Abstract
Metabolic disorders represent a growing worldwide health challenge due to their dramatically increasing prevalence. The gut microbiota is a crucial actor that can interact with the host by the production of a diverse reservoir of metabolites, from exogenous dietary substrates or endogenous host compounds. Metabolic disorders are associated with alterations in the composition and function of the gut microbiota. Specific classes of microbiota-derived metabolites, notably bile acids, short-chain fatty acids, branched-chain amino acids, trimethylamine N-oxide, tryptophan and indole derivatives, have been implicated in the pathogenesis of metabolic disorders. This review aims to define the key classes of microbiota-derived metabolites that are altered in metabolic diseases and their role in pathogenesis. They represent potential biomarkers for early diagnosis and prognosis as well as promising targets for the development of novel therapeutic tools for metabolic disorders.
Keywords: intestinal microbiology, bile acid metabolism, obesity
Key messages.
Metabolic disorders, a growing worldwide health challenge, are associated with alterations in the composition and function of the gut microbiota.
Microbial metabolites are key factors in host-microbiota cross-talk.
Specific classes of microbiota-derived metabolites, notably bile acids, short-chain fatty acids, branched-chain amino acids, trimethylamine N-oxide, tryptophan and indole derivatives, have been strongly implicated in the pathogenesis of metabolic disorders.
Gut microbiota-derived metabolites represent potential biomarkers for the early diagnosis and show promise for identifying targets for the development of novel therapeutic tools for metabolic disorders.
Introduction
The human intestine harbours a complex and diverse system of mutualistic microorganisms, consisting of bacteria, fungi, viruses, archaea and protozoa. This rich ecosystem contributes to a large number of physiological functions: fermentation of indigestible dietary components and vitamin synthesis, defenses against pathogens, host immune system maturation and maintenance of gut barrier function.1 2 Thus, this central regulator, sometimes qualified as the ‘second brain’, plays a significant role in maintaining host physiology and homeostasis. All the species interconnected in the gut produce an extremely diverse reservoir of metabolites from exogenous dietary components and/or endogenous compounds generated by microorganisms and the host. Notably, while food is generally examined for calories and macronutrients and micronutrients, microbial metabolism (and even human enzymes) recognises food molecules and transforms them into metabolites. These microbial metabolites are key actors in host-microbiota cross-talk. The beneficial or detrimental effect of specific microbiota-derived metabolites depends on the context and the host state, suggesting the primordial nature of the symbiotic microbiota in ensuring optimal health in humans.3
With the widespread westernisation of lifestyles, alteration of the gut microbiota composition and functions has become a worldwide phenomenon. Despite the difficulty to distinct a direct causal relationship and an association between dysbiosis and diseases, several lines of evidence demonstrate that the alteration of the gut microbiota is involved in the pathogenesis of multiple diseases affecting the GI tract, such as IBD4 or colorectal cancer,5 as well as many non-digestive systems. Metabolic disorders have been recognised to be massively impacted by gut microbiota.6 In the last two decades, increasing calorie intake and decreasing levels of physical activity have contributed to a progression in the prevalence of metabolic disorders. Metabolic disorders represent a group of disorders with the clustering of various inter-related pathological conditions combining obesity, non-alcoholic steatohepatitis (NASH), dyslipidaemia, glucose intolerance, insulin resistance, hypertension and diabetes that, when occurring together, strongly increase the incidence of cardiovascular diseases and mortality.7 8 Deciphering the mechanisms of host-intestinal microbiota interactions represents a major public health challenge in the development of new preventive or curative therapeutic strategies. In the present review, we will focus on the results from the most significant studies dealing with the role of microbiota-derived metabolites in metabolic disorders.
Disrupted equilibrium of the gut microbiome-host interactions in metabolic disorders
The gut microbiota plays a crucial role in maintaining the physiological functions of the host. A disruption of the fragile host-microbiota interaction equilibrium can play a role in the onset of several metabolic diseases. The gut microbiota can interact with the host by producing metabolites, which are small molecules (<1500 Da) representing intermediates or end-products of microbial metabolism. These metabolites can derive directly from bacteria or the transformation of dietary or host-derived substrates.9
Gut microbiota incrimination
The implication of the gut microbiota in the regulation of host metabolic balance has been demonstrated in the last decade.10 Studies conducted both in animal models and humans revealed a significant role of the gut microbiota in the pathogenesis of metabolic disorders, strongly influenced by diet and lifestyle modifications.
Evidence from animal experiments
The gut microbiota modulates energy expenditure and homeostasis in several animal models, including germ-free mice (GF mice) and genetically induced mice with obesity (ob/ob mice). GF mice are protected against obesity in a Western diet setting.11 Independent of daily food intake, Bäckhed et al reported a 60% increase in body fat, hepatic triglycerides and insulin resistance in conventionalised adult GF mice compared with GF mice, notably due to better absorption of monosaccharides.12 Interestingly, the transfer of gut microbiota from ob/ob mice to GF mice results in a significant increase in body weight and fat mass compared with colonisation with a lean microbiota, showing a causal relationship.13 The gut microbiota composition is unique to each individual. Caecal microbiota transplantation, from two mice with different responses to high-fat diet (HFD), into GF mice leads to the transmission of the donor’s responder (RR) or non-responder (NR) phenotype. The gut microbiota of severely hyperglycaemic RR mice is enriched in Firmicutes, whereas NR is dominated by Bacteroidetes and Actinobacteria.14 Moreover, the transplantation of faecal microbiota from human twin pairs, discordant for obesity, into GF mice led to the acquisition of lean and obese phenotypes according to the donor. This phenotype transmission is strongly diet-dependent and notably favoured by a low-fat diet enriched in vegetables and fruits and thus enriched in fibre.15 The effect of the gut microbiota seems to occur even before birth, as the maternal gut microbiota, through short-chain fatty acid (SCFAs), triggers embryonic GPR41 and GPR43 and influences prenatal development of neural, enteroendocrine and pancreatic systems of the offspring to maintain postnatal energy homeostasis and eventually prevent metabolic disorder development.16
Overall, these animal studies demonstrate the tight interconnection between diet and the gut microbiome in the pathogenesis of metabolic disorders as well as in its vertical transmissibility.
Evidence from human studies
Alterations in the gut microbiome composition and functions are associated with various traits observed in metabolic disorders. Although there are some conflicting results, the obesity-associated gut microbiota has been characterised by a decline in Bacteroidetes and a compensatory expansion of the Firmicutes phylum17 and by a reduction in microbial diversity and richness.18 There is notably a negative correlation between the severity of metabolic markers and the richness of the gut microbiota. Individuals with low microbiota gene content present more adiposity, insulin resistance and dyslipidaemia than high bacterial richness populations.19 Even in severe obesity conditions, those with diminished gut microbiota richness have a more severe metabolic condition.20
In patients with diabetes, the higher proximity of the altered microbiota to epithelial cells could promote pro-inflammatory signals, contributing to the development of aggravated metabolic alterations.21 In humans, faecal microbiota transplantation (FMT) demonstrated some positive but moderate effects in patients with metabolic syndrome traits, proving the involvement of the gut microbiota in the pathogenesis and its potential therapeutic role.22–24 However, the efficiency of FMT in improving metabolic amelioration was dependent on the recipient gut microbiota profile, with low baseline richness promoting gut microbiota engraftment.
Gut microbiota-derived metabolite implications in metabolic diseases
The gut metabolome
Metabolomics, which consists of the study of the small molecules present in any type of biological sample, has proven to be helpful in enriching the knowledge on microbiota-host interactions.25 Several hundred faecal or serum metabolites have been associated with clinical features associated with metabolic disorders.26 Moreover, a combination of metagenomics and metabolomics was used to elucidate the associations between gut microbiota imbalances and metabolic disturbances. This field is still in its infancy and, for some metabolites, it remains difficult to determine whether they are fully microbiota-derived or if other sources are involved, including diet or the host itself.
Metagenome and metabolome studies led to the discovery of new associations between microbial-derived metabolites and metabolic syndrome, but additional arguments are needed to establish a potential causality link. Notably, the decreased abundance of Bacteroides thetaiotaomicron, a glutamate fermenting commensal, in subjects with obesity is inversely correlated with serum glutamate.27 Furthermore, positive correlations between insulin resistance and microbial functions are driven mainly by a few species, such as Prevotella copri and Bacteroides vulgatus, suggesting that they may directly impact host metabolism.28 Metabolomics studies in plasma, saliva or urine identified different biochemical classes of metabolites that may be altered in metabolic disorders in association with gut microbiota perturbations. Dysregulation of lipolysis, fatty acid oxidation and aminogenesis and ketogenesis, as well as changes in the levels of triglycerides, phospholipids and trimethylamine N-oxide (TMAO) are described in samples from humans with metabolic disorders,29–32 and more recently, imidazole propionate (IMP) was discovered as being involved in insulin resistance.33 Shotgun metagenomics data suggest that hepatic steatosis and metabolic alterations are associated with dysregulated aromatic and branched-chain amino acid (BCAA) metabolism.34 The dysregulation of SCFA35 and bile acid (BA)36 metabolism are also associated with metabolic diseases, including obesity, type 2 diabetes mellitus and non-alcoholic fatty liver diseases.
Bile acids
BAs are small molecules synthesised in hepatocytes from cholesterol. The primary BAs chenodeoxycholic acid (CDCA) and cholic acid (CA), conjugated to glycine or taurine, are essential for lipid/vitamin digestion and absorption. Ninety-five per cent of them are reabsorbed actively from the terminal ileum and are recycled in the liver (enterohepatic circulation). Primary BAs are also transformed into secondary BAs and deconjugated by gut microbiota. They can be either passively reabsorbed to reenter the circulating BA pool or excreted in the faeces (figure 1).37
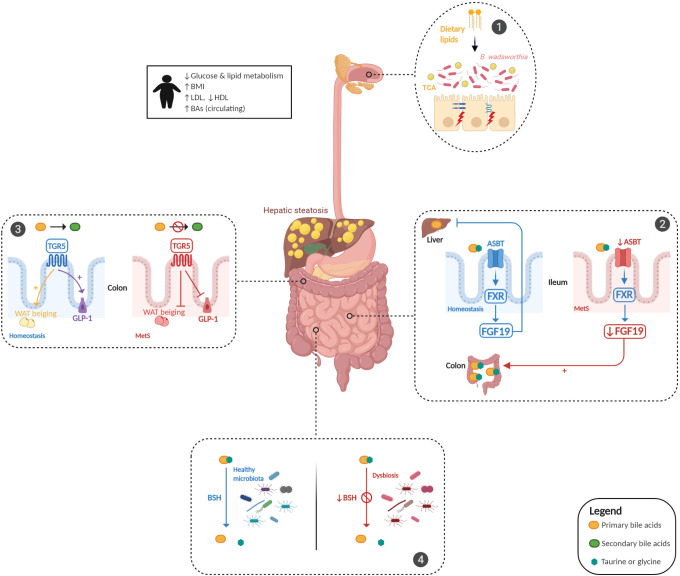
Figure 1.
Bile acid (BA) dysmetabolism in metabolic syndrome. BA metabolism is altered in patients with metabolic syndrome (MetS) and is associated with hepatic steatosis and glucose and lipid dysmetabolism. Dietary animal fat consumption promotes taurocholic acid (TCA) production, which favours the proliferation of sulfite-reducing bacteria, Bilophila wadsworthia, leading to an increase in intestinal permeability and inflammation (panel 1). Gut microbiota alterations induce an impairment in the ileal absorption of BAs, which occurs normally via the apical-sodium BA transporter (ASBT). This induces a decrease in the expression of nuclear Farnesoid-X receptor (FXR) and fibroblast growth factor 19 (FGF19) in intestinal epithelial cells and the abundance of colonic primary conjugated BAs (panel 2). Gut microbiota dysfunction leads to a decreased transformation of primary conjugated BAs to secondary BAs in the colon, leading to defective activation of Takeda-G-protein-receptor-5 (TGR5). The effect of TGR5 activation on the increase in glucagon-like peptide 1 (GLP-1) and white adipose tissue (WAT) browning was thus inhibited (panel 3). Gut microbiota alterations impair bile salt hydrolase (BSH) activity, leading to primary conjugated BA accumulation in the colon (panel 4). BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Increased total circulating BA levels in individuals with obesity positively correlate with body mass index and serum triglycerides in patients with hyperlipidaemia.38 BAs regulate their synthesis through FGF19/FGF15, but they also have metabolic effects through their receptors Farnesoid-X receptor (FXR) and Takeda-G-protein-receptor-5 (TGR5). Activation of FXR and TGR5 (1) promotes glycogen synthesis and insulin sensitivity in the liver; (2) increases insulin secretion by the pancreas; (3) facilitates energy expenditure, especially in the liver, brown adipose tissue and muscles (browning); (4) favours thermogenesis, resulting in a decrease in body weight and (5) mediates satiety in the brain.39 BAs also impact lipid metabolism, especially by exerting profound effects on triacylglycerol. The perturbations of the intestinal microbiota composition in metabolic disorders strongly impact BA metabolism, especially characterised by a failure to metabolise primary BAs, thus leading to their accumulation. Indeed, an increase in primary CDCA levels induces a decrease in very low-density lipoprotein production and plasma triglyceride concentrations. Short-term antibiotic supplementation in mice induces a decrease in secondary BA-producing bacteria and a reduction in hepatic deoxycholic acid (DCA) and lithocholic acid concentrations as well as serum triglyceride levels, suggesting that secondary BAs can act as regulators to maintain metabolic host homeostasis.40 Moreover, this alteration in the primary to secondary BA pool in metabolic disorders might play a role in the observed low-grade intestinal inflammation, as conjugated primary BAs exhibit pro-inflammatory effects on intestinal epithelial cells. Conversely, secondary BAs have anti-inflammatory properties.41 In addition, Parséus et al showed that the promoting effect of the gut microbiome on obesity and hepatic steatosis is dependent on the FXR pathway.42 However, the FXR-dependent role of secondary BAs in the regulation of glucose and lipid metabolism is debated and might be context-dependent. The accumulation of hepatic lipids, triglycerides and cholesterol has been observed in FXR-deficient mice on a normal chow diet,43 while in HFD-fed mice or an obese background, FXR deficiency improves glucose homeostasis and decreases body weight,42 44 possibly a consequence of different basal gut microbiota. The effects of FXR in the pathogenesis of metabolic disorders are also likely to be different from one tissue to the other, as demonstrated by studies in conditional knockout mice.45 46 FXR induces the transcription of fibroblast growth factor 19 (FGF19) in intestinal epithelial cells, which reach the liver and inhibit BA synthesis in a feedback loop. Mice overexpressing FGF19 exhibit increased metabolic activity and energy expenditure by increasing brown adipose tissue and decreasing liver expression of acetyl coenzyme A carboxylase 2, thus leading to protection against HFD-induced metabolic injury.47 Gut microbiota perturbations induce impairment in the ileal absorption of BAs, which normally occurs via the apical-sodium bile acid transporter, resulting in decreased expression of FXR and FGF19 and an imbalance of BAs, notably characterised by an increase in colonic primary conjugated BAs.48 Transgenic mice overexpressing TGR5 exhibit improved glucose tolerance with increased secretion of glucagon-like peptide 1 (GLP-1) and insulin.49 This BA-TGR5 axis elicits beige remodelling in subcutaneous white adipose tissue and may contribute to improvement in whole-body energy homeostasis.50 The alteration of gut microbiota-dependent BA metabolism, through qualitative (primary vs secondary and conjugated vs deconjugated BAs) or quantitative modification of the BA pool, is likely to participate in the pathogenesis of metabolic disorders. Moreover, BAs have an important impact on intestinal epithelium function. Primary BAs, such as CA and CDCA, and some secondary deconjugated BAs, such as DCA, increase epithelial permeability through the phosphorylation of occludin in intestinal Caco-2 cells.51 52 Some correlations have been observed between BA levels and intestinal permeability in mouse models.53 The effect of the BA-microbiota dialogue is massively impacted by diet. High consumption of animal fat promotes taurocholic acid production, leading to a shift in microbiota composition with a bloom of sulfite-reducing microorganisms such as Bilophila wadsworthia and to increased susceptibility to colitis in IL-10−/− mice and more severe liver steatosis, barrier dysfunction and glucose metabolism alteration in HFD-fed mice.54 55 Moreover, bile salt hydrolase (BSH) activity, which is responsible for BA deconjugation in the normal gut microbiota, is impaired in metabolic disorders and likely plays a role in the accumulation of primary conjugated BAs in the colon of these patients. In mouse models, correcting BSH defects by the administration of BSH-overexpressing Escherichia coli improved lipid metabolism, homeostasis and circadian rhythm in the liver and GI tract, resulting in protection against metabolic disorders.56
Short-chain fatty acids
SCFAs, such as butyrate, propionate and acetate, are end-products of microbial fermentation implicated in a multitude of physiological functions.57 SCFAs participate in the maintenance of intestinal mucosa integrity,58 improve glucose and lipid metabolism,59 control energy expenditure60 and regulate the immune system and inflammatory responses (figure 2).35 They act through different mechanisms, including specific G protein-coupled receptor family (GPCR)61 and epigenetic effects.
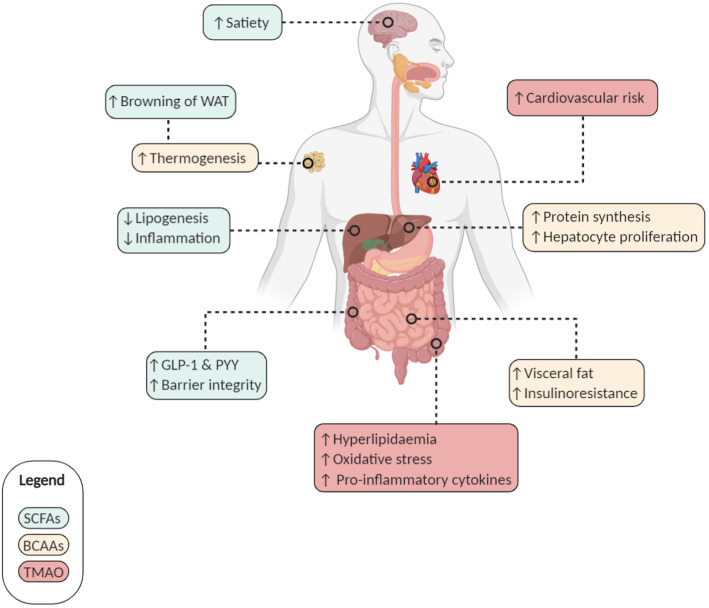
Figure 2.
Short-chain fatty acids (SCFAs), branched-chain amino acids (BCAAs) and Trimethylamine N-oxide (TMAO): relevant effects for metabolic syndrome on the host. Microbiota-derived metabolites mediate diverse effects on host metabolism. SCFAs (green frame): (i) increase satiety and browning of white adipose tissue (WAT); (ii) induce a decrease in lipogenesis and associated inflammation; (iii) increase the secretion of glucagon-like peptide 1 (GLP-1) and peptide YY (PYY) and (iv) participate in the maintenance of intestinal barrier integrity. BCAAs (yellow frame): (i) increase thermogenesis, protein synthesis and hepatocyte proliferation but (ii) are also associated with insulin resistance and visceral fat accumulation. TMAO (red frame): increases cardiovascular risks by inducing hyperlipidaemia, oxidative stress and pro-inflammatory cytokines.
The amount of SCFA-producing bacteria and SCFAs is reduced in faecal samples of dysmetabolic mice62 and in humans with obesity and diabetes.63 In rodents with diabetes and obesity, supplementation with SCFAs improves the metabolic phenotype by increasing energy expenditure, glucose tolerance and homeostasis.64 Adding back fermentable fibres (inulin) to an HFD seems to be enough to protect against metabolic alterations.65 In humans, SCFA administration (inulin-propionate ester, acetate or propionate) stimulates the production of GLP-1 and PYY, leading to a reduction in weight gain.59 66 The protective effects of SCFAs on metabolic alterations might occur as early as in utero. In mice, high-fibre diet-induced propionate from the maternal microbiota crosses the placenta and confers resistance to obesity in offspring through the SCFA-GPCR axis.16
Branched-chain amino acids
The most abundant BCAAs, valine, isoleucine and leucine, are essential amino acids synthesised by plants, fungi and bacteria, particularly by members of the gut microbiota. They play a critical role in maintaining homeostasis in mammals by regulating protein synthesis, glucose and lipid metabolism, insulin resistance, hepatocyte proliferation and immunity.67 BCAA catabolism is essential in brown adipose tissue (BAT) to control thermogenesis. It occurs in mitochondria via SLC25A44 transporters and contributes to an improvement in metabolic status.68 Moreover, supplementation of mice with a mixture of BCAAs promotes a healthy microbiota with an increase in Akkermansia and Bifidobacterium and a decrease in Enterobacteriaceae.69 However, the potential positive effects of BCAAs are controversial. Elevated systemic BCAA levels are associated with obesity and diabetes, probably a consequence of the 20% increased consumption of calories over the last 50 years.70 In genetically obese mice (ob/ob mice), BCAA accumulation induces insulin resistance.71 The gut microbiota is a modulator of BCAA levels, as it can both produce and use BCAAs. Prevotella copri and B. vulgatus are potent producers of BCAAs, and their amounts correlate positively with BCAA levels and insulin resistance. In parallel, a reduced abundance of bacteria able to take up BCAAs, such as Butyrivibrio crossotus and Eubacterium siraeum, occurs in patients with insulin resistance.28 Further studies are needed to more precisely elucidate the effects of BCAAs in the pathogenesis of metabolic disorders.
Trimethylamine N-oxide
The gut microbiota can metabolise choline and L-carnitine from dietary sources (eg, red meat, eggs and fish) to produce trimethylamine (TMA). This gut microbiota-derived TMA is then absorbed and reaches the liver where it is converted into TMAO72 through the enzymatic activity of hepatic flavin monooxygenases 3.
In humans, the level of TMAO increases in patients with diabetes73 or at risk of diabetes74 and in obesity.72 Increasing evidence demonstrates that the gut microbiota-dependent metabolite TMAO is also associated with a higher risk of developing cardiovascular disease and kidney failure. In mice, dietary supplementation with TMAO, carnitine or choline alters the caecal microbial composition, leading to TMA/TMAO production that increases the atherosclerosis risk. This effect is dependent on the gut microbiota, as it is lost in antibiotic-treated mice.75 Moreover, transferring the gut microbiota of high-TMAO mice recapitulates atherosclerosis susceptibility in recipient low-TMAO mice.76 Importantly, the role of the gut microbiota in the production of TMAO from TMA has also been demonstrated in humans.77 Overall, in metabolic disorders, the altered microbiota associated with an increased intake of choline and L-carnitine from dietary sources leads to an increase in plasma levels of TMAO, which is directly involved in the pathogenesis of metabolic disease comorbidities and particularly cardiovascular disorders. However, detailed investigations are needed in populations from different countries to understand the interaction between food consumption patterns, TMAO production and cardiovascular risks.
Tryptophan and indole-derivative metabolites
Tryptophan is an essential aromatic amino acid acquired through common diet sources, including oats, poultry, fish, milk and cheese. In addition to its role in protein synthesis, tryptophan is a precursor for crucial metabolites. Dietary tryptophan can follow two main pathways in host cells, namely, the kynurenine78 79 and serotonin80 routes. The third pathway implicates gut microorganisms in the direct metabolism of tryptophan into several molecules, such as indole and its derivatives, with some of them acting as aryl hydrocarbon receptor (AhR) ligands (figure 3).81 82
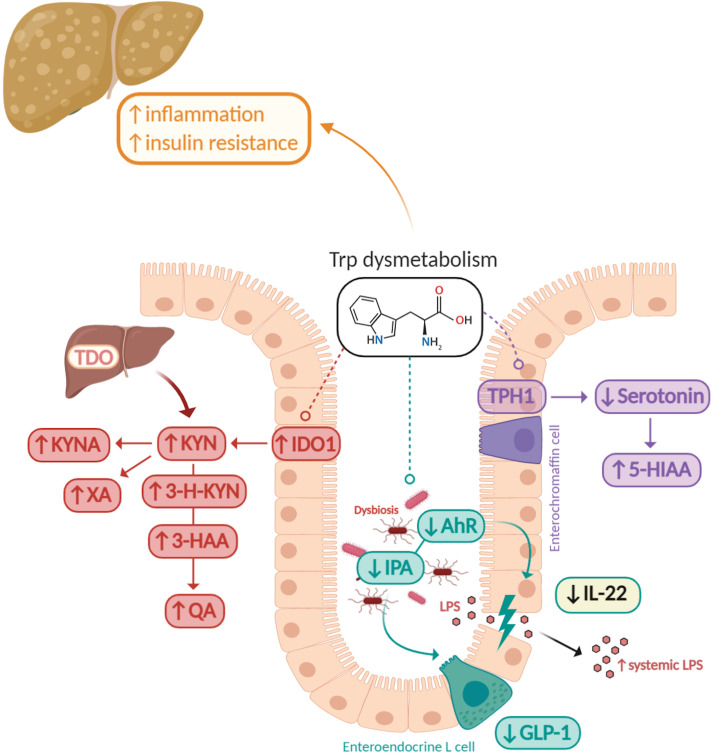
Figure 3.
Tryptophan metabolism alterations in metabolic syndrome. Tryptophan dysmetabolism is associated with liver inflammation, steatosis and insulin resistance. In metabolic syndrome (MetS), the inflammatory state is associated with kynurenine (KYN) production through the activation of indoleamine 2,3-dioxygenase 1 (IDO1). This leads to an increase in kynurenine-derived metabolites, such as kynurenic acid (KYNA), xanthurenic acid (XA), 3-hydroxykynurenine (3-H-KYN), 3-hydroxyanthranilic acid (3-HAA) and quinolinic acid (QA). In parallel, the gut microbiota presents a defect in the production of aryl hydrocarbon receptor (AhR) ligands such as indole-3-propionic acid (IPA). The incretin hormone glucagon-like peptide 1 (GLP-1) secretion from intestinal enteroendocrine L cells and interleukin (IL)-22 production are decreased, altering gut permeability and promoting lipopolysaccharide (LPS) translocation. Serotonin (5-HT) biosynthesis from intestinal enterochromaffin cells is also reduced in the context of MetS due to a decrease in the production of microbiota-derived metabolites inducing the production of host 5-HT.
We have identified in a previous study, in both preclinical and clinical settings, that metabolic disorders are characterised by a reduced capacity of the microbiota to metabolise tryptophan into AhR agonists.83 Defective activation of the AhR pathway leads to decreased production of GLP-1 and IL-22, which contribute to intestinal permeability and lipopolysaccharide (LPS) translocation, resulting in inflammation, insulin resistance and liver steatosis.84 In this context, treatment with AhR agonists or administration of Lactobacillus reuteri, which naturally produces AhR ligands, can reverse metabolic dysfunction.83 Similarly, indole prevents LPS-induced alterations of cholesterol metabolism and alleviates liver inflammation in mice.85 Moreover, exploring human jejunum samples from patients with severe obesity led to the observation that a low AhR tone correlated with a high inflammatory score. Interestingly, the use of the AhR ligand is able to prevent damage to barrier integrity and inflammation in Caco-2/TC7 cells.86
We and others also showed strong activation of the kynurenine pathway in metabolic diseases.83 87 Genetic or pharmacological approaches inhibiting the activity of indoleamine 2,3-dioxygenase (IDO), the rate-limiting enzyme of the kynurenine pathway, are protective against HFD-induced obesity and metabolic alterations.88 The mechanism is likely to be mediated by the microbiota and AhR. The increased amount of available tryptophan, due to the inactivation of IDO, can be converted by the microbiota in AhR agonists.89 Conversely, in obesity, the overactivation of IDO, associated with an increase in plasma levels of downstream metabolites such as kynurenic acid, xanthurenic acid, 3-hydroxykynurenine, 3-hydroxyanthranilic acid and quinolinic acid,90 decreases the tryptophan pool, which is less available for the production of AhR agonists by the microbiota.91 The third pathway of tryptophan metabolism, serotonin (5-HT), is also involved, as it affects feeding behaviour and satiety and is thus important for obesity development.92 The gut microbiota, and primarily indigenous spore-forming bacteria, represent an essential modulator of the intestinal production of 5-HT in enterochromaffin cells that represents >80% of the whole body 5-HT synthesis.80 These effects are notably mediated by SCFAs and BAs. Mice deficient for the production of peripheral serotonin are protected from HFD-induced obesity. Mechanistically, 5-HT inhibits brown adipose tissue thermogenesis, thus leading to fat accumulation.93 Human data support these results, as elevated plasma levels of 5-hydroxyindole-3-acetic acid, an end-product of serotonin metabolism, are increased in patients with metabolic disorders.94
Imidazole propionate
Exploring the interaction between food intake, gut microbiota and derived metabolites might be of interest to discover metabolites impacting metabolic health. As such, it was recently shown that IMP, a metabolite produced by histidine utilisation of gut microbiota, was enhanced in type 2 diabetes and associated with insulin resistance.33 In the liver, IMP appeared to affect the insulin signalling pathway via mammalian target of rapamycin complex 1 (mTORC1). The examination of IMP in large human cohorts also links it with metabolic health and lifestyle. IMP was elevated in subjects with prediabetes and diabetes in the MetaCardis cohort and in subjects with low bacterial gene richness and Bacteroides 2 enterotype in this cohort. Associations between IMP levels and markers of low-grade inflammation were also identified. Importantly, relationships were observed between serum IMP levels and unhealthy diet measured by dietary quality scores emphasising the importance of nutrition in this context. Thus, this study confirms that in type 2 diabetes, the intestinal microbiota may is switched towards IMP production, which can impact host inflammation and metabolism.95
Therapeutic relevance
The mechanistic links between gut microbiota-derived metabolites and metabolic disorders make these interactions a promising therapeutic target in these complex diseases.
Lessons from faecal microbiota transplantation
FMT is a drastic strategy to modify the gut microbiome. It is highly effective in the treatment of recurring Clostridioides difficile infections and has been evaluated in small trials in metabolic syndrome and obesity.22–24 The clinical efficacy of this strategy is so far mild, with mostly some positive effects on insulin sensitivity in subgroups of patients. However, these studies had several limitations, including small size and limited duration of intervention. Nevertheless, they provide relevant information to identify the critical molecules involved in biological effects. Following successful FMT, both the microbiota composition and metabolomics, such as BA and SCFA profiles, can be restored. In patients with obesity, FMT can induce engraftment of the butyrate-producing and bile-hydrolysing genus Faecalibacterium, leading to a restoration of the BA profile and microbiota BSH activity.96 FMT increases the relative abundance of SCFA-producing bacteria such as Roseburia intestinalis and the protective strain Akkermansia muciniphila,97 with a possible role in the improvement in insulin sensitivity through regulation of GLP-1.98 A. muciniphila supplementation alone improves metabolic parameters in overweight/obese insulin-resistant volunteers characterised by better insulin sensitivity and a reduction in plasma total cholesterol levels and fat mass.99 In mice, A. muciniphila promotes the production of SCFAs100 and the restoration of HFD-induced alterations in tryptophan metabolism.101 These data highlight the key family of microbiota-derived metabolites with potential therapeutic effects.
Synthetic agonists of bile acid receptors
Given their potential benefits in metabolic diseases, BAs and synthetic FXR and TGR5 agonists are currently under development in the metabolic field. Preclinical trials based on in vitro and in vivo studies identified potent synthetic FXR and TGR5 agonists, which are currently being investigated in phase II or III clinical trials.36 102 Due to the regulatory roles of FXR and TGR5 receptors on glucose and lipid metabolism, multiple specific agonists have been designed. Obeticholic acid (OCA), one of the best-characterised FXR agonists, protects the liver from damage in mice with a reduction in hepatic steatosis and inflammation36 102 and is currently being evaluated in a phase III trial in patients with NASH.103 The synthetic FXR agonist GW4064 improves hyperglycaemic and hyperlipidaemia in mice with diabetes104 and is able to correct BA dysmetabolism and alleviate liver toxicity in rodents with short bowel.105 The intestine-restricted FXR agonist fexaramine can also promote adipose tissue browning and GLP-1 secretion in wild type (WT) and leptin receptor-deficient diabetic mice.106 Finally, a TGR5 agonist ameliorated insulin resistance and glucose homeostasis in mice with diabetes by the cyclic AMP/protein kinase A pathway in skeletal muscles.107
Short-chain fatty acid and branched-chain amino acid treatment
Dietary supplementation with fermentable fibres, such as inulin in HFD-fed mice or inulin-propionate ester in overweight humans, protects against metabolic disturbances by restoring the gut microbial composition and the action of the IL-22-mediated axis.65 108 Oral SCFA treatment in obese mice can modulate lipid synthesis and insulin receptors by upregulating peroxisome proliferator-activated receptor-γ.109 It also improves intestinal barrier functions with a lower serum LPS concentration.110 SCFAs exert their beneficial effects partly through specific G-protein-coupled receptors, and their activation by specific agonists is an attractive strategy in the treatment of MetS. GPR40/FFA1,111 GPR41/FFA3,112 GPR43/FFA2113 and GPR120/FFA4114 agonists induce protection against diet-induced obesity in mice through the improvement in insulin, GLP-1 and incretin secretion and anti-inflammatory effects. In addition, a link between dietary BCAAs and energy balance was noted in animals with obesity, and reducing the proportion of dietary BCAAs was associated with a restoration of metabolic health.115
Concluding remarks
Gut microbiota-derived metabolites have a central role in the physiology and physiopathology of metabolic disorders. The microbial metabolites described above, specifically BAs, SCFAs, BCAAs, TMAO, tryptophan and indole derivatives, are implicated in the pathogenesis of these complex disorders and represent potential biomarkers for the early diagnosis and prognosis of these diseases.116 117 Moreover, microbiota-derived metabolites and their host receptors, possibly in combination with dietary intervention, represent promising targets for the development of novel therapeutic tools for metabolic disorders.
Acknowledgments
The authors would like to thank BioRender, for its revolutionised tool to create custom scientific figures (https://biorender.com/).
Footnotes
Twitter: @h_sokol
Contributors: AA, KC and HS designed the systematic review, did the literature search and assessed data quality. AA drafted the manuscript and figures. KC and HS critically revised the review. All authors approved the final submitted version for publication.
Funding: Grant supports in this field were obtained by Ministry of Health and Solidarity (Assistance Publique-Hôpitaux de Paris, to KC/PHRC Microbaria), by European Union (Metacardis to KC HEALTH-F4-2012-305312 to KC), JPI MICRODIET Grant (5290510105) to KC, EU Horizon 2020 grant (LITMUS 777377) to KC and by LeDucq Foundation consortium grant (17CVD01) to KC. HS received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Programme (ERC-2016-StG-71577).
Competing interests: HS received unrestricted study grants from Danone, Biocodex and Enterome; board membership, consultancy or lecture fees from Carenity, AbbVie, Astellas, Danone, Ferring, Mayoly Spindler, MSD, Novartis, Roche, Tillots, Enterome, Maat, BiomX, Biose, Novartis and Takeda and a co-founder of Exeliom Biosciences.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1. Alam A, Neish A. Role of gut microbiota in intestinal wound healing and barrier function. Tissue Barriers 2018;6:1539595. 10.1080/21688370.2018.1539595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheng H-Y, Ning M-X, Chen D-K, et al. Interactions between the gut microbiota and the host innate immune response against pathogens. Front Immunol 2019;10:607. 10.3389/fimmu.2019.00607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sittipo P, Shim J-W, Lee YK. Microbial metabolites determine host health and the status of some diseases. Int J Mol Sci 2019;20:5296. 10.3390/ijms20215296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ni J, Wu GD, Albenberg L, et al. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol 2017;14:573–84. 10.1038/nrgastro.2017.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alhinai EA, Walton GE, Commane DM. The role of the gut microbiota in colorectal cancer causation. Int J Mol Sci 2019;20:5295. 10.3390/ijms20215295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ding R-X, Goh W-R, Wu R-N, et al. Revisit gut microbiota and its impact on human health and disease. J Food Drug Anal 2019;27:623–31. 10.1016/j.jfda.2018.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diehl AM, Day C, Cause DC. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med 2017;377:2063–72. 10.1056/NEJMra1503519 [DOI] [PubMed] [Google Scholar]
- 8. Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest 2019;129:4050–7. 10.1172/JCI129194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lamichhane S, Sen P, Dickens AM, et al. Gut metabolome meets microbiome: a methodological perspective to understand the relationship between host and microbe. Methods 2018;149:3–12. 10.1016/j.ymeth.2018.04.029 [DOI] [PubMed] [Google Scholar]
- 10. Tilg H, Zmora N, Adolph TE, et al. The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol 2020;20:40–54. 10.1038/s41577-019-0198-4 [DOI] [PubMed] [Google Scholar]
- 11. Bäckhed F, Manchester JK, Semenkovich CF, et al. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A 2007;104:979–84. 10.1073/pnas.0605374104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A 2004;101:15718–23. 10.1073/pnas.0407076101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–31. 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 14. Le Roy T, Llopis M, Lepage P, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut 2013;62:1787–94. 10.1136/gutjnl-2012-303816 [DOI] [PubMed] [Google Scholar]
- 15. Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013;341:1241214. 10.1126/science.1241214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kimura I, Miyamoto J, Ohue-Kitano R, et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science 2020;367:eaaw8429. 10.1126/science.aaw8429 [DOI] [PubMed] [Google Scholar]
- 17. Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: human gut microbes associated with obesity. Nature 2006;444:1022–3. 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- 18. Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature 2013;500:585–8. 10.1038/nature12480 [DOI] [PubMed] [Google Scholar]
- 19. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013;500:541–6. 10.1038/nature12506 [DOI] [PubMed] [Google Scholar]
- 20. Aron-Wisnewsky J, Prifti E, Belda E, et al. Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut 2019;68:70–82. 10.1136/gutjnl-2018-316103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chassaing B, Raja SM, Lewis JD, et al. Colonic microbiota Encroachment correlates with dysglycemia in humans. Cell Mol Gastroenterol Hepatol 2017;4:205–21. 10.1016/j.jcmgh.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Groot P, Scheithauer T, Bakker GJ, et al. Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut 2020;69:502–12. 10.1136/gutjnl-2019-318320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kootte RS, Levin E, Salojärvi J, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab 2017;26:611–9. 10.1016/j.cmet.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 24. Yu EW, Gao L, Stastka P, et al. Fecal microbiota transplantation for the improvement of metabolism in obesity: the FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med 2020;17:e1003051. 10.1371/journal.pmed.1003051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen MX, Wang S-Y, Kuo C-H, et al. Metabolome analysis for investigating host-gut microbiota interactions. J Formos Med Assoc 2019;118(Suppl 1):S10–22. 10.1016/j.jfma.2018.09.007 [DOI] [PubMed] [Google Scholar]
- 26. Monnerie S, Comte B, Ziegler D, et al. Metabolomic and lipidomic signatures of metabolic syndrome and its physiological components in adults: a systematic review. Sci Rep 2020;10:669. 10.1038/s41598-019-56909-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu R, Hong J, Xu X, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med 2017;23:859–68. 10.1038/nm.4358 [DOI] [PubMed] [Google Scholar]
- 28. Pedersen HK, Gudmundsdottir V, Nielsen HB, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016;535:376–81. 10.1038/nature18646 [DOI] [PubMed] [Google Scholar]
- 29. Fiamoncini J, Rundle M, Gibbons H, et al. Plasma metabolome analysis identifies distinct human metabotypes in the postprandial state with different susceptibility to weight loss-mediated metabolic improvements. Faseb J 2018;32:5447–58. 10.1096/fj.201800330R [DOI] [PubMed] [Google Scholar]
- 30. Surowiec I, Noordam R, Bennett K, et al. Metabolomic and lipidomic assessment of the metabolic syndrome in Dutch middle-aged individuals reveals novel biological signatures separating health and disease. Metabolomics 2019;15:23. 10.1007/s11306-019-1484-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Org E, Blum Y, Kasela S, et al. Relationships between gut microbiota, plasma metabolites, and metabolic syndrome traits in the METSIM cohort. Genome Biol 2017;18:70. 10.1186/s13059-017-1194-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang C, Yin A, Li H, et al. Dietary modulation of gut microbiota contributes to alleviation of both genetic and simple obesity in children. EBioMedicine 2015;2:968–84. 10.1016/j.ebiom.2015.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koh A, Molinaro A, Ståhlman M, et al. Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell 2018;175:947–61. e17. 10.1016/j.cell.2018.09.055 [DOI] [PubMed] [Google Scholar]
- 34. Hoyles L, Fernández-Real J-M, Federici M, et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat Med 2018;24:1070–80. 10.1038/s41591-018-0061-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ratajczak W, Rył A, Mizerski A, et al. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim Pol 2019;66:1–12. 10.18388/abp.2018_2648 [DOI] [PubMed] [Google Scholar]
- 36. McGlone ER, Bloom SR. Bile acids and the metabolic syndrome. Ann Clin Biochem 2019;56:326–37. 10.1177/0004563218817798 [DOI] [PubMed] [Google Scholar]
- 37. Thomas C, Pellicciari R, Pruzanski M, et al. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov 2008;7:678–93. 10.1038/nrd2619 [DOI] [PubMed] [Google Scholar]
- 38. Ma H, Patti ME. Bile acids, obesity, and the metabolic syndrome. Best Pract Res Clin Gastroenterol 2014;28:573–83. 10.1016/j.bpg.2014.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ðanić M, Stanimirov B, Pavlović N, et al. Pharmacological applications of bile acids and their derivatives in the treatment of metabolic syndrome. Front Pharmacol 2018;9:1382. 10.3389/fphar.2018.01382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kuno T, Hirayama-Kurogi M, Ito S, et al. Reduction in hepatic secondary bile acids caused by short-term antibiotic-induced dysbiosis decreases mouse serum glucose and triglyceride levels. Sci Rep 2018;8:1253. 10.1038/s41598-018-19545-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Duboc H, Rajca S, Rainteau D, et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut 2013;62:531–9. 10.1136/gutjnl-2012-302578 [DOI] [PubMed] [Google Scholar]
- 42. Parséus A, Sommer N, Sommer F, et al. Microbiota-induced obesity requires farnesoid X receptor. Gut 2017;66:429–37. 10.1136/gutjnl-2015-310283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lambert G, Amar MJA, Guo G, et al. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem 2003;278:2563–70. 10.1074/jbc.M209525200 [DOI] [PubMed] [Google Scholar]
- 44. Prawitt J, Abdelkarim M, Stroeve JHM, et al. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes 2011;60:1861–71. 10.2337/db11-0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schmitt J, Kong B, Stieger B, et al. Protective effects of farnesoid X receptor (FXR) on hepatic lipid accumulation are mediated by hepatic FXR and independent of intestinal FGF15 signal. Liver Int 2015;35:1133–44. 10.1111/liv.12456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jiang C, Xie C, Lv Y, et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun 2015;6:10166. 10.1038/ncomms10166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tomlinson E, Fu L, John L, et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 2002;143:1741–7. 10.1210/endo.143.5.8850 [DOI] [PubMed] [Google Scholar]
- 48. Molinaro A, Wahlström A, Marschall H-U. Role of bile acids in metabolic control. Trends Endocrinol Metab 2018;29:31–41. 10.1016/j.tem.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 49. van Nierop FS, Scheltema MJ, Eggink HM, et al. Clinical relevance of the bile acid receptor TGR5 in metabolism. Lancet Diabetes Endocrinol 2017;5:224–33. 10.1016/S2213-8587(16)30155-3 [DOI] [PubMed] [Google Scholar]
- 50. Velazquez-Villegas LA, Perino A, Lemos V, et al. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat Commun 2018;9:245. 10.1038/s41467-017-02068-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Raimondi F, Santoro P, Barone MV, et al. Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. Am J Physiol Gastrointest Liver Physiol 2008;294:G906–13. 10.1152/ajpgi.00043.2007 [DOI] [PubMed] [Google Scholar]
- 52. Suzuki T, Hara H. Dietary fat and bile juice, but not obesity, are responsible for the increase in small intestinal permeability induced through the suppression of tight junction protein expression in LETO and OLETF rats. Nutr Metab 2010;7:19. 10.1186/1743-7075-7-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stenman LK, Holma R, Korpela R. High-fat-induced intestinal permeability dysfunction associated with altered fecal bile acids. World J Gastroenterol 2012;18:923–9. 10.3748/wjg.v18.i9.923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Devkota S, Wang Y, Musch MW, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in IL10-/- mice. Nature 2012;487:104–8. 10.1038/nature11225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Natividad JM, Lamas B, Pham HP, et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat Commun 2018;9:2802. 10.1038/s41467-018-05249-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Joyce SA, MacSharry J, Casey PG, et al. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc Natl Acad Sci U S A 2014;111:7421–6. 10.1073/pnas.1323599111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016;7:189–200. 10.1080/19490976.2015.1134082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim CH. Microbiota or short-chain fatty acids: which regulates diabetes? Cell Mol Immunol 2018;15:88–91. 10.1038/cmi.2017.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chambers ES, Morrison DJ, Frost G. Control of appetite and energy intake by SCFA: what are the potential underlying mechanisms? Proc Nutr Soc 2015;74:328–36. 10.1017/S0029665114001657 [DOI] [PubMed] [Google Scholar]
- 60. Hu J, Lin S, Zheng B, et al. Short-chain fatty acids in control of energy metabolism. Crit Rev Food Sci Nutr 2018;58:1243–9. 10.1080/10408398.2016.1245650 [DOI] [PubMed] [Google Scholar]
- 61. Priyadarshini M, Kotlo KU, Dudeja PK, et al. Role of short chain fatty acid receptors in intestinal physiology and pathophysiology. Compr Physiol 2018;8:1091–115. 10.1002/cphy.c170050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhao L, Zhang F, Ding X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018;359:1151–6. 10.1126/science.aao5774 [DOI] [PubMed] [Google Scholar]
- 63. Makki K, Deehan EC, Walter J, et al. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018;23:705–15. 10.1016/j.chom.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 64. De Vadder F, Kovatcheva-Datchary P, Goncalves D, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014;156:84–96. 10.1016/j.cell.2013.12.016 [DOI] [PubMed] [Google Scholar]
- 65. Zou J, Chassaing B, Singh V, et al. Fiber-Mediated Nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host Microbe 2018;23:41–53. 10.1016/j.chom.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Freeland KR, Wolever TMS. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-alpha. Br J Nutr 2010;103:460–6. 10.1017/S0007114509991863 [DOI] [PubMed] [Google Scholar]
- 67. Tajiri K, Shimizu Y. Branched-chain amino acids in liver diseases. Transl Gastroenterol Hepatol 2018;3:47. 10.21037/tgh.2018.07.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yoneshiro T, Wang Q, Tajima K, et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 2019;572:614–9. 10.1038/s41586-019-1503-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yang Z, Huang S, Zou D, et al. Metabolic shifts and structural changes in the gut microbiota upon branched-chain amino acid supplementation in middle-aged mice. Amino Acids 2016;48:2731–45. 10.1007/s00726-016-2308-y [DOI] [PubMed] [Google Scholar]
- 70. Arany Z, Neinast M. Branched chain amino acids in metabolic disease. Curr Diab Rep 2018;18:76. 10.1007/s11892-018-1048-7 [DOI] [PubMed] [Google Scholar]
- 71. Zhou M, Shao J, Wu C-Y, et al. Targeting BCAA catabolism to treat obesity-associated insulin resistance. Diabetes 2019;68:1730–46. 10.2337/db18-0927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dehghan P, Farhangi MA, Nikniaz L, et al. Gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) potentially increases the risk of obesity in adults: an exploratory systematic review and dose-response meta- analysis. Obes Rev 2020;21:e12993. 10.1111/obr.12993 [DOI] [PubMed] [Google Scholar]
- 73. Shan Z, Sun T, Huang H, et al. Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am J Clin Nutr 2017;106:888–94. 10.3945/ajcn.117.157107 [DOI] [PubMed] [Google Scholar]
- 74. Zhuang R, Ge X, Han L, et al. Gut microbe-generated metabolite trimethylamine N-oxide and the risk of diabetes: a systematic review and dose-response meta-analysis. Obes Rev 2019;20:883–94. 10.1111/obr.12843 [DOI] [PubMed] [Google Scholar]
- 75. Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–85. 10.1038/nm.3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gregory JC, Buffa JA, Org E, et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem 2015;290:5647–60. 10.1074/jbc.M114.618249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tang WHW, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–84. 10.1056/NEJMoa1109400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Badawy AA-B. Kynurenine pathway and human systems. Exp Gerontol 2020;129:110770. 10.1016/j.exger.2019.110770 [DOI] [PubMed] [Google Scholar]
- 79. Comai S, Bertazzo A, Brughera M, et al. Tryptophan in health and disease. Adv Clin Chem 2020;95:165–218. 10.1016/bs.acc.2019.08.005 [DOI] [PubMed] [Google Scholar]
- 80. Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015;161:264–76. 10.1016/j.cell.2015.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2020;17:223–37. 10.1038/s41575-019-0258-z [DOI] [PubMed] [Google Scholar]
- 82. Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018;23:716–24. 10.1016/j.chom.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 83. Natividad JM, Agus A, Planchais J, et al. Impaired aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metab 2018;28:737–49. 10.1016/j.cmet.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 84. Taleb S. Tryptophan dietary impacts gut barrier and metabolic diseases. Front Immunol 2019;10:2113. 10.3389/fimmu.2019.02113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Beaumont M, Neyrinck AM, Olivares M, et al. The gut microbiota metabolite indole alleviates liver inflammation in mice. Faseb J 2018:fj201800544:6681–93. 10.1096/fj.201800544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Postal BG, Ghezzal S, Aguanno D, et al. AhR activation defends gut barrier integrity against damage occurring in obesity. Mol Metab 2020;39:101007. 10.1016/j.molmet.2020.101007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mallmann NH, Lima ES, Lalwani P. Dysregulation of tryptophan catabolism in metabolic syndrome. Metab Syndr Relat Disord 2018;16:135–42. 10.1089/met.2017.0097 [DOI] [PubMed] [Google Scholar]
- 88. Moyer BJ, Rojas IY, Kerley-Hamilton JS, et al. Inhibition of the aryl hydrocarbon receptor prevents Western diet-induced obesity. model for AhR activation by kynurenine via oxidized-LDL, TLR2/4, TGFβ, and IDO1. Toxicol Appl Pharmacol 2016;300:13–24. 10.1016/j.taap.2016.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Laurans L, Venteclef N, Haddad Y, et al. Genetic deficiency of indoleamine 2,3-dioxygenase promotes gut microbiota-mediated metabolic health. Nat Med 2018;24:1113–20. 10.1038/s41591-018-0060-4 [DOI] [PubMed] [Google Scholar]
- 90. Liu J-J, Movassat J, Portha B. Emerging role for kynurenines in metabolic pathologies. Curr Opin Clin Nutr Metab Care 2019;22:82–90. 10.1097/MCO.0000000000000529 [DOI] [PubMed] [Google Scholar]
- 91. Galligan JJ. Beneficial actions of microbiota-derived tryptophan metabolites. Neurogastroenterol Motil 2018;30:e13283. 10.1111/nmo.13283 [DOI] [PubMed] [Google Scholar]
- 92. Young RL, Lumsden AL, Keating DJ. Gut serotonin is a regulator of obesity and metabolism. Gastroenterology 2015;149:253–5. 10.1053/j.gastro.2015.05.020 [DOI] [PubMed] [Google Scholar]
- 93. Crane JD, Palanivel R, Mottillo EP, et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat Med 2015;21:166–72. 10.1038/nm.3766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fukui M, Tanaka M, Toda H, et al. High plasma 5-hydroxyindole-3-acetic acid concentrations in subjects with metabolic syndrome. Diabetes Care 2012;35:163–7. 10.2337/dc11-1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Molinaro A, Bel Lassen P, Henricsson M, et al. Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nat Commun 2020;11:5881. 10.1038/s41467-020-19589-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Allegretti JR, Kassam Z, Mullish BH, et al. Effects of fecal microbiota transplantation with oral capsules in obese patients. Clin Gastroenterol Hepatol 2020;18:855–63. 10.1016/j.cgh.2019.07.006 [DOI] [PubMed] [Google Scholar]
- 97. Dao MC, Everard A, Aron-Wisnewsky J, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 2016;65:426–36. 10.1136/gutjnl-2014-308778 [DOI] [PubMed] [Google Scholar]
- 98. Zhang T, Li Q, Cheng L, et al. Akkermansia muciniphila is a promising probiotic. Microb Biotechnol 2019;12:1109–25. 10.1111/1751-7915.13410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Depommier C, Everard A, Druart C, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med 2019;25:1096–103. 10.1038/s41591-019-0495-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bian X, Wu W, Yang L, et al. Administration of Akkermansia muciniphila ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front Microbiol 2019;10:2259. 10.3389/fmicb.2019.02259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wu F, Guo X, Zhang M, et al. An Akkermansia muciniphila subtype alleviates high-fat diet-induced metabolic disorders and inhibits the neurodegenerative process in mice. Anaerobe 2020;61:102138. 10.1016/j.anaerobe.2019.102138 [DOI] [PubMed] [Google Scholar]
- 102. Lazarević S, Đanić M, Goločorbin-Kon S, et al. Semisynthetic bile acids: a new therapeutic option for metabolic syndrome. Pharmacol Res 2019;146:104333. 10.1016/j.phrs.2019.104333 [DOI] [PubMed] [Google Scholar]
- 103. Ratziu V, Sanyal AJ, Loomba R, et al. Regenerate: design of a pivotal, randomised, phase 3 study evaluating the safety and efficacy of obeticholic acid in patients with fibrosis due to nonalcoholic steatohepatitis. Contemp Clin Trials 2019;84:105803. 10.1016/j.cct.2019.06.017 [DOI] [PubMed] [Google Scholar]
- 104. Zhang Y, Lee FY, Barrera G, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A 2006;103:1006–11. 10.1073/pnas.0506982103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cao Y, Xiao Y, Zhou K, et al. FXR agonist GW4064 improves liver and intestinal pathology and alters bile acid metabolism in rats undergoing small intestinal resection. Am J Physiol Gastrointest Liver Physiol 2019;317:G108–15. 10.1152/ajpgi.00356.2017 [DOI] [PubMed] [Google Scholar]
- 106. Pathak P, Xie C, Nichols RG, et al. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 2018;68:1574–88. 10.1002/hep.29857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Huang S, Ma S, Ning M, et al. TGR5 agonist ameliorates insulin resistance in the skeletal muscles and improves glucose homeostasis in diabetic mice. Metabolism 2019;99:45–56. 10.1016/j.metabol.2019.07.003 [DOI] [PubMed] [Google Scholar]
- 108. Chambers ES, Byrne CS, Morrison DJ, et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: a randomised cross-over trial. Gut 2019;68:1430–8. 10.1136/gutjnl-2019-318424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. den Besten G, Bleeker A, Gerding A, et al. Short-Chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes 2015;64:2398–408. 10.2337/db14-1213 [DOI] [PubMed] [Google Scholar]
- 110. Fang W, Xue H, Chen X, et al. Supplementation with sodium butyrate modulates the composition of the gut microbiota and ameliorates high-fat diet-induced obesity in mice. J Nutr 2019;149:747–54. 10.1093/jn/nxy324 [DOI] [PubMed] [Google Scholar]
- 111. Nagasumi K, Esaki R, Iwachidow K, et al. Overexpression of GPR40 in pancreatic beta-cells augments glucose-stimulated insulin secretion and improves glucose tolerance in normal and diabetic mice. Diabetes 2009;58:1067–76. 10.2337/db08-1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Schmidt J, Smith NJ, Christiansen E, et al. Selective orthosteric free fatty acid receptor 2 (FFA2) agonists: identification of the structural and chemical requirements for selective activation of FFA2 versus FFA3. J Biol Chem 2011;286:10628–40. 10.1074/jbc.M110.210872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hudson BD, Due-Hansen ME, Christiansen E, et al. Defining the molecular basis for the first potent and selective orthosteric agonists of the FFA2 free fatty acid receptor. J Biol Chem 2013;288:17296–312. 10.1074/jbc.M113.455337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Milligan G, Alvarez-Curto E, Hudson BD, et al. FFA4/GPR120: pharmacology and therapeutic opportunities. Trends Pharmacol Sci 2017;38:809–21. 10.1016/j.tips.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Cummings NE, Williams EM, Kasza I, et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J Physiol 2018;596:623–45. 10.1113/JP275075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Luo L, Aubrecht J, Li D, et al. Assessment of serum bile acid profiles as biomarkers of liver injury and liver disease in humans. PLoS One 2018;13:e0193824. 10.1371/journal.pone.0193824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ma Z, Wang X, Yin P, et al. Serum metabolome and targeted bile acid profiling reveals potential novel biomarkers for drug-induced liver injury. Medicine 2019;98:e16717. 10.1097/MD.0000000000016717 [DOI] [PMC free article] [PubMed] [Google Scholar]