Abstract
Repeat-associated non-AUG (RAN) translation was discovered in 2011 in spinocerebellar ataxia type 8 (SCA8) and myotonic dystrophy type 1 (DM1). This non-canonical form of translation occurs in all reading frames from both coding and non-coding regions of sense and antisense transcripts carrying expansions of trinucleotide to hexanucleotide repeat sequences. RAN translation has since been reported in 7 of the 53 known microsatellite expansion disorders which mainly present with neurodegenerative features. RAN translation leads to the biosynthesis of low-complexity polymeric repeat proteins with aggregating and cytotoxic properties. However, the molecular mechanisms and protein factors involved in assembling functional ribosomes in absence of canonical AUG start codons remain poorly characterised while secondary repeat RNA structures play key roles in initiating RAN translation. Here, we briefly review the repeat expansion disorders, their complex pathogenesis and the mechanisms of physiological translation initiation together with the known factors involved in RAN translation. Finally, we discuss research challenges surrounding the understanding of pathogenesis and future directions that may provide opportunities for the development of novel therapeutic approaches for this group of incurable neurodegenerative diseases.
Keywords: microsatellite repeat expansion disorders, pathophysiology, RAN translation
Introduction
Microsatellite expansions have been characterised in a large number of incurable neurodegenerative diseases subdivided into polyglutamine (polyQ) and non-polyglutamine (non-polyQ) disorders [1]. Autosomal-dominant glutamine-encoding CAG repeat expansions in the Huntingtin gene (HTT) cause Huntington's disease (HD) [2,3] while CAG repeats in the coding regions of various unrelated ataxin genes lead to spinocerebellar ataxias (SCA) [4,5]. Non-polyQ expansion disorders are caused by various lengths of trinucleotide to hexanucleotide repeat sequences mostly contained within non-coding regions of genes (5′-/3′-untranslated regions (UTR) and introns). These most commonly include: CGG repeats in fragile X mental retardation 1 (FMR1) gene in Fragile X-associated syndromes [6]; thousands of CTG/CCTG repeats in the myotonic dystrophies (DM1 and DM2) [7,8] and trinucleotide, pentanucleotide or hexanucleotide repeats in non-polyQ SCAs [9]; GAA repeat expansions in Friedreich's ataxia [10]; thousands of GGGGCC repeats in chromosome 9 open reading frame 72 (C9ORF72) in the most common genetic forms of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) [11,12]. Altogether, we compiled a list of 53 expansion disorders that mainly present with neurodegenerative conditions (Table 1).
Table 1. Microsatellite repeat expansion disorders.
| Disorder | Gene | Sense repeat | Antisense repeat | Disease length | Location in gene | RAN translated proteins | References |
|---|---|---|---|---|---|---|---|
| PolyQ microsatellite repeat expansion disorders: | |||||||
| Dentatorubropallidoluysian Atrophy (DRPLA) | ATN1/DRPLA | CAG | Unknown | 49–88 | Exon 5 | Unknown | [127] |
| Schizophrenia/migraines | KCNN3 | CAG | Unknown | >28 | Exon 1 | Unknown | [128] |
| Prostate/breast Cancer | AIB/SRC-3 | CAG/CAA | Unknown | >23 | Exon 20 | Unknown | [129] |
| Huntington's Disease (HD) | HTT | CAG | CTG | 36–250 | Exon 1 | polyS, polyA, polyC, polyL in human brains & in vitro3 | [2,52] |
| Spinal and Bulbar Muscular Atrophy (SBMA) | AR | CAG | Unknown | 38–62 | Exon 1 | Unknown | [130] |
| Spinocerebellar Ataxia Type 1 (SCA1) | ATXN1 | CAG | Unknown | 49–88 | Exon 8 | Unknown | [131] |
| Spinocerebellar Ataxia Type 2 (SCA2) | ATXN2 | CAG | CTG | 33–77 | Exon 1 | polyQ, polyA, polyS in vitro3 | [132,133] |
| Spinocerebellar Ataxia Type 3 (SCA3) or Machado-Joseph Disease (MJD) | ATXN3/MJD | CAG | CTG | 55–86 | Exon 10 | polyQ, polyA, polyS in vitro3 | [45,108,134] |
| Spinocerebellar Ataxia Type 6 (SCA6) | CACNA1A | CAG | Unknown | 21–30 | Exon 47 | Unknown | [135] |
| Spinocerebellar Ataxia Type 7 (SCA7) | ATXN7 | CAG | Unknown | 28–120 | Exon 3 | Unknown | [136] |
| Spinocerebellar Ataxia Type 17 (SCA17) | TBP | CAG/CAA | Unknown | 47–63 | Exon 3 | Unknown | [137] |
| Non-polyQ microsatellite repeat expansion disorders: | |||||||
| Amyotrophic lateral sclerosis (ALS)/Frontotemporal Dementia (FTD) | C9ORF72 | GGGGCC | CCCCGG | 30–4400 | Intron 1 | polyGA, polyGP, polyGR, polyPA, polyPR in human brains & in vitro3 | [11,12,49–51,120] |
| Baratela-Scott Syndrome | XYLT1 | GGC | Untranscribed expansion | >100 | Promoter | Unknown | [138] |
| Blepharophimosis-Ptosis-Epicanthus Inversus Syndactylyl | FOXL2 | GCG | Unknown | 22–24 | Exon 1 | Unknown | [139] |
| Cerebellar Ataxia, Neuropathy, Vestibular Areflexia Syndrome (CANVAS) | RFC1 | AAGGG | Unknown | 400–2000 | Intron 2 | Unknown | [140] |
| Cleidocranial Dysplasia | RUNX2/CBFA1 | GCG | Unknown | >20 | Exon 1 | Unknown | [141] |
| Congenital Central Hypoventilation/Haddad Syndrome | PHOX2B | GCG | Unknown | 5–13 | Exon 3 | Unknown | [142] |
| Familial adult myoclonic epilepsy (FAME1/BAFME1) | SAMD12 | TTTCA/TTTTA | Unknown | 440–3680 | Intron 4 | Unknown | [143] |
| Fragile X syndrome (FRAXA/FXS) | FMR1 | CGG | CCG | >230 | 5′-UTR | Unknown | [6] |
| Fragile X-associated tremor/ataxia syndrome (FXTAS) | FMR1 | CGG | CCG | 55–200 | 5′-UTR | polyG, polyP, polyA, polyR in vitro3 and Drosophila | [46,144,145] |
| Fragile X-associated Primary Ovary Insufficiency (FXPOI) | FMR1 | CGG | Not found1 | 55–200 | 5′-UTR | polyG in biopsied human ovarian stromal cells | [146,147] |
| Fragile XE mental retardation (FRAXE) | AFF2/FMR2 | CGG/CCG | Untranscribed expansion | >200 | Promoter | Unknown | [17] |
| Fragile XF syndrome (FRAXF) | TMEN185A | GCC | Unknown | 300–500 | 5′-UTR | Unknown | [148] |
| FRA2A-associated mental retardation | AFF3 | CGG | Unknown | >300 | 5′-UTR | Unknown | [19] |
| FRA7A-associated autism spectrum disorder | ZNF713 | CGG | Unknown | >85 | Intron 1 | Unknown | [19] |
| FRA10A-associated mental retardation | FRA10AC1 | CGG | Unknown | >200 | 5′-UTR | Unknown | [20] |
| FRA11A-associated mental retardation | C11ORF80 | CGG | Unknown | 500 | 5′UTR | Unknown | [149] |
| FRA12A-associated mental retardation | DIP2B | CGG | Unknown | >50 | 5′-UTR | Unknown | [21] |
| FRA16A-associated mental retardation | LOC109617027 | CGG | Unknown | 1000–1900 | 5′-UTR | Unknown | [150] |
| Friedreich's ataxia (FRDA) | FXN/X25 | GAA | TTC | >100 | Intron 1 | Unknown | [10] |
| Fuchs’ Endothelial Corneal Dystrophy (FECD) | TCF4 | CTG | CAG | >50 | Intron 3 | polyC in human corneal endothelium + polyA, polyQ, polyS in vitro3 | [151,152] |
| Hand-Foot-Genital Syndrome | HOXA13 | GCG | Unknown | 24–26 | Exon 1 | Unknown | [153] |
| Holoprosencephaly | ZIC2 | GCG | Unknown | >25 | Exon 3 | Unknown | [154] |
| Huntington Disease-Like 2 (HDL2) | JPH3 | CAG | CTG | >41 | 3′-terminal exon | polyQ, polyA, polyS in vitro3 | [45,155] |
| Jacobsen Syndrome | FRA11B/CBL2 | CGG | Not found1 | 100–1000 | 5′-UTR | Unknown | [156] |
| Myoclonus Epilepsy of the Unverricht-Lundborg Type | CYSTB | CCCCGCCCCGCG | Untranscribed expansion | 12–13 | Promoter | Unknown | [18] |
| Congenital Myotonic Dystrophy (CDM)/Steinert's Disease | DMPK | CTG | CAG | 50–10000 | 3′-UTR | Unknown | [157] |
| Myotonic dystrophy (DM1) | DMPK | CTG | CAG | 50–10000 | 3′-UTR | polyQ in human muscle/blood + polyA, polyS in vitro3 | [7,8,45,157] |
| Myotonic dystrophy type 2 (DM2) | ZNF9 | CCTG | GGAC | 75–1100 | Intron 1 | polyQAGRpolyLPAC in human brains & in vitro3 | [106,158] |
| Neuronal Intranuclear Inclusion Disease (NIID) & Amyotrophic lateral Sclerosis (ALS) | NOTCH2NLC | GGC | Unknown | >71 | 5′-UTR | Unknown | [159,160] |
| Oculopharyngeal Musclar Dystrophy | PABPN1/PABP2 | GCG | Unknown | 12–17 | Exon 1 | Unknown | [161] |
| Pseudoachrondroplasia and Multple Epiphyseal Displaysia (PSACH/MED) | COMP | GAC | Not found1 | >6 | Exon 13 | Unknown | [162] |
| Spinocerebellar Ataxia Type 8 (SCA8) | ATXN8OS & ATXN8 | CTG | CAG | 110–250 | 3′-UTR ATXN8OS; 5′UTR ATXN8 | polyA in human brains + polyS, polyQ in vitro3 | [15,45] |
| Spinocerebellar ataxia Type 10 (SCA10) | ATXN10 | ATTCT | Not found1 | 32–4000 | Intron 9 | Unknown | [163] |
| Spinocerebellar ataxia Type 12 (SCA12) | PPP2R2B | CAG | CTG | 66–78 | 5′-UTR | Unknown | [164] |
| Spinocerebellar ataxia Type 31 (SCA31) | BEAN1 | TGGAA | TTCCA | >110 | Intron 1 | polyWNGME?2 in vitro3 | [165] |
| Spinocerebellar ataxia Type 36 (SCA36) | NOP56 | GGCCTG | Unknown | >100 | Intron 1 | polyGP, polyPR in human brains + polyGL, polyWA in vitro3 | [166,167] |
| Spinocerebellar ataxia Type 37 (SCA37)4 | DAB1 | ATTTC | GAAAT | 31–75 | 5′-UTR | Unknown | [168] |
| Synpolydactylyl Type II (SPD) | HOXD13 | GCG | Unknown | 22–29 | Exon 1 | Unknown | [169] |
| X-Linked Dystonia-Parkinsonism (XPD)5 | TAF1 | CCCTCT | Unknown | 35–52 | Intron 32 | Unknown | [170] |
| X-Linked Mental Retardation and Abnormal Genitalia (XLAG) | ARX | GCN | Unknown | 20 | Exon 2 | Unknown | [171] |
| X-Linked Mental Retardation (XMLR) | ARX | GCN | Unknown | 18–23 | Exon 2 | Unknown | [172] |
| X-linked Mental Retardation with Growth Hormone Deficiency (XLMRGHD) | SOX3 | GCN | Unknown | 15–26 | Exon 1 | Unknown | [173] |
Not found indicates that antisense transcripts were not detected;
The polypeptide polyWNGME is produced from the intronic repeat expansion, however it can not be confirmed as a RAN translation product due to the presence of an ATG sequence encoding a canonical AUG start codon within the repeat expansion;
In vitro indicates that the RAN-translated proteins were detected from reporter constructs in transfected cell model of diseases;
Not classical expansions but insertions due to replication/recombination/duplication events;
Not classical expansion but insertion due to retrotransposon event.
Pathogenic mechanisms induced by microsatellite repeat expansions
The transcription of repeat expansions located in coding and non-coding regions of genes generates pathological transcripts with polymorphic RNA-repeat sequences. The microsatellite loci are moreover bi-directionally transcribed in HD, DM, C9ORF72-ALS/FTD and in some SCAs and Fragile X-associated syndromes leading to expression of both sense and antisense repeat transcripts. These are thought to cause neuronal injury through complex intertwined mechanisms involving: (i) translation of proteins with expanded stretches of glutamine in poly-Q disorders; (ii) protein gain-of-functions caused by repeat-associated non-AUG (RAN) translation of toxic repeat proteins; (iii) RNA toxic gain-of-functions through the sequestration of RNA-binding proteins within RNA foci and onto repeat transcripts; (iv) protein loss-of-functions via haploinsufficiency (reviewed in [13,14]).
Translation of protein with expanded poly-glutamine domains
Polymorphic CAG repeat expansions in HD and poly-Q SCAs encode long stretches of poly-glutamine and the translation of proteins with polyQ domains. These promote misfolding/ aggregation, inhibit interactions with physiological binding protein partners and generate abnormal interactions with other proteins, mediating thus both toxic protein loss- and gain-of-functions [4]. The non-polyQ disorder SCA8 was initially shown to express expanded CUG repeats in the 3′-UTR of the ATXN8OS (ATXN8 Opposite Strand) gene [15]. Later, bidirectional expression of CAG expansion transcripts from ATXN8 were reported and shown to result in the expression and accumulation of a polyQ protein that forms neuronal inclusions [9].
Haploinsufficiency
Loss-of-function of the genes harbouring the repeat expansions can directly contribute to the pathophysiology of the microsatellite repeats. Over 200 CGG repeats in the 5′-UTR of FMR1 cause Fragile X syndrome (FXS) [16], the most common inherited form of intellectual disability, due to transcriptional silencing induced by DNA methylation of the CGG trinucleotides and loss of the FMRP protein which has roles in synaptic plasticity. A contributory loss-of-function is the likely pathological cause of diseases where the repeat expansions are found in promotors, e.g. fragile-XE mental retardation (FMR2 gene; [17]) and myoclonus epilepsy of the Unverricht-Lundborg type (CYSTB gene; [18]). Loss-of-function is also associated with folate sensitive fragile sites harbouring CGG repeats (FRA7A, FRA10A and FRA12A) through DNA methylation of the repeat expansions [19–21]. Hexanucleotide-repeat expansions in the 5′-UTR region of C9ORF72 lead to decreased expression levels of C9ORF72 mRNAs, encoding a protein involved in autophagy regulation [22–25], vesicle trafficking [26,27] and immune response in mice [28,29] in several in vitro and in vivo models and post-mortem brains [11,30–33]. However, the direct contribution of reduced levels of C9ORF72 protein to disease pathogenesis is still debated.
Formation of RNA foci and RNA-repeat sequestration of proteins
RNA-mediated cellular toxicity results in either protein gain- and loss- of-functions via sequestration of RNA-processing proteins on repeat transcripts which may either be co-transcriptionally processed or aggregated into RNA foci. Protein loss-of-functions have been implicated in a wide range of expansion disorders via RNA-repeat sequestration of mRNA-binding proteins which may loose their normal cellular functions including: muscleblind-like splicing regulator (MBLN) and CUG-binding protein and ETR3-like factor (CELF) families of proteins in myotonic dystrophy [34–36]; MBLN and other RNA-binding proteins in polyQ disorders [37,38]; Sam68 [39], PUR-alpha, hnRNP A2/B1, CUGBP1 [40,41] in fragile X-associated tremor ataxia syndrome (FXTAS); PUR-alpha, heterogeneous nuclear ribonucleoproteins (hnRNPs) and SR-rich splicing factors (SRSFs) among others in C9ORF72-ALS/FTD [42,43]. On the other hand, toxic protein gain-of-function also occurs through RNA-repeat sequestration of SRSF1 which triggers the nuclear export and subsequent RAN translation of sense and antisense C9ORF72-repeat transcripts retaining pathological expansions in intron-1 [44].
RAN translation of toxic repeat proteins
In 2011, Laura Ranum's group demonstrated that CAG-repeat transcripts lacking canonical AUG start codons are remarkably translated into homo-polymeric proteins in all frames (poly-glutamine, poly-serine and poly-alanine) by repeat-associated non-AUG (RAN) translation [45]. RAN-translated poly-alanine proteins driven from ATXN8 CAG-repeat transcripts were also characterised in SCA8 mice and human brain tissue [45]. Interestingly, the poly-alanine repeat proteins can also be produced by RAN translation of the 5′UTR-sense ATXN8OS CUG-repeat transcripts in transfected cells. Since this discovery, RAN translation of non-coding transcript regions was highlighted to occur from CGG repeats in FXTAS which produce toxic poly-glycine FMRpolyG and poly-alanine FMRpolyA proteins [46–48] and from bi-directionally transcribed GGGGCC repeats in all frames in C9ORF72-ALS/FTD to generate five cytotoxic sense and antisense dipeptide-repeat proteins (DPRs) (poly-glycine-alanine, poly-glycine-arginine, poly-glycine-proline, poly-proline-alanine and poly-proline-arginine) [49–51]. Moreover, RAN translation also occurs through the coding CAG-repeat expansions in the HTT open reading frame leading overall to both canonical translation of the polyQ-expanded HTT mutant protein and to four RAN-translated sense and antisense homo-polymeric repeat proteins in HD (poly-alanine, poly-serine, poly-leucine, poly-cysteine) [52]. To date, RAN translation has been evidenced from repeat transcripts expressed in human disease samples in seven expansion disorders (Table 1).
The recent discovery of RAN translation challenged the initial hypothesis that non-coding repeat expansion disorders are primarily caused by RNA foci and protein loss-of functions due to sequestration of RNA-binding proteins since polymeric repeat proteins exhibit aggregating properties and high levels of cytotoxicity in multiple cell and animal models of repeat expansion disorders. A range of polypeptides are produced through these mechanisms from the homo-polymeric proteins derived from trinucleotide repeat expansions through to dipeptide repeat proteins found in C9ORF72-ALS/FTD and SCA36 to more complex polypeptide repeat proteins expressed in transfected reporter cell models in SCA31 and DM2 (Table 1). Repeat expansions can be translated from sense, e.g. Jacobsen Syndrome, or sense and antisense strands, e.g. C9ORF72-ALS/FTD, SCA8, HD and FXTAS. The pathophysiological properties of C9ORF72-ALS/FTD DPRs are the most characterised. Increasing evidence has associated very high cytotoxicity to the arginine-containing DPRs (poly-glycine-arginine and poly-proline-arginine) in Drosophila, mice, patient-derived neurons and other cell models [53–59] while poly-GA toxicity was also reported in chicks and mice [60–62]. Mechanisms of DPR-mediated cytoxicity include nucleolar dysfunction [53], transcriptional silencing [63], broad disruption of gene expression through interaction with low complexity domain-containing proteins such as RNA Recognition Motif proteins [64], altered splicing [65] and nucleocytoplasmic transport [44,66,67], impairment of DNA repair [68], mitochondrial defects [59,69] and global alteration of translation [56,70,71] together with alterations of ubiquitin/proteasome mediated proteolysis [72,73].
Physiological mechanisms of eukaryotic translation
Translation involves three distinct mechanisms in Eukaryotes: (i) canonical AUG-driven cap-dependent initiation of translation for the vast majority of mRNAs; (ii) IRES-mediated cap-independent translation and (iii) canonical translation using alternative near-cognate codons.
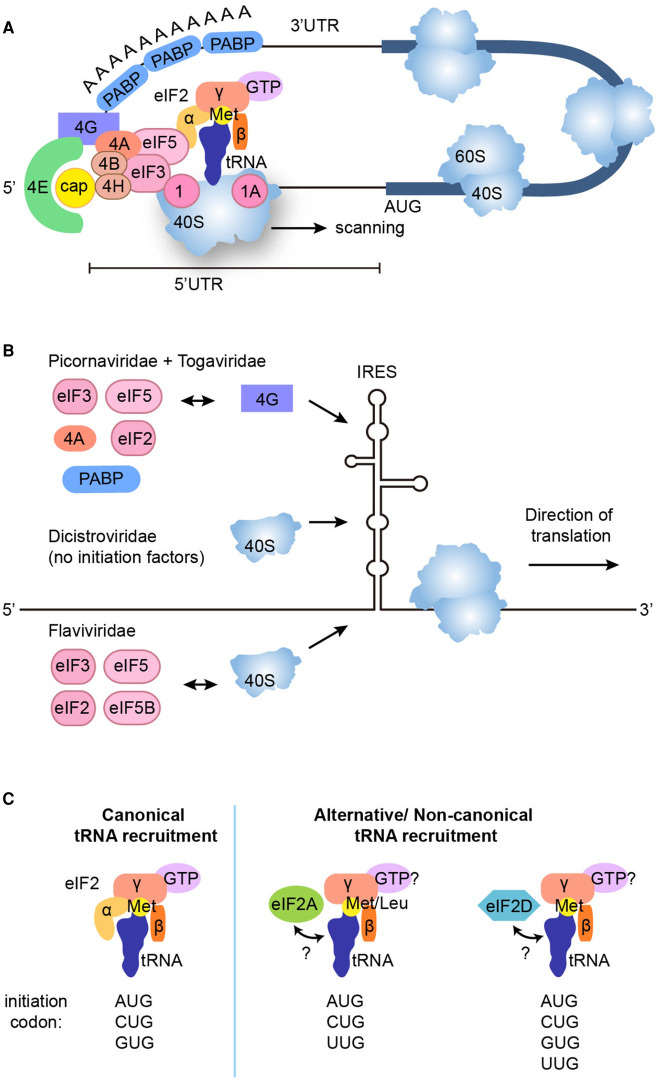
Translation initiation of canonical mRNAs is a complex process which requires many eukaryotic initiation factors (eIFs) and is one of the key rate-limiting steps for the regulation of gene expression [74]. Translation of canonical mRNAs has been shown to occur through the formation of a closed loop complex, with eIF4G forming a bridge between the m7cap-binding protein eIF4E and the poly(A) tail binding protein PABP although the closed loop formation does not explain initiation of all cellular mRNAs [75]. Briefly, the 40S ribosomal subunit is recruited to mRNAs upstream of the translation start site via multiple initiation factors and an incorporated eIF2α-bound Met-tRNAiMet to form the 48S pre-initiation complex, which scans along the mRNA 5′-UTR with the RNA-helicase eIF4A and its cofactors eIF4B and eIF4H unwinding any secondary structures until the AUG codon is reached. Further initiation proteins facilitate the joining of the 60S subunit to produce the initiating 80S complex [74]. Regulation of translation is predominantly exerted at the initiation stage where the AUG start codon is identified by eIF2α-bound methionyl-tRNA and start codon selection efficiency is tightly influenced by the surrounding nucleotide sequence known as the Kozak consensus element [76]. A schematic of canonical AUG-driven translation initiation is provided in Figure 1A.
Figure 1. Canonical and physiological translation initiation mechanisms.
(A) Canonical initiation involves the eIF4F complex and the poly(A) tail binding protein PABP binding to the mRNA and subsequently interacting with the 43S complex (eIF5, eIF3, eIF2 and the 40S ribosome) to form the 48S complex. eIF4E and PABP both interact with eIF4G to create a ‘closed loop complex'. eIF4A, with its cofactors eIF4B and eIF4H, interact with eIF4G and eIF4E to provide helicase activity to unwind secondary structures present in the 5′UTR. The 48S complex scans the mRNA for an AUG start codon, where the 60S ribosomal subunit is recruited through eIF5B and several of the initiator factors are displaced and recycled to initiate a new round of translation. (B) IRES mediated translation involves a strong secondary or tertiary structure within the 5′UTR. The precise mechanisms vary between viruses but the IRES element interacts with either the 40S subunit or eIF4G, which recruit any other required factors to initiate translation. (C) Canonical and alternative physiological initiator tRNA-binding eIF factors recognise different start codons. Canonical translation occurs through eIF2α, delivering Met-tRNAiMet to the P site of the 40S ribosomal subunit in a GTP-dependent manner, through interaction with both the canonical AUG start codon and near cognate start codons CUG and GCG. Both eIF2A and eIF2D are also able to initiate translation, however this can occur in a GTP-dependent or independent manner, binding either charged or uncharged tRNAiMet. eIF2A can additionally bind Leu-tRNACUG to initiate translation. eIF2A can initiate translation at AUG, CUG and UUG codons, while eIF2D can initiate at AUG, CUG, GCG and UUG codons.
Alternative initiation mechanisms using internal ribosome entry site (IRES) elements are utilised by many viral and a growing number of cellular mRNAs [77]. IRES elements drive translation in a cap-independent manner via distinct secondary or tertiary RNA structures that directly bind either the initiation factor eIF4G (picornaviridae and togaviridae) or the 40S ribosome (dicistroviridae and flaviviridae) to initiate translation [78,79] (Figure 1B). With picornaviridae, togaviridae and flaviviridae, eIF4G or the 40S ribosome subsequently recruits other initiation factors to facilitate translation of the IRES-harbouring RNAs while IRES from dicistroviridae require no initiation factors directing translation solely via its binding of the 40S ribosome (Figure 1B).
Finally, near-cognate start codons (typically CUG, GUG and UUG), which differ from the AUG start codon by one nucleotide, initiate translation in mammalian cells at a much lower efficiency, using the non-AUG initiator tRNAiMet and methionine as the initiating amino acid [80] or an elongator Leu-tRNALeu at a CUG codon in the case of the major histocompatibility complex class I molecules [81,82]. Near-cognate initiation sites can be used by mismatch recognition of eIF2α-bound Met-tRNAiMet [80], when fidelity of start codon usage is affected depending on secondary structures downstream of the initiation codon and expression levels of other initiation factors such as eIF1 or eIF5 which respectively increases or decreases the fidelity of AUG recognition. Two other initiation factors (eIF2A and eIF2D) can also be used at non-AUG codons to initiate translation in either a GTP-dependent manner through initiator tRNAiMet or a GTP-independent manner through Leu-tRNALeu [83] (Figure 1C).
Mechanisms of RAN translation
RAN translation involves the translation of short repeated RNA sequences in sense and/or antisense transcripts in an AUG-independent manner and in multiple frames. However, how RAN translation occurs and which sets of factors are required for initiation, elongation and potential regulatory controls remains largely unknown, although it is clearly emerging that some features are shared with canonical and/or IRES-mediated initiation [84].
The roles of initiation factors and RNA structures in RAN translation
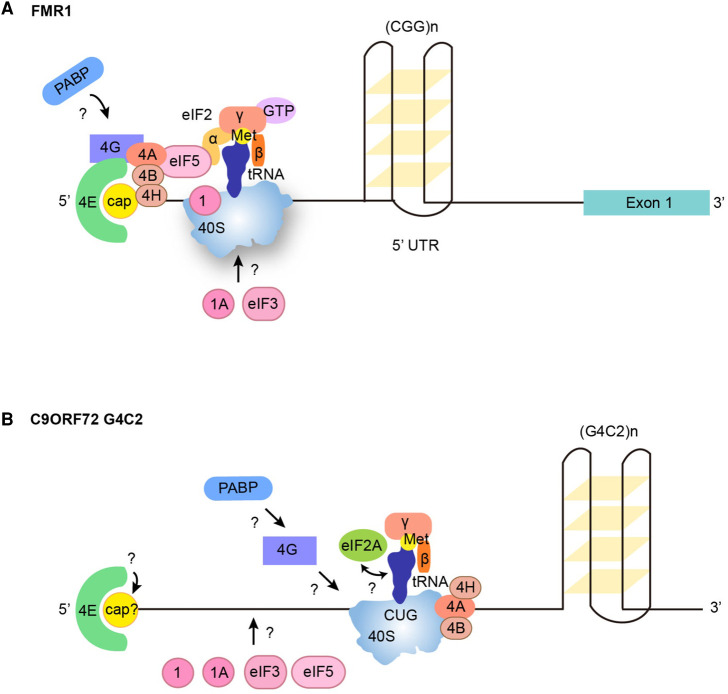
An improved understanding of the mechanisms driving RAN translation has begun to emerge with a clear role for the general translation initiation factor eIF4A, a DEAD-box RNA helicase, identified in stimulating the canonical translation of mRNAs containing complex secondary structures such as G-quadruplexes in the 5′-UTR of oncogenes [85]. The identification of inhibitors of eIF4A highlighted that the RNA helicase activity of eIF4A plays an essential role in unwinding secondary structures during ribosome scanning [86–88]. G-quadruplex structures are formed in GGGGCC-repeat [89] and CGG-repeat [90] RNAs. The eIF4A inhibitor hippuristanol showed that eIF4A is required for the RAN translation of CGG-repeat expansions in FMR1 in FXTAS [47] (Figure 2A) and GGGGCC-repeat transcripts in C9ORF72-ALS/FTD [91] (Figure 2B). The eIF4A inhibitor FL3 [92] further confirmed the role of eIF4A in the RAN translation of sense C9ORF72-repeat transcripts. However, the RAN translation mechanisms of antisense C9ORF72-repeats which form a double RNA helix [93] remain completely unknown.
Figure 2. Known RNA structures and protein factors involved in RAN translation.
(A) RAN translation of FMR1 occurs in a Cap-, eIF4E- and eIF4A-dependent manner along with eIF4A cofactors eIF4H and eIF4B, recruiting the 40S ribosome and eIF2α-bound Met-tRNAiMet to the near-cognate ACG start codon upstream of the CGG repeat expansion. Regulation of start codon fidelity through eIF1 and eIF5 is important. Any potential role of the translation initiation factors PABP, eIF4G, eIF1A and eIF3 remain unknown. (B) RAN translation of the GGGGCC repeat expansion from C9ORF72 occurs in an eIF4A-dependent manner to recruit the 40S ribosome subunit and eIF2A-bound Met-tRNAiMet to the near cognate CUG start codon upstream of the hexanucleotide repeat expansion. The eIF4A cofactors eIF4B and eIF4H have been shown to be disease modifiers and are involved. Contradictory evidence for the role of the m7cap and eIF4E factor indicates that further elucidation of their roles is required. Any potential role of the translation initiation factors PABP, eIF4G, eIF1, eIF1A, eIF3 and eIF5 still remain unknown. The mechanisms involved in the RAN translation of C9ORF72 antisense CCCCGG-repeat transcripts have not yet been explored.
The RNA helicase activity of eIF4A is significantly enhanced by two cofactors, eIF4B and eIF4H [94–96], and eIF4B is essential for the translation of mRNAs with long-structured 5-UTRs independently of eIF4A [97]. Interestingly, recent Drosophila screens involving sense C9ORF72-repeat [98] and FXTAS CGG-repeat [99] transcripts identified eIF4B and eIF4H as disease modifiers of the RAN translation with down-regulation of eIF4B or eIF4H leading to reduced RAN translation and associated toxicity, ameliorating Drosophila eye neurodegenerative phenotypes and life span (Figure 2A,B). Interestingly, sequestration of eIF4H by GGGGCC-repeat sequences was previously reported [43]. DDX3X, another RNA helicase which is required for the resolution of RNA–RNA structures in long GC-rich 5′-UTRs, is also implicated in the RAN translation of FXTAS CGG-repeats, with suppression of this helicase inhibiting RAN translation and rescuing associated toxicity in Drosophila and primary neurons [99]. However, the role of DDX3X in RAN translation appears sequence-specific since the depletion and overexpression of DDX3X respectively lead to increased and reduced DPR levels in C9ORF72-ALS lymphoblasts [100]. Interestingly, the ribosomal protein RPS25, involved in IRES translation [101], also behaves differently during RAN translation of FXTAS CGG-repeats [99] and sense C9ORF72-repeats [102]. Suppression of RPS25 in a FXTAS Drosophila model enhanced RAN-translated protein production and associated toxicity [99], while suppression of RPS25 reduced DPR production and rescued associated toxicity in yeast, Drosophila and human C9ORF72-ALS/FTD models [102].
RAN translation of reporter constructs require both a m7G-cap and eIF4E for FXTAS CGG-repeats [47] and sense C9ORF72-repeats [91]. Accordingly, the eIF4E competitive inhibitor m7G-cap analogue (m7GpppG) prevented RAN translation of FXTAS CGG-repeats [47] and sense C9ORF72-repeats [91,92]. However, another study using a bicistronic reporter construct with all-frame stop codons prior to the initiating start codon reported that RAN translation of C9ORF72-repeat transcripts still occurred, suggesting recruitment of ribosomes in a cap-independent manner [103]. eIF4E was shown to be important for the RAN translation of sense C9ORF72-repeat transcripts using the 4EIRCat inhibitor [92] however, it was also reported that depletion of eIF4E does not result in a reduction in RAN translation [103] (Figure 2A,B). It thus clearly appears that the sequence-specific context surrounding repeat expansions regulate the mechanisms of RAN translation.
Additional translation initiation factors involved in start codon fidelity are implicated in RAN translation. eIF1 is important in increasing AUG start codon fidelity and overexpression reduces RAN translation and associated toxicity in FXTAS Drosophila [99]. eIF5, on the other hand, relaxes start codon fidelity and suppression of eIF5 reduces RAN translation and associated toxicity in FXTAS Drosophila [99] (Figure 2A). SCA8 has been shown to require eIF3f during RAN translation of poly-serine and poly-alanine proteins [104]. eIF3f is a non-core component of eIF3 which plays a role in regulating both canonical and IRES-dependent translation [105]. Interestingly, the poly-S proteins produced by RAN translation in SCA8 [104] and HD [52] and poly-QAGR/LPAC in DM2 [106] accumulate in white matter brain regions, where eIF3f levels are elevated compared with grey matter [107], further supporting a potential role of eIF3f in the RAN translation and/or its regulation for some repeat expansions.
The roles of near-cognate and non-cognate initiation codons in non-AUG translation initiation
Complex secondary RNA-repeat structures such as G-quadruplexes appear to play direct initiating roles through altered ribosome scanning and/or recruitment of ribosomes at near-cognate initiation codons, which differ from AUG by only one nucleotide, and non-cognate initiation codons that differ by more than one nucleotide. FXTAS CGG-repeats within the 5′-UTR of FMR1 initiate RAN translation of FMRpolyG at a near-cognate ACG codon embedded in a putative Kozak element 32 nucleotides upstream of the repeats in the +1 reading frame, while FMRpolyA initiates at a non-cognate GCG codon within the repeats in a +2 reading frame [47,48]. Mass spectrometry analysis also indicated that polyA RAN-translated proteins from the SCA8 CAG repeats are initiated at non-cognate GCA codons throughout the repeat tract [45] while non-cognate CUU and ACU codons are used to initiate RAN translation of polyQ upstream of the SCA3 CAG repeats and polyA proteins likely within the repeats [108] in transfected cell models. Sense C9ORF72-repeat transcripts initiate RAN translation with a Met-tRNAiMet through eIF2A at a Kozak-embedded CUG codon located 24 nucleotides upstream of the repeat sequence in the +1 reading frame of transfected reporter constructs [91,92,109].
The integrated stress response enhances non-canonical translation
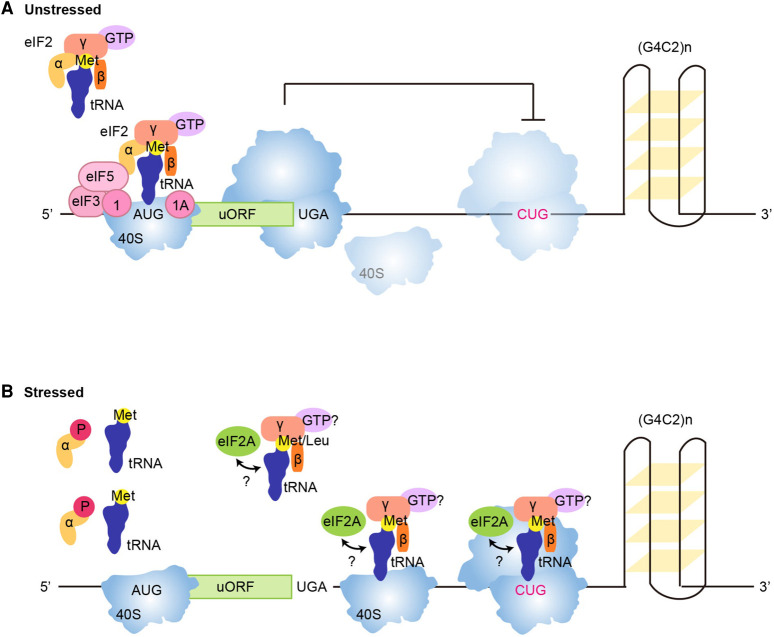
Under non-stressed conditions, RAN translation of sense C9ORF72-repeats is strongly inhibited by an upstream open reading frame (uORF) of 55 nucleotides which is located in intron 1 and flanked by an AUG start codon and 2 downstream stop codons (UGA and UAA) [92] (Figure 3A). However, the integrated stress response (ISR), which is stimulated during disease progression, leads to phosphorylation of eIF2α and down-regulation of canonical translation due to poor recruitment of the 60S subunit of the ribosome and inhibition of translation initiation. This results in read-through of the uORF and subsequent initiation at the downstream CUG codon responsible for RAN translation of the hexanucleotide-repeats [92] (Figure 3B). In neurons, eIF2α phosphorylation is important for synaptic plasticity and rapid activity-dependent alterations of synaptic proteins [110,111]. Following cellular stress and activation of the ISR, a shift in translation occurs towards a subset of transcripts with 5′-UTRs containing uORFs, e.g. ATF4 and CHOP [112], cellular IRES e.g. HIAP2, HIF1α and VEGF [113] and non-AUG start codons e.g. EPRS and major histocompatibility class I antigens [81,114], allowing these transcripts to escape eIF2α-phosphorylated translational inhibition. Phosphorylation of eIF2α increases non-canonical translation and increasing ISR occur concomitantly with increased RAN translation of both FMR1 and C9ORF72 repeats in a positive feedback loop [91,103,109,115]. It is noteworthy that ISR-resistant translation involves eIF2D, eIF2A and eIF5B [116–119] and as aforementioned, RAN translation of sense C9ORF72-repeats initiates at a CUG codon through eIF2A [109] (Figure 3B). Taken together, these studies strongly support a model in which increased ISR upon disease progression down-regulates canonical translation of the uORF to stimulate RAN translation in a positive disease-enhancing feedback loop.
Figure 3. The role of a uORF in the translation of RAN proteins from pathological sense GGGGCC C9ORF72 repeat expansions.
(A) In unstressed cellular conditions, a uORF of 55 nucleotides in length within intron 1 of C9ORF72 inhibits RAN translation of the downstream repeat expansion. The uORF is translated through the canonical translation machinery and ribosomes are unable to reassemble on the mRNA for initiation at the downstream CUG RAN initiation codon. (B) Following cellular stress, phosphorylation of eIF2α prevents its binding of Met-tRNAiMet results in an inhibition of eIF2-driven canonical translation. Consequently, the alternative tRNA recruiting factor eIF2A is able to initiate non-canonical translation. The scanning 40S ribosomal complex scans through the uORF and eIF2A initiates RAN translation at the downstream CUG codon.
Conclusions
The pathogenesis driven by nucleic acid repeat expansions and RAN-translated products is complex and still poorly understood. Multiple mechanisms of neuronal injury involve toxic RNA gain-of-functions, haploinsufficiency as well as protein gain-of-functions via canonical translation of proteins with extended polyQ domains and/or RAN translation of toxic repeat polypeptides which have been characterised in vitro in 13 reporter repeat expansion cell models and in patient bio-samples from seven diseases, including SCA8, DM1 [45,106], C9ORF72-ALS [49–51,120] and HD [52]. However, the molecular mechanisms involved in RAN translation remain poorly understood, hindering thus the development of therapeutic approaches for this incurable group of diseases. In addition, it has remained challenging to dissect how the life-long expression of repeat transcripts and RAN-translated proteins contribute to the pathogenesis of these progressive adult-onset diseases. In the past few years, growing evidence has implicated RAN translation as one of the main drivers of neurotoxicity in C9ORF72-ALS/FTD models. On the other hand, FXTAS was initially thought to be caused by intranuclear retention of transcripts and sequestration of splicing factors [40,41] however, the later discovery of RAN translation in the same model challenged this view [46]. Similarly in C9ORF72-ALS, increasing the number of intranuclear RNA foci does not appear to alter neuronal survival or global RNA processing while expression of DPRs drives the neurodegeneration process [44,54,121]. Sequestration of RNA-binding factors on repeat transcripts might not only necessarily implicate loss-of-function mechanisms, as often hypothesised, as cells can use compensatory mechanisms to up-regulate the transcription/translation of the sequestered proteins. So far, there has not been any evidence demonstrating that RNA foci and sequestration of proteins on RNA-repeats trigger protein loss-of-function mechanisms. In contrast, RNA-repeat sequestration of SRSF1 produces protein gain-of-function by driving the nuclear export of intron-retaining C9ORF72-repeat pre-mRNAs and subsequent RAN translation of cytotoxic DPRs in the cytoplasm [44].
If expression of polymeric repeat proteins can kill cells and animal models, it is difficult to evaluate which levels are RAN-translated in patients and which expression levels trigger toxicity depending on the nature of the repeat expansion, disease and cell type. Understanding the molecular mechanisms of RAN translation will be key to improving our understanding of pathogenesis. Additional mechanisms such as ribosome frameshifting events also need to be explored. For example, frameshifting was suggested to occur during translation of CAG-repeat expanded transcripts in the −1 frame in SCA3 [122–124] as well as into the −1 [125] and +1 [126] directions in HD, however, the production of chimeric repeat proteins was not directly evidenced. RAN translation of three C9ORF72-related DPRs encoded from the three reading frames of sense repeat transcripts suggested that RAN translation initiates at multiple initiation sites [91,109], however, mutation of a near-cognate CUG start codon also inhibited the production of all sense DPRs, suggesting the occurrence of potential frameshifting mechanisms that remain to be demonstrated [92].
Perspectives
Mechanisms of RAN translation and RAN-translated proteins/peptides still remain poorly characterised despite discovery in DM1/SCA8 patient samples in 2011, and later in C9ORF72-ALS and HD in 2013 and 2015, respectively.
RAN-translation occurs in absence of the canonical methionine start codon, in all frames, and from coding and non-coding regions of transcripts encoding proteins of various functions. So far, it is known to involve RNA secondary structures formed by repeat sequences and general translation initiation factors, which exhibit/stimulate RNA-helicase activities, or play a role in the fidelity of start codon recognition.
In the future, it will be fundamental to fully identify the RAN-translation machinery components and mechanisms in the context of the sequences flanking each microsatellite repeat expansions, as well as examine further the pathological contribution of RAN-translated products for the future development of much-needed disease treatments.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- C9ORF72
chromosome 9 open reading frame 72
- DM1
dystrophy type 1
- DPRs
dipeptide-repeat proteins
- FMR1
fragile X mental retardation 1
- FTD
frontotemporal dementia
- FXS
Fragile X syndrome
- FXTAS
fragile X-associated tremor ataxia syndrome
- HD
Huntington's disease
- IRES
internal ribosome entry site
- ISR
integrated stress response
- RAN
repeat-associated non-AUG
- SCA8
spinocerebellar ataxia type 8
- UTR
untranslated regions
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
Royal Society International Exchanges 2017 Cost Share Grant IEC\R3\170103 (G.M.H. and K.Y.C.) and MOST-RS International Exchange Scheme: MOST 108-2911-I-005-504 (K.Y.C. and G.M.H.). G.M.H. also acknowledge support from the MRC (Medical Research Council) New Investigator grant MR/R024162/1 and Biotechnology and Biological Sciences Research Council (BBSRC) grant BB/S005277/1.
Open Access Statement
Open access for this article was enabled by the participation of University of Sheffield in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with JISC.
Author Contributions
L.M.C. and G.M.H. wrote the review. W.P.H. designed the figures. All authors contributed to the proofreading and editing of the manuscript.
References
- 1.Paulson, H. (2018) Repeat expansion diseases. Handb. Clin. Neurol. 147, 105–123 10.1016/B978-0-444-63233-3.00009-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacDonald, M.E., Ambrose, C.M., Duyao, M.P., Myers, R.H., Lin, C., Srinidhi, L.et al. (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72, 971–983 10.1016/0092-8674(93)90585-E [DOI] [PubMed] [Google Scholar]
- 3.La Spada, A.R., Paulson, H.L. and Fischbeck, K.H. (1994) Trinucleotide repeat expansion in neurological disease. Ann. Neurol. 36, 814–822 10.1002/ana.410360604 [DOI] [PubMed] [Google Scholar]
- 4.Orr, H.T. and Zoghbi, H.Y. (2007) Trinucleotide repeat disorders. Annu. Rev. Neurosci. 30, 575–621 10.1146/annurev.neuro.29.051605.113042 [DOI] [PubMed] [Google Scholar]
- 5.Whaley, N.R., Fujioka, S. and Wszolek, Z.K. (2011) Autosomal dominant cerebellar ataxia type I: a review of the phenotypic and genotypiccharacteristics. Orphanet J. Rare Dis. 6, 33 10.1186/1750-1172-6-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verkerk, A.J., Pieretti, M., Sutcliffe, J.S., Fu, Y.H., Kuhl, D.P., Pizzuti, A.et al. (1991) Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65, 905–914 10.1016/0092-8674(91)90397-H [DOI] [PubMed] [Google Scholar]
- 7.Brook, J.D., McCurrach, M.E., Harley, H.G., Buckler, A.J., Church, D., Aburatani, H.et al. (1992) Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 68, 799–808 10.1016/0092-8674(92)90154-5 [DOI] [PubMed] [Google Scholar]
- 8.Fu, Y.H., Pizzuti, A., Fenwick, R.G., King, J., Rajnarayan, S., Dunne, P.W.et al. (1992) An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science 255, 1256–1258 10.1126/science.1546326 [DOI] [PubMed] [Google Scholar]
- 9.Moseley, M.L., Zu, T., Ikeda, Y., Gao, W., Mosemiller, A.K., Daughters, R.S.et al. (2006) Bidirectional expression of CUG and CAG expansion transcripts and intranuclear polyglutamine inclusions in spinocerebellar ataxia type 8. Nat. Genet. 38, 758–769 10.1038/ng1827 [DOI] [PubMed] [Google Scholar]
- 10.Campuzano, V., Montermini, L., Molto, M.D., Pianese, L., Cossee, M., Cavalcanti, F.et al. (1996) Friedreich's ataxia: Autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271, 1423–1427 10.1126/science.271.5254.1423 [DOI] [PubMed] [Google Scholar]
- 11.DeJesus-Hernandez, M., Mackenzie, I.R., Boeve, B.F., Boxer, A.L., Baker, M., Rutherford, N.J.et al. (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-Linked FTD and ALS. Neuron 72, 245–256 10.1016/j.neuron.2011.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renton, A.E., Majounie, E., Waite, A., Simón-Sánchez, J., Rollinson, S., Gibbs, J.R.et al. (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72, 257–268 10.1016/j.neuron.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rohilla, K.J. and Gagnon, K.T. (2017) RNA biology of disease-associated microsatellite repeat expansions. Acta Neuropathol. Commun. 5, 63–22 10.1186/s40478-017-0468-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sznajder, Ł.J and Swanson, M.S. (2019) Short tandem repeat expansions and RNA-mediated pathogenesis in myotonic dystrophy. Int. J. Mol. Sci. 20, 3365 10.3390/ijms20133365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koob, M.D., Moseley, M.L., Schut, L.J., Benzow, K.A., Bird, T.D., Day, J.W.et al. (1999) An untranslated CTG expansion causes a novel form of spinocerebellar ataxia (SCA8). Nat. Genet. 21, 379–384 10.1038/7710 [DOI] [PubMed] [Google Scholar]
- 16.McLennan, Y., Polussa, J., Tassone, F. and Hagerman, R. (2011) Fragile X syndrome. Curr. Genomics 12, 216–224 10.2174/138920211795677886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gecz, J., Gedeon, A.K., Sutherland, G.R. and Mulley, J.C. (1996) Identification of the gene FMR2, associated with FRAXE mental retardation. Nat. Genet. 13, 105–108 10.1038/ng0596-105 [DOI] [PubMed] [Google Scholar]
- 18.Lalioti, M.D., Scott, H.S., Buresi, C., Rossier, C., Bottani, A., Morris, M.A.et al. (1997) Dodecamer repeat expansion in cystatin B gene in progressive myoclonus epilepsy. Nature 386, 847–851 10.1038/386847a0 [DOI] [PubMed] [Google Scholar]
- 19.Metsu, S., Rainger, J.K., Debacker, K., Bernhard, B., Rooms, L., Grafodatskaya, D.et al. (2014) A CGG-repeat expansion mutation in ZNF713 causes FRA7A: association with autistic spectrum disorder in two families. Hum. Mutat. 35, 1295–1300 10.1002/humu.22683 [DOI] [PubMed] [Google Scholar]
- 20.Sarafidou, T., Kahl, C., Martinez-Garay, I., Mangelsdorf, M., Gesk, S., Baker, E.et al. (2004) Folate-sensitive fragile site FRA10A is due to an expansion of a CGG repeat in a novel gene, FRA10AC1, encoding a nuclear protein. Genomics 84, 69–81 10.1016/j.ygeno.2003.12.017 [DOI] [PubMed] [Google Scholar]
- 21.Winnepenninckx, B., Debacker, K., Ramsay, J., Smeets, D., Smits, A., FitzPatrick, D.R.et al. (2007) CGG-repeat expansion in the DIP2B gene is associated with the fragile site FRA12A on chromosome 12q13.1. Am. J. Hum. Genet. 80, 221–231 10.1086/510800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sellier, C., Campanari, M.-L., Julie Corbier, C., Gaucherot, A., Kolb-Cheynel, I., Oulad-Abdelghani, M.et al. (2016) Loss of C9ORF72 impairs autophagy and synergizes with polyQ Ataxin-2 to induce motor neuron dysfunction and cell death. EMBO J. 35, 1276–1297 10.15252/embj.201593350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan, P.M., Zhou, X., Robins, A.M., Paushter, D.H., Kim, D., Smolka, M.B.et al. (2016) The ALS/FTLD associated protein C9orf72 associates with SMCR8 and WDR41 to regulate the autophagy-lysosome pathway. Acta Neuropathol. Commun. 4, 51–16 10.1186/s40478-016-0324-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webster, C.P., Smith, E.F., Bauer, C.S., Moller, A., Hautbergue, G.M., Ferraiuolo, L.et al. (2016) The C9orf72 protein interacts with Rab1a and the ULK1 complex to regulate initiation of autophagy. EMBO J. 35, 1656–1676 10.15252/embj.201694401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang, M., Liang, C., Swaminathan, K., Herrlinger, S., Lai, F., Shiekhattar, R.et al. (2016) A C9ORF72/SMCR8-containing complex regulates ULK1 and plays a dual role in autophagy. Sci Adv 2, e1601167 10.1126/sciadv.1601167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farg, M.A., Sundaramoorthy, V., Sultana, J.M., Yang, S., Atkinson, R.A.K., Levina, V.et al. (2014) C9ORF72, implicated in amytrophic lateral sclerosis and frontotemporal dementia, regulates endosomal trafficking. Hum. Mol. Genet. 23, 3579–3595 10.1093/hmg/ddu068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aoki, Y., Manzano, R., Lee, Y., Dafinca, R., Aoki, M., Douglas, A.G.L.et al. (2017) C9orf72 and RAB7L1 regulate vesicle trafficking in amyotrophic lateral sclerosis and frontotemporal dementia. Brain 140, 887–897 10.1093/brain/awx024 [DOI] [PubMed] [Google Scholar]
- 28.O'Rourke, J.G., Bogdanik, L., Yanez, A., Lall, D., Wolf, A.J., Muhammad, A.K.M.G.et al. (2016) C9orf72 is required for proper macrophage and microglial function in mice. Science 351, 1324–1329 10.1126/science.aaf1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atanasio, A., Decman, V., White, D., Ramos, M., Ikiz, B., Lee, H.-C.et al. (2016) C9orf72 ablation causes immune dysregulation characterized by leukocyte expansion, autoantibody production, and glomerulonephropathy in mice. Sci. Rep. 6, 23204–23214 10.1038/srep23204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belzil, V.V., Bauer, P.O., Prudencio, M., Gendron, T.F., Stetler, C.T., Yan, I.K.et al. (2013) Reduced C9orf72 gene expression in c9FTD/ALS is caused by histone trimethylation, an epigenetic event detectable in blood. Acta Neuropathol. 126, 895–905 10.1007/s00401-013-1199-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciura, S., Lattante, S., Le Ber, I., Latouche, M., Tostivint, H., Brice, A.et al. (2013) Loss of function of C9orf72 causes motor deficits in a zebrafish model of amyotrophic lateral sclerosis. Ann. Neurol. 74, 180–187 10.1002/ana.23946 [DOI] [PubMed] [Google Scholar]
- 32.Waite, A.J., Bäumer, D., East, S., Neal, J., Morris, H.R., Ansorge, O.et al. (2014) Reduced C9orf72 protein levels in frontal cortex of amyotrophic lateral sclerosis and frontotemporal degeneration brain with the C9ORF72 hexanucleotide repeat expansion. Neurobiol. Aging 35, 1779.e5–1779.e13 10.1016/j.neurobiolaging.2014.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi, Y., Lin, S., Staats, K.A., Li, Y., Chang, W.-H., Hung, S.-T.et al. (2018) Haploinsufficiency leads to neurodegeneration in C9ORF72 ALS/FTD human induced motor neurons. Nat. Med. 24, 313–325 10.1038/nm.4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooper, T.A., Wan, L. and Dreyfuss, G. (2009) RNA and disease. Cell 136, 777–793 10.1016/j.cell.2009.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Batra, R., Charizanis, K., Manchanda, M., Mohan, A., Li, M., Finn, D.J.et al. (2014) Loss of MBNL leads to disruption of developmentally regulated alternative polyadenylation in RNA-mediated disease. Mol. Cell 56, 311–322 10.1016/j.molcel.2014.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, K.-Y., Chang, H.-C., Seah, C. and Lee, L.-J. (2019) Deprivation of muscleblind-like proteins causes deficits in cortical neuron distribution and morphological changes in dendritic spines and postsynaptic densities. Front. Neuroanat. 13, 75 10.3389/fnana.2019.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li, L.-B., Yu, Z., Teng, X. and Bonini, N.M. (2008) RNA toxicity is a component of ataxin-3 degeneration in drosophila. Nature 453, 1107–1111 10.1038/nature06909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsoi, H. and Chan, H.Y.E. (2013) Expression of expanded CAG transcripts triggers nucleolar stress in huntington's disease. Cerebellum 12, 310–312 10.1007/s12311-012-0447-6 [DOI] [PubMed] [Google Scholar]
- 39.Sellier, C., Rau, F.E.D.E.R., Liu, Y., Tassone, F., Hukema, R.K., Gattoni, R.et al. (2010) Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients. EMBO J. 29, 1248–1261 10.1038/emboj.2010.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin, P., Duan, R., Qurashi, A., Qin, Y., Tian, D., Rosser, T.C.et al. (2007) Pur α binds to rCGG repeats and modulates repeat-mediated neurodegeneration in a drosophila model of fragile X tremor/Ataxia syndrome. Neuron 55, 556–564 10.1016/j.neuron.2007.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sofola, O.A., Jin, P., Qin, Y., Duan, R., Liu, H., de Haro, M.et al. (2007) RNA-Binding Proteins hnRNP A2/B1 and CUGBP1 suppress fragile X CGG premutation repeat-induced neurodegeneration in a drosophila model of FXTAS. Neuron 55, 565–571 10.1016/j.neuron.2007.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee, Y.-B., Chen, H.-J., Peres, J.N., Gomez-Deza, J., Attig, J., Štalekar, M.et al. (2013) Hexanucleotide repeats in ALS/FTD form length-dependent RNA foci, sequester RNA binding proteins, and are neurotoxic. Cell Rep. 5, 1178–1186 10.1016/j.celrep.2013.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cooper-Knock, J., Walsh, M.J., Higginbottom, A., Robin Highley, J., Dickman, M.J., Edbauer, D.et al. (2014) Sequestration of multiple RNA recognition motif-containing proteins by C9orf72 repeat expansions. Brain 137, 2040–2051 10.1093/brain/awu120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hautbergue, G.M., Castelli, L.M., Ferraiuolo, L., Sanchez-Martinez, A., Cooper-Knock, J., Higginbottom, A.et al. (2017) SRSF1-dependent nuclear export inhibition of C9ORF72 repeat transcripts prevents neurodegeneration and associated motor deficits. Nat. Commun 8, 16063 10.1038/ncomms16063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zu, T., Gibbens, B., Doty, N.S., Gomes-Pereira, M., Huguet, A., Stone, M.D.et al. (2011) Non-ATG-initiated translation directed by microsatellite expansions. Proc. Natl Acad. Sci. U.S.A. 108, 260–265 10.1073/pnas.1013343108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Todd, P.K., Oh, S.Y., Krans, A., He, F., Sellier, C., Frazer, M.et al. (2013) CGG Repeat-associated translation mediates neurodegeneration in fragile X tremor ataxia syndrome. Neuron 78, 440–455 10.1016/j.neuron.2013.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kearse, M.G., Green, K.M., Krans, A., Rodriguez, C.M., Linsalata, A.E., Goldstrohm, A.C.et al. (2016) CGG Repeat-Associated Non-AUG translation utilizes a Cap-Dependent scanning mechanism of initiation to produce toxic proteins. Mol. Cell 62, 314–322 10.1016/j.molcel.2016.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sellier, C., Buijsen, R.A.M., He, F., Natla, S., Jung, L., Tropel, P.et al. (2017) Translation of expanded CGG repeats into FMRpolyG Is pathogenic and may contribute to fragile X tremor ataxia syndrome. Neuron 93, 331–347 10.1016/j.neuron.2016.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mori, K., Weng, S.-M., Arzberger, T., May, S., Rentzsch, K., Kremmer, E.et al. (2013) The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science 339, 1335–1338 10.1126/science.1232927 [DOI] [PubMed] [Google Scholar]
- 50.Ash, P.E.A., Bieniek, K.F., Gendron, T.F., Caulfield, T., Lin, W.-L., DeJesus-Hernandez, M.et al. (2013) Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron 77, 639–646 10.1016/j.neuron.2013.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zu, T., Liu, Y., Baez-Coronel, M., Reid, T., Pletnikova, O., Lewis, J.et al. (2013) RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc. Natl Acad. Sci. U.S.A. 110, E4968–E4977 10.1073/pnas.1315438110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bañez-Coronel, M., Ayhan, F., Tarabochia, A.D., Zu, T., Perez, B.A., Tusi, S.K.et al. (2015) RAN translation in huntington disease. Neuron 88, 667–677 10.1016/j.neuron.2015.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwon, I., Xiang, S., Kato, M., Wu, L., Theodoropoulos, P., Wang, T.et al. (2014) Poly-dipeptides encoded by the C9orf72 repeats bind nucleoli, impede RNA biogenesis, and kill cells. Science 345, 1139–1145 10.1126/science.1254917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mizielinska, S., Gronke, S., Niccoli, T., Ridler, C.E., Clayton, E.L., Devoy, A.et al. (2014) C9orf72 repeat expansions cause neurodegeneration in drosophila through arginine-rich proteins. Science 345, 1192–1194 10.1126/science.1256800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boeynaems, S., Bogaert, E., Kovacs, D., Konijnenberg, A., Timmerman, E., Volkov, A.et al. (2017) Phase separation of C9orf72 dipeptide repeats perturbs stress granule dynamics. Mol. Cell 65, 1044–1045 10.1016/j.molcel.2017.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang, Y.-J., Gendron, T.F., Ebbert, M.T.W., O'Raw, A.D., Yue, M., Jansen-West, K.et al. (2018) Poly(GR) impairs protein translation and stress granule dynamics in C9orf72-associated frontotemporal dementia and amyotrophic lateral sclerosis. Nat. Med. 24, 1136–1142 10.1038/s41591-018-0071-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hao, Z., Liu, L., Tao, Z., Wang, R., Ren, H., Sun, H.et al. (2019) Motor dysfunction and neurodegeneration in a C9orf72 mouse line expressing poly-PR. Nat. Commun. 10, 2906–2911 10.1038/s41467-019-10956-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moens, T.G., Niccoli, T., Wilson, K.M., Atilano, M.L., Birsa, N., Gittings, L.M.et al. (2019) C9orf72 arginine-rich dipeptide proteins interact with ribosomal proteins in vivo to induce a toxic translational arrest that is rescued by eIF1A. Acta Neuropathol. 137, 487–500 10.1007/s00401-018-1946-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi, S.Y., Lopez-Gonzalez, R., Krishnan, G., Phillips, H.L., Li, A.N., Seeley, W.W.et al. (2019) C9ORF72-ALS/FTD-associated poly(GR) binds Atp5a1 and compromises mitochondrial function in vivo. Nat. Neurosci. 22, 851–862 10.1038/s41593-019-0397-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang, Y.-J., Gendron, T.F., Grima, J.C., Sasaguri, H., Jansen-West, K., Xu, Y.-F.et al. (2016) C9ORF72 poly(GA) aggregates sequester and impair HR23 and nucleocytoplasmic transport proteins. Nat. Neurosci. 19, 668–677 10.1038/nn.4272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee, Y.-B., Baskaran, P., Gomez-Deza, J., Chen, H.-J., Nishimura, A.L., Smith, B.N.et al. (2017) C9orf72 poly GA RAN-translated protein plays a key role in amyotrophic lateral sclerosis via aggregation and toxicity. Hum. Mol. Genet. 26, 4765–4777 10.1093/hmg/ddx350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schludi, M.H., Becker, L., Garrett, L., Gendron, T.F., Zhou, Q., Schreiber, F.et al. (2017) Spinal poly-GA inclusions in a C9orf72 mouse model trigger motor deficits and inflammation without neuron loss. Acta Neuropathol. 134, 241–254 10.1007/s00401-017-1711-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schludi, M.H., May, S., Grässer, F.A., Rentzsch, K., Kremmer, E., Küpper, C.et al. (2015) Distribution of dipeptide repeat proteins in cellular models and C9orf72 mutation cases suggests link to transcriptional silencing. Acta Neuropathol. 130, 537–555 10.1007/s00401-015-1450-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin, Y., Mori, E., Kato, M., Xiang, S., Wu, L., Kwon, I.et al. (2016) Toxic PR poly-Dipeptides encoded by the C9orf72 repeat expansion target LC domain polymers. Cell 167, 789–802.e12 10.1016/j.cell.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin, S., Lopez-Gonzalez, R., Kunz, R.C., Gangopadhyay, J., Borufka, C., Gygi, S.P.et al. (2017) Evidence that C9ORF72 dipeptide repeat proteins associate with U2 snRNP to cause mis-splicing in ALS/FTD patients. Cell Rep. 19, 2244–2256 10.1016/j.celrep.2017.05.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang, K., Donnelly, C.J., Haeusler, A.R., Grima, J.C., Machamer, J.B., Steinwald, P.et al. (2015) The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature 525, 56–61 10.1038/nature14973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boeynaems, S., Bogaert, E., Michiels, E., Gijselinck, I., Sieben, A., Jovičić, A.et al. (2016) Drosophila screen connects nuclear transport genes to DPR pathology in c9ALS/FTD. Sci. Rep. 6, 20877–8 10.1038/srep20877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker, C., Herranz-Martin, S., Karyka, E., Liao, C., Lewis, K., Elsayed, W.et al. (2017) C9orf72 expansion disrupts ATM-mediated chromosomal break repair. Nat. Neurosci. 20, 1225–1235 10.1038/nn.4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lopez-Gonzalez, R., Lu, Y., Gendron, T.F., Karydas, A., Tran, H., Yang, D.et al. (2016) Poly(GR) in C9ORF72-Related ALS/FTD compromises mitochondrial function and increases oxidative stress and DNA damage in iPSC-Derived motor neurons. Neuron 92, 383–391 10.1016/j.neuron.2016.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanekura, K., Yagi, T., Cammack, A.J., Mahadevan, J., Kuroda, M., Harms, M.B.et al. (2016) Poly-dipeptides encoded by the C9ORF72 repeats block global protein translation. Hum. Mol. Genet. 25, 1803–1813 10.1093/hmg/ddw052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hartmann, H., Hornburg, D., Czuppa, M., Bader, J., Michaelsen, M., Farny, D.et al. (2018) Proteomics and C9orf72 neuropathology identify ribosomes as poly-GR/PR interactors driving toxicity. Life Sci. Alliance 1, e201800070 10.26508/lsa.201800070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gupta, R., Lan, M., Mojsilovic-Petrovic, J., Choi, W.H., Safren, N., Barmada, S.et al. (2017) The proline/Arginine dipeptide from hexanucleotide repeat expanded C9ORF72 inhibits the proteasome. eNeuro 4, ENEURO.0249–16.2017 10.1523/ENEURO.0249-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo, Q., Lehmer, C., Martínez-Sánchez, A., Rudack, T., Beck, F., Hartmann, H.et al. (2018) In situ structure of neuronal C9orf72 poly-GA aggregates reveals proteasome recruitment. Cell 172, 696–705.e12 10.1016/j.cell.2017.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hinnebusch, A.G. (2017) Structural insights into the mechanism of scanning and start codon recognition inEukaryotic translation initiation. Trends Biochem. Sci. 42, 589–611 10.1016/j.tibs.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 75.Costello, J., Castelli, L.M., Rowe, W., Kershaw, C.J., Talavera, D., Mohammad-Qureshi, S.S.et al. (2015) Global mRNA selection mechanisms for translation initiation. Genome Biol 16, 10 10.1186/s13059-014-0559-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kozak, M. (1986) Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell 44, 283–292 10.1016/0092-8674(86)90762-2 [DOI] [PubMed] [Google Scholar]
- 77.Komar, A.A. and Hatzoglou, M. (2014) Cellular IRES-mediated translation. Cell Cycle 10, 229–240 10.4161/cc.10.2.14472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Filbin, M.E. and Kieft, J.S. (2009) Toward a structural understanding of IRES RNA function. Curr. Opin. Struct. Biol. 19, 267–276 10.1016/j.sbi.2009.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jackson, R.J., Hellen, C.U.T. and Pestova, T.V. (2010) The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 11, 113–127 10.1038/nrm2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peabody, D.S. (1989) Translation initiation at non-AUG triplets in mammalian cells. J. Biol. Chem. 264, 5031–5035 10.1016/S0021-9258(18)83694-8 [DOI] [PubMed] [Google Scholar]
- 81.Schwab, S.R., Shugart, J.A., Horng, T., Malarkannan, S. and Shastri, N. (2004) Unanticipated antigens: translation initiation at CUG with leucine. PLoS Biol. 2, e366 10.1371/journal.pbio.0020366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Starck, S.R., Jiang, V., Pavon-Eternod, M., Prasad, S., McCarthy, B., Pan, T.et al. (2012) Leucine-tRNA initiates at CUG start codons for protein synthesis and presentation by MHC class I. Science 336, 1719–1723 10.1126/science.1220270 [DOI] [PubMed] [Google Scholar]
- 83.Kearse, M.G. and Wilusz, J.E. (2017) Non-AUG translation: a new start for protein synthesis in eukaryotes. Genes Dev. 31, 1717–1731 10.1101/gad.305250.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nguyen, L., Cleary, J.D. and Ranum, L.P.W. (2019) Repeat-associated Non-ATG translation: Molecular mechanisms and contribution to neurological disease. Annu. Rev. Neurosci. 42, 227–247 10.1146/annurev-neuro-070918-050405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wolfe, A.L., Singh, K., Zhong, Y., Drewe, P., Rajasekhar, V.K., Sanghvi, V.R.et al. (2017) RNA G-quadruplexes cause eIF4A- dependent oncogene translation in cancer. Nature 513, 65–70 10.1038/nature13485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bordeleau, M.-E., Cencic, R., Lindqvist, L., Oberer, M., Northcote, P., Wagner, G.et al. (2006) RNA-mediated sequestration of the RNA helicase eIF4A by pateamine A inhibits translation initiation. Chem. Biol. 13, 1287–1295 10.1016/j.chembiol.2006.10.005 [DOI] [PubMed] [Google Scholar]
- 87.Cruz-Migoni, A., Hautbergue, G.M., Artymiuk, P.J., Baker, P.J., Bokori-Brown, M., Chang, C.-T.et al. (2011) A Burkholderia pseudomallei toxin inhibits helicase activity of translation factor eIF4A. Science 334, 821–824 10.1126/science.1211915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boussemart, L., Malka-Mahieu, H., Girault, I., Allard, D., Hemmingsson, O., Tomasic, G.et al. (2014) eIF4F is a nexus of resistance to anti-BRAF and anti-MEK cancer therapies. Nature 513, 105–109 10.1038/nature13572 [DOI] [PubMed] [Google Scholar]
- 89.Reddy, K., Zamiri, B., Stanley, S.Y.R., Macgregor, Jr, R.B. and Pearson, C.E. (2013) The disease-associated r(GGGGCC) nRepeat from the C9orf72Gene forms tract length-dependent Uni- and multimolecular RNA G-quadruplex structures. J. Biol. Chem. 288, 9860–9866 10.1074/jbc.C113.452532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blice-Baum, A.C. and Mihailescu, M.-R. (2014) Biophysical characterization of G-quadruplex forming FMR1 mRNA and of its interactions with different fragile X mental retardation protein isoforms. RNA 20, 103–114 10.1261/rna.041442.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Green, K.M., Glineburg, M.R., Kearse, M.G., Flores, B.N., Linsalata, A.E., Fedak, S.J.et al. (2017) RAN translation at C9orf72-associated repeat expansions is selectively enhanced by the integrated stress response. Nat. Commun. 8, 2005 10.1038/s41467-017-02200-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tabet, R., Schaeffer, L., Freyermuth, F., Jambeau, M., Workman, M., Lee, C.-Z.et al. (2018) CUG initiation and frameshifting enable production of dipeptide repeat proteins from ALS/FTD C9ORF72 transcripts. Nat. Commun. 9, 152–114 10.1038/s41467-017-02643-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dodd, D.W., Tomchick, D.R., Corey, D.R. and Gagnon, K.T. (2016) Pathogenic C9ORF72 antisense repeat RNA forms a double helix with tandem C:C mismatches. Biochemistry 55, 1283–1286 10.1021/acs.biochem.6b00136 [DOI] [PubMed] [Google Scholar]
- 94.Sun, Y., Atas, E., Lindqvist, L., Sonenberg, N., Pelletier, J. and Meller, A. (2012) The eukaryotic initiation factor eIF4H facilitates loop-binding, repetitive RNA unwinding by the eIF4A DEAD-box helicase. Nucleic Acids Res. 40, 6199–6207 10.1093/nar/gks278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harms, U., Andreou, A.Z., Gubaev, A. and Klostermeier, D. (2014) eIF4B, eIF4G and RNA regulate eIF4A activity in translation initiation by modulating the eIF4A conformational cycle. Nucleic Acids Res. 42, 7911–7922 10.1093/nar/gku440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.García-García, C., Frieda, K.L., Feoktistova, K., Fraser, C.S. and Block, S.M. (2015) RNA.BIOCHEMISTRY. Factor-dependent processivity in human eIF4A DEAD-box helicase. Science 348, 1486–1488 10.1126/science.aaa5089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sen, N.D., Zhou, F., Harris, M.S., Ingolia, N.T. and Hinnebusch, A.G. (2016) eIF4B stimulates translation of long mRNAs with structured 5′ UTRs and low closed-loop potential but weak dependence on eIF4G. Proc. Natl Acad. Sci. U.S.A. 113, 10464–10472 10.1073/pnas.1612398113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goodman, L.D., Prudencio, M., Srinivasan, A.R., Rifai, O.M., Lee, V.M.Y., Petrucelli, L.et al. (2019) eIF4B and eIF4H mediate GR production from expanded G4C2 in a drosophila model for C9orf72-associated ALS. Acta Neuropathol. Commun. 7, 62 10.1186/s40478-019-0711-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Linsalata, A.E., He, F., Malik, A.M., Glineburg, M.R., Green, K.M., Natla, S.et al. (2019) DDX3X and specific initiation factors modulate FMR1 repeat-associated non-AUG-initiated translation. EMBO Rep. 20, e47498 10.15252/embr.201847498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheng, W., Wang, S., Zhang, Z., Morgens, D.W., Hayes, L.R., Lee, S.et al. (2019) CRISPR-Cas9 Screens identify the RNA helicase DDX3X as a repressor of C9ORF72 (GGGGCC)n repeat-Associated Non-AUG translation. Neuron 104, 885–888 10.1016/j.neuron.2019.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hertz, M.I., Landry, D.M., Willis, A.E., Luo, G. and Thompson, S.R. (2013) Ribosomal protein S25 dependency reveals a common mechanism for diverse internal ribosome entry sites and ribosome shunting. Mol. Cell. Biol. 33, 1016–1026 10.1128/MCB.00879-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamada, S.B., Gendron, T.F., Niccoli, T., Genuth, N.R., Grosely, R., Shi, Y.et al. (2019) RPS25 is required for efficient RAN translation of C9orf72 and other neurodegenerative disease-associated nucleotide repeats. Nat. Neurosci. 22, 1383–1388 10.1038/s41593-019-0455-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheng, W., Wang, S., Mestre, A.A., Fu, C., Makarem, A., Xian, F.et al. (2018) C9ORF72 GGGGCC repeat-associated non-AUG translation is upregulated by stress through eIF2α phosphorylation. Nat. Commun. 9, 51–12 10.1038/s41467-017-02495-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ayhan, F., Perez, B.A., Shorrock, H.K., Zu, T., Bañez-Coronel, M., Reid, T.et al. (2018) SCA8 RAN polySer protein preferentially accumulates in white matter regions and is regulated by eIF3F. EMBO J. 37, 1–15 10.15252/embj.201899023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hashem, Y., Georges des, A., Dhote, V., Langlois, R., Liao, H.Y., Grassucci, R.A.et al. (2013) Hepatitis-C-virus-like internal ribosome entry sites displace eIF3 to gain access to the 40S subunit. Nature 503, 539–543 10.1038/nature12658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zu, T., Cleary, J.D., Liu, Y., Bañez-Coronel, M., Bubenik, J.L., Ayhan, F.et al. (2017) RAN translation regulated by muscleblind proteins in myotonic dystrophy type 2. Neuron 95, 1292–1295 10.1016/j.neuron.2017.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mills, J.D., Kavanagh, T., Kim, W.S., Chen, B.J., Kawahara, Y., Halliday, G.M.et al. (2013) Unique transcriptome patterns of the white and grey matter corroborate structural and functional heterogeneity in the human frontal lobe. PLoS ONE 8, e78480 10.1371/journal.pone.0078480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jazurek-Ciesiolka, M., Ciesiolka, A., Komur, A.A., Urbanek-Trzeciak, M.O., Krzyzosiak, W.J. and Fiszer, A. (2020) RAN translation of the expanded CAG repeats in the SCA3 disease context. J. Mol. Biol. 432, 166699 10.1016/j.jmb.2020.10.033 [DOI] [PubMed] [Google Scholar]
- 109.Sonobe, Y., Ghadge, G., Masaki, K., Sendoel, A., Fuchs, E. and Roos, R.P. (2018) Translation of dipeptide repeat proteins from the C9ORF72 expanded repeat is associated with cellular stress. Neurobiol. Dis. 116, 155–165 10.1016/j.nbd.2018.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Costa-Mattioli, M., Sossin, W.S., Klann, E. and Sonenberg, N. (2009) Translational control of long-lasting synaptic plasticity and memory. Neuron 61, 10–26 10.1016/j.neuron.2008.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bellato, H.M. and Hajj, G.N.M. (2016) Translational control by eIF2alpha in neurons: beyond the stress response. Cytoskeleton (Hoboken) 73, 551–565 10.1002/cm.21294 [DOI] [PubMed] [Google Scholar]
- 112.Barbosa, C., Peixeiro, I. and Romão, L. (2013) Gene expression regulation by upstream open reading frames and human disease. PLoS Genet. 9, e1003529 10.1371/journal.pgen.1003529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Holcik, M. and Sonenberg, N. (2005) Translational control in stress and apoptosis. Nat. Rev. Mol. Cell Biol. 6, 318–327 10.1038/nrm1618 [DOI] [PubMed] [Google Scholar]
- 114.Young, S.K., Baird, T.D. and Wek, R.C. (2016) Translation regulation of the glutamyl-prolyl-tRNA synthetase gene EPRS through bypass of upstream open reading frames with noncanonical initiation codons. J. Biol. Chem. 291, 10824–10835 10.1074/jbc.M116.722256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Westergard, T., McAvoy, K., Russell, K., Wen, X., Pang, Y., Morris, B.et al. (2019) Repeat-associated non-AUG translation in C9orf72-ALS/FTD is driven by neuronal excitation and stress. EMBO Mol. Med 11, 1–14 10.15252/emmm.201809423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pestova, T.V., de Breyne, S., Pisarev, A.V., Abaeva, I.S. and Hellen, C.U.T. (2008) eIF2-dependent and eIF2-independent modes of initiation on the CSFV IRES: a common role of domain II. EMBO J. 27, 1060–1072 10.1038/emboj.2008.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dmitriev, S.E., Terenin, I.M., Andreev, D.E., Ivanov, P.A., Dunaevsky, J.E., Merrick, W.C.et al. (2010) GTP-independent tRNA delivery to the ribosomal P-site by a novel eukaryotic translation factor. J. Biol. Chem. 285, 26779–26787 10.1074/jbc.M110.119693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Skabkin, M.A., Skabkina, O.V., Dhote, V., Komar, A.A., Hellen, C.U.T. and Pestova, T.V. (2010) Activities of ligatin and MCT-1/DENR in eukaryotic translation initiation and ribosomal recycling. Genes Dev. 24, 1787–1801 10.1101/gad.1957510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Starck, S.R., Tsai, J.C., Chen, K., Shodiya, M., Wang, L., Yahiro, K.et al. (2016) Translation from the 5′ untranslated region shapes the integrated stress response. Science 351, aad3867 10.1126/science.aad3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gendron, T.F., Bieniek, K.F., Zhang, Y.-J., Jansen-West, K., Ash, P.E.A., Caulfield, T.et al. (2013) Antisense transcripts of the expanded C9ORF72 hexanucleotide repeat form nuclear RNA foci and undergo repeat-associated non-ATG translation in c9FTD/ALS. Acta Neuropathol. 126, 829–844 10.1007/s00401-013-1192-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tran, H., Almeida, S., Moore, J., Gendron, T.F., Chalasani, U., Lu, Y.et al. (2015) Differential toxicity of nuclear RNA foci versus dipeptide repeat proteins in a drosophila model of C9ORF72 FTD/ALS. Neuron 87, 1207–1214 10.1016/j.neuron.2015.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gaspar, C., Jannatipour, M., Dion, P., Laganière, J., Sequeiros, J., Brais, B.et al. (2000) CAG tract of MJD-1 may be prone to frameshifts causing polyalanine accumulation. Hum. Mol. Genet. 9, 1957–1966 10.1093/hmg/9.13.1957 [DOI] [PubMed] [Google Scholar]
- 123.Toulouse, A., Au-Yeung, F., Gaspar, C., Roussel, J., Dion, P. and Rouleau, G.A. (2005) Ribosomal frameshifting on MJD-1 transcripts with long CAG tracts. Hum. Mol. Genet. 14, 2649–2660 10.1093/hmg/ddi299 [DOI] [PubMed] [Google Scholar]
- 124.Stochmanski, S.J., Therrien, M., Laganière, J., Rochefort, D., Laurent, S., Karemera, L.et al. (2012) Expanded ATXN3 frameshifting events are toxic in drosophila and mammalian neuron models. Hum. Mol. Genet. 21, 2211–2218 10.1093/hmg/dds036 [DOI] [PubMed] [Google Scholar]
- 125.Girstmair, H., Saffert, P., Rode, S., Czech, A., Holland, G., Bannert, N.et al. (2013) Depletion of cognate charged transfer RNA causes translational frameshifting within the expanded CAG stretch in huntingtin. Cell Rep. 3, 148–159 10.1016/j.celrep.2012.12.019 [DOI] [PubMed] [Google Scholar]
- 126.Saffert, P., Adamla, F., Schieweck, R., Atkins, J.F. and Ignatova, Z. (2016) An expanded CAG repeat in huntingtin causes +1 frameshifting. J. Biol. Chem. 291, 18505–18513 10.1074/jbc.M116.744326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Koide, R., Ikeuchi, T., Onodera, O., Tanaka, H., Igarashi, S., Endo, K.et al. (1994) Unstable expansion of CAG repeat in hereditary dentatorubral-pallidoluysian atrophy (DRPLA). Nat. Genet. 6, 9–13 10.1038/ng0194-9 [DOI] [PubMed] [Google Scholar]
- 128.Chandy, K.G., Fantino, E., Wittekindt, O., Kalman, K., Tong, L.L., Ho, T.H.et al. (1998) Isolation of a novel potassium channel gene hSKCa3 containing a polymorphic CAG repeat: a candidate for schizophrenia and bipolar disorder? Mol. Psychiatry 3, 32–37 10.1038/sj.mp.4000353 [DOI] [PubMed] [Google Scholar]
- 129.Hsing, A.W., Chokkalingam, A.P., Gao, Y.-T., Wu, G., Wang, X., Deng, J.et al. (2002) Polymorphic CAG/CAA repeat length in the AIB1/SRC-3 gene and prostate cancer risk: a population-based case-control study. Cancer Epidemiol. Biomarkers Prev. 11, 337–341 PMID: [PubMed] [Google Scholar]
- 130.La Spada, A.R., Wilson, E.M., Lubahn, D.B., Harding, A.E. and Fischbeck, K.H. (1991) Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352, 77–79 10.1038/352077a0 [DOI] [PubMed] [Google Scholar]
- 131.Orr, H.T., Chung, M.Y., Banfi, S., Kwiatkowski, T.J., Servadio, A., Beaudet, A.L.et al. (1993) Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat. Genet. 4, 221–226 10.1038/ng0793-221 [DOI] [PubMed] [Google Scholar]
- 132.Pulst, S.M., Nechiporuk, A., Nechiporuk, T., Gispert, S., Chen, X.N., Lopes-Cendes, I.et al. (1996) Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat. Genet. 14, 269–276 10.1038/ng1196-269 [DOI] [PubMed] [Google Scholar]
- 133.Scoles, D.R., Ho, M.H.T., Dansithong, W., Pflieger, L.T., Petersen, L.W., Thai, K.K.et al. (2015) Repeat associated Non-AUG translation (RAN translation) dependent on sequence downstream of the ATXN2 CAG repeat. PLoS ONE 10, e0128769 10.1371/journal.pone.0128769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kawaguchi, Y., Okamoto, T., Taniwaki, M., Aizawa, M., Inoue, M., Katayama, S.et al. (1994) CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat. Genet. 8, 221–228 10.1038/ng1194-221 [DOI] [PubMed] [Google Scholar]
- 135.Zhuchenko, O., Bailey, J., Bonnen, P., Ashizawa, T., Stockton, D.W., Amos, C.et al. (1997) Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat. Genet. 15, 62–69 10.1038/ng0197-62 [DOI] [PubMed] [Google Scholar]
- 136.Lindblad, K., Savontaus, M.L., Stevanin, G., Holmberg, M., Digre, K., Zander, C.et al. (1996) An expanded CAG repeat sequence in spinocerebellar ataxia type 7. Genome Res. 6, 965–971 10.1101/gr.6.10.965 [DOI] [PubMed] [Google Scholar]
- 137.Zühlke, C.H., Spranger, M., Spranger, S., Voigt, R., Lanz, M., Gehlken, U.et al. (2003) SCA17 caused by homozygous repeat expansion in TBP due to partial isodisomy 6. Eur. J. Hum. Genet. 11, 629–632 10.1038/sj.ejhg.5201018 [DOI] [PubMed] [Google Scholar]
- 138.LaCroix, A.J., Stabley, D., Sahraoui, R., Adam, M.P., Mehaffey, M., Kernan, K.et al. (2019) GGC repeat expansion and exon 1 methylation of XYLT1 Is a common pathogenic variant in Baratela-Scott syndrome. Am. J. Hum. Genet. 104, 35–44 10.1016/j.ajhg.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Crisponi, L., Deiana, M., Loi, A., Chiappe, F., Uda, M., Amati, P.et al. (2001) The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat. Genet. 27, 159–166 10.1038/84781 [DOI] [PubMed] [Google Scholar]
- 140.Cortese, A., Simone, R., Sullivan, R., Vandrovcova, J., Tariq, H., Yau, W.Y.et al. (2019) Biallelic expansion of an intronic repeat in RFC1 is a common cause of late-onset ataxia. Nat. Genet. 51, 649–658 10.1038/s41588-019-0372-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mundlos, S., Otto, F., Mundlos, C., Mulliken, J.B., Aylsworth, A.S., Albright, S.et al. (1997) Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell 89, 773–779 10.1016/S0092-8674(00)80260-3 [DOI] [PubMed] [Google Scholar]
- 142.Amiel, J., Laudier, B., Attié-Bitach, T., Trang, H., de Pontual, L., Gener, B.et al. (2003) Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat. Genet. 33, 459–461 10.1038/ng1130 [DOI] [PubMed] [Google Scholar]
- 143.Ishiura, H., Doi, K., Mitsui, J., Yoshimura, J., Matsukawa, M.K., Fujiyama, A.et al. (2018) Expansions of intronic TTTCA and TTTTA repeats in benign adult familial myoclonic epilepsy. Nat. Genet. 50, 581–590 10.1038/s41588-018-0067-2 [DOI] [PubMed] [Google Scholar]
- 144.Hagerman, R.J., Leehey, M., Heinrichs, W., Tassone, F., Wilson, R., Hills, J.et al. (2001) Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology 57, 127–130 10.1212/WNL.57.1.127 [DOI] [PubMed] [Google Scholar]
- 145.Hagerman, P.J. and Hagerman, R.J. (2015) Fragile X-associated tremor/ataxia syndrome. Ann. N.Y. Acad. Sci. 1338, 58–70 10.1111/nyas.12693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sherman, S.L. (2000) Premature ovarian failure in the fragile X syndrome. Am. J. Med. Genet. 97, 189–194 [DOI] [PubMed] [Google Scholar]
- 147.Buijsen, R.A.M., Visser, J.A., Kramer, P., Severijnen, E.A.W.F.M., Gearing, M., Charlet-Berguerand, N.et al. (2016) Presence of inclusions positive for polyglycine containing protein, FMRpolyG, indicates that repeat-associated non-AUG translation plays a role in fragile X-associated primary ovarian insufficiency. Hum. Reprod. 31, 158–168 10.1093/humrep/dev280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Parrish, J.E., Oostra, B.A., Verkerk, A.J., Richards, C.S., Reynolds, J., Spikes, A.S.et al. (1994) Isolation of a GCC repeat showing expansion in FRAXF, a fragile site distal to FRAXA and FRAXE. Nat. Genet. 8, 229–235 10.1038/ng1194-229 [DOI] [PubMed] [Google Scholar]
- 149.Debacker, K., Winnepenninckx, B., Longman, C., Colgan, J., Tolmie, J., Murray, R.et al. (2007) The molecular basis of the folate-sensitive fragile site FRA11A at 11q13. Cytogenet. Genome Res. 119, 9–14 10.1159/000109612 [DOI] [PubMed] [Google Scholar]
- 150.Nancarrow, J.K., Kremer, E., Holman, K., Eyre, H., Doggett, N.A., Le Paslier, D.et al. (1994) Implications of FRA16A structure for the mechanism of chromosomal fragile site genesis. Science 264, 1938–1941 10.1126/science.8009225 [DOI] [PubMed] [Google Scholar]
- 151.Wieben, E.D., Aleff, R.A., Tosakulwong, N., Butz, M.L., Highsmith, W.E., Edwards, A.O.et al. (2012) A common trinucleotide repeat expansion within the transcription factor 4 (TCF4, E2-2) gene predicts fuchs corneal dystrophy. PLoS ONE 7, e49083 10.1371/journal.pone.0049083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Soragni, E., Petrosyan, L., Rinkoski, T.A., Wieben, E.D., Baratz, K.H., Fautsch, M.P.et al. (2018) Repeat-Associated Non-ATG (RAN) translation in fuchs’ endothelial corneal dystrophy. Invest. Ophthalmol. Vis. Sci. 59, 1888–1896 10.1167/iovs.17-23265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Goodman, F.R., Bacchelli, C., Brady, A.F., Brueton, L.A., Fryns, J.P., Mortlock, D.P.et al. (2000) Novel HOXA13 mutations and the phenotypic spectrum of hand-foot-genital syndrome. Am. J. Hum. Genet. 67, 197–202 10.1086/302961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Brown, S.A., Warburton, D., Brown, L.Y., Yu, C.Y., Roeder, E.R., Stengel-Rutkowski, S.et al. (1998) Holoprosencephaly due to mutations in ZIC2, a homologue of Drosophila odd-paired. Nat. Genet. 20, 180–183 10.1038/2484 [DOI] [PubMed] [Google Scholar]
- 155.Holmes, S.E., O'Hearn, E., Rosenblatt, A., Callahan, C., Hwang, H.S., Ingersoll-Ashworth, R.G.et al. (2001) A repeat expansion in the gene encoding junctophilin-3 is associated with Huntington disease-like 2. Nat. Genet. 29, 377–378 10.1038/ng760 [DOI] [PubMed] [Google Scholar]
- 156.Jones, C., Penny, L., Mattina, T., Yu, S., Baker, E., Voullaire, L.et al. (1995) Association of a chromosome deletion syndrome with a fragile site within the proto-oncogene CBL2. Nature 376, 145–149 10.1038/376145a0 [DOI] [PubMed] [Google Scholar]
- 157.Mahadevan, M., Tsilfidis, C., Sabourin, L., Shutler, G., Amemiya, C., Jansen, G.et al. (1992) Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science 255, 1253–1255 10.1126/science.1546325 [DOI] [PubMed] [Google Scholar]
- 158.Liquori, C.L., Ricker, K., Moseley, M.L., Jacobsen, J.F., Kress, W., Naylor, S.L.et al. (2001) Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science 293, 864–867 10.1126/science.1062125 [DOI] [PubMed] [Google Scholar]
- 159.Sone, J., Mitsuhashi, S., Fujita, A., Mizuguchi, T., Hamanaka, K., Mori, K.et al. (2019) Long-read sequencing identifies GGC repeat expansions in NOTCH2NLC associated with neuronal intranuclear inclusion disease. Nat. Genet. 51, 1215–1221 10.1038/s41588-019-0459-y [DOI] [PubMed] [Google Scholar]
- 160.Yuan, Y., Liu, Z., Hou, X., Li, W., Ni, J., Huang, L.et al. (2020) Identification of GGC repeat expansion in the NOTCH2NLC gene in amyotrophic lateral sclerosis. Neurology 95, e3394–e3405 10.1212/WNL.0000000000010945 [DOI] [PubMed] [Google Scholar]
- 161.Brais, B., Bouchard, J.P., Xie, Y.G., Rochefort, D.L., Chrétien, N., Tomé, F.M.et al. (1998) Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nat. Genet. 18, 164–167 10.1038/ng0298-164 [DOI] [PubMed] [Google Scholar]
- 162.Délot, E., King, L.M., Briggs, M.D., Wilcox, W.R. and Cohn, D.H. (1999) Trinucleotide expansion mutations in the cartilage oligomeric matrix protein (COMP) gene. Hum. Mol. Genet. 8, 123–128 10.1093/hmg/8.1.123 [DOI] [PubMed] [Google Scholar]
- 163.Matsuura, T., Yamagata, T., Burgess, D.L., Rasmussen, A., Grewal, R.P., Watase, K.et al. (2000) Large expansion of the ATTCT pentanucleotide repeat in spinocerebellar ataxia type 10. Nat. Genet. 26, 191–194 10.1038/79911 [DOI] [PubMed] [Google Scholar]
- 164.Holmes, S.E., O'Hearn, E.E., McInnis, M.G., Gorelick-Feldman, D.A., Kleiderlein, J.J., Callahan, C.et al. (1999) Expansion of a novel CAG trinucleotide repeat in the 5′ region of PPP2R2B is associated with SCA12. Nat. Genet. 23, 391–392 10.1038/70493 [DOI] [PubMed] [Google Scholar]
- 165.Sato, N., Amino, T., Kobayashi, K., Asakawa, S., Ishiguro, T., Tsunemi, T.et al. (2009) AR TICLESpinocerebellar ataxia type 31 is associated with ‘Inserted’ penta-nucleotide repeats containing (TGGAA). Am. J. Hum. Genet. 85, 544–557 10.1016/j.ajhg.2009.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kobayashi, H., Abe, K., Matsuura, T., Ikeda, Y., Hitomi, T., Akechi, Y.et al. (2011) REPOR t expansion of intronic GGCCTG hexanucleotide repeatin NOP56 causes SCA36, a type of spinocerebellar ataxia accompanied by motor neuron involvement. Am. J. Hum. Genet. 89, 121–130 10.1016/j.ajhg.2011.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Todd, T.W., McEachin, Z.T., Chew, J., Burch, A.R., Jansen-West, K., Tong, J.et al. (2020) Hexanucleotide repeat expansions in c9FTD/ALS and SCA36 confer selective patterns of neurodegeneration In vivo. Cell Rep. 31, 107616 10.1016/j.celrep.2020.107616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Seixas, A.I., Loureiro, J.R., Costa, C., Ordóñez-Ugalde, A., Marcelino, H., Oliveira, C.L.et al. (2017) A pentanucleotide ATTTC repeat insertion in the non-coding region of DAB1, mapping to SCA37, causes spinocerebellar ataxia. Am. J. Hum. Genet. 101, 87–103 10.1016/j.ajhg.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Akarsu, A.N., Stoilov, I., Yilmaz, E., Sayli, B.S. and Sarfarazi, M. (1996) Genomic structure of HOXD13 gene: a nine polyalanine duplication causes synpolydactyly in two unrelated families. Hum. Mol. Genet. 5, 945–952 10.1093/hmg/5.7.945 [DOI] [PubMed] [Google Scholar]
- 170.Bragg, D.C., Mangkalaphiban, K., Vaine, C.A., Kulkarni, N.J., Shin, D., Yadav, R.et al. (2017) Disease onset in X-linked dystonia-parkinsonism correlates with expansion of a hexameric repeat within an SVA retrotransposon in TAF1. Proc. Natl Acad. Sci. U.S.A. 114, E11020–E11028 10.1073/pnas.1712526114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kato, M., Das, S., Petras, K., Kitamura, K., Morohashi, K.-I., Abuelo, D.N.et al. (2004) Mutations of ARX are associated with striking pleiotropy and consistent genotype-phenotype correlation. Hum. Mutat. 23, 147–159 10.1002/humu.10310 [DOI] [PubMed] [Google Scholar]
- 172.Strømme, P., Mangelsdorf, M.E., Shaw, M.A., Lower, K.M., Lewis, S.M.E., Bruyere, H.et al. (2002) Mutations in the human ortholog of aristaless cause X-linked mental retardation and epilepsy. Nat. Genet. 30, 441–445 10.1038/ng862 [DOI] [PubMed] [Google Scholar]
- 173.Laumonnier, F., Ronce, N., Hamel, B.C.J., Thomas, P., Lespinasse, J., Raynaud, M.et al. (2002) Transcription factor SOX3 is involved in X-linked mental retardation with growth hormone deficiency. Am. J. Hum. Genet. 71, 1450–1455 10.1086/344661 [DOI] [PMC free article] [PubMed] [Google Scholar]