Key Points
Question
Is there a strong association of rotavirus vaccines and preventing rotavirus gastroenteritis (RVGE)?
Findings
Meta-analysis revealed that Rotarix and RotaTeq reduced RVGE in children younger than 5 years by 68.4% and 63.6%, respectively, and this was confirmed in case-control studies (65.3% and 72.8%, respectively). Adjusted indirect comparisons indicated no significant differences in the protection of Rotarix and RotaTeq; other rotavirus vaccines, including Rotavac, Rotasiil, and Lanzhou lamb rotavirus vaccine, also showed positive associations with reduced RVGE risk.
Meaning
The findings favor the worldwide introduction of rotavirus vaccines to prevent RVGE, but head-to-head comparisons are needed to compare the benefit and risk of different rotavirus vaccines.
This meta-analysis synthesizes randomized clinical trials and observational studies to evaluate the comparative benefit, risk, and immunogenicity of different rotavirus vaccines.
Abstract
Importance
Rotavirus vaccines have been introduced worldwide, and the clinical association of different rotavirus vaccines with reduction in rotavirus gastroenteritis (RVGE) after introduction are noteworthy.
Objective
To evaluate the comparative benefit, risk, and immunogenicity of different rotavirus vaccines by synthesizing randomized clinical trials (RCTs) and observational studies.
Data Sources
Relevant studies published in 4 databases: Embase, PubMed, the Cochrane Library, and Web of Science were searched until July 1, 2020, using search terms including “rotavirus” and “vaccin*.”
Study Selection
Randomized clinical trials and cohort and case-control studies involving more than 100 children younger than 5 years that reported the effectiveness, safety, or immunogenicity of rotavirus vaccines were included.
Data Extraction and Synthesis
A random-effects model was used to calculate relative risks (RRs), odds ratios (ORs), risk differences, and 95% CIs. Adjusted indirect treatment comparison was performed to assess the differences in the protection of Rotarix and RotaTeq.
Main Outcomes and Measures
The primary outcomes were RVGE, severe RVGE, and RVGE hospitalization. Safety-associated outcomes involved serious adverse events, intussusception, and mortality.
Results
A meta-analysis of 20 RCTs and 38 case-control studies revealed that Rotarix (RV1) significantly reduced RVGE (RR, 0.316 [95% CI, 0.224-0.345]) and RVGE hospitalization risk (OR, 0.347 [95% CI, 0.279-0.432]) among children fully vaccinated; RotaTeq (RV5) had similar outcomes (RVGE: RR, 0.350 [95% CI, 0.275-0.445]; RVGE hospitalization risk: OR, 0.272 [95% CI, 0.197-0.376]). Rotavirus vaccines also demonstrated higher protection against severe RVGE. Additionally, no significant differences in the protection of RV1 and RV5 against rotavirus disease were noted in adjusted indirect comparisons. Moderate associations were found between reduced RVGE risk and Rotavac (RR, 0.664 [95% CI, 0.548-0.804]), Rotasiil (RR, 0.705 [95% CI, 0.605-0.821]), and Lanzhou lamb rotavirus vaccine (RR, 0.407 [95% CI, 0.332-0.499]). All rotavirus vaccines demonstrated no risk of serious adverse events. A positive correlation was also found between immunogenicity and vaccine protection (eg, association of RVGE with RV1: coefficient, −1.599; adjusted R2, 99.7%).
Conclusions and Relevance
The high protection and low risk of serious adverse events for rotavirus vaccines in children who were fully vaccinated emphasized the importance of worldwide introduction of rotavirus vaccination. Similar protection provided by Rotarix and RotaTeq relieves the pressure of vaccines selection for health care authorities.
Introduction
Worldwide, diarrhea, accounting for approximately 70.6 deaths per 100 000 population and 1.75 episodes per child in 2016, is the fifth leading cause of death among children younger than 5 years.1 Rotavirus gastroenteritis, which results in 28.8% of the deaths from diarrhea, is the leading causative mechanism for diarrhea in children younger than 5 years.1,2 The mortality and morbidity of RVGE varies by location, with the highest in sub-Saharan Africa, Southeast Asia, and South Asia.2 Fortunately, the mortality of RVGE decreased by 43.6% from 2005 to 2015,3 which most likely owing to the introduction of rotavirus vaccines.
In 2018, 101 countries have introduced rotavirus vaccine into their national immunization programs, with global coverage at 53%.4 At present, 6 oral rotavirus vaccines have been widely used. Two live attenuated oral rotavirus vaccines, Rotarix (RV1), a 2-dose monovalent (G1P[8]) vaccine and RotaTeq [RV5]), a 3-dose pentavalent (G1, G2, G3, G4, and P[8]) vaccine, are globally introduced.5 Another 2 novel vaccines, Rotasiil (BRV-PV) and Rotavac (116E), are currently licensed in India only.6 Besides, domestically licensed rotavirus vaccines are also available in China (Lanzhou lamb rotavirus [LLR] vaccine) and Vietnam (Rotavin). Although a decline in the morbidity and mortality of RVGE has been reported in many countries following the introduction of rotavirus vaccines, concerns about serious adverse events still exist. Furthermore, little is known about the comparative benefit and risk of different rotavirus vaccines because of the lack of powerful head-to-head comparisons. Therefore, by synthesizing randomized clinical trials (RCTs) and case-control and cohort studies, we undertook a systematic review and meta-analysis to evaluate the association of different rotavirus vaccines with RVGE in aspects of benefit, risk, and immunogenicity and analyzed comparative protection of different vaccines by indirect comparisons.
Methods
Search Strategy and Selection Criteria
We searched for relevant studies published until July 1, 2018, and further updated until July 1, 2020, in 4 databases: Embase, PubMed, the Cochrane Library, and Web of Science, using search terms including “rotavirus” and “vaccin*.” Randomized clinical trials and cohort and case-control studies reporting the efficacy, effectiveness, safety, or immunogenicity of rotavirus vaccine were included. Studies with fewer than 100 enrolled participants were excluded. The selection criteria are further outlined in detail in eTable 1 in the Supplement.
Procedures
The study selection and data collection process were explained in detail in the eMethods in the Supplement. Using EndNote X8 (Clarivate), 2 reviewers (Z.-W.S. and Y.F.) independently screened all obtained articles for relevance, with a third reviewer (H.L.L.) consulted when necessary. We developed a standardized data extraction form, and Z.-W.S. rechecked the extracted data of included trials identified by Y.F. The quality of RCTs and observational studies were accessed in accordance with the Cochrane Reviewers’ Handbook and the Newcastle-Ottawa Scales (eTable 2 in the Supplement). For 10% of included studies, data were doubly extracted by a third author (H.L.L.). The procedure was supervised and arbitrated by a fourth author (H.G.X.).
Statistical Analysis
In preliminary analyses, estimates of relative risks (RRs), odds ratios (ORs), and risk differences using raw data were similar to reported results; thus, we opted to perform more detailed statistical analyses with raw data, using Stata version 12.0 (StataCorp) and Revman version 5.3 (Cochrane Library). The RRs, ORs, and risk differences were calculated in a Mantel-Haenszel random-effects model. We used the per-protocol estimates in RCTs and combined control groups, including both hospital and community controls, in case-control studies. Considering the diminished vaccine efficacy in low-income countries (LICs) compared with middle-income countries and high-income countries, included studies were stratified by the economic development of countries, using the World Bank’s classification (eTable 3 in the Supplement).7 For multicenter RCTs, we included each individual country as a separate observation point whenever possible. If not, we used the sample size in each site to calculate a weighted level of economic development and used this estimate to assign the trial to a specific stratum.
Adjusted indirect treatment comparison was performed to assess the differences in vaccine protection between different subgroups, adopting P < .05 as the level of statistical significance. We performed a metaregression model to estimate the association between vaccine protection in 1 to 2 years of follow-up and the rate of seropositivity at 1 to 2 months after the last dose (IgA antibody concentration ≥20 units/mL or ≥3-fold increase from baseline), and the adjusted R2 index was used to quantify the proportion of variance explained by the covariates. For the outcomes obtained from fewer than 3 studies, we conducted a systematic review. A sensitivity analysis was performed by excluding each study to identify the stability and consistency of our results. The Q test and I2 statistic was applied to determine heterogeneity (P < .10 or I2 > 50% indicated significant heterogeneity). Publication bias was assessed using funnel plots (eFigure 6 in the Supplement).
Results
Study Selection and Characteristics
Initial literature retrieval produced 3998 articles, and 241 full-text articles were assessed for eligibility. Seventeen studies were further identified from updated literature retrieval, and 121 studies were finally included. The selection process is summarized in eFigure 1 in the Supplement. Included studies varied by study design (57 RCTs,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64 50 case-control studies,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114 and 14 cohort studies115,116,117,118,119,120,121,122,123,124,125,126,127,128), rotavirus vaccine type (74 for RV18,9,11,12,15,16,19,20,21,22,23,24,25,26,31,32,34,35,40,41,42,43,44,48,49,50,51,52,58,65,66,72,73,74,75,76,77,78,79,80,81,82,83,84,85,87,88,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,113,114,118,119,120,121,122,124,125,126,127,128; 45 for RV510,13,14,17,18,27,28,29,33,45,53,54,55,56,57,59,60,61,62,63,64,65,66,67,68,69,70,71,76,78,81,84,86,88,93,94,95,96,111,112,115,116,117,122,123; 5 for LLR30,89,90,91,92; and 3 each for Rotavac36,37,38 and Rotasiil39,46,47), or study population. The characteristics of included studies are reported in eTable 4 in the Supplement.
Benefits and Risks of Rotavirus Vaccines, Stratified by Vaccine Type
During the first year of follow-up, more children in placebo groups developed RVGE compared with children vaccinated with full-dose RV1 (RR, 0.316 [95% CI, 0.224-0.345]) or RV5 (RR, 0.350 [95% CI, 0.275-0.445]) (Figure 1 and Figure 2). In case-control studies, a low risk of RVGE hospitalization was also estimated among children fully vaccinated with RV1 (OR, 0.347 [95% CI, 0.279-0.432]) or RV5 (OR, 0.272 [95% CI, 0.197-0.376]). A systemic review of cohort studies revealed RRs of 0.125 (95% CI, 0.086-0.182) for RV1 and 0.049 (95% CI, 0.028-0.083) for RV5 for the prevention of RVGE hospitalization, regardless of the cohort year (eTable 5 in the Supplement). Rotavirus vaccines demonstrated higher protection against severe RVGE but less against severe all-cause gastroenteritis. A clear gradient in vaccine protection was noted by country income level, with the highest in high-income countries and the lowest in LICs (RVGE hospitalization, P = .002; RVGE, P < .001; eTables 6-12 in the Supplement).
Figure 1. Random-Effects Model of Rotavirus Vaccine Protection Against Rotavirus Gastroenteritis (RVGE) and RVGE Hospitalization, by Country Income Level, in Randomized Clinical Trials.
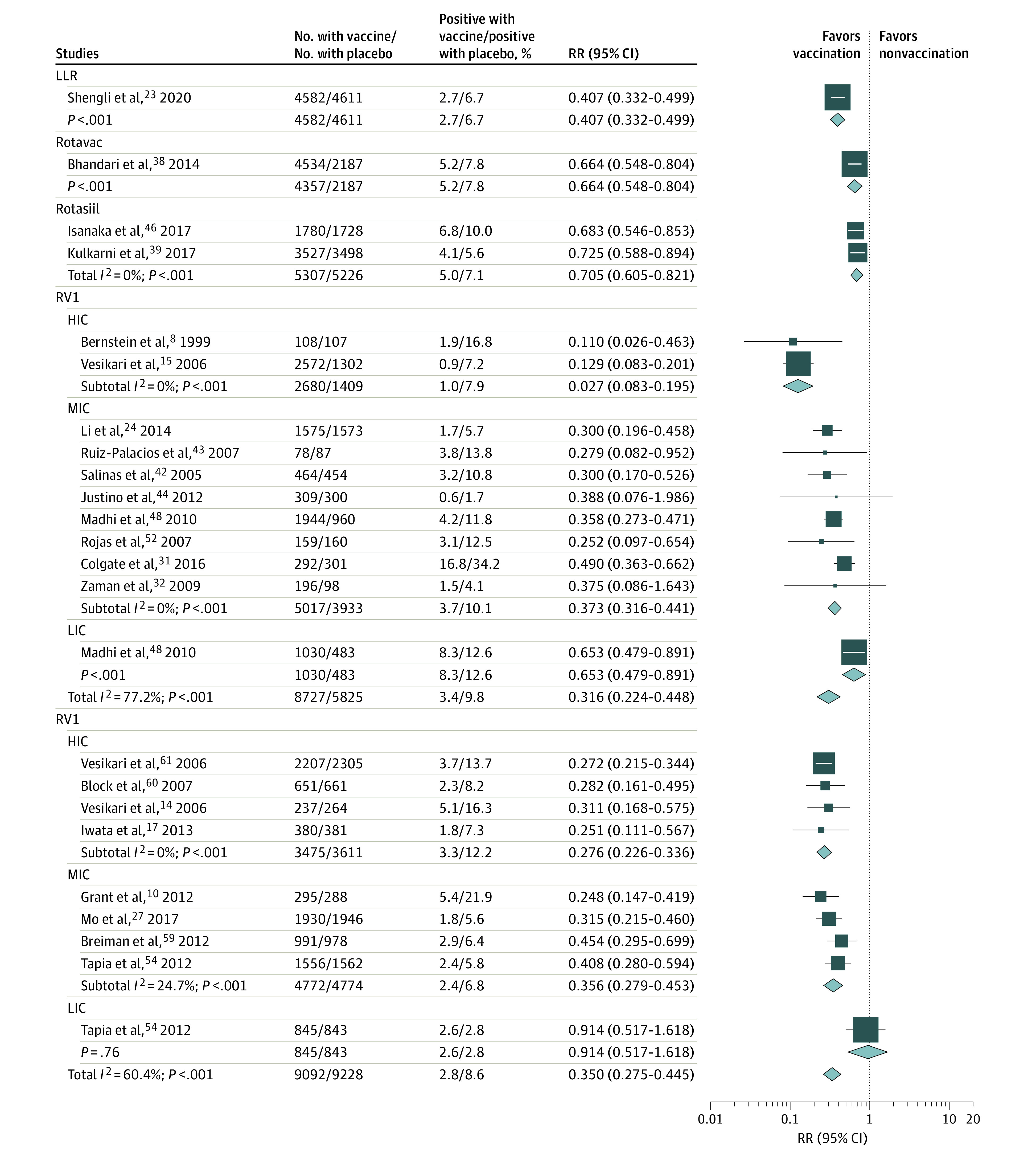
HIC indicates high-income countries; LIC, low-income countries; LLR, Lanzhou lamb rotavirus; MIC, middle-income countries; RR, relative risk; RV1, monovalent rotavirus vaccine.
Figure 2. Random-Effects Model of Rotavirus Vaccine Protection against Rotavirus Gastroenteritis (RVGE) and RVGE Hospitalization, by Country Income Level, in Case-Control Studies.
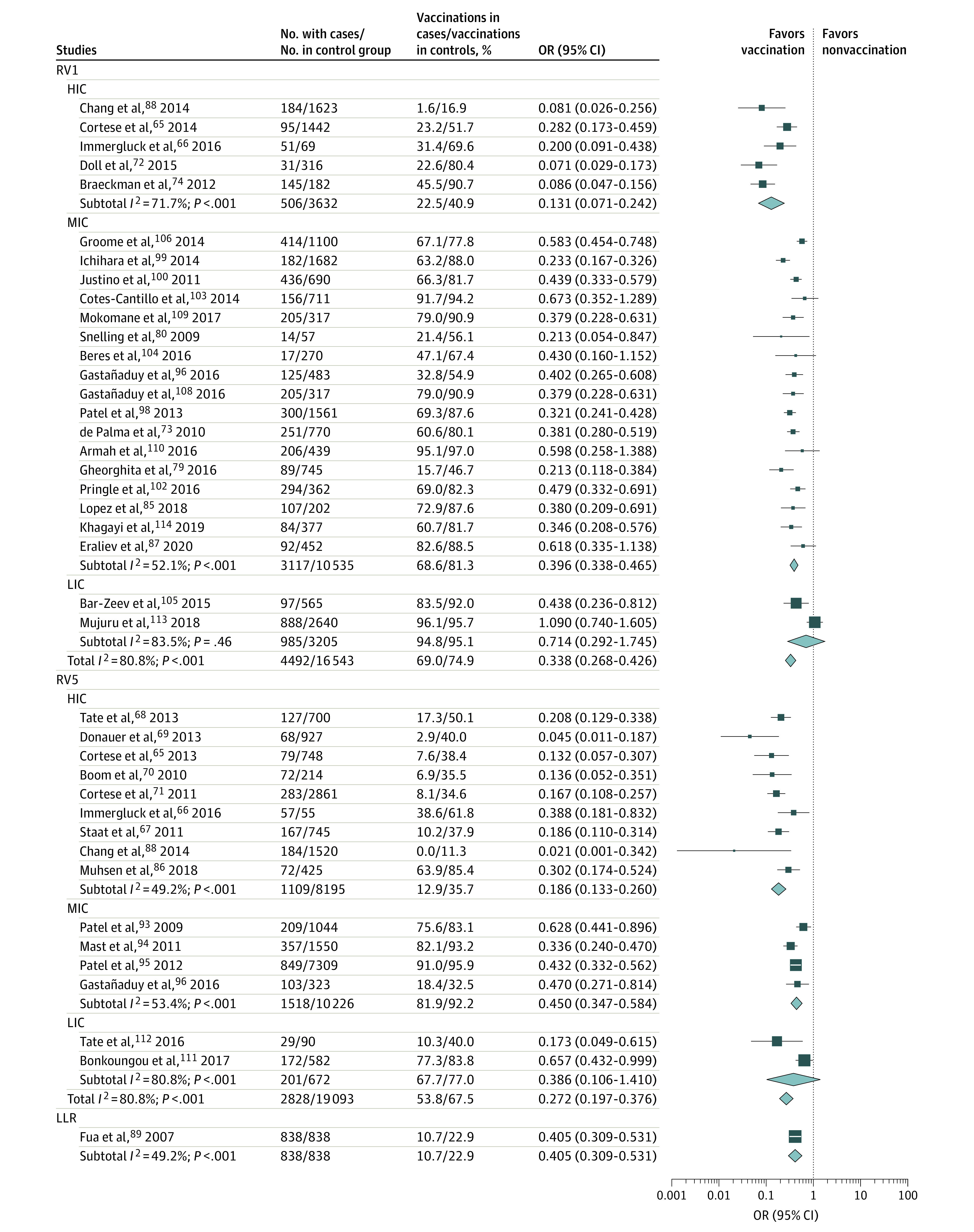
HIC indicates high-income countries; LIC, low-income countries; LLR, Lanzhou lamb rotavirus; MIC, middle-income countries; OR, odds ratio; RV1, monovalent rotavirus vaccine; RV5, pentavalent rotavirus vaccine.
Rotavac reduced RVGE and severe RVGE risk in India by 33.6% (95% CI, 19.6%-45.2%) and 56.0% (95% CI, 37.3%-69.2%), respectively. Rotasiil reduced RVGE and severe RVGE risk by 29.5% (95% CI, 17.9%-39.5%) and 52.2% (95% CI, 12.1%-74.0%) in India and Niger, respectively. In China, LLR was associated with a decrease in RVGE (RR, 0.407 [95% CI, 0.332-0.499]; OR, 0.348 [95% CI, 0.121-0.999]), severe RVGE (RR, 0.248 [95% CI, 0.144-0.427]), and RVGE hospitalization (OR, 0.405 [95% CI, 0.309-0.531]).
In indirect treatment comparisons, no significant differences were noted in the protection of RV1 and RV5 against RVGE (RR, 0.865 [95% CI, 0.565-1.325]; P = .51; OR, 1.264 [95% CI, 0.866-1.844]; P = .23) or severe RVGE (RR, 0.768 [95% CI, 0.335-1.758]; P = .53; OR, 0.944 [95% CI, 0.603-1.476]; P = .80) (eTable 13 in the Supplement). When stratified by the World Bank classification, there were also no significant differences in vaccine protection between RV1 and RV5. Furthermore, to alleviate the bias of sociodemographic factors, only studies conducted in the same region were included to perform adjusted indirect treatment comparison, and the results also indicated little difference in vaccine protection between RV1 and RV5 (eTable 14 in the Supplement).
We identified 36 RCTs,9,11,12,13,14,16,17,18,19,20,23,24,26,27,29,30,32,33,34,35,37,38,39,40,41,42,43,45,47,48,50,53,58,60,61,63 4 case-control studies,71,81,101,107 and 2 cohort studies117,119 evaluating the safety of rotavirus vaccines (eFigures 2-4 in the Supplement). The incidence of serious adverse events in the vaccine group was similar to that of the placebo group. The overall estimate of risk differences showed no increased risk of intussusception and death in children vaccinated with RV1, RV5, Rotavac, Rotasiil, or LLR during 1 or 2 years of follow-up.
Stratified Analyses of Rotavirus Vaccine Benefit by Duration and Vaccination Schedule
In stratified analyses of the duration of vaccine protection, we found that the protection of RV1 or RV5 against RVGE was lower in the second year of follow-up (RV1: RR, 0.494 [95% CI, 0.255-0.955]; RV5: RR, 0.622 [95% CI, 0.388-0.996]) in comparisons with the first year of follow-up (RV1: RR, 0.297 [95% CI, 0.207-0.425]; RV5: RR, 0.344 [95% CI, 0.271-0.436]), while similar in 2 years of follow-up (RV1: RR, 0.348 [95% CI, 0.196-0.618]; RV5: RR, 0.500 [95% CI, 0.288-0.869]; Figure 3). Also, the reductions in vaccine protection during the second year of follow-up were small in the high-income countries (RV1: RR, 0.281 [95% CI, 0.207-0.381]; RV5: RR, 0.497 [95% CI, 0.353-0.699]) but pronounced in the LICs (RV1: RR, 1.288 [95% CI, 0.738-2.248]; RV5: RR, 0.815 [95% CI, 0.659-1.007]) (eTable 9 in the Supplement). Here, estimates should be interpreted with caution, because there was only 1 study for an LIC.48 Similar results were observed for Rotavac and Rotasiil in India. In case-control studies, RV1 and RV5 provided similar protection among children aged younger than 12 months, 12-24 months, or ≥12 months. By contrast, the OR of RV1 vs control in the LIC and lower- and middle-income countries was significantly lower among children aged 12 to 24 months (OR, 0.528 [95% CI, 0.249-1.120]; P < .001) or those 12 months or older (OR, 0.526 [95% CI, 0.370-0.750]; P = .008), compared with children younger than 12 months (OR, 0.356 [95% CI, 0.266-0.476]).
Figure 3. Subgroup Analysis for Rotavirus Vaccine Protection, Stratified by Follow-up Duration and Vaccination Schedule.
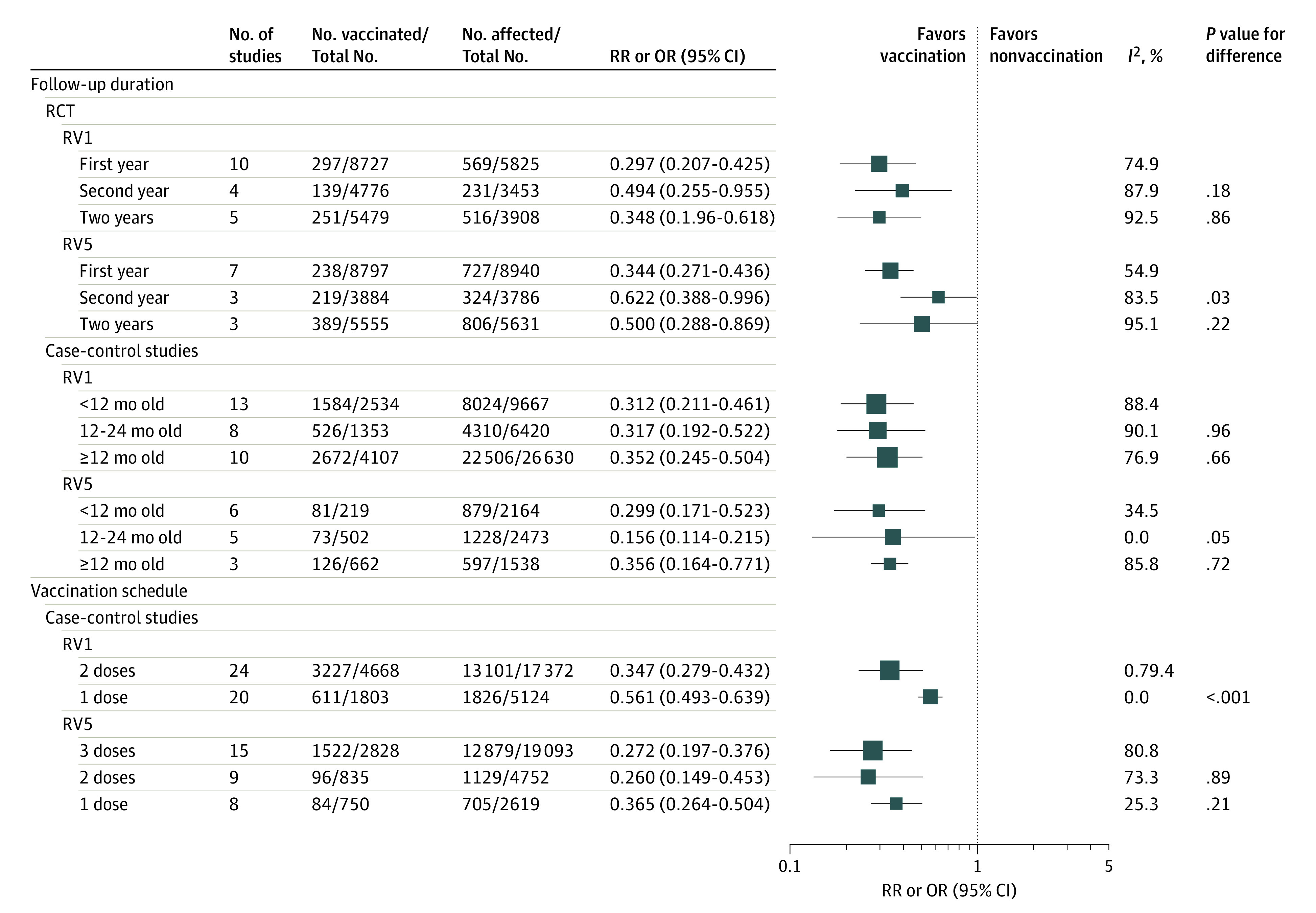
In randomized clinical trials (RCTs), the first column shows the number of cases in the vaccine group and the total population in the vaccine group. In case-control studies, these numbers denote the number of vaccinated children in the case group and the sum of children with no vaccination plus those receiving full doses in the case group. In RCTs, the second column shows the number of cases in the placebo group and the total population in placebo group. In case-control studies, this column shows the number of vaccinated children in the control group and the sum of children with no vaccination plus those receiving full doses in the control group. Odds ratios (ORs) were used for case-control studies; relative risks (RRs), for RCTs. RV1 indicates monovalent rotavirus vaccine; RV5, pentavalent rotavirus vaccine.
In the second comparison, we divided 35 case-control studies65,66,67,68,69,70,71,72,73,74,79,80,85,86,87,88,93,94,95,96,98,99,100,102,103,104,105,106,108,109,110,111,112,113,114 depending on whether the enrolled children received complete vaccination (Figure 3). Studies reported a nonsignificantly lower risk of RVGE hospitalization among children vaccinated with 3-dose RV5 compared with 1 dose, but a similar risk between 2 doses and 3 doses. When stratified by the World Bank classification, no significant differences in vaccine protection between 3 doses, 2 doses, and 1 dose of RV5 were observed. Two-dose RV1 showed stronger association with reduced risk of RVGE hospitalization than only 1 dose (OR, 0.347 [95% CIs, 0.279-0.432] vs 0.561 [95% CIs, 0.493-0.639]; P < .001), especially in the middle-income countries (OR, 0.396 [95% CIs, 0.338-0.465] vs 0.559 [95% CIs, 0.489-0.640]; P = .001).
Strain-Specific Protection of Rotavirus Vaccine
Pooled data from 13 RCTs11,16,20,24,27,40,41,42,48,49,54,59,61 suggested that RV1 conferred protection against severe RVGE caused by G1, G2, G3, G4, G9, and P[8] strains, respectively, whereas the protection of RV5 was low (and nonsignificant) against G1, G2, and G3 strain (eTable 15 in the Supplement). The Table showed the strain-specific protection of RV1,129 and no significant differences were noted in vaccine protection against partly heterotypic or fully heterotypic strains compared with homotypic strains in middle-income countries. There were also no significant differences in vaccine protection against single-antigen vaccine type and single-antigen nonvaccine type strains for RV1 and RV5. However, higher protection of RV1 against homotypic strains (OR, 0.116 [95% CIs, 0.065-0.217]) than heterotypic strains (OR, 0.457, [95% CIs, 0.264-0.579]) was estimated in case-control studies in middle-income countries (P = .005).
Table. Strain-Stratified Vaccine Protection During the 2-Year Efficacy Period.
| Characteristic | RV1 | RV5 | ||||
|---|---|---|---|---|---|---|
| Observations, No. | RR or OR (95% CI)a | P valueb | Observations, No. | RR or OR (95% CI)a | P valueb | |
| RCTsc | ||||||
| High-income countries | ||||||
| Single-antigen vaccine type strain | 4 | 0.054 (0.022-0.130) | NA | 4 | 0.056 (0.036-0.088) | NA |
| Single-antigen nonvaccine type strain | 9 | 0.123 (0.076-0.198) | .11 | 2 | 0.098 (0.010-0.945) | .63 |
| Middle-income countries | ||||||
| Homotypic strain | 3 | 0.248 (0.139-0.444) | NA | NA | NA | NA |
| Partly heterotypic strain | 7 | 0.200 (0.131-0.306) | .56 | NA | NA | NA |
| Fully heterotypic strain | 3 | 0.333 (0.191-0.581) | .47 | NA | NA | NA |
| Single-antigen vaccine type strain | 4 | 0.293 (0.196-0.438) | NA | 13 | 0.533 (0.423-0.672) | NA |
| Single-antigen nonvaccine type strain | 10 | 0.204 (0.151-0.276) | .16 | 8 | 0.443 (0.285-0.690) | .47 |
| Low-income countries | ||||||
| Single-antigen vaccine type strain | 2 | 0.558 (0.315-0.991) | NA | NA | NA | NA |
| Single-antigen nonvaccine type strain | 6 | 0.485 (0.336-0.701) | .69 | NA | NA | NA |
| Case-control studiesd | ||||||
| High-income countries | ||||||
| Homotypic strain | 3 | 0.096 (0.030-0.313) | NA | 2 | 0.156 (0.091-0.268) | NA |
| Partly heterotypic strain | 3 | 0.188 (0.063-0.555) | .41 | 3 | 0.135 (0.041-0.445) | .83 |
| Fully heterotypic strain | 3 | 0.178 (0.107-0.295) | .35 | NA | NA | NA |
| Single-antigen vaccine type strain | NA | NA | NA | 3 | 0.165 (0.101-0.271) | NA |
| Single-antigen nonvaccine type strain | NA | NA | NA | 2 | 0.215 (0.105-0.441) | .56 |
| Middle-income countries | ||||||
| Homotypic strain | 2 | 0.116 (0.065-0.217) | NA | NA | NA | NA |
| Partly heterotypic strain | 4 | 0.457 (0.264-0.579) | .001 | NA | NA | NA |
| Fully heterotypic strain | 6 | 0.335 (0.197-0.569) | .005 | NA | NA | NA |
| Single-antigen vaccine type strain | 2 | 0.458 (0.147-1.427) | NA | 5 | 0.354 (0.249-0.503) | NA |
| Single-antigen nonvaccine type strain | 4 | 0.592 (0.310-1.129) | .70 | 3 | 0.176 (0.039-0.793) | .38 |
| Low-income countries | ||||||
| Single-antigen vaccine type strain | 1 | 0.201 (0.065-0.617) | NA | NA | NA | NA |
| Single-antigen nonvaccine type strain | 2 | 0.516 (0.232-1.147) | .21 | NA | NA | NA |
| Cohort studiese | ||||||
| Middle-income countries | ||||||
| Homotypic strain | 2 | 0.276 (0.096-0.794) | NA | NA | NA | NA |
| Partly heterotypic strain | 3 | 0.436 (0.183-1.039) | .25 | NA | NA | NA |
| Fully heterotypic strain | 4 | 0.429 (0.185-0.996) | .26 | NA | NA | NA |
Abbreviations: NA, not applicable; OR, odds ratio; RCTs, randomized clinical trials; RR, relative risk; RV1, monovalent rotavirus vaccine; RV5, pentavalent rotavirus vaccine; RVGE, rotavirus gastroenteritis.
Relative risks are used in the RCT portion of this Table, and ORs are used in the case-control studies portion.
P values were the differences of vaccine protection against partly heterotypic or fully heterotypic strains compared with homotypic strains; the differences of vaccine protection against single-antigen nonvaccine type strains compared with single-antigen vaccine type strains.
Metaregression Between Immunogenicity and Protection of Rotavirus Vaccine
Circulating antirotavirus IgA antibodies have been used as the early proxy for vaccine uptake,130,131 which is a convenient method to monitor vaccine effectiveness at the population level. Pooled data from 24 RCTs8,9,12,14,15,16,18,19,22,24,28,29,31,32,33,34,35,42,43,48,53,57,60,61 showed that the percentage of seropositivity among children who were fully vaccinated was 69.3% (95% CIs, 60.0%-78.6%) for RV1 and 89.5% (95% CIs, 84.5%-94.5%) for RV5, much higher than in placebo group (11.9% [95% CIs, 8.5%-15.2%]). Moreover, the percentage of vaccinated children with seropositivity exhibited a positive association with vaccine protection (R2 >0; (eg, association of RVGE with Rotarix: coefficient, −1.599; adjusted R2, 99.7%; Figure 4). In metaregression analyses, with the difference of the rate of seropositivity between vaccine groups and placebo groups as the abscissa, the adjusted R2 values for the associations between immunogenicity and vaccine protection were 86.5% for RV1 and 15.6% for RV5 against severe RVGE and 99.7% for RV1 against RVGE. We did not evaluate the association between immunogenicity and vaccine protection of RV5 against RVGE because only 3 studies15,60,61 were included.
Figure 4. Metaregression Between Immunogenicity and Vaccine Protection.
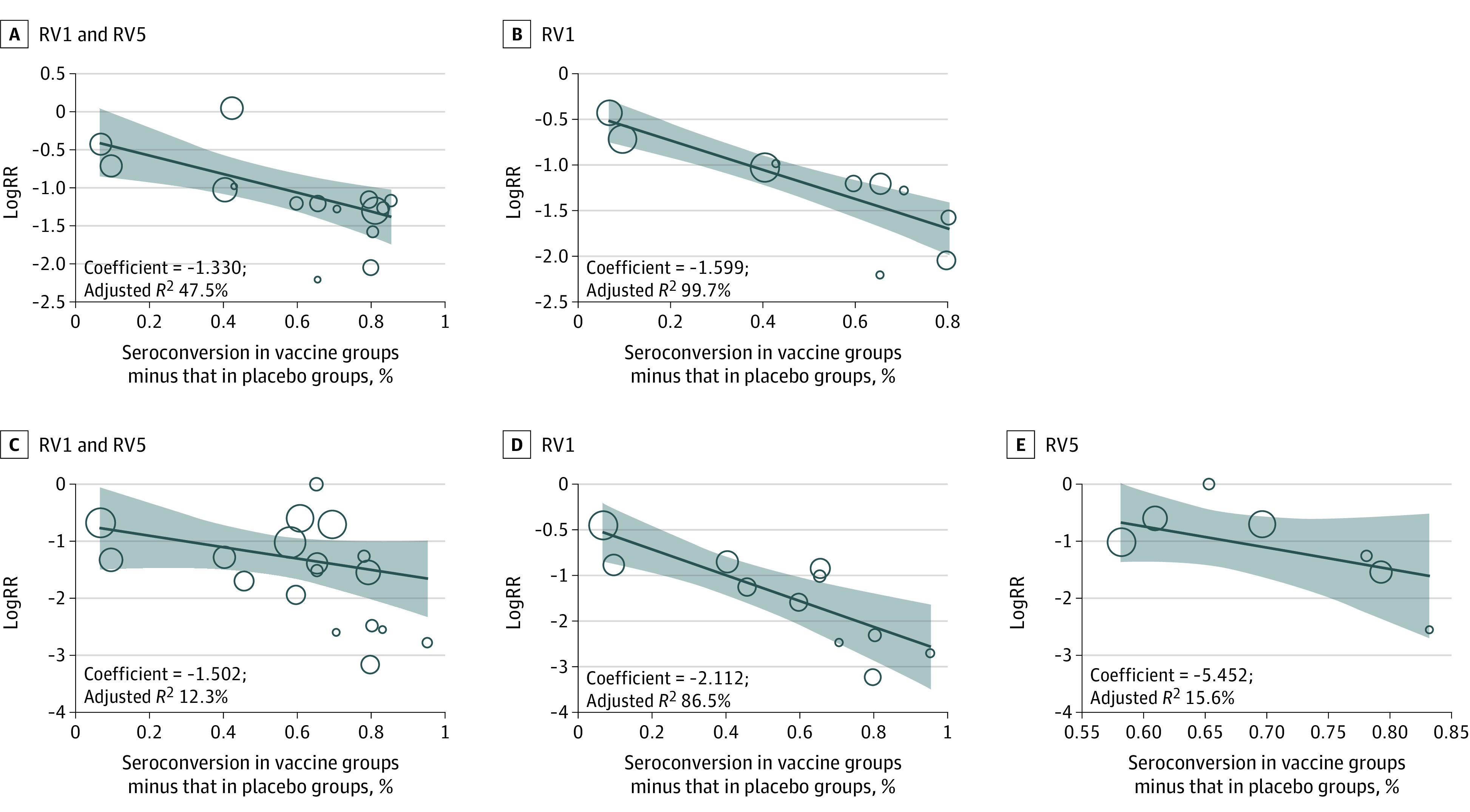
The coefficient is the regression correlation coefficient, and the adjusted R2 is the proportion of between-study variance explained. Metaregression between immunogenicity and logRR against rotavirus gastroenteritis (RVGE) for RV1 and RV5 (A), RVGE for RV1 (B), severe RVGE for RV1 and RV5 (C), severe RVGE for RV1 (D), and severe RVGE for RV5 (E). RV1 indicates monovalent rotavirus vaccine; RV5, pentavalent rotavirus vaccine.
Sensitivity and Heterogeneity Analyses
Our systematic review found considerable heterogeneity between included studies. To investigate the potential sources of heterogeneity, a subgroup analysis was performed using economic development as a variable, and the heterogeneity was subsequently shown to be dealt with in varying degrees when I2 was greater than 50%. Sensitivity analyses for all outcomes did not identify any substantial effects resulting from differences in study quality (eFigure 5 in the Supplement).
Discussion
Our findings from RCTs, case-control studies, and cohort studies corroborated that RV1 and RV5 have substantial and sustained protection against rotavirus disease, especially against severe RVGE, which is in line with previously reported data.132,133,134,135 Considering that vaccine administrations are not always followed by recommendations, comorbidities may be present, and sociodemographic factors vary in real world, the consistency of results from observational studies and RCTs reconfirmed the high protection of rotavirus vaccination. Moreover, pooled data showed no increased risk of serious adverse events including intussusception among children who were vaccinated.136,137 However, a study about intussusception conducted in Australia reported a smaller increased risk of intussusception after RV1 and RV5 vaccination.138 Therefore, continuous surveillance of the benefits and adverse effects of rotavirus vaccines is required after vaccination.
The protection against rotavirus diseases varied by time interval after vaccination, and rotavirus vaccines, particularly RV5, provided lower protection against RVGE in the second efficacy period. Although our results indicated that rotavirus vaccines can provide substantial protection against RVGE during the first 2 years of life,139 more studies following up the vaccine efficacy for more than 2 years are required. The reduced vaccine protection might be caused by declining vaccine-induced antibodies, acquisition of protection against RVGE through indirect effects of vaccine, or exposure to natural asymptomatic and mild infections among control populations who are unvaccinated.140 The wane of vaccine protection over time highlights the importance of monitoring the morbidity of rotavirus diarrhea after vaccination; more children may become infected at older ages, and evaluation of alternative vaccination schedules is useful.
During the subgroup analyses, vaccination schedule may affect vaccine performance. Data from case-control studies identified that a partial vaccination provided considerable protection, but not to the same level as a full series.133,141 Several phase 3 RCTs also showed that RV1 and RV5 conferred early protection against RVGE before completion of a 2-dose or 3-dose schedule.11,142 This finding is encouraging so that numerous children who are partly vaccinated in LICs and children vaccinated during the periods of intensive rotavirus circulation can receive protection. Nonetheless, the protection of partial vaccination was lower than full vaccination, and the duration of protection from partial vaccination was not clear. Therefore, more efforts should be made to ensure full vaccination as recommended to achieve optimal protection.
The wide variety of rotavirus strain is a challenge for improving vaccine effectiveness. It is encouraging that RV1 and RV5 work well against heterotypic strains. The heterotypic protective immunity is important for low-income and lower middle–income countries, where greater strain diversity and concurrent circulation of several strains is a common phenomenon.143 However, prevalent rotavirus strain varied by time and region, and the dominance of 1 strain was often followed by the replacement with other strains.144 The changes of serotype distribution was also reported in some countries after vaccine introduction.145,146,147 Therefore, the characterization of rotavirus strains after vaccination should be monitored to avoid population-based selection of so-called escape strains, especially fully heterotypic strains and new strains, because of the long-term pressure of vaccine immunity.148
A clear gradient in rotavirus vaccine protection was noted by country income level, with the highest in high-income countries.11,33,48,53,149 Possible reasons for weaker vaccine protection in LICs include host characteristics, such as malnutrition150; environmental enteropathy151; concomitant enteric infections152; poor maternal health151; high titers of RV-specific maternal antibodies in breast milk153,154; and interference by coadministration of oral poliovirus vaccine.155 Besides, most children in LICs were not vaccinated as per the recommended schedule and subsequently received lower protection from partial vaccination. The lower-than-expected rotavirus protection in LICs can also be explained by high natural rotavirus infections before vaccination, which confer protection against subsequent RVGE and may cause a biased outcome.43,156,157 Furthermore, the scarcity of clean water can increase the risk of rotavirus spread by fecal-oral transmission in LICs. However, since the greater burden of severe RVGE in middle-income countries and LICs, the cases of severe RVGE prevented by rotavirus vaccines seem to be more in these settings. In addition, rotavirus vaccination was found to be cost-effective in LICs, suggesting a potential benefit of vaccination.158,159
Currently there are 6 rotavirus vaccines licensed in the market, but little was known about the interchangeability of these vaccines. Therefore, we performed adjusted indirect comparisons, which showed similar protection of RV1 and RV5, Rotavac, and Rotasiil, particularly at the same economic level or in the same country. This relieves the pressure of vaccine selection and suggests that health care authorities should weigh not only vaccine effectiveness but also economic factors associated with vaccine procurement and introduction, such as unit price, cold-chain volume, the cost of storage, and wastage. Cost-effectiveness models in Kenya and Palestine have indicated that 2-dose RV1 vaccinations seems to be more cost-effective and create less strain on a cold chain than 3-dose RV5 vaccinations.160,161 Additionally, the duration of high vaccine protection and the reduced vaccine protection resulted from partial vaccination should be taken into account. Furthermore, herd effects induced by rotavirus vaccination should be estimated to further compare the social benefits of different vaccines for children who are unvaccinated. It has been reported in Europe and the US that a herd effect of the rotavirus vaccine may enhance its clinical performance when implemented at a large scale under routine conditions.162,163 Considering the inherent limitations of indirect comparisons, a well-designed head-to-head study should be conducted to further compare the efficacy, cost-effectiveness, and strain-specific protection of different vaccines.
Limitations
There are several limitations of our meta-analysis. First, despite a systematic search of published studies, the final estimates were identified in only 45 settings, and an exhaustive review of gray literature was not included. Especially in stratified analyses, sparse data in some subgroups limit generalizability; for example, there was only 1 available study conducted in an LIC. But the number of children enrolled in our meta-analysis was more than 100 000, suggesting the value and reliability of our results. Second, considering that the introduction and protection of rotavirus vaccines vary by regions, it may prevent a fair comparison of RV1 and RV5 at a global level. So, we also performed indirect comparisons in the same region. The most accurate method, head-to-head comparisons, to evaluate the comparative efficacy of different vaccines is required in further studies. Third, the missing data and low quality in some included studies may influence our results, although we have excluded studies with small enrolled populations (<100 children). Well-designed observational trials and RCTs are still required to evaluate the clinical performance of rotavirus vaccines.
Conclusions
In conclusion, based on a large worldwide data set, we identified reasonable evidence of sustained high protection and low risk of adverse effects for rotavirus vaccines in children aged 2 years or younger, which is important to combat vaccine hesitancy. Also, the differences in vaccine performance between 4 licensed rotavirus vaccines were not surprising. Although the global introduction of rotavirus vaccines faces many scientific, programmatic, and financial challenges, these licensed vaccines hold promise to have immediate and measurable effectiveness to improve child health and survival from rotavirus disease. Our findings and prelicensing evidence reinforce the importance of optimizing uptake rates of rotavirus vaccines worldwide. Continued surveillance after vaccine introduction is also required to monitor the long-term changes in rotavirus incidence and the potential emergence of heterotypic strains.
eMethods. Methods and Statistical Analysis
eTable 1. Included Criteria During Selection Process
eTable 2. The Criteria for grading methodological quality
eTable 3. The basis for grouping in sub-analysis
eTable 4. Characteristics of the Included RCTs, Case-control and Cohort studies
eTable 5. Estimated Pooled Vaccine Protection in Cohort Studies
eTable 6. Estimated Pooled Vaccine Protection against Severe RVGE Stratified by Income Development at Different Follow-up Times
eTable 7. Estimated Pooled Vaccine Protection against Severe RVGE Stratified by Income Development
eTable 8. Estimated Pooled Vaccine Protection against Severe AGE Stratified by Income Development at Different Follow-up Times
eTable 9. Estimated Pooled Vaccine Protection against RVGE Stratified by Income Development at Different Follow-up Times
eTable 10. Estimated Pooled Vaccine Protection against RVGE Hospitalization Stratified by Income Development at Different Follow-up Times
eTable 11. Estimated Pooled Vaccine Protection against RVGE Stratified by Income Development
eTable 12. Estimated Pooled Vaccine Protection against RVGE Hospitalization stratified by income development
eTable 13. Indirect Comparisons between RV1 and RV5 Stratified by Income Development
eTable 14. Indirect Comparisons between Different Vaccines in the Same Region
eTable 15. Random-effect Model of Strain-specific Rotavirus Vaccine Protection
eFigure 1. Flow Diagram
eFigure 2. The Risk of Serious Adverse Events Between Vaccine and Placebo Groups at Different Follow-up Times
eFigure 3. The Risk of Intussusception Between Vaccine and Placebo Groups at Different Follow-up Times
eFigure 4. All-Cause Mortality in Vaccine and Placebo Groups During the Follow-up Period
eFigure 5. Sensitivity Analysis
eFigure 6. Funnel Plots
eReferences.
References
- 1.GBD 2016 Diarrhoeal Disease Collaborators . Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1211-1228. doi: 10.1016/S1473-3099(18)30362-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troeger C, Khalil IA, Rao PC, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018;172(10):958-965. doi: 10.1001/jamapediatrics.2018.1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaborators GDD; GBD Diarrhoeal Diseases Collaborators . Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17(9):909-948. doi: 10.1016/S1473-3099(17)30276-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peck M, Gacic-Dobo M, Diallo MS, Nedelec Y, Sodha SV, Wallace AS. Global routine vaccination coverage, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(42):937-942. Correction published in MMWR Morb Mortal Wkly Rep. 2019;68(44):1010. doi: 10.15585/mmwr.mm6842a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . Rotavirus vaccines: WHO position paper, January 2013. Wkly Epidemiol Rec. 2013;88(5):49-64. [PubMed] [Google Scholar]
- 6.World Health Organization . Immunization, vaccines and biologicals. Updated October 2020. Accessed February 26, 2019. https://www.who.int/immunization/diseases/rotavirus/en/
- 7.Collaborators GM; GBD 2016 Mortality Collaborators . Global, regional, and national under-5 mortality, adult mortality, age-specific mortality, and life expectancy, 1970-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1084-1150. doi: 10.1016/S0140-6736(17)31833-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernstein DI, Sack DA, Rothstein E, et al. Efficacy of live, attenuated, human rotavirus vaccine 89-12 in infants: a randomised placebo-controlled trial. Lancet. 1999;354(9175):287-290. doi: 10.1016/S0140-6736(98)12106-2 [DOI] [PubMed] [Google Scholar]
- 9.Dennehy PH, Brady RC, Halperin SA, et al. ; North American Human Rotavirus Vaccine Study Group . Comparative evaluation of safety and immunogenicity of two dosages of an oral live attenuated human rotavirus vaccine. Pediatr Infect Dis J. 2005;24(6):481-488. doi: 10.1097/01.inf.0000164763.55558.71 [DOI] [PubMed] [Google Scholar]
- 10.Grant LR, Watt JP, Weatherholtz RC, et al. Efficacy of a pentavalent human-bovine reassortant rotavirus vaccine against rotavirus gastroenteritis among American Indian children. Pediatr Infect Dis J. 2012;31(2):184-188. doi: 10.1097/INF.0b013e3182435afe [DOI] [PubMed] [Google Scholar]
- 11.Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370(9601):1757-1763. doi: 10.1016/S0140-6736(07)61744-9 [DOI] [PubMed] [Google Scholar]
- 12.Vesikari T, Karvonen A, Prymula R, et al. Immunogenicity and safety of the human rotavirus vaccine Rotarix co-administered with routine infant vaccines following the vaccination schedules in Europe. Vaccine. 2010;28(32):5272-5279. doi: 10.1016/j.vaccine.2010.05.057 [DOI] [PubMed] [Google Scholar]
- 13.Vesikari T, Itzler R, Karvonen A, et al. RotaTeq, a pentavalent rotavirus vaccine: efficacy and safety among infants in Europe. Vaccine. 2009;28(2):345-351. doi: 10.1016/j.vaccine.2009.10.041 [DOI] [PubMed] [Google Scholar]
- 14.Vesikari T, Karvonen A, Bouckenooghe A, Suryakiran PV, Smolenov I, Han HH. Immunogenicity, reactogenicity and safety of the human rotavirus vaccine RIX4414 oral suspension (liquid formulation) in Finnish infants. Vaccine. 2011;29(11):2079-2084. doi: 10.1016/j.vaccine.2011.01.004 [DOI] [PubMed] [Google Scholar]
- 15.Vesikari T, Clark HF, Offit PA, et al. Effects of the potency and composition of the multivalent human-bovine (WC3) reassortant rotavirus vaccine on efficacy, safety and immunogenicity in healthy infants. Vaccine. 2006;24(22):4821-4829. doi: 10.1016/j.vaccine.2006.03.025 [DOI] [PubMed] [Google Scholar]
- 16.Kawamura N, Tokoeda Y, Oshima M, et al. Efficacy, safety and immunogenicity of RIX4414 in Japanese infants during the first two years of life. Vaccine. 2011;29(37):6335-6341. doi: 10.1016/j.vaccine.2011.05.017 [DOI] [PubMed] [Google Scholar]
- 17.Iwata S, Nakata S, Ukae S, et al. Efficacy and safety of pentavalent rotavirus vaccine in Japan: a randomized, double-blind, placebo-controlled, multicenter trial. Hum Vaccin Immunother. 2013;9(8):1626-1633. doi: 10.4161/hv.24846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim DS, Lee TJ, Kang JH, et al. Immunogenicity and safety of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine in healthy infants in Korea. Pediatr Infect Dis J. 2008;27(2):177-178. doi: 10.1097/INF.0b013e31815aba79 [DOI] [PubMed] [Google Scholar]
- 19.Kim JS, Bae CW, Lee KY, et al. Immunogenicity, reactogenicity and safety of a human rotavirus vaccine (RIX4414) in Korean infants: a randomized, double-blind, placebo-controlled, phase IV study. Hum Vaccin Immunother. 2012;8(6):806-812. doi: 10.4161/hv.19853 [DOI] [PubMed] [Google Scholar]
- 20.Phua KB, Lim FS, Lau YL, et al. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double-blind, controlled study. Vaccine. 2009;27(43):5936-5941. doi: 10.1016/j.vaccine.2009.07.098 [DOI] [PubMed] [Google Scholar]
- 21.Phua KB, Lim FS, Lau YL, et al. Rotavirus vaccine RIX4414 efficacy sustained during the third year of life: a randomized clinical trial in an Asian population. Vaccine. 2012;30(30):4552-4557. doi: 10.1016/j.vaccine.2012.03.030 [DOI] [PubMed] [Google Scholar]
- 22.Phua KB, Lim FS, Quak SH, et al. Efficacy, immunogenicity and safety of a human rotavirus vaccine RIX4414 in Singaporean infants. Ann Acad Med Singap. 2016;45(2):44-50. [PubMed] [Google Scholar]
- 23.Phua KB, Quak SH, Lee BW, et al. Evaluation of RIX4414, a live, attenuated rotavirus vaccine, in a randomized, double-blind, placebo-controlled phase 2 trial involving 2464 Singaporean infants. J Infect Dis. 2005;192(suppl 1):S6-S16. doi: 10.1086/431511 [DOI] [PubMed] [Google Scholar]
- 24.Li RC, Huang T, Li Y, et al. Human rotavirus vaccine (RIX4414) efficacy in the first two years of life: a randomized, placebo-controlled trial in China. Hum Vaccin Immunother. 2014;10(1):11-18. doi: 10.4161/hv.26319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li RC, Huang T, Li Y, et al. Immunogenicity and reactogenicity of the human rotavirus vaccine, RIX4414 oral suspension, when co-administered with routine childhood vaccines in Chinese infants. Hum Vaccin Immunother. 2016;12(3):785-793. doi: 10.1080/21645515.2015.1085143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lau Y-L, Nelson EAS, Poon K-H, et al. ; Hong Kong Rotarix Study Group . Efficacy, safety and immunogenicity of a human rotavirus vaccine (RIX4414) in Hong Kong children up to three years of age: a randomized, controlled trial. Vaccine. 2013;31(18):2253-2259. doi: 10.1016/j.vaccine.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 27.Mo Z, Mo Y, Li M, et al. Efficacy and safety of a pentavalent live human-bovine reassortant rotavirus vaccine (RV5) in healthy Chinese infants: a randomized, double-blind, placebo-controlled trial. Vaccine. 2017;35(43):5897-5904. doi: 10.1016/j.vaccine.2017.08.081 [DOI] [PubMed] [Google Scholar]
- 28.Mo Z, Ma X, Luo P, et al. ; V260-024 Study Group . Immunogenicity of pentavalent rotavirus vaccine in Chinese infants. Vaccine. 2019;37(13):1836-1843. doi: 10.1016/j.vaccine.2019.02.018 [DOI] [PubMed] [Google Scholar]
- 29.Chang CC, Chang MH, Lin TY, Lee HC, Hsieh WS, Lee PI. Experience of pentavalent human-bovine reassortant rotavirus vaccine among healthy infants in Taiwan. J Formos Med Assoc. 2009;108(4):280-285. doi: 10.1016/S0929-6646(09)60067-X [DOI] [PubMed] [Google Scholar]
- 30.Xia S, Du J, Su J, et al. Efficacy, immunogenicity and safety of a trivalent live human-lamb reassortant rotavirus vaccine (LLR3) in healthy Chinese infants: a randomized, double-blind, placebo-controlled trial. Vaccine. 2020;38(46):7393-7400. doi: 10.1016/j.vaccine.2020.04.038 [DOI] [PubMed] [Google Scholar]
- 31.Colgate ER, Haque R, Dickson DM, et al. Delayed dosing of oral rotavirus vaccine demonstrates decreased risk of rotavirus gastroenteritis associated with serum zinc: a randomized controlled trial. Clin Infect Dis. 2016;63(5):634-641. doi: 10.1093/cid/ciw346 [DOI] [PubMed] [Google Scholar]
- 32.Zaman K, Sack DA, Yunus M, et al. ; Bangladeshi Rotavirus Vaccine study group . Successful co-administration of a human rotavirus and oral poliovirus vaccines in Bangladeshi infants in a 2-dose schedule at 12 and 16 weeks of age. Vaccine. 2009;27(9):1333-1339. doi: 10.1016/j.vaccine.2008.12.059 [DOI] [PubMed] [Google Scholar]
- 33.Zaman K, Dang DA, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):615-623. doi: 10.1016/S0140-6736(10)60755-6 [DOI] [PubMed] [Google Scholar]
- 34.Anh DD, Carlos CC, Thiem DV, et al. Immunogenicity, reactogenicity and safety of the human rotavirus vaccine RIX4414 (Rotarix™) oral suspension (liquid formulation) when co-administered with expanded program on immunization (EPI) vaccines in Vietnam and the Philippines in 2006-2007. Vaccine. 2011;29(11):2029-2036. doi: 10.1016/j.vaccine.2011.01.018 [DOI] [PubMed] [Google Scholar]
- 35.Narang A, Bose A, Pandit AN, et al. Immunogenicity, reactogenicity and safety of human rotavirus vaccine (RIX4414) in Indian infants. Hum Vaccin. 2009;5(6):414-419. doi: 10.4161/hv.5.6.8176 [DOI] [PubMed] [Google Scholar]
- 36.Bhandari N, Sharma P, Taneja S, et al. ; Rotavirus Vaccine Development Group . A dose-escalation safety and immunogenicity study of live attenuated oral rotavirus vaccine 116E in infants: a randomized, double-blind, placebo-controlled trial. J Infect Dis. 2009;200(3):421-429. doi: 10.1086/600104 [DOI] [PubMed] [Google Scholar]
- 37.John J, Kawade A, Rongsen-Chandola T, et al. Active surveillance for intussusception in a phase III efficacy trial of an oral monovalent rotavirus vaccine in India. Vaccine. 2014;32(suppl 1):A104-A109. doi: 10.1016/j.vaccine.2014.03.036 [DOI] [PubMed] [Google Scholar]
- 38.Bhandari N, Rongsen-Chandola T, Bavdekar A, et al. ; India Rotavirus Vaccine Group . Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial. Lancet. 2014;383(9935):2136-2143. doi: 10.1016/S0140-6736(13)62630-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kulkarni PS, Desai S, Tewari T, et al. ; SII BRV-PV author group . A randomized phase III clinical trial to assess the efficacy of a bovine-human reassortant pentavalent rotavirus vaccine in Indian infants. Vaccine. 2017;35(45):6228-6237. doi: 10.1016/j.vaccine.2017.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tregnaghi MW, Abate HJ, Valencia A, et al. ; Rota-024 Study Group . Human rotavirus vaccine is highly efficacious when coadministered with routine expanded program of immunization vaccines including oral poliovirus vaccine in Latin America. Pediatr Infect Dis J. 2011;30(6):e103-e108. doi: 10.1097/INF.0b013e3182138278 [DOI] [PubMed] [Google Scholar]
- 41.Linhares AC, Velázquez FR, Pérez-Schael I, et al. ; Human Rotavirus Vaccine Study Group . Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371(9619):1181-1189. doi: 10.1016/S0140-6736(08)60524-3 [DOI] [PubMed] [Google Scholar]
- 42.Salinas B, Pérez Schael I, Linhares AC, et al. Evaluation of safety, immunogenicity and efficacy of an attenuated rotavirus vaccine, RIX4414: a randomized, placebo-controlled trial in Latin American infants. Pediatr Infect Dis J. 2005;24(9):807-816. doi: 10.1097/01.inf.0000178294.13954.a1 [DOI] [PubMed] [Google Scholar]
- 43.Ruiz-Palacios GM, Guerrero ML, Bautista-Márquez A, et al. Dose response and efficacy of a live, attenuated human rotavirus vaccine in Mexican infants. Pediatrics. 2007;120(2):e253-e261. doi: 10.1542/peds.2006-2630 [DOI] [PubMed] [Google Scholar]
- 44.Justino MCA, Araújo EC, van Doorn L-J, et al. Oral live attenuated human rotavirus vaccine (Rotarix™) offers sustained high protection against severe G9P[8] rotavirus gastroenteritis during the first two years of life in Brazilian children. Mem Inst Oswaldo Cruz. 2012;107(7):846-853. doi: 10.1590/S0074-02762012000700002 [DOI] [PubMed] [Google Scholar]
- 45.Christie CDC, Duncan ND, Thame KA, et al. Pentavalent rotavirus vaccine in developing countries: safety and health care resource utilization. Pediatrics. 2010;126(6):e1499-e1506. doi: 10.1542/peds.2010-1240 [DOI] [PubMed] [Google Scholar]
- 46.Isanaka S, Guindo O, Langendorf C, et al. Efficacy of a low-cost, heat-stable oral rotavirus vaccine in Niger. N Engl J Med. 2017;376(12):1121-1130. doi: 10.1056/NEJMoa1609462 [DOI] [PubMed] [Google Scholar]
- 47.Coldiron ME, Guindo O, Makarimi R, et al. Safety of a heat-stable rotavirus vaccine among children in Niger: data from a phase 3, randomized, double-blind, placebo-controlled trial. Vaccine. 2018;36(25):3674-3680. doi: 10.1016/j.vaccine.2018.05.023 [DOI] [PubMed] [Google Scholar]
- 48.Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362(4):289-298. doi: 10.1056/NEJMoa0904797 [DOI] [PubMed] [Google Scholar]
- 49.Steele AD, Neuzil KM, Cunliffe NA, et al. Human rotavirus vaccine Rotarix™ provides protection against diverse circulating rotavirus strains in African infants: a randomized controlled trial. BMC Infect Dis. 2012;12:213. doi: 10.1186/1471-2334-12-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steele AD, Reynders J, Scholtz F, et al. Comparison of 2 different regimens for reactogenicity, safety, and immunogenicity of the live attenuated oral rotavirus vaccine RIX4414 coadministered with oral polio vaccine in South African infants. J Infect Dis. 2010;202(suppl):S93-S100. doi: 10.1086/653550 [DOI] [PubMed] [Google Scholar]
- 51.Cunliffe NA, Witte D, Ngwira BM, et al. Efficacy of human rotavirus vaccine against severe gastroenteritis in Malawian children in the first two years of life: a randomized, double-blind, placebo controlled trial. Vaccine. 2012;30(suppl 1):A36-A43. doi: 10.1016/j.vaccine.2011.09.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rojas OL, Caicedo L, Guzmán C, et al. Evaluation of circulating intestinally committed memory B cells in children vaccinated with attenuated human rotavirus vaccine. Viral Immunol. 2007;20(2):300-311. doi: 10.1089/vim.2006.0105 [DOI] [PubMed] [Google Scholar]
- 53.Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):606-614. doi: 10.1016/S0140-6736(10)60889-6 [DOI] [PubMed] [Google Scholar]
- 54.Tapia MD, Armah G, Breiman RF, et al. Secondary efficacy endpoints of the pentavalent rotavirus vaccine against gastroenteritis in sub-Saharan Africa. Vaccine. 2012;30(suppl 1):A79-A85. doi: 10.1016/j.vaccine.2012.01.022 [DOI] [PubMed] [Google Scholar]
- 55.Sow SO, Tapia M, Haidara FC, et al. Efficacy of the oral pentavalent rotavirus vaccine in Mali. Vaccine. 2012;30(suppl 1):A71-A78. doi: 10.1016/j.vaccine.2011.11.094 [DOI] [PubMed] [Google Scholar]
- 56.Laserson KF, Nyakundi D, Feikin DR, et al. Safety of the pentavalent rotavirus vaccine (PRV), RotaTeq(®), in Kenya, including among HIV-infected and HIV-exposed infants. Vaccine. 2012;30(suppl 1):A61-A70. doi: 10.1016/j.vaccine.2011.09.026 [DOI] [PubMed] [Google Scholar]
- 57.Georges-Courbot MC, Monges J, Siopathis MR, et al. Evaluation of the efficacy of a low-passage bovine rotavirus (strain WC3) vaccine in children in Central Africa. Res Virol. 1991;142(5):405-411. doi: 10.1016/0923-2516(91)90008-Q [DOI] [PubMed] [Google Scholar]
- 58.Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. ; Human Rotavirus Vaccine Study Group . Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11-22. doi: 10.1056/NEJMoa052434 [DOI] [PubMed] [Google Scholar]
- 59.Breiman RF, Zaman K, Armah G, et al. Analyses of health outcomes from the 5 sites participating in the Africa and Asia clinical efficacy trials of the oral pentavalent rotavirus vaccine. Vaccine. 2012;30(suppl 1):A24-A29. doi: 10.1016/j.vaccine.2011.08.124 [DOI] [PubMed] [Google Scholar]
- 60.Block SL, Vesikari T, Goveia MG, et al. ; Pentavalent Rotavirus Vaccine Dose Confirmation Efficacy Study Group . Efficacy, immunogenicity, and safety of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine at the end of shelf life. Pediatrics. 2007;119(1):11-18. doi: 10.1542/peds.2006-2058 [DOI] [PubMed] [Google Scholar]
- 61.Vesikari T, Matson DO, Dennehy P, et al. ; Rotavirus Efficacy and Safety Trial (REST) Study Team . Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23-33. doi: 10.1056/NEJMoa052664 [DOI] [PubMed] [Google Scholar]
- 62.Goveia MG, DiNubile MJ, Dallas MJ, Heaton PM, Kuter BJ; REST Study Team . Efficacy of pentavalent human-bovine (WC3) reassortant rotavirus vaccine based on breastfeeding frequency. Pediatr Infect Dis J. 2008;27(7):656-658. doi: 10.1097/INF.0b013e318168d29e [DOI] [PubMed] [Google Scholar]
- 63.Dennehy PH, Goveia MG, Dallas MJ, Heaton PM. The integrated phase III safety profile of the pentavalent human-bovine (WC3) reassortant rotavirus vaccine. Int J Infect Dis. 2007;11(suppl 2):S36-S42. doi: 10.1016/S1201-9712(07)60020-4 [DOI] [PubMed] [Google Scholar]
- 64.Vesikari T, Itzler R, Matson DO, et al. Efficacy of a pentavalent rotavirus vaccine in reducing rotavirus-associated health care utilization across three regions (11 countries). Int J Infect Dis. 2007;11(suppl 2):S29-S35. doi: 10.1016/S1201-9712(07)60019-8 [DOI] [PubMed] [Google Scholar]
- 65.Cortese MM, Immergluck LC, Held M, et al. Effectiveness of monovalent and pentavalent rotavirus vaccine. Pediatrics. 2013;132(1):e25-e33. doi: 10.1542/peds.2012-3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Immergluck LC, Parker TC, Jain S, et al. Sustained effectiveness of monovalent and pentavalent rotavirus vaccines in children. J Pediatr. 2016;172:116-120.e1. doi: 10.1016/j.jpeds.2016.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Staat MA, Payne DC, Donauer S, et al. ; New Vaccine Surveillance Network (NVSN) . Effectiveness of pentavalent rotavirus vaccine against severe disease. Pediatrics. 2011;128(2):e267-e275. doi: 10.1542/peds.2010-3722 [DOI] [PubMed] [Google Scholar]
- 68.Tate JE, Mijatovic-Rustempasic S, Tam KI, et al. Comparison of 2 assays for diagnosing rotavirus and evaluating vaccine effectiveness in children with gastroenteritis. Emerg Infect Dis. 2013;19(8):1245-1252. doi: 10.3201/eid1908.130461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Donauer S, Payne DC, Edwards KM, et al. Determining the effectiveness of the pentavalent rotavirus vaccine against rotavirus hospitalizations and emergency department visits using two study designs. Vaccine. 2013;31(24):2692-2697. doi: 10.1016/j.vaccine.2013.03.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boom JA, Tate JE, Sahni LC, et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics. 2010;125(2):e199-e207. doi: 10.1542/peds.2009-1021 [DOI] [PubMed] [Google Scholar]
- 71.Cortese MM, Leblanc J, White KE, et al. Leveraging state immunization information systems to measure the effectiveness of rotavirus vaccine. Pediatrics. 2011;128(6):e1474-e1481. doi: 10.1542/peds.2011-1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Doll MK, Buckeridge DL, Morrison KT, et al. Effectiveness of monovalent rotavirus vaccine in a high-income, predominant-use setting. Vaccine. 2015;33(51):7307-7314. doi: 10.1016/j.vaccine.2015.10.118 [DOI] [PubMed] [Google Scholar]
- 73.de Palma O, Cruz L, Ramos H, et al. Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: case-control study. BMJ. 2010;340:c2825. doi: 10.1136/bmj.c2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Braeckman T, Van Herck K, Meyer N, et al. ; RotaBel Study Group . Effectiveness of rotavirus vaccination in prevention of hospital admissions for rotavirus gastroenteritis among young children in Belgium: case-control study. BMJ. 2012;345:e4752. doi: 10.1136/bmj.e4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matthijnssens J, Zeller M, Heylen E, et al. ; RotaBel study group . Higher proportion of G2P[4] rotaviruses in vaccinated hospitalized cases compared with unvaccinated hospitalized cases, despite high vaccine effectiveness against heterotypic G2P[4] rotaviruses. Clin Microbiol Infect. 2014;20(10):O702-O710. doi: 10.1111/1469-0691.12612 [DOI] [PubMed] [Google Scholar]
- 76.Castilla J, Beristain X, Martínez-Artola V, et al. Effectiveness of rotavirus vaccines in preventing cases and hospitalizations due to rotavirus gastroenteritis in Navarre, Spain. Vaccine. 2012;30(3):539-543. doi: 10.1016/j.vaccine.2011.11.071 [DOI] [PubMed] [Google Scholar]
- 77.Walker JL, Andrews NJ, Atchison CJ, et al. Effectiveness of oral rotavirus vaccination in England against rotavirus-confirmed and all-cause acute gastroenteritis. Vaccine X. 2019;1:100005. doi: 10.1016/j.jvacx.2019.100005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oberle D, Hoffelner M, Pavel J, et al. Retrospective multicenter matched case-control study on the risk factors for intussusception in infants less than 1 year of age with a special focus on rotavirus vaccines—the German Intussusception Study. Hum Vaccin Immunother. 2020;16(10):2481-2494. doi: 10.1080/21645515.2020.1726679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gheorghita S, Birca L, Donos A, et al. Impact of rotavirus vaccine introduction and vaccine effectiveness in the Republic of Moldova. Clin Infect Dis. 2016;62(suppl 2):S140-S146. doi: 10.1093/cid/civ1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Snelling TL, Schultz R, Graham J, et al. Rotavirus and the indigenous children of the Australian outback: monovalent vaccine effective in a high-burden setting. Clin Infect Dis. 2009;49(3):428-431. doi: 10.1086/600395 [DOI] [PubMed] [Google Scholar]
- 81.Carlin JB, Macartney KK, Lee KJ, et al. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia’s National Immunization Program. Clin Infect Dis. 2013;57(10):1427-1434. doi: 10.1093/cid/cit520 [DOI] [PubMed] [Google Scholar]
- 82.Maguire JE, Glasgow K, Glass K, et al. Rotavirus epidemiology and monovalent rotavirus vaccine effectiveness in Australia: 2010-2017. Pediatrics. 2019;144(4):e20191024. doi: 10.1542/peds.2019-1024 [DOI] [PubMed] [Google Scholar]
- 83.Araki K, Hara M, Tsugawa T, et al. Effectiveness of monovalent and pentavalent rotavirus vaccines in Japanese children. Vaccine. 2018;36(34):5187-5193. doi: 10.1016/j.vaccine.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 84.Araki K, Hara M, Shimanoe C, Nishida Y, Matsuo M, Tanaka K. Case-control study of rotavirus vaccine effectiveness compared to test-negative controls or hospital controls. J Epidemiol. 2019;29(8):282-287. doi: 10.2188/jea.JE20180054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lopez AL, Daag JV, Esparagoza J, et al. Effectiveness of monovalent rotavirus vaccine in the Philippines. Sci Rep. 2018;8(1):14291. doi: 10.1038/s41598-018-32595-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Muhsen K, Anis E, Rubinstein U, et al. Effectiveness of rotavirus pentavalent vaccine under a universal immunization programme in Israel, 2011-2015: a case-control study. Clin Microbiol Infect. 2018;24(1):53-59. doi: 10.1016/j.cmi.2017.04.018 [DOI] [PubMed] [Google Scholar]
- 87.Eraliev U, Latipov R, Tursunova D, et al. Rotavirus vaccine effectiveness and impact in Uzbekistan, the first country to introduce in central Asia. Hum Vaccin Immunother. 2021;17(2):503-509. doi: 10.1080/21645515.2020.1776034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang WC, Yen C, Wu FT, et al. Effectiveness of 2 rotavirus vaccines against rotavirus disease in Taiwanese infants. Pediatr Infect Dis J. 2014;33(3):e81-e86. doi: 10.1097/INF.0000000000000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fu C, Wang M, Liang J, He T, Wang D, Xu J. Effectiveness of Lanzhou lamb rotavirus vaccine against rotavirus gastroenteritis requiring hospitalization: a matched case-control study. Vaccine. 2007;25(52):8756-8761. doi: 10.1016/j.vaccine.2007.10.036 [DOI] [PubMed] [Google Scholar]
- 90.Fu C, He Q, Xu J, et al. Effectiveness of the Lanzhou lamb rotavirus vaccine against gastroenteritis among children. Vaccine. 2012;31(1):154-158. doi: 10.1016/j.vaccine.2012.10.078 [DOI] [PubMed] [Google Scholar]
- 91.Zhen SS, Li Y, Wang SM, et al. Effectiveness of the live attenuated rotavirus vaccine produced by a domestic manufacturer in China studied using a population-based case-control design. Emerg Microbes Infect. 2015;4(10):e64. doi: 10.1038/emi.2015.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li J, Zhang Y, Yang Y, et al. Effectiveness of Lanzhou lamb rotavirus vaccine in preventing gastroenteritis among children younger than 5 years of age. Vaccine. 2019;37(27):3611-3616. doi: 10.1016/j.vaccine.2019.03.069 [DOI] [PubMed] [Google Scholar]
- 93.Patel M, Pedreira C, De Oliveira LH, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA. 2009;301(21):2243-2251. doi: 10.1001/jama.2009.756 [DOI] [PubMed] [Google Scholar]
- 94.Mast TC, Khawaja S, Espinoza F, et al. Case-control study of the effectiveness of vaccination with pentavalent rotavirus vaccine in Nicaragua. Pediatr Infect Dis J. 2011;30(11):e209-e215. doi: 10.1097/INF.0b013e31822a8527 [DOI] [PubMed] [Google Scholar]
- 95.Patel M, Pedreira C, De Oliveira LH, et al. Duration of protection of pentavalent rotavirus vaccination in Nicaragua. Pediatrics. 2012;130(2):e365-e372. doi: 10.1542/peds.2011-3478 [DOI] [PubMed] [Google Scholar]
- 96.Gastañaduy PA, Contreras-Roldán I, Bernart C, et al. Effectiveness of monovalent and pentavalent rotavirus vaccines in Guatemala. Clin Infect Dis. 2016;62(suppl 2):S121-S126. doi: 10.1093/cid/civ1208 [DOI] [PubMed] [Google Scholar]
- 97.Correia JB, Patel MM, Nakagomi O, et al. Effectiveness of monovalent rotavirus vaccine (Rotarix) against severe diarrhea caused by serotypically unrelated G2P[4] strains in Brazil. J Infect Dis. 2010;201(3):363-369. doi: 10.1086/649843 [DOI] [PubMed] [Google Scholar]
- 98.Patel MM, Patzi M, Pastor D, et al. Effectiveness of monovalent rotavirus vaccine in Bolivia: case-control study. BMJ. 2013;346:f3726. doi: 10.1136/bmj.f3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ichihara MYT, Rodrigues LC, Teles Santos CAS, et al. Effectiveness of rotavirus vaccine against hospitalized rotavirus diarrhea: a case-control study. Vaccine. 2014;32(23):2740-2747. doi: 10.1016/j.vaccine.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 100.Justino MCA, Linhares AC, Lanzieri TM, et al. Effectiveness of the monovalent G1P[8] human rotavirus vaccine against hospitalization for severe G2P[4] rotavirus gastroenteritis in Belém, Brazil. Pediatr Infect Dis J. 2011;30(5):396-401. doi: 10.1097/INF.0b013e3182055cc2 [DOI] [PubMed] [Google Scholar]
- 101.Patel MM, López-Collada VR, Bulhões MM, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011;364(24):2283-2292. doi: 10.1056/NEJMoa1012952 [DOI] [PubMed] [Google Scholar]
- 102.Pringle KD, Patzi M, Tate JE, et al. Sustained effectiveness of rotavirus vaccine against very severe rotavirus disease through the second year of life, Bolivia 2013-2014. Clin Infect Dis. 2016;62(suppl 2):S115-S120. doi: 10.1093/cid/civ1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cotes-Cantillo K, Paternina-Caicedo A, Coronell-Rodríguez W, et al. Effectiveness of the monovalent rotavirus vaccine in Colombia: a case-control study. Vaccine. 2014;32(25):3035-3040. doi: 10.1016/j.vaccine.2014.03.064 [DOI] [PubMed] [Google Scholar]
- 104.Beres LK, Tate JE, Njobvu L, et al. A preliminary assessment of rotavirus vaccine effectiveness in Zambia. Clin Infect Dis. 2016;62(suppl 2):S175-S182. doi: 10.1093/cid/civ1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bar-Zeev N, Kapanda L, Tate JE, et al. ; VacSurv Consortium . Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: an observational and case-control study. Lancet Infect Dis. 2015;15(4):422-428. doi: 10.1016/S1473-3099(14)71060-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Groome MJ, Page N, Cortese MM, et al. Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhoea in South African children: a case-control study. Lancet Infect Dis. 2014;14(11):1096-1104. doi: 10.1016/S1473-3099(14)70940-5 [DOI] [PubMed] [Google Scholar]
- 107.Groome MJ, Tate JE, Arnold M, et al. Evaluation of intussusception after oral monovalent rotavirus vaccination in South Africa. Clin Infect Dis. 2020;70(8):1606-1612. doi: 10.1093/cid/ciz431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gastañaduy PA, Steenhoff AP, Mokomane M, et al. Effectiveness of monovalent rotavirus vaccine after programmatic implementation in Botswana: a multisite prospective case-control study. Clin Infect Dis. 2016;62(suppl 2):S161-S167. doi: 10.1093/cid/civ1207 [DOI] [PubMed] [Google Scholar]
- 109.Mokomane M, Tate JE, Steenhoff AP, et al. Evaluation of the influence of gastrointestinal coinfections on rotavirus vaccine effectiveness in Botswana. Pediatr Infect Dis J. 2018;37(3):e58-e62. doi: 10.1097/INF.0000000000001828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Armah G, Pringle K, Enweronu-Laryea CC, et al. Impact and effectiveness of monovalent rotavirus vaccine against severe rotavirus diarrhea in Ghana. Clin Infect Dis. 2016;62(suppl 2):S200-S207. doi: 10.1093/cid/ciw014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bonkoungou IJO, Aliabadi N, Leshem E, et al. Impact and effectiveness of pentavalent rotavirus vaccine in children <5 years of age in Burkina Faso. Vaccine. 2018;36(47):7170-7178. doi: 10.1016/j.vaccine.2017.12.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tate JE, Ngabo F, Donnen P, et al. Effectiveness of pentavalent rotavirus vaccine under conditions of routine use in Rwanda. Clin Infect Dis. 2016;62(suppl 2):S208-S212. doi: 10.1093/cid/civ1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mujuru HA, Burnett E, Nathoo KJ, et al. Monovalent rotavirus vaccine effectiveness against rotavirus hospitalizations among children in Zimbabwe. Clin Infect Dis. 2019;69(8):1339-1344. doi: 10.1093/cid/ciy1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khagayi S, Omore R, Otieno GP, et al. Effectiveness of monovalent rotavirus vaccine against hospitalization with acute rotavirus gastroenteritis in Kenyan children. Clin Infect Dis. 2020;70(11):2298-2305. doi: 10.1093/cid/ciz664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang FT, Mast TC, Glass RJ, Loughlin J, Seeger JD. Effectiveness of the pentavalent rotavirus vaccine in preventing gastroenteritis in the United States. Pediatrics. 2010;125(2):e208-e213. doi: 10.1542/peds.2009-1246 [DOI] [PubMed] [Google Scholar]
- 116.Wang FT, Mast TC, Glass RJ, Loughlin J, Seeger JD. Effectiveness of an incomplete RotaTeq (RV5) vaccination regimen in preventing rotavirus gastroenteritis in the United States. Pediatr Infect Dis J. 2013;32(3):278-283. doi: 10.1097/INF.0b013e318275328f [DOI] [PubMed] [Google Scholar]
- 117.Shui IM, Baggs J, Patel M, et al. Risk of intussusception following administration of a pentavalent rotavirus vaccine in US infants. JAMA. 2012;307(6):598-604. doi: 10.1001/jama.2012.97 [DOI] [PubMed] [Google Scholar]
- 118.Krishnarajah G, Kageleiry A, Korves C, Lefebvre P, Duh MS. Public health impact of Rotarix vaccination among commercially insured children in the United States. Vaccine. 2017;35(37):5065-5072. doi: 10.1016/j.vaccine.2017.06.034 [DOI] [PubMed] [Google Scholar]
- 119.Hoffman V, Abu-Elyazeed R, Enger C, et al. Safety study of live, oral human rotavirus vaccine: a cohort study in United States health insurance plans. Hum Vaccin Immunother. 2018;14(7):1782-1790. doi: 10.1080/21645515.2018.1450123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gosselin V, Généreux M, Gagneur A, Petit G. Effectiveness of rotavirus vaccine in preventing severe gastroenteritis in young children according to socioeconomic status. Hum Vaccin Immunother. 2016;12(10):2572-2579. doi: 10.1080/21645515.2016.1189038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mrozek-Budzyn D, Kieltyka A, Majewska R, Augustyniak M. The effectiveness of rotavirus vaccine in preventing acute gastroenteritis during rotavirus seasons among Polish children. Arch Med Sci. 2016;12(3):614-620. doi: 10.5114/aoms.2016.59935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pérez-Vilar S, Díez-Domingo J, López-Lacort M, Martínez-Úbeda S, Martinez-Beneito MA. Effectiveness of rotavirus vaccines, licensed but not funded, against rotavirus hospitalizations in the Valencia region, Spain. BMC Infect Dis. 2015;15:92. doi: 10.1186/s12879-015-0811-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gagneur A, Nowak E, Lemaitre T, et al. ; IVANHOE investigators . Impact of rotavirus vaccination on hospitalizations for rotavirus diarrhea: the IVANHOE study. Vaccine. 2011;29(21):3753-3759. doi: 10.1016/j.vaccine.2011.03.035 [DOI] [PubMed] [Google Scholar]
- 124.Muhsen K, Chodick G, Goren S, Shalev V, Cohen D. The uptake of rotavirus vaccine and its effectiveness in preventing acute gastroenteritis in the community. Vaccine. 2010;29(1):91-94. doi: 10.1016/j.vaccine.2010.10.010 [DOI] [PubMed] [Google Scholar]
- 125.Zaman K, Sack DA, Neuzil KM, et al. Effectiveness of a live oral human rotavirus vaccine after programmatic introduction in Bangladesh: a cluster-randomized trial. PLoS Med. 2017;14(4):e1002282. doi: 10.1371/journal.pmed.1002282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tharmaphornpilas P, Jiamsiri S, Boonchaiya S, et al. Evaluating the first introduction of rotavirus vaccine in Thailand: moving from evidence to policy. Vaccine. 2017;35(5):796-801. doi: 10.1016/j.vaccine.2016.12.043 [DOI] [PubMed] [Google Scholar]
- 127.Vieira SCF, Gurgel RQ, Kirby A, et al. Acute diarrhoea in a community cohort of children who received an oral rotavirus vaccine in Northeast Brazil. Mem Inst Oswaldo Cruz. 2011;106(3):330-334. doi: 10.1590/S0074-02762011000300012 [DOI] [PubMed] [Google Scholar]
- 128.Bar-Zeev N, King C, Phiri T, et al. ; VacSurv Consortium . Impact of monovalent rotavirus vaccine on diarrhoea-associated post-neonatal infant mortality in rural communities in Malawi: a population-based birth cohort study. Lancet Glob Health. 2018;6(9):e1036-e1044. doi: 10.1016/S2214-109X(18)30314-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Leshem E, Lopman B, Glass R, et al. Distribution of rotavirus strains and strain-specific effectiveness of the rotavirus vaccine after its introduction: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(9):847-856. doi: 10.1016/S1473-3099(14)70832-1 [DOI] [PubMed] [Google Scholar]
- 130.Grimwood K, Lund JC, Coulson BS, Hudson IL, Bishop RF, Barnes GL. Comparison of serum and mucosal antibody responses following severe acute rotavirus gastroenteritis in young children. J Clin Microbiol. 1988;26(4):732-738. doi: 10.1128/JCM.26.4.732-738.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Velázquez FR, Matson DO, Guerrero ML, et al. Serum antibody as a marker of protection against natural rotavirus infection and disease. J Infect Dis. 2000;182(6):1602-1609. doi: 10.1086/317619 [DOI] [PubMed] [Google Scholar]
- 132.Soares-Weiser K, Bergman H, Henschke N, Pitan F, Cunliffe N. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev. 2019;2019(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hungerford D, Smith K, Tucker A, et al. Population effectiveness of the pentavalent and monovalent rotavirus vaccines: a systematic review and meta-analysis of observational studies. BMC Infect Dis. 2017;17(1):569. doi: 10.1186/s12879-017-2613-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lamberti LM, Ashraf S, Walker CL, Black RE. A systematic review of the effect of rotavirus vaccination on diarrhea outcomes among children younger than 5 Years. Pediatr Infect Dis J. 2016;35(9):992-998. doi: 10.1097/INF.0000000000001232 [DOI] [PubMed] [Google Scholar]
- 135.Burnett E, Parashar UD, Tate JE. Global impact of rotavirus vaccination on diarrhea hospitalizations and deaths among children <5 years old: 2006-2019. J Infect Dis. 2020;222(10):1731-1739. doi: 10.1093/infdis/jiaa081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lu HL, Ding Y, Goyal H, Xu HG. Association between rotavirus vaccination and risk of intussusception among neonates and infants: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(10):e1912458. doi: 10.1001/jamanetworkopen.2019.12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.WHO . Global advisory committee on vaccine safety, 11–12 December 2013. wkly epidemiol rec. 2014;89(7):53-60. [PubMed] [Google Scholar]
- 138.Carlin JB, Macartney KK, Lee KJ, et al. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia’s National Immunization Program. Clin Infect Dis. 2013;57(10):1427-1434. doi: 10.1093/cid/cit520 [DOI] [PubMed] [Google Scholar]
- 139.Hasso-Agopsowicz M, Ladva CN, Lopman B, et al. ; Global Rotavirus Surveillance Network and Rotavirus Age Study Collaborators . Global review of the age distribution of rotavirus disease in children aged <5 years before the introduction of rotavirus vaccination. Clin Infect Dis. 2019;69(6):1071-1078. doi: 10.1093/cid/ciz060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Clark A, van Zandvoort K, Flasche S, et al. Efficacy of live oral rotavirus vaccines by duration of follow-up: a meta-regression of randomised controlled trials. Lancet Infect Dis. 2019;19(7):717-727. doi: 10.1016/S1473-3099(19)30126-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jonesteller CL, Burnett E, Yen C, Tate JE, Parashar UD. Effectiveness of rotavirus vaccination: a systematic review of the first decade of global postlicensure data, 2006-2016. Clin Infect Dis. 2017;65(5):840-850. doi: 10.1093/cid/cix369 [DOI] [PubMed] [Google Scholar]
- 142.Dennehy PH, Vesikari T, Matson DO, et al. Efficacy of the pentavalent rotavirus vaccine, RotaTeq® (RV5), between doses of a 3-dose series and with less than 3 doses (incomplete regimen). Hum Vaccin. 2011;7(5):563-568. doi: 10.4161/hv.7.5.15406 [DOI] [PubMed] [Google Scholar]
- 143.Todd S, Page NA, Duncan Steele A, Peenze I, Cunliffe NA. Rotavirus strain types circulating in Africa: review of studies published during 1997-2006. J Infect Dis. 2010;202(suppl):S34-S42. doi: 10.1086/653555 [DOI] [PubMed] [Google Scholar]
- 144.Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol. 2005;15(1):29-56. doi: 10.1002/rmv.448 [DOI] [PubMed] [Google Scholar]
- 145.Nakagomi T, Cuevas LE, Gurgel RG, et al. Apparent extinction of non-G2 rotavirus strains from circulation in Recife, Brazil, after the introduction of rotavirus vaccine. Arch Virol. 2008;153(3):591-593. doi: 10.1007/s00705-007-0028-z [DOI] [PubMed] [Google Scholar]
- 146.Carvalho-Costa FA, Araújo IT, Santos de Assis RM, et al. Rotavirus genotype distribution after vaccine introduction, Rio de Janeiro, Brazil. Emerg Infect Dis. 2009;15(1):95-97. doi: 10.3201/eid1501.071136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hungerford D, Allen DJ, Nawaz S, et al. Impact of rotavirus vaccination on rotavirus genotype distribution and diversity in England, September 2006 to August 2016. Euro Surveill. 2019;24(6). doi: 10.2807/1560-7917.ES.2019.24.6.1700774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Matthijnssens J, Bilcke J, Ciarlet M, et al. Rotavirus disease and vaccination: impact on genotype diversity. Future Microbiol. 2009;4(10):1303-1316. doi: 10.2217/fmb.09.96 [DOI] [PubMed] [Google Scholar]
- 149.Vesikari T, Matson DO, Dennehy P, et al. ; Rotavirus Efficacy and Safety Trial (REST) Study Team . Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23-33. doi: 10.1056/NEJMoa052664 [DOI] [PubMed] [Google Scholar]
- 150.Perez-Schael I, Salinas B, Tomat M, et al. Efficacy of the human rotavirus vaccine RIX4414 in malnourished children. J Infect Dis. 2007;196(4):537-540. doi: 10.1086/519687 [DOI] [PubMed] [Google Scholar]
- 151.Naylor C, Lu M, Haque R, et al. ; PROVIDE study teams . Environmental enteropathy, oral vaccine failure and growth faltering in infants in Bangladesh. EBioMedicine. 2015;2(11):1759-1766. doi: 10.1016/j.ebiom.2015.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Taniuchi M, Platts-Mills JA, Begum S, et al. Impact of enterovirus and other enteric pathogens on oral polio and rotavirus vaccine performance in Bangladeshi infants. Vaccine. 2016;34(27):3068-3075. doi: 10.1016/j.vaccine.2016.04.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang H, Luo G, Zeng Y, et al. The distinct impact of maternal antibodies on the immunogenicity of live and recombinant rotavirus vaccines. Vaccine. 2019;37(30):4061-4067. doi: 10.1016/j.vaccine.2019.05.086 [DOI] [PubMed] [Google Scholar]
- 154.Sindhu KN, Cunliffe N, Peak M, et al. Impact of maternal antibodies and infant gut microbiota on the immunogenicity of rotavirus vaccines in African, Indian and European infants: protocol for a prospective cohort study. BMJ Open. 2017;7(3):e016577. doi: 10.1136/bmjopen-2017-016577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Soares-Weiser K. Rotavirus vaccine schedules: a systematic review of safety and efficacy from randomized controlled trials and observational studies of childhood schedules using RV1 and RV5 vaccines, report to WHO/Initiative for Vaccine Research 2012. Published 2012. Accessed March 5, 2021. https://www.who.int/immunization/sage/meetings/2012/april/Soares_K_et_al_SAGE_April_rotavirus.pdf
- 156.Lopman BA, Pitzer VE, Sarkar R, et al. Understanding reduced rotavirus vaccine efficacy in low socio-economic settings. PLoS One. 2012;7(8):e41720. doi: 10.1371/journal.pone.0041720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gladstone BP, Ramani S, Mukhopadhya I, et al. Protective effect of natural rotavirus infection in an Indian birth cohort. N Engl J Med. 2011;365(4):337-346. doi: 10.1056/NEJMoa1006261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bar-Zeev N, Tate JE, Pecenka C, et al. ; VACSURV Consortium . Cost-effectiveness of monovalent rotavirus vaccination of infants in Malawi: a postintroduction analysis using individual patient-level costing data. Clin Infect Dis. 2016;62(suppl 2):S220-S228. doi: 10.1093/cid/civ1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bruijning-Verhagen P, van Dongen JAP, Verberk JDM, et al. Updated cost-effectiveness and risk-benefit analysis of two infant rotavirus vaccination strategies in a high-income, low-endemic setting. BMC Med. 2018;16(1):168. doi: 10.1186/s12916-018-1134-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.van Hoek AJ, Ngama M, Ismail A, et al. A cost effectiveness and capacity analysis for the introduction of universal rotavirus vaccination in Kenya: comparison between Rotarix and RotaTeq vaccines. PLoS One. 2012;7(10):e47511. doi: 10.1371/journal.pone.0047511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Debellut F, Jaber S, Bouzya Y, et al. Introduction of rotavirus vaccination in Palestine: An evaluation of the costs, impact, and cost-effectiveness of Rotarix and Rotavac. PLoS One. 2020;15(2):e0228506. doi: 10.1371/journal.pone.0228506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Karafillakis E, Hassounah S, Atchison C. Effectiveness and impact of rotavirus vaccines in Europe, 2006-2014. Vaccine. 2015;33(18):2097-2107. doi: 10.1016/j.vaccine.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 163.Rha B, Tate JE, Payne DC, et al. Effectiveness and impact of rotavirus vaccines in the United States: 2006-2012. Expert Rev Vaccines. 2014;13(3):365-376. doi: 10.1586/14760584.2014.877846 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Methods and Statistical Analysis
eTable 1. Included Criteria During Selection Process
eTable 2. The Criteria for grading methodological quality
eTable 3. The basis for grouping in sub-analysis
eTable 4. Characteristics of the Included RCTs, Case-control and Cohort studies
eTable 5. Estimated Pooled Vaccine Protection in Cohort Studies
eTable 6. Estimated Pooled Vaccine Protection against Severe RVGE Stratified by Income Development at Different Follow-up Times
eTable 7. Estimated Pooled Vaccine Protection against Severe RVGE Stratified by Income Development
eTable 8. Estimated Pooled Vaccine Protection against Severe AGE Stratified by Income Development at Different Follow-up Times
eTable 9. Estimated Pooled Vaccine Protection against RVGE Stratified by Income Development at Different Follow-up Times
eTable 10. Estimated Pooled Vaccine Protection against RVGE Hospitalization Stratified by Income Development at Different Follow-up Times
eTable 11. Estimated Pooled Vaccine Protection against RVGE Stratified by Income Development
eTable 12. Estimated Pooled Vaccine Protection against RVGE Hospitalization stratified by income development
eTable 13. Indirect Comparisons between RV1 and RV5 Stratified by Income Development
eTable 14. Indirect Comparisons between Different Vaccines in the Same Region
eTable 15. Random-effect Model of Strain-specific Rotavirus Vaccine Protection
eFigure 1. Flow Diagram
eFigure 2. The Risk of Serious Adverse Events Between Vaccine and Placebo Groups at Different Follow-up Times
eFigure 3. The Risk of Intussusception Between Vaccine and Placebo Groups at Different Follow-up Times
eFigure 4. All-Cause Mortality in Vaccine and Placebo Groups During the Follow-up Period
eFigure 5. Sensitivity Analysis
eFigure 6. Funnel Plots
eReferences.


