Abstract
Hydrogels prepared via self-assembly offer scalable and tunable platforms for drug delivery applications. Molecular-scale self-assembly leverages an interplay of attractive and repulsive forces; drugs and other active molecules can be incorporated into such materials by partitioning in hydrophobic domains, affinity-mediated binding, or covalent integration. Peptides have been widely used as building blocks for self-assembly due to facile synthesis, ease of modification with bioactive molecules, and precise molecular-scale control over material properties through tunable interactions. Additional opportunities are manifest in stimuli-responsive self-assembly for more precise drug action. Hydrogels can likewise be fabricated from macromolecular self-assembly, with both synthetic polymers and biopolymers used to prepare materials with controlled mechanical properties and tunable drug release. These include clinical approaches for solubilization and delivery of hydrophobic drugs. To further enhance mechanical properties of hydrogels prepared through self-assembly, recent work has integrated self-assembly motifs with polymeric networks. For example, double-network hydrogels capture the beneficial properties of both self-assembled and covalent networks. The expanding ability to fabricate complex and precise materials, coupled with an improved understanding of biology, will lead to new classes of hydrogels specifically tailored for drug delivery applications.
Keywords: Biomaterials, Molecular Engineering, Peptide Self-Assembly, Supramolecular Chemistry, Block Copolymers
1. Introduction
A number of hydrogel biomaterials have been explored for various applications in tissue engineering, regenerative medicine, and drug delivery.[1, 2] Hydrogels are commonly used in a variety of biomedical applications, including as topical or oral drug formulations, injectable drug depots, in situ forming biomaterials, and contact lenses.[3-6] This class of materials is characterized by an ability to imbibe water or physiologic fluid in quantities which may be upwards of 1000 times the dry weight of the material.[6] Many common hydrogels are constructed from hydrophilic polymers which are held together by some type of crosslinking to yield a percolated network.[7] Of the different modes of crosslinking, chemical crosslinks are characterized by typically permanent, non-reversible covalent bonds between polymer chains. Meanwhile, physical crosslinks are characterized by transient and reversible interactions which give rise hydrogel networks often exhibiting these same dynamic properties.[8, 9] Regardless of the specific mode of crosslinking, the material comprising the hydrogel is itself rendered insoluble; this is in spite of its construction from highly soluble polymeric precursors as well as typically a high degree of favorable hydrogen bonding or ion-dipole interactions between water and the polymer backbone.
Due to their highly hydrated and porous architecture, these materials have been extensively explored for the encapsulation and controlled release of therapeutic drugs and proteins. Both small molecules and macromolecules can be modified with a range of chemistries that enable interaction with drugs, either through covalent linkages or non-covalent binding. The delivery of drugs can be controlled using strategies which leverage diffusion, swelling, and/or erosion-based release mechanisms.[10, 11] The soft and compliant mechanics of hydrogels, combined with their extensive hydration, enables uses in soft tissue regeneration as cell-supporting scaffolds which match the typical mechanics of native tissue and enable facile nutrient and waste transport.[12-14] These features are often enhanced by strategies to facilitate minimally invasive (e.g., syringe-injectable) administration through in situ gelation mechanisms or mechanically responsive and self-healing interactions.[15-18] In their applications for drug delivery, it may furthermore be desirable for these hydrogels to respond to environmental stimuli with changes in their chemical, microscopic, or bulk mechanical properties.[19-21]
The phenomenon of self-assembly, defined as the autonomous organization of components into patterns or structures without intervention, is a powerful tool to create a diversity of functional materials.[22-24] Examples from the living world support the many uses of self-assembly as a bio-inspired design tool.[25, 26] A variety of biomaterials have thus been designed to leverage self-assembly phenomena in their formation and stability.[27-29] While a subset of self-assembling biomaterials include specific motifs rooted in supramolecular chemistry which give rise to molecular-scale organization,[30] non-specific hydrophobic association is the prevailing underlying mechanism by which order is realized for self-assembly in a water environment. The use of hydrophobic association as a directive cue in the formation of self-assembled hydrogels is primarily an entropically driven phenomenon. Whereas the dispersal of typically non-polar hydrophobic building blocks may have long-range impact on the mobility and free orientation of bulk water molecules, the association of these same building blocks so as to limit the surface area available for unfavorable contact with the bulk water phase affords significant increase in solvent entropy.[31, 32] Hydrophobic domains within hydrogels can thus serve a dual role to both stabilize the network, while offering a favorable environment to solvate and sequester hydrophobic drugs. This same phenomenon underlies many structures in nature; the lipid bilayer of a cell forms a semi-permeable barrier through the association of long aliphatic tails and outward solvent presentation of polar/charged head-groups, while the native tertiary structures of many proteins arise spontaneously and reliably in part by preferentially concealing amino acids with non-polar side-chains. It is furthermore important to note that self-assembly is a dynamic and concentration-driven phenomenon. Accordingly, many building blocks which are designed to self-assemble exhibit concentration-dependent association, whereby assemblies arise above a critical concentration (e.g., a critical micellar concentration). Self-assembled building blocks also exhibit dynamic exchange between associated and free conformations, the nature of which is often tunable as a function of molecular design or environmental parameters such as temperature, pH, or osmolarity. Importantly, the spontaneous and non-covalent nature of self-assembly typically enables reversibility and “healing” of these interactions in response to a disruptive force.
Self-assembly is a useful tool for forming hydrogels. Utilizing self-assembly for the non-covalent organization of small molecules generates hydrogels which exhibit traits consistent with a physically crosslinked network. Namely, these materials form by reversible and dynamic associations of certain constituents, allowing the material to respond to mechanical forces by reorganizing to dissipate stress, and to self-heal in response to destructive mechanical insult. The transition from liquid to gel can be done using only chemical moieties found on native biomolecules, and doesn’t require unnatural functional groups. In certain cases, self-assembly drives formation of high aspect-ratio structures, which themselves can physically entangle and/or bundle to form hydrogel networks. Herein, a variety of different self-assembly strategies are discussed which have used molecular and/or macromolecular building blocks to create hydrogels for drug delivery. Routes based on molecular-scale self-assembly have been explored from both hydrophobic association and/or supramolecular interactions to yield hydrogels prepared from molecular or oligopeptides building blocks. Oligopeptides in particular offer opportunities to include enzyme-responsive functionality in their use for drug delivery. Macromolecular building blocks such as block (co)polymers and proteins have also been designed to self-assemble and form hydrogels through hydrophobic interactions, which in many cases are also supplemented by supramolecular or electrostatic interactions. For added control of properties, the covalent character of self-assembled materials can be subsequently modulated. One such route would seek to couple both self-assembled and covalent networks, wherein covalent bonding imbues a hydrogel with elasticity and resistance to permanent deformation, while dynamic non-covalent bonds increase hydrogel toughness and allow for energy dissipation within the material. With these many varied approaches, new materials have been realized which afford function as new tools for drug delivery as well as enable more accurate structural mimicry of viscoelastic biological matrices.
2. Self-Assembly of Low Molecular Weight Gelators
The controlled assembly of small molecules proceeds through non-covalent intermolecular interactions between adjacent molecules.[33] In many systems the concentration of a gelator is less than 10 milligrams per gram of water (<1% by weight).[34] A variety of intermolecular forces have been explored to drive association of molecules, and the balance of these interactions serves to not only dictate the properties of the assemblies but also their functional utility in the context of both sequestering and releasing bioactive molecules.
2.1. Motifs in Small-Molecule and Peptide Self-Assembly
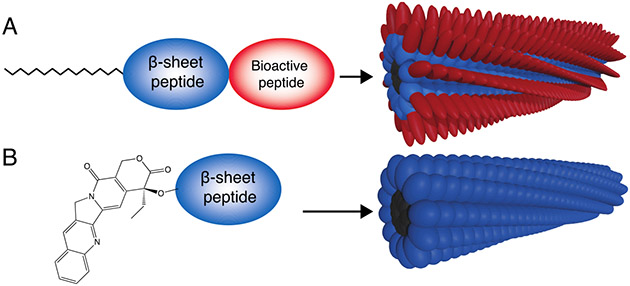
Small molecule self-assembly is often driven through the incorporation of groups which initiate hydrophobic association in aqueous environments and, in doing so, effectively reduce the entropic penalty entailed in adopting defined conformations (Figure 1).[35] Hydrophobic modification of peptides and other biomolecules is used in a native biological context to control their circulation half-life and localization.[36] Some of these moieties, such as palmitoyl groups, have been used to drive self-assembly of synthetic small molecules.[37] For instance, peptide amphiphiles are a class of supramolecular polymers in which an alkyl tail is appended to a peptide, giving rise to high aspect-ratio assemblies which form physically entangled hydrogel networks under physiological conditions.[37] Other hydrophobic moieties, such as fluorenylmethyloxycarbonyl (Fmoc) protecting groups common in peptide synthesis, can also be easily added to small molecules to induce self-assembly.[38] Finally, hydrophobic drugs may be covalently coupled to the peptide to induce hydrophobic collapse while also possessing therapeutic capabilities.[39]
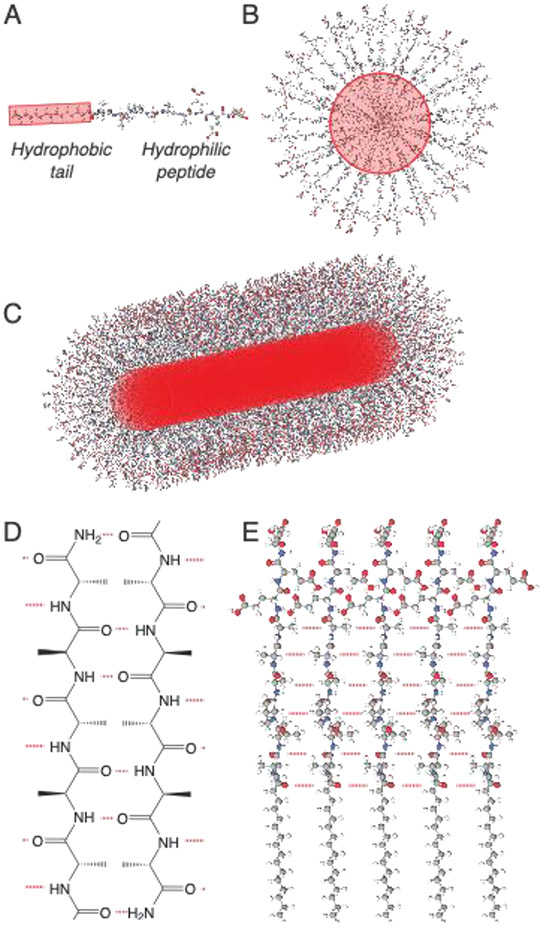
Figure 1:
(A) Non-polar motifs can be coupled to peptides to (B) drive self-assembly through hydrophobic association. (C) Directional non-covalent interactions between adjacent molecules, such as the formation of β-sheet hydrogen bonding networks, drives the formation of high-aspect ratio nanostructures. (D) Peptides can form ordered arrangements of hydrogen bonds (in red), most often resembling β-sheet secondary structures, by positioning adjacent proton donors and acceptors on their amide backbone. (E) Hydrogen bond networks drive axial organization are are a common driving force for the formation of high-aspect ratio nanostructures.
Beyond hydrophobic interactions, non-covalent hydrogen bonds, which typically form between a carbonyl group and a hydrogen coupled to either a nitrogen or oxygen, are also useful in driving self-assembly in an aqueous environment. Hydrogen bonding is especially prevalent in self-assembly arising from peptides due to the presence of hydrogen bond donors and acceptors in the peptide backbone (Figure 1D).[40] Unlike hydrophobic interactions, hydrogen bonds are directional and are often a primary driving force underlying the formation of high-aspect ratio structures from molecular self-assembly.[41] Fortunately, a common secondary structure found in proteins, the β-sheet, features extensive hydrogen bonding between adjacent strands. As such, β-sheet interactions are often incorporated into self-assembling peptides, serving to template axial assembly into high aspect-ratio structures (Figure 1E).[40, 42, 43]
Another directional non-covalent force which is common in molecular self-assembly is the π-π stacking of aromatic groups. This special class of electrostatic interaction arises most commonly from conjugated ring structures which are hydrophobic, enabling these motifs to serve a dual role by both inducing hydrophobic association in water and driving axial assembly through stacking with adjacent moieties.[44] Aromaticity can be added through the use of specific amino acids, including phenylalanine,[45] as well as with non-peptidic motifs including Fmoc protecting groups,[38] to aromatic fluorophores,[46] and hydrophobic drugs (Figure 2).[39]
Figure 2:
(A) Peptides can be modified with hydrophobic aromatic groups both as terminal prosthetic groups and through the inclusion of aromatic amino acids like phenylalanine. (B) This design enables directional π-π stacking, (C) leading to axial organization and nanofibril formation.
Though many chemistries have been used to develop self-assembling small molecule gelators, peptides are pervasive in the use of such systems for drug delivery.[47] Peptides are composed of amino acids which offer both a diversity of chemical functionalities to drive self-assembly as well assembling the opportunity to encode biological functionality in the peptide sequence. For these reasons, peptides offer many advantages from a pharmaceutical, drug delivery, and biomaterials perspective. Peptides are also synthesized by economical automated methodologies to yield high purity products with minimal synthetic expertise required.[48] There are 20 canonical amino acids found in nature, yet solid-phase peptide synthesis is compatible with an array of non-natural amino acids and functional groups to enable stimuli-responsiveness or afford sites for specific bioconjugation.[49] The backbone of peptides contains repeating amides, each of which can participate in two hydrogen bonds, once as an acceptor and once as a donor. Interestingly, converting the amide bond to a urea increases backbone rigidity and hydrogen bond propensity and leads to a significant increase in mechanical properties of materials prepared from self-assembling peptide gelators.[50] While individual hydrogen bonds are weak, peptides offer a very high density of hydrogen bonds; proteinaceous materials in nature such as silk fibroin[51] and collagen[52] offer inspirational examples for the exceptional mechanical properties possible from hydrogen bonding of a peptide backbone.
Peptides undergo proteolytic degradation into their amino acid substituents; as these are already present in the body, degradation typically does not yield toxic side products. Many self-assembling peptides do not generate a significant immune response,[53] and are primarily excreted through kidney clearance.[54] Since proteins and peptides are involved in many natural biological processes, peptide-based biomaterials can be harnessed to recreate such functions through binding proteins or activating cell surface receptors.[55] Peptides are also the substrates for a variety of enzymes, including proteases which cleave specific peptide sequences,[56] or kinases which transfer phosphate groups to hydroxyl-containing amino acids.[57]
2.2. Mechanisms in Small-Molecule and Peptide Hydrogel Formation
Self-assembling molecules can undergo gelation, transitioning from a solution to a solid hydrogel. Early hydrogel theory described the behavior of highly hydrated covalently crosslinked polymer networks which do not flow once equilibrated.[58] Hydrogel networks featuring only covalent crosslinks are elastic and the mechanical properties are not typically dependent upon shear rate.[59] Non-covalent self-assembled hydrogels are both capable of flowing under high shear (e.g., shear-thinning) and generally have an observed storage modulus (stiffness) which is non-linear and increases with oscillatory frequency.[60] For these systems, rheological gelation is often defined as the point at which the measure of “elastic” portion of the modulus (G’, storage modulus) is greater than the viscous component (G”, loss modulus).[61] The specific values for storage and loss moduli will depend upon both the magnitude and frequency at which the strain is applied. However, a gel should have a storage modulus that is greater than the loss modulus for a range of frequencies relevant to biomedical applications, such as 0.1-100 Hz. Furthermore, the strain at which rheology is performed should be within the linear viscoelastic region to ensure mechanical properties of the hydrogel are not compromised during measurement.
Hydrogels typically arise in molecular-scale self-assembly through one of two routes. In the first route, small molecules or oligomers transition into larger elongated aggregates known as supramolecular polymers which form entanglements and contribute to a network with elastic behavior.[62] The second route by which gelation is induced arises from an increase interactions between nanostructures which lead to an enhancement in the density of physical crosslinks and often drive increased nanostructure bundling.[63] In both of these gelation mechanisms, forces within and between self-assembled structures becomes more attractive (or less repulsive). Often, such a phenomenon is induced by modulating charge states, either by adjusting the pH or by adding counterions, such as calcium, to screen repulsive electrostatic interactions (Figure 3). Interactions between self-assembled nanostructures can be tuned at the molecular level through controlling surface charge,[64, 65] introducing host–guest interactions between fibers,[66] or adding multivalent binding groups to non-covalently crosslink multiple fibers simultaneously.[67]
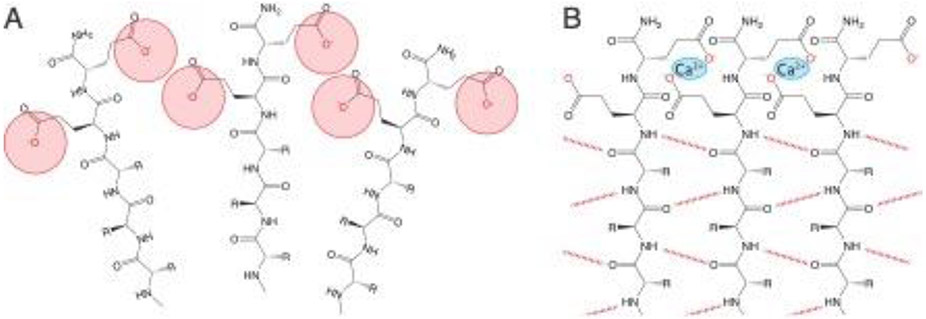
Figure 3:
(A) Self-assembling peptides typically contain hydrophilic domains comprised of charged amino acids, leading to electrostatic repulsion which may disfavor self-assembly under physiological conditions. (B) These repulsive forces can be overcome to drive self-assembly and/or stabilize assembled nanostructures by changing pH or adding ions, like calcium, to screen and/or bridge charged groups.
The pH of the body is typically around 7.4, though there are regions where pH may be significantly higher or lower. pH can also be dependent on specific pathologies , as a hallmark of cancer is an increase in metabolic activity which can decrease the local pH below 7.0.[68] Accordingly, pH presents a useful and biologically relevant trigger to induce gelation. Molecules which are designed to be sensitive to a relevant pH change often present functional groups with pKa values near those of the physiological scenario where gelation is desired. The side-chain of the amino acid histidine, for example, is mostly uncharged at physiological pH, but becomes positively charge as pH drops below 6. This has been used to induce a transition from sheet-like nanostructures at pH 4.5 to short, twisted fibers at pH 7.4.[69] Another key challenge in drug delivery arises in transitioning therapeutic payloads from the extracellular space into the cytoplasm, and this often requires escape from an endosome/lysosome.[70] In one example, a β-sheet forming peptide coupled to an oligoarginine was designed to be in an unassembled monomeric state at pH 7 but form nanostructures in the acidic pH of the lysosome to promote intracellular escape.[71]
2.3. Controlling Hydrogel Properties in Self-Assembling Peptides
Self-assembly results from a balance of both attractive and repulsive intermolecular forces. Amphiphilic molecules that lack axial directives such as hydrogen bonding or π-π stacking will form a variety of nanostructures, from spherical micelles to sheets, depending on the ratio of their hydrophilic and hydrophobic components.[72] For most systems, the nanostructure will depend upon the molecular architecture and composition of the molecule. The alkyl tails on peptide amphiphiles are capable of crystallizing within nanostructures,[73] which promotes the formation planar structures, while β-sheets are naturally curved,[74] and have a propensity to form cylindrical micelles. Supramolecular peptide assemblies prepared from shorter peptide domains are more likely to form flat or belt-like structures,[75, 76] while those with longer β-sheet sequences typically form cylindrical micelles.[73]
The structure of amphiphilic peptide gelators can also be tuned by modifying the length and order of amino acids in the β-sheet domain, while keeping the composition the same. Since β-sheets are naturally curved, molecules with a higher propensity to form β-sheets typically result in cylindrical assemblies in which the β-sheets twist around a central axis, while those in which the β-sheet domains are weaker form flat structures.[77-79] The resulting nanostructure has also been controlled through incorporation of multiple hydrophobic components within a single nanostructure. This approach has been utilized in peptide-drug conjugates, for example by placing between 1-4 camptothecin groups per molecule as a way to both modulate the drug loading capacity and afford control of nanostructure from filaments, to fibers, to tubes.[80] In self-assembling systems which don’t rely on hydrophobic domains, hierarchical structures can form in which the twisted β-sheets stack to form high aspect-ratio fibers.[81] While most self-assembled structures form on the nanometer length scale, a challenge in the field of biomaterials is to recapitulate the function of human tissues which have structures organized across multiple length scales.[82, 83] Heating peptide amphiphile solutions can lead to the formation of highly aligned monodomain gels by strengthening β-sheet interactions and inducing liquid-crystalline behavior and nanostructure bundling;[84] these massively aligned fibrillar hydrogels are able to guide the growth of encapsulated neurons.[85]
A benefit of using peptides is that the common β-sheet secondary structures offers extensive intermolecular hydrogen bonding between adjacent strands. As a result, many self-assembled peptide systems have rheological properties that are surprisingly stiff, with storage moduli that can exceed 100 kPa for gels that are less than 2% peptide by weight.[73] This affords a variety of different features which can be tailored to modify the mechanical properties of hydrogels arising from self-assembled peptide materials. The control of self-assembled structures can lead to a diversity of morphologies which can then influence the mechanical properties of a material,[86] its cellular or biologic response,[87] or even its in vivo circulation time.[88] Even within a specific morphology, the mechanical properties can be tuned through molecular design. The number of hydrogen bonds between peptides will largely depend upon the length of the peptide sequence, with each additional amino acid being capable of providing two hydrogen bonds (Figure 5). The β-sheets themselves can have varying degrees of twisting within nanostructures, which can lead to sub-optimal hydrogen bond distances and hydrogels with lower storage moduli.[73] In another example, native chemical ligation has been used to form covalent bonds between self-assembling peptides in situ, leading to a five-fold increase in hydrogel stiffness.[89] Independent of added bioactivity, self-assembled hydrogels exhibit “plug flow” which shields cells from shear forces experienced during injection or other high-shear operations, improving cell viability.
Figure 5:
Self-assembled nanostructures are able to sequester drugs through (A) encapsulation due to hydrophobic partitioning or (B) covalent conjugation to the peptide sequence.
2.4. Peptide Self-Assembly in Drug Delivery
Among the simplest ways to endow self-assembling peptide materials with therapeutic action is to use peptide sequences which can signal cells (Figure 4A). Supramolecular peptide hydrogels can thus be modified with amino acid sequences that improve cell adhesion,[90-93] mimic growth factors,[94, 95] selectively differentiate stem cells,[96] inhibit angiogenesis,[97] act as an immune adjuvant,[98] bind growth factors,[99] or provide anti-inflammatory signals.[100] The process of making materials via self-assembly affords an added benefit of co-assembling multiple bioactive peptides into a single nanofiber,[101, 102] while the spacing of these presented cues can be controlled to improve selectivity in binding to cell-surface proteins.[103] Peptide self-assembly has been used to present epitopes at defined density to tune both T-cell response[104] and humoral antibody response.[105] The surface of peptide nanofibers can also be conjugated to full length protein antigens.[106]
Figure 4:
Bioactivity can be incorporated into self-assembled hydrogels by (A) the addition of cell-signaling peptides on the exterior of nanostructures or (B) the inclusion of binding sequences for bioactive proteins.
Protein-mimetic oligopeptide sequences typically have inferior binding compared to the use of full-length proteins.[107] However, supramolecular systems can compensate for this effect by leveraging polyvalent ligand display with tunable and high density.[91, 103] The presentation of bioactive sequences on an assembled nanostructure, which dictates the efficiency of binding, can also be controlled by changing of the length and flexibility of the linker.[108] Ligand concentration and orientation are thus tunable parameters that can control cell signaling.[109]
The hydrophobic domains present in many self-assembled peptides can serve a dual role by both inducing self-assembly and acting to solubilize and/or sequester hydrophobic drugs (Figure 5A).[110] Compared to direct drug conjugation, this approach is compatible with the delivery of unmodified drugs and does not require the drug possess specific chemical groups amenable to functionalization. The chemotherapeutic camptothecin has been loaded into self-assembling peptides, where the nanofiber-encapsulated drug was found to inhibit tumor growth.[111] Low molecular weight peptide gelators featuring Fmoc protecting groups and a single phenylalanine were used to deliver the non steroidal anti-inflammatory drug diclofenac.[112] Self-assembling peptides that do not contain hydrophobic protecting groups, such as the β-hairpin peptide assemblies, have also been used as injectable depots for the delivery of hydrophobic drugs like curcumin.[113] More recently, multifunctional delivery systems have been reported, for example by encapsulating the anti-cancer drug paclitaxel in the interior of nanofibers while presenting cell-targeting sequences on the periphery of the assembly.[114]
Non-covalent encapsulation of drugs within self-assembled peptides via hydrophobic partitioning has challenges in terms of encapsulation of drug and leakage once introduced to the body, where the drug may be just as likely to partition into other hydrophobic regions.[115] Covalently coupling a drug to the gelator provides a useful way to obtain consistent loading and ensure that the drug remains stably encapsulated in the self-assembled delivery material (Figure 5B). Gelator design is a crucial feature in this regard, as the release of a hydrolyzable drug from a peptide assembly was found to be dependent on the location within the nanostructure in which the drug was tethered.[116] The loading efficiency is also readily tuned through controlling the number of drugs coupled per molecule. Self-assembling nanofibers have been made from gelators with up to four camptothecin units per monomer.[117] This general strategy to design self-assembling systems which contain drugs as explicit components of their design has been termed as “one component nanomedicine.”[118]
The percolated mesh arising from self-assembled nanostructures can also be used for controlled release of encapsulated proteins or other macromolecular payloads. For instance, a hydrogel explored for the repair of periodontal bone defects released was designed to release both stromal cell derived factor-1 (SDF-1) to attract stem cells and bone morphogenetic protein 2 (BMP-2) to differentiate these cells into osteoblasts.[119] Generally, the hydrogels which arise from peptide self-assembly are capable of tunable protein release, with rates controlled by varying the concentration of the hydrogel.[120] An alternate route to the controlled release of biological drugs has sought to present binding sequences on the surface of assemblies. This approach can be tailored towards a specific biomedical application, and binding sequences have been explored for active agents including BMP-2,[121] fibroblast growth factor (FGF),[122] and transforming growth factor beta-2 (TGF-β2).[123] These bioactive factors do not have to directly bind to a signal presented directly on the assembly, as several designs have utilized an intermediate tether to simultaneously bind both the self-assembling peptide and the molecule of interest. For instance, tetravalent streptavidin can bind biotin molecules presented from a self-assembled nanostructure and appended to biomolecules.[124] Many biomolecules of interest have native binding sites for biopolymers, like heparin. Accordingly, nanofibers have been designed to bind heparin, which can then in turn bind growth factors including vascular endothelial growth factor (VEGF) and FGF for their prolonged release from a hydrogel.[125-127] Release systems have also been designed in which proteins are covalently tethered to self-assembling peptide, which can be fabricated into gradients within the hydrogel.[128]
2.5. Stimuli-Responsive Self-Assembly for Drug Delivery
The delivery of active therapeutics often leverages pharmacologically inactive prodrugs with sensitivity to a particular stimulus in order to trigger therapeutic activation at the desired site of drug action.[129] The mechanism by which this transformation occurs depends on the linkage used, with the availability of such linkages dependent on the presence of certain functional groups on the therapeutic, and require specific conditions to cleave this covalent bond. For instance, pH responsive linkers have been incorporated to release drugs at acidic pH in several systems.[130] Disulfide bonds are also common in prodrug designs, since this bond is stable in the extracellular space, but is ruptured through disulfide reduction in the cytoplasm due to millimolar quantities of glutathione.[131] Reduction-sensitive bonds have thus been used in a variety of self-assembled delivery systems wherein cleavage of the disulfide bond induces disassembly of the hydrogel or coverts a pro-drug to the active form upon internalization into the cell.[80, 132, 133]
A key goal of drug delivery is to increase the targeting specificity to desired cells or tissues within the heterogenous body environment. Targeted cells may differ in many features, including size, shape, metabolic rate, and/or protein expression. Enzyme expression is particularly useful as a trigger for drug delivery as enzymes-catalyze chemical reactions can be utilized to locally release bioactive payloads or convert non-active molecules to active drugs.[134] Enzymes catalyze a range of reactions, many of which have been used to both convert a pro-drug to its active form, as well as modulate the properties of the self-assembled systems. In one example ascorbyl palmitate, which is generally regarded as safe (GRAS) by the FDA, forms self-assembled hydrogels in which the corticosteroid dexamethasone can be loaded.[135] These gels are degraded by enzymes released as part of inflammatory bowel disease, locally releasing the drug to reduce inflammation. Esterases have been leveraged as the target stimuli to release the non-steroidal anti-inflammatory drug naproxen from self-assembling peptide amphiphile nanofibers.[136]
2.6. Enzyme-Responsive Self-Assembly for Drug Delivery
A benefit of molecular self-assembly is that modification at a single site can trigger significant nanostructural transformations. Enzyme-induced self-assembly (EISA) often utilizes phosphatases in their role as biological catalysts to convert charged phosphate-containing amino acids to uncharged alcohols.[137] Drug-containing low molecular weight gelators have the added benefit that enzymatic catalysis rates can depend upon the self-assembled structure, with molecules in the assembled state being shielded from enzymatic activity.[138] Self-assembling peptide systems have therefore been designed as substrates for both phosphates and kinases, which convert alcohols to phosphates. These systems undergo enzyme-reversible sol-gel transformations in the presence of physiologically relevant enzymes.[139] One phosphatase, alkaline phosphatase (ALP), has also been used to simultaneously trigger gelation and induce chemiluminescence in a dual-responsive system.[140] Immune cell-secreted protein tyrosine kinase (PTK) has also been used to locally deliver tacrolimus, a drug used to prevent organ rejection after transplantation (Figure 6).[141]
Figure 6:
(A) Self-assembling peptides can be designed as substrates for cell-secreted enzymes such as kinases, (B) which enables these enzymes to be cues for disassembly and drug release from peptide-based carriers. This figure illustrates concepts from reference 129.
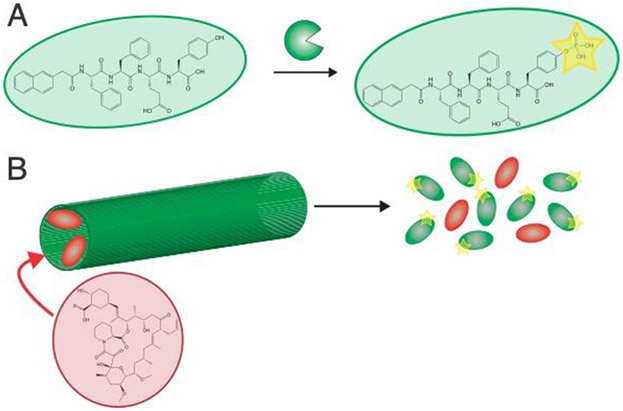
Proteases are subset of enzymes that cleave peptide bonds and constitute a class with over 580 members.[56] These proteins play important roles in many physiological processes, including cell migration,[142] tissue regeneration,[143] and cancer progression.[144] MMP-2, for example, is over-expressed in a variety of cancers,[145] and peptide sequences containing MMP-2 substrates have been incorporated into several self-assembling peptide systems to afford either proteolytically induced degradation[146, 147] or conversion from high aspect-ratio nanofibers into micelles.[87] The rate at which proteases hydrolyze peptide bonds depends on the peptide sequence, and incorporating specific peptides can both dictate the proteases which cleave the sequence and tune the degradation rate.[148] Supramolecular chemistry offers another level of control, as using the same sequence in different self-assembled geometries significantly influences enzyme kinetics.[149, 150] Proteolytic degradation of MMP-2 sensitive peptide have been used to deliver the chemotherapeutic agents cisplatin,[151] paclitaxel,[152] and release growth factors to improve the regeneration of dental tissue (Figure 7).[153] Interestingly, the enzyme enteropeptidase has been used to remove a branch from a self-assembling peptide which converts the self-assembled structure from spherical micelles to a nanofibrillar hydrogel.[154] A challenge in using protease-sensitive linkers to covalently couple drugs to self-assembling moieties is that most substrate sequences will leave some amino acids attached the drug after cleavage. To overcome this challenge and realize the release of the authentic and unmodified drugs, self-immolative linkers have been developed such that a proteolytic cleavage initializes a reaction which releases a drug coupled to the molecule by a primary amine.[155] For example, a tri-phenylalanine peptide gelator was designed with a boronic acid-phenyl group on the N-terminus such that removal of the boronic acid from the aromatic ring by hydrogen peroxide cleaved the phenyl ester from the N-terminus, leading to dissolution of the hydrogel.[156] Hydrogen peroxide can be generated in situ with localized glucose oxidase such that these hydrogels disassemble in the presence of glucose.
Figure 7:
(A) Self-assembling peptides can be mixed with biopolymers and biopolymer-binding growth factors. (B) Self-assembly of these components creates protein-loaded nanofibrous hydrogels. (C) These peptides contain a substrate for MMP-2, with this protease promoting hydrogel degradation and growth factor release. This figure illustrates concepts from reference 138.
3. Self-Assembly of Macromolecular Gelators
Macromolecules, typically consisting of polymeric or biopolymeric constructs, offer an alternate design paradigm by which to achieve self-assembling building blocks for the creation of hydrogels. The design space for macromolecular gelators is quite broad and largely under-explored, owing to the plethora of synthetic approaches and commercially available reagents for polymerization, as well as a variety of bio-derived or bio-inspired sources of natural biopolymeric materials. Herein, we describe generally the creation and use of hydrogels based on self-assembling macromolecules for various possible applications in drug delivery.
3.1. Mechanisms and Motifs in Synthetic Polymer Self-Assembly
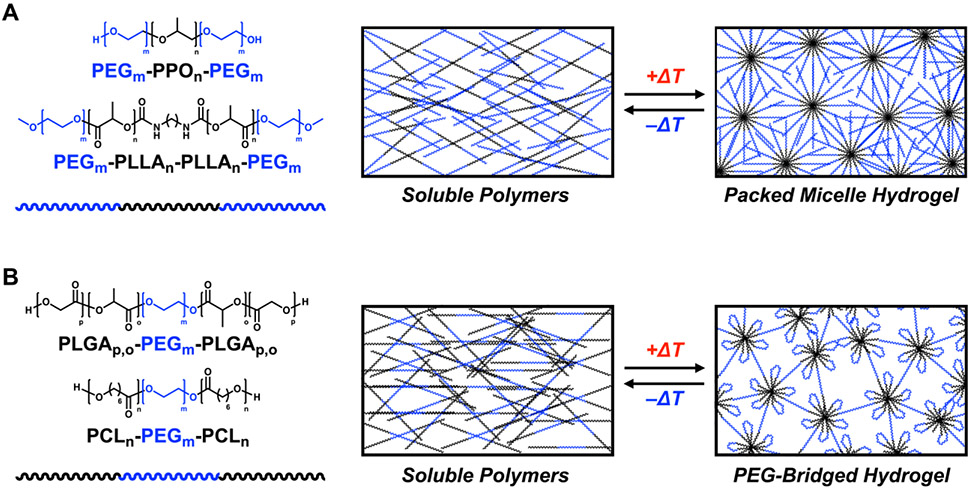
While various synthetic polymers have been explored for the design of self-assembled materials (e.g., micelles) and hydrogels, the majority fall into the category of block copolymers which bear hydrophilic and hydrophobic blocks.[157-163] A prevailing class of block copolymers with a long history of biomedical and pharmaceutical use is poly(ethylene glycol)-b-poly(propylene oxide)-b-poly(ethylene glycol) triblock copolymers (PEG-PPO-PEG), marketed as Pluronic® or Poloxamer® surfactants (Figure 10A).[164, 165] Different variations of PEG-PPO-PEG copolymers are used for an assortment of biomedical applications including as solubility enhancers or as stabilizers for protein drugs, earning these a place on the US FDA GRAS list for their role as excipients. A key benefit of PEG-PPO-PEG materials is temperature-dependent micelle formation, with the PPO mid-block desolvating and transitioning from hydrophilic to hydrophobic at temperatures experienced when traversing a transition from ambient to physiologic conditions.[166, 167] Above a critical concentration of ~15-20 wt% in water, these thermally induced micelles can pack to form hydrogel networks (Figure 8A).[168, 169] At lower concentrations, these micelles can still be induced to form temperature-responsive hydrogels by synthetic end-modification with recognition motifs to facilitate micelle crosslinking.[170, 171] Pluronics have been modified with a variety of polymer functionalizations to control both their thermoresponsive behavior and drug release. Poly(PEG/PPG/PCL urethane) materials were shown to have thermogelling behavior above 3 wt% and sustain paclitaxel delivery for several weeks.[172] Other modifications, including poly(polytetrahydrofuran carbonate) blocks[173] and polyhedral oligomeric silsesquioxane (POSS) blocks[174] indicate that pluronic-based hydrogels are a robust platform whose properties can be tailored with a variety of polymer chemistries.
Figure 10:
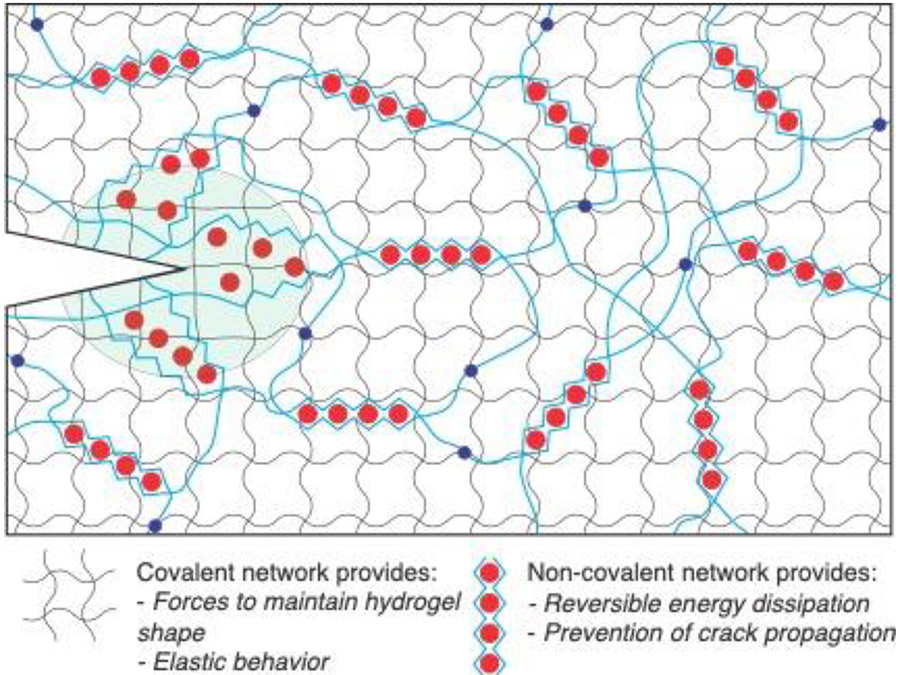
Hydrogel networks with coupled covalent and non-covalent interactions have exceptional mechanical properties, including stretchability and fracture toughness. Figure based on concepts from reference 352.
Figure 8:
(A) Example of thermoresponsive ABA block copolymer gelators which feature two hydrophilic PEG blocks flanking a midblock prepared from a polymer which transitions to hydrophobic according to a temperature stimulus. Shown are the structures of PEG-PPO-PEG and PEG-PLLA-PEG copolymers. These polymers transition from a soluble sol state to a hydrogel state characterized by packed micelle structures. (B) Example of thermoresponsive BAB block copolymer gelators which feature a PEG midblock flanked by two blocks prepared from a polymer which transitions to hydrophobic according to a temperature stimulus. Shown are the structures of PLGA-PEG-PLGA and PCL-PEG-PCL copolymers. These copolymers transition from a soluble sol state to a hydrogel state characterized by self-associating aggregates of the ‘A’ blocks which are bridged by soluble PEG chains.
A related family of block copolymers explored for hydrogel formation and drug delivery are PEG/polyesters, and like with the Pluronics these materials also frequently take the form of an ABA triblock copolymer (Figure 8A).[175] Early work on self-assembling hydrogel variants of these materials for uses in drug delivery explored diblock and triblock copolymers prepared from a hydrophilic PEO block and a hydrophobic poly-L-lactic acid (PLLA) block.[176] Unlike with the Pluronics, these PEG-PLLA copolymers are a sol at an elevated temperatures of 45°C where they can be loaded with drug, and immediately gel upon introduction into the reduced temperature of the body. Features of this system, including the balance and lengths of the PEG and PLLA blocks and the hydrophobicity of the hydrophobic polyester block were found to be useful parameters to tune the sol-gel transition temperature.[177, 178] A similar design replacing the polyester mid-block with polylactide (PLA) has been shown,[179] along with others replacing the hydrophobic mid-block with poly(D,L-lactide-co-glycolide) (PLGA).[180] The hydrophobic block can also be comprised of poly(ε-caprolactone) (PCL) which can be combined with PEG to form diblock or PEG-PCL-PEG triblock copolymers.[181-183] In each of these systems, gelation arises from close packing of micelles which assemble due to temperature-governed hydrophobicity of the polyester mid-blocks upon heating, with some variations even able to achieve a sol-gel transition upon reaching body temperature. Self-assembling PEG/polyester diblock copolymer hydrogels, which typically arise from packing of PEG-coated micelles, have also been extensively explored for applications in drug delivery.[184, 185]
Another PEG/polyester block copolymer motif has explored BAB triblock designs where instead two hydrophobic blocks flank a hydrophilic PEO mid-block (Figure 8B). Designs based on this general motif include PLGA-PEG-PLGA triblocks.[186, 187] This motif self-assembles via the formation of close-packed bridged micelles upon temperature-induced increase in hydrophobicity of the PLGA blocks, with associated PLGA chains forming the micelle core and PEG mid-blocks bridging these aggregates to form a network. The PLGA-PEG-PLGA design results in hydrogel formation which is very sensitive to the chemistry of the terminal groups (e.g., hydroxyl, acetyl, propionate, or butanoyl) on the PLGA blocks.[188] PLGA-PEG-PLGA hydrogelation is also sensitive to the block ratio, and mixtures of copolymers with different blocks can be prepared to control the gelation temperature.[189] These systems also exhibit gelation which which can be controlled by stereocomplexation of triblocks prepared from different enantiomeric hydrophobic blocks.[190] PCL-PEG-PCL triblocks have also been reported to form thermo-sensitive hydrogels.[191] In this case, the design based on PCL-PEG-PCL results in ~100x increase in the storage modulus (G’) of the hydrogels compared to PEG-PCL-PEG designs due to the altered network topology which arises from intra-micellar association of PCL end blocks and micelle bridging by PEG chains.[182, 191]
Polymers based on poly(N-isopropylacrylamide) (NIPAAm) constitute another commonly explored class of synthetic polymeric materials which have been used to create hydrogels via temperature-induced self-assembly.[192] This polymer has a particularly well-defined lower critical solution temperature (LCST) wherein it undergoes a hydrophilic to hydrophobic transition upon heating from (nominally) ambient to physiologic temperatures, though the precise transition temperature can be tuned by copolymerization and control of polymer molecular weight.[193] Accordingly, this polymer has been routinely used for numerous biomedical applications as a component of thermosensitive materials and coatings.[194, 195] Though a majority of uses for NIPAAm have explored this moiety within crosslinked polymer networks to afford temperature-responsive swelling, systems arising from self-assembly have also been reported. For example, copolymerization of NIPAAm with acrylic acid monomers succeeded in fusing hydrophilic carboxylate groups with NIPAAM to achieve thermoresponsive hydrogels via physical entanglements of aggregated structures above the LCST.[196] Subsequent work instead copolymerized NIPAAm with propylacrylic acid and enabled pH-responsive gelation owing to the elevated pKa for the propylacrylic acid sidechain relative to acrylic acid.[197]
Self-assembled hydrogels have also been prepared via interactions between synthetic polymers and colloidal particles, as well as from electrostatic interactions among networks of colloidal particles themselves.[198-201] Many examples in this class have relied on inorganic nanoparticles which either interact with other similar colloids to form networks or are fused by surface-adsorbed polymers bridging multiple colloids; the reader is encouraged to also explore this body of literature. In the specific context of amphiphilic macromolecules which self-assemble in water, a class of polymer-colloid materials has been created from PEG-PLA block co-polymer assemblies through interaction with surface-adsorbed hydrophobically modified polymers.[202] In this system, crosslinking of PEG-PLA assemblies by bridging of a modified cellulose polymer requires a strong interaction greater than or equal to that of thermal fluctuation (i.e., kT), while the creation of a percolated network requires polymer persistence length in excess of the particle diameter to enable the polymer to bridge ≥2 particles rather than wrapping around a single particle. The hydrophobic association in this system can be replaced with electrostatic associations between polymer and PEG-PLA colloid to also achieve a hydrogel.[203] A related class of materials has been created from cylodextrin-mediated bridging of PEG-PLA diblock co-polymer assemblies.[204]
A broad array of hydrogels have also been prepared from ordered supramolecular interactions and crosslinking within hydrogel networks.[8, 9] Supramolecular interactions of this sort form spontaneously, driven by a mixture of hydrophobic interactions, hydrogen bonding, and ion-dipole interactions. Synthetic polymers can thus be designed to present supramolecular motifs to facilitate dynamic associations which serve to crosslink a percolated hydrogel network. The nature of these supramolecular interactions means that they are equilibrium-governed, concentration-driven, and temperature-dependent and can furthermore be engineered to respond to a variety of stimuli.[205-208] Thus, when these motifs are present at concentrations around or above the KD for the motif being used, the spontaneous organization of these motifs yields a dynamic physical crosslink that can give rise to a hydrogel network. For example, multi-arm PEG macromers and other synthetic polymers have been modified with macrocyclic host molecules and their corresponding guests to form dynamic points of crosslinking in a hydrogel.[209-217] Similarly, multi-dentate hydrogen bonding motifs which form complementary or self-complementary interactions can also be incorporated within a synthetic polymer backbone or at its termini to facilitate crosslinking via motif dimerization.[218] The design of such polymeric materials from a varied and extensive number of supramolecular motifs could be the topic of its own review of this sort; the reader is encouraged to explore the number of reviews cited herein for more detailed discussion of supramolecular hydrogels prepared from synthetic polymers and specifically designed recognition motifs.
3.2. Drug Delivery from Synthetic Macromolecular Hydrogels
There are specific considerations for this class of synthetic macromolecular materials which are important to keep in mind when envisioning their use in drug delivery applications. It has been thought desirable to use designs which afford in situ gelation for ease of injection-based administration; this avoids the high pressure or large needle required to extrude a pre-formed hydrogel through a syringe. In this regard, temperature, pH, or osmolarity are useful triggers to promote a rapid hydrophobic transition once the polymer is introduced into the body.[163] The kinetics of gelation for an in situ triggered process introduce risk of a burst release if the delivered payload is not encapsulated quickly enough by the hydrogelation event. In the case where hydrogelation is triggered upon delivery to the body, the often high molecular weight of many macromolecular precursors may still present a high viscosity sol which may prove difficult to integrate with facile injection-based administration. Finally, wherein small molecule gelators afford a discrete molecular entity, synthetic polymeric building blocks are characterized by some degree of molecular weight dispersity which can lead to batch-dependent variability and may complicate regulatory approval in terms of the thorough characterization required for both the desired polymer and its degradation products.
There are many examples of synthetic block-copolymer hydrogels for applications in drug delivery. The Pluronic/Poloxamer class of block copolymers has seen extensive use in the context of drug delivery applications.[219, 220] While in their more dilute form these polymers serve the role of excipients to stabilize and solubilize pharmaceutical and biopharmaceutical agents, gels prepared from these materials have also been explored. Applications have included the use of injectable thermosensitive hydrogels for localized release of bioactive proteins from within the hydrated polymer mesh of the hydrogel,[221, 222] as well as release of hydrophobic antibiotic and chemotherapeutic drugs from within hydrophobic micellar cores of the hydrogel network.[223-225] The rapid formation of these gels upon introduction in the body has also been explored clinically, for example, using and FDA-approved Poloxamer-407 gel marketed as LeGoo® to achieve rapid clampless vascular occlusion during surgery.[226] Variants of these materials have shown bioactivity on their own; Vepoloxamer is a purified Poloxamer-188 which has been explored for a variety of clinical applications due to its anti-inflammatory and cytoprotective function resulting from native activation of tissue plasminogen.[227] Materials based on dynamic crosslinking of Pluronic micelles also have been used as easily injectable hydrogel depots which can encapsulate a payload of interest for controlled release,[171] or alternatively localize systemically administered small molecule drugs to the hydrogel site through drug affinity for a presented macrocycle.[170]
Hydrogels prepared from PEG/polyester ABA block copolymers have also been extensively explored for applications in drug delivery.[228] PEG-PLGA-PEG has been shown to be a useful hydrogel depot for controlled release, persisting for a tunable timeframe of up to 2 months upon subcutaneous injection.[229, 230] As an injectable depot, the release of both small molecule drugs and larger therapeutic proteins has been explored,[231] with the controlled delivery of TGF-β demonstrating enhanced wound healing.[232] The delivery of plasmid DNA has also been explored with this thermoresponsive hydrogel platform.[233] Compared to Pluronic hydrogels, which typically exhibit short tissue retention and rapid clearance, technology based on PEG-PLGA-PEG offers a more sustained presence in tissue.[234] Systems based on PLGA-PEG-PLGA and related BAB polyesters have also been used to deliver a variety of small molecule and protein drugs, with these systems typically encapsulating a hydrophobic drug within the associated its phase and proteins within its hydrated mesh.[235-238] One particular example of clinical use is found in a product, OngoGel™, which was explored in clinical trials for the delivery of paclitaxel to inoperable solid tumors.[239]
Hydrogels prepared from polymer-mediated crosslinking of self-assembled PEG-PLA diblock copolymers have likewise been explored for drug delivery applications. Multi-modal drug release is possible from such a system, wherein a protein encapsulated within the hydrophilic mesh and a small molecule encapsulated within the PLA core can be simultaneously released with differential rates.[202] The polymers used to create these nanoparticle assemblies can also be varied to control release rate of encapsulated therapeutics.[240] These materials have been explored for applications as shear-thinning supports for injectable delivery of cells in tissue engineering applications,[241-243] as well being applied to tissue in order to prevent pericardial adhesions following surgical intervention.[244] Recent work has furthermore explored the release of chemotactic proteins and immune-stimulating adjuvants for applications in immune cell recruitment and vaccination.[245-248]
Hydrogels formed through spontaneous recognition of supramolecular motifs appended from synthetic polymers also offer a useful approach to the creation of injectable biomaterials and drug delivery devices.[249, 250] These materials afford three-dimensional networks which can encapsulate and control the release of bioactive macromolecules, with the porosity offering a size-selective control over release.[251] The nature of these dynamic supramolecular recognition groups may themselves give rise to specific function, with the release of an encapsulated model macromolecule therapeutic and the rate of immune cell infiltration following injection both dictated by the dynamics of supramolecular crosslink exchange in these materials.[215] Likewise, the dynamic and reversible formation of supramolecular interactions enables synthetic hydrogels to be injected by minimally invasive catheter delivery into deep tissue sites such as the myocardium, where interactions can rapidly self-heal to afford tissue retention and controlled release for bioactive signals encapsulated within the gel.[252-255]
3.3. Mechanisms and Motifs in Biomacromolecular Self-Assembly
Nature achieve remarkable structure and concomitant function through organized assemblies of biological macromolecules. Proteins constitute a prime example of self-assembling building blocks found in nature, underlying both structure and function in the formation of extracellular matrices and cytoskeletal components. In this regard, naturally sourced collagen is one of the most commonly explored biomacromolecular hydrogels for applications in tissue engineering and drug delivery.[256] Type-1 collagen has a primary sequence that consists predominantly of tripeptide repeats of Proline–Hydroxyproline–Glycine, enabling the extended chains to present an interface which can register with two other primary sequences to form triple-helical bundles.[257] Isolates of native type-1 collagen also self-assemble into triple-helical fibrils which further bundle to form long fibers of 12-120 nm in diameter and which then entangle to form a physically crosslinked hydrogel network upon heating. This gel formation is typically irreversible on relevant timescales with temperature cycling. However, thermoresponsive collagen hydrogels can be realized by methacrylation of lysine side-chains in the protein to enable hydrogels which form at physiological temperatures and revert to a sol upon cooling.[258] Efforts to engineer synthetic replicates of collagen structures from synthesized or recombinant building blocks have likewise been explored for the creation collagen-mimetic assemblies which can give rise to self-assembled fibrils that bundle to form hydrogels.[259, 260]
Silk is another self-assembling protein which has been extensively explored as hydrogels for biomedical applications.[261, 262] Silk is typically derived from the cocoons of the Bombyx mori silkworm, though spider silk and recombinant silk proteins have also been explored. Like with collagen, silk also arises from primary chains with significantly repeating amino acid sequences which encodes efficient β-sheet formation between chains.[263] These β-sheets form highly aligned domains yielding a crystalline character which contributes to silk having a remarkable combination of strength and toughness.[264] The crystalline nature of these β-sheet assemblies makes silk typically insoluble in water, and thus the process of making hydrogels from silk protein extracts can be challenging. However, with control over the concentration, temperature, pH, and osmolarity of silk processing, hydrogels can be realized by enabling the random coil to β-sheet transition in the protein to be controlled.[265, 266] The high stability and crystallinity of the β-sheets which underly the structure of silk lead to self-assembled hydrogels which have effectively irreversible formation.
Elastin inspires another class of biopolymer-based materials which have been used to form self-assembling materials and hydrogels for biomedical applications.[267, 268] Elastin-like polypeptides (ELPs) consist of pentapeptide repeats of Valine–Proline–Glycine–X–Glycine, where X is a guest residue that can be any amino acid other than proline. ELPs undergo an inverse temperature phase transition, similar to that described above for NIPAAm, transitioning from a soluble state to a water-excluding coacervate phase upon heating.[269] This transition temperature can be tuned by varying the guest residue, controlling the chain length, varying concentration, or altering the concentration or identity of ion species.[270] The creation of ELP diblock copolymers by altering the guest residue in each block can be used to vary the transition temperature in each block to afford micelle assemblies.[271] While most uses of ELPs have explored their temperature transition in the context of chemically crosslinked networks, uncrosslinked ELPs can also form networks with rheological properties similar to a collagen hydrogel upon reversible hydrophobic association arising above its transition temperature.[272]
Other bio-inspired materials have been explored in a similar context. For example, natural resilin is found in specialized compartments of most arthropods and gives rise to rubber-like properties of low stiffness, high resilience and effective energy storage; this has likewise motivated exploration of polypeptide hydrogels possessing similar properties.[273] Several works have also evaluated hybrid engineered proteins consisting of multiple different naturally inspired motifs. For example, multistimuli-responsive hydrogels can be realized from silk-elastin-like polypeptides, coupling the thermal sensitivity of elastin motifs to induce association of the polypeptides with the β-sheet propensity of silk motifs to stabilize these assemblies and resist phase separation.[274-277] Recombinant silk-collagen hybrids have also been explored which couple the self-association of each motif to yield hydrogels.[278, 279] Another class of protein-based hydrogel materials has been demonstrated from the use of naturally derived recognition motifs, resembling synthetic supramolecular interactions in their use to physically crosslink polymer or biopolymer precursors.[30, 280] By this way, self-associating motifs derived from protein binding pairs have been used to form dynamic hydrogel materials with useful shear-thinning and self-healing character, using either fully recombinant or peptide-polymer conjugates as building blocks.[281-284] The creation of synthetic or recombinant polypeptides thus has proven as powerful strategy to capture one or a number of protein-based assembly motifs for creation of functional hydrogel materials.
Polysaccharides constitute a broad class of biomacromolecules — including common biomaterials like alginate, chitosan, hyaluronic acid, cellulose, dextran, and carrageenan — which have been extensively explored in the creation of hydrogels.[285] Some of these, such as alginate and chitosan, are able to self-assemble into hydrogels through ionic crosslinking with certain oppositely charged multivalent ions.[286, 287] However, their chain flexibility and often hydrophilic character means that most polysaccharides must be synthetically modified in order to self-assemble and form hydrogels. Early work in this regard explored hydrophobic modification of polysaccharides with groups such as cholesterol to enable physical crosslinking mediated by self-association of the prosthetic groups.[288-290] In another approach, polysaccharides may be modified with supramolecular (e.g., host–guest) recognition motifs to facilitate physical crosslinking and hydrogel formation.[291] As such, the modification of polysaccharides constitutes another rich and biologically relevant design strategy to fabricate self-assembling hydrogels for drug delivery applications.
3.4. Drug Delivery from Biomacromolecular Hydrogels
Hydrogels arising from association and self-assembly of isolated collagen have a long history of clinical use, with several examples approved for use as wound dressings.[292] Hydrogels prepared from pure isolated collagen, recombinant collagen analogues, and collagen blended with other material components have also been used in various applications for the encapsulation and controlled release of therapeutics.[293-296] For example, the encapsulation and controlled release of growth factors from in situ-forming collagen hydrogel assemblies has been explored in the context of cardiovascular regeneration.[297] In another common use, the natural origins of collagen present a logical choice as a support matrix in the context of injectable cell-based therapies.[298] Self-assembling hydrogels prepared from synthetic collagen mimics and analogues have also been explored for biomedical applications, including one demonstration of inherent platelet activation and homeostasis for applications in controlling bleeding.[299]
Silk-based materials and hydrogels have also been readily explored for applications in drug delivery.[300] Like with collagen, the material itself may serve to activate regenerative processes following administration.[301] While processing to produce self-assembled hydrogels from silk can be laborious, their compact network structure and high extent of hydrophobic regions afford many opportunities to deliver both molecular-scale and protein payloads. For example, silk-based hydrogels have been explored to encapsulate a small molecule drug and control its release with zero-order kinetics over a period of weeks to drive vascularization at an injection site.[302] Others have demonstrated the use of sonication-induced self-assembly and hydrogel formation of silk for the controlled release of antibiotics.[303] Protein drug delivery has also been achieved using silk-based hydrogels, for example in preparing materials for neural regeneration through the delivery of active signaling proteins,[304] or for the encapsulation and controlled release of antibodies.[305]
Recombinant ELPs are also of great interest as self-assembling materials for injectable drug delivery applications.[306-308] The formation of hydrophobic domains upon exposure to body temperatures serves as both a rapid trigger for hydrogelation and affords the ability to partition certain drugs for controlled release.[309, 310] For example, these materials may be used as injectable localized depots for the controlled release of antibiotics or anti-inflammatory agents.[311, 312] Peptide and protein therapeutics may also be encapsulated for sustained release from within these in situ gelling materials.[313] Hydrogels prepared form self-assembly of ELPs have also been explored for the support of cells useful for cartilage regeneration.[314]
There are also a number of examples of recombinant hybrids combining multiple protein-derived assembly motifs being used for drug delivery. Silk-elastin hydrogels have been extensively explored in this regard.[315, 316] One of the most explored directions for these materials has been in gene therapy applications for hydrogel-mediated delivery of plasmid DNA and related genetic cargo.[317-319] These hydrogels have also been evaluated for the encapsulation and controlled release of small molecule drugs, with one example exploring an ophthalmic formulation for use in addressing glaucoma.[320] Silk-elastin hydrogels have also been explored as cell supports for applications in tissue regeneration.[321] In terms of engineering materials with protein-derived associating domains to form physical hydrogels via motif association, there are a variety of examples using these injectable materials to encapsulate therapeutic cells and bioactive molecules for tissue regeneration applications.[322-326]
Hydrogels based on polysaccharide self-assembly have also been widely studied, with a plethora of examples from the ionic crosslinking of alginate and chitosan to prepare hydrogels with encapsulated drugs, cells, and other therapeutic cargo.[327, 328] In this regard, the breadth of this topic extends beyond the scope of this review. However, materials prepared through self-assembly arising from designed interactions of modified polysaccharides have also been explored. Hydrophobic modification of certain polysaccharides offers one route to create hydrogels via self-assembly.[290] The gelation of hydrophobically modified polysaccharides has been explored for the encapsulation and controlled release of a variety of small molecule and protein drugs.[329-331] Supramolecular interactions used to crosslink modified hyaluronic acid (HA) have also been used for a range of application as injectable drug-releasing depots.[332-334] For example, modified HA crosslinked by a macrocyclic ternary complex was used for controlled delivery of active therapeutics upon depot injection into tumors.[335] Modified cellulose which was designed to form hydrogels via a similar crosslinking mechanism has also been explored for the oral delivery of active agents to address ulcerative colitis.[336] As such, the engineering of associative interactions on polysaccharide offers a tool to enable the use a variety of abundant and compatible biopolymers as hydrogels for drug delivery.
4. Manipulating Covalent Bonds to Modulate Self-Assembled Hydrogels
Human tissues, such as cartilage, have exceptional mechanical properties with an ability to withstand cyclic loads orders of magnitude better than most single component hydrogel systems.[337] It is likewise well understood that cells are able to sense their local mechanical environment.[338] Accordingly, there is significant interest in developing hydrogels with mechanical properties that more closely mimic those of the local matrix microenvironment. In this section we will describe how a combination of covalent and non-covalent interactions can be coupled to influence the material properties of hydrogels prepared through self-assembled components.
4.1. Covalent Stabilization of Self-Assembled Hydrogels
Underlying self-assembled systems are an array of dynamic non-covalent interactions which give rise to an assortment of nanostructures. This process of material fabrication offers many benefits, such as the ability to control the properties of resulting hydrogels, reliably include bioactive components, and controllably release drugs through a variety of previously described mechanisms. However, these hydrogels are derived from extensive non-covalent interactions which serve to promote axial elongation or dynamic physical crosslinking. These features result in networks which are more dynamic than covalently bound polymers. Moreover, hydrogels arising from one-dimensional self-assembly of low molecular weight gelators can also be significantly more rigid than covalent polymer networks,[339] leading to materials that are unable to deform like crosslinked polymer networks with significant degradation of mechanical properties often occurring above only 2% strain.[73, 340, 341] The interactions present in self-assembled systems, comprising non-covalent hydrophobic interactions, hydrogen bonds, and electrostatic interactions, are also susceptible to competition from species existing within the body. Thus, their mechanical properties and stability in vitro may not be representative of how these materials behave in vivo. While While covalently crosslinked hydrogels can persist in the body for years,[342] self-assembled hydrogels have typically degraded within months in vivo.[343] Materials which contain both covalent and non-covalent interactions thus offer an opportunity to couple the desirable properties of both classes of materials to realize exceptional properties.
Systems combining covalent and non-covalent interactions can broadly be divided into two different classes: those which feature non-covalent/supramolecular crosslinks of polymeric systems, and those which have two distinct networks. Non-covalently crosslinking of covalent polymers can be achieved by attaching chemical recognition motifs as pendants or terminal groups. For example, reports have explored polymers which are terminated with self-complementary ureidopyrimidinone (UPy) groups,[344, 345] host–guest chemistries,[346-348] coiled-coil peptide motifs,[349, 350] and complementary nucleic acid sequences.[351, 352] Since many of these interactions can be tuned, such as by the length of the nucleic acid sequence[353, 354] or the affinity of host–guest motif,[355] these non-covalent crosslinks not only stabilize the resulting hydrogels but can used to endow the material with the desired mechanical properties.
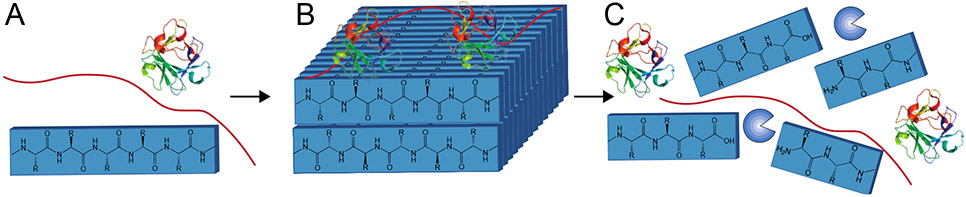
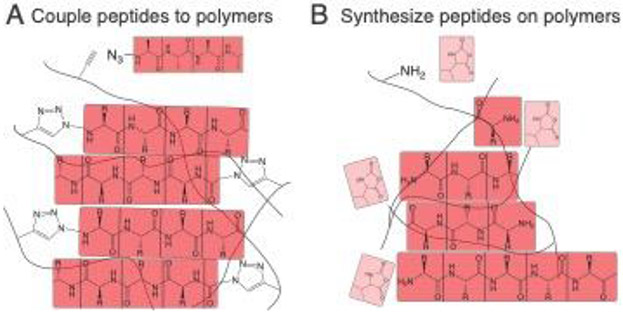
Another route to combine covalent/non-covalent systems would seek a hybrid material comprised of two separate interpenetrating networks. Such materials are inspired by work in double-network hydrogels, wherein the presence of two discrete polymers systems leads to hydrogels with exceptional mechanical properties.[356] The ability of the non-covalent network to dynamically break and reform allows these materials to dissipate energy without inducing permanent deformation.[337] Hybrid hydrogel systems with both covalent and non-covalent networks arise from a variety of polymer architectures, to include systems with distinct supramolecular and polymer networks,[357] systems which engineer non-covalent interactions between the networks,[358] and systems in which the self-assembling molecule is covalently bound to the polymer network (Figure 9).[359, 360] The myriad of design choices in these systems, such as whether the polymer network itself is cross linked,[361] enables a more tailored approach to achieving desired mechanical properties for a variety of biomedical applications.[362]
Figure 9:
The mechanical properties of self-assembling hydrogels can be improved through conjugation to a covalent polymer network. These hybrid-hydrogel networks and be made through (A) direct conjugation of peptides to the polymer network or (B) growing peptides from functional groups on the polymer backbone. Panel A is based on concepts from reference 340 and Panel B is based on concepts from reference 341.
4.1.2. Bioconjugation and Double-Network Formation
A number of polymers have been utilized as hydrogels and drug delivery, including those based on PEG, hyaluronic acid, poly(acrylic acid), and others.[363] These polymers are typically not capable of inherent non-covalent bonding that is sufficient to induce gelation at reasonable concentrations. As such, these are typically modified with other chemical groups to afford addition interactions and drive hydrogel formation.[8] One route to combinine these common biomedical polymers with additional bonds is to create two distinct networks within the resulting material. For instance, hydrogel-forming Fmoc-FF dipeptides have been mixed with unmodified polymers including hyaluronic acid,[357] alginate,[364] konjac glucomannan,[365] poly-L-lysine,[366] and dextran.[367] The Fmoc-FF peptides have a negatively charged carboxylate on their C-terminus, affording some opportunity for electrostatic interaction between the peptide assembly and a positively charged polymer such as poly-L-lysine. However, many of the polymers which have been combined with self-assembling peptides do not introduce electrostatic interactions. Other self-assembling peptides, such as peptide amphiphiles, have also been mixed with polymers including alginate[368] and PEG,[369] to modulate their mechanical properties.
The covalent polymer component in double-network hydrogels can be modified with a range of chemistries.[370] Moreover, supramolecular recognition motifs can often be coupled for polymer presentation.[371] In systems utilizing biopolymers the most widely used bioconjugation route for modification is to form an amide bond between a primary amine and a carboxylic acid using a standard coupling agent.[372] This approach has been used to couple self-assembling Fmoc-FF gelators to polymer backbones.[373] Combining biopolymers with oppositely charged self-assembling peptides can lead to strong interfacial interactions and the formation of a peptide-polymer membrane.[374] The mechanical properties and nanostructure of these membranes can be tuned by both modulating the concentration of the negatively charged hyaluronic acid biopolymer and the time at which the membranes are incubated in a solution of positively charged self-assembling peptide amphiphiles.[375] More complex systems can be made in which a second negatively charged biopolymer, heparin, is added to release growth factors.[376] This system demonstrates multiple biopolymers can be incorporated with distinct roles, from increasing mechanical properties to improving bioactivity.
Many biopolymers and peptide gelators present groups that can participate in unwanted side reactions; various “click” reactions have been explored which occur under mild conditions and offer greater selectivity for these conjugations.[377] Accordingly, a variety of different chemistries have been used to couple biomolecules to polymers,[378] including thiol-maleimide,[379] thiol-vinyl sulfone,[380] copper catalyzed azide-alkyne cycloadditions,[359] and copper-free azide-alkyne cycloadditions.[381] Technologies have also been developed to enable local hydrogel modification through the use of photo-reactive chemistries.[382-384]
The formation of self-assembled materials can also be used to template the synthesis of covalent polymer networks. For instance, diacetylene groups have been included in gelator design to localized to the interior of self-assembling peptide systems and these can be subsequently polymerized.[385, 386] Modifying self-assembling peptides with polymerizable groups and adding free polymer can be used to create networks in which supramolecular assemblies are covalently coupled, a useful approach for reinforced 3D-printed gels.[387] Biopolymers such as hyaluronic acid can also be modified with both supramolecular host–guest chemistries to stabilize the hydrogel during 3D printing, and acrylate groups for post-printing covalent crosslinking.[388] The templated polymer networks can have functions beyond improving the mechanical properties of the hydrogels; including light-activated spyropyran molecules enables the use of these hydrogels as stimuli-responsive mechanical actuators.[389]
4.1.2. Impact on Properties and Function in Drug Delivery
The mechanical properties of double-network hydrogels are often greater than the sum of their parts, with properties often greatly surpassing either network in isolation.[390] Synthetic hydrogel materials typically lack the fracture toughness of human tissues, as covalent bonds at the tip of propagating cracks are highly strained which focuses the stresses on a small area and reduces resistance to fracture.[391] Pioneering efforts in 2003 demonstrated the use of a second interpenetrating network, or “double-network” which was lightly crosslinked and coupled to the first network, significantly increasing the energy required to propagate cracks through the hydrogel compared to single-network hydrogels.[356] These initial double-network hydrogels were purely covalent, but in 2012 significant progress was realized by using an alginate network to introduce reversible non-covalent crosslinks.[371] In this system, these non-covalent interactions dissipate energy, while the covalent network provides a force to restore the hydrogel to its original shape (Figure 10).[337, 392] Double-network hydrogels have been developed for a range of biomedical applications,[393] and their combination of stretchability, wet adhesion, and transparency have made double network materials promising candidates for hydrogels that interface with skin.[371, 394]
While most interpenetrating network (IPN) hydrogels are composed of two distinct covalent polymer networks, other systems have also been developed in which one or both networks are supramolecular. An advantage of this approach is that the formation of an optimized covalent network generally requires multiple polymerization steps,[356] while small molecule self-assembly is orthogonal to most covalent polymerizations or crosslinking reactions. A self-assembling peptide was used to synthesize a one-pot IPN in combination with PEG-crosslinked chitosan.[395] These IPN hydrogels had superior mechanical properties and promoted increased expression of type-2 collagen in culture chondrocytes. Other self-assembling molecules have also been mixed with polymers to improve hydrogel mechanical properties. A non-covalent structure consisting of the nucleic acid guanosine, boronic acid, and potassium hydroxide was formed with an interpenetrating covalently cross-linked poly(N,N′-dimethyacrylamide) network.[340] These hydrogels exhibited impressive mechanical properties in tension, and were rapidly self-healing. The covalent polymer can also contribute to enhanced mechanical properties through electrostatic interactions with the surfaces of self-assembled structures. For instance, the introduction of heparin bound to the surface of a peptide nanostructure, intended to bind and release growth factors, at the same time was found to increase the storage modulus of the resulting heparin-binding peptide-amphiphile hydrogels.[396]
The Fmoc-FF hydrogelator is the most widely used self-assembling peptide system explored for double network systems. Adding alginate to Fmoc-FF hydrogels increased the storage modulus several fold, even without the addition of calcium to gel the alginate.[364] The Fmoc-FF peptide has also been modified with a negatively charged C-terminal phosphotyrosine (Fmoc-FFpY) and co-assembled with a positively charged polymer poly(allylamine hydrochloride) (PAH).[358] Whereas the Fmoc-FFpY, does not form a hydrogel on its own, the addition of PAH resulted in hydrogels with superior mechanical properties to Fmoc-FFY by both increasing the storage modulus and increased maintenance of mechanical properties upon induction of strain. In some cases double-network hydrogels themselves can elicit a biological response in vivo without any added bioactivity. Self-assembling Fmoc-FF monomers were mixed with sulfhydryl-modified poly-L-lysine and injected into the tumors of mice to activate a T-cell response and reduce tumor volume.[366]
Chemical groups which form non-covalent crosslinks serve dual roles by both improving the mechanical properties within the hydrogel and also acting as depots to bind hydrophobic drugs which interact with these groups. For example, polyurethane was functionalized with an Fmoc-FF dipeptide to induce hydrogel formation.[373] The associated Fmoc-FF groups which formed crosslinks in these networks were able to both bind and release the drug curcumin, and interestingly the mechanical properties of the hydrogel were improved upon the addition of the drug. These hydrogels are injectable using a syringe, and improved wound healing in a rat model. The combination of Fmoc-FF peptide and the polysaccharide konjac glucomannan (KGM) formed hydrogels with two distinct networks that were stiffer than Fmoc-FF peptides alone, and KGM slowed the release of the chemotherapeutic drug docetaxel.[365] Double-network gels have also been fabricated with supramolecular systems combining two separate gelators.[397] In one case, one self-assembling molecule was used to endow the hydrogel with mechanical strength, while another was covalently bound to the non-steroidal anti-inflammatory drug Naproxen for pH-dependent release.[397] Gels containing both peptide amphiphiles and a second low molecular weight gelator were stiffer than either single component gel.[398]
4.2. Covalent Bond Rupture in Self-Assembled Hydrogels
The combination of both self-assembled networks and covalent polymer networks not only expands the hydrogel design space, but affords new mechanisms by which these gels can be degraded. In peptide amphiphile hydrogels with an interpenetrating PEG network that contains MMP-sensitive crosslinks, the presence of the covalent network serves to both increase the stiffness of the hydrogel and link its degradation to the presence of cell-secreted proteases.[399] Most biopolymers used in double-network hydrogels are not polypeptides, and thus not commonly substrates for proteases. However, a variety of other enzymes exist that degrade an assortment of other bonds found in the body, including many used for the construction of biomaterials. When hyaluronic acid is mixed with calixpyridinium it forms nanostructures that are responsive to both temperature and the enzyme hyaluronidase.[400] Aside from degrading polymeric backbones, enzymes can also cleave crosslinks between macromolecular chains. Self-healing hydrogels have been crosslinked by modifying the polymer with a host and a difunctional crosslinking molecule with guests, enabling the host–guest crosslinker to include a substrate for the bacterial enzyme β-Lactamase.[401] These hydrogels are stable in media, but degrade within 72 hours in the presence of bacteria. Drug release can also be tied to enzymatic activity in hybrid hydrogels. In the Fmoc-FF/konjac glucomannan system, the release of docetaxel after 24h was three times higher in hydrogels incubated with β-mannanase, which degrades the konjac glucomannan, than those with no enzyme.[365]
5. Conclusion and future prospects
The human body is a complex and dynamic environment and creating materials for a particular therapeutic application requires balancing a variety different design criteria. Hydrogels prepared from self-assembly offer many useful properties in this regard, including the ability be delivered non-invasively through a syringe,[18] the extensive tunabilty of their different properties and features,[402] and more straightforward routes of biodegradation and clearance owing to their molecular-scale construction.[403] Accordingly, this broad and diverse class of materials have many useful properties which can be more thoroughly exploited in developing strategies to address many biomedical applications.
Many self-assembling molecules are modular by nature, and over decades of research into various systems specific motifs have been developed to induce gelation. Further efforts have demonstrated a robust capacity to modulate the biological, chemical, or mechanical properties of the resulting hydrogels. For applications specific to drug delivery, strategies have also been explored to enable the controlled release of a variety of different pharmaceutical and biopharmaceutical agents. Small molecules which form gels are often based on peptides, affording inherent advantages in their ease of synthesis and opportunities to use these as substrates for biological enzymes or ligands for protein binding. These features can enable improved therapeutic targeting as drug release can be designed with specificity for conditions presented by a desired cell, tissue, or physiologic state.
The rich field of small molecule self-assembly draws inspiration from many natural systems. The assembly process, arising from a balance of attractive and repulsive forces, gives rise to a diversity of nanostructures and corresponding organization of these to yield hydrogel networks. A number of design features of these systems can be used to incorporate therapeutic activity, including the encapsulation of hydrophobic drugs, the incorporation of bioactive ligands, and the inherent bioactivity of the material mechanical properties. The release of drugs from such systems can be further directed by stimuli-directed transformations in nanostructure and/or through environmentally sensitive cleavage of labile bonds.
The self-assembly of macromolecular species have also been explored in the formation of hydrogels. The various platforms have included synthetic block copolymers in which individual sections have distinct chemical properties to promote phase segregation in an aqueous environment, as well as natural biopolymers and protein-based designs which are not only useful in forming networks but can be rich with bioactivity. Both of these types of systems can be modified with functional groups that participate in non-covalent interactions to modulate hydrogel properties and control the release rates of drugs and proteins. The capabilities in both polymer synthesis and recombinant protein design and expression continue to expand, allowing for increasingly controlled designer macromolecular matrices tailored to specific biomedical applications.
Most self-assembling hydrogels are held together with weak hydrogen bonds, which typically have poor mechanical properties compared to human tissues and are also prone to degradation and clearance over accelerated time scales. One way to improve on these features is through the fabrication of hybrid materials, including double-network hydrogels, which are designed to include both covalent and non-covalent components. Hydrogels which are able to dissipate energy by restricting non-covalent interactions, yet have a covalent network to maintain the original material form offer enabling traits for a number of applications. Due to current network designs, many hydrogels with exceptional mechanical properties have been targeted towards applications on the skin, such as strain monitoring. However, as our understanding of hydrogel mechanics and fabrication improve, these materials offer a number of promising directions for biomedical applications, such as cartilage and tendon repair where physical demands have thus far prevented successful clinical translation.
Improving the efficacy of therapeutic biomaterials will require systems that can dynamically interact with the local environment and deliver a desired signal at a specific time. As improvements in genomics, proteomics, and super-resolution microscopy allow for the interrogation of tissues at the sub-cellular level, this information can be incorporated into the design considerations for novel materials, especially cell-responsive materials. Developments in both covalent click reactions, and non-covalent interactions, such as host–guest interactions, offer a growing repertoire of orthogonal reactions for creating complex supramolecular hydrogels, and increasingly allows for in vivo targeting. The combination of these advancements will enable the fabrication of clinically-relevant hydrogels created through self-assembly toward advancing health and quality of life globally.
6. Acknowledgements
ETP would like to acknowledge funding support from Lehigh University. MJW acknowledges support from the National Science Foundation (BMAT, 1944875), the National Institutes of Health (R35GM137987), and the University of Notre Dame “Advancing our Vision” initiative.
7. References Cited:
- 1.Drury JL and Mooney DJ, Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials, 2003. 24(24): p. 4337–51. [DOI] [PubMed] [Google Scholar]
- 2.Peppas NA, et al. , Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Advanced Materials, 2006. 18(11): p. 1345–1360. [Google Scholar]
- 3.Gutowska A, et al. , Squeezing hydrogels for controlled oral drug delivery. Journal of Controlled Release, 1997. 48(2-3): p. 141–148. [Google Scholar]
- 4.Jeong B, Bae YH, and Kim SW, Drug release from biodegradable injectable thermosensitive hydrogel of PEG-PLGA-PEG triblock copolymers. Journal of Controlled Release, 2000. 63(1-2): p. 155–163. [DOI] [PubMed] [Google Scholar]
- 5.Nicolson PC and Vogt J, Soft contact lens polymers: an evolution. Biomaterials, 2001. 22(24): p. 3273–3283. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman AS, Hydrogels for biomedical applications. Advanced Drug Delivery Reviews, 2012. 64: p. 18–23. [DOI] [PubMed] [Google Scholar]
- 7.Peppas NA, et al. , Physicochemical, foundations and structural design of hydrogels in medicine and biology. Annual Review of Biomedical Engineering, 2000. 2: p. 9–29. [DOI] [PubMed] [Google Scholar]
- 8.Appel EA, et al. , Supramolecular polymeric hydrogels. Chemical Society Reviews, 2012. 41(18): p. 6195–6214. [DOI] [PubMed] [Google Scholar]
- 9.Mantooth SM, Munoz-Robles BG, and Webber MJ, Dynamic Hydrogels from Host-Guest Supramolecular Interactions. Macromolecular Bioscience, 2019. 19(1). [DOI] [PubMed] [Google Scholar]
- 10.Peppas NA and Khare AR, Preparation, Structure and Diffusional Behavior of Hydrogels in Controlled-Release. Advanced Drug Delivery Reviews, 1993. 11(1-2): p. 1–35. [Google Scholar]
- 11.Lin CC and Metters AT, Hydrogels in controlled release formulations: Network design and mathematical modeling. Advanced Drug Delivery Reviews, 2006. 58(12-13): p. 1379–1408. [DOI] [PubMed] [Google Scholar]
- 12.Engler AJ, et al. , Matrix elasticity directs stem cell lineage specification. Cell, 2006. 126(4): p. 677–689. [DOI] [PubMed] [Google Scholar]
- 13.Seliktar D, Designing Cell-Compatible Hydrogels for Biomedical Applications. Science, 2012. 336(6085): p. 1124–1128. [DOI] [PubMed] [Google Scholar]
- 14.Rosales AM and Anseth KS, The design of reversible hydrogels to capture extracellular matrix dynamics. Nature Reviews Materials, 2016. 1(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen KT and West JL, Photopolymerizable hydrogels for tissue engineering applications. Biomaterials, 2002. 23(22): p. 4307–4314. [DOI] [PubMed] [Google Scholar]
- 16.Yang J-A, et al. , In situ-forming injectable hydrogels for regenerative medicine. Progress in Polymer Science, 2014. 39(12): p. 1973–1986. [Google Scholar]
- 17.Dimatteo R, Darling NJ, and Segura T, In situ forming injectable hydrogels for drug delivery and wound repair. Advanced Drug Delivery Reviews, 2018. 127: p. 167–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahoo JK, VandenBerg MA, and Webber MJ, Injectable network biomaterials via molecular or colloidal self-assembly. Advanced drug delivery reviews, 2018. 127: p. 185–207. [DOI] [PubMed] [Google Scholar]
- 19.Qiu Y and Park K, Environment-sensitive hydrogels for drug delivery. Advanced Drug Delivery Reviews, 2012. 64: p. 49–60. [DOI] [PubMed] [Google Scholar]
- 20.Culver HR, Clegg JR, and Peppas NA, Analyte-Responsive Hydrogels: Intelligent Materials for Biosensing and Drug Delivery. Accounts of Chemical Research, 2017. 50(2): p. 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xian S and Webber MJ, Temperature-responsive supramolecular hydrogels. J Mater Chem B, 2020. 8(40): p. 9197–9211. [DOI] [PubMed] [Google Scholar]
- 22.Philp D and Stoddart JF, Self-assembly in natural and unnatural systems. Angewandte Chemie-International Edition, 1996. 35(11): p. 1154–1196. [Google Scholar]
- 23.Whitesides GM and Grzybowski B, Self-assembly at all scales. Science, 2002. 295(5564): p. 2418–2421. [DOI] [PubMed] [Google Scholar]
- 24.Mai YY and Eisenberg A, Self-assembly of block copolymers. Chemical Society Reviews, 2012. 41(18): p. 5969–5985. [DOI] [PubMed] [Google Scholar]
- 25.Mendes AC, et al. , Self-assembly in nature: using the principles of nature to create complex nanobiomaterials. Wiley Interdisciplinary Reviews-Nanomedicine and Nanobiotechnology, 2013. 5(6): p. 582–612. [DOI] [PubMed] [Google Scholar]
- 26.Wang HM, Feng ZQQ, and Xu B, Bioinspired assembly of small molecules in cell milieu. Chemical Society Reviews, 2017. 46(9): p. 2421–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang SG, Fabrication of novel biomaterials through molecular self-assembly. Nature Biotechnology, 2003. 21(10): p. 1171–1178. [DOI] [PubMed] [Google Scholar]
- 28.Kyle S, et al. , Production of self-assembling biomaterials for tissue engineering. Trends in Biotechnology, 2009. 27(7): p. 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephanopoulos N, Ortony JH, and Stupp SI, Self-assembly for the synthesis of functional biomaterials. Acta Materialia, 2013. 61(3): p. 912–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webber MJ, et al. , Supramolecular biomaterials. Nature Materials, 2016. 15(1): p. 13–26. [DOI] [PubMed] [Google Scholar]
- 31.Meyer EE, Rosenberg KJ, and Israelachvili J, Recent progress in understanding hydrophobic interactions. Proceedings of the National Academy of Sciences of the United States of America, 2006. 103(43): p. 15739–15746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez-Iglesias A, et al. , Hydrophobic Interactions Modulate Self-Assembly of Nanoparticles. Acs Nano, 2012. 6(12): p. 11059–11065. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, et al. , Peptide self-assembly: thermodynamics and kinetics. Chemical Society Reviews, 2016. 45(20): p. 5589–5604. [DOI] [PubMed] [Google Scholar]
- 34.Tomasini C and Castellucci N, Peptides and peptidomimetics that behave as low molecular weight gelators. Chemical Society Reviews, 2013. 42(1): p. 156–172. [DOI] [PubMed] [Google Scholar]
- 35.Mason TO and Buell AK, The Kinetics, Thermodynamics and Mechanisms of Short Aromatic Peptide Self-Assembly, in Biological and Bio-inspired Nanomaterials. 2019, Springer. p. 61–112. [DOI] [PubMed] [Google Scholar]
- 36.Resh MD, Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1999. 1451(1): p. 1–16. [DOI] [PubMed] [Google Scholar]
- 37.Hartgerink JD, Beniash E, and Stupp SI, Self-assembly and mineralization of peptide-amphiphile nanofibers. Science, 2001. 294(5547): p. 1684–1688. [DOI] [PubMed] [Google Scholar]
- 38.Smith AM, et al. , Fmoc-diphenylalanine self assembles to a hydrogel via a novel architecture based on π–π interlocked β-sheets. Advanced materials, 2008. 20(1): p. 37–41. [Google Scholar]
- 39.Li J, et al. , The conjugation of nonsteroidal anti-inflammatory drugs (NSAID) to small peptides for generating multifunctional supramolecular nanofibers/hydrogels. Beilstein journal of organic chemistry, 2013. 9(1): p. 908–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paramonov SE, Jun H-W, and Hartgerink JD, Self-assembly of peptide− amphiphile nanofibers: the roles of hydrogen bonding and amphiphilic packing. Journal of the American Chemical Society, 2006. 128(22): p. 7291–7298. [DOI] [PubMed] [Google Scholar]
- 41.Yang P-P, et al. , Reorganization of self-assembled supramolecular materials controlled by hydrogen bonding and hydrophilic–lipophilic balance. Journal of Materials Chemistry B, 2016. 4(15): p. 2662–2668. [DOI] [PubMed] [Google Scholar]
- 42.Schneider JP, et al. , Responsive hydrogels from the intramolecular folding and self-assembly of a designed peptide. Journal of the American Chemical Society, 2002. 124(50): p. 15030–15037. [DOI] [PubMed] [Google Scholar]
- 43.Dong H, et al. , Self-assembly of multidomain peptides: balancing molecular frustration controls conformation and nanostructure. Journal of the American Chemical Society, 2007. 129(41): p. 12468–12472. [DOI] [PubMed] [Google Scholar]
- 44.Orbach R, et al. , The rheological and structural properties of Fmoc-peptide-based hydrogels: the effect of aromatic molecular architecture on self-assembly and physical characteristics. Langmuir, 2012. 28(4): p. 2015–2022. [DOI] [PubMed] [Google Scholar]
- 45.Reches M and Gazit E, Casting metal nanowires within discrete self-assembled peptide nanotubes. Science, 2003. 300(5619): p. 625–627. [DOI] [PubMed] [Google Scholar]
- 46.Jandl B, et al. , Peptide–Fluorophore Hydrogel as a Signal Boosting Approach in Rapid Detection of Cancer DNA. ACS omega, 2019. 4(9): p. 13889–13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sis MJ and Webber MJ, Drug delivery with designed peptide assemblies. Trends in pharmacological sciences, 2019. 40(10): p. 747–762. [DOI] [PubMed] [Google Scholar]
- 48.Pedersen SL, et al. , Microwave heating in solid-phase peptide synthesis. Chemical Society Reviews, 2012. 41(5): p. 1826–1844. [DOI] [PubMed] [Google Scholar]
- 49.O'Donnell MJ, Zhou C, and Scott WL, Solid-phase unnatural peptide synthesis (UPS). Journal of the American Chemical Society, 1996. 118(25): p. 6070–6071. [Google Scholar]
- 50.Basavalingappa V, et al. , Expanding the Functional Scope of the Fmoc-Diphenylalanine Hydrogelator by Introducing a Rigidifying and Chemically Active Urea Backbone Modification. Advanced Science, 2019. 6(12): p. 1900218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keten S, et al. , Nanoconfinement controls stiffness, strength and mechanical toughness of β-sheet crystals in silk. Nature materials, 2010. 9(4): p. 359–367. [DOI] [PubMed] [Google Scholar]
- 52.Wenger MP, et al. , Mechanical properties of collagen fibrils. Biophysical journal, 2007. 93(4): p. 1255–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rudra JS, et al. , Modulating adaptive immune responses to peptide self-assemblies. Acs Nano, 2012. 6(2): p. 1557–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chung EJ, et al. , In vivo biodistribution and clearance of peptide amphiphile micelles. Nanomedicine: Nanotechnology, Biology and Medicine, 2015. 11(2): p. 479–487. [DOI] [PubMed] [Google Scholar]
- 55.Varanko A, Saha S, and Chilkoti A, Recent trends in protein and peptide-based biomaterials for advanced drug delivery. Advanced Drug Delivery Reviews, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pérez-Silva JG, et al. , The Degradome database: expanding roles of mammalian proteases in life and disease. Nucleic acids research, 2015. 44(D1): p. D351–D355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zelzer M, et al. , Enzyme responsive materials: design strategies and future developments. Biomaterials Science, 2013. 1(1): p. 11–39. [DOI] [PubMed] [Google Scholar]
- 58.Schultz KM, et al. , Gelation of covalently cross-linked PEG− heparin hydrogels. Macromolecules, 2009. 42(14): p. 5310–5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kavanagh GM and Ross-Murphy SB, Rheological characterisation of polymer gels. Progress in Polymer Science, 1998. 23(3): p. 533–562. [Google Scholar]
- 60.Yan C and Pochan DJ, Rheological properties of peptide-based hydrogels for biomedical and other applications. Chemical Society Reviews, 2010. 39(9): p. 3528–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dawn A and Kumari H, Low molecular weight supramolecular gels under shear: Rheology as the tool for elucidating structure–function correlation. Chemistry–A European Journal, 2018. 24(4): p. 762–776. [DOI] [PubMed] [Google Scholar]
- 62.Veerman C, et al. , Gelation kinetics of β-hairpin peptide hydrogel networks. Macromolecules, 2006. 39(19): p. 6608–6614. [Google Scholar]
- 63.Stendahl JC, et al. , Intermolecular forces in the self-assembly of peptide amphiphile nanofibers. Advanced Functional Materials, 2006. 16(4): p. 499–508. [Google Scholar]
- 64.Lee NR, Bowerman CJ, and Nilsson BL, Effects of varied sequence pattern on the self-assembly of amphipathic peptides. Biomacromolecules, 2013. 14(9): p. 3267–3277. [DOI] [PubMed] [Google Scholar]
- 65.Sahoo JK, et al. , Electrostatic-driven self-sorting and nanostructure speciation in self-assembling tetrapeptides. Nanoscale, 2019. 11(35): p. 16534–16543. [DOI] [PubMed] [Google Scholar]
- 66.Redondo-Gómez C, et al. , Self-assembling hydrogels based on a complementary host–guest peptide amphiphile pair. Biomacromolecules, 2019. 20(6): p. 2276–2285. [DOI] [PubMed] [Google Scholar]
- 67.Zhang X, et al. , Rational design of a tetrameric protein to enhance interactions between self-assembled fibers gives molecular hydrogels. Angewandte Chemie International Edition, 2012. 51(18): p. 4388–4392. [DOI] [PubMed] [Google Scholar]
- 68.Gerweck LE and Seetharaman K, Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer research, 1996. 56(6): p. 1194–1198. [PubMed] [Google Scholar]
- 69.Hatip Koc M, et al. , Hierarchical self-assembly of histidine-functionalized peptide amphiphiles into supramolecular chiral nanostructures. Langmuir, 2017. 33(32): p. 7947–7956. [DOI] [PubMed] [Google Scholar]
- 70.Varkouhi AK, et al. , Endosomal escape pathways for delivery of biologicals. Journal of Controlled Release, 2011. 151(3): p. 220–228. [DOI] [PubMed] [Google Scholar]
- 71.Waqas M, et al. , pH-dependent in-cell self-assembly of peptide inhibitors increases the anti-prion activity while decreasing the cytotoxicity. Biomacromolecules, 2017. 18(3): p. 943–950. [DOI] [PubMed] [Google Scholar]
- 72.Israelachvili JN, Mitchell DJ, and Ninham BW, Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. Journal of the Chemical Society, Faraday Transactions 2: Molecular and Chemical Physics, 1976. 72: p. 1525–1568. [Google Scholar]
- 73.Pashuck ET, Cui H, and Stupp SI, Tuning supramolecular rigidity of peptide fibers through molecular structure. Journal of the American Chemical Society, 2010. 132(17): p. 6041–6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koh E, Kim T, and Cho H.-s., Mean curvature as a major determinant of β-sheet propensity. Bioinformatics, 2006. 22(3): p. 297–302. [DOI] [PubMed] [Google Scholar]
- 75.Cui H, et al. , Self-assembly of giant peptide nanobelts. Nano letters, 2009. 9(3): p. 945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pashuck ET and Stupp SI, Direct observation of morphological tranformation from twisted ribbons into helical ribbons. Journal of the American Chemical Society, 2010. 132(26): p. 8819–8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cui H, et al. , Amino acid sequence in constitutionally isomeric tetrapeptide amphiphiles dictates architecture of one-dimensional nanostructures. Journal of the American Chemical Society, 2014. 136(35): p. 12461–12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sahoo JK, et al. , Self-assembly of amphiphilic tripeptides with sequence-dependent nanostructure. Biomater Sci, 2017. 5(8): p. 1526–1530. [DOI] [PubMed] [Google Scholar]
- 79.Sahoo JK, et al. , Aromatic identity, electronic substitution, and sequence in amphiphilic tripeptide self-assembly. Soft Matter, 2018. 14(45): p. 9168–9174. [DOI] [PubMed] [Google Scholar]
- 80.Cheetham AG, et al. , Supramolecular nanostructures formed by anticancer drug assembly. Journal of the American Chemical Society, 2013. 135(8): p. 2907–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aggeli A, et al. , Hierarchical self-assembly of chiral rod-like molecules as a model for peptide β-sheet tapes, ribbons, fibrils, and fibers. Proceedings of the National Academy of Sciences, 2001. 98(21): p. 11857–11862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan J and Saltzman WM, Biomaterials with hierarchically defined micro-and nanoscale structure. Biomaterials, 2004. 25(17): p. 3593–3601. [DOI] [PubMed] [Google Scholar]
- 83.VandenBerg MA, et al. , Divergent Self-Assembly Pathways to Hierarchically Organized Networks of Isopeptide-Modified Discotics under Kinetic Control. ACS Nano, 2020. 14(5): p. 5491–5505. [DOI] [PubMed] [Google Scholar]
- 84.Zhang S, et al. , A self-assembly pathway to aligned monodomain gels. Nature materials, 2010. 9(7): p. 594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Berns EJ, et al. , Aligned neurite outgrowth and directed cell migration in self-assembled monodomain gels. Biomaterials, 2014. 35(1): p. 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clarke DE, Parmenter CD, and Scherman OA, Tunable Pentapeptide Self-Assembled β-Sheet Hydrogels. Angewandte Chemie International Edition, 2018. 57(26): p. 7709–7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi Y, et al. , Enzymatic activation of cell-penetrating peptides in self-assembled nanostructures triggers fibre-to-micelle morphological transition. Chemical Communications, 2017. 53(52): p. 7037–7040. [DOI] [PubMed] [Google Scholar]
- 88.Geng Y, et al. , Shape effects of filaments versus spherical particles in flow and drug delivery. Nature nanotechnology, 2007. 2(4): p. 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jung JP, et al. , Modulating the mechanical properties of self-assembled peptide hydrogels via native chemical ligation. Biomaterials, 2008. 29(13): p. 2143–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Storrie H, et al. , Supramolecular crafting of cell adhesion. Biomaterials, 2007. 28(31): p. 4608–4618. [DOI] [PubMed] [Google Scholar]
- 91.Webber MJ, et al. , Development of bioactive peptide amphiphiles for therapeutic cell delivery. Acta biomaterialia, 2010. 6(1): p. 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Castelletto V, et al. , New RGD-peptide amphiphile mixtures containing a negatively charged diluent. Faraday Discussions, 2013. 166: p. 381–397. [DOI] [PubMed] [Google Scholar]
- 93.Sur S, et al. , Synergistic regulation of cerebellar Purkinje neuron development by laminin epitopes and collagen on an artificial hybrid matrix construct. Biomater Sci, 2014. 2(6): p. 903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Webber MJ, et al. , Supramolecular nanostructures that mimic VEGF as a strategy for ischemic tissue repair. Proceedings of the National Academy of Sciences, 2011. 108(33): p. 13438–13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu C, et al. , Bioactive self-assembling peptide hydrogels functionalized with brain-derived neurotrophic factor and nerve growth factor mimicking peptides synergistically promote peripheral nerve regeneration. ACS Biomaterials Science & Engineering, 2018. 4(8): p. 2994–3005. [DOI] [PubMed] [Google Scholar]
- 96.Silva GA, et al. , Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science, 2004. 303(5662): p. 1352–1355. [DOI] [PubMed] [Google Scholar]
- 97.Zha RH, et al. , Supramolecular assembly of multifunctional maspin-mimetic nanostructures as a potent peptide-based angiogenesis inhibitor. Acta biomaterialia, 2015. 12: p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rudra JS, et al. , A self-assembling peptide acting as an immune adjuvant. Proceedings of the National Academy of Sciences, 2010. 107(2): p. 622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shah RN, et al. , Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proceedings of the National Academy of Sciences, 2010. 107(8): p. 3293–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bury MI, et al. , The promotion of functional urinary bladder regeneration using anti-inflammatory nanofibers. Biomaterials, 2014. 35(34): p. 9311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Niece KL, et al. , Self-assembly combining two bioactive peptide-amphiphile molecules into nanofibers by electrostatic attraction. Journal of the American Chemical Society, 2003. 125(24): p. 7146–7147. [DOI] [PubMed] [Google Scholar]
- 102.Jain R and Roy S, Designing a bioactive scaffold from coassembled collagen–laminin short peptide hydrogels for controlling cell behaviour. RSC Advances, 2019. 9(66): p. 38745–38759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pashuck ET, et al. , Controlled sub-nanometer epitope spacing in a three-dimensional self-assembled peptide hydrogel. ACS nano, 2016. 10(12): p. 11096–11104. [DOI] [PubMed] [Google Scholar]
- 104.Pompano RR, et al. , Titrating T-cell epitopes within self-assembled vaccines optimizes CD4+ helper T cell and antibody outputs. Advanced healthcare materials, 2014. 3(11): p. 1898–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mora-Solano C, et al. , Active immunotherapy for TNF-mediated inflammation using self-assembled peptide nanofibers. Biomaterials, 2017. 149: p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hudalla GA, et al. , A self-adjuvanting supramolecular vaccine carrying a folded protein antigen. Advanced healthcare materials, 2013. 2(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martins IM, Reis RL, and Azevedo HS, Phage display technology in biomaterials engineering: progress and opportunities for applications in regenerative medicine. ACS Chemical Biology, 2016. 11(11): p. 2962–2980. [DOI] [PubMed] [Google Scholar]
- 108.Sur S, et al. , Epitope topography controls bioactivity in supramolecular nanofibers. Biomaterials science, 2015. 3(3): p. 520–532. [DOI] [PubMed] [Google Scholar]
- 109.Satav T, Huskens J, and Jonkheijm P, Effects of variations in ligand density on cell signaling. Small, 2015. 11(39): p. 5184–5199. [DOI] [PubMed] [Google Scholar]
- 110.Webber MJ and Langer R, Drug delivery by supramolecular design. Chemical Society Reviews, 2017. 46(21): p. 6600–6620. [DOI] [PubMed] [Google Scholar]
- 111.Soukasene S, et al. , Antitumor activity of peptide amphiphile nanofiber-encapsulated camptothecin. ACS nano, 2011. 5(11): p. 9113–9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Raymond DM, et al. , Low-Molecular-Weight Supramolecular Hydrogels for Sustained and Localized in Vivo Drug Delivery. ACS Applied Bio Materials, 2019. 2(5): p. 2116–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Altunbas A, et al. , Encapsulation of curcumin in self-assembling peptide hydrogels as injectable drug delivery vehicles. Biomaterials, 2011. 32(25): p. 5906–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moyer TJ, et al. , Self-Assembled Peptide Nanostructures Targeting Death Receptor 5 and Encapsulating Paclitaxel As a Multifunctional Cancer Therapy. ACS Biomaterials Science & Engineering, 2019. 5(11): p. 6046–6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cheetham AG, et al. , Self-assembling prodrugs. Chemical Society Reviews, 2017. 46(21): p. 6638–6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Matson JB, et al. , Nanostructure-templated control of drug release from peptide amphiphile nanofiber gels. Soft Matter, 2012. 8(13): p. 3586–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lock LL, et al. , Self-assembly of natural and synthetic drug amphiphiles into discrete supramolecular nanostructures. Faraday discussions, 2013. 166: p. 285–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Su H, Koo JM, and Cui H, One-component nanomedicine. J Control Release, 2015. 219: p. 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tan J, et al. , Sustained release of two bioactive factors from supramolecular hydrogel promotes periodontal bone regeneration. Acs Nano, 2019. 13(5): p. 5616–5622. [DOI] [PubMed] [Google Scholar]
- 120.Koutsopoulos S, et al. , Controlled release of functional proteins through designer self-assembling peptide nanofiber hydrogel scaffold. Proceedings of the National Academy of Sciences, 2009. 106(12): p. 4623–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Behanna HA, et al. , Coassembly of amphiphiles with opposite peptide polarities into nanofibers. Journal of the American Chemical Society, 2005. 127(4): p. 1193–1200. [DOI] [PubMed] [Google Scholar]
- 122.Yayon A, et al. , Isolation of peptides that inhibit binding of basic fibroblast growth factor to its receptor from a random phage-epitope library. Proceedings of the National Academy of Sciences, 1993. 90(22): p. 10643–10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li L, et al. , Peptide ligands that use a novel binding site to target both TGF-β receptors. Molecular BioSystems, 2010. 6(12): p. 2392–2402.20890540 [Google Scholar]
- 124.Davis ME, et al. , Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proceedings of the National Academy of Sciences, 2006. 103(21): p. 8155–8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rajangam K, et al. , Heparin binding nanostructures to promote growth of blood vessels. Nano letters, 2006. 6(9): p. 2086–2090. [DOI] [PubMed] [Google Scholar]
- 126.Chow LW, et al. , Self-assembling nanostructures to deliver angiogenic factors to pancreatic islets. Biomaterials, 2010. 31(24): p. 6154–6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Webber MJ, et al. , Capturing the stem cell paracrine effect using heparin-presenting nanofibres to treat cardiovascular diseases. J Tissue Eng Regen Med, 2010. 4(8): p. 600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hudalla GA, et al. , Gradated assembly of multiple proteins into supramolecular nanomaterials. Nature materials, 2014. 13(8): p. 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang Y.-h., et al. , Enzyme-mediated hydrolytic activation of prodrugs. Acta Pharmaceutica Sinica B, 2011. 1(3): p. 143–159. [Google Scholar]
- 130.Sonawane SJ, Kalhapure RS, and Govender T, Hydrazone linkages in pH responsive drug delivery systems. European Journal of Pharmaceutical Sciences, 2017. 99: p. 45–65. [DOI] [PubMed] [Google Scholar]
- 131.Montero D, et al. , Intracellular glutathione pools are heterogeneously concentrated. Redox biology, 2013. 1(1): p. 508–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang C, et al. , Disulfide bond reduction-triggered molecular hydrogels of folic acid–Taxol conjugates. Organic & biomolecular chemistry, 2013. 11(40): p. 6946–6951. [DOI] [PubMed] [Google Scholar]
- 133.Zhu Y, et al. , Injectable pH and redox dual responsive hydrogels based on self-assembled peptides for anti-tumor drug delivery. Biomaterials Science, 2020. [DOI] [PubMed] [Google Scholar]
- 134.De La Rica R, Aili D, and Stevens MM, Enzyme-responsive nanoparticles for drug release and diagnostics. Advanced drug delivery reviews, 2012. 64(11): p. 967–978. [DOI] [PubMed] [Google Scholar]
- 135.Zhang S, et al. , An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease. Science translational medicine, 2015. 7(300): p. 300ra128–300ra128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Conda-Sheridan M, et al. , Esterase-activated release of naproxen from supramolecular nanofibres. Chemical Communications, 2014. 50(89): p. 13757–13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.He H, et al. , Enzymatic Noncovalent Synthesis. Chemical Reviews, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.van Bommel KJ, et al. , Two-stage enzyme mediated drug release from LMWG hydrogels. Organic & biomolecular chemistry, 2005. 3(16): p. 2917–2920. [DOI] [PubMed] [Google Scholar]
- 139.Webber MJ, et al. , Switching of self-assembly in a peptide nanostructure with a specific enzyme. Soft Matter, 2011. 7(20): p. 9665–9672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hai Z, et al. , Alkaline phosphatase-triggered simultaneous hydrogelation and chemiluminescence. Journal of the American Chemical Society, 2017. 139(3): p. 1041–1044. [DOI] [PubMed] [Google Scholar]
- 141.Wu J, et al. , Immune responsive release of tacrolimus to overcome organ transplant rejection. Advanced Materials, 2018. 30(45): p. 1805018. [DOI] [PubMed] [Google Scholar]
- 142.Itoh Y, MT1-MMP: A key regulator of cell migration in tissue. IUBMB life, 2006. 58(10): p. 589–596. [DOI] [PubMed] [Google Scholar]
- 143.Ortega N, et al. , How proteases regulate bone morphogenesis. Annals of the New York Academy of Sciences, 2003. 995(1): p. 109–116. [DOI] [PubMed] [Google Scholar]
- 144.Deryugina EI and Quigley JP, Matrix metalloproteinases and tumor metastasis. Cancer and metastasis reviews, 2006. 25(1): p. 9–34. [DOI] [PubMed] [Google Scholar]
- 145.Hadler-Olsen E, Winberg J-O, and Uhlin-Hansen L, Matrix metalloproteinases in cancer: their value as diagnostic and prognostic markers and therapeutic targets. Tumor Biology, 2013. 34(4): p. 2041–2051. [DOI] [PubMed] [Google Scholar]
- 146.Chau Y, et al. , Incorporation of a matrix metalloproteinase-sensitive substrate into self-assembling peptides–a model for biofunctional scaffolds. Biomaterials, 2008. 29(11): p. 1713–1719. [DOI] [PubMed] [Google Scholar]
- 147.Galler KM, et al. , Self-assembling multidomain peptide hydrogels: designed susceptibility to enzymatic cleavage allows enhanced cell migration and spreading. Journal of the American Chemical Society, 2010. 132(9): p. 3217–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Patterson J and Hubbell JA, Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials, 2010. 31(30): p. 7836–7845. [DOI] [PubMed] [Google Scholar]
- 149.Shi Y, et al. , Tuning the matrix metalloproteinase-1 degradability of peptide amphiphile nanofibers through supramolecular engineering. Biomaterials Science, 2019. 7(12): p. 5132–5142. [DOI] [PubMed] [Google Scholar]
- 150.Son J, et al. , Customizing morphology, size, and response kinetics of matrix metalloproteinase-responsive nanostructures by systematic peptide design. ACS nano, 2019. 13(2): p. 1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim J-K, et al. , Self-assembling peptide amphiphile-based nanofiber gel for bioresponsive cisplatin delivery. Molecular pharmaceutics, 2009. 6(3): p. 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hua D, et al. , Potent tumor targeting drug release system comprising MMP-2 specific peptide fragment with self-assembling characteristics. Drug design, development and therapy, 2014. 8: p. 1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Galler KM, et al. , A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue Engineering Part A, 2012. 18(1-2): p. 176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.He H, et al. , Branched peptides for enzymatic supramolecular hydrogelation. Chemical Communications, 2018. 54(1): p. 86–89. [DOI] [PubMed] [Google Scholar]
- 155.Sáez JA, Escuder B, and Miravet JF, Supramolecular hydrogels for enzymatically triggered self-immolative drug delivery. Tetrahedron, 2010. 66(14): p. 2614–2618. [Google Scholar]
- 156.Yoshii T, et al. , Chemically reactive supramolecular hydrogel coupled with a signal amplification system for enhanced analyte sensitivity. Journal of the American Chemical Society, 2015. 137(9): p. 3360–3365. [DOI] [PubMed] [Google Scholar]
- 157.Kazunori K, et al. , Block copolymer micelles as vehicles for drug delivery. Journal of Controlled Release, 1993. 24(1): p. 119–132. [Google Scholar]
- 158.Kwon GS and Okano T, Polymeric micelles as new drug carriers. Advanced Drug Delivery Reviews, 1996. 21(2): p. 107–116. [Google Scholar]
- 159.Kataoka K, Harada A, and Nagasaki Y, Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev, 2001. 47(1): p. 113–31. [DOI] [PubMed] [Google Scholar]
- 160.Rosler A, Vandermeulen GW, and Klok HA, Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv Drug Deliv Rev, 2001. 53(1): p. 95–108. [DOI] [PubMed] [Google Scholar]
- 161.He C, Kim SW, and Lee DS, In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J Control Release, 2008. 127(3): p. 189–207. [DOI] [PubMed] [Google Scholar]
- 162.Huynh CT, Nguyen MK, and Lee DS, Injectable Block Copolymer Hydrogels: Achievements and Future Challenges for Biomedical Applications. Macromolecules, 2011. 44(17): p. 6629–6636. [Google Scholar]
- 163.Singh NK and Lee DS, In situ gelling pH- and temperature-sensitive biodegradable block copolymer hydrogels for drug delivery. J Control Release, 2014. 193: p. 214–27. [DOI] [PubMed] [Google Scholar]
- 164.Akash MSH and Rehman K, Recent progress in biomedical applications of Pluronic (PF127): Pharmaceutical perspectives. Journal of Controlled Release, 2015. 209: p. 120–138. [DOI] [PubMed] [Google Scholar]
- 165.Zarrintaj P, et al. , Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta Biomaterialia, 2020. 110: p. 37–67. [DOI] [PubMed] [Google Scholar]
- 166.Alexandridis P, Holzwarth JF, and Hatton TA, Micellization of Poly(Ethylene Oxide)-Poly(Propylene Oxide)-Poly(Ethylene Oxide) Triblock Copolymers in Aqueous-Solutions - Thermodynamics of Copolymer Association. Macromolecules, 1994. 27(9): p. 2414–2425. [Google Scholar]
- 167.Bohorquez M, et al. , A study of the temperature-dependent micellization of pluronic F127. Journal of Colloid and Interface Science, 1999. 216(1): p. 34–40. [DOI] [PubMed] [Google Scholar]
- 168.Malmsten M and Lindman B, Self-Assembly in Aqueous Block Copolymer Solutions. Macromolecules, 1992. 25(20): p. 5440–5445. [Google Scholar]
- 169.Jalaal M, et al. , On the rheology of Pluronic F127 aqueous solutions. Journal of Rheology, 2017. 61(1): p. 139–146. [Google Scholar]
- 170.Zou L, Braegelman AS, and Webber MJ, Spatially Defined Drug Targeting by in Situ Host-Guest Chemistry in a Living Animal. ACS Cent Sci, 2019. 5(6): p. 1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zou L, et al. , Temperature-Responsive Supramolecular Hydrogels by Ternary Complex Formation with Subsequent Photo-Cross-linking to Alter Network Dynamics. Biomacromolecules, 2019. 20(12): p. 4512–4521. [DOI] [PubMed] [Google Scholar]
- 172.Loh XJ, Yee BJH, and Chia FS, Sustained delivery of paclitaxel using thermogelling poly (PEG/PPG/PCL urethane) s for enhanced toxicity against cancer cells. Journal of Biomedical Materials Research Part A, 2012. 100(10): p. 2686–2694. [DOI] [PubMed] [Google Scholar]
- 173.Loh XJ, et al. , New thermogelling poly (ether carbonate urethane) s based on pluronics F127 and poly (polytetrahydrofuran carbonate). Journal of Applied Polymer Science, 2014. 131(5). [Google Scholar]
- 174.Dou Q, Abdul Karim A, and Loh XJ, Modification of thermal and mechanical properties of PEG-PPG-PEG copolymer (F127) with MA-POSS. Polymers, 2016. 8(9): p. 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jeong B, et al. , New biodegradable polymers for injectable drug delivery systems. J Control Release, 1999. 62(1-2): p. 109–14. [DOI] [PubMed] [Google Scholar]
- 176.Jeong B, et al. , Biodegradable block copolymers as injectable drug-delivery systems. Nature, 1997. 388(6645): p. 860–862. [DOI] [PubMed] [Google Scholar]
- 177.Choi SW, et al. , Thermoreversible gelation of poly(ethylene oxide) biodegradable polyester block copolymers. II. Journal of Polymer Science Part A: Polymer Chemistry, 1999. 37(13): p. 2207–2218. [Google Scholar]
- 178.Jeong B, et al. , Thermoreversible gelation of poly(ethylene oxide) biodegradable polyester block copolymers. Journal of Polymer Science Part A: Polymer Chemistry, 1999. 37(6): p. 751–760. [Google Scholar]
- 179.Li F, et al. , Synthesis and Gelation Properties of PEG–PLA–PEG Triblock Copolymers Obtained by Coupling Monohydroxylated PEG–PLA with Adipoyl Chloride. Langmuir, 2007. 23(5): p. 2778–2783. [DOI] [PubMed] [Google Scholar]
- 180.Jeong B, Bae YH, and Kim SW, Thermoreversible Gelation of PEG–PLGA–PEG Triblock Copolymer Aqueous Solutions. Macromolecules, 1999. 32(21): p. 7064–7069. [Google Scholar]
- 181.Kim MS, et al. , Preparation of poly(ethylene glycol)-block-poly(caprolactone) copolymers and their applications as thermo-sensitive materials. Journal of Biomedical Materials Research Part A, 2004. 70A(1): p. 154–158. [DOI] [PubMed] [Google Scholar]
- 182.Hwang MJ, et al. , Caprolactonic poloxamer analog: PEG-PCL-PEG. Biomacromolecules, 2005. 6(2): p. 885–90. [DOI] [PubMed] [Google Scholar]
- 183.Gong C, et al. , Synthesis and characterization of PEG-PCL-PEG thermosensitive hydrogel. Int J Pharm, 2009. 365(1-2): p. 89–99. [DOI] [PubMed] [Google Scholar]
- 184.Kim MS, et al. , Preparation of methoxy poly(ethylene glycol)/polyester diblock copolymers and examination of the gel-to-sol transition. Journal of Polymer Science Part a-Polymer Chemistry, 2004. 42(22): p. 5784–5793. [Google Scholar]
- 185.Kim MS, et al. , Preparation of thermosensitive diblock copolymers consisting of MPEG and polyesters. Macromolecules, 2006. 39(9): p. 3099–3102. [Google Scholar]
- 186.Zentner GM, et al. , Biodegradable block copolymers for delivery of proteins and water-insoluble drugs. J Control Release, 2001. 72(1-3): p. 203–15. [DOI] [PubMed] [Google Scholar]
- 187.Wang P, et al. , Modified PLGA-PEG-PLGA thermosensitive hydrogels with suitable thermosensitivity and properties for use in a drug delivery system. J Mater Chem B, 2017. 5(8): p. 1551–1565. [DOI] [PubMed] [Google Scholar]
- 188.Yu L, Zhang H, and Ding J, A subtle end-group effect on macroscopic physical gelation of triblock copolymer aqueous solutions. Angew Chem Int Ed Engl, 2006. 45(14): p. 2232–5. [DOI] [PubMed] [Google Scholar]
- 189.Yu L, et al. , Mixing a sol and a precipitate of block copolymers with different block ratios leads to an injectable hydrogel. Biomacromolecules, 2009. 10(6): p. 1547–53. [DOI] [PubMed] [Google Scholar]
- 190.Abebe DG and Fujiwara T, Controlled Thermoresponsive Hydrogels by Stereocomplexed PLA-PEG-PLA Prepared via Hybrid Micelles of Pre-Mixed Copolymers with Different PEG Lengths. Biomacromolecules, 2012. 13(6): p. 1828–1836. [DOI] [PubMed] [Google Scholar]
- 191.Bae SJ, et al. , Thermogelling Poly(caprolactone-b-ethylene glycol-b-caprolactone) Aqueous Solutions. Macromolecules, 2005. 38(12): p. 5260–5265. [Google Scholar]
- 192.Haq MA, Su Y, and Wang D, Mechanical properties of PNIPAM based hydrogels: A review. Mater Sci Eng C Mater Biol Appl, 2017. 70(Pt 1): p. 842–855. [DOI] [PubMed] [Google Scholar]
- 193.Schild HG, Poly(N-isopropylacrylamide): experiment, theory and application. Progress in Polymer Science, 1992. 17(2): p. 163–249. [Google Scholar]
- 194.Loh XJ, et al. , Surface Coating with a Thermoresponsive Copolymer for the Culture and Non-Enzymatic Recovery of Mouse Embryonic Stem Cells. Macromolecular bioscience, 2009. 9(11): p. 1069–1079. [DOI] [PubMed] [Google Scholar]
- 195.Lanzalaco S and Armelin E, Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels, 2017. 3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Han CK and Bae YH, Inverse thermally-reversible gelation of aqueous N-isopropylacrylamide copolymer solutions. Polymer, 1998. 39(13): p. 2809–2814. [Google Scholar]
- 197.Garbern JC, Hoffman AS, and Stayton PS, Injectable pH- and temperature-responsive poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers for delivery of angiogenic growth factors. Biomacromolecules, 2010. 11(7): p. 1833–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zaccarelli E, Colloidal gels: equilibrium and non-equilibrium routes. Journal of Physics: Condensed Matter, 2007. 19(32): p. 323101. [Google Scholar]
- 199.Wang Q, et al. , Biodegradable Colloidal Gels as Moldable Tissue Engineering Scaffolds. Advanced Materials, 2008. 20(2): p. 236–239. [Google Scholar]
- 200.Sahoo JK, VandenBerg MA, and Webber MJ, Injectable network biomaterials via molecular or colloidal self-assembly. Adv Drug Deliv Rev, 2017. [DOI] [PubMed] [Google Scholar]
- 201.Freitas B, et al. , Self-Assembled Bioactive Colloidal Gels as Injectable Multiparticle Shedding Platforms. ACS Appl Mater Interfaces, 2020. 12(28): p. 31282–31291. [DOI] [PubMed] [Google Scholar]
- 202.Appel EA, et al. , Self-assembled hydrogels utilizing polymer–nanoparticle interactions. Nature communications, 2015. 6: p. 6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Appel EA, et al. , Exploiting Electrostatic Interactions in Polymer–Nanoparticle Hydrogels. ACS Macro Letters, 2015. 4(8): p. 848–852. [DOI] [PubMed] [Google Scholar]
- 204.Poudel AJ, et al. , Supramolecular hydrogels based on poly (ethylene glycol)-poly (lactic acid) block copolymer micelles and alpha-cyclodextrin for potential injectable drug delivery system. Carbohydrate Polymers, 2018. 194: p. 69–79. [DOI] [PubMed] [Google Scholar]
- 205.Yan XZ, et al. , Stimuli-responsive supramolecular polymeric materials. Chemical Society Reviews, 2012. 41(18): p. 6042–6065. [DOI] [PubMed] [Google Scholar]
- 206.Webber MJ, Engineering responsive supramolecular biomaterials: Toward smart therapeutics. Bioeng Transl Med, 2016. 1(3): p. 252–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Braegelman AS and Webber MJ, Integrating Stimuli-Responsive Properties in Host-Guest Supramolecular Drug Delivery Systems. Theranostics, 2019. 9(11): p. 3017–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hoque J, Sangaj N, and Varghese S, Stimuli-Responsive Supramolecular Hydrogels and Their Applications in Regenerative Medicine. Macromolecular Bioscience, 2019. 19(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yoshinori T, et al. , Complex Formation and Gelation between Copolymers Containing Pendant Azobenzene Groups and Cyclodextrin Polymers. Chemistry Letters, 2004. 33(7): p. 890–891. [Google Scholar]
- 210.Appel EA, et al. , Supramolecular Cross-Linked Networks via Host-Guest Complexation with Cucurbit[8]uril. Journal of the American Chemical Society, 2010. 132(40): p. 14251–14260. [DOI] [PubMed] [Google Scholar]
- 211.Kakuta T, Takashima Y, and Harada A, Highly Elastic Supramolecular Hydrogels Using Host-Guest Inclusion Complexes with Cyclodextrins. Macromolecules, 2013. 46(11): p. 4575–4579. [Google Scholar]
- 212.Harada A, Takashima Y, and Nakahata M, Supramolecular Polymeric Materials via Cyclodextrin-Guest Interactions. Accounts of Chemical Research, 2014. 47(7): p. 2128–2140. [DOI] [PubMed] [Google Scholar]
- 213.Chen XY, et al. , Supramolecular hydrogels cross-linked by preassembled host-guest PEG cross-linkers resist excessive, ultrafast, and non-resting cyclic compression. Npg Asia Materials, 2018. 10: p. 788–799. [Google Scholar]
- 214.Liu GT, et al. , Cyclodextrin-based host-guest supramolecular hydrogel and its application in biomedical fields. Polymer Chemistry, 2018. 9(25): p. 3436–3449. [Google Scholar]
- 215.Zou L, Braegelman AS, and Webber MJ, Dynamic Supramolecular Hydrogels Spanning an Unprecedented Range of Host-Guest Affinity. ACS Appl Mater Interfaces, 2019. 11(6): p. 5695–5700. [DOI] [PubMed] [Google Scholar]
- 216.Zou L and Webber MJ, Reversible hydrogel dynamics by physical-chemical crosslink photoswitching using a supramolecular macrocycle template. Chem Commun (Camb), 2019. 55(67): p. 9931–9934. [DOI] [PubMed] [Google Scholar]
- 217.Sinawang G, et al. , Supramolecular self-healing materials from non-covalent cross-linking host-guest interactions. Chemical Communications, 2020. 56(32): p. 4381–4395. [DOI] [PubMed] [Google Scholar]
- 218.Guo M, et al. , Tough stimuli-responsive supramolecular hydrogels with hydrogen-bonding network junctions. J Am Chem Soc, 2014. 136(19): p. 6969–77. [DOI] [PubMed] [Google Scholar]
- 219.Ruel-Gariepy E and Leroux JC, In situ-forming hydrogels - review of temperature-sensitive systems. European Journal of Pharmaceutics and Biopharmaceutics, 2004. 58(2): p. 409–426. [DOI] [PubMed] [Google Scholar]
- 220.Pitto-Barry A and Barry NPE, Pluronic (R) block-copolymers in medicine: from chemical and biological versatility to rationalisation and clinical advances. Polymer Chemistry, 2014. 5(10): p. 3291–3297. [Google Scholar]
- 221.Katakam M, Ravis WR, and Banga AK, Controlled release of human growth hormone in rats following parenteral administration of poloxamer gels. Journal of Controlled Release, 1997. 49(1): p. 21–26. [Google Scholar]
- 222.Barichello JM, et al. , Absorption of insulin from Pluronic F-127 gels following subcutaneous administration in rats. International Journal of Pharmaceutics, 1999. 184(2): p. 189–198. [DOI] [PubMed] [Google Scholar]
- 223.Veyries ML, et al. , Controlled release of vancomycin from Poloxamer 407 gels. International Journal of Pharmaceutics, 1999. 192(2): p. 183–193. [DOI] [PubMed] [Google Scholar]
- 224.Amiji MM, et al. , Intratumoral administration of paclitaxel in an in situ gelling poloxamer 407 formulation. Pharmaceutical Development and Technology, 2002. 7(2): p. 195–202. [DOI] [PubMed] [Google Scholar]
- 225.Wenzel JGW, et al. , Pluronic (R) F127 gel formulations of Deslorelin and GnRH reduce drug degradation and sustain drug release and effect in cattle. Journal of Controlled Release, 2002. 85(1-3): p. 51–59. [DOI] [PubMed] [Google Scholar]
- 226.Bouchot O, et al. , Clinical Experience With a Novel Thermosensitive Temporary Coronary Artery Occluder (LeGoo). Annals of Thoracic Surgery, 2010. 89(6): p. 1912–1917. [DOI] [PubMed] [Google Scholar]
- 227.Dansdill D, et al. , Synthetic, organic compound vepoloxamer (P-188) potentiates tissue plasminogen activator. Journal of Vascular Surgery, 2018. 67(1): p. 294–299. [DOI] [PubMed] [Google Scholar]
- 228.Basu A, et al. , Poly(lactic acid) based hydrogels. Advanced Drug Delivery Reviews, 2016. 107: p. 192–205. [DOI] [PubMed] [Google Scholar]
- 229.Jeong B, Bae YH, and Kim SW, In situ gelation of PEG-PLGA-PEG triblock copolymer aqueous solutions and degradation thereof. J Biomed Mater Res, 2000. 50(2): p. 171–7. [DOI] [PubMed] [Google Scholar]
- 230.Jeong B, Bae YH, and Kim SW, Drug release from biodegradable injectable thermosensitive hydrogel of PEG-PLGA-PEG triblock copolymers. J Control Release, 2000. 63(1-2): p. 155–63. [DOI] [PubMed] [Google Scholar]
- 231.Zentner GM, et al. , Biodegradable block copolymers for delivery of proteins and water-insoluble drugs. Journal of Controlled Release, 2001. 72(1-3): p. 203–215. [DOI] [PubMed] [Google Scholar]
- 232.Lee PY, Li ZH, and Huang L, Thermosensitive hydrogel as a Tgf-beta 1 gene delivery vehicle enhances diabetic wound healing. Pharmaceutical Research, 2003. 20(12): p. 1995–2000. [DOI] [PubMed] [Google Scholar]
- 233.Li ZH, et al. , Controlled gene delivery system based on thermosensitive biodegradable hydrogel. Pharmaceutical Research, 2003. 20(6): p. 884–888. [DOI] [PubMed] [Google Scholar]
- 234.Singh NK and Lee DS, In situ gelling pH- and temperature-sensitive biodegradable block copolymer hydrogels for drug delivery. Journal of Controlled Release, 2014. 193: p. 214–227. [DOI] [PubMed] [Google Scholar]
- 235.Choi S and Kim SW, Controlled release of insulin from injectable biodegradable triblock copolymer depot in ZDF rats. Pharmaceutical Research, 2003. 20(12): p. 2008–2010. [DOI] [PubMed] [Google Scholar]
- 236.Choi S, Baudys M, and Kim SW, Control of blood glucose by novel GLP-1 delivery using biodegradable triblock copolymer of PLGA-PEG-PLGA in type 2 diabetic rats. Pharmaceutical Research, 2004. 21(5): p. 827–831. [DOI] [PubMed] [Google Scholar]
- 237.Samlowski WE, et al. , ReGel (R) polymer-based delivery of interleukin-2 as a cancer treatment. Journal of Immunotherapy, 2006. 29(5): p. 524–535. [DOI] [PubMed] [Google Scholar]
- 238.Ma HC, et al. , Localized Co-delivery of Doxorubicin, Cisplatin, and Methotrexate by Thermosensitive Hydrogels for Enhanced Osteosarcoma Treatment. Acs Applied Materials & Interfaces, 2015. 7(49): p. 27040–27048. [DOI] [PubMed] [Google Scholar]
- 239.Elstad NL and Fowers KD, OncoGel (ReGel/paclitaxel) - Clinical applications for a novel paclitaxel delivery system. Advanced Drug Delivery Reviews, 2009. 61(10): p. 785–794. [DOI] [PubMed] [Google Scholar]
- 240.Maikawa CL, et al. , Block copolymer composition drives function of self-assembled nanoparticles for delivery of small-molecule cargo. Journal of Polymer Science Part a-Polymer Chemistry, 2019. 57(12): p. 1322–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hernandez HL, et al. , Non-Newtonian Polymer-Nanoparticle Hydrogels Enhance Cell Viability during Injection. Macromolecular Bioscience, 2019. 19(1). [DOI] [PubMed] [Google Scholar]
- 242.Steele AN, et al. , A Biocompatible Therapeutic Catheter-Deliverable Hydrogel for In Situ Tissue Engineering. Advanced Healthcare Materials, 2019. 8(5). [DOI] [PubMed] [Google Scholar]
- 243.Grosskopf AK, et al. , Injectable supramolecular polymer-nanoparticle hydrogels enhance human mesenchymal stem cell delivery. Bioengineering & Translational Medicine, 2020. 5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Stapleton LM, et al. , Use of a supramolecular polymeric hydrogel as an effective post-operative pericardial adhesion barrier. Nature Biomedical Engineering, 2019. 3(8): p. 611–620. [DOI] [PubMed] [Google Scholar]
- 245.Fenton OS, et al. , Injectable Polymer-Nanoparticle Hydrogels for Local Immune Cell Recruitment. Biomacromolecules, 2019. 20(12): p. 4430–4436. [DOI] [PubMed] [Google Scholar]
- 246.Gale EC, et al. , A Nanoparticle Platform for Improved Potency, Stability, and Adjuvanticity of Poly(I:C). Advanced Therapeutics, 2020. 3(1). [Google Scholar]
- 247.Roth GA, et al. , Injectable Hydrogels for Sustained Codelivery of Subunit Vaccines Enhance Humoral Immunity. ACS Central Science, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Smith AAA, et al. , Nanoparticles Presenting Potent TLR7/8 Agonists Enhance Anti-PD-L1 Immunotherapy in Cancer Treatment. Biomacromolecules, 2020. 21(9): p. 3704–3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Webber MJ, et al. , Supramolecular biomaterials. Nat Mater, 2016. 15(1): p. 13–26. [DOI] [PubMed] [Google Scholar]
- 250.Webber MJ and Langer R, Drug delivery by supramolecular design. Chem Soc Rev, 2017. 46(21): p. 6600–6620. [DOI] [PubMed] [Google Scholar]
- 251.Appel EA, et al. , Sustained release of proteins from high water content supramolecular polymer hydrogels. Biomaterials, 2012. 33(18): p. 4646–4652. [DOI] [PubMed] [Google Scholar]
- 252.Bastings MMC, et al. , A Fast pH-Switchable and Self-Healing Supramolecular Hydrogel Carrier for Guided, Local Catheter Injection in the Infarcted Myocardium. Advanced Healthcare Materials, 2014. 3(1): p. 70–78. [DOI] [PubMed] [Google Scholar]
- 253.Pape ACH, et al. , An Injectable and Drug-loaded Supramolecular Hydrogel for Local Catheter Injection into the Pig Heart. Jove-Journal of Visualized Experiments, 2015(100). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Eding JEC, et al. , Supramolecular hydrogel for local cardiac delivery of antimiR therapeutics. Cardiovascular Research, 2018. 114: p. S25–S25. [Google Scholar]
- 255.Mol E, et al. , Slow release of cardiac progenitor cell-derived extracellular vesicles from a pH-switchable hydrogel. Cardiovascular Research, 2018. 114: p. S53–S53. [Google Scholar]
- 256.Antoine EE, Vlachos PP, and Rylander MN, Review of collagen I hydrogels for bioengineered tissue microenvironments: characterization of mechanics, structure, and transport. Tissue Eng Part B Rev, 2014. 20(6): p. 683–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Shoulders MD and Raines RT, Collagen structure and stability. Annu Rev Biochem, 2009. 78: p. 929–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Drzewiecki KE, et al. , Methacrylation induces rapid, temperature-dependent, reversible self-assembly of type-I collagen. Langmuir, 2014. 30(37): p. 11204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Fallas JA, O'Leary LE, and Hartgerink JD, Synthetic collagen mimics: self-assembly of homotrimers, heterotrimers and higher order structures. Chem Soc Rev, 2010. 39(9): p. 3510–27. [DOI] [PubMed] [Google Scholar]
- 260.O'Leary LE, et al. , Multi-hierarchical self-assembly of a collagen mimetic peptide from triple helix to nanofibre and hydrogel. Nat Chem, 2011. 3(10): p. 821–8. [DOI] [PubMed] [Google Scholar]
- 261.Altman GH, et al. , Silk-based biomaterials. Biomaterials, 2003. 24(3): p. 401–16. [DOI] [PubMed] [Google Scholar]
- 262.Kapoor S and Kundu SC, Silk protein-based hydrogels: Promising advanced materials for biomedical applications. Acta Biomaterialia, 2016. 31: p. 17–32. [DOI] [PubMed] [Google Scholar]
- 263.Zhou CZ, et al. , Silk fibroin: structural implications of a remarkable amino acid sequence. Proteins, 2001. 44(2): p. 119–22. [DOI] [PubMed] [Google Scholar]
- 264.Perez-Rigueiro J, et al. , Mechanical properties of single-brin silkworm silk. Journal of Applied Polymer Science, 2000. 75(10): p. 1270–1277. [Google Scholar]
- 265.Kim UJ, et al. , Structure and properties of silk hydrogels. Biomacromolecules, 2004. 5(3): p. 786–92. [DOI] [PubMed] [Google Scholar]
- 266.Yucel T, Cebe P, and Kaplan DL, Vortex-induced injectable silk fibroin hydrogels. Biophys J, 2009. 97(7): p. 2044–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.MacEwan SR and Chilkoti A, Elastin-Like Polypeptides: Biomedical Applications of Tunable Biopolymers. Biopolymers, 2010. 94(1): p. 60–77. [DOI] [PubMed] [Google Scholar]
- 268.Acosta S, et al. , Elastin-Like Recombinamers: Deconstructing and Recapitulating the Functionality of Extracellular Matrix Proteins Using Recombinant Protein Polymers. Advanced Functional Materials, 2020. [Google Scholar]
- 269.Meyer DE and Chilkoti A, Quantification of the effects of chain length and concentration on the thermal behavior of elastin-like polypeptides. Biomacromolecules, 2004. 5(3): p. 846–851. [DOI] [PubMed] [Google Scholar]
- 270.Varanko AK, Su JC, and Chilkoti A, Elastin-Like Polypeptides for Biomedical Applications. Annu Rev Biomed Eng, 2020. 22: p. 343–369. [DOI] [PubMed] [Google Scholar]
- 271.Wright ER and Conticello VP, Self-assembly of block copolymers derived from elastin-mimetic polypeptide sequences. Adv Drug Deliv Rev, 2002. 54(8): p. 1057–73. [DOI] [PubMed] [Google Scholar]
- 272.Betre H, et al. , Characterization of a genetically engineered elastin-like polypeptide for cartilaginous tissue repair. Biomacromolecules, 2002. 3(5): p. 910–6. [DOI] [PubMed] [Google Scholar]
- 273.Li LQ, et al. , Resilin-like polypeptide hydrogels engineered for versatile biological function. Soft Matter, 2013. 9(3): p. 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Xia XX, et al. , Tunable Self-Assembly of Genetically Engineered Silk-Elastin-like Protein Polymers. Biomacromolecules, 2011. 12(11): p. 3844–3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Huang WW, et al. , Design of Multistimuli Responsive Hydrogels Using Integrated Modeling and Genetically Engineered Silk-Elastin-Like Proteins. Advanced Functional Materials, 2016. 26(23): p. 4113–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ibanez-Fonseca A, et al. , Influence of the Thermodynamic and Kinetic Control of Self-Assembly on the Microstructure Evolution of Silk-Elastin-Like Recombinamer Hydrogels. Small, 2020. 16(28). [DOI] [PubMed] [Google Scholar]
- 277.Parker RN, et al. , Smart Material Hydrogel Transfer Devices Fabricated with Stimuli-Responsive Silk-Elastin-Like Proteins. Advanced Healthcare Materials, 2020. 9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Golinska MD, et al. , Dilute Self-Healing Hydrogels of Silk-Collagen-Like Block Copolypeptides at Neutral pH. Biomacromolecules, 2014. 15(3): p. 699–706. [DOI] [PubMed] [Google Scholar]
- 279.Wlodarczyk-Biegun MK, et al. , Genetically engineered silk-collagen-like copolymer for biomedical applications: Production, characterization and evaluation of cellular response. Acta Biomaterialia, 2014. 10(8): p. 3620–3629. [DOI] [PubMed] [Google Scholar]
- 280.Wang Y, Katyal P, and Montclare JK, Protein-Engineered Functional Materials. Advanced Healthcare Materials, 2019. 8(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Foo CTSWP, et al. , Two-component protein-engineered physical hydrogels for cell encapsulation. Proceedings of the National Academy of Sciences of the United States of America, 2009. 106(52): p. 22067–22072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Mulyasasmita W, Lee JS, and Heilshorn SC, Molecular-Level Engineering of Protein Physical Hydrogels for Predictive Sol-Gel Phase Behavior. Biomacromolecules, 2011. 12(10): p. 3406–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Lu HD, et al. , Injectable shear-thinning hydrogels engineered with a self-assembling Dock-and-Lock mechanism. Biomaterials, 2012. 33(7): p. 2145–2153. [DOI] [PubMed] [Google Scholar]
- 284.Wu JH, et al. , Rationally designed synthetic protein hydrogels with predictable mechanical properties. Nature Communications, 2018. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Coviello T, et al. , Polysaccharide hydrogels for modified release formulations. Journal of Controlled Release, 2007. 119(1): p. 5–24. [DOI] [PubMed] [Google Scholar]
- 286.Rowley JA, Madlambayan G, and Mooney DJ, Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials, 1999. 20(1): p. 45–53. [DOI] [PubMed] [Google Scholar]
- 287.Berger J, et al. , Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. European Journal of Pharmaceutics and Biopharmaceutics, 2004. 57(1): p. 19–34. [DOI] [PubMed] [Google Scholar]
- 288.Nishikawa T, Akiyoshi K, and Sunamoto J, Supramolecular Assembly between Nanoparticles of Hydrophobized Polysaccharide and Soluble-Protein Complexation between the Self-Aggregate of Cholesterol-Bearing Pullulan and Alpha-Chymotrypsin. Macromolecules, 1994. 27(26): p. 7654–7659. [Google Scholar]
- 289.Akiyoshi K, et al. , Microscopic structure and thermoresponsiveness of a hydrogel nanoparticle by self-assembly of a hydrophobized polysaccharide. Macromolecules, 1997. 30(4): p. 857–861. [Google Scholar]
- 290.Hassani LN, Hendra F, and Bouchemal K, Auto-associative amphiphilic polysaccharides as drug delivery systems. Drug Discovery Today, 2012. 17(11-12): p. 608–614. [DOI] [PubMed] [Google Scholar]
- 291.Rodell CB, Kaminski AL, and Burdick JA, Rational Design of Network Properties in Guest-Host Assembled and Shear-Thinning Hyaluronic Acid Hydrogels. Biomacromolecules, 2013. 14(11): p. 4125–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Koehler J, Brandl FP, and Goepferich AM, Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. European Polymer Journal, 2018. 100: p. 1–11. [Google Scholar]
- 293.Olsen D, et al. , Recombinant collagen and gelatin for drug delivery. Advanced Drug Delivery Reviews, 2003. 55(12): p. 1547–1567. [DOI] [PubMed] [Google Scholar]
- 294.Sano A, et al. , Atelocollagen for protein and gene delivery. Advanced Drug Delivery Reviews, 2003. 55(12): p. 1651–1677. [DOI] [PubMed] [Google Scholar]
- 295.Wallace DG and Rosenblatt J, Collagen gel systems for sustained delivery and tissue engineering. Advanced Drug Delivery Reviews, 2003. 55(12): p. 1631–1649. [DOI] [PubMed] [Google Scholar]
- 296.Zhu SC, et al. , Self-assembly of collagen-based biomaterials: preparation, characterizations and biomedical applications. Journal of Materials Chemistry B, 2018. 6(18): p. 2650–2676. [DOI] [PubMed] [Google Scholar]
- 297.Fan CX, et al. , Myocardial-Infarction-Responsive Smart Hydrogels Targeting Matrix Metalloproteinase for On-Demand Growth Factor Delivery. Advanced Materials, 2019. 31(40). [DOI] [PubMed] [Google Scholar]
- 298.Liu M, et al. , Injectable hydrogels for cartilage and bone tissue engineering. Bone Research, 2017. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Kumar VA, et al. , A Nanostructured Synthetic Collagen Mimic for Hemostasis. Biomacromolecules, 2014. 15(4): p. 1484–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Yucel T, Lovett ML, and Keplan DL, Silk-based biomaterials for sustained drug delivery. Journal of Controlled Release, 2014. 190: p. 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Fini M, et al. , The healing of confined critical size cancellous defects in the presence of silk fibroin hydrogel. Biomaterials, 2005. 26(17): p. 3527–3536. [DOI] [PubMed] [Google Scholar]
- 302.Ding ZZ, et al. , Injectable Silk Nanofiber Hydrogels for Sustained Release of Small-Molecule Drugs and Vascularization. Acs Biomaterials Science & Engineering, 2019. 5(8): p. 4077–4088. [DOI] [PubMed] [Google Scholar]
- 303.Pritchard EM, et al. , Antibiotic-Releasing Silk Biomaterials for Infection Prevention and Treatment. Advanced Functional Materials, 2013. 23(7): p. 854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Hopkins AM, et al. , Silk Hydrogels as Soft Substrates for Neural Tissue Engineering. Advanced Functional Materials, 2013. 23(41): p. 5140–5149. [Google Scholar]
- 305.Guziewicz NA, et al. , Mechanisms of monoclonal antibody stabilization and release from silk biomaterials. Biomaterials, 2013. 34(31): p. 7766–7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Nettles DL, Chilkoti A, and Setton LA, Applications of elastin-like polypeptides in tissue engineering. Advanced Drug Delivery Reviews, 2010. 62(15): p. 1479–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.MacEwan SR and Chilkoti A, Applications of elastin-like polypeptides in drug delivery. Journal of Controlled Release, 2014. 190: p. 314–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Rodriguez-Cabello JC, et al. , Elastin-like polypeptides in drug delivery. Advanced Drug Delivery Reviews, 2016. 97: p. 85–100. [DOI] [PubMed] [Google Scholar]
- 309.Herrero-Vanrell R, et al. , Self-assembled particles of an elastin-like polymer as vehicles for controlled drug release. Journal of Controlled Release, 2005. 102(1): p. 113–122. [DOI] [PubMed] [Google Scholar]
- 310.Betre H, et al. , A thermally responsive biopolymer for intra-articular drug delivery. Journal of Controlled Release, 2006. 115(2): p. 175–182. [DOI] [PubMed] [Google Scholar]
- 311.Adams SB, et al. , Sustained Release of Antibiotics From Injectable and Thermally Responsive Polypeptide Depots. Journal of Biomedical Materials Research Part B-Applied Biomaterials, 2009. 90b(1): p. 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Sinclair SM, et al. , A genetically engineered thermally responsive sustained release curcumin depot to treat neuroinflammation. Journal of Controlled Release, 2013. 171(1): p. 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Shamji MF, et al. , An injectable and in situ-gelling biopolymer for sustained drug release following perineural administration. Spine, 2008. 33(7): p. 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Betre H, et al. , Characterization of a genetically engineered elastin-like polypeptide for cartilaginous tissue repair. Biomacromolecules, 2002. 3(5): p. 910–916. [DOI] [PubMed] [Google Scholar]
- 315.Megeed Z, Cappello J, and Ghandehari H, Genetically engineered silk-elastinlike protein polymers for controlled drug delivery. Advanced Drug Delivery Reviews, 2002. 54(8): p. 1075–1091. [DOI] [PubMed] [Google Scholar]
- 316.Huang WW, Rollett A, and Kaplan DL, Silk-elastin-like protein biomaterials for the controlled delivery of therapeutics. Expert Opinion on Drug Delivery, 2015. 12(5): p. 779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Megeed Z, Cappello J, and Ghandehari H, Controlled release of plasmid DNA from a genetically engineered silk-elastinlike hydrogel. Pharmaceutical Research, 2002. 19(7): p. 954–959. [DOI] [PubMed] [Google Scholar]
- 318.Megeed Z, et al. , In vitro and in vivo evaluation of recombinant silk-elastinlike hydrogels for cancer gene therapy. Journal of Controlled Release, 2004. 94(2-3): p. 433–445. [DOI] [PubMed] [Google Scholar]
- 319.Gustafson JA, et al. , Silk-Elastin-like Hydrogel Improves the Safety of Adenovirus-Mediated Gene-Directed Enzyme-Prodrug Therapy. Molecular Pharmaceutics, 2010. 7(4): p. 1050–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Fernandez-Colino A, et al. , Self-Assembling Elastin-Like Hydrogels for Timolol Delivery: Development of an Ophthalmic Formulation Against Glaucoma. Molecular Pharmaceutics, 2017. 14(12): p. 4498–4508. [DOI] [PubMed] [Google Scholar]
- 321.Cipriani F, et al. , Cartilage Regeneration in Preannealed Silk Elastin-Like Co-Recombinamers Injectable Hydrogel Embedded with Mature Chondrocytes in an Ex Vivo Culture Platform. Biomacromolecules, 2018. 19(11): p. 4333–4347. [DOI] [PubMed] [Google Scholar]
- 322.Parisi-Amon A, et al. , Protein-Engineered Injectable Hydrogel to Improve Retention of Transplanted Adipose-Derived Stem Cells. Advanced Healthcare Materials, 2013. 2(3): p. 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Greenwood-Goodwin M, Teasley ES, and Heilshorn SC, Dual-stage growth factor release within 3D protein-engineered hydrogel niches promotes adipogenesis. Biomaterials Science, 2014. 2(11): p. 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Mulyasasmita W, et al. , Avidity-Controlled Delivery of Angiogenic Peptides from Injectable Molecular-Recognition Hydrogels. Tissue Engineering Part A, 2014. 20(15-16): p. 2102–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Soranno DE, et al. , Immunotherapy with injectable hydrogels to treat obstructive nephropathy. Journal of Biomedical Materials Research Part A, 2014. 102(7): p. 2173–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Cai L, Dewi RE, and Heilshorn SC, Injectable Hydrogels with In Situ Double Network Formation Enhance Retention of Transplanted Stem Cells. Advanced Functional Materials, 2015. 25(9): p. 1344–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Bhattarai N, Gunn J, and Zhang MQ, Chitosan-based hydrogels for controlled, localized drug delivery. Advanced Drug Delivery Reviews, 2010. 62(1): p. 83–99. [DOI] [PubMed] [Google Scholar]
- 328.Gombotz WR and Wee SF, Protein release from alginate matrices. Advanced Drug Delivery Reviews, 2012. 64: p. 194–205. [DOI] [PubMed] [Google Scholar]
- 329.Akiyoshi K, et al. , Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: Complexation and stabilization of insulin. Journal of Controlled Release, 1998. 54(3): p. 313–320. [DOI] [PubMed] [Google Scholar]
- 330.Cerchiara T, et al. , Physically cross-linked chitosan hydrogels as topical vehicles for hydrophilic drugs. Journal of Pharmacy and Pharmacology, 2002. 54(11): p. 1453–1459. [DOI] [PubMed] [Google Scholar]
- 331.Martin L, et al. , The release of model macromolecules may be controlled by the hydrophobicity of palmitoyl glycol chitosan hydrogels. Journal of Controlled Release, 2002. 80(1-3): p. 87–100. [DOI] [PubMed] [Google Scholar]
- 332.Mealy JE, Rodell CB, and Burdick JA, Sustained small molecule delivery from injectable hyaluronic acid hydrogels through host-guest mediated retention. Journal of Materials Chemistry B, 2015. 3(40): p. 8010–8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Rodell CB, et al. , Selective Proteolytic Degradation of Guest-Host Assembled, Injectable Hyaluronic Acid Hydrogels. Acs Biomaterials Science & Engineering, 2015. 1(4): p. 277–286. [DOI] [PubMed] [Google Scholar]
- 334.Loebel C, et al. , Shear-thinning and self-healing hydrogels as injectable therapeutics and for 3D-printing. Nature Protocols, 2017. 12(8): p. 1521–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Rowland MJ, et al. , An adherent tissue-inspired hydrogel delivery vehicle utilised in primary human glioma models. Biomaterials, 2018. 179: p. 199–208. [DOI] [PubMed] [Google Scholar]
- 336.Ding Y-F, et al. , Oral Colon-Targeted Konjac Glucomannan Hydrogel Constructed through Noncovalent Cross-Linking by Cucurbit[8]uril for Ulcerative Colitis Therapy. ACS Applied Bio Materials, 2020. 3(1): p. 10–19. [DOI] [PubMed] [Google Scholar]
- 337.Zhao X, Multi-scale multi-mechanism design of tough hydrogels: building dissipation into stretchy networks. Soft matter, 2014. 10(5): p. 672–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Discher DE, Janmey P, and Wang Y.-l., Tissue cells feel and respond to the stiffness of their substrate. Science, 2005. 310(5751): p. 1139–1143. [DOI] [PubMed] [Google Scholar]
- 339.Cui H, et al. , Spontaneous and x-ray–triggered crystallization at long range in self-assembling filament networks. Science, 2010. 327(5965): p. 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Chen F, et al. , General strategy to fabricate strong and tough low-molecular-weight gelator-based supramolecular hydrogels with double network structure. Chemistry of Materials, 2018. 30(5): p. 1743–1754. [Google Scholar]
- 341.Koch F, et al. , Mechanical characteristics of beta sheet-forming peptide hydrogels are dependent on peptide sequence, concentration and buffer composition. Royal Society open science, 2018. 5(3): p. 171562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Kobayashi M, Chang Y-S, and Oka M, A two year in vivo study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus. Biomaterials, 2005. 26(16): p. 3243–3248. [DOI] [PubMed] [Google Scholar]
- 343.Ghanaati S, et al. , Dynamic in vivo biocompatibility of angiogenic peptide amphiphile nanofibers. Biomaterials, 2009. 30(31): p. 6202–6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Sijbesma RP, et al. , Reversible polymers formed from self-complementary monomers using quadruple hydrogen bonding. Science, 1997. 278(5343): p. 1601–1604. [DOI] [PubMed] [Google Scholar]
- 345.Chirila TV, et al. , Hydrogen-bonded supramolecular polymers as self-healing hydrogels: Effect of a bulky adamantyl substituent in the ureido-pyrimidinone monomer. Journal of Applied Polymer Science, 2014. 131(4). [Google Scholar]
- 346.Huan X, et al. , Supramolecular ABC miktoarm star terpolymer based on host–guest inclusion complexation. Macromolecules, 2012. 45(15): p. 5941–5947. [Google Scholar]
- 347.Rodell CB, Kaminski AL, and Burdick JA, Rational design of network properties in guest–host assembled and shear-thinning hyaluronic acid hydrogels. Biomacromolecules, 2013. 14(11): p. 4125–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Mantooth SM, Munoz-Robles BG, and Webber MJ, Dynamic hydrogels from host–guest supramolecular interactions. Macromolecular Bioscience, 2019. 19(1): p. 1800281. [DOI] [PubMed] [Google Scholar]
- 349.Daånmark S, Aronsson C, and Aili D, Tailoring Supramolecular Peptide–Poly (ethylene glycol) Hydrogels by Coiled Coil Self-Assembly and Self-Sorting. Biomacromolecules, 2016. 17(6): p. 2260–2267. [DOI] [PubMed] [Google Scholar]
- 350.Selegård R, et al. , Folding driven self-assembly of a stimuli-responsive peptide-hyaluronan hybrid hydrogel. Scientific reports, 2017. 7(1): p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Lin DC, Yurke B, and Langrana NA, Mechanical properties of a reversible, DNA-crosslinked polyacrylamide hydrogel. J. Biomech. Eng, 2004. 126(1): p. 104–110. [DOI] [PubMed] [Google Scholar]
- 352.Chu T-W, et al. , Hybrid polymeric hydrogels via peptide nucleic acid (PNA)/DNA complexation. Journal of Controlled Release, 2015. 220: p. 608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Lin D, Yurke B, and Langrana N, Inducing reversible stiffness changes in DNA-crosslinked gels. Journal of materials research, 2005. 20(6): p. 1456–1464. [Google Scholar]
- 354.Culver HR, et al. , Click Nucleic Acid-DNA Binding Behavior: Dependence on Length, Sequence, and Ionic Strength. Biomacromolecules, 2020. [DOI] [PubMed] [Google Scholar]
- 355.Zou L, Braegelman AS, and Webber MJ, Dynamic supramolecular hydrogels spanning an unprecedented range of host–guest affinity. ACS applied materials & interfaces, 2019. 11(6): p. 5695–5700. [DOI] [PubMed] [Google Scholar]
- 356.Gong JP, et al. , Double-network hydrogels with extremely high mechanical strength. Advanced materials, 2003. 15(14): p. 1155–1158. [Google Scholar]
- 357.Aviv M, et al. , Improving the mechanical rigidity of hyaluronic acid by integration of a supramolecular peptide matrix. ACS applied materials & interfaces, 2018. 10(49): p. 41883–41891. [DOI] [PubMed] [Google Scholar]
- 358.Criado-Gonzalez M, et al. , Supramolecular Hydrogel Induced by Electrostatic Interactions between Polycation and Phosphorylated-Fmoc-Tripeptide. Chemistry of Materials, 2020. 32(5): p. 1946–1956. [Google Scholar]
- 359.Clarke DE, et al. , Self-healing, self-assembled β-sheet peptide–poly (γ-glutamic acid) hybrid hydrogels. Journal of the American Chemical Society, 2017. 139(21): p. 7250–7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Chan NJ-A, et al. , Spider-silk inspired polymeric networks by harnessing the mechanical potential of β-sheets through network guided assembly. Nature communications, 2020. 11(1): p. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Sun W, et al. , Polymer-supramolecular polymer double-network hydrogel. Advanced Functional Materials, 2016. 26(48): p. 9044–9052. [Google Scholar]
- 362.Radvar E and Azevedo HS, Supramolecular peptide/polymer hybrid hydrogels for biomedical applications. Macromolecular bioscience, 2019. 19(1): p. 1800221. [DOI] [PubMed] [Google Scholar]
- 363.Liechty WB, et al. , Polymers for drug delivery systems. Annual review of chemical and biomolecular engineering, 2010. 1: p. 149–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Ghosh M, et al. , Injectable alginate-peptide composite hydrogel as a scaffold for bone tissue regeneration. Nanomaterials, 2019. 9(4): p. 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Huang R, et al. , Self-assembling peptide–polysaccharide hybrid hydrogel as a potential carrier for drug delivery. Soft Matter, 2011. 7(13): p. 6222–6230. [Google Scholar]
- 366.Xing R, et al. , Self-assembled injectable peptide hydrogels capable of triggering antitumor immune response. Biomacromolecules, 2017. 18(11): p. 3514–3523. [DOI] [PubMed] [Google Scholar]
- 367.Abraham JN, et al. , Injectable dextran-fluorenylmethoxycarbonyl phenylalanine composite hydrogels with improved mechanical properties. Polymer International. [Google Scholar]
- 368.Borges J, et al. , Nanoengineering Hybrid Supramolecular Multilayered Biomaterials Using Polysaccharides and Self-Assembling Peptide Amphiphiles. Advanced Functional Materials, 2017. 27(17): p. 1605122. [Google Scholar]
- 369.Goktas M, et al. , Self-assembled peptide amphiphile nanofibers and PEG composite hydrogels as tunable ECM mimetic microenvironment. Biomacromolecules, 2015. 16(4): p. 1247–1258. [DOI] [PubMed] [Google Scholar]
- 370.Spicer CD, Pashuck ET, and Stevens MM, Achieving Controlled Biomolecule–Biomaterial Conjugation. Chemical reviews, 2018. 118(16): p. 7702–7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Sun J-Y, et al. , Highly stretchable and tough hydrogels. Nature, 2012. 489(7414): p. 133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Valeur E and Bradley M, Amide bond formation: beyond the myth of coupling reagents. Chemical Society Reviews, 2009. 38(2): p. 606–631. [DOI] [PubMed] [Google Scholar]
- 373.Zhang F, et al. , Peptide-/Drug-Directed Self-Assembly of Hybrid Polyurethane Hydrogels for Wound Healing. ACS applied materials & interfaces, 2019. 11(40): p. 37147–37155. [DOI] [PubMed] [Google Scholar]
- 374.Capito RM, et al. , Self-assembly of large and small molecules into hierarchically ordered sacs and membranes. Science, 2008. 319(5871): p. 1812–1816. [DOI] [PubMed] [Google Scholar]
- 375.Carvajal D, et al. , Physical properties of hierarchically ordered self-assembled planar and spherical membranes. Soft Matter, 2010. 6(8): p. 1816–1823. [Google Scholar]
- 376.Chow LW, et al. , A bioactive self-assembled membrane to promote angiogenesis. Biomaterials, 2011. 32(6): p. 1574–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.McKay CS and Finn M, Click chemistry in complex mixtures: bioorthogonal bioconjugation. Chemistry & biology, 2014. 21(9): p. 1075–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Tang W and Becker ML, “Click” reactions: a versatile toolbox for the synthesis of peptide-conjugates. Chemical Society Reviews, 2014. 43(20): p. 7013–7039. [DOI] [PubMed] [Google Scholar]
- 379.Chow LW, et al. , Peptide-Directed Spatial Organization of Biomolecules in Dynamic Gradient Scaffolds. Advanced healthcare materials, 2014. 3(9): p. 1381–1386. [DOI] [PubMed] [Google Scholar]
- 380.Lutolf M and Hubbell J, Synthesis and physicochemical characterization of end-linked poly (ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules, 2003. 4(3): p. 713–722. [DOI] [PubMed] [Google Scholar]
- 381.Barker K, et al. , Biodegradable DNA-enabled poly (ethylene glycol) hydrogels prepared by copper-free click chemistry. Journal of Biomaterials Science, Polymer Edition, 2016. 27(1): p. 22–39. [DOI] [PubMed] [Google Scholar]
- 382.Gelmi A and Schutt CE, Stimuli-Responsive Biomaterials: Scaffolds for Stem Cell Control. Advanced Healthcare Materials: p. 2001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Khoshakhlagh P and Moore MJ, Photoreactive interpenetrating network of hyaluronic acid and Puramatrix as a selectively tunable scaffold for neurite growth. Acta Biomaterialia, 2015. 16: p. 23–34. [DOI] [PubMed] [Google Scholar]
- 384.Ruskowitz ER and DeForest CA, Photoresponsive biomaterials for targeted drug delivery and 4D cell culture. Nature Reviews Materials, 2018. 3(2): p. 1–17. [Google Scholar]
- 385.Hsu L, Cvetanovich GL, and Stupp SI, Peptide amphiphile nanofibers with conjugated polydiacetylene backbones in their core. Journal of the American Chemical Society, 2008. 130(12): p. 3892–3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.van den Heuvel M, Löwik DW, and van Hest JC, Self-assembly and polymerization of diacetylene-containing peptide amphiphiles in aqueous solution. Biomacromolecules, 2008. 9(10): p. 2727–2734. [DOI] [PubMed] [Google Scholar]
- 387.Wei Q, et al. , Printable hybrid hydrogel by dual enzymatic polymerization with superactivity. Chemical science, 2016. 7(4): p. 2748–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Highley CB, Rodell CB, and Burdick JA, Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels. Advanced Materials, 2015. 27(34): p. 5075–5079. [DOI] [PubMed] [Google Scholar]
- 389.Li C, et al. , Supramolecular–covalent hybrid polymers for light-activated mechanical actuation. Nature materials, 2020. 19(8): p. 900–909. [DOI] [PubMed] [Google Scholar]
- 390.Gong JP, Why are double network hydrogels so tough? Soft Matter, 2010. 6(12): p. 2583–2590. [Google Scholar]
- 391.Shull KR, A hard concept in soft matter. Nature, 2012. 489(7414): p. 36–37. [DOI] [PubMed] [Google Scholar]
- 392.Creton C, 50th anniversary perspective: Networks and gels: Soft but dynamic and tough. Macromolecules, 2017. 50(21): p. 8297–8316. [Google Scholar]
- 393.Dhand AP, Galarraga JH, and Burdick JA, Enhancing Biopolymer Hydrogel Functionality through Interpenetrating Networks. Trends in Biotechnology, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Zhao X, et al. , Physical Double-Network Hydrogel Adhesives with Rapid Shape Adaptability, Fast Self-Healing, Antioxidant and NIR/pH Stimulus-Responsiveness for Multidrug-Resistant Bacterial Infection and Removable Wound Dressing. Advanced Functional Materials, 2020. 30(17): p. 1910748. [Google Scholar]
- 395.Ishikawa S, et al. , Interpenetrating Polymer Network Hydrogels via a One-Pot and in Situ Gelation System Based on Peptide Self-Assembly and Orthogonal Cross-Linking for Tissue Regeneration. Chemistry of Materials, 2020. 32(6): p. 2353–2364. [Google Scholar]
- 396.Rajangam K, et al. , Peptide amphiphile nanostructure–heparin interactions and their relationship to bioactivity. Biomaterials, 2008. 29(23): p. 3298–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Patterson AK and Smith DK, Two-component supramolecular hydrogel for controlled drug release. Chemical Communications, 2020. [DOI] [PubMed] [Google Scholar]
- 398.Okesola BO, et al. , Supramolecular self-assembly to control structural and biological properties of multicomponent hydrogels. Chemistry of Materials, 2019. 31(19): p. 7883–7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Zhan H and Löwik DW, A hybrid peptide amphiphile fiber PEG hydrogel matrix for 3D cell culture. Advanced Functional Materials, 2019. 29(16): p. 1808505. [Google Scholar]
- 400.Wang K, et al. , A hyaluronidase/temperature dual-responsive supramolecular assembly based on the anionic recognition of calixpyridinium. Chemical Communications, 2017. 53(54): p. 7517–7520. [DOI] [PubMed] [Google Scholar]
- 401.Yu C, Alkekhia D, and Shukla A, β-Lactamase Responsive Supramolecular Hydrogels with Host–Guest Self-Healing Capability. ACS Applied Polymer Materials, 2019. 2(1): p. 55–65. [Google Scholar]
- 402.Pashuck ET, Designing self-assembling biomaterials with controlled mechanical and biological performance, in Self-assembling biomaterials. 2018, Elsevier. p. 7–26. [Google Scholar]
- 403.Webber MJ, et al. , Supramolecular biomaterials. Nature materials, 2016. 15(1): p. 13–26. [DOI] [PubMed] [Google Scholar]