Abstract
The COVID-19 pandemics has created unprecedented challenges and threats to patients and healthcare systems worldwide. Acute respiratory complications that require intensive care unit (ICU) management are a major cause of morbidity and mortality in COVID-19 patients. Among other important risk factors for severe COVID-19 outcomes, obesity has emerged along with undernutrition-malnutrition as a strong predictor of disease risk and severity. Obesity-related excessive body fat may lead to respiratory, metabolic and immune derangements potentially favoring the onset of COVID-19 complications. In addition, patients with obesity may be at risk for loss of skeletal muscle mass, reflecting a state of hidden malnutrition with a strong negative health impact in all clinical settings. Also importantly, obesity is commonly associated with micronutrient deficiencies that directly influence immune function and infection risk. Finally, the pandemic-related lockdown, deleterious lifestyle changes and other numerous psychosocial consequences may worsen eating behaviors, sedentarity, body weight regulation, ultimately leading to further increments of obesity-associated metabolic complications with loss of skeletal muscle mass and higher non-communicable disease risk. Therefore, prevention, diagnosis and treatment of malnutrition and micronutrient deficiencies should be routinely included in the management of COVID-19 patients in the presence of obesity; lockdown-induced health risks should also be specifically monitored and prevented in this population. In the current document, the European Society for Clinical Nutrition and Metabolism (ESPEN) aims at providing clinical practice guidance for nutritional management of COVID-19 patients with obesity in various clinical settings.
Keywords: COVID-19, Obesity, Nutritional management
1. Introduction
1.1. COVID-19 and malnutrition
COVID-19 is a primarily respiratory disease caused by SARS-CoV-2 infection that can spread from upper to lower airways, leading to respiratory insufficiency requiring respiratory support and intensive care, where it may be fatal [[1], [2], [3], [4], [5]]. Patient groups with pre-existing comorbidities ranging from diabetes and cardiovascular disease to cancer and chronic organ failures, as well as older age, are burdened with higher risk for complications and COVID-19 mortality [[1], [2], [3], [4], [5]]. Importantly, both older age and pre-existing chronic diseases in polymorbid individuals are per se associated with high risk and prevalence of undernutrition (which will be hereafter referred to as MALNUTRITION, to align with the utilization of this term in clinical practice as supported by clinical nutrition Societies) [6,7], due to catabolic derangements, low food intake and low physical activity whose various combinations result in loss of body and skeletal muscle mass and muscle function [[6], [7], [8]]. In COVID-19, infection with related inflammation and potential organ failure with systemic complications and immobilization may further contribute to enhance muscle loss and malnutrition risk. Also notably, malnutrition is an independent major cause of morbidity and mortality in most disease conditions, through mechanisms including high risk of infections or superinfection [9], caused by its deleterious impact on immune function [10] as well as respiratory and cardiac muscle function [11,12]. Malnutrition with loss of skeletal muscle mass may also directly lead to poor quality of life, disability and morbidities long after discharge also in disease survivors [[6], [7], [8],[13], [14], [15]]. Consistent with these concepts, in the past several months evidence has demonstrated: 1) a high prevalence of malnutrition in COVID-19 patient cohorts at hospital admission [[16], [17], [18], [19], [20], [21], [22]], thereby confirming a likely high risk of infection in malnourished individuals; 2) a negative impact of malnutrition on COVID-19 prognosis [[16], [17], [18], [19],[21], [22], [23]], thereby confirming the potential negative impact of malnutrition to enhance COVID-19 severity; 3) a negative impact of COVID-19 on body weight in both hospitalized and non-hospitalized patients [24], thereby confirming the potential negative impact of COVID-19 on nutritional status.
1.2. Obesity and COVID-19: an additional risk factor with nutritional implications
In addition to polymorbid and older adults, robust evidence has shown that persons with obesity are also at high risk for severe disease and reduced survival in COVID-19 [[25], [26], [27], [28], [29], [30]]. Obesity-induced metabolic derangements causally associated with adipose tissue and systemic inflammation [such as hypertension, insulin resistance, hyperglycemia and type 2 diabetes that are commonly clustered in the metabolic syndrome (MS)] have been also reported to worsen COVID-19 severity and prognosis [[31], [32], [33]]. Pre-existing activation of systemic and tissue inflammation may also contribute to impaired immune function while enhancing organ damage [34]. One common feature in individuals highly susceptible to suffer from a more severe form of COVID-19 is the presence of a chronic low-grade inflammatory state, that has also been associated to alterations of the gut microbiota (dysbiosis). Studies in small patient cohorts have reported that the baseline gut microbiome was associated with COVID-19 severity; for example Faecalibacterium prausnitzii, that plays a role in control of inflammation, is lowered in obese patients and is inversely correlated with COVID-19 disease severity [35].
Although the development of obesity with fat mass accumulation is commonly associated with variable increments of skeletal muscle mass, obese individuals with metabolic complications, comorbidities and older age are also at high risk for loss of skeletal muscle mass and function, thereby developing a double burden of overnutrition and malnutrition [36]. Metabolic derangements primarily associated with excess fat, leading to obesity-associated insulin resistance and diabetes, may specifically lead to muscle anabolic resistance and catabolism, which may conversely directly impair muscle mass preservation [37]. Under these conditions, decreased muscle protein synthesis may result from lower activation of the translation initiation signaling pathways in response to insulin [38], in conjunction with a low-grade inflammation state, muscle disuse [39], and commonly a higher muscle lipid ectopic deposition [40]. Recent data have indeed indicated that the high intramuscular fat deposition that may characterize MS in persons with obesity independently predicts critical illness in patients with SARS-CoV-2 infection [41]. Also importantly, obesity is a strong risk factor for many diseases and for chronic and acute organ failures [42], with additional independent negative impact on muscle mass and nutritional status [[43], [44], [45], [46]]. It should be further pointed out that obesity is commonly associated with micronutrient deficiencies that enhance the malnutrition burden, with negative impact on skeletal muscle metabolism and immune function [47,48], leading to higher risk of infection and potentially loss of skeletal muscle mass. Finally, persons with obesity may be particularly exposed to the consequences of deleterious lifestyle changes during pandemic-related lockdowns that may worsen body weight and composition, leading to further increments of obesity-associated non-communicable disease risk [49].
Altogether, immune dysfunction, adipose tissue inflammation and insulin resistance-induced metabolic alterations, comorbidities and organ failures including respiratory dysfunction with obstructive sleep apnea may be converging mechanisms that potentially contribute to the severity of COVID-19 in persons with obesity. Malnutrition and particularly low skeletal muscle mass should be considered as an additional factor contributing to the severity of disease, particularly in patients at high nutritional risk, such as older adults and polymorbid individuals, but this appears to have been so far neglected [36]. Obesity is defined by WHO as the presence of excess body fat with negative impact on health [50]. In clinical practice, obesity is however commonly defined by body mass index (BMI) above 30 kg/m2 or 27 kg/m2 for Caucasian or East Asian populations, respectively. Important limitations of BMI-based diagnosis include lack of information on 1) potential changes in body composition with relative changes in body fat and skeletal muscle mass, which limit the ability to identify malnutrition in this setting and 2) body fat distribution, with particular regard to visceral abdominal fat which is known to increase the risk of developing metabolic complications and non-communicable disease [51,52]. While keeping in mind that BMI-diagnosed obesity per se has indeed emerged as a risk factor for SARS-CoV-2 infection and COVID-19 complications, persons with obesity represent a highly heterogeneous patient group also with respect to potential nutritional needs.
Early after the onset of the COVID-19 pandemic, the European Society for Clinical Nutrition and Metabolism (ESPEN) has published a paper in collaboration with experts from the World Health Organization-Europe to provide concise statements and guidance on nutritional management of COVID-19 patients, with particular focus on those with pre-existing conditions associated with malnutrition risk and on those in the intensive care unit (ICU) [53]. In the current paper, we aim at providing additional statements and guidance, specifically for the prevention, diagnosis and treatment of malnutrition and micronutrient deficiencies in the management of COVID-19 patients in the presence of obesity. Common metabolic complications of obesity such as MS and type 2 diabetes mellitus (T2DM), that have emerged as strong independent risk factors for poor COVID-19 outcomes [[31], [32], [33]] and may be affected by nutritional treatment will be also discussed. As there are no comprehensive studies on nutrition management in COVID-19 in persons with obesity, the following statements will largely be based on the best available knowledge and clinical experience. Lockdown-induced health risks with increased prevalence of unhealthy eating patterns and sedentarity, which may induce changes in body composition through fat gain and muscle loss, are also considered and addressed, although we will not detail recommendations on lifestyle and pharmacological approaches that may be largely referred to a large body of evidence and existing guidelines. In particular, we will discuss in different sections: the impact of lifestyle changes on nutritional status during lockdown and social restriction conditions; nutritional management of persons with obesity for prevention of SAR-CoV2 infection and potential poor COVID-19 outcomes; nutritional management of persons with obesity and COVID-19 at home, in the non-ICU and in the ICU hospital setting; and nutritional management of persons with obesity during recovery from COVID-19.
2. Nutritional management of persons with obesity in the COVID-19 pandemic
-
a)Negative impact of lifestyle changes on nutritional status during lockdown and social restriction conditions
-
1.In order to reduce the risk of fat gain with muscle mass loss, persons with obesity undergoing lockdown conditions and social restrictions should be encouraged to maintain healthy eating patterns and physical activity.
- Lifestyle changes during lockdown and social restriction conditions are conducive to negative consequences on nutritional status in persons with obesity. Negative lifestyle changes may include unhealthy eating patterns and behaviors as well as reduced physical activity. Reduced access to healthcare services and psychological consequences of social isolation may contribute to establish negative lifestyle changes. Most importantly, such changes may directly lead to negative changes in body composition with fat gain and muscle loss, resulting in malnutrition or sarcopenic obesity, particularly in persons with obesity and older age or polymorbidity.
- Eating Behavior - Public health recommendations and governmental measures during the COVID-19 pandemic have resulted in numerous restrictions on daily living, including social distancing, isolation and home confinement. The impact of these restrictions and related stress on health behaviors and lifestyles at home is huge. In the “Effects of home Confinement on multiple Lifestyle Behaviors during the COVID-19 outbreak (ECLB-COVID19)”, a large international electronic survey involving 1046 respondents, food consumption and meal patterns (the type of food, eating out of control, snacks between meals, number of main meals) were more unhealthy during confinement, with only alcohol binge drinking decreasing significantly [54]. A systematic review including 128,292 subjects from 34 Countries worldwide investigated changes in specific dietary habits (snacking, fast-foods, ordered food) and alcohol consumption during the COVID-19 lockdown period [55]. It has to be noted that despite considerable heterogeneity in the definition of “snacks” (including desserts, candy, chips, nuts, crackers, popcorns, etc.), most of such food items are rich in calories and poor in nutrients [55]. In the above review [55], although snacking behavior remained unchanged during lockdowns for the majority of the population, an increased consumption of both salty and sugar snacks was reported for 18.9–45.1% of individuals. These negative changes that are influenced by internal and external factors, such as reduced foods availability, may affect the general population but may bear more negative consequences for people living with obesity. Increased episodes of secret eating or binge eating, and high frequency of snacking have been reported in patients within the Obesity UK support groups associated with lower physical activity during lockdown [56,57]. In a study including only persons with obesity, one third of participants reported an increase in daily snack consumption during lockdown [58]. This finding may suggest that for persons with obesity control of snacking behavior may be complex under lockdown and social confinement conditions.
- Physical Activity - Also from the ECLB-COVID19 survey, home confinement had a negative effect on physical activity levels and daily sitting time increased from 5 to 8 h per day [59]. A systematic review has reported that physical activity, assessed by means of questionnaires, decreased during the COVID-19 pandemic in many studies, although a few reports also found an increase [59]. The determinants of changes in physical activity have not yet been clarified but the decrease in physical activity are likely to be directly related to lockdown conditions, with limited ability to exercise outdoors, and a deteriorated mental health. A decrease in physical activity has been shown especially in young and active men [60,61] but also in people with obesity and other chronic diseases [62]. One study showed that being overweight or the perception of weight increase was associated with an OR of 1.8 and 2.0 of decrease in physical activity, respectively [63]. A low physical activity level during the COVID-19 pandemics was also associated with a low quality of life [57]. Conversely, it has been demonstrated that the physical active individuals, whether older [64] or young [61], experience a better well-being. In view of these findings, it appears essential to promote physical activity in persons with obesity, not only for potential control of body weight, body composition and metabolic complications but also for quality of life.
- Several papers have published recommendations regarding physical activity, in order to maintain health, avoid functional limitations and improve immune function [65] but also to improve the sleep and limit psychological stress [66]. While the modalities (frequencies, intensity, type, duration) differ, they all advocate the combination of endurance and resistance exercise. The WHO for instance recommends 150 min of moderate to intensity or 75 min of vigorous intensity per week [67]. In case of lockdown, potential strategies to promote physical activity during confinement could be videos, live workouts through physical exercise classes on TV or internet or exercise apps. Although none of the recommendations focus specifically on persons with obesity, there is no reason to modify recommendations of physical activity in this group in the period of COVID-19 pandemics, and persons with obesity should stay as physically active as possible.
- Weight Changes - A systematic review and meta-analysis, including 59,711 subjects from 32 countries investigated the impact of the first lockdown period (March–May 2020) on body weight (BW) and on BMI. The authors found that increments in BW should be considered an alarming negative effect of lockdown, as they might lead to a higher prevalence of overweight, obesity and related health-risks [68]. In a study including only obese subjects, more than one third reported an increase in their body weight during/after lockdown [69]. This finding is consistent with enhanced unhealthy eating patterns and sedentarity, indicating that this group may find it more difficult to control BW during a lockdown. Another study reported that persons with obesity were among those experiencing the largest weight variability during quarantine [70]. Finally, a large study including people with BMI of all ranges showed that those who gained weight during the confinement were also those with the largest decrease in physical activity [71]. No studies have reported effects of lockdown-related weight changes on body composition and on muscle mass and function. It is however likely that body weight changes in persons with obesity may also affect body composition by favoring fat gain and muscle loss, particularly in those with older age and polymorbidity. Studies should be performed to directly test this hypothesis.
- Healthcare Access - Other factors with potential negative impact on persons with obesity during COVID-19-related lockdowns and social restrictions may include social determinants of health, access to care, weight bias, and obesity stigma [[72], [73], [74]]. Intersecting vulnerabilities such as socio-economic status, ethnicity, language, gender, immigration status, and past experiences of weight bias and obesity stigma may prevent persons with obesity from accessing COVID-19 information and health care services. The isolation of people living with obesity during the COVID-19 pandemic has been further exacerbated by the reduction of the contacts with weight-management services during the lockdown. In particular during the first wave of the epidemic in Europe, the shortage of health care professionals (HCPs) in the ICUs and medical wards dedicated to the care of COVID-19 adult patients, caused by the rapid increase in the number of cases, required the diversion of HCPs from other services to COVID-19-dedicated ones. This shift heavily affected obesity medical services. According to a survey conducted by the European Association for the Study of Obesity in their affiliated centers (COMs), 61% of the responding centers had their staff directly involved in treating COVID-19 patients and diverted away from routine work at the obesity clinic. These figures suggest a substantial reduction in the number of HCPs available for obesity care [75,76]. This lack of appropriate in-person contacts has been partly compensated by an increase in the use of telemedicine and online consultations and support [75,76].
- In summary, the difficulty in maintaining a healthy lifestyle in persons with obesity may be increased by confinement and isolation imposed by the COVID-19 pandemic, including reduced availability of continuous obesity care. Patients with obesity should be encouraged to retain healthy eating patterns and behaviors even in these difficult conditions and helped by implementing online consultations and contacts.
-
1.
-
b)Estimate of energy and protein requirements in obesity
- Energy - measurement of energy requirements should be ideally based on accurate measurements of energy expenditure (EE), including both its basal and activity-related components [77]. This implies the use of indirect calorimetry as gold standard for basal EE [77] while accurate recording of activity EE is difficult, particularly in outpatient settings. In non-obese individuals, equations based on actual body weight with correction for height, age and gender are therefore often used as surrogates [77]. Recommendations for calorie and protein intake may also be directly based on measured body weight in clinical practice in several disease conditions [6,7,14,78]. However, this approach may not be appropriate for persons with obesity, when excess body weight overwhelmingly includes relatively low-energy consuming fat tissue. Although discrepancies remain in available literature, in the absence of indirect calorimetry Harris–Benedict equations introduced over 100 years ago may provide a reasonably accurate estimate of EE in persons with obesity [77,79]. For weight-based recommendations, the discrepancy between actual and ideal body weight (IBW: Men 50 + [0.91 × (height in centimeters − 152.4)]; Women 45.5 + [0.91 × (height in centimeters − 152.4)]) may however lead to substantial overestimation of energy requirements. From the perspective of prevention and treatment of malnutrition in obesity, it should be kept in mind that the use of IBW ignores the energy expenditure of excess body mass, which may also include not negligible lean components. Literature approaches have varied in recommendations for weight-based calorie requirements for persons with obesity particularly in the ICU [14,80,81]. A potential approach to use actual body weight with prescription of fewer calories for BMIs up to 50 kg/m2, and reverting to IBW and higher calorie per kg IBW for higher BMI values has been proposed, particularly for the ICU setting [[80], [81], [82]]. In its most recent ICU guidelines, ESPEN suggested the pragmatic utilization of Adjusted Body Weight [ABW: IBW + 25% Excess Body Weight] to calculate energy and protein requirements [14]. This approach may allow for a more physiological consideration of actual energy expenditure from the predominant fat but also the lean components of Excess Body Weight. In addition it may allow a more gradual adjustment of calorie delivery across large BMI ranges.
- Protein - protein requirements should ideally be based on accurate estimates of skeletal muscle mass [6], i.e. the major protein reservoir in the body whose preservation should be a major goal of nutritional care in obesity. Techniques assessing body composition estimate muscle mass but they are not commonly available for outpatient evaluation. Nitrogen balance may also accurately assess protein losses and therefore protein needs [14], but this approach is also not available for outpatients and is cumbersome in routine hospital practice. Protein requirements are therefore commonly expressed as gram per kg body weight per day in non-obese individuals. Similar to the estimation of energy needs, this approach may result in substantial overestimation of protein requirements in persons with obesity [83]. For practical purposes, when more accurate assessments of muscle mass are not available, calculating total protein requirements based on grams per kg ABW may also be suggested in obesity [14]. ABW may allow to take into account the lean and skeletal muscle fraction of excess body weight, that could be approximately estimated at 10% [6]. In disease situations with high risk of muscle loss and in malnourished individuals, this approach may therefore contribute to deliver appropriate protein doses to stimulate skeletal muscle anabolism.
-
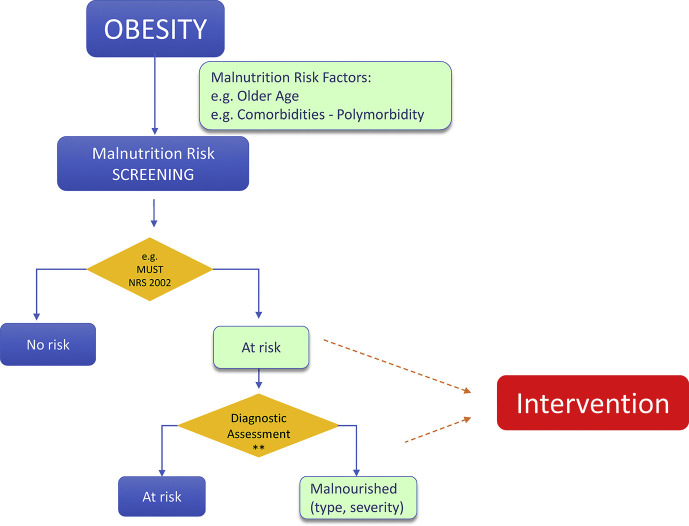
c)Nutritional management of persons with obesity for prevention ofSARS-CoV-2infection and potential poorCOVID-19outcomes (Fig. 1 )
-
2.Persons with both obesity and additional risk factors for both malnutrition and severeSARS-CoV-2infection, such as obese older adults and obese patients with polymorbidity, should be screened for malnutrition risk by using validated tools, followed by malnutrition diagnosis and assessment if at risk.
- Poor nutritional status and malnutrition increase the risk of developing viral respiratory infections including influenza, and they are risk factors for poor outcomes including mortality [[84], [85], [86], [87], [88]]. High prevalence of malnutrition at hospital admission has clearly emerged also in various ICU and non-ICU COVID-19 patient cohorts [[16], [17], [18], [19], [20], [21], [22]]. Malnutrition risk reached 77% in some studies, with higher prevalence in ICU patients [20], where established malnutrition was reported in up to 50% of patients [20], consistent with the concept that malnutrition may be a pre-existing risk factor for COVID-19 infection. Although no studies specifically aimed at investigating the prevalence of malnutrition risk in persons with obesity and COVID-19, in one report of hospitalized COVID-19 patients with average BMI 28,8 kg/m2 [16] malnutrition defined by weight loss or high nutritional risk index from plasma biomarkers were also often present, with 38 and 83% prevalence respectively [16]. Malnutrition is also a major risk factor for negative outcomes in most disease conditions, particularly in groups such as older adults and polymorbid patients, in which malnutrition is a common consequence of multifactorial derangements in energy balance and enhanced muscle catabolism [6,7,14,78]. Malnutrition was accordingly also associated with disease severity and poor outcomes, including mortality, in available literature [[16], [17], [18], [19],21,22], also in an overweight COVID-19 cohort [16].
- Based on the GLIM global malnutrition diagnostic criteria [[89], [90], [91]], involuntary weight loss or low skeletal muscle mass indicate the presence of malnutrition independently of BMI, when associated with reduced food intake or inflammation and disease burden. Non-volitional weight loss [92,93] is also independently reported to exert a negative impact on patient prognosis. In the large cohort of the ESPEN nutritionDay survey in hospitalized patients, self-reported weight loss prior to hospitalization was notably associated with one-month mortality, with superimposable negative impact in both non-obese and obese patients [92]. In persons with obesity, altered body composition with low fat-free mass [sarcopenic obesity [94]] is associated with reduced survival compared to higher fat-free mass in chronic disease conditions, including chronic kidney disease and cancer [44,95,96]. Particularly in older adults, but potentially at any age, low skeletal muscle mass as well as function are associated with higher risk of frailty [97], which in turn is an important predictor or poor outcomes in the presence of acute diseases and hospitalization including respiratory viral infections [98]. It is therefore of utmost importance to screen for malnutrition all persons with obesity at risk for SARS-CoV-2 infection with poor outcomes, with particular regard to those with older age and polymorbidity. Validated tools such as MUST can be used (https://www.bapen.org.uk/screening-andmust/must-calculator). Such screening can be performed by non-nutrition specialists and could therefore be widely implemented to allow for identification of high-risk individuals. At-risk individuals should then undergo diagnostic assessment using validated tools. As mentioned above, the GLIM criteria for simple malnutrition diagnosis have been recently published in a consensus paper by all major global clinical nutrition Societies [89]. Studies have provided initial support for validity and applicability of the GLIM diagnostic approach [99,100]. Available validated tools for comprehensive nutritional assessment to diagnose malnutrition in the outpatient setting also include SGA [8] and MNA for geriatric populations [7].
-
3.Persons with both obesity and malnutrition should be advised to optimize their nutritional status, ideally by the support of experienced healthcare professionals. Oral nutritional supplements should be considered when dietary intake is unable to meet nutritional targets.
- Persons with obesity and malnutrition should be ideally referred to experienced professionals such as dietitian, clinical nutritionist or medical specialists for clinical nutrition and obesity, for full nutritional assessment and diet optimization due to risk of developing obesity-related severe forms of the disease [[25], [26], [27], [28], [29], [30]] with a potential malnutrition-induced synergistic negative impact on outcomes [[16], [17], [18], [19], [20], [21], [22]]. Given the prominent role of loss of skeletal muscle mass in obesity-associated malnutrition [89], ensuring adequate dietary protein is a primary goal of intervention, along with muscle strength exercises as applicable. The following general guidance is suggested for nutritional management of outpatient persons with obesity and malnutrition:
-
i.Protein requirements: >1 g/kg Adjusted Body Weight (ABW)·day
- Recently published ESPEN guidelines for both older adults and polymorbid individuals recommend a dietary intake of at least 1 g of protein/kg body weight·day for the general, non-obese geriatric and polymorbid population [6,7]. Even higher protein intake has been recommended for other patient groups with malnutrition in various disease settings such as chronic liver disease [78]. Based on the above considerations on use of body weight for dietary prescriptions in the presence of obesity, we suggest the use of ABW as a reasonable approach in calculating protein requirements for persons with obesity and malnutrition [14], and the dose of 1 g/kg ABW·day in the absence of advanced chronic kidney disease in conservative treatment [101]. High quality studies on this topic are advocated as a clinical research priority to collect direct evidence.
-
ii.Calorie requirements: 25 kcal/kg ABW·day UNLESS WEIGHT LOSS INDICATED
- In persons with obesity, ESPEN geriatrics guidelines recommend that weight-loss programmes be considered only when clear indications are established, based on risk-benefit assessment and in the presence of weight-related health problems [7]. Any dietary intervention should aim at a slow, gradual weight loss to minimize loss of skeletal muscle mass, using balanced diets with limited calorie deficits up to 500 calories/day from measured or estimated requirements [7]. We suggest extending this approach also to persons with obesity and malnutrition at risk of SARS-CoV-2 infection with poor outcomes, thereby limiting weight-loss interventions to patients with health problems caused by excess weight, after careful individual risk-benefit evaluation. Particular consideration should be given to the risk of inducing or aggravating loss of skeletal muscle mass and its clinical consequences; supervision and re-assessment of nutritional status by expert nutrition healthcare professionals should be implemented. ABW is suggested for calculation of calorie requirements, with initial indication for 25 kcal/kg ABW·day.
- Calorie quality- besides quantity, quality of dietary carbohydrate and fat is of well-recognized pivotal importance in dietary management of obesity and its complications in outpatient settings [102,103]. Detailed discussion of this well-established topic is beyond the scope of the current work. Implementation of dietary recommendations from established obesity guidelines should be sought with appropriate amounts of complex carbohydrates and dietary fiber, as well as limitation of saturated fat and cholesterol with higher dietary content of mono- and polyunsaturated fatty acids [102,103]. Dietary patterns and habits leading to higher intake of quality nutrients such as those characterizing a traditional Mediterranean diet should be also considered for long-term improvement of metabolic and nutritional risk [104,105].
-
i.
- Exercise- various guidelines recommend physical activity and exercise programmes for patients at risk or with malnutrition, in order to promote anabolic nutrient utilization and reduce catabolic derangements associated with aging and disease [6,7,14,78]. We suggest that physical activity and exercise should be recommended to all persons with obesity and malnutrition at risk for SARS-CoV-2 infection with poor outcomes, particularly in older adults and in the presence of polymorbidity and metabolic complications, in order to prevent further skeletal muscle loss and promote muscle mass and function [6,7,106]. Besides its beneficial impact on nutritional and metabolic status, regular physical activity is reported to be associated with lower severity and symptomatology of upper respiratory tract infections other than SARS-COV2 when performed at moderate intensity [107] although with higher severity if performed at high intensity and for a prolonged time [108]. By analogy to these findings, we hypothesize that this may be true in case of SARS-COV2 infection. Indeed, regular physical activity leads to anti-inflammatory responses that may protect against severe outcome of COVID-19 [109]. A population study found that besides smoking, physical inactivity (along with obesity) was a risk factors for COVID-19 serious enough to require hospitalization. The combination of smoking, inactivity and the addition of alcohol consumption led to a 4 times higher risk or hospitalization, after adjustments for age, sex, education, and cardiovascular risk factors and diseases [110]. Potential health- and performance status-related contraindications or limitations for exercise should be assessed before the initiation of activity or exercise programmes, and individualized recommendations should be provided as much as possible with regard to exercise type, frequency, intensity and duration by experienced healthcare professionals [7].
-
4.Since persons with obesity are also at risk for micronutrient deficiencies, particularly in the presence of malnutrition, daily dietary allowance of vitamins and other micronutrients should be ensured.
- Micronutrient deficiencies and infectious disease risk - Micronutrients, including several vitamins (vitamin A, B6, B12, folate, C, D, E) and trace elements (Zinc, Selenium, Copper), play important roles in the immune system supporting the innate and adaptive immune response, and thus their deficiencies could increase the susceptibility to infectious diseases [111]. Besides, vitamin A, C and D play an important role modulating the inflammatory response. A link between selenium deficiency and human disease was first demonstrated by the discovery in China that the etiology of Keshan disease involved both Coxsackievirus infection and a low intake of the micronutrient selenium [112]. Vitamin D deficiency has been also associated with a number of different viral diseases including influenza, HIV and hepatitis C, while other studies questioned such a relation for influenza. A systematic review and meta-analysis on acute respiratory tract infections reported a modest protective effect of vitamin D supplementation compared with placebo [113]. Although not all reports agree [114], several studies from different countries suggest a possible correlation between vitamin D deficiency and susceptibility to SARS-CoV-2 infection. A meta-analysis including 10 articles with 361.934 participants showed that vitamin D deficiency or insufficiency was associated with increased risk of COVID-19, although significant heterogeneity and publication bias were noted [115]. In another meta-analysis that included 27 selected articles, vitamin D deficiency was not associated with a higher chance of infection by COVID-19.
- Micronutrient deficiencies in obesity - Obesity is a risk factor for micronutrient deficiencies due to multiple factors that can include: consumption of unhealthy foods, increased needs due to the low-grade chronic inflammatory state and increased oxidative stress, and decreased intestinal absorption in patients with bariatric surgery, among other reasons [47,48]. Common micronutrient deficiencies may be even more frequent in persons with obesity and malnutrition or in patients with sarcopenic obesity [6,7,116]. Studies have revealed reduced serum zinc levels in up to 28% of obese individuals prior to bariatric surgery and in 36–51% of patients, afterwards [117]. The use of drugs for obesity comorbidities, such us hypotensive drugs (thiazides, ACE inhibitors and ARA-II drugs) and statins, can affect zinc balance, worsening zinc homeostasis in already mild zinc–deficient individuals [117]. A recent systematic review confirmed both that mean concentrations of selenium in blood/serum were lower in the obese compared with non-obese adult subjects and that mean blood GPX activity was decreased in obese individuals [118]. Obesity associates inversely with both B12 and folate levels, and directly with homocysteine levels [[119], [120], [121]]. Vitamin D deficiency is also more prevalent in individuals living with overweight and obesity compared with individuals of normal weight, with some research suggesting that deficiency prevalence is 25–35% in these groups [122]. Multiple factors are considered to lead to obesity-associated vitamin D deficiency, including reduced synthesis from sunlight exposure, sequestering of vitamin D in excess adipocyte cells, impaired activation in the body due to fatty liver, altered parathyroid hormone concentrations and signaling, and lifestyle factors. In addition, pharmacological agents such as antibiotics, anti-inflammatory agents, antihypertensive medications, endocrine drugs, and herbal medications commonly taken in response to weight related comorbidities can affect sunlight synthesis of vitamin D3 and reduce serum 25(OH)D concentrations available for activation in the body.
- In conclusion, micronutrients play an important role in the defense against infections, enhancing immunity and modulating the inflammatory response. As micronutrient deficiencies are more frequent in persons with obesity and malnutrition, nutritional assessment should include micronutrient status according to practical possibilities. At least recommended dietary allowances (RDA) – dietary reference intakes (DRI) should be covered by diet, and additional supplementation may be necessary in case of proven deficiency.
-
5.Since metabolic complications of obesity are associated withCOVID-19severity, persons with obesity at risk ofSARS-CoV-2infection with poor outcomes (such as those with older age and polymorbidity) should be checked for metabolic complications, including measurement of plasma lipid profile, plasma glucose, blood pressure as well as waist circumference for metabolic syndrome (MS) and T2DM.
-
6.Persons with obesity at risk ofSARS-CoV-2infection and poor outcomes with MS or T2DM should be advised by healthcare professionals including experienced nutrition teams to optimize their cardiometabolic risk profile.
- MS and T2DM are common complications of obesity that underlie a large fraction of obesity-associated cardiovascular risk [42]. Their prevalence and incidence notably increase with age and are causally associated with obesity- and aging-related muscle-catabolic derangements, including systemic inflammation and insulin resistance [123]. Consistent with this concept, skeletal muscle mass reduction has been reported in both MS and T2DM, particularly in aging individuals and in the presence of disease conditions [[123], [124], [125], [126], [127], [128]]. Both MS and T2DM, as well as hyperglycemia in individuals with no previous diagnosis of T2DM, have been notably reported to have a negative impact on COVID-19 outcomes upon hospitalization [[31], [32], [33],129]. In addition and also importantly, cardiovascular risk factors may contribute to COVID-19 mortality by increasing cardiovascular complications that in turn represent a significant component of COVID-19 associated mortality [130].
- Based on these observations, we suggest that persons with obesity and MS or T2DM are advised to undergo not only nutritional screening for malnutrition, but also full evaluation of MS-associated cardiometabolic risk profile. Metabolic syndrome components, as well as glucose control for persons with T2DM, should be optimized to minimize risk of poor outcomes and complications in the presence of COVID-19. To this aim, persons with obesity and metabolic complications should continue regular metabolic evaluation during the COVID-19 pandemic, also using telemedicine tools whenever possible. Discussion of the pharmacological treatment of MS components and T2DM is beyond the scope of this work, except for glucose monitoring in hospitalized patients receiving medical nutrition treatment that will be discussed below. Dietary approach to MS and T2DM also cannot be described in detail; we suggest that adequate protein (1 g/kg ABW·day) and daily allowances of micronutrient intake should be secured in persons with obesity and MS or T2DM with malnutrition risk or established malnutrition, in the absence of advanced chronic kidney disease undergoing conservative treatment. Dietary patterns providing low glycemic index foods, higher amounts of unsaturated fat, dietary fibers and antioxidant and anti-inflammatory compounds such as a traditional MD should be implemented as much as possible [102,104,105]. Particularly in the presence of malnutrition, and in general in older or polymorbid patients, any weight loss programme with moderate calorie restriction to reduce insulin resistance and metabolic derangements should be carefully considered against the risk of further loss of skeletal muscle mass [7]. Under such conditions weight loss may potentially lead to worse outcomes in general and in case of SARS-CoV-2 infection, and should be only implemented with supervision and monitoring from expert nutrition healthcare professionals.
-
2.
-
d)
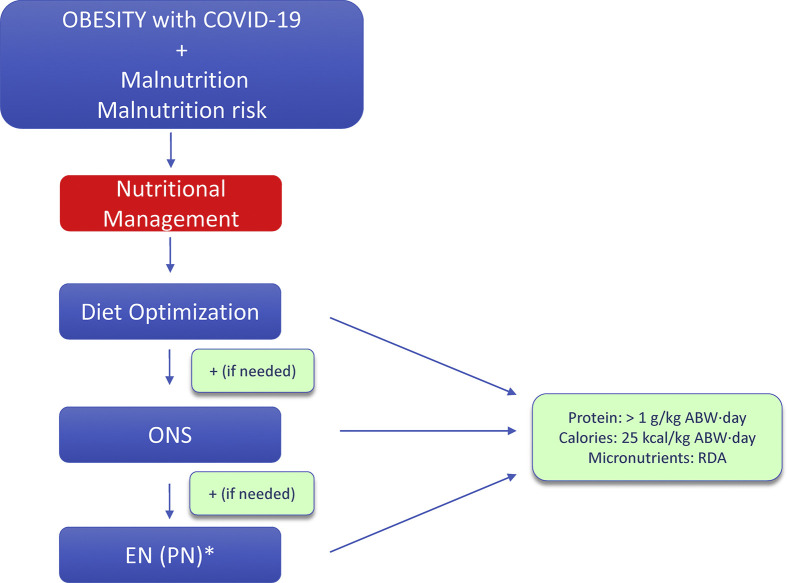
Nutritional management of persons with obesity andCOVID-19 (Fig. 1, Fig. 2 )
- Home setting
-
7.Persons with obesity diagnosed with COVID-19 undergoing home care and quarantine, particularly but not limited to obese older adults and with polymorbidity, should be checked for malnutrition risk and advised on potential needs for nutritional optimization by experienced personnel; telemedicine tools should be implemented as much as possible.
- Regardless of pre-existing nutritional derangements, COVID-19 may cause or worsen systemic inflammation while leading to reduced physical activity or immobilization, thereby resulting in involuntary weight loss with reduction of skeletal muscle mass. Gastrointestinal symptoms may also occur with diarrhea and vomiting which may reduce food assimilation [[1], [2], [3], [4], [5]]. Ageusia and anorexia may further reduce food intake [[1], [2], [3], [4], [5]]. Persons with obesity and COVID-19 disease undergoing home quarantine, particularly but not limited to those with risk factors for poor outcomes such as older adults and those with polymorbidity, should therefore undergo malnutrition screening. In the presence of malnutrition risk or full-blown malnutrition, we suggest that guidance for persons with obesity at risk for COVID-19 may be also applied for nutritional management of those with COVID-19 and undergoing home care and quarantine. Since COVID-19 is an acute intercurrent catabolic condition, and potential COVID-19-induced weight loss is likely to include substantial proportions of lean mass and skeletal muscle, balanced calorie and adequate protein intake should be implemented, as follows:
-
i.Protein: >1 g/kg ABW·day
-
ii.Calorie: 25 kcal/kg ABW·day
- Calorie targets may be revised in case of weight gain in the absence of fluid retention, especially in the presence of metabolic complications, with the goal to maintain stable body weight while preventing muscle loss. Daily micronutrient dietary allowance should also be secured. Use of ONS should be considered as described below (Statements 9–10) if dietary intake is insufficient to meet nutritional targets. Physical activity may be encouraged, compatible with health conditions and the quarantine environment.
- Organization of healthcare services and allocation of adequate resources obviously represent difficult challenges in times of the pandemic. Provision of nutritional screening, diagnosis, assessment as well as continuation of chronic nutritional care also in the home setting pose huge organization and logistical problems [[131], [132], [133], [134], [135], [136]]. Various papers addressing these issues emphasize the importance of telemedicine implementation, based on the logical assumption that lack of nutritional evaluation and care due to limited access to in-person evaluation will cause negative health effects and potentially increase COVID-19 infections and complications. Algorythms for malnutrition risk screening have been recently proposed, also including nutritional advice in case of positive testing [134], although no specific tools have been proposed for persons with obesity. For persons with obesity, optimized nutritional care should be associated with continuing management of potential metabolic complications, that should also involve telemedicine approaches; initial reports are available for hyperglycemia and T2DM [137] and their implementation is being discussed.
-
7.
- Non-ICU hospital setting
-
8.Persons with obesity hospitalized for COVID-19, particularly those at highest risk of poor outcomes due to older age and comorbidities, should be screened for malnutrition by using validated tools such as NRS-2002
- As summarized above, emerging evidence shows that the prevalence of nutritional risk or full-blown malnutrition is high at hospital admission in COVID-19 patients [[16], [17], [18], [19], [20], [21], [22]]. Studies also reported high prevalence of malnutrition in patients with BMI in the overweight or obese range [16,138]. The NUTRICOV study reported a malnutrition prevalence of almost 40% based on GLIM criteria in a population with average BMI in the overweight range [138]. In the same study, self-reported dysgeusia, dysphagia, anorexia, and >50% decrease of oral food intake were reported to occur at least one week before hospitalization in up to 60% of malnourished individuals [138]. In order to identify malnutrition risk, persons with obesity hospitalized for COVID-19 should therefore be screened upon admission using validated tools, and those with positive screening should undergo diagnostic assessment. NRS 2002 is a simple validated screening tool preferred in hospital settings (https://www.mdcalc.com/nutrition-riskscreening-2002-nrs-2002). The GLIM criteria can also be used in hospitalized patients for malnutrition diagnosis after positive risk screening [89]. Additional tools are available for full nutritional diagnostic assessment including SGA, MNA for geriatric populations [7] or Nutric Score for ICU, which has also shown predictive value for negative outcomes in COVID-19 patients in this setting [22].
-
9.Persons with obesit y hospitalized for COVID-19 with malnutrition should undergo nutritional and dietary assessment by an experienced professional and should receive dietary counseling for diet optimization as well as oral nutritional supplements when needed.
-
10.Enteral nutrition (EN) should be started in persons with obesity hospitalized for COVID-19 when oral intake including ONS is not sufficient to meet nutritional targets. If EN is contraindicated or not sufficient to meet targets, parenteral nutrition (PN) can be considered.
- For hospitalized COVID-19 patients, higher disease severity compared to those undergoing home care and quarantine may imply more intense catabolic inflammatory responses, lower spontaneous food intake and sustained immobilization, with overall higher risk for weight and muscle loss. One study has indeed provided direct evidence that weight loss is common in both hospitalized and non-hospitalized COVID-19 patients, with negative impact on outcome in terms of length-of-stay in the hospital group [24]. In addition, muscle damage has been reported in subgroups of hospitalized COVID-19 patients, associated with severe disease course and higher pro-inflammatory mediators, as well as plasma biochemical markers such as elevated creatine kinase that directly reflect skeletal muscle injury and potential loss of tissue mass and function [139]. Medical nutrition treatment should therefore be implemented in hospitalized persons with obesity and malnutrition with COVID-19. Early start within 24–48 h from admission should be also sought, since early compared to late nutrition support in the course of hospital stay may provide protection from infectious complications [140,141] and reduce sarcopenia, particularly when associated with in-hospital rehabilitation for various disease conditions [142].
- We suggest that previous recommendations for patient groups at high-risk of malnutrition, such as older adults and polymorbid patients, are also applicable to malnourished hospitalized persons with obesity and COVID-19 with respect to calorie and protein requirements. For persons with compromised nutritional status and suspicion for long-standing malnutrition, nutritional treatment should reach targets gradually in order to prevent refeeding syndrome.
-
i.Protein requirements: >1 g/kg ABW·day
-
ii.Calorie requirements: 25 kcal/kg ABW·day
- For calorie requirements we further suggest that, in the presence of highly catabolic stimuli provided by COVID-19 infection and hospitalization, body weight loss should not be sought and should be instead prevented, due to high risk of profound muscle mass depletion
- Also for persons with obesity, we suggest that general guidance on use of medical nutrition should be applicable to the context of COVID-19 infection [6,7,53]. The oral route should be always chosen when possible. ONS provide energy-dense alternatives to regular meals and may be specifically enriched to meet targets in terms of protein as well as micronutrients (vitamins and trace elements) whose daily estimated requirements should be regularly provided. Several large meta-analyses and systematic reviews have provided evidence for positive impact of ONS treatment on nutritional status with preservation of muscle mass, quality of life, readmissions and in selected studies reduced mortality was also demonstrated [143]. Also based on existing literature and one large systematic review [144], daily target intakes from ONS should be 400 calorie and 30 g protein. Monitoring of treatment should be performed after continuation for at least one month, while more frequent evaluation was advised when compliance is questioned (e.g. weekly). Importantly, nutritional treatment should continue also after discharge from the hospital with individualized nutritional plans, in order to prevent or limit the likely further worsening of nutritional status, which may result from pre-existing derangements and acute disease- and hospitalization-induced catabolic stimuli.
- EN should be implemented when nutritional needs cannot be met by the oral route while monitoring for EN-related potential complications [6,7]. There are no limitations to the use of enteral or parenteral nutrition based on patient age or diagnosis, in the presence of reasonable expectation to improve nutritional status.
-
11.Hospitalized persons with obesity with COVID-19 should be ensured their recommended daily allowance of vitamins and micronutrients with oral diet or medical nutrition treatm ents.
- The negative impact of vitamin and micronutrient deficiencies on general risk of viral infections has been discussed in comments to Statement 3. We will now discuss available evidence on their impact on disease severity and outcomes as well as available studies investigating their potential therapeutic utilization in COVID-19 patients.
- Micronutrient deficiencies and infectious disease severity – In general, low circulating levels or low intakes of micronutrients such as vitamins A, D, E, B6 and B12, zinc and selenium have been associated with adverse clinical outcomes during viral infections [111]. For instance, vitamin A deficiency is involved in measles and diarrhea, and measles can become severe in vitamin A deficient children [145]. Vitamin A supplementation also may offer some protection against the complications of other life-threatening infections, including malaria, HIV, viral pneumonia caused by an avian coronavirus, influenza A, rotavirus, and Newcastle disease virus [48]. The increased knowledge about the role of zinc in viral immunity has resulted in clinical studies, showing therapeutic effect of zinc supplementation in viral infections like common cold and herpes simplex. Episodes of acute diarrhea were characterized by shorter duration and less severity in zinc-supplemented groups. Other studies indicate that the incidence of acute lower respiratory tract infections and malaria may also be reduced by zinc supplementation [117,145]. Presently, zinc is recommended by WHO as first–line treatment, with oral rehydration solution, for acute gastroenteritis in children. Also the deficiency of selenium may increase the severity of HIV and hepatitis C infections and hemolytic anemias [145].
- Micronutrients as treatment for COVID-19: experimental models - Regarding the COVID-19 pandemic, several micronutrients have been tested to combat SARS-CoV-2 infection in vitro and in animal models. A significant portion of the SARS-Cov-2 genome codes for a main polypeptide protease (Mpro), a key enzyme for viral replication and thus an attractive target for potential anti-viral drugs. Vitamin B12 is a potent inhibitor of Mpro and it potentially inhibits the non-structural coronavirus protein12 (an RNA-dependent-RNA polymerase that is also vital for viral replication) [146]. A selenium derivative, ebselen, also inhibits Mpro and has demonstrated strong antiviral activity in cell-based assays [147]. Furthermore, the vitamin A derivate isotretinoin may interfere with the cellular uptake of SARS-CoV-2 and its lung-directed pathogenicity by inhibiting the ACE2, the SARS-CoV-2 receptor [48]. Besides immune-enhancing and anti-inflammatory properties, vitamin D also interferes with the renin-angiotensin pathway. Xu et al. demonstrated that calcitriol is able to upregulate ACE2 and downregulate renin and angiotensin II in the lung tissue of rats exposed to lipopolysaccharide as a model of ARDS [148]. In this scenario, recent studies have suggested that vitamin D can act by targeting ACE2 down-regulation in SARS-CoV-2 infection, which could be a potential therapeutic approach to COVID-19 and induced ARDS [149]. However, further studies must be performed to understand the impact of ACE2 modulation for COVID-19.
- Micronutrients as treatment for COVID-19: initial clinical studies - Based on experimental findings, and lack of specific antiviral treatment for COVID-19, several micronutrients have been tested alone or in combination with other drugs in patients with SARS-Cov2 infection, in the context of clinical practice and in some clinical studies. Most of these studies have been of very low scientific quality, and without differentiation between correcting micronutrient deficiencies, or administration as a supplemental dose. As an example, high dose oral supplementation of zinc salts was reported to reduce respiratory clinical symptoms of COVID-19 in a series of four patients without any adverse effect [150]. Conversely, a prospective study did not find a significant correlation between zinc supplementation and reduced COVID-19-related mortality [151]. In a retrospective study Zhang et al. found a positive correlation between selenium status and the cure rate from COVID-19, and the authors suggested a positive clinical impact of higher selenium intake and selenium status [152]. Two studies point to defective one-carbon metabolism and poor COVID-19 outcomes. In the first study a combination of vitamin D, magnesium and vitamin B12 lead to significantly lower odds of need of oxygen therapy and/or intensive care support [153]. In the second one, lower folate levels were consistently associated with increasing stages of COVID-19 severity [154]. Interestingly, in the latter study higher levels of vitamin B12 were associated with increased severity of COVID-19, even though the authors did not publish multivariable analyses nor did they statistically explore potential interaction between folate and B12. Vitamin C, alone or in combinations with other nutraceuticals (curcumin and glycyrrhizic acid, quercetin), has been extensively used in COVID-19 patients with promising results on the inflammatory response, in observational studies and clinical cases reports [[155], [156], [157]]. High-dose intravenous vitamin C in moderately to severely ill COVID-19 patients was reported to improve the oxygenation index with all patients eventually cured and discharged [158]. In COVID-19 patients in need of high-dose oxygen treatment 1 g of vitamin C intravenously was reported to reduce anti-inflammatory markers such as D-dimer and ferritin. However, this study had a small number of participants, had a short three-day duration and did not investigate the effects of using vitamin C alone [159].
- Vitamin D has attracted most interest among micronutrients for treatment of COVID-19. Vitamin D deficiency was reported to increase hospitalization and mortality from COVID-19 [160] and Vitamin D deficiency has been reported in a small cohort of COVID-19 ICU patients, indirectly confirming a link between low Vitamin D and risk of severe disease [161]. Few studies analyzed the efficacy of vitamin D treatment in improving COVID-19 outcomes. As previously mentioned, Tan et al. conducted a small-cohort observational study to evaluate the effect of vitamin D and vitamin B12, with magnesium in combination on clinical outcomes. In multivariate analysis (after separately adjusting for age or hypertension), intervention retained a protective effect [153]. In a randomized open-label, double-masked clinical pilot trial treatment with calcifediol (25(OH)D) reduced required admission to ICU [162]. In support of this finding, in a prospective cohort study in nursing homes among residents with suspected or diagnosed COVID-19 the non-vitamin D arm had higher mortality [163].
-
-In summary, micronutrient deficiencies may lead to worse outcomes of several viral infectious diseases and a worse outcome of COVID-19 has been observed in studies in patients with micronutrient deficiencies (especially vitamin D). Therefore, we suggest the need to cover RDA/DRI needs in all hospitalized patients with COVID-19, particularly in persons with obesity due to high risk of deficiency, with additional regard to those with malnutrition or with older age and polymorbidity. The added value of micronutrient supplementation above RDA/DRI in patients with COVID-19 is not clear so far, and we need more studies to define its role in the clinical outcome of these patients. In patients diagnosed with micronutrient deficiencies through direct measurement of their plasma concentration, supplementation can be considered.
-
12.Hospitalized persons with obesity with COVID-19 should be checked for metabolic complications including measurement of blood glucose, plasma lipid profile, blood pressure as well as waist circumference.
-
13.Hospitalized persons with obesity with COVID-19 with MS or T2DM should be offered medical and nutritional treatment by experienced professionals to optimize their cardiometabolic risk profile.
- In addition to the following comments to Statements 12–13 also see Statements 5–6.
- Measurement of blood pressure, blood glucose and plasma lipid profile is commonly part of routine work-up for all hospitalized patients. For hospitalized COVID-19 patients with and without malnutrition, screening for these parameters is of additional value since they represent additional risk factors for worse COVID-19 outcomes [[31], [32], [33],129,130]. Also in the hospital setting for COVID-19 infection, due to higher risk of metabolic syndrome and type 2 diabetes, it is important that persons with obesity as well as overweight undergo both nutritional screening for malnutrition and evaluation of MS-associated cardiometabolic risk factors, including waist circumference. Control and ideally optimization of metabolic syndrome components may contribute to minimize risk of poor COVID-19 outcomes and complications [[31], [32], [33],129,130], and it should therefore be pursued through pharmacological and nutritional approaches as described for Statement 6.
- Use of disease-specific formula - Nutritional support can cause or exacerbate hyperglycemia, especially in persons with obesity that are at higher risk of insulin resistance or T2DM. Since hyperglycemia is associated with severe COVID-19 and poor outcomes also in non-critically ill COVID-19 patients [32,129,130], monitoring blood glucose and limiting its elevations and fluctuations is an important target in the context of nutritional care. In the medical nutrition setting, nutritional enteral formula for people with diabetes (Diabetes-Specific Formula: DSF) are available, aiming at limiting glycemic peaks and variability. Systematic reviews and meta-analyses in non-critically ill patients have indicated that DSF may improve hyperglycemia and glucose excursions in patients with T2DM [164,165]. It should be pointed out that no demonstration of improved hard outcomes, including mortality, is available, and that evidence does not include sufficient rigorous randomized controlled studies [164,165]. Improvement of hyperglycemia may however potentially improve outcomes in COVID-19 patients [32,129,130]. We therefore suggest that DSF may be considered for enteral nutrition in persons with obesity and hyperglycemia, particularly when blood glucose control is poor (e.g. majority of values above 180–200 mg/dl) and after individual evaluation. Further randomized controlled studies are advocated to identify optimal formula composition to control blood glucose and improve outcomes in various disease conditions. Also notably, regarding quality of dietary fat, PUFA have well-recognized action on inflammation and coagulation and higher intake of omega 3 might be suggested, but evidence of their clinical impact in various clinical settings is still incomplete [6,7,14].
-
8.
-
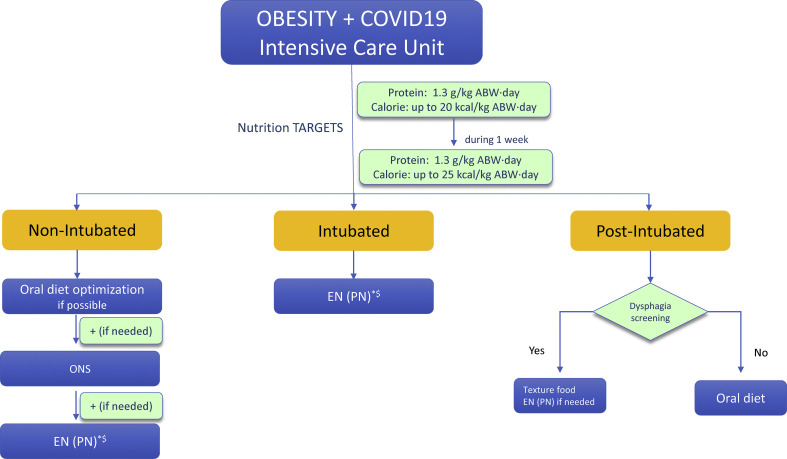
e)Nutritional management of persons with obesity andCOVID-19in the intensive care unit (ICU) (Fig. 3 )
- A large body of evidence has demonstrated that patients with COVID-19 with any degree of obesity had a significantly higher risk of hospitalization and admission to ICU, mechanical ventilation or death compared to patients without obesity [25,26,[28], [29], [30],166]. Most studies identified severe obesity as an independent risk factor for disease severity and the need for invasive ventilation. Interestingly, bariatric surgery with subsequent weight loss and improvement of metabolic abnormalities was associated with lower need of hospitalization and ICU admission, lower rate of intubation and dialysis than patients with obesity who became infected with SARS-CoV-2 [166]. An upregulation and greater release of inflammatory cytokines such as interleukin (IL)-6 could explain this phenomenon. It has also been suggested that adipose tissue in people with obesity may act as a reservoir for more extensive viral spread and increased shedding [167]. We provide here statements based on the recent ESPEN guidelines on nutritional therapy in the ICU [14] at the different stages guided by the patient condition.
- Pre intubation period
-
14.In non-intubated ICU patients with obesity and COVID-19, ONS or EN treatment should be implemented if indicated to reach protein and energy targets; PN should be considered if oral end enteral treatment are contraindicated or insufficient to reach targets.
- Patients with severe obesity treated by high flow nasal cannula oxygen therapy may experience difficulties to ingest oral food. The approach to severe respiratory failure has evolved during the past year from an early intubation strategy; High-Flow Nasal Cannulae (HFNC) oxygen therapy has been administered much longer and the exact timing for intubation has been debated, with recent proposals for decision-support algorithms [168,169]. If the oral/enteral route is limited by risk of aspiration, it could be advised to prescribe parenteral nutrition in the population not reaching energy-protein target. EN may be challenging during non-invasive respiratory support as pointed out in our previous statements [53]. However, a new mask device allowing introduction of a nasogastric tube has been tested successfully and may be considered to allow administration of enteral nutrition during NIV [170]. High rates of failure for NIV have been unfortunately overall described in persons with obesity with COVID-19-induced respiratory failure [171]. Some centers succeed to prone despite the high weight and body mass and there are no contraindications to pronation due to obesity per se; patients however frequently need mechanical ventilation after intubation.
-
14.
- Intubated patients
-
15.In intubated ICU patients with obesity and COVID-19, EN should be started to gradually reach energy targets while securing high-protein delivery. The prone position does not represent per se a limitation or contraindication for EN also in persons with obesity.
-
16.Due to high risk of loss of skeletal muscle mass with consequent malnutrition and disabilities, persons with obesity should be provided opportunities for mobilization and/or controlled physical activity during ICU stay to optimize anabolic effects of nutritional treatment and reduce muscle protein catabolism.
- Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 seem to be comparable to those caused by other conditions [172]. However, obesity and mainly excess visceral adipose tissue are independent risk factors for ICU admission when compared to those not admitted to ICU [25,26,[28], [29], [30]]. Severe hypoxemia and hypercapnia are contraindications to medical nutritional support. When the patient is stabilized with acceptable oxygenation (PaO2> 60 mmHg) and ventilation (pH> 7.2), nutritional support can be started and based on ESPEN guidelines on nutrition in the ICU [14]. Progressive enteral feeding should be started, even if prone position is required [173]. The prone position being one of the most frequent approaches used to improve oxygenation, ICU teams may be reluctant to administer enteral nutrition due to concerns for regurgitation and vomiting. Reigner et al. showed that prone position is not increasing risks of aspiration and should allow safe enteral feeding [174]. To prevent risks of regurgitation, the bed should be placed in a Trendelenburg position of maximum 30°, prokinetic agents may be used, and close monitoring of gastric residual volume should be performed. PN may be utilized in patients who cannot receive EN or are unable to reach their nutrition goals by the end of the first week. Parenteral nutrition is also used in case of severe diarrhea. The increasing utilization of extra corporeal membrane oxygenation (ECMO) in persons with obesity is not a contraindication to the use of enteral feeding that has been found to be safe. However, in patients in shock or severe gastrointestinal dysfunction, enteral feeding should not be administered [175]. The 2019 ESPEN guidelines recommend early EN if possible in patients treated with ECMO [14].
- Calorie-protein targets - Defining energy and protein targets in persons with obesity and COVID-19 may be challenging also in the ICU, since, as discussed, actual body weight in persons with obesity may lead to substantial overestimation of energy and protein requirements. The effects of lockdowns and deleterious lifestyle changes, as well as acute disease per se, may lead to further alterations in body composition in persons with obesity, with loss of skeletal muscle mass and concomitant further fat accumulation, thereby resulting in malnutrition and sarcopenic obesity. Ideal definition of energy requirements in ICU should be based on direct measurement by indirect calorimetry. Indirect calorimetry could be performed with precautions despite the COVID 19 restrictions, and recommendations have been proposed to this regard [176]. A longitudinal study [177] assessed resting energy expenditure in COVID 19 patients over more than 3 weeks. Interestingly, measured REE was higher than indicated by predictive equations, and it increased even more after 10–14 days of ICU stay, thereby potentially challenging the concept of REE decrease after the early acute phase of the disease. In the absence of indirect calorimetry and when predictive equations need to be used, we suggest to extend the recommendations provided in the 2019 ESPEN ICU Guidelines also to persons with obesity with COVID-19 in the ICU, with moderate hypocaloric feeding up to 70% of estimated needs for the first 3–7 days of ICU stay [14], in order to avoid overfeeding and its associated metabolic and clinical risks during the hyper-acute ICU phase. 20 kcal/kg ABW·day may represent an approximated reasonable estimate for initial calorie provision. Calories may be gradually increased up to estimated isocaloric needs of approximately 25 kcal/kg ABW·day after the first week, following individual evaluation based on clinical and hemodynamic conditions, as well as metabolic status and complications. A recent study notably provided initial direct evidence for the positive impact of preventing early caloric deficit to improve clinical outcomes in mechanically ventilated COVID-19 patients including overweight and obese BMI levels [178].
- Optimal protein targets should be also ideally guided by nitrogen excretion measurement [14]. In the absence of this information, protein recommendations provided in the ESPEN ICU guidelines should be followed, to provide at least 1.3 g/kg ABW·day. Similar to non-ICU settings, mobilization and physical activity when possible should be implemented with structured individualized protocols aimed at enhancing protein anabolism [14,179].
- Calorie quality, formula and blood glucose control- Nutrition formula for ICU patients should provide high calorie density (1.5 kcal/ml) and high protein content. A large fraction of persons with obesity in the ICU may present with pre-existing metabolic syndrome or T2DM, or develop severe insulin resistance and stress hyperglycemia during the course of COVID-19 [14]. In addition, administration of beneficially proven dexamethasone has been accepted in most centers to treat pneumonia [180], but it may lead to, or contribute to, severe glucose intolerance. Strict glucose control protocols with insulin treatment should be implemented avoiding severe hyperglycemia (above 180–200 mg/dl) and hypoglycemia, as well as glucose variability, e.g. aiming at keeping more than 80% of glucose measurements in the proposed target (for instance 100–180 mg/dl). Algorythms have been recently proposed specifically for glucose control and stabilization in critically ill patients with COVID-19, based on s.c. or i.v. insulin administration according to glucose measurements, suggesting that action be taken to improve glycemia when blood glucose exceeds 200 mg/dl [181]. In the same study, the authors propose 180 mg/dl as acceptable blood glucose threshold with targeted values between 150 and 180 mg/dl, and up to 200 mg/dl for labile glycemic control [181]. Similar insulin treatment protocols could be considered for glucose control in patients undergoing medical nutrition to limit hyperglycemia. It should kept in mind that glucose variability and particularly hypoglycemia are also dangerous and were associated with higher mortality also in COVID-19 patients [129] and should therefore be prevented. There is no sufficient evidence to recommend a disease-specific formula higher in fat content and lower in slower digesting carbohydrates in ICU settings. Research in this field is a strong priority.
-
15.
- Post-intubation
-
17.Oral nutrition should be reintroduced to reach nutritional targets after extubation. When post-extubation dysphagia is present, texture-adapted food should be administered or EN should be considered when swallowing is unsafe with aspiration risk.
- After extubation, dysphagia is of major concern with particularly high prevalence in older adults and following prolonged intubation that may be common in COVID-19 patients [53]. Severe dysphagia is reported to be associated with worse outcomes in terms of pneumonia, need for reintubation and mortality [53]. No direct evidence regarding post-extubation dysphagia in COVID-19 patients is however yet available. The European Society of Swallowing Disorders [182] provided considerations related to the COVID-19 pandemic: “Assessment and treatment of patients with oropharyngeal dysphagia should be provided, while at the same time balancing risk of oropharyngeal complications with that of infection of patients and healthcare professionals involved in their management. Assessment and management of oropharyngeal dysphagia is a high-risk situation as it must be considered an aerosol-generating procedure. Personal protective equipment (PPE) should be used. Elective, non-urgent assessment may be temporarily postponed and patients are triaged to decide whether dysphagia assessment is necessary; instrumental assessment of swallowing is performed only if processing of the instruments can be guaranteed and clinical assessment has not provided enough diagnostic information for treatment prescription. Telepractice is encouraged and compensatory treatments are recommended”. For extubation in the post COVID-19 period, standard tests can be recommended including instrumental dysphagia diagnostic aerosol generation procedures. In practice, remote evaluation should be performed. Two-meter distance should be maintained and reflex cough testing should be avoided. Videofluoroscopy and other instrumentations should be avoided and test food should be self-administered by the patient. In case of dysphagia a diet with suitable texture should be provided, and when aspiration risk is present EN should be started [173]. If EN is not possible (e.g. if swallowing rehabilitation may require removal of the feeding tube), temporary PN may be considered. In case of tracheostomy, fractional, enriched feeding with oral nutritional supplements should be used. If energy and protein needs are not covered (<70% of the needs), supplemental PN may be also considered to meet energy and protein targets [173].
-
17.
-
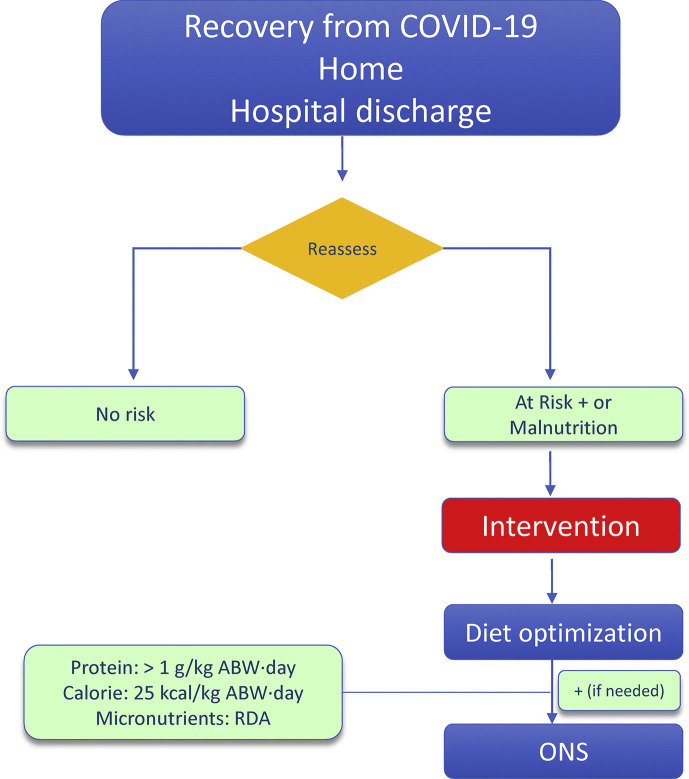
f)Nutritional management of persons with obesity during recovery fromCOVID-19 (Fig. 4 )
-
18.Due to high risk of loss of skeletal muscle mass with ensuing malnutrition, sarcopenia and disabilities, persons with obesity recovering fromCOVID-19should be checked for malnutrition andfollowed-upwith dietary counseling by experienced professionals, particularly in the presence of older age, polymorbidity or previous ICU stay.
-
19.Due to high risk of loss of skeletal muscle mass with ensuing malnutrition and sarcopenia, persons with obesity recovering fromCOVID-19should be encouraged to perform safe and adequate physical activity including exercise programmes, particularly in the presence of older age, comorbidities or previous ICU stay, ideally within individualized programmes established by experienced professionals.
- There is increasing awareness that persons recovering from COVID-19 may present numerous clinical sequelae ranging from fatigue and weakness to psychological distress [183,184]. The term “long COVID” or “post COVID” has been proposed to define persistence of complaints and symptoms following negativization of viral testing [185,186]. Among other general symptoms, the high prevalence of fatigue and weakness directly suggests involvement of negative changes in skeletal muscle mass and function [183,184]. Persistent, residual alterations in taste and smell may also interfere with food intake [131,133]. These complaints importantly are reported not only in persons recovering from severe COVID-19 disease and ICU stays, but also in non-ICU and non-hospitalized patients [183,184]. The above combined observations are fully consistent with a negative impact of COVID-19 on nutritional status that may specifically involve disease-induced lean tissue and skeletal muscle catabolism [53]. In addition, for ICU survivors the emerging long COVID syndrome is also in full agreement with the well-established concept that weakness and fatigue may persist for several months after ICU discharge, due to multifactorial issues, described as ICU-acquired weakness (ICUAW) or chronical critical illness [187]. In particular, one study demonstrated COVID-19 induced weight loss at discharge in both hospitalized and non-hospitalized patients, although no information was available on skeletal muscle and body composition [24]. In another report, reduced skeletal muscle strength and performance were found following COVID-19 in a patient cohort with average BMI in the overweight range [188]. In elderly polymorbid patients high risk of severe frailty, including impaired mobility and potential poor nutritional status, was reported following hospitalization for COVID-19 in sub-acute setting [189].
- Although no studies are specifically available on long COVID symptoms including fatigue and weakness in the obesity setting, persons with obesity recovering from COVID-19 should continue to be monitored for malnutrition risk and nutritional status, particularly in the presence of reported fatigue and weakness and in high-risk groups such as older adults and polymorbid individuals. Malnutrition risk should be checked at discharge in hospitalized patients, and upon recovery from positive testing on patients quarantined at home. In patients with malnutrition, adequate dietary intake of protein and calorie should be ensured, with use of ONS when nutritional targets cannot be reached through fortified diet. In the absence of clinical indication for weight loss, in the presence of malnutrition risk or overt malnutrition we suggest calorie provision of 25 kcal/kg ABW·day with >1 g/kg ABW·day protein. In the absence of malnutrition with fatigue and weakness, protein provision >1 g/kg ABW·day protein should be ensured along with physical activity and exercise appropriate for general conditions and after exclusion of any contraindications. The impact of a physical exercise program after a COVID-19 disease has not yet been studied. A recent review suggests that people who recover from mild COVID-19 should probably slowly and progressively resume to pre-COVID physical activity once they are asymptomatic, in order to recover their physical fitness [109]. In general, physical activity and individualized exercise programs including both aerobic and resistance modalities should be introduced or resumed ideally with supervision and monitored by experienced professionals in terms of type, intensity and duration in persons with obesity recovering from COVID-19. In case of cardiac or respiratory symptoms arising during physical activity, cardiac investigations may be indicated before increasing the intensity of physical activity.
- Besides the use of tele-monitoring and telemedicine tools for nutritional screening and assessment, the use of tele-rehabilitation programmes is also important in order to implement guided physical activity not only for people with established disabilities but also potentially to provide metabolic and nutritional benefits in the presence of nutritional risk or malnutrition [[190], [191], [192]].
-
20.Persons with obesity recovering fromCOVID-19,particularly in the presence of older age or polymorbidity should resume (or start) multiprofessional support for appropriate treatment and nutritional management of obesity and related comorbidities and metabolic complications as soon as possible.
- As a complex chronic condition posing severe health threats and often burdened by severe comorbidities [50,103], obesity requires sustained management by multiprofessional team approach. Persons with obesity recovering from SARS-CoV-2 infection should resume regular checks by the obesity team in charge also using telemedicine tools, in order to reassess treatment goals, metabolic profile and comorbidities status. Any need for changes in dietary and lifestyle goals as well as need and indications for pharmacological treatment should be monitored and reassessed, also in the light of potential catabolic bouts during the course of COVID-19 with worsening of nutritional status, and worsening or new onset of malnutrition. Harmonizing nutritional treatment goals should be a priority.
-
18.
Fig. 1.
Algorythm for malnutrition risk screening, malnutrition diagnosis and assessment in persons with obesity. The algorythm may apply both for 1) prevention of SARS-CoV-2 infection with potential poor COVID-19 outcomes and 2) identification of indications for nutritional treatment in patients with established COVID-19. (∗Consider different cut-off points in Caucasian and East Asian people, ∗∗e.g. GLIM criteria, SGA, MNA).
Fig. 2.
Algorythm of nutritional treatment in persons with obesity with COVID-19 and malnutrition or at risk of malnutrition. (∗Algorythm potentially applicable for both HOME and HOSPITAL care; EN (or PN when needed) to be initiated in most cases in the hospital setting for practical reasons). ONS: Oral Nutritional Supplements, EN: Enteral Nutrition, PN: Parenteral Nutrition, ABW: Adjusted Body Weight, RDA: Recommended Daily Allowances.
Fig. 3.
Algorythm of nutritional treatment in critically ill obese patients with COVID-19. (∗Reach targets gradually, $ Contraindicated if: 1) shock or hemodynamic instability; 2) unstable respiratory failure or acidosis). ONS: Oral Nutritional Supplements, EN: Enteral Nutrition, PN: Parenteral Nutrition, ABW: Adjusted Body Weight, RDA: Recommended Daily Allowances).
Fig. 4.
Algorythm of nutritional monitoring and treatment for COVID-19 patients during recovery and after hospital discharge. ONS: Oral Nutritional Supplements, EN: Enteral Nutrition, PN: Parenteral Nutrition, ABW: Adjusted Body Weight, RDA: Recommended Daily Allowances.
3. Conclusions
Obesity has a negative impact on SARS-CoV-2 infection and severity of COVID-19 disease. Multifactorial obesity- and adiposity-associated metabolic derangements may causally contribute to this relationship. Since malnutrition has emerged in the past year as an important risk factor for infection and poor outcomes of COVID-19, we have here discussed the importance of raising awareness on preventing and treating malnutrition in at risk persons with obesity. Particular care should be placed to screen, prevent and treat malnutrition in persons with obesity and older age, as well as those with polymorbidity in both outpatient and hospitalized settings, with specific indications for ICU. The role of nutritional screening and care should also be emphasized in the emerging challenge of long-term COVID-19 sequelae. Metabolic complications of obesity that are also reported to be independently associated with COVID-19 severity and poor outcomes should also be screened, and alterations should be minimized. Particular attention should be finally paid to the negative lifestyle changes potentially induced by lockdowns and general restrictions during the pandemic, which may worsen obesity, its metabolic complications and the general obesity-associated non-communicable disease risk, with potential negative impact both in the short- and long-term.
Conflict of interest
The authors declare that they have no competing interests for the content of this paper.
Acknowledgements
The authors gratefully acknowledge the useful discussions on the manuscript with drs Joao Breda (Division of Country Health Policies and Systems, WHO Regional Office for Europe) and Kremlin Wickramasinghe (WHO European Office for Prevention and Control of Noncommunicable Diseases, WHO Regional Office for Europe, Moscow, Russian Federation) and their thoughtful comments.
References
- 1.Bouadma L., Lescure F.X., Lucet J.C., Yazdanpanah Y., Timsit J.F. Severe SARS-CoV-2 infections: practical considerations and management strategy for intensivists. Intensive Care Med. 2020;46(4):579–582. doi: 10.1007/s00134-020-05967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomes F., Schuetz P., Bounoure L., Austin P., Ballesteros-Pomar M., Cederholm T., et al. ESPEN guidelines on nutritional support for polymorbid internal medicine patients. Clin Nutr. 2018;37(1):336–353. doi: 10.1016/j.clnu.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Volkert D., Beck A.M., Cederholm T., Cruz-Jentoft A., Goisser S., Hooper L., et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10–47. doi: 10.1016/j.clnu.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 8.Cederholm T., Barazzoni R., Austin P., Ballmer P., Biolo G., Bischoff S.C., et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36(1):49–64. doi: 10.1016/j.clnu.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Schneider S.M., Veyres P., Pivot X., Soummer A.M., Jambou P., Filippi J., et al. Malnutrition is an independent factor associated with nosocomial infections. Br J Nutr. 2004;92(1):105–111. doi: 10.1079/BJN20041152. [DOI] [PubMed] [Google Scholar]
- 10.Calder P.C. Feeding the immune system. Proc Nutr Soc. 2013;72(3):299–309. doi: 10.1017/S0029665113001286. [DOI] [PubMed] [Google Scholar]
- 11.Arora N.S., Rochester D.F. Respiratory muscle strength and maximal voluntary ventilation in undernourished patients. Am Rev Respir Dis. 1982;126(1):5–8. doi: 10.1164/arrd.1982.126.1.5. [DOI] [PubMed] [Google Scholar]
- 12.Heymsfield S.B., Bethel R.A., Ansley J.D., Gibbs D.M., Felner J.M., Nutter D.O. Cardiac abnormalities in cachectic patients before and during nutritional repletion. Am Heart J. 1978;95(5):584–594. doi: 10.1016/0002-8703(78)90300-9. [DOI] [PubMed] [Google Scholar]
- 13.Hiesmayr M., Frantal S., Schindler K., Themessl-Huber M., Mouhieddine M., Schuh C., et al. The patient- and nutrition-derived outcome risk assessment score (PANDORA): development of a simple predictive risk score for 30-day in-hospital mortality based on demographics, clinical observation, and nutrition. PloS One. 2015;10(5) doi: 10.1371/journal.pone.0127316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singer P., Blaser A.R., Berger M.M., Alhazzani W., Calder P.C., Casaer M.P., et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 15.Souza T.T., Sturion C.J., Faintuch J. Is the skeleton still in the hospital closet? A review of hospital malnutrition emphasizing health economic aspects. Clin Nutr. 2015;34(6):1088–1092. doi: 10.1016/j.clnu.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Allard L., Ouedraogo E., Molleville J., Bihan H., Giroux-Leprieur B., Sutton A., et al. Malnutrition: percentage and association with prognosis in patients hospitalized for coronavirus disease 2019. Nutrients. 2020;12(12) doi: 10.3390/nu12123679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu X., Deng H., Wang Y., Chen L., Gu X., Wang X. Predictive value of the prognostic nutritional index for the severity of coronavirus disease 2019. Nutrition. 2020;84:111123. doi: 10.1016/j.nut.2020.111123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li G., Zhou C.L., Ba Y.M., Wang Y.M., Song B., Cheng X.B., et al. Nutritional risk and therapy for severe and critical COVID-19 patients: a multicenter retrospective observational study. Clin Nutr. 2020;40(4):2154–2161. doi: 10.1016/j.clnu.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li T., Zhang Y., Gong C., Wang J., Liu B., Shi L., et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020;74(6):871–875. doi: 10.1038/s41430-020-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pironi L., Sasdelli A.S., Ravaioli F., Baracco B., Battaiola C., Bocedi G., et al. Malnutrition and nutritional therapy in patients with SARS-CoV-2 disease. Clin Nutr. 2020;40(3):1330–1337. doi: 10.1016/j.clnu.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Y., Ye J., Chen M., Jiang C., Lin W., Lu Y., et al. Malnutrition prolongs the hospitalization of patients with COVID-19 infection: a clinical epidemiological analysis. J Nutr Health Aging. 2021;25(3):369–373. doi: 10.1007/s12603-020-1541-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang P., He Z., Yu G., Peng D., Feng Y., Ling J., et al. The modified NUTRIC score can be used for nutritional risk assessment as well as prognosis prediction in critically ill COVID-19 patients. Clin Nutr. 2021;40(2):534–541. doi: 10.1016/j.clnu.2020.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendes A., Serratrice C., Herrmann F.R., Gold G., Graf C.E., Zekry D., et al. Clinical Nutrition; Edinburgh, Scotland): 2021. Nutritional risk at hospital admission is associated with prolonged length of hospital stay in old patients with COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Filippo L., De Lorenzo R., D’Amico M., Sofia V., Roveri L., Mele R., et al. COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: a post-hoc analysis of a prospective cohort study. Clin Nutr. 2020;40(4):2420–2426. doi: 10.1016/j.clnu.2020.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foldi M., Farkas N., Kiss S., Dembrovszky F., Szakacs Z., Balasko M., et al. Visceral adiposity elevates the risk of critical condition in COVID-19: a systematic review and meta-analysis. Obesity. 2021;29(3):521–528. doi: 10.1002/oby.23096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y., Lu Y., Huang Y.M., Wang M., Ling W., Sui Y., et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metab Clin Exp. 2020;113:154378. doi: 10.1016/j.metabol.2020.154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muscogiuri G., Pugliese G., Barrea L., Savastano S., Colao A. 2020. Commentary: Obesity: the "Achilles Heel" for COVID-19? Metabolism: Clinical and Experimental; p. 154251. 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma A., Garg A., Rout A., Lavie C.J. Association of obesity with more critical illness in COVID-19. Mayo Clin Proc. 2020;95(9):2040–2042. doi: 10.1016/j.mayocp.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh S., Bilal M., Pakhchanian H., Raiker R., Kochhar G.S., Thompson C.C. Impact of obesity on outcomes of patients with coronavirus disease 2019 in the United States: a multicenter electronic health records network study. Gastroenterology. 2020;159(6):2221–2225. doi: 10.1053/j.gastro.2020.08.028. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collard D., Nurmohamed N.S., Kaiser Y., Reeskamp L.F., Dormans T., Moeniralam H., et al. Cardiovascular risk factors and COVID-19 outcomes in hospitalised patients: a prospective cohort study. BMJ Open. 2021;11(2) doi: 10.1136/bmjopen-2020-045482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coppelli A., Giannarelli R., Aragona M., Penno G., Falcone M., Tiseo G., et al. Hyperglycemia at hospital admission is associated with severity of the prognosis in patients hospitalized for COVID-19: the pisa COVID-19 study. Diabetes Care. 2020;43(10):2345–2348. doi: 10.2337/dc20-1380. [DOI] [PubMed] [Google Scholar]
- 33.Racine-Brzostek S.E., Yang H.S., Jack G.A., Chen Z., Chadburn A., Ketas T.J., et al. Post convalescent SARS-CoV-2 IgG and neutralizing antibodies are elevated in individuals with poor metabolic health. J Clin Endocrinol Metab. 2021;106(5):e2025–e2034. doi: 10.1210/clinem/dgab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huizinga G.P., Singer B.H., Singer K. The collision of meta-inflammation and SARS-CoV-2 pandemic infection. Endocrinol. 2020;161(11) doi: 10.1210/endocr/bqaa154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daoust L., Pilon G., Marette A. Perspective: nutritional strategies targeting the gut microbiome to mitigate COVID-19 outcomes. Adv Nutr. 2021 doi: 10.1093/advances/nmab031. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barazzoni R., Bischoff S.C., Boirie Y., Busetto L., Cederholm T., Dicker D., et al. Sarcopenic obesity: time to meet the challenge. Clin Nutr. 2018;37(6 Pt A):1787–1793. doi: 10.1016/j.clnu.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 37.Guillet C., Masgrau A., Walrand S., Boirie Y. Impaired protein metabolism: interlinks between obesity, insulin resistance and inflammation. Obes Rev : Off J Int Ass Study Obes. 2012;13(Suppl 2):51–57. doi: 10.1111/j.1467-789X.2012.01037.x. [DOI] [PubMed] [Google Scholar]
- 38.Guillet C., Delcourt I., Rance M., Giraudet C., Walrand S., Bedu M., et al. Changes in basal and insulin and amino acid response of whole body and skeletal muscle proteins in obese men. J Clin Endocrinol Metab. 2009;94(8):3044–3050. doi: 10.1210/jc.2008-2216. [DOI] [PubMed] [Google Scholar]
- 39.Wall B.T., Cruz A.M., Otten B., Dunlop M.V., Fulford J., Porter C., et al. The impact of disuse and high-fat overfeeding on forearm muscle amino acid metabolism in humans. J Clin Endocrinol Metab. 2020;105(7) doi: 10.1210/clinem/dgaa184. [DOI] [PubMed] [Google Scholar]
- 40.Tardif N., Salles J., Guillet C., Tordjman J., Reggio S., Landrier J.F., et al. Muscle ectopic fat deposition contributes to anabolic resistance in obese sarcopenic old rats through eIF2alpha activation. Aging Cell. 2014;13(6):1001–1011. doi: 10.1111/acel.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y., Ding L., Zou X., Shen Y., Hu D., Hu X., et al. Visceral adiposity and high intramuscular fat deposition independently predict critical illness in patients with SARS-CoV-2. Obesity. 2020;28(11):2040–2048. doi: 10.1002/oby.22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bischoff S.C., Boirie Y., Cederholm T., Chourdakis M., Cuerda C., Delzenne N.M., et al. Towards a multidisciplinary approach to understand and manage obesity and related diseases. Clin Nutr. 2017;36(4):917–938. doi: 10.1016/j.clnu.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Atkins J.L., Wannamathee S.G. Sarcopenic obesity in ageing: cardiovascular outcomes and mortality. Br J Nutr. 2020;124(10):1102–1113. doi: 10.1017/S0007114520002172. [DOI] [PubMed] [Google Scholar]
- 44.Montano-Loza A.J., Angulo P., Meza-Junco J., Prado C.M., Sawyer M.B., Beaumont C., et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cache Sarcop Musc. 2016;7(2):126–135. doi: 10.1002/jcsm.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Aller C., Lara J., Stephan B.C.M., Donini L.M., Heymsfield S., Katzmarzyk P.T., et al. Sarcopenic obesity and overall mortality: results from the application of novel models of body composition phenotypes to the National Health and Nutrition Examination Survey 1999-2004. Clin Nutr. 2019;38(1):264–270. doi: 10.1016/j.clnu.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X., Xie X., Dou Q., Liu C., Zhang W., Yang Y., et al. Association of sarcopenic obesity with the risk of all-cause mortality among adults over a broad range of different settings: a updated meta-analysis. BMC Geriatr. 2019;19(1):183. doi: 10.1186/s12877-019-1195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fedele D., De Francesco A., Riso S., Collo A. Obesity, malnutrition, and trace element deficiency in the coronavirus disease (COVID-19) pandemic: an overview. Nutrition. 2021;81:111016. doi: 10.1016/j.nut.2020.111016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sahin E., Orhan C., Uckun F.M., Sahin K. Clinical impact potential of supplemental nutrients as adjuncts of therapy in high-risk COVID-19 for obese patients. Front Nutr. 2020;7:580504. doi: 10.3389/fnut.2020.580504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clemmensen C., Petersen M.B., Sorensen T.I.A. Will the COVID-19 pandemic worsen the obesity epidemic? Nat Rev Endocrinol. 2020;16(9):469–470. doi: 10.1038/s41574-020-0387-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization ROfE Data and statistics on obesity. http://wwweurowhoint/en/health-topics/noncommunicable-diseases/obesity/data-and-statistics. 2020
- 51.Barazzoni R., Gortan Cappellari G., Semolic A., Ius M., Zanetti M., Gabrielli A., et al. Central adiposity markers, plasma lipid profile and cardiometabolic risk prediction in overweight-obese individuals. Clin Nutr. 2019;38(3):1171–1179. doi: 10.1016/j.clnu.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 52.Krotkiewski M., Bjorntorp P., Sjostrom L., Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest. 1983;72(3):1150–1162. doi: 10.1172/JCI111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barazzoni R., Bischoff S.C., Breda J., Wickramasinghe K., Krznaric Z., Nitzan D., et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39(6):1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ammar A., Brach M., Trabelsi K., Chtourou H., Boukhris O., Masmoudi L., et al. Effects of COVID-19 home confinement on eating behaviour and physical activity: results of the ECLB-COVID19 international online survey. Nutrients. 2020;12(6) doi: 10.3390/nu12061583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bakaloudi D.R.J.D., Jayawardena R., Chourdakis M. The impact of COVID-19 lockdown on snacking habits, fast-food and alcohol consumption: a systematic review of the evidence. Clin Nutr. 2021 doi: 10.1016/j.clnu.2021.04.020. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le Brocq S., Clare K., Bryant M., Roberts K., Tahrani A.A., writing group form Obesity Uk, et al. Obesity and COVID-19: a call for action from people living with obesity. Lancet Diab Endocrinol. 2020;8(8):652–654. doi: 10.1016/S2213-8587(20)30236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robinson E., Boyland E., Chisholm A., Harrold J., Maloney N.G., Marty L., et al. Obesity, eating behavior and physical activity during COVID-19 lockdown: a study of UK adults. Appetite. 2021;156:104853. doi: 10.1016/j.appet.2020.104853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pellegrini M., Ponzo V., Rosato R., Scumaci E., Goitre I., Benso A., et al. Changes in weight and nutritional habits in adults with obesity during the "lockdown" period caused by the COVID-19 virus emergency. Nutrients. 2020;12(7):1–11. doi: 10.3390/nu12072016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chew H.S.J., Lopez V. Global impact of COVID-19 on weight and weight-related behaviors in the adult population: a scoping review. Int J Environ Res Publ Health. 2021;18(4) doi: 10.3390/ijerph18041876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Castaneda-Babarro A., Arbillaga-Etxarri A., Gutierrez-Santamaria B., Coca A. Physical activity change during COVID-19 confinement. Int J Environ Res Publ Health. 2020;17(18) doi: 10.3390/ijerph17186878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faulkner J., O'Brien W.J., McGrane B., Wadsworth D., Batten J., Askew C.D., et al. Physical activity, mental health and well-being of adults during initial COVID-19 containment strategies: a multi-country cross-sectional analysis. J Sci Med Sport. 2021;24(4):320–326. doi: 10.1016/j.jsams.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rogers N.T., Waterlow N.R., Brindle H., Enria L., Eggo R.M., Lees S., et al. Behavioral change towards reduced intensity physical activity is disproportionately prevalent among adults with serious health issues or self-perception of high risk during the UK COVID-19 lockdown. Front Publ Heal. 2020;8:575091. doi: 10.3389/fpubh.2020.575091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reyes-Olavarria D., Latorre-Roman P.A., Guzman-Guzman I.P., Jerez-Mayorga D., Caamano-Navarrete F., Delgado-Floody P. Positive and negative changes in food habits, physical activity patterns, and weight status during COVID-19 confinement: associated factors in the Chilean population. Int J Environ Res Publ Health. 2020;17(15) doi: 10.3390/ijerph17155431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carriedo A., Cecchini J.A., Fernandez-Rio J., Mendez-Gimenez A. COVID-19, psychological well-being and physical activity levels in older adults during the nationwide lockdown in Spain. Am J Geriatr Psychiatr : Off J Am Ass Geriatr Psychiatr. 2020;28(11):1146–1155. doi: 10.1016/j.jagp.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sepulveda-Loyola W., Rodriguez-Sanchez I., Perez-Rodriguez P., Ganz F., Torralba R., Oliveira D.V., et al. Impact of social isolation due to COVID-19 on health in older people: mental and physical effects and recommendations. J Nutr Health Aging. 2020;24(9):938–947. doi: 10.1007/s12603-020-1500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al-Musharaf S., McTernan P.G., Hussain S.D., Aleisa K.A., Alnaami A.M., Wani K., et al. Prevalence and indicators of vitamin B12 insufficiency among young women of childbearing age. Int J Environ Res Publ Health. 2020;18(1) doi: 10.3390/ijerph18010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.World Health Organization ROfE How to stay physically active during COVID-19 self-quarantine. https://wwweurowhoint/en/health-topics/disease-prevention/physical-activity/news/news/2020/3/how-to-stay-physically-active-during-covid-19-self-quarantine. 2020
- 68.Bakaloudi D.R.B.R., Bischoff S.C., Breda J., Wickramasinghe K., Chourdakis M. Impact of the first COVID-19 lockdown on body weight: a combined systematic review and a meta-analysis. Clin Nutr. 2021 doi: 10.1016/j.clnu.2021.04.015. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Luis Roman D.A., Izaola O., Primo Martin D., Gomez Hoyos E., Torres Torres B., Lopez Gomez J.J. Efecto del confinamiento por COVID-19 sobre la ganancia de peso corporal autorreportada en una muestra de pacientes obesos, Effect of lockdown for COVID-19 on self-reported body weight gain in a sample of obese patients. Nutr Hosp. 2020;37(6) doi: 10.20960/nh.03307. (in press) [DOI] [PubMed] [Google Scholar]
- 70.Fernandez-Rio J., Cecchini J.A., Mendez-Gimenez A., Carriedo A. Weight changes during the COVID-19 home confinement. Effects on psychosocial variables. Obes Res Clin Pract. 2020;14(4):383–385. doi: 10.1016/j.orcp.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 71.Flanagan E.W., Beyl R.A., Fearnbach S.N., Altazan A.D., Martin C.K., Redman L.M. The impact of COVID-19 stay-at-home orders on health behaviors in adults. Obesity. 2021;29(2):438–445. doi: 10.1002/oby.23066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abrams E.M., Szefler S.J. COVID-19 and the impact of social determinants of health. Lancet Respirat Med. 2020;8(7):659–661. doi: 10.1016/S2213-2600(20)30234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alberga A.S., Edache I.Y., Forhan M., Russell-Mayhew S. Weight bias and health care utilization: a scoping review. Prim Health Care Res Dev. 2019;20:e116. doi: 10.1017/S1463423619000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sutin A.R., Stephan Y., Terracciano A. Weight discrimination and risk of mortality. Psychol Sci. 2015;26(11):1803–1811. doi: 10.1177/0956797615601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Amicis R., Cancello R., Capodaglio P., Gobbi M., Brunani A., Gilardini L., et al. Patients with severe obesity during the COVID-19 pandemic: how to maintain an adequate multidisciplinary nutritional rehabilitation program? Obesity Facts. 2021:1–9. doi: 10.1159/000513283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dicker D., Bettini S., Farpour-Lambert N., Fruhbeck G., Golan R., Goossens G., et al. Obesity and COVID-19: the two sides of the coin. Obesity Facts. 2020;13(4):430–438. doi: 10.1159/000510005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bendavid I., Lobo D.N., Barazzoni R., Cederholm T., Coeffier M., de van der Schueren M., et al. The centenary of the Harris-Benedict equations: how to assess energy requirements best? Recommendations from the ESPEN expert group. Clin Nutr. 2020;40(3):690–701. doi: 10.1016/j.clnu.2020.11.012. [DOI] [PubMed] [Google Scholar]
- 78.Plauth M., Bernal W., Dasarathy S., Merli M., Plank L.D., Schutz T., et al. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38(2):485–521. doi: 10.1016/j.clnu.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jesus P., Achamrah N., Grigioni S., Charles J., Rimbert A., Folope V., et al. Validity of predictive equations for resting energy expenditure according to the body mass index in a population of 1726 patients followed in a Nutrition Unit. Clin Nutr. 2015;34(3):529–535. doi: 10.1016/j.clnu.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 80.Choban P., Dickerson R., Malone A., Worthington P., Compher C., American Society for P., et al. A.S.P.E.N. Clinical guidelines: nutrition support of hospitalized adult patients with obesity. J Parenter Enteral Nutr. 2013;37(6):714–744. doi: 10.1177/0148607113499374. [DOI] [PubMed] [Google Scholar]
- 81.McClave S.A., Taylor B.E., Martindale R.G., Warren M.M., Johnson D.R., Braunschweig C., et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of critical care medicine (SCCM) and American society for parenteral and enteral nutrition (A.S.P.E.N.) J Parenter Enteral Nutr. 2016;40(2):159–211. doi: 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]
- 82.Elke G., Hartl W.H., Kreymann K.G., Adolph M., Felbinger T.W., Graf T., et al. Clinical nutrition in critical care medicine - guideline of the German society for nutritional medicine (DGEM) Clin Nutr ESPEN. 2019;33:220–275. doi: 10.1016/j.clnesp.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 83.Bhasin S., Apovian C.M., Travison T.G., Pencina K., Moore L.L., Huang G., et al. Effect of protein intake on lean body mass in functionally limited older men: a randomized clinical trial. JAMA Int Med. 2018;178(4):530–541. doi: 10.1001/jamainternmed.2018.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grantz K.H., Rane M.S., Salje H., Glass G.E., Schachterle S.E., Cummings D.A. Disparities in influenza mortality and transmission related to sociodemographic factors within Chicago in the pandemic of 1918. Proc Natl Acad Sci USA. 2016;113(48):13839–13844. doi: 10.1073/pnas.1612838113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maruyama T., Fujisawa T., Suga S., Nakamura H., Nagao M., Taniguchi K., et al. Outcomes and prognostic features of patients with influenza requiring hospitalization and receiving early antiviral therapy: a prospective multicenter cohort study. Chest. 2016;149(2):526–534. doi: 10.1378/chest.14-2768. [DOI] [PubMed] [Google Scholar]
- 86.Papadimitriou-Olivgeris M., Gkikopoulos N., Wust M., Ballif A., Simonin V., Maulini M., et al. Predictors of mortality of influenza virus infections in a Swiss Hospital during four influenza seasons: role of quick sequential organ failure assessment. Eur J Intern Med. 2020;74:86–91. doi: 10.1016/j.ejim.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 87.Reyes L., Arvelo W., Estevez A., Gray J., Moir J.C., Gordillo B., et al. Population-based surveillance for 2009 pandemic influenza A (H1N1) virus in Guatemala, 2009. Influ Other Resp Virus. 2010;4(3):129–140. doi: 10.1111/j.1750-2659.2010.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Short K.R., Kedzierska K., van de Sandt C.E. Back to the future: lessons learned from the 1918 influenza pandemic. Front Cell Inf Microb. 2018;8:343. doi: 10.3389/fcimb.2018.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cederholm T., Jensen G.L., Correia M., Gonzalez M.C., Fukushima R., Higashiguchi T., et al. GLIM criteria for the diagnosis of malnutrition - a consensus report from the global clinical nutrition community. Clin Nutr. 2019;38(1):1–9. doi: 10.1016/j.clnu.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 90.Cederholm T., Jensen G.L., Correia M., Gonzalez M.C., Fukushima R., Higashiguchi T., et al. GLIM criteria for the diagnosis of malnutrition - a consensus report from the global clinical nutrition community. J Cach Sarcop Musc. 2019;10(1):207–217. doi: 10.1002/jcsm.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jensen G.L., Cederholm T., Correia M., Gonzalez M.C., Fukushima R., Higashiguchi T., et al. GLIM criteria for the diagnosis of malnutrition: a consensus report from the global clinical nutrition community. J Parenter Enteral Nutr. 2019;43(1):32–40. doi: 10.1002/jpen.1440. [DOI] [PubMed] [Google Scholar]
- 92.Barazzoni R., Sulz I., Schindler K., Bischoff S.C., Gortan Cappellari G., Hiesmayr M., et al. A negative impact of recent weight loss on in-hospital mortality is not modified by overweight and obesity. Clin Nutr. 2020;39(8):2510–2516. doi: 10.1016/j.clnu.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 93.Doehner W., Gerstein H.C., Ried J., Jung H., Asbrand C., Hess S., et al. Obesity and weight loss are inversely related to mortality and cardiovascular outcome in prediabetes and type 2 diabetes: data from the ORIGIN trial. Eur Heart J. 2020;41(28):2668–2677. doi: 10.1093/eurheartj/ehaa293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Donini L.M., Busetto L., Bauer J.M., Bischoff S., Boirie Y., Cederholm T., et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin Nutr. 2020;39(8):2368–2388. doi: 10.1016/j.clnu.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 95.Lin T.Y., Lim P.S., Hung S.C. Impact of misclassification of obesity by body mass index on mortality in patients with CKD. Kidn Int Repor. 2018;3(2):447–455. doi: 10.1016/j.ekir.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Prado C.M., Lieffers J.R., McCargar L.J., Reiman T., Sawyer M.B., Martin L., et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 97.Baumgartner R.N., Wayne S.J., Waters D.L., Janssen I., Gallagher D., Morley J.E. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12(12):1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 98.Lees C., Godin J., McElhaney J.E., McNeil S.A., Loeb M., Hatchette T.F., et al. Frailty hinders recovery from influenza and acute respiratory illness in older adults. J Infect Dis. 2020;222(3):428–437. doi: 10.1093/infdis/jiaa092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cederholm T., Barazzoni R. A year with the GLIM diagnosis of malnutrition - does it work for older persons? Curr Opin Clin Nutr Metab Care. 2021;24(1):4–9. doi: 10.1097/MCO.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 100.Zhang X., Tang M., Zhang Q., Zhang K.P., Guo Z.Q., Xu H.X., et al. The GLIM criteria as an effective tool for nutrition assessment and survival prediction in older adult cancer patients. Clin Nutr. 2020;40(3):1224–1232. doi: 10.1016/j.clnu.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 101.Deutz N.E., Bauer J.M., Barazzoni R., Biolo G., Boirie Y., Bosy-Westphal A., et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33(6):929–936. doi: 10.1016/j.clnu.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barazzoni R., Deutz N.E.P., Biolo G., Bischoff S., Boirie Y., Cederholm T., et al. Carbohydrates and insulin resistance in clinical nutrition: recommendations from the ESPEN expert group. Clin Nutr. 2017;36(2):355–363. doi: 10.1016/j.clnu.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 103.Yumuk V., Tsigos C., Fried M., Schindler K., Busetto L., Micic D., et al. European guidelines for obesity management in adults. Obesity facts. 2015;8(6):402–424. doi: 10.1159/000442721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Estruch R., Ros E. The role of the Mediterranean diet on weight loss and obesity-related diseases. Rev Endocr Metab Disord. 2020;21(3):315–327. doi: 10.1007/s11154-020-09579-0. [DOI] [PubMed] [Google Scholar]
- 105.Estruch R., Ros E., Salas-Salvado J., Covas M.I., Corella D., Aros F., et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 106.Gine-Garriga M., Roque-Figuls M., Coll-Planas L., Sitja-Rabert M., Salva A. Physical exercise interventions for improving performance-based measures of physical function in community-dwelling, frail older adults: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95(4):753–769 e3. doi: 10.1016/j.apmr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 107.Nieman D.C., Henson D.A., Austin M.D., Sha W. Upper respiratory tract infection is reduced in physically fit and active adults. Br J Sports Med. 2011;45(12):987–992. doi: 10.1136/bjsm.2010.077875. [DOI] [PubMed] [Google Scholar]
- 108.Nieman D.C. Exercise, upper respiratory tract infection, and the immune system. Med Sci Sports Exerc. 1994;26(2):128–139. doi: 10.1249/00005768-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 109.Filgueira T.O., Castoldi A., Santos L.E.R., de Amorim G.J., de Sousa Fernandes M.S., Anastacio W., et al. The relevance of a physical active lifestyle and physical fitness on immune defense: mitigating disease burden, with focus on COVID-19 consequences. Front Immunol. 2021;12:587146. doi: 10.3389/fimmu.2021.587146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hamer M., Kivimaki M., Gale C.R., Batty G.D. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: a community-based cohort study of 387,109 adults in UK. Brain Behav Immun. 2020;87:184–187. doi: 10.1016/j.bbi.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Calder P.C., Carr A.C., Gombart A.F., Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12(4) doi: 10.3390/nu12041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Manzanares W., Moreira E., Hardy G. Pharmaconutrition revisited for critically ill patients with coronavirus disease 2019 (COVID-19): does selenium have a place? Nutrition. 2021;81:110989. doi: 10.1016/j.nut.2020.110989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jolliffe D.A., Camargo C.A., Sluyter J.D., Aglipay M., Aloia J.F., Ganmaa D., et al. Vitamin D supplementation to prevent acute respiratory infections: systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021;9(5):276–292. doi: 10.1016/S2213-8587(21)00051-6. [DOI] [PubMed] [Google Scholar]
- 114.Marik P.E., Kory P., Varon J. Does vitamin D status impact mortality from SARS-CoV-2 infection? Medicine in drug discovery. 2020;6:100041. doi: 10.1016/j.medidd.2020.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu N., Sun J., Wang X., Zhang T., Zhao M., Li H. Low vitamin D status is associated with coronavirus disease 2019 outcomes: a systematic review and meta-analysis. Int J Infect Dis : IJID : Off Publ Int Soc Infect Dis. 2021;104:58–64. doi: 10.1016/j.ijid.2020.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Biesalski H.K. Obesity, vitamin D deficiency and old age a serious combination with respect to coronavirus disease-2019 severity and outcome. Curr Opin Clin Nutr Metab Care. 2021;24(1):18–24. doi: 10.1097/MCO.0000000000000700. [DOI] [PubMed] [Google Scholar]
- 117.Mossink J.P. Zinc as nutritional intervention and prevention measure for COVID-19 disease. BMJ Nutr Prev Heal. 2020;3(1):111–117. doi: 10.1136/bmjnph-2020-000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tinkov A.A., Ajsuvakova O.P., Filippini T., Zhou J.C., Lei X.G., Gatiatulina E.R., et al. Selenium and selenoproteins in adipose tissue physiology and obesity. Biomolecules. 2020;10(4) doi: 10.3390/biom10040658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim H., Hwang J.Y., Kim K.N., Ha E.H., Park H., Ha M., et al. Relationship between body-mass index and serum folate concentrations in pregnant women. Eur J Clin Nutr. 2012;66(1):136–138. doi: 10.1038/ejcn.2011.160. [DOI] [PubMed] [Google Scholar]
- 120.Lind M.V., Lauritzen L., Vestergaard H., Hansen T., Pedersen O., Kristensen M., et al. One-carbon metabolism markers are associated with cardiometabolic risk factors. Nutrition, metabolism, and cardiovascular diseases. Nutr Metabol Cardiovasc Dis. 2018;28(4):402–410. doi: 10.1016/j.numecd.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 121.Sun Y., Sun M., Liu B., Du Y., Rong S., Xu G., et al. Inverse association between serum vitamin B12 concentration and obesity among adults in the United States. Front Endocrinol. 2019;10:414. doi: 10.3389/fendo.2019.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.DeLuccia R., Clegg D., Sukumar D. The implications of vitamin D deficiency on COVID-19 for at-risk populations. Nutr Rev. 2021;79(2):227–234. doi: 10.1093/nutrit/nuaa092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tanaka M., Okada H., Hashimoto Y., Kumagai M., Nishimura H., Oda Y., et al. Relationship between metabolic syndrome and trunk muscle quality as well as quantity evaluated by computed tomography. Clin Nutr. 2020;39(6):1818–1825. doi: 10.1016/j.clnu.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 124.Andreasen A.S., Kelly M., Berg R.M., Moller K., Pedersen B.K. Type 2 diabetes is associated with altered NF-kappaB DNA binding activity, JNK phosphorylation, and AMPK phosphorylation in skeletal muscle after LPS. PloS One. 2011;6(9) doi: 10.1371/journal.pone.0023999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kalyani R.R., Metter E.J., Egan J., Golden S.H., Ferrucci L. Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care. 2015;38(1):82–90. doi: 10.2337/dc14-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kalyani R.R., Tra Y., Egan J.M., Ferrucci L., Brancati F. Hyperglycemia is associated with relatively lower lean body mass in older adults. J Nutr Health Aging. 2014;18(8):737–743. doi: 10.1007/s12603-014-0445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee C.G., Boyko E.J., Barrett-Connor E., Miljkovic I., Hoffman A.R., Everson-Rose S.A., et al. Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care. 2011;34(11):2381–2386. doi: 10.2337/dc11-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pupim L.B., Flakoll P.J., Majchrzak K.M., Aftab Guy D.L., Stenvinkel P., Ikizler T.A. Increased muscle protein breakdown in chronic hemodialysis patients with type 2 diabetes mellitus. Kidney Int. 2005;68(4):1857–1865. doi: 10.1111/j.1523-1755.2005.00605.x. [DOI] [PubMed] [Google Scholar]
- 129.Klonoff D.C., Messler J.C., Umpierrez G.E., Peng L., Booth R., Crowe J., et al. Association between achieving inpatient glycemic control and clinical outcomes in hospitalized patients with COVID-19: a multicenter, retrospective hospital-based analysis. Diabetes Care. 2021;44(2):578–585. doi: 10.2337/dc20-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pareek M., Singh A., Vadlamani L., Eder M., Pacor J., Park J., et al. Relation of cardiovascular risk factors to mortality and cardiovascular events in hospitalized patients with coronavirus disease 2019 (from the yale COVID-19 cardiovascular registry) Am J Cardiol. 2021;146:99–106. doi: 10.1016/j.amjcard.2021.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Allan P.J., Pironi L., Joly F., Lal S., Van Gossum A., Home Artificial N., et al. An international survey of clinicians' experience caring for patients receiving home parenteral nutrition for chronic intestinal failure during the COVID-19 pandemic. J Parenter Enteral Nutr. 2021;45(1):43–49. doi: 10.1002/jpen.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cawood A.L., Walters E.R., Smith T.R., Sipaul R.H., Stratton R.J. A review of nutrition support guidelines for individuals with or recovering from COVID-19 in the community. Nutrients. 2020;12(11) doi: 10.3390/nu12113230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Holdoway A. Nutritional management of patients during and after COVID-19 illness. Br J Community Nurs. 2020;25(Sup8):S6–S10. doi: 10.12968/bjcn.2020.25.Sup8.S6. [DOI] [PubMed] [Google Scholar]
- 134.Krznaric Z., Bender D.V., Laviano A., Cuerda C., Landi F., Monteiro R., et al. A simple remote nutritional screening tool and practical guidance for nutritional care in primary practice during the COVID-19 pandemic. Clin Nutr. 2020;39(7):1983–1987. doi: 10.1016/j.clnu.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Matras P., Klek S., Folwarski M., Zmarzly A., Bartoszewska L., Cebulski W., et al. Home medical nutrition during SARS-CoV-2 pandemic - a position paper. Clin Nutr ESPEN. 2020;38:196–200. doi: 10.1016/j.clnesp.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Viana Bagni U., da Silva Ribeiro K.D., Soares Bezerra D., Cavalcante de Barros D., de Magalhaes Fittipaldi A.L., Pimenta da Silva Araujo R.G., et al. Anthropometric assessment in ambulatory nutrition amid the COVID-19 pandemic: possibilities for the remote and in-person care. Clin Nutr ESPEN. 2021;41:186–192. doi: 10.1016/j.clnesp.2020.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Al-Sofiani M.E., Alyusuf E.Y., Alharthi S., Alguwaihes A.M., Al-Khalifah R., Alfadda A. Rapid implementation of a diabetes telemedicine clinic during the coronavirus disease 2019 outbreak: our protocol, experience, and satisfaction reports in Saudi arabia. J Diab Sci Techn. 2020;15(2):329–338. doi: 10.1177/1932296820947094. 1932296820947094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rouget A., Vardon-Bounes F., Lorber P., Vavasseur A., Marion O., Marcheix B., et al. Prevalence of malnutrition in coronavirus disease 19: the NUTRICOV study. Br J Nutr. 2020:1–8. doi: 10.1017/S0007114520005127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA neurology. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dennis M.S., Lewis S.C., Warlow C., Collaboration F.T. Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): a multicentre randomised controlled trial. Lancet. 2005;365(9461):764–772. doi: 10.1016/S0140-6736(05)17983-5. [DOI] [PubMed] [Google Scholar]
- 141.Li J.Y., Yu T., Chen G.C., Yuan Y.H., Zhong W., Zhao L.N., et al. Enteral nutrition within 48 hours of admission improves clinical outcomes of acute pancreatitis by reducing complications: a meta-analysis. PloS One. 2013;8(6) doi: 10.1371/journal.pone.0064926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hegerova P., Dedkova Z., Sobotka L. Early nutritional support and physiotherapy improved long-term self-sufficiency in acutely ill older patients. Nutrition. 2015;31(1):166–170. doi: 10.1016/j.nut.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 143.Deutz N.E., Matheson E.M., Matarese L.E., Luo M., Baggs G.E., Nelson J.L., et al. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: a randomized clinical trial. Clin Nutr. 2016;35(1):18–26. doi: 10.1016/j.clnu.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 144.Cawood A.L., Elia M., Stratton R.J. Systematic review and meta-analysis of the effects of high protein oral nutritional supplements. Ageing Res Rev. 2012;11(2):278–296. doi: 10.1016/j.arr.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 145.Joint F. 1998. WHO Expert Consultation on Human vitamin and mineral requirements. Vitamin and mineral requirements in human nutrition: report of a joint FAO/WHO expert consultation, Bangkok, Thailand; p. 2. [Google Scholar]
- 146.Wee A.K.H. COVID-19's toll on the elderly and those with diabetes mellitus - is vitamin B12 deficiency an accomplice? Med Hypotheses. 2021;146:110374. doi: 10.1016/j.mehy.2020.110374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bermano G., Meplan C., Mercer D.K., Hesketh J.E. Selenium and viral infection: are there lessons for COVID-19? Br J Nutr. 2021;125(6):618–627. doi: 10.1017/S0007114520003128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu J., Yang J., Chen J., Luo Q., Zhang Q., Zhang H. Vitamin D alleviates lipopolysaccharideinduced acute lung injury via regulation of the reninangiotensin system. Mol Med Rep. 2017;16(5):7432–7438. doi: 10.3892/mmr.2017.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kumar D., Gupta P., Letter Banerjee D. does vitamin D have a potential role against COVID-19? Aliment Pharmacol Ther. 2020;52(2):409–411. doi: 10.1111/apt.15801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Finzi E. Treatment of SARS-CoV-2 with high dose oral zinc salts: a report on four patients. Int J Infect Dis : IJID : Off Publi Int Soc Infect Dis. 2020;99:307–309. doi: 10.1016/j.ijid.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yao J.S., Paguio J.A., Dee E.C., Tan H.C., Moulick A., Milazzo C., et al. The minimal effect of zinc on the survival of hospitalized patients with COVID-19: an observational study. Chest. 2021;159(1):108–111. doi: 10.1016/j.chest.2020.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang J., Taylor E.W., Bennett K., Saad R., Rayman M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am J Clin Nutr. 2020;111(6):1297–1299. doi: 10.1093/ajcn/nqaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tan C.W., Ho L.P., Kalimuddin S., Cherng B.P.Z., Teh Y.E., Thien S.Y., et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19) Nutrition. 2020;79–80:111017. doi: 10.1016/j.nut.2020.111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Itelman E., Wasserstrum Y., Segev A., Avaky C., Negru L., Cohen D., et al. Clinical characterization of 162 COVID-19 patients in Israel: preliminary report from a large tertiary center. Isr Med Assoc J : IMAJ. 2020;22(5):271–274. [PubMed] [Google Scholar]
- 155.Chen L., Hu C., Hood M., Zhang X., Zhang L., Kan J., et al. A novel combination of vitamin C, curcumin and glycyrrhizic acid potentially regulates immune and inflammatory response associated with coronavirus infections: a perspective from system biology analysis. Nutrients. 2020;12(4) doi: 10.3390/nu12041193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Colunga Biancatelli R.M.L., Berrill M., Catravas J.D., Marik P.E. Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19) Front Immunol. 2020;11:1451. doi: 10.3389/fimmu.2020.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ding H., Deng W., Ding L., Ye X., Yin S., Huang W. Glycyrrhetinic acid and its derivatives as potential alternative medicine to relieve symptoms in nonhospitalized COVID-19 patients. J Med Virol. 2020;92(10):2200–2204. doi: 10.1002/jmv.26064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cheng R.Z. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med Drug Discov. 2020;5:100028. doi: 10.1016/j.medidd.2020.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hiedra R., Lo K.B., Elbashabsheh M., Gul F., Wright R.M., Albano J., et al. The use of IV vitamin C for patients with COVID-19: a case series. Expert Rev Anti-infect Ther. 2020;18(12):1259–1261. doi: 10.1080/14787210.2020.1794819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pereira M., Dantas Damascena A., Galvao Azevedo L.M., de Almeida Oliveira T., da Mota Santana J. Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2020:1–9. doi: 10.1080/10408398.2020.1841090. [DOI] [PubMed] [Google Scholar]
- 161.Notz Q., Herrmann J., Schlesinger T., Kranke P., Sitter M., Helmer P., et al. Vitamin D deficiency in critically ill COVID-19 ARDS patients. Clin Nutr. 2021 doi: 10.1016/j.clnu.2021.03.001. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Entrenas Castillo M., Entrenas Costa L.M., Vaquero Barrios J.M., Alcala Diaz J.F., Lopez Miranda J., Bouillon R., et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Annweiler C., Hanotte B., Grandin de l'Eprevier C., Sabatier J.M., Lafaie L., Celarier T. Vitamin D and survival in COVID-19 patients: a quasi-experimental study. J Steroid Biochem Mol Biol. 2020;204:105771. doi: 10.1016/j.jsbmb.2020.105771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Laesser C.I., Cumming P., Reber E., Stanga Z., Muka T., Bally L. Management of glucose control in noncritically ill, hospitalized patients receiving parenteral and/or enteral nutrition: a systematic review. J Clin Med. 2019;8(7) doi: 10.3390/jcm8070935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ojo O., Weldon S.M., Thompson T., Crockett R., Wang X.H. The effect of diabetes-specific enteral nutrition formula on cardiometabolic parameters in patients with type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Nutrients. 2019;11(8) doi: 10.3390/nu11081905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Aminian A., Fathalizadeh A., Tu C., Butsch W.S., Pantalone K.M., Griebeler M.L., et al. Association of prior metabolic and bariatric surgery with severity of coronavirus disease 2019 (COVID-19) in patients with obesity. Sur Obes Rel Dis: Off J Am Soc Bariat Sur. 2021;17(1):208–214. doi: 10.1016/j.soard.2020.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Scheen A.J., Marre M., Thivolet C. Prognostic factors in patients with diabetes hospitalized for COVID-19: findings from the CORONADO study and other recent reports. Diabetes Metabol. 2020;46(4):265–271. doi: 10.1016/j.diabet.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Amit M., Sorkin A., Chen J., Cohen B., Karol D., Tsur A.M., et al. Clinical course and outcomes of severe covid-19: a national scale study. J Clin Med. 2020;9(7) doi: 10.3390/jcm9072282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.de Watteville A., Genton L., Barcelos G.K., Pugin J., Pichard C., Heidegger C.P. Easy-to-prescribe nutrition support in the intensive care in the era of COVID-19. Clin Nutr ESPEN. 2020;39:74–78. doi: 10.1016/j.clnesp.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lemyze M., Mascot M., Orfi A. Enteral feeding and non-invasive ventilation during the COVID-19 crisis: a new snorkeling mask specially-fit to provide both concomitantly. Clin Nutr. 2020;39(12):3852–3853. doi: 10.1016/j.clnu.2020.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lemyze M., Courageux N., Maladobry T., Arumadura C., Pauquet P., Orfi A., et al. Implications of obesity for the management of severe coronavirus disease 2019 pneumonia. Crit Care Med. 2020;48(9):e761–e767. doi: 10.1097/CCM.0000000000004455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ferrando C., Suarez-Sipmann F., Mellado-Artigas R., Hernandez M., Gea A., Arruti E., et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. IntenCare Med. 2020;46(12):2200–2211. doi: 10.1007/s00134-020-06192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Thibault R., Seguin P., Tamion F., Pichard C., Singer P. Nutrition of the COVID-19 patient in the intensive care unit (ICU): a practical guidance. Crit Care. 2020;24(1):447. doi: 10.1186/s13054-020-03159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Reignier J., Thenoz-Jost N., Fiancette M., Legendre E., Lebert C., Bontemps F., et al. Early enteral nutrition in mechanically ventilated patients in the prone position. Crit Care Med. 2004;32(1):94–99. doi: 10.1097/01.CCM.0000104208.23542.A8. [DOI] [PubMed] [Google Scholar]
- 175.Al-Dorzi H.M., Arabi Y.M. Enteral nutrition safety with advanced treatments: extracorporeal membrane oxygenation, prone positioning, and infusion of neuromuscular blockers. Nutr Clin Pract : Off Publ Am Soc Parent Ent Nutr. 2021;36(1):88–97. doi: 10.1002/ncp.10621. [DOI] [PubMed] [Google Scholar]
- 176.Singer P., Pichard C., De Waele E. Practical guidance for the use of indirect calorimetry during COVID 19 pandemic. Clin Nutr Exp. 2020;33:18–23. doi: 10.1016/j.yclnex.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Whittle J., Molinger J., MacLeod D., Haines K., Wischmeyer P.E., Group L.-C.S. Persistent hypermetabolism and longitudinal energy expenditure in critically ill patients with COVID-19. Crit Care. 2020;24(1):581. doi: 10.1186/s13054-020-03286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cereda E., Guzzardella A., Klersy C., Belliato M., Pellegrini A., Sciutti F., et al. Clinical Nutrition; Edinburgh, Scotland): 2021. Early caloric deficit is associated with a higher risk of death in invasive ventilated COVID-19 patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Trethewey S.P., Brown N., Gao F., Turner A.M. Interventions for the management and prevention of sarcopenia in the critically ill: a systematic review. J Crit Care. 2019;50:287–295. doi: 10.1016/j.jcrc.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 180.Noreen S., Maqbool I., Madni A. Dexamethasone: therapeutic potential, risks, and future projection during COVID-19 pandemic. Eur J Pharmacol. 2021;894:173854. doi: 10.1016/j.ejphar.2021.173854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gianchandani R., Esfandiari N.H., Ang L., Iyengar J., Knotts S., Choksi P., et al. Managing hyperglycemia in the COVID-19 inflammatory storm. Diabetes. 2020;69(10):2048–2053. doi: 10.2337/dbi20-0022. [DOI] [PubMed] [Google Scholar]
- 182.Schindler A., Baijens L.W.J., Clave P., Degen B., Duchac S., Dziewas R., et al. ESSD commentary on dysphagia management during COVID pandemia. Dysphagia. 2020 doi: 10.1007/s00455-020-10194-z. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Carfi A., Bernabei R., Landi F., Gemelli Against C-P-ACSG Persistent symptoms in patients after acute COVID-19. Jama. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Norton A., Olliaro P., Sigfrid L., Carson G., Hastie C., Kaushic C., et al. Long COVID: tackling a multifaceted condition requires a multidisciplinary approach. Lancet Infect Dis. 2021;21(5):601–602. doi: 10.1016/S1473-3099(21)00043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.The L. Facing up to long COVID. Lancet. 2020;396(10266):1861. doi: 10.1016/S0140-6736(20)32662-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Morgan A. Long-term outcomes from critical care. Surgery. 2021;39(1):53–57. doi: 10.1016/j.mpsur.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Paneroni M., Simonelli C., Saleri M., Bertacchini L., Venturelli M., Troosters T., et al. Muscle strength and physical performance in patients without previous disabilities recovering from COVID-19 pneumonia. Am J Phys Med Rehab. 2021;100(2):105–109. doi: 10.1097/PHM.0000000000001641. [DOI] [PubMed] [Google Scholar]
- 189.Mandora E., Comini L., Olivares A., Fracassi M., Cadei M.G., Paneroni M., et al. Patients recovering from COVID-19 pneumonia in sub-acute care exhibit severe frailty: role of the nurse assessment. J Clin Nurs. 2021;7-8:952–960. doi: 10.1111/jocn.15637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mukaino M., Tatemoto T., Kumazawa N., Tanabe S., Katoh M., Saitoh E., et al. An affordable, user-friendly telerehabilitation system Assembled using existing technologies for individuals isolated with COVID-19: development and feasibility study. JMIR Rehab Ass Techn. 2020;7(2) doi: 10.2196/24960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Turcinovic M., Singson R., Harrigan M., Ardito S., Ilyas A., Sinvani L., et al. Physical Therapy for Hospitalized COVID-19 Patients in Isolation: feasibility and pilot implementation of telehealth for delivering individualized therapy. Arch Rehab Res Cli Trans. 2021:100113. doi: 10.1016/j.arrct.2021.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhu S., Zhang L., Xie S., He H., Wei Q., Du C., et al. Reconfigure rehabilitation services during the Covid-19 pandemic: best practices from Southwest China. Disabil Rehabil. 2021;43(1):126–132. doi: 10.1080/09638288.2020.1853828. [DOI] [PubMed] [Google Scholar]