Abstract
This study was conducted to evaluate the prebiotic effects of dietary xylooligosaccharide (XOS) supplementation on performance, nutrient digestibility, intestinal morphology, and gut microbiota in laying hens. In a 12-wk experiment, a total of 288 Hy-Line Brown layers at 50 wk of age were randomly assigned into 3 dietary treatments supplemented with XOS at 0, 200 or 400 mg/kg. Each treatment had 8 replicates with 12 birds each. Hens fed XOS diets showed a lower feed-to-egg ratio during wk 7 to 12 and a higher egg yolk color value in wk 12 compared with those fed the control diet (P < 0.05). Dietary XOS supplementation improved the apparent total tract digestibility of gross energy and nitrogen at the end of the 12th wk (P < 0.05). In addition, a higher villus height-to-crypt depth ratio of the ileum was observed in XOS-added groups (P < 0.05). The high throughput sequencing analysis of bacterial 16S rRNA revealed that dietary XOS supplementation at 200 mg/kg altered cecal microbiota. Alpha diversity analysis illustrated a higher cecal bacterial richness in birds fed with XOS at 200 mg/kg. The composition of cecal microbiota modulated by the XOS addition was characterized by an increased abundance of Firmicutes along with a reduced abundance of Bacteroidetes. At the genus level, dietary XOS supplementation triggered decreases in Bacteroides and Campylobacter concurrent with increases in Lactobacillus and several short chain fatty acid producers including Desulfovibrio, Faecalitalea, Faecalicoccus, and 5 genera of family Lachnospiraceae. Collectively, dietary XOS addition improved the feed conversion ratio by modulating nutrient digestibility and ileal morphology in laying hens, which could be attributed to the enhancement of bacterial diversity and alteration of microbial composition.
Keywords: Gut microbiota, Ileal morphology, Laying hen, Nutrient digestibility, Xylooligosaccharide
1. Introduction
The gastrointestinal microbiota plays an important role in nutrition absorption, immune system development, and resistance to pathogens, thus contributing to chicken growth and health (Pandit et al., 2018). Numerous studies have demonstrated improvements in performance and intestinal health in chickens as a result of modulating gut microbiota (Crisol-Martínez et al., 2017; Peng et al., 2016; Salaheen et al., 2017; Wu et al., 2019; Zhu et al., 2019; Zou et al., 2019). In the past, antibiotics and other medicinal products were broadly utilized to modify the alimentary microbiota and to boost productivity of animals (Markowiak and Śliżewska, 2018). However, problems caused by the overuse of antibiotics, such as resistance of pathogens to antibiotics and accumulation of antibiotics in poultry products, resulted in severe restriction or total ban of the use of antibiotics and medicinal products in animals in many countries. For example, the MOARA (Ministry of Agriculture and Rural Affairs of the People's Republic of China) proposed a deadline of December 2020 to complete the exit plan of medicated feed additives in feed production. Consequently, there has been increased interest in exploitation of antibiotic alternatives and investigation into their potential mechanisms.
Prebiotics have been suggested as alternatives to antibiotics in animal production (Saad et al., 2013). Xylooligosaccharide (XOS) is one of the common type of prebiotics, which consists of xylose units, linked through β-1,4-glycosidic bonds (Aachary and Prapulla, 2008). Chickens lack enzymes required to degrade the glycoside link between xylose units, therefore XOS is available for fermentation in the distal intestine by xylanolytic bacteria (Pourabedin and Zhao, 2015). The composition of the intestinal microbiota in broilers could be shifted by fermenting XOS towards a relative increase in probiotics and a decrease in pathogenic bacteria (De Maesschalck et al., 2015; Eeckhaut et al., 2008). Additionally, fermentation of XOS in the cecum of broilers leads to formation of short-chain fatty acids (SCFA) (Lin et al., 2018) which was implicated in energy absorption, alleviation of gut inflammation, and maintenance of intestinal epithelial integrity (Lin et al., 2018; Postler and Ghosh, 2017; Topping and Clifton, 2001; Weese et al., 2015). Recently, several studies have indicated that dietary XOS supplementation could enhance feed efficiency of broilers probably through improving nutrient digestibility (Craig et al., 2020; Ribeiro et al., 2018), as well as exerting an effect on gut immune system via the stimulation on the specific bacteria (Lin et al., 2018; Pourabedin et al., 2017).
However, gut microbial community structures between broilers and laying hens are somewhat different (Videnska et al., 2014a). The cecal microbiota of broilers was dominated by Firmicutes (76.2%) followed by Proteobacteria (14%) and Bacteroidetes (6.5%) (Videnska et al., 2014b), whereas a two-thirds microbiota of aged laying hens were formed by the representative of Bacteroidetes (Callaway et al., 2009). To the best of our knowledge, there is little publication on the effect of XOS on gut microbiota of laying hens beside a recent report by Ding et al., 2018, in which only 3 specific bacteria in cecum were determined following XOS addition. However, it is still largely unknown whether or how dietary XOS supplementation alter the bacterial diversity, regulate microbial structure and remodel gut microbial composition in laying hens. There is also very limited information concerning the linkage between intestinal bacterial phylotypes and physiological phenotypes following XOS addition in laying hens. Therefore, it is necessary to investigate the diversity and composition of intestinal microbiota in order to explore the potential benefits of dietary XOS supplementation on the performance and intestinal health in laying hens.
The objective of this study was to assess the effects of XOS on performance, nutrient digestibility, and intestinal morphology in laying hens. Subsequently, the changes to cecal microbiota compositions were characterized by high throughput sequencing, and the correlations between phylotypes and phenotypes were established using correlation analysis. This integrative investigation may provide new evidence for the microbial mechanism of action of XOS in laying hens.
2. Materials and methods
All experimental protocols were approved by the Animal Care and Use Committee of the Feed Research Institute of the Chinese Academy of Agricultural Sciences (ACE-CAAS-20180704), and the methods were carried out in accordance with the relevant guidelines and regulations.
2.1. Birds and management
Laying hens for the trial were allocated to 3-tier battery cages with 3 hens per cage (cage size: 40 cm × 40 cm × 35 cm) and exposed to light (16 h/d) with an intensity of 20 lx. Temperature was between 24 and 28 °C throughout the experiment. The cages for excreta collection were equipped with excreta collection trays. Diets and water were offered ad libitum in mash form and by nipple drinkers, respectively. All hens remained in good health during the feeding period. There were no culled birds and medical intervention was not applied to any bird.
2.2. Experimental design and diets
A total of 288 Hy-Line Brown layers aged 50 wk were randomly allocated into 3 treatments. They were fed a corn-soybean meal-based diet (Table 1) supplemented with XOS at 0 (control group), 200 and 400 mg/kg. Each treatment consisted of 8 replicates with 12 birds in 4 adjacent cages as a replicate. The basal diet was formulated according to Chinese Ministry of Agriculture (2004) and National Research Council (1994). The XOS (XOS95P) added in the diets was purchased from a commercial supplier (Jinan Longlive Biology Co., Ltd., Shandong, China). It was extracted from corncob and contained 95% XOS with the degree of polymerization from 2 to 7. During the last week, TiO2 was added into the experimental diets at a dose of 5 g/kg as an indigestible marker.
Table 1.
Composition and nutrient levels of the basal diet (air-dry basis, g/kg).
| Ingredient | Content | Nutrient level | Content1 |
|---|---|---|---|
| Corn | 627.8 | AME, MJ/kg | 11.64 |
| Soybean meal | 240.0 | Crude protein | 165.0 (162.1) |
| Soybean oil | 20.0 | Calcium | 34.8 |
| Salt | 3.0 | Nonphytate phosphorus | 3.3 |
| Dicalcium phosphate | 10.0 | Lysine | 7.9 |
| Calcium carbonate | 93.4 | Methionine | 3.7 |
| dl-methionine | 1.2 | Methionine + Cysteine | 6.5 |
| Choline chloride | 1.0 | Threonine | 6.0 |
| Premix2 | 3.3 | ||
| Phytase | 0.3 | ||
| XOS95P3 | +/−4 |
The value in parentheses was analyzed, and others are calculated.
The premix supplied the following per kilogram of diet: vitamin A, 12,500 IU; vitamin D3, 4,125 IU; vitamin E, 15 IU; vitamin K3, 2 mg; thiamine, 1 mg; riboflavin, 8.5 mg; pyridoxine, 8 mg; vitamin B12, 5 mg; biotin, 2 mg; folic acid, 5 mg; Ca-pantothenate, 50 mg; niacin, 32.5 mg; Cu, 8 mg; Zn, 65 mg; Fe, 60 mg; Mn, 65 mg; Se, 0.3 mg; I, 1 mg.
XOS95P, a mixture of 95% xylooligosaccharide (XOS) with the degree of polymerization 2 to 7 and 5% xylose.
XOS was supplemented at 0 (control group), 200, and 400 mg/kg at the expense of corn.
2.3. Performance and egg quality parameters
Mortality was recorded as it occurred. Daily egg number, total egg weight and biweekly feed consumption were recorded and calculated as hen-day egg production (EP), average egg weight (AEW), average daily feed intake (ADFI), feed conversion ratio (FCR) on a biweekly basis. Egg production, AEW, ADFI and FCR were calculated for wk 1 to 6, wk 7 to 12, and wk 1 to 12.
Six eggs from each replicate with the weight close to replicate average were collected for interior and exterior quality tests at the end of wk 6 and 12. Eggshell thickness was a mean value of the measurements at 3 locations on the surface (air cell, equator, and sharp end) using the Eggshell Thickness Gauge (ESTG-1, ORKA Technology Ltd, Ramat HaSharon, Israel). The eggshell breaking strength was measured using the Egg Force Reader (ORKA Technology Ltd, Ramat HaSharon, Israel). Albumen height, Haugh unit, and yolk color were measured using the Egg Analyzer (ORKA Food Technology Ltd, Ramat HaSharon, Israel). The determination of yolk color value was based on the “Roche yolk color fan” (15 grades) system, which consists of 15 color samples corresponding to values 1 to 15. Haugh unit was calculated from the height of the albumen and the egg weight using the simplified (Eisen et al., 1962) Haugh unit formula:
| HU = 100 log (H − 1.7W0.37 + 7.57), |
where HU is the Haugh unit, H (mm) is the albumen height, W (g) is the weight of egg.
2.4. Apparent total tract digestibility of nutrients
To determine the apparent total tract digestibility (ATTD) of gross energy (GE), total nitrogen (N), ether extract (EE), and dry matter (DM), 3 hens were selected randomly from each replicate and allocated to another cage equipped with excreta collection tray at the end of the 12th wk. Excreta from each cage were collected 2 times daily (at 12 h intervals) for 3 d and stored in sealed bags at −20 °C. Remaining feed and feathers in the excreta trays were carefully removed. Excreta collected per cage during the 3-d collection period were pooled and represented one replicate, resulting in 8 samples for each treatment. Before chemical analysis, excreta samples were thawed and dried at 70 °C for 72 h, and finely ground to pass through a 0.5-mm screen. The following formula was used to calculated the ATTD of the nutrients:
| ATTD = 1 − [(TiDiet/TiExcreta) × (NUTRExcreta/NUTRDiet)], |
where TiDiet and TiExreta (g/kg DM) are the contents of element Titanium in the diet and excreta, respectively, and NUTRDiet and NUTRExcreta (g/kg DM) are nutrient concentrations in diet and excreta, respectively.
2.5. Morphology analyses of jejunal and ileal mucosa
Segments (approximately 2 cm in length) of the middle portion of the jejunum (about 30 cm from the point of entry of the bile ducts) and ileum (about 5 cm from Meckel's diverticulum) were collected at the end of the experiment, washed with PBS and fixed in 10% neutral-buffered formalin for histology. Samples were washed, dehydrated, clarified, and embedded in paraffin. Serial sections were cut at 5 μm thickness, placed on glass slides, deparaffinized in xylene, rehydrated, stained with hematoxylin and eosin, fixed with neutral balsam, and examined by light microscopy (BX51, Olympus Co., Tokyo, Japan). All reagents used were analytical grade (Sinopharm Chemical Reagent Co., Ltd. Beijing, China). The morphometric indices evaluated were villus height (from the tip of villus to the villus–crypt junction), crypt depth (from the base up to the crypt–villus transition region) and the villus height-to-crypt depth ratio (Forte et al., 2016). The number of goblet cells was counted on 100 columnar cells of villus mucosa at 400× magnification.
2.6. DNA extraction, 16S rRNA amplification, and high throughput sequencing
Both addition of XOS at 200 and 400 mg/kg positively affected ATTD and ileal morphology compared with the control, and no significant difference were observed between the 2 groups. These results suggested no more improvement when dietary XOS addition was more than 200 mg/kg. We then investigated the effects of dietary XOS addition on cecal microbiota at this level in order to explore the potential benefits of XOS on performance and intestinal morphology.
Microbial DNA was extracted from cecal content samples using the E.Z.N.A Soil DNA Kit (Omega Bio-tek, Norcross, GA, USA) according to manufacturer's instructions. Using the isolated DNA as a template, the v3-v4 hypervariable region of the bacterial 16S rRNA was amplified with universal primers: 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The PCR reaction conditions were: 3 min of denaturation at 95 °C, followed by 25 cycles of 30 s at 94 °C (denaturation), annealing at 55 °C for 30 s, and extension at 72 °C for 30 s, with a final extension of 5 min at 72 °C. Amplicons were extracted from 2% agarose gels and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer's instructions. Purified amplicons were qualified and sequenced using MiSeq platform with MiSeq Reagent Kit v3 at Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China).
2.7. Statistical analysis
Data analyses of performance, egg quality, nutrient digestibility, and intestinal morphology were performed using SPSS version 19.0 for Windows (SPSS, Chicago, IL). The normality of data was initially tested using the Shapiro–Wilk test. Data were then analyzed using one-way ANOVA, and means were compared using Duncan's multiple range test. Differences were considered statistically significant at P ≤ 0.05. Data are expressed as the means and pooled SEM.
For microbial community profiling, raw pair-end sequences were demultiplexed and quality-filtered using the Quantitative Insights Into Microbial Ecology (QIIME, version 1.17) (Edgar, 2010). Only sequences that overlap by more than 10 bp were assembled according to their overlap sequence. Operational taxonomic units (OTU) were clustered with 97% sequence identity using UPARSE (version 7.1), and the chimera sequences were identified and removed to obtain effective tags using UCHIME. Mann–Whitney U-test was used to assess statistical significance of measures derived from alpha diversity metrics. Principal coordinate analysis (PCoA) and partial least squares discriminant analysis (PLS-DA) were conducted to compare the bacterial community structures across all samples. The significant differentiation of microbial structure between 2 groups was statistically tested by analysis of similarity (ANOSIM), and non-parametric multivariate ANOVA (Adonis). Calculations of the Bray–Curtis matrix, PCoA, PLS-DA, ANOSIM, and Adonis were all performed using the Vegan package (Dixon, 2003). The statistical significance of comparison in bacterial community composition between 2 groups was assessed using Student's t test. Statistical tests for differentially abundant taxa were performed using the linear discriminant analysis (LDA) effect size (LEfSe) method with an alpha value of 0.05 for the Kruskal–Wallis test among classes. The LDA was used to estimate the effect size of each differentially abundant feature, and the threshold on the LDA score (log10 LDA) was set to 2.0. Finally, correlations were estimated by Pearson correlation using the Pheatmap package in R. Correlations were considered significantly different at P ≤ 0.05.
3. Results
3.1. Performance and egg quality
The effects of dietary supplementation with XOS on performance of layers (50 to 62 wk of age) are presented in Table 2. No significant difference in laying performance was found among all treatments in wk 1 to 6 and wk 1 to 12 (P > 0.05). During wk 7 to 12, dietary XOS supplementation did not affect the EP, AEW and ADFI but affected the FCR (P < 0.05). Hens fed the 200 or 400 mg/kg XOS diet showed a better FCR than those fed the control diet (P < 0.05).
Table 2.
Effect of dietary xylooligosaccharide (XOS) supplementation on performance of laying hens1.
| Item | XOS level, mg/kg of feed |
SEM | P-value | ||
|---|---|---|---|---|---|
| 0 | 200 | 400 | |||
| Wk 1 to 6 | |||||
| EP, % | 92.61 | 93.26 | 93.17 | 0.554 | 0.883 |
| AEW, g | 66.45 | 68.21 | 67.25 | 0.342 | 0.100 |
| ADFI, g | 117.84 | 119.69 | 120.81 | 0.692 | 0.206 |
| FCR2, g/g | 1.96 | 1.92 | 1.93 | 0.010 | 0.263 |
| Wk 7 to 12 | |||||
| EP, % | 85.84 | 86.71 | 87.96 | 0.632 | 0.405 |
| AEW, g | 64.27 | 64.87 | 64.67 | 0.276 | 0.674 |
| ADFI, g | 112.47 | 111.47 | 113.24 | 0.721 | 0.624 |
| FCR2, g/g | 2.00b | 1.91a | 1.92a | 0.016 | 0.022 |
| Wk 1 to 12 | |||||
| EP, % | 89.03 | 89.79 | 90.45 | 0.520 | 0.555 |
| AEW, g | 65.36 | 66.50 | 65.96 | 0.297 | 0.294 |
| ADFI, g | 116.59 | 115.58 | 117.03 | 0.741 | 0.749 |
| FCR2, g/g | 1.98 | 1.91 | 1.92 | 0.012 | 0.184 |
EP = egg production; AEW = average egg weight; ADFI = average daily feed intake.
a, b Within a row, means with no common superscript differ significantly (P < 0.05).
n = 8 replicates per treatment.
FCR is feed-to-eggs ratio.
Table 3 shows the egg quality indices of hens fed with XOS. There were no differences in overall egg quality (i.e., eggshell thickness, eggshell strength, albumen height, yolk color value, or Haugh unit) among all groups at wk 6 (P > 0.05). At wk 12, birds in XOS-supplemented groups showed an increased yolk color value in comparison with that in the control (P < 0.05). No differences were found in the other indices among all treatments at wk 12 (P > 0.05).
Table 3.
Effect of dietary xylooligosaccharide (XOS) supplementation on egg quality of laying hens1.
| Item | XOS level, mg/kg of feed |
SEM | P-value | ||
|---|---|---|---|---|---|
| 0 | 200 | 400 | |||
| Wk 6 | |||||
| Eggshell thickness, × 0.01 mm | 42.07 | 42.34 | 42.58 | 0.169 | 0.487 |
| Eggshell strength, N | 38.56 | 38.53 | 39.88 | 0.408 | 0.317 |
| Albumen height, mm | 7.08 | 7.16 | 7.19 | 0.053 | 0.711 |
| Egg-yolk color | 5.82 | 6.33 | 6.32 | 0.103 | 0.069 |
| Haugh unit | 83.45 | 84.05 | 84.08 | 0.369 | 0.754 |
| Wk 12 | |||||
| Eggshell thickness, × 0.01 mm | 42.21 | 42.64 | 42.50 | 0.157 | 0.534 |
| Eggshell strength, N | 37.70 | 37.36 | 37.73 | 0.318 | 0.878 |
| Albumen height, mm | 6.61 | 6.87 | 6.83 | 0.072 | 0.285 |
| Egg-yolk color | 5.78b | 6.50a | 6.23a | 0.113 | 0.022 |
| Haugh unit | 78.98 | 81.39 | 80.55 | 0.513 | 0.152 |
a, b Within a row, means with no common superscript differ significantly (P < 0.05).
Means were calculated using 8 replicates (6 eggs/replicate) per treatment.
3.2. Apparent total tract digestibility of nutrients
Table 4 shows the effects of dietary XOS supplementation on ATTD of nutrients in laying hens at 62 wk of age. The ATTD of GE and N were significantly affected by dietary XOS supplementation (P < 0.05). Significant increases were observed in the ATTD of GE in XOS-supplemented groups (200 and 400 mg/kg) (P < 0.05). Compared with the control, dietary supplementation with XOS at 200 and 400 mg/kg increased the ATTD of N by 24.0% and 23.7%, respectively (P < 0.05). Supplementing the diet with XOS did not affect the ATTD of DM or EE (P > 0.05).
Table 4.
Effect of dietary xylooligosaccharide (XOS) supplementation on coefficient of apparent total tract digestibility of laying hens at 62 wk of age1.
| Item | XOS level, mg/kg of feed |
SEM | P-value | ||
|---|---|---|---|---|---|
| 0 | 200 | 400 | |||
| Gross energy (GE) | 0.80b | 0.82a | 0.82a | 0.003 | 0.042 |
| Dry matter (DM) | 0.59 | 0.60 | 0.61 | 0.003 | 0.080 |
| Nitrogen (N) | 0.44b | 0.55a | 0.55a | 0.015 | 0.001 |
| Ether extract (EE) | 0.77 | 0.82 | 0.81 | 0.018 | 0.530 |
a, b Within a row, means with no common superscript differ significantly (P < 0.05).
Eight replicates per treatment with one mixed sample from 3 laying hens each replicate.
3.3. Intestinal morphology
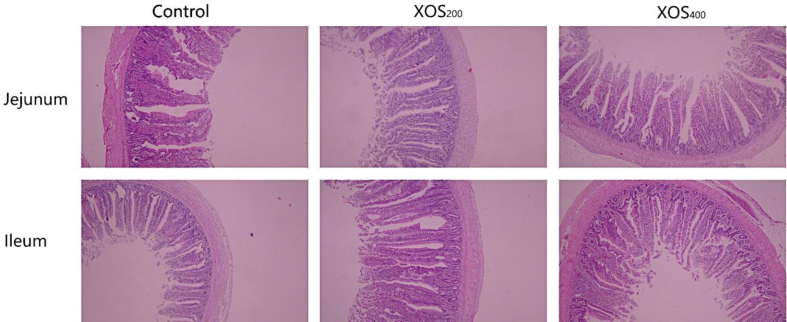
The results regarding the effects of dietary XOS supplementation on intestinal morphology of laying hens are presented in Table 5 and Fig. 1. No significant differences were detected in villus height, crypt depth, villus height-to-crypt depth ratio, or goblet cell number of the jejunum among all groups (P > 0.05). Hens fed diets added XOS at 200 or 400 mg/kg showed no differences in villus height, crypt depth or goblet cell number but exhibited a higher villus height-to-crypt depth ratio in the ileum compared with those in the control (P < 0.05).
Table 5.
Effect of dietary xylooligosaccharide (XOS) supplementation on intestinal morphology of laying hens at 62 wk of age1.
| Item | XOS level, mg/kg of feed |
SEM | P-value | ||
|---|---|---|---|---|---|
| 0 | 200 | 400 | |||
| Jejunum | |||||
| Villus height, μm | 791.36 | 776.43 | 782.09 | 27.941 | 0.978 |
| Crypt depth, μm | 211.90 | 226.33 | 249.95 | 10.404 | 0.335 |
| Villus height-to-crypt depth ratio | 3.61 | 3.52 | 3.34 | 0.100 | 0.580 |
| Goblet cell number | 29.78 | 27.43 | 25.25 | 1.465 | 0.471 |
| Ileum | |||||
| Villus height, μm | 571.96 | 643.60 | 645.65 | 14.568 | 0.070 |
| Crypt depth, μm | 173.50 | 157.78 | 166.60 | 4.120 | 0.300 |
| Villus height-to-crypt depth ratio | 3.40b | 4.10a | 3.87a | 0.089 | 0.002 |
| Goblet cell number | 20.23 | 17.17 | 20.40 | 0.437 | 0.488 |
a, b Within a row, means with no common superscript differ significantly (P < 0.05).
Eight replicates per treatment.
Fig. 1.
Morphology of jejunum and ileum of laying hens fed control and xylooligosaccharide (XOS) diets. Hematoxylin and erosion (H&E) staining, 40 × magnification. XOS200, supplementation at 200 mg/kg; XOS400, supplementation at 400 mg/kg.
3.4. Intestinal microbial diversity and community
A total of 892,597 valid sequences were obtained after quality filtering and chimeras checking. The maximum, mean and minimum sequence reads were respectively 74,974, 55,787, and 35,410 across all cecal samples. On the OTU level, 1,083 OTU were matched, among which 16 phyla and 177 genera of intestinal microbes were annotated.
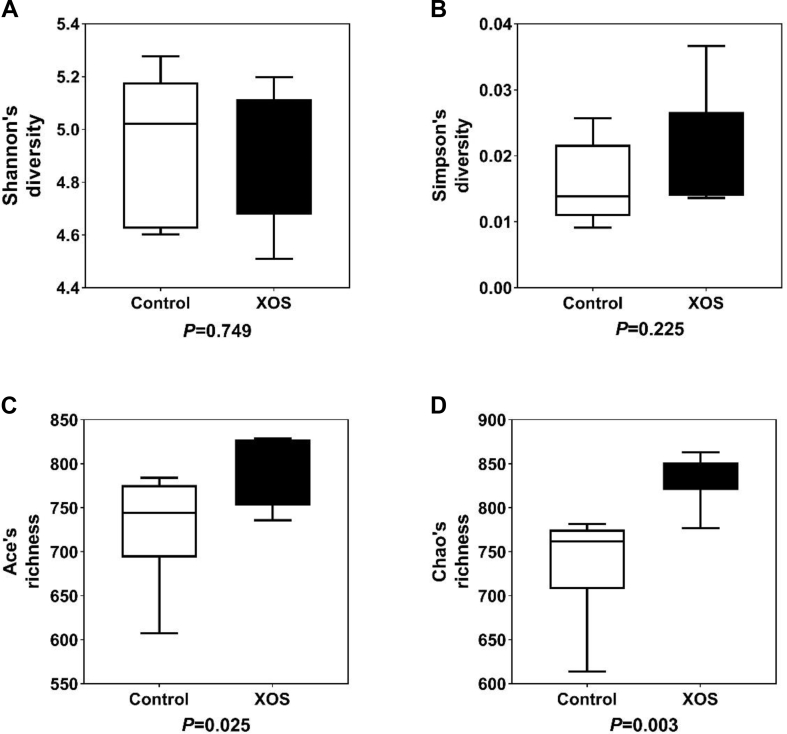
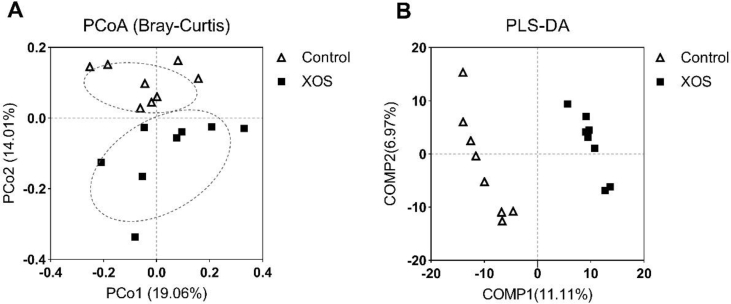
Bacterial alpha diversity in cecal microbiota was estimated using Shannon, Simpson, Ace, and Chao indices of diversity and richness (Fig. 2). No significant differences in Shannon or Simpson indices were found between the 2 groups (P > 0.05). Ace and Chao indices in XOS-supplemented group at 200 mg/kg were significantly higher than those in the control (P < 0.05), suggesting that the overall bacterial richness of cecal microbiota was increased by XOS treatment (200 mg/kg). Principal coordinate analysis based on Bray–Curtis distances revealed statistically significant discrimination between the XOS-supplemented group and the control (PCo1, 19.06%; PCo2, 14.01%; Fig. 3A). PLS-DA plot defined groups where samples from different groups occupied distinct positions (COMP1, 11.11%; COMP2, 6.97%; Fig. 3B). Results were also supported by statistics obtained from ANOSIM (R = 0.180, P = 0.01) and Adonis (R2 = 0.117, P = 0.004) analyses.
Fig. 2.
Influence of dietary xylooligosaccharide (XOS) supplementation on bacterial alpha diversity. Four different alpha diversity metrics, Shannon's diversity (A), Simpson's diversity (B), Ace's richness (C), and Chao's richness (D) indices, were compared between the control and XOS-supplemented (200 mg/kg) groups. P-values given below each boxplot were estimated by Mann–Whitney U-test.
Fig. 3.
Effect of dietary xylooligosaccharide (XOS) (200 mg/kg) supplementation on bacterial beta diversity. (A) Principal coordinate analysis (PCoA) based on Bray–Curtis dissimilarity indices calculated from OTU abundance matrix. The horizontal axis represents the first principal coordinate and the vertical axis represents the second one. (B) Partial least squares discriminant analysis (PLS-DA). The horizontal axis represents the first component (COMP1) of PLS-DA and the vertical axis represents the second one (COMP2). The percentages in parentheses represent the explanatory values of the principal coordinates or the components for the difference in sample composition. Triangles, the control group; rectangles, XOS-supplemented group (200 mg/kg).
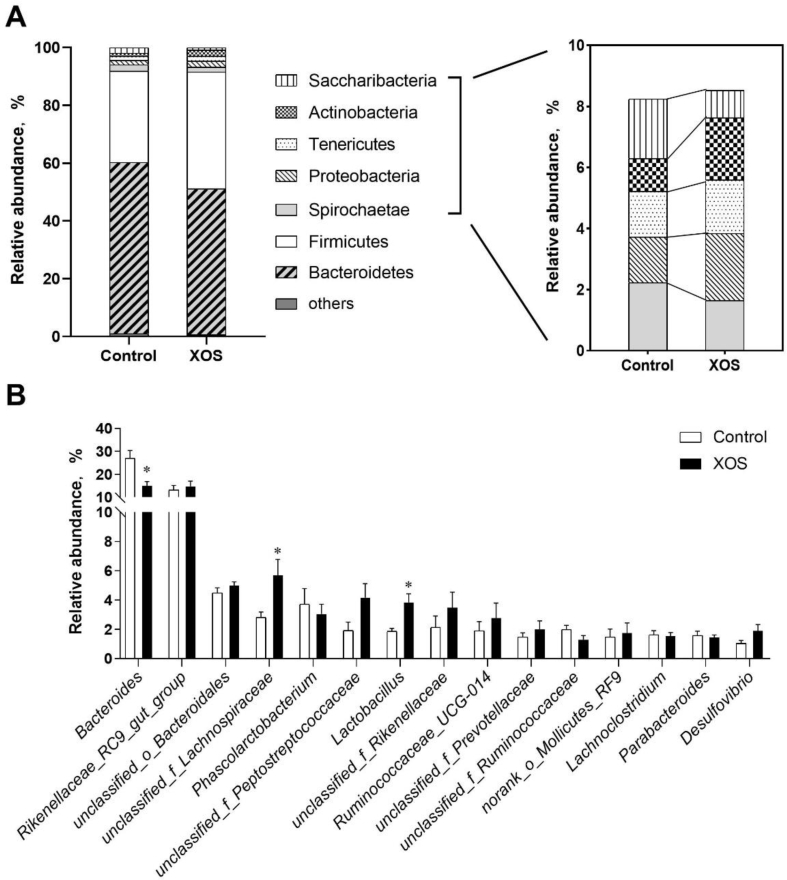
Taxonomic compositions of the microbiota were analyzed at the phylum and genus levels using the RDA classifier. The results, shown in Fig. 4A, revealed average relative abundance (above 1%) at level of the phylum. Overall, the microbiota were dominated by the phylum Bacteroidetes (54.9% ± 11.1% of total reads) and Firmicutes (36.0% ± 8.9%), followed by Spirochaetae, Proteobacteria, Tenericutes, Actinobacteria, Saccharibacteria, and others. Hens fed with XOS diet were characterized by a higher relative abundance of Firmicutes (40.4% to 31.5%) and lower abundance of Bacteroidetes (50.5% to 59.3%), thus leading to a higher Firmicutes-to-Bacteroidetes ratio. At the genus level, the sequences from samples were matched with 177 genera. The top 15 most abundant genera are listed in Fig. 4B, in which, Bacteroides exhibited lower abundance in XOS-supplemented group (200 mg/kg), whereas the abundances of unclassified_f_Lachnospiraceae, Lactobacillus, and Desulfovibrio were higher in XOS-supplemented group (200 mg/kg) than those in the control.
Fig. 4.
Bacterial community compositions of the cecum of hens in xylooligosaccharide (XOS)-supplemented (200 mg/kg) and the control groups. (A) Relative abundance of bacterial phyla detected in the samples. Those abundance below 1% were classified into “others”. The relative abundance values represent the averages of 8 samples in each group. (B) Relative abundance of the most abundant 15 bacterial genera in 2 treatment groups. Bars with asterisks mean that the genera in the XOS-supplemented group were significant different compared with the control group (P < 0.05), and bars with no asterisks mean no significant difference in the genera between 2 groups (P > 0.05). Differential abundance was statistically tested using Student's t test.
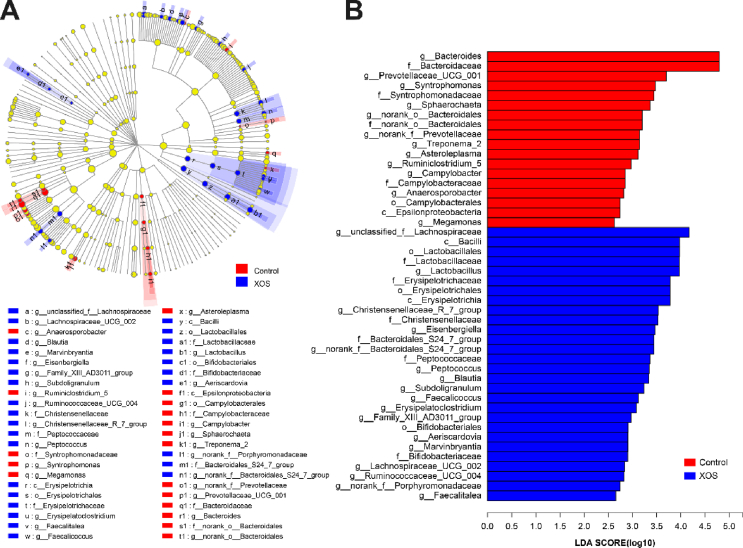
The significant differentially abundant OTU for entire microbiota at levels from phylum to genus were analyzed by LEfSe (LDA > 2.0; Fig. 5A and B). Increased abundance of members of the dominant Firmicutes phylum was evident in response to XOS supplementation at 200 mg/kg, including Bacilli, Lactobacillales, Lactobacillaceae, Lactobacillus, and Erysipelotrichia, Erysipelotrichales, Erysipelotrichaceae (Erysipelatoclostridium, Faecalitalea, Faecalicoccus), as well as genera of unclassified_f_Lachnospiraceae, Lachnospiraceae_UCG_002, Blautia, Marvinbryantia, and Eisenbergiella, all belonging to family Lachnospiraceae. Besides, order Bifidobacteriales and its derivatives (Bifidobacteriaceae and Aeriscardovia) were also enriched in XOS-supplemented group. In the control, LEfSe highlights the greater differential abundance of class Epsilonproteobacteria and its derivatives (Campylobacterales, Campylobacteraceae, Campylobacter) and the family Bacteroidaceae with its derivative Bacteroides.
Fig. 5.
Linear discriminant analysis effect size (LEfSe) method identified the most differentially abundant taxa enriched in cecal microbiota of xylooligosaccharide (XOS)-supplemented (200 mg/kg) and control groups. (A) Cladogram generated from LEfSe analysis, where red circles represent taxa of greater abundance in hens in the control, blue circles for those of hens fed with XOS (200 mg/kg), and yellow for non-significant differences. The diameters of the circles are proportional to the taxon's abundance. (B) Histogram of the linear discriminant analysis (LDA) scores computed for features differentially abundant in XOS-supplemented (200 mg/kg) group (blue bars) and the control (red bars) group. Species with significant difference that have an LDA score greater than 2.0 are presented. The length of the histogram represents the LDA score, which can be interpreted as the effect size of each differentially abundant feature.
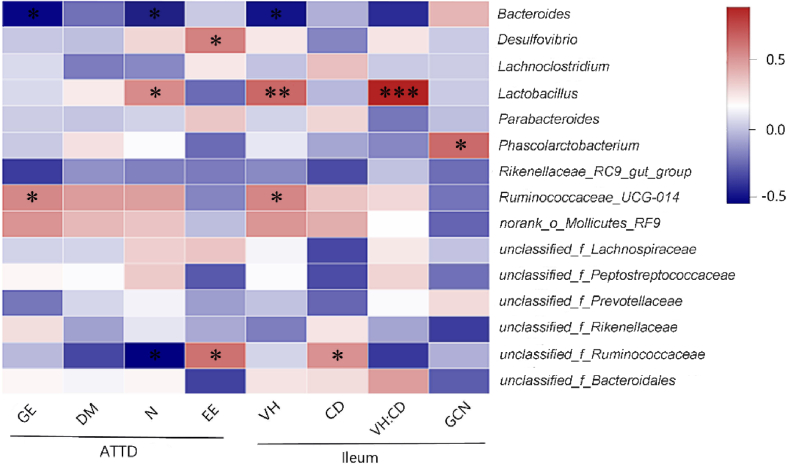
Pearson correlation analysis was conducted to estimate the association between the phylotypes in the gut microbiota and phenotypes of hens (Fig. 6). As shown in the Heatmap, the abundance of Bacteroides had negative relationships with the ATTD of GE and N (P < 0.01; R = −0.68; P < 0.05; R = −0.59) and the villus height of ileum (P < 0.05; R = −0.50). Conversely, the abundance of Lactobacillus was correlated positively with the ATTD of N (P < 0.05; R = 0.52), ileal villus height (P < 0.01; R = 0.63) and the villus height-to-crypt depth ratio (P < 0.001; R = 0.84). Significantly positive relationships were also identified between the abundance of Ruminococcaceae_UCG-014 and the ATTD of GE (P < 0.05; R = 0.53) and ileal villus height (P < 0.05; R = 0.54). Moreover, one unclassified member of Ruminococcaceae family exhibited a negative correlation with the ATTD of N (P < 0.05; R = −0.52), but positive relationships with the ATTD of EE (P < 0.05; R = 0.60) and ileal crypt depth (P < 0.05; R = 0.50). These results indicate that intestinal morphology and function are related with gut microbiota in laying hens.
Fig. 6.
Associations of 15 key phylotypes (at genus level) with phenotypes. The depth of colors ranging from blue to red represents the magnitude of correlation. The OTU were organized according to their Pearson correlation coefficient. Significant correlations are noted by: ∗, 0.01 < P ≤ 0.05; ∗∗, 0.001 < P ≤ 0.01; ∗∗∗, P ≤ 0.001. GE = gross energy; DM = dry mater; N = total nitrogen; EE = ether extract; VH = villus height; CD = crypt depth; VH:CD = the ratio of villus height to crypt depth; GCN = goblet cell number; ATTD = apparent total tract digestibility.
4. Discussion
Beneficial effects of XOS on performance have already been described in poultry (Craig et al., 2020; Courtin et al., 2008; Lin et al., 2018). In the present study, hens receiving the corn-soybean meal-based diet supplemented with XOS presented a better FCR than those in the control during wk 7 to 12 of the trial, indicating that a period longer than 6-wk was needed for dietary XOS to exert the positive effects. Similar data were reported by De Maesschalck et al. (2015), who found that unbranched XOS displayed an improvement of feed conversion ratio in broilers. A possible action mechanism of XOS on FCR of broilers was reported by Ribeiro et al. (2018) that XOS could activate specific bacteria by the release of signal to ferment non-digestible carbohydrate and interact with the digestive tract, leading to a higher digestive efficiency. Intestinal morphology is an important indicator of intestinal mucosal function and has been considered to be associated with animal performance (Montagne et al., 2003). In our study, hens fed the basal diet with XOS supplementation exhibited a higher ileal villus height and presented a higher villus height-to-crypt depth ratio, which was consistent with previous studies (Ding et al., 2018; Min et al., 2016). Dietary XOS supplementation could stimulate butyrate-producing bacteria to produce butyrate (Broekaert et al., 2011; Scott et al., 2014) that may improve the intestinal morphology due to its role in fueling epithelial cells (Kumar et al., 2015). A healthier intestinal condition can lead to a stronger nutrient digestibility on account of larger absorption area and higher epithelial cell turnover. Similar to a previous report (Ribeiro et al., 2018), our current study showed that birds fed diets supplemented with 200 and 400 mg/kg XOS presented higher ATTD of GE and N when compared with non-supplemented animals, suggesting that XOS improved energy and protein utilization. Dietary XOS addition increased the cecal SCFA concentration in birds (Craig et al., 2020; Ding et al., 2018), which has been reported to involved in energy and nutrition absorption (Xue et al., 2016; Yan and Charles, 2017). In the current study, no further improvements in performance, intestinal morphology, and nutrient digestibility were observed when XOS addition was more than 200 mg/kg. The possible reason was that a high level of prebiotics can be fermented rapidly and increases gas production, which impairs the gut mucosal barrier (Mikkelsen et al., 2004; Bruggencate et al., 2005). The higher egg yolk color value observed in XOS-supplemented groups might be connected with the interference of XOS on lipid metabolism (Bederskałojewska et al., 2019; Li et al., 2017), thus influenced carotenoid absorption or deposition in the yolk. Further studies are needed to reveal the effects of XOS on yolk color.
Data presented above suggest that dietary XOS supplementation results in elevating nutrient digestibility and improving ileal morphology, which could contribute to an improvement in FCR. The alteration of cecal microbiota triggered by prebiotics has been reported to be involved in the improvement of intestinal morphology and nutrient utilization (Li et al., 2018; Pourabedin et al., 2014). In attempts to better understand the mechanism and validate our hypothesis, we analyzed the cecal microbiota which contains the most detailed information regarding chicken gut microbiota and is the key region for bacterial fermentation of non-digestible carbohydrate (Pourabedin and Zhao, 2015). Data from analyses of α-diversity and β-diversity corroborate our initial hypothesis that XOS improved microbial richness and altered microbiota structure (Dotsenko et al., 2018). The greater diversity of the intestinal tract microbiota community is believed to have a positive effect on the welfare and productivity of the host bird (Janczyk et al., 2009). The gut micro-ecosystem of XOS fed birds was also shifted at the phylum level by favoring Firmicutes at expense of Bacteroidetes, thus leading to a higher Firmicutes-to-Bacteroidetes ratio. Numerous studies in mice and humans indicated that the higher ratio of Firmicutes to Bacteroidetes may play an important role in energy uptake and ultimately increased body weight (Bervoets et al., 2013; Ley et al., 2006; Paola et al., 2010). Similar relationship was also observed in chickens (Singh et al., 2013). The abundance of Firmicutes has been proved to be positively correlated with energy and nutrient absorption, whereas an increase in fecal Bacteroidetes is associated with poor nutrient digestibility (Jumpertz et al., 2011; Turnbaugh et al., 2006). Thereby, the increased abundance of Firmicutes along with the reduced abundance of Bacteroidetes might contribute to the nutrient utilization of laying hens.
Further analyses revealed more differential species at various taxonomic levels between 2 groups. For example, there was an increase in abundance of Bifidobacteriaceae in XOS-supplemented group (200 mg/kg). Bifidobacteriaceae is considered to be an important probiotic not only in human gut (Sekirov et al., 2010), but also in chicken intestinal tract (Yang et al., 2019), which exerts a wide number of benefits, including inhibiting the colonization of pathogens and positively improving the host gut health (Greene and Klaenhammer, 1994). At the genus level, there was an increased abundance of Lactobacillus in cecum of XOS-supplemented birds. As one of the predominant genera in the chicken gut, Lactobacillus can offer protection to intestinal barrier by antagonistic activities against pathogens (Servin, 2004). Besides, lactate, produced from fermentation of Lactobacillus, could be utilized by butyrate-producers through cross-feeding pattern, resulting in the beneficial effects of butyrate on nutrient digestibility and intestinal morphology (De Maesschalck et al., 2015). This possible pattern was also evidenced by enrichment of Faecalicoccus and Faecalitalea in XOS-supplemented group (200 mg/kg). Faecalicoccus and Faecalitalea were 2 of the 5 novel genera of family Erysipelotrichaceae which was proposed as a key butyrate-producer and considered more important in the chicken cecum than those in the human colon (Eeckhaut et al., 2011; Liu et al., 2019). Genera unclassified_f_Lachnospiraceae, Lachnospiraceae_UCG_002, Blautia, Marvinbryantia, and Eisenbergiella, all belonging to family Lachnospiraceae, were also more abundant in XOS-supplemented group (200 mg/kg). The majority of acetogens are affiliated with the Lachnospiraceae (Gagen et al., 2015), especially Blautia (Pérez-Burillo et al., 2019). Acetate might be implicated in protein synthesis since it could be absorbed and converted to aspartate and glutamate at the distal portion of animals’ gut (Bergman, 1990; Marty and Vernay, 1984; Vernay, 1989). Besides, Liu et al. (2018) reported that Lachnospiraceae was strongly correlated with adipokine levels, indicating the role of Lachnospiraceae in energy metabolism. Elevated Desulfovibrio was also confirmed to be implicated in energy absorption of host by aiding propionic acid production (Macfabe et al., 2011; Wang et al., 2019). In the current study, dietary XOS addition triggered decreases in the abundances of Bacteroides and Campylobacter. The linkage between Bacteroides and SCFA-related energy utilization was considered to be complex since the role of Bacteroides in polysaccharide decomposition and subsequent generation of SCFA was controversial (Postler and Ghosh, 2017; Murphy et al., 2010). However, the abundance of Bacteroides were proved to be positively related with the expression of ileal pro-inflammatory cytokines interleukin (IL)-1β, IL-8 and tumor necrosis factor-α in laying hens (Wang et al., 2019), which could disrupt the epithelial barrier function (Matthias et al., 2003). Campylobacter species were established as harmful bacteria that had gained prominence as aetiological agents of a range of gastrointestinal diseases (Kaakoush et al., 2016; Nielsen et al., 2014). To sum up, alterations of these bacterial phylotypes compared with the control suggested that cecal microbiota were modulated by XOS to be more efficient in maintaining intestinal morphology and assisting nutrient utilization, leading to a better performance in laying hens.
Corresponding to the alterations in cecal microbiota, Pearson correlation analysis further identified that genera Bacteroides, Desulfovibrio, Lactobacillus, Ruminococcaceae_UCG-014, and unclassified_f_Ruminococcaceae exhibited markedly positive or negative correlation with nutrient digestibility and/or ileal morphology, supporting that dietary XOS supplementation mediated intestinal functions by targeting gut microbiota. However, our observations were inconsistent with some studies that Bifidobacterium spp. were much more efficient in utilizing XOS than Lactobacillus spp. in vitro or in broilers (Crittenden et al., 2010; Eeckhaut et al., 2008; Moura et al., 2007). Ribeiro et al. (2018) reported that broilers receiving the wheat-soybean meal-based diet supplemented with XOS presented higher abundances of Bifidobacterium spp. in cecum. Contradictorily, two other studies on broilers demonstrated the increased abundances of Lactobacillus and butyrate-producers in cecum and colon following XOS addition, contributing to the improvement of gut function and performance through cross-feeding pattern (De Maesschalck et al., 2015; Pourabedin et al., 2017). The above studies highlighted that the microbial variation by dietary intervention might depend on specific conditions (Dethlefsen and Relman, 2011). In the current study, the abundance of Lactobacillus was significantly increased by XOS supplementation at 200 mg/kg and elicited positive correlations with the ATTD of N, ileal villus height and the villus height to crypt depth ratio, whereas the abundance of Bacteroides was significantly reduced following XOS addition at 200 mg/kg and had negative relationships with the ATTD of GE and N, and ileal villus height. Thus, Lactobacillus and Bacteroides might play essential roles in XOS modulating intestinal morphology and nutrient digestibility in laying hens.
5. Conclusion
XOS supplementation improved FCR by modulating nutrient digestibility and ileal morphology in laying hens, which could be attributed to the enhancement of bacterial richness and alteration of microbial composition, especially the enrichment of Lactobacillus and SCFA producers and the decrease of abundance of Bacteroides. This study can expand our understanding concerning the microbial mechanism of action of XOS in laying hens.
Author contributions
Jianmin Zhou: conceptualization, methodology, investigation, writing - original draft. Shugeng Wu: project administration, supervision. Guanghai Qi: writing - review & editing, resources, formal analysis, supervision. Yu Fu: writing - review & editing, data curation, methodology. Weiwei Wang: writing - review & editing, data curation. Haijun Zhang: writing: review & editing, data curation. Jing Wang: conceptualization, writing: review & editing.
Conflict of interest
We declare that we have no financial or personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This study was supported by National Key R&D Program of China (2018YFD0500603), China Agricultural Research System (CARS40-K12), the China Agriculture Research System-Beijing Team for Poultry Industry and the Agricultural Science and Technology Innovation Program (ASTIP) of the Chinese Academy of Agricultural Sciences.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Haijun Zhang, Email: zhanghaijun@caas.cn.
Jing Wang, Email: wangjing@caas.cn.
References
- Aachary A.A., Prapulla S.G. Corncob-induced endo-1,4-beta-d-xylanase of Aspergillus oryzae MTCC 5154: production and characterization of xylobiose from glucuronoxylan. J Agric Food Chem. 2008;56:3981–3988. doi: 10.1021/jf073430i. [DOI] [PubMed] [Google Scholar]
- Bederskałojewska D., Arczewskawlosek A., Świątkiewicz S., Orczewskadudek S., Schwarz T., Puchala M. The effect of different dietary levels of hybrid rye and xylanase addition on the performance and egg quality in laying hens. Br Poultry Sci. 2019;60:423–430. doi: 10.1080/00071668.2019.1605149. [DOI] [PubMed] [Google Scholar]
- Bergman E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990;70:567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- Bervoets L., Hoorenbeeck K.V., Kortleven I., Noten C.V., Hens N., Vael C. Differences in gut microbiota composition between obese and lean children: a cross-sectional study. Gut Pathog. 2013;5:10. doi: 10.1186/1757-4749-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekaert W.F., Courtin C.M., Verbeke K., De Wiele T.V., Verstraete W., Delcour J.A. Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit Rev Food Sci Nutr. 2011;51:178–194. doi: 10.1080/10408390903044768. [DOI] [PubMed] [Google Scholar]
- Bruggencate S.J., Boveeoudenhoven I.M., Lettinkwissink M.L., Der Meer R.V. Dietary fructooligosaccharides increase intestinal permeability in rats. J Nutr. 2005;135:837–842. doi: 10.1093/jn/135.4.837. [DOI] [PubMed] [Google Scholar]
- Callaway T.R., Dowd S.E., Wolcott R.D., Sun Y., Mcreynolds J.L., Edrington T.S. Evaluation of the bacterial diversity in cecal contents of laying hens fed various molting diets by using bacterial tag-encoded FLX amplicon pyrosequencing. Poultry Sci. 2009;88:298–302. doi: 10.3382/ps.2008-00222. [DOI] [PubMed] [Google Scholar]
- Craig A.D., Khattak F., Hastie P., Bedford M.R., Olukosi O.A. Xylanase and xylo-oligosaccharide prebiotic improve the growth performance and concentration of potentially prebiotic oligosaccharides in the ileum of broiler chickens. Br Poultry Sci. 2020;61:70–78. doi: 10.1080/00071668.2019.1673318. [DOI] [PubMed] [Google Scholar]
- Crisol-Martínez E., Stanley D., Geier M.S., Hughes R.J., Moore R.J. Understanding the mechanisms of zinc bacitracin and avilamycin on animal production: linking gut microbiota and growth performance in chickens. Appl Microbiol Biotechnol. 2017;101:4547–4559. doi: 10.1007/s00253-017-8193-9. [DOI] [PubMed] [Google Scholar]
- Crittenden R., Karppinen S., Ojanen S., Tenkanen M., Fagerström R., Mättö J. In vitro fermentation of cereal dietary fibre carbohydrates by probiotic and intestinal bacteria. J Sci Food Agric. 2010;82:781–789. [Google Scholar]
- Courtin C.M., Broekaert W.F., Swennen K., Lescroart O., Onagbesan O., Buyse J. Dietary inclusion of wheat bran arabinoxylooligosaccharides induces beneficial nutritional effects in chickens. Cereal Chem. 2008;85:607–613. [Google Scholar]
- De Maesschalck C., Eeckhaut V., Maertens L., De Lange L., Marchal L., Nezer C. Effects of xylo-oligosaccharides on broiler chicken performance and microbiota. Appl Environ Microbiol. 2015;81:5880–5888. doi: 10.1128/AEM.01616-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen L., Relman D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108:4554–4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X.M., Li D.D., Bai S.P., Wang J.P., Zeng Q.F., Su Z.W. Effect of dietary xylooligosaccharides on intestinal characteristics, gut microbiota, cecal short-chain fatty acids, and plasma immune parameters of laying hens. Poultry Sci. 2018;97:874–881. doi: 10.3382/ps/pex372. [DOI] [PubMed] [Google Scholar]
- Dixon P.M. VEGAN, a package of R functions for community ecology. J Veg Sci. 2003;14:927–930. [Google Scholar]
- Dotsenko G.S., Meyer A.S., Canibe N., Thygesen A., Nielsen M.K., Lange L. Enzymatic production of wheat and ryegrass derived xylooligosaccharides and evaluation of their in vitro effect on pig gut microbiota. Biomass Convers Biorefin. 2018;8:497–507. [Google Scholar]
- Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2641. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Eeckhaut V., Van Immerseel F., Dewulf J., Pasmans F., Haesebrouck F., Ducatelle R. Arabinoxylooligosaccharides from wheat bran inhibit Salmonella colonization in broiler chickens. Poultry Sci. 2008;87:2329–2334. doi: 10.3382/ps.2008-00193. [DOI] [PubMed] [Google Scholar]
- Eeckhaut V., Van Immerseel F., Croubels S., De Baere S., Haesebrouck F., Ducatelle R. Butyrate production in phylogenetically diverse Firmicutes isolated from the chicken caecum. Microb Biotechnol. 2011;4:503–512. doi: 10.1111/j.1751-7915.2010.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen E.J., Bohren B.B., Mckean H.E. Haugh unit as a measure of egg albumen quality. Poultry Sci. 1962;41:1461–1468. [Google Scholar]
- Forte C., Acuti G., Manuali E., Proietti P.C., Pavone S., Trabalzamarinucci M. Effects of two different probiotics on microflora, morphology, and morphometry of gut in organic laying hens. Poultry Sci. 2016;95:2528–2535. doi: 10.3382/ps/pew164. [DOI] [PubMed] [Google Scholar]
- Gagen E.J., Jagadish P., Denman S.E., Mcsweeney C.S. Hydrogenotrophic culture enrichment reveals rumen Lachnospiraceae and Ruminococcaceae acetogens and hydrogen-responsive Bacteroidetes from pasture-fed cattle. FEMS Microbiol Lett. 2015;362:fnv104. doi: 10.1093/femsle/fnv104. [DOI] [PubMed] [Google Scholar]
- Greene J.D., Klaenhammer T.R. Factors involved in adherence of lactobacilli to human Caco-2 cells. Appl Environ Microbiol. 1994;60:4487–4494. doi: 10.1128/aem.60.12.4487-4494.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczyk P., Halle B., Souffrant W.B. Microbial community composition of the crop and ceca contents of laying hens fed diets supplemented with Chlorella vulgaris. Poultry Sci. 2009;88:2324–2332. doi: 10.3382/ps.2009-00250. [DOI] [PubMed] [Google Scholar]
- Jumpertz R., Le D.S., Turnbaugh P.J., Trinidad C., Bogardus C., Gordon J.I. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaakoush N.O., Thomas D.S., Ruzayqat M.M., Lynch D., Leach S.T., Lemberg D.A. Campylobacter concisus utilizes blood but not short chain fatty acids despite showing associations with Firmicutes taxa. Microbiology. 2016;162:1388–1397. doi: 10.1099/mic.0.000328. [DOI] [PubMed] [Google Scholar]
- Kumar A., Alrefai W.A., Borthakur A., Dudeja P.K. Lactobacillus acidophilus counteracts enteropathogenic E. coli-induced inhibition of butyrate uptake in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2015;309:G602–G607. doi: 10.1152/ajpgi.00186.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R.E., Turnbaugh P.J., Samuel K., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Li B., Leblois J., Taminiau B., Schroyen M., Backers Y., Bindelle J. The effect of inulin and wheat bran on intestinal health and microbiota in the early life of broiler chickens. Poultry Sci. 2018;97:3156–3165. doi: 10.3382/ps/pey195. [DOI] [PubMed] [Google Scholar]
- Li D.D., Ding X.M., Zhang K.Y., Bai S.P., Wang J.P., Zeng Q.F. Effects of dietary xylooligosaccharides on the performance, egg quality, nutrient digestibility and plasma parameters of laying hens. Anim Feed Sci Technol. 2017;225:20–26. [Google Scholar]
- Lin Y., Li W.L., Huo Q.Q., Du C.H., Wang Z.X., Yi B.D. Effects of xylo-oligosaccharide and flavomycin on the immune function of broiler chickens. PeerJ. 2018;6:e4435. doi: 10.7717/peerj.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Zhang J.Y., Li M.L., Zhao L.H., Ji C., Ma Q.G. Alterations and structural resilience of the gut microbiota under dietary fat perturbations. J Nutr Biochem. 2018;61:91–100. doi: 10.1016/j.jnutbio.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Liu S.M., Li E.Y., Sun Z.Y., Fu D.J., Duan G.Q., Jiang M.M. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci Rep. 2019;9:287–295. doi: 10.1038/s41598-018-36430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfabe D.F., Cain N.E., Francis B., Klaus-Peter O., Cain D.P. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: relevance to autism spectrum disorder. Behav Brain Res. 2011;217:47–54. doi: 10.1016/j.bbr.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Markowiak P., Śliżewska K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018;10:21. doi: 10.1186/s13099-018-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty J., Vernay M. Absorption and metabolism of the volatile fatty acids in the hind-gut of the rabbit. Br J Nutr. 1984;51:265–277. doi: 10.1079/bjn19840031. [DOI] [PubMed] [Google Scholar]
- Matthias B., Andreas L., Torsten K., Parkos C.A., Madara J.L., Hopkins A.M. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- Mikkelsen L.L., Knudsen K.E.B., Jensen B.B. In vitro fermentation of fructo-oligosaccharides and transgalacto-oligosaccharides by adapted and unadapted bacterial population from the gastrointestinal tract of piglets. Anim Feed Sci Technol. 2004;116:225–238. [Google Scholar]
- Min Y.N., Yang H.L., Xu Y.X., Gao Y.P. Effects of dietary supplementation of synbiotics on growth performance, intestinal morphology, sIgA content and antioxidant capacities of broilers. J Anim Physiol Anim Nutr. 2016;100:1073–1080. doi: 10.1111/jpn.12479. [DOI] [PubMed] [Google Scholar]
- Ministry of Agriculture . Agricultural Standard Council, Ministry of Agriculture; Beijing, China: 2004. Feeding standard of chicken (NY/T 33-2004) [Google Scholar]
- Montagne L., Crévieu-Gabriel I., Toullec R., Lallès J.P. Influence of dietary protein level and source on the course of protein digestion along the small intestine of the veal calf. J Dairy Sci. 2003;86:934–943. doi: 10.3168/jds.S0022-0302(03)73676-5. [DOI] [PubMed] [Google Scholar]
- Moura P., Barata R., Carvalheiro F., Gírio F., Loureiro-Dias M.C., Esteves M.P. In vitro fermentation of xylo-oligosaccharides from corn cobs autohydrolysis by Bifidobacterium and Lactobacillus strains. Food Sci Technol. 2007;40:963–972. [Google Scholar]
- Murphy E.F., Cotter P.D., Healy S., Marques T.M., O’Sullivan O., Fouhy F. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut. 2010;59:1635–1642. doi: 10.1136/gut.2010.215665. [DOI] [PubMed] [Google Scholar]
- National Research Council . ninth revised ed. The National Academies Press; Washington, DC: 1994. Nutrient requirements of poultry. [Google Scholar]
- Nielsen H.L., Engberg J., Ejlertsen T., Nielsen H. Psychometric scores and persistence of irritable bowel after Campylobacter concisus infection. Scand J Gastroenterol. 2014;49:545–551. doi: 10.3109/00365521.2014.886718. [DOI] [PubMed] [Google Scholar]
- Pandit R.J., Hinsu A.T., Patel N.V., Koringa P.G., Jakhesara S.J., Thakkar J.R. Microbial diversity and community composition of caecal microbiota in commercial and indigenous indian chickens determined using 16s rDNA amplicon sequencing. Microbiome. 2018;6:115–121. doi: 10.1186/s40168-018-0501-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paola M.D., Filippo C.D., Cavalieri D., Ramazzotti M., Poullet J.B., Massart S. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q., Zeng X.F., Zhu J.L., Wang S., Liu X.T., Hou C.L. Effects of dietary Lactobacillus plantarum B1 on growth performance, intestinal microbiota, and short chain fatty acid profiles in broiler chickens. Poultry Sci. 2016;95:893–900. doi: 10.3382/ps/pev435. [DOI] [PubMed] [Google Scholar]
- Pérez-Burillo S., Pastoriza S., Fernández-Arteaga A., Luzón G., Jiménez-Hernández N., Dauria G. Spent coffee grounds extract, rich in mannooligosaccharides, promotes a healthier gut microbial community in a dose-dependent manner. J Agric Food Chem. 2019;67:2500–2509. doi: 10.1021/acs.jafc.8b06604. [DOI] [PubMed] [Google Scholar]
- Postler T.S., Ghosh S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metabol. 2017;26:110–130. doi: 10.1016/j.cmet.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourabedin M., Chen Q.L., Yang M.M., Zhao X. Mannan- and xylo-oligosaccharides modulate cecal microbiota and expression of inflammatory related cytokines and reduce cecal Salmonella enteritidis colonization in young chickens. FEMS Microbiol Ecol. 2017;93:fiw226. doi: 10.1093/femsec/fiw226. [DOI] [PubMed] [Google Scholar]
- Pourabedin M., Xu Z.X., Baurhoo B., Chevaux E., Zhao X. Effects of mannan oligosaccharide and virginiamycin on the cecal microbial community and intestinal morphology of chickens raised under suboptimal conditions. Can J Microbiol. 2014;60:255–266. doi: 10.1139/cjm-2013-0899. [DOI] [PubMed] [Google Scholar]
- Pourabedin M., Zhao X. Prebiotics and gut microbiota in chickens. FEMS Microbiol Lett. 2015;362:fnv122. doi: 10.1093/femsle/fnv122. [DOI] [PubMed] [Google Scholar]
- Ribeiro T., Cardoso V., Ferreira L.M.A., Lordelo M.M.S., Coelho E., Moreira A.S.P. Xylo-oligosaccharides display a prebiotic activity when used to supplement wheat or corn-based diets for broilers. Poultry Sci. 2018;97:4330–4341. doi: 10.3382/ps/pey336. [DOI] [PubMed] [Google Scholar]
- Saad N., Delattre C., Urdaci M., Schmitter J., Bressollier P. An overview of the last advances in probiotic and prebiotic field. LWT - Food Sci Technol (Lebensmittel-Wissenschaft -Technol) 2013;50:1–16. [Google Scholar]
- Salaheen S., Kim S.W., Haley B.J., Van Kessel J.A., Biswas D. Alternative growth promoters modulate broiler gut microbiome and enhance body weight gain. Front Microbiol. 2017;8:2088. doi: 10.3389/fmicb.2017.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirov I., Russell S.L., Antunes L.C.M., Finlay B.B. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- Scott K.P., Martin J.C., Duncan S.H., Flint H.J. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol Ecol. 2014;87:30–40. doi: 10.1111/1574-6941.12186. [DOI] [PubMed] [Google Scholar]
- Servin A.L. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev. 2004;28:405–440. doi: 10.1016/j.femsre.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Singh P., Karimi A., Devendra K., Waldroup P.W., Cho K.K., Kwon Y.M. Influence of penicillin on microbial diversity of the cecal microbiota in broiler chickens. Poultry Sci. 2013;92:272–276. doi: 10.3382/ps.2012-02603. [DOI] [PubMed] [Google Scholar]
- Topping D.L., Clifton P.M. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- Turnbaugh P.J., Ley R.E., Mahowald M.A., Vincent M., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Vernay M. Incidence of the circadian rhythm of the excretion pattern on acetate absorption and metabolism in the rabbit hind-gut. Reprod Nutr Dev. 1989;29:185–196. doi: 10.1051/rnd:19890206. [DOI] [PubMed] [Google Scholar]
- Videnska P., Sedlar K., Lukac M., Faldynova M., Gerzova L., Cejkova D. Succession and replacement of bacterial populations in the caecum of egg laying hens over their whole life. PloS One. 2014;9:e115142. doi: 10.1371/journal.pone.0115142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnska P., Rahman M.M., Faldynova M., Babak V., Matulova M.E., Prukner-Radovcic E. Characterization of egg laying hen and broiler fecal microbiota in poultry farms in Croatia, Czech Republic, Hungary and Slovenia. PloS One. 2014;9 doi: 10.1371/journal.pone.0110076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.W., Jia H.J., Zhang H.J., Wang J., Lv H.Y., Wu S.G. Supplemental plant extracts from flos ionicerae in combination with baikal skullcap attenuate intestinal disruption and modulate gut microbiota in laying hens challenged by salmonella pullorum. Front Microbiol. 2019;10:1681. doi: 10.3389/fmicb.2019.01681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese J.S., Holcombe S.J., Embertson R.M., Kurtz K.A., Roessner H.A., Jalali M. Changes in the faecal microbiota of mares precede the development of post partum colic. Equine Vet J. 2015;47:641–649. doi: 10.1111/evj.12361. [DOI] [PubMed] [Google Scholar]
- Wu S.R., Li T.H., Niu H.F., Zhu Y.F., Liu Y.L., Duan Y.L. Effects of glucose oxidase on growth performance, gut function, and cecal microbiota of broiler chickens. Poultry Sci. 2019;98:828–841. doi: 10.3382/ps/pey393. [DOI] [PubMed] [Google Scholar]
- Xue B., Xie J., Huang J., Chen L., Gao L., Ou S. Plant polyphenols alter a pathway of energy metabolism by inhibiting fecal Bacteroidetes and Firmicutes in vitro. Food Funct. 2016;7:1501–1507. doi: 10.1039/c5fo01438g. [DOI] [PubMed] [Google Scholar]
- Yan J., Charles J.F. Gut microbiome and bone: to build, destroy, or both? Curr Osteoporos Rep. 2017;15:376–384. doi: 10.1007/s11914-017-0382-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G.Q., Zhang P., Liu H.Y., Zhu X., Dong W.G. Spatial variations in intestinal skatole production and microbial composition in broilers. Anim Sci J. 2019;90:412–422. doi: 10.1111/asj.13164. [DOI] [PubMed] [Google Scholar]
- Zhu N., Wang J., Yu L., Zhang Q., Chen K., Liu B. Modulation of growth performance and intestinal microbiota in chickens fed plant extracts or virginiamycin. Front Microbiol. 2019;10:1333. doi: 10.3389/fmicb.2019.01333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X., Ji J., Qu H.X., Wang J., Shu D.M., Wang Y. Effects of sodium butyrate on intestinal health and gut microbiota composition during intestinal inflammation progression in broilers. Poultry Sci. 2019;98:4449–4456. doi: 10.3382/ps/pez279. [DOI] [PubMed] [Google Scholar]