Abstract
Obstructive sleep apnea syndrome is caused by upper airway collapse during inspiration, causing intermittent hypoxemia, hypercapnia, acidosis, sympathetic nervous system activation, and arousal from sleep. Nighttime blood pressure is higher, but unexpectedly, daytime hypertension occurs. The prevalence of hypertension is very high and the incidence of hypertension increases as the number of apneic and hypopneic events per hour rises. Obesity is a major predisposing factor for the development of obstructive sleep apnea. Daytime sleepiness, snoring, and breathing pauses are important symptoms to elicit from the patient or sleep partner. Resistant hypertension is an important clue. Overnight polysomnography is required for diagnosis. Weight loss, avoidance of nocturnal sedatives, cessation of evening alcohol ingestion, and avoidance of the supine position during sleep are initial therapeutic actions in mild obstructive sleep apnea syndrome. Continuous positive airway pressure is the treatment of choice for patients unable to find relief from lifestyle changes. Blood pressure modestly improves with treatment.
Obstructive sleep apnea (OSA) syndrome is a variety of sleep‐disordered breathing commonly associated with daytime sleepiness. Oxygen desaturation (Figure 1) and carbon dioxide retention during sleep activate the sympathetic nervous system and elevate blood pressure (BP). OSA is considered a secondary cause of hypertension that can be screened with home overnight oximetry. Cyclic desaturation may indicate the presence of OSA, although the absence of desaturation does not rule out OSA. Apnea is defined as cessation of breathing for 10 seconds or longer, and hypopnea is a reduction in airflow with a concomitant reduction in oxygen saturation. A sleep study is diagnostic. 1
Figure 1.
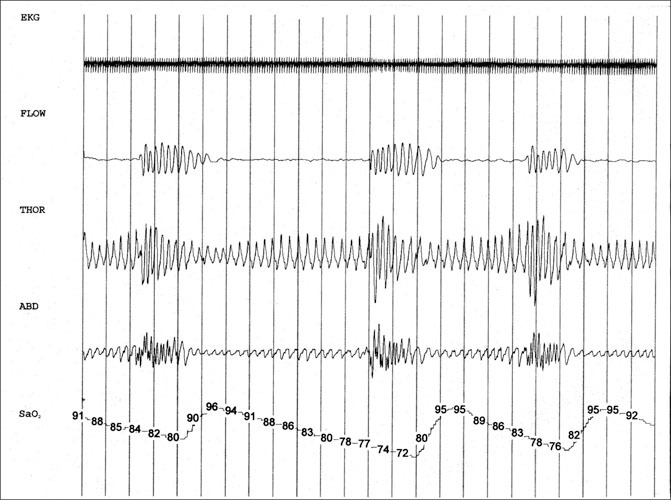
Polysomnogram of a 55‐year‐old male hypertensive patient with sleep apnea. Each vertical line indicates 10 seconds. The top tracing (EKG) shows the electrocardiographic rhythm. A few ectopic beats can be seen in the latter half of the tracing. There is slightly reduced amplitude of the QRS complex with respiration. The second tracing (FLOW) shows airflow from the nose and mouth by temperature probe (thermistor). The 4 periods with no airflow constitute apneas, the longest lasting over a minute. The third (THOR) and fourth (ABD) tracings show the tidal breathing pattern of the chest and abdomen. Respiratory efforts continue throughout apneas, indicative of obstructive apnea events. During apnea, expansion of the chest and abdomen decreases due to lack of airflow despite effort to breathe. The expansion increases with opening of the airway. The phase relationship between chest and abdomen changes with opening and closing of the airway. The bottom tracing (SaO2) shows oxyhemoglobin saturation by finger pulse oximetry. The oximeter tracing lags behind respiratory events due to circulation time. The worst desaturation dips to 72% but recovers to 95% with airway opening. Gradual lowering of the baseline saturation may occur with hypercapnia that persists between apneas.
PATHOPHYSIOLOGY
Upper airway patency is maintained by genioglossus contraction and lung volume expansion. 2 Airway patency can be compromised by a small or posteriorly placed mandible, redundant soft palate, tonsil‐lar hypertrophy, macroglossia, and pharyngeal fat deposition. During sleep, muscle tone is lost, and upper airway collapse during inspiration occurs, causing intermittent hypoxemia, hypercapnia, acidosis, sympathetic nervous system activation, and arousal from sleep. BP increases during each cycle of sleep‐disordered breathing. The normal 20% decline in nocturnal BP is diminished or absent. 3
Since sleep‐disordered breathing is also associated with daytime hypertension, other mechanisms must be active (Figure 2). 4 , 5 Obesity, which causes pharyngeal fat deposition, is a major factor. 6 Levels of leptin, a hormone that decreases appetite, promotes energy utilization, and controls ventilation, 7 are paradoxically higher in obesity, but leptin is ineffective (ie, leptin resistance). Chronic hyperleptinemia increases BP. Leptin levels are higher in patients with OSA compared with obese patients without sleep apnea. 8 Other factors promoting daytime hypertension include increased sympathetic nervous system activity, 9 insulin resistance, 10 activation of the renin‐angiotensin‐aldosterone system, systemic inflammation, 11 altered oxidative stress, endothelial dysfunction, and impaired arterial baroreflex function. 6 , 12
Figure 2.
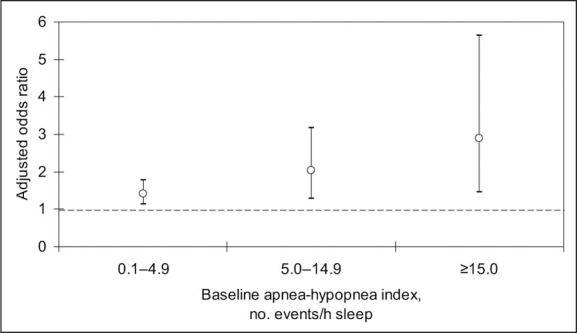
Adjusted odds ratios of incident hypertension. The odds ratio was adjusted for baseline hypertension status, nonmodifiable risk factors, habitus, and the use of alcohol and tobacco. P for trend =.002. Figure derived from data of Peppard et al. 5
Apolipoprotein E e4 elevates total cholesterol, reduces high‐density lipoprotein cholesterol, and is implicated in coronary atherosclerosis and Alzheimer's disease. In obese and nonobese patients younger than 65 years, the apolipoprotein E e4 allele is associated with an increased risk of OSA, but the mechanism for this association has not yet been elucidated. 13
EPIDEMIOLOGY
The Sleep Heart Health Study 4 is a community‐based, multicenter trial of 6132 individuals 40 years or older. This prospective cohort study was designed to evaluate the relationship between sleep‐disordered breathing and the development of cardiovascular disease. Participants were fitted with portable polysomnography equipment that recorded an electroencephalogram, electrooculogram, chin electromyogram, electrocardiogram, oxygen saturation, nasal/oral airflow, chest wall and abdominal movement, and body position. Sleep‐disordered breathing was quantified using the apnea‐hypopnea index (AHI), which refers to the average number of apneic and hypopneic episodes per hour. Higher AHI values were observed with male gender, self‐reported snoring, increasing body mass index (BMI) and neck circumference, higher BP, and an increased number of sleep arousals per hour after correction for BMI. The odds ratio for hypertension after adjustment for BMI, neck circumference, waist/hip ratio, alcohol use, and smoking increased with an increasing AHI and the percentage of sleep time with <90% oxygen saturation. A subsequent report observed no relationship of sleep‐disordered breathing with isolated systolic hypertension or with hypertension among patients 60 years or older. 14
In a 1993 report from the Wisconsin Sleep Cohort Study, the prevalence of sleep‐disordered breathing was estimated at 9% for women and 24% for men. 15 It was predicted that 2% of women and 4% of men in a middle‐aged work force met the minimal criteria for the sleep apnea syndrome. The Wisconsin Sleep Cohort Study prospectively performed 18‐channel polysomnography at baseline and after 4 years in 709 patients. 5 The incidence of hypertension increased as AHI rose (Figure 2). After adjustment for risk factors, age, gender, body habitus, and tobacco and alcohol use, the incidence of hypertension among individuals with 0.1–4.9 events per hour was 42% compared with those patients with no episodes.
Cardiovascular conditions associated with OSA include arrhythmias (atrial fibrillation, nonsustained ventricular tachycardia, and complex ventricular ectopy), sudden death, heart failure, myocardial infarction, and stroke. 16 , 17 Sudden death occurs more commonly between midnight and 6 AM and correlates with worsening AHI (hypoxemia). 18
CLINICAL HISTORY
Excessive daytime sleepiness is a cardinal symptom of OSA. 19 This can be subjectively quantified using the Epworth Sleepiness Scale, which asks the patient to estimate the likelihood (0 = never, 1 = slight, 2 = moderate, 3 = high) of dozing during various situations: (1) sitting and reading, (2) watching television, (3) sitting inactive in a public place (eg, a theater or a meeting), (4) as a passenger in a car for an hour without a break, (5) lying down to rest in the afternoon when circumstances permit, (6) sitting and talking to someone, (7) sitting quietly after a lunch without alcohol consumption, and (8) in a car, while stopped for a few minutes in traffic. 20 A score of 11 or more suggests hypersomnolence and may indicate an underlying sleep disorder (or insufficient sleep). In general, symptoms of daytime sleepiness increase as AHI worsens (Figure 3). 19 Some patients with milder OSA may present with insomnia.
Figure 3.
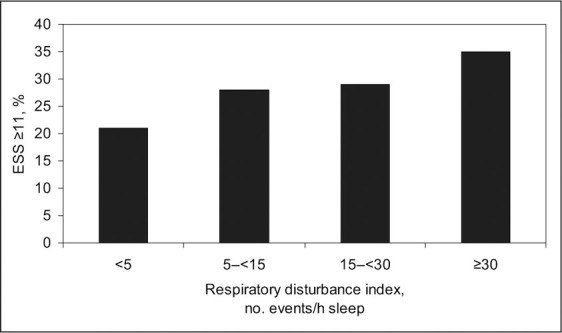
Relationship of sleepiness to respiratory disturbance index. Sleepiness is defined as a score of 11 or more using the Epworth Sleepiness Scale (ESS). The respiratory disturbance index is defined as the number of apneas plus hypopneas per hour of sleep time; apnea is defined as a reduction in the thermocouple signal to ≤25% of baseline for ≥10 seconds; and hypopnea is defined as a decrease in the thermocouple signal or thoracoabdominal excursion ≤70% of baseline for ≥10 seconds accompanied by a 4% decrease in oxygen saturation. Figure derived from data of Gottlieb et al. 19
Frequent breathing pauses, loud snoring, and habitual snoring are 3‐4 times more likely to be associated with an AHI of 15 or greater. 21 The sleep partner often reports the snoring, choking, gasping, and breathing pauses. Fatigue, irritability, difficulty concentrating, memory and judgment change, and personality problems may be present, although there is controversy concerning whether some of these symptoms result from OSA. 22 , 23 Automobile accidents and work‐related injuries do occur. Neurocognitive studies from the Sleep Heart Health Study document that processing and motor speed performance correlate with the severity of hypoxemia and the degree of sleep fragmentation resulting from respiratory events. 22
Obesity and hypertension are common in the OSA syndrome. The index of suspicion for OS A should be high in any hypertensive patient whose weight exceeds 120% of ideal body weight. Resistant hypertension appears to be more common among patients with sleep apnea. 23 In comparison with OSA patients with controlled hypertension, patients with resistant hypertension have significantly higher AHI (44 vs 33 events/h; P<.0005), despite comparable nocturnal oxygenation. 24
A neck circumference of >17 inches in men and >16 inches in women increases the risk of sleep apnea. Macroglossia (seen in acromegaly or amyloidosis) and craniofacial abnormalities such as retrognathia (abnormal posterior position of one or both jaws, particularly the mandible) predispose to sleep apnea.
DIAGNOSIS
Overnight polysomnography performed in a certified sleep laboratory is the optimum test for diagnosis. OSA syndrome is present if the AHI is >15, or >5 in the presence of hypertension, stroke, sleepiness, ischemic heart disease, insomnia, or mood disorders. A staging system is displayed in the Table.
TREATMENT
Weight loss, avoidance of nocturnal sedatives, cessation of evening alcohol ingestion, and avoidance of the supine position during sleep are initial therapeutic actions. In mild cases, severely increased AHI or severe desaturation should be treated immediately with continuous positive airway pressure (CPAP) to avoid increased morbidity and mortality. A 10% reduction in weight (Figure 4) is predicted to reduce AHI by 18%–34%. 25 Additional benefits of weight reduction include improvements in BP, insulin sensitivity, and lipids.
Figure 4.
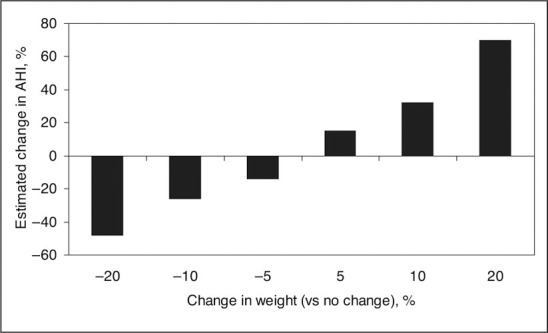
Estimated percentage change in apnea‐hypopnea index (AHI) per gains or losses of percentage body weight. Adjusted for gender, tobacco use change, baseline body mass index, and baseline age. P <.001. Figure derived from data of Peppard et al. 25
Angiotensin converting enzyme (ACE) inhibitors can cause an intractable cough and angioneurotic edema. 26 A small series reported a higher AHI among OSA patients with an ACE inhibitor‐related cough. 27 AHI improved after drug withdrawal. More data are required before a firm recommendation can be made about avoiding this drug class in hypertensive patients with OSA syndrome.
CPAP provides sustained and effective treatment of OSA by maintaining a patent airway throughout the respiratory cycle. It reduces daytime somnolence 28 and lowers nighttime and daytime BP. 29 One study compared subtherapeutic and therapeutic nasal CPAP in 118 men with OSA using ambulatory BP monitoring before and after CPAP. 30 Therapeutic CPAP significantly reduced 24‐hour BP (−3.4/−3.3 mm Hg). Unfortunately, patients do not always tolerate this therapy.
SUMMARY
OSA is considered a remediable cause of hypertension, although studies with CPAP show only modest benefits. The hypertension specialist should strive to make the diagnosis, since treatment may favorably reduce cardiovascular events and prevent the occurrence of right‐sided heart failure.
Refrences
- 1. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 2. Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–245. [DOI] [PubMed] [Google Scholar]
- 3. Prisant LM. Blunted nocturnal decline in blood pressure. J Clin Hypertens (Greenwich). 2004;6:594–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nieto FJ, Young TB, Lind BK, et al. Association of sleep‐disordered breathing, sleep apnea, and hypertension in a large community‐based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. [DOI] [PubMed] [Google Scholar]
- 5. Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep‐disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. [DOI] [PubMed] [Google Scholar]
- 6. Wolk R, Shamsuzzaman AS, Somers VK. Obesity, sleep apnea, and hypertension. Hypertension. 2003;42:1067–1074. [DOI] [PubMed] [Google Scholar]
- 7. Atwood CW. Sleep‐related hypoventilation: the evolving role of leptin. Chest. 2005;128:1079–1081. [DOI] [PubMed] [Google Scholar]
- 8. Phillips BG, Kato M, Narkiewicz K, et al. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279: H234–H237. [DOI] [PubMed] [Google Scholar]
- 9. Narkiewicz K, Van De Borne PJ, Cooley RL, et al. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98:772–776. [DOI] [PubMed] [Google Scholar]
- 10. Punjabi NM, Shahar E, Redline S, et al. Sleep‐disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–530. [DOI] [PubMed] [Google Scholar]
- 11. Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C‐reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–2464. [DOI] [PubMed] [Google Scholar]
- 12. Narkiewicz K, Pesek CA, Kato M, et al. Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension. 1998;32:1039–1043. [DOI] [PubMed] [Google Scholar]
- 13. Gottlieb DJ, DeStefano AL, Foley DJ, et al. APOE epsilon4 is associated with obstructive sleep apnea/hypopnea: the Sleep Heart Health Study. Neurology. 2004;63:664–668. [DOI] [PubMed] [Google Scholar]
- 14. Haas DC, Foster GL, Nieto FJ, et al. Age‐dependent associations between sleep‐disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation. 2005;111:614–621. [DOI] [PubMed] [Google Scholar]
- 15. Young T, Palta M, Dempsey J, et al. The occurrence of sleep‐disordered breathing among middle‐aged adults. N Engl J Med. 1993;328:1230–1235. [DOI] [PubMed] [Google Scholar]
- 16. Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep‐disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shahar E, Whitney CW, Redline S, et al. Sleep‐disordered breathing and cardiovascular disease: cross‐sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. [DOI] [PubMed] [Google Scholar]
- 18. Gami AS, Howard DE, Olson EJ, et al. Day‐night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–1214. [DOI] [PubMed] [Google Scholar]
- 19. Gottlieb DJ, Whitney CW, Bonekat WH, et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159:502–507. [DOI] [PubMed] [Google Scholar]
- 20. Johns MW A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 21. Young T, Shahar E, Nieto FJ, et al. Predictors of sleep‐disordered breathing in community‐dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. [DOI] [PubMed] [Google Scholar]
- 22. Quan SF, Wright R, Baldwin CM, et al. Obstructive sleep apnea‐hypopnea and neurocognitive functioning in the Sleep Heart Health Study. Sleep Med [serial online]. June 30, 2006. [DOI] [PubMed] [Google Scholar]
- 23. Boland LL, Shahar E, Iber C, et al. Measures of cognitive function in persons with varying degrees of sleep‐disordered breathing: the Sleep Heart Health Study. J Sleep Res. 2002;11:265–272. [DOI] [PubMed] [Google Scholar]
- 24. Lavie P, Hoffstein V. Sleep apnea syndrome: a possible contributing factor to resistant hypertension. Sleep. 2001;24:721–725. [DOI] [PubMed] [Google Scholar]
- 25. Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep‐disordered breathing. JAMA. 2000;284:3015–3021. [DOI] [PubMed] [Google Scholar]
- 26. Prisant LM. Angioneurotic edema. J Clin Hypertens (Greenwich). 2001;3:262–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cicolin A, Mangiardi L, Mutani R, et al. Angiotensin‐converting enzyme inhibitors and obstructive sleep apnea. Mayo Clin Proc. 2006;81:53–55. [DOI] [PubMed] [Google Scholar]
- 28. Barnes M, Houston D, Worsnop CJ, et al. A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:773–780. [DOI] [PubMed] [Google Scholar]
- 29. Faccenda JF, Mackay TW, Boon NA, et al. Randomized placebo‐controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea‐hypopnea syndrome. Am J Respir Crit Care Med. 2001;163:344–348. [DOI] [PubMed] [Google Scholar]
- 30. Pepperell JCT, Ramdassingh‐Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and sub‐therapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204–210. [DOI] [PubMed] [Google Scholar]


