Abstract
Background
Left ventricular (LV) aneurysms complicate anterior myocardial infarctions (MIs) in 8–15% of cases. In case of associated LV dysfunction, rapidly evolving heart failure may follow, and urgent surgery becomes life-saving.
Case summary
Following an acute anterior MI treated by percutaneous coronary intervention, which resulted in apical hypokinesis, depressed LV function, and moderate mitral regurgitation, a 70-year-old male patient kept in contact with our cardiology department through phone calls. Over 6 weeks, the patient's conditions worsened. For fear of contracting COVID-19, he refused to attend to the Emergency Room. Conditions did not improve despite medical therapy adjustments, and he was admitted to hospital following a syncope. Computed tomography scan revealed pneumonia, and he was placed in a ‘grey’ ward while waiting for nose-swab results for COVID-19. A rapid escalation of treatment was necessary as conditions did not improve with low-dose inotropes, and he required invasive ventilation. An Impella 5.0 was implanted as support prior to surgery, was maintained during the procedure and as a means of weaning off extracorporeal circulation. Surgery was successful and Impella 5.0 was removed on postoperative Day 5.
Discussion
To date, Impella use in cardiothoracic surgery has been described in case of ventricular septal rupture or as a bridge to permanent LV assist device. In our case, Impella 5.0 was implanted, used as a bridge to surgery, and as postoperative support in a patient with evolving cardiogenic shock due to LV aneurysm and depressed LV ejection fraction following acute MI, in the difficult setting of the COVID-19 pandemic.
Keywords: Case report, COVID-19, Cardiogenic shock, Ventricular aneurysm, Impella
Learning points
Safe, clean, pathways for high-risk patients must be created even in critical situations such as the ongoing COVID-19 pandemic to prevent increase in morbidity and mortality in patients not affected by the virus itself.
Left ventricular (LV) remodelling with apical aneurysm and consequent LV dysfunction is a complication of acute anterior myocardial infarction which must be recognized and promptly treated to prevent evolution to acute heart failure and cardiogenic shock.
In case of ongoing cardiogenic shock, support with a short-term mechanical circulatory device is adequate to both support the patient in the acute phase and to optimize the heart–lung system for high-risk surgery.
Introduction
Left ventricular (LV) aneurysms complicate 8–15% of ST-elevation myocardial infarctions (MIs).1 Global ventricular function may be compromised to various extents, and consequent functional mitral regurgitation may further complicate haemodynamics.2 From a clinical standpoint, patients may present with signs of progressive heart failure, or with rapidly evolving cardiogenic shock.3 In the latter cases, urgent cardiac surgery becomes life-saving.
This report describes a case of rapidly evolving cardiogenic shock in a patient with LV aneurysm, severely depressed LV function, and functional mitral regurgitation, in which short-term mechanical circulatory support was used as a bridge to surgery, kept during surgery itself, and as means to aid weaning off extracorporeal circulation, in the complex setting of the ongoing COVID-19 pandemic.
Timeline
Table 1.
Biochemical and haemodynamic parameters and pharmacological support at arrival, after Impella 5.0 positioning
| Parameter | Arrival | Post-Impella 5.0 | Post-surgery |
|---|---|---|---|
| Central venous pressure (mmHg) | 20 | 12 | 12 |
| Lactates (mmol/L) | 3.5 | 1.3 | 2.6 |
| Creatinine (mg/dL) | 2.39 | 1.52 | 1.8 |
| Brain natriuretic peptide (pg/mL) | 2557 | 865 | 685 |
| Tricuspid annular plane systolic excursion (mm) | 17 | 20 | 20 |
| Inferior vena cava (mm) | 24 | 18 | 20 |
| IVC (%) | 0 | 50 | 50 |
| Pulomary artery pressure (mmHg) | 75 | 45 | 35 |
| Noradrenalin (μg/kg/min) | 0.5 | 0.02 | 0.02 |
| Adrenalin (μg/kg/min) | 0.5 | 0.01 | 0.01 |
| Furosemide (mg/h) | 10 | 20 | 20 |
ER, emergency room; IABP, intra-aortic balloon pump; LV, left ventricular.
Case presentation
Following an acute anterior MI treated with angioplasty on proximal left anterior descending artery (LAD) in mid-February, a 70-year-old male patient with no past relevant medical history kept in contact with our cardiology department through regular phone calls. Pre-discharge echocardiography showed normal LV dimensions, severely depressed LV function (ejection fraction 35%) due to apical and anterior hypokinesis, normal right ventricular dimensions, and function. Medication consisted of metoprolol, torasemide, ramipril, spironolactone, acetylsalicylic acid, clopidogrel, and transdermic nitroglycerine.
Over the course of approximately 6 weeks post-discharge, the patients’ general conditions progressively worsened, and he complained of dyspnoea, fatigue, and ongoing hypotension. For fear of contracting COVID-19 pneumonia, the patient repeatedly refused to attend the Emergency Room, despite medical advice to do so. Conditions did not improve despite medical therapy adjustments (increase in oral diuretics and start on ranolazine), and the patient was finally admitted to hospital following a syncope. Physical examination revealed hypotension, mild apical systolic murmur, bilateral lung crackles, and ankle oedema. Blood exams showed elevated BNP (1500 pg/mL) and creatinin (2.39 ml/dL), and high-sensitivity Troponin I within normal limits. Due to a computed tomography scan which revealed bilateral pleural effusion and possible bilateral pneumonia (Figure 1), he was placed in a general ‘grey’ ward while waiting for nose-swab results, as he could not be placed in the ‘red’ intensive care unit (ICU), dedicated to COVID patients for his own safety, but could not also be considered COVID-free and thus admitted to the ‘white’ (clean) ICU.
Figure 1.
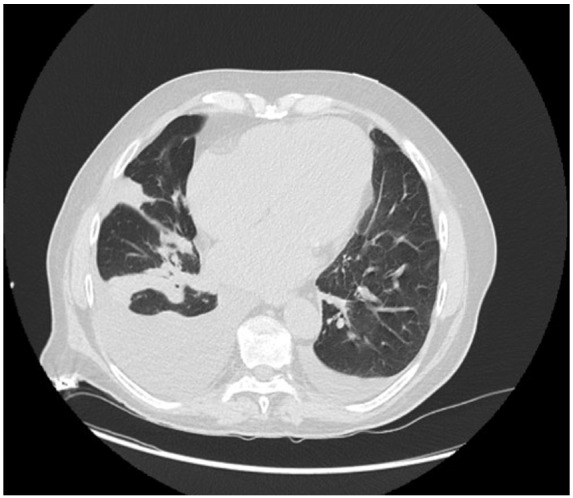
Chest computed tomography scan showing bilateral pleural effusion and possible underlying pneumonia.
Overnight, a rapid escalation of treatment was necessary as conditions did not improve with low-dose inotropes (Dobutamine and Dopamine), and he required intubation and admittance to the white ICU once the nose-swab came back clean (after ∼12 h). Emergency coronary angiography was performed, which confirmed absence of restenosis, and Intra-Aortic Ballon Pump was placed as emergency circulatory support. High-dose inotropic support (Adrenalin and Noradrenalin) was necessary to maintain sufficient haemodynamics. Bedside echocardiography was performed, and confirmed a severely dilated [end-diastolic volume (EDV) = 198 ml, indicized end-diastolic volume (iEDV) = 94 ml/m2], hypokinetic left ventricle with apical aneurysm (distal part of the cavity, inferior to papillary muscle implant, Figure 2), ejection fraction of 30%, no LV thrombus, moderate mitral regurgitation due to multiple, central jets and annular dilatation and increased LV filling pressures. Right ventricular dimensions and function were within normal range, with severely increased pulmonary artery pressures (60 + 15 mmHg).
Figure 2.
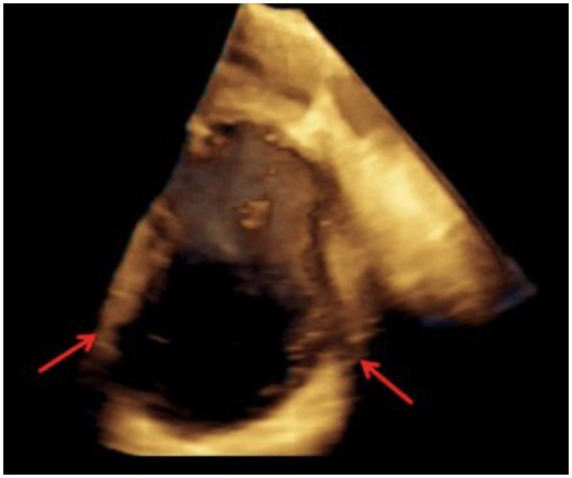
Preoperative trans-oesophageal echocardiogram revealing left ventricular apical aneurysm (arrows).
Chest X-ray revealed severe pulmonary congestion. Furthermore, the patient developed sustained ventricular arrhythmias.
An emergency Heart Team was arranged, where it appeared clear that surgical correction of the apical aneurysm and mitral regurgitation were mandatory to allow clinical stabilization and patient discharge. However, pulmonary congestion and consequent hypertension, associated to the high inotropic dosages required to maintain haemodynamic stability, posed a severe threat to postoperative management. It was thus decided to support the patient with a short-term mechanical circulatory support, to allow optimal bridging to cardiac surgery.
An Impella 5.0 (via surgical cut-down of the subclavian artery4) was implanted, with the aim of reducing pulmonary congestion and treating the ventricular arrhythmias prior to scheduled ventriculoplasty and mitral valve repair. Intraprocedural trans-oesophageal echocardiography confirmed correct positioning of the device and no interference with the aortic valve function.
Following Impella 5.0 implantation, we immediately observed (Table 1): resolution of sustained ventricular arrhythmias; reduction of pulmonary congestion; reduction of pulmonary artery pressure by about 20 mmHg, although PCWP (Pulmonary Capillary Wedge Pressure) remained high, around 20 mmHg. Right ventricular dimensions and function remained within normal limits.
| Discharge after anterior myocardial infarction treated with percutaneous coronary angioplasty on left anterior descending artery (LAD) |
Complained of fatigue, dyspnoea, hypotension. Refused access to emergency room (ER) because of fear of contracting COVID-19. Down-titration of medical therapy |
| Access to the ER following syncope |
Evidence of interstitial pneumonia on computed tomography scan. Rapid evolution of Cardiogenic Shock with need for intra-aortic balloon pump (IABP) implant, high-dose inotropes. |
| Twelve hours later—nose-swab tested negative for COVID-19 |
Access to ‘clean’ intensive care unit (ICU), evidence of left ventricular (LV) aneurysm and dysfunction, mitral regurgitation, pulmonary congestion, and hypertension, sustained ventricular arrhythmias. Heart Team: decision to implant Impella 5.0 before cardiac surgery of ventriculoplasty and mitral valve repair. |
| Forty-eight hours after Impella 5.0 support |
Reduction in pulmonary congestion, mitral regurgitation, LV filling pressures, termination of arrhythmias. Cardiac surgery with Impella 5.0 support during surgery and weaning off by-pass with minimal inotropic support. |
| Postoperative Day 5 | Weaning off Impella 5.0 with aid of IABP. |
Amelioration of general haemodynamic status which allowed a down-titration of inotropes to minimal levels; anticoagulation was maintained through continuous heparin infusion with a target activated partial thromboplastin time (aPTT) of 50–70 s.
After 48 h of support, the patient’s conditions appeared optimal to undergo surgical correction of the LV apical aneurysm and mitral regurgitation. The Impella 5.0 was maintained during the procedure (Figure 3), after being advanced forward to allow aortic cross-clamping despite the 9F catheter in the aortic lumen. The aneurysmatic segment was excluded with a Dacron Velour patch (with reduction of the LV cavity from 198 to 110 mL), mitral valve was repaired with annuloplasty and cryoablation of both the aneurysmatic neck and pulmonary veins was performed (Figure 4).
Figure 3.
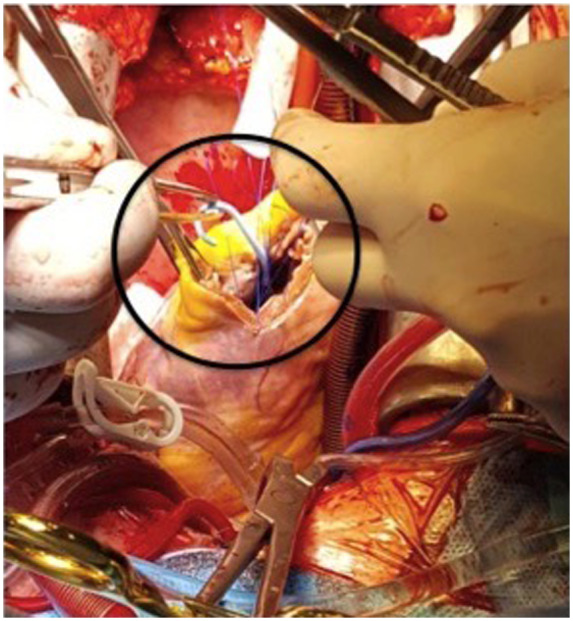
Intraoperative image of Impella 5.0 in the left ventricular apex.
Figure 4.
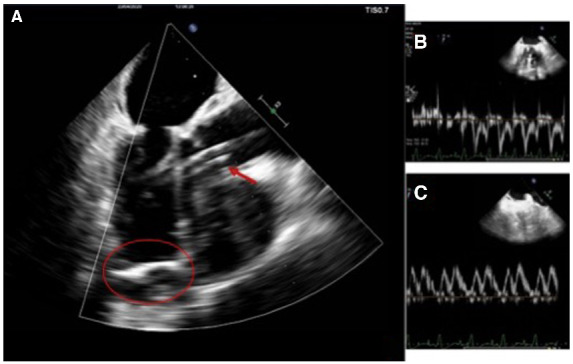
Intraoperative trans-oesophageal echocardiogram showing final surgical results: (A) Left ventricular pericardial patch (circled) and Impella 5.0 support (arrow); (B) Mitral inflow; and (C) PVF (Pulmonary Vein Filling) which shows non-restrictive left ventricular filling pattern.
Surgery was successful and the patient was weaned off the Impella 5.0 with the aid of intra-aortic balloon pump (IABP) on postoperative Day 5. The intermediate step of IABP positioning was felt necessary due to the development of non-negligible aortic regurgitation and the significant and constant increase in PCWP that threatened haemodynamic stability at Impella flow lower than 2.1 L/min. The patient was weaned off the ventilator on postoperative Day 10 and discharged from the ICU in the cardiac rehabilitation ward on postoperative Day 21. He was discharged home after a 1-month rehabilitation programme and is currently being regularly followed in the cardiological outpatient clinic: his condition appears stable.
Discussion
Short-term mechanical support devices (mainly Impella 2.5 and Impella-CP) have previously been used as a bridge to surgery, as a bridge to definitive mechanical circulatory support or in the setting of ventricular septal rupture following acute MI (in the latter setting, its use is in fact debated as LV unloading might cause right to left shunt).5 Maintenance of the device during surgery has never been reported yet.
In our case, with rapidly evolving cardiogenic shock due to late hospitalization subsequent to the COVID-19 pandemic, it appeared mandatory to both support patient haemodynamics in the acute phase and optimally prepare the patient for high-risk cardiac surgery, through reduction of pulmonary congestion, right ventricular overload, and LV filling pressures.
The option of percutaneous Impella (2.5 or CP) was evaluated but felt inadequate to the situation. Moreover, different trials and meta-analysis have tried unsuccessfully to prove that minor Impella is better than IABP,6,7 triggering multiple access of scepticism.
Paracorporeal LVAD implant was considered but excluded because of suboptimal access to the LV apex due to the aneurysm. Furthermore, this approach precluded postoperative support once the apex was remodelled. Extra-corporeal membrane oxygenation (ECMO) was deemed unsuitable as it would not allow LV unloading and thus would be ineffective in reducing pulmonary congestion and ventricular arrhythmias.
The most interesting option thus appeared to be supporting the patient through an Impella 5.0 device.
In this patient, the Impella 5.0 proved to be effective in the pre-surgical phase, by supporting haemodynamics, reducing pulmonary congestion, and pulmonary artery pressure, and terminating sustained ventricular arrhythmias.
It was also optimally tolerated in the surgical phase, as it was forwarded in the ventricle, allowing aortic cross-clamping despite the 9F catheter in the aortic lumen.
In the postoperative setting, it facilitated weaning off extracorporeal circulation, and haemodynamic parameters were optimal with only minimal amounts of inotropes.
Overall, the solution of using Impella 5.0 as a means of supporting the patient in the pre, intra, and postoperative setting proved to be extremely feasible and valuable, and could be considered for future use in similar situations.
Additionally, this case is an unfortunate example of the collateral damage of the COVID-19 pandemic and underlines how, in a difficult moment in which the majority of healthcare resources are dedicated to facing the SARS-CoV-2 infection, it is mandatory to create safe, clean pathways to provide optimal care to patients not affected by the SARS-CoV-2 virus but who still require dedicated medical attention.
Lead author biography

Martina Briani was born in 1989 in Rome, Italy. She received her medical training in ‘La Sapienza’ University of Rome. In 2018, she completed her training as a Cardiologist in ‘Humanitas Clinical and Research Hospital’, Milan, where she is currently working as an Intensive Care Unit Cardiologist.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this casa and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: Publication costs related to this manuscript were provided by Abiomed.
Supplementary Material
References
- 1. Cohn LH, Adams DH.. Cardiac Surgey in the Adult. 3rd ed.McGraw-Hill Professional; 2007. [Google Scholar]
- 2. Castelvecchio S, Pappalardo OA, Menicanti L.. Myocardial reconstruction in ischaemic cardiomyopathy. Eur J Cardio-Thorac Surg 2019;155:i49–i56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kouchoukos NT, Blackstone EH, Hanley Kirklin JK.. Cardiac Surgery. Kirklin/Baratt-Boyes Cardiac Surgery. 4th ed.Elsevier; 2012. [Google Scholar]
- 4. Mohite PH, Umakumar K, Sàez DG, Simon AR.. Right Subclavian Artery Cutdown for Implantation of Impella 5.0. CTSNet; 2018.
- 5. Via G, Buson S, Tavazzi G, Halasz G, Quagliana A, Moccetti M. et al. Early cardiac unloading with Impella CP in acute myocardial infarction with ventricular septal defect. ESC Heart Fail 2020;7:708–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rios SA, Bravo CA, Weinreich M, Olmedo W, Villablanca P, Alvarez Villela M. et al. Meta-analysis and trial sequential analysis comparing percutaneous ventricular assist devices versus intra-aortic balloon pump during high-risk percutaneous coronary intervention or cardiogenic shock. Am J Cardiol 2018;122:1330–1338. [DOI] [PubMed] [Google Scholar]
- 7. Alushi B, Douedari A, Froehlig G, Knie W, Wurster TH, Leistner DM. et al. Skurk CImpella versus IABP in acute myocardial infarction complicated by cardiogenic shock. Open Heart 2019;6:e000987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


