Abstract
Precision medicine models for personalizing achieving sustained behavior change are largely outside of current clinical practice. Yet, changing self-regulatory behaviors is fundamental to the self-management of complex lifestyle-related chronic conditions such as depression and obesity - two top contributors to the global burden of disease and disability. To optimize treatments and address these burdens, behavior change and self-regulation must be better understood in relation to their neurobiological underpinnings. Here, we present the conceptual framework and protocol for a novel study, “Engaging self-regulation targets to understand the mechanisms of behavior change and improve mood and weight outcomes (ENGAGE)”. The ENGAGE study integrates neuroscience with behavioral science to better understand the self-regulation related mechanisms of behavior change for improving mood and weight outcomes among adults with comorbid depression and obesity. We collect assays of three self-regulation targets (emotion, cognition, and self-reflection) in multiple settings: neuroimaging and behavioral lab-based measures, virtual reality, and passive smartphone sampling. By connecting human neuroscience and behavioral science in this manner within the ENGAGE study, we develop a prototype for elucidating the underlying self-regulation mechanisms of behavior change outcomes and their application in optimizing intervention strategies for multiple chronic diseases.
Keywords: Self-regulation, Neuroimaging, Virtual reality, Depression, Obesity, Behavior change
1. Introduction
Effective and personalized precision medicine models for achieving sustained behavior change have not yet been implemented in routine clinical practice. Depression and obesity are two of the top contributors to the global burden of disease and disability (GBD 2015 Disease and Injury Incidence and Prevalence Collaborators, 2016; GBD 2015 Risk Factors Collaborators, 2016; The Global BMI Mortality Collaboration, 2016). Given their chronic and disabling impact, we are in urgent need of personalized and precise models for achieving sustained behavior change for depression and obesity. Depression and obesity are complex in their own right. They also commonly co-occur (Atlantis & Baker, 2008; Carpenter, Hasin, Allison, & Faith, 2000; Dragan & Akhtar-Danesh, 2007; Faith, Matz, & Jorge, 2002; Markowitz, Friedman, & Arent, 2008; Friedman & Brownell, 1995; Heo, Pietrobelli, Fontaine, Sirey, & Faith, 2006; Istvan, Zavela, & Weidner, 1992; Stunkard, Faith, & Allison, 2008; Onyike, Crum, Lee, Lyketsos, & Eaton, 2003; Simon et al., 2008; Strine et al., 2008), and their combined disease burden is exacerbated and often intractable (Bjerkesset, Romundstad, Evans, & Gunnell, 2008; Blaine, 2008; de Wit et al., 2010; Ma & Xiao, 2010; Simon et al., 2008; Strine et al., 2008).
Framed within the precision medicine paradigm, our central premise is that behavior change can be better understood and optimized, when defined in relation to its underlying brain functions. With an anchor in brain functions we can conceptualize how these functions express themselves in daily living choices, and how they are influenced by targeted interventions. With enhanced knowledge of the nature and variability of brain-behavior relations both between and within individuals, we have the foundations for developing interventions that are precise, proactive, and personalized – and consequently more effective. Such an approach reflects the National Institute of Health’s Science of Behavior Change (SOBC) initiative (https://commonfund.nih.gov/behaviorchange/index; http://scienceofbehaviorchange.org/). SOBC is pioneering an experimental medicine approach to build our knowledge about how individuals achieve behavior change to promote positive health outcomes.
A specific focus of our premise is that the construct of “self-regulation” offers a framework for understanding how brain functions relate to behavior change in people experiencing depression and obesity. What do we mean by “self-regulation”? Our perspective is that self-regulation requires ongoing adjustment of emotional reactions, the contents of cognition, and self-directed reflection, in order to maximize adaptive – and minimize maladaptive – outcomes (Lövdén, Wenger, Mårtensson, Lindenberger, & Bäckman, 2013; Williams et al., 2008). The overarching concept of self-regulation has been articulated in several disciplines, including medicine (e.g., as homeostasis) and psychology (e.g., as self-control and emotion regulation). In common, these concepts highlight a reliance on self-directed or endogenous processing of stimulus, rather than exogenous, stimulus-response behaviors. Reflecting our specific perspective, we focus on regulation of emotion, cognition, and self-reflection as three key domains of impaired self-regulation implicated in depression and obesity (Lindquist & Barrett, 2012; Oosterwijk et al., 2012; Yeo et al., 2011). We operationalize these domains by brain and behavior targets that are assayed in multiple lab, virtual and naturalistic settings.
Our brain targets are grounded in evidence from functional neuroimaging studies that have defined large-scale neural circuits engaged by the regulation of emotion and cognition and by self-reflection (Buckner, Krienen, & Yeo, 2013; Cole, Bassett, Power, Braver, & Peterson, 2014; Fox et al., 2005; Gordon et al., 2014; Lindquist & Barrett, 2012; Oosterwijk et al., 2012; Power et al., 2011; Seeley et al., 2007; Spreng, Sepulcre, Turner, Stevens, & Schacter, 2013; Williams, 2016; Williams, 2017; Yeo et al., 2011). We use the term “large-scale neural circuit” to refer to the macroscale of neural organization. At this macroscale, vast numbers of interconnected neurons and their associated elements constitute functional circuits that comprise correlated regions of activation at rest or during task-evoked situations (Williams, 2016, 2017). These neural circuits are commonly referred to as “networks” and may be probed using non-invasive brain imaging. Affective neural circuit targets for regulation of emotion, and reactivity to emotion stimuli, can be probed by specific emotion tasks, and can be, further delineated by the use of negative versus positively valenced stimuli within these tasks (Dichter, Kozink, McClernon, & Smoski., 2012; Etkin, Prater, Hoeft, Menon, & Schatzberg, 2010; Haber & Knutson, 2010; Keedwell, Andrew, Williams, Brammer, & Phillips, 2005; Kober et al., 2008; Robinson et al., 2014; Treadway & Zald, 2011; Williams, Das et al., 2006; Williams, Liddell et al., 2006) (Fig. 1). Neural circuit targets for cognitive regulation are referred to as “cognitive control” circuits, and can be probed by tasks that require selection of stimulus-relevant responses and inhibition of stimulus-irrelevant ones (Cole & Schneider, 2007; Niendam et al., 2012; Roalf et al., 2014) (Fig. 1).1 Neural circuit targets for self-reflection are operationalized by the “default mode” network. The default mode network is prominent during self-reflective functions and self-directed thought (Cole et al., 2014; Fox et al., 2005) (Fig. 1).
Fig. 1.
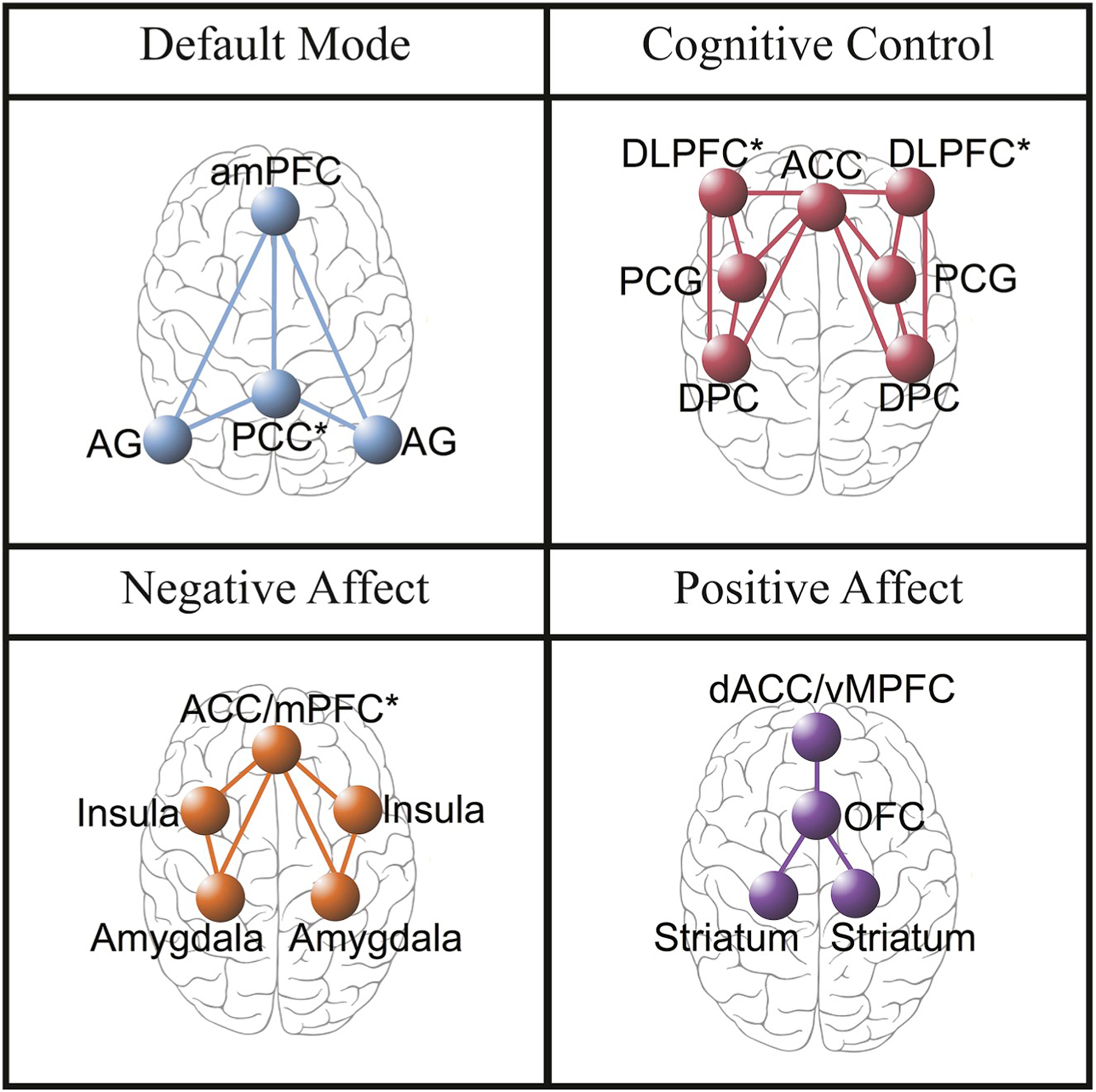
Brain circuits in which the authors theorize plasticity mediates long-term behavior change in regulation of self-focused reflection (Default Mode Network), regulation of cognition (Cognitive Control Network), and regulation of emotion (Negative Affect and Positive Affect Network). aMPFC = anterior medial prefrontal cortex. AG = angular gyrus. PCC = posterior cingulate cortex (includes precuneus). DLPFC = dorsolateral prefrontal cortex (includes anterior prefrontal cortex and inferior frontal cortex). ACC = anterior cingulate cortex. PCG = precentral gyrus. DPC = dorsal parietal cortex. ACC/MPFC = dorsal medial prefrontal cortex (includes dorsal ACC and vMPFC, including ventral—subgenual and pregenual—and rostral ACC). dACC = dorsal anterior cingulate cortex. OFC = orbitofrontal cortex.
Affective, cognitive control and default mode circuits have been implicated in both depression and obesity (Williams et al., 2015; Stoeckel et al., 2008; Nummenmaa et al., 2012; Cole & Schneider, 2007; Tuulari et al., 2015; DelParigi et al., 2007; Falconer et al., 2008; Gyurak et al., 2016; Supplementary Materials Section 1). Functioning of these circuits has also been implicated in the day-to-day behaviors associated with depression and obesity, including managing diet, physical activity and mood reactivity (Heatherton, 2011; Oosterwijk et al., 2012). Convergent information from lab-based behavioral, virtual reality-elicited and self-reported proxies of these circuits, have also been linked to behavior change outcomes (Achtziger, Gollwitzer, & Sheeran, 2008; Bailenson et al., 2008; Fox, Bailenson, & Ricciardi, 2012; Oaten & Cheng, 2006; Rothman, Sheeran, & Wood, 2009). A persistent failure to self-regulate is implicated in a variety of negative behaviors and outcomes, including overeating, sedentary lifestyle, and psychopathology. Conversely, those who are better able to persistently self-regulate demonstrate improved weight management and mental health, along with better function at work and in relationships (Gillison et al., 2015; Gross & John, 2003; Tangney, Baumeister, & Boone, 2004; Wing, Tate, Gorin, Raynor, & Fava, 2006). Yet, the exact nature of relations among neural circuits and behaviors, and their expression in the natural world has not yet been articulated. We do not yet have a mechanistic understanding of how neural circuits are expressed in overt behavior and subjective experience, and which brain-behavior relations explain why some people can change their behavior and others cannot. With such a mechanistic and detailed understanding, we lack the foundation for developing more effective interventions based on a precision medicine approach.
Grounded in this perspective, we present the conceptual framework and protocol for a novel study, “Engaging self-regulation targets to understand the mechanisms of behavior change and improve mood and weight outcomes (ENGAGE).” The ENGAGE study is embedded within and operationally linked to “Research Aimed at Improving Both Mood and Weight (RAINBOW),” an ongoing randomized controlled trial (RCT) (Ma et al., 2015). RAINBOW (and thus ENGAGE) draws on the existing evidence base for interventions for comorbid depression and obesity (Jensen, Ryan, & Apovian, 2014). The RAINBOW treatment model pragmatically integrates the core components of two behavioral interventions previously proven efficacious in studies that focused on depression and obesity separately (Ma et al., 2015). By expanding RAINBOW into the ENGAGE study, we can further address why these interventions are effective only for some individuals, how to tailor them to comorbidity of conditions, or how to optimize them for each person. For example, through this integrative therapeutic approach, we have the capacity to assess important health behaviors (including problem solving, self-monitoring, and adherence to medical regimens such as healthy eating, increased physical activity and antidepressant medications) and health outcomes (such as mood and weight).
In the ENGAGE study we integrate human neuroscience with behavioral science in order to better understand the self-regulation related mechanisms of behavior change for improving mood and weight outcomes. Our assays of the three self-regulation targets of interest span multiple settings. In the lab-based setting, neural circuits are assayed using functional neuroimaging and cognitive-behavioral functioning is assayed via computerized testing. During immersive virtual reality (VR) we assay ratings of experience and behavior. In the natural world, we assay moment-by-moment behaviors using passive smartphone sampling (Table 1). By taking assays across multiple settings, from lab to the natural world, we can ultimately translate our lab-based mechanistic knowledge into the real life of the individual, and to explain individual trajectories of change. This approach has the potential to advance a precision model that has immediate relevance for improving our population’s health and for the science of behavior change.
Table 1.
Overview of self-regulation targets, assays to assess these targets and the settings in which assays were undertaken.
| Target | Assay settings | Assay Domains | |||
|---|---|---|---|---|---|
| MRI | Behavior | Physiology | Self-Report | ||
| Regulation of Emotion | Lab-based | “Affective circuit” reactivity and connectivity elicited by emotional regulation and viewing of facial expression tasks | Reaction time biases generated by priming from the emotional content of images | Heart rate variability, respiration rate, and skin conductivity recorded with fMRI during emotion tasks | Emotion Regulation Questionnaire (ERQ) for self-reported regulation Psychological strategies for cognitive coping using the COPE Inventory: emotion regulation subscales BRISC questionnaire for emotional resilience and self-efficacy |
| Virtual Reality | Screen recording of virtual reality headset, coded for behavior Head orientation data collected multiple times per second during emotional regulation environments |
Perceived strength of emotions, immersion, felt before and after regulation | |||
| Naturalistic | Emotional experience relevant passive smartphone sampling | ||||
| Regulation of Cognition | Lab-based | “Cognitive Control” circuit reactivity and connectivity elicited during a Go-NoGo inhibition task | Errors of omission and commission during the Go- NoGo task | Heart rate variability, respiration rate, and skin conductivity recorded with fMRI during the Go-NoGo cognitive task | Psychological strategies for cognitive coping using the COPE Inventory: cognitive regulation subscales |
| Virtual Reality | Screen recording of virtual reality headset, coded for behavior, “Score” on VR version of GoNoGo, “hits (non-wasps)’ and “misses (wasps)” Head orientation data collected during cognitive control environments |
Index of immersion in cognitive challenges | |||
| Naturalistic | Passive smartphone sampling of cognitive metrics | ||||
| Regulation of Self- Focused Reflection | Lab-based | “Default Mode circuit” reactivity and connectivity elicited during rest | Heart rate variability, respiration rate, and skin conductivity recorded with fMRI during rest | Psychological strategies for cognitive coping using the COPE Inventory: self-focused regulation subscales | |
| Virtual Reality | Screen recording from virtual reality headset, coded for behavior Head orientation data during self-reflection environment |
Self-reported strength of emotional immersion | |||
| Naturalistic | Passive smartphone sampling of movement |
2. Methods
2.1. Study aims and hypotheses
The primary objective of the ENGAGE study is to identify assays that engage emotion, cognition, and self-reflection aspects of self-regulation within lab-based, VR and naturalistic settings (Table 1), to understand the relations between assays taken at different levels of measurement, and to evaluate the extent to which these assayed self-regulation targets predict and/or mediate adherence to the intervention and mood and weight outcomes.
The ENGAGE project focuses on the following specific aims and these aims are summarized in Table 2:
Table 2.
ENGAGE project aims organized according to the self-regulation targets of focus, the assays used to assess each target and the settings in which the assays were undertaken.
| Target | Assay settings | AIMS | ||
|---|---|---|---|---|
| AIM 1: Target Engagement | AIM 2: Target Malleability | AIM 3: Target Prediction | ||
| Includes evaluation of convergent validity | Includes evaluation of concurrent validity | Includes evaluation of predictive validity | ||
| Regulation of Emotion | Lab-based Virtual Reality Naturalistic |
Activation on emotion fMRI tasks will correlate with emotion identification behavior and with self-reported emotion regulation. | Across timepoints, changes in emotion task fMRI activation will correlate with changes in self-reported emotion and behavior, VR, and smartphone sampling of mood-related word usage. | Emotion task baseline measures and assay changes over time will predict changes in health behaviors and outcomes. |
| Regulation of Cognition | Lab-based Virtual Reality Naturalistic |
Activation on cognitive control fMRI tasks will correlate with scores of omissions/commissions on cognitive behavioral paradigms and self-report coping strategies. | Across timepoints, changes in cognitive task fMRI activation will correlate with changes in cognitive self-report, behavior, VR, and smartphone sampling of cognitive metrics. | Cognitive task baseline measures and assay changes over time will predict changes in health behaviors and outcomes. |
| Regulation of Self-Focused Reflection | Lab-based Virtual Reality Naturalistic |
Resting state fMRI activation and connectivity will correlate with virtual reality mindfulness and self-reported reflection measures. | Across timepoints, changes in resting activation and connectivity will correlate with changes in reflection self-report, behavior, VR, and smartphone sampling. | Resting state baseline assays and assay changes over time will predict changes in health behaviors and outcomes. |
| Across all Targets | Across Settings | Within each target domain, lab-based assays will correlate with VR measures & naturalistic passive assays. | Changes in lab-based assays will correlate with changes in VR and naturalistic passive sampling assays within target domains (within subject). | Changes in lab-based assays will differentiate treatment responders and nonresponders. Treatment-related changes in Lab-based assays will correlate with those on Virtual Reality and Naturalistic assays. |
Aim 1:
To establish target engagement at baseline for each of our assays for each of our targets of interest for each setting.
Within each setting the assays encompass different types of measurement which we refer to as different “assay domains.” The specific assays and assay domains used to assess self-regulation targets within each setting are detailed in Table 1.
As a secondary aspect of this aim, we will establish criteria for evaluating the internal consistency and convergent validity of assays at baseline.
Our hypotheses related to this aim are as follows:
-
i
Within the lab-based setting, baseline assays will represent at least three latent domains of self-regulation targets including emotion (affect), cognition (cognitive control) and self-reflection.
-
ii
Across settings, lab-based assays will correlate with VR and naturalistic passive sampling assays within domains.
Aim 2:
To verify target malleability over time for each assay of our self-regulation targets of interest across the multiple settings, and the extent to which malleability (change) in one assay correlates with change in another. Under this aim we will evaluate concurrent validity of the assays.
Our hypotheses (Table 1) related to this aim are as follows:
-
iii
Lab-based assays will change significantly from baseline to each post-intervention point, from short-term through maintenance and end of follow-up.
-
iv
Changes in lab-based assays will correlate with changes in VR and naturalistic passive sampling assays.
Aim 3:
Assess target prediction regarding whether the baseline levels of target engagement and their changes over time predict changes in health behaviors (problem solving, self-monitoring, and adherence to healthy eating and active living recommendations and antidepressant medications) and outcomes (depression and weight) at 2 (end of initial treatment), 6 (end of intensive treatment), 12 (end of maintenance), and 24 months (end of follow-up). A secondary aspect of this aim will be to establish criteria for evaluating predictive validity using these same outcome criteria.
Our hypotheses related to this aim are as follows:
-
v
Lab-based assays at baseline and changes in response to initial treatment will predict changes in the health behaviors and outcomes of interest, and will differentiate treatment responders and nonresponders.
-
vi
VR and naturalistic passive sampling assays will replicate the predictive and discriminant validity of the lab-based assays.
2.2. Participants
ENGAGE participants (minimum n = 100) will be adult patients receiving primary care at Palo Alto Medical Foundation (PAMF), who have comorbid depression and obesity and are eligible. All participants are randomized to receive either the experimental or control condition of the RAINBOW study. The full RAINBOW trial protocol can be found elsewhere (Ma et al., 2015; See Supplementary protocol paper). Briefly, RAINBOW applies a type 1 hybrid design (Curran, Bauer, Mittman, Pyne, & Stetler, 2012) to evaluate the clinical and cost effectiveness and the implementation potential of an integrated, technology-enhanced, collaborative care model for treating comorbid obesity and depression in primary care. Obese depressed adults with no significant medical (e.g., diabetes or cardiovascular disease) or psychiatric comorbidities (e.g., psychotic or bipolar disorders) will be recruited through primary care clinics of PAMF. Eligible and consenting participants will be randomly assigned to receive usual care enhanced with the provision of a pedometer and information about the health system’s available services for mood or weight management (control), or with the Integrated Coaching for Better Mood and Weight (I-CARE) program (intervention). The I-CARE program combines standard behavioral weight-loss treatment and problem-solving therapy for depression, with as-needed antidepressant medication intensification. Follow-up assessments will occur every 6 months through 24 months post randomization. The RAINBOW target enrollment is 404 obese depressed adults.
A minimum of 100 RAINBOW participants meeting additional eligibility criteria (i.e., weight < 350 pounds due to scanner constraints, no MRI contraindications, no traumatic brain injuries, and no tumor or any other known structural abnormality in brain) will also be enrolled in ENGAGE to complete additional assessments. These assessments are detailed in the subsequent sections and are completed at timepoints of baseline, 2, 6, 12, and 24 months. The timeframes set for each visit are based on the participant’s original randomization date. With the exception of 2 months, all other assessment time points coincide with a RAINBOW assessment. During each visit, participants will be scanned during conditions and tasks designed to engage the self-regulation targets of interest. The order of the tasks will remain as constant as possible. Participants may complete their self-report items and computerized cognitive-behavioral assessments from home within 2 days of their in-person visit. If they are unable to, they can also complete these either immediately before, or after their in-person visit at the lab. When in lab, participants will undergo the fMRI neuroimaging tasks in scanner, followed by VR tasks.
The participants will be asked to provide written informed consent to take part in the ENGAGE study. All study procedures have been reviewed and approved by the respective Institutional Review Boards of Stanford, the Palo Alto Medical Foundation Research Institute, and the University of Illinois at Chicago.
2.3. Assay settings & targets
The rationale for our choice of assays and targets is as follows. Our motivation was to select assays that would enable us to shed light on how neural systems involved in laboratory assays of self-regulation are engaged in the brief and repeated everyday battles to modify and control behavior and solve problems. The goals of the ENGAGE project are to test directly the relations between assays acquired in multiple contexts. Thus, our rationale is necessarily theoretically motivated, with the choice of each assay anchored in data from “within assay” studies.
To address our aims, we selected a hierarchy of putative targets which provides us with gold standard lab-based measures and the opportunity to validate these measures with more ecologically applicable assays. Within this approach our acquisition design was structured so that we can acquire multiple different types of lab-based and ecologically-valid assays simultaneously, and thus control for the impact of other contextual factors.
Our choice to focus on functional imaging of large scale circuits was based on the premise that circuit properties represent the right measurement scale to delineate mechanistic targets of self-regulation. Circuits integrate across different levels and measures of brain function, but still reflect the complexity of the brain and its behavioral outputs. Due to their role in self-regulation and their implication in obesity and depression, as outlined in the introduction, we target 3 large-scale circuits: the “Affective,” “Cognitive Control” and “Default Mode” networks (Fig. 1; Supplementary Materials). In the lab setting, we make the working assumption that individual differences in neural circuits implicated in emotional, cognitive and self-reflective aspects of self-regulation will be reflected in corresponding individual differences in overt behavior, physiology and self-report that can be readily measured in the lab. For example, variations in the engagement of the neural circuit for cognitive control are also expressed in errors of omission and commission during behavioral performance, differences in heart rate variability, and self-reported psychological traits of impulsivity (Cape, Whittington, Buszewicz, Wallace, & Underwood, 2010). Our perspective is that self-report measures assess the individual difference factors that predispose us to use particular neural and behavioral styles of self-regulation. For example, when dealing with negative emotions we tend to use adaptive regulation strategies such as reappraising the emotion or we use less adaptive (and short-term) strategies such as suppression (Gross & John, 2003).
For the virtual reality setting, we chose environments with face validity for engaging the same regulation of emotion, cognition and self-reflection challenges as probed by lab-based assays.
To assay targets in the naturalistic setting, we selected a particular app for passive and ecological assessment of behavior because it has previously been shown to correlate with lab-based behavioral assays.
We recognize that there are more comprehensive ways that neural circuits can be defined, and that our choice of assays across all settings is constrained by our theoretical perspective. Thus, our choices are intended as a step toward consensus building around assays for self-regulation targets that have translational relevance across lab to natural world settings.
2.3.1. Lab-based assays for target: regulation of emotion
2.3.1.1. MRI assays for regulation of emotion
2.3.1.1.1. Viewing of facial expressions of emotion.
These tasks reliably engage affective circuits and has been well established by the investigators’ previous research (Goldstein-Piekarski et al., 2016; Liddell et al., 2005; Williams, Das et al., 2006; Williams et al., 2004; Williams et al., 2006; Williams et al., 2015). Stimuli were selected from a standardized series of facial expressions of threat-related emotions (fear, anger), loss-related emotions (sadness), reward-related emotions (happiness), and neutral emotions, modified such that the eyes are presented in the central position of the image.
Stimuli are presented under two conditions, one designed to engage implicit regulation of emotional reactivity and one to engage explicit appraisal. In the implicit condition, faces are presented below the sensory threshold for conscious discrimination of emotion, followed immediately by a neutral face perceptual mask. Mask stimuli are offset by 1° in the direction of each diagonal, randomly, to control for the potential effects of priming due to the perceptual difference between emotion-neutral and neutral-neutral target-mask pairs. Using a blocked design, stimuli are grouped with eight faces expressing the same emotion per block and repeated 5 times in a pseudorandom order.
In the explicit condition the same face stimuli are also presented in a blocked design. The stimulus appears with sufficient time to allow for conscious elaborative processing of the emotion stimulus. Facial expressions of emotion consistently elicit a contagious effect of experiencing the emotion signaled by the stimulus at the duration used, 500 ms (Wild, Erb, & Bartels, 2001). We create a context for participants to continuously view the faces by instructing them that they would be asked post-scan questions about these faces.
2.3.1.1.2. Emotional regulation task.
We will employ a modified version of an effortful emotion regulation task based on the psychological principles of strengthening, weakening and appraising emotional states (Kim & Hamann, 2007; Supplementary Materials Section 2.1.). The task will display sets of negative, positive, and neutral pictures from the International Affective Picture System (Lang, Bradley, & Cuthbert, 2008). Following every individual picture display, an instruction will be shown to either “increase”, “decrease”, or “watch. For “increase” instructions, participants are instructed to think about the picture in a way that elicits their emotions more intensely. In the “decrease” instructions, participants are told to think about the image in a way that reduced the intensity of their emotions (Kim & Hamann, 2007). The task is designed to induce positive and negative emotional states, as well as examine participants’ abilities to increase positive and decrease negative emotions when instructed. At the baseline visit, participants will be instructed on how to regulate their emotions in accordance with protocol used by Kim and Hamann (2007). At follow-up visits, participants will be asked if they need a reminder on the appropriate emotional regulation strategies, and given one if requested. For each trial, participants will be presented a white fixation cross, followed by a photograph, then a prompt to either “increase”, “decrease”, or “watch” will be overlaid on the image. The overlay will then be removed, and the participant will modulate their emotion. Finally, the participant will rate how strong their emotion is regarding the photo, post modulation, on a Likert scale from 1 to 4. Anchors for this scale are “Weak” for 1, and “Strong” for 4. The task will consist of nine repetitions of the five conditions (i.e. decrease negative, watch negative, increase positive, watch positive, and watch neutral). The watch conditions will represent a baseline emotional experience, when the participant is to be instructed to not attempt to modulate their emotions. The neutral condition will be included to control for the image viewing and button pressing, so that effects of mood-induction and emotional regulation processes can be isolated. The photo sets for each condition will be selected from the International Affective Picture Set (Lang et al., 2008) to achieve equivalent absolute values of valence and values of arousal, except for the neutral condition. Two different sets of pictures equated for valence and arousal will be alternated between visits, to minimize emotional habituation to the stimuli. Participants will be asked to identify their emotion regulation strategies after the scan to confirm proper strategies were used.
2.3.1.1.3. MRI assay acquisition parameters.
During each of the above two fMRI paradigms, blood oxygenation level-dependent contrast functional images will be acquired with echo-planar T1*-weighted imaging using 3.0 T GE Discovery MR750 scanner (GE Healthcare, Milwaukee, Wisconsin) with a 32-channel head coil. Each whole brain volume will consist of 45 interleaved 3 mm thick axial/oblique slices (74 × 74 matrix; TR, 2000 ms;; TE, 27.5 ms; size, 3 × 3 × 3 mm; FOV, 222 mm; flip angle, 77°). For the facial emotion viewing task we will acquire 154 vol over 5 min and 8 s. For the emotion regulation task, 350 vol will be acquired over 11 min and 40 s. To ensure BOLD saturation, three dummy scans will be acquired at the start of each acquisition.
Participants will have a heart rate monitor attached to their toe, a respiration belt attached around their waist, and two skin conductivity readers attached to their left middle and ring finger. Output will be recorded from the beginning to the end of each individual task.
A high-resolution T1-weighted structural scan will be acquired using a 3D spoiled gradient echo (SPGR) sequence at the end of the imaging session for use in normalization of the fMRI data into standard space. Furthermore, a diffusion scan will also be acquired, when time permits, to quantify white matter integrity.
2.3.1.1.4. MRI assay preprocessing steps.
Preprocessing and data analysis will be performed using Statistical Parametric Mapping software implemented in Matlab (SPM8; Wellcome Department of Cognitive Neurology) and the R statistical language in a manner similar to that of our prior publications (Goldstein-Piekarski et al., 2016; Supplementary Materials Section 2.3.). Specifically, images will be motion corrected by realigning and unwarping. Next, as in Power et al. (2014), we will conduct quality control by censoring volumes associated with large movement and/or changes in BOLD signal intensity. A temporal mask will then be created for each censored volume (as well as subsequent volume) and used as regressors of no interest in the first-level statistical models (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012, 2014). Images will be normalized to the stereotactic space of the Montreal Neurological Institute (MNI) template (Ashburner & Friston, 2005). T1-weighted data will be normalized to standard space using nonlinear registration, and the fMRI data will be coregistered to the T1 data using linear registration. Normalization warps from these two steps will be stored for use in functional to standard space transformations. Confounding signal will then be estimated within the ventricles and white matter and will be removed from the motion-corrected time series. Data will be smoothed using an 8 mm full-width at half-maximum Gaussian kernel and high-pass filtered.
2.3.1.1.5. MRI assay definition of affective circuits.
To define circuit-based constructs we will focus first on specific nodes in the circuit of interest using a region of interest (ROI) approach. With the ROI approach, we identify BOLD-dependent signal change in the defining nodes of the negative affect circuit, including the amygdala, insula, and anterior cingulate cortex/ventral and dorsal medial prefrontal cortex. We define the complementary positive affect circuit by the striatal nucleus accumbens and ventral tegmental areas and their projections to the orbitofrontal cortex and dorsal anterior cingulate cortex/ventromedial prefrontal cortex (Fig. 1). Beta values for each ROI will be extracted for each subject for regression analyses. We will also use functional connectivity analyses to quantify the functional relationships between regions. Further, exploratory whole brain, voxel-wise analyses will be conducted using a significance threshold of p < 0.05 corrected for multiple comparisons. In parallel we will study additional regions as part of the exploratory goals of the study.
2.3.1.2. Behavioral assays for regulation of emotion.
Behavioral data will be obtained from the browser-based tasks designed to evaluate the identification of facial emotions and emotional biases. Similar to the fMRI face-viewing task, faces are presented on a computer screen (96 stimuli, 8 different individuals), however, this paradigm will not be linked to the fMRI task. Identification will be recorded by the verbal labeling of the expressions and reaction time. Implicit priming of reaction time to “old/new” memory recognition of faces, primed by prior exposure to facial expressions of threat versus neutral, will be used to elicit biases to threat using an established procedure (Watters & Williams, 2011). The bias to fear will be the reaction time difference (in milliseconds) for priming due to threat minus neutral.
2.3.1.3. Self-report assays for regulation of emotion.
The Emotion Regulation Questionnaire (ERQ) has 10 items assessing individual differences in the habitual use of two emotion regulation strategies: cognitive reappraisal and expressive suppression (Gross & John, 2003). Cognitive reappraisal is a strategy that involves interpreting an emotion-eliciting situation in a way that changes its emotional impact, whereas expressive suppression is a form of response modulation that involves restraining emotion-expressive behavior (Gross & John, 2003).
The BRIEF COPE is a 28-item multidimensional coping inventory to assess the different ways in which people report responding to stress (Carver, Scheier, & Weintraub, 1989). There are several subscales that can describe an individual’s reported coping strategy, including the use of self-distraction, active coping, denial, substance use, use of emotional support, use of instrumental support, behavioral disengagement, venting, positive reframing, planning, humor, acceptance, religion, and self-blame (Carver et al., 1989). Depending on an individual’s coping strategy in response to a stressor, he or she may actively approach or avoid problems. Carver et al. (1989) have reported differences between an individual’s tendency to use approach-focused coping, (dealing directly with the stressor) or avoidance-focused coping (trying to avoid dealing with the stressor).
Some subscales most closely assay self-reported regulation of emotion. This includes the use of emotional support, venting, positive reframing, and humor.
The Brief Risk-resilience Index for Screening (BRISC) is a 45-item scale assessing emotional health and coping capacity (Williams et al., 2012). This scale will measure participants’ self-reported negativity bias, emotional resilience, and social skills.
2.3.2. Lab-based assays for target: regulation of cognition
2.3.2.1. MRI assays for regulation of cognition
2.3.2.1.1. Go-NoGo paradigm.
We will use the Go-NoGo paradigm that has been established as a robust probe of the cognitive control circuit relevant to regulation of cognitive functions (Korgaonkar, Cooper, Williams, & Grieve, 2012; Falconer, Allen, Felmingham, Williams, & Bryant, 2013; Falconer et al., 2008; Supplementary Materials Section 2.2.). The Go-NoGo paradigm allows for event-related analysis and is used to assess impulsivity (automatically-generated ‘Go’ responses) versus inhibition (‘NoGo’ responses). In the ‘Go’ trials, participants are required to press a button on GREEN stimuli (the word “press” displayed in green); in the ‘NoGo’ trials, participants withhold presses on RED stimuli (the word “press” displayed in red). The probability of NoGo stimuli is 0.33. There is a total of 180 Go and 60 NoGo stimuli presented in a pseudorandom order with a constraint to ensure that NoGo stimuli are not repeated more than 3 times in a row. Participants are asked to respond via button press as quickly as possible to the Go stimuli and inhibit their response for the NoGo stimuli. Reaction times and number of errors on task are used to evaluate task performance.
2.3.2.1.2. MRI assay acquisition parameters.
The MRI acquisition parameters will be the same as those outlined in for the MRI assays for regulation of emotion (Section 2.3.1.1.), including the duration of scan and number of volumes collected.
2.3.2.1.3. MRI assay preprocessing steps.
The MRI assay preprocessing parameters will be the same as those outlined for the MRI assays for regulation of emotion (Section 2.3.1.1.).
2.3.2.1.4. MRI assay definition of cognitive control circuit.
Using the ROI approach, we will define the cognitive control circuit by regions in the dorsolateral prefrontal cortex, anterior cingulate cortex, dorsal parietal cortex (DPC) and posterior cingulate gyrus (Fig. 1). Together these regions and their interconnectivity are implicated in the support of higher cognitive functions such as working memory and selective attention (Cole & Schneider, 2007; for meta-analysis; Niendam et al., 2012). We will also use functional connectivity analyses to quantify the functional relationships between regions and undertake exploratory analyses of additional regions.
2.3.2.2. Behavioral assays for regulation of cognition.
Behavioral assessments of cognitive regulation will be evaluated from performance on the following computerized cognitive tasks that are part of the previously established “WebNeuro” test battery (Mathersul et al., 2009). This includes the following:
2.3.2.2.1. Go-NoGo.
To assess impulsivity and response inhibition we will use the same task we do in the scanner, a previously established task in which participants respond quickly to green stimuli and withhold responses to red stimuli.
2.3.2.2.2. Digit span.
To assess working memory participants will be asked to hold online a span of 2–9 digits and then repeat these digits in order.
2.3.2.2.3. Verbal interference test.
Colored words with incongruent color–word combinations will be presented, and name (Part I) and color (Part II) of each word will be identified by the participant. This test assesses aspects of inhibition and interference corresponding to those indexed by the Stroop test (Golden, 1978).
2.3.2.2.4. Maze.
A total of 24 consecutive correct moves are required to identify a hidden path within an 8 × 8 “maze.” Incorrect moves elicit a red cross at bottom of screen, and correct moves a green tick. The test ends with two error-free completions (or time-out after 7 min). This is a computerized variation assessing constructs similar to those assessed by the Austin Maze (Walsh, 1985).
2.3.2.3. Self-report assays for regulation of cognition.
The BRIEF COPE is the same self-report inventory outlined in the Self-Report assays for Regulation of Emotion (Section 2.3.1.3.). Some subscales most closely assay self-reported regulation of cognition. This includes active coping, substance use, use of instrumental support, behavioral disengagement, planning, acceptance, and religion.
2.3.3. Lab-based assays for target: regulation of self-focused reflection
2.3.3.1. MRI assays for the regulation of self-focused reflection
2.3.3.1.1. Resting condition.
We will extract resting state fMRI data from the resting periods within our MRI assays, using a previously established procedure (Korgaonkar, Ram, Williams, Gatt, & Grieve, 2014). This method of obtaining resting connectivity from task fMRI data has been previously established and validated against non-task-derived resting state data (Korgaonkar et al., 2014).
2.3.3.1.2. MRI assay acquisition parameters.
The MRI acquisition parameters for extracted resting state fMRI data will be the same as those outlined for the MRI assays for regulation of emotion (Section 2.3.1.1.).
2.3.3.1.3. MRI assay preprocessing steps.
The MRI assay preprocessing parameters will be similar as those outlined for the MRI assays for regulation of emotion (Section 2.3.1.1.) with a few exceptions. First, in addition to the steps previously outlined, the connectivity data will further be slice time corrected. Second, since movement related artifacts have been shown to impact volumes acquired before and several seconds after a movement spike, to reduce the influence of movement related artifacts a total of four temporal masks will be created for each movement spike (an additional volume before and 2 vol after the movement spike) (Power et al., 2014). Third, white mater and CSF removal procedure will be replaced by the resting state extraction procedure described below.
2.3.3.1.4. Resting state extraction.
For each fMRI task, the BOLD responses for each experimental condition will be modeled in the general linear model framework. These effects will be removed and the remaining (residual) time-series signal is analyzed using SPM8 software. Motion effects will also be modeled for each task using the Volterra expansion of the realignment parameters proposed in Friston, Williams, Howard, Frackowiak, & Turner, 1996 (24 regressors).8 Additional covariates for each task will include the mean signal time course extracted from ventricle and white matter masks as well as the temporal masks of censored volumes. The resting state signal (task-derived resting activity) will then be extracted by removing variance by modeling the BOLD signal for each of the stimuli as covariates and residual images created after removing these effects (Supplementary Materials Section 2.4.). After this, the data will be band-pass filtered.
2.3.3.1.5. MRI assay definition of default mode circuit.
Using the ROI approach, we will define the default mode circuit by regions in the anterior medial prefrontal cortex (amPFC), anterior gyrus (AG), and posterior cingulate cortex (PCC) (Fig. 1). MR studies have demonstrated that these regions activate and show significant functional connectivity during periods of rest, deactivate during cognitive tasks (Greicius, Krasnow, Reiss, & Menon, 2003), and that the circuit is engaged during periods of rest between task stimuli (Korgaonkar et al., 2014). In the same fashion as the affective and cognitive control circuit analysis, we will evaluate ROI activation as well as within-circuit connectivity.
2.3.3.2. Self-report assays for regulation of self-focused reflection.
The BRIEF COPE is the same self-report inventory outlined in the Self-Report assays for Regulation of Emotion (Section 2.3.1.3.). Some subscales most closely assay self-reported regulation of self-focused reflection. This includes self-distraction, denial, and self-blame.
2.3.4. Immersive virtual reality assays for target: regulation of emotion
After the scan, participants will move to an adjacent room in the scanning facility for immersive virtual reality assays. We will plan to keep up to date with Oculus VR technology as it develops, and refine our assays to remain consistent with the goals of the project to refine our assays. We will commence with using the Oculus DK2 (Oculus, 2014) and plan to upgrade it with its successor (Oculus CV1; Supplementary Materials Section 3.1), once available for research use, to improve the screen resolution and head tracking (thereby producing greater feelings of immersion). Participants will undergo six different virtual environments selected for valence equivalence through in-house pilot-testing. These environments are intended to target the four circuits in a fixed order, Regulation of Self-Focused Reflection, Regulation of Emotion, and Regulation of Cognition; each of these is detailed in its corresponding section. Please see supplementary materials for additional information on our setup and virtual environments.
We will assess behavior in two negative emotion regulation environments for the negative affect circuit, and two positive emotion regulation environments for the positive affect circuit. Each visit time point corresponds to a different set of emotional regulation environments.
Emotional regulation virtual environments were selected from various online video databases, and were matched for positive and negative valence indicated from pilot testing to avoid leaving participants in a negative emotional state upon visit completion (Supplementary Materials Section 3.2.). Throughout each environment, participants will receive the same instructions and conditions they do in the fMRI emotional regulation task: “Increase” for the last 2/3rds of positive videos, “Decrease” for the last 2/3rds of negative videos, and “Watch” for the first 1/3rd of every mood-induction video.
2.3.4.1. Behavioral assays for regulation of emotion.
All participant-environment interactions will be recorded through FRAPS screen capture software setup on the headset (FRAPS, 2016). Additionally, pitch, yaw, and roll of the headset over the duration of each emotion regulation environment will be captured, as well as coordinates of the headset in space relative to the origin.
2.3.4.2. Self-report assays for regulation of emotion.
Twice throughout each virtual environment - two negative environments for the negative affect circuit, and two positive emotion regulation environments for the positive affect circuit - participants will be prompted to verbally rate the strength of their emotions from 0 to 8. When prompted to by the screen instruction “How strong? 0–8”, participants will state their ratings aloud for the experimenter to manually record.
After each environment, participants will fill out a self-report form reporting the strength of various emotions across multiple scales including amusement, anger, confusion, contempt, disgust, fear, happiness, pain, relief, sadness, tension, relaxation, nausea/discomfort, and strength of immersion. They will also rate how nauseous and uncomfortable each environment made them feel to monitor for motion sickness. After each environment, participants will also be asked to rate how successful they thought they were at increasing and decreasing their emotions.
2.3.5. Immersive virtual reality assays for target: regulation of cognition
All participants will also complete one cognitive control virtual environment relating to the cognitive control circuit (Supplementary Materials Section 3.3.). The cognitive control virtual environment will not vary as a function of visit time points.
VROG (Illusion Walk, 2014) was selected to assess cognitive control within VR (Oculus DK2) because of its conceptual similarity to Go-NoGo. Similar to the inhibitory action needed to avoid pressing during a red “PRESS,” participants (the “frogs” in this environment) will be told to avoid ‘eating’ wasps placed through the scene while trying to ‘eat’ as many non-wasp bugs as quickly and accurately as possible to give them the highest score. A research coordinator will record scores, non-wasp bugs eaten (“Go”), and wasps eaten (“NoGo”).
To anticipate the rapid upgrading of the Oculus system, we have also developed two other environments. The first we have ready is a second cognitive control environment using Crystal Rift (Psytec Games, 2016). Crystal Rift features a map editor, in which we have created a maze-like dungeon environment. Also intended to be parallel to Go-NoGo, participants will be instructed to run to the end of the several hallways as quickly as possible, but also to pause and wait if they encounter an open trap door. Open trap doors will close upon a few thousand milliseconds of waiting, increasing non-linearly by the end of the course. Automatic response inhibition will be assessed via frequency of premature advancement over these trap doors, which will result in the player’s avatar falling in. A research coordinator will record “fall-ins”.
Our third cognitive control environment is based on Fruit Ninja VR (Halfbrick Studios, 2016). The participant’s controllers will appear as swords in the participant’s virtual environment, and they will be instructed to use the controllers to cut through various fruits as they shoot up from the ground. If fruits hit the ground without being sliced, a “strike” is recorded. The round ends after three “strikes” or if the participant accidentally slices a bomb, which are visually distinct from the fruits but spatially intermixed with them. A research coordinator will record these strikes, bombs hit, and score per round.
We posit refraining from eating wasps in VROG, inability to override the automatic motion of forward movement over an open trap door in Crystal Rift, and refraining from striking a bomb in Fruit Ninja VR measure the same or a similar psychological construct that is responsible for inhibitory failures in Go-NoGo.
2.3.5.1. Behavioral assays for regulation of cognition.
The behavioral assays for regulation of cognition will be the same as those outlined for behavioral assays for regulation of emotion (Section 2.3.4.1.), with the addition of the score metrics listed above.
2.3.5.2. Self-report assays for regulation of cognition.
After each environment, participants will fill out a self-report form reporting the strength of reported immersion in cognitive challenges.
2.3.6. Immersive virtual reality assays for target: regulation of self-focused reflection
All virtual reality assay collections, will begin with the baseline/Mindfulness environment for the default mode circuit. This initial environment is intended to both target the Default Mode circuit and allow the participant to acclimate to VR. These environments are all 360° still images of various relaxing natural settings. This is intended to minimize sensory stimulation and engage self-reflective processes relevant to the Default Mode circuit.
At all follow-up visits, participants will also be allowed to choose one of two baseline/Mindfulness environments. This choice can ensure that participants are exposed to an environment they prefer, and consequently can experience the intended relaxing effects of the baseline/mindfulness environments. Point of view screenshots and hyperlinks to each environment are available in Supplementary Materials (Section 3.4.).
2.3.6.1. Behavioral assays for regulation of self-focused reflection.
The behavioral assays for regulation of self-focused reflection will be the same as those outlined for behavioral assays for regulation of emotion (Section 2.3.4.1.).
2.3.6.2. Self-report assays for regulation of self-focused reflection.
After each environment, participants will fill out a self-report form reporting the strength of immersion within the cognitive control virtual reality environment. They will also rate how nauseous and uncomfortable each environment made them feel to monitor for motion sickness.
2.3.7. Naturalistic assays (passive) for target: regulation of emotion
At baseline visits, the Mindstrong data collection application is installed on a participant’s smart phone and will provide continuous passive naturalistic sampling of 288 phone-use feature variables throughout the two-year study period. These features are unobtrusive, continuous and ecological. We will begin with 45 keyboard and scroll patterns, such as latency between a space and character or between a successive delete. From there, we will create a time-series of performance measures from each of the 45 patterns, and finally apply 23 signal processing transforms to each time-series to derive 1035 potential digital biomarkers. Mindstrong’s technology and algorithms were validated in two longitudinal cohort studies demonstrating that a user’s day-to-day smartphone keyboard and swipe-tap interactions contained repeated psychometric challenges capable of approximating gold-standard neuropsychological tests across a battery of domains.
Correlations of the prediction of the cognitive test scores were good (e.g., comparable to cognitive test-retest variability), with most correlation values ranging from 0.71 to 0.85 (Kerchner, Dougherty, & Dagum, 2015, pp. P272–P273). We apply machine learning on the cognitive features from this application to predict measures of several valuable constructs that are used in the study; each is described below.
2.3.7.1. Behavioral assays for regulation of emotion.
Several variables are obtained from participants’ smartphones using the Mindstrong app that relate to emotional states and regulation. The application will record word frequencies collected from input text in emails, text messages, or search terms as well as punctuation usage indicative of emotional states. Additionally, behavioral changes will be inferred from changes in social activity, such as incoming and outgoing calls and messages.
2.3.8. Naturalistic assays (passive) for target: regulation of cognition
The passive sampling acquisition parameters for cognitive self-regulation data will be the same as those outlined for the naturalistic assays for regulation of emotion. (Section 2.3.7.1).
2.3.8.1. Behavioral assays for regulation of cognition.
Using the Mindstrong features we will measure the ecologic day-to-day effect of emotional regulation on cognition and cognitive regulation. Variables indicative of cognitive regulation include typing latencies, reaction times, and phone stimuli detection.
2.3.9. Naturalistic assays (passive) for target: regulation of self-focused reflection
The passive sampling acquisition parameters for self-reflective regulation data will be the same as those outlined for the naturalistic assays for regulation of emotion (Section 2.3.7.1.).
2.3.9.1. Behavioral assays for regulation of self-focused reflection.
We will attempt to infer self-reflection related behavior from various relevant smartphone variables in GPS-indicated resting periods. Furthermore, these changes will be tracked over time and monitored for changes that may relate to participants’ diurnal behavior patterns.
2.4. Data analysis addressing study aims
We will use (1) unsupervised and (2) supervised data analysis to address our aims related to establishing target engagement within each setting and for each assay, and the relations between assays and settings, target malleability over time and how assays of self-regulation targets predict behavior change and outcomes. Because of our within-subject, repeated-measure design we will have rich data for each participant within each setting on each assay. This will give us the power to tease out relations not only at the cross-sectional baseline time point but to determine the extent to which assays and the relations between them change in a cohesive way over time. In regard to Aim 1, we will first assess whether our targets are engaged by each of the assays at baseline; for example, we will determine whether regions of the negative affect circuit are activated significantly by the facial emotion viewing task for the contrast of negative emotion minus neutral. We will investigate the extent to which assays of each target correlate with each other within and between settings, and whether specific targets or combination of targets explain more variance within the sample than others.
In regard to Aim 2, we will investigate the extent to which the assays of each target change at repeat testing sessions, and also have the opportunity to combine assays at multiple time points to investigate if the change in a target assay is related to change in another target assay as well as to behaviors and outcome measures. Furthermore, we will use sparse regression approaches to develop predictive models of how a combination of assays capturing the activity of a self-regulation circuit predicts target malleability and changes in health behaviors and outcomes. Finally, we will use regular linear regression with and without regularization when needed to reduce the number of predictors and make a sparse model. Regularized linear models acts like a stretchable fishing net to remove the irrelevant predictors and retain “all the big fish” (Zou & Hastie, 2005).
To address Aim 3, we will assess whether our baseline target assays and their early response within 2 months of treatment predict subsequent healthy behaviors and outcomes, including the moderation of these relationships by the intervention. For Aim 3 we will model change in the behavior or outcome of interest as a function of baseline measurements and change at time point 1 (i.e., 2 months), the treatment and interactions between treatment and baseline measurements. We will use regular linear regression (as we will for Aim 2) with and without regularization when needed to reduce the number of predictors and make a sparse model. In addition to conventional supervised approach, we will use more advanced unsupervised analysis in the form of hierarchical clustering to investigate the correlation structure of all assays designed to capture a single self-regulation target. This provides a more high-level multivariate overview of the structure of our three targets considered together. We will also explore deeper dives into the assays, particularly those with high dimensionality such as the MRI assays. For example, we will analyze the unbiased imaging data to identify potential novel subgroups by looking at finer-grained anatomical brain structures and use a data driven approach to define brain structures. In this case, we start from the raw high-dimensional imaging feature vectors in combination with consensus clustering analysis to investigate the existence of novel pathways defining the self-regulation circuits.
In our prediction models for addressing Aim 3, we will use classification approaches investigated in parallel such that they can inform each other. To limit overfitting, we will pursue cross-validation approaches to estimate the performance of supervised models. To address our goals of establishing a prototype for a personalized medicine of behavior change, we will use goodness-of-fit statistics to develop a taxonomy that classifies each subject according to his/her primary dysfunction on each self-regulation target and, where relevant, secondary and tertiary dysfunctions. This taxonomy will form the basis for a mechanistic understanding of why each individual participant responded or did not response to the intervention, as assessed by matching their profile of self-regulation to the extent of their behavior change.
2.5. Data and computational management; reproducibility
The data acquired from the MR scanner will be directly transferred into a secure data and computation management system (Flywheel.io). In addition to the MRI data, behavioral and physiological measurements will be uploaded to the system. All of the data can be searched according to subject and metadata properties (e.g., age, sex, dates, and so forth). The system also simplifies user rights management; access can be easily granted subject to IRB requirements. This makes it straightforward to share both the data and computations.
The Flywheel system also automates several computational methods for file conversion, quality assessment, and basic analyses. Custom analyses can also be written and added to the system as Flywheel Gears. In addition to the integrated analysis tools, the raw data or analysis results can be downloaded via the Flywheel API and further analyzed using Python, Matlab, R, and other programming languages. The system is capable of recording the parameters and results of a computational method, supporting reproducibility of the analyses.
3. Discussion
The SOBC experimental medicine approach is spearheading developments to increase our understanding of how individuals achieve behavior change to advance positive health outcomes. Following a precision medicine perspective, the ENGAGE study seeks to address the staggering gap between the fields of human neuroscience and behavioral science, and their application in optimizing interventions. To bridge this gap, we ground our study in a theoretical perspective focused on constructs of self-regulation that may be understood and quantified from both neuroscience-informed and behavioral points of view. Within the ENGAGE study we develop one approach to integrating these viewpoints. We have identified three target aspects of self-regulation: emotion regulation, cognitive control and self-reflection. These self-regulation targets will be assayed in a rich, longitudinal within-subjects design that enables us to identify which targets relate most to behavioral and health outcomes, which change together, and which predict who will achieve behavioral change following an intervention and why. In order to lay the foundations for translation of the findings into real-world practice, we integrate lab-based neuroimaging assays of neural circuits with virtual-reality induced behaviors and naturalistic smartphone sampling of daily choices. Because depression and obesity are such rapidly growing areas of serious global health concern (GBD 2015 Disease and Injury Incidence and Prevalence Collaborators, 2016; GBD 2015 Risk Factors Collaborators, 2016; The Global BMI Mortality Collaboration, 2016), we focus on these health issues. Our mechanistically-oriented ENGAGE study benefits from its operational linkage to a clinical trial (Ma et al., 2015) in which interventions are aimed at both depression and obesity. Our behavioral and health outcomes of focus include medical regimen adherence as well as mood and weight change, in order to ultimately prevent morbidity and mortality and reduce the spike in lifetime disability caused by comorbid depression and obesity. By directly linking a complex neuroscience study to a rigorous behavioral intervention trial, we will be in a position to immediately utilize the knowledge of the self-regulation targets and assays we will gain to refine and enable target-driven individual tailoring of our intervention. This is precision medicine for behavior change.
We are encouraged by the feasibility of such mechanism-focused individual intervention tailoring based on our prior research in depressed people, work that informed the selection of the assays for ENGAGE. For example, using lab-based brain imaging and behavioral measures, we have found that profiles of over- or under-regulation on targets assessing emotional, cognitive, and intrinsic (resting) aspects of self-regulation, predict with high accuracy which patients with depression will or will not benefit from antidepressant treatments (McRae, Rekshan, Williams, Cooper, & Gross, 2014; Williams et al., 2015; Gyurak et al., 2016; Goldstein-Piekarski et al., 2016; Shilyansky et al., 2016; Goldstein-Piekarski et al., in submission). We have also found that weight, determined by body mass index is an important predictor of personalized treatment outcomes in depression (Green et al., 2016). Thus, we foresee the capacity to use self-regulation profiles identified by ENGAGE assays to personalize the precise combination and implementation of intervention strategies that are based on an integrated behavioral-pharmacological approach. Based on the findings from ENGAGE, we will define a classification taxonomy of self-regulation profiles based on individual differences in engagement of self-regulation targets at baseline and over time, in particular in response to early treatment. We envision developing a “menu” of intervention strategies tailored to these self-regulation profiles. The intervention tailoring strategies could include augmentation, reduction, or elimination of existing intervention components as well as addition or replacement with additional components. Ultimately, tailoring based on the self-regulation taxonomy will incorporate an individual’s emotional, cognitive, and self-reflective regulation profiles to customize the intervention strategies for optimal potency and efficiency for the individual.
Our study has broad relevance, given the prevalence and burden from depression and obesity, the scope of the targeted health behaviors, and the proven efficacy of the integrated interventions that comprise the RAINBOW trial. ENGAGE will be a prototype experimental personalized medicine study for understanding and optimizing behavioral treatment for co-occurring chronic conditions, with excellent generalizability to other clinical areas (e.g., diabetes, cardiovascular disease, and cancer). ENGAGE exemplifies a mechanism-focused experimental approach to developing precision medicine, with the potential for a profound impact on the science of behavior change and on population health.
Supplementary Material
Acknowledgements
This work is supported by the National Institutes of Health (NIH) Science of Behavior Change Common Fund Program through an award administered by the National Heart, Lung, and Blood Institute grant number UH2HL132368. The views presented here are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
The RAINBOW-ENGAGE study is supported by NIH Grant 1UH2AG052163-01. We thank our consultants and scientific collaborators for their expert input on self-regulation task development (James Gross, Ph.D.) and on VR environment development (Benjy Li, Ph.D.).
Funding
This work was supported by the National Institutes of Health [grant number UH2HL132368]; Science of Behavior Change (SOBC) project: “Engaging self-regulation targets to understand the mechanisms of behavior change and improve mood and weight outcomes”.
Footnotes
Disclosures
LMW: Received direct (non-salary) research funding from Brain Resource Pty Ltd. as cross-site Academic Principal Investigator lead for the ISPOT-D study (2008–2013).
TS: Consulting fees from A/S H. Lundbeck, Sunovion, Merck & Co, and Astra Zeneca.
AP, ANG-P, LGS, MK, MDS, OG, JM, PWL, PD, BW, CC, WG, LMP, JMSm, MAL, EMV, MS, JMSi, JM: Nothing to disclose.
Trial registration
Trial Registry Name: ClinicalTrials.gov.
Registration Number: NCT02246413.
URL: https://clinicaltrials.gov/ct2/show/NCT02246413.
Open Science Framework URL: https://osf.io/u37e9/
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.brat.2017.09.012.
Sometimes known as a central executive circuit.
References
- Achtziger A, Gollwitzer PM, & Sheeran P (2008). Implementation intentions and shielding goal striving from unwanted thoughts and feelings. Personality & Social Psychology Bulletin, 34(3), 381–393. [DOI] [PubMed] [Google Scholar]
- Ashburner J, & Friston KJ (2005). Unified segmentation. Neuroimage, 26, 839–851. [DOI] [PubMed] [Google Scholar]
- Atlantis E, & Baker M (2008). Obesity effects on depression: Systematic review of epidemiological studies. International Journal of Obesity, 32, 881–891. [DOI] [PubMed] [Google Scholar]
- Bailenson JN, Pontikakis ED, Mauss IB, Gross JJ, Jabon ME, Hutcherson CAC, et al. (2008). Real-time classification of evoked emotions using facial feature tracking and physiological responses. International Journal of Human-computer Studies, 66(5), 303–317. [Google Scholar]
- Bjerkesset O, Romundstad P, Evans J, & Gunnell D (2008). Association of adult body mass index and height with anxiety, depression, and suicide in the general population: The HUNT study. American Journal of Epidemiology, 167(2), 193–202. [DOI] [PubMed] [Google Scholar]
- Blaine B (2008). Does depression cause obesity?: A meta-analysis of longitudinal studies of depression and weight control. Journal of Health Psychology, 13(8), 1190–1197. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, & Yeo BT (2013). Opportunities and limitations of intrinsic functional connectivity MRI. Natural Neuroscience, 16(7), 832–837. [DOI] [PubMed] [Google Scholar]
- Cape J, Whittington C, Buszewicz M, Wallace P, & Underwood L (2010). Brief psychological therapies for anxiety and depression in primary care: meta-analysis and meta-regression. BMC Medicine, 8(1), 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter KM, Hasin DS, Allison DB, & Faith MS (2000). Relationships between obesity and DSM-IV major depressive disorder, suicide ideation, and suicide attempts: Results from a general population study. American Journal of Public Health, 90, 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Scheier MF, & Weintraub JK (1989). Assessing coping strategies: A theoretically based approach. Journal of Personality and Social Psychology, 56, 267–283. [DOI] [PubMed] [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, & Peterson SE (2014). Intrinsic and task-evoked network architectures of the human brain. Neuron, 83(1), 238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, & Schneider W (2007). The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage, 37(1), 343–360. [DOI] [PubMed] [Google Scholar]
- Curran GM, Bauer M, Mittman B, Pyne JM, & Stetler C (2012). Effectiveness-implementation hybrid designs: Combining elements of clinical effectiveness and implementation re- search to enhance public health impact. Medical Care, 50, 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit L, Luppino F, van Straten A, Penninx B, Zitman F, & Cuijpers P (2010). Depression and obesity: A meta-analysis of community-based studies. Psychiatry Research, 178(2), 230–235. [DOI] [PubMed] [Google Scholar]
- DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, et al. (2007). Successful dieters have increased neural activity in cortical areas involved in the control of behavior. International Journal of Obesity (London), 31(3), 440–448. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Kozink RV, McClernon FJ, & Smoski MJ (2012). Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. Journal of Affective Disorders, 136(3), 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragan A, & Akhtar-Danesh N (2007). Relation between body mass index and depression: A structural equation modeling approach. BMC Medical Research Methodology, 7, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Hoeft F, Menon V, & Schatzberg AF (2010). Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. American Journal of Psychiatry, 167(5), 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith MS, Matz PE, & Jorge MA (2002). Obesity-depression associations in the population. Journal of Psychosomatic Research, 53, 935–942. [DOI] [PubMed] [Google Scholar]
- Falconer E, Allen A, Felmingham KL, Williams LM, & Bryant RA (2013). Inhibitory neural activity predicts response to cognitive-behavioral therapy for posttraumatic stress disorder. Journal of Clinical Psychiatry, 74, 895–901. [DOI] [PubMed] [Google Scholar]
- Falconer E, Bryant R, Felmingham KL, Kemp AH, Gordon E, Peduto A, et al. (2008). The neural networks of inhibitory control in posttraumatic stress disorder. Journal of Psychiatry Neuroscience, 33, 413–422. [PMC free article] [PubMed] [Google Scholar]
- Fox J, Bailenson JN, & Ricciardi T (2012). Physiological responses to virtual selves and virtual others. Journal of CyberTherapy & Rehabilitation, 5(1), 69–72. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, & Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102(27), 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAPS (2016). Computer software. FRAPS real-time video capture and benchmarking. Vers. 3.5.99. N.p., n.d. Web. 27 Sept. 2016.
- Friedman MA, & Brownell KD (1995). Psychological correlates of obesity: Moving to the next research generation. Psychological Bulletin, 117, 3–20. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, & Turner R (1996). Movement-related effects in fMRI time-series. Magnetic Resonance Medicine, 35(3), 346–355. [DOI] [PubMed] [Google Scholar]
- Gillison F, Stathi A, Reddy P, Perry R, Taylor G, Bennett P, et al. (2015). Processes of behavior change and weight loss in a theory-based weight loss intervention program: A test of the process model for lifestyle behavior change. International Journal of Behavioral Nutrition and Physical Activity, 12(1), 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Disease 2015 Disease and Injury Incidence and Prevalence Collaborators (2016). Global, regional, and national incidence, prevalence, and years lived with disability for 310 disease and injuries, 1990–2015: A systematic analysis for the global burden of disease study 2015. The Lancet, 388(10053), 1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Disease 2015 Risk Factors Collaborators (2016). Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the global burden of disease study 2015. The Lancet, 388(10053), 1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden C (1978). A manual for the clinical and experimental use of the Stroop color and word test. College of Psychology: Faculty Books and Book Chapters Retrieved from http://nsuworks.nova.edu/cps_facbooks/47. [Google Scholar]
- Goldstein-Piekarski AN, Korgaonkar MS, Green E, Suppes T, Schatzberg AF, Hastie T, et al. (2016). Human amygdala engagement moderated by early life stress exposure is a biobehavioral target for predicting recovery on antidepressants. Proceedings of the National Academy of Sciences of the United States of America, 113(42), 11955–11960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, & Petersen SE (2014). Generation and evaluation of a cortical area parcellation from resting-state correlations. Cerebral Cortex, 26(1), 288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E, Goldstein-Piekarski AN, Schatzberg AF, Rush AJ, Ma J, & Williams LM (2016). Personalizing antidepressant choice by sex, body mass index, and symptom profile: An iSPOT-D report. Personalized Medicine in Psychiatry, 1–2, 65–73. [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, & Menon V (2003). Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Science of the United States of America, 100(1), 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, & John OP (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85(2), 348. [DOI] [PubMed] [Google Scholar]
- Gyurak A, Patenaude B, Korgaonkar MS, Grieve SM, Williams LM, & Etkin A (2016). Fronto-parietal activation during response inhibition predicts remission to antidepressants in patients with major Depression: Outcomes from iSPOT-D a randomized trial. Biological Psychiatry, 79(4), 274–281. [DOI] [PubMed] [Google Scholar]
- Haber SN, & Knutson B (2010). The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology, 35(1), 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfbrick Studios Pty Ltd (2016). Fruit Ninja VR (version 1.0). Available from:https://vr.fruitninja.com/.
- Heatherton TF (2011). Neuroscience of self and self-regulation. Annual Review of Psychology, 62, 363–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo M, Pietrobelli A, Fontaine KR, Sirey JA, & Faith MS (2006). Depressive mood and obesity in US adults: Comparison and moderation by sex, age, and race. International Journal of Obesity, 30(3), 513–519. [DOI] [PubMed] [Google Scholar]
- Walk Illusion (2014). VROG. Available from: http://vrog-game.com/.
- Istvan J, Zavela K, & Weidner G (1992). Body weight and psychological distress in NHANES I. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity, 16(12), 999–1003. [PubMed] [Google Scholar]
- Jensen MD, Ryan DH, & Apovian CM (2014). 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. Journal of the American College of Cardiology, 63(25), 2985–3023. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, & Phillips ML (2005). The neural correlates of anhedonia in major depressive disorder. Biological Psychiatry, 58(11), 843–853. [DOI] [PubMed] [Google Scholar]
- Kerchner GA, Dougherty RF, & Dagum P (2015). Unobtrusive neuropsychological monitoring from smart phone use behavior. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 11(7), P272–P273. [Google Scholar]
- Kim SH, & Hamann S (2007). Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience, 19(5), 776–798. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, & Wager TD (2008). Functional grouping and cortical-subcortical interactions in emotion: A meta-analysis of neuroimaging studies. Neuroimage, 42(2), 998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar MS, Cooper NJ, Williams LM, & Grieve SM (2012). Mapping inter-regional connectivity of the entire cortex to characterize major depressive disorder: A whole-brain diffusion tensor imaging tractography study. Neuroreport, 23(9), 566–571. [DOI] [PubMed] [Google Scholar]
- Korgaonkar MS, Ram K, Williams LM, Gatt JM, & Grieve SM (2014). Establishing the resting state default mode network derived from functional magnetic resonance imaging tasks as an endophenotype: A twins study. Human Brain Mapping, 35(8), 3893–3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report, A-8. [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, … Williams LM (2005). A direct brainstem–amygdala–cortical ‘alarm’system for subliminal signals of fear. Neuroimage, 24(1), 235–243. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, & Barrett LF (2012). A functional architecture of the human brain: Emerging insights from the science of emotion. Trends in Cognitive Sciences, 16(11), 533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lövdén M, Wenger E, Mårtensson J, Lindenberger U, & Bäckman L (2013). Structural brain plasticity in adult learning and development. Neuroscience and Biobehavioral Reviews, 37(9 Pt B), 2296–2310. [DOI] [PubMed] [Google Scholar]
- Markowitz S, Friedman MA, & Arent SM (2008). Understanding the relation between obesity and depression: Causal mechanisms and implications for treatment. Clinical Psychology: Science and Practice, 15(1), 1–20. [Google Scholar]
- Mathersul D, Palmer DM, Gur RC, Gur RE, Cooper N, Gordon E, et al. (2009). Explicit identification and implicit recognition of facial emotions: II. Core domains and relationships with general cognition. Journal of Clinical and Experimental Neuropsychology, 31(3), 278–291. [DOI] [PubMed] [Google Scholar]
- Ma J, & Xiao L (2010). Obesity and depression in US women: Results from the 2005–2006 national health and nutritional examination survey. Obesity, 18(2), 348–353. [DOI] [PubMed] [Google Scholar]
- Ma J, Yank V, Lv N, Goldhaber-Fiebert JD, Lewis MA, Kramr MK, et al. (2015). Research aimed at improving both mood and weight (RAINBOW) in primary care: A type 1 hybrid design randomized controlled trial. Contemporary Clinical Trials, 43, 260–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Rekshan W, Williams LM, Cooper N, & Gross JJ (2014). Effects of antidepressant medication on emotion regulation in depressed patients: An iSPOT-D report. Journal of Affective Disorders, 159, 127–132. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, & Carter CS (2012). Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive Affective Behavioral Neuroscience, 12(2), 241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, Hirvonen J, Hannukainen JC, Immonen H, Lindroos MM, Salminen P, et al. (2012). Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PLoS One, 7(2), e31089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaten M, & Cheng K (2006). Improved self-control: The benefits of a regular program of academic study. Basic and Applied Social Psychology, 28(1), 1–16. [Google Scholar]
- Oculus VR (2014): Announcing the Oculus Rift Development Kit 2 (DK2). Weblog post, 19 Mar. http://www.oculus.com/blog/announcing-the-oculus-rift-development-kit-2-dk2/. Accessed 27 September 2016.
- Onyike CU, Crum RM, Lee HB, Lyketsos CG, & Eaton WW (2003). Is obesity associated with major depression? Results from the third national health and nutrition examination survey. American Journal of Epidemiology, 158(12), 1139–1147. [DOI] [PubMed] [Google Scholar]
- Oosterwijk S, Lindquist KA, Anderson E, Dautoff R, Moriguchi Y, & Barrett LF (2012). States of mind: Emotions, body feelings, and thoughts share distributed neural networks. NeuroImage, 62(3), 2110–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, & Petersen SE (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage, 59(3), 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. (2011). Functional network organization of the human brain. Neuron, 72(4), 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, & Petersen SE (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage, 84, 320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psytec Games Ltd (2016). Crystal Rift (Version 1.2.9) [Crystal Rift]. Available from http://store.steampowered.com/app/345140.
- Roalf DR, Ruparel K, Gur RE, Bilker W, Gerraty R, Elliot MA, et al. (2014). Neuroimaging predictors of cognitive performance across a standardized neurocognitive battery. Neuropscyhology, 28(2), 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Krimsky M, Lieberman L, Allen P, Vytal K, & Grillon C (2014). The dorsal medial prefrontal (anterior cingulate) cortex-amygdala aversive amplification circuit in unmedicated generalised and social anxiety disorders: An observational study. Lancet Psychiatry, 1(4), 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman AJ, Sheeran P, & Wood W (2009). Reflective and automatic processes in the initiation and maintenance of dietary change. Annals of Behavioral Medicine, 38(Suppl 1), S4–S17. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilyansky C, Williams LM, Gyurak A, Harris A, Usherwood T, & Etkin A (2016). Effect of antidepressant treatment on cognitive impairments associated with depression: A randomised longitudinal study. The Lancet Psychiatry, 3(5), 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GE, Ludman EJ, Linde JA, Operskalski BH, Ichikawa L, Rohde P, et al. (2008). Association between obesity and depression in middle-aged women. General Hospital Psychiatry, 30(1), 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, & Schacter DL (2013). Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. Journal of Cognitive Neuroscience, 25(1), 74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW 3rd, Twieg DB, Knowlton RC, & Cox JE (2008). Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage, 41(2), 636–647. [DOI] [PubMed] [Google Scholar]
- Strine TW, Mokdad AH, Dube SR, Balluz LS, Gonzalez O, Berry JT, et al. (2008). The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. General Hospital Psychiatry, 30(2), 127–137. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Faith MS, & Allison KC (2003). Depression and obesity. Biological Psychiatry, 54(3), 330–337. [DOI] [PubMed] [Google Scholar]
- Tangney JP, Baumeister RF, & Boone AL (2004). High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. Journal of Personality, 72(2), 271–324. [DOI] [PubMed] [Google Scholar]
- The Global BMI Mortality Collaboration (2016). Body-mass index and all-cause mortality: Individual participant-data meta-analysis of 239 prospective studies in four continents. The Lancet, 388(10046), 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, & Zald DH (2011). Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neuroscience and Biobehavioral Reviews, 35(3), 537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuulari JJ, Karlsson HK, Hirvonen J, Salminen P, Nuutila P, & Nummenmaa L (2015). Neural circuits for cognitive appetite control in healthy and obese individuals: An FMRI study. PLoS One, 10(2), e0116640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh KW (1985). Understanding brain damage: A primer of neuropsychological evaluation. Churchill Livingstone. [Google Scholar]
- Watters AJ, & Williams LM (2011). Negative biases and risk for depression; integrating self-report and emotion task markers. Depression and Anxiety, 28(8), 703–718. [DOI] [PubMed] [Google Scholar]
- Wild B, Erb M, & Bartels M (2001). Are emotions contagious? Evoked emotions while viewing emotionally expressive faces: Quality, quantity, time course and gender differences. Psychiatry Research, 102(2), 109–124. [DOI] [PubMed] [Google Scholar]
- Williams LM (2016). Precision psychiatry: A neural circuit taxonomy for depression and anxiety. Lancet Psychiatry, 3(5), 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM (2017). Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: A theoretical review of the evidence and future directions for clinical translation. Depression and Anxiety, 34(1), 9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Liddell BJ, Rathjen J, Brown KJ, Gray J, Phillips M, et al. (2004). Mapping the time course of nonconscious and conscious perception of fear: An integration of central and peripheral measures. Human Brain Mapping, 21(2), 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Das P, Liddell BJ, Kemp AH, Rennie CJ, & Gordon E (2006a). Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. Journal of Neuroscience, 26(36), 9264–9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Liddell BJ, Kemp AH, Bryant RA, Meares RA, Peduto AS, et al. (2006b). Amygdala–prefrontal dissociation of subliminal and supraliminal fear. Human Brain Mapping, 27(8), 652–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Gatt JM, Hatch A, Palmer DM, Nagy M, Rennie C, et al. (2008). The integrate model of emotion, thinking and self regulation: An application to the “paradox of aging”. Journal of Integrative Neuroscience, 7(3), 367–404. [DOI] [PubMed] [Google Scholar]
- Williams LM, Cooper NJ, Wisniewski SR, Gatt JM, Koslow SH, Kulkarni J, et al. (2012). Sensitivity, specificity, and predictive power of the “Brief Risk-resilience Index for SCreening,” a brief pan-diagnostic web screen for emotional health. Brain and Behavior, 2(5), 576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Korgaonkar MS, Song YC, Paton R, Eagles S, Goldstein-Piekarski A, et al. (2015). Amygdala reactivity to emotional faces in the prediction of general and medication-specific responses to antidepressant treatment in the randomized iSPOT-D trial. Neuropsychopharmacology, 40(10), 2398–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing RR, Tate DF, Gorin AA, Raynor HA, & Fava JL (2006). A self-regulation program for maintenance of weight loss. The New England Journal of Medicine, 355(15), 1563–1571. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, & Hastie T (2005). Regularization and variable selection via the elastic net. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 67(2), 301–320. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


