This study summarizes current understanding of the role of microglia and P2X4 receptor in chronic pain including neuropathic pain and of their therapeutic potential.
Keywords: Microglia, P2X4 receptors, Chronic pain, Neuropathic pain, Pain mechanism, Drug discovery
Abstract
Pain plays an indispensable role as an alarm system to protect us from dangers or injuries. However, neuropathic pain, a debilitating pain condition caused by damage to the nervous system, persists for a long period even in the absence of dangerous stimuli or after injuries have healed. In this condition, pain becomes a disease itself rather than the alarm system and is often resistant to currently available medications. A growing body of evidence indicates that microglia, a type of macrophages residing in the central nervous system, play a crucial role in the pathogenesis of neuropathic pain. Whenever microglia in the spinal cord detect a damaging signal within the nervous system, they become activated and cause diverse alterations that change neural excitability, leading to the development of neuropathic pain. For over a decade, several lines of molecular and cellular mechanisms that define microglial activation and subsequently altered pain transmission have been proposed. In particular, P2X4 receptors (a subtype of purinergic receptors) expressed by microglia have been investigated as an essential molecule for neuropathic pain. In this review article, we describe our understanding of the mechanisms by which activated microglia cause neuropathic pain through P2X4 receptors, their involvement in several pathological contexts, and recent efforts to develop new drugs targeting microglia and P2X4 receptors.
1. Introduction
Nociception is a sensory process caused by noxious stimuli in the nervous system. Nociceptive signals from peripheral tissues are conveyed to the brain through primary afferents and spinal dorsal horn (SDH) neurons and are frequently perceived as pain. Such acute nociceptive pain functions as an important defense system to protect the organisms from injury or potentially dangerous stimuli. By contrast, chronic pain often persists for a long time even in the absence of any dangers or even after the primary tissue damage has already been repaired. Neuropathic pain, a debilitating chronic pain, is caused by damage to the nervous system after diabetes, cancer, chemotherapy, infection, and trauma. Symptoms of neuropathic pain are characterized by spontaneous pain, hyperalgesia, and tactile allodynia, the last of which is a pain caused by innocuous stimuli.29 In this condition, pain no longer functions as a defense system and becomes simply an unpleasant sensation. Moreover, neuropathic pain is often refractory to current available analgesics.43 Malfunctions in the pain transmission pathway caused by injury within the peripheral or central nervous system (CNS) characterize this debilitating disorder.
Somatosensory stimuli from the periphery are converted to electrical excitation at the endings of primary afferents. Primary afferents are broadly divided into 2 classes: nociceptive and non-nociceptive, which respond to noxious and innocuous stimuli, respectively.9 The nociceptive afferents consist mainly of thin, myelinated Aδ fibers and unmyelinated C fibers, which transmit to the superficial SDH (laminae I and II). The non-nociceptive afferents, such as large-diameter, thick, myelinated Aβ fibers, detect innocuous mechanical stimuli and transmit to the deeper laminae of the SDH. Spinal neural circuit consists of projection neurons, which convey sensory information to higher brain regions, and a large number of excitatory and inhibitory interneurons.9,105,127 The electrical excitation in nociceptors caused by nociceptive stimuli is conveyed to the SDH, where it excites the projection neurons through complex circuits. This excitation is further conveyed into various brain regions. Although Aβ fibers convey innocuous stimuli such as touch to the skin and anatomically connect to the nociceptive projection neurons, the excitation of Aβ fibers does not cause pain sensation. This can be explained by the gate-control theory.94 As per this theory, feed-forward inhibition prevents the pain-projection neurons to be fired by innocuous stimuli through γ-aminobutyric acid (GABA) or glycine-containing inhibitory interneurons which are also excited by Aβ fibers. In short, a balance between synaptic excitation and inhibition determines the output of pain-projection neurons.15 It should be properly coordinated for the normal pain transmission.
When the nervous system is injured, the inhibitory inputs within the SDH are reduced (disinhibition), altering the excitatory and inhibitory balance, and shifting the balance towards excitation.95,117 Artificial disinhibition of inhibitory interneurons causes nocifensive behavior or mechanical hypersensitivity in which the innocuous stimuli can elicit abnormal pain.40,75,82,106,108 The excitatory shift of the balance is also mediated by enhancement of the excitatory inputs. In pathological conditions, glutamatergic excitatory inputs are potentiated through activation of glutamate receptors including n-methyl-d-aspartate receptors (NMDARs) or α-amino-3-hydroxy5-methyl-4-isoxazolepropionic acid receptors.14,55,150 These synaptic alterations have been extensively studied using rodent models of peripheral nerve injury (PNI). In the SDH of these models, not only neuronal cells but also microglia, which are resident immune cells in the CNS, undergo functional alterations.
Microglia are originally derived from erythromyeloid progenitors in the embryonic yolk sac.47–49 These erythromyeloid progenitors migrate into the CNS and differentiate to form microglia in a stepwise manner, after which the microglia persist to stay in the CNS throughout life.49,92,110 Microglia are necessary for the proper development of neural circuits or myelin sheaths during their developmental stages52,104 and are also used for surveying the surrounding environment using their ramified and motile processes during adulthood.35,99 In pathological conditions, there is activation of microglia characterized by increase in their number and change in their morphology and transcriptional activity.20,61,114 Microglia in the SDH also become activated in response to PNI.38,76 Although PNI-induced activation of SDH microglia was described in 1970s46 and later confirmed by several other studies,27,28,33,45 the role of the activated microglia had not been elucidated for long.60 In 2003, 2 studies demonstrated a causal link between activated microglia and neuropathic pain by showing the role of P2X4 receptors (P2X4Rs), a subtype of ionotropic purinergic receptors, and p38 mitogen-activated protein kinases (p38MAPKs) which are upregulated and activated specifically in microglia after PNI.65,135 Thereafter, activated microglia and P2X4R have been recognized as critical players for the pathogenesis of neuropathic pain.61,134
In this review article, we summarize our current insights into the mechanism by which microglial P2X4Rs cause neuropathic pain, focusing on their downstream signaling leading to neuronal hyperexcitability and upstream factors increasing P2X4Rs on microglia. In addition, we also describe the role of microglia and P2X4R in several pathological conditions and their therapeutic potential, based on recent advances in the development of new drugs targeting microglia.
2. Activation of P2X4Rs on activated microglia causes neuropathic pain
Since Burnstock proposed new roles of nucleotides as neurotransmitters,18 there is an increasing body of evidence for important and interesting roles of extracellular adenosine-5′-triphosphate (ATP) in cell-to-cell signaling through purinergic receptors in health and disease.58,112 ATP activates P2 receptors, which are divided into ionotropic (P2XRs) and metabotropic receptors (P2YRs). Seven subunits of P2XRs (P2X1R–P2X7R) have been identified, and these are assembled as trimeric channels. P2XRs are cation-selective channels with almost equal permeability to Na+, K+ and, in some including P2X4R, Ca2+. The flow of ions through P2XRs, altering transmembrane potential and intracellular ion concentrations, is a key machinery for the cell-to-cell signaling.72,73 P2X4Rs are expressed by various tissues in the body12,44,145 including the nervous systems16,151 and have been implicated in physiological role and disease.19,73 In the context of PNI, a selective upregulation of P2X4Rs in microglia activated in the SDH was seen.135 Furthermore, it was found that pharmacological blockade or antisense-oligonucleotide knockdown of P2X4Rs suppresses the PNI-induced mechanical pain hypersensitivity without any effects on acute nociceptive behavioral responses. Further research showed that P2X4R-knockout mice failed to develop the PNI-induced mechanical hypersensitivity.133,139 These reports indicate the role of P2X4R in neuropathic pain. Moreover, intrathecal administration of ATP-stimulated cultured microglia, but not unstimulated microglia, evoked mechanical hypersensitivity in naive animals,128,135 indicating a sufficient role of microglial P2X4R activation in neuropathic pain development.
3. Downstream signaling of P2X4R activation
Findings that non-neuronal cells cause pain hypersensitivity had indicated a possible interaction between microglia and neurons in the SDH. Brain-derived neurotrophic factor (BDNF) was identified as a signaling molecule that is released from microglia stimulated by activation of P2X4Rs. Brain-derived neurotrophic factor acts on tropomyosin-related kinase B (TrkB) expressed on nociceptive projection neurons located in lamina I31,71,139 and decreases expression of K+-Cl– cotransporter 2 (KCC2) that maintains transmembrane Cl– gradient required for GABA or glycine-induced hyperpolarization. The BDNF-induced downregulation of KCC2 led to a depolarizing shift in the anion reversal potential and rendered lamina I neurons excitatory by GABA or glycine.31,32,71 In addition, BDNF also induces potentiation of NMDAR responses through phosphorylation of the NMDAR subunit GluN2B.55 Importantly, this phenomenon can be observed not only in rodents but also in human SDH neurons.36 These changes might collectively contribute to the abnormal excitation of lamina I neurons and subsequently to hypersensitive pain behaviors (Fig. 1). ATP stimulation of microglia through P2X4Rs evokes release of BDNF followed by de novo synthesis and further release of BDNF.129 These are released by exocytosis depending on extracellular Ca2+ and phosphorylation of p38MAPK. Although BDNF is also upregulated in dorsal root ganglion (DRG) neurons after PNI,100 a conditional knockout of BDNF in Nav1.8-positive primary afferent neurons does not affect pain hypersensitivity induced by PNI.160 However, a recent study demonstrated a significant contribution of BDNF derived from DRG neurons, for processing of chronic pain120 This study also found that mice lacking BDNF in all DRG neurons showed a normal development of pain hypersensitivity after PNI. However, a significant recovery of the hypersensitivity was observed only in the conditional BDNF-knockout mice 5 days after PNI, indicating the contribution of DRG-derived BDNF to the chronification of neuropathic pain.120 The critical role of microglial BDNF has also been confirmed by using microglia-selective BDNF-knockout mice.121
Figure 1.
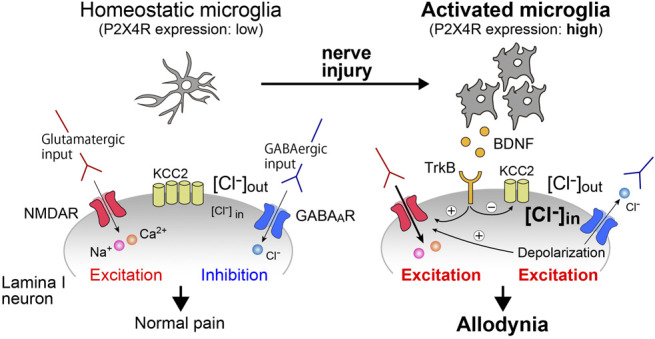
Activated microglia cause an aberrant neuronal excitability that underlies mechanical pain hypersensitivity. The excitability of SDH neurons is determined by a balance between excitatory and inhibitory inputs. After PNI, BDNF released from activated microglia act on TrkB expressed by the neurons, causing down regulation of KCC2 and phosphorylation of NMDA. The alterations result in an aberrant neuronal excitation and mechanical pain hypersensitivity (allodynia). PNI, peripheral nerve injury; [Cl−]i, intracellular Cl− concentration; [Cl−]o, extracellular Cl− concentration, NMDAR, n-methyl-d-aspartate receptor.
Besides BDNF, activated microglia after PNI also release proinflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α, which contribute to neuropathic pain by modulating neuronal excitability.61,64 Released IL-1β acted on SDH neurons and rapidly enhanced the strength of excitatory synaptic transmission through NMDAR phosphorylation and decreased GABA- and glycine-mediated synaptic inhibition.24,70,113,144 Tumor necrosis factor-α rapidly increased glutamatergic transmission within the SDH through tumor necrosis factor-α receptors without changing GABA- or glycine-evoked responses.70 P2X7Rs, fractalkine receptors (CX3CR1), or toll-like receptors on microglia have been extensively studied as key regulators of production and release of the proinflammatory cytokines.25,74,125,132 P2X4R has been reported to regulate inflammation in cultured microglia79,141 and other cell types (macrophages or kidney cells),22,69 but there is so far no direct evidence, indicating that microglial P2X4Rs are necessary for the production of proinflammatory cytokines in the SDH after PNI. Although a recent study reported that CORM-2 (carbon monoxide–releasing molecule), a compound that was used as a P2X4R antagonist in this study, reduces the production of proinflammatory cytokines in the SDH and pain hypersensitivity after PNI,67 another study indicated that antinociceptive effects of CORM-2 are independent of the blockade of P2X4Rs.54 P2X4R has been shown to interact with P2X7R5,13 and to augment the P2X7R-mediated IL-1β release,69 raising the possibility that P2X4R may participate in microglial inflammatory responses through an interaction with P2X7R. Thus, P2X4Rs on activated microglia enhance neuronal excitability and pain transmission primarily through BDNF-TRKB-KCC2 axis-mediated disinhibition61 and also presumably through P2X7R-mediated proinflammatory cytokine production (Fig. 2).
Figure 2.
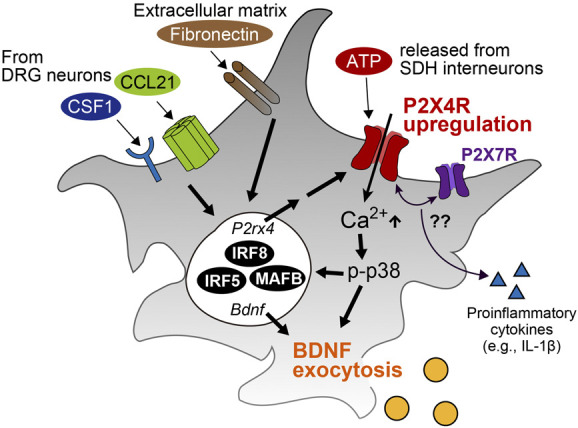
Molecular mechanisms of P2X4R activation leading BDNF and other factor secretion. Extracellular factors (CCL21, CSF1, and fibronectin) and transcriptional factors (IRF8, IRF5, and MAFB) contribute to P2X4R upregulation. ATP released by SDH interneurons activate P2X4Rs and result in BDNF exocytosis. P2X4R may also have a role in production or release of proinflammatory cytokines (eg, IL-1β) through an interaction with P2X7R. IL-1β, interleukin-1β; BDNF, brain-derived neurotrophic factor; DRG, dorsal root ganglion; SDH, spinal dorsal horn.
4. Mechanisms underlying P2X4R upregulation and activation
As mentioned above, microglia proliferate and increase their cell number in response to PNI. The expression of P2X4Rs per cell was upregulated in activated microglia.135 Thus, the number of microglia that have high levels of P2X4Rs increases in the SDH after PNI. Several extracellular factors, intracellular signaling, and transcriptional factors, which are involved in the increase of cell number, upregulation P2X4R, and subsequent pain hypersensitivity, have been identified (Fig. 2).
4.1. Extracellular factors
Chemokine (C-C motif) ligand 21 (CCL21) was found to be induced in the injured DRG neurons and transported to central terminals of the SDH.11 CCL21 increased the expression of P2X4Rs in microglia both under in vitro and in vivo conditions. Peripheral nerve injury–induced upregulation of P2X4Rs was blunted in mice lacking CCL21. CCL21-deficient mice also showed no development of mechanical hypersensitivity after PNI, indicating that CCL21 in DRG neurons contributes to neuropathic pain by upregulating microglial P2X4Rs. On the other hand, CCL21 deficiency did not affect microglial proliferation and morphological changes.11
Recent studies revealed that colony stimulating factor 1 (CSF1) is also a crucial factor for inducing microglial activation.51,102 Like CCL21, CSF1 is also upregulated in DRG neurons after PNI and transported to the SDH, where its receptor (CSF1R) is specifically expressed in microglia. Conditional deletion of CSF1 from DRG neurons suppressed the increase of microglial cell number and development of pain hypersensitivity.51 Moreover, intrathecal administration of CSF1 induced microglial activation (microgliosis and upregulation of pain-related gene expression) and pain hypersensitivity. These results indicate that CSF1 is necessary and sufficient for activation of spinal microglia after PNI. Colony stimulating factor 1 was also found to increase P2X4R expression,51 assuming a possible involvement of CSF1 in producing P2X4R-expressing activated microglia. Although P2X4R deficiency did not suppress CSF1-induced mechanical hypersensitivity at 2 hours after administration, it is conceivable that a P2X4R-independent mechanism may be involved in acute mechanical pain behavior caused by CSF1 at that time point.51
Fibronectin was also shown to be considered as a key regulator of P2X4R expression.98 Fibronectin levels were elevated in the ipsilateral side of the SDH after PNI.98 Exogenous treatment with fibronectin induced the upregulation of microglial P2X4R both in vitro and in vivo.136 Inhibition of fibronectin binding with β1 integrins by echistatin or genetic knockout of Lyn (one of the Src family kinases) resulted in reduced expression of P2X4Rs and impaired pain hypersensitivity,136,137 suggesting the contribution of fibronectin to neuropathic pain through microglial P2X4R upregulation.
4.2. Transcriptional factors
Microglia alter their gene expression pattern along with development of disease processes in the nervous system.90 The profile of gene expression is controlled by transcriptional factors in a context-dependent manner.56 After PNI, interferon regulatory factor (IRF) 8, IRF5, and v-maf avian musculoaponeurotic fibrosarcoma oncogene homolog B (MAFB) critically contribute to the regulation of P2X4R expression in microglia.61 It was found that IRF8 is upregulated in the SDH after PNI, and its expression is restricted to microglia.91 Forced expression of IRF8 in cultured microglia promoted P2X4R expression as well as other pain-related molecules including TLR2, P2Y12R, cathepsin S (CTSS), and CX3CR1. Interferon regulatory factor 8 deficiency blunted pain hypersensitivity and P2X4R upregulation after PNI. Finally, intrathecal administration of microglia overexpressing IRF8, but not mutant IRF8, was sufficient to produce pain hypersensitivity. These results indicate that IRF8 is a critical transcriptional factor to regulate pain-evoking microglia. Interferon regulatory factor 8 was also shown to regulate IRF5 expression by directly binding to its promoter regions.87 Thereafter, it was identified that IRF5 binds to putative promoter region of P2X4R and promotes its expression.87 In this process, fibronectin stimulation of microglia mediated the translocation of IRF5 into the nucleus, which could be involved in the fibronectin-induced P2X4R upregulation. Consistently, genetic ablation of IRF5 dampened the upregulation of P2X4R and development of mechanical pain hypersensitivity.87 In addition to IRF5, IRF1 was also controlled by IRF8. IRF1 regulated the expression of IL-1β,86 which led to pain hypersensitivity by enhancing excitatory synaptic transmission of SDH neurons.70 As the mRNA level of IRF1 was increased in the SDH as well as IL-1β levels after PNI, IRF1 may also be involved in neuropathic pain by enhancing inflammatory responses. Musculoaponeurotic fibrosarcoma oncogene homolog B has been implicated as a critical regulator to maintain the transcriptional profile of homeostatic microglia in adulthood.92 Although MAFB expression was not specific to microglia, PNI-induced upregulation of MAFB protein was selectively observed in microglia.128 Musculoaponeurotic fibrosarcoma oncogene homolog B knockdown by intrathecal treatment with its small interfering RNA prevented the development of mechanical pain behavior and the upregulation of P2X4R expression. Furthermore, conditional knockout of microglial MAFB demonstrated suppression of neuropathic pain development, indicating its contribution to P2X4R-dependent neuropathic pain.128
4.3. Extracellular adenosine-5′-triphosphate
Activation of P2X4Rs (and other purinergic receptors) expressed on the plasma membrane of microglia would need extracellular ATP. Adenosine-5′-triphosphate can be released from a broad range of cell types in the CNS through vesicular release- and/or pannexin or connexin hemichannel-dependent mechanisms.23 By focusing on the role of vesicular nucleotide transporter (VNUT) that was identified as being crucial for ATP exocytosis,116 its essential role in neuropathic pain was revealed.88 Extracellular ATP content was increased in supernatant of spinal cord slices from PNI mice. The ATP increase was suppressed in VNUT-deficient mice. These mice also exhibited reduction of PNI-induced pain. Moreover, these attenuations of biochemical and behavioral alterations observed in VNUT global knockout mice were also observed in mice whose VNUT was selectively deleted from the SDH neurons, but not from astrocytes, microglia, or DRG neurons. Thus, VNUT expressed in the SDH neurons are involved in the increased levels of the extracellular ATP and subsequent pain hypersensitivity.88
5. Role of P2X4R and microglia in other models of chronic pain
Other than PNI models in which peripheral nerves are directly constricted or transected, a variety of other neuropathic pain models has been developed. Although the mechanisms underlying microglial P2X4R-dependent neuropathic pain (described in above sections) have been mainly studied in PNI models, the role of microglial P2X4Rs has also been investigated in other models. Herpes zoster is a viral disease whose symptoms are clustered blisters and mechanical allodynia. In a model of herpetic pain in which herpes simplex virus-1 is inoculated on the skin of mice, mechanical allodynia was developed after the inoculation, and the time course of allodynia was found to correlate with that of P2X4R upregulation in microglia.93 Intrathecal administration of NP-1815-PX, a P2X4R-selective antagonist developed in the study, attenuated the herpetic mechanical allodynia.
Chronic migraine is a disabling headache defined as having more than 15 days of headache per month over a 3-month period. Repeated dural infusions with inflammatory soups induce trigeminal allodynia (decreased periorbital withdrawal threshold to von Frey filaments), which is used as a migraine model, and describes underlying neuronal hyperexcitability in the trigeminal nucleus caudalis.39 In this region, expression of P2X4Rs was upregulated in microglia, and blocking P2X4Rs by 2′,3′-O-(2,4,6-Trinitrophenyl)ATP (TNP-ATP), a nonselective P2X4R antagonist, repressed the BDNF protein upregulation and trigeminal allodynia.80 The P2X4R upregulation was also observed in a model of bone cancer pain, where P2X4R knockdown by its small interfering RNA attenuated pain behavior and reduced expression of BDNF.66
An increase of P2X4R expression was also observed in an experimental autoimmune neuritis model,159 which mirrors pathologies such as mechanical allodynia of acute inflammatory demyelinating polyradiculoneuropathy. The increased P2X4R expression was mainly localized in CD68+ microglia and temporally correlated with the development of pain hypersensitivity, suggesting an involvement of microglial P2X4Rs in mechanical allodynia induced by experimental autoimmune neuritis. A similar correlation between P2X4R expression and pain hypersensitivity has been also reported in a spinal cord injury model118 or an experimental autoimmune encephalomyelitis model.141,156 Thus, P2X4Rs in activated microglia might contribute to neuropathic pain in diverse models.
An important role of microglial P2X4R-BDNF-TrkB-KCC2 pathway was also demonstrated in morphine hyperalgesia, a paradoxical increase in pain sensitivity which hampers an efficient pain control and leads to dose escalation of morphine. Repeated administration of morphine caused a decrease in pain threshold to thermal and mechanical stimuli, increase of nocifensive behavior and microglial activation in the SDH. Ferrini et al. showed that chronic morphine treatment also caused an upregulation of P2X4R expression in vivo and in cultured microglia.42 Furthermore, morphine induced a depolarizing shift in lamina I neurons, which was prevented by coincubation of a TrkB-blocking antibody. These observations indicate that morphine hyperalgesia is attributed to microglial P2X4R-BDNF-TrkB-KCC2 pathway. Indeed, P2X4R-deficient mice did not show morphine hyperalgesia, and pharmacological blockade of P2X4Rs attenuated morphine-induced BDNF release from microglia. Consistently, microglia-selective BDNF knockout abolished morphine hyperalgesia without affecting an antinociceptive response to a single dose of morphine.42 Moreover, it was demonstrated that spinal microglia activated by repeated morphine treatment also play a crucial role for morphine withdrawal.17 Although morphine directly affects microglial activation status and pain transmission, the pathway how it activates microglia remains unclear. A study demonstrated that microglia do not express μ-opioid receptors.30 In addition, the chronic morphine-induced microglial activation (increased cell number and hypertrophic morphology) was evident even in μ-opioid receptor–knockout mice, suggesting that an unidentified receptor is responsible for their activation.30 This is an important question that should be clarified by further investigations.
Besides P2X4R, many molecules expressed by activated microglia have been implicated in pain hypersensitivity caused by some neuropathic pain models. Diabetes mellitus is one of the primary causes of neuropathic pain, and a considerable portion of diabetic patients describe mechanical allodynia. In animal models of diabetes that are developed by streptozotocin (type 1) or by obese (type 2), painful behaviors including allodynia are accompanied by microglia activation which is characterized by the increased cell number and the upregulated marker gene expression in the spinal cord.131 Treatment with minocycline (a tetracycline antibiotic),96 gabapentin (an anticonvulsant drug),149 or inhibitors of an intracellular kinase, which are selectively activated in microglia,138 attenuated allodynia as well as microglial activation, suggesting microglial contribution to diabetic pain hypersensitivity. Spinal activation of microglia was also shown to occur in other neuropathic pain models such as fibromyalgia,3 chemotherapy,101 and chronic fatigue syndrome.153 Pain hypersensitivity observed in these models was attenuated by interfering with microglial activation. In summary, spinal microglia undergo activation in a variety of neuropathic pain models and may contribute to their pathologies.
6. Sex differences
In rodent models of neuropathic pain, it is still under debate that whether there is a sex difference in the role of microglia in pain hypersensitivity or not. Reports have shown that minocycline (a microglial inhibitor),8 by deletion of microglia (and macrophages),107 or genetic removal of microglia-selective molecules (including P2Y12R,50 CX3CR1,122 and transmembrane protein 16F [TMEM16F]10) can suppress PNI-induced pain hypersensitivity in both females and males. In other rodent models of chronic pain, it has demonstrated that there is no obvious sexual dimorphism in the analgesic effects of microglia targeted treatments.61 On the other hand, sex difference in the contribution of spinal microglia to pain has also been shown.53 It was found that although PNI produces microglial activation and pain behaviors to the same extent between the female and male in rodents, the suppressive effect of genetic or pharmacological inhibition of microglial P2X4Rs or its downstream molecules (BDNF and TrkB) in the PNI-induced pain hypersensitivity was observed only in male mice but not in female mice.121 A similar sex difference has also been reported in rats.84 The sexual dimorphism might be dependent on a failure of the PNI-induced upregulation of P2X4R expression in female microglia.84,121 A difference in chromatin accessibility may underlie the transcriptional difference of P2X4R. Interferon regulatory factor 5 and transcriptional factors, which drive microglial P2X4Rs,87 were increased to an equivalent degree in both female and male; however, IRF5 silencing prevented P2X4R upregulation only in male-derived microglial culture. Chromatin immunoprecipitation-quantitative polymerase chain reaction revealed that IRF5 binding affinities at the P2X4R region were increased by PNI only in males but not in females.84 Instead of microglia, adaptive immune cells, such as T cells, function in females to induce pain hypersensitivity at a comparable level that in males.121 However, in a model of herpetic pain, microglial P2X4Rs were found to be increased in female mice,93 suggesting that the sexual dimorphism seems to be dependent on pathological contexts.
7. Pain modulation by P2X4R on other cells
Beside microglia, P2X4R has been reported to be expressed by Schwann cells, peripheral macrophages, and DRG neurons which contribute to chronic pain induced by tissue inflammation. Global knockout of P2X4Rs attenuated pain behavior after intraplantar injection with complete Freund's adjuvant (CFA) is a well-established model of inflammatory pain.133,140 It was also reported that CFA-induced neuronal plasticity within the SDH is a P2X4R-dependent manner.2 Wide-dynamic-range (WDR) neurons located in deeper laminae of the SDH, which respond to both noxious and innocuous stimuli, are known to project into brain. Wide-dynamic-range neurons display a form of sensitization after repetitive inputs from primary afferents, referred as wind-up.115 Complete Freund's adjuvant injection to wild-type mice decreased the threshold for C-fiber-evoked responses and enhanced wind-up amplitude in wide-dynamic-range neurons, which caused facilitated pain transmission. These alterations were absent in P2X4R-deficient mice, and CFA-induced pain was blunted in these mice, suggesting the role of P2X4R in inflammatory pain.2
In the context of inflammatory pain, P2X4Rs on peripheral macrophages also participate in the pathology.140 Macrophages stimulated by ATP released prostaglandin E2 (PGE2), a potent mediator of inflammation triggering pain hypersensitivity and enhancing neuronal excitability, through P2X4Rs and their downstream intracellular Ca2+- and p38MAPK-dependent mechanisms. P2X4Rs were expressed by macrophages in the inflamed hind paw, and an increase in PGE2 contents in the hind paw was suppressed in mice lacking P2X4Rs. Thus, macrophage in inflammatory peripheral tissue may drive sensitization of nociceptors and subsequent pain hypersensitivity by releasing PGE2.140 Macrophage P2X4R in the muscle was shown to be involved in activity-induced muscle pain.103 In PNI models of neuropathic pain, a pharmacological ablation of peripheral macrophages, which accumulated in the DRG or the site of injured nerve, was found to attenuate pain development.26,119,155 Thus, P2X4Rs in macrophages may have a role in PNI-induced pain hypersensitivity, but this remains to be determined.
On the other hand, P2X4Rs on Schwann cells play a beneficial role in remyelination of the sciatic nerve after crush injury. Overexpression of P2X4Rs in Schwann cells resulted in an enhanced recovery of injured nerves. In contrast, pharmacological blockade of P2X4Rs hampered the functional recovery.123 Putting these reports together, P2X4Rs play various functions in a cell-type and context-dependent manner. Further characterization of the role of P2X4R in other cell types will be an important issue for clinical application of drugs targeting P2X4R.
8. Discovery of drug for P2X4R and its signaling
Based on the basic studies described above, microglia and P2X4R have been getting attention as potential therapeutic targets of chronic pain.61 Although there are so far no drugs selectively targeting microglia and their expressing molecules that have been approved for treating neuropathic pain, microglia-selective drug discovery research is in progress.
Several P2X4R antagonists have been developed and are used in basic research.1,158 NP-1815-PX was shown to be a potent selective inhibitor of functioning of P2X4Rs in rodents and human and to attenuate pain hypersensitivity induced by PNI or herpes inoculation.93 More recently, a new antagonist, BAY-1797, was also found to inhibit P2X4R.147 This compound when given orally produced anti-inflammatory and antinociceptive effect in CFA-induced pain. Unfortunately, the development of BAY-1797 was stopped because of its non-negligible effect on CYP3A4 induction.147
In terms of the specificity and selectivity, antibody drugs which bind to target antigen are superior to conventional small molecule drugs. A series of various monoclonal antibodies to mouse and human P2X4R has also been developed.148 Several antibodies inhibited the function of P2X4R channels, while the others potentiated it. Among them, IgG#151-LO inhibited ATP-induced responses through human P2X4Rs expressed on HEK293 cells, and a similar inhibition was observed in native P2X4Rs expressed in human monocytes. Intrathecal administration of IgG#191 that inhibited mouse P2X4Rs reduced pain hypersensitivity caused by PNI in mice. Moreover, systemic administration of IgG#191-Bbbt0626, a CNS-permeable antibody, produced dose-dependent and long-lasting analgesia. However, the CNS-impermeable antibody IgG#191 did not produce any analgesic effects, suggesting that central, but not peripheral, P2X4Rs could be a primary target for its analgesic effect on neuropathic pain.148
As another strategy to discover drugs for chronic pain, much effort has been made to seek new effects of existing drugs. Antidepressants are frequently used to alleviate chronic pain43 presumably through their inhibitory effects on serotonin and/or noradrenaline transporters, which potentiate endogenous descending pain inhibition. However, it was found that paroxetine and duloxetine have an inhibitory effect on P2X4R and that their analgesic effect on the PNI-induced pain hypersensitivity persisted even after serotonin and/or noradrenaline signaling was inhibited by its receptor antagonists or spinal depletion of these neurotransmitters.97,152 Thus, it is possible that these antidepressants produce pain relieving effect, at least in part, through inhibiting microglial P2X4Rs.
More recently, a first-generation bisphosphonate clodronate was shown to have analgesic effects through a mechanism inhibiting VNUT,68 which are responsible for ATP release required for activation of purinergic receptors such as P2X4R and neuropathic pain.88 Clodronate reversed pain hypersensitivity in models of inflammatory and neuropathic pain. The analgesic effect was dependent on inhibition of VNUT because it was occluded in VNUT-deficient mice.68
Preclinical studies have shown that ablating microglia or inhibiting their activation attenuate pain hypersensitivity. For instance, minocycline was used to suppress activated microglia.109,154 Although it can also affect other types of cells including neurons,83 preemptive administrations of minocycline prevented neuropathic pain and microglial activation after perturbation.77,111 Although some clinical studies reported that perioperative administration of minocycline does not improve pain resolution after surgery,34,85 other smaller trials reported slight efficacy of minocycline.124,142 However, further large-scale trials will be needed. Chronic treatments of CSF1R inhibitor, which specifically depletes microglia from the CNS,41 can also alleviate tactile allodynia.78,126 Because clinical trials to evaluate the efficacy of CSF1R inhibitors in cancer are currently underway, depleting activated microglia by such CSF1R inhibitors can be a new treatment for neuropathic pain in the future.
It is also particularly important to determine whether microglia are indeed activated in patients with neuropathic pain. A histological study using the postmortem spinal cord from a patient with long-standing complex regional pain syndrome reported an increased number of CD68-immunoreactive microglia in the posterior horn compared with healthy subjects.37 Positron emission tomography (PET) imaging targeting 18-kD translocator protein (TSPO), which can be expressed by activated microglia, has been used as a technique to detect microglia in the CNS.62 A study using rats with PNI clearly showed the availability of PET imaging with [11C] PK11195, a radiolabeled ligand to TSPO, to quantify glial activation in the spinal cord.59 Accumulation of TSPO was observed in some brain regions of patients with limb denervation,7 complex regional pain syndrome,63 and chronic low back pain.81 One limitation of PET imaging using TSPO is its low specificity to microglia.62 However, a recent study showed that cortical microglia, but not astrocytes, were activated in the brain of patients with fibromyalgia by combining 2 PET tracers, [11C] PBR28 and [11C]-L-deprenyl-D24: the former binds to TSPO, which is expressed on both of microglia and astrocytes, and the latter is considered to reflect astrocytic signals. [11C] PBR28 signal was elevated within some cortical regions in fibromyalgia patients compared with healthy control subjects, whereas no difference between the 2 groups was observed in [11C]-L-deprenyl-D2 signals. These studies suggest that microglia are activated in the CNS of patients with chronic pain.
Several new radiotracers targeting molecules expressed on microglia other than TSPO have been developed for the purpose of conquering the shortcomings of TSPO ligands.62,130,157 There is an article reporting a radiolabeled P2X4R antagonist as a candidate PET tracer, but it did not show adequate selectivity and affinity to P2X4Rs.146 More recently, [11C] CPPC, a radiolabeled ligand that specifically binds to CSF1R, was developed.57 Further investigations using these tracers or development of new tracers are needed to characterize microglial activation in patients with neuropathic pain. Moreover, the improvement of PET imaging technique may allow us to use it as a new diagnostic tool.
9. Conclusion
In 2003, the critical role of P2X4R on microglia was identified as being necessary and sufficient for neuropathic pain. Since then, a large number of studies have revealed the mechanisms underlying P2X4R upregulation and the downstream pathway that leads the hyperexcitability of neurons in the pain transmission pathway.21,61 Furthermore, the P2X4R-BDNF-TrkB-KCC2 pathway has been implicated in several pain models. Because pharmacological or genetical inhibition of these molecules and other microglial genes that are implicated to neuropathic pain result in attenuation of pain hypersensitivity without any major effects in normal nociception in animals, targeting microglia may be a promising strategy for refractory chronic pain. A role of P2X4Rs expressed by DRG neurons or peripheral macrophages in inflammatory pain extends its therapeutic application. On the other hand, microglial P2X4Rs also plays a beneficial role as antidepressants and remyelination associated with a late phase of ischemic stroke and multiple sclerosis, respectively.143,156 A longer term analysis of neuropathic pain pathology and potential involvement of activated microglia in later phase is needed. Recent studies using a single-cell RNA sequencing technique have identified heterogenous subpopulations of microglia in the rodent and human brain.6,89,90 A heterogeneity of activated microglia in the SDH and its transcriptome signature in PNI models and patients with neuropathic pain will help us to develop new therapeutics or diagnostic criteria for neuropathic pain.
Disclosures
The authors have no conflicts of interest to declare.
This work was supported by JSPS KAKENHI Grant Numbers JP19H05658 (M.T.), by the Core Research for Evolutional Science and Technology (CREST) program from AMED under Grant Number JP20gm0910006 (M.T.), and by Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research [BINDS]) from AMED under Grant Number JP20am0101091 (M.T.).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Abdelrahman A, Namasivayam V, Hinz S, Schiedel AC, Köse M, Burton M, El-Tayeb A, Gillard M, Bajorath J, de Ryck M, Müller CE. Characterization of P2X4 receptor agonists and antagonists by calcium influx and radioligand binding studies. Biochem Pharmacol 2017;125:41–54. [DOI] [PubMed] [Google Scholar]
- [2].Aby F, Whitestone S, Landry M, Ulmann L, Fossat P. Inflammatory-induced spinal dorsal horn neurons hyperexcitability is mediated by P2X4 receptors. Pain Rep 2018;3:e660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Akagi T, Matsumura Y, Yasui M, Minami E, Inoue H, Masuda T, Tozaki-Saitoh H, Tamura T, Mizumura K, Tsuda M, Kiyama H, Inoue K. Interferon regulatory factor 8 expressed in microglia contributes to tactile allodynia induced by repeated cold stress in rodents. J Pharmacol Sci 2014;126:172–6. [DOI] [PubMed] [Google Scholar]
- [4].Albrecht DS, Forsberg A, Sandström A, Bergan C, Kadetoff D, Protsenko E, Lampa J, Lee YC, Höglund CO, Catana C, Cervenka S, Akeju O, Lekander M, Cohen G, Halldin C, Taylor N, Kim M, Hooker JM, Edwards RR, Napadow V, Kosek E, Loggia ML. Brain glial activation in fibromyalgia—a multi-site positron emission tomography investigation. Brain Behav Immun 2019;75:72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Antonio LS, Stewart AP, Xu XJ, Varanda WA, Murrell-Lagnado RD, Edwardson JM. P2X4 receptors interact with both P2X2 and P2X7 receptors in the form of homotrimers. Br J Pharmacol 2011;163:1069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Böttcher C, Schlickeiser S, Sneeboer MAM, Kunkel D, Knop A, Paza E, Fidzinski P, Kraus L, Snijders GJL, Kahn RS, Schulz AR, Mei HE, Hol EM, Siegmund B, Glauben R, Spruth EJ, de Witte LD, Priller J, Psy NBB. Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat Neurosci 2019;22:78–90. [DOI] [PubMed] [Google Scholar]
- [7].Banati RB, Cagnin A, Brooks DJ, Gunn RN, Myers R, Jones T, Birch R, Anand P. Long-term trans-synaptic glial responses in the human thalamus after peripheral nerve injury. NeuroReport 2001;12:3439–42. [DOI] [PubMed] [Google Scholar]
- [8].Barragán-Iglesias P, Pineda-Farias JB, Cervantes-Durán C, Bravo-Hernández M, Rocha-González HI, Murbartián J, Granados-Soto V. Role of spinal P2Y6 and P2Y11 receptors in neuropathic pain in rats: possible involvement of glial cells. Mol Pain 2014;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 2009;139:267–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Batti L, Sundukova M, Murana E, Pimpinella S, De Castro Reis F, Pagani F, Wang H, Pellegrino E, Perlas E, Di Angelantonio S, Ragozzino D, Heppenstall Paul A. TMEM16F regulates spinal microglial function in neuropathic pain states. Cell Rep 2016;15:2608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Biber K, Tsuda M, Tozaki-Saitoh H, Tsukamoto K, Toyomitsu E, Masuda T, Boddeke H, Inoue K. Neuronal CCL21 up-regulates microglia P2X4 expression and initiates neuropathic pain development. EMBO J 2011;30:1864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bo X, Kim M, Nori SL, Schoepfer R, Burnstock G, North RA. Tissue distribution of P2X4 receptors studied with an ectodomain antibody. Cell Tissue Res 2003;313:159–65. [DOI] [PubMed] [Google Scholar]
- [13].Boumechache M, Masin M, Edwardson JM, Górecki DC, Murrell-Lagnado R. Analysis of assembly and trafficking of native P2X4 and P2X7 receptor complexes in rodent immune cells. J Biol Chem 2009;284:13446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bourinet E, Altier C, Hildebrand ME, Trang T, Salter MW, Zamponi GW. Calcium-permeable ion channels in pain signaling. Physiol Rev 2014;94:81–140. [DOI] [PubMed] [Google Scholar]
- [15].Braz J, Solorzano C, Wang X, Basbaum Allan I. Transmitting pain and itch messages: a contemporary view of the spinal cord circuits that generate gate control. Neuron 2014;82:522–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Buell G, Lewis C, Collo G, North RA, Surprenant A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO J 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- [17].Burma NE, Bonin RP, Leduc-Pessah H, Baimel C, Cairncross ZF, Mousseau M, Shankara JV, Stemkowski PL, Baimoukhametova D, Bains JS, Antle MC, Zamponi GW, Cahill CM, Borgland SL, De Koninck Y, Trang T. Blocking microglial pannexin-1 channels alleviates morphine withdrawal in rodents. Nat Med 2017;23:355–60. [DOI] [PubMed] [Google Scholar]
- [18].Burnstock G. Purinergic nerves. Pharmacol Rev 1972;24:509–81. [PubMed] [Google Scholar]
- [19].Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov 2008;7:575–90. [DOI] [PubMed] [Google Scholar]
- [20].Butovsky O, Weiner HL. Microglial signatures and their role in health and disease. Nat Rev Neurosci 2018;19:622–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron 2018;100:1292–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen K, Zhang J, Zhang W, Zhang J, Yang J, Li K, He Y. ATP-P2X4 signaling mediates NLRP3 inflammasome activation: a novel pathway of diabetic nephropathy. Int J Biochem Cell Biol 2013;45:932–43. [DOI] [PubMed] [Google Scholar]
- [23].Cisneros-Mejorado A, Perez-Samartin A, Gottlieb M, Matute C. ATP signaling in brain: release, excitotoxicity and potential therapeutic targets. Cell Mol Neurobiol 2015;35:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Clark AK, Gruber-Schoffnegger D, Drdla-Schutting R, Gerhold KJ, Malcangio M, Sandkühler J. Selective activation of microglia facilitates synaptic strength. J Neurosci 2015;35:4552–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Clark AK, Malcangio M. Fractalkine/CX3CR1 signaling during neuropathic pain. Front Cell Neurosci 2014;8:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cobos EJ, Nickerson CA, Gao F, Chandran V, Bravo-Caparrós I, González-Cano R, Riva P, Andrews NA, Latremoliere A, Seehus CR, Perazzoli G, Nieto FR, Joller N, Painter MW, Ma CHE, Omura T, Chesler EJ, Geschwind DH, Coppola G, Rangachari M, Woolf CJ, Costigan M. Mechanistic differences in neuropathic pain modalities revealed by correlating behavior with global expression profiling. Cell Rep 2018;22:1301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmunol 1997;79:163–75. [DOI] [PubMed] [Google Scholar]
- [28].Colburn RW, Rickman AJ, DeLeo JA. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp Neurol 1999;157:289–304. [DOI] [PubMed] [Google Scholar]
- [29].Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C, Kalso E, Bennett DL, Dworkin RH, Raja SN. Neuropathic pain. Nat Rev Dis Primers 2017;3:17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Corder G, Tawfik VL, Wang D, Sypek EI, Low SA, Dickinson JR, Sotoudeh C, Clark JD, Barres BA, Bohlen CJ, Scherrer G. Loss of mu opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nat Med 2017;23:164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Coull JAM, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005;438:1017–21. [DOI] [PubMed] [Google Scholar]
- [32].Coull JAM, Boudreau D, Bachand K, Prescott SA, Nault F, Sík A, Koninck PD, Koninck YD. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 2003;424:938–42. [DOI] [PubMed] [Google Scholar]
- [33].Coyle DE. Partial peripheral nerve injury leads to activation of astroglia and microglia which parallels the development of allodynic behavior. Glia 1998;23:75–83. [PubMed] [Google Scholar]
- [34].Curtin CM, Kenney D, Suarez P, Hentz VR, Hernandez-Boussard T, Mackey S, Carroll IR. A double-blind placebo randomized controlled trial of minocycline to reduce pain after carpal tunnel and trigger finger release. J Hand Surg 2017;42:166–74. [DOI] [PubMed] [Google Scholar]
- [35].Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan W-B. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 2005;8:752–8. [DOI] [PubMed] [Google Scholar]
- [36].Dedek A, Xu J, Kandegedara CM, Lorenzo LÉ, Godin AG, De Koninck Y, Lombroso PJ, Tsai EC, Hildebrand ME. Loss of STEP61 couples disinhibition to N-methyl-d-aspartate receptor potentiation in rodent and human spinal pain processing. Brain 2019;142:1535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Del Valle L, Schwartzman RJ, Alexander G. Spinal cord histopathological alterations in a patient with longstanding complex regional pain syndrome. Brain Behav Immun 2009;23:85–91. [DOI] [PubMed] [Google Scholar]
- [38].Denk F, Crow M, Didangelos A, Lopes Douglas M, McMahon Stephen B. Persistent alterations in microglial enhancers in a model of chronic pain. Cell Rep 2016;15:1771–81. [DOI] [PubMed] [Google Scholar]
- [39].Dodick D, Silberstein S. Central sensitization theory of migraine: clinical implications. Headache 2006;46(suppl 4):S182–191. [DOI] [PubMed] [Google Scholar]
- [40].Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, Ross Sarah E, Lowell Bradford B, Wang Y, Goulding M, Ma Q. Identification of spinal circuits transmitting and gating mechanical pain. Cell 2014;159:1417–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Elmore MRP, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 2014;82:380–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ferrini F, Trang T, Mattioli TAM, Laffray S, Del'Guidice T, Lorenzo LE, Castonguay A, Doyon N, Zhang W, Godin AG, Mohr D, Beggs S, Vandal K, Beaulieu JM, Cahill CM, Salter MW, De Koninck Y. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl− homeostasis. Nat Neurosci 2013;16:183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice ASC, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Garcia-Guzman M, Soto F, Gomez-Hernandez JM, Lund PE, Stühmer W. Characterization of recombinant human P2X4 receptor reveals pharmacological differences to the rat homologue. Mol Pharmacol 1997;51:109–18. [DOI] [PubMed] [Google Scholar]
- [45].Gehrmann J, Monaco S, Kreutzberg GW. Spinal cord microglial cells and DRG satellite cells rapidly respond to transection of the rat sciatic nerve. Restor Neurol Neurosci 1991;2:181–98. [DOI] [PubMed] [Google Scholar]
- [46].Gilmore SA, Skinner RD. Intraspinal non-neuronal cellular responses to peripheral nerve injury. Anat Rec 1979;194:369–87. [DOI] [PubMed] [Google Scholar]
- [47].Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010;330:841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ginhoux F, Prinz M. Origin of microglia: current concepts and past controversies. Cold Spring Harb Perspect Biol 2015;7:a020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald H-R. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015;518:547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Gu N, Eyo UB, Murugan M, Peng J, Matta S, Dong H, Wu LJ. Microglial P2Y12 receptors regulate microglial activation and surveillance during neuropathic pain. Brain Behav Immun 2016;55:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S, Guan AK, Evans-Reinsch Z, Braz J, Devor M, Abboud-Werner SL, Lanier LL, Lomvardas S, Basbaum AI. Injured sensory neuron–derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci 2016;19:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hagemeyer N, Hanft KM, Akriditou MA, Unger N, Park ES, Stanley ER, Staszewski O, Dimou L, Prinz M. Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol 2017;134:441–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Halievski K, Ghazisaeidi S, Salter MW. Sex-dependent mechanisms of chronic pain: a focus on microglia and P2X4R. J Pharmacol Exp Ther 2020;375:202–9. [DOI] [PubMed] [Google Scholar]
- [54].Hervera A, Leanez S, Negrete R, Motterlini R, Pol O. Carbon monoxide reduces neuropathic pain and spinal microglial activation by inhibiting nitric oxide synthesis in mice. PLoS One 2012;7:e43693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hildebrand ME, Xu J, Dedek A, Li Y, Sengar AS, Beggs S, Lombroso PJ, Salter MW. Potentiation of synaptic GluN2B NMDAR currents by fyn kinase is gated through BDNF-mediated disinhibition in spinal pain processing. Cell Rep 2016;17:2753–65. [DOI] [PubMed] [Google Scholar]
- [56].Holtman IR, Skola D, Glass CK. Transcriptional control of microglia phenotypes in health and disease. J Clin Invest 2017;127:3220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Horti AG, Naik R, Foss CA, Minn I, Misheneva V, Du Y, Wang Y, Mathews WB, Wu Y, Hall A, LaCourse C, Ahn HH, Nam H, Lesniak WG, Valentine H, Pletnikova O, Troncoso JC, Smith MD, Calabresi PA, Savonenko AV, Dannals RF, Pletnikov MV, Pomper MG. PET imaging of microglia by targeting macrophage colony-stimulating factor 1 receptor (CSF1R). Proc Natl Acad Sci U S A 2019;116:1686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature 2014;509:310–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Imamoto N, Momosaki S, Fujita M, Omachi S, Yamato H, Kimura M, Kanegawa N, Shinohara S, Abe K. [11C]PK11195 PET imaging of spinal glial activation after nerve injury in rats. Neuroimage 2013;79:121–8. [DOI] [PubMed] [Google Scholar]
- [60].Inoue K, Tsuda M. Microglia and neuropathic pain. Glia 2009;57:1469–79. [DOI] [PubMed] [Google Scholar]
- [61].Inoue K, Tsuda M. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci 2018;19:138–52. [DOI] [PubMed] [Google Scholar]
- [62].Janssen B, Vugts DJ, Windhorst AD, Mach RH. PET imaging of microglial activation-beyond targeting TSPO. Molecules (Basel, Switzerland) 2018;23:607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Jeon SY, Seo S, Lee JS, Choi SH, Lee DH, Jung YH, Song MK, Lee KJ, Kim YC, Kwon HW, Im HJ, Lee DS, Cheon GJ, Kang DH. [11C]-(R)-PK11195 positron emission tomography in patients with complex regional pain syndrome: a pilot study. Medicine 2017;96:e5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 2014;13:533–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci 2003;23:4017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Jin XH, Wang LN, Zuo JL, Yang JP, Liu Sl. P2X4 receptor in the dorsal horn partially contributes to brain-derived neurotrophic factor oversecretion and toll-like receptor-4 receptor activation associated with bone cancer pain. J Neurosci Res 2014;92:1690–702. [DOI] [PubMed] [Google Scholar]
- [67].Jurga AM, Piotrowska A, Makuch W, Przewlocka B, Mika J. Blockade of P2X4 receptors inhibits neuropathic pain-related behavior by preventing MMP-9 activation and, consequently, pronociceptive interleukin release in a rat model. Front Pharmacol 2017;8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kato Y, Hiasa M, Ichikawa R, Hasuzawa N, Kadowaki A, Iwatsuki K, Shima K, Endo Y, Kitahara Y, Inoue T, Nomura M, Omote H, Moriyama Y, Miyaji T. Identification of a vesicular ATP release inhibitor for the treatment of neuropathic and inflammatory pain. Proc Natl Acad Sci U S A 2017;114:E6297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kawano A, Tsukimoto M, Mori D, Noguchi T, Harada H, Takenouchi T, Kitani H, Kojima S. Regulation of P2X7-dependent inflammatory functions by P2X4 receptor in mouse macrophages. Biochem Biophys Res Commun 2012;420:102–7. [DOI] [PubMed] [Google Scholar]
- [70].Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1β, interleukin-6, and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci 2008;28:5189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Keller AF, Beggs S, Salter MW, De Koninck Y. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol Pain 2007;3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature 2006;442:527–32. [DOI] [PubMed] [Google Scholar]
- [73].Khakh Baljit S, North RA. Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron 2012;76:51–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kim D, Kim MA, Cho IH, Kim MS, Lee S, Jo EK, Choi SY, Park K, Kim JS, Akira S, Na HS, Oh SB, Lee SJ. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J Biol Chem 2007;282:14975–83. [DOI] [PubMed] [Google Scholar]
- [75].Koga K, Kanehisa K, Kohro Y, Shiratori-Hayashi M, Tozaki-Saitoh H, Inoue K, Furue H, Tsuda M. Chemogenetic silencing of GABAergic dorsal horn interneurons induces morphine-resistant spontaneous nocifensive behaviours. Sci Rep 2017;7:4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kohno K, Kitano J, Kohro Y, Tozaki-Saitoh H, Inoue K, Tsuda M. Temporal kinetics of microgliosis in the spinal dorsal horn after peripheral nerve injury in rodents. Biol Pharm Bull 2018;41:1096–102. [DOI] [PubMed] [Google Scholar]
- [77].Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. PAIN 2005;115:71–83. [DOI] [PubMed] [Google Scholar]
- [78].Lee S, Shi XQ, Fan A, West B, Zhang J. Targeting macrophage and microglia activation with colony stimulating factor 1 receptor inhibitor is an effective strategy to treat injury-triggered neuropathic pain. Mol Pain 2018;14:1744806918764979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Li F, Wang L, Li JW, Gong M, He L, Feng R, Dai Z, Li SQ. Hypoxia induced amoeboid microglial cell activation in postnatal rat brain is mediated by ATP receptor P2X4. BMC Neurosci 2011;12:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Liu C, Zhang Y, Liu Q, Jiang L, Li M, Wang S, Long T, He W, Kong X, Qin G, Chen L, Zhang Y, Zhou J. P2X4-receptor participates in EAAT3 regulation via BDNF-TrkB signaling in a model of trigeminal allodynia. Mol Pain 2018;14:1744806918795930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, Hill E, Hsu S, Izquierdo-Garcia D, Ji RR, Riley M, Wasan AD, Zürcher NR, Albrecht DS, Vangel MG, Rosen BR, Napadow V, Hooker JM. Evidence for brain glial activation in chronic pain patients. Brain 2015;138(pt 3):604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lu Y, Dong H, Gao Y, Gong Y, Ren Y, Gu N, Zhou S, Xia N, Sun YY, Ji RR, Xiong L. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J Clin Invest 2013;123:4050–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Möller T, Bard F, Bhattacharya A, Biber K, Campbell B, Dale E, Eder C, Gan L, Garden GA, Hughes ZA, Pearse DD, Staal RG, Sayed FA, Wes PD, Boddeke HW. Critical data-based re-evaluation of minocycline as a putative specific microglia inhibitor. Glia 2016;64:1788–94. [DOI] [PubMed] [Google Scholar]
- [84].Mapplebeck JCS, Dalgarno R, Tu Y, Moriarty O, Beggs S, Kwok CHT, Halievski K, Assi S, Mogil JS, Trang T, Salter MW. Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. PAIN 2018;159:1752–63. [DOI] [PubMed] [Google Scholar]
- [85].Martinez V, Szekely B, Lemarié J, Martin F, Gentili M, Ben Ammar S, Lepeintre JF, Garreau de Loubresse C, Chauvin M, Bouhassira D, Fletcher D. The efficacy of a glial inhibitor, minocycline, for preventing persistent pain after lumbar discectomy: a randomized, double-blind, controlled study. PAIN 2013;154:1197–203. [DOI] [PubMed] [Google Scholar]
- [86].Masuda T, Iwamoto S, Mikuriya S, Tozaki-Saitoh H, Tamura T, Tsuda M, Inoue K. Transcription factor IRF1 is responsible for IRF8-mediated IL-1β expression in reactive microglia. J Pharmacol Sci 2015;128:216–20. [DOI] [PubMed] [Google Scholar]
- [87].Masuda T, Iwamoto S, Yoshinaga R, Tozaki-Saitoh H, Nishiyama A, Mak TW, Tamura T, Tsuda M, Inoue K. Transcription factor IRF5 drives P2X4R+-reactive microglia gating neuropathic pain. Nat Commun 2014;5:3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Masuda T, Ozono Y, Mikuriya S, Kohro Y, Tozaki-Saitoh H, Iwatsuki K, Uneyama H, Ichikawa R, Salter MW, Tsuda M, Inoue K. Dorsal horn neurons release extracellular ATP in a VNUT-dependent manner that underlies neuropathic pain. Nat Commun 2016;7:12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Masuda T, Sankowski R, Staszewski O, Böttcher C, Amann L, Sagar, Scheiwe C, Nessler S, Kunz P, van Loo G, Coenen VA, Reinacher PC, Michel A, Sure U, Gold R, Grün D, Priller J, Stadelmann C, Prinz M. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 2019;566:388–92. [DOI] [PubMed] [Google Scholar]
- [90].Masuda T, Sankowski R, Staszewski O, Prinz M. Microglia heterogeneity in the single-cell era. Cell Rep 2020;30:1271–81. [DOI] [PubMed] [Google Scholar]
- [91].Masuda T, Tsuda M, Yoshinaga R, Tozaki-Saitoh H, Ozato K, Tamura T, Inoue K. IRF8 is a critical transcription factor for transforming microglia into a reactive phenotype. Cell Rep 2012;1:334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S, Ben-Yehuda H, David E, Zelada González F, Perrin P, Keren-Shaul H, Gury M, Lara-Astaiso D, Thaiss CA, Cohen M, Bahar Halpern K, Baruch K, Deczkowska A, Lorenzo-Vivas E, Itzkovitz S, Elinav E, Sieweke MH, Schwartz M, Amit I. Microglia development follows a stepwise program to regulate brain homeostasis. Science 2016;353:aad8670. [DOI] [PubMed] [Google Scholar]
- [93].Matsumura Y, Yamashita T, Sasaki A, Nakata E, Kohno K, Masuda T, Tozaki-Saitoh H, Imai T, Kuraishi Y, Tsuda M, Inoue K. A novel P2X4 receptor-selective antagonist produces anti-allodynic effect in a mouse model of herpetic pain. Sci Rep 2016;6:32461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150:971–9. [DOI] [PubMed] [Google Scholar]
- [95].Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci 2002;22:6724–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Morgado C, Pereira-Terra P, Cruz CD, Tavares I. Minocycline completely reverses mechanical hyperalgesia in diabetic rats through microglia-induced changes in the expression of the potassium chloride co-transporter 2 (KCC2) at the spinal cord. Diabetes Obes Metab 2011;13:150–9. [DOI] [PubMed] [Google Scholar]
- [97].Nagata K, Imai T, Yamashita T, Tsuda M, Tozaki-Saitoh H, Inoue K. Antidepressants inhibit P2X4 receptor function: a possible involvement in neuropathic pain relief. Mol Pain 2009;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Nasu-Tada K, Koizumi S, Tsuda M, Kunifusa E, Inoue K. Possible involvement of increase in spinal fibronectin following peripheral nerve injury in upregulation of microglial P2X4, a key molecule for mechanical allodynia. Glia 2006;53:769–75. [DOI] [PubMed] [Google Scholar]
- [99].Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005;308:1314–18. [DOI] [PubMed] [Google Scholar]
- [100].Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K. The effect of site and type of nerve injury on the expression of brain-derived neurotrophic factor in the dorsal root ganglion and on neuropathic pain behavior. Neuroscience 2006;137:961–70. [DOI] [PubMed] [Google Scholar]
- [101].Ochi-ishi R, Nagata K, Inoue T, Tozaki-Saitoh H, Tsuda M, Inoue K. Involvement of the chemokine CCL3 and the purinoceptor P2X7 in the spinal cord in paclitaxel-induced mechanical allodynia. Mol Pain 2014;10:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Okubo M, Yamanaka H, Kobayashi K, Dai Y, Kanda H, Yagi H, Noguchi K. Macrophage-colony stimulating factor derived from injured primary afferent induces proliferation of spinal microglia and neuropathic pain in rats. PLoS One 2016;11:e0153375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Oliveira-Fusaro MC, Gregory NS, Kolker SJ, Rasmussen L, Allen LAH, Sluka KA. P2X4 receptors on muscle macrophages are required for development of hyperalgesia in an animal model of activity-induced muscle pain. Mol Neurobiol 2020;57:1917–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science 2011;333:1456–8. [DOI] [PubMed] [Google Scholar]
- [105].Peirs C, Seal RP. Neural circuits for pain: recent advances and current views. Science 2016;354:578–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Peirs C, Williams S-Paul G, Zhao X, Walsh Claire E, Gedeon Jeremy Y, Cagle Natalie E, Goldring Adam C, Hioki H, Liu Z, Marell Paulina S, Seal Rebecca P. Dorsal horn circuits for persistent mechanical pain. Neuron 2015;87:797–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Peng J, Gu N, Zhou L, B Eyo U, Murugan M, Gan WB, Wu LJ. Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury. Nat Commun 2016;7:12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Petitjean H, Pawlowski SA, Fraine SL, Sharif B, Hamad D, Fatima T, Berg J, Brown CM, Jan LY, Ribeiro-da-Silva A, Braz JM, Basbaum AI, Sharif-Naeini R. Dorsal horn parvalbumin neurons are gate-keepers of touch-evoked pain after nerve injury. Cell Rep 2015;13:1246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Plane JM, Shen Y, Pleasure DE, Deng W. Prospects for minocycline neuroprotection. Arch Neurol 2010;67:1442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Prinz M, Jung S, Priller J. Microglia biology: one century of evolving concepts. Cell 2019;179:292–311. [DOI] [PubMed] [Google Scholar]
- [111].Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther 2003;306:624–30. [DOI] [PubMed] [Google Scholar]
- [112].Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 1998;50:413–92. [PubMed] [Google Scholar]
- [113].Reeve AJ, Patel S, Fox A, Walker K, Urban L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur J Pain 2000;4:247–57. [DOI] [PubMed] [Google Scholar]
- [114].Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med 2017;23:1018–27. [DOI] [PubMed] [Google Scholar]
- [115].Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev 2009;89:707–58. [DOI] [PubMed] [Google Scholar]
- [116].Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci U S A 2008;105:5683–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, Moore KA, Decosterd I, Coggeshall RE, Woolf CJ. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci 2005;25:7317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Schwab JM, Guo L, Schluesener HJ. Spinal cord injury induces early and persistent lesional P2X4 receptor expression. J Neuroimmunol 2005;163:185–9. [DOI] [PubMed] [Google Scholar]
- [119].Shepherd AJ, Mickle AD, Golden JP, Mack MR, Halabi CM, de Kloet AD, Samineni VK, Kim BS, Krause EG, Gereau RW, Mohapatra DP. Macrophage angiotensin II type 2 receptor triggers neuropathic pain. Proc Natl Acad Sci U S A 2018;115:E8057–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Sikandar S, Minett MS, Millet Q, Santana-Varela S, Lau J, Wood JN, Zhao J. Brain-derived neurotrophic factor derived from sensory neurons plays a critical role in chronic pain. Brain 2018;141:1028–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 2015;18:1081–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Staniland AA, Clark AK, Wodarski R, Sasso O, Maione F, D'Acquisto F, Malcangio M. Reduced inflammatory and neuropathic pain and decreased spinal microglial response in fractalkine receptor (CX3CR1) knockout mice. J Neurochem 2010;114:1143–57. [DOI] [PubMed] [Google Scholar]
- [123].Su WF, Wu F, Jin ZH, Gu Y, Chen YT, Fei Y, Chen H, Wang YX, Xing LY, Zhao YY, Yuan Y, Tang X, Chen G. Overexpression of P2X4 receptor in Schwann cells promotes motor and sensory functional recovery and remyelination via BDNF secretion after nerve injury. Glia 2019;67:78–90. [DOI] [PubMed] [Google Scholar]
- [124].Syngle A, Verma I, Krishan P, Garg N, Syngle V. Minocycline improves peripheral and autonomic neuropathy in type 2 diabetes: MIND study. Neurol Sci 2014;35:1067–73. [DOI] [PubMed] [Google Scholar]
- [125].Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A 2005;102:5856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Thompson ML, Jimenez-Andrade JM, Chartier S, Tsai J, Burton EA, Habets G, Lin PS, West BL, Mantyh PW. Targeting cells of the myeloid lineage attenuates pain and disease progression in a prostate model of bone cancer. PAIN 2015;156:1692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 2010;11:823–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Tozaki-Saitoh H, Masuda J, Kawada R, Kojima C, Yoneda S, Masuda T, Inoue K, Tsuda M. Transcription factor MafB contributes to the activation of spinal microglia underlying neuropathic pain development. Glia 2019;67:729–40. [DOI] [PubMed] [Google Scholar]
- [129].Trang T, Beggs S, Wan X, Salter MW. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci 2009;29:3518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Tronel C, Largeau B, Santiago Ribeiro MJ, Guilloteau D, Dupont AC, Arlicot N. Molecular targets for PET imaging of activated microglia: the current situation and future expectations. Int J Mol Sci 2017;18:802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Tsuda M. Microglia in the spinal cord and neuropathic pain. J Diabetes Investig 2016;7:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Tsuda M. P2 receptors, microglial cytokines and chemokines, and neuropathic pain. J Neurosci Res 2017;95:1319–29. [DOI] [PubMed] [Google Scholar]
- [133].Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, Inoue K. Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain 2009;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Tsuda M, Masuda T, Tozaki-Saitoh H, Inoue K. P2X4 receptors and neuropathic pain. Front Cell Neurosci 2013;7:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 2003;424:778–83. [DOI] [PubMed] [Google Scholar]
- [136].Tsuda M, Toyomitsu E, Komatsu T, Masuda T, Kunifusa E, Nasu-Tada K, Koizumi S, Yamamoto K, Ando J, Inoue K. Fibronectin/integrin system is involved in P2X4 receptor upregulation in the spinal cord and neuropathic pain after nerve injury. Glia 2008;56:579–85. [DOI] [PubMed] [Google Scholar]
- [137].Tsuda M, Toyomitsu E, Kometani M, Tozaki-Saitoh H, Inoue K. Mechanisms underlying fibronectin-induced up-regulation of P2X4R expression in microglia: distinct roles of PI3K–Akt and MEK–ERK signalling pathways. J Cell Mol Med 2009;13:3251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Tsuda M, Ueno H, Kataoka A, Tozaki-Saitoh H, Inoue K. Activation of dorsal horn microglia contributes to diabetes-induced tactile allodynia via extracellular signal-regulated protein kinase signaling. Glia 2008;56:378–86. [DOI] [PubMed] [Google Scholar]
- [139].Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci 2008;28:11263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Ulmann L, Hirbec H, Rassendren F. P2X4 receptors mediate PGE2 release by tissue-resident macrophages and initiate inflammatory pain. EMBO J 2010;29:2290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Vázquez-Villoldo N, Domercq M, Martín A, Llop J, Gómez-Vallejo V, Matute C. P2X4 receptors control the fate and survival of activated microglia. Glia 2014;62:171–84. [DOI] [PubMed] [Google Scholar]
- [142].Vanelderen P, Van Zundert J, Kozicz T, Puylaert M, De Vooght P, Mestrum R, Heylen R, Roubos E, Vissers K. Effect of minocycline on lumbar radicular neuropathic pain: a randomized, placebo-controlled, double-blind clinical trial with amitriptyline as a comparator. Anesthesiology 2015;122:399–406. [DOI] [PubMed] [Google Scholar]
- [143].Verma R, Cronin CG, Hudobenko J, Venna VR, McCullough LD, Liang BT. Deletion of the P2X4 receptor is neuroprotective acutely, but induces a depressive phenotype during recovery from ischemic stroke. Brain Behav Immun 2017;66:302–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1β enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci 2003;23:8692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Wang CZ, Namba N, Gonoi T, Inagaki N, Seino S. Cloning and pharmacological characterization of a fourth P2X receptor subtype widely expressed in brain and peripheral tissues including various endocrine tissues. Biochem Biophys Res Commun 1996;220:196–202. [DOI] [PubMed] [Google Scholar]
- [146].Wang M, Gao M, Meyer JA, Peters JS, Zarrinmayeh H, Territo PR, Hutchins GD, Zheng QH. Synthesis and preliminary biological evaluation of radiolabeled 5-BDBD analogs as new candidate PET radioligands for P2X4 receptor. Bioorg Med Chem 2017;25:3835–44. [DOI] [PubMed] [Google Scholar]
- [147].Werner S, Mesch S, Hillig RC, ter Laak A, Klint J, Neagoe I, Laux-Biehlmann A, Dahllöf H, Bräuer N, Puetter V, Nubbemeyer R, Schulz S, Bairlein M, Zollner TM, Steinmeyer A. Discovery and characterization of the potent and selective P2X4 inhibitor N-[4-(3-Chlorophenoxy)-3-sulfamoylphenyl]-2-phenylacetamide (BAY-1797) and structure-guided amelioration of its CYP3A4 induction profile. J Med Chem 2019;62:11194–217. [DOI] [PubMed] [Google Scholar]
- [148].Williams WA, Linley JE, Jones CA, Shibata Y, Snijder A, Button J, Hatcher JP, Huang L, Taddese B, Thornton P, Schofield DJ, Thom G, Popovic B, Dosanjh B, Wilkinson T, Hughes J, Dobson CL, Groves MA, Webster CI, Billinton A, Vaughan TJ, Chessell I. Antibodies binding the head domain of P2X4 inhibit channel function and reverse neuropathic pain. PAIN 2019;160:1989–2003. [DOI] [PubMed] [Google Scholar]
- [149].Wodarski R, Clark AK, Grist J, Marchand F, Malcangio M. Gabapentin reverses microglial activation in the spinal cord of streptozotocin-induced diabetic rats. Eur J Pain 2009;13:807–11. [DOI] [PubMed] [Google Scholar]
- [150].Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science 2000;288:1765–8. [DOI] [PubMed] [Google Scholar]
- [151].Xu J, Bernstein AM, Wong A, Lu X-H, Khoja S, Yang XW, Davies DL, Micevych P, Sofroniew MV, Khakh BS. P2X4 receptor reporter mice: sparse brain expression and feeding-related presynaptic facilitation in the arcuate nucleus. J Neurosci 2016;36:8902–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Yamashita T, Yamamoto S, Zhang J, Kometani M, Tomiyama D, Kohno K, Tozaki-Saitoh H, Inoue K, Tsuda M. Duloxetine inhibits microglial P2X4 receptor function and alleviates neuropathic pain after peripheral nerve injury. PLoS One 2016;11:e0165189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Yasui M, Yoshimura T, Takeuchi S, Tokizane K, Tsuda M, Inoue K, Kiyama H. A chronic fatigue syndrome model demonstrates mechanical allodynia and muscular hyperalgesia via spinal microglial activation. Glia 2014;62:1407–17. [DOI] [PubMed] [Google Scholar]
- [154].Yrjänheikki J, Keinänen R, Pellikka M, Hökfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A 1998;95:15769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Yu X, Liu H, Hamel KA, Morvan MG, Yu S, Leff J, Guan Z, Braz JM, Basbaum AI. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun 2020;11:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Zabala A, Vazquez-Villoldo N, Rissiek B, Gejo J, Martin A, Palomino A, Perez-Samartín A, Pulagam KR, Lukowiak M, Capetillo-Zarate E, Llop J, Magnus T, Koch-Nolte F, Rassendren F, Matute C, Domercq M. P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis. EMBO Mol Med 2018;10:e8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Zarrinmayeh H, Territo PR. Purinergic receptors of the central nervous system: biology, PET ligands, and their applications. Mol Imaging 2020;19:1536012120927609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Zhang WJ, Zhu ZM, Liu ZX. The role of P2X4 receptor in neuropathic pain and its pharmacological properties. Pharmacol Res 2020;158:104875. [DOI] [PubMed] [Google Scholar]
- [159].Zhang Z, Zhang ZY, Fauser U, Schluesener HJ. Mechanical allodynia and spinal up-regulation of P2X4 receptor in experimental autoimmune neuritis rats. Neuroscience 2008;152:495–501. [DOI] [PubMed] [Google Scholar]
- [160].Zhao J, Seereeram A, Nassar MA, Levato A, Pezet S, Hathaway G, Morenilla-Palao C, Stirling C, Fitzgerald M, McMahon SB, Rios M, Wood JN, London Pain C. Nociceptor-derived brain-derived neurotrophic factor regulates acute and inflammatory but not neuropathic pain. Mol Cell Neurosci 2006;31:539–48. [DOI] [PubMed] [Google Scholar]


