Abstract
The Kronos Early Estrogen Prevention Study (KEEPS) was a randomized, double-blind, placebo-controlled trial designed to determine the effects of hormone treatments (menopausal hormone treatments; MHT) on the progression of carotid intima-medial thickness (CIMT) in recently menopausal women. Participants less than 3 years from menopause and without a history of overt cardiovascular disease (CVD), defined as no clinical CVD events and coronary artery calcium < 50 Agatston Units, received either oral conjugated equine estrogens (0.45 mg/day), transdermal 17β-estradiol (50 μg/day), both with progesterone (200 mg/day for 12 days/month), or placebo pills and patches for 4 years. Although MHT did not decrease the age-related increase in CIMT, KEEPS provided other important insights about MHT effects. Both MHTs versus placebo reduced the severity of menopausal symptoms, and maintained bone density, but differed in efficacy regarding mood/anxiety, sleep, sexual function and deposition of β-amyloid in the brain. Additionally, genetic variants in enzymes for metabolism and uptake of estrogen affected the efficacy of MHT for some aspects of symptom relief. KEEPS provides important information for use of MHT in clinical practice, including: type, dose, and mode of delivery of MHT recently after menopause, and how genetic variants in hormone metabolism may affect MHT efficacy on specific outcomes.
Keywords: Conjugated equine estrogens, estradiol, hormone treatments, menopause
Introduction
The Kronos Early Estrogen Prevention Study (KEEPS; NCT00154180) was initiated in the wake of the results from the Women’s Health Initiative (WHI), which, surprisingly, did not support the hypothesis generated from observational studies that use of menopausal hormone treatments (MHT) would reduce the risk of cardiovascular disease (CVD)1,2. Importantly, the design of the WHI was criticized as rather irrelevant to clinical practice as the majority of WHI participants where well beyond the age at which women typically seek treatment for menopausal symptom relief (average age was 63 years and 12 years past menopause), and indeed, about 61% of women did not have menopausal symptoms of hot flashes. In addition, the WHI included many women at high risk for cardiovascular disease (CVD)3–6. KEEPS was designed to mitigate these limitations of the WHI design by recruiting women within 3 years of menopause and by excluding those with known clinical and subclinical atherosclerosis. Participant characteristics and the major outcomes for KEEPS have been reported in detail elsewhere5,7,8. This review will highlight the major findings from the study, but primarily will report new observations, point to where further research is needed, and provide insight into how the findings from KEEPS can be applied to the care of menopausal women.
Study participants
Nine centers for KEEPS across the United States recruited 728 participants who were within 3 years of menopause, of whom (n = 544) were identified as Central European (81%), Asian (3%), Black (Yoruba; 7%), or Hispanic (7%) ancestry through DNA analysis9. One major advantage of KEEPS over other prospective clinical trials of MHT was the exclusion of women with a history of clinically defined CVD or CVD risk factors, including having an arterial calcification score (CAC) ≥ 50 Agatston Units, current smoking [more than ten cigarettes (half pack)/day by self-report], a body mass index (BMI) > 35 kg/m2, dyslipidemia (low density lipoprotein cholesterol > 190 mg/dl or fasting triglycerides > 400 mg/dl), uncontrolled hypertension (systolic blood pressure >150 mmHg and/or diastolic blood pressure > 95 mmHg) or likely diabetes (fasting blood glucose > 126 mg/dl)2. Thus, KEEPS participants were relatively healthy, recently menopausal women (see Table 1). Although highly selected, and thus less widely generalizable, these well-characterized women represent an ideal group to examine the effects of MHT on multiple physiological systems in addition to CVD processes. Using a randomized, double-blind, placebo-controlled trial design, women were treated with either oral conjugated equine estrogen (oCEE, 0.45 mg/day), or transdermal 17β-estradiol (tE2, 50 μg/day) both with oral progesterone (200 mg/day for 12 days/month) or placebo pills and patches for 4 years.
Table 1.
Clinical characteristics of KEEPS participants prior to randomization.
| Variable | Mean ± SD (n = 727) |
|---|---|
| Age (years) | 52.7 ± 2.6 |
| BMI (kg/m2) | 26.2 ± 4.3 |
| Systolic blood pressure (mmHg) | 118.6 ± 14.9 |
| Total cholesterol (mg/dl) | 208.1 ± 33.7 |
| LDL cholesterol (mg/dl) | 110.9 ± 27.8 |
| HDL cholesterol (mg/dl) | 72.0 ± 14.6 |
| Triglycerides (mg/dl) | 87.0 ± 55.9 |
| Fasting glucose (mg/dl) | 79.6 ± 10.0 |
| HOMA-IR score (units) | 1.27 ± 2.32 |
Derived from data reported in Table 1 of reference 7. SD, standard deviation; BMI, body mass index; LDL, low density lipoprotein; HDL, high density lipoprotein; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance.
Hormonal metabolism and pharmacological outcomes
While the transdermal patch delivers17β-estradiol (E2) directly into the systemic circulation, oCEE contains more estrone (E1) and E1 sulfate than E2 and, due to absorption into the portal circulation, is metabolized in the liver before entry into the systemic circulation. Thus, the concept of dose-equivalency cannot be applied to the interpretations of these data due to these differences in product formulation, absorption, and metabolism. However, the doses and formulations of both hormonal treatments were the same as those used in clinical practice. As expected, circulating levels of E2 and E1, although both increased with both treatments vs. placebo, differed between women assigned to tE2 or oCEE (Table 2).
Table 2.
Hormone levels by treatment assignment at baseline and month 48 in KEEPS participants who were compliant to treatment.
| Treatment | Baseline | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FSH (IU/l) | LH (IU/l) | Testosterone (ng/dl) | Estrone (pg/ml) | Estradiol (pg/ml) | ||||||
| Mean (SD) | Med (IQR) | Mean (SD) | Med (IQR) | Mean (SD) | Med (IQR) | Mean (SD) | Med (IQR) | Mean (SD) | Med (IQR) | |
| Placebo (n=148) | 84.1 (34) | 79.2 (50.0) | 38.8 (16) | 36.7 (21.9) | 17.1 (11) | 15.0 (9.0) | 35.3 (27) | 28.0 (17.0) | 18.7 (38) | 7.0 (7.0) |
| tE2 (n=107) | 94.1 (41) | 88.4 (50.5) | 42.0 (16) | 40.9 (20.3) | 16.6 (8) | 15.0 (9.0) | 32.3 (21) | 26.0 (20.0) | 18.2 (32) | 6.8 (5.4) |
| oCEE (n=109) | 92.2 (34) | 86.2 (46.2) | 41.1 (15) | 40.0 (20.3) | 17.6 (10) | 17.0 (9.0) | 33.3 (18) | 28.0 (20.5) | 13.8 (21) | 6.5 (7.0) |
| Month 48 | ||||||||||
| Placebo (n=148) | 84.6 (33) | 80.0 (47.1) | 38.5 (13) | 31.75 (15.6) | 18.1 (18) | 15.0 (10) | 28.5 (11) | 28.0 (15.0) | 7.2 (4) | 6.6 (4.0) |
| tE2 (n=107) | 65.2 (30) | 60.5 (41.6) | 31.2 (11) | 31.3 (15.0) | 17.0 (9) | 15.0 (10.0) | 52.4 (26) | 50.0 (35.0) | 47.1 (40) | 41 (53.7) |
| oCEE (n=109) | 69.5 (29) | 68.0 (39.4) | 35.6 (14) | 31.8 (20.6) | 20.4 (10) | 18.0 (12.0) | 106.2 (86) | 88.0 (90.0) | 15.5 (10) | 13.0 (12.3) |
tE2, transdermal estradiol; oCEE, oral conjugated equine estrogens; FSH, follicle stimulating hormone; LH, luteinizing hormone; SD, standard deviation; Med, Median; IQR, interquartile range.
Genetic variability in metabolism of estrogen among individuals is known to affect the availability of ligands for the estrogen receptors and subsequent receptor-mediated responses that could affect the onset of menopause, the severity of menopausal symptoms, and the response to MHT. Both E1 and E2 are ligands for the estrogen receptors, but these receptors have higher affinity for E2 than for E1. In addition, both are substrates for SULT1A1 which sulfonates both steroids. The gene encoding SULT1A1 is polymorphic with copy number variation as well as multiple single nucleotide polymorphisms (SNPs). Both the copy number and SNPs affect enzyme activity. In KEEPS participants, the number of gene copies with G alleles at rs92822861, resulting in increased enzymatic activity, was associated with a younger age of menopause10. Increases in enzymatic activity, resulting in greater sulfonation of circulating E1 and E2, reduce the bioavailable E1 and E2, which could lower MHT efficacy and perhaps contribute to differences in outcomes between the two formulations of MHT. The relative effectiveness of the various estrogen metabolites on specific physiological parameters remains to be clarified.
Cardiovascular outcomes
The primary cardiovascular outcome for KEEPS was the change in carotid intima medial thickness (CIMT) measured by B-mode ultrasound. Prior to randomization, CIMT averaged 0.72 mm, which is comparable to baseline values for women of similar age who participated in the Early versus Late Intervention Trail with Estrogen (ELITE; Table 3)11. CIMT increased comparably in all three groups over the 4 years of treatment (Table 2)7. Interestingly, however, SNPs within genes of the innate immunity pathway correlated with the treatment effect on the change in CIMT over the 4 years9,12. The genetic analysis included 13,229 SNPs within 764 genes from the anticoagulant, procoagulant, fibrinolytic or innate immunity pathways. Prior to randomization, two SNPs, one on chromosome 2 for MAP4K4 gene (rs2236935), and one on chromosome 5 for IL5 gene (rs739318), associated positively with CIMT; two SNPs on chromosome 17 for CCL5 (rs4796119, rs2291299) associated negatively with CIMT. However, although 20 SNPs within the innate immunity pathway associated with CIMT after treatment, none were among those that associated with CIMT prior to treatment9,12. These observations emphasize the potential impact of genetic variants in assessing CVD outcomes that are considered ‘complex traits’ and the interaction of hormones with genetic variants associated with those traits, i.e. pharmacogenomics effects.
Table 3.
Baseline measures and changes in carotid intima-thickening during KEEPS.
| Treatment | Baseline Mean (mm) (95% CI) | Progression over 4 years (mm/year) (95% CI) |
|---|---|---|
| Placebo | 0.72132 (0.7106–0.7319) | 0.0072 (0.0058–0.0086) |
| oCEE | 0.7368 (0.7152–0.7384) | 0.0080 (0.0065–0.0095) |
| tE2 | 0.7176 (0.7058–0.7295) | 0.0077 (0.0061–0.0092) |
Derived from Table 2 of reference 7. oCEE, oral conjugated equine estrogens; tE2, transdermal 17β-estradiol; CI, confidence interval.
E2 and E1 also could influence CIMT through activation of vascular endothelial cells, and through activation of circulating cells (monocytes, lymphocytes, platelets), which are associated with inflammation and innate immunity. These circulating cells interacting with the vascular wall are considered initiating steps in development of atherosclerosis13–15. Quantification of microvesicles derived from endothelial cells, platelets, leukocytes, senescent cells, and adipocytes can provide a type of measure for a general state of ‘vascular inflammation’. Indeed, in a subset of KEEPS participants, the average increase in CIMT was associated with a measure of inflammation defined by the quantity of leukocyte-derived microvesicles and microvesicles positive for the vascular cell adhesion molecule (VCAM)-116,17.
In addition to activated leukocytes and endothelium, biologically active substances released from platelets, such as thromboxane, prostacyclin and 5-hydroxytryptamine (serotonin) have the potential to affect vascular reactivity, thrombotic risk, and vascular remodeling associated with atherosclerosis. Both thromboxane and prostacyclin are present in lysate from platelets but were lower in women randomized to oCEE compared to lysate derived from women randomized to tE2. In the tE2 group, there was a positive correlation between thromboxane in platelet lysate and serum with serum levels of thromboxane associating positively with increases in CIMT18.
A secondary cardiovascular outcome for KEEPS was coronary artery calcification (CAC). Of the 570 women for whom both baseline and 48-month CAC scores were obtained, 57.3% showed an increase in CAC scores. Although there was a trend for smaller increases in CAC in the MHT groups compared to the placebo group, these differences did not reach statistical significance [risk difference: oCEE vs. placebo = −3.6 percentage points (95% confidence interval, CI −11.4 to 4.1); tE2 vs. placebo = −2.1 percentage points (95% CI −10.0 to 5.7)7. It is important to note that the lack of a significant reduction in coronary calcification in KEEPS may not invalidate the pre-study hypothesis, but rather may simply reflect three study limitations: (1) the healthy status of participants with most having an Agatston score of zero at entry, (2) the relatively small number of participants, and, perhaps of greatest importance, (3) the relatively short duration of the trial compared to the many years generally required for progression of atherosclerosis19–21.
Brain–body connection
An important ancillary study to KEEPS was the KEEPS-Cognitive and Affective Study, designed to measure the effects of MHT on global cognition, visual and verbal learning and memory, language, attention and executive function, and mood22. Consistent with other studies which have showed an inverse correlation between systolic blood pressure and cognition (see reference 23), KEEPS-Cognitive also found that systolic blood pressure was inversely associated with performance in auditory attention and working memory at study baseline, although all of the scores were within the normative range24. During the study, neither oCEE nor tE2 affected scores on any of the tested cognitive domains, and the scores on all of the tests remained within the normative range25. This lack of apparent effect on cognition may reflect that the women were relatively young and not in an age range expected to show cognitive decline or that the tests may lack the sensitivity to detect subtle changes in cognitive performance over the 4 years of the study. Despite this neutral result on cognition, both MHT treatments did affect processes in the brain: autonomic menopausal symptoms (hot flashes and night sweats) were reduced, while quality of sleep was improved26. There were interesting and potentially important differences in efficacy between oCEE and tE2 in the brain, such that the domain of sleep disturbances was reduced more with tE2 than oCEE27, while mood was improved and anxiety decreased more with oCEE than tE2, an effect that was associated with increasing concentrations of serotonin in platelets28 and serum levels of E1 sulfate25. In women with a genetic variant resulting in reduced transporter function of E2 sulfate into the liver, the decrease in night sweats was greater than in women with normal transporter function29. This result suggests that the sulfonated forms of E1 and E2 have biological activity on the central and perhaps peripheral autonomic nervous system. Whether these actions are due to local conversion of the sulfates to E1 or E2 in the tissue remains to be determined.
As expected, both hormonal preparations reduced circulating levels of follicular stimulating hormone (FSH) and luteinizing hormone (LH), a response likely due to the down-regulated release of both hormones from the pituitary gland (negative feedback). Also, as might be expected, lower levels of FSH were observed with higher levels of E2 in women randomized to tE2, and also with slightly higher levels of E1 in women randomized to placebo. Interestingly, these associations were weaker in women randomized to oCEE, in whom, however, there was a significant inverse association between circulating total testosterone and FSH30. The differences in components of oCEE (E1 and E1 sulfate) of feedback regulation of FSH might explain, in part, the beneficial effects of oCEE on improving mood and reducing anxiety compared to tE230.
Decreases in FSH were associated with smaller increases in white matter hyperintensities (WMH) in the brain more with tE2 than with oCEE. Changes in LH did not correlate with WMH30. FSH may also have direct influence on systemic functions associated with menopause including obesity, bone mineral density, and cardiovascular risk31. The interesting and potentially important associations of FSH levels with various parameters in KEEPS have yet to be further explored.
Other brain structures affected by MHT include accumulation of β-amyloid in the cerebral cortex, a hallmark of Alzheimer’s dementia pathology. Amyloid plaques also occur in blood vessels of aging women32. Women who are carriers for the APOE ε4 genetic variant are at particular risk for β-amyloid deposition in the brain. Among a subset of women randomized to tE2 who were positive for the APOE ε4, accumulation of β-amyloid in the brain was significantly lower compared to women randomized to oCEE or placebo33. Improvements in sleep were also associated with less accumulation of β-amyloid, lower volumes of WMH, and thicker parietal cortex, but only in women randomized to tE234.
Peripheral physical factors that influence cerebral blood flow and vascular activation affect development of WMH. In the subset of KEEPS participants in whom it was measured, modest increases in aortic blood pressure, even within the normal range (which may presage increases in brachial pressure) were associated with increases in WMH35,36 as were the quantity of monocyte- and endothelium-derived microvesicles37. Despite the fact that MHT appeared to affect both sets of parameters, the correlations between aortic pressure and activated microvesicles with WMH were independent of study treatments36,38. Recall that baseline systolic blood pressure was inversely associated with both auditory attention and working memory in KEEPS participants prior to randomization. Further assessment of peripheral blood biomarkers, structural changes in the brain, and cognitive function is being performed in KEEPS participants 7 years after cessation of treatment in the KEEPS-Continuation study, which is being conducted through 2023.
Additional effects: lipid metabolism/fat deposition, bone, and sexual function
Lipid metabolism/fat deposition
The concept that the protective cardiovascular effects of MHT are mediated through alterations in lipid metabolism was proposed in the mid 1980s39,40. As expected in KEEPS, low density lipoprotein (LDL) decreased and high density lipoproteins (HDL) increased compared to placebo in the oCEE group7. The reasons for the difference in effect of the two treatments on lipoprotein is thought to reflect the greater effect of the oral agent, oCEE, on the liver, although it might also reflect perhaps a synergistic effect of E1, or other compounds in CEE compared to E2 on hepatic lipid metabolism. Other studies showing effects of estradiol on lipids used an oral preparation which would undergo first-pass metabolism in the liver due to absorption into the portal circulation, and women enrolled in those studies included some with dyslipidemia11,41. Importantly, in KEEPS both treatments demonstrated trends toward reducing insulin resistance7,8.
Epicardial and pericardial fat may influence development of cardiovascular disease. In KEEPS, increases in epicardial adipose tissue were less with oCEE than with tE242,43, but changes in epicardial adipose tissue did not correlate with changes in either CIMT or CAC in either treatment group. On the contrary, increases in pericardial adipose tissue were similar between the hormone treatment groups. However, the change in CIMT per change in pericardial adipose tissue was less in the oCEE than in the tE2 group42, whereas increases in CAC showed a positive correlation increase in the pericardial adipose tissue only in the tE2 group43. Underlying mechanisms of factors contributing to these differences in cardiac adipose tissue depositions and vascular remodeling in the carotid compared to coronary arteries require clarification.
Bone
Previous studies provided evidence that MHT maintained bone mineral density, and MHT had been recommended for prevention and treatment of osteoporosis44. Consistent with previous studies, and as expected, both MHT formulations similarly maintained bone mineral density compared to placebo at the wrist, hip and spine in a subset of participants at one site who had undergone dual-energy X-ray absorptiometry (DXA) throughout the study45,46.
Sexual function
Complaints associated with decreased sexual function are common among postmenopausal women. Assessing the effect of MHT on sexual function is complicated as MHT may impact related physiological as well as psychological processes. Using the validated Female Sexual Function Inventory (FSFI)47, which includes psychological components of desire, arousal, and satisfaction, and physiological responses of lubrication, pain, and orgasm, tE2, but not oCEE, was associated with improvement in the overall FSFI score. As for particular domains of sexual function, both tE2 and oCEE increased satisfaction, lubrication and reduced pain compared to placebo. However, tE2 improved libido (desire and arousal) more than did oCEE, an effect that was significant especially in women who were considered to have low sexual function prior to randomization48. Moreover, given that oCEE, but not tE2, was associated with improvements in mood, it is likely that the improvements in sexual function occurred independent of mood alterations. The improvement in lubrication and associated decrease in pain with both treatments may reflect the distribution of estrogen receptors in the vagina49–51. These data may help clinicians choose among MHT options as they may improve specific aspects of sexual dysfunction after the menopause.
Summary and perspectives
The KEEPS participants are a well-characterized cohort of recently menopausal women at relatively low risk for CVD in whom the effects of two types of MHT have been investigated on multiple systems (Figure 1). No major adverse cardiovascular or cognitive events were observed, nor were there differences in incidence of breast cancers among groups7. This lack of evidence of adverse effects is encouraging, but not definitive due to: (1) the short duration of the trial, (2) the lack of baseline clinical or even sub-clinical vascular disease at the time of randomization, and (3) the small number of participants relative to other studies. KEEPS did not show significantly less progression of atherosclerosis as measured by CIMT or CAC with MHT compared to placebo, despite improvement in some metabolic risk factors. Studies of larger participant groups over longer duration have shown that oral estradiol can slow progression of atherosclerosis when started soon after the menopause11,41. KEEPS differed from these studies in that KEEPS participants were younger (within 3 years of natural menopause), and were not dyslipidemic or using a statin, and the treatments differed by type and dose11,41.
Figure 1.
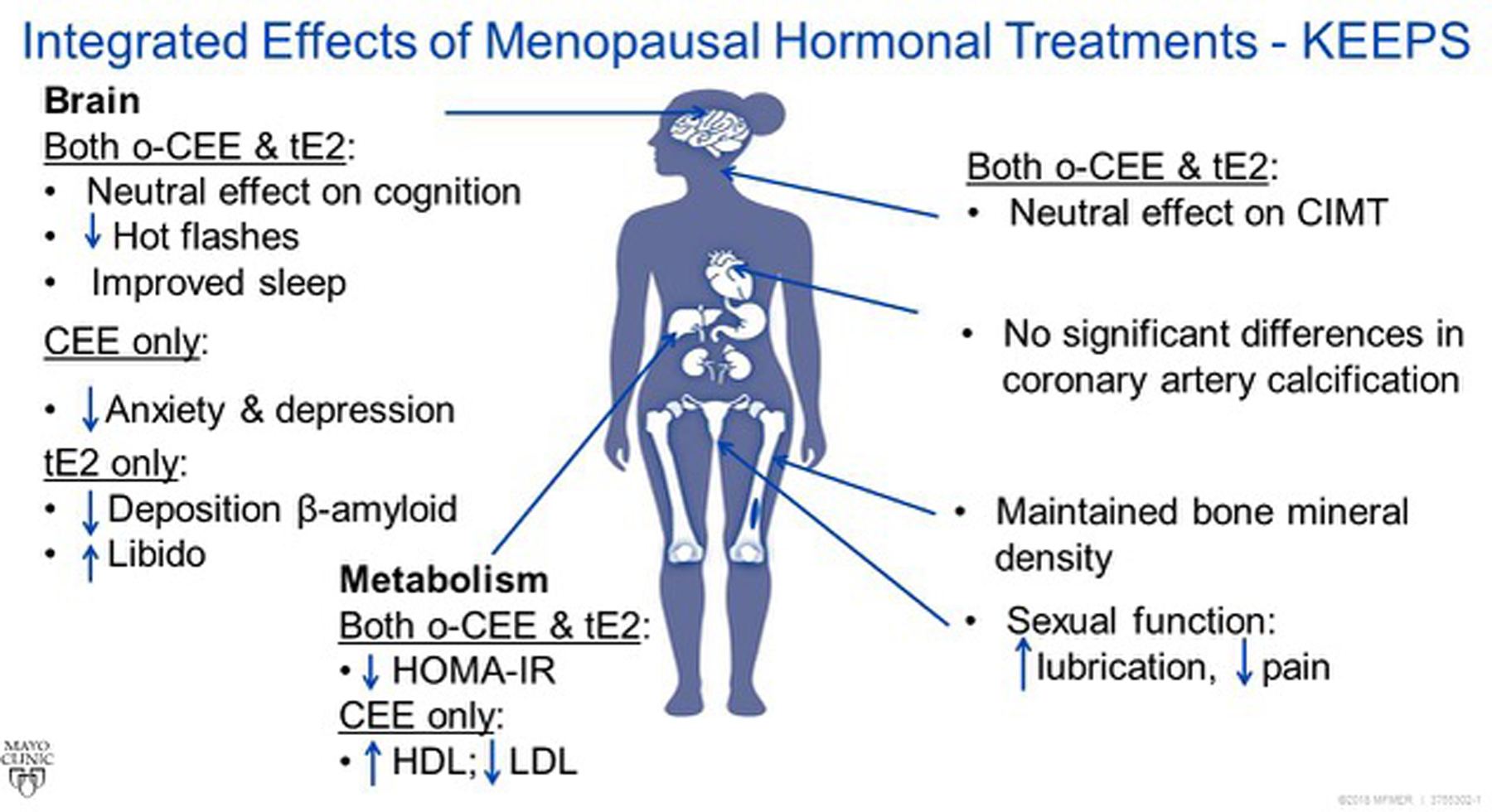
Schematic summary of effects of KEEPS hormonal treatments in recently menopausal women. CIMT, carotid intima-medial thickness; HDL, high density lipoprotein cholesterol; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; LDL, low density lipoprotein cholesterol; oCEE oral conjugated equine estrogens; tE2, transdermal 17β-estradiol.
In general, KEEPS data provide reassurance regarding the efficacy and safety of these specific doses of oCEE (0.45 mg/day), or tE2 (50 μg/day) both with oral progesterone (200 mg/day for 12 days/month) for women who may be considering use of MHT to reduce postmenopausal symptoms. As with any randomized clinical trial, the results may not be generalizable to patients outside the study population such as older women or those at higher cardiovascular risk. KEEPS results do, however, appear to corroborate accumulating data suggesting generally favorable outcomes with MHT, and, in particular, with these two formulations and doses of MHT used in clinical practice, when initiated within 3 years of menopause. These favorable outcomes include: decreased general postmenopausal symptoms, improved bone health and sexual function. Vaginal bleeding outside of the expected time was reported for 78 participants assigned to oCEE, 92 to those assigned to tE2, and 25 to those assigned to placebo. Numbers of neoplasia/hyperplasia were low and did not differ among treatment groups [breast cancer: three with oCEE, three with tE2, two with placebo; endometrial cancer: two with oCEE, one with tE2, none with placebo; endometrial hyperplasia: two with oCEE, one with tE2, and one with placebo]7.
There are several areas requiring additional mechanistic investigations into actions of MHT. The various apparent differences between the effects of oCEE compared to tE2 on several parameters should raise caution about generalizations regarding benefit and risk of MHT. The type of treatment, dose, and mode of delivery, the outcomes of interest, and the cardiovascular risk of the woman all need to be considered when choosing among the many MHT options. In the future, it may be possible to consider genetic variations in estrogen metabolism and transport to help direct the most efficacious dose and type of hormonal formulation for individual women.
Finally, the holistic approach used to assess KEEPS participants provides a model for the design of future clinical studies and points to how new technologies and information might be implemented to tailor the type and dose of MHT to the individual needs, and health of the patient.
Acknowledgements
The authors gratefully acknowledge the dedicated efforts of all the investigators and staff at the KEEPS clinical centers, the KEEPS Data Coordinating Center at KLRI, and the NIH Institutes supporting ancillary studies. Above all, they recognize and thank the KEEPS participants for their dedication and commitment to the KEEPS research program.
Source of funding KEEPS was funded by grants from the Aurora Foundation to the Kronos Longevity Research Institute; by National Institutes of Health (NIH) HL90639, P50 AG44170 to VMM; RF1 AG057547 and R21 NS066147 to KK; by the Mayo Foundation and the Mayo CTSA UL1 RR024150; by the Harvard Medical School CTSA UL1 RR024139; by the UCSF CTSA UL1 RR024131 from the National Center for Research Resources (NCRR); and by the Veterans Health Administration of the US Department of Veterans Affairs. The manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR, NIH, or VA. Some study medications (Climara® patches and Prometrium®) were kindly donated by Bayer Health Care and by Abbott Pharmaceuticals. FN received support from Pfizer, Inc., for assistance with hormone measurement. The Aurora Foundation did not have input into the design or conduct of the study or the review or approval of any presentations or publications.
Footnotes
Potential conflict of interest Nothing to disclose.
References
- 1.Writing Group for the Women’s Health Initiative randomized controlled trial. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321–33 [DOI] [PubMed] [Google Scholar]
- 2.Harman SM, Brinton EA, Cedars M, et al. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric 2005;8:3–12 [DOI] [PubMed] [Google Scholar]
- 3.Anderson G, Cummings S, Freedman LS, et al. Design of the Women’s Health Initiative clinical trial and observational study. Controlled Clin Trials 1998;19:61–109 [DOI] [PubMed] [Google Scholar]
- 4.Tan O, Harman S, Naftolin F. What we can learn from design faults in the Women’s Health Initiative randomized clinical trial? Bull NYU Hosp Jt Dis 2009;67:226–9 [PubMed] [Google Scholar]
- 5.Miller VM, Black DM, Brinton EA, et al. Using basic science to design a clinical trial: baseline characteristics of women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS). J Cardiovasc Transl Res 2009;2:228–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langer RD. The evidence base for HRT: what can we believe? Climacteric 2017;20:91–6 [DOI] [PubMed] [Google Scholar]
- 7.Harman SM, Black DM, Naftolin F, et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: A randomized trial. Ann Intern Med 2014;161:249–60 [DOI] [PubMed] [Google Scholar]
- 8.Miller VM, Naftolin F, Asthana S, et al. The Kronos Early Estrogen Prevention Study (KEEPS): what have we learned? Menopause 2019;26:1071–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller VM, Petterson TM, Jeavons EN, et al. Genetic polymorphisms associated carotid artery intima-media thickness and coronary artery calcification in women of the Kronos Early Estrogen Prevention Study. Physiol Genomics 2013;45:79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moyer AM, de Andrade M, Weinshilboum RM, Miller VM. Influence of SULT1A1 genetic variation on age at menopause, estrogen levels, and response to hormone therapy in recently postmenopausal white women. Menopause 2016;23:863–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodis HN, Mack WJ, Henderson VW, et al. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med 2016;374:1221–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller VM, Jenkins GD, Biernacka JM, et al. Pharmacogenomics of estrogens on changes in carotid artery intima-medial thickness and coronary arterial calcification: Kronos Early Estrogen Prevention Study. Physiol Genomics 2016;48:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mason JC, Libby P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J 2015;36:482–9c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011;473:317–25 [DOI] [PubMed] [Google Scholar]
- 15.Naftolin F, Mehr H, Fadiel A. Sex steroids block the initiation of atherosclerosis. Reprod Sci 2016;23:1620–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller VM, Lahr BD, Bailey KR, Hodis HN, Mulvagh SL, Jayachandran M. Specific cell-derived microvesicles: linking endothelial function to carotid artery intima-media thickness in low cardiovascular risk menopausal women. Atherosclerosis 2016;246:21–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jayachandran M, Garovic VD, Mielke MM, Bailey KR, Lahr BD, Miller VM. Characterization of intravascular cellular activation in relationship to subclinical atherosclerosis in postmenopausal women. PLoS One 2017;12:e0183159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raz L, Hunter LW, Jayachandran M, Heit JA, Miller VM. Differential effects of oral and transdermal menopausal hormone therapy on prostacyclin and thromboxane in platelets. Physiol Rep 2014;2:e00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease. Arch Intern Med 2006;166:357–65 [DOI] [PubMed] [Google Scholar]
- 20.Manson J, Allison M, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med 2007;356:2591–602 [DOI] [PubMed] [Google Scholar]
- 21.Harman SM, Vittinghoff E, Brinton EA, et al. Timing and duration of menopausal hormone treatment may affect cardiovascular outcomes. Am J Med 2011;124:199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wharton W, Gleason CE, Miller VM, Asthana S. Rationale and design of the Kronos Early Estrogen Prevention Study (KEEPS) and the KEEPS cognitive and affective sub study (KEEPS Cog). Brain Res 2013;1514:12–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papademetriou V. Hypertension and cognitive function. Blood pressure regulation and cognitive function: a review of the literature. Geriatrics 2005;60:20–2, 4 [PubMed] [Google Scholar]
- 24.Wharton W, Gleason CE, Dowling NM, et al. The KEEPS-Cognitive and Affective Study: baseline associations between vascular risk factors and cognition. J Alzheimers Dis 2014;40:331–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med 2015;12:e1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santoro N, Allshouse A, Neal-Perry G, et al. Longitudinal changes in menopausal symptoms comparing women randomized to low-dose oral conjugated estrogens or transdermal estradiol plus micronized progesterone versus placebo: the Kronos Early Estrogen Prevention Study. Menopause 2017;24:238–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cintron D, Lahr BD, Bailey KR, et al. Effects of oral versus transdermal menopausal hormone treatments on self-reported sleep domains and their association with vasomotor symptoms in recently menopausal women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS). Menopause 2018;25:145–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raz L, Hunter LV, Dowling NM, et al. Differential effects of hormone therapy on serotonin, vascular function and mood in the KEEPS. Climacteric 2016;19:49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moyer AM, de Andrade M, Faubion SS, et al. SLCO1B1 genetic variation and hormone therapy in menopausal women. Menopause 2018;25:877–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kling JM, Dowling NM, Bimonte-Nelson HA, et al. Impact of menopausal hormone formulations on pituitary-ovarian regulatory feedback. Am J Physiol Regul Integr Comp Physiol 2019;317:R912–R20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lizneva D, Rahimova A, Kim SM, et al. FSH beyond fertility. Front Endocrinol (Lausanne) 2019;10:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naftolin F, Silva I, Orley A. Reproductive hormones and dementia. In: Schenker JG, Sciarra JJ, Mettler L, Genazzani AR, Birkhauser M, eds. Reproductive Medicine for Clinical Practice: Springer, Cham Switzerland; 2018:191–201 [Google Scholar]
- 33.Kantarci K, Lowe VJ, Lesnick TG, et al. Early postmenopausal transdermal 17beta-estradiol therapy and amyloid-beta deposition. J Alzheimers Dis 2016;53:547–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeydan B, Lowe VJ, Tosakulwong N, et al. Sleep quality and beta-amyloid deposition in recently menopausal women participating in the Kronos Early estrogen Prevention Study (KEEPS). Alzheimer’s & Dementia 2019;15:551 [Google Scholar]
- 35.Barnes JN, Harvey RE, Zuk SM, et al. Aortic hemodynamics and white matter hyperintensities in normotensive postmenopausal women. J Neurol 2017;264:938–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harvey RE, Johnson MC, Ranadive SM, et al. Aortic hemodynamics in postmenopausal women following cessation of hormone therapy. Physiol Rep 2017;5:e13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raz L, Jayachandran M, Tosakulwong N, et al. Thrombogenic microvesicles and white matter hyperintensities in postmenopausal women. Neurology 2013;80:911–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jayachandran M, Lahr BD, Bailey KR, Miller VM, Kantarci K. Menopausal hormone therapy, blood thrombogenicity, and development of white matter hyperintensities in women of the Kronos Early Estrogen Prevention Study. Menopause 2020;27:305–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bush TL, Barrett-Connor E. Noncontraceptive estrogen use and cardiovascular disease. Epidemiol Rev 1985;7:89–104 [PubMed] [Google Scholar]
- 40.Bush TL, Cowan LD, Barrett-Connor E, et al. Estrogen use and all-cause mortality. Preliminary results from the Lipid Research Clinics Program Follow-Up Study. JAMA 1983;249:903–6 [DOI] [PubMed] [Google Scholar]
- 41.Hodis H, Mack W, Lobo R, et al. Estrogen in the prevention of atherosclerosis. a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2001;135:939–53 [DOI] [PubMed] [Google Scholar]
- 42.El Khoudary SR, Zhao Q, Manson J, et al. Effects of hormone therapy on heart fat and atherosclerosis progression in recently postmenopausal women from the KEEPS Trial. Menopause 2018;25:1484 (abstract S1) [Google Scholar]
- 43.El Khoudary SR, Zhao Q, Venugopal V, et al. Effects of hormone therapy on heart fat and coronary artery calcification progression: secondary analysis From the KEEPS Trial. J Am Heart Assoc 2019;8:e012763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bush TL, Wells HB, James MK, et al. Effects of hormone therapy on bone mineral density - Results from the postmenopausal estrogen/progestin interventions (PEPI) trial. JAMA 1996;276:1389–96 [PubMed] [Google Scholar]
- 45.Farr JN, Khosla S, Miyabara Y, Miller VM, Kearns AE. Effects of estrogen with micronized progesterone on cortical and trabecular bone mass and microstructure in recently postmenopausal women. J Clin Endocrinol Metab 2013;98:E249–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyabara Y, Camp J, Holmes D, et al. Coronary arterial calcification and thoracic spine mineral density in early menopause. Climacteric 2011;14:438–44 [DOI] [PubMed] [Google Scholar]
- 47.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 2000;26:191–208 [DOI] [PubMed] [Google Scholar]
- 48.Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early postmenopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med 2017;177:1471–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen GD, Oliver RH, Leung BS, Lin LY, Yeh J. Estrogen receptor alpha and beta expression in the vaginal walls and uterosacral ligaments of premenopausal and postmenopausal women. Fertil Steril 1999;71:1099–102 [DOI] [PubMed] [Google Scholar]
- 50.Fuermetz A, Schoenfeld M, Ennemoser S, Muetzel E, Jeschke U, Jundt K. Change of steroid receptor expression in the posterior vaginal wall after local estrogen therapy. Eur J Obstet Gynecol Reprod Biol 2015;187:45–50 [DOI] [PubMed] [Google Scholar]
- 51.Xie Z, Shi H, Zhou C, Dong M, Hong L, Jin H. Alterations of estrogen receptor-alpha and -beta in the anterior vaginal wall of women with urinary incontinence. Eur J Obstet Gynecol Reprod Biol 2007;134:254–8 [DOI] [PubMed] [Google Scholar]


