Abstract
Phosphatidylinositol 3-kinases (PI3Ks) are critical regulators of many cellular processes including cell survival, proliferation, migration, cytoskeletal reorganization, and intracellular vesicular trafficking. They are a family of lipid kinases that phosphorylate membrane phosphoinositide lipids at the 3′ position of their inositol rings, and in mammals they are divided into three classes. The role of the class III PI3K Vps34 is well-established, but recent evidence suggests the physiological significance of class II PI3K isoforms in vesicular trafficking. This review focuses on the recently discovered functions of the distinct PI3K-C2α and PI3K-C2β class II PI3K isoforms in clathrin-mediated endocytosis and consequent endosomal signaling, and discusses recently reported data on class II PI3K isoforms in different physiological contexts in comparison with class I and III isoforms.
Keywords: clathrin-mediated endocytosis, endosomal signaling, phosphatidylinositol 3-kinase (PI3K), phosphoinositide, vesicular trafficking
Introduction
Endocytosis is an essential process in which proteins and lipids are internalized as membrane-bound cargo in forms such as clathrin-coated vesicles, which are regulated by phosphoinositides (PIs), small G-proteins including Rabs and other proteins [1, 2]. Subcellular localization patterns of PIs are tightly controlled by the regulation of lipid kinases and lipid phosphatases. Among these, phosphatidylinositol 3-kinases (PI3Ks) catalyze the transfer of the γ-phosphate group of adenosine triphosphates to the D3 position of their inositol ring and control diverse processes including cell proliferation, migration, cytoskeletal reorganization, and vesicular trafficking [1–3]. Yeasts have a single PI3K homolog called Vps34 which mainly regulates autophagy [4, 5], whereas higher eukaryotes have multiple PI3K isoforms.
In mammals, PI3Ks are categorized into three classes based on their substrate specificity [3]. Class I PI3Ks directly engage in signaling downstream of plasma membrane-bound receptors, whereas class II and III PI3Ks primarily regulate vesicular trafficking and subsequently regulate cellular signaling. Ligand binding triggers the activation and internalization of signaling receptors from the plasma membrane into early endosomes, where receptors are sorted to the late endosomes/lysosomes for degradation or recycling back to the plasma membrane [6–8]. Numerous recent studies indicate that receptor signaling continues on endosomes after receptor endocytosis [9–11]. We recently demonstrated that PI3K-C2α and PI3K-C2β have specific redundant cellular functions pertaining to clathrin-mediated endocytosis, endosomal signaling, and the regulation of Rho-dependent smooth muscle contraction [12–16].
Herein we discuss emerging data on the isoform-specific regulation of class II PI3Ks, the coordination of membrane composition, and the regulation of intracellular signaling of class II PI3Ks. Recent reviews have mentioned an increasing relating to their intracellular functions [2, 17–19]. The current review also focuses on emerging evidence that class II PI3Ks could be used as therapeutic targets, particularly in vascular diseases.
Structure and substrate specificities of class II PI3K isoforms
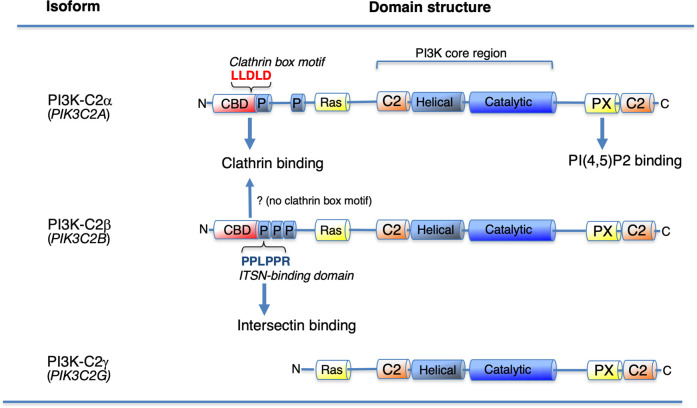
Class I PI3Ks have been intensely investigated, their fundamental roles have been identified, and physiological insights into class I PI3K activation and regulation have been reviewed [1–3, 20, 21]. The PI3K isoform Vps34 is conserved in yeasts and humans. It is the single class III isoform and is responsible for regulating autophagy and endo-lysosomal sorting via the respective production of phosphatidylinositol-3-monophosphate (PI(3)P) in autophagosomes and endo-lysosomes [2, 4, 5]. In mammals class II PI3Ks include PI3K-C2α, PI3K-C2β, and PI3K-C2γ, which remain the least characterized PI3K subfamily [22, 23]. Class II PI3Ks have strong resistance against the pan-PI3K inhibitors wortmannin and LY294002 [24–26], and selective inhibitors of class II PI3K isoforms have not yet been developed. PI3K-C2α and PI3K-C2β are expressed ubiquitously, whereas PI3K-C2γ exhibits a more restricted pattern of expression, mainly in hepatocytes [27–29]. Class II PI3Ks have a conserved C-terminal extension with the PX and C2 domains that is unique to the class II isoforms and is probably responsible for the association with PI(4,5)P2-containing plasma membranes [30, 31] (Figure 1). Class II PI3Ks also have an extended N-terminal region with additional protein-binding regions, such as the clathrin-binding domain in PI3K-C2α and the unique proline-rich motif in PI3K-C2β [32, 33]. It has been suggested that clathrin can bind directly to PI3K-C2α but not to PI3K-C2β, which contains the ‘clathrin box motif’ consensus sequence (L[LI][DEN][LF][DE]) [34, 35] (Figure 1), although previous analysis indicates that PI3K-C2β has an affinity for the recombinant clathrin protein in vitro [33, 36].
Figure 1. Domain structures of the Class II phosphoinositide 3-kinase (PI3K) isoforms.
PI3K-C2α has a clathrin-binding domain (CBD) containing the clathrin box motif consensus sequence, whereas the N-terminal proline-rich region of PI3K-C2β directly binds to the SH3 domain of intersectin-1 (ITSN) via its ITSN-binding domain. PI3K-C2α also present a PX-domain that is responsible to the binding of PI(4,5)P2 in plasma membranes. C2, protein kinase C conserved region 2 (C2 domain), Catalytic, catalytic domain; CBD, clathrin-binding domain; Helical, helical domain; P, proline-rich region; PX, phox homology (PX domain). Ras, Ras-binding domain.
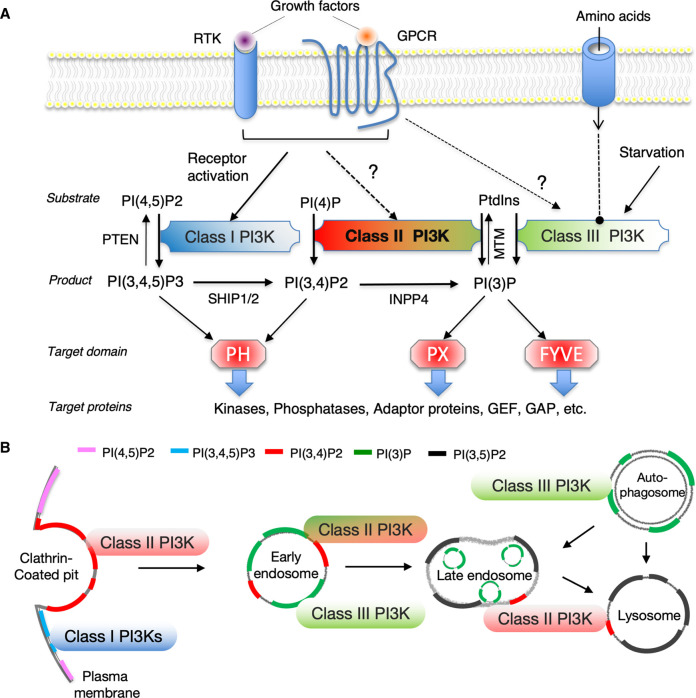
The lipid products of class II PI3Ks have been a subject of discussion, and it is now accepted that they phosphorylate both PI and PI(4)P resulting in the respective synthesis of PI(3)P and PI(3,4)P2 [37, 38] (Figure 2A). These 3′-phosphoinositides can regulate various membrane trafficking processes and are key membrane identity markers (Figure 2B). Recent studies have demonstrated that PI3K-C2α becomes fully active at the clathrin-coated pits (CCPs) by changing its conformation when the N-terminal clathrin-binding domain and the C-terminal PX-C2 domains, which are associated with clathrin and membrane-bound PI(4,5)P2, respectively [31, 32]. This supports the contention that PI3K-C2α generates PI(3,4)P2 and primarily functions in the endocytic pits in a kinase-dependent manner. It has also been proposed that PI3K-C2α regulates cilia formation by producing PI(3)P via the recycling endosomes, and that it activates Rab11 which is an important regulator of endosome recycling [37]. The endosomal PI(3)P pools are also involved in cellular signaling including growth factor receptor responses [12, 39], cell migration [13, 40], and insulin stimulation responses. Vps34 contributes to the production of basal cellular PI(3)P in many cell types [2]. It is believed that Vps34 is one of the main sources of cellular PI(3)P, though it is not the only source. Class II PI3Ks and lipid phosphatases presumably contribute to the maintenance of the PI(3)P pool in a cell context-dependent manner. Their distinct subcellular localization and/or complex regulation of upstream and downstream targets may affect localized PI(3)P production and represent part of a distinct PI(3)P pool that controls different types of cell signaling. The substrate specificity of PI3Ks is difficult to determine precisely, particularly given that PI(3)P and PI(3,4)P2 are only present in trace amounts in normal resting cells and exhibit rapid turnover. Local endosomal PI(3)P levels may be derived from PI(3,4)P2 dephosphorylation by 4′-phosphatase INPP4 during endocytosis [41]. In a recent report we postulated that the formation of PI(3,4)P2 by PI3K-C2α followed by 5′-phosphatase synaptojanin-1-mediated PI(4)P production from PI(4,5)P2 at CCPs mediates TGFβ1 receptor endocytosis and TGFβ1-induced activation of Smad2/3 on endosomes [42]. The intracellular role of PI3K-C2α in clathrin-mediated endocytosis demonstrates how sequential phosphoinositide conversion can transmit CCP formation to clathrin-coated vesicle identity.
Figure 2. Substrate specificity and subcellular localization of phosphoinositides produced by PI3K isoforms.
(A) Upstream activating input into distinct classes of PI3Ks and their downstream effector domains/proteins are shown. (B) Subcellular distribution of the distinct PI3K isoform-generated phosphoinositides is shown. The three classes of PI3K specifically generate sub-compartmentally localized 3′-phosphoinositide pools. Only PI(3,4,5)P3 is produced by class I PI3Ks in the plasma membrane, whereas both PI(3,4)P2 and PI(3)P are generated by class II and III PI3Ks, forming distinct cellular pools mainly involved in endo-lysosomal and autophagy pathways.
Cellular functions of class II PI3K isoforms
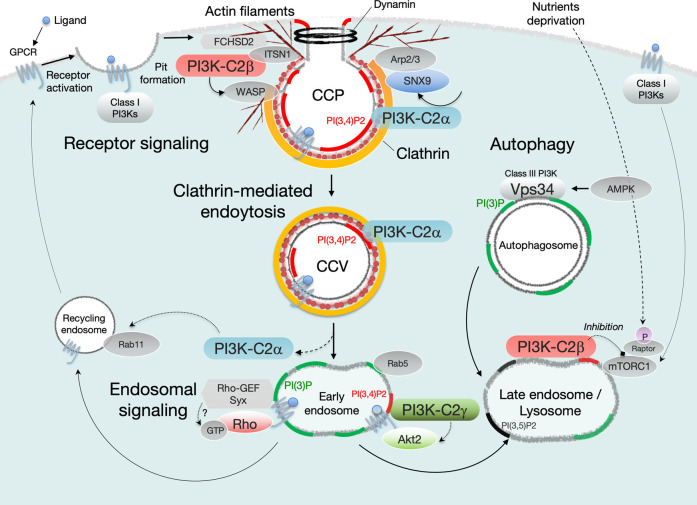
At the clathrin lattice, PI3K-C2α metabolizes PI(4,5)P2 to PI(3,4)P2 in cooperation with the PI-5′-phosphatases ORCL and synaptojanin-1 [35, 42]. Localized enrichments in PI(3,4)P2 enable the recruitment of endocytic accessory proteins such as sorting nexin-9, which interact with actin-branching activator Arp2/3 and dynamin, and ultimately generate constricting force at the neck of the CCPs [43–46] (Figure 3). Endocytosis is considered an important mechanism involved in down-regulation of receptor signaling events via the internalization of ligand–receptor complexes. Notably however, evidence reported in the last two decades indicates that endocytosis can contribute to a form of intracellular signal transduction dubbed ‘endosomal signaling’ [47, 48] (Figure 3).
Figure 3. Intracellular functions of the distinct class II PI3K isoforms.
PI3K-C2α is recruited to clathrin-coated pits (CCPs) and clathrin-coated vesicles (CCVs) via direct binding to clathrin, and produces a local PI(3,4)P2 pool. The PI3K-C2α-mediated production of PI(3,4)P2 triggers maturation of the CCPs and pinches off the neck of membrane invagination in cooperation with the sorting nexin-9 (SNX9), Arp2/3, and dynamin. Alternatively, PI3K-C2α-mediated endocytic pools of PI(3)P facilitate endosomal signaling including RhoA, Rac1, and Rap1. PI3K-C2β is also required for the formation and maturation of CCPs through its recruitment to CCPs via direct interaction with intersectin-1 (ITSN1). ITSN1 can recruit FCHSD2, which stimulates WASP-dependent actin filament formation at CCPs. PI3K-C2β inhibits mTORC1 activation on late endosome/lysosome compartments under nutrient-starved conditions. In hepatocytes, PI3K-C2γ mediates insulin-dependent production of endosomal PI(3,4)P2 pools that can prolong endosomal Akt2 activation.
With respect to vascular endothelial cells, PI3K-C2α is evidently involved in critical angiogenic signaling pathways via VEGF-A [12], S1P [13], TGFβ1 [14], and Notch1 (unpublished data) receptors. In PI3K-C2α-deficient endothelial cells impaired receptor endocytosis results in various signaling defects; e.g. the impaired endosomal RhoA, Rac1, and Rap1 activation lead to defective VE-cadherin delivery to the cell–cell junction and subsequent defective adherence junction assembly [12]. It is therefore possible that PI3K-C2α regulates the clathrin-mediated endocytosis that is highly integrated into signaling pathways, and in this regard several studies have demonstrated the existence of signaling-capable clathrin-coated structures on plasma membranes [49–51]. The endothelial function of PI3K-C2α appears to be associated with its regulatory role in receptor endocytosis. The relevance of PI3K-C2α in cancer biology has recently been demonstrated in studies in which the inactivation of PI3K-C2α lead to delayed mitosis and subsequent reduced proliferation of breast cancer cells [52]. Surprisingly this process can serve a scaffold function that is not dependent on its kinase activity. The scaffold function of PI3K-C2α may contribute to the alternative clathrin-dependent intracellular processes in which it regulates microtubule stabilization in kinetochore fibers during mitosis [53]. More detailed investigation is necessary to further elucidate the role of PI3K-C2α in cancer progression.
Unlike PI3K-C2α, the roles of PI3K-C2β in the endocytic pathway are poorly understood; however, a critical role of the isoform in clathrin-mediated endocytosis has been reported. The multifunctional scaffold protein intersectin-1 has been identified as a binding partner of PI3K-C2β via interaction between its SH3 domain and the proline-rich region of PI3K-C2β [54]. A recent study demonstrated that the intersectin-1 also recruits the F-BAR domain-containing protein FCHSD2, which stimulates actin polymerization via activation of a WASP family protein, resulting in the formation of actin patches around the CCPs [55] (Figure 3). Cell migration is an actin remodeling-related cellular process that is also reportedly regulated by PI3K-C2β [29, 40, 56, 57], suggesting that it may contribute to actin polymerization in the endocytic site via FCHSD2 recruitment. Consistent with this, we have demonstrated that the class II PI3K isoforms C2α and C2β, but not class I or III isoforms, are required for clathrin-dependent fluid-phase endocytosis ‘pinocytosis’ in endothelial cells [58]. These observations indicate that PI3K-C2α and PI3K-C2β play different indispensable roles in clathrin-mediated endocytosis (Figure 3). Interestingly, PI3K-C2β is also found to localize in late endosomes and lysosomes under starved conditions, where it suppresses the activity of mTORC1 [59]. A recent study demonstrated that protein kinase N regulates mTORC1 signaling by controlling PI3K-C2β activity and localization [60], suggesting that functionally PI3K-C2β counters the action of class I PI3K, which activates mTORC1. In addition, it has been demonstrated that the PI(3,4)P2 produced by PI3K-C2γ regulates long-termed early endosomal Akt activation during insulin signaling [30]. Class II PI3K isoforms may therefore generate spatially distinct pools of PI(3)P or PI(3,4)P2, which are linked to endocytic events and endosomal signal transduction in a context-dependent manner (Figure 3), although several reports indicate that class II PI3Ks are involved in autophagy regulation [61–63].
Distinct physiological roles of class II PI3K isoforms
The generation of PI3K-C2α-targeted mice in 2012 [12] rapidly yielded insights into the physiological roles of class II PI3Ks at the organism level. Independently generated PI3K-C2α knockout (KO) [37, 38, 64] or kinase-dead mutant [41] mice exhibit embryonic lethality at midgestation (E8.5 to 11.5), indicating that PI3K-C2α has a non-redundant kinase-dependent role in murine development. PI3K-C2α null-mice display significant roles in developmental angiogenesis and vascular barrier integrity [12], as well as primary cilia function [37]. Smooth muscle-specific and cardiomyocyte-specific deletion of PI3K-C2α does not affect embryonic development or survival, but endothelial cell-specific deletion of PI3K-C2α results in delayed death around E16.5–18.5, suggesting that unknown causes of death account for the observed embryonic lethality [12]. Although its postnatal physiological functions remain poorly understood, several studies implicate PI3K-C2α in postnatal pathophysiology [12, 65, 41, 64]. A murine gene-trapped PI3K-C2α mutant that expresses a truncated protein lacking a C-terminus is reportedly abnormally small and exhibits severe glomerulonephritis [65].
Heterozygous PI3K-C2α-deficient mice develop normally and are fertile, and adults reportedly exhibit no obvious histological abnormalities in any organs examined [12, 64]. Notably, however, adult tamoxifen-inducible endothelial PI3K-C2α conditional KO mice exhibit a greater incidence of severe dissecting aortic aneurysm formation in response to systemic infusion of angiotensin-II [12]. Pathophysiological defects are due to the impairment of vascular barrier integrity in mice with genetic loss of PI3K-C2α. In other studies investigating the therapeutic potential of targeting class II PI3Ks, in an Mtm1-deficient mouse model of X-linked myotubular myopathy, muscle-specific deletion of PI3K-C2β, but not Vps34, can fully ameliorate their defective muscle morphology, reduce PI(3)P levels, and shortened survival [66]. X-linked myotubular myopathy is caused by mutations in the 3′-phosphatase MTM1 gene that lead to impaired elimination of endosomal PI(3)P and result in defective endosomal trafficking. In contrast, PI3K-C2β-null mice are reportedly normal and fertile [67, 68], indicating that PI3K-C2β is not required for normal development.
Mountford J.K. et al. reported that heterozygous PI3K-C2α (Pik3c2a+/–) and homozygous PI3K-C2β (Pik3c2b–/–) double mutant mice are born at expected Mendelian ratios, exhibit no gross abnormalities, and have normal numbers of standard-sized platelets, although the constitutive knock-down of PI3K-C2α resulted in altered platelet morphology and impaired membrane shear-dependent platelet adhesion [64, 69]. Notably however, smooth muscle cell-specific PI3K-C2α deletion in PI3K-C2β null background mice resulted in delayed parturition and reduced blood pressure due to impaired smooth muscle cell contraction in the uterus and blood vessels [15, 16], but this was not evident in mice with single KOs of each genes. This suggests that at least one isoform of class II PI3K-C2α and PI3K-C2β is essential for maintaining arterial blood pressure and normal parturition. It has been established that smooth muscle contraction is mediated by two major pathways, Ca2+-dependent myosin light-chain kinase activation, and small G-protein Rho and Rho-kinase-dependent myosin light-chain phosphatase (MLCP) inhibition [70–74]. In double KO cells the Rho pathway is inhibited and consequently MLCP activity is enhanced, suggesting that it leads to reduced contraction [15, 16].
The direct visualization of Rho activation in uterine myometrial cells [15] and aortic vascular smooth muscle cells [16] via using a Förster resonance energy transfer (FRET) imaging technique results in agonist-induced Rho activation, mainly in the early endosomes, and it is significantly reduced in double KO cells. Consistently, in previous studies the activation of endosomal small G-proteins including Rac1 and Rap1 was observed in vascular endothelial cells [12, 13], as was Rab11 activation [75]. This further emphasizes how class II PI3K-C2α can regulate the endosomal signaling via modulation of receptor endocytosis. Ngok et al. [76] reported that the unique Rho-guanine nucleotide exchange factor (GEF) Syx is involved in endosomal Rho activation. It is possible that PI3K-C2α facilitates receptor endocytosis and subsequent signaling in which ligand-bound receptors and their associated molecule Syx are assembled. Further investigation is necessary to identify a signaling molecule involved in endosomal signaling, and confirm its mechanistic roles.
Surprisingly, homozygous loss-of-function mutation of PI3K-C2α has been reported in a small number of patients with a phenotype that skeletal abnormalities, short stature, cataract formation with glaucoma, and neurological manifestations [77], indicating a functional significance of PI3K-C2α in humans. Cultured fibroblasts derived from these patients exhibit compensatory increases in PI3K-C2β mRNA expression, raising the possibility of a compensatory mechanism similar to that observed in murine smooth muscle. The physiological significance and potential therapeutic implications of these observations remain to be determined.
Perspectives
Three class II PI3K isoforms share functional roles in the regulation of vesicular trafficking events, and influence context-dependent specific cell signaling. Among them, PI3K-C2α has a non-redundant role in clathrin-mediated endocytosis in mouse development.
PI3K-C2α and PI3K-C2β play context-dependent compensatory or cooperative roles in smooth muscle tissues. Clathrin-mediated endocytosis is regulated by the class II PI3K isoforms PI3K-C2α and PI3K-C2β via distinct but at least partially redundant mechanisms, however the precise roles of three class II PI3K isoforms have not been elucidated.
A full understanding of the physiological roles of three class II PI3K isoforms in vesicular trafficking remains a distant prospect. Knowledge pertaining to these roles and their impairment may provide insight into disease pathophysiologies. A better understanding of these mechanisms requires faster live-cell imaging with super-resolution microscopy and quantitative microscopy to investigate spatio-temporal dynamics of phosphoinositide turnover mediated by class II PI3Ks.
Acknowledgements
I thank Chiemi Hirose for secretarial assistance and was supported by grants from the Japan Society for the Promotion of Science [17K08532].
Abbreviations
- Arp2/3
actin-related protein 2/3
- C2
protein kinase C conserved region 2
- CBD
clathrin-binding domain
- CCP
clathrin-coated pit
- CCV
clathrin-coated vesicle
- FCHSD2
FCH and double SH3 domain 2
- INPP
Inositol polyphosphate-1-phosphatase
- ITSN1
intersectin-1
- KO
knockout
- mTORC1
mammalian target of rapamycin complex 1
- PI
phosphoinositide
- PX
phox homology
- S1P
sphingosine-1-phosphate
- SH3
src-homology 3
- SNX
sorting nexin
- TGFβ1
transforming growth factor-β1
- VEGF
vascular growth factor
- Vps34
vacuolar protein sorting 3
Competing Interests
The author declares that there are no competing interests associated with this manuscript.
References
- 1.Schink, K.O., Tan, K.-W. and Stenmark, H. (2016) Phosphoinositides in control of membrane dynamics. Annu. Rev.Cell Dev. Biol. 32, 143–171 10.1146/annurev-cellbio-111315-125349 [DOI] [PubMed] [Google Scholar]
- 2.Bilanges, B., Posor, Y. and Vanhaesebroeck, B. (2019) PI3K isoforms in cell signaling and vesicle trafficking. Nat. Rev. Mol. Cell Biol. 20, 515–534 10.1038/s41580-019-0129-z [DOI] [PubMed] [Google Scholar]
- 3.Fruman, D.A., Chiu, H., Hopkins, B.D., Bagrodia, S., Cantley, L.C., Abraham, R.T. (2017) The PI3K pathway in human disease. Cell 170, 605–635 10.1016/j.cell.2017.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Backer, J.M. (2016) The intricate regulation and complex functions of the class III phosphoinositide 3-kinase Vps34. Biochem. J. 473, 2251–2271 10.1042/BCJ20160170 [DOI] [PubMed] [Google Scholar]
- 5.Mizushima, N. and Murphy, L.O. (2020) Autophagy assays for biological discovery and therapeutic development. Trends Biochem. Sci. 45, 1080–1093 10.1016/j.tibs.2020.07.006 [DOI] [PubMed] [Google Scholar]
- 6.Balla, T. (2013) Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol. Rev. 93, 1019–1137 10.1152/physrev.00028.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohdanowicz, M. and Grinstein, S. (2013) Role of phospholipids in endocytosis, phagocytosis and micropinocytosis. Physiol. Rev. 93, 69–106 10.1152/physrev.00002.2012 [DOI] [PubMed] [Google Scholar]
- 8.Redpath, G.M., Betzler, V.M., Rossatti, P. and Rossy, J. (2020) Membrane heterogeneity controls cellular endocytic trafficking. Front. Cell Dev. Biol. 8, 757 10.3389/fcell.2020.00757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villasenor, R., Kalaidzidis, Y. and Zerial, M. (2016) Signal processing by the endosomal system. Curr. Opin. Cell Biol. 29, 53–60 10.1016/j.ceb.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 10.Palfy, M., Remenyi, A. and Korcsmaros, T. (2012) Endosomal crosstalk: meeting points for signaling pathways. Trends Cell Biol. 22, 447–456 10.1016/j.tcb.2012.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mettlen, M., Chen, P.-H., Srinivasan, S., Danuser, G. and Schmid, S.L. (2018) Regulation of clathrin-mediated endocytosis. Annu. Rev. Biochem. 87, 871–895 10.1146/annurev-biochem-062917-012644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshioka, K., Yoshida, K., Cui, H., Wakayama, T., Takuwa, N., Okamato, Y.et al. (2012) Endothelial PI3K-C2α, a class II PI3K, has an essential role in angiogenesis and vascular barrier function. Nat. Med. 18, 1560–1569 10.1038/nm.2928 [DOI] [PubMed] [Google Scholar]
- 13.Biswas, K., Yoshioka, K., Asanuma, K., Okamoto, Y., Takuwa, N., Sasaki, T.et al. (2013) Essential role of class II phosphatidylinositol-3-kinase-C2α in sphingosine 1-phosphate receptor-1-mediated signaling and migration in endothelial cells. J. Biol. Chem. 288, 2325–2339 10.1074/jbc.M112.409656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aki, S., Yoshioka, K., Okamoto, Y., Takuwa, N. and Takuwa, Y. (2015) Phosphatidylinositol 3-kinase class II α-isoform PI3K-C2α is required for transforming growth factor β-induced smad signaling in endothelial cells. J. Biol. Chem. 290, 6086–6105 10.1074/jbc.M114.601484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarkar, M.A.K., Aki, S., Yoshioka, K., Kuno, K., Okamoto, Y., Ishimaru, K.et al. (2018) Class II PI3Ks α and β are required for Rho-dependent uterine smooth muscle contraction and parturition in mice. Endocrinology 160, 235–248 10.1210/en.2018-00756 [DOI] [PubMed] [Google Scholar]
- 16.Islam, S., Yoshioka, K., Aki, S., Ishimaru, K., Yamada, H., Takuwa, N.et al. (2019) Class II phosphatidylinositol 3-kinase α and β isoforms are required for vascular smooth muscle Rho activation, contraction and blood pressure regulation in mice. J. Physiol. Sci. 70, 18 10.1186/s12576-020-00745-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margaria, J.P., Ratto, E., Gozzelino, L.et al. (2019) Class II PI3Ks at the intersection between signal transduction and membrane trafficking. Biomolecules 9, 104 10.3390/biom9030104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulluni, F., De Santis, M.C., Margaria, J.P., Martini, M. and Hirsch, E. (2019) Class II PI3K functions in cell biology and disease. Trends Cell Biol. 29, 339–359 10.1016/j.tcb.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 19.Wallroth, A. and Haucke, V. (2018) Phosphoinositide conversion in endocytosis and the endosomal system. J. Biol. Chem. 293, 1526–1535 10.1074/jbc.R117.000629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke, J.E. (2018) Structure basis for regulation of phosphoinositide kinases and their involvement in human diseases. Mol. Cell 71, 653–673 10.1016/j.molcel.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 21.Dornan, G.L. and Burke, J.E. (2018) Molecular mechanisms of human disease mediated by oncogenic and primary immunodeficiency mutants in class IA phosphoinositide 3-kianses. Front. Immunol. 9, 575 10.3389/fimmu.2018.00575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maffucci, T. and Falasca, M. (2014) New insight into the intracellular roles of class II phosphoinositide 3-kinases. Biochem. Soc. Trans. 42, 1378–1382 10.1042/BST20140140 [DOI] [PubMed] [Google Scholar]
- 23.Conduit, S.E. and Vanhaesebroeck, B. (2020) Phosphoinositide lipids in primary cilia biology. Biochem. J. 477, 3541–3565 10.1042/BCJ20200277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Virbasius, J.V., Guilherme, A. and Czech, M.P. (1996) Mouse p170 is a novel phosphatidylinositol 3-kinase containing a C2 domain. J. Biol. Chem. 271, 13304–13307 10.1074/jbc.271.23.13304 [DOI] [PubMed] [Google Scholar]
- 25.Domin, J., Pages, F., Volinia, S., Rittenhouse, S.E., Zvelebil, M.J., Stein, R.C.et al. (1997) Cloning of a human phosphoinositide 3-kinase with a C2 domain that display reduced sensitivity to the inhibitor wortmannin. Biochem. J. 326, 139–147 10.1042/bj3260139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, Y., Yoshioka, K., Azam, M.A., Takuwa, N., Sakurada, S., Kayaba, Y.et al. (2006) Class II phosphoinositide 3-kinase α-isoform regulates Rho, myosin phosphatase and contraction in vascular smooth muscle. Biochem. J. 394, 581–592 10.1042/BJ20051471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshioka, K., Sugimoto, N., Takuwa, N. and Takuwa, Y. (2007) Essential role for class II phosphoinositide 3-kinase α-isoform in Ca2+-induced, Rho- and Rho kinase-dependent regulation of myosin phosphatase and contraction in isolated vascular smooth muscle cells. Mol. Pharmacol. 71, 912–920 10.1124/mol.106.032599 [DOI] [PubMed] [Google Scholar]
- 28.Rozycka, M., Lu, Y.J., Brown, R.A., Lau, M.R., Shipley, J.M. and Fry, M.J. (1998) cDNA cloning of third human C2 domain-containing class II phosphoinositide 3-kinase, PI3K-C2gamma, and chromosomal assignment of this gene (PIK3C2G) to 12p12. Genomics 54, 569–574 10.1006/geno.1998.5621 [DOI] [PubMed] [Google Scholar]
- 29.Maffucci, T., Cooke, F.T., Foster, F.M., Traer, C.J., Fry, M.J., Falasca, M. (2005) Class II phosphoinositide 3-kinase defines a novel signaling pathway in cell migration. J. Cell. Biol. 169, 789–799 10.1083/jcb.200408005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braccini, L., Ciraolo, E., Campa, C.C., Perino, A., Longo, D.L., Tibolla, G. (2015) PI3K-C2γ is a Rab5 effector selectively controlling endosomal Akt2 activation downstream of insulin signalling. Nat. Commun. 6, 7400 10.1038/ncomms8400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen, K.-E., Vikas, A., Tillu, V.A., Chandra, M. and Collins, B.M. (2018) Molecular basis for membrane recruitment by the PX and C2 domains of class II phosphoinositide 3-kianse-C2α. Structure 26, 1612–1625 10.1016/j.str.2018.08.010 [DOI] [PubMed] [Google Scholar]
- 32.Wang, H., Lo, W-.T., Žagar, A.V., Gulluni, F., Lehmann, M., Scapozza, L. (2018) Autoregulation of class II alpha PI3K activity by its lipid-binding PX-C2 domain module. Mol. Cell 71, 343–351 10.1016/j.molcel.2018.06.042 [DOI] [PubMed] [Google Scholar]
- 33.Domin, J., Gaidarov, I., Smith, M.E., Keen, J.H. and Waterfield, M.D. (2000) The class II phosphoinositide 3-kinase PI3K-C2alpha is concentrated in the trans-Golgi network and present in clathrin-coated vesicles. J. Biol. Chem. 275, 11943–11950 10.1074/jbc.275.16.11943 [DOI] [PubMed] [Google Scholar]
- 34.Dell'Angelica, E.C., Klumperman, J., Stoorvogeland, W. and Bonifacino, J.S. (1998) Association of the AP-3 adaptor complex with clathrin. Science 280, 431–434 10.1126/science.280.5362.431 [DOI] [PubMed] [Google Scholar]
- 35.Dell'Angelica, E.C. (2001) Clathrin-binding proteins: Got a motif? Join the network! Trends Cell Biol. 11, 315–318 10.1016/S0962-8924(01)02043-8 [DOI] [PubMed] [Google Scholar]
- 36.Wheeler, M. and Domin, J. (2006) The N-terminus of phosphoinositide 3-kinase-C2β regulates lipid kinase activity and binding to clathrin. J. Cell. Physiol. 206, 586–593 10.1002/jcp.20507 [DOI] [PubMed] [Google Scholar]
- 37.Franco, I., Gulluni, F., Campa, C.C., Costa, C., Margaria, J.P., Ciraolo, E.et al. (2014) PI3K class II α controls spatially restricted endosomal PtdIns3P and Rab11 activation to promote primary cilium function. Dev. Cell 28, 647–658 10.1016/j.devcel.2014.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Posor, Y., Eichhorn-Gruenig, M., Puchkov, D., Schöneberg, J., Ullrich, A., Lampe, A.et al. (2013) Spatiotemporal control of endocytosis by phosphatidylinositol-3,4-bisphosphate. Nature 499, 233–237 10.1038/nature12360 [DOI] [PubMed] [Google Scholar]
- 39.Banfic, H., Visnjic, D., Mise, D., Balakrishnan, S., Deplano, S., Korchev, Y.E.et al. (2009) Epidermal growth factor stimulates translocation of the class II phosphoinositide 3-kinase PI3K-C2beta top the nucleus. Biochem. J. 422, 53–60 10.1042/BJ20090654 [DOI] [PubMed] [Google Scholar]
- 40.Domin, J., Harper, L., Aubyn, D., Balakrishnan, S., Deplano, S., Korchev, Y.E.et al. (2005) The phosphoinositide 3-kinase PI3K-C2beta regulates cell migration by a PtdIns3P dependent mechanism. J. Cell Physiol. 205, 452–462 10.1002/jcp.20478 [DOI] [PubMed] [Google Scholar]
- 41.Alliouachene, S., Bilangas, B., Chaussade, C., Pearce, W., Foukas, L.C., Scudamore, C.L.et al. (2016) Inactivation of class II PI3K-C2α induces leptin resistance, age-dependent insulin resistance and obesity in male mice. Diabetologia 59, 1503–1512 10.1007/s00125-016-3963-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aki, S., Yoshioka, K., Takuwa, N. and Takuwa, Y. (2020) TGFβ receptor endocytosis and Smad signaling require synaptojanin1-, PI3K-C2α-, and INPP4B mediated phosphoinositide conversions. Mol. Biol. Cell. 31, 360–372 10.1091/mbc.E19-11-0662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lo, W.-T., Žagar, A. V., Gerth, S., Lehmann, M., Puchkov, D., Krylova, O. (2017) A coincidence detection mechanism controls PX-BAR domain-mediated endocytic membrane remodeling via an allosteric structural switch. Dev. Cell 43, 522–529 10.1016/j.devcel.2017.10.019 [DOI] [PubMed] [Google Scholar]
- 44.Schöneberg, J., Lehmann, M., Ullrich, A., Posor, Y., Lo, W-.T., Lichtner, G.et al. (2017) Lipid-mediated PX-BAR domain recruitment couples local membrane constriction to endocytic vesicle fission. Nat.Commun. 8, 15873 10.1038/ncomms15873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shin, N., Ahn, N., Chang-Ileto, B., Park, J., Takei, K., Ahn, S.et al. (2008) SNX9 regulates tubular invagination of the plasma membrane through interaction with actin cytoskeleton and dynamin 2. J. Cell. Sci. 121, 1252–1263 10.1242/jcs.016709 [DOI] [PubMed] [Google Scholar]
- 46.Ferguson, S., Raimondi, A., Paradise, S., Shen, H., Mesaki, K., Ferguson, A.et al. (2009) Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev. Cell 17, 811–822 10.1016/j.devcel.2009.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miaczynska, M., Pelkmans, L. and Zerial, M. (2004) Not just a sink: endosomes in control of signal transduction. Curr. Opin. Cell Biol. 16, 400–406 10.1016/j.ceb.2004.06.005 [DOI] [PubMed] [Google Scholar]
- 48.Le Roy, C. and Wrana, J.L. (2005) Clathrin- and non-clathrin-mediated endocytic regulation of cell signaling. Nat. Rev. Mol. Cell Biol. 6, 112–126 10.1038/nrm1571 [DOI] [PubMed] [Google Scholar]
- 49.Garay, C., Judge, G., Lucarelli, S., Bautista, S., Pandey, R., Singh, T.et al. (2015) Epidermal growth factor-stimulated Akt phosphorylation requires clathrin or ErbB2 but not receptor endocytosis. Mol. Biol. Cell 26, 3504–3519 10.1091/mbc.E14-09-1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosselli-Murai, L.K., Yates, J.A., Yoshida, S., Bourg, J., Ho, K.K.Y., White, M.et al. (2018) Loss of PTEN promotes formation of signaling-capable clathrin-coated pits. J. Cell Sci. 131, jcs208926 10.1242/jcs.208926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eichel, K., Jullie, D., Barsi-Rhyne, B., Latorraca, N.R., Masureei, M., Sibarita, J-.B.et al. (2018) Catalytic activation of b-arrestin by GPCRs. Nature 557, 381–386 10.1038/s41586-018-0079-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gulluni, F., Martini, M., DeSantis, M., Campa, C.C., Ghigo, A., Margaria, J.P.et al. (2017) Mitotic spindle assembly and genomic stability in breast cancer require PI3K-C2α scaffold function. Cancer Cell 32, 444–459 10.1016/j.ccell.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 53.Royle, S.J. (2013) Protein adaptation: mitotic functions for membrane trafficking proteins. Nat. Rev. Mol. Cell Biol. 14, 592–559 10.1038/nrm3641 [DOI] [PubMed] [Google Scholar]
- 54.Russo, A. and O'Bryan, J.O. (2012) Intersectin 1 is required for neuroblastoma tumorigenesis. Oncogne 31, 4828–4834 10.1038/onc.2011.643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Almeida-Souza, L., Frank, R.A.W., Garcia-Nafria, J., Colussi, A., Gunawardana, N., Johnson, C.M.et al. (2018) A flat BAR protein promotes actin polymerization at the base of clathrin-coated pits. Cell 174, 325–337 10.1016/j.cell.2018.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katso, R.M., Pardo, O.E., Palamidessi, A., Reanz, C.M., Marinov, M., De Laurentiis, A.et al. (2006) Phosphoinositide 3-Kinase C2β regulates cytoskeletal organization and cell migration via Rac-dependent mechanisms. Mol. Biol. Cell 17, 3729–3744 10.1091/mbc.E05-11-1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mooren, O.L., Galletta, B.J. and Cooper, J.A. (2012) Roles for actin assembly in endocytosis. Annu. Rev. Biochem. 81, 661–686 10.1146/annurev-biochem-060910-094416 [DOI] [PubMed] [Google Scholar]
- 58.Aung, K.T., Yoshioka, K., Aki, S., Ishimaru, K., Takuwa, N. and Takuwa, Y. (2019) The class II phosphoinositide 3-kinases PI3K-C2α and PI3K-C2β differentially regulate clathrin-dependent pinocytosis in human vascular endothelial cells. J. Physiol. Sci. 69, 263–280 10.1007/s12576-018-0644-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marat, A.L., Wallroth, A., Lo, W.T., Muller, R., Norata, G.D., Falasca, M.et al. (2017) mTORC1 activity repression by late endosomal phosphatidylinositol 3,4-bisphosphate. Science 356, 968–972 10.1126/science.aaf8310 [DOI] [PubMed] [Google Scholar]
- 60.Wallroth, A., Koch, P.A., Marat, A.L., Krause, E. and Haucke, V. (2019) Protein kinase N controls a lysosomal lipid switch to facilitate nutrient signaling via mTORC1. Nat. Cell Biol. 21, 1093–1101 10.1038/s41556-019-0377-3 [DOI] [PubMed] [Google Scholar]
- 61.Devereaux, K., Dall'Armi, C., Alcazar-Roman, A., Ogasawara, Y., Zhou, X., Wang, F.et al. (2013) Regulation of mammalian autophagy by class II and III PI 3-kinases through PI3P synthesis. PLoS One 8, e76405 10.1371/journal.pone.0076405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu, N., Shen, Q., Mahoney, T.R., Neukomm, L.J., Wang, Y. and Zhou, Z. (2012) Two PI 3-kinases and one PI 3-phosphatase together establish the cyclic waves of phagosomal PtdIns(3)P critical for the degradation of apoptotic cells. PLoS Biol. 10, e1001245 10.1371/journal.pbio.1001245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Merrill, N.M., Schipper, J., Karnes, J.B., Kauffman, A.L., Martin, K.R. and MacKeigan, J.P (2017) PI3K-C2α knockdown decreases autophagy and maturation of endocytic vesicles. PLoS One 12, e0184909 10.1371/journal.pone.0184909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mountford, J.K., Petitjean, C., Putra, H.W.K., McCafferty, J.A., Setiabakti, N.M., Lee, H.et al. (2015) The class II PI 3-kinase, PI3KC2α, links platelet internal membrane structure to shear-dependent adhesive function. Nat Commun. 6, 6535 10.1038/ncomms7535 [DOI] [PubMed] [Google Scholar]
- 65.Harris, D.P., Vogal, P., Wims, M., Moberg, K., Humphries, J., Jhaver, K.G.et al. (2011) Requirement for class II phosphoinositide 3-kinase C2alpha in maintenance of glomerular structure and functions. Mol. Cell Biol. 31, 63–80 10.1128/MCB.00468-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sabha, N., Volpatti, J.R., Gonorazky, H., Reifler, A., Davidson, A.E., Li, X.et al. (2016) PIK3C2β inhibition improves function and prolongs survival in myotubular myopathy animal models. J. Clin. Invest. 126, 3613–3625 10.1172/JCI86841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alliouachene, S., Bilanges, B., Chicanne, G., Anderson, K.E., Pearce, W., Ali, K.et al. (2015) Inactivation of the class II PI3K-C2β potentiates insulin signaling and sensitivity. Cell Rep. 13, 1881–1894 10.1016/j.celrep.2015.10.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harada, K., Truong, A.B., Cai, T. and Khavari, P.A. (2005) The class II phosphoinositide 3-kinase C2beta is not essential for epidermal differentiation. Mol. Cell Biol. 25, 11122–11130 10.1128/MCB.25.24.11122-11130.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valet, C., Chicanne, G., Severac, C., Chaussade, C., Whitehead, M.A., Cabou, C.et al. (2015) Essential role of class II PI3K-C2α in platelet membrane morphology. Blood 126, 1128–1137 10.1182/blood-2015-03-636670 [DOI] [PubMed] [Google Scholar]
- 70.Azam, M.A., Yoshioka, K., Ohkura, S., Takuwa, N., Sugimoto, N., Sato, K.et al. (2007) Ca2+-independent, inhibitory effects of cyclic adenosine 5′-monophosphate on Ca2+ regulation of phosphoinositide 3-kinase C2α, Rho, and myosin phosphatase in vascular smooth muscle. J. Pharmacol. Exp. Ther. 320, 907–916 10.1124/jpet.106.111443 [DOI] [PubMed] [Google Scholar]
- 71.Seok, Y.M., Azam, M.A., Okamoto, Y., Sato, A., Yoshioka, K., Maeda, M.et al. (2010) Enhanced Ca2+-dependent activation of phosphoinositide 3-kinase class II α isoform-Rho axis in blood vessels of spontaneously hypertensive rats. Hypertension 56, 934–941 10.1161/HYPERTENSIONAHA.110.160853 [DOI] [PubMed] [Google Scholar]
- 72.Sakurada, S., Okamoto, H., Takuwa, N., Sugimoto, N. and Takuwa, Y. (2001) Rho activation in excitatory agonist-stimulated vascular smooth muscle. Am. J. Physiol. Cell Physiol. 281, 571–578 10.1152/ajpcell.2001.281.2.C571 [DOI] [PubMed] [Google Scholar]
- 73.Sakurada, S., Takuwa, N., Sugimoto, N., Wang, Y., Seto, M., Sasaki, Y.et al. (2003) Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ. Res. 93, 548–556 10.1161/01.RES.0000090998.08629.60 [DOI] [PubMed] [Google Scholar]
- 74.Somlyo, A.P. and Somlyo, A.V. (2003) Ca2+-sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, and myosin phosphatase. Physiol. Rev. 83, 1325–1358 10.1152/physrev.00023.2003 [DOI] [PubMed] [Google Scholar]
- 75.Campa, C.C., Margaria, J.P., Derle, A., Giudice, M.D., De Santis, M.C., Gozzelino, L.et al. (2018) Rab11 activity and ptdIns(3)P turnover removes recycling cargo from endosomes. Nat. Chem. Biol. 14, 801–810 10.1038/s41589-018-0086-4 [DOI] [PubMed] [Google Scholar]
- 76.Ngok, S.P., Geyer, R., Liu, M., Kourtidis, A., Agrawal, S., Wu, C.et al. (2012) VEGF and angiopoietin-1 exert opposing effects on cell junctions by regulating the Rho GEF Syx. J. Cell Biol. 199, 1103–1115 10.1083/jcb.201207009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tiosano, D. (2019) Mutations in PIK3C2A cause syndromic short stature, skeletal abnormalities, and cataracts associated with ciliary dysfunction. PLoS Genet. 15, e1008088 10.1371/journal.pgen.1008088 [DOI] [PMC free article] [PubMed] [Google Scholar]