Abstract
Oxidative stress has been implicated in both the functional and cognitive decline associated with neuropsychiatric diseases and aging. A master regulator of the body’s defense mechanism against oxidative stress is nuclear factor erythroid 2-related factor (NRF2). Here we investigated the effects of NRF2 deletion on motor and cognitive performance in “Aged” mice (17–25 months old) as compared to “Mature” mice (3–15 months old). We observed that the Aged Nrf2−/− mice were hyperactive and exhibited impaired acquisition of an active avoidance response. Furthermore, the Mature mice also displayed a hyperactive phenotype and had impaired working memory in the probe trial of the water radial arm maze. Overall, it appears that NRF2 may be implicated in memory and activity functions and its deletion exacerbates deficits associated with aging. These observations provide a model for assessing the role of oxidative stress in age-related disorders.
Keywords: Oxidative stress, Nrf2, Active avoidance, Hyperactivity
1. Introduction
Oxidative stress has been implicated in several disease states ranging from cancer to neurodegenerative disorders. Endogenously produced reactive oxygen species (ROS), the oxidative stressors, have the potential to cause dysfunction in various mechanisms of cellular activity and, therefore, require regular removal. A key mechanism for ROS clearance is via nuclear factor erythroid 2-related factor (NFE2L2) encoding a protein, NRF2, which functions as a leucine-zipper transcription factor responsible for the downstream expression of multiple antioxidant proteins that detoxify ROS molecules (Lee and Johnson, 2004; Lambros and Plafker, 2015; Itoh et al., 1997; Hayes and Dinkova-Kostova, 2014; Wang et al., 2017). NRF2 binds to antioxidant response elements (ARE) which encode for genes such as NADPH:quinone oxidoreducatase-1, superoxide dismutase, heme oxygenase-1, catalase, sulforedoxin, thioredoxin, peroxiredoxin, and glutathione enzymes, all of which combat ROS (Lee and Johnson, 2004; Itoh et al., 1997; Hayes and Dinkova-Kostova, 2014). Under reduced conditions, NRF2 is sequestered in a complex in the cytoplasm by Kelch-like ECH associated protein 1 (Keap1) and Phosphoglycerate Mutase Family Member 5 (PGAM5) and is marked for degradation by ubiquitination, giving it a half-life of only 15 min (Lee and Johnson, 2004; Lo and Hannink, 2008; Kobayashi et al., 2004; Itoh et al., 1997; Hayes and Dinkova-Kostova, 2014). In the presence of ROS, Keap1 dissociates from NRF2, thereby allowing its translocation into the nucleus where it forms a heterodimer with small Maf proteins and binds to the ARE promoters initiating transcription of antioxidant genes (Itoh et al., 1997; Hayes and Dinkova-Kostova, 2014).
NRF2 has been previously implicated in neuropsychiatric diseases such as depression, autism, and Alzheimer’s disease (Bouvier et al., 2016; Furnari et al., 2014; Lipton et al., 2016; Prasad, 2016; Xu et al., 2017). For example, NRF2 deletion altered neurobehavioral development following exposure to valproic acid in motor tasks and has been shown to result in an increased depression-like phenotype (Bouvier et al., 2016; Furnari et al., 2014). NRF2 has also been linked to reducing neuroinflammation and dendritic spine loss (Buendia et al., 2016; Martín-de-Saavedra et al., 2013). Deletion of NRF2 or inhibition of the pathway results in reduced dopamine and serotonin levels in the prefrontal cortex, as well as, vascular endothelial growth factor (VEGF) reductions in the hippocampus (Martín-de-Saavedra et al., 2013). Additionally, in a chronic stress paradigm, NRF2 was linked to a mechanism for antidepressant response following fluoxetine treatment (Tritschler et al., 2015). Furthermore, activation of the NRF2 pathway through compounds like sulforaphane can reduce amyloid plaque accumulation and reduce the working memory deficits in Alzheimer’s models (Lipton et al., 2016; Prasad, 2016; Xu et al., 2017). Interestingly, a novel role of NRF2 in neurogenesis and neural cell fate was recently identified where NRF2 deletion resulted in impaired long-term potentiation, reduced neurogenesis, and neural differentiation (Robledinos-Antón et al., 2017).
Oxidative stress has been implicated in functional deficits associated with aging (Muller et al., 2007). There is an age-dependent reduction in NRF2 and a resulting reduction of downstream antioxidant genes with increases in oxidative damage in proteins and DNA, culminating in apoptosis (Shih and Yen, 2006; Miller et al., 2012; Ames et al., 1993). Using accelerated aging mouse models (SAMP8, SOD1), oxidative stress accumulation increased circulating levels of pro-inflammatory cytokines (IL-6), activation of Nf-kappaB pathway, and cell senescence (Zhang et al., 2017; Farr et al., 2012). Furthermore, SAMP8 mice showed working memory deficits and reduced NRF2 levels in the brain, demonstrating the link between oxidative stress and cognitive function. However, both the number of mouse models of aging and understanding how neural effects contribute to deterioration of cognitive function is lacking. The present study further investigates the role of NRF2 in motor and cognitive function as well as a potential age-dependent phenotype.
2. Results
2.1. Mature & aged mice
There was no statistical difference in the spread of age ranges between Mature Nrf2+/+ (9.82 ± 2.40 mnths, mean & stdev. respectively) and Nrf2−/− (11.4 ± 2.45 mnths, mean & stdev. respectively) (t (36) = 1.82, p = 0.0768). Similar was true for Aged Nrf2+/+ (23.6 ± 2.32 mnths, mean & stdev. respectively) and Nrf2−/− (22.5 ± 2.9 mnths, mean & stdev. respectively) (t (21) = 0.945, p = 0.355).
2.2. Rotarod
Nrf2+/+ and Nrf2−/− mice exhibited differential performance on the rotarod over the three days of training (F (2, 116) = 6.742, p = 0.002; Fig. 1), with differences between each group (F (3, 58) = 18.23, p < 0.001; Fig. 1) and an interaction effect between days of training and group (F (6, 116) = 4.566, p < 0.001). Furthermore, the Mature Nrf2−/− mice exhibited a significantly longer latency to fall as compared to the Mature Nrf2+/+ counterparts on day 1 (t (1 7 4) = 3.935, p = 0.002, Bonferroni-corrected), as well as Mature Nrf2−/− mice compared to Aged Nrf2+/+mice (t (1 7 4) = 6.431, p < 0.001, Bonferroni-corrected) and Aged Nrf2−/− (t (1 7 4) = 6.34, p < 0.001, Bonferroni-corrected) mice. On the second day of training an age effect was observed between Mature Nrf2+/+ and Aged Nrf2+/+ (t (1 7 4) = 3.431, p = 0.014, Bonferroni-corrected), and Mature and Aged Nrf2−/− mice (t (1 7 4) = 3.498, p = 0.011, Bonferroni-corrected), and lastly an age and genotype difference was found between Mature Nrf2−/− and Aged Nrf2+/+ (t (5.734) = 5.734, p < 0.001, Bonferroni-corrected) mice. Mature Nrf2−/− mice had the best performance over each day, but declined in performance over days whereas all other groups increased performance over days. Aged Nrf2−/− mice did not show this pattern which suggests an age-dependent phenotype.
Fig. 1.
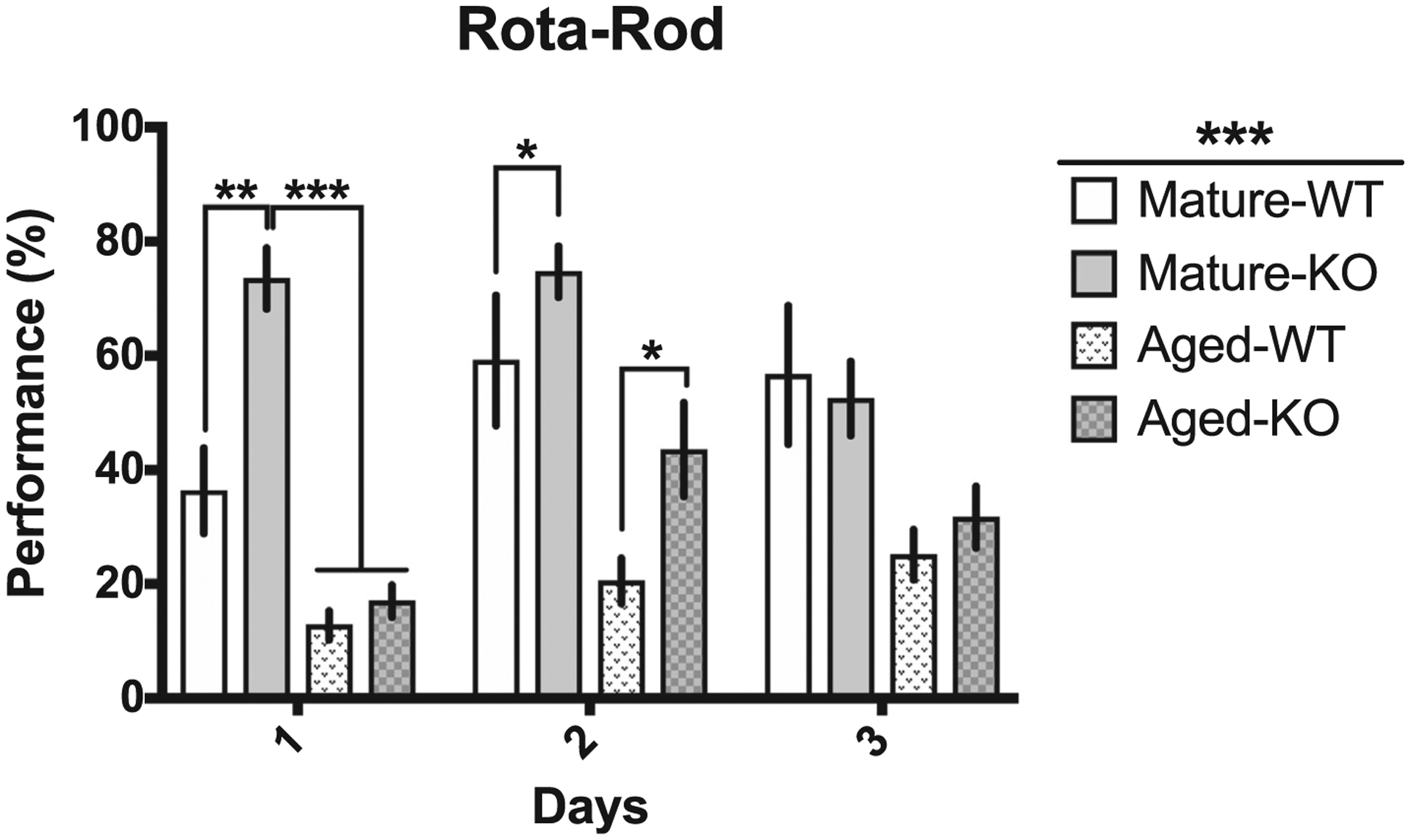
Nrf2 deletion results in increased performance in the rotarod in both Mature and Aged mice. A) Average rotarod performance over 3-day trials in Mature and Aged mice (Mature, Nrf2+/+ n = 13, Nrf2−/− n = 27; Aged, Nrf2+/+ n = 4, Nrf2−/− n = 12). Bars shown are mean with S.E.M. *indicates p < 0.05, **indicates p < 0.01, ***indicates p < 0.001.
2.3. Mature Adult mice
2.3.1. Motor activity
To test whether Nrf2−/− mice have general higher locomotor activity levels, they were assessed in an activity chamber. Nrf2−/− mice demonstrated higher locomotor activity for horizontal movements (t (47) = 3.361, p = 0.002) than Nrf2+/+ mice (Fig. 2A). Additionally, Nrf2−/− mice displayed more stereotypic movements as compared to controls (t (47) = 3.717, p < 0.001; Fig. 2B). Therefore, Nrf2−/− mice likely performed better than controls in the rotarod test because of a general higher activity rather than a learned motor ability over the trials. Furthermore, since Nrf2−/− mice demonstrated higher stereotyped movements (i.e. fewer new directional movements were made) these data appear to reflect a hyperactive state.
Fig. 2.

Mature Nrf2−/− mice display hyperactivity with increased stereotyped movements. A) Horizontal motor activity measured over 30 min (Nrf2+/+ n = 13, Nrf2−/− n = 27). B) Stereotypic behavior measured by percentage of new directional movements during motor activity task. Bars shown are mean with S.E.M. **indicates p < 0.01, ***indicates p < 0.001.
2.3.2. Anxiogenic Phenotyping
Open Field: There were no group differences in terms of time spent in the periphery or open areas of the open field chamber (Fig. 3A) between Mature Nrf2+/+ and Nrf2−/− mice.
Fig. 3.

No Anxiety-Related Behavior was detected in Mature Nrf2−/−’s. A) open field time measurements in peripheral and center locations (Nrf2+/+ n = 10, Nrf2−/− n = 16). B) elevated-plus maze measures; times, entries (Nrf2+/+ n = 18, Nrf2−/− n = 8), C) closed and open arms (Nrf2+/+ n = 10, Nrf2−/− n = 16). Bars shown are group mean with S.E.M.
Elevated Plus Maze: There were no group differences in terms of percent of time spent in either arm or number of entries (Fig. 3B & 3C). These two behavioral measures suggest that the hyperactivity seen in previous testing was not due to a more anxiogenic phenotype.
2.3.3. Spatial navigation learning Tasks
Morris Water Maze (MWM): Since Nrf2−/− mice appeared to have higher activity states compared to Nrf2+/+ mice, we sought to determine whether their learning was similar or impaired compared to Nrf2+/+ mice in the MWM. During the hidden platform trials, both Nrf2+/+ and Nrf2−/− mice were able to learn the task and showed significantly improved performance over four days (F (3, 93) = 26.41, p < 0.001, Fig. 3A), with no differences between groups. However, during visible platform trials Nrf2−/− mice performed better than the Nrf2+/+ mice (unpaired two-tailed t-test, t (31) = 2.623, p = 0.013; Fig. 3B), which likely is the result of their hyperactive state. Both Nrf2+/+ and Nrf2−/− mice were able to learn the maze and Nrf2−/− mice were better than Nrf2+/+ mice in finding the visible platform, which suggests there was no impairment in spatial navigation.
Water Radial Arm Maze (wRAM): Over the ten days of hidden platform trials all mice demonstrated ability to learn the task with decreased latency to find the platform (F (9, 225) = 11.38, p < 0.001), with only a statistical difference between genotypes on day 7 (t (2 5 0) = 2.837, p = 0.049, Bonferroni corrected; Fig. 4E). Additionally, there was no statistically significant differences when comparing distance traveled over the trials between groups (Fig. 4D). During visible platform trials both groups were able to find the platform with no difference between groups (Fig. 4C). However, Nrf2−/− mice spent significantly less time in the goal arm (previously containing the hidden platform) during the probe trial (t (17) = 2.231, p = 0.039; Fig. 4F), which suggests that Nrf2−/− mice may be randomly sampling each arm looking for the hidden platform, rather than using the contextual clues for spatial navigation.
Fig. 4.

Differences in Spatial Navigation Learning Tasks. A) Morris water maze hidden platform training over 4 days (Nrf2+/+ n = 13, Nrf2−/− n = 20), followed by B) visible platform testing, C) water radial arm maze visible platform latencies, D) distance travelled over hidden platform training days (Nrf2+/+ n = 13, Nrf2−/− n = 15) and E) latency to find platform. F) probe trial testing on 11th day of testing with percentage of time spent in goal arm previously containing hidden platform. Bars and line graph shown are group means with S.E.M. *indicates p < 0.05.
2.3.4. Passive avoidance
There was no significant difference between raw calculated Nrf2+/+ and Nrf2−/− latencies during either the training or test trial (despite Nrf2+/+ mice having a higher test latency) in passive avoidance learning between first and second trial (Fig. 5A). However, upon further analysis of z-scores to understand individual differences in learning (comparing the difference in latency of each mouse between the test trial compared to the training trial) revealed a significant difference between Nrf2+/+ and Nrf2−/− mice (t (42) = 4.22, p < 0.001; Fig. 5B). Results suggest that the memory for the shock compartment is more robust in Nrf2+/+ mice compared to Nrf2−/− mice.
Fig. 5.

Mature Nrf2−/− mice begin to show cognitive decline in passive avoidance. A) passive avoidance latencies to enter shock zone on training and test trials (Nrf2+/+ n = 22, Nrf2−/− n = 22). B) Z-score differences between Nrf2+/+ and Nrf2−/− relative to individual changes in latencies in test and training trials. Bars and line graph shown are group means with S.E.M. ***indicates p < 0.001.
2.4. Aged Mice:
2.4.1. Active avoidance
Nrf2+/+ and Nrf2−/− mice both demonstrated ability to make escape responses immediately during first trials and this improved throughout testing (F (6, 113) = 6.301, p < 0.001) with a statistically significant genotype effect (F (1, 113) = 16.34, p < 0.001; Fig. 6A). Assessment of escape latency also revealed that both Nrf2+/+ and Nrf2−/− mice exhibited significant improvement over trials (F (6, 115) = 5.138, p < 0.001) with a significant trial by genotype interaction where Nrf2−/− mice made faster escape responses (F (6, 115) = 2.687, p = 0.018; Fig. 6B). Additionally, on day 2, Nrf2−/− mice made significantly faster responses compared to Nrf2+/+ mice (t (1 1 5) = 3.457, p = 0.005, Bonferroni corrected).
Fig. 6.

Active Avoidance reveals Nrf2−/− memory deficits. A) Average number of escape responses per group over 10 trials per day for 7 days (Nrf2+/+ n = 6, Nrf2−/− n = 13), and B) latencies to escape. C) Average number of avoidance responses per group and D) latencies. E) Z-scores showing learning differences between genotypes comparing last to first trial. Line graph shows group means per 10 trials with S.E.M. *indicates p < 0.01, **indicates p < 0.01, ***indicates p < 0.001.
When measuring the number of avoidance responses, Nrf2+/+ and Nrf2−/− mice showed acquisition with a significant trial effect (F (6, 115) = 6.496, p < 0.001) as well as a genotype effect (F (6, 115) = 13.54, p < 0.001; Fig. 6C). Nrf2+/+ mice, on average, made more avoidance responses than Nrf2−/− mice, which was significant with a trial by genotype interaction (F (4, 68) = 3.292, p = 0.016). Latency for avoidance responses was significant with a trial effect (F (6, 116) = 3.674, p = 0.002) with a difference between genotypes (F (1,116) = 8.647, p = 0.004; Fig. 6D). Additionally, Nrf2+/+ and Nrf2−/− mice did not show a difference in total number of escapes plus avoidance responses (making either escapes or avoidances; data not shown) indicating mice could learn either response. Then together, this test demonstrates that in these Aged mice, NRF2 deletion resulted in decreased cognitive function for learning the avoidance task with no perturbation (but an actual potentiation) in escape responses.
2.5. Brain chemistry
Mature Nrf2−/− mice exhibited a higher turnover rate of dopamine in the hippocampus that was statistically significant as compared to Mature wild type mice (t (7) = 3.922, p = 0.006) (Table 1). Additionally, Mature Nrf2−/− mice had higher serotonin levels in cerebellum (t (9) = 2.62, p = 0.029). Aged Nrf2−/− mice exhibited less DOPAC levels in the hypothalamus compared to their age matched controls (t (12) = 3.169, p = 0.008). Aged Nrf2+/+ mice also had elevated levels of serotonin in the cerebellum compared to their Mature wild type counterparts (t (12) = 2.693, p = 0.046, Bonferroni-corrected).
Table 1.
Brain chemistry. Catecholamine levels and their metabolites measured via HPLC across Frontal Cortex, Striatum, Hippocampus, Hypothalamus, and Cerebellum in Mature Nrf2+/+ (n = 7), Mature Nrf2−/− (n = 4), Aged Nrf2+/+ (n = 7), and Aged Nrf2−/− (n = 6) mice.
| Mature-WT | Mature-KO | Aged-WT | Aged-KO | |||||
|---|---|---|---|---|---|---|---|---|
| NT: | MEAN | S.E.M. | MEAN | S.E.M. | MEAN | S.E.M. | MEAN | S.E.M. |
| NE | 0.42 | 0.05 | 0.35 | 0.06 | 0.48 | 0.07 | 0.50 | 0.06 |
| DA | 6.98 | 1.11 | 7.31 | 2.32 | 5.81 | 1.33 | 7.81 | 1.25 |
| DOPAC | 1.11 | 0.29 | 0.85 | 0.37 | 1.10 | 0.21 | 1.91 | 0.50 |
| 5HT | 0.42 | 0.06 | 0.54 | 0.15 | 0.54 | 0.17 | 1.00 | 0.50 |
| 5HIAA | 0.03 | 0.00 | 0.24 | 0.22 | 0.06 | 0.02 | 0.37 | 0.29 |
| HVA | 0.34 | 0.06 | 0.65 | 0.23 | 0.71 | 0.26 | 2.69 | 1.41 |
| DOPAC/DA | 0.17 | 0.05 | 0.12 | 0.03 | 1.00 | 0.85 | 0.23 | 0.05 |
| HVA/DA | 0.05 | 0.01 | 0.10 | 0.04 | 1.84 | 1.77 | 0.38 | 0.23 |
| 5-HIAA/5HT | 0.08 | 0.01 | 0.39 | 0.34 | 0.23 | 0.14 | 0.23 | 0.07 |
| STRIATUM | ||||||||
| Mature-WT | Mature-KO | Aged-WT | Aged-KO | |||||
| NT: | MEAN | S.E.M. | MEAN | S.E.M. | MEAN | S.E.M. | MEAN | S.E.M. |
| NE | 0.26 | 0.03 | 0.38 | 0.14 | 0.31 | 0.02 | 0.43 | 0.12 |
| DA | 7.78 | 0.86 | 11.16 | 2.67 | 10.01 | 1.21 | 9.98 | 1.90 |
| DOPAC | 0.69 | 0.04 | 1.73 | 1.00 | 1.31 | 0.43 | 1.53 | 0.31 |
| 5HT | 0.52 | 0.05 | 2.27 | 1.72 | 1.48 | 0.67 | 2.21 | 1.17 |
| 5HIAA | 0.05 | 0.01 | 0.98 | 0.60 | 0.07 | 0.03 | 0.12 | 0.06 |
| HVA | 1.06 | 0.10 | 1.75 | 0.56 | 1.63 | 0.39 | 1.79 | 0.30 |
| DOPAC/DA | 0.09 | 0.01 | 0.15 | 0.07 | 0.13 | 0.05 | 0.15 | 0.01 |
| HVA/DA | 0.14 | 0.02 | 0.17 | 0.04 | 0.17 | 0.05 | 0.19 | 0.03 |
| 5-HIAA/5HT | 0.10 | 0.01 | 1.59 | 1.01 | 0.10 | 0.05 | 0.11 | 0.03 |
| HIPPOCAMPUS | ||||||||
| Mature-WT | Mature-KO | Aged-WT | Aged-KO | |||||
| NT: | MEAN | S.E.M. | MEAN | S.E.M. | MEAN | S.E.M. | MEAN | S.E.M. |
| NE | 0.68 | 0.09 | 0.62 | 0.10 | 0.51 | 0.10 | 0.76 | 0.22 |
| DA | 9.77 | 1.38 | 6.92 | 0.97 | 9.10 | 2.06 | 8.29 | 1.21 |
| DOPAC | 0.83 | 0.10 | 0.96 | 0.21 | 0.79 | 0.14 | 0.98 | 0.23 |
| 5HT | 0.52 | 0.09 | 0.64 | 0.22 | 0.55 | 0.14 | 0.90 | 0.16 |
| 5HIAA | 0.08 | 0.02 | 0.13 | 0.05 | 0.09 | 0.03 | 0.36 | 0.13 |
| HVA | 0.19 | 0.07 | 0.33 | 0.19 | 0.38 | 0.11 | 1.44 | 0.66 |
| DOPAC/DA | 0.09 | 0.00 | 0.14** | 0.02 | 0.13 | 0.05 | 0.11 | 0.01 |
| HVA/DA | 0.02 | 0.01 | 0.05 | 0.03 | 0.10 | 0.06 | 0.15 | 0.07 |
| 5-HIAA/5HT | 0.16 | 0.02 | 0.21 | 0.03 | 0.24 | 0.10 | 0.47 | 0.20 |
| HYPOTHALAMUS | ||||||||
| Mature-WT | Mature-KO | Aged-WT | Aged-KO | |||||
| NT: | MEAN | S.E.M. | MEAN | S.E.M. | MEAN | S.E.M. | MEAN | S.E.M. |
| NE | 1.19 | 0.11 | 1.12 | 0.10 | 1.53 | 0.22 | 1.48 | 0.26 |
| DA | 6.07 | 1.36 | 13.31 | 8.28 | 10.41 | 3.22 | 6.55 | 1.12 |
| DOPAC | 1.11 | 0.18 | 1.33 | 0.79 | 1.82 | 0.42 | 0.65** | 0.16 |
| 5HT | 0.67 | 0.12 | 1.39 | 0.83 | 1.52 | 0.58 | 2.15 | 0.57 |
| 5HIAA | 0.07 | 0.01 | 0.05 | 0.03 | 0.15 | 0.09 | 0.32 | 0.11 |
| HVA | 0.41 | 0.17 | 1.32 | 0.89 | 1.25 | 0.50 | 0.98 | 0.29 |
| DOPAC/DA | 0.49 | 0.34 | 0.10 | 0.01 | 0.21 | 0.07 | 0.11 | 0.03 |
| HVA/DA | 0.06 | 0.02 | 0.09 | 0.00 | 0.19 | 0.10 | 0.18 | 0.06 |
| 5-HIAA/5HT | 0.12 | 0.01 | 0.07 | 0.04 | 0.11 | 0.05 | 0.29 | 0.19 |
| CEREBELLUM | ||||||||
| Mature-WT | Mature-KO | Aged-WT | Aged-KO | |||||
| NT: | MEAN | S.E.M. | MEAN | S.E.M. | MEAN | S.E.M. | MEAN | S.E.M. |
| NE | 0.51 | 0.05 | 0.54 | 0.10 | 0.52 | 0.08 | 0.60 | 0.09 |
| DA | 9.43 | 2.47 | 9.97 | 3.28 | 13.78 | 6.28 | 8.67 | 1.78 |
| DOPAC | 0.43 | 0.06 | 0.85 | 0.62 | 1.47 | 0.54 | 0.70 | 0.18 |
| 5HT | 0.05 | 0.00 | 0.49* | 0.23 | 0.59# | 0.21 | 0.47 | 0.11 |
| 5HIAA | 0.24 | 0.09 | 0.03 | 0.01 | 0.10 | 0.04 | 0.13 | 0.03 |
| HVA | 0.18 | 0.07 | 0.65 | 0.60 | 0.55 | 0.18 | 1.19 | 0.69 |
| DOPAC/DA | 0.05 | 0.01 | 0.06 | 0.03 | 0.18 | 0.08 | 0.09 | 0.04 |
| HVA/DA | 0.02 | 0.01 | 0.04 | 0.03 | 0.10 | 0.05 | 0.05 | 0.02 |
| 5-HIAA/5HT | 5.15 | 2.05 | 0.15 | 0.07 | 1.26 | 1.12 | 1.03 | 0.78 |
indicates p < 0.05 effect of genotype,
indicates p < 0.01 effect of genotype.
indicates p < 0.05 effect of age, Bonferroni-corrected.
3. Discussion
Mature NRF2 knockout mice displayed a hyperactive state with enhanced motor activity, enhanced rotarod performance, and shortened visible platform latency in the Morris water maze. Interestingly, others have reported that pharmacological activation of NRF2 or NRF2 deletion resulted in no differences in locomotion but reductions in measures of stress behavior in the Tail Suspension Test and Forced Swim Test following autoimmune activation with lipopolysaccharide (Martín-de-Saavedra et al., 2013; Muramatsu et al., 2013; Yao et al., 2016; Zhang et al., 2017). This reveals an interesting difference with genetic removal of NRF2 increasing levels of motor movements whereas activation of NRF2 following immune challenge increased depressive-like behaviors. It would be interesting to see whether NRF2 activation alone decreases motor behaviors and how NRF2 may play a part in regulating affective behaviors as well as how they may change across age.
Additionally, this enhanced motor performance was still present in Aged knockouts during rotarod testing. Others have shown no change in rotarod performance when using knockout mice 9–13 weeks of age (Muramatsu et al., 2013) while we found that Mature and Aged mice exhibited enhanced performance. Furthermore, this study found a hyperactivity state observed in the motor activity chamber that was accompanied by an increase in stereotypic movements. Additionally, our data show that aging influences the rotarod task where Mature mice perform better than Aged mice.
Deficits in probe trial performance in the water radial arm maze suggests that their ability to find the platforms in both the Morris water maze and water radial arm maze was more consequent to their hyperactivity rather than cognitive capability. That is, their hyperactive state may have masked a cognitive deficit which was then revealed by poor probe trial performance. Similarly, although there was no difference in the latency to re-enter the dark chamber in the passive avoidance paradigm, the negative z-score of the Nrf2−/− mice reflects their tendency to re-enter faster than the Nrf2+/+ mice. Behaviorally, Nrf2−/− mice did not show elevated anxiety levels as measured by time in center of the open field or time in the open arms of the elevated plus maze. These observations are consistent with previous data showing no baseline changes in open field tests in terms of distance or time spent. (Martín-de-Saavedra et al., 2013). Therefore, the evidence suggests that hyperlocomotion demonstrated by the Nrf2−/− mice is not due to increased anxiety.
Interestingly, Aged Nrf2−/− mice also showed deficits in active avoidance, as evidenced by a significant reduction in the number of avoidance responses during acquisition. Knockout mice also showed an increased latency to make avoidance responses, but made fewer avoidances overall and made increased numbers of escape responses with no change in the latency to make escape responses. Therefore, Nrf2−/− mice demonstrated no overt motor deficit but rather a cognitive deficit compared to their Nrf2+/+ counterparts. Impairments in active avoidance training demonstrate that Nrf2 may play a role in learning and memory, warranting further investigation into how it affects brain circuitry in areas such as the hippocampus and prefrontal cortex. Also, the need of rescue experiments can demonstrate reversal of deficits can be accomplished with administration of antioxidants or reversing deficits of monoamine dysregulation.
Mature Nrf2−/− adults had increased dopamine turnover in the hippocampus and increased serotonin levels in the cerebellum, which may relate to hyperactivity seen in these mice. They also exhibited a dysregulation of dopamine signaling in the hippocampus, resulting in a deficit in memory tasks. Previous studies using 2–3 month old Nrf2−/− mice have shown increases in 5-HIAA levels in the cortex and brainstem, increased HVA levels in brainstem, and increased dopamine turnover in striatum, hippocampus, and brainstem. Another study using 3–4 month-old Swiss mice found a reduction in serotonin, dopamine, and increased glutamate in prefrontal cortex (Martín-de-Saavedra et al., 2013; Muramatsu et al., 2013). These observations are in concert with our present study demonstrating monoamine metabolism dysregulation in key areas associated with motor behavior, learning, and affective cognition. An effect of aging was also found in Nrf2+/+ mice where Aged mice had increased serotonin in the cerebellum, an effect which was not seen in the knockout mice. This effect may reflect an age-dependent change in serotonin that occurs normally during aging and the deletion of NRF2 accelerates serotonin changes. As with the motor behaviors, more evidence needs to be collected to better understand the mechanism behind changes in neurotransmitter levels in conjunction with age and how this functionally regulates behavior.
NRF2 helps regulate the response to oxidative stress and has been implicated in several disease states, including the cognitive decline associated with aging. Here, we describe that NRF2 deletion in both Mature (age 3–15 months) and Aged (Aged 17–25 months) mice results in changes in motor performance and a cognitive dysregulation. These observations may open new avenues for understanding the role of oxidative stress, both natural and pathological, in aging. Furthermore, these present findings indicate that a careful investigation of the mechanism of both NRF2′s function in relation to behavioral changes is warranted.
4. Experimental procedure
All procedures were approved by the Rutgers University Institutional Animal Care and Use Committee. Adult C57 male wild type (+/+) and NRF2-knockout (−/−) mice were generated as described previously (Moi et al., 1994; Shen et al., 2005; Chan et al., 1996). Mice were single housed with free access to food and water on a 12:12 light/dark cycle. All behavioral testing was done during the light cycle. “Mature” Nrf2+/+ and Nrf2−/− were defined as being between 3 and 15 months old while “Aged” mice of the same genotypes were 17 to 25 months old at testing. Behavioral tests were conducted sequentially with at least 2 days between tests and brain collection was at least 1 week post last behavioral trial. Ordering of testing follows is shown by ordering of behavioral procedure as follows below.
4.1. Mature adult mice
4.1.1. Rotarod
Each mouse was placed onto a 3.75 cm diameter rod rotating at 16 rpm and located 10 cm above a padded area. The latency of the mice to fall was recorded for a maximum of 30 s per trial for a total of 3 trials per day for 3 consecutive days. Data are reported as percent of the 30-sec maximum.
4.1.2. Morris water maze
Testing as previously described with minor modifications (Ames et al., 1993). The maze consisted of a circular tub measuring 60 cm in diameter and 26 cm in height. The tub was painted white on the interior and was filled 3/4 full of water maintained at 23–26 °C and made opaque with white non-toxic latex paint. A starting point was determined randomly from one of four equally spaced quadrants. In the visible platform version, a platform measuring 8 cm in diameter and painted black was placed in one quadrant of the maze 1.5 cm above the surface. In the hidden platform trials, an identical platform painted white sat 2 cm below the surface of the water. Animals received five trials each day and each animal were allowed a maximum of 60 sec to reach the escape platform. The position of the hidden platform remained constant throughout the experiment and extra maze cues were present. If the animal did not reach the platform in 60 secs, a score of 60 was recorded and the animal was gently guided to and placed on the platform. During the inter-trial interval, all mice rested atop the platform until the next trial began.
4.1.3. Water radial arm maze
The procedure was described elsewhere (Hyde et al., 1997). Briefly, mice were placed into one arm (each arm measuring 11 × 30 cm) of an 8-arm maze filled with opaque water with a hidden platform in a different arm (the start arm was selected in a pseudorandomized fashion) for 3 consecutive trials with a maximum of 60 s/trial for 10 days. On the 11th day, mice received a one-trial probe test with no platform and then received three trials with a visible platform in a different arm (not the same goal arm- that was used for the past 10 days) with a maximum of 60 sec. Latency to find the platform was recorded. The maze was surrounded by a curtain with external cues.
4.1.4. Motor activity
Mice were placed in a new cage which was then placed inside a Plexiglas box (43 × 42 × 9 cm) activity chamber with photocells placed 7 cm apart and surrounding the cage. Photobeam breaks were recorded over 30 min with respect to horizontal (movement in any direction) and ambulatory (movement in a new direction) movements. Stereotypy was measured by percentage difference in ambulatory and horizontal movements normalized to each individual animal, then averaged per group to assess percent of new movements.
4.1.5. Passive avoidance
Passive avoidance was assessed in a shuttle box divided into two 15 × 12 × 9 compartments, one well-lit and the other rendered dark. The floor consisted of stainless steel bars 0.75 cm apart. Mice were placed in the illuminated start compartment. Upon entering the dark compartment, mice received a scrambled foot-shock of 0.8 mA that continued until they reentered the light compartment (training trial); 24 h later mice were retested to measure memory for the shock compartment (test trial). Latency to reenter the dark box was then recorded with a maximum of 90 sec. Difference in latencies between test and training trial were calculated and z-scores were taken compared to the control group; these calculations are described elsewhere (Guilloux et al., 2011).
4.1.6. Elevated plus maze
Procedure described in more detail elsewhere (Furnari et al., 2014). In brief, the elevated plus maze, 60 in. above the floor, had two closed arms measuring 65 × 8 cm, and two open arms measuring 30 cm long and 9 cm wide with a central area of 5 cm × 5 cm. Each mouse was placed in the center square and allowed to explore the maze for 10 min with number of entries and time spent recorded for each arm.
4.1.7. Open field
Mice were placed in the center of a chamber measuring 55 × 6 × 27 cm and video recorded for five minutes. AnyMaze software (Stoelting Co.) recorded movement within the inner area of the box compared to the outer area of the box, as well as time spent in each compartment. Data are represented as percent time and distance within the center area of the chamber.
4.2. Aged mice
4.2.1. Rotarod
Each mouse was placed onto a rotating rod (3.75 cm diameter) that was rotating at 24 rpm and located 105 cm above a padded area. The latency of the mice to fall was recorded for a maximum of 30 sec per trial for a total of 3 trials per day for 3 consecutive days. Data are reported as percent of the 30 s maximum.
4.2.2. Active avoidance
Similar to previously described (Halladay et al., 2000). Briefly, each mouse was placed into a Plexiglas T-maze with a 30 × 9 × 11 cm start box, a 11 × 9 × 22 cm runway and two 11 × 9 × 9 arms, one of which served as the goal box. The maze had a stainless-steel grid floor (0.75 cm apart). A 70 dB, 10 sec maximum cue served as the conditioned stimulus (CS), after which a 10-sec maximum, 0.8 mA, scrambled foot-shock was delivered as the unconditioned stimulus (US). The US was followed by a 20 × s inter-trial interval (ITI) in the start box. Mice could avoid the shock by entering the goal box during the CS or escape the shock by entering to goal box during the US. Mice were tested for 10 trials per day for 7 days. Data recorded were average latency and number of responses for each avoidance or escape responses, per 10 trial bins.
4.3. Brain chemistry
At the completion of behavioral studies mice were rapidly decapitated and brains were dissected for the following brain regions: frontal cortex, hypothalamus, striatum, hippocampus, cerebellum, and tegmentum, then flash frozen in liquid nitrogen. Samples were then homogenized in 0.3 ml of 0.4 N perchloric acid (Fischer Scientific), centrifuged at 16,000×g for 12 min at 4 °C, and supernatant was collected and stored in −80 °C until analysis. Analysis was run through HPLC-FLD setup as follows: samples were delivered through high-pressure (Rheodyne) valve fitted with a 20-microliter sample loop onto a Biophase ODS C-18 reverse-phase column (5 mm, 250×4.6 mm i.d.) at 0.7 ml per minute flow rate, then excited at 280 nm and emission at 315 nm with a HP 1100 series fluorescence detector (Allegiant Technologies). Mobile phase consisted of 0.1375 M sodium phosphate (dibasic), 0.0625 M citric acid, 5.0 mg EDTA, and 10% methanol. A reference mix standard at 25 pM concentration for each neurotransmitter and metabolite was used for quantitative analysis. Data are reported as micrograms of neurotransmitter per gram of wet tissue.
4.4. Statistics
Data were analyzed using Prism software 7.0 (GraphPad Software). Repeated measures, two-way, one-way ANOVA’s, z-score, and multiple t-tests were run on data with Bonferroni correction when appropriate. Statistical significance was determined with an alpha value of 0.05.
HIGHLIGHTS.
Deletion of NRF2 resulted in enhanced motor performance.
Deletion of NRF2 resulted in impaired cognitive performance with age.
Deletion of NRF2 resulted in subtle changes in brain monoamines.
Acknowledgements
We would like to thank Casey Lee and Andy Tran for their efforts in collecting the final data set for these studies.
Funding
Funding was provided by Aresty Research Funds (MG, AM, SS), and Graduate Vice Chair Funds (GCW).
Footnotes
Conflicts of interest
The authors do not declare any conflicts of interest.
References
- Lee JM, Johnson JA, 2004. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J. Biochem. Mol. Biol 37 (2), 139–143. 10.5483/BMBRep. 2004, 37(2), pp. 139. [DOI] [PubMed] [Google Scholar]
- Buendia I, Michalska P, Navarro E, Gameiro I, Egea J, León R, 2016. Nrf2–ARE pathway: an emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol. Ther 157, 84–104. 10.1016/j.pharmthera.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Lo S-C, Hannink M, 2008. PGAM5 tethers a ternary complex containing Keap1 and Nrf2 to mitochondria. Exp. Cell Res 314, 1789–1803. 10.1016/j.yexcr.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M, 2004. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol 24, 7130–7139. 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier E, Brouillard F, Molet J, Claverie D, Cabungcal J-H, Cresto N, Doligez N, Rivat C, Do KQ, Bernard C, Benoliel J-J, Becker C, 2016. Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Nat. Publish. Group 22, 1701–1713. 10.1038/mp.2016.144. [DOI] [PubMed] [Google Scholar]
- Furnari MA, Saw CL-L, Kong A-N, Wagner GC, 2014. Altered behavioral development in Nrf2 knockout mice following early postnatal exposure to valproic acid. Brain Res. Bull 109, 132–142. 10.1016/j.brainresbull.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA, Rezaie T, Nutter A, Lopez KM, Parker J, Kosaka K, Satoh T, McKercher SR, Masliah E, Nakanishi N, 2016. Therapeutic advantage of pro-electrophilic drugs to activate the Nrf2/ARE pathway in Alzheimer’s disease models. Nat. Publish. Group 7, 1–8. 10.1038/cddis.2016.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KN, 2016. Simultaneous activation of Nrf2 and elevation of antioxidant compounds for reducing oxidative stress and chronic inflammation in human Alzheimer’s disease. Mech. Ageing Dev 153, 41–47. 10.1016/j.mad.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Xu P, Wang K, Lu C, Dong L, Gao L, Yan M, Aibai S, Yang Y, Liu X, 2017. Protective effects of linalool against amyloid beta-induced cognitive deficits and damages in mice. Life Sci. 174, 21–27. 10.1016/j.lfs.2017.02.010. [DOI] [PubMed] [Google Scholar]
- Martín-de-Saavedra MD, Budni J, Cunha MP, Gómez-Rangel V, Lorrio S, del Barrio L, Lastres-Becker I, Parada E, Tordera RM, Rodrigues ALS, Cuadrado A, López MG, 2013. Nrf2 participates in depressive disorders through an anti-inflammatory mechanism. Psychoneuroendocrinology 38, 2010–2022. 10.1016/j.psyneuen.2013.03.020. [DOI] [PubMed] [Google Scholar]
- Tritschler L, El Ali Z, Damiens M-H, Pallardy M, David DJ, Kerdine-Römer S, Gardier AM, 2015. Nrf2-signaling and BDNF: a new target for the antidepressant-like activity of chronic fluoxetine treatment in a mouse model of anxiety/depression. Neurosci. Lett 597, 121–126. 10.1016/j.neulet.2015.04.036. [DOI] [PubMed] [Google Scholar]
- Robledinos-Antón N, Rojo AI, Ferreiro E, Núñez Á, Krause K-H, Jaquet V, Cuadrado A, 2017. Transcription factor NRF2 controls the fate of neural stem cells in the subgranular zone of the hippocampus. Redox Biol. 13, 393–401. 10.1016/j.redox.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H, 2007. Trends in oxidative aging theories. Free Rad. Biol. Med 43, 477–503. 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Shih P-H, Yen G-C, 2006. Differential expressions of antioxidant status in aging rats: the role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontology 8, 71–80. 10.1007/s10522-006-9033-y. [DOI] [PubMed] [Google Scholar]
- Miller CJ, Gounder SS, Kannan S, Goutam K, Muthusamy VR, Firpo MA, Symons JD, Paine III R, Hoidal JR, Rajasekaran NS, 2012. Disruption of Nrf2/ARE signaling impairs antioxidant mechanisms and promotes cell degradation pathways in Aged skeletal muscle. BBA – Mol. Basis Dis 1822, 1038–1050. 10.1016/j.bbadis.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Ames BN, Shigenaga MK, Hagen TM, 1993. Oxidants, antioxidants, and the degenerative diseases of aging. PNAS 90, 7915.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Unnikrishnan A, Deepa SS, Liu Y, Li Y, Ikeno Y, Sosnowska D, Van Remmen H, Richardson A, 2017. A new role for oxidative stress in aging. The accelerated aging phenotype in Sod1−/− mice is correlated to increased cellular senescence. Redox Biol. 11, 30–37. 10.1016/j.redox.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu H, Katsuoka F, Toide K, Shimizu Y, Furusako S, Yamamoto M, 2013. Nrf2 deficiency leads to behavioral, neurochemical and transcriptional changes in mice. n/a–n/a. Genes Cells 173. 10.1111/gtc.12083. [DOI] [PubMed] [Google Scholar]
- Yao W, Zhang J-C, Ishima T, Ren Q, Yang C, Dong C, Ma M, Saito A, Honda T, Hashimoto K, 2016. Antidepressant effects of TBE-31 and MCE-1, the novel Nrf2 activators, in an inflammation model of depression. Eur. J. Pharmacol 793, 21–27. 10.1016/j.ejphar.2016.10.037. [DOI] [PubMed] [Google Scholar]
- Zhang J-C, Yao W, Dong C, Yang C, Ren Q, Ma M, Han M, Wu J, Ushida Y, Suganuma H, Hashimoto K, 2017. Prophylactic effects of sulforaphane on depression-like behavior and dendritic changes in mice after inflammation. J. Nutrit. Biochem 39, 134–144. 10.1016/j.jnutbio.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Lambros ML, Plafker SM, 2015. Oxidative Stress and the Nrf2 Anti-Oxidant Transcription Factor in Age-Related Macular Degeneration. In Retinal Degenerative Diseases. Springer International Publishing, Cham: 10.1007/978-3-319-17121-0_10 pp. 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LA, Hoplight BJ, Denenberg VH, 1997. Water version of the radial-arm maze: Learning in three inbred strains of mice. Brain Res. 785, 236–244. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y, 1997. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun 236, 313–322. [DOI] [PubMed] [Google Scholar]
- Halladay AK, Coyne T, Sharifi J, Seto J, Wagner GC, 2000. Avoidance responding following amphetamine-induced dopamine depletion. Pharamcol. Toxicol 87, 211–217. 10.1034/j.1600-0773.2000. [DOI] [PubMed] [Google Scholar]
- Guilloux J-P, Seney M, Edgar N, Sibille E, 2011. Integrated behavioral z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: Relevance to emotionality and sex. J. Neurosci. Methods 197, 21–31. 10.1016/j.jneumeth.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, Dinkova-Kostova AT, 2014. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci 9, 199–218. 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhu J, Li L, Li Y, Lv H, Xu Y, Sun G, Pi J, 2017. Effects of Nrf2 deficiency on arsenic metabolism in mice. Toxicol. Appl. Pharmacol 337, 111–119. 10.1016/j.taap.2017.11.001. [DOI] [PubMed] [Google Scholar]
- Farr SA Price TO Banks WA Ercal N Morley JE 2012. Effect of alpha-lipoic acid on memory, oxidation, and lifespan in SAMP8 mice. J. Alzheimers Dis 32 (2), 447–455, Epub 2012/07/13. doi: 10.3233/JAD-2012-120130. [DOI] [PubMed] [Google Scholar]
- Moi P, Chan K, Asunis I, Cao A, Kan YW, 1994. Isolation of NF-E2-related factor 2(Nrf2), a NF-E2 like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. U.S.A 91 (21), 9926–9930. 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen G, Xu C, Hu R, Jain MR, Nair S, Lin W, Yang CS, Chan JY, Kong AN, 2005. Comparison of (−)-epigallocatechin-3-gallate elicited liver and small intestine gene expression profiles between C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Pharm. Res 22 (11), 1805–1820. 10.1007/s11095-005-7546-8. [DOI] [PubMed] [Google Scholar]
- Chan K, Lu R, Chang JC, Kan YW, 1996. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc. Natl. Acad. Sci. U.S.A 93 (24), 13943–13948. 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]


