Abstract
Coronavirus disease 2019 (CoVID-19) caused by Severe Acute Respiratory Syndrome Coronavirus 2 has affected more than 100 million lives. Severe CoVID-19 infection may lead to acute respiratory distress syndrome and death of the patient, and is associated with hyperinflammation and cytokine storm. The broad spectrum immunosuppressant corticosteroid, dexamethasone, is being used to manage the cytokine storm and hyperinflammation in CoVID-19 patients. However, the extensive use of corticosteroids leads to serious adverse events and disruption of the gut-lung axis. Various micronutrients and probiotic supplementations are known to aid in the reduction of hyperinflammation and restoration of gut microbiota. The attenuation of the deleterious immune response and hyperinflammation could be mediated by short chain fatty acids produced by the gut microbiota. Butyric acid, the most extensively studied short chain fatty acid, is known for its anti-inflammatory properties. Additionally, butyric acid has been shown to ameliorate hyperinflammation and reduce oxidative stress in various pathologies, including respiratory viral infections. In this review, the potential anti-inflammatory effects of butyric acid that aid in cytokine storm depletion, and its usefulness in effective management of critical illness related to CoVID-19 have been discussed.
Keywords: Butyrate, Butyric acid, CoVID-19, Cytokine storm, Dexamethasone, Gut microbiota, Hyperinflammation, Probiotics
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative factor for the Coronavirus disease 2019 (CoVID-19) 1. SARS-CoV-2 has affected nearly 100 million people around the globe 2. SARS-CoV-2 enters the host by binding to its receptor, angiotensin converting enzyme 2 (ACE2), which is expressed mainly in the lungs and intestine 3– 5. Upon infection, SARS-CoV-2 causes mild to severe inflammation, which disrupts homeostasis and the integrity of infected organs 6, 7. Furthermore, severe infection of CoVID-19 results in systemic inflammation, thrombosis, acute respiratory distress syndrome (ARDS) and multiple organ failure, which may lead to death 8– 10. The corticosteroid immunosuppressant, dexamethasone, which attenuates hyperinflammation and cytokine storm, is being used to treat seriously ill CoVID-19 patients and has been found to improve survival in hospitalised patients 11, 12. However, the prolonged usage of dexamethasone causes serious adverse effects and gut dysbiosis 13, 14. Besides, the hyperinflammation and thrombotic complications associated with CoVID-19 can also be alleviated by various nutrients including vitamins, polyunsaturated fatty acids, minerals, and even amino acids 15– 20. The growing number of studies indicate the potential role of nutritional supplement, probiotics, and gut microbiome in mitigating the inflammation and in preventing viral infections including respiratory viral infections 21. Alterations of the gut microbiome has been observed during SARS-CoV-2 infection, which significantly reduces the abundance of beneficial microbiome and its metabolites, such as short chain fatty acids (SCFAs) including butyric acid 22– 24.
Butyric acid or butyrate can act primarily as an anti-inflammatory molecule and various studies have reported its role in mitigating hyperinflammation via several mechanisms 25– 27. For the past several years, our group has worked on the role of proinflammatory regulators in the pathogenesis of various inflammatory disorders and identified that the role of histone deacetylase (HDAC) inhibitor in activating anti-inflammatory molecules. Further, this leads to the simultaneous down regulation of proinflammatory membrane receptors, downstream signalling molecules and respective cytokines, resulting in inflammatory homeostasis. Our in vitro preliminary experiments using various cell lines have revealed that the molecular mechanism of butyrate in neutralising inflammatory devastation, induction of anti-inflammatory molecular expression and its translocation to the site of action, is almost similar to dexamethasone 28. Consequently, we hypothesise if the SCFA, butyric acid, a HDAC inhibitor, which is synthesized by the gut microbiota, could have strong anti-inflammatory functions with anti-fibrotic properties. Therefore, this article reviews the anti-inflammatory properties of butyric acid or butyrate and its associated molecular pathways involved in controlling the cytokine storm and hyperinflammation associated with SARS-CoV-2 infection. Based on the various positive reports, we presume that butyric acid possesses potent anti-inflammatory activity, which suggests it as an alternative to dexamethasone for the preventive management of primary and secondary complications related to CoVID-19.
Coronavirus disease 2019
The CoVID-19 pandemic is caused by SARS-Cov-2, which belongs to genera β-coronaviruses and is the seventh known coronavirus to infect humans 4. Spike protein of SARS-CoV-2 binds to ACE2, a type I membrane protein 29 expressed in the lung, heart, kidney, and intestine 3, 30, 31. The majority of SARS-CoV-2 infected cases present with mild symptoms like dry cough, sore throat, fatigue and fever 9, 10, 32. Less common symptoms such as myalgia, expectoration, pharyngalgia, dizziness, nausea, headache, haemoptysis, diarrhoea, abdominal pain, and vomiting have also been reported. Lymphocytopenia along with elevated expression of C-reactive proteins (CRP) and inflammatory cytokines are also common 9, 10, 32, 33. Infection can progress into severe disease with dyspnoea, grinding glass-like abnormalities and patchy consolidation areas in lungs observed upon imaging; viral pneumonia usually appears after 2–3 weeks of infection 9, 10, 32, 33. However, some patients have developed organ failure, septic shock, myocarditis, acute cardiac injury, arrhythmia, pulmonary oedema, severe pneumonia, acute kidney injury, and ARDS. Inflammation, oxidative stress, and fibrosis associated with CoVID-19 is perhaps partially mediated by angiotensin II (AngII), a substrate for ACE2, which degrades it to anti-inflammatory angiotensin (1–7). The accumulation of AngII results in hyperinflammation induced by nucleotide-binding oligomerization domain (NOD) like receptors family pyrin domain-containing 3 (NLRP3) inflammasome and nuclear factor kappa B (NF-κB) activation 34. Disrupted immune response in CoVID-19 is further characterized by decreased expression of human leukocyte antigen D related (HLA-DR) on CD(cluster of differentiation)14 monocytes, accompanied by decrease in number of CD4 and CD19 lymphocytes, and natural killer cells along with the continuous production of proinflammatory tumour necrosis factor (TNF)-α and interleukin (IL)-6 secreted by circulating monocytes, subsequently leading to cytokine storm and hyperinflammation 7. Hypercytokinemia and hyperinflammation associated with CoVID-19 results in acute lung injury, ARDS and death of the patients 8, 35, 36. Furthermore, the SARS-CoV-2 infection may lead to liver injury and its dysfunction, and dysbiosis in the gut, where high expression of ACE2 is observed. Myocardial damage by interaction of SARS-CoV-2 with ACE2 expressed in cardiac pericytes has also been observed 37. In addition, the high complication of disseminated intravascular coagulation is known to be associated with the severe form of CoVID-19 38.
SARS-CoV2 infection significantly alters gut microbiota, increasing the number of opportunistic pathogens such as Clostridium hathewayi, Actinomyces viscosus, Bacteroides nordii, Streptococcus, Rothia, Erysipelatoclostridium and Veillonella along with significant reduction in beneficial bacteria such as Lachnospiraceae bacterium 5_1_63FAA, Eubacterium rectale, Ruminococcus obeum, Fusicatenibacter, Eubacterium hallii, Anaerostipes, Agathobacter, Roseburia, Dorea formicigenerans, Clostridium butyricum, Clostridium leptum and Faecalibacterium prausnitzii, which includes butyric acid producing bacteria (BPB). Abundance of BPB is negatively correlated with inflammatory and thrombosis markers including CRP, Procalcitonin and D-dimer . However, the plethora of opportunistic Coprobacillus species, Clostridium ramosum and C. hathewayi are positively associated with the CoVID-19 severity, but beneficial species Alistipes onderdonkii and Faecalibacterium prausnitzii show negative correlation. In addition, the probiotic bacteria, Lactobacillus and Bifidobacterium are also decreased in CoVID-19 patients 22, 24, 39. The resulting gut dysbiosis may lead to aberrant inflammation and increased severity of CoVID-19 due to the disruption in the gut-lung axis 34, 39– 41. The reduction in BPB may impact lung inflammation and subsequent injury associated with CoVID-19 42, 43.
Nutrients in mitigating the Covid-19 pathogenesis
Nutrition and nutrients play a vital role in enhancing immune response along with reduction of inflammation and oxidative stress 44– 46. Better nutritional status of CoVID-19 patients is associated with less adverse outcomes 18, 47– 52. Vitamin D is involved in reducing respiratory infections, such as influenza, and a reduced plasma 25-hydroxyvitamin D (25(OH)D) concentration in SARS-CoV-2 patients has been observed 53. Moreover, people with vitamin D deficiency are at higher risk of getting infected with SARS-CoV-2 54, 55. Co-supplementation of vitamin D along with glutathione precursor L-cysteine significantly increases serum 25(OH)D levels and augments vitamin D regulatory gene expression, which in turn reduces the oxidative stress and inflammatory responses in CoVID-19 patients 56. Vitamin D supplementation in SARS-CoV-2 infected patients attenuates the production of proinflammatory cytokines like Interferon (IFN)-γ, IL-6, IL-2 and TNF-α by inhibiting NF-κB and other pathways 57– 59. CoVID-19 associated inflammatory signalling pathways including NF-κB, Mitogen-Activated Protein Kinase (MAPK) and phosphatidylinositol 3-kinase/ protein kinase B (PI3K/AKT) and innate immune response pathways, such as Toll-like signalling and NOD-like signalling modulation and regulation can be mediated by the combination of curcumin, vitamin C, and glycyrrhizic acid 60. Vitamin C has been known to improve the immune condition by enhancing differentiation and proliferation of B- and T-cells, but severe vitamin C deficiency is associated with pneumonia and respiratory tract infections 61. Intravenous administration of vitamin C can significantly decrease IL-6 levels 62, 63. Glycyrrhizic acid and curcumin exhibits anti-viral, anti-inflammation, anti-cancer, and immune system benefits 60. The combination of vitamin D/magnesium/vitamin B12 significantly reduced the subsequent need for oxygen therapy and/or intensive care support in older CoVID-19 patients 57. Vitamin B12 is crucial in maintaining the healthy gut microbiome which plays a vital role in immune responses 57. Fat soluble vitamin E acts as an antioxidant that scavenges Reactive Oxygen species (ROS) and inhibits devastating effects of hyperinflammation 64. Moreover, the supplementation of vitamin E stimulates T cell function and confers protection against upper respiratory infections 65.
Selenium is one of the key micronutrients known to positively impact CoVID-19 patient recovery 66, 67. Selenium status regulates the expression of glutathione peroxidase 1 (GPX1), a cytosolic selenoenzyme known for its antioxidative properties. The antioxidant enzyme GPX1 mitigates the production of ROS and further leading to mutations in the viral genome 68. In addition, attenuating ROS also helps in the inhibition of proinflammatory NF-κB activation and further nuclear translocation 69. Severe endothelial injury and widespread pulmonary micro thromboses are accompanied with platelet activation and aggregation in patients with severe CoVID-19 manifestations. The synthetic Rupatadine (histamine1 receptor antagonist) and natural flavonoids with anti-inflammatory properties are known to inhibit the platelet activating factor 70. Elderly individuals with deficiency of nutrients, such as vitamin C, vitamin D, calcium, folate, and zinc are prone to increase severity of SARS-CoV-2 infection 71. Folic acid may inhibit furin protease and inactivates chymotrypsin-like protease (3CL pro) 72. Zinc (Zn 2+) deficiency contributes to impaired cell mediated immune response and increased susceptibility to various infections. However, increased intracellular levels of Zn 2+ disrupt viral RNA replication including SARS-CoV-2, where Zn 2+ inhibits RNA (Ribonucleic acid) dependent RNA polymerase (RdRp) elongation and template binding 73. Among CoVID-19 patients, iron deficiency is strongly associated with increased inflammation and longer stay in hospitals 74.
Health beneficial compounds, including minerals, antioxidants, phytochemicals, vitamins, and minerals present in fruits and vegetables, can exert antioxidative, anti-inflammatory and antiviral effects during various non-infectious and infectious disease 71. Alliin, an S-allyl cysteine sulfoxide compound present in garlic has shown to have inhibitory action on 3CL pro, a protease that plays a vital role in SARS-CoV-2 replication 75. Salvianolic acid A and curcumin have the potential to bind to 3CL pro with greater affinity 76. Resveratrol acts as an anti-inflammatory molecule that inhibits the NFκB pathway and thereby reduces circulatory cytokines, such as IL-6 and TNF-α levels, which are observed in severe SARS-CoV-2 infection 77. Sea cucumber ( Stichopus japonicus) derived sulphated polysaccharide showed significant anti-viral activity against SARS-CoV-2 infection 78. Omega-3 polyunsaturated fatty acids, including eicosapentaenoic acid and docosahexaenoic acid have been shown to exhibit anti-inflammatory effects by downregulation of the NF-κB pathway 71, 79, 80. Free fatty acids such as oleic acid, arachidonic acid and linoleic acid have shown antiviral activity at micromolar concentrations 81. Dietary fibre intake alters the intestinal microflora and enhances relative proportion of SCFAs, which exhibit anti-inflammatory properties through fatty acid receptors like G-protein-coupled receptor (GPCR) 41 and 43 82– 84.
Probiotics: suppressors of respiratory tract infections and inflammation
Probiotics are living microorganisms that provide health benefits to the host upon administration at appropriate doses 85. Probiotics exert a wide range of beneficial effects such as host microbiome balancing, stimulation of immune system, enhancement of intestinal barrier function or inhibiting pathogens by direct interactions 40, 46, 86, 87 ( Table 1). Several microorganisms belonging to the family of Enterococcus species ( E. fecalis, E. faecium), Bifidobacterium species ( B. bifidum, B. longum, B. lactis), Lactobacillus species ( L. acidophilus, L. casei, L. rhamnosus), and Saccharomyces ( S. boulardii, S. cerevisiae) are considered as probiotics 40. Probiotic supplementation causes significant reduction in the incidence of oral and respiratory tract infections 88, 89. Dietary supplementation of cow’s milk and fermented rice with L. paracasei CBA L74 helps in prevention of common infectious disease including upper respiratory tract infections in children 90. Daily intake of fermented milk containing probiotic L. casei strain ‘Shirota’ has been shown to reduce the incidence and duration of respiratory tract infections in healthy middle aged office workers and young children via modulation of the immune system 91, 92. Daily ingestion of the probiotic L. paracasei ST11 can reduce the degree of virus replication and dissemination thereby attenuating lung inflammation and subsequent death in mice infected with vaccinia virus 95. L. gasseri SBT2055 exhibits antiviral activity against human respiratory syncytial virus (RSV) by silencing SWI2/SNF2-related cAMP Response Element-Binding Protein (CREB)-binding protein activator protein, which is involved in RSV replication. L. gasseri SBT2055 reduced the expression of proinflammatory cytokines in lungs upon RSV infection 96. CC chemokine receptor 2 acts as a receptor for monocyte chemoattractant protein-1 (MCP-1), which induces increased lung inflammation and subsequently decreases survival associated with influenza virus infection. Prophylactic oral administration of heat-killed E. faecalis can protect mice from influenza virus infection and subsequent lung inflammation by modulation of MCP-1 production. Alternatively, lipoteichoic acid of E. faecalis binds to toll like receptor 2 and exerts antiviral and anti-inflammatory activity during influenza infection 97. Oral administration of probiotics L. paracasei, L. gasseri, and B. longum improved immune response and reduced mortality in influenza infected mice 105 by reducing the inflammation and oxidative stress associated with it 106, 107.
Probiotics, in combination with enteral nutrition, given to post-operative gastric cancer patients aids in increased production of antibodies and reduction of inflammatory cytokines 108. Oral administration of L. plantarum ameliorates intestinal inflammation and lipid metabolism disorders by modulating gut microbiota in turn producing more SCFAs in high-fat diet induced obese mice 100. This disrupted enterohepatic immunoregulation, which can be ameliorated by intervention of Clostridium butyricum B1 via its metabolite butyric acid 99. Probiotic mixture of Lactobacillus and Bifidobacterium prevents the non-alcoholic fatty liver disease by suppressing systemic adiposity and inflammation through butyric acid and its receptor GPR109A 98. Treatment with probiotic strain L. acidophilus DDS-1 upsurges the abundance of beneficial bacteria such as Lactobacillus spp and Akkermansia spp and also the levels of butyrate, while downregulating the production of inflammatory cytokines IL-6, IL-1β, IL-1α, MCP-1, Macrophage Inflammatory Protein (MIP)-1α, MIP-1β, IL-12 and IFN-γ in aging mice 101. L. paracasei KW3110 suppresses hyperinflammation via activation of M2 macrophages and exhibit anti-inflammatory effects via suppression of IL-β production and caspase 1 activation by promoting IL-10 production 103. Probiotic complex of L. acidophilus, L. casei, L. fermentum, L. paracasei, Streptococcus thermophilus, Bifidobacterium longum, B. bifidum, B. breve, L. rhamnosus, L. plantarum, L. helveticus, and L. salivarius in combination with zinc and coenzyme Q10 can improve autoimmune arthritis via downregulation of proinflammatory cytokines including IL-6, IL-17 and TNF-α and inhibition of T-helper cell 17 (Th17) cell differentiation 109– 111. Oral administration of B. infantis suppresses allergic inflammation in lungs by significantly reducing serum levels of Immunoglobulin (Ig)E, IgG1, IL-4 and IL-13 102 . Daily administration of L. plantarum DR7 for 12 weeks can prevent development of upper respiratory tract infections among young adults through various mechanisms including inhibition of respiratory infection causing bacteria such as Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes and Streptococcus mutans, stimulation of proinflammatory cytokine production such as IL-10 and IL-4, and enhancement of antioxidant potential of RBC membrane 93. Significant reduction in the number of Bifidobacteria and Lactobacilli along with increased number of Escherichia coli is observed in the gut of children with recurrent respiratory tract infections. Oral probiotic supplement containing Bifidobacterium infantis, L. acidophilus, E. faecalis and Bacillus cereus restored the intestinal flora along with reduction in incidence of respiratory tract infections and use of antibiotics 23.
Table 1. Ameliorative role of probiotics in suppressing respiratory tract infections and hyperinflammation.
| Organism | Dose and Duration | Type of study | Outcome | Reference | |
|---|---|---|---|---|---|
|
S. salivarius K12,
S. salivarius M18, L. reuteri, L. sakei, and L. paracasei |
First month: 3 tablets/day,
Next two months: one tablet/day |
double-blind,
randomized, placebo- controlled trial |
↓ RTIs in paediatric population | 88 | |
| L. paracasei CBA L74 | For 3 Months: 5.9 × 10
11
CFU/day dietary product deriving from cow's milk or rice fermentation |
double-blind,
randomized, placebo- controlled trial |
↓ incidence of URTIs in children
attending day care or preschool |
90 | |
| L. casei Shirota | For 12 weeks: 1× 10
11
CFU/day |
randomized controlled
trial |
↓ incidence of URTIs in healthy
middle aged office workers |
91 | |
| For 12 weeks: 65 mL/day
fermented milk, containing 10 8 CFU/mL |
controlled open trial | ↓ acute RTIs in young
Vietnamese children |
92 | ||
| L. plantarum DR7 | For 12 weeks: 1 × 10
9
CFU/day |
randomized, double-
blind, placebo-controlled study |
↓ duration and frequency URTIs
↓ TNF-α and IFN-γ ↓oxidative stress ↑ IL-10 and IL-14 |
93 | |
| L. gasseri A5 | For 4 weeks: 1 × 10
7
CFU/day |
In vivo (Female BALB/c
and C57BL/6 mice) |
↓mite induced allergic
inflammation |
94 | |
| L. paracasei ST11 | For 9 days: 10 8 CFU/day | In vivo study (mice) | ↓vaccinia virus replication,
dissemination and infection associated lung inflammation |
95 | |
|
Lactobacillus gasseri
SBT2055 |
For 24h: 50 μg/ml |
In vitro (HEp-2 human
laryngeal epithelial cells and MLE12 mouse lung epithelial cells) |
↓ RSV replication and associated
lung inflammation |
96 | |
| For 21 days: 2 × 10
9
CFU/day |
In vivo (mice) | ||||
| E. faecalis (heat killed) | For 12 days: 8.5 × 10
10
CFU/kg/ day |
pre-treatment,
in vivo
(CCR2-deficient and C57BL/6 mice) |
↓ monocyte chemoattractant
protein-1 in influenza infection |
97 | |
| Probiotic mixture
containing 6 Lactobacillus and 3 Bifidobacterium |
For 16 weeks: 0.6 g/kg/day
(6 billion CFU/g) |
In vivo (male SD rats, 6
weeks old) |
↓systemic adiposity and
inflammation |
98 | |
| C. butyricum B1 | For 8 weeks: 1×10
9 cells/
day |
In vivo (male C57BL/6
mice) |
↓ Non-alcoholic steatohepatitis
and inflammation. ↔ enterohepatic immunoregulation |
99 | |
| L. plantarum Y44 | For 12 weeks : 4×10
7
CFU/mL/ day or 4×10 9 CFU/mL/day |
In vivo (C57BL/6 obese
mice) |
↓intestinal inflammation
↑gut bacteria and SCFAs production |
100 | |
| L. acidophilus DDS-1 | 3 × 10 9 CFU/g |
In vivo (C57BL/6 obese
mice) |
↓proinflammatory cytokine levels
↑gut microbiota and SCFAs |
101 | |
|
B. infantis
CGMCC313-02 |
0.2 mL/day
(5 × 10 10 CFU/mL) |
In vivo (Male BALB/c
mice) |
↓ allergen induced secretion of
IgE, IgG1 and proinflammatory cytokines. |
102 | |
| L. paracasei KW3110 | 1.25–5
μg/mL |
For 24
hours |
J774A.1 cells | ↓ cytokine IL-1β via IL-10
activation and signalling |
103 |
| 100 μg/mL | human monocytes | ||||
|
S. thermophilus DSM
32345, L. acidophilus DSM 32241, L. helveticus DSM 32242, L. paracasei DSM 32243, L. plantarum DSM 32244, L. brevis DSM 27961, B. lactis DSM 32246, and B. lactis DSM 32247 |
For 21 days: 2.4×10
9/day in
3 equal doses/day |
cohort study | 8 – fold decrease in risk of
developing respiratory failure associated with CoVID-19. |
104 | |
RTIs-Respiratory tract infections; URTIs- Upper respiratory tract infections; CFU-Colony forming unit; RSV-Respiratory syncytial virus; CCR2- C-C chemokine receptor type 2; IL-Interleukin; IFN-Interferon; TNF-Tumour necrosis factor, SCFAs-short chain fatty acids; CoVID19- Coronavirus disease 2019. ↓-Reduce; ↑-Enhance; ↔- Balance.
Exopolysaccharides produced during milk fermentation by probiotic L. paracasei acts as a substrate for the gut microbiome. Fermentation of this exopolysaccharide increases the number of beneficial microbiomes belonging to phyla Firmicutes and Lentisphaerae, accompanied by the decrease in Actinobacteria, Proteobacteria and Bacteroidetes. Fermentation of exopolysaccharide enhances SCFAs production mainly butyric acid 112. Aqueous probiotic supplements containing L. acidophilus NCIMB 30175, L. plantarum NCIMB 30173, L. rhamnosus NCIMB 30174 and E. faecium NCIMB 30176 induces an increase in butyric acid producing bacteria resulting in increased production of butyric acid exhibiting immunomodulatory activity via downregulation of proinflammatory cytokines such as MCP-1, Chemokine (C-X-C motif) ligand (CXCL)-10 and IL-8 in vitro 113. Oral administration of multistrain probiotic mixture containing L. helveticus DSM 32242, B. lactis DSM 32246, L. paracasei DSM 32243, L. plantarum DSM 32244, L. brevis DSM 27961, L. acidophilus DSM 32241, Streptococcus thermophilus DSM 32345 and B. lactis DSM 32247 decreased development of respiratory failure associated with CoVID-19 by 8 times along with reduction in other symptoms such as diarrhoea, fever, asthenia, headache, myalgia, and dyspnoea 104, 114. Use of probiotics may restore the healthy gut microbiome in CoVID-19 patients and exhibit antiviral effects through gut-lung axis. The immunomodulatory role of probiotics helps in viral shedding, regulation of hypercytokinemia and associated multiple organ failure in severe CoVID-19 cases 40, 115– 117.
Is butyrate an alternative to dexamethasone?
Dexamethasone is a synthetic corticosteroid that acts as an anti-inflammatory agent, widely affecting innate and acquired immune system via glucocorticoid receptor 118, 119. Low dose dexamethasone treatment significantly supresses neutrophil infiltration and subsequent pulmonary inflammation and significantly improves lung function in early phase of ARDS 120. Lower respiratory tract transcriptomic profiling of patients with CoVID-19 associated ARDS shows dysregulated immunoregulation and inflammation. This dysregulated immune response can be modulated by dexamethasone 121. A short course of dexamethasone significantly reduces CRP levels and accelerates recovery 122. Dexamethasone treatment in CoVID-19 patients who were receiving mechanical ventilation support results in lower mortality rate 123. Severe CoVID-19 cases have been brought to remission state after 6 mg once a day intravenous administration of dexamethasone 124.
Dexamethasone is indicated as a therapeutic option for immune thrombocytopenic purpura associated with CoVID-19 124, 125. Administration of dexamethasone before 30 hours of ARDS onset can significantly reduce the period of mechanical ventilation and mortality 12. Dexamethasone provides an excellent protective effect against hypoxia associated with CoVID-19 118. Intravenous dexamethasone treatment for CoVID-19 patients along with standard care significantly decreases the number of ventilator dependent days over 28 days 11. High dose pulse therapy of dexamethasone increased the survival rate in CoVID-19 patients presented with hyperinflammation 126. However, dexamethasone, a broad spectrum immunosuppressant, inhibits lymphocytes function and prevents macrophage mediated removal of apoptotic cells, which leads to reduced viral shedding and increases subsequent viremia in mild to moderately ill CoVID-19 patients 124, 127, 128. Prolonged use of corticosteroids is associated with serious adverse effects such as short-term hyperglycaemia, cataracts, glaucoma, hypertension, psychological effects, weight gain, increased risk of secondary infections and osteoporosis 13, 129. Use of such corticosteroids may induce gut dysbiosis 14.
Intestinal microflora widely affects host health and alterations in the gut microbiome is correlated with several disease including respiratory disease 130. Commensal gut microbiome and its metabolites can modulate host immunity and can also impact on pro inflammatory and immune-regulatory response 131. Increased production of microbiome metabolite SCFAs may improve health condition 132. Depletion of SCFA production makes mice more susceptible for allergic lung inflammation. Biological effects exerted by SCFAs is dependent mainly on two mechanisms: SCFA mediated (i) activation of GPCRs and (ii) inhibition of HDAC. SCFAs, via HDAC inhibition, positively impacts the functions and numbers of T-helper 1 cells, T-regulatory cells, and Th17 effector cells resulting in reduced inflammatory response in airway diseases 130. The short chain fatty acid, butyrate or butyric acid is produced in the colon by anaerobic bacteria such as Roseburia intestinalis, Faecalibacterium prausnitzii, Clostridium butyricum, Megasphaera elsdenii, Mitsuokella multiacida, Eubacterium spp., Fusobacterium spp., Butyrivibrio spp. and Eubacterium hallii 133. Butyrate concentration in the colon can reach from 10 to 20 mM and serves as major source of energy for colonocytes. Sodium butyrate supplementation enhances the abundance of beneficial bacteria such as Coprococcus, Lachnospiraceae, Ruminococcus, Bifidobacteriaceae and Actinobacteria improving intestinal barrier integrity in obese mice 134.
Primarily, butyric acid exhibits anti-inflammatory and tissue protective function in the large intestine 135. Butyric acid is a potential inhibitor of pro-inflammatory molecule NF-κB 135– 137 ( Figure 1). Tight junction protein expression in intestinal epithelial cells is also influenced by butyrate mediated regulation 138. Butyrate treatment on epithelial colon cells significantly downregulated the proinflammatory molecules including Toll-like receptor (TLR)2, TLR4, IL-6, IL-12A, IL-1β, IL-18, TNF, MAPK13, MAPK10, MAPK3, AKT1, AKT2, AKT3, NF-κB1A, NF-κB1, CXCL1, CXCL2, CXCL3, CXCL6, CXCL8, Chemokine ligands (CCL)2, Serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 1 (SERPINA1), SERPINA2, Colony Stimulating Factor (CSF) 3, Intercellular Adhesion Molecule 1 (ICAM1), Vascular Endothelial Growth Factor A (VEGFA), Major Vault Protein (MVP), Cathelicidin Antimicrobial Peptide (CAMP) and insulin-like growth factor binding protein (IGFBP)3, along with inhibition of proinflammatory pathways, including (i) triggering receptor expressed on myeloid cells (TREM-1) signalling, (ii) production of nitric oxide (NO) and ROS, (iii) high-mobility group box-1 (HMGB1) signalling, (iv) IL-6 signalling, and (v) acute phase response signalling 25. Pre-treatment with butyric acid can attenuate heart depression along with reduction in inflammation and oxidative stress associated with septic shock in mice 139. Acute lung injury along with ARDS characterized by excessive inflammation can be induced by various factors such as endotoxins, infections, hypoxia and complement activation. Lipopolysaccharide (LPS) induced acute lung injury (ALI) and inflammation can be attenuated by 4-phenyl butyric acid (4-PBA), a derivative of butyric acid and also by sodium butyrate 26, 140.
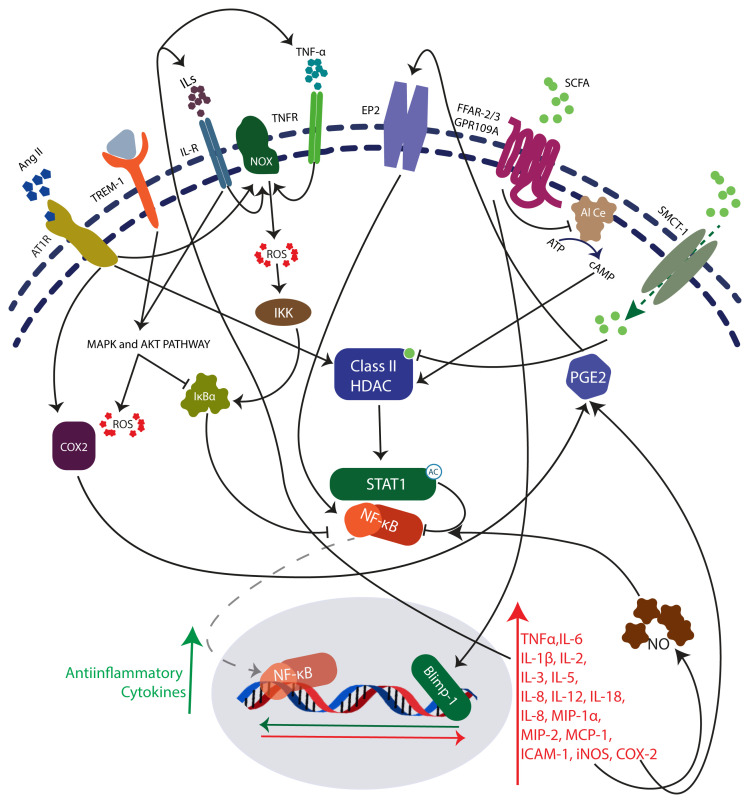
Figure 1. Proinflammatory Angiotensin II, Interleukins, Tumour necrosis factor-α and Triggering receptor expressed on myeloid cells 1 (TREM-1) mediates the activation of Mitogen-activated protein kinase (MAPK), Extracellular signal-regulated kinase (ERK1/2) and Phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) intracellular signalling pathways.
The downstream activators of these pathways induces the reactive oxygen species (ROS) generation and transcription factor, NF-κB dependent expression of proinflammatory molecules. HDACs, which deacetylates Signal transducer and activator of transcription 1 (STAT1), and promotes the nuclear translocation and subsequent activity of NF-κB. Target genes of NF-κB, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 increases the NF-κB activity via positive feedback loop. Histone deacetylase (HDAC) inhibitor, butyrate mediates its effects through GPCRs: Free fatty acid receptors 2/3 and GPCR 109A or by directly binding to HDAC active sites. Inhibition of NF-κB activity by butyrate attenuates inflammation and oxidative stress associated with various pathologies including CoVID-19. Butyrate also activates the transcription factor B lymphocyte-induced maturation protein-1 (BLIMP-1) and enhances the production of anti-inflammatory cytokines.
Prophylactic treatment of sodium butyrate significantly reduces myeloperoxidase activity and inflammatory cell infiltration into lungs which is correlated with the inhibition of proinflammatory cytokine, HMGB1 expression and NFκB 26. The TLR 4/NF-κB pathway involved in the LPS is targeted by sodium butyrate, which attenuates the LPS induced lung injury 27. Hyaluronan ester with butyric acid treatment induces apoptosis in mesangial cells after exposure to oxidative stress and thereby reducing cell proliferation via p38 MAPK pathway 141. N-(1-carbamoyl-2-phenyl-ethyl) butyramide (FBA), a butyrate releasing compound, confers protection to mice from colitis induced by dextran sodium sulphate by suppressing neutrophils recruitment and subsequent release of pro-inflammatory molecules mediated by HDAC-9/NF-κB inhibition and peroxisome proliferator-activated receptor gamma (PPAR-γ) upregulation 142. Butyrate inhibits IL-13 and IL-15 production by Type 2 innate lymphoid cells. Butyrate downregulates various RNA binding proteins and thereby post transcriptionally downregulating the expression of inflammatory genes 143. Sodium butyrate attenuates AngII induced hypertension, cardiac hypertrophy, cardiac fibrosis, and inflammation by inhibiting Cyclooxygenase-2 (COX2)/ Prostaglandin E2 (PGE2) pathway in a HDAC5/ HDAC6 dependent manner 144. Butyrate reduces AngII induced endothelial dysfunction 145. Sodium butyrate attenuates lung inflammation by promoting forkhead box P3 (FOXP3) expression and suppression of IL-9 expression. Butyrate also reduces the infiltration of proinflammatory Th9 cells and eosinophils into lungs 146. Mice treated with butyrate exhibited a significant reduction of inflammatory infiltrates in the airways, tissue, and vascular disruption, and subsequently less haemorrhaging in the lungs induced by influenza infection 82. HDAC inhibitor sodium butyrate can suppress ACE2 expression in gut epithelial cells which can help in reducing gastrointestinal symptoms associated with CoVID-19 147.
Pancreatitis and associated fibrosis induced by L-Arginine can be attenuated by sodium butyrate, which reduces collagen deposition and nitric oxide along with inhibition of profibrotic pancreatic stellate cells 148. Butyric acid ameliorates bleomycin induced pulmonary fibrosis by attenuating leukocytes infiltration, oxidative stress and NF-κB activation 149.
Consequently, based on the evidence presented, the potential anti-inflammatory and tissue protective effects of butyric acid on lungs and gut, along with its ability to modulate gut microbiome diversity, enhancing production of endogenous butyric acid could be a better preventive approach to manage CoVID-19 over dexamethasone ( Figure 2). However, there is a need for more detailed studies and clinical trials to determine the potency and long-term effect of butyric acid in the preventive management of seriously ill CoVID-19 patients.
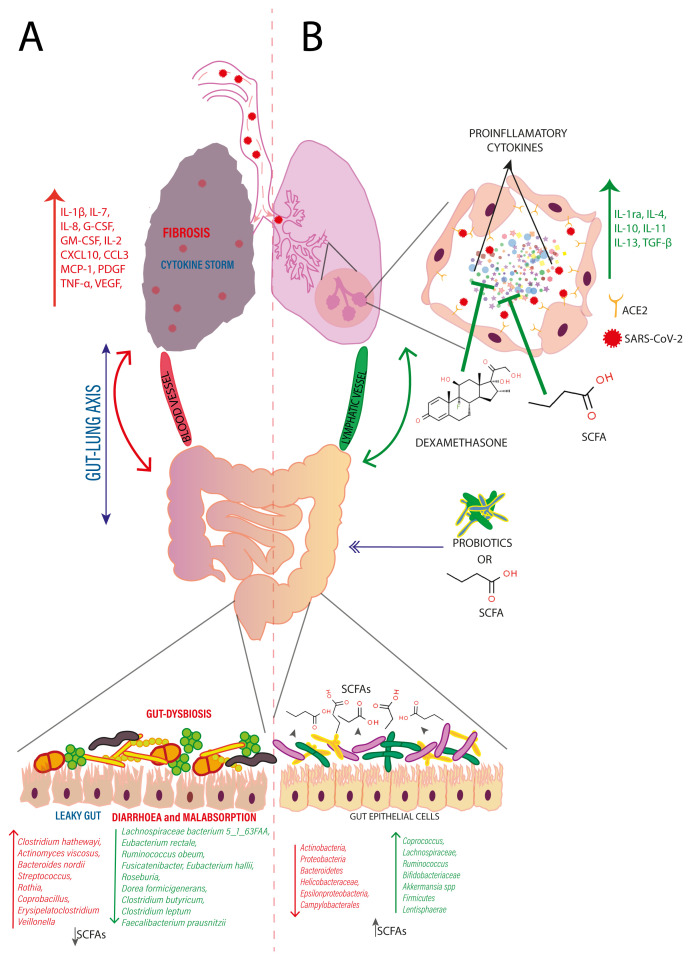
Figure 2.
A. SARS-CoV-2 transmitted through aerosols reach the lungs via respiratory tract and enters the host cell by binding to its receptor, ACE2 present on the surface of pneumocytes. Followed by endosome mediated internalization, SARS-CoV-2 causes cell injury and subsequent hyperinflammation and cytokine storm, resulting in fibrosis of lungs. These cytokines reach the gut via blood and lymphatic vessels that instigates local inflammation in gut, ushering to leaky gut and gut dysbiosis, resulting in diarrhoea and malabsorption together with reduced production of short chain fatty acids. B. Dexamethasone a synthetic broad-spectrum immunosuppressant can inhibit cytokine storm associated with CoVID-19. As an alternative, oral administration of probiotics or gut microbiome metabolite, SCFAs may ameliorate gut inflammation, restore gut integrity, and gut microbiome. This enhances the production of endogenous SCFAs and reaches the lungs via blood and lymphatic vessels, and may inhibit hyperinflammation and cytokine storm along with induction of anti-inflammatory cytokines production which recovers the lung from injury and the acute respiratory distresses associated with CoVID-19.
Conclusion
Seriously ill CoVID-19 patients are succumbing to respiratory distress syndrome due to significant hyperinflammation and cytokine storm. A broad-spectrum immunosuppressant, dexamethasone, is widely used to treat such cases. However, the prolonged use of this corticosteroid leads to severe adverse events and disrupted immune responses. There are growing number of advanced research studies in search of an alternative to dexamethasone for the better management of critical CoVID-19 patients. Hence, this review extensively searched for evidence to show the anti-inflammatory properties of butyric acid or butyrate and its associated molecular pathways involved in preventing SARS-CoV-2 infected patients from cytokine storm and hyperinflammation. It has been observed that the SARS-CoV-2 infection significantly decreases butyric acid producing bacteria in the host gut. Further, previous research shows that a histone deacetylase inhibitor, butyric acid has proven to be anti-inflammatory in lung inflammation including inflammation associated with respiratory viral infection. Therefore, based on the various positive reports, we presume that butyric acid possesses potent anti-inflammatory activity, making it a suitable alternative candidate for the preventive management of primary and secondary complications related to CoVID-19.
Data availabilty
No data is associated with this article.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; peer review: 3 approved]
References
- 1. Zu ZY, Di Jiang M, Xu PP, et al. : Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology. 2020;296(2):E15–E25. 10.1148/radiol.2020200490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. University JH: COVID-19 Case Tracker.Johns Hopkins University;2019; [cited 2020 12 April]. This website is a resource to help advance the understanding of the virus, inform the public, and brief policymakers in order to guide a response, improve care, and save lives.]. Reference Source [Google Scholar]
- 3. Hoffmann M, Kleine-Weber H, Schroeder S, et al. : SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng ZJ, Shan J: 2019 Novel coronavirus: where we are and what we know. Infection. 2020;48(2):155–63. 10.1007/s15010-020-01401-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma C, Cong Y, Zhang H: COVID-19 and the Digestive System. Am J Gastroenterol. 2020;115(7):1003–6. 10.14309/ajg.0000000000000691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martines RB, Ritter JM, Matkovic E, et al. : Pathology and Pathogenesis of SARS-CoV-2 Associated with Fatal Coronavirus Disease, United States. Emerg Infect Dis. 2020;26(9):2005–15. 10.3201/eid2609.202095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. : Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020;27(6):992–1000.e3. 10.1016/j.chom.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Badawi A: Hypercytokinemia and Pathogen-Host Interaction in COVID-19. J Inflamm Res. 2020;13:255–61. 10.2147/JIR.S259096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang D, Hu B, Hu C, et al. : Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang C, Wang Y, Li X, et al. : Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tomazini BM, Maia IS, Cavalcanti AB, et al. : Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA. 2020;324(13):1307–16. 10.1001/jama.2020.17021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Villar J, Ferrando C, Martínez D, et al. : Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267–76. 10.1016/S2213-2600(19)30417-5 [DOI] [PubMed] [Google Scholar]
- 13. Mattos-Silva P, Felix NS, Silva PL, et al. : Pros and cons of corticosteroid therapy for COVID-19 patients. Respir Physiol Neurobiol. 2020;280:103492. 10.1016/j.resp.2020.103492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Din AU, Mazhar M, Waseem M, et al. : SARS-CoV-2 microbiome dysbiosis linked disorders and possible probiotics role. Biomed Pharmacother. 2021;133:110947. 10.1016/j.biopha.2020.110947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. BourBour F, Dahka SM, Gholamalizadeh M, et al. : Nutrients in prevention, treatment, and management of viral infections; special focus on Coronavirus. Arch Physiol Biochem. 2020;1–10. 10.1080/13813455.2020.1791188 [DOI] [PubMed] [Google Scholar]
- 16. Richardson DP, Lovegrove JA: Nutritional status of micronutrients as a possible and modifiable risk factor for COVID-19: a UK perspective. Br J Nutr. 2021;125(6):678–684. 10.1017/S000711452000330X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fedele D, De Francesco A, Riso S, et al. : Obesity, malnutrition, and trace element deficiency in the coronavirus disease (COVID-19) pandemic: An overview. Nutrition. 2021;81:111016. 10.1016/j.nut.2020.111016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Calder PC, Carr AC, Gombart AF, et al. : Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients. 2020;12(4):1181. 10.3390/nu12041181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsoupras A, Lordan R, Zabetakis I: Thrombosis and COVID-19: The Potential Role of Nutrition. Front Nutr. 2020;7:583080. 10.3389/fnut.2020.583080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pecora F, Persico F, Argentiero A, et al. : The Role of Micronutrients in Support of the Immune Response against Viral Infections. Nutrients. 2020;12(10):3198. 10.3390/nu12103198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu L, Zhai Q, Yin R, et al. : Lactobacillus plantarum CCFM639 Alleviate Trace Element Imbalance-Related Oxidative Stress in Liver and Kidney of Chronic Aluminum Exposure Mice. Biol Trace Elem Res. 2017;176(2):342–9. 10.1007/s12011-016-0843-8 [DOI] [PubMed] [Google Scholar]
- 22. Zuo T, Zhang F, Lui GCY, et al. : Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology. 2020;159(3):944–55.e8. 10.1053/j.gastro.2020.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li KL, Wang BZ, Li ZP, et al. : Alterations of intestinal flora and the effects of probiotics in children with recurrent respiratory tract infection. World J Pediatr. 2019;15(3):255–61. 10.1007/s12519-019-00248-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gu S, Chen Y, Wu Z, et al. : Alterations of the Gut Microbiota in Patients With Coronavirus Disease 2019 or H1N1 Influenza. Clin Infect Dis. 2020;71(10):2669–2678. 10.1093/cid/ciaa709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Elce A, Amato F, Zarrilli F, et al. : Butyrate modulating effects on pro-inflammatory pathways in human intestinal epithelial cells. Benef Microbes. 2017;8(5):841–7. 10.3920/BM2016.0197 [DOI] [PubMed] [Google Scholar]
- 26. Li N, Liu XX, Hong M, et al. : Sodium butyrate alleviates LPS-induced acute lung injury in mice via inhibiting HMGB1 release. Int Immunopharmacol. 2018;56:242–8. 10.1016/j.intimp.2018.01.017 [DOI] [PubMed] [Google Scholar]
- 27. Liu J, Chang G, Huang J, et al. : Sodium Butyrate Inhibits the Inflammation of Lipopolysaccharide-Induced Acute Lung Injury in Mice by Regulating the Toll-Like Receptor 4/Nuclear Factor κB Signaling Pathway. J Agric Food Chem. 2019;67(6):1674–82. 10.1021/acs.jafc.8b06359 [DOI] [PubMed] [Google Scholar]
- 28. Haridas V, Shetty P, Sarathkumar E, et al. : Reciprocal regulation of pro-inflammatory Annexin A2 and anti-inflammatory Annexin A1 in the pathogenesis of rheumatoid arthritis. Mol Biol Rep. 2019;46(1):83–95. 10.1007/s11033-018-4448-5 [DOI] [PubMed] [Google Scholar]
- 29. Wang R, Simoneau CR, Kulsuptrakul J, et al. : Genetic Screens Identify Host Factors for SARS-CoV-2 and Common Cold Coronaviruses. Cell. 2021;184(1):106–119.e14. 10.1016/j.cell.2020.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jin Y, Yang H, Ji W, et al. : Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses. 2020;12(4):372. 10.3390/v12040372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Letko M, Marzi A, Munster V: Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5(4):562–9. 10.1038/s41564-020-0688-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun P, Lu X, Xu C, et al. : Understanding of COVID-19 based on current evidence. J Med Virol. 2020;92(6):548–551. 10.1002/jmv.25722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Velavan TP, Meyer CG: The COVID-19 epidemic. Trop Med Int Health. 2020;25(3):278–80. 10.1111/tmi.13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Terruzzi I, Senesi P: Does intestinal dysbiosis contribute to an aberrant inflammatory response to severe acute respiratory syndrome coronavirus 2 in frail patients? Nutrition. 2020;79–80:110996. 10.1016/j.nut.2020.110996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gustine JN, Jones D: Immunopathology of Hyperinflammation in COVID-19. Am J Pathol. 2021;191(1):4–17. 10.1016/j.ajpath.2020.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meng J, Ma Y, Jia J, et al. : Cytokine Storm in Coronavirus Disease 2019 and Adult-Onset Still's Disease: Similarities and Differences. Front Immunol. 2021;11:603389. 10.3389/fimmu.2020.603389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Azevedo RB, Botelho BG, Hollanda JVG, et al. : Covid-19 and the cardiovascular system: a comprehensive review. J Hum Hypertens. 2021;35(1):4–11. 10.1038/s41371-020-0387-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Asakura H, Ogawa H: COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol. 2021;113(1):45–57. 10.1007/s12185-020-03029-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yeoh YK, Zuo T, Lui GC, et al. : Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70(4):698–706. 10.1136/gutjnl-2020-323020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Angurana SK, Bansal A: Probiotics and COVID-19: Think about the link. Br J Nutr. 2020;1–26. 10.1017/S000711452000361X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ahlawat S, Asha, Sharma KK: Immunological co-ordination between gut and lungs in SARS-CoV-2 infection. Virus Res. 2020;286:198103. 10.1016/j.virusres.2020.198103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tang L, Gu S, Gong Y, et al. : Clinical Significance of the Correlation between Changes in the Major Intestinal Bacteria Species and COVID-19 Severity. Engineering (Beijing). 2020;6(10):1178–84. 10.1016/j.eng.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baghbani T, Nikzad H, Azadbakht J, et al. : Dual and mutual interaction between microbiota and viral infections: a possible treat for COVID-19. Microb Cell Fact. 2020;19(1):217. 10.1186/s12934-020-01483-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alwarawrah Y, Kiernan K, MacIver NJ: Changes in Nutritional Status Impact Immune Cell Metabolism and Function. Front Immunol. 2018;9:1055. 10.3389/fimmu.2018.01055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Niedzwiecki: Essential nutrients suppress inflammation by modulating key inflammatory gene expression. Int J Mol Med. 1998. [PubMed] [Google Scholar]
- 46. Singh P, Tripathi MK, Yasir M, et al. : Potential Inhibitors for SARS-CoV-2 and Functional Food Components as Nutritional Supplement for COVID-19: A Review. Plant Foods Hum Nutr. 2020;75(4):458–466. 10.1007/s11130-020-00861-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhou J, Ma Y, Liu Y, et al. : A Correlation Analysis between the Nutritional Status and Prognosis of COVID-19 Patients. J Nutr Health Aging. 2021;25(1):84–93. 10.1007/s12603-020-1457-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mehta S: Nutritional status and COVID-19: an opportunity for lasting change? Clin Med (Lond). 2020;20(3):270–273. 10.7861/clinmed.2020-0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yanowsky-Escatell FG, Osuna-Padilla IA: Nutritional therapy optimization in COVID-19 critically ill patients. Gac Med Mex. 2020;156(4):360–362. 10.24875/GMM.20000177 [DOI] [PubMed] [Google Scholar]
- 50. Morais AHD, Aquino JD, da Silva-Maia JK, et al. : Nutritional status, diet and viral respiratory infections: perspectives for severe acute respiratory syndrome coronavirus 2. Br J Nutr. 2020;125(8):851–862. 10.1017/S0007114520003311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bedock D, Bel Lassen P, Mathian A, et al. : Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin Nutr ESPEN. 2020;40:214–219. 10.1016/j.clnesp.2020.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Briguglio M, Pregliasco FE, Lombardi G, et al. : The Malnutritional Status of the Host as a Virulence Factor for New Coronavirus SARS-CoV-2. Front Med (Lausanne). 2020;7:146. 10.3389/fmed.2020.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. D'Avolio A, Avataneo V, Manca A, et al. : 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients. 2020;12(5):1359. 10.3390/nu12051359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pizzini A, Aichner M, Sahanic S, et al. : Impact of Vitamin D Deficiency on COVID-19-A Prospective Analysis from the CovILD Registry. Nutrients. 2020;12(9):2775. 10.3390/nu12092775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Meltzer DO, Best TJ, Zhang H, et al. : Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Netw Open. 2020;3(9):e2019722. 10.1001/jamanetworkopen.2020.19722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jain SK, Parsanathan R: Can Vitamin D and L-Cysteine Co-Supplementation Reduce 25(OH)-Vitamin D Deficiency and the Mortality Associated with COVID-19 in African Americans? J Am Coll Nutr. 2020;39(8):694–699. 10.1080/07315724.2020.1789518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tan CW, Ho LP, Kalimuddin S, et al. : Cohort study to evaluate the effect of vitamin D magnesium, and vitamin B 12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19). Nutrition. 2020;79-80:111017. 10.1016/j.nut.2020.111017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Orru B, Szekeres-Bartho J, Bizzarri M, et al. : Inhibitory effects of Vitamin D on inflammation and IL-6 release. A further support for COVID-19 management? Eur Rev Med Pharmacol Sci. 2020;24(15):8187–8193. 10.26355/eurrev_202008_22507 [DOI] [PubMed] [Google Scholar]
- 59. Quesada-Gomez JM, Entrenas-Castillo M, Bouillon R: Vitamin D receptor stimulation to reduce acute respiratory distress syndrome (ARDS) in patients with coronavirus SARS-CoV-2 infections: Revised Ms SBMB 2020_ 166. J Steroid Biochem Mol Biol. 2020;202:105719. 10.1016/j.jsbmb.2020.105719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen L, Hu C, Hood M, et al. : A Novel Combination of Vitamin C, Curcumin and Glycyrrhizic Acid Potentially Regulates Immune and Inflammatory Response Associated with Coronavirus Infections: A Perspective from System Biology Analysis. Nutrients. 2020;12(4):1193. 10.3390/nu12041193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Carr AC: Micronutrient status of COVID-19 patients: a critical consideration. Crit Care. 2020;24(1):349. 10.1186/s13054-020-03085-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Carr AC, Rowe S: The Emerging Role of Vitamin C in the Prevention and Treatment of COVID-19. Nutrients. 2020;12(11):3286. 10.3390/nu12113286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Feyaerts AF, Luyten W: Vitamin C as prophylaxis and adjunctive medical treatment for COVID-19? Nutrition. 2020;79–80:110948. 10.1016/j.nut.2020.110948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cervantes-Perez E, Cervantes-Guevara G, Martinez-Soto Holguin MC, et al. : Medical Nutrition Therapy in Hospitalized Patients With SARS-CoV-2 (COVID-19) Infection in a Non-critical Care Setting: Knowledge in Progress. Curr Nutr Rep. 2020;9(4):309–15. 10.1007/s13668-020-00337-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bencivenga L, Rengo G, Varricchi G: Elderly at time of COronaVIrus disease 2019 (COVID-19): possible role of immunosenescence and malnutrition. Geroscience. 2020;42(4):1089–92. 10.1007/s11357-020-00218-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bermano G, Meplan C, Mercer DK, et al. : Selenium and viral infection: are there lessons for COVID-19? Br J Nutr. 2020;125(6):618–627. 10.1017/S0007114520003128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Moghaddam A, Heller RA, Sun Q, et al. : Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients. 2020;12(7):2098. 10.3390/nu12072098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Seale LA, Torres DJ, Berry MJ, et al. : A role for selenium-dependent GPX1 in SARS-CoV-2 virulence. Am J Clin Nutr. 2020;112(2):447–8. 10.1093/ajcn/nqaa177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang J, Saad R, Taylor EW, et al. : Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020;37:101715. 10.1016/j.redox.2020.101715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Theoharides TC, Antonopoulou S, Demopoulos CA: Coronavirus 2019, Microthromboses, and Platelet Activating Factor. Clin Ther. 2020;42(10):1850–2. 10.1016/j.clinthera.2020.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zabetakis I, Lordan R, Norton C, et al. : COVID-19: The Inflammation Link and the Role of Nutrition in Potential Mitigation. Nutrients. 2020;12(5):1466. 10.3390/nu12051466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Acosta-Elias J, Espinosa-Tanguma R: The Folate Concentration and/or Folic Acid Metabolites in Plasma as Factor for COVID-19 Infection. Front Pharmacol. 2020;11:1062. 10.3389/fphar.2020.01062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Quiles JL, Rivas-Garcia L, Varela-Lopez A, et al. : Do nutrients and other bioactive molecules from foods have anything to say in the treatment against COVID-19? Environ Res. 2020;191:110053. 10.1016/j.envres.2020.110053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Junaid K, Ejaz H, Abdalla AE, et al. : Effective Immune Functions of Micronutrients against SARS-CoV-2. Nutrients. 2020;12(10):2992. 10.3390/nu12102992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Khubber S, Hashemifesharaki R, Mohammadi M, et al. : Garlic ( Allium sativum L.): a potential unique therapeutic food rich in organosulfur and flavonoid compounds to fight with COVID-19. Nutr J. 2020;19(1):124. 10.1186/s12937-020-00643-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ibrahim MAA, Abdelrahman AHM, Hussien TA, et al. : In silico drug discovery of major metabolites from spices as SARS-CoV-2 main protease inhibitors. Comput Biol Med. 2020;126:104046. 10.1016/j.compbiomed.2020.104046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Benedetti F, Sorrenti V, Buriani A, et al. : Resveratrol, Rapamycin and Metformin as Modulators of Antiviral Pathways. Viruses. 2020;12(12):1458. 10.3390/v12121458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Song S, Peng H, Wang Q, et al. : Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 2020;11(9):7415–20. 10.1039/d0fo02017f [DOI] [PubMed] [Google Scholar]
- 79. Weill P, Plissonneau C, Legrand P, et al. : May omega-3 fatty acid dietary supplementation help reduce severe complications in Covid-19 patients? Biochimie. 2020;179:275–80. 10.1016/j.biochi.2020.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rogero MM, Leao MC, Santana TM, et al. : Potential benefits and risks of omega-3 fatty acids supplementation to patients with COVID-19. Free Radic Biol Med. 2020;156:190–9. 10.1016/j.freeradbiomed.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Miyazawa D: SARS-CoV-2 in saliva may be deactivated with fatty acids or emulsifiers. Authorea. 2020. 10.22541/au.159493480.06361071/v2 [DOI] [Google Scholar]
- 82. Trompette A, Gollwitzer ES, Pattaroni C, et al. : Dietary Fiber Confers Protection against Flu by Shaping Ly6c - Patrolling Monocyte Hematopoiesis and CD8 + T Cell Metabolism. Immunity. 2018;48(5):992–1005 e8. 10.1016/j.immuni.2018.04.022 [DOI] [PubMed] [Google Scholar]
- 83. Conte L, Toraldo DM: Targeting the gut-lung microbiota axis by means of a high-fibre diet and probiotics may have anti-inflammatory effects in COVID-19 infection. Ther Adv Respir Dis. 2020;14:1753466620937170. 10.1177/1753466620937170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Halnes I, Baines KJ, Berthon BS, et al. : Soluble Fibre Meal Challenge Reduces Airway Inflammation and Expression of GPR43 and GPR41 in Asthma. Nutrients. 2017;9(1):57. 10.3390/nu9010057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Shinde T, Hansbro PM, Sohal SS, et al. : Microbiota Modulating Nutritional Approaches to Countering the Effects of Viral Respiratory Infections Including SARS-CoV-2 through Promoting Metabolic and Immune Fitness with Probiotics and Plant Bioactives. Microorganisms. 2020;8(6):921. 10.3390/microorganisms8060921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lehtoranta L, Pitkaranta A, Korpela R: Probiotics in respiratory virus infections. Eur J Clin Microbiol Infect Dis. 2014;33(8):1289–302. 10.1007/s10096-014-2086-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wang Y, Li X, Ge T, et al. : Probiotics for prevention and treatment of respiratory tract infections in children: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2016;95(31):e4509. 10.1097/MD.0000000000004509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Campanella V, Syed J, Santacroce L, et al. : Oral probiotics influence oral and respiratory tract infections in pediatric population: a randomized double-blinded placebo-controlled pilot study. Eur Rev Med Pharmacol Sci. 2018;22(22):8034–41. 10.26355/eurrev_201811_16433 [DOI] [PubMed] [Google Scholar]
- 89. Kanauchi O, Andoh A, AbuBakar S, et al. : Probiotics and Paraprobiotics in Viral Infection: Clinical Application and Effects on the Innate and Acquired Immune Systems. Curr Pharm Des. 2018;24(6):710–717. 10.2174/1381612824666180116163411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nocerino R, Paparo L, Terrin G, et al. : Cow's milk and rice fermented with Lactobacillus paracasei CBA L74 prevent infectious diseases in children: A randomized controlled trial. Clin Nutr. 2017;36(1):118–25. 10.1016/j.clnu.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 91. Shida K, Sato T, Iizuka R, et al. : Daily intake of fermented milk with Lactobacillus casei strain Shirota reduces the incidence and duration of upper respiratory tract infections in healthy middle-aged office workers. Eur J Nutr. 2017;56(1):45–53. 10.1007/s00394-015-1056-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mai TT, Thi Thu P, Thi Hang H, et al. : Efficacy of probiotics on digestive disorders and acute respiratory infections: a controlled clinical trial in young Vietnamese children. Eur J Clin Nutr. 2020;75(3):513–520. 10.1038/s41430-020-00754-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chong HX, Yusoff NAA, Hor YY, et al. : Lactobacillus plantarum DR7 improved upper respiratory tract infections via enhancing immune and inflammatory parameters: A randomized, double-blind, placebo-controlled study. J Dairy Sci. 2019;102(6):4783–97. 10.3168/jds.2018-16103 [DOI] [PubMed] [Google Scholar]
- 94. Hsieh MH, Jan RL, Wu LSH, et al. : Lactobacillus gasseri attenuates allergic airway inflammation through PPARγ activation in dendritic cells. J Mol Med (Berl). 2018;96(1):39–51. 10.1007/s00109-017-1598-1 [DOI] [PubMed] [Google Scholar]
- 95. Dos Santos Pereira Andrade AC, Lima MT, Oliveira GP, et al. : Daily ingestion of the probiotic Lactobacillus paracasei ST11 decreases Vaccinia virus dissemination and lethality in a mouse model. Benef Microbes. 2017;8(1):73–80. 10.3920/BM2016.0074 [DOI] [PubMed] [Google Scholar]
- 96. Eguchi K, Fujitani N, Nakagawa H, et al. : Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Sci Rep. 2019;9(1):4812. 10.1038/s41598-019-39602-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chen MF, Weng KF, Huang SY, et al. : Pretreatment with a heat-killed probiotic modulates monocyte chemoattractant protein-1 and reduces the pathogenicity of influenza and enterovirus 71 infections. Mucosal Immunol. 2017;10(1):215–227. 10.1038/mi.2016.31 [DOI] [PubMed] [Google Scholar]
- 98. Liang Y, Lin C, Zhang Y, et al. : Probiotic mixture of Lactobacillus and Bifidobacterium alleviates systemic adiposity and inflammation in non-alcoholic fatty liver disease rats through Gpr109a and the commensal metabolite butyrate. Inflammopharmacology. 2018;26(4):1051–5. 10.1007/s10787-018-0479-8 [DOI] [PubMed] [Google Scholar]
- 99. Zhou D, Pan Q, Liu XL, et al. : Clostridium butyricum B1 alleviates high-fat diet-induced steatohepatitis in mice via enterohepatic immunoregulation. J Gastroenterol Hepatol. 2017;32(9):1640–1648. 10.1111/jgh.13742 [DOI] [PubMed] [Google Scholar]
- 100. Liu Y, Gao Y, Ma F, et al. : The ameliorative effect of Lactobacillus plantarum Y44 oral administration on inflammation and lipid metabolism in obese mice fed with a high fat diet. Food Funct. 2020;11(6):5024–5039. 10.1039/d0fo00439a [DOI] [PubMed] [Google Scholar]
- 101. Vemuri R, Gundamaraju R, Shinde T, et al. : Lactobacillus acidophilus DDS-1 Modulates Intestinal-Specific Microbiota, Short-Chain Fatty Acid and Immunological Profiles in Aging Mice. Nutrients. 2019;11(6):1297. 10.3390/nu11061297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Liu MY, Yang ZY, Dai WK, et al. : Protective effect of Bifidobacterium infantis CGMCC313-2 on ovalbumin-induced airway asthma and beta-lactoglobulin-induced intestinal food allergy mouse models. World J Gastroenterol. 2017;23(12):2149–58. 10.3748/wjg.v23.i12.2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yamazaki T, Ohshio K, Sugamata M, et al. : Lactic acid bacterium, Lactobacillus paracasei KW3110, suppresses inflammatory stress-induced caspase-1 activation by promoting interleukin-10 production in mouse and human immune cells. PLoS One. 2020;15(8):e0237754. 10.1371/journal.pone.0237754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. d'Ettorre G, Ceccarelli G, Marazzato M, et al. : Challenges in the Management of SARS-CoV2 Infection: The Role of Oral Bacteriotherapy as Complementary Therapeutic Strategy to Avoid the Progression of COVID-19. Front Med (Lausanne). 2020;7:389. 10.3389/fmed.2020.00389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lehtoranta L, Latvala S, Lehtinen MJ: Role of Probiotics in Stimulating the Immune System in Viral Respiratory Tract Infections: A Narrative Review. Nutrients. 2020;12(10):3163. 10.3390/nu12103163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Vaghef-Mehrabany E, Homayouni-Rad A, Alipour B, et al. : Effects of Probiotic Supplementation on Oxidative Stress Indices in Women with Rheumatoid Arthritis: A Randomized Double-Blind Clinical Trial. J Am Coll Nutr. 2016;35(4):291–9. 10.1080/07315724.2014.959208 [DOI] [PubMed] [Google Scholar]
- 107. Tamtaji OR, Kouchaki E, Salami M, et al. : The Effects of Probiotic Supplementation on Gene Expression Related to Inflammation, Insulin, and Lipids in Patients With Multiple Sclerosis: A Randomized, Double-Blind, Placebo-Controlled Trial. J Am Coll Nutr. 2017;36(8):660–665. 10.1080/07315724.2017.1347074 [DOI] [PubMed] [Google Scholar]
- 108. Xie H, Lu Q, Wang H, et al. : Effects of probiotics combined with enteral nutrition on immune function and inflammatory response in postoperative patients with gastric cancer. J BUON. 2018;23(3):678–83. [PubMed] [Google Scholar]
- 109. Lee SY, Lee SH, Jhun J, et al. : A Combination with Probiotic Complex, Zinc, and Coenzyme Q10 Attenuates Autoimmune Arthritis by Regulation of Th17/Treg Balance. J Med Food. 2018;21(1):39–46. 10.1089/jmf.2017.3952 [DOI] [PubMed] [Google Scholar]
- 110. Jia H, Ren S, Wang X: Heat-killed probiotic regulates the body's regulatory immunity to attenuate subsequent experimental autoimmune arthritis. Immunol Lett. 2019;216:89–96. 10.1016/j.imlet.2019.10.009 [DOI] [PubMed] [Google Scholar]
- 111. Vaghef-Mehrabany E, Alipour B, Homayouni-Rad A, et al. : Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition. 2014;30(4):430–5. 10.1016/j.nut.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 112. Bengoa AA, Dardis C, Gagliarini N, et al. : Exopolysaccharides From Lactobacillus paracasei Isolated From Kefir as Potential Bioactive Compounds for Microbiota Modulation. Front Microbiol. 2020;11:583254. 10.3389/fmicb.2020.583254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Moens F, Van den Abbeele P, Basit AW, et al. : A four-strain probiotic exerts positive immunomodulatory effects by enhancing colonic butyrate production in vitro. Int J Pharm. 2019;555:1–10. 10.1016/j.ijpharm.2018.11.020 [DOI] [PubMed] [Google Scholar]
- 114. Walton GE, Gibson GR, Hunter KA: Mechanisms linking the human gut microbiome to prophylactic and treatment strategies for COVID-19. Br J Nutr. 2020;1–9. 10.1017/S0007114520003980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mahooti M, Miri SM, Abdolalipour E, et al. : The immunomodulatory effects of probiotics on respiratory viral infections: A hint for COVID-19 treatment? Microb Pathog. 2020;148:104452. 10.1016/j.micpath.2020.104452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gohil K, Samson R, Dastager S, et al. : Probiotics in the prophylaxis of COVID-19: something is better than nothing. 3 Biotech. 2021;11(1):1. 10.1007/s13205-020-02554-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Baindara P, Chakraborty R, Holliday ZM, et al. : Oral probiotics in coronavirus disease 2019: connecting the gut-lung axis to viral pathogenesis, inflammation, secondary infection and clinical trials. New Microbes New Infect. 2021;40:100837. 10.1016/j.nmni.2021.100837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hosseinzadeh MH, Shamshirian A, Ebrahimzadeh MA: Dexamethasone vs COVID-19: An experimental study in line with the preliminary findings of a large trial. Int J Clin Pract. 2020;e13943. 10.1111/ijcp.13943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Andreakos E, Papadaki M, Serhan CN: Dexamethasone, pro-resolving lipid mediators and resolution of inflammation in COVID-19. Allergy. 2021;76(3):626–628. 10.1111/all.14595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mikolka P, Kosutova P, Kolomaznik M, et al. : Effect of different dosages of dexamethasone therapy on lung function and inflammation in an early phase of acute respiratory distress syndrome model. Physiol Res. 2019;68(Suppl 3):S253–S63. 10.33549/physiolres.934364 [DOI] [PubMed] [Google Scholar]
- 121. Sarma A, Christenson S, Mick E, et al. : COVID-19 ARDS is characterized by a dysregulated host response that differs from cytokine storm and is modified by dexamethasone. Res Sq. 2021;rs.3.rs–141578. 10.21203/rs.3.rs-141578/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Selvaraj V, Dapaah-Afriyie K, Finn A, et al. : Short-Term Dexamethasone in Sars-CoV-2 Patients. R I Med J (2013). 2020;103(6):39–43. [PubMed] [Google Scholar]
- 123. RECOVERY Collaborative Group; Horby P, Lim WS, et al. : Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693–704. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hassan ME, Hasan HM, Sridharan K, et al. : Dexamethasone in severe COVID-19 infection: A case series. Respir Med Case Rep. 2020;31:101205. 10.1016/j.rmcr.2020.101205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lévesque V, Millaire É, Corsilli D, et al. : Severe immune thrombocytopenic purpura in critical COVID-19. Int J Hematol. 2020;112(5):746–50. 10.1007/s12185-020-02931-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Zúñiga MÁL, Moreno-Moral A, Ocaña-Granados A, et al. : High-dose corticosteroid pulse therapy increases the survival rate in COVID-19 patients at risk of hyper-inflammatory response. PLoS One. 2021;16(1):e0243964. 10.1371/journal.pone.0243964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Waterer GW, Rello J: Steroids and COVID-19: We Need a Precision Approach, Not One Size Fits All. Infect Dis Ther. 2020;9(4):701–5. 10.1007/s40121-020-00338-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Sharun K, Tiwari R, Dhama J, et al. : Dexamethasone to combat cytokine storm in COVID-19: Clinical trials and preliminary evidence. Int J Surg. 2020;82:179–81. 10.1016/j.ijsu.2020.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Salem MA: A Response to the Recommendations for Using Dexamethasone for the Treatment of COVID-19: The Dark Side of Dexamethasone. J Pharm Pract. 2021;34(2):179–180. 10.1177/0897190020979608 [DOI] [PubMed] [Google Scholar]
- 130. He LH, Ren LF, Li JF, et al. : Intestinal Flora as a Potential Strategy to Fight SARS-CoV-2 Infection. Front Microbiol. 2020;11:1388. 10.3389/fmicb.2020.01388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhou X, Du L, Shi R, et al. : Early-life food nutrition, microbiota maturation and immune development shape life-long health. Crit Rev Food Sci Nutr. 2019;59(sup1):S30–S8. 10.1080/10408398.2018.1485628 [DOI] [PubMed] [Google Scholar]
- 132. Tayyeb JZ, Popeijus HE, Mensink RP, et al. : Butyric Acid Added Apically to Intestinal Caco-2 Cells Elevates Hepatic ApoA-I Transcription and Rescues Lower ApoA-I Expression in Inflamed HepG2 Cells Co-Cultured in the Basolateral Compartment. Biomolecules. 2021;11(1):71. 10.3390/biom11010071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Pituch A, Walkowiak J, Banaszkiewicz A: Butyric acid in functional constipation. Prz Gastroenterol. 2013;8(5):295–8. 10.5114/pg.2013.38731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Fang W, Xue H, Chen X, et al. : Supplementation with Sodium Butyrate Modulates the Composition of the Gut Microbiota and Ameliorates High-Fat Diet-Induced Obesity in Mice. J Nutr. 2019;149(5):747–754. 10.1093/jn/nxy324 [DOI] [PubMed] [Google Scholar]
- 135. Liu J, Zhu H, Li B, et al. : Beneficial effects of butyrate in intestinal injury. J Pediatr Surg. 2020;55(6):1088–1093. 10.1016/j.jpedsurg.2020.02.036 [DOI] [PubMed] [Google Scholar]
- 136. Bachmann M, Meissner C, Pfeilschifter J, et al. : Cooperation between the bacterial-derived short-chain fatty acid butyrate and interleukin-22 detected in human Caco2 colon epithelial/carcinoma cells. Biofactors. 2017;43(2):283–292. 10.1002/biof.1341 [DOI] [PubMed] [Google Scholar]
- 137. Johnstone M, Bennett N, Standifer C, et al. : Characterization of the Pro-Inflammatory Cytokine IL-1β on Butyrate Oxidation in Colorectal Cancer Cells. J Cell Biochem. 2017;118(6):1614–1621. 10.1002/jcb.25824 [DOI] [PubMed] [Google Scholar]
- 138. Yin J, Zhou C, Yang K, et al. : Mutual regulation between butyrate and hypoxia-inducible factor-1α in epithelial cell promotes expression of tight junction proteins. Cell Biol Int. 2020;44(6):1405–1414. 10.1002/cbin.11336 [DOI] [PubMed] [Google Scholar]
- 139. Wang F, Jin Z, Shen K, et al. : Butyrate pretreatment attenuates heart depression in a mice model of endotoxin-induced sepsis via anti-inflammation and anti-oxidation. Am J Emerg Med. 2017;35(3):402–409. 10.1016/j.ajem.2016.11.022 [DOI] [PubMed] [Google Scholar]
- 140. Zeng M, Sang W, Chen S, et al. : 4-PBA inhibits LPS-induced inflammation through regulating ER stress and autophagy in acute lung injury models. Toxicol Lett. 2017;271:26–37. 10.1016/j.toxlet.2017.02.023 [DOI] [PubMed] [Google Scholar]
- 141. Baraldi O, Bianchi F, Menghi V, et al. : An in vitro model of renal inflammation after ischemic oxidative stress injury: nephroprotective effects of a hyaluronan ester with butyric acid on mesangial cells. J Inflamm Res. 2017;10:135–142. 10.2147/JIR.S138431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Simeoli R, Raso GM, Pirozzi C, et al. : An orally administered butyrate-releasing derivative reduces neutrophil recruitment and inflammation in dextran sulphate sodium-induced murine colitis. Br J Pharmacol. 2017;174(11):1484–1496. 10.1111/bph.13637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Torun A, Enayat S, Sheraj I, et al. : Butyrate mediated regulation of RNA binding proteins in the post-transcriptional regulation of inflammatory gene expression. Cell Signal. 2019;64:109410. 10.1016/j.cellsig.2019.109410 [DOI] [PubMed] [Google Scholar]
- 144. Zhang L, Deng M, Lu A, et al. : Sodium butyrate attenuates angiotensin II-induced cardiac hypertrophy by inhibiting COX2/PGE2 pathway via a HDAC5/HDAC6-dependent mechanism. J Cell Mol Med. 2019;23(12):8139–8150. 10.1111/jcmm.14684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Robles-Vera I, Toral M, de la Visitacion N, et al. : Protective Effects of Short-Chain Fatty Acids on Endothelial Dysfunction Induced by Angiotensin II. Front Physiol. 2020;11:277. 10.3389/fphys.2020.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. de Souza Vieira R, Castoldi A, Basso PJ, et al. : Butyrate Attenuates Lung Inflammation by Negatively Modulating Th9 Cells. Front Immunol. 2019;10:67. 10.3389/fimmu.2019.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Takahashi Y, Hayakawa A, Sano R, et al. : Histone deacetylase inhibitors suppress ACE2 and ABO simultaneously, suggesting a preventive potential against COVID-19. Sci Rep. 2021;11(1):3379. 10.1038/s41598-021-82970-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kanika G, Khan S, Jena G: Sodium Butyrate Ameliorates L-Arginine-Induced Pancreatitis and Associated Fibrosis in Wistar Rat: Role of Inflammation and Nitrosative Stress. J Biochem Mol Toxicol. 2015;29(8):349–59. 10.1002/jbt.21698 [DOI] [PubMed] [Google Scholar]
- 149. Kabel AM, Omar MS, Elmaaboud MAA: Amelioration of bleomycin-induced lung fibrosis in rats by valproic acid and butyrate: Role of nuclear factor kappa-B, proinflammatory cytokines and oxidative stress. Int Immunopharmacol. 2016;39:335–342. 10.1016/j.intimp.2016.08.008 [DOI] [PubMed] [Google Scholar]