Abstract
Hematopoietic stem and progenitor cell (HSPC) functions are regulated by a specialized microenvironment in the bone marrow - the hematopoietic stem cell niche - of which the extracellular matrix (ECM) is an integral component. We describe here the localization of ECM molecules, in particular the laminin α4, α3 and α5 containing isoforms in the bone marrow. Laminin 421 (composed of laminin α4, β2, γ1 chains) is identified as a major component of the bone marrow ECM, occurring abundantly surrounding venous sinuses and in a specialized reticular fiber network of the intersinusoidal spaces of murine bone marrow (BM) in close association with HSPC. Bone marrow from Lama4 −/− mice is significantly less efficient in reconstituting the hematopoietic system of irradiated wildtype (WT) recipients in competitive bone marrow transplantation assays and shows reduced colony formation in vitro. This is partially due to retention of Lin−c-kit+Sca-1+CD48− long-term and short-term hematopoietic stem cells (LT-HSC/ST-HSC) in the G0 phase of the cell cycle in Lama4 −/− bone marrow and hence a more quiescent phenotype. In addition, the extravasation of WT BM cells into Lama4 −/− bone marrow is impaired, influencing the recirculation of HSPC. Our data suggest that these effects are mediated by a compensatory expression of laminin α5 containing isoforms (laminin 521/522) in Lama4−/− bone marrow. Collectively, these intrinsic and extrinsic effects lead to reduced HSPC numbers in Lama4 −/− bone marrow and reduced hematopoietic potential.
Keywords: Laminin, Bone marrow, Hematopoietic stem and progenitor cells (HSPC)
Introduction
Hematopoietic stem cells (HSC) reside in the specialized bone marrow microenvironment, which consists of various cell types and extracellular matrix molecules (ECM). Molecular information provided by cell-cell and cell-matrix interactions, combined with biophysical forces, regulate the survival and self-renewal of hematopoietic stem and progenitor cells (HSPC) [1,2]. Work to date has mainly focused on the cellular components of HSC niches, with little attention to the ECM despite its abundant presence in the bone marrow. One important HSC niche is considered to be the endosteal surface, where several adhesion molecules, such as CD44 and the ECM molecules, osteopontin and osteonectin, have been shown to play a role in HSC retention and survival [3]. However, there is evidence for additional HSC niches involving other cell types including endothelial cells, adipocytes and fibroblasts and indeed several micro-niches are now proposed to exist for different progenitor cell types [4–6]. Apart from osteonectin and osteopontin, various other ECM molecules have been implicated in hematopoietic progenitor cell adhesion and differentiation, in particular fibronectin and the laminins [7,8], both of which can induce intracellular signaling pathways in progenitors and can also influence biophysical aspects of the microenvironment [9]. Recently, it was shown that megakaryocytes are surrounded by a peri-cellular matrix, consisting of fibronectin, type IV collagen and laminin, which regulates megakaryocyte development and homeostasis [10].
Laminins are large (500–1000 kDA) glycoproteins that are secreted as α-β-γ heterotrimers and constitute one of the main components of all basement membranes. Five α (α1–α5), three β (β1–β3) and three γ (γ1–γ3) chains exist that can assemble to generate at least 15 different laminin isoforms [11]. The laminin α chains are responsible for binding cell surface receptors such as integrins, dystroglycan, Lutheran glycoproteins, MCAM, or sulfated glycolipids [11–16] and are therefore considered to be responsible for the biological function of laminins. In the bone marrow, expression of laminin α4 and laminin α5 containing laminins was reported in basement membranes of sinusoids and larger blood vessels and within the intersinusoidal space in mouse and in man [17–21]. In vitro studies using hematopoietic cell lines have shown that both laminin α4 and α5 containing isoforms can support hematopoietic cell adhesion, however, to different degrees. In general, the adhesion of hematopoietic stem and progenitor cells as well as the more committed hematopoietic precursor cells is higher to laminin α5 than α4 containing laminins [8,19]. The differential binding capacities of the laminin α4 and α5 containing isoforms raise the possibility of specific roles for different laminin isoforms in interaction with hematopoietic stem, progenitor and precursor cells as has been shown for embryonic stem cells. Integrin α6β1 mediated binding of murine embryonic stem cells to laminin 511 in vitro was shown to maintain pluripotency via induction of the PI3K/Akt pathway, while binding to laminin α4 containing laminins favored differentiation [22]. In a previous study we have shown that the bone marrow from laminin α4 deficient mice (Lama4−/−) [23] has reduced capacity to reconstitute the hematopoietic system of a lethally irradiated recipient [24], specifically implicating laminin α4 containing isoforms in development and/or maintenance of HSPC.
Laminin α4 deletion in mice results in hemorrhage during the embryonic period leading to normochromic, normocytic anemia at birth. This phenotype is not observed in adult Lama4−/− mice as laminin α5 containing laminins are deposited in the endothelial basement membranes during the postnatal period and probably compensate for the defect [23]. In the present study, we describe the different types of ECM structures in the bone marrow and use the Lama4−/− mouse to investigate how laminin α4-containing laminins affect hematopoietic stem and progenitor cells in vivo. We describe a laminin α4 rich peri-sinus matrix and a reticular fiber network in the intersinusoidal space to which HSPC localize upon intravenous injection. Our data show that laminin α4 deletion leads to a more quiescent cell cycle status of HSPC without affecting their lineage output. In vivo engraftment experiments further show reduced homing of WT HSPC to Lama4−/− bone marrow, raising the possibility of an additional defect in migration of HSPC across bone marrow vessels into the bone marrow niche.
Results
ECM of the bone marrow
Immunofluorescence staining of bone marrow preparations and confocal microscopy was used to investigate the distribution and localization of laminin isoforms and other ECM molecules in the bone marrow (Table 1). Double staining with a pan-laminin antibody, which recognizes laminin α1, β1 and γ1 chains and therefore a broad range of different laminin isoforms [25], and the endothelial marker endoglin [26] revealed basement membranes of larger blood vessels and surrounding sinusoids within the bone marrow cavity as previously described [19], as well as a filigree fiber network in the intersinusoidal space (Fig. 1A; Movie 1). A similar staining pattern was observed for laminin γ1 (Fig. 1B) and β2 (Fig. 1C), and for other basement membrane molecules, including perlecan, collagen type IV and nidogen-1 (Fig. 1D; Suppl. Fig. S1). Laminin β1 or γ3 were not detected and antibody to laminin β3 weakly stained the sinuses only, while anti-laminin γ2 showed a slightly broader localization in sinuses, the fiber network in the intersinusoidal space and arteries (Table 1).
Table 1.
Localization of ECM molecules and reticular fibroblast markers in murine bone marrow.
| Large blood vessels | Sinusoidal BMs | Fiber network | |
|---|---|---|---|
| Collagen I | − | − | − |
| Collagen III | ++ | ++ | ++ |
| Collagen IV | ++ | ++ | ++ |
| PanLM | ++ | ++ | ++ |
| Laminin α1 | − | − | − |
| Laminin α2a | − | − | − |
| Laminin α3 | + | + | + |
| Laminin α4 | ++ | ++ | + |
| Laminin α5 | ++ | ++ | − |
| Laminin β1 | − | − | − |
| Laminin β2 | ++ | ++ | ++ |
| Laminin β3 | − | + | − |
| Laminin γ1 | ++ | ++ | ++ |
| Laminin γ2 | + | + | + |
| Laminin γ3 | − | − | − |
| Perlecan | ++ | ++ | ++ |
| Nidogen-1 | ++ | ++ | ++ |
| Fibronectin | ++ | ++ | ++ |
| ER-TR7 | − | − | + |
| Fibrillin-1 | − | − | − |
| gp38 | − | − | − |
| Desmin | − | − | − |
| SMA | − | − | − |
strongly detectable
weakly detectable
absent;
SMA is smooth muscle actin.
Detected in nerves associated with arteries.
Fig. 1.
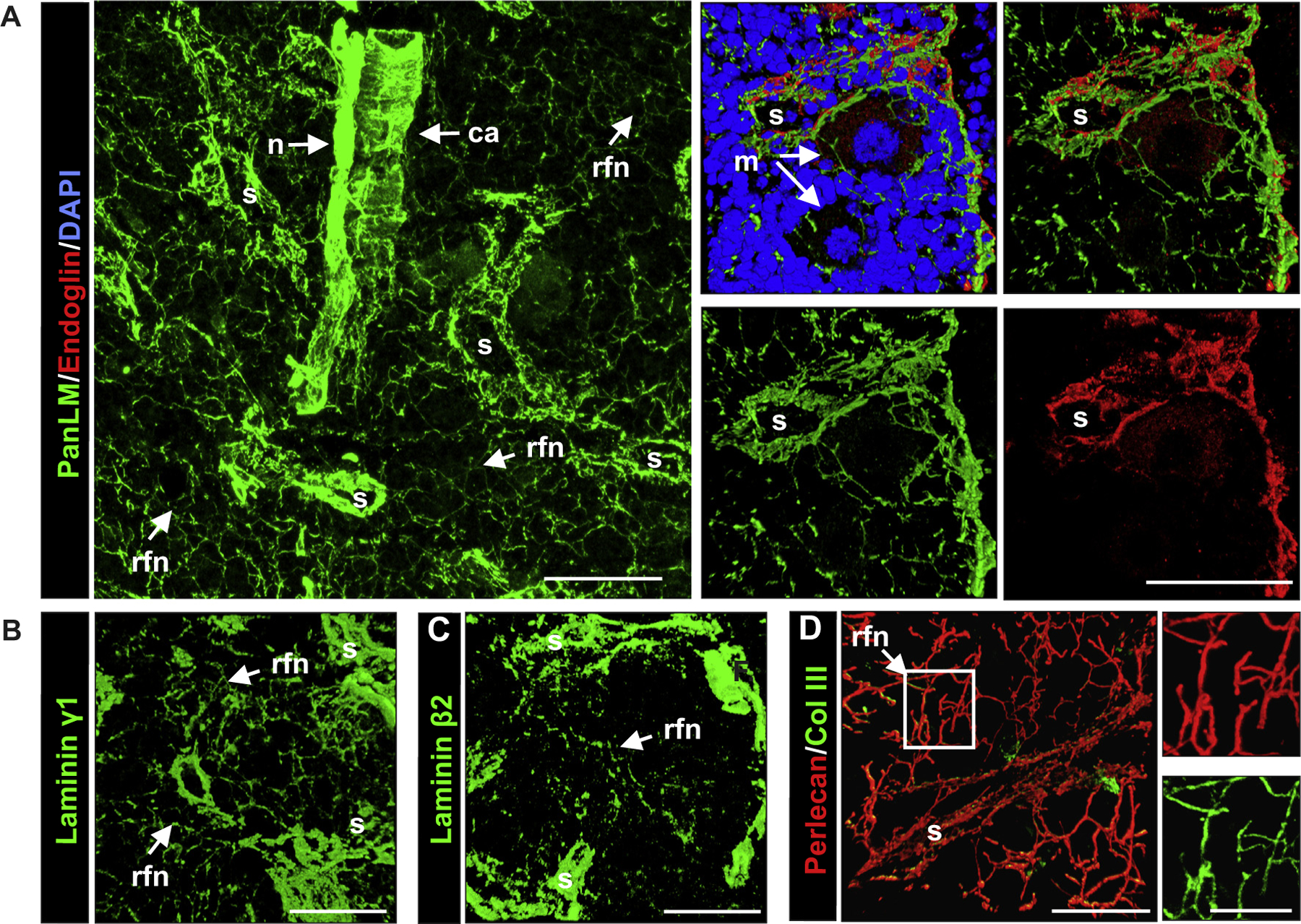
ECM localization in WT bone marrow. (A) Immunofluorescence staining of whole mount bone marrow preparations for pan-laminin (PanLM) provides an overview of the central region of the bone marrow, which contains the central artery (ca), nerves (n), the sinusoids (s) and the reticular fiber-like network (rfn) between sinusoids. Costaining of PanLM with endoglin, an endothelial cell marker (shown in right panels) reveals laminin in basement membranes underlying the endothelial layer of sinusoids. The reticular fibers form a net-like structure around the megakaryocytes (m) that reside in close vicinity to the outer surface of sinusoidal wall. (B) Laminin γ1 and (C) laminin β2 occur in the reticular fibers of the intersinusoidal network and in the sinusoidal basement membranes. (D) Double staining for perlecan and collagen type III shows colocalization in the reticular fiber-like structures, where collagen type III forms the cores of the fibers and is ensheathed by perlecan. Boxed area in D is shown at higher magnification to the right. Scale bars are 50 μm in A–C, 25 μm in D, and 10 μm in the insert of D. Data shown are representative of at least 6 mice analyzed.
Double staining for basement membrane markers and fibrillar collagens or fibronectin revealed colocalization around sinuses and in the intersinusoidal fiber network, which showed similarities to the reticular fiber network of secondary lymphoid organs [27–29]: all basement membrane molecules tested were found to ensheath a central core of collagen type III and fibronectin (Fig. 1D; Suppl. Fig. S1); collagen type I was not detected (Table 1). However, the diameter of each fiber (approximately 0.5 μm) was considerably smaller than that of reticular fibers and, of several common reticular fibroblast markers tested (Table 1), only ER-TR7 was detected on the outer surface of the fibers (Table 1; Suppl. Fig. S1). This result together with the very small diameter of the intersinusoidal fibers suggests that, while they share some similarities to the reticular fibers of secondary lymphoid organs, they are structurally distinct.
Laminin isoform localization in the bone marrow
Laminin α2, α3, α4 and α5 but not laminin α1 were detected in wild type (WT) murine bone marrow; laminin α2 was restricted to basement membranes around nerve fibers (not shown); while laminin α4 and α5 chain double staining revealed colocalization in basement membranes of larger blood vessels and of the sinusoids (Fig. 2A). Only laminin α4 was clearly detectable in the intersinusoidal fiber network (Fig. 2A) with some laminin α5 occurring exclusively in the thicker fibers immediately subjacent to the sinusoidal wall (Fig. 2A). Expression of laminin β2 but not laminin β1, suggests the existence of laminin 421 in the intersinusoidal fiber network, and laminins 421 and 521 at sinusoidal and blood vessel basement membranes. The presence of laminin γ2 which has been reported to form a heterotrimer with laminin α5 and β2 chains in human bone marrow stromal cells [18], also raises the possibility of laminin 522 around larger blood vessels and venus sinuses.
Fig. 2.
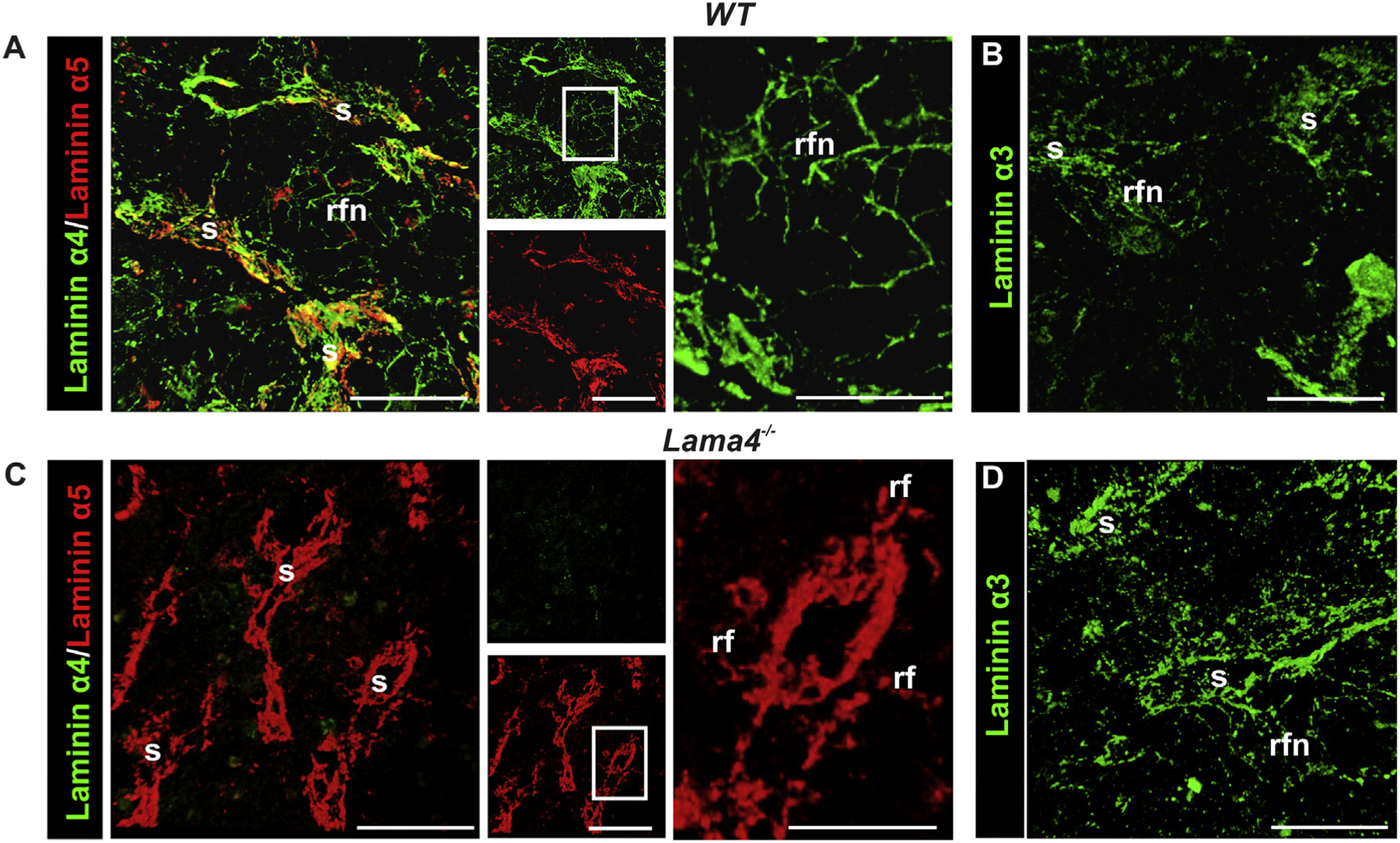
Localization of laminin α chains in the bone marrow of WT (A, B) and Lama4−/− mice (C, D). (A) Double immunofluorescence staining of WT bone marrow sections for laminin α4 and α5, showing colocalization in basement membranes of sinusoids (s), and filigree staining for laminin α4 throughout the bone marrow between sinusoids in a reticular fiber-like network (rfn); laminin α5 staining is restricted to the reticular fibers (rf) in close proximity to the sinusoids. (B) Laminin α3 staining is weak in the reticular fibers and basement membranes of sinusoids of WT mice. (C) In Lama4−/− bone marrow, laminin α5 distribution is slightly broader in the reticular fibers around the sinusoids compared to the WT bone marrow, while (D) laminin α3 staining is more pronounced in the reticular fibers and sinusoids. Boxed areas are shown at higher magnifications to the right. Scale bars are 50 μm, except in high magnifications in A and C where it is 25 μm. Data shown are representative of at least 6 WT mice and 6 Lama4−/− mice.
Laminin α3 staining was sparce and occurred mainly in association with the central artery, and weakly in the reticular fiber network and surrounding sinuses (Fig. 2B), showing a similar pattern to that observed for laminin γ2. Weak staining for laminin β3 (data not shown) only around sinuses suggests low levels of laminin 332 at sinuses and not elsewhere. The presence of laminin γ1 and, to a lesser extent, laminin γ2 but not β3 in the intersinusoidal fiber network and around arteries suggests the existence of low levels of laminin 321, a rare isoform previously only reported in human amnion [30], and potentially also laminin 322 at these sites. The antibody employed for laminin α3 detection recognizes both the short form, typical of the skin, and the less well investigated long form of this chain [31]. Data is summarized in Table 1.
All hematopoietic stem and progenitor cells occur in the so-called lineage negative (Lin−) population of bone marrow cells, which lack expression of cell-surface markers present on mature hematopoietic cells [32], including CD3, CD11b, CD41, B220, Ter119 and Gr-1. Therefore, to investigate the localization of hematopoietic precursor cells in relation to the bone marrow ECM, Lin− enriched bone marrow cells isolated from green fluorescence protein (GFP)-mice were injected intravenously (i.v.) into WT recipients, and the bone marrow was removed 16 h later and was examined by immunofluorescence staining and confocal microscopy. Hematopoietic precursor cells possess the ability to re-enter the bone marrow, a process referred to as ‘homing’. The majority of the GFP+ donor cells were found scattered throughout the bone marrow cavity as previously reported [4], in close association to the intersinusoidal fiber network (Suppl. Fig. S2).
ECM of Lama4−/− bone marrow
The distribution of most of the analyzed ECM molecules was similar in WT and Lama4−/− mice. Only laminin α3 staining was broader and more intense in Lama4−/− mice, becoming detectable in the venous sinuses and in the intersinusoidal fiber network (Fig. 2C, D), while laminin α5 showed a slightly broader expression around venous sinuses (Fig. 2C) and remained intense around larger blood vessels and undetectable in the intersinusoidal fiber network (Fig. 2C). Up-regulation of other laminin α chains or ECM molecules within the bone marrow could not be detected by immunofluorescence analyses (Suppl. Fig. S3).
Flow cytometry of hematopoietic stem and progenitor cell numbers in WT and Lama4−/− bone marrow
As previous studies suggested a slower reconstitution of the hematopoietic system by Lama4−/− bone marrow compared to WT bone marrow (Suppl. Fig. S4) [24], we assessed the relative amounts of different hematopoietic stem, progenitor and precursor cells present in the bone marrow by flow cytometry using common HSPC marker combinations.
Within the Lin− population, Lin−Sca-1+c-kit+ (LSK) cells contain those with stem cell activity [33,34] and consist of long-term HSC (LT-HSC), short-term HSC (ST-HSC) and multipotent progenitor cells (MPP), which can be differentiated on the basis of the cell surface markers listed in Table 2 [35,36]. LT-HSC have a repopulation capacity of >16 weeks, ST-HSC of <10 weeks [33,37], and MPP can give rise to more than one cell type but have a more limited self-renewal capacity than the pluripotent HSC populations [35,38]. Common myeloid progenitor cells give rise to more lineage-restricted cell populations. Using these cell markers, no differences were detected in proportions of the different stem and progenitor cell populations in Lama4−/− mice compared to WT mice: 0.19% of the WT and 0.17% of the Lama4−/− bone marrow cells were Lin−Sca-1+ckit+ (LSK) (Fig. 3A), of which approximately 5.6% were LT-HSC, 41% ST-HSC and 52% MPP in both Lama4−/− and WT mice according to CD34/flt3 marker combinations (Fig. 3B). Use of the alternative CD150/CD48, so-called SLAM, marker combination, revealed the same pattern of results with no differences between Lama4−/− and WT mice, but with slightly different values for the ST-HSC and MPP populations (Suppl. Fig. S5), which is probably due to some CD48 expression by ST-HSCs [39]. The proportions of these different hematopoietic cell populations measured in WT mice with the two marker combinations are consistent with data from other groups [35,36].
Table 2.
Cell surface markers employed to identify hematopoietic cell populations.
| Progenitor Type | Cell Type | Markers§ | |
|---|---|---|---|
 |
Lin−Sca–1+c–kit+ (LSK) | LT–HSC | CD34− flt3− CD150+ CD48− |
| ST–HSC | CD34+ flt3− CD150− CD48−/low |
||
| MPP | CD34+ flt3+ CD150− CD48+ |
||
| Lin−Sca–1−c–kit+ | CMP | CD150− CD48+ |
Fig. 3.
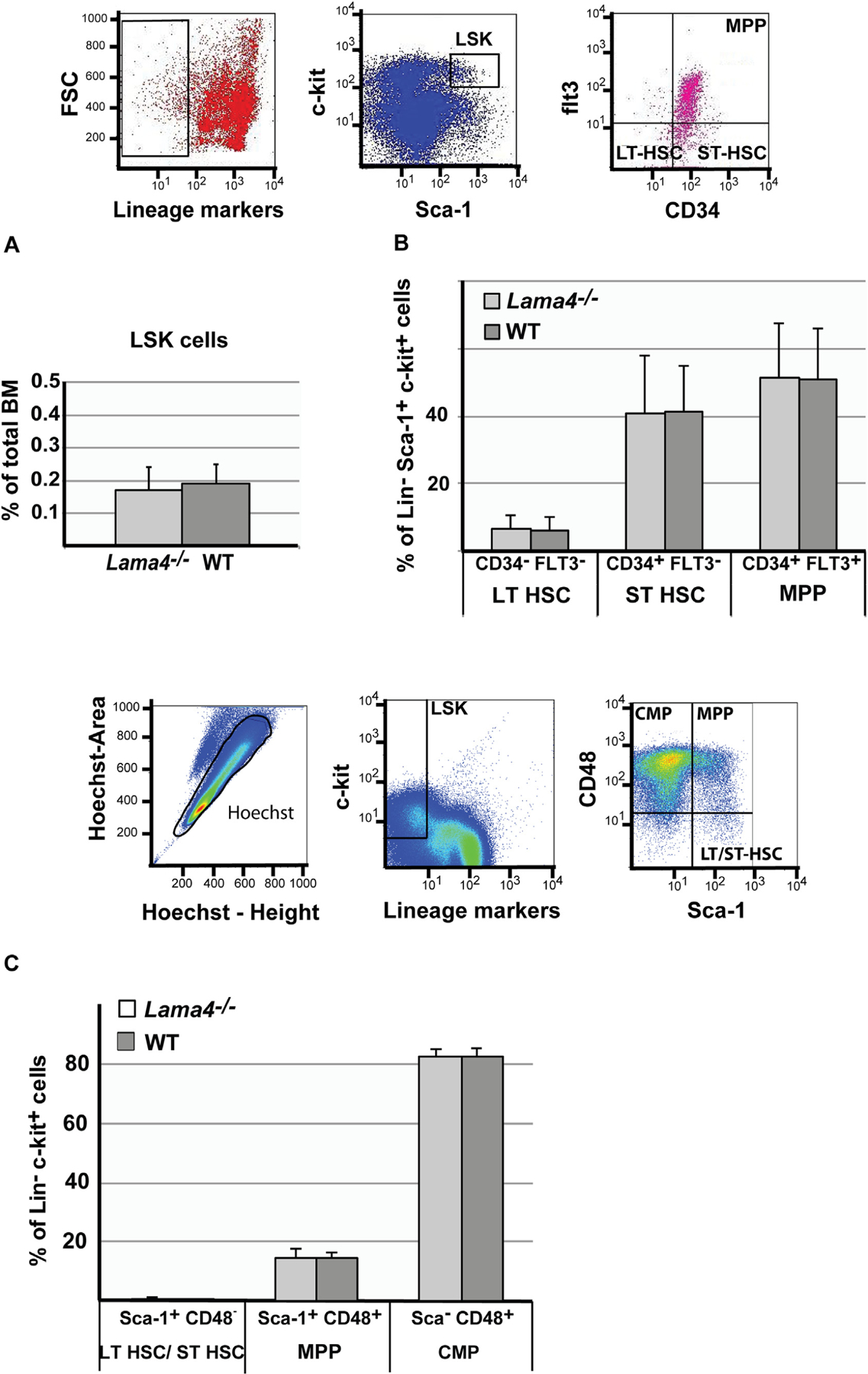
Hematopoietic stem and progenitor cell numbers in Lama4−/− bone marrow. Proportions of hematopoietic stem and progenitor cells in murine bone marrow of WT and Lama4−/− mice were quantified by flow cytometry. The upper panel shows flow cytometry gatings for graphs A and B. (A) LSK cells are lineage−Sca-1+c-kit+; (B) LSK cells from (A) were further subdivided by expression of CD34 and Flt3 expression: Lin−c-kit+Sca-1+CD34+Flt3+ are multipotent progenitor cells (MPP), Lin−c-kit+Sca-1+CD34+Flt3− are short-term repopulating HSC (ST-HSC), Lin−c-kit+Sca-1+CD34−Flt3− are long-term repopulating HSC (LT-HSC). (C) Flow cytometry gatings employed are shown. These cell populations were further used for cell cycle analysis (Fig. 5). All nucleated live single cells are found within the gate Hoechst peak height versus Hoechst peak area. Further classification into different cell populations is based on expression of lineage markers, c-kit, Sca-1 and CD48. Lin−c-kit+Sca-1+CD48− are LT-HSC/ST-HSC, Lin−c-kit+Sca-1+CD48+ are MPP and Lin−c-kit+Sca-1−CD48+ are common myeloid progenitors (CMP) quantification of which is show in the bar graph. Data are mean values ± SD obtained from at least 3 experiments with 2–4 WT and Lama4−/− mice/experiment.
To assess more committed progenitor cells, Sca-1 expression was examined within the Lin−c-Kit+ population (Table 2). During early hematopoietic development Sca-1 expression is down-regulated, so that more committed progenitor cells such as common myeloid cells (CMP) are Sca-1− [40]. The proportions of common myeloid progenitor cells were comparable in WT (82.23%) and Lama4–/– mice (82.5%). Also, the proportions of LT-HSC/ST-HSC and MPP did not vary between WT and Lama4–/– mice within the Lin−c-Kit+ cell population (Fig. 3C); which is in accordance to the data presented above.
Competitive bone marrow reconstitution assays for HSPC quantification
To more precisely quantify hematopoietic stem and progenitor cells from Lama4−/− and WT bone marrow competitive reconstitution assays were performed in vivo. Different ratios of WT and Lama4 −/− cells carrying distinct CD45 allelic markers were transplanted into lethally irradiated WT recipients, and the blood was analyzed by flow cytometry at 4, 8, 12 and 16 weeks for CD45.1+ WT versus CD45.2+ Lama4−/− immune cells. Transfer of equal numbers of WT and Lama4−/− cells resulted in 4 times higher numbers of WT derived cells in the peripheral blood (Fig. 4A). This difference was statistically significant at 4, 8 and 12 weeks, but not at 16 weeks, suggesting reduced numbers of ST-HSC in Lama4−/− bone marrow. Although not statistically significant, even injection of ten times more Lama4−/− bone marrow cells resulted in slightly higher numbers of WT derived leukocytes in the blood, i.e. a ratio of 1.5 WT:Lama4−/− cells (Fig. 4B). Only 100 times more Lama4−/− bone marrow cells led to higher numbers of Lama4−/− derived leukocytes in the blood (Fig. 4C).
Fig. 4.
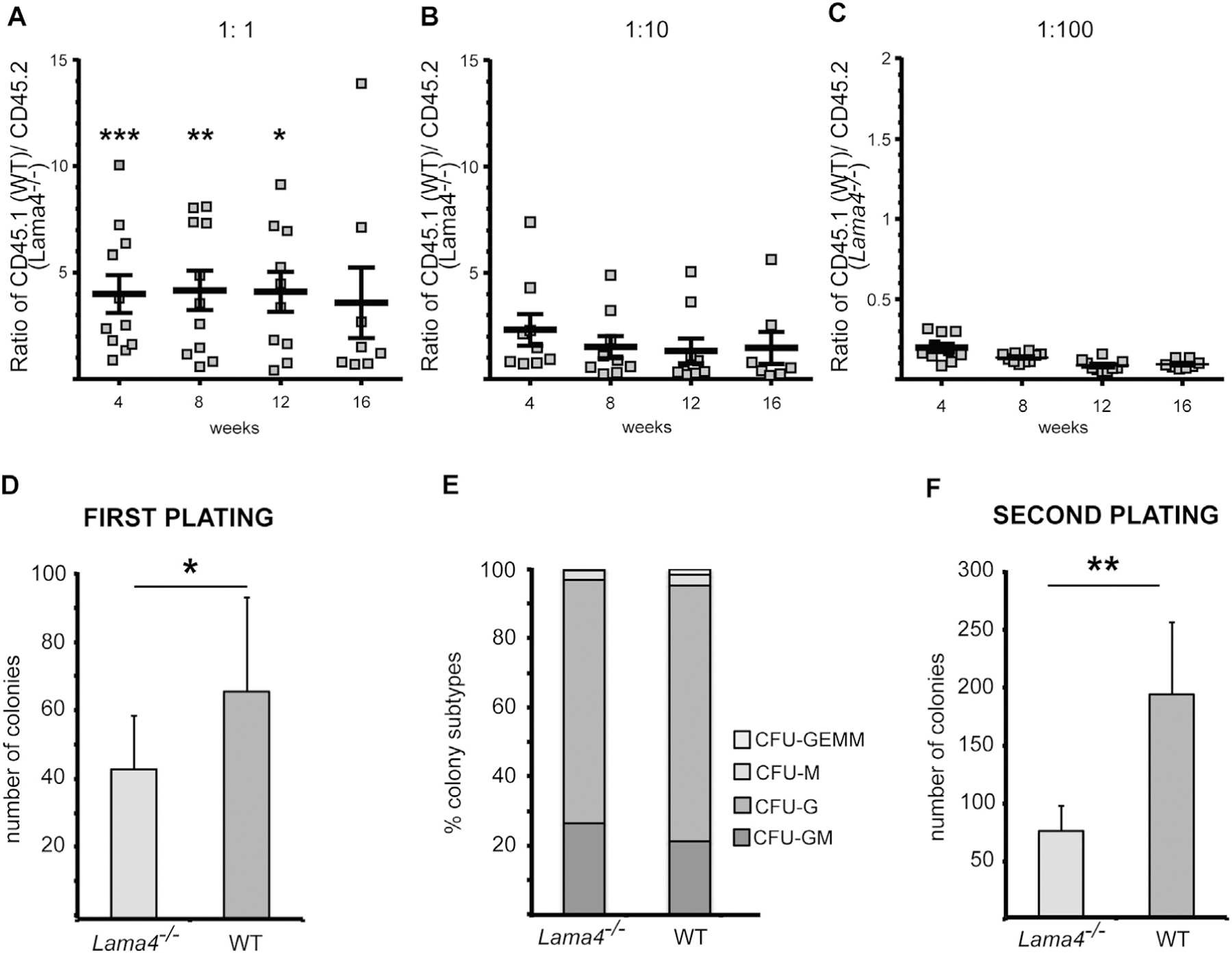
Competitive bone marrow repopulation assays (A–C) and colony forming assays (D–F) suggest reduced HSPC cells in Lama4−/− bone marrow but no defects in differentiation. Ratios of (A) 1:1 (B) 1:10 and (C) 1:100 WT (CD45.1): Lama4−/− (CD45.2) bone marrow cells were injected i.v. into irradiated CD45.1+/CD45.2+ recipient mice and CD45.1 versus CD45.2 cells in the peripheral blood were determined by flow cytometry at 4, 8, 12 and 16 weeks. At an equal transplantation ratio, fewer Lama4−/− bone marrow derived peripheral blood cells were measured at 4, 8 and 12 weeks, suggesting a competitive repopulation deficit. Data are mean values ± s.e.m., each dot represents one recipient mouse; at least 4 experiments were performed with 3–4 mice/experiment; *P < .05; **P < .01; ***P < .001. (D) WT and Lama4−/− bone marrow cells were seeded in semi-solid medium and the resulting colonies were counted at 8 days, revealing reduced colonies derived from Lama4−/− bone marrow at first platings (D), but no differences in the proportions of different myeloid cell types that develop from WT or Lama4−/− bone marrow. (F) Second platings of colonies derived from the first plating and analyzed at day 8 show fewer Lama4−/−-derived colonies. Data in D–E are mean values ± SD obtained from 3 experiments with 3 WT and 3 Lama4−/− mice/experiment. **P < .01, ***P < .001.
Colony forming assays for assessment of ST- and LT-HSCs and lineage specification
Bone marrow from WT and Lama4−/− mice was plated in semi-solid medium and the number of colonies that formed at 8 days was counted. As only cells with stem cell characteristics can survive and grow in these culture conditions, and not stromal cells that could secrete ECM molecules, this method assesses the number of hematopoietic stem cells in the donor bone marrow and does not contain secreted laminins in the cultures. The first plating allows analysis of the more mature hematopoietic precursor cells, whereas subsequent replatings reveal information about the differentiation and cell division ability of earlier hematopoietic precursor cells. Plating of total primary bone marrow cells showed a significant reduction in colony numbers derived from Lama4−/− mice compared to WT mice (Fig. 4D). The proportions of BFU-E, CFU-G, CFU-M, and CFU-GEMM, which give rise to erythrocytes, granulocytes, monocytes and macrophages, and all three groups of myeloid cells, respectively, in the first plating did not differ in cultures derived from WT or Lama4−/− bone marrow (Fig. 4E). The second plating revealed even lower numbers of colonies in cultures derived from Lama4−/− bone marrow cells compared to WT bone marrow with mean colony numbers of 200 colonies/mouse from WT BM compared to 77 colonies/mouse from Lama4−/− bone marrow (Fig. 4F). Taken together this confirms reduced hematopoietic stem and progenitor cell numbers in the Lama4−/− bone marrow but no defect in differentiation capacity.
Steady state hematopoiesis analyses of bone marrow and blood
To further investigate whether loss of laminin α4 affects lineage differentiation of hematopoietic stem and progenitor cells, flow cytometry was performed for Gr-1+CD11bhigh monocytes, Gr1+CD11blow granulocytes, CD3+ T-lymphocytes, B220+ B-lymphocytes, CD41 + megakaryocytes and TER119 + erythroid cells, revealing no statistically significant differences between WT and Lama4−/− mice either in the bone marrow (Suppl. Fig. S6A) or the spleen (Suppl. Fig. S6B). Accordingly, blood counts revealed similar numbers of erythroid, lymphoid and megakaryocytic lineage cells in WT and Lama4−/− mice. The total lymphocyte, granulocyte and monocyte numbers did not reveal a quantitative difference between WT and Lama4−/− mice (Suppl. Fig. S6C). Platelet numbers were slightly elevated in the blood of Lama4−/− mice compared to WT mice, however, this was not statistically significant even though the mean platelet volume (MPV) was statistically significantly lower in Lama4−/− mice compared to WT mice (Suppl. Fig. S6D, Suppl. Table 1). Also, the red blood cell (RBC) count and relevant RBC parameters were not altered between WT and Lama4−/− mice (Suppl. Fig. S6E, Suppl. Table 1).
Lama4 and Lama5 expression by LSK cells
As immune cells and hematopoietic cells have been reported to express ECM molecules, including laminins [10], we investigated whether bone marrow LSK cells can express laminin α4 mRNA (Lama4). As laminin α5 is reported to compensate for loss of laminin α4 in some tissues [23,24], we also investigate expression of laminin α5 mRNA (Lama5). Compared to b.End3 endothelial cells, which express both Lama4 and Lama5 mRNA [41], LSK cells sorted by flow cytometry from the bone marrow of WT and Lama4−/− mice expressed little or no Lama4 and Lama5 as defined by real-time PCR, and importantly no differences were detected between WT and Lama4 −/− mice (Suppl. Fig. S7), excluding the possibility of cell intrinsic effects.
Cell cycling analyses of HSPCs
The results from the competitive reconstitution assays suggest reduced hematopoietic stem and progenitor cell numbers in the Lama4−/− bone marrow compared to WT. As laminins have been implicated in stem cell turnover [42], we performed a flow cytometry cell cycle analysis of bone marrow for the Lin−c-kit+Sca-1 +CD48 − hematopoietic stem cells with short-and long-term repopulating activity (LT-HSC/ST-HSC), as well as Lin−c-kit+Sca-1+CD48+ multipotent progenitor cells and Lin−c-kit+Sca-1−CD48+ common myeloid progenitor cells. Significantly more Lin−c-kit+Sca-1+CD48− LT-HSC/ST-HSCs accumulated in the G0 phase of the cell cycle in Lama4−/− mice, and correspondingly fewer in G1, compared to WT mice (Fig. 5A). Although statistically not significant, the same tendency was observed for multipotent progenitor cells (Lin−c-kit+Sca-1+CD48+) (Fig. 5B) and common myeloid progenitors (Lin−c-kit+Sca-1−CD48+) (Fig. 5C), suggesting that the absence of laminin α4 slows cycling of both stem and progenitor cells.
Fig. 5.
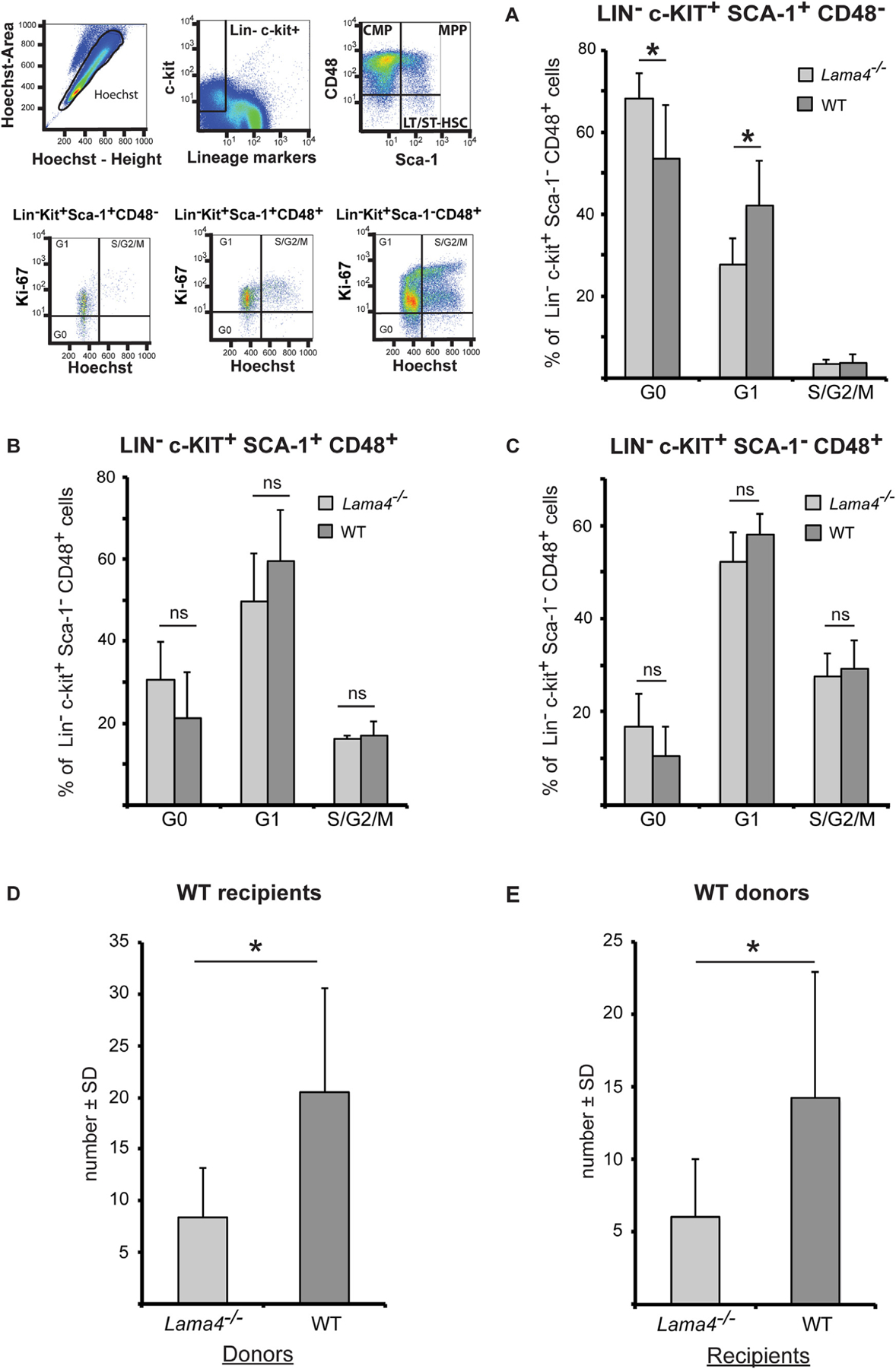
Cycling (A–C) of HSPC from Lama4−/− mice is slower and their homing to the bone marrow (D–E) is reduced. The cell cycle status of (A) Lin−c-kit+Sca-1+CD48− multipotent progenitors and HSC, (B) Lin−c-kit+Sca-1−CD48+ myeloid progenitors and (C) Lin−c-kit+Sca-1+CD48+ lineage restricted hematopoietic progenitors was analyzed by flow cytometry. Data shown are mean values ± s.e.m. obtained from 3 experiments with 3 WT and 3 Lama4−/− mice/experiment. *P < .05). (D, E) Bone marrow cells were injected i.v. into irradiated recipient mice and the bone marrow of recipient mice analyzed at 16 h in colony forming assays. D) Transplantation of Lama4−/− bone marrow to WT recipients reveals lower numbers of colonies compared to WT bone marrow; E) transplantation of WT bone marrow to Lama4−/− recipients also reveals fewer colonies than when WT bone marrow cells are transferred. Data shown are mean values ± SD from 3 experiments with 2–3 recipient mice/experiment. *P < .05.
HSPC homing to WT and Lama4−/− bone marrow
Given that reconstitution of the bone marrow hematopoietic system after irradiation requires extravasation of hematopoietic stem and progenitor cells from the blood to the bone marrow niche, we employed homing assays to investigate whether migration to and entry of hematopoietic stem and progenitor cells into the bone marrow was altered in Lama4−/− mice. First, the same number of bone marrow cells from WT or Lama4−/− mice was injected i.v. into WT recipients and, secondly, the same number of bone marrow cells from WT mice was injected i.v. into WT or Lama4−/− recipients. Sorted CD45+ bone marrow cells were isolated and plated in semi-solid medium 16 h after injection and the number of colonies that formed over 8 days was counted. This permitted estimation of colony forming units (CFU) per defined number of donor bone marrow cells. Colony formation was significantly reduced in WT recipients that received bone marrow transplantations from Lama4 −/− mice (Fig. 5D), consistent with lower numbers of hematopoietic stem and progenitor cells in Lama4−/− bone marrow. However, colony formation was also significantly reduced when WT bone marrow cells were injected into Lama4−/− recipients compared to WT recipients (Fig. 5E). Here, the capacity of WT bone marrow cells to induce colony formation in WT recipients was two-fold higher that in Lama4 −/− recipients. Control mice that did not receive bone marrow transplantation showed no colony formation. This suggests an additional defect in hematopoietic stem and progenitor cell entry into the bone marrow hematopoietic niche in Lama4−/− mice.
Discussion
The data presented here indicate that laminin α4 isa major constituent of bone marrow ECM and that in its absence hematopoietic stem and progenitor cell cycling is reduced, but not differentiation into different hematopoietic lineages. Additionally, reduced homing of WT hematopoietic stem and progenitor cells to the bone marrow of Lama4−/− mice suggests a defect in extravasation across bone marrow vessels and, thereby, impaired hematopoietic stem and progenitor cell circulation.
We demonstrate that only laminin α3, α4 and α5 chains occur in the bone marrow, with laminin α4 showing the most extensive distribution, occurring in basement membranes of larger vessels and underlying the venous sinusoids, as previously reported [19], but also in a reticular fiber-like network in the intersinusoidal space. Costaining for laminin β and γ chains suggests the occurrence of predominantly laminin 421 in the intersinusoidal fibers, and laminin 421 and 521 around larger vessels and venous sinuses. In the absence of laminin α4, the only notable alteration was a slightly broader expression of laminin α3, most likely in the form of laminin 321 but possibly also as laminin 322, in the intersinusoidal fiber network. While we have previously shown laminin α4 to be a major constituent of the mouse and human bone marrow [17,19], we have not previously analyzed whole mount bone marrow samples, which has revealed a laminin α4-rich intersinusoidal fiber network that interconnects arterioles and venous sinuses, two potential sites of HSC localization [43]. We show here that this intersinusoidal fiber network is a unique structure, comparable to the reticular fiber network of secondary lymphoid organs, but because of its dimensions, extracellular matrix composition and absence of common reticular fibroblast markers it is not likely to have a conduit function as in secondary lymphoid organs [29], but rather a structural role or a role in maintaining specialized adhesive niches for HSC [44]. In particular this intersinusoidal fiber network was altered in the Lama4−/− mice, suggesting that some of the effects observed on hematopoietic stem and progenitor cell cycling may be due to a role of this structure in maintaining these cell populations.
Previous data from our laboratory showed slower reconstitution of the hematopoietic system by Lama4 −/− than WT bone marrow [24], which is probably explained by the combined effects of slower stem and progenitor cell cycling and the reduced extravasation of stem cells into the bone marrow cavity, resulting in reduced hematopoietic and progenitor cell numbers. This was suggested by the results of the in vivo competitive reconstitution assays, which, in particular, showed reduced short-term HSPC numbers in the Lama4 −/− bone marrow. Flow cytometry could not confirm reduced short-term HSPC numbers in Lama4−/− bone marrow, probably due to the lower sensitivity of this method compared to in vivo competitive reconstitution assays and the low numbers of HSPC in the bone marrow. The statistically significant accumulation of Lin−c-kit+Sca-1+CD48− HSC with long-term and short-term repopulation activity in the G0 phase in Lama4−/− mice suggests that the reduced HSPC numbers in these mice is due to their slower cycling. Although not significant, Lin−c-kit+Sca-1−CD48+ common myeloid progenitor cells and Lin−c-kit+Sca-1+CD48+ multipotent progenitor cells showed the same tendency towards reduced cycling in the Lama4−/− mice, suggesting that the effects are not specific for stem cells and early progenitor cells but also for more differentiate populations.
The colony forming assays, which showed fewer colonies in both first and second platings of Lama4−/− bone marrow versus WT bone marrow, further support reduced progenitor cell numbers in Lama4−/− bone marrow. However, in such experiments it is not HSC but rather myeloid-restricted progenitors and precursors that are assessed and their clonal expansion, i.e. a single progenitor cell that clonally expands into a colony. The first plating reflects mature progenitor numbers, whereas the second plating addresses earlier progenitors. In both platings, the number of colonies was significantly reduced in the case of Lama4−/− bone marrow, suggesting lower numbers of both early and mature myeloid progenitors. However, as the proportions of different CFU types did not differ between WT and Lama4−/− bone marrow, i.e. the proportion of cells differentiating towards granulocyte, monocyte/macrophage or erythrocyte lineage, this indicates no defects in differentiation capacity. This was supported by unaltered peripheral blood cell counts and numbers of mature immune cell populations including granulocytes, lymphocytes, megakaryocytes and erythrocytes in the bone marrow and spleens of Lama4−/− and WT mice. Taken together, these results suggest that although stem and progenitor cells are reduced in the bone marrow of Lama4−/− mice they have normal differentiation capacity. In addition, the CFU and in vivo limiting dilution data suggest that laminin α4/α5 probably exert their effects upstream of the myeloid progenitors, at the level of the multipotent progenitors or the short-term HSC. The slower cycling of these stem cells may lead to reduced numbers of progenitors cells committed to myeloid, and possibly also lymphoid lineages, although the latter remains to be investigated.
Since Lama4−/− mice still express laminin α5, at least surrounding sinuses and larger blood vessels, and show a broader expression of laminin α3, whether reduced numbers and cycling of stem and progenitor cell populations is due to the absence of laminin α4 or to the presence of laminin α5 and/or α3 cannot be distinguished from the experiments performed here. However, previous studies have reported that only laminin α5 and not laminin α4 or α3 containing isoforms inhibit embryonic stem cell cycling and maintain pluripotency, consistent with our data [22,42,45]. Additionally, laminin α5 containing isoforms support high levels of high affinity binding of bone marrow cells and stem cell lines, while laminin α4 containing isoforms are considerably weaker substrates and laminin α3 containing isoforms have not been shown to support binding [8,17,19]. Taken together, this suggests that the reduced HSPC numbers and cycling observed here is most likely due to inhibitory effects induced by strong adhesion of stem cell and progenitor cell to laminin 521 and potentially also laminin 522 [18]. Whether weak interactions with laminin 421 stimulate differentiation, however, remains an open question.
Differential effects of laminin α4 and α5 containing isoforms on HSPC formation and function are likely, given differences in their abilities to self-polymerize into networks and to engage different cell surface receptors. Laminin 411 is an isoform with severe N-terminal truncation of one of the three short arms needed to polymerize, it binds to heparin and sulfatides, and is reported to exhibit weak integrin and dystroglycan binding [46,47]. Laminin 421, of which little is known, can be predicted to have the same polymerization and dystroglycan-binding attributes but might bind differently to integrins [12,48]. By contrast, α5-laminins bind strongly to a variety of integrins, including α6β1 [46], α5β1 and αvβ3 [49], all of which are expressed by HSPC [50] and, of which, α6β1 has been shown to mediate binding of primary and hematopoietic progenitor cell lines to laminin 511 in a 2D system [17,19]. In support of integrin-laminin induced signaling in HSPC numbers and cycling, kindlin-3-mediated integrin activation was recently reported to be essential for the retention of short-term repopulating HSC (ST-HSC) but not the long-term repopulating HSC [51] in the bone marrow, resulting in reduced ST-HSCs in the circulation and exhaustion of the hematopoietic system. Additionally, short-term homing of LSK into the bone marrow was impaired in these kindlin 3-mutant mice [51]. While the phenotypes described in this study are more extreme than those described here for the Lama4−/− mice they show the same pattern, supporting the possibility that the bone marrow laminins (probably laminin 521/522) represent at least one of the integrin substrates controlling HSPC cycling and extravasation.
In addition to effects on HSPC numbers and cycling, experiments addressing HSPC homing to the bone marrow suggested defects in the hematopoietic niche and/or in HSPC extravasation into the bone marrow niche. The analysis of WT mice transferred with Lama4−/− donor cells resulted in 67% fewer colonies than in the case of WT donor cells, consistent with reduced HSPC cells in the Lama4−/− bone marrow. However, the converse experiment, where WT cells were transferred to Lama4−/− recipients, showed a similar reduced number of colonies in the Lama4−/− versus WT recipients. Given that bone marrow was excised at 16 h after transfer this suggests either a defect in HSC survival in the Lama4−/− bone marrow or, as has been shown for immune cell infiltration into the brain of Lama4−/− mice [24], impaired extravasation from the blood into the bone marrow. As in inflammatory situations, in bone marrow transplantations the HSC must exit from the blood at the level of venules in order to enter into the bone marrow niche. Even though the venous sinuses and their underlying basement membrane are considered to have a fenestrated phenotype, these fenestrations are roughly 200 nm in diameter [52], hence, extravasating cells must still interact both with the endothelium and the basement membrane. As laminin α5, which is still present in the blood vessel basement membranes of Lama4−/− mice, inhibits this transmigration process in the case of T lymphocytes and neutrophils during inflammation [24,53], it may have a similar effect on HSC extravasation. As a small population of HSC constantly circulates in the peripheral blood [54] such an effect would reduce both entry into and exit from the bone marrow niche, reducing HSC peripheral circulation. A further factor crucial for stem cell homing and survival in the bone marrow is the chemokine CXCL12/SDF-1, the localization of which within the bone marrow has recently been shown to affect HSC function [4,44]. Whether laminin α4 and/or laminin α5 containing isoforms can differentially bind and/or present this chemokine at specific sites within the bone marrow is a possibility that requires future investigation.
In conclusion, while laminin α4 and α5 containing isoforms have been previously identified in the bone marrow and show to interact with HSCs in vitro, an in vivo function for such interactions has not been shown to date. We provide evidence here that the balance of laminin α4 and α5 containing isoforms in the bone marrow influence self-replication of hematopoietic stem and progenitor cells without affecting lineage output, and influences their recirculation between blood and bone marrow. Our data support an inhibitory role for laminin α5 containing isoforms (laminin 521/522) in both these functions. Such knowledge about the bone marrow extracellular matrix is important for tissue engineering strategies that focus on the generation of extracellular matrix substrates that promote in vitro expansion of HSPC. Future studies will have to address the in vivo signals originating from laminin α4 and α5 containing isoforms, and their effects on stem and progenitor cell expansion and survival in 3D systems reflecting the in vivo architecture.
Material and methods
Animals
C57BL/6 mice (Charles River Laboratories) and Lama4 −/− mice [23] of 8–16 weeks were employed. C57BL/6 mice carrying CD45.1 were crossed with C57BL/6 mice carrying CD45.2 to create CD45.1/CD45.2 mice. Experiments were conducted according to German animal welfare guidelines and approved by ‘Landesamt fuer Natur, Umwelt und Verbrau-cherschutz Nordrhein-Westfalen’.
Antibodies
Antibodies against the following extracellular matrix molecules were employed in immunofluorescence staining: perlecan (clones C11L1, A7L6) [55], collagen type I (Chemicon, catalog number AB765P), collagen type III (Southern Biotech, catalog number 1330–01), collagen type IV (Chemicon, catalog number AB765P), nidogen-1 [56], laminin 111 (455/pan-laminin), laminin α1 (clone 200) [57], laminin α2 (clone 4H8–2) [58], laminin β1 (clone 3A4) [59], laminin β2 (antibody name 409) [60], laminin γ1 (clone 3E10) [59], laminin α5 (clone 4G6) [41], fibronectin (Sigma, catalog number F3648), monoclonal antibody ER-TR7 [61] (Dianova/BMA catalog number T-2109), laminin α4 domain III (antibody name 377) [41], laminin α3A (a3 LEc1110) [31], laminin β3 VI/V, laminin γ2, laminin γ3 (kind gift from T. Sasaki) [49,62], fibrillin-1 [63], desmin (Progene, catalog number 10570), α-SMA (alpha smooth muscle actin) (Sigma, Catalog number C6198), gp38 (clone 8.1.1, Developmental Studies Hybridoma Bank, University of Iowa). Rat anti-mouse endoglin (clone MJ7/18) [26] was employed as an endothelial marker in immunofluorescence stainings.
Antibodies against the following surface markers were used in flow cytometry: CD3 (clone 145–2C11), PE-cy5.5-anti-CD3 (clone 7D6) [64], CD4 (clone GK1.5), PE-Cy5.5-anti-CD4 (clone RM4–5, Invitrogen), CD8 (clone 5H10), CD8a (clone 53–6.7, Invitrogen), CD11b (clone M1/70) [65], CD19 (clone 6D5), B220 (clone RA3–6B2), Gr-1 (clone RB6–8C5) [66], CD41 (clone MWreg30) [67], TER-119 (BD Bioscience, catalog number 553673) [68], c-kit (clone 2B8) [69], Sca-1 (clone E13–161.7) [70], CD34 (clone RAM34) [71], flt3 (clone A2F10.1), CD45.1 (clone A20) [72], CD45.2 (clone 30G12) [73], CD48 (BioLegend, clone HM48–1) [74] (all purchased from BD Biosciences, unless otherwise stated).
Additional reagents used in flow cytometry were: PE-Cy7 streptavidin (catalog number 557598; BD Bioscience), anti-Ki67 (clone B56; BD Pharmingen) and Hoechst33342 (catalog number 14533, Sigma).
Tissue collection
Mice between 8 and 16 weeks of age were employed. Murine bone marrow cells were collected from femur and tibia. Lysis of enucleated red blood cells was achieved by adding RBC lysis buffer (BD Pharma Lyse™, 555899) according to the manufacturer’s protocol.
Immunohistochemistry
Femurs were embedded in 8% gelatin/PBS and immediately frozen at −80 °C; 20 μm or 40 μm cryosections were used for immunofluorescence staining. Sections were air-dried at room temperature and fixed in ice cold methanol for 5 min at −20 °C. Subsequent blocking with 1% bovine serum albumin (BSA) in PBS was performed in a humidified chamber at room temperature for 1 h. Incubation with primary antibody followed overnight at 4 °C or for 2 h at room temperature, after washing, secondary antibodies were incubated for 1 h at room temperature. Secondary antibodies were diluted in PBS containing 1 μg/ml DAPI to visualize nuclei.
In the case of pan-laminin and endoglin costaining, bone marrow was removed from the bone and stained as a whole mount as described above, with the exception that 1% Triton-×100 was included in all solutions.
Sections were washed with PBS and mounted in Elvanol. Slides were analyzed using a Zeiss AxioImager microscope equipped with epifluorescence (Zeiss) or a LSM700 confocal laser-scanning microscope (Zeiss). Images from Zeiss AxioImager microscope were captured using an ORCA-1 camera (Hamamatsu) and Volocity 6.0.1. software (Improvision).
Blood analyses
Blood was taken from the tip of the tail and collected into a heparinized tube. Blood parameters were measured using the veterinary blood cell counter Vet abc (Horiba).
Flow cytometry (FACS) and cell cycle analyses
Single cell suspensions of bone marrow or spleen cells were obtained by sieving through a 70-μm filter. The cell surface marker combination, Lin−Sca−1+c-kit+ (LSK), was used to identify a population of bone marrow cells enriched for hematopoietic stem and progenitor cells (Table 2). This subpopulation was further subdivided into long-term HSC (LT-HSC), short-term HSC (ST-HSC), multipotent progenitor cells (MPP), lineage-restricted hematopoietic precursor cells (HPC) or myeloid cells based on their differential expression of CD34, flt3, CD48 or CD150 (Table 2). Cell cycle analysis was performed as described previously [75]. Briefly, all single nucleated cells were identified by flow cytometry using the fluorescent vital dye Hoechst 33342 and subsequent gating on lineage−c-kit+ cells, which represent a population enriched for HSPC and more committed progenitors. Sca-1 and CD48 expression was used to subdivide cells into LT-HSC/ST-HSC, MPP and common myeloid progenitors (CMP). Classification into G0, G1 and S/G2/M phase was performed based on Hoechst33342 fluorescence intensity and Ki67 analyses. Flow cytometry was performed using a Calibur™ flow cytometer with CellQuest (BD Bioscience) software or Becton Dickinson FACS Canto™ flow cytometer with Becton FACS Diva (BD Bioscience) software or FlowJow software. Cell sorting was performed with Becton Dickinson FACS Aria™ flow cytometer (BD Bioscience).
Colony forming unit assay (CFU assay)
7 × 104 bone marrow cells were added to 3.5 ml sterile methylcellulose medium in triplicate and colony forming unit assays were performed according to the manufacturer’s instructions (MethoCult M3434, Stem Cell Technologies, Vancouver, BC, Canada). Colonies were counted on day 8 after plating. Colony types were classified as burst forming units erythrocytes (BFU-E), colony-forming units granulocytes (CFU-G), colony-forming units monocytes (CFU-M), colony-forming units granulocytes/monocytes (CFU-GM) and colony-forming units granulocytes/erythrocytes/monocytes/macrophages (CFU-GEMM) according to their morphology. After counting, cells were replated: 7 × 104 cells were used for second platings, and analyzed as for the first plating.
Competitive reconstitution assay
CD45.1+/CD45.2+ mice between 12 and 16 weeks of age were employed as recipient mice and irradiated with 11 Gy 4 h prior to bone marrow transplantation. Donor bone marrow cells from CD45.1+ WT mice and CD45.2+ Lama4−/− mice were harvested and three different cell suspensions were prepared. Suspension one contained 100000 CD45.1+, 100000 CD45.2+; suspension two contained 10000 CD45.1+, 100000 CD45.2+ and suspension three contained 10000 CD45.1+, 1000000 CD45.2+ cells. WT CD45.1+/CD45.2 + donor bone marrow cells were co-transferred with suspension 1 and 2 to make up the total number of cells transferred to 1 × 106 in order to guarantee the survival of irradiated recipients. Cells were transferred in 200 μl PBS injected i. v. into the tail veins of recipient mice. The reconstitution of the hematopoietic system was analyzed by flow cytometry quantification of the single positive populations only, i.e. CD45.1 WT versus CD45.2 Lama4 −/− cells, at 4, 8, 12 and 16 weeks after transfer. Data were expressed as the CD45.1 WT/CD45.2 Lama4−/− ratio.
Bone marrow homing assays
4 h after irradiation of recipient mice with 11 Gy 5 × 106 donor bone marrow cells were injected into the tail vein of recipient mice. Control mice did not receive bone marrow transplantations. Recipient mice were sacrificed 16 h after injection and 1 × 106 bone marrow cells were gained to perform the CFU assay as described above.
Statistical analyses
Statistical analyses were performed using GraphPad Prism. All values are expressed as means ± standard deviation (SD). Data were tested for normality (Kolmogorov–Smirnov test in GraphPad Prism). If there was no normal distribution, then we used the non-parametric Mann–Whitney test, otherwise we employed the two-sided Student’s t-test. Pearson’s R value and mean fluorescent intensity were analyzed by ImageJ.
Supplementary Material
Acknowledgements
The authors thank Stefan Luetke-Enking for help with animal breeding, Sophie Loismann for technical assistance; Isabel Schulze for help with colony forming assays and bone marrow, spleen and blood analyses, and Thorsten König for help with sorting of HSC.
Funding
This work was partially funded by grants from the German Research Foundation to LS (CRC1009/A02, EXE1003) and the medical faculty of the University of Muenster, Germany. K.S. received funding from Innovative Medizinische Forschung (IMF SU611008) of the University of Muenster, Germany.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.matbio.2018.01.007.
Declaration of interest
The authors declare to have no competing interests.
References
- [1].Mercier FE, Ragu C, Scadden DT, The bone marrow at the crossroads of blood and immunity, Nat. Rev. Immunol 12 (2012) 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Smith JN, Calvi LM, Concise review: current concepts in bone marrow microenvironmental regulation of hematopoietic stem and progenitor cells, Stem Cells 31 (2013) 1044–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Levesque JP, Helwani FM, Winkler IG, The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization, Leukemia 24 (2010) 1979–1992. [DOI] [PubMed] [Google Scholar]
- [4].Asada N, Takeishi S, Frenette PS, Complexity of bone marrow hematopoietic stem cell niche, Int. J. Hematol 106 (2017) 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang L, Benedito R, Bixel MG, Zeuschner D, Stehling M, Savendahl L, Haigh JJ, Snippert H, Clevers H, Breier G, Kiefer F, Adams RH, Identification of a clonally expanding haematopoietic compartment in bone marrow, EMBO J 32 (2013) 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kiel MJ, Morrison SJ, Uncertainty in the niches that maintain haematopoietic stem cells, Nat. Rev. Immunol 8 (2008) 290–301. [DOI] [PubMed] [Google Scholar]
- [7].Sagar BM, Rentala S, Gopal PN, Sharma S, Mukhopadhyay A, Fibronectin and laminin enhance engraftibility of cultured hematopoietic stem cells, Biochem. Biophys. Res. Commun 350 (2006) 1000–1005. [DOI] [PubMed] [Google Scholar]
- [8].Gu YC, Kortesmaa J, Tryggvason K, Persson J, Ekblom P, Jacobsen SE, Ekblom M, Laminin isoform-specific promotion of adhesion and migration of human bone marrow progenitor cells, Blood 101 (2003) 877–885. [DOI] [PubMed] [Google Scholar]
- [9].Domogatskaya A, Rodin S, Tryggvason K, Functional diversity of laminins, Annu. Rev. Cell Dev. Biol 28 (2012) 523–553. [DOI] [PubMed] [Google Scholar]
- [10].Malara A, Currao M, Gruppi C, Celesti G, Viarengo G, Buracchi C, Laghi L, Kaplan DL, Balduini A, Megakaryocytes contribute to the bone marrow-matrix environment by expressing fibronectin, type IV collagen, and laminin, Stem Cells 32 (2014) 926–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Durbeej M, Laminins, Cell Tissue Res 339 (2010) 259–268. [DOI] [PubMed] [Google Scholar]
- [12].Li S, Qi Y, McKee K, Liu J, Hsu J, Yurchenco PD, Integrin and dystroglycan compensate each other to mediate laminin-dependent basement membrane assembly and epiblast polarization, Matrix Biol 57–58 (2017) 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Flanagan K, Fitzgerald K, Baker J, Regnstrom K, Gardai S, Bard F, Mocci S, Seto P, You M, Larochelle C, Prat A, Chow S, Li L, Vandevert C, Zago W, Lorenzana C, Nishioka C, Hoffman J, Botelho R, Willits C, Tanaka K, Johnston J, Yednock T, Laminin-411 is a vascular ligand for MCAM and facilitates TH17 cell entry into the CNS, PLoS One 7 (2012), e40443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Timpl R, Tisi D, Talts JF, Andac Z, Sasaki T, Hohenester E, Structure and function of laminin LG modules, Matrix Biol 19 (2000) 309–317. [DOI] [PubMed] [Google Scholar]
- [15].Li S, Harrison D, Carbonetto S, Fassler R, Smyth N, Edgar D, Yurchenco PD, Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation, J. Cell Biol 157 (2002) 1279–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kikkawa Y, Miner JH, Review: Lutheran/B-CAM: a laminin receptor on red blood cells and in various tissues, Connect. Tissue Res 46 (2005) 193–199. [DOI] [PubMed] [Google Scholar]
- [17].Siler U, Seiffert M, Puch S, Richards A, Torok-Storb B, Muller CA, Sorokin L, Klein G, Characterization and functional analysis of laminin isoforms in human bone marrow, Blood 96 (2000) 4194–4203. [PubMed] [Google Scholar]
- [18].Siler U, Rousselle P, Muller CA, Klein G, Laminin gamma2 chain as a stromal cell marker of the human bone marrow microenvironment, Br. J. Haematol 119 (2002) 212–220. [DOI] [PubMed] [Google Scholar]
- [19].Gu Y, Sorokin L, Durbeej M, Hjalt T, Jonsson JI, Ekblom M, Characterization of bone marrow laminins and identification of alpha5-containing laminins as adhesive proteins for multipotent hematopoietic FDCP-Mix cells, Blood 93 (1999) 2533–2542. [PubMed] [Google Scholar]
- [20].Vogel W, Kanz L, Brugger W, Berndt A, Kosmehl H, Expression of laminin beta2 chain in normal human bone marrow, Blood 94 (1999) 1143–1145. [PubMed] [Google Scholar]
- [21].Di Russo J, Hannocks MJ, Luik AL, Song J, Zhang X, Yousif L, Aspite G, Hallmann R, Sorokin L, Vascular laminins in physiology and pathology, Matrix Biol 57–58 (2017) 140–148. [DOI] [PubMed] [Google Scholar]
- [22].Domogatskaya A, Rodin S, Boutaud A, Tryggvason K, Laminin-511 but not −332, −111, or −411 enables mouse embryonic stem cell self-renewal in vitro, Stem Cells 26 (2008) 2800–2809. [DOI] [PubMed] [Google Scholar]
- [23].Thyboll J, Kortesmaa J, Cao R, Soininen R, Wang L, Iivanainen A, Sorokin L, Risling M, Cao Y, Tryggvason K, Deletion of the laminin alpha4 chain leads to impaired microvessel maturation, Mol. Cell. Biol 22 (2002) 1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wu C, Ivars F, Anderson P, Hallmann R, Vestweber D, Nilsson P, Robenek H, Tryggvason K, Song J, Korpos E, Loser K, Beissert S, Georges-Labouesse E, Sorokin LM, Endothelial basement membrane laminin alpha5 selectively inhibits T lymphocyte extravasation into the brain, Nat. Med 15 (2009) 519–527. [DOI] [PubMed] [Google Scholar]
- [25].Aumailley M, The laminin family, Cell Adhes. Migr 7 (2013) 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ge AZ, Butcher EC, Cloning and expression of a cDNA encoding mouse endoglin, an endothelial cell TGF-beta ligand, Gene 138 (1994) 201–206. [DOI] [PubMed] [Google Scholar]
- [27].Song J, Lokmic Z, Lammermann T, Rolf J, Wu C, Zhang X, Hallmann R, Hannocks MJ, Horn N, Ruegg MA, Sonnenberg A, Georges-Labouesse E, Winkler TH, Kearney JF, Cardell S, Sorokin L, Extracellular matrix of secondary lymphoid organs impacts on B-cell fate and survival, Proc. Natl. Acad. Sci. U. S. A 110 (2013) E2915–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lokmic Z, Lammermann T, Sixt M, Cardell S, Hallmann R, Sorokin L, The extracellular matrix of the spleen as a potential organizer of immune cell compartments, Semin. Immunol 20 (2008) 4–13. [DOI] [PubMed] [Google Scholar]
- [29].Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, Pabst R, Lutz MB, Sorokin L, The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node, Immunity 22 (2005) 19–29. [DOI] [PubMed] [Google Scholar]
- [30].Champliaud MF, Lunstrum GP, Rousselle P, Nishiyama T, Keene DR, Burgeson RE, Human amnion contains a novel laminin variant, laminin 7, which like laminin 6, covalently associates with laminin 5 to promote stable epithelial-stromal attachment, J. Cell Biol 132 (1996) 1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chang YC, Sabourin CL, Lu SE, Sasaki T, Svoboda KK, Gordon MK, Riley DJ, Casillas RP, Gerecke DR, Upregulation of gamma-2 laminin-332 in the mouse ear vesicant wound model, J. Biochem. Mol. Toxicol 23 (2009) 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Weissman IL, Shizuru JA, The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases, Blood 112 (2008) 3543–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Morrison SJ, Weissman IL, The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype, Immunity 1 (1994) 661–673. [DOI] [PubMed] [Google Scholar]
- [34].Spangrude GJ, Heimfeld S, Weissman IL, Purification and characterization of mouse hematopoietic stem cells, Science 241 (1988) 58–62. [DOI] [PubMed] [Google Scholar]
- [35].Yang L, Bryder D, Adolfsson J, Nygren J, Mansson R, Sigvardsson M, Jacobsen SE, Identification of Lin(−)Sca1(+) kit(+)CD34(+)Flt3-short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients, Blood 105 (2005) 2717–2723. [DOI] [PubMed] [Google Scholar]
- [36].Oguro H, Ding L, Morrison SJ, SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors, Cell Stem Cell 13 (2013) 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Morrison SJ, Wandycz AM, Hemmati HD, Wright DE, Weissman IL, Identification of a lineage of multipotent hematopoietic progenitors, Development 124 (1997) 1929–1939. [DOI] [PubMed] [Google Scholar]
- [38].Osawa M, Nakamura K, Nishi N, Takahasi N, Tokuomoto Y, Inoue H, Nakauchi H, In vivo self-renewal of c-Kit+ Sca-1+ Lin (low/−) hemopoietic stem cells, J. Immunol 156 (1996) 3207–3214. [PubMed] [Google Scholar]
- [39].Boles NC, Lin KK, Lukov GL, Bowman TV, Baldridge MT, Goodell MA, CD48 on hematopoietic progenitors regulates stem cells and suppresses tumor formation, Blood 118 (2011) 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Holmes C, Stanford WL, Concise review: stem cell antigen-1: expression, function, and enigma, Stem Cells 25 (2007) 1339–1347. [DOI] [PubMed] [Google Scholar]
- [41].Frieser M, Nockel H, Pausch F, Roder C, Hahn A, Deutzmann R, Sorokin LM, Cloning of the mouse laminin alpha 4 cDNA. Expression in a subset of endothelium, Eur. J. Biochem 246 (1997) 727–735. [DOI] [PubMed] [Google Scholar]
- [42].Rodin S, Antonsson L, Hovatta O, Tryggvason K, Monolayer culturing and cloning of human pluripotent stem cells on laminin-521-based matrices under xeno-free and chemically defined conditions, Nat. Protoc 9 (2014) 2354–2368. [DOI] [PubMed] [Google Scholar]
- [43].Richter R, Forssmann W, Henschler R, Current developments in mobilization of hematopoietic stem and progenitor cells and their interaction with niches in bone marrow, Transfus. Med. Hemother 44 (2017) 151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Addington CP, Dharmawaj S, Heffernan JM, Sirianni RW, Stabenfeldt SE, Hyaluronic acid-laminin hydrogels increase neural stem cell transplant retention and migratory response to SDF-1alpha, Matrix Biol 60–61 (2017) 206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Miyazaki T, Futaki S, Suemori H, Taniguchi Y, Yamada M, Kawasaki M, Hayashi M, Kumagai H, Nakatsuji N, Sekiguchi K, Kawase E, Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells, Nat. Commun 3 (2012) 1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nishiuchi R, Takagi J, Hayashi M, Ido H, Yagi Y, Sanzen N, Tsuji T, Yamada M, Sekiguchi K, Ligand-binding specificities of laminin-binding integrins: a comprehensive survey of laminin-integrin interactions using recombinant alpha3beta1, alpha6beta1, alpha7beta1 and alpha6beta4 integrins, Matrix Biol 25 (2006) 189–197. [DOI] [PubMed] [Google Scholar]
- [47].Talts JF, Sasaki T, Miosge N, Gohring W, Mann K, Mayne R, Timpl R, Structural and functional analysis of the recombinant G domain of the laminin alpha4 chain and its proteolytic processing in tissues, J. Biol. Chem 275 (2000) 35192–35199. [DOI] [PubMed] [Google Scholar]
- [48].Sasaki T, Takagi J, Giudici C, Yamada Y, Arikawa-Hirasawa E, Deutzmann R, Timpl R, Sonnenberg A, Bachinger HP, Tonge D, Laminin-121—recombinant expression and interactions with integrins, Matrix Biol 29 (2010) 484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sasaki T, Timpl R, Domain IVa of laminin alpha5 chain is cell-adhesive and binds beta1 and alphaVbeta3 integrins through Arg-Gly-Asp, FEBS Lett 509 (2001) 181–185. [DOI] [PubMed] [Google Scholar]
- [50].Klamer S, Voermans C, The role of novel and known extracellular matrix and adhesion molecules in the homeostatic and regenerative bone marrow microenvironment, Cell Adhes. Migr 8 (2014) 563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ruppert R, Moser M, Sperandio M, Rognoni E, Orban M, Liu WH, Schulz AS, Oostendorp RA, Massberg S, Fassler R, Kindlin-3-mediated integrin adhesion is dispensable for quiescent but essential for activated hematopoietic stem cells, J. Exp. Med 212 (2015) 1415–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM, Expression and function of laminins in the embryonic and mature vasculature, Physiol. Rev 85 (2005) 979–1000. [DOI] [PubMed] [Google Scholar]
- [53].Song J, Zhang X, Buscher K, Wang Y, Wang H, Di Russo J, Li L, Lutke-Enking S, Zarbock A, Stadtmann A, Striewski P, Wirth B, Kuzmanov I, Wiendl H, Schulte D, Vestweber D, Sorokin L, Endothelial basement membrane laminin 511 contributes to endothelial junctional tightness and thereby inhibits leukocyte transmigration, Cell Rep 18 (2017) 1256–1269. [DOI] [PubMed] [Google Scholar]
- [54].Bhattacharya D, Czechowicz A, Ooi AG, Rossi DJ, Bryder D, Weissman IL, Niche recycling through division-independent egress of hematopoietic stem cells, J. Exp. Med 206 (2009) 2837–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Couchman JR, Ljubimov AV, Mammalian tissue distribution of a large heparan sulfate proteoglycan detected by monoclonal antibodies, Matrix Biol 9 (1989) 311–321. [DOI] [PubMed] [Google Scholar]
- [56].Fox JW, Mayer U, Nischt R, Aumailley M, Reinhardt D, Wiedemann H, Mann K, Timpl R, Krieg T, Engel J, et al. , Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV, EMBO J 10 (1991) 3137–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sorokin LM, Conzelmann S, Ekblom P, Battaglia C, Aumailley M, Timpl R, Monoclonal antibodies against laminin A chain fragment E3 and their effects on binding to cells and proteoglycan and on kidney development, Exp. Cell Res 201 (1992) 137–144. [DOI] [PubMed] [Google Scholar]
- [58].Schuler F, Sorokin LM, Expression of laminin isoforms in mouse myogenic cells in vitro and in vivo, J. Cell Sci 108 (Pt 12) (1995) 3795–3805. [DOI] [PubMed] [Google Scholar]
- [59].Sixt M, Hallmann R, Wendler O, Scharffetter-Kochanek K, Sorokin LM, Cell adhesion and migration properties of beta 2-integrin negative polymorphonuclear granulocytes on defined extracellular matrix molecules. Relevance for leukocyte extravasation, J. Biol. Chem 276 (2001) 18878–18887. [DOI] [PubMed] [Google Scholar]
- [60].Sasaki T, Mann K, Miner JH, Miosge N, Timpl R, Domain IV of mouse laminin beta1 and beta2 chains, Eur. J. Biochem 269 (2002) 431–442. [DOI] [PubMed] [Google Scholar]
- [61].Van Vliet E, Melis M, Foidart JM, Van Ewijk W, Reticular fibroblasts in peripheral lymphoid organs identified by a monoclonal antibody, J. Histochem. Cytochem 34 (1986) 883–890. [DOI] [PubMed] [Google Scholar]
- [62].Koch M, Olson PF, Albus A, Jin W, Hunter DD, Brunken WJ, Burgeson RE, Champliaud MF, Characterization and expression of the laminin gamma3 chain: a novel, non-basement membrane-associated, laminin chain, J. Cell Biol 145 (1999) 605–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Tiedemann K, Batge B, Muller PK, Reinhardt DP, Interactions of fibrillin-1 with heparin/heparan sulfate, implications for microfibrillar assembly, J. Biol. Chem 276 (2001) 36035–36042. [DOI] [PubMed] [Google Scholar]
- [64].Coulie PG, Uyttenhove C, Wauters P, Manolios N, Klausner RD, Samelson LE, Van Snick J, Identification of a murine monoclonal antibody specific for an allotypic determinant on mouse CD3, Eur. J. Immunol 21 (1991) 1703–1709. [DOI] [PubMed] [Google Scholar]
- [65].Ault KA, Springer TA, Cross-reaction of a rat-anti-mouse phagocyte-specific monoclonal antibody (anti-Mac-1) with human monocytes and natural killer cells, J. Immunol 126 (1981) 359–364. [PubMed] [Google Scholar]
- [66].Fleming TJ, Fleming ML, Malek TR, Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6–8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family, J. Immunol 151 (1993) 2399–2408. [PubMed] [Google Scholar]
- [67].Nieswandt B, Echtenacher B, Wachs FP, Schroder J, Gessner JE, Schmidt RE, Grau GE, Mannel DN, Acute systemic reaction and lung alterations induced by an antiplatelet integrin gpIIb/IIIa antibody in mice, Blood 94 (1999) 684–693. [PubMed] [Google Scholar]
- [68].Kina T, Ikuta K, Takayama E, Wada K, Majumdar AS, Weissman IL, Katsura Y, The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage, Br. J. Haematol 109 (2000) 280–287. [DOI] [PubMed] [Google Scholar]
- [69].Perez C, Moreno S, Summerfield A, Domenech N, Alvarez B, Correa C, Alonso F, Ezquerra A, Dominguez J, Revilla C, Characterisation of porcine bone marrow progenitor cells identified by the anti-c-kit (CD117) monoclonal antibody 2B8/BM, J. Immunol. Methods 321 (2007) 70–79. [DOI] [PubMed] [Google Scholar]
- [70].Spangrude GJ, Klein J, Heimfeld S, Aihara Y, Weissman IL, Two monoclonal antibodies identify thymic-repopulating cells in mouse bone marrow, J. Immunol 142 (1989) 425–430. [PubMed] [Google Scholar]
- [71].Morel F, Szilvassy SJ, Travis M, Chen B, Galy A, Primitive hematopoietic cells in murine bone marrow express the CD34 antigen, Blood 88 (1996) 3774–3784. [PubMed] [Google Scholar]
- [72].Spencer JS, Kubo RT, Mixed isotype class II antigen expression. A novel class II molecule is expressed on a murine B cell lymphoma, J. Exp. Med 169 (1989) 625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ralph SJ, Berridge MV, Expression of antigens of the ‘T200’ family of glycoproteins on hemopoietic stem cells: evidence that thymocyte cell lineage antigens are represented on ‘T200’, J. Immunol 132 (1984) 2510–2514. [PubMed] [Google Scholar]
- [74].Kato K, Koyanagi M, Okada H, Takanashi T, Wong YW, Williams AF, Okumura K, Yagita H, CD48 is a counter-receptor for mouse CD2 and is involved in T cell activation, J. Exp. Med 176 (1992) 1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Barbier V, Nowlan B, Levesque JP, Winkler IG, Flow cytometry analysis of cell cycling and proliferation in mouse hematopoietic stem and progenitor cells, Methods Mol. Biol 844 (2012) 31–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


