Abstract
Background and Aims:
Despite advances in haemophilia care, inhibitor development remains a significant complication. Although viable treatment options exist, there is some divergence of opinion in the appropriate standard approach to care and goals of treatment. The aim of this study was to assess consensus on United Kingdom (UK) standard of care for child and adult haemophilia patients with inhibitors.
Methods:
A modified Delphi study was conducted using a two-round online survey. A haemophilia expert steering committee and published literature informed the Round 1 questionnaire. Invited participants included haematologists, haemophilia nurses and physiotherapists who had treated at least one haemophilia patient with inhibitors in the past 5 years. Consensus for 6-point Likert scale questions was pre-defined as ⩾70% participants selecting 1–2 (disagreement) or 5–6 (agreement).
Results:
In all, 46.7% and 35.9% questions achieved consensus in Rounds 1 (n = 41) and 2 (n = 34), respectively. Consensus was reached on the importance of improving quality of life (QoL) and reaching clinical goals such as bleed prevention, eradication of inhibitors and pain management. There was agreement on criteria constituting adequate/inadequate responses to immune tolerance induction (ITI) and the appropriate factor VIII dose to address suboptimal ITI response. Opinions varied on treatment aims for adults and children/adolescents, when to offer prophylaxis with bypassing agents and expectations of prophylaxis. Consensus was also lacking on appropriate treatment for mild/moderate patients with inhibitors.
Conclusion:
UK healthcare professionals appear to be aligned on the clinical goals and role of ITI when managing haemophilia patients with inhibitors, although novel treatment developments may require reassessment of these goals. Lack of consensus on prophylaxis with bypassing agents and management of mild/moderate cases identifies a need for further research to establish more comprehensive, evidence-based treatment guidance, particularly for those patients who are unable/prefer not to receive non-factor therapies.
Keywords: adult, children, consensus, Delphi panel, haemophilia, inhibitors
Introduction
Haemophilia is an X-linked bleeding disorder that is typically managed through factor replacement therapy. In 5–7% of all haemophilia A and B patients, and ~30% of severe haemophilia A cases, antibodies known as ‘inhibitors’ can form in response to regular treatment with exogenous clotting factors.1,2 Inhibitors impair the effectiveness of factor replacement therapy by reducing or fully neutralising the efficacy of infused factor concentrates.3,4 Available treatment options include immune tolerance induction (ITI) and bypassing agents (BPAs), such as activated prothrombin complex concentrates (aPCC) or recombinant activated factor VIIa (rFVIIa), either on demand or prophylactically.5,6 Furthermore, since initiation of this study, the non-factor therapy emicizumab has been licensed, and other treatments such as anticoagulant inhibitors (e.g. fiturisan in phase III trials) and anti-tissue factor pathway therapies (e.g. concizumab and marstacimab) are in development for the treatment of haemophilia A patients with FVIII inhibitors.6–8
Whilst aspects of multidisciplinary care for haemophilia patients with inhibitors have been discussed in both international and European guidelines as well as the United Kingdom Haemophilia Centre Doctors’ Organisation (UKHCDO) guidance,9–12 some factors relating to the clinical standard of care for UK patients remain unclear. Treatment decisions are complicated by differing clinical and social needs of patient subpopulations, for example adults versus children/adolescents.13 As novel therapies emerge, reassessment of these unmet needs of haemophilia patients with inhibitors is necessary, to determine optimal management strategies.
The Delphi technique offers a systematic, robust and reproducible methodology, using an iterative process to gather consensus from a group of experts via a series of questionnaires.14 We sought to establish consensus on the UK standard of care for adult and child/adolescent haemophilia patients with inhibitors using a modified Delphi study conducted between May 2017 and November 2018.
Materials and methods
Steering committee
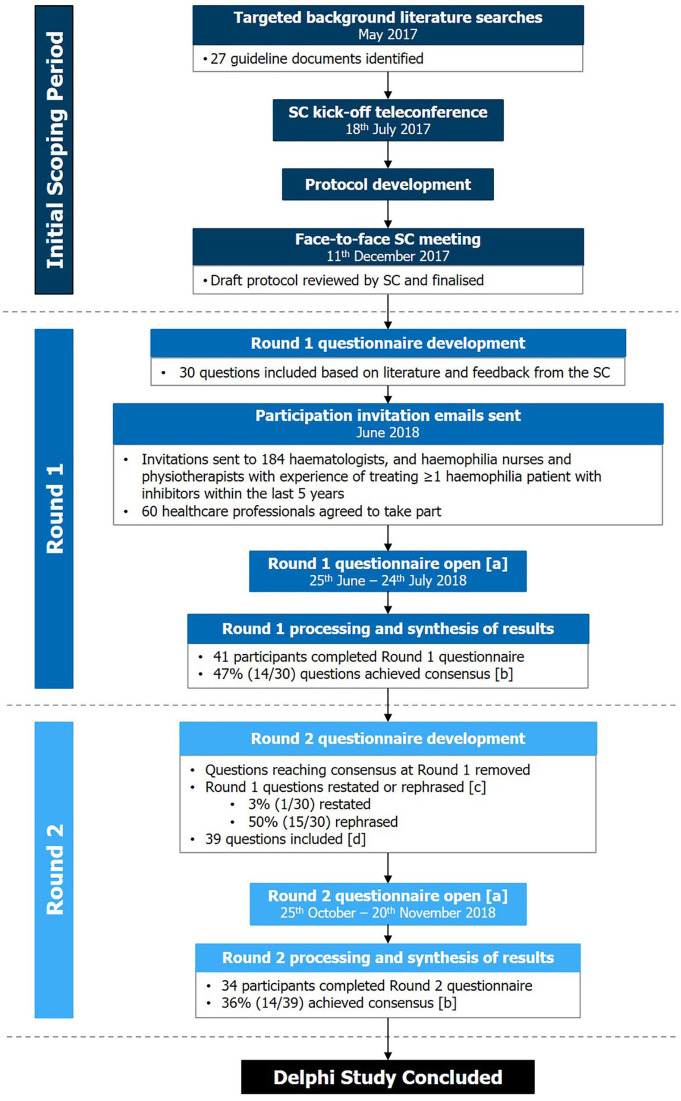
A multidisciplinary steering committee (SC) of healthcare professionals (HCPs) with expertise in treating haemophilia patients with inhibitors was selected by an employee of Roche Products Ltd (GT). SC members were selected based on experience in treating haemophilia patients, irrespective of the treatments used in their practice. The SC comprised two Consultant Haematologists (PC, GD), a Consultant Paediatric Haematologist (EC), a Clinical Academic (KK) and a Haemophilia Physiotherapist (TF). The SC scoped key themes/questions to be explored and assisted with recruitment of Delphi panel participants. The committee also critically reviewed questionnaire results and supported development of subsequent rounds (coordinated by AG, FR and GT; Figure 1). To avoid bias, the SC did not complete the questionnaires.
Figure 1.
Delphi study design. (a) SurveyMonkey® questionnaire distributed as a web link via email to haematologists and haemophilia nurses/physiotherapists with experience of treating at least one haemophilia patient with inhibitors in the last 5 years. (b) Consensus: ⩾70% agreement/disagreement. (c) Restated if achieved between ⩾60% and <70% agreement/disagreement or rephrased if <60% agreement/disagreement. (d) Number of questions asked in the Round 2 questionnaire increased due to some Round 1 questions being rephrased as multiple questions.
SC, steering committee.
Delphi panel participants
Participants were invited via email using convenience sampling and a person-to-person cascade approach,15–17 with a focus on ensuring representation of different multidisciplinary roles and UK regions. Eligible participants had experience treating at least one haemophilia patient with inhibitors within the last 5 years. Individuals wishing to participate were asked to respond to the invitation email and confirm their eligibility. All responses remained anonymous to other participants and to the SC throughout.
Study design
This study used a modified Delphi method, differing from the classical Delphi method in several ways, for example, an initial exploratory questionnaire containing mainly open-ended response questions to inform the next round was not conducted.17 In addition, classical Delphi studies are typically designed to continue until consensus is achieved for all questions.17 However, the protocol for this study specified three or fewer rounds, with the exact number decided by the SC on the basis of results from previous rounds and the feasibility of obtaining further information of value.
Written consent was obtained from all participants prior to their completion of the Delphi questionnaires. As no patients were involved in the study, ethical approval was not required.
Round 1 questionnaire development and distribution
A targeted literature review (TLR) was performed in May 2017 to identify existing haemophilia treatment guidelines (TLR strategy and results described in Supplemental Tables S1 and S2, respectively).4,10,18–20 A preliminary teleconference call was held with SC members to discuss TLR results and to determine key objectives of the study. SC members attended a face-to-face meeting in December 2017 to provide feedback on a draft study protocol and to select overarching themes to be investigated. Committee feedback on potential questions and TLR results informed the Round 1 questionnaire. Questions were stratified according to whether they related to adult care only, care of children/adolescents only or both (referred to as ‘general care’). In the surveys, ‘general care’ questions were posed to all respondents, and participants could then choose to exclusively answer ‘adult care’ questions or ‘child/adolescent care’ questions only, or both sets, based on their clinical experience. Participants opting to answer ‘adult care’ questions only were unable to record a response for ‘child/adolescent care’ questions in that questionnaire round (and vice versa). Throughout, participants were asked to consider their answers in relation to both haemophilia A and B patients unless specified otherwise. Participants were also given the option to select ‘do not wish to answer (DNW)’. A pilot of the initial questionnaire was not conducted, as this was expected to delay initiation of the study,21 thereby decreasing the relevance of results given the rapidly evolving haemophilia treatment landscape.7
Questionnaires were delivered to participants through SurveyMonkey® (SurveyMonkey Inc., San Mateo, CA, USA, www.surveymonkey.com; Supplemental Information file 1) using a weblink via email.
Question types and pre-specified consensus thresholds
Five question types were included (Table 1): Likert scale, ranking, single-option, numerical and free-text questions. Questions were deemed to have reached consensus if ⩾70% participants agreed/disagreed (Likert scale), if a Kendall’s W stat (W) ⩾0.7 was achieved (ranking), or if ⩾70% participants selected the same option (single-option). Questions that did not achieve consensus were rephrased or restated in the subsequent round, depending on levels of agreement/disagreement (rephrasing/restating thresholds detailed in Table 1). Consensus was not assessed for open-ended numerical and free-text questions, but responses were instead used to inform questions for the subsequent round.
Table 1.
Question types and consensus thresholds used in questionnaires.
| Question type | Definition | Example questions | Consensus threshold | Agreement threshold for rephrasing the question | Agreement threshold for restating the question |
|---|---|---|---|---|---|
| 6-Point Likert scale | Assessing agreement through options from ‘1’ (strongly disagree) to ‘6’ (strongly agree).22 Agreement was defined as a participant choosing options ‘5’ or ‘6’ whilst disagreement was defined as a choice of ‘1’ or ‘2’. 6-Point scales were used to ensure participants indicated a preference towards either agreement or disagreement17 | ‘Joint health should be regularly measured in routine comprehensive care visits by a suitably trained physiotherapist using a validated tool’ (1 = strongly disagree; 6 = strongly agree)a | ⩾70% participants agreeing/disagreeing | ⩾60% but <70% participants agreeing/disagreeing | <60% participants agreeing/disagreeing |
| Ranking | Participants asked to rank multiple options in terms of importance from ‘1’ (most important) to ‘4’ (least important) in Round 1 or from ‘1’ (most important) to ‘3’ (least important) in Round 217 | ‘Please rank the following recommendations in terms of their importance when offering a further round of ITI to patients who inadequately respond to their first round of ITI at the full dose of 200 IU/kg/day (1 = most important; 3 = least important) pdFVIII alone pdFVIII and immunosuppression Immunosuppression alonea | Kendall’s W statistic (W) ⩾0.7 | 0.6 ⩽ W < 0.7 | W < 0.6 |
| Single-option | Participants asked to select one answer from multiple options23,24 | ‘What annual bleed rate do you feel justifies prophylaxis in adults?’a | ⩾70% participants choosing the same option | ⩾60% but <70% participants choosing the same option | <60% participants choosing the same option |
| Numerical | A free-text, open-ended question where participants were asked to provide a numerical response17 | ‘Based on your response to the previous question, what percentage reduction in joint bleeds (any severity) on prophylaxis would you then consider to be a clinically significant improvement in adults?’b | Consensus not assessedc | Responses used to inform questions in subsequent round | Responses used to inform questions in subsequent round |
| Free-text questions | Optional free-text, open-ended questions both in the survey, and at the end of each questionnaire section (to allow participants to provide context to their responses)17 | ‘How should mild/moderate haemophilia A patients with inhibitors be treated to eradicate their inhibitors?’b‘If you have any additional comments related to prophylaxis, please add them to this text box’d | Consensus not assessedc | Responses used to inform questions in subsequent round | Responses used to inform questions in subsequent round |
Participants could also select DNW for any questions they did not wish to answer, and in Round 2 an IE option was also added.
Example question from the Round 2 questionnaire.
Example question from the Round 1 questionnaire.
Responses instead used to inform the questions in subsequent round.
Example question from the Round 2 questionnaire whereby participants could add additional details at the end of a questionnaire section.
DNW, do not wish to answer; IE, insufficient expertise; ITI, immune tolerance induction; IU, international units; pdFVIII, plasma-derived factor VIII.
Round 2 questionnaire development and distribution
Questions reaching consensus in Round 1 were removed from Round 2. Some questions/options were rephrased with a view to increasing the likelihood of achieving consensus in the subsequent round, whilst others were split into multiple questions to gather more detailed information. Open-ended numerical questions in Round 1 were used to inform single-option questions in Round 2.25 In response to free-text feedback, additional questions were added, as well as an ‘insufficient expertise (IE)’ option for all questions.
The Round 2 questionnaire (Supplemental Information 2) was delivered to participants via email, accompanied by a reminder of their response and overall results from the previous round, to encourage achievement of consensus.17
Processing and synthesis of results
All results were exported from SurveyMonkey® and analyses conducted in Microsoft Excel® 2016. Round 1 and Round 2 questionnaire responses were collated and analysed to assess consensus achievement, by calculating response distribution. DNW responses were considered neither agreement or disagreement and IE responses were removed prior to analysis. The proportion of participants selecting each option was calculated using the total number with sufficient experience (deducting IE responses) as the denominator. One participant answered ‘adult care’ questions only in Round 1 but answered ‘adult care’ and ‘child/adolescent care’ questions in Round 2. For consistency, this participant’s Round 2 child/adolescent-related responses were not included in analyses.
Round 2 results, presented to the SC via email/teleconference call, suggested that further rounds would be unlikely to elicit consensus in light of free-text responses received, as well as due to high and persistent variability in responses. The SC therefore decided to conclude the study after two questionnaire rounds.
Results
Delphi study participation
Round 1 and 2 questionnaires were open from 25 June to 24 July 2018, and 25 October to 20 November 2018, respectively. Of 184 HCPs invited to participate in the Delphi study, 60 agreed to take part and 41 completed the Round 1 questionnaire (Figure 1). These included haemophilia physicians, nurses and physiotherapists affiliated to UK haemophilia treatment centres (Table 2). The Round 2 questionnaire was distributed to all 41 participants who completed Round 1 and 34 responses were received (Figure 1).
Table 2.
Participant demographics.
| Professional roles of participants | Number of participants, n (%) | Answered questions on adult care only, n (%) | Answered questions on child/adolescent care only, n (%) | Answered questions on adult care and child/adolescent care, n (%) |
|---|---|---|---|---|
| Consultant haematologist | 12 (29.3) | 7 (58.3) | 1 (8.3) | 4 (33.3) |
| Consultant paediatric haematologist | 4 (9.8) | 0 (0.0) | 4 (100.0) | 0 (0.0) |
| Haemophilia nurse | 13 (31.7) | 4 (30.8) | 7 (53.8) | 2 (15.4) |
| Haemophilia physiotherapist | 10 (24.4) | 4 (40.0) | 4 (40.0) | 2 (20.0) |
| Othera | 2 (4.9) | 2 (100.0) | 0 (0.0) | 0 (0.0) |
| Number of haemophilia patients with inhibitors participants were currently treating/had treated in the past 5 yearsb | Number of participants, n (%) | |||
| 0 Inhibitor patients | 0 (0.0) | |||
| 1–2 Inhibitor patients | 8 (19.5) | |||
| 3–5 Inhibitor patients | 16 (39.0) | |||
| >5 Inhibitor patients | 17 (41.5) | |||
| Do not wish to answer | 0 (0.0) | |||
| Location of institutions to which participants were affiliated | Number of participants, n (%) | |||
| London | 9 (22.0) | |||
| North West of England | 4 (9.8) | |||
| Scotland | 3 (7.3) | |||
| South East of England | 3 (7.3) | |||
| South West of England | 7 (17.1) | |||
| West Midlands | 4 (9.8) | |||
| Yorkshire | 3 (7.3) | |||
| Do not wish to answer | 0 (0.0) | |||
| Other | 8 (19.5) | |||
Demographics of participants that responded to the Round 1 questionnaire.
Other roles: n = 1 Haemophilia Research Nurse; n = 1 Associate Specialist Haematology.
All respondents were required to have experience of treating ⩾1 haemophilia patient with inhibitors; any individuals without experience, or who chose not to answer this question, were excluded from the Delphi study.
Questionnaire results
In Round 1, 46.7% (14/30) of questions achieved consensus. Following Round 1 analysis, 39 questions were asked in Round 2, and 35.9% (14/39) reached consensus. Further details on individual questions, the round in which they were asked, and consensus levels achieved are available in Table 3 and Supplemental Figure S1 (general care), Table 4 and Supplemental Figure S2 (adult care) and Table 5 and Supplemental Figure S3 (child/adolescent care); additional detail in Supplemental Tables S3–S7.
Table 3.
Likert scale questions relating to the general care of haemophilia patients with inhibitors.
| Question | Percentage agreement/disagreement (%) | Number of participants’ responses included in analysis (n)a | Delphi survey round |
|---|---|---|---|
| Joint health should be regularly measured in routine comprehensive care visits by a suitably trained physiotherapist using a validated tool | 90.2 | 41 | Round 1 |
| Infusion requirements (both volume and frequency) must be considered when selecting a therapyb | 84.4 | 32 | Round 2 |
| Quality of life should be regularly measured in routine comprehensive care visits using a validated tool | 82.9 | 41 | Round 1 |
| The number of major bleeds should be specifically considered when deciding whether to offer prophylaxis with bypassing agents to a mild or moderate haemophilia patients with inhibitorsc | 82.4 | 34 | Round 2 |
| Inadequate response to ITI is best defined as an upward trend in inhibitor titre or <20% reduction in inhibitor titre over a 6-month periodb | 82.1 | 28 | Round 2 |
| The number of joint bleeds should be specifically considered when deciding whether to offer prophylaxis with bypassing agents to a mild or moderate haemophilia patient with inhibitorsc | 79.4 | 34 | Round 2 |
| If inadequate response to ITI is observed at <200 IU/kg/day, the dose can be increased to this levelb | 79.3 | 29 | Round 2 |
| The avoidance of allergic reactions is a key factor which should be considered when selecting a therapy | 70.7 | 41 | Round 1 |
| The aims of treatment in haemophilia patients with inhibitors are completely different from the aims of treatment in haemophilia patients without inhibitors [b] | 67.6 | 34 | Round 2 |
| Eradicating inhibitors is a priority in mild or moderate haemophilia patients with inhibitorsb | 62.5 | 32 | Round 2 |
| Anamnesis is an important consideration when selecting a therapy prior to ITI or during ITIb | 60.0 | 30 | Round 2 |
| Anamnesis is an important consideration when selecting a therapy for a patient who has failed ITIb | 56.7 | 30 | Round 2 |
| Moderate haemophilia patients with inhibitors should not be routinely offered prophylaxis with bypassing agentsb | 46.9 | 32 | Round 2 |
| For patients who inadequately respond to ITI, ITI should be terminatedd | 43.3 | 30 | Round 2 |
| Infusion requirements should be specifically considered when deciding whether to offer prophylaxis with bypassing agents to a mild or moderate haemophilia patients with inhibitorsb | 42.4 | 33 | Round 2 |
| Baseline factor activity levels should be specifically considered when deciding whether to offer prophylaxis with bypassing agents to a mild or moderate haemophilia patients with inhibitorsb | 37.9 | 29 | Round 2 |
| Mild haemophilia patients with inhibitors should not be routinely offered prophylaxis with bypassing agentsb | 37.9 | 29 | Round 2 |
A total of 41 participants answered questions on general care in Round 1, and 34 in Round 2. Questions achieving consensus agreement and disagreement (⩾70% participants agreeing/disagreeing with the statement) are highlighted in dark blue and light blue, respectively. Where questions did not achieve consensus in Round 1 and were carried forward to Round 2, only the Round 2 results are shown here.
In Round 2, ‘IE’ responses were removed prior to analysis.
Rephrased from question in Round 1.
New question added based on free-text response in Round 1.
Round 1 question restated in Round 2.
IE, insufficient expertise; ITI, immune tolerance induction; IU, international units.
Table 4.
Likert scale questions relating to the care of adults with haemophilia and inhibitors.
| Question | Percentage agreement/disagreement (%) | Number of participants’ responses included in analysis (n)a | Delphi survey round |
|---|---|---|---|
| Tolerance to factor therapy can be demonstrated in adults when a half-life of >7 h is observedb | 94.4 | 18 | Round 2 |
| When treating adults with newly-developed inhibitors, the priority is to eradicate the inhibitorsb | 90.0 | 20 | Round 2 |
| High dose factor prophylaxis is justified in adults who are partially tolerised to ITIc | 90.0 | 20 | Round 2 |
| Pain in adults should be regularly measured in routine comprehensive care visits using a validated tool | 88.0 | 25 | Round 1 |
| Prophylaxis with bypassing agents is justified in adults who require joint preservation | 84.0 | 25 | Round 1 |
| Prophylaxis with bypassing agents is justified in adults who have had a single life-threatening bleed | 84.0 | 25 | Round 1 |
| In adults who have failed ITI, prophylaxis with bypassing therapy should be offered, if not already initiated | 80.0 | 25 | Round 1 |
| Restoring/maintaining an adult’s independence should be the main priority | 76.0 | 25 | Round 1 |
| When treating adults with long-standing inhibitors who are unresponsive to ITI, the aim is for them to not have any bleedsb | 70.0 | 20 | Round 2 |
| If an inhibitor is no longer detected in adults (negative Bethesda assay), this indicates a positive response to ITIb | 70.0 | 20 | Round 2 |
| When treating adults with long-standing inhibitors, the priority is not to eradicate the inhibitorsb | 55.0 | 20 | Round 2 |
| Adults with haemophilia A and inhibitors should be treated with ITI to eradicate their inhibitorsd | 31.6 | 19 | Round 2 |
A total of 25 participants answered questions on care of adults in Round 1, and 20 in Round 2. Questions achieving consensus agreement and disagreement (⩾70% participants agreeing/disagreeing with the statement) are highlighted in dark blue and light blue, respectively. Where questions did not achieve consensus in Round 1 and were carried forward to Round 2, only the Round 2 results are shown here.
In Round 2, ‘IE’ responses were removed prior to analysis.
Rephrased from question in Round 1.
Round 1 question restated in Round 2.
New question added based on free-text response in Round 1.
IE, insufficient expertise; ITI, immune tolerance induction.
Table 5.
Likert scale questions relating to the care of children/adolescents with haemophilia and inhibitors.
| Question | Percentage agreement/disagreement (%) | Number of participants’ responses included in analysis (n)a | Delphi survey round |
|---|---|---|---|
| When treating children and adolescents with inhibitors on ITI, the aim is for them to not have any bleedsb | 90.9 | 22 | Round 2 |
| In children and adolescents who have failed ITI, prophylaxis with bypassing therapy should be offered, if not already initiated | 83.3 | 24 | Round 1 |
| Prophylaxis with bypassing agents is justified in children and adolescents who require joint protection | 79.2 | 24 | Round 1 |
| Restoring/maintaining a child’s or an adolescent’s lifestyle, in terms of their everyday activities, should be the main priority | 75.0 | 24 | Round 1 |
| A key aim of treatment in children and adolescents with inhibitors is to eradicate the inhibitor | 70.8 | 24 | Round 1 |
| Prophylaxis with bypassing agents is justified in children and adolescents who have had a single life-threatening bleed | 70.8 | 24 | Round 1 |
| High dose factor prophylaxis is justified in children and adolescents who are partially tolerised to ITI | 70.8 | 24 | Round 1 |
| Children and adolescents with haemophilia A and inhibitors should be treated with ITI to eradicate their inhibitors, regardless of severityc | 70.0 | 20 | Round 2 |
A total of 24 participants answered questions on paediatric care in Round 1, and 22 in Round 2. Questions achieving consensus agreement and disagreement (⩾70% participants agreeing/disagreeing with the statement) are highlighted in dark blue and light blue, respectively. Where questions did not achieve consensus in Round 1 and were carried forward to Round 2, only the Round 2 results are shown here. One participant answered the ‘adult care only’ questions in Round 1 but answered questions relating to the care of both children/adolescents and adults in Round 2. For consistency with Round 1, this participant’s Round 2 child-related responses were not included in analyses.
In Round 2, ‘IE’ responses were removed prior to analysis.
Rephrased from question in Round 1.
New question added based on free-text response in Round 1.
IE, insufficient expertise; ITI, immune tolerance induction.
Clinical goals
Restoring/maintaining an adult’s independence and a child’s/adolescent’s lifestyle were considered main priorities. Participants agreed that joint health, pain (in adults) and quality of life (QoL) should be measured regularly in routine comprehensive care visits using validated tools. It was agreed that in children/adolescents with inhibitors and adults with newly developed inhibitors, the priority is to eradicate the inhibitors. Similarly, participants agreed that for adults with long-standing inhibitors who are unresponsive to ITI, and for children/adolescents on ITI, the aim is for them to not have any bleeds.
Consensus was not reached on whether treatment aims for adults with and without inhibitors are the same, or whether inhibitor eradication is the priority for adults with long-standing inhibitors.
Role of ITI
Participants agreed that, in adults, tolerance is indicated when a half-life of >7 h is observed. If an inhibitor is no longer detected (via a negative Bethesda assay), it was agreed that this indicates a positive response to ITI, whilst an inadequate response is best defined as an upward trend or <20% reduction in inhibitor titre over 6 months. Participants agreed that if inadequate response to ITI is observed with a dose of <200 international units (IU)/kg/day, the dose should be increased to 200 IU/kg/day. However, opinions around ITI termination for patients responding inadequately tended to differ.
Results indicated ‘plasma-derived FVIII (pdFVIII) alone’ as the most important recommendation when offering a further round of ITI to patients who inadequately respond to their first round at the full dose of 200 IU/kg/day (62.5% ranked pdFVIII as highest priority). This was most commonly followed by ‘pdFVIII and immunosuppression’ and lastly ‘immunosuppression alone’, although this order of priority did not achieve consensus (W = 0.16).
Bypassing agents
Consensus on whether anamnesis is an important consideration when selecting a therapy for a patient prior to/during ITI, or for a patient who has failed ITI, was not achieved. However, when selecting a therapy, participants agreed that infusion requirements (volume and frequency) and the avoidance of allergic reactions must be considered.
Prophylaxis
Participants agreed that prophylaxis with BPAs should be offered, if not already initiated, to patients that have failed ITI. They also agreed that prophylaxis with BPAs is justified in adults and children/adolescents who have experienced a single life-threatening bleed and for joint protection. High-dose factor prophylaxis was also considered justifiable in patients partially tolerised to ITI.
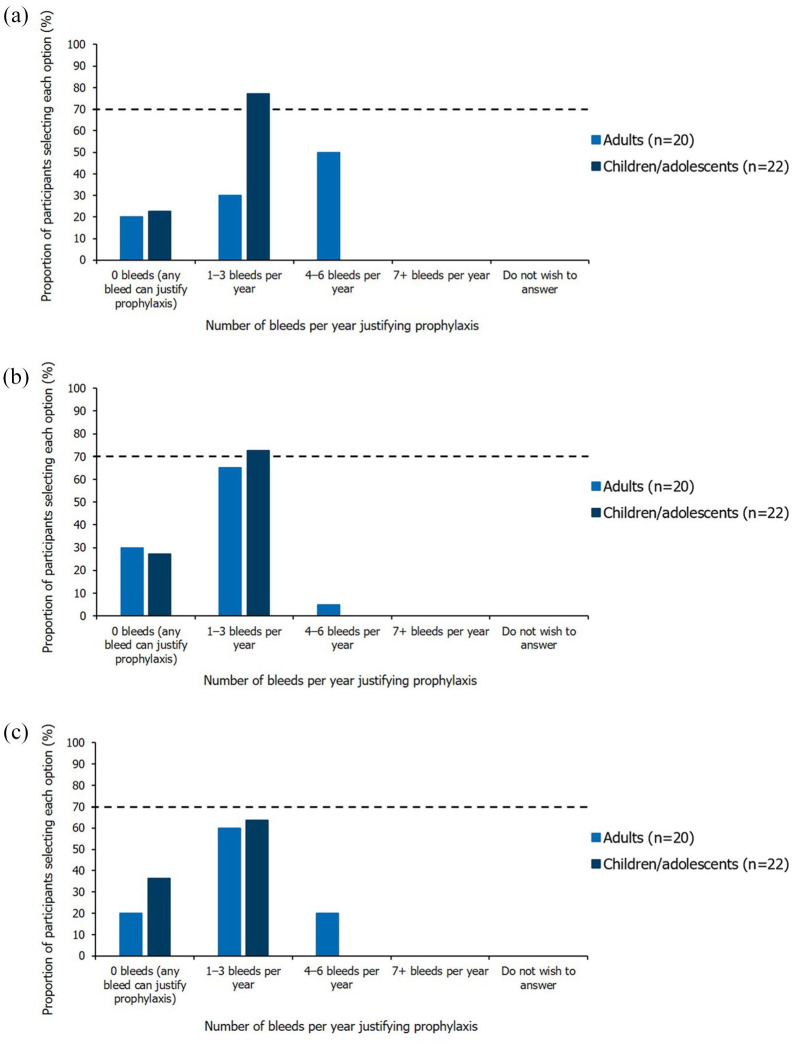
For children/adolescents with inhibitors, participants agreed that an annual bleed rate (ABR) of 1–3 bleeds per year of any type or major bleeds (joint or muscle) justified prophylaxis with BPAs (Figure 2). In adults, no consensus was reached on the ABR or number of major bleeds per year (joint or muscle) justifying prophylaxis with BPAs. Similarly, the number of joint bleeds per year (any severity) justifying prophylaxis, did not reach consensus for either adults or children.
Figure 2.
Single-option questions relating to the number of bleeds per year justifying prophylaxis. Results shown are from the Round 2 questionnaire (questions were informed by numerical questions in Round 1), excluding participants who indicated they had insufficient expertise. The threshold for achieving consensus (⩾70% participants selecting the same option), is marked by the dashed horizontal line. (a) Annual bleed rate. Question worded as ‘What annual bleed rate do you feel justifies prophylaxis?’. (b) Number of major bleeds per year (joint or muscle). Question worded as ‘What number of major bleeds per year (joint or muscle) justifies prophylaxis?’. (c) Number of joint bleeds per year. Question worded as ‘What number of joint bleeds per year (any severity) justifies prophylaxis?’.
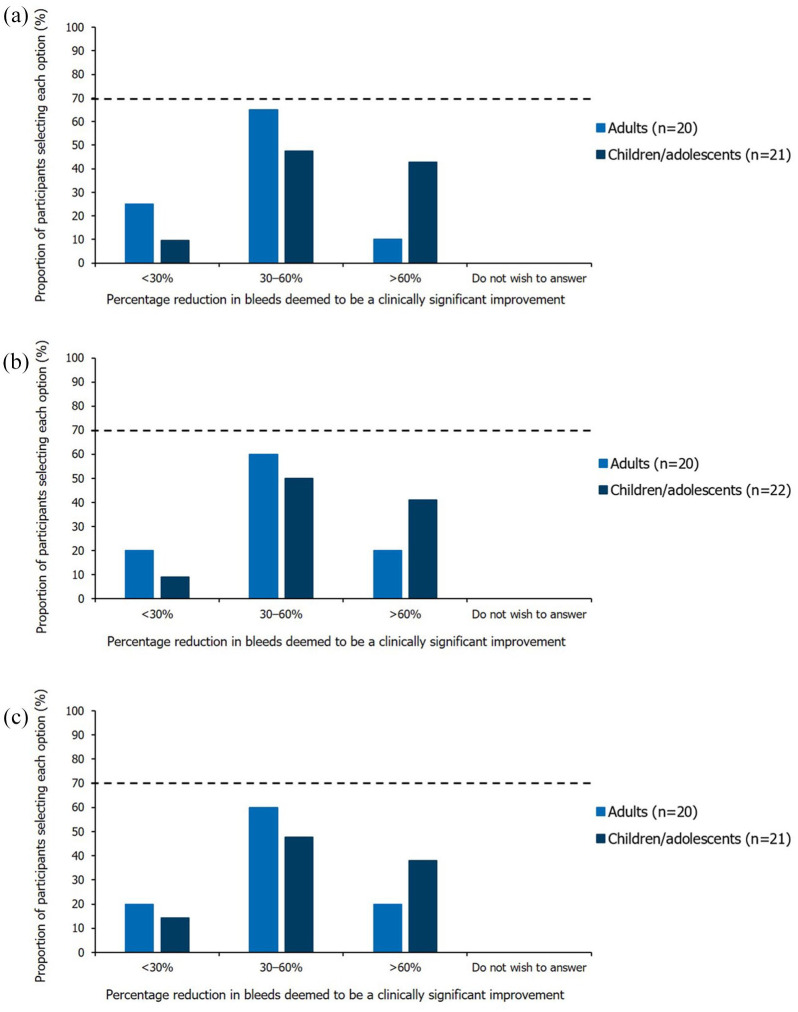
Opinions varied regarding the change in number of major and joint bleeds that would be considered a clinically significant improvement. A 30–60% bleed reduction (any severity) was most commonly selected for adults and children, although these questions did not achieve consensus (Figure 3).
Figure 3.
Single-option questions relating to the percentage reduction in bleeds deemed to be a clinically significant improvement. Results shown are from the Round 2 questionnaire (questions informed by numerical questions in Round 1), excluding participants who indicated they had insufficient expertise. The threshold for achieving consensus (⩾70% participants selecting the same option), is marked by the dashed horizontal line. (a) Bleeds per year (any severity). Question worded as ‘Based on your response to the previous questions, what percentage reduction in bleeds per year (any severity) on prophylaxis would you then consider to be a clinically significant improvement?’. (b) Joint bleeds per year (any severity). Question worded as ‘Based on your response to the previous question, what percentage reduction in joint bleeds per year (any severity) on prophylaxis would you then consider to be a clinically significant improvement?’. (c) Major bleeds per year (joint or muscle). Question worded as ‘Based on your response to the previous question, what percentage reduction in major bleeds per year (joint or muscle) on prophylaxis would you then consider to be a clinically significant improvement?’.
Although consensus was not reached, the most common treatment recommendation when an improvement with prophylaxis with BPAs was not observed in adults was to ‘increase frequency of prophylactic treatment alone’ [63.2% (12/19)]. Conversely, the favoured recommendation in children/adolescents was to ‘increase both dose and frequency of prophylactic treatment’ [61.9% (13/21)].
Patients with mild or moderate haemophilia
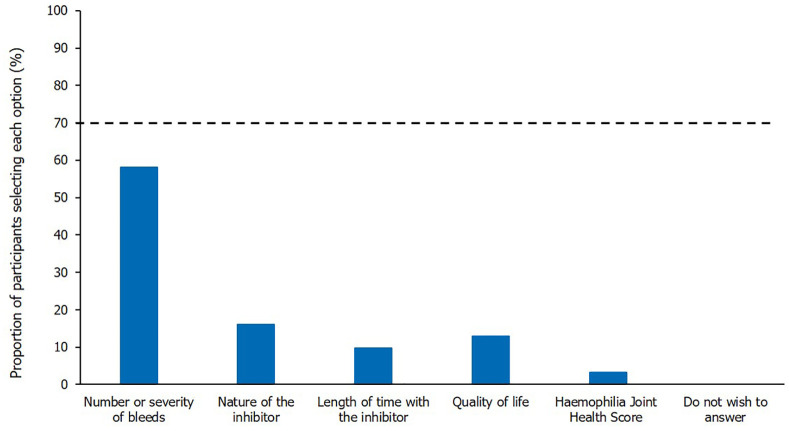
There were differing opinions about whether adults with mild or moderate haemophilia A should be treated with ITI to eradicate inhibitors, regardless of bleeding phenotype. Participants did agree, however, that children/adolescents with mild/moderate haemophilia A should be treated with ITI to eradicate inhibitors. Opinions differed as to the most important factor to be considered when treating mild/moderate patients with inhibitors, when the aim was to eradicate their inhibitors; whilst consensus was not reached, ‘number or severity of bleeds’ was most commonly selected (Figure 4).
Figure 4.
The most important factor when treating mild/moderate patients with inhibitors, when aiming to eradicate their inhibitors. Single-option question; results shown are from the Round 2 questionnaire, excluding participants who indicated they had insufficient expertise; n = 31. The threshold for achieving consensus (⩾70% participants selecting the same option), is marked by the dashed horizontal line. Question worded as ‘Please select the most important factor to consider when treating mild/moderate haemophilia A patients with inhibitors, when the aim is to eradicate their inhibitors’.
There were mixed opinions on whether mild or moderate haemophilia patients with inhibitors should be offered prophylaxis with BPAs and, if so, whether infusion requirements and baseline factor activity levels should be specifically considered. However, participants agreed that number of bleeds (any type) and joint bleeds should be considered when deciding whether to offer prophylaxis with BPAs to the subpopulation of mild or moderate haemophilia patients with inhibitors.
Discussion
Inhibitor development represents a significant complication in the treatment of haemophilia, yet uncertainty around some areas of best practice for this patient population continues to exist in the UK. This Delphi study elicited expert consensus on aspects of standard care for adults and children/adolescents with inhibitors, with 46.7% (14/30) and 35.9% (14/39) questions achieving consensus in Rounds 1 and 2, respectively.
Participants agreed that joint health, pain (in adults) and QoL should be measured regularly. Although instruments for these outcomes are available, often their use is impractical or has not been standardised.26 Many outcome measures fail to allow for the complexities of treating inhibitor patients such as variation in bleeding phenotype, or the differing treatment priorities of adults and children.13,27,28
Generally, treatment goals were more ambitious for children/adolescents than adults, with aims for children/adolescents including inhibitor eradication and lower ABRs whilst on prophylaxis with BPAs. This aligns with the more intensive treatment approach associated with paediatric care and is likely due to the increased importance of maintaining children’s joint health.13,29 Participants were also better aligned on the numbers of major and joint bleeds justifying prophylaxis with BPAs in children compared with adults. The lack of consensus pertaining to treatment of adults suggests that further efforts should be made to establish standard of care in this population. The professional role of participants and, subsequently, the likelihood that they would regularly work with adults and/or children, should also be considered as a potential factor in this variance. For example the majority of Consultant Haematologists chose to answer questions on adult care only, whilst Haemophilia Nurses generally answered on child/adolescent care only (Table 2). This may have potentially influenced whether consensus was reached for individual questions, and more broadly, for questionnaire sections relating to different treatment areas. It is also worth noting that the questionnaires did not specifically refer to decisions pertaining to newly diagnosed/acute presentation of inhibitors compared with patients who have chronic inhibitors, which typically require different approaches for management. Although it is generally considered to be more likely that adults will have had inhibitors for longer periods of time than children, this is not always the case and subsequently stratifying by these two approaches may be of interest in future studies.
Participants agreed on criteria constituting an adequate/inadequate response to ITI, suggesting that UKHCDO guidelines describing these principles at the time of study initiation were well regarded.10 However, a lack of consensus around best practice for patients who inadequately respond to their first round of ITI at full dose was demonstrated. Since the conclusion of this study, updated guidance has been published by the World Federation of Hemophilia (WFH) in light of the introduction of emicizumab, which suggests that it may be possible for patients with persistent inhibitors to delay or avoid ITI altogether with emicizumab prophylaxis, which may resolve the existing uncertainty around the use of ITI.12 However, the WFH notes that there is a paucity of data in this area, and subsequently ITI, and its use with non-factor therapies such as emicizumab remains a topic of debate.12 Uncertainty remains as to the appropriate ITI dosing regimen and when this should be initiated, although studies ongoing at the time of publication seek to investigate this topic further.30–35
Although prophylaxis with BPAs was considered to be a key treatment for haemophilia patients with inhibitors who had failed ITI at the time of the study,36 the point at which it should be offered was contentious. Whilst it was agreed that the number of joint and major bleeds should be considered, opinions differed on the number of these bleeds per year justifying prophylaxis, and to what extent improvements are considered clinically significant. Inter-individual differences have been highlighted as a key reason for the lack of consensus on prophylactic treatment and, in the advent of newer therapies,37 treatment expectations are likely to change further. Responses may also have been complicated by variations in how bleeding is defined.38 It should be noted that in the advent of non-factor therapies, use of prophylaxis with BPAs in treating patients with inhibitors, is likely to become less common.11 However, for those patients who are unable/prefer not to receive non-factor therapies, further guidance in the use of prophylaxis would still be valuable.
Responses regarding care of patients with mild or moderate haemophilia and inhibitors showed considerable variation, with few questions reaching consensus. Participants were not asked specifically about the extent of their experience with treatment of patients with mild/moderate haemophilia in the questionnaires and, subsequently, any variation in experience between participants may have contributed towards differing opinions/approaches in the management of this patient group. In addition, treatment decisions may also be complicated by heterogeneity in bleeding phenotypes of non-severe haemophilia patients,4 and the fact that many mild/moderate patients clear their inhibitors spontaneously, causing clinicians to delay treatment.39,40 Furthermore, since inhibitor development in mild/moderate haemophilia is less common than in severe haemophilia,41 there are limited data on ITI efficacy for this group, and HCPs are likely to have less therapeutic experience with this subpopulation.42 The lack of consensus in our study may also be reflective of the previously published recommendation for management of non-severe haemophilia patients on a case-by-case basis, with close follow up.43,44
More generally, the absence of consensus in more than half of the questions in Rounds 1 and 2, may suggest a need for more specific guidelines on standard of care in this population. In rare conditions like haemophilia, where clinicians may have limited experience treating patients due to the relatively small population, consensus on standard of care is often less easily achieved. In our study, 58.5% of participants had treated five or fewer haemophilia patients with inhibitors in the past 5 years, suggesting that limited experience could have contributed to the variability in responses to some questions. Furthermore, participants likely had differing clinical experiences due to their diverse job roles, which may have impacted responses to individual questions, contributing to the lack of consensus.
The Delphi technique has many key methodological advantages when eliciting expert consensus. This method allows a variety of opinions to be collected from a heterogeneous sample of experts, allowing questionnaires to evolve based on results/feedback by combining participants’ selected answers with optional free-text responses. Anonymity of responses and the avoidance of participants meeting face-to-face, ensured any external bias was minimised. In our study, utilisation of an online tool to gather participant responses allowed rapid collection and analysis of results from a large number of geographically dispersed experts.
However, the Delphi method is not without limitation; research has shown that social-psychological factors may lead to experts with divergent views feeling pressure to conform.17 Furthermore, the Delphi method, unlike standard surveys, requires ongoing time commitment from participants. This may lead to survey attrition, although here we attempted to mitigate participant drop-out by designing questionnaires to take <30 min to complete. In light of this time commitment, it is important to balance the feasibility of obtaining information of value in subsequent rounds against the implications of using further expert resource. The potential for participant drop-out in a third questionnaire round, in addition to analysis of free-text responses indicating that consensus was very unlikely to be achieved in a further round, led the SC to decide to conclude the study after Round 2. Whilst this decision avoided further requirements of experts’ time, it should be acknowledged as a limitation of the study given that in a classical Delphi study, further rounds are typically conducted until consensus is achieved.17
As eluded to above, capturing consensus of opinion as HCPs adapt treatment decisions in an evolving treatment landscape presented difficulties, and although this study aimed to establish optimal management of the UK inhibitor population, clinical opinions may have changed during the 18-month process (May 2017–November 2018). Emicizumab was licensed by the EMA in January 2018 following initiation of this study.7 Although no questions on emicizumab were included in the questionnaires, the treatment was mentioned several times in free-text comments as a factor that influenced participants’ responses. It is therefore important to understand the current and future impact novel therapies may have within their licensed indications, and what areas of unmet need will remain once the impact of these treatments for haemophilia patients with inhibitors has been evaluated. It would also be important to understand how emicizumab, and other novel treatments, can be used in combination with alternative interventions, such as ITI. Finally, it cannot be assumed that all patients will receive non-factor therapies in the future and, subsequently, it is crucial that relevant guidance is available in these situations.
Looking to the future, there are many opportunities to address outstanding areas of unmet need in the field of haemophilia, including those areas that did not achieve consensus in this study. Future consensus studies could be expanded to assess how treatment decisions differ for both newly diagnosed and chronic inhibitors, as well as to include the haemophilia population without inhibitors, to understand how their treatment expectations differ from patients with inhibitors (particularly since treatment approaches for the former tend to be more ambitious, focussing on elimination of bleeding using prophylaxis).45 Furthermore, this study collected opinions exclusively from HCPs and therefore future research should consider collecting patients/carers’ opinion in order to further understand the impact of these decisions on patient/carer QoL.
Conclusion
This study demonstrated that UK healthcare professionals were aligned on appropriate clinical goals for haemophilia patients with inhibitors and confirmed differences in treatment expectations for adults and children/adolescents. There was lack of consensus on factors relating to the use of prophylaxis in this patient population, with mixed opinions about the number of bleeds justifying its use and the extent to which improvements in outcomes should be considered clinically significant. Opinion was also divided on best practice when ITI has failed as well as the use of prophylaxis with BPAs when treating mild and moderate patients with inhibitors. This suggests that, as emerging novel therapies become available, further research is required to establish better treatment guidance.
Supplemental Material
Supplemental material, sj-jpg-1-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-jpg-2-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-jpg-3-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-pdf-4-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-pdf-5-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-pdf-6-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-pdf-7-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-pdf-8-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-pdf-9-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-xlsx-10-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-xlsx-11-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-xlsx-12-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-xlsx-13-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-xlsx-14-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Acknowledgments
The authors thank the Delphi panel participants who took part in the study and steering committee members including Gerard Dolan, St Thomas’ Hospital, London (identified as GD within the manuscript). The authors also acknowledge Eleanor Thurtle, Costello Medical, Cambridge, UK and Emma Warnants, Costello Medical, London, UK, for writing and editorial assistance and submission support (funded by Roche Products Ltd) and Bethan Du-Mont and Aimée Hall, Costello Medical, Cambridge, UK, for their contributions to study design and analysis.
Footnotes
Author contributions: KK, EC, TF, AG, GT and PC made substantial contributions to the conception and design of the study, as well as to acquisition of data. KK, EC, TF, AG, FR, GT and PC made substantial contributions to data interpretation. GT (employee of Roche Products Ltd) selected the steering committee, provided assistance with development of the Round 1 questionnaire and undertook adverse event monitoring during the study.
Conflict of interest statement: KK: Research support from: Pfizer, Shire, Sobi; Speaker fees from Bayer, CSL Behring, Novo Nordisk, Roche, Shire and Sobi; EC: Research support from: Boehringer Ingelheim, CSL Behring, Grifols, Roche, Shire and Sobi; Speaker’s fees: Boehringer Ingelheim, CSL Behring, Grifols, Roche, Shire and Sobi; Education support: Boehringer Ingelheim, CSL Behring, Grifols, Roche, Shire and Sobi; TF: Education and quality improvement grants from: Sobi; Honoraria from: Bayer; AG: Employee of Costello Medical; FR: Employee of Roche Products Ltd; GT: Employee of Roche Products Ltd; PC: Research support from: Bayer, CSL Behring, Novo Nordisk, Pfizer and Sobi; Consulting fees from: Baxalta/Shire, Bayer, Biogen Idec, CSL Behring, Chugai, Freeline, Novo Nordisk, Pfizer, Roche and Sobi; Speaker’s bureau from: Baxalta/Shire, Biogen Idec, CSL Behring, Novo Nordisk, Pfizer, Roche and Sobi.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Roche Products Ltd and Chugai Pharma UK Ltd.
This study was funded and initiated by Roche Products Ltd and Chugai Pharma UK Ltd. Adverse event monitoring was undertaken by Guillermo Tobaruela (employee of Roche Products Ltd) and supported by Alice Marshall (employee of Chugai Pharma UK). Dr Kate Khair’s research at Great Ormond Street Hospital is funded by the Biomedical Research Centre/National Institute for Health Research.
ORCID iD: Pratima Chowdary  https://orcid.org/0000-0002-6690-8586
https://orcid.org/0000-0002-6690-8586
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Kate Khair, Centre for Outcomes and Experience Research in Children’s Health Illness and Disability (ORCHID), NIHR Biomedical Research Centre Great Ormond Street Hospital for Children, London, UK; Haemnet, London, UK.
Elizabeth Chalmers, Paediatric Haemophilia Comprehensive Care Centre, Royal Hospital for Sick Children, Glasgow, UK.
Thuvia Flannery, Leeds Haemophilia Centre, St James’ University Hospital, Leeds, UK.
Annabel Griffiths, Costello Medical, Cambridge, UK.
Felicity Rowley, Roche Products Ltd., Welwyn Garden City, UK.
Guillermo Tobaruela, Roche Products Ltd., Welwyn Garden City, UK.
Pratima Chowdary, Katharine Dormandy Haemophilia and Thrombosis Centre, Royal Free Hospital, Pond Street, London, NW3 2QG, UK.
References
- 1. Oldenburg J, Brackmann HH, Schwaab R. Risk factors for inhibitor development in hemophilia A. Haematologica 2000; 85: 7–13. [PubMed] [Google Scholar]
- 2. Mancuso ME, Mannucci PM, Rocino A, et al. Source and purity of factor VIII products as risk factors for inhibitor development in patients with hemophilia A. J Thromb Haemost 2012; 10: 781–790. [DOI] [PubMed] [Google Scholar]
- 3. Ingerslev J. Hemophilia. Strategies for the treatment of inhibitor patients. Haematologica 2000; 85: 15–20. [PubMed] [Google Scholar]
- 4. Srivastava A, Brewer A, Mauser-Bunschoten E, et al. Guidelines for the management of hemophilia. Haemophilia 2013; 19: e1–e47. [DOI] [PubMed] [Google Scholar]
- 5. Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia. Hematology Am Soc Hematol Educ Program 2014; 1: 364–371. [DOI] [PubMed] [Google Scholar]
- 6. Carcao M, Goudemand J. Inhibitors in hemophila: a primer. World Fed Haemoph 2018; 7: 1–24. [Google Scholar]
- 7. European Medicines Agency. Hemlibra: summary of product characteristics, https://www.ema.europa.eu/en/documents/product-information/hemlibra-epar-product-information_en.pdf (2018, accessed February 2021).
- 8. Chowdary P. Anti-tissue factor pathway inhibitor (TFPI) therapy: a novel approach to the treatment of haemophilia. Int J Hematol 2020; 111: 42–50. [DOI] [PubMed] [Google Scholar]
- 9. Giangrande PLF, Hermans C, O’Mahony B, et al. European principles of inhibitor management in patients with haemophilia. Orphanet J Rare Dis 2018; 13: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collins P, Chalmers E, Hart D, et al. Diagnosis and treatment of factor VIII and IX inhibitors in congenital haemophilia: (4th edition). Br J Haematol 2013; 160: 153–170. [DOI] [PubMed] [Google Scholar]
- 11. Rayment R, Chalmers E, Forsyth K, et al. Guidelines on the use of prophylactic factor replacement for children and adults with Haemophilia A and B. Br J Haematol 2020; 190: 684–695. [DOI] [PubMed] [Google Scholar]
- 12. Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia 2020; 26: 1–158. [DOI] [PubMed] [Google Scholar]
- 13. Hermans C, Auerswald G, Benson G, et al. Outcome measures for adult and pediatric hemophilia patients with inhibitors. Eur J Haematol 2017; 99: 103–111. [DOI] [PubMed] [Google Scholar]
- 14. Hsu CC, Sandford BA. The Delphi technique: making sense of consensus. Pract Assess Res Eval 2007; 12: 1–8. [Google Scholar]
- 15. Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs 2000; 32: 1008–1015. [PubMed] [Google Scholar]
- 16. Skinner D, Nelson RR, Chin W, et al. The Delphi method research strategy in studies of information systems. Commun Assoc Inf Syst 2015; 37: 31–63. [Google Scholar]
- 17. Keeney S, Hasson F, McKenna H. The Delphi technique in nursing and health research, 1st ed. West Sussex: Wiley-Blackwell, 2011. [Google Scholar]
- 18. European Haemophilia Consortium. Event report: EHC round table of stakeholders on ‘Inhibitors in Haemophilia A’, https://www.ehc.eu/wp-content/uploads/EHC-Report-Round-Table-2016-02-Inhibitors-in-Haemophilia-A.pdf (2016, accessed February 2021).
- 19. López-Fernández MF, Roca CA, Álvarez-Román MT, et al. Spanish consensus guidelines on prophylaxis with bypassing agents in patients with haemophilia and inhibitors. Thromb Haemost 2016; 115: 872–895. [DOI] [PubMed] [Google Scholar]
- 20. Collins P, Chalmers E, Alamelu J, et al. First-line immune tolerance induction for children with severe haemophilia A: a protocol from the UK Haemophilia Centre Doctors’ Organisation Inhibitor and Paediatric Working Parties. Haemophilia 2017; 23: 654–659. [DOI] [PubMed] [Google Scholar]
- 21. Clibbens N, Walters S, Baird W. Delphi research: issues raised by a pilot study. Nurse Res 2012; 19: 37–44. [DOI] [PubMed] [Google Scholar]
- 22. McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm 2016; 38: 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huisstede BMA, Miedema HS, Verhagen AP, et al. Multidisciplinary consensus on the terminology and classification of complaints of the arm, neck and/or shoulder. Occup Environ Med 2007; 64: 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kleynen M, Braun SM, Bleijlevens MH, et al. Using a Delphi technique to seek consensus regarding definitions, descriptions and classification of terms related to implicit and explicit forms of motor learning. PLoS One 2014; 9: e100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hsu C-C, Sandford BA. Minimizing non-response in the Delphi process: how to respond to non-response. Pract Assess Res Eval 2007; 12: 1-6. [Google Scholar]
- 26. Fischer K, Poonnoose P, Dunn AL, et al. Choosing outcome assessment tools in haemophilia care and research: a multidisciplinary perspective. Haemophilia 2017; 23: 11–24. [DOI] [PubMed] [Google Scholar]
- 27. Riley RR, Witkop M, Hellman E, et al. Assessment and management of pain in haemophilia patients. Haemophilia 2011; 17: 839–845. [DOI] [PubMed] [Google Scholar]
- 28. Humphries TJ, Kessler CM. The challenge of pain evaluation in haemophilia: can pain evaluation and quantification be improved by using pain instruments from other clinical situations? Haemophilia 2013; 19: 181–187. [DOI] [PubMed] [Google Scholar]
- 29. Santagostino E, Morfini M, Auerswald GK, et al. Paediatric haemophilia with inhibitors: existing management options, treatment gaps and unmet needs. Haemophilia 2009; 15: 983–989. [DOI] [PubMed] [Google Scholar]
- 30. ClinicalTrials.gov. The hemophilia inhibitor eradication trial (NCT04303572), https://clinicaltrials.gov/ct2/show/NCT04303572 (2021, accessed February 2021).
- 31. ClinicalTrials.gov. Treatment of hemophilia A patients with FVIII inhibitors (MOTIVATE) (NCT04023019), https://www.clinicaltrials.gov/ct2/show/NCT04023019 (2020, accessed February 2021).
- 32. ClinicalTrials.gov. Preventing inhibitor recurrence indefinitely (PRIORITY) (NCT04621916), https://www.clinicaltrials.gov/ct2/show/NCT04621916 (2020, accessed February 2021).
- 33. ClinicalTrials.gov. Immune tolerance induction in haemophilia A patients using Wilate or Nuwiq (PREVAIL) (NCT03344003), https://www.clinicaltrials.gov/ct2/show/NCT03344003 (accessed February 2021).
- 34. ClinicalTrials.gov. Evaluation of safety following immune tolerance induction treatment with Turoctocog alfa in patients with Haemophilia A following inhibitor development in NN7170-4213 trial (NCT03588741), https://clinicaltrials.gov/ct2/show/NCT03588741 (2021, accessed February 2021).
- 35. ClinicalTrials.gov. Emicizumab PUPs and Nuwiq ITI study (NCT04030052), https://www.clinicaltrials.gov/ct2/show/NCT04030052 (2020, accessed February 2021).
- 36. Meeks SL, Batsuli G. Hemophilia and inhibitors: current treatment options and potential new therapeutic approaches. Hematology Am Soc Hematol Educ Program 2016; 2016: 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gomez K, Klamroth R, Mahlangu J, et al. Key issues in inhibitor management in patients with haemophilia. Blood Transfus 2014; 12(Suppl. 1): S319-S329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chai-Adisaksopha C, Hillis C, Thabane L, et al. A systematic review of definitions and reporting of bleeding outcome measures in haemophilia. Haemophilia 2015; 21: 731–735. [DOI] [PubMed] [Google Scholar]
- 39. Kempton CL, Allen G, Hord J, et al. Eradication of factor VIII inhibitors in patients with mild and moderate hemophilia A. Am J Hematol 2012; 87: 933–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Andersson NG, Auerswald G, Barnes C, et al. Intracranial haemorrhage in children and adolescents with severe haemophilia A or B—the impact of prophylactic treatment. Br J Haematol 2017; 179: 298–307. [DOI] [PubMed] [Google Scholar]
- 41. Benson G, Auerswald G, Dolan G, et al. Diagnosis and care of patients with mild haemophilia: practical recommendations for clinical management. Blood Transfus 2018; 16: 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Giuffrida AC, Genesini S, Franchini M, et al. Inhibitors in mild/moderate haemophilia A: two case reports and a literature review. Blood Transfus 2008; 6: 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van Velzen AS, Eckhardt CL, Hart DP, et al. Inhibitors in nonsevere haemophilia A: outcome and eradication strategies. Thromb Haemost 2015; 114: 46–55. [DOI] [PubMed] [Google Scholar]
- 44. Eckhardt CL, Loomans JI, Van Velzen AS, et al. Inhibitor development and mortality in non-severe hemophilia A. J Thromb Haemost 2015; 13: 1217–1225. [DOI] [PubMed] [Google Scholar]
- 45. Oldenburg J. Optimal treatment strategies for hemophilia: achievements and limitations of current prophylactic regimens. Blood 2015; 125: 2038–2044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-jpg-1-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-jpg-2-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-jpg-3-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-pdf-4-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-pdf-5-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-pdf-6-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-pdf-7-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-pdf-8-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-pdf-9-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-xlsx-10-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-xlsx-11-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-xlsx-12-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-xlsx-13-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology
Supplemental material, sj-xlsx-14-tah-10.1177_20406207211007058 for Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study by Kate Khair, Elizabeth Chalmers, Thuvia Flannery, Annabel Griffiths, Felicity Rowley, Guillermo Tobaruela and Pratima Chowdary in Therapeutic Advances in Hematology