Abstract
Background: Thyrotropin (TSH) is well known as the hormone of the anterior pituitary thyrotrophs responsible for acting in the thyroid gland, where it stimulates synthesis and release of thyroid hormones through Gs and Gq/11 protein coupled TSH receptors (TSHRs).
Methods: In this study, we examined whether the functional TSHRs are also expressed in cultured rat pituitary cells, using double immunocytochemistry, quantitative reverse transcription-polymerase chain reaction analysis, cAMP and hormone measurements, and single-cell calcium imaging.
Results: Double immunocytochemistry revealed the expression of TSHRs in cultured corticotrophs and melanotrophs, in addition to previously identified receptors in folliculostellate cells. The functional coupling of these receptors to the Gq/11 signaling pathway was not observed, as demonstrated by the lack of TSH activation of IP3-dependent calcium mobilization in these cells when bathed in calcium-deficient medium. However, TSH increased cAMP production in a time- and concentration-dependent manner and facilitated calcium influx in single corticotrophs and melanotrophs, indicating their coupling to the Gs signaling pathway. Consistent with these findings, TSH stimulated adrenocorticotropin and β-endorphin release in male and female pituitary cells in a time- and concentration-dependent manner without affecting the expression of proopiomelanocortin gene.
Conclusions: These results indicate that TSH is a potential paracrine modulator of anterior pituitary corticotrophs and melanotrophs, controlling the exocytotic but not the transcriptional pathway in a cAMP/calcium influx-dependent manner.
Keywords: TSH receptors, corticotrophs, melanotrophs, calcium signaling, Pomc, ACTH, β-endorphin
Introduction
Thyrotropin (TSH) is a member of the glycoprotein family of hormones, synthesized and released by anterior pituitary thyrotrophs. Two other members of this family are follicle-stimulating hormone and luteinizing hormone, which are produced by anterior pituitary gonadotrophs. Only in primates and equines, a fourth member of this family, named chorionic gonadotropin, is secreted by the placenta. Glycoprotein hormones are known to consist of the common α subunit and the hormone-specific β subunit that are noncovalently associated (1). These hormones signal through a subfamily of G-protein-coupled receptors, known as glycoprotein hormone receptors, which are members of the class A of these receptors. All glycoprotein hormone receptors stimulate adenylyl cyclase when activated, which suggests involvement of Gs heterotrimeric proteins. At higher agonist concentrations, they could also stimulate the phospholipase C-dependent signaling pathway via Gq/11 proteins (2).
It is well established that TSH regulates thyroid hormone synthesis and release by the thyroid gland via activation of TSH receptors (TSHRs) expressed in the thyroid follicular cells (3). In human thyroid gland, TSHRs interact not only with Gs and Gq/11 proteins, leading to the activation of adenylyl cyclase and phospholipase C, respectively, but are also capable of signaling through Gi/o and G12/13 proteins (4). Several extrathyroidal expression sites of TSHRs have also been reported (5). These include fibroblasts and preadipocytes of the orbit (6,7) and abdominal adipose cells, where TSHRs control interleukin-6 release (8,9), stimulate lipolysis in adipocytes in culture, and raise serum free fatty acid levels (10). The presence of TSHRs has also been reported in osteoblastic lineage of bones (11–13), as well as in the heart, kidney, immune cells, digestive tract, and several regions of the brain (14). Furthermore, high-affinity TSH binding sites have been detected in human fat, testicular, and adrenal membranes (15). TSHRs were also found in normal and adenomatous human pituitary, and receptors were identified in folliculostellate cells (FSCs) (16,17) as well as in the FSC line TtT/GF (18). It has also been suggested that TSHR autoantibodies can suppress intrapituitary TSH levels independent of circulating thyroid hormone levels, suggesting that these receptors are functional (19).
In this study, we examined the expression and role of TSHRs in cultured rat pituitary cells. Our double immunocytochemical analysis not only confirmed the expression of TSHR in FSCs but also showed that these receptors were expressed in pituitary corticotrophs and melanotrophs. The functionality of TSHRs was evaluated by analyzing the proopiomelanocortin gene (Pomc) expression, cAMP production, adrenocorticotropin (ACTH) and β-endorphin release in mixed pituitary cells, and calcium signaling in single identified corticotrophs and melanotrophs. These investigations indicate that, in addition to FSCs, TSH is a paracrine modulator of POMC-producing pituitary cells, controlling the exocytotic but not the transcriptional pathway in a cAMP/calcium influx-dependent manner.
Materials and Methods
All experiments with animals were approved by the National Institute of Child Health and Human Development Animal Use and Care Committee (19-041). The details of the experimental methods are described in the Supplementary Material and Methods.
Results
TSH stimulates the release of POMC-derived hormones in a sex-independent manner
A preliminary study with cultured rat pituitary cells revealed that TSH significantly stimulates ACTH release, which prompted experiments summarized in this article. Because corticotrophs and melanotrophs are sister cells expressing a common marker gene Pomc, which encodes the 239 amino acid-containing POMC peptide (20), we also included β-endorphin measurements in our experiments. Corticotrophs express prohormone convertase-1 and the predominant POMC cleavage products are ACTH and β-lipotropin, whereas melanotrophs express prohormone convertase-1 and -2, generating three forms of melanocyte-stimulating hormone, α, β, and γ, as well as β-endorphin (21).
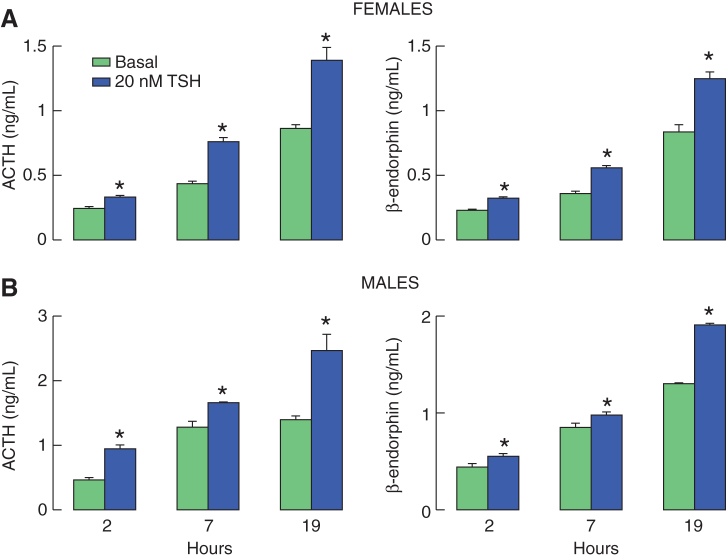
Initially, we examined the time course effect of TSH on ACTH and β-endorphin release in cultured rat pituitary cells from both female and male rats. As shown in Figure 1, 20 nM of TSH was able to significantly elevate ACTH and β-endorphin secretion in all three time points examined, 2, 7, and 19 hours. The stimulatory effects of TSH on ACTH and β-endorphin release were not sex-specific. In untreated cells, hormone secretion increased with time of incubation in both cell preparations, reflecting dependence of basal secretion on spontaneous electrical activity. Together, these results raised the possibility that TSHR could be expressed in POMC-producing corticotrophs and melanotrophs. To clarify this issue, in further experiments, we performed immunocytochemical analysis in cultured pituitary cells, as stated below.
FIG. 1.
Time course of TSH-induced ACTH and β-endorphin release in pituitary cells in static cultures. Experiments were performed with cells derived from postpubertal female (A) and male (B) rats 20 hours after cell dispersion. Notice a progressive increase in basal hormone release with incubation time. If not otherwise specified, in this and following figures, data shown are mean ± SEM values from four to six replicates in one from three similar experiments, and asterisks indicate significant differences between pairs, with p < 0.01. ACTH, adrenocorticotropin; SEM, standard error of the mean; TSH, thyrotropin. Color images are available online.
TSHR is expressed in corticotrophs, melanotrophs, and FSCs
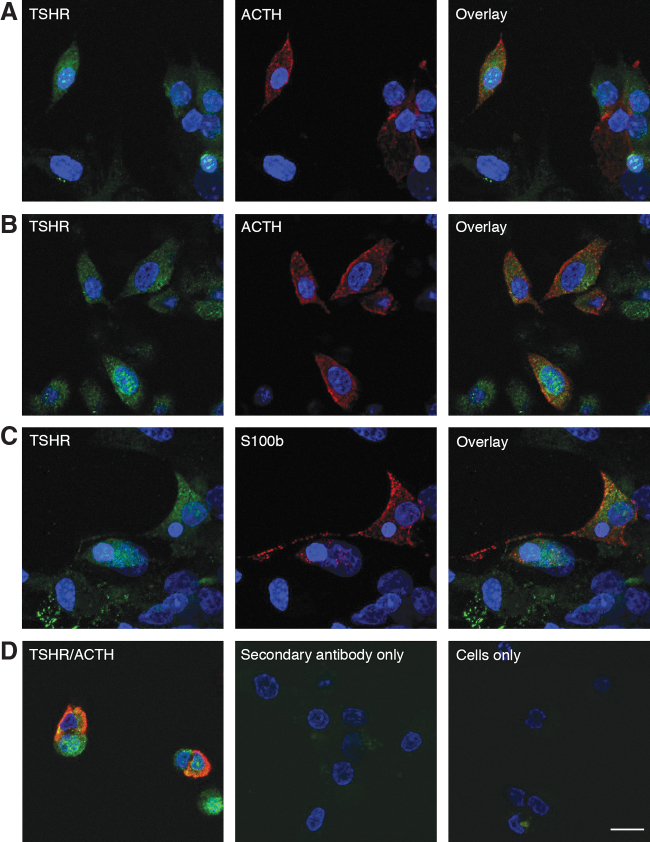
Corticotrophs and melanotrophs are localized in the anterior pituitary gland; the former are situated in the anterior lobe and the latter are situated in the intermediate lobe of anterior pituitary gland (21). However, after separation and dispersion of rat anterior and posterior glands, the majority of melanotrophs were found in the posterior gland preparation and only 7% of Pomc-expressing cells in the anterior pituitary preparation were identified as melanotrophs (22). Cells from both preparations were cultured for 48 hours in horse serum containing M199, washed in the morning, incubated with TSHR antibody, followed by incubation with POMC/ACTH antibody, as described in the Immunocytochemistry section in the Supplementary Material and Methods, to identify melanotrophs and corticotrophs. As control, S100b antibody was used to identify FSCs.
The TSHR-positive cells were detected in a fraction of posterior and anterior pituitary cells. The TSHR-positive cells from posterior pituitary were positive with POMC/ACTH antibody, a finding consistent with expression of this receptor in melanotrophs. Of 688 melanotrophs, 395 (57%) were also TSHR-positive (Fig. 2A). In anterior pituitary cells, of 419 ACTH-positive cells, 299 expressed both ACTH and TSHR (71%). The majority of these cells should be corticotrophs (Fig. 2B). These results confirmed that in vitro activation of these receptors by TSH accounts for elevation in ACTH and β-endorphin secretion, respectively. Among anterior pituitary cells, a fraction of S100b-positive FSCs (66 of 454) were also TSHR-positive (15%). Finally, cells incubated with only secondary antibodies or staining solution indicated specificity of the reaction (Fig. 2D).
FIG. 2.
Immunofluorescence analysis of TSHR expression in cultured rat pituitary cells. (A, B) Representative images for immunostaining of TSHR (green, left), POMC/ACTH (red, center), and their overlay (right) in melanotrophs (A), derived from dispersion of posterior pituitary gland, and corticotrophs (B), derived from dispersion of anterior pituitary gland. (C) Expression of TSHR (green, left) in S100b-positive anterior pituitary FSCs (red, center) and their overlay (right). (D) Cells stained with both ACTH and TSHR antibody (left), cells incubated with only secondary antibodies (center), and cells incubated with only staining solution (right). Scale bars (applies to all images), 10 μm. If not otherwise specified, in this and following figures, pituitary cells from postpubertal females were used. FSCs, folliculostellate cells; TSHR, TSH receptor. Color images are available online.
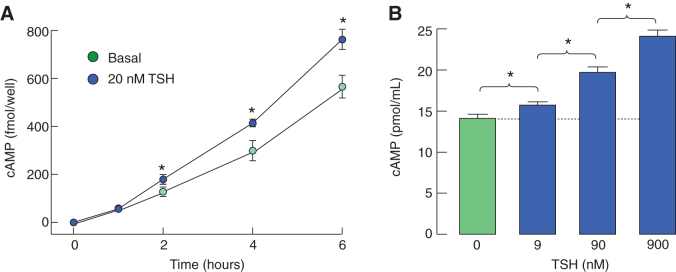
Pituitary TSHRs are coupled to the Gs signaling pathway
To evaluate the Gs-dependent signaling functions of pituitary TSHR, we performed two experiments. In the first experiment, pituitary cells in static cultures were treated with 20 nM of TSH for 1, 2, 4, and 6 hours in the presence of 1 mM IBMX, medium was collected, and released cAMP content was measured using radioimmunoassay. Figure 3A shows significant elevation in released cAMP levels in TSH-treated cells for 2, 4, and 6 hours. In the second experiment, concentration-dependent effects of TSH on intracellular cAMP accumulation were studied using an enzyme-linked immunosorbent assay (Fig. 3B). These experiments confirmed that the activation of TSHR in anterior pituitary led to the activation of adenylyl cyclase independent of TSH source, method of cAMP detection, and cell number per well.
FIG. 3.
Coupling of TSHRs to the Gs-dependent signaling pathway in cultured pituitary cells. (A) Time course of TSH-induced cAMP release in pituitary cells from males. (B) Concentration dependence of TSH on intracellular cAMP accumulation in pituitary cells from females. Before TSH application, medium of cells in static cultures was replaced with 0.1% BSA containing medium 199 supplemented with 1 mM IBMX. At the end of incubation with TSH, medium was collected to measure released cAMP by radioimmunoassay, or cells were lysed, and content collected to measure cAMP intracellular accumulation by ELISA as described in the Materials and Methods section. BSA, bovine serum albumin; ELISA, enzyme-linked immunosorbent assay. Color images are available online.
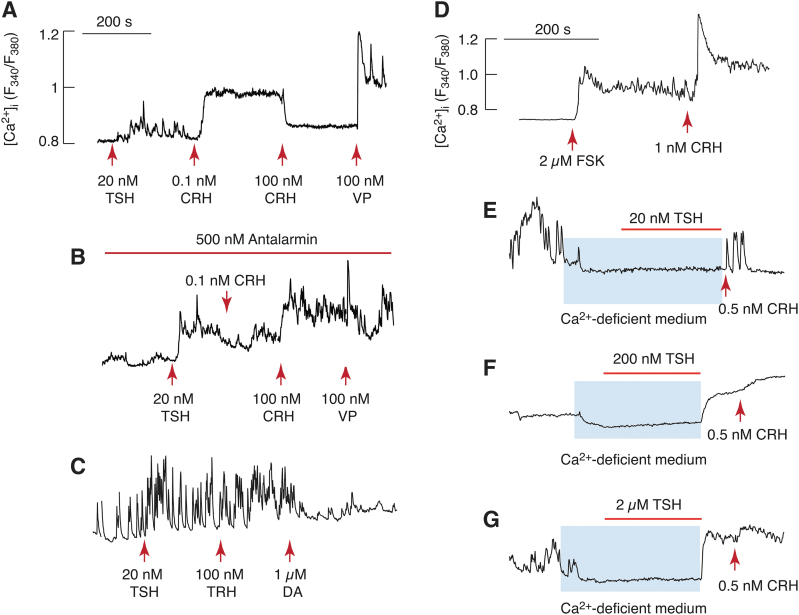
TSH stimulates calcium influx in single corticotrophs and melanotrophs
We used single-cell calcium measurements to evaluate the potential coupling of TSHR to calcium signaling pathway(s). Two types of experiments were performed. In the first experiment, we studied the effects of TSH on calcium signaling in cells bathed in 2 mM Ca2+-containing medium and quiescent cells (not exhibiting spontaneous calcium transients) were selected. In such cells, we consistently observed that the application of TSH initiated calcium transients (Fig. 4A). At physiological concentrations, corticotropin-releasing hormone (CRH) further stimulated calcium influx, whereas at pharmacological concentrations, it blocked calcium influx. Furthermore, arginine vasopressin (VP) elevated [Ca2+]i, confirming that cells responding to TSH were corticotrophs (23). In cells treated with a CRH antagonist, antalarmin, TSH also facilitated Ca2+ influx, indicating that the effect of TSH is not mediated by CRH receptors (Fig. 4B). Consistent with this, the stimulatory action of CRH in a physiological concentration was abolished in the presence of antalarmin, and inhibitory effect of high CRH dose was replaced with elevation in [Ca2+]i. TSH also increased the amplitude and frequency of spontaneous calcium transients in posterior pituitary cells. Identification of melanotrophs was carried out by application of dopamine, which abolished Ca2+ influx (Fig. 4C). The stimulatory effect of TSH was mimicked by forskolin, an allosteric activator of adenylyl cyclase (Fig. 4D).
FIG. 4.
Calcium signaling functions of TSHRs in pituitary corticotrophs and melanotrophs: single-cell recording. (A–C) Effects of TSH on calcium signaling in corticotrophs (A, B) and melanotrophs (C). Cells were bathed in calcium-containing medium, and arrows indicate the moment of application of hormones. TSH (20 nM) induced calcium transients in quiescent cells (A, B) or increased the amplitude and frequency of spontaneous calcium transients in spontaneously active cells (C). Corticotrophs were identified by stimulatory effects of CRH in physiological concentrations and inhibitory effects in pharmacological concentrations on calcium influx, as well as by arginine VP-induced calcium mobilization (A). TSH effect on calcium influx was not affected by 500 nM antalarmin, a CRH receptor antagonist, in contrast to CRH effects (B). Melanotrophs were identified by blockade of TSH-stimulated calcium transients by dopamine and the lack of TRH response on calcium signaling (C). (D) Stimulatory effect of CRH on calcium signaling was mimicked by application of forskolin, an adenylyl cyclase allosteric activator. (E–G) Calcium signaling function of TSHR in corticotrophs was dependent on calcium influx. Blue areas indicate duration of application of Ca2+-deficient medium (to exclude Ca2+ influx from extracellular medium), and horizontal bars indicate duration of TSH application in three concentrations. Arrows indicate the moment of CRH application, which was added to identify corticotrophs. Notice abolition of spontaneous calcium transients by removal of bath Ca2+. All experiments were performed 20 hours after cell dispersion. CRH, corticotropin-releasing hormone; VP, vasopressin. Color images are available online.
In the second set of experiments, cells were bathed in Ca2+-deficient medium and stimulated with TSH to dissociate whether the rise in [Ca2+]i reflects Ca2+ influx or mobilization. In general, the activation of pituitary Gq/11-coupled receptors elevates [Ca2+]i in cells bathed in Ca2+-deficient medium due to IP3-dependent calcium release from the endoplasmic reticulum (24). However, 20 nM of TSH was unable to elevate [Ca2+]i (Fig. 4E), clearly indicating that TSH-induced calcium signaling reflected Ca2+ influx from extracellular medium. Furthermore, none of corticotrophs stimulated with 200 and 2000 nM of TSH responded (Fig. 4F, G). These results argue against the hypothesis that TSHR expressed in corticotrophs cross-couple to the Gq/11 signaling pathway. Thus, it is reasonable to postulate that the activation of the Gs signaling pathway is associated with the promotion of Ca2+ influx.
TSH stimulates hormone secretion in a PKA-independent manner
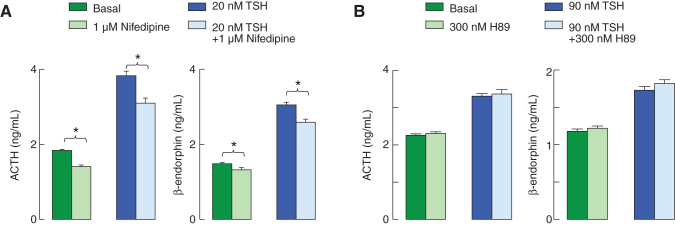
In corticotrophs and melanotrophs, Ca2+ influx is partially dependent on L-type voltage-gated calcium channels (23). In further experiments, cells were bathed in calcium-containing medium with and without nifedipine, a blocker of L-type channels, to evaluate the role of calcium influx in spontaneous and TSH-stimulated hormone secretion. In the absence and presence of nifedipine, TSH elevated ACTH and β-endorphin secretion significantly, and basal and stimulated hormone secretion were reduced but not abolished in the presence of nifedipine (Fig. 5A). These results indicate that, at least in part, basal and TSH-stimulated hormone secretion were dependent on Ca2+ influx.
FIG. 5.
Partial dependence of TSH-induced hormone release on calcium influx through L-type calcium channels but not on protein kinase A in cultured pituitary cells. (A) Basal and TSH-stimulated ACTH (left) and β-endorphin (right) release was evaluated during 7 hours of incubation in the presence and absence of 1 μM nifedipine, a blocker of L-type calcium channels. (B) The lack of effects of H89, a protein kinase A inhibitor, on basal and TSH-stimulated ACTH (left) and β-endorphin (right) release. Color images are available online.
To evaluate a potential role of cAMP-dependent protein kinase (PKA) in basal and TSH-induced hormone release, we used two inhibitors of this enzyme: KT5720 and H89. The first compound exhibited nonspecific stimulatory effects on basal and TSH-stimulated hormone secretion when used at 1 μM concentration (data not shown). The second compound did not affect basal and TSH-stimulated hormone secretion, arguing against the role of PKA in ACTH and β-endorphin release (Fig. 5B).
TSH stimulates the release of POMC-derived peptides without affecting Pomc expression
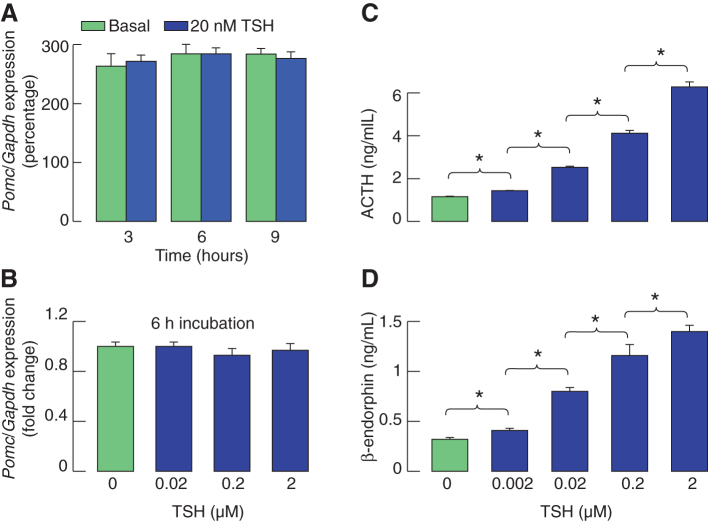
To clarify whether TSH-induced cAMP/calcium signaling is coupled to Pomc expression, we examined the expression of this gene in static cultures of pituitary cells treated with 20 nM of TSH for 3, 6, and 9 hours. For this purpose, TaqMan assays were used, and values were expressed as relative to glyceraldehyde 3-phosphate dehydrogenase gene (Gapdh) expression, which was recently shown as a valid reference gene for pituitary tissue and cultured pituitary cells (25). Figure 6A shows the lack of effect of TSH treatment on Pomc expression. We also examined the concentration dependence of TSH on Pomc expression during 6 hours of incubation. Again, we did not observe changes in Pomc expression when cells were stimulated with a wide range of TSH concentrations for 6 hours (Fig. 6B).
FIG. 6.
TSH stimulates hormone release in a concentration-dependent manner without affecting basal Pomc expression in pituitary cells static cultures. (A) Quantitative reverse transcription-polymerase chain reaction analysis of Pomc expression in pituitary cells static cultures under the presence and absence of TSH for variable times. (B) The lack of TSH effects in 0.02, 0.2, and 2 μM concentrations during 6 hours of treatment on Pomc expression. At the end of incubation periods, total mRNA was extracted, and Pomc and Gapdh expression was evaluated using TaqMan assays. (C, D) Concentration dependence of TSH on ACTH (C) and β-endorphin (D) release during 6 hours of treatment. Gapdh, glyceraldehyde 3-phosphate dehydrogenase gene; mRNA, messenger RNA; Pomc, proopiomelanocortin gene. Color images are available online.
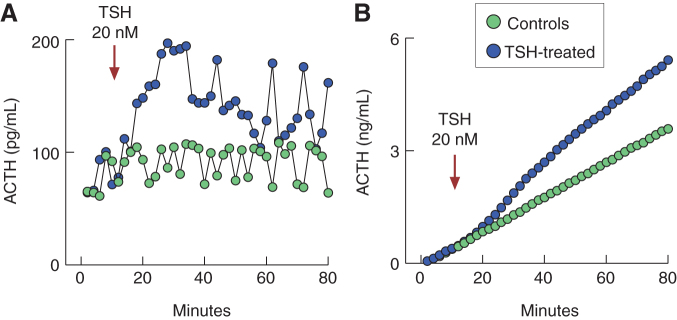
In contrast, we recorded a progressive increase in ACTH and β-endorphin release with elevation in TSH concentration (Fig. 6C, D), indicating that TSH-induced hormone release did not reflect elevation in Pomc expression. To confirm independence of TSH-induced ACTH release of the status of Pomc expression, in further work, we used a perifusion system (Fig. 7). Cells attached on beads were cultured for 40 hours before experiments, and transferred in 0.5 mL volume chambers and perifused at 0.5 mL/min flow rate at 37°C for 2 hours. After that, samples were collected every 2 minutes during an 80-minute period and 20 nM of TSH was applied from 10 to 80 minutes (Fig. 7A). Basal ACTH release fluctuated between 60 and 100 pg/mL, whereas the application of TSH rapidly elevated secretion to about 200 pg/mL, with the peak in stimulation reached 14 minutes after the start of TSH application, followed by establishment of a pulsatile-like secretion above basal levels during the next 40 minutes. Figure 7B shows cumulative ACTH release with time in controls and TSH-treated cells, indicating that basal ACTH release occurs at constant rate and that TSH enhances the rate of hormone secretion. Such rapid effects of TSH on ACTH release demonstrate that prestored hormone is secreted by regulated exocytosis.
FIG. 7.
Stimulatory effect of TSH on ACTH release in perifused pituitary cells. (A) A rapid effect of TSH on ACTH release. (B) Calculated cumulative effects of TSH on ACTH release. Anterior pituitary cells were attached on beads and cultured for 40 hours before experiments. Perifusion was performed in 0.5 mL volume chambers at a flow rate of 0.5 mL/min. Arrow indicates the beginning of application of TSH (blue circles) and solvent (green circles). Samples were collected every 2 minutes during 80 minutes. Color images are available online.
Discussion
Using cultured rat pituitary cells and double immunocytochemistry, we show for the first time the presence of immunoreactive TSHR in sister cells: corticotrophs and melanotrophs. These receptors were functional, as shown by TSH-induced rise in cAMP production in amplitude sufficient enough to allow the release of this messenger in extracellular medium. The function of this receptor was further confirmed in single-cell calcium measurements in identified corticotrophs and melanotrophs. Finally, we show here that in static cultures and perifused pituitary cells, TSH stimulates ACTH and β-endorphin secretion.
For in vitro experiments, 20 nM of TSH was considered as low concentration, 200 nM as medium concentration, and 2 μM as high concentration (26). This concentration range is consistent with measurements of TSH levels in rat portal blood of around 360 nM (27). In our experiments, 20 nM of TSH was sufficient to stimulate cAMP production, elevate [Ca2+]i, and stimulate ACTH and β-endorphin release in cultured cells bathed in calcium-containing medium. Secretory studies also revealed that 2 nM of TSH is the threshold concentration to trigger ACTH and β-endorphin release. We also observed concentration dependence of cAMP production and hormone secretion in this concentration range, with 2 μM probably reflecting a pharmacological dose.
TSH-stimulated cAMP production indicates the coupling of pituitary TSHR to the Gs signaling pathway. The cross-coupling of this receptor to the Gq/11 signaling pathway is well established in the thyroid gland (28). Low concentrations of TSH are known to activate the Gs signaling pathway, whereas medium and high concentrations activate both the Gq/11 and Gi/o signaling pathways (26,29). If these receptors cross-couple to the Gq/11/phospholipase C signaling pathway, the rise in [Ca2+]i, reflecting the phospholipase C-mediated IP3 production and IP3-dependent release of calcium from the endoplasmic reticulum (20), should also be observed in cells bathed in calcium-deficient medium. However, in several independent experiments, with TSH doses ranging from 20 nM to 2 μM, we were unable to observe it.
Thus, it is reasonable to conclude that the stimulation of calcium influx in corticotrophs and melanotrophs bathed in Ca2+-containing medium reflects the coupling of TSHR to the Gs signaling pathway (3) and cAMP-dependent facilitation of voltage-gated calcium influx (30). In general, cAMP can regulate excitability of pituitary cells and calcium-dependent hormone secretion directly by activating hyperpolarization-activated cyclic nucelotide-gated channels. These channels and their activation by cAMP have been identified in immortalized mouse corticotrophs (31). cAMP could also regulate excitability and Ca2+ influx indirectly through EPAC and PKA signaling pathways. The role of EPAC in CRH-induced signaling via cAMP-dependent mechanism has been described in corticotroph cell lines (32) as well as the role of PKA in cAMP-dependent control of excitability in corticotrophs (33). The cAMP-dependent PKA-independent mechanism involves the activation of Rap1 and B-Raf in the pathway of MAP kinase stimulation by TSH (34). G-protein-coupled receptors could also signal independently of G-proteins through β-arrestin signaling pathway, which is operative in AtT-20 cells (35), and TSHR is known to signal through this pathway (36,37). Our results argue against the role of PKA in these processes, but future experiments are needed to clarify which pathway is utilized in TSH-stimulated corticotrophs and melanotrophs.
In general, the rise in [Ca2+]i in corticotrophs and melanotrophs could be coupled to stimulation of Pomc expression and/or activation of the regulated exocytotic pathway. In our experiments, we did not observe changes in Pomc expression in TSH-stimulated cells within 20–2000 nM concentration range during variable times of treatment. CRH also activates the Gs signaling pathway in corticotrophs but stimulates Pomc expression, probably reflecting the specific feature of this receptor to stimulate the MAPK signaling pathway as well (38). This finding indicates that TSH-stimulated calcium influx triggers ACTH and β-endorphin secretion by the exocytotic pathway. There was a rapid (within 2–4 minutes) ACTH release, further confirming independence of hormone release of de novo protein synthesis, and a sustained (at least 19 hours) release of ACTH, suggesting that these receptors do not desensitize. In all cases, stimulation of hormone release was significant, but the amplitude of response was smaller relative to CRH and VP-induced ACTH release (39), which is typical for paracrine hormonal actions.
The expression of functional TSHR in corticotrophs and melanotrophs implicates the potential physiological role of this signaling pathway. It is reasonable to speculate that intrapituitary TSH elevates basal secretory activity of these two cell types. In corticotrophs, this should modulate ACTH-dependent actions. Consistent with this hypothesis, it has been reported that elevated TSH is associated with elevated cortisol among young people (40). The existence of positive relationship between serum TSH and cortisol levels in a healthy population is an interesting finding that is consistent with observation that hypothyroid patients frequently have elevated cortisol levels (41). Furthermore, it is unlikely that acute elevation in cortisol levels influences TSH secretion; the inhibitory effect was observed only during prolonged hypercortisolism, that is, Cushing's syndrome (42).
Previous work has established the expression of TSHRs in normal and adenomatous human anterior pituitary, and receptors were identified in FSCs (16,17) and the FSC line TtT/GF (18). Consistent with these findings, we also observed the expression of TSHR in FSCs, but only in a small fraction (15%) of these cells. Genetically, FSCs are a highly heterogeneous population of cells (22), which could indicate that a subfraction of these cells express TSHR. In parallel to our observations with corticotrophs and melanotrophs, the activation of TSHR in TtT/GF is also not coupled to the Gq/11/phospholipase C signaling pathway in FSC line. In contrast to secretory pituitary cells, in TtT/GF cells, TSHR is not coupled to the Gs signaling pathway but probably signals through the JAK/STAT5a pathway (18). Further studies are needed to characterize whether the rat TSHR in FSC exhibits similar signaling properties and functions.
In conclusion here, we show that pituitary corticotrophs and melanotrophs from both female and male rats express TSHR, which activation by TSH leads to an increase in cAMP production and facilitation of Ca2+ influx in corticotrophs and melanotrophs. The rise in [Ca2+]i is accompanied with increase in ACTH and β-endorphin secretion, whereas Pomc expression was not altered. The effects of TSH on hormone release were rapid and sustained, indicating that pituitary TSHRs do not desensitize at that agonist concentration. These findings indicate that thyrotrophs influence corticotroph and melanotroph functions through TSH, which is of potential physiological and clinical relevance, but this needs to be addressed thoroughly in future investigations.
Acknowledgments
We are thankful to Melanija Tomić for help with calcium measurements. Confocal imaging was performed at the National Institute of Child Health and Human Development (NICHD) Microscopy and Imaging Core of the National Institutes of Health, with the kind assistance of Lynne Holtzclaw and Vincent Schram.
Author Disclosure Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Information
Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD): Z01 HD000195-25 (R.M.P., K.W., K.S., M.M.J., and S.S.S.); FAPESP Grant 2013/05629-4 (M.T.N.). R.M.P. was a recipient of a CAPES Scholarship, process number: 99999.000399/2015-09.
Supplementary Material
Supplementary Material and Methods
References
- 1. Jiang X, Dreano M, Buckler DR, Cheng S, Ythier A, Wu H, Hendrickson WA, el Tayar N. 1995. Structural predictions for the ligand-binding region of glycoprotein hormone receptors and the nature of hormone-receptor interactions. Structure 3:1341–1353 [DOI] [PubMed] [Google Scholar]
- 2. Kleinau G, Worth CL, Kreuchwig A, Biebermann H, Marcinkowski P, Scheerer P, Krause G. 2017. Structural-functional features of the thyrotropin receptor: a class A G-protein-coupled receptor at work. Front Endocrinol (Lausanne) 8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vassart G, Dumont JE. 1992. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev 13:596–611 [DOI] [PubMed] [Google Scholar]
- 4. Laugwitz KL, Allgeier A, Offermanns S, Spicher K, Van Sande J, Dumont JE, Schultz G. 1996. The human thyrotropin receptor: a heptahelical receptor capable of stimulating members of all four G protein families. Proc Natl Acad Sci U S A 93:116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davies TF, Latif R. 2019. Editorial: TSH receptor and autoimmunity. Front Endocrinol (Lausanne) 10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Place RF, Krieger CC, Neumann S, Gershengorn MC. 2017. Inhibiting thyrotropin/insulin-like growth factor 1 receptor crosstalk to treat Graves' ophthalmopathy: studies in orbital fibroblasts in vitro. Br J Pharmacol 174:328–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neumann S, Pope A, Geras-Raaka E, Raaka BM, Bahn RS, Gershengorn MC. 2012. A drug-like antagonist inhibits thyrotropin receptor-mediated stimulation of cAMP production in Graves' orbital fibroblasts. Thyroid 22:839–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Antunes TT, Gagnon A, Chen B, Pacini F, Smith TJ, Sorisky A. 2006. Interleukin-6 release from human abdominal adipose cells is regulated by thyroid-stimulating hormone: effect of adipocyte differentiation and anatomic depot. Am J Physiol Endocrinol Metab 290:E1140–E1144 [DOI] [PubMed] [Google Scholar]
- 9. Antunes TT, Gagnon A, Langille ML, Sorisky A. 2008. Thyroid-stimulating hormone induces interleukin-6 release from human adipocytes through activation of the nuclear factor-kappaB pathway. Endocrinology 149:3062–3066 [DOI] [PubMed] [Google Scholar]
- 10. Gagnon A, Antunes TT, Ly T, Pongsuwan P, Gavin C, Lochnan HA, Sorisky A. 2010. Thyroid-stimulating hormone stimulates lipolysis in adipocytes in culture and raises serum free fatty acid levels in vivo. Metabolism 59:547–553 [DOI] [PubMed] [Google Scholar]
- 11. Abe E, Marians RC, Yu WQ, Wu XB, Ando T, Li YN, Iqbal J, Eldeiry L, Rajendren G, Blair HC, Davies TF, Zaidi M. 2003. TSH is a negative regulator of skeletal remodeling. Cell 115:151–162 [DOI] [PubMed] [Google Scholar]
- 12. Boutin A, Neumann S, Gershengorn MC. 2016. Multiple transduction pathways mediate thyrotropin receptor signaling in preosteoblast-like cells. Endocrinology 157:2173–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baliram R, Latif R, Zaidi M, Davies TF. 2017. Expanding the role of thyroid-stimulating hormone in skeletal physiology. Front Endocrinol 8:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Williams GR 2011. Extrathyroidal expression of TSH receptor. Ann Endocrinol (Paris) 72:68–73 [DOI] [PubMed] [Google Scholar]
- 15. Trokoudes KM, Sugenoya A, Hazani E, Row VV, Volpe R. 1979. Thyroid-stimulating hormone (TSH) binding to extrathyroidal human tissues: TSH binding to extrathyroidal human tissues: TSH and thyroid-stimulating immunoglobulin effects on adenosine 3′,5′-monophosphate in testicular and adrenal tissues. J Clin Endocrinol Metab 48:919–923 [DOI] [PubMed] [Google Scholar]
- 16. Prummel MF, Brokken LJS, Meduri G, Misrahi M, Bakker O, Wiersinga WM. 2000. Expression of the thyroid-stimulating hormone receptor in the folliculo-stellate cells of the human anterior pituitary. J Clin Endocrinol Metab 85:4347–4353 [DOI] [PubMed] [Google Scholar]
- 17. Theodoropoulou M, Arzberger T, Gruebler Y, Korali Z, Mortini P, Joba W, Heufelder AE, Stalla GK, Schaaf L. 2000. Thyrotrophin receptor protein expression in normal and adenomatous human pituitary. J Endocrinol 167:7–13 [DOI] [PubMed] [Google Scholar]
- 18. Brokken LJ, Bakker O, Wiersinga WM, Prummel MF. 2005. Functional thyrotropin receptor expression in the pituitary folliculo-stellate cell line TtT/GF. Exp Clin Endocrinol Diabetes 113:13–20 [DOI] [PubMed] [Google Scholar]
- 19. Brokken LJS, Scheenhart JWC, Wiersinga WM, Prummel MF. 2001. Suppression of serum TSH by Graves' Ig: evidence for a functional pituitary TSH receptor. J Clin Endocrinol Metab 86:4814–4817 [DOI] [PubMed] [Google Scholar]
- 20. Stojilkovic SS, Tabak J, Bertram R. 2010. Ion channels and signaling in the pituitary gland. Endocr Rev 31:845–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goodman HM 2009. Basic Medical Endocrinology. Fourth edition. Elsevier, Academic Press, New York, NY [Google Scholar]
- 22. Fletcher PA, Smiljanic K, Maso Previde R, Iben JR, Li T, Rokic MB, Sherman A, Coon SL, Stojilkovic SS. 2019. Cell type- and sex-dependent transcriptome profiles of rat anterior pituitary cells. Front Endocrinol (Lausanne) 10:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zemkova H, Tomic M, Kucka M, Aguilera G, Stojilkovic SS. 2016. Spontaneous and CRH-induced excitability and calcium signaling in mice corticotrophs involves sodium, calcium, and cation-conducting channels. Endocrinology 157:1576–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stojilkovic SS 2012. Molecular mechanisms of pituitary endocrine cell calcium handling. Cell Calcium 51:212–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Janjic MM, Previde RM, Fletcher PA, Sherman A, Smiljanic K, Abebe D, Bjelobaba I, Stojilkovic SS. 2019. Divergent expression patterns of pituitary gonadotropin subunit and GnRH receptor genes to continuous GnRH in vitro and in vivo. Sci Rep 9:20098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neumann S, Malik SS, Marcus-Samuels B, Eliseeva E, Jang DS, Klubo-Gwiezdzinska J, Krieger CC, Gershengorn MC. 2020. Thyrotropin causes dose-dependent biphasic regulation of cAMP production mediated by G(s) and G(i/o) proteins. Mol Pharmacol 97:2–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oliver C, Mical RS, Porter JC. 1977. Hypothalamic-pituitary vasculature: evidence for retrograde blood flow in the pituitary stalk. Endocrinology 101:598–604 [DOI] [PubMed] [Google Scholar]
- 28. Kero J, Ahmed K, Wettschureck N, Tunaru S, Wintermantel T, Greiner E, Schutz G, Offermanns S. 2007. Thyrocyte-specific Gq/G11 deficiency impairs thyroid function and prevents goiter development. J Clin Invest 117:2399–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laurent E, Van Sande J, Ludgate M, Corvilain B, Rocmans P, Dumont JE, Mockel J. 1991. Unlike thyrotropin, thyroid-stimulating antibodies do not activate phospholipase C in human thyroid slices. J Clin Invest 87:1634–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stojilkovic SS, Kretschmannova K, Tomic M, Stratakis CA. 2012. Dependence of the excitability of pituitary cells on cyclic nucleotides. J Neuroendocrinol 24:1183–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tian L, Shipston MJ. 2000. Characterization of hyperpolarization-activated cation currents in mouse anterior pituitary, AtT20 D16:16 corticotropes. Endocrinology 141:2930–2937 [DOI] [PubMed] [Google Scholar]
- 32. Van Kolen K, Dautzenberg FM, Verstraeten K, Royaux I, De Hoogt R, Gutknecht E, Peeters PJ. 2010. Corticotropin releasing factor-induced ERK phosphorylation in AtT20 cells occurs via a cAMP-dependent mechanism requiring EPAC2. Neuropharmacology 58:135–144 [DOI] [PubMed] [Google Scholar]
- 33. Lee AK, Tse A. 1997. Mechanism underlying corticotropin-releasing hormone (CRH) triggered cytosolic Ca2+ rise in identified rat corticotrophs. J Physiol 504 (Pt 2):367–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iacovelli L, Capobianco L, Salvatore L, Sallese M, D'Ancona GM, De Blasi A. 2001. Thyrotropin activates mitogen-activated protein kinase pathway in FRTL-5 by a cAMP-dependent protein kinase A-independent mechanism. Mol Pharmacol 60:924–933 [DOI] [PubMed] [Google Scholar]
- 35. Kageyama K, Hagiwara R, Niioka K, Takayasu S, Daimon M. 2020. Differential effects of beta-arrestin1 and beta-arrestin2 on somatostatin receptors in murine AtT-20 corticotroph tumor cells. Endocr J [Epub ahead of print]; DOI: 10.1507/endocrj.EJ20-0251 [DOI] [PubMed] [Google Scholar]
- 36. Krieger CC, Boutin A, Jang D, Morgan SJ, Banga JP, Kahaly GJ, Klubo-Gwiezdzinska J, Neumann S, Gershengorn MC. 2019. Arrestin-beta-1 physically scaffolds TSH and IGF1 receptors to enable crosstalk. Endocrinology 160:1468–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boutin A, Eliseeva E, Gershengorn MC, Neumann S. 2014. beta-Arrestin-1 mediates thyrotropin-enhanced osteoblast differentiation. FASEB J 28:3446–3455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Drouin J 2016. 60 Years of POMC: transcriptional and epigenetic regulation of POMC gene expression. J Mol Endocrinol 56:T99-T112 [DOI] [PubMed] [Google Scholar]
- 39. Abou-Samra AB, Catt KJ, Aguilera G. 1987. Calcium-dependent control of corticotropin release in rat anterior pituitary cell cultures. Endocrinology 121:965–971 [DOI] [PubMed] [Google Scholar]
- 40. Walter KN, Corwin EJ, Ulbrecht J, Demers LM, Bennett JM, Whetzel CA, Klein LC. 2012. Elevated thyroid stimulating hormone is associated with elevated cortisol in healthy young men and women. Thyroid Res 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Iranmanesh A, Lizarralde G, Johnson ML, Veldhuis JD. 1990. Dynamics of 24-hour endogenous cortisol secretion and clearance in primary hypothyroidism assessed before and after partial thyroid hormone replacement. J Clin Endocrinol Metab 70:155–161 [DOI] [PubMed] [Google Scholar]
- 42. Rubello D, Sonino N, Casara D, Girelli ME, Busnardo B, Boscaro M. 1992. Acute and chronic effects of high glucocorticoid levels on hypothalamic-pituitary-thyroid axis in man. J Endocrinol Invest 15:437–441 [DOI] [PubMed] [Google Scholar]