Abstract
J Clin Hypertens (Greenwich). 2012; 14:644–649. © 2012 Wiley Periodicals, Inc.
Phosphodiesterase 5 (PDE‐5) inhibitors are selective blockers of PDE‐5, which catalyzes the hydrolysis of cyclic guanosine monophosphate (cGMP) to its corresponding monophosphates. cGMP is a potent vasodilator and nitric oxide donor. Since PDE‐5 is widely distributed in the body, it was hypothesized that inhibition of its actions could lead to significant vasodilation, which could benefit patients with coronary artery disease. This hypothesis led to the development of PDE‐5 inhibitors, the first being sildenafil citrate. Studies of sildenafil in patients with coronary artery disease demonstrated a modest cardiovascular effect but a potent action on penile erection in men, resulting in sildenafil becoming first‐line treatment of erectile dysfunction. Two more PDE‐5 inhibitors are now US Food and Drug Administration–approved (vardenafil and tadalafil) for the treatment of erectile dysfunction. Recent studies have demonstrated several beneficial pleiotropic cardiovascular effects of PDE‐5 inhibitors in patients with erectile dysfunction and multiple comorbidities, including coronary artery disease, heart failure, hypertension, and diabetes mellitus. Treatment of these conditions with PDE‐5 inhibitors has been very effective, safe, and well tolerated. Drug interactions have been minimal with the exception of nitrates, where coadministration may result in severe vasodilation and hypotension. These beneficial pleiotropic and safe cardiovascular effects of PDE‐5 inhibitors will be discussed in this concise review.
The phosphodiesterases (PDEs) are a super family of enzymes that catalyze the hydrolysis of the nucleotide monophosphates, cyclic adenosine monophosphate (cAMP), and cyclic guanosine monophosphate (cGMP) to the corresponding nucleoside monophosphates. 1 Several of the PDEs have already been identified and characterized, including PDE‐5, PDE‐6, PDE‐8, PDE‐9, PDE‐10, and PDE‐11, to name a few. 2 , 3 , 4 , 6 However, the major interest of research was focused on PDE‐5 because of its specificity in catalyzing the hydrolysis of cGMP. 1 , 7 , 8 PDE‐5 is expressed in various tissues of the body, such as the corpora cavernosa of the penis, the systemic arteries and veins, the pulmonary arteries, the skeletal muscles, and the platelets. 8 , 9 It was hypothesized that blocking the action of PDE‐5 will result in an increase in the intracellular levels and prolongation of action of cGMP, which is a nitric oxide (NO) donor and potent vasodilator. Thus, the initial research concentrated on discovering PDE‐5 blockers for the treatment of coronary artery disease (CAD). The discovery in 1989 of sildenafil citrate, a highly selective PDE‐5 inhibitor, was the result of extensive research on chemical agents targeting PDE‐5 inhibitors that might, potentially, be used for the treatment of CAD. Interestingly, initial studies with sildenafil in patients with CAD were not promising for the treatment of CAD, but they demonstrated a “beneficial side effect” in male participants by enhancing penile erection. This observation led to further studies in patients with erectile dysfunction (ED). These studies resulted in the approval by the US Food and Drug Administration (FDA) in 1998 of sildenafil citrate for the treatment of ED. Since the approval of sildenafil, two more PDE‐5 inhibitors, vardenafil and tadalafil, were developed and approved by the FDA for the treatment of ED. It is now well recognized that ED is a vascular disease, is quite common, and is age and disease dependent. It accounts for 39% of men 40 years old and for 67% of those 70 and older and affects about 30 million men in the United States and more than 100 million worldwide. 10 Since ED coexists with cardiovascular diseases, hypertension, heart failure (HF), and diabetes mellitus (DM), the treatment of ED has important clinical functional and safety implications related mostly to the interactions of PDE‐5 inhibitors with other drugs these patients might be taking. Recent studies have also indicated that PDE‐5 inhibitors possess several beneficial pleiotropic cardiovascular effects in addition to their action on ED. All of these actions will be discussed in this review in the context of the American College of Cardiology/American Heart Association (ACC/AHA) guidelines 11 and the recent developments in this field.
Mechanism of Action of PDE‐5 Inhibitors
The vascular tone, systemic vasodilation, and circulation are principally regulated through the endothelial production of NO from the systemic arteries and veins. After its production, NO diffuses into the adjacent smooth muscle cells and enhances the production of cGMP, which, in turn, increases the production of NO and leads to both vascular smooth muscle relaxation and an increase in systemic vasodilation. Since cGMP is catabolized by the PDE‐5 enzyme, its inhibition by the PDE‐5 inhibitors (sildenafil, vardenafil, and tadalafil) will result in an increase in the intracellular levels of cGMP and prolong the duration of its action. The chemical structures of the three PDE‐5 inhibitors approved by the FDA are depicted in Figure 1. The chemical structures of sildenafil and vardenafil are very similar, their half‐lives are approximately 4 hours and their action is dissipated 24 hours later. In contrast, the chemical structure of tadalafil is different, wherein its half‐life is longer (approximately 17.5 hours) and its action is dissipated 48 hours later. 12 These actions of PDE‐5 inhibitors could have clinical implications in patients with ED and CAD receiving nitrates, which are also NO donors and could lead to significant vasodilation and lowering of blood pressure (BP). In addition, they could have interactions with other drugs taken by patients with CAD, hypertension, HF, and DM. These drug interactions are important to know by physicians managing these patients. In the past, patients with ED were treated exclusively by urologists, but due to recent developments and understanding of the pathophysiology of ED, the care of these patients has now been shifted to the general physicians and cardiologists, putting more burden on their knowledge and understanding of the treatment of ED. The several pleiotropic effects of PDE‐5 inhibitors on various disease states will be discussed in subsequent sections of this review.
Figure 1.
The chemical structure of phosphodiesterase 5 inhibitors sildenafil, vardenafil, and tadalafil. The chemical structures of sidenafil and vardenafil are similar, whereas that of tadalafil is different. Adapted with permission from Zusman. 10
Cardiovascular Disease
Several clinical and experimental studies have demonstrated a cardioprotective effect of PDE‐5 inhibitors. In experimental animal studies, sildenafil provided a cardioprotective effect given immediately, 13 at 30 minutes, 14 at 24 hours, 14 , 15 and at 4 weeks before coronary artery occlusion 16 or reperfusion. 17 Likewise, vardenafil 18 and tadalafil 19 reduced infarct size when given 30 to 120 minutes before coronary artery occlusion in animal models. In addition to reducing infarct size, PDE‐5 inhibitors have been shown to increase the endothelial and inducible NO synthase in animals, to reduce cardiac hypertrophy and apoptosis to preserve myocardial fractional shortening, and to improve survival. 20
In studies of patients with ED and stable CAD, the administration of sildenafil 50 mg to 100 mg 21 or vardenafil 10 mg 22 while the patients were off nitrates was well tolerated and improved cardiac function and ED significantly more than placebo. These patients were previously exercised on a Bruce protocol to symptom‐limited exercise and ≥1 mm ST depression and also to a level of exercise equivalent to the effort produced by sexual activity. In one study, the tests were repeated 1 hour after the administration of vardenafil. 22 Compared with placebo, vardenafil did not alter the exercise time (P=.39) or time to awareness of angina (P=.59), but it significantly prolonged the time to ischemic threshold (P=.0004) as depicted in Figure 2. In addition, at peak exercise, vardenafil did not significantly affect BP, heart rate (HR), or rate pressure product relative to placebo. The most common side effects from the administration of sildenafil and vardenafil were facial flushing and headache, which were mild to moderate in severity and were short‐lived. Similar findings have been reported by other investigators. 23 , 24 In one study, the hemodynamic effects of sildenafil 100 mg and isosorbide mononitrate (ISMN) 40 mg were compared against placebo in 31 men with ED and stable CAD. 25 Compared with baseline, sildenafil increased slightly, the cardiac and stroke indices by 0.29 L/min/m2 and 4.4 mL/min/m2, respectively compared with ISMN, which decreased these indices by −0.12 L/min/cm2 and −5.4 mL/min/cm2, respectively. The mean arterial pressure was decreased more with ISMN than sildenafil (−22 vs −10 mm Hg, respectively). The systemic and pulmonary vascular resistance was decreased more with sildenafil than ISMN. Since the PDE‐5 inhibitors and nitrates are both NO donors, their combination could produce large decreases in BP with dire consequences and therefore their coadministration is prohibited in patients with ED and CAD. 11
Figure 2.
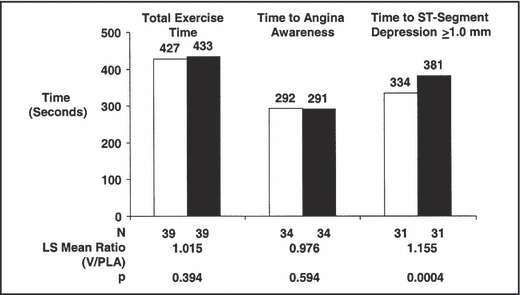
The effects of vardenafil (V) on exercise parameters of total exercise time, time to angina, and time to ≥1‐mm ST depression in patients with stable coronary artery disease. PLA indicates placebo. Adapted with permission from Thadani et al. 22
Hypertension
Several lines of evidence suggest that there is an increased prevalence of ED in hypertensive patients compared with normotensive controls, which has been estimated to be between 15% and 46% depending on the characteristics of the studied populations. 26 Endothelial dysfunction, which is present in hypertension, represents a major pathologic process that leads to abnormal vasoreactivity and vasoconstriction that affects the balance between vasodilating and vasoconstrictive factors. The reduced bioavailability of NO in hypertension presents a link between the pathophysiology of hypertension and ED, since ED is considered a vascular disease and NO is a major mediator of smooth muscle relaxation necessary for penile erection. Besides hypertension itself, ED is a common adverse effect of antihypertensive drugs, among which the most important are the diuretics, central sympatholytics, peripheral sympatholytics, aldosterone antagonists, and β‐blockers. Calcium channel blockers (CCBs), angiotensin‐converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs) are rarely implicated as a cause of ED. 27 , 28 Efficacy data were analyzed from 4274 patients 18 years and older with hypertension and ED from 18 different double‐blind, placebo‐controlled studies. 29 Of these patients, 2881 were not taking any antihypertensive medication and were randomized to sildenafil 50 mg to 200 mg (n=1837) or placebo (n=1044). The other 1094 patients were taking ≥1 antihypertensive drug and were also randomized to sildenafil 50 mg to 200 mg (n=704) or placebo (n=390). All patients were followed for 6 weeks to 6 months. The drug classes involved in these studies were diuretics, β‐blockers, α1‐blockers, ACE inhibitors, and CCBs. All patients included in these studies were in a stable relationship with a female partner and did not have any anatomic defects of their penis. Patients with BP >170/110 mm Hg or <90/50 mm Hg at screening were excluded from participation. The incidence rates of treatment‐related adverse events in patients taking sildenafil in combination with antihypertensive drugs were not different from patients taking sildenafil with placebo. These adverse events included hypotension (<1%) and (0%) for cardiovascular events. 29 With respect to ED, the success rate was 70% vs 72% for those taking combination therapy with sildenafil or placebo with sildenafil, respectively (P=.93). Similar results have been reported from a review of 19 studies of hypertensive patients with multiple comorbidities on various treatment regimens randomized to sildenafil, vardenafil, or tadalafil. 30 In this meta‐analysis, PDE‐5 inhibitors were shown to be effective, safe, and well tolerated regardless of the cause and severity of comorbid conditions. In another study, 371 hypertensive patients taking single or multiple antihypertensive drugs randomized to tadalafil 10 mg or placebo 31 did not show any adverse events or BP reductions different than placebo (Table I). In addition, in another double‐blind, placebo‐controlled, multicenter trial, 568 hypertensive patients with ED taking single or multiple antihypertensive drugs were randomized to sildenafil 50 mg to 100 mg (n=281) or placebo (n=281) and were followed for 12 weeks. 32 Sildenafil significantly improved patient ED and was not associated with any serious adverse events that were different from placebo.
Table I.
Mean Changes From Baseline in SBP and DBP With TadalaChanges From Baseline Between Sildenafil and Placebo and Between Sildenafil afil or Placebo in Patients Taking ≥1 Antihypertensive Drug
| One Drug | Tadalafil (n=140) | Placebo (n=59) |
| Baseline SBP, mm Hg | 143 | 142 |
| Mean change, mm Hg | −5.7 | −3.4 |
| Baseline DBP, mm Hg | 83 | 85 |
| Mean change, mm Hg | −2.2 | −2.1 |
| ≥2 Drugs | Tadalafil (n=140) | Placebo (n=137) |
| Baseline SBP, mm Hg | 126 | 44 |
| Mean change, mm Hg | −2.6 | +0.9 |
| Baseline DBP, mm Hg | 83 | 82 |
| Mean change, mm Hg | −2.7 | −2.2 |
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure. Adapted and modified from Kloner et al. 31
In a proof of concept study, the acute effects of sildenafil 50 mg given alone or in combination with ISMN 10 mg on brachial and central BP were tested in 6 patients with resistant hypertension taking ≥3 antihypertensive drugs. 33 The baseline brachial and central BPs were 169/93 mm Hg and 135/82 mm Hg. The addition of sildenafil to baseline treatment resulted in further reductions in mean brachial and central BP of 10/8 mm Hg and 8/7 mm Hg compared with placebo (P<.05). The greatest reductions in brachial and central BPs were seen with the combinations of sildenafil and ISMN, accounting for a mean reduction in brachial and central BP of 20/14 mm Hg and 22/14 mm Hg (P=.002) compared with placebo. The HR‐adjusted augmentation index and the arterial wave reflection were both significantly reduced with the combination of the two drugs (P<.0020). There was no significant effect on HR and pulse wave velocity with sildenafil alone or its combination with ISMN. Also, there were no significant adverse effects noted with either drug alone or in combination.
Heart Failure
The prevalence of ED is high in patients with congestive HF (CHF) and yet PDE‐5 inhibitors are not given in these patients. The reason for this is the fear of complications from these drugs due to the lack of experience from large, long‐term, placebo‐controlled clinical trials regarding the safety of these drugs in such patients. However, several small, short‐term studies have demonstrated a beneficial effect of PDE‐5 inhibitors with respect to ED and cardiac function in such patients. 34 , 35 In one small study, 20 patients aged 48 to 88 years with stable CHF and an ejection fraction <35% were randomized to sildenafil 50 mg or placebo in a double‐blind, two‐way crossover study after a 7‐day interval between the two phases of the study while the patients were off their regular therapy for at least 12 hours. 35 Sildenafil improved the cardiac index by 0.37 L/min/m2 from a baseline of 2.3 L/min/m2 (P<.0001) as well as the other study parameters (Table II). Also, in other small studies, sildenafil reduced systemic vascular resistance and aortic stiffness and improved exercise time, 6‐minutes walk distance, and quality of life. 36 , 37 Interestingly, the presence of PDE‐5 enzyme is minimal in the normal myocardium and is upregulated in the diseased myocardium. 37 In this study, myocardial PDE‐5 enzyme expression and its cellular distribution were determined in left ventricular samples from patients with end‐stable CHF and from normal heart donors. Compared with the donor hearts, the myocardial PDE‐5 protein was increased about 4.5‐fold in heart samples from patients with CHF and the PDE‐5 enzyme expression was significantly correlated with the expression of myocardial oxidative stress markers of 3‐nitrotyrosine and 4‐hydroxynomenal. In these studies, histological examination showed that the PDE‐5 enzyme was mainly expressed in the vascular smooth muscle of the normal donor hearts, but its expression in CHF hearts was increased in both the cardiac myocytes and vascular smooth muscle. 38
Table II.
Hemodynamic Effects of Sildenafil 50 mg and Placebo in Patients With Congestive Heart Failure
| Baseline Parameters (Mean±Standard Deviation) | Study Parameters (Difference) | P Value | |||
|---|---|---|---|---|---|
| Sildenafil | Placebo | Sildenafil | Placebo | ||
| Arm SBP, mm Hg | 131 | 130 | −8.6 | +4.9 | <.0001 |
| Arm DBP, mm Hg | 67 | 67 | −4.8 | +3.8 | <.0001 |
| Aortic SBP, mm Hg | 117 | 117 | −7.5 | +6.4 | <.0001 |
| Aortic DBP, mm Hg | 67 | 67 | −5.1 | +4.0 | <.0001 |
| Augmentation pressure mm Hg | 14 | 13 | −0.8 | +2.1 | <.0001 |
| AIx, % | 27 | 26 | −0.7 | +2.9 | <.0001 |
| SVR, dynes/s/cm5 | 1705 | 1660 | −326 | +153 | <.0001 |
Abbreviations: AIx, augmentation index; DBP, diastolic blood pressure; SBP, systolic blood pressure; SVR, systemic vascular resistance. Revised from Hirata et al. 34
The beneficial effects of PDE‐5 inhibitors in patients with CHF have been attributed to the increased production of cAMP. In diseased hearts, the protein kinase G activity induced by cGMP is inhibited, so cGMP shifts its action preferentially to the production of cAMP, which activates protein kinase A, leading to increased intracellular calcium and myocardial contractility. In addition, sildenafil induces other mechanisms that prevent the adverse cardiovascular remodeling in a myocardial infarction mouse model. 39 Compared with placebo, sildenafil preserved left ventricular function and decreased fibrosis, apoptosis, and left ventricular hypertrophy. These beneficial effects were mediated through inhibition of the RhoA/Rho‐kinase pathway. This pathway is a pathogenic one and its inhibition has been associated with improved atherosclerosis, post–myocardial infarction remodeling, and cardiac hypertrophy. 40
In all of these short‐term human studies, PDE‐5 inhibitors were well tolerated, caused minimal adverse effects, and improved cardiac function, ED, and quality of life. In chronic CHF studies, the best results were seen in patients with CHF and secondary pulmonary hypertension; however, this subject is beyond the scope of the current review.
Discussion
From the data presented in the previous sections of this paper, it appears that the PDE‐5 inhibitors sildenafil, vardenafil, and tadalafil are effective and safe for the treatment of ED in men with multiple comorbidities and therapies. 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 These studies have served to alleviate some of the anxiety produced by the ACC/AHA consensus document regarding the use of PDE‐5 inhibitors in patients with cardiovascular diseases and multiple therapies and especially the coadministration of PDE‐5 inhibitors and nitrates in patients with CAD. 11 The prevalence of ED is quite high, accounting for 10 to 30 million men in the United States and more than 100 million worldwide and is age‐, disease‐, and treatment‐dependent. 10 It has been estimated that the prevalence of ED is 39% and 67% in men aged 40 to 70 years, respectively. 10 In addition, the treatment of ED has shifted lately from the urologists to the primary care physicians, which requires an understanding on their part on its management, mainly with respect to the interactions of PDE‐5 inhibitors and the drugs that these patients might be taking. The PDE‐5 inhibitors exert their beneficial effects in patients with ED through inhibition of catabolism of cGMP, which is a potent NO donor that leads to vasodilation and increased blood flow to the corpora cavernosa of the penis, which facilitate penile erection. Since nitrates are also NO donors, their coadministration with PDE‐5 inhibitors could cause severe vasodilation and severe decrease of BP with dire consequences. For this reason, their coadministration, especially in patients with ED and CAD, is prohibited. 11 However, they can be safely administered 24 hours after the administration of sildenafil or vardenafil and 48 hours after the administration of tadalafil. 12 Admittedly, these restrictions can be serious in the need of emergent administration of nitrates. On the other hand, in the absence of nitrate administration, PDE‐5 inhibitors can be safely administered to patients taking other drugs, with some precaution in those taking α1‐blockers or other vasodilators, since these drugs are not NO donors and the additional BP‐lowering effect of PDE‐5 inhibitors is modest and not significantly different from placebo. 29 , 30 , 31 , 32 In these studies, the success rate on ED improvement was independent of disease or treatment, and in patients with hypertension while taking ≥1 antihypertensive drug, it was 70% compared with 72% in those taking no drugs or placebo (P=.93). Recent studies have also shown that PDE‐5 inhibitors, besides their effects on ED, possess several pleiotropic effects that might benefit patients with CAD, 7 , 13 , 14 , 15 , 16 , 17 , 18 , 19 CHF, 34 , 35 , 36 , 37 , 38 , 39 and hypertension. 20 , 26 , 27 , 28 , 29 , 30 , 31 , 32 In addition to these studies, they have also been very effective in improving ED in patients with DM without disturbing blood glucose control. 40 In treating patients with these comorbidities and treatments, caution should be exercised with respect to baseline BP levels and whether some of the medications they are taking are metabolized through the cytochrome P450 3A4 pathway because they might increase the blood levels of PDE‐5 inhibitors, since these drugs are also metabolized through the same pathway. 11 With respect to baseline BP, this should be >90/50 mm Hg, because in most studies with PDE‐5 inhibitors, patients with a baseline BP ≤90/50 mm Hg were excluded from participation. In a proof of concept study in patients with resistant hypertension, the addition of sildenafil 50 mg alone or in combination with ISMN 10 mg to baseline treatment resulted in further significant reduction in brachial and central BP. In addition, the augmentation index was significantly decreased with sildenafil alone and in combination with ISMN. These effects of PDE‐5 inhibitors are significant, especially regarding central BP, since central BP is more directly responsible for the cardiovascular and stroke complications of hypertension. Thus, a long‐term study evaluating these effects of PDE‐5 inhibitors will be of great scientific and clinical interest.
Conclusions
The PDE‐5 inhibitors are still considered first‐line treatment for men with ED and are effective, safe, and well tolerated. In addition, they can be safely administered in patients with multiple comorbidities and therapies without fear of causing serious clinical or metabolic adverse events, with the exception of patients who have CAD taking long‐acting nitrates. In such patients, the administration of PDE‐5 inhibitors is prohibited. In patients with CAD not taking nitrates on a regular basis, their administration in case of need should be 24 hours after the administration of sildenafil or vardenafil and 48 hours after the administration of tadalafil.
References
- 1. Conti M, Jin SL. The molecular biology of cyclic nucleotide phosphodiesterases. Prog Nucleic Acid Res Mol Biol. 1999;63:1–38. [DOI] [PubMed] [Google Scholar]
- 2. Stacey P, Rulten S, Dapling A, Phillips SC. Molecular cloning and expression of human cGMP‐binding cGMP‐specific phosphodiesterase (PDE‐5). Biochem Biophys Res Commun. 1998;247:249–254. [DOI] [PubMed] [Google Scholar]
- 3. Soderling SH, Bayuga SJ, Beavo JA. Isolation and characterization of a dual substrate phosphodiesterase gene family: PDE 10A. Proc Natl Acad Sci U S A. 1999;96:7071–7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fisher DA, Smith JF, Pillar JS. Isolation and characterization of PDE8A, a novel human cAMP‐specific phosphodiesterase. Biochem Biophys Res Commun. 1998;246:570–577. [DOI] [PubMed] [Google Scholar]
- 5. Fisher DA, Smith JF, Pillar JS, et al. Isolation and characterization of PDE 9A, a novel human cGMP‐specific phosphodiesterase. J Biol Chem. 1998;273:15557–15564. [DOI] [PubMed] [Google Scholar]
- 6. Yuasa K, Kotera J, Fujishige K, et al. Isolation and characterization of two novel phosphodiesterase PDE 11A variants showing unique structure and tissue specific expression. J Biol Chem. 2000;275:31469–31479. [DOI] [PubMed] [Google Scholar]
- 7. Reffelman T, Kloner RA. Therapeutic potential of phosphodiesterase 5 inhibition for cardiovascular disease. Circulation. 2003;108:239–244. [DOI] [PubMed] [Google Scholar]
- 8. Senzaki H, Smith CJ, Juang CJ, et al. Cardiac phosphodiesterase 5 (cGMP‐specific) modulates b‐adrenergic signaling in vivo and is down‐regulated in heart failure. FASEB J. 2001;15:1718–1726. [DOI] [PubMed] [Google Scholar]
- 9. Wallis RM, Corbin JD, Francis SH, et al. Tissue distribution of phosphodiesterase families and the effect of sildenafil on tissue cyclic nucleotides, platelet function and the contractile responses of trabecular carneae and aortic rings in vitro . Am J Cardiol. 1999;83:3C–12C. [DOI] [PubMed] [Google Scholar]
- 10. Zusman RM. Therapeutic potential of phosphodiesterase 5 inhibition for cardiovascular disease. Am J Cardiol. 1999;83:1C–2C. [DOI] [PubMed] [Google Scholar]
- 11. Cheitlin MD, Hutter AM, Brindis RG, et al. Use of sildenafil (Viagra) in patients with cardiovascular disease. J Am Coll Cardiol. 1999;33:273–282. [DOI] [PubMed] [Google Scholar]
- 12. Kloner RA. Cardiovascular effects of the 3 phosphodiesterase‐5 inhibitors approved for the treatment of erectile dysfunction. Circulation. 2004;110:3149–3155. [DOI] [PubMed] [Google Scholar]
- 13. Jamnicki‐Abegg M, Weihrauch D, Chiari PC, et al. Diabetes abolishes sildenafil‐induced cGMP‐dependent protein kinase‐1 expression and cardioprotection. J Cardiovasc Pharmacol. 2007;50:670–676. [DOI] [PubMed] [Google Scholar]
- 14. Okaili R, Salloum F, Hawkins J, et al. Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial K (ATP) channels in rabbits. Am J Physiol Heart Circ Physiol. 2002;283:H1263–H1269. [DOI] [PubMed] [Google Scholar]
- 15. Wang X, Fisher PW, Xi L, Kukreja RC. Essential role of mitochondrial Ca2+ activated and ATP‐sensitive k+ channels in sildenafil‐induced late cardioprotection. J Mol Cell Cardiol. 2008;44:105–113. [DOI] [PubMed] [Google Scholar]
- 16. Salloum FN, Abbate A, Das A, et al. Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol Heart Circ Physiol. 2008;294:H1398–H1406. [DOI] [PubMed] [Google Scholar]
- 17. Madhani M, Hall AR, Cuello F, et al. Phospholemman Ser69 phosphorylation contributes to sildenafil‐induced cardioprotection against reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;299:H827–H836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salloum FN, Okaili RA, Wittkamp M, et al. Vardenafil: a novel type 5 phosphodiesterase inhibitor reduces myocardial infarct size following ischemia‐reperfusion injury via opening mitochondrial K(ATP) channels in rabbits. J Mol Cell Cardiol. 2006;40:405–411. [DOI] [PubMed] [Google Scholar]
- 19. Sesti C, Florio V, Johnson EG, Kloner RA. The phosphodiesterase 5 inhibitor tadalafil reduces myocardial infarct size. Int J Impot Res. 2007;19:55–61. [DOI] [PubMed] [Google Scholar]
- 20. Schwartz BG, Levine LA, Comstock G, et al. Cardiac uses of phosphodiesterase 5 inhibitors. J Am Coll Cardiol. 2012;59:9–15. [DOI] [PubMed] [Google Scholar]
- 21. DeBusk RF, Pepine CJ, Glasser DB, et al. Efficacy and safety of sildenafil citrate in men with erectile dysfunction and stable coronary artery disease. Am J Cardiol. 2004;93:147–153. [DOI] [PubMed] [Google Scholar]
- 22. Thadani U, Smith W, Nash S, et al. The effect of vardenafil: a potent highly selective phosphodiesterase‐5 inhibitor for the treatment of cardiovascular response to exercise in patients with coronary artery disease. J Am Coll Cardiol. 2002;40:2006–2012 [DOI] [PubMed] [Google Scholar]
- 23. Fox KM, Thadani U, Ma TS, et al. Sildenafil citrate does not reduce exercise tolerance in men with erectile dysfunction and chronic stable angina. Eur Heart J. 2003;24:2206–2212. [DOI] [PubMed] [Google Scholar]
- 24. Herrmann H, Chang G, Klugher BD, Mahoney PD. Hemodynamic effects of sildenafil in men with severe coronary artery disease. N Engl J Med. 2000;342:1622–1626. [DOI] [PubMed] [Google Scholar]
- 25. Jackson G, Kelta M, Csanady M, et al. Hemodynamic effects of sildenafil citrate and isosorbide mononitrate in men with coronary artery disease and erectile dysfunction. J Sex Med. 2005;2:407–414. [DOI] [PubMed] [Google Scholar]
- 26. Papatsoris AG, Karantzopoulos PG. Hypertension, antihypertensive therapy and erectile dysfunction. Angiology. 2006;57:47–52. [DOI] [PubMed] [Google Scholar]
- 27. Fogari R, Zoppi A. Effects of antihypertensive therapy on sexual activity in hypertensive men. Curr Hypertens Rep. 2002;4:202–210. [DOI] [PubMed] [Google Scholar]
- 28. Grimm RH, Grandits GA, Prineas RJ, et al. Long‐term effects on sexual dysfunction of five antihypertensive drugs and nutritional treatment in hypertensive men and women Treatment of Mild Hypertension Study(TOMHS). Hypertension. 1997;29:8–14. [DOI] [PubMed] [Google Scholar]
- 29. Kloner RA, Brown M, Prisant LM, et al. Effects of sildenafil in patients with erectile dysfunction taking antihypertensive therapy. Am J Hypertens. 2001;14:70–73. [DOI] [PubMed] [Google Scholar]
- 30. Nehra A. Erectile dysfunction and cardiovascular disease: efficacy and safety of phosphodiesterase type 5 inhibitors in men with both conditions. Mayo Clin Proc. 2009;84:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kloner RA, Mitchell M, Emmick JT. Cardiovascular effects of tadalafil in patients with common antihypertensive therapies. Am J Cardiol. 2003;91(suppl):47M–57M. [DOI] [PubMed] [Google Scholar]
- 32. Pickering TG, Shepherd AM, Puddey I, et al. Sildenafil citrate for erectile dysfunction in men receiving multiple antihypertensive agents. Am J Hypertens. 2004;17:1135–1142. [DOI] [PubMed] [Google Scholar]
- 33. Oliver JJ, Dear JW, Webb DJ. Clinical potential of combined organic nitrate and phosphodiesterase type 5 inhibitor in treatment‐resistant hypertension. Hypertension. 2010;56:62–67. [DOI] [PubMed] [Google Scholar]
- 34. Lewis GD, Shah R, Shahzal K, et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–1562. [DOI] [PubMed] [Google Scholar]
- 35. Hirata K, Adji A, Vlachopoulos C, et al. Effects of sildenafil on cardiac performance in patients with heart failure. Am J Cardiol. 2005;96:1436–1440. [DOI] [PubMed] [Google Scholar]
- 36. Bocchi EA, Guimaraes G, Mocelin A, et al. Sildenafil effects on exercise, neurohormonal activation and erectile dysfunction in congestive heart failure. A double‐blind, placebo‐controlled, randomized study followed by a prospective treatment for erectile dysfunction. Circulation. 2002;106:1097–1103. [DOI] [PubMed] [Google Scholar]
- 37. Webster LJ, Michelakis ED, Davis T, Archer SL. Use of sildenafil for safe improvement of erectile function and quality of life in men with New York Heart Association classes II and III congestive heart failure. Arch Intern Med. 2004;164:514–520. [DOI] [PubMed] [Google Scholar]
- 38. Lu Z, Xu X, Hu X, et al. Oxidative stress regulates left ventricular PDE‐5 expression in the failing heart. Circulation. 2010;121:1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chau VQ, Salloum FN, Hoke NN, et al. Mitigation of the progression of heart failure with sildenafil involves inhibition of Rho/Rho‐kinase pathway. Am J Physiol Heart Circ Physiol. 2011;300:H2272–H2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goldstein I, Young JM, Fischer J, et al. Vardenafil Diabetes Study Group . Vardenafil, a new phosphodiesterase type 5 inhibitor, in the treatment of erectile dysfunction in men with diabetes: a multicenter double‐blind placebo‐controlled fixed‐dose study. Diabetes Care. 2003;26:777–783. [DOI] [PubMed] [Google Scholar]


