Abstract
Introduction
The COVID-19 pandemic had an unprecedented global socioeconomic impact. Responses to pandemics include strategies to accumulate vast stockpiles of vital medical equipment. In such times of desperation, 3D-printing could be a life-saving alternative.
Methods
We undertook a PRISMA systematic review of 3D printing solutions in response to COVID-19 utilising the PICO methodology. The objectives were to identify the uses of 3D printing during the COVID-19 pandemic, determine the extent of preclinical testing, comparison to commercial alternatives, presence of regulatory approvals and replicability regarding the description of the printing parameters and the availability of the print file.
Results
Literature searches of MEDLINE (OVID interface)/ PubMed identified 601 studies. Of these, 10 studies fulfilled the inclusion and exclusion criteria. Reported uses of 3D printing included personal protective equipment (PPE), nasopharyngeal swabs and adjunctive anaesthetic equipment. Few studies undertook formal safety and efficacy testing before clinical use with only one study comparing to the commercial equivalent. Six articles made their model print files available for wider use.
Conclusion
We describe a protocol for a systematic review of 3D-printed healthcare solutions in response to COVID-19. This remains a viable method of producing vital healthcare equipment when supply chains are exhausted. We hope that this will serve as a summary of innovative 3D-printed solutions during the peak of the pandemic and also highlight concerns and omissions regarding safety and efficacy testing that should be addressed urgently in preparation for a subsequent resurgences and future pandemics.
Keywords: COVID-19, 3D printing, Pandemic, Resources alternatives, 3D solutions, Innovation
1. Introduction
Despite the accumulation of large stockpiles in preparation for potential influenza pandemics by the UK government and many governments around the world, there have still been significant shortages of personal protective equipment (PPE) for healthcare workers and essential medical supplies [1]. The ‘Operating Framework for Managing the Response to Pandemic Influenza’ (2017) outlines the UK government's policy for such an eventuality [2]. Pandemics of novel viruses such as COVID-19, however, provide unique challenges that cannot be planned for. The three main principles underlying the government strategy are 1) precautionary, 2) proportionality and 3) flexibility. The precautionary aspect delineates the need for preparedness and the ability to mount an effective response to mitigate early spread. Key to this strategy is the need for adherence to infection control procedures, protection of healthcare workers and the distribution of a vaccine or treatment if one exists. The proportionality of the response outlines the ability to mobilise the correct volume of resources commensurate to the scale of the threat, whilst flexibility defines the need to adapt to new challenges and spread of the disease.
Healthcare systems throughout the world have been put under immense strain and sometimes overrun by the surge of critically ill patients [3]. The technology of 3D printing can transcend and contribute to each of the main strategic principles in a meaningful way. Communities of 3D printing enthusiasts [4], as well as industry (Noble B., 2020; UCL, 2020), have been able to leverage the technology to rapidly design and manufacture replacements for essential healthcare equipment in response to the depletion of vital medical supplies[5].
We undertook a Preference Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) [6] review of 3D printing applications in response to healthcare supply shortages during the COVID-19 pandemic. This systematic review aims to evaluate the emergence of novel 3D printing applications during the peak of the pandemic. Accordingly, the objectives of the systematic review are to:
-
i)
Describe the current uses of 3D printing that have been published within the medical literature to date.
-
ii)
Determine the extent of preclinical testing before distribution of the devices.
-
iii)
The extent to which the devices were compared to the products that they replaced and their relative effectiveness.
-
iv)
Adherence to safety testing, reporting standards and regulatory approvals.
-
v)
The extent to which the printing and filament parameters, as well as cost, are described to allow the printed items to be replicated.
-
vi)
Whether the print file is made freely available for widespread use.
2. Methods
We utilised the PICO criteria to identify all study designs describing the use of 3D printing including case reports and case series. The identified population included patients of all ages with confirmed or suspected COVID-19. Interventions were defined as the use of any 3D printed model in response to local shortages of essential medical supplies. Comparators included commercially available equivalents or lack of any device. The outcomes for assessment in line with the aforementioned objectives of the review included a description of the uses, the pre- and post-clinical testing, evaluations of effectiveness, description of printing parameters and availability of the models for wider use. No time frame for the publications was placed. Review articles and non-English language studies were excluded.
Literature searches of MEDLINE (OVID interface)/ PubMed were undertaken and the references of relevant review articles were scanned to improve the completeness of the search capture. The date of the last search was 25/05/20. The search strategy included the following medical subject headings (MeSH) terms:
"COVID-19″[All Fields] AND “3D print*” OR “additive manufacturing” OR “rapid prototyping” OR “fused deposition modelling” OR “stereolithograph*” OR “selective laser sint*”.
The search results were filtered using a two-layered approach. In the first layer, two senior authors (VNV and LW) reviewed the titles and abstracts independently utilising a standardised protocol. Disagreements between the reviewers were resolved by consensus. Once the publications for the full manuscript review were identified, this was performed using a predefined set of eligibility and exclusion criteria as defined above. Data extraction was undertaken independently by FG, KM and SK and reviewed by VNV for accuracy utilising an extraction sheet with predefined column headings.
Data extraction included the publication type, subjects enroled, use of the technology, sample size and extent of preclinical and clinical testing (see Table 1 ). Further information regarding the safety testing, use of reporting guidelines, regulatory approvals and printer/filament parameters as well as the availability of the described models were documented (see Table 2 ). Where there was ambiguity resulting from missing or insufficient information this was marked as ‘Not specified’. Where it was clear that a particular action was not undertaken this was marked as ‘Not assessed’. The quality of evidence and risk of bias was assessed using the GRADE tool [7]
Table 1.
Summary of described uses of 3D –printing and preclinical testing.
| Authors | Publication date | Subjects | Publication Type | Uses described | Sample size (n) | Pre-clinical testing prior to clinical use | Comparator | Effectiveness | Clinical use |
|---|---|---|---|---|---|---|---|---|---|
| Hung et al | 21-May-20 | Patients | Letter to editor | 3D printed connector for the application of suction to a negative pressure tent to prevent droplet exposure during extubation | Not specified | Simulation testing | Nil | Simulated aerosols generated through a nebuliser were visibly seen to be directed towards the endotracheal tube. | Used during the extubation of presumed COVID-19 patients but further descriptive information not provided |
| Jacob et al | 11-May-20 | Patients | Letter to editor | 3D printed disposable endotracheal tube clamp to prevent droplet exposure during extubation | Not specified | Not specified | Nil | Not assessed | Used during the extubation of presumed COVID-19 patients but further descriptive information not provided |
| Cox et al | 09-May-20 | Patients | Case report | Naso-pharyngeal swabs for testing of COVID19 | 24 | Yes (n = 2) | Commercial equivalent | No significant difference in RNAse-P detection between 3D printed and commercially available swab | 5500 swabs prepared and delivered for use over a 20 day period |
| Khoo et al | 07-May-20 | Healthcare professionals | Letter to editor | PPE - 3D-printed adaptor to convert commercial snorkel masque into a powered air purifying respirator (PAPR) masque | 7 clinicians | Oxygen saturations, respiratory rate, fraction of inspired 02 and CO2 and end-tidal CO2 were monitored during standing (10 mins), jogging (3–10 mins) and during chest compressions (2 min) | Informal comparison to commercially available PAPR models (PAPR models 3M™ Jupiter™ Powered Air Turbo and Bullard EVA™) | Tested through physiological monitoring parameters and qualitative assessment of ease of assembly, ease of wear, comfort, noise level, weight and subjective safety | Not used clinically |
| Amin et al | 01-May-20 | Healthcare professionals | Case report | PPE - face shields | Not specified | Not specified | Nil | Not assessed | 100 face shields distributed to various departments |
| Sapoval et al | 18-Apr-20 | Healthcare professionals | Case series | PPE - face shield 'Oxyframe' during interventional radiology procedures | 38 | Not specified | Nil | Not assessed | Face shields during interventional radiology procedures |
| Maracaja et al | 13-Apr-20 | Healthcare professionals | Letter to editor | PPE - Face visors and plastic hoods | Not specified | Not specified | Nil | Not assessed | Compressed air is delivered through the frame at 15 L/min when used in combination with a hood |
| Erickson et al | 18-Apr-20 | Healthcare professionals | Case report | PPE - modification of the Stryker Flyte helmet to a PAPR masque | Not specified | CO2 monitoring for 30 min and particle flow testing | N/A | System meets HEPA filter standards | Used by anaesthesia staff and surgeons for higher risk surgeries/procedures in COVID-19 suspected or positive patients |
| Liu et al | 29-Apr-20 | Healthy volunteers | Case report | PPE - modification of commercially available 3 M masks with an anaesthesia circuit filter | 8 | Leak testing using the PortaCount Pro = 8038 fit tester, C02 monitoring for one hour and respiratory rate. | N/A | Tested through physiological monitoring parameters and comfort | Not specified |
| Swennen et al | 02-Apr-20 | Healthcare professionals | Technical note | PPE - Personalised respirators | Not specified | Not specified | N/A | Not assessed | Not specified |
Table 2.
Summary of safety testing, reporting and 3D- printing parameters.
| Authors | Publication date | Safety testing | Use of SQUIRE2.0 guidelines | Regulatory approvals | Printer used | Material used | Printing parameters / post-processing | Print time / cost | Model print file availability |
|---|---|---|---|---|---|---|---|---|---|
| Hung et al | 21-May-20 | Not described | No | No | Not described | Not described | Not described | Not specified | STL file available from chris.r.hung@gmail.com |
| Jacob et al | 11-May-20 | Not described | No | No | Not described | Polylactic acid | Not described | Not specified | Provided by author as online supplementary file |
| Cox et al | 09-May-20 | Before clinical use | No | No | Prusa®, MK3s | Polyethylene terephthalate glycol | Layer height 0.15 mm; nozzle temperature: 250 °C, bed temperature: 90 °C. | 5 min for one or fifty in 3 h and 40 min | By request from: www.unemed.com, NIN No.: 20,081 |
| Khoo et al | 07-May-20 | Tested using an adapted version of the National Institute of Occupational Safety and Health guidelines for PARP | No | No | Not described | Acrylonitrile Butadiene Styrene | Not described | £17 for 3D printed parts | Not provided |
| Amin et al | 01-May-20 | Not performed | No | No | Prusa®, RC2 | Polylactic acid or Acrylonitrile Butadiene Styrene | Printer speed 80–100 mm/s | 5 h to print 200 frames at a cost of $7.30 each | https://airwolf3d.com/2020/03/27/covid-19-face-shield-3d-printable-file/ |
| Sapoval et al | 18-Apr-20 | Qualitatitve assessment of ability to perform task, visual comfort and tolerability | No | No | Commercially available three-dimensional (3D) printers. | Not described | Not described | Not specified | Not provided |
| Maracaja et al | 13-Apr-20 | Not performed | No | No | Formlabs2 | Tough 1500 and Draft resin | Requires alcohol wash for 10–20 min and UV curing for 60 min | 10 frames in 3.5 h; 1 L of the resin could generate approximately 100 frames | Not provided |
| Erickson et al | 18-Apr-20 | Final quality control testing performed | No | No | Formlabs printers and their “durable” material | Durable Formlabs material | Not described | Not specified | Available through Duke's office of Licensing and Ventures |
| Liu et al | 29-Apr-20 | Not performed | No | No | Ultimaker S5 3D printer | Polylactic acid | Printing parameters described in Online Appendix S1 of authors manuscript | £3 / $3.73 / 3.5 euros for each 3D printed adaptor | Not described |
| Swennen et al | 02-Apr-20 | Not performed | No | No | Selective laser sintering (SLS) 3D printer (Prodways, Les Mureaux, France; https://prodways.com) | Polyamide composite (PA11-SX 1450 Prodways,Les Mureaux,France) | Requires sandblasting and vacuum cleaning after printing | 4 masks with filter supports took 11 h with an additional 12 h of post-processing. | Provided by author as online supplementary file |
Due to the heterogeneity in the described uses of the 3D printed models and relative lack of reported quantitative data, a quantitative assessment of consistency was not performed and only a qualitative systematic review was undertaken.
3. Results
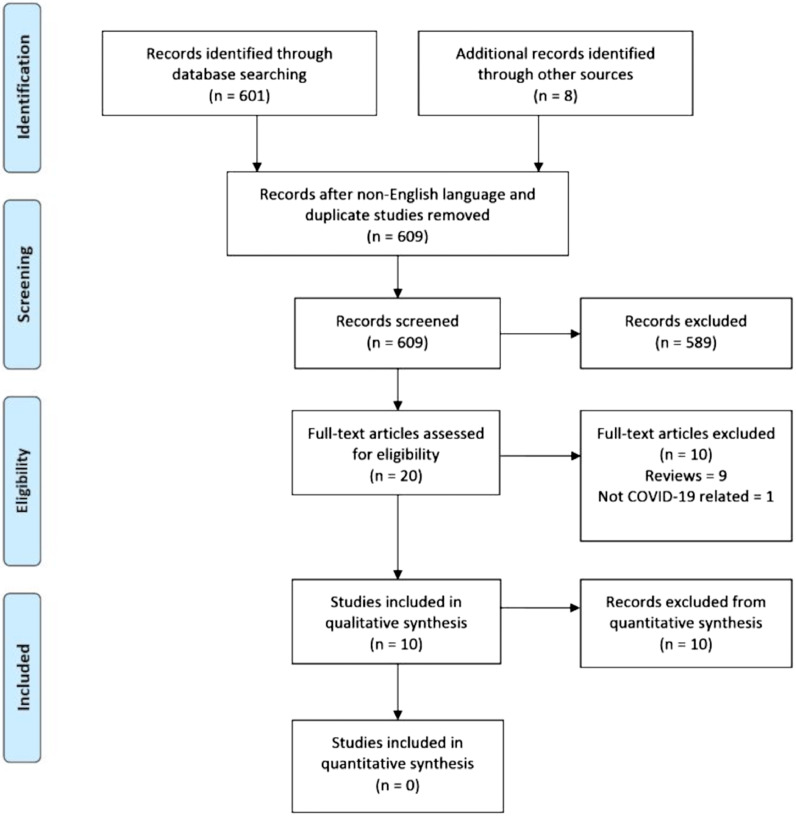
A total of 10 studies were included in the qualitative systematic review. 601 studies were initially identified through the search criteria and an additional 8 studies identified through scanning of the publication references. Of these, 589 were excluded after reviewing the titles or abstracts as they did not meet the eligibility criteria, were not published in English or could not be translated into English. A full-text review was undertaken on the remaining 20 manuscripts. Nine manuscripts were excluded as they were reviews of the topic that did not describe a novel use. A further study was excluded as it was not related to the COVID-19 pandemic (see Fig. 1 ).
Fig. 1.
Legend: PRISMA flowchart summarising the number of studies identified utilising the search criteria, identification of eligible studies for full text review and number of studies included in the qualitative synthesis.
All of the manuscripts were published in 2020. Publications were in the form of letters to the editor (n = 4)[8], [9], [10], [11], case reports [12], [13], [14], [15], [16] or series (n = 5) or technical notes (n = 1) [17]. The majority of the studies included healthcare professionals, whilst a minority were undertaken on patients. Only a single study described using healthy volunteers. Reported uses of 3D printing included personal protective equipment (PPE), nasopharyngeal swabs and adjunctive anaesthetic equipment. Of the 7 studies describing PPE, 3 studies described face shields, a further 3 described powered air-purifying respirator (PARP) masks and a final technical note described a workflow for printing personalised respirators contoured to the face of the wearer to improve the comfort and seal of the masque. Sample sizes were not specified in 6 of the publications and the largest sample size in the remaining reports was 38. Preclinical testing was not performed or not specified in half of the publications before clinical use, most of which were reporting on the production of face shields. All of the studies describing PARP did, however, undertake preclinical testing in the form of physiological monitoring, the most consistent of which was monitoring the end-tidal and fraction of inspired CO2 as well as respiratory rates. Duration of testing varied from 2 min of strenuous exercise to one hour of normal activity. A single study undertook specialised particle load testing and reported that their system met high-efficiency particular air (HEPA) filter standards. There were no formal comparisons to commercially available PARP products but a single report did survey satisfaction ratings from healthcare professionals in comparison to their previous experience of commercial equivalents.
Two studies described the use of ancillary anaesthetic equipment, the first of which was a novel disposable endotracheal tube clamp to prevent droplet exposure during extubation of patients with presumed or confirmed COVID-19 to protect healthcare staff. No pre-clinical testing of efficacy was performed in this study before use on patients. A similar study with the same goal of preventing droplet exposure during extubation through the use of a clear plastic tent that covered the patient utilised a 3D-printed adaptor that when connected to a suction device generated a negative pressure within the tent. Simulation testing of this device with aerosols generated through a nebuliser provided visual confirmation of the effectiveness of the negative pressure tent in capturing the aerosols before clinical use in patients. Finally, a single study reported the use of 3D-printed nasopharyngeal swabs for COVID-19 testing in 24 patients. Preclinical testing was initially performed in 2 patients with comparison to the established commercial equivalent. Microscopic analysis revealed a sufficient yield of cells from the 3D-printed swabs. RNAse-P detection was then compared with the commercially available swab in a further 24 patients and revealed no statistical difference with the 3D-printed version. Based on this the authors described printing and distributing a further 5500 swabs over 20 days to make up for the shortfall in commercial swab kits.
In 4 of the 10 reports, safety testing to established clinical standards was undertaken, one of which tested their PARP to an adapted version of the National Institute of Occupational Safety and Health guidelines for PARP, whilst a further study undertook final quality control testing procedures. None of the studies utilised the Revised Standards for Quality Improvement Reporting Excellence (SQUIRE2.0) as a structured framework for reporting new knowledge or systems to improve reporting of interventions, as well as assessing methods to establish that reported outcomes were due to the interventions. 3D printing technologies described include fused filament deposition in 5 cases, stereolithography in 3 cases and selective laser sintering in one case. The material used was described in 8 of the manuscripts but sufficient printing parameter detail to replicate the prints was only provided in 4 reports. Despite this, 6 reports provided the STL with the manuscript or provided details on how it could be acquired.
Due to the varying and disparate nature of the uses described the quality of evidence was assessed using the GRADE tool [7]. Based on the lack of preclinical efficacy testing of face shield the GRADE of evidence provided was deemed to be ‘very low’ certainty. Regarding the PARP devices in some cases, a high standard of quality assurance testing was undertaken and the authors believe a ‘moderate’ certainty could be applied in which the true effect is probably close to the estimated effect.
4. Discussion
The global COVID-19 pandemic has resulted in the greatest worldwide surge in demand for critical supplies such as ventilators and PPE. In the UK, this challenge was exacerbated by the recall of 16 million ‘tiger eye’ protective goggles purchased by the government for the national pandemic stockpile in 2009, which were found not to meet the clinical standards for splash protection needed to protect from COVID-19. Similarly, in April 2020, 400 000 gowns were imported from Turkey but were deemed unsuitable for use in the NHS. It is unsurprising, therefore, that a British Medical Association survey revealed that almost half of England's doctors had sourced their own PPE or relied on donations when none was available through normal NHS channels.
3D printing has key manufacturing advantages over traditional processes. These include:
-
1.
Rapid prototyping – 3D printing facilitates rapid development of physical prototypes, which can be evaluated and tested and near-immediate adjustments to the design made if necessary
-
2.
Low upfront manufacturing and tooling costs – which is of particular benefit for low-volume production
-
3.
Complex and innovative designs – 3D printing can produce complex shapes without the limitations of cutting and moulding technologies
These advantages enable rapid, decentralized manufacturing to compensate for the initial shortage of critical supplies. Hence many individuals and organisations across the globe, including those unrelated to healthcare, focused on redeploying 3D printing capacity to ramp up procurement of scarce medical supplies[5].
Preclinical testing was not carried out in most of the publications in this review. Additionally, only four of the studies evaluated their 3D-printed equipment to clinical safety guidelines. This poses a challenge in terms of balancing the risk of inadequate 3D-printed equipment versus the increased demand for such equipment during the COVID-19 pandemic. Therefore, we highlight that there is still a need for organisations to adhere to regulatory processes to assess the quality of their equipment. The CE certification of 3D-printed face masks mark adherence to European safety standards, however, Pecchia et al. [18] argue that the stringent process of CE approval might be a constraint to rapid production in future emergencies. Non-certified 3D face shields have also been accepted by NHS hospitals and other healthcare settings across the UK already. In the face of such issues, Pecchia et al. [18] have provided their subset of suggested tests that meet a balance between ensuring a minimum standard of medical device safety and providing a rapid supply of PPE during the COVID-19 pandemic.
Additionally, governments worldwide have adapted their regulatory frameworks to meet the mismatch in supply and demand for novel forms of PPE and medical devices. The FDA declared a no-objection policy to the individual use of improvised PPE in situations where FDA-approved surgical masks are unavailable [19]. The UK government also published guidance into fast-tracking applications for 3D printed medical devices and PPE [20]. This highlights the UK government's flexibility in the approach to 3D-printed devices, and recognises their significance, carving a landscape in which such technology can provide rapid solutions in emergencies.
The FDA recommends that some face masks without FDA approval can be used by medical personnel, as long as they meet certain criteria: they are not to be used in a high-risk setting, must be accurately labelled and made from a non-flammable material [19]. However, the FDA still recommends that N95 respirators, which are typically used in high-risk settings, should be FDA-approved before use by healthcare professionals. This is in response to concerns regarding the performance of unregulated N95 respirators. The IDEAL collaboration suggests a five-step process for surgical innovation [21,22]. This could be modified for healthcare professionals wishing to structure their approach into producing novel 3D-printed medical equipment.
None of the identified studies utilised a standardised reporting guideline. Although no guideline exists for novel and expedient medical device reporting, the SQUIRE 2.0 guidelines aim to provide a standardised framework for analysing new knowledge about healthcare interventions and consequently apply a systematic approach to improving the quality, safety and value of healthcare [23]. We suggest that structured reporting following the SQUIRE 2.0 guidelines should be utilised in future publications. Only two of the publications describing face-shield makers [13,17] mentioned guidance on sterilisation for equipment re-use. Different strategies outlining sterilisation of face-shields exist [24,25] and ultimately further testing and evaluation of sterilisation protocols for 3D-printed face shields need to be carried out.
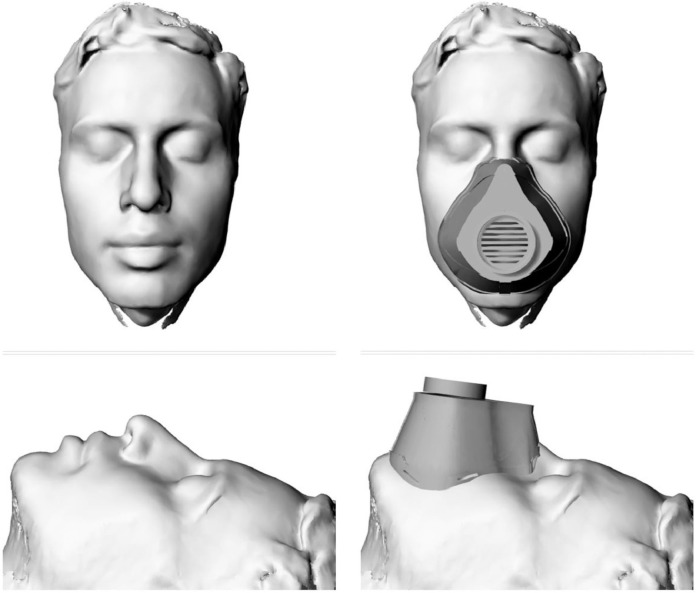
A single publication reported the use of 3D printing for the development of personalised PPE. (See Fig. 2 ). This offers the unique potential to contour the masque to the user's face to improve the fit and efficacy of the seal. It has also been suggested that this would improve the comfort for the wearer, especially when wearing PPE for prolonged periods, such as in healthcare settings. Practically, with the aid of photogrammetry apps available on smartphones, the morphological data of the user's face can be captured in high fidelity and predesigned masks can be custom-fit [26].
Fig. 2.
Legend: Example of a 3D facial scan acquired using a freely available photogrammetry app on a personal smartphone. The PPE masque can then be contoured to face to ensure a custom fit around the nose, cheeks and chin to improve the seal and comfort for the wearer. The filter module, in this design, is screwed to the front of the masque and can be replaced as needed. The masque can also be decontaminated for repeated use.
Manufacturers and designers have worked closely with healthcare institutions to rapidly scale production. A notable example of this collaborative approach to 3D printing includes Detroit-based Ford Motor Company (amongst several other automakers) collaborating with medical equipment manufacturers such as 3 M Co. and GE Healthcare to increase production by ten-fold of their powered air-purifying respirators, ventilators and PEEP masks [27]. The effort, code-named "Apollo 13″, placed Ford engineers inside 3 M and GE plants to utilise their assembly lines by opening supply channels, simplifying the devices to their basic elements and identifying high-volume components to use, such as the already-available fans used in F-150 trucks, which they adapted and 3D-printed at scale for use in respirator masks worn by healthcare professionals. Crucially, the automotive industry has extremely tight safety and manufacturing regulations, making it ideal for translation into the biomedical devices industry, whilst simultaneously acting as a central resource to allow rapid dissemination of critical supplies to the frontline.
5. Conclusion
In this systematic review, we show that within a very short period 3D printing has been used to produce a variety of personal protective equipment, anaesthetic adjuncts and nasopharyngeal swabs. Clinical testing was variable and comparative studies of efficacy to commercial alternatives were lacking. In times of desperation, however, when supply chains have failed 3D printing was able to meaningfully bridge the shortfall. Looking forward, as traditional manufacturing processes in the developed world scale to cope with increased demand, we expect 3D printing manufacturers to migrate from disposables to more complex, longer-term applications such as parts for outdated ventilators, components for modifying existing ventilators and complex parts for new equipment designs. 3D printing may also enable the replacement of reusable parts that are sterilized between procedures with disposable parts that are discarded after each use (such as disposable laryngoscope blades). In the event of another pandemic, this will allow these devices to be used far more often each day without any downtime. The COVID-19 pandemic has indeed forced the world to adapt quickly and spurred innovation that would have otherwise taken years to materialize. Also, 3D printing has demonstrated its ability to scale up quickly in a decentralized fashion, a necessity that is even more compelling in the developing world. Local 3D printing in these regions was truly life-saving.
Resources should now focus on developing and maintaining a central repository of 3D print files for critical equipment that is likely to be in short supply in future pandemics. Clinical testing, validation and identification of optimal printing materials and parameters must be determined as part of the rigorous preparation strategies for future pandemics or resurgences of COVID-19.
Data sharing
All data extracted for this publication is provided within the Tables.
Funding
No financial support was provided for the production of this manuscript. The researchers are independent from any funders.
Ethical approval
Ethical approval was not sought for this review.
Guarantor
VNV and DC are the guarantors of the work.
Contributorship
All authors aided with the drafting of the manuscript and development of the selection criteria. VNV and LW developed the search strategy. Data extraction was performed by SK, KM and TG. VNV provided expertise on 3D printing. All authors contributed to, read, provided critical feedback and approved the final manuscript.
Amendments
No previous systematic reviews on this topic were identified. No amendments were made to the review protocol during or after the review process.
Declaration of competing interest
All authors declare: no support from any organisation or financial relationships with any organisations that might have an interest in the submitted work.
Acknowledgements
Nil
References
- 1.Ranney M.L., Griffeth V., Jha A.K. Critical supply shortages — the need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med. 2020;382:e41. doi: 10.1056/NEJMp2006141. [DOI] [PubMed] [Google Scholar]
- 2.Operations NHS England Operating framework for managing the response to pandemic influenza. NHS England. 2017:1–25. [Google Scholar]
- 3.Armocida B., Formenti B., Ussai S., et al. The Italian health system and the COVID-19 challenge. Lancet. Public Health. 2020;5:e253. doi: 10.1016/S2468-2667(20)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flanagan S.T., Ballard D.H. 3D printed face shields: a community response to the COVID-19 global pandemic. Acad Radiol. 2020;27:905–906. doi: 10.1016/j.acra.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daoulas T., Bizaoui V., Dubrana F., et al. The role of three-dimensional printing in coronavirus disease-19 medical management: a French nationwide survey. Ann 3D Print Med. 2021;1 doi: 10.1016/j.stlm.2020.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Guyatt G.H., Oxman A.D., Vist G.E., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hung O., Hung D., Hung C., et al. A simple negative-pressure protective barrier for extubation of COVID-19 patients. Canad J Anaesthesia=J Canadien d'anesthesie. 2020:1–3. doi: 10.1007/s12630-020-01720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khoo D., Yen C.-.C., Chow W.T., et al. Ultra-portable low-cost improvised powered air-purifying respirator: feasibility study. Br J Anaesth. 2020 doi: 10.1016/j.bja.2020.04.082. Epub ahead of print May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maracaja L., Blitz D., Maracaja D.L .V, et al. How 3D printing can prevent spread of COVID-19 among healthcare professionals during times of critical shortage of protective personal equipment. J Cardiothorac Vasc Anesth. 2020 doi: 10.1053/j.jvca.2020.04.004. Epub ahead of print10.1053/j.jvca.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacob M., Ruivo E., Portela I., et al. An innovative endotracheal tube clamp for use in COVID-19. Canad J Anaesthesia=J Canadien d'anesthesie. 2020:1–3. doi: 10.1007/s12630-020-01703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox J.L., Koepsell S.A. 3D-printing to address COVID-19 testing supply shortages. Lab Med. 2020;51:e45–e46. doi: 10.1093/labmed/lmaa031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amin D., Nguyen N., Roser S.M., et al. 3D printing of face shields during COVID-19 pandemic: a technical note. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. 2020 doi: 10.1016/j.joms.2020.04.040. Epub ahead of print May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erickson M.M., Richardson E.S., Hernandez N.M., et al. Helmet modification to PPE with 3D printing during the COVID-19 pandemic at duke university medical center: a novel technique. J Arthroplasty. 2020;35:S23–S27. doi: 10.1016/j.arth.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu D.C.Y., Koo T.H., Wong J.K.K., et al. Adapting re-usable elastomeric respirators to utilise anaesthesia circuit filters using a 3D-printed adaptor - a potential alternative to address N95 shortages during the COVID-19 pandemic. Anaesthesia. 2020 doi: 10.1111/anae.15108. Epub ahead of print April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sapoval M., Gaultier A.L., Del Giudice C., et al. 3D-printed face protective shield in interventional radiology: evaluation of an immediate solution in the era of COVID-19 pandemic. Diagn Interv Imag. 2020;101:413–415. doi: 10.1016/j.diii.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swennen G.R.J., Pottel L., Haers P.E. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg. 2020;49:673–677. doi: 10.1016/j.ijom.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pecchia L., Piaggio D., Maccaro A., et al. The inadequacy of regulatory frameworks in time of crisis and in low-resource settings: personal protective equipment and COVID-19. Health Technol (Berl) 2020:1–9. doi: 10.1007/s12553-020-00429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.FDA. Enforcement policy for face masks and respirators during the coronavirus disease (COVID-19) public health emergency (Revised), https://www.fda.gov/media/136449/(2020).
- 20.GOV.UK. Exemptions from devices regulations during the coronavirus (COVID-19) outbreak, https://www.gov.uk/guidance/exemptions-from-devices-regulations-during-the-coronavirus-covid-19-outbreak (2020).
- 21.McCulloch P., Cook J.A., Altman D.G., et al. IDEAL framework for surgical innovation 1: the idea and development stages. BMJ. 2013;346 doi: 10.1136/bmj.f3012. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ergina P.L., Barkun J.S., McCulloch P., et al. IDEAL framework for surgical innovation 2: observational studies in the exploration and assessment stages. BMJ. 2013:346. doi: 10.1136/bmj.f3011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogrinc G., Davies L., Goodman D., et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. Am J Med Qual. 2015;30:543–549. doi: 10.1177/1062860615605176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan M.M., Parab S.R. Simple economical solution for personal protection equipment (Face Mask/Shield) for health care staff during COVID 19. Indian J Otolaryngol Head Neck Surg Off Publ Assoc Otolaryngol India. 2020:1–5. doi: 10.1007/s12070-020-01863-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mostaghimi A., Antonini M.-.J., Plana D., et al. Rapid prototyping and clinical testing of a reusable face shield for health care workers responding to the COVID-19 pandemic. medRxiv: Preprint Serv Health Sci. 2020 doi: 10.1101/2020.04.11.20061960. Epub ahead of print April. [DOI] [Google Scholar]
- 26.NIH 3D Print Exchange. Mask Fitter by bellus3D, https://3dprint.nih.gov/builds/bellus3d/mask-fitter-bellus3d (2020).
- 27.Noble B. Ford partners with 3M, GE to make respirators, ventilators and face shields. Detroit News Website. 2020 https://www.detroitnews.com/story/business/autos/ford/2020/03/24/ford-partners-3-m-ge-healthcare-respirators-ventilators-face-shields/2905986001/ [Google Scholar]