Abstract
It is widely accepted that the pathogen Clostridioides difficile exploits an intestinal environment with an altered microbiota, but the details of these microbe–microbe interactions are unclear. Adherence and colonization of mucus has been demonstrated for several enteric pathogens and it is possible that mucin-associated microbes may be working in concert with C. difficile. we showed that C. difficile ribotype-027 adheres to MUC2 glycans and using fecal bioreactors, we identified that C. difficile associates with several mucin-degrading microbes. C. difficile was found to chemotax toward intestinal mucus and its glycan components, demonstrating that C. difficile senses the mucus layer. Although C. difficile lacks the glycosyl hydrolases required to degrade mucin glycans, coculturing C. difficile with the mucin-degrading Akkermansia muciniphila, Bacteroides thetaiotaomicron, and Ruminococcus torques allowed C. difficile to grow in media that lacked glucose but contained purified MUC2. Collectively, these studies expand our knowledge on how intestinal microbes support C. difficile.
Keywords: Clostridioides difficile, MUC2, glycans, Akkermansia, Rumminococcus, Bacteroides
Graphical Abstract

In the United States, Clostridioides difficile infection (CDI) affects millions of patients each year and represents an annual cost of over $1 billion.1–5 Despite the immense human health burden of this antibiotic-associated disease and significant research effort dedicated to fighting CDI, the various mechanisms C. difficile employs to colonize the host and promote disease remain ill-defined. CDI typically results from a disruption in the gut microbiota following broad-spectrum antibiotic use,6 whereby C. difficile produces toxins that result in the clinical manifestations of CDI, which include diarrhea, inflammation, epithelial damage, and pseudomembranous colitis.1–5 While much progress has been made on understanding the primary virulence factors produced by C. difficile, questions still remain in terms of colonization and C. difficile–microbe interactions.
The ability to interact with intestinal mucus constitutes an important colonization factor for several enteric pathogens.7–13 In vitro, C. difficile has been shown to adhere to intestinal mucins,14–18 and in vivo C. difficile has been observed within the mucus of mice19–21 and humans.22,23 C. difficile and other mucosa-associated microbes grow as microcolonies or intestinal biofilms in the mucus layer, indicating that mucus is likely the colonization site for C. difficile.21,24–46 Intestinal mucus is primarily composed of a MUC2 core protein that is secreted from goblet cells.47,48 MUC2 is highly O-glycosylated, which accounts for up to 80% of the mucin molecular weight. O-Glycans contain branched carbohydrates including N-acetylgalactosamine (GalNAc), N-acetyl-glucosamine (GlcNAc), galactose, fucose, and sialic acid (NANA).49–53 Additionally, MUC2 is N-glycosylated, consisting primarily of linked mannose residues.54 These O- and N-glycosylated residues on MUC2 could provide an environment rich in nutrients capable of supporting C. difficile growth.
Numerous bacterial species can use host-derived mucin glycans as growth substrates.19,55–61 Mucin-degrading specialists such as Akkermansia muciniphila, Bacteroides thetaiotaomicron, B. fragilis, and Ruminococcus gnavus possess a large repertoire of glycosyl hydrolase enzymes that can degrade mucin glycans.61–69 These released glycan oligosaccharides, such as NANA, GlcNAc, GalNAc, galactose, mannose, and fucose, can then be metabolized by the degrading microbe or by other resident microbes.67,70–73 Enteric pathogens, in particular, are well-adapted to use mucin carbohydrates to colonize specific niches.7,74–77 As a result, MUC2 glycans can serve as a nutrient source for bacteria that can utilize the mucus-derived sugars even if they lack mucin-degrading enzymes. Since glycans function as a nutrient source, multiple enteric pathogens including Vibrio cholera,10,78,79 Campylobacter jejuni,8,9,80 and Salmonella typhimurium11,13,81 chemotax toward intestinal mucus and mucin oligosaccharides. Similar to other pathogens, C. difficile has also been shown to utilize carbohydrates,82–86 but the precise interactions between C. difficile, mucins, and mucin-degrading microbes remain unclear. Here we examined C. difficile (ribotype 027)–human mucin interactions in vitro and identify the role of mucin-degrading microbes in promoting C. difficile growth.
RESULTS
C. difficile Adheres to Human MUC2 Glycans.
Highly O-glycosylated mucin proteins can serve as ligands for bacterial adhesins.87 To examine if mucin glycans could serve as ligands for C. difficile, we examined the localization of fluorescently labeled C. difficile R20291 in the human mucin-producing cell line HT29-MTX (Figure 1A). C. difficile R20291 was fluorescently labeled with the cell-permeable compound carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) and incubated with confluent layers of HT29-MTX for 1 h prior to immunostaining for MUC2. Fluorescence microscopy showed C. difficile (green) colocalized with MUC2 (purple) among HT29-MTX cells (nuclei blue) in Figure 1A–C. At high magnification (100×), a direct interaction between C. difficile and MUC2 was observed in vitro (Figure 1C), showing that C. difficile could interact closely with mucin ligands along the gut epithelium.
Figure 1.
C. difficile R20291 adheres to the glycans human and mouse MUC2. (A) A 3D projection of C. difficile R20291 fluorescently tagged with CFDA-SE (green) colocalized with human MUC2 (purple) in mucin-producing HT29-MTX cells by immunostaining. Nuclei are marked with Hoechst dye (blue) (n = 3 replicates). (B, C) Representative images of C. difficile R20291 at higher magnifications (40×, 100×). Scale bar = 10 μm (n = 3 replicates). (D) Representative images of CFDA-SE tagged C. difficile R20291 (green) adhering to human LS174T-derived MUC2 coated coverslips after 1 h incubation (scale bar = 100 μm) (n = 3 replicates). (E) Representative images of CFDA-SE tagged C. difficile R20291 (green) adhering to human LS174T-derived MUC2 coated coverslips with glycans removed by beta-elimination and acid hydrolysis (scale bar = 100 μm) (n = 4 replicates). (F) Mouse colon tissue sections stained with Hoechst (blue) and incubated with CFDA-SE tagged C. difficile (green) for 1 h with costaining of mucin glycans (UEA-1, purple) (scale bar = 100 μm)6666 (n = 3 mice). (G) Mouse colon tissue sections with glycans removed stained with Hoechst (blue) and incubated with CFDA-SE tagged C. difficile (green) for 1 h with costaining of mucin glycans (UEA-1, purple) (scale bar = 100 μm) (n = 3 mice). (H) Normal human colon incubated with CFDA-SE tagged C. difficile (green) for 1 h with costaining of mucin glycans (UEA-1, purple) (n = 72 patients; 3 patients shown). Yellow arrows highlight C. difficile within the mucus layer. Scale bar = 100 μm.
To identify the role of glycans in adherence, MUC2-coated coverslips were oxidized and underwent acid-hydrolysis and beta-elimination, effectively removing all N- and O-linked glycans.88 Fluorescently labeled C. difficile was then incubated with control (untreated) or glycan-removed MUC2 coverslips for 1 h (Figure 1D, E). Robust adhesion of C. difficile was observed in untreated MUC2 coverslips (Figure 1D), while glycan removal greatly diminished the ability of C. difficile to adhere to MUC2 (Figure 1E). To confirm the adhesion of C. difficile to mucin glycans, we incubated slides containing 7 μm sections of fixed tissue from C57BL/6J mouse colons with fluorescently labeled C. difficile under anaerobic conditions at 37 °C for 1 h and examined glycan colocalization using the lectin Ulex Europaeus Agglutinin-1 (UEA-1), which recognizes fucose residues. We observed C. difficile within the UEA-1 positive mucus layer (Figure 1F). Interestingly, we rarely found C. difficile on the epithelium, despite the access of C. difficile to the tissue using this technique. To further demonstrate specificity for mucin glycans, tissue sections were oxidized and underwent acid-hydrolysis and beta-elimination as described above. Incubation of fluorescently labeled C. difficile with these glycan-removed slides resulted in little to no adhesion (Figure 1G). These data indicate that C. difficile adheres to the glycan component of purified MUC2 and murine intestinal MUC2. Finally, we employed a human normal colon array (US Biomax) to identify adhesion of C. difficile in human colon (Figure 1H). Although these biopsy specimens were fixed in 10% formalin, which compresses the mucus layer, we still observed C. difficile localized in mucus above goblet-cell-lined crypts. Consistent with our mouse colon staining, we observed limited number of C. difficile cells associated with the epithelium. These data suggest that C. difficile preferentially adheres to mucin glycans which may facilitate intestinal colonization.
C. difficile Colonizes Mucus in Antibiotic-Treated Human Fecal Bioreactors.
Once C. difficile adheres to the mucus layer, it likely interacts with other mucus-associated microbes to form a stable biofilm community.19,21,23,42,89 Fecal minibioreactor arrays (MBRAs) have been used to model C. difficile colonization of antibiotic-disrupted microbial communities using the antibiotic clindamycin and fecal samples of healthy adult donors.85,90 We modified the MBRA model for mucus-coated coverslips and evaluated C. difficile colonization of mucus-associated microbial communities when treated with broad-spectrum antibiotics (Figure 2). We used 50 mL fecal bioreactors to test whether C. difficile-colonized coverslips coated with purified mucin (MUC2) from LS174T (n = 2 bioreactors) and HT29-MTX (n = 2) cells compared to coverslips with no MUC2 coating (mock, n = 2). Coverslips were suspended into established microbial communities after clindamycin treatment at the time of C. difficile inoculation. After 4 days, coverslips were removed and evaluated microscopically for biofilm formation. Imaging of the coverslips revealed biofilms established on MUC2-coated and mock-coated coverslips (Figure 2A), as determined by acridine orange staining. C. difficile within biofilm communities was identified by fluorescence in situ hybridization (FISH) staining. As seen in Figure 2B, C. difficile was observed in greater abundance in MUC2-coated coverslips compared to mock-coated coverslips.
Figure 2.
C. difficile interacts with other mucus-associated microbes in bioreactors. Bioreactors seeded with stool from healthy donors and treated with clindamycin (250 μg/mL), followed by C. difficile (106 CFU). Bioreactors were fitted with inserts harboring mock or MUC2-APTS-coated coverslips. MUC2 was purified from human LS174T and HT29-MTX cell lines. (A) Biofilm status was confirmed by staining with acridine orange in mock and MUC2-coated coverslips (scale bar = 50 μm) (mock, n = 2; MUC2, n = 4). (B) The presence of C. difficile within biofilm communities was determined by FISH with a C. difficile specific probe (red) and counterstained with Hoechst (blue) (mock, n = 2; MUC2, n = 4). Community structure of the MUC2-coated coverslips were assessed by 16S rDNA sequencing at the level of (C) family and (D) genus (mock, n = 2; MUC2, n = 4). (E) CFUs of C. difficile in bioreactor supernatant overtime, confirming C. difficile invasion. (F) Abundance of Peptostreptococcaceae OTUs detected by 16S rRNA gene sequencing of mock and MUC2-coated coverslips (BLAST analysis indicates sequence of Peptostreptococcaceae OTU is 100% identical to C. difficile). (G) qPCR analysis of C. difficile housekeeping gene rpoA, back calculated to CFU by four-parameter logistic curve in mock and MUC2-coated coverslips. Student’s t test, *p < 0.05.
C. difficile Interacts with Mucin-Degrading Microbes in Human Fecal Bioreactors.
To evaluate the effects of mucus on microbial community composition and the gut microbes that aid in C. difficile mucus colonization, we sequenced the V4 variable region of 16S rRNA genes from DNA isolated from bioreactor coverslips. Family level (Figure 2C) and genus level (Figure 2D) relative abundances of microbial communities from mock coverslips were compared to that of mucus-coated coverslips. The dominant microbes of both mock and MUC2-coated coverslips were Bacteroidaceae (mock, 23.5%; MUC2, 30.6%), Fusobacteriaceae (mock, 21%; MUC2, 20.5%) and Clostridiaceae (mock, 16.7%; MUC2, 9.4%). In addition to Bacteroides, within MUC2 biofilms we identified several known mucin-degrading containing genera, including Parabacteroides, Porphyromonas, Ruminococcaceae, and Akkermansia. We speculated that the presence of these microbes within the mucus layer may promote the colonization of C. difficile.
Despite successful invasion of C. difficile in all bioreactors (Figure 2E), we observed varying levels of C. difficile in the inserted coverslips. Consistent with our FISH staining, we found that the overall abundance of C. difficile associated with mucus-coated coverslips was 7.4-fold greater than that for mock coverslips (Figure 2F). Importantly, the concentrations of C. difficile did not vary between mucus isolated from LS174T or MTX (LS174T, 70 ± 9.9 sequences per Peptostreptococcaceae OTUs vs HT29-MTX, 60.5 ± 6.4 sequences). These findings were confirmed using quantitative PCR (qPCR) with primers specificto C. difficile (Figure 2G), further confirming the preferential colonization of C. difficile to mucus-coated coverslips within a mixed microbial community.
Mucin Monosaccharides Chemoattract C. difficile.
Since we observed C. difficile in higher abundance in the MUC2-coated coverslips, we reasoned that C. difficile was able to sense mucins and chemotax toward the MUC2-coated coverslip. Chemotaxis is the directed movement of an organism in response to a chemical gradient that facilitates survival by enabling cells to move toward favorable growth conditions or away from toxic environments.80,91 Chemotaxis toward intestinal mucus is thought to be crucial in the life cycle of motile enteric pathogens7 and could play a role in C. difficile infection. Mucin-degrading bacteria in biofilms release mucin glycan oligosaccharides, which can provide a key source of nutrients to other bacteria in the community, including C. difficile. Using a standard capillary chemotaxis assay,92 we investigated the ability of CFDA-SE-labeled C. difficile R20291 to migrate up the concentration gradient of the mucin oligosaccharides glucose, fucose, galactose, mannose, N-acetylglucosamine (GlcNAc), N-acetylgalactosamine (Gal-Nac), or N-acetylneuraminic acid (NANA) (Figure 3A). Chemotaxis of C. difficile up the monosaccharide concentration gradient would result in increased fluorescence intensity due to the accumulation of the CFDA-SE-labeled C. difficile. Capillary tubes containing chemotaxis buffer alone (negative control) or chemotaxis buffer with 100 mM glucose (positive control) or mucin monosaccharides were placed upright in the wells of a 96-well plate containing CFDA-SE-labeled C. difficile. After a 2 h anaerobic incubation at 37 °C, the capillary tubes were removed and fluorescence measured. Increased fluorescence intensity was observed in capillary tube contents for all monosaccharides compared to buffer control; however, the amount of fluorescence was monosaccharide-specific(Figure 3A). The mucin monosaccharide NANA provided the least fluorescence compared to buffer control (2.8-fold differences) and was 3.3-fold lower than glucose suggesting NANA is not a preferred chemoattractant for C. difficile. The greatest fluorescence resulted from C. difficile chemotaxis toward mannose (21-fold greater than glucose) followed closely by GlcNAc (18-fold greater than glucose). Galactose also elicited a greater chemotactic response from C. difficile than glucose (14-fold difference). In addition to monosaccharides, we also tested intact pig stomach mucus (MUC5AC/MUC6) and purified human MUC2 from HT29-MTX and LS174T cell lines. Fluorescent readings indicate that human-derived mucus served as a stronger chemoattractant for C. difficile than pig mucus (Figure 3A). Notably, human-derived mucus resulted in C. difficile chemotaxis comparable to glucose, while pig-derived mucus performed similarly to the mucin monosaccharides NANA and GalNAc. Addition of purified O-linked glycans from LS174T MUC2 also served a strong chemoattractant for C. difficile (13.7-fold increase). These data support that mucin glycan monosaccharides serve as chemoattractants for C. difficile.
Figure 3.
C. difficile R20291 chemotaxes toward and utilizes mucin components. (A) Capillary chemotaxis assay with C. difficile R20291 in chemotaxis buffer (10 M potassium phosphate, 0.1 mM EDTA) with or without 100 mM sugars, 1 mg/mL mucus (pig stomach MUC5AC/6, HT29-MTX derived MUC2, LS174T derived MUC2), or 1 mg/mL O-linked glycans isolated from LS174T MUC2. The amount of C. difficile in the capillaries following incubation was assessed by transferring the capillary contents to 96-well plates where fluorescence was read by a plate reader at excitation 428 nm/emission 528 nm. n = 8 wells per sugar, repeated four independent times. *p < 0.05, one-way ANOVA. (B, C) Live cell imaging analysis of ibidi chemotaxis slide using the ibidi “Motility” FIJI plugin. (C) Velocity and (D) distance traveled was measured from traces of individual C. difficile cells. n = 3 replicates, repeated five independent times. *p < 0.05, Student’s t test. (D) C. difficile R20291 was inoculated in the fully defined minimal medium CDMM at an OD600 nm = 0.1. Samples were analyzed for OD600nm at 1, 3, 6, 9, 12, and 24 h. CDMM was prepared without sugars or with 10 mM sugars (glucose, fucose, galactose, mannose, N-acetylglucosamine (GlcNAc), N-acetylgalactocosamine (GalNAc), or N-acetylneuraminic acid (NANA)). (E) Growth of C. difficile after 24 h in CDMM lacking sugars (0 mM) or containing 1, 10, or 100 mM of glucose or mannose. (F) Levels of AI-2 were determined by measuring V. harveyi luminescnence. V. harveyi was incubated with cell-free supernatant of C. difficile grown for 24 h in CDMM with 10 mM glucose or 10 mM mannose. Control represents cell-free supernatant AB media alone. (G) C. difficile inoculated at OD600nm = 0.1 in CDMM containing glucose or mannose (1, 10, 100 mM) and incubated for 72 h in a MUC2 (LS174T)-coated 96-well plate. Crystal violet staining of mucin-based biofilms was assessed at A600nm. (H, I) Vero cells were grown as 2D monolayers and incubated for 4 h with supernatant from 48 h cultures of C. difficile R20291 grown in CDMM with various carbohydrate sources (glucose, fucose, galactose, mannose, GlcNAc, GalNAc, NANA) or LS174T purified MUC2. Cell rounding was visualized by microscopy on a Nikon TiE with a 20× Plan Apo (NA 0.75) differential interference contrast objective, using a SPECTRA X LED light source and ORCA-Flash 4.0 sCMOS camera. Cell diameter (μm) was calculated from representative images of Vero cells following treatment (scale bar = 100 μm) (n = 3 per experiment, repeated two independent experiments). *p < 0.05, one-way ANOVA.
We further characterized the positive chemotactic response of C. difficile to mannose, the strongest chemoattractant tested, using live-cell imaging of ibidi μ-Slide chemotaxis slides. These slides are designed to generate a chemoattractant gradient for live imaging. CFDA-SE-labeled C. difficile R20291 were added in a chemotaxis agar to the channel and then chemotaxis buffer with or without an increasing mannose gradient was added to the flanking reservoirs. When C. difficile was exposed to chemotaxis buffer lacking mannose, cells displayed random unbiased movement within the chamber. However, when seeded with a mannose gradient increasing in concentration to the right-hand side of the chamber, imaging verified C. difficile cells exhibited directed motion toward mannose. Using the ibidi motility FIJI software, we tracked individual bacterial cells and showed that in the presence of a mannose gradient C. difficile cells moved with a higher velocity (PBS, 0.9 ± 0.4; mannose, 4.0 ± 2.1 μm/min) and traveled longer distances (PBS, 135.2 ± 88.6; mannose, 294.7 ± 128.7 μm) compared to chambers without mannose (Figure 3B, C). These data support the finding that mucin components, particularly mannose, behave as chemoattractants for C. difficile.
Mucin Monosaccharides Serve as Primary Carbon Sources for C. difficile.
Positive chemotactic behavior drives bacterial cells toward greater concentrations of beneficial chemicals and often these chemoattractants support bacterial growth. Having established that C. difficile can chemotax to mucin monosaccharides, we hypothesized that these sugars might serve as a nutrient source for C. difficile. To test this, we cultured C. difficile in CDMM supplemented with either no carbon source (negative control), 10 mM glucose (positive control), or 10 mM fucose, galactose, mannose, GlcNac, GalNAc, or NANA (Figure 3D). With 10 mM glucose as the primary carbon source in CDMM, C. difficile reached the stationary phase by 12 h with a peak OD600 = 0.5 (Figure 3D, gray squares). C. difficile had only minor growth in the absence of glucose (Figure 3D, black circles), and the addition of mucin monosaccharides was sufficient to support C. difficile growth as individual primary carbon sources in CDMM. At 12 h, growth on all mucin monosaccharides was lower than that observed on glucose and cells had not reached stationary phase. However, cell density reached OD600 values greater than 0.7 at 24 h for mucin monosaccharides mannose (green diamonds), GlcNAc (light green squares), and NANA (light blue squares), 1.4-fold greater than glucose (Figure 3D). Along with being the preferred chemoattractant, mannose also led to the greatest levels of total growth among mucin monosaccharides (Figure 3D). We further characterized C. difficile growth on mannose over a range of concentrations (1–100 mM) (Figure 3E). After 24 h, C. difficile growth increased with increasing concentrations of mannose and continued to produce greater cell density than glucose at the same concentration.
Our data indicated that C. difficile exhibits optimal growth in the presence of mannose as the primary carbon source; therefore, we examined if the presence of mannose had any effect on quorum-sensing compounds, because chemoattractant may increase bacterial numbers and thus AI-2 production. C. difficile secretes the universal quorum-sensing compound AI-293 and specific dietary sources are known to regulate AI-2 production.94 Analysis of the Vibrio harveyi reporter strain BAA-1121, a mutant sensor strain that only senses autoinducer AI-2, revealed that C. difficile secretes AI-2 in the presence of glucose and mannose (Figure 3F). Interestingly, mannose did not elevate the levels of AI-2 above those observed for glucose. This data suggests that C. difficile does synthesize AI-2 in the presence of mannose as the sole carbon source. Finally, we questioned whether mannose influenced C. difficile colonization of mucus. To mimic the intestine, we coated 96-well plates with purified MUC2 from LS174T cells and examined C. difficile colonization by crystal violet staining after 72 h in CDMM with glucose or mannose (Figure 3G). The presence of glucose and mannose both enhanced C. difficile colonization of MUC2-coated wells compared to the CDMM no glucose/mannose controls. However, a significant increase in the density of colonized C. difficile was observed with 100 mM mannose compared to the equivalent glucose levels, indicating that mannose can bolster bacterial density and thus colonization. These data provide new evidence that mucin oligosaccharides can be a potent carbon source to promote C. difficile growth and colonization of the intestinal mucus layer.
Finally, we examined toxin production in CDMM with various carbohydrate sources after 48 h. To assess toxin activity, we incubated Vero cells with 1:1 C. difficile CDMM in DMEM for 4 h and collected images. Cell diameter calculations (Figure 3H) from representative Vero cell images (Figure 3I) revealed that all 48 h CDMM cultures harbored toxins. In this assay, no differences were observed between carbohydrate sources.
Mucin Degraders Promote C. difficile Growth When Mucin Is the Sole Carbon Source.
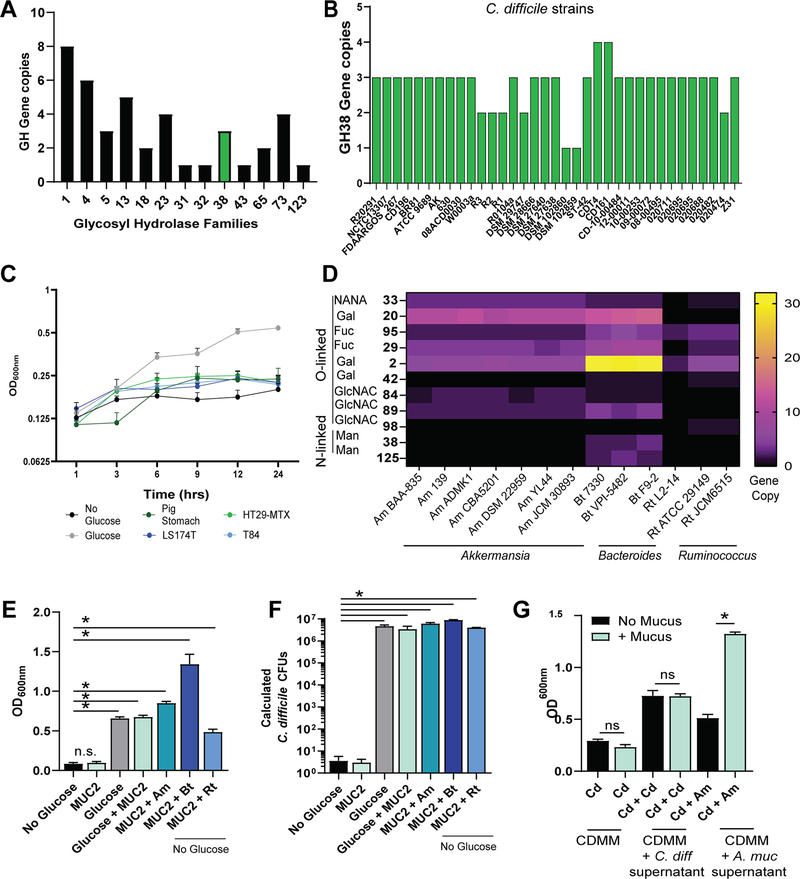
Bacteria must harbor the specific glycosyl hydrolase (GH) to enzymatically cleave mucin glycan structures and use mucin as a carbohydrate source. GH families cleave O-linked mucin-glycans including NANA (GH 33), GalNAc (GH 101, 129), GlcNAc (GH 84, 85, 89, 98), galactose (GH 2, 20, 42), or fucose (GH 29, 85). Additionally, the N-linked mucin glycans mannose can be removed with GH families 38 and 125. We queried the C. difficile R20291 genome for GH families using the Carbohydrate-Active enZYmes Database (CAZy). C. difficile R20291 harbored 13 GH families, only one of which was involved in mucin glycan degradation (Figure 4A). C. difficile R20291 was found to contain three gene copies of GH 38, which removes N-linked mannose residues. We next investigated whether these genes were conserved among C. difficile strains. When we examined 35 C. difficile genomes, we found that all genomes only harbored GH 38. Additionally, we found that all 35 genomes contained one to four gene copies of GH 38 (Figure 4B), indicating that the ability to remove mannose is a conserved function across all examined C. difficile members. Secreted mucins are covered with O-linked glycans, and the 3D structure of mucus often masks N-linked glycans. As a result, we speculated that GH38 from C. difficile would not be able to readily access N-linked glycans. Based on the lack of mucin-O-glycan degrading GHs, we predicted that C. difficile would be unable to grow in CDMM if mucus were the only carbon source. Consistent with this hypothesis, we found that C. difficile had minimal growth in CDMM without glucose, even when supplemented with mucus from pig stomach or human mucin-producing cell lines LS174T, HT29-MTX, or T84 (Figure 4C) (starting OD600nm = 0.1; final OD600nm = 0.18–0.22). These data indicate that C. difficile is able to use available mucin monosaccharides, but is unable to free these sugars from mucin on its own.
Figure 4.
C. difficile cannot enzymatically cleave intestinal MUC2, but can use oligosaccharides cleaved by A. muciniphila, B. thetaiotaomicron, and R. torques. (A) C. difficile R20291 genome analysis for glycosyl hydrolase (GH) families using CAZY. Y-axis represents the number of genes in each family. The green bar indicates GH families involved in mucin degradation. (B) Gene copies for GH Family 38 following genome analysis of 35 C. difficile genomes using CAZY. (C) C. difficile R20291 was inoculated in the fully defined minimal medium CDMM containing 1 mg/mL mucus (pig stomach, human LS174T, human HT29-MTX, human T84) or 10 mM glucose at OD600nm = 0.1. OD600nm was measured after 1, 3, 6, 9, 12, and 24 h of incubation. (D) A. muciniphila, B. thetaiotaomicron, R. torques, and R. gnavus genome analysis for mucin-degrading glycosyl hydrolase (GH) families using CAZy. (E) C. difficile R20291 was inoculated in CDMM at an OD600nm = 0.1 with or without 1 mg/mL MUC2 (LS174T), with or without glucose. Separate cultures of A. muciniphila BAA-835, B. thetaiotaomicron ATCC 29148, and R. torques ATCC 2775 were coinoculated with C. difficile R20291 in CDMM containing 1 mg/mL MUC2 and no glucose at an OD600nm = 0.1. Data represents OD600 nm at 24 h growth. (F) qPCR analysis of C. difficile housekeeping gene rpoA, back calculated to CFU by four-parameter logistic curve from the same experiment. (G) C. difficile R20291 and A. muciniphila BAA-835 were inoculated in CDMM containing glucose at an OD600nm = 0.1 with or without 1 mg/mL MUC2 (LS174T). After overnight incubation, cell-free supernatant was collected (“spent medium”) and used to supplement new cultures of CDMM with no glucose at 25%. Data represents OD600 nm at 24 h growth. *p < 0.05, one-way ANOVA.
Mucin degradation has been shown to be a cooperative event by members of the gut microbiome.55 Akkermansia, Bacteroides, and Ruminococcus have been well documented for their mucin-degrading ability.55,68,95–98 We queried seven A. muciniphila genomes, three Bacteroides thetaioatiomicron genomes, one Ruminococcus torques, and two Ruminococcus gnavus genomes using CAZy (Figure 4D). These genomes contained multiple genes for glycosyl hydrolases involved in mucin degradation (Figure 4D), with the highest gene copies of mucin-degrading GHs observed in Bacteroides. To identify whether mucin-degrading bacteria were capable of liberating mucin-glycans and cross-feeding C. difficile, we grew C. difficile in CDMM containing LS174T MUC2 as the sole carbon source in the presence or absence of A. muciniphila BAA-835, B. thetaiotaomicron ATCC 29148, and R. torques ATCC 2775 (Figure 4E). Consistent with our previous findings, we observed that C. difficile was unable to grow on MUC2 alone. However, addition of mucin-degrading microbes to media containing MUC2 resulted in increased optical density that was similar to or greater than that observed when C. difficile was grown in CDMM + glucose or CDMM + glucose + MUC2. Using qPCR, we verified increased C. difficile growth in mucin-containing CDMM with A. muciniphila (calculated CFU: 6.1 × 106 ± 1.1 × 106), B. thetaiotaomicron (CFU: 8.9 × 106 ± 5.4 × 105), and R. torques (CFU: 4.1 × 106 ± 9.3 × 104) compared to the CDMM with MUC2 alone (CFU 3.0 ± 5.2) (Figure 4F).
To confirm that degradation of mucin was responsible for the observed increase in C. difficile growth and not bacterial metabolites, we focused our experiments on characterizing interactions with A. muciniphila. We cultured C. difficile R20291 and A. muciniphila separately in CDMM containing 10 mM glucose with or without MUC2 overnight (Figure 4G) and used the filtered cell-free supernatants to assess whether hydrolyzed MUC2 byproducts produced by A. muciniphila could influence the growth of C. difficile. The following day, we added 25% of this cell-free supernatant from the overnight culture medium to new CDMM containing no sugars and inoculated with C. difficile at an OD600nm of 0.1. As expected, C. difficile was unable to grow in CDMM without glucose, but it grew at moderate levels when cultured in the presence of 25% cell-free supernatant from C. difficile grown in CDMM with glucose. The addition of A. muciniphila cell-free supernatant previously cultured in CDMM with glucose supported C. difficile similarly to that of C. difficile cell-free supernatant containing glucose. However, cell-free supernatant from A. muciniphila cultured in CDMM with glucose and MUC2 increased the OD600nm of C. difficile by 2.6-fold, indicating that mucin degradation and not simply A. muciniphila metabolites were responsible for increased C. difficile growth. These data point to the role of mucin-degrading microbes in promoting C. difficile growth and colonization of the intestinal mucus layer.
MUC2 and Mucin-Degrading Microbes Influence C. difficile Gene Expression.
Finally, we examined if the presence of mucin or mucin degradation by microbes could influence C. difficile gene expression. To address this question, C. difficile was cultured overnight in CDMM with 10 mM glucose, 10 mM mannose, or 10 mM glucose in the presence of 1 mg/mL LS174T purified MUC2. Alternatively, C. difficile was cocultured with A. muciniphila, B. thetaiotaomicron, or R. torques in CDMM containing MUC2 as the only carbon source (Figure 5). The presence of MUC2 or MUC2 and mucin-degrading microbes significantly increased expression of the cell wall protein 84 (cwp84) and surface layer protein A (slpA), both of which are involved in adhesion (Figure 5A, B). No differences in slpA or cwp84 were observed between C. difficile grown in 10 mM glucose and 10 mM mannose in the absence of MUC2. Additionally, MUC2 and to a greater degree (>20-fold increase) MUC2 with mucin-degrading microbes elevated gene expression of fliC and flgG that encode components of flagella (Figure 5C, D). Increased expression of genes important for chemotaxis (fliC and flgG) in C. difficile cells cultured in the presence of MUC2 and mucin-degrading microbes led us to hypothesize that cleavage of mucin glycans by A. muciniphila, B. thetaiotaomicron, or R. toques may increase concentrations of these chemoattractants and promote C. difficile chemotaxis. To test this, we exposed chemotaxis buffer to MUC2-treated A. muciniphila, B. thetaiotaomicron, or R. toques and allowed cells to secrete metabolites or mucin-degradation products into the buffer. After 2 h exposure, we used the filtered chemotaxis buffer to monitor C. difficile chemotaxis (Figure 5E). As a control, intact LS174T MUC2 in buffer was used. We observed that C. difficile chemotaxed toward intact MUC2, but that chemotaxis was enhanced by 1.5-fold and 1.9-fold in response to MUC2 preincubated with either A. muciniphila or B. thetaiotaomicron. We observed a trend toward elevated chemotaxis with R. torques and MUC2 compared to MUC2 alone, but it did not reach significance. With fewer GHs, it is possible that R. torques is less efficient at mucin monosaccharide cleavage than A. muciniphila or B. thetaiotaomicron, leaving the filtered chemotaxis buffer from R. torques less concentrated in mucin monosaccharides and therefore less efficient at chemoattracting C. difficile. These findings suggest that C. difficile is responsive to both intact MUC2 and monosaccharaides cleaved from MUC2 by mucin-degrading gut microbes. These data lead us to propose that mucin degradation by the gut microbiome promotes C. difficile chemotaxis, mucin adhesion, and colonization. Collectively, these data highlight the interplay between intestinal mucus, C. difficile, and other mucus-associated microbes.
Figure 5.
Intestinal MUC2 influences C. difficile gene expression C. difficile R20291 was inoculated in the fully defined minimal medium CDMM with glucose, mannose, 1 mg/mL human LS174T MUC2, and/or A. muciniphila BAA-835, B. thetaiotaomicron ATCC 29148, and R. torques ATCC 2775. After 16 h of incubation, samples were collected for RNA isolation and qPCR analysis. qPCR analysis of adhesion- related genes, (A) splA and (B) cwp84 or flagella-related genes (C) flgG and (D) fliC. (E) Chemotaxis analysis of CFDA-SE labeled C. difficile toward buffer controls, 1 mg/mL MUC2 (LS174T), or supernatant from A. muciniphila, B. thetaiotaomicron, or R. torques incubated with MUC2 in PBS for 2 h. n = 6 replicates, repeated three independent times. *p < 0.05, One-Way ANOVA.
DISCUSSION
Herein, we demonstrate the importance of intestinal mucins as a chemoattractant and energy source for C. difficile. Our work indicates that C. difficile is responsive to nutrient bioavailability and that C. difficile readily metabolizes mucin monosaccharides. However, we demonstrate that C. difficile does not harbor the GHs required for extensive degradation of mucin glycans and thus is not capable of generating these monosaccharides without the aid of other members of the gut microbiota. This work points to the role of other microbes in supporting C. difficile colonization. A major question in the field is which microbes play a supportive role in CDI and which ones simply co-occur with C. difficile.99 In the antibiotic-treated gut, we propose that mucin-degrading microbes such as Akkermansia, Ruminoccocus, and Bacteroides create a gradient of mucin monosaccharides which chemoattracts C. difficile to its colonization site. Once at the intestinal mucus layer, C. difficile metabolizes mucin monosaccharides cleaved by members of this shifted microbiota.
Antibiotic treatment is known to perturb the microbiota and thus modulates mucin carbohydrate availability. An expansion of MUC2 into the lumen following antibiotic treatment has been documented.100,101 Additionally, the concentrations of free fucose and sialic acid have been reported to reach high levels during antibiotic treatment.102 This may be due in part to an increased abundance of mucin-degrading Bacteroides and Akkermansia which have been shown to increase following antibiotic administration.101,103 Additionally, postantibiotic luminal contents possess more mucin and host carbohydrate-degrading genetic modules than strains isolated before antibiotic treatment.101 The importance of carbohydrates in C. difficile infection has been shown in vivo, where a decreased abundance of murine cecal carbohydrates over time correlated with the expression of C. difficile genes for carbohydrate utilization.104 Moreover, inoculation of mouse intestinal microbes into growth medium made from the cecal contents of gnotobiotic mice depleted carbohydrates and prevented C. difficile replication.105 This same study demonstrated that supplementation with the monosaccharides glucose, GlcNAc, or NANA was sufficient to overcome this nutrient-depravation and enable C. difficile growth.105 Based upon these previous results and the work presented here, we propose that C. difficile uses released mucin glycans (GlcNAc, NANA, and mannose) as a fuel source to drive its colonization in antibiotic-treated individuals.
Previous studies have found that mucin degradation is a community process.52,55,96,98,106,107 Bacterial species which do not harbor neuraminidases (enzymes that remove sialic acid/NANA from mucin) must rely on species that do contain these enzymes. Our in silico findings revealed that C. difficile does not harbor GH 33 neuraminidases or any other O-linked glycan removing enzymes. As a result, C. difficile must rely on other members of the gut microbiome to provide this function. Antibiotic exposure, a prerequisite for most C. difficile infection, shifts the microbiome structure.86,104,108 Patients with C. difficile infection harbor mucin-degrading Escherichia, Ruminococcus, Streptococcus, and Akkermansia.27,55,109–113 Thus, it is possible that mucin degradation is involved in the pathogenesis of C. difficile. Conflicting data exists on the levels of Bacteroidetes in C. difficile infection. Some groups have observed increased Bacteroidetes levels in C. difficile infected patients, while other groups have observed decreased Bacteroidetes levels.22,23,109,111,114–116 In B. thetaiotaomicron monocolonized mice, B. thetaiotaomicron was shown to promote C. difficile infection likely through release of mucin-derived monosaccharides.102 These studies suggest that bacterial mucin degradation and mucin monosaccharides likely influence C. difficile infection. Another example of a microbe likely aided by mucin degradation is vancomycin-resistant Enterococcus. Similar to our studies with C. difficile, Enterococcus is unable to grow on purified mucin alone, but it has been reported to grow on mucin predigested by the mucin-degrader R. torques.117 Enterococcus dominated our bioreactor MUC2-biofilms (Figure 2). As a result, we speculate that like C. difficile, Enterococcus may take advantage of mucin-degradation to promote its expansion.
Removal of mucin glycans by microbes can destabilize the mucus gel, allowing microbes to come in closer proximity to the host.118–120 However, in addition to the secreted mucins, like MUC2, epithelial cells are covered in adherent mucins.121 Similar to MUC2, adherent mucins like MUC1, MUC4, and MUC13 are also glycosylated and can serve as additional decoys for pathogens. Adherent mucins like MUC1 have been shown to be critical for C. jejuni infection by acting as a releasable decoy.82 Therefore, even if MUC2 levels are decreased, we would speculate that C. difficile could adhere to adherent mucins, which can be cleaved and released from the epithelium.
Our work highlights the potential importance of mucin-degrading microbes in the initial stages of C. difficile pathogenesis. We demonstrate that A. muciniphila, B. thetaiotaomicron, and R. torques can grow with and cross-feed C. difficile mucin glycans, indicating a level of synergy between these microbes. In general, A. muciniphila is considered a commensal microbe. Evidence of a dose-dependent benefitof A. muciniphila on human health is lacking, but several positive attributes have been attributed to A. muciniphila. Favorable effects of A. muciniphila on the host include improved glucose tolerance, prevention of obesity, amelioration of experimental alcoholic liver disease, and immune modulation.122–127 However, overgrowth of A. muciniphila is speculated to cause mucin degradation and may promote tissue damage.128 Additionally, A. muciniphila may inadvertently promote infection with enteric pathogens. A. muciniphila has been reported to stimulate epithelial access and initiate lethal colitis by the mucosal pathogen Citrobacter rodentium.129 We similarly find that A. muciniphila may promote C. difficile colonization of the intestinal mucus layer.
Competition for nutrients among microbial communities has been suggested as a key barrier to enteric infection. When the microbiome is disrupted by antibiotics, as is commonly the case in C. difficile infection, nutrient competition is reduced. As a result, nutrients are more freely available, allowing C. difficile to establish a niche and cause infection. The ability of C. difficile to use carbohydrates as a nutrient source is well documented. Using Biolog Phenotype MicroArrays, Scaria et al. examined the ability of C. difficile strains 630, CD196, QCD-32g58, 54634, QCD-23m63, and R20291 to grow in rich BHI broth with various carbon sources.130 All C. difficile strains were found to use simple sugars to support enhanced growth. The authors identified that all six strains had the highest levels of growth (OD600nm > 2.2) in mannose, GlcNAc, and sialic acid, with moderate growth (OD600nm = 1) with GalNAc and fucose. However, these studies were not performed with minimal media and glucose was present. We recently published work examining C. difficile 2015 carbohydrate utilization in our fully defined medium CDMM using Biolog Phenotype MicroArrays.131 Similar to the C. difficile R20291 data present herein, we found that C. difficile 2015 exhibited the highest growth with mannose and GlcNAC (OD600 nm ~ 1), with lower growth observed with fucose and galactose. In these assays, we did not examine GalNAc or sialic acid. In another study using a fully defined medium and microarrays, C. difficile 2015 and 630 were found to have >1.5 fold growth advantage with mannose, GlcNAc, and sialic aicd.132 These studies confirm our present work and demonstrate that a variety of C. difficile strains and ribotypes can utilize mucin-related sugars, particularly mannose and GlcNAc. In the future, in vivo studies would be valuable to confirm the ability of C. difficile to use preferentially use sugars and examine whether mucin-degrading microbes can promote C. difficile in the setting of complex microbiota. Collectively, these findings provide an additional link between intestinal mucus, mucin degrading microbes, and C. difficile.
METHODS
Bacterial Strains and Culture Conditions.
Routine culturing of Clostridioides difficile strains R20291 (ribotype 027) and CD2015 (ribotype 027), B. thetaioatomicron ATCC 29148, and Ruminococcus torques ATCC 2775 was performed in BHIS (Brain Heart Infusion medium supplemented with 2% yeast extract and 0.2% cysteine). Akkermansia muciniphila ATCC BAA-835 was cultured in BHIS supplemented with 0.4% pig stomach mucin (Sigma # M2378). All cultures were grown in an Anaerobe Systems AS-580 anaerobic chamber supplied with a mixture of 10% CO2, 5% H2, and 85% N2 at 37 °C. Routine culturing of Vibrio harveyi BAA-1121 in Marine medium and Escherichia coli strains K12 and DH5α in LB medium were carried out aerobically at 30 and 37 °C, respectively.
To assess utilization of mucin-related carbohydrates, BHIS overnight cultures of C. difficile strains were subcultured to an optical density (OD600nm) of 0.1 into the fully defined minimal media, CDMM133 with varied carbon sources. CDMM composition contained 10 mM of either glucose, fucose, galactose, mannose, N-acetyl-glucosamine (GlcNAc), N-acetylgalactosamine (GalNAc), or N-acetyl-neuraminic acid (NANA) (all purchased from Carbsynth). Anaerobic growth of cultures at 37 °C was monitored over time (0, 3, 6, 9, 12, and 24 h) through measurement of OD600 nm on a Smartspec Plus spectrophotometer (Biorad Laboratories Inc.).
Chemotaxis and Live Imaging.
Chemotaxis of C. difficile strains toward monosaccharides was evaluated. Overnight cultures were diluted 1:50 in fresh BHIS, incubated anaerobically at 37 °C for 3 h, and then washed in PBS. Cells were fluorescently tagged with 10 μM CFDA-SE (ThermoFisher) in PBS anaerobically at 37 °C for 1 h, washed in PBS, and resuspended to an OD600nm = 2.0 in chemotaxis buffer (10 M potassium phosphate, 0.1 mM EDTA at pH 7.0) and incubated anaerobically for 1 h at 37 °C as previously described.92 Fluorescent C. difficile was then distributed into 96-well plates (200 μL/well) prior to the addition of capillary tubes (one tube per well, n = 8 tubes per group) containing chemotaxis buffer with or without 100 mM monosaccharides (glucose, fucose, galactose, mannose, GlcNAc, GalNAc, and NANA). This capillary tube setup was incubated anaerobically at 37 °C for 2 h,92 and then the capillary tube contents were collected and measured by a fluorescence plate reader (excitation 485 nm/emission 528 nm). Fluorescent microbes were also confirmed by microscopy.
For live imaging assays, CFDA-SE fluorescently tagged C. difficile was added to cooled chemotaxis buffer containing 0.05% agar and added to ibidi Chemotaxis slides (ibidi). Chemotaxis slides were placed in an Okolabs stage-top incubation chamber with CO2 mixing and humidity control. The incubation chamber was placed on a Nikon TiE inverted wide field epifluorescence microscope (Nikon) with a motorized X, Y, and Z stage for software-controlled multi-position imaging. Cells were imaged with widefield epifluorescence using a 20× PlanFluor (NA 0.45) phase contrast objective or a 20× Plan Apo (NA 0.75) differential interference contrast objective, using a SPECTRA X LED light source (Lumencor). Images were recorded using an ORCA-Flash 4.0 sCMOS camera (Hamamatsu). Videos were analyzed with the “Chemotaxis and Migration Tool,” a plugin used with the NIH ImageJ processing system (FIJI).
Quorum Sensing AI-2 Assay.
The AI-2 bioluminescence assay was performed as previously described.93,134 Briefly, V. harveyi BAA-1121 overnight cultures were diluted 1:5000 in fresh Autoinducer Bioassay (AB) liquid medium and aliquoted into a 96-well plate (100 μL/well). C. difficile cell-free conditioned CDMM (10% v/v) was added. Negative controls included uninoculated CDMM, uninoculated LB, and E. coli DH5α cell-free conditioned LB. E. coli K12 cell-free conditioned LB served as a positive control. Reactions were incubated at 30 °C shaking on a Synergy H1 microplate reader (BioTek) and luminescence measured every 15 min. Induction of luminescence was taken at the time when there was maximal difference between the positive and negative controls (3–5 h).
RNA Isolation, cDNA Preparation, and qPCR.
For gene expression studies, C. difficile was cultured anaerobically at 37 °C in CDMM (starting OD600nm = 0.1) for 16 h. Cells (1 mL) were fixed in an equal volume of ice-cold methanol, pelleted at 16 000g for 30 s, and stored at −80°C until RNA isolation. RNA was extracted from bacterial pellets using the Zymo QuickRNA kit (Zymo) as previously described.135 Briefly, pellets were resuspended in 100 μL of STE buffer (100 mM NaCl, 10 mM Tris-HCl, pH 8.0, 1 mM EDTA) and bead beaten with ~100 μL of 0.1 mm glass beads on a FastPrep bead homogenizer (MP Biologicals) for 20 s at 4.0 m/s. Next, Zymo Quick RNA lysis buffer was added and samples bead beaten as before. Beads were removed by centrifugation at 10 000g for 30 s. RNA was isolated from supernatants according to the manufacturer’s protocol. Complementary strands of DNA (cDNA) were prepared from 500 ng RNA using a SensiFast cDNA kit per manufacturer’s instructions (manufacturer). Quantitative real time PCR (qPCR) of cDNA (1 μg) was performed using FAST SYBR green (ThermoFisher) on a QuantStudio3 qPCR machine (Applied Biosystems). For quantification of C. difficile within fecal-bioreactor MUC2 biofilms, cycle of threshold values (CT) for C. difficile-specific primers were matched to a standard curve of C. difficile CFUs.
Mammalian Cell Culture.
Human colonic mucusproducing adenocarcinoma cell line LS174T ATCC CL-188, T84 (ATCC CCL-248), HT29-MTX-E12 (Sigma 12040401), and T84 (ATCC CCL-248) were maintained in DMEM medium (ATCC) supplemented with heat-inactivated 10% fetal bovine serum (FBS) (Invitrogen) at 37 °C, 5% CO2. Additionally, Vero (ATCC CCL-81) cells were grown in Dulbecco’s modified Eagle’s medium (ThermoFisher) supplemented with heat-inactivated 10% fetal bovine serum (FBS) at 37 °C, 5% CO2. All cell lines were incubated in a humidified atmosphere at 37 °C, 5% CO2 and routinely tested for Mycoplasma (Mycoplasma Detection Kit Lonza, cat# LT07–518).
Vero cells were used to assess C. difficile toxin activity as previously described.131 Briefly, Vero cells were grown to confluency on 96-well plates and treated with C. difficile CDMM supernatant (after 48 h growth) in DMEM at final concentration of 1:1 C. difficile supernatant to DMEM. Vero cells were imaged after 4 h incubation on a Nikon TiE inverted wide field epifluorescence microscope (Nikon) using a SPECTRAX LED light source (Lumencor), ORCA-Flash 4.0 sCMOS camera (Hamamatsu), and Nikon Elements Advanced Research v4.5 software. To determine cell rounding, FIJI (Formerly ImageJ; National Institutes of Health) was used to define cell diameter. Cell rounding was defined as a >50% reduction in Vero cell diameter. Data was collected in three regions/well, n = 3 wells; repeated two independent times.
MUC2 Purification.
MUC2 was collected from human mucin-producing LS174T and HT29-MTX cells as previously described.136,137 Briefly, supernatant was collected from T300 flasks of cells and incubated with phenylmethylsulfonyl fluoride, EDTA, and iodoacetamide to prevent further degradation. Ice cold ethanol was added to precipitate crude mucin protein, and crude mucin was purified by guanidine chloride and cesium chloride isopycnic density gradient high-speed ultracentrifugation (70 Ti rotor, Beckman Coulter Inc.).138,139 MUC2 fractions were identified by slot blot with a mouse anti-MUC2 antibody (Santa Cruz #sc-515032). Pooled MUC2 fractions were dialyzed, lyophilized, and resuspended in Hanks’ salt solution. MUC2 was adhered to glass coverslips (18 mm) using 3-aminopropyltriethoxysilane (APTS) as previously described.140,141 APTS-treated coverslips were incubated with 1 mg/mL purified MUC2 overnight at 4 °C and air-dried. For MUC2 adhesion assays, C. difficile was fluorescently tagged with 10 μM CFDA-SE (ThermoFisher) in PBS anaerobically at 37 °C for 1 h, washed thoroughly and then incubated with MUC2-coated coverslips anaerobically at 37 °C for 1 h. Coverslips were washed and mounted, and C. difficile localization was examined by microscopy. O-Linked glycans were removed from purified MUC2 by beta-elimination (0.5 M NaBH4 in 50 mM KOH, 50 °C overnight). MUC2 protein was isolated by the addition of ethanol, and the resulting supernatant containing O-linked glycans was lyophilized.
Human Fecal Bioreactors.
Fecal samples were collected from consenting adults who self-identified as healthy using protocols reviewed and approved by the Institutional Review Boards of Michigan State University and Baylor College of Medicine. The colonization of mucus coated coverslips by fecal microbial communities and C.difficile was tested in bioreactors. Replicate bioreactors and bioreactor medium (BRM2) were prepared as previously described (60) with the following modifications. Arabinogalactan, d-cellobiose, maltose, potassium phosphate monobasic, and potassium phosphate dibasic were added to BRM2. The reactors (independent 50 mL reactors in 100 mL media bottles) were inoculated with an anaerobic preparation of a 20% fecal slurries (final concentration, 3.5% [wt/vol]) and were operated in an anaerobic chamber with a 5% H2,5% CO2, 90% N2 atmosphere. The fecal slurries were pooled from two different healthy donors. After 16 h of outgrowth in batch culture mode, continuous-flow cultivation was initiated at a flow rate of 6.25 mL/h (8 h retention time). After 24 h of flow, the reactor communities were treated twice daily with clindamycin (final concentration, 250 μg/mL) for 4 days. The reactors were transitioned to fresh BRM2 medium supplemented with a arabinogalactan, d-cellobiose, and maltose mixture on day 4 of clindamycin treatment and continued with this medium throughout the duration of the experiment. Simultaneously, coverslips coated with mucus isolated from either LS174T or HT29-MTX, or not coated were introduced into reactors (mock, n = 2; human MUC2 (LS174T and HT29-MTX), n = 4). All six reactors were challenged with vegetative cells of C. difficile CD2015 (clinical ribotype 027) that had been propagated in BRM2 prior to inoculation. After 3 days of coincubation, mucus-coated coverslips were removed for microbial community analysis as described below.
Bioreactor 16S rDNA Sequencing and Data Analysis.
DNA from MUC2-coated coverslips was extracted using the Zymo gDNA isolation kit (Zymo) according to the manufacturer’s instructors with the addition of two rounds of bead beating. For microbiome characterization, the V4 region of the 16S rRNA gene was amplified using the NEXTflex 16S V4 Amplicon-Seq Kit 2.0 (Bioo Scientific, Austin, TX) as previously described.142 Sequencing of the pooled 16S libraries was performed using a 500 cycle v2 chemistry kit on an Illumina MiSeq instrument (Illumina, San Diego, CA) at the Texas Children’s Microbiome Center following the standard Illumina sequencing protocol.143 The barcode and primer sequences in the raw reads were trimmed using cutadapt (v1.10). Next, PhiX and human-derived sequences were removed using Bowtie2.144 Sequences were then quality filtered using the LotuS pipeline (v1.462)145 and processed as previously described.146 OTUs that fail to classify as bacteria at the kingdom level and unclassified OTUs at the phylum level were excluded from the analysis, along with the mitochondrial and cyanobacterial sequences. The median sequencing depth was 22 866 reads (range, 1960–38 207). Resultant sequences were analyzed using standard bioinformatics approaches within the TCMC.
Next generation sequencing data were analyzed by several metagenomic methods including determination of bacterial diversity, evenness, richness, and relative abundance of the bacteria identified in each sample. Relative abundance of a bacterial taxon in a sample was calculated using the statistical analysis of metagenomic profiles (STAMP v12.3).147 ANOVA was performed for multiple group comparisons. The p-values were corrected for multiple testing with the Benjamini–Hochberg method to control for false discovery rate. Diversity indices were computed using QIIME (Quantitative Insights Into Microbial Ecology, v1.9.1) software.147 Sequences are available online on the NCBI BioSample database (accession numbers: SAMN15790567, SAMN15790568, SAMN15790569, SAMN15790570, SAMN15790571, and SAMN15790572).
Immunostaining.
For live cell adhesion assays, HT29-MTX cells were seeded at 5 × 105 cells/well into Corning Costar 12-well cell culture plates (Corning, Sigma-Aldrich) containing polylysine coated coverslips and grown to confluence. HT29-MTX cells on coverslips were stained with Hoechst 33342 nuclear dye and incubated with live CFDA-SE-tagged C. difficile for 1 h at 37 °C, 5% CO2. Following incubation, coverslips were gently washed 2× with PBS and fixed with Clark’s fixative for 30 min at RT. Mucin staining was performed with an anti-MUC2 antibody (dilution: 1:200, rabbit anti-MUC2, cat # sc-15334) incubated overnight at 4 °C. Donkey anti-rabbit-Alexa Fluor 488 diluted at 1:1000 (Life Technologies) was added and incubated for 1 h at RT. Alternatively, APTS-adhered MUC2 coverslips were incubated with CFDA-labeled C. difficile for 1 h at 37 °C and fixed in Clark’s fixative. HT29-MTX and MUC2-coated coverslips were mounted to slides with mounting media (Life Technologies). Slides were imaged on an upright wide field epifluorescence Nikon Eclipse 90i microscope (Nikon, Tokyo, Japan).
Fluorescent In Situ Hybridization (FISH).
C. difficile localization was examined in bioreactor coverslips with a C. difficile-specific FISH probe [6FAM] CATCCTGTACTGGCTCAC (Integrated DNA Technologies, IDT). Coverslips were hybridized for 45 min at 45 °C in a dark humidifying chamber. To identify other bacteria, Hoechst 33342 (Invitrogen) nuclear dye was added and incubated at RT for 10 min. Mounted coverslips were imaged on an upright wide field epifluorescence Nikon Eclipse 90i (Nikon, Tokyo, Japan).
Ex Vivo C. difficile Adherence Assays.
Carnoy’s fixed paraffin-embedded mouse colons were obtained from C57BL6/J mice (6–8 weeks of age) purchased from Jackson Laboratories and housed in the BCM animal facility (n = 3 mice). Animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee at BCM (Protocol #AN-914). Mice were humanely euthanized by CO2 with thoracotomy as a secondary method. Paraffin-embedded human colon samples were obtained from US Biomax (Colon normal tissue array #BN05014; n = 72 patients). Mouse and human colon sections were deparaffinized through a series of ethanol washes. CFDA-labeled C. difficile R20291 was incubated with deparaffinized tissue for 1 hat 37 °C. Epithelial cells were identified by staining nuclei with Hoechst 33342 for 10 min at room temperature. Mucus glycans were identified by staining with the lectin UEA-1 (Ulex Europaeus Agglutinin-1; Vector Laboratories, Rhodamine labeled #RL-1062; 1:200 dilution) for 30 min at room temperature.
To remove glycans from tissue and APTS-MUC2 coverslips, samples were processed as previously described.88 Briefly, deparaffinized slides or coverslips were incubated with 0.1 M NaOH for 30 min at room temperature and oxidized by adding 100 mM NaIO4 in 100 mM acetate buffer (pH 4.5) overnight at 4 °C. Reactive aldehydes were neutralized by incubating with 2% glycine solution for 30 min and beta-elimination was performed by adding 0.1 M NaOH for 30 min at room temperature. These slides and coverslips were then incubated with CFDA-labeled C. difficile and imaged on a Nikon Eclipse 90i instrument.
Identification of Microbial Glycosyl Hydrolases using the Carbohydrate Active Enzymes database (CAZy).
Bacterial glycosyl hydrolases (GH) were examined using the Carbohydrate-Active Enzyme (CAZy) database (http://www.cazy.org) as previously described.148–151 GH copy numbers were collected from selected annotated genomes. Only GHs known to be involved in mucin degradation (GH 2, 20, 29, 33, 38 42, 84, 85, 89, 95, 101, 125, 129) were included for analysis.55
Statistical Analysis.
GraphPad Prism software (ver. 8) (GraphPad Inc., La Jolla, CA) was used to generate all graphs. One-way or two-way ANOVA with a Bonferroni posthoc test was used for all assessment, except for ibidi chemotaxis analysis, which was performed by Student’s t test. The data are presented as mean ± SEM, with P < 0.05 (*).
Acknowledgments
The authors declare the following competing financial interest(s): J.V. serves on the scientific advisory boards of Biomica, Plexus Worldwide, and Seed Health, and J.V. and J.K.S. have received unrestricted research support from Biogaia, AB. The remaining authors have no commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acsinfecdis.0c00634
Contributor Information
Melinda A. Engevik, Department of Pathology & Immunology, Baylor College of Medicine, Houston, Texas 77030, United States; Department of Pathology, Texas Children’s Hospital, Houston, Texas 77030, United States.
Amy C. Engevik, Department of Surgery and Epithelial Biology Center, Vanderbilt University School of Medicine, Nashville, Tennessee 37232, United States
Kristen A. Engevik, Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas 77030, United States.
Jennifer M. Auchtung, Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas 77030, United States; Department of Food Science and Technology, University of Nebraska-Lincoln, Lincoln, Nebraska 68588, United States
Alexandra L. Chang-Graham, Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas 77030, United States
Wenly Ruan, Department of Pathology & Immunology, Baylor College of Medicine, Houston, Texas 77030, United States; Department of Pathology, Texas Children’s Hospital, Houston, Texas 77030, United States.
Ruth Ann Luna, Department of Pathology & Immunology, Baylor College of Medicine, Houston, Texas 77030, United States; Department of Pathology, Texas Children’s Hospital, Houston, Texas 77030, United States.
Joseph M. Hyser, Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas 77030, United States
Jennifer K. Spinler, Department of Pathology & Immunology, Baylor College of Medicine, Houston, Texas 77030, United States; Department of Pathology, Texas Children’s Hospital, Houston, Texas 77030, United States
James Versalovic, Department of Pathology & Immunology, Baylor College of Medicine, Houston, Texas 77030, United States; Department of Pathology, Texas Children’s Hospital, Houston, Texas 77030, United States.
REFERENCES
- (1).Savidge TC, Pan WH, Newman P, O’Brien M, Anton PM, and Pothoulakis C (2003) Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology 125, 413–420. [DOI] [PubMed] [Google Scholar]
- (2).Campbell RJ, Giljahn L, Machesky K, Cibulskas-White K, Lane LM, Porter K, Paulson JO, Smith FW, and McDonald LC (2009) Clostridium difficile infection in Ohio hospitals and nursing homes during 2006. Infection control and hospital epidemiology: the official journal of the Society of Hospital Epidemiologists of America 30, 526–533. [DOI] [PubMed] [Google Scholar]
- (3).Dubberke ER, Butler AM, Reske KA, Agniel D, Olsen MA, D’Angelo G, McDonald LC, and Fraser VJ (2008) Attributable outcomes of endemic Clostridium difficile-associated disease in nonsurgical patients. Emerging Infect. Dis. 14, 1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).O’Brien JA, Lahue BJ, Caro JJ, and Davidson DM (2007) The emerging infectious challenge of clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infection control and hospital epidemiology: the official journal of the Society of Hospital Epidemiologists of America 28, 1219–1227. [DOI] [PubMed] [Google Scholar]
- (5).Redelings MD, Sorvillo F, and Mascola L (2007) Increase in Clostridium difficile-related mortality rates, United States, 1999–2004. Emerging Infect. Dis. 13, 1417–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Koenigsknecht MJ, Theriot CM, Bergin IL, Schumacher CA, Schloss PD, and Young VB (2015) Dynamics and establishment of Clostridium difficile infection in the murine gastrointestinal tract. Infect. Immun. 83, 934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Alemka A, Corcionivoschi N, and Bourke B (2012) Defense and adaptation: the complex inter-relationship between Campylobacter jejuni and mucus. Front. Cell. Infect. Microbiol. 2, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Lee A, O’Rourke JL, Barrington PJ, and Trust TJ (1986) Mucus colonization as a determinant of pathogenicity in intestinal infection by Campylobacter jejuni: a mouse cecal model. Infect. Immun. 51, 536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Tu QV, McGuckin MA, and Mendz GL (2008) Campylobacter jejuni response to human mucin MUC2: modulation of colonization and pathogenicity determinants. J. Med. Microbiol. 57, 795–802. [DOI] [PubMed] [Google Scholar]
- (10).Reddi G, Pruss K, Cottingham KL, Taylor RK, and Almagro-Moreno S (2018) Catabolism of mucus components influences motility of Vibrio cholerae in the presence of environmental reservoirs. PLoS One 13, e0201383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Furter M, Sellin ME, Hansson GC, and Hardt WD (2019) Mucus Architecture and Near-Surface Swimming Affect Distinct Salmonella Typhimurium Infection Patterns along the Murine Intestinal Tract. Cell Rep. 27, 2665–2678.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Linden SK, Florin TH, and McGuckin MA (2008) Mucin dynamics in intestinal bacterial infection. PLoS One 3, e3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).McCormick BA, Stocker BA, Laux DC, and Cohen PS (1988) Roles of motility, chemotaxis, and penetration through and growth in intestinal mucus in the ability of an avirulent strain of Salmonella typhimurium to colonize the large intestine of streptomycin-treated mice. Infect. Immun. 56, 2209–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Mora-Uribe P, Miranda-Cardenas C, Castro-Cordova P, Gil F, Calderon I, Fuentes JA, Rodas PI, Banawas S, Sarker MR, and Paredes-Sabja D (2016) Characterization of the Adherence of Clostridium difficile Spores: The Integrity of the Outermost Layer Affects Adherence Properties of Spores of the Epidemic Strain R20291 to Components of the Intestinal Mucosa. Front. Cell. Infect. Microbiol. 6, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Eveillard M, Fourel V, Bare MC, Kerneis S, Coconnier MH,Karjalainen T, Bourlioux P, and Servin AL(1993) Identification and characterization of adhesive factors of Clostridium difficile involved in adhesion to human colonic enterocyte-like Caco-2 and mucus-secreting HT29 cells in culture. Mol. Microbiol. 7, 371–381. [DOI] [PubMed] [Google Scholar]
- (16).Karjalainen T, Barc MC, Collignon A, Trolle S, Boureau H, Cotte-Laffitte J, and Bourlioux P (1994) Cloning of a genetic determinant from Clostridium difficile involved in adherence to tissue culture cells and mucus. Infect. Immun. 62, 4347–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Olson A, Diebel LN, and Liberati DM (2013) Effect of host defenses on Clostridium difficile toxin-induced intestinal barrier injury. J. Trauma Acute Care Surg. 74, 983–989, discussion 989–990.. [DOI] [PubMed] [Google Scholar]
- (18).Diebel LN, and Liberati DM (2014) Reinforcement of the intestinal mucus layer protects against Clostridium difficile intestinal injury in vitro. J. Am. Coll Surg 219, 460–468. [DOI] [PubMed] [Google Scholar]
- (19).Tasteyre A, Barc MC, Collignon A, Boureau H, and Karjalainen T (2001) Role of FliC and FliD flagellar proteins of Clostridium difficile in adherence and gut colonization. Infect. Immun. 69, 7937–7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Gomez Trevino M, Boureau H, Karjalainen T, and Bourlioux P (1996) Clostridium dificile Adherence to Mucus: Results of an in vivo and ex vivo assay. Microb. Ecol. Health Dis. 9, 329–334. [Google Scholar]
- (21).Semenyuk EG, Poroyko VA, Johnston PF, Jones SE, Knight KL, Gerding DN, and Driks A (2015) Analysis of Bacterial Communities during Clostridium difficile Infection in the Mouse. Infect. Immun. 83, 4383–4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Engevik MA, Engevik KA, Yacyshyn MB, Wang J, Hassett DJ, Darien B, Yacyshyn BR, and Worrell RT (2015) Human Clostridium difficile infection: inhibition of NHE3 and microbiota profile. Am. J. Physiol Gastrointest Liver Physiol 308, G497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Engevik MA, Yacyshyn MB, Engevik KA, Wang J, Darien B, Hassett DJ, Yacyshyn BR, and Worrell RT (2015) Human Clostridium difficile infection: altered mucus production and composition. Am. J. Physiol Gastrointest Liver Physiol 308, G510–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Ahmed S, Macfarlane GT, Fite A, McBain AJ, Gilbert P, and Macfarlane S (2007) Mucosa-associated bacterial diversity in relation to human terminal ileum and colonic biopsy samples. Appl. Environ. Microbiol. 73, 7435–7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Macfarlane GT, Furrie E, and Macfarlane S (2008) Bacterial milieu and mucosal bacteria in ulcerative colitis. Novartis Found Symp. 263, 57–64, discussion 64–70, 211–218.. [DOI] [PubMed] [Google Scholar]
- (26).Kleessen B, and Blaut M (2005) Modulation of gut mucosal biofilms. Br. J. Nutr. 93, S35–40. [DOI] [PubMed] [Google Scholar]
- (27).Macfarlane S, Woodmansey EJ, and Macfarlane GT (2005) Colonization of mucin by human intestinal bacteria and establishment of biofilm communities in a two-stage continuous culture system. Appl. Environ. Microbiol. 71, 7483–7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Crowther GS, Chilton CH, Todhunter SL, Nicholson S, Freeman J, Baines SD, and Wilcox MH (2014) Comparison of planktonic and biofilm-associated communities of Clostridium difficile and indigenous gut microbiota in a triple-stage chemostat gut model. J. Antimicrob. Chemother. 69, 2137–2147. [DOI] [PubMed] [Google Scholar]
- (29).Crowther GS, Chilton CH, Todhunter SL, Nicholson S, Freeman J, Baines SD, and Wilcox MH (2014) Development and validation of a chemostat gut model to study both planktonic and biofilm modes of growth of Clostridium difficile and human microbiota. PLoS One 9, e88396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Dapa T, and Unnikrishnan M (2013) Biofilm formation by Clostridium difficile. Gut Microbes 4, 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Dawson LF, Valiente E, Faulds-Pain A, Donahue EH, and Wren BW (2012) Characterisation of Clostridium difficile biofilm formation, a role for Spo0A. PLoS One 7, e50527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Thapa T, Leuzzi R, Ng YK, Baban ST, Adamo R, Kuehne SA, Scarselli M, Minton NP, Serruto D, and Unnikrishnan M (2013) Multiple factors modulate biofilm formation by the anaerobic pathogen Clostridium difficile. J. Bacteriol. 195, 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Gosteva VV, Klitsunova NV, and Bondarenko VM (2009) [Interaction of Clostridium difficile with bacterial associations of parietal biofilm in colon of mice]. Zh. Mikrobiol., Epidemiol. Immunobiol, 3–6. [PubMed] [Google Scholar]
- (34).Hammond EN, Donkor ES, and Brown CA (2014) Biofilm formation of Clostridium difficile and susceptibility to Manuka honey. BMC Complementary Altern. Med. 14, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Jain S, Smyth D, O’Hagan BMG, Heap JT, McMullan G, Minton NP, and Ternan NG (2017) Inactivation of the dnaK gene in Clostridium difficile 630 Deltaerm yields a temperature-sensitive phenotype and increases biofilm-forming ability. Sci. Rep. 7, 17522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Maldarelli GA, Piepenbrink KH, Scott AJ, Freiberg JA, Song Y, Achermann Y, Ernst RK, Shirtliff ME, Sundberg EJ, Donnenberg MS, and von Rosenvinge EC (2016) Type IV pili promote early biofilm formation by Clostridium difficile. Pathog. Dis. 74, ftw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Pantaleon V, Soavelomandroso AP, Bouttier S, Briandet R, Roxas B, Chu M, Collignon A, Janoir C, Vedantam G, and Candela T (2015) The Clostridium difficile Protease Cwp84 Modulates both Biofilm Formation and Cell-Surface Properties. PLoS One 10, e0124971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Pickering DS, Wilcox MH, and Chilton CH (2018) Biofilm-derived spores of Clostridioides (Clostridium) difficile exhibit increased thermotolerance compared to planktonic spores. Anaerobe 54, 169–171. [DOI] [PubMed] [Google Scholar]
- (39).Piotrowski M, Karpinski P, Pituch H, van Belkum A, and Obuch-Woszczatynski P (2017) Antimicrobial effects of Manuka honey on in vitro biofilm formation by Clostridium difficile. Eur. J. Clin. Microbiol. Infect. Dis. 36, 1661–1664. [DOI] [PubMed] [Google Scholar]
- (40).Poquet I, Saujet L, Canette A, Monot M, Mihajlovic J, Ghigo JM, Soutourina O, Briandet R, Martin-Verstraete I, and Dupuy B (2018) Clostridium difficile Biofilm: Remodeling Metabolism and Cell Surface to Build a Sparse and Heterogeneously Aggregated Architecture. Front. Microbiol. 9, 2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Purcell EB, McKee RW, Courson DS, Garrett EM, McBride SM, Cheney RE, and Tamayo R (2017) A Nutrient-Regulated Cyclic Diguanylate Phosphodiesterase Controls Clostridium difficile Biofilm and Toxin Production during Stationary Phase. Infect. Immun. 85, e00347–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Soavelomandroso AP, Gaudin F, Hoys S, Nicolas V, Vedantam G, Janoir C, and Bouttier S (2017) Biofilm Structures in a Mono-Associated Mouse Model of Clostridium difficile Infection. Front. Microbiol. 8, 2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Valiente E, Bouche L, Hitchen P, Faulds-Pain A, Songane M, Dawson LF, Donahue E, Stabler RA, Panico M, Morris HR, Bajaj-Elliott M, Logan SM, Dell A, and Wren BW (2016) Role of Glycosyltransferases Modifying Type B Flagellin of Emerging Hypervirulent Clostridium difficile Lineages and Their Impact on Motility and Biofilm Formation. J. Biol. Chem. 291, 25450–25461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Vuotto C, Donelli G, Buckley A, and Chilton C (2018) Clostridium difficile Biofilm. Adv. Exp. Med. Biol. 1050, 97–115. [DOI] [PubMed] [Google Scholar]
- (45).Vuotto C, Moura I, Barbanti F, Donelli G, and Spigaglia P (2016) Subinhibitory concentrations of metronidazole increase biofilm formation in Clostridium difficile strains. Pathog. Dis. 74, ftv114. [DOI] [PubMed] [Google Scholar]
- (46).Walter BM, Cartman ST, Minton NP, Butala M, and Rupnik M (2015) The SOS Response Master Regulator LexA Is Associated with Sporulation, Motility and Biofilm Formation in Clostridium difficile. PLoS One 10, e0144763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Johansson ME, Larsson JM, and Hansson GC (2011) The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc. Natl. Acad. Sci. U. S. A. 108, 4659–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, and Hansson GC (2008) The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. U. S. A. 105, 15064–15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Engevik MA, Aihara E, Montrose MH, Shull GE, Hassett DJ, and Worrell RT (2013) Loss of NHE3 alters gut microbiota composition and influences Bacteroides thetaiotaomicron growth. Am. J. Physiol Gastrointest Liver Physiol 305, G697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Engevik MA, Hickerson A, Shull GE, and Worrell RT (2013) Acidic conditions in the NHE2(–/–) mouse intestine result in an altered mucosa-associated bacterial population with changes in mucus oligosaccharides. Cell. Physiol. Biochem. 32, 111–128. [DOI] [PubMed] [Google Scholar]
- (51).Jensen PH, Kolarich D, and Packer NH (2010) Mucin-type O-glycosylation–putting the pieces together. FEBS J. 277, 81–94. [DOI] [PubMed] [Google Scholar]
- (52).Crost EH, Tailford LE, Le Gall G, Fons M, Henrissat B, and Juge N (2013) Utilisation of mucin glycans by the human gut symbiont Ruminococcus gnavus is strain-dependent. PLoS One 8, e76341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Holmén Larsson JM, Karlsson H, Sjovall H, and Hansson GC (2009) A complex, but uniform O-glycosylation of the human MUC2 mucin from colonic biopsies analyzed by nanoLC/MSn. Glycobiology 19, 756–766. [DOI] [PubMed] [Google Scholar]
- (54).van Klinken BJ, Einerhand AW, Buller HA, and Dekker J (1998) The oligomerization of a family of four genetically clustered human gastrointestinal mucins. Glycobiology 8, 67–75. [DOI] [PubMed] [Google Scholar]
- (55).Tailford LE, Crost EH, Kavanaugh D, and Juge N (2015) Mucin glycan foraging in the human gut microbiome. Front. Genet. 6, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Ruas-Madiedo P, Gueimonde M, Fernandez-Garcia M, de los Reyes-Gavilan CG, and Margolles A (2008) Mucin degradation by Bifidobacterium strains isolated from the human intestinal microbiota. Appl. Environ. Microbiol. 74, 1936–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Turroni F, Bottacini F, Foroni E, Mulder I, Kim JH, Zomer A, Sanchez B, Bidossi A, Ferrarini A, Giubellini V, Delledonne M, Henrissat B, Coutinho P, Oggioni M, Fitzgerald GF, Mills D, Margolles A, Kelly D, van Sinderen D, and Ventura M (2010) Genome analysis of Bifidobacterium bifidum PRL2010 reveals metabolic pathways for host-derived glycan foraging. Proc. Natl. Acad. Sci. U. S. A. 107, 19514–19519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Crociani F, Alessandrini A, Mucci MM, and Biavati B (1994) Degradation of complex carbohydrates by Bifidobacterium spp. Int. J. Food Microbiol. 24, 199–210. [DOI] [PubMed] [Google Scholar]
- (59).Salyers AA, West SE, Vercellotti JR, and Wilkins TD (1977) Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. Appl. Environ. Microbiol. 34, 529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Ruiz L, Gueimonde M, Coute Y, Salminen S, Sanchez JC, de los Reyes-Gavilan CG, and Margolles A (2011) Evaluation of the ability of Bifidobacterium longum to metabolize human intestinal mucus. FEMS Microbiol. Lett. 314, 125–130. [DOI] [PubMed] [Google Scholar]
- (61).Hoskins LC, and Boulding ET (1981) Mucin degradation in human colon ecosystems. Evidence for the existence and role of bacterial subpopulations producing glycosidases as extracellular enzymes. J. Clin. Invest. 67, 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Koropatkin NM, Cameron EA, and Martens EC (2012) How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 10, 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Ouwerkerk JP, de Vos WM, and Belzer C (2013) Glycobiome: bacteria and mucus at the epithelial interface. Best Pract Res. Clin Gastroenterol 27, 25–38. [DOI] [PubMed] [Google Scholar]
- (64).Sicard JF, Le Bihan G, Vogeleer P, Jacques M, and Harel J (2017) Interactions of Intestinal Bacteria with Components of the Intestinal Mucus. Front. Cell. Infect. Microbiol. 7, 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Rho JH, Wright DP, Christie DL, Clinch K, Furneaux RH, and Roberton AM (2005) A novel mechanism for desulfation of mucin: identification and cloning of a mucin-desulfating glycosidase (sulfoglycosidase) from Prevotella strain RS2. J. Bacteriol. 187, 1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, Hooper LV, and Gordon JI (2003) A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science 299, 2074–2076. [DOI] [PubMed] [Google Scholar]
- (67).Sonnenburg JL, Xu J, Leip DD, Chen CH, Westover BP, Weatherford J, Buhler JD, and Gordon JI (2005) Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307, 1955–1959. [DOI] [PubMed] [Google Scholar]
- (68).Png CW, Linden SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, and Florin TH (2010) Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am. J. Gastroenterol. 105, 2420–2428. [DOI] [PubMed] [Google Scholar]
- (69).Huang JY, Lee SM, and Mazmanian SK (2011) The human commensal Bacteroides fragilis binds intestinal mucin. Anaerobe 17, 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Johansson ME, Jakobsson HE, Holmen-Larsson J, Schutte A, Ermund A, Rodriguez-Pineiro AM, Arike L, Wising C, Svensson F, Backhed F, and Hansson GC (2015) Normalization of Host Intestinal Mucus Layers Requires Long-Term Microbial Colonization. Cell Host Microbe 18, 582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Arike L, and Hansson GC (2016) The Densely O-Glycosylated MUC2Mucin Protects the Intestine and Provides Food for the Commensal Bacteria. J. Mol. Biol. 428, 3221–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Bjursell MK, Martens EC, and Gordon JI (2006) Functional genomic and metabolic studies of the adaptations of a prominent adult human gut symbiont, Bacteroides thetaiotaomicron, to the suckling period. J. Biol. Chem. 281, 36269–36279. [DOI] [PubMed] [Google Scholar]
- (73).Martens EC, Chiang HC, and Gordon JI (2008) Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe 4, 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Fabich AJ, Jones SA, Chowdhury FZ, Cernosek A, Anderson A, Smalley D, McHargue JW, Hightower GA, Smith JT, Autieri SM, Leatham MP, Lins JJ, Allen RL, Laux DC, Cohen PS, and Conway T (2008) Comparison of carbon nutrition for pathogenic and commensal Escherichia coli strains in the mouse intestine. Infect. Immun. 76, 1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Bertin Y, Chaucheyras-Durand F, Robbe-Masselot C, Durand A, de la Foye A, Harel J, Cohen PS, Conway T, Forano E, and Martin C (2013) Carbohydrate utilization by enterohaemorrhagic Escherichia coli O157:H7 in bovine intestinal content. Environ. Microbiol. 15, 610–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Almagro-Moreno S, Pruss K, and Taylor RK (2015) Intestinal Colonization Dynamics of Vibrio cholerae. PLoS Pathog. 11, e1004787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Hoyer LL, Hamilton AC, Steenbergen SM, and Vimr ER (1992) Cloning, sequencing and distribution of the Salmonella typhimurium LT2 sialidase gene, nanH, provides evidence for interspecies gene transfer. Mol. Microbiol. 6, 873–884. [DOI] [PubMed] [Google Scholar]
- (78).Millet YA, Alvarez D, Ringgaard S, von Andrian UH, Davis BM, and Waldor MK (2014) Insights into Vibrio cholerae intestinal colonization from monitoring fluorescently labeled bacteria. PLoS Pathog. 10, e1004405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Jones GW, and Freter R (1976) Adhesive properties of Vibrio cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect. Immun. 14, 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Hugdahl MB, Beery JT, and Doyle MP (1988) Chemotactic behavior of Campylobacter jejuni. Infect. Immun. 56, 1560–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Stecher B, Hapfelmeier S, Muller C, Kremer M, Stallmach T, and Hardt WD (2004) Flagella and chemotaxis are required for efficient induction of Salmonella enterica serovar Typhimurium colitis in streptomycin-pretreated mice. Infect. Immun. 72, 4138–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).McAuley JL, Linden SK, Png CW, King RM, Pennington HL, Gendler SJ, Florin TH, Hill GR, Korolik V, and McGuckin MA (2007) MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J. Clin. Invest. 117, 2313–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Lei XH, and Bochner BR (2013) Using phenotype microarrays to determine culture conditions that induce or repress toxin production by Clostridium difficile and other microorganisms. PLoS One 8, e56545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Scaria J, Suzuki H, Ptak CP, Chen JW, Zhu Y, Guo XK, and Chang YF (2015) Comparative genomic and phenomic analysis of Clostridium difficile and Clostridium sordellii, two related pathogens with differing host tissue preference. BMC Genomics 16, 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Robinson CD, Auchtung JM, Collins J, and Britton RA (2014) Epidemic Clostridium difficile strains demonstrate increased competitive fitness compared to nonepidemic isolates. Infect. Immun. 82, 2815–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Theriot CM, Koenigsknecht MJ, Carlson PE Jr., Hatton GE, Nelson AM, Li B, Huffnagle GBJZL, and Young VB (2014) Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat. Commun. 5, 3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Juge N (2012) Microbial adhesins to gastrointestinal mucus. Trends Microbiol. 20, 30–39. [DOI] [PubMed] [Google Scholar]
- (88).Hong JC, and Kim YS (2000) Alkali-catalyzed beta-elimination of periodate-oxidized glycans: a novel method of chemical deglycosylation of mucin gene products in paraffin embedded sections. Glycoconjugate J. 17, 691–703. [DOI] [PubMed] [Google Scholar]
- (89).Almeida MG, Kiss DR, Zilberstein B, Quintanilha AG, Teixeira MG, and Habr-Gama A (2008) Intestinal mucosa-associated microflora in ulcerative colitis patients before and after restorative proctocolectomy with an ileoanal pouch. Dis. Colon Rectum 51, 1113–1119. [DOI] [PubMed] [Google Scholar]
- (90).Auchtung JM, Robinson CD, and Britton RA (2015) Cultivation of stable, reproducible microbial communities from different fecal donors using minibioreactor arrays (MBRAs). Microbiome 3, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Borriello SP (1998) Pathogenesis of Clostridium difficile infection. J. Antimicrob. Chemother. 41, 13–19. [DOI] [PubMed] [Google Scholar]
- (92).Bainer R, Park H, and Cluzel P (2003) A high-throughput capillary assay for bacterial chemotaxis. J. Microbiol. Methods 55, 315–319. [DOI] [PubMed] [Google Scholar]
- (93).Carter GP, Purdy D, Williams P, and Minton NP (2005) Quorum sensing in Clostridium difficile: analysis of a luxS-type signalling system. J. Med. Microbiol. 54, 119–127. [DOI] [PubMed] [Google Scholar]
- (94).Yun B, Oh S, Song M, Hong YS, Park S, Park DJ, Griffiths MW, and Oh S (2015) Inhibitory Effect of Epigallocatechin Gallate on the Virulence of Clostridium difficile PCR Ribotype 027. J. Food Sci. 80, M2925–2931. [DOI] [PubMed] [Google Scholar]
- (95).Derrien M, Vaughan EE, Plugge CM, and de Vos WM (2004) Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 54, 1469–1476. [DOI] [PubMed] [Google Scholar]
- (96).Hoskins LC, Agustines M, McKee WB, Boulding ET, Kriaris M, and Niedermeyer G (1985) Mucin degradation in human colon ecosystems. Isolation and properties of fecal strains that degrade ABH blood group antigens and oligosaccharides from mucin glycoproteins. J. Clin. Invest. 75, 944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Hoskins LC, and Zamcheck N (1968) Bacterial degradation of gastrointestinal mucins. I. Comparison of mucus constituents in the stools of germ-free and conventional rats. Gastroenterology 54, 210–217. [PubMed] [Google Scholar]
- (98).Miller RS, and Hoskins LC (1981) Mucin degradation in human colon ecosystems. Fecal population densities of mucin-degrading bacteria estimated by a ″most probable number″ method. Gastroenterology 81, 759–765. [PubMed] [Google Scholar]
- (99).Hryckowian AJ, Pruss KM, and Sonnenburg JL (2017) The emerging metabolic view of Clostridium difficile pathogenesis. Curr. Opin. Microbiol. 35, 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Croswell A, Amir E, Teggatz P, Barman M, and Salzman NH (2009) Prolonged impact of antibiotics on intestinal microbial ecology and susceptibility to enteric Salmonella infection. Infect. Immun. 77, 2741–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Ng KM, Aranda-Diaz A, Tropini C, Frankel MR, Van Treuren W, OĽaughlin CT, Merrill BD, Yu FB, Pruss KM, Oliveira RA, Higginbottom SK, Neff NF, Fischbach MA, Xavier KB, Sonnenburg JL, and Huang KC (2019) Recovery of the Gut Microbiota after Antibiotics Depends on Host Diet, Community Context, and Environmental Reservoirs. Cell Host Microbe 26, 650–665.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, Gopinath S, Naidu N, Choudhury B, Weimer BC, Monack DM, and Sonnenburg JL (2013) Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 502, 96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Rodrigues RR, Greer RL, Dong X, DSouza KN, Gurung M, Wu JY, Morgun A, and Shulzhenko N (2017) Antibiotic-Induced Alterations in Gut Microbiota Are Associated with Changes in Glucose Metabolism in Healthy Mice. Front. Microbiol. 8, 2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Fletcher JR, Erwin S, Lanzas C, and Theriot CM (2018) Shifts in the Gut Metabolome and Clostridium difficile Transcriptome throughout Colonization and Infection in a Mouse Model. mSphere 3, e00089–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Wilson KH, and Perini F (1988) Role of competition for nutrients in suppression of Clostridium difficile by the colonic microflora. Infect. Immun. 56, 2610–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Pudlo NA, Urs K, Kumar SS, German JB, Mills DA, and Martens EC (2015) Symbiotic Human Gut Bacteria with Variable Metabolic Priorities for Host Mucosal Glycans. mBio 6, e01282–01215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Lozupone CA, Hamady M, Cantarel BL, Coutinho PM, Henrissat B, Gordon JI, and Knight R (2008) The convergence of carbohydrate active gene repertoires in human gut microbes. Proc. Natl. Acad. Sci. U. S. A. 105, 15076–15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Nguyen D, Joshi-Datar A, Lepine F, Bauerle E, Olakanmi O, Beer K, McKay G, Siehnel R, Schafhauser J, Wang Y, Britigan BE, and Singh PK (2011) Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science 334, 982–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Perez-Cobas AE, Artacho A, Ott SJ, Moya A, Gosalbes MJ, and Latorre A (2014) Structural and functional changes in the gut microbiota associated to Clostridium difficile infection. Front. Microbiol. 5, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Sangster W, Hegarty JP, Schieffer KM, Wright JR, Hackman J, Toole DR, Lamendella R, and Stewart DB Sr. (2016) Bacterial and Fungal Microbiota Changes Distinguish C. difficile Infection from Other Forms of Diarrhea: Results of a Prospective Inpatient Study. Front. Microbiol. 7, 789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Skraban J, Dzeroski S, Zenko B, Mongus D, Gangl S, and Rupnik M (2013) Gut microbiota patterns associated with colonization of different Clostridium difficile ribotypes. PLoS One 8, e58005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Derrien M, van Passel MW, van de Bovenkamp JH, Schipper RG, de Vos WM, and Dekker J (2010) Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes 1, 254–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, Schmidt TM, and Young VB (2008) Decreased diversity of the fecal Microbiome in recurrent Clostridium difficile-associated diarrhea. J. Infect. Dis. 197, 435–438. [DOI] [PubMed] [Google Scholar]
- (114).Amrane S, Hocquart M, Afouda P, Kuete E, Pham TP, Dione N, Ngom II, Valles C, Bachar D, Raoult D, and Lagier JC (2019) Metagenomic and culturomic analysis of gut microbiota dysbiosis during Clostridium difficile infection. Sci. Rep. 9, 12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Manges AR, Labbe A, Loo VG, Atherton JK, Behr MA, Masson L, Tellis PA, and Brousseau R (2010) Comparative metagenomic study of alterations to the intestinal microbiota and risk of nosocomial Clostridum difficile-associated disease. J. Infect. Dis. 202, 1877–1884. [DOI] [PubMed] [Google Scholar]
- (116).Milani C, Ticinesi A, Gerritsen J, Nouvenne A, Lugli GA, Mancabelli L, Turroni F, Duranti S, Mangifesta M, Viappiani A, Ferrario C, Maggio M, Lauretani F, De Vos W, van Sinderen D, Meschi T, and Ventura M (2016) Gut microbiota composition and Clostridium difficile infection in hospitalized elderly individuals: a metagenomic study. Sci. Rep. 6, 25945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Pultz NJ, Hoskins LC, and Donskey CJ (2006) Vancomycin-resistant Enterococci may obtain nutritional support by scavenging carbohydrate fragments generated during mucin degradation by the anaerobic microbiota of the colon. Microb. Drug Resist. 12, 63–67. [DOI] [PubMed] [Google Scholar]
- (118).Corfield AP, Wagner SA, Clamp JR, Kriaris MS, and Hoskins LC (1992) Mucin degradation in the human colon: production of sialidase, sialate O-acetylesterase, N-acetylneuraminate lyase, arylesterase, and glycosulfatase activities by strains of fecal bacteria. Infect. Immun. 60, 3971–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Haider K, Hossain A, Wanke C, Qadri F, Ali S, and Nahar S (1993) Production of mucinase and neuraminidase and binding of Shigella to intestinal mucin. J. Diarrhoeal Dis. Res. 11, 88–92. [PubMed] [Google Scholar]
- (120).Corfield AP, Wagner SA, O’Donnell LJ, Durdey P, Mountford RA, and Clamp JR (1993) The roles of enteric bacterial sialidase, sialate O-acetyl esterase and glycosulfatase in the degradation of human colonic mucin. Glycoconjugate J. 10, 72–81. [DOI] [PubMed] [Google Scholar]
- (121).McGuckin MA, Linden SK, Sutton P, and Florin TH (2011) Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 9, 265–278. [DOI] [PubMed] [Google Scholar]
- (122).Ijssennagger N, Belzer C, Hooiveld GJ, Dekker J, van Mil SW, Muller M, Kleerebezem M, and van der Meer R (2015) Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc. Natl. Acad. Sci. U. S. A. 112, 10038–10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Greer RL, Dong X, Moraes AC, Zielke RA, Fernandes GR, Peremyslova E, Vasquez-Perez S, Schoenborn AA, Gomes EP, Pereira AC, Ferreira SR, Yao M, Fuss IJ, Strober W, Sikora AE, Taylor GA, Gulati AS, Morgun A, and Shulzhenko N (2016) Akkermansia muciniphila mediates negative effects of IFNgamma on glucose metabolism. Nat. Commun. 7, 13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Gomes JMG, Costa JA, and Alfenas RCG (2017) Metabolic endotoxemia and diabetes mellitus: A systematic review. Metab., Clin. Exp. 68, 133–144. [DOI] [PubMed] [Google Scholar]
- (125).Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, and Bae JW (2014) An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 63, 727–735. [DOI] [PubMed] [Google Scholar]
- (126).Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, and Cani PD (2013) Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. U. S. A. 110, 9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Grander C, Adolph TE, Wieser V, Lowe P, Wrzosek L, Gyongyosi B, Ward DV, Grabherr F, Gerner RR, Pfister A, Enrich B, Ciocan D, Macheiner S, Mayr L, Drach M, Moser P, Moschen AR, Perlemuter G, Szabo G, Cassard AM, and Tilg H (2018) Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 67, 891–901. [DOI] [PubMed] [Google Scholar]
- (128).Wang Q, Huang SQ, Li CQ, Xu Q, and Zeng QP (2017) Akkermansia muciniphila May Determine Chondroitin Sulfate Ameliorating or Aggravating Osteoarthritis. Front. Microbiol. 8, 1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A, Young VB, Henrissat B, Wilmes P, Stappenbeck TS, Nunez G, and Martens EC (2016) A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 167, 1339–1353.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Scaria J, Chen JW, Useh N, He H, McDonough SP, Mao C, Sobral B, and Chang YF (2014) Comparative nutritional and chemical phenome of Clostridium difficile isolates determined using phenotype microarrays. Int. J. Infect. Dis. 27, 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Engevik MA, Danhof HA, Shrestha R, Chang-Graham AL, Hyser JM, Haag AM, Mohammad MA, Britton RA, Versalovic J, Sorg JA, and Spinler JK (2020) Reuterin disrupts Clostridioides difficile metabolism and pathogenicity through reactive oxygen species generation. Gut Microbes 12, 1788898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Collins J, Robinson C, Danhof H, Knetsch CW, van Leeuwen HC, Lawley TD, Auchtung JM, and Britton RA (2018) Dietary trehalose enhances virulence of epidemic Clostridium difficile. Nature 553, 291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Karasawa T, Ikoma S, Yamakawa K, and Nakamura S (1995) A defined growth medium for Clostridium difficile. Microbiology 141 (Pt 2), 371–375. [DOI] [PubMed] [Google Scholar]
- (134).Bassler BL, Wright M, Showalter RE, and Silverman MR (1993) Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol. Microbiol. 9, 773–786. [DOI] [PubMed] [Google Scholar]
- (135).Engevik MA, Morra CN, Roth D, Engevik K, Spinler JK, Devaraj S, Crawford SE, Estes MK, Kalkum M, and Versalovic J (2019) Microbial Metabolic Capacity for Intestinal Folate Production and Modulation of Host Folate Receptors. Front. Microbiol. 10, 2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Ouwehand AC, Isolauri E, Kirjavainen PV, and Salminen SJ (1999) Adhesion of four Bifidobacterium strains to human intestinal mucus from subjects in different age groups. FEMS Microbiol. Lett. 172, 61–64. [DOI] [PubMed] [Google Scholar]
- (137).Juntunen M, Kirjavainen PV, Ouwehand AC, Salminen SJ, and Isolauri E (2001) Adherence of probiotic bacteria to human intestinal mucus in healthy infants and during rotavirus infection. Clin. Diagn. Lab. Immunol. 8, 293–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Tkachenko A, Da Silva L, Hearne J, Parveen S, and Waguespack Y (2013) An assay to screen bacterial adhesion to mucus biomolecules. Lett. Appl. Microbiol. 56, 79–82. [DOI] [PubMed] [Google Scholar]
- (139).Starkey BJ, Snary D, and Allen A (1972) Fractionation of water-soluble pig gastric mucus by equilibrium density-gradient centrifugation in caesium chloride. Biochem. J. 128, 123P. [PMC free article] [PubMed] [Google Scholar]
- (140).Landry RM, An D, Hupp JT, Singh PK, and Parsek MR (2006) Mucin-Pseudomonas aeruginosa interactions promote biofilm formation and antibiotic resistance. Mol. Microbiol. 59, 142–151. [DOI] [PubMed] [Google Scholar]
- (141).Gargano AF, Lammerhofer M, Lonn H, Schoenmakers PJ, and Leek T (2014) Mucin-based stationary phases as tool for the characterization of drug-mucus interaction. J. Chromatogr A 1351, 70–81. [DOI] [PubMed] [Google Scholar]
- (142).Luna RA, Oezguen N, Balderas M, Venkatachalam A, Runge JK, Versalovic J, Veenstra-VanderWeele J, Anderson GM, Savidge T, and Williams KC (2017) Distinct Microbiome-Neuroimmune Signatures Correlate With Functional Abdominal Pain in Children With Autism Spectrum Disorder. Cellular and molecular gastroenterology and hepatology 3, 218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (143).Kozich JJ, Westcott SL, Baxter NT, Highlander SK, and Schloss PD (2013) Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl. Environ. Microbiol. 79, 5112–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Langmead B, and Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Hildebrand F, Tadeo R, Voigt AY, Bork P, and Raes J (2014) LotuS: an efficient and user-friendly OTU processing pipeline. Microbiome 2, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Pammi M, Thapa S, Balderas M,Runge JK, Venkatachalam A, and Luna RA (2020) Microbiome signatures in neonatal central line associated bloodstream infections. PLoS One 15, e0227967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Parks DH, Tyson GW, Hugenholtz P, and Beiko RG (2014) STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 30, 3123–3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, and Henrissat B (2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res. 37, D233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).El Kaoutari A, Armougom F, Gordon JI, Raoult D, and Henrissat B (2013) The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 11, 497–504. [DOI] [PubMed] [Google Scholar]
- (150).Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, and Henrissat B (2014) The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 42, D490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (151).Park BH, Karpinets TV, Syed MH, Leuze MR, and Uberbacher EC (2010) CAZymes Analysis Toolkit (CAT): web service for searching and analyzing carbohydrate-active enzymes in a newly sequenced organism using CAZy database. Glycobiology 20, 1574–1584. [DOI] [PubMed] [Google Scholar]