD v2) in CACNA1D.6 Notably, the patient was heterozygous for a variant, c.89 G > A (p.Arg30Gln; 9/282,688 on gnomAD v2), in TRPM8. Given the expression of TRPM8 within trigeminal ganglia7–9 and reported association of TRPM8 with pain,10–12 we asked whether this variant might produce any functional changes in the TRPM8 channel or within cells expressing it.
Plasmid, Cell Culture, and Transfection
Patch-clamp studies were carried out in human embryonic kidney (HEK) 293 cells transiently transfected with vectors that encode human TRPM8 or the Arg30Gln variant (referred to in the single letter amino acid code R30Q hereinafter). The wild-type TRPM8 plasmid was obtained from GenScript, in which the insert was cloned in-frame with 2A-GFP at the C-terminus of the channel in the vector pcDNA3.1. The single TRPM8-2AGFP transcript is translated into the separate channel protein and the transfection marker GFP protein.13,14 The R30Q mutation was introduced into the TRPM8 channel using QuikChange Lightning site-directed mutagenesis (Agilent Technologies). For heterologous expression, cells were plated in 6-well cell culture dishes with 2-mL growth medium (DMEM, 10% FBS, 2 mM l-glutamine, 2 U/mL penicillin, and 2 mg/mL streptomycin) 24 hours before transfection. Transfection was performed using the Lipofectamine LTX Reagent (Invitrogen) as previously described.15
Electrophysiology
Patch-clamp recordings of HEK293 cells were carried out at room temperature using an EPC-9 amplifier (HEKA Elektronik, Lambrecht, Germany) controlled by PatchMaster software (HEKA Elektronik, Lambrecht, Germany). Patch-clamp electrodes were pulled and fire polished to 4.0 ± 0.5 MΩ resistance when filled with intracellular solution using a DMZ-Universal Puller (Zeitz Instruments, Munich, Germany). An AgCl wire was used as a reference electrode. To elicit I-V whole-cell currents, repetitive 400 ms voltage ramps (at 2 seconds intervals) from −100 to +100 mV were applied, from a holding potential of 0 mV. Currents were sampled at 20 kHz and digitally filtered at 2.9 kHz. For statistical analysis, the currents were normalized to cell capacitance. Solutions were applied to the cells via a custom-built gravity-fed perfusion system, connected by a 5-way manifold, to a RC25 perfusion chamber (Warner Instruments, Hamden, CT). For whole cell recordings, the standard extracellular solution had the following composition (in mM): 150 NaCl, 5 KCl, 1 MgCl2, 1.8 CaCl2, 10 glucose, and 10 HEPES, buffered at pH 7.4 with NaOH. The osmolarity of this solution was 320 ± 5 mOsm/kgH2O. The intracellular solution had the following composition (in mM): 140 CsCl, 0.3 CaCl2, 2 MgATP, 10 EGTA and 10 HEPES, adjusted to pH 7.2 with CsOH (290 ± 5 mOsm).
To analyze the effects of the mutation R30Q on the voltage dependence of TRPM8, we applied voltage step protocols from −100 to +200 mV. At each data point, we calculated G/Gmax, where Gmax is the maximal steady state conductance in the presence of 1 mM menthol. Therefore, we fit the G/Gmax values using a Boltzmann function of the form:
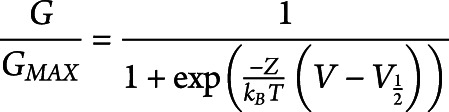 |
where z is the gating charge, V is voltage, V1/2 is the voltage for half-maximal activation, kB is the Boltzmann constant, and T is the absolute temperature. For simplicity, we assume for our calculations a two-state model of channel gating.16
Ca2+ Imaging
Loading of the HEK293 cells with Fura2-AM was achieved at room temperature in a Krebs medium with 5 μM fura-2-AM and 0.01% pluronic acid for 1 hours [Ca2+]i was measured in 10–15 individual cells for each experiment using an alternative excitation of Fura2-AM (0.5 Hz) at 340 and 380 nm using a Lambda DG-4 Ultra High Speed Wavelength Switcher (Sutter Instrument, Novato, CA). Images were acquired with a Zeiss Axiocam camera coupled to a 510-nm emission filter and analyzed with Axiovision software. Ca2+ imaging experiments were performed in a Krebs solution (NaCl 135 mM, KCl 5.9 mM, MgCl2 1.2 mM, CaCl2 1.8 mM, HEPES 11.6 mM, glucose 11.5 mM, pH 7.35).
Data Availability
The raw data are available upon request.
Results
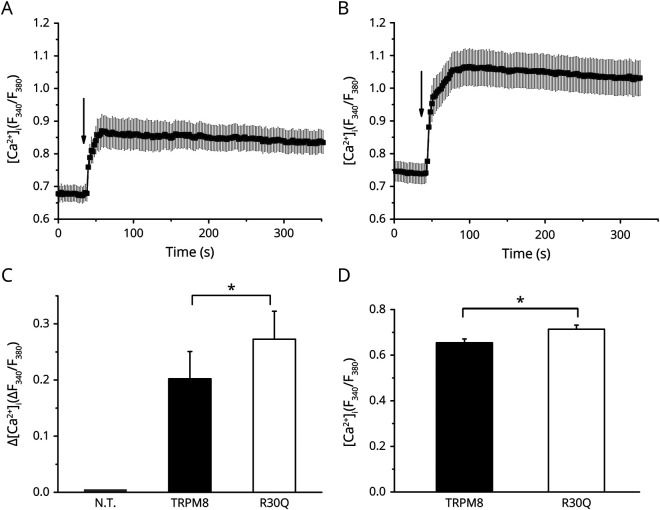
To investigate the effect of the R30Q variant on TRPM8 activity, wild-type and mutant human TRPM8 were expressed in HEK293 cells. In cultured cells transiently transfected with either wild-type or R30Q TRPM8 channels, exposure to 100 μM menthol, added in the bath at the time indicated by the arrow and continuously perfused until the end of the experiment, evoked a robust increase in intracellular calcium, measured as a change in the Fura-2 fluorescence signal ratio (figure 1, A and B). In comparison with cells expressing wild-type TRPM8, the R30Q mutation yielded a larger Ca2+ increase in response to menthol (figure 1C). Moreover, the initial Fura-2 fluorescence signal ratio, which reflects the basal intracellular [Ca2+], was slightly but significantly higher in cells expressing R30Q compared with the wild-type TRPM8 (figure 1D). In vitro cotransfections of mutant and wild-type to model in vivo heterozygosity revealed a slight increase of Ca2+ uptake in response to menthol (Supplementary figure 1A, links.lww.com/NXG/A359) and a significant increase of basal Ca2+ (Supplementary figure 1B, links.lww.com/NXG/A359) compared to wild-type TRPM8 transfected cells.
Figure 1. The TRPM8-R30Q Enhances Calcium Entry in Response to Stimulation by Menthol.
Average changes in the Fura-2 ratio of human embryonic kidney 293 cells transfected with wild-type TRPM8 (A) and R30Q mutant (B), in the continued presence of menthol (100 μM; arrow indicates time of addition). (C) Average increase in Fura-2 ratio in response to menthol (100 μM) in nontransfected cells (NT), wild-type TRPM8, and R30Q transfected cells (n = 10 experiments, 4 transfections). *p < 0.05, unpaired Student's t test. (D) Basal fura-2 ratio in wild-type TRPM8 and R30Q mutant transfected cells (n = 15 experiments, 4 transfections). *p < 0.05, unpaired Student t test. TRPM8 = transient receptor potential melastatin 8.
To further characterize the R30Q mutation under basal conditions, we recorded steady-state evoked currents during voltage steps from −100 mV to +200 mV from a holding potential of 0 mV (figure 2A). The basal current amplitude of the R30Q mutant was significantly higher compared with TRPM8 wild-type at potentials greater than 150 mV (figure 2B). Normalized conductance for each cell, referred to as G/Gmax, was plotted for the given voltages and fitted with a Boltzmann function (figure 2C), Gmax being the maximal steady state conductance in the presence of 1 mM menthol. We found that the R30Q mutation left-shifted the activation curve toward physiologic voltages, from 153 ± 8 mV to 115 ± 5 mV (**p < 0.01, unpaired Student's t test). Because saturating effects in the presence of 1 mM menthol could not be obtained for the R30Q mutant, values for V1/2 and slope factor should be considered as approximates.
Figure 2. Voltage-Clamp Recordings Show Increased Basal Activity of TRPM8-R30Q Channels.
(A) Representative whole-cell current traces through wild-type TRPM8 and R30Q transfected cells, in response to the indicated voltage step protocol. (B) Steady state current-voltage relationships of the basal whole-cell currents for wild-type TRPM8 and R30Q mutant (n = 12, **p < 0.01, ***p < 0.001, 2-way analysis of variance with Bonferroni post hoc test). (C) Steady-state activation curves of wild-type TRPM8 and R30Q transfected cells. The normalized conductance (G/Gmax) was plotted against voltage and fitted with a Boltzmann function, giving rise to V1/2 and slope factor as follows: TRPM8, 153 ± 8 mV and 30 ± 3 mV (n = 12 cells, 3 transfections); R30Q, 115 ± 5 mV and 28 ± 2 mV (n = 12 cells, 3 transfections). **p < 0.01, unpaired Student's t test. (D) Representative time courses (left) recorded at +80 mV and −80 mV (black and grey curves, respectively) and I-V traces (right) of whole cell currents through wild-type TRPM8 transfected cells, in the presence of 100 μM menthol, at the indicated time intervals. (E) Same as D), except that time courses and I-V traces are recorded from R30Q transfected cells. (F) Pooled data of whole-cell current (at +80 mV and −80 mV) evoked by 100 μM menthol, from wild-type TRPM8 and R30Q transfected cells. Each column represents mean ± SEM of n = 8 cells, 3 independent experiments. *p < 0.05 (unpaired Student's t-test). TRPM8 = transient receptor potential melastatin 8.
Finally, because menthol is well-known to be an agonist for TRPM8, we tested menthol-induced responses of mutant and wild-type TRPM8 channels using patch clamp recordings. Menthol at nonsaturating concentration of 100 μM32 evoked robust currents with strong outward rectification in cells expressing both R30Q and wild-type TRPM8 (figure 2, D and E). The current is characterized by a reversal potential near 0 mV, suggesting that TRPM8 is a nonselective Ca2+ channel. In particular, when activated, it allows the entry of Na+ and Ca2+ ions into the cell. At positive membrane potential (+80 mV) the amplitude of the current in response to 100 μM menthol was significantly higher in R30Q transfected cells compared with wild-type TRPM8 expressing cells (figure 2F).
Discussion
Trigeminal neuralgia is characterized by recurrent excruciating pain in affected individuals, which is thought to arise from episodes of hyperactivity of primary trigeminal afferents.17–19 Despite intense study, the pathophysiologic basis of this trigeminal ganglion neuron hyperexcitability remains incompletely understood. Trigeminal neuralgia is unilateral, and in many cases, although not all cases, it is associated with neurovascular compression of the trigeminal root.2
Although trigeminal neuralgia tends to occur in a sporadic manner, familial occurrence has been reported.3–5 Together with the occurrence of trigeminal neuralgia in the absence of neurovascular compression, the occurrence of familial trigeminal neuralgia supports a genetic contribution to this pain disorder. Here, we build on a study which demonstrated positive family history for 12 subjects, within a series of 88 patients with trigeminal neuralgia.6 Whole-exome sequencing of genes within the electrogenisome of trigeminal ganglion neurons demonstrated variants of ion channels including sodium channels, potassium channels, chloride channels, calcium channels, and TRP channels.
In addition, to a mutation of TRPM8, the patient described in this report carried a rare missense variant c.6032 G > A (p.Arg2011Gln) in CACNA1D. Gain-of-function mutations of CACNA1D, encoding pore-forming Cav1.3 α1-subunit, have been reported to contribute to primary aldosteronism, seizures, and multiple psychiatric and neurologic syndromes.20,21 The current patient also carried rare variants in 3 potassium channel genes, consisting of a 9-bp duplication (c.642_650dup) in KCNC3, a synonymous splice site variant (c.1467 G > A) in KCND2 predicted to reduce splicing efficiency, and a novel missense variant p.1489 G > A (p.Gly497Ser) in KCNH2. Among these potassium channel genes, KCND2 encodes Kv4.2, a rapidly inactivating voltage-gated potassium channel, and Kv4.2 knockout mice display increased neuronal excitability and hypersensitivity to nociceptive stimuli.22 Missense variants of KCND2 gene have been found possibly associated with infantile-onset severe refractory epilepsy23 and persistent breast pain after breast cancer surgery.24 Loss-of-function mutations of KCNC3, encoding Kv3.3, have been linked to cerebellar ataxia, accompanied with hyperreflexia, deep sensory loss, seizures, involuntary movement, and cognitive impairment,25,26 whereas loss-of-function mutations of KCNH2, encoding Kv11.1, are found in approximately 30% of patients with Long QT syndrome.27,28 Within transcriptome data sets of mouse or human trigeminal/dorsal root ganglion, RNA expression levels of KCNC3, KCNH2, and CACNA1D were higher than for KCND2, but no linkage or association between the former 3 genes and pain had been described.29–31 (Supplementary table 1, links.lww.com/NXG/A359). Nevertheless, we can not exclude a contribution of these variants to trigeminal neuralgia.
In this study, we focused on a mutation in TRPM8, a gene of which is known to be expressed in the trigeminal and dorsal root ganglia.7–9 TRPM8 is an outwardly rectifying nonselective Ca2+ channel, first characterized as a neuronal detector of cold.32,33 It is activated by cold temperatures (<26°C) and compounds that produce a cooling sensation such as menthol, icilin, and eucalyptol.33,34 TRPM8 is also activated by voltage. In the absence of chemical agonists or at high temperature, the channel is gated by strong depolarizations. In contrast, cold temperature or agonist application shift the steady-state activation curve of TRPM8 to more negative potentials, increasing the open probability of the channel at physiologic membrane potentials.35,36
Although the role of TRPM8 as cold transducer is well-established, its role in pain sensation is not clear. In fact it is still under debate whether TRPM8 amplifies and/or reduces pain sensation. A number of studies suggest that the activation of TRPM8 exaggerates pain sensation.11,12 In support of these results, selective TRPM8 antagonists reduced chronic pain and allodynia in animal models.10,37 In contrast, other studies report that TRPM8 agonists have analgesic properties.38,39 A possible explanation for these apparently disparate results, where the activation of TRPM8 seems to cause cold pain after injury while simultaneously reducing mechanical and heat pain, could be that TRPM8-expressing afferent fibers have the ability to either enhance or attenuate pain, but the outcome is determined by context.40
We studied the effect of the substitution of the Arg30, located in the N-terminus, by glutamine on the biophysical properties of TRPM8. Our results indicate that the R30Q mutation in TRPM8 enhances channel activation, increases basal current amplitude and intracellular [Ca2+] in cells carrying the mutant channel, and enhances the channel response to menthol. Interestingly, coexpression of mutant and wild type to model in vivo heterozygosity revealed a significant increase of basal Ca2+ suggesting a dominant effect of the R30Q mutation. It was previously observed that deletion and substitution of the first 40 residues yielded TRPM8 channels with augmented responses to cold and menthol.41 In particular, electrophysiologic analysis of the S26P and S27P mutants revealed that the enhanced sensitivity to agonists was related to a strong leftward shift in the voltage dependence of activation. Because this phenotype occurs only when a proline is introduced, without a significant effect of alanine or aspartate mutations, the authors suggest that the augmented response of the channels is because of the alteration of the structure of TRPM8. They speculated that in wild-type TRPM8 channels, the interaction of the N-terminal region with other TRPM8 domains or with a partner protein reduces channel activity. As a consequence, specific deletion or substitution of the first 40 amino acids of TRPM8 could compromise the interaction with modulatory proteins or internal regions that constitutively inhibits TRPM8 function. In the light of our findings, we can hypothesize that a mutation of the Arg30, which is close to Ser26 and Ser27 residues, could alter the stability of the N-terminus of TRPM8, similarly, to the effect observed for S26P and S27P mutations, leading to its gain-of-function enhancement.
Small persistent Na+ currents42 are known to trigger reverse Na+/Ca2+ exchange that can lead to increased intracellular Ca2+, which can produce axonal injury43; gain-of-function mutations of Na+ channels impair axonal integrity and ionic homeostasis in vitro44,45 and have been linked to painful human neuropathies.46,47 Although the predicted increase in intracellular Ca2+ resulting from the R30Q mutation at physiologic membrane potentials would be expected to be small, there is evidence that the cumulative effects of small increases in intracellular Ca2+ can predispose axons to time-dependent injury, i.e., adult-onset axonal disease.48 Consistent with the view that mutations underlie a slow influx of Na+ or Ca2+ can predispose trigeminal axons to mechanical injury, a gain-of-function mutation of sodium channel Nav1.6 has been described in a patient with adult onset trigeminal neuralgia and ipsilateral neurovascular compression of the trigeminal root.49 Alternatively, it is possible that vascular compression causes demyelination of the trigeminal root. Electrophysiologic recordings and computer simulations show that focally demyelinated axons can become hyperexcitable50,51 and the enhanced TRPM8 channel activation increased basal current amplitude and intracellular [Ca2+] in cells carrying the mutant channel could summate with this hyperexcitability to produce ectopic activity.
The familial occurrence of trigeminal neuralgia in some patients, together with the presence of unilateral pain and adult onset, suggest a multihit model in which genetic factors can contribute to pathophysiology. Our results provide support for the idea that genetic factors can predispose axons to injury in response to mechanical insults and specifically for the hypothesis that variants of ion channels within the trigeminal neuron electrogenisome can predispose to the development of trigeminal neuralgia in response to microvascular compression.
Acknowledgment
The authors thank Fadia B. Dib-Hajj for technical assistance.
Glossary
- DMEM
Dulbecco's Modified Eagle's Medium
- EGTA
ethylene glycol tetraacetic acid
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HEK
human embryonic kidney
- TN
trigeminal neuralgia
- TRPM8
transient Receptor Potential Melastatin 8
Appendix. Authors
Contributor Information
Roberta Gualdani, Email: roberta.gualdani@uclouvain.be.
Jun-Hui Yuan, Email: jun-hui.yuan@yale.edu.
Philip R. Effraim, Email: philip.effraim@yale.edu.
Giulia Di Stefano, Email: giulia.distefano@uniroma1.it.
Andrea Truini, Email: andrea.truini@uniroma1.it.
Giorgio Cruccu, Email: giorgio.cruccu@uniroma1.it.
Sulayman D. Dib-Hajj, Email: sulayman.dib-hajj@yale.edu.
Philippe Gailly, Email: philippe.gailly@uclouvain.be.
Study Funding
This work was supported in part by Merit Award (I01 RX003201) from the Rehabilitation Research and Development Service, U.S. Department of Veterans Affairs; by the Queen Elisabeth Medical Foundation (Belgium); and the Concerted Research Action from the General Direction of Scientific Research of the French Community of Belgium (ARC17/22-083).
Disclosure
R. Gualdani, J.H. Yuan, P.R. Effraim, G. Di Stefano, A. Truini, G. Cruccu, and S.D. Dib-Hajj report no disclosures relevant to the manuscript. P. Gailly was funded by the Queen Elisabeth Medical Foundation (Belgium) and the Concerted Research Action from the General Direction of Scientific Research of the French Community of Belgium (ARC17/22-083). S.G. Waxman was funded by Merit Award (I01 RX003201) from the Rehabilitation Research and Development Service, U.S. Department of Veterans Affairs. Go to Neurology.org/NG for full disclosures.
References
- 1.Cruccu G, Di Stefano G, Truini A. Trigeminal neuralgia. New Engl J Med 2020;383:754–762. [DOI] [PubMed] [Google Scholar]
- 2.Cruccu G, Finnerup NB, Jensen TS, et al. Trigeminal neuralgia: new classification and diagnostic grading for practice and research. Neurology 2016;87:220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleetwood IG, Innes AM, Hansen SR, Steinberg GK. Familial trigeminal neuralgia. Case report and review of the literature. J Neurosurg 2001;95:513–517. [DOI] [PubMed] [Google Scholar]
- 4.Maarbjerg S, Gozalov A, Olesen J, Bendtsen L. Trigeminal neuralgia--a prospective systematic study of clinical characteristics in 158 patients. Headache 2014;54:1574–1582. [DOI] [PubMed] [Google Scholar]
- 5.Panchagnula S, Sularz AK, Kahle KT. Familial trigeminal neuralgia cases implicate genetic factors in disease pathogenesis. JAMA Neurol 2019;76:9–10. [DOI] [PubMed] [Google Scholar]
- 6.Di Stefano G, Yuan JH, Cruccu G, Waxman SG, Dib-Hajj SD, Truini A. Familial trigeminal neuralgia - a systematic clinical study with a genomic screen of the neuronal electrogenisome. Cephalalgia 2020;40:767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caudle RM, Caudle SL, Jenkins AC, Ahn AH, Neubert JK. Sex differences in mouse transient receptor potential cation channel, subfamily M, member 8 expressing trigeminal ganglion neurons. PLoS One 2017;12:e0176753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Mikrani R, He Y, et al. TRPM8 channels: a review of distribution and clinical role. Eur J Pharmacol 2020;882:173312. [DOI] [PubMed] [Google Scholar]
- 9.Pina R, Ugarte G, Campos M, et al. Role of TRPM8 channels in altered cold sensitivity of corneal primary sensory neurons induced by axonal damage. J Neurosci 2019;39:8177–8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Caro C, Cristiano C, Avagliano C, et al. Characterization of new TRPM8 modulators in pain perception. Int J Mol Sci 2019;20:5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gavva NR, Sandrock R, Arnold GE, et al. Reduced TRPM8 expression underpins reduced migraine risk and attenuated cold pain sensation in humans. Sci Rep 2019;9:19655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling YH, Chen SP, Fann CS, Wang SJ, Wang YF. TRPM8 genetic variant is associated with chronic migraine and allodynia. J Headache Pain 2019;20:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atkins JF, Wills NM, Loughran G, et al. A case for “StopGo”: reprogramming translation to augment codon meaning of GGN by promoting unconventional termination (Stop) after addition of glycine and then allowing continued translation (Go). RNA 2007;13:803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luke GA, de Felipe P, Lukashev A, Kallioinen SE, Bruno EA, Ryan MD. Occurrence, function and evolutionary origins of “2A-like” sequences in virus genomes. J Gen Virol 2008;89:1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gualdani R, Ceruti S, Magni G, et al. Lipoic-based TRPA1/TRPV1 antagonist to treat orofacial pain. ACS Chem Neurosci 2015;6:380–385. [DOI] [PubMed] [Google Scholar]
- 16.Voets T, Owsianik G, Janssens A, Talavera K, Nilius B. TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat Chem Biol 2007;3:174–182. [DOI] [PubMed] [Google Scholar]
- 17.Burchiel KJ, Baumann TK. Pathophysiology of trigeminal neuralgia: new evidence from a trigeminal ganglion intraoperative microneurographic recording. Case Report J Neurosurg 2004;101:872–873. [DOI] [PubMed] [Google Scholar]
- 18.Devor M, Amir R, Rappaport ZH. Pathophysiology of trigeminal neuralgia: the ignition hypothesis. Clin J Pain 2002;18:4–13. [DOI] [PubMed] [Google Scholar]
- 19.Rappaport ZH, Devor M. Trigeminal neuralgia: the role of self-sustaining discharge in the trigeminal ganglion. Pain 1994;56:127–138. [DOI] [PubMed] [Google Scholar]
- 20.Pinggera A, Striessnig J. Cav 1.3 (CACNA1D) L-type Ca(2+) channel dysfunction in CNS disorders. J Physiol 2016;594:5839–5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scholl UI, Goh G, Stolting G, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet 2013;45:1050–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu HJ, Carrasquillo Y, Karim F, et al. The kv4.2 potassium channel subunit is required for pain plasticity. Neuron 2006;50:89–100. [DOI] [PubMed] [Google Scholar]
- 23.Lee H, Lin MC, Kornblum HI, Papazian DM, Nelson SF. Exome sequencing identifies de novo gain of function missense mutation in KCND2 in identical twins with autism and seizures that slows potassium channel inactivation. Hum Mol Genet 2014;23:3481–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langford DJ, Paul SM, West CM, et al. Variations in potassium channel genes are associated with distinct trajectories of persistent breast pain after breast cancer surgery. Pain 2015;156:371–380. [DOI] [PubMed] [Google Scholar]
- 25.Tada Y, Kume K, Matsuda Y, et al. Genetic screening for potassium channel mutations in Japanese autosomal dominant spinocerebellar ataxia. J Hum Genet 2020;65:363–369. [DOI] [PubMed] [Google Scholar]
- 26.Waters MF, Minassian NA, Stevanin G, et al. Mutations in voltage-gated potassium channel KCNC3 cause degenerative and developmental central nervous system phenotypes. Nat Genet 2006;38:447–451. [DOI] [PubMed] [Google Scholar]
- 27.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell 1995;80:795–803. [DOI] [PubMed] [Google Scholar]
- 28.Tester DJ, Ackerman MJ. Genetics of long QT syndrome. Methodist Debakey Cardiovasc J 2014;10:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flegel C, Schobel N, Altmuller J, et al. RNA-seq analysis of human trigeminal and dorsal root ganglia with a focus on chemoreceptors. PLoS One 2015;10:e0128951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopes DM, Denk F, McMahon SB. The molecular fingerprint of dorsal root and trigeminal ganglion neurons. Front Mol Neurosci 2017;10:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Usoskin D, Furlan A, Islam S, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 2015;18:145–153. [DOI] [PubMed] [Google Scholar]
- 32.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002;416:52–58. [DOI] [PubMed] [Google Scholar]
- 33.Peier AM, Moqrich A, Hergarden AC, et al. A TRP channel that senses cold stimuli and menthol. Cell 2002;108:705–715. [DOI] [PubMed] [Google Scholar]
- 34.Chuang HH, Neuhausser WM, Julius D. The super-cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel. Neuron 2004;43:859–869. [DOI] [PubMed] [Google Scholar]
- 35.Brauchi S, Orio P, Latorre R. Clues to understanding cold sensation: thermodynamics and electrophysiological analysis of the cold receptor TRPM8. Proc Natl Acad Sci U S A 2004;101:15494–15499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 2004;430:748–754. [DOI] [PubMed] [Google Scholar]
- 37.Knowlton WM, Daniels RL, Palkar R, McCoy DD, McKemy DD. Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLoS One 2011;6:e25894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu B, Fan L, Balakrishna S, Sui A, Morris JB, Jordt SE. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain 2013;154:2169–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel R, Goncalves L, Leveridge M, et al. Anti-hyperalgesic effects of a novel TRPM8 agonist in neuropathic rats: a comparison with topical menthol. Pain 2014;155:2097–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weyer AD, Lehto SG. Development of TRPM8 antagonists to treat chronic pain and migraine. Pharmaceuticals (Basel) 2017;10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pertusa M, Gonzalez A, Hardy P, Madrid R, Viana F. Bidirectional modulation of thermal and chemical sensitivity of TRPM8 channels by the initial region of the N-terminal domain. J Biol Chem 2014;289:21828–21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stys PK, Sontheimer H, Ransom BR, Waxman SG. Noninactivating, tetrodotoxin-sensitive Na+ conductance in rat optic nerve axons. Proc Natl Acad Sci U S A 1993;90:6976–6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stys PK, Waxman SG, Ransom BR. Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na(+)-Ca2+ exchanger. J Neurosci 1992;12:430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Estacion M, Vohra BP, Liu S, et al. Ca2+ toxicity due to reverse Na+/Ca2+ exchange contributes to degeneration of neurites of DRG neurons induced by a neuropathy-associated Nav1.7 mutation. J Neurophysiol 2015;114:1554–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Persson AK, Liu S, Faber CG, Merkies IS, Black JA, Waxman SG. Neuropathy-associated Nav1.7 variant I228M impairs integrity of dorsal root ganglion neuron axons. Ann Neurol 2013;73:140–145. [DOI] [PubMed] [Google Scholar]
- 46.Faber CG, Hoeijmakers JG, Ahn HS, et al. Gain of function Nanu1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol 2012;71:26–39. [DOI] [PubMed] [Google Scholar]
- 47.Faber CG, Lauria G, Merkies IS, et al. Gain-of-function Nav1.8 mutations in painful neuropathy. Proc Natl Acad Sci U S A 2012;109:19444–19449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Persson AK, Hoeijmakers JGJ, Estacion M, Black JA, Waxman SG. Sodium channels, mitochondria, and axonal degeneration in peripheral neuropathy. Trends Mol Med 2016;22:377–390. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka BS, Zhao P, Dib-Hajj FB, et al. A gain-of-function mutation in Nav1.6 in a case of trigeminal neuralgia. Mol Med 2016;22:338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rasminsky M. Ectopic generation of impulses and cross-talk in spinal nerve roots of “dystrophic” mice. Ann Neurol 1978;3:351–357. [DOI] [PubMed] [Google Scholar]
- 51.Waxman SG, Brill MH. Conduction through demyelinated plaques in multiple sclerosis: computer simulations of facilitation by short internodes. J Neurol Neurosurg Psychiatry 1978;41:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data are available upon request.