Abstract
Background: Levothyroxine (LT4) is one of the most commonly prescribed medications. Although considered a life-long replacement therapy, LT4 therapy can be discontinued for some patients. This study aims at: (i) reviewing the evidence on clinical outcomes of patients undergoing thyroid hormone replacement discontinuation, (ii) identifying the predictors of successful discontinuation, and (iii) systematically appraising frameworks used for deprescribing thyroid hormone.
Methods: We searched multiple bibliographic databases, including Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus, from inception to February 2020 for studies in which thyroid hormone replacement was discontinued. Clinical outcomes assessed included: proportion of patients that remained euthyroid or needed to restart thyroid hormone replacement after discontinuation and frequency of clinical symptoms of hypothyroidism and adverse effects. We also evaluated predictors for discontinuation and deprescribing frameworks. Reviewers (F.J.K.T., N.B., N.M.S.O., S.M.) evaluated studies for inclusion, extracted data, and assessed methodological quality independently and in duplicate.
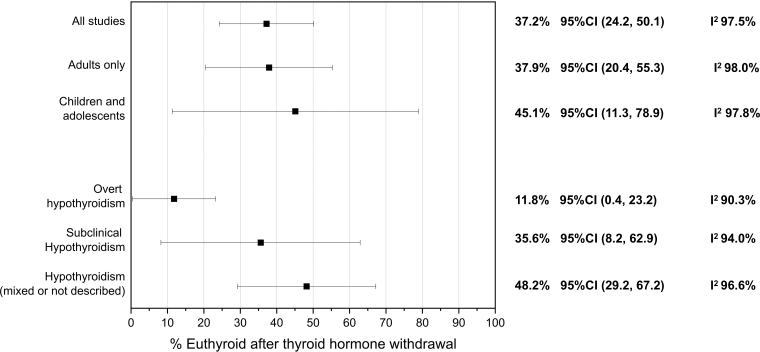
Results: Seventeen observational studies at moderate to high risk of bias met inclusion criteria, including a total of 1103 patients (86% women) with an age range of 2–81 years. Approximately a third of patients undergoing thyroid hormone discontinuation remained euthyroid at follow-up (37.2%, 95% confidence interval [CI 24.2–50.1%], I2 97.5%). Subgroup analysis showed that patients with a previous diagnosis of overt hypothyroidism (OH) were less likely to remain euthyroid (11.8% [CI 0.4–23.2%], I2 90.3%) than patients with a prior diagnosis of subclinical hypothyroidism (SCH) (35.6% [CI 8.2–62.9%], I2 94.0%). No study followed a framework for systematically deprescribing LT4.
Conclusions: Low-quality evidence suggests that up to a third of patients remained euthyroid after thyroid hormone discontinuation, with a higher proportion of patients with an initial diagnosis of SCH remaining euthyroid than patients with an initial diagnosis of OH. A deprescribing framework focusing on adequate selection of patients for deprescribing LT4 and a systematic process is warranted to guide clinicians in re-evaluating the need for LT4 in their patients.
Keywords: subclinical hypothyroidism, deprescribing, levothyroxine, thyroid dysfunction, medication withdrawal
Introduction
Thyroid hormone medications may be prepared synthetically or be derived from animal sources and contain thyroxine (T4), triiodothyronine (T3), or both. These medications are used to restore thyroid hormone levels in patients with hypothyroidism (1). Levothyroxine (LT4), a synthetic form of T4, is the second-most prescribed drug in the United States. The global market for the treatment of thyroid disorders, dominated by hypothyroidism, was valued at $2057 million in 2017, and it is estimated to reach $2771 million by 2025 at a compound annual growth rate of 3.8% from 2018 to 2025 (2,3). Although many factors likely play a role in the extensive prescription of LT4, an increase in the treatment of subclinical hypothyroidism (SCH) is a contributing factor (4).
SCH is a common biochemical diagnosis, which affects ∼10% of adults (5) and is accompanied by either nonspecific symptoms (2/3 of cases) or no symptoms at all (5,6). Once thyroid hormone replacement is started, 9 out of 10 patients with SCH continue thyroid hormone therapy indefinitely (7). Although the benefits of LT4 use for patients with overt hypothyroidism (OH) are clear, no benefits have been demonstrated with respect to quality of life or thyroid-related symptoms for patients with SCH (7–11). Observational studies have shown an association of untreated SCH with increased mortality (12,13), but randomized trials have not found that LT4 therapy decreases the risk of death (9). In addition to the treatment burden associated with thyroid hormones use, ∼50% of patients older than 65 years who take thyroid hormones develop iatrogenic hyperthyroidism, increasing their risk for arrhythmias, angina pectoris, bone loss, and fractures (14–16). After an extensive review of the available evidence, a guideline panel recently concluded that almost all adults with SCH do not benefit from thyroid hormone treatment (17). Thus, many patients currently on LT4 may not experience any significant benefit, while being exposed to its potential harms.
Deprescribing refers to the “thoughtful and systematic process of identifying problematic medications and either reducing the dose or stopping these medications in a manner that is safe, effective, and helps patients maximize their wellness and goals of care” (18). Deprescribing has been shown to reduce potentially inappropriate or unnecessary medications (19,20) and may be successful and effective in selected classes of drugs (21). Deprescribing LT4 has the potential to reduce medication burden and avoid LT4 adverse effects. The goal of this study was to summarize the clinical outcomes of patients for whom thyroid hormone replacement was discontinued, identify the predictors of successful discontinuation, and evaluate the frameworks used to deprescribe thyroid hormone replacement.
Methods
We conducted a systematic review of academic databases and meta-analysis of included studies. This review follows the recommendations of the Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) (22).
Eligibility criteria
We sought randomized controlled trials and observational studies that included patients on thyroid hormone replacement (regardless of the indication for therapy) who underwent treatment discontinuation, with no restrictions in terms of participants' age or type of hormone replacement (LT4, liothyronine, thyroid extracts, combination therapy). Studies reporting on the following were included: (i) thyroid function assessment after discontinuation of thyroid hormone replacement, (ii) predictors and procedures related to thyroid hormone discontinuation, (iii) the frequency of symptoms or adverse events as a result of thyroid hormone discontinuation, and/or (iv) description of a framework for thyroid hormone discontinuation. We excluded studies of patients undergoing thyroid hormone withdrawal in preparation for radioactive iodine treatment, patients whose indication for therapy was central or congenital hypothyroidism, postpartum thyroiditis, or thyroid cancer, and patients exposed to recent interventions that could affect thyroid hormone requirements (e.g., bariatric surgery). Finally, we excluded studies with inadequate information to determine eligibility and those for whom there was no response from the authors seeking that information.
Data sources and search strategies
An experienced librarian (L.C.H.) designed and performed a comprehensive search that ran from database inception through February 27, 2020. Supplementary Appendix SA1 describes the included databases, the search terms, and how they were combined. The search excluded animal studies, had no language limits, and used controlled vocabulary supplemented with keywords. The reference list of the included studies was reviewed to identify any additional relevant studies.
Study selection
The search results were uploaded into the systematic review software DistillerSR (Evidence Partners, Ottawa, Canada). Reviewers working independently and in duplicate reviewed all abstracts and titles for inclusion. After abstract screening and retrieval of potentially eligible studies, the full text publications were assessed for eligibility. Duplicate studies were excluded. The kappa statistic for full text screening was 0.77. Disagreements were resolved by consensus.
Data collection and management
Reviewers working independently and in duplicate by using a standardized form collected the following from eligible studies: (i) baseline clinical features: age, type of hypothyroidism (etiology), degree of hypothyroidism/thyroid status (euthyroid, subclinical, overt), type of hormone replacement, sex, thyroid hormone replacement dose at withdrawal, treatment duration, goiter presence, family history of thyroid disease, thyroid antibody positivity (thyroid peroxidase and/or thyroglobulin antibody), thyroid gland heterogeneity on ultrasound, and body mass index; (ii) thyrotropin (TSH) and free T4 levels at baseline and after withdrawal; (iii) clinical outcomes and predictors (thyroid status after withdrawal [e.g., euthyroid], thyroid hormone levels after withdrawal, symptoms, and side effects during follow-up); and (iv) features of deprescribing strategies. We extracted TSH and/or free T4 levels cut-offs used to define euthyroidism, SCH and OH, as well as definitions for goiter and thyroid gland heterogeneity.
Risk-of-bias assessment
Reviewers working independently and in duplicate used the Newcastle-Ottawa risk-of-bias tool for observational studies to evaluate the methodological quality of included studies (23). This tool determines the comparability of cohorts, their representativeness, and the ascertainment of exposure and outcomes. Disagreements were resolved by consensus.
Author contact
To reduce reporting bias, we contacted the authors of studies in which clarification or more information was needed to determine eligibility or to complete analysis. Four of the seven contacted authors replied (24–27).
Meta-analysis and subgroup analysis
A random-effects meta-analysis was conducted to evaluate the percentage of patients who were euthyroid at follow-up and those who needed to restart thyroid hormone replacement after thyroid hormone discontinuation (OpenMeta Analyst, Brown University Evidence-Based Practice Center). We performed subgroup analyses according to the type of hypothyroidism and age group. Similarly, we evaluated the mean differences in TSH measurements before and after thyroid hormone discontinuation. Inconsistency was assessed by using I2 statistic, with values >75% indicative of high inconsistency not due to chance (28).
Results
Study identification
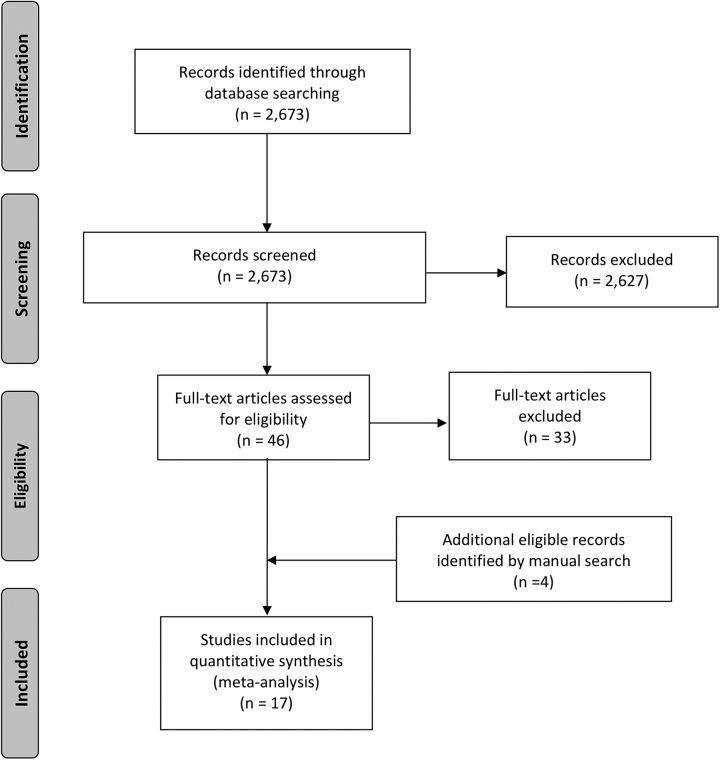
We identified 2673 potentially eligible studies; 17 observational studies, including patients undergoing discontinuation of thyroid hormone replacement therapy, were deemed eligible (24–27,29–41). Figure 1 describes the study selection process.
FIG. 1.
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic reviews and Meta-analysis.
Study characteristics
Table 1 summarizes the characteristics of the included studies. Sixteen studies evaluated thyroid hormone status categorically (e.g., proportion of euthyroid patients after discontinuation), eight evaluated thyroid hormone status numerically (e.g., TSH levels), and four evaluated clinical symptoms. Four studies evaluated children and adolescents, 3 studies evaluated mixed populations (children, adolescents, and adults), and 10 studies evaluated adults. The etiology and degree of hypothyroidism was also variable, including autoimmune and idiopathic, and SCH and OH. In addition, some studies included patients who were euthyroid at baseline (time of initial diagnosis). Table 2 summarizes patient characteristics.
Table 1.
Characteristics of Studies Included in the Systematic Review
| Author (reference) | Year | Country | Age group | Etiology of hypothyroidism | Degree of hypothyroidism | Outcomes | Follow-up time (months) |
|---|---|---|---|---|---|---|---|
| Wasniewska et al. (36) | 2012 | Italy | Children Adolescents |
Idiopathic | Subclinical | Clinical symptoms Thyroid hormone status categorical Thyroid hormone status numerical |
3 |
| Battelino et al. (25) | 1994 | Slovenia | Children Adolescents |
Autoimmune | Mixed | Thyroid hormone status categorical | 0.33 |
| Radetti et al. (26) | 2017 | Italy | Children Adolescents |
Autoimmune | Mixed | Thyroid hormone status categorical Thyroid hormone status numerical |
12 2, 6, 12, 24 |
| Sklar (37) | 1986 | United States | Children Adolescents |
Autoimmune | Euthyroid Subclinical Overt |
Thyroid hormone status categorical | Variable (28–36) |
| Fava et al. (38) | 2009 | Italy | Children Adolescents Adults |
Autoimmune | Subclinical | Thyroid hormone status categorical | 1.3 |
| Takasu et al. (35) | 1992 | Japan | Children Adolescents Adults |
Autoimmune | Overt | Clinical symptoms Thyroid hormone status categorical |
12 |
| Takasu et al. (39) | 1990 | Japan | Adolescents Adults |
Autoimmune | Overt | Thyroid hormone status categorical | 3 |
| Carlwe et al. (40) | 2013 | Sweden | Adults | No description | Overt | Thyroid hormone status numerical | 0.25 |
| Rizzolo et al. (41) | 1986 | United States | Adults | No description | No description | Thyroid hormone status categorical | 0.75 |
| Krugman et al. (29) | 1975 | United States | Adults | Mixed | No description | Thyroid hormone status categorical | 2 |
| Rosario and Calsolari (27) | 2016 | Brazil | Adults | Mixed | Subclinical | Thyroid hormone status categorical Thyroid hormone status numerical |
24–120 Median: 60 |
| Ohsawa et al. (30) | 1981 | Japan | Adults | Autoimmune | No description | Thyroid hormone status categorical Thyroid hormone status numerical |
2 |
| Comtois et al. (32) | 1995 | Canada | Adults | Autoimmune | Mixed | Clinical symptoms Thyroid hormone status categorical Thyroid hormone status numerical |
0.75 |
| Höfling et al. (31) | 2013 | Brazil | Adults | Autoimmune | Overt | Thyroid hormone status categorical Thyroid hormone status numerical |
9 1 |
| Rieu et al. (33) | 1995 | France | Adults | Autoimmune | Euthyroid Hypothyroid |
Thyroid hormone status categorical | 2 |
| Livadas et al. (24) | 2018 | Greece | Adults | Other (mixed) | No description | Clinical symptoms Thyroid hormone status categorical Thyroid hormone status numerical |
1.5–2 |
| Nikolai (34) | 1989 | United States | Adults | Primary | Overt | Thyroid hormone status categorical |
6 12 36 |
Table 2.
Patient Characteristics of the Studies Included in the Systematic Review
| Study | Sample size (n) | Age (years) | Women (%) | Were all patients euthyroid at TH discontinuation | LT4 dose at discontinuation (mcg/day) | Treatment duration (years) | Thyroid antibodies TPO and/or anti-TG Ab (%) | Goiter (%) | Heterogeneous gland (%) | Family history of thyroid disease (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Children and adolescents | ||||||||||
| Sklar (37) | 16 | — | — | Unclear | Median 2.2 | — | 100 | — | — | |
| Battelino et al. (25) | 29 | Mean 14.9 Range 8.5–19.7 |
79 | Yes | — | — | — | — | — | — |
| Wasniewska et al. (36) | 69 | 9.4 (4.0) Range 2.1–14.9 |
75.4 | Yes | — | 2 | 7.2a | 0 | 7.2 | — |
| Radetti et al. (26) | 148 | — | 82.6 | No | — | 4.1 (2.6) Range 1–12 |
100 | — | 100 | 47.7 |
| Children, adolescents, and adults | ||||||||||
| Takasu et al. (39) | 92 | Range 14–68 | 90.2 | Yes | — | Mean 3.8 Range 1–8 |
100 | 100 | — | — |
| Takasu et al. (35) | 21 | 31 [12–72]b | 85.7 | Yes | — | Range 1.5–7 | — | 33.3 | — | — |
| Fava et al. (38) | 14 | 18 [8–26]b | 78.6 | Yes | 1.26 (0.3)c | 6.4 [2.8–12.4]b | — | 42.9 | 100 | 21.4 |
| Adults | ||||||||||
| Krugman et al. (29) | 14 | Range 17–68 | 71 | Unknown | — | Range 0.4–22 | — | 21.4 | — | — |
| Ohsawa et al. (30) | 24 | — | 100 | Unknown | LT4: 200 LT3: 50 |
Range 0.1–5 | — | 100 | — | — |
| Rizzolo et al. (41) | 22 | — | — | Unknown | — | — | — | — | — | — |
| Nikolai (34) | 49 | Mean 52 Range 23–81 |
79.6 | Unclear | — | Range 0.5–3 | — | 38.8 | — | — |
| Rieu et al. (33) | 20 | — | — | Yes | — | 1 | — | 100 | — | — |
| Comtois et al. (32) | 79 | 39 (13) Range 17–64 |
91 | Yes | 110 (30) Range 50–150 |
1 | — | 55.7 | — | 44 |
| Carlwe et al. (40) | 13 | Mean 43 Range 28–60 |
92 | Yes | 109 [75–162)] | — | — | — | — | — |
| Höfling et al. (31) | 20 | 42.1 (11.6) | 100 | Yes | Mean 90.00 [CI 70.87–109.13] | 4.52 (3.47) | 90a 80d |
25 | 100 | — |
| Rosario and Calsolari (27) | 182 | 42 [19–63]b | 100 | Yes | Range 25–100 Range 0.3–1.5c |
3 [2–6.8]b | 55a | — | 23.1 | — |
| Livadas et al. (24) | 291 | 48 (16.4) | 84 | Yes | 0.92 (0.42)c | Mean 8.1 Range 1–37 |
58 | 0 | 62 | 63 |
Results are mean (SD), unless otherwise indicated.
TPO Ab only.
Results are median [range].
Results are mcg/kg/day.
Anti-TG Ab only.
anti-TG Ab, anti-thyroglobulin antibody; CI, 95% confidence interval; LT3, liothyronine; LT4, levothyroxine; TH, thyroid hormone; TPO, thyroid peroxidase; TPO Ab, thyroid peroxidase antibody.
In 14 studies, thyroid hormone replacement therapy consisted of LT4. Ohsawa et al. studied an LT4 and a liothyronine group; Krugman et al. studied patients on variable regimens (LT4/liothyronine combination, LT4, and thyroid extract); and in the study by Nikolai, the type of thyroid hormone replacement was unspecified (29,30,34). Three studies discontinued therapy following a tapering regimen. Tapering regimens included: discontinuing therapy within two weeks, first halving dose at week one, and discontinuing the remaining dose at week two (26); halving LT4 dose successively every four weeks until a dose ≤12.5 mcg/day was reached and therapy was then discontinued (27); or either halving dose and eliminating the remaining dose in two months or by 25 mcg reductions until discontinuation (34).
No study formally used the term “deprescribing” when referring to the process of discontinuing thyroid hormone replacement therapy, and none described a systematic process of deprescribing thyroid hormone in clinical practice.
Study quality
We judged the observational studies to be at moderate to high risk of bias on the basis of representativeness of the exposed cohort (most were a selected group of users), lack of blinding, and lack of assessment of cofounders (Supplementary Table S1).
Meta-analysis
Proportion of patients who remained euthyroid
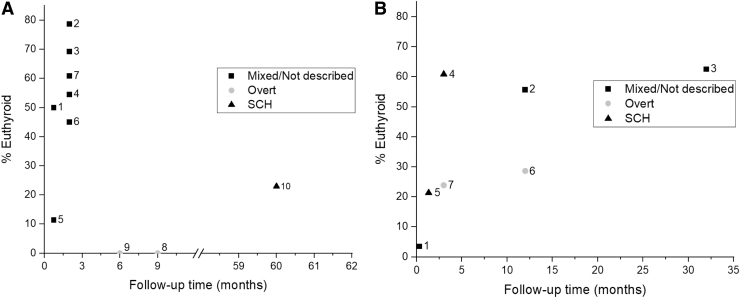
Sixteen studies evaluated the proportion of patients who remained euthyroid after thyroid hormone discontinuation (Fig. 2). A total of 1082 patients were included, and the time of outcome assessment ranged from 10 days to a median follow-up of five years. Most studies (n = 10) included adults only or participants with no description of the degree of hypothyroidism and/or a mix of overt/subclinical/euthyroid patients (n = 9). Table 3 describes the proportion of patients with euthyroidism according to the indication for thyroid hormone therapy. The definitions for euthyroidism for each study are summarized in Supplementary Table S2.
FIG. 2.
(A) Percentage of euthyroid adult patients by type of hypothyroidism and follow-up time. 1: Rizzolo et al. (41); 2: Krugman et al. (29); 3: Ohsawa et al.–LT4 arm (30); 4: Ohsawa et al.–LT3 arm (30); 5: Comtois et al. (32); 6: Rieu et al. (33); 7: Livadas et al. (24); 8: Höfling et al. (31); 9: Nikolai (34); 10: Rosario and Calsolari (27). (B) Percentage of euthyroid patients by type of hypothyroidism and follow-up time in studies with mixed patient age groups (children, adolescents, and adults). 1: Battelino (25); 2: Radetti et al. (26); 3: Sklar (37); 4: Wasniewska et al. (36); 5: Fava et al. (38); 6: Takasu et al. (35); 7: Takasu et al. (39). SCH, subclinical hypothyroidism; Overt, overt hypothyroidism.
Table 3.
Percent of Euthyroid Patients After Thyroid Hormone Discontinuation by Prior Indication for Therapy
| Population | Study | Indication for treatment | n | % Euthyroid |
|---|---|---|---|---|
| Children and adolescents | Battelino et al. (25) | Autoimmune hypothyroidism (overt or subclinical) | 29 | 3.5 |
| Wasniewska et al. (36) | Idiopathic SCH | 69 | 60.9 | |
| Radetti et al. (26) | Autoimmune hypothyroidism (overt or subclinical) | 140 | 55.7 | |
| Sklar (37) | Autoimmune euthyroid | 7 | 86 | |
| Autoimmune SCH | 4 | 65 | ||
| Autoimmune OH | 5 | 20 | ||
| Children, adolescents, and adults | Fava et al. (38) | Autoimmune hypothyroidism (overt or subclinical) | 14 | 21.4 |
| Takasu et al. (39) | Autoimmune OH | 92 | 23.9 | |
| Takasu et al. (35) | Autoimmune OH | 21 | 28.6 | |
| Adults | Rizzolo et al. (41) | Hypothyroidism: clinical diagnosis without adequate laboratory confirmation | 7 | 86 |
| Hypothyroidism: diagnosis based on laboratory tests | 7 | 14 | ||
| Hypothyroidism: diagnosis based on symptoms and the presence of goiter | 8 | 50 | ||
| Comtois et al. (32) | Autoimmune hypothyroidism (overt or subclinical) | 79 | 11 | |
| Krugman et al. (29) | OH | 10 | 70 | |
| Goiter | 1 | 100 | ||
| Hashimoto's thyroiditis | 2 | 100 | ||
| Nontoxic nodular goiter | 1 | 100 | ||
| Rieu et al. (33) | Euthyroid Goitrous Hashimoto's disease | 9 | 100 | |
| Autoimmune OH | 11 | 0 | ||
| Ohsawa et al. (30) | Euthyroid Hashimoto's thyroiditis | 13 11 |
69 55a |
|
| Livadas et al. (24) | Presence of thyroid nodules but not on suppression therapy | 96 | 63.5b | |
| Unknown reason for LT4 supplementation or no evidence of past thyroid dysfunction provided | 78 | 46.2b | ||
| Therapy initiated postpregnancy without reassessment | 15 | 73.3b | ||
| Hashimoto's thyroiditis or hypothyroidism-like or related symptoms | 102 | 67.6b | ||
| Nikolai (34) | OH | 49 | 0 | |
| Höfling et al. (31) | Autoimmune OH | 20 | 0 | |
| Rosario and Calsolari (27) | SCH with presence of TPO Ab with or without goiter, symptoms of hypothyroidism, dyslipidemia, depression, infertility, or unknown reasons | 182 | 23 |
% euthyroid after liothyronine discontinuation.
% hypothyroid (overt/subclinical) was reported for subgroups, and % euthyroid was derived from this report.
OH, overt hypothyroidism; SCH, subclinical hypothyroidism.
When all studies were included, the pooled estimate for euthyroidism at follow-up was 37.2% (95% confidence interval [CI 24.2–50.1%], I2 97.5%). The pooled estimate for euthyroidism was lower for those with OH, 11.8% ([CI 0.4–23.2%], I2 90.3%) and for adults it was 37.9% ([CI 20.4–55.3%], I2 98%). Figure 3 shows the results of this meta-analysis.
FIG. 3.
Meta-analysis of euthyroidism (%) after thyroid hormone discontinuation, including all studies and subgroup analysis by degree of hypothyroidism and age of participants. CI, 95% confidence interval.
Proportion of patients who reinitiated thyroid hormone treatment
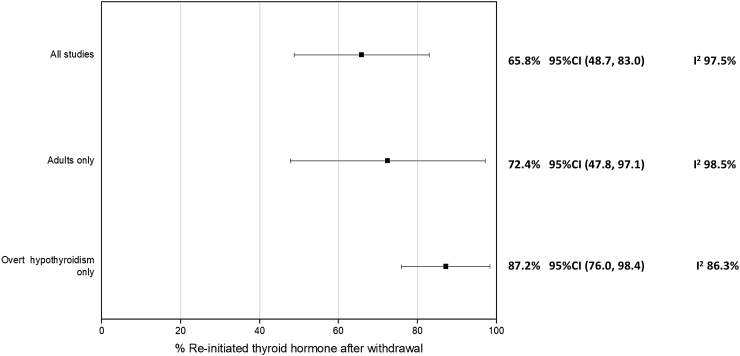
Nine studies (24,26,27,31,34,35,37,39,41) evaluated the percentage of patients in whom thyroid hormone was restarted during follow-up. A total of 833 patients were included, and the time of outcome assessment ranged from three weeks to a median of five years. The criteria for reinitiating thyroid hormone therapy varied across studies. Most studies reinitiated treatment when laboratory evidence of SCH was present, applying different TSH thresholds for treatment between 4.5 and 10 mIU/L or above the reference value.
When all studies were included, the pooled estimate for restarting thyroid hormone during follow-up was 65.8% ([CI 48.7–83.0%], I2 97.5%). The pooled estimate for patients with OH was higher, 87.2% ([CI 76.0–98.4%], I2 86.3%). Figure 4 shows the results of this meta-analysis.
FIG. 4.
Meta-analysis of re-initiation of thyroid hormone (% X axis), including all studies and subgroup analysis according to degree of hypothyroidism and age of participants.
TSH changes at follow-up
In four studies (27,31,36,40), the mean TSH values before and after LT4 withdrawal were available. Three of the studies included adults. Two studies included patients with SCH and two with OH. Time of outcome assessment ranged from one week to five years of median follow-up. The mean TSH difference (increase) was 9.4 mIU/L ([CI 5.0–12.8], I2 97.4%).
Predictors for developing hypothyroidism after LT4 withdrawal
Data regarding predictors for the development of hypothyroidism after LT4 withdrawal were insufficient for meta-analysis; however, statistically significant predictors are summarized in Table 4.
Table 4.
Predictors for Development of Euthyroidism and Hypothyroidism After Thyroid Hormone Discontinuation
| Population | Predictors for euthyroidism |
|---|---|
| Adults | Positive family history of thyroid disease (32) |
| Children and adolescents |
TSH at diagnosis <10 mIU/L (26) |
| Population |
Predictors for hypothyroidism |
| Adults |
Heterogeneous thyroid on ultrasound (24) |
| TPO Ab and ultrasound with diffuse hypoechogenicity (27) | |
| TSH ≥8 mIU/L at diagnosis (27) | |
| Children and adolescents | Baseline TSH >9 mIU/L (36) |
| Age at diagnosis (younger) (26) | |
| Anti-TG Ab at diagnosis (26) | |
| Age at withdrawal (younger) (26) | |
| TPO Ab level at withdrawal (26) | |
| Goiter at thyroid hormone withdrawal (26) |
TSH, thyrotropin.
Symptoms and adverse events after LT4 withdrawal
Four studies evaluated the development of symptoms after LT4 withdrawal. In two studies, no patients developed symptoms; Livadas et al. reported no changes in quality of life after LT4 discontinuation (24); and Wasniewska et al. found no clinical signs or symptoms of hypothyroidism (36). Two studies reported development of symptoms after LT4 withdrawal; Comtois et al. reported fatigue in 15.2% of patients, however whether these patients became hypothyroid was not reported (32). Takasu et al. reported “symptoms of hypothyroidism” in 71.4% of patients, all of whom were biochemically hypothyroid (35). These four studies relied on self-reporting, and none included a systematic assessment of symptoms. Rizzolo et al. assessed eight hypothyroidism-related symptoms at baseline and 21 days after thyroid hormone discontinuation; however, they included patient populations not meeting our inclusion criteria (e.g., subtotal thyroidectomy), and thus they are not reported here (41). Reporting on the development of symptoms after abrupt versus tapered LT4 discontinuation was unavailable.
Three studies evaluated adverse events after LT4 withdrawal. Livadas et al. reported no “adverse events”(24) and Radetti et al. found no adverse effects on growth, lipid profile, glucose homeostasis, and the development or worsening of goiter (26). Carlwe et al. reported intolerable fatigue in one participant (7.7%) who dropped out with unknown thyroid function status (40).
Discussion
We performed a systematic review and meta-analysis to summarize the available evidence on clinical outcomes after thyroid hormone discontinuation that could guide LT4 deprescription in clinical practice. We found that 37% of patients remained euthyroid at follow-up when including all studies; with a lower proportion (12%) for patients initially diagnosed with OH. Similarly, most patients (66%) were restarted on thyroid hormone replacement, although different criteria, including the development of SCH, were used to restart therapy. Moreover, heterogenous echogenicity on thyroid ultrasound, elevated TSH ≥8–9 mIU/L, and presence of thyroid antibodies were negatively associated with the rate of euthyroidism after thyroid hormone discontinuation in individual studies. Data pertinent to patient-centered outcomes (adverse effects, development of symptoms) were scarce. No study reported a systematic process/framework for deprescribing LT4. These findings suggest that deprescribing LT4 could be successful for carefully selected patients and highlight the need for studies at low risk of bias that include evaluation of patient-important outcomes.
Despite decades-old controversies related to treatment thresholds for SCH, current guidelines (1,17,42) do not recommend the continuous evaluation of the need for thyroid hormone replacement therapy. However, in clinical practice, patients may be overtreated with LT4 if therapy is initiated without a well-documented hypothyroidism diagnosis, if clinicians start therapy based solely on the nonspecific symptoms of hypothyroidism, and depending on thresholds used to start treatment for SCH (7,24,43).
The term “deprescribing” usually refers to the process of withdrawal of an inappropriate medication, supervised by a health care professional with the goal of managing polypharmacy and improving outcomes. Therefore, deprescribing is a more complex process than just stopping a medication (44–46). This systematic process, starts with an accurate evaluation of the medication list, followed by identification of potentially inappropriate medications, collaboration between patients and clinicians to decide whether deprescribing would be appropriate, and establishing a supportive plan to safely deprescribe the medication (44,45). Shared decision making is fundamental for a successful deprescribing intervention, as patients are more likely to consider deprescription if they: (i) understand why the medication is inappropriate, (ii) have their concerns related to stopping the medication addressed, and (iii) understand the deprescribing plan and feel supported by the clinical team. Deprescribing conversations should focus on raising awareness about alternatives, discussing the risks and benefits of deprescribing, and understanding the patient's preferences (44,47).
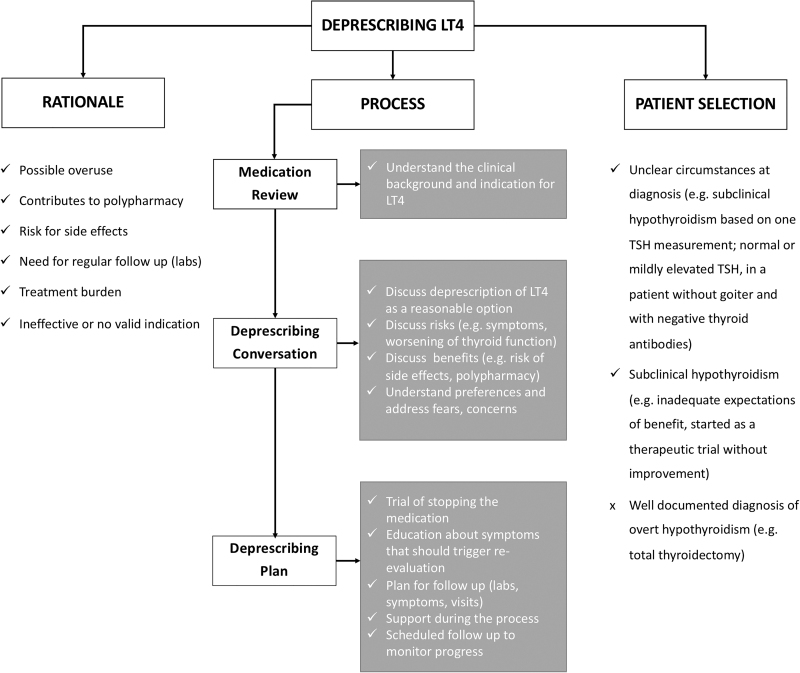
The rationale, indications, and a proposed process for deprescribing LT4 are summarized in Figure 5. Briefly, LT4 deprescribing is reasonable in patients for whom the benefits of treatment do not outweigh the risks. This could be the case for patients diagnosed with SCH based on a single TSH measurement or for whom LT4 was started as part of a therapeutic trial and was never discontinued despite the lack of clinical improvement (4,48). As summarized in Figure 5, there is a strong rationale to consider LT4 discontinuation and preliminary clinical evidence guiding patient selection and process to deprescribe LT4.
FIG. 5.
Algorithm for approaching LT4 deprescribing. LT4, levothyroxine; TSH, thyrotropin.
After patients and physicians decide to deprescribe LT4, a plan for discontinuation and follow-up should be made. The likelihood of developing symptoms while using an abrupt versus tapering discontinuation regimen has yet to be elucidated. Once LT4 has been discontinued, it might be reasonable to follow up a patient's symptoms and thyroid function tests every four to six weeks (49) and less frequently after six months of follow-up if the patient remains clinically and biochemically euthyroid. The patient–physician discussion should also delineate what criteria would merit LT4 re-initiation.
Though research on deprescribing is increasing, data on deprescribing thyroid hormone are scarce (50). Available studies have evaluated changes in thyroid function status or the need to re-prescribe thyroid hormone replacement; however, long-term effects and patient-centered outcomes are yet to be determined. Future studies should consider measuring quality of life, adverse drug withdrawal events, and reduction in cardiovascular or bone health events (50,51). Noninferiority study designs may be helpful to evaluate deprescribing interventions (50,52). This design was developed from the need to evaluate similar efficacy as compared with the established treatment while offering greater safety, convenience, or lower cost. Although these trial designs may be more complex than those used to establish superiority, they can help determine that an intervention is not worse than the control treatment (53). Therefore, the use of noninferiority study designs evaluating the absence of change in clinical status after medication withdrawal has been proposed in the development of deprescribing trials (50). Studies focusing on the process of deprescribing (e.g., selection of patients, conversation about deprescribing and deprescribing plan) are needed to support safe and likely beneficial deprescribing of LT4 in practice. Further, the field will need to develop and test multi-level strategies for deprescribing that are context specific but feasible, cost-effective, adaptable, and generalizable across settings. Such strategies will need to specifically target the unique barriers to the deprescribing of thyroid hormone therapy. Future research should investigate potential unintended negative consequences of deprescribing for patients, clinicians, and health care systems.
Incomplete searching and arbitrary study selection represent potential limitations of systematic reviews. However, the rigorous and comprehensive nature of our overlapping search strategies with a medical librarian's input minimize the possibility that we missed studies that could have substantially changed the inferences drawn from this study (54). The risk of reporting bias is high, particularly when the body of evidence is based on small observational studies. We attempted to decrease the chances of reporting bias by contacting authors (55). Although it would have been clinically meaningful to evaluate the effects of important patient characteristics on thyroid function after LT4 withdrawal (e.g., thyroid autoimmunity status) or perform subgroup analysis according to time of follow-up, this was not possible due to insufficient data. Due to their uncontrolled and observational nature, and the lack of adjustment for confounders, the included studies were at moderate- to high risk of bias. In addition, the meta-analysis results showed high heterogeneity and imprecision. Studies included patients with variable characteristics, limiting the direct application of the results to specific patients. In all, low-quality evidence suggests that deprescribing LT4 could be successful, but patient selection is important. Although these limitations could not be overcome methodologically, our review exhibited important strengths, including synthesis of the totality of the available evidence following a predetermined protocol, with reproducible judgments about study selection and quality and focused analyses assessing the effects of LT4 discontinuation, which has not been previously performed (56).
In summary, low-quality evidence suggests that up to a third of patients remained euthyroid after thyroid hormone discontinuation, with a higher proportion of patients with an initial diagnosis of SCH remaining euthyroid than patients with an initial diagnosis of OH. Data regarding patient-centered outcomes remain sparse. Nonetheless, for some patients, deprescribing LT4 is likely reasonable. Patients and physicians can use this information when discussing whether discontinuation of LT4 is a reasonable consideration.
Supplementary Material
Authors' Contributions
N.M.S.O., J.P.B., and S.M. conceived and designed the study, with input from all the co-authors. L.C.H. designed and performed the literature search with input from N.M.S.O. and S.M. N.B., F.J.K.T., N.M.S.O., and S.M. carried out the data collection and statistical analysis, with input from J.P.B. All co-authors contributed to critical appraisal and review of the results and the article. All authors reviewed and agreed on the final version of the article.
Author Disclosure Statement
The authors have nothing to disclose. No competing financial interests exist.
Funding Information
Dr. Maraka receives support from the Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000. Dr. Singh Ospina was supported by the National Cancer Institute of the National Institutes of Health under Award Number K08CA248972. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Material
References
- 1. Garber JR, Cobin RH, Gharib H. 2013. American Association of Clinical Endocrinologists and American Thyroid Association taskforce on hypothyroidism in adults 2012 clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists a. Endocr Pract 19:175. [DOI] [PubMed] [Google Scholar]
- 2. Fuentes A, Pineda M, Venkata K. 2018. Comprehension of top 200 prescribed drugs in the US as a resource for pharmacy teaching, training and practice. Pharmacy 6:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kunsel T, Chandra G 2018 Thyroid Gland Disorder Treatment Market by Disease Type (Hyperthyroidism and Hypothyroidism), Drug Type (Levothyroxine, Liothyronine, Propylthiouracil, Imidazole-based Compound, and Others), Route of Administration (Oral, Intravenous, and Others), and Distribution/Sales Channel (Wholesaler/Distributor, Retailer, Mail-order Pharmacy, and Others)—Global Opportunity Analysis and Industry Forecast, 2017–2025. p 243. Available at https://www.alliedmarketresearch.com/thyroid-gland-disorder-treatment-market (accessed June9, 2020)
- 4. Rodriguez-Gutierrez R, Maraka S, Ospina NS, Montori VM, Brito JP. 2017. Levothyroxine overuse: time for an about face? Lancet Diabetes Endocrinol 5:246–248 [DOI] [PubMed] [Google Scholar]
- 5. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. 2000. The colorado thyroid disease prevalence study. Arch Intern Med 160:526–534 [DOI] [PubMed] [Google Scholar]
- 6. Cooper DS, Biondi B. 2012. Subclinical thyroid disease. Lancet 379:1142–1154 [DOI] [PubMed] [Google Scholar]
- 7. Taylor PN, Iqbal A, Minassian C, Sayers A, Draman MS, Greenwood R, et al. . 2014. Falling threshold for treatment of borderline elevated thyrotropin levels—balancing benefits and risks evidence from a large community-based study. JAMA Intern Med 174:32–39 [DOI] [PubMed] [Google Scholar]
- 8. Mooijaart SP, Du Puy RS, Stott DJ, Kearney PM, Rodondi N, Westendorp RGJ, et al. . 2019. Association between levothyroxine treatment and thyroid-related symptoms among adults aged 80 years and older with subclinical hypothyroidism. JAMA 322:1977–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stott DJ, Rodondi N, Kearney PM, Ford I, Westendorp RGJ, Mooijaart SP, et al. . 2017. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med 376:2534–2544 [DOI] [PubMed] [Google Scholar]
- 10. Andersen MN, Olsen AMS, Madsen JC, Kristensen SL, Faber J, Torp-Pedersen C, et al. . 2016. Long-term outcome in levothyroxine treated patients with subclinical hypothyroidism and concomitant heart disease. J Clin Endocrinol Metab 101:4170–4177 [DOI] [PubMed] [Google Scholar]
- 11. Grossman A, Feldhamer I, Meyerovitch J. 2018. Treatment with levothyroxin in subclinical hypothyroidism is associated with increased mortality in the elderly. Eur J Intern Med 50:65–68 [DOI] [PubMed] [Google Scholar]
- 12. Lillevang-Johansen M, Abrahamsen B, Jørgensen HL, Brix TH, Hegedüs L. 2018. Over- and under-treatment of hypothyroidism is associated with excess mortality: a register-based cohort study. Thyroid 28:566–574 [DOI] [PubMed] [Google Scholar]
- 13. Thayakaran R, Adderley NJ, Sainsbury C, Torlinska B, Boelaert K, Šumilo D, et al. . 2019. Thyroid replacement therapy, thyroid stimulating hormone concentrations, and long term health outcomes in patients with hypothyroidism: longitudinal study. BMJ 366:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sawin CT, Geller A, Wolf PA, Belanger AJ, Baker E, Bacharach P, et al. . 1994. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation. N Engl J Med 221:1249–1252 [DOI] [PubMed] [Google Scholar]
- 15. Somwaru LL, Arnold AM, Joshi N, Fried LP, Cappola AR. 2009. High frequency of and factors associated with thyroid hormone over-replacement and under-replacement in men and women aged 65 and over. J Clin Endocrinol Metab 94:1342–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flynn RW, Bonellie SR, Jung RT, MacDonald TM, Morris AD, Leese GP. 2010. Serum thyroid-stimulating hormone concentration and morbidity from cardiovascular disease and fractures in patients on long-term thyroxine therapy. J Clin Endocrinol Metab 95:186–193 [DOI] [PubMed] [Google Scholar]
- 17. Bekkering GE, Agoritsas T, Lytvyn L, Heen AF, Feller M, Moutzouri E, et al. . 2019. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ 365:1–9 [DOI] [PubMed] [Google Scholar]
- 18. U.S. Deprescribing Research Network. What is deprescribing?. 2020. Available at https://deprescribingresearch.org/about-us/what-is-deprescribing/ (accessed July18, 2020)
- 19. Pruskowski J, Handler SM. 2017. The DE-PHARM project: a pharmacist- driven deprescribing initiative in a nursing facility. Consult Pharm 32:468–478 [DOI] [PubMed] [Google Scholar]
- 20. Wouters H, Scheper J, Koning H, Brouwer C, Twisk JW, van der Meer H, et al. . 2017. Discontinuing inappropriate medication use in nursing home residents. Ann Intern Med 167:609–617 [DOI] [PubMed] [Google Scholar]
- 21. Dills H, Shah K, Messinger-Rapport B, Bradford K, Syed Q. 2018. Deprescribing medications for chronic diseases management in primary care settings: a systematic review of randomized controlled trials. J Am Med Dir Assoc 19:923–935.e2. [DOI] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, Altman DG. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62:1006–1012 [DOI] [PubMed] [Google Scholar]
- 23. Wells GS, O'Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available at http://www.ohri.ca/programs/clinical_epidemiology/nos_manual.pdf (accessed July17, 2020)
- 24. Livadas S, Bothou C, Androulakis I, Boniakos A, Angelopoulos N, Duntas L. 2018. Levothyroxine replacement therapy and overuse: a timely diagnostic approach. Thyroid 28:1580–1586 [DOI] [PubMed] [Google Scholar]
- 25. Battelino T, Krzisnik C, Gottschalk ME, Zeller WP. 1994. Testing for thyroid function recovery in children and adolescents with Hashimoto thyroiditis. Ann Clin Lab Sci 24:489–494 [PubMed] [Google Scholar]
- 26. Radetti G, Salerno M, Guzzetti C, Cappa M, Corrias A, Cassio A, et al. . 2017. Thyroid function in children and adolescents with Hashimoto's thyroiditis after L-thyroxine discontinuation. Endocr Connect 6:206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosario PW, Calsolari MR. 2016. Levothyroxine therapy in the subclinical hypothyroidism: a lifelong therapy? A long-term study. Clin Endocrinol (Oxf) 85:819–820 [DOI] [PubMed] [Google Scholar]
- 28. Higgins JP, Thompson SG, Deeks JJ, Altman DG. 2003. Measuring inconsistency in meta-analyses. BMJ 327:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krugman LG, Hershman JM, Chopra IJ. 1975. Patterns of recovery of the hypothalamic pituitary thyroid (HPT) axis in patients taken off chronic thyroid therapy. Clin Res 23:70–80 [DOI] [PubMed] [Google Scholar]
- 30. Ohsawa N, Kobayashi I, Suwa K, Kamio N, Maruta S, Ohshima K, et al. . 1981. TSH and prolactin secretions in Hashimoto' s following withdrawal of thyroid hormone thyroiditis therapy. Endocrinol Jpn 28:329–334 [DOI] [PubMed] [Google Scholar]
- 31. Höfling DB, Chavantes MC, Juliano AG, Cerri GG, Knobel M, Yoshimura EM, et al. . 2013. Low-level laser in the treatment of patients with hypothyroidism induced by chronic autoimmune thyroiditis: a randomized, placebo-controlled clinical trial. Lasers Med Sci 28:743–753 [DOI] [PubMed] [Google Scholar]
- 32. Comtois R, Faucher L, Laflèche L. 1995. Outcome of hypothyroidism caused by Hashimoto's thyroiditis. Arch Intern Med 155:1404–1408 [PubMed] [Google Scholar]
- 33. Rieu M, Richard A, Sadoudi R, Berrod JL. 1995. Effects of thyroid status on thyroid autoimmunity expression in surgically induced hypothyroid patients with graves' disease. Horm Res Paediatr 44:29–34 [DOI] [PubMed] [Google Scholar]
- 34. Nikolai TF 1989. Recovery of thyroid function in primary hypothyroidism. Am J Med Sci 297:18–21 [DOI] [PubMed] [Google Scholar]
- 35. Takasu N, Yamada T, Takasu M, Komiya I, Nagasawa Y, Asawa T, et al. . 1992. Disappearance of thyrotropin-blocking antibodies and spontaneous recovery from hypothyroidism in autoimmune thyroiditis. N Engl J Med 326:513–518 [DOI] [PubMed] [Google Scholar]
- 36. Wasniewska M, Corrias A, Aversa T, Valenzise M, Mussa A, De Martino L, et al. . 2012. Comparative evaluation of therapy with l-thyroxine versus no treatment in children with idiopathic and mild subclinical hypothyroidism. Horm Res Paediatr 77:376–381 [DOI] [PubMed] [Google Scholar]
- 37. Sklar CA 1986. Juvenile autoimmune thyroiditis. Am J Dis Child 140:877. [DOI] [PubMed] [Google Scholar]
- 38. Fava A, Oliverio R, Giuliano S, Parlato G, Michniewicz A, Indrieri A, et al. . 2009. Clinical evolution of autoimmune thyroiditis in children and adolescents. Thyroid 19:361–367 [DOI] [PubMed] [Google Scholar]
- 39. Takasu N, Komiya I, Asawa T, Nagasawa Y, Yamada T. 1990. Test for recovery from hypothyroidism during thyroxine therapy in Hashimoto's thyroiditis. Lancet 336:1084–1086 [DOI] [PubMed] [Google Scholar]
- 40. Carlwe M, Schaffer T, Sjöberg S. 2013. Short-term withdrawal of levothyroxine, induced increase of thyroid-stimulating hormone and an increase ratio of triiodothyronine to thyroxine. Eur Endocrinol 9:37–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rizzolo PJ, Porr D, Fisher PC. 1986. Reevaluation of patients on thyroxine therapy. J Fam Pract 22:241–244 [PubMed] [Google Scholar]
- 42. Pearce SHS, Brabant G, Duntas LH, Monzani F, Peeters RP, Razvi S, et al. . 2013. 2014 ETA guideline: management of Subclinical Hypothyroidism. Eur Thyroid J 2:215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Negro R, Attanasio R, Nagy E V., Papini E, Perros P, Hegedüs L. 2020. Use of thyroid hormones in hypothyroid and euthyroid patients; the 2019 Italian survey. Eur Thyroid J 9:25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reeve E, To J, Hendrix I, Shakib S, Roberts MS, Wiese MD. 2013. Patient barriers to and enablers of deprescribing: a systematic review. Drugs Aging 30:793–807 [DOI] [PubMed] [Google Scholar]
- 45. Machado-Alba JE, Gaviria-Mendoza A, Machado-Duque ME, Chica L. 2017. Deprescribing: a new goal focused on the patient. Expert Opin Drug Saf 16:111–112 [DOI] [PubMed] [Google Scholar]
- 46. Reeve E, Gnjidic D, Long J, Hilmer S. 2015. A systematic review of the emerging definition of “deprescribing” with network analysis: implications for future research and clinical practice. Br J Clin Pharmacol 80:1254–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jansen J, Naganathan V, Carter SM, McLachlan AJ, Nickel B, Irwig L, et al. . 2016. Too much medicine in older people? Deprescribing through shared decision making. BMJ 353:1–6 [DOI] [PubMed] [Google Scholar]
- 48. Nixon M, Westendorp RGJ. 2017. When subclinical hypothyroidism becomes clinically diagnosed. Eur J Intern Med 46:34–35 [DOI] [PubMed] [Google Scholar]
- 49. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. . 2014. Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid 24:1670–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thompson W, Reeve E, Moriarty F, Maclure M, Turner J, Steinman MA, et al. . 2019. Deprescribing: future directions for research. Res Soc Adm Pharm 15:801–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reeve E, Thompson W, Farrell B. 2017. Deprescribing: a narrative review of the evidence and practical recommendations for recognizing opportunities and taking action. Eur J Intern Med 38:3–11 [DOI] [PubMed] [Google Scholar]
- 52. Anderson K, Stowasser D, Freeman C, Scott I. 2014. Prescriber barriers and enablers to minimising potentially inappropriate medications in adults: a systematic review and thematic synthesis. BMJ Open 4:e006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mauri L, D'Agostino RB. 2017. Challenges in the design and interpretation of noninferiority trials. N Engl J Med 377:1357–1367 [DOI] [PubMed] [Google Scholar]
- 54. Rethlefsen ML, Farrell AM, Osterhaus Trzasko LC, Brigham TJ. 2015. Librarian co-authors correlated with higher quality reported search strategies in general internal medicine systematic reviews. J Clin Epidemiol 68:617–626 [DOI] [PubMed] [Google Scholar]
- 55. Meursinge Reynders R, Ladu L, Di Girolamo N. 2017. Contacting of authors by systematic reviewers: protocol for a cross-sectional study and a survey. Syst Rev 6:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Siddaway AP, Wood AM, Hedges LV. 2018. How to do a systematic review: a best practice guide for conducting and reporting narrative reviews, meta-analyses, and meta-syntheses. Annu Rev Psychol 70:747–770 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.