Abstract
Background
Individuals admitted to hospital for COVID-19 might have persisting symptoms (so-called long COVID) and delayed complications after discharge. However, little is known regarding the risk for those not admitted to hospital. We therefore examined prescription drug and health-care use after SARS-CoV-2 infection not requiring hospital admission.
Methods
This was a population-based cohort study using the Danish prescription, patient, and health insurance registries. All individuals with a positive or negative RT-PCR test for SARS-CoV-2 in Denmark between Feb 27 and May 31, 2020, were eligible for inclusion. Outcomes of interest were delayed acute complications, chronic disease, hospital visits due to persisting symptoms, and prescription drug use. We used data from non-hospitalised SARS-CoV-2-positive and matched SARS-CoV-2-negative individuals from 2 weeks to 6 months after a SARS-CoV-2 test to obtain propensity score-weighted risk differences (RDs) and risk ratios (RRs) for initiation of 14 drug groups and 27 hospital diagnoses indicative of potential post-acute effects. We also calculated prior event rate ratio-adjusted rate ratios of overall health-care use. This study is registered in the EU Electronic Register of Post-Authorisation Studies (EUPAS37658).
Findings
10 498 eligible individuals tested positive for SARS-CoV-2 in Denmark from Feb 27 to May 31, 2020, of whom 8983 (85·6%) were alive and not admitted to hospital 2 weeks after their positive test. The matched SARS-CoV-2-negative reference population not admitted to hospital consisted of 80 894 individuals. Compared with SARS-CoV-2-negative individuals, SARS-CoV-2-positive individuals were not at an increased risk of initiating new drugs (RD <0·1%) except bronchodilating agents, specifically short-acting β2-agonists (117 [1·7%] of 6935 positive individuals vs 743 [1·3%] of 57 206 negative individuals; RD +0·4% [95% CI 0·1–0·7]; RR 1·32 [1·09–1·60]) and triptans (33 [0·4%] of 8292 vs 198 [0·3%] of 72 828; RD +0·1% [0·0–0·3]; RR 1·55 [1·07–2·25]). There was an increased risk of receiving hospital diagnoses of dyspnoea (103 [1·2%] of 8676 vs 499 [0·7%] of 76 728; RD +0·6% [0·4–0·8]; RR 2·00 [1·62–2·48]) and venous thromboembolism (20 [0·2%] of 8785 vs 110 [0·1%] of 78 872; RD +0·1% [0·0–0·2]; RR 1·77 [1·09–2·86]) for SARS-CoV-2-positive individuals compared with negative individuals, but no increased risk of other diagnoses. Prior event rate ratio-adjusted rate ratios of overall general practitioner visits (1·18 [95% CI 1·15–1·22]) and outpatient hospital visits (1·10 [1·05–1·16]), but not hospital admission, showed increases among SARS-CoV-2-positive individuals compared with SARS-CoV-2-negative individuals.
Interpretation
The absolute risk of severe post-acute complications after SARS-CoV-2 infection not requiring hospital admission is low. However, increases in visits to general practitioners and outpatient hospital visits could indicate COVID-19 sequelae.
Funding
None.
Introduction
COVID-19 caused by SARS-CoV-2 is an urgent threat to global public health, with 140·3 million cases registered worldwide and 245 761 cases in Denmark as of April 23, 2021.1 Of these cases, more than 80% are expected to be mild or asymptomatic.2 Although an increasing body of evidence shows that individuals admitted to hospital for COVID-19 might have delayed complications such as myocarditis,3 pulmonary fibrosis,4 encephalitis,5 thromboembolic events,6, 7 and psychiatric illness,8 as well as persisting symptoms9 such as dyspnoea, cough, and fatigue, little is known about how often such post-acute complications occur in individuals with mild or asymptomatic disease.10, 11 Survey-based estimates of symptom persistence vary widely—eg, dyspnoea after recovery from primary SARS-CoV-2 infection has been reported in 10–20% of patients in one study11 and up to 75% of patients in another study,12 but these studies were based on selected patient samples and did not have a control group. Therefore, we aimed to examine the occurrence of post-acute effects 2 weeks to 6 months after SARS-CoV-2 infection not requiring hospital admission, by assessing initiation of specific drugs, hospital diagnoses, and overall frequency of health-care encounters in a population-based cohort of SARS-CoV-2-positive individuals who were not admitted to hospital compared with SARS-CoV-2-negative individuals.
Research in context.
Evidence before this study
To identify existing studies on post-acute effects, delayed complications, or long-term effects of mild or asymptomatic SARS-CoV-2 infection, we searched PubMed from inception to Sept 25, 2020, (ie, the time of the public registration of the study protocol). We used the following search string: (COVID-19[title] OR SARS-CoV-2[title]) AND (((long-term[title/abstract] OR delayed[title/abstract]) AND complications[title/abstract]) OR sequelae[title/abstract] OR ((persistent[title/abstract] OR persistence[title/abstract]) AND symptoms[title/abstract])).
After the registration of the study protocol, up to Jan 22, 2021, additional literature was identified in relevant medical journals and preprint servers—eg, medRxiv. We mainly considered observational studies and reviews concerning post-acute or long-term effects of SARS-CoV-2 infection, but also considered case reports if they reported potential serious complications. Observational studies regarding post-acute complications of moderate to severe COVID-19—ie, in individuals admitted to hospital, reported a modest to high prevalence of severe late complications such as ischaemic stroke (1·6–2·5%), venous thromboembolism (1·5–21%), and reduced lung function (11–22%). We did not identify any studies investigating severe post-acute complications of mild or asymptomatic SARS-CoV-2 infection. We identified three published studies and one large, as of yet unpublished, patient-initiated survey investigating symptom persistence after SARS-CoV-2 infection in individuals not requiring hospital admission. Overall, the three published studies reported a high prevalence of symptoms such as persisting dyspnoea (10–30%) and fatigue (30–40%) up to 6 months after SARS-CoV-2 infection. In the patient-initiated survey, which recruited individuals from “long COVID” support groups on social media, more than 95% had fatigue and more than 75% had dyspnoea. The main limitation for all studies was the absence of a control group of people without COVID-19.
Added value of this study
Our population-based cohort study includes all Danish residents who, during the first wave of the COVID-19 pandemic, tested positive for SARS-CoV-2 yet were not admitted to hospital for COVID-19 (8983 individuals). As a reference, we identified a reference cohort, matched by age, sex, and calendar time, of 80 894 individuals who tested negative for SARS-CoV-2 and were not admitted to hospital. We observed no increased risk (absolute risk difference <0·1%) of initiating 11 selected drug therapies or receiving one of 25 selected new hospital diagnoses considered potential post-acute effects of SARS-CoV-2 infection, in the 2 weeks to 6 months after a SARS-CoV-2 test, for individuals who tested positive for SARS-CoV-2 compared with those who tested negative. Slightly increased risks were identified for initiating bronchodilating agents (risk difference [RD] +0·3%), specifically short-acting β-2 agonists (RD +0·4%), and triptans (+0·1%), and for new hospital diagnoses of dyspnoea (+0·6%) and venous thromboembolism (+0·1%). We found a low risk (<0·4%) of hospital contacts for previously reported persisting symptoms (anosmia, fatigue, and non-specific pain). This finding might indicate that these symptoms are primarily managed by general practitioners, whom positive individuals visited 1·18 times more often from 2 weeks after the SARS-CoV-2 test than negative individuals.
Implications of all the available evidence
The absolute risk of delayed acute complications after SARS-CoV-2 infection not requiring hospital admission is low, although late venous thromboembolism might occur. SARS-CoV-2-positive individuals are at an increased risk of initiating bronchodilator therapy and being seen at hospital with dyspnoea 2 weeks to 6 months after primary infection. The increased rate of general practitioner and outpatient hospital visits could indicate persistent symptoms.
Methods
Study design
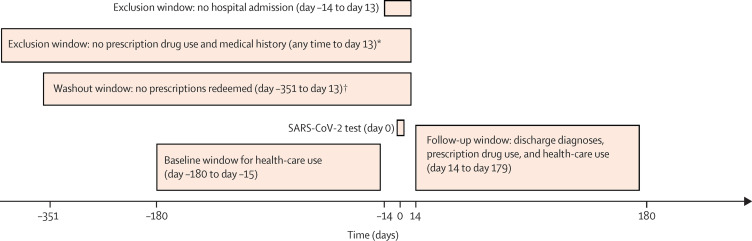
This Danish population-based cohort study used the Danish national health registries and the Danish COVID-19 cohort.13 We examined incident drug use, hospital diagnoses, and overall health-care use extending from 2 weeks to 6 months after a positive SARS-CoV-2 test in individuals who did not require hospital admission. For initiation of drugs and first hospital diagnoses representing potential late complications of SARS-CoV-2 infection, we obtained risks, risk differences, and risk ratios compared with individuals who tested negative for SARS-CoV-2. For the total burden of general practitioner visits, outpatient clinic visits, emergency department visits, and hospital admissions, we calculated rates and prior event rate ratio (PERR)-adjusted rate ratios from 2 weeks to 6 months after a SARS-CoV-2 test for positive versus negative individuals (figure 1 ). This study was reported according to the Reporting of Studies Conducted Using Observational Routinely Collected Health Data statement. All source code used in this study is available online.
Figure 1.
Study design for non-hospitalised individuals
*Analyses regarding new use of prescription drugs and first-ever diagnoses. †Analyses regarding reinitiation of prescription drugs.
The institutional data protection board at the University of Southern Denmark and the Danish Health Data Authority approved the research project. According to Danish law, studies based entirely on registry data do not require approval from an ethics review board.14
Study population
All individuals with a positive or negative RT-PCR test for SARS-CoV-2 in Denmark between Feb 27 and May 31, 2020, were eligible for inclusion. Individuals who had less than 1 year of residency in Denmark, had inconclusive test results, or died in the 2 weeks after their test were excluded.
Setting
All tests during the study period were RT-PCR tests. During the inclusion period, Denmark was affected by the global shortage of reagents for SARS-CoV-2 RT-PCR tests, which led to the following test strategy: until March 12, only symptomatic individuals with a travel history to mainland China or northern Italy were tested. From March 12 to April 21, testing was available for all individuals who had moderate to severe symptoms of respiratory tract infection. From April 21, testing of individuals with mild symptoms and asymptomatic contacts was gradually introduced and on May 18, testing for SARS-CoV-2 was made available for anyone free of charge.13
The Danish hospital system operated below maximum capacity during the entire study period. The highest strain placed on the hospital system was April 1, 2020, when 34% of intensive care beds (146 patients in intensive care of a normal capacity of 433, which could have been escalated to 925) and 3·3% of all hospital beds (535 patients of about 15 000 hospital beds) were occupied by patients with COVID-19. All elective hospital outpatient visits were canceled from March 11 to April 18, after which normal activity resumed.
Cohorts
Three cohorts were included in the study. The main exposed cohort comprised individuals with SARS-CoV-2 infection not requiring hospital admission, assessed at 2 weeks after their first positive SARS-CoV-2 test. The comparison cohort comprised individuals who tested negative for SARS-CoV-2 and were not admitted to hospital. For every non-hospitalised SARS-CoV-2-positive individual, we randomly sampled up to ten individuals with negative test results, without replacement, matching birth year, sex, and week of test.
To contextualise findings from the main analyses of non-hospitalised, SARS-CoV-2-infected individuals, we identified a cohort of SARS-CoV-2-infected individuals who were admitted to hospital. These individuals were hospitalised on the day of the positive test or during the 2 weeks after, were discharged alive, and were not readmitted in the 2 weeks after discharge. Individuals who were discharged after May 31 were excluded from further analyses.
Outcomes
Outcomes of interest were delayed acute complications, chronic disease, persisting symptoms, and prescription drug use (appendix pp 7–9). We focused on health outcomes that have been suggested to be related to SARS-CoV-2 beyond the acute infection.15
We identified initiation of prescription drugs representing possible late or delayed complications or persistent symptoms6, 8, 10, 16 of SARS-CoV-2 infection using the Danish Prescription Registry,17 including bronchodilators, cough preparations, analgesics, platelet inhibitors, anticoagulants, glucose-lowering drugs, antidepressants, anxiolytics, and antipsychotics. Information on diagnoses representing possible delayed complications, new onset of chronic disease, and persisting symptoms leading to hospital contact after SARS-CoV-2 infection3, 4, 6, 8, 18, 19, 20, 21, 22 was obtained from inpatient and outpatient hospital diagnoses recorded in the Danish National Patient Registry.23 For the outcome of acute kidney injury (appendix p 3), data on creatinine measurements were obtained from the Danish National Laboratory database.24
Regarding health-care use, we established overall event rates of general practitioner visits, hospital outpatient visits, emergency department visits, and hospital admissions per 1000 individuals between 6 months and 2 weeks before a SARS-CoV-2 test and 2 weeks to 6 months after the test, counting multiple visits per individual. Data on general practitioner visits was obtained from the Danish National Health Insurance Register.14
Statistical analysis
We assessed risks for initiation of new medications and for hospital contacts with first-ever diagnoses of interest from 2 weeks to 6 months after a SARS-CoV-2 test. Individuals with a history of the prescription drug or hospital diagnosis of interest were excluded from respective analyses. Because the number of individuals in our cohort who emigrated during follow-up was considered negligible (24 [0·3%] of 8983 test-positive and 165 [0·2%] of 80 894 test-negative individuals), these individuals were included in the calculation of risks.
For each outcome, risk differences (RDs) and risk ratios (RRs) comparing SARS-CoV-2-positive to SARS-CoV-2-negative non-hospitalised individuals were estimated using generalised linear models (binomial distribution and an identity or log link). To control for potential confounding, propensity score-weighted25 risk estimates with robust 95% CIs were obtained. SARS-CoV-2-negative individuals were weighted according to the propensity score odds, whereas SARS-CoV-2 positive individuals were assigned a weight of 1. The propensity score model included prespecified confounders that might be associated with the likelihood of having a positive versus negative SARS-CoV-2 test and with the risk of subsequent late health outcomes (appendix p 10). Covariate balance was assessed using standardised mean differences. Differences less than 0·1 were considered negligible.26
For overall health-care use outcomes, we obtained rate ratios for each observation period (pre-baseline [6 months to 2 weeks before a SARS-CoV-2 test] and follow-up [2 weeks to 6 months after a SARS-CoV-2 test]) using Poisson regression, comparing non-hospitalised SARS-CoV-2-positive individuals to SARS-CoV-2-negative individuals. To adjust for differences in baseline health-care use, we calculated PERR-adjusted rate ratios27 by dividing the rate ratio during follow-up by the pre-baseline rate ratio. We used bootstrapping techniques with 200 replications per estimate to obtain normal approximation 95% CIs.
To assess whether SARS-CoV-2 could aggravate pre-existing disease, risks of reinitiation of medication and readmission for a given diagnosis 2 weeks to 6 months after a SARS-CoV-2 test were estimated among former users of a drug (defined as not having filled a prescription for the drug during the last 12 months) or individuals with a history of the diagnosis of interest.
To identify potential late complications of SARS-CoV-2 infection outside drugs and diagnoses specified a priori, we did a hypothesis-free screening analysis. We calculated risks of initiating any individual drug (specified at the single substance level) corresponding to the fifth level of the Anatomical Therapeutical Chemical classification for drug substances, or receiving any new (first ever) hospital diagnosis (specified at the second level of the International Statistical Classification of Diseases and Related Health Problems tenth revision).
As a post-hoc sensitivity analysis, we ran analyses excluding individuals hospitalised for COVID-19 3–4 weeks after the infection—ie, early in our a priori-defined post-acute infection period.
To identify risks that might only be increased shortly after recovery from SARS-CoV-2 infection, we obtained risk estimates for outcomes in a shorter period 2–14 weeks after a test.
Statistical analyses were done using Stata MP, version 16.1. This study is registered in the EU Electronic Register of Post-Authorisation Studies (EUPAS37658).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
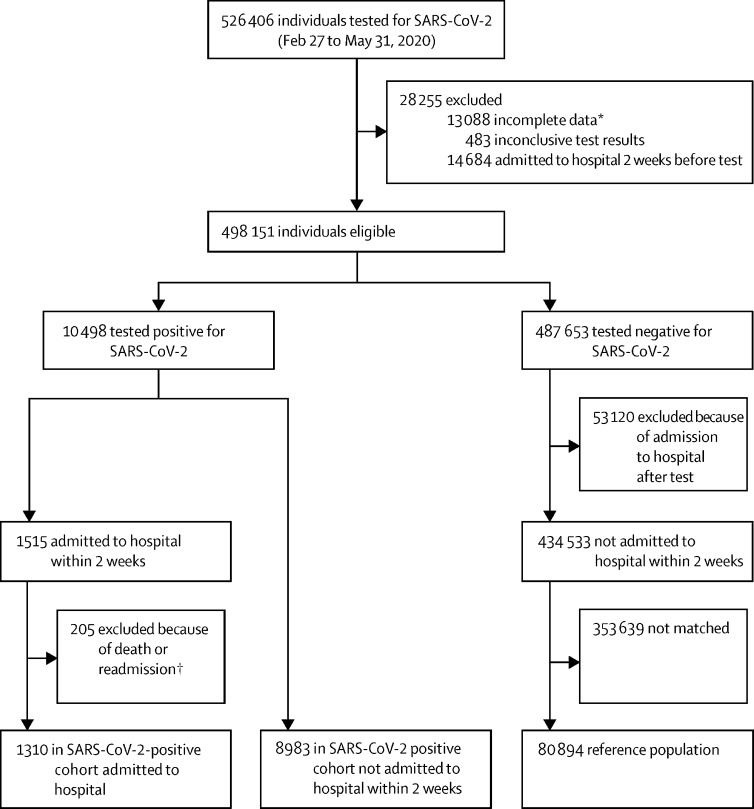
Of 10 498 eligible individuals who tested positive for SARS-CoV-2 in Denmark from Feb 27 to May 31, 2020, 8983 (85·6%) were alive and not admitted to hospital 2 weeks after their positive test. They were matched to 80 894 eligible SARS-CoV-2-negative individuals (figure 2 ). Of the 10 498 with a positive test result, 1310 individuals were admitted to hospital within 2 weeks of their test and were included as the cohort of hospitalised SARS-CoV-2-positive individuals.
Figure 2.
Cohort selection
*Death within 2 weeks of a SARS-CoV-2 test (N=3663) or migration 1 year before or up to 14 days after a SARS-CoV-2 test. †Death during admission or within 2 weeks of discharge or readmission within 2 weeks of discharge.
The non-hospitalised cohorts had a median age of 43 years (IQR 29–56), 57 102 (63·5%) of 89 877 were female, and 32 775 (36·5%) were male (table ). SARS-CoV-2-positive individuals were similar to SARS-CoV-2-negative individuals, albeit with a lower burden of comorbidity and lower use of drugs for the treatment of chronic conditions. These differences between cohorts were eliminated after propensity score weighting, with propensity score distributions exhibiting satisfying overlap (appendix p 4) between positive and negative individuals. Crude mortality during follow-up was 0·6% (58 of 8983) among non-hospitalised individuals with SARS-CoV-2 infection and 0·6% (449 of 80 894) in those who were SARS-CoV-2 negative. After propensity score weighting, the risk of death among negative individuals decreased slightly (0·5% [42 of 8977], 95% CI 0·4–0·5).
Table.
Baseline characteristics of SARS-CoV-2-positive and SARS-CoV-2-negative individuals not requiring admission to hospital
| SARS-CoV-2 positive (n=8983) | SARS-CoV-2 negative (n=80 894) | Standardised mean difference | SARS-CoV 2-negative, weighted (n=8977)* | Standardised mean difference, weighted | ||
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, median (IQR) | 43 (30–56) | 43 (29–56) | 0·02 | 44 (30–56) | 0·00 | |
| Age <18 years | 584 (6·5%) | 5662 (7·0%) | 0·02 | 567 (6·3%) | 0·01 | |
| Sex | ||||||
| Female | 5471 (60·9%) | 51 631 (63·8%) | 0·06 | 5479 (61·0%) | 0·00 | |
| Male | 3512 (39·1%) | 29 263 (36·2%) | 0·06 | 3498 (39·0%) | 0·00 | |
| Tested before April 11, 2020 | 4342 (48·3%) | 34 946 (43·2%) | 0·10 | 4440 (49·5%) | 0·02 | |
| Prescription drug use† | ||||||
| Bronchodilating agents | 757 (8·4%) | 10 984 (13·6%) | 0·17 | 1039 (11·6%) | 0·10 | |
| Inhaled corticosteroids | 531 (5·9%) | 7160 (8·9%) | 0·11 | 667 (7·4%) | 0·06 | |
| Paracetamol | 2257 (25·1%) | 22 769 (28·1%) | 0·07 | 2279 (25·4%) | 0·01 | |
| NSAIDs | 1485 (16·5%) | 15 532 (19·2%) | 0·07 | 1598 (17·8%) | 0·03 | |
| Opioids and opioid-like drugs | 591 (6·6%) | 7340 (9·1%) | 0·09 | 612 (6·8%) | 0·01 | |
| Antidepressants | 634 (7·1%) | 8896 (11·0%) | 0·14 | 798 (8·9%) | 0·07 | |
| Benzodiazapines and Z-drugs | 362 (4·0%) | 4738 (5·9%) | 0·08 | 419 (4·7%) | 0·03 | |
| Antipsychotics | 168 (1·9%) | 2453 (3·0%) | 0·08 | 163 (1·8%) | 0·00 | |
| Platelet inhibitors | 396 (4·4%) | 4592 (5·7%) | 0·06 | 425 (4·7%) | 0·02 | |
| Anticoagulants | 214 (2·4%) | 2245 (2·8%) | 0·02 | 209 (2·3%) | 0·00 | |
| Loop diuretics | 171 (1·9%) | 2386 (2·9%) | 0·07 | 180 (2·0%) | 0·01 | |
| Lipid-lowering drugs | 794 (8·8%) | 7846 (9·7%) | 0·03 | 790 (8·8%) | 0·00 | |
| Lifestyle-related diagnoses | ||||||
| Hospital diagnosis of obesity | 682 (7·6%) | 7745 (9·6%) | 0·07 | 682 (7·6%) | 0·00 | |
| Markers of smoking | 246 (2·7%) | 4548 (5·6%) | 0·14 | 246 (2·7%) | 0·00 | |
| Alcohol-related disorders | 184 (2·0%) | 3151 (3·9%) | 0·11 | 184 (2·1%) | 0·00 | |
| Mental health | ||||||
| Depression | 99 (1·1%) | 1278 (1·6%) | 0·04 | 78 (0·9%) | 0·02 | |
| Anxiety disorders | 197 (2·2%) | 3240 (4·0%) | 0·10 | 211 (2·3%) | 0·01 | |
| Psychosis | 39 (0·4%) | 650 (0·8%) | 0·05 | 35 (0·4%) | 0·01 | |
| Frailty-related diagnoses | ||||||
| Cancer | 398 (4·4%) | 4139 (5·1%) | 0·03 | 399 (4·4%) | 0·00 | |
| Dementia | 69 (0·8%) | 426 (0·5%) | 0·03 | 71 (0·8%) | 0·00 | |
| Chronic conditions | ||||||
| Diabetes (type 1 or 2)‡ | 526 (5·9%) | 4910 (6·1%) | 0·01 | 529 (5·9%) | 0·00 | |
| Hypertension† | 2127 (23·7%) | 21 808 (27·0%) | 0·08 | 2132 (23·7%) | 0·00 | |
| Cardiovascular disease | 837 (9·3%) | 9120 (11·3%) | 0·06 | 839 (9·3%) | 0·00 | |
| Peripheral vascular disease | 44 (0·5%) | 662 (0·8%) | 0·04 | 56 (0·6%) | 0·02 | |
| Pulmonary disease | 788 (8·8%) | 10 173 (12·6%) | 0·12 | 788 (8·8%) | 0·00 | |
| Kidney disease§ | 49 (0·5%) | 555 (0·7%) | 0·02 | 49 (0·5%) | 0·00 | |
Data are n (%) unless otherwise stated. Data on race are not available from our data sources. NSAIDs=non-steroidal anti-inflammatory drugs.
Counts in the weighted cohort are rounded to whole numbers for presentation; proportions were calculated using the exact decimal numbers.
Defined as having redeemed a prescription for the drug of interest during the year before the start of follow-up.
Defined as having redeemed a prescription for a drug used to treat this condition.
Defined as having received a kidney disease-related hospital diagnosis.
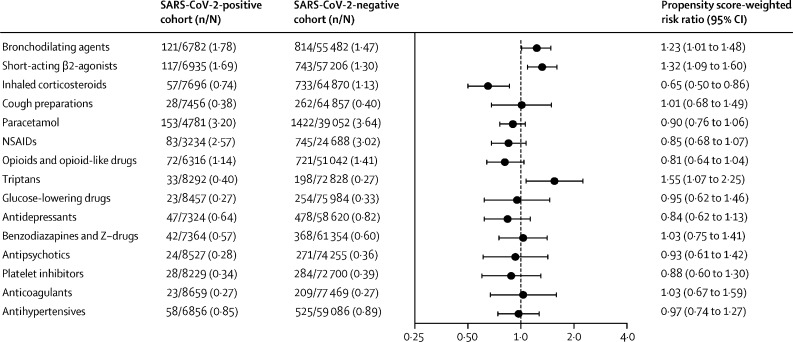
Among the 8983 SARS-CoV-2-positive individuals, 2757 (30·7%) initiated new drug treatments during follow-up compared with 28 525 (35·3%) of 80 894 SARS-CoV-2-negative individuals. Individuals who tested positive for SARS-CoV-2 were at an increased risk of initiating bronchodilating agents (121 [1·8%] initiators of 6782 non-users) compared with SARS-CoV-2-negative individuals (814 [1·5%] of 55 482; adjusted RD +0·3% [95% CI 0·0–0·7]; adjusted RR 1·23 [95% CI 1·01–1·48]), specifically short-acting β2-agonists (117 [1·7%] of 6935 positive individuals vs 743 [1·3%] of 57 206 negative individuals; adjusted RD +0·4% [0·1–0·7]; adjusted RR 1·32 [1·09–1·60]) and triptans (33 [0·4%] of 8292 vs 198 [0·3%] of 72 828; adjusted RD +0·1% [0·0–0·3]; adjusted RR 1·55 [1·07–2·25]). We found no increased risk of initiation of any of the remaining 11 drug groups when comparing non-hospitalised individuals with and without SARS-CoV-2 infection (figure 3 ; appendix p 11).
Figure 3.
Risks and risk ratios for the initiation of new medication 2 weeks to 6 months after a SARS-CoV-2 test in individuals not admitted to hospital
NSAIDs=non-steroidal anti-inflammatory drugs.
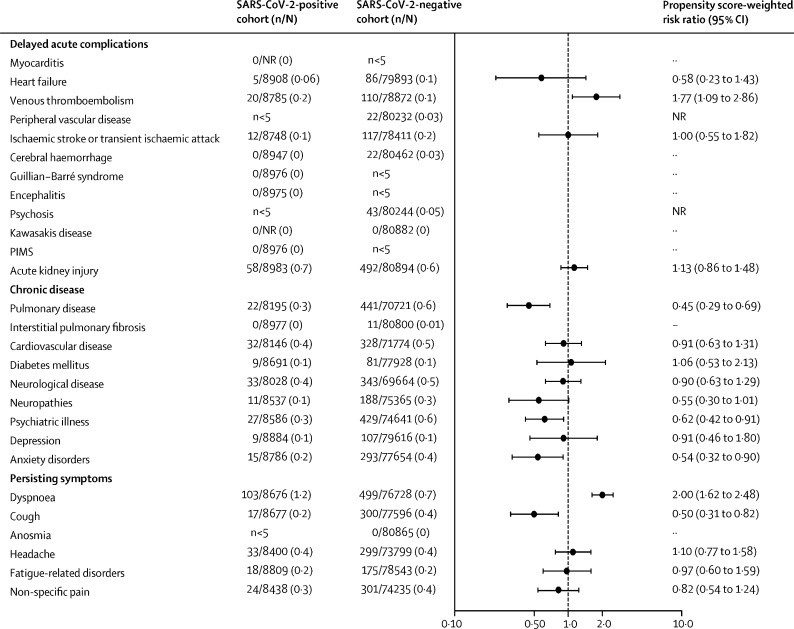
The risks of receiving any new hospital diagnosis during follow-up were 26·3% (2362 of 8983) among SARS-CoV-2-positive individuals and 28·8% (23 314 of 80 894) among SARS-CoV-2-negative individuals. SARS-CoV-2-positive individuals were at an increased risk of receiving a first diagnosis of dyspnoea (103 [1·2%] of 8676) compared with negative individuals (499 [0·7%] of 76 728; adjusted RD +0·6% [95% CI 0·4–0·8]; adjusted RR 2·00 [1·62–2·48]) and venous thromboembolism (20 [0·2%] of 8785 positive individuals vs 110 [0·1%] of 78 872 negative individuals; adjusted RD +0·1% [0·0–0·2]; adjusted RR 1·77 [1·09–2·86]). We identified no increased risk of suggested serious complications of SARS-CoV-2 infection—eg, ischaemic stroke, encephalitis, psychoses, or diagnoses related to paediatric inflammatory multisystemic syndrome (figure 4 ; appendix p 12). Furthermore, we did not identify an increased risk of receiving a first hospital diagnosis of persisting symptoms except for dyspnoea, including anosmia.
Figure 4.
Risks and risk ratios for receiving first hospital diagnoses 2 weeks to 6 months after a SARS-CoV-2 test in individuals not admitted to hospital
Counts less than five cannot be reported because of Danish legislation. PIMS=diagnoses related to paediatric inflammatory multisystemic syndrome. NR=not reported because of Danish data protection laws.
By the end of follow-up, 6557 (73·0%) of 8983 non-hospitalised individuals with SARS-CoV-2 infection and 62 391 (77·1%) of 80 894 SARS-CoV-2-negative individuals had visited their general practitioner, were seen at a hospital outpatient clinic, or were admitted to hospital (appendix p 5). Comparing overall health-care use between SARS-CoV-2-positive and SARS-CoV-2-negative individuals, we observed increased PERR-adjusted rate ratios for general practitioner visits (1·18 [95% CI 1·15–1·22]) and outpatient clinic visits (1·10 [1·05–1·16]) among SARS-CoV-2-positive individuals. We found no material difference between cohorts for emergency department visits (1·07 [0·88–1·30]) or inpatient hospitalisations (1·00 [0·87–1·14]; appendix pp 14–15). Among health-care users, most individuals had a single visit to the general practitioner or hospital outpatient clinic, and few individuals had five or more visits (appendix p 6).
We identified no increased risk of reinitiating any drug of interest or being readmitted for any of the examined diagnoses after SARS-CoV-2 infection among SARS-CoV-2-positive individuals (appendix pp 16–18).
In our hypothesis-free screening, we found an increased risk of initiating short-acting β2-agonists (salbutamol RR 1·25 [95% CI 1·03–1·53]; terbutaline RR 1·49 [1·05–2·11]) and sumatriptan (RR 1·37 [0·94–1·99]) (appendix pp 19–20) among SARS-CoV-2-positive individuals. When investigating the risk of receiving any single hospital diagnosis, we identified increased risks of receiving diagnoses related to potentially persisting symptoms of COVID-19 (ICD-10 R06—abnormalities of breathing RR 1·69 [1·36–2·09]; ICD-10 R43—disturbances of smell and taste RR 6·43 [2·04–20·3]) but also an increased risk of receiving a first hospital diagnosis of other venous embolism and thrombosis (ICD-10 I82; RR 3·15 [1·33–7·45]; appendix pp 21–23). Other signals identified were an increased risk of receiving a hospital diagnosis of unspecified viral disease, chest and throat pain, and fall-related injuries.
Individuals admitted to hospital after their SARS-CoV-2 infection (N=1310) were more burdened by comorbidities and more often used drugs for chronic conditions than those not requiring admission to hospital (appendix p 24). When comparing hospitalised and non-hospitalised SARS-CoV-2-positive individuals, we identified generally increased risks beyond 2 weeks of initiating new drug treatments (appendix p 25) and receiving new hospital diagnoses (appendix pp 26–27) among the individuals admitted to hospital.
Exclusion of primarily non-hospitalised individuals who were admitted to hospital early in the post-acute period, 3–4 weeks after the SARS-CoV-2 test, resulted in attenuated risk estimates for venous thromboembolism (adjusted RD 0·0 [95% CI −0·1 to 0·1]; adjusted RR 1·33 [0·77 to 2·29]) whereas risk estimates for a diagnosis of dyspnoea (adjusted RD 0·6 [0·3 to 0·8]; adjusted RR 1·93 [1·56 to 2·40]) remained unchanged.
We identified no increased risks of initiating new drug therapies during the shorter period 2–14 weeks after a SARS-CoV-2 test (appendix p 28), whereas risks of receiving a first hospital diagnosis of dyspnoea (RR 1·66 [95% CI 1·24–2·22]) or venous thromboembolism (RR 1·82 [1·02–3·25]) were also increased when only considering these first 3 months of follow-up (appendix pp 29–30). PERR-adjusted rate ratios during the first 3 months were similar to risk estimates for the full duration of follow-up (appendix p 31).
Discussion
We did a nationwide cohort study investigating the occurrence of post-acute effects of SARS-CoV-2 infection in individuals who were not admitted to hospital for the primary infection. Comparing SARS-CoV-2-positive with SARS-CoV-2-negative individuals, we generally identified no increased risk of delayed severe acute complications and new onset of chronic disease, except for a slightly increased absolute risk of venous thromboembolism. We did identify an increased risk of health-care encounters caused by dyspnoea, but no increased risk of hospital contacts for other symptoms previously reported to persist after SARS-CoV-2 infection. In accordance with these findings, SARS-CoV-2-positive individuals were more likely than SARS-CoV-2-negative individuals to initiate treatment with short-acting β-2 agonists 2 weeks to 6 months after the SARS-CoV-2 test. Furthermore, we identified an increased risk of initiating treatment with triptans after SARS-CoV-2 infection than in those not infected. We also observed that SARS-CoV-2-positive individuals overall visit their general practitioner more often after the infection than SARS-CoV-2-negative individuals, which could indicate that persisting symptoms of SARS-CoV-2 infection are managed in general practice.
Most of the literature regarding post-acute complications of SARS-CoV-2 infection is based on follow-up of individuals admitted to hospital for COVID-19,3, 5, 6, 9, 18, 28 and is therefore not generalisable to all individuals infected with SARS-CoV-2. Because most SARS-CoV-2-infected individuals are managed in the community,2 it is of major public health importance to better understand the risk of delayed effects in non-hospitalised individuals.
The strengths of our study are related to the nationwide coverage of the Danish health registries and the universal tax-funded health insurance: using data from the population-based Danish COVID-19 cohort13 allowed us to follow all individuals tested for SARS-CoV-2 in Denmark, regardless of test location, severity of symptoms, or access to health-care services, minimising selection biases and increasing the generalisability of our results. SARS-CoV-2 infection status has been established by highly sensitive and specific RT-PCR tests29 for every individual, eliminating any potential risk of major misclassification of infection status. Still, no tests and sampling techniques are 100% sensitive or specific, so false-negative or false-positive results might have occurred.
In our study, follow-up was limited to 6 months after a test for SARS-CoV-2, which might not yet account for all long-term complications and persisting symptoms after COVID-19. Some individuals with complications might have been referred to hospital specialists, but might not be seen in clinics before the end of follow-up, because of low capacity during the COVID-19 pandemic. Also, because of the registry-based nature of our study, information on the indication for testing was not available. This missing information might have resulted in an imbalanced prevalence of symptomatic individuals between cohorts.
Compared with previous studies, we found lower absolute risks for most severe post-acute effects of SARS-CoV-2 infection. Previous studies on serious complications of SARS-CoV-2 infection such as myocarditis,3 reduced lung function,18 venous thromboembolism,6 encephalitis,5 stroke,30 and paediatric inflammatory multisystemic syndrome22 were usually done in individuals admitted to hospital with severe COVID-19 without control groups, or by screening individuals with these conditions for SARS-CoV-2 infection or vice versa, conferring a high risk of selection and surveillance bias. Furthermore, we did not identify any events of myocarditis, cerebral haemorrhage, encephalitis, or diagnoses related to paediatric inflammatory multisystemic syndrome in individuals with SARS-CoV-2 infection not requiring hospital admission, indicating that these complications might be exceedingly rare in these SARS-CoV-2 cases. We did find an increased risk of post-acute venous thromboembolism (adjusted RR 1·77), and the fact that the risk estimates were attenuated (adjusted RR 1·33) when excluding individuals admitted to hospital for COVID-19 during week 3 and 4 after the SARS-CoV-2 test suggests that venous thromboembolism events might occur with a delay in some patients with COVID-19. In support of this hypothesis, we also found an increased risk of receiving a hospital diagnosis of other venous embolism and thrombosis (appendix pp 21–23) when comparing individuals with SARS-COV-2 infection not requiring hospital admission with SARS-CoV-2-negative individuals.
Persistence of symptoms such as fatigue, cough, dyspnoea, anosmia, headache, and joint pain have been well described after primary SARS-CoV-2 infection, both in hospitalised9, 28 and non-hospitalised10, 11, 12 individuals. In our study, we identified an increased risk beyond 2 weeks of receiving a hospital diagnosis of dyspnoea (adjusted RR 2·00), but no increased risk of hospital visits for other symptom-related diagnoses, compared with SARS-CoV-2-negative individuals. The increased risk of initiating short-acting β-2 agonists might be related to dyspnoea, as might be the increased number of general practitioner and outpatient hospital clinic visits (appendix pp 14–15) after SARS-CoV-2 infection not requiring hospital admission. Information on the reason for these visits was not available. We could assume that excess general practitioner and outpatient visits were due to persisting symptoms from SARS-CoV-2 infection not leading to initiation of specific drugs or acute treatment. A large, international patient-initiated survey done in individuals who had persisting symptoms after SARS-CoV-2 infection reported a high prevalence of fatigue, dyspnoea, and cognitive issues at any point from the primary SARS-CoV-2 infection and up to 7 months after.12 Our analysis only captures specific symptoms leading to hospital contacts, and can therefore not be used as a measure of the overall prevalence of these symptoms. It is expected that absolute risks of persisting symptoms such as dyspnoea or fatigue would be vastly underestimated, because only a fraction of individuals with these symptoms will be seen at a hospital. Still, the discrepancy between patient-reported symptoms10, 11, 12 and quantification of health-care encounters is important knowledge, because it might indicate that a presumably large share of patients with persisting symptoms after SARS-CoV-2 infection might have unmet health-care needs.
Our study provides new evidence that the absolute risk of delayed acute complications such as venous thromboembolism, ischaemic stroke, and psychoses after SARS-CoV-2 infection not requiring hospital admission is low. Furthermore, the measured burden placed on the secondary health-care sector by post-acute effects of primarily non-hospitalised individuals with SARS-CoV-2 infection might be lower than expected, possibly because of persisting symptoms being managed in general practice or not all persisting symptoms leading to health-care encounters.
Future large, population-based, controlled studies making use of patient-reported symptoms and planned study visits need to be done to fully assess the duration and spectrum of any persisting symptoms after SARS-CoV-2 infection.
In conclusion, the absolute risk of delayed acute complications, new onset of chronic disease, and hospital encounters for persisting symptoms 2 weeks to 6 months after SARS-CoV-2 infection not requiring hospital admission is low. However, among those not admitted to hospital, SARS-CoV-2-positive individuals are at a slightly increased risk of venous thromboembolism, receiving a hospital diagnosis of dyspnoea, initiating bronchodilator therapy, and initiating triptans compared with individuals who tested negative for SARS-CoV-2. Moreover, SARS-CoV-2-positive individuals visited their general practitioner and outpatient hospital clinics more often after the primary infection than those who tested negative, which could indicate persistent symptoms that do not lead to specific drug treatment or hospital admission.
Data sharing
Because of data protection regulation, data cannot be shared directly by the authors. Data is accessible to authorised researchers after application to the Danish Health Data Authority. To apply for data and help with the application process, please apply through the Danish Health and Medicines Authority.
Declaration of interests
AP and JH report funds paid to their institution for participation in research projects funded by Alcon, Almirall, Astellas, AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Servier, and LEO Pharma, outside the the current work. LCL reports funds paid to his institution for participation in research projects funded by Menarini Pharmaceuticals and LEO Pharma, outside the current work. HN reports funds paid to their institution for participation in research projects funded by Merck Sharp & Dohme and Novo Nordisk Foundation, outside the current work. The institution of CFC and RWT is involved in studies with funding from various companies as research grants to (and administered by) Aarhus University, outside the current work. AK, SHM, and NCB report no competing interests.
Acknowledgments
Acknowledgments
We thank Kasper Bruun Kristensen and Martin Thomsen Ernst from the University of Southern Denmark for having validated the source code used in this study.
Contributors
LCL, JH, and AP conceived the study. LCL did the data analysis. LCL and AP had full access to and verified all data. LCL and AP drafted the original manuscript. All authors provided important input to methods of the study, revised the manuscript, and approved the final version. Database data cleaning and identification of the individuals tested for SARS-CoV-2 was done by the Danish Health Data Agency and Statens Serum Institut (Copenhagen, Denmark). LCL and AP are the guarantors, had full access to the primary data, and verify that this manuscript is an honest, accurate, and transparent account of the study that has been conducted. All authors had full access to study results and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.WHO Weekly epidemiological update on COVID-19—20. April 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19-20-april-2021
- 2.Reilev M, Kristensen KB, Pottegård A, et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol. 2020;49:1468–1481. doi: 10.1093/ije/dyaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ojo AS, Balogun SA, Williams OT, Ojo OS. Pulmonary fibrosis in COVID-19 survivors: predictive factors and risk reduction strategies. Pulm Med. 2020;2020 doi: 10.1155/2020/6175964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pilotto A, Masciocchi S, Volonghi I, et al. Clinical presentation and outcomes of severe acute respiratory syndrome coronavirus 2-related encephalitis: the ENCOVID multicentre study. J Infect Dis. 2021;223:28–37. doi: 10.1093/infdis/jiaa609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalager-Pedersen M, Lund LC, Mariager T, et al. Venous thromboembolism and major bleeding in patients with COVID-19: a nationwide population-based cohort study. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab003. published online Jan 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8:130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network—United States, March–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G. Persistent symptoms 1·5–6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax. 2020;76:405–407. doi: 10.1136/thoraxjnl-2020-216377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. medRxiv. 2020 doi: 10.1101/2020.12.24.20248802. published online Dec 27. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pottegård A, Kristensen KB, Reilev M, et al. Existing data sources in clinical epidemiology: the Danish COVID-19 cohort. Clin Epidemiol. 2020;12:875–881. doi: 10.2147/CLEP.S257519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thygesen LC, Daasnes C, Thaulow I, Brønnum-Hansen H. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health. 2011;39(suppl):12–16. doi: 10.1177/1403494811399956. [DOI] [PubMed] [Google Scholar]
- 15.Datta SD, Talwar A, Lee JT. A proposed framework and timeline of the spectrum of disease due to SARS-CoV-2 infection: illness beyond acute infection and public health implications. JAMA. 2020;324:2251–2252. doi: 10.1001/jama.2020.22717. [DOI] [PubMed] [Google Scholar]
- 16.Poncet-Megemont L, Paris P, Tronchere A, et al. High prevalence of headaches during Covid-19 infection: a retrospective cohort study. Headache. 2020;60:2578–2582. doi: 10.1111/head.13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, Sørensen HT, Hallas J, Schmidt M. Data resource profile: the danish national prescription registry. Int J Epidemiol. 2017;46:798. doi: 10.1093/ije/dyw213. 798f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.You J, Zhang L, Ni-Jia-Ti MY, et al. Anormal pulmonary function and residual CT abnormalities in rehabilitating COVID-19 patients after discharge. J Infect. 2020;81:e150–e152. doi: 10.1016/j.jinf.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellosta R, Luzzani L, Natalini G, et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72:1864–1872. doi: 10.1016/j.jvs.2020.04.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollstein T, Schulte DM, Schulz J, et al. Autoantibody-negative insulin-dependent diabetes mellitus after SARS-CoV-2 infection: a case report. Nat Metab. 2020;2:1021–1024. doi: 10.1038/s42255-020-00281-8. [DOI] [PubMed] [Google Scholar]
- 21.Vijayan A, Humphreys BD. SARS-CoV-2 in the kidney: bystander or culprit? Nat Rev Nephrol. 2020;16:703–704. doi: 10.1038/s41581-020-00354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pouletty M, Borocco C, Ouldali N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79:999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arendt JFH, Hansen AT, Ladefoged SA, Sørensen HT, Pedersen L, Adelborg K. Existing data sources in clinical epidemiology: laboratory information system databases in Denmark. Clin Epidemiol. 2020;12:469–475. doi: 10.2147/CLEP.S245060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ. 2019;367 doi: 10.1136/bmj.l5657. [DOI] [PubMed] [Google Scholar]
- 26.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiner MG, Xie D, Tannen RL. Replication of the Scandinavian Simvastatin Survival Study using a primary care medical record database prompted exploration of a new method to address unmeasured confounding. Pharmacoepidemiol Drug Saf. 2008;17:661–670. doi: 10.1002/pds.1585. [DOI] [PubMed] [Google Scholar]
- 28.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Kasteren PB, van der Veer B, van den Brink S, et al. Comparison of seven commercial RT-PCR diagnostic kits for COVID-19. J Clin Virol. 2020;128 doi: 10.1016/j.jcv.2020.104412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benussi A, Pilotto A, Premi E, et al. Clinical characteristics and outcomes of inpatients with neurologic disease and COVID-19 in Brescia, Lombardy, Italy. Neurology. 2020 doi: 10.1212/WNL.0000000000009848. 9x0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Because of data protection regulation, data cannot be shared directly by the authors. Data is accessible to authorised researchers after application to the Danish Health Data Authority. To apply for data and help with the application process, please apply through the Danish Health and Medicines Authority.