Abstract
Animal-derived dietary protein ingestion and physical activity stimulate myofibrillar protein synthesis rates in older adults. We determined whether a non-animal-derived diet can support daily myofibrillar protein synthesis rates to the same extent as an omnivorous diet. Nineteen healthy older adults (age 66±1 y; BMI 24±1 kg·m−2; m=12, f=7) participated in a randomised, parallel-group, controlled trial during which they consumed a 3-day isocaloric high-protein (1.8 g·kg body mass−1·d−1) diet, where the protein was provided from predominantly (71%) animal (OMNI; n=9; m=6, f=3) or exclusively vegan (VEG; n=10; m=6, f=4; mycoprotein providing 57% of daily protein intake) sources. During the dietary control period participants conducted a daily bout of unilateral resistance-type leg extension exercise. Prior to the dietary control period participants ingested 400 mL deuterated water, with 50 mL doses consumed daily thereafter. Saliva samples were collected throughout to determine body water deuterium (2H) enrichments, and muscle samples were collected from rested and exercised muscle to determine daily myofibrillar protein synthesis rates. Deuterated water dosing resulted in body water 2H enrichments of ~0.78±0.03%. Daily myofibrillar protein synthesis rates were 13±8 (P=0.169) and 12±4% (P=0.016) greater in the exercised compared with rested leg (1.59±0.12 vs 1.77±0.12 %·d−1 and 1.76±0.14 vs 1.93±0.12 %·d−1) in OMNI and VEG groups, respectively. Daily myofibrillar protein synthesis rates did not differ between OMNI and VEG in either rested or exercised muscle (P>0.05). Over the course of a three day intervention, omnivorous or vegan derived dietary protein sources can support equivalent rested and exercised daily myofibrillar protein synthesis rates in healthy older adults consuming a high-protein diet.
Keywords: Sarcopenia, skeletal muscle, muscle protein synthesis, dietary protein, mycoprotein
Introduction
Ageing is associated with a progressive loss of skeletal muscle mass, termed sarcopenia (1). The association between muscle loss and increased incidence of falls, fractures, and metabolic disease indicates that the burden of our ageing society on health-care systems will increase dramatically in the coming decades (1; 2; 3; 4). Importantly, it also underlines the critical role that muscle mass and quality play in healthy ageing.
Muscle mass is regulated by the dynamic balance between daily muscle protein synthesis (MPS) and breakdown (MPB) rates. Ageing muscle displays a blunted responsiveness to the major acute anabolic stimuli; dietary protein ingestion (5; 6; 7; 8) and physical activity (particularly resistance exercise) (9; 10). This ‘anabolic resistance’ is now generally accepted as a key physiological mechanism responsible for age-related sarcopenia (11). Consuming dietary protein and performing physical activity in close temporal proximity can synergistically augment muscle protein synthesis rates in older adults (12; 13). Moreover, by increasing the per meal dose of protein (14; 15; 16), consuming high quality or fortified protein sources (17; 18), or strategic timing of protein ingestion (19), the muscle protein synthesis response to each meal can be augmented, though how this translates to daily muscle protein synthesis rates has received less attention.
Our understanding of how dietary protein and physical activity regulate muscle protein turnover in older adults is largely derived from studies using animal-derived protein sources. Indeed, studies evaluating the anabolic potential of animal versus non-animal-derived proteins typically compare meat, milk, casein and/or whey with soy (15; 20) or wheat (21) proteins only. Despite limited non-animal protein sources having been investigated, there is the widespread assumption that animal-derived proteins are more anabolic compared with plant-based proteins (22). Mycoprotein is a sustainably produced protein-rich whole food source, cultivated by the continuous flow fermentation of the filamentous fungus Fusarium venenatum, that is relatively high in protein (45% protein, 20.9% EAA, 24.6% NEAA, 9% BCAA, 3.9% leucine; Supplementary Table 1), high in fiber (25 %; two-thirds b-glucan and one-third chitin), and with a relatively low-energy-density (23). A full description of the nutritional properties of mycoprotein can be found in the review by Coelho and colleagues (2019) (24). We recently reported that an ingested bolus of mycoprotein is effectively digested and its amino acids absorbed (25), which results in a robust stimulation of muscle protein synthesis rates in rested and exercised muscle of young men (26). This suggests that mycoprotein may be a suitable alternative to animal or plant derived proteins to incorporate within the diet of older adults to support daily muscle protein synthesis rates.
In the present work, we applied an oral deuterated water approach (27) to determine whether a mycoprotein-based high-protein vegan diet could support rested and exercised daily muscle protein synthesis in older adults to the same extent as an isonitrogenous omnivorous protein diet. We hypothesised that in older adults consuming a high-protein diet, exercise would increase daily muscle protein synthesis rates compared with rested muscle, and by a similar extent irrespective of whether dietary protein was primarily obtained from animal or non-animal sources.
Methods
Participants
Nineteen healthy older adults (age: 66±1 y, BMI: 24±1 kg·m−2, 7 f and 12 m) were included in the study, and their characteristics are presented in Table 1. Participants (within the age range of 55–75 y) attended the laboratory for a medical screening, where height, body mass and blood pressure were measured, a fasting venous blood sample collected, and a general medical questionnaire was completed, all to assess their eligibility for participation and to ensure no adverse health conditions were present. Exclusion criteria included; a (family) history of deep vein thrombosis/cardiovascular disease, metabolic disorders (e.g. type-2 diabetes), musculoskeletal/orthopedic disorders, a body mass index of above 30 kg·m−2 or below 18 kg·m−2, hypertension (defined as >150/90 mmHg), participation in a structured resistance training program within 6 months prior to the study, any musculoskeletal injury of the legs within 12 months prior to the study, habitual use of anticoagulants, and consumption of any nutritional supplement shortly prior to the study. Blood markers were screened to exclude any participants who displayed evidence of impaired renal function, blood/clotting, autoimmune disorders or high glycated haemoglobin. Overall 34 participants were screened of which 1 was excluded based on the above criteria, and 14 either did not take part or did not complete the study. Participants completed the International Physical Activity Questionnaire (IPAQ) (28), and were provided with a diet diary to record habitual nutritional intake for 3 days (two weekdays and one weekend day). Detailed instructions from a member of the research team were provided to assist participants in collecting these data. Dietary analyses for the calculation of energy and macronutrient intakes were completed using specialized nutrition software (Nutritics Professional Nutritional Analysis Software; Swords, Co. Dublin). All subjects were informed of the nature and possible risks of the experimental procedures before providing written informed consent. This study was registered as a clinical trial with clinicaltrials.gov (NCT04325178). This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Sport and Health Sciences Ethics committee of the University of Exeter (180314/B/01).
Table 1.
Participants’ characteristics and work done during three consecutive days of unilateral resistance-type exercise (15 sets of 30 maximal isokinetic extension contractions).
| OMNI | VEG | P Value | |
|---|---|---|---|
| (n = 9) 3♀ / 6♂ |
(n = 10) 4♀ / 6♂ |
||
| Age (y) | 64±2 | 68±2 | P=0.21 |
| Body mass (kg) | 70±3 | 69±3 | P=0.86 |
| Height (cm) | 172±3 | 166±3 | P=0.17 |
| BMI (kg·m−2) | 23.6±0.6 | 25.1±0.7 | P=0.16 |
| Fat (% body mass) | 20±3 | 25±3 | P=0.27 |
| Lean mass (kg) | 56±4 | 52±4 | P=0.45 |
| Total work done (J) | 32,457±3,450 | 26,657±2,809 | P=0.30 |
Values represent mean ± SEM. OMNI, omnivorous diet; VEG, non-animal-derived diet.
Pre-testing
Following screening and admittance, all participants underwent a single pre-testing session, which took place ≥5 days before the start of the experimental period. Participants reported to the laboratory for familiarisation with the exercise equipment and unilateral resistance-type exercise protocol to be used, and to determine body composition (using Air Displacement Plethysmography; BodPod, Life Measurement, Inc. Concord, CA, USA). The exercise protocol consisted of 5 sets of 30 repetitions of maximal concentric isokinetic leg extension contractions, with 90 seconds rest between each set, on a Biodex System 3 isokinetic dynamometer (Biodex Medical Systems, Shirley, New York, USA) at a speed of 60° per second over a central 80° range of motion using their dominant leg. The dominant leg was selected as we deemed it likely that they would be better able to execute contractions in a leg that they were more accustomed to loading, and therefore create more mechanical tension (29). The exercise protocol was selected as we have previously shown that it stimulates muscle protein synthesis rates (30), allowing us to model the effect on rested tissue alongside exercised tissue. As the exercise is maximally concentric in nature, we would expect it to largely obviate any muscle damage, and therefore represent a protein accretional stimulus as opposed to one directed at correcting muscle damage (31). In addition, the lack of muscle damage permitted maximal exercise on three consecutive mornings, was practical for the population in question, and allowed us to stimulate cumulative myofibrillar protein synthesis rates over the three days. Verbal encouragement was provided throughout the familiarisation and experimental testing to encourage maximal effort. Work done (J) was recorded for each completed set, and fatigue was calculated as the percentage decrement in work done between the first and last set.
Experimental design
Participants were randomly assigned to two parallel-groups, A or B, by the lead investigator and completed a single condition. The study was an open-label design, with it not practically possible to blind the participants or the investigators. We took a theoretically optimal approach to stimulating daily muscle protein synthesis rates, providing a high protein diet distributed throughout the day, and with protein consumed post resistance exercise (32; 33; 34). This necessitated that we clamped both protein and energy intake across groups. Participants received one of two dietary interventions which differed with respect to the primary sources of dietary protein consumed: predominantly animal-derived protein sources including milk protein supplementation (OMNI; n=9), or exclusively vegan-derived protein sources including mycoprotein supplementation (VEG; n=10). Three participants were vegetarian, and were therefore allocated to the VEG group. A graphical representation of the experimental study design can be seen in Figure 1. All participants were asked to refrain from alcohol and caffeine consumption, and from strenuous exercise (except for the exercise prescribed within the protocol) for 2 days prior and throughout the experimental protocol, though to keep all other daily habitual activities as normal.
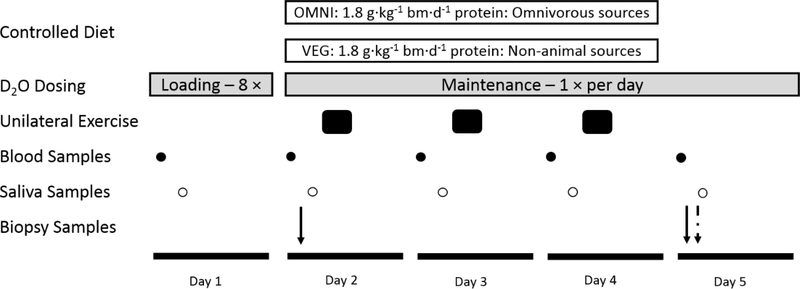
Figure 1.
Schematic representation of the experimental protocol. Nineteen healthy older adults (age, 66±1 y) consumed a 3-day fully controlled, eucaloric and high-protein (1.8 g·kg−1 body mass−1·d−1) diet, where the protein was provided predominantly from animal (OMNI; n=9) or exclusively non-animal (VEG; n=10) sources. During the dietary control period (Days 2–4) participants conducted a single bout of unilateral isokinetic knee extension exercise (5 x 30 contractions) each morning. On Day 1 participants loaded 400 mL deuterated water, with 50 mL maintenance doses consumed daily thereafter. Saliva (open circles) and blood samples (closed circles) were collected daily, and muscle biopsies were collected from both the rested (straight arrow) and exercised (dashed arrow) legs to determine daily myofibrillar protein synthesis rates.
Participants attended the laboratory each day for a 5-day (Mon-Fri) experimental period with days 2–4 (i.e. 3 days, Tue-Thu, inclusive) involving the dietary control. Each day of the 3-day dietary control period participants attended the laboratory to conduct a single bout of unilateral leg extension exercise as described above. Immediately afterwards volunteers received a protein-rich breakfast (~20 g protein), and were then provided with their food for the remainder of the day. To measure daily myofibrillar protein synthesis rates participants underwent a deuterium oxide dosing protocol (described below), in line with our previous work (27), and muscle biopsies were collected before commencing the controlled diet (i.e. Tue ~0800 h; single muscle biopsy from the [to-be] rested leg) and following (i.e. Fri ~0800 h; bilateral biopsies from the rested and exercised legs). We chose a duration of three days to reduce the burden on participants, in terms of sampling and dietary imposition, whilst also remaining confident that this allowed sufficient time to robustly measure daily muscle protein synthesis rates (35) and detect differences between groups.
Muscle biopsies were obtained under local anaesthesia, using a percutaneous Bergstrom biopsy needle technique (6), from the m. vastus lateralis ~15 cm above the patella and ~3 cm below the fascia. Muscle tissue was quickly assessed and any blood or non-muscle tissue was dissected and discarded. The muscle samples were immediately frozen in liquid nitrogen and stored at −80°C until further analysis.
Dietary intervention
Basal metabolic rate (BMR) was estimated using the Henry equations based on age, gender, and weight (36). The IPAQ was used to calculate a physical activity level (PAL) factor (37). Individual energy requirements were then calculated by multiplying the participant’s BMR by their PAL factor. Thereafter, an individual 3-day meal plan was designed for each participant with all food prepared, weighed, and packaged in-house in the Nutritional Physiology Unit’s research kitchen facility. Nutritional information for the two diets is provided in Table 2 and 3. Subjects consumed a diet containing 1.8 g of protein per kg of body mass (bm) per day (g·kg bm−1·d−1, with 23–26% of energy being provided by fat and 49–54% from carbohydrates in OMNI, and with 23–28% and 42–52% of energy being provided by fat and carbohydrates, respectively, in VEG (variation due to different energy requirements and the matching of protein intake). The meals were identical between the two groups, aside from meat or dairy providing the primary protein source in lunches and dinners for the OMNI group and this being replaced by Quorn Foods™ mycoprotein containing products or supplementary mycoprotein in the VEG group (provided by Marlow Foods Ltd, Stokesly, North Yorkshire, UK). The OMNI group received 39 g supplemental milk protein daily (31 g protein, 2 g carbohydrate, <1 g fat, 131 kcal) to drink prior to sleep, which was replaced with 70 g of supplementary mycoprotein (31 g protein, 7 g carbohydrate, 9 g fat, 232 kcal) to drink in the VEG group. A small amount of mycoprote in was also added to the breakfast in the VEG group to more closely equate the protein in the breakfast meal across groups. Breakfast was consumed within 1 hour of completing the unilateral resistance-type exercise, and provided 19±1 and 21±1 g protein per day in OMNI and VEG, respectively. The OMNI group consumed meals based on chicken, pork, and dairy. In the VEG group, this was substituted for Quorn deli ham, Quorn pieces, Quorn burgers, Quorn BBQ strips, Quorn sausages, and Quorn nuggets from their vegan range (which does not contain any egg products). A document and diary detailing the plan were provided to the subjects to log mealtimes and provide recipe information/instructions. Compliance to the intervention was ascertained verbally on each morning of the intervention during a detailed discussion with the researcher. There were no major deviations from the diet and no major incidents of GI distress reported by participants who completed the study. Three out of the four participants who withdrew from the intervention (all in VEG) did so because they disliked the diet with one citing bloating as the primary reason. Participants body mass was measured wearing light clothing at the start and end of the three-day control diet period (seca 703 column scale, seca GmbH & Co. KG, Hamburg, Germany). Each morning the researchers discussed with the participants any questions or issues that may have arisen, before the next day of food was provided.
Table 2.
The nutritional content of the participants’ habitual diets and of the intervention diets. In OMNI, participants consumed an omnivorous diet with the majority of their protein coming from animal-derived sources. In VEG, participants consumed a diet derived from non-animal sources with the majority of their protein coming from Quorn products and mycoprotein. Both groups received 1.8 g·kg bm−1·day−1 protein.
| OMNI | VEG | P Value | |
|---|---|---|---|
| (n = 9) 3♀ / 6♂ |
(n = 10) 4♀ / 6♂ |
||
| Habitual Diet | |||
| Energy (MJ·day−1 (kcal·day−1)) | 9.7±0.7 (2,314±164) | 8.2±0.6 (1,949±145) | 0.12 |
| Protein (g·day−1) | 81±6 | 82±6 | 0.90 |
| Protein (g·kg bm−1·day−1) | 1.17±0.08 | 1.27±0.10 | 0.42 |
| Carbohydrate (g·day−1) | 264±32 | 222±20 | 0.31 |
| Fat (g·day−1) | 94±6 | 72±8 | 0.04* |
| Fibre (g·day−1) | 27±2 | 31±2 | 0.22 |
| Intervention Diet | |||
| Energy (MJ·day−1 (kcal·day−1)) | 10±0.6 (2,382±139) | 9.6±0.6 (2,296±136) | 0.67 |
| Protein (g·day−1) | 127±5 | 125±6 | 0.79 |
| Protein (g·kg bm−1·day−1) | 1.8-±0.00 | 1.8-±0.00 | N/A |
| Carbohydrate (g·day−1) | 305±20 | 274±20 | 0.28 |
| Fat (g·day−1) | 65±4 | 67±4 | 0.60 |
| Fibre (g·day−1) | 32±2 | 68±3 | <0.0001* |
Values represent mean ± SEM. OMNI, omnivorous diet; VEG, non-animal-derived diet.
indicates a difference between groups (P<0.05).
Table 3.
Dietary intake, meal-by-meal, during the intervention. In OMNI, participants consumed an omnivorous diet with the majority of their protein coming from animal-derived sources. In VEG, participants consumed a diet derived from non-animal sources with the majority of their protein coming from Quorn products and mycoprotein. Both groups received 1.8 g·kg bm−1·day−1 protein.
| Energy | Protein | CHO | Fat | Fibre | |||||
|---|---|---|---|---|---|---|---|---|---|
| Kcal | kcal/kg | g | g/kg | g | g/kg | g | g/kg | g | |
| OMNI | |||||||||
| Breakfast | 500 ± 27 | 7.1 ± 0.3 | 19 ± 1 | 0.3 ± 0.0 | 75 ± 4 | 1.1 ± 0.0 | 11 ± 1 | 0.2 ± 0.0 | 11 ± 1 |
| Lunch | 493 ± 35 | 6.9 ± 0.4 | 33 ± 2 | 0.5 ± 0.0 | 67 ± 6 | 0.9 ± 0.1 | 9 ± 1 | 0.1 ± 0.0 | 4 ± 0 |
| Dinner | 917 ± 55 | 13.0 ± 0.5 | 40 ± 3 | 0.6 ± 0.0 | 91 ± 6 | 1.3 ± 0.1 | 41 ± 3 | 0.6 ± 0.0 | 11 ± 1 |
| Snacks | 473 ± 56 | 6.6 ± 0.5 | 35 ± 1 | 0.5 ± 0.0 | 73 ± 10 | 1.0 ± 0.1 | 3 ± 1 | 0.0 ± 0.0 | 6 ± 1 |
| Total | 2382 ± 139 | 33.6 ± 0.7 | 127 ± 5 | 1.8 ± 0.0 | 305 ± 20 | 4.3 ± 0.1 | 65 ± 4 | 0.9 ± 0.0 | 32 ± 2 |
| VEG | |||||||||
| Breakfast | 448 ± 26 | 6.5 ± 0.3 | 21 ± 1 | 0.3 ± 0.0 | 61 ± 4 | 0.9 ± 0.1 | 11 ± 1 | 0.2 ± 0.0 | 15 ± 1 |
| Lunch | 590 ± 35 | 8.5 ± 0.3 | 33 ± 2 | 0.5 ± 0.0 | 74 ± 5 | 1.1 ± 0.0 | 15 ± 1 | 0.2 ± 0.0 | 13 ± 1 |
| Dinner | 842 ± 58 | 12.1 ± 0.5 | 39 ± 3 | 0.6 ± 0.0 | 95 ± 7 | 1.4 ± 0.1 | 30 ± 2 | 0.4 ± 0.0 | 19 ± 1 |
| Snacks | 416 ± 46 | 5.9 ± 0.5 | 33 ± 1 | 0.5 ± 0.0 | 45 ± 8 | 0.6 ± 0.1 | 11 ± 1 | 0.2 ± 0.0 | 21 ± 1 |
| Total | 2296 ± 136 | 33.0 ± 0.9 | 125 ± 5 | 1.8 ± 0.0 | 274 ± 20 | 3.9 ± 0.2 | 67 ± 4 | 1.0 ± 0.0 | 68 ± 3 |
Values represent mean ± SEM. OMNI, omnivorous diet; VEG, non-animal-derived diet.
Deuterated water dosing protocol
The deuterated water dosing protocol was based on our previous work (27). Day 1 (Mon) of the experimental protocol acted as a D2O loading day where participants consumed 400 mL of 70% D2O separated over the day as 8 x 50 mL boluses (CK Isotopes Ltd, Leicestershire, UK). Upon arrival at the laboratory (0730 h) background saliva samples were collected before the first bolus of D2O was ingested. The first dose of D2O was consumed at ~0800 h with the remaining loading day doses being consumed every 1 hour (doses 2 and 3) and then every 1.5 h thereafter. Participants stayed at the laboratory until 4 out of the 8 loading day D2O doses had been consumed, with the remaining D2O doses being consumed at home under instruction of timings (i.e. leaving 1.5 h between each). Every day following the loading day participants consumed a maintenance dose of D2O (50 mL) upon waking (~0800 h). A small proportion of participants reported mild feelings of dizziness during the latter part of the loading day, which subsided by the following morning (4/19). At least 90 minutes (~09:30 h) after the daily D2O maintenance dose a daily saliva sample was collected using a cotton mouth swab (Celluron, Hartmann, Germany) which the participant lightly chewed for ~1 min until saturated with saliva. The saturated sponge was placed into an empty syringe where the swab was squeezed to release the saliva into a collection tube, and stored at −80°C until further analysis. The saliva samples were used to assess the body water 2H enrichment. To ensure uniformity and compliance with the D2O protocol, participants were provided with bottles of D2O labelled with the specific time and date to be taken, and were required to return bottles each day.
Body water deuterium enrichment
Body water deuterium enrichment was measured using the saliva samples collected daily throughout the study, at the University of Texas Medical Branch. A ThermoFisher Delta V Advantage Isotope Ratio mass spectrometer (IRMS) (Bremen, Germany), equipped with a Finnigan GasBench II (Thermo Fisher Scientific, Waltham, MA, USA), was used for stable hydrogen isotope ratio measurements. After uncapping a 12-mL Exetainer (Labco Limited, Lampeter, UK), 5 mg of activated charcoal (Thermo Fisher Scientific) and 200 mg of copper powder (Thermo Fisher Scientific) were introduced into the Exetainer followed with a platinum catalytic rod (Thermo Fisher Scientific). The activated charcoal and copper powder were added to remove any potential contaminants in the samples that might poison the platinum catalyst. After putting 200uL of sample into the Exetainer, the Exetainer was recapped and placed into the GasBench II and flushed with 2% H2 in helium for 7 min. The sample was allowed to equilibrate with the flushed gas at room temperature (~4–6 hours). At the end of the equilibration, an aliquot of the headspace in the Exetainer was injected into the Thermo Delta V Advantage mass spectrometer system for stable hydrogen isotope ratio measurement against the reference gas H2. A standard calibration curve was prepared using 99.9% deuterium-enriched water (Sigma-Aldrich, St. Louis, MO, USA), and the deuterium (2H) enrichment in duplicate saliva samples was determined (38).
Myofibrillar bound 2H alanine enrichments
Myofibrillar protein-enriched fraction was extracted from ~50 mg of wet weight muscle tissue by hand-homogenization on ice using a pestle in a standard homogenisation buffer (TRIS-HCL 50 mM, EDTA 1 mM, EGTA 1 mM, β-glycerophosphate 10 mM, NaF 50 mM, activated sodium orthovanadate 0.5mM, cOmplete protease inhibitor cocktail tablet (Roche Holding AG, Basel, Switzerland)) (7.5 μL/mg). The samples were centrifuged at 2,200 g for 10 min at 4°C, the pellet was then washed with 500 μl of homogenisation buffer, and centrifuged at 700 g for 10 min at 4°C. The myofibrillar protein was solubilized by adding 750 μl of 0.3 M NaOH and heating for 30 min at 50°C with samples being vortexed every 10 min. Samples were then centrifuged for 10 min at 10,000 g and 4°C, the supernatant containing the myofibrillar protein was kept and the collagen protein pellet was discarded. The myofibrillar proteins were precipitated by the addition of 500 μL of 1 M PCA and centrifuged at 700 g and 4°C for 10 min. Myofibrillar proteins were then washed with 70% ethanol twice and hydrolyzed overnight in 2 mL of 6 M HCL at 110°C. The free amino acids from the hydrolyzed myofibrillar protein pellet were dried under vacuum with a Speed-Vac rotary dryer (Savant Instruments, Farmingdale, NY, USA) for 3 h at 80°C radiant cover. The free amino acids were subsequently dissolved in 1.5 mL 25% acetic acid solution and passed over cation exchange AG 50W-X8 resin columns (mesh size: 100–200, ionic form: hydrogen; Bio-Rad Laboratories, Hercules, CA) and eluted with 6 M NH4OH. Following this, the purified amino acids were dried and derivatized to tert-butyldimethylsilyl derivatives via the addition of 50 μl MTBSTFA + 1% tert-butyl-dimethylchlorosilane and 50 μl acetonitrile, which was then vortex mixed and heated at 95°C for 40 min. The samples were transferred to a GC vial. The level of enrichment of D4-alanine was analyzed using a ThermoFisher Delta V Advantage Isotope Ratio mass spectrometer (IRMS) fitted with a Trace 1310 GC with an on-line high-temperature thermal conversion oven (HTC) at 1420°C. The sample (1μl) was injected in splitless mode at an injection port temperature of 250°C. The peaks were resolved on a 30m × 0.25mm ID × 0.25μm film Agilent Technologies DB-5 capillary column (temperature program: 110°C for 1 min; 10°C∙min-1 ramp to 180°C; 5°C∙min-1 ramp to 220°C; 20°C∙min-1 ramp to 300°C; hold for 2 min) prior to pyrolysis. Helium was used as the carrier gas with a constant flow of 1ml/min. Any amino acid eluting from the gas chromatograph was converted to H2 before entry into the IRMS. The enrichment of the alanine tracer was measured by monitoring the Ion masses 2 and 3 to determine the 2H/1H ratios in the samples and referenced to the calibration curve. The calibration curve consisted of a series of known concentrations of d4-alanine and was applied to assess both the linearity of the mass spectrometer and to control for the loss of tracer. The isotopic abundances were expressed as the delta notation, δ2H per mil (‰) deviation from VSMOW (Vienna Standard Mean Ocean Water) standard (38). Values of delta per mil given by the IRMS were transformed into MPE.
Calculations
Myofibrillar fractional synthesis rates (FSR) were calculated based on the incorporation of the mean body water deuterium enrichment over the 3-day intervention as a precursor pool into myofibrillar bound proteins. We and others have previously shown the body water deuterium pool is a valid precursor pool for the calculation of myofibrillar protein synthesis rates (corrected by a factor of 3.7 based on deuterium labelling of alanine during de novo synthesis) which shows excellent agreement with either plasma or muscle free [2H4] alanine as alternative precursor pool selections (27; 32; 39). FSR was calculated using the standard precursor-product method and expressed as daily rates as follows:
where Em1 and Em2 are the myofibrillar muscle protein-bound enrichments pre- (one leg only) and post- (either the rested or exercised leg) the dietary intervention. Eprecursor represents mean body water deuterium enrichment corrected by a factor of 3.7. t represents the time between biopsies (i.e. 3 days).
Statistics
A two-sided power analysis based on previous research (32) showed that n= 8 per groups was sufficient to detect expected differences in myofibrillar protein synthesis rates between rested and exercised legs when using a two-factor ANOVA (P < 0.05, 95% power, f = 1.68; G*power version 3.1.9.2). Our primary measure was myofibrillar protein synthesis rates, with all other measures representing secondary measures. All data are presented as mean ± SEM and all statistical analyses were conducted in GraphPad Prism version 7.0 (GraphPad Software, San Diego, CA, USA). An independent samples t-test was used to compare each of the participants’ characteristics across groups. A two-way (OMNI vs VEG and time [pre-post]) ANOVA was used to compare body mass during the nutritional intervention. A two-way (OMNI vs VEG and time [days 1–4]) ANOVA was used to compare changes in body water deuterium enrichments. A three-way (OMNI vs VEG, pre vs post and rested vs exercised leg) mixed-effects model was used to compare myofibrillar protein-bound [2H] alanine enrichments. A two-way (OMNI vs VEG and rested vs exercised leg) ANOVA, independent samples t-tests, and paired t-tests were used to compare myofibrillar protein synthesis rates. When a significant interaction was found, Sidak or Tukey post hoc tests were applied to locate individual differences. Statistical significance was set at P<0.05.
Results
Participants’ characteristics and daily exercise protocol
No differences in age, weight, height, BMI, or body composition were detected between groups (all P>0.05) and groups were also well balanced for sex (Table 1). Data relating to work done during the exercise protocol are displayed in Table 1. No differences in total work performed during the experimental resistance exercise bouts (32,547±3,450 J in OMNI vs 27,851±2,809 J in VEG; P=0.302), or in fatigue during each trial (all P>0.05) or over the week (P=0.392) were detected between groups. Total work done was lower on day 2 than day 3 (P=0.026), with no differences detected between days 1 and 2 (P=0.503), or 1 and 3 (P=0.934). Body mass did not change in response to the nutritional intervention in either group (P=0.703 and P=0.175 in OMNI and VEG, respectively).
Nutritional intervention
Habitual diet did not differ between groups for energy intake, protein intake or carbohydrate intake (all P>0.05), although fat intake was higher in the OMNI group than VEG (P=0.039; Table 2). Energy intake did not change between participants’ habitual diets and the diet they received during the intervention (P=0.142). Daily protein intake was higher (by design) during the intervention diet than in participants’ habitual diets (1.8±0.0 vs 1.2±0.1 g.day−1, respectively; P<0.0001). Daily carbohydrate intake was also higher during the intervention diet than in participants’ habitual diets (P=0.037). Time and interaction effects were detected (both P<0.05) such that fat intake decreased from habitual levels during the intervention diet in the OMNI group only (P<0.0001).
During the intervention diet participants consumed 10±0.6 and 9.6±0.6 MJ (0.14±0.00 and 0.14±0.00 MJ/kg; 2382±139 and 2296±137 kcal) per day in OMNI and VEG, respectively, with no differences between groups (P=0.667). By design, daily protein intake (i.e. 127±5 and 125±5 g per day, respectively) was identical between groups during the intervention. Participants consumed 305±20 and 274±20 g carbohydrate, and 65±4 and 67±4 g fat per day in OMNI and VEG, respectively, with no differences between groups (both P>0.05). Fibre intake was higher in the VEG intervention diet than the OMNI intervention diet (68±3 g vs 32±2 g; P<0.0001) as a result of the high natural fibre content of mycoprotein (Table 3).
In VEG, of the 125±6 g protein consumed per day, 71±2 g was derived from mycoprotein (30±2 g from mycoprotein within 329±23 g of Quorn products, and 41±1 g protein from supplementary isolated mycoprotein) corresponding to 57±1 % total protein intake. Remaining protein was provided by wheat and potato protein in Quorn products, and from the protein present in the other elements of the diet. Overall, Quorn products provided 50±3 g daily protein and accounted for 39±2 % total daily protein intake.
In OMNI, of the 127±5 g protein consumed per day, 90±3 g was provided by animal-derived sources and 38±3 g from non-animal sources, corresponding to 71±1 % and 29±1 % from animal and non-animal-derived sources, respectively. Meat products provided 40±0 g and dairy products (including the milk protein supplement) provided 50±3 g protein per day, corresponding to 32±1 and 39±1 % of total protein, respectively. The milk protein supplement alone provided 31±0 g protein per day, 25±1 % of total protein.
Body water deuterium enrichments
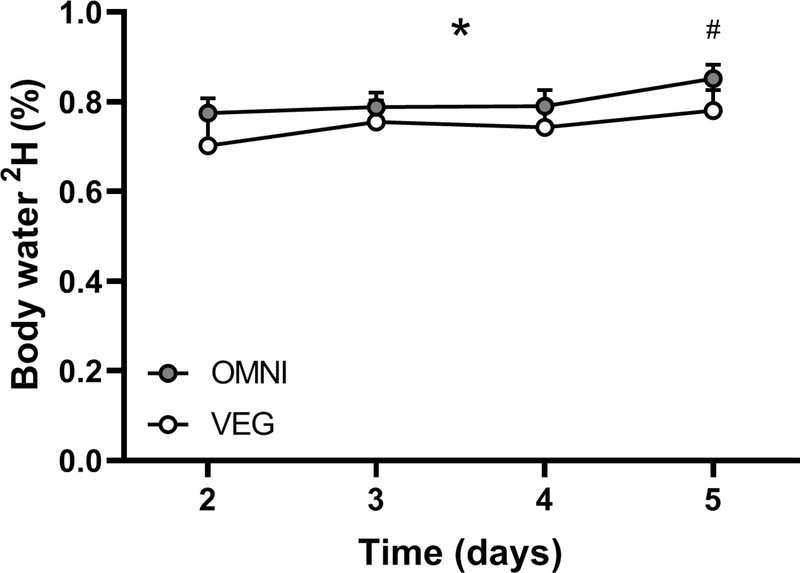
Saliva deuterium enrichments throughout the experimental protocol are depicted in Figure 2. From baseline (i.e. background) enrichments of 0.00044±0.00017 and 0.00030±0.00010% in OMNI and VEG, respectively, body water deuterium enrichments increased (P<0.0001; effect of time) and reached 0.77±0.03, 0.79±0.03, 0.79±0.04 and 0.85±0.03% on days 2–5 in OMNI, and 0.70±0.06, 0.75±0.05, 0.74±0.05 and 0.78±0.05% on days 2–5 in VEG, with no differences between groups (treatment and treatment × time interaction both P>0.05).
Figure 2.
Saliva deuterium enrichments over time during oral deuterated water dosing in nineteen healthy older adults (age, 66±1 y) consuming a 3-day fully controlled eucaloric high-protein (1.8 g·kg bm−1·d−1) diet, where the protein was provided predominantly from animal (OMNI; n=9) or exclusively non-animal (VEG; n=10) sources. During the dietary control period (Day 2–4) participants conducted a single bout of maximal unilateral concentric isokinetic knee extension exercise (5 x 30 contractions) each morning. On Day 1 participants were loaded with a total of 400 mL deuterated water, with 50 mL maintenance doses consumed daily thereafter. Data were analysed with mixed-effects ANOVA. Values are means, with their standard errors represented by vertical bars. * indicates a main effect of time (P<0.05). # indicates a significant difference to preceding time points (P<0.05). Treatment × time interaction effect; P= 0.5565, Treatment; P= 0.3847, Time; P= 0.0026.
Daily myofibrillar protein synthesis rates
Myofibrillar protein-bound [2H] alanine enrichments increased over time (P<0.0001) and to a greater extent in the exercised compared with control leg (time x leg interaction; P=0.015). Myofibrillar protein-bound [2H] alanine enrichments increased in the OMNI group by 358±76 % (from 0.058±0.014 to 0.206±0.027 MPE) in rested and 394±74 % (from 0.058 ±0.014 to 0.216±0.023 MPE) in exercised muscle, and in the VEG group by 263±42 % (from 0.070±0.014 to 0.229±0.0.031 MPE) in rested and 299±44 % (from 0.070±0.014 to 0.235±0.028 MPE) in exercised muscle, with no differences between groups (all OMNI vs VEG interactions; P>0.05)
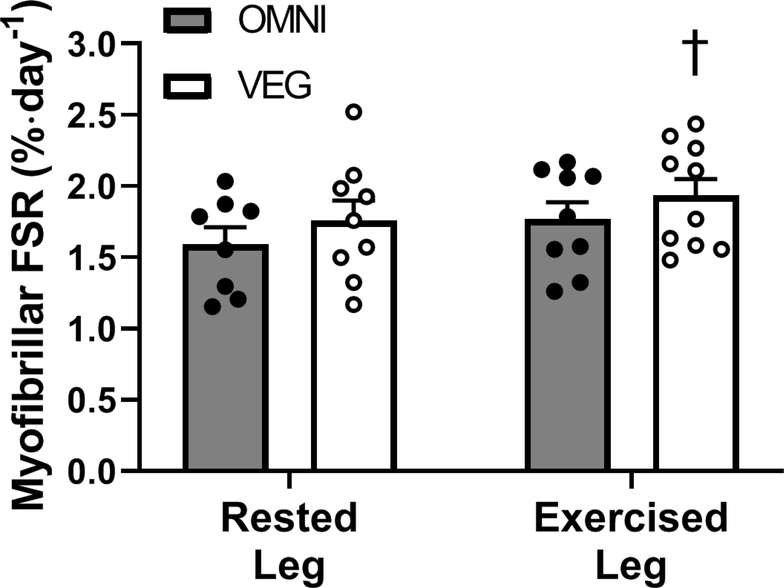
Saliva deuterium enrichments were used as a precursor pool and myofibrillar protein-bound [2H] alanine enrichments as the product to calculate daily myofibrillar fractional synthetic rates (FSRs) (Figure 3). Daily myofibrillar FSRs were 13±8 (P=0.169) and 12±4% (P=0.016) greater in the exercised compared with rested leg (1.59±0.12 vs 1.77±0.12 %·d−1 and 1.75±0.14 vs 1.93±0.12 %·d−1) in OMNI and VEG groups, respectively. Daily myofibrillar protein synthesis rates did not differ between groups in either rested (P=0.38) or exercised (P=0.33) muscle.
Figure 3.
Daily free-living myofibrillar protein fractional synthesis rates (FSRs) calculated from the body water deuterium precursor pool in nineteen healthy older adults (age, 66±1 y) consuming a 3-day fully controlled eucaloric high-protein (1.8 g·kg bm−1·d−1) diet, where the protein was provided predominantly from animal (OMNI; n=9) or exclusively non-animal (VEG; n=10) sources, in rested and exercised (single bout of 5 x 30 maximal unilateral isokinetic knee extension contractions on three consecutive days) muscle. Data were analysed with two-way ANOVA, with paired t-tests used to detect differences between rested and exercised legs in each respective groups. Values are means, with their standard errors represented by vertical bars. † indicates an effect of exercise in the VEG groups (P<0.05). Treatment × exercise interaction effect; P= 0.9917, treatment; P= 0.1874, Exercise; P= 0.1632. OMNI paired t-test, rested leg vs exercised leg, P= 0.1694. VEG paired t-test, rested leg vs exercised leg, P= 0.0162†.
Discussion
The present study demonstrates that performing a single bout of unilateral knee extensor resistance exercise daily for a 3-day period modestly stimulates daily myofibrillar protein synthesis rates compared with the rested control leg in older adults consuming a high-protein (1.8 g·kg bm−1·d−1) diet. Importantly, we report the novel finding that daily myofibrillar protein synthesis rates in both rested and exercised muscle of older adults during the dietary intervention period were equivalent regardless of whether dietary protein was obtained primarily from animal or exclusively from non-animal sources.
The continuous infusion of stable isotopically labelled amino acids into human volunteers to measure hour-to-hour muscle protein synthesis rates has revealed important insights concerning the physiological aetiology of age-related sarcopenia. Specifically, a failure of senescent muscle tissue to respond appropriately to the anabolic properties of bolus dietary protein ingestion (‘anabolic resistance’) is now widely accepted as a key physiological mechanism responsible for sarcopenia (5; 11; 40). Such experimental approaches have also demonstrated that anabolic resistance can effectively be overcome (or compensated for) on a meal-by-meal basis by consuming higher amounts of protein (14; 41) and/or consuming protein in close temporal proximity to resistance-type exercise (12; 41). However, such studies have relied on single time point measurements made over only a few hours and confined to laboratory conditions. Fewer data are available exploring whether increased dietary protein intake with concurrent physical activity in older adults translates to improvements in muscle protein synthesis rates throughout multiple days (including basal, postprandial and overnight periods), and thus encompassing all free-living influences (e.g. multiple meals, habitual physical activity, diurnal metabolic fluctuations, sleep etc.). In the present work, we applied a deuterated water stable isotope approach in older adults consuming a fully controlled high-protein (1.8 g·kg bm−1·d−1) diet and daily unilateral resistance exercise (with the non-exercised leg serving as a rested, within-subject control) to determine daily and free-living myofibrillar protein synthesis rates over a 3-day experimental period. In agreement with our previous work in young adults (27), we observed the deuterated water dosing regimen rapidly increased body water deuterium enrichments to near steady-state levels of ~0.78%, with a slight increase over time. The steady-state enrichment of body water served as a precursor for the calculation of daily myofibrillar protein synthesis rates from muscle biopsy samples (Figure 2). We report a single bout of unilateral resistance exercise performed daily each morning for 3 days increased daily myofibrillar protein synthesis rates by ~13% in healthy older men and women (Figure 3), though this effect only reached statistical significance in the vegan group (though numerically similar across groups).
The absence of myofibrillar protein synthesis data collected under habitual (i.e. lower; 1.2 g·kg bm−1·d−1 for the present volunteers) protein intake conditions precludes us from confirming that the high protein dietary intervention per se (i.e. irrespective of protein source) had a stimulatory effect. However, recent work has reported that higher protein diets in the absence of exercise (1.8 vs 1.0 g·kg bm−1·d−1; achieved via protein supplementation, and in line with the intervention vs habitual intakes of the present volunteers) elevates daily, free-living myofibrillar protein synthesis rates in older adults (42). Further, such studies have also demonstrated that resistance exercise can elevate daily muscle protein synthesis rates across a range of low-moderate or protein supplemented protein intakes (1.0–1.8 g·kg bm−1·d−1) in older adults of varying health statuses (42; 43; 44; 45). Our data, therefore, support and extend on these observations by demonstrating that daily resistance exercise retains the capacity to augment daily myofibrillar protein synthesis rates (though perhaps more modestly) in healthy older adults consuming a strategically timed (i.e. protein consumed directly after exercise, distributed throughout the day and before sleep (19; 46; 47)) high-protein diet designed to nutritionally maximize daily myofibrillar protein synthesis rates. Collectively, therefore, the data indicate that chronic maintenance of higher daily muscle protein synthesis rates mechanistically underpin the cross-sectional and longitudinal observational studies that reliably show more active (48; 49; 50) and/or higher protein consuming (at least above the currently accepted RDA/RDIs) (51; 52) older adults experience slower rates of annual muscle loss.
Aside from total daily (or per meal) dietary protein intake, an important contemporary research focus is from where dietary protein should (or could) be obtained. Government and societal priorities are increasing the demand to reduce animal-derived protein consumption in favour of sustainable alternatives (53). This is particularly pertinent for older adults where the scientific consensus is advocating for a near 50% increase in the UK/US RDI for dietary protein (11; 54; 55). Limited comparisons to date have suggested that, on a gram-for-gram basis, plant-based protein sources are inferior to animal-derived protein sources with respect to their capacity to stimulate muscle protein synthesis rates upon ingestion, attributed to their typically lower leucine contents (15; 20; 21). This implies that more total protein would be required within a vegan diet to support equivalent daily muscle protein synthesis rates, a circumstance itself that has implications for environmental sustainability and dietary feasibility. Indeed, by supplementing with more acutely anabolic proteins (whey vs collagen) it has been shown that daily muscle protein synthesis rates can be augmented to a greater degree, even under isonitrogenous conditions (42). We have recently shown that the fungal-derived protein source, mycoprotein, robustly stimulates muscle protein synthesis rates in young men. In the present work, we therefore hypothesized that the incorporation of mycoprotein within a vegan, high-protein diet, would support daily myofibrillar protein synthesis rates to the same extent as a protein-matched diet based (more typically) upon animal-derived protein consumption in older men and women. In support of this hypothesis, comparable daily free-living rates of myofibrillar protein synthesis between omnivorous and vegan diets were observed in both rested (1.59 vs 1.76 %, respectively) and exercised (1.77 vs 1.93 %, respectively) muscle (Figure 3). Our data therefore provide the proof-of-concept that higher protein vegan diets can be adopted in healthy older adults without compromising rested myofibrillar protein synthesis rates, implying vegan diets can be equivalently capable of supporting the maintenance of muscle mass during ageing. Further, our data also indicate that for older adults living more active lifestyles and/or participating in structured (resistance) exercise training, implementing a higher protein vegan diet would not compromise prolonged muscle tissue adaptive responses. We therefore show that non-animal derived dietary proteins (the vast majority of which have not been investigated in relation to their impact on muscle protein synthesis) are not necessarily inferior in their capacity to stimulate daily myofibrillar protein synthesis rates when incorporated into the daily diet, even in older adults. Our work also captures the total muscle anabolism achieved with two divergent diets in a free-living scenario over multiple days, thereby incorporating the diurnal variation and multiple feeding-fasting cycles that are potentially missed by intravenous isotope infusion studies. The necessarily short-term nature of such metabolic studies as presently reported (i.e. 3 days) means we cannot rule out the possibility that a longer duration of study (or increased statistical power) may have yielded significant differences across groups. However, it is also true that numerically our data indicated greater rates of myofibrillar protein synthesis in the vegan group. Irrespective, it is of clear importance that future work is performed to confirm our mechanistic findings as to the longer-term impact of such diets on muscle mass and strength in older adults.
Some important design aspects and limitations of this study require further consideration and context. We chose to provide a dietary protein intake (amount, type, timing and distribution) that we considered (close to) ‘optimal’ for maximizing daily myofibrillar protein synthesis rates. While this amount (i.e. 1.8 g·kg bm−1·d−1) is well above the RDA, it is in line with the currently asserted optimal dietary protein intakes to support active and healthy muscle ageing (56; 57), and is only ~50% above habitual intakes typically reported by healthy and active UK adults (58), including those within this study (see Table 2). Though the feasibility of applying such a diet in community-dwelling older adults can be debated, the approach allowed us to perform a proof-of-concept experiment investigating whether manipulating the type of protein only would impair daily myofibrillar rates under these (‘optimal’) conditions. As a result, we can conclude that protein type may become less relevant when consuming a high-protein diet, where the amount and timing of protein is at an ‘optimal’ level. Further research is necessary to establish whether such findings hold true under lower and/or less optimal protein intake conditions. Indeed, given the typically lower leucine (and total EAA/BCAA) contents of non-animal protein sources (59), it may be expected that differences across sources would become most apparent when protein intake is suboptimal. However, due to the relatively high leucine content of mycoprotein and the proportion of protein across groups that was obtained from identical sources (i.e. the control group was omnivorous), we estimated that daily leucine intakes were sufficient (and perhaps surplus) and comparable across groups (~10 g leucine a day, an average of ~2.5 g split between four meals, in both groups). The latter may, at least in part, explain the lack of differences between diets in daily myofibrillar protein synthesis rates.
The inclusion of mycoprotein as the basis for the vegan dietary intervention is also of relevance. To date, mycoprotein is the only vegan protein source that’s bolus ingestion has been shown to acutely stimulate postprandial muscle protein synthesis rates to a comparable extent as an animal-derived control (26). Accordingly, it cannot be assumed that our current data are generalizable to vegan diets predicated on other protein sources, particularly those lower in leucine and/or other essential amino acids. Future work is required to investigate the impact of a range of vegan protein sources both on acute postprandial and daily muscle protein synthesis rates. Finally, we employed unilateral isodynamic maximal concentric contractions as a model of exercise to ensure a daily stimulation of muscle protein synthesis rates in line with our previous work (30). We reasoned that this would increase the cumulative stimulatory effect across the 3-day intervention without inducing excessive muscle damage. While effective for the experimental purposes herein, there will be value in translating our data to multiple modalities of exercise to further the ability to make applied recommendations to support active and healthy ageing.
To conclude, the present work reports that a single bout of resistance exercise performed daily each morning increases daily myofibrillar protein synthesis rates in older men and women consuming a high-protein diet. Obtaining the majority of dietary protein from animal-derived sources compared with exclusively vegan-based sources (primarily mycoprotein) did not modulate daily myofibrillar protein synthesis rates in rested or exercised muscle. Our data indicate that obtaining dietary protein from animal-derived sources is not an essential prerequisite to support daily myofibrillar protein synthesis rates in older adults.
Supplementary Material
Acknowledgements
The project was sponsored by Marlow Foods Ltd (BTW as grant holder). The University of Exeter (BTW) were responsible for the study design, data collection and analysis, decision to publish and preparation of the manuscript. The private partners have contributed to the project through regular discussion. AJM and MOCC are supported from a studentship grant in collaboration with Marlow Foods. The authors’ contributions were as follows: AJM, BTW, and FBT designed the research; AJM, MVD, BTW, DJM and MOCC conducted the research; AJM, GFP and DRA performed the biological analysis; AJM and BTW analysed the data and wrote the manuscript. BTW has primary responsibility for the final content. All authors have read and approved the final content. BTW, MLD, FBS and MVD are employees of the University of Exeter. Aside from those mentioned above, the authors declare that there are no conflicts of interest. AJM and DRA were supported by a pilot award to AJM from the Claude D. Pepper Older Americans Independence Center (Grant NIH/NIA AG024832).
References
- 1.Evans W (1995) What is sarcopenia? J Gerontol A Biol Sci Med Sci 50, 5–8. [DOI] [PubMed] [Google Scholar]
- 2.WHO (2008) [http://www.who.int/topics/ageing]. webcite.
- 3.Janssen I, Shepard DS, Katzmarzyk PT et al. (2004) The Healthcare Costs of Sarcopenia in the United States. Journal of the American Geriatrics Society 52, 80–85. [DOI] [PubMed] [Google Scholar]
- 4.Sousa AS, Guerra RS, Fonseca I et al. (2016) Financial impact of sarcopenia on hospitalization costs. Eur J Clin Nutr 70, 1046–1051. [DOI] [PubMed] [Google Scholar]
- 5.Cuthbertson D, Smith K, Babraj J et al. (2005) Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19, 422–424. [DOI] [PubMed] [Google Scholar]
- 6.Wall B, Gorissen S, Pennings B et al. (2016) Aging Is Accompanied by a Blunted Muscle Protein Synthetic Response to Protein Ingestion. PLOS one 10, e0140903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guillet C, Prod’homme M, Balage M et al. (2004) Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J 18, 1586–1587. [DOI] [PubMed] [Google Scholar]
- 8.Katsanos C, Kobayashi H, Sheffield-Moore M et al. (2005) Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr 82, 1065–1073. [DOI] [PubMed] [Google Scholar]
- 9.Kumar V, Selby A, Rankin D et al. (2009) Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 587, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fry C, Drummond M, Glynn E et al. (2011) Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle 1, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wall B, Cermak N, van Loon L (2014) Dietary protein considerations to support active aging. Sports Med 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pennings B, Koopman R, Beelen M et al. (2011) Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr 93, 322–331. [DOI] [PubMed] [Google Scholar]
- 13.Holwerda A, Kouw I, Trommelen J et al. (2016) Physical Activity Performed in the Evening Increases the Overnight Muscle Protein Synthetic Response to Presleep Protein Ingestion in Older Men. J Nutr 146, 1307–1314. [DOI] [PubMed] [Google Scholar]
- 14.Pennings B, Groen B, de Lange A et al. (2012) Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men Am J Physiol Endocrinol Metab 302, E992–999. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Churchward-Venne T, Burd N et al. (2012) Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr Metab (Lond) 9, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore D, Churchward-Venne T, Witard O et al. (2014) Protein Ingestion to Stimulate Myofibrillar Protein Synthesis Requires Greater Relative Protein Intakes in Healthy Older Versus Younger Men. J Gerontol A Biol Sci Med Sci 70, 57–62. [DOI] [PubMed] [Google Scholar]
- 17.Pennings B, Boirie Y, Senden J et al. (2011) Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr 93, 997–1005. [DOI] [PubMed] [Google Scholar]
- 18.Wall B, Hamer H, de Lange A et al. (2013) Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin Nutr 32, 412–419. [DOI] [PubMed] [Google Scholar]
- 19.Kouw I, Holwerda A, Trommelen J et al. (2017) Protein Ingestion before Sleep Increases Overnight Muscle Protein Synthesis Rates in Healthy Older Men: A Randomized Controlled Trial. J Nutr 147, 2252–2261. [DOI] [PubMed] [Google Scholar]
- 20.Tang J, Moore D, Kujbida G et al. (2009) Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol 107, 987–992. [DOI] [PubMed] [Google Scholar]
- 21.Gorissen S, Horstman A, Franssen R et al. (2016) Ingestion of Wheat Protein Increases In Vivo Muscle Protein Synthesis Rates in Healthy Older Men in a Randomized Trial. J Nutr 146, 1651–1659. [DOI] [PubMed] [Google Scholar]
- 22.van Vliet S, Burd N, van Loon L (2015) The Skeletal Muscle Anabolic Response to Plant- versus Animal-Based Protein Consumption. J Nutr 145, 1981–1991. [DOI] [PubMed] [Google Scholar]
- 23.Finnigan T (2011) Mycoprotein: origins, production and properties. In Handbook of Food Proteins, pp. 335–352: Elsevier. [Google Scholar]
- 24.Coelho MOC, Monteyne AJ, Dunlop MV et al. (2019) Mycoprotein as a possible alternative source of dietary protein to support muscle and metabolic health. Nutrition Reviews. [DOI] [PubMed] [Google Scholar]
- 25.Dunlop MV, Kilroe SP, Bowtell JL et al. (2017) Mycoprotein represents a bioavailable and insulinotropic non-animal-derived dietary protein source: a dose–response study. British Journal of Nutrition 118, 673–685. [DOI] [PubMed] [Google Scholar]
- 26.Monteyne A, Coleho M, Porter C et al. (2020) Mycoprotein ingestion stimulates protein synthesis rates to a greater extent than milk protein in rested and exercised skeletal muscle of healthy young men AJCN (under second review). [DOI] [PubMed]
- 27.Kilroe S, Fulford J, Holwerda A et al. (2019) Short-term muscle disuse induces a rapid and sustained decline in daily myofibrillar protein synthesis rates under review. [DOI] [PubMed]
- 28.Craig CL, Marshall AL, Sjorstrom M et al. (2003) International physical activity questionnaire: 12-country reliability and validity. Medicine and science in sports and exercise 35, 1381–1395. [DOI] [PubMed] [Google Scholar]
- 29.Tang JE, Perco JG, Moore DR et al. (2008) Resistance training alters the response of fed state mixed muscle protein synthesis in young men. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 294, R172–R178. [DOI] [PubMed] [Google Scholar]
- 30.Monteyne AJ, Coelho MOC, Porter C et al. (2020) Branched-Chain Amino Acid Fortification Does Not Restore Muscle Protein Synthesis Rates following Ingestion of Lower- Compared with Higher-Dose Mycoprotein. The Journal of nutrition. [DOI] [PubMed] [Google Scholar]
- 31.Damas F, Phillips SM, Libardi CA et al. (2016) Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. The Journal of physiology 594, 5209–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holwerda A, Paulussen K, Overkamp M et al. (2018) Daily resistance-type exercise stimulates muscle protein synthesis in vivo in young men. Journal of Applied Physiology 124, 66–75. [DOI] [PubMed] [Google Scholar]
- 33.Murphy CH, Churchward-Venne TA, Mitchell CJ et al. (2015) Hypoenergetic diet-induced reductions in myofibrillar protein synthesis are restored with resistance training and balanced daily protein ingestion in older men. American Journal of Physiology-Endocrinology and Metabolism 308, E734–E743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mamerow MM, Mettler JA, English KL et al. (2014) Dietary Protein Distribution Positively Influences 24-h Muscle Protein Synthesis in Healthy Adults. The Journal of nutrition 144, 876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kilroe SP, Fulford J, Jackman S et al. (2020) Dietary protein intake does not modulate daily myofibrillar protein synthesis rates or loss of muscle mass and function during short-term immobilization in young men: a randomized controlled trial. The American Journal of Clinical Nutrition. [DOI] [PubMed] [Google Scholar]
- 36.Henry C (2005) Basal metabolic rate studies in humans: measurement and development of new equations. Public health nutrition 8, 1133–1152. [DOI] [PubMed] [Google Scholar]
- 37.Westerterp K (1999) Obesity and physical activity. International Journal of Obesity 23, S59. [DOI] [PubMed] [Google Scholar]
- 38.Wong WW, Clarke LL (2012) A Hydrogen Gas-Water Equilibration Method Produces Accurate and Precise Stable Hydrogen Isotope Ratio Measurements in Nutrition Studies. The Journal of nutrition 142, 2057–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dufner D, Bederman I, Brunengraber D et al. (2005) Using 2 H 2 O to study the influence of feeding on protein synthesis: effect of isotope equilibration in vivo vs. in cell culture. Am J Physiol 288. [DOI] [PubMed] [Google Scholar]
- 40.Burd N, Gorissen S, van Loon L (2013) Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev 41, 169–173. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, Breen L, Burd N et al. (2012) Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr 7, 1–9. [DOI] [PubMed] [Google Scholar]
- 42.Oikawa S, Kamal M, Webb E et al. (2020) Whey protein but not collagen peptides stimulate acute and longer-term muscle protein synthesis with and without resistance exercise in healthy older women: a randomized controlled trial. Am J Clin Nutr [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy C, Saddler N, Devries M et al. (2016) Leucine supplementation enhances integrative myofibrillar protein synthesis in free-living older men consuming lower- and higher-protein diets: a parallel-group crossover study. Am J Clin Nutr 104, 1594–1606. [DOI] [PubMed] [Google Scholar]
- 44.Murphy C, Shankaran M, TA C-V et al. (2018) Effect of resistance training and protein intake pattern on myofibrillar protein synthesis and proteome kinetics in older men in energy restriction. J Physiol 596, 2091–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKendry J, Shad B, Smeuninx B et al. (2019) Comparable Rates of Integrated Myofibrillar Protein Synthesis Between Endurance-Trained Master Athletes and Untrained Older Individuals. Front Physiol 10, doi: 10.3389/fphys. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mamerow M, Mettler J, English K et al. (2014) Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J Nutr 144, 876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Areta J, Burke L, Ross M et al. (2013) Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J Physiol 591, 2319–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strasser B, Volaklis K, Fuchs D et al. (2018) Role of Dietary Protein and Muscular Fitness on Longevity and Aging. Aging Dis 9, 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKendry J, Joanisse S, Baig S et al. (2019) Superior Aerobic Capacity and Indices of Skeletal Muscle Morphology in Chronically Trained Master Endurance Athletes Compared with Untrained Older Adults. J Gerontol A Biol Sci Med Sci [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 50.Mckendry J, Breen L, Shad B et al. (2018) Muscle morphology and performance in master athletes: A systematic review and meta-analyses. Ageing Res Rev 45. [DOI] [PubMed] [Google Scholar]
- 51.Isanejad M, Mursu J, Sirola J et al. (2015) Association of protein intake with the change of lean mass among elderly women: The Osteoporosis Risk Factor and Prevention - Fracture Prevention Study (OSTPRE-FPS). J Nutr Sci 4:e41. doi: 10.1017/jns.2015.31. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beasley J, Wertheim B, LaCroix A et al. (2013) Biomarker-calibrated protein intake and physical function in the Women’s Health Initiative. J Am Geriatr Soc 61, 1863–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allès B, Baudry J, Méjean C et al. (2017) Comparison of Sociodemographic and Nutritional Characteristics between Self-Reported Vegetarians, Vegans, and Meat-Eaters from the NutriNet-Santé Study. Nutrients 9, 1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phillips S (2017) Nutrition in the elderly: a recommendation for more (evenly distributed) protein? Am J Clin Nutr 106, 12–13. [DOI] [PubMed] [Google Scholar]
- 55.Paddon-Jones D, Campbell W, Jacques P et al. (2015) Protein and healthy aging. Am J Clin Nutr pii: ajcn084061. [DOI] [PubMed] [Google Scholar]
- 56.Lancha AH Jr, Zanella R Jr, Tanabe SGO et al. (2017) Dietary protein supplementation in the elderly for limiting muscle mass loss. Amino Acids 49, 33–47. [DOI] [PubMed] [Google Scholar]
- 57.Traylor DA, Gorissen SHM, Phillips SM (2018) Perspective: Protein Requirements and Optimal Intakes in Aging: Are We Ready to Recommend More Than the Recommended Daily Allowance? Advances in Nutrition 9, 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smeuninx B, Greig CA, Breen L (2020) Amount, Source and Pattern of Dietary Protein Intake Across the Adult Lifespan: A Cross-Sectional Study. Frontiers in Nutrition 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Vliet S, Burd NA, van Loon LJ (2015) The Skeletal Muscle Anabolic Response to Plant- versus Animal-Based Protein Consumption. The Journal of nutrition 145, 1981–1991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.